Low to moderate doses of 100% fruit juice have shown protective associations with cardiovascular disease [1], stroke [2, 3], stroke mortality [3], metabolic syndrome [4] and hypertension [5] in prospective cohort studies. The nutritional value of 100% fruit juice is comparable to whole fruits [6] and its consumption has been recognized as an option to meet recommendations for fruit and vegetable intake in several nutrition guidelines [7, 8]. However, the definition for 100% fruit juice is often unclear in dietary assessment questionnaires which may group together both non-defined sources of fruit juice and 100% fruit juice. These assessments may not capture the true association with 100% fruit juice, as non-defined sources of fruit juice may include fruit drinks with very little fruit juice and added sugars resembling more sugar sweetened beverages, which have shown the opposite associations with cardiovascular disease [9], type 2 diabetes [10], metabolic syndrome [11], and hypertension [12, 13] in many of the same prospective cohort studies.
The recent draft European Food Safety Authority (EFSA) assessment of the safety of dietary sugars concluded that the intake of 100% fruit juice had a positive causal relationship with type 2 diabetes and adiposity outcomes [14]. A major limitation of the EFSA assessment was that it included sources of fruit juice which did not differentiate between 100% fruit juice and non-defined sources of fruit juice. This misclassification error derives from the food frequency questionnaires used by several of the included prospective cohort studies which only asked participants questions about fruit juice and not 100% fruit juice [15–17]. Another limitation was that the EFSA assessors did not quantify the relationship between reported 100% fruit juice intake and adiposity outcomes, instead using vote counting (i.e., counting how many studies were significant versus those that were not)—an invalid approach for evidence synthesis [18].
To address the issues of misclassification of fruit juice and vote counting, we undertook a re-analysis of the prospective cohort studies identified by the EFSA assessment. We performed a quantitative meta-analysis to account for the differential weights and precision of included studies.
We stratified the included studies by the author reported definition of fruit juice into two categories. Studies that reported 100% fruit juice and pure fruit juice were categorized as 100% fruit juice. Studies that did not specify the type of fruit juice were categorized as non-specified fruit juice. We assessed the relation of the two different categories of fruit juice (100% fruit juice and non-specified fruit juice) separately with incident type 2 diabetes and adiposity outcomes (incident abdominal obesity, body weight in adults, and BMI z-scores in children). Meta-analyses were conducted by pooling beta-coefficients for continuous outcomes and log-relative risks (RRs) for incident outcomes with 95% confidence intervals (CIs) using the generic inverse variance method with DerSimonian-Laird random-effects models [19]. Heterogeneity was assessed by the Cochran Q test (significance at P < 0.1) and quantified by the I2 statistic, with I2 ≥ 50% and P < 0.1 considered evidence of substantial heterogeneity [20]. We assessed subgroup differences by fruit juice definition using the Cochrane Handbook’s recommended standard Q-test for subgroup differences (significance at P < 0.1) [21–23]. We used one-stage random effects meta-analysis to assess dose response for both a linear trend and a non-linear trend using restricted cubic splines with three knots [24, 25]. We tested for departure from linearity using the Wald test [26].
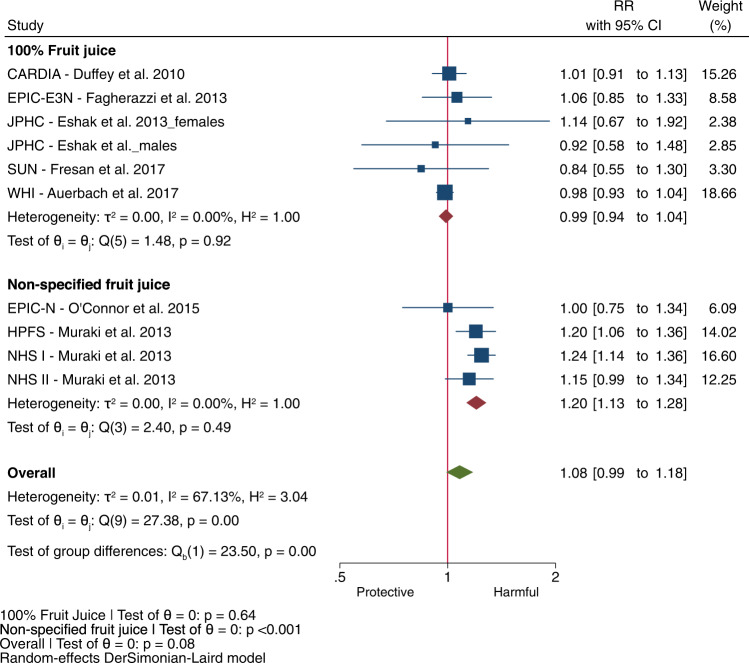
We included all 10 of the EFSA identified prospective cohort comparisons assessing the association of fruit juice with incident type 2 diabetes in our meta-analysis [5, 15, 16, 27–30]. There was no association of total fruit juice with incident type 2 diabetes (Fig. 1, RR: 1.08, 95% CI: 0.99–1.18) with substantial heterogeneity (I2 = 67%, Phet < 0.001) over a median follow-up of 12.4 years. This heterogeneity was fully explained by fruit juice definition (100% fruit juice versus non-specified fruit juice) in subgroup analyses (P < 0.001 for subgroup difference). 100% Fruit juice was not associated with type 2 diabetes incidence in 6 cohort comparisons (Fig. 1, RR: 0.99, 95% CI: 0.94 to 1.04, P = 0.64); whereas, non-specified fruit juice was associated with increased type 2 diabetes incidence in 4 cohort comparisons (Fig. 1, RR: 1.20, 95% CI: 1.13–1.28, P < 0.001), with no evidence of heterogeneity (I2 = 0%, Phet > 0.05) in either group. There was a lack of a dose-response relationship between 100% fruit juice and incident type 2 diabetes (Fig. 2a, P = 0.63) in the subset of 3 cohort comparisons identified by EFSA for dose-response analysis (Supplementary Table 1). In contrast, non-specified fruit juice showed a linear dose response gradient with incident type 2 diabetes (Fig. 2b, P < 0.001) in the subset of 4 cohort comparisons identified by EFSA for dose-response analysis (Supplementary Table 2).
Fig. 1. Relation of fruit juice with incident type 2 diabetes by fruit juice definition (100% fruit juice or non-specified fruit juice) for every increase in serving (250 mL) in adults in 10 prospective cohort comparisons identified by EFSA.
Effect estimates for each subgroup and overall effect are represented by the diamonds. Data are expressed as relative risks with 95% confidence intervals using the generic inverse variance method with DerSimonian-Laird random-effects model. Inter-study heterogeneity was assessed using the Cochrane Q statistic and quantified using the I2 statistic, with significance set at P < 0.10 and I2 ≥ 50% considered to be evidence of substantial heterogeneity. Subgroup differences were tested using the standard Q-test with significance set at P < 0.10. CARDIA Coronary Artery Risk Development in Young Adults Study, CI confidence interval, EPIC-E3N European Prospective Investigation into Cancer and Nutrition-Etude Epidémiologique auprès des femmes de la Mutuelle Générale de l’Education National, EPIC-N European Prospective Investigation into Cancer and Nutrition-Norfolk, HPFS Health Professionals Follow-up Study, JPHC Japan Public Health Centre-based Prospective Study, NHS Nurses’ Health Study, RR relative risk, SUN Seguimiento Universidad de Navarra, WHI Women’s Health Initiative.
Fig. 2. Dose response meta-analysis of 100% fruit juice and non-specified fruit juice with incident type 2 diabetes.
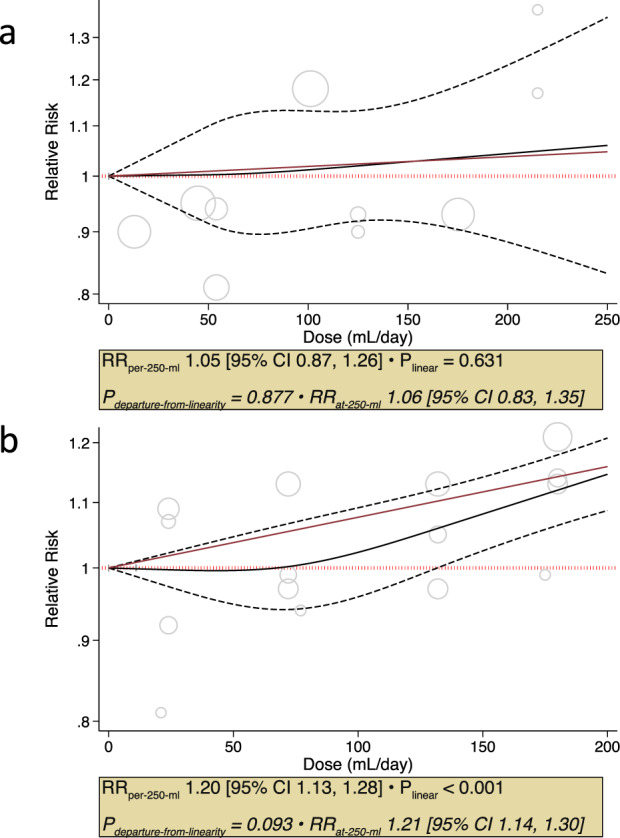
Dose-response meta-analysis on the relation of (a) 100% fruit juice with incident type 2 diabetes in adults in 3 prospective cohort comparisons identified by EFSA (b) non-specified fruit juice with incident type 2 diabetes in 4 prospective cohort comparisons identified by EFSA. Individual comparisons are represented by the circles, with the weight of the comparison in the analysis represented by the size of the circle. The solid red line represents the linear dose response assessed by one stage linear mixed effects meta-analysis. The solid black line and outer black dashed lines represent the non-linear dose response and 95% confidence intervals, respectively, which were modelled with restricted cubic splines with 3 knots. Departure from linearity was assessed using the Wald’s test, with significance set at P < 0.05. CI confidence interval, RR relative risk.
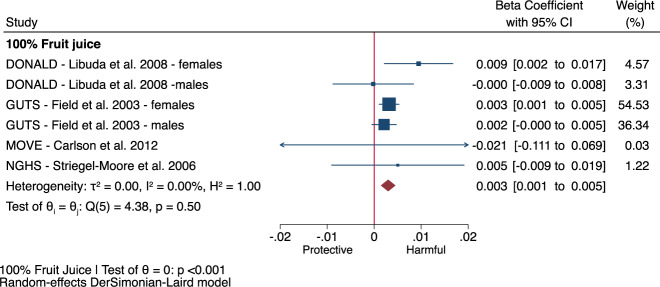
The 2 prospective cohort comparisons [27, 31] identified by EFSA for the assessment of incident abdominal obesity, both of which assessed the exposure to 100% fruit juice only, showed no association of 100% fruit juice with incident abdominal obesity (Fig. 3a, RR: 0.92, 95% CI: 0.73–1.16, P = 0.47; moderate heterogeneity, I2 = 41%, Phet = 0.19) over a median follow-up of 15 years. The 4 prospective cohort comparisons [17, 32] for the assessment of change in body weight in adults showed total fruit juice was associated with an increase in body weight in adults (Fig. 3b, beta-coefficient: 0.08 kg/250 mL/yr, 95% CI: 0.06–0.10, P < 0.001; substantial heterogeneity, I2 = 79%, Phet < 0.001) over a median follow-up of 4 years with no effect modification by fruit juice definition (P = 0.86). Similarly, the 4 prospective cohort comparisons [33–36] for the assessment of change in BMI z-scores in children, all of which assessed the exposure to 100% fruit juice only, showed 100% fruit juice was associated with an increase in BMI z-scores (Fig. 4, beta-coefficient: 0.003, 95% CI: 0.001–0.005, P < 0.001; no heterogeneity, I2 = 0%, Phet = 0.50) over a median follow-up of 3 years, although the increases in both BMI z-scores in children and body weight in adults were clinically trivial (a change of 0.25 BMI z-score [37] and 2.5% body weight [38] or ~2 kg for 80 kg person is considered the minimally important difference in metabolic health).
Fig. 3. Fruit juice with incident abdominal obesity and change in body weight by fruit juice definition.
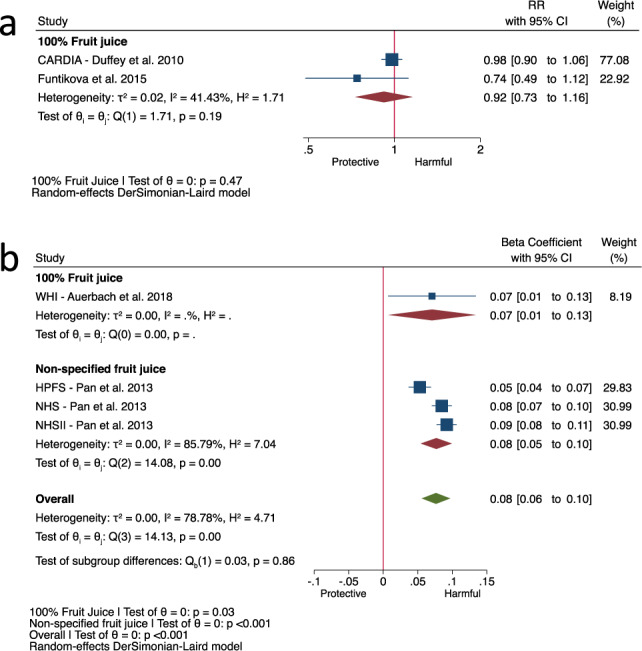
Relation of 100% fruit juice with (a) incident abdominal obesity for every increase in serving (250 mL) in adults in 2 prospective cohort comparisons identified by EFSA and (b) change in body weight by fruit juice definition (100% fruit juice or non-specified fruit juice) for every increase in serving (250 mL) per year in adults in 4 prospective cohort comparisons identified by EFSA. Effect estimates for each subgroup and overall effect are represented by the diamonds. Data are expressed as relative risks or beta-coefficients with 95% confidence intervals using the generic inverse variance method with DerSimonian-Laird random-effects model. Inter-study heterogeneity was assessed using the Cochrane Q statistic and quantified using the I2 statistic, with significance set at P < 0.10 and I2 ≥ 50% considered to be evidence of substantial heterogeneity. Subgroup differences were tested using the standard Q-test with significance set at P < 0.10. CARDIA Coronary Artery Risk Development in Young Adults Study, CI confidence interval, HPFS Health Professionals Follow-up Study, NHS Nurses’ Health Study, RR relative risk, WHI Women’s Health Initiative.
Fig. 4. Relation of 100% fruit juice with change in BMI z-score for every increase in serving (250 mL) per year in children in 6 prospective cohort comparisons identified by EFSA.
Effect estimates for each subgroup and overall effect are represented by the diamonds. Data are expressed as beta-coefficients with 95% confidence intervals using the generic inverse variance method with DerSimonian-Laird random-effects model. Inter-study heterogeneity was assessed using the Cochrane Q statistic and quantified using the I2 statistic, with significance set at P < 0.10 and I2 ≥ 50% considered to be evidence of substantial heterogeneity. Subgroup differences were tested using the standard Q-test with significance set at P < 0.10. BMI body mass index, CI confidence interval, DONALD Dortmund Nutritional and Longitudinal Designed Study, GUTS Growing Up Today Study, NGHS National Heart, Lung, and Blood Institute Growth and Health Study, RR relative risk.
Our results are in contrast with the EFSA assessment which concludes that the available evidence suggests a positive and causal relationship between the consumption of 100% fruit juice and risk of type 2 diabetes. We show that the association between total fruit juice and incident type 2 diabetes is dependent on fruit juice definition where any association with harm is only observed in studies with non-specified fruit juice. For adiposity outcomes, we show no association of 100% fruit juice and incident abdominal obesity. In prospective cohort studies which reported change in body weight (adults) or BMI z-score (children), regardless of definition of fruit juice, there was a positive association with intake of fruit juice. However, the association between 100% fruit juice and change in BMI z-score in children in our analysis was driven by one study [34] which represented 90% of the overall weight in the analysis and the increases were clinically trivial. The association was no longer significant after removal of this study (Supplementary Fig. 1).
Strengths of our reanalysis include our stratification by 100% and non-specified fruit juice, quantitative syntheses and dose-response analysis for the type 2 diabetes outcome. Our reanalysis also revealed several limitation. There was a lack of relevant data to perform dose response analyses for adiposity outcomes. Additionally, although prospective cohort studies represent the highest quality observational studies, the inability to remove unmeasured and residual confounding is inherent in all observational studies. Finally, fruit juice is generally defined as 100% fruit juice which is squeezed directly from fruit, reconstituted from concentrate [39], and labelled as such. We categorized studies in this category when they reported the intake as 100% fruit juice or pure fruit juice. Cohorts that reported only “juice” intake may have captured a wide-range of juice categories including 100% fruit juice but also those that contain little fruit juice e.g., fruit drinks, cocktails, punches, and juice beverages. The implication of this misclassification is that it is unclear exactly how much fruit juice is present in the non-defined category; therefore such studies should not be combined with those that report 100% fruit juice intake.
In conclusion, the association of fruit juice with type 2 diabetes and obesity appears dependent on the definition of fruit juice. Whereas non-specified fruit juice does show an association with increased risk of type 2 diabetes, there is no reliable association of 100% fruit juice with incident diabetes or incident abdominal obesity. More research is needed to understand the clinical importance of the small increases in body weight in adults or BMI z-scores in children associated with 100% fruit juice in the absence of adverse associations with downstream adiposity-related complications.
Supplementary information
Acknowledgements
Aspects of this work were included in a submission made by the European Fruit Juice Association to the Public Consultation for the EFSA Draft scientific opinion on the Tolerable Upper Intake Level for Dietary Sugars [14].
Author contributions
JLS conceived and designed the research. VC, TAK, LC, AA, DL and JLS acquired, analyzed and interpreted the data. VC and TAK drafted the manuscript. TAK and JLS provided supervision. All authors critically revised the manuscript and approved the final version of the manuscript.
Funding
This work was supported by the Canadian Institutes of Health Research (funding reference number, 129920) through the Canada-wide Human Nutrition Trialists’ Network (NTN). The Diet, Digestive tract, and Disease (3-D) Centre, funded through the Canada Foundation for Innovation (CFI) and the Ministry of Research and Innovation’s Ontario Research Fund (ORF), provided the infrastructure for the conduct of this project. VC was funded by a Toronto 3D Summer Student Award. TAK was funded by a Toronto 3D Postdoctoral Fellowship Award. LC was funded by a Mitacs Elevate Postdoctoral Fellowship Award. AA was funded by a Toronto 3D MSc Scholarship Award. DL was funded by a St. Michael’s Hospital Research Training Centre MSc Scholarship Award. JLS was funded by a Diabetes Canada Clinician Scientist Award.
Data availability
All data generated or analyzed during this study are included in this published articles (and its supplementary information files).
Competing interests
VC has received research support from the University of Toronto and Toronto 3D Knowledge Synthesis and Clinical Trials foundation. TAK has received research support from the Canadian Institutes of Health Research (CIHR), the International Life Science Institute (ILSI), and National Honey Board. He has been an invited speaker at the Calorie Control Council Annual meeting for which he has received an honorarium. He has received funding from the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. LC was a Mitacs-Elevate post-doctoral fellow jointly funded by the Government of Canada and the Canadian Sugar Institute (Sep 2019-Aug 2021). AA has received research support from the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. DL has received research support from a University of Toronto Department of Nutritional Sciences Graduate Student Fellowship, University of Toronto Fellowship in Nutritional Sciences, Dairy Farmers of Canada Graduate Student Fellowship, and St. Michael’s Hospital Research Training Centre MSc Award. CWCK has received grants or research support from the Advanced Food Materials Network, Agriculture and Agri-Foods Canada (AAFC), Almond Board of California, Barilla, Canadian Institutes of Health Research (CIHR), Canola Council of Canada, International Nut and Dried Fruit Council, International Tree Nut Council Research and Education Foundation, Loblaw Brands Ltd, the Peanut Institute, Pulse Canada and Unilever. He has received in-kind research support from the Almond Board of California, Barilla, California Walnut Commission, Kellogg Canada, Loblaw Companies, Nutrartis, Quaker (PepsiCo), the Peanut Institute, Primo, Unico, Unilever, WhiteWave Foods/Danone. He has received travel support and/or honoraria from the Barilla, California Walnut Commission, Canola Council of Canada, General Mills, International Nut and Dried Fruit Council, International Pasta Organization, Lantmannen, Loblaw Brands Ltd, Nutrition Foundation of Italy, Oldways Preservation Trust, Paramount Farms, the Peanut Institute, Pulse Canada, Sun-Maid, Tate & Lyle, Unilever and White Wave Foods/Danone. He has served on the scientific advisory board for the International Tree Nut Council, International Pasta Organization, McCormick Science Institute and Oldways Preservation Trust. He is a founding member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD), is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the EASD and is a Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. JLS has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, Canadian Institutes of health Research (CIHR), Diabetes Canada, PSI Foundation, Banting and Best Diabetes Centre (BBDC), American Society for Nutrition (ASN), INC International Nut and Dried Fruit Council Foundation, National Honey Board (the U.S. Department of Agriculture [USDA] honey “Checkoff” program), Institute for the Advancement of Food and Nutrition Sciences (IAFNS; formerly ILSI North America), Pulse Canada, Quaker Oats Centre of Excellence, The United Soybean Board (the USDA soy “Checkoff” program), The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), The Plant Protein Fund at the University of Toronto (a fund which has received contributions from IFF), and The Nutrition Trialists Fund at the University of Toronto (a fund established by an inaugural donation from the Calorie Control Council). He has received food donations to support randomized controlled trials from the Almond Board of California, California Walnut Commission, Peanut Institute, Barilla, Unilever/Upfield, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, WhiteWave Foods/Danone, Nutrartis, and Dairy Farmers of Canada. He has received travel support, speaker fees and/or honoraria from ASN, Danone, Dairy Farmers of Canada, FoodMinds LLC, International Sweeteners Association, Nestlé, Abbott, General Mills, Comité Européen des Fabricants de Sucre (CEFS), Nutrition Communications, International Food Information Council (IFIC), Calorie Control Council, and International Glutamate Technical Committee. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate & Lyle, and Inquis Clinical Research. He is a member of the European Fruit Juice Association Scientific Expert Panel and former member of the Soy Nutrition Institute (SNI) Scientific Advisory Committee. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada/Canadian Association of Bariatric Physicians and Surgeons. He serves or has served as an unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Technical Committee on Carbohydrates of IAFNS (formerly ILSI North America). He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His spouse is an employee of AB InBev.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41430-023-01258-y.
References
- 1.Khan TA, Chiavaroli L, Zurbau A, Sievenpiper JL. A lack of consideration of a dose-response relationship can lead to erroneous conclusions regarding 100% fruit juice and the risk of cardiometabolic disease. Eur J Clin Nutr. 2019;73:1556–60.. doi: 10.1038/s41430-019-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheffers FR, Boer JMA, Verschuren WMM, Verheus M, van der Schouw YT, Sluijs I, et al. Pure fruit juice and fruit consumption and the risk of CVD: the European Prospective Investigation into Cancer and Nutrition-Netherlands (EPIC-NL) study. Br J Nutr. 2019;121:351–9. doi: 10.1017/S0007114518003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zurbau A, Au-Yeung F, Blanco Mejia S, Khan TA, Vuksan V, Jovanovski E, et al. Relation of different fruit and vegetable sources with incident cardiovascular outcomes: a systematic review and meta-analysis of prospective cohort studies. J Am Heart Assoc. 2020;9:e017728. doi: 10.1161/JAHA.120.017728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira-Pêgo C, Babio N, Bes-Rastrollo M, Corella D, Estruch R, Ros E, et al. Frequent consumption of sugar- and artificially sweetened beverages and natural and bottled fruit juices is associated with an increased risk of metabolic syndrome in a mediterranean population at high cardiovascular disease risk. J Nutr. 2016;146:1528–36. doi: 10.3945/jn.116.230367. [DOI] [PubMed] [Google Scholar]
- 5.Auerbach BJ, Littman AJ, Tinker L, Larson J, Krieger J, Young B, et al. Associations of 100% fruit juice versus whole fruit with hypertension and diabetes risk in postmenopausal women: Results from the Women’s Health Initiative. Prev Med. 2017;105:212–8. doi: 10.1016/j.ypmed.2017.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemens R, Drewnowski A, Ferruzzi MG, Toner CD, Welland D. Squeezing fact from fiction about 100% fruit juice. Adv Nutr. 2015;6:236s–43s. doi: 10.3945/an.114.007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Health Service. 5 A Day: what counts? 2018 [Available from: https://www.nhs.uk/live-well/eat-well/5-a-day-what-counts/.
- 8.U.S. Department of Agriculture and U.S. Department of Helath and Human Services. Dietary Guidelines for Americans, 2020-2025 2020 [9th Edition]: Available from: https://www.dietaryguidelines.gov/.
- 9.Yin J, Zhu Y, Malik V, Li X, Peng X, Zhang FF, et al. Intake of sugar-sweetened and low-calorie sweetened beverages and risk of cardiovascular disease: a meta-analysis and systematic review. Adv Nutr. 2021;12:89–101. doi: 10.1093/advances/nmaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin P, Li Q, Zhao Y, Chen Q, Sun X, Liu Y, et al. Sugar and artificially sweetened beverages and risk of obesity, type 2 diabetes mellitus, hypertension, and all-cause mortality: a dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2020;35:655–71. doi: 10.1007/s10654-020-00655-y. [DOI] [PubMed] [Google Scholar]
- 11.Semnani-Azad Z, Khan TA, Blanco Mejia S, de Souza RJ, Leiter LA, Kendall CWC, et al. Association of major food sources of fructose-containing sugars with incident metabolic syndrome: a systematic review and meta-analysis. JAMA Netw Open. 2020;3:e209993. doi: 10.1001/jamanetworkopen.2020.9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Q, Ayoub-Charette S, Khan TA, Au-Yeung F, Blanco Mejia S, de Souza RJ, et al. Important food sources of fructose-containing sugars and incident hypertension: a systematic review and dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc. 2019;8:e010977. doi: 10.1161/JAHA.118.010977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayalath VH, de Souza RJ, Ha V, Mirrahimi A, Blanco-Mejia S, Di Buono M, et al. Sugar-sweetened beverage consumption and incident hypertension: a systematic review and meta-analysis of prospective cohorts. Am J Clin Nutr. 2015;102:914–21. doi: 10.3945/ajcn.115.107243. [DOI] [PubMed] [Google Scholar]
- 14.European Food Safety Authority. Scientific opinion on the Tolerable Upper Intake Level for dietary sugars. 2021.
- 15.O’Connor L, Imamura F, Lentjes MA, Khaw KT, Wareham NJ, Forouhi NG. Prospective associations and population impact of sweet beverage intake and type 2 diabetes, and effects of substitutions with alternative beverages. Diabetologia. 2015;58:1474–83. doi: 10.1007/s00125-015-3572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muraki I, Imamura F, Manson JE, Hu FB, Willett WC, van Dam RM, et al. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. doi: 10.1136/bmj.f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan A, Malik VS, Hao T, Willett WC, Mozaffarian D, Hu FB. Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int J Obes (Lond) 2013;37:1378–85. doi: 10.1038/ijo.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR Chapter 28: vote counting–a new name for an old problem. Introduction to meta-analysis.: Chichester: John Wiley & Sons; 2009.
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J, Thomas J, Chandler J Cochrane Handbook for Systematic Reviews of Interventions version 6.1. 2019. [DOI] [PMC free article] [PubMed]
- 21.Borenstein M, Hedges LV, Higgins JP, Rothstein HR Introduction to Meta-Analysis: UK: John Wiley & Sons; 2009.
- 22.Borenstein M, Higgins JP. Meta-analysis and subgroups. Prev Sci. 2013;14:134–43. doi: 10.1007/s11121-013-0377-7. [DOI] [PubMed] [Google Scholar]
- 23.Deeks JJ, Higgins JP, Altman DG, Group obotCSM. Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions 2019. p. 241-84.
- 24.Orsini N. Weighted mixed-effects dose–response models for tables of correlated contrasts. Stata J. 2021;21:320–47.. doi: 10.1177/1536867X211025798. [DOI] [Google Scholar]
- 25.Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res. 2019;28:1579–96.. doi: 10.1177/0962280218773122. [DOI] [PubMed] [Google Scholar]
- 26.Harrell FEJ Regression Modeling Strategies-with Applications to Linear Models, Logistic Regression, and Survival Analysis: Springer Series in Statistics. Springer. 2001.
- 27.Duffey KJ, Gordon-Larsen P, Steffen LM, Jacobs DR, Jr, Popkin BM. Drinking caloric beverages increases the risk of adverse cardiometabolic outcomes in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2010;92:954–9. doi: 10.3945/ajcn.2010.29478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fagherazzi G, Vilier A, Saes Sartorelli D, Lajous M, Balkau B, Clavel-Chapelon F. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidemiologique aupres des femmes de la Mutuelle Generale de l’Education Nationale-European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr. 2013;97:517–23. doi: 10.3945/ajcn.112.050997. [DOI] [PubMed] [Google Scholar]
- 29.Eshak ES, Iso H, Mizoue T, Inoue M, Noda M, Tsugane S. Soft drink, 100% fruit juice, and vegetable juice intakes and risk of diabetes mellitus. Clin Nutr. 2013;32:300–8. doi: 10.1016/j.clnu.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Fresan U, Gea A, Bes-Rastrollo M, Basterra-Gortari FJ, Carlos S, Martinez-Gonzalez MA. Substitution of water or fresh juice for bottled juice and type 2 diabetes incidence: The SUN cohort study. Nutr Metab Cardiovasc Dis. 2017;27:874–80. doi: 10.1016/j.numecd.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Funtikova AN, Subirana I, Gomez SF, Fitó M, Elosua R, Benítez-Arciniega AA, et al. Soft drink consumption is positively associated with increased waist circumference and 10-year incidence of abdominal obesity in Spanish adults. J Nutr. 2015;145:328–34. doi: 10.3945/jn.114.205229. [DOI] [PubMed] [Google Scholar]
- 32.Auerbach BJ, Littman AJ, Krieger J, Young BA, Larson J, Tinker L, et al. Association of 100% fruit juice consumption and 3-year weight change among postmenopausal women in the in the Women’s Health Initiative. Prev Med. 2018;109:8–10. doi: 10.1016/j.ypmed.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Libuda L, Alexy U, Sichert-Hellert W, Stehle P, Karaolis-Danckert N, Buyken AE, et al. Pattern of beverage consumption and long-term association with body-weight status in German adolescents-results from the DONALD study. Br J Nutr. 2008;99:1370–9. doi: 10.1017/S0007114507862362. [DOI] [PubMed] [Google Scholar]
- 34.Field AE, Gillman MW, Rosner B, Rockett HR, Colditz GA. Association between fruit and vegetable intake and change in body mass index among a large sample of children and adolescents in the United States. Int J Obes Relat Metab Disord. 2003;27:821–6. doi: 10.1038/sj.ijo.0802297. [DOI] [PubMed] [Google Scholar]
- 35.Carlson JA, Crespo NC, Sallis JF, Patterson RE, Elder JP. Dietary-related and physical activity-related predictors of obesity in children: a 2-year prospective study. Child Obes. 2012;8:110–5. doi: 10.1089/chi.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Striegel-Moore RH, Thompson D, Affenito SG, Franko DL, Obarzanek E, Barton BA, et al. Correlates of beverage intake in adolescent girls: the national heart, lung, and blood institute growth and health study. J Pediatr. 2006;148:183–7. doi: 10.1016/j.jpeds.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 37.Ford AL, Hunt LP, Cooper A, Shield JP. What reduction in BMI SDS is required in obese adolescents to improve body composition and cardiometabolic health? Arch Dis Child. 2010;95:256–61. doi: 10.1136/adc.2009.165340. [DOI] [PubMed] [Google Scholar]
- 38.Williamson DA, Bray GA, Ryan DH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obes (Silver Spring) 2015;23:2319–20. doi: 10.1002/oby.21358. [DOI] [PubMed] [Google Scholar]
- 39.O’Neil CE, Nicklas TA, Zanovec M, Fulgoni VL., 3rd Diet quality is positively associated with 100% fruit juice consumption in children and adults in the United States: NHANES 2003-2006. Nutr J. 2011;10:17. doi: 10.1186/1475-2891-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published articles (and its supplementary information files).