Abstract
Vascular smooth muscle cell (VSMC) migration plays an important role in cardiovascular diseases, including atherosclerotic plaque formation and restenosis after vascular intervention. The mechanisms involved in VSMC migration are complex and have not been fully elucidated. Recently, we discovered a novel interaction, direct binding of active Fyn-paxillin at focal adhesions, which plays an important role in actin stress fiber formation and migration in VSMCs. In this review, we highlight paxillin as an intermediate signaling molecule that mediates actin stress fiber formation and VSMC migration through the Fyn/paxillin/Rho-kinase signaling pathway by directly binding to active Fyn. We also discuss the inhibition of VSMC migration by blocking the active Fyn-paxillin interaction and the potential of this interaction as a therapeutic target for cardiovascular diseases.
Keywords: vascular smooth muscle cell, migration, Fyn, paxillin, Rho-kinase
Introduction
Vascular smooth muscle cell (VSMC) migration is a normally occurring process that plays an important role in vascular development and tissue repair after vascular injury. The migration of VSMCs involves a complex series of processes, including detachment from the extracellular matrix, polarization, protrusion formation, and translocation (1, 2). Aberrant VSMC migration contributes to the development of various vascular diseases, such as atherosclerosis and restenosis (2). To date, the exact role of VSMC migration and its regulatory mechanism have not been fully elucidated. Therefore, understanding the mechanisms underlying VSMC migration is essential for developing new therapeutic strategies to treat these diseases.
Vascular Smooth Muscle Cell Migration in Pathological Conditions
Vascular smooth muscle cells (VSMCs) have two phenotypes, the contractile phenotype and the synthetic phenotype (2, 3). Under physiological conditions, VSMCs are of the contractile phenotype and perform the functions of controlling vascular contractility to maintain blood pressure and regulate blood flow in healthy vessels (4, 5). During the repair response to vascular injury, cells with a contractile phenotype can transform into cells with a synthetic phenotype, causing cell proliferation and migration (6, 7).
VSMC migration is an important process that occurs in various pathological conditions, including atherosclerosis (8, 9), restenosis (9,10,11), and aneurysm formation (12, 13). Under these pathological conditions, VSMCs undergo a phenotypic switch from a contractile phenotype to a synthetic phenotype, and cells of the synthetic phenotype can migrate toward the intima of vessels under the regulation of multiple signaling pathways, promoting atherosclerotic plaques, restenosis, and aneurysm formation (14,15,16). Multiple factors and mechanisms contribute to VSMC migration. A large number of studies have shown that epidermal growth factor (1, 17), fibroblast growth factor (18, 19), insulin-like growth factor (1, 20), platelet-derived growth factor (PDGF) (21, 22), transforming growth factor (TGF) (22), vascular endothelial growth factor (VEGF) (23, 24), angiotensin II (Ang II) (25, 26), and sphingosylphosphorylcholine (SPC) (27, 28) are involved in the migration of VSMCs. In atherosclerosis, the migration of VSMCs is associated with the activation of the Rho GTPase/Rho-kinase pathway (29, 30) and the upregulation of cell adhesion molecules (31) and matrix metalloproteinases (32, 33). In restenosis, the proliferation and migration of VSMCs following injury to the arterial wall is regulated by the PDGF pathway (34). In aneurysm formation, VSMC migration and proliferation are regulated by the TGF-β pathway (35).
Rho-kinase and Vascular Smooth Muscle Cell Migration
Rho-kinase regulates the various functions of VSMCs, including VSMC contraction (36,37,38,39) and migration (38, 40). During cell migration, actin stress fibers are thought to play a role in this process by linking the leading edge of the cell to the substrate, helping to generate traction and maintain cell shape (41). Actin stress fibers are structures formed by the polymerization of actin filaments in the cytoplasm of cells (42) and are anchored to focal adhesions, which connect the extracellular matrix to the actin cytoskeleton (43). Small GTPases such as RhoA, Rac1, and Cdc42 regulate the formation and dynamics of actin stress fibers and play important roles in cell migration (44). Rho-kinase, a downstream effector of RhoA, is activated to lead to actin stress fiber formation (45) and trigger cell migration (41). The Rho-kinase-mediated formation of bundled stress fibers and cell migration also require Src tyrosine kinase activity (46). The mDia family of Rho effectors can bind Src and cooperate with Rho-kinase to form bundled stress fibers, and the synergistic effect of mDia on stress fiber formation requires Src kinase activity (47). In addition, Rho-kinase is reported to be involved in VSMC migration through myosin light chain phosphorylation-dependent and phosphorylation-independent pathways (48). Rho-kinase is therefore considered an important therapeutic target for cardiovascular diseases, including atherosclerosis, aortic aneurysm, and vascular stenosis (38, 49,50,51,52,53).
Active Fyn-paxillin Interaction regulates Vascular Smooth Muscle Cell Migration
Fyn is a type of Src family kinase that was discovered in the regulation of VSMC contraction in our previous study (54). Our studies have also shown that Fyn activation can stimulate the formation of actin stress fibers (55, 56) and promote VSMC migration (57). Activation of Fyn has been shown to increase RhoA activity (55), which is a key regulator of actin cytoskeleton organization. Fyn activation can also regulate the expression of genes involved in cell migration, such as matrix metalloproteinases and adhesion molecules (58). Additionally, Fyn has been shown to interact with other signaling pathways that play a role in cell migration, such as the PI3K/Akt pathway and the MAPK pathway (59, 60). This interaction may allow Fyn to integrate signals from multiple pathways and regulate the behavior of VSMCs in response to various stimuli. Our previous study demonstrated that constitutively active Fyn is located at the ends of actin stress fibers and regulates the formation of actin stress fibers (27, 55, 56).
Paxillin is a cytoskeleton-associated protein involved in the regulation of various cellular processes, including cell adhesion (61, 62), migration (62, 63), and polarity (62, 64). The N-terminal region of paxillin contains five LD motifs that facilitate interactions with other proteins (61, 63, 65, 66), while the C-terminal region includes four LIM domains that are essential for targeting paxillin to focal adhesion sites (63, 66). Paxillin binds to multiple signaling and structural proteins through different domains, thereby mediating signal transduction and participating in various cellular functions. Paxillin associates with Raf and MEK at focal adhesions, forming a complex that facilitates localized ERK activation (67). Additionally, paxillin acts as a scaffold for ERK recruitment, coordinating the activation of FAK and Rac (68). Paxillin also mediates Rho signaling through the paxillin-p42/44MAPK-GEF-H1 complex (69). Therefore, paxillin is recognized as a key platform for multiple signaling and structural proteins, acting as a mediator for the transmission of signals in cellular communication pathways.
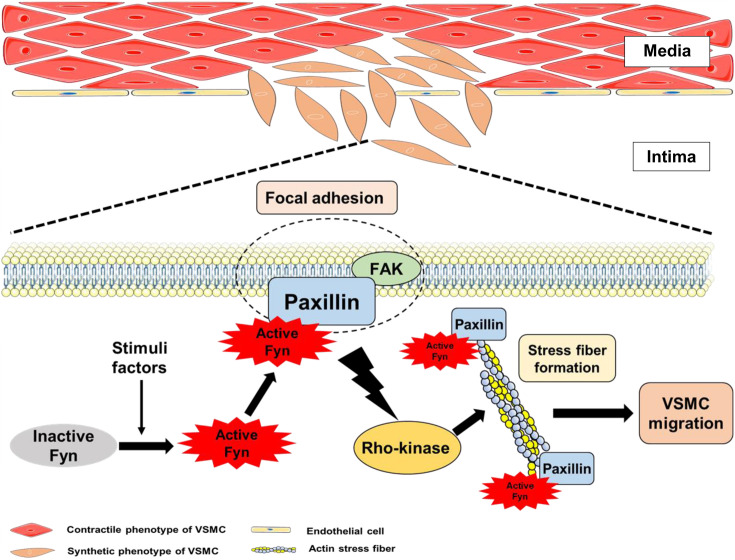
The mechanism underlying the migration of VSMCs into the intimal layer in pathological conditions remains incompletely understood. Recently, we discovered that the Fyn/paxillin/Rho-kinase signaling pathway regulates the migration of VSMCs (27). Paxillin, a protein that directly binds to active Fyn, regulates Rho-kinase-mediated actin stress fiber formation and VSMC migration (Fig. 1).
Fig. 1.
Direct active Fyn-paxillin interaction regulates Rho-kinase-mediated actin stress fiber formation and vascular smooth muscle cell (VSMC) migration.
Our findings demonstrate that the interaction between active Fyn and paxillin regulates the migration of VSMCs through two main mechanisms: (1) direct binding between active Fyn and the N-terminus of paxillin (N-pax) and (2) localization of the active Fyn-paxillin complex at the ends of actin stress fibers (27). The direct binding of active Fyn and N-pax is critical for actin stress fiber formation and cell migration. The downregulation of the expression of paxillin reduces active Fyn-paxillin binding, leading to impaired actin stress fiber formation and cell migration. Similarly, the downregulation of Fyn or a dominant-negative Fyn mutant inhibits VSMC migration (57). While the overexpression of constitutively active Fyn alone in paxillin knockdown cells cannot induce actin stress fiber formation and migration, the coexpression of constitutively active Fyn and paxillin results in successful stress fiber formation and cell migration. Additionally, the inability of the C-terminus of paxillin to bind to active Fyn prevented the rescue of actin stress fiber formation and cell migration. These results indicate that direct binding of active Fyn and paxillin is necessary for actin stress fiber formation and subsequent cell migration. Interestingly, our results also showed that the overexpression of N-pax alone is insufficient to rescue actin stress fiber formation and cell migration (27). This led us to investigate the second necessary condition for actin stress fiber formation and migration in VSMCs, which is the localization of Fyn-paxillin at the ends of actin stress fibers. We observed that constitutively active Fyn and full-length paxillin colocalize at the ends of actin stress fibers, whereas constitutively active Fyn and N-pax colocalize in the cytoplasm near the nucleus (27), suggesting that the localization of the Fyn-paxillin complex at the ends of actin stress fibers is another key mechanism of VSMC migration. Further studies revealed that overexpressed N-pax competitively binds to active Fyn, reducing active Fyn-paxillin binding and leading to impaired actin stress fiber formation and cell migration (27). These findings indicate that the direct interaction between active Fyn and paxillin could be a potential target for regulating the migration of VSMCs.
The Fyn/Paxillin/Rho-kinase Signaling Pathway Mediates VSMC Migration
Rho-kinase is a well-known regulatory factor that mediates the formation of actin stress fibers and cell migration (45, 70, 71). Fyn knockdown and dominant negative-Fyn inhibit Rho-kinase activity and Rho-kinase-mediated actin stress fiber formation (55,56,57), indicating that Fyn acts as an upstream signaling molecule of Rho-kinase. Paxillin knockdown also inhibits Rho-kinase activity and Rho-kinase-mediated actin stress fiber formation, and paxillin re-expression rescues these effects (27), indicating that paxillin is required for Rho-kinase activation. On the other hand, the association of active Fyn and paxillin suggests that paxillin, as a downstream molecule of active Fyn, regulates Fyn-mediated VSMC migration. Additionally, our recent data showed that paxillin knockdown had no impact on Fyn activation (unpublished data). Taken together, these results indicate that paxillin acts as an intermediate signaling molecule, mediating actin stress fiber formation and VSMC migration via the Fyn/paxillin/Rho-kinase signaling pathway.
Conclusions and Perspectives
Active Fyn-paxillin binding at focal adhesions plays an important role in Rho-kinase-mediated actin stress fiber formation and migration in VSMCs. Targeting of the active Fyn-paxillin interaction represents a promising therapeutic avenue for various vascular diseases. By regulating this interaction, it may be possible to modulate the behavior of VSMCs and enhance treatment outcomes. However, additional research is required to fully realize the therapeutic potential of this approach and to develop safe and effective drugs that can specifically target this interaction.
Conflict of Interest
The authors declare no conflicts of interest in association with the present study.
Acknowledgment
The authors thank Dr. Kazuhiro Kohama for his generous encouragement and support to our research.
References
- 1.Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res. 2007; 100(5): 607–21. doi: 10.1161/01.RES.0000258492.96097.47 [DOI] [PubMed] [Google Scholar]
- 2.Louis SF, Zahradka P. Vascular smooth muscle cell motility: from migration to invasion. Exp Clin Cardiol. 2010; 15(4): e75–85. [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshiyama S, Chen Z, Okagaki T, Kohama K, Nasu-Kawaharada R, Izumi T, et al. Nicotine exposure alters human vascular smooth muscle cell phenotype from a contractile to a synthetic type. Atherosclerosis. 2014; 237(2): 464–70. doi: 10.1016/j.atherosclerosis.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 4.Jaminon A, Reesink K, Kroon A, Schurgers L. The role of vascular smooth muscle cells in arterial remodeling: focus on calcification-related processes. Int J Mol Sci. 2019; 20(22): 5694. doi: 10.3390/ijms20225694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brozovich FV, Nicholson CJ, Degen CV, Gao YZ, Aggarwal M, Morgan KG. Mechanisms of vascular smooth muscle contraction and the basis for pharmacologic treatment of smooth muscle disorders. Pharmacol Rev. 2016; 68(2): 476–532. doi: 10.1124/pr.115.010652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grootaert MOJ, Bennett MR. Vascular smooth muscle cells in atherosclerosis: time for a re-assessment. Cardiovasc Res. 2021; 117(11): 2326–39. doi: 10.1093/cvr/cvab046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allahverdian S, Chaabane C, Boukais K, Francis GA, Bochaton-Piallat ML. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc Res. 2018; 114(4): 540–50. doi: 10.1093/cvr/cvy022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan J, Lu L, Wang X, Liu D, Tian J, Liu H, et al. AIM2 regulates vascular smooth muscle cell migration in atherosclerosis. Biochem Biophys Res Commun. 2018; 497(1): 401–9. doi: 10.1016/j.bbrc.2018.02.094 [DOI] [PubMed] [Google Scholar]
- 9.Schwartz SM. Perspectives series: cell adhesion in vascular biology. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest. 1997; 99(12): 2814–6. doi: 10.1172/JCI119472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vorpahl M, Schönhofer-Merl S, Michaelis C, Flotho A, Melchior F, Wessely R. The Ran GTPase-activating protein (RanGAP1) is critically involved in smooth muscle cell differentiation, proliferation and migration following vascular injury: implications for neointima formation and restenosis. PLoS One. 2014; 9(7): e101519. doi: 10.1371/journal.pone.0101519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casscells W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation. 1992; 86(3): 723–9. doi: 10.1161/01.CIR.86.3.723 [DOI] [PubMed] [Google Scholar]
- 12.Patel MI, Ghosh P, Melrose J, Appleberg M. Smooth muscle cell migration and proliferation is enhanced in abdominal aortic aneurysms. Aust N Z J Surg. 1996; 66(5): 305–8. doi: 10.1111/j.1445-2197.1996.tb01192.x [DOI] [PubMed] [Google Scholar]
- 13.Goodall S, Porter KE, Bell PR, Thompson MM. Enhanced invasive properties exhibited by smooth muscle cells are associated with elevated production of MMP-2 in patients with aortic aneurysms. Eur J Vasc Endovasc Surg. 2002; 24(1): 72–80. doi: 10.1053/ejvs.2002.1675 [DOI] [PubMed] [Google Scholar]
- 14.Tang HY, Chen AQ, Zhang H, Gao XF, Kong XQ, Zhang JJ. Vascular smooth muscle cells phenotypic dwitching in cardiovascular diseases. Cells. 2022; 11(24): 4060. doi: 10.3390/cells11244060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao G, Xuan X, Hu J, Zhang R, Jin H, Dong H. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun Signal. 2022; 20(1): 180. doi: 10.1186/s12964-022-00993-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashraf JV, Al Haj Zen A. Role of vascular smooth muscle cell phenotype switching in arteriogenesis. Int J Mol Sci. 2021; 22(19): 10585. doi: 10.3390/ijms221910585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makki N, Thiel KW, Miller FJ, Jr. The epidermal growth factor receptor and its ligands in cardiovascular disease. Int J Mol Sci. 2013; 14(10): 20597–613. doi: 10.3390/ijms141020597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schröder K, Helmcke I, Palfi K, Krause KH, Busse R, Brandes RP. Nox1 mediates basic fibroblast growth factor-induced migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007; 27(8): 1736–43. doi: 10.1161/ATVBAHA.107.142117 [DOI] [PubMed] [Google Scholar]
- 19.Pickering JG, Uniyal S, Ford CM, Chau T, Laurin MA, Chow LH, et al. Fibroblast growth factor-2 potentiates vascular smooth muscle cell migration to platelet-derived growth factor: upregulation of alpha2beta1 integrin and disassembly of actin filaments. Circ Res. 1997; 80(5): 627–37. doi: 10.1161/01.RES.80.5.627 [DOI] [PubMed] [Google Scholar]
- 20.Stratton MS, Yang X, Sreejayan N, Ren J. Impact of insulin-like growth factor-I on migration, proliferation and Akt-ERK signaling in early and late-passages of vascular smooth muscle cells. Cardiovasc Toxicol. 2007; 7(4): 273–81. doi: 10.1007/s12012-007-9006-7 [DOI] [PubMed] [Google Scholar]
- 21.Kohno T, Urao N, Ashino T, Sudhahar V, Inomata H, Yamaoka-Tojo M, et al. IQGAP1 links PDGF receptor-β signal to focal adhesions involved in vascular smooth muscle cell migration: role in neointimal formation after vascular injury. Am J Physiol Cell Physiol. 2013; 305(6): C591–600. doi: 10.1152/ajpcell.00011.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi YX, Jiang J, Jiang XH, Wang XD, Ji SY, Han Y, et al. PDGF-BB and TGF-beta1 on cross-talk between endothelial and smooth muscle cells in vascular remodeling induced by low shear stress. Proc Natl Acad Sci USA. 2011; 108(5): 1908–13. doi: 10.1073/pnas.1019219108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerjee S, Mehta S, Haque I, Sengupta K, Dhar K, Kambhampati S, et al. VEGF-A165 induces human aortic smooth muscle cell migration by activating neuropilin-1-VEGFR1-PI3K axis. Biochemistry. 2008; 47(11): 3345–51. doi: 10.1021/bi8000352 [DOI] [PubMed] [Google Scholar]
- 24.Grosskreutz CL, Anand-Apte B, Dupláa C, Quinn TP, Terman BI, Zetter B, et al. Vascular endothelial growth factor-induced migration of vascular smooth muscle cells in vitro. Microvasc Res. 1999; 58(2): 128–36. doi: 10.1006/mvre.1999.2171 [DOI] [PubMed] [Google Scholar]
- 25.Yaghini FA, Song CY, Lavrentyev EN, Ghafoor HU, Fang XR, Estes AM, et al. Angiotensin II-induced vascular smooth muscle cell migration and growth are mediated by cytochrome P450 1B1-dependent superoxide generation. Hypertension. 2010; 55(6): 1461–7. doi: 10.1161/HYPERTENSIONAHA.110.150029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M, Xu Y, Qiu Z, Jiang L. Sulforaphane attenuates angiotensin II-induced vascular smooth muscle cell Migration via suppression of NOX4/ROS/Nrf2 signaling. Int J Biol Sci. 2019; 15(1): 148–57. doi: 10.7150/ijbs.28874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Kishi H, Morita T, Kobayashi S. Paxillin controls actin stress fiber formation and migration of vascular smooth muscle cells by directly binding to the active Fyn. FASEB J. 2021; 35(12): e22012. doi: 10.1096/fj.202101035RR [DOI] [PubMed] [Google Scholar]
- 28.Wang HH, Nakamura A, Matsumoto A, Yoshiyama S, Qin X, Ye LH, et al. Nonkinase activity of MLCK in elongated filopodia formation and chemotaxis of vascular smooth muscle cells toward sphingosylphosphorylcholine. Am J Physiol Heart Circ Physiol. 2009; 296(5): H1683–93. doi: 10.1152/ajpheart.00965.2008 [DOI] [PubMed] [Google Scholar]
- 29.Li M, Jiao Q, Xin W, Niu S, Liu M, Song Y, et al. The emerging role of rho guanine nucleotide exchange factors in cardiovascular disorders: insights into atherosclerosis: a mini review. Front Cardiovasc Med. 2022; 8: 782098. doi: 10.3389/fcvm.2021.782098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Afewerki T, Ahmed S, Warren D. Emerging regulators of vascular smooth muscle cell migration. J Muscle Res Cell Motil. 2019; 40(2): 185–96. doi: 10.1007/s10974-019-09531-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Libby P, Li H. Vascular cell adhesion molecule-1 and smooth muscle cell activation during atherogenesis. J Clin Invest. 1993; 92(2): 538–9. doi: 10.1172/JCI116620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson JL, Dwivedi A, Somerville M, George SJ, Newby AC. Matrix metalloproteinase (MMP)-3 activates MMP-9 mediated vascular smooth muscle cell migration and neointima formation in mice. Arterioscler Thromb Vasc Biol. 2011; 31(9): e35–44. doi: 10.1161/ATVBAHA.111.225623 [DOI] [PubMed] [Google Scholar]
- 33.Newby AC. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc Res. 2006; 69(3): 614–24. doi: 10.1016/j.cardiores.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Han Y, Lin C, Zhen Y, Song X, Teng S, et al. PDGF-D contributes to neointimal hyperplasia in rat model of vessel injury. Biochem Biophys Res Commun. 2005; 329(3): 976–83. doi: 10.1016/j.bbrc.2005.02.062 [DOI] [PubMed] [Google Scholar]
- 35.Lu H, Du W, Ren L, Hamblin MH, Becker RC, Chen YE, et al. Vascular smooth muscle cells in aortic aneurysm: from genetics to mechanisms. J Am Heart Assoc. 2021; 10(24): e023601. doi: 10.1161/JAHA.121.023601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Zhang M, Lyu B, Kishi H, Kobayashi S. Omega-3 and omega-6 DPA equally inhibit the sphingosylphosphorylcholine-induced Ca2+-sensitization of vascular smooth muscle contraction via inhibiting Rho-kinase activation and translocation. Sci Rep. 2017; 7: 36368. doi: 10.1038/srep36368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swärd K, Mita M, Wilson DP, Deng JT, Susnjar M, Walsh MP. The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction. Curr Hypertens Rep. 2003; 5(1): 66–72. doi: 10.1007/s11906-003-0013-1 [DOI] [PubMed] [Google Scholar]
- 38.Satoh K, Fukumoto Y, Shimokawa H. Rho-kinase: important new therapeutic target in cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2011; 301(2): H287–96. doi: 10.1152/ajpheart.00327.2011 [DOI] [PubMed] [Google Scholar]
- 39.Li N, Zhang Y, Morita T, Kishi H, Kobayashi S. Inhibitory mechanism of tangeretin, a citrus flavone on the sphingosylphosphorylcholine (SPC)-induced vascular smooth muscle contraction. J Pharmacol Sci. 2022; 149(4): 189–97. doi: 10.1016/j.jphs.2022.05.002 [DOI] [PubMed] [Google Scholar]
- 40.Miyata K, Hitomi H, Guo P, Zhang GX, Kimura S, Kiyomoto H, et al. Possible involvement of Rho-kinase in aldosterone-induced vascular smooth muscle cell remodeling. Hypertens Res. 2008; 31(7): 1407–13. doi: 10.1291/hypres.31.1407 [DOI] [PubMed] [Google Scholar]
- 41.Pellegrin S, Mellor H. Actin stress fibres. J Cell Sci. 2007; 120(Pt 20): 3491–9. doi: 10.1242/jcs.018473 [DOI] [PubMed] [Google Scholar]
- 42.Tojkander S, Gateva G, Lappalainen P. Actin stress fibers—assembly, dynamics and biological roles. J Cell Sci. 2012; 125(Pt 8): 1855–64. [DOI] [PubMed] [Google Scholar]
- 43.Naumanen P, Lappalainen P, Hotulainen P. Mechanisms of actin stress fibre assembly. J Microsc. 2008; 231(3): 446–54. doi: 10.1111/j.1365-2818.2008.02057.x [DOI] [PubMed] [Google Scholar]
- 44.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995; 81(1): 53–62. doi: 10.1016/0092-8674(95)90370-4 [DOI] [PubMed] [Google Scholar]
- 45.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, et al. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997; 275(5304): 1308–11. doi: 10.1126/science.275.5304.1308 [DOI] [PubMed] [Google Scholar]
- 46.Timpson P, Jones GE, Frame MC, Brunton VG. Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr Biol. 2001; 11(23): 1836–46. doi: 10.1016/S0960-9822(01)00583-8 [DOI] [PubMed] [Google Scholar]
- 47.Tominaga T, Sahai E, Chardin P, McCormick F, Courtneidge SA, Alberts AS. Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol Cell. 2000; 5(1): 13–25. doi: 10.1016/S1097-2765(00)80399-8 [DOI] [PubMed] [Google Scholar]
- 48.Ai S, Kuzuya M, Koike T, Asai T, Kanda S, Maeda K, et al. Rho-Rho kinase is involved in smooth muscle cell migration through myosin light chain phosphorylation-dependent and independent pathways. Atherosclerosis. 2001; 155(2): 321–7. doi: 10.1016/S0021-9150(00)00585-2 [DOI] [PubMed] [Google Scholar]
- 49.Hartmann S, Ridley AJ, Lutz S. The function of Rho-associated kinases ROCK1 and ROCK2 in the pathogenesis of cardiovascular disease. Front Pharmacol. 2015; 6: 276. doi: 10.3389/fphar.2015.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimokawa H, Sunamura S, Satoh K. RhoA/Rho-kinase in the cardiovascular system. Circ Res. 2016; 118(2): 352–66. doi: 10.1161/CIRCRESAHA.115.306532 [DOI] [PubMed] [Google Scholar]
- 51.Abdel-Magid AF. Rho kinase inhibitors: potentially versatile therapy for the treatment of cardiovascular diseases and more. ACS Med Chem Lett. 2015; 6(4): 371–2. doi: 10.1021/acsmedchemlett.5b00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Q, Liao JK. Rho kinase: an important mediator of atherosclerosis and vascular disease. Curr Pharm Des. 2009; 15(27): 3108–15. doi: 10.2174/138161209789057986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seasholtz TM, Majumdar M, Kaplan DD, Brown JH. Rho and Rho kinase mediate thrombin-stimulated vascular smooth muscle cell DNA synthesis and migration. Circ Res. 1999; 84(10): 1186–93. doi: 10.1161/01.RES.84.10.1186 [DOI] [PubMed] [Google Scholar]
- 54.Nakao F, Kobayashi S, Mogami K, Mizukami Y, Shirao S, Miwa S, et al. Involvement of Src family protein tyrosine kinases in Ca2+ sensitization of coronary artery contraction mediated by a sphingosylphosphorylcholine-Rho-kinase pathway. Circ Res. 2002; 91(10): 953–60. doi: 10.1161/01.RES.0000042702.04920.BF [DOI] [PubMed] [Google Scholar]
- 55.Xu D, Kishi H, Kawamichi H, Kajiya K, Takada Y, Kobayashi S. Sphingosylphosphorylcholine induces stress fiber formation via activation of Fyn-RhoA-ROCK signaling pathway in fibroblasts. Cell Signal. 2012; 24(1): 282–9. doi: 10.1016/j.cellsig.2011.09.013 [DOI] [PubMed] [Google Scholar]
- 56.Xu D, Kishi H, Kawamichi H, Kajiya K, Takada Y, Kobayashi S. Involvement of Fyn tyrosine kinase in actin stress fiber formation in fibroblasts. FEBS Lett. 2007; 581(27): 5227–33. doi: 10.1016/j.febslet.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 57.Ginnan R, Zou X, Pfleiderer PJ, Mercure MZ, Barroso M, Singer HA. Vascular smooth muscle cell motility is mediated by a physical and functional interaction of Ca2+/calmodulin-dependent protein kinase IIδ2 and Fyn. J Biol Chem. 2013; 288(41): 29703–12. doi: 10.1074/jbc.M113.477257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ortiz MA, Mikhailova T, Li X, Porter BA, Bah A, Kotula L. Src family kinases, adaptor proteins and the actin cytoskeleton in epithelial-to-mesenchymal transition. Cell Commun Signal. 2021; 19(1): 67. doi: 10.1186/s12964-021-00750-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xue F, Jia Y, Zhao J. Overexpression of FYN suppresses the epithelial-to-mesenchymal transition through down-regulating PI3K/AKT pathway in lung adenocarcinoma. Surg Oncol. 2020; 33: 108–17. doi: 10.1016/j.suronc.2020.02.002 [DOI] [PubMed] [Google Scholar]
- 60.Xie YG, Yu Y, Hou LK, Wang X, Zhang B, Cao XC. FYN promotes breast cancer progression through epithelial-mesenchymal transition. Oncol Rep. 2016; 36(2): 1000–6. doi: 10.3892/or.2016.4894 [DOI] [PubMed] [Google Scholar]
- 61.Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol. 2000; 2(12): E231–6. doi: 10.1038/35046659 [DOI] [PubMed] [Google Scholar]
- 62.Yu JA, Deakin NO, Turner CE. Emerging role of paxillin-PKL in regulation of cell adhesion, polarity and migration. Cell Adhes Migr. 2010; 4(3): 342–7. doi: 10.4161/cam.4.3.11406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.López-Colomé AM, Lee-Rivera I, Benavides-Hidalgo R, López E. Paxillin: a crossroad in pathological cell migration. J Hematol Oncol. 2017; 10(1): 50. doi: 10.1186/s13045-017-0418-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dubois F, Alpha K, Turner CE. Paxillin regulates cell polarization and anterograde vesicle trafficking during cell migration. Mol Biol Cell. 2017; 28(26): 3815–31. doi: 10.1091/mbc.e17-08-0488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schaller MD. Paxillin: a focal adhesion-associated adaptor protein. Oncogene. 2001; 20(44): 6459–72. doi: 10.1038/sj.onc.1204786 [DOI] [PubMed] [Google Scholar]
- 66.Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004; 84(4): 1315–39. doi: 10.1152/physrev.00002.2004 [DOI] [PubMed] [Google Scholar]
- 67.Ishibe S, Joly D, Zhu X, Cantley LG. Phosphorylation-dependent paxillin-ERK association mediates hepatocyte growth factor-stimulated epithelial morphogenesis. Mol Cell. 2003; 12(5): 1275–85. doi: 10.1016/S1097-2765(03)00406-4 [DOI] [PubMed] [Google Scholar]
- 68.Ishibe S, Joly D, Liu ZX, Cantley LG. Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol Cell. 2004; 16(2): 257–67. doi: 10.1016/j.molcel.2004.10.006 [DOI] [PubMed] [Google Scholar]
- 69.Gawlak G, Tian Y, O’Donnell JJ, 3rd, Tian X, Birukova AA, Birukov KG. Paxillin mediates stretch-induced Rho signaling and endothelial permeability via assembly of paxillin-p42/44MAPK-GEF-H1 complex. FASEB J. 2014; 28(7): 3249–60. doi: 10.1096/fj.13-245142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Katoh K, Kano Y, Amano M, Kaibuchi K, Fujiwara K. Stress fiber organization regulated by MLCK and Rho-kinase in cultured human fibroblasts. Am J Physiol Cell Physiol. 2001; 280(6): C1669–79. doi: 10.1152/ajpcell.2001.280.6.C1669 [DOI] [PubMed] [Google Scholar]
- 71.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken). 2010; 67(9): 545–54. doi: 10.1002/cm.20472 [DOI] [PMC free article] [PubMed] [Google Scholar]