Abstract
Pulmonary sequestration is a rare congenital bronchopulmonary malformation with an estimated incidence of less than 6%. It is described as the abnormal formation of nonfunctional lung tissue that receives its blood supply from systemic circulation rather than the bronchial tree. Most are unilateral, while a miniscule proportion is bilateral. Delayed diagnosis can result in recurrent pneumonia, failure to thrive, regular hospital visits, morbidity and even fatality. Thus, it is important to raise awareness of this condition. Herein, we present a case of a 42-year-old patient with bilateral pulmonary sequestration (BPS) on a triple rule out CT angiography (TRO-CTA).
Keywords: Bilateral lung sequestration, Case report, Congenital abnormality, Radiology
Introduction
Pulmonary sequestration is a rare condition, accounting for 0.1%-6.4% of all congenital bronchopulmonary malformations [1]. This pathologic process was first identified by Huber in 1777 and the term “sequestration” was coined by Pryce in 1946 [2]. It consists of lung tissue that does not have an identifiable connection with the bronchial system and instead receives its arterial blood supply from anomalous systemic arteries such as the aorta or aortic branches [3]. Most sequestrations are unilateral, while a rare minority are bilateral [4]. Herein, we report a case of BPS on a TRO-CTA in a 42-year-old patient presenting with chest tightness and shortness of breath.
Case presentation
A 42-year-old male, with relevant past medical history of asthma and pneumonia, presented to the emergency department with complaints of chest tightness and shortness of breath. He reports taking antibiotics for pneumonia 8 days prior with no relief.
The physical examination revealed normal vital signs. Physical examinations, including respiratory and cardiovascular, were normal. Due to the chest discomfort, he underwent standard cardiac work-up which failed to demonstrate any specific abnormality. In particular, the EKG demonstrated nonspecific T wave inversions, chest X-Ray was negative for any acute pulmonary disease, and laboratory data was unremarkable, including negative serum troponin levels.
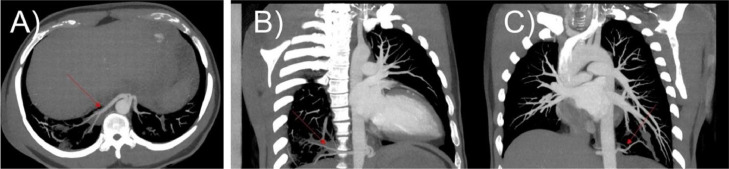
An EKG-gated TRO-CTA for simultaneous noninvasive evaluation of the coronary arteries, pulmonary arteries and thoracic aorta was then performed. In addition to showing normal coronary arteries, bilateral lower lobes sequestrations seen on prior chest CT from 2019 were redemonstrated (Fig. 1, Fig. 2). Now, small regions of mucous plugging within the sequestrations were visible, possibly related to new infection and pneumonia (Fig. 3). The overall TRO-CTA appearance of the abnormality was consistent with BPS showing right and left arterial supply arising from the descending thoracic aorta.
Fig. 1.
TRO-CTA examination. (A) Maximum intensity (MIP) image and (B) 3D Volume Rendering (VR) image showing 2 side-by-side arteries arising from the distal descending thoracic aorta. The vessel on the right supplies the right sequestration (long red arrow). The vessel on the left bifurcates close to its origin, giving a right branch supplying the right sequestration (short red arrow) and a left branch supplying the left sequestration (yellow arrow).
Fig. 2.
TRO CTA examination. Maximum intensity (MIP) images (Figure A - Axial, Figures B and C - Coronal obliques) showing the right (arrows - Figures A and B) and left (arrow - Figure C) systemic arterial supplies originating from the descending thoracic aorta.
Fig. 3.
Multiplanar (MPR) images (Figure A - Axial and Figure B - Coronal) using lung window showing lower attenuation of the bilateral sequestrations due to air trapping (Figures A and B - red arrows). Notice absence of clear demarcation between sequestrations and the rest of the lung parenchyma consistent with intralobar sequestrations. Notice a region of mucous plugging in the right sequestration (Figure B – yellow arrow).
An hour later, patient was re-evaluated and reexamined. Patient denied chest pain, shortness of breath, and palpitations at this time. No additional abnormalities were discovered following a comprehensive cardio-pulmonary work-up including physical examination and CTA. Patient was then discharged home.
It remains unclear whether there was a causal relationship between the findings of BPS and patient's acute asthmatic exacerbation, as his symptoms have spontaneously resolved. Therefore, it is possible that the discovery of bilateral pulmonary sequestrations was purely coincidental.
Discussion
Pulmonary sequestration is a rare congenital abnormality accounting for less than 6% of all reported congenital bronchopulmonary cases [1]. It consists of aberrant and nonfunctional lung tissue that is not connected with the normal bronchial system but rather fed by a systemic vessel, generally originating at the level of the thoracic aorta [3]. There are 2 subtypes of bronchopulmonary sequestrations: intralobar and extralobar. Intralobar bronchopulmonary sequestrations, such as the one presented in this case report, are invested within the visceral pleura of the normal lung [5]. Due to proximity, they may form abnormal connections to either the bronchi or gastrointestinal (GI) tract, increasing the risk of infections such as pneumonia. This may have contributed to the patient's bout of pneumonia a week prior. On the other hand, extralobar bronchopulmonary sequestrations are completely disconnected from the lung parenchyma by a pleural separation [5]. Therefore, anomalous connections with the bronchi or GI tract do not occur.
Although the mechanism of BPS remains unclear, the current literature suggests its origination in the early stages of embryogenesis [6]. Many internal organ capillaries are initially connected with the dorsal aorta around the archenteron and lung bud. During the fourth week of embryogenesis, they normally separate from the lung tissues by absorption. However, in patients with BPS, the internal capillary does not get absorbed entirely and thus, an abnormal aortic branch of blood vessels remains. This residual vasculature leads to the development of lung isolation due to the spreading apart of the embryonic lung tissue.
BPS can be asymptomatic or present with symptoms that range from neonatal heart failure secondary to left-right shunting to respiratory distress and recurrent infections [7]. Although typically asymptomatic at birth, BPS with a large systemic feeding vessel may lead to high out-put heart failure [8]. Other complications include fetal hydrothorax and hydrops [9]. Respiratory symptoms including persistent cough and expectoration after birth are uncommon, but when present, typically occur in conjunction with larger BPS lesions, hydrothorax, or hydrops. When misdiagnosed or undiagnosed, cases may present with recurrent infections and hemoptysis [10]. As mentioned earlier regarding the anatomy, interlobar BPS in particular carries an increased risk for infection. Other potential complications include failure to thrive, regular hospital visits, morbidity and even fatality.
The optimal management approach for BPS continues to be debated [8]. Asymptomatic patients can be observed continuously since most cases of BPS spontaneously regress [11]. Symptomatic cases are treated surgically [5]. This classically involves surgical resection of sequestrated segments [10]. Embolization and radiofrequency ablation of the feeding systemic artery have also been explored as 2 other potential surgical strategies [11,12].
Conclusion
Herein, we report a case of bilateral BPS in an adult patient. This case report is rare and unique due its bilateral nature. On TRO-CTA, the systemic arterial supply of both sequestrations originate in very close proximity to each other from the distal descending aorta. Moreover, one of the feeding arteries bifurcates, providing arterial supply to both sequestrations. In a retrospective study of 126 BPS cases, only 1 was bilateral, and in 2007, only 16 bilateral BPS cases were reported [4,6]. To the best of our knowledge, ours is the first report of a bilateral BPS showing a bifurcating feeding artery supplying both sequestrations.
Patient consent
A written informed consent for the publication of this case report was obtained from the patient.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Taylor JA, Laor T, Warner BW. Extralobar pulmonary sequestration. Surgery. 2008;143:833–834. doi: 10.1016/j.surg.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Pryce DM. Lower accessory pulmonary artery with intralobar sequestration of lung; a report of seven cases. J Pathol Bacteriol. 1946;58:457–467. doi: 10.1002/PATH.1700580316. [DOI] [PubMed] [Google Scholar]
- 3.Erden ES, Bayarogullari H, Bilgic H, Yetim T, Buyukkaya E. Bilateral pulmonary sequestration in the elderly adult. Multidiscip Respir Med. 2012;7:36. doi: 10.1186/2049-6958-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stern R, Berger S, Casaulta C, Raio L, Abderhalden S, Zachariou Z. Bilateral intralobar pulmonary sequestration in a newborn, case report and review of the literature on bilateral pulmonary sequestrations. J Pediatr Surg. 2007;42:E19–E23. doi: 10.1016/j.jpedsurg.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 5.Zobel M, Gologorsky R, Lee H, Vu L. Congenital lung lesions. Semin Pediatr Surg. 2019;28 doi: 10.1053/J.SEMPEDSURG.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Hong C, Yu G, Tang J, Liu QL, Xia B. Risk analysis and outcomes of bronchopulmonary sequestrations. Pediatr Surg Int. 2017;33:971–975. doi: 10.1007/S00383-017-4097-0/TABLES/2. [DOI] [PubMed] [Google Scholar]
- 7.Yoon HM, Kim EAR, Chung SH, Kim SO, Jung AY, Cho YA, et al. Extralobar pulmonary sequestration in neonates: the natural course and predictive factors associated with spontaneous regression. Eur Radiol. 2017;27:2489–2496. doi: 10.1007/S00330-016-4594-X/FIGURES/5. [DOI] [PubMed] [Google Scholar]
- 8.Hall NJ, Stanton MP. Long-term outcomes of congenital lung malformations. Semin Pediatr Surg. 2017;26:311–316. doi: 10.1053/J.SEMPEDSURG.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Riley JS, Urwin JW, Oliver ER, Coleman BG, Khalek N, Moldenhauer JS, et al. Prenatal growth characteristics and pre/postnatal management of bronchopulmonary sequestrations. J Pediatr Surg. 2018;53:265. doi: 10.1016/J.JPEDSURG.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shafiq M, Ali A, Dawar U, Setty N. Rare cause of haemoptysis: bronchopulmonary sequestration. BMJ Case Rep. 2021;14 doi: 10.1136/BCR-2020-239140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curros F, Chigot V, Emond S, Sayegh N, Revillon Y, Scheinmann P, et al. Role of embolization in the treatment of bronchopulmonary sequestration. Pediatr Radiol. 2000;30:769–773. doi: 10.1007/S002470000332. [DOI] [PubMed] [Google Scholar]
- 12.Dadhwal V, Sharma KA, Sahay N, Rana A, Chitkara A. Successful treatment of bronchopulmonary sequestration by radiofrequency ablation of feeding artery. Fetal Diagn Ther. 2022;49:502–505. doi: 10.1159/000528176. [DOI] [PubMed] [Google Scholar]