Abstract
Background
One emergency that occurs frequently and has high morbidity is carbon monoxide poisoning. After a time of lucidity, some victims who recover from the incident of acute carbon monoxide exposure may later suffer delayed neuropsychiatric sequelae (DNS), which can include cognitive impairments, a wide range of neurological abnormalities, and affective disorders. Below, we report the first documented incident of DNS after carbon monoxide poisoning in Ethiopia.
Case presentation
A 40-year-old male patient who developed a coma after he was exposed to burning charcoal in a closed room was admitted to Debre Tabor Comprehensive Specialized Hospital in the northwest region of Ethiopia. He stayed for 12 days during his first admission and was discharged with improvement. One month after discharge, he developed delayed neuropsychiatric sequelae, which manifested as bizarre behavior, being unable to walk unsupported, loss of concentration, and failure to control urine and feces. Brain MRI showed bilateral periventricular and frontoparietal deep white matter subcortical abnormal T1 and T2 prolongation with no restriction of diffusivity, possibly due to demyelinating disorders. He was managed with fluid therapy, benzhexol, risperidone, and rehabilitation therapy. He stayed for two weeks in our hospital and was discharged with improvement.
Conclusion
Despite the paucity of delayed neuropsychiatric sequelae from carbon monoxide poisoning, physicians should be highly suspicious in the right situations. The precise mechanisms of delayed toxic effects, prevention measures, and treatment modalities have not yet been determined; therefore, more research and attention are required.
Keywords: Ethiopia, Delayed neuropsychiatric sequelae, Carbon monoxide poisoning
Graphical Abstract
Highlights
-
•
Delayed neuropsychiatric sequelae from carbon monoxide poisoning are a rare occurrence.
-
•
Due to the diagnostic dilemma of delayed neuropsychiatric sequelae, physicians should be highly suspicious.
-
•
The precise mechanisms of delayed toxic effects, prevention measures, and treatment modalities have not yet been determined.
1. Background
Inhalation of carbon monoxide (CO), a toxic colorless gas, is one of the main reasons for emergency department (ED) visits around the world. Because the symptoms of CO poisoning are vague, they often go unrecognized and unreported, and the actual diagnosis is difficult for doctors to make. Therefore, the true incidence is generally understated. Every year, it causes more than 50,000 admissions to emergency rooms in the United States [1]. Parkinsonism, cortical blindness, mutism, chorea, choreoathetosis, hemiplegia, and peripheral neuropathy are delayed, rare manifestations of CO poisoning. Occasionally, patients who seem to fully recover subsequently show symptoms and physical evidence of brain injury to the white matter. In almost all instances, this delayed syndrome is characterized by apathy, disorientation, amnesia, and hypokinesia. Less frequently seen symptoms include apraxia, irritability, increased distractibility, urinary and/or fecal incontinence, and behavioral abnormalities [2]. Even though DNS is one of the clinical signs of CO poisoning, making the diagnosis in a clinical setting is challenging. Nevertheless, even in the international literature, delayed neurological sequelae from CO poisoning remain a rare occurrence [3]. We provide the first case report of delayed neurological sequelae from CO poisoning in Ethiopia.
2. Case presentation
A 40-year-old male patient presented to the emergency department of Debre Tabor Comprehensive Specialized Hospital in the northwest region of Ethiopia after a 6-hour loss of consciousness. He was found unconscious by his neighbors in a closed room. He was with his sister, who was found dead in the same room. There was burning charcoal inside the room, which they used for cooking purposes.
On arrival, his blood pressure was 120/80 mmHg, his pulse rate was 140 beats per minute, his respiratory rate was 36 per minute, his blood carboxyhemoglobin (COHb) was 52%, and his point of care glucose was 123 mg/dl. His Glasgow Coma Scale (GCS) was 3/15. Pupils were dilated bilaterally but reactive. Immediately after arrival, he was put on a 15 L/min face mask of oxygen with a partial rebreather mask and maintenance fluid, and he was intubated for an unprotected airway due to low GCS. Hyperbaric oxygen therapy was not performed since it was not available in our setup. Then, he was transferred to the medical Intensive Care Unit (MICU) to continue supportive treatment.
Subsequently, his consciousness improved, and on the 9th day of MICU admission, he was extubated and transferred to the medical ward for observation. He stayed at the medical ward for 3 days and was discharged with improvement after 12 days of hospitalization. His laboratory results were grossly normal, except his liver enzymes were elevated, which later normalized as summarized below (Table 1).
Table 1.
Laboratory, ECG, and chest X-ray results during the stay at Debre Tabor Comprehensive Specialized Hospital from September 11 to September 23, 2022.
| Investigations | 1st day | 2nd day | 5th day | 10th day | Reference value |
|---|---|---|---|---|---|
| White blood cell | 9.79 * 103 | ---------- | 9.87 * 103 | 13.97 * 103 | 4.5–11 * 103 /Ul |
| Hemoglobin | 16 g/dl | ---------- | 15.9 | 14.3 | 13.5–17.5 gm/dl |
| Platelets | 150 * 103 | ---------- | 189 * 103 | 139 * 103 | 150–350 * 103/uL |
| Urine analysis | Non-revealing | ---------- | --------- | ----------- | |
| Serum sodium | 141 mmol/L | ---------- | 141 | ----------- | 136–145 mmol/L |
| Potassium | 4.4 mmol/L | ---------- | 3.4 | ----------- | 3.5–5.1 mmol/L |
| Chloride | 107 mmol/L | ---------- | 107 | ----------- | 98–107 mmol/L |
| Calcium | 7.8 mg/dl | ---------- | 7.2 | ----------- | 8.5–10.1 mg/dl |
| Magnesium | 3 mg/dl | ---------- | 2.2 | ----------- | 1.8–2.4 mg/dl |
| BUN | 45 mg/dl | ---------- | 20 | ----------- | 7–18 mg/dl |
| Creatinine | 1.2 mg/dl | --------- | 0.91 | ---------- | 0.55–1.3 mg/dl |
| AST | 159 | ---------- | 198 | 87 | 15–37 U/L |
| ALT | 81 | ---------- | 134 | 68 | 14–63 U/L |
| PT | 12.4 | ----------- | --------- | ---------- | 11–13.5 s |
| aPTT | 26.6 | ----------- | --------- | ---------- | 25–40 s |
| INR | 1.3 | ----------- | ---------- | ---------- | 2–3 |
| Troponin | --------- | 0.1 | ---------- | ---------- | 0.1–0.3 ng/ml |
| ECG | Normal | Normal | Normal | Normal | |
| Chest x-ray | Normal | --------- | ---------- | ----------- |
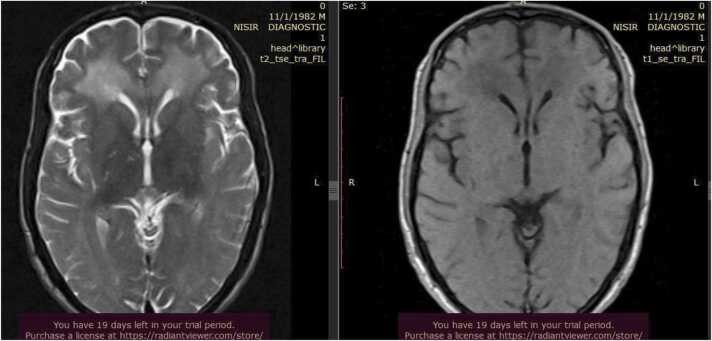
One month after discharge, he developed bizarre behavior, was unable to walk unsupported, lost concentration, and failed to control urine and feces for 3 days. He was doing his routine activities before these complaints, but this time he was unable to perform his daily routines. For the above complaints, he visited our emergency department. On arrival, his vital signs were: blood pressure 110/80 mmHg, pulse rate 88, respiratory rate 24, temperature 36.3 °C, saturation of oxygen 95% with room air, and random blood glucose 140 mg/dl. His GCS was 13/15 (E = 4, V = 5, M = 4). Pupils were dilated and sluggish bilaterally. On motor examination, he had a continuous repetitive movement of the extremities, and strength was reduced to 3/5 on the lower extremities. This time, a brain MRI was sent (Fig. 1), and it showed bilateral periventricular and frontoparietal deep white matter subcortical abnormal T1 and T2 prolongation with no restriction of diffusivity, possibly due to demyelinating disorders, with no significant change seen on the ADC map (Fig. 2). There was no significant mass effect. On the post-contrast scan, there was no significant enhancement. The rest of the cerebral structures, cerebellum, and vascular structures appear normal.
Fig. 1.
Brain MRI showing bilateral periventricular and frontoparietal deep white matter subcortical abnormal T1 and T2 prolongation with no restriction of diffusivity due to demyelinating disorders.
Fig. 2.
Brain MRI showing bilateral periventricular and frontoparietal deep white matter subcortical T2 high signal intensity and T1 low signal intensity lesions with no significant difference seen on the ADC map.
Considering his clinical presentation and MRI findings, delayed neuropsychiatric sequelae due to post-anoxic brain injury were entertained. He was managed with fluid therapy, benzhexol, risperidone, and rehabilitation therapy. But later on, as his clinical manifestations improved, he only continued with rehabilitation therapy. He stayed for two weeks in our hospital and was discharged with improvement. Upon discharge, he was advised to continue rehabilitation and to have regular follow-ups at the nearby hospital.
3. Discussion
Carbon monoxide poisoning results from breathing in a toxic, colorless gas that damages organs by depriving cells of oxygen, which causes a variety of systemic and neurological issues and eventually death [4]. Gas heaters, furnaces, water heaters, wood- or charcoal-burning stoves, and kerosene heaters are typical sources of CO poisoning. Other common sources are house fires, poorly aired cars, and gas heaters. In our situation, CO was released into the air by charcoal being burned in a closed space inside the victim's shelter.
The clinical manifestation of CO poisoning mimics other prevalent ailments, making it very difficult to identify unless suspected. The diagnosis is therefore commonly missed. Moreover, patients' symptoms may be ambiguous, fluctuating, and generic, making a diagnosis more challenging [5], [6], [7].
The clinical course of CO poisoning can be monophasic or biphasic. In the biphasic course, individuals may experience delayed neurological sequelae following an apparent normal period of lucidity (from days to weeks, typically within one month) [4], [8], [9]. A study conducted in Korea found that 75 patients out of 2759 cases experienced DNS [4]. Most people who suffer from acute CO poisoning fully recover without any further complications, while between 10% and 30% of sufferers are readmitted to a facility with DNS [8], [10]. Thus, DNS is a serious issue for those who survived acute CO poisoning. Our patient, who suffered delayed neurological consequences, experienced a biphasic clinical course. Before experiencing delayed neurological effects, this patient experienced over a month of lucidity.
Compared to oxygen, CO has a far higher affinity for hemoglobin; it attaches to hemoglobin 240 times more strongly than oxygen does. When exposed to CO, there is a competitive coupling of CO to hemoglobin, which in turn lowers the hemoglobin's ability to deliver oxygen. Because of this, the oxygen-hemoglobin dissociation curve shifts to the left, impairing oxygen release at the tissue level and resulting in hypoxic stress at the cellular level [11].
Although cellular hypoxia is one of the channels by which CO poisoning induces an aberrant inflammatory response, the precise pathogenic mechanism of CO's acute toxicity and, notably, the delayed toxic effects and subsequent DNS are still poorly understood [12]. Further research is thus required to close the gaps in our understanding of the pathophysiological mechanisms behind the acute and long-term effects of CO poisoning.
DNS can present with a wide range of neurological abnormalities, including Parkinsonism, fecal and urine incontinence, confusion, cognitive difficulties, and psychosis [8], [12]. The deep white matter and periventricular regions exhibit hyperintense signals by brain MRI in CO poisoning [13]. Our patient's diagnosis of delayed neuropsychiatric sequelae due to carbon monoxide toxicity was determined in light of the patient's medical history, physical examination results, and brain MRI findings.
Even though a few factors have been linked to DNS, the development of DNS has a weak correlation with the victim's initial clinical presentation. Older age, duration of CO exposure, longer time to treatment, brief loss of consciousness, coma, and increased serum levels of neuron-specific enolase (NSE) are some of the factors that have been shown to predict the development of DNS [8], [10], [14]. Hence, in our case, loss of consciousness, coma, and a prolonged time to therapy can be seen as indicators of DNS.
While DNS had no known treatment [15], we managed his condition with hydration therapy, benzhexol, risperidone, and physical therapy. But, as time went on and his clinical symptoms became better, he merely proceeded with rehabilitation therapy.
We learned from the extensive literature review that although CO poisoning is common, the precise mechanisms of acute and delayed toxic effects, preventive measures, and suggested treatments have not yet been fully understood [16], [17]. Despite the paucity of delayed neuropsychiatric sequelae from carbon monoxide poisoning, physicians should be highly suspicious in the right situations.
The reporting of such cases is crucial for improving our knowledge of the toxicities related to CO poisoning. Also, it might contribute to raising awareness among the general public and medical professionals, which would ultimately contribute to a decrease in the prevalence and complications of CO poisoning.
Ethical approval and consent to participate
Our institution's research review board waived ethical approval for this study. Because the study of the case reports is exempt from ethical approval in circumstances where the patient gives consent or a guarantee, we received written informed consent from the patient, and, upon request, a copy of that consent will be provided.
Funding
Not applicable.
CRediT authorship contribution statement
B.D.K., N.M., M.W.K., and A.A.Y. analyzed and interpreted patient data, conducted a literature review, and were involved in writing the original draft. B. D. K., Y. G. K., and Y. F. M. gathered the materials, assisted the other authors in reporting this case, and edited the final draft. Y.F.M. interpreted the MRI of the brain. The final paper was read and approved by all authors, who were also involved throughout the patient's care.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
All authors would like to thank the patient for kindly agreeing to have his case published as a case report.
Consent for publication
Our patient offered his written permission for the publication of this case report and any related images. On request, a copy of the written informed consent will be provided.
Handling Editor: Prof. L.H. Lash
Data Availability
Data will be made available on request.
References
- 1.Hampson N.B., Weaver L.K. Carbon monoxide poisoning: a new incidence for an old disease. Undersea Hyperb. Med J. Undersea Hyperb. Med Soc. Inc. 2007;34(3):163–168. [PubMed] [Google Scholar]
- 2.Bateman D.N. Carbon Monoxide. Medicine. 2003;31(10):41–42. Oct 1. [Google Scholar]
- 3.Kumarihamy P., Kularatne S.A.M., Pathirage M., Gunaratne W.M.S.N., Waduge R. A case of delayed neurological manifestation following carbon monoxide poisoning in Sri Lanka: epidemiology of exposure and literature review. BMC Pharm. Toxicol. 2019;20(1):17. doi: 10.1186/s40360-019-0295-9. Apr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi I.S. Carbon monoxide poisoning: systemic manifestations and complications. J. Korean Med Sci. 2001;16(3):253–261. doi: 10.3346/jkms.2001.16.3.253. (Jun) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong J.R., Hou S.W., Shu H.T., Chen H.T., Chong C.F., Chong C.F. Diagnostic pitfall: carbon monoxide poisoning mimicking hyperventilation syndrome. Am. J. Emerg. Med. 2005;23(7):903–904. doi: 10.1016/j.ajem.2005.07.008. (Nov) [DOI] [PubMed] [Google Scholar]
- 6.Balzan M.V., Agius G., Galea, Debono A. Carbon monoxide poisoning: easy to treat but difficult to recognize. Post. Med J. 1996;72(850):470–473. doi: 10.1136/pgmj.72.850.470. (Aug) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright J. Chronic and occult carbon monoxide poisoning: we don’t know what we’re missing. Emerg. Med J. EMJ. 2002;19(5):386–390. doi: 10.1136/emj.19.5.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi I.S. Delayed neurologic sequelae in carbon monoxide intoxication. Arch. Neurol. 1983;40(7):433–435. doi: 10.1001/archneur.1983.04050070063016. [DOI] [PubMed] [Google Scholar]
- 9.Adir Y., Bentur Y., Melamed Y. [Hyperbaric oxygen for neuropsychiatric sequelae of carbon monoxide poisoning] Harefuah. 1992;122(9) [PubMed] [Google Scholar]
- 10.Thom S.R., Taber R.L., Mendiguren I.I., Clark J.M., Hardy K.R., Fisher A.B. Delayed neuropsychologic sequelae after carbon monoxide poisoning: prevention by treatment with hyperbaric oxygen. Ann. Emerg. Med. 1995;25(4):474–480. doi: 10.1016/s0196-0644(95)70261-x. (Apr) [DOI] [PubMed] [Google Scholar]
- 11.Roughton F.J.W., Darling R.C. The effect of carbon monoxide on the oxyhemoglobin dissociation curve. Am. J. Physiol. -Leg. Content. 1944;141(1):17–31. [Google Scholar]
- 12.Ernst A., Zibrak J.D. Carbon monoxide poisoning. N. Engl. J. Med. 1998;339(22):1603–1608. doi: 10.1056/NEJM199811263392206. Nov 26. [DOI] [PubMed] [Google Scholar]
- 13.Kim H.Y., Kim B.J., Moon S.Y., Kwon J.C., Shon Y.M., Na D.G., et al. Serial diffusion-weighted MR Imaging in delayed postanoxic encephalopathy. A case study. J Neuroradiol. 2002;29(3):211–215. [PubMed] [Google Scholar]
- 14.Carbon Monoxide | Goldfrank’s Toxicologic Emergencies, 11e | AccessPharmacy | McGraw Hill Medical [Internet]. [cited 2023 Feb 27]. Available from: https://accesspharmacy.mhmedical.com/content.aspx?bookid=2569§ionid=210264419
- 15.Choi I.S. Parkinsonism after carbon monoxide poisoning. Eur. Neurol. 2002;48(1):30–33. doi: 10.1159/000064954. [DOI] [PubMed] [Google Scholar]
- 16.L.M. Yee, G.K. Brandon. Successful reversal of presumed carbon monoxide-induced semicoma - PubMed [Internet]. [cited 2023 Feb 27]. Available from: https://pubmed.ncbi.nlm.nih.gov/6882333/. [PubMed]
- 17.Iwamoto K., Ikeda K., Mizumura S., Tachiki K., Yanagihashi M., Iwasaki Y. Combined treatment of methylprednisolone pulse and memantine hydrochloride prompts recovery from neurological dysfunction and cerebral hypoperfusion in carbon monoxide poisoning: a case report. J. Stroke Cereb. Dis. J. Natl. Stroke Assoc. 2014;23(3):592–595. doi: 10.1016/j.jstrokecerebrovasdis.2013.05.014. (Mar) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.