Background:
Ultrasound-assisted liposuction (UAL) is a popular and minimally invasive cosmetic procedure. Third-generation devices such as the vibration amplification of sound energy at resonance (VASER)lipo system are used for body contouring with enhanced tissue specificity. Despite the widespread use of VASER UAL, published guidelines and recent expert consensus recommendations are lacking. The objective of this study is to develop an expert consensus on the recommendations for use of VASER UAL.
Methods:
In a modified Delphi process, a panel of five US-based, expert plastic surgeons participated in three rounds of consensus building that spanned 4 months to align on guidance statements for the use of VASER as an adjunct to liposuction.
Results:
After the experts responded to an online questionnaire that assessed device settings, postoperative instructions, side effects or complications, and best practices, 32 initial consensus statements were developed. By round 3, these consensus statements for VASER UAL had been reduced and refined to a total of 18.
Conclusions:
To improve patient outcomes, clinicians must understand key factors and best practices when using VASER UAL, including device settings, provider technique, managing side effects, potential complications, and postoperative care. The consensus statements developed herein aim to provide clinicians with expert-backed recommendations for the use of VASER UAL.
Takeaways
Question: Despite literature supporting the clinical use and success of VASER, no practical treatment guidelines have been published.
Findings: Using a modified Delphi method, five experts in cosmetic surgery developed 18 consensus statements regarding the use of VASER.
Meaning: These consensus statements aim to provide clinicians with expert-backed recommendations for the use of VASER, including device settings, provider technique, managing side effects, potential complications, and postoperative care.
INTRODUCTION
Liposuction is a surgical and minimally invasive access procedure that involves body contouring for volume loss, body fat reduction or augmentation, and harvesting of viable fat for soft tissue augmentation.1,2 Relative to other surgical procedures, advantages of liposuction include typically shorter surgery and recovery times, low complication rates, and generally permanent results. The current therapeutic landscape of liposuction comprises numerous techniques, including traditional suction-assisted, laser-assisted, water-jet-assisted, radiofrequency-assisted, power-assisted, and ultrasound-assisted.1,3,4
Ultrasound-assisted liposuction (UAL) was initially developed in the late 1980s.5 This technique utilizes ultrasonic waves to emulsify fat in the target area, which can then be aspirated with less tissue trauma relative to other forms of lipoplasty. Indeed, third-generation ultrasound improves on the limitations of previous generation devices by delivering less energy with greater efficacy and can be customized to meet clinical requirements for full body contouring (infiltration, emulsification, and aspiration).2,5–7 This technique also selectively targets fat while preserving vasculature and minimizing blood loss,5,8 and it has been used to harvest viable adipocytes.2
Third-generation UAL is represented by the vibration amplification of sound energy at resonance (VASER)lipo system (Solta Medical, Bothell, Wash.).5 VASER UAL is an ultrasound system intended for fragmentation, emulsification, and aspiration of subcutaneous fatty tissue for aesthetic body contouring.9 VASER utilizes small-diameter, solid, multiringed probes that deliver power in a pulsed mode at 36 kHz, the level necessary for targeting and disrupting fatty tissues.2 In addition to lipoplasty, VASER UAL is indicated for use in numerous surgical specialties, including neurologic, urologic, general, gynecologic, laparoscopic, orthopedic, plastic and reconstructive, and thoracic surgery, for the fragmentation, emulsification, and aspiration of soft tissues.9 Although VASER UAL has been used by plastic surgeons since the early 2000s,6 there is currently a lack of up-to-date published guidance for this technology. Therefore, we have developed a set of clinician-led consensus statements on the use of third-generation VASER UAL.
METHODS
An online survey of 77 questions was developed based on a modified Delphi process published for a related device, with clinical input and review from authors.10 Topics included device settings, postoperative instructions, side effects or complications, and best practices for VASER UAL. Concurrently, a panel of five US plastic surgeons were invited to develop consensus statements. The experts were all board-certified plastic surgeons with clinical experience treating patients with VASER UAL (across surgeons, between ~200 and 360 VASER cases performed per year). Experts varied in their experience with VASER; three experts had 5 years or less of experience using VASER and two had more than 15 years of experience.
The electronic questionnaire was completed by all panelists. On the basis of expert responses to the survey, 32 consensus statements were developed. These statements were then distributed and reviewed by the panelists across multiple rounds in accordance with a modified Delphi technique adapted previously (Fig. 1).10 In subsequent rounds, additional surveys were presented to the panelists in which they could anonymously review, comment, and align on the statements.
Fig. 1.
Overview of consensus statement development and the Delphi technique adapted from the work of Chapas et al. J Drugs Dermatol. 2020.10
Table 1 shows the algorithm through which statements were analyzed for inclusion and/or modification.10 All participants were asked to judge whether each statement was clear (yes or no) and if the statement should be included (1 “definitely do not include” to 9 “definitely include”), as well as to provide any optional comments to improve the clarity of the statement. In subsequent surveys, where a statement was modified, participants were asked to rerate the revised statements. Deleted statements were not presented to the panel in subsequent rounds.
Table 1.
Delphi Technique Consensus Statement Inclusion Criteria
| Statement Result | Threshold Applied |
|---|---|
| Definitely include | (i) >80% of consensus panel rate statement as =9 OR (ii) Median rating of >8 |
| Maybe include | (i) >70% of consensus panel rate statement as =9 OR (ii) Median rating of >7 |
| Definitely exclude | (i) <70% of consensus panel rate statement as =9 AND 100% of consensus panel said statement was clear OR (ii) Median rating of <6 AND 100% of consensus panel said statement was clear* |
| Revise | (i) Major revisions suggested OR (ii) <70% of panel rate statement as =9 AND <100% of consensus panel said statement was clear* |
Suggesting that low scores were not due to lack of understanding of proposed consensus statement.
All online surveys were built and distributed using SurveyMonkey software. Data collection for all three rounds took place over 4 months. All five clinicians participated in electronic correspondence for rounds 1 and 2. Four of five panelists participated in the subsequent conference call to align on final statements, with electronic follow-up for the remaining participant.
RESULTS
Experts’ responses to the round 0 online questionnaire resulted in the initial development of 32 statements. After the round 1 review of these 32 statements, nine were listed as definitely include, 12 were listed as maybe include, three were listed as revise, and eight were deleted. Of these 24 statements entering round 2, four statements were rated definitely include without modification and were retained for inclusion in the final statement list; thus, a total of 20 statements were submitted to the panelists for revision and rerating in round 2.
Of the 20 statements entering round 3, six were listed as definitely include, five as maybe include, and three as revise; six were deleted. After this round, a conference call was conducted with four of the five experts to review and discuss the five maybe include and three revise statements. Minor text revisions were also made to the wording of prior definitely include without modification statements accepted from rounds 1 and 2 to align with the VASER label. An additional statement was added as a result of revisions during the conference call that split subject matter from one previous statement into two new statements.
The conference call and subsequent electronic follow-ups resulted in the acceptance of 18 final consensus statements.
Final Consensus Statements
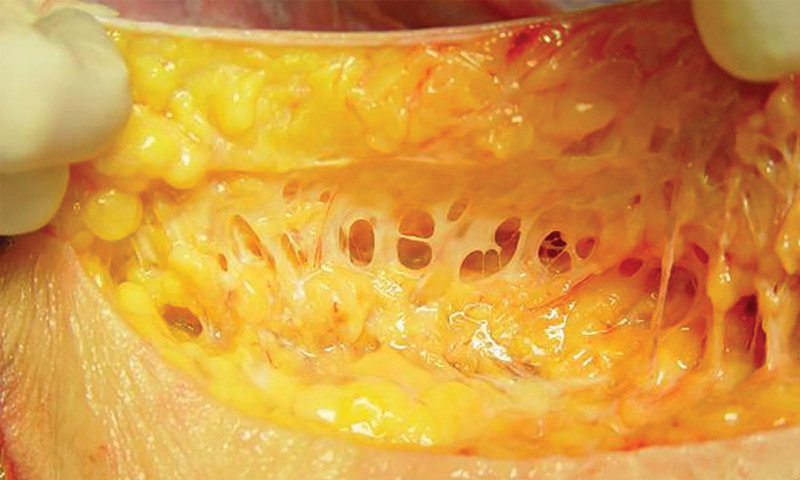
Best practice recommendations to follow when utilizing VASER UAL include preservation of the subdermal fibroseptal network (Fig. 2), utilizing enough suction pressure to efficiently aspirate, guiding hand monitoring of depth, evenly pacing strokes, and using two or more port sites in an area to optimize effectiveness of fat removal.
Use of full-day compression garments is preferred for approximately 1.5 months after VASER UAL, the length of which is determined by degree of edema; best practices for compression garments are similar between VASER UAL and traditional liposuction.
Compression may minimize the risk of seromas, and serial drainage is recommended for follow-up treatment of seromas.
Significant blood loss after VASER UAL is very rare and occurs less often than with traditional liposuction.
VASER UAL does not compromise the architecture and proliferative capacity of adipose-associated tissues harvested at clinically relevant settings and ultrasound exposure times.
Manual lymphatic massage after use of VASER liposuction technology should optimally be performed on a daily or weekly basis for six to 15 sessions or until drainage has resolved.
Relative to traditional liposuction, side effects of VASER UAL (eg, bruising and pain) are less severe and shorter in duration.
Suction-pressure settings vary based on different body areas, such as the face, neck, breast, chest, abdomen, or flanks.
VASER UAL may be used alongside other liposuction technologies, including power-, suction-, or radio frequency–assisted liposuction at the discretion of the treating physician.
VASER UAL may be used after reconstructive procedures, at the discretion of the treating physician.
The suggested ratio of VASER ultrasound time to wetting solution or tumescent for most body areas is 1 minute to 200 to 250 cc, with the expected endpoint of loss of resistance. Factors, such as physician experience, presence of fibrous tissue, or prior surgery or liposuction, may alter suggested protocols for ratios of wetting solution or tumescent to VASER ultrasound time.
Other uses of VASER UAL for experienced users include pretunneling, male breast reduction, and predissections for cosmetic body and facial procedures.
Pulsed mode (V) is preferred versus continuous mode (C).
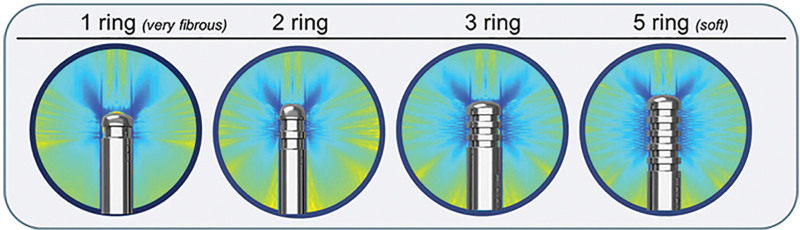
The three-ring and five-ring probes can be used on most body areas, and both are effective for all subcutaneous tissues.
In cases of more fibrous tissue, consider use of two- or three-ring probe; use of one-ring probe is not warranted in most situations.
VASER UAL may be used in combination with excisional procedures and energy-based technologies, at the discretion of the treating physician.
VASER UAL has a demonstrated safety profile and a history of efficacy in treating large volumes and multiple body areas, including the face, neck, arms, chest, abdomen, back/flanks, dorsal hump, lower extremities, and buttocks.
With proper training, advanced techniques using VASER, such as high-definition liposculpture, may be performed on the arms, chest, abdomen, back/flanks, buttocks, thighs, and calves.
Fig. 2.
Tissue matrix. Image courtesy of William W. Cimino.
DISCUSSION
The consensus panel method is integral to the field of liposuction, because it provides expert-backed recommendations for proper use and safety of VASER UAL, which may ultimately improve patient outcomes. This method also helps to promote communication and collaboration and establishes a foundation for future, large-scale guideline- and recommendation-development projects.
Liposuction is a procedure that entails removing excess fat from the body to achieve the desired contour.1 According to a survey of members of the American Society for Aesthetic Plastic Surgery, liposuction has been the most commonly performed aesthetic surgical procedure since these data began being formally collected in 1997.3 One form of liposculpting, UAL, utilizes ultrasonic energy to precisely and selectively thin subcutaneous adipose tissue.4 Third-generation UAL devices (eg, VASER) improve upon previous generations by using pulsed energy from a small-diameter probe to fragment the fatty matrix at lower energy settings, thereby reducing potential complications and increasing efficiency.5,6,11
The VASER generator/amplifier supplies electrical energy and controls ultrasound vibration frequency (36 kHz) and amplitude; the VASER handpiece contains a piezoelectric transducer that generates sound waves.2,5,11 When an electric current is applied to piezoelectric ceramic crystals, the resulting sonic energy vibrates in resonance with the handpiece.5 The forward (compressing) and backward (pulling) motion of the probe tip creates an expanding spherical wave of ultrasound energy.11 The probe design efficiently disperses energy transmission and coupling for varying properties of tissue (Fig. 3).6,9,11
Fig. 3.
Representative visual of pressure field visualization and probe design. Images adapted from Solta Medical Engineering.
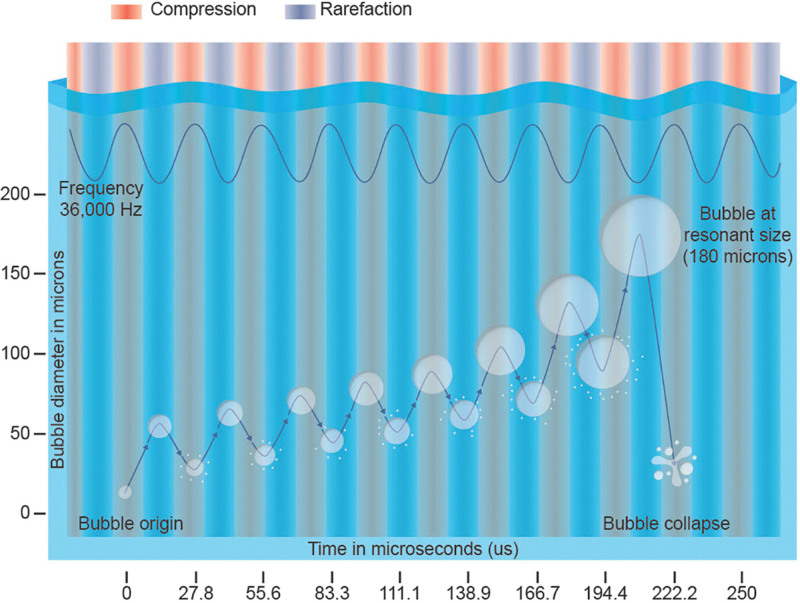
Tumescent fluid, which contains small gas bubbles (5–10 µm), is infused throughout targeted subcutaneous fat, which is more malleable than connective tissue (ie, muscle or bone).11 Sound waves from the probe exert a push/pull force on the gas microbubbles, producing a cavitation effect that allows bubbles to reach a resonant size of 180 µm and then collapse (Fig. 4).5,11 Collapsed bubbles act as wedges between the fat cells, loosening the tissue matrix, as they reach resonant size and explode. Strong, localized, fluid forces from the probe further separate dislodged fat clusters into smaller lipocyte packets, which mix with tumescent fluid and form an emulsion. Because VASER UAL produces less shear force and tissue matrix trauma than suction-assisted liposuction, nonadipose tissue is preferentially preserved. By using optimal ultrasound delivery to specifically target fat cells and spare surrounding tissue, VASER UAL reduces patient discomfort, limits blood loss, and at clinically relevant settings, may preserve fat cells that are suitable for transfer.4,8,11,12 VASER achieved FDA approval for use in liposuction in 2002. Despite its advanced design, probes, and energy application and its potential for protecting and preserving tissue, VASER remains an underutilized tool in body contouring.
Fig. 4.
Effects of cavitation.11 Illustration by Travis Vermilye.
Despite literature supporting the clinical use and success of VASER UAL, no practical treatment guidelines have been published for this technology. These 18 consensus statements aim to educate practitioners and to assist them in achieving successful patient outcomes. However, these recommendations do have limitations. Although expert plastic surgeons followed a transparent methodology to develop these statements, the process was less rigorous than for evidence-based clinical guidelines. Another important limitation is that these recommendations are general in nature and may not apply for individual patients. Other limitations relate to the methodology of the Delphi method, including online collaboration (versus in-person) and increased potential for participant fatigue as the number of rounds increases. Finally, future recommendations and guidelines should leverage the input of a larger, more global team of experts in plastic surgery who utilize VASER UAL. Although additional work is needed to develop evidence-based guidelines for the use of VASER UAL, the recommendations presented herein represent an important step toward standardized guidance for this commonly performed aesthetic surgical procedure.
CONCLUSIONS
Despite nearly two decades of widespread VASER UAL use, up-to-date published guidance is lacking regarding the use of this technology. Indeed, further education regarding optimal device settings, provider technique, and best practices for the management of side effects, complications, and postoperative care may help to improve patient outcomes and reduce downtime. The clinician-led consensus statements presented here aim to assist clinicians in understanding and implementing these key factors while using third-generation VASER UAL.
DISCLOSURES
Dr. Ruff has served as a Medical Advisory Board Member for Apyx Medical and Cartessa, and as a trainer for Allergan, Galderma, and VASER. Dr. Garcia has served as a consultant and speaker for BD Bard (Galatea), Mentor, Solta Medical, and MTF Biologics, and serves on the Board of Directors of the Aesthetic Surgery Education and Research Foundation. Dr. Nykiel has served as a key opinion leader and trainer for VASER and Renuvion. Dr. Galanis has served as a paid speaker for Solta Medical.
ACKNOWLEDGMENTS
Editorial assistance was provided under the direction of the author(s) by MedThink SciCom with support from Deirdre Rodeberg, PhD, CMPP. The authors gratefully acknowledge Wendie I. Grunberg, DO, FACS, for her expert review of the statements, as well as Catherine Parker, NP, MSN, a former employee of Solta Medical.
Footnotes
Disclosure statements are at the end of this article, following the correspondence information.
Funding: Solta Medical is the sole sponsor for this project and provided funding to invited participants, including honoraria for the participation in the survey process and attendance at the final consensus meeting. Solta Medical provided funding to a third-party vendor (MedThink SciCom) for survey development, drafting of statements, logistical support at the final consensus meeting, and medical writing assistance for the article. Solta Medical personnel attended the meetings to provide logistical support. Solta Medical personnel were given opportunities to review draft versions of final statements and this article. No payments were made to the authors for the development of this article.
REFERENCES
- 1.Wu S, Coombs DM, Gurunian R. Liposuction: concepts, safety, and techniques in body-contouring surgery. Cleve Clin J Med. 2020;87:367–375. [DOI] [PubMed] [Google Scholar]
- 2.Schafer ME, Hicok KC, Mills DC, et al. Acute adipocyte viability after third-generation ultrasound-assisted liposuction. Aesthet Surg J. 2013;33:698–704. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad J, Eaves FF, III, Rohrich RJ, et al. The American Society for Aesthetic Plastic Surgery (ASAPS) survey: current trends in liposuction. Aesthet Surg J. 2011;31:214–224. [DOI] [PubMed] [Google Scholar]
- 4.Jewell ML. Ultrasonic-assisted liposuction: introduction and historic perspectives. In Garcia MO, ed. Ultrasound Assisted Liposuction. Cham, Switzerland: Springer Nature Switzerland; 2020:3–8. [Google Scholar]
- 5.Cimino WW. Ultrasound-assisted lipoplasty: basic physics, tissue interactions, and related results/complications. In Prendergast PM, Shiffman MA, eds. Aesthetic Medicine: Art and Techniques. Berlin: Springer; 2011:519–528. [Google Scholar]
- 6.Jewell ML, Fodor PB, de Souza Pinto EB, et al. Clinical application of VASER-assisted lipoplasty: a pilot clinical study. Aesthet Surg J. 2002;22:131–146. [DOI] [PubMed] [Google Scholar]
- 7.Hoyos AE, Millard JA. VASER-assisted high-definition liposculpture. Aesthet Surg J. 2007;27:594–604. [DOI] [PubMed] [Google Scholar]
- 8.Garcia O, Jr, Nathan N. Comparative analysis of blood loss in suction-assisted lipoplasty and third-generation internal ultrasound-assisted lipoplasty. Aesthet Surg J. 2008;28:430–435. [DOI] [PubMed] [Google Scholar]
- 9.Solta Medical, Inc. The VASERlipoTM System: VASER and VASER pro amplifier user’s guide [user manual]. Bothell, WA: Solta Medical, Inc; 2013. [Google Scholar]
- 10.Chapas A, Biesman BS, Chan HHL, et al. Consensus recommendations for 4th generation non-microneedling monopolar radiofrequency for skin tightening: a Delphi consensus panel. J Drugs Dermatol. 2020;19:20–26. [DOI] [PubMed] [Google Scholar]
- 11.Schafer ME. Basic science of ultrasound in body contouring. In Garcia MO, ed. Ultrasound Assisted Liposuction. Cham, Switzerland: Springer Nature Switzerland; 2020:9–20. [Google Scholar]
- 12.Nagy MW, Vanek PF, Jr. A multicenter, prospective, randomized, single-blind, controlled clinical trial comparing VASER-assisted lipoplasty and suction-assisted lipoplasty. Plast Reconstr Surg. 2012;129:681e–689e. [DOI] [PubMed] [Google Scholar]