Abstract
Oysters (Crassostrea virginica) were screened for 12 phycotoxins over two years in nearshore waters to collect baseline phycotoxin data and to determine prevalence of phycotoxin co-occurrence in the commercially and ecologically-relevant species. Trace to low concentrations of azaspiracid-1 and -2 (AZA1, AZA2), domoic acid (DA), okadaic acid (OA), and dinophysistoxin-1 (DTX1) were detected, orders of magnitude below seafood safety action levels. Microcystins (MCs), MC-RR and MC-YR, were also found in oysters (maximum: 7.12 μg MC-RR/kg shellfish meat wet weight), warranting consideration of developing action levels for freshwater phycotoxins in marine shellfish. Oysters contained phycotoxins that impair shellfish health: karlotoxin1-1 and 1–3 (KmTx1-1, KmTx1-3), goniodomin A (GDA), and pectenotoxin-2 (PTX2). Co-occurrence of phycotoxins in oysters was common (54%, n = 81). AZAs and DA co-occurred most frequently of the phycotoxins investigated that are a concern for human health (n = 13) and PTX2 and KmTxs co-occurred most frequently amongst the phycotoxins of concern for shellfish health (n = 9). Various harmful algal bloom (HAB) monitoring methods and tools were assessed for their effectiveness at indicating levels of phycotoxins in oysters. These included co-deployed solid phase adsorption toxin tracking (SPATT) devices, toxin levels in particulate organic matter (POM, >1.5 μm) and whole water samples and cell concentrations from water samples as determined by microscopy and quantitative real-time PCR (qPCR). The dominant phycotoxin varied between SPATTs and all other phycotoxin sample types, and out of the 11 phycotoxins detected in oysters, only four and seven were detected in POM and whole water respectively, indicating phycotoxin profile mismatch between ecosystem compartments. Nevertheless, there were correlations between DA in oysters and whole water (simple linear regression [LR]: R2 = 0.6, p < 0.0001, n = 40), and PTX2 in oysters and SPATTs (LR: R2 = 0.3, p = 0.001, n = 36), providing additional monitoring tools for these phycotoxins, but oyster samples remain the best overall indicators of seafood safety.
Keywords: Domoic acid, Pectenotoxin, Azaspiracid, Okadaic acid, Karlotoxin, Microcystin
Graphical abstract
Highlights
-
•
Oysters (Crassostrea virginica) screened for 12 phycotoxins in nearshore waters.
-
•
Co-occurrence of phycotoxins in oysters was common (54%, n = 81).
-
•
Human health toxins, azaspiracids and domoic acid, co-occurred most frequently.
-
•
Shellfish health toxins, pectenotoxins and karlotoxins, also co-occurred frequently.
-
•
Phycotoxin profiles were mismatched between some sample types.
1. Introduction
Along with fresh seafood consumption per capita, market demand for oysters in the USA has generally increased since 2000 (Botta et al., 2020; NMFS 2021). The USA commercial oyster fishery was valued over $250 M in 2019 (NMFS 2021). In the same year, the ex-vessel dollar value (i.e., dollars received at time of first sale) of oyster (Crassostrea virginica) landings in the USA Middle Atlantic fishery region was $51 M, with Virginia making up about $39 M of that amount (NOAA Fisheries 2020). Virginia is home to a lucrative oyster fishery, ranking first in oyster production along the US East Coast (Hudson 2019). Much of this oyster production occurs in the waters of Chesapeake Bay.
Chesapeake Bay is also home to an array of phycotoxins that have recently been detected via passive samplers, or solid phase adsorption toxin tracking devices (SPATTs, Onofrio et al., 2021). The same study found a high prevalence of phycotoxin co-occurrence in the region, with 76% of SPATT samples detecting more than one phycotoxin. Furthermore, phycotoxins were detected year-round throughout the Virginia portion of Chesapeake Bay (Onofrio et al., 2021), suggesting that oysters in the area are exposed to dissolved phycotoxins throughout grow-out and up to harvest.
Phycotoxins in Chesapeake Bay are produced by a range of dinoflagellates, diatoms, raphidophytes, and cyanobacterial harmful algal bloom (HAB) species (Marshall 1996; Marshall and Egerton 2009). Some of these species are associated with seafood safety, while others are associated with shellfish health. Comprehensive, multi-year baseline data are currently lacking for the accumulation of these phycotoxins in regional shellfish. These data are necessary to assess risks to human health and to identify phycotoxins that may impact resource sustainability through accumulation and deleterious impacts on shellfish.
Some regional phycotoxins are associated with global human health syndromes that can occur when shellfish with high concentrations of these phycotoxins are consumed. These phycotoxins and their associated syndromes include: azaspiracids (AZAs)—azaspiracid shellfish poisoning (AZP), domoic acid (DA)—amnesic shellfish poisoning (ASP), and diarrhetic shellfish toxins (DSTs)—diarrhetic shellfish poisoning (DSP). AZAs are produced by the Amphidomataceae family of dinoflagellates (Tillmann et al., 2017), but a causative organism has yet to be identified in the Chesapeake Bay (Onofrio et al., 2021). Diatoms from the DA-producing genus Pseudo-nitzschia have been documented in Chesapeake Bay (Thessen and Stoecker 2008), as have DST-producing dinoflagellates Dinophysis spp. and Prorocentrum lima (Barbier et al., 1999; Marshall et al., 2005; Wolny et al., 2020a). In addition to these marine phycotoxins, the traditionally freshwater phycotoxins, microcystins (MCs) have been detected in Chesapeake Bay along with MC-producing Microcystis aeruginosa (Tango and Butler 2008; Wood et al., 2014; Bukaveckas et al., 2017, 2018; Onofrio et al., 2021). MCs are hepatotoxins produced by several marine and freshwater cyanobacteria, including Anabaena, Aphanizomenon, Nostoc, Microcystis, and Planktothrix cyanobacteria (Eriksson et al., 1990; Dawson 1998; Campos and Vasconcelos 2010; Huang and Zimba 2019 and references therein). Multiple research groups have raised concern about MCs and shellfish in relation to seafood safety, as shellfish can concentrate MCs (Miller et al., 2010; Mulvenna et al., 2012; Vareli et al., 2013; Preece et al., 2017; Camacho-Muñoz et al., 2021) and in addition to hepatotoxicity, the toxin class has been linked to tumor promotion and reproductive toxicity in vertebrate models (Nishiwaki-Matsushima et al., 1992; Li et al. 2008, 2009; Wang et al., 2013).
Current HAB monitoring in Virginia shellfish growing areas is coordinated by the Virginia Department of Health (VDH) and consists of routine water sampling at over 60 stations throughout the region. The abundances of phycotoxin-producing species are monitored using microscopy and/or DNA analysis, with subsequent testing of seawater samples and/or shellfish tissues for phycotoxins when elevated HAB cell numbers are detected. To date, there have been no documented human illnesses caused by the consumption of Virginia shellfish contaminated with phycotoxins, and only one precautionary shellfish harvest closure occurred due to an elevated cell concentration of Dinophysis in the Potomac River in 2002, though only trace concentrations of DSTs were detected in exposed shellfish in that event (Tango et al., 2004).
In addition to phycotoxins associated with human health syndromes, other phycotoxins found in Chesapeake Bay can have negative impacts on shellfish health. Pectenotoxin-2 (PTX2) is a phycotoxin produced by Dinophysis spp. that has been documented in Chesapeake Bay (Onofrio et al., 2021) and is harmful to shellfish health, reducing oyster fertilization success (Gaillard 2020; Gaillard et al., 2020) and causing inactivity and mortality in oyster larvae under laboratory settings (Pease et al., 2021). In Chesapeake Bay, goniodomin A (GDA) is produced by the dinoflagellate Alexandrium monilatum(Hsia et al., 2006; Wolny et al., 2020b). Exposure to live or lysed A. monilatum has been shown to have negative impacts on shellfish health, inducing valve closure, reducing clearance rates, and causing mortality in oysters, clams, and mussels (Ray and Aldrich 1966; Sievers 1969, May et al., 2010; Pease 2016). While the precise mechanism of toxicity remains undetermined, GDA was detected in whelks that died during an A. monilatum bloom (Harding et al., 2009). Karlodinium veneficum is a dinoflagellate in Chesapeake Bay that produces karlotoxins (KmTxs), including KmTx1-1 and KmTx1-3 (Brownlee et al., 2008; Stoecker et al., 2008; Bachvaroff et al., 2008, 2009; Adolf et al., 2009). After exposure to KmTx-producing K. veneficum, reduced clearance rates, reduced growth, and/or mortality have been reported in oysters, clams, mussels, and scallops (Abbott and Ballantine 1957; Nielsen and Strømgren 1991; Galimany et al., 2008; Brownlee et al., 2008; Place et al., 2008).
The current study is a continuation of the work from a collaboration between the Virginia Institute of Marine Science (VIMS) and the VDH Division of Shellfish Safety and Waterborne Hazards to collect baseline data on phycotoxin spatiotemporal distribution in the Virginia portion of Chesapeake Bay (Onofrio et al., 2021). Efficient methods for monitoring a suite of phycotoxins in shellfish are needed to assess risks to seafood safety and shellfish health and productivity. The objectives of this study were, therefore, to (1) establish baseline phycotoxin co-occurrence and spatiotemporal distribution in oysters (C. virginica) in the Virginia portion of Chesapeake Bay, and (2) assess whether oyster phycotoxin concentrations could be monitored or predicted using other metrics by (a) examining relationships between phycotoxin concentrations in oysters, SPATTs, particulate organic matter (POM, >1.5 μm), and whole water, and (b) comparing HAB cell concentrations determined by microscopy and by quantitative real-time PCR (qPCR) with amounts of phycotoxins detected in oysters and SPATTs.
2. Materials & methods
2.1. Field study design
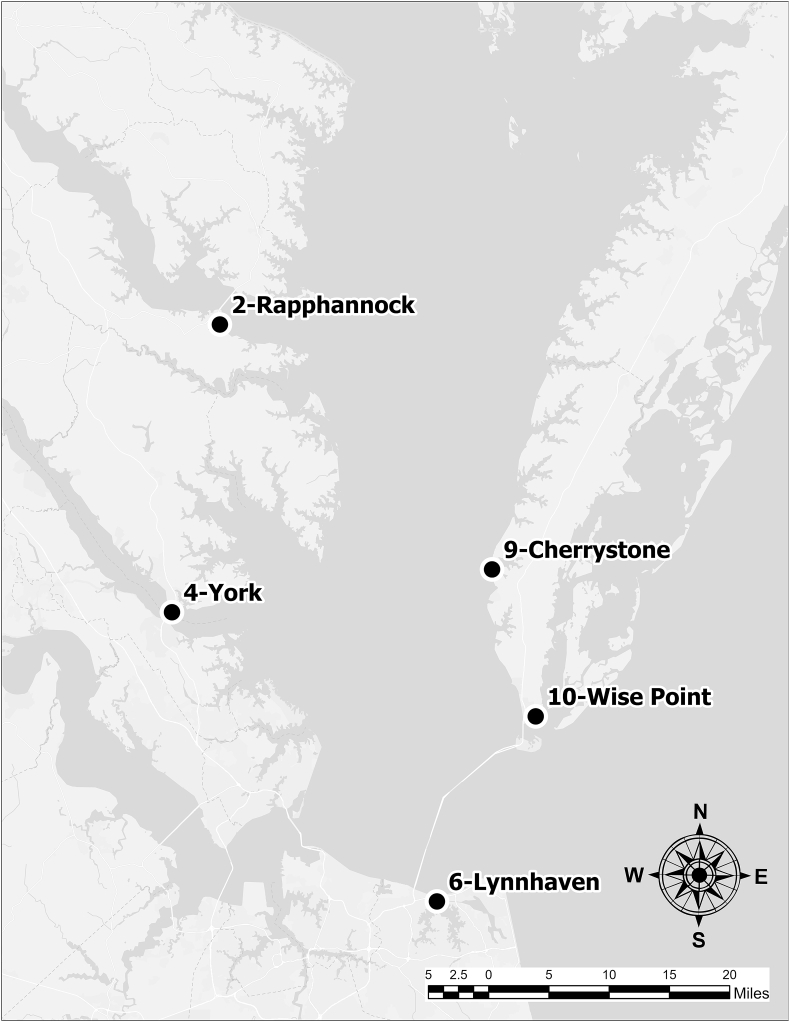
A field study was performed over six months in 2019 (January through June), and six months in 2020 (March through August) in nearshore waters of the Virginia-portion of Chesapeake Bay. Sampling periods focused on late winter – early summer due to expected seasonality of regional HABs of concern; sampling period shifted later in 2020 due to onset of sampling restrictions from COVID19 pandemic. Four stations were sampled each year, selected based on their proximity to shellfish growing areas and geographic distribution around the Bay (Fig. 1, Onofrio et al., 2021). Due to COVID19 constraints in 2020, station 9 was replaced by station 10. The stations sampled during the current study were previously characterized by Onofrio et al. (2021); briefly, the stations were mesohaline (S = 5–18, station 2 Rappahannock) to polyhaline (S = 18–30, stations 4 York River, 6 Lynnhaven Inlet, 9 Cherrystone Inlet, and station 10 Wise Point), with respect to chlorophyll, stations ranged from low (0 - < 0.5 μg/L, station 10) to medium (5–20 μg/L, stations 2, 4, 6, and 9) on the Chl a eutrophic index, and all stations were shallow (≤ 2 m), with intermediate to high flushing rates. Geomorphic settings of each station were classified: station 2 is a tidal creek, station 4 is a tidal river, stations 6 and 9 are tidal inlets, and station 10 is a strait.
Fig. 1.
Locations of the five sampling stations in the lower Chesapeake Bay, Virginia, USA: station 2 Rappahannock, station 4 York River, station 6 Lynnhaven Inlet, station 9 Cherrystone Inlet, and station 10 Wise Point.
Adult oysters (30–134 mm, C. virginica) were deployed once per year at each station, approximately two weeks before the start of the sampling period. Oysters were deployed in bottom cages that held them 0.3 m off the bottom, and SPATTs were attached to the top of the oyster cage, roughly 0.6 m from the bottom. Sampling occurred approximately every other week throughout the sampling period in each year for phycotoxin quantification in oyster meat (6–15 oysters pooled) and SPATTs. Discrete surface water samples were collected in each year, in 2019 for phycotoxin quantification in POM (>1.5 μm) and whole water, and in 2020 for enumeration of HAB cells by microscopy and qPCR.
2.2. Sample preparation and phycotoxin extraction
After sampling, oysters were shucked, rinsed with ultrapure water to remove salts, and stored at −20 °C until phycotoxin extraction. Extraction followed McNabb et al. (2005); briefly, 18 mL of 90% methanol (MeOH) was used to extract 2 g of pooled oyster homogenate, crude extract was centrifuged (3234×g, 10 min, 4 °C), and the supernatant was aliquoted for clean-up with hexane (2 mL - original) and alkaline hydrolysis (1 mL - hydrolysis). Alkaline hydrolysis converted esterified DSTs in the aliquot into the free, parent toxins, e.g., okadaic acid (OA), dinophysistoxin-1 (DTX1), and dinophysistoxin-2 (DTX2), following an adaptation of Villar-González et al. (2008). Briefly, 125 μL of 2.5 N NaOH was added to 1 mL of oyster extract; the mixture was heated to 76 °C for 40 min and then neutralized with 125 μL of 2.5 N AcOH. Both oyster extracts: original and hydrolysis extracts, were subsequently analyzed by mass spectrometry.
SPATTs were constructed and prepared with Diaion® HP-20 resin (Fux et al., 2008). This resin readily adsorbs phycotoxins with a range of different polarities and sizes (Lane et al., 2010; Kudela 2011; McCarthy et al., 2014; Roué et al., 2018; Onofrio et al., 2021). SPATTs were stored, deployed, and extracted as described in Onofrio et al. (2021). Briefly, during extraction, SPATTs were rinsed with ultrapure water to remove salts, resin was transferred to a 0.45-μm PVDF spin-filter centrifuge tube (Thermo Fisher Scientific, Waltham, MA, USA), and the resin underwent sequential extractions with 10 mL of 35% MeOH and 2 × 10 mL of 100% MeOH, as described in detail in Onofrio et al. (2021). The 35% MeOH extracts were collected and analyzed by enzyme linked immunosorbent assay (ELISA); 100% MeOH extracts were pooled and analyzed by mass spectrometry.
POM samples were prepared by filtering 200 mL of sample water through a Whatman 934-AH GFF filter (nominal pore size 1.5 μm). Filters were stored at −20 °C until extraction. Filters were extracted in 2.0 mL of 100% MeOH, bath-sonicated for 30 min, centrifuged (3200×g, 10 min, 4 °C), and the supernatant was collected and analyzed by mass spectrometry.
Whole water samples were thawed, bath-sonicated at < 20 °C for 30 min, and 75 mL of sample was acidified to 0.5% formic acid before being extracted using solid-phase extraction (SPE). SPE cartridges (Waters Oasis HLB 3-cc, 60 mg; P/N WAT094226) were equilibrated with 3 mL MeOH and water, and loaded with the acidified sample. Cartridges were then washed with 3 mL 0.5% aqueous formic acid, blown dry, and eluted twice with 0.75-mL aliquots of 0.5% formic acid in MeOH. Eluents were pooled and analyzed by mass spectrometry.
2.3. Phycotoxin analysis
Sample extracts (90–100% MeOH) from oyster, SPATT, POM, and whole water were analyzed for 12 phycotoxins (AZA1, AZA2, DA, MC-LR, MC-RR, MC-YR, KmTx1-1, KmTx1-3, GDA, PTX2, OA, DTX1) at VIMS using ultra-performance liquid chromatography-tandem mass spectrometry, with a trapping dimension and at-column dilution (UPLC-MS/MS with trap/ACD) and mass spectrometer and chromatography conditions that were previously described in Onofrio et al. (2020). Phycotoxins were selected based on recent work that demonstrated occurrence in the region (Onofrio et al., 2021; Sanderson et al., 2023) and a subset of analogs were chosen to represent the toxin classes. Toxin classes and analogs that have thus far been absent, and therefore were excluded from the current study, include DTX2, brevetoxins, and yessotoxins. Parent > daughter transitions, as presented in Onofrio et al. (2021), were used for quantitation, with the addition of a transition for DA: m/z 312.0 > 266.1, 30 V, 15eV (Onofrio, 2020). All extracts were 0.22-μm syringe filtered (PVDF, 13-mm, Millipore Sigma, Burlington, MA, USA) and stored at −20 °C for a maximum of two weeks before phycotoxin analysis. Injection volumes for each sample were 50 μL for oyster samples, and 100 μL for SPATTs, POM, and whole water samples, unless otherwise noted.
Standard curves were prepared in 100% MeOH using a series of 9 calibration points between 0.1 and 50 μg/L for all phycotoxins except AZA1 and AZA2, which were calibrated between 0.004 and 2 μg/L. SPATTs from 2019 were run with the higher standard curve (0.1–50 μg/L) for AZAs, and any samples with detectable levels of AZA2 were rerun with an injection volume of 200 μL and a standard curve between 0.003 and 2 μg AZA2/L. KmTxs were not included in the standard curve due to limited purified material; a check standard of unknown concentration (∼2 μg/L) containing KmTx1-1 and KmTx1-3 was run with each sample set to determine presence/absence. Instrument limits of detection (LOD) for the majority of phycotoxins were between 0.01 and 0.39 μg/L (Onofrio et al., 2020); the LOD for DA was 0.18 μg/L (Onofrio unpublished). Lower LODs for AZAs and GDA, 0.003–0.004 μg/L and 0.1 μg/L, respectively, were associated with oyster, whole water, and some SPATT samples, and quantification utilized the lowest points on their respective standard curves (this study). Blank injections of 100% MeOH were run after each set of 15 SPATTs, POM, or whole water extracts, and after every 3 oyster extracts, to confirm that carryover was not occurring. The inlet method of Onofrio et al. (2020) was modified with the addition of 2 min of isocratic flow, 95% acetonitrile, to the end of each oyster run to provide better cleanup between injections. To confirm that retention times remained consistent, check standards (5 μg/L for each phycotoxin, except 0.12 μg/L of AZA1 and AZA2) or full standard curves were run after every 15–16 injections of extracts. Non-detects, samples with S/N < 10, and POM and whole water samples without confirmatory peaks, were represented as < LOD and/or 0. SPATT, POM, and whole water phycotoxin concentrations less than the lowest point on the respective standard curve, i.e., the limit of quantitation (LOQ), but with S/N ≥ 10, were represented as ½ LOD. Oyster phycotoxin concentrations less than the LOQ but with a S/N ≥ 10 and a parent peak of S/N ≥ 3, were represented as ½ LOD. Phycotoxin results in oysters were also presented as the percentage of samples that tested positive within the 81 extracts evaluated across all sites and time points.
The 2019, 35% MeOH extracts from SPATTs were analyzed for DA as described in Onofrio et al. (2021). Briefly, DA (ASP) ELISA kits (Abraxis Inc., Warminster, PA, USA) and an Abraxis plate reader were used to detect and quantify DA.
Phycotoxin standards were purchased from the National Research Council Canada: CRM-AZA1-b, CRM-AZA2-b, CRM-DA-g, CRM-PTX2-b, CRM-OA-d, CRM-DTX1-b. A mixed solution of MC-LR, MC-RR, and MC-YR was purchased from Sigma Aldrich (33,578-1 ML). KmTx1-1 and KmTx1-3 were purified from K. veneficum and provided by Dr. Allen Place (UMCES, Maryland). GDA was purified from A. monilatum and provided by Drs. Thomas and Constance Harris (Harris et al., 2020).
The prevalence of each phycotoxin in oysters was reported, i.e., the percentage of oyster samples with phycotoxin > LOD out of all oyster samples. The phycotoxin profile, or relative proportion of each toxin group, was also determined for each sample type (i.e., oysters, SPATTs, POM, and whole water). Proportions were calculated by summing the concentrations of a phycotoxin group across all 2019 samples and then dividing by the total concentration of all phycotoxins detected across 2019 samples of that sample type. Phycotoxin concentrations below the LOQ were represented by ½ LOD for the method associated with that sample type and all phycotoxin concentration units were normalized within sample type (μg/kg for oysters and SPATTs, and μg/L for POM and whole water).
2.4. Percent response from oyster matrix and percent recovery from POM and whole water phycotoxin samples
Percent response was determined for ten phycotoxins in oyster matrix (AZA1, AZA2, DA, MC-LR, MC-RR, MC-YR, GDA, PTX2, OA, DTX1). This looks at ion enhancement or suppression of toxin analysis due to matrix effects. Triplicate oyster matrices and triplicate 90% MeOH controls were spiked to a final concentration of 5 ng phycotoxin/mL, or 0.2 ng phycotoxin/mL for AZAs. Spiked samples were analyzed as described above by UPLC-MS/MS with trap/ACD (section 2.3) and the following equation was used to calculate percent response:
Recovery efficiency was determined for the bulk extraction of seven phycotoxins (AZA1, AZA2, DA, GDA, PTX2, OA, DTX1) from POM samples and ten phycotoxins (AZA1, AZA2, DA, MC-LR, MC-RR, MC-YR, GDA, PTX2, OA, DTX1) from whole water samples. Recoveries have already been reported in the other two matrices: SPATT (Onofrio et al., 2021) and oyster tissue (McNabb et al., 2005), and so were not repeated as part of the present work. Microcystins, MC-LR, MC-RR, and MC-YR, were excluded from present recovery trials in POM because previous studies have already confirmed success using the utilized extraction method in field and laboratory samples (Gjølme and Utkilen, 1996; Barco et al., 2005, Silva-Stenico et al., 2009; Pestana et al., 2014), with recovery efficiency between 80 and 125% for MC-RR and 81–129% for MC-LR (Lawton et al., 1994). KmTxs were excluded due to a limited amount of available purified material.
Recovery efficiency for the POM extraction method was carried out as follows. Six 200-mL samples of seawater, S = 20, from the York River, Chesapeake Bay, Virginia, USA, were filtered as described above (see section 2.2), 2 mL of MeOH was added to the filters, and half of the filters were then spiked to a final concentration of 5 μg phycotoxin/L for each phycotoxin, or 0.2 μg/L for the AZAs. Filters were extracted as described in section 2.2, above.
To determine recovery efficiency for the whole water extraction method, six 75-mL samples of unfiltered seawater, S = 20, from the York River, Chesapeake Bay, Virginia, USA were collected. Half of the samples (n = 3) were spiked to a final concentration of 5 ng phycotoxin/mL (or 0.2 ng phycotoxin/mL for AZAs). All six samples (spiked and unspiked) were extracted as described in section 2.2 above and analyzed by UPLC-MS/MS with trap/ACD as described above in section 2.3. Esterified forms of OA and DTX1 were added to non-esterified forms of OA and DTX1, respectively, and the following equation was used to calculate the recovery efficiency for each phycotoxin:
2.5. HAB cell enumeration
Surface water samples collected in 2020 were used for enumeration of HAB cells using two different methods. Microscopic analysis for HAB species was carried out in Lugol's preserved water samples as described in Onofrio et al. (2021), with the exception that 5-mL aliquots were enumerated. Briefly, HAB cells were identified and enumerated in well plates (Cellvis P12-1.5H–N, Mountainview, CA, USA) using light microscopy at 100–400X (Olympus CKX41).
qPCR was used to enumerate certain HAB species using template DNA isolated from 3-μm-filtered, 100-mL water samples as previously described (Pease et al., 2021). Previously published qPCR assays were used for detection and quantification of members of the Amphidomataceae family, A. monilatum, and K. veneficum (Smith et al., 2016; Vandersea et al., 2017; Pease et al., 2021). A previously described primer set designed to detect Pseudo-nitzschia spp. (Penna et al., 2007) was used for a SYBR Green-based qPCR assay for detection and quantification of this genus. A Dinophysis spp. TaqMan assay was developed based on Chesapeake Bay Dinophysis spp. sequences to target the internal transcribed spacer region of the rRNA gene region. The Dinophysis spp. primers are DinoITS_52 F (5′-CATGTGGAAGCTCGAGGGTA-3′) and DinoITS_130 R (5′-GTGAGCCAAGCAGACGGTAG-3′). The probe is DinoITS_82 P R (5′FAM-AGCAGTGTGGTCTTGCTGTT-3′BHQ). Stocks of A. monilatum, K. veneficum, and Dinophysis acuminata are maintained at VIMS for positive controls and were used to generate standard curves for qPCR. Pseudo-nitzschia multiseriesfiltered cells were received from NOAA Fisheries, Northwest Fisheries Science Center, Seattle, WA for use as control material and for standard curves in the Pseudo-nitzschia spp. assay. Azadinium dexteroporum filtered cells were received from the Algal Resources Collection at the University of North Carolina at Wilmington, Wilmington, NC for use as control material and for standard curves in the Amphidomataceae family assay. Cell counts for the control stock cultures were determined by light microscopy. DNA was extracted from a known number of cells to generate standard curves through serial dilution of the DNA to achieve a range of cell number equivalents. DNA from the filtered water samples were run against these standard curves to quantify cell concentrations for each sample. qPCR assays were performed on 7500 Fast, QuantStudio 6, or QuantStudio 3 Real-Time PCR systems (Applied Biosystems™, ThermoFisher, Waltham, Massachusetts, USA) using the following cycling parameters: an initial denaturation step at 95 °C for 20 s followed by 40 cycles of 95 °C for 3 s to denature and 60 °C for 30 s to anneal and extend. All reactions were performed in duplicate. Reagent concentrations for the TaqMan assays were 0.4 mg/mL BSA, 0.9 μM for each primer, 0.1 μM for the probe and 1X concentration of the TaqMan® Fast Advanced Master Mix (Applied Biosystems™, ThermoFisher, Waltham, Massachusetts, USA) in a 10 μL final volume. Reagent concentrations for the SYBR Green assays were 0.9 μM for each primer and 1X concentration of PowerUp™ SYBR™ Green Master Mix (Applied Biosystems™, ThermoFisher, Waltham, Massachusetts, USA) in a 10 μL volume. A dissociation step was added to the SYBR Green assays so that the correct melting temperature could be verified for the qPCR product.
2.6. Statistical analysis
In 2019, phycotoxin concentrations in SPATTs, POM, and whole water were assessed as predictors of phycotoxin concentrations in oysters using linear regressions. In 2020, phycotoxin concentrations in SPATTs, and HAB cell concentrations determined by microscopy and by qPCR were assessed as predictors of phycotoxin concentrations in oysters using linear regressions. Within each year, only phycotoxins that were quantifiable (>LOQ) in ≥ 3 oyster samples, and that had predictor data to compare to, were analyzed. All data were log10-transformed to meet assumptions of normality and centered. All linear regressions were re-run with a two-week (i.e., one sampling event) lag time, to see if predictor data from two weeks prior to oyster sampling improved model fit. Statistical tests were performed in R Studio using R version 3.6.2. Raw data for these analyses can be found in Tables S1 and S2.
3. Results
3.1. Percent response and recovery
Ion enhancement and suppression of 10 analytes were examined in oyster extracts by comparing spiked matrix to 90% methanolic controls. Overall, percent responses were above 100% in oyster matrix relative to methanolic controls for all tested phycotoxins, indicating ion enhancement (102–179%, Table S3). The exception was OA which demonstrated slight suppression (92% response). Signal enhancement potentially led to overestimation of the amounts of these phycotoxins in oysters. The phycotoxin with the greatest signal enhancement, MC-LR (227%), was not detected in oysters in this study.
The bulk extraction methods used for POM and whole water samples were successful in the co-extraction and recovery of phycotoxins, resulting in percent recoveries >78% for all tested phycotoxins except DA in POM samples, and percent recoveries >89% for all tested phycotoxins in whole water samples (Table S3). Recovery for DA from POM was 50%, indicating that reported amounts of DA underestimate the actual amount of DA in POM samples. Recoveries were adequate for multi-toxin screening of samples in this study.
3.2. Phycotoxins in oysters
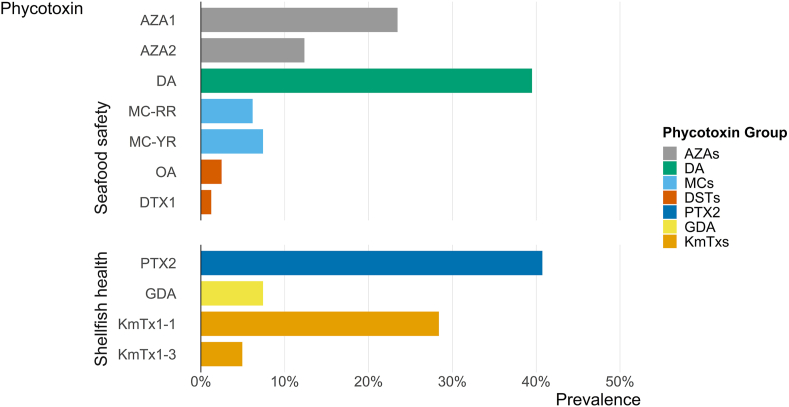
Most oyster samples (84%, n = 81), contained at least one of the 12 phycotoxins assessed in this study (Table 1). Of the phycotoxins measured, 11 were detected in oysters, including AZA1, AZA2, DA, OA, DTX1, MC-RR, MC-YR, GDA, PTX2, KmTx1-1, and KmTx1-3. The phycotoxin MC-LR was not detected in oysters. Phycotoxin results are separated into two categories: phycotoxin groups associated with seafood safety, i.e., AZAs, DA, MCs, and DSTs (OA and DTX1), and phycotoxin groups associated with shellfish health, i.e., PTX2, GDA, and KmTxs. Co-occurrence of phycotoxins from two or more phycotoxin groups was common (54% of oyster samples). The most prevalent phycotoxins in oysters were PTX2 and DA, and the least prevalent phycotoxins were the DSTs (Fig. 2). The most widespread phycotoxins in oyster meat were AZA1 and PTX2, which were found at all stations between 2019 and 2020 (Table 1). In all sampled months, January through June 2019, and March through August 2020, at least one oyster sample contained detectable phycotoxin (Table S4). Phycotoxin concentrations are presented in the same units as the U.S. Food and Drug Administration action levels for that phycotoxin as it is regulated in seafood where applicable (USFDA 2019).
Table 1.
Spatial distribution of 12 phycotoxins across five stations in the lower Chesapeake Bay.
| Sample type (years collected) | Station IDc(sample size) | Seafood safety phycotoxinb maximum |
Shellfish health phycotoxinb maximum |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AZA1 | AZA2 | DA | MC-LRd | MC-RRd | MC-YRd | OAe | DTX1e | PTX2 | GDA | KmTx1-1 | KmTx1-3 | ||
| Oysters | Units |
μg/kg |
μg/kg |
mg/kg |
μg/kg |
μg/kg |
μg/kg |
μg/kg | μg/kg | μg/kg | mg/kg | +/− | +/− |
| (2019, 2020) | SM w.w. | SM w.w. | SM w.w. | SM w.w. | SM w.w. | SM w.w. | SM w.w. | SM w.w. | SM w.w. | SM w.w. | |||
| Action levelf | 160 | 160 | 20 | NA | NA | NA | 160 | 160 | NA | NA | NA | NA | |
| Station 2 (n = 17) | 0.36 | 0.38 | 0 | 0 | 0 | 1.1a | 0 | 0.50a | 0.18a | 0.00045a | + | + | |
| Station 4 (n = 22) | 0.35 | 0 | 0.028 | 0 | 7.1 | 0 | 0 | 0 | 2.2 | 0 | + | + | |
| Station 6 (n = 21) | 0.80 | 0.33 | 0.58 | 0 | 7.1 | 1.1a | 0.45a | 0 | 4.4 | 0.00045a | + | + | |
| Station 9 (n = 11) | 0.29 | 0 | 0.075 | 0 | 0 | 0 | 0 | 0 | 0.18a | 0.00045a | + | + | |
| Station 10 (n = 10) | 0.28 | 0.42 | 0.086 | 0 | 0.32a | 0 | 0.45a | 0 | 6.2 | 0 | – | – | |
| SPATTs | Units | μg/kg | μg/kg | μg/kg | μg/kg | μg/kg | μg/kg | μg/kg | μg/kg | μg/kg | μg/kg | +/− | +/− |
| (2019, 2020) | resin | resin | resin | resin | resin | resin | resin | resin | resin | resin | |||
| Station 2 (n = 21) | 0.067a | 0 | 0 | 0 | 0 | 0 | 22 | 16 | 52 | 14 | – | – | |
| Station 4 (n = 21) | 0 | 0.20 | 2.2 | 0 | 0 | 0 | 43 | 34 | 73 | 16 | – | – | |
| Station 6 (n = 21) | 0 | 0.26 | 6.0 | 0 | 0 | 0 | 73 | 66 | 110 | 84 | – | – | |
| Station 9 (n = 11) | 0 | 0 | 2.4 | 0 | 0 | 0 | 58 | 9.9 | 6.8 | 0 | – | – | |
| Station 10 (n = 10) | 0 | 0.23 | 2.8 | 0 | 0 | 0 | 75 | 240 | 140 | 0.33a | – | – | |
| POM | Units | μg/L | μg/L | μg/L | μg/L | μg/L | μg/L | μg/L | μg/L | μg/L | μg/L | +/− | +/− |
| (2019) | Station 2 (n = 10) | 0 | 0 | 0.0017 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – |
| Station 4 (n = 11) | 0 | 0 | 0.15 | 0 | 0 | 0 | 0 | 0 | 0.0036 | 0 | – | – | |
| Station 6 (n = 11) | 0 | 0 | 0.94 | 0 | 0 | 0 | 0 | 0 | 0.0027 | 0 | – | – | |
| Station 9 (n = 11) | 0 | 0.000072 | 0.22 | 0 | 0.0040 | 0 | 0 | 0 | 0.0021 | 0 | – | – | |
| Whole water | Units | μg/L | μg/L | μg/L | μg/L | μg/L | μg/L | μg/L | μg/L | μg/L | μg/L | +/− | +/− |
| (2019) | Station 2 (n = 10) | 0 | 0 | 0.074 | 0 | 0 | 0 | 0 | 0 | 0.0015 | 0 | + | + |
| Station 4 (n = 10) | 0.000040a | 0 | 0.38 | 0.0022 | 0 | 0.0047 | 0.0060 | 0.0011a | 0.014 | 0 | + | + | |
| Station 6 (n = 10) | 0.000040a | 0.000040a | 1.8 | 0.0027 | 0 | 0.0038 | 0.0085 | 0.0011a | 0.048 | 0 | – | – | |
| Station 9 (n = 10) | 0.000040a | 0 | 0.99 | 0 | 0.00070a | 0 | 0 | 0 | 0.010 | 0 | – | – | |
Within each phycotoxin and sample type across sites, the phycotoxin maximum is bolded. Values below the limit of detection (LOD) are shown as zeroes. Karlotoxins are reported as presence/absence (+/-).
Phycotoxin value below the limit of quantitation (LOQ) and represented by 1/2 LOD for that method.
AZA1 = azaspiracid-1, AZA2 = azaspiracid-2, DA = domoic acid, MC-LR = microcystin-LR, MC-RR = microcystin-RR, MC-YR = microcystin-YR, OA = okadaic acid, DTX1 = dinophysistoxin-1, PTX2 = pectenotoxin-2, GDA = goniodomin A, KmTx1-1 = karlotoxin1-1, KmTx1-3 = karlotoxin1-3.
Station 9 was only sampled in 2019 and Station 10 was only sampled in 2020.
While hepatotoxic shellfish poisoning is currently theoretical (Miller et al., 2010; Mulvenna et al., 2012; Vareli et al., 2013; Preece et al., 2017; Camacho-Muñoz et al., 2021; Straquadine et al., 2022), microcystins are included in this study as seafood safety phycotoxins because they are known hepatotoxins (Eriksson et al., 1990; Dawson 1998; Campos and Vasconcelos 2010).
Okadaic acid and dinophysistoxin-1 concentrations in oysters are the result of alkaline hydrolysis, and therefore, represent esterified and free toxins combined.
U.S. Food and Drug Administration (FDA) action levels for phycotoxins regulated in seafood (USFDA 2019); NA indicates no action level is available for that phycotoxin.
Fig. 2.
Prevalence of each phycotoxin detected in oyster samples (n = 81); i.e., for each phycotoxin, the percentage of oyster samples that it was detected in, in an amount greater than the limit of detection (LOD) for that phycotoxin. Microcystin-LR was not detected in oysters. Extracts underwent alkaline hydrolysis, and therefore, okadaic acid (OA) and dinophysistoxin-1 (DTX1) concentrations represent esterified and free toxins combined. AZA1 = azaspiracid-1, AZA2 = azaspiracid-2, AZAs = azaspiracids, DA = domoic acid, MC-RR = microcystin-RR, MC-YR = microcystin-YR, MCs = microcystins, PTX2 = pectenotoxin-2, GDA = goniodomin A, KmTx1-1 = karlotoxin1-1, KmTx1-3 = karlotoxin1-3, KmTxs = karlotoxins.
DA was observed in the highest concentration in oysters (Table 1), reaching 0.58 mg/kg SM w. w. In one sample, followed by MC-RR (7.1 μg/kg SM w. w.), PTX2 (6.2 μg/kg SM w. w.), AZA1 (0.80 μg/kg SM w. w.), and AZA2 (0.42 μg/kg SM w. w.). In oysters, MC-YR, OA, DTX1, and GDA were detected in amounts below their respective limits of quantitation, while KmTx1-1 and KmTx1-3 were detected but not quantified in this study due to lack of reference material.
3.2.1. Seafood safety
Most oyster samples (67%, n = 81), contained at least one phycotoxin associated with seafood safety: AZAs, DA, DSTs, and MCs. Seafood safety phycotoxins co-occurred in 23% of oyster samples (n = 81) and at least once at each sampling site (Table S4). Of these co-occurrences (n = 19), 68% were between AZAs and DA.
Both AZA1 and AZA2 were detected in oysters. AZA1 prevalence in oyster samples (23%) was almost double that of AZA2 (12%, n = 81, Fig. 2). No co-occurrences of these phycotoxins were detected in oysters. Between 2019 and 2020, AZA1 was detected at all five stations, while AZA2 was detected at only three stations (2, 6, and 10) (Table 1). AZA1 was detected in oysters in February through March, and May 2019, with a maximum concentration of 0.80 μg/kg SM w. w. (station 6, 5/15/19), and in May through August 2020, with a maximum concentration of 0.40 μg/kg SM w. w. (station 6, 6/24/20, Fig. 3). In 2019, AZA2 was detected in oysters at two stations, but only in April, with a maximum concentration of 0.22 μg/kg SM w. w. (station 6, 4/29/19). In 2020, AZA2 was detected in March, and May through August, with a maximum concentration of 0.42 μg/kg SM w. w. (station 10, 8/3/20, Fig. 3).
Fig. 3.
Oyster phycotoxin data (μg/kg shellfish meat [SM] w. w.) for azaspiracid-1 and -2 (AZA1 and AZA2, respectively) across five stations from January to June 2019 and from March to August 2020. Station 9 was only sampled in 2019 and station 10 was only sampled in 2020. Hollow circles are below the limit of quantitation (<LOQ) and are represented as ½ the method limit of detection (LOD). Samples below the LOD are indicated by plus signs (+).
DA was prevalent in 40% of oyster samples (n = 81, Fig. 2). Between 2019 and 2020, DA was detected at every site, except station 2 (Table 1). DA was detected in oysters in January through May 2019, with a maximum concentration of 0.58 mg/kg SM w. w. (station 6, 1/25/19), and in March through May, and July through August 2020, with a maximum concentration of 0.086 mg/kg SM w. w. (station 10, 3/31/20, Fig. 4).
Fig. 4.
Oyster phycotoxin data (mg/kg shellfish meat [SM] w. w.) for domoic acid (DA) across five stations from January to June 2019 and from March to August 2020. Station 9 was only sampled in 2019 and station 10 was only sampled in 2020. Hollow circles are below the limit of quantitation (<LOQ) and are represented as ½ the method limit of detection (LOD). Samples below the LOD are indicated by plus signs (+).
Both MC-RR and MC-YR were detected in oysters. MC-RR prevalence in oyster samples (6%) was similar to MC-YR (7%, n = 81, Fig. 2). No co-occurrences of these phycotoxins were detected in oysters. Between 2019 and 2020, MC-RR was detected at three stations (4, 6, and 10), while MC-YR was detected at two stations (2 and 6) (Table 1). MC-RR was detected once in oysters in April 2019, with a concentration of 7.1 μg/kg SM w. w. (station 4, 4/1/19), and in May through June 2020, with a maximum concentration of 7.1 μg/kg SM w. w. (station 6, 5/26/20, Fig. 5). MC-YR was detected in oysters in March through May 2019, and in June and August 2020, and was always detected in low concentrations (<LOQ, Fig. 5).
Fig. 5.
Oyster phycotoxin data (μg/kg shellfish meat [SM] w. w.) for microcystin-RR and -YR (MC-RR and MC-YR, respectively) across five stations from January to June 2019 and from March to August 2020. Microcystin-LR was not detected in oysters. Station 9 was only sampled in 2019 and station 10 was only sampled in 2020. Hollow circles are below the limit of quantitation (<LOQ) and are represented as ½ the method limit of detection (LOD). Samples below the LOD are indicated by plus signs (+).
Alkaline hydrolysis was performed on all oyster extracts, therefore, reported DST concentrations (OA or DTX1) represent both esterified and free toxins combined. DSTs, OA and DTX1 and their esterified forms, were rarely detected in oyster samples (2 and 1%, respectively, n = 81) (Fig. 2). No co-occurrences of these phycotoxins were detected in oysters. Neither of these phycotoxins was detected in 2019. In 2020, OA and esterified forms were detected at two stations: station 6 (5/26/20) and station 10 (6/10/20), while DTX1 was detected at station 2 (6/25/20, Table 1); both phycotoxins were detected only in low concentrations (<LOQ, Fig. 6).
Fig. 6.
Oyster phycotoxin data (μg/kg shellfish meat [SM] w. w.) for diarrhetic shellfish toxins (DSTs) okadaic acid and dinophysistoxin-1 (OA and DTX1, respectively) as determined using alkaline hydrolysis, across five stations from January to June 2019 and from March to August 2020. Station 9 was only sampled in 2019 and station 10 was only sampled in 2020. Hollow circles are below the limit of quantitation (<LOQ) and are represented as ½ the method limit of detection (LOD). Samples below the LOD are indicated by plus signs (+).
3.2.2. Shellfish health
At least one phycotoxin group associated with shellfish health, GDA, PTX2, or KmTxs, was detected in most oyster samples (64%, n = 81) (Table S4). Shellfish health phycotoxins co-occurred in 12% of oyster samples (n = 81), from three stations (4, 6, 9). Of these co-occurrences (n = 10), 90% were between PTX2 and KmTxs (Table S4).
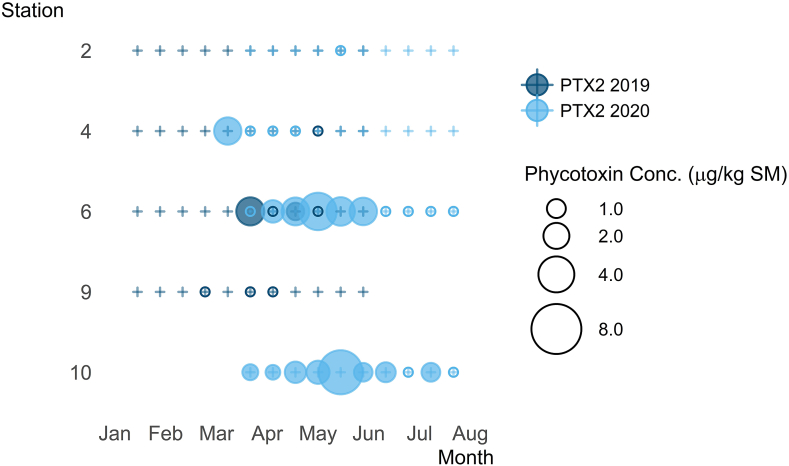
PTX2 was the most prevalent phycotoxin detected in oyster samples (41%, n = 81, Fig. 2, Table S4). Between 2019 and 2020, PTX2 was detected at every station (Table 1). PTX2 was detected in oysters in March through May 2019, with a maximum concentration of 2.4 μg/kg SM w. w. (station 6, 4/1/19), and in March through August 2020, with a maximum concentration of 6.2 μg/kg SM w. w. (station 10, 5/26/20, Fig. 7). In 2020, every oyster sample collected from stations 6 and 10 had detectable concentrations of PTX2 (Fig. 7).
Fig. 7.
Oyster phycotoxin data (μg/kg shellfish meat [SM] w. w.) for pectenotoxin-2 (PTX2) across five stations from January to June 2019 and from March to August 2020. Station 9 was only sampled in 2019 and station 10 was only sampled in 2020. Hollow circles are below the limit of quantitation (<LOQ) and are represented as ½ the method limit of detection (LOD). Samples below the LOD are indicated by plus signs (+).
GDA was detected in 7% of oyster samples (n = 81, Fig. 2, Table S4). Between 2019 and 2020, GDA was detected at three stations (2, 6, 9) (Table 1). GDA was detected in oysters in June 2019, at stations 2 and 9, and in June through July 2020, at stations 2 and 6, but was always detected in low concentrations (<LOQ, Fig. 8).
Fig. 8.
Oyster phycotoxin data (mg/kg shellfish meat [SM] w. w.) for goniodomin A (GDA) across five stations from January to June 2019 and from March to August 2020. Station 9 was only sampled in 2019 and station 10 was only sampled in 2020. Hollow circles are below the limit of quantitation (<LOQ) and are represented as ½ the method limit of detection (LOD). Samples below the LOD are indicated by plus signs (+).
KmTxs, KmTx1-1 and KmTx1-3 were both detected in oysters. KmTx1-1 prevalence in oyster samples (28%) was greater than that of KmTx1-3 (5%, n = 81, Fig. 2, Table S4). KmTx1-3 was only detected in oyster samples in which KmTx1-1 was also present (Table S4). Between 2019 and 2020, both KmTxs were detected at four stations (2, 4, 6, and 9) (Table 1). KmTx1-1 was detected in oysters in January through May 2019, at those four stations, and in March through April 2020, at station 4 (Fig. 9). KmTx1-3 was detected in oysters once at each of the four stations in April 2019 and was not detected in oysters in 2020 (Fig. 9).
Fig. 9.
Oyster phycotoxin presence/absence data for karlotoxin1-1 and -3 (KmTx1-1 and KmTx1-3, respectively) across five stations from January to June 2019 and from March to August 2020. Station 9 was only sampled in 2019 and station 10 was only sampled in 2020. Phycotoxin presence is denoted by hollow circles; phycotoxin absence is denoted by plus signs (+).
3.3. Predictors of phycotoxins in oysters
Phycotoxin profiles varied between sample types. The SPATT phycotoxin profile was dominated by DSTs (79% of all phycotoxins, n = 44), while DSTs made up 0% of the phycotoxin profile of oysters and POM (n = 44 and 43, respectively), and 0.8% of the phycotoxin profile in whole water (n = 40, Fig. 10). In contrast, the phycotoxin profiles of oysters, POM, and whole water were dominated by DA, making up 98, 99, and 97% of the respective profiles, while DA made up only 0.6% of the SPATT phycotoxin profile (Fig. 10).
Fig. 10.
Comparison of phycotoxin profiles within oyster, solid phase adsorption toxin tracking devices (SPATTs), particulate organic matter (POM, > 1.5 µm), and whole water across four stations for all 2019 sampling dates (n = 40-44). Proportions were calculated by summing the concentrations of a phycotoxin group across all 2019 samples and then dividing by the total concentration of all phycotoxins detected across all 2019 samples of that sample type. Karlotoxins (KmTxs) were not included as they were not quantified in this study, however, KmTxs were present in oysters and whole water samples, and absent from SPATTs and POM samples. AZAs = azaspiracids, DA = domoic acid, MCs = microcystins, DSTs = diarrhetic shellfish toxins (okadaic acid and dinophysistoxin-1), PTX2 = pectenotoxin-2, GDA = goniodomin A.
3.3.1. Seafood safety
Of the seven seafood safety phycotoxins detected in the current study, all were detected in oysters and whole water (AZA1, AZA2, DA, MC-RR, MC-YR, OA, DTX1), while SPATTs picked up five of these phycotoxins (AZA1, AZA2, DA, OA, DTX1), and POM picked up only three (AZA2, DA, MC-RR, Table 1).
AZAs, AZA1 and AZA2, were detected in oysters, SPATTs, and whole water; AZA2 was detected in POM, while AZA1 was not (Table 1). In 2019, SPATTs and whole water samples collected concurrently or two weeks prior to the oyster sample, were poor predictors of oyster AZA1 concentrations (Table 2). AZA2 oyster concentration data from 2019 was inadequate for assessing phycotoxin predictors (see section 2.6). In 2020, Amphidomataceae cell concentrations enumerated by qPCR from concurrent samples, or samples collected two weeks prior to oysters, were not a good predictor of AZA1 or AZA2 concentrations in oysters (Table 3). Additionally, AZA2 concentrations in concurrent SPATTs were not a good predictor of AZA2 concentrations in 2020 oyster samples (Table 3). Amphidomataceaecell concentrations enumerated by qPCR from 2020 samples can be found in Table S5, Amphidomataceae cells were too small to enumerate by the microscopy methods used.
Table 2.
Linear regressions with 2019 data for phycotoxins in oysters versus phycotoxins in solid phase adsorption toxin tracking devices (SPATT), particulate organic matter (POM, >1 μm), and whole water samples.
| Lag Time | Predictor | AZA1a | DA |
|---|---|---|---|
| No lag | SPATT | −0.02 | 0.1 |
| (p = 0.6, n = 44) | (p = 0.02, n = 44) | ||
| POM | – | 0.3 | |
| – | (p = 0.0001, n = 43) | ||
| Whole water | −0.02 | 0.6 | |
| (p = 0.8, n = 40) | (p < 0.0001, n = 40) | ||
| Two-week lag | SPATT | −0.02 | 0.1 |
| (p = 0.6, n = 40) | (p = 0.02, n = 40) | ||
| POM | – | 0.3 | |
| – | (p = 0.0001, n = 39) | ||
| Whole water | −0.009 | 0.4 | |
| (p = 0.4, n = 36) | (p < 0.0001, n = 36) |
The R2 is reported with the p-value and sample size. Significant linear regressions are bolded.
AZA1 = azaspiracid-1, DA = domoic acid.
Azaspiracid-1 (AZA1) was not detected in POM samples.
Table 3.
Linear regressions with 2020 data for phycotoxins in oysters versus phycotoxins in solid phase adsorption toxin tracking devices (SPATT), cell concentrations determined by microscopy (scope), or by quantitative real-time PCR (qPCR).
| Lag Time | Predictor | AZA1a,b | AZA2b | DAc | PTX2d |
|---|---|---|---|---|---|
| No lag | SPATT | – | 0.005 | 0.4 | 0.3 |
| – | (p = 0.3, n = 36) | (p < 0.0001, n = 36) | (p = 0.001, n = 36) | ||
| Scope | – | – | 0.2 | 0.3 | |
| – | – | (p = 0.002, n = 36) | (p = 0.0002, n = 36) | ||
| qPCR | −0.04 | 0.01 | 0.1 | −0.04 | |
| (p = 0.6, n = 23) | (p = 0.3, n = 23) | (p = 0.05, n = 23) | (p = 0.8, n = 23) | ||
| Two-week lag | SPATT | – | −0.03 | 0.2 | 0.2 |
| – | (p = 1, n = 33) | (p = 0.004, n = 33) | (p = 0.005, n = 33) | ||
| Scope | – | – | 0.04 | 0.007 | |
| – | – | (p = 0.1, n = 33) | (p = 0.3, n = 33) | ||
| qPCR | 0.08 | −0.03 | 0.3 | −0.05 | |
| (p = 0.1, n = 20) | (p = 0.5, n = 20) | (p = 0.009, n = 20) | (p = 0.7, n = 20) |
The R2 is reported with the p-value and sample size. Significant linear regressions are bolded.
AZA1 = azaspiracid-1, AZA2 = azaspiracid-2, DA = domoic acid, PTX2 = pectenotoxin-2.
AZA1 was not detected on SPATTs in 2020.
AZA-producing cells from the family Amphidomataceae, were too small to be detected by microscopy.
Pseudo-nitzschia spp. cells were enumerated by microscopy and by qPCR (cells/mL) to compare with DA concentrations in oyster samples.
Dinophysis spp. cells were enumerated by microscopy and by qPCR (cells/mL) to compare with PTX2 concentrations in oyster samples.
DA was detected in oysters, SPATTs, POM, and whole water (Table 1). In 2019, DA concentrations in oysters were significantly correlated with DA concentrations in concurrent SPATTs, POM, and whole water (LR SPATTs: R2 = 0.1, p = 0.02, n = 44; LR POM: R2 = 0.3, p = 0.0001, n = 43, LR Whole water: R2 = 0.6, p < 0.0001, n = 40, Table 2), and in SPATTs, POM, and whole water collected two weeks before the oysters (LR SPATTs: R2 = 0.1, p = 0.02, n = 40; LR POM: R2 = 0.3, p = 0.0001, n = 39, LR Whole water: R2 = 0.4, p < 0.0001, n = 36, Table 2). In 2020, DA concentrations in oysters were significantly correlated with DA concentrations in concurrent SPATT, and with Pseudo-nitzschia spp. cell concentrations enumerated by microscopy from concurrent water samples (LR SPATTs: R2 = 0.4, p < 0.0001, n = 36; LR Microscopy: R2 = 0.2, p = 0.002, n = 36, Table 3), and in SPATTs and Pseudo-nitzschia spp. cell concentrations enumerated by qPCR collected two weeks before the oysters (LR SPATTs: R2 = 0.2, p = 0.004, n = 33; LR qPCR: R2 = 0.3, p = 0.009, n = 20, Table 3). Concurrent Pseudo-nitzschia spp. cell concentrations enumerated by qPCR, and Pseudo-nitzschia spp. cell concentrations by microscopy from two weeks prior to collecting the oyster sample, were not good predictors of DA concentration in oysters (Table 3). Pseudo-nitzschia spp. cell concentrations enumerated by microscopy and by qPCR from 2020 samples can be found in Table S5.
MCs, MC-RR and MC-YR were detected in oysters and whole water; MC-RR was detected in POM, while MC-YR was not, and neither were detected in SPATTs. MC-LR was detected only in whole water (Table 1). MC-LR, MC-RR, and MC-YR oyster concentration data from 2019 to 2020 were inadequate for assessing phycotoxin predictors (see section 2.6). MC-producing cells were not enumerated in this study, as estuarine water samples are traditionally not screened for freshwater cells.
DSTs, OA and DTX1, were detected in oysters, SPATTs, and whole water, but not in POM (Table 1). Phycotoxin predictors for OA and DTX1 could not be tested in 2019 as these phycotoxins were not detected in oysters in that year, and in 2020, OA and DTX1 oyster concentration data was inadequate for assessing phycotoxin predictors (see section 2.6). Dinophysis spp. cell concentrations enumerated by microscopy and by qPCR from 2020 samples can be found in Table S5.
3.3.2. Shellfish health
Of the shellfish health phycotoxins detected in the current study, four were detected in oysters (PTX2, GDA, KmTx1-1, KmTx1-3), three in whole water (PTX2, KmTx1-1, KmTx1-3), two in SPATTs (PTX2, GDA), and only one in POM (PTX2, Table 1).
PTX2 was detected in oysters, SPATTs, POM, and whole water (Table 1). PTX2 oyster concentration data from 2019 was inadequate for assessing phycotoxin predictors (see section 2.6). In 2020, PTX2 concentration in oysters was significantly correlated to PTX2 in concurrent SPATTs and Dinophysis spp. cell concentrations enumerated by microscopy (LR SPATTs: R2 = 0.3, p = 0.001, n = 36; LR Microscopy: R2 = 0.3, p = 0.0002, n = 36, Table 3). SPATTs collected two weeks before the oysters also exhibited a significant correlation with oyster PTX2 concentrations (LR: R2 = 0.2, p = 0.005, n = 33, Table 3). Concurrent Dinophysis spp. cell concentrations enumerated by qPCR, and Dinophysisspp. cell concentrations by either method from two weeks prior to collecting the oyster sample, were not good predictors of PTX2 concentration in oysters (Table 3). Dinophysis spp. cell concentrations enumerated by microscopy and by qPCR from 2020 samples can be found in Table S5.
GDA was detected in oysters and SPATTs, but not in POM or whole water (Table 1). GDA oyster concentration data from 2019 to 2020 was inadequate for assessing phycotoxin predictors (see section 2.6). Alexandrium monilatum cell concentrations enumerated by microscopy and by qPCR from 2020 samples can be found in Table S5; no A. monilatumwas detected in this study.
KmTxs, KmTx1-1 and KmTx1-3, were detected in oysters and whole water, but not in SPATTs or POM (Table 1). KmTx1-1 and KmTx1-3 oyster concentration data from 2019 to 2020 did not meet the criteria set for assessing phycotoxin predictors (see section 2.6). Karlodinium veneficumcell concentrations enumerated by microscopy and by qPCR from 2020 samples can be found in Table S5.
4. Discussion
This is the first comprehensive examination of phycotoxin concentrations in oysters in the Chesapeake Bay, and the first known report of co-occurrence of phycotoxins in Eastern oysters. Co-occurrence of phycotoxins in oysters was common. Eleven phycotoxins were detected in oysters, with most oyster samples, 84%, containing at least one phycotoxin, and 54% of samples containing more than one phycotoxin at the same time. While most seafood safety phycotoxins were found at relatively low levels, i.e., well below action levels (USFDA 2019), domoic acid (DA) was the most prevalent seafood safety phycotoxin in oyster meat, and had the maximum concentration (0.58 mg/kg SM w. w.) when compared across phycotoxins. Phycotoxins that are known to negatively impact shellfish health in laboratory studies, such as pectenotoxins, karlotoxins, and goniodomin A, were also detected in oysters, providing the first report of accumulation in the Bay.
4.1. Seafood safety phycotoxins
Most oyster samples contained at least one phycotoxin associated with seafood safety; however, phycotoxin concentrations in oysters were well below action levels set to protect public health (USFDA 2019) and do not pose a current risk to the region. The majority of these phycotoxins are associated with well-known human health syndromes like AZP (AZAs), ASP (DA), and DSP (DSTs), while hepatotoxic MCs have been flagged as a potential seafood safety concern (Miller et al., 2010; Butler et al., 2012; Gibble et al., 2016, Chorus and Welker, 2021). This study also showed that oysters can co-accumulate low concentrations of multiple seafood safety phycotoxins. These findings support the need for further research on chronic, sub-acute exposure of vertebrate models to phycotoxins, as well as a risk assessment of the combined effects of phycotoxins for seafood safety. Some phycotoxins classified herein under seafood safety (i.e., human health) have not yet been investigated for effects in invertebrate models (e.g., AZAs).
4.1.1. Azaspiracids (AZAs)
This is the first known report of AZAs (AZA1 and AZA2) in shellfish on the US East Coast. There are few reports of AZAs or AZA-producing species in North America (Twiner et al., 2008; Trainer et al., 2013; Luo et al., 2016; Kim et al., 2017; Adams et al., 2020). Compared to other phycotoxins detected, AZA concentrations were low (Table 1), especially considering that AZA signals were enhanced due to the methodology (Table S3). The highest concentration of AZAs detected (0.80 μg AZA1/kg SM w. w., Fig. 3) was at least two orders of magnitude below the action level for AZAs in shellfish (160 μg/kg SM w. w., USFDA 2019), suggesting there is no current risk to seafood safety, but providing justification for regional monitoring of these phycotoxins, and for including them in regional marine biotoxin contingency plans.
AZA1 predominated in oyster samples and was more widespread over time and space in oysters than AZA2, showing no clear seasonality (Fig. 3). The AZA profile of oysters differs from the AZA profile of SPATTs in this study (Table 1) and in a previous study (Onofrio et al., 2021), both of which found that AZA2 was more prevalent on SPATTs. This observation may reflect differences in production, chemical stability, and/or uptake or detoxification rates between these analogs. AZA1 and AZA2 did not co-occur in oysters or SPATTs in the current study, a finding which contrasts with a previous report of AZA1 and AZA2 co-occurrence in SPATT extracts (Onofrio et al., 2021). Currently, the AZA-producing family Amphidomataceae, is being monitored within Virginia waters using qPCR, as there are no commercially available AZA toxin test kits, and the cells are too small to be identified by traditional microscopy. Overall, this study did not identify good predictors (e.g., AZAs in water, POM, or SPATT, or cells via qPCR) for AZA concentrations in oysters (Table 2, Table 3), suggesting that shellfish testing for azaspiracids should take a prominent role in monitoring for these phycotoxins.
4.1.2. Domoic acid (DA)
DA was the most abundant phycotoxin detected in oysters in the lower Chesapeake Bay (Table 1), and one of the most prevalent phycotoxins in oyster samples (40%, n = 81, Fig. 2). Generally, DA concentrations in oysters were at least an order of magnitude higher than other phycotoxins. Phycotoxin accumulation of DA was comparable between oysters, SPATTs, POM, and whole water. For DA, SPATTs, POM, whole water, and Pseudo-nitzschia spp. enumerated by qPCR from two weeks prior to oyster sampling also showed significant correlations with oyster phycotoxin concentrations (Table 2, Table 3), indicating that these metrics could potentially be used for early warning of DA accumulation in oysters.
The current study found that oysters in the lower Chesapeake Bay can accumulate low amounts of DA, even when water temperatures are below 10 °C (Fig. 4, Tables S1 and S6). Furthermore, water temperatures were lowest in late January 2019, reaching < 5 °C, coinciding with the highest DA concentration detected in this study (0.58 mg/kg SM w. w., Table S1). This DA concentration is still well below the action level for DA (20 mg/kg SM w. w., USFDA 2019), indicating there was no concern for seafood safety at the time. However, the presence of this phycotoxin in oysters at such low temperatures challenges the theory that cold conditions favor Pseudo-nitzschia blooms but limit filter-feeding rates of oysters, thereby reducing the risk of oysters accumulating unsafe concentrations of DA. This theory relies on evidence that (1) C. virginica oysters feed inefficiently at temperatures lower than 16 °C (Loosanoff 1958), and (2) the optimum temperature for growth of Pseudo-nitzschia is around 9–10 °C (Comeau et al., 2008; Anderson et al., 2010). The current study demonstrates that low water temperatures do not inhibit the accumulation of DA by oysters, and that Pseudo-nitzschia spp. cell concentrations enumerated by microscopy do correlate to oyster DA concentrations (Table 3). The question remains, however, how oysters would react to larger blooms of Pseudo-nitzschia during these colder months and if they could reach DA concentrations of concern to seafood safety under those conditions.
4.1.3. Microcystins (MCs)
MCs – traditionally freshwater phycotoxins – were detected in oysters in estuarine waters (Fig. 5). This study adds to a growing body of literature on MCs detected in estuarine and marine environments both in Virginia (Tango and Butler 2008; Wood et al., 2014; Bukaveckas et al., 2017, 2018; Onofrio et al., 2021) and globally (De Pace et al., 2014; Gibble and Kudela 2014; Preece et al., 2015; Gibble et al., 2016; Peacock et al., 2018), raising questions about potential human health impacts (Mulvenna et al., 2012; Vareli et al., 2013; Preece et al., 2017; Camacho-Muñoz et al., 2021; Straquadine et al., 2022) and the possibility of hepatotoxic shellfish poisoning (HSP, Miller et al., 2010). Note that the current study may underestimate MCs in oysters as (1) only free MCs were quantified as extraction methods excluded the less-toxic, covalently bound MCs (Chorus and Welker, 2021 and references therein), and (2) only three MC analogs out of 200+ identified thus far (Bouaïcha et al., 2019) were quantified as part of this study.
A recent study by Straquadine et al. (2022) demonstrated that eastern oysters can readily bioaccumulate MCs to levels of seafood safety concern, and depurate the phycotoxins slowly, making eastern oysters a potential vector for HSP in estuaries influenced by upstream Microcystis blooms. Uptake can occur through both particulate or dissolved routes of exposure (Gibble et al., 2016) and shellfish have been found to biomagnify MCs to concentrations more than 100X that of ambient water (Miller et al., 2010). The sporadic occurrence of MCs in oysters from March through August in the current study (7%, n = 81, Fig. 2, Fig. 5) supports the theory that these phycotoxins entered the estuarine environment during or after episodic summer bloom events in upstream freshwaters, or from spillover from freshwater ponds during rain events. In the current study, relationships could not be detected between MC presence in oysters and that in particulate (POM) or dissolved fractions (SPATT or whole water) within the environment, even when a two-week lag was considered in the analyses. It is, therefore, unclear whether oysters in the current study accumulated particulate and/or dissolved MCs, or which HAB species produced the MCs.
The concentrations of MCs in oyster samples in the current study suggest that strategies for monitoring both free and covalently bound MCs in shellfish be developed and implemented, and that research on seafood safety pertaining to MCs should be a priority. While recreational and drinking water criteria exist for these phycotoxins, MCs are currently not regulated in shellfish at the federal level in the US. The California Environmental Protection Agency (Butler et al., 2012) has proposed an action level for MCs of 10 μg/kg w. w. For sport fish and shellfish, while the Victorian Department of Health in Australia has calculated an MC health guideline value (for people 16 years and younger) of 51 μg/kg in mollusks (Mulvenna et al., 2012). Testai et al. (2016) summarized current established advisory levels of MCs in recreational fish and shellfish in several US states (i.e., California, Illinois, Nebraska, Ohio, Oregon, and Washington), and other countries, including Australia, Canada, and France. Shellfish with concentrations of MCs well above these values have been found on the US West Coast (Gibble et al., 2016). While the highest concentration of MC detected in the current study (7.12 μg MC-RR/kg SM w. w.) was below the range of existing guidance values, the limited scope of this project, and the concentrations recorded elsewhere in the country suggest more data should be acquired from shellfish harvesting areas using adequately validated methods.
4.1.4. Diarrhetic shellfish toxins (DSTs)
Concentrations of DSTs were low (approximately 5.63 μg DST/kg SM w. w.), more than an order of magnitude below action levels for DSTs in shellfish (160 μg DST/kg SM w. w., USFDA 2019), and were rarely detected (3%, n = 81). Additionally, DSTs were only found in oyster samples in May and June and were detected at three of the five stations (Fig. 6). This puts the sparse occurrence of DST in oysters at the end of the timeframe when Dinophysis cells were typically detected in water samples (March to June, Table S5). While uncommon in oysters, dissolved DSTs were detected on all SPATTs in the current study, even when Dinophysis cells were not detected (Table S5). Together this demonstrates (1) the persistence of these lipophilic phycotoxins extracellularly in the estuarine environment over the course of a year (Onofrio et al., 2021), and (2) an uncoupling between dissolved DSTs (as measured by SPATT) and shellfish accumulation.
This latter finding contrasts with a study in another Mid-Atlantic estuarine system (Long Island, NY, USA) where DSTs in extracts of SPATT and in shellfish were correlated; shellfish exceeded the USFDA criteria, warranting harvest closures (Hattenrath-Lehmann et al., 2018). Interestingly, these two estuarine systems both harbor the DST-producer, D. acuminata, with a similar phycotoxin profile (Hattenrath-Lehmann et al., 2018; Fiorendino et al., 2020; Wolny et al., 2020a), but currently appear to present different risk levels for DSP. The apparent difference in risk between estuaries may be explained by the types of shellfish harvested (Mafra et al., 2015), as mussels (Mytilus edulis and Geukensia demissa) accumulated much higher amounts of DSTs than oysters (C. virginica) or clams (Mya arenaria, Hattenrath-Lehmann et al., 2018) in New York, and only oysters were included in the current study in Virginia. As such, SPATTs do not provide an adequate understanding of DST concentrations in oysters in Chesapeake Bay and further investigation into regional differences in DSP risk in the US is warranted.
4.2. Shellfish health phycotoxins: PTX2, KmTxs, GDA
Most oyster samples (64%, n = 81) contained at least one phycotoxin associated with shellfish health: PTX2, KmTxs, or GDA. These phycotoxins are not expected to pose a risk to seafood safety (Miles et al., 2004; Place et al., 2014; Boundy et al., 2020), but have been shown to be detrimental to at least one species of shellfish (Place et al., 2008; Harding et al., 2009; Gaillard et al., 2020; Pease et al., 2022). When co-occurrence was detected in oysters (12%, n = 81), KmTxs and PTX2 were most commonly found together. While this demonstrates a need for research focused on co-exposures in shellfish to understand ecological consequences, it is also important to point out that a large mortality event was not recorded in any of the deployed cages/bags during the study, suggesting levels reported herein are not causes of acute toxicity to market-size oysters.
Complementary metrics (i.e., phycotoxins in SPATTs, POM, whole water, and cell concentrations by microscopy or qPCR) were analyzed as potential predictors of oyster exposure or accumulation in case the need should arise to protect the sustainability of this important commercial and ecological shellfish species. SPATTs and Dinophysis spp. cell concentrations by microscopy were the best predictors of PTX2 concentrations in oysters (Table 3) and could potentially be used to indicate PTX2 exposure to oysters. This is relevant as PTX2 was the most prevalent, and second-most abundant, phycotoxin detected during this study (Fig. 2, Table 1) and studies have demonstrated the deleterious effects of PTX2 on early life stages of two species of oysters (Gaillard et al., 2020; Pease et al., 2022). No studies as of yet have investigated the effects of PTX2 on adult shellfish. It is important to note that the exclusion of the PTX2 seco acid from the current study's detection method may have underestimated PTXs, especially in shellfish where conversion has been well documented (Miles et al., 2004).
This is the first study to detect KmTxs in oysters; KmTxs were detected (5–28%, n = 81, January through May, Fig. 9) during the timeframe when blooms of K. veneficum have typically occurred in the Chesapeake Bay (Li et al., 2000; Glibert et al., 2007; Marshall and Egerton 2009). The presence of KmTxs in oysters, however, was not correlated with cell abundance in this study (Table S5). A relationship was also lacking between oysters and other metrics investigated, i.e., KmTxs were not detected in any SPATT or POM samples, demonstrating that a metric has not yet been identified for predicting oyster exposure to these chemicals.
Similarly, GDA was detected in oyster samples (7%, n = 81, June and July, Fig. 8) in this study; however, GDA was not concurrently detected in POM or whole water samples, and cells of A. monilatum were not detected by microscopy or by qPCR. The detection of GDA in oysters was earlier than expected given that, historically, blooms of A. monilatum occur in Chesapeake Bay in the late summer (Mulholland et al., 2018; Wolny et al., 2020b; Onofrio et al., 2021), with dissolved GDA detectable in SPATTs from August to October (Onofrio et al., 2021). This first report of GDA accumulation in field oysters is still of note as this lipophilic phycotoxin was previously implicated in mortality of whelks (Rapana venosa, Harding et al., 2009) in a flow-through system. Concentrations in whelk foot tissue were as high as 8.77 mg/kg SM w. w. It is possible that both KmTxs and GDA were underestimated in water or SPATT samples, as both chemicals are relatively unstable in water (A. Place, unpublished; Onofrio, 2020).
4.3. Implications for seafood safety phycotoxin monitoring and management
This study utilized a sensitive method, UPLC-MS/MS with trap/ACD, for detecting a suite of seafood safety phycotoxins, AZAs, DA, DSTs, MCs, in shellfish tissue that can be used for monitoring and early warning. Phycotoxins currently regulated in shellfish in the US (AZAs, DA, DSTs) were co-detected at concentrations at least an order of magnitude below current action levels (USFDA 2019) demonstrating the ability of the method to screen for multiple phycotoxins at trace levels. This method, therefore, creates opportunities for early warning and mitigation via detection in oysters well before shellfish meat reaches concentrations that pose a risk to consumers.
Co-occurrence of phycotoxins was documented in oysters at all sites, and in both sampling years. Of the seafood safety phycotoxins, AZAs and DA co-occurred most commonly in oysters (Fig. 4). Co-occurrence of seafood safety phycotoxins in shellfish has been reported in mussels for DSTs and PSTs (Gago-Martinez et al., 1996; García et al., 2004; Hattenrath-Lehmann et al., 2018), DSTs and DA (Jester et al., 2009), DSTs, PSTs, DA, and MCs (Peacock et al., 2018), as well as DSTs, YTX, and AZAs in mussels, Pacific oysters (Crassostrea gigas), and Littleneck clams (Leukoma staminea, Trainer et al., 2013). A better understanding of how shellfish bioaccumulation of multiple phycotoxins may impact seafood safety is needed.
With the exception of DA, there was a general lack of agreement in phycotoxin profiles between sample types; some phycotoxins found in oysters were not detected in complementary SPATTs, POM, or whole water samples (Fig. 10). SPATTs failed to detect the MCs that were detected in oysters (MC-RR and MC-YR). POM samples failed to detect AZA1, as well as any seafood safety phycotoxins that were detected in only low concentrations in oysters (MC-YR, OA, DTX1), likely because POM only detected instantaneous phycotoxin content of the particulate fraction. SPATTs and POM, therefore, did not accurately reflect the range of phycotoxins accumulated by oysters. Furthermore, SPATTs in this and a previous study (Onofrio et al., 2021), primarily accumulated OA (Table 1); in contrast, oysters, POM, and whole water phycotoxin profiles were dominated by DA (Fig. 10).
Given the overall variability in profiles (Fig. 10), it was not surprising that SPATTs, POM, and whole water were not good predictors of most oyster phycotoxins when examining for relationships at the level of individual phycotoxins. POM and whole water samples provided discrete phycotoxin concentrations at the time of sampling. Whole water detected any phycotoxins present in the sample, while POM detected the particulate fraction of phycotoxins in a water sample, including intracellular phycotoxins as well as phycotoxins associated with particles > 1.5 μm. SPATTs and oysters, conversely, provided semi-cumulative phycotoxin concentrations over the time deployed. SPATTs and oysters accumulated phycotoxins from the water column in fundamentally different ways. SPATTs were passive, adsorptive samplers that accumulated dissolved phycotoxins from the water based on the chemical affinity and stability between those phycotoxins and the resin. Oysters were active samplers, feeding, respiring, and interacting with substantial portions of water through time. Oysters were exposed to both dissolved and particulate phycotoxins, may accumulate phycotoxins in different tissue types with varying affinities for those phycotoxins, and are known to both biotransform and depurate phycotoxins over time. To address this general mismatch between phycotoxin concentration in oysters versus phycotoxin concentrations in SPATTs, POM or whole water, or versus HAB cell concentrations, region-, phycotoxin-, and shellfish species-specific methods must be utilized for effective monitoring and prediction of shellfish phycotoxin accumulation.
Finally, MCs present additional challenges for monitoring and management of phycotoxins in shellfish. Human health effects of MCs have been well-documented (Mulvenna et al., 2012; Vareli et al., 2013; Preece et al., 2017; Camacho-Muñoz et al., 2021), and the possibility of MC-transfer to humans through shellfish consumption (HSP) has been discussed, but so far remains theoretical (Miller et al., 2010; Straquadine et al., 2022). As the highest MC concentrations in the current study were approaching the action level proposed in California (Butler et al., 2012), it is recommended that Virginia and similar estuarine systems consider a broader monitoring for freshwater phycotoxins, including MCs, in shellfish. Additionally, the development of a federal action level for MCs in shellfish is recommended.
Ethical statement
No human subjects were involved in this work.
Funding
This research was funded by the National Oceanic and Atmospheric Administration Sea Grant Aquaculture Research Program under award NA14OAR4170093 to J.L.S, K.S.R. (VIMS), and T.A.E. (VDH). This work was also funded in part by an MOU from VDH to K.S.R. (MOU#-VDH-20-102-0046) and a grant from the National Oceanic and Atmospheric Administration, National Centers for Coastal Ocean Science Competitive Research, under award NOAA–NOS–NCCOS-2012-2,002,987 to A.R.P. (IMET). The APC was funded by NA14OAR4170093.
CRediT authorship contribution statement
Sarah K.D. Pease: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Investigation, Writing - original draft, Writing - review & editing. Todd A. Egerton: Conceptualization, Funding acquisition, Investigation, Supervision, Visualization, Writing – review & editing. Kimberly S. Reece: Conceptualization, Funding acquisition, Supervision, Methodology, Writing - review & editing. Marta P. Sanderson: Data curation, Formal analysis, Investigation, Methodology, Writing - review & editing. Michelle D. Onofrio: Conceptualization, Investigation, Methodology, Writing – review & editing. Evan Yeargan: Investigation, Writing – review & editing. Adam Wood: Investigation, Writing – review & editing. Amanda Roach: Investigation, Writing – review & editing. I-Shuo Wade Huang: Methodology, Validation, Investigation, Writing - review & editing. Gail P. Scott: Data curation, Methodology, Writing – review & editing. Allen R. Place: Funding acquisition, Resources, Writing – review & editing. Amy M. Hayes: Resources, Writing – review & editing. Juliette L. Smith: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Tommy Leggett, M.S., of Chessie Seafood for supplying oysters for this study. Thanks to Rappahannock Oyster Company, Chesapeake Bay Foundation – Brock Environmental Center, and Cherrystone Aqua Farms for providing access to field sites for this research. The authors would like to acknowledge Bill Jones, Clara Robison, and Alanna MacIntyre (VIMS) for their valuable laboratory contributions. Thanks to Dr. Claude Mallet (Waters, Inc.) for his generous donation of the analytical instrumentation used in phycotoxin analyses. The authors also express their gratitude to Jeffrey O'Brien (William & Mary), Caroline DeMent (William & Mary), Madison Powell (William & Mary), Jessica Klinklam (William & Mary), Jaclyn Friedman, M.S. (VIMS), Dr. Gao Han (VIMS), Karina Donahoe (University of Mary Washington), Gabrielle Campbell (University of Lynchburg), and Katrina Napora (Christopher Newport University) for their assistance in the laboratory and field. The authors appreciate Dr. Roger Mann (VIMS), Dr. Kathi Lefebvre (NOAA), and Dr. Marjy Friedrichs (VIMS) for their constructive comments on this project and earlier versions of this manuscript. This publication was prepared by Sarah K. D. Pease using Federal funds under award [NOAA Award/Grant #NA22OAR417004], Virginia Sea Grant College Program Project [VASG Project #E/724120], from the NOAA National Sea Grant College Program, U.S. Department of Commerce. The statements, findings, conclusions, and recommendations are those of the authors and do not necessarily reflect the views of VASG, NOAA, or the U.S. Department of Commerce. This is Contribution No. 23-103 from the Institute of Marine and Environmental Technology.
Handling Editor: Ray Norton
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.toxcx.2023.100166.
Contributor Information
Sarah K.D. Pease, Email: skpease@wm.edu.
Todd A. Egerton, Email: todd.egerton@vdh.virginia.gov.
Kimberly S. Reece, Email: kreece@vims.edu.
Marta P. Sanderson, Email: mps@vims.edu.
Michelle D. Onofrio, Email: mdonofrio@email.wm.edu.
Evan Yeargan, Email: evan.yeargan@vdh.virginia.gov.
Adam Wood, Email: adam.wood@vdh.virginia.gov.
Amanda Roach, Email: roach.amanda@epa.gov.
I-Shuo Wade Huang, Email: ishuo.huang@fda.hhs.gov.
Gail P. Scott, Email: gpscott@vims.edu.
Allen R. Place, Email: place@umces.edu.
Amy M. Hayes, Email: amy.hayes@vdh.virginia.gov.
Juliette L. Smith, Email: jlsmith@vims.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- Abbott B.C., Ballantine D. The toxin from Gymnodinium veneficum Ballantine. J. Mar. Biol. Assoc. U. K. 1957;36:169–189. doi: 10.1017/S0025315400017173. [DOI] [Google Scholar]
- Adams N.G., Tillmann U., Trainer V.L. Temporal and spatial distribution of Azadinium species in the inland and coastal waters of the Pacific northwest in 2014-2018. Harmful Algae. 2020;98 doi: 10.1016/j.hal.2020.101874. [DOI] [PubMed] [Google Scholar]
- Adolf J.E., Bachvaroff T.R., Place A.R. Environmental modulation of karlotoxin levels in strains of the cosmopolitan dinoflagellate, Karlodinium veneficum (Dinophyceae) J. Phycol. 2009;45:176–192. doi: 10.1111/j.1529-8817.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- Anderson C.R., Sapiano M.R.P., Prasad M.B.K., Long W., Tango P.J., Brown C.W., Murtugudde R. Predicting potentially toxigenic Pseudo-nitzschia blooms in the Chesapeake Bay. Mar. Sys. 2010;83:127–140. doi: 10.1016/j.jmarsys.2010.04.003. [DOI] [Google Scholar]
- Bachvaroff T.R., Adolf J.E., Squier A.H., Harvey H.R., Place A.R. Characterization and quantification of karlotoxins by liquid chromatography–mass spectrometry. Harmful Algae. 2008;7(4):473–484. doi: 10.1016/j.hal.2007.10.003. [DOI] [Google Scholar]
- Bachvaroff T.R., Adolf J.E., Place A.R. Strain variation in Karlodinium veneficum (Dinophyceae) toxin profiles, pigments, and growth characteristics. J. Phycol. 2009;45:137–153. doi: 10.1111/j.1529-8817.2008.00629.x. [DOI] [PubMed] [Google Scholar]
- Barbier M., Amzil Z., Mondeguer F., Bhaud Y., Soyer-Gobillard M., Lassus P. Okadaic acid and PP2A cellular immunolocalization in Prorocentrum lima (Dinophyceae) Phycologia. 1999;38(1):41–46. doi: 10.2216/i0031-8884-38-1-41.1. [DOI] [Google Scholar]
- Barco M., Lawton L.A., Rivera J., Caixach J. Optimization of intracellular microcystin extraction for their subsequent analysis by high-performance liquid chromatography. J. Chromatogr. A. 2005;1074:23–30. doi: 10.1016/j.chroma.2005.03.087. [DOI] [PubMed] [Google Scholar]
- Botta R., Asche F., Borsum J.S., Camp E.V. A review of global oyster aquaculture production and consumption. Mar. Pol. 2020;117 doi: 10.1016/j.marpol.2020.103952. [DOI] [Google Scholar]
- Bouaïcha N., Miles C.O., Beach D.G., Labidi Z., Djabri A., Benayache N.Y., Nguyen-Quang T. Structural diversity, characterization and toxicology of microcystins. Toxins. 2019;11:714. doi: 10.3390/toxins11120714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boundy M.J., Harwood D.T., Kiermeier A., McLeod C., Nicolas J., Finch S. Risk assessment of pectenotoxins in New Zealand bivalve molluscan shellfish, 2009-2019. Toxins. 2020;12:776. doi: 10.3390/toxins12120776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee E.F., Sellner S.G., Sellner K.G., Nonogaki H., Adolf J.E., Bachvaroff T.R., Place A.R. Responses of Crassostrea virginica (Gmelin) and C. ariakensis (Fujita) to bloom-forming phytoplankton including ichthyotoxic Karlodinium veneficum (Ballantine) J. Shellfish Res. 2008;27(3):581–591. [Google Scholar]
- Bukaveckas P.A., Lesutiene J., Gasiunaite Z.R., Lozys L., Olenina I., Pilkaityte R., Putys Z., Tassone S., Wood J. Microcystin in aquatic food webs of the Baltic and Chesapeake Bay regions. Estuar. Coast Shelf Sci. 2017;191:50–59. doi: 10.1016/j.ecss.2017.04.016. [DOI] [Google Scholar]
- Bukaveckas P.A., Franklin R., Tassone S., Trache B., Egerton T.A. Cyanobacteria and cyanotoxins at the river-estuarine transition. Harmful Algae. 2018;76:11–21. doi: 10.1016/j.hal.2018.04.012. [DOI] [PubMed] [Google Scholar]
- Butler N., Carlisle J., Linville R. (Office of Environmental Health Hazard Assessment California Environmental Protection Agency) Toxicological summary and suggested action levels to reduce potential adverse health effects of six cyanotoxins. 2012 https://www.waterboards.ca.gov/water_issues/programs/tmdl/records/state_board/2016/ref4294.pdf Final report. Final report. [Google Scholar]
- Camacho-Muñoz D., Waack J., Turner A.D., Lewis A.M., Lawton L.A., Edwards C. Rapid uptake and slow depuration: health risks following cyanotoxin accumulation in mussels? Environ. Pollut. 2021;271 doi: 10.1016/j.envpol.2020.116400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos A., Vasconcelos V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010;11:268–287. doi: 10.3390/ijms11010268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorus I., Welker M., editors. Toxic Cyanobacteria in Water. second ed. CRC Press, Boca Raton (FL), on behalf of the World Health Organization; Geneva, CH: 2021. [Google Scholar]
- Comeau L.A., Pernet F., Tremblay R., Bates S.S., LeBlanc A. Comparison of eastern oyster (Crassostrea virginica) and blue mussel (Mytilus edulis) filtration rates at low temperatures. Can. Tech. Rep. Fish. Aquat. Sci. 2008;2810 vii + 17 pp. [Google Scholar]
- Dawson R.M. The toxicology of microcystins. Toxicon. 1998;36(7):953–962. doi: 10.1016/S0041-0101(97)00102-5. [DOI] [PubMed] [Google Scholar]
- De Pace R., Vita V., Bucci M.S., Gallo P., Bruno M. Microcystin contamination in sea mussel farms from the Italian southern Adriatic coast following cyanobacterial blooms in an artificial reservoir. Ecosys. 2014;2014 doi: 10.1155/2014/374027. [DOI] [Google Scholar]
- Eriksson J.E., Toivola D., Meriluoto J.A.O., Karaki H., Han Y.-G., Hartshorne D. Hepatocyte deformation induced by cyanobacterial toxins reflects inhibition of protein phosphatases. Biochem. Bioph. Res. Co. 1990;173(3):1347–1353. doi: 10.1016/S0006-291X(05)80936-2. [DOI] [PubMed] [Google Scholar]
- Fiorendino J.M., Smith J.L., Campbell L. Growth response of Dinophysis, Mesodinium, and Teleaulax cultures to temperature, irradiance, and salinity. Harmful Algae. 2020;98 doi: 10.1016/j.hal.2020.101896. [DOI] [PubMed] [Google Scholar]
- Fux E., Marcaillou C., Mondeguer F., Bire R., Hess P. Field and mesocosm trials on passive sampling for the study of adsorption and desorption behaviour of lipophilic toxins with a focus on OA and DTX1. Harmful Algae. 2008;7(5):574–583. doi: 10.1016/j.hal.2007.12.008. [DOI] [Google Scholar]
- Gago-Martinez A., Rodriguez-Vazquez J.A., Thibault P., Quilliam M.A. Simultaneous occurrence of diarrhetic and paralytic shellfish poisoning toxins in Spanish mussels in 1993. Nat. Toxins. 1996;4:72–79. doi: 10.1002/19960402nt3. [DOI] [PubMed] [Google Scholar]
- Gaillard S. Ecophysiological studies on Dinophysisand its food chain, and in vitro effects of the dinoflagellate and its toxins on early life stages of two models of marine animals (oyster and fish) PhD Thesis. Université de Nantes. 2020 https://archimer.ifremer.fr/doc/00666/77807/ [Google Scholar]
- Gaillard S., Le Goïc N., Malo F., Boulais M., Fabioux C., Zaccagnini L., Carpentier L., Sibat M., Réveillon D., Séchet V., Hess P., Hégaret H. Cultures of Dinophysis sacculus, D. acuminata and pectenotoxin 2 affect gametes and fertilization success of the Pacific oyster, Crassostrea gigas. Environ. Pollut. 2020;265(Pt B) doi: 10.1016/j.envpol.2020.114840. [DOI] [PubMed] [Google Scholar]
- Galimany E., Place A.R., Ramón M., Jutson M., Pipe R.K. The effects of feeding Karlodinium veneficum (PLY # 103; Gymnodinium veneficum Ballantine) to the blue mussel Mytilus edulis. Harmful Algae. 2008;7:91–98. doi: 10.1016/j.hal.2007.05.004. [DOI] [Google Scholar]
- García C., Mardones P., Sfeir A., Lagos N. Simultaneous presence of paralytic and diarrheic shellfish poisoning toxins in Mytilus chilensis samples collected in the chiloe Island, austral Chilean fjords. Biol. Res. 2004;37:721–731. doi: 10.4067/s0716-97602004000500002. [DOI] [PubMed] [Google Scholar]
- Gibble C.M., Kudela R.M. Detection of persistent microcystin toxins at the land-sea interface in Monterey Bay, California. Harmful Algae. 2014;39:146–153. doi: 10.1016/j.hal.2014.07.004. [DOI] [Google Scholar]
- Gibble C.M., Peacock M.B., Kudela R.M. Evidence of freshwater algal toxins in marine shellfish: implications for human and aquatic health. Harmful Algae. 2016;59:59–66. doi: 10.1016/j.hal.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Gjølme N., Utkilen H. The extraction and stability of microcystin-RR in different solvents. Phycologia. 1996;35(sup6):80–82. [Google Scholar]
- Glibert P.M., Alexander J., Merritt D.W., North E.W., Stoecker D.K. Harmful algae pose additional challenges for oyster restoration: impacts of the harmful algae Karlodinium veneficum and Prorocentrum minimum on early life stages of the oysters Crassostrea virginica and Crassostrea ariakensis. J. Shellfish Res. 2007;26(4):919–925. doi: 10.2983/0730-8000(2007)26. [DOI] [Google Scholar]
- Harding J.M., Mann R., Moeller P., Hsia M.S., Road F.J., Carolina S. Mortality of the veined rapa whelk, Rapana venosa, in relation to a bloom of Alexandrium monilatum in the York River, United States. J. Shellfish Res. 2009;28(2):363–367. doi: 10.2983/035.028.0219. [DOI] [Google Scholar]
- Harris C.M., Reece K.S., Stec D.F., Scott G.P., Jones W.M., Hobbs P.L., Harris T.M. The toxin goniodomin, produced by Alexandrium spp., is identical to goniodomin A. Harmful Algae. 2020;92 doi: 10.1016/j.hal.2019.101707. [DOI] [PubMed] [Google Scholar]
- Hattenrath-Lehmann T.K., Lusty M.W., Wallace R.B., Haynes B., Wang Z., Broadwater M., Deeds J.R., Morton S.L., Hastback W., Porter L., Chytalo K., Gobler C.J. Evaluation of rapid, early warning approaches to track shellfish toxins associated with Dinophysis and Alexandriumblooms. Mar. Drugs. 2018;16:28. doi: 10.3390/md16010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia M.H., Morton S.L., Smith L.L., Beauchesne K.R., Huncik K.M., Moeller P.D.R. Production of goniodomin A by the planktonic, chain-forming dinoflagellate Alexandrium monilatum (Howell) Balech isolated from the Gulf Coast of the United States. Harmful Algae. 2006;5:290–299. doi: 10.1016/j.hal.2005.08.004. [DOI] [Google Scholar]
- Huang I.-S., Zimba P.V. Cyanobacterial bioactive metabolites—a review of their chemistry and biology. Harmful Algae. 2019;83:42–94. doi: 10.1016/j.hal.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Hudson K. Virginia shellfish aquaculture situation and outlook report. Results of the 2018 Virginia Shellfish Aquaculture Crop Reporting Survey. VIMS Mar. Resour. Rep. No. 2019-8. Virginia Sea Grant VSG-19-3: 20. Virginia Sea Grant Marine Extension Program, Virginia Institute of Marine Science. 2019 doi: 10.25773/jc19-y847. [DOI] [Google Scholar]
- Jester R., Lefebvre K., Langlois G., Vigilant V., Baugh K., Silver M.W. A shift in the dominant toxin-producing algal species in central California alters phycotoxins in food webs. Harmful Algae. 2009;8:291–298. doi: 10.1016/j.hal.2008.07.001. [DOI] [Google Scholar]
- Kim J.H., Tillmann U., Adams N.G., Krock B., Stutts W.L., Deeds J.R., Han M.S., Trainer V.L. Identification of Azadinium species and a new azaspiracid from Azadinium poporum in Puget Sound, Washington State, USA. Harmful Algae. 2017;68:152–167. doi: 10.1016/j.hal.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Kudela R.M. Characterization and deployment of Solid Phase Adsorption Toxin Tracking (SPATT) resin for monitoring of microcystins in fresh and saltwater. Harmful Algae. 2011;11:117–125. doi: 10.1016/j.hal.2011.08.006. [DOI] [Google Scholar]
- Lane J.Q., Roddam C.M., Langlois G.W., Kudela R.M. Application of Solid Phase Adsorption Toxin Tracking (SPATT) for field detection of the hydrophilic phycotoxins domoic acid and saxitoxin in coastal California. Limnol Oceanogr. Methods. 2010;8(11):645–660. doi: 10.4319/lom.2010.8.0645. [DOI] [Google Scholar]
- Lawton L.A., Edwards C., Codd G.A. Extraction and high-performance liquid chromatographic method for the determination of microcystins in raw and treated waters. Analyst. 1994;119:1525–1530. doi: 10.1039/an9941901525. [DOI] [PubMed] [Google Scholar]
- Li A., Stoecker D.K., Coats D.W. Spatial and temporal aspects of Gyrodinium galatheanum in Chesapeake Bay: distribution and mixotrophy. J. Plankton Res. 2000;22(11):2105–2124. doi: 10.1093/plankt/22.11.2105. [DOI] [Google Scholar]
- Li H., Xie P., Li G., Hao L., Xiong Q. In vivo study on the effects of microcystin extracts on the expression profiles of proto-oncogenes (c-fos, c-jun, and c-myc) in liver, kidney and testis of male Wistar rats injected i.v. with toxins. Toxicon. 2009;53:169–175. doi: 10.1016/j.toxicon.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Li Y., Sheng J., Sha J., Han X. The toxic effects of microcystin-LR on the reproductive system of male rats in vivo and in vitro. Reprod. Toxicol. 2008;26(3–4):239–245. doi: 10.1016/j.reprotox.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Loosanoff V.L. Some aspects of behavior of oysters at different temperatures. Biol. Bull. 1958;114(1):57–70. doi: 10.2307/1538965. [DOI] [Google Scholar]
- Luo Z., Krock B., Mertens K.N., Price A.M., Turner R.E., Rabalais N.N., Gu H. Morphology, molecular phylogeny and azaspiracid profile of Azadinium poporum (Dinophyceae) from the Gulf of Mexico. Harmful Algae. 2016;55:56–65. doi: 10.1016/j.hal.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Mafra L.L., Jr., Ribas T., Alves T.P., Proença L.A.O., Schramm M.A., Uchida H., Suzuki T. Differential okadaic acid accumulation and detoxification by oysters and mussels during natural and simulated Dinophysisblooms. Fish. Sci. 2015;81:749–762. doi: 10.1007/s12562-015-0882-7. [DOI] [Google Scholar]
- Marshall H.G. Toxin producing phytoplankton in Chesapeake Bay. Va. J. Sci. 1996;47(1):29–37. [Google Scholar]
- Marshall H.G., Burchardt L., Lacouture R. A review of phytoplankton composition within Chesapeake Bay and its tidal estuaries. J. Plankton Res. 2005;27(11):1083–1102. doi: 10.1093/plankt/fbi079. [DOI] [Google Scholar]
- Marshall H.G., Egerton T.A. Phytoplankton blooms: their occurrence and composition within Virginia's tidal tributaries. Va. J. Sci. 2009;60:149–164. doi: 10.25778/3KCS-7J11. [DOI] [Google Scholar]
- May S.P., Burkholder J.M., Shumway S.E., Hégaret H., Wikfors G.H., Frank D. Effects of the toxic dinoflagellate Alexandrium monilatum on survival, grazing and behavioral response of three ecologically important bivalve molluscs. Harmful Algae. 2010;9:281–293. doi: 10.1016/j.hal.2009.11.005. [DOI] [Google Scholar]
- McCarthy M., van Pelt F.N., Bane V., O'Halloran J., Furey A. Application of passive (SPATT) and active sampling methods in the profiling and monitoring of marine biotoxins. Toxicon. 2014;89:77–86. doi: 10.1016/j.toxicon.2014.07.005. [DOI] [PubMed] [Google Scholar]
- McNabb P., Selwood A.I., Holland P.T., Aasen J., Aune T., Eaglesham G., Hess P., Igarishi M., Quilliam M., Slattery D., Van de Riet J., Van Egmond H., Van den Top H., Yasumoto T. Multiresidue method for determination of algal toxins in shellfish: single-laboratory validation and interlaboratory study. J. AOAC Int. 2005;88(3):761–772. doi: 10.1093/jaoac/88.3.761. [DOI] [PubMed] [Google Scholar]
- Miles C.O., Wilkins A.L., Munday R., Dines M.H., Hawkes A.D., Briggs L.R., Sandvik M., Jensen D.J., Cooney J.M., Holland P.T., Quilliam M.A., MacKenzie A.L., Beuzenberg V., Towers N.R. Isolation of pectenotoxin-2 from Dinophysis acuta and its conversion to pectenotoxin-2 seco acid, and preliminary assessment of their acute toxicities. Toxicon. 2004;43:1–9. doi: 10.1016/j.toxicon.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Kudela R.M., Mekebri A., Crane D., Oates S.C., Tinker M.T., Staedler M., Miller W.A., Toy-Choutka S., Dominik C., Hardin D., Langlois G., Murray M., Ward K., Jessup D.A. Evidence for a novel marine harmful algal bloom: cyanotoxin (microcystin) transfer from land to sea otters. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland M.R., Morse R., Egerton T.A., Bernhardt P.W., Filippino K.C. Blooms of dinoflagellate mixotrophs in a lower Chesapeake Bay tributary: carbon and nitrogen uptake over diurnal, seasonal, and interannual timescales. Estuar. Coast. 2018;41:1744–1765. doi: 10.1007/s12237-018-0388-5. [DOI] [Google Scholar]
- Mulvenna V., Dale K., Priestly B., Mueller U., Humpage A., Shaw G., Allinson G., Falconer I. Health risk assessment for cyanobacterial toxins in seafood. Int. J. Environ. Res. Publ. Health. 2012;9(3):807–820. doi: 10.3390/ijerph9030807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Marine Fisheries Service (NMFS) U.S. Department of Commerce; 2021. Fisheries of the United States, 2019.https://www.fisheries.noaa.gov/national/sustainable-fisheries/fisheries-united-states NOAA Current Fishery Statistics No. 2019. Available at: [Google Scholar]
- Nielsen M.V., Strømgren T. Shell growth response of mussels (Mytilus edulis) exposed to toxic microalgae. Mar. Biol. 1991;108:263–267. doi: 10.1007/BF01344341. [DOI] [Google Scholar]
- Nishiwaki-Matsushima R., Ohta T., Nishiwaki S., Suganuma M., Kohyama K., Ishikawa T., Carmichael W.W., Fujiki H. Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. J. Cancer Res. Clin. Oncol. 1992;118:420–424. doi: 10.1007/BF01629424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOAA Fisheries. National Oceanic and Atmospheric Administration 2020. https://www.fisheries.noaa.gov/foss/f?p=215:200:6136995510764::NO Landings (3.5.0.0) [Data Set].
- Onofrio M.D., Mallet C.R., Place A.R., Smith J.L. A screening tool for the direct analysis of marine and freshwater phycotoxins in organic SPATT extracts from the Chesapeake Bay. Toxins. 2020;12(5):322. doi: 10.3390/toxins12050322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onofrio M.D. Spatial and temporal distribution of phycotoxins in lower Chesapeake Bay: method development and application. Master’s Thesis. William & Mary. 2020 doi: 10.25773/v5-z6gg-jz22. [DOI] [Google Scholar]
- Onofrio M.D., Egerton T.A., Reece K.S., Pease S.K.D., Sanderson M.P., Jones W., III, Yeargan E., Roach A., DeMent C., Wood A., Reay W.G., Place A.R., Smith J.L. Spatiotemporal distribution of phycotoxins within nearshore waters of the Chesapeake Bay and Virginia coastal bays. Harmful Algae. 2021;103 doi: 10.1016/j.hal.2021.101993. [DOI] [PubMed] [Google Scholar]
- Peacock M.B., Gibble C.M., Senn D.B., Cloern J.E., Kudela R.M. Blurred lines: multiple freshwater and marine algal toxins at the land-sea interface of San Francisco Bay, California. Harmful Algae. 2018;73:138–147. doi: 10.1016/j.hal.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Pease S.K.D. Alexandrium monilatum in the Lower Chesapeake Bay: sediment cyst distribution and potential health impacts on Crassostrea virginica. Master’s Thesis. William & Mary. 2016 doi: 10.21220/V5C30T. [DOI] [Google Scholar]
- Pease S.K.D., Reece K.S., O’Brien J., Hobbs P.L.M., Smith J.L. Oyster hatchery breakthrough of two HABs and potential effects on larval eastern oysters (Crassostrea virginica) Harmful Algae. 2021;101 doi: 10.1016/j.hal.2020.101965. [DOI] [PubMed] [Google Scholar]
- Pease S.K.D., Brosnahan M.L., Sanderson M.P., Smith J.L. Effects of two toxin-producing harmful algae, Alexandrium catenella and Dinophysis acuminata (Dinophyceae), on activity and mortality of larval shellfish. Toxins. 2022;14:335. doi: 10.3390/toxins14050335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna A., Bertozzini E., Battocchi C., Galluzzi L., Giacobbe M.G., Vila M., Garces E., Lugliè A., Magnani M. Monitoring of HAB species in the Mediterranean Sea through molecular methods. J. Plankton Res. 2007;29:19–38. doi: 10.1093/plankt/fbl053. [DOI] [Google Scholar]
- Pestana C.J., Reeve P.J., Newcombe G. Extraction method for total microcystins in cyanobacteria-laden sludge. Journal of Chromatography B. 2014;965:61–64. doi: 10.1016/j.jchromb.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Place A.R., Brownlee E.F., Nonogaki H., Adolf J.E., Bachvaroff T.R., Sellner S.G., Sellner K.G. International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO; Copenhagen: 2008. Responses of bivalve molluscs to the ichthyotoxic dinoflagellate Karlodinium veneficum; pp. 5–8. [Google Scholar]
- Place A.R., Munday R., Munday J.S. Acute toxicity of karlotoxins to mice. Toxicon. 2014;90:184–190. doi: 10.1016/j.toxicon.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preece E.P., Moore B.C., Hardy F.J., Deobald L.A. First detection of microcystin in Puget Sound, Washington, mussels (Mytilus trossulus) Lake Reservoir Manag. 2015;31(1):50–54. doi: 10.1080/10402381.2014.998398. [DOI] [Google Scholar]
- Preece E.P., Hardy F.J., Moore B.C., Bryan M. A review of microcystin detections in estuarine and marine waters: environmental implications and human health risk. Harmful Algae. 2017;61:31–45. doi: 10.1016/j.hal.2016.11.006. [DOI] [Google Scholar]
- Ray S.M., Aldrich D.V. Animal Toxins. 1966. Ecological interactions of toxic dinoflagellates and molluscs in the Gulf of Mexico; pp. 75–83. [Google Scholar]
- Roué M., Darius H.T., Chinain M. Solid phase adsorption toxin tracking (SPATT) technology for the monitoring of aquatic toxins: a review. Toxins. 2018;10(4):167. doi: 10.3390/toxins10040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson M.P., Hudson K.L., Gregg L.S., Chesler-Poole A.B., Small J.M., Reece K.S., Carnegie R.B., Smith J.L. Detection of toxins and harmful algal bloom cells in shellfish hatcheries and efforts toward removal. Aquaculture. 2023;562 doi: 10.1016/j.aquaculture.2022.738714. [DOI] [Google Scholar]
- Sievers A.M. Comparative toxicity of Gonyaulax monilataand Gymnodinium breve to annelids, crustaceans, molluscs and a fish. J. Protozool. 1969;16:401–404. doi: 10.1111/j.1550-7408.1969.tb02288.x. [DOI] [PubMed] [Google Scholar]
- Silva-Stenico M.E., Cantúsio Neto R., Alves I.R., Moraes L.A.B., Shishido T.K., Fiore M.F. Hepatotoxin microcystin-LR extraction optimization. Journal of the Brazilian Chemical Society. 2009;20:535–542. [Google Scholar]
- Smith K.F., Rhodes L., Harwood D.T., Adamson J., Moisan C., Munday R., Tillmann U. Detection of Azadinium poporum in New Zealand: the use of molecular tools to assist with species isolations. J. Appl. Phycol. 2016;28:1125–1132. doi: 10.1007/s10811-015-0667-5. [DOI] [Google Scholar]
- Stoecker D., Adolf J.E., Place A.R., Glibert P., Meritt D. Effects of the dinoflagellates Karlodinium veneficum and Prorocentrum minimumon early life history stages of the eastern oyster (Crassostrea virginica) Mar. Biol. 2008;154:81–90. doi: 10.1007/s00227-007-0901-z. [DOI] [Google Scholar]
- Straquadine N.R.W., Kudela R.M., Gobler C.J. Hepatotoxic shellfish poisoning: accumulation of microcystins in Eastern oysters (Crassostrea virginica) and Asian clams (Corbicula fluminea) exposed to wild and cultured populations of the harmful cyanobacteria, Microcystis. Harmful Algae. 2022;115 doi: 10.1016/j.hal.2022.102236. [DOI] [PubMed] [Google Scholar]
- Tango P., Butler W., Lacouture R., Goshorn D., Magnien R., Michael B., et al. In: Harmful Algae 2002. Steidinger K.A., Landsberg J.H., Tomas C.R., Vargo G.A., editors. Florida Fish and Wildlife Conservation Commission); Tallahassee, FL: 2004. An unprecedented bloom of Dinophysis acuminata in Chesapeake Bay; pp. 358–360. [Google Scholar]
- Tango P.J., Butler W. Cyanotoxins in tidal waters of Chesapeake Bay. Northeast. Nat. 2008;15(3):403–416. doi: 10.1656/1092-6194-15.3.403. [DOI] [Google Scholar]
- Testai E., Buratti F.M., Funari E., Manganelli M., Vichi S., Arnich N., Biré R., Fessard V., Sialehaamoa A. Review and analysis of occurrence, exposure and toxicity of cyanobacteria toxins in food. EFSA Supporting Publications. 2016;13(2):309. doi: 10.2903/sp.efsa.2016.EN-998. EN-998. [DOI] [Google Scholar]
- Thessen A.E., Stoecker D.K. Distribution, abundance and domoic acid analysis of the toxic diatom genus Pseudo-nitzschia from the Chesapeake Bay. Estuar. Coast. 2008;31:664–672. doi: 10.1007/s12237-008-9053-8. [DOI] [Google Scholar]
- Tillmann U., Jaén D., Fernández L., Gottschling M., Witt M., Blanco J., Krock B. Amphidoma languida (Amphidomatacea, Dinophyceae) with a novel azaspiracid toxin profile identified as the cause of molluscan contamination at the Atlantic coast of southern Spain. Harmful Algae. 2017;62:113–126. doi: 10.1016/j.hal.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Trainer V.L., Moore L., Bill B.D., Adams N.G., Harrington N., Borchert J., Da Silva D.A., Eberhart B.T.L. Diarrhetic shellfish toxins and other lipophilic toxins of human health concern in Washington State. Mar. Drugs. 2013;11(6):1815–1835. doi: 10.3390/md11061815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twiner M.J., Rehmann N., Hess P., Doucette G.J. Azaspiracid Shellfish Poisoning: a review on the chemistry, ecology, and toxicology with an emphasis on human health impacts. Mar. Drugs. 2008;6:39–72. doi: 10.3390/md20080004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration (USFDA) 2019. National Shellfish Sanitation Program Guide for the Control of Molluscan Shellfish.https://www.fda.gov/food/federalstate-food-programs/national-shellfish-sanitation-program-nssp [Google Scholar]
- Vandersea M.W., Kibler S.R., Van Sant S.B., Tester P.A., Sullivan K., Eckert G., Cammarata C., Reece K., Scott G., Place A., Holderied K., Hondolero D., Litaker R.W. qPCR assays for Alexandrium fundyense and A. ostenfeldii (Dinophyceae) identified from Alaskan waters and a review of species-specific Alexandrium molecular assays. Phycologia. 2017;56:303–320. doi: 10.2216/16-41.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vareli K., Jaeger W., Touka A., Frillingos S., Briasoulis E., Sainis I. Hepatotoxic Seafood Poisoning (HSP) due to microcystins: a threat from the ocean? Mar. Drugs. 2013;11:2751–2768. doi: 10.3390/md11082751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar-González A., Rodríguez-Velasco M.L., Ben-Gigirey B., Yasumoto T., Botana L.M. Assessment of the hydrolysis process for the determination of okadaic acid-group toxin ester: presence of okadaic acid 7-O-acyl-ester derivates in Spanish shellfish. Toxicon. 2008;51(5):765–773. doi: 10.1016/j.toxicon.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Wang X., Chen Y., Zuo X., Ding N., Zeng H., Zou X., Hab X. Microcystin (-LR) induced testicular cell apoptosis via up-regulating apoptosis-related genes in vivo. Food Chem. Toxicol. 2013;60:309–317. doi: 10.1016/j.fct.2013.07.039. [DOI] [PubMed] [Google Scholar]
- Wolny J., Egerton T.A., Handy S.M., Stutts W.L., Smith J.L., Whereat E.B., Bachvaroff T.R., Henrichs D.W., Campbell L., Deeds J.R. Characterization of Dinophysis spp. (Dinophyceae, Dinophysiales) from the mid-Atlantic region of the United States. J. Phycol. 2020;56:404–424. doi: 10.1111/jpy.12966. [DOI] [PubMed] [Google Scholar]
- Wolny J.L., Tomlinson M.C., Schollaert Uz S., Egerton T.A., McKay J.R., Meredith A., Reece K.S., Scott G.P., Stumpf R.P. Current and future remote sensing of harmful algal blooms in the Chesapeake Bay to support the shellfish industry. Front. Mar. Sci. 2020;7:337. doi: 10.3389/fmars.2020.00337. [DOI] [Google Scholar]
- Wood J.D., Franklin R.B., Garman G., McIninch S., Porter A.J., Bukaveckas P.A. Exposure to the cyanotoxin microcystin arising from interspecific differences in feeding habits among fish and shellfish in the James River Estuary, Virginia. Environ. Sci. Technol. 2014;48(9):5194–5202. doi: 10.1021/es403491k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.