INTRODUCTION
Myocarditis is an inflammation of the heart muscle due to infiltration of cardiac tissue with proinflammatory blood cells.[1] It is well established that SARS-CoV-2 can cause a wide range of cardiovascular problems, including myocarditis.[2] Additionally, there have been recent reports about myocarditis associated with SARS-CoV-2 mRNA COVID-19 vaccines.[3] We present a case of myocarditis with severe left ventricular (LV) dysfunction in a young man, which was temporally associated with the BNT162b2 mRNA COVID-19 vaccine, as well as a review and summary of the available literature on this condition.
CASE DESCRIPTION
A 34-year-old man presented with a three-day history of central chest pain. He recalled several weeks of progressive exertional dyspnoea associated with palpitations prior to admission. He denied any fever or exposure to patients with COVID-19 infection. There was no preceding viral illness. He had a history of mild childhood asthma and was an ex-smoker with a five pack-year history. He had received the second dose of BNT162b2 mRNA COVID-19 vaccine ten weeks prior to presentation.
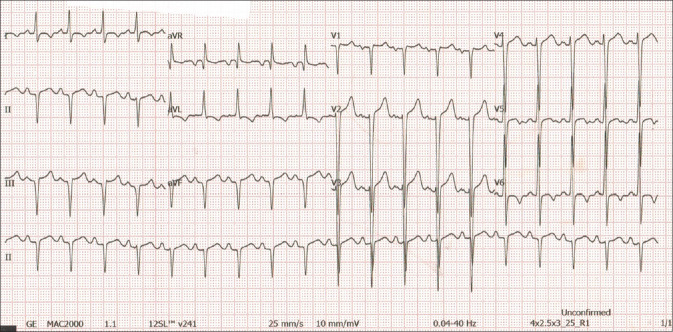
On admission, the patient’s blood pressure was 153/95 mmHg and heart rate was 117/min. His lungs were clear on auscultation and there was no pedal oedema. Heart sounds were normal. Electrocardiography (ECG) showed ST-segment and T wave changes, predominantly over the anterior and lateral leads [Figure 1]. Blood investigation revealed the following: creatine kinase (CK) 221 u/L (46–171 u/L); CK-MB 14.9 ng/mL (<5 ng/mL); troponin I 4.09 ng/mL (<0.06 ng/mL); and brain natriuretic peptide 1,150 pg/mL. Treatment with dual antiplatelet medication and anticoagulation was commenced for presumed acute coronary syndrome.
Figure 1.
ECG upon arrival shows sinus tachycardia, left axis deviation, ST elevations in the anterior and lateral leads, inferior Q waves and T wave inversions in the lateral leads.
Echocardiography performed at the bedside showed a large left ventricular thrombus (46 mm × 11 mm), a dilated left ventricle (LV) and globally reduced LV function (ejection fraction 22%) [Figure 2 and Supplementary materials 1–2, Appendix]. Computed tomography (CT) of the coronary arteries showed patent coronary vessels, a calcium score of zero and no evidence of atherosclerotic disease. There was mild atelectasis at the lung bases. Antiplatelet medications were withdrawn based on CT and echocardiographic findings, and the patient was commenced on warfarin for the LV thrombus. Comprehensive medical therapy for LV failure, comprising sacubitril/valsartan, bisoprolol, spironolactone and dapagliflozin, was rapidly introduced and up-titrated.
Figure 2.
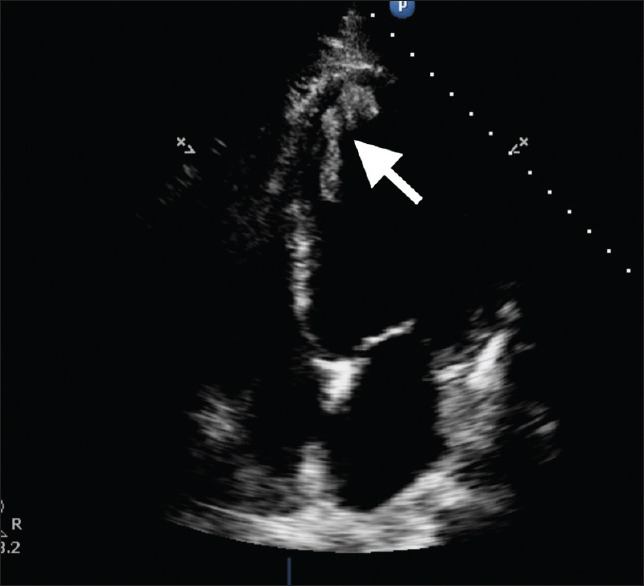
Apical 4-chamber echocardiography image shows a dilated left ventricle with thrombus (arrow).
Cardiac magnetic resonance (MR) imaging performed nine days after admission confirmed the echocardiographic findings of a globally reduced LV function; LV end-diastolic volume (LVEDV) was 293 mL and LV EF was 20%. The right ventricular function was also reduced, although to a lesser extent. There was late gadolinium enhancement of the apical inferior segment in a non-ischaemic pattern [Figure 3]. The large intraventricular thrombus had mostly resolved by the time the MR imaging was done. A small apical residual thrombus measuring 0.4 cm was still noted. No oedema was seen on T2-weighted imaging.
Figure 3.
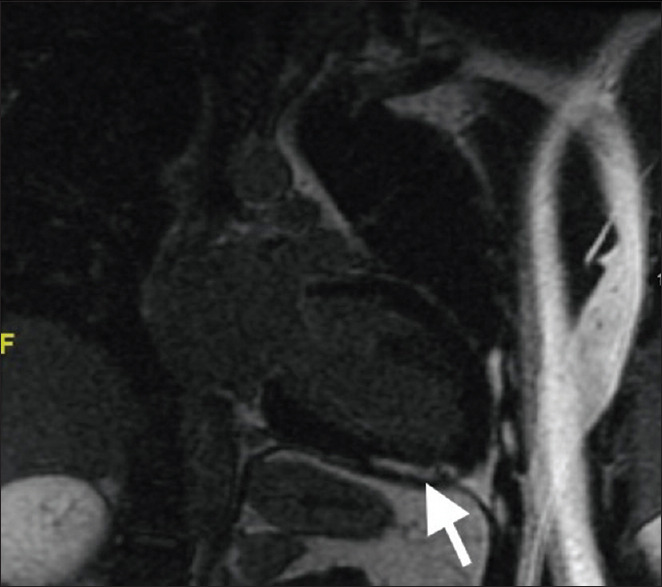
Two-chamber MR image shows late gadolinium enhancement (arrow), with diffuse epicardial and midwall delayed enhancement at the apical inferior segment.
Autoimmune screening, comprising anti-nuclear-factor, antineutrophil cytoplasmic antibodies, anti-dsDNA and cardiolipin antibodies, was normal. C-reactive protein (CRP) was 13.84 mg/L (<5.00). Protein C and Protein S were normal. There was no eosinophilia. Renal function, liver function and full blood count were also normal. SARS-CoV-2 polymerase chain reaction test of a nasopharyngeal and oropharyngeal swab was negative. A BIOFIRE® Respiratory 2.1 plus Panel of a nasopharyngeal and oropharyngeal swab was negative for the following pathogens: adenovirus, coronavirus 229E, coronavirus HKU1, coronavirus NL63, coronavirus OC43, Middle East respiratory syndrome coronovirus, SARS-CoV-2, human metapneumovirus, human rhinovirus/enterovirus, influenza A virus, influenza B virus, parainfluenza virus types 1–4, respiratory syncytial virus, Bordetella parapertussis, Bordetella pertussis, Chlamydia pneumoniae and Mycoplasma pneumoniae. Screening for hepatitis B and C, human immunodeficiency virus, syphilis, cytomegalovirus, Mycoplasma pneumoniae, Epstein-Barr virus and coxsackievirus was negative. The patient remained well and was discharged in a stable condition after ten days.
The patient was asymptomatic during follow-up in an outpatient clinic three months later. He denied any exertional dyspnoea or chest pain. LV systolic function had normalised with an EF of 62%. There was evidence of positive cardiac remodelling with reduced left ventricular and left atrial size. There was no residual LV thrombus [Supplementary materials 3–5, Appendix]. His blood pressure was 123/74 mmHg and heart rate was 68/min. He tolerated guideline-directed pharmacotherapy at target doses: sacubitril/valsartan 200 mg twice daily, bisoprolol 7.5 mg once daily, spironolactone 12.5 mg once daily and dapagliflozin 10 mg once daily, without side effects.
Our report on the occurrence of myocarditis with a transient severe LV dysfunction with intraventricular thrombus formation following a recent BNT162b2 mRNA COVID-19 vaccination could represent an adverse reaction following immunisation.
DISCUSSION
For more than 1.5 years, the COVID-19 pandemic has caused enormous human suffering, in terms of loss of lives, chronic health impairment and economic devastation. The way out of the pandemic appears to be through mass vaccination. There is, however, a rising concern regarding the safety of the novel m-RNA vaccines. Their risks and benefits need to be weighed carefully to sustain acceptance of the vaccines. It is of paramount importance to thoroughly investigate reports of side effects.
Our literature search for SARS-CoV-2 vaccine-associated myocarditis yielded eight publications with a total of 32 cases described [Table 1].[4,5,6,7,8,9,10,11] The characteristics of these patients are presented in Table 2. From the studies, it appears that predominantly young patients developed post-vaccine myocarditis. The average age was 26 years (14–56 years), and only male patients were affected. One patient had a documented comorbidity, obesity. Out of the 32 patients who developed myocarditis, 31 patients had received mRNA vaccines: BNT162b2 from Pfizer (n = 25) or ModernaTX mRNA-1273 (n = 6). One patient received the Johnson and Johnson’s vector-based vaccine (Ad.26.COV2.S). 88% of the patients developed symptoms after the second dose. Most patients developed symptoms early, with 23 (75%) patients showing symptoms within 72 hours. All patients had chest pain at presentation, and one-fifth complained of dyspnoea. Elevated cardiac markers are required for a diagnosis of myocarditis — all 32 patients had increased troponin levels. Reflecting the inflammatory nature of the problem, CRP was elevated in 93% of patients. Only 10% of patients had a normal ECG. The most common ECG abnormality was ST-segment changes (80% of cases). Most patients (88%) had preserved ejection fraction on echocardiography. Mean EF was 54% ±7.8% (range 34%–65%. Imaging of the coronary arteries was not routinely performed. Only about a third of the patients underwent CT coronary angiography and all had patent vessels. Cardiac MR imaging is the gold standard for diagnosing myocarditis in the absence of a myocardial biopsy. All patients showed evidence of late gadolinium enhancement and 90% of patients had MR imaging features of myocardial oedema. Treatment was focused on the inflammatory component — non-steroidal anti-inflammatory drugs (NSAIDs) (72%), colchicine (41%) and corticosteroids (25%) were most commonly used. Heart failure medications were given to four patients. Only one patient required hospitalisation beyond one week, and there were no reported deaths.
Table 1.
| Study | Age (yr)/gender | Type of vaccine/dose/duration of symptoms onset (day) | Presenting symptoms/troponin T | ECG changes/type of ECG changes | Echocardiogram, EF/cardiac MRI | Management | Outcome (duration of admission/survival) |
|---|---|---|---|---|---|---|---|
| Mouch et al.[11] | 24/M | Pfizer BNT/second/3 | Chest pain/elevated | Yes/diffuse ST elevation | Normal, 63%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID + colchicine) | <7 days/Yes |
|
| |||||||
| 20/M | Pfizer BNT/second/<1 | Chest pain/elevated | Yes/ST elevation V2-6 | Normal, 55%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID + colchicine) | <7 days/Yes | |
|
| |||||||
| 29/M | Pfizer BNT/second/2 | Chest pain/elevated | Yes/diffuse ST elevation, diffuse PR depression | Normal, 61%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID + colchicine) | <7 days/Yes | |
|
| |||||||
| 45/M | Pfizer BNT/ first/16 | Chest pain/elevated | Yes/ST elevation and depression | Normal, 50%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID + colchicine) | >7 days/Yes | |
|
| |||||||
| 16/M | Pfizer BNT/second/<1 | Chest pain/elevated | Yes/ST elevation | Normal, 59%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID + colchicine) | <7 days/Yes | |
|
| |||||||
| 17/M | Pfizer BNT/second/3 | Chest pain, abdominal pain/elevated | Yes/ST elevation | Normal, NA/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID + colchicine) | <7 days/Yes | |
|
| |||||||
| Albert et al.[10] | 24/M | Moderna mRNA/second/4 | Chest pain | Yes/sinus tachycardia | Normal, 65%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID); Anti-failure (B-blocker) | NA/Yes |
|
| |||||||
| Rosner et al.[9] | 28/M | Johnson/ first/5 | Chest pain, shortness of breath/elevated | Yes/ST elevation | Normal, 51%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (aspirin); Anti-failure (B-blocker, ACEI) | <7 days/Yes |
|
| |||||||
| 39/M | Pfizer BNT/second/3 | Chest pain, shortness of breath/elevated | Yes/PR depression | Reduced, 35%/suggestive MRI with myocardial oedema and LGE | Anti-failure (B-Blocker, ARB) | <7 days/Yes | |
|
| |||||||
| 39/M | Moderna mRNA/second/4 | Chest pain, shortness of breath/elevated | No/- | Normal, 61%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (IV steroid) | <7 days/Yes | |
|
| |||||||
| 24/M | Pfizer BNT/ first/7 | Chest pain/elevated | No/- | Normal, 53%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (ibuprofen, colchicine) | <7 days/Yes | |
|
| |||||||
| 19/M | Pfizer BNT/second/2 | Chest pain/elevated | No/- | Normal, 55%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (ibuprofen, colchicine) | <7 days/Yes | |
|
| |||||||
| 20/M | Pfizer BNT/second/3 | Chest pain/elevated | Yes/ST elevation | Normal, 50%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (ibuprofen) | <7 days/Yes | |
|
| |||||||
| 23/M | Pfizer BNT/second/3 | Chest pain/elevated | Yes/diffuse ST elevation | Normal, 58%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (colchicine); Anti-failure (B-blocker) | <7 days/Yes | |
|
| |||||||
| Marshall et al.[8] | 16/M | Pfizer BNT/second/2 | Chest pain/elevated | Yes/ST elevation, AV dissociation | Normal, NA/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID, methylprednisolone, IVIG) | <7 days/Yes |
|
| |||||||
| 19/M | Pfizer BNT/second/3 | Chest pain/elevated | Yes/diffuse ST elevation | Normal, NA/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID, colchicine, aspirin) | <7 days/Yes | |
|
| |||||||
| 17/M | Pfizer BNT/second/2 | Chest pain/elevated | Yes/diffuse ST elevation, nonspecific T changes | Normal, NA/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID) | <7 days/Yes | |
|
| |||||||
| 18/M | Pfizer BNT/second/2 | Chest pain/elevated | Yes/ST elevation | Normal, NA/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID, methylprednisolone, IVIG) | <7 days/Yes | |
|
| |||||||
| 17/M | Pfizer BNT/second/4 | Chest pain, shortness of breath/elevated | Yes/nonspecific T changes | Normal, NA/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID, aspirin, methylprednisolone, IVIG) | <7 days/Yes | |
|
| |||||||
| 16/M | Pfizer BNT/second/3 | Chest pain, palpation, shortness of breath/elevated | Yes/ST elevation | Normal, NA/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (prednisolone, IVIG) | <7 days/Yes | |
|
| |||||||
| 14/M | Pfizer BNT/second/2 | Chest pain, shortness of breath/elevated | Yes/ST elevation | Reduced, 47%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID); Anti-failure (furosemide) | <7 days/Yes | |
|
| |||||||
| McLean et al.[7] | 16/M | Pfizer BNT/second/2 | Chest pain/elevated | Yes/ST elevation | Normal, 61%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID, IVIG) | <7 days/Yes |
|
| |||||||
| Muthukumar et al.[6] | 52/M | Moderna mRNA/second/3 | Chest pain/elevated | Yes/right bundle branch block | Normal, 54%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID); Anti-failure (carvedilol, lisinopril) | <7 days/Yes |
|
| |||||||
| D’Angelo et al.[5] | 30/M | Pfizer BNT/second/3 | Chest pain/elevated | Yes/ST elevation, nonspecific T changes | Normal, NA/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (aspirin); Anti-failure (bisoprolol, prednisolone) | <7 days/Yes |
|
| |||||||
| Larson et al.[4] | 22/M | Moderna mRNA/second/3 | Chest pain/elevated | Yes/diffuse ST elevation | Normal, 50%/suggestive MRI with myocardial LGE | Anti-inflammatory (NASID, prednisolone) | <7 days/Yes |
|
| |||||||
| 31/M | Moderna mRNA/second/3 | Chest pain, shortness of breath/elevated | No/- | Reduced, 34%/suggestive MRI with myocardial LGE | – | <7 days/Yes | |
|
| |||||||
| 40/M | Pfizer BNT/ first/2 | Chest pain/elevated | Yes/diffuse ST elevation | Reduced, 47%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (colchicine, prednisolone) | <7 days/Yes | |
|
| |||||||
| 56/M | Pfizer BNT/second/3 | Chest pain/elevated | Yes/nonspecific T changes | Normal, 60%/suggestive MRI with myocardial oedema and LGE | – | <7 days/Yes | |
|
| |||||||
| 26/M | Pfizer BNT/second/3 | Chest pain/elevated | Yes/ST elevation | Normal, 60%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (colchicine) | <7 days/Yes | |
|
| |||||||
| 35/M | Pfizer BNT/second/2 | Chest pain/elevated | Yes/diffuse ST elevation | Normal, 50%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID) | <7 days/Yes | |
|
| |||||||
| 21/M | Pfizer BNT/second/4 | Chest pain/elevated | Yes/diffuse ST elevation | Normal, 54%/suggestive MRI with myocardial oedema and LGE | Anti-inflammatory (NASID) | <7 days/Yes | |
|
| |||||||
| 22/M | Moderna mRNA/second/2 | Chest pain/elevated | Yes/ST elevation | Normal, 53%/suggestive MRI with myocardial oedema and LGE | – | <7 days/Yes | |
ACEI: angiotensin-converting enzyme inhibitor, ARB: angiotensin receptor blocker, AV: atrial ventricle, IVIG: intravenous immunoglobulin, Johnson: Johnson & Johnson (Ad.26.COV2.S), LGE: late gadolinium enhancement, M: male, Moderna mRNA: ModernaTX (mRNA-1273), MRI: magnetic resonance imaging, NA: not available, NASID: non-steroidal anti-inflammatory drug, Pfizer BNT: Pfizer-BioNTech mRNA vaccine (BNT162b2)
Table 2.
Characteristics of patients with SARS-CoV-2 vaccine-associated myocarditis.
| Characteristic | n (%) |
|---|---|
| Age group (yr) (n=32) | |
|
| |
| Adolescents (0–17) | 8 (25.0) |
|
| |
| Younger adults (18–54) | 24 (75.0) |
|
| |
| Older adults (≥55) | 0 |
|
| |
| Gender (n=32) | |
|
| |
| Male | 32 (100) |
|
| |
| Female | 0 |
|
| |
| Underlying comorbidities (n=32) | |
|
| |
| Yes | 1 (3.1) |
|
| |
| No | 31 (96.9) |
|
| |
| COVID-19 vaccine (n=32) | |
|
| |
| Type of vaccine | |
|
| |
| Pfizer- BioNTech mRNA vaccine (BNT162b2) | 25 (78.1) |
|
| |
| ModernaTX (mRNA-1273) | 6 (18.8) |
|
| |
| Johnson & Johnson (Ad.26.COV2.S) | 1 (3.1) |
|
| |
| Dose of vaccine | |
|
| |
| First dose | 4 (12.5) |
|
| |
| Second dose | 28 (87.5) |
|
| |
| Duration to develop symptoms after vaccine (h) | |
|
| |
| <24 | 2 (6.3) |
|
| |
| 24–48 | 9 (28.1) |
|
| |
| 49–72 | 12 (40.6) |
|
| |
| >72 | 8 (25.0) |
|
| |
| COVID-19 status (n=32) | |
|
| |
| Negative COVID-19 PCR test | 32 (100) |
|
| |
| Clinical presentation (n=32) | |
|
| |
| Chest Pain | 32 (100) |
|
| |
| Shortness of breath | 7 (21.9) |
|
| |
| Laboratory investigations (n=32) | |
|
| |
| Elevated C-reactive protein | 30 (93.8) |
|
| |
| Elevated troponin T | 32 (100) |
|
| |
| Electrocardiogram (ECG) | |
|
| |
| ECG changes (n=32) | |
|
| |
| Yes | 29 (90.6) |
|
| |
| No | 3 (9.4) |
|
| |
| Type of ECG changes (n=29) | |
|
| |
| ST segment elevation or depression | 20 (69.0) |
|
| |
| PR interval depression | 2 (6.8) |
|
| |
| Sinus tachycardia | 1 (3.5) |
|
| |
| Nonspecific T wave changes | 4 (13.7) |
|
| |
| Bundle branch block | 1 (3.5) |
|
| |
| Atrial ventricle dissociation | 1 (3.5) |
|
| |
| Echocardiogram | |
|
| |
| Left ventricular ejection fraction (LVEF) (n=32) | |
|
| |
| Normal LVEF ≥50% | 28 (87.5) |
|
| |
| Reduced LVEF <50% | 4 (12.5) |
|
| |
| Mean LVEF (%) (n=24)a | 54±7.8 (EF max 65%, min 34%) |
|
| |
| Percutaneous/CT coronary angiogram (n=32) | |
|
| |
| Yes | 11 (34.4) |
|
| |
| No | 21 (65.6) |
|
| |
| Normal coronary angiogram (n=11)b | 11 (100) |
|
| |
| Cardiac MRI (n=32) | |
|
| |
| Evidence of cardiac oedema (epicardial/myocardial) | |
|
| |
| Yes | 30 (93.8) |
|
| |
| No | 2 (6.3) |
|
| |
| Evidence of late gadolinium enhancement | |
|
| |
| Yes | 32 (100) |
|
| |
| Management | |
|
| |
| ICU admission (n=15)c | |
|
| |
| Yes | 3 (20.0) |
|
| |
| No | 12 (80.0) |
|
| |
| Requirement of supplementary oxygen (n=24)d | |
|
| |
| Yes | 4 (16.7) |
|
| |
| No | 20 (83.3) |
|
| |
| Anti-inflammatory drugs (n=32) | |
|
| |
| Non-steroidal anti-inflammatory drugs | 23 (71.9) |
|
| |
| Colchicine | 13 (40.6) |
|
| |
| Corticosteroids | 8 (25.0) |
|
| |
| Intravenous immunoglobulin | 5 (15.6) |
|
| |
| Aspirin | 4 (12.5) |
|
| |
| Anti-failure drugs (n=32) | |
|
| |
| Beta-blocker | 4 (12.5) |
|
| |
| Angiotensin-converter enzyme inhibitor | 2 (6.3) |
|
| |
| Prognosis | |
|
| |
| Duration of admission (day) (n=31) | |
|
| |
| <7 | 30 (96.8) |
|
| |
| ≥8 | 1 (3.2) |
|
| |
| Survival (n=32) | |
|
| |
| Yes | 32 (100) |
a8 subjects were reported as normal LVEF with no quantitative EF stated. Data presented as mean ± standard deviation. bAll 11 subjects who underwent coronary angiogram had normal coronary angiogram. cData for 17 subjects were not available. dData for 8 subjects were not available. CT: computed tomography, ICU: intensive care unit, MRI: magnetic resonance imaging, PCR: polymerase chain reaction
Post-vaccination myocarditis is a well-recognised phenomenon. Smallpox, streptococcal, meningococcal C, tetanus and hepatitis B vaccines have all been associated with post-vaccination myocarditis.[12,13,14] It is often subclinical and only identified with active screening measures for myocardial injury. In keeping with that experience, most of the reported cases of COVID-19 vaccine-related myocarditis had mild disease. It is reasonable to assume that many subclinical cases avoid detection and that the true incidence of myocarditis/pericarditis after immunisation is underestimated. Studies with active screening measures to quantify the true extent of myocardial injury would be required to establish the real extent of the problem.
In addition to the published case reports, the Center for Disease Control and Prevention (CDC) is investigating 226 cases of verified vaccine-related myocarditis/pericarditis in young m-RNA vaccine recipients that have been reported to the Vaccine Adverse Event Reporting System (VAERS) at the time of writing of this paper. It is concerning that predominantly young males seem to be affected, while most countries still prioritise elderly people in their vaccination efforts. The CDC estimates that young people aged 12–24 years account for more than 50% of myocarditis cases, whereas they received less than 10% of the vaccines administered.[3]
At the time of this writing, Malaysia has vaccinated about 2 million people with two doses, representing about 6% of the population. Most vaccine recipients were in the older age groups. Younger persons would only receive vaccination at this stage if they were considered frontliners, such as our patient. The number of frontliners is estimated to be around 500,000.
Despite the seemingly benign course with mild left ventricular impairment and quick recovery, the long-term effects of COVID-19 vaccine-related myocarditis are currently unknown. The patient reported herein differs in two important ways from the patient population in the reviewed literature. First, his myocarditis was diagnosed at a later point after the vaccination and secondly, his disease was much more severe. Although cardiac MR imaging demonstrated late gadolinium enhancement, the absence of oedema is in keeping with the theory that his inflammation had started weeks earlier following vaccination and subsided by the time the diagnosis was made. Another concern is the patient’s large LV thrombus. Although, it is commonly seen in extensive anterior myocardial infarctions or hyper-eosinophilia, it is extremely rare to encounter LV thrombus in myocarditis. There have been reports of COVID-19-vaccination-associated thrombosis, specifically with the AstraZeneca COVID-19 vaccine (AZD1222).[15] Our patient made a complete recovery within three months despite the severity of his LV dysfunction at diagnosis. Cardioembolic phenomena were not observed. The observed positive outcome could be a consequence of prompt and aggressive treatment, but likewise might reflect the natural progression of vaccine related myocarditis which appears mild and transient in almost all cases reported to date.
Most patients reported in the literature received NSAIDs, colchicine or corticosteroids despite negative trials of immunosuppression for acute myocarditis. The role of NSAIDS in the acute phase of myocarditis is controversial and has been associated with impaired myocardial healing and increased mortality. Treatment of myocarditis should include supportive therapy for symptoms of acute heart failure and chronic heart failure with evidence-based pharmacotherapy.[16]
There are currently no guideline changes with regard to the use of mRNA vaccines in younger males. Only the Pfizer-BioNTech vaccine has emergency use authorisation from the US Food and Drug Administration for adolescents aged 12–15 years. Clinicians are encouraged to consider myocarditis in patients who develop acute chest pain or dyspnoea within days of vaccination.
It is important to interpret our current case and analysis of previously published data with caution. The benefits of COVID-19 vaccination clearly outweigh the risk of suffering a rare and usually mild complication. Public trust in vaccines is of paramount importance for controlling the COVID-19 pandemic. Hence, physicians must be seen to investigate and report and put into context any potential side effects of the vaccines without fear or favour.
In conclusion, we present a case of myocarditis with resulting severe LV dysfunction and LV thrombus following the BNT162b2 mRNA COVID-19 vaccine. Our case differed from previously documented cases, which were predominantly mild. Reassuringly, the patient made full functional recovery with resolution of the LV clot within three months. Prospective studies are essential to elucidate the natural progression of this vaccine-related disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
APPENDIX
The videos (supplementary materials 15) are available online
Supplementary material 1: (ECHO Pre).
Supplementary material 2: (ECHO Pre).
Supplementary material 3: (ECHO Post).
Supplementary material 4: (ECHO Post).
Supplementary material 5: (ECHO Post).
REFERENCES
- 1.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–48. doi: 10.1093/eurheartj/eht210. 2648a-48d. [DOI] [PubMed] [Google Scholar]
- 2.Vasquez-Bonilla WO, Orozco R, Argueta V, Sierra M, Zambrano LI, Muñoz-Lara F, et al. A review of the main histopathological findings in coronavirus disease 2019. Hum Pathol. 2020;105:74–83. doi: 10.1016/j.humpath.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenco M. CDC confirms 226 cases of myocarditis after COVID-19 vaccination in people 30 and under. AAP News June 10. 2021. [[Last accessed on 2021 Jun 10]]. Available from: https://publications.aap.org/aapnews/news/17152/CDC-confirms-226-cases-of-myocarditis-after-COVID .
- 4.Larson KF, Ammirati E, Adler ED, Cooper LT, Jr, Hong KN, Saponara G, et al. Myocarditis after BNT162b2 and mRNA-1273 Vaccination. Circulation. 2021;144:506–8. doi: 10.1161/CIRCULATIONAHA.121.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Angelo T, Cattafi A, Carerj ML, Booz C, Ascenti G, Cicero G, et al. Myocarditis after SARS-CoV-2 vaccination: A vaccine-induced reaction? Can J Cardiol. 2021;37:1665–7. doi: 10.1016/j.cjca.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muthukumar A, Narasimhan M, Li QZ, Mahimainathan L, Hitto I, Fuda F, et al. In depth evaluation of a case of presumed myocarditis following the second dose of COVID-19 mRNA vaccine. Circulation. 2021;144:487–98. doi: 10.1161/CIRCULATIONAHA.121.056038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLean K, Johnson TJ. Myopericarditis in a previously healthy adolescent male following COVID-19 vaccination: A case report. Acad Emerg Med. 2021;28:918–21. doi: 10.1111/acem.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS, et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021;148:e2021052478. doi: 10.1542/peds.2021-052478. [DOI] [PubMed] [Google Scholar]
- 9.Rosner CM, Genovese L, Tehrani BN, Atkins M, Bakhshi H, Chaudhri S, et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021;144:502–5. doi: 10.1161/CIRCULATIONAHA.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albert E, Aurigemma G, Saucedo J, Gerson DS. Myocarditis following COVID-19 vaccination. Radiol Case Rep. 2021;16:2142–5. doi: 10.1016/j.radcr.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu Mouch S, Roguin A, Hellou E, Ishai A, Shoshan U, Mahamid L, et al. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021;39:3790–3. doi: 10.1016/j.vaccine.2021.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makaryus AN, Revere DJ, Steinberg B. Recurrent reversible dilated cardiomyopathy secondary to viral and streptococcal pneumonia vaccine-associated myocarditis. Cardiol Rev. 2006;14:e1–4. doi: 10.1097/01.crd.0000174803.68564.65. [DOI] [PubMed] [Google Scholar]
- 13.Barton M, Finkelstein Y, Opavsky MA, Ito S, Ho T, Ford-Jones LE, et al. Eosinophilic myocarditis temporally associated with conjugate meningococcal C and hepatitis B vaccines in children. Pediatr Infect Dis J. 2008;27:831–5. doi: 10.1097/INF.0b013e31816ff7b2. [DOI] [PubMed] [Google Scholar]
- 14.Dilber E, Karagoz T, Aytemir K, Ozer S, Alehan D, Oto A, et al. Acute myocarditis associated with tetanus vaccination. Mayo Clin Proc. 2003;78:1431–3. doi: 10.4065/78.11.1431-a. [DOI] [PubMed] [Google Scholar]
- 15.Thakur KT, Tamborska A, Wood GK, McNeill E, Roh D, Akpan IJ, et al. Clinical review of cerebral venous thrombosis in the context of COVID-19 vaccinations: Evaluation, management, and scientific questions. J Neurol Sci. 2021;427:117532. doi: 10.1016/j.jns.2021.117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, et al. Heart Failure Society of America. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1: (ECHO Pre).
Supplementary material 2: (ECHO Pre).
Supplementary material 3: (ECHO Post).
Supplementary material 4: (ECHO Post).
Supplementary material 5: (ECHO Post).