Abstract
The large majority of patients with HER2-positive metastatic breast cancer (MBC) will eventually develop resistance to anti-HER2 therapy and die of this disease. Despite, relatively high levels of stromal tumor infiltrating lymphocytes (sTILs), PD1-blockade has only shown modest responses. Monalizumab targets the inhibitory immune checkpoint NKG2A, thereby unleashing NK- and CD8 T cells. We hypothesized that monalizumab synergizes with trastuzumab by promoting antibody-dependent cell-mediated cytotoxicity.
In the phase II MIMOSA-trial, HER2-positive MBC patients were treated with trastuzumab and 750 mg monalizumab every two weeks. Following a Simon's two-stage design, 11 patients were included in stage I of the trial.
Treatment was well tolerated with no dose-limiting toxicities. No objective responses were observed. Therefore, the MIMOSA-trial did not meet its primary endpoint.
In summary, despite the strong preclinical rationale, the novel combination of monalizumab and trastuzumab does not induce objective responses in heavily pre-treated HER2-positive MBC patients.
Keywords: Breast cancer, Immunotherapy, Checkpoint blockade
Highlights
-
•
The MIMOSA trial investigated the novel combination of monalizumab and trastuzumab for HER2+ MBC.
-
•
The novel combination was well-tolerated, but no clinical responses were observed.
-
•
Combination strategies are warranted to induce a more inflamed tumor microenvironment.
-
•
Enrichment of inflamed HER2+ MBC with high TIL is likely to increase response to ICB.
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- BC
Breast cancer
- CD8 T cell
Cytotoxic T cell
- CR
Complete response
- CTCAE
Common terminology criteria for adverse events
- DLT
Dose limiting toxicity
- ER
Estrogen receptor
- HER2
Human epidermal receptor 2
- HLA-E
Human leukocyte antigen E
- ICI
Immune checkpoint inhibition
- IF
Immunofluorescence
- IHC
Immunohistochemistry
- LDH
Lactate dehydrogenase
- LVEF
Left ventricular ejection fraction
- MBC
Metastatic breast cancer
- NK cell
Natural killer cell
- NKG2A
Natural killer cell receptor group 2 A
- PD-1
Programmed death-1
- PD-L1
Programmed death ligand-1
- PR
Progesterone receptor
- PR
Partial response
- SCCHN
Squamous cell carcinoma of the head and neck
- SD
Stable disease
- T-DM1
Trastuzumab emtansine
- T-Dxd
Trastuzumab deruxtecan
- TILs
Tumor infiltrating lymphocytes
- TMB
Tumor mutational burden
- WHO-score
World Health Organization performance score
1. Background
Approximately 15–20% of all breast malignancies overexpress the human epidermal growth factor receptor 2 (HER2) [1]. This breast cancer subtype is known for its aggressive phenotype and patients used to have a poor survival [1]. The addition of anti-HER2 directed agents to standard therapies has greatly improved survival and is nowadays the foundation of treatment in the early and advanced setting. Currently, the treatment landscape for HER2-positive metastatic breast cancer (MBC) is rapidly expanding with antibody-drug conjugates (ADC) trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd), and tyrosine kinase inhibitor (TKI) tucatinib [[2], [3], [4]]. Nevertheless, once metastasized, patients will die of this disease, emphasizing the clinical need for novel therapeutic strategies [5].
One of the main mechanisms of action of trastuzumab is antibody-dependent cell-mediated cytotoxicity (ADCC), in which effector immune cells actively lyse target cells coated with antibodies [6]. Trastuzumab-mediated ADCC mainly involves natural killer (NK) cells, which recognize the Fc-portion of a trastuzumab antibody bound to the cell surface via the CD16 receptor [7]. Upon activation, NK cells will produce inflammatory cytokines thereby recruiting additional effector immune cells. Inhibition of effector immune cells, including NK cells and T cells might result in impaired ADCC contributing to trastuzumab resistance [8]. Unleashing T and especially NK cells could be a strategy to overcome trastuzumab resistance and enhance an endogenous anti-cancer immune response.
Immune checkpoint inhibition (ICI) has revolutionized treatment of various solid tumors by releasing the breaks on immune cells to induce anti-tumor immunity. HER2-positive breast cancer is considered an immunogenic breast cancer subtype with relatively high levels of stromal tumor infiltrating lymphocytes (sTILs) and a high tumor mutational burden (TMB), providing a rationale for immunotherapy [9,10]. However, results from clinical trials evaluating PD1-blockade in HER2-positive breast cancer have been disappointing. The phase I JAVELIN trial showed no responses upon treatment with avelumab (anti-PDL1) without anti-HER treatment, whereas responses in the phase II PANACEA and KATE2-trials with anti-PD1 combined with trastuzumab or T-DM1 respectively were limited to patients with PD-L1 positive tumors [[11], [12], [13]]. The combination of anti-PD1 and T-DM1 failed to improve progression free survival (PFS) compared to T-DM1 alone. The addition of anti-PD1 to neoadjuvant chemotherapy and dual HER2-blockade did not improve pathological complete response (pCR)-rates in patients with early-stage high-risk HER2-positive breast cancer [14]. Novel approaches to enhance the anti-cancer immune response in HER2-positive breast cancer are thus highly needed.
The inhibitory checkpoint NKG2A is expressed on NK cells and a subset of CD8 T cells and interacts with the non-classical HLA-molecule HLA-E upon tumor cells [15]. HLA-E is expressed on approximately 50% of the primary breast tumors and its expression is preserved throughout disease progression [16,17]. The monoclonal antibody monalizumab binds to NKG2A, thereby blocking the NKG2A-HLA-E axis and unleashing NK and CD8 T cells. When combined with tumor-specific monoclonal antibody cetuximab (anti-EGFR), it was shown that monalizumab enhanced the ADCC-capacity of cetuximab [18]. Moreover, monalizumab induced clinical activity in a phase I trial in patients with head and neck cancer when combined with cetuximab [18].
We hypothesize that combining monalizumab with trastuzumab enhances ADCC and can promote anti-tumor immunity by unleashing NK as well as CD8 T cells, thereby inducing clinical activity. In the MIMOSA trial, we investigated efficacy and safety of this novel combination for patients with HER2-positive MBC.
2. Methods
2.1. Trial design
The MIMOSA-trial was a single-center phase II trial in which we evaluated efficacy and safety of 4 mg/kg trastuzumab (loading dose 6 mg/kg) and 750 mg monalizumab administered once every two weeks in patients with HER2-positive MBC. Clinical efficacy was assessed following a Simons's-two stage design. Primary endpoint was ORR (defined as complete response CR or partial response PR) according to RECIST1.1. Secondary endpoints included clinical benefit (defined as CR, PR or stable disease (SD) for at least six months), progression-free survival according to RECIST1.1, overall survival and safety.
Key eligibility criteria included patients with HER2-positive MBC, age 18 years or older, World Health Organization (WHO)- performance status score of 0–1, progressive disease during previous trastuzumab-based therapy, 1–3 prior lines of palliative chemotherapy, left ventricular ejection fraction (LVEF) at least 50%, LDH serum levels < 2x upper limit of normal, no symptomatic brain metastases, no chronic infections or auto-immune disease requiring corticosteroids and no prior treatment with ICI. In addition, a metastatic lesion had to be accessible for a biopsy on which HER2-positive breast cancer was histologically confirmed, defined as 3+ on immunohistochemistry, or 2+ with gene amplification determined by in-situ hybridization. The trial protocol, informed consent form and amendments were approved by the medical-ethical committee of the Netherlands Cancer Institute. The trial protocol was written during ECCO-AACR-ESMO-EORTC course ‘Methods in Clinical Cancer Research 2019’. All patients provided written informed consent prior to study procedures and the trial was conducted in accordance with the declaration of Helsinki and following the standards of Good Clinical Practice.
2.2. Assessments
At baseline, after one cycle and after four cycles of treatment, a biopsy of a metastatic lesion was obtained and additional blood was taken for translational research purposes. Preferably, biopsies were taken from the same lesion at all time points. A biopsy was considered to be of good quality if the biopsy contained at least 100 tumor cells. Estrogen receptor (ER)-, progesterone receptor (PR)- and HER2-expression as well as levels of sTILs were determined on the baseline biopsy. The percentage of sTILs was assessed on an H&E slide by trained pathologists and scored according to the International Immuno-Oncology Biomarker Working Group guideline (tilsinbreastcancer.org).
2.3. Immunohistochemistry (IHC) and immunofluorescence (IF)
ER-, PR- and HER2-expression was assessed on formalin-fixed paraffin embedded tissue biopsies. ER-positivity was defined as an ER expression of at least 10%. HER2-positivity was defined as 3+ on IHC or 2+ on IHC with SISH gene amplification.
2.4. Safety
Safety was assessed using the Pocock-type boundary rules for continuous monitoring of toxicity throughout the trial. Sequential boundaries were used to monitor the rate of dose-limiting toxicities (DLTs). In case of excessive numbers of DLTs, accrual was stopped. Adverse events were graded via CTCAE version 5.0. Safety analyses were performed in all patients that received at least one cycle of trastuzumab and monalizumab.
2.5. Statistical considerations
In patients progressing on prior trastuzumab-based therapy, the expected response rate with trastuzumab monotherapy was expected to be 0%. The null hypothesis was defined as a response rate of 10% in patients treated with the combination of trastuzumab and monalizumab. The null hypothesis of a true response rate of 10% was tested against a one-sided alternative of 35% with a type I error of 0.1 and power of 0.9. In the first stage of the trial, eleven patients were included. If two or more responders were observed, accrual of another eight patients was allowed in stage II. The null hypothesis was rejected if four or more responses were observed in nineteen patients. The trial was originally designed with one cohort for patients with sTILS <5% and one cohort for patients with sTILS ≥5%. Since the first seven enrolled patients had sTILs below 5%, it was decided to merge the cohorts for a total of n = 11 patients.
Assessment of baseline characterterics and efficacy analyses were performed in the per protocol population, which included all patients who had received at least 1 cycle of treatment and were evaluable for response.
3. Results
3.1. Baseline characteristics
From January 2021 until April 2022, 15 patients were screened, of which 11 patients were eligible for response evaluation (Table 1). Screening failures were due to the absence of HER2-overexpression or gene amplification in the baseline biopsy (N = 3), and absence of measurable disease according to RECIST1.1 (N = 1). Patients had received a median of two lines of prior palliative chemotherapy with an anti-HER2 agent, or the antibody-drug conjugate (ADC) trastuzumab-emtansine (T-DM1) for MBC. 82% of the patients had hormone receptor-positive BC. The majority had visceral metastases. Median level of sTILs observed was 5%, ranging from 1% to 20%, with 9 patients having sTILs below 5%. 2 patients had sTILs-levels over 5%, respectively 8 and 20%.
Table 1.
Baseline characteristics of evaluable patients in MIMOSA trial.
| Total N=11 | |
|---|---|
| Age, median (range) | 55,1y, (44–62) |
| WHO performance status, no. (%) | |
| 0 | 10 (91) |
| 1 | 1 [9] |
| Germline BRCA1/2, no. (%) | |
| Pathogenic variant | 1 [9] |
| Wildtype | 2 [18] |
| Unknown | 8 (73) |
| Hormone receptor status, no. (%) | |
| ER+a | 9 (82) |
| ER- | 2 [18] |
| TIL-score, median (range) | 1% [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]] |
| LDH at baseline, no. | |
| ≤1x ULN | 7 (64) |
| ≤2x ULN | 4 (36) |
| Disease-free interval | |
| De novo metastatic disease | 4 (36) |
| DFS ≥12 months | 7 (64) |
| No. of metastatic sites, no. (%) | |
| 1-2 | 3(27) |
| ≥3 | 8 (73) |
| Location of metastasis, no. (%) | |
| Lymph node only | 0 (0) |
| Visceral metastasis | 9 (82) |
| Other metastatic sitesb | 4 (36) |
| No. of previous lines of therapy for MBCc, no. (%) | |
| 1 | 3 [27] |
| 2-3 | 8 (73) |
| Previous anti-HER2 therapy, no. (%) | |
| Trastuzumab | 11 (100) |
| Pertuzumab | 9 (82) |
| TDM-1 | 6 (55) |
| Lapatinib | 1 [9] |
Abbreviations: WHO performance status: World Health Organization performance status; LVEF: left ventricular ejection fraction; BRCA: Breast Cancer-1 or -2 gene; ER: estrogen receptor, TIL: tumor-infiltrating lymphocytes; LDH: lactate-dehydrogenase; MBC: metastatic breast cancer; TDM-1: trastuzumab emtansine.
ER-positivity is defined as ER-expression of ≥10%.
Other metastatic sites include bone, brain, skin and subcutaneous tissue.
Prior lines of chemotherapy with/without anti-HER2 agent and/or ADCs.
3.2. Efficacy
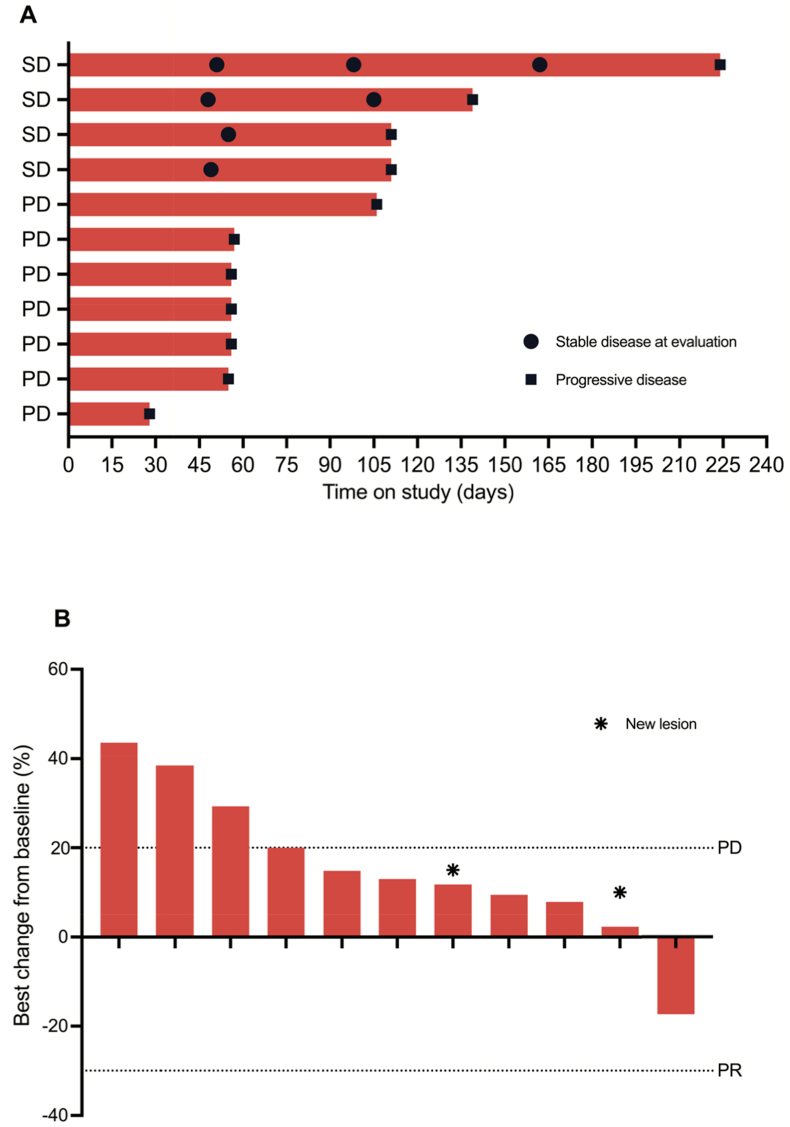
Median duration of treatment from administration of first cycle until progressive disease was 57 days, ranging from 28 to 224 days on study. Patients received a median of four cycles of trastuzumab and monalizumab (Fig. 1, panel A). No objective responses (CR, PR) were observed (Fig. 1, panel B). Four patients showed stable disease, however, only one patient had clinical benefit with SD for >6 months. Seven patients had progressive disease at first evaluation. The primary endpoint of the MIMOSA trial was not met and continuation of the trial into stage II was not supported.
Fig. 1.
Anti-tumor activity of trastuzumab and monalizumab in the per protocol population
Panel A: Swimmersplot. Duration of response according to RECIST1.1. Two patients showed stable disease at first evaluation, two patients showed stable disease at both first and second evaluation. All patients are off-study (data cut-off 19-01-2023). Panel B: Waterfall plot. Best change (%) over the course of treatment of the sum of the diameters of target lesion(s) as compared to baseline. Radiological assessment of target lesions, defined by RECIST1.1 criteria.
3.3. Safety
Treatment was well tolerated with no dose-limiting toxicities observed during the course of treatment in the safety population which involves the twelve patients who received at least 1 cycle of treatment. 55% of the patients experienced grade 1 or 2 adverse events, of which five patients experienced monalizumab-related adverse events (Table 2). One patient was admitted with progressive dyspnea within three months after the last cycle of trastuzumab and monalizumab. CT-scan showed multiple pulmonary embolisms and significant increase in pleural fluid. In addition, ground glass opacities that can either indicate immune-mediated pneumonitis or a viral infection, however, no adequate COVID19-testing was performed. Because of the lack of therapeutic options for her MBC best supportive care was initiated including steroids and unfractionated heparin. Her condition deterioted rapidly and she died a couple of days later.
Table 2.
Safety summary; No of patients. No grade 3–4 AE observed. One case of pneumonitis, in which an immune-mediated pneumonitis could not be ruled out.
| Any toxicitiy |
Monalizumb-related AE |
|||
|---|---|---|---|---|
| Grade 1-2 | Grade ≥3 | Grade 1-2 | Grade ≥3 | |
| Diarrhea | 2 (14%) | 2 (14%) | ||
| Fatigue | 2 (14%) | 2 (14%) | ||
| Allergic reaction | 1 (7%) | 1 (7%) | ||
| Bladder infection | 1 (7%) | |||
| Dyspnea | 1 (7%) | |||
| Headache | 1 (7%) | 1 (7%) | ||
| Infections and infestations, other | 1 (7%) | |||
| Nausea | 1 (7%) | 1 (7%) | ||
| Peripheral motor neuropathy | 1 (7%) | |||
| Pneumonitis | 1 (7%) | 1 (7%) | 1 (7%) | 1 (7%) |
| Soft tissue infection | 1 (7%) | |||
| Weight loss | 1 (7%) | 1 (7%) | ||
4. Discussion
In the phase II MIMOSA trial, efficacy and safety of the combination of trastuzumab with monalizumab, an immune checkpoint inhibitor targeting NKG2A on CD8+ T cells and NK cells, was evaluated for patients with HER2-positive MBC. This novel combination was found to be well-tolerated, but did not induce objective responses.
Previous clinical trials investigating the efficacy of T cell based ICI for HER2-positive breast cancer also showed limited efficacy. In the advanced disease setting, no responders were observed upon anti-PD1 monotherapy, whereas anti-PD1 combined with an anti-HER2 agent resulted in responses predominantly in patients with PD-L1 positive disease with an ORR of 15% in the PANACEA trial [[11], [12], [13]]. ORR in the KATE2-trial was 54%, but no improvement in PFS was observed. The low response rates in unselected patient populations can partly be explained by characteristics favoring immune escape in advanced disease stages including a less inflamed TME with low levels of sTILs [17]. Also in the early-stage setting, the addition of atezolizumab to dual HER2-blockade and chemotherapy for early stage HER2-positive breast cancer in the IMpassion050-trial failed to improve pCR-rates [14]. However, event free survival (EFS)-data are still awaiting and might still show benefit despite lack of significant improvement in pCR-rates, similar as seen with durvalumab for early TNBC in the GeparNuevo-trial [19]. Together, clinical data on ICI for HER2-positive breast cancer suggests that more complex immune interactions underly the immune escape mechanism requiring alternative immunomodulatory approaches.
The lack of responders in the MIMOSA-trial could be due to the enrollment of patients with unfavorable immunological characteristics. Most patients were heavily pre-treated, had hormone receptor (HR) positive disease and low levels of sTILs. Although HER2-positive breast cancer is considered an immunogenic breast cancer subtype, it is also known for its biological heterogeneity [20]. Hormone receptor positivity is thought to be one of the drivers of heterogeneity associated with reduced immune activity [21]. Additionally, the level of immune cell infiltrate diminishes towards more advanced disease and after multiple lines of treatment [22]. The CLEOPATRA-trial was the first to provide insight in the immune cell infiltrate in advanced HER2-positive breast cancer with a median of 10% sTILs observed in freshly obtained metastatic samples [22], contrasting our observation of a median of 1% sTILs. Of note, patients in the CLEOPATRA-trial had received no prior treatment for advanced disease and only 48% of the patients had HR-positive breast cancer. However, in the pretreated patient population of the PANACEA-trial, a median of 1.5% sTILs was observed, in line with our observations [11]. Additional evaluation of TILs is necessary to assess the predictive and prognostic value of TILs in advanced HER2+ BC and optimize its clinical utility [23].
NK cells gained renewed interest as target for immunotherapeutic strategies since these immune cells present some preferred immunogenic properties over T cells. In particular, NK cells stimulate crosstalk between the innate and adaptive immune system upon activation via the production of inflammatory cytokines and cytotoxicity of NK cells does not rely on antigen recognition [24,25]. Our data and data by others suggest that inhibition of the NKG2A-HLA-E axis to induce anti-tumor immunity and to prevent immune escape is tumor-type dependent [18,[26], [27], [28], [29]]. Although the capacity of monalizumab to enhance ADCC via cetuximab showed promising results in a phase I trial in patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN), the phase III trial failed to confirm the clinical activity of this combination resulting in early withdrawal of the trial [18,28,29]. The combination of durvalumab with monalizumab for patients with stage III unresectable or metastatic NSCLC showed an ORR of 35.5% in the phase II COAST trial [30]. Also, the triplet durvalumab, monalizumab and cetuximab as first line treatment for patients with R/M SCCHN resulted in an ORR of 32.5% [31]. These data suggest that monalizumab might be more effective while combined with ICIs blocking other inhibitory pathways rather than stimulation of ADCC only.
Novel immunomodulatory strategies capable of inducing more robust immune responses are warranted for HER2-positive MBC. HER2 is an attractive tumor-associated antigen for immune modulatory approaches. Chimeric antigen receptor (CAR) T cells can induce anti-tumor immunity in mouse models, however, clinical data is still lacking due to high costs and potential occurrence of serious off-target effects in healthy tissue [32]. In addition, bispecific antibodies simultaneously bind to CD3 on T cells and HER2 thereby bridging immune cells and tumor cells, which have shown anti-tumor activity in preclinical models, but induces toxicity in healthy tissues expressing HER2 [33]. The novel bispecific antibody CD3 x p95HER2 circumvents toxicity in healthy tissues, since p95HER2 is not expressed in healthy tissues but in approximately 40% of HER2-positive breast tumors [34]. Despite successes in preclinical models, clinical data regarding novel immune modulatory approaches for HER2-positive breast cancer is still limited.
The MIMOSA-trial has several limitations. First, althought a Simon's two-stage design prevents the exposure of many patients to a potentially ineffective therapy, consequently, only a small patient population was included. Second, the majority of the included patients had unfavorable characteristics concerning immunogenicity illustrated by a median TIL levels of 1%. Third, although sTILs were assessed at baseline, tissue sections were not stained for NKG2A + cells or HLA-E expression on tumor cells. However, the association between presence of NK cells and response to monalizumab has not yet been established.
In summary, despite ongoing developments in the treatment landscape of HER2-positive breast cancer patients, optimal treatment after progression on dual anti-HER2 therapy, TKIs and ADCs in the advanced setting is not yet established. Identification of patients with highly immunogenic breast tumors is essential to select patients that might benefit from ICI. For patients with a less immunogenic tumor and limited pre-existing immunity, alternative immunomodulatory strategies are required to induce a more favorable TME. Moreover, biomarkers to discriminate upfront between high and less inflamed tumors are key to increase response rates to immunotherapy for patients with HER2-positive MBC.
Declarations
Ethics approval and consent to participate: All patients provided written informed consent prior to study procedures and the trial was conducted in accordance with the declaration of Helsinki and followed the standards of Good Clinical Practice.
Contributors: Conception and design: LV, MD, IM, MH, WS, JH, GS, MK. Screening & enrollment of patients: VG, MD, IK, GS, MK. Pathological assessments: RS, KV and HH. Statistical analyses: SB. VG & MK wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by funding from AstraZeneca.
Declaration of competing interest
VCM Geurts, L Voorwerk, S Balduzzi, I Kemper, IAM Mandjes, M Heuver-Mes: no disclosures.
MGJ van Dongen: advisory role for Relay Therapeutics.
R Salgado: Research support from: Merck, Puma Biotechnology and Roche. Non-financial support from Merck and BMS. Advisory roles for: BMS, Exact Sciences and Roche outside the submitted work.
K van de Vijver: Advisory roles for/consultancy fee paid to the institute: AstraZeneca, Exact Sciences, GSK, outside the submitted work.
W Sparreboom: employment: AstraZeneca.
JBAG Haanen: advisory roles for Achilles Tx, BioNTech, BMS, GSK, Iovance Bio, Instil Bio, Ipsen, MSD, Molecular Partners, Novartis, Neogene Tx, Pfizer, Roche, Sanofie, Scenic, T-Knife. Received grant support from Amgen, BioNTech, BMS, MSD, Novartis and has stock options in Neogene Tx.
GS Sonke: Received funding paid to the institute from: Agendia, AstraZeneca, Merck, Novartis and Roche. Advisory roles for: Biovica, Seagen outside the submitted work.
HM Horlings: consultancy fee paid to institute: Roche, and is advisor for SlideScore and Ellogon, outside the submitted work.
M Kok: Received funding paid to the institute from BMS, Roche, AstraZeneca. Advisory roles for: Daiichi Sankyo, BMS, MSD, Roche outside the submitted work. Speakers’ fee from: Roche, BMS and Gilead.
Handling Editor: Giuseppe Curigliano
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2023.06.007.
Contributor Information
V.C.M. Geurts, Email: V.Geurts@nki.nl.
L. Voorwerk, Email: L.Voorwerk@nki.nl.
S. Balduzzi, Email: S.Balduzzi@nki.nl.
R. Salgado, Email: Roberto@salgado.be.
K. Van de Vijver, Email: koen.vandevijver@uzgent.nl.
M.G.J. van Dongen, Email: mg.v.dongen@nki.nl.
I. Kemper, Email: I.Kemper@nki.nl.
I.A.M. Mandjes, Email: I.Mandjes@nki.nl.
M. Heuver, Email: M.Mes@nki.nl.
W. Sparreboom, Email: wouter.sparreboom@astrazeneca.com.
J.B.A.G. Haanen, Email: J.Haanen@nki.nl.
G.S. Sonke, Email: G.Sonke@nki.nl.
H.M. Horlings, Email: H.Horlings@nki.nl.
M. Kok, Email: M.Kok@nki.nl.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Loibl S., Gianni L. HER2-positive breast cancer. Lancet. 2017;389(10087):2415–2429. doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 2.Cortés J., Kim S., Chung W., Im S., Park Y., Hegg R., et al. LBA1 Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (Pts) with HER2+ metastatic breast cancer (mBC): results of the randomized phase III DESTINY-Breast03 study. Ann Oncol. 2021;32:S1287–S1288. [Google Scholar]
- 3.Cameron D., Casey M., Oliva C., Newstat B., Imwalle B., Geyer C.E. Lapatinib plus capecitabine in women with HER-2–positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncol. 2010;15(9):924–934. doi: 10.1634/theoncologist.2009-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murthy R.K., Loi S., Okines A., Paplomata E., Hamilton E., Hurvitz S.A., et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 5.Yardley D., Tripathy D., Brufsky A., Rugo H., Kaufman P., Mayer M., et al. Long-term survivor characteristics in HER2-positive metastatic breast cancer from registHER. Br J Cancer. 2014;110(11):2756–2764. doi: 10.1038/bjc.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchini G., Gianni L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014;15(2):e58–e68. doi: 10.1016/S1470-2045(13)70477-7. [DOI] [PubMed] [Google Scholar]
- 7.Park S., Jiang Z., Mortenson E.D., Deng L., Radkevich-Brown O., Yang X., et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18(2):160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida R., Tazawa H., Hashimoto Y., Yano S., Onishi T., Sasaki T., et al. Mechanism of resistance to trastuzumab and molecular sensitization via ADCC activation by exogenous expression of HER2-extracellular domain in human cancer cells. Cancer Immunology. Immunotherapy. 2012;61:1905–1916. doi: 10.1007/s00262-012-1249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denkert C., von Minckwitz G., Darb-Esfahani S., Lederer B., Heppner B.I., Weber K.E., et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 10.Koboldt D.C., Fulton R.S., McLellan M.D., Schmidt H., Kalicki-Veizer J., McMichael J.F., et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loi S., Giobbie-Hurder A., Gombos A., Bachelot T., Hui R., Curigliano G., et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b–2 trial. Lancet Oncol. 2019;20(3):371–382. doi: 10.1016/S1470-2045(18)30812-X. [DOI] [PubMed] [Google Scholar]
- 12.Dirix L.Y., Takacs I., Jerusalem G., Nikolinakos P., Arkenau H.-T., Forero-Torres A., et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018;167(3):671–686. doi: 10.1007/s10549-017-4537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emens L.A., Esteva F.J., Beresford M., Saura C., De Laurentiis M., Kim S.-B., et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020;21(10):1283–1295. doi: 10.1016/S1470-2045(20)30465-4. [DOI] [PubMed] [Google Scholar]
- 14.Huober J., Barrios C.H., Niikura N., Jarząb M., Chang Y.-C., Huggins-Puhalla S.L., et al. Atezolizumab with neoadjuvant anti–human epidermal growth factor receptor 2 therapy and chemotherapy in human epidermal growth factor receptor 2–positive early breast cancer: primary results of the randomized phase III IMpassion050 trial. J Clin Oncol. 2022;40(25):2946–2956. doi: 10.1200/JCO.21.02772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borst L., van der Burg S.H., van Hall T. The NKG2A–HLA-E Axis as a novel checkpoint in the tumor MicroenvironmentNKG2A as a novel checkpoint in the tumor microenvironment. Clin Cancer Res. 2020;26(21):5549–5556. doi: 10.1158/1078-0432.CCR-19-2095. [DOI] [PubMed] [Google Scholar]
- 16.de Kruijf E.M., Sajet A., van Nes J.G., Natanov R., Putter H., Smit V.T., et al. HLA-E and HLA-G expression in classical HLA class I-negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J Immunol. 2010;185(12):7452–7459. doi: 10.4049/jimmunol.1002629. [DOI] [PubMed] [Google Scholar]
- 17.Szekely B., Bossuyt V., Li X., Wali V., Patwardhan G., Frederick C., et al. Immunological differences between primary and metastatic breast cancer. Ann Oncol. 2018;29(11):2232–2239. doi: 10.1093/annonc/mdy399. [DOI] [PubMed] [Google Scholar]
- 18.André P., Denis C., Soulas C., Bourbon-Caillet C., Lopez J., Arnoux T., et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175(7):1731. doi: 10.1016/j.cell.2018.10.014. 43. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loibl S., Schneeweiss A., Huober J., Braun M., Rey J., Blohmer J.-U., et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann Oncol. 2022;33(11):1149–1158. doi: 10.1016/j.annonc.2022.07.1940. [DOI] [PubMed] [Google Scholar]
- 20.Griguolo G., Pascual T., Dieci M.V., Guarneri V., Prat A. Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. Journal for immunotherapy of cancer. 2019;7(1):1–14. doi: 10.1186/s40425-019-0548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung Y.R., Kim H.J., Jang M.H., Park S.Y. Prognostic value of tumor infiltrating lymphocyte subsets in breast cancer depends on hormone receptor status. Breast Cancer Res Treat. 2017;161(3):409–420. doi: 10.1007/s10549-016-4072-9. [DOI] [PubMed] [Google Scholar]
- 22.Luen S.J., Salgado R., Fox S., Savas P., Eng-Wong J., Clark E., et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol. 2017;18(1):52–62. doi: 10.1016/S1470-2045(16)30631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valenza C., Taurelli Salimbeni B., Santoro C., Trapani D., Antonarelli G., Curigliano G. Tumor infiltrating lymphocytes across breast cancer subtypes: current issues for biomarker assessment. Cancers. 2023;15(3):767. doi: 10.3390/cancers15030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guillerey C., Huntington N.D., Smyth M.J. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17(9):1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 25.Bald T., Krummel M.F., Smyth M.J., Barry K.C. The NK cell–cancer cycle: advances and new challenges in NK cell–based immunotherapies. Nat Immunol. 2020;21(8):835–847. doi: 10.1038/s41590-020-0728-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Montfoort N., Borst L., Korrer M.J., Sluijter M., Marijt K.A., Santegoets S.J., et al. NKG2A blockade potentiates CD8 T cell immunity induced by cancer vaccines. Cell. 2018;175(7):1744. doi: 10.1016/j.cell.2018.10.028. 55. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor B.C., Sun X., Balko J., Gonzalex-Ericsson P., Sanders M. SABCS; 2022. Implications of heterogeneity in breast tumor cell MHC-I expression on immunity and therapeutic resistance. [Google Scholar]
- 28.Cohen R.B., Bauman J.R., Salas S., Colevas A.D., Even C., Cupissol D., et al. American Society of Clinical Oncology; 2020. Combination of monalizumab and cetuximab in recurrent or metastatic head and neck cancer patients previously treated with platinum-based chemotherapy and PD-(L) 1 inhibitors. [Google Scholar]
- 29.Fayette J., Seiwert T., Ferris R.L., Harrington K., Haddad R., Tahara M., et al. Abstract CT236: INTERLINK-1: a phase 3, randomized, double-blind, placebo-controlled, multicenter, global study of monalizumab in combination with cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma previously treated with an immune checkpoint inhibitor. Cancer Res. 2022;82(12_Supplement):CT236–CT. [Google Scholar]
- 30.Herbst R.S., Majem M., Barlesi F., Carcereny E., Chu Q., Monnet I., et al. COAST: an open-label, phase II, multidrug platform study of durvalumab alone or in combination with oleclumab or monalizumab in patients with unresectable, stage III non–small-cell lung cancer. J Clin Oncol. 2022;40(29):3383–3393. doi: 10.1200/JCO.22.00227. [DOI] [PubMed] [Google Scholar]
- 31.Colevas D., Misiukiewicz K., Pearson A., Fayette J., Bauman J., Cupissol D., et al. 123MO Monalizumab, cetuximab and durvalumab in first-line treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): a phase II trial. Ann Oncol. 2021;32:S1432. [Google Scholar]
- 32.Tchou J., Zhao Y., Levine B.L., Zhang P.J., Davis M.M., Melenhorst J.J., et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancerc-met-CAR T cells for breast cancer. Cancer immunology research. 2017;5(12):1152–1161. doi: 10.1158/2326-6066.CIR-17-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staflin K., de Zafra C.L.Z., Schutt L.K., Clark V., Zhong F., Hristopoulos M., et al. Target arm affinities determine preclinical efficacy and safety of anti-HER2/CD3 bispecific antibody. JCI insight. 2020;5(7) doi: 10.1172/jci.insight.133757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rius Ruiz I., Vicario R., Morancho B., Morales C.B., Arenas E.J., Herter S., et al. p95HER2–T cell bispecific antibody for breast cancer treatment. Sci Transl Med. 2018;10(461) doi: 10.1126/scitranslmed.aat1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.