Abstract
Introduction:
We aimed to describe the extrapulmonary manifestations of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, including their frequency, onset with respect to respiratory symptoms, pathogenesis and association with disease severity.
Methods:
We searched the MEDLINE and Embase databases for SARS-CoV-2-related studies. Meta-analysis, observational studies, case series and case reports published in English or Chinese between 1 January 2020 and 1 May 2020 were included. Reports with only paediatric or obstetric cases were excluded.
Results:
169 articles were included. Early manifestations (preceding respiratory symptoms until Day 6 of onset) included olfactory and gustatory disturbance (self-reported in up to 68% and 85% of cases, respectively), gastrointestinal symptoms (up to 65.9%) and rash (up to 20.4%). From Day 7 onwards, hypercytokinaemia, paralleled multi-organ complications including acute cardiac injury (pooled incidence of 17.7% in 1,412 patients, mostly with severe disease and 17.4% mortality), kidney and liver injury (up to 17% and 33%, respectively) and thrombocytopenia (up to 30%). Hypercoagulability resulted in venous thromboembolic events in up to 31% of all patients. Uncommon disease presentation and complications comprised Guillain-Barré syndrome, rhabdomyolysis, otitis media, meningoencephalitis and spontaneous pneumomediastinum.
Conclusion:
Although the systemic manifestations of SARS-CoV-2 infection are variegated, they are deeply interwoven by shared mechanisms. Two phases of extrapulmonary disease were identified: (a) an early phase with possible gastrointestinal, ocular and cutaneous involvement; and (b) a late phase characterised by multiorgan dysfunction and clinical deterioration. A clear, multidisciplinary consensus to define and approach thromboinflammation and cytokine release syndrome in SARS-CoV-2 is needed.
Keywords: Complications, COVID-19, extrapulmonary, SARS-CoV-2
INTRODUCTION
As COVID-19 decimates healthcare systems worldwide, our understanding of the disease has extended to manifestations beyond those of the respiratory system. The ubiquitous expression of angiotensin-converting enzyme 2 (ACE-2) receptors in multiple organs, including the lungs, kidneys, endothelium, skin, brain and the gastrointestinal tract,[1] portends the diverse and multifaceted presentation of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. Additionally, endocytosis of ACE-2 during viral entry in the lungs has been implicated as a driver of cytokine release syndrome (CRS), with resultant endotheliopathy, thromboinflammation and systemic organ dysfunction.[2] As COVID-19 emerges as a complex disease with wide-ranging symptoms, we performed a systematic review with the aim of comprehensively delineating the myriad of extrapulmonary manifestations and complications. In this study, we abstracted the frequency of each manifestation and described their pathogenesis and association with disease severity.
METHODS
We searched the MEDLINE and Embase databases for meta-analyses, observational studies, case series and case reports on laboratory-confirmed or clinically diagnosed COVID-19 in English or Chinese, published between 1 January 2020 and 1 May 2020. We used the search terms ‘COVID-19’, ‘coronavirus infection’, ‘coronavirus disease 2019’ and ‘SARS-CoV-2’. Reports with only paediatric or obstetric cases were excluded. For each manifestation, we abstracted the following: (a) prevalence; (b) description, including onset with respect to respiratory symptoms; (c) correlation with overall disease course; and (d) effect on prognosis.
Two reviewers independently screened studies for inclusion by reading the title and abstract. Each reviewer was blinded to the decision of the other reviewer. The reviewers resolved disagreements by discussion and by reading the full text if necessary. Selected articles were divided among the study team members for Level 2 screening and data extraction. Relevant secondary references were included. Two members of the team cross-checked extracted data. We omitted formal quality assessment, as we aimed to include case reports and case series and to capture the full spectrum of clinical manifestations. However, key limitations of selected studies were highlighted.
The frequency of manifestation was reported as a range across the studies. We abstracted the description of the manifestation from the first study and added information from each subsequent study until saturation was achieved. Effects on the prognosis were reported using estimates for mortality, requirement of intensive care or overall severe disease. Data was extracted and described as per the Synthesis Without Meta-analysis reporting guidelines. This non-funded review has been registered on PROSPERO (registration no. CRD42020184524).
RESULTS
We identified 3,157 articles from the literature search and 49 articles from secondary references. After removing duplicates, 2,797 articles remained for first-level screening. We reviewed the full text of 336 articles, of which 169 were included [Figure 1]. Disease severity was stratified into mild to moderate versus severe and critical, as defined by the WHO-China Joint Mission. The definition of severe disease comprised dyspnoea, respiratory frequency ≥30/minute, blood oxygen saturation ≤93%, PaO2/FiO2 (arterial oxygen partial pressure/fraction of inspired oxygen) ratio <300 and/or lung infiltrates >50% of the lung field. Critical disease was characterised by respiratory failure, septic shock and/or multiple organ dysfunction.[3]
Figure 1.
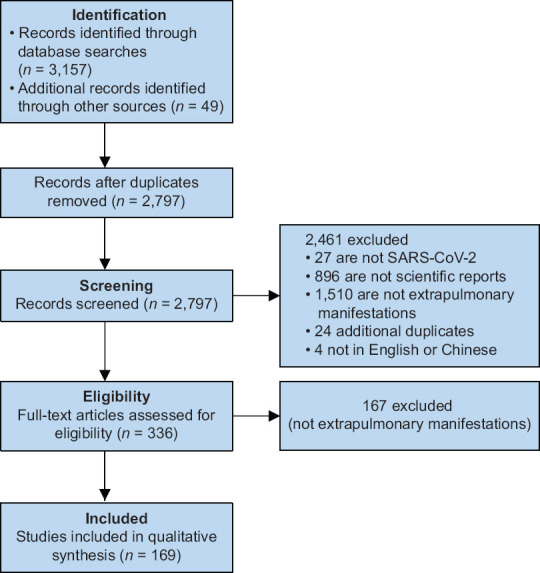
PRISMA flow diagram shows the inclusion process for the study.
Due to a lack of a universally accepted definition of CRS and the lack of routine checks of cytokine levels, the incidence of CRS in SARS-CoV-2 infection remains poorly characterised. Five reports from China (21–452 patients) demonstrated elevated levels of pro-inflammatory cytokines, mainly interleukin-6 (IL-6), interleukin-8, interleukin-10 and interferon-γ, which were associated with indices of inflammation such as leucopenia, lymphopenia, and elevated C-reactive protein (CRP), ferritin and D-dimer levels.[4,5,6,7,8] Also, in a study of 191 patients, the onset of acute respiratory distress syndrome (ARDS) paralleled an increase in IL-6 on Day 12 of illness.[9] Interestingly, in a study that performed daily transcriptome studies on a patient, expression of pro-inflammatory genes peaked on Day 6 of illness, but only the expression of IL-1A and IL-1B preceded the peak in oxygen requirement.[10] Hypercytokinaemia was also associated with acute cardiac and liver injury and higher mortality.[4,5,6,7,8] These findings were corroborated in a meta-analysis of 21 studies including 3,377 patients and 33 laboratory parameters, where elevated IL-6, IL-10 and serum ferritin levels were strong discriminators for severe disease.[11] Seven patients displayed laboratory features of hemophagocytic lymphohistiocytosis, based on the H-score in one study.[12]
Underlying immune dysregulation accounting for the hypercytokinaemia was characterised by increased pathogenic T-helper (Th) 1 cells and CD14+CD16+ monocytes[13] but reduced memory Th (CD3+CD4+CD45RO+) and regulatory (CD3+CD4+CD25+CD127low+) T cells.[6] Declining absolute CD4+ and CD8+ counts and increased functional exhaustion markers (including PD-1 and Tim-3 expression) portended elevation of IL-6, IL-10 and tumour necrosis factor-α, and the requirement for intensive care unit care.[14,15,16]
Haematologic manifestations
Hypercoagulability
As a result of thromboinflammation, thrombotic events occurred at a higher rate in SARS-CoV-2-infected individuals than in matched patients with influenza, particularly in patients with more severe disease.[17] Whole blood thromboelastography of 30 patients with elevated CRP levels demonstrated elevated levels of D-dimer, fibrinogen, factor VIII and von Willebrand factor.[18] In addition, in 16 patients with ARDS, elevation in D-dimer and fibrinogen levels paralleled the rise in IL-6.[19] Another study of 94 patients showed lower antithrombin and higher fibrinogen levels as well as reduced prothrombin and thrombin time in patients with severe disease.[20] Other mechanisms for the excess thrombotic risk include endotheliitis via direct viral invasion,[21,22] complement-mediated endothelial injury[22] and induction of antiphospholipid antibodies.[23,24,25]
Venous thromboembolism (VTE) was the most common form of thrombotic complication and afflicts 10%–31% of patients (seven studies, sample size 81–184).[17,26,27,28,29,30,31] These events usually presented within the first two weeks of disease[28,32,33,34,35,36] in spite of prophylactic anticoagulation.[17,28,29,34,35] Predictors included elevated D-dimer level,[27,29] age and coagulopathy (defined as spontaneous prolongation of prothrombin time >3 seconds or activated partial thromboplastin time >5 seconds).[28] VTE and disseminated intravascular coagulation were associated with severe disease and mortality.[26,30] Other forms of thrombosis, including ischaemic stroke,[24,25,28,37,38,39,40,41,42] cerebral venous sinus thrombosis,[43,44] acute myocardial infarction,[21,28] systemic arterial embolism,[28] arteriosclerosis obliterans,[45] mesenteric ischaemia,[21] glomerular fibrin thrombi,[46] and digital and skin necrosis,[22,24,47] have been reported. Small fibrinous thrombi were also found in the pulmonary arterioles of ten patients on autopsy.[48]
Blood count anomalies
Lymphopenia was found in 26.1%–85%[4,9,16,29,49,50,51,52,53,54,55,56,57,58,59,60] of patients. Low lymphocyte count at baseline, decreasing lymphocyte count over time[4,9,11,16,54,55,57,61,62,63,64] and reduced lymphocyte-to-leucocyte ratio[65] were associated with severe disease and mortality. A lymphoplasmacytoid subset of reactive lymphocyte population was reported in 69% of patients.[50] The mechanisms for lymphopenia include: (a) hypercytokinaemia-induced apoptosis[6,16]; (b) direct viral destruction via ACE-2 receptor[66]; and (c) translocation into affected organs.[67] In addition, leucopenia (9%–41%),[4,9,29,50,51,52,53,55,57,64,68,69] leucocytosis (1.9%–44.7%),[11,51,55,56,57,58,63,64,68] neutrophilia (38%–60%)[4,11,51,57,58,63,65,68] and eosinopenia (52.9%–81.2%)[11,54,68] were commonly observed. Leucocytosis was associated with severe disease (27.7% vs. 9.3% in mild disease).[51] Thrombocytopenia was seen as a late manifestation in 11.4%–30% of patients, with the nadir observed on Day 15–20 of illness,[51,70,71] and was associated with mortality.[70,72]
Neurologic manifestations
Strokes
Based on the literature review censored on 1 May 2020, strokes were reported in 32 patients (27 ischemic strokes, three haemorrhagic strokes and two cerebral venous sinus thrombosis).[24,25,37,38,39,40,41,42,43,44,73] Strokes occurred synchronously with respiratory symptoms until 33 days after symptom onset. Additionally, strokes have been reported in pre-symptomatic patients [Table 1]. Although most patients were elderly with cardiovascular risk factors, a case series and one case report described strokes in patients <50 years old.[38,43] Positive antiphospholipid antibodies (lupus anticoagulant, anticardiolipin immunoglobulin M [IgM] and immunoglobulin A [IgA], anti-β2-glycoprotein-I IgM, IgA and immunoglobulin G [IgG]) were described in nine patients, eight of whom suffered multiple cerebral infarcts.[23,24,25] Table 1 summarises the characteristics of SARS-CoV-2 patients with acute stroke.
Table 1.
Reports of strokes in patients with severe acute respiratory syndrome coronavirus-2.
| Study | n | Type of event | Presentation | Radiological anatomy/vascular territory | Time from disease onset (day) | Anti-phospholipid antibody status | Coagulation parameters |
|---|---|---|---|---|---|---|---|
| Mao et al.[37] | 5 | 4 ischaemic strokes, 1 haemorrhagic stroke | Not described | Not described | 1–18 (median 9) | Not described | Not described |
|
| |||||||
| Helms et al.[41] | 3 | All ischaemic strokes | Incidental finding on MR imaging done for encephalopathy | Small hyperintensity lesions on MR imaging | Not described | Not described | Not described |
|
| |||||||
| Zhang et al.[24] | 3 | All ischaemic strokes | Aged 65–70 yr, headache and digital ischaemia | Multifocal infarcts including frontal, parietal, occipital lobes, basal ganglia, brainstem and cerebellum | 10, 18, 33 | Positive anticardiolipin IgA, anti-β2-glycoprotein-I IgA and IgG in all patients | Elevated PT, aPTT, D-dimer and reduced platelets |
|
| |||||||
| Oxley et al.[38] | 5 | All ischaemic strokes | Aged 33–49 yr, focal neurological deficits NIHSS score 13-23 | 1 internal carotid artery, 3 MCA, 1 posterior cerebral artery | Not described | Not described | Prolonged PT or aPTT in 3 patients, elevated D-dimer and fibrinogen in 3 patients |
|
| |||||||
| Beyrouti et al.[25] | 6 | All ischaemic strokes | Aged 53–85 yr, acute confusion with focal neurological deficits | 5 multifocal infracts, 2 patients developed ischaemic stroke despite therapeutic anticoagulation | −2 to 24 (median 12.5) | 5 patients with lupus anticoagulant, 1 patient with medium-titre IgM anticardiolipin | Prolonged PT or aPTT in 2 patients, elevated fibrinogen in 4 patients, elevated D-dimer in all patients |
|
| |||||||
| Avula et al.[39] | 4 | All ischaemic strokes | Aged 73–88 yr, focal neurological deficits, NIHSS score 2-36 | 2 MCA, 1 frontal lobe, 1 medial temporal lobe | 1–3 (median 2) | Not described | Prolonged PT or aPTT in 2 patients, elevated D-dimer in 2 patients |
|
| |||||||
| Gu et al.[74] | 1 | Ischaemic stroke | Aged 78 yr with right-sided weakness, NIHSS score 2. A physician involved in treatment contracted COVID-19 3 days later | CT brain unremarkable | −2 | Not described | Normal PT and aPTT |
|
| |||||||
| He et al.[40] | 1 | Ischaemic stroke with concurrent subarachnoid hemorrhage | Aged 70 yr with anisocoria | Not described | 28 | Not described | Severe thrombocytopenia and elevated D-dimer |
|
| |||||||
| Zhou et al.[42] | 1 | Ischaemic stroke | Aged 75 yr with bilateral deep vein thrombosis | Bilateral cerebral infarcts involving the middle and anterior cerebral artery on the right and anterior cerebral artery on the left | 15 | Not described | Normal coagulation profile, elevated D-dimer |
|
| |||||||
| Sharifi- Razavi et al.[73] | 1 | Haemorrhagic stroke | Aged 75 yr, drowsiness and extensor plantars | Massive intracerebral haemorrhage in the right hemisphere, accompanied by intraventricular and subarachnoid haemorrhage | 3 | Not described | Prolonged aPTT, normal platelet count |
|
| |||||||
| Hughes et al.[44] | 1 | Cerebral venous sinus thrombosis | Aged 59 yr with headache and weakness | Cerebral venous sinus thrombosis | Not described | Not described | Not described |
|
| |||||||
| Garaci et al.[43] | 1 | Cerebral venous sinus thrombosis | Aged 44 yr with drowsiness, aphasia and hemiparesis with concurrent pulmonary embolism | Empty delta sign in the vein of Galen, straight sinus and in the torcular herophili due to dural sinus thrombosis with poor representation of left internal cerebral vein | 14 | Absence of anticardiolipin and anti-β2 glycoprotein IgM and IgG | Severe thrombocytopenia and elevated D-dimer |
aPTT: activated partial thromboplastin time, CT: computed tomography, IgA: immunoglobulin A, IgG: immunoglobulin G, IgM: immunoglobulin M, MCA: middle cerebral artery, MR: magnetic resonance, NIHSS: National Institutes of Health Stroke Scale, PT: prothrombin time
Guillain-Barré syndrome and Miller Fisher syndrome
Five Italian patients with Guillain-Barré syndrome (GBS) developed flaccid weakness on Day 5–10 of COVID-19 infection. Electrophysiologic studies revealed a mix of axonal or demyelinating patterns of injury. Antiganglioside antibodies were negative in those who were tested.[75] One patient developed fever and cough eight days after the GBS diagnosis. The nasopharyngeal swab was positive for SARS-CoV-2. In this study, the authors evoked the possibility of a parainfectious mechanism for GBS that deviated from the usual post-infectious autoimmune aetiology.[76] Two patients were diagnosed with Miller Fisher syndrome (MFS) on Days 3 and 5 of illness. One carried anti-GD1b IgG antibodies. Both patients reported ageusia that accompanied the typical symptoms of MFS, such as diplopia, ataxia and areflexia.[77]
Seizures and encephalitis
Seizures were uncommon and reported in only four patients in the included studies.[37,60,78,79] One patient with fever, headache and sore throat suffered generalised seizures nine days later. Brain magnetic resonance (MR) imaging showed right mesial temporal lobe and hippocampal hyperintense signal changes. Lumbar puncture revealed SAR-CoV-2 in the cerebrospinal fluid.[79] Acute necrotising encephalopathy was reported in another patient three days after the initial fever and cough, and brain MR imaging demonstrated bilateral haemorrhagic rim-enhancing lesions in the thalami and medial temporal lobes.[80]
Elevated creatine kinase and rhabdomyolysis
In a meta-analysis of 3,600 patients, elevated creatine kinase (CK) afflicted 28.6% of patients with severe disease, compared to 16.7% of patients with mild disease.[51] Only 0.2% of 1,099 patients suffered rhabdomyolysis in one study.[49] A case report described a patient with CK of 13,581 U/L who presented with fever and respiratory symptoms.[81] Muscle involvement by SARS-CoV-2 could be mediated by viral binding to the skeletal muscle ACE-2 receptor and circulating viral toxins that destroy muscle cell membranes.[37]
Cardiac manifestations
Acute cardiac injury
The pooled incidence of acute cardiac injury, defined as troponin rise or cardiac dysfunction, was 17.7% from a total of 1,412 COVID-19 patients (ten cohort studies, sample sizes 41–416).[4,59,62,82,83,84,85,86,87,88] In this sample, 70.8% (n = 1,000) had severe disease and 17.4% died. In another meta-analysis of 1,527 patients, 8.0% of patients suffered acute cardiac injury.[89] Acute cardiac injury was more prevalent in patients with underlying cardiovascular disease[86,88,89] and non-survivors.[9,59,62,90] The mean mortality rate was 54.4% (87/150) in patients with acute cardiac injury compared to 9.0% (35/386) in those without.[86,87,88] Patients with acute cardiac injury were at higher risk of malignant arrhythmias[86] and tended to require intensive care and mechanical ventilation.[89] There were limited reports on the proportion of patients requiring percutaneous coronary intervention or who developed acute myocardial infarction from atherosclerotic plaque rupture. Postmortem histology of a patient with ST-segment elevation myocardial infarction revealed lymphocytic endotheliitis of the submucosal cardiac vessels.[21]
Heart failure and myocarditis
Heart failure was described in 6.5%–23% of all patients[9,51,60] and up to 52% of non-survivors.[9,90] SARS-CoV-2 infection exacerbated pre-existing heart failure in a case series.[91] Myocarditis was observed in 12.5% of 112 patients (diagnosis based on raised cardiac enzymes, electrocardiogram and echocardiogram).[85] Seven patients with viral myocarditis or myopericarditis presented with cardiogenic shock and/or severe left ventricular hypokinesia.[92,93,94,95,96,97,98] Cardiac MR imaging demonstrated myocardial oedema and transmural late gadolinium enhancement.[93,95,98] One patient with acute myocarditis presented with reverse Takotsubo syndrome.[98] Two patients with myocarditis received endomyocardial biopsy. In the first patient, cytopathic interstitial inflammatory cells containing coronavirus particles were seen on electron microscopy.[96] In the other patient, T lymphocytes diffusely infiltrated the myocardial interstitium despite the absence of SARS-CoV-2 on molecular analysis.[98] Pericardial effusion was reported in 18.8% of 112 patients, particularly those with severe disease or myocarditis.[93,94]
Gastrointestinal manifestations
Gastrointestinal symptoms
Due to heterogeneity in patient populations and symptom definitions, the prevalence of gastrointestinal symptoms in COVID-19 patients ranged widely, from 12.8% to 65.9%.[65,82,99,100,101,102,103,104] However, in a meta-analysis that included 4,243 patients, the pooled prevalence of all gastrointestinal symptoms was only 17.6% (anorexia 26.8%, diarrhoea 12.5%, nausea and vomiting 10.2%, abdominal pain 9.2%).[105] Gastrointestinal symptoms were the only presenting symptoms in 28.4% of 74 patients in one study.[106] A patient developed respiratory symptoms only nine days after the onset of haemorrhagic colitis.[107] Interestingly, viral shedding in stools was reported in up to 48.1% of patients, including patients without diarrhoea.[105] Also, 70.3% of patients continued to have positive stool viral RNA even after demonstrating negative results in respiratory samples.[105] Viral RNA was detected in the oesophagus, stomach, duodenum and rectum in three out of six patients with gastrointestinal symptoms who received endoscopy. One patient bled from multiple herpetic ulcers in the oesophagus.[108] Enteric infection by SARS-CoV-2 occurs via ACE-2 receptors expressed in epithelial cells.[109] A postmortem case series reported small bowel endotheliitis in patients with mesenteric ischaemia.[21]
Acute liver injury
Elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were observed in 10%–36.6% and 7.7%–33.3% of patients, respectively.[4,8,49,57,100,103,110,111,112,113,114,115] Hyperbilirubinaemia was seen in 2.1%–18.2% of patients.[49,57,110,113,114,116,117] Gross elevation to more than three times the upper limit of normal was reported in 37% of patients for ALT, 20% of patients for AST, and 10% of patients for total bilirubin.[118] Rare cases of severe acute hepatitis have also been reported.[119] Elevation in AST, ALT and bilirubin levels were associated with ARDS, shock and other extrahepatic complications.[49] Hepatic injury in COVID-19 infection occurs via (a) direct viral cytopathic effect via ACE-2 receptors in biliary and liver epithelial cells; (b) systemic inflammation; and (c) ischaemia from distributive shock.[120] Drug-induced liver injury from the use of lopinavir/ritonavir, remdesivir and tocilizumab should be considered.[117] Histological assessment of COVID-19 associated liver injury is non-specific,[67] although one postmortem examination described lymphocytic endotheliitis in the liver.[21]
Renal manifestations
Acute kidney injury (AKI) was observed 0.5%–7% of all patients and up to 17% of patients in cohorts with higher prevalence of cardiovascular diseases.[4,9,49,53,57,82,86,103,121] Proteinuria and haematuria were detected in 44% and 27% of patients on admission to hospital, respectively,[90] suggesting renal involvement early in the disease course. AKI is postulated to be driven by a direct viral cytopathic effect, mediated through the ACE-2 receptor and cellular transmembrane serine proteases expressed on podocytes and tubular epithelial cells.[122] This was supported by the postmortem renal histopathology of 26 patients, which showed direct viral invasion of the tubular epithelium and podocytes. Erythrocyte aggregation in tubular capillary loops, along with glomerular fibrin thrombi and ischaemic contraction, was also seen.[46]
While the kidneys may be affected early in the course of infection, severe AKI seemed to manifest only later as part of a systemic inflammatory response. In critically ill patients, rates of AKI were increased up to 29%,[4,49,53,59,82] and AKI was observed in up to 50% of patients who eventually died.[9,65,68,90] In a study of 191 patients, ARDS developed at a median of 12 days, while the onset of AKI closely followed at a median of 15 days,[9] suggesting the clustering of AKI at the period of critical illness and mechanical ventilation. The overall rate of renal replacement therapy was 5% in all hospitalised patients, increasing to 17% in the critically ill.[9,68] Proteinuria, haematuria, elevated baseline serum urea levels, and creatinine and peak serum creatinine levels >133 μmol/L were associated with mortality.[122]
Olfactory and gustatory dysfunction
Smell and taste disturbances are early manifestations of SARS-CoV-2 infection and were the presenting symptoms in 11.8%–18.1% of patients.[123,124] Smell disturbance occurred after 4.4 ± 1.9 days of symptom onset among 114 patients. Anosmia lasted a mean of 8.9 ± 6.3 days.[125] Four studies that captured self-reported symptoms reported smell and taste disturbance in 20%–68% and 24%–85% of patients, respectively.[125,126,127,128] Three other studies that utilised objective measures reported a higher incidence of olfactory and gustatory dysfunction (82.6%–98% and 49.3%–88%, respectively).[123,124,129] Table 2 describes the observational studies on smell and taste dysfunction in patients with SARS-CoV-2. Mechanisms for olfactory dysfunction include reactive inflammation of the olfactory cleft causing conductive smell dysfunction[130,131] and SARS-CoV-2 directly invading ACE-2-expressing olfactory epithelial cells.[130,131]
Table 2.
Observational studies on olfactory and gustatory dysfunction.
| Study | n | Country | Confirmed SARS-CoV-2 diagnosis (%) | Method of assessing smell and/or taste | Dysfunction (%) | Remarks | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Olfactory | Gustatory | ||||||
| Lechien et al.[123] | 417 | 12 European hospitals | 100 | Questionnaires based on the smell and taste component of the National Health and Nutrition Examination Survey and the short version of the Questionnaire of Olfactory Disorders-Negative Statements | 85.6 | 88.0 | 11.8% had smell dysfunction appearing before other symptoms. Among the 18.2% of patients without nasal obstruction or rhinorrhoea, 79.7% were hyposmic or anosmic. |
|
| |||||||
| Moein et al.[129] | 60 | Iran | 100 | University of Pennsylvania Smell Identification Test | 98 (58 severe) | NA | 18% smell disturbance in controls |
|
| |||||||
| Vaira et al.[124] | 72 | Italy | 100 | Odour identification test and the Connecticut Chemosensory Clinical Research Center olfactory test and a standardised test of gustatory function that examined the ability to perceive four primary tastes | 82.6 (8.3 severe) | 49.3 (22.9 severe) | 61.1% subjectively reported olfactory dysfunction |
|
| |||||||
| Yan et al.[126] | 59 | USA | 100 | Patient-reported via Internet platform | 68 | 71 | |
|
| |||||||
| Giacomelli et al.[127] | 59 | Italy | 100 | Questionnaire about the presence or absence of olfactory and taste disturbance | 33.9 with either taste or olfactory disorder, 18.6 with both taste and olfactory disorder | 20.3% presented with symptoms of smell or taste disturbance before hospital admission | |
|
| |||||||
| Klopfenstein et al.[125] | 114 | France | 100 | Patient-reported symptoms | 47 | 85 | Mean duration of anosmia was 8.9±6.3 days. Duration was ≥7 days for 55% and ≥14 days for 20%. Tinnitus, and hearing loss were uncommon (<15%) |
|
| |||||||
| Bénézit et al.[128] | 259 | France | 26 | Web-based questionnaire comprising four questions | 20 | 24 | |
NA: not applicable, SARS-CoV-2: severe acute respiratory syndrome coronavirus-2
Cutaneous manifestations
Cutaneous manifestations were found in up to 20.4% of SARS-CoV-2 infected patients.[132] In a study of 375 patients (COVID-19 infection confirmed in 62.4%), five distinct patterns were discerned: (a) maculopapular eruption (47%); (b) urticaria (19%); (c) vesicular eruption (9%); (d) livedoid lesions or necrosis (9%); and (e) chilblain-like lesions (19%).[133] Maculopapular eruption, vesicular rash and urticaria were also observed in a study of 18 patients[132] and eight case series/reports.[134,135,136,137,138,139,140,141] Variants of maculopapular eruption include widespread confluent lesions of up to 10 cm,[142] symmetrical drug-related intertriginous and flexural exanthema,[143] petechiae and purpura.[144,145,146] Skin histopathology in patients with a maculopapular or vesicular eruption showed varying features including perivascular spongiotic dermatitis, nests of Langerhans cells, eosinophils, lymphocytic vasculitis, microthrombi and dyskeratotic keratinocytes.[147]
Most characteristically, chilblain-like lesions that present as erythematous-to-purplish macules and plaques distributed over acral sites (‘COVID toes’) were reported predominantly in patients who had asymptomatic or mild disease.[133,148,149,150,151] Histopathology showed superficial and deep lichenoid, perivascular and perieccrine lymphocytic infiltrates with vacuolar interface dermatitis and necrotic keratinocytes.[149] While transient livedo may accompany mild disease,[152] fixed and necrotic lesions were described in patients with hypercoagulability.[22,24,45,47] In these patients, skin histopathology demonstrated complement-mediated thrombogenic vasculopathy and epidermal necrosis without vasculitis. Notably, SARS-CoV-2 glycoprotein was found in the skin microvasculature.[22]
Other manifestations
Auditory and ocular manifestations of the disease, arthralgia, spontaneous pneumomediastinum and mediastinal lymphadenopathy are described in Table 3.
Table 3.
Summary of extrapulmonary manifestations of SARS-CoV-2.
| Manifestation | Described frequency | Day of onset after fever or respiratory symptoms | Pathologic mechanism | Association with severe disease or mortality |
|---|---|---|---|---|
| Immunologic manifestations | ||||
|
| ||||
| Cytokine release syndrome | 1. Not defined and not precisely described in observational studies 2. Likely mirrors proportion of severe and critical cases (19.9%)[3] |
Day 7–19[4,9,59,82] | 1. Increased pathogenic Th1 cells and CD14+CD16+ monocytes[13] 2. Reduced memory Th (CD3+CD4+CD45RO+) and regulatory (CD3+CD4+CD25+ CD127low+) Tcells[6] 3. Depletion of absolute numbers and functional exhaustion of CD4+ and CD8+ T cells[14,15,16] |
Not precisely described. ARDS and multiorgan dysfunction accompany higher mortality, as described below |
|
| ||||
| Induction of antiphospholipid antibodies | 3 case reports/series, total n=9 [23,24,25] | Not reported | 1. Unknown for SARS-CoV-2 2. APLs induced by other viruses were related to endothelial exposure of phospholipid-binding viral peptides[153] |
Not known |
|
| ||||
| Haematologic manifestations | ||||
|
| ||||
| Hypercoagulability | Venous thromboembolism in 10%–31% of patients[17,16,27,28,29,30,31] | Although D-dimer increases on Day 5–7,[82] venous thromboembolism was reported on Day 1–21[28,32,33,34,35,36] | 1. Thromboinflammation with elevated cytokines, such as IL-6, causing endothelial injury[19] 2. Direct invasion of endothelial cells by the SARS-CoV-2 virus[21] 3. Complement-mediated endothelial injury[22] 4. Elevations in circulating prothrombotic factors, e.g., factor VIII, fibrinogen, prothrombotic microparticles[18,19,20] 5. Possible role of induced antiphospholipid antibodies[23,24,25] |
Disseminated intravascular coagulation was seen in 71.4% of non-survivors vs. 0.6% survivors in one study[26] |
|
| ||||
| Leucocytosis | 1.9%–44.7%[11,51,55,56,57,58,63,64,68] | Peaks on Day 15–17[82] | 1. Increased leucocyte mobilisation from hypercytokinaemia 2. Secondary bacterial or fungal infections |
1. ICU-admitters had a higher WBC count on admission[82] 2. 46% of non-survivors had leucocytosis vs. 11% of survivors[9] 3. In a meta-analysis of 3600 patients, leucocytosis was associated with severe disease (27.7% vs. 9.3% in mild disease)[51] |
|
| ||||
| Lymphopenia | 26.1%–85%[4,9,16,29,49,50,51,52,53,54,55,56,57,58,59,60] | Nadir on Day 5-10[82] | 1. Apoptosis induced by hypercytokinaemia[6,16] 2. Direct viral destruction via ACE-2 receptor[66] 3. Translocation into affected organs[67] |
Lymphocyte count at baseline, lymphocyte count over time[4,9,11,16,54,55,57,61,62,63,64] as well as lymphocyte-to-leucocyte ratio[65] were associated with severe disease and mortality |
|
| ||||
| Thrombocytopenia | Thrombocytopenia was seen in 11.4%–30% of patients[51,70,71] | Nadir on Day 17–19[70] | 1. Reduction of platelet production from direct infection of bone marrow or cytokine storm 2. Increased platelet destruction from increased immune clearance 3. Increased platelet consumption from microthrombi or pulmonary damage[154] |
In-hospital mortality of 92.1% was observed for patients with a nadir platelet count of <50×10[70] |
|
| ||||
| Eosinopenia | 52.9%–81.2%[11,54,68] | Not known | Not known | Not predictive of disease severity in a meta-analysis of 294 patients[155] |
|
| ||||
| Neutrophilia | 38%–60%[4,11,51,57,58,63,65,68] | Peaks on Day 15–17[82] | 1. Increase mobilisation as a result of hypercytokinaemia[10] 2. Possible secondary bacterial infection |
HR 1.14 for death or ARDS in bivariable analysis in one study of 201 patients[58] |
|
| ||||
| Sickle cell crisis | Case series of 2 patients who presented with painful vaso-occlusive crisis and acute chest syndrome that preceded fever and respiratory symptoms[156] | Day −3 to 1[156] | Similar process to other viral infections | Not known |
|
| ||||
| Cardiac manifestations | ||||
|
| ||||
| Acute cardiac injury | 1. 17.7% from a total of 1,412 COVID-19 patients (10 cohort studies, sample sizes 41–416).[4,59,62,82,83,84,85,86,87,88] 2. In another meta-analysis of 1,527 patients, 8.0% of patients suffered acute cardiac injury[89] |
Day 4–21, peaking on Day 15[9] | 1. Hypercytokinaemia driving increased sympathetic activity and resulting in demand ischaemia[7,157] 2. Over-representation of patients with underlying cardiovascular diseases[86,88] 3. Myocarditis with direct invasion of ACE-2-expressing myocytes[98] 4. Endotheliitis and endotheliopathy impairing perfusion of submucosal cardiac vessels[21] 5. Hyperinflammation increasing atherosclerotic plaque vulnerability and rupture[21,158] 6. Hypercoagulability leading to pulmonary embolism or microthrombi and right heart strain[17,27,28,29,32] |
Mean mortality rate is 54.4% in patients with acute cardiac injury compared to 9.0% in those without[86,87,88] |
|
| ||||
| Heart failure | 6.5%–23% of all patients[9,51,60] | Not known but likely parallels cardiac injury | 1. Ischaemia secondary to endotheliitis and endotheliopathy[21] 2. Key cytokines implicated in SARS-CoV-2 hypercytokinaemia, such as IL-6, may promote myocardial dysfunction[159] 3. Downregulation of ACE-2 has been demonstrated in SARS-CoV and may reduce cardioprotection by angiotensin 1-7[160] 4. Myocarditis |
Seen in 52% of non-survivors vs. 12% of survivors[9] |
|
| ||||
| Myocarditis | 12.5% of 112 patients[85] 7 case reports[92,93,94,95,96,97,98] | Not known | Direct invasion of myocytes via ACE-2[98] | Not known |
|
| ||||
| Arrhythmias | 10.9%–16.7% of patients[53,82] Rapid ventricular tachycardia and ventricular fibrillation causing haemodynamic instability were seen in 7.0% of 187 patients.[86] Reports of transient complete heart block[161] and Brugada syndrome[162] | Not known but likely parallels cardiac injury | 1. Acute cardiac injury 2. Myocarditis 3. Electrolyte disturbance from acute kidney injury or diarrhoea 4. Contribution from medications such as hydroxychloroquine |
Seen in 40% of severe cases vs. 1.2% of non-severe cases[53] |
|
| ||||
| Gastrointestinal manifestations | ||||
|
| ||||
| Gastrointestinal symptoms (including nausea, diarrhoea, anorexia and abdominal pain) | 1. 12.8%-65.9% of all patients[65,82,99,100,101,102,103,104] 2. In a meta-analysis of 4,243 patients, the pooled prevalence of the symptoms was: anorexia 26.8%, diarrhoea 12.5%, nausea and vomiting 10.2%, abdominal pain 9.2%[105] |
Predate respiratory symptoms by up to 9 days before, up till 11 days after[65,82,99,100,101,102,103,104] | 1. Enteric infection is proposed to be mediated by ACE-2 receptors, which are abundantly expressed in epithelial cells[109] 2. Small bowel endotheliitis in patients with mesenteric ischaemia.[21] |
1. Higher mortality and need for ventilatory support reported in 181 patients with high comorbidity burden[163] 2. No difference in outcomes in another multicentre, cross-sectional study of 204 patients[109] |
|
| ||||
| Acute liver injury | 1. Elevated aspartate aminotransferase and alanine aminotransferase were observed in 10%–36.6% and 7.7%–33.3% of patients, respectively.[4,8,49,57,100,103,110,111,112,113,114,115] 2. Hyperbilirubinaemia was seen in 2.1%–18.2%.[49,57,110,113,114,116,117] |
Day 4–11, peak on Day 10[114] | 1. Direct virus-induced effect via ACE-2 receptors expressed in biliary, liver epithelial cells or endothelial cells 2. Systemic inflammation and immune injury 3. Sepsis from secondary bacterial infection 4. Ischaemia secondary to hypoxia, heart failure or hypotension 5. Drug-induced liver injury from lopinavir/ritonavir, remdesivir and tocilizumab |
More common in severely ill patients[4,49,51] |
|
| ||||
| Neurologic manifestations | ||||
|
| ||||
| Stroke | 1. 5.7% in severely ill patients[37] 2. Case series and case reports in Table 1 |
Day −2 to 33, appear to peak on Day 9–15 [Table 1] | Ischaemic strokes may be related to mechanisms of hypercoagulability, endotheliopathy and over-representation of cardiovascular comorbidities, as outlined above | 5.7% in severely ill patients vs. 0.8% in mild cases[37] |
|
| ||||
| Meningoencephalitis | 1. Isolated case reports[79,80] 2. Leptomeningeal enhancement on brain imaging was reported in 62% of severe patients with ARDS and encephalopathy[41] |
Diagnosed on Day 3 and Day 9 in 2 case reports[79,80] | 1. Direct SARS-CoV-2 invasion into CNS[79] 2. Para-infectious mechanism with blood-brain barrier breakdown due to cytokine storm[75,79] |
Not known |
|
| ||||
| Elevated creatine kinase and rhabdomyolysis | 1. 28.6% and 16.7% of patients with severe and mild clinical disease, respectively[51] 2. 0.2% rhabdomyolysis[49] |
Not known. Reports of rhabdomyolysis as initial presentation[81] | 1. Direct viral invasion by binding to ACE-2 receptor in skeletal muscle 2. Immune response resulting in cytokine storms and damaging muscle tissues 3. Circulating viral toxins may directly destroy muscle cell membranes[25,37] |
More common in severely ill patients[51] |
|
| ||||
| Guillain-Barré syndrome and Miller Fisher syndrome | Estimated 0.4%–0.5% from an Italian case series of 5 Guillain-Barré syndrome patients, with approximately 1,000−1,200 COVID-19 patients admitted during the study period[77] | Day 3−10[75,77] (1 report preceding SARS-CoV-2 by 8 days)[76] | 1. Post-infectious autoimmune mechanism with presentation 3-10 days after COVID-19 symptoms[77,79] 2. Para-infectious mechanism proposed in view of an early presentation of Guillain-Barré syndrome[76] |
Not known |
|
| ||||
| Non-specific findings including headache, giddiness, delirium and corticospinal tract signs | 36.4% of 214 patients in China, including headache (13.1%), giddiness (16.8%) and drowsiness (7.5%).[37] Similarly, in 58 French patients with ARDS, ICU delirium (65%) and corticospinal tract signs (67%) were the main neurological findings. A high incidence of leptomeningeal enhancement and bilateral frontotemporal hypoperfusion was reported on imaging. SARS-CoV-2 was negative in the CSF of all 7 patients who underwent lumbar puncture.[41] | Not known | Possibly related to general unwell state, systemic inflammation or drugs | Delirium and corticospinal tract signs are common in severely ill patients in ICU[41] |
|
| ||||
| Renal manifestations | ||||
|
| ||||
| Acute kidney injury | 0.5%–7% of all patients and up to 17% of patients in cohorts with higher prevalence of cardiovascular diseases[4,9,49,53,57,82,86,103,121] | Day 11–17[82] | 1. Direct viral cytopathic effect, mediated through the ACE-2 receptor and cellular transmembrane serine proteases expressed on podocytes and tubular epithelial cells.[122] 2. Severe AKI in later course of disease in tandem with ARDS and other organ dysfunction, likely related to hypercytokinaemia[9,82] |
In critically-ill patients, rates of AKI increased up to 29%,[4,49,53,59,82] and AKI was observed in up to 50% of patients who eventually died[9,65,68,90] |
|
| ||||
| Collapsing glomerulopathy | 1 case report in a patient who presented with fever, vomiting and acute renal failure. SARS-CoV-2 was absent in the kidney.[164] | Presented with other symptoms | Patient was homozygous for the APOL1 genotype[164] | Not known |
|
| ||||
| Olfactory, gustatory and auditory manifestations | ||||
|
| ||||
| Olfactory dysfunction | 1. Studies using self-reported symptoms: 20%–68% 2. Studies using objective measures: 82.6%–98% [Table 2] |
Day 2–7[125]; could precede respiratory symptoms | 1. Reactive inflammation of the olfactory cleft causing conductive smell dysfunction[130,131] 2. Direct viral invasion of ACE-2-expressing olfactory epithelial cells[130,131] |
9% of 54 patients needed ICU care, 4% died[125] |
|
| ||||
| Gustatory dysfunction | 1. Studies using self-reported symptoms: 24%−85% 2. Studies using objective measures: 49.3%−88% [Table 2] |
Day 2-7[125]; could precede respiratory symptoms | 1. Inactivation of ACE-2 receptors in the oral cavity resulting in sodium channel inactivation in the taste receptors[131] 2. Viral binding to sialic acid receptors in the taste pores[131] 3. Smell dysfunction altering taste perception[130,131] |
Not known |
|
| ||||
| Cutaneous manifestations | ||||
|
| ||||
| Maculopapular eruption | 47% of cutaneous manifestations[133] | Day 3–8[134,136,138,140,142,144,146] | Non-specific and could be related to both virus and drugs | 12% needed ICU or non-invasive ventilation[133] |
|
| ||||
| Urticaria | 19% of cutaneous manifestations[133] | Day −2 to 6[135,137,138] | Non-specific and could be related to both virus and drugs | 11% needed ICU or invasive ventilation[133] |
|
| ||||
| Vesicular eruption | 9% of cutaneous manifestations[133] | Day −2 to 12[139] | Non-specific and could be related to both virus and drugs | 1. 6% of patients needed ICU or invasive ventilation[133] 2. 3/22 patients died in one series[139] |
|
| ||||
| Livedoid lesions or necrosis | 6% of cutaneous manifestations[133] | Day 7–10 for livedo reticularis[156] Day 14–21 for skin necrosis[22] | Endotheliitis, endotheliopathy and hypercoagulability (outlined above). Transient processes may result in livedo, while fulminant intravascular coagulation may lead to fixed necrosis. | 33% required ICU or invasive ventilation[133] |
|
| ||||
| Chilblain-like lesions | 19% of cutaneous manifestations[133] | Day 6–15 (median 10 days)[150] | Not known. Likely related to microangiopathic process and a continuous spectrum with livedo | 1. Only 3% required ICU or invasive ventilation.[133] 2. Case reports of generally well patients.[148,149,151] |
|
| ||||
| Other manifestations | ||||
|
| ||||
| Conjunctivitis | 19 patients (1 observation study and 3 case series/reports)[165,166,167,168] Symptoms included conjunctival hyperaemia, chemosis, epiphora and increased secretions[167] | Was the only symptom in 7 patients and often preceded other symptoms[165,166,167,168] | Viral invasion of conjunctival cells that express ACE-2 | Not known. Observed in asymptomatic as well as critically ill patients |
|
| ||||
| Mediastinal lymphadenopathy | 1%–6% of patients who received chest computed tomography in 2 small observational studies (described to be subcarinal and voluminous)[169,170] | Not clear and depended on time of imaging | Likely similar to other infection-associated lymphadenopathy | Not known |
|
| ||||
| Spontaneous pneumomediastinum | 3 case reports in patients with bilateral pneumonia (one received positive pressure ventilation)[171,172,173] | Day 10–12[171,172,173] | Not known | Not known |
|
| ||||
| Arthralgia | 2 case reports, one in a patient with fever and thrombocytopenia[174]; another in a patient with urticaria[135] | Presenting symptom in both patients | 1. Not known for SARS-CoV-2 2. Possibly similar to other viral arthritides: mechanisms include complement activation and infiltration of inflammatory cells into synovium 3. Key cytokines in the cytokine release of SARS-CoV-2 (e.g. IL-6 and MCP1) have been implicated in pathogenesis of acute arthritis for other viruses[175] |
Not known |
|
| ||||
| Auditory manifestations | Tinnitus (11%) and hearing loss (4%) were reported among 54 patients with anosmia.[125] A study showed that the high frequency pure-tone threshold and the transient evoked otoacoustic emissions amplitudes were significantly worse in a group of 20 young patients with confirmed SARS-CoV-2 who were asymptomatic.[176] One patient with otitis media presented with otalgia along with bilateral pneumonia[177] | Not known for tinnitus and hearing loss. Report of otitis media was synchronous with other symptoms[177] | Postulated to be due to direct viral damage to the hair cells, organ of Corti, stria vascularis, or spiral ganglion[176] | Not known |
|
| ||||
| Testicular pain | One patient presented with stabbing testicular pain and fever[178] | Same as other symptoms | Not known | Not known |
ACE-2: Angiotensin-converting enzyme 2, AKI: acute kidney injury, APL: anti-phospholipid antibodies, ARDS: acute respiratory distress syndrome, CSF: cerebrospinal fluid, CNS: central nervous system, HR: hazard ratio, ICU: intensive care unit, IL-6: interleukin-6, MCP1: monocyte chemoattractant protein-1, SARS-CoV-2: severe acute respiratory syndrome coronavirus-2, Th1: T-helper 1, WBC: white blood cell count
DISCUSSION
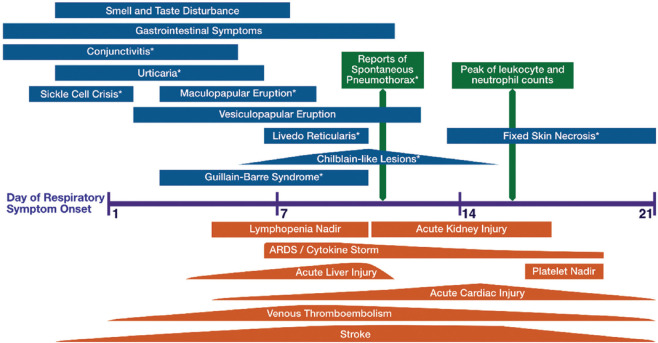
We undertook a multidisciplinary approach to systematically consolidate the wide range of manifestations and complications of SARS-CoV-2 infection [Figure 2 and Table 3]. Referencing included studies, we assembled a timeline to demonstrate the distribution of these manifestations with respect to the onset of respiratory symptoms [Figure 3].
Figure 2.
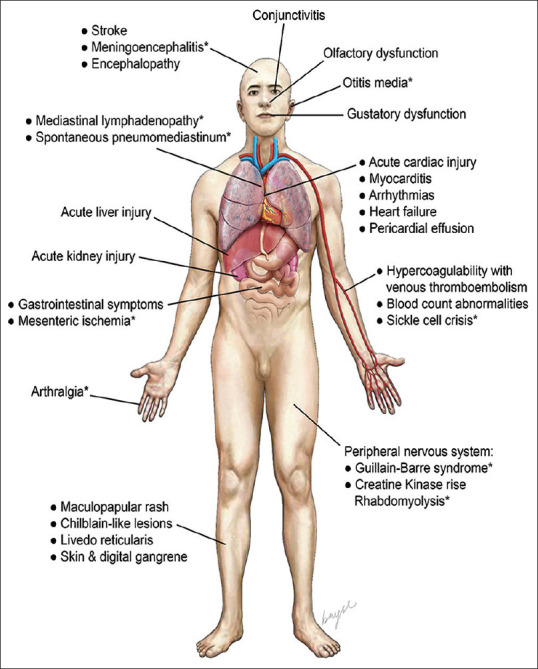
Illustration shows extrapulmonary manifestations and complications of SARS-CoV-2 infection. *Manifestations described only in case series or case reports.
Figure 3.
Time course of extrapulmonary manifestations of SARS-CoV-2 infection with respect to respiratory symptoms shows early manifestations (blue bars), late manifestations (orange bars) and single day events (green bars). Some manifestations (*) were only from case series/reports.
While some manifestations occurred early, including olfactory and gustatory disturbance and gastrointestinal symptoms, late manifestations on Day 7–19 were dominated by multiorgan sequelae, mirroring the early ‘viraemic’ and late ‘acute’ phases of disease that were previously described.[179]
Multiorgan involvement in SARS-CoV-2 occur via the following key mechanisms: (a) direct cytopathic effect from viral binding to ACE-2 receptors[2,66,122]; (b) endothelial dysfunction and thromboinflammation[19,21,22]; and (c) CRS.[4,5,6,7,8] Yet, these processes are not separate and exist on a continuum. Viral entry via ACE-2 receptors presages CRS by increasing angiotensin II and activating NF-κB and the IL-6 amplifier. IL-1 and IL-6, in turn, promote endotheliopathy and thromboinflammation.[2,180] Parenchymal injury at various organs occur when circulating leucocytes migrate into the interstitium or when resident histiocytes are activated.[181] Concurrently, pro-inflammatory cytokines induce the release of ultralarge von Willebrand factor multimers and the overexpression of tissue factor.[182] As a result, microthromboses, found in the histologic samples in the skin and kidneys of patients with SARS-CoV-2,[22,46,48] lead to further ischaemic damage.
While immunomodulation in SARS-CoV-2 CRS with IL-6 blockade has been described, patient selection and timing are crucial for safety and success.[183] Extrapulmonary symptoms in the early phase of illness could be attributed to direct viral invasion in sites such as the gastrointestinal tract and conjunctivae. In the later phase, however, multiorgan dysfunction reflects the hypercytokinaemia heralded by endotheliopathy, in which activated leucocytes infiltrate organ interstitium.[181] Biomarkers that identify patients with early endotheliopathy could inform physicians about the patients who are destined to do poorly, but who could still be intervened upon within this narrow window of opportunity.
Our description of the extrapulmonary manifestations and complications of SARS-CoV-2 has been limited by differences in patient sampling and definitions used for extrapulmonary symptoms in observational studies, resulting in a wide range of incidence of complications quoted in this review. Also, as patients with SARS-CoV-2 were less likely to receive invasive procedures or radiological investigations, many of the manifestations were likely under-reported. In addition, we did not examine the validity of the observational studies, so as to exhaustively describe the range of manifestations. Importantly, after the time of censoring of this review on 1 May 2020, there have been many important observational studies and meta-analyses of COVID-19-related complications such stroke and AKI that demonstrated a different prevalence or natural history from the reports in this review. In addition, new manifestations, such as thromboembolism in asymptomatic individuals and electrolyte disturbances, have been reported. Unfortunately, these studies were not included based on the design of this review. However, we believe that we have taken the most comprehensive approach to delineate the systemic manifestations and complications of SARS-CoV-2 infection and outline their clinical course.
In conclusion, although the systemic manifestations of SARS-CoV-2 infection are variegated, they are deeply interwoven by shared pathological mechanisms. Two phases of extrapulmonary disease were identified: (a) an early phase with gastrointestinal, ocular and cutaneous involvement and (b) a late phase characterised by multiorgan dysfunction and clinical deterioration. A clear, multidisciplinary consensus to define and approach thromboinflammation and CRS in SARS-CoV-2 is needed to identify patients in need of early intervention.
Financial support and sponsorship
Nil.
Conflicts of interest
Sia CH and Tan BYQ are members of the SMJ Editorial Board, and were thus not involved in the peer review and publication decisions of this article.
Acknowledgement
We acknowledge Ms Song Lin Bay (Yong Loo Lin School of Medicine, National University of Singapore) and Mr Tianwen Ng (Beary Naise Co) for illustrating Figures 2 and 3, respectively.
REFERENCES
- 1.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–7. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirano T, Murakami M. COVID-19: A new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52:731–3. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19) [[Last accessed on 2020 May 01]]. Available from: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf .
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–8. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–9. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769–77. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ong EZ, Chan YFZ, Leong WY, Lee NMY, Kalimuddin S, Haja Mohideen SM, et al. A dynamic immune response shapes COVID-19 progression. Cell Host Microbe. 2020;27:879–82.e2. doi: 10.1016/j.chom.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin Chem Lab Med. 2020;58:1021–8. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 12.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020;17:541–3. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533–5. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, et al. Pulmonary embolism in patients with COVID-19: Awareness of an increased prevalence. Circulation. 2020;142:184–6. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 18.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–42. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranucci M, Ballotta A, Di Dedda U, Baryshnikova E, Dei Poli M, Resta M, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–51. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han H, Yang L, Liu R, Liu F, Wu KL, Li J, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–20. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 21.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–8. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escher R, Breakey N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91:889–91. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–7. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–4. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–7. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Léonard-Lorant I, Delabranche X, Séverac F, Helms J, Pauzet C, Collange O, et al. Acute pulmonary embolism in patients with COVID-19 on CT angiography and relationship to D-dimer levels. Radiology. 2020;296:E189–91. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;296:E186–8. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louhaichi S, Allouche A, Baili H, Jrad S, Radhouani A, Greb D, et al. Features of patients with 2019 novel coronavirus admitted in a pneumology department: The first retrospective Tunisian case series. Tunis Med. 2020;98:261–5. [PubMed] [Google Scholar]
- 32.Casey K, Iteen A, Nicolini R, Auten J. COVID-19 pneumonia with hemoptysis: Acute segmental pulmonary emboli associated with novel coronavirus infection. Am J Emerg Med. 2020;38:1544.e1–3. doi: 10.1016/j.ajem.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: A random association? Eur Heart J. 2020;41:1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rotzinger DC, Beigelman-Aubry C, von Garnier C, Qanadli SD. Pulmonary embolism in patients with COVID-19: Time to change the paradigm of computed tomography. Thromb Res. 2020;190:58–9. doi: 10.1016/j.thromres.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poggiali E, Bastoni D, Ioannilli E, Vercelli A, Magnacavallo A. Deep vein thrombosis and pulmonary embolism: Two complications of COVID-19 pneumonia? Eur J Case Rep Intern Med. 2020;7:001646. doi: 10.12890/2020_001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cellina M, Oliva G. Acute pulmonary embolism in a patient with COVID-19 pneumonia. Diagn Interv Imaging. 2020;101:325–6. doi: 10.1016/j.diii.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–90. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S, et al. COVID-19 presenting as stroke. Brain Behav Immun. 2020;87:115–9. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He J, Cheng G, Xu W, Zhang L, Zeng Z. [Diagnosis and treatment of an elderly patient with secondary cerebral infarction caused by COVID-19. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40:351–2. doi: 10.12122/j.issn.1673-4254.2020.03.10. Chinese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–70. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou B, She J, Wang Y, Ma X. A case of coronavirus disease 2019 with concomitant acute cerebral infarction and deep vein thrombosis. Front Neurol. 2020;11:296. doi: 10.3389/fneur.2020.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garaci F, Di Giuliano F, Picchi E, Da Ros V, Floris R. Venous cerebral thrombosis in COVID-19 patient. J Neurol Sci. 2020;414:116871. doi: 10.1016/j.jns.2020.116871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes C, Nichols T, Pike M, Subbe C, Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med. 2020;7:001691. doi: 10.12890/2020_001691. doi: 10.12890/2020_001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou B, She J, Wang Y, Ma X. Venous thrombosis and arteriosclerosis obliterans of lower extremities in a very severe patient with 2019 novel coronavirus disease: A case report. J Thromb Thrombolysis. 2020;50:229–32. doi: 10.1007/s11239-020-02084-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–27. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Cao W, Xiao M, Li YJ, Yang Y, Zhao J, et al. [Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006. doi: 10.3760/cma.j.issn.0253-2727.2020.0006. Chinese. [DOI] [PubMed] [Google Scholar]
- 48.Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, da Silva LFF, de Oliveira EP, Saldiva PHN, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. 2020;18:1517–9. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou C, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan BE, Chong VCL, Chan SSW, Lim GH, Lim KGE, Tan GB, et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95:E131–4. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 51.Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect. 2020;80:656–65. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiong Y, Sun D, Liu Y, Fan Y, Zhao L, Li X, et al. Clinical and high-resolution CT features of the COVID-19 infection: Comparison of the initial and follow-up changes. Invest Radiol. 2020;55:332–9. doi: 10.1097/RLI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang G, Hu C, Luo L, Fang F, Chen Y, Li J, et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–41. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 55.Zheng F, Tang W, Li H, Huang YX, Xie YL, Zhou ZG. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24:3404–10. doi: 10.26355/eurrev_202003_20711. [DOI] [PubMed] [Google Scholar]
- 56.Zhu J, Ji P, Pang J, Zhong Z, Li H, He C, et al. Clinical characteristics of 3,062 COVID-19 patients: A meta-analysis. J Med Virol. 2020;92:1902–14. doi: 10.1002/jmv.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–43. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323:1612–4. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, et al. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peng YD, Meng K, Guan HQ, Leng L, Zhu RR, Wang BY, et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:450–5. doi: 10.3760/cma.j.cn112148-20200220-00105. Chinese. [DOI] [PubMed] [Google Scholar]
- 63.Zhang G, Zhang J, Wang B, Zhu X, Wang Q, Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A retrospective analysis. Respir Res. 2020;21:74. doi: 10.1186/s12931-020-01338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in critically ill patients in the Seattle region-case series. N Engl J Med. 2020;382:2012–22. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deng Y, Liu W, Liu K, Fang YY, Shang J, Zhou L, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: A retrospective study. Chin Med J (Engl) 2020;133:1261–7. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–9. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng Y, Xu H, Yang M, Zeng Y, Chen H, Liu R, et al. Epidemiological characteristics and clinical features of 32 critical and 67 noncritical cases of COVID-19 in Chengdu. J Clin Virol. 2020;127:104366. doi: 10.1016/j.jcv.2020.104366. doi: 10.1016/j.jcv.2020.104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469–72. doi: 10.1111/jth.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou M, Qi J, Li X, Zhang Z, Yao Y, Wu D, et al. The proportion of patients with thrombocytopenia in three human-susceptible coronavirus infections: A systematic review and meta-analysis. Br J Haematol. 2020;189:438–41. doi: 10.1111/bjh.16655. [DOI] [PubMed] [Google Scholar]
- 72.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta. 2020;506:145–8. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharifi-Razavi A, Karimi N, Rouhani N. COVID-19 and intracerebral haemorrhage: Causative or coincidental? New Microbes New Infect. 2020;35:100669. doi: 10.1016/j.nmni.2020.100669. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gu Y, Tao M, Chen K, Zhou G, He SF. [Nursing management of acute chest pain in patients with COVID-19 infection. Nurs Res. 2020;34:945–6. Chinese. [Google Scholar]
- 75.Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain-Barrésyndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–6. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barrésyndrome associated with SARS-CoV-2 infection: Causality or coincidence? Lancet Neurol. 2020;19:383–4. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Mañas R, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–5. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 78.Vollono C, Rollo E, Romozzi M, Frisullo G, Servidei S, Borghetti A, et al. Focal status epilepticus as unique clinical feature of COVID-19: A case report. Seizure. 2020;78:109–12. doi: 10.1016/j.seizure.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–8. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: Imaging features. Radiology. 2020;296:E119–20. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suwanwongse K, Shabarek N. Rhabdomyolysis as a presentation of 2019 novel coronavirus disease. Cureus. 2020;12:e7561. doi: 10.7759/cureus.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–8. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen C, Chen C, Yan JT, Zhou N, Zhao JP, Wang DW. [Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:567–71. doi: 10.3760/cma.j.cn112148-20200225-00123. Chinese. [DOI] [PubMed] [Google Scholar]
- 85.Deng Q, Hu B, Zhang Y, Wang H, Zhou X, Hu W, et al. Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116–21. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He XW, Lai JS, Cheng J, Wang MW, Liu YJ, Xiao ZC, et al. [Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:456–60. doi: 10.3760/cma.j.cn112148-20200228-00137. Chinese. [DOI] [PubMed] [Google Scholar]
- 88.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–10. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–8. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease. 2019: Retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dong N, Cai J, Zhou Y, Liu J, Li F. End-stage heart failure with COVID-19: Strong evidence of myocardial injury by 2019-nCoV. JACC Heart Fail. 2020;8:515–7. doi: 10.1016/j.jchf.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2021 doi: 10.1093/eurheartj/ehaa190. doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–24. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Irabien-Ortiz Á, Carreras-Mora J, Sionis A, Pàmies J, Montiel J, Tauron M. Fulminant myocarditis due to COVID-19. Rev Esp Cardiol. 2020;73:503–4. doi: 10.1016/j.recesp.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim IC, Kim JY, Kim HA, Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41:1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–5. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX, et al. First case of COVID-19 complicated with fulminant myocarditis: A case report and insights. Infection. 2020;48:773–7. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–2. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luo S, Zhang X, Xu H. Don't overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19) Clin Gastroenterol Hepatol. 2020;18:1636–7. doi: 10.1016/j.cgh.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen T, Dai Z, Mo P, Li X, Ma Z, Song S, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (|y2019): A single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. 2020;75:1788–95. doi: 10.1093/gerona/glaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lei S, Jiang F, Su W, Chen C, Chen J, Mei W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. E Clinical Medicine. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2021;73:e4208–13. doi: 10.1093/cid/ciaa270. doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang X, Fang J, Zhu Y, Chen L, Ding F, Zhou R, et al. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang hospital. Clin Microbiol Infect. 2020;26:1063–8. doi: 10.1016/j.cmi.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort: Systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–9. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carvalho A, Alqusairi R, Adams A, Paul M, Kothari N, Peters S, et al. SARS-CoV-2 gastrointestinal infection causing hemorrhagic colitis: Implications for detection and transmission of COVID-19 disease. Am J Gastroenterol. 2020;115:942–6. doi: 10.14309/ajg.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 109.Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: A descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–73. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang R, Pan M, Zhang X, Han M, Fan X, Zhao F, et al. Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, Anhui, China. Int J Infect Dis. 2020;95:421–8. doi: 10.1016/j.ijid.2020.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qian GQ, Yang NB, Ding F, Ma AHY, Wang ZY, Shen YF, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: A retrospective, multi-centre case series. QJM. 2020;113:474–81. doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–93. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–6. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim ES, Chin BS, Kang CK, Kim NJ, Kang YM, Choi JP, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: A preliminary report of the first 28 patients from the Korean cohort study on COVID-19. J Korean Med Sci. 2020;35:e142. doi: 10.3346/jkms.2020.35.e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen L, Liu HG, Liu W, Liu J, Liu K, Shang J, et al. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005. Chinese. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095–103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 118.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566–74. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–9. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li J, Fan JG. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J Clin Transl Hepatol. 2020;8:13–7. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–38. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: A study based on single-cell transcriptome analysis. Intensive Care Med. 2020;46:1114–6. doi: 10.1007/s00134-020-06026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–61. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vaira LA, Deiana G, Fois AG, Pirina P, Madeddu G, De Vito A, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck. 2020;42:1252–8. doi: 10.1002/hed.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Klopfenstein T, Kadiane-Oussou NJ, Toko L, Royer PY, Lepiller Q, Gendrin V, et al. Features of anosmia in COVID-19. Med Mal Infect. 2020;50:436–9. doi: 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10:806–13. doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory syndrome coronavirus 2 infection: A cross-sectional study. Clin Infect Dis. 2020;71:889–90. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bénézit F, Le Turnier P, Declerck C, Paillé C, Revest M, Dubée V, et al. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect Dis. 2020;20:1014–5. doi: 10.1016/S1473-3099(20)30297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moein ST, Hashemian SMR, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: A biomarker for COVID-19. Int Forum Allergy Rhinol. 2020;10:944–50. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ralli M, Di Stadio A, Greco A, de Vincentiis M, Polimeni A. Defining the burden of olfactory dysfunction in COVID-19 patients. Eur Rev Med Pharmacol Sci. 2020;24:3440–1. doi: 10.26355/eurrev_202004_20797. [DOI] [PubMed] [Google Scholar]
- 131.Vaira LA, Salzano G, Fois AG, Piombino P, De Riu G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int Forum Allergy Rhinol. 2020;10:1103–4. doi: 10.1002/alr.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Recalcati S. Cutaneous manifestations in COVID-19: A first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212–3. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 133.Galván Casas C, Català A, Carretero Hernández G, Rodríguez-Jiménez P, Fernández-Nieto D, Rodríguez-Villa Lario A, et al. Classification of the cutaneous manifestations of COVID-19: A rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71–7. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hunt M, Koziatek C. A case of COVID-19 pneumonia in a young male with full body rash as a presenting symptom. Clin Pract Cases Emerg Med. 2020;4:219–21. doi: 10.5811/cpcem.2020.3.47349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Henry D, Ackerman M, Sancelme E, Finon A, Esteve E. Urticarial eruption in COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34:e244–5. doi: 10.1111/jdv.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Avellana Moreno R, Estela Villa LM, Avellana Moreno V, Estela Villa C, Moreno Aparicio MA, Avellana Fontanella JA. Cutaneous manifestation of COVID-19 in images: A case report. J Eur Acad Dermatol Venereol. 2020;34:e307–9. doi: 10.1111/jdv.16531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, Pindado-Ortega C, Prieto-Barrios M, Jimenez-Cauhe J. Comment on: Cutaneous manifestations in COVID-19: A first perspective. Safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol. 2020;34:e252–4. doi: 10.1111/jdv.16470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hedou M, Carsuzaa F, Chary E, Hainaut E, Cazenave-Roblot F, Masson Regnault M. Comment on ‘Cutaneous manifestations in COVID-19: A first perspective’ by Recalcati S. J Eur Acad Dermatol Venereol. 2020;34:e299–300. doi: 10.1111/jdv.16519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marzano AV, Genovese G, Fabbrocini G, Pigatto P, Monfrecola G, Piraccini BM, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83:280–5. doi: 10.1016/j.jaad.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sachdeva M, Gianotti R, Shah M, Bradanini L, Tosi D, Veraldi S, et al. Cutaneous manifestations of COVID-19: Report of three cases and a review of literature. J Dermatol Sci. 2020;98:75–81. doi: 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tammaro A, Adebanjo GAR, Parisella FR, Pezzuto A, Rello J. Cutaneous manifestations in COVID-19: The experiences of Barcelona and Rome. J Eur Acad Dermatol Venereol. 2020;34:e306–7. doi: 10.1111/jdv.16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Najarian DJ. Morbilliform exanthem associated with COVID-19. JAAD Case Rep. 2020;6:493–4. doi: 10.1016/j.jdcr.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mahé A, Birckel E, Krieger S, Merklen C, Bottlaender L. A distinctive skin rash associated with coronavirus disease 2019? J Eur Acad Dermatol Venereol. 2020;34:e246–7. doi: 10.1111/jdv.16471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, Pindado-Ortega C, Selda-Enriquez G, Bea-Ardebol S, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820–2. doi: 10.1001/jamadermatol.2020.1741. [DOI] [PubMed] [Google Scholar]
- 145.Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82:e177. doi: 10.1016/j.jaad.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, Moreno-Arrones OM, Fernandez-Nieto D. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: Petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol. 2020;83:e141–2. doi: 10.1016/j.jaad.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gianotti R, Zerbi P, Dodiuk-Gad RP. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the Northern part of Italy. J Dermatol Sci. 2020;98:141–3. doi: 10.1016/j.jdermsci.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Landa N, Mendieta-Eckert M, Fonda-Pascual P, Aguirre T. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739–43. doi: 10.1111/ijd.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kolivras A, Dehavay F, Delplace D, Feoli F, Meiers I, Milone L, et al. Coronavirus (COVID-19) infection-induced chilblains: A case report with histopathologic findings. JAAD Case Rep. 2020;6:489–92. doi: 10.1016/j.jdcr.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Piccolo V, Neri I, Filippeschi C, Oranges T, Argenziano G, Battarra VC, et al. Chilblain-like lesions during COVID-19 epidemic: A preliminary study on 63 patients. J Eur Acad Dermatol Venereol. 2020;34:e291–3. doi: 10.1111/jdv.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alramthan A, Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: First report from the Middle East. Clin Exp Dermatol. 2020;45:746–8. doi: 10.1111/ced.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Manalo IF, Smith MK, Cheeley J, Jacobs R. A dermatologic manifestation of COVID-19: Transient livedo reticularis. J Am Acad Dermatol. 2020;83:700. doi: 10.1016/j.jaad.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Asherson R, Cervera R. Antiphospholipid antibodies and infections. Ann Rheum Dis. 2003;62:388–93. doi: 10.1136/ard.62.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99:1205–8. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lippi G, Henry BM. Eosinophil count in severe coronavirus disease 2019. QJM. 2020;113:511–2. doi: 10.1093/qjmed/hcaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nur E, Gaartman AE, van Tuijn CFJ, Tang MW, Biemond BJ. Vaso-occlusive crisis and acute chest syndrome in sickle cell disease due to 2019 novel coronavirus disease (COVID-19) Am J Hematol. 2020;95:725–6. doi: 10.1002/ajh.25821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Babapoor-Farrokhran S, Gill D, Walker J, Rasekhi RT, Bozorgnia B, Amanullah A. Myocardial injury and COVID-19: Possible mechanisms. Life Sci. 2020;253:117723. doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med. 2015;278:483–93. doi: 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pathan N, Hemingway CA, Alizadeh AA, Stephens AC, Boldrick JC, Oragui EE, et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004;363:203–9. doi: 10.1016/S0140-6736(03)15326-3. [DOI] [PubMed] [Google Scholar]
- 160.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–8. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 161.Azarkish M, Laleh Far V, Eslami M, Mollazadeh R. Transient complete heart block in a patient with critical COVID-19. Eur Heart J. 2020;41:2131. doi: 10.1093/eurheartj/ehaa307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chang D, Saleh M, Garcia-Bengo Y, Choi E, Epstein L, Willner J. COVID-19 infection unmasking Brugada syndrome. HeartRhythm Case Rep. 2020;6:237–40. doi: 10.1016/j.hrcr.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wan Y, Li J, Shen L, Zou Y, Hou L, Zhu L, et al. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol Hepatol. 2020;5:534–5. doi: 10.1016/S2468-1253(20)30118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19) Kidney Int Rep. 2020;5:935–9. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Salducci M, La Torre G. COVID-19 emergency in the cruise's ship: A case report of conjunctivitis. Clin Ter. 2020;171:e189–91. doi: 10.7417/CT.2020.2212. [DOI] [PubMed] [Google Scholar]
- 166.Scalinci SZ, Trovato Battagliola E. Conjunctivitis can be the only presenting sign and symptom of COVID-19. IDCases. 2020;20:e00774. doi: 10.1016/j.idcr.2020.e00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–8. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92:589–94. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect Dis. 2020;20:425–34. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xu X, Yu C, Qu J, Zhang L, Jiang S, Huang D, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47:1275–80. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sun R, Liu H, Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol. 2020;21:541–4. doi: 10.3348/kjr.2020.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang J, Su X, Zhang T, Zheng C. Spontaneous pneumomediastinum: A probable unusual complication of coronavirus disease 2019 (COVID-19) pneumonia. Korean J Radiol. 2020;21:627–8. doi: 10.3348/kjr.2020.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhou C, Gao C, Xie Y, Xu M. COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis. 2020;20:510. doi: 10.1016/S1473-3099(20)30156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Joob B, Wiwanitkit V. Arthralgia as an initial presentation of COVID-19: Observation. Rheumatol Int. 2020;40:823. doi: 10.1007/s00296-020-04561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Assunção-Miranda I, Cruz-Oliveira C, Da Poian AT. Molecular mechanisms involved in the pathogenesis of alphavirus-induced arthritis. Biomed Res Int 2013. 2013:973516. doi: 10.1155/2013/973516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mustafa MWM. Audiological profile of asymptomatic Covid-19 PCR-positive cases. Am J Otolaryngol. 2020;41:102483. doi: 10.1016/j.amjoto.2020.102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fidan V. New type of corona virus induced acute otitis media in adult. Am J Otolaryngol. 2020;41:102487. doi: 10.1016/j.amjoto.2020.102487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim J, Thomsen T, Sell N, Goldsmith AJ. Abdominal and testicular pain: An atypical presentation of COVID-19. Am J Emerg Med. 2020;38:1542.e1–3. doi: 10.1016/j.ajem.2020.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-A review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9:727–32. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19: Implications for the cardiovascular system. Circulation. 2020;142:68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 181.Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med. 2008;26:711–5. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 182.Joly BS, Siguret V, Veyradier A. Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID-19. Intensive Care Med. 2020;46:1603–6. doi: 10.1007/s00134-020-06088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang S, Li L, Shen A, Chen Y, Qi Z. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin Drug Investig. 2020;40:511–8. doi: 10.1007/s40261-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]