Abstract
Amyloid-beta (Aβ) is produced from amyloid precursor protein (APP) primarily after APP is internalised by endocytosis and clathrin-mediated endocytic processes are altered in Alzheimer’s disease (AD). There is also evidence that cholesterol and flotillin affect APP endocytosis. We hypothesised that endocytic protein expression would be altered in the brains of people with AD compared to non-diseased subjects which could be linked to increased Aβ generation. We compared protein expression in frontal cortex samples from men with AD compared to age-matched, non-diseased controls. Soluble and insoluble Aβ40 and Aβ42, the soluble Aβ42/Aβ40 ratio, βCTF, BACE1, presenilin-1 and the ratio of phosphorylated:total GSK3β were significantly increased while the insoluble Aβ42:Aβ40 ratio was significantly decreased in AD brains. Total and phosphorylated tau were markedly increased in AD brains. Significant increases in clathrin, AP2, PICALM isoform 4, Rab-5 and caveolin-1 and 2 were seen in AD brains but BIN1 was decreased. However, using immunohistochemistry, caveolin-1 and 2 were decreased. The results obtained here suggest an overall increase in endocytosis in the AD brain, explaining, at least in part, the increased production of Aβ during AD.
Keywords: Alzheimer’s disease, Amyloid precursor protein, Amyloid-beta, Endocytosis, Human brain, Male sex
Introduction
Alzheimer’s Disease (AD) is the most common form of dementia, accounting for 60–80% of diagnoses [6]. There are two main types, late-onset or sporadic AD, accounting for about 90–95% of cases where the causes are still not understood and early onset AD, with a significant genetic component [72]. There are a number of risk factors for developing AD including age and associated conditions, environment and being female [6].
Two proteins are key to the pathogenesis of AD, amyloid-beta (Aβ) and tau. Amyloid precursor protein (APP) is metabolised by either the amyloidogenic or non-amyloidogenic pathways. In the amyloidogenic pathway, APP is cleaved by β-secretase APP-cleaving enzyme (BACE1) to form soluble APPβ (sAPPβ) and an intracellular carboxy-terminal fragment (βCTF) [15], [29]). γ-Secretase then cleaves βCTF to produce various fragments of Aβ and a C-terminal intracellular fragment [15]. Aβ peptides range from 37 to 49 amino acids [8] with the most abundant isoforms being Aβ40 and Aβ42 [70]. The non-amyloidogenic pathway involves the α-secretase enzyme which metabolises APP within the Aβ region to release soluble APPα (sAPPα) precluding the production of Aβ [2]. Once produced, Aβ aggregates to form soluble oligomeric assemblies, leading to neuroinflammation, tau hyperphosphorylation and neurodegeneration according to the amyloid hypothesis [1], [30]. Aβ aggregates then form extracellular insoluble plaques [24]. Tau is a microtubule protein and essential for normal transport in neurones and neuronal microtubules stabilisation [36]. In AD, hyperphosphorylated tau accumulates as intracellular neurofibrillary tangles (NFTs) in neurones leading to cell death [36].
Aβ is primarily produced after APP has undergone endocytosis [78]. Changes in endocytosis are one of the earliest reported abnormalities in AD [73] and inducing endosomal dysfunction by overexpression of Rab5 independently of APP recapitulates changes seen in AD underlying the importance of changes in endocytosis to the disease process [57]. The majority of studies in AD have focused on clathrin-mediated endocytosis (CME) as this is the best understood endocytic pathway [61]. However, the expression of proteins involved in clathrin-independent endocytosis (CIE) such as caveolins and flotillins is increased in AD brains [21], [23], [56]. Flotillin-1 and −2, highly expressed in neurones, appear to establish their own CIE pathway [5], [50]. Flotillin-2, a lipid raft protein, promoted the clustering of APP at the cell surface which increased its endocytosis and the interaction of flotillin with APP was dependent on cholesterol [69]. Cholesterol also promoted the clustering of APP and BACE1 in lipid rafts leading to endocytosis and Aβ production [48]. Caveolae and caveolins were suggested to be important for α-secretase cleavage of APP in non-neuronal cells [34]. Caveolin-1 has also been proposed to have various roles in neurones independent of caveolae including in CIE [32], [71]. Various GWAS have identified several genes of small risk involved in endocytic trafficking including PICALM, BIN1, CD2AP and SORLA which have been shown to affect APP endocytosis and Aβ production [26]. In addition, endocytosis has been found to contribute 19.2% to the total polygenic risk of AD [74]. In our previous study, we showed that ageing affects the expression of several endocytic proteins involved in CME or CIE in human male cortical brain samples [3]. We saw significant increases in clathrin, dynamin-1, AP180, Rab-5, caveolin-2 and flotillin-2 with ageing. Our results suggest that ageing may cause up-regulation of endocytosis which could increase APP internalisation and Aβ generation in the ageing human brain leading to an increased development of AD. This idea is supported by data showing upregulation of APP endocytosis in aged primary neurones and the suggestion that this is an important mechanism contributing to brain ageing and the subsequent development of AD [10]. Having seen changes in endocytic proteins with ageing, we then wanted to understand how the presence of AD affected endocytic protein expression. We hypothesised that we would also see changes in endocytic protein expression in samples from people with AD compared to non-diseased control subjects as the increased endocytosis could be involved in increased Aβ generation and hence in the progression of the disease. We have investigated this hypothesis here using brain samples from male AD patients and age-matched non-diseased subjects to examine the expression of a range of endocytic proteins involved in CME and CIE and metabolites of APP. Our data provide evidence of wide-ranging changes in several endocytic proteins during AD, suggesting that our hypothesis is indeed correct and that alterations in the process of endocytosis could underlie the development of AD.
Materials and methods
Human brain samples
Fresh frozen human frontal cortex brain samples and 6 μm formalin-fixed, paraffin-embedded sections from subjects with or without AD were obtained from the Newcastle Brain Tissue Resource (Table 1). All subjects were male and were either patients with late-onset sporadic AD (n = 6, mean age ± SD, 77.3 ± 5.6 years) or age-matched, non-diseased (ND) control subjects (n = 6, mean age ± SD, 77.2 ± 4.1 years). The AD patients had advanced AD (Braak V-VI), while controls were Braak 0-II so were considered as non-diseased subjects. The mean ± SD post-mortem intervals were 42 ± 21.2 and 47.5 ± 21.1 h for the ND and AD subjects, respectively (Table 1). There were no significant differences in age, post-mortem interval or brain pH between the AD and ND groups (data not all shown) so we do not believe that our findings can be explained by these factors.
Table 1.
Summary of the sample identifiers, disease status, ages, gender and post-mortem intervals of the human frontal cortex brain samples and paraffin-embedded brain sections.
|
Brain Bank Sample identifier |
MRCa identifier |
Disease code |
Age (years) |
Gender |
Braak staging |
Post-mortem interval (hours) |
|---|---|---|---|---|---|---|
| 20030116 | BBN_7340 | ADb | 83 | M | 5 | 48 |
| 20070062 | BBN_2606 | AD | 76 | M | 6 | 64 |
| 20090026 | BBN_2612 | AD | 83 | M | 6 | 12 |
| 20100483 | BBN_7509 | AD | 78 | M | 6 | 37 |
| 20121029 | BBN_12991 | AD | 68 | M | 6 | 71 |
| 20130871 | BBN_19191 | AD | 76 | M | 6 | 53 |
| 20030123 | BBN_7341 | Control | 64 | M | 0 | 64 |
| 20040091 | BBN_7386 | Control | 75 | M | 1 | 64 |
| 20050087 | BBN_7111 | Control | 68 | M | 0 | 54 |
| 20130894 | BBN_19192 | Control | 80 | M | 2 | 16 |
| 20131187 | BBN_19217 | Control | 88 | M | 2 | 26 |
| 20140411 | BBN_24265 | Control | 88 | M | 1 | 28 |
MRC – Medical Research Council.
AD – Alzheimer’s disease.
All frozen samples were stored at −80 °C prior to use while paraffin-embedded samples were stored at room temperature (RT). All procedures were performed in accordance with the U.K. Human Tissue Act (2004).
Tissue processing
Soluble and insoluble proteins were extracted from cortex samples to allow quantification of Aβ40 and Aβ42 from each fraction. All other proteins were investigated in the soluble fraction. Proteins were extracted using 2% sodium dodecyl sulphate with protease inhibitor cocktail III (Roche) adapted from Rees et al. [3], [60]. Total protein concentration was determined with the BCA Protein Assay Kit (Thermo Scientific, Waltham, USA).
Western blotting
Western blotting (WB) was performed using standard methods. Briefly, samples were resolved on 7.5 or 10% polyacrylamide gels, transferred on to 0.45 µm nitrocellulose membranes (Amersham Biosciences, Little Chalfont, U.K.), incubated with the relevant primary antibody (see Materials) and detected as previously described [3].
The work described below in this study was limited by the financial, staff and time resources available and therefore the Western blots were only performed once for each protein. All antibodies had been optimised in our previous paper [3] so concentrations and exposures required for the AD and ND samples were already known and thus we are confident in the validity of our results.
Quantification of APP, Aβ, βCTF, sAPPα and sAPPβ
ELISA quantification was performed to detect APP (APP DuoSet ELISA, R&D Systems, Abingdon, Oxon., UK), Aβ40, Aβ42, βCTF, sAPPα and sAPPβ (IBL International GmbH, Hamburg, Germany) according to the suppliers’ guidelines and as described previously [3]. Data were presented for Aβ as pg/mg total protein concentration, APP, sAPPα and sAPPβ as ng/mg total protein concentration and βCTF as pmol/mg total protein concentration.
Immunohistochemistry (IHC)
Sections were deparaffinised and rehydrated through xylene, graded ethanol (100%, 95%, 70%, 50%) × 3 min each and distilled water 2 × 3 min. All stages were carried out at room temperature unless otherwise specified. Antigen retrieval was performed by immersing sections in boiled 0.01 M citric acid at 95 °C for 20 min. Sections were cooled under running tap water for 5 min and rinsed in 0.1 M PBS for 10 min. For Aβ analysis, sections were incubated in 80% formic acid for 10 min and then distilled water for 5 min before 0.1 M PBS. Endogenous peroxidases were deactivated by incubation for 30 min in 20% MeOH/1.5% H2O2 followed by washing in PBS with 0.3% Tween (PBST) 3 × 5 min. Non-specific binding was blocked by incubation in 1% BSA in 0.1 M PBS, 3% serum (from the species the secondary antibody was raised in) and 0.1% Triton X-100 for 30 min. The sections were incubated in primary antibody in blocking buffer overnight at 4 °C in a humidified container at the optimal concentration for each protein (see Materials). Sections were then incubated in biotinylated secondary antibody (1:100) in blocking buffer for 2 h and then in the Avidin-Biotin complex (ABC) reagent in 0.1 M PBS and 0.5% Triton X-100 for 45 min. After washing with 0.05 M TNS (Tris-buffered non-saline), sections were stained for 5 min in 0.5 mg/ml 3′3diaminobenzidine (DAB) in 0.05 M TNS with 0.0018% H2O2. Sections were then immersed in distilled water for 5 min, followed by 0.5% CuSO4 for 10 min to increase the strength of the DAB signal if required and distilled water for 5 min before drying overnight. The sections were counterstained with haematoxylin for 30 sec-1 min, 96% ethanol/3% HCl for 5 s and Scott’s alkaline tap water substitute (0.02 M KHCO3, 0.08 M MgSO4 per litre distilled water) for 15 min before dehydration in distilled water 2 × 3 min, graded ethanol (50%, 70%, 95%, 100%) 3 min each, xylene 2 × 6 min and coverslipped with DPX mountant.
Materials
All chemicals and reagents were purchased from Sigma–Aldrich (Poole, UK), Fisher Scientific (Leicester, UK) or Vector Laboratories Ltd (Peterborough, UK) unless specified. Antibodies used were: anti N-APP (22C11; WB 1:500, IHC 1:20) and total and phospho anti-GSK-3α/β (1:750) (Millipore, Watford, UK); anti-Aβ (6E10, BioLegend, San Diego, CA, USA); anti-clathrin heavy chain (CHC) (Clone 23; WB 1:1200, IHC 1;20), anti-caveolin-2 (Clone 65; WB 1:200, IHC 1:50), anti-flotillin-1 (Clone 18; 1:300) and anti-dynamin-2 (1:500) (BD Biosciences, Oxford, UK); anti-BACE1 (1:500, Merck Chemicals Ltd. Nottingham, UK); anti-AP180 (LP2D11; 1:750), anti-PICALM (WB 1:700, IHC 1:100), anti-flotillin-2 (1:500) and anti-AP-2 (1:500) (Novus Biologicals, Littleton, CO, USA); anti-caveolin-1 (WB 1:800, IHC 1:100, Cell Signalling Technology, Beverly, MA, USA); anti-caveolin-3 (1:200), anti-presenilin-1 (PS-1, 1:250), anti-ADAM10 (1:5000), anti-SORLA (EPR14670; 1:400), anti-dynamin-1 (1:300) and anti-BIN-1 (1:100) (Abcam, Cambridge, MA); anti-Rab5 (S-19; WB 1:300, IHC 1:30, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and total tau (1:2000, Dako, Hamburg, Germany). Monoclonal anti-phospho-tau Ser396/Ser404 (PHF-1, 1:100) was a generous gift from Prof. Peter Davies, Albert Einstein College of Medicine, Bronx, NY, USA.
Data and statistical analysis
ELISA data were quantified using standard curves with GraphPad Prism5 and normalised to total protein concentrations. Western blots were quantified using ImageJ (https://www.imagej.nih.gov). All protein bands were expressed as the relative density of the same human brain sample and then normalised for relative GAPDH levels. The density of the endocytic proteins for the immunohistochemistry was determined by measuring the optical density of the DAB staining on a Leica bright-field microscope. At least 20 images of individual neurons or blood vessels were taken in the grey matter or the grey and white matter, respectively, for each section and quantified using ImageJ. For each image, the optical density of the background was measured and subtracted from the optical density of the cell/vessel being analysed. ELISA results, Western blot data and immunohistochemistry data were analysed using unpaired Student’s t-tests at the two-tailed significance level to determine whether levels of the relevant protein differed significantly between AD and the ND groups. Data are presented as mean ± SEM. p values < 0.05 were considered significant. Where necessary, data were transformed to fit the assumptions of normality and equal variances.
Results
APP metabolites were altered in people with AD
The level of APP was measured by ELISA (Fig. 1A), immunohistochemistry (Fig. 1B) and immunoblotting (data not shown) and was not affected by disease status. APP was found to have a wide-spread distribution in the cytoplasm of neurones in the brain (Fig. 1B).
Fig. 1.
Comparison of (A) APP (ELISA), (B) APP immunohistochemistry (IHC) with representative sections, (C) soluble and insoluble Aβ40, (D) soluble and insoluble Aβ42, (E) soluble and insoluble Aβ42/Aβ40 ratios, (F) βCTF and (G) sAPPα and sAPPβ in male human frontal cortex samples from AD patients with age-matched, non-diseased (ND) subjects. IHC was carried out on 4 μm paraffin-embedded sections from all individuals following antigen retrieval. Optical density was measured in at least 20 neurones in the grey matter for each subject and analysed using ImageJ. (A), (B) and (G) Levels of APP, sAPPα and sAPPβ were not altered in AD brains. (C-F) Levels of soluble and insoluble Aβ40 and Aβ42, the soluble Aβ42/Aβ40 ratio and βCTF were significantly increased in AD brains compared to ND subjects. (E) The insoluble Aβ42/Aβ40 ratio was significantly decreased in AD brains compared to ND controls. Data are represented as mean ± S.E.M. *p < 0.05, **p < 0.01, ***p < 0.001 with Student's t-test. n = 6. White arrows indicate neurones in each IHC section.
Levels of soluble and insoluble Aβ40 and Aβ42 (p < 0.01, Fig. 1C,D) and the soluble Aβ42/40 ratio (p < 0.05, Fig. 1E) were significantly higher in AD brains compared to ND controls. However, the insoluble Aβ42/40 ratio was significantly greater in ND controls compared to AD brains (p < 0.05, Fig. 1E).
βCTF levels were also significantly increased by AD status (p < 0.001, Fig. 1F). Neither the absolute levels of sAPPα and sAPPβ (Fig. 1G) nor the sAPPα/sAPPβ ratio (data not shown) were significantly affected by disease status.
The expression of the three secretases essential for APP processing and Aβ production, ADAM10 for α-secretase, BACE1 (mature and immature) and presenilin-1 (PS-1, representative of γ–secretase) were not all affected by disease status. Levels of mature BACE (62 kDa, p < 0.05, Fig. 2A) and the cleaved 26 and 19 kDa derivatives of PS-1 (p < 0.05, p < 0.01, Fig. 2C; corresponding to the N- and C-terminal fragments of PS-1, respectively [75]) were increased in AD brains compared to ND controls. In contrast, the level of full-length PS-1 (50 kDa) was significantly decreased in AD brains compared to ND controls (p < 0.05, Fig. 2C). The level of immature BACE-1 (55.5 kDa, Fig. 2A) was not altered in AD brains. However, although the ‘t’ test for the ratios of mature to immature BACE-1 was not significantly different between AD and ND samples, the variances were significantly different (Fig. 2B, F value 398.2, p < 0.0001). Neither the expression of mature (73 kDa) and immature (83 kDa) ADAM10 individually nor the ratio of the mature to the immature protein were affected by disease status (Fig. 2B).
Fig. 2.
Comparison of mature and immature BACE-1 (A), mature and immature ADAM10 (B) and full-length and cleaved derivatives of PS-1 (C) in male human frontal cortex samples from AD patients compared to age-matched, non-diseased (ND) subjects. Representative immunoblots and densitometric analysis are shown for each protein. (A) and (C) Levels of mature (62 kDa) BACE and the 19 and 26 kDa cleaved derivatives of PS-1 were increased in AD brains compared to ND controls. (C) The level of full-length (50 kDa) PS-1 was significantly decreased in AD brains compared to ND controls. (A) and (B) The levels of immature (55.5 kDa) BACE-1 and mature (73 kDa) and immature (83 kDa) ADAM10 were not altered in AD brains. Data are represented as mean ± S.E.M. *p < 0.05, **p < 0.01 with Student's t-test; ****p < 0.0001 with F test. n = 6.
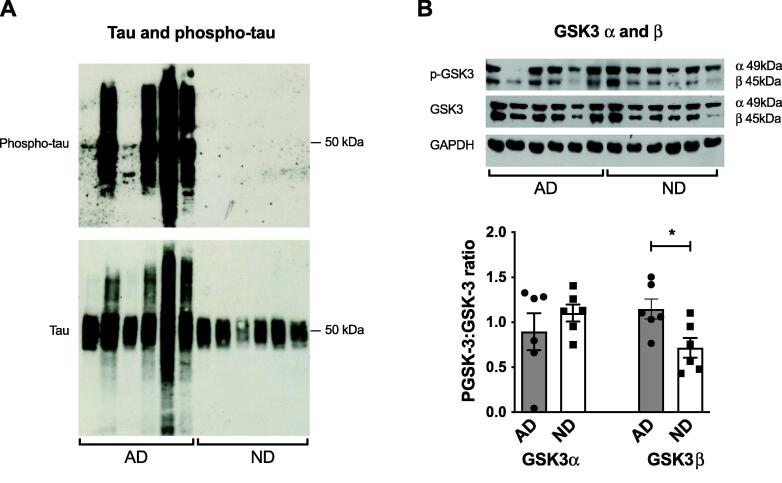
Levels of phosphorylated-tau and phosphorylated-GSK-3β were significantly increased in AD brains
Total tau was detected at approximately 45–60 kDa due to multiple tau isoforms in AD and ND samples (Fig. 3A). Total tau expression was not quantified due to the multiple bands for the different isoforms, however, it was clearly increased in 4 out of the 6 AD brain samples compared to ND controls (Fig. 3A). Phosphorylated tau was examined using the well-established PHF-1 antibody (pSer396/pSer404) [59] and extensive expression was detected in all AD brain samples but none was seen in the control ND brains (Fig. 3A). Again, the expression of phosphorylated tau was not quantified due to the multiple bands seen. The ratios of phosphorylated to total GSK-3α and GSK-3β, one of the major enzymes involved in the phosphorylation of tau, were also measured [36]. The ratio of phospho-GSK-3α:GSK-3α did not differ between AD brains and ND controls but the phospho-GSK-3β:GSK-3β ratio was significantly increased in AD brains compared to ND subjects (p < 0.05, Fig. 3B).
Fig. 3.
Comparison of phospho- and total-tau (A) and the phospho-GSK-3α:GSK-3α and phospho-GSK-3β:GSK-3β ratios (B) in male human frontal cortex samples of AD patients compared to age-matched, non-diseased (ND) subjects. Representative immunoblots and densitometric analysis are shown for each protein. (A) Phospho-tau was only detected in AD brains. (B) The phospho-GSK-3β:GSK-3β ratio was significantly increased in AD brains compared to ND controls. The phospho-GSK-3α:GSK-3α ratio was unchanged in AD brains. Data are represented as mean ± S.E.M. *p < 0.05 with Student's t-test. n = 6.
Levels of several CME-related proteins were upregulated in AD brains
Clathrin Adaptor Protein 2 (AP-2) expression was significantly higher in AD brains compared to ND subjects (p < 0.001, Fig. 4A). The punctate expression of clathrin heavy chain (CHC), measured using IHC, was significantly increased in AD brains compared to ND subjects (p < 0.01, Fig. 4B), but was not altered using Western blotting although a trend for an increase was seen (Supplementary Fig. 1C). Three bands were detected for PICALM with molecular masses of 70.5, 63.5 and 55.5 kDa (Fig. 4C). As we have previously reported in the human brain [3], it is likely that the 70.5 kDa band equates to isoform 1 with a predicted mass of 70.6 kDa, the 63.5 kDa band to isoform 2 with a predicted mass of 66.3 kDa and the 55.05 band to isoform 4 with a predicted mass of 59.7 kDa. The band with a predicted mass of 69.9 kDa, representing isoform 3, was not well resolved from that of isoform 1 due to their similar sizes and thus was analysed with isoform 1. The level of PICALM isoform 4 was significantly higher in AD compared to ND brains (p < 0.01, Fig. 4C) but the levels of PICALM isoforms 1 and 2 were not affected by disease status (Fig. 4C). The punctate expression of PICALM detected with IHC was also significantly higher in AD brains compared to ND subjects (Fig. 4D). There are at least 15 different isoforms of BIN1, most of which are expressed in the brain including the largest isoform (isoform 1) which is believed to be expressed exclusively in neurons [33]. Here, as in our previous study [3], BIN-1 was detected as multiple bands (Fig. 4E) with the three clearest bands at 77 kDa, equating to isoform 1 and ∼ 65 kDa, equating to the smaller isoforms [33]. The expression of BIN-1 isoform1 was significantly lower in AD brains compared to ND controls (p < 0.05, Fig. 4E) but the other isoforms were not affected by disease status (Fig. 4E). The expression of Rab5 was significantly increased in AD brains compared to ND subjects measured with both Western blotting and IHC where a distinctive punctate distribution was seen throughout the neuronal cytoplasm (p < 0.05, Fig. 4F,G).
Fig. 4.
Comparison of CME-related proteins in male human frontal cortex samples of AD patients compared to age-matched, non-diseased (ND) subjects. Representative immunoblots and densitometric analysis or immunohistochemistry (IHC) and analysis are shown for each protein. IHC was carried out on 4 μm paraffin-embedded sections from all individuals following antigen retrieval. Optical density was measured in at least 20 neurones in the grey matter for each subject and analysed using ImageJ. (A), (C) and (G) Levels of AP-2, PICALM (isoform 4) and Rab-5 were increased in AD brains compared to ND controls. (E) Levels of BIN-1 (isoform 1) significantly decreased in AD compared to ND brains. (C) and (E) Levels of PICALM (isoforms 1 & 2) and the other isoforms of BIN-1 were not altered in AD brains. (B), (D) and (F) The expression of clathrin, PICALM and Rab-5 was significantly increased in AD sections. Data are represented as mean ± S.E.M. *p < 0.05, **p < 0.01, ***p < 0.001 with Student's t-test. n = 6. White arrows indicate neurones in each IHC section.
The levels of dynamin-1 (Supplementary Fig. 1A), dynamin-2 (Supplementary Fig. 1B) and SORLA (Supplementary Fig. 1D) did not differ between AD brains and ND controls. Clathrin coat assembly protein AP180 exists in multiple isoforms in the human brain as previously shown [3]. The level of AP180 was not altered by disease status (Supplementary Fig. 1E).
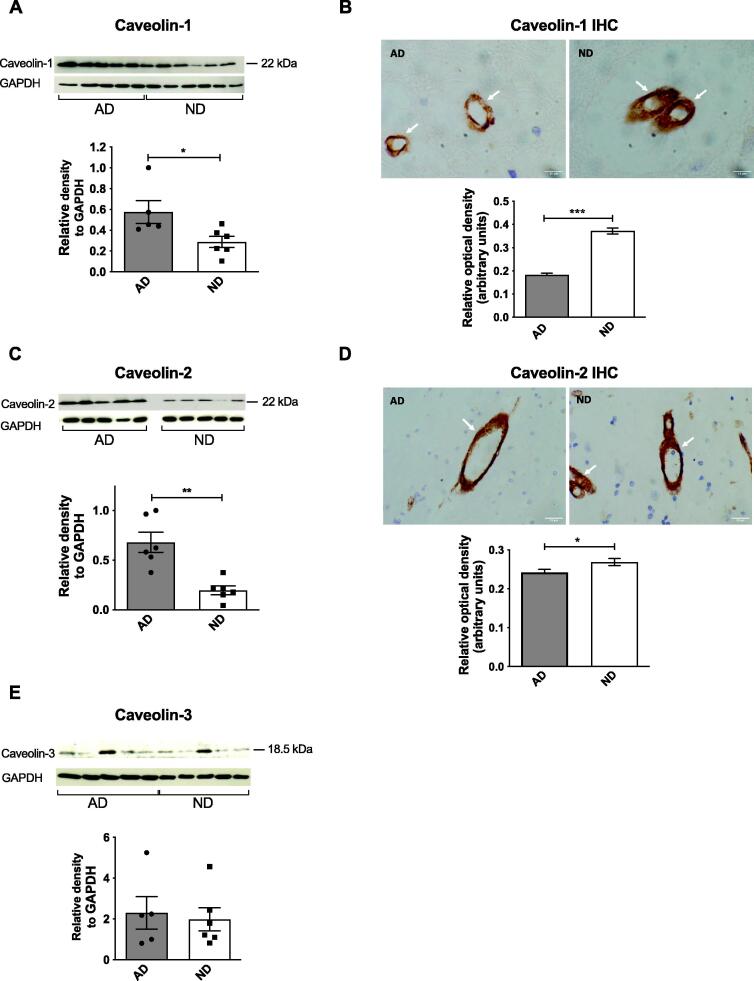
Expression of several CIE-related proteins was altered in AD brains
Using Western blotting, the levels of caveolin-1 (p < 0.05, Fig. 5A) and caveolin-2 (p < 0.01, Fig. 5C) increased significantly in AD brains compared to ND controls, while the level of caveolin-3 was not affected by disease status (Fig. 5E). The predominant IHC staining for caveolin-1 and caveolin-2 was in blood vessels in the brain but, in contrast to the blotting studies, the expression of caveolin-1 (p < 0.001, Fig. 5B) and caveolin-2 (p < 0.05, Fig. 5D) was significantly lower in AD brains compared to ND subjects (Fig. 5B). The expression of flotillin-1 and flotillin-2 measured with Western blotting was not altered by disease status (Supplementary Fig. 1F,G).
Fig. 5.
Comparison of CIE-related proteins in male human frontal cortex samples of AD patients compared to age-matched, non-diseased (ND) subjects. Representative immunoblots and densitometric analysis or immunohistochemistry (IHC) and analysis are shown for each protein. IHC was carried out on 4 μm paraffin-embedded sections from all individuals following antigen retrieval. Optical density was measured in at least 20 blood vessels in the grey and white matter for each subject and analysed using ImageJ. (A) and (C) Levels of caveolin-1 and caveolin-2 were significantly increased in AD brains compared to ND controls. (E) Levels of caveolin-3 were unchanged in AD brains. (B) and (D) The expression of caveolin-1 and caveolin-2 was significantly decreased in AD sections compared to ND controls. Data are represented as mean ± S.E.M. *p < 0.05, **p < 0.01, ***p < 0.001 with Student's t-test. n = 6. White arrows indicate blood vessels in each IHC section.
Discussion
This study has shown that the levels of several endocytic proteins were significantly increased in AD brains compared to age-matched ND controls. Furthermore, as expected there was an increase in the levels of βCTF, soluble and insoluble Aβ40 and Aβ42 and phosphorylated tau in AD brains, while APP and the sAPP metabolites were unchanged. Expression of mature BACE1, the functional units of PS-1 and the proportion of active GSK-3β were also increased in AD brains.
Previous studies have shown that the levels of Aβ40 and Aβ42 are significantly higher in familial or sporadic AD brains as compared to control individuals [16], [62], [65], [79] and we replicated this observation. Increased levels of Aβ40 have been shown to inhibit Aβ deposition and toxicity in vivo [41], [49], hence the high level of insoluble Aβ40 in AD brains here could reflect a compensatory mechanism to decrease Aβ deposition in plaques. The increased level of soluble Aβ42 is consistent with the Aβ oligomer hypothesis where soluble Aβ oligomer levels correlate much better than insoluble forms with the extent of synaptic loss and severity of cognitive impairment [8], [28]. Toxic oligomers could be formed from the high levels of soluble Aβ42 found in the AD brains and later deposited as plaques, reflected in the high levels of insoluble Aβ42. It is possible that increased endocytosis and processing of APP leading to accumulation of intracellular Aβ40 and Aβ42 [10] could explain the increased levels seen here.
Few studies have compared APP levels in sporadic AD cases to control subjects, but our results showing no change in APP expression are in agreement with other studies [42], [76]. The increase we saw in βCTF in AD brains was expected given the increases seen in Aβ40 and Aβ42 and agrees with others showing a significant increase in βCTF in sporadic AD brains [42]. Accumulation of βCTF occurs early in the pathogenesis of AD and has been shown to recruit APPL1 leading to overactivation of Rab5 which is involved in the development of endosomal dysfunction including the enlarged endosomes seen in AD and Down Syndrome [37], [42], [57]. It may induce toxicity resulting in synaptic loss and cell death, hence contributing to AD pathology [38], [42]. There are contradictions in the literature regarding sAPPα and sAPPβ levels in the nervous system in AD [27], but generally our findings of sAPPα and sAPPβ being unaltered are similar to results from CSF [55], [63]. Since sAPPα and sAPPβ are not known to have a direct involvement in the pathogenesis of AD, it is not surprising that their expression was unaffected.
The unchanged levels of ADAM10, one of the most important α-secretases in the brain [64], suggest that the increases in Aβ seen in AD brains may not be due to alterations in the activity of α-secretase. However, as we did not measure enzyme activity, we cannot be sure that this is the case. In contrast, the increased levels of mature BACE1 in the AD brains agree with other findings [58] and suggest increased β-secretase activity which could account for the increased production of Aβ and βCTF. While the expression of full-length PS-1 decreased significantly in AD brains compared to the controls, its cleaved fragments were significantly increased. These NTF and CTF fragments have been shown to represent the functional units of PS-1, essential for γ-secretase activity [18], [80] and their elevation in AD brains strongly suggests increased activity of PS-1. Thus, the likely increased activity of both β-secretase and γ-secretase would result in the higher levels of Aβ observed in the AD brains.
We only detected hyper-phosphorylated tau in AD brains with no expression in the ND controls, confirming their Braak staging of 0-II. GSK-3β has been shown to play a critical role in in the pathogenesis of AD by inducing tau hyperphosphorylation, phosphorylating tau at multiple AD-associated sites [36], [46]. In agreement with a previous study, we observed a significant increase in the proportion of active phosphorylated GSK-3β at Tyr216 in AD brains [47] but the proportion of active phosphorylated GSK-3α at Tyr279 was unaltered. Thus, it is likely that the phosphorylated tau seen in the AD brains is due, at least in part, to upregulated GSK-3β activity.
There is now much evidence to suggest that alterations in endocytosis are causal in AD as described above and we tested this hypothesis by investigating whether levels of endocytic proteins were altered in AD brains.
The level of clathrin, a major CME protein, was significantly increased in AD brains compared to ND subjects when measured with immunohistochemistry but not with immunoblotting although we saw a trend towards an increase. This difference is probably due to the ability to measure cellular localisation more precisely with the former technique. We have previously shown that CHC expression was significantly higher in the brains of old compared to young and middle-aged ND subjects (Alsaqati. et al. 2018). Thus, it appears that clathrin levels continue to increase in AD patients, suggesting possible upregulation of endocytosis and involvement in increased Aβ levels. Closely linked to clathrin, dynamin-1 expression has been reported to decrease in AD patients compared to controls in the hippocampus and entorhinal cortex but not in the frontal or temporal cortex [11], but an increase in the insoluble fraction in the frontal cortex [40] and a decrease in the dynamin gene in the prefrontal cortex [56] were also described. Our data for dynamin-1 agree with Cao et al. [11] and could be explained by stable protein expression but are surprising given the increase we saw in clathrin in AD brains suggesting increased CME. However, previously we saw a significant increase in dynamin-1 expression with age [3], and it is possible that this is sufficient to support the increase in clathrin expression seen in the AD patients.
BIN-1, thought to be one of the most important susceptibility genes for sporadic AD, has been suggested to be involved in different mechanisms linked to AD [20]. Here, the level of BIN-1 isoform 1 or neuronal BIN-1 was significantly lower in AD brains compared to ND controls, agreeing with other findings using frontal cortex [17], [33]. Overall BIN-1 expression was also found to be reduced in sporadic but not familial AD and suggested to be mainly involved in the pathogenesis of sporadic AD [25]. In contrast, another study [14] found increased BIN1 mRNA in the frontal cortex of AD patients, but their data still support disrupted BIN1 processing in AD. Consistent with the changes in BIN1 and clathrin suggesting disrupted CME in AD, AP-2 levels were significantly higher in AD brains in relation to ND controls but were not affected by age in our previous study [3]. However, the AP-2 gene was found to be downregulated in the prefrontal cortex [56]. In contrast, AP180 levels were unaltered here but have been found to be decreased using IHC in the prefrontal, entorhinal and temporal cortex and hippocampus [11], [81]. The differences for AP-2 and AP180 could be due to compensation by other adaptor proteins [51] or changes may be in specific cells not detectable with immunoblotting.
PICALM is involved in AD as a link to the development of tau pathology, a susceptibility gene for AD and is crucial for the regulation of Aβ transcytosis and clearance across the blood–brain barrier [4], [31], [45], [52], [84]. We found that PICALM isoform 4 was significantly increased in AD brains as was total PICALM staining in IHC, while isoforms 1 and 2 were not altered. Our finding is consistent with increased PICALM isoform 4 levels in the hippocampus in AD cases in relation to controls [4]. However, unlike Ando et al. [4] we did not observe changes in isoforms 1 and 2 or abnormal cleavage of PICALM in AD brains, possibly due to our use of a different brain region. It has been suggested that cleavage of PICALM could be involved in the promotion of tau fibrillisation [4], supported by the increased level of isoform 4 here. Our finding, coupled with the alterations in clathrin, BIN1 and AP-2, supports disruption of CME in AD which could contribute to increased Aβ levels.
Various findings have linked Rab5 to AD [7] including increased Rab5 expression [12], [13], [22], [44] and enlarged Rab-5-positive endosomes in early-stage AD brains [13]. Our findings confirm this increased expression of Rab-5 in AD brains suggesting that both endocytic uptake and recycling are increased. Importantly, overactivation of Rab5 in the absence of any influence of βCTF has been shown to recapitulate the endosomal dysfunction seen in AD, thus confirming the importance of changes in endocytic pathways in the pathogenesis of the disease [57] and supporting our findings that alterations in endocytic pathway proteins in AD are important for disease development.
Decreased expression of SORLA, an important risk factor for AD due to its role and genetic variants [68], was found in AD brains [19], [67], [83] and here, although no significant change was seen in SORLA levels in AD brains, there was a trend for a decrease supporting the earlier studies.
Caveolin-1 has been shown to be associated with APP and APP was enriched in caveolae [34], [39]. In AD brains, increases were seen in the caveolin gene in the prefrontal cortex [56] and caveolin-1 protein in the hippocampus and caveolin-1 mRNA in the frontal cortex [21]. Our results for caveolin-1 and caveolin-2 depended on the technique used with increases in AD brains seen with immunoblotting while decreased blood vessel staining was seen with IHC, the latter likely to be more reflective of caveolin levels in the brain. Previous IHC studies identified caveolin-1 and caveolin-2 expression in endothelial cells of cortical blood vessels [35], [77], reflecting our localisation, but their expression was not altered in AD brains [77] in contrast to our study. There are marked changes in the blood–brain barrier including the endothelium in AD brains which could explain our findings [82]. Interestingly, a study on the temporal lobe from Type 2 diabetes patients showed a large decrease in caveolin-1 with a concomitant increase in Aβ compared to healthy controls [9]. Caveolin-3 immunoreactivity was decreased in astroglial cells surrounding senile plaques in AD brains [54] but, here, it was not affected by AD, possibly due to the measurement of total protein.
Flotillin has been linked with AD most recently as a potential biomarker [5]. Flotillin-1 accumulated in the lysosomes of NFT-containing neurons in AD brains [23], [53] and Aβ was found in flotillin-1-containing exosomes from AD brains [66]. Both flotillin-1 and −2 expression were increased as plaque numbers increased compared to subjects with no plaques [43]. However, here, neither flotillin-1 nor flotillin-2 were affected in the AD brains.
Interestingly, in our previous study on ageing in male brains with immunoblotting [3], we saw no changes in caveolin-1 or flotillin-1, an increase in caveolin-2 and flotillin-2 and a decrease in caveolin-3 with age. Overall, our data suggest that caveolins and flotillins are differentially modulated by age and the presence of disease and more detailed investigations at the cellular level are needed to understand how these proteins are affected by disease status.
Conclusions
In conclusion, this study provides evidence for changes in both CME and CIE proteins in the male AD brain. Increases in several of the CME proteins suggest an upregulation in endocytosis in male AD brains, leading to increased internalisation of APP, more metabolism by β- and γ-secretase and increased generation of Aβ. This increased metabolism of APP in the amyloidogenic pathway would be augmented by the higher expression of β- and γ-secretase, thus further increasing Aβ production. We note that, although we controlled for the effects of biological sex as we only used male brain samples, as we did not include any female brains in our study it is possible that our results cannot be generalised to everyone with AD. Further studies are needed to determine whether there are sex-related changes in endocytic proteins in ND and AD brains. The results obtained here, combined with our previous study on increased endocytic protein expression with ageing, lend support to an increase in endocytosis explaining, at least in part, the increased production of Aβ during AD. Our study sheds light on several endocytic proteins that could be targeted to slow down the progression of AD.
Funding
This work and MA were supported by Bristol Research into Alzheimer's and Care of Elderly (BRACE).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Prof. Peter Davies for the gift of the PHF-1 antibody. The authors gratefully acknowledge the Newcastle Brain Tissue Resource which provided the brain samples for this study. The Resource is funded in part by a grant from the UK Medical Research Council (G0400074), by NIHR Newcastle Biomedical Research Centre and Unit awarded to the Newcastle upon Tyne NHS Foundation Trust and Newcastle University, and by a grant from Alzheimer's Society and Alzheimer's Research Trust as part of the Brains for Dementia Research Project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nbas.2023.100084.
Contributor Information
Mouhamed Alsaqati, Email: Mouhamed.alsaqati@newcastle.ac.uk.
Rhian S. Thomas, Email: rhian.thomas@swansea.ac.uk.
Emma J. Kidd, Email: KiddEJ@cf.ac.uk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ahmed M., Davis J., Aucoin D., Sato T., Ahuja S., Aimoto S., et al. Structural conversion of neurotoxic amyloid-[beta] 1–42 oligomers to fibrils. Nat Struct Mol Biol. 2010;17:561–567. doi: 10.1038/nsmb.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allinson T.M., Parkin E.T., Turner A.J., Hooper N.M. ADAMs family members as amyloid precursor protein α-secretases. J Neurosci Res. 2003;74:342–352. doi: 10.1002/jnr.10737. [DOI] [PubMed] [Google Scholar]
- 3.Alsaqati M., Thomas R.S., Kidd E.J. Proteins involved in endocytosis are upregulated by ageing in the normal human brain: Implications for the development of Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2018;73:289–298. doi: 10.1093/gerona/glx135. [DOI] [PubMed] [Google Scholar]
- 4.Ando K., Brion J.-P., Stygelbout V., Suain V., Authelet M., Dedecker R., et al. Clathrin adaptor CALM/PICALM is associated with neurofibrillary tangles and is cleaved in Alzheimer’s brains. Acta Neuropathol. 2013;125(6):861–878. doi: 10.1007/s00401-013-1111-z. [DOI] [PubMed] [Google Scholar]
- 5.Angelopoulou E., Paudel Y.N., Shaikh M.F., Piperi C. Flotillin: A promising biomarker for Alzheimer's disease. J Pers Med. 2020;10:20. doi: 10.3390/jpm10020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alzheimer’s Association 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;(18):700–789. doi: 10.1002/alz.12638. [DOI] [PubMed] [Google Scholar]
- 7.Behl T., Kaur D., Sehgal A., Singh S., Makeen H.A., Albratty M., et al. Exploring the potential role of rab5 protein in endo-lysosomal impairment in Alzheimer's disease. Biomed Pharmacother. 2022;148:112773. doi: 10.1016/j.biopha.2022.112773. [DOI] [PubMed] [Google Scholar]
- 8.Benilova I., Karran E., De Strooper B. The toxic A [beta] oligomer and Alzheimer's disease: An emperor in need of clothes. Nat Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 9.Bonds J.A., Shetti A., Bheri A., Chen Z., Disouky A., Tai L., et al. Depletion of caveolin-1 in type 2 diabetes model induces Alzheimer's disease pathology precursors. J Neurosci. 2019;39(43):8576–8583. doi: 10.1523/JNEUROSCI.0730-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrinha T., Martinsson I., Gomes R., Terrasso A.P., Gouras G.K., Almeida C.G. Upregulation of APP endocytosis by neuronal aging drives amyloid-dependent synapse loss. J Cell Sci. 2021;134 doi: 10.1242/jcs.255752. [DOI] [PubMed] [Google Scholar]
- 11.Cao Y., Xiao Y., Ravid R., Guan Z.-Z. Changed clathrin regulatory proteins in the brains of Alzheimer's disease patients and animal models. J Alzheimers Dis. 2010;22(1):329–342. doi: 10.3233/JAD-2010-100162. [DOI] [PubMed] [Google Scholar]
- 12.Cataldo A.M., Barnett J.L., Pieroni C., Nixon R.A. Increased neuronal endocytosis and protease delivery to early endosomes in sporadic Alzheimer’s disease: neuropathologic evidence for a mechanism of increased β-amyloidogenesis. J Neurosci. 1997;17(16):6142–6151. doi: 10.1523/JNEUROSCI.17-16-06142.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cataldo A.M., Hamilton D.J., Barnett J.L., Paskevich P.A., Nixon R.A. Abnormalities of the endosomal-lysosomal system in Alzheimer's disease: Relationship to disease pathogenesis. Adv Exp Med Biol. 1996;389:271–280. [PubMed] [Google Scholar]
- 14.Chapuis J., Hansmannel F., Gistelinck M., Mounier A., Van Cauwenberghe C., Kolen K.V., et al. Increased expression of BIN1 mediates Alzheimer genetic risk by modulating tau pathology. Mol Psychiatry. 2013;18(11):1225–1234. doi: 10.1038/mp.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Citron M. Strategies for disease modification in Alzheimer's disease. Nat Rev Neurosci. 2004;5(9):677–685. doi: 10.1038/nrn1495. [DOI] [PubMed] [Google Scholar]
- 16.Collins-Praino L.E., Francis Y.I., Griffith E.Y., Wiegman A.F., Urbach J., Lawton A., et al. Soluble amyloid beta levels are elevated in the white matter of Alzheimer’s patients, independent of cortical plaque severity. Acta Neuropathol Commun. 2014;2:83. doi: 10.1186/s40478-014-0083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Rossi P., Buggia-Prevot V., Clayton B.L., Vasquez J.B., Van Sanford C., Andrew R.J., et al. Predominant expression of Alzheimer's disease-associated BIN1 in mature oligodendrocytes and localization to white matter tracts. Mol Neurodegener. 2016;11:59. doi: 10.1186/s13024-016-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Strooper B. Aph-1, Ren-2, and nicastrin with presenilin generate an active γ-secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 19.Dodson S.E., Gearing M., Lippa C.F., Montine T.J., Levey A.I., Lah J.J. Lr11/SorLA expression is reduced in sporadic Alzheimer disease but not in familial Alzheimer disease. J Neuropathol Exp Neurol. 2006;65(9):866–872. doi: 10.1097/01.jnen.0000228205.19915.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao P., Ye L., Cheng H., Li H. The mechanistic role of bridging integrator 1 (BIN1) in Alzheimer's disease. Cell Mol Neurobiol. 2021;41(7):1431–1440. doi: 10.1007/s10571-020-00926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaudreault S.B., Dea D., Poirier J. Increased caveolin-1 expression in Alzheimer’s disease brain. Neurobiol Aging. 2004;25(6):753–759. doi: 10.1016/j.neurobiolaging.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Ginsberg S.D., Mufson E.J., Counts S.E., Wuu J., Alldred M.J., Nixon R.A., et al. Regional selectivity of rab5 and rab7 protein upregulation in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2010;22(2):631–639. doi: 10.3233/JAD-2010-101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girardot N., Allinquant B., Langui D., Laquerriere A., Dubois B., Hauw J.J., et al. Accumulation of flotillin-1 in tangle-bearing neurones of Alzheimer's disease. Neuropathol Appl Neurobiol. 2003;29:451–461. doi: 10.1046/j.1365-2990.2003.00479.x. [DOI] [PubMed] [Google Scholar]
- 24.Glenner G.G., Wong C.W. Alzheimer's disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 25.Glennon E.B.C., Whitehouse I.J., Miners J.S., Kehoe P.G., Love S., Kellett K.A.B., et al. BIN1 is decreased in sporadic but not familial Alzheimer’s disease or in aging. PLoS One. 2013;8(10):e78806. doi: 10.1371/journal.pone.0078806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guimas Almeida C., Sadat Mirfakhar F., Perdigão C., Burrinha T. Impact of late-onset Alzheimer's genetic risk factors on beta-amyloid endocytic production. Cell Mol Life Sci. 2018;75(14):2577–2589. doi: 10.1007/s00018-018-2825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habib A., Sawmiller D., Tan J. Restoring soluble amyloid precursor protein alpha functions as a potential treatment for Alzheimer's disease. J Neurosci Res. 2017;95:973–991. doi: 10.1002/jnr.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hampel H., Hardy J., Blennow K., Chen C., Perry G., Kim S.H., et al. The amyloid-beta pathway in Alzheimer’s disease. Mol Psychiatry. 2021;26:5481–5503. doi: 10.1038/s41380-021-01249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hampel H., Vassar R., De Strooper B., Hardy J., Willem M., Singh N., et al. The beta-secretase BACE1 in Alzheimer's disease. Biol Psychiatry. 2021;89:745–756. doi: 10.1016/j.biopsych.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 31.Harold D., Abraham R., Hollingworth P., Sims R., Gerrish A., Hamshere M.L., et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41(10):1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Head B.P., Insel P.A. Do caveolins regulate cells by actions outside of caveolae? Trends Cell Biol. 2007;17(2):51–57. doi: 10.1016/j.tcb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Holler C.J., Davis P.R., Beckett T.L., Platt T.L., Webb R.L., Head E., et al. Bridging integrator 1 (BIN1) protein expression increases in the Alzheimer’s disease brain and correlates with neurofibrillary tangle pathology. Journal of Alzheimer's Disease: JAD. 2014;42(4):1221–1227. doi: 10.3233/JAD-132450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikezu T., Trapp B.D., Song K.S., Schlegel A., Lisanti M.P., Okamoto T. Caveolae, plasma membrane microdomains for α-secretase-mediated processing of the amyloid precursor protein. J Biol Chem. 1998;273(17):10485–10495. doi: 10.1074/jbc.273.17.10485. [DOI] [PubMed] [Google Scholar]
- 35.Ikezu T., Ueda H., Trapp B.D., Nishiyama K., Sha J.F., Volonte D., et al. Affinity-purification and characterization of caveolins from the brain: Differential expression of caveolin-1, -2, and-3 in brain endothelial and astroglial cell types. Brain Res. 1998;804(2):177–192. doi: 10.1016/s0006-8993(98)00498-3. [DOI] [PubMed] [Google Scholar]
- 36.Jiang J., Wang Z.-H., Qu M., Gao D., Liu X.-P., Zhu L.-Q., et al. Stimulation of EphB2 attenuates tau phosphorylation through PI3K/Akt-mediated inactivation of glycogen synthase kinase-3β. Sci Rep. 2015;5:11765. doi: 10.1038/srep11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Y., Mullaney K.A., Peterhoff C.M., Che S., Schmidt S.D., Boyer-Boiteau A., et al. Alzheimer’s-related endosome dysfunction in Down syndrome is Aβ-independent but requires APP and is reversed by BACE-1 inhibition. Proc Natl Acad Sci. 2010;107(4):1630–1635. doi: 10.1073/pnas.0908953107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin L.-W., Hua D.H., Shie F.-S., Maezawa I., Sopher B., Martin G.M. Novel tricyclic pyrone compounds prevent intracellular APP C99-induced cell death. J Mol Neurosci. 2002;19(1-2):57–61. doi: 10.1007/s12031-002-0011-9. [DOI] [PubMed] [Google Scholar]
- 39.Kang M., Chung Y.H., Hwang C., Murata M., Fujimoto T., Mook-Jung I., et al. Caveolin-1 upregulation in senescent neurons alters amyloid precursor protein processing. Exp Mol Med. 2006;38:126–133. doi: 10.1038/emm.2006.16. [DOI] [PubMed] [Google Scholar]
- 40.Kepchia D., Huang L., Dargusch R., Rissman R.A., Shokhirev M.N., Fischer W., et al. Diverse proteins aggregate in mild cognitive impairment and Alzheimer's disease brain. Alzheimers Res Ther. 2020;12:75. doi: 10.1186/s13195-020-00641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J., Onstead L., Randle S., Price R., Smithson L., Zwizinski C., et al. Aβ40 inhibits amyloid deposition in vivo. J Neurosci. 2007;27(3):627–633. doi: 10.1523/JNEUROSCI.4849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S., Sato Y., Mohan P.S., Peterhoff C., Pensalfini A., Rigoglioso A., et al. Evidence that the rab5 effector APPL1 mediates APP-[beta]CTF-induced dysfunction of endosomes in Down syndrome and Alzheimer's disease. Mol Psychiatry. 2016;21:707–716. doi: 10.1038/mp.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kokubo H., Lemere C.A., Yamaguchi H. Localization of flotillins in human brain and their accumulation with the progression of Alzheimer's disease pathology. Neurosci Lett. 2000;290(2):93–96. doi: 10.1016/s0304-3940(00)01334-3. [DOI] [PubMed] [Google Scholar]
- 44.Laifenfeld D., Patzek L.J., McPhie D.L., Chen Y., Levites Y., Cataldo A.M., et al. Rab5 mediates an amyloid precursor protein signaling pathway that leads to apoptosis. J Neurosci. 2007;27(27):7141–7153. doi: 10.1523/JNEUROSCI.4599-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lambert J.-C., Heath S., Even G., Campion D., Sleegers K., Hiltunen M., et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009;41(10):1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 46.Lauretti E., Dincer O., Praticò D. Glycogen synthase kinase-3 signaling in Alzheimer's disease. Biochim Biophys Acta Mol Cell Res. 2020;1867(5):118664. doi: 10.1016/j.bbamcr.2020.118664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leroy K., Yilmaz Z., Brion J.P. Increased level of active GSK-3beta in Alzheimer's disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol Appl Neurobiol. 2007;33:43–55. doi: 10.1111/j.1365-2990.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- 48.Marquer C., Devauges V., Cossec J.C., Liot G., Lecart S., Saudou F., et al. Local cholesterol increase triggers amyloid precursor protein-Bace1 clustering in lipid rafts and rapid endocytosis. FASEB J. 2011;25:1295–1305. doi: 10.1096/fj.10-168633. [DOI] [PubMed] [Google Scholar]
- 49.McGowan E., Pickford F., Kim J., Onstead L., Eriksen J., Yu C., et al. Aβ42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47(2):191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meister M., Tikkanen R. Endocytic trafficking of membrane-bound cargo: A flotillin point of view. Membranes (Basel) 2014;4:356–371. doi: 10.3390/membranes4030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moshkanbaryans L., Chan L.-S., Graham M.E. The biochemical properties and functions of CALM and AP180 in clathrin mediated endocytosis. Membranes. 2014;4:388–413. doi: 10.3390/membranes4030388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naj A.C., Jun G., Beecham G.W., Wang L.-S., Vardarajan B.N., Buros J., et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet. 2011;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishikawa T., Takahashi T., Nakamori M., Yamazaki Y.u., Kurashige T., Nagano Y., et al. Phosphatidylinositol-4,5-bisphosphate is enriched in granulovacuolar degeneration bodies and neurofibrillary tangles. Neuropathol Appl Neurobiol. 2014;40(4):489–501. doi: 10.1111/nan.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishiyama K., Trapp B.D., Ikezu T., Ransohoff R.M., Tomita T., Iwatsubo T., et al. Caveolin-3 upregulation activates β-secretase–mediated cleavage of the amyloid precursor protein in Alzheimer’s disease. J Neurosci. 1999;19(15):6538–6548. doi: 10.1523/JNEUROSCI.19-15-06538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olsson A., Höglund K., Sjögren M., Andreasen N., Minthon L., Lannfelt L., et al. Measurement of α-and β-secretase cleaved amyloid precursor protein in cerebrospinal fluid from Alzheimer patients. Exp Neurol. 2003;183(1):74–80. doi: 10.1016/s0014-4886(03)00027-x. [DOI] [PubMed] [Google Scholar]
- 56.Omtri R.S., Thompson K.J., Tang X., Gali C.C., Panzenboeck U., Davidson M.W., et al. Differential effects of Alzheimer’s disease Aβ40 and 42 on endocytosis and intraneuronal trafficking. Neuroscience. 2018;373:159–168. doi: 10.1016/j.neuroscience.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Pensalfini A., Kim S., Subbanna S., Bleiwas C., Goulbourne C.N., Stavrides P.H., et al. Endosomal dysfunction induced by directly overactivating Rab5 recapitulates prodromal and neurodegenerative features of Alzheimer's disease. Cell Rep. 2020;33(8):108420. doi: 10.1016/j.celrep.2020.108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pera M., Alcolea D., Sánchez-Valle R., Guardia-Laguarta C., Colom-Cadena M., Badiola N., et al. Distinct patterns of APP processing in the CNS in autosomal-dominant and sporadic Alzheimer disease. Acta Neuropathol. 2013;125(2):201–213. doi: 10.1007/s00401-012-1062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petry F.R., Pelletier J., Bretteville A., Morin F., Calon F., Hébert S.S., et al. Specificity of anti-tau antibodies when analyzing mice models of Alzheimer's disease: problems and solutions. PLoS One. 2014;9(5):e94251. doi: 10.1371/journal.pone.0094251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rees T., Hammond P.I., Soreq H., Younkin S., Brimijoin S. Acetylcholinesterase promotes beta-amyloid plaques in cerebral cortex. Neurobiol Aging. 2003;24:777–787. doi: 10.1016/s0197-4580(02)00230-0. [DOI] [PubMed] [Google Scholar]
- 61.Rennick J.J., Johnston A.P.R., Parton R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat Nanotechnol. 2021;16(3):266–276. doi: 10.1038/s41565-021-00858-8. [DOI] [PubMed] [Google Scholar]
- 62.Roberts B.R., Lind M., Wagen A.Z., Rembach A., Frugier T., Li Q.X., et al. Biochemically-defined pools of amyloid-beta in sporadic Alzheimer's disease: correlation with amyloid PET. Brain. 2017;140:1486–1498. doi: 10.1093/brain/awx057. [DOI] [PubMed] [Google Scholar]
- 63.Rosen C., Andreasson U., Mattsson N., Marcusson J., Minthon L., Andreasen N., et al. Cerebrospinal fluid profiles of amyloid beta-related biomarkers in Alzheimer's disease. Neuromolecular Med. 2012;14:65–73. doi: 10.1007/s12017-012-8171-4. [DOI] [PubMed] [Google Scholar]
- 64.Saftig P., Lichtenthaler S.F. The alpha secretase ADAM10: A metalloprotease with multiple functions in the brain. Prog Neurobiol. 2015;135:1–20. doi: 10.1016/j.pneurobio.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 65.Sandebring A., Welander H., Winblad B., Graff C., Tjernberg L.O., Fernandez-Funez P. The pathogenic Aβ43 is enriched in familial and sporadic Alzheimer disease. PLoS One. 2013;8(2):e55847. doi: 10.1371/journal.pone.0055847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sardar Sinha M., Ansell-Schultz A., Civitelli L., Hildesjö C., Larsson M., Lannfelt L., et al. Alzheimer's disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018;136(1):41–56. doi: 10.1007/s00401-018-1868-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scherzer C.R., Offe K., Gearing M., Rees H.D., Fang G., Heilman C.J., et al. Loss of apolipoprotein E receptor LR11 in Alzheimer disease. Arch Neurol. 2004;61:1200–1205. doi: 10.1001/archneur.61.8.1200. [DOI] [PubMed] [Google Scholar]
- 68.Schmidt V., Subkhangulova A., Willnow T.E. Sorting receptor SORLA: Cellular mechanisms and implications for disease. Cell Mol Life Sci. 2017;74(8):1475–1483. doi: 10.1007/s00018-016-2410-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneider A., Rajendran L., Honsho M., Gralle M., Donnert G., Wouters F., et al. Flotillin-dependent clustering of the amyloid precursor protein regulates its endocytosis and amyloidogenic processing in neurons. J Neurosci. 2008;28(11):2874–2882. doi: 10.1523/JNEUROSCI.5345-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Selkoe D.J. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 71.Shikanai M., Nishimura Y.V., Sakurai M., Nabeshima Y.-I., Yuzaki M., Kawauchi T. Caveolin-1 promotes early neuronal maturation via caveolae-independent trafficking of N-cadherin and L1. iScience. 2018;7:53–67. doi: 10.1016/j.isci.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sirkis D.W., Bonham L.W., Johnson T.P., La Joie R., Yokoyama J.S. Dissecting the clinical heterogeneity of early-onset Alzheimer's disease. Mol Psychiatry. 2022;27(6):2674–2688. doi: 10.1038/s41380-022-01531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tate B.A., Mathews P.M. Targeting the role of the endosome in the pathophysiology of Alzheimer's disease: A strategy for treatment. SAGE KE. 2006;2006(10) doi: 10.1126/sageke.2006.10.re2. [DOI] [PubMed] [Google Scholar]
- 74.Tesi N., Van Der Lee S.J., Hulsman M., Jansen I.E., Stringa N., Van Schoor N.M., et al. Immune response and endocytosis pathways are associated with the resilience against Alzheimer's disease. Translational. Psychiatry. 2020;10:332. doi: 10.1038/s41398-020-01018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thinakaran G., Borchelt D.R., Lee M.K., Slunt H.H., Spitzer L., Kim G., et al. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17(1):181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 76.Vaillant-Beuchot L., Mary A., Pardossi-Piquard R., Bourgeois A., Lauritzen I., Eysert F., et al. Accumulation of amyloid precursor protein C-terminal fragments triggers mitochondrial structure, function, and mitophagy defects in Alzheimer's disease models and human brains. Acta Neuropathol. 2021;141:39–65. doi: 10.1007/s00401-020-02234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Helmond Z.K., Miners J.S., Bednall E., Chalmers K.A., Zhang Y., Wilcock G.K., et al. Caveolin-1 and-2 and their relationship to cerebral amyloid angiopathy in Alzheimer's disease. Neuropathol Appl Neurobiol. 2007;33(3):317–327. doi: 10.1111/j.1365-2990.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- 78.Vassar R., Bennett B.D., Babu-Khan S., Kahn S., Mendiaz E.A., Denis P., et al. β-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 79.Wang J., Dickson D.W., Trojanowski J.Q., Lee V.M. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer's disease from normal and pathologic aging. Exp Neurol. 1999;158:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- 80.Wolfe M.S. Inhibition and modulation of γ-secretase for Alzheimer's disease. Neurotherapeutics. 2008;5(3):391–398. doi: 10.1016/j.nurt.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yao P.J., Morsch R., Callahan L.M., Coleman P.D. Changes in synaptic expression of clathrin assembly protein AP180 in Alzheimer's disease analysed by immunohistochemistry. Neuroscience. 1999;94(2):389–394. doi: 10.1016/s0306-4522(99)00360-7. [DOI] [PubMed] [Google Scholar]
- 82.Zenaro E., Piacentino G., Constantin G. The blood-brain barrier in Alzheimer's disease. Neurobiol Dis. 2017;107:41–56. doi: 10.1016/j.nbd.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao Y., Cui J.G., Lukiw W.J. Reduction of sortilin-1 in Alzheimer hippocampus and in cytokine-stressed human brain cells. Neuroreport. 2007;18:1187–1191. doi: 10.1097/WNR.0b013e32821c56c4. [DOI] [PubMed] [Google Scholar]
- 84.Zhao Z., Sagare A.P., Ma Q., Halliday M.R., Kong P., Kisler K., et al. Central role for PICALM in amyloid-[beta] blood-brain barrier transcytosis and clearance. Nat Neurosci. 2015;18:978–987. doi: 10.1038/nn.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.