Sir,
The genus Prevotella is a group of obligate anaerobic Gram-negative bacteria that includes more than 50 species, many of them associated with the human microbiota, where they are commensals of the upper respiratory tract and the genitourinary system. Prevotella oris was isolated for the first time in 1982 in a patient with periodontitis being named Bacteroides oris and reclassified years later as P. oris [1]. In this manuscript, we describe two cases of serious pleuropulmonary infections, a necrotizing pneumonia and a pneumonia associated with pleural empyema.
Case 1
A 4-year-old patient with a history of sickle cell anemia presented with fever up to 39ºC associated with cough and purulent expectoration of 19 days of evolution. At the beginning of the clinical picture, the patient was diagnosed as a respiratory viriasis due to Influenza A virus by PCR. On examination, no alarm signs were observed and the blood analysis showed 20,680 leukocytes with 81% neutrophils and a C-reactive protein of 210 mg/L. A Pulmonary ultrasound showed condensation in the right upper lobe (Figure 1A) and the patient was admitted for study and IV antibiotic treatment with 1 g/6 h of ampicillin, obtaining bronchoaspirate, bronchoalveolar lavage and bronchial brushing samples, ruling out Mycobacterium tuberculosis by PCR with the GeneXpert MTB/RIF panel (Cepheid, California, USA).
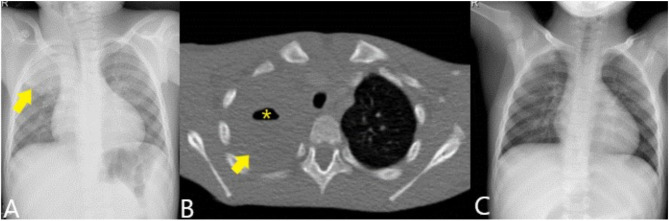
Figure 1.
Chest X-ray (A) showing condensation in the right upper lobe (arrow). Contrast-enhanced CT (B) shows the condensation (arrow) with the presence of an air level suggestive of cavitary pneumonia (asterisk). The control radiograph (C) shows complete resolution of the picture.
After two days of hospitalization, a computed tomography scan was conducted with data consistent with necrotizing pneumonia (Figure 1B). In the aerobic culture of the broncho-aspirate sample, Lautropia mirabillis (oral cavity commensal previously isolated from children with HIV) was isolated, while Prevotella oris was identified by 16S rRNA gene sequencing of bronchial brushing and bronchoalveolar lavage samples with an homology percentage of 99.50% and 99.82%, respectively. The second sequence was registered in GenBank with accession number OP601405. After switching to IV 1 g/8 h cefotaxime and 260 mg/6 h vancomycin, the patient improved until resolution of the infection 18 days after admission (Figure 1C).
Case 2
A 13-year-old patient with a history of Glanzmann’s thrombasthenia and allergic asthma came to the emergency department for left flank pain radiating to the shoulder and respiratory distress. Examination showed hypoventilation in the left lung base and blood tests showed a C-reactive protein of 180 mg/L, 25,340 leukocytes/µL with 22,280 neutrophils/µL. Chest X-ray (Figure 2A) showed condensation in the left lung base, being admitted with IV antibiotic treatment with 2 g/6 h of ampicillin. The patient had daily febrile fever for 5 days until reaching a fever of 39ºC, at which time lung ultrasound was performed (Figure 2B) and the presence of bilateral pleural effusion was confirmed. The pleural effusion was drained by evacuating thoracentesis and sent to the laboratory for micro-biological studies and cultures. The treatment was changed to IV 2 g/8 h of cefotaxime, 1 g/8 h of vancomycin and 500 mg/8 h of clindamycin.
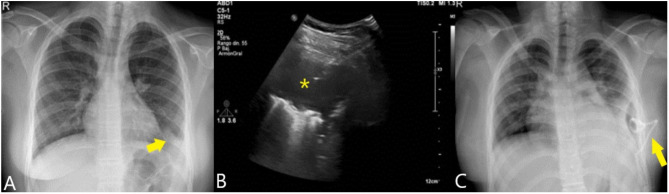
Figure 2.
Chest X-ray (A) showing a condensation in the left pulmonary base (arrow). Ultrasound (B) showed presence of empyema (asterisk), which was drained with a trocar (arrow). The control radiograph (C) shows the placement of the drainage catheter (arrow) achieving complete resolution of the picture.
The fluid was compatible with empyema presenting 396,000 leukocytes/µL with 74% neutrophils, LDH of 5,447 U/L and glucose of 0 mg/dL. In microbiological cultures there was no growth after 5 days, being performed sequencing of the 16S rRNA gene with P. oris with a homology percentage of 98.94%. The sequence was registered in GenBank with accession number OP601436. After 5 more days of treatment the patient was discharged with good evolution (Figure 2C).
The occurrence of oral commensals as a cause of pulmonary infections is invariably related to aspiration of food or other secretions, which together with dental procedures constitute the most likely cause of these infections. In addition, poor oral hygiene or periodontitis increases the growth of mouth commensals such as P. oris, favoring severe opportunistic infections to occur [2]. Previously, P. oris has been associated with clinical pictures such as abscesses or bacteremia, among others [3,4]. Although rare, it has also been described causing pleuropulmonary infections in the form of pneumonia and pleural empyema in both adult and pediatric populations [2,5-8]. Both the article by Viswanath LS et al. and Cobo F et al. describe cases in which P. oris are an unequivocal cause of pleural pathology. These cases and the ones presented here show that P. oris should be taken into account in the etiology of pleuropulmonary infections after bronchoaspiration or when there is a history of previous birth procedures with consequent antibiotic coverage.
In terms of diagnosis, the MALDI-TOF mass spectrometer is a rapid tool of great help for the identification of numerous species, however, in the case of Prevotella spp. there may be discrepancies. This idea is stated in both the articles by Wybo I et al. and Gürsoy M et al., in which they collect statistics concerning the percentage of strains identified by MALDI-TOF MS at the genus and species level of Prevotella spp. [9, 10]. In the first article, out of 102 isolates, the genus was identified in 73.5% and the species in 62.7%, while in the second one, out of 123 isolates, the genus was identified in 100% and the species in 88%. Although MALDI-TOF MS is of great help, the identification should be confirmed by another more accurate technique such as 16S rRNA gene sequencing and expand the MALDI-TOF database to increase the accuracy of identification as stated in the articles of Wybo I et al and Gürsoy M et al. Furthermore, in our experience, we believe that confirmation is necessary in order to avoid misidentifications of some species of Prevotella. In fact, in one case, our Bruker® MALDI-TOF MS identified the isolate as Prevotella bivia with a value greater than 2, while 16S rRNA gene sequencing identified it as P. oris with a homology percentage greater than 99% (with a reference strain studied through complete genomic sequence).
In terms of treatment, control of the focus by draining the collections associated with antibiotics would be of choice [5-8]. In recent years, antimicrobial resistance is increasing among anaerobic anaerobic bacteria worldwide. Prevotella spp. have traditionally been considered susceptible to penicillin, but an increasing rate of resistance to this drug has been lately documented [11-13]. Prevotella spp. are generally susceptible to beta-lactams associated with beta-lactamase inhibitor, metronidazole, tigecycline or cefoxitin. Some strains could be resistant to clindamycin, tetracyclines or moxifloxacin as can be seen, for example, in the multicenter study by Ulger Toprak N et al [13]. On the other hand, metronidazole has been reported as resistant in a case of bacteremia caused by Prevotella spp. although this is exceptional, retaining good activity against most strains [14]. Therefore, empirical treatment with penicillin should not be recommended in infections caused by species of the genus Prevotella, whereas beta-lactams associated with beta-lactamase inhibitors, carbapenems or anaerobicides such as clindamycin or metronidazole could be used. In our cases, it was not possible to study the susceptibility of the strains as they were identified only by sequencing of the 16S rRNA gene. This may be due to the fact that the patients had received intravenous antibiotics in the days prior to sampling.
FUNDING
None to declare.
CONFLICT OF INTEREST
Authors declare no have conflict of interest.
References
- 1.Holdeman LV, Moore WEC, Churn PJ, Johnson JL. Bacteroides oris and Bacteroides buccae New Species from Human Periodontitis and Other Human Infections. Int J Syst Evol Microbiol . 1982; 32, 125-131. doi: 10.1099/00207713-32-1-125. [DOI] [Google Scholar]
- 2.Brook I. Anaerobic pulmonary infections in children. Pediatr Emerg Care. 2004;20:636-40. doi: 10.1097/01.pec.0000139751.63624.0b. [DOI] [PubMed] [Google Scholar]
- 3.Frat, J. P.; Godet, C.; Grollier, G.; Blanc, J. L.; Robert, R.. Cervical spinal epidural abscess and meningitis due to Prevotella oris and Peptostreptococcus micros after retropharyngeal surgery. Intensive Care Med 2004, 30, 1695. doi: 10.1007/s00134-004-2265-x. [DOI] [PubMed] [Google Scholar]
- 4.Cobo F, Pérez-Carrasco V, Pérez-Rosillo MÁ, García-Salcedo JA, Navarro-Marí JM. Bacteremia due to Prevotella oris of probable hepatic origin. Anaerobe. 2022;76:102586. doi: 10.1016/j.anaerobe.2022.102586. [DOI] [PubMed] [Google Scholar]
- 5.Civen R, Jousimies-Somer H, Marina M, Borenstein L, Shah H, Finegold SM. A retrospective review of cases of anaerobic empyema and update of bacteriology. Clin Infect Dis. 1995. Jun;20 Suppl 2:S224-9. doi: 10.1093/clinids/20.supplement_2.s224. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett JG. Anaerobic bacterial infections of the lung and pleural space. Clin Infect Dis. 1993;16 Suppl 4:S248-55. doi: 10.1093/clinids/16.supplement_4.s248. [DOI] [PubMed] [Google Scholar]
- 7.Viswanath LS, Gunalan A, Jamir I, S B, S A, K A, Biswas R. Prevotella oris: A lesser known etiological agent of pleural effusion. Anaerobe. 2022;78:102644. doi: 10.1016/j.anaerobe.2022.102644. [DOI] [PubMed] [Google Scholar]
- 8.Cobo F, Lara-Oya A, Correa I, Rodríguez-Guerrero E, Pérez-Carrasco V, García-Salcedo JA, Navarro-Marí JM. Two rare cases of pleural infection due to Prevotella species. Rev Esp Quimioter 2022; 35(5):503-505 doi: 10.37201/req/046.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wybo I, Soetens O, De Bel A, Echahidi F, Vancutsem E, Vandoorslaer K, Piérard D. Species identification of clinical Prevotella isolates by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2012;50:1415-8. doi: 10.1128/JCM.06326-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gürsoy M, Harju I, Matomäki J, Bryk A, Könönen E. Performance of MALDI-TOF MS for identification of oral Prevotella species. Anaerobe. 2017;47:89-93. doi: 10.1016/j.anaerobe.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Jeverica, S.; Kolenc, U.; Mueller-Premru, M.; Papst, L. Evaluation of the routine antimicrobial susceptibility testing results of clinically significant anaerobic bacteria in a Slovenian tertiary-care hospital in 2015. Anaerobe 2017, 47, 64-69. DOI: 10.1016/j.anaerobe.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Boyanova L, Kolarov R, Gergova G, Dimitrova L, Mitov I. Trends in antibiotic resistance in Prevotella species from patients of the University Hospital of Maxillofacial Surgery, Sofia, Bulgaria, in 2003-2009. Anaerobe. 2010;16:489-92. doi: 10.1016/j.anaerobe.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Ulger Toprak, N.; Velooy, A. C. M.; Urbán, E.; Wybo, I.; Justesen, U. S.; Jean-Pierre, H. l. n.; Morris, T.; Akgul, O.; Kulekci, G.; Soyletir, G.; et al. A multicenter survey of antimicrobial susceptibility of Prevotella species as determined by E-test methodology. Anaerobe. 2018;52, 9-15. doi: 10.1016/j.anaerobe.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Mory, F.; Carlier, J. P.; Alauzet, C.; Thouvenin, M.; Schuhmacher, H.; Lozniewski, A.. Bacteremia caused by a metronidazole-resistant Prevotella spp. strain. J Clin Microbiol. 2005;43, 5380-5383. doi: 10.1128/JCM.43.10.5380-5383.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]