Abstract
Anhedonia is a core symptom of depression and of several psychiatric disorders. Anhedonia has however expanded from its original definition to encompass a spectrum of reward processing deficits that received much interest in the last decades. It is a relevant risk factor for possible suicidal behaviors, and that it may operate as an independent risk factor for suicidality apart from the episode severity. Anhedonia has also been linked to inflammation with a possible reciprocal deleterious effect on depression. Its neurophysiological bases mainly include alterations in striatal and prefrontal areas, with dopamine being the most involved neurotransmitter. Anhedonia is thought to have a significant genetic component and polygenic risk scores are a possible tool for predicting an individual’s risk for developing anhedonia. Traditional antidepressants, such as selective serotonin reuptake inhibitors, showed a limited benefit on anhedonia, also considering their potential pro-anhedonic effect in some subjects. Other treatments may be more effective in treating anhedonia, such as agomelatine, vortioxetine, ketamine and transcranial magnetic stimulation. Psychotherapy is also widely supported, with cognitive-behavioral therapy and behavioral activation both showing benefit. In conclusion, a large body of evidence suggests that anhedonia is, at least partially, independent from depression, therefore it needs careful assessment and targeted treatment.
Keywords: Major depressive disorder, Anhedonia, Psychopharmacology, Antidepressants
INTRODUCTION
Anhedonia is commonly defined as the inability to experience pleasure or enjoyment from activities that would normally be pleasurable. It is a core symptom of depression and is often used to diagnose the disorder. However the concept of anhedonia and its role for the diagnosis changed in the past hundred years. Originally it was described as an independent disorder [1], but since the Diagnostic and Statistical Manual of Mental Disorders 3rd edition (DSM-III) it was included as a symptom for the diagnosis of a major depressive episode [2]. After that, for some time it has been considered just a symptom of the depressive disorder [3], but in the recent decades converging evidence suggested that it should be considered independent from depression itself [4-8].
The aim of the present review is to summarize the present knowledge on anhedonia in terms of clinical features, neurophysiological basis, predictive role and possible treatments.
CLINICAL FEATURES OF ANHEDONIA
In the DSM-5 criteria for major depressive episode, anhedonia is defined as a “Markedly diminished interest or pleasure in all, or almost all, activities” [9]. However anhedonia is a common symptom of several psychiatric disorders, including schizophrenia, substance dependence, posttraumatic stress disorder, anxiety disorders, eating disorders, autism and neurodegenerative disorders [10]. Anhedonia has been originally characterized by a reduced ability to experience pleasure or interest in normally rewarding stimuli. However, anhedonia has expanded from its original definition (loss of pleasure or interest) to encompass a spectrum of reward processing deficits [11-14]. Current conceptualizations, such as the positive valence systems outlined by the Research Domain Criteria [15], include reward components such as interest, motivation, effort expenditure, valuation, reward anticipation, learning, pleasure, and satiation [4,5].
This broadening raised much interest and discussion, in the present paper it is possible to summarize the various facets in two broad concepts: anticipatory anhedonia, which refers to a reduced ability to experience pleasure in anticipation of rewarding events, and consummatory anhedonia, which refers to a reduced ability to experience pleasure from activities [6,16]. Individuals with anticipatory anhedonia may have difficulties in imagining or anticipating future pleasurable events. This difficulty can manifest in several ways, such as reduced motivation to engage in pleasurable activities, difficulty planning and initiating activities, and a lack of enthusiasm or excitement about future events.
Consummatory anhedonia refers to a reduced ability to experience pleasure during enjoyable activities. This can also manifest in several ways, such as reduced pleasure from social and recreational activities, a lack of enjoyment during activities, and a reduced frequency of engaging in pleasurable activities. These individuals have difficulty experiencing positive emotions during rewarding events, such as social interaction, sport, sexual activity, or eating.
Anhedonia is not a stable trait, it may also change, typically it is more severe in acute phases of the disorder, and it may decrease or disappear after recovery. This raised the issue of state vs trait anhedonia. In some cases there may be a decrease in the ability to derive pleasure, that is, not to enjoy a particular activity and was enjoyed it in the past, this is typical of acute depressive episodes [17]. Alternatively, other subjects present a low general impairment to experience pleasure, without a temporal com-ponent. This may be typical of dysthymia or substance abuse.
Another perspective is on the target of anhedonia feelings, as an example for social or physical activities. Social anhedonia is defined as getting little or no pleasure from interpersonal situations, whereas physical anhedonia is defined as getting little or no pleasure from nonsocial physical sensations like smell, taste, touch, or sound [18].
PREVALENCE OF ANHEDONIA
The prevalence of anhedonia in depression is generally quite high, however it varies depending on the study and the population being studied. Up to 70% of people with depression may experience anhedonia [19]. Without evidence of variation in the prevalence based on the subtype of depression [20]. Other psychiatric disorders have also shown a substantial prevalence of anhedonia, though lower [21]. It may also persist after treatment in a number of cases [22]. There is no reported gender difference. Despite the known higher rate of depression in women, there is no evidence that anhedonia is more common in women than in men. On the contrary, some evidence suggests a higher prevalence in men, at least regarding social anhedonia [23]. Age is also correlated with increased rates of anhedonia, though only in general population studies [23].
ASSESSMENT OF ANHEDONIA
Assessment of anhedonia can be challenging because it is a subjective experience. However, several measures have been developed to assess anhedonia in clinical and research settings. One of the most common scales is the Temporal Experience of Pleasure Scale (TEPS), it is a 18- item self-report questionnaire that assesses anticipatory and consummatory pleasure in social and physical activities [24]. The TEPS has been shown to have good reliability and validity in both clinical and non-clinical popu-lations.
Another common instrument is the Snaith-Hamilton Pleasure Scale is a 14-item self-report questionnaire that assesses anhedonia in the context of social, physical, and recreational activities [25]. The scale has been largely used and it was designed for use in major depressive disorder. A number of other instruments have been developed, at times with focus on specific aspects of anhedonia, but they have generally been validated in fewer samples [10].
NEUROPHYSIOLOGICAL BASIS
The neurophysiological basis has been repeatedly investigated in depressed patients, but also in healthy controls, with some evidence coming from animal studies. The prefrontal cortex, including the ventromedial prefrontal cortex and the dorsolateral prefrontal cortex, plays a critical role in reward processing and decision-making [26,27]. But probably the most relevant mechanism at the basis of anhedonia is the striatal hypoactivation for anticipatory anhedonia while prefrontal areas for consum-matory anhedonia [5,8,28].
Other areas have been involved in various studies: the nucleus accumbens, striatum, ventral pallidum, ventral tegmental area, amygdala, insula, anterior cingulate cortex, orbitofrontal cortex, and prefrontal cortex have all been involved in multiple facets of reward deficits in both animal models and depressed patients. As expected, dopamine is the neurotransmitter that has been studied the most extensively in preclinical studies with some clinical support for its role in regulating the various facets of anhedonia such as anticipation, motivation, effort, and learning. Some evidence also involves the other neurotransmitters. Serotonin, epinephrine, opioid, glutamate, gamma-aminobutyric acid, and acetylcholine may modulate consummatory pleasure and motivation [5,8,29-31].
GENETIC STUDIES
Genetic studies may boost our knowledge on the physiopathological basis of anhedonia and suggest new treat-ments. Anhedonia is thought to have a significant genetic component and recent reports suggest a substantial heritability, that is inherent genetic predisposition [32-38]. The largest genome wide analysis to date on 375,275 subjects reported 11 loci explaining 5.6% of anhedonia [39], even if the percentage may look modest, it is promising. In fact a deeper genetic analysis including more variants and other genetic variations is likely to explain a larger part of the variance.
Polygenic risk scores are a promising approach for predicting an individual’s risk for developing a particular disorder based on their genetic makeup, therefore they may be useful in clinical practice to predict which subjects are at most risk of developing anhedonia. They are calculated by summing the effects of multiple genetic variants that have been associated with the disorder of interest, with the weights of each variant determined by their effect sizes from previous genome-wide association studies. Interestingly, polygenic risk for anhedonia (the cumulative measure of genetic risk) was associated with poorer brain white matter integrity, smaller total grey matter volume, and smaller volumes of brain regions linked to reward and pleasure processing [40]. This is a convergent finding linking brain imaging findings with genetics.
In addition to genetic studies, researchers have also looked at the role of epigenetic modifications in anhedonia. Epigenetic modifications are chemical changes to DNA that do not alter the underlying sequence, but can affect gene expression. It has been reported that epigenetic modifications to genes involved in reward processing and mood regulation are associated with anhedonia [41].
While genetic and epigenetic studies have identified a number of candidate genes and pathways associated with anhedonia, it is important to note that these findings are often complex and context-dependent. For example, the same gene may have different effects in different populations or under different environmental conditions. Moreover, many genes and pathways are likely to interact with each other to contribute to the development of an-hedonia.
Despite these complexities, the study of the genetics of anhedonia holds promise for improving our understanding of the underlying mechanisms and developing more targeted treatments. For example, the identification of specific genetic variants or pathways could help identify individuals who are at higher risk for developing anhedonia, and may help guide early interventions or treatment strategies. Moreover, understanding the specific genes and pathways involved in anhedonia could lead to the development of novel pharmacological or behavioral interventions that target these pathways.
PROGNOSTIC VALUE
In the context of mood disorders, convergent lines of evidence imply that anhedonia is linked with a more severe and recurrent disease. Higher levels of anhedonia in depression have been related with a larger number of preceding depressive episodes, longer depressive episode length, and greater overall disease severity. Similarly, longitudinal studies suggest that severity of anhedonia predicts a persistence of depression 12 months later. There is therefore wide support to the view that the presence of more severe anhedonia may lead to a more severe disease course [42-44].
Anhedonia has often been associated with an increased risk for suicidal behavior [45] though not unequivocally [46].
However the majority of studies suggest that anhedonia should be considered a relevant risk factor for possible suicidal behaviors, this is an issue considering the persistence of suicidal ideation also after treatment [47-49]. Higher levels of anhedonia in depression have been linked to higher suicidal ideation both in cross-sectional and longitudinal studies. There is also evidence of an increase of higher risk for suicide attempts. Interestingly, these correlations survive after adjustment for the overall symptomatology severity, indicating that anhedonia may operate as an independent risk factor for suicidality apart from the episode severity [50-54].
Several studies have investigated the relationship between anhedonia and inflammation in depression. As an example, increased plasma C-reactive protein has been associated with anhedonia, through decreased connectivity between ventral striatum and ventromedial prefrontal cortex and mediated by left basal ganglia glutamate, this was independent from clinical and non clinical confounders [55-59]. The relationship between anhedonia and inflammation may be bidirectional, meaning that inflammation may contribute to the physiopathology of anhedonia, and anhedonia may also influence inflam-mation by reducing engagement in anti inflammatory behaviors, such as exercise and social interactions.
The role of inflammation in anhedonia is also supported by research on the efficacy of anti-inflammatory treatments in reducing anhedonia. For example, one study found that treatment with the anti-inflammatory drug infliximab significantly improved anhedonia in individuals with treatment-resistant depression [60], despite a non- significant effect on the overall depressive symptoma-tology. While the relationship between anhedonia and inflammation is still being studied, it is clear that inflam-mation plays an important role in the pathophysiology of depression [61] and may contribute to the development and persistence of anhedonia in a vicious circle of poor prognosis.
TREATMENT OF ANHEDONIA
Traditional antidepressants, such as selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors, are first line treatments for depression and its associated symptoms, including anhedonia. However, their effectiveness in treating anhedonia is limited, with evidence of partial or no improvement in anhedonia with antidepressant medication alone [19,62,63]. Further, antidepressants have been reported to possibly lead to emotional detachment in some subjects, this may result in perceived anhedonia [64,65].
Nonetheless, some antidepressants may be more effective in treating anhedonia than others. Given the involvement of dopamine in the pathophysiology of anhedonia, bupropion is a likely candidate for treatment. A randomized, double-blind, placebo-controlled clinical trial reported positive effects of bupropion (200−300 mg) on anhedonia [66], and, interestingly, the newly reported combination of dextromethorphan with bupropion may be effective as well [67]. The benefit is also supported by animal studies [68,69]. However, opposite effects have been observed [70]. Similar drugs such as pramipexole have also shown positive effects, particularly in the context of Parkinson disease [71].
Selective serotonin reuptake inhibitors, as previously stated, do not seem very beneficial on anhedonia, as an example escitalopram resulted less effective than cognitive behavioral therapy or agomelatine in anhedonia improvement [72,73]. However, selective serotonin reuptake inhibitors with some effect on the dopaminergic system such as sertraline and fluoxetine seem to be more effective [74,75]. Similarly, venlafaxine, which targets also noradrenergic and dopaminergic systems, was beneficial [76].
Agomelatine is probably the compound that received most interest in treating anhedonia, a number of inde-pendent studies suggested a relevant benefit both in the short and medium term [42,77-81]. Finally, the most recent multimodal antidepressant vortioxetine was more effective than agomelatine [82]. The effectiveness of vortioxetine is also supported by a number of other studies [83-85], also in mild cognitive impairment [86].
Ketamine is reaching a wide clinical use due to the rapid antidepressant effects [87-89], the preliminary evidence available suggest that it has a relevant anti-anhedonic effect with a rapid onset of action [50,90], thus suggesting its use in specific clinical contexts where rapid effect is needed. Within a similar drug class, much interest is now focusing on psilocybin, which has the further benefit of one point administration [91]. Studies are ongoing, but preliminary evidence suggests a significant and specific benefit on anhedonia, which persists up to 3 months after treatment [92].
Stimulants, such as methylphenidate, are currently used for the treatment of attention deficit disorder. Based on their mechanism of action it is possible to hypothesize a benefit on anhedonia as well, in fact a randomized placebo controlled trial reported a significant benefit of methylphenidate on anhedonia together with the antidepressant treatment [93].
Given the knowledge deriving from imaging studies about the brain regions involved in anhedonia, there is a growing interest in possible circuit-targeted neuromodu-lation techniques [10]. One of the most promising is transcranial magnetic stimulation. It is a well known treatment for depression, but there is evidence also that it may benefit anhedonia. Pilot studies reported some benefit in anhedonia [94-97]. These early findings have been recently confirmed in a large naturalistic study which reported a substantial benefit from transcranial magnetic stimulation [98]. Interestingly, the improvement in anhedonia was correlated to the improvement of depressive symptoms, as expected, but with a correlation far from complete, suggesting that a number of subjects improved in anhedonia much more than in depressive symptoms.
Psychotherapy’s usefulness in depression and resistant depression, in combination with antidepressants, is well known [99], therefore it has been investigated for the treatment of anhedonia. Psychotherapy is an important component of the treatment of anhedonia in depression. The most common psychotherapy, cognitive-behavioral therapy, and its component alone behavioral activation, both demonstrated benefit on anhedonia [100], cognitive-behavioral therapy targets and challenges negative thought patterns that may contribute to anhedonia, while behavioral activation focuses on increasing engagement in rewarding activities, for this reason behavioral activation has been repeatedly suggested for treating anhedonia. Moreover a large number of less studied treatments have been suggested as well [10].
It is important to note that the effectiveness of psychotherapy in treating anhedonia in depression may vary depending on individual factors, such as the severity of depression, the presence of other symptoms, and personal preferences. A personalized approach to treatment that takes these factors into account is therefore recommended [101].
CONCLUSION
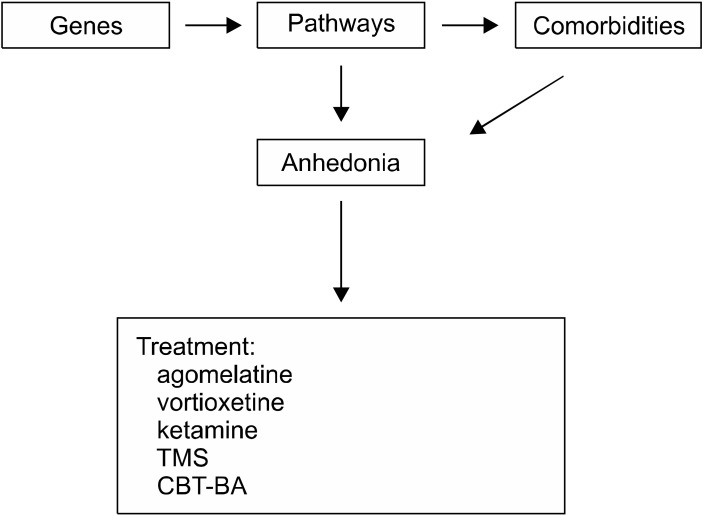
Anhedonia is one of the hallmark symptoms of depression as well as a number of other mental conditions. The term “anhedonia” now has been expanded and it refers to a spectrum of reward processing deficiencies, which have garnered a lot of attention over the last several decades. This is a significant expansion from the term’s initial meaning, and it boosted research on anhedonia itself. It has then been shown that anhedonia is a meaningful risk factor for prospective suicidal behaviours, and that it may function as a potentially independent risk factor for suicidality separate from the depression itself. Anhedonia has also been connected to inflammation, which may have a detrimental influence on depression as a result of the reciprocal relationship. Dopamine is the neurotransmitter that is most heavily implicated in this condition’s neurophysiological basis, which mostly entails abnormalities in the striatal and prefrontal parts of the brain. An important role for genetics is assumed to play in the development of anhedonia, and polygenic risk scores may provide in the future a potentially useful method for predicting an individual’s likelihood of acquiring the condition (Fig. 1). Conventional antidepressants, such as selective serotonin reuptake inhibitors, demonstrated a modest benefit on anhedonia. This is especially important when one takes into account the fact that these medications may actually have a positive impact on anhedonia in certain patients along with the depressive symptoms. Anhedonia may be more effectively treated with a variety of different antidepressants, such as bupropion, dextromethorphan combined with bupropion, agomelatine, ketamine and transcranial magnetic stimulation (Table 1). Some of these treatments may be more successful than others in specific subjects but this is still under investigation. Moreover, there is a lot of evidence for psychotherapy, particularly cognitive behavioral therapy and behavioral activation, both of which have shown to be beneficial. In conclusion, a substantial body of data demonstrates that anhedonia is at least somewhat separate from depression. As a result, clinicians should focus on this aspect specifically and not disregard anhedonia as a simple symptom of the depressive episode.
Fig. 1.
Anhedonia causes and main treatments.
Table 1.
Level of evidence for Anhedonia treatments
| Treatment | Level of evidence |
|---|---|
| Agomelatine [42,77-81] | *** |
| Vortioxetine [82-86] | *** |
| Ketamine [50,90] | *** |
| Transcranial magnetic stimulation [94-98] | *** |
| Psylocibine [92] | ** |
| Bupropion (with or without dextromethorphan) [66,67,70] | ** |
| Sertraline and fluoxetine [74,75] | ** |
| Venlafaxine [76] | ** |
| Methylphenidate [93] | ** |
| Cognitive-behavioral therapy, behavioral activation [100] | ** |
| Selective serotonin reuptake inhibitors except sertraline and fluoxetine [19,62,63,72,73] | * |
| Pramipexole [71] | * |
Ranked from * to *** based on empirical evidence.
Funding Statement
Funding None.
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Lewis WB. A text-book of mental diseases: With special reference to the pathological aspects of insanity. Griffin; 1889. p. 552. [Google Scholar]
- 2.American Psychiatric Association, author. Diagnostic and statistical manual of mental disorders. 3rd ed. American Psychiatric Association; 1980. [Google Scholar]
- 3.Carlson GA, Kashani JH. Phenomenology of major depression from childhood through adulthood: Analysis of three studies. Am J Psychiatry. 1988;145:1222–1225. doi: 10.1176/ajp.145.10.1222. [DOI] [PubMed] [Google Scholar]
- 4.Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. Assessing anhedonia in depression: Potentials and pitfalls. Neurosci Biobehav Rev. 2016;65:21–35. doi: 10.1016/j.neubiorev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S, Leri F, Rizvi SJ. Anhedonia as a central factor in depression: Neural mechanisms revealed from preclinical to clinical evidence. Prog Neuropsychopharmacol Biol Psychiatry. 2021;110:110289. doi: 10.1016/j.pnpbp.2021.110289. [DOI] [PubMed] [Google Scholar]
- 6.Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitton AE, Pizzagalli DA. Anhedonia in depression and bipolar disorder. Curr Top Behav Neurosci. 2022;58:111–127. doi: 10.1007/7854_2022_323. [DOI] [PubMed] [Google Scholar]
- 8.Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Psychiatric Association, author. Diagnostic and statistical manual of mental disorders: DSM-5-TR. American Psychiatric Association Publishing; 2022. p. 1050. [DOI] [Google Scholar]
- 10.Pizzagalli DA. Anhedonia: Preclinical, translational, and clinical integration. Springer Nature; 2022. p. 3.p. 515. [DOI] [Google Scholar]
- 11.Watson CG, Klett WG, Lorei TW. Toward an operational definition of anhedonia. Psychol Rep. 1970;26:371–376. doi: 10.2466/pr0.1970.26.2.371. [DOI] [PubMed] [Google Scholar]
- 12.Phillips AG, Ahn S. Anticipation: An essential feature of anhedonia. Curr Top Behav Neurosci. 2022;58:305–323. doi: 10.1007/7854_2022_317. [DOI] [PubMed] [Google Scholar]
- 13.Kieslich K, Valton V, Roiser JP. Pleasure, reward value, prediction error and anhedonia. Curr Top Behav Neurosci. 2022;58:281–304. doi: 10.1007/7854_2021_295. [DOI] [PubMed] [Google Scholar]
- 14.Thomsen KR. Measuring anhedonia: Impaired ability to pursue, experience, and learn about reward. Front Psychol. 2015;6:1409. doi: 10.3389/fpsyg.2015.01409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 16.Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology (Berl) 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- 17.Gandhi A, Mote J, Fulford D. A transdiagnostic meta-analysis of physical and social Anhedonia in major depressive disorder and schizophrenia spectrum disorders. Psychiatry Res. 2022;309:114379. doi: 10.1016/j.psychres.2021.114379. [DOI] [PubMed] [Google Scholar]
- 18.Winer ES, Jordan DG, Collins AC. Conceptualizing anhedonias and implications for depression treatments. Psychol Res Behav Manag. 2019;12:325–335. doi: 10.2147/PRBM.S159260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao B, Zhu J, Zuckerman H, Rosenblat JD, Brietzke E, Pan Z, et al. Pharmacological interventions targeting anhedonia in patients with major depressive disorder: A systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:109–117. doi: 10.1016/j.pnpbp.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher K, Parker G, Paterson A, Fava M, Iosifescu D, Pizzagalli DA. Anhedonia in melancholic and non-melancholic depressive disorders. J Affect Disord. 2015;184:81–88. doi: 10.1016/j.jad.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trøstheim M, Eikemo M, Meir R, Hansen I, Paul E, Kroll SL, et al. Assessment of anhedonia in adults with and without mental illness: A systematic review and meta-analysis. JAMA Netw Open. 2020;3:e2013233. doi: 10.1001/jamanetworkopen.2020.13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Nicola M, De Risio L, Battaglia C, Camardese G, Tedeschi D, Mazza M, et al. Reduced hedonic capacity in euthymic bipolar subjects: A trait-like feature? J Affect Disord. 2013;147:446–450. doi: 10.1016/j.jad.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Dodell-Feder D, Germine L. Epidemiological dimensions of social anhedonia. Clin Psychol Sci. 2018;6:735–743. doi: 10.1177/2167702618773740. [DOI] [Google Scholar]
- 24.Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 26.Ciaramelli E, De Luca F, Kwan D, Mok J, Bianconi F, Knyagnytska V, et al. The role of ventromedial prefrontal cortex in reward valuation and future thinking during intertemporal choice. Elife. 2021;10:e67387. doi: 10.7554/eLife.67387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staudinger MR, Erk S, Walter H. Dorsolateral prefrontal cortex modulates striatal reward encoding during reappraisal of reward anticipation. Cereb Cortex. 2011;21:2578–2588. doi: 10.1093/cercor/bhr041. [DOI] [PubMed] [Google Scholar]
- 28.Borsini A, Wallis ASJ, Zunszain P, Pariante CM, Kempton MJ. Characterizing anhedonia: A systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cogn Affect Behav Neurosci. 2020;20:816–841. doi: 10.3758/s13415-020-00804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treadway MT. The neurobiology of motivational deficits in depression--an update on candidate pathomechanisms. Curr Top Behav Neurosci. 2016;27:337–355. doi: 10.1007/7854_2015_400. [DOI] [PubMed] [Google Scholar]
- 30.Tejeda HA, Bonci A. Dynorphin/kappa-opioid receptor control of dopamine dynamics: Implications for negative affective states and psychiatric disorders. Brain Res. 2019;1713:91–101. doi: 10.1016/j.brainres.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 31.Morales M, Margolis EB. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017;18:73–85. doi: 10.1038/nrn.2016.165. [DOI] [PubMed] [Google Scholar]
- 32.Bondy E, Bogdan R. Understanding anhedonia from a genomic perspective. Curr Top Behav Neurosci. 2022;58:61–79. doi: 10.1007/7854_2021_293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berenbaum H, Oltmanns TF, Gottesman II. Hedonic capacity in schizophrenics and their twins. Psychol Med. 1990;20:367–374. doi: 10.1017/S0033291700017682. [DOI] [PubMed] [Google Scholar]
- 34.Berenbaum H, McGrew J. Familial resemblance of schizotypic traits. Psychol Med. 1993;23:327–333. doi: 10.1017/S0033291700028427. [DOI] [PubMed] [Google Scholar]
- 35.Clementz BA, Grove WM, Katsanis J, Iacono WG. Psycho-metric detection of schizotypy: Perceptual aberration and physical anhedonia in relatives of schizophrenics. J Abnorm Psychol. 1991;100:607–612. doi: 10.1037/0021-843X.100.4.607. [DOI] [PubMed] [Google Scholar]
- 36.Franke P, Maier W, Hardt J, Hain C. Cognitive functioning and anhedonia in subjects at risk for schizophrenia. Schizophr Res. 1993;10:77–84. doi: 10.1016/0920-9964(93)90079-X. [DOI] [PubMed] [Google Scholar]
- 37.Hay DA, Martin NG, Foley D, Treloar SA, Kirk KM, Heath AC. Phenotypic and genetic analyses of a short measure of psychosis-proneness in a large-scale Australian twin study. Twin Res. 2001;4:30–40. doi: 10.1375/twin.4.1.30. [DOI] [PubMed] [Google Scholar]
- 38.Kendler KS, Ochs AL, Gorman AM, Hewitt JK, Ross DE, Mirsky AF. The structure of schizotypy: A pilot multitrait twin study. Psychiatry Res. 1991;36:19–36. doi: 10.1016/0165-1781(91)90114-5. [DOI] [PubMed] [Google Scholar]
- 39.Ward J, Lyall LM, Bethlehem RAI, Ferguson A, Strawbridge RJ, Lyall DM, et al. Novel genome-wide associations for anhedonia, genetic correlation with psychiatric disorders, and polygenic association with brain structure. Transl Psychiatry. 2019;9:327. doi: 10.1038/s41398-019-0635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu X, Ward J, Cullen B, Lyall DM, Strawbridge RJ, Lyall LM, et al. Phenotypic and genetic associations between anhedonia and brain structure in UK Biobank. Transl Psychiatry. 2021;11:395. doi: 10.1038/s41398-021-01522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bang M, Kang JI, Kim SJ, Park JY, Kim KR, Lee SY, et al. Reduced DNA methylation of the oxytocin receptor gene is associated with anhedonia-asociality in women with recent-onset schizophrenia and ultra-high risk for psychosis. Schizophr Bull. 2019;45:1279–1290. doi: 10.1093/schbul/sbz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vinckier F, Gourion D, Mouchabac S. Anhedonia predicts poor psychosocial functioning: Results from a large cohort of patients treated for major depressive disorder by general practitioners. Eur Psychiatry. 2017;44:1–8. doi: 10.1016/j.eurpsy.2017.02.485. [DOI] [PubMed] [Google Scholar]
- 43.Gabbay V, Johnson AR, Alonso CM, Evans LK, Babb JS, Klein RG. Anhedonia, but not irritability, is associated with illness severity outcomes in adolescent major depression. J Child Adolesc Psychopharmacol. 2015;25:194–200. doi: 10.1089/cap.2014.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spijker J, Bijl RV, de Graaf R, Nolen WA. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: Results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Acta Psychiatr Scand. 2001;103:122–130. doi: 10.1034/j.1600-0447.2001.103002122.x. [DOI] [PubMed] [Google Scholar]
- 45.Auerbach RP, Pagliaccio D, Kirshenbaum JS. Anhedonia and suicide. Curr Top Behav Neurosci. 2022;58:443–464. doi: 10.1007/7854_2022_358. [DOI] [PubMed] [Google Scholar]
- 46.Bonanni L, Gualtieri F, Lester D, Falcone G, Nardella A, Fiorillo A, et al. Can anhedonia be considered a suicide risk factor? A review of the literature. Medicina (Kaunas) 2019;55:458. doi: 10.3390/medicina55080458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pompili M. Persistent suicidal ideation in major depressive disorder patients: Still in need of empathic understanding. Int Clin Psychopharmacol. 2022;37:279–280. doi: 10.1097/YIC.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 48.Olgiati P, Serretti A. Persistence of suicidal ideation within acute phase treatment of major depressive disorder: Analysis of clinical predictors. Int Clin Psychopharmacol. 2022;37:193–200. doi: 10.1097/YIC.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 49.Olgiati P, Fanelli G, Serretti A. Clinical correlates and prognostic implications of severe suicidal ideation in major depressive disorder. Int Clin Psychopharmacol 2023 Feb 27. doi: 10.1097/YIC. 2023 Feb 27; doi: 10.1097/YIC.0000000000000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ballard ED, Wills K, Lally N, Richards EM, Luckenbaugh DA, Walls T, et al. Anhedonia as a clinical correlate of suicidal thoughts in clinical ketamine trials. J Affect Disord. 2017;218:195–200. doi: 10.1016/j.jad.2017.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ducasse D, Dubois J, Jaussent I, Azorin JM, Etain B, Gard S, et al. Association between anhedonia and suicidal events in patients with mood disorders: A 3-year prospective study. Depress Anxiety. 2021;38:17–27. doi: 10.1002/da.23072. [DOI] [PubMed] [Google Scholar]
- 52.Ducasse D, Loas G, Dassa D, Gramaglia C, Zeppegno P, Guillaume S, et al. Anhedonia is associated with suicidal ideation independently of depression: A meta-analysis. Depress Anxiety. 2018;35:382–392. doi: 10.1002/da.22709. [DOI] [PubMed] [Google Scholar]
- 53.Fawcett J, Scheftner WA, Fogg L, Clark DC, Young MA, Hedeker D, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry. 1990;147:1189–1194. doi: 10.1176/ajp.147.9.1189. [DOI] [PubMed] [Google Scholar]
- 54.Sagud M, Šimunić L, Tudor L, Jezernik D, Madžarac Z, Jakšić N, et al. Physical and social anhedonia are associated with suicidality in major depression, but not in schizophrenia. Suicide Life Threat Behav. 2021;51:446–454. doi: 10.1111/sltb.12724. [DOI] [PubMed] [Google Scholar]
- 55.Felger JC, Treadway MT. Inflammation effects on motivation and motor activity: Role of dopamine. Neuropsychopharma-cology. 2017;42:216–241. doi: 10.1038/npp.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21:1358–1365. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haroon E, Fleischer CC, Felger JC, Chen X, Woolwine BJ, Patel T, et al. Conceptual convergence: Increased inflam-mation is associated with increased basal ganglia glutamate in patients with major depression. Mol Psychiatry. 2016;21:1351–1357. doi: 10.1038/mp.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee Y, Subramaniapillai M, Brietzke E, Mansur RB, Ho RC, Yim SJ, et al. Anti-cytokine agents for anhedonia: Targeting inflammation and the immune system to treat dimensional disturbances in depression. Ther Adv Psychopharmacol. 2018;8:337–348. doi: 10.1177/2045125318791944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vichaya EG, Dantzer R. Inflammation-induced motivational changes: Perspective gained by evaluating positive and negative valence systems. Curr Opin Behav Sci. 2018;22:90–95. doi: 10.1016/j.cobeha.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benedetti F, Zanardi R, Mazza MG. Antidepressant psychopharmacology: Is inflammation a future target? Int Clin Psychopharmacol. 2022;37:79–81. doi: 10.1097/YIC.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 62.Ho N, Sommers M. Anhedonia: A concept analysis. Arch Psychiatr Nurs. 2013;27:121–129. doi: 10.1016/j.apnu.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buckner JD, Joiner TE, Jr, Pettit JW, Lewinsohn PM, Schmidt NB. Implications of the DSM's emphasis on sadness and anhedonia in major depressive disorder. Psychiatry Res. 2008;159:25–30. doi: 10.1016/j.psychres.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment ap-proaches. Lancet Psychiatry. 2017;4:409–418. doi: 10.1016/S2215-0366(17)30015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serretti A, Calati R, Goracci A, Di Simplicio M, Castrogiovanni P, De Ronchi D. Antidepressants in healthy subjects: What are the psychotropic/psychological effects? Eur Neuropsy-chopharmacol. 2010;20:433–453. doi: 10.1016/j.euroneuro.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 66.Tomarken AJ, Dichter GS, Freid C, Addington S, Shelton RC. Assessing the effects of bupropion SR on mood dimensions of depression. J Affect Disord. 2004;78:235–241. doi: 10.1016/S0165-0327(02)00306-3. [DOI] [PubMed] [Google Scholar]
- 67.Majeed A, Xiong J, Teopiz KM, Ng J, Ho R, Rosenblat JD, et al. Efficacy of dextromethorphan for the treatment of depression: A systematic review of preclinical and clinical trials. Expert Opin Emerg Drugs. 2021;26:63–74. doi: 10.1080/14728214.2021.1898588. [DOI] [PubMed] [Google Scholar]
- 68.Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psycho-pharmacology (Berl) 2003;168:347–358. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- 69.Paterson NE, Balfour DJ, Markou A. Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell. Eur J Neurosci. 2007;25:3099–3108. doi: 10.1111/j.1460-9568.2007.05546.x. [DOI] [PubMed] [Google Scholar]
- 70.Walsh AEL, Huneke NTM, Brown R, Browning M, Cowen P, Harmer CJ. A dissociation of the acute effects of bupropion on positive emotional processing and reward processing in healthy volunteers. Front Psychiatry. 2018;9:482. doi: 10.3389/fpsyt.2018.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lemke MR, Brecht HM, Koester J, Reichmann H. Effects of the dopamine agonist pramipexole on depression, anhedonia and motor functioning in Parkinson's disease. J Neurol Sci. 2006;248:266–270. doi: 10.1016/j.jns.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 72.Farabaugh A, Fisher L, Nyer M, Holt D, Cohen M, Baer L, et al. Similar changes in cognitions following cognitive-behavioral therapy or escitalopram for major depressive disorder: Implications for mechanisms of change. Ann Clin Psychiatry. 2015;27:118–126. [PubMed] [Google Scholar]
- 73.Corruble E, de Bodinat C, Belaïdi C, Goodwin GM agomelatine study group, author. Efficacy of agomelatine and escitalopram on depression, subjective sleep and emotional experiences in patients with major depressive disorder: A 24-wk randomized, controlled, double-blind trial. Int J Neuropsychophar-macol. 2013;16:2219–2234. doi: 10.1017/S1461145713000679. [DOI] [PubMed] [Google Scholar]
- 74.Boyer P, Tassin JP, Falissart B, Troy S. Sequential improvement of anxiety, depression and anhedonia with sertraline treatment in patients with major depression. J Clin Pharm Ther. 2000;25:363–371. doi: 10.1046/j.1365-2710.2000.00302.x. [DOI] [PubMed] [Google Scholar]
- 75.Light SN, Heller AS, Johnstone T, Kolden GG, Peterson MJ, Kalin NH, et al. Reduced right ventrolateral prefrontal cortex activity while inhibiting positive affect is associated with improvement in hedonic capacity after 8 weeks of antidepres-sant treatment in major depressive disorder. Biol Psychiatry. 2011;70:962–968. doi: 10.1016/j.biopsych.2011.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martinotti G, Sepede G, Gambi F, Di Iorio G, De Berardis D, Di Nicola M, et al. Agomelatine versus venlafaxine XR in the treatment of anhedonia in major depressive disorder: A pilot study. J Clin Psychopharmacol. 2012;32:487–491. doi: 10.1097/JCP.0b013e31825d6c25. [DOI] [PubMed] [Google Scholar]
- 77.Di Giannantonio M, Di Iorio G, Guglielmo R, De Berardis D, Conti CM, Acciavatti T, et al. Major depressive disorder, anhedonia and agomelatine: An open-label study. J Biol Regul Homeost Agents. 2011;25:109–114. [PubMed] [Google Scholar]
- 78.Martinotti G, Pettorruso M, De Berardis D, Varasano PA, Lucidi Pressanti G, De Remigis V, et al. Agomelatine increases BDNF serum levels in depressed patients in correlation with the improvement of depressive symptoms. Int J Neuropsychopharmacol. 2016;19:pyw003. doi: 10.1093/ijnp/pyw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gargoloff PD, Corral R, Herbst L, Marquez M, Martinotti G, Gargoloff PR. Effectiveness of agomelatine on anhedonia in depressed patients: An outpatient, open-label, real-world study. Hum Psychopharmacol. 2016;31:412–418. doi: 10.1002/hup.2597. Erratum in: Hum Psychopharmacol 2017 May. doi: 10.1002/hup.2597. [DOI] [PubMed] [Google Scholar]
- 80.De Berardis D, Fornaro M, Orsolini L, Iasevoli F, Tomasetti C, de Bartolomeis A, et al. Effect of agomelatine treatment on C-reactive protein levels in patients with major depressive disorder: An exploratory study in "real-world," everyday clinical practice. CNS Spectr. 2017;22:342–347. doi: 10.1017/S1092852916000572. [DOI] [PubMed] [Google Scholar]
- 81.di Giannantonio M, Montemitro C, Sepede G, Brunetti M, Baroni G, Corbo M, et al. Agomelatine effectiveness, tolerability, and impact on anhedonia in major depression: A pooled analysis. J Clin Psychopharmacol. 2019;39:288–290. doi: 10.1097/JCP.0000000000001038. [DOI] [PubMed] [Google Scholar]
- 82.McIntyre RS, Loft H, Christensen MC. Efficacy of vortioxetine on anhedonia: Results from a pooled analysis of short- term studies in patients with major depressive disorder. Neuropsychiatr Dis Treat. 2021;17:575–585. doi: 10.2147/NDT.S296451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mattingly GW, Necking O, Schmidt SN, Reines E, Ren H. Long-term safety and efficacy, including anhedonia, of vortioxetine for major depressive disorder: Findings from two open-label studies. Curr Med Res Opin. 2023;39:613–619. doi: 10.1080/03007995.2023.2178082. [DOI] [PubMed] [Google Scholar]
- 84.Watanabe K, Fujimoto S, Marumoto T, Kitagawa T, Ishida K, Nakajima T, et al. Therapeutic potential of vortioxetine for anhedonia-like symptoms in depression: A post hoc analysis of data from a clinical trial conducted in Japan. Neuropsy-chiatr Dis Treat. 2022;18:363–373. doi: 10.2147/NDT.S340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cao B, Park C, Subramaniapillai M, Lee Y, Iacobucci M, Mansur RB, et al. The efficacy of vortioxetine on anhedonia in patients with major depressive disorder. Front Psychiatry. 2019;10:17. doi: 10.3389/fpsyt.2019.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tan SN, Tan C. Vortioxetine improves cognition in mild cognitive impairment. Int Clin Psychopharmacol. 2021;36:279–287. doi: 10.1097/YIC.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tham JCW, Do A, Fridfinnson J, Rafizadeh R, Siu JTP, Budd GP, et al. Repeated subcutaneous racemic ketamine in treatment-resistant depression: Case series. Int Clin Psychophar-macol. 2022;37:206–214. doi: 10.1097/YIC.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 88.Bahji A, Vazquez GH, Zarate CA., Jr Comparative efficacy of racemic ketamine and esketamine for depression: A systematic review and meta-analysis. J Affect Disord. 2021;278:542–555. doi: 10.1016/j.jad.2020.11.103. Erratum in: J Affect Disord 2021;281:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–811. doi: 10.1038/mp.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goodwin GM, Aaronson ST, Alvarez O, Arden PC, Baker A, Bennett JC, et al. Single-dose psilocybin for a treatment- resistant episode of major depression. N Engl J Med. 2022;387:1637–1648. doi: 10.1056/NEJMoa2206443. [DOI] [PubMed] [Google Scholar]
- 92.Carhart-Harris RL, Bolstridge M, Rucker J, Day CM, Erritzoe D, Kaelen M, et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry. 2016;3:619–627. doi: 10.1016/S2215-0366(16)30065-7. [DOI] [PubMed] [Google Scholar]
- 93.Rizvi SJ, Geraci J, Ravindran A, Kennedy SH. Predictors of response to adjunctive osmotic-release methylphenidate or placebo in patients with major depressive disorder: Effects of apathy/anhedonia and fatigue. J Clin Psychopharmacol. 2014;34:755–759. doi: 10.1097/JCP.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 94.Pettorruso M, Spagnolo PA, Leggio L, Janiri L, Di Giannantonio M, Gallimberti L, et al. Repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex may improve symptoms of anhedonia in individuals with cocaine use disorder: A pilot study. Brain Stimul. 2018;11:1195–1197. doi: 10.1016/j.brs.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 95.Light SN, Bieliauskas LA, Taylor SF. Measuring change in anhedonia using the "Happy Faces" task pre- to post-repetitive transcranial magnetic stimulation (rTMS) treatment to left dorsolateral prefrontal cortex in Major Depressive Disorder (MDD): Relation to empathic happiness. Transl Psychiatry. 2019;9:217. doi: 10.1038/s41398-019-0549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Spano MC, Lorusso M, Pettorruso M, Zoratto F, Di Giuda D, Martinotti G, et al. Anhedonia across borders: Transdiagnostic relevance of reward dysfunction for noninvasive brain stimulation endophenotypes. CNS Neurosci Ther. 2019;25:1229–1236. doi: 10.1111/cns.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang X, He K, Chen T, Shi B, Yang J, Geng W, et al. Thera-peutic efficacy of connectivity-directed transcranial magnetic stimulation on anticipatory anhedonia. Depress Anxiety. 2021;38:972–984. doi: 10.1002/da.23188. [DOI] [PubMed] [Google Scholar]
- 98.Fukuda AM, Kang JWD, Gobin AP, Tirrell E, Kokdere F, Carpenter LL. Effects of transcranial magnetic stimulation on anhedonia in treatment resistant major depressive disorder. Brain Behav. 2021;11:e2329. doi: 10.1002/brb3.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Bronswijk S, Moopen N, Beijers L, Ruhe HG, Peeters F. Effectiveness of psychotherapy for treatment-resistant depression: A meta-analysis and meta-regression. Psychol Med. 2019;49:366–379. doi: 10.1017/S003329171800199X. [DOI] [PubMed] [Google Scholar]
- 100.Alsayednasser B, Widnall E, O'Mahen H, Wright K, Warren F, Ladwa A, et al. How well do cognitive behavioural therapy and behavioural activation for depression repair anhedonia? A secondary analysis of the COBRA randomized controlled trial. Behav Res Ther. 2022;159:104185. doi: 10.1016/j.brat.2022.104185. [DOI] [PubMed] [Google Scholar]
- 101.Serretti A. Advances in the treatment of depression. Int Clin Psychopharmacol. 2022;37:183–184. doi: 10.1097/YIC.0000000000000424. [DOI] [PubMed] [Google Scholar]