Abstract
To commercialize functional foods, probiotics must exhibit high resistance and acceptable stability under various unfavorable conditions to maintain the quality of fruit juices. This study will provide an insight into fortification of orange juice with a plant probiotic Kocuria flava Y4 by microencapsulation. Therefore, this study investigated the colony release, physicochemical and phytochemical parameters, and antioxidant activity of the orange juice exposed to microencapsulated probiotics and the one without probiotics (control). Evaluation of orange juice on the growth of probiotic bacteria showed that the fortification with alginate and psyllium micro-particles showed highest encapsulation efficiency (99.01%) and acceptable viability of probiotic cells (8.12 ± 0.077 CFU/mL) during five weeks storage at 4 °C. The morphology and functional properties of beads was studied by SEM, Zeta-potential and FTIR analysis. The sucrose and organic acids concentrations decreased significantly during fortification period (0–72 h) except ascorbic acid. Furthermore, glucose, pH, acidity, TSS were maintained. The results affirm the suitability and feasibility of developing a plant probiotic beverage using orange juice by encapsulation method.
Keywords: Fortification, Probiotics, Encapsulation, Storage, Orange juice
Graphical abstract
Highlights
-
•
Development of a novel functional beverage (orange juice) through the incorporation of a plant probiotic Kocuriaflava Y4 encapsulated into psyllium-alginate microparticles, with proven increase of glucose, phytochemical properties and antioxidant activity.
-
•
The functional beverage formulations presented counts of 8.12 ± 0.07 log CFU/mL after 5 weeks at 4 °C. Therefore, orange juice can act as an ideal food matrix for probiotic beverage of K. flava Y4.
-
•
This is the first study that evaluated the impact of plant probiotic on fortification of beverage to increase consumer acceptability.
Abbreviation
- PG
Persian Gum
- EE
Encapsulation efficiency
- TSS
Total soluble solids
- TTA
Total titratable acidity
- HPLC
High performance liquid chromatography
- TFC
Total flavonoid content
- TPC
Total phenolic content
- DPPH
2,2-diphenyl-1-picryl-hydrazyl-hydrate
1. Introduction
Consumers are currently interested in functional foods and beverages that are both very nutritious and healthy, thus incorporating live probiotic bacteria into dairy and non-dairy diets is one possibility. Fermented milks, pharmaceutical, and dairy products typically provide probiotics. In addition, intolerance of lactose and changing way of life (vegetarians and vegans) have raised interest in non-dairy probiotic foods like granolas, meats, juices, dry fruits, jams, and some plant-based materials for transportation of probiotics [1,2]. Non-dairy beverages have recently been identified as potential dietary matrices for probiotics. Additionally, plant-based materials contain functional components [3]. In this context, beverages from fruits are gaining popularity because they provide a simple and comfortable way to ingest fruits that are high in health-promoting components [4]. Thus, the demand for high-quality, fresh fruit juices are constantly expanding around the world, especially as there is a growing awareness of the benefits of integrating healthy juices and fluids in the human diet instead of soft drinks and sweetened beverages [5]. Consumption of citrus fruits and their products has been reported to be inversely related to various diseases. Moreover, orange juice is an ideal transporter of probiotic microbes since it contains vital components, has a pleasant flavor, and is popular among people of various ages. However, research has identified significant challenges for production of these food products, including the problem of live probiotics survival in harsh environments. Similarly, drinks of non-dairy probiotics currently in the market have a relatively short shelf life [6]. Additionally, viability and functional activity of probiotics are readily deteriorated when exposed to numerous adverse circumstances that occur during processing of food (e.g., high degree of heat), preservation (e.g. acids, oxygen and other specific components in food) and transportation. To prevent such viability losses, effective probiotic microencapsulation into a matrix of polymer as a protective layer is a potential strategy that also acts as a probiotic micro vehicle [[7], [8], [9]]. The above approach has been proven to promote not just to cell viability in harsh surrounding environments, but also growth of cell and fermentation efficiency. Particularly, most commonly used assisting material is calcium alginate because it is simple, non-toxic, biocompatible and inexpensive [10]. However, owing of its poor permanence due to existence of Ca2+ and anti-gelling removing cations (magnesium ions and sodium), its employment as a micro vehicle system may be limited. Furthermore, alginate gels have elevated porosity and are susceptible to environment of low pH, which might result in an unwanted faster and earlier cell leakage. Earlier studies indicated that either adding other polymer matrices to alginate beads or coating them by poly cations (e.g., poly-l-lysine, chitosan) could enhance the permanence and safety of the alginate capsule. Poly-catalytic hydrogels can make microencapsulated beads with outstanding stability and durability through ion exchange among a negatively charged alginate structure. Poly cationic compounds (like poly-l-lysine, chitosan, and gelatine) have demonstrated the potential to make ion-exchange relationships with alginate structures having negative charge; hence performance of the encapsulating matrix improves. Alginate in combination with the other biopolymers (having properties of prebiotic) can provide the significance of probiotics. Prebiotic properties are generally present in numerous herbal-based polymers, like psyllium [11].
The extracted psyllium from Plantago genus, in its physiological state acts as a cationic herbal gel. The structure of psyllium gel is arabinoxylan and composed with arabinose (side chains) and xylose (backbone). Furthermore, herbal-based hydrogels like Persian Gum (PG) are inexpensive, innovative, and have prebiotic and growth-promoting characteristics [12]. Persian Gum is a hydrogel produced by mountain almond (Amygdaluss coparia Spach) plants trunk bark under physiological conditions. The gel is a polysaccharide, and is made up of rhamnose, galactose and (1 → 3) β-D–Galp. As a result, if it is correctly adapted for poly (AAm) utilization of N, N-MBAAm and ammonium persulfate (APS), it could be a leading contender for microencapsulation [13].
Despite the fact that certain research on the fortification of orange juice are accessible in the literature, we still don't have much idea about the viability and survival of individual probiotic strain during fortification and the storage period. This study will provide an insight into fortification of orange juice with a plant probiotic Kocuria flava Y4 (isolated and identified in our previous study) by microencapsulation. It is the first report on analysis of physicochemical and phytochemical properties of the functional beverage exposed to probiotic K. flava Y4.
2. Materials and methods
2.1. Microorganisms and inoculum preparation
The Kocuria flava Y4 strain isolated from Dioscorea villosa L. leaves and its characterization made in our previous study [14] has good acid and bile tolerance along with good hydrophobicity. This strain was maintained at 35 °C and stored at 4 °C on nutrient agar slants. It was inoculated in nutrient broth to grow the culture for overnight, and the pellet and supernatant were separated by centrifugation at 10,000 rpm for 15 min. The pellets were then stored at 4 °C for further analysis. Moreover, it is a functional probiotic with robust fermentation capability and produces rich metabolites.
2.2. Extraction of Persian Gum (PG)
Simas- Tosin's method [15] was used with certain changes for gel purification formed from PG. In a nutshell, 30 g of dehydrated PG was dissolved in 1000 mL of distilled water at 70 °C (pH 8) and kept for 12 h. To eliminate insoluble residues, the fumes in the gel were exacerbated by centrifugation with high-speed at 18000×g for 20 min. The purified gel was left to dry at 40 °C in the oven (Bionic scientific, India) and finely grounded before used in the microencapsulation process.
2.3. Psyllium gel extraction
The psyllium gel was extracted using a process explained by Guo et al. [16] with little modifications. In brief, 10 g of dried psyllium husk was dissolved in 200 mL of water at 80 °C and shaken vigorously up to 18 h till the gel was formed. The gel phase was separated by centrifugation at 18000×g for 15 min. Then, 2 M NaOH solutions were dissolved in the separated gel and centrifuged (15 min, 18000×g) after being shaken for 120 min at 37 °C. In the alkaline phase, HCl (2 M) was added to neutralize the solution and the mixture was centrifuged (15 min, 18000×g). As an encapsulation matrix, a yellow colour gel was kept separate and rinsed with distilled water.
2.4. Microencapsulation of probiotics
With slight modifications, probiotic capsules were made using the procedure described by Chaikham et al. [17]. A sterile sodium alginate (3% v/v) solution was mixed with various gums (psyllium and PG) (SRL, India) and cell pellets (20% v/v). The solution was exposed dropwise into a 0.1 M sterilized calcium chloride solution (Himedia, India) by a sterile needle of 0.5 mm (Nipro, Japan) and kept for 30 min for bead formation. The sterile beads were then cleaned with 0.85% (w/v) sterilized saline water and used for fortification of orange juice.
2.5. Orange juice
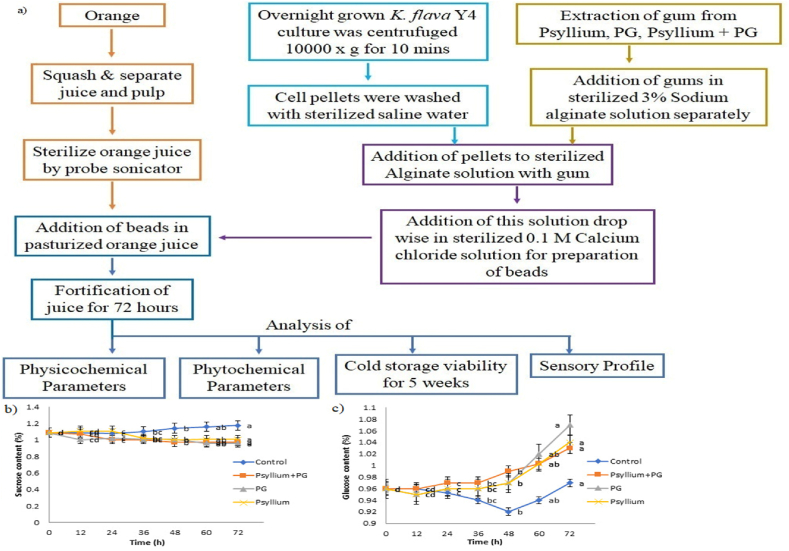
Sweet orange [Citrus sinensis (Mill.) Pers.] a hybrid between pomelo [Citrus maxima (Burm.) Merr.] and mandarin (Citrus reticulata Blanco) locally called as Nagpur santra (generally present all over India and when fully ripened shows brilliant orange colour) were purchased from local market of Bhubaneswar, Odisha, India. The fruits were washed and peeled. The oranges were then squashed, separating the juice and pulp. The juice (100 mL) was sterilized in three Schotts Duran bottle for 15 min by a probe sonicator at 80% amplitude (on/off per 3 s). Following this, the pasteurized juices were inoculated with Kocuria flava Y4 entrapped in three different encapsulants (Psyllium, PG, Psyllium + PG). Pasteurized orange juices with three different encapsulants exhibited a microbiological load (2.63 CFU/mL) within permissible range. These samples were further fortified for 72 h at 37 °C in an incubator (Fig. 2a). The living cells in the samples were tallied using the conventional process. In this process a total of 0.5 g of microcapsules were kept in 10 mL of sterile phosphate buffer (pH 7.0) for 1 h at 30 °C. After grinding the microcapsules into a solution, the encapsulated bacteria were released. Following serial dilutions, the bacteria were cultured on nutrient agar plates to check their viability.
Fig. 2.
a) Processing flow chart of fortified orange juice b) Sucrose utilization during fortification of orange juice c) Glucose utilization during fortification of orange juice. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.6. Physicochemical properties
2.6.1. TTA, pH and total soluble solids (TSS) determination
After diluting the juice (1:10), TTA was determined by titrating against 0.1 N NaOH solution to a pH 8.5 endpoint, then results were expressed as percentiles of total acidity. A pH meter (Esico, India) was used to determine the pH of the juice at 25 °C. The TSS (°Brix) in the juices was measured using a benchtop digital refractometer (HI96801 refractometer, India).
2.6.2. Determination of sugars and organic acid
The HPLC system (LC-10A HPLC Series, Shimadzu, Kyoto, Japan) incorporated by a pump was used for sugar such as glucose and sucrose (Sigma Aldrich) analysis by a detector of RID-10A (refractive index) and organic acid by a sensor of UV/Vis (SPD-20A) operating at 210 nm. Simultaneously organic acids and sugars were evaluated on a Bio-Rad Aminex HPX-87H column (300 × 7.8 mm) at 55 °C. The following analytical conditions were used: 0.3 mL min−1 flow rate, 0.045 N H2SO4 eluent with 6% acetonitrile (v/v).
2.7. Phytochemical properties
2.7.1. Determination of total anthocyanin content
The pH differential absorbance process was used to calculate total anthocyanin content of orange juice [18]. Diluting the samples with potassium chloride (pH 1.0) and sodium acetate (pH 4.5) buffers resulted in absorbance less than 1.00 at wavelengths of 510 and 700 nm respectively. The orange juice absorbance was calculated using the following equation (1):
| (1) |
The anthocyanin concentration was observed by cyanidin-3-glucoside, and the outcomes were represented as total anthocyanin in mg present per liter of sample.
| (2) |
Where, A = orange juice absorbance, MW = Cyanidin3-glucoside molecular weight (449.2 g/mol), DF = Dilution Factor of orange juice (150), L = Path length (1 cm).
2.7.2. Total flavonoid content (TFC)
The process used to determine the TFC was proposed by Viacava et al. [19]. The reaction mixture with composition of 1.28 mL of deionized H2O and 0.06 mL of NaNO2 (50 g/L) (SRL, India) were added with 0.2 mL of ethanolic extracts. Adding 60 μL of AlCl3 (100 g/L) (SRL, India) after 5 min at 20 °C was accompanied by the inclusion of 0.4 mL of NaOH (40 g/L) (SRL, India) 6 min later under same conditions. After mixing the mixtures, the absorbance at 510 nm was observed using a microplate reader. The concentration of total flavonoid was found in mg of quercetin equals 100/mL (SRL, India) of orange juice. The standard curve for quercetin was constructed in the range of 0.05–1.2 g/L.
2.7.3. Total phenolic content (TPC)
The analysis of TPC was performed in accordance with Cassani et al. [20], including slight changes. In brief, an extract of 15 μL was added in a well along with 75 μL of water-diluted folin-ciocalteu reagent (1:10). Following after 3 min of incubation at room temperature, 60 μL of 7.5% sodium carbonate solution was mixed to the reaction solution and kept for 120 min incubation. Following incubation period, every sample was monitored in triplicate at 765 nm using a spectrophotometric microplate reader (BIO RAD, USA). To compare absorbance values, a calibration curve of gallic acid was employed. The findings are represented by mg gallic acid equivalent (GAE) per 100 g of fresh product.
2.7.4. DPPH radical scavenging activity determination
The orange juice DPPH radical scavenging activity was monitored using the procedure given by Mousavi et al. [21] with some modification. 2.9 mL of DPPH solution was added to a 0.1 mL volume of sample. After vigorous agitation, the mixed solution was placed in a dark condition for 30 min. The decrease of DPPH in the mixed solution was evaluated using UV–Vis spectrophotometer (Thermo Fisher Scientific, USA) to measure the samples absorbance against a control (DPPH solution without sample) as blank at 517 nm. The inhibition percentage (%) was computed by using the following equation (3):
| (3) |
2.8. Encapsulated probiotic microcapsule analysis
-
a)
Morphological analysis
Surface morphology of K. flava Y4 beads encapsulated with psyllium and sodium alginate was analyzed under SEM (Hitachi, Flex SEM 1000) at 10 kV and magnification 37× and 1000×. Samples were prepared for SEM carefully by lyophilization.
Using a Zetasizer (Nano ZS, Malvern Instruments, UK), the zeta potentials of the microencapsulated beads were measured for confirmation of psyillium and sodium alginate surrounding layers. After washing the beads with sterile distilled water, they were loaded into the capillary cell containing a small amount of deionized water. Then the analysis was carried out at 25 °C with 90°scattering angle.
-
b)
FTIR Analysis
In order to identify the functional groups associated with K. flava Y4 encapsulated with psyllium and sodium alginate beads, FTIR spectroscopic analysis was performed. The analysis was conducted using attenuated total reflectance-Fourier transform infrared spectroscopy (ATR/FT-IR-4600, JASCO, Japan). Peaks represent IR transmittance at specified frequencies, and IR spectra tables were used to analyze them.
2.9. Effect of storage conditions on probiotic cell viability in orange juice
Probiotic-fortified orange juice was kept at 4 ± 1 °C for five weeks. At seven-day intervals, the viability of probiotics (log CFU/mL) in juice sample was investigated until the cell load reached 6 log CFU/mL.
2.10. Sensory profile
The sensory profile study was carried out to determine the consumer acceptability of fortified orange juice prototypes encapsulated with various encapsulants (pysillum, PG, pysllium + PG). The sensory evaluation study was conducted through hedonic tests for inexperienced participants to evaluate a product. The sensory analysis included 160 inexperienced people, mostly students and staff from Centre for Biotechnology, Siksha 'O' Anusandhan (Deemed to be University), Bhubaneswar. Participants were asked to complete a questionnaire that included demographic and questions regarding familiarity with beverage, as well as a consent form. The 9-point hedonic scale and the food action rating scale test were used to evaluate the acceptance by consumers. Using a 9-point hedonic scale, participants rated the prototype on attributes such as aroma, taste, colour, sweetness, and overall preference. The food action rating scale test used a 9-point scale to assess the frequency of drinking. After collecting the data, the consumer acceptability was assessed.
2.11. Statistical analysis
All experimental studies were carried out in triplicate. The outcomes of this study are mean values with standard errors. SPSS statistical software (Version 22.0, IBM, USA) was used to analyze the experimental data. The analysis of variance (ANOVA) (p < 0.05) has been used, and the Tukey Kramer comparison test was used to estimate the significant difference between treatments (p < 0.05).
3. Results
3.1. Characterization of Kocuria flava Y4 as probiotic
From our previous study it was observed that Kocuria flava Y4 has the ability to tolerate low pH environments in the human GIT at 0.3% and 1% concentration of bile, so it was chosen as a potential probiotic. As a result, it exhibited resistance under both aerobic and anaerobic conditions to varying concentrations of bile salt (0.15%, 0.3%, 0.5%, and 1%). It displayed the survivability after 30–50 min of exposure to lysozyme. Pepsin resistance was also observed at pH 2 and pH 3 when exposed for 1 h and 3 h, but less growth was seen at pH 2 than pH 3. The pancreatin resistance was also observed under pH 8 after 3 h and 4 h of exposure.
3.2. Efficiency of encapsulation (EE)
As demonstrated by our results, some vital differences were observed for efficient encapsulation of K. flava Y4 by using different encapsulant gum matrices (psyllium, PG and psyllium + PG). A high entrapment efficiency (99.01%) signifies that viable cells were effectively trapped in the prepared beads, but viable cells are not trapped by only sodium alginate encapsulation due to leakage. Thus, by using psyllium + PG, PG, psyllium gum as encapsulation matrix along with sodium alginate beads efficiently traps the viable cells. The EE for psyllium + PG, PG and psyllium gum were observed to be 97.15%, 97.96% and 99.01% respectively. From the above findings, it is concluded that psyllium gum encapsulation along with alginate beads showed highest EE (99.01%) for viable cells.
3.3. Changes in physicochemical properties during probiotification
3.3.1. Determination of pH, TTA and TSS
Fig. 1 displays the physicochemical parameters of fortified orange juices. The pH and TTA of orange juices were not changed in fortified and non-fortified orange juice during 0–72 h incubation. In the fortified orange juices (containing probiotic K. flava Y4 encapsulated with three encapsulant gums: psyllium, PG and psyllium + PG), the observed range of pH (3.12 ± 0.014 to 3.44 ± 0.028); titratable acidity (0.55 ± 0.094 to 1.64 ± 0.16%) and TSS (3.43 ± 0.42 to 4.4 ± 0.31 °Brix) values were similar to the orange juice without probiotics. The encapsulated probiotic K. flava Y4 maintains pH, acidity and TSS of orange juice as per the control sample.
Fig. 1.
a) a) pH profile during fortification of orange juice b) Total tritable acidity during fortification of orange juice c) TSS profile during fortification of orange juice. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3.2. Sugar contents of the orange juice
Fig. 2 bc depicts the changes in concentration of sugars, namely sucrose and glucose, in each of the four samples (exposed to different encapsulants: psyllium, PG, pysillm + PG, and control) during fortification period. Glucose concentration increased while concentration of sucrose significantly decreased. The initial concentration of sucrose and glucose in the orange juice was 1.09 ± 0.076% and 0.96 ± 0.033% respectively. The sucrose content of orange juices treated with psyllium, PG, psyllium + PG encapsulant matrices and control were observed to be 1.006 ± 0.01%, 0.96 ± 0.028%, 0.976 ± 0.038% and 1.176 ± 0.04% respectively after 72 h of incubation. The concentration of glucose was higher than sucrose with 1.04 ± 0.01%, 1.07 ± 0.074%, 1.03 ± 0.033% and 0.973 ± 0.045% in orange juice treated with psyllium, PG, psyllium + PG encapsulants and control respectively. Thus, it can be concluded that sucrose serves as the primary carbon source for K. flavaY4 during fortification of orange juice. Except control, there is no noticeable difference in sugar consumption by all the samples.
3.3.3. Organic acid content of orange juice
Organic acids make up the majority of non-carbohydrate soluble solids in citrus juices. In orange juice: citric, ascorbic, and malic acids were isolated and identified as organic acids. The most abundant organic acid in orange juice was revealed to be citric acid. The orange juice had an initial citric acid concentration of 5426.41 ± 1.19 mg/L. The concentration of citric acid in orange juice treated with microencapsulated K. flava Y4 in three different gel formulations containing gum matrices (psyllium, PG, pysillium + PG) and without K. flava Y4 (control) for 72 h incubation were found to be 3613.57 ± 2.9 mg/L, 4617.85 ± 1.45 mg/L, 6064 ± 1.5 mg/Land 5577.27 ± 1.04 mg/L respectively. Thus the findings reveal that the encapsulant of psyllium gum shows least concentration of citric acid in comparison to other encapsulants and the control (Fig. 3a). The second most abundant organic acid in orange juice was malic acid. Malic acid in orange juice had an initial concentration of 19.26 ± 0.118 mg/L. The malic acid concentration of orange juice treated with designed gel formulations for K. flavaY4 encapsulation (Psyllium, PG, Psyllium + PG) and control (orange juice without probiotic K. flava Y4) were 14.36 ± 0.038 mg/L, 15.21 ± 0.02 mg/L, 19.46 ± 0.062 mg/L and 19.52 ± 0.057 mg/L respectively after completion of incubation for 72 h. In accordance to the findings, the encapsulant of psyllium gum with K. flava Y4 produced malic acid in small amounts than other formulations and control (Fig. 3b). Ascorbic acid, an important antioxidant, is abundant in citrus juices, particularly orange juice. Its concentration is a good indicator of orange juice quality. The initial concentration of ascorbic acid in orange juice was found to be 4.84 ± 0.051 g/L in our study. Ascorbic acid concentrations in orange juice after fortification with K. flava Y4 encapsulated with three different gums matrixes (psyllium, PG, psyllium + PG) and control (orange juice without probiotics) were observed to be 8.98 ± 0.085 g/L, 5.046 ± 0.039 g/L, 4.26 ± 0.033 g/L and 5.35 ± 0.098 g/L, respectively. On the basis of our results, psyllium gum encapsulant for K. flava Y4 produces more ascorbic acid in orange juice than other encapsulants and control (Fig. 3c).
Fig. 3.
a) Citric acid utilization during fortification of orange juice b) Malic acid utilization during fortification of orange juice c) Ascorbic acid utilization during fortification of orange juice. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. Changes in phytochemical properties during probiotification
3.4.1. Total anthocyanin content of orange juice
The concentration of total anthocyanin in orange juice increases during fortification period. The fortified orange juice with probiotics, K. flava Y4 encapsulated in different gums (psyllium, PG, PG + psyllium) as encapsulation matrices and without probiotic K. flavaY4 (control) increased progressively (Fig. 4a). From the results, it was observed that the psyllium gum encapsulation served as best matrix as the anthocyanin concentration of the fortified orange juice (containing psyllium gum encapsulants) enhanced significantly till 72 h. However, no significant difference in anthocyanin concentration of fortified juice exposed to K. flava Y4 (encapsulated with PG and psyllium + PG matrices) and control was observed after 36 h of fermentation. This result could be due to the poor growth of K. flavaY4 after 36 h. As per result, orange juice with K. flava Y4 (encapsulated with psyllium encapsulant matrices) has a greater ability to increase anthocyanine concentration than orange juice treated with K. flava Y4 (encapsulated with PG and psyllium + PG encapsulant matrices).
Fig. 4.
a) Total anthocyanin profile of orange juice during fortification b) Total flavonoid profile of orange juice during fortification c) Total phenolic content of orange juice during fortification. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4.2. Total flavonoid content orange juice
Orange juice fermented with K. flava Y4 probiotics encapsulated in different encapsulated gum matrix (psyllium, PG, psyllium + PG) and without probiotic K. flava Y4 (control) showed enhancement in the total flavonoid content with increase in time (Fig. 4b). After 36 h, the total flavonoid content in fortified juice with probiotic K. flava Y4 encapsulated with psyllium gum matrix increased significantly followed by PG encapsulant, while there was no significant rise in flavonoid content of the fortified juice with K. flava Y4 encapsulated in psyllium + PG mixed gum matrices and control. This result could be attributed to slow growth of K. flava Y4 after 36 h. Moreover, the juice containing probiotic K. flava Y4 encapsulated in psyllium gum matrices has more flavonoid content (73.2 ± 1.23 mg equivalent to quercetin/100 mL) when compared to other encapsulants (PG and psyllium + PG gum matrices), and the control.
3.4.3. Total phenolic content of orange juice
Fig. 4c depicts the total phenolic concentration of orange juice fortified with the probiotic K. flava Y4 encapsulated in different gums (psyllium, PG and psyllium + PG) and without probiotic K. flava Y4 (control). The phenol concentration of orange juice exposed to the probiotic K. flavaY4, encapsulated in psyllium was highest (260.33 ± 1.18 mg gallic acid equivalent/100 mL) at 36 h followed by 154.5 ± 0.47 (12 h), 147.7 ± 1.19 (72 h), 135.66 ± 1.18 (48 h), 126 ± 1.55 (60 h), 107 ± 0.77 (24 h) and 100 ± 0.77 (0 h). It was also observed that the total phenol content of fortified orange juice (exposed to probiotic K. flava Y4 encapsulated with psyllium + PG) was highest (163 ± 1.24 mg of gallic acid equivalent/100 mL) in 36 h followed by 121 ± 0.93 (48 h), 119 ± 1.18 (12 h), 100 ± 0.77 (0 h) 68.53 ± 1.02 (24 h), 57.2 ± 0.84 (60 h) and 34.43 ± 1.12 (72 h). Fortification of orange juice with probiotic K. flava Y4 (encapsulated in PG) displayed highest phenol concentration (152.56 ± 0.98 mg gallic acid equivalent/100 mL) at 24 h followed by 76.46 ± 1.04 (36 h), 120 ± 0.77 (48 h), 16.8 ± 0.85 (60 h), 146.33 ± 1.18 (72 h) 100 ± 0.77 (0 h) and 109.5 ± 1.12 (12 h). Although, the concentration of phenol in the control was highest (163 ± 1.24 mg gallic acid equivalent/100 mL juice) at 36 h followed by 121 ± 0.93 (48 h), 119 ± 1.18 (12 h), 57.2 ± 0.84 (60 h), 100 ± 0.77 (0 h), 68.53 ± 1.02 (24 h) and 34.43 ± 1.12 (72 h). From the above findings, it is concluded that the encapsulation of the probiotic K. flava Y4 using the psyllium gum encapsulant gives the best results. The growth of bacteria comes to the declining stage after 36 h of fortification, resulting in an insignificant increase in total phenols.
3.4.4. DPPH radical scavenging activity of orange juice
The DPPH radical scavenging activity represents the antioxidant property of orange juice. In our study, the DPPH radical scavenging activity of orange juice exposed to probiotic K. flavaY4 encapsulated with three different gums (psyllium, PG and psyllium + PG) and without probiotic K. flavaY4 (control) are shown in Table 1. The control shows same antioxidant activity at different time periods [68.46 ± 0.93 (0 h), 67.62 ± 0.57 (12 h), 68.43 ± 1.01 (24 h), 68.43 ± 1.01 (36 h), 68.62 ± 0.94 (48 h), 68.43 ± 1.01 (60 h), 68.11 ± 0.58 (72 h)]. The orange juice with probiotic K. flavaY4 encapsulated in psyllium + PG gum matrices showed initial decrease in DPPH radical scavenging activity [68.46 ± 0.93 (0 h), 61.23 ± 0.62 (12 h), 56.29 ± 0.59 (24 h)], but it started increasing from 36 h: 62.55 ± 0.47 (36 h), 65.91 ± 0.44 (48 h), 65.35 ± 0.67 (60 h), 68.6 ± 0.55 (72 h). The antioxidant activity of the fortified orange juice containing micro encapsulants of PG gum matrices remained almost same at different time intervals [68.46 ± 0.93 (0 h), 67.77 ± 0.83 (12 h), 67.77 ± 0.83 (24 h), 68.37 ± 0.78 (36 h), 68.62 ± 0.47 (48 h), 68.86 ± 0.31 (60 h)] but increased to 70.25 ± 0.62 at 72 h. Whereas the orange juice with probiotic K. flava Y4 encapsulated within psyllium gum matrices demonstrated increased antioxidant activity as follows: 68.46 ± 0.93 (0 h), 67.7 ± 0.41 (12 h), 68.5 ± 0.67 (24 h), 70.23 ± 0.68 (36 h), 70.43 ± 0.63 (48 h), 71.6 ± 0.58 (60 h), 72.61 ± 0.44 (72 h). Thus it can be concluded that psyllium gum encapsulants has shown highest antioxidant properties as compared to encapsulants of PG, psyllium + PG gum and control.
Table 1.
Changes in DPPH radical scavenging (Antioxidant) activity of fortified orange juice.
| Time | Control | Psyllium + PG | PG | Psyllium |
|---|---|---|---|---|
| 0 h | 68.46 ± 0.93a | 68.46 ± 0.93a | 68.46 ± 0.93a | 68.46 ± 0.93a |
| 12 h | 67.62 ± 0.57a | 61.23 ± 0.62b | 67.77 ± 0.83a | 67.7 ± 0.41a |
| 24 h | 68.43 ± 1.01a | 56.29 ± 0.59b | 67.77 ± 0.83ab | 68.5 ± 0.67a |
| 36 h | 68.43 ± 1.01ab | 62.55 ± 0.47b | 68.37 ± 0.78ab | 70.23 ± 0.68a |
| 48 h | 68.62 ± 0.94ab | 65.91 ± 0.44b | 68.62 ± 0.47ab | 70.43 ± 0.63a |
| 60 h | 68.43 ± 1.01ab | 65.35 ± 0.67b | 68.86 ± 0.31ab | 71.6 ± 0.58a |
| 72h | 68.11 ± 0.58b | 68.6 ± 0.55b | 70.25 ± 0.62ab | 72.61 ± 0.44a |
Above data represents the mean ± standard deviation (p < 0.05) by taking triplicate samples with significant and non-significant value.
ab Within a row, means without a common superscript differ (p < 0.05).
3.5. Encapsulated probiotic microcapsules analysis
-
a)
Morphological analysis
The SEM analysis was performed to study the surface morphology of beads along with the cells in it (Fig. 5a). It was observed that the microbeads had rough surface with visible wrinkles and presence of K. flava Y4 cells inside the beads. The elemental composition of microbeads was evaluated by EDS analysis and the spectra displayed sodium, chloride and calcium peaks (Fig. 5b.)
Fig. 5.
a) Representing the SEM image of psyllium and sodium alginate beads with K. flava Y4 b) EDS spectra of beads c) Representing the FTIR spectra of psyllium and sodium alginate beads with K. flava Y4.
In order to confirm the bi-layering of the beads, the zeta potential was used to measure their electrochemical properties [22]. The Zeta potential of psyllium with sodium alginate bead was found to be −16.6 mV which demonstrated stability of the bead surface making it desirable for fortification of orange juice.
-
b)
FTIR analysis
Chemical structure and characteristics of functional groups were determined by FTIR analysis. The FTIR spectra in Fig. 5c indicated the presence of functional groups in K. flava Y4 microcapsules encapsulated with psyllium and sodium alginate like alkyne, amine at 3322.75 cm−1, 2115.53 cm− 1and 1623.77 cm− 1with strong sharp band with C–H stretching, strong band with C≡C stretching and medium band with N–H bending respectively. The spectra at 1297.86 cm−1 showed functional group such as aromatic ester having strong band with C–O stretching. The spectra at 1083.8 cm-1 and 1029.8 cm-1 demonstrated one functional group (fluoro compound) with medium band with C–N stretching. The other spectra at 1418.39 cm-1 showed only medium band with O–H bending but no functional groups and at 2359.48 no band and functional groups were observed.
3.6. Effect of storage conditions on the viability of probiotic cells in orange juice
For maximum health benefits, the cell viability score of the probiotic beverages must be greater than 6 log CFU/mL [23]. The fortified orange juices with K. flava Y4 encapsulated in sodium alginate by using different gums (psyllium, PG, psyllium + PG) to prevent leakage and were preserved in cold condition at 4 °C for five weeks. The viability of probiotic cells were investigated at one-week interval. Table 2 showed the viability of K. flava Y4 encapsulated with various encapsulant matrices in orange juice and the highest viability of K. flava Y4 was noticed with encapsulant of psyllium gum. The data confirmed that the probiotic K. flava Y4 can survive in fortified orange juice for five weeks at 4 ± 1 °C. The viability of K. flava Y4 cells in alginate and gum encapsulations were unaffected by the cold storage. The viability K. flava Y4 encapsulated with psyllium gum remained same (8.12 ± 0.077 log CFU/mL) throughout the five weeks cold storage conditions. However, K. flava Y4 encapsulated with PG and psyllium + PG showed a substantial decline in cell viability during storage at cold condition. The above decrease in cell load could be attributed to the poor nutrient accessibility, oxygen level, and pH of the sample.
Table 2.
Total microbial count during fortification and cold storage of orange juice on viability (Log CFU/mL) of K. flava Y4.
| Time (h) | Psyllium + PG | PG | Psyllium |
|---|---|---|---|
| 0 | 2.63 ± 0.27a | 2.63 ± 0.27a | 2.63 ± 0.27a |
| 12 | 3.14 ± 0.089b | 3.37 ± 0.027b | 4.66 ± 0.24a |
| 24 | 3.73 ± 0.054b | 4.32 ± 0.18ab | 5.54 ± 0.18a |
| 36 | 4.66 ± 0.11b | 5.89 ± 0.18ab | 6.24 ± 0.134a |
| 48 | 5.84 ± 0.19b | 6.53 ± 0.25ab | 7.82 ± 0.15a |
| 60 | 6.57 ± 0.19b | 7.35 ± 0.14ab | 8.36 ± 0.14a |
| 72 | 6.81 ± 0.13b | 7.55 ± 0.16ab | 8.58 ± 0.24a |
| Time (Weeks) | |||
| 0 | 6.81 ± 0.13b | 7.55 ± 0.16ab | 8.58 ± 0.24a |
| 1 | 6.45 ± 0.054b | 7.55 ± 0.19ab | 8.37 ± 0.11a |
| 2 | 6.36 ± 0.073b | 7.27 ± 0.13ab | 8.34 ± 0.113a |
| 3 | 5.9 ± 0.102b | 6.59 ± 0.16ab | 8.31 ± 0.12a |
| 4 | 5.48 ± 0.12b | 6.64 ± 0.17ab | 8.25 ± 0.1a |
| 5 | 4.95 ± 0.089c | 5.7 ± 0.25b | 8.12 ± 0.077a |
Above data represents the mean ± standard deviation (p < 0.05) by taking triplicate samples with significant and non-significant value.
a-cWithin a row, least squares means without a common superscript differ (p < 0.05) due to microbial count and conditions (fortification and cold storage of orange juice).
3.7. Sensory profile
The sensory value of fortified orange juice exposed to K. flava Y4 in various encapsulation matrices and control (without K. flava Y4) was evaluated. K. flava Y4 with psyllium showed maximum acceptability for the natural beverage, followed by modest permissibility for other encapsulation matrices. Based on a Hedonics scale of 1–9, the K. flava Y4 with psyllium beverage received an overall rating of 8.67 ± 0.031, with others receiving ratings of 7.9 ± 0.042 (PG), 6.82 ± 0.039 (pysillum ± PG) and 8.14 ± 0.046 (control). Other features of juice with K. flava Y4 encapsulated with psyllium rated highest in aroma 7.27 ± 0.001, sweetness 7.31 ± 0.054,taste 7.56 ± 0.018 and colour 6.59 ± 0.046. The other encapsulants showed 7.09 ± 0.085 (PG), 6.33 ± 0.035 (psyllium + PG), 7.01 ± 0.07 (control) for aroma; 7.15 ± 0.039 (PG), 6.67 ± 0.046 (psyllium + PG) and 7.046 ± 0.042 (control) for sweetness; 6.82 ± 0.005 (PG), 6.42 ± 0.054 (psyllium + PG) and 6.94 ± 0.046 (control) for colour and 7.01 ± 0.054 (PG), 5.31 ± 0.042 (psyllium + PG) and 7.15 ± 0.043 (control) for taste. The fortified orange beverage with K. flava Y4 encapsulated with psyllium received a 6.82 ± 0.023 rating on the Food Action Rating Scale Test (1–9), indicating “I like it and would drink it very often.” As a result, it was possible to conclude that orange juice fortified with K. flava Y4 encapsulated by psyllium had preferable nutritional and organoleptic properties for consuming.
4. Discussion
From our findings, psyllium gum encapsulation along with alginate beads showed highest efficiency for viable cells compared to other encapsulant gums. Likewise, other researchers have studied high rates of encapsulation efficiency (nearly 100%) by various encapsulation processes, which proves our results [24].
The K. flava Y4 strain was characterized as a probiotic in our previous study. Some Kocuria sp. plays important role in other field such as Kocuria flava HO-9041 and K. flava CR1 are used for removal of heavy metals [25,26], K. rhizophila Y1 strain act as plant growth promoting rhizobacteria (PGPR) to enhance crop production [27], Kocuria marina strains have more antimicrobial activity and halotolerant ability [28,29].
The encapsulated probiotic K. flava Y4 maintains pH, acidity and TSS of orange juice as per control sample. Our results were found to be similar with those reported by many other authors [2]. The strong buffering capacity of orange juice is connected to the maintenance of pH and titratable acidity [30]. Moreover, the strong buffering capacity of the product may be attributed to the information that several strains, like Lactobacillus casei, can metabolize citric acid. The retention of acidity in juices is intriguing from a sensory standpoint, since rising acidity may lead to a reduction in consumer acceptance of the juices. Moreover, high acidity has been linked to a reduction in the viability of probiotic cultures in products of food [31]. Generally, the probiotic microorganisms break down the simple sugars in the juice producing small amount of organic acids and lowering the pH which reduces the approval of fortified products through the consumers. Whereas, our probiotic K. flava Y4 do not break the sugars in the juice into organic acids thus maintains pH and acidity. TSS maintenance is linked to the fact that sucrose hydrolysis to monomers (glucose and fructose) may take place during the probiotification period [32]. These simple sugars are preferentially devoured through cultures of probiotic, allowing the TSS to be maintained [33]. Other studies also proved our study that the formulations of orange juice acted likewise, with the TSS and pH values maintained by the addition of L. casei and L. paracasei [2,34].
As a result, in orange juice fortification, sucrose serves as the primary carbon source for K. flava Y4. Glucose concentrations increased while sucrose concentrations decreased significantly in all treated orange juice except control. Similar outcomes were obtained when Lactobacillus acidophilus and Lactobacillus plantarum were used to ferment pomegranate juice. After 32 h of fermentation, the rate of degradation of both sugars was dawdling [21]. The ratios and profile of sugars for specific sugars have been proposed as indicator for deciding juice authenticity.
As per the outcomes, psyllium gel plans with K. flava Y4 produced less citric acid and malic acid while production of ascorbic acid was more than other encapsulants and the control. It is indicated that K. flava Y4 with psyllium encapsulant produces high-quality orange juice when compared to others. Similarly, citric acid levels in fresh, hand-squeezed Navelina juice from various areas ranged from 8400 to 12600 mg/L in previous studies, confirming our findings that our juices are less acidic [35]. In this study, the ascorbic acid content of the juices were found to be very similar to that of Navel orange juices produced in Australia (498 mg/L), though higher than that of Valencia orange juices (406 mg/L) and others manufactured in the Mediterranean region from various orange varieties, with concentrations ranging from 386.2 to 620.0 mg/L [36]. Probiotics can generate organic acids by using citric acid and/or sugars, or through amino acids catabolism [37,38].
The anthocyanin contents were increased in fortified juice containing probiotic K. flava Y4 (encapsulated with psyllium encapsulant matrices) during the fortification period. As a result, orange juice with K. flava Y4 (encapsulated with psyllium encapsulant matrices) has a greater ability to increase anthocyanine concentration than orange juice treated with K. flava Y4 (encapsulated with PG and psyllium + PG encapsulant matrices). Similarly [39], found that after fermentation with probiotic L. plantarum, mulberry juice's total anthocyanin concentration increases. Some probiotic lactic acid bacteria exhibit activity of β-glucosidase, which involves the glycosidic linkage cleavage in the mother anthocyanin compound (glycosylated anthocyanins), resulting in the output of glycone compounds (anthocyanidins) [40].
The flavonoid content of orange juice with probiotic K. flava Y4 encapsulated in psyllium matrices is higher than that of other encapsulants (PG and psyllium + PG) used for encapsulation of probiotic K. flava Y4 and the control. Based on our findings, we conclude that encapsulating the probiotic K. flava Y4 with the psyllium gum yields the best result. Likewise, it is observed flavonoids in other fruit juices like pineapple, litchi, watermelon, black jamun, and carambola, with amounts of 12.50 mg/100 mL, 14.50 mg/100 mL, 5.50 mg/100 mL, 58.38 mg/100 mL, and 23.25 mg/100 mL respectively [41]. It was also found in another study that the flavonoid content of lemon [Citrus limon (L.) Burm.] juices ranged from 757 to 847 mg/L [42] and orange juice contained flavonoids ranging from 39.5 to 64.3 mg/100 mL [43]. The advantageous characteristics of lemon juice flavonoids recommend consuming this product on a regular basis to keep a healthy standard of living. There are similar findings for the juices of mandarin [44], bergamot [45], blood orange juice [46] and grapefruit [47]. Flavonoids are almost entirely found in plants as β-glycosides. The glycosidic linkage is generally not cleaved during industrial and domestic food processing. Therefore, flavonoids are generally present in foods as glycosides.
The results concluded that encapsulating probiotic K. flava Y4 in psyllium gum matrix produced higher phenolic content in comparison to other encapsulants (PG and psyllium + PG) and the control. As per our findings, it is confirmed that encapsulant of psyllium gum delivered the best performance. Similarly, in another experiment observed that polyphenolic content increased in the juice of mangoes and sapotas when fermented with the bacteria Lactobacillus plantarum[1].
There are a number of proven benefits to human health associated with phenolic compounds, including antioxidant activity and a role in the color and sensory characteristics of food products [48]. The antioxidant effects of phenols include inhibiting lipid peroxidation [49], decreasing blood platelet aggregation, and increasing vasodilation [50].
Our findings proved that, the encapsulant psyllium gum demonstrated the highest antioxidant properties compared to other encapsulants PG, psyllium + PG and control for encapsulation of K. flava Y4. Similar findings were reported for juices of pomegranate [21], noni [51] and jussara [52]. The improvement in antioxidant potential could be attributed to anthocyanin acylation with phenolic acid or anthocyanin diacylation [53].
SEM analysis determined the morphology of beads and presence of K. flava Y4 cells inside the beads. Similar study was observed by Reddy et al. [54] that the rough surface with visible wrinkles have been displayed on microbeads. Another investigation reported that the viable cells were present inside the beads and also suggested encapsulation is important because encapsulated beads can transfer nutrients and execratory products to cell for viability [55].
In our study the zeta potential of psyllium with sodium alginate bead was found to be −16.6 mV, but previous studies report that the zeta potential of psyllium and sodium alginate to be −7.5 mV and −10.1 mV, respectively [56].
The K. flava Y4 encapsulated with psyllium encapsulant gum had some chemical structures such as C–H, C≡C, C–O, C–N, O–H and functional groups such as alkyne, amine, aromatic ester and fluoro compound at different IR. It has been reported that similar peak was observed at 3342.63 cm−1 corresponding to the hydroxyl group [57]. Similar observations have been drawn by previous studies which showed broad absorption band at 3307.9 cm−1 attributing to –OH stretching in alcohols and 2891.2 cm−1 to –CH stretching in alkanes of psyllium husk (Ph) [58].
The K. flava Y4 encapsulated with psyllium encapsulant gum had the highest cell load (8.12 ± 0.077 log CFU/mL), whereas K. flava Y4 encapsulated with PG and psyllium + PG had lower cell viability at cold temperatures. Alike outcomes were demonstrated for bitter gourd [23], bottle gourd, carrot and beet juices [59]. Maximum health benefits can be obtained whenever the finished product contains a large number of live cells [31]. Another study by Shigematsu et al. (2018) found probiotic viability to be higher than 7 logs during 19 days of refrigerated storage of carrot slices covered with alginate comprising Lactobacillus acidophilus La-14 [60].
In comparison to other encapsulation matrices, the overall acceptability of K. flava Y4 encapsulated with alginate and psyllium gum had highest sensory profile. Similar findings were reported for juices of sapota and mango, with no evident divergence among fortified and non-fortified juice samples [1]. Similar findings has been reported in a previous study which demonstrated that by significantly lowering the pH (6.45–5.65), Bifidobacterium animalis BB-12 maintained microbial quality in cashew milk during refrigerated storage without affecting sensory properties or whiteness index, two of the most important quality parameters for milk [61].
5. Conclusion
The probiotic Kocuria flava Y4 cells were successfully microencapsulated using three encapsulant gums (psyllium, PG, PG + psyllium). Based on the results, the highest encapsulation efficiency of natural gum psyllium in combination with alginate can be used as a suitable scaffold and ideal matrix for the encapsulation of probiotics. These natural gums as prebiotic also promote the growth of probiotic cells in the food environment. The viability of encapsulated probiotic bacteria enhanced (above 6 log CFU/mL) in the orange juice during the storage period (72 h) and further storage at 4 ± 1 °C for five weeks. The antioxidant properties, TPC, TFC and anthocyanin contents increased significantly during probiotification and acidity of juice was maintained, suggesting suitability of the juice for developing a probiotic product with high consumer acceptance. The advantageous characteristics of orange juice flavonoids recommend consuming this product on a regular basis to keep a healthy standard of living. The identification and isolation of bioactive phenolic and flavonoid compounds with an antioxidant activity present in the functional beverage prepared using plant probiotic bacteria can lead to a step ahead in future.
Author contribution statement
Adyasa Barik, Preeti Pallavi, Sudip Kumar Sen, Geetanjali Rajhans, Anindya Bose, Sangeeta Raut: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
Authors have no conflict of interest.
Acknowledgement
The authors express their gratitude towards Center for Biotechnology, Siksha O Anusandhan (Deemed to be University), Bhubaneswar, for providing lab facilities for conducting the research. The authors would like to express their gratitude to Dr. Kundan Kishor for analyzing TSS of our sample at IIHR, CHES, Bhubaneswar.
References
- 1.Kumar B.V., Sreedharamurthy M., Reddy O.V.S. Probiotication of mango and sapota juices using Lactobacillus plantarum NCDC LP 20. Nutrafoods. 2015;14:97–106. doi: 10.1007/s13749-015-0002-4. [DOI] [Google Scholar]
- 2.da Costa G.M., de Carvalho Silva J.V., Mingotti J.D., Barão C.E., Klososki S.J., Pimentel T.C. Effect of ascorbic acid or oligofructose supplementation on L. paracasei viability, physicochemical characteristics and acceptance of probiotic orange juice. LWT. 2017;75:195–201. doi: 10.1016/j.lwt.2016.08.051. [DOI] [Google Scholar]
- 3.Ta L.P., Bujna E., Antal O., Ladányi M., Juhász R., Szécsi A., Kun S., Sudheer S., Gupta V.K., Nguyen Q.D. Effects of various polysaccharides (alginate, carrageenan, gums, chitosan) and their combination with prebiotic saccharides (resistant starch, lactosucrose, lactulose) on the encapsulation of probiotic bacteria Lactobacillus casei 01 strain. Int. J. Biol. Macromol. 2021;183:1136–1144. doi: 10.1016/j.ijbiomac.2021.04.170. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez-Roque M.J., de Ancos B., Sánchez-Moreno C., Cano M.P., Elez-Martínez P., Martín-Belloso O. Impact of food matrix and processing on the in vitro bioaccessibility of vitamin C, phenolic compounds, and hydrophilic antioxidant activity from fruit juice-based beverages. J. Funct.Foods. 2015;14:33–43. doi: 10.1016/j.jff.2015.01.020. [DOI] [Google Scholar]
- 5.Ephrem E., Najjar A., Charcosset C., Greige-Gerges H. Encapsulation of natural active compounds, enzymes, and probiotics for fruit juice fortification, preservation, and processing: an overview. J. Funct.Foods. 2018;48:65–84. doi: 10.1016/j.jff.2018.06.021. [DOI] [Google Scholar]
- 6.B Haffner F., Diab R., Pasc A. Encapsulation of probiotics: insights into academic and industrial approaches. AIMS Mater. Sci. 2016;3:114–136. doi: 10.3934/matersci.2016.1.114. [DOI] [Google Scholar]
- 7.Heidebach T., Först P., Kulozik U. Microencapsulation of probiotic cells for food applications. Crit. Rev. Food Sci. Nutr. 2012;52:291–311. doi: 10.1080/10408398.2010.499801. [DOI] [PubMed] [Google Scholar]
- 8.Shori A.B. Microencapsulation improved probiotics survival during gastric transit. Hayati J. Biosci. 2017;24:1–5. doi: 10.1016/j.hjb.2016.12.008. [DOI] [Google Scholar]
- 9.Cook M.T., Tzortzis G., Charalampopoulos D., Khutoryanskiy V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Contr. Release. 2012;162:56–67. doi: 10.1016/j.jconrel.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Mishra M. Handb. Encapsulation Control. Release. CRC Press; 2015. Overview of encapsulation and controlled release; pp. 3–19. [DOI] [Google Scholar]
- 11.Lotfipour F., Mirzaeei S., Maghsoodi M. Preparation and characterization of alginate and psyllium beads containing Lactobacillus acidophilus. Sci. World J. 2012:1–8. doi: 10.1100/2012/680108. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haghshenas B., Abdullah N., Nami Y., Radiah D., Rosli R., Yari Khosroushahi A. Microencapsulation of probiotic bacteria Lactobacillus plantarum 15HN using alginate-psyllium-fenugreek polymeric blends. J. Appl. Microbiol. 2015;118:1048–1057. doi: 10.1111/jam.12762. [DOI] [PubMed] [Google Scholar]
- 13.Rahimi S.A.S. Fractionation and determination of some structural properties of Persian gum. J. Food Biosci. Technol. 2018;8:81–90. [Google Scholar]
- 14.Barik A., Patel G.D., Sen S.K., Rajhans G., Nayak C., Raut S. Probiotic characterization of indigenous Kocuria flava Y4 strain isolated from Dioscorea villosa leaves. Probiotics Antimicrob. Proteins. 2021 doi: 10.1007/s12602-021-09877-2. [DOI] [PubMed] [Google Scholar]
- 15.Simas-Tosin F.F., Wagner R., Santos E.M.R., Sassaki G.L., Gorin P.A.J., Iacomini M. Polysaccharide of nectarine gum exudate: comparison with that of peach gum. Carbohydr. Polym. 2009;76:485–487. doi: 10.1016/j.carbpol.2008.11.013. [DOI] [Google Scholar]
- 16.Guo Q., Cui S.W., Wang Q., Christopher Young J. Fractionation and physicochemical characterization of psyllium gum. Carbohydr. Polym. 2008;73:35–43. doi: 10.1016/j.carbpol.2007.11.001. [DOI] [Google Scholar]
- 17.Chaikham P., Apichartsrangkoon A., Worametrachanon S., Supraditareporn W., Chokiatirote E., Van der Wiele T. Activities of free and encapsulated Lactobacillus acidophilus LA5 or Lactobacillus casei 01 in processed longan juices on exposure to simulated gastrointestinal tract. J. Sci. Food Agric. 2013;93:2229–2238. doi: 10.1002/jsfa.6030. [DOI] [PubMed] [Google Scholar]
- 18.Sariburun E., Şahin S., Demir C., Türkben C., Uylaşer V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J. Food Sci. 2010;75:C328–C335. doi: 10.1111/j.1750-3841.2010.01571.x. [DOI] [PubMed] [Google Scholar]
- 19.Viacava G.E., Roura S.I. Principal component and hierarchical cluster analysis to select natural elicitors for enhancing phytochemical content and antioxidant activity of lettuce sprouts. Sci. Hortic. (Amsterdam) 2015;193:13–21. doi: 10.1016/j.scienta.2015.06.041. [DOI] [Google Scholar]
- 20.Cassani L., Gerbino E., Moreira M. del R., Gómez-Zavaglia A. Influence of non-thermal processing and storage conditions on the release of health-related compounds after in vitro gastrointestinal digestion of fiber-enriched strawberry juices. J. Funct.Foods. 2018;40:128–136. doi: 10.1016/j.jff.2017.11.005. [DOI] [Google Scholar]
- 21.Mousavi Z.E., Mousavi S.M., Razavi S.H., Hadinejad M., Emam-Djomeh Z., Mirzapour M. Effect of fermentation of pomegranate juice by Lactobacillus plantarum and Lactobacillus acidophilus on the antioxidant activity and metabolism of sugars, organic acids and phenolic compounds. Food Biotechnol. 2013;27:1–13. doi: 10.1080/08905436.2012.724037. [DOI] [Google Scholar]
- 22.Haidar Z.S., Hamdy R.C., Tabrizian M. Protein release kinetics for core–shell hybrid nanoparticles based on the layer-by-layer assembly of alginate and chitosan on liposomes. Biomaterials. 2008;29:1207–1215. doi: 10.1016/j.biomaterials.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Sharma V., Mishra H.N. Fermentation of vegetable juice mixture by probiotic lactic acid bacteria. Nutrafoods. 2013;12:17–22. doi: 10.1007/s13749-012-0050-y. [DOI] [Google Scholar]
- 24.Shi L.-E., Li Z.-H., Li D.-T., Xu M., Chen H.-Y., Zhang Z.-L., Tang Z.-X. Encapsulation of probiotic Lactobacillus bulgaricus in alginate–milk microspheres and evaluation of the survival in simulated gastrointestinal conditions. J. Food Eng. 2013;117:99–104. doi: 10.1016/j.jfoodeng.2013.02.012. [DOI] [Google Scholar]
- 25.Achal V., Pan X., Zhang D. Remediation of copper-contaminated soil by Kocuria flava CR1, based on microbially induced calcite precipitation. Ecol. Eng. 2011;37:1601–1605. doi: 10.1016/j.ecoleng.2011.06.008. [DOI] [Google Scholar]
- 26.Zhou M., Zhang Y., Li X., Wang Z., Tang J., Mu Y., Fang C., Chen X., Dai J. Complete genome sequence of Kocuria flava strain HO-9041, a heavy metal removal bacterium from Xinjiang. J. Biotechnol. 2016;220:21–22. doi: 10.1016/j.jbiotec.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Li X., Sun P., Zhang Y., Jin C., Guan C. A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maize by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ. Exp. Bot. 2020;174 doi: 10.1016/j.envexpbot.2020.104023. [DOI] [Google Scholar]
- 28.Sarafin Y., Donio M.B.S., Velmurugan S., Michaelbabu M., Citarasu T. Kocuria marina BS-15 a biosurfactant producing halophilic bacteria isolated from solar salt works in India. Saudi J. Biol. Sci. 2014;21:511–519. doi: 10.1016/j.sjbs.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uzair B., Menaa F., Khan B.A., Mohammad F.V., Ahmad V.U., Djeribi R., Menaa B. Isolation, purification, structural elucidation and antimicrobial activities of kocumarin, a novel antibiotic isolated from actinobacterium Kocuria marina CMG S2 associated with the brown seaweed Pelvetia canaliculata. Microbiol. Res. 2018;206:186–197. doi: 10.1016/j.micres.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Nualkaekul S., Deepika G., Charalampopoulos D. Survival of freeze dried Lactobacillus plantarum in instant fruit powders and reconstituted fruit juices. Food Res. Int. 2012;48:627–633. doi: 10.1016/j.foodres.2012.06.003. [DOI] [Google Scholar]
- 31.Pimentel T.C., Madrona G.S., Garcia S., Prudencio S.H. Probiotic viability, physicochemical characteristics and acceptability during refrigerated storage of clarified apple juice supplemented with Lactobacillus paracasei ssp. paracasei and oligofructose in different package type. LWT - Food Sci. Technol. 2015;63:415–422. doi: 10.1016/j.lwt.2015.03.009. [DOI] [Google Scholar]
- 32.Garcia E.F., de Oliveira Araújo A., Luciano W.A., de Albuquerque T.M.R., de Oliveira Arcanjo N.M., Madruga M.S., dos Santos Lima M., Magnani M., Saarela M., de Souza E.L. The performance of five fruit-derived and freeze-dried potentially probiotic Lactobacillus strains in apple, orange, and grape juices. J. Sci. Food Agric. 2018;98:5000–5010. doi: 10.1002/jsfa.9034. [DOI] [PubMed] [Google Scholar]
- 33.Fonteles T.V., Costa M.G.M., de Jesus A.L.T., Fontes C.P.M.L., Fernandes F.A.N., Rodrigues S. Stability and quality parameters of probiotic cantaloupe melon juice produced with sonicated juice. Food Bioproc. Technol. 2013;6:2860–2869. doi: 10.1007/s11947-012-0962-y. [DOI] [Google Scholar]
- 34.Miranda R.F., de Paula M.M., da Costa G.M., Barão C.E., da Silva A.C.R., Raices R.S.L., Gomes R.G., Pimentel T.C. Orange juice added with L. casei: is there an impact of the probiotic addition methodology on the quality parameters? LWT. 2019;106:186–193. doi: 10.1016/j.lwt.2019.02.047. [DOI] [Google Scholar]
- 35.Saavedra L., Rupérez F.J., Barbas C. Capillary electrophoresis for evaluating orange juice authenticity: a study on Spanish oranges. J. Agric. Food Chem. 2001;49:9–13. doi: 10.1021/jf0004762. [DOI] [PubMed] [Google Scholar]
- 36.Meléndez-Martínez A.J., Vicario I.M., Heredia F.J. Carotenoids, color, and ascorbic acid content of a novel frozen-marketed orange juice. J. Agric. Food Chem. 2007;55:1347–1355. doi: 10.1021/jf063025b. [DOI] [PubMed] [Google Scholar]
- 37.Batista A.L.D., Silva R., Cappato L.P., Ferreira M.V.S., Nascimento K.O., Schmiele M., Esmerino E.A., Balthazar C.F., Silva H.L.A., Moraes J., Pimentel T.C., Freitas M.Q., Raices R.S.L., Silva M.C., Cruz A.G. Developing a synbiotic fermented milk using probiotic bacteria and organic green banana flour. J. Funct.Foods. 2017;38:242–250. doi: 10.1016/j.jff.2017.09.037. [DOI] [Google Scholar]
- 38.Mousavi Z.E., Mousavi S.M., Razavi S.H., Emam-Djomeh Z., Kiani H. Fermentation of pomegranate juice by probiotic lactic acid bacteria. World J. Microbiol. Biotechnol. 2011;27:123–128. doi: 10.1007/s11274-010-0436-1. [DOI] [Google Scholar]
- 39.Kwaw E., Ma Y., Tchabo W., Apaliya M.T., Wu M., Sackey A.S., Xiao L., Tahir H.E. Effect of Lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018;250:148–154. doi: 10.1016/j.foodchem.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Faria A., Fernandes I., Norberto S., Mateus N., Calhau C. Interplay between anthocyanins and gut microbiota. J. Agric. Food Chem. 2014;62:6898–6902. doi: 10.1021/jf501808a. [DOI] [PubMed] [Google Scholar]
- 41.Saikia S., Mahnot N.K., Mahanta C.L. Optimisation of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze drying. Food Chem. 2015;171:144–152. doi: 10.1016/j.foodchem.2014.08.064. [DOI] [PubMed] [Google Scholar]
- 42.Gil-Izquierdo A., Riquelme M.T., Porras I., Ferreres F. Effect of the rootstock and interstock grafted in lemon tree (Citrus limon (L.) Burm.) on the flavonoid content of lemon juice. J. Agric. Food Chem. 2004;52:324–331. doi: 10.1021/jf0304775. [DOI] [PubMed] [Google Scholar]
- 43.Vanamala J., Cobb G., Turner N.D., Lupton J.R., Yoo K.S., Pike L.M., Patil B.S. Bioactive compounds of grapefruit (Citrus paradisi Cv. Rio Red) respond differently to postharvest irradiation, storage, and freeze drying. J. Agric. Food Chem. 2005;53:3980–3985. doi: 10.1021/jf048167p. [DOI] [PubMed] [Google Scholar]
- 44.Zhang H., Yang Y., Zhou Z. Phenolic and flavonoid contents of Mandarin (Citrus reticulata Blanco) fruit tissues and their antioxidant capacity as evaluated by DPPH and ABTS methods. J. Integr. Agric. 2018;17:256–263. doi: 10.1016/S2095-3119(17)61664-2. [DOI] [Google Scholar]
- 45.Maria G.A., Riccardo N. Citrus bergamia, Risso: the peel, the juice and the seed oil of the bergamot fruit of Reggio Calabria (South Italy) Emir. J. Food Agric. 2020;32(7):522–532. doi: 10.9755/ejfa.2020.v32.i7.2128. [DOI] [Google Scholar]
- 46.Giuffrè A.M., Zappia C., Capocasale M. Physicochemical stability of blood orange juice during frozen storage. Int. J. Food Prop. 2017;20:1930–1943. doi: 10.1080/10942912.2017.1359184. [DOI] [Google Scholar]
- 47.Castro-Vazquez L., Alañón M.E., Rodríguez-Robledo V., Pérez-Coello M.S., Hermosín-Gutierrez I., Díaz-Maroto M.C., Jordán J., Galindo M.F., Arroyo-Jiménez M. del M. Bioactive flavonoids, antioxidant behaviour, and cytoprotective effects of dried grapefruit peels (Citrus paradisi Macf.) Oxid. Med. Cell. Longev. 2016:1–12. doi: 10.1155/2016/8915729. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maria Rita Alaniz Porto S.H., Okina V.S., Pimentel T.C., Garcia S. Beet and orange mixed juices added with Lactobacillus acidophilus. Nutr. Food Sci.48. 2018:76–87. [Google Scholar]
- 49.Lizcano L.J., Viloria-Bernal M., Vicente F., Berrueta L.A., Gallo B., Martínez-Cañamero M., Ruiz-Larrea M.B., Ruiz-Sanz J.I. Lipid oxidation inhibitory effects and phenolic composition of aqueous extracts from medicinal plants of Colombian amazonia. Int. J. Mol. Sci. 2012;13:5454–5467. doi: 10.3390/ijms13055454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barona J., Aristizabal J.C., Blesso C.N., Volek J.S., Fernandez M.L. Grape polyphenols reduce blood pressure and increase flow-mediated vasodilation in men with metabolic syndrome. J. Nutr. 2012;142:1626–1632. doi: 10.3945/jn.112.162743. [DOI] [PubMed] [Google Scholar]
- 51.Wang C.-Y., Lin P.-R., Ng C.-C., Shyu Y.-T. Probiotic properties of Lactobacillus strains isolated from the feces of breast-fed infants and Taiwanese pickled cabbage. Anaerobe. 2010;16:578–585. doi: 10.1016/j.anaerobe.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Braga A.R.C., Mesquita L.M. de S., Martins P.L.G., Habu S., de Rosso V.V. Lactobacillus fermentation of jussara pulp leads to the enzymatic conversion of anthocyanins increasing antioxidant activity. J. Food Compos. Anal. 2018;69:162–170. doi: 10.1016/j.jfca.2017.12.030. [DOI] [Google Scholar]
- 53.Khoo H.E., Azlan A., Tang S.T., Lim S.M. Food Nutr.; Roma): 2017. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits; p. 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sreekanth Reddy O., Subha M.C.S., Jithendra T., Madhavi C., Chowdoji Rao K. Curcumin encapsulated dual cross linked sodium alginate/montmorillonite polymeric composite beads for controlled drug delivery. J. Pharm. Anal. 2021;11:191–199. doi: 10.1016/j.jpha.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patra T., Gupta M.K. Evaluation of sodium alginate for encapsulation-vitrification of testicular Leydig cells. Int. J. Biol. Macromol. 2020;153:128–137. doi: 10.1016/j.ijbiomac.2020.02.233. [DOI] [PubMed] [Google Scholar]
- 56.Kumar D., Gautam A., Rohatgi S., Kundu P.P. Synthesis of vildagliptin loaded acrylamide-g-psyllium/alginate-based core-shell nanoparticles for diabetes treatment. Int. J. Biol. Macromol. 2022;218:82–93. doi: 10.1016/j.ijbiomac.2022.07.066. [DOI] [PubMed] [Google Scholar]
- 57.Bhatia M., Ahuja M. Thiol modification of psyllium husk mucilage and evaluation of its mucoadhesive applications. Sci. World J. 2013;2013:1–7. doi: 10.1155/2013/284182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Surekha R S.T. An efficient encapsulation of thymoquinone using solid lipid nanoparticle for brain targeted drug delivery: physicochemical characterization, pharmacokinetics and bio-distribution studies. Int. J. Pharm. Clin. Res. 2016;8(12):1616–1624. [Google Scholar]
- 59.Yoon K.Y., Woodams E.E., Hang Y.D. Fermentation of beet juice by beneficial lactic acid bacteria. LWT - Food Sci. Technol. 2005;38:73–75. doi: 10.1016/j.lwt.2004.04.008. [DOI] [Google Scholar]
- 60.Shigematsu E., Dorta C., Rodrigues F.J., Cedran M.F., Giannoni J.A., Oshiiwa M., Mauro M.A. Edible coating with probiotic as a quality factor for minimally processed carrots. J. Food Sci. Technol. 2018;55:3712–3720. doi: 10.1007/s13197-018-3301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruno L.M., Lima J.R., Wurlitzer N.J., Rodrigues T.C. Non-dairy cashew nut milk as a matrix to deliver probiotic bacteria. Food Sci. Technol. 2020;40:604–607. doi: 10.1590/fst.14219. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.