Abstract
Vascular nitric oxide (NO•) resistance, manifested by an impaired vasodilator function of NO• in both the macro- and microvessels, is a common state in type 2 diabetes (T2D) associated with developing cardiovascular events and death. Here, we summarize experimental and human evidence of vascular NO• resistance in T2D and discuss its underlying mechanisms. Human studies indicate a ~ 13-94% decrease in the endothelium (ET)-dependent vascular smooth muscle (VSM) relaxation and a 6-42% reduced response to NO• donors, i.e., sodium nitroprusside (SNP) and glyceryl trinitrate (GTN), in patients with T2D. A decreased vascular NO• production, NO• inactivation, and impaired responsiveness of VSM to NO• [occurred due to quenching NO• activity, desensitization of its receptor soluble guanylate cyclase (sGC), and/or impairment of its downstream pathway, cyclic guanosine monophosphate (cGMP)-protein kinase G (PKG)] are the known mechanisms underlying the vascular NO• resistance in T2D. Hyperglycemia-induced overproduction of reactive oxygen species (ROS) and vascular insulin resistance are key players in this state. Therefore, upregulating vascular NO• availability, re-sensitizing or bypassing the non-responsive pathways to NO•, and targeting key vascular sources of ROS production may be clinically relevant pharmacological approaches to circumvent T2D-induced vascular NO• resistance.
Subject terms: Valvular disease, Diabetes
Facts
Current evidence implies on the presence of vascular nitric oxide (NO•) resistance, an independent risk factor for cardiovascular events, in patients with type 2 diabetes (T2D).
Vascular NO• resistance in T2D is stage-dependent and displays a progressive spectrum, initially manifested by an augmented or preserved vascular NO• production and/or vascular smooth muscle (VSM) response to NO•, followed by a reduced NO• bioavailability and/or partial to almost entirely impaired NO• function in VSM, in both the macro- and microvessels.
Quenching NO• activity, desensitization of soluble guanylate cyclase (sGC), and/or impairment of cyclic guanosine monophosphate (cGMP)-protein kinase G (PKG) are the known mechanisms underlying the vascular NO• resistance in T2D.
Questions
Which mechanism(s) is/are initiator(s) and key player(s) in developing vascular NO• resistance in T2D?
Do different vessels display diverse phenotypes of vascular NO• resistance?
Which vessels are more sensitive to and affected earlier by NO• resistance in T2D?
Which pharmaceutical approaches could be effective in preventing and retarding the progression of T2D-induced vascular NO• resistance?
Introduction
The global prevalence of diabetes in adults was reported to be 10.5% (536.6 million people) in 2021, reaching up to 12.2% (783.2 million) in 2045 [1]. Type 2 Diabetes (T2D), accounting for approximately 90% of diabetes cases, is related to macrovascular and microvascular complications, with an overall prevalence of 32.2% and 12.0%, respectively [2, 3]. In addition, cardiovascular disease (CVD) mortality is estimated to constitute 50.3% of all deaths in patients with T2D [2]. Both endothelium (ET) and vascular smooth muscle (VSM) layer are functionally impaired in diabetic vessels [4, 5], and vascular complications account for the most significant part of diabetes-associated morbidity and mortality [6].
Nitric oxide (NO•) is the most critical vasodilator produced by vascular ET [7]. Hyperglycemia-induced overproduction of reactive oxygen species (ROS) in T2D, which triggers several biochemical pathways [i.e., polyol and hexosamine pathway, advanced glycation end products (AGEs) production, activation of protein kinase C (PKC) and its downstream targets, especially nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX)], and vascular insulin resistance result in impaired metabolism of NO• (a crucial player in vascular homeostasis) and development of vascular dysfunction [8, 9].
The NO• resistance syndrome, a state of decreased NO• production by the ET [10–12], enhanced NO• inactivation [10–12], and impaired responsiveness to NO• at receptor level or its subsequent signal transduction [10–15], has been documented in the VSM in T2D [11, 14, 16–18]. Furthermore, the vascular NO• resistance presented as impaired vasodilator function of NO• manifests in both the macro- [i.e., large elastic and muscular arteries] [19–21] and micro- [i.e., vessels with a diameter <150 μm, including arterioles and venules] [22–25] vessels. The vascular NO• resistance is associated with future cardiovascular events (i.e., myocardial infarction, definite angina, coronary revascularization, stroke, resuscitated cardiac arrest, and CVD mortality), independent of the other well-known risk factors [26, 27]. Here, we summarize evidence of vascular NO• resistance from animal and human studies and discuss the underlying mechanisms of vascular NO• resistance in T2D.
Evidence of vascular NO• resistance in T2D
Human studies
As shown in Table 1, impairment of ET-dependent VSM relaxation has been consistently reported in both prediabetes [23, 28–30] and established T2D [12, 22, 23, 25, 28–47]. Impairment of ET-dependent VSM relaxation in T2D has been documented mostly in the brachial artery [11, 12, 30–45] and to a lesser extent in thoracic [10, 19, 46] and femoral [28, 29] arteries, as well as in saphenous vein [46] and skin microvessels [22, 23, 25, 48]. To assess ET-dependent VSM relaxation, flow-mediated dilatation (FMD) [34], a noninvasive technique for measuring NO•-mediated vascular function [49], as well as infusion of serotonin [40] and cholinergic agonists, mainly acetylcholine (ACh) [22, 23, 25, 31–33, 35–39, 41, 42, 44–47] and methacholine [28, 29], have been used in different studies. Overall, results indicate a ~ 13-94% decrease in ET-dependent VSM relaxation in T2D patients. ET-independent, NO•-dependent relaxation has been reported to be decreased in some prediabetes subjects by 26-33% [23, 30], but it preserves in others [28, 29]. The same is true in the case of established T2D, where responses to NO• donors, sodium nitroprusside (SNP), and glyceryl trinitrate (GTN) are decreased by 6-42% [21–23, 25, 31–33, 36, 37, 40, 41, 43–45, 47] or preserved [19, 28, 29, 34, 35, 38, 39, 42, 50].
Table 1.
Impaired endothelium (ET)-dependent and ET-independent, nitric oxide (NO•)-dependent vascular smooth muscle (VSM) relaxation in patients with type 2 diabetes (T2D) or impaired glucose and insulin homeostasis.
| Study | Condition | Age (years) | Duration of T2D (years) | HbA1C (%) | Vessel type | ET-dependent VSM relaxation | ET-independent, NO•-dependent VSM relaxation |
|---|---|---|---|---|---|---|---|
| Steinberg et al. [28] | Obese-IR | 35 | – | NR | Femoral a. | ↓Meth (40%) | ↔SNP |
| Steinberg et al.a [29] | Obese-IR | 34 | – | NR | Femoral a. | ↓Meth (52%) | ↔SNP |
| Steinberg et al.b [29] | Obese-IR | 34 | – | NR | Femoral a. | ↓Meth (41%) | ↔SNP |
| Caballero et al. [23] | IGT | 50 | – | 5.7 | FSM | ↓ACh (23%) | ↓SNP (26%) |
| Sivitz et al. [30] | IFG | 56 | – | 7.6 | Brachial a. | ↓ACh (31%) | ↓SNP (33%) |
| McVeigh et al. [31] | T2D | 53 | 5.2 | NR | Brachial a. | ↓ACh (94%) | ↓GTN (32%) |
| Ting et al. [12] | T2D | 47 | 3.5 | 7.9 | Brachial a. | ↓Meth (37%) | NR |
| Watts et al. [32] | T2D | 55 | 3.6 | NR | Brachial a. | ↓ACh (52%) | ↓SNP (25%) |
| Goodfellow et al. [50] | T2D | 50 | 3.8 | 9.7 | Brachial a. | NR | ↔GTN |
| Hogikyan et al. [33] | T2D | 57 | 5.6 | 10.3 | Brachial a. | ↓ACh (50%) | ↓SNP (21%) |
| Enderle et al. [34] | T2D | 57 | 7.4 | 9.1 | Brachial a. | ↓FMD (50%) | ↔GTN |
| Mäkimattila et al. [35] | T2D | 51 | 3.5 | NR | Brachial a. | ↓ACh (37%) | ↔SNP |
| Gazis et al. [36] | T2D | 57 | 4.6 | 6.9 | Brachial a. | ↓ACh (28%) | ↓SNP (10%) |
| Preik et al. [37] | T2D | 60 | 10.0 | 9.6 | Brachial a. | ↓ACh (41%) | ↓SNP (6%) |
| Heitzer et al. [38] | T2D | 52 | 5.3 | 7.8 | Brachial a. | ↓ACh (47%) | ↔SNP |
| Kimura et al. [39] | T2D | 70 | 7.0 | 8.0 | Brachial a. | ↓ACh (31%) | ↔GTN |
| van Etten et al. [40] | T2D | 58 | NR | NR | Brachial a. | ↓Ser (50%) | ↓SNP (30%) |
| Vehkavaara and Yki-Järvinen [41] | T2D | 59 | > 3.0 | 9.1 | Brachial a. | ↓ACh (28%) | ↓SNP (15%) |
| Ifrim and Vasilescu et al. [42] | T2D | 56 | 5.6 | 9.2 | Brachial a. | ↓ACh (20%) | ↔GTN |
| Natali et al. [43] | T2D | 56-58 | 5-8 | 7.6-8.1 | Brachial a. | ↓ACh | ↓SNP |
| Woodman et al. [44] | T2D | 55 | NR | NR | Brachial a. | ↓ACh (44%) | ↓SNP (33%) |
| Sivitz et al. [30] | T2D | 56 | NR | 5.9 | Brachial a. | ↓ACh (42%) | ↓SNP (42%) |
| Steinberg et al. [28] | T2D | 40 | NR | NR | Femoral a. | ↓Meth (55%) | ↔SNP |
| Steinberg et al.a [29] | T2D | 39 | NR | NR | Femoral a. | ↓Meth (40%) | ↔SNP |
| Steinberg et al.b [29] | T2D | 36 | NR | NR | Femoral a. | ↓Meth (70%) | ↔SNP |
| Karasu et al.a [46] | T2D | 55 | 11.0 | NR | Thoracic a. | ↓ACh (54%) | ↔SNP |
| Tawa et al. [19] | T2D | 73 | 7.1 | 7.0 | Thoracic a. | NR | ↔GTN |
| Karasu et al.a [46] | T2D | 55 | 11.0 | NR | Saphenous v. | ↓ACh (62%) | ↔SNP |
| Caballero et al. [23] | T2D | 53 | 4.3 | 8.0 | FSM | ↓ACh (29%) | ↓SNP (33%) |
| Morris et al.a [22] | T2D | 59 | 9.1 | 6.5 | FSM | ↓ACh (38%) | ↓SNP (21%) |
| Beer et al. [25] | T2D | 58 | 10.8 | 8.2 | FSM | ↓ACh (15%) | ↓SNP (21%) |
| Brooks et al. [47] | T2D | 57 | 9.9 | 6.7 | FSM | ↓ACh (44%) | ↓SNP (47%) |
| Natali et al. [45] | T2D | 59 | 5.0 | 8.0 | Brachial a. | ↓ACh (52%) | ↓SNP (27%) |
| Vavuranakis et al. [21] | T2D | 53 | 4.2 | 9.1 | Coronary a. | NR | ↓GTN (29%) |
| Preik et al. [37] | T2D | 57 | 9.0 | 9.8 | Brachial a. | ↓ACh (58%) | ↓SNP (42%) |
| Beer et al. [25] | T2D | 54 | 6.4 | 7.3 | FSM | ↓ACh (13%) | ↓SNP (16%) |
| Brooks et al. [47] | T2D | 58 | 10.2 | 8.3 | FSM | ↓ACh (23%) | ↓SNP (32%) |
Data are reported for both sexes otherwise indicated (amen, bwomen).
a artery, v vein, ACh acetylcholine, FMD flow-mediated dilatation, FSM forearm skin microcirculation, GTN glyceryl trinitrate, HbA1C glycated hemoglobin, IFG impaired fasting glucose, IGT impaired glucose tolerance, IR insulin resistance, Meth methacholine, Ser serotonin, SNP sodium nitroprusside, NR not reported.
A meta-analysis of published data evaluating ET-dependent VSM relaxation and ET-independent, NO•-dependent VSM relaxation in T2D patients compared with aged-matched controls reported a significantly impaired ET-dependent and-independent vascular functions [standardized mean difference (SMD)= −0.89 and −0.69) that were stronger in micro rather macrocirculation [5].
Likely explanations for discrepancy in the presented results are the presence of other comorbidities, such as dyslipidemia [23, 32] or diabetes complications [51], including neuropathy [52], severity and duration of T2D [45], and good management of the T2D to meet the therapeutic targets [20]. These factors may affect the vessel response to nitrovasodilators [20, 23, 32, 35, 44] and are estimated to account for about 32-37% of the variation in the VSM response to NO• [23]. Furthermore, impaired vasodilatory response to ACh in patients with T2D is negatively correlated with serum triglycerides and positively correlated with high-density lipoprotein-cholesterol [23, 32]. On the other hand, ET-dependent and—independent vascular functions are preserved in the brachial artery of complication-free T2D patients [51]. In addition, SNP-induced vasodilatation was significantly reduced only in the neuropathic T2D patients, compared to either the non-neuropathic diabetic or the non-diabetic controls. In contrast, ACh-induced vasodilation was comparable with non-neuropathic diabetic patients [52].
Some evidence supports the notion that vascular NO• resistance depends on the disease’s severity and treatment modality. Decreased ET-dependent VSM relaxation has been reported to be 22%, 40%, and 52% in insulin-sensitive, intermediate insulin-resistant, and insulin-resistant T2D patients, respectively [45]; in this study, decreased ET-independent, NO•-dependent VSM relaxation was 3%, 7%, and 27%, respectively [45]. In addition, in people with T2D who had glycated hemoglobin (HbA1C)<7%, skeletal muscle ET function was similar to non-diabetic subjects, whereas it was lower by ~30% in T2D patients with HbA1C > 7% [20]. Insulin therapy can significantly affect nitrovasodilatory response in T2D; a 6-month and a 3.5-y follow-up of T2D patients indicated that insulin therapy resulted in ACh- and SNP-induced vasodilation to return to normal levels and even higher than the controls [41].
Animal studies
As shown in Table 2, an impaired ET-dependent VSM relaxation has consistently been documented in established T2D [53–64], whereas for the prediabetes state, both preserved [59, 63–65] and decreased (20–89%) [59, 64, 66–68] ET-dependent VSM relaxation have been reported. The impaired ET-dependent VSM relaxation in T2D has been reported mostly in mesenteric arteries (5–66%) [53, 55–58, 60–62, 64]; however, it has also been documented in other vessels, including the aorta [59, 64], pulmonary [59], thoracic [62], coronary [64], femoral [63], and uterine [54] arteries. ET-independent, NO•-dependent VSM relaxation has mainly been preserved [64, 66–68] or upregulated [64, 65] in prediabetes. The augmented or preserved VSM response to NO• in prediabetes may be attributed to increased compensatory NO• production and/or increased sensitivity and activity of VSM soluble guanylate cyclase (sGC). In the case of established T2D, ET-independent, NO•-dependent VSM relaxation is mainly preserved in mesenteric [53, 55, 56, 58, 60–62, 64], thoracic [62], coronary [64], and femoral [63] arteries; however, a decreased VSM response to NO• has been reported in mesenteric [57] and uterine arteries [54].
Table 2.
Impaired endothelium (ET)-dependent and ET-independent, nitric oxide (NO•)-dependent vascular smooth muscle (VSM) relaxation in animal models with prediabetes and type 2 diabetes (T2D).
| Study | Model/species | Vascular region assessed | ET-dependent VSM relaxation | ET-independent, NO•-dependent VSM relaxation |
|---|---|---|---|---|
| Prediabetes | ||||
| Oltman et al. [64] | Zucker obese rats | Aorta | ↔ ACh | ↔ SNP |
| Oltman et al. [64] | Zucker obese rats | Mesenteric a. | ↔ ACh | ↑ SNP (56%) |
| Mourmoura et al. [65] | Zucker obese rats | Coronary a. | ↔ ACh | ↑ SNP (19%) |
| Lu et al. [63] | Zucker obese rats | Femoral a. | ↔ ACh | NR |
| Melo et al. [59] | HFD-fed rats | Pulmonary a. | ↔ ACh | NR |
| Melo et al. [59] | HFD-fed rats | Aorta | ↓ ACh (44%) | NR |
| Belin et al. [66] | Zucker obese rats | Mesenteric a. | ↓ ACh (20%) | ↔ SNP |
| Qiu et al. [68] | db/db obese mice | Mesenteric a. | ↓ ACh (46%) | ↔ SNP |
| Oltman et al. [64] | Zucker obese rats | Coronary a. | ↓ ACh (89%) | ↔ SNP |
| Stablished T2D | ||||
| Mishra et al. [56] | Goto-Kakizaki rats | Mesenteric a. | ↓ ACh (34%) | ↔ SNP |
| Belin et al. [61] | Zucker obese HFD-fed rats | Mesenteric a. | ↓ ACh (44%) | ↔ SNP |
| Wang et al. [62] | Zucker obese rats | Mesenteric a. | ↓ ACh (13%) | ↔ SNP |
| Oltman et al. [64] | Zucker obese rats | Mesenteric a. | ↓ ACh (25%) | ↔ SNP |
| Pannirselvam et al. [58] | db/db obese mice | Mesenteric a. | ↓ ACh (34%) | ↔ SNP |
| Leo et al. [60] | Zucker obese rats | Mesenteric a. | ↓ ACh (5%) | NR |
| Oniki et al. [57] | Goto-Kakizaki rats | Mesenteric a. | ↓ ACh (66%) | ↓ SNP (71%) |
| Melo et al. [59] | HFD + HSD-fed rats | Aorta | ↓ ACh (56%) | NR |
| Oltman et al. [64] | Zucker obese rats | Aorta | ↓ ACh (12%) | NR |
| Melo et al. [59] | HFD + HSD-fed rats | Pulmonary a. | ↓ ACh (34%) | NR |
| Wang et al. [62] | Zucker obese rats | Thoracic a. | ↓ ACh (29%) | ↔ SNP |
| Oltman et al. [64] | Zucker obese rats | Coronary a. | ↓ ACh (70%) | ↔ SNP |
| Lu et al. [63] | Zucker obese rats | Femoral a. | ↓ ACh (15%) | ↔ SNP |
| Goulopoulou et al. [54] | Goto-Kakizaki ratsa | Uterine a. | ↓ ACh (14%) | ↓ SNP (11%) |
All studies were conducted in males except one that has been identified (aFemale).
a artery, ACh acetylcholine, HFD high-fat diet, HSD high-sucrose diet, SNP sodium nitroprusside, NR not reported.
Vascular NO• production in normal conditions
All three layers of the vessel wall (i.e., tunica intima, tunica media, and tunica adventitia) and its supportive components, including perivascular adipose tissue (pVAT), nerve fibers, mast cells, and macrophages, contribute to the bioavailable pool of NO• within the vessel wall [69]. In addition, NO• may penetrate the vascular wall from the vessel lumen; red blood cells (RBCs) and other circulating cells (i.e., monocytes, neutrophils, lymphocytes, and platelets) contribute to the circulating pool of NO• reaching the vessel wall [70].
Endothelial NO• synthase (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS) are differently localized and involved in NO• production within the hierarchy (i.e., tree-like hierarchical branching structure, from larger to smaller branches) of blood vessels in different organs [71, 72]. Traditionally, eNOS was considered the primary source of bioavailable vascular NO•; this view has been changed by studying the genetically-modified animal models (lacking either nNOS or eNOS) demonstrated that nNOS is as important as eNOS in arteriolar relaxation [73] or those indicated that nNOS is the predominant source of NO• in the microvasculature of eNOS-lacking animals [74]. Evidence of iNOS expression in vessels under normal conditions is controversial [72, 75, 76]; however, it is significantly expressed under inflammatory conditions in all three layers of the vessels [75]. The vascular ET expresses all NOS isoforms. ET-derived NO• is released abluminal toward VSMCs and, to a lesser extent, as a spillover into the blood at its luminal side [77]. About 40-60% whole-body NO• production in the human body [~411–661 of 1100 μmol/day [78]] is produced by endothelial cells (ECs) [79]. More than 60% of ET-derived NO• in the vessel wall is detectable in VSMC after about 20 seconds [80].
Human VSMCs express all NOS isoforms [72], however, nNOS seems to be the predominant isoform in VSMCs. The physiologically-relevant vasodilatory role of VSM nNOS-derived NO• was confirmed by evidence showing that potassium chloride (KCl)-induced contraction was significantly elevated in the de-endothelialized aortic rings of nNOS knockout mice compared to controls (151% vs. 131% of reference KCl contraction) [81]. The nNOS-derived NO• is involved in autoregulating local vascular tone via direct effects on VSM [82].
pVAT significantly expresses eNOS, which produces measurable amounts of NO• [83]. pVAT-originated NO• seems directly targets VSMCs to induce vessel relaxation via the sGC-cyclic guanosine monophosphate (cGMP)-protein kinase G (PKG) [84]. The attributable effect size of pVAT on ACh-induced relaxation of the aorta has been estimated to be ~40% [85]. The effect is entirely mediated by NO•, given that it was fully blocked by NG-Methyl-l-arginine (L-NMMA) [85]. pVAT-derived NO• is suggested to travel to the tunica media or even the intima and modulate vascular function [86]. The contribution of the adventitia and other components of the vessel wall in vascular NO• availability is less documented. The adventitia seems to be a potent source of NO• than VSMC under an inflammatory condition [87]. Supplementary Table 1 summarizes available data regarding the expression/activity NOS isoforms in different vessels in humans and animals.
Circulating or blood-born NO• [i.e., estimated to be ~15-38 µM [7, 88, 89], derived from RBC-eNOS, free NO•, nitrate (NO3–), NO2–, S-nitrosothiols (SNOs), S-nitrosoalbumin (SNO-albumin), and SNO-hemoglobin (Hb-SNO)] may significantly contribute to the vascular NO• pool [77, 90–92]. In macro- and micro-vessels, the contribution of circulating NO• to available NO• within the vessel wall is estimated about 60% [93] and 7%, respectively. A small fraction (~3.4 ± 0.58 nM) of circulating NO• [94], diffuses through RBC-free zone of laminar flowing blood and can be transported into the vascular wall [95]. RBC-eNOS produces 4.6 µmol NO• per day [90], and that NO• has an essential and independent contribution to VSM relaxation [77]. NO• delivered by cell-free Hb and Hb-SNO to the vascular wall is estimated ~0.02 and 0.25-6 pM [96, 97], respectively. SNO-albumin releases NO• ~1.4 pmol NO•/min [88].
Vascular NO• production in T2D
Changes in whole-body NO• production in T2D depend on the disease’s duration. An enhanced NO• production occurs in the initial stages of T2D; this idea is supported by several lines of evidence, e.g., an elevated serum NO• concentration in T2D patients at the initial stages (5 years of the onset), compared to its reduced level in patients with prolonged T2D [98]. On the other hand, a decreased whole-body [99, 100] and vascular NO• production [101, 102] have been documented in established T2D. A reduced fractional synthesis rate (FSR) of NO• (i.e., percent of circulating pool newly synthesized from l-arginine) (19.3 ± 3.9% vs. 22.9 ± 4.5% per day) and absolute synthesis rate (ASR) of NO• (320 vs. 890 μmol per day) were observed in patients with established T2D compared to normal subjects [99]. In addition, a 50% decreased NO• production from plasma l-arginine turnover (0.009 ± 0.002 vs. 0.019 ± 0.007 µmole/kg/h, 0.19% vs. 0.35%), and a 16% decrease of the total NO• synthesis rate (0.52 ± 0.16 vs. 0.62 ± 0.16 µmole/kg/h) have been observed in established T2D compared to healthy subjects [100].
Decreased NO• production in diabetes is attributed to diminished eNOS expression [99], overexpression of negative regulators of eNOS activity [i.e., the membrane-associated scaffolding protein caveolin-1 (Cav-1) and phosphatase and tensin homolog (PTEN)] [103–105], substrate deficiency for NOSs [due to increased arginase activity and decreased l-arginine availability [106, 107]], eNOS uncoupling [due to elevated ROS, oxidation of tetrahydrobiopterin (BH4), l-arginine depletion, and accumulation of methylarginines] resulting in superoxide anion instead of NO production [108].
Changes in vascular NO• levels in T2D are also dependent on the duration of the disease, with increased production in initial stages and decreased production in later stages (Fig. 1); initial stages of T2D may induce compensatory mechanisms, leading to adaptation and relative normalization of NO• vascular output, whereas a more extended duration towards established T2D results in a state of vascular NO• deficiency. In Goto-Kakizaki (GK) rats, ACh-induced NO• release (in the thoracic aorta) was enhanced at 12 weeks but decreased at 36 weeks [109]. At the initial stages of T2D in GK rats (17-week old, in the presence of hyperglycemia and hyperinsulinemia), basal NO• bioavailability in the abdominal aorta was more likely to be elevated compared with aged-matched controls [110]. In streptozotocin (STZ)-induced diabetic rats, the aorta showed significantly elevated NO• levels after 3 weeks of diabetes onset, which remained high after 7 weeks [111]. The mesenteric arteries pVAT has also been reported to undergo an adaptive NO• overproduction (by 2-fold compared to controls) during the initial stages of insulin resistance (8-week high-fat diet induced-obese mice, with hyperinsulinemia) which contribute to preserving vascular function [112]. Evidence of augmented blood flow at early stages of diabetes may also reflect higher NO• production [102, 113].
Fig. 1. Increased and decreased vascular nitric oxide (NO•) production in the early and late stages of type 2 diabetes (T2D), respectively.
ACh, acetylcholine; ADMA, asymmetric dimethyl arginine; AEG, advanced end-glycation products; BH4, tetrahydrobiopterin; DDAH, dimethylarginine dimethylaminohydrolase; NOS, NO• synthase; NOX, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.
An upregulated NOSs expression/activity may explain the elevated NO• production in the initial stages of T2D. In the aorta of GK rats, increased eNOS mRNA expression was observed in young (12-week-old) compared to age-matched controls, whereas eNOS, iNOS, and nNOS mRNAs were lower in older rats (70-week old) compared to the younger (12-week old) rats [114]. Acute exposure of ET to high-glucose concentrations upregulates eNOS expression via activation of PKC [115]. Both eNOS and iNOS gene expressions were up-regulated in ECs in short-term exposure to high-glucose concentrations (10 and 50 mM), resulting in increased NO• concentration in the media (from 8 to 11.5 and 12.5 µM) [98]. An up-regulated eNOS expression in the ET of pre-glomerular and post-glomerular vessels was also evident in the early stages of diabetes in rats [116].
Different mechanisms are involved in decreased vascular NO• production in established diabetes (Fig. 1), including (1) decreased eNOS activity [117] and expression [118], (2), increased arginase activity [83, 119], and l-arginine deficiency [83], and (3) decreased eNOS sensitivity to ACh [120]. Due to decreased activity and expression of eNOS, diabetic patients displayed a decreased conversion of l-arginine to NO•, and ET of human diabetic vessels cannot generate enough NO• to regulate blood flow [10, 100]. Decreased vascular eNOS activity/expression in T2D is due to (A) increased dimethylarginine dimethylaminohydrolase (DDAH) activity, which increases asymmetric dimethyl arginine (ADMA) [117], (B) increased AEGs, which decreases eNOS mRNA stability and eNOS phosphorylation [118], (C) increased NOX, which uncouples eNOS and oxidizes BH4 [121], (D) impaired insulin signaling [122], (F) increased arginase activity [120], and (G) decreased NOS sensitivity to ACh [120].
During the establishment of T2D, DDAH (the enzyme that metabolizes the endogenous competitive inhibitor of NOS enzymes, ADMA) activity decreased by about 44% in the abdominal aorta of T2D rats. Incubation of human ET cells and rat VSMCs in high-glucose resulted in ADMA accumulation, decreased eNOS activity, and reduced cGMP levels [117]. Hyperglycemia inhibits ET-eNOS activity through post-translational modification, that is, by increasing O-linked N-acetylglucosamine modification of eNOS and decreasing phosphorylation at O-linked serine residue 1177 [123]. Prolonged exposure of vascular ET with AGEs (i.e., glucose-derived moieties that are produced non-enzymatically through glycation reaction between glucose and the amino groups of proteins) under hyperglycemic conditions significantly reduce eNOS expression, eNOS mRNA stability, and eNOS phosphorylation (at Ser1177) and its activity, and cellular NO• levels [118].
T2D also initiates a cascade of events in the vessel wall, including NOX-induced ROS over-production, oxidation of BH4, and uncoupling of eNOS in vascular ET, leading to decreased NO• availability [121]. In addition, T2D impairs phosphatidylinositol 3-kinase (PI3K)-protein kinase B (PKB/Akt) insulin signaling pathway in the vascular ET; this pathway stimulates eNOS and thus NO production; therefore, its impairment decreases NO• production in T2D vessels [122].
High-glucose concentrations in vascular ET caused a 66.7% increase in arginase activity, leading to a 27% decreased NO• production [119]. Furthermore, elevated circulating free heme in T2D impairs l-arginine transport across RBC membranes, increases l-arginine consumption by arginase, and reduces l-arginine availability for NO• production by RBC [124]; in this state, RBCs tend to catabolize l-arginine to ornithine, citrulline, and urea [125]. Elevated RBC-arginase activity in patients with T2D causes decreased NO• bioavailability and ET dysfunction [126].
A progressive decreased eNOS sensitivity to ACh was also shown to be worsened with the diabetes duration; EC50 for ACh in diabetic arteries was increased from 13.5 nM after 6 weeks to 63 and 100 nM after 16 and 24 weeks of diabetes [120].
NO• storage pool within VSM
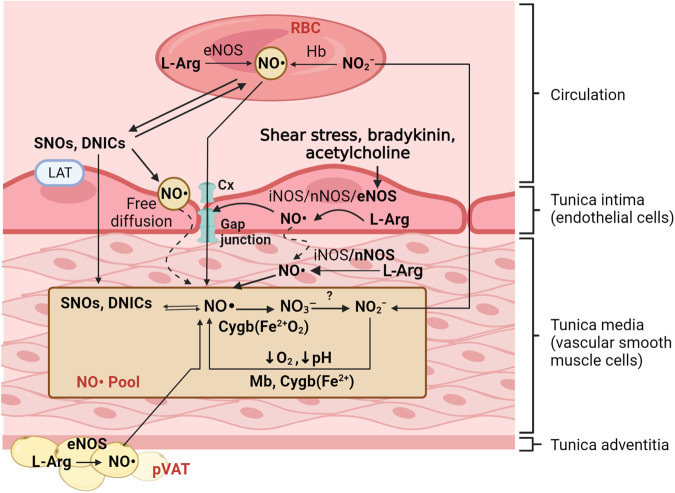
VSMC is proposed to contain a NO• storage pool comprised of NO• and NO•-equivalents, including NO2–, SNOs [i.e., S-nitrosocysteine (cysNO), GSNO], and dinitrosyl iron complexes (DNICs, a non-heme-iron nitrosyl species). NO• and NO•-equivalents enter the VSMCs from different sources, including ET (in response to shear stress and agonists like ACh), pVAT, nerve fibers, mast cells, and circulation [127, 128]. The predicted NO• concentration in the VSM ranges from 20–100 pM to 400 nM [129]. However, depending on the vessel diameter, NO•-RBC reaction rate constant (KNO•-RBC), and blood flow velocity, NO• concentration in the VSM may reach up to ~1100 nM (e.g., 281–1163 nM for 50 μm arteriole over the blood velocity range of 0.5-4.0 cm/s and KNO•-RBC of 0.2 × 105 M/s) [93].
A diffusion constant of 3.3 × 10−5 cm2/s in all directions to a distance of 150–600 μm enables NO• to diffuse from its sources simply (e.g., ET, pVAT, or circulation) to VSMCs [80, 128] (Fig. 2). Connexins (Cx37, Cx40, Cx43, Cx46) are involved in NO• diffusion across the plasma membranes of ET [130]; S-nitrosylation of cysteine residues of connexins by NO• derivates activates the opening of the connexins [131]. The expression pattern and activity of connexins are changed in T2D [132, 133]; thus, decreased vascular NO• availability can be attributed partly to impaired delivery of circulating NO• to the vessel wall.
Fig. 2. Main sources of nitric oxide (NO•) pool within the vascular smooth muscle cells (VSMCs).
The pool of NO• within the VSMCs is comprised of NO synthase (NOS)-derived NO• [produced within VSMC, transporting from endothelial cells, perivascular adipose tissue (pVAT) or other vascular components], and NO•/NO•-equivalents [i.e., nitrite (NO2–), S-nitrosothiols [SNOs, i.e., S-nitrosocysteine (cysNO), nitrosoglutathione (GSNO), dinitrosyl iron complexes (DNICs, a non-heme-iron nitrosyl species)] that are derived from red blood cells (RBCs) and other circulating NO• sources. Cygb, cytoglobin; eNOS, endothelial NOS; Hb, hemoglobin; iNOS, inducible NOS; l-Arg, l-arginine; LAT, L-type amino acid transporter; Mb, myoglobin; nNOS, neural NOS.
Circulating NO• penetrates the vessel wall despite very effective scavenging by Hb. The RBC-free layer near the vascular ET reduces the rate of free NO• trapping by RBCs-Hb. It allows it to escape scavenging by Hb and reach the VSM in physiologically significant concentrations to induce vasorelaxation [134]. The NO• delivery from circulating sources into the vessel wall is governed by a dynamic cycle between circulatory Hb-NO and Hb-SNO [95], enabling RBCs to act as SNOs reactor, regulating plasma SNOs levels [135] and delivery of NO• to its target [95, 136]. This dynamic cycle is impaired in hyperglycemic conditions like T2D; glycosylated RBCs are likely to be dysfunctional compared to normal-glycosylated RBCs [137]. NO• is trapped within the glycosylated-Hb and cannot be transferred into the vascular cells, a condition that results in a reduced NO• bioavailability within the diabetic vessels; compared to normal-glycosylated Hb, NO• mainly exists in the form of Hb-NO• (0.044 vs. 0.013 percent NO• per Hb mol/L) rather Hb-SNO (0.025 vs. 0.032 percent NO/Hb mol/L) within the highly-glycosylated RBCs (indicated as HbA1C > 10.7%) [137]. The rate of NO• release from Hb-NO• is extremely low [138].
Cellular DNICs [i.e., Fe(RS)2(NO•)2 complexes produced via interaction of NO• with iron-sulfur clusters of proteins] deliver NO• into the cytosol of VSMC; they are important NO• storage form in the VSMC [139]. DNICs and SNOs are suggested to release NO• outside the VSMC (because they are membrane impermeable) [127]; however, selective transport of SNOs via L-type amino acid transporters (i.e., LAT1 and LAT2, in vascular ET and SMCs) [140], and dipeptide transporters (PEPT2) (as documented in other cells like macrophages) [141] may occur. Handoff of NO• from extracellular SNOs to the plasma membrane and VSM thiols via transnitrosylation with subsequent transport of the NO• to the cytoplasm has also been proposed [127].
The NO• storage pool in VSMC seems to release NO• in a controlled manner upon extracellular stimulations [127]. NO• release from the VSMC store (DNICs and SNOs) is light- [142] and thiol-[143] sensitive. NO2 likely contributes to the NO• stores via conversion into NO•, probably via the action of metalloproteins, including Hb, myoglobin (Mb), cytoglobin ([Cygb), xanthine oxidase, cytochrome c oxidase, and eNOS, that is favored by hypoxia and low pH [127]. Cygb [i.e., a globin expressed at μM levels (~3.5–5.0 μM [144]) and co-localized with myosin heavy chain [145]] regulates NO• bioavailability within the VSM [146]; under a normal O2 level, Cygb metabolizes excessive amount of NO• by dioxygenation (converting NO• to NO3, a rate of 11.6 ± 0.6 nM/s in mouse aorta); in contrast, under a hypoxic condition, it generates NO• from NO2 (referred to as O2-dependent NO•-dioxygenase and NO2-reductase, respectively) [146], and that NO• binds to sGC in the VSM, making the vessel to be relaxed [147]. About 78% of NO• metabolism in VSMCs is Cygb-dependent [144]. In human VSMCs, Cygb-mediated NO• production from NO2 (at the physiological intracellular level of NO2 ~ 10 μM) is estimated to be ~7 and 35 pM/s in VSM, at pH 7.0 and 5.5, respectively); an amount that can rise to 10-fold ( ~ 350 pM/s) under acidic condition (pH=5.5) and chronic hypoxia (i.e., intracellular Cygb concentration of ~350 μM) [147]. In human VSMCs, Cygb-mediated NO• release corresponds to about 40% of cGMP activation under hypoxic conditions [147].
VSMCs Mb is another important vascular NO2 reductase; deletion of Mb significantly decreased NO2-NO•-cGMP mediated vasorelaxation (~57% decrease in cGMP production, from 1300 to 550 fmol/mg in mice aorta), indicating that Mb is also a bioconvertor of NO2 to NO• in VSM [148]. Chronic hyperglycemia, resulting in a non-enzymatic reaction of glucose with an amino group of Mb and changes in the Mb’s structure and function, may decrease NO• availability in the VSM in T2D [149].
NO• actions in VSM in normal conditions and T2D
Role of NO• in VSM contraction
Mechanisms underlying VSM contraction are illustrated in Fig. 3. Signal transduction of NO• in VSM involves two major pathways: (1) the indirect pathway of NO•-sGC-cGMP-PKG [128, 150, 151] and (2) the direct pathway of protein S-nitrosylation [128, 150, 151], also called cGMP-dependent and -independent pathways, respectively [152] (Fig. 3). Although both pathways are found in the VSM, NO•-mediated vasorelaxation is mostly cGMP-dependent at a normal oxygen pressure [152]. The NO• concentrations eliciting a physiological response in VSM have been estimated to fall from 100 pM to 5 nM [153].
Fig. 3. The main mechanisms of vascular smooth muscle (VSM) contraction and underlying mechanisms of NO•-mediated VSM relaxation.
VSM contraction is initiated by increasing [Ca2+]i via an influx of Ca2+ from extracellular fluid Ca2+ channels (i.e., voltage-dependent, receptor-operated, transient receptor potential (TRP), store-operated, and stretch-activated channels) or Ca2+ release from the sarcoplasmic reticulum (via PLC-IP3-IP3R pathway and Ca2+-induced Ca2+release pathway via RyR). Cytosolic Ca2+ binds to CaM, and the Ca2+–CaM complex activates MLCK, which phosphorylates MLC and causes contraction. Increased force of VSM contraction is mediated via Rho-Rho kinase (ROK)- and PKC-dependent MLCP inhibition. As indicated in the right section, NO• signaling in the VSM includes two major pathways, i.e., NO•-sGC-cGMP-PKG pathway and protein S-nitrosylation of target proteins. The downstream cGMP-dependent pathways may be Ca2+-dependent (i.e., decreasing intracellular Ca2+ and Ca2+ desensitization) or Ca2+-independent. The cGMP-dependent pathway decreases Ca2+ influx and increases Ca2+ efflux from the cytosol of VSM. NO• decreases Ca2+ sensitivity by phosphorylating MLCP, thereby increasing MLCP activity, reducing agonist-induced Ca2+ sensitization of contraction, and inhibiting PKC activation; PKC promotes contraction of VSM by phosphorylating CaD, which in its non-phosphorylated form inhibits actin-myosin interaction, and by inhibiting MLCP and thereby increase in Ca2+ sensitivity. S-nitrosylation of RhoA, inhibiting the Rho-ROK-MLCP pathway, is the cGMP-independent pathway for NO•-induced vasodilation. BKCa; Ca2+-activated K+-channels; CaM, calmodulin; CaD, caldesmon; CaV, voltage-gated Ca2+channels; DAG; diacylglycerol; GEFs, guanine nucleotide exchange factors; GTP, guanosine triphosphate; GDP, guanosine diphosphate; IP3, inositol triphosphate; IP3R, IP3 receptor; MLCP, myosin light chain phosphatase; MLC, myosin light chain; MLCK myosin light chain kinase; NCX, Na+–Ca2+ exchanger; PDE, phosphodiesterase enzyme; PKC, protein kinase C; PKG, protein kinase G; sGC, soluble guanylate cyclase; PLC, phospholipase C; PMCA, plasma membrane Ca2+-ATPase; RYR, ryanodine receptors; SERCA, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase; TRPC4, transient receptor potential channel 4; VASP; vasodilator-stimulated phosphoprotein.
At low nM concentrations, NO• binds to ferrous heme residue (the NO•-biding site) of its own receptor, sGC, and activates it [150, 154]. Activated sGC converts GTP to second messenger cGMP, which leads to tissue-specific biological effects of NO• [128, 150]. cGMP exerts its effects in VSMCs by cGMP-dependent protein kinase (PKG, or cGK) and by affecting cGMP-binding phosphodiesterase [154]. PKG, the principal mediator of cGMP-induced VSM relaxation, acts via Ca2+-dependent mechanisms, i.e., decreasing [Ca2+]i [152, 154, 155] and Ca2+ desensitization [152, 154], as well as Ca2+-independent mechanisms [156].
NO•-sGC-cGMP-PKG pathway decreases Ca2+ influx and increases Ca2+ efflux from the cytosol of VSMCs. PKG increases the activity of big conductance Ca2+-activated K+-channels (BKCa), and cGMP activates ATP-sensitive K+ (KATP) channels in VSMCs [157], both of which hyperpolarize cell membrane and inhibit Ca2+ entry through voltage-gated Ca2+channels (VGCCs or CaV) [154, 155]. cGMP inhibits PLC and IP3 generation [154]; in addition, PKG phosphorylates the IP3 receptor and decreases its activity, leading to decreased Ca2+ release from the SR [154]. Furthermore, PKG increases Ca2+ efflux by activating PMCA and SERCA [154].
In the case of Ca2+ desensitization, PKG phosphorylates MBS of MLCP, thereby increasing MLCP activity and reducing agonist-induced Ca2+ sensitization [154, 155]. In addition, cGMP-PKG inhibits PKC activation [154]. PKC promotes contraction of VSM by phosphorylating caldesmon (CaD), which in its non-phosphorylated form inhibits actin-myosin interaction, and by inhibiting MLCP and thereby increasing Ca2+ sensitivity [154].
Vasodilator-stimulated phosphoprotein (VASP), a downstream molecule of NO• signaling, is found in VSMCs [158] and is phosphorylated at serine 239 by PKG [158, 159]. In rabbit thoracic aorta, SNP increases Ser239-phosphorylated VASP, and L-NG-Nitro arginine methyl ester (L-NAME) and 1h-Oxadiazoloquinoxalin-1-one (ODQ) decrease it by about 80% and 85%, respectively, indicating dependency of this phosphorylation to NO•-cGMP-PKG pathway [158]. However, the role of VASP in regulating vascular tone remains to be elucidated [158]. Aortic rings of wild-type and VASP null mice showed similar relaxant responses to cGMP, ACh, and SNP, indicating that VASP is not essential for vasodilator-induced relaxation of VSM [160]. However, in mesangial cells, SNP via the NO•-cGMP-PKG-Iα pathway increases Ser239-phosphorylated VASP and inhibits store-operated Ca2+ entry [161]. In addition, in mesenteric arteries of Sprague-Dawley rats, incubation of mesenteric vessels with NONOate, a NO• donor, caused Ser239 phosphorylation and colocalization of VASP with TRP channel 4 (TRPC4); 1,1-Diethyl-3-oxotriazane-2-ol (NONOate) and 8p-CPT-cGMP also blocked cyclopiazonic acid (CPA, a selective inhibitor of SERCA)-induced increase in Ca2+ entry [159]. It has been suggested that Ser239-phosphorylated VASP decreases Ca2+ entry through SOCCs in VSMCs and leads to vasorelaxation [159].
Protein S-nitrosylation, the binding of a NO• moiety to a thiol group of a cysteine residue to form SNO [128], is a specific, reversible, and enzymatic reaction leading to specific protein modification [151, 162–164]. For example, in rat aortic VSMCs, S-nitrosylation of RhoA by S-nitrosocystein inhibits the Rho-ROK-MLCP pathway, suggesting a cGMP-independent pathway for vasodilation [165]. In addition, S-nitrosylated actin at Cysteine-374 produces a dose-dependent high potency (EC50 ≈ 9.37 nM) relaxation in rat abdominal aorta strips, which may be sGC-dependent or independent [166].
The underlying mechanisms of impaired NO• action on VSM in T2D
The main cause of vascular hypo-responsiveness to NO• is impairment of the main physiologically relevant NO• signaling cascade, i.e., the NO•-cGMP-PKG pathway; this may occur at the receptor level (i.e., quenching of NO• and/or sGC desensitization [53, 167]) and post-receptor level [i.e., cGMP generation by sGC, cGMP degradation by phosphodiesterase (PDE), and/or cGMP-induced activation of PKG] [14, 18]. The T2D-induced mechanisms of impaired NO• action on VSM relaxation are illustrated in Fig. 4.
Fig. 4. Proposed underlying mechanisms of vascular smooth muscle (VSM) hypo-responsiveness to nitric oxide (NO•) in type 2 diabetes (T2D).
The vascular hypo-responsiveness to NO• is evident at the receptor level (soluble guanylate cyclase, sGC) and post-receptor level (sGC-downstream pathway). Mechanisms acting at the receptor level include: (a) quenching of NO• by advanced glycation end products (AGEs) and reactive oxygen species (ROSs), and (b) sGC desensitization via ROS-induced sGC oxidation, and augmented cGMP-induced negative feedback on sGC [because of reduced phosphodiesterase (PDE) activity and increased cGMP level]. Mechanisms acting at the post-receptor level include (a) defective cGMP catabolism and (b) hyperglycemia-induced downregulation of protein kinase G (PKG).
The NO• bioavailability (extent NO• becomes available to its targets) reduces in diabetic vessels regardless of high NO• production at early stages of diabetes [115, 121], evidence indicating NO• quenching. In hyperglycemic states, high levels of AGEs are accumulated within the subendothelial collagen, quench NO• activity and impair its vasodilatory effect [168]. Quenching occurs by a direct reaction between the NO• and the AGEs within less than 5 seconds [168]. The apparent paradox of diabetes-induced eNOS upregulation at early stages concomitantly with a decreased vascular NO• bioavailability is attributed to the elevated formation of superoxide anion O2• – and peroxynitrite (ONOO-) by vascular ET [121]. Furthermore, endothelial NOX (NOX1, NOX2, and NOX5), potent stimulators of ROS production in ECs in hyperglycemia, reduce NO• availability by its conversion to ONOO- [169, 170]. Incubation of human umbilical vein endothelial cells (HUVEC) in high-glucose media resulted in upregulated NO• production (~2-3 fold), decreased NO• availability by 52%, and increased ONOO- levels by 240% within 30 min [170]. Other ROS generators (e.g., mitochondria, uncoupled eNOS, xanthine oxidase, and cyclooxygenase-1) under hyperglycemic conditions may accelerate NO• quenching within the diabetic vessels [171].
Desensitization of sGC is the transition to a state in which sGC response to a new NO• molecule is reduced or abolished [172]. Both the potency and efficacy of NO• to activate sGC (i.e., measured as cGMP production) were lower in the aorta of GK rats [167] and T2D patients [173]. In addition, SNP-induced increase in cGMP was lower in VSMCs from insulin-resistant obese Zucker rats (OZR) than controls; this response was maintained in the presence of IBMX (unselective PDE inhibitor), indicating the impaired ability of NO• to activate sGC [18]. In addition, it was not due to different protein expressions of sGC subunits (α1 and β1) [18]. These data indicate sGC desensitization as an underlying mechanism for vascular NO• resistance in T2D.
Although not entirely determined, two mechanisms seem to result in sGC desensitization: 1) ROS-induced sGC oxidation, and 2) defective cGMP catabolism. The binding of NO• to reduced ferrous (Fe2+) heme residue of sGC increases its catalytic activity and cGMP production from GTP. The reduced sGC response to NO• seems to be due to the reduced enzyme’s heme content and/or oxidation of the heme iron under hyperglycemic conditions because the expression of sGC was reported to be preserved [167] or even increased in the diabetic vessels [53]. High levels of ROS can oxidize the sGC heme iron to the ferric form (Fe3+), rendering sGC insensitive to normal levels of NO• and developing NO• resistance [174, 175]. Indeed, one of the heterodimeric redox-sensitive sites of sGC is a prosthetic heme group bound to the β subunit, and oxidation of its ferrous heme inhibits the NO•-mediated activation of the receptor [176]. Heme-dependent hypo-responsiveness of sGC to NO• is supported by evidence showing that response to BAY 41-2272 (an sGC stimulator, i.e., can bind directly to the reduced-form of heme-containing sGC) was reduced in diabetic resistance arteries, while heme-independent sGC activation (using BAY 58-2667, an sGC activator, i.e., binds directly to oxidized-form of heme-containing sGC) was relatively preserved [53]. Likewise, other vascular beds, e.g., aorta derived from diabetic GK rats and isolated vessels from T2D humans, exhibited a preserved and enhanced relaxation response to sGC activators (i.e., heme-independent activator protoporphyrin-IX and BAY 58–2667, respectively) [167, 177]. This phenomenon has been introduced as a sub-phenotype of ET dysfunction, characterized by NO• resistance at the receptor level in the blood vessels of patients with T2D [13, 177].
Defective cGMP catabolism may also contribute to sGC desensitization in diabetic vessels. PDEs control the abundance of cGMP [128]. Among 11 families of PDE that have been identified [128], PDE1, PDE3, and PDE5 degrade cGMP in VSM [156], and PDE5 is the main one involved in cGMP catabolism in the smooth muscle [178]. cGMP binds to PDE5 and increases its activity which means PDE5 activity increases following increased cGMP production [156]. In addition, PKG-I phosphorylates PDE5 and prolongs its activation by increasing its affinity for cGMP [156]. This provides a negative feedback mechanism to preserve the sensitivity of the NO•-sGC-cGMP-PKG signaling pathway [156]. In VSMCs isolated from the aorta, baseline cGMP concentrations were about 3 times (1.83 ± 0.08 vs. 0.67 ± 0.07 pmol/mg protein) higher in insulin-resistant-obese Zucker rats than controls [18]. This was not affected by the NOS inhibitor L-NMMA, which rules out sGC hyperactivation [18]. In addition, sGC inhibitors (ODQ and Ambroxol) decreased baseline cGMP in VSMCs from OZR but not controls. Still, baseline cGMP remained higher in VSMCs from OZR, suggesting defective cGMP catabolism [18]. Baseline PDE5 activity was lower in VSMCs from OZR, and the IBMX-induced increase in cGMP was smaller in VSMCs from OZR than in controls, suggesting defective PDE activity in VSMCs from OZR [18].
Some evidence supports the notion that vascular hypo-responsiveness to NO• is at least in part due to post-receptor events in the NO•-sGC-cGMP-PKG signaling pathway. Hyperglycemia downregulates mRNA and protein levels of PKG-I in VSMCs through altered NOX signaling [179]. In diabetic vessels, the ability of cGMP to activate PKG and PKG-dependent activation of PDE5 are also impaired [18]. No further evidence is available regarding T2D-induced changes in sGC downstream signaling.
Conclusion and perspectives
Both animal and human studies provide evidence for the presence of vascular NO• resistance in T2D patients, which is an independent risk factor for cardiovascular events. Human studies indicate a 13-94% decrease in ET-dependent VSM relaxation and a 6–42% decresae in the vasodilatory action of exogenous NO• in different vascular beds of patients with T2D (see Table 1). Vascular NO• resistance in T2D is stage-dependent and displays a progressive spectrum, initially manifested by an augmented or preserved vascular NO• production and/or VSM response to NO•, followed by a reduced NO• bioavailability and/or partial to almost entirely impaired NO• function in VSM, in both the macro- and microvessels. Because various vessels may have different capacities of NO• production [180] and heterogeneously respond to vasodilatory action of NO• (probably due to their different morphology, functions, and diverse receptor and ion channel populations) [181–183], the vascular NO• resistance seems to be progressed in a time-course manner with a different magnitude in the vessels. Although it needs to be proved, small coronary arteries seem to be affected by T2D-induced vascular NO• resistance earlier and to a greater extent compared to mesenteric resistance arteries and large elastic vessels like the aorta [64]. These observations may imply that different vessels probably exhibit diverse phenotypes of NO• resistance in T2D.
The mechanisms underlying vascular NO• resistance in T2D are not fully understood; however, a leading hypothesis proved in several vascular beds is that hyperglycemia-induced overproduction of ROS is a key player initiating a cascade of events (i.e., polyol and hexosamine pathway, AGEs production, induction of PKC-dependent pathways and NOX activity) in the vessel wall resulting in decreased NO• availability (by inhibiting NOS expression/activity and quenching NO• activity), sGC desensitization (by oxidizing its heme residue, and/or nitrosylating its cysteine residues) and impaired cGMP-PKG pathway (by decreasing cGMP catabolism, and/or inhibiting PKG expression/activity) in VSM.
New pharmacological approaches that upregulate vascular NO• availability, re-sensitize or bypass the non-responsive pathways to NO•, and target key vascular sources of ROS production are potentially relevant strategies in preventing and retarding the progression of T2D-induced vascular NO• resistance. Beneficial effects of some pharmaceutical agents, including angiotensin-converting enzyme inhibitors (e.g., ramipril, perindopril), the anti-anginal agent perhexiline, insulin (by decreasing oxidative stress and superoxide production), statins (by upregulating eNOS expression and activity), NO2, and sGC activators, have been documented for attenuating vascular NO• resistance [14, 15]. Furthermore, the use of nitroxyl donors, e.g., Angeli’s salt, is now proposed as an effective strategy for overcoming NO• resistance in T2D [15] because of its antioxidant properties and potential ability to target alternative biological molecules [e.g., calcitonin gene-related peptide (CGRP), thiol residues] and distinct pathways [i.e., cyclic AMP(cAMP)-protein kinase A (PKA), rather than cGMP-PKG] involved in vasodilation.
Quantifying the contribution of the impaired pathways (i.e., reduced NO• synthesis/availability, sGC desensitization, and impaired cGMP-PKG) and determining their chronology in developing vascular NO• resistance in T2D may drive therapeutic approaches to design specified and timely interventions. Furthermore, considering the diverse phenotypes of NO• resistance in different vessels may help develop specific vessel-targeted drug delivery platforms to overcome vascular NO• resistance in T2D. Concurrent management of hyperglycemia, insulin resistance, and oxidative stress is also essential to ameliorate vascular NO• resistance in T2D patients.
Supplementary information
Author contributions
Study concept and design: Zahra Bahadoran and Asghar Ghasemi. Drafting of the manuscript: Zahra Bahadoran and Asghar Ghasemi. Critical revision of the manuscript for important intellectual content: Khosrow Kashfi and Parvin Mirmiran.
Funding
This study was supported by the Shahid Beheshti University of Medical Sciences (Grant number: 43004304), Tehran, Iran.
Competing interests
The authors declare no competing interests.
Footnotes
Edited by Alessandro Finazzi-Agrò
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41419-023-05935-5.
References
- 1.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovascular Diabetol. 2018;17:83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aikaeli F, Njim T, Gissing S, Moyo F, Alam U, Mfinanga SG, et al. Prevalence of microvascular and macrovascular complications of diabetes in newly diagnosed type 2 diabetes in low-and-middle-income countries: A systematic review and meta-analysis. PLOS Glob Public Health. 2022;2:e0000599. doi: 10.1371/journal.pgph.0000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag. 2007;3:853–76. [PMC free article] [PubMed] [Google Scholar]
- 5.Montero D, Walther G, Pérez-Martin A, Vicente-Salar N, Roche E, Vinet A. Vascular smooth muscle function in type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetologia. 2013;56:2122–33. doi: 10.1007/s00125-013-2974-1. [DOI] [PubMed] [Google Scholar]
- 6.Forbes JM, Fotheringham AK. Vascular complications in diabetes: old messages, new thoughts. Diabetologia. 2017;60:2129–38. doi: 10.1007/s00125-017-4360-x. [DOI] [PubMed] [Google Scholar]
- 7.Kelm M. Nitric oxide metabolism and breakdown. Biochim Biophys Acta. 1999;1411:273–89. doi: 10.1016/S0005-2728(99)00020-1. [DOI] [PubMed] [Google Scholar]
- 8.Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34:2436–43. doi: 10.1093/eurheartj/eht149. [DOI] [PubMed] [Google Scholar]
- 9.Mather KJ, Steinberg HO, Baron AD. Insulin resistance in the vasculature. J Clin Investig. 2013;123:1003–4. doi: 10.1172/JCI67166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okon EB, Chung AW, Rauniyar P, Padilla E, Tejerina T, McManus BM, et al. Compromised arterial function in human type 2 diabetic patients. Diabetes. 2005;54:2415–23. doi: 10.2337/diabetes.54.8.2415. [DOI] [PubMed] [Google Scholar]
- 11.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–74. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 12.Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Investig. 1996;97:22–8. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gladwin MT. Deconstructing endothelial dysfunction: soluble guanylyl cyclase oxidation and the NO resistance syndrome. J Clin Investig. 2006;116:2330–2. doi: 10.1172/JCI29807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chirkov YY, Horowitz JD. Impaired tissue responsiveness to organic nitrates and nitric oxide: a new therapeutic frontier? Pharmacol Therapeutics. 2007;116:287–305. doi: 10.1016/j.pharmthera.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Velagic A, Qin C, Woodman OL, Horowitz JD, Ritchie RH, Kemp-Harper BK. Nitroxyl: a novel strategy to circumvent diabetes associated impairments in nitric oxide signaling. Front Pharmacol. 2020;11:727. doi: 10.3389/fphar.2020.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson R, Ellis G, Chirkov Y, Holmes A, Payne N, Blackman D, et al. Determinants of platelet responsiveness to nitric oxide in patients with chronic heart failure. Eur J Heart Fail. 2004;6:47–54. doi: 10.1016/S1388-9842(03)00038-2. [DOI] [PubMed] [Google Scholar]
- 17.Young ME, Leighton B. Evidence for altered sensitivity of the nitric oxide/cGMP signalling cascade in insulin-resistant skeletal muscle. Biochemical J. 1998;329:73–9. doi: 10.1042/bj3290073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo I, Del Mese P, Doronzo G, Mattiello L, Viretto M, Bosia A, et al. Resistance to the nitric oxide/cyclic guanosine 5'-monophosphate/protein kinase G pathway in vascular smooth muscle cells from the obese Zucker rat, a classical animal model of insulin resistance: role of oxidative stress. Endocrinology. 2008;149:1480–9. doi: 10.1210/en.2007-0920. [DOI] [PubMed] [Google Scholar]
- 19.Tawa M, Kinoshita T, Asai T, Suzuki T, Imamura T, Okamura T. Impact of type 2 diabetes on vascular reactivity to cGMP generators in human internal thoracic arteries. Vasc Pharmacol. 2017;91:36–41. doi: 10.1016/j.vph.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Bock JM, Hughes WE, Ueda K, Feider AJ, Hanada S, Casey DP. Glycemic management is inversely related to skeletal muscle microvascular endothelial function in patients with type 2 diabetes. Physiological Rep. 2021;9:e14764. doi: 10.14814/phy2.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vavuranakis M, Stefanadis C, Triandaphyllidi E, Toutouzas K, Toutouzas P. Coronary artery distensibility in diabetic patients with simultaneous measurements of luminal area and intracoronary pressure: evidence of impaired reactivity to nitroglycerin. J Am Coll Cardiol. 1999;34:1075–81. doi: 10.1016/S0735-1097(99)00331-9. [DOI] [PubMed] [Google Scholar]
- 22.Morris SJ, Shore AC, Tooke JE. Responses of the skin microcirculation to acetylcholine and sodium nitroprusside in patients with NIDDM. Diabetologia. 1995;38:1337–44. doi: 10.1007/BF00401767. [DOI] [PubMed] [Google Scholar]
- 23.Caballero AE, Arora S, Saouaf R, Lim SC, Smakowski P, Park JY, et al. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48:1856–62. doi: 10.2337/diabetes.48.9.1856. [DOI] [PubMed] [Google Scholar]
- 24.Sokolnicki LA, Roberts SK, Wilkins BW, Basu A, Charkoudian N. Contribution of nitric oxide to cutaneous microvascular dilation in individuals with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2007;292:E314–8. doi: 10.1152/ajpendo.00365.2006. [DOI] [PubMed] [Google Scholar]
- 25.Beer S, Feihl F, Ruiz J, Juhan-Vague I, Aillaud MF, Wetzel SG, et al. Comparison of skin microvascular reactivity with hemostatic markers of endothelial dysfunction and damage in type 2 diabetes. Vasc Health Risk Manag. 2008;4:1449–58. doi: 10.2147/VHRM.S4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–906. doi: 10.1161/01.CIR.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 27.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–8. doi: 10.1161/01.CIR.0000025404.78001.D8. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Investig. 1996;97:2601–10. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinberg HO, Paradisi G, Cronin J, Crowde K, Hempfling A, Hook G, et al. Type II diabetes abrogates sex differences in endothelial function in premenopausal women. Circulation. 2000;101:2040–6. doi: 10.1161/01.CIR.101.17.2040. [DOI] [PubMed] [Google Scholar]
- 30.Sivitz WI, Wayson SM, Bayless ML, Sinkey CA, Haynes WG. Obesity impairs vascular relaxation in human subjects: hyperglycemia exaggerates adrenergic vasoconstriction arterial dysfunction in obesity and diabetes. J Diabetes Complications. 2007;21:149–57. doi: 10.1016/j.jdiacomp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 31.McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, et al. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:771–6. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- 32.Watts GF, O'Brien SF, Silvester W, Millar JA. Impaired endothelium-dependent and independent dilatation of forearm resistance arteries in men with diet-treated non-insulin-dependent diabetes: role of dyslipidaemia. Clin Sci. 1996;91:567–73. doi: 10.1042/cs0910567. [DOI] [PubMed] [Google Scholar]
- 33.Hogikyan RV, Galecki AT, Pitt B, Halter JB, Greene DA, Supiano MA. Specific impairment of endothelium-dependent vasodilation in subjects with type 2 diabetes independent of obesity. J Clin Endocrinol Metab. 1998;83:1946–52. doi: 10.1210/jcem.83.6.4907. [DOI] [PubMed] [Google Scholar]
- 34.Enderle MD, Benda N, Schmuelling RM, Haering HU, Pfohl M. Preserved endothelial function in IDDM patients, but not in NIDDM patients, compared with healthy subjects. Diabetes Care. 1998;21:271–7. doi: 10.2337/diacare.21.2.271. [DOI] [PubMed] [Google Scholar]
- 35.Mäkimattila S, Liu M-L, Vakkilainen J, Schlenzka A, Lahdenperä S, Syvänne M, et al. Impaired endothelium-dependent vasodilation in type 2 diabetes. Relation to LDL size, oxidized LDL, and antioxidants. Diabetes Care. 1999;22:973–81. doi: 10.2337/diacare.22.6.973. [DOI] [PubMed] [Google Scholar]
- 36.Gazis A, White DJ, Page SR, Cockcroft JR. Effect of oral vitamin E (alpha-tocopherol) supplementation on vascular endothelial function in Type 2 diabetes mellitus. Diabet Med: J Br Diabet Assoc. 1999;16:304–11. doi: 10.1046/j.1464-5491.1999.00049.x. [DOI] [PubMed] [Google Scholar]
- 37.Preik M, Kelm M, Rösen P, Tschöpe D, Strauer BE. Additive effect of coexistent type 2 diabetes and arterial hypertension on endothelial dysfunction in resistance arteries of human forearm vasculature. Angiology. 2000;51:545–54. doi: 10.1177/000331970005100703. [DOI] [PubMed] [Google Scholar]
- 38.Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000;43:1435–8. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 39.Kimura Y, Matsumoto M, Miyauchi E, Deng YB, Iwai K, Hattori H. Noninvasive detection of endothelial dysfunction in elderly with NIDDM by ultrasonography. Echocardiogr. 2001;18:559–64. doi: 10.1046/j.1540-8175.2001.00559.x. [DOI] [PubMed] [Google Scholar]
- 40.van Etten RW, de Koning EJ, Verhaar MC, Gaillard CA, Rabelink TJ. Impaired NO-dependent vasodilation in patients with Type II (non-insulin-dependent) diabetes mellitus is restored by acute administration of folate. Diabetologia. 2002;45:1004–10. doi: 10.1007/s00125-002-0862-1. [DOI] [PubMed] [Google Scholar]
- 41.Vehkavaara S, Yki-Järvinen H. 3.5 years of insulin therapy with insulin glargine improves in vivo endothelial function in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2004;24:325–30. doi: 10.1161/01.ATV.0000113817.48983.c5. [DOI] [PubMed] [Google Scholar]
- 42.Ifrim S, Vasilescu R. Early detection of atherosclerosis in type 2 diabetic patients by endothelial dysfunction and intima-media thickness. Rom J Intern Med = Rev Roum Med interne. 2004;42:343–54. [PubMed] [Google Scholar]
- 43.Natali A, Toschi E, Baldeweg S, Casolaro A, Baldi S, Sironi AM, et al. Haematocrit, type 2 diabetes, and endothelium-dependent vasodilatation of resistance vessels. Eur Heart J. 2005;26:464–71. doi: 10.1093/eurheartj/ehi113. [DOI] [PubMed] [Google Scholar]
- 44.Woodman RJ, Playford DA, Watts GF. Basal production of nitric oxide (NO) and non-NO vasodilators in the forearm microcirculation in Type 2 diabetes: associations with blood pressure and HDL cholesterol. Diabetes Res Clin Pract. 2006;71:59–67. doi: 10.1016/j.diabres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Natali A, Toschi E, Baldeweg S, Ciociaro D, Favilla S, Saccà L, et al. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55:1133–40. doi: 10.2337/diabetes.55.04.06.db05-1076. [DOI] [PubMed] [Google Scholar]
- 46.Karasu C, Soncul H, Altan VM. Effects of non-insulin dependent diabetes mellitus on the reactivity of human internal mammary artery and human saphenous vein. Life Sci. 1995;57:103–12. doi: 10.1016/0024-3205(95)00251-Z. [DOI] [PubMed] [Google Scholar]
- 47.Brooks BA, Franjic B, Ban CR, Swaraj K, Yue DK, Celermajer DS, et al. Diastolic dysfunction and abnormalities of the microcirculation in type 2 diabetes. Diabetes, Obes Metab. 2008;10:739–46. doi: 10.1111/j.1463-1326.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 48.Sokolnicki LA, Strom NA, Roberts SK, Kingsley-Berg SA, Basu A, Charkoudian N. Skin blood flow and nitric oxide during body heating in type 2 diabetes mellitus. J Appl Physiol. 2009;106:566–70. doi: 10.1152/japplphysiol.91289.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green DJ, Dawson EA, Groenewoud HM, Jones H, Thijssen DH. Is flow-mediated dilation nitric oxide mediated?: A meta-analysis. Hypertension. 2014;63:376–82. doi: 10.1161/HYPERTENSIONAHA.113.02044. [DOI] [PubMed] [Google Scholar]
- 50.Goodfellow J, Ramsey MW, Luddington LA, Jones CJ, Coates PA, Dunstan F, et al. Endothelium and inelastic arteries: an early marker of vascular dysfunction in non-insulin dependent diabetes. BMJ (Clin Res Ed) 1996;312:744–5. doi: 10.1136/bmj.312.7033.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Avogaro A, Piarulli F, Valerio A, Miola M, Calveri M, Pavan P, et al. Forearm nitric oxide balance, vascular relaxation, and glucose metabolism in NIDDM patients. Diabetes. 1997;46:1040–6. doi: 10.2337/diab.46.6.1040. [DOI] [PubMed] [Google Scholar]
- 52.Pitei DL, Watkins PJ, Edmonds ME. NO-dependent smooth muscle vasodilatation is reduced in NIDDM patients with peripheral sensory neuropathy. Diabet Med: J Br Diabet Assoc. 1997;14:284–90. doi: 10.1002/(SICI)1096-9136(199704)14:4<284::AID-DIA348>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 53.Goulopoulou S, Hannan JL, Matsumoto T, Ogbi S, Ergul A, Webb RC. Reduced vascular responses to soluble guanylyl cyclase but increased sensitivity to sildenafil in female rats with type 2 diabetes. Am J Physiol Heart Circ Physiol. 2015;309:H297–H304. doi: 10.1152/ajpheart.00079.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goulopoulou S, Hannan JL, Matsumoto T, Ergul A, Webb RC. Augmented dilation to nitric oxide in uterine arteries from rats with type 2 diabetes: implications for vascular adaptations to pregnancy. Am J Physiol Heart Circ Physiol. 2014;306:H610–8. doi: 10.1152/ajpheart.00588.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oniki H, Goto K, Fujii K, Kansui Y, Murakami N, Ohtsubo T, et al. Effects of the superoxide dismutase mimetic tempol on impaired endothelium-dependent and endothelium-independent relaxations in type II diabetic rats. Clin Exp Hypertension. 2013;35:112–9. doi: 10.3109/10641963.2012.702829. [DOI] [PubMed] [Google Scholar]
- 56.Mishra RC, Kyle BD, Kendrick DJ, Svystonyuk D, Kieser TM, Fedak PWM, et al. KCa channel activation normalizes endothelial function in Type 2 Diabetic resistance arteries by improving intracellular Ca(2+) mobilization. Metab: Clin Exp. 2021;114:154390. doi: 10.1016/j.metabol.2020.154390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oniki H, Fujii K, Kansui Y, Goto K, Iida M. Effects of angiotensin II receptor antagonist on impaired endothelium-dependent and endothelium-independent relaxations in type II diabetic rats. J Hypertension. 2006;24:331–8. doi: 10.1097/01.hjh.0000200518.34980.cc. [DOI] [PubMed] [Google Scholar]
- 58.Pannirselvam M, Verma S, Anderson TJ, Triggle CR. Cellular basis of endothelial dysfunction in small mesenteric arteries from spontaneously diabetic (db/db -/-) mice: role of decreased tetrahydrobiopterin bioavailability. Br J Pharmacol. 2002;136:255–63. doi: 10.1038/sj.bjp.0704683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Melo BF, Prieto-Lloret J, Cabral MD, Martins FO, Martins IB, Sacramento JF, et al. Type 2 diabetes progression differently affects endothelial function and vascular contractility in the aorta and the pulmonary artery. Sci Rep. 2021;11:6052. doi: 10.1038/s41598-021-85606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leo CH, Hart JL, Woodman OL. 3',4'-Dihydroxyflavonol restores endothelium-dependent relaxation in small mesenteric artery from rats with type 1 and type 2 diabetes. Eur J Pharmacol. 2011;659:193–8. doi: 10.1016/j.ejphar.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 61.Belin de Chantemèle EJ, Vessières E, Guihot AL, Toutain B, Maquignau M, Loufrani L, et al. Type 2 diabetes severely impairs structural and functional adaptation of rat resistance arteries to chronic changes in blood flow. Cardiovasc Res. 2009;81:788–96. doi: 10.1093/cvr/cvn334. [DOI] [PubMed] [Google Scholar]
- 62.Wang H, Zhou Y, Guo Z, Dong Y, Xu J, Huang H, et al. Sitagliptin attenuates endothelial dysfunction of zucker diabetic fatty rats: implication of the antiperoxynitrite and autophagy. J Cardiovasc Pharm Ther. 2018;23:66–78. doi: 10.1177/1074248417715001. [DOI] [PubMed] [Google Scholar]
- 63.Lu X, Guo X, Karathanasis SK, Zimmerman KM, Onyia JE, Peterson RG, et al. Rosiglitazone reverses endothelial dysfunction but not remodeling of femoral artery in Zucker diabetic fatty rats. Cardiovasc Diabetol. 2010;9:19. doi: 10.1186/1475-2840-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oltman CL, Richou LL, Davidson EP, Coppey LJ, Lund DD, Yorek MA. Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2006;291:H1780–7. doi: 10.1152/ajpheart.01297.2005. [DOI] [PubMed] [Google Scholar]
- 65.Mourmoura E, Vial G, Laillet B, Rigaudière JP, Hininger-Favier I, Dubouchaud H, et al. Preserved endothelium-dependent dilatation of the coronary microvasculature at the early phase of diabetes mellitus despite the increased oxidative stress and depressed cardiac mechanical function ex vivo. Cardiovasc Diabetol. 2013;12:49. doi: 10.1186/1475-2840-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belin de Chantemèle EJ, Vessières E, Guihot AL, Toutain B, Loufrani L, Henrion D. Cyclooxygenase-2 preserves flow-mediated remodelling in old obese Zucker rat mesenteric arteries. Cardiovasc Res. 2010;86:516–25. doi: 10.1093/cvr/cvp411. [DOI] [PubMed] [Google Scholar]
- 67.Vessières E, Belin De Chantemèle EJ, Toutain B, Guihot A-L, Jardel A, Loufrani L, et al. Cyclooxygenase-2 inhibition restored endothelium-mediated relaxation in old obese zucker rat mesenteric arteries. Front Physiol. 2010;1:145. doi: 10.3389/fphys.2010.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qiu S, Mintz JD, Salet CD, Han W, Giannis A, Chen F, et al. Increasing muscle mass improves vascular function in obese (db/db) mice. J Am Heart Assoc. 2014;3:e000854. doi: 10.1161/JAHA.114.000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen K, Pittman RN, Popel AS. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal. 2008;10:1185–98. doi: 10.1089/ars.2007.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wood KC, Cortese-Krott MM, Kovacic JC, Noguchi A, Liu VB, Wang X, et al. Circulating blood endothelial nitric oxide synthase contributes to the regulation of systemic blood pressure and nitrite homeostasis. Arterioscler Thromb Vasc Biol. 2013;33:1861–71. doi: 10.1161/ATVBAHA.112.301068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kashiwagi S, Kajimura M, Yoshimura Y, Suematsu M. Nonendothelial source of nitric oxide in arterioles but not in venules. Circulation Res. 2002;91:e55–e64. doi: 10.1161/01.RES.0000047529.26278.4D. [DOI] [PubMed] [Google Scholar]
- 72.Buchwalow IB, Podzuweit T, Bocker W, Samoilova VE, Thomas S, Wellner M, et al. Vascular smooth muscle and nitric oxide synthase. FASEB J. 2002;16:500–8. doi: 10.1096/fj.01-0842com. [DOI] [PubMed] [Google Scholar]
- 73.Grange RW, Isotani E, Lau KS, Kamm KE, Huang PL, Stull JT. Nitric oxide contributes to vascular smooth muscle relaxation in contracting fast-twitch muscles. Physiol Genomics. 2001;5:35–44. doi: 10.1152/physiolgenomics.2001.5.1.35. [DOI] [PubMed] [Google Scholar]
- 74.Al-Shabrawey M, El-Remessy A, Gu X, Brooks SS, Hamed MS, Huang P, et al. Normal vascular development in mice deficient in endothelial NO synthase: possible role of neuronal NO synthase. Mol Vis. 2003;9:549–58. [PubMed] [Google Scholar]
- 75.Boulanger CM, Heymes C, Benessiano J, Geske RS, Lévy BI, Vanhoutte PM. Neuronal nitric oxide synthase is expressed in rat vascular smooth muscle cells: activation by angiotensin II in hypertension. Circulation Res. 1998;83:1271–8. doi: 10.1161/01.RES.83.12.1271. [DOI] [PubMed] [Google Scholar]
- 76.Wilcox JN, Subramanian RR, Sundell CL, Tracey WR, Pollock JS, Harrison DG, et al. Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Arterioscler Thromb Vasc Biol. 1997;17:2479–88. doi: 10.1161/01.ATV.17.11.2479. [DOI] [PubMed] [Google Scholar]
- 77.Leo F, Suvorava T, Heuser SK, Li J, LoBue A, Barbarino F, et al. Red blood cell and endothelial eNOS independently regulate circulating nitric oxide metabolites and blood pressure. Circulation. 2021;144:870–89. doi: 10.1161/CIRCULATIONAHA.120.049606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghasemi A. Quantitative aspects of nitric oxide production from nitrate and nitrite. EXCLI J. 2022;21:470. doi: 10.17179/excli2022-4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghasemi A, Jeddi S. Quantitative aspects of nitric oxide production in the heart. Mol Biol Rep. 2022:49;11113–22. [DOI] [PubMed]
- 80.Malinski T, Taha Z, Grunfeld S, Patton S, Kapturczak M, Tomboulian P. Diffusion of nitric oxide in the aorta wall monitored in situ by porphyrinic microsensors. Biochem Biophys Res Commun. 1993;193:1076–82. doi: 10.1006/bbrc.1993.1735. [DOI] [PubMed] [Google Scholar]
- 81.Cheah LS, Gwee M, Das R, Ballard H, Yang YF, Daniel EE, et al. Evidence for the existence of a constitutive nitric oxide synthase in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2002;29:725–7. doi: 10.1046/j.1440-1681.2002.03707.x. [DOI] [PubMed] [Google Scholar]
- 82.Melikian N, Seddon MD, Casadei B, Chowienczyk PJ, Shah AM. Neuronal nitric oxide synthase and human vascular regulation. Trends Cardiovasc Med. 2009;19:256–62. doi: 10.1016/j.tcm.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xia N, Horke S, Habermeier A, Closs EI, Reifenberg G, Gericke A, et al. Uncoupling of endothelial nitric oxide synthase in perivascular adipose tissue of diet-induced obese mice. Arteriosclerosis Thrombosis Vasc Biol. 2016;36:78–85. doi: 10.1161/ATVBAHA.115.306263. [DOI] [PubMed] [Google Scholar]
- 84.Withers SB, Simpson L, Fattah S, Werner ME, Heagerty AM. cGMP-dependent protein kinase (PKG) mediates the anticontractile capacity of perivascular adipose tissue. Cardiovasc Res. 2014;101:130–7. doi: 10.1093/cvr/cvt229. [DOI] [PubMed] [Google Scholar]
- 85.Nakladal D, Sijbesma JWA, Visser LM, Tietge UJF, Slart R, Deelman LE, et al. Perivascular adipose tissue-derived nitric oxide compensates endothelial dysfunction in aged pre-atherosclerotic apolipoprotein E-deficient rats. Vasc Pharmacol. 2022;142:106945. doi: 10.1016/j.vph.2021.106945. [DOI] [PubMed] [Google Scholar]
- 86.Nava E, Llorens S. The local regulation of vascular function: from an inside-outside to an outside-inside model. Front Physiol. 2019;10:729. [DOI] [PMC free article] [PubMed]
- 87.Kleschyov AL, Muller B, Schott C, Stoclet JC. Role of adventitial nitric oxide in vascular hyporeactivity induced by lipopolysaccharide in rat aorta. Br J Pharmacol. 1998;124:623–6. doi: 10.1038/sj.bjp.0701916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rassaf T, Bryan NS, Kelm M, Feelisch M. Concomitant presence of N-nitroso and S-nitroso proteins in human plasma. Free Radic Biol Med. 2002;33:1590–6. doi: 10.1016/S0891-5849(02)01183-8. [DOI] [PubMed] [Google Scholar]
- 89.Rassaf T, Preik M, Kleinbongard P, Lauer T, Heiß C, Strauer BE, et al. Evidence for in vivo transport of bioactive nitric oxide in human plasma. J Clin Investig. 2002;109:1241–8. doi: 10.1172/JCI0214995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robinson JM, Lancaster JR., Jr Hemoglobin-mediated, hypoxia-induced vasodilation via nitric oxide: mechanism(s) and physiologic versus pathophysiologic relevance. Am J Respiratory Cell Mol Biol. 2005;32:257–61. doi: 10.1165/rcmb.F292. [DOI] [PubMed] [Google Scholar]
- 91.Ford E, Hughes MN, Wardman P. The reaction of superoxide radicals with S-nitrosoglutathione and the products of its reductive heterolysis. J Biol Chem. 2002;277:2430–6. doi: 10.1074/jbc.M109310200. [DOI] [PubMed] [Google Scholar]
- 92.Singh RJ, Hogg N, Joseph J, Kalyanaraman B. Mechanism of nitric oxide release from S-nitrosothiols. J Biol Chem. 1996;271:18596–603. doi: 10.1074/jbc.271.31.18596. [DOI] [PubMed] [Google Scholar]
- 93.Bohlen HG, Zhou X, Unthank JL, Miller SJ, Bills R. Transfer of nitric oxide by blood from upstream to downstream resistance vessels causes microvascular dilation. Am J Physiol Heart Circ Physiol. 2009;297:H1337–46. doi: 10.1152/ajpheart.00171.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stamler JS, Jaraki O, Osborne J, Simon DI, Keaney J, Vita J, et al. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci. 1992;89:7674–7. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rassaf T, Feelisch M, Kelm M. Circulating NO pool: assessment of nitrite and nitroso species in blood and tissues. Free Radic Biol Med. 2004;36:413–22. doi: 10.1016/j.freeradbiomed.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 96.Chen K, Piknova B, Pittman RN, Schechter AN, Popel AS. Nitric oxide from nitrite reduction by hemoglobin in the plasma and erythrocytes. Nitric Oxide. 2008;18:47–60. doi: 10.1016/j.niox.2007.09.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen K, Pittman RN, Popel AS. Vascular smooth muscle NO exposure from intraerythrocytic SNOHb: a mathematical model. Antioxid Redox Signal. 2007;9:1097–110. doi: 10.1089/ars.2007.1594. [DOI] [PubMed] [Google Scholar]
- 98.Adela R, Nethi SK, Bagul PK, Barui AK, Mattapally S, Kuncha M, et al. Hyperglycaemia enhances nitric oxide production in diabetes: a study from South Indian patients. PLoS ONE. 2015;10:e0125270. doi: 10.1371/journal.pone.0125270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tessari P, Cecchet D, Cosma A, Vettore M, Coracina A, Millioni R, et al. Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy. Diabetes. 2010;59:2152–9. doi: 10.2337/db09-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Avogaro A, Toffolo G, Kiwanuka E, de Kreutzenberg SV, Tessari P, Cobelli C. L-arginine-nitric oxide kinetics in normal and type 2 diabetic subjects: a stable-labelled 15N arginine approach. Diabetes. 2003;52:795–802. doi: 10.2337/diabetes.52.3.795. [DOI] [PubMed] [Google Scholar]
- 101.Salvatore T, Galiero R, Caturano A, Vetrano E, Loffredo G, Rinaldi L, et al. Coronary microvascular dysfunction in diabetes mellitus: pathogenetic mechanisms and potential therapeutic options. Biomedicines. 2022;10:2274. doi: 10.3390/biomedicines10092274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial dysfunction. Hypertension. 1998;31:1047–60. doi: 10.1161/01.HYP.31.5.1047. [DOI] [PubMed] [Google Scholar]
- 103.Bonini MG, Dull RO, Minshall RD. Caveolin-1 regulation of endothelial nitric oxide synthase (eNOS) function and oxidative stress in the endothelium. In: Laher I, editor. Systems biology of free radicals and antioxidants. Berlin, Heidelberg: Springer; 2014. p. 1343–63.
- 104.Haddad D, Al Madhoun A, Nizam R, Al-Mulla F. Role of Caveolin-1 in diabetes and its complications. Oxid Med Cell Longev. 2020;2020:9761539. doi: 10.1155/2020/9761539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Butler M, McKay RA, Popoff IJ, Gaarde WA, Witchell D, Murray SF, et al. Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes. 2002;51:1028–34. doi: 10.2337/diabetes.51.4.1028. [DOI] [PubMed] [Google Scholar]
- 106.Caldwell RB, Zhang W, Rojas M, Bartoli M, El-Remessy AB, Tsai N-T, et al. Decreased nitric oxide bioavailability in diabetic retinopathy: involvement of arginase activity. Investigative Ophthalmol Vis Sci. 2009;50:32. [Google Scholar]
- 107.Kövamees O, Shemyakin A, Checa A, Wheelock CE, Lundberg JO, Östenson C-G, et al. Arginase inhibition improves microvascular endothelial function in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101:3952–8. doi: 10.1210/jc.2016-2007. [DOI] [PubMed] [Google Scholar]
- 108.Faria AM, Papadimitriou A, Silva KC, Lopes de Faria JM, Lopes de Faria JB. Uncoupling endothelial nitric oxide synthase is ameliorated by green tea in experimental diabetes by re-establishing tetrahydrobiopterin levels. Diabetes. 2012;61:1838–47. doi: 10.2337/db11-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kobayashi T, Matsumoto T, Ooishi K, Kamata K. Differential expression of alpha2D-adrenoceptor and eNOS in aortas from early and later stages of diabetes in Goto-Kakizaki rats. Am J Physiol Heart Circ Physiol. 2004;287:H135–43. doi: 10.1152/ajpheart.01074.2003. [DOI] [PubMed] [Google Scholar]
- 110.Halvorson BD, Whitehead SN, McGuire JJ, Wiseman RW, Frisbee JC. Endothelium-dependent impairments to cerebral vascular reactivity with type 2 diabetes mellitus in the Goto-Kakizaki rat. Am J Physiol Regulatory Integr Comp Physiol. 2019;317:R149–r59. doi: 10.1152/ajpregu.00088.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stadler K, Jenei V, von Bölcsházy G, Somogyi A, Jakus J. Increased nitric oxide levels as an early sign of premature aging in diabetes. Free Radic Biol Med. 2003;35:1240–51. doi: 10.1016/S0891-5849(03)00499-4. [DOI] [PubMed] [Google Scholar]
- 112.Gil-Ortega M, Stucchi P, Guzmán-Ruiz RO, Cano V, Arribas S, González MC, et al. Adaptative nitric oxide overproduction in perivascular adipose tissue during early diet-induced obesity. Endocrinology. 2010;151:3299–306. doi: 10.1210/en.2009-1464. [DOI] [PubMed] [Google Scholar]
- 113.Jaap AJ, Tooke JE. Pathophysiology of microvascular disease in non-insulin-dependent diabetes. Clin Sci. 1995;89:3–12. doi: 10.1042/cs0890003. [DOI] [PubMed] [Google Scholar]
- 114.Kazuyama E, Saito M, Kinoshita Y, Satoh I, Dimitriadis F, Satoh K. Endothelial dysfunction in the early- and late-stage type-2 diabetic Goto-Kakizaki rat aorta. Mol Cell Biochem. 2009;332:95–102. doi: 10.1007/s11010-009-0178-2. [DOI] [PubMed] [Google Scholar]
- 115.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–22. doi: 10.1161/01.RES.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 116.De Vriese AS, Stoenoiu MS, Elger M, Devuyst O, Vanholder R, Kriz W, et al. Diabetes-induced microvascular dysfunction in the hydronephrotic kidney: role of nitric oxide. Kidney Int. 2001;60:202–10. doi: 10.1046/j.1523-1755.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- 117.Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, Kimoto M, et al. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation. 2002;106:987–92. doi: 10.1161/01.CIR.0000027109.14149.67. [DOI] [PubMed] [Google Scholar]
- 118.Ren X, Ren L, Wei Q, Shao H, Chen L, Liu N. Advanced glycation end-products decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Cardiovasc Diabetol. 2017;16:52. doi: 10.1186/s12933-017-0531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shatanawi A, Momani MS. Plasma arginase activity is elevated in type 2 diabetic patients. Biomed Res. 2017;28:4102–6. [Google Scholar]
- 120.Diederich D, Skopec J, Diederich A, Dai FX. Endothelial dysfunction in mesenteric resistance arteries of diabetic rats: role of free radicals. Am J Physiol. 1994;266:H1153–61. doi: 10.1152/ajpheart.1994.266.3.H1153. [DOI] [PubMed] [Google Scholar]
- 121.Bitar MS, Wahid S, Mustafa S, Al-Saleh E, Dhaunsi GS, Al-Mulla F. Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur J Pharmacol. 2005;511:53–64. doi: 10.1016/j.ejphar.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 122.Pierce GL. Targeting vascular endothelial cell insulin resistance in type 2 diabetes mellitus. Circulation. 2013;127:16–8. doi: 10.1161/CIRCULATIONAHA.112.150813. [DOI] [PubMed] [Google Scholar]
- 123.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Investig. 2001;108:1341–8. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Omodeo-Sale F, Cortelezzi L, Vommaro Z, Scaccabarozzi D, Dondorp AM. Dysregulation of L-arginine metabolism and bioavailability associated to free plasma heme. Am J Physiol Cell Physiol. 2010;299:C148–54. doi: 10.1152/ajpcell.00405.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ramírez-Zamora S, Méndez-Rodríguez ML, Olguín-Martínez M, Sanchez-Sevilla L, Quintana-Quintana M, García-García N, et al. Increased erythrocytes by-products of arginine catabolism are associated with hyperglycemia and could be involved in the pathogenesis of type 2 diabetes mellitus. PloS ONE. 2013;8:e66823. doi: 10.1371/journal.pone.0066823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mahdi A, Tengbom J, Alvarsson M, Wernly B, Zhou Z, Pernow J. Red blood cell peroxynitrite causes endothelial dysfunction in type 2 diabetes mellitus via arginase. Cells. 2020;9:1712. doi: 10.3390/cells9071712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu T, Schroeder H, Power GG, Blood AB. A physiologically relevant role for NO stored in vascular smooth muscle cells: a novel theory of vascular NO signaling. Redox Biol. 2022;53:102327. doi: 10.1016/j.redox.2022.102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Farah C, Michel LYM, Balligand JL. Nitric oxide signalling in cardiovascular health and disease. Nat Rev Cardiol. 2018;15:292–316. doi: 10.1038/nrcardio.2017.224. [DOI] [PubMed] [Google Scholar]
- 129.Kavdia M, Popel AS. Contribution of nNOS- and eNOS-derived NO to microvascular smooth muscle NO exposure. J Appl Physiol. 2004;97:293–301. doi: 10.1152/japplphysiol.00049.2004. [DOI] [PubMed] [Google Scholar]
- 130.Figueroa XF, Lillo MA, Gaete PS, Riquelme MA, Sáez JC. Diffusion of nitric oxide across cell membranes of the vascular wall requires specific connexin-based channels. Neuropharmacology. 2013;75:471–8. doi: 10.1016/j.neuropharm.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 131.García IE, Sánchez HA, Martínez AD, Retamal MA. Redox-mediated regulation of connexin proteins; focus on nitric oxide. Biochim Biophys Acta (BBA)—Biomembranes. 2018;1860:91–5. doi: 10.1016/j.bbamem.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 132.Wright JA, Richards T, Becker DL. Connexins and diabetes. Cardiol Res Pract. 2012;2012:496904. doi: 10.1155/2012/496904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Looft-Wilson RC, Billaud M, Johnstone SR, Straub AC, Isakson BE. Interaction between nitric oxide signaling and gap junctions: Effects on vascular function. Biochim Biophys Acta (BBA)—Biomembranes. 2012;1818:1895–902. doi: 10.1016/j.bbamem.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vaughn MW, Kuo L, Liao JC. Effective diffusion distance of nitric oxide in the microcirculation. Am J Physiol-Heart Circulatory Physiol. 1998;274:H1705–H14. doi: 10.1152/ajpheart.1998.274.5.H1705. [DOI] [PubMed] [Google Scholar]
- 135.Doctor A, Spinella P. Effect of processing and storage on red blood cell function in vivo. Semin Perinatol. 2012;36:248–59. doi: 10.1053/j.semperi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McMahon TJ, Doctor A. Extrapulmonary effects of inhaled nitric oxide: role of reversible S-nitrosylation of erythrocytic hemoglobin. Proc Am Thorac Soc. 2006;3:153–60. doi: 10.1513/pats.200507-066BG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.James PE, Lang D, Tufnell-Barret T, Milsom AB, Frenneaux MP. Vasorelaxation by red blood cells and impairment in diabetes: reduced nitric oxide and oxygen delivery by glycated hemoglobin. Circulation Res. 2004;94:976–83. doi: 10.1161/01.RES.0000122044.21787.01. [DOI] [PubMed] [Google Scholar]
- 138.Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arteriosclerosis Thrombosis Vasc Biol. 2006;26:697–705. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]
- 139.Pectol DC. Toward the optimization of dinitrosyl iron complexes as therapeutics for smooth muscle. Cells. 2019;16:3178–87. doi: 10.1021/acs.molpharmaceut.9b00389. [DOI] [PubMed] [Google Scholar]
- 140.Li S, Whorton AR. Functional characterization of two S-nitroso-L-cysteine transporters, which mediate movement of NO equivalents into vascular cells. Am J Physiol Cell Physiol. 2007;292:C1263–71. doi: 10.1152/ajpcell.00382.2006. [DOI] [PubMed] [Google Scholar]
- 141.Brahmajothi MV, Sun NZ, Auten RL. S-nitrosothiol transport via PEPT2 mediates biological effects of nitric oxide gas exposure in macrophages. Am J Respiratory Cell Mol Biol. 2013;48:230–9. doi: 10.1165/rcmb.2012-0305OC. [DOI] [PubMed] [Google Scholar]
- 142.Kubaszewski E, Peters A, McClain S, Bohr D, Malinski T. Light-activated release of nitric oxide from vascular smooth muscle of normotensive and hypertensive rats. Biochem Biophys Res Commun. 1994;200:213–8. doi: 10.1006/bbrc.1994.1436. [DOI] [PubMed] [Google Scholar]
- 143.Alencar JL, Chalupsky K, Sarr M, Schini-Kerth V, Vanin AF, Stoclet JC, et al. Inhibition of arterial contraction by dinitrosyl-iron complexes: critical role of the thiol ligand in determining rate of nitric oxide (NO) release and formation of releasable NO stores by S-nitrosation. Biochem Pharmacol. 2003;66:2365–74. doi: 10.1016/j.bcp.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 144.Ilangovan G, Khaleel SA, Kundu T, Hemann C, El-Mahdy MA, Zweier JL. Defining the reducing system of the NO dioxygenase cytoglobin in vascular smooth muscle cells and its critical role in regulating cellular NO decay. J Biol Chem. 2021;296:100196. doi: 10.1074/jbc.RA120.016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mahgoup EM, Khaleel SA, El-Mahdy MA, Abd-Allah AR, Zweier JL. Role of cytoglobin in cigarette smoke constituent-induced loss of nitric oxide bioavailability in vascular smooth muscle cells. Nitric Oxide. 2022;119:9–18. doi: 10.1016/j.niox.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zweier JL, Ilangovan G. Regulation of nitric oxide metabolism and vascular tone by cytoglobin. Antioxid Redox Signal. 2020;32:1172–87. doi: 10.1089/ars.2019.7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li H, Hemann C, Abdelghany TM, El-Mahdy MA, Zweier JL. Characterization of the mechanism and magnitude of cytoglobin-mediated nitrite reduction and nitric oxide generation under anaerobic conditions. J Biol Chem. 2012;287:36623–33. doi: 10.1074/jbc.M112.342378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ormerod JOM, Ashrafian H, Maher AR, Arif S, Steeples V, Born GVR, et al. The role of vascular myoglobin in nitrite-mediated blood vessel relaxation. Cardiovasc Res. 2010;89:560–5. doi: 10.1093/cvr/cvq299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Roy A, Sil R, Chakraborti AS. Non-enzymatic glycation induces structural modifications of myoglobin. Mol Cell Biochem. 2010;338:105–14. doi: 10.1007/s11010-009-0343-7. [DOI] [PubMed] [Google Scholar]
- 150.Marletta MA. Revisiting nitric oxide signaling: where was it, and where is it going? Biochemistry. 2021;60:3491–96. [DOI] [PMC free article] [PubMed]
- 151.Gusarov I, Nudler E. Protein S-nitrosylation: enzymatically controlled, but intrinsically unstable, post-translational modification. Mol Cell. 2018;69:351–3. doi: 10.1016/j.molcel.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 152.Resta TC. Hypoxic regulation of nitric oxide signaling in vascular smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2003;285:L293–5. doi: 10.1152/ajplung.00133.2003. [DOI] [PubMed] [Google Scholar]
- 153.Hall CN, Garthwaite J. What is the real physiological NO concentration in vivo? Nitric Oxide. 2009;21:92–103. doi: 10.1016/j.niox.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol. 2000;184:409–20. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 155.Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol. 2001;91:1421–30. doi: 10.1152/jappl.2001.91.3.1421. [DOI] [PubMed] [Google Scholar]
- 156.Mergia E, Stegbauer J. Role of phosphodiesterase 5 and Cyclic GMP in hypertension. Curr hypertension Rep. 2016;18:39. doi: 10.1007/s11906-016-0646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Miyoshi H, Nakaya Y, Moritoki H. Nonendothelial-derived nitric oxide activates the ATP-sensitive K+ channel of vascular smooth muscle cells. FEBS Lett. 1994;345:47–9. doi: 10.1016/0014-5793(94)00417-X. [DOI] [PubMed] [Google Scholar]
- 158.Ibarra-Alvarado C, Galle J, Melichar VO, Mameghani A, Schmidt HH. Phosphorylation of blood vessel vasodilator-stimulated phosphoprotein at serine 239 as a functional biochemical marker of endothelial nitric oxide/cyclic GMP signaling. Mol Pharm. 2002;61:312–9. doi: 10.1124/mol.61.2.312. [DOI] [PubMed] [Google Scholar]
- 159.Banday AA, Lokhandwala MF. Oxidative stress impairs cGMP-dependent protein kinase activation and vasodilator-stimulated phosphoprotein serine-phosphorylation. Clin Exp hypertension. 2019;41:5–13. doi: 10.1080/10641963.2018.1433197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aszódi A, Pfeifer A, Ahmad M, Glauner M, Zhou XH, Ny L, et al. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO J. 1999;18:37–48. doi: 10.1093/emboj/18.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang X, Pluznick JL, Settles DC, Sansom SC. Association of VASP with TRPC4 in PKG-mediated inhibition of the store-operated calcium response in mesangial cells. Am J Physiol Ren Physiol. 2007;293:F1768–76. doi: 10.1152/ajprenal.00365.2007. [DOI] [PubMed] [Google Scholar]
- 162.Zheng H, Wu J, Jin Z, Yan LJ. Protein modifications as manifestations of hyperglycemic glucotoxicity in diabetes and its complications. Biochem Insights. 2016;9:1–9. doi: 10.4137/BCI.S36141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kruszelnicka O. Nitric oxide vs insulin secretion, action and clearance. Diabetologia. 2014;57:257–8. doi: 10.1007/s00125-013-3082-y. [DOI] [PubMed] [Google Scholar]
- 164.Mannick JB, Schonhoff CM. Nitrosylation: the next phosphorylation? Arch Biochem Biophys. 2002;408:1–6. doi: 10.1016/S0003-9861(02)00490-3. [DOI] [PubMed] [Google Scholar]
- 165.Lin L, Xu C, Carraway MS, Piantadosi CA, Whorton AR, Li S. RhoA inactivation by S-nitrosylation regulates vascular smooth muscle contractive signaling. Nitric Oxide. 2018;74:56–64. doi: 10.1016/j.niox.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 166.Dalle-Donne I, Milzani A, Giustarini D, Di Simplicio P, Colombo R, Rossi R. S-NO-actin: S-nitrosylation kinetics and the effect on isolated vascular smooth muscle. J muscle Res Cell Motil. 2000;21:171–81. doi: 10.1023/A:1005671319604. [DOI] [PubMed] [Google Scholar]
- 167.Witte K, Jacke K, Stahrenberg R, Arlt G, Reitenbach I, Schilling L, et al. Dysfunction of soluble guanylyl cyclase in aorta and kidney of Goto-Kakizaki rats: influence of age and diabetic state. Nitric Oxide. 2002;6:85–95. doi: 10.1006/niox.2001.0363. [DOI] [PubMed] [Google Scholar]
- 168.Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Investig. 1991;87:432–8. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Patel H, Chen J, Das KC, Kavdia M. Hyperglycemia induces differential change in oxidative stress at gene expression and functional levels in HUVEC and HMVEC. Cardiovasc Diabetol. 2013;12:142. doi: 10.1186/1475-2840-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Potdar S, Kavdia M. NO/peroxynitrite dynamics of high glucose-exposed HUVECs: chemiluminescent measurement and computational model. Microvasc Res. 2009;78:191–8. doi: 10.1016/j.mvr.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circulation Res. 2017;120:713–35. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 172.Sayed N, Baskaran P, Ma X, van den Akker F, Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc Natl Acad Sci USA. 2007;104:12312–7. doi: 10.1073/pnas.0703944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Witte K, Hachenberger J, Castell MF, Vahl CF, Haller C. Nitric oxide-sensitive soluble guanylyl cyclase activity is preserved in internal mammary artery of type 2 diabetic patients. Diabetes. 2004;53:2640–4. doi: 10.2337/diabetes.53.10.2640. [DOI] [PubMed] [Google Scholar]
- 174.Shah RC, Sanker S, Wood KC, Durgin BG, Straub AC. Redox regulation of soluble guanylyl cyclase. Nitric Oxide. 2018;76:97–104. doi: 10.1016/j.niox.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stuehr DJ, Misra S, Dai Y, Ghosh A. Maturation, inactivation, and recovery mechanisms of soluble guanylyl cyclase. J Biol Chem. 2021;296:100336. doi: 10.1016/j.jbc.2021.100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schrammel A, Behrends S, Schmidt K, Koesling D, Mayer B. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol Pharm. 1996;50:1–5. [PubMed] [Google Scholar]
- 177.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, H S AK, Meurer S, et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Investig. 2006;116:2552–61. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rybalkin SD, Rybalkina IG, Feil R, Hofmann F, Beavo JA. Regulation of cGMP-specific phosphodiesterase (PDE5) phosphorylation in smooth muscle cells. J Biol Chem. 2002;277:3310–7. doi: 10.1074/jbc.M106562200. [DOI] [PubMed] [Google Scholar]
- 179.Liu S, Ma X, Gong M, Shi L, Lincoln T, Wang S. Glucose down-regulation of cGMP-dependent protein kinase I expression in vascular smooth muscle cells involves NAD(P)H oxidase-derived reactive oxygen species. Free Radic Biol Med. 2007;42:852–63. doi: 10.1016/j.freeradbiomed.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 180.Bohlen HG. Nitric oxide and the cardiovascular system. Compr Physiol. 2015;5:808–23. doi: 10.1002/cphy.c140052. [DOI] [PubMed] [Google Scholar]
- 181.Houghton JL, Davison CA, Kuhner PA, Torossov MT, Strogatz DS, Carr AA. Heterogeneous vasomotor responses of coronary conduit and resistance vessels in hypertension. J Am Coll Cardiol. 1998;31:374–82. doi: 10.1016/S0735-1097(97)00505-6. [DOI] [PubMed] [Google Scholar]
- 182.Clark SG, Fuchs LC. Role of nitric oxide and Ca++-dependent K+ channels in mediating heterogeneous microvascular responses to acetylcholine in different vascular beds. J Pharmacol Exp Therapeutics. 1997;282:1473–9. [PubMed] [Google Scholar]
- 183.Gao Y, Tolsa J-F, Raj JU. Heterogeneity in endothelium-derived nitric oxide-mediated relaxation of different sized pulmonary arteries of newborn lambs. Pediatr Res. 1998;44:723–9. doi: 10.1203/00006450-199811000-00015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.