Abstract
Aims
Examine the association between (1) admission frailty and (2) frailty changes during cardiac rehabilitation (CR) with 5-year outcomes (i.e. time to mortality, first hospitalization, first emergency department (ED) visit, and number of hospitalizations, hospital days, and ED visits).
Methods and results
Data from patients admitted to a 12-week CR programme in Halifax, Nova Scotia, from May 2005 to April 2015 (n = 3371) were analysed. A 25-item frailty index (FI) estimated frailty levels at CR admission and completion. FI improvements were determined by calculating the difference between admission and discharge FI. CR data were linked to administrative health data to examine 5-year outcomes [due to all causes and cardiovascular diseases (CVDs)]. Cox regression, Fine–Gray models, and negative binomial hurdle models were used to determine the association between FI and outcomes. On average, patients were 61.9 (SD: 10.7) years old and 74% were male. Mean admission FI scores were 0.34 (SD: 0.13), which improved by 0.07 (SD: 0.09) by CR completion. Admission FI was associated with time to mortality [HRs/IRRs per 0.01 FI increase: all causes = 1.02(95% CI 1.01,1.04); CVD = 1.03(1.02,1.05)], hospitalization [all causes = 1.02(1.01,1.02); CVD = 1.02(1.01,1.02)], ED visit [all causes = 1.01(1.00,1.01)], and the number of hospitalizations [all causes = 1.02(95% CI 1.01,1.03); CVD = 1.02(1.00,1.04)], hospital days [all causes = 1.01(1.01,1.03)], and ED visits [all causes = 1.02(1.02,1.03)]. FI improvements during CR had a protective effect regarding time to all-cause hospitalization [0.99(0.98,0.99)] but were not associated with other outcomes.
Conclusion
Frailty status at CR admission was related to long-term adverse outcomes. Frailty improvements during CR were associated with delayed all-cause hospitalization, in which a larger effect was associated with a greater chance of improved outcome.
Keywords: Frailty, Cardiac rehabilitation, Mortality, Hospitalization
Graphical Abstract
Graphical Abstract.
See the editorial comment for this article ‘The many uses of frailty assessments in cardiac rehabilitation programs’, by D. B. Hogan, https://doi.org/10.1093/eurjpc/zwad065.
Introduction
Cardiovascular diseases (CVDs) accounted for a third of global deaths in 20191 and cost hundreds of billions of dollars to European and North American countries.2–4 The prevalence of CVDs increases with older age. In conjunction with the rising global life expectancy,5,6 the burden of CVDs is expected to escalate. Furthermore, because CVDs rarely exist on their own, their management requires careful consideration of various other age-related health problems.7 Variability in health outcomes at a given age means that some individuals will have more favourable prognoses from CVD than others.8
Frailty is the age-associated and graded increase in the risk of adverse outcomes and explains the variability in health amongst people of the same age.9 Considering frailty as age-related vulnerability recognizes and quantifies that some people accumulate health problems more slowly while others rapidly develop health problems as they get older. Frailty is more common in people who live with CVDs than in those without (50–54% vs. 14–24%).10,11 Importantly, people who live with frailty have an increased risk of CVD-related morbidity and mortality compared with those who do not.12 Thus, effective management and treatment of patients who live with CVD and frailty require an individualized approach that addresses both conditions at the same time.
One individualized approach to treating CVD and frailty is cardiac rehabilitation (CR).13–17 CR is multi-dimensional and implements nutritional counselling, risk factor modification, psychosocial management, patient education, and exercise training to improve patient outcomes and quality of life. Indeed, multi-component interventions have been shown to improve frailty,18–22 suggesting that CR may be well-equipped to address both CVD and frailty. In fact, there is a call to action by the European Association of Preventive Cardiology (EAPC) to address frailty in CR.23–25 Indeed, the global ageing climate requires clinicians, researchers, and policy makers to better understand the implications of frailty in the care of older patients living with CVD. Although most patients of varying ages enter CR with high degrees of frailty,26–36 there is sparse evidence showing how frailty in CR will impact long-term outcomes such as mortality and hospitalizations. Importantly, while completion of CR is associated with frailty improvements,26,28,31,35,37 there are no investigations on whether such improvements are associated with long-term outcomes.
Here, we explore the association between frailty at CR admission, frailty changes during CR, and long-term outcomes of people who attend CR. Long-term outcomes include time to mortality, first hospitalization, first emergency department (ED) visit, and number of hospitalizations, hospital days, and ED visits over a 5-year follow-up due to both all-causes and CVDs. Specifically, our objectives were to (1) examine the association between admission frailty and long-term outcomes and to (2) investigate the association between frailty changes during CR and long-term outcomes.
Methods
Design
This study was a secondary analysis of data from Nova Scotia residents who enrolled in a CR programme called Cardiovascular & Pulmonary Health in Motion offered within Nova Scotia Health (NSH) at a single centre in Halifax, NS, Canada. This database contains information on participants from the central zone of the NSH management zones (Halifax area, Eastern Shore, and West Hants) who enrolled in the CR programme from 1995 to 2015. Data from 2005 to 2015 were used for the current project due to the lack of information required to construct the FI prior to 2005. All participants experienced an adverse cardiovascular event (e.g. a myocardial infarction) prior to their CR referral. Data collected as part of the CR programme were linked to administrative health databases to examine outcomes. Patients consented to have their data entered and used for research purposes at the CR admission appointment. This study was approved by the NSH research ethics board (REB identifier number: #1023328).
Cardiac rehabilitation
The CR programme was a group-based 12-week exercise and education programme delivered at a single centre in Halifax, Canada. CR staff included a medical director, a programme lead, nurses, dietitians, and physiotherapists with a 7 : 1 patient-to-staff ratio. Prior to starting CR, all patients were assessed by the CR nurse, dietitian, and physiotherapist with access to the medical director for consultation as needed. The CR programme aimed to improve CVD risk factors, functional (exercise) capacity, and patients’ understanding of CVD risk factor management by modifying health behaviours including physical activity, diet, medication management, and smoking. Patients received two weekly centre-based exercise sessions (60-min duration) and one weekly education session. The exercise sessions were led by a licensed physiotherapist and individualized according to exercise stress testing and prescription; each session included warm-ups and cool-downs, 40 min of continuous or interval aerobic training (treadmill, cycling, or arm ergometer), and 10 min of resistance training. Aerobic training progressed by increasing treadmill speed or incline or ergometer resistance while maintaining revolution speed. Resistance training focused on major muscle groups (legs, back, chest, shoulders, and core) with the use of body weight exercises (wall push-ups, sit-to-stand chair exercises, and leg lifts), resistance bands, and hand weights. Patients were also prescribed an individualized home-based CR programme consisting of moderate aerobic and resistance training with the frequency and duration adjusted according to individual needs. CR staff provided group-based education sessions focusing on how to manage CVD risk factors through health behaviour changes to diet, exercise safety, and medication management. Completion of CR was defined as completion of the outtake CR assessment appointment at 12 weeks after admission and attendance of more than 70% of exercise sessions. Patients were given discharge plans to help continue healthy habits developed during the program and follow-up was managed by their family doctor. Baseline and discharge measures were also sent to the family doctor and referring specialist.
Frailty index and frailty index change
We used a validated FI from the CR database,26,38 developed following standard protocols39 from routinely collected CR data at both admission and completion (12 weeks after admission). The FI included 25 variables in multiple domains: cardiovascular biomarkers, symptoms, quality of life, cardiovascular fitness, body composition, and diet. The full list of variables, coding, and prevalence is available in Supplementary material online, Table S1. Further information on FI score calculations is available in the Expanded methods section of the Appendix. Patients who had <30% (8/25) of variables missing were considered to have sufficient data for calculating a FI score.40 The FI was divided into five groups: <0.2 = non-frail/very mildly frail, 0.2 ≤ FI < 0.3 = mildly frail, 0.3 ≤ FI < 0.4 = moderately frail, 0.4 ≤ FI < 0.5 = severely frail, and 0.5 ≤ FI = very severely frail.26
A continuous value for the frailty change was calculated by subtracting the CR admission FI by the CR completion FI; a positive FI change value represents an improvement in the FI from CR admission to CR completion (e.g. 0.20admission—0.10completion = 0.10 improvement). Conversely, a negative FI change represents a worsening in the FI from CR admission to CR completion. Only patients who completed CR had available FI change data. Clinically meaningful changes in the FI, defined as a change of at least 0.03 in the FI,41,42 were used: improvement—FI decreased by at least 0.03; stable—FI changed by <0.03; and worsened—FI increased by at least 0.03.
Linking to administrative databases
To investigate associations of admission frailty and frailty changes from CR with long-term adverse outcomes, we linked data sets managed by Health Data Nova Scotia (see Supplementary material online, Figure S1), including the Canadian Institutes for Health Information’s (CIHI) Discharge Abstract Database (DAD—hospital use; linked from 1 January 2009 to 1 December 2020), CIHI’s National Ambulatory Care Reporting System (NACRS—emergency service use; linked from 1 April 2011 to 1 December 2020) Metadata, and Vital Statistics Canada (mortality; linked from 1 May 2005 to 31 December 2019). To ensure that participants had a minimum follow-up of 5 years, we excluded patients from analyses with DAD and NACRS data who were admitted to CR before 1 January 2009 and 1 April 2011, respectively. Our data linkage application was approved by Health Data Nova Scotia on 24 March 2021. Supplementary material online, Table S2, provides a list of all outcomes used and their associated database.
Outcomes
All outcomes were censored at a maximum follow-up of 5 years (see Supplementary material online, Table S2). A small sub-sample of participants (n = 102/3371) who began CR after 31 December 2014 had a maximum follow-up period of <5 years (range: 4.7–5.0 years). The DAD and NACRS database contained information for health service usages across all of Nova Scotia.
Mortality
Time to mortality from CR admission was determined using the Vital Statistics database and the Cardiac Rehabilitation Database. CVD as the underlying cause of mortality was discerned from all-cause mortality by using the International Classification of Diseases 10th Revision (ICD-10) codes I00 to I99.
Hospitalization data
Time to first hospitalization from CR admission, total number of hospital days, and total number of hospitalizations were determined using the CIHI-DAD. The total number of hospital days and hospitalizations was summed from the beginning of CR admission to a maximum of 5 years post-CR admission. Patients who did not have a hospital stay in this period had their total number of hospital days and hospitalizations set to zero. The CIHI-DAD identifies the ICD-10 code that represents the most responsible diagnosis (MRDx) for each hospitalization. This MRDx was determined to have been responsible for the greatest portion of the patient’s length of stay. All ICD-10 codes from I00 to I99 were used to discern CVD hospitalizations from all-cause hospitalizations.
Emergency department data
The outcomes of time to first ED visit from CR admission and total number of ED visits were determined using the CIHI-NACRS. The total number of ED visits was summed from the beginning of CR admission to 5 years post-CR admission. ED visits due to CVDs were not explored due to high levels of missingness (44%) in the cause of ED visit variable available in this database.
Analysis
Summary statistics were presented as mean (standard deviation) or frequency (%). Regression models were used to examine the association between admission FI and FI change with mortality, hospitalizations, and ED visits in people who attend CR. Cox regression was used for time to all-cause mortality and Fine–Gray43 competing risk models for time to CVD-related mortality, all-cause and CVD-related first hospitalization, and all-cause ED. Proportional hazard assumption was checked with Schoenfeld residuals and graphical diagnostics. Negative binomial hurdle regression models were used for all-cause and CVD-related total hospital length of stay, all-cause and CVD-related total number of hospital visits, and all-cause total number of ED visits to account for the zero-inflated nature of hospital-based outcomes (see Supplementary material online, Figure S2). To account for death as a competing risk for hospitalizations and ED visits, we excluded patients who died within the 5-year follow-up from CR admission in negative binomial hurdle regression models [ndied = 157/2422 (6.5%) for DAD data; ndied = 100/1602 (6.2%) for NACRS data for objective 1; ndied = 72/1469 (4.9%) for DAD data; and ndied = 42/996 (4.2%) for NACRS data for objective 2]. We checked for 3-way (admission FI-age-sex) and 2-way (admission FI-sex, admission FI-age, and admission FI-referring diagnosis) interactions (objective 1). The same approach was used for checking interactions in frailty change models (objective 2), with an additional term for FI change by percent exercise sessions. We found that the 2-way admission FI and FI change interactions with referring diagnosis were significant for several models and performed a sensitivity analysis which excluded patients who were referred to CR due to a percutaneous coronary intervention (PCI). We also explored non-linear relationships (quadratic, cubic) between admission FI and FI change with all outcomes in additional models. All analyses were adjusted for sex, age, referring diagnosis, exercise sessions attended, education, employment, smoking status, marital status, year of CR, and CR completion. Models with admission FI did not adjust for exercise sessions attended. Models with FI change did not adjust for CR completion as all patients with a valid FI change measure completed CR. An alpha value of <0.05 was considered as statistically significant. Cell sizes < 5 were suppressed in compliance with Health Data Nova Scotia policy. The statistical software ‘R’ version 4.0.544 was used to perform all analyses. Packages ‘cmprsk2’, ‘survival’, and ‘pscl’ were used for competing risk, survival, and hurdle models.
Results
Participant characteristics
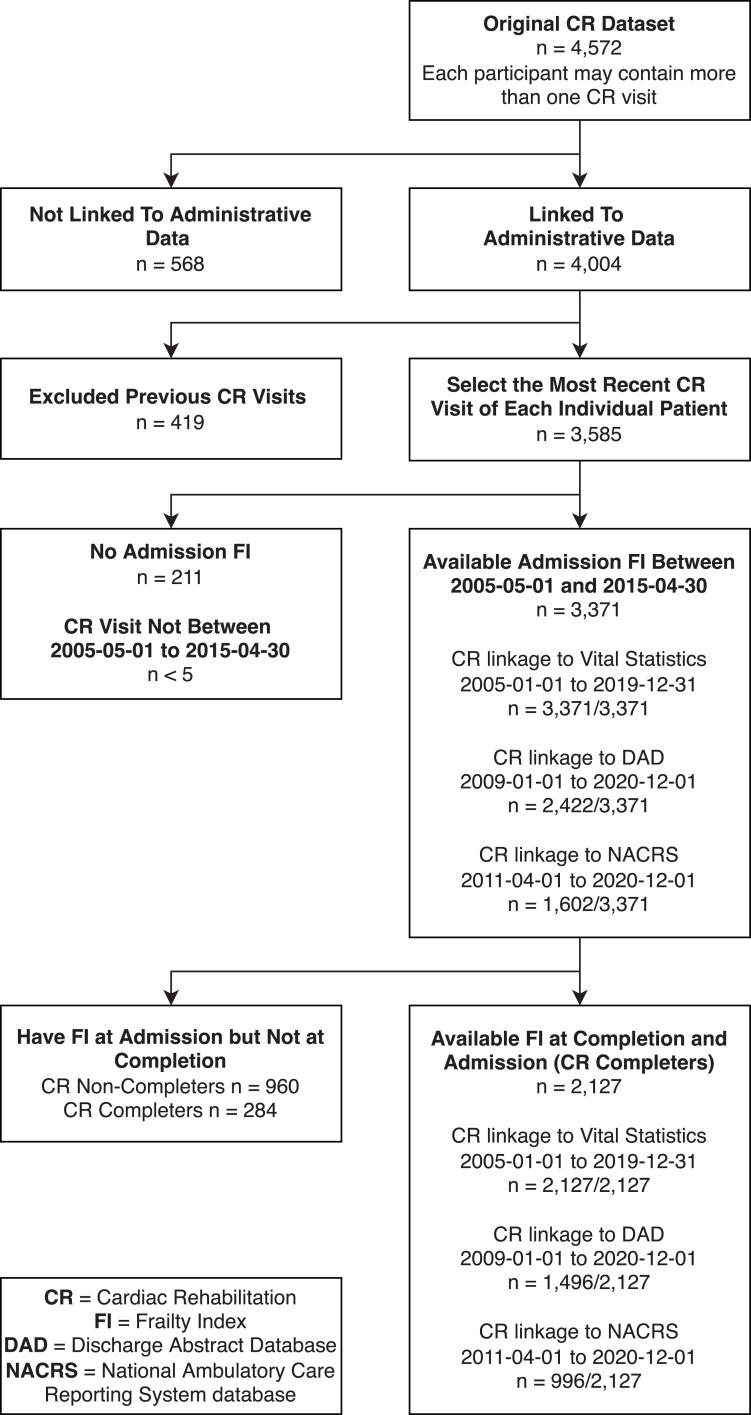
Of 3585 patients who enrolled in CR, 3371 (94.0%) patients had sufficient data for calculating a FI at CR admission. Sample sizes for mortality, hospital, and ED outcomes varied due to different time periods for which each data set was available for linkage (Figure 1). Patients with available CR admission FI were 61.9 (SD: 10.7) years old on average and 74.2% (n = 2503) were male (Table 1). On average, patients attended nearly 80% of all exercise sessions. The mean admission FI was 0.34 (0.13). The most common referring diagnoses to CR were coronary artery disease and myocardial infarction. For patients admitted to CR, a lower education level, unemployment, long-term disability, currently or formerly smoking, lower exercise sessions attended, and a referring diagnosis of coronary artery disease were associated with higher frailty levels (Table 1). From 3371 patients who had a FI at CR admission, 2411 (71.5%) patients completed CR (Figure 1). Among those who completed CR, 88.2% (2127/2411) had sufficient data to calculate FI change (Figure 1). For these patients, the FI improved by 0.07 (0.09) on average from CR admission to completion (see Supplementary material online, Table S3). A third of patients had a clinically meaningful improvement in FI (improvement of at least 0.03) during CR (see Supplementary material online, Table S4).
Figure 1.
Flowchart of participants.
Table 1.
Admission characteristics of people who attended cardiac rehabilitation by admission FI
| Full sample | Frailty levels | |||||
|---|---|---|---|---|---|---|
| <0.2 | 0.2–0.3 | 0.3–0.4 | 0.4–0.5 | 0.5+ | ||
| n (%) | 3371 (100) | 529 (15.7) | 872 (25.9) | 964 (28.6) | 650 (19.3) | 356 (10.6) |
| Age, mean (SD; range) | 61.9 (10.7; 21–94) | 62.2 (10.7; 21–93) | 62.0 (10.8; 23–88) | 62.7 (10.6; 26–90) | 60.8 (10.9; 30–94) | 60.7 (9.8; 35–86) |
| Sex, n male (%) | 2503 (74.2) | 424 (80.2) | 681 (78.1) | 724 (75.1) | 439 (67.5) | 235 (66.0) |
| Education, n (%) | ||||||
| <grade 12 | 709 (21.0) | 66 (12.5) | 149 (17.1) | 202 (21.0) | 171 (26.3) | 121 (34.0) |
| Grade 12/GED | 605 (17.9) | 93 (17.6) | 153 (17.6) | 195 (20.2) | 111 (17.1) | 53 (14.9) |
| Technical college | 1073 (31.8) | 157 (29.7) | 263 (30.2) | 296 (30.7) | 232 (35.7) | 125 (35.1) |
| Bachelor’s degree | 626 (18.6) | 126 (23.8) | 196 (22.5) | 173 (18.0) | 85 (13.1) | 46 (12.9) |
| Post-graduate education | 358 (10.6) | 87 (16.5) | 111 (12.7) | 98 (10.2) | 51 (7.9) | 11 (3.1) |
| Employment, n (%) | ||||||
| Long-term disability | 223 (6.6) | 13 (2.5) | 30 (3.4) | 54 (5.6) | 72 (11.1) | 54 (15.2) |
| Unemployed | 89 (2.6) | 13 (2.5) | 16 (1.8) | 26 (2.7) | 21 (3.2) | 13 (3.7) |
| Part-time | 186 (5.5) | 27 (5.1) | 61 (7.0) | 56 (5.8) | 32 (4.9) | 10 (2.8) |
| Full-time | 712 (21.1) | 137 (25.9) | 224 (25.7) | 181 (18.8) | 112 (17.2) | 58 (16.3) |
| Retired | 1538 (45.6) | 254 (48.0) | 398 (45.6) | 453 (47.0) | 279 (42.9) | 154 (43.3) |
| Other | 623 (18.5) | 85 (16.1) | 143 (16.4) | 194 (20.1) | 134 (20.6) | 67 (18.8) |
| Smoking status, n (%) | ||||||
| Non-smoker | 997 (29.6) | 224 (42.3) | 267 (30.6) | 265 (27.5) | 163 (25.1) | 78 (21.9) |
| Former smoker | 2007 (59.5) | 264 (49.9) | 508 (58.3) | 607 (63.0) | 407 (62.6) | 221 (62.1) |
| Current smoker | 367 (10.9) | 41 (7.8) | 97 (11.1) | 92 (9.5) | 80 (12.3) | 57 (16.0) |
| Marital status, n (%) | ||||||
| Divorced/separated | 308 (9.1) | 35 (6.6) | 66 (7.6) | 84 (8.7) | 72 (11.1) | 51 (14.3) |
| Widowed | 226 (6.7) | 20 (3.8) | 62 (7.1) | 67 (7.0) | 50 (7.7) | 27 (7.6) |
| Single | 256 (7.6) | 32 (6.1) | 63 (7.2) | 68 (7.1) | 56 (8.6) | 37 (10.4) |
| Married/living with a partner | 2581 (76.6) | 442 (83.6) | 681 (78.1) | 745 (77.3) | 472 (72.6) | 241 (67.7) |
| Referring diagnosis, n (%) | ||||||
| Coronary artery disease | 900 (26.7) | 122 (23.1) | 208 (23.9) | 266 (27.6) | 183 (28.2) | 121 (34.0) |
| Percutaneous coronary intervention | 516 (15.3) | 80 (15.1) | 141 (16.2) | 132 (13.7) | 104 (16.0) | 59 (16.6) |
| Cardiac surgery | 629 (18.7) | 119 (22.5) | 193 (22.1) | 178 (18.5) | 95 (14.6) | 44 (12.4) |
| Heart failure | 222 (6.6) | 23 (4.4) | 48 (5.5) | 57 (5.9) | 60 (9.2) | 34 (9.6) |
| Myocardial infarction | 955 (28.3) | 158 (29.9) | 241 (27.6) | 289 (30.0) | 183 (28.2) | 84 (23.6) |
| Other | 149 (4.4) | 27 (5.1) | 41 (4.7) | 42 (4.4) | 25 (3.9) | 14 (3.9) |
| Year of CR admission, n (%) | ||||||
| 2005–2008 | 949 (28.1) | 155 (29.3) | 232 (26.6) | 253 (26.2) | 190 (29.2) | 119 (33.4) |
| 2009–2012 | 1533 (45.5) | 227 (42.9) | 384 (44.0) | 464 (48.1) | 293 (45.1) | 165 (46.4) |
| 2013–2015 | 889 (26.4) | 147 (27.8) | 256 (29.4) | 247 (25.6) | 167 (25.7) | 72 (20.2) |
| Exercise sessions attended, % (SD) | 79.5 (27.4) | 84.6 (22.6) | 81.7 (25.7) | 80.72 (26.7) | 73.9 (30.4) | 70.2 (32.78) |
| Admission FI, mean (SD) | 0.34 (0.13) | 0.15 (0.04) | 0.26 (0.03) | 0.35 (0.03) | 0.45 (0.03) | 0.57 (0.03) |
| DBP, mean mmHg (SD) | 68.7 (9.0) | 69.2 (7.5) | 69.0 (8.0) | 68.4 (9.1) | 68.5 (9.6) | 68.6 (11.6) |
| SBP, mean mmHg (SD) | 118.7 (17.3) | 116.8 (13.3) | 118.1 (15.1) | 118.1 (16.9) | 119.7 (18.9) | 122.9 (24.1) |
| MAP, mean mmHg (SD) | 85.4 (10.2) | 85.1 (8.2) | 85.3 (8.9) | 85.0 (10.2) | 85.5 (11.0) | 86.8 (14.0) |
| Resting HR, mean bpm (SD) | 63.9 (9.3) | 63.9 (8.1) | 63.9 (9.25) | 63.9 (9.6) | 63.7 (9.0) | 64.5 (10.6) |
| Cholesterol, mean mmol/L (SD) | 4.1 (1.2) | 3.9 (0.9) | 4.0 (1.1) | 4.1 (1.1) | 4.3 (1.2) | 4.4 (1.4) |
| Triglycerides, mean mmol/L (SD) | 1.5 (1.0) | 1.1 (0.7) | 1.4 (0.8) | 1.5 (0.9) | 1.8 (1.0) | 2.3 (1.6) |
| LDL, mean mmol/L (SD) | 2.4 (1.0) | 2.2 (0.8) | 2.4 (1.0) | 2.4 (1.0) | 2.5 (1.1) | 2.5 (1.2) |
| HDL, mean mmol/L (SD) | 1.0 (0.3) | 1.2 (0.4) | 1.1 (0.3) | 1.0 (0.3) | 1.0 (0.3) | 0.9 (0.3) |
| Glucose, mean mmol/L (SD) | 6.4 (2.0) | 5.7 (1.4) | 6.0 (1.6) | 6.5 (1.9) | 6.9 (2.3) | 7.7 (2.7) |
| BMI, mean kg/m2 (SD) | 29.9 (5.9) | 25.5 (3.1) | 27.8 (4.4) | 30.4 (5.7) | 32.6 (5.6) | 35.2 (6.4) |
| NYHA, mean class (SD) | 1.6 (0.8) | 1.2 (0.5) | 1.4 (0.6) | 1.6 (0.8) | 1.9 (0.9) | 2.4 (0.9) |
| METS, mean (SD) | 7.7 (2.8) | 9.1 (2.6) | 8.4 (2.6) | 7.2 (2.6) | 6.4 (2.4) | 5.1 (2.1) |
BMI, body mass index; DBP, diastolic blood pressure; HDL, high-density lipoprotein; FI, frailty index; HR, heart rate; LDL, low-density lipoprotein; MAP, mean arterial pressure; METS, metabolic equivalents; NYHA, New York Heart Association Classifications—The Stages of Heart Failure; SD, standard deviation; SBP, systolic blood pressure. Glucose measurements are done during fasting.
Frailty at CR admission and adverse outcomes
There were no significant 3-way or 2-way interactions found between admission FI, age, and sex (P > 0.05) for all outcomes considered. Patients who were frailer at CR admission had higher mortality rates (Figure 2), number of hospitalizations, number of days in hospital, and number of ED visits regardless of the cause (Figure 3).
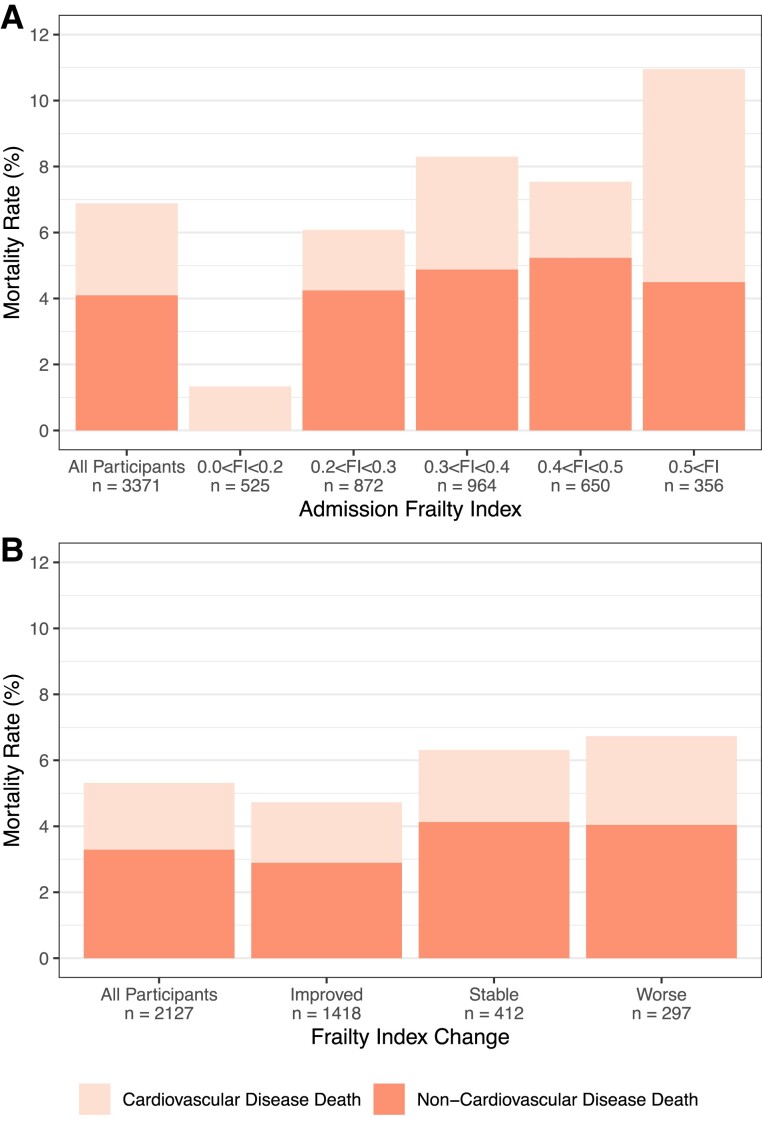
Figure 2.
Mortality rates by (A) admission frailty index and (B) frailty index change. Improvement—frailty index decreased by at least 0.03; stable—frailty index changed by <0.03; worsened—frailty index increased by at least 0.03 during cardiac rehabilitation.
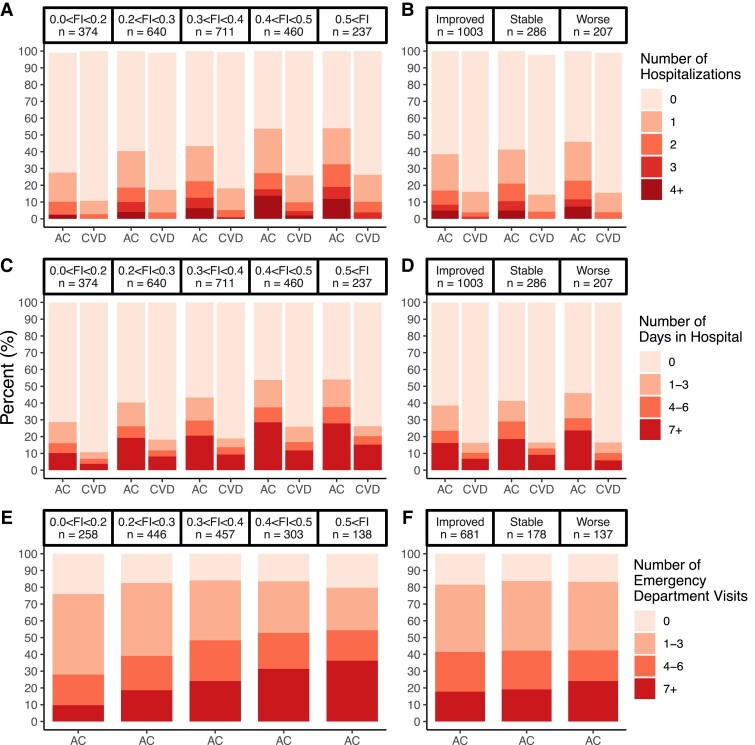
Figure 3.
Total number of hospitalizations, hospital days, and emergency department visits by admission frailty index (panels A, C, and E) and by frailty index change categories (panels B, D, and F). Improvement—frailty index decreased by at least 0.03; stable—frailty index changed by <0.03; worsened—frailty index increased by at least 0.03 during cardiac rehabilitation. AC, all causes; CVD, cardiovascular disease.
Mortality
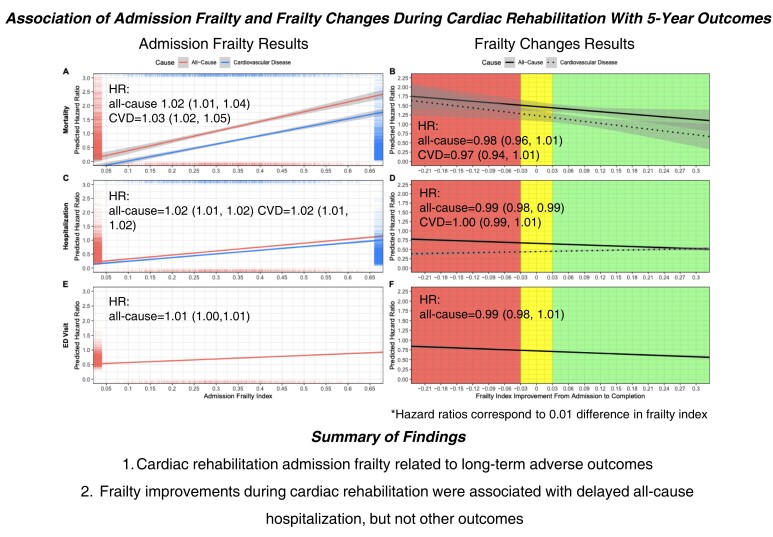
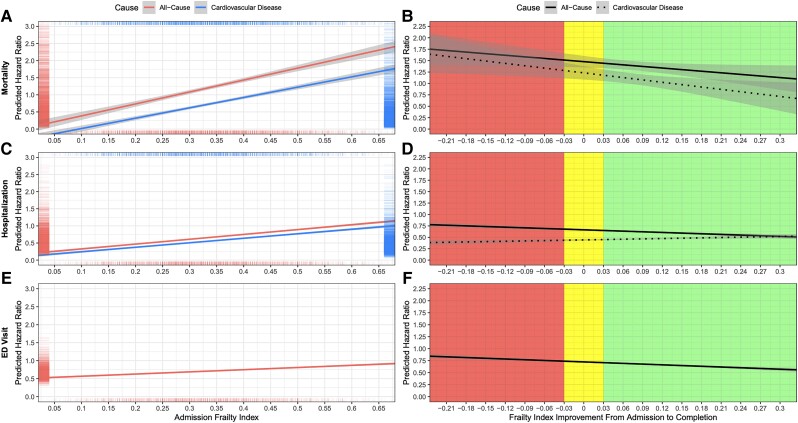
The 5-year all-cause and CVD-related mortality rates were 6.9% (232/3371) and 2.8% (94/3371), respectively (see Supplementary material online, Table S5 and Figure 2). CVD mortality rates increased according to admission frailty status, from 1.3% in patients who were non-frail/mildly frail (FI < 0.2) to 6.5% in those who were very severely frail (FI > 0.5) (Figure 1). A 0.01 higher admission FI was significantly associated with a 2% (95% CI: 1–4%) greater risk of all-cause mortality and 3% (2–5%) greater risk of CVD mortality (Table 2 and Figure 4A).
Table 2.
Association between admission FI, FI change, and outcome measures up to 5 years after cardiac rehabilitation admission
| Outcome | Cause | Admission FI | FI Change | ||
|---|---|---|---|---|---|
| Per 0.01 greater admission FI | Per 0.01 improvement in FI | ||||
| HR or SHR (CI) | HR or SHR (CI) | ||||
| Time to mortality | All causes | 1.02 (1.01, 1.04) | 0.98 (0.96, 1.01) | ||
| (n = 3371) | CVD | 1.03 (1.02, 1.05) | 0.97 (0.94, 1.01) | ||
| Time to first hospitalization | All causes | 1.02 (1.01, 1.02) | 0.99 (0.98, 0.99) | ||
| (n = 2422) | CVD | 1.02 (1.01, 1.02) | 1.00 (0.99, 1.01) | ||
| Time to first ED visit (n = 1602) | All causes | 1.01 (1.00, 1.01) | 0.99 (0.98, 1.01) | ||
| IRR (CI) | OR (CI) | IRR (CI) | OR (CI) | ||
| Number of hospitalizations | All causes | 1.02 (1.01, 1.03) | 1.02 (1.02, 1.03) | 0.99 (0.98, 1.01) | 0.98 (0.97, 0.99) |
| (n = 2422) | CVD | 1.02 (1.00, 1.04) | 1.02 (1.01, 1.03) | 0.99 (0.96, 1.02) | 1.00 (0.98, 1.02) |
| Number of hospital days | All causes | 1.01 (1.01, 1.03) | 1.02 (1.02, 1.03) | 0.98 (0.97, 1.01) | 0.98 (0.97, 0.99) |
| (n = 2422) | CVD | 1.01 (0.99, 1.02) | 1.02 (1.01, 1.03) | 1.00 (0.97, 1.03) | 1.00 (0.98, 1.02) |
| Number of Times in ED | All-Cause | 1.02 (1.02, 1.03) | 1.00 (0.99, 1.01) | 0.99 (0.98, 1.01) | 0.98 (0.96, 1.01) |
| (n = 1602) | |||||
Outcomes correspond to 0.01 greater admission FI or FI improvement (+0.01 change in FI indicates improvement in FI from admission to completion of cardiac rehabilitation). All models were adjusted for sex, age, referring diagnosis, education, employment, smoking status, marital status, and year of cardiac rehabilitation. The admission FI model was also adjusted for completion of cardiac rehabilitation status and the FI change model for admission FI score and exercise sessions attended. There were no significant three-way and two-way interactions. Underlined text represents significance at <0.05. CI, 95% confidence interval; CVD, cardiovascular disease; ED, emergency department; FI, frailty index; HR, hazard ratio; IRR, incidence rate ratio; OR, odds ratio; SHR, sub-distributional hazard ratio.
Figure 4.
Predicted hazard ratios for risk of mortality (panels A and B), hospitalization (panels C and D), and ED visit (panels E and F) by frailty index score at cardiac rehabilitation admission (panels A, C, and E) and improvement during cardiac rehabilitation (panels B, D, and F). Admission frailty index was a significant predictor of all-cause and cardiovascular disease risk of mortality, hospitalization, and emergency department visit (P < 0.001). Frailty index improvement was a significant predictor of all-cause risk of hospitalization (P = 0.016) but not for all other outcomes. Panels A, C, and E contains ‘rug’ plots which visualize the distribution of predicted hazard ratios and admission frailty index scores. The areas in panels B, D, and F x-axes indicate different levels of clinically important changes in frailty index score during cardiac rehabiliation: less than −0.03 = worsening, −0.03 to 0.03 = stable, and greater than 0.03 = improvement. Grey bands represent 95% confidence intervals. All models were adjusted for sex, age, referring diagnosis, education, employment, smoking status, marital status, and year of cardiac rehabilitation. The admission frailty index model was also adjusted for completion of cardiac rehabilitation status and the frailty index improvement model for admission frailty index score and per cent of exercise sessions attended. ED, emergency department.
Hospitalization
Within 5 years of CR admission, 43.3% (1049/2422) and 19.4% (471/2422) of patients were hospitalized at least once due to all causes and CVDs, respectively (see Supplementary material online, Table S5). The total number of all-cause and CVD hospitalizations was generally greater in patients who were frailer at CR admission. For instance, only 28.6% of non-frail/very mildly frail (FI < 0.20) patients were hospitalized at least once compared with almost double the proportion (54.0%) in patients who were very severely frail (FI > 0.5). A 0.01 higher admission FI was significantly associated with a 2% (1–2%) greater risk of all-cause and CVD hospitalization (Table 2 and Figure 4C). Similarly, a 0.01 higher admission FI was significantly associated with a 2% (1–3%) greater number of all-cause and 2% (0–4%) greater number of CVD hospitalizations over 5 years of follow-up (Table 2).
Days in hospital
The mean (SD) number of days spent in the hospital was 7.3 (22.3) and 2.3 (9.2) days due to all causes and CVDs, respectively. In the 5 years after their CR admission, 20.8% (504/2422) and 9.2% (222/2422) of all CR patients spent 7 or more days (in one or multiple visits) in the hospital due to all causes and CVDs, respectively (see Supplementary material online, Table S5). Patients who were frailer at CR admission typically spent a greater number of days in hospitals regardless of the cause (Figure 3C). A 0.01 higher admission FI was significantly associated with a 1% (1–3%) greater number of days spent in hospitals due to all causes over the follow-up period (Table 2). The FI at CR admission was not significantly associated with the number of days in the hospital due to CVD.
ED visits
Four in five CR patients had at least one visit to the ED in the 5-year follow-up (see Supplementary material online, Table S5). About one-third of patients living with severe or very severe frailty (FI > 0.4) visited EDs at least seven times during the follow-up period, compared with <10% for patients in the non-frail/very mildly frail groups (FI < 0.2). A 0.01 higher admission FI was significantly associated with 1% (0–1%) greater risk of visiting the ED (Table 2 and Figure 4E).
Frailty change during CR and adverse outcomes
FI change analyses were done with data from participants who completed CR (63.1%; 2127/3371). There were no significant 3-way or 2-way interactions found between FI change, age, sex, and exercise sessions (P > 0.05) for all outcomes considered.
Mortality
Patients who had FI improvements during CR (FI reduced by at least 0.03) had the lowest mortality rates (all causes = 4.7%; CVD = 1.8%) compared with patients whose FI remained stable (FI changed by <0.03) (all causes = 6.3%; CVD = 2.2%) or worsened (FI increased by more than 0.03) (all causes = 6.7%; CVD = 2.7%) (Figure 2B). Even so, improvements in the FI from CR admission to completion were not significantly associated with lower risks of all-cause and CVD mortality (Table 2 and Figure 4B).
Hospitalizations
Patients whose FI improved during CR had fewer all-cause hospitalizations in the follow-up period than those whose FI remained stable or worsened (Figure 3B). A 0.01 higher improvement in FI from CR admission to completion was significantly associated with a 1% (1–2%) lower risk of all-cause hospitalization but not CVD hospitalization (Table 2 and Figure 4D). In addition, improvements in FI during CR were not significantly associated with the number of hospitalizations due to all causes or CVDs (Table 2).
Days in the hospital
Patients whose FI improved during CR had fewer hospital days due to all causes than those whose FI remained stable or worsened (Figure 3D). For example, 16.2% of patients whose FI improved spent at least 7 days in the hospital vs. 23.7% in patients whose FI worsened (Figure 3D). There were little differences across FI change categories for hospital days due to CVDs (Figure 3D). With the hurdle regression models, improvements in FI were not significantly associated with the total number of hospital days due to all causes or CVDs over the 5-year follow-up period (Table 2).
ED visits
Patients whose FI improved during CR had a lower percentage of at least seven visits to the ED compared with those whose FI worsened (17.8% vs. 24.1%, respectively) (Figure 3F). Changes in FI during CR were not significantly associated with the risk of visiting the ED (Table 2 and Figure 3F). In addition, changes in FI during CR were not significantly associated with the total number of ED visits over the 5-year follow-up period (Table 2).
Non-linear relationships between FI and outcomes
Quadratic and cubic terms were included in additional models for both objectives 1 (admission FI) and 2 (FI improvements; Supplementary material online, Table S6, and Supplementary material online, Table S7). Although statistically significant, the effect sizes for these relationships were consistently small (HRs, IRRs, and ORs < 1.0001) and were thus not presented in the primary results.
Sensitivity analysis
We found that the 2-way admission FI and FI change interaction with referring diagnosis was significant for several models (see Supplementary material online, Table S8). The referring diagnosis that interacted with frailty most frequently was PCI. Since patients who recently underwent a PCI (treatment) may follow a distinct recovery frailty trajectory compared with other diagnoses,45 we re-analysed all models and excluded patients who were referred to CR due to a PCI [nexcluded = 516/3371 (15.3%) from admission FI models and 319/2127 (15.0%) from FI change models]. Results regarding admission FI mirrored the main analysis. Models with FI change showed that the association between FI change and the risk of all-cause hospitalization was no longer significant (P = 0.072 without PCI, P = 0.014 with PCI).
Discussion
Summary of results
Here, we demonstrated that higher FI at CR admission was associated with a greater 5-year risk of mortality and hospitalization due to all causes and CVDs and ED visits due to all causes. In addition, higher FI at CR admission was also associated with a greater number of hospitalizations due to all causes and CVDs and greater numbers of days in the hospital and ED visits due to all causes over a 5-year follow-up (Table 2 and Figure 4). We also demonstrated that FI improvements during CR were associated with a lower 5-year risk of all-cause hospitalization, but not with other outcomes (Table 2 and Figure 4).
Admission FI and 5-year outcomes
The mortality and hospitalization results are consistent with a recent secondary analysis of the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial46 which found that a higher admission FI score was associated with greater risk of mortality and hospitalization due to all causes and CVDs. However, another secondary analysis31 of the EJECTION-HF (Exercise Joins Education, Combined Therapies to Improve Outcomes in Newly Discharged HF) trial showed that admission FI was not associated with the 1-year composite outcome of all-cause mortality or all-cause hospitalization for patients in a CR programme. These differences may be attributable to the (1) relatively small sample size of the EJECTION-HF study vs. the HF-ACTION and the current study (27831 vs. 213046 and 3371 in the current study), (2) shorter follow-up time for outcome (1 year31 vs. 2.9 years46 and 5 years in the current study), and (3) exclusive recruitment of patients with heart failure in the EJECTION-HF study. In addition, two CR studies in Japan also showed that admission frailty (abbreviated frailty phenotype32, 19-item FI47) was associated with the composite of all-cause mortality and CVD hospitalization.32,47
Here, we demonstrate that the admission frailty status in a CR setting was associated with the number of all-cause and CVD hospital visits, all-cause days in hospital, and all-cause ED visits over a 5-year follow-up (Table 2 and Figure 4). Our work adds to a body of literature which shows that FI scores predict the number of hospital admissions and days in hospital in a nursing home setting,48 community-dwelling populations,49 and adult home-care patients.50 Indeed, a greater burden of frailty at CR admission translates to more time spent in care facilities and thus greater use of healthcare resources.
Overall, these results outline the importance of considering the degree of frailty in the CR setting.9 Not only was frailty important for understanding the risk of several long-term outcomes but incremental differences in admission frailty levels represented valuable information for understanding long-term clinical outcomes (Table 2). In addition, the significant association of the CR admission frailty status with both all-cause and CVD outcomes, independent of several prognostic factors, positions frailty as a key indicator of both overall and cardiovascular health. Here, we demonstrated that the frailest patients admitted to CR are predisposed to health complications which, in combination with CVDs and a diminished ability to recover from their disease, result in poor long-term outcomes.
FI change and 5-year outcomes
Here, we show that FI improvements during a 12-week CR programme were associated with delayed time to all-cause hospitalization independent of admission FI (Table 2 and Figure 4). This is consistent with data showing that a one-point increase in the frailty phenotype was related to a 2.1% greater rate of hospital usage in older adults.51 Our study, along with others,32,46,47 outlines the prognostic value of frailty status at CR admission. Indeed, if high frailty burdens equate to reduced physiological reserves and heightened vulnerability to stressors, then, reductions in the frailty burden should translate into greater multi-system resilience. Such changes may reflect improvements in overall health, physical function, and quality of life. Patients whose health status changed during CR may experience corresponding changes in their healthcare use patterns—greater improvements in frailty may delay the patient’s next hospitalization. Even so, FI changes were not associated with the total number of hospitalizations, hospital days, and ED visits due to any cause during the 5-year follow-up (Table 2). While greater improvements in frailty may delay the next all-cause hospitalization, the frequency of healthcare usage over 5 years was not dependent on frailty changes. Readmittances to hospitals may be inevitable for patients with CVDs who require follow-up visits as part of routine care for their condition. For instance, severely frail older adults who received comprehensive geriatric assessments and early rehabilitation had lower rates of rehospitalization than usual care 1 month after discharge, but not 3 months after discharge.52
FI changes were also not associated with mortality, regardless of cause. These findings are inconsistent with longitudinal studies in community-dwelling populations which have shown that increases in FI were associated with greater mortality risk.53–55 This may be attributable to differences in study populations (CR participants vs. normal health). FI changes may be more sporadic due to the heterogeneity in health of patients living with a recent CVD event, while observed FI changes in community populations over longer periods of time reflect ageing processes that are related to greater mortality risk. For example, the duration over which FI changes took place was longer for previous works. Stolz et al.53 and Shi et al.54 used 1-year changes and Thompson et al.55 used 4.5-year changes; we used 12-week change from CR. Here, significant results with mortality may be partially driven by the increasing trend of FIs over longer intervals than 12 weeks.56–58 In addition, as frailty is dynamic and likely susceptible to measurement errors,53 a longer interval between frailty measures may offer more stable estimates which can be explored through frequent, long-term serial assessments of the frailty status during and after completion of CR.
Our observation that incremental frailty changes in a 12-week CR programme only inform the risk of all-cause hospitalization, but not other long-term outcomes, suggests that a more nuanced approach may be needed when evaluating frailty changes in relation to long-term outcomes. To better understand the relationship between FI change during CR and long-term outcomes, future studies need to consider why frailty changed. FI changes reflect the influences of a combination of the beneficial effects of a multi-component CR programme, together with the natural trajectory of illness, changes to CVD treatment, or due to a new CVD or other diagnosis that could either acutely increase their FI or prove fatal. Clinical trials show that exercise and multi-component interventions similar to CR can reduce frailty levels;59,60 a number of cohort studies show that frailty improved during CR.26,28,31,35,37 In addition, patients who recently received treatment for CVD (PCIs and coronary artery bypass graft) exhibit U-shaped frailty trajectories,45 meaning that they improve initially but worsen after 6 months post-treatment which is consistent with our population where 66.7% of patients improved in frailty during CR (see Supplementary material online, Table S4). However, we did not monitor longer-term change in frailty as in the mentioned study.
Importance of program adherence
A recent consensus statement by the EAPC emphasized the importance of treatment adherence as a major determinant of patient outcomes.61 Our findings echo this statement as non-completion of CR was detrimental for almost all long-term outcomes considered (see Supplementary material online, Tables S9 and S10). The percentage of exercise sessions attended was not associated with long-term outcomes which outline the importance of CR's holistic nature (see Supplementary material online, Tables S11 and S12). Previous work in the same database26 found that greater frailty at CR admission was related to non-completion of CR. Indeed, understanding patient frailty levels and its’ implications on CR adherence may help staff to identify patients who may benefit from additional supports or interventions. This aligns with the EAPC statement that CR interventions for older adults should be tailored towards frailty prevention.61 In addition, although patients in the current CR program were provided a discharge plan and follow-up with their primary care provider, future efforts to integrate and monitor follow-up data as part of CR research data would enable investigations on the long-term adherence to healthy habits developed during CR and their impacts on frailty and adverse outcomes.
Limitations
Our study has several limitations. First, there were large numbers of missing data (17.6%; 593/3371) for the exercise sessions attended, variable in the objective 1 sample; thus, we were not able to control for any dose-response effect of CR exercise training on long-term outcomes for objective 1. Second, it was unclear how long patients waited from their CR referral to CR admission as the referral date was not available. However, the median time from referral to enrolment was 27 days62 for this programme (n = 4443 from 1999 to 2012). Third, although we found some significant 2-way interactions between admission frailty, frailty change, and referring diagnosis (see Supplementary material online, Table S8), we were unable to explore each diagnosis independently further due to low sample sizes in each diagnosis category which only worsens when using the lower sample sizes from DAD and NACRS. Fourth, we were unable to calculate a FI score for 6.0% of people who attended CR (see Supplementary material online, Table S13) due to missing data. People with missing data were on average older and potentially frailer, and thus, prevalence estimates of demographic and outcome data may be biased. Lastly, the FI used in the current study contained 25 items, five fewer than the minimum recommended by standard procedures.39 At 30 items or more, the estimates of mortality are more stable. Even so, the creators of the FI reported that a minimum of 20 items may be acceptable63 and that estimates are only unstable when there are fewer than 10 items.39
Conclusion
Here, we highlight the importance of the frailty status at admission of a CR programme for informing the 5-year risk of mortality, hospitalization, and ED visit, as well as the total number of hospital visits, hospital days, and ED visits over 5 years post-CR. In addition, incremental frailty improvements during CR were related to a lower risk of hospitalization regardless of admission frailty status. Future work should investigate the benefits of targeting frailty reductions as an additional component of CR.
Authors’ contributions
J.Q., D.S.K., and O.T. conceived the presented idea. J.Q., D.S.K., N.G., C.M., and O.T. developed and designed the project methodology. J.Q. performed data acquisition, data analysis, computations, and manuscript preparation. D.S.K. and O.T. co-supervised the findings of this work. N.G. and W.F. provided clinical consultation and facilitated data acquisition from the CR programme. All authors discussed the results and contributed to the final manuscript. All gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Jack Quach, Dustin Scott Kehler (PhD), Nicholas Giacomantonio (MD), Caitlin McArthur (PhD), Chris Blanchard (PhD), Wanda Firth, Kenneth Rockwood (MD), and Olga Theou (PhD)
Supplementary material
Supplementary material is available at European Journal of Preventive Cardiology online.
Supplementary Material
Acknowledgements
Portions of the data used in this report were made available by Health Data Nova Scotia of Dalhousie University. Although this research analysis is based on data obtained from the Nova Scotia Department of Health and Wellness, the observations and opinions expressed are those of the authors and do not represent those of either Health Data Nova Scotia or the Department of Health and Wellness.
Appendices
Expanded methods
Participants and setting
This study used de-identified data of patients entering a CR programme from the Cardiac Rehabilitation Database. This database contains information from participants who previously enrolled in the CR programme from 1995 to 2015. Data from 2005 to 2015 was used for the current project due to the lack of information required to construct the FI in the data from 1995 to 2004. The Cardiac Rehabilitation Database recorded information on a patient’s medical history, life satisfaction, physical and mental quality of life, laboratory variables including bloodwork and blood pressure, and health behaviours during CR attendance. It is housed within the NSH Hearts and Health in Motion CR programme located at the Mumford Professional Center in Halifax, NS. All participants consented for their data to be entered into a database and to be collected and used for research purposes. Participants were not excluded based on age. All participants experienced an adverse cardiovascular event (e.g. a myocardial infarction, cardiac surgery, or heart failure) prior to their CR referral. Patients were automatically referred if they were recently an inpatient at the QEII hospital in the central zone due to heart-related conditions. Otherwise, a family physician or another health care professional would refer the patient to the CR programme.
Cardiac rehabilitation and additional database information
From 2005 to 2015, the programme experienced an expansion to the CR team and an increase in the number of classes offered per week to increase the capacity of the programme. In addition, the programme location moved from NS Rehabilitation to the Mumford Professional Center in 2009. These changes allowed the CR programme to reduce wait times, thus enrolling more patients. The database was collected as part of the Queen Elizabeth II Hospital’s CR program with funding from NSH (previously known as Capital Health and Nova Scotia Health Authority). The purpose of the database was to evaluate patient outcome measures, patient statistics, quality measures, and support research and report generation. The data input process was handled by a dedicated CR team member responsible for data entry. To ensure data quality, ongoing reviews and evaluations were conducted through regular queries, abstracts, and research projects. Additionally, the database was continuously updated and evaluated by the data programmer to maintain integrity.
Frailty index information
The FI included 25 variables in multiple domains: cardiovascular biomarkers (triglycerides; total, low-density lipoprotein, and high-density lipoprotein cholesterol; fasting blood glucose; systolic and diastolic blood pressure; resting pulse rate; pulse pressure; and mean arterial pressure) and symptoms (New York Heart Association functional class); quality of life according to the SF-36 questionnaire in physical, mental, and general health domains; cardiovascular fitness from an exercise stress test; body composition according to the body mass index; waist circumference; bioelectrical impedance (percentage fat mass and percentage lean mass); and diet as determined by the use of the Food Frequency Questionnaire. Variables were recoded so to have scores that were either binary (0 = no deficit present, 1 = deficit present) or ordinal (e.g. New York Heart Association functional class increased in 0.33 increments: 0, 0.33, 0.66, and 1). Once all variables were recoded, the FI was calculated by dividing the sum of the deficits present in the patient by the total number of deficits considered. For example, if someone had five out of a possible 25 deficits, their FI score was 0.2. Higher scores indicate higher frailty levels. Any patients who were missing at least 30% of variables were excluded from the study.40
Other
We used the 7+ days cut point for describing and illustrating prolonged hospital length of stay. We chose to use this cut point as several previous works (PubMed IDs: 25660316 and 30591055) have also defined prolonged length of stay as spending 7+ days in hospitals: similarly, we decided to use 7 days as a cut point to describe frequent ED visits.
Contributor Information
Jack Quach, School of Physiotherapy, Dalhousie University, 5869 University Ave, Halifax, NS B3H 4R2, Canada; Division of Geriatric Medicine, Dalhousie University, 5955 Veterans Memorial Lane, Halifax, NS B3H 2E1, Canada.
Dustin Scott Kehler, School of Physiotherapy, Dalhousie University, 5869 University Ave, Halifax, NS B3H 4R2, Canada; Division of Geriatric Medicine, Dalhousie University, 5955 Veterans Memorial Lane, Halifax, NS B3H 2E1, Canada.
Nicholas Giacomantonio, Division of Cardiology, Dalhousie University, 1796 Summer Street, Halifax, NS B3H 3A7, Canada; Department of Medicine, Dalhousie University, 1276 South Park Street, Halifax, NS B3H 2Y9, Canada.
Caitlin McArthur, School of Physiotherapy, Dalhousie University, 5869 University Ave, Halifax, NS B3H 4R2, Canada.
Chris Blanchard, Department of Medicine, Dalhousie University, 1276 South Park Street, Halifax, NS B3H 2Y9, Canada.
Wanda Firth, Queen Elizabeth II Health Sciences Centre, Heart Health, 1276 South Park St, Halifax, NS B3H 2Y9, Canada.
Kenneth Rockwood, Division of Geriatric Medicine, Dalhousie University, 5955 Veterans Memorial Lane, Halifax, NS B3H 2E1, Canada.
Olga Theou, School of Physiotherapy, Dalhousie University, 5869 University Ave, Halifax, NS B3H 4R2, Canada; Division of Geriatric Medicine, Dalhousie University, 5955 Veterans Memorial Lane, Halifax, NS B3H 2E1, Canada.
Funding
J.Q. is supported by the Canadian Institutes for Health Research—Canada Graduate Scholarships, the Nova Scotia Graduate Scholarship, the Heart and Stroke Foundation of Canada BrightRed Award, and the Killam Predoctoral Scholarship. K.R. is supported by the Dalhousie Medical Research Fund as the Kathryn Allen Weldon Professor of Alzheimer Research.
Data availability
The data underlying this article cannot be shared publicly to preserve the privacy of individuals that participated in the study.
References
- 1. World Health Organization . Cardiovascular Diseases (CVDs): Geneva, Switzerland. World Health Organization; 2021. [Google Scholar]
- 2. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang NY, Tsao CW. . Heart disease and stroke statistics—2021 update. Circulation 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 3. Tarride JE, Lim M, DesMeules M, Luo W, Burke N, O’Reilly D, Bowen J, Goeree R. . A review of the cost of cardiovascular disease. Can J Cardiol 2009;25:e195–e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leal J, Luengo-Fernández R, Gray A, Petersen S, Rayner M. Economic burden of cardiovascular diseases in the enlarged European Union. Eur Heart J 2006;27:1610–1619. [DOI] [PubMed] [Google Scholar]
- 5. Kanasi E, Ayilavarapu S, Jones J. The aging population: demographics and the biology of aging. Periodontol 2000 2016;72:13–18. [DOI] [PubMed] [Google Scholar]
- 6. Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature 2018;561:45–56. [DOI] [PubMed] [Google Scholar]
- 7. Forman DE, Rich MW, Alexander KP, Zieman S, Maurer MS, Najjar SS, Cleveland JC, Krumholz HM, Wenger NK. . Cardiac care for older adults: time for a new paradigm. J Am Coll Cardiol 2011;57:1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heckman GA, Rockwood K. Frailty, risk, and heart failure care: commission or omission? J Am Coll Cardiol 2022;80:1144–1146. [DOI] [PubMed] [Google Scholar]
- 9. Howlett SE, Rutenberg AD, Rockwood K. The degree of frailty as a translational measure of health in aging. Nat Aging 2021;1:651–665. [DOI] [PubMed] [Google Scholar]
- 10. Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol 2009;103:1616–1621. [DOI] [PubMed] [Google Scholar]
- 11. Shamliyan T, Talley KMC, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev 2013;12:719–736. [DOI] [PubMed] [Google Scholar]
- 12. Veronese N, Cereda E, Stubbs B, Solmi M, Luchini C, Manzato E, Sergi G, Manu P, Harris T, Fontana L, Strandberg T, Amieva H, Dumurgier J, Elbaz A, Tzourio C, Eicholzer M, Rohrmann S, Moretti C, D’Ascenzo F, Quadri G, Polidoro A, Lourenço RA, Moreira VG, Sanchis J, Scotti V, Maggi S, Correll CU. . Risk of cardiovascular disease morbidity and mortality in frail and pre-frail older adults: results from a meta-analysis and exploratory meta-regression analysis. Ageing Res Rev 2017;35:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stone JA, Arthur HM; Canadian Association of Cardiac Rehabilitation Guidelines Writing Group . Canadian Guidelines for Cardiac Rehabilitation and Cardiovascular Disease Prevention: Translating Knowledge into Action. 3rd ed. Markham, Canada: Canadian Association of Cardiovascular Prevention and Rehabilitation; 2009. [Google Scholar]
- 14. Cowie A, Buckley J, Doherty P, Furze G, Hayward J, Hinton S, Jones J, Speck L, Dalal H, Mills J. . Standards and core components for cardiovascular disease prevention and rehabilitation. Heart 2019;105:510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson L, Sharp GA, Norton RJ, Dalal H, Dean SG, Jolly K, Cowie A, Zawada A, Taylor RS. . Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2017;6:CD007130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Santiago de Araújo Pio C, Beckie TM, Varnfield M, Sarrafzadegan N, Babu AS, Baidya S, Buckley J, Chen SY, Gagliardi A, Heine M, Khiong JS, Mola A, Radi B, Supervia M, Trani MR, Abreu A, Sawdon JA, Moffatt PD, Grace SL. . Promoting patient utilization of outpatient cardiac rehabilitation: a joint international council and Canadian Association of Cardiovascular Prevention and Rehabilitation position statement. J Cardiopulm Rehabil Prev 2020;40:79–86. [DOI] [PubMed] [Google Scholar]
- 17. Grace SL, Turk-Adawi KI, Contractor A, Atrey A, Campbell N, Derman W, Melo Ghisi GL, Oldridge N, Sarkar BK, Yeo TJ, Lopez-Jimenez F, Mendis S, Oh P, Hu D, Sarrafzadegan N. . Cardiac rehabilitation delivery model for low-resource settings. Heart 2016;102:1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan DCD, Tsou HH, Yang RS, Tsauo JY, Chen CY, Hsiung CA, Kuo KN. . A pilot randomized controlled trial to improve geriatric frailty. BMC Geriatr 2012;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim H, Suzuki T, Kim M, Kojima N, Ota N, Shimotoyodome A, Hase T, Hosoi E, Yoshida H. . Effects of exercise and milk fat globule membrane (MFGM) supplementation on body composition, physical function, and hematological parameters in community-dwelling frail Japanese women: a randomized double blind, placebo-controlled, follow-up trial. PloS One 2015;10:e0116256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tarazona-Santabalbina FJ, Gómez-Cabrera MC, Pérez-Ros P, Martínez-Arnau FM, Cabo H, Tsaparas K, Salvador-Pascual A, Rodriguez-Mañas L, Viña J. . A multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J Am Med Dir Assoc 2016;17:426–433. [DOI] [PubMed] [Google Scholar]
- 21. Serra-Prat M, Sist X, Domenich R, Jurado L, Saiz A, Roces A, Palomera E, Tarradelles M, Papiol M. . Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing 2017;46:401–407. [DOI] [PubMed] [Google Scholar]
- 22. Ng TP, Feng L, Nyunt MSZ, Feng L, Niti M, Tan BY, Chan G, Khoo SA, Chan SM, Yap P, Yap KB. . Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: a randomized controlled trial. Am J Med 2015;128:1225–1236.e1. [DOI] [PubMed] [Google Scholar]
- 23. Vigorito C, Abreu A, Ambrosetti M, Belardinelli R, Corrà U, Cupples M, Davos CH, Hoefer S, Iliou MC, Schmid JP, Voeller H, Doherty P. . Frailty and cardiac rehabilitation: a call to action from the EAPC Cardiac Rehabilitation Section. Eur J Prev Cardiol 2017;24:577–590. [DOI] [PubMed] [Google Scholar]
- 24. Richter D, Guasti L, Walker D, Lambrinou E, Lionis C, Abreu A, Savelieva I, Fumagalli S, Bo M, Rocca B, Jensen MT, Pierard L, Sudano I, Aboyans V, Asteggiano R. . Frailty in cardiology: definition, assessment and clinical implications for general cardiology. A consensus document of the Council for Cardiology Practice (CCP), Association for Acute Cardio Vascular Care (ACVC), Association of Cardiovascular Nursing and Allied Professions (ACNAP), European Association of Preventive Cardiology (EAPC), European Heart Rhythm Association (EHRA), Council on Valvular Heart Diseases (VHD), Council on Hypertension (CHT), Council of Cardio-Oncology (CCO), Working Group (WG) Aorta and Peripheral Vascular Diseases, WG e-Cardiology, WG Thrombosis, of the European Society of Cardiology, European Primary Care Cardiology Society (EPCCS). Eur J Prev Cardiol 2022;29:216–227. [DOI] [PubMed] [Google Scholar]
- 25. Lettino M, Mascherbauer J, Nordaby M, Ziegler A, Collet JP, Derumeaux G, Hohnloser SH, Leclercq C, O’Neill DE, Visseren F, Weidinger F, Richard-Lordereau I. . Cardiovascular disease in the elderly: proceedings of the European Society of Cardiology-Cardiovascular Round Table. Eur J Prev Cardiol 2022; 29:1412–1424. [DOI] [PubMed] [Google Scholar]
- 26. Kehler DS, Giacomantonio N, Firth W, Blanchard CM, Rockwood K, Theou O. Association between cardiac rehabilitation and frailty. Can J Cardiol 2020;36:482–489. [DOI] [PubMed] [Google Scholar]
- 27. Nishitani-Yokoyama M, Shimada K, Yamada M, Honzawa A, Kunimoto M, Sugita Y, Fujiwara K, Matsubara T, Matsumori R, Abulimiti A, Shimada A, Yamamoto T, Asai T, Amano A, Saitoh M, Morisawa T, Takahashi T, Daida H, Minamino T. . Association between constipation and frailty components in patients undergoing late phase II cardiac rehabilitation. Cardiol Res 2021;12:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lutz AH, Delligatti A, Allsup K, Afilalo J, Forman DE. Cardiac rehabilitation is associated with improved physical function in frail older adults with cardiovascular disease. J Cardiopulm Rehabil Prev 2020;40:310–318. [DOI] [PubMed] [Google Scholar]
- 29. Arai Y, Kimura T, Takahashi Y, Hashimoto T, Arakawa M, Okamura H. Preoperative frailty is associated with progression of postoperative cardiac rehabilitation in patients undergoing cardiovascular surgery. Gen Thorac Cardiovasc Surg 2019;67:917–924. [DOI] [PubMed] [Google Scholar]
- 30. Ushijima A, Morita N, Hama T, Yamamoto A, Yoshimachi F, Ikari Y, Kobayashi Y. . Effects of cardiac rehabilitation on physical function and exercise capacity in elderly cardiovascular patients with frailty. J Cardiol 2021;77:424–431. [DOI] [PubMed] [Google Scholar]
- 31. Mudge AM, Pelecanos A, Adsett JA. Frailty implications for exercise participation and outcomes in patients with heart failure. J Am Geriatr Soc 2021;69:2476–2485. [DOI] [PubMed] [Google Scholar]
- 32. Aida K, Kamiya K, Hamazaki N, Matsuzawa R, Nozaki K, Ichikawa T, Nakamura T, Yamashita M, Maekawa E, Yamaoka-Tojo M, Matsunaga A, Ako J. . Usefulness of the simplified frailty scale in predicting risk of readmission or mortality in elderly patients hospitalized with cardiovascular disease. Int Heart J 2020;61:571–578. [DOI] [PubMed] [Google Scholar]
- 33. Honzawa A, Nishitani-Yokoyama M, Shimada K, Kunimoto M, Yamada M, Matsubara T, Matsumori R, Fujiwara K, Abulimiti A, Aikawa T, Ouchi S, Shimizu M, Sugita Y, Shimada A, Yamamoto T, Amano A, Asai T, Saito M, Morisawa T, Takahashi T, Fujiwara T, Daida H, Minamino T. . Relationship between Kihon checklist score and anxiety levels in elderly patients undergoing early phase II cardiac rehabilitation. Cardiol Res 2020;11:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kunimoto M, Shimada K, Yokoyama M, Matsubara T, Aikawa T, Ouchi S, Shimizu M, Fukao K, Miyazaki T, Kadoguchi T, Fujiwara K, Honzawa A, Yamada M, Shimada A, Yamamoto T, Amano A, Daida H. . Relationship between the Kihon checklist and the clinical parameters in patients who participated in cardiac rehabilitation. Geriatr Gerontol Int 2019;19:287–292. [DOI] [PubMed] [Google Scholar]
- 35. Mathew A, Youngson E, Wirzba B, Graham M. The trajectory of frailty scores over the course of cardiac rehabiliation. Can J Cardiol 2019;35:S50. [Google Scholar]
- 36. Nozaki K, Hamazaki N, Kamiya K, Ichikawa T, Nakamura T, Yamashita M, Maekawa E, Matsunaga A, Yamaoka-Tojo M, Ako J. . Rising time from bed in acute phase after hospitalization predicts frailty at hospital discharge in patients with acute heart failure. J Cardiol 2020;75:587–593. [DOI] [PubMed] [Google Scholar]
- 37. Fonteles Ritt L, Matos E Oliveira F, Santos Pereira Ramos J, et al. Impact of a cardiovascular rehabilitation program on frailty indicators in elderly patients with heart disease. Eur J Prev Cardiol 2021;28:zwab061.037. [Google Scholar]
- 38. Dafoe W, Arthur H, Stokes H, Morrin L, Beaton L. Universal access: but when? Treating the right patient at the right time: access to cardiac rehabilitation. Can J Cardiol 2006;22:905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Howlett SE, Rockwood MRH, Mitnitski A, Rockwood K. Standard laboratory tests to identify older adults at increased risk of death. BMC Med 2014;12:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Theou O, van der Valk AM, Godin J, Andrew MK, McElhaney JE, McNeil SA, Rockwood K. . Exploring clinically meaningful changes for the frailty index in a longitudinal cohort of hospitalized older patients. J Gerontol A Biol Sci Med Sci 2020;75:1928–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jang IY, Jung HW, Lee HY, Park H, Lee E, Kim DH. Evaluation of clinically meaningful changes in measures of frailty. J Gerontol Ser A 2020;75:1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 2017;36:4391–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. R Core Team . R: A Language and Environment for Statistical Computing; Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 45. Freiheit EA, Hogan DB, Patten SB, Wunsch H, Anderson T, Ghali WA, Knudtson M, Maxwell CJ. . Frailty trajectories after treatment for coronary artery disease in older patients. Circ Cardiovasc Qual Outcomes 2016;9:230–238. [DOI] [PubMed] [Google Scholar]
- 46. Pandey A, Segar MW, Singh S, Reeves G, O’Connor C, Pina I, Whellan D, Kraus W, Mentz R, Kitzman D. . Frailty status modifies the efficacy of exercise training among patients with chronic heart failure and reduced ejection fraction: an analysis from the HF-ACTION trial. Circulation 2022;146:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kamiya K, Sato Y, Takahashi T, Tsuchihashi-Makaya M, Kotooka N, Ikegame T, Takura T, Yamamoto T, Nagayama M, Goto Y, Makita S, Isobe M. . Multidisciplinary cardiac rehabilitation and long-term prognosis in patients with heart failure. Circ Heart Fail 2020;13:e006798. [DOI] [PubMed] [Google Scholar]
- 48. Simo N, Cesari M, Tchiero H, Rolland Y, de Souto Barreto P, Dartigues JF, Vellas B, Tabue-Teguo M. . Frailty index, hospital admission and number of days spent in hospital in nursing home residents: results from the incur study. J Nutr Health Aging 2021;25:155–159. [DOI] [PubMed] [Google Scholar]
- 49. Hastings SN, Purser JL, Johnson KS, Sloane RJ, Whitson HE. A frailty index predicts some but not all adverse outcomes in older adults discharged from the emergency department. J Am Geriatr Soc 2008;56:1651–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sinn CLJ, Heckman G, Poss JW, Onder G, Vetrano DL, Hirdes J. A comparison of 3 frailty measures and adverse outcomes in the intake home care population: a retrospective cohort study. Can Med Assoc Open Access J 2020;8:E796–E809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sirven N, Rapp T. The dynamics of hospital use among older people evidence for Europe using SHARE data. Health Serv Res 2017;52:1168–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ekerstad N, Dahlin Ivanoff S, Landahl S, Östberg G, Johansson M, Andersson D, Husberg M, Alwin J, Karlson BW. . Acute care of severely frail elderly patients in a CGA-unit is associated with less functional decline than conventional acute care. Clin Interv Aging 2017;12:1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stolz E, Hoogendijk EO, Mayerl H, Freidl W. Frailty changes predict mortality in four longitudinal studies of aging. J Gerontol A Biol Sci Med Sci 2020;76(9):1619-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shi SM, Olivieri-Mui B, McCarthy EP, Kim DH. Changes in a frailty index and association with mortality. J Am Geriatr Soc 2021;69:1057–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thompson MQ, Theou O, Tucker GR, Adams RJ, Visvanathan R. Recurrent measurement of frailty is important for mortality prediction: findings from the North West Adelaide Health Study. J Am Geriatr Soc 2019;67:2311–2317. [DOI] [PubMed] [Google Scholar]
- 56. Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ Can Med Assoc J 2011;183:E487–E494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chamberlain AM, Finney Rutten LJ, Manemann SM, Yawn BP, Jacobson DJ, Fan C, Grossardt BR, Roger VL, St. Sauver JL. . Frailty trajectories in an elderly population-based cohort. J Am Geriatr Soc 2016;64:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hwang AC, Lee WJ, Huang N, Chen LY, Peng LN, Lin MH, Chou YJ, Chen LK. . Longitudinal changes of frailty in 8 years: comparisons between physical frailty and frailty index. BMC Geriatr 2021;21:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Theou O, Stathokostas L, Roland KP, Jakobi JM, Patterson C, Vandervoort AA, Jones GR. . The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res 2011;2011:569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pérez-Zepeda MU, Martínez-Velilla N, Kehler DS, Izquierdo M, Rockwood K, Theou O. The impact of an exercise intervention on frailty levels in hospitalised older adults: secondary analysis of a randomised controlled trial. Age Ageing 2022;51:afac028. [DOI] [PubMed] [Google Scholar]
- 61. Pedretti RFE, Hansen D, Ambrosetti M, Back M, Berger T, Ferreira MC, Cornelissen V, Davos CH, Doehner W, de Pablo y Zarzosa C, Frederix I, Greco A, Kurpas D, Michal M, Osto E, Pedersen SS, Salvador RE, Simonenko M, Steca P, Thompson DR, Wilhelm M, Abreu A. . How to optimize the adherence to a guideline-directed medical therapy in the secondary prevention of cardiovascular diseases: a clinical consensus statement from the European Association of Preventive Cardiology (EAPC). Eur J Prev Cardiol 2023;30(2):149-166. [DOI] [PubMed] [Google Scholar]
- 62. Yeung C, Giacomantonio N, Firth W. Adherence to quality indicators and temporal trends in a cardiac rehabilitation program. Can J Cardiol 2014;30:S306–S307. [Google Scholar]
- 63. Rockwood K, Mitnitski A. How might deficit accumulation give rise to frailty? J Frailty Aging 2012;1:8–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly to preserve the privacy of individuals that participated in the study.