Abstract
Purpose
Purpose: Radiation therapy (RT) and the antibody-drug conjugate brentuximab vedotin (BV) are standard-of-care treatment options for patients with certain B and T-cell lymphomas; however, there are limited data exploring the safety of concurrent BV and RT (BVRT).
Methods and Materials
We performed a single institutional retrospective review of 44 patients who received BVRT.
Results
Twenty percent of patients (9/44) developed new grade 2 or higher (G2+) hematologic toxicity (HT) after BVRT, which was associated with radiation dose (median dose of 35 Gy in those with new G2+ HT compared with 15 Gy in those without; P < .001). Acute G2+ elevation in aspartate transaminase or alanine transaminase level was associated with administration of concurrent chemotherapy with BVRT (57% vs 21%; P = .047) but was not associated with any RT factors. Local control (LC) was achieved in 24 of 42 patients (57%) with available follow-up. Ten patients (23%) proceeded to stem cell transplant or cellular therapy after BVRT at a median of 48 days (interquartile range, 27-188 days). At last follow-up, 10 patients (23%) remained without evidence of disease.
Conclusions
Our analysis demonstrates that the combination of BV and RT is well tolerated, though care should be taken during RT planning to reduce the risk of HT. This combination can be considered for patients in need of both local and systemic disease control.
Introduction
Brentuximab vedotin (BV) is an antibody-drug conjugate with a chimeric antibody targeting CD30 conjugated to the antimicrotubule agent monomethyl auristatin E.1 BV is approved for the treatment of classical Hodgkin lymphoma (cHL), anaplastic large-cell lymphoma (ALCL), and mycosis fungoides (MF),2 and it has also shown activity in B-cell non-Hodgkin lymphomas.3, 4, 5, 6 Although BV is generally well tolerated, alone or with other systemic therapy (ST) agents,5,7 there is a paucity of data to inform the use of concurrent BV and radiation therapy (RT) (BVRT).8, 9, 10
Methods and Materials
We conducted an institutional review board approved single institutional retrospective review of 44 patients who received BVRT from May 2018 to February 2022, defined as an infusion within 3 weeks before or concurrent with RT. Toxicity (Common Terminology Criteria for Adverse Events v5.0) was classified as pre-RT (within 1 month before RT), acute (start of RT to 1 month post-RT), or subacute (2-3 months after RT), censored at subsequent ST. Statistics were performed using Mann-Whitney U and χ2 tests using SPSS (v26; IBM, Armonk, NY).
Results
Of 44 patients, 16 (36%) were women. Median age at BVRT was 52 years (interquartile range [IQR], 42-65). The most common diagnoses were MF (22 patients, 50%; 17 with large cell transformation), relapsed or refractory (R/R) cHL (6 patients, 14%), and ALCL (6 patients, 14%) (Table 1). Twenty-eight patients (64%) had stage III or IV disease. Patients received a median of 3 lines of prior ST (IQR, 1-4) and a median of 1 prior course of RT (IQR, 0-3). The median duration from prior chemotherapy to BVRT was 5 months (IQR, 1-8). Twenty-three patients (52%) received BV before BVRT, with a median of 4 cycles (IQR, 2-9).
Table 1.
Patient characteristics
| Characteristic | No. (% of 44) or median (IQR) |
|---|---|
| Age at diagnosis (y) | 48 (37-61) |
| Age at BVRT (y) | 52 (42-65) |
| Female sex | 16 (36%) |
| Diagnosis | |
| Mycosis fungoides | 22 (50%) |
| Classical Hodgkin lymphoma | 6 (14%) |
| Anaplastic large cell lymphoma (primary cutaneous or systemic) | 6 (14%) |
| Primary cutaneous T-cell lymphoma, other | 4 (9%) |
| B-cell lymphoma, other* | 4 (9%) |
| T-cell lymphoma, other† | 2 (5%) |
| Stage | |
| I | 7 (16%) |
| II | 9 (20%) |
| III | 3 (7%) |
| IV | 25 (57%) |
| Number of lines of prior systemic therapy | 3 (1-4) |
| Prior courses of RT | 1 (0-3) |
| Prior chemotherapy | 31 (70%) |
| Months from last chemo to BVRT | 5 (1-8) |
| RT dose (Gy) | 20 (12-30) |
| RT fractions | 8 (4-12) |
| Electrons only | 21 (48%) |
| Photons +/– electrons | 23 (52%) |
| Concurrent systemic therapy with BVRT | |
| Cyclophosphamide | 5 (11%) |
| Nivolumab | 3 (7%) |
| Romidepsin | 1 (2%) |
| Pralatrexate | 1 (2%) |
| Ibrutinib | 1 (2%) |
| Intrathecal methotrexate/cytarabine | 1 (2%) |
| Therapy after BVRT | |
| Additional RT | 19 (43%)‡ |
| Systemic therapy | 627 (1%) |
| Stem cell transplant | 7 (16%) |
| Cellular therapy | 3 (7%) |
Abbreviations: BV = brentuximab vedotin; BVRT = concurrent RT with BV; IQR = interquartile range; RT = radiation therapy.
Includes gray zone lymphoma, B-cell plasmablastic lymphoma, primary mediastinal B-cell lymphoma.
Includes peripheral T-cell lymphoma, Human T-lymphotropic virus T-cell lymphoblastic leukemia/lymphoma.
Percentages exceed 100 as patients could have received multiple different treatment modalities after BVRT.
Percentages may not add to 100 because of rounding.
Twenty-one patients (48%) received skin-directed RT exclusively with electrons, and 23 patients (52%) were treated with photons, with or without electrons. The median RT dose was 20 Gy (IQR, 12-30) in 8 fractions (IQR, 4-12). Eight patients (18%) received additional concurrent ST with BVRT (Table 1).
Nine patients (20%) had new grade 2+ (G2+) hematologic toxicity (HT) in the acute setting. New acute G2+ HT was associated with RT dose, with a median dose of 35 Gy in patients with new G2+ HT (95% CI, 23-43 Gy) compared with 15 Gy without (95% CI, 14-20 Gy) (P < .001) (Fig 1). There was a trend toward increased new acute G2+ HT with RT courses that included photons at 32% (7/22 patients) compared with electrons alone (2/20 or 10%; P = .09). There was no significant association with concurrent chemotherapy (P = .61), prior lines of ST (P = .14), months from last chemotherapy to BVRT (P = .34), age at BVRT (P = .32), or RT to the spine (P = .11) or pelvis (P = .44).
Figure 1.
Association of radiation therapy dose and new acute grade 2+ hematologic toxicity (HT).
Median dose of 15 Gy (95% confidence interval, 14-20 Gy) in those without new grade 2+ HT compared with a median dose of 35 Gy in those with new grade 2+ HT (95% confidence interval, 23-43) (P < .001).
Eleven of 41 patients had new G2+ acute aspartate transaminase/alanine transaminase elevation. New G2+ aspartate transaminase/alanine transaminase elevation was associated with concurrent administration of chemotherapy with BVRT (57% [4/7] with vs 21% [7/34] without; P = .047). It was not associated with photon-based RT (P = .42), RT dose (P = .70), RT fields that included the abdomen (P = .48), or prior lines of ST (P = .19).
After BVRT, there was a decrease in G2+ pain in the subacute (P = .02) but not acute setting (Table 2). There was no significant difference in G2+ fatigue, neuropathy, diarrhea, or grade 3+ HT in the acute or subacute setting (Table 2).
Table 2.
Toxicity before and after BVRT
| G2+ pain | G2+ fatigue | G2+ neuropathy | G2+ diarrhea | G2+ AST/ALT elevation | G2+ HT | G3+ HT | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Timing of toxicity | Yes | No | P value⁎ | Yes | No | P value⁎ | Yes | No | P value⁎ | Yes | No | P value⁎ | Yes | No | P value⁎ | Yes | No | P value⁎ | Yes | No | P value⁎ | |
| Pre- BVRT | 21 | 23 | 15 | 29 | 9 | 35 | 4 | 40 | 1 | 33 | 24 | 20 | 14 | 30 | ||||||||
| Acute (1 mo after BVRT) | 14 | 29 | .15 | 18 | 25 | .56 | 12 | 31 | .42 | 5 | 37 | .67 | 11 | 30 | .005 | 30 | 11 | .07 | 17 | 24 | .36 | |
| Subacute (2-3 mo after BVRT) | 4 | 18 | .02 | 10 | 12 | .37 | 5 | 16 | .76 | 2 | 17 | .86 | 3 | 24 | .20 | 14 | 9 | .62 | 7 | 16 | .91 | |
Abbreviations: ALT = alanine transaminase; AST = aspartate transaminase; BV = brentuximab vedotin; BVRT = concurrent RT with BV; HT = hematologic toxicity; RT = radiation therapy.
Bolding indicates statistical signficance (P<0.05).
P values: rate of toxicity pre-BVRT compared with the acute or subacute setting after BVRT.
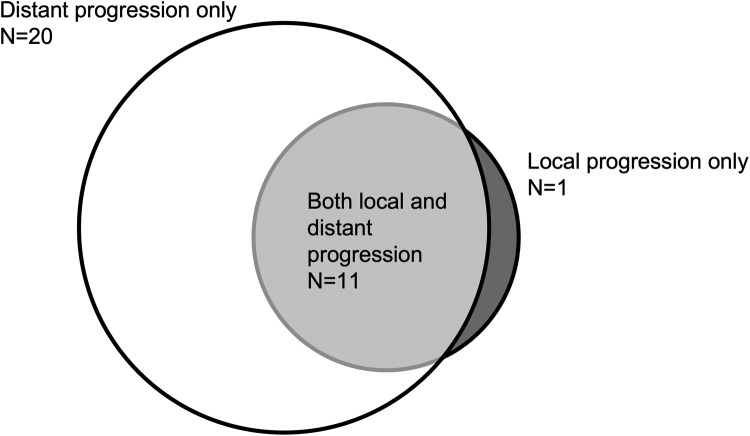
At last follow-up, 21 patients (48%) were deceased, with median time from the start of BVRT to death of 6.1 months (IQR, 3.7-8.1 months). In surviving patients, the median follow-up from BVRT to last follow-up was 6.2 months (IQR, 2.6-14.4 months). Twenty-four of 42 patients (57%) with follow-up data achieved a complete response in the treatment field after BVRT. Six patients (25%) had improvement but persistent disease after BVRT, 4 of whom received additional focal radiation. At last follow-up, 10 patients (23%) remained without evidence of disease. Patients experienced the following patterns of progression: distant progression only (20, 45%), local and distant progression (11, 25%), and local progression only (1, 2%) (Fig 2). Nineteen patients (43%) received subsequent RT courses. Twenty-seven patients (61%) received new ST after BVRT at a median of 28 days (IQR, 15-94), most commonly for progression (19 patients, 43%) or planned bridging therapy (5 patients, 11%). After BVRT, 7 patients (16%) proceeded to stem cell transplant at a median of 54 days (IQR, 19-169); 6 had no evidence of disease on positron emission tomography (PET)/computed tomography (CT) and 1 patient had improvement on CT (PET/CT denied by insurance). After BVRT, 2 patients with a partial response and 1 patient with progressive disease on PET/CT proceeded to cellular therapy at a median of 124 days (IQR, 80-185).
Figure 2.
Local and distant progression in patients at last follow-up.
Discussion
To our knowledge, this is the largest reported series of patients treated with concurrent BV and RT. BVRT resulted in low rates of new G2+ toxicity, with no unexpected toxicities related to concurrent therapy identified. Our data suggest that concurrent ST, RT dose, and photon-based treatment may affect HT.
Knowledge of the safety profile of concurrent BVRT is important, as both BV and RT are effective agents in the treatment of patients with lymphoma. Especially in the R/R setting, it is common to encounter patients who require both systemic disease control (addressed with BV and other STs) as well as more intensive local therapy (provided by RT).
BV has produced promising results even when given as a single agent in the treatment of R/R cHL (overall response rates [ORR], 57%-75% alone or with nivolumab11, 12, 13, 14, 15), R/R CD30+ non-Hodgkin lymphomas (ORR, 36%-44%; complete response rate [CRR], 17%-24%),4,5 and R/R ALCL (ORR, 67%-86%; CRR, 56%-66%).12,16 Although designed to target CD30, the efficacy of BV has also been shown in lymphomas with variable5,6 or absent17,18 CD30 expression, such as non-Hodgkin B-cell lymphoma or non-ALCL T-cell lymphomas.
RT is perhaps the most effective single agent therapy in the treatment of patients with lymphoma.19 Responses to RT vary greatly based on histology. Cutaneous T-cell lymphomas are quite radiation sensitive, with 3-year local control approaching 100% for ALCL treated to doses of less than 20 Gy.20 In patients with MF, 8 Gy in 2 fractions has been associated with a CRR over 90% without significant side effects.21 In contrast, systemic lymphomas such as cHL and diffuse large B-cell lymphoma often require doses in excess of 40 Gy to secure local control in the R/R setting.22
In our study, we sought to recognize both the exacerbation of known toxicities as well as identification of any novel, unexpected toxicity with BVRT. The most commonly reported BV-related toxicities included peripheral sensory neuropathy and neutropenia; nausea, fatigue, and rash can also occur. Hepatoxicity has been reported; however, it is uncommon, seen in 1.4% of patients on clinical trials.23 Fortunately, prior trials have shown that toxicity improves or resolves for the majority of patients with therapy cessation.16
Possible RT toxicity relates to the anatomic structures included in the RT field. Patients treated with higher doses of RT had higher rates of G2+ HT. Dose-dependent RT-induced myelosuppression is well-documented.24, 25, 26 Photon-based RT, compared with electrons, which deposit their dose superficially, is more likely to affect the bone marrow or blood volume and thus lead to cytopenias.27,28 Patients requiring treatment with photons, often with more aggressive histologies such as DLBCL, are also at higher risk of HT because of numerous factors, including heavy pretreatment status, more aggressive disease course, and need for higher RT doses. This work is reassuring as it included even heavily pretreated patients. However, it must be noted that the RT doses in this study were moderate, and additional caution may be necessary with higher doses. A high proportion of patients in this series received skin-directed RT with BV; in prior studies , up to 5% of patients treated with BV alone experienced G3 skin toxicity, with treatment discontinuation in 3%.29 When used in combination, bendamustine and BV second line for R/R cHL has been associated with skin reaction in 65% of patients.30 Although short-lived, with a median duration of 6 days, this resulted in treatment discontinuation in 15% of patients (6/40).
This analysis is limited by a heterogeneous patient population, with a variety of histologic diagnoses and diverse prior/concurrent ST regimens. This analysis is retrospective, and we were limited in our ability to grade toxicity using clinical documentation, particularly for subjective symptoms such as pain, neuropathy, and fatigue. Laboratory values were more readily available; however, they were not routinely obtained for outpatients receiving skin-directed RT. Assessment of bone marrow involvement or cellularity before BVRT, which may also affect HT, was limited. Given the small sample size, we were limited in power to detect more nuanced relationships.
Conclusion
Concurrent RT and BV resulted in limited new G2+ toxicities. We did observe expected HT, and care should be taken during RT planning to reduce this risk, potentially by using lower doses or smaller fields, when appropriate. BVRT may be an effective therapeutic option with a reasonable toxicity profile for patients with lymphoma in need of both systemic and local disease control. Further prospective investigation is warranted.
Disclosures
Susan Wu reports Rad Onc Questions, outside submitted work. Sairah Ahmed reports research support to institution for clinical trials from Seattle Genetics, Merck, Xencor, Chimagen, and Tessa Therapeutics, has membership on Tessa Therapeutic's and Chimagen scientific advisory committee, serves on the Data Safety Monitoring Board for Myeloid Therapeutics; she is a consultant for ADC therapeutics and KITE/Gilead. Paolo Strati reports being a consultant for Roche-Genentech, Kite-Gilead, Hutchinson MediPharma, Astrazeneca-Acerta, ADC Therapeutics, Sobi, and TG Therapeutics; he has received research funds from Sobi, Astrazeneca-Acerta, ALX Oncology, and ADC Therapeutics. Raphael E. Steiner reports research funding from Seagen, BMS (Bristol Myers Squibb), GSK (GlaxoSmithKline), and Rafael Pharmaceuticals. Bouthaina S. Dabaja reports research support from Seattle Genetics. The other authors report no relevant disclosures/conflicts of interest.
Footnotes
Sources of support: This work is supported by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30 CA016672).
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Francisco JA, Cerveny CG, Meyer DL, et al. cAC10-vcMMAE, an anti-CD30–monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102:1458–1465. doi: 10.1182/blood-2003-01-0039. [DOI] [PubMed] [Google Scholar]
- 2.Duvic M, Tetzlaff MT, Gangar P, Clos AL, Sui D, Talpur R. Results of a phase II trial of brentuximab vedotin for CD30+ cutaneous T-cell lymphoma and lymphomatoid papulosis. J Clin Oncol. 2015;33:3759. doi: 10.1200/JCO.2014.60.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zinzani PL, Santoro A, Gritti G, et al. Nivolumab combined with brentuximab vedotin for relapsed/refractory primary mediastinal large B-cell lymphoma: Efficacy and safety from the phase II checkmate 436 study. J Clin Oncol. 2019;37:3081–3089. doi: 10.1200/JCO.19.01492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SJ, Yoon DH, Kim JS, et al. Efficacy of brentuximab vedotin in relapsed or refractory high-CD30-expressing non-Hodgkin lymphomas: Results of a multicenter, open-labeled phase II trial. Cancer Res Treat. 2020;52:374–387. doi: 10.4143/crt.2019.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobsen ED, Sharman JP, Oki Y, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015;125:1394–1402. doi: 10.1182/blood-2014-09-598763. [DOI] [PubMed] [Google Scholar]
- 6.Kim YH, Prince HM, Whittaker S, et al. Response to brentuximab vedotin versus physician's choice by CD30 expression and large cell transformation status in patients with mycosis fungoides: An ALCANZA sub-analysis. Eur J Cancer. 2021;148:411–421. doi: 10.1016/j.ejca.2021.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horwitz S, O'Connor OA, Pro B, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet. 2019;393:229–240. doi: 10.1016/S0140-6736(18)32984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu S-Y, Mills M, Figura N, Kim S. Clinical outcomes for patients with lymphoma treated with radiation therapy and brentuximab: Tolerability and efficacy. Presented at the Lymphoma Leukemia and Myeloma Congress. October 21-24, 2020; virtual event.
- 9.Floyd EG, Burns TF, Linos K, et al. Combined modality treatment with brentuximab vedotin and radiation therapy for primary cutaneous anaplastic large cell lymphoma: A case report. J Hematol. 2019;8:132–136. doi: 10.14740/jh534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montana W, Buck DA, Smith T. Near complete response in a patient with classical hodgkin lymphoma treated with brentuximab vedotin concurrent with radiation therapy. Case Rep Oncol. 2017;10:795–801. doi: 10.1159/000479224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fedorova LV, Lepik KV, Volkov NP, et al. Efficacy and safety of nivolumab combined with brentuximab vedotin after nivolumab monotherapy failure in patients with relapsed and refractory classic Hodgkin lymphoma. Int J Clin Oncol. 2022;27:626–632. doi: 10.1007/s10147-021-02085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song Y, Guo Y, Huang H, et al. Phase II single-arm study of brentuximab vedotin in Chinese patients with relapsed/refractory classical Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Expert Rev Hematol. 2021;14:867–875. doi: 10.1080/17474086.2021.1942831. [DOI] [PubMed] [Google Scholar]
- 13.Moskowitz AJ, Schoder H, Yahalom J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin's lymphoma: A non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol. 2015;16:284–292. doi: 10.1016/S1470-2045(15)70013-6. [DOI] [PubMed] [Google Scholar]
- 14.Rothe A, Sasse S, Goergen H, et al. Brentuximab vedotin for relapsed or refractory CD30+ hematologic malignancies: The German Hodgkin Study Group experience. Blood. 2012;120:1470–1472. doi: 10.1182/blood-2012-05-430918. [DOI] [PubMed] [Google Scholar]
- 15.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30:2183. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pro B, Advani R, Brice P, et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2017;130:2709–2717. doi: 10.1182/blood-2017-05-780049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horwitz SM, Advani RH, Bartlett NL, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123:3095–3100. doi: 10.1182/blood-2013-12-542142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jagadeesh D, Horwitz SM, Bartlett NL, et al. Response to brentuximab vedotin by CD30 expression: Results from five trials in PTCL, CTCL, and B-cell lymphomas. J Clin Oncol. 2019;15 7543-7543. [Google Scholar]
- 19.Imber BS, Yahalom J. Radiotherapy for non-Hodgkin lymphomas. Cancer J. 2020;26:217–230. doi: 10.1097/PPO.0000000000000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith GL, Duvic M, Yehia ZA, et al. Effectiveness of low-dose radiation for primary cutaneous anaplastic large cell lymphoma. Adv Radiat Oncol. 2017;2:363–369. doi: 10.1016/j.adro.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neelis KJ, Schimmel EC, Vermeer MH, Senff NJ, Willemze R, Noordijk EM. Low-dose palliative radiotherapy for cutaneous B- and T-cell lymphomas. Int J Radiat Oncol Biol Phys. 2009;74:154–158. doi: 10.1016/j.ijrobp.2008.06.1918. [DOI] [PubMed] [Google Scholar]
- 22.Ng AK, Yahalom J, Goda JS, et al. Role of radiation therapy in patients with relapsed/refractory diffuse large B-cell lymphoma: Guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2018;100:652–669. doi: 10.1016/j.ijrobp.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Fanale MA, Whiting NC, Neylon E. Treatment strategies to optimize outcomes with brentuximab vedotin: Management of common and rare toxicities. J Target Ther Cancer. 2015;4 [Google Scholar]
- 24.Parker RG, Berry HC. Late effects of therapeutic irradiation on the skeleton and bone marrow. Cancer. 1976;37:1162–1171. doi: 10.1002/1097-0142(197602)37:2+<1162::aid-cncr2820370827>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Blomlie V, Rofstad EK, Skjønsberg A, Tverå K, Lien HH. Female pelvic bone marrow: Serial MR imaging before, during, and after radiation therapy. Radiology. 1995;194:537–543. doi: 10.1148/radiology.194.2.7824737. [DOI] [PubMed] [Google Scholar]
- 26.Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: Acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–1339. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 27.McDermott PN, Orton CG. Medical Physics Publishing; Madison, WI: 2010. The Physics & Technology of Radiation Therapy. [Google Scholar]
- 28.Schaff EM, Rosenberg SA, Olson SJ, Howard SP, Bradley KA. Bone marrow suppression as a complication of total skin helical tomotherapy in the treatment of mycosis fungoides. Radiat Oncol. 2018;13:67. doi: 10.1186/s13014-018-1013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh Y, Bang A, Kurtansky N, et al. Dermatologic adverse events of brentuximab vedotin: Characteristics, management, and their relationship with dose regimen. J Clin Oncol. 2021;39:3049. [Google Scholar]
- 30.Broccoli A, Argnani L, Botto B, et al. First salvage treatment with bendamustine and brentuximab vedotin in Hodgkin lymphoma: A phase 2 study of the Fondazione Italiana Linfomi. Blood Cancer J. 2019;9:100. doi: 10.1038/s41408-019-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]