OBJECTIVES:
Patients with COVID-19–associated acute respiratory distress syndrome (ARDS) have a high risk for developing acute kidney injury (AKI) which is associated with an increased risk of death and persistent renal failure. Early prediction of AKI is crucial in order to implement preventive strategies. The purpose of this study was to investigate the predictive performance of tissue inhibitor of metalloproteinases 2 and insulin like growth factor binding protein 7 (TIMP-2) × (IGFBP7) in critically ill patients with COVID-19–associated ARDS.
DESIGN:
Multicenter, prospective, observational study.
SETTING:
Twelve centers across Europe and United Kingdom.
PATIENTS:
Patients with moderate or severe COVID-19–associated ARDS were included and serial measurements of (TIMP-2) × (IGFBP7) were performed.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
The primary endpoint was the development of moderate or severe AKI according to the Kidney Disease: Improving Global Outcomes definition. Three hundred patients were available for the primary analysis, and 39 met the primary endpoint. At enrollment, urinary (TIMP-2) × (IGFBP7) had high predictive value for the primary endpoint with an area under the receiver operating characteristic curve of 0.89 (95% CI, 0.84–0.93). (TIMP-2) × (IGFBP7) was significantly higher in endpoint-positive patients at enrollment and at 12 hours.
CONCLUSIONS:
Urinary (TIMP-2) × (IGFBP7) predicts the occurrence of AKI in critically ill patients with COVID-19–associated ARDS.
Keywords: acute kidney injury, biomarkers, cell cycle arrest biomarkers, COVID-19, lung injury, tissue inhibitor of metalloproteinases 2 and insulin like growth factor binding protein 7
KEY POINTS
Question: The purpose of this study was to investigate whether the two biomarkers tissue inhibitor of metalloproteinases 2 and insulin like growth factor binding protein 7 (TIMP-2) × (IGFBP7) can predict the development of acute kidney injury (AKI) in critically ill patients with COVID-19–associated acute respiratory distress syndrome (ARDS).
Findings: Urinary (TIMP-2) × (IGFBP7) showed good predictive performance for AKI development with an area under the receiver operating characteristic curve of 0.89 (95% CI, 0.84–0.93). (TIMP-2) × (IGFBP7) was significantly higher in endpoint-positive patients at enrollment and at 12 hours.
Meaning: Urinary (TIMP-2) × (IGFBP7) predicts the occurrence of AKI in critically ill patients with COVID-19–associated ARDS.
Recent evidence demonstrates that COVID-19 is associated with a high risk of developing acute kidney injury (AKI), in particular, in patients with acute respiratory distress syndrome (ARDS) (1, 2). A recently published study including 3,993 hospitalized COVID-19 patients showed that the frequency of COVID-19–associated AKI was 46% and that mortality rates were six times higher compared with patients without AKI (3). Furthermore, in 35% of survivors, renal function had not recovered to baseline at discharge (3). In another multicenter study including 67 ICUs in the United States, 20% of ICU patients developed AKI and received renal replacement therapy (RRT) (4). Approximately 63% of these patients died while one third of the survivors remained RRT-dependent upon discharge, and 15% of the survivors were still RRT-dependent on day 60 (4). A recent meta-analysis including 15 studies concluded that the development of AKI in COVID-19 was associated with a 19-fold increased odds of in-hospital death (5).
Given the large impact of COVID-19–associated AKI on patient outcomes, especially among those treated with RRT, special attention is required to identify patients at higher risk for developing AKI early in the course of the syndrome, thus, enabling timely implementation of supportive measures. According to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, AKI is diagnosed based on changes in kidney function such as rise in serum creatinine. However, serum creatinine has a low sensitivity; in patients with normal underlying kidney function, a significant proportion of the glomerular filtration rate has to be lost before a change in serum creatinine is detectable. Urine output has a low specificity as it can be effected by volume status, anti-diuretic hormone secretion, and medications, without actual change in kidney function (6). The recognition of AKI is unacceptably delayed in up to 43% of hospitalized patients (7), leading to potential loss of therapeutic opportunities.
Increased urinary biomarkers of kidney stress can identify patients at high risk for AKI (8). In the cardiac surgical setting, the implementation of supportive measures in these high-risk patients might reduce the occurrence or severity of AKI (9, 10). In this context, tissue inhibitor of metalloproteinases 2 (TIMP-2) and the insulin-like growth factor binding protein 7 (IGFBP7) have been identified as possible AKI biomarkers, given that both molecules are released early following various insults to the kidney, resulting in reversible G1 cell cycle arrest (11–13).
This study aimed to explore the role of the urinary biomarkers TIMP-2 and IGFBP7 to predict AKI in critically ill patients with COVID-19.
METHODS
Study Design and Ethics
We conducted a multicenter, multinational, observational study in 12 centers across Europe and the United Kingdom. Institutional review board approval was obtained from the Research Ethics Committee of the Chamber of Physicians Westfalen-Lippe and the Westfalian Wilhelms University Muenster (2020-265-f-S) as well as the corresponding institutional review boards of all participating centers. The study was registered prior to patient enrollment at ClinicalTrials.gov (NCT04406688, date of registration May 28, 2020). In the United Kingdom, it was approved as a National Institute for Health and Care Research (NIHR) portfolio study (NIHR Central Portfolio Management System Identificator 45824). The study was conducted in accordance with the Declaration of Helsinki (Version Fortaleza, 2013). Written informed consent was obtained from all participating patients or representatives according to local requirements and legislation.
Participants
Eligible patients included adult patients with moderate or severe ARDS (according to the Berlin definition: acute onset of hypoxemia [moderate, Pao2/Fio2 = 101–200 mm Hg with positive end-expiratory pressure (PEEP) ≥ 5 cm H2O and severe, Pao2/Fio2 ≤ 100 mm Hg with PEEP ≥ 5 cm H2O] with bilateral infiltrates on frontal chest radiograph, with no evidence of left atrial hypertension) due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (positive polymerase chain reaction test). Patients were excluded if any of the following criteria were present: preexisting AKI, severe chronic kidney disease (CKD) with an estimated glomerular filtration rate (eGFR) less than 20 mL/min/1.73 m2, chronic dialysis dependency, kidney transplant within the last 12 months, pregnancy or breastfeeding.
Study Procedures
Patients admitted with moderate or severe ARDS within 7 days due to COVID-19 infection were included in this study. Patients or their legally authorized representatives were approached by members of the study team and invited to give written informed consent/assent for participation in the study. Consent processes followed local guidelines. With informed consent in place, blood and urine samples were collected at different time points: at study inclusion, 12 hours, and 24 hours later. Urinary (TIMP-2) × (IGFBP7) was measured using the NephroCheck test (BioMérieux, Lyon, France).
Outcomes
The purpose of this study was to analyze the predictive performance of (TIMP-2) × (IGFBP7) for moderate or severe AKI in patients with SARS-CoV-2–associated ARDS. Accordingly, the primary endpoint of the study was the rate of moderate or severe AKI (KDIGO stage 2/3). within 7 days. Secondary endpoints included the rate of transient (< 72 hr) and persistent AKI (≥ 72 hr), treatment with RRT during hospital stay, duration of RRT, mortality during hospital stay, duration of mechanical ventilation, duration of vasopressor administration, and ICU and hospital length of stay.
Statistical Analysis
In the primary statistical analysis, the distribution of biomarker values (TIMP-2) × (IGFBP7) at study inclusion, 12 hours, and 24 hours after inclusion was compared between those patients who did and did not reach the primary endpoint using two-sided Wilcoxon-Mann-Whitney (WMW) tests . Sample size considerations were based on previously published data (14). At 12 hours after inclusion, median biomarker values of 0.2 and 0.5 (ng/mL)2/1,000, respectively, were expected in patients who did not and did reach the primary endpoint, with sds of logarithmized values 1 and 3, respectively. Five percent of the recruited patients are assumed to have missing data, and among the remaining patients, 25% are assumed to reach the primary endpoint. Under these assumptions the power of the WMW test is greater than85%.
Multivariable predictive analyses of the occurrence of the primary endpoint were performed by logistic regression. An initial model includes the baseline clinical variables sex, age, weight, hypertension, congestive heart failure, diabetes, CKD (eGFR < 60 mL/min), severe ARDS, peripheral vascular disease, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers. Based on the initial model stepwise variable selection was applied to establish a clinical reference model, as well as a new model with the additional biomarker (TIMP-2) × (IGFBP7). The predictive performance regarding the occurrence of the primary endpoint was evaluated by receiver operating characteristic (ROC) analyses, the Youden index, the integrated discrimination improvement (IDI), and the category-free net reclassification improvement (cfNRI).
Statistical analyses were performed using the SAS software (Version 9.4 for Windows; SAS Institute, Cary, NC) and IBM SPSS Statistics 28 for Windows (IBM Corporation, Somers, NY). All statistical analyses are exploratory, not confirmatory. p values are regarded noticeable (“significant”) in case p value of less than or equal to 0.05 without adjustment for multiple testing. An overall significance level was not determined and cannot be calculated.
RESULTS
Patients
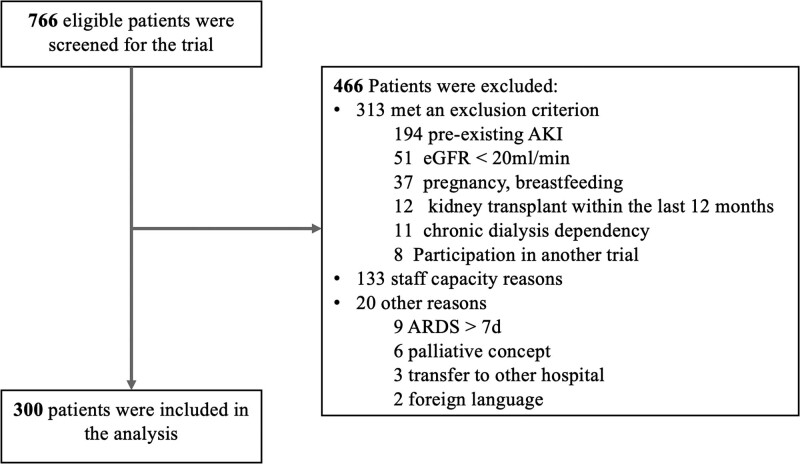
The study was conducted between June 2020 and December 2021 in 12 centers in Germany, Spain, Italy, Portugal, and the United Kingdom. A total of 766 patients were screened and 300 patients were enrolled (Fig. 1). Mean age was 57 years (sd, 14 yr) and 71% were males. Baseline serum creatinine was 0.83 mg/dL (sd, 0.21 mg/dL). Median Sequential Organ Failure Assessment score was 7 (Q1–Q3, 3–10) and median Acute Physiology And Chronic Health Evaluation II score 16 (Q1–Q3, 11–25). Further baseline characteristics are shown in Table 1.
Figure 1.
Participant flow. AKI = acute kidney injury, ARDS = acute respiratory distress syndrome, eGFR = estimated glomerular filtration rate.
TABLE 1.
Baseline and Patient Characteristics
| Characteristics | Total (n = 300) | No Moderate/Severe AKI (n = 261) | Moderate/Severe AKI (n = 39) | p |
|---|---|---|---|---|
| Age, mean (sd), yr | 56.9 (13.9) | 56.2 (14.0) | 61.3 (12.9) | 0.034 |
| Male sex, n (%) | 213 (71.0) | 185 (70.9) | 28 (71.8) | 0.907 |
| Weight, mean (sd), kg | 89.5 (20.9) | 89.1 (21.0) | 91.7 (20.2) | 0.481 |
| Sequential Organ Failure Assessment score, median (Q1–Q3) | 7 (3–10) | 7 (3–9) | 10 (5–12) | < 0.001 |
| Acute Physiology And Chronic Health Evaluation score, median (Q1–Q3) | 16 (11–25) | 15 (10–23) | 26 (13–32) | < 0.001 |
| Baseline creatinine, mean (sd), mg/dLa | 0.83 (0.21) | 0.83 (0.2) | 0.82 (0.2) | 0.709 |
| Time acute respiratory distress syndrome to inclusion, median (Q1–Q3), d | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.120 |
| Time COVID-19 positivity to inclusion (Q1–Q3), d | 6 (3–10) | 6 (3–10) | 5 (4–12) | 0.778 |
| COVID-19 symptoms at hospital admission, n (%) | ||||
| Fever/shivering | 204 (68.0) | 183 (78.5) | 21 (56.8) | 0.004 |
| Dry cough | 167 (55.7) | 152 (65.0) | 15 (40.5) | 0.005 |
| Loss of taste or smell | 28 (9.3) | 25 (10.8) | 3 (8.1) | 0.616 |
| Respiratory distress | 228 (76.0) | 194 (80.2) | 34 (89.5) | 0.170 |
| Headache | 44 (14.7) | 41 (17.8) | 3 (8.1) | 0.139 |
| Gastrointestinal symptoms | 50 (16.7) | 44 (18.8) | 6 (16.7) | 0.759 |
| Lowest Horovitz index at inclusion, median (Q1–Q3), mm Hg | 103.0 (79.0–143.0) | 107.0 (82.5–144.0) | 88.5 (68.8–120.0) | 0.185 |
| Extracorporeal membrane oxygenation therapy, n (%) | 59 (19.7) | 47 (8.0) | 12 (30.8) | 0.061 |
| Comorbidities, n (%) | ||||
| Hypertension | 123 (41.0) | 103 (39.8) | 20 (51.3) | 0.173 |
| Congestive heart failure | 11 (3.7) | 11 (4.2) | 0 (0) | 0.191 |
| Diabetes | 59 (19.7) | 51 (19.7) | 8 (20.5) | 0.904 |
| Chronic obstructive pulmonary disease | 19 (6.3) | 16 (6.2) | 3 (7.7) | 0.718 |
| Chronic kidney disease | 5 (1.7) | 4 (1.5) | 1 (2.6) | 0.641 |
| Myocardial infarction | 14 (4.7) | 11 (4.3) | 3 (7.7) | 0.346 |
| Atrial fibrillation | 17 (5.7) | 14 (5.4) | 3 (7.7) | 0.562 |
| Stroke | 9 (3.0) | 7 (2.8) | 2 (5.1) | 0.430 |
| Peripheral vascular disease | 10 (3.3) | 5 (1.9) | 5 (12.8) | < 0.001 |
| Medication, n (%) | ||||
| ß-blockers | 48 (16.0) | 39 (15.0) | 9 (23.7) | 0.174 |
| Statins | 71 (23.7) | 60 (23.1) | 11 (28.9) | 0.428 |
| Diuretics | 45 (15.0) | 38 (14.7) | 7 (18.4) | 0.547 |
| Angiotensin-converting enzyme inhibitors | 59 (19.7) | 50 (19.3) | 9 (23.7) | 0.527 |
| Angiotensin receptor blockers | 42 (14.0) | 36 (13.8) | 6 (15.8) | 0.748 |
AKI = acute kidney injury.
This value represents patients premorbid kidney function.
Patients who met the primary endpoint were older, more likely to suffer from peripheral vascular disease and had higher severity of illness scores. (Table 1)
Outcomes
Of 300 included patients, 86 developed AKI (28.7%) of which 46 episodes were transient and 29 persistent (Table 2). Thirty-nine of 300 patients (13%) met the primary endpoint moderate/severe AKI within 7 days (15 patients [5.0%] KDIGO stage 2 and 24 patients [8.0%] KDIGO stage 3). The median time to development of AKI was 1 day (Q1–Q3, 0–2 d). Among patients meeting the primary endpoint, 17 of 39 received RRT (43.6%) and 32 died (82.1%) (Table 2).
TABLE 2.
Outcome Parameters
| Outcome | No Moderate/Severe AKIa (n = 261) | Moderate/Severe AKIa (n = 39) | p a |
|---|---|---|---|
| AKI all, n (%) | 47 (18.2) | 39 (100) | < 0.001 |
| Transientb | 32 (80.0) | 14 (40.0) | |
| Persistentb | 8 (20.0) | 21 (60.0) | |
| Renal replacement therapy, n (%) | 8 (3.1) | 17 (43.6) | < 0.001 |
| Mortality during hospital stay, n (%) | 63 (24.7) | 32 (82.1) | < 0.001 |
| Duration of mechanical ventilation, median (Q1–Q3), d | 11 (6–30) | 42 (19–68) | 0.004c |
| Duration of vasopressor administration, median (Q1–Q3), d | 5 (2–19) | 46 (33–46) | < 0.001c |
| ICU length of stay, median (Q1–Q3), d | 15 (8–42) | 48 (29–70) | 0.001c |
| Hospital length of stay, median (Q1–Q3), d | 26 (17–47) | 77 (30–91) | 0.015c |
AKI = acute kidney injury.
Referring to the primary endpoint moderate/severe AKI within 7 d.
Missing values 11.
Log rank.
Biomarker Performance
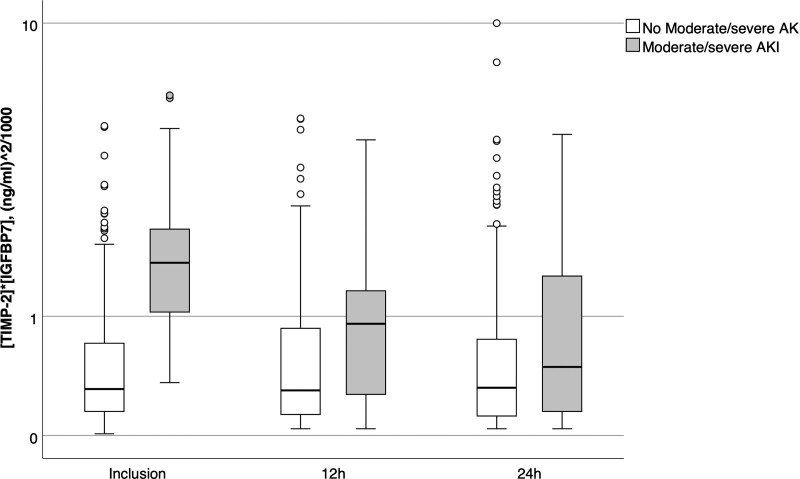
Patients who met the primary endpoint had significantly higher urinary (TIMP-2) × (IGFBP7) concentrations at the time of inclusion and 12 hours later compared with endpoint-negative patients (inclusion: median 1.73 [Q1–Q3, 1.0–2.48] [ng/mL]2/1,000 vs 0.31 [Q1–Q3, 0.15–0.71] [ng/mL]2/1,000; p < 0.001; 12 hr: median 0.92 [Q1–Q3, 0.25–1.44] [ng/mL]2/1,000 vs 0.30 [Q1–Q3, 0.13–0.87] [ng/mL]2/1,000; p = 0.003). No significant differences in (TIMP-2) × (IGFBP7) levels were detectable at 24 hours (Fig. 2).
Figure 2.
Urinary tissue inhibitor of metalloproteinases 2 and insulin like growth factor binding protein 7 (TIMP-2) × (IGFBP7) levels at different time points. The box represents the 25th/75th percentile. Whiskers are drawn from the ends of the box to the largest and smallest observed values within 1.5 times the interquartile range. Outliers beyond the whiskers are represented by circles. AKI = acute kidney injury.
At inclusion, the optimal cutoff value (maximizing the Youden index) for the prediction of the primary endpoint was 0.90 (ng/mL)2/1,000 (Youden index, 0.67; sensitivity, 85%; specificity, 82%) (eTable 1, http://links.lww.com/CCM/H320).
The median lead time provided by the positive biomarker result was 24 hours (Q1–Q3, 0–24 hr).
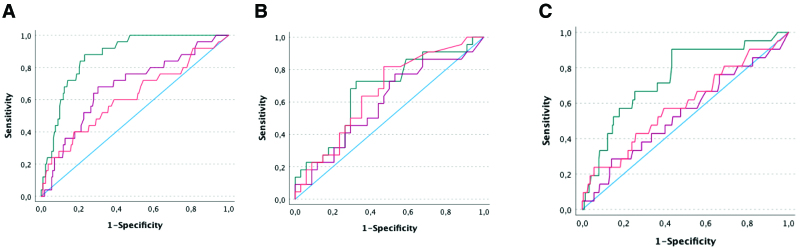
The ROC analyses for prediction of AKI resulted in an area under the receiver operating characteristic curve (AUC) of 0.89 (95% CI, 0.84–0.93) at inclusion, 0.67 (95% CI, 0.56–0.78) at 12 hours and 0.58 (95% CI, 0.46–0.70) at 24 hours after inclusion (Fig. 3A). For prediction of persistent AKI, the AUC was 0.81 (95% CI, 0.73–0.90) at inclusion, AUC 0.60 (95% CI, 0.46–0.73) at 12 hours and AUC 0.60 (95% CI, 0.47–0.73) at 24 hours (Fig. 3B). For prediction of RRT, the AUC was 0.76 (95% CI, 0.66–0.86) at inclusion, 0.58 (95% CI, 0.45–0.72) at 12 hours and 0.57 (95% CI, 0.44–0.70) at 24 hours (Fig. 3C).
Figure 3.
Predictive performance of (tissue inhibitor of metalloproteinases 2 [TIMP-2]) × (insulin-like growth factor binding protein 7 [IGFBP7]) for kidney outcomes. Receiver operating characteristic curves of urinary TIMP-2 and IGFBP7 for prediction of moderate/severe acute kidney injury (AKI) (A), prediction of persistent AKI (B), and for prediction of renal replacement therapy (C) (green line: at inclusion, red line: 12 hr after inclusion, orange line: 24 hr after inclusion).
Additional Predictive Ability of (TIMP-2) × (IGFBP7) Over Clinical Variables
Urinary (TIMP-2) × (IGFBP7) significantly improved risk prediction when added to the clinical model for the primary endpoint, using ROC, AUC, IDI, and cfNRI analyses (eTable 2, http://links.lww.com/CCM/H320).
DISCUSSION
The Acute Disease Quality Initiative consensus statement on COVID-19–associated AKI recommended to investigate novel biomarkers for the diagnosis and prognosis of AKI in the setting of COVID-19. Urinary (TIMP-2) × (IGFBP7) concentrations have been analyzed in non-COVID-19 settings with good predictive ability to detect patients at risk for AKI (11, 13, 15). In a biomarker-based approach, these two G1 cell cycle arrest markers guided the implementation of a bundle of supportive measures which was associated with a significantly reduced occurrence of AKI (9). Such a patient individualized approach using novel biomarkers could also be beneficial for COVID-19 patients in order to improve outcomes. However, first it is important to investigate whether (TIMP-2) × (IGFBP7) is also predictive for AKI in this specific setting. In this study, we demonstrated that urinary (TIMP-2) × (IGFBP7) has an even better ability to predict AKI than in other settings (15, 16). When adding (TIMP-2) × (IGFBP7) to a clinical model, risk prediction increased significantly compared with only using clinical variables. In other settings, it has been demonstrated that the implementation of the KDIGO bundle (discontinuation of all nephrotoxic agents, ensure volume status and perfusion pressure, consideration of a functional hemodynamic monitoring, monitoring of serum creatinine and urine output, avoidance of hyperglycemia, and consideration of alternatives to radiocontrast agents) in patients at high risk for AKI can reduce the occurrence of AKI (9, 10, 17). Transferring the new data to COVID-19 patients, patients with elevated (TIMP-2) × (IGFBP7) levels should also be treated according to the KDIGO guidelines by implementing the KDIGO bundle.
To our best knowledge, only few small studies have investigated the performance of (TIMP-2) × (IGFBP7) in critically ill patients with COVID-19. One small study demonstrated that patients with COVID-19 associated AKI and high levels of (TIMP-2) × (IGFBP7) were more prone to receive RRT compared with those with low levels of (TIMP-2) × (IGFBP7) (18). Another small observational study showed that urinary (TIMP-2) × (IGFBP7) greater than or equal to 0.2 was a risk factor for AKI (hazard ratio 7.23; 95% CI, 0.99–52.4; p = 0.050) (19). Both studies did not specifically focus on COVID-19 patients with ARDS.
Interestingly, we found that urinary (TIMP-2) × (IGFBP7) did not persisted for very long after enrollment. Although levels at 12 hours were still significantly higher in patients who met the endpoint, the levels were, on average, lower than at enrollment (Fig. 2). The predictive value of (TIMP-2) × (IGFBP7) is dependent on the kinetic of the biomarkers in the context of the course of the disease. We know from other studies that (TIMP-2) × (IGFBP7) sharply increases after an insult and then rapidly decreases. Obviously, the first time point we measured (TIMP-2) × (IGFBP7) was at the peak of the kinetic curve and therefore the predictive value was very good. Therefore, it is possible that the two later time points were too late. We speculate that hypoxemia may have been the trigger for kidney stress which was then reflected by (TIMP-2) and (IGFBP7) release. Once the patient was admitted to the hospital, the hypoxemic stress was presumably reduced or eliminated completely. Further studies will need to explore this hypothesis further.
Important strengths of this study are the multicenter study design as well as the large sample size and the application of both, serum creatinine and urine output criteria to diagnose AKI. However, we also acknowledge some limitations. First, we cannot account for the virus type. Most of the patients were included during the Delta variant interval. However, due to the international collaboration, we cannot preclude that some patients may have been infected with other variants. Whether the risk of AKI varies with different virus variants or (unlikely) impacts the expression of (TIMP-2) × (IGFBP7) is unknown. Second, we focused on COVID-19 patients with moderate and severe ARDS who are particularly susceptible for AKI. It remains unknown whether the results are generalizable to other COVID-19 cohorts. Third, as the biomarker levels within the first 24 hours of enrollment were not different, the timing of the measurement in the course of the COVID-19 infection is very important. In this study, the median time from moderate/severe ARDS to inclusion was 0 days. The results of this study may not be transferable to patients meeting the ARDS criteria days earlier. Fourth, confounding is a frequent issue in observational studies. To control for this, we per-formed multivariable analyses and confirmed the additional value of (TIMP-2) × (IGFBP7) for early identifying AKI. However, residual effects from unmeasured confounders upon the findings cannot be ruled out.
CONCLUSIONS
In patients with COVID-19–associated moderate or severe ARDS, urinary (TIMP-2) × (IGFBP7) demonstrated a high predictive ability for moderate or severe AKI. Future studies need to investigate whether (TIMP-2) × (IGFBP7) based implementation of supportive measures helps to reduce the occurrence of AKI.
ACKNOWLEDGMENTS
We would like to thank the study nurses at all participating centers for patient recruitment and data entry and the staff in the participating ICUs for supporting the study. Also, we acknowledge the support by the Open Access Publication Fund of the University of Münster, Germany.
Supplementary Material
Footnotes
Drs. Meersch and Zarbock had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Meersch, Gerss, and Zarbock designed the study and study concept. Drs. Weiss and von Groote, Ms. Leidereiter, and Drs. Ostermann, Lumlertgul, Weerapolchai, Garcia, Cano, del Corral, Broch-Porcar, Perez Carrasco, De la Vega Sanchez, Sousa, Catarino, Roig, Martinez de Irujo, de Rosa, de la Peña, Tomasa, Brivio, De Molina, Wempe, Meersch, and Zarbock were involved in acquisition of data. Drs. Meersch, Gerss, Kellum, and Zarbock were involved in analysis and interpretation of data. Drs. Meersch, Kellum, Gerss, and Zarbock were involved in drafting of the article. Drs. Weiss and von Groote, Ms. Leidereiter, and Drs. Ostermann, Lumlertgul, Weerapolchai, Garcia, Cano, del Corral, Broch-Porcar, Perez Carrasco, De la Vega Sanchez, Sousa, Catarino, Roig, Martinez de Irujo, Silvia De Rosa, de la Peña, Tomasa, Brivio, De Molina, and Wempe were involved in critical revision of the article for important intellectual content. Drs. Gerss and Meersch were involved in statistical analysis. Drs. Meersch and Zarbock were involved in obtained funding. All authors read and approved the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
The study was supported by the German Research Foundation (KFO342-1, ZA428/18-1, and ZA428/21-1 to Dr. Zarbock and ME5413/1-1 to Dr. Meersch) and an unrestricted research grant from BioMerieux.
Dr. Ostermann received research funding from BioMérieux and Baxter. Dr. Garcia received funding from Edwards Lifesciences. Dr. Cano disclosed work for hire. Dr. Broch-Porcar disclosed that she participated in a lecture in Nephroweek with economical compensation from BioMereiux. Drs. de la Peña, Meersch, and Zarbock received funding from Baxter. Dr. Brivio received funding from Jafron and Bbraun. Dr. de Rosa received lectures fees from ESTOR SpA. Drs. Kellum’s and Zarbock’s institutions received funding from BioMereiux. Drs. Kellum, Meersch, and Zarbock received funding from BioMereiux. Dr. Meersch received funding from FMC and Baxter. Dr. Gerss received honoraria from TESARO, QUIRIS Healthcare, Ecker+Ecker, Dr August Wolff, Roche, University Clinics Schleswig-Holstein, and RWTH Aachen University. Dr. von Groote was supported by a rotational position of KFO 342, funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)–ZA428/18-1. Dr. Zarbock’s institution received funding from DFG, Baxter, and Fresenius; he received funding from AM Pharma, Novartis, Alexion, Bayer, Guard Therapeutics, Paion, and Fresenius as well as independent research grants from the German Research Foundation. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Drs. Meersch and Zarbock contributed equally and share authorship.
Trial registration: The study was registered prior to patient enrollment at ClinicalTrials.gov (NCT04406688, date of registration May 28, 2020).
REFERENCES
- 1.Nadim MK, Forni LG, Mehta RL, et al. : COVID-19-associated acute kidney injury: Consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020; 16:747–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alenezi FK, Almeshari MA, Mahida R, et al. : Incidence and risk factors of acute kidney injury in COVID-19 patients with and without acute respiratory distress syndrome (ARDS) during the first wave of COVID-19: A systematic review and meta-analysis. Ren Fail. 2021; 43:1621–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan L, Chaudhary K, Saha A, et al. ; on behalf of the Mount Sinai COVID Informatics Center (MSCIC): AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021; 32:151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S, Coca SG, Chan L, et al. ; and the STOP-COVID Investigators: AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol. 2021; 32:161–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheruiyot I, Henry B, Lippi G, et al. : Acute kidney injury is associated with worse prognosis in COVID-19 patients: A systematic review and meta-analysis. Acta Biomed. 2020; 91:e2020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronco C, Bellomo R, Kellum JA: Acute kidney injury. Lancet. 2019; 394:1949–1964 [DOI] [PubMed] [Google Scholar]
- 7.MacLeod A: NCEPOD report on acute kidney injury-must do better. Lancet. 2009; 374:1405–1406 [DOI] [PubMed] [Google Scholar]
- 8.Ostermann M, Zarbock A, Goldstein S, et al. : Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: A consensus statement. JAMA Netw Open. 2020; 3:e2019209. [DOI] [PubMed] [Google Scholar]
- 9.Meersch M, Schmidt C, Hoffmeier A, et al. : Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial. Intensive Care Med. 2017; 43:1551–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarbock A, Kullmar M, Ostermann M, et al. : Prevention of cardiac surgery-associated acute kidney injury by implementing the KDIGO guidelines in high-risk patients identified by biomarkers: The PrevAKI-multicenter randomized controlled trial. Anesth Analg. 2021; 133:292–302 [DOI] [PubMed] [Google Scholar]
- 11.Kashani K, Al-Khafaji A, Ardiles T, et al. : Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013; 17:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoste EA, McCullough PA, Kashani K, et al. ; Sapphire Investigators: Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant. 2014; 29:2054–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meersch M, Schmidt C, Van Aken H, et al. : Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014; 9:e93460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nusshag C, Rupp C, Schmitt F, et al. : Cell cycle biomarkers and soluble urokinase-type plasminogen activator receptor for the prediction of sepsis-induced acute kidney injury requiring renal replacement therapy: A prospective, exploratory study. Crit Care Med. 2019; 47:e999–e1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honore PM, Nguyen HB, Gong M, et al. ; Sapphire and Topaz Investigators: Urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 for risk stratification of acute kidney injury in patients with sepsis. Crit Care Med. 2016; 44:1851–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su LJ, Li YM, Kellum JA, et al. : Predictive value of cell cycle arrest biomarkers for cardiac surgery-associated acute kidney injury: A meta-analysis. Br J Anaesth. 2018; 121:350–357 [DOI] [PubMed] [Google Scholar]
- 17.Gocze I, Jauch D, Gotz M, et al. : Biomarker-guided intervention to prevent acute kidney injury after major surgery: The prospective randomized BigpAK study. Ann Surg. 2018; 267:1013–1020 [DOI] [PubMed] [Google Scholar]
- 18.Husain-Syed F, Wilhelm J, Kassoumeh S, et al. : Acute kidney injury and urinary biomarkers in hospitalized patients with coronavirus disease-2019. Nephrol Dial Transplant. 2020; 35:1271–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casas-Aparicio G, Alvarado-de la Barrera C, Escamilla-Illescas D, et al. : Role of urinary kidney stress biomarkers for early recognition of subclinical acute kidney injury in critically ill COVID-19 patients. Biomolecules. 2022; 12:275. [DOI] [PMC free article] [PubMed] [Google Scholar]