Key Points
-
•
The life expectancy among Medicare and Medicaid beneficiaries with sickle cell disease was 52.6 years.
-
•
Individuals insured by Medicare for disabilities or end-stage renal disease and dually insured by Medicare and Medicaid had worse survival.
Visual Abstract
Abstract
To our knowledge, we report the first population-based period life table, the expected lifetime survival for Medicare and Medicaid beneficiaries with sickle cell disease (SCD), and the disparities in survival by insurance types in the United States. We constructed a retrospective cohort of individuals with diagnosed SCD receiving common care (any real-world patterns of care except transplant) based on nationwide Medicare and Medicaid claim data (2008-2016), covering beneficiaries in all 50 states. We analyzed lifetime survival probabilities using Kaplan-Meier curves and projected life expectancies at various ages for all, stratified by sex and insurance types. Our analysis included 94 616 individuals with SCD that have not undergone any transplant. Life expectancy at birth was 52.6 years (95% confidence interval: 51.9-53.4). Compared with the adults covered by Medicaid only, those covered by Medicare for disabilities or end-stage renal disease and those dually insured by Medicare and Medicaid had significantly worse life expectancy. Similarly, for beneficiaries aged ≥65 years, these 2 insurance types were associated with significantly shorter life expectancy than those enrolled in Medicare old age and survivor’s insurance. Our study underscores the persistent life expectancy shortfall for patients with SCD, the burden of premature mortality during adulthood, and survival disparities by insurance status.
Introduction
Sickle cell disease (SCD) is a group of genetically inherited disorders of hemoglobin that affects ∼100 000 people in the United States.1 The prevalence of SCD is disproportionately higher among people of African descent than other ethnicities.1 An individual with SCD is prone to a wide range of acute and chronic life-threatening complications, such as acute pain crisis, stroke, acute chest syndrome, chronic pain, symptomatic anemia, and an increased risk of infections and organ damage.1,2
Owing to the widespread adoption of several medical interventions over the past few decades, including hydroxyurea, newborn screening, pneumococcal vaccination, and prophylactic antibiotics, the survival rate of children with SCD in the United States has considerably improved.3, 4, 5 However, the complications of SCD can still lead to premature mortality during adulthood, and thereby the life expectancy of individuals with SCD remains well below the general population average.3, 4, 5 According to a recent study, the life expectancy for a US birth cohort with SCD is ∼2 decades shorter than that of those without SCD.6 However, these projections were made based on a simulation model informed via summary statistics rather than an empirical estimation from individual data. No population-level individual data–based periodic life table exists for individuals with SCD.
Most people living with SCD in the United States are covered by public insurance, with Medicaid as the major source of coverage.7,8 Insurance coverage plays a vital role in patients’ access to necessary and appropriate health care management, thus affecting their mortality outcomes.8, 9, 10 Prior studies examined the mortality of Medicaid or Medicaid beneficiaries with SCD.11, 12, 13, 14 Yet, these studies were limited to a few states11 or hospital settings,14 focused on a single public insurance type,11, 12, 13 or did not report age-specific information.11,13,14
Moreover, the survival of individuals with SCD may differ across the types of public insurance. For example, some Medicare beneficiaries are also enrolled in Medicaid. Those dual-eligible individuals tend to experience a higher burden of chronic diseases and poorer survival outcomes than single eligibles.15 In addition, evidence has shown that younger people entitled to Medicare because of disabilities or end-stage renal diseases (ESRD) face an increased mortality risk.16,17 Although the disparity in survival by public insurance type has been explored in the general population, little is known about the SCD population.
To address the identified gaps in knowledge, we aimed to estimate period life tables among Medicare and Medicaid beneficiaries with SCD and examine the potential disparities in survival by insurance type based on US nationwide claims data.
Methods
Data source
Data for this study were obtained from the Medicaid Analytic eXtract files and Medicare Part A and B Fee-for-Service claims covering enrollees from 2008 to 2016. The data contain demographic information, insurance enrollment status, and administrative claims for 100% of the individuals with SCD covered by Medicaid or Medicare in all 50 states. In addition, information on the exact date of death was derived from death certificates provided through the linkage with the National Death Index. The details about the data files can be found on the website of the Centers for Medicare and Medicaid Services Research Data Assistance Center.18 This study was approved by the University of Washington Institutional Review Board.
Study population
We conducted a retrospective cohort study of Medicare and Medicaid beneficiaries with SCD in the United States. They were identified based on the SCD indicator developed by the Centers for Medicare & Medicaid Services Chronic Conditions Data Warehouse (CCW). The CCW’s algorithm requires 3 or more nondrug claims of any service type with diagnosis codes for SCD (supplemental Table 1) during a 5-year “look-back” period.19 We included individuals who were continuously enrolled for 12 months after the entry date.20 Further, we restricted the follow-up of our cohort to those receiving “common care,” consisting of all-comers who either are receiving no treatment or hydroxyurea or transfusions (but did not receive hematopoietic stem cell transplant).20 This common care cohort was identified to serve as a control group for individuals who would be eligible for novel disease-modifying or gene therapies in SCD.20 The insurance statuses for individuals in our common care cohort were classified as Medicare old age and survivor’s insurance (OASI) only, Medicare disability insurance benefits (DIB) or ESRD only, Medicaid only, and dual-eligible individuals (defined as those who are enrolled in both Medicare and Medicaid at least some time during their lives). We excluded the individuals aged <65 years whose insurance status was classified as Medicare OASI but not DIB or ESRD (Figure 1). The Centers for Medicare and Medicaid Services obtained information about the original reason for Medicare entitlement (OASI, DIB, or ESRD) from the Social Security Administration and Railroad Retirement Board record systems.18 Individuals were followed until death, the end of enrollment, or receiving hematopoietic stem cell transplantation, whichever occurred first. Only the longest enrollment period was included in the analysis for those with multiple distinct enrollment periods during the study period.
Figure 1.
Patient selection flowchart.
Study variables
The outcome variable was time to event, from the age of enrollment to death. The end of enrollment or receiving hematopoietic stem cell transplantation were considered censoring events. We also summarized the following demographic characteristics by insurance status: age at entry and exit, sex, region (Northeast, Midwest, South, and West), race (White, Black, Hispanic, and others), and birth cohort (classified according to the year of birth: 1900-1939, 1940-1959, 1960-1979, 1980-1999, 2000-2004, 2005-2009, and 2010-2016, based on population size as the number of individuals with SCD decreases rapidly with age). The demographic characteristics were ascertained at the index date, when the first use with a diagnosis of SCD occurred.
Analysis
We used the Kaplan-Meier approach to estimate period life tables and lifetime survival curves for individuals with SCD. Using these period life tables, we computed expected life expectancies at birth and ages 18, 35, 45, 65, and 85 years old. The analysis was further stratified by sex and insurance status. The risk set accounted for delayed entry (left truncation) and right censoring of individuals enrolled in the insurance program. Clustered bootstrapped replicates (1000 deviates) were used to generate 95% confidence intervals (CIs) for the survival probabilities and life expectancies.
Furthermore, we estimated the life expectancies for the Black and non-Black groups separately to identify the potential racial disparity. Finally, because there might be a disparity between individuals with Medicare DIB and those with Medicare ESRD, additional analyses were performed with Medicare DIB or ESRD being further classified as Medicare DIB only and Medicare ESRD only.
The Kaplan-Meier curves were generated using the package ggplot2 in Rstudio 2022.02.0, and the data manipulation was performed in Stata/MP 17.0.
Results
We included 94 616 individuals with SCD between 2008 and 2016, of whom 5% had Medicare OASI, 4% had Medicare DIB or ESRD, 48% had Medicaid, and 43% were dually eligible for Medicare and Medicaid (Table 1). The mean entry ages were 73.4 (standard deviation [SD]: 7.5), 50.2 (SD: 13.5), 15.4 (SD: 14.9), and 31.4 (SD: 21.2) years for Medicare OASI, Medicare DIB or ESRD, Medicaid, and dual-eligible cohorts, respectively. The mean entry age overall was 26.6 years (SD: 22.5) across all the insurance types. The mean and median follow-up times were 6 (SD: 3) and 7 (interquartile range: 4-9) years, respectively. Most of the included individuals were Black (74%) and most resided in the South (53%) at the index date. Similar racial and regional distribution was found across the insurance types, except that 53% of the Medicare OASI beneficiaries were White.
Table 1.
Descriptive characteristics for individuals with SCD by insurance status
| Total (N = 94 616) | Medicare OASI (N= 4 475) | Medicare DIB or ESRD (N = 4 153) | Medicaid (N = 45 068) | Dual eligibles (N = 40 920) | |
|---|---|---|---|---|---|
| Mean age at entry (y) | 26.6 (22.6) | 73.4 (7.5) | 50.2 (13.5) | 15.4 (14.9) | 31.4 (21.2) |
| Mean age at exit (y) | 33.0 (22.6) | 79.9 (7.9) | 56.7 (13.9) | 21.4 (14.7) | 38.1 (21.3) |
| Claims | 7 331 038 | 357 086 | 330 363 | 3 331 488 | 3 312 101 |
| Sex | |||||
| Male | 40 877 (43%) | 1 737 (39%) | 1 857 (45%) | 20 452 (45%) | 16 831 (41%) |
| Female | 53 739 (57%) | 2 738 (61%) | 2 296 (55%) | 24 616 (55%) | 24 089 (59%) |
| Race | |||||
| White | 6 476 (7%) | 2 389 (53%) | 478 (12%) | 1 319 (3%) | 2 290 (6%) |
| Black | 70 064 (74%) | 1 869 (42%) | 3 486 (84%) | 31 651 (70%) | 33 058 (81%) |
| Hispanic | 4 069 (4%) | 97 (2%) | 84 (2%) | 2 189 (5%) | 1 699 (4%) |
| Other | 14 007 (15%) | 120 (3%) | 105 (3%) | 9 909 (22%) | 3 873 (10%) |
| Region | |||||
| Northeast | 18 630 (20%) | 951 (21%) | 694 (17%) | 10 054 (22%) | 6 931 (17%) |
| Midwest | 17 460 (19%) | 798 (18%) | 627 (15%) | 7 428 (17%) | 8 607 (21%) |
| South | 50 428 (53%) | 2 150 (48%) | 2 526 (61%) | 23 592 (52%) | 22 160 (54%) |
| West | 8 098 (9%) | 576 (13%) | 306 (7%) | 3 994 (9%) | 3 222 (8%) |
| Birth cohort | |||||
| 1900-1939 | 6 140 (7%) | 3 107 (69%) | 317 (8%) | 38 (0%) | 2 678 (7%) |
| 1940-1959 | 11 317 (12%) | 1 368 (31%) | 2 057 (50%) | 1 837 (4%) | 6 055 (15%) |
| 1960-1979 | 19 306 (20%) | 0 (0%) | 1 443 (35%) | 6 355 (14%) | 11 508 (28%) |
| 1980-1999 | 33 478 (35%) | 0 (0%) | 336 (8%) | 18 720 (42%) | 14 422 (35%) |
| 2000-2004 | 8 281 (9%) | 0 (0%) | 0 (0%) | 6 289 (14%) | 1 992 (5%) |
| 2005-2009 | 9 959 (11%) | 0 (0%) | 0 (0%) | 7 433 (17%) | 2 526 (6%) |
| 2010-2016 | 6 135 (7%) | 0 (0%) | 0 (0%) | 4 396 (10%) | 1 739 (4%) |
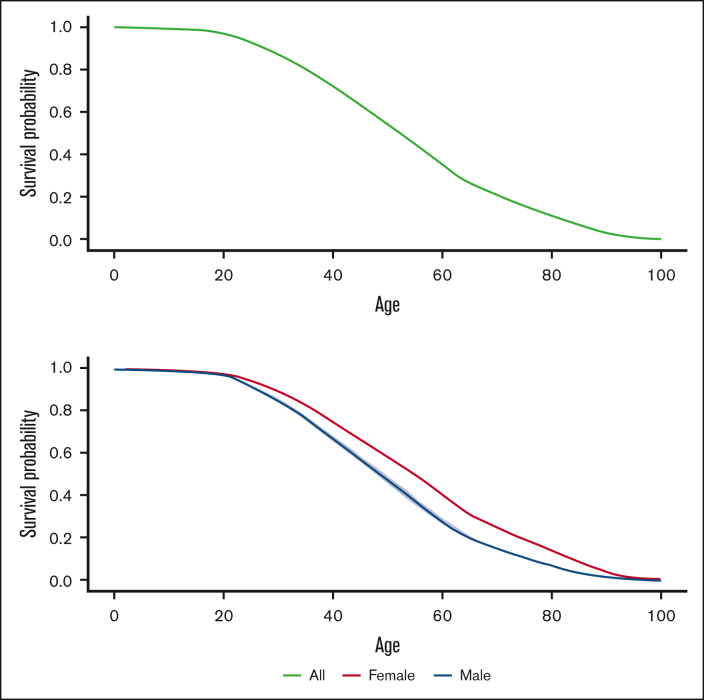
The Kaplan-Meier curve in Figure 2 depicts the survival probabilities over age for all the included individuals. They maintained high survival with a steady decline during childhood; the survival probability at 18 years old was 0.980 (95% CI: 0.977-0.984). After transitioning into adulthood, however, their survival declined rapidly. The survival probabilities were 0.804 (95% CI: 0.795-0.815), 0.628 (95% CI: 0.616-0.641), 0.267 (95% CI: 0.255-0.279), and 0.070 (95% CI: 0.064-0.075) at 30, 45, 65, and 85 years old, respectively. Females had significantly greater survival likelihoods than males during adulthood.
Figure 2.
Survival curves for all the individuals with SCD (top panel) and by sex (bottom panel).
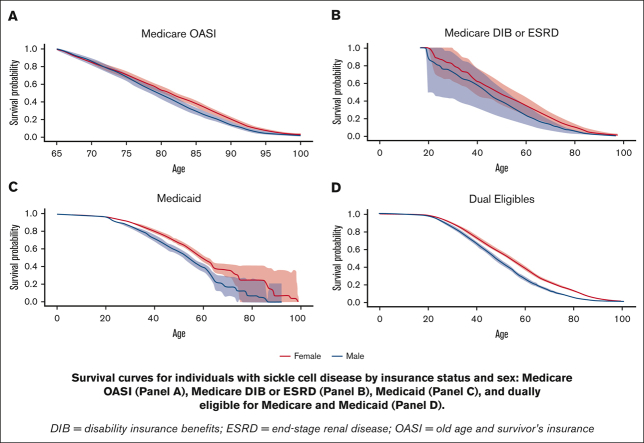
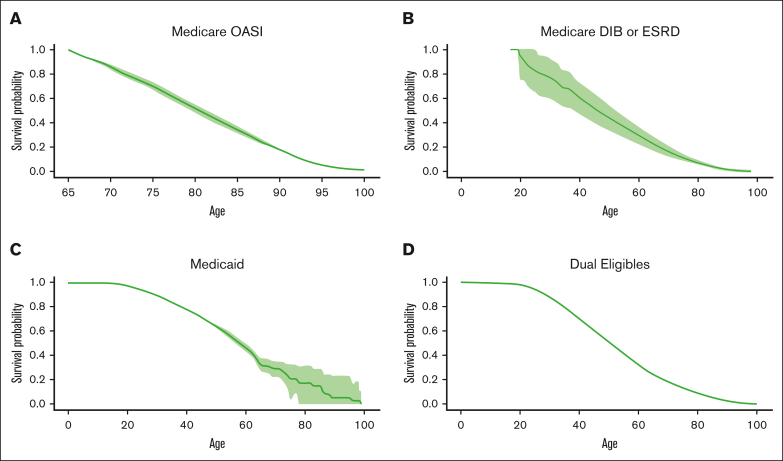
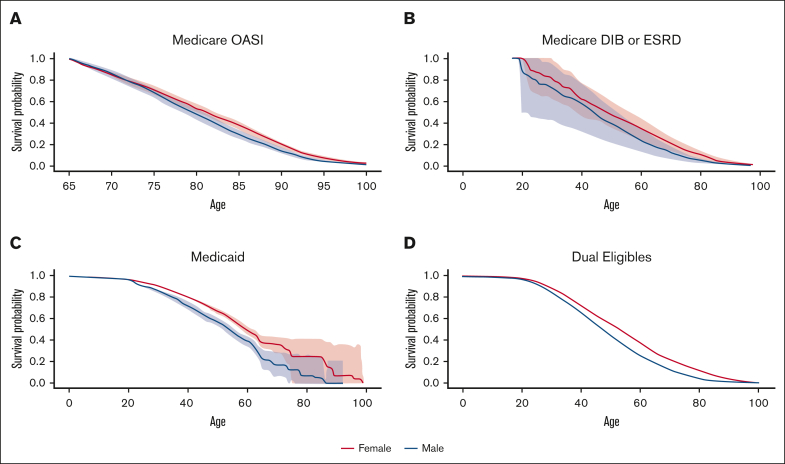
Figure 3 presents the Kaplan-Meier curve for each insurance type. The Medicaid and dual-eligible cohorts had a similar probability of survival till 18 years of age, with 0.979 (95% CI: 0.975-0.983) for the former and 0.984 (0.978-0.989) for the latter. The probability of survival to 45 years old was highest among individuals with Medicaid across the insurance types, estimated to be 0.707 (95% CI: 0.688-0.728). At the age of 85 years, the survival probability was 0.343 (95% CI: 0.318-0.371) for the elderly cohort covered by Medicare OASI, which was better than the other cohorts. The survival curve for each insurance cohort by sex can be found in Figure 4. The females who were covered by Medicaid or were dually eligible experienced greater survival probabilities than the males for most of their lifetime. The supplementary curves showing the cumulative mortality over age can be found in supplemental Appendices 1-3.
Figure 3.
Survival curves for individuals with SCD by insurance status. Medicare OASI (A), Medicare DIB or ESRD (B), Medicaid (C), and dually eligible for Medicare and Medicaid (D).
Figure 4.
Survival curves for individuals with SCD by insurance status and sex. Medicare OASI (A), Medicare DIB or ESRD (B), Medicaid (C), and dually eligible for Medicare and Medicaid (D).
The life expectancy of all the Medicare and Medicaid beneficiaries with SCD was 52.6 years (95% CI: 51.9-53.4) at birth, 35.4 years (95% CI: 34.9-36.1) at 18 years old, 24.1 years (95% CI: 23.7-24.6) at 35 years old, 19.6 years (95% CI: 19.2-20.0) at 45 years old, 13.2 years (95% CI: 13.0-13.5) at 65 years old, and 5.4 years (95% CI: 5.1-5.7) at 85 years old (Table 2). Similar to the difference by sex in survival probability observed above, the life expectancy at birth for females (55.0 [95% CI: 54.1-56.1]) was significantly longer compared with that of males (49.3 [95% CI: 48.2-50.4]). The Medicaid cohort had a life expectancy of 39.8 years (95% CI: 35.9-44.9) at 18 years old, better than the Medicare DIB or ESRD and dual-eligible cohorts. At 65 years old, the life expectancy for individuals with Medicare OASI (15.2 [95% CI: 14.5-16.1]) was significantly longer than for those with Medicare DIB or ESRD and dual-eligible patients.
Table 2.
Life expectancies (95% CI) at different ages for individuals with SCD by insurance status
| Age | All | Female | Male |
|---|---|---|---|
| Total | |||
| Birth | 52.6 (51.9-53.4) | 55.0 (54.1-56.1) | 49.3 (48.2-50.4) |
| 18 | 35.4 (34.9-36.1) | 37.8 (37.0-38.6) | 32.2 (31.3-33.1) |
| 35 | 24.1 (23.7-24.6) | 26.0 (25.4-26.6) | 21.4 (20.7-22.1) |
| 45 | 19.6 (19.2-20.0) | 21.3 (20.8-21.8) | 17.1 (16.6-17.6) |
| 65 | 13.2 (13.0-13.5) | 14.0 (13.7-14.4) | 11.9 (11.6-12.2) |
| 85 | 5.4 (5.1-5.7) | 5.6 (5.2-6.0) | 4.9 (4.5-5.4) |
| Medicare OASI | |||
| 65 | 15.2 (14.5-16.1) | 15.8 (14.7-16.9) | 14.5 (13.2-15.7) |
| 85 | 5.4 (5.0-5.8) | 5.6 (5.2-6.1) | 5.0 (4.4-5.5) |
| Medicare DIB or ESRD | |||
| 18 | 29.7 (23.0-35.7) | 32.5 (25.0-39.5) | 27.0 (15.4-35.7) |
| 35 | 23.0 (22.5-23.8) | 24.4 (23.4-25.5) | 21.4 (20.7-22.5) |
| 45 | 18.9 (18.4-19.5) | 20.7 (19.9-21.6) | 16.7 (15.9-17.5) |
| 65 | 11.2 (10.7-11.7) | 12.1 (11.0-13.2) | 10.1 (9.4-11.0) |
| 85 | 4.7 (3.5-6.1) | 5.0 (3.4-7.0) | 4.2 (2.3-6.0) |
| Medicaid | |||
| Birth | 56.8 (52.8-62.0) | 60.0 (54.3-67.4) | 51.8 (48.4-57.8) |
| 18 | 39.8 (35.9-44.9) | 43.0 (37.4-50.1) | 34.8 (31.6-40.6) |
| 35 | 28.0 (24.0-33.5) | 30.7 (24.8-38.3) | 23.5 (20.4-29.6) |
| 45 | 22.3 (17.7-28.4) | 24.7 (18.3-32.9) | 18.1 (14.7-24.8) |
| 65 | 16.1 (8.1-25.3) | 18.2 (7.4-29.8) | 11.3 (5.6-22.2) |
| 85 | 4.8 (0.0-9.9) | 4.6 (0.0-11.6) | 0.0 (0.0-5.2) |
| Dual eligibles | |||
| Birth | 51.1 (50.1-52.0) | 53.4 (52.1-54.7) | 47.9 (46.6-49.3) |
| 18 | 33.7 (33.1-34.5) | 36.0 (35.1-37.0) | 30.7 (29.6-31.7) |
| 35 | 22.5 (22.0-23.1) | 24.4 (23.8-25.1) | 19.8 (19.1-20.6) |
| 45 | 18.4 (17.9-18.8) | 20.1 (19.6-20.7) | 15.7 (15.0-16.4) |
| 65 | 12.1 (11.8-12.5) | 13.0 (12.6-13.5) | 9.9 (9.4-10.4) |
| 85 | 5.3 (4.9-5.8) | 5.4 (5.0-5.9) | 5.0 (4.3-5.8) |
The results of the additional analyses can be found in supplemental Tables 2 and 3. Black individuals had a significantly shorter life expectancy at birth (52.2 [95% CI: 51.4-53.1]) than non-Black individuals (55.1 [95% CI: 53.2-57.0]). In the second analysis, the survival was significantly worse for adults with Medicare ESRD and those with both Medicare DIB and ESRD compared with those with Medicare DIB.
Discussion
Using a Medicare and Medicaid administrative database linked to the National Death Index, we estimated the survival of individuals with SCD and projected their life expectancies at various ages. We found that this population's life expectancy at birth was 52.6 years, with females presenting superior survival to males. Furthermore, adults aged 18 to 64 years enrolled in Medicare DIB or ESRD or dually eligible for Medicare and Medicaid had a higher death rate than those covered by Medicaid. Similarly, for enrollees aged ≥65 years, these 2 insurance types were associated with significantly worse survival relative to Medicare OASI.
Previous studies reported the mortality of individuals with SCD in the United States.6,11, 12, 13, 14 However, these analyses were conducted based on aggregated statistics or limited individual-level data. To the best of our knowledge, this is the first United States nationwide investigation into lifetime survival for individuals with SCD covered by Medicare and Medicaid and disparities in survival by public insurance type, using comprehensive claims data collected from all 50 states.
A recent study simulated the life trajectory and mortality outcomes of a United States SCD birth cohort based on data from the US Centers for Disease Control and Prevention, the National Newborn Screening Information System, and published mortality literature.6 Consistent with our estimate, they projected a life expectancy of 54 years at birth. One discrepancy is that their projections did not differ between males and females with SCD. We could not explain this discrepancy because their article did not provide details about the sex-specific mortality inputs used. However, the published mortality study they relied on21 and other prior studies22,23 did estimate a higher mean age of death in females than males with SCD.
Based on the 2016 US Centers for Disease Control and Prevention life table, the life expectancy of the general Black population was 75 years.24 Similarly, Lubeck et al6 estimated a life expectancy of 76 years for a non-SCD population matched on age, sex, and race or ethnicity with the SCD population. Both are substantially longer than our estimate for the Medicare and Medicaid beneficiaries with SCD. Evidently, the life expectancy gap persists among patients with SCD, even though they are protected by public insurance. Furthermore, the survival rates of individuals with SCD remained high during childhood but declined significantly after adulthood, echoing the general recognition that survivorship into adulthood for children with SCD is now a less problematic issue but early mortality among adults remains concerning.3 Our study also suggests that Black beneficiaries may experience worse survival outcomes than non-Black beneficiaries. However, the potential measurement error of race coding in claims data warrants caution in interpreting the race-specific results.
Another critical study finding is that the survival of adults with SCD varies across public insurance types. We demonstrate that ESRD, disability (an eligible criterion for Medicare regardless of age), or dual-eligible status remains a significant predictor of worse survival among adults with SCD.
Chronic kidney disease is a common complication among SCD individuals, and a fraction of them will progress to ESRD.25 It has been shown that patients with SCD with ESRD have a remarkably inferior prognosis, associated with a mortality rate of 26% within the first year of initiating renal replacement therapy.25 Although the premature death attributable to ESRD seems well understood, more research would be beneficial for characterizing and explaining premature death for disabled or dual-eligible adults with SCD. The existing literature has extensively explored excess deaths among disabled individuals in the general community and in other disease areas, which can be explained by clinical comorbidities, unhealthy behaviors, poor access to preventive services and high-quality health care, and low socioeconomic status,26, 27, 28 yet there is little knowledge about the mechanism in the SCD population. Similarly, it is well recognized that the dual-eligible individuals are a vulnerable population with some of the most complex and expensive health care needs.29 They are more likely to have fewer socioeconomic resources, more chronic conditions, and poorer survival outcomes than single eligibles.15,30, 31, 32 Future studies should uncover factors influencing survival outcomes and explore policy options to address the unmet needs of the disabled or dually eligible population with SCD.
The major strength of our study is that, with the claims data from all 50 states, we provide the US nationwide estimates of survival among both Medicare and Medicaid beneficiaries with SCD. Moreover, leveraging this large database covering ∼100 000 individuals, we created a comprehensive catalog of survival profiles at various ages for all the individuals and by sex and insurance type. Additionally, the Medicare and Medicaid claims data was linked to the National Death Index, providing accurate death data for these individuals. Finally, appropriate statistical methods were used to produce the survival estimates.
Our study is not without limitations. First, our analyses relied on Medicare and Medicare beneficiaries and thus may not be generalizable to other SCD populations such as commercially insured or uninsured individuals. Studies have shown that individuals with commercial insurance may experience life-threatening complications (eg, vaso-occlusive crisis) less frequently and have greater access to health care than those covered by Medicaid.33,34 We also should not ignore the possibility that mortality risk may be even higher among uninsured individuals because of health care access issues.34 Second, CCW identified the individuals with SCD using an administrative claims-based definition. Misclassifications may be inevitable, although the definition has been validated with a sensitivity of 0.96.35 Third, although it is well recognized that the disease severity may differ based on the genotypes of SCD, we did not stratify the analysis by genotype because of the limited sample size and the accuracy of identifying these subsets in claims data. Future studies could close this knowledge gap using appropriate genotype-specific data sets. Finally, albeit the use of recently available Medicare and Medicaid data, the treatment pattern among the older individuals might not reflect the evolvements in medical interventions over the past decades, such as the use of penicillin prophylaxis and medications like hydroxyurea. Also, our analysis did not capture the possible change in health outcomes due to the uptake of recently approved emerging therapies, which have been phased in over recent years. We recommend future research to investigate the latest survival trends in the SCD population, reflecting the quickly evolving landscape of SCD treatments.
Conclusions
We provide US nationwide long-term survival estimates for Medicare and Medicaid beneficiaries with SCD. Our findings highlight the persistent life expectancy gap for patients with SCD and the enduring premature mortality throughout adulthood. This gap has improved little in recent decades. We also identify the inferior survival among those with Medicare DIB or ESRD and those dually eligible for Medicare and Medicaid. Further research should explore the clinical and socioeconomic factors that can explain the disparity across the insurance types.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
The authors gratefully acknowledge the National Heart, Lung and Blood Institute, participants from Emmes, the CureSCi Expert Panel, and the CureSCi Initiative.
National Heart, Lung and Blood Institute, Cure Sickle Cell initiative. This research was funded in part by the National Institutes of Health Agreements OT3HL152448 and OT3HL151434. Support for data access and analyses for this research came from the UW’s Population Health Initiative, UW’s Student Technology Fee program, the UW’s Provost’s office, and a Eunice Kennedy Shriver National Institute of Child Health and Human Development research infrastructure grant, P2C HD042828, to the Center for Studies in Demography & Ecology at the University of Washington.
The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the NIH.
Authorship
Contribution: A.B., S.D.R., B.D., and M.A.B. conceived of the study; B.J., K.M.J., and A.B. conducted the analyses; B.J. wrote the first draft of the manuscript; all authors critically commented on the manuscript and approved the final version; and A.B. is the guarantor of the manuscript.
Footnotes
Data are available on request from the corresponding author, Anirban Basu (basua@uw.edu).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.National Heart, Lung, and Blood Institute Sickle Cell Disease. National Institutes of Health. 2022 https://www.nhlbi.nih.gov/health-topics/education-and-awareness/sickle-cell [Google Scholar]
- 2.Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390(10091):311–323. doi: 10.1016/S0140-6736(17)30193-9. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi S, DeBaun MR. Evolution of sickle cell disease from a life-threatening disease of children to a chronic disease of adults: the last 40 years. Am J Hematol. 2016;91(1):5–14. doi: 10.1002/ajh.24235. [DOI] [PubMed] [Google Scholar]
- 4.Lanzkron S, Carroll CP, Haywood C., Jr. Mortality rates and age at death from sickle cell disease: US, 1979-2005. Public Health Rep. 2013;128(2):110–116. doi: 10.1177/003335491312800206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maitra P, Caughey M, Robinson L, et al. Risk factors for mortality in adult patients with sickle cell disease: a meta-analysis of studies in North America and Europe. Haematologica. 2017;102(4):626–636. doi: 10.3324/haematol.2016.153791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lubeck D, Agodoa I, Bhakta N, et al. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Netw Open. 2019;2(11):e1915374. doi: 10.1001/jamanetworkopen.2019.15374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kayle M, Valle J, Paulukonis S, et al. Impact of Medicaid expansion on access and healthcare among individuals with sickle cell disease. Pediatr Blood Cancer. 2020;67(5):e28152. doi: 10.1002/pbc.28152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee L, Smith-Whitley K, Banks S, Puckrein G. Reducing health care disparities in sickle cell disease: a review. Public Health Rep. 2019;134(6):599–607. doi: 10.1177/0033354919881438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perimbeti SP, Hou KY, Ramanathan S, et al. The effect of health care disparities on complications and mortality in sickle cell disease. Blood. 2018;132(suppl 1):5886. [Google Scholar]
- 10.Cintron-Garcia J, Ajebo G, Kota V, Guddati AK. Mortality trends in sickle cell patients. Am J Blood Res. 2020;10(5):190–197. [PMC free article] [PubMed] [Google Scholar]
- 11.Grady A, Fiori A, Patel D, Nysenbaum J. Profile of Medicaid enrollees with sickle cell disease: a high need, high cost population. PLoS One. 2021;16(10):e0257796. doi: 10.1371/journal.pone.0257796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai RJ, Mahesri M, Globe D, et al. Clinical outcomes and healthcare utilization in patients with sickle cell disease: a nationwide cohort study of Medicaid beneficiaries. Ann Hematol. 2020;99(11):2497–2505. doi: 10.1007/s00277-020-04233-w. [DOI] [PubMed] [Google Scholar]
- 13.Shah N, Bhor M, Xie L, et al. Evaluation of vaso-occlusive crises in United States sickle cell disease patients: a retrospective claims-based study. J Health Econ Outcomes Res. 2019;6(3):106–117. doi: 10.36469/9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCavit TL, Lin H, Zhang S, Ahn C, Quinn CT, Flores G. Hospital volume, hospital teaching status, patient socioeconomic status, and outcomes in patients hospitalized with sickle cell disease. Am J Hematol. 2011;86(4):377–380. doi: 10.1002/ajh.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadhera RK, Wang Y, Figueroa JF, Dominici F, Yeh RW, Joynt Maddox KE. Mortality and hospitalizations for dually enrolled and nondually enrolled Medicare beneficiaries aged 65 years or older, 2004 to 2017. JAMA. 2020;323(10):961–969. doi: 10.1001/jama.2020.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meseguer J. How does mortality among disability-program beneficiaries compare with that of the general population? A summary of actuarial estimates. Soc Sec Bull. 2021;81:19. [Google Scholar]
- 17.Ziemba R, Campbell KN, Yang TH, et al. Excess death estimates in patients with end-stage renal disease—United States, February–August 2020. Am J Transplant. 2021;21(8):2900–2904. doi: 10.1111/ajt.16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Medicare & Medicaid Services ResDAC: find, request and use CMS data. https://resdac.org/
- 19.Chronic Conditions Data Warehouse Other chronic health, mental health, and potentially disabling conditions: sickle cell disease. https://www2.ccwdata.org/condition-categories-other
- 20.Johnson KM, Jiao B, Bender MA, Ramsey SD, Devine B, Basu A. Development of a conceptual model for evaluating new non-curative and curative therapies for sickle cell disease. PLoS One. 2022;17(4):e0267448. doi: 10.1371/journal.pone.0267448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulukonis ST, Eckman JR, Snyder AB, et al. Defining sickle cell disease mortality using a population-based surveillance system, 2004 through 2008. Public Health Rep. 2016;131(2):367–375. doi: 10.1177/003335491613100221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crosby LE, Quinn CT, Kalinyak KA. A biopsychosocial model for the management of patients with sickle-cell disease transitioning to adult medical care. Adv Ther. 2015;32(4):293–305. doi: 10.1007/s12325-015-0197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease--life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 24.Arias E, Xu J, Kochanek K. United States Life Tables, 2016. 2019. https://stacks.cdc.gov/view/cdc/78186 Centers for Disease Control and Prevention. [PubMed]
- 25.McClellan AC, Luthi JC, Lynch JR, et al. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br J Haematol. 2012;159(3):360–367. doi: 10.1111/bjh.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forman-Hoffman VL, Ault KL, Anderson WL, et al. Disability status, mortality, and leading causes of death in the United States community population. Med Care. 2015;53(4):346–354. doi: 10.1097/MLR.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo YF, Raji MA, Goodwin JS. Association of disability with mortality from opioid overdose among US Medicare adults. JAMA Netw Open. 2019;2(11):e1915638. doi: 10.1001/jamanetworkopen.2019.15638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennessy S, Kurichi JE, Pan Q, et al. Disability stage is an independent risk factor for mortality in Medicare beneficiaries aged 65 years and older. Pharm Manag PM R. 2015;7(12):1215–1225. doi: 10.1016/j.pmrj.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson K, Becker M, Tewarson H, Mosey A. Improving Care and Managing Costs for Dual Eligibles. https://www.nga.org/wp-content/uploads/2020/02/Improving-Care-and-Managing-Costs-for-Dual-Eligibles.pdf National Governors Association Center for Best Practices. Accessed 15 July 2022.
- 30.Pierre-Louis YS, Perla KMR, Perez GM, et al. The insurance coverage paradox - characterizing outcomes among dual-eligible hemorrhagic stroke patients. J Clin Neurosci. 2022;97:99–105. doi: 10.1016/j.jocn.2021.12.023. [DOI] [PubMed] [Google Scholar]
- 31.Chen JS, Corcoran Ruiz KM, Rivera Perla KM, Liu Y, Nwaiwu CA, Moreira CC. Health disparities attributed to Medicare-Medicaid dual-eligible status in patients with peripheral arterial disease. J Vasc Surg. 2022;75(4):1386–1394.e3. doi: 10.1016/j.jvs.2021.11.069. [DOI] [PubMed] [Google Scholar]
- 32.Koressel JE, Perez BA, Kerbel YE, DeAngelis RD, Israelite CL, Nelson CL. Does dual-eligible Medicare/Medicaid insurance status as a surrogate for socioeconomic status compromise total knee arthroplasty outcomes? J Arthroplasty. 2022;37(6S):S32–S36. doi: 10.1016/j.arth.2022.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Shah NR, Bhor M, Latremouille-Viau D, et al. Vaso-occlusive crises and costs of sickle cell disease in patients with commercial, Medicaid, and Medicare insurance–the perspective of private and public payers. J Med Econ. 2020;23(11):1345–1355. doi: 10.1080/13696998.2020.1813144. [DOI] [PubMed] [Google Scholar]
- 34.Dampier C, Kanter J, Howard R, et al. Access to care for Medicaid and commercially-insured United States patients with sickle cell disease. Blood. 2017;130:4660. [Google Scholar]
- 35.Snyder AB, Zhou M, Theodore R, Quarmyne MO, Eckman J, Lane PA. Improving an administrative case definition for longitudinal surveillance of sickle cell disease. Public Health Rep. 2019;134(3):274–281. doi: 10.1177/0033354919839072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.