Abstract
Cigarette smoke exposure is an important factor in chronic inflammation in patients with allergic rhinitis (AR); however, the relationship between cigarette smoke and AR-related glucocorticoid resistance requires further study. In mice, calpeptin significantly reduces inflammation of the lower respiratory tract caused by cigarette smoke, but whether it can treat glucocorticoid-resistant AR caused by cigarette smoke requires further research. In this study, we confirmed that cigarette smoke exposure can aggravate the Th2 inflammatory response in AR leading to glucocorticoid resistance. The underlying mechanism may be related to decreased expression of DNA methyltransferase 3a (Dnmt3a), and increased expression of interferon regulatory factor 1 (IRF1). In addition, we found that calpeptin can inhibit the expression of IRF1 and thus treat AR-associated glucocorticoid resistance in rats exposed to cigarette smoke. These data suggest that calpeptin may downregulate IRF1 and therefore treat glucocorticoid resistance in AR-associated with cigarette smoke exposure.
Keywords: Allergic rhinitis, Calpeptin, Cigarette smoke, Glucocorticoid resistance, Rats, Regulatory factor 1
1. Introduction
Allergic rhinitis (AR) is a common chronic immunoglobulin E (IgE)-mediated respiratory disease characterised by itching, sneezing, and runny and stuffy nose. Intranasal corticosteroids are the main drugs used to treat AR [1]. Their function is to reduce the inflow of inflammatory cells and inhibit the release of cytokines, thus reducing inflammation of the nasal mucosa [2]. However, some patients with AR who were treated with inhaled steroids did not effectively experience symptom relief or respond to the treatment. Evidently, these patients exhibited steroid-resistant AR [3]. The emergence of steroid-resistant AR poses a severe challenge to clinical treatment.
Etiologically, various infections and environmental factors, such as cigarette smoke exposure, trigger nasal congestion and chronic inflammation in patients with AR and are risk factors for upper respiratory diseases [4]. Studies have shown that cigarette smoke exposure increases the number of eosinophils in the nasal mucosa and aggravates AR inflammation [5]. Smoking is an important independent risk factor for asthma in adult patients with AR. Some smokers have a lower AR risk after quitting smoking [6]. Among patients with asthma and allergic airway diseases, patients who smoke are more likely to develop corticosteroid resistance than are those who do not smoke [7]. However, whether patients with AR who smoke are more likely to develop steroid resistance remains unclear.
Calpeptin is a calpain inhibitor that can pass through cell membranes and inhibit membrane-associated phosphatase activity [8]. Some studies have shown that calpeptin can reduce inflammatory responses, cell death, and axonal injury in rats with multiple sclerosis, and also reduce apoptosis and inflammation in muscle cells [9]. Calpeptin treatment provided protective effects against cerebral ischaemia-reperfusion injury, mechanically ventilated diaphragm injury, and atrophy in animal experiments, indicating that it may be developed as a drug for clinical applications [10]. In addition, our previous studies showed that calpeptin can inhibit the expression of μ-calpain, m-calpain, and IκBα in mouse lung and human bronchial epithelial BEAS-2B cells induced by cigarette smoke, significantly reducing lower respiratory tract inflammation. This finding suggests that calpeptin may have a potentially therapeutic effect in airway diseases related to cigarette smoke exposure [11].
Some studies have reported on the mechanisms of glucocorticoid resistance in allergic diseases. However, the relationship between cigarette smoke exposure and glucocorticoid insensitivity in AR, and the mechanism of action as well as the effect of calpeptin on glucocorticoid-resistant AR, is not clear. Therefore, we evaluated the effect of cigarette smoke on inflammation in AR and rat AR models, as well as the mechanism of glucocorticoid resistance induced by cigarette smoke in AR rats. In this study, we also assessed the effect of calpeptin on rat AR models exposed to cigarette smoke by using an in vitro experiment where RPMI-2650 cells were treated with calpeptin to explore the specific mechanism of its effect in glucocorticoid-resistant AR.
2. Materials and methods
2.1. Collection and testing of human samples
2.1.1. Collection and processing of human samples
Patients who underwent endoscopic surgery to correct nasal septum deviation in our hospital between January and December of 2021 were divided into three groups: 1) The control group, which included patients who did not have AR; 2) The AR group, which included patients who met the diagnostic criteria of AR, that is, those who exhibited nasal hyper-reaction symptoms, had nasal examination results indicating AR, and had a positive serum-specific IgE test result (at least one allergen-specific IgE ≥0.35 IU/mL or total IgE ≥100 IU/mL); and 3) The Smoke-exposed AR group, which included patients meeting the diagnostic criteria of AR, and who had actively smoked for more than 5 years. Patients with infectious nasal diseases, systemic diseases, and diseases with tumours, such as acute and chronic sinusitis, were excluded. Five patients were included in each group. Perioperatively, the inferior turbinate mucosa was collected, and the tissue was divided into three sections. After washing, one section was placed in an Eppendorf tube containing 10% paraformaldehyde, fixed for 24 h, and embedded in paraffin. During observation of the paraffin-embedded tissue section, eosinophils were counted and averaged over five high-power visual fields. The remaining two sections were placed in Eppendorf tubes and cryopreserved at −80 °C for subsequent western blotting and transcriptome sequencing. This study was approved by the Ethics Committee of the Renmin Hospital of Wuhan University (WDRY2018-K052), and all patients signed informed consent forms.
2.1.2. Collection and testing of serum samples
Serum samples (3 mL) were collected from all patients in the three groups before surgery, and the total IgE and Th2 cell-related cytokines interleukin (IL)-4 and IL-5 were measured using enzyme-linked immunosorbent assays (ELISAs) in strict accordance with the manufacturer's instructions.
2.1.3. Nasal mucosa transcriptome sequencing and bioinformatics analysis
Transcriptome sequencing was performed using an Illumina sequencing platform. First, RNA was extracted from the nasal mucosa, and the mRNA was enriched. The mRNA was then randomly interrupted by divalent cations in the NEB (Ipswich, Massachusetts, England) fragmentation buffer (New England Biolabs, USA), and a library was built according to the common or chain-specific method of building the NEB library. After passing inspection, different libraries were sequenced using the Illumina platform after pooling according to the effective concentration and target off-machine data demand. Gene Ontology (GO) functional enrichment analysis included possible participation in biological processes, cell composition, and differentiation. Kyoto Encyclopedia of Genes and Genomes (KEGG) signal pathway enrichment analysis predicted the signalling pathways in which all proteins might participate. The STRING website (https://stringdb.org/) for protein-protein interactions was used for analysis, and the protein-protein interaction network was drawn using Cytoscape 3.9.1 software, with the protein pairs as points and edges.
2.2. Animal experiments
Sprague Dawley (SD) rats were obtained from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. (licence number SCXK2016-0006). Rats (4–6 weeks old) were housed and fed at the Animal Experimental Centre of Renmin Hospital of Wuhan University (licence number SYXK2015-0027) at room temperature (18–22 °C). Rats were fed adaptively for 1 week before beginning the experiment. All experimental animal procedures used in this study were approved by the Animal Ethics Committee of the Renmin Hospital of Wuhan University (ethics approval number WDRY2018-K052).
2.2.1. Establishment and grouping of AR rat model exposed to cigarette smoke
Male SD rats (n = 25) were randomly divided into Control group, AR group, smoke-exposed AR group (Smoke + AR), AR group treated with dexamethasone (DEX + AR) and cigarette smoke exposed AR group treated with dexamethasone (DEX + Smoke + AR) with 5 rats in each group. The AR group was injected intraperitoneally with a 1 mL saline suspension containing 300 μg ovalbumin (OVA) and 30 mg aluminium hydroxide on days 1, 3, 5, 7, 9, 11, and 13. Starting on day 15, 10% ovalbumin saline was injected intranasally (once per day, 50 μL on each side) for seven consecutive days. During the modelling period of the other AR groups, basic sensitisation and nasal stimulation were repeated twice.
For Smoke + AR group, rats were placed in a plexiglas's fumigation box made for a previous study [11]. Three days after the successful establishment of AR, AR rats were placed on the partition and a cigarette was lit in one-half of the space under the plexiglass box until the cigarette had burned completely and the smoke had almost disappeared. After approximately 15 min, the lid was lifted for 10 min. This smoking regimen was continued (one cigarette at a time, five cigarettes per day) for 54 consecutive days. Basic sensitisation and nasal stimulation were repeated twice during the smoke exposure.
For DEX + AR group, the preparation was the same as for the AR group. After the establishment of the model, AR rats were intraperitoneally injected with dexamethasone (1.0 mg/kg) once every other day [12], 28 times in total.
For DEX + Smoke + AR group, the preparation was the same as for the Smoke + AR group. AR rats were intraperitoneally injected with dexamethasone (1.0 mg/kg) before each smoke exposure once every other day, 28 times in total.
For the control group, normal saline was used instead of ovalbumin in the intraperitoneal injection and nasal drip, and the treatment time was the same as that of the AR group.
2.2.2. Establishment of AR rat model treated with calpeptin
Male SD rats (n = 25) were randomly divided into five groups. The control, AR, Smoke + AR, and DEX + Smoke + AR groups were established as described above. For the fifth group, Calpeptin treated cigarette smoke exposed AR group (CP + Smoke + AR), the rats in the AR group were injected intraperitoneally with calpeptin (200 μg/kg) before each smoke exposure [13], once every other day, 28 times in total.
2.2.3. Detection of serum cytokines in rats
Twenty-four hours after the last nasal drip, rats were anaesthetised using isoflurane, and blood samples were collected from the inferior vena cava. The serum was collected and stored in a freezer at −80 °C. The serum levels of IgE, IL-4, IL-5 and IL-13 were detected using an ELISA kit.
(R&D Systems, Minneapolis, MN, USA).
2.2.4. Morphological observation of nasal mucosa in rats
Twenty-four hours after the last challenge, blood was collected from the inferior vena cava of rats anaesthetised with isoflurane. The skin on the heads of the rats was separated from the skulls, and the complete nasal bones were dissected and fixed in paraformaldehyde, and subsequently (24 h later) treated with a 10% EthyleneDiamineTetraaceticAcid (EDTA) decalcification solution. Two weeks later, samples were embedded in paraffin and sliced into sections. Morphological changes in the nasal mucosa were observed using hematoxylin-eosin (HE) that stained eosinophil cytoplasm red or pink and nuclei blue. The quantifications of HE staining were conducted by an observer blind to the treatments. Eosinophils were counted and averaged over five high-power visual fields [14].
2.2.5. Detection of ILC2 and Th2 cells in the rat spleens
On the same day, the rat spleens were removed and the splenic lymphocytes were separated with rat peripheral blood lymphocyte separation solution (Tianjin Haoyang Biological Products Technology Co. Ltd, China) and stained. The proportions of ILC2 and Th2 cells in the spleens were determined using flow cytometry. Lin-CD45 + CD90 + CD25 + cells were identified as ILC2 cells [15] (Lin includes CD3, CD45R, CD49b, and CD11b/c), and CD4 + IL4 + cells were identified as Th2 cells [16]. The flow antibodies FITC-CD3, FITC-CD45R, FITC-CD49b, FITC-11b/c, PE-CD45, APC-CD90, APC-cy7-FVS780, PE-IL-4, and FITC-CD4 (BD Biosciences, Franklin Lake, New Jersey, USA), and the flow antibody Percp-cy5.5-CD25 were used (eBioscience, San Diego, California, USA) in the flow cytometry experiments.
2.3. Cell culture in vitro
2.3.1. Preparation of cigarette smoke extract (CSE)
One end of a rubber tube was connected to a cigarette, and the other end was connected to a 50 mL syringe. A volume of 50 mL cigarette smoke was collected six times, and the smoke (300 mL) was placed in a glass bottle containing 25 mL aseptic RPMI 1640 medium without foetal bovine serum (Hyclone, Logan, Utah, USA), adjusted to pH 7.4, and filtered through a 0.22-μm microporous membrane. The solution obtained at the end of this procedure was considered 100% CSE [11].
2.3.2. Cell culture
The human nasal epithelial cell line, RPMI-2650, was purchased from the Cell Bank of the Chinese Academy of Sciences. The cells were cultured in RPMI 1640 medium (Hyclone, Logan, Utah, USA) supplemented with 10% foetal bovine serum (Gibco, CA, USA) and 1% penicillin-streptomycin solution (Biosharp, China), and placed in a 5% CO2 humidified environment at 37 °C. The RPMI-2650 cells were inoculated in six-well plates at a density of 1.0 × 105 cells per well. Either CSE (18.02%) or control medium was added to the cell cultures, which were grown to 60–70% confluence. After 24 h, the cell culture medium was changed, and the cells were stimulated overnight with TNF-α (10 ng/mL; PeproTech, New Jersey, USA) [17]. Next, the cells were divided into four groups: 1) control medium + TNF-α stimulation; 2) CSE medium + TNF-α stimulation; 3) CSE medium + TNF-α stimulation + dexamethasone; and 4) CSE medium + TNF-α stimulation + calpeptin. Dexamethasone (2.51 × 10−6 M) [17] and calpeptin (50 nmol, MedChemExpress, China) [11] were each administered for 1 h.
2.4. Western blotting
After the human and rat nasal mucosa samples were sectioned into slices, a radioimmunoprecipitation assay cleavage buffer containing protease and phosphatase inhibitors was added to fully cleave the tissue in a liquid nitrogen grinder. The protein in the supernatant was collected by centrifugation and stored at −80 °C for later use. A total of 40 μg from each group was loaded onto a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis was complete, the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was subsequently placed in a tris buffer solution containing 5% skim milk powder and sealed at room temperature for 1 h. After washing, the membranes were incubated overnight with primary antibodies against glucocorticoid receptor (GR)-α (Novus, China), GR-β (Abcam, Cambridge, UK), p38 mitogen-activated protein kinase (P38MAPK) (Affinity, China), DNA methyltransferase 3a (Dnmt3a) (Abcam, Cambridge, UK), interferon regulatory factor 1 (IRF1) (Cell Signaling Technology, CST, Boston, USA), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Abcam, Cambridge, UK) at 4 °C. The corresponding secondary antibodies were then added and the membrane was incubated at room temperature for 1 h. After washing the membrane, the bands were analysed using an enhanced chemiluminescence (ECL) detection reagent on the gel imaging system. Quantitative analysis was performed using ImageJ software (Rawak Software Inc., Germany). The ratio of the target band to the internal reference GAPDH band was calculated and reported as the expression value. The composition of all buffers used in the study are shown in a Supplement 2. All material informations in the study are shown in STAR methods format table.
2.5. Statistical analyses
Data were analysed and plotted using GraphPad Prism 8 (La Jolla, CA, USA). We utilised descriptive analysis and analysis of variance. The results are expressed as mean ± standard deviation for data that were consistent with a normal distribution and homogeneity of variance. The Student's t-test was used to compare the two groups. Single-factor analysis of variance was used for comparisons among groups, and Tukey's test was used for pairwise comparisons. Statistical significance was set at P < 0.05. All the accurate statistical values of this paper are shown in a Supplement table 1. More raw data and unprocessed images can be seen in https://doi.org/10.6084/m9.figshare.22004057.v1.
3. Results
3.1. Cigarette smoke exposure aggravates the inflammatory response in patients with AR
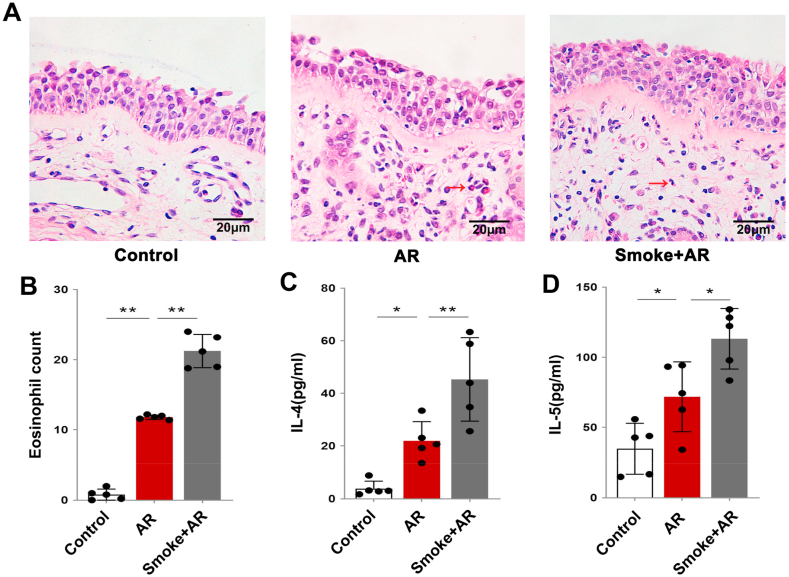
The nasal mucosa of patients with AR showed an increase in eosinophils when compared with those of the controls, and a further increase when compared with those from patients with AR and a history of smoking. The differences were statistically significant (Fig. 1A and B; P < 0.01). The serum levels of Th2 cytokines IL-4 and IL-5 in patients with AR were significantly higher than those in control patients, and were further increased in patients with AR and a history of smoking (Fig. 1C and D; P < 0.05). It has been suggested that cigarette smoke exposure can aggravate the Th2 inflammatory response in patients with AR.
Fig. 1.
A: Hematoxylin-eosin (HE) staining of the nasal mucosa in each group. The eosinophil is indicated by the red arrow. B: Statistical analysis of the eosinophil count in nasal mucosa. C–D: Statistical analysis of the expression levels of IL-4 and IL-5 in the serum of patients in each group. *P < 0.05, **P < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
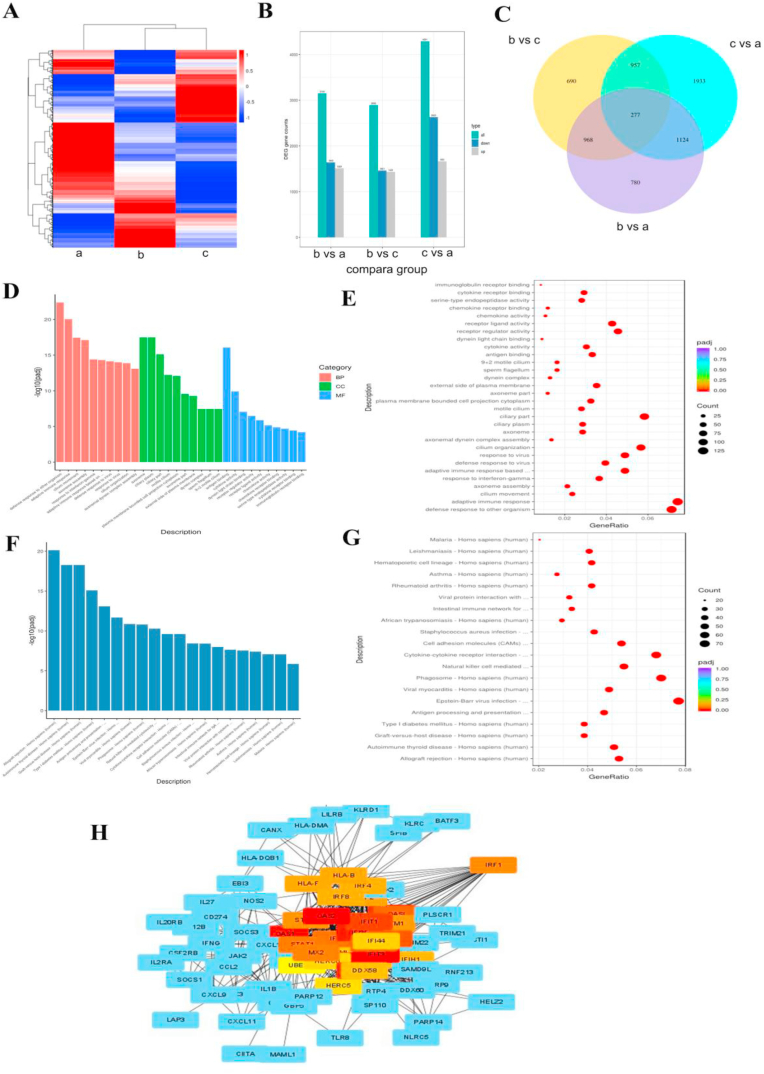
Additionally, the nasal mucosa of the three groups was collected for transcriptional group-sequencing analysis. KEGG pathway enrichment and GO were analysed for differentially expressed genes (DEGs). Fig. 2A and B shows the differential gene clustering of three groups. A Wayne diagram shows the overlap of the DEGs among the three groups (Fig. 2C). As shown in Fig. 2D–G, when compared with AR patients who smoked, DEGs in the nasal mucosa of patients with AR (who did not smoke) were mainly concentrated in immune-related biological processes, such as adaptive immune response, cytokine activity, chemokine receptor binding, and asthma. Fig. 2H shows that IRF1, IRF4, IRF8, oligoadenylate synthetases2 (OAS2), interferon-induced protein with tetratricopeptide repeats (IFIT)1, IFIT3, and other proteins were associated with the DEGs in patients with AR and also patients with AR who smoked.
Fig. 2.
A: Differential gene clustering. B: Statistics of differential genes among the three groups. C: Differential gene Wayne diagram. D: GO enrichment analysis histogram. BP, biological process; CC, cellular component; MF, molecular function. E: GO enrichment analysis scatter plot. F: KEGG enrichment analysis histogram. G: KEGG enrichment analysis scatter plot. H: Protein interaction network. a, control group; b, allergic rhinitis (AR) group; c, Smoke + AR group.
3.2. Cigarette smoke exposure aggravates Th2 inflammation in AR rats and causes insensitivity to dexamethasone treatment
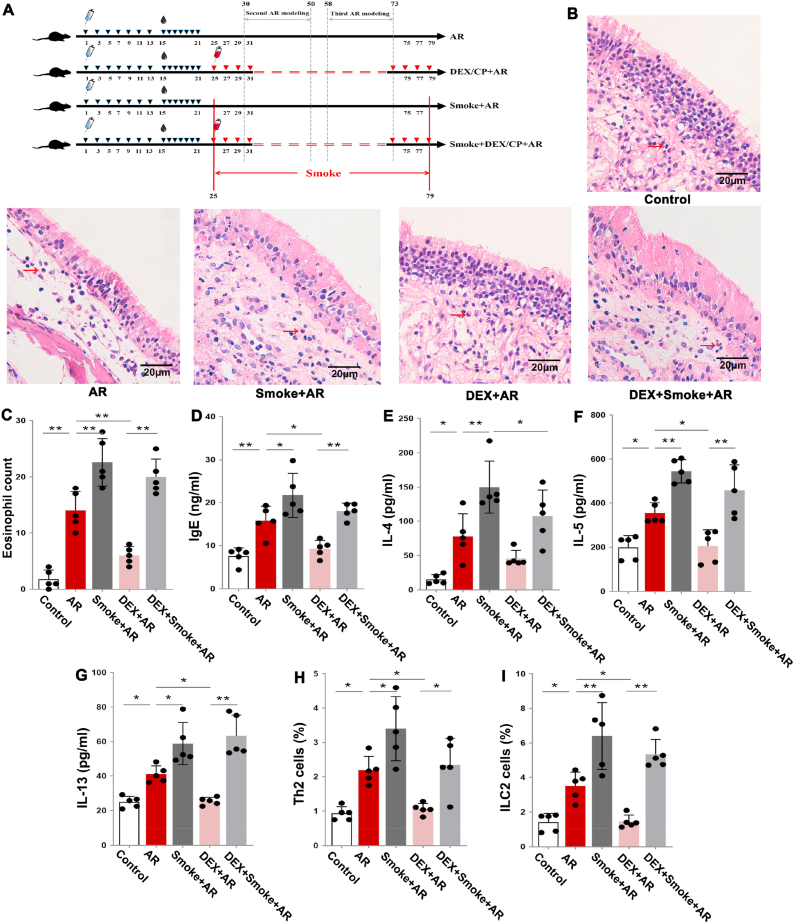
To clarify the relationship between cigarette smoke exposure and AR glucocorticoid resistance, we established an AR rat model and treated the rats with dexamethasone only or cigarette smoke exposure combined with dexamethasone. Fig. 3A shows the animal models. When compared with the control group, the number of eosinophils in the nasal mucosa, and the levels of Th2 cytokines IL-4, IL-5 and IL-13 and IgE were increased in the serum of AR rats and the Smoke + AR group (Fig. 3B–G; P < 0.05). Additionally, the proportion of Th2 cells and ILC2 cells in the spleen gradually increased in the control group, AR group and Smoke + AR group (Fig. 3H and I; P < 0 05). These results in a rat model further suggest that cigarette smoke exposure aggravates Th2 inflammation in AR rats.
Fig. 3.
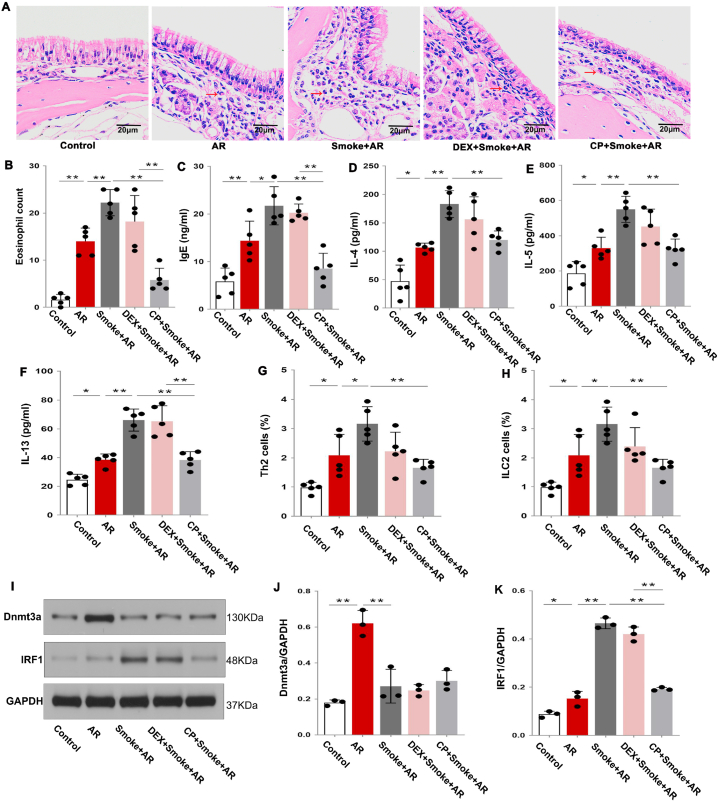
A: Flow chart of animal models. B: Hematoxylin-eosin (HE) staining of the nasal mucosa of rats in each group. Eosinophils are indicated by the red arrow. C: Statistical analysis of the eosinophil count in nasal mucosa. D–G: Statistical analysis of the expression levels of IgE, IL-4, IL-5 and IL-13 in the serum of rats in each group. H–I: Statistical analysis of the percentage of Th2 cells and ILC2 cells in lymphocytes in the spleen of rats in each group. *P < 0.05, **P < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
When compared with the AR group, AR rats treated with dexamethasone had significantly fewer eosinophils, decreased serum IgE, IL-5 and IL-13 levels, and a lower percentage of splenic Th2 and ILC2 cells (Fig. 3C–I; P < 0.05), indicating that dexamethasone treatment could reduce the inflammatory reaction in AR. However, when compared with smoke-exposed AR rats, we found no significant changes in eosinophils, serum IgE, IL-5 and IL-13 levels, or spleen Th2 and ILC2 cells in smoke-exposed AR rats treated with dexamethasone (Fig. 3C–I; P < 0.05), suggesting that cigarette smoke exposure led to dexamethasone treatment resistance in AR.
3.3. Cigarette smoke exposure changes the expression of GR and its related proteins in dexamethasone-treated AR rats
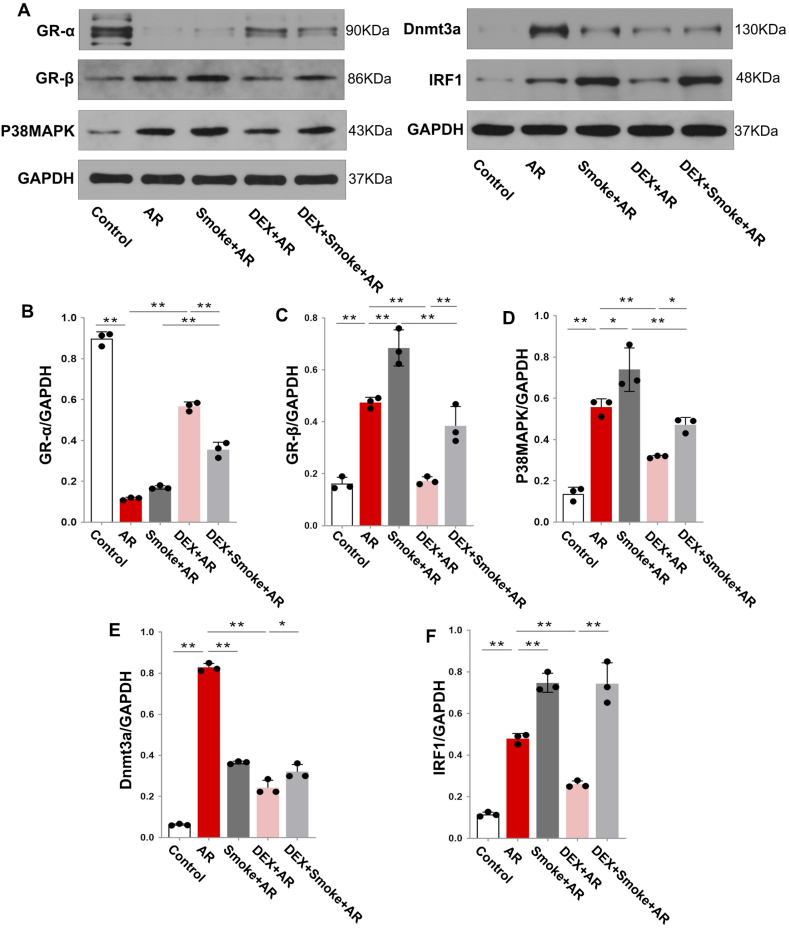
Next, we explored the mechanism underlying AR glucocorticoid resistance induced by cigarette smoke exposure. The GR-α expression in the nasal mucosa of the AR group was decreased when compared with that in the control group. However, the expression of GR-β, P38MAPK, Dnmt3a, and IRF1 in the AR group was increased when compared with that in the control group (Fig. 4A–F; P < 0.05). When compared with the AR group, the expression of GR-β, P38MAPK, and IRF1 in the Smoke + AR group was increased, whereas the expression of Dnmt3a was decreased (Fig. 4A–F; P < 0.05; The uncropped versions of Fig. 4A is shown in supplement 3). This suggested that cigarette smoke exposure might possibly lead to glucocorticoid resistance in AR through the differential expression of GR-β, P38MAPK, Dnmt3a, and IRF1 proteins.
Fig. 4.
A: Expression of GR-α, GR-β, P38MAPK, Dnmt3a, and IRF1 proteins in the nasal mucosa of rats in each group. B: Statistical analysis of protein expression of GR-α, GR-β, P38MAPK, Dnmt3a, and IRF1. *P < 0.05, **P < 0.01.
The expression of GR-β and P38MAPK in the nasal mucosa of the DEX + Smoke + AR group was decreased when compared with that of the Smoke + AR group (Fig. 4C and D; P < 0.05). However, we found no significant difference between the Smoke + AR and the DEX + Smoke + AR groups in the nasal mucosa expression of Dnmt3a and IRF1 (Fig. 4E and F). These results further suggest that glucocorticoid resistance in Smoke + AR rats may be related to the decreased expression of Dnmt3a and increased expression of IRF1, but not to GR-β and P38MAPK.
3.4. Calpeptin can reverse the inflammatory state induced by Th2 in cigarette smoke-exposed AR rats
To clarify the specific role of calpeptin in AR glucocorticoid resistance associated with cigarette smoke exposure, we treated cigarette smoke-exposed rats with dexamethasone and calpeptin. The eosinophil infiltration in the nasal mucosa, the levels of serum IgE, IL-4, IL-5, IL-13, and the percentage of Th2 and ILC2 cells in AR rats were significantly higher than those in the control group, and were further increased in the Smoke + AR group. Notably, the levels of eosinophils, serum IgE, IL-4, IL-5, IL-13, and the percentage of Th2 and ILC2 cells in the nasal mucosa of the DEX + Smoke + AR group were not significantly decreased when compared with those of the Smoke + AR group. However, the serum IgE, IL-4, IL-5, IL-13 levels and the percentage of Th2 and ILC2 cells were significantly decreased in the CP + Smoke + AR group when compared with those in the Smoke + AR group (Fig. 5A–H; P < 0.05). The expression of Dnmt3a and IRF1 in the nasal mucosa of AR rats was significantly increased when compared with that in the control group, and the expression of IRF1 was further increased in the Smoke + AR group. When compared, the expressions of Dnmt3a and IRF1 in the nasal mucosa of rats in the Smoke + AR group and the DEX + Smoke + AR group were not significantly different. Also, the expression of Dnmt3a in the nasal mucosa of rats in the CP + Smoke + AR group was not significantly different from that in the Smoke + AR group and the DEX + Smoke + AR group, whereas the IRF1 expression in CP + Smoke + AR group was lower than that in Smoke + AR group (Fig. 5 I–K; The uncropped versions of Fig. 5I is shown in supplement 3). These results indicate that calpeptin can treat Th2 inflammation in rats with glucocorticoid-resistant AR induced by cigarette smoke exposure, and that the mechanism may be related to the decreased expression of IRF1.
Fig. 5.
A: Hematoxylin-eosin (HE) staining of the nasal mucosa of rats in each group. Eosinophils are indicated by the red arrow. B: Statistical analysis of the eosinophil count in nasal mucosa. C–F: Statistical analysis of the expression levels of IgE, IL-4, IL-5 and IL-13 in the serum of rats in each group. G–H: Statistical analysis of the percentage of Th2 cells and ILC2 cells in the spleen of rats in each group. I: Expression of Dnmt3a and IRF1 proteins in rat nasal mucosa of each group. J–K: Statistical analysis of Dnmt3a and IRF1 protein expression. *P < 0.05, **P < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5. Calpeptin can treat the glucocorticoid-insensitive pathway in RPMI-2650 cells
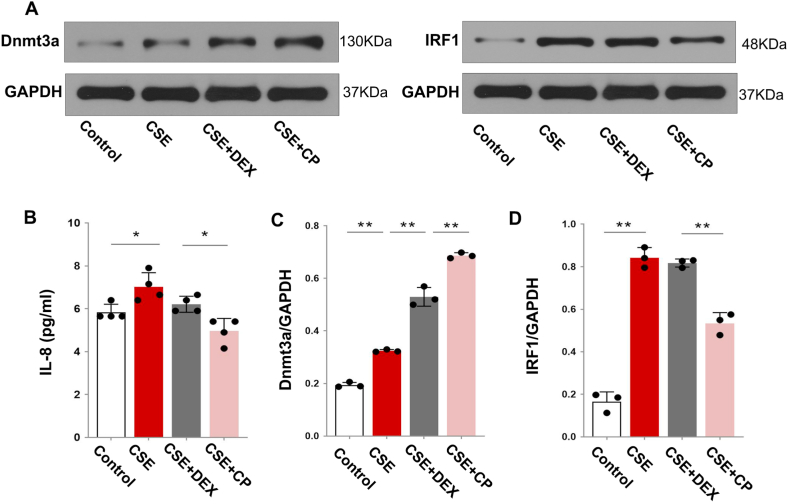
Finally, we evaluated the effect and mechanism of calpeptin action on glucocorticoid sensitivity in RPMI-2650 nasal epithelial cells induced by TNF-α to produce IL-8. We found that IL-8 expression was increased in the CSE group when compared to that in the control group. Moreover, the IL-8 expression in the CSE group was not significantly different from that in the CSE + DEX group. Furthermore, the IL-8 expression in the CSE + CP group was significantly decreased when compared with that in the CSE group indicating that CSE in RPMI-2650 cells increased glucocorticoid resistance, which could be effectively treated by calpeptin (Fig. 6B; P < 0.05).
Fig. 6.
A: Expression of Dnmt3a and IRF1 proteins in RPMI-2650 cells of each group. B: Expression of IL-8 in the supernatant of RPMI-2650 cells. C–D: Statistical analysis of Dnmt3a and IRF1 protein expression. *P < 0.05, **P < 0.01.
The expression of Dnmt3a and IRF1 in the CSE group was higher than that in the control group (Fig. 6A–G; P < 0.05). Also, the expression of Dnmt3a in the CSE + DEX group was increased when compared with that in the CSE group (Fig. 6A, C; P < 0 05; The uncropped versions of Fig. 6A is shown in supplement 3), but there was no significant change in IRF1 expression (Fig. 6A, D). Moreover, the IRF1 expression in the CSE + DEX + CP group was lower than that in the CSE + DEX group (Fig. 6A, D; P < 0 05). The reversal of the glucocorticoid-insensitive pathway by calpeptin may be related to the decreased expression of IRF1.
4. Discussion/Conclusion
Approximately six million people worldwide die from cigarette use every year, approximately 600,000 of whom die from second-hand smoke. In addition to contributing to chronic cardiovascular diseases, smoking is a recognised risk factor for many chronic systemic inflammatory diseases. However, the effect of cigarette smoke on the immune system remains a focus area that has not been fully studied [18]. The association between cigarette smoke exposure and AR is worthy of further study. Although passive cigarette smoke exposure is associated with increased nasal congestion and symptoms of rhinitis in children, other studies have produced contradictory results. Consequently, this study further investigated the effects of cigarette smoke exposure on AR inflammation in humans and rat models.
Independent evidence suggests a positive correlation between cigarette smoke exposure and allergy development. Yao et al. reported that exposure to cigarette smoke was positively correlated with increased IgE levels and skin prick sensitisation in children, showing a dose-dependent relationship [19]. Mechanically, exposure to tobacco smoke can distort the immune response by weakening the Th1 type and enhancing the Th2-dependent response, mainly by altering the function of various immune cells and aggravating allergic inflammation and allergenicity [20]. The previous evidence supports the results of the present study. Specifically, this study demonstrated that in humans and in rat models, IgE levels and Th2 inflammatory factors in the Smoke + AR group were increased compared with those in the AR group, whereas Th1 inflammatory factors were decreased.
Regarding the effect of cigarette smoke on AR, in addition to aggravating allergic inflammation, recent studies suggested that cigarette smoke can cause glucocorticoid insensitivity in the treatment of other allergic diseases. Cigarette smoke consists of a complex mixture of gases and particles, and there is ample evidence that it can lead to glucocorticoid resistance in patients with asthma and chronic obstructive pulmonary disease [21]. Dexamethasone increases lung tissue resistance, airway hyper-responsiveness, and neutrophil inflammation in asthmatic mice exposed to cigarette smoke [22]. However, whether cigarette smoke can cause glucocorticoid insensitivity in AR remains to be determined. In this study, allergy-related inflammatory indices decreased significantly in the DEX + AR group when compared with those in the AR group, suggesting the therapeutic effectiveness of dexamethasone. Moreover, the number of eosinophils in the nasal mucosa, the serum IgE level, and the percentage of splenic Th2 cells and ILC2 cells in lymphocytes did not decrease significantly in the DEX + Smoke + AR group when compared with those in the Smoke + AR group. These results suggest that cigarette smoke exposure reduces the sensitivity of AR rats to dexamethasone treatment. In a previous study, we explored the possible mechanisms of cigarette smoke-induced dexamethasone resistance in AR rats. Dexamethasone is a long-acting glucocorticoid. The anti-inflammatory effect of glucocorticoids is mediated by the activation of the cytoplasmic GR. As a transcriptional factor activated by glucocorticoids, the GR inhibits or induces glucocorticoid target genes by directly binding to the DNA sequence (trans-activation) or by transferring it to the nucleus [23]. In this study, we found that the expression of Dnmt3a in the Smoke + AR group was lower than that in the AR group, suggesting that cigarette smoke could lead to a decrease in Dnmt3a expression in AR rats. However, the expression of Dnmt3a in the DEX + Smoke + AR group was not significantly different from that in the Smoke + AR group, further suggesting that dexamethasone insensitivity in AR rats exposed to cigarette smoke might be related to the decreased expression of Dnmt3a. This finding may be related to the regulation of GR expression by DNA methylation. Widespread DNA methylation occurs in the promoter region of the GR gene [24], and the expression level of GR is negatively correlated with the level of DNA methylation in its promoter region [25]. The methylation level of the GR gene promoter region is regulated by DNMT, and a decrease in DNMT is thought to lead to hypomethylation and gene activation [26]. Dnmt3a, has a stronger ab initio methylation ability than Dnmt1 and can selectively cause changes in the methylation status of different target genes [27]. In this study, we also found that expression of IRF1 in the Smoke + AR group was higher than that in the AR group, and that IRF1 expression in the DEX + Smoke + AR group was not significantly different from that in the Smoke + AR group, indicating that dexamethasone insensitivity in AR rats exposed to cigarette smoke may also be related to the increased expression of IRF1. In previous studies, a significant positive association was found between the full allele distribution of IRF1 and atopic asthma [28]. Some studies have shown that expression of IRF1 is closely related to glucocorticoid resistance, indicating that IRF1 can induce early steroid resistance in airway smooth muscle cells [29]. In addition, glucocorticoids can downregulate the activity of MAPK by inducing the expression of DUSP, while MAPK negatively regulates the expression of IRF1-and/or IRF1-dependent genes [30]. DUSP1 maintains IRF1 and increases the expression of IRF1-dependent genes, promoting glucocorticoid insensitivity [31]. In this study, protein interaction analysis revealed that IRF1 was differentially expressed between the AR and Smoke + AR groups, and that it also interacted with IRF4, IRF8, OAS2, IFIT1, IFIT3, and other proteins. These studies further support the finding that increased IRF1 expression is associated with glucocorticoid resistance.
We also found that GR-β and P38MAPK expression in the Smoke + AR group was higher than that in the AR group, but GR-β and P38MAPK expression in the nasal mucosa of the DEX + Smoke + AR group was lower than that in the Smoke + AR group. This finding suggested that dexamethasone could reduce the expression of GR-β and P38MAPK protein in AR rats exposed to cigarette smoke, but that this did not affect dexamethasone resistance. However, this result is not entirely consistent with the results of other studies showing that the overexpression of GR-β may also weaken the response to corticosteroids by inhibiting the effect of ligand-activated GR-α [32]. Notably, the phosphorylation level of P38MAPK identified in peripheral blood mononuclear cells was significantly increased in corticosteroid-resistant asthma, the phosphorylation of GR-α was enhanced, and the affinity of GR-α to ligand was decreased [33]. These findings suggest that different factors cause AR glucocorticoid resistance via different mechanisms. Mechanisms of glucocorticoid resistance include a decrease in the number of GRs, the binding affinity of GR to glucocorticoids, and the binding ability of the GR complex to DNA [34]. The above results suggest that the glucocorticoid resistance induced by cigarette smoke is not related to a change in the number of GRs but may be related to the influence of GR function.
Calpeptin is a calpain inhibitor that can pass through cell membranes and inhibit the activity of membrane-associated phosphatase [35]. Our previous studies showed that calpeptin could reduce the proliferation and accumulation of eosinophils and inhibit the expression of μ-calpain, m-calpain, and IκBα in mouse lung and human bronchial epithelial BEAS-2B cells induced by cigarette smoke, significantly reducing inflammation of the lower respiratory tract [11]. In this study, the Th2 inflammatory factors and IRF1 of rats in the DEX + Smoke + AR group were not significantly different from those in the Smoke + AR group, whereas the Th2 inflammatory factors and IRF1 expression of rats in CP + Smoke + AR group were significantly decreased. This suggests that calpeptin significantly reduced eosinophil infiltration in the nasal mucosa, Th2 inflammatory factors, and IRF1 in serum, thus ameliorating cigarette smoke-induced AR in rats, which was not affected by dexamethasone. In addition, the role of calpeptin in nasal mucosal epithelial cells cultured with CSE was assessed. TNF-α plays an important role in chronic inflammation by promoting the Th1 immune response [36], especially in the recruitment of neutrophils and eosinophils [37]. IL-8 is a chemotactic and inflammatory cytokine produced by phagocytes and mesenchymal cells exposed to TNF-α, which activates and induces local aggregation of neutrophils [38]. Howarth et al. [39] found that blocking TNF-α may restore glucocorticoid sensitivity. In a study by Luo et al. [17], TNF-α induced BEAS-2B cells that produced IL-8 were used to evaluate glucocorticoid sensitivity.
In the RPMI-2650 cells used in this study, we also found that the IL-8 induced by TNF-α in the CSE group was significantly higher than that in the control group. Moreover, the level of IL-8 in the supernatant was not significantly different in the CSE group after the addition of dexamethasone, showing that it had no effect, but after further addition of calpeptin, the IL-8 level was effectively reduced. These results suggest that calpeptin can treat the decrease in glucocorticoid sensitivity induced by CSE. Finally, IRF1 expression in the CSE group was significantly higher than that in the control group, although there was no significant difference in IRF1 expression between the CSE + DEX group and the CSE group. Furthermore, the expression of IRF1 in the CSE + CP group was lower than that in the CSE + DEX group. This result confirmed that cigarette smoke promoted glucocorticoid insensitivity in vitro, and that calpeptin may treat the glucocorticoid-insensitive pathway by reducing the expression of IRF1.
This study had some limitations. First, we evaluated only the effect of cigarette smoke on the quantity of GR, but did not study the function of GR. Second, the treatment of AR with calpeptin for cigarette smoke-induced glucocorticoid resistance may be related to a variety of pathways, but in this study, we found that it was only related to the decline of IRF1 protein. Therefore, other mechanisms are worthy of further study.
In this study, we explored glucocorticoid insensitivity induced by cigarette smoke exposure in AR rat models. We verified that calpeptin can treat the glucocorticoid insensitivity induced by cigarette smoke in AR rat models and BEAS-2B cells, which may be related to differences in the expression of IRF1 protein.
Statement of ethics
This study was approved by the Ethics Committee of the Renmin Hospital of Wuhan University, and all patients signed informed consent forms. All experimental animal procedures used in the study were approved by the Animal Ethics Committee of the Renmin Hospital of Wuhan University (ethics approval number WDRY2018-K052).
Author contribution statement
Wen-Xuan Zhua and Yang Xi: Conceived and designed the experiments; Performed the experiments; Analysed and interpreted the data; Wrote the paper.
Fen Li, Jing-Jing Zuo and Ze-Zhang Tao: Conceived and designed the experiments.
Wo-Er Jiao: Conceived and designed the experiments; Wrote the paper.
Zi-Jing Li: Performed the experiments; Analysed and interpreted the data.
Yu-Qin Deng, Shi-Ming Chen, Yong-Gang Kong, Yu Xu: Contributed reagents, materials, analysis tools or data.
Data availability statement
Data associated with this study has been deposited at Figshare; https://doi.org/10.6084/m9.figshare.22004057.v1.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgements
We thank all colleagues who assisted with this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17316.
Contributor Information
Jing-Jing Zuo, Email: zj.rm@163.com.
Ze-Zhang Tao, Email: taozezhang696@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Greiner A.N., Hellings P.W., Rotiroti G., Scadding G.K. Allergic rhinitis. Lancet. 2011;378(9809):2112–2122. doi: 10.1016/S0140-6736(11)60130-X. [DOI] [PubMed] [Google Scholar]
- 2.Sur D.K., Plesa M.L. Treatment of allergic rhinitis. Am. Fam. Physician. 2015;92(11):985–992. [PubMed] [Google Scholar]
- 3.Wu J., Lu A.D., Zhang L.P., Zuo Y.X., Jia Y.P. [Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia] Zhonghua Xue Ye Xue Za Zhi. 2019;40(1):52–57. doi: 10.3760/cma.j.issn.0253-2727.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brindisi G., Marazzato M., Brunetti F., De Castro G., Loffredo L., Carnevale R., et al. Allergic rhinitis, microbiota and passive smoke in children: a pilot study. Pediatr. Allergy Immunol. 2022;33(Suppl 27):22–26. doi: 10.1111/pai.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montano-Velazquez B.B., Flores-Rojas E.B., Garcia-Vazquez F.J., Jurado-Hernandez S., Venancio Hernandez M.A., Alanis Flores A.K., et al. Effect of cigarette smoke on counts of immunoreactive cells to eotaxin-1 and eosinophils on the nasal mucosa in young patients with perennial allergic rhinitis. Braz J Otorhinolaryngol. 2017;83(4):420–425. doi: 10.1016/j.bjorl.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polosa R., Knoke J.D., Russo C., Piccillo G., Caponnetto P., Sarva M., et al. Cigarette smoking is associated with a greater risk of incident asthma in allergic rhinitis. J. Allergy Clin. Immunol. 2008;121(6):1428–1434. doi: 10.1016/j.jaci.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 7.Stapleton M., Howard-Thompson A., George C., Hoover R.M., Self T.H. Smoking and asthma. J. Am. Board Fam. Med. 2011;24(3):313–322. doi: 10.3122/jabfm.2011.03.100180. [DOI] [PubMed] [Google Scholar]
- 8.Ugurel E., Kisakurek Z.B., Aksu Y., Goksel E., Cilek N., Yalcin O. Calcium/protein kinase C signaling mechanisms in shear-induced mechanical responses of red blood cells. Microvasc. Res. 2021;135 doi: 10.1016/j.mvr.2020.104124. [DOI] [PubMed] [Google Scholar]
- 9.Guyton M.K., Das A., Samantaray S., Wallace GC Th, Butler J.T., Ray S.K., et al. Calpeptin attenuated inflammation, cell death, and axonal damage in animal model of multiple sclerosis. J. Neurosci. Res. 2010;88(11):2398–2408. doi: 10.1002/jnr.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin I.J., Zhou Z., Crusselle-Davis V.J., Moghimi B., Gandhi K., Anantharaman A., et al. Calpeptin increases the activity of upstream stimulatory factor and induces high level globin gene expression in erythroid cells. J. Biol. Chem. 2009;284(30):20130–20135. doi: 10.1074/jbc.M109.001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuo J., Hu Z., Liu T., Chen C., Tao Z., Chen S., et al. Calpeptin attenuates cigarette smoke-induced pulmonary inflammation via suppressing calpain/IkappaBalpha signaling in mice and BEAS-2B cells. Pathol. Res. Pract. 2018;214(8):1199–1209. doi: 10.1016/j.prp.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Khodir A.E., Ghoneim H.A., Rahim M.A., Suddek G.M. Montelukast attenuates lipopolysaccharide-induced cardiac injury in rats. Hum. Exp. Toxicol. 2016;35(4):388–397. doi: 10.1177/0960327115591372. [DOI] [PubMed] [Google Scholar]
- 13.Wei X., Yan F., Ming E., Zhang C., Li G., Yang X., et al. Neuroprotective effect of calpeptin on acrylamide-induced neuropathy in rats. Neurochem. Res. 2015;40(11):2325–2332. doi: 10.1007/s11064-015-1722-y. [DOI] [PubMed] [Google Scholar]
- 14.Jiao W.E., Wei J.F., Kong Y.G., Xu Y., Tao Z.Z., Chen S.M. Notch signaling promotes development of allergic rhinitis by suppressing Foxp3 expression and treg cell differentiation. Int. Arch. Allergy Immunol. 2019;178(1):33–44. doi: 10.1159/000493328. [DOI] [PubMed] [Google Scholar]
- 15.Matsuki A., Takatori H., Makita S., Yokota M., Tamachi T., Suto A., et al. T-bet inhibits innate lymphoid cell-mediated eosinophilic airway inflammation by suppressing IL-9 production. J. Allergy Clin. Immunol. 2017;139(4):1355–1367. doi: 10.1016/j.jaci.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Stachowski J., Barth C., Michalkiewicz J., Krynicki T., Jarmolinski T., Runowski D., et al. Th1/Th2 balance and CD45-positive T cell subsets in primary nephrotic syndrome. Pediatr. Nephrol. 2000;14(8–9):779–785. doi: 10.1007/PL00013437. [DOI] [PubMed] [Google Scholar]
- 17.Luo Q., Lin J., Zhang L., Li H., Pan L. The anti-malaria drug artesunate inhibits cigarette smoke and ovalbumin concurrent exposure-induced airway inflammation and might reverse glucocorticoid insensitivity. Int. Immunopharm. 2015;29(2):235–245. doi: 10.1016/j.intimp.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Strzelak A., Ratajczak A., Adamiec A., Feleszko W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int. J. Environ. Res. Publ. Health. 2018;15(5) doi: 10.3390/ijerph15051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao T.C., Chang S.W., Hua M.C., Liao S.L., Tsai M.H., Lai S.H., et al. Tobacco smoke exposure and multiplexed immunoglobulin E sensitization in children: a population-based study. Allergy. 2016;71(1):90–98. doi: 10.1111/all.12775. [DOI] [PubMed] [Google Scholar]
- 20.Van Hove C.L., Moerloose K., Maes T., Joos G.F., Tournoy K.G. Cigarette smoke enhances Th-2 driven airway inflammation and delays inhalational tolerance. Respir. Res. 2008;9:42. doi: 10.1186/1465-9921-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rider C.F., Carlsten C. Air pollution and resistance to inhaled glucocorticoids: evidence, mechanisms and gaps to fill. Pharmacol. Ther. 2019;194:1–21. doi: 10.1016/j.pharmthera.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Kawagoe J., Maeda Y., Kikuchi R., Takahashi M., Fuchikami J.I., Tsuji T., et al. Differential effects of dexamethasone and roflumilast on asthma in mice with or without short cigarette smoke exposure. Pulm. Pharmacol. Ther. 2021;70 doi: 10.1016/j.pupt.2021.102052. [DOI] [PubMed] [Google Scholar]
- 23.Thomson N.C., Chaudhuri R., Livingston E. Asthma and cigarette smoking. Eur. Respir. J. 2004;24(5):822–833. doi: 10.1183/09031936.04.00039004. [DOI] [PubMed] [Google Scholar]
- 24.Filiberto A.C., Maccani M.A., Koestler D., Wilhelm-Benartzi C., Avissar-Whiting M., Banister C.E., et al. Birthweight is associated with DNA promoter methylation of the glucocorticoid receptor in human placenta. Epigenetics. 2011;6(5):566–572. doi: 10.4161/epi.6.5.15236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbeck Y.E., Gulevich R.G., Amelkina O.A., Plyusnina I.Z., Oskina I.N. Conserved methylation of the glucocorticoid receptor gene exon 1(7) promoter in rats subjected to a maternal methyl-supplemented diet. Int. J. Dev. Neurosci. 2010;28(1):9–12. doi: 10.1016/j.ijdevneu.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Lamothe J., Khurana S., Tharmalingam S., Williamson C., Byrne C.J., Khaper N., et al. The role of DNMT and HDACs in the fetal programming of hypertension by glucocorticoids. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/5751768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filiberto A.C., Maccani M.A., Koestler D., Wilhelm-Benartzi C., Avissar-Whiting M., Banister C.E., et al. Birthweight is associated with DNA promoter methylation of the glucocorticoid receptor in human placenta. Epigenetics. 2011;6(5):566–572. doi: 10.4161/epi.6.5.15236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakao F., Ihara K., Kusuhara K., Sasaki Y., Kinukawa N., Takabayashi A., et al. Association of IFN-gamma and IFN regulatory factor 1 polymorphisms with childhood atopic asthma. J. Allergy Clin. Immunol. 2001;107(3):499–504. doi: 10.1067/mai.2001.113051. [DOI] [PubMed] [Google Scholar]
- 29.Tliba O., Damera G., Banerjee A., Gu S., Baidouri H., Keslacy S., et al. Cytokines induce an early steroid resistance in airway smooth muscle cells: novel role of interferon regulatory factor-1. Am. J. Respir. Cell Mol. Biol. 2008;38(4):463–472. doi: 10.1165/rcmb.2007-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newton R., Shah S., Altonsy M.O., Gerber A.N. Glucocorticoid and cytokine crosstalk: feedback, feedforward, and co-regulatory interactions determine repression or resistance. J. Biol. Chem. 2017;292(17):7163–7172. doi: 10.1074/jbc.R117.777318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah S., King E.M., Mostafa M.M., Altonsy M.O., Newton R. DUSP1 maintains IRF1 and leads to increased expression of IRF1-dependent genes: a mechanism promoting glucocorticoid insensitivity. J. Biol. Chem. 2016;291(41):21802–21816. doi: 10.1074/jbc.M116.728964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnes P.J. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2013;131(3):636–645. doi: 10.1016/j.jaci.2012.12.1564. [DOI] [PubMed] [Google Scholar]
- 33.Mei D., Tan W., Wong W. Pharmacological strategies to regain steroid sensitivity in severe asthma and COPD. Curr. Opin. Pharmacol. 2019;46:73–81. doi: 10.1016/j.coph.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Ito K., Chung K.F., Adcock I.M. Update on glucocorticoid action and resistance. J. Allergy Clin. Immunol. 2006;117(3):522–543. doi: 10.1016/j.jaci.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida M., Miyasaka Y., Ohuchida K., Okumura T., Zheng B., Torata N., et al. Calpain inhibitor calpeptin suppresses pancreatic cancer by disrupting cancer-stromal interactions in a mouse xenograft model. Cancer Sci. 2016;107(10):1443–1452. doi: 10.1111/cas.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Britt R.J., Thompson M.A., Sasse S., Pabelick C.M., Gerber A.N., Prakash Y.S. Th1 cytokines TNF-alpha and IFN-gamma promote corticosteroid resistance in developing human airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2019;316(1):L71–L81. doi: 10.1152/ajplung.00547.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang K., Gharaee-Kermani M., McGarry B., Remick D., Phan S.H. TNF-alpha-mediated lung cytokine networking and eosinophil recruitment in pulmonary fibrosis. J. Immunol. 1997;158(2):954–959. [PubMed] [Google Scholar]
- 38.Baggiolini M., Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992;307(1):97–101. doi: 10.1016/0014-5793(92)80909-z. [DOI] [PubMed] [Google Scholar]
- 39.Howarth P.H., Babu K.S., Arshad H.S., Lau L., Buckley M., McConnell W., et al. Tumour necrosis factor (TNFalpha) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax. 2005;60(12):1012–1018. doi: 10.1136/thx.2005.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study has been deposited at Figshare; https://doi.org/10.6084/m9.figshare.22004057.v1.