Abstract
Further applications of electric vehicles (EVs) and energy storage stations are limited because of the thermal sensitivity, volatility, and poor durability of lithium-ion batteries (LIBs), especially given the urgent requirements for all-climate utilization and fast charging. This study comprehensively reviews the thermal characteristics and management of LIBs in an all-temperature area based on the performance, mechanism, and thermal management strategy levels. At the performance level, the external features of the batteries were analyzed and compared in cold and hot environments. At the mechanism level, the heat generation principles and thermal features of LIBs under different temperature conditions were summarized from the perspectives of thermal and electrothermal mechanisms. At the strategy level, to maintain the temperature/thermal consistency and prevent poor subzero temperature performance and local/global overheating, conventional and novel battery thermal management systems (BTMSs) are discussed from the perspective of temperature control, thermal consistency, and power cost. Moreover, future countermeasures to enhance the performance of all-climate areas at the material, cell, and system levels are discussed. This study provides insights and methodologies to guarantee the performance and safety of LIBs used in EVs and energy storage stations.
Keywords: lithium-ion battery, all-temperature area, thermal characteristics, thermal management, electric vehicle, energy storage
Graphical abstract

Public summary
-
•
Mechanism-temperature map reveals all-temperature area battery reaction evolution.
-
•
Battery performance and safety issues are clarified from material, cell, and system levels.
-
•
Strategy-temperature map proposes multilevel solutions for battery applications.
-
•
Future perspectives guide next generation high performance and safety battery design.
Introduction
The carbon neutrality proposal has promoted clean energy development in recent years.1,2 Electric vehicles (EVs) are investigated as the appropriate replacement for the conventional internal combustion engine-based vehicle to reduce greenhouse gas emissions and pollution, such as carbon dioxide (CO2).3 As a renewable power source, batteries make the EV efficient and environmentally friendly.4,5 Different types of battery technologies have been proposed, such as lithium-ion batteries (LIBs), lead acid batteries, nickel-based batteries, and sodium-based batteries.6 However, with the worldwide electrification trend, LIB has become predominant in electronics, EVs, and energy storage stations, with superior properties of higher energy density and long lifespan.7,8,9 These industries call for better batteries with lower cost, fast charging rates, and enhanced subzero temperature performance/thermal stability in next-generation applications.10,11,12
However, LIB performance is sensitive to certain factors in the operating environment, such as temperature,13,14 pressure,15 and vibration.16 The ambient temperature has a considerable influence on the overall performance of LIBs, such as capacity, available power, charging/discharging efficiency, safety, and lifespan. The temperature sensitivity of LIBs stems primarily from the temperature sensitivity of the physical and chemical properties of the material. LIBs exhibits higher efficiency and a longer lifetime under ideal operational temperatures.17 The thermal issue attracts attention to the precise battery thermal management system (BTMS) and current control to maintain the cell/module/pack temperature within the acceptable range (0–40°C). Considering the thermal safety and operational efficiency,18,19 the cell body temperature should be maintained within 15°C–35°C. The system temperature deviation should be controlled below 5°C to enhance the lifespan and operational performance.20,21,22
BTMSs are proposed to satisfy these operating requirements, especially in extreme working conditions such as fast charging, severe high-temperature environments, or subzero cold zones.23 Moreover, with higher requirements proposed for pure EVs, the energy density of LIB cells will be increased to extend the driving range. Fast charging technology will be widely employed to enhance long-term driving convenience.24,25 Therefore, battery thermal management is crucial for solving emerging problems in the all-temperature area EV industry, such as severe lithium plating, overheating, and even thermal runaway (TR).26,27 Moreover, more research is needed to enhance thermal management efficiency, thermal uniformity, and energy cost to cope with the future harsh requirements of BTMSs. Most existing reviews are organized for pure thermal management methods or structures.28,29,30 This study provides a comprehensive overview and perspectives of LIB characteristics and thermal management strategies under all temperature areas and future requirements. As depicted in Figure 1, the basic idea behind this review is to give out the thermal performance, mechanisms, and strategies for the LIBs under all-temperature areas (1, low-temperature area [<0°C]; 2, normal temperature area [0°C–60°C]; 3 high-temperature area [>60°C]), from the performance, mechanism, and thermal management strategies levels.
Figure 1.
Performance, mechanism and strategies of lithium-ion batteries under an all-temperature area
Low-temperature area
Performance level
Subzero temperatures result in a negative impact on LIBs: (1) lower charge/discharge ability,31 (2) less available energy and power capacity,32 and (3) shorter lifespan.23,33,34 The LIB output voltage decreases, causing lower energy density and power fading.35 Consequently, the available energy loss under subzero temperatures reduces the EVs’ driving mileages and available energy of the energy storage devices (ESDs). Moreover, the available power loss under subzero temperatures limits the quick acceleration and power frequency modulation of ESDs. Previous studies demonstrated that the 18650 LiPF6 battery cell could only provide 5% and 1.25% of the power capacity (20°C) and energy capacity (20°C), respectively, under −40°C.32 The driving range of the 2012 Nissan LEAF was reported to drop significantly from 138 miles under the ideal operational condition to 63 miles under −10°C. The usable charge/discharge capacity was calculated under low-temperature constant current charging/discharging tests.32,36 Even in recent studies, with the development of battery technology, lithium-ion phosphate (LFP)/graphite-based battery cells could only provide available 70% and 60% capacities (refer to the room temperatures) under −10°C and −20°C, respectively. Furthermore, LIB rapidly degrade at subzero temperatures. You et al.37 revealed that battery capacity fades nonlinearly after 500 cycles, under −5°C to 0.5°C cycling.
Under increasing charging rate requirements, lithium plating has more potential to occur, especially under a high state of charge (SOC) and subzero temperatures. Lithium plating facilitates gas generation, forming gas pockets on the electrodes, accelerating degradation, and even failure.38
Mechanism level
Low temperatures cause LIB deterioration (decreased ionic diffusivity, electrolyte conductivity, and anode lithium-ion diffusivity). In addition, the lithium-ion intercalation capacity of graphite decreases.39,40 Moreover, the available capacity decreases with increasing internal resistance.5,41,42,43 The charging/discharging capacity and efficiency are worse than those in typical applications. Furthermore, lithium evolution may occur inside the battery cell during high-current charging, harming the charging efficiency, lifespan, and safety.
Similarly, suppose that the LIB operates at subzero temperatures. The activity of the active material inside a battery is considerably limited. In this case, the internal resistance and polarization voltage increase.44 Furthermore, the charging/discharging power and capacity are inevitably reduced, causing irreversible degradation of the LIB capacity and buried safety hazards. During the charging process, under an electric field applied by the charging equipment, lithium ions are extracted from the cathode material into the electrolyte, move to the anode, and enter the anode material composed of graphite to form an LC compound. Lithium ions cannot enter the anode in a timely manner to form an LC compound while charging at high current rates and low temperatures. Lithium ions close to the negative electrode trap electrons, become metallic lithium, and aggregate to form lithium dendrites, which can grow. Piercing the diaphragm creates an internal short circuit.
Some existing research has proposed that the performance degradation of LIBs under subzero temperatures results from material property changes, which turns the stored energy into an unusable state.45
As shown in Figure 2, the intrinsic low-temperature degradation mechanism includes the following aspects.
-
(1)
Limited electrolyte conductivity and increased viscosity affect the Li-ion transport rate between the two electrodes. Moreover, it has been reported that inadequate electrode activity results in limited performance. Electrode activity implies the combined effect of marginalized Li-ion transport through the solid-electrolyte interphase (SEI) film, increased charge-transfer resistance, intrinsic loss of ionic conductivity, and slower lithium-ion diffusivity within the anode materials.46,47,48
-
(2)
The chemical composition and physical characteristics of the SEI film are also critical, such as the resistance to lithium intercalation, which depends on the salt-forming electrolyte, anode material quality, SEI formation mode, and temperature.37,49,50 The SEI is the surface film on LIB electrodes, consisting of organic and inorganic components that maintain the electrolyte kinetically stable under an anode potential of less than 0.8 V. The SEI film thickness varies by approximately 0.5–80 nm, depending on the degree of anode graphitization.51,52,53 However, the anodic film is less permissible for lithium-ion transportation at the electrolyte/electrode interphase, significantly increasing the battery impedance.6,54,55
-
(3)
The diffusion of solid-state lithium ions decreases significantly, especially during the charging process (lithiation),56 which contributes to the polarization. The graphite anode was more likely to be affected by lithium plating during charging.
-
(4)
For the cathode material, the charge-transfer resistance increases by more than 200% compared with the normal charge-transfer resistance during the discharging process (in the lithiated state).57 Besides, high grain boundary resistance and slow solid-state diffusion contribute to the sluggish lithium transport and poor electronic conductivity of the cathode material.
Figure 2.
Battery degradation mechanisms under a subzero temperature area
Strategy level
Owing to the broad geographical applicability of automobiles, EVs inevitably operate in subzero temperature environments. Although a massive amount of heat is generated during the charging and discharging processes, the heat generated is insufficient for maintaining the cell within a suitable operating temperature range. Considering the limited charging and discharging performance in cold climates and high-altitude drone applications, preheating approaches are required to enhance battery performance at subzero temperatures.58,59
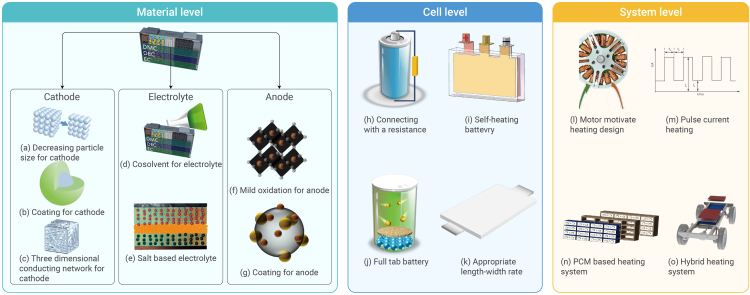
Low-temperature performance enhancement strategies can be summarized at the material, cell, and system levels.
Material level
Cathode modification
Decreasing particle size
As depicted in Figures 3A and 4I, decreasing the particle size provides a shorter diffusion path length for lithium ions and electrons and a broader electrolyte/electrode surface area for lithium-ion insertion and extraction, which results in enhanced electrochemical kinetics and low-temperature performance.60,61 However, nanoparticles lead to more side reactions considering their high surface area and reactivity.62 In addition, many binders are needed to glue these small particles together, inevitably decreasing the capacity. The low-temperature performance of other cathode materials such as LiCoO2, LiMn2O4, and V2O5 has also been enhanced by decreasing the particle size.63
Figure 3.
Strategies to enhance low-temperature performance of Li-ion batteries from different levels
Figure 4.
Schematic diagram of strategies to enhance low temperature performance
(A) Decreasing cathode particles. (B) Coated cathode for enhanced low temperature performance (adapted from Wu et al.64). (C) Graphene embedded LiFePO4 (GLFP).65 (D) Li[NixCoyMn1-x-y]O2-based cathode material.66 (E) Li salt-based additives. (F) Mild oxidized anode (adapted from Wu et al.67). (G) Mixed anode (adapted from Nobili et al.68). (H) Coated anode (adapted from Gao et al.69). (I) Multi-layer crystalline graphene-based anode.70
To avoid the nanoparticle electrode issues of reactive surfaces, limited particle contact, and retention of the merits of nanostructures, extensive studies have been conducted focusing on conductive coating materials and forming nanostructured electrodes.
Coating
As depicted in Figure 3B, similar to the carbon anode, electrolyte decomposition layers (EDL) still exist on the cathode surfaces.71 The electrochemical behavior on cathode surfaces depends on the EDL properties.72,73,74 Electrolyte component decomposition on the cathode is temperature sensitive75 and depends on the electrolyte material.73,76 Commercial cathode components are LiF, Li2CO3, ROCO2M, ROCO2Li, ROLi, MCO3, MF2 (M = transition metal), and polycarbonates,72 respectively. Owing to its pronounced stability, EDL formation on the LiFePO4 cathode differs from that on the LixMOy host material (M = Ni, Mn).77 Generally, the EDL thickness on LiFePO4 is less than 5 nm in a 1 M LiPF6 mixture of EC and DEC. An LiFePO4 cathode coated with a carbonaceous material prevents undesirable reactions and enhances EDL stability.78,79,80,81 Cathode coating significantly prevents contamination from HF and water.72 Compared with the pristine LiFePO4, a carbon-coated cathode capacity retention increases from 53.4% to 66.1% at −25°C.64
As Figures 3B and 4II show, an appropriately thin carbon coating is beneficial for balancing sufficient conductivity and Li+ penetration,82 especially for the superior electronic conductivity of carbon. Graphite is desirable for coating.83,84 Uniform carbon coating for LiFePO4 particles provides access for electrons from all directions to achieve low polarization.85 Organic carbon pyrolysis produces a more uniform carbon film,86 and increasing the porous carbon film surface area improves the conductivity.87 However, excessive carbon significantly decreases the tap density of LiFePO4 and generates some by-products, such as Fe2P.81,88 Therefore, the desirable carbon content in LiFePO4/C is less than 5% wt.89
3D conduction network
As demonstrated in Figures 3G and 4III, to further enhance the conductivity and electrochemical characteristics, a three-dimensional (3D) conduction network was proposed.90 Chang et al.91 proposed a cathode material without any supplementary conduction agent. These results indicate that the prepared LiFePO4 nanographite platelet) heterostructure enhances electron transport. Wu et al.64 proposed 3D carbon-decorated LiFePO4 nanocomposites with ultrahigh rate capability and enhanced subzero-temperature performance. Kim et al.65 proposed a catalyst-assisted self-assembly approach to embed graphene onto LiFePO4 as the cathode material, which exhibited superior cycling performance and rate capability.
Novel cathode material
To enhance the cycling performance under low temperatures.92 As in Figure 4IV, Yoon et al.66 investigated the subzero-temperature performance of Li[NixCoyMn1-x-y]O2 (NCM) cathodes with a 1.2 M EC-EMC (3:7 by volume) electrolyte. The results indicated that the low-temperature capacity of the NCM cathode was dependent on its chemical composition, which increased with increasing Ni content. Since high Ni content causes a high electrical conductivity, Li[Ni0.85Co0.075Mn0.075]O2 provides 127 mAh g−1 under a −20°C environment. Smart et al.93 investigated the low-temperature performance of Li1+x(Co1/3Ni1/3Mn1/3)1-xO2 cathode with a 1.2 M EC-EMC (20:80) electrolyte, which exhibited a 55% capacity retention at −40°C under high current rate discharging. The results indicated that a low ethylene carbonate content helped achieve a high discharge rate owing to its low viscosity and high ionic conductivity. Rui et al.94 proved that an Li3V2(PO4)3/C (LVP/C)-based battery material provided a high discharging capacity of 108.1 mAh g−1 under −20°C environment. Its low-temperature performance was enhanced by the low activation energy of LVP (6.57 kJ mol−1), causing more access to Li+ extraction/intercalation in LVP.
Electrolyte modification
More researchers have recently focused on enhancing the electrolyte stability with effective SEI film formation. These studies can be summarized as follows:
-
(1)
utilization of cosolvents with lower viscosity and lower freezing temperature, such as glymes, esters, and lactones.95,96,97,98
-
(2)
changing the lithium salt LiPF6 with new mixtures improved the charge transfer resistance and other characteristics of the SEI film.99,100,101,102,103,104
-
(3)
formulation of new electrolyte additives to lower the freezing temperature.105,106,107,108,109
-
(4)
novel electrolyte design to maintain the ionic conductivity within a desirable range.110
-
(5)
LTO||LTO symmetric battery design to prevent high interface resistance owing to lithium-ion desolvation.111,112
The electrolyte determines the ionic mobility and participates in the reaction of SEI film formation on the anode.113 The SEI film permits lithium-ion conduction, protects the electrodes, and prevents further electrolyte reduction, affecting the low-temperature performance and cyclic life.47 Low-temperature performance can be achieved by improving the electrolyte conductivity and SEI stability at the electrolyte/electrode interface. The experimental results indicated that the electrolyte system can be optimized by adding suitable cosolvents and improving the Li salts.106,114,115
Adding cosolvent
The electrolyte solution conductivity drops rapidly in a low-temperature environment owing to the high freezing temperatures of conventional solvents (EC, DMC).115 As depicted in Figure 3D, to reach a low freezing temperature but high conductivity under low temperatures, adding suitable cosolvents into the electrolyte has been proposed as a reliable method, which is selected considering some critical factors: (1) dielectric constant, (2) viscosity, (3) coordination behavior, (4) liquid range, and (5) pronounced chemical compatibility.
Salt selection
As Figures 3E and 4V demonstrate, Li salt selection also affects the lithium-ion conductivity and SEI stability, which is significant for enhancing the low-temperature performance.116 LiPF6 is a commonly utilized Li salt. However, it spontaneously decomposes into LiF and PF5, thereby inducing structural changes and capacity fading. The selection of LiPF6 with lithium tetrafluoroborate (LiBF4) or lithium bis(oxalato)borate (LiBOB) enhances low-temperature performance because LiBF4 or LiBOB-based electrolytes demonstrate low charge-transfer resistance.117,118,119
Anode modification
Limited Li diffusion in the electrodes and higher charge-transfer resistance at high charge/discharge current rates result in higher polarization and limited performance.63
The kinetic limitation of lithium-ion transfer at the electrode/electrolyte interface induces primary performance limitations at low temperatures. The electrochemical (faradic) reaction can be optimized by reducing Rct and maintaining SEI stability.
Graphite is the most commonly used anode material for LIBs. However, its principal limitations are high Rct values at the electrolyte-electrode interface, SEI instability, and reduced solid-state lithium diffusivity. Effective methods for improving the anode include the discussion of the following.32,56,120
Mild oxidation
As shown in Figures 3F and 4VI, mild oxidation of the graphite anode decreases the number of unsaturated carbon atoms at the edge planes, resulting in a smaller mean particle size.121 Furthermore, this approach causes the formation of nanovoids, nanochannels, and chemically bonded SEI.122 Consequently, the electrochemical impedance decreases, hindering the co-intercalation of solvated lithium ions and electrolyte decomposition. Mild oxidation can be achieved through thermal treatment or wet chemical oxidation.67,123,124
Mixing
Mildly oxidized graphite mixed with metal nanoparticles demonstrates enhanced low-temperature performance.125 As Figure 4VII depicts, the oxidized graphite anodes mixed with 1% Cu and Sn in a 1 M LiPF6 EC-DEC-DMC (1:1:1) electrolyte were proved to have the capacities of 130 mAh g−1 122 and 94 mAh g−1 68 at −30°C, respectively. The superior low-temperature performance resulted from the enhanced lithium-ion desolvation, increase in SEI conductivity, and internal conductivity of the metal-dispersed powder bulk electrode.68
Coating
Anode coating has been proven an alternative method to enhance electrochemical performance.126 As shown in Figures 3G and 4VIII, the oxidized graphite anodes coated with 50 Å Cu and Sn layers in a 1 M LiPF6 EC-DEC-DMC (1:1:1) electrolyte was tested to have the capacity of 103 mAh g−1 123 and 152 mAh g−1 68 at −30°C, respectively. Gao et al.69 prepared a Cu-coated graphitic carbon anode via plating. The stable SEI suppresses the electrolyte decomposition (1 M LiClO4 PC-DMC, 1:1 by volume) and achieves promising LIB performance at −60°C.69 Therefore, the metal layers on the anode cause a remarkable decrease in Rct, a stable SEI, and an increased lithium conductivity.68,69,127
Ag-Fe2O3/carbon nanofibers (CNFs) anode materials demonstrated pronounced electrochemical performances at −5°C due to their synergistic effects on the CNF matrix and the conducting Ag.128 Li et al.129 proposed Fe/Fe3C-CNF materials with a high capacity of 250 mAh g−1 after 55 cycles at −5°C in a 1 M LiPF6 EC-EMC-DMC (1:1:1 in volume) electrolyte.
Novel anode material
Raccichini et al.70 synthesized a multi-layer graphene anode by combining microwave irradiation and ultrasonication in an ionic liquid. The low-temperature characteristics of multi-layer crystalline graphene (GRAL) and the commonly utilized graphite SLP30 in a 1 M LiClO4 PC-DMC (1:1 by volume) electrolyte were compared. The results indicate that the GRAL capacity was higher than that of SLP30 at low temperatures. As shown in Figure 4IX, the low-temperature performance is enhanced owing to its high active surface area, which results in a higher anodic electrochemical reaction efficiency and improved lithium-ion diffusion kinetics.130
Li4Ti5O12 is another promising low-temperature anode material with a high cycling stability. However, its sluggish lithium-ion and electron conductivities limit its electrochemical performance. Reducing the particle size of Li4Ti5O12 and coating are typical methods to enhance electrochemical performance.131,132 Yuan et al.133 prepared nanosized rutile TiO2 via a solegel approach, which provides a capacity of 77 mAh g−1 at −40°C with a C/5 rate in a 1 M LiClO4 PC-DMC (1:1 by volume) electrolyte.
Cell level
Connecting with a resistance
As depicted in Figure 3H, the battery cell can be connected to an external resistor, resulting in a closed loop. The current increased along the path inside the battery material. In addition, external resistance heats the battery surface. However, it is challenging to utilize and precisely control this heating approach in real applications, especially for massive battery cells in battery packs or energy storage stations, owing to local overheating and low heating efficiency.
Self-heating battery
As shown in Figure 3I, Wang et al.39 proposed a self-heating battery cell by inserting an insulating polymer-coated metal foil. The switch between the activated and negative terminals can be closed to preheat the LIB to a low-temperature environment. Experimental results suggested that the battery temperature could be heated from −30°C to 0°C within 30 s through Ohmic heat generation. The switch was turned off until it reached 0°C, with 5.5% of the battery capacity consumed during the preheating process. Zhang et al.40 concluded that nickel foil can accelerate spontaneous heat generation. There was a linear relationship between the inner temperature and the nickel foil resistance, which could be utilized as a temperature sensor to detect the internal temperature.
To enhance battery cell consistency, temperature gradients should be avoided.134 However, a single nickel foil-based self-heating cell cannot ensure uniformity. Zhang et al.40 proposed a self-heating cell with two or three pieces of nickel foil to address the issue. Moreover, the heating time was shortened, and the energy cost was reduced by 25%–30%. Compared with the slow heating rate issue of some external heating approaches, the self-heating rate reached 1°C–2°C/s. However, internal resistance heating efficiency may be limited by the electrothermal conversion efficiency.58 Furthermore, considering the internal short-circuit-based mechanism and the complex operational conditions of EVs, the safety issue of changing the Li-ion battery cell’s inner structure should be paid more attention to.135,136
Full tab battery
As demonstrated in Figure 3J, a full-tap battery has been proposed to achieve a trade-off between energy density and heat dissipation in recent years.137 Conventional cylindrical batteries are commonly wound based on anode copper foil, cathode aluminum foil, and separator superposition. A pole lug was welded to both ends of the copper and aluminum foils for the outside electrode design. The winding length of a conventional 18650 cylindrical battery was 800 mm. To ensure low resistance, a long copper foil has high thickness and consistency requirements.
Taking the Tesla 4680 battery as an example, the full-tab battery converts the entire current collector into a tab, and the conductive path no longer depends on the tab. The current changes from the transverse transmission of the TS collector to the longitudinal transmission of the current collector. The entire conductive length decreased to 80 mm (battery height). The resistance is reduced from about 20 to 2 mΩ, calculated by the copper resistance formula. According to the ohmic heat theory, the internal resistance power cost can be reduced from 2 to 0.2 W.
Previous research has indicated that more tab numbers result in a less apparent internal temperature rise, and the current density of the battery tab increases, increasing the heat generation rate at the tab and causing a significant local temperature rise. The full-tab design effectively reduces the local current density and solves the thermal uniformity issue, which improves heat dissipation and preheating efficiency. Therefore, the preheating efficiency, lifespan, and overall performance were enhanced when the batteries were heated using current.
Appropriate length-width rate for battery
As Figure 3K shows, large-scale battery cell schemes have been commonly used in recent years to achieve higher energy densities in battery systems. However, from the perspective of the current path, it is difficult to maintain thermal uniformity using a BTMS. In an inappropriate battery design, the current concentration around the tabs is different, which induces different heat accumulation issues, significantly affects the thermal uniformity and preheating rate, and can even cause overheating. In addition, a large cross-sectional area results in insufficient preheating efficiency. Therefore, an appropriate length-width ratio is important for battery design, especially in cold areas.
System level
As shown in Table S1, the preheating strategies utilized at the system level were evaluated and compared from various perspectives (heating rate, thermal consistency, safety, cost, and applicability).
Air heating
The air heating system flows hot air through the battery system.22,138 The battery cells can be warmed by heat exchange between the airflow and battery surfaces.18 Figure 5H shows that the air-cooling structure is designed based on air convection combined with heaters and control elements. Song et al.139 found that the cell capacity was enhanced by 3.1% under a −3°C environment with a 5 kW heater-based air heating structure. Zhang et al.140 utilized the EV’s air conditioning system combined with pentadecane (C15H31) as the heat transfer medium under an ambient temperature lower than 0°C. The results demonstrated that the preheating efficiency, based on the phase change of pentadecane, was higher than that of the refrigerant. This strategy can be combined with an air-cooling system by combining the BTMS with heater control.
Figure 5.
Scheme of thermal management strategies
(A) Air cooling, (B) liquid cooling, (C) phase change material cooling, (D) heat pipe cooling, (E) PCM heating, (F) self-heating battery, (G) liquid heating, and (H) air heating.
Liquid heating
Liquid heating is an approach for heating the cooling liquid to a specific temperature through the heating components of the vehicle, and the bump can be utilized for cycling the heated coolant in the battery module/pack.141 To achieve all climate applications with low volume and weight costs, the liquid heating loop is commonly incorporated in the liquid cooling system. As shown in Figure 5F, a direct liquid heating system is proposed based on nonconductive transformer oil, a heating film, and a heat insulation layer. Nelson et al.142 concluded that a silicone-oil-based liquid preheating system is effective for the cold start of LIBs. Wang et al.143 inserted an L-shaped heating plate into the cell gap, and the fluid temperature of the heating plate evaporator was set to 40°C. Simulation results indicated that the preheating time of the battery module from −20°C to 0°C was controlled within 500 s.
PCM heating
Phase-change materials (PCMs) have received extensive attention owing to their latent heat without an extra power supply, which is a typical active heating/cooling approach.144 As demonstrated in Figure 5E, latent heat can also be utilized for preheating under subzero temperatures. Zhong et al.145 proposed a PCM-resistant wire-based BTMS with a fin for −20°C application. The central part’s temperature of the battery module can be increased by 40°C within 300 s. Moreover, the fin structure can be utilized to prevent the thermal saturation prevention. Ling et al.146 compared the heating effects of various composite PCMs. However, the proposed PCM did not have sufficient thermal conductivity, which resulted in a severe temperature deviation, uneven voltage distribution, and capacity loss. Therefore, to fulfill the requirements of fast charging and all-climate applications in next-generation BTMSs, PCM cannot achieve the desired thermal conductivity, volume, weight, latent heat density, and price.
PTC coupled heating
Positive temperature coefficient (PTC) resistance is a typical external heater based on the principle of Joule heat generation.147 The metal heating wire is generally encapsulated in an insulating layer.148 Li and Huang149 proposed an aluminum plate scheme twinned with a PTC resistance wire for battery preheating. Fan et al.150 conducted a simulation study to analyze the influential factors of a heating-plate-based BTMS. The results indicated that the heating efficiency increased with the coolant flow rate and coolant temperature. However, the maximum temperature exceeded 40°C when the inlet temperature was above 50°C. Chen et al.33 conducted a thermal analysis to compare the preheating efficiency of two PTC-liquid heating systems under a −40°C environment. It was demonstrated that the preheating speed, thermal uniformity, and power consumption could be enhanced through appropriate structural design and preheating interval settings.
Alternating current heating
The feasibility of alternating current (AC) preheating in battery systems has been verified by setting alternative pulse currents through batteries.151,152 Joule heat can be generated for preheating. Previous experimental results indicated that a high current amplitude was beneficial for temperature uniformity, and that the LIB performance did not degrade significantly.153
Zhang et al.154 developed a sinusoidal AC heating structure with thermal insulation design. Repeated experimental results indicated that the preheating speed was positively correlated with the amplitude and heat insulation and was proven to have a negative correlation with frequency. Moreover, no significant capacity loss was observed after the tests. Furthermore, a lumped energy conservation model was proposed to predict the temperature rise for real-time preheating control.134 Zhao et al.155 compared the preheating efficiency of pulse heating and constant-current/voltage charging and found that the charging time could be shortened by 36 min (23.4%) and the charging capacity could be increased by 7.1% with pulse heating under the same operational conditions.
Ruan et al.156 proposed an electrothermal coupling model-based internal heating system. A constant polarization voltage was controlled during heating to achieve a trade-off between the preheating interval and minor capacity loss. The heating efficiency of variable-frequency preheating was found to be consistent with that of constant-frequency heating. Therefore, constant-frequency heating can be selected for real applications, considering the feasibility of variable-frequency preheating.
A DC-DC converter can be used to amplify the output voltage to reach the desired charging voltage. Although external heating elements are not required for mutual pulse heating, their unique circuit and control elements increase the cost of the preheating system.157
Heating preservation
In summer, external high temperatures increase the LIB temperature. However, in severely low temperatures or winter, the internal temperature of the LIBs drops rapidly after parking outside for a long time, which affects the recharging, starting, and speed performance of the EV. Therefore, it is necessary to eliminate the impact of external high temperatures in summer or low temperatures in winter on the battery system through a thermal insulation design.158 Insulation materials are employed to insulate and reduce the impact of external environmental factors.
A heat preservation system is commonly integrated with a BTMS to enhance cooling/heating efficiency, and energy consumption can also be controlled.
Normal temperature area
Performance level
Ambient temperature directly affects the activity and conductivity of the electrode material, the insertion and deintercalation of lithium ions on the electrode, and the lithium-ion permeability of the separator. Furthermore, ambient and internal temperatures affect the electrochemical reactions inside the battery cell. Therefore, LIBs have a normal operating temperature range without severe heat generation.
As the temperature increases within this range, the activity of the internal active materials is enhanced, and the charging/discharging voltage, efficiency, and capacity of the battery increase accordingly, resulting in a corresponding reduction in the internal resistance. However, as Figure 6 depicts, the internal side reactions accelerated when the battery temperature exceeded a specific range. These side reactions consume lithium ions, solvents, and electrolytes, degrading the battery performance. Previous studies demonstrated that LIB cycle life is significantly reduced when the battery works above 60°C. This phenomenon is more evident during high-rate charging and discharging, and can lead to accidents.
Figure 6.
Schematic diagram of the temperature-current coupled mechanism and BTMS design for lithium-ion batteries
The calendar lifespan of EVs and hybrid electric vehicles was defined as 10 or 15 years.159 However, the LIB temperature can be easily elevated to a relatively higher value, such as 40°C, which accelerates the ageing process, resulting in the loss of the retention capacity.
LIB performance is also influenced by temperature rise and deviation resulting from heat generation, ambient temperature, and different degrees of electrochemical reactions that occur at all times.
Existing studies have proven that the heat-generating rate of LIBs has a quadratic relationship with the charging/discharging current. At high ambient temperatures or during high-rate charging/discharging, the corresponding heat dissipation methods should be utilized to control the increasing temperature. Otherwise the cell will be overheated, causing performance degradation, a shortened lifespan, or even a dangerous state of TR.
Mechanism level
Almost all main/side reactions are related to temperature. The side reaction rate increases at high temperatures. Moreover, self-heating and thermal accidents can occur when the temperature exceeds a certain threshold. At subzero temperatures, the polarization increases owing to the increase in internal resistance, causing other side reactions. Charging at low temperatures triggers lithium deposition, accelerates degradation, and causes safety issues. Furthermore, material embrittlement under subzero temperatures limits battery cycle life. Therefore, maintaining battery temperature within the above-mentioned temperature range (15°C–35°C) is significant for the overall performance and cycle life.
In the normal temperature range, batteries exhibit desirable operational efficiency. The lithium ions were smoothly inserted and extracted from the anode. Only the degradation (loss of active material/lithium inventory/conductivity) and heat generation mechanisms during the cycling process affect the battery thermal performance, rather than the other side reactions.160 The heat generation mechanism under the normal temperature range is discussed in the supplemental information.
Strategy level
Under normal temperature conditions, LIBs do not require heat or fire suppression. However, heat generation during conventional charging/discharging cannot be neglected because it may exceed the threshold of some side reactions. The cooling component of the BTMS is used to prevent overheating. Because of the characteristics of the battery system, thermal consistency should be maintained to guarantee the desired performance and cycle life of the battery system.161
According to the heat transfer media, the commonly used cooling methods in the EV market can be divided into three main categories (1) air cooling,162 (2) liquid cooling,163 and (3) PCM cooling.164 In addition to being distinguished based on the cooling medium, cooling is often classified as active or passive based on whether additional energy is consumed in the cooling process. Cooling efficiency is mainly characterized by the convective heat transfer coefficient. Generally, the convective heat transfer coefficient required by the BTMS is determined by the operational environment of the EV, LIB characteristics, and other factors, such as weight, volume, and cost.
As shown in Figure 6, the selection of cooling approaches included the following steps: (1) confirmation of cooling system objectives, (2) calculation of heat generation power, (3) establishment of a battery model, (4) thermal fluid dynamic simulation analysis, (5) convection coefficient analysis, and (6) cooling method selection.
Table S2 summarizes the critical technical standards for the typical cooling strategies for battery systems. A detailed discussion of the specific cooling approaches is provided in the supplemental information.
High-temperature area
Performance level
Fire behavior
As demonstrated in Figure 1, when the battery temperature exceeds 60°C, high temperature triggers SEI film decomposition and self-heating. Massive heat is released under high-temperature areas or abuse operations owing to chemical reactions and ISC, and the battery temperature rises rapidly.165 Simultaneously, the safety valve opens when the internal pressure reaches a threshold, or the battery casing swells and breaks. Furthermore, aerosols and flammable gases are ejected at high speeds and temperatures.166 These flammable materials tend to ignite in air when the air-fuel mixture ratio is within the flammability range, resulting in combustion.167
Ping et al.168 carried out a full-scale burning test for a 50 Ah LFP/graphite battery pack. The combustion behavior of a 100% SOC pack can be divided into several stages: (1) battery expansion, (2) jet flame, (3) stable combustion, (4) second flame jet, (5) stable combustion, (6) third flame jet, (7) stable combustion, and (8) abatement and extinguishment.
Thermal runaway propagation
An increasing number of battery cells are tightly connected in series or parallel to meet the demand for capacity and power in EV battery packs and energy storage stations.169 As in the Tesla Model S, the battery pack is equipped with seven thousand 18650-format LIBs, and the total energy reaches 85 kWh. However, the total heat released from the battery module is not the sum of the combustion energies of each cell. The proportion is proven to be 1.26.170 Therefore, TR and fire may propagate throughout the module/pack. A TR fire promotes the combustion of other cells to a higher fire intensity, resulting in catastrophic disaster.171
Generally, the thermal runaway propagation (TRP) from the center cell through the entire pack is faster than TR initiation from the corner.172 In addition, the electrical connection mode influences the TRP characteristics within a battery module.173 Ten 18650-format cylindrical LIBs were connected as an equilateral triangle module, and the center cell was penetrated to trigger TRP. The results indicated that the parallel configuration resulted in an external short circuit with other cells. The surrounding cells were triggered in the parallel-connection mode rather than in the series-connection mode.
The battery format (cylindrical/prismatic/pouch) also affects the TR dynamics. Owing to the limited physical contact between neighboring cylindrical battery cells, the cylindrical battery module was less prone to TRP than the pouch cell module.173,174,175 Heat transfer between neighboring pouch cells was identified as the primary driving force for TRP.176
There are three heat transfer paths within the battery module during the TRP process: (1) heat conduction through the battery shells, (2) heat conduction through the pole connectors, and (3) convection and radiation from the flame to the battery cells. Feng et al.177 concluded that heat was mainly transferred through the shell, which mainly depended on the TRP behavior, and was approximately ten times higher than the heat transfer through the pole connectors. The heat that triggers the neighboring cell for TRP is less than 12% of the total heat released during the TR process. The thermal resistance between the neighboring cells and the triggering methods determine the TRP characteristics within a battery module.177,178,179 However, flame impingement determines the fire propagation within a battery module. The flame and convection/radiation from the flame significantly affect the temperature of the LIB in contact.180
Mechanism level
The self-heat generation of LIBs occurs in high-temperature environments or under electrical/mechanical abuse. When the accumulated heat from the exothermic reactions cannot be dissipated into the environment, the battery temperature increases continually. Furthermore, the impervious SEI film breaks down and dissolves, exposing the anode surface to electrolytic corrosion accompanied by irreversible lithium loss. Moreover, SEI film dissolution disturbs the physical balance state of the SEI metastable organic components, which form a more stable inorganic material such as lithium carbonate.181 The ionic conductivity and permeability of the SEI film gradually decrease as the inorganic carbonate ratio increases, causing a significant reduction in the LIB energy capacity and output power.182,183
As shown schematically in Figure 7, the meltdown process of the cell consisted of three steps. The first step is the electrochemical breakdown of the anode SEI film, which starts at around 70°C.184,185,186 Besides, the intercalated lithium reacts with (CH2OCO2Li)2. When the heat is insufficiently dissipated from the LIB during this step, the governing electrochemical reaction becomes self-sustained.
Figure 7.
Thermal runaway scheme of lithium-ion under extreme high temperatures
(Triggering mode; Mechanism; Fire resources; Thermal runaway propagation paths).
Without protection from the SEI film, the following reaction is between the electrolyte and carbon anode.187 Furthermore, if the self-heat generation rate exceeds 0.2 °C/min, the defined TR phenomenon occurs.188,189 The melting temperatures of the PE, PP, and ceramic-coated separators are around 135°C, 166°C, and 200°C, respectively.190 The second step of the separator meltdown process commonly starts at around 130°C, with the symbol of exothermic activity on the cathode. In this step, the self-heat generation rate of the cell increased to approximately 5°C/min. The cathode and anode layers are in direct contact with each other, resulting in a large-scale internal short circuit,191 which is a critical inducement of TR. The energy released during the TR process is approximately equal to the energy stored in the battery cell.192 However, LIB TR can also be triggered without ISC, and may be induced by chemical crosstalk.193
The final step began at temperatures greater than 200°C. Oxygen is rapidly released during the cathode decomposition,193 and the self-heat generating rate of the cell increases to higher than 10°C/min with the decomposition of the cathode, the oxidization/decomposition of the electrolyte. The temperature increases to 100°C/min in some severe meltdown processes,184,194,195,196 releasing carbon dioxide, fluoride gas, and hydrocarbons.197,198,199,200
The driving temperature for the exothermic TR reaction depends on the chemical components and the SOC.201,202 Generally speaking, with a higher voltage or SOC value, the onset temperature for the LIBs, TR is lower. However, even for specific battery types with the same chemical components, the onset temperature varies with load history and abuse events.184,203
Strategy level
As summarized in the previous analysis, heat and flammable gas generation directly determine the TR hazard. Therefore, battery system safety can be enhanced through the following approaches: (1) preventing or alleviating heat and gas generation and (2) managing heat and gas generation. Safer battery cells can be fabricated by modifying the materials, inner structures, or safety devices. The incorporated safety equipment can be classified as a safety vent, current interrupt device, shutdown separator, detection component, BMS, BTMS, or fire suppression device.204,205,206 Furthermore, from a thermal management perspective, battery system safety can be enhanced by equipping an efficient BTMS and fire suppression devices.
Material level
Based on a previous study on the TR mechanism, the quantified TR mitigation target was to increase the self-heat generation temperature (T1) and TR onset temperature (T2) to inhibit TR triggering and to decrease the maximum temperature (T3), which decreases the heat released during the TR process.205
Cathode modification
Oxygen block and capture
In addition to the flammable gases generated during the TR process, oxygen released from the cathode has been proven to be a significant TR factor, resulting from the cathode-anode chemical crosstalk.193 Therefore, as demonstrated in Figure 8B, a cathode coating is proposed as an intuitive method to block the release pathway for O2.207,208 However, a specific solution is needed for the cathode coating to immediately capture the active O2 release with reliable electrical performance and cycle life. Feng et al.205 proposed replacing the polycrystalline secondary structure with a single-crystal morphology to reduce the O2 release surface area.
Figure 8.
Thermal runaway mitigation strategies for Li-ion batteries from different levels205
Separator modification
As depicted in Figures 8C and 9I, to postpone the occurrence of large-scale ISC, a high-temperature resistance separator should be designed against shrinkage in a hot environment,209 such as by selecting a thermal-resistant base material and coating with ceramic or other materials that do not break up under high temperatures. Rahman et al.210 prepared a separator coated with boron nitride nanotubes. Results indicated that the proposed separator enhances the thermal stability up to 150°C without blocking the lithium-ion diffusion.
Figure 9.
Schematic diagram of safe battery material design
(I) Cathode-electrolyte interphase [CEI] design.211 (II) Thermal-resistant separator.210 (III) Al2O3-coated anode,212 and (IV) flame-retardant electrolyte.213
Anode modification
The coated layer on the anode surface also remained intact during cycling, preventing lithium metal deposition on the anode-electrolyte interphase. Therefore, there is no massive irreversible lithium plating on the anode to induce large-scale ISC. As depicted in Figure 9II, Friesen et al.212 prepared an anode material coated with Al2O3, which suppressed Li dendrite growth and demonstrated no deterioration in the thermal and mechanical safety behavior, despite thick Li deposition on the anode surface.
Electrolyte modification
Electrolyte selection
As Figure 8D shows, a more stable SEI film formation is required to postpone the self-heat generation state, which can be achieved by adding electrolyte additives.214 Ma et al.215 investigated the impact of electrolyte additives (vinylene carbonate [VC], fluoroethylene carbonate, and vinyl ethylene carbonate) on electrode/electrolyte reactions. Results indicated that adding VC decreases the lithiated graphite-electrolyte reactivity below about 200°C. Liu et al.216 proved that electrolyte additives enhance SEI uniformity and suppress dendrite growth.
As depicted in Figures 8E and 9III, flame-retardant electrolyte additives have also been considered as alternative mitigation approaches in recent years. Hou et al.217 proved that flame-retardant fluorinated electrolytes reduce heat release during the TR process, which guides safer LIB design through appropriate electrolyte design.
Cathode-electrolyte interphase design
To pursue higher energy density, the nickel content in the cathode material has been increased in recent years.218 As Figures 8A and 9IV demonstrate, in situ robust cathode-electrolyte interphase formation and control are considered the promising cathode enhancement strategy to address the aggressive thermal reaction by forming a protective layer on the cathode, which has been investigated through appropriate electrolyte selection (conventional carbonate-based electrolyte, fluorinated electrolyte, concentrated electrolytes, or solid-state electrolyte) and adding additives.211 Besides, the operational environment, such as temperature and pressure, should be precisely controlled during pristine and post-cycling to achieve a stable and uniform interphase formation.
Cell level
Blade battery cell
As shown in Figure 8H, a blade battery cell is proposed with a trend of higher system energy density and larger scale. Zhang et al.219 observed the TR front phenomenon in a long-shaped battery, which propagated at a moving rate. In real applications, an ISC failure after lithium planting or a defect inside it generally triggers TR accidents. A severe TRP temperature increase within a cell or battery system can be detected by a BMS and stopped by fire-distinguishing devices. In addition, thin, long battery cells were designed with fewer electrode layers. The energy released from the ISC is less than that from conventional batteries, especially when the LIB is penetrated/squeezed/triggered by a defeat ISC/partially overheated. The heat that propagates to other areas or neighboring cells is less and can even be cooled with proper heat dissipation or other mitigation countermeasures. Therefore, a long battery design is beneficial for TRP mitigation.
Self-poisoned battery cell
As Figure 8I shows, to reduce the heat released during the TR process, neutralizing the oxidant and reductant is considered a promising strategy for mild reactions,220 which can be achieved using the following methods.
-
(1)
application of thermoresponsive materials to block the cathode-anode contact.221,222
-
(2)
structural design of current collector to isolate damaged area.223
Liu et al.224 prepared an electrospun separator with a core-shell structure containing a flame-retardant liquid within the microfibers. Lai et al.225 utilized a poison agent to mitigate the TR process, and results indicated that the energy release was significantly reduced and the maximum temperature was decreased by more than 300°C. However, it is necessary to guarantee the normal operation of LIBs. A reliable packaging material is urgently needed for the poisoning material to prevent the long-term corrosive effect from the electrolyte, the suppression behavior due to the LIB “breathing effect.” In addition, avoiding internal structural changes in ISC is critical for safety during long-term cycling.
Safety valve design and gas extraction
As depicted in Figure 8J, proper design of the battery vent valve is significant for controlling the rupture moment at an appropriate temperature level, which might help diminish the venting damage and TRP.226
System level
Detection devices
To avoid abnormal temperature environments or electrical loads, a BMS can be incorporated with a battery system for battery state monitoring.227 With timely detection and reporting of abnormal battery states, it is helpful to avoid overheating,228 overcharging, or overdischarging.229 In addition, BMSs utilize effective methods (such as timely discharge) to alleviate abuse conditions, even preventing TRP disasters.230 However, it is challenging for BMSs to detect ISC, which results from various factors, including manufacturing.179
Apart from the temperature, current, and voltage detection of LIBs,230 gas and smoke detection231 during the TRP process is also significant, especially within a battery system with a progressively larger size and higher energy density.232
TR propagation mitigation design for oxygen
Oxygen extraction
As shown in Figure 8K, oxygen extraction using a mini-bump may be helpful for TR fire mitigation, even if oxygen is generated from the cathode during the TR process. Continuous gas extraction cannot provide sufficient oxygen for the flame reaction, and TR propagation may stop.
Inertia gas environment
As Figure 8L demonstrates, wrapping the battery system in an inert gas environment helps suppress the TR fire propagation.233,234 Diluting the flammable gases and oxygen to a lean level might eliminate the flame.
Fire-proof cover
As shown in Figure 8M, attaching a thick fireproof cover on top of the battery system prevents fire from spreading. In addition, when a fireproof cover is designed with reliable sealing ability, oxygen can be decreased to a certain level with continuous consumption during the flame reaction. Furthermore, to protect passengers, a fireproof cover is essential to insulate the battery system from the cabins.
TR propagation mitigation design for fuel
Pack valve design and fast spread
As depicted in Figure 8N, when the pressure resistivity of the pack vent valve is lower than that of the pack wrap, expected venting occurs, and gases emerge from the vent valve. The gases can vent spontaneously once the pressure requirements are satisfied.
Flammable gas exhausting
As shown in Figure 8O, the gases inside the battery system can be extracted from the pack by using a bump with an appropriate gas channel design. Therefore, without sufficient flammable gases and oxygen, the TR fire cannot spread to the LIB even if the first cell is fired during the TR process.
TR propagation mitigation design for heat
Cooing
As Figure 8P shows, the heat conduction and dissipation efficiency of the commonly utilized liquid cooling system can be reinforced to prevent TR propagation.206 Moreover, PCM barriers can be combined to absorb the massive heat during the TR process.27 To cope with the dilemma of high energy density and thermal safety; higher heat capacity and composite PCM barriers are considered promising solutions for TR propagation prevention in high-nickel ternary material battery systems.
Heat insulation
The heat transfer pathway through the surfaces between adjacent cells is the primary heat source, as shown in Figure 8Q. Setting thermal insulation barriers between neighboring cells and battery modules is a cost-effective method for mitigating TRP.
The proposed heat-insulation materials include ceramic plates, rock wool panels, glass fibers, perlite, calcium silicates, silica aerogel,192 Al extrusion,192 and graphite composite sheets. However, thermal barriers utilized for TRP prevention should be thermal resistant (>600°C) and have low thermal conductivity (<0.1 W m−1 K−1).235 Besides, a reliable thermal barrier should maintain the heat insulation area under high-temperature burning and fire impact.236
Fire extinguishing
As shown in Figure 8R, the typical approaches to fire suppression can be divided into (1) isolation, (2) smothering, (3) cooling, and (4) chemical suppression. Unlike typical fires, some battery fires result from direct reactions between the components. These reactions do not require external oxygen.
A detailed discussion of the fire extinguishing studies is provided in the supplemental information. Table S3 compares the characteristics and critical standards of commonly utilized extinguishing agents for battery fire disasters that guide fire suppression designs for battery systems. Table S4 summarizes the TR/TRP mitigation strategies for high-temperature hazards at the material, cell, and system levels.
Conclusion
As the primary obstacle for large-scale LIB applications, thermal issues remain challenging to address, especially for all-climate applications and severe operations, such as fast charging. This study comprehensively reviews the thermo-electric characteristics and mechanisms in all climate areas. To enhance performance and ensure safety in all-temperature areas, advanced thermal management and fire suppression strategies for LIBs have been introduced from the perspectives of subzero, normal, and high temperatures. In addition, as demonstrated in Figure 10, to further address the all-temperature area thermal issues, promising future strategies are provided from the perspectives of the material, cell, and system levels, which include novel material preparation, battery cell design, battery thermal management, warning, and fire suppression technologies (provided in the supplemental information).
Figure 10.
Future strategies to enhance the all-temperature area performance and safety
Acknowledgments
This work is supported by National Natural Science Foundation of China (NSFC) (nos. U20A20310, 52176199, and 52076121), sponsored by Program of Shanghai Academic/Technology Research Leader(22XD1423800).
Author contributions
H.D. and X.F. supervised the manuscript. S.C. wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
Declaration of interests
The authors declare no competing interests.
Published Online: June 21, 2023
Footnotes
It can be found online at https://doi.org/10.1016/j.xinn.2023.100465.
Contributor Information
Xuning Feng, Email: fxn17@mail.tsinghua.edu.cn.
Haifeng Dai, Email: tongjidai@tongji.edu.cn.
Lead contact website
Supplemental information
References
- 1.Sun J., Chen S., Shen K., et al. Liquid cooling system optimization for a cell-to-pack battery module under fast charging. Int. J. Energy Res. 2022;46:12241–12253. [Google Scholar]
- 2.Wang H., Chen S., Du Z. Side plate-based cell-to-pack LiNi0.5Co0.2Mn0.3O2 lithium battery module design with internal temperature acquisition and precise thermal modeling. Int. J. Energy Res. 2021;45:21254–21263. [Google Scholar]
- 3.Chen S., Zhang G., Zhu J., et al. Multi-objective optimization design and experimental investigation for a parallel liquid cooling-based Lithium-ion battery module under fast charging. Appl. Therm. Eng. 2022;211:118503. [Google Scholar]
- 4.Roveda A.C., Comin M., Caires A.R.L., et al. Thermal stability enhancement of biodiesel induced by a synergistic effect between conventional antioxidants and an alternative additive. Energy. 2016;109:260–265. [Google Scholar]
- 5.Wang X., Wei X., Zhu J., et al. A review of modeling, acquisition, and application of lithium-ion battery impedance for onboard battery management. eTransportation. 2021;7:100093. [Google Scholar]
- 6.Zhang S.S., Xu K., Jow T.R. Electrochemical impedance study on the low temperature of Li-ion batteries. Electrochim. Acta. 2004;49:1057–1061. [Google Scholar]
- 7.Fuller T.F., Doyle M., Newman J. Simulation and optimization of the dual lithium ion insertion cell. J. Electrochem. Soc. 1994;141:1–10. [Google Scholar]
- 8.Feng X., Merla Y., Weng C., et al. A reliable approach of differentiating discrete sampled-data for battery diagnosis. eTransportation. 2020;3:100051. [Google Scholar]
- 9.Han X., Lu L., Zheng Y., et al. A review on the key issues of the lithium ion battery degradation among the whole life cycle. eTransportation. 2019;1:100005. [Google Scholar]
- 10.Chen S., Bao N., Peng X., et al. A Thermal Design and Experimental Investigation for the Fast Charging Process of a Lithium-Ion Battery Module With Liquid Cooling. J. Electrochem. Energy Convers. Storage. 2020;17:021109. [Google Scholar]
- 11.Tomaszewska A., Chu Z., Feng X., et al. Lithium-ion battery fast charging: A review. eTransportation. 2019;1:100011. [Google Scholar]
- 12.Tanim T.R., Dufek E.J., Walker L.K., et al. Advanced diagnostics to evaluate heterogeneity in lithium-ion battery modules. eTransportation. 2020;3:100045. [Google Scholar]
- 13.Wang Q., Jiang B., Li B., et al. A critical review of thermal management models and solutions of lithium-ion batteries for the development of pure electric vehicles. Renew. Sustain. Energy Rev. 2016;64:106–128. [Google Scholar]
- 14.Yi Y., Xia C., Feng C., et al. Digital twin-long short-term memory (LSTM) neural network based real-time temperature prediction and degradation model analysis for lithium-ion battery. J. Energy Storage. 2023;64:107203. [Google Scholar]
- 15.Jamal-Abad M.T., Zamzamian A., Dehghan M. Experimental studies on the heat transfer and pressure drop characteristics of Cu-water and Al-water nanofluids in a spiral coil. Exp. Therm. Fluid Sci. 2013;47:206–212. [Google Scholar]
- 16.Zhang Y., Chen S., Shahin M.E., et al. Multi-objective optimization of lithium-ion battery pack casing for electric vehicles: Key role of materials design and their influence. Int. J. Energy Res. 2020;44:9414–9437. [Google Scholar]
- 17.Bandhauer T.M., Garimella S., Fuller T.F. A critical review of thermal issues in lithium-ion batteries. J. Electrochem. Soc. 2011;158:R1. [Google Scholar]
- 18.Jaguemont J., Van Mierlo J. A comprehensive review of future thermal management systems for battery-electrified vehicles. J. Energy Storage. 2020;31:101551. [Google Scholar]
- 19.Hales A., Prosser R., Bravo Diaz L., et al. The cell cooling coefficient as a design tool to optimise thermal management of lithium-ion cells in battery packs. eTransportation. 2020;6:100089. [Google Scholar]
- 20.Shiao H.C., Chua D., Lin H.-p., et al. Low temperature electrolytes for Li-ion PVDF cells. J. Power Sources. 2000;87:167–173. [Google Scholar]
- 21.Li W., Chen S., Peng X., et al. A comprehensive approach for the clustering of similar-performance cells for the design of a lithium-ion battery module for electric vehicles. Engineering. 2019;38:795–803. [Google Scholar]
- 22.Qin P., Sun J., Yang X., et al. Battery thermal management system based on the forced-air convection: a review. eTransportation. 2021;7:100097. [Google Scholar]
- 23.Jaguemont J., Boulon L., Dubé Y. A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures. Appl. Energy. 2016;164:99–114. [Google Scholar]
- 24.Wu W., Wang S., Wu W., et al. A critical review of battery thermal performance and liquid based battery thermal management. Energy Convers. Manag. 2019;182:262–281. [Google Scholar]
- 25.Abdel-Monem M., Trad K., Omar N., et al. Influence analysis of static and dynamic fast-charging current profiles on ageing performance of commercial lithium-ion batteries. Energy. 2017;120:179–191. [Google Scholar]
- 26.Peng X., Chen S., Garg A., et al. A review of the estimation and heating methods for lithium-ion batteries pack at the cold environment. Energy Sci. Eng. 2019;7:645–662. [Google Scholar]
- 27.Li L., Xu C., Chang R., et al. Thermal-responsive, super-strong, ultrathin firewalls for quenching thermal runaway in high-energy battery modules. Energy Storage Mater. 2021;40:329–336. [Google Scholar]
- 28.Kim J., Oh J., Lee H. Review on battery thermal management system for electric vehicles. Appl. Therm. Eng. 2019;149:192–212. [Google Scholar]
- 29.Iten M., Liu S., Shukla A. A review on the air-PCM-TES application for free cooling and heating in the buildings. Renew. Sustain. Energy Rev. 2016;61:175–186. [Google Scholar]
- 30.Xia G., Cao L., Bi G. A review on battery thermal management in electric vehicle application. J. Power Sources. 2017;367:90–105. [Google Scholar]
- 31.Burow D., Sergeeva K., Calles S., et al. Inhomogeneous degradation of graphite anodes in automotive lithium ion batteries under low-temperature pulse cycling conditions. J. Power Sources. 2016;307:806–814. [Google Scholar]
- 32.Nagasubramanian G. Electrical characteristics of 18650 Li-ion cells at low temperatures. J. Appl. Electrochem. 2001;31:99–104. [Google Scholar]
- 33.Chen S., Zhang G., Wu C., et al. Multi-objective optimization design for a double-direction liquid heating system-based Cell-to-Chassis battery module. Int. J. Heat Mass Tran. 2022;183:122184. [Google Scholar]
- 34.Zhang G., Wei X., Han G., et al. Lithium plating on the anode for lithium-ion batteries during long-term low temperature cycling. J. Power Sources. 2021;484:229312. [Google Scholar]
- 35.Väyrynen A., Salminen J. Lithium ion battery production. J. Chem. Therm. 2012;46:80–85. [Google Scholar]
- 36.Zhang S.S., Xu K., Jow T.R. The low temperature performance of Li-ion batteries. J. Power Sources. 2003;115:137–140. [Google Scholar]
- 37.You H., Jiang B., Zhu J., et al. In-situ quantitative detection of irreversible lithium plating within full-lifespan of lithium-ion batteries. J. Power Sources. 2023;564:232892. [Google Scholar]
- 38.Ng B., Coman P.T., Faegh E., et al. Low-temperature lithium plating/corrosion hazard in lithium-ion batteries: electrode rippling, variable states of charge, and thermal and nonthermal runaway. ACS Appl. Energy Mater. 2020;3:3653–3664. [Google Scholar]
- 39.Wang C.-Y., Zhang G., Ge S., et al. Lithium-ion battery structure that self-heats at low temperatures. Nature. 2016;529:515–518. doi: 10.1038/nature16502. [DOI] [PubMed] [Google Scholar]
- 40.Zhang G.Y., Yang M., Liu B., et al. Rapid self-heating and internal temperature sensing of lithium-ion batteries at low temperatures. Electrochim. Acta. 2016;313:149–161. [Google Scholar]
- 41.Park G., Gunawardhana N., Nakamura H., et al. The study of electrochemical properties and lithium deposition of graphite at low temperature. J. Power Sources. 2012;199:293–299. [Google Scholar]
- 42.Foss C.E.L., Svensson A.M., Gullbrekken Ø., et al. Temperature effects on performance of graphite anodes in carbonate based electrolytes for lithium ion batteries. J. Energy Storage. 2018;17:395–402. [Google Scholar]
- 43.Senyshyn A., Mühlbauer M., Dolotko O., et al. Low-temperature performance of Li-ion batteries: the behavior of lithiated graphite. J. Power Sources. 2015;282:235–240. [Google Scholar]
- 44.Zhu J., Knapp M., Liu X., et al. Low-temperature separating lithium-ion battery interfacial polarization based on distribution of relaxation times (DRT) of impedance. Ann. Transl. Med. 2021;9:410–421. [Google Scholar]
- 45.Hu X., Zheng Y., Howey D.A., et al. Battery warm-up methodologies at subzero temperatures for automotive applications: Recent advances and perspectives. Prog. Energy Combust. Sci. 2020;77:100806. [Google Scholar]
- 46.Smart M.C., Ratnakumar B.V., Surampudi S. Electrolytes for low-temperature lithium batteries based on ternary mixtures of aliphatic carbonates. J. Electrochem. Soc. 1999;146:486–492. [Google Scholar]
- 47.Ratnakumar B.V., Smart M.C., Surampudi S. Effects of SEI on the kinetics of lithium intercalation. J. Power Sources. 2001;97–98:137–139. [Google Scholar]
- 48.Lin H.P., Chua D., Salomon M., et al. Low-temperature behavior of Li-ion cells. Electrochem. Solid State Lett. 2001;4:A71–A73. [Google Scholar]
- 49.Blomgren G.E. Electrolytes for advanced batteries. J. Power Sources. 1999;81–82:112–118. [Google Scholar]
- 50.Joho F., Rykart B., Imhof R., et al. Key factors for the cycling stability of graphite intercalation electrodes for lithium-ion batteries. J. Power Sources. 1999;81–82:243–247. [Google Scholar]
- 51.Amatucci G., Du Pasquier A., Blyr A., et al. The elevated temperature performance of the LiMn2O4/C system: failure and solutions. Electrochim. Acta. 1999;45:255–271. [Google Scholar]
- 52.Abe K., Yoshitake H., Kitakura T., et al. Additives-containing functional electrolytes for suppressing electrolyte decomposition in lithium-ion batteries. Electrochim. Acta. 2004;49:4613–4622. [Google Scholar]
- 53.Zhu J., Wang Y., Huang Y., et al. Data-driven capacity estimation of commercial lithium-ion batteries from voltage relaxation. Nat. Commun. 2022;13:2261. doi: 10.1038/s41467-022-29837-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vetter J., Novák P., Wagner M.R., et al. Ageing mechanisms in lithium-ion batteries. J. Power Sources. 2005;147:269–281. [Google Scholar]
- 55.Waldmann T., Wilka M., Kasper M., et al. Temperature dependent ageing mechanisms in Lithium-ion batteries – A Post-Mortem study. J. Power Sources. 2014;262:129–135. [Google Scholar]
- 56.Zhang S.S., Xu K., Jow T.R. Low temperature performance of graphite electrode in Li-ion cells. Electrochim. Acta. 2002;48:241–246. [Google Scholar]
- 57.Gao F., Tang Z. Kinetic behavior of LiFePO4/C cathode material for lithium-ion batteries. Electrochim. Acta. 2008;53:5071–5075. [Google Scholar]
- 58.Yang X.-G., Liu T., Wang C.-Y. Innovative heating of large-size automotive Li-ion cells. J. Power Sources. 2017;342:598–604. [Google Scholar]
- 59.Dai H., Jiang B., Hu X., et al. Advanced battery management strategies for a sustainable energy future: Multilayer design concepts and research trends. Renew. Sustain. Energy Rev. 2021;138:110480. [Google Scholar]
- 60.Aricò A.S., Bruce P., Scrosati B., et al. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005;4:366–377. doi: 10.1038/nmat1368. [DOI] [PubMed] [Google Scholar]
- 61.Sides C.R., Martin C.R. Nanostructured electrodes and the low-temperature performance of Li-ion batteries. Adv. Mater. 2005;17:125–128. [Google Scholar]
- 62.Bruce P.G., Scrosati B., Tarascon J.-M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. Engl. 2008;47:2930–2946. doi: 10.1002/anie.200702505. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y., Cao G. Developments in nanostructured cathode materials for high-performance lithium-ion batteries. Adv. Mater. 2008;20:2251–2269. [Google Scholar]
- 64.Wu X.-L., Guo Y.-G., Su J., et al. Carbon-nanotube-decorated nano-LiFePO4 @C cathode material with superior high-rate and low-temperature performances for lithium-ion batteries. Adv. Energy Mater. 2013;3:1155–1160. [Google Scholar]
- 65.Kim W., Ryu W., Han D., et al. Fabrication of graphene embedded LiFePO4 using a catalyst assisted self assembly method as a cathode material for high power lithium-ion batteries. ACS Appl. Mater. Interfaces. 2014;6:4731–4736. doi: 10.1021/am405335k. [DOI] [PubMed] [Google Scholar]
- 66.Yoon S.-J., Myung S.-T., Sun Y.-K. Low temperature electrochemical properties of Li[NixCoyMn1-x-y]O-2 cathode materials for lithium-ion batteries. J. Electrochem. Soc. 2014;161:A1514–A1520. [Google Scholar]
- 67.Wu Y., Jiang C., Wan C., et al. Modified natural graphite as anode material for lithium ion batteries. J. Power Sources. 2002;111:329–334. [Google Scholar]
- 68.Nobili F., Mancini M., Dsoke S., et al. Low-temperature behavior of graphite–tin composite anodes for Li-ion batteries. J. Power Sources. 2010;195:7090–7097. [Google Scholar]
- 69.Gao J., Fu L.J., Zhang H.P., et al. Suppression of PC decomposition at the surface of graphitic carbon by Cu coating. Electrochem. Commun. 2006;8:1726–1730. [Google Scholar]
- 70.Raccichini R., Varzi A., Chakravadhanula V.S.K., et al. Enhanced low-temperature lithium storage performance of multilayer graphene made through an improved ionic liquid-assisted synthesis. J. Power Sources. 2015;281:318–325. [Google Scholar]
- 71.Edström K., Gustafsson T., Thomas J.O. The cathode–electrolyte interface in the Li-ion battery. Electrochim. Acta. 2004;50:397–403. [Google Scholar]
- 72.Aurbach D., Zinigrad E., Cohen Y., et al. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ionics. 2002;148:405–416. [Google Scholar]
- 73.Churikov A.V. Transfer mechanism in solid-electrolyte layers on lithium: influence of temperature and polarization. Electrochim. Acta. 2001;46:2415–2426. [Google Scholar]
- 74.Zhong Z., Chen L., Zhu C., et al. Nano LiFePO4 coated Ni rich composite as cathode for lithium ion batteries with high thermal ability and excellent cycling performance. J. Power Sources. 2020;464:228235. [Google Scholar]
- 75.Marinaro M., Pfanzelt M., Kubiak P., et al. Low temperature behaviour of TiO2 rutile as negative electrode material for lithium-ion batteries. J. Power Sources. 2011;196:9825–9829. [Google Scholar]
- 76.Negi R.S., Celik E., Pan R., et al. Insights into the positive effect of post-annealing on the electrochemical performance of Al2O3-coated Ni-Rich NCM cathodes for lithium-ion batteries. ACS Appl. Energy Mater. 2021;4:3369–3380. [Google Scholar]
- 77.Andersson A.M., Henningson A., Siegbahn H., et al. Electrochemically lithiated graphite characterised by photoelectron spectroscopy. J. Power Sources. 2003;119–121:522–527. [Google Scholar]
- 78.Ji H., Zhang L., Pettes M.T., et al. Ultrathin graphite foam: a three-dimensional conductive network for battery electrodes. Nano Lett. 2012;12:2446–2451. doi: 10.1021/nl300528p. [DOI] [PubMed] [Google Scholar]
- 79.Wang L., Wang H., Liu Z., et al. A facile method of preparing mixed conducting LiFePO4/graphene composites for lithium-ion batteries. Solid State Ionics. 2010;181:1685–1689. [Google Scholar]
- 80.Ravet N., Chouinard Y., Magnan J.F., et al. Electroactivity of natural and synthetic triphylite. J. Power Sources. 2001;97–98:503–507. [Google Scholar]
- 81.Ji H.-X., Wu X.-L., Fan L.-Z., et al. Self-wound composite nanomembranes as electrode materials for lithium ion batteries. Adv. Mater. 2010;22:4591–4595. doi: 10.1002/adma.201001422. [DOI] [PubMed] [Google Scholar]
- 82.Chang Z.-R., Lv H.-J., Tang H.-W., et al. Synthesis and characterization of high-density LiFePO4/C composites as cathode materials for lithium-ion batteries. Electrochim. Acta. 2009;54:4595–4599. [Google Scholar]
- 83.Hu Y., Doeff M.M., Kostecki R., et al. Electrochemical performance of sol-gel synthesized LiFePO4 in lithium batteries. J. Electrochem. Soc. 2004;151:A1279–A1285. [Google Scholar]
- 84.Doeff M., Wilcox J., Kostecki R., et al. Optimization of carbon coatings on LiFePO4. Meet. Abstr. 2006;163:180–184. [Google Scholar]
- 85.Park O.K., Cho Y., Lee S., et al. Who will drive electric vehicles, olivine or spinel? Energy Environ. Sci. 2011;4:1621–1633. [Google Scholar]
- 86.Nien Y.-H., Carey J.R., Chen J.-S. Physical and electrochemical properties of LiFePO4/C composite cathode prepared from various polymer-containing precursors. J. Power Sources. 2009;193:822–827. [Google Scholar]
- 87.Wu X.-L., Jiang L.-Y., Cao F.-F., et al. LiFePO4 nanoparticles embedded in a nanoporous carbon matrix: superior cathode material for electrochemical energy-storage devices. Adv. Mater. 2009;21:2710–2714. doi: 10.1002/adma.200802998. [DOI] [PubMed] [Google Scholar]
- 88.Ellis B., Subramanya Herle P., Rho Y.H., et al. Nanostructured materials for lithium-ion batteries: Surface conductivity vs. bulk ion/electron transport. Faraday Discuss. 2007;134:119–141. doi: 10.1039/b602698b. discussion 215-233, 415-419. [DOI] [PubMed] [Google Scholar]
- 89.Yuan L.X., Wang Z.H., Zhang W.X., et al. Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ. Sci. 2011;4:269–284. [Google Scholar]
- 90.Li W.-H., Li Y.-M., Liu X.-F., et al. All-climate and ultrastable dual-ion batteries with long life achieved via synergistic enhancement of cathode and anode interfaces. Adv. Funct. Mater. 2022;32:2201038. [Google Scholar]
- 91.Chang Y.-C., Peng C.-T., Hung I.M. Effects of particle size and carbon coating on electrochemical properties of LiFePO4/C prepared by hydrothermal method. J. Mater. Sci. 2014;49:6907–6916. [Google Scholar]
- 92.Song X., Liu G., Yue H., et al. A novel low-cobalt long-life LiNi0.88Co0.06Mn0.03Al0.03O2 cathode material for lithium ion batteries. Chem. Eng. J. 2021;407:126301. [Google Scholar]
- 93.Smart M.C., Whitacre J.F., Ratnakumar B.V., et al. Electrochemical performance and kinetics of Li1+x(Co1/3Ni1/3Mn1/3)1−xO2 cathodes and graphite anodes in low-temperature electrolytes. J. Power Sources. 2007;168:501–508. [Google Scholar]
- 94.Rui X.H., Jin Y., Feng X.Y., et al. A comparative study on the low-temperature performance of LiFePO4/C and Li3V2(PO4)3/C cathodes for lithium-ion batteries. J. Power Sources. 2011;196:2109–2114. [Google Scholar]
- 95.Petibon R., Harlow J., Le D.B., et al. The use of ethyl acetate and methyl propanoate in combination with vinylene carbonate as ethylene carbonate-free solvent blends for electrolytes in Li-ion batteries. Electrochim. Acta. 2015;154:227–234. [Google Scholar]
- 96.Smart M.C., Ratnakumar B.V., Chin K.B., et al. Lithium-ion electrolytes containing ester cosolvents for improved low temperature performance. J. Electrochem. Soc. 2010;157:A1361. [Google Scholar]
- 97.Zhou L., Xu M., Lucht B.L. Performance of lithium tetrafluorooxalatophosphate in methyl butyrate electrolytes. J. Appl. Electrochem. 2013;43:497–505. [Google Scholar]
- 98.Deng X., Zhang S., Chen C., et al. Rational design of electrolytes operating at low temperatures: does the co-solvent with a lower melting point correspond to better performance? Electrochim. Acta. 2022;415:140268. [Google Scholar]
- 99.Smart M.C., Lucht B.L., Dalavi S., et al. The effect of additives upon the performance of MCMB/LiNixCo1−xO2Li-ion cells containing methyl butyrate-based wide operating temperature range electrolytes. J. Electrochem. Soc. 2012;159:A739–A751. [Google Scholar]
- 100.Niedzicki L., Grugeon S., Laruelle S., et al. New covalent salts of the 4+V class for Li batteries. J. Power Sources. 2011;196:8696–8700. [Google Scholar]
- 101.Xu K., Zhang S., Jow T.R. LiBOB as additive in LiPF[sub 6]-based lithium ion electrolytes. Electrochem. Solid State Lett. 2005;8:A365. [Google Scholar]
- 102.Lazar M.L., Lucht B.L. Carbonate free electrolyte for lithium ion batteries containing γ-butyrolactone and methyl butyrate. J. Electrochem. Soc. 2015;162:A928–A934. [Google Scholar]
- 103.Li S., Zhao W., Zhou Z., et al. Studies on electrochemical performances of novel electrolytes for wide-temperature-range lithium-ion batteries. ACS Appl. Mater. Interfaces. 2014;6:4920–4926. doi: 10.1021/am405973x. [DOI] [PubMed] [Google Scholar]
- 104.Zhang N., Deng T., Zhang S., et al. Critical review on low-temperature Li-ion/metal batteries. Adv. Mater. 2022;34:2107899. doi: 10.1002/adma.202107899. [DOI] [PubMed] [Google Scholar]
- 105.Herreyre S., Huchet O., Barusseau S., et al. New Li-ion electrolytes for low temperature applications. J. Power Sources. 2001;97–98:576–580. [Google Scholar]
- 106.Plichta E.J., Behl W.K. A low-temperature electrolyte for lithium and lithium-ion batteries. J. Power Sources. 2000;88:192–196. [Google Scholar]
- 107.Ji Y., Zhang Y., Wang C.-Y. Li-ion cell operation at low temperatures. J. Electrochem. Soc. 2013;160:A636–A649. [Google Scholar]
- 108.Yang B., Zhang H., Yu L., et al. Lithium difluorophosphate as an additive to improve the low temperature performance of LiNi0.5Co0.2Mn0.3O2/graphite cells. Electrochimica Acta. 2016;221:107–114. [Google Scholar]
- 109.Liu Y.-K., Zhao C.-Z., Du J., et al. Research progresses of liquid electrolytes in lithium-ion batteries. Small. 2023;19:2205315. doi: 10.1002/smll.202205315. [DOI] [PubMed] [Google Scholar]
- 110.Goodenough J.B., Kim Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010;22:587–603. [Google Scholar]
- 111.Li Q., Lu D., Zheng J., et al. Li+-desolvation dictating lithium-ion battery's low-temperature performances. ACS Appl. Mater. Interfaces. 2017;9:42761–42768. doi: 10.1021/acsami.7b13887. [DOI] [PubMed] [Google Scholar]
- 112.Li Q., Jiao S., Luo L., et al. Wide-temperature electrolytes for lithium-ion batteries. ACS Appl. Mater. Interfaces. 2017;9:18826–18835. doi: 10.1021/acsami.7b04099. [DOI] [PubMed] [Google Scholar]
- 113.Zhang S.S., Xu K., Jow T.R. EIS study on the formation of solid electrolyte interface in Li-ion battery. Electrochim. Acta. 2006;51:1636–1640. [Google Scholar]
- 114.Liao X.-Z., Ma Z.-F., Gong Q., et al. Low-temperature performance of LiFePO4/C cathode in a quaternary carbonate-based electrolyte. Electrochem. Commun. 2008;10:691–694. [Google Scholar]
- 115.Mandal B.K., Padhi A.K., Shi Z., et al. New low temperature electrolytes with thermal runaway inhibition for lithium-ion rechargeable batteries. J. Power Sources. 2006;162:690–695. [Google Scholar]
- 116.Lin S., Hua H., Lai P., et al. A multifunctional dual-salt localized high-concentration electrolyte for fast dynamic high-voltage lithium battery in wide temperature range. Adv. Energy Mater. 2021;11:2101775. [Google Scholar]
- 117.Zhang S.S., Xu K., Jow T.R. A new approach toward improved low temperature performance of Li-ion battery. Electrochem. Commun. 2002;4:928–932. [Google Scholar]
- 118.Jow T.R., Ding M.S., Xu K., et al. Nonaqueous electrolytes for wide-temperature-range operation of Li-ion cells. J. Power Sources. 2003;119–121:343–348. [Google Scholar]
- 119.Abraham D.P., Heaton J.R., Kang S.H., et al. Investigating the low-temperature impedance increase of lithium-ion cells. J. Electrochem. Soc. 2008;155:A41. [Google Scholar]
- 120.Huang C.K., Sakamoto J.S., Wolfenstine J., et al. Limits of low-temperature performance of Li-ion cells. J. Electrochem. Soc. 2000;147:2893–2896. [Google Scholar]
- 121.Du Y.-F., Sun G.-H., Li Y., et al. Pre-oxidation of lignin precursors for hard carbon anode with boosted lithium-ion storage capacity. Carbon. 2021;178:243–255. [Google Scholar]
- 122.Nobili F., Dsoke S., Mecozzi T., et al. Metal-oxidized graphite composite electrodes for lithium-ion batteries. Electrochim. Acta. 2005;51:536–544. [Google Scholar]
- 123.Mancini M., Nobili F., Dsoke S., et al. Lithium intercalation and interfacial kinetics of composite anodes formed by oxidized graphite and copper. J. Power Sources. 2009;190:141–148. [Google Scholar]
- 124.Cao X., Kim J.H., Oh S.M. The effects of oxidation on the surface properties of MCMB-6-28. Electrochim. Acta. 2002;47:4085–4089. [Google Scholar]
- 125.Collins G.A., Geaney H., Ryan K.M. Alternative anodes for low temperature lithium-ion batteries. J. Mater. Chem. A Mater. 2021;9:14172–14213. [Google Scholar]
- 126.Shi H.W., Yang J.G., Wang Y., et al. In situ fabrication of dual coating structured SiO/1D-C/a-C composite as high-performance lithium ion battery anode by fluidized bed chemical vapor deposition. Carbon. 2020;168:113–124. [Google Scholar]
- 127.Wang G.J., Gao J., Fu L.J., et al. Preparation and characteristic of carbon-coated Li4Ti5O12 anode material. J. Power Sources. 2007;174:1109–1112. [Google Scholar]
- 128.Zou M., Li J., Wen W., et al. Silver-incorporated composites of Fe2O3 carbon nanofibers as anodes for high-performance lithium batteries. J. Power Sources. 2014;270:468–474. [Google Scholar]
- 129.Li J., Wen W., Xu G., et al. Fe-added Fe3C carbon nanofibers as anode for Li ion batteries with excellent low-temperature performance. Electrochim. Acta. 2015;153:300–305. [Google Scholar]
- 130.Yang M., Sun Z., Nie P., et al. SbPS4: A novel anode for high-performance sodium-ion batteries. Chin. Chem. Lett. 2022;33:470–474. [Google Scholar]
- 131.Yuan T., Cai R., Wang K., et al. Combustion synthesis of high-performance Li4Ti5O12 for secondary Li-ion battery. Ceram. Int. 2009;35:1757–1768. [Google Scholar]
- 132.Wen Z., Yang X., Huang S. Composite anode materials for Li-ion batteries. J. Power Sources. 2007;174:1041–1045. [Google Scholar]
- 133.Aurbach D. Review of selected electrode–solution interactions which determine the performance of Li and Li ion batteries. J. Power Sources. 2000;89:206–218. [Google Scholar]
- 134.Piao N., Gao X., Yang H., et al. Challenges and development of lithium-ion batteries for low temperature environments. eTransportation. 2022;11:100145. [Google Scholar]
- 135.Lei Z., Zhang Y., Lei X. Improving temperature uniformity of a lithium-ion battery by intermittent heating method in cold climate. Int. J. Heat Mass Tran. 2018;121:275–281. [Google Scholar]
- 136.Chen S., Peng X., Bao N., et al. A comprehensive analysis and optimization process for an integrated liquid cooling plate for a prismatic lithium-ion battery module. Appl. Therm. Eng. 2019;156:324–339. [Google Scholar]
- 137.Li S., Kirkaldy N., Zhang C., et al. Optimal cell tab design and cooling strategy for cylindrical lithium-ion batteries. J. Power Sources. 2021;492:229594. [Google Scholar]
- 138.Lin J., Liu X., Li S., et al. A review on recent progress, challenges and perspective of battery thermal management system. Int. J. Heat Mass Tran. 2021;167:120834. [Google Scholar]
- 139.Song H.S., Jeong J.B., Lee B.H., et al. 2012 IEEE Vehicle Power and Propulsion Conference. VPPC; 2012. Experimental study on the effects of pre-heating a battery in a low-temperature environment. [Google Scholar]
- 140.Zhang X., Kong X., Li G., et al. Thermodynamic assessment of active cooling/heating methods for lithium-ion batteries of electric vehicles in extreme conditions. Energy. 2014;64:1092–1101. [Google Scholar]
- 141.Zhang X., Li Z., Luo L., et al. A review on thermal management of lithium-ion batteries for electric vehicles. Energy. 2022;238:121652. [Google Scholar]
- 142.Nelson P., Dees D., Amine K., et al. Modeling thermal management of lithium-ion PNGV batteries. J. Power Sources. 2002;110:349–356. [Google Scholar]
- 143.Wang Q., Jiang B., Xue Q.F., et al. Experimental investigation on EV battery cooling and heating by heat pipes. Appl. Therm. Eng. 2015;88:54–60. [Google Scholar]
- 144.Luo M., Song J., Ling Z., et al. Phase change material coat for battery thermal management with integrated rapid heating and cooling functions from −40 °C to 50 °C. Mater. Today Energy. 2021;20:100652. [Google Scholar]
- 145.Zhong G., Zhang G., Yang X., et al. Researches of composite phase change material cooling/resistance wire preheating coupling system of a designed 18650-type battery module. Appl. Therm. Eng. 2017;127:176–183. [Google Scholar]
- 146.Ling Z., Wen X., Zhang Z., et al. Thermal management performance of phase change materials with different thermal conductivities for Li-ion battery packs operated at low temperatures. Energy. 2018;144:977–983. [Google Scholar]
- 147.Pan S., Zheng Y., Lu L., et al. Neural network PID-based preheating control and optimization for a li-ion battery module at low temperatures. World Electric Vehicle J. 2023;14:83. [Google Scholar]
- 148.Lin C.T., Lai K.L., Tian Y., et al. Heating lithium-ion batteries at low temperatures for onboard applications: recent progress, challenges and prospects. J. Med. Ultrasound. 2022;30:3–5. [Google Scholar]
- 149.Li J., Huang W. In: Towards Mesoscience: The Principle of Compromise in Competition. Li J., Huang W., editors. Springer Berlin Heidelberg; 2014. Background: correlating microscale and macroscale; pp. 1–6. [Google Scholar]
- 150.Fan R., Zhang C., Wang Y., et al. Numerical study on the effects of battery heating in cold climate. J. Energy Storage. 2019;26:100969. [Google Scholar]
- 151.Huang R., Wang X., Jiang B., et al. Revealing the electrochemical impedance characteristics of lithium-ion battery (nickel-cobalt-aluminum vs. graphite) under various alternating current amplitudes. J. Power Sources. 2023;566:232929. [Google Scholar]
- 152.Yue Q.L., He C.X., Wu M.C., et al. Advances in thermal management systems for next-generation power batteries. Int. J. Heat Mass Tran. 2021;181:121853. [Google Scholar]
- 153.Qu Z.G., Jiang Z.Y., Wang Q. Experimental study on pulse self–heating of lithium–ion battery at low temperature. Int. J. Heat Mass Tran. 2019;135:696–705. [Google Scholar]
- 154.Zhang J., Ge H., Li Z., et al. Internal heating of lithium-ion batteries using alternating current based on the heat generation model in frequency domain. J. Power Sources. 2015;273:1030–1037. [Google Scholar]
- 155.Zhao X.W., Zhang G.Y., Yang L., et al. A new charging mode of Li-ion batteries with LiFePO4/C composites under low temperature. J. Therm. Anal. Calorim. 2011;104:561–567. [Google Scholar]
- 156.Ruan H., Jiang J., Sun B., et al. A rapid low-temperature internal heating strategy with optimal frequency based on constant polarization voltage for lithium-ion batteries. Appl. Energy. 2016;177:771–782. [Google Scholar]
- 157.Ji Y., Wang C.Y. Heating strategies for Li-ion batteries operated from subzero temperatures. Electrochim. Acta. 2013;107:664–674. [Google Scholar]
- 158.Wu H., Zhang X., Cao R., et al. An investigation on electrical and thermal characteristics of cylindrical lithium-ion batteries at low temperatures. Energy. 2021;225:120223. [Google Scholar]
- 159.Brodd R.J. Springer Science & Business Media; 2012. Batteries for Sustainability: Selected Entries from the Encyclopedia of Sustainability Science and Technology. [Google Scholar]
- 160.Chen S., Bao N., Garg A., et al. A fast charging–cooling coupled scheduling method for a liquid cooling-based thermal management system for lithium-ion batteries. Engineering. 2021;7:1165–1176. [Google Scholar]
- 161.Chen S., Bao N., Gao L., et al. An experimental investigation of liquid cooling scheduling for a battery module. Int. J. Energy Res. 2020;44:3020–3032. [Google Scholar]
- 162.Chen S., Wei X., Garg A., et al. A comprehensive flowrate optimization design for a novel air-liquid cooling coupled battery thermal management system. J. Electrochem. Energy Convers. Storage. 2021;18:021008. [Google Scholar]
- 163.Shen K., Sun J., Zheng Y., et al. A comprehensive analysis and experimental investigation for the thermal management of cell-to-pack battery system. Appl. Therm. Eng. 2022;211:118422. [Google Scholar]
- 164.Chen S., Garg A., Gao L., et al. An experimental investigation for a hybrid phase change material-liquid cooling strategy to achieve high-temperature uniformity of Li-ion battery module under fast charging. Int. J. Energy Res. 2021;45:6198–6212. [Google Scholar]
- 165.Wang Y., Feng X., Peng Y., et al. Reductive gas manipulation at early self-heating stage enables controllable battery thermal failure. Joule. 2022;6:2810–2820. [Google Scholar]
- 166.Kong D., Wang G., Ping P., et al. A coupled conjugate heat transfer and CFD model for the thermal runaway evolution and jet fire of 18650 lithium-ion battery under thermal abuse. eTransportation. 2022;12:100157. [Google Scholar]
- 167.Wang G., Kong D., Ping P., et al. Revealing particle venting of lithium-ion batteries during thermal runaway: a multi-scale model toward multiphase process. eTransportation. 2023;16:100237. [Google Scholar]
- 168.Ping P., Wang Q., Huang P., et al. Study of the fire behavior of high-energy lithium-ion batteries with full-scale burning test. J. Power Sources. 2015;285:80–89. [Google Scholar]
- 169.Li K., Xu C., Wang H., et al. Investigation for the effect of side plates on thermal runaway propagation characteristics in battery modules. Appl. Therm. Eng. 2022;201:117774. [Google Scholar]
- 170.Chen M., He Y., De Zhou C., et al. Experimental study on the combustion characteristics of primary lithium batteries fire. Fire Technol. 2016;52:365–385. [Google Scholar]
- 171.Wang Y., Song Z., Wang H., et al. Experimental research on flammability characteristics and ignition conditions of hybrid mixture emissions venting from a large format thermal failure lithium-ion battery. J. Energy Storage. 2023;59:106466. [Google Scholar]
- 172.Ouyang D., Liu J., Chen M., et al. An experimental study on the thermal failure propagation in lithium-ion battery pack. J. Electrochem. Soc. 2018;165:A2184–A2193. [Google Scholar]
- 173.Lamb J., Orendorff C.J., Steele L.A.M., et al. Failure propagation in multi-cell lithium ion batteries. J. Power Sources. 2015;283:517–523. [Google Scholar]
- 174.Hu J., Liu T., Wang X., et al. Investigation on thermal runaway of 18,650 lithium ion battery under thermal abuse coupled with charging. J. Energy Storage. 2022;51:104482. [Google Scholar]
- 175.Liu T., Hu J., Zhu X., et al. A practical method of developing cooling control strategy for thermal runaway propagation prevention in lithium ion battery modules. J. Energy Storage. 2022;50:104564. [Google Scholar]
- 176.Xu C., Wang H., Jiang F., et al. Modelling of thermal runaway propagation in lithium-ion battery pack using reduced-order model. Energy. 2023;268:126646. [Google Scholar]
- 177.Feng X., Lu L., Ouyang M., et al. A 3D thermal runaway propagation model for a large format lithium ion battery module. Energy. 2016;115:194–208. [Google Scholar]
- 178.Feng X., Sun J., Ouyang M., et al. Characterization of penetration induced thermal runaway propagation process within a large format lithium ion battery module. J. Power Sources. 2015;275:261–273. [Google Scholar]
- 179.Wang Q., Mao B., Stoliarov S.I., et al. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019;73:95–131. [Google Scholar]
- 180.Huang P., Ping P., Li K., et al. Experimental and modeling analysis of thermal runaway propagation over the large format energy storage battery module with Li4Ti5O12 anode. Appl. Energy. 2016;183:659–673. [Google Scholar]
- 181.Zhang W., Wu J., Li Y., et al. High stability and high performance nitrogen doped carbon containers for lithium-ion batteries. J. Colloid Interface Sci. 2022;625:692–699. doi: 10.1016/j.jcis.2022.06.062. [DOI] [PubMed] [Google Scholar]
- 182.MacNeil D.D., Larcher D., Dahn J.R. Comparison of the reactivity of various carbon electrode materials with electrolyte at elevated temperature. J. Electrochem. Soc. 1999;146:3596–3602. [Google Scholar]
- 183.Richard M.N., Dahn J.R. Accelerating rate calorimetry study on the thermal stability of lithium intercalated graphite in electrolyte. I. experimental. J. Electrochem. Soc. 1999;146:2068–2077. [Google Scholar]
- 184.Yang H., Amiruddin S., Bang H.J., et al. A review of Li-ion cell chemistries and their potential use in hybrid electric vehicles. J. Ind. Eng. Chem. 2006;12:12–38. [Google Scholar]
- 185.Abraham D.P., Roth E.P., Kostecki R., et al. Diagnostic examination of thermally abused high-power lithium-ion cells. J. Power Sources. 2006;161:648–657. [Google Scholar]
- 186.Wang Q., Sun J., Yao X., et al. Thermal behavior of lithiated graphite with electrolyte in lithium-ion batteries. J. Electrochem. Soc. 2006;153:A329–A333. [Google Scholar]
- 187.Wang Q., Sun J., Yao X., et al. Micro calorimeter study on the thermal stability of lithium-ion battery electrolytes. J. Loss Prev. Process. Ind. 2006;19:561–569. [Google Scholar]
- 188.Al Hallaj S., Maleki H., Hong J.S., et al. Thermal modeling and design considerations of lithium-ion batteries. J. Power Sources. 1999;83:1–8. [Google Scholar]
- 189.Arora S., Shen W., Kapoor A. Review of mechanical design and strategic placement technique of a robust battery pack for electric vehicles. Renew. Sustain. Energy Rev. 2016;60:1319–1331. [Google Scholar]
- 190.Mao B., Chen H., Cui Z., et al. Failure mechanism of the lithium ion battery during nail penetration. Int. J. Heat Mass Tran. 2018;122:1103–1115. [Google Scholar]
- 191.Zhang G., Wei X., Chen S., et al. Comprehensive investigation of a slight overcharge on degradation and thermal runaway behavior of lithium-ion batteries. ACS Appl. Mater. Interfaces. 2021;13:35054–35068. doi: 10.1021/acsami.1c06029. [DOI] [PubMed] [Google Scholar]
- 192.Feng X., He X., Ouyang M., et al. Thermal runaway propagation model for designing a safer battery pack with 25Ah LiNixCoyMnzO2 large format lithium ion battery. Appl. Energy. 2015;154:74–91. [Google Scholar]
- 193.Liu X., Ren D., Hsu H., et al. Thermal runaway of lithium-ion batteries without internal short circuit. Joule. 2018;2:2047–2064. [Google Scholar]
- 194.Lisbona D., Snee T. A review of hazards associated with primary lithium and lithium-ion batteries. Process Saf. Environ. Protect. 2011;89:434–442. [Google Scholar]
- 195.Wu C., Wu Y., Feng X., et al. Ultra-high temperature reaction mechanism of LiNi0.8Co0.1Mn0.1O2 electrode. J. Energy Storage. 2022;52:104870. [Google Scholar]
- 196.Wang Y., Ren D., Feng X., et al. Thermal runaway modeling of large format high-nickel/silicon-graphite lithium-ion batteries based on reaction sequence and kinetics. Appl. Energy. 2022;306:117943. [Google Scholar]
- 197.Yang H., Shen X.-D. Dynamic TGA–FTIR studies on the thermal stability of lithium/graphite with electrolyte in lithium-ion cell. J. Power Sources. 2007;167:515–519. [Google Scholar]
- 198.Kawamura T., Kimura A., Egashira M., et al. Thermal stability of alkyl carbonate mixed-solvent electrolytes for lithium ion cells. J. Power Sources. 2002;104:260–264. [Google Scholar]
- 199.Wang Q., Sun J., Yao X., et al. Thermal stability of LiPF6/EC+DEC electrolyte with charged electrodes for lithium ion batteries. Thermochim. Acta. 2005;437:12–16. [Google Scholar]
- 200.Gnanaraj J.S., Zinigrad E., Asraf L., et al. The use of accelerating rate calorimetry (ARC) for the study of the thermal reactions of Li-ion battery electrolyte solutions. J. Power Sources. 2003;119–121:794–798. [Google Scholar]
- 201.Xu C., Zhang F., Feng X., et al. Experimental study on thermal runaway propagation of lithium-ion battery modules with different parallel-series hybrid connections. J. Clean. Prod. 2021;284:124749. [Google Scholar]
- 202.Zhang G., Wei X., Chen S., et al. Revealing the impact of slight electrical abuse on the thermal safety characteristics for lithium-ion batteries. ACS Appl. Energy Mater. 2021;4:12858–12870. [Google Scholar]
- 203.Wang Y., Ren D., Feng X., et al. Thermal kinetics comparison of delithiated Li[NixCoyMn1-x-y]O2 cathodes. J. Power Sources. 2021;514:230582. [Google Scholar]
- 204.Balakrishnan P.G., Ramesh R., Prem Kumar T. Safety mechanisms in lithium-ion batteries. J. Power Sources. 2006;155:401–414. [Google Scholar]
- 205.Feng X., Ren D., He X., et al. Mitigating thermal runaway of lithium-ion batteries. Joule. 2020;4:743–770. [Google Scholar]
- 206.Rui X., Feng X., Wang H., et al. Synergistic effect of insulation and liquid cooling on mitigating the thermal runaway propagation in lithium-ion battery module. Appl. Therm. Eng. 2021;199:117521. [Google Scholar]
- 207.Xu G.-L., Liu Q., Lau K.K.S., et al. Building ultraconformal protective layers on both secondary and primary particles of layered lithium transition metal oxide cathodes. Nat. Energy. 2019;4:484–494. [Google Scholar]
- 208.Hou P., Zhang H., Deng X., et al. Stabilizing the electrode/electrolyte interface of LiNi0.8Co0.15Al0.05O2 through tailoring aluminum distribution in microspheres as long-life, high-rate, and safe cathode for lithium-ion batteries. ACS Appl. Mater. Interfaces. 2017;9:29643–29653. doi: 10.1021/acsami.7b05986. [DOI] [PubMed] [Google Scholar]
- 209.Zhang G., Wei X., Tang X., et al. Internal short circuit mechanisms, experimental approaches and detection methods of lithium-ion batteries for electric vehicles: A review. Renew. Sustain. Energy Rev. 2021;141:110790. [Google Scholar]
- 210.Rahman M.M., Mateti S., Cai Q., et al. High temperature and high rate lithium-ion batteries with boron nitride nanotubes coated polypropylene separators. Energy Storage Mater. 2019;19:352–359. [Google Scholar]
- 211.Wu X., Wang Z., He Z., et al. Development of cathode-electrolyte-interphase for safer lithium batteries. Energy Storage Mater. 2021;37:77–86. [Google Scholar]
- 212.Friesen A., Hildebrand S., Horsthemke F., et al. Al2O3 coating on anode surface in lithium ion batteries: impact on low temperature cycling and safety behavior. J. Power Sources. 2017;363:70–77. [Google Scholar]
- 213.Hou J., Wang L., Feng X., et al. Thermal runaway of lithium-ion batteries employing flame-retardant fluorinated electrolytes. Energy Environ. Mater. 2023;6:e12297. [Google Scholar]
- 214.Wu C., Wu Y., Yang X., et al. Thermal runaway suppression of high-energy lithium-ion batteries by designing the stable interphase. J. Electrochem. Soc. 2021;168:090563. [Google Scholar]
- 215.Ma L., Xia J., Xia X., et al. The impact of vinylene carbonate, fluoroethylene carbonate and vinyl ethylene carbonate electrolyte additives on electrode/electrolyte reactivity studied using accelerating rate calorimetry. J. Electrochem. Soc. 2014;161:A1495–A1498. [Google Scholar]
- 216.Liu K., Liu Y., Lin D., et al. Materials for lithium-ion battery safety. Sci. Adv. 2018;4:eaas9820. doi: 10.1126/sciadv.aas9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hou J., Wang L., Feng X., et al. Thermal runaway of lithium-ion batteries employing flame-retardant fluorinated electrolytes. Energy Environm. Mater. 2023;6:e12297. [Google Scholar]
- 218.Wang H., Du Z., Rui X., et al. A comparative analysis on thermal runaway behavior of Li (NixCoyMnz) O2 battery with different nickel contents at cell and module level. J. Hazard Mater. 2020;393:122361. doi: 10.1016/j.jhazmat.2020.122361. [DOI] [PubMed] [Google Scholar]
- 219.Zhang F., Feng X., Xu C., et al. Thermal runaway front in failure propagation of long-shape lithium-ion battery. Int. J. Heat Mass Tran. 2022;182:121928. [Google Scholar]
- 220.Noelle D.J., Shi Y., Wang M., et al. Aggressive electrolyte poisons and multifunctional fluids comprised of diols and diamines for emergency shutdown of lithium-ion batteries. J. Power Sources. 2018;384:93–97. [Google Scholar]
- 221.Ji W., Jiang B., Ai F., et al. Temperature-responsive microspheres-coated separator for thermal shutdown protection of lithium ion batteries. RSC Adv. 2015;5:172–176. [Google Scholar]
- 222.Shi Y., Noelle D.J., Wang M., et al. Mitigating thermal runaway of lithium-ion battery through electrolyte displacement. Appl. Phys. Lett. 2017;110:063902. [Google Scholar]
- 223.Naguib M., Allu S., Simunovic S., et al. Limiting internal short-circuit damage by electrode partition for impact-tolerant Li-ion batteries. Joule. 2018;2:155–167. [Google Scholar]
- 224.Liu K., Liu W., Qiu Y., et al. Electrospun core-shell microfiber separator with thermal-triggered flame-retardant properties for lithium-ion batteries. Sci. Adv. 2017;3:e1601978. doi: 10.1126/sciadv.1601978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lai X., Meng Z., Zhang F., et al. Mitigating thermal runaway hazard of high-energy lithium-ion batteries by poison agent. J. Energy Chem. 2023;83:3–15. [Google Scholar]
- 226.Hong Y., Zhang Y., Li C., et al. High-security prismatic battery with cover filled agent. J. Energy Storage. 2023;64:107133. [Google Scholar]
- 227.Lu L., Han X., Li J., et al. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources. 2013;226:272–288. [Google Scholar]
- 228.Jin C., Sun Y., Wang H., et al. Model and experiments to investigate thermal runaway characterization of lithium-ion batteries induced by external heating method. J. Power Sources. 2021;504:230065. [Google Scholar]
- 229.Qian L., Yi Y., Zhang W., et al. Revealing the impact of high current overcharge/overdischarge on the thermal safety of degraded Li-ion batteries. Int. J. Energy Res. 2023;2023:8571535. [Google Scholar]
- 230.Wu H., Chen S., Chen J., et al. Dimensionless normalized concentration based thermal-electric regression model for the thermal runaway of lithium-ion batteries. J. Power Sources. 2022;521:230958. [Google Scholar]
- 231.Huang W., Feng X., Pan Y., et al. Early warning of battery failure based on venting signal. J. Energy Storage. 2023;59:106536. [Google Scholar]
- 232.Cai T., Valecha P., Tran V., et al. Detection of Li-ion battery failure and venting with carbon dioxide sensors. eTransportation. 2021;7:100100. [Google Scholar]
- 233.Li W., Wang H., Zhang Y., et al. Flammability characteristics of the battery vent gas: A case of NCA and LFP lithium-ion batteries during external heating abuse. J. Energy Storage. 2019;24:100775. [Google Scholar]
- 234.Yang X., Wang H., Li M., et al. Experimental study on thermal runaway behavior of lithium-ion battery and analysis of combustible limit of gas production. Batteries. 2022;8:250. [Google Scholar]
- 235.Li L., Jia C., Liu Y., et al. Nanograin-glass dual-phasic, elasto-flexible, fatigue-tolerant, and heat-insulating ceramic sponges at large scales. Mater. Today. 2022;54:72–82. [Google Scholar]
- 236.Shen K., Sun J., Xu C., et al. Experimental investigation for the phase change material barrier area effect on the thermal runaway propagation prevention of cell-to-pack batteries. Batteries. 2023;9:206. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.