Abstract
Background
Epstein-Barr virus (EBV) usually leads to latent infection and is reported mostly in infectious mononucleosis, lymphoma, and cancer in adolescents and adults, but pneumonitis due to EBV infection in adults is rare.
Case presentation
We hereby reported a case of a 52-year-old woman with breast cancer who developed acute pneumonia during neoadjuvant chemotherapy. Her serologic workup revealed a low CD4+ count and positive anti-EBV antibodies. Chest computed tomography (CT) shows multiple patchy ground-glass shadows in the bilateral lung. Microscopic examination of stained sputum and bronchoalveolar lavage fluid (BALF) smear specimens did not find any pathogens. Metagenomic next-generation sequencing (mNGS) of BALF indicated a large number of EBV reads, allowing to confirm the diagnosis of EBV induced pneumonitis. The patient was then treated with ganciclovir with subsequent dramatic clinical and radiological improvement.
Conclusions
This case highlights the combined application of mNGS and traditional tests in the clinical diagnosis of invasive pulmonary infection. In the meanwhile, clinicians should be aware neoadjuvant chemotherapy for breast cancer carries a risk of EBV induced pneumonitis, so that EBV induced pneumonitis could be considered in differential diagnosis while similar patients present, to orchestrate improvements in diagnosis, treatment, and prognosis.
Keywords: Breast cancer, Neoadjuvant chemotherapy, Epstein-Barr virus, Viral pneumonitis, Metagenomic next-generation sequencing
1. Introduction
Epstein-Barr virus (EBV) usually leads to latent infection and is reported mostly in infectious mononucleosis, lymphoma, and cancer in adolescents and adults [1,2]. Under certain conditions, such as immunosuppression, EBV may be reactivated and becomes a pathogenic factor [3]. But pneumonitis due to EBV infection in adults is rarely reported [4]. The lack of characteristic clinical manifestations and imaging features in the early stages has made the diagnosis of EBV induced pneumonitis difficult and highly susceptible to misdiagnosis and underdiagnosis [5].
Besides, Pulmonary infections can be caused by a wide variety of microorganisms, including bacteria, fungi, and viruses. Especially for immunocompromised individuals, it is necessary to consider mixed pulmonary infections. Metagenomic next-generation sequencing (mNGS) shows good diagnostic value in the pathogen detection of pulmonary infections, and it is a highly sensitive, and culture-independent method. One of the advantages of mNGS test is useful for rapid and accurate detection and identification of co-infections [6,7].
At present, EBV induced pneumonitis has not been reported in breast cancer patients receiving neoadjuvant chemotherapy in China and abroad, we present such a case to raise awareness of the diagnosis and management of EBV induced pneumonitis infection in immunodeficient patients.
2. Case report
A 52-year-old woman was admitted to our department because of a 3-day history of irritant dry cough. She had been previously diagnosed with grade III of infiltrating carcinoma on the right breast. Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2) were negative. The patient was started on neoadjuvant chemotherapy consisting of four courses of epirubicin (100 mg/m2) and cyclophosphamide (600 mg/m2) every 2 weeks, followed by four courses of paclitaxel (220 mg/m2) every 2 weeks. She had received 5 courses of treatment, and the chemotherapy response was mild. However, 10 days after the fifth course of chemotherapy she had begun a dry cough.
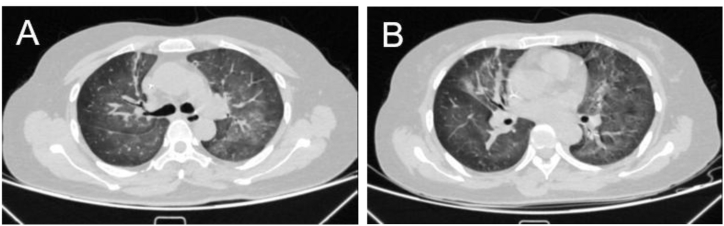
On admission, the patient was mentally good. Physical examination found a body temperature of 36.3 °C, blood pressure of 113/67 mm Hg, pulse of 116 beats per minute, respiration rate of 22 breaths per minute, and O2 saturation (SpO2) was 97% (no oxygen inhalation). The bilateral lung presented coarse breathing sounds. Laboratory examinations showed the following: blood routine: white blood cell count 9.2 × 109/L, the hemoglobin level was 80.0g/L, red blood cells count was 2.73 × 1012/L, the neutrophil count was 7.80 × 109/L, lymphocyte count was 0.7 × 109/L, with 0.8% lymphocytes; C-reactive protein (CRP) 7.9 mg/L; lactate dehydrogenase content increased to 372 U/L, and α-hydroxybutyrate dehydrogenase content increased to 295 U/L, as detailed in Table 1. The nucleic acid detection of COVID-19 was negative. There were no obvious abnormalities in urine routine, fecal routine, liver and kidney function, myocardial enzymes, infectious diseases, and coagulation function. The electrocardiogram and echocardiography were also normal, with an ejection fraction of 62%. Chest computed tomography (CT) shows multiple patchy ground-glass shadows in the bilateral lung, partially solid shadows, with scattered fiber bar shadows (Fig. 1A and B). In the meanwhile, the patient's serological tests showed negative results for antibodies to common respiratory pathogens (including influenza A and B, parainfluenza virus, Adenovirus, respiratory syncytial virus, cytomegalovirus, Mycoplasma pneumonia, and Chlamydia pneumonia). The 1,3-beta-D-glucan test (G test) result and the galactomannan test (GM test) were negative. The nucleic acid of EBV in the blood was reported at 4.78 × 103 copies/mL by quantitative PCR. EBV nuclear IgG antibody and Epstein-Barr viral capsid IgG antibody were positive.
Table 1.
Main laboratory test results before treatment.
| Laboratory Parameters | Normal Range | |
|---|---|---|
| White blood cell count/L | 9.2 × 109 | 3.5–9.5 × 109 |
| Red blood cell count/L | 2.73 × 1012 | 3.8–5.1 × 109 |
| Hemoglobin, g/L | 80.0 | 115–150 |
| Platelet count/L | 239 × 109 | 125–350 × 109 |
| neutrophil count/L | 7.80 × 109 | 1.8–6.3 × 109 |
| lymphocyte count/L | 0.7 × 109 | 1.1–3.2 × 109 |
| Neutrophil % | 84.6 | 40–75 |
| Lymphocyte % | 8.0 | 20–50 |
| Alanine aminotransferase, U/L | 13 | 7–45 |
| Aspartate aminotransferase, U/L | 22 | 13–35 |
| Serum albumin, g/L | 30.2 | 40–55 |
| Lactate dehydrogenase, U/L | 372 | 125–243 |
| α-hydroxybutyrate dehydrogenase, U/L | 295 | 74–199 |
| Calcium, mmol/L | 2.01 | 2.11–2.52 |
| Creatinine, umol/L | 53.8 | 49–90 |
| C-reactive protein, mg/L | 7.9 | <5 |
| Procalcitonin, ng/mL | 0.09 | <0.05 |
| 1,3-beta-D-glucan, pg/mL | 41.546 | <70 |
| Galactomannan | <0.5 | <0.5 |
| Total T lymphocyte count/μL | 707 | 955–2860 |
| Total B lymphocyte count/μL | 19 | 90–560 |
| CD4+ T cell count/μL | 435 | 550–1440 |
| CD8+ T cell count/μL | 212 | 320–1250 |
| CD4+/CD8+ ratio | 2.05 | 0.96–2.05 |
| EBV EA IgA, AU/mL | 1.942 | <3.0 |
| EBV EA IgG, AU/mL | 0.855 | <2.0 |
| EBV NA IgG, AU/mL | 17.11 | <3.0 |
| EBV VCA IgA, AU/mL | 2.986 | <4.0 |
| EBV VCA IgM, AU/mL | 0.010 | <3.0 |
| EBV VCA IgG, AU/mL | >50.00 | <2.0 |
Notes: *Abnormal values are indicated in bold face.
Fig. 1.
Fuzzy texture of double lungs, multiple patchy ground-glass shadows, some solid shadow, with scattered fiber cable shadow.
Her peripheral blood lymphocyte subsets revealed a low CD4+ lymphocyte count. Considering that the patient is an immunodeficiency individual after receiving chemotherapy for malignancy, and combined with the relevant adjuvant examination, the initial consideration is the possibility of EBV induced pneumonitis, but mixed pulmonary infections cannot be ruled out, such as Pneumocystis jirovecii pneumonia (PCP). Based on these findings, patients empirically received intravenous antibacterial (cefotaxime 3g, bid), antifungal (compound sulfamethoxazole 0.48g, tid), and antiviral (ganciclovir 0.25g bid) treatment.
To investigate pathogen-induced pneumonitis, we further performed bronchoalveolar lavage (BAL). An analysis of the BAL fluid (BALF) showed no malignant cells. The Gram stain and acid-fast stain of BALF were negative. Both the G test and GM test of BALF were negative. Furthermore, no bacteria or fungi were grown in the BALF culture. The mNGS result of BALF indicated that human herpesvirus type 4 (1088 in sequence). Some other nonspecific microorganisms are found by NGS, but they were not considered that they can cause a symptomatic lung infection. Combining these findings, we finally diagnosed EBV induced pneumonitis and excluded PCP. Therefore, cefotaxime and compound sulfamethoxazole were stopped, and antiviral treatment of ganciclovir was continued.
One week after the patient had received ganciclovir treatment, the patient's clinical symptoms were completely relieved, and the reexamination of chest CT showed significant absorption of the infectious lesion (Fig. 2A and B). The patient was followed up 2 weeks after discharge, and the reexamination of chest CT showed no exudation in both lungs (Fig. 3A and B).
Fig. 2.
Compare Fig. 1, significant absorption of multiple patchy and high-density shadows in the bilateral lung.
Fig. 3.
Compare Fig. 2, little change from before.
3. Discussion
EBV is usually acquired asymptomatically during infancy or early childhood and thereafter as a lifelong carrier of asymptomatic infections of the B-lymphatic system [8,9]. EBV in adults mainly presents as a latent infection, however, many conditions can disrupt the virus-host balance, like immunosuppression, which leads to viral activation of its pathogenic potential [3,10]. EBV infection is controlled by the immune system, and reactivation is considered to be the most common pathogenesis of infection in an immunodeficient state [11]. Long-term use of chemotherapy drugs disrupts the immune function of lymphocytes in the peripheral blood and damages the patient's immune system. Puts the patient in a susceptibility state. For patients with an impaired immune system, the immune response can lose control of EBV replication, EBV reactivate and rapidly replicate, which is a rare but reasonable pathogenic factor of lung infections [8,12].
EBV infection in adults induces pulmonary involvement is rare but can be fatal [13]. Currently, EBV infection is rarely regarded as responsible for invasive pulmonary infection. We believe that, as with most viral infections, EBV causes immediate and rapid damage to the lungs. During viral infection, alveolar inflammation enhances pulmonary host defenses and macrophages play a synergistic role, but promote infection regression while activating the immune system to cause immune-mediated injury. Large amounts of pro-inflammatory and pro-fibrotic factors are released, and the combination of the virus and these factors continues to induce, thereby promoting pulmonary fibrosis and causing fatal and substantial lung injury [[14], [15], [16]].
The early onset of EBV pneumonitis lacks characteristic clinical manifestations, mostly presenting with cough, expectoration, fever, and other symptoms. In addition, there is a lack of clear radiological features, most chest CT show ground-glass shadow, which is easily misdiagnosed and delays the treatment [5,17]. Currently, the main diagnosis of EBV infection relies on EBV-specific antibody tests, heterophile antibody tests, quantitative EBV DNA measurement, and EBER in situ hybridization [18]. EBER in situ hybridization is deemed the gold standard for the detection of EBV in tissue samples but has limited clinical use due to invasive manipulation. In the present case, the diagnosis of EBV pneumonitis was made based on the patient's symptoms, chest CT findings, EBV seropositive, and high circulating viral load of EBV in blood and BALF. At the same time, the long-term use of chemotherapy drugs is a high-risk group for opportunistic infection, and combined with mNGS to exclude other pathogens ultimately supports our diagnosis of EBV-induced pneumonitis.
In recent years, mNGS has been gradually applied in clinical practice, and when many conventional testing methods cannot be diagnosed, mNGS can simultaneously detect various bacterial, viral, fungal, or parasitic DNA/RNA sequences with high accuracy, providing strong evidence for clinical treatment [6,7,19]. In immunocompromised patients, mNGS also shows high sensitivity in the pathogen detection of pulmonary infection, which can accurately identify mixed infection as early as possible, which is crucial for timely targeted etiological treatment [19,20]. Furthermore, mNGS can also recognize the colonized bacteria in the host body, so the correct interpretation of the mNGS results is also quite challenging [21]. Clinicians need to combine the patient's clinical manifestations and laboratory examination results to obtain the final diagnosis and give precise treatment.
Notably, in this case, chest CT findings are non-specific and we need to differentiate them from multiple diseases. First, normal cardiac performance values combined with the absence of features of cardiac failure make pulmonary edema less likely to be a contributing factor. Secondly, given the timing of the last chemotherapy and the similarity of symptoms and chest CT findings to drug-induced pneumonitis in this case, it is difficult to completely rule out the contribution of drug toxicity caused by paclitaxel [22]. Unfortunately, no diagnostic test can adjudicate drug-induced pneumonitis, as it is indeed a diagnosis of exclusion [22,23]. However, there was ample evidence that this patient had known immunosuppression predisposing to a severe course of EBV infection. Based on these findings, as a presumptive diagnosis, we suspected EBV induced pneumonitis over drug-induced pneumonitis on admission. Furthermore, After receiving ganciclovir, the patient's clinical symptoms as well as radiological findings were significantly improved. Although the patient did not undergo lung tissue biopsy and lacked pathologic information, these clinical findings and outcomes were consistent with EBV induced pneumonitis. Based on these considerations, we thought that the final diagnosis of EBV induced pneumonitis is reasonable.
Although there are few reports of symptomatic pulmonary involvement directly attributable to EBV infection, EBV-induced pneumonitis should be considered in this new era of suffering from viral pneumonitis, especially in the immunocompromised host receiving chemotherapeutic agents, when appearing respiratory symptoms and high EBV viremia. Besides, it is necessary to consider multiple etiologies. Pulmonary toxicity is a common complication of some chemotherapeutic agents, but a definitive diagnosis relies on the exclusion of other causes of respiratory deterioration. Bronchoalveolar lavage is used for this purpose, and mNGS is recommended as early as possible to help clarify or exclude infection. In this case, assessment of disease status by serological examination combined with mNGS played a crucial role in the diagnosis of the disease and deserves to be promoted in the clinic. There are no specific antiviral drugs for EBV induced pneumonitis, but in the relevant literature, ganciclovir, acyclovir, and hormone therapy have shown good efficacy [4,5,24].In this case, the patient also received ganciclovir therapy for one week and then completely improved, accumulating evidence-based experience for the future clinical treatment of EBV induced pneumonitis.
In conclusion, clinicians should be aware neoadjuvant chemotherapy for breast cancer carries a risk of EBV induced pneumonitis. Which can be diagnosed based on the combined application of mNGS and traditional tests, and that antiviral treatment of ganciclovir is effective.
Declaration of competing interest
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
Contributor Information
Huibin Liao, Email: liaohuibin2021@outlook.com.
Zhenshun Cheng, Email: zhenshun_cheng@126.com.
References
- 1.Epstein M.A., Achong B.G., Barr Y.M. Virus particles in cultured lymphoblasts from burkitt's lymphoma. Lancet. 1964;1(7335):702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 2.Ok C.Y., Li L., Young K.H. EBV-driven B-cell lymphoproliferative disorders: from biology, classification and differential diagnosis to clinical management. Exp. Mol. Med. 2015;47(1):e132. doi: 10.1038/emm.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor G.S., Long H.M., Brooks J.M., Rickinson A.B., Hislop A.D. The immunology of Epstein-Barr virus-induced disease. Annu. Rev. Immunol. 2015;33:787–821. doi: 10.1146/annurev-immunol-032414-112326. [DOI] [PubMed] [Google Scholar]
- 4.Niazi M.R., Iqbal Q.Z., Mishiyev D., Narula N., Abdul Sattar S.B., Zia Z., et al. Epstein-Barr virus (EBV) induced pneumonitis in an immunocompetent adult: a case report. Respir. Med. Case Rep. 2020;31 doi: 10.1016/j.rmcr.2020.101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McManus T.E., Coyle P.V., Lawson J., Elborn J.S., Kidney J.C. Epstein - Barr virus pneumonitis. Ulster Med. J. 2009;78(2):137–138. [PMC free article] [PubMed] [Google Scholar]
- 6.Gu W., Miller S., Chiu C.Y. Clinical metagenomic next-generation sequencing for pathogen detection. Ann. Rev. Pathol. 2019;14:319–338. doi: 10.1146/annurev-pathmechdis-012418-012751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simner P.J., Miller S., Carroll K.C. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin. Infect. Dis. 2018;66(5):778–788. doi: 10.1093/cid/cix881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbott R.J., Pachnio A., Pedroza-Pacheco I., Leese A.M., Begum J., Long H.M., et al. Asymptomatic primary infection with Epstein-Barr virus: observations on young adult cases. J. Virol. 2017;91(21) doi: 10.1128/JVI.00382-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jayasooriya S., de Silva T.I., Njie-jobe J., Sanyang C., Leese A.M., Bell A.I., et al. Early virological and immunological events in asymptomatic Epstein-Barr virus infection in African children. PLoS Pathog. 2015;11(3) doi: 10.1371/journal.ppat.1004746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr J.R. Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J. Clin. Pathol. 2019;72(10):651–658. doi: 10.1136/jclinpath-2019-205822. [DOI] [PubMed] [Google Scholar]
- 11.AbuSalah M.A.H., Gan S.H., Al-Hatamleh M.A.I., Irekeola A.A., Shueb R.H., Yean Yean C. Recent advances in diagnostic approaches for Epstein-Barr virus. Pathogens. 2020;9(3):226. doi: 10.3390/pathogens9030226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrei G., Trompet E., Snoeck R. Novel therapeutics for Epstein-Barr virus. Molecules. 2019;24(5):997. doi: 10.3390/molecules24050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui Y., Zhang Y., Wang F., Zhu Y., Chen R., Xu L. Lung injury associated with severe Epstein-Barr virus (EBV) infection. Zhonghua er ke za zhi. 2015;53(8):586–591. [PubMed] [Google Scholar]
- 14.Huang W.J., Tang X.X. Virus infection induced pulmonary fibrosis. J. Transl. Med. 2021;19(1):496. doi: 10.1186/s12967-021-03159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malizia A.P., Keating D.T., Smith S.M., Walls D., Doran P.P., Egan J.J. Alveolar epithelial cell injury with Epstein-Barr virus upregulates TGFbeta1 expression. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295(3):L451–L460. doi: 10.1152/ajplung.00376.2007. [DOI] [PubMed] [Google Scholar]
- 16.Sides M.D., Klingsberg R.C., Shan B., Gordon K.A., Nguyen H.T., Lin Z., et al. The Epstein-Barr virus latent membrane protein 1 and transforming growth factor--β1 synergistically induce epithelial--mesenchymal transition in lung epithelial cells. Am. J. Respir. Cell Mol. Biol. 2011;44(6):852–862. doi: 10.1165/rcmb.2009-0232OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koo H.J., Lim S., Choe J., Choi S.H., Sung H., Do K.H. Radiographic and CT features of viral pneumonia. Radiographics. 2018;38(3):719–739. doi: 10.1148/rg.2018170048. [DOI] [PubMed] [Google Scholar]
- 18.Gulley M.L., Tang W. Laboratory assays for Epstein-Barr virus-related disease. J. Mol. Diagn. 2008;10(4):279–292. doi: 10.2353/jmoldx.2008.080023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J., Han Y., Feng J. Metagenomic next-generation sequencing for mixed pulmonary infection diagnosis. BMC Pulm. Med. 2019;19(1):252. doi: 10.1186/s12890-019-1022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin P., Chen Y., Su S., Nan W., Zhou L., Zhou Y., et al. Diagnostic value of metagenomic next-generation sequencing of bronchoalveolar lavage fluid for the diagnosis of suspected pneumonia in immunocompromised patients. BMC Infect. Dis. 2022;22(1):416. doi: 10.1186/s12879-022-07381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller S., Chiu C., Rodino K.G., Miller M.B. Point-counterpoint: should we Be performing metagenomic next-generation sequencing for infectious disease diagnosis in the clinical laboratory? J. Clin. Microbiol. 2020;58(3) doi: 10.1128/JCM.01739-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mo H., Jazieh K.A., Brinzevich D., Abraham J. A review of treatment-induced pulmonary toxicity in breast cancer. Clin. Breast Cancer. 2022;22(1):1–9. doi: 10.1016/j.clbc.2021.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Omarini C., Thanopoulou E., Johnston S.R. Pneumonitis and pulmonary fibrosis associated with breast cancer treatments. Breast Cancer Res. Treat. 2014;146(2):245–258. doi: 10.1007/s10549-014-3016-5. [DOI] [PubMed] [Google Scholar]
- 24.Ankermann T., Claviez A., Wagner H.J., Krams M., Riedel F. Chronic interstitial lung disease with lung fibrosis in a girl: uncommon sequelae of Epstein-Barr virus infection. Pediatr. Pulmonol. 2003;35(3):234–238. doi: 10.1002/ppul.10244. [DOI] [PubMed] [Google Scholar]