Abstract
Introduction
A learning health network is a type of learning health system in which stakeholders use network organization to improve health and health care. Building on existing resources in the cystic fibrosis (CF) community, the Cystic Fibrosis Learning Network (CFLN) was designed to improve medical outcomes and quality of life through an intentional focus on achieving reliable evidence‐based chronic care delivery and creating a system for data‐driven collaborative learning.
Methods
We describe the development and growth of the CFLN considering six domains of a Network Maturity Grid: system leadership; governance and policy management; quality improvement (QI); engagement and community building; data and analytics; and research. We illustrate the impact of the CFLN experience on chronic care processes and indicators of collaborative infrastructure.
Results
The CFLN represents 36 accredited care centers in the CF Foundation Care Center Network caring for over 6300 patients. Of 6779 patient clinical care visits/quarter, 77% are entered into the CF Foundation Patient Registry within 30 days, providing timely means to track outcomes. Collaborative visit planning is occurring in 93% of clinical care visits to share agenda setting with patients and families. Almost all CFLN teams (94%, n = 34) have a patient/family partner (PFP), and 74% of PFPs indicate they are actively participating, taking ownership of, or leading QI initiatives with the interdisciplinary care team. In 2022, 97% of centers reported completing 1–13 improvement cycles per month, and 82% contributed to monthly QI progress reports to share learning.
Conclusion
The CFLN is a maturing, collaborative infrastructure. CFLN centers practice at an advanced level of coproduction. The CFLN fosters interdisciplinary and PFP leadership and the performance of consistent data‐driven improvement cycles. CFLN centers are positioned to respond to rapid changes in evidence‐based care and advance the practice of QI and implementation science on a broader scale.
Keywords: coproduction, cystic fibrosis, learning networks, quality improvement
Abbreviations
- CF
cystic fibrosis
- CFFPR
cystic fibrosis foundation patient registry
- CFLN
cystic fibrosis learning network
- FEV1
forced expiratory volume in 1 second
- FIES
FEV1‐indicated exacerbation signal
- IDC
interdisciplinary care
- LHN
learning health network
- LHS
learning health system
- NLT
network leadership team
- NMG
network maturity grid
- PFPs
patient and family partners
- PwCF
people with CF
- QI
quality improvement
- QIL
quality improvement leaders
- SC
stewardship committee
- SPC
statistical process control
1. INTRODUCTION
Cystic fibrosis (CF) is a genetic, multisystem, chronic disease that impacts nearly 40 000 children and adults in the United States. 1 , 2 , 3 Major advances include the discovery of medications that address CF source protein defects, patient registries that facilitate global research and process improvement, and the widespread use of quality improvement (QI) methods to reduce variation in care processes. 4 Over 80% of people with CF (PwCF) in the United States receive care at one of 286 CF Foundation‐accredited care centers staffed by an interdisciplinary team of healthcare professionals. 2 , 5 The CF Foundation also supports a clinical trials network to expand research. 6 , 7 Together these structures have dramatically advanced the quality and length of life for PwCF. 8
The delivery of CF care is patterned after the chronic care model to promote co‐productive partnerships with PwCF/families. 8 , 9 , 10 In the last 30 years, the focus of CF care has transformed from a life‐shortening disease in young children to an adult, chronic disease. The discovery and access to modulator medications and a forthcoming pipeline of therapies that address the basic genetic defect of CF are changing the lives of PwCF through further improvement in health outcomes. As with most chronic conditions, health systems are challenged to support PwCF/families in ways that respect and serve their needs and priorities. 10 , 11 , 12 Health priorities and care needs among PwCF are shifting as the result of aging and more diverse subpopulations, improving health status, and rapid changes to care delivery from the SARS‐CoV‐2 pandemic. Traditional care standards require ongoing scrutiny to provoke learning of what is best practice. 13
The National Academy of Medicine proposes the model of a learning health system (LHS) to address such care delivery challenges, advance improvements in the quality of care, and facilitate research relevant to patients, clinicians, and researchers. 14 One type of LHS is a Collaborative Learning Health System, also called a Learning Health Network (LHN). An LHN uses a network organizational architecture to engage all stakeholders in collaborating, at scale, to improve health and health care. 15 , 16 , 17 A set of processes across six domains—systems of leadership, governance and management, QI, engagement and community building, data and analytics, and research—support the architecture. These domains, organized in a Network Maturity Grid (NMG), characterize the growth and capacity of an LHN. 18
Seeking to build on the success of prior QI collaboratives and test LHS principles as a novel way to organize improvement and learning in the community, 8 the CF Foundation spearheaded the design, pilot, and implementation of the CF Learning Network (CFLN). In this report, we describe network‐level interventions across the domains of the NMG 18 and indicators of collaborative infrastructure—shared purpose, processes, and resources in the CFLN.
2. METHODS
2.1. Setting
Between July 2014 and December 2015, a diverse group of PwCF/families, interdisciplinary clinicians, researchers, and CF Foundation leaders used a structured complex systems design process to co‐design the CFLN. 19 In 2016, 13 care centers (healthcare professionals and PwCF/families) and PwCF/families from the community at large (Community Innovators) (Cohort 1) were invited and supported by the CF Foundation to initiate a pilot phase. The focus of the pilot phase was to provide a proof‐of‐concept approach in a community structure that facilitated shared learning. In 2017, 16 additional centers joined the pilot phase (Cohort 2). In 2020, 17 more centers (Cohort 3) joined, and the CFLN transitioned to an implementation phase (Appendix A). The implementation phase focused on formal structures for innovation testing.
2.2. Network maturity grid
An NMG has previously been developed from literature review, content theory from existing LHNs, and expert opinion to establish six domains: systems of leadership, governance and management, QI, engagement and community building, data and analytics, and research. 18 Each domain includes a set of processes to operate an LHN. Each process is rated on a 5‐point scale, with descriptors at each point detailing behaviors that denote increasing process maturity. This tool is designed to be used to assess the status of core network processes or functions and as a framework for strategic planning. As network goals and interventions are intended to increase shared purpose, processes, and resources, 20 the CFLN sought to advance along each domain in the NMG. 18 The CFLN did not attempt to achieve maturity on all components of the NMG. Rather, strategic planning focused on advancing those processes within each NMG domain is thought to be key to increasing the number of members willing and able to self‐organize, develop structures, protocols, and processes that facilitate collaboration, and build a communication and resource exchange (Commons) where members could create and share knowledge.
2.3. Goals and processes
We describe the six NMG domains and detail the infrastructure and activities relevant to each domain as prioritized by CFLN strategic planning. 18
2.3.1. Systems of leadership
Processes in this domain enable the network to perform as a system. Defining and communicating a common purpose and developing leaders increases shared purpose. Drawing from the mission of the CF Foundation, the shared purpose of the CFLN is that all PwCF live fulfilling lives. This purpose is propagated in the global and SMART aims organized in a key driver diagram (Figure 1). To reinforce the principles of the Chronic Care Model and key underpinnings of an LHS/LHN, 10 , 17 early CFLN activities included ensuring timely data entry into the CF Foundation Patient Registry (CFFPR) and reinforcing processes of patient/clinician collaborative visit planning.
FIGURE 1.
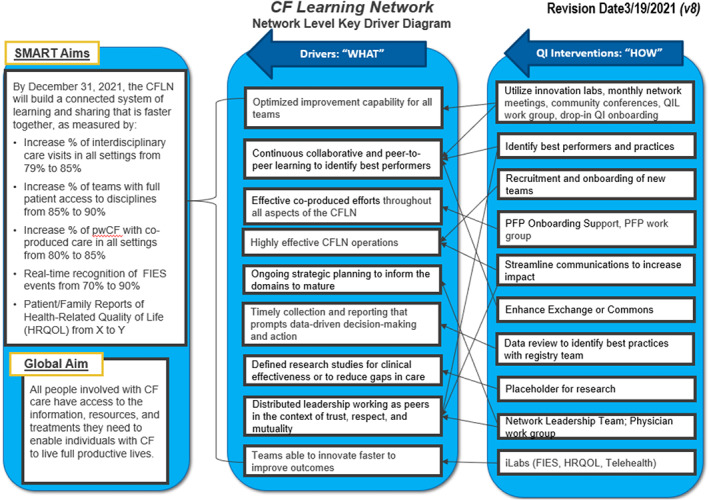
CF Learning Network Key Driver Diagram 2021
The Network Leadership Team (NLT) guides the strategic direction of the CFLN. It is co‐chaired by a physician and a parent partner and consists of five adult and pediatric interdisciplinary clinicians and four patient and family partners (PFPs). A Stewardship Committee (SC) connects the NLT to leaders at the CF Foundation. The SC facilitates resources, removes barriers, ensures alignment between the CFLN and CF Foundation strategic objectives, and raises awareness of the CFLN to the wider CF community. Role‐specific, peer workgroups (QI Leaders [QILs], PFPs, and physicians) are led by peers and open to all teams. CFLN leaders operate in four functional systems: (i) QI System (ii) Community Engagement System, (iii) Data System, and (iv) Research System (Figure 2). Leaders are supported through processes and activities within each functional system, for example, education for QI skills or contributing to the data system through team progress reports.
FIGURE 2.
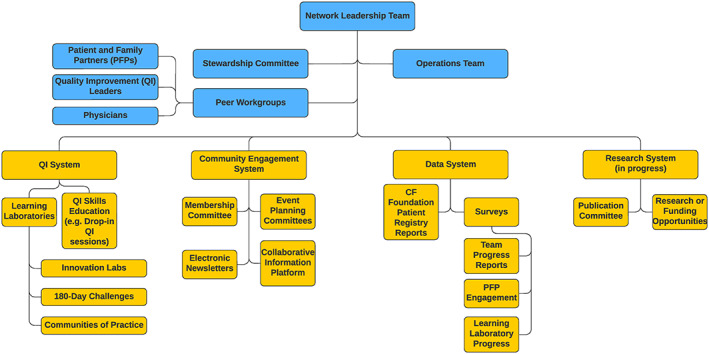
Cystic Fibrosis Learning Network Organizational Chart. Members are delineated in blue and functions and activities are in yellow
A network charter delineates the CFLN as a system. The charter shares the network theory of change, displayed as a Key Driver Diagram (Figure 1) and focused aims of CFLN leadership structures (Appendix B). A family of measures (Appendix C) reflects participant engagement, processes of chronic care, initiative‐specific measures, and outcomes important to data‐driven leadership.
2.3.2. Governance and management
This domain includes the way policies, processes, norms, and actions are structured, sustained, regulated, and accounted. Defining membership, including policies pertaining to PFP inclusion and network participation in governance, increases the number of members willing and able to self‐organize. Centers commit to identifying and developing a local team that includes a physician leader, a QIL (responsible for project planning, data collection, and submission), PFPs, and clinical team members. The CFLN adopted the concept of a triad leadership structure to formalize a central leadership role between a physician, QIL, and PFP. Formal role descriptions for each member of the triad were developed and annually distributed to centers to affirm network leadership values (Appendix D).
Teams commit annually to a scope of work that outlines expectations for participation in network‐wide activities, support for PFPs and clinical team member participation, and monthly progress reporting. The CF Foundation provides a grant to the principal investigator at Cincinnati Children's Hospital Medical Center who in turn organizes subawards to participating centers to cover CFLN‐related travel for the team and PFPs, honoraria for PFPs, and salary support for the QIL (0.26 full‐time equivalents) and physician leader (0.05 full‐time equivalents) (Appendix E). To facilitate CFLN operations, the CF Foundation also supports approximately 7.0 full‐time equivalents for faculty, QI specialists, and project management staff (Operations Team) based in the Anderson Center at Cincinnati Children's Hospital Medical Center (Appendix E).
2.3.3. Quality improvement
The processes in this domain ensure a systematic approach to improvement. Having a common framework for QI enhances shared processes, making it easier to collaborate. The CFLN utilizes the Model for Improvement. 21 A QI curriculum is offered to onboard and support all network members. It allows members to attend sessions for QI topics on a drop‐in basis depending on gaps in knowledge and areas of interest. Centers complete a QI skills assessment used to tailor session content.
Collaborative learning structures, including Community Conferences, All‐Network calls, and “Learning Laboratories” serve to create, test, and share approaches and tools. The CFLN uses different types of “Learning Laboratories” which vary in purpose, support, and time commitment (Table 1). Lessons and tested interventions from collaborative learning are collated and disseminated as change packages. 22
TABLE 1.
Description of learning laboratory types and topics addressed in the CFLN
| Learning lab type | Purpose | Network QI support | Network data support | Team commitment | Topics in CFLN (year launched; N, participating teams) |
|---|---|---|---|---|---|
| 180‐day challenge | Test feasibility in multiple teams; if feasible, move to innovation lab | QI specialists guide teams in discovery to set the QI plan | Biweekly process measures | Weekly to biweekly collaborative meetings, measures, and PDSAs for a definitive time period; 2‐4 participating teams |
|
| Innovation lab | Test innovations to meet reliable processes; spread to more teams in the network if successful | Innovation Lab leaders guide key drivers and innovation testing to set the QI plan | Weekly outcome and process data; frequency tapers as process measures are consistently met; Measures can expand to all teams in the network | Weekly collaborative meetings, measures, and PDSAs; 6‐12 participating teams |
|
| Community of practice | Improve reliable measures and processes which are known; adapt and revise change packages; and onboard new teams | QI specialists support the Practice leaders to set the QI plan | Use of existing measures and data within CFLN | Biweekly collaborative meetings and tasks; 2–4 participating teams or multiple small groups |
|
The early goals and interventions of timely data entry and collaborative visit planning are foundational both for the way the CFLN seeks to practice data‐driven learning and partner with patients and families to coproduce care. These goals continue to be reinforced through collaborative QI learning structures and support newer initiatives to foster partnership skills with PFPs and clinical teams and expand goals to assess health‐related quality of life and improve interdisciplinary care coordination.
2.3.4. Engagement and community building
The structures and processes in this domain promote member involvement in an LHN. Setting expectations and establishing a culture of partnership increases the number of people involved. The CFLN by design emphasizes co‐creation and coproduction between PwCF/families, clinicians, and researchers as equal partners. Clinicians and PFPs are encouraged and supported to co‐design and co‐lead all aspects of the CFLN, including peer workgroups and collaborative learning structures.
Workgroups (physician, QIL, and PFP) also serve to foster engagement and community. These groups nurture a sense of belonging, provide space to teach QI skills, and facilitate peer‐to‐peer engagement. These groups are led by designated co‐chairs with support from the Operations Team. The PFP workgroup meets monthly and the physician and QIL workgroups meet every 6–8 weeks. Community Innovators engage and contribute via Community Conferences and All‐Network calls.
A Commons to share information, knowledge, and know‐how facilitates the growth of shared resources. In the CFLN, the Operations Team supports network‐wide communication through email, a monthly newsletter, and a Twitter account. The CFLN trialed several commons platforms including “The Learning Exchange,” 23 HIVE (Hive Networks, Inc, Cincinnati, OH), Slack (Slack Technologies, LLC, San Francisco, CA), DropBox (San Francisco, CA), and an email listserv.
2.3.5. Data and analytics
LHS/LHNs depend on data and analytics to measure and determine the results of actions relevant to goals. 24 , 25 The activities in this domain make data available, understandable, and usable for all members.
The CFLN uses multiple data sources to drive improvement, including visit information entered in the CFFPR. 1 CFFPR data includes over 80% of all PwCF who receive care in the United States and data are accessible to all centers in the CF Foundation Care Center Network through a cloud‐based platform known as CFSmartReports. 26
CFLN centers report QI measures, such as the number of Plan‐Do‐Study‐Act (PDSA) cycles performed and satisfaction with collaboration, through monthly progress reports. Surveys are also used to monitor defined measures in learning laboratories. CFLN survey data are analyzed by a dedicated specialist on the Operations Team and presented in run charts or statistical process control (SPC) charts. 27 Data are reviewed by the NLT at least quarterly and biannually with all CFLN centers.
2.3.6. Research
Activities in this domain facilitate the use of research methods to drive new knowledge in the clinical domain and network improvement. The CF Foundation supports a robust profile of basic science, clinical, and real‐world research, including established networks for early‐phase drug discovery and the Therapeutics Development Network (TDN). 7 Given this existing infrastructure, the CFLN focused on opportunities to contribute to real‐world research and participated in study planning of the CF Foundation first at‐home observational research study, HERO‐2. 28 Leaders in the CFLN also drafted and disseminated QI and research publication guidance to promote inclusive authorship with emphasis on PFPs and interdisciplinary clinicians and to ensure equity in sharing findings from collaborative learning.
2.4. Measures
2.4.1. Chronic care
Chronic care process measures include timely data entry and collaborative clinic visit planning between PwCF and interdisciplinary clinicians. Timely data entry is defined as clinical data entered in the CFFPR within 30 days of a clinic visit. Interdisciplinary care (IDC) is defined as a visit with the physician and at least one other discipline team member. Collaborative visit planning is defined as an opportunity for PwCF and families to participate in shared agenda setting prior to or during the clinic visit.
2.4.2. Shared purpose—members leading and taking ownership
In addition to tracking the number and roles of members and participating centers, teams report on their program's satisfaction with the support received through participating in the CFLN on a scale of 1–5 (5 = extremely satisfied). Coproduction is also measured monthly using a 1–7 scale completed by the center (1 = no PFP involvement and 7 = multiple PFPs taking QI leadership roles) and a PFP self‐rating scale of 1–7 (1 = passive to QI work, listening and learning, 5 = active participant in the team QI work, 6 = ownership of a QI project[s], and 7 = QI expert or leader) (Appendix F).
2.4.3. Shared processes—making it easier to collaborate
Engagement in shared processes for collaboration across centers is measured through participation in Community Conferences, All‐Network calls, and submission of monthly reports. We measure the number of PDSAs completed per month reported by centers to estimate overall QI process engagement.
2.4.4. Shared resources—broadcasting knowledge and knowhow
The number of change packages and scientific products (abstracts and manuscripts) are tracked. Teams have access to a DropBox maintained by the Operations team as a central repository for change packages and education materials. Awareness of materials available is promoted through meetings such as community conferences and community practices and newsletter communications.
3. RESULTS
3.1. Growth and engagement
As of 2022, the CFLN includes 23 pediatric and 13 adult centers representing 13% (n = 36) of the CF Foundation Care Center Network and approximately 24% of PwCF in the CFFPR (Appendix A). The CFLN centers care for a diverse group of patients, evenly distributed throughout the United States. Two centers report on their adult and pediatric results jointly, fluctuating the number of centers between 34 and 36 teams in 2022. Of current teams, 97% (n = 33) have participated in at least one or more innovation learning laboratories, involving weekly to biweekly meetings, sharing experiences and improvement cycles, and submitting data for common measures (Table 1).
3.2. Chronic care
Timeliness of clinical data entry into the CFFPR across CFLN sites has increased from 65% to 77% of a mean of 6779 patient clinical care visits/quarter‐year (Figure 3). In comparison, centers not participating in the CFLN have remained at approximately 66% of visits (mean 25 912 clinical care visits/quarter‐year in 2021). SPC charts show a shift in the reliability of providing IDC at clinic visits from 80% to 86% (Figure 4A) and of performing collaborative visit planning from 72% to 93% (Figure 4B). These data suggest the success of the CFLN approach to enhance chronic care processes.
FIGURE 3.
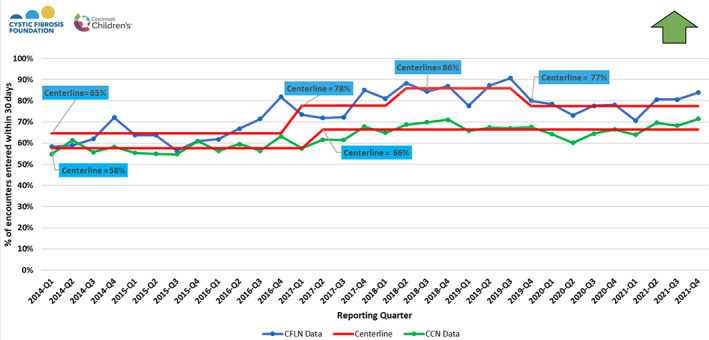
Timeliness of data entry: percentage of clinic visit encounters entered in the CF Foundation Patient registry within 30 days of the encounter date per quarter‐year from centers in the CF Learning Network (CFLN) and the non‐CFLN centers in the CF Foundation Care Center Network
FIGURE 4.
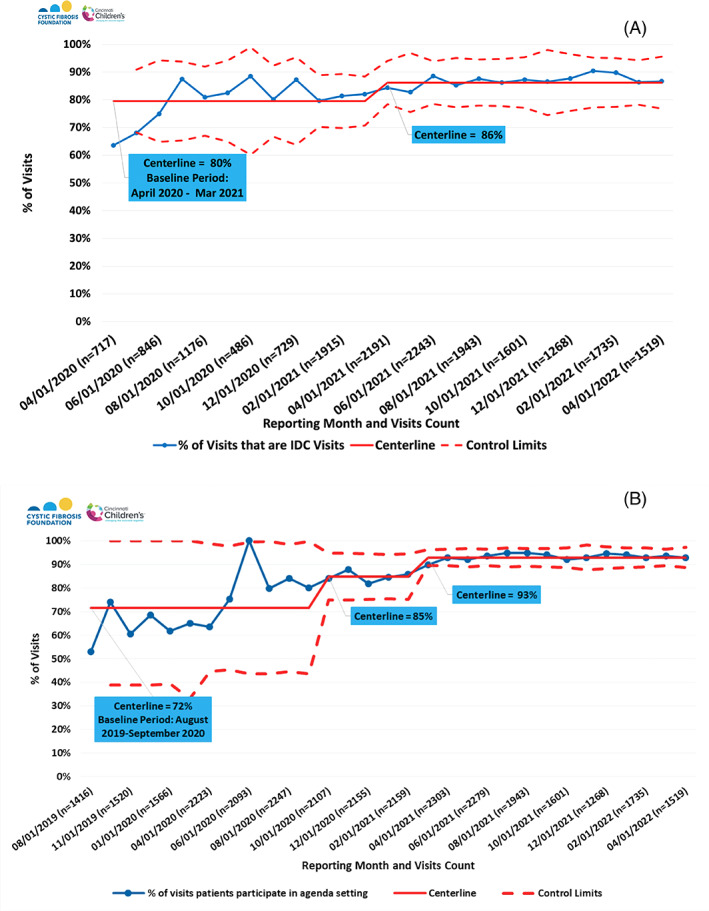
Reliability of Chronic Care Delivery in Clinic Visits for (A) Interdisciplinary Care (IDC) and (B) Collaborative Visit Planning. IDC visits involve a physician and at least one other care team member. Collaborative visit planning is defined as an opportunity for patients and families to participate in shared agenda setting for the clinic visit
3.3. Shared purpose
Centers are represented by a wide variety of disciplines and participate at multiple levels of leadership. Most centers (86%) are overall satisfied with the support they receive from participating in the CFLN (Figure 5).
FIGURE 5.
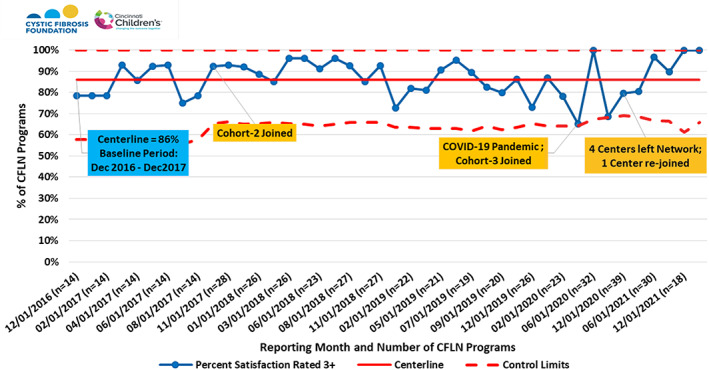
Percentage of CFLN programs reporting 3 or higher satisfaction scores with CFLN support. Likert Scale ranking from 1 to 5 was used, where 5 = highest satisfaction. Cohorts 2 and 3 were additional waves of recruited centers that joined the original 13 CFLN centers at noted intervals
There are currently 54 QILs (one to two identified per center), 40 physician leaders, and 75 PFPs (23% (n = 17) adults with CF, 77% (n = 58) family members) with an average of 2.2 PFPs per team. Of 36 CFLN programs, 30% (n = 11) are represented across committees providing network‐level governance (eg, NLT, membership, and publication) with similar representation between pediatric (n = 7) and adult centers (n = 4). Diverse roles are represented in these structures: 7 PFPs, 1 dietitian, 1 QIL, 2 advanced practice providers, 8 physicians, 1 psychologist, 1 health systems doctorate, and 5 Operations Team members.
In the QIL workgroup, there is an average of 19 participants per meeting, ranging from 29% to 74% (mean 47%) of centers represented. In the physician leader workgroup, meeting participation ranges from 33% to 48% (mean 41%, n = 16). Five PFPs lead the PFP monthly workgroup meetings. Since January 2020, more than half (51%) of centers have at least 1 PFP attending PFP workgroup meetings (range 35%–59%).
The proportion of programs that reported PFPs are actively participating within the QI team (coproduction scale of 5+ or higher) grew from 88% to 91% of teams. The average coproduction scale from teams has been 5.5 of 7, consistent over time as new centers joined (Figure 6A). There has been a fluctuation over time in the percentage of PFP self‐rating at 5 and above (Figure 6B). Since 2021, 74% of PFPs indicate they are actively participating, taking ownership of, or leading QI work at their center.
FIGURE 6.
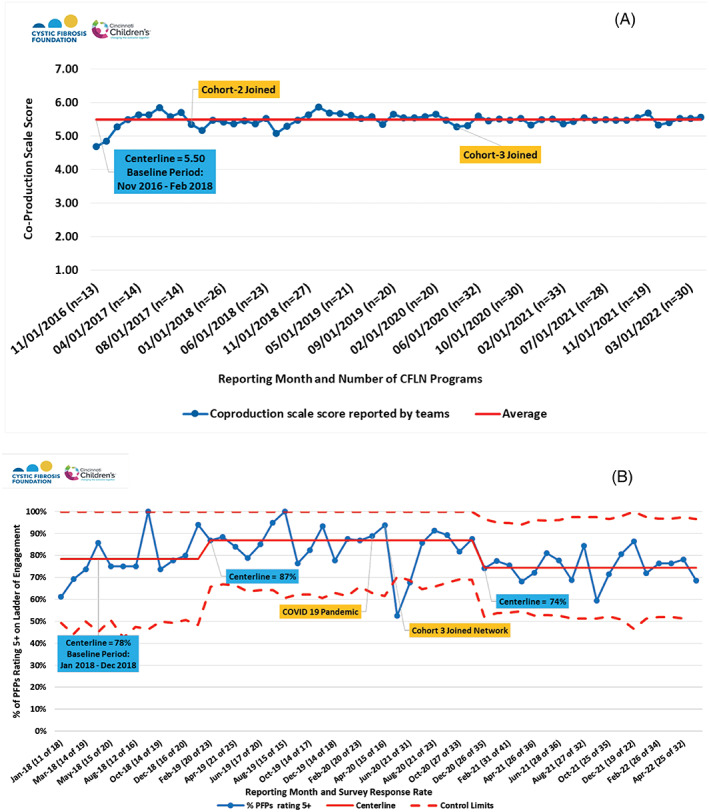
Coproduction scales over time are rated by (A) CFLN programs and (B) patient and family partners (PFPs). Team scores were rated on a scale from 1 to 7, 1 = no PFP involvement and 7 = multiple PFPs taking QI leadership roles. PFP scores rated 1–7, where percent report 5 or higher represent active participation, ownership, or leading QI work. Centerline data is reported in the blue box. Cohorts 2 and 3 were additional waves of recruited centers that joined the original 13 CFLN centers at noted intervals
3.4. Shared processes
Center participation in CFLN meetings ranged between 38% and 100% (Figure 7A). All‐Network meeting frequency was reduced from monthly to quarterly through a portion of the COVID‐19 pandemic to accommodate fluctuations in centers' availability. Despite the pandemic and four centers leaving the CFLN in 2022, recent meeting participation has been over 70% of centers. Since 2019, an average of 82% of centers contribute monthly data through QI surveys (Figure 7B). These surveys include centers' progress on QI activities that reflect center‐specific or CFLN‐focused goals. Centers are encouraged to follow the Model for Improvement framework and scope tests of single interventions in small, frequent PDSA cycles for action‐oriented learning. Although there may be variation in how centers define the PDSAs and the time frame needed per cycle, centers report completing an average of 2.5 PDSAs per month (Figure 8). As of 2022, 97% of centers reported completing a range of 1–13 PDSA cycles per month.
FIGURE 7.
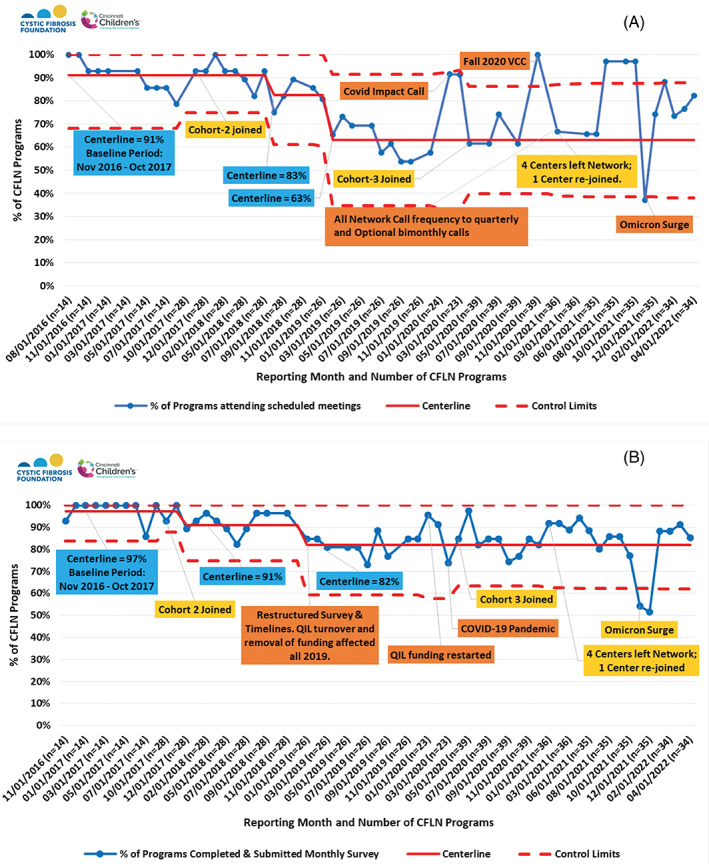
CFLN Program Participation 2016‐2022, (A) Meeting Participation and (B) Survey Participation. Annotations in blue boxes represent centerline data; in yellow boxes, programs entering or leaving CFLN; and in orange boxes, other key events. Virtual Community Conference, VCC; Quality Improvement Leader, QIL
FIGURE 8.
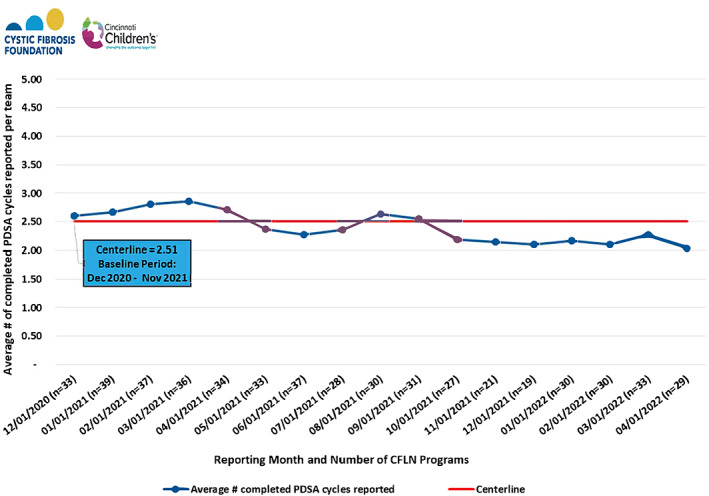
Monthly average number of PDSA (Plan‐Do‐Study‐Act) cycles reported per program 2020–2022. Centerline data is reported in the blue box
3.5. Shared resources
To date, the CFLN has supported nine learning laboratories (Table 1). Three change packages summarizing interventions for coproduction, timely data entry, and telehealth are available to CFLN centers. Packages are shared through DropBox and promoted through All‐Network Calls, community conferences, and newsletters or other email communications. Change packages are referenced as a core curriculum within organized learning laboratories and QI specialists within the CFLN to emphasize foundational learning, such as in a community of practice. The packages can also be used by teams asynchronously without an organized QI learning structure. Teams may independently choose to test and adapt practices to their centers, such as recruiting and onboarding a new patient partner when the timing is right for them. Change packages, coupled with high QI capability among teams regardless of an available learning laboratory structure, increase flexibility to implement interventions at local centers.
Multiple CFLN centers have published because of their network participation. 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 Approximately 40 QI abstracts were presented by CFLN centers at the 2021 North American Cystic Fibrosis Conference. These reports represent a growing body of multicenter and single‐center QI projects and include both self‐organized and CFLN‐led groups showcasing shared CFLN measures.
4. DISCUSSION
The purpose of this report was to describe how the CFLN developed network‐level interventions in domains of the NMG and its progress across indicators of the resulting collaborative infrastructure. The maturity of key CFLN features is highlighted: PwCF/families' roles in coproduction; inclusive, trans‐disciplinary leadership distributed across centers; collaborative QI infrastructure; and data‐driven learning and improvement. CFLN centers have had outstanding success in timely data and interdisciplinary care to support the Chronic Care Model, a mainstay of CF care. 10 We highlight lessons learned and challenges in the CFLN that may serve as a paradigm to further develop an LHS.
4.1. Lessons learned
4.1.1. Distributing leadership with patients and families
The CFLN intentionally designed structures and processes to ensure patient and family involvement at the care center and network level. Every care center team is expected to involve at least one PFP, and funding from the CFF supports PFP honoraria and travel to community conferences. Patients and families are actively integrated to lead QI efforts at the care center level. At the network level, PFPs represent and co‐chair within the NLT, direct the PFP peer workgroup, and lead QI design across multiple centers as co‐chairs of the learning laboratories. Distributed leadership of patients and families at the level of the centers and the network models opportunity and engagement to coproduce improvement.
4.1.2. Measurement of coproduction
An equally important process within the CFLN is its measure of collaboration and active partnership with patients and families from the perspective of the clinical teams and the patients and families. Although self‐report scales have inherent limitations, these measures offer unique insights into collaboration and engagement in the CFLN as well as how they may change or stay stable through changes in the team and network‐level membership. We strongly suggest such a measure for all developing networks to uphold a cultural expectation of patient and family partnerships as reflected in other LHNs. 40 , 41 , 42
4.1.3. Financial investment
The CF Foundation has consistently invested in the adoption and practice of QI methods and promoted PwCF/families as full partners to facilitate the rate of improvement in CF care across care centers. 8 , 9 , 11 , 12 The CF Foundation recognizes that financial systems in health care often do not allocate enough professional time or support for learning and practicing improvement in an interdisciplinary setting. The funds specific for financial recognition of PFPs align with the Patient‐Centered Outcomes Research Institute compensation statement. 44 , 45
4.1.4. Collaborative QI infrastructure
The CFLN, through systems design, 16 , 17 , 19 augments QI capacity and supports organized learning laboratory structures in the form of 180‐day challenges, innovation labs, and community of practices. These mechanisms promote active discovery learning, sharing best practices, and working to scale practices across centers. Pivotal to implementing these structures is the use of a central framework for improvement and an alignment of priorities within a governance plan. The CF Foundation committed to stewardship of the CFLN, providing a persistent alignment of goals and assessment of strategic priorities as the CFLN matured. Multiple examples of collaborative LHNs show success to test innovations, even with the demands of the pandemic. 29 , 46 , 47
4.1.5. Data and analytics
An up‐to‐date data source is critical to LHNs to detect changes rapidly in desired aims. 24 , 25 Data in the CFFPR is available to clinicians and researchers as a resource for data‐driven improvement. 48 , 49 While the CF Foundation provides national and center‐level reports and near real‐time access to data aggregated by patient cohorts via CFSmartReports, 26 the CFLN spurred the development of multi‐center displays in run and SPC charts to report improvement. Data from CFLN centers can be benchmarked with data from the entire CF Care Center Network. Focused first on the foundations of timely data entry, CFLN centers comparatively have more up‐to‐date information available than other centers in the CF Care Network, but these processes remain predominantly manual and time‐consuming. 34
4.2. Challenges
The CFLN successfully compiled learning and interventions in “change packages” and published articles, but experienced barriers to disseminating interventions. One difficulty was sustaining a virtual community commons. 23 , 45 The commons platforms that were trialed varied in features like options to post tools or communicate with smaller groups. However, users did not find these options consistently viable, cumbersome to support document‐sharing, or facilitate communication. Most community users defaulted to more mainstream methods of communication like email and shared drives. CF community stakeholders in a prior study acknowledged technological limitations and the burden of data collection to support shared platforms and other digital resources in an LHS. 50 , 51 Nontechnical solutions for LHSs have been reported as strategies to promote community and share ideas. 52 Opportunities to spread improvements to the CF Care Center Network are valued by members in the CFLN and warrant future models of study. 43
Larger system issues have posed restrictions to fully engage patients and families as equal partners. Centers faced barriers from their institutions to provide financial recognition to patients and families. CFFPR data reports are only accessible to clinical CFLN members. PwCF/families and the Operational team must rely on clinical leaders or the CFFPR team to share these data. The CF Foundation is working to address this barrier in future versions of the CFFPR.
4.3. Future directions
For over 20 years, the CF Foundation has been successful in QI initiatives and looked to pilot and test an LHN as a new way to organize improvement. 8 CFLN structures are organized to advance care delivery and outcomes, promote multi‐site testing and learning through a collaborative infrastructure, integrate enduring data use, and augment coproduction. The perceived benefits to the CF Foundation and community are in its potential to mature further as a testing ground for innovations and address social factors, new models of care delivery, and real‐world research.
CFLN members are interested in identifying and responding to care disparities, in particular defining collaborative care delivery responsive to those not eligible for disease‐modifying medications and inclusive of Hispanic and Black PwCF. 53 , 54 , 55 Other initiatives include pragmatic considerations for facets of telehealth and remote monitoring to enhance clinical care and patient experience. 29 , 56 , 57 , 58 This work will be vital to contribute to the evolution of the care model. 13
The CFLN's participation in planning for the HERO‐2 real‐world research study offered both the CFLN and TDN the opportunity to begin collaborating on the research. 28 As the CF community continues to expand its research portfolio to include more real‐world studies, especially in the realm of tools and processes for remote monitoring and telehealth, the ability to leverage a broader range of scientific methods, including improvement science and clinical and quality research will advance the provision of high quality, specialized care to ensure all PwCF have the opportunity to lead long, fulfilling lives.
5. CONCLUSION
The CFLN represents a maturing LHN with organized aims and processes for centers to collaborate, practice at advanced levels of coproduction, and share learning and resources. The achieved growth of the CFLN has the potential to advance improvement science to respond to the evolving chronic care model and study implementation and effectiveness on a broader scale across the CF community.
CONFLICT OF INTEREST
Kathryn A. Sabadosa is an employee of the Cystic Fibrosis Foundation. Other authors have no conflicts of interest to disclose.
Supporting information
Appendix A Characteristics of Participating CF Programs
Appendix B Examples of shared purpose/aims across CFLN systems of leadership.
Appendix C Table of family of measures
Appendix D Role descriptions for patient and family partner lead, quality improvement leader, and physician leader
Appendix E Financial construct provided by the CF Foundation
Appendix F Coproduction scales. Ladder for Engagement for Patient and Family Partner Rating and Coproduction Scale for Team Rating.
ACKNOWLEDGMENTS
We would like to acknowledge Aaron Dawson, George Dellal, Clifford Gammon, Sarah Gomez, Nancy Griffin, Paige Krack, Sarah Noyes, Drew Warmin, Karen Zeribi, and the Operations Team members at the Cincinnati Children's Hospital Medical Center Anderson Center for their contributions to the development and growth of the CF Learning Network. We thank Alexander Elbert, Tom O'Neil, Kristofer Petren, and the Cystic Fibrosis Foundation Patient Registry Team for their partnership. We thank Dr. Bruce Marshall and the Cystic Fibrosis Foundation.
This work was funded by the Cystic Fibrosis Foundation (SEID15A0, SEID19AB0). TO was supported by the Cystic Fibrosis Foundation (CFF ONG20A0‐KB) and the AHRQ‐PCORI funded PEDSnet Scholars Training Program (5K12HS026393‐03), which is a national faculty development program that trains individuals in the competencies of learning health systems science. Cystic Fibrosis Learning Network Group information: The following physician leaders, patient and family partners, and quality improvement leaders are acknowledged collaborators in the Cystic Fibrosis Learning Network (CFLN): Cori Daines, MD, Glenda Drake, RT, Amy Lucero, (Banner Children's, Diamond Children's Medical Center, Tucson, Arizona); David Miller, MD, Amanda Sharpe (Banner University Medical Center, Tucson, Arizona); Gregory Sawicki, MD MPH, Kate Barnico, BSN RN, Rachel Gordon, MHA, Cindy Murphy, Amanda Lemieux, Georgia Dangel, Lillian O'Leary (Boston Children's Hospital, Boston, Massachusetts); Ahmet Uluer, DO MPH, Lindsey McMahon, MPH, Melanie Lawrence, Meghan Murray (Brigham & Women's Hospital, Boston, Massachusetts); Danielle Goetz, MD, Danielle Woerner, Megan Whelan PhD, RDN, CDN, Katelyn Violanti, MS RDN CDN (CF Center of Western New York, Buffalo, New York); Meghana Sathe, MD, Preeti Sharma, MD, Susan Attel, NP, Traci Liberto, Prigi Varghese, APRN CPNP (Children's Health of Dallas, Dallas, Texas); Rachel Linnemann, MD, Alexia Hernández Cargal, Kelly Clute, MPA, Olivia Ries, BS MS (Children's Healthcare of Atlanta, Emory University, Atlanta, Georgia); Pornchai Tirakitsoontorn, MD, Susan Gage, Bridget Kominek, Kristin Lawrence, Danielle Poulin, MSN CPNP, Maivy Sou, MSN RN CPNP (Children's Hospital of Orange County, Orange, California); Michael Schechter, MD MPH, Andrea Molzhon, PhD, Catherine Hopkins, Megan Martin, Kevin Martin (Children's Hospital of Richmond at VCU, Richmond, Virginia); Nicholas Antos, MD, Laura Roth, CRC, Karen Wunschel (Children's Hospital of Wisconsin, Milwaukee, Wisconsin); Christopher M. Siracusa, MD, Jessica Roach, APRN, Lisa A Mullen, MHSA, Kyle Traver, Travis Burgett (Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio); Errin Newman, MD, Phillip Vaden, JD, Esther Giezendanner, MPH RD, Marsha Triana, RN (Cook Children's Medical Center, Fort Worth, Texas); Danielle Beachler, MD, Sujal Rangwalla, DO, Shine‐Ann Pai, BSRC RRT, Stephanie Robbins (Dell Children's Medical Center of Central Texas, Austin, Texas); Sarah Dykes, DNP CPNP‐PC, Ben McCullar RN BSN, Lindsay Deveaux, Meghann Weil (Doernbecher Children's Hospital, Oregon Health & Science University, Portland, Oregon); Randy Hunt, MD, Emily Walker, MS RD LD, Caroline Starnes, Kendra Adderhold, Megan Barker (Emory University Hospital, Atlanta, Georgia); Johanna Zea‐Hernandez, MD, Beth Debri, RN, Ann Kaiser (Helen DeVos Children's Hospital, Grand Rapids, Michigan); Cindy Brown, MD, Pi Chun Cheng, MD MS, Jana Yeley, NP, Laura Jay‐Ballinger (Indiana University School of Medicine, Indianapolis, Indiana); Christian Merlo, MD MPH, Lauren Mitchell, PT DPT, Andrew Scaljon, Julian McConnie, Meghana Malapaka, Perry Aulie (Johns Hopkins University, Baltimore, Maryland); Rebekah Brown, MD, Stefanie Rushing, RN MSN, Ginger Birnbaum, Cynthia Driskill CPNP (Monroe Carell Jr. Children's Hospital at Vanderbilt, Nashville, Tennessee); Hossein Sadeghi, MD, Golnar Raissi, MD, Bean Corcoran, Janerisa Encarnacion (Morgan Stanley Children's Hospital (Columbia), New York City, New York); Fadi Asfour MD, Stacy Allen, Amanda Oswald, Stephanie Fullmer, NP (Primary Children's Cystic Fibrosis Center, Salt Lake City, Utah); Don B. Sanders, MD MS, Erin Newbill, RN, Misty Thompson, BS CCRC (Riley Hospital for Children, Indianapolis, Indiana); Robert Balk, MD, Anthony Fashoda, Laura Steinhaus, Maureen Tinley, Kristen Nowak, RDN (Rush University Medical Center, Chicago, Illinois); Jame' Vajda, BS, Janine Cassidy MSN ARNP PCNS‐BC, Megan Akers, Lisa Greene, Susan Whitmore (Seattle Children's Hospital, Seattle, Washington); Catherine Kier, MD, Teresa Carney, NP, Sandy Corr, Barbara Leyva, Jillian Salvatore, Michelle Roberts, Christian Santaniello (Stony Brook Children's Hospital, Stony Brook, New York); Robert Abdullah, MD (Stony Brook University, Stony Brook, New York); Bryan Garcia, MD, Cameron Crenshaw, Kandice Amos, James Lawlor (University of Alabama at Birmingham, Alabama); Veronica Indihar, MD, Lisa Shively, Anissa Hostetter, Angela Oder, MSN APRN ACHPN (University of Cincinnati Medical Center, Cincinnati, Ohio); Samya Z Nasr, MD, Rebekah Raines, Catherine Enochs, BSN RN AE‐C, Brandi Morgan, Amy Filbrun, MD MS (University of Michigan Health System, Ann Arbor, Michigan); Jordan Dunitz, MD, Kristen Jesse, RN, Kayla Hubley, RN, Mackenzie Wharram, (University of Minnesota, Minneapolis, Minnesota); Deborah K. Froh, MD, Holly Carroll‐Owen, Rhonda List, Lauren Miller Ahrens, RN BSN CPN (University of Virginia Children's Hospital, Charlottesville, Virginia); Lindsay Somerville, MD, Rhonda List, Brielle Evangelista, Lucy Gettle, Lauren Williamson (University of Virginia Health System, Charlottesville, Virginia); James Tolle, MD, Susan Eastman, FNP, Tracie O'Sullivan, Autumn Bonstein (Vanderbilt University Medical Center, Nashville, Tennessee); Nauman Chaudary, MD, Mahsa Farsad, RD, Kimberly Wingo, Stacey Miller (Virginia Commonwealth University, Richmond, Virginia); Kathryn Moffett, MD, Erin Brozik, RN APRN FNP‐BC, Angela Bender, Billie Jo Bennett (West Virginia University, Morgantown, West Virginia); Alex Gifford, MD, Nicola Felicetti, RN, MSN, Heidi Dolan, Tracey Gendreau (Dartmouth Hitchcock Medical Center, Dartmouth, Lebanon, New Hampshire); Michelle Prickett, MD, Rachel Nelson, MPH, Joanne Cullina, APRN (Northwestern University, Chicago, Illinois, USA); Stacy Bichl, APN, John Palla, MD, Cathy O'Malley, RT, Maria Dowell, Allison Fitch‐Markham, Chloe Ford, Carolyn Heyman, RN, Terri Laguna, MD (Ann & Robert H. Lurie Children's Hospital of Chicago); Debbie Benitez, APN, Lynn Fukushima, RC, Mary Lester, RT, Martha Markovitz, LMSW, Adupa Rao, MD, Gregory Storm, Vai Jun Lam, RD, John Mercer, Joshua Wang, PharmD (Keck Medicine Center of USC); Peter Michelson, Sara Renschen, Mike Price, Betsy Price (St. Louis Children's Hospital, St. Louis, Missouri); Cori Muirhead, PharmD, Jeff Gold, MD, Aaron Trimble, MD, Gopal Allada, MD, Wendy Palmrose, Sue Sullivan, RN, Kim Keeling, RT (Oregon Health & Science University); Rob, Shradar, Jill Fliege, APN, Heidi Klasna, RD, Janelle Sorensen, RN, Stacy Millikan, RN, Dave Davison, Peter J Murphy, MD, John Dickinson, MD, Joe Poler, PhD, Jill Rollins, RT, Sandy Wahl, RT, Cristy Batten, LSW, Laura Romero, LSW (University of Nebraska Medical Center); Whitney Gore, APN, Thomas Keens, MD, Kimberly Morse, LSW, Rocio Munter, Danieli Salinas, MD, Sylvia Sanchez (Children's Hospital of Los Angeles); Virginia Anderson, RD, Jami Dunn, RN, Stephanie Gamble, RT, Hector Gutierrez, MD, Kelli Lachowicz, LSW, Isabel Lowell, MD, Cathy Mims, RN, LaShonna Stodghill, RT, Gabriela Oates, PhD, Amanda Phillips, PT, Linda Russo, Staci Self, LSW (Children's of Alabama); Adrienne Savant, MD, Clement Ren, MD, MBA, George M. Solomon, MD, Julie Desch, MD, David Hansen, Ilene Hollin, PhD, Emily Kramer‐Golinkoff, Pamela Mertz, Erin Moore
Ong T, Albon D, Amin RS, et al. Establishing a Cystic Fibrosis Learning Network: Interventions to promote collaboration and data‐driven improvement at scale. Learn Health Sys. 2023;7(3):e10354. doi: 10.1002/lrh2.10354
The physician leaders, patient and family partners, and quality improvement leaders who participated in the Cystic Fibrosis Learning Network (CFLN) are collaborators listed following acknowledgments.
REFERENCES
- 1. Knapp EA, Fink AK, Goss CH, et al. The Cystic Fibrosis Foundation patient registry. Design and methods of a National Observational Disease Registry. Ann Am Thorac Soc. 2016;13(7):1173‐1179. doi: 10.1513/AnnalsATS.201511-781OC [DOI] [PubMed] [Google Scholar]
- 2. Dowd C, Van Citters AD, Dieni O, Willis A, Powell L, Sabadosa KA. Design and methods for understanding the state of cystic fibrosis care amid the COVID‐19 pandemic. J Cyst Fibros. 2021;20(Suppl 3):3‐8. doi: 10.1016/j.jcf.2021.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foundation CF . CF Foundation estimates increase in CF population. Updated July 28, 2022. Accessed 12 Aug, 2022. https://www.cff.org/news/2022-07/cf-foundation-estimates-increase-cf-population
- 4. Bell SC, Mall MA, Gutierrez H, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med. 2020;8(1):65‐124. doi: 10.1016/S2213-2600(19)30337-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mogayzel PJ Jr, Dunitz J, Marrow LC, Hazle LA. Improving chronic care delivery and outcomes: the impact of the cystic fibrosis care center network. BMJ Qual Saf. 2014;23(Suppl 1):i3‐i8. doi: 10.1136/bmjqs-2013-002363 [DOI] [PubMed] [Google Scholar]
- 6. Rowe SM, Borowitz DS, Burns JL, et al. Progress in cystic fibrosis and the CF therapeutics development network. Thorax. 2012;67(10):882‐890. doi: 10.1136/thoraxjnl-2012-202550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goss CH, Mayer‐Hamblett N, Kronmal RA, Ramsey BW. The cystic fibrosis therapeutics development network (CF TDN): a paradigm of a clinical trials network for genetic and orphan diseases. Adv Drug Deliv Rev. 2002;54(11):1505‐1528. doi: 10.1016/s0169-409x(02)00163-1 [DOI] [PubMed] [Google Scholar]
- 8. Marshall BC, Nelson EC. Accelerating implementation of biomedical research advances: critical elements of a successful 10 year Cystic Fibrosis Foundation healthcare delivery improvement initiative. BMJ Qual Saf. 2014;23(Suppl 1):i95‐i103. doi: 10.1136/bmjqs-2013-002790 [DOI] [PubMed] [Google Scholar]
- 9. Boyle MP, Sabadosa KA, Quinton HB, Marshall BC, Schechter MS. Key findings of the US Cystic Fibrosis Foundation's clinical practice benchmarking project. BMJ Qual Saf. 2014;23(Suppl 1):i15‐i22. doi: 10.1136/bmjqs-2013-002369 [DOI] [PubMed] [Google Scholar]
- 10. Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood). 2001;20(6):64‐78. doi: 10.1377/hlthaff.20.6.64 [DOI] [PubMed] [Google Scholar]
- 11. Batalden M, Batalden P, Margolis P, et al. Coproduction of healthcare service. BMJ Qual Saf. 2016;25(7):509‐517. doi: 10.1136/bmjqs-2015-004315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sabadosa KA, Batalden PB. The interdependent roles of patients, families and professionals in cystic fibrosis: a system for the coproduction of healthcare and its improvement. BMJ Qual Saf. 2014;23(Suppl 1):i90‐i94. doi: 10.1136/bmjqs-2013-002782 [DOI] [PubMed] [Google Scholar]
- 13. Prickett MH, Flume PA, Sabadosa KA, Tran QT, Marshall BC. Telehealth and CFTR modulators: accelerating innovative models of cystic fibrosis care. J Cyst Fibros. 2022;S1569‐1993(22)00600‐2. doi: 10.1016/j.jcf.2022.07.002 [DOI] [PubMed] [Google Scholar]
- 14. Institute of Medicine (US) Roundtable on Evidence‐Based Medicine . The Learning Healthcare System: Workshop Summary. Washington DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 15. Fjeldstad OD, Johnson JK, Margolis PA, Seid M, Hoglund P, Batalden PB. Networked health care: rethinking value creation in learning health care systems. Learn Health Syst. 2020;4(2):e10212. doi: 10.1002/lrh2.10212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Britto MT, Fuller SC, Kaplan HC, et al. Using a network organisational architecture to support the development of learning healthcare systems. BMJ Qual Saf. 2018;27(11):937‐946. doi: 10.1136/bmjqs-2017-007219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seid M, Hartley DM, Margolis PA. A science of collaborative learning health systems. Learn Health Syst. 2021;5(3):e10278. doi: 10.1002/lrh2.10278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lannon C, Schuler CL, Seid M, et al. A maturity grid assessment tool for learning networks. Learn Health Syst. 2021;5(2):e10232. doi: 10.1002/lrh2.10232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fore D, Goldenhar LM, Margolis PA, Seid M. Using goal‐directed design to create a novel system for improving chronic illness care. JMIR Res Protoc. 2013;2(2):e43. doi: 10.2196/resprot.2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fjeldstad ØD, Snow CC, Miles RE, Lettl C. The architecture of collaboration. Strateg Manag J. 2012;33(6):734‐750. doi: 10.1002/smj.1968 [DOI] [Google Scholar]
- 21. Picarillo AP. Introduction to quality improvement tools for the clinician. J Perinatol. 2018;38(7):929‐935. doi: 10.1038/s41372-018-0100-4 [DOI] [PubMed] [Google Scholar]
- 22. Langley GJ, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing a Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco, CA: Jossey Bass; 2009. [Google Scholar]
- 23. McLinden D, Myers S, Seid M, Busch M, Davis D, Murphy J. The learning exchange, a community knowledge commons for learning networks: qualitative evaluation to test acceptability, feasibility, and utility. JMIR Form Res. 2019;3(1):e9858. doi: 10.2196/formative.9858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deans KJ, Sabihi S, Forrest CB. Learning health systems. Semin Pediatr Surg. 2018;27(6):375‐378. doi: 10.1053/j.sempedsurg.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 25. Marsolo K, Margolis PA, Forrest CB, Colletti RB, Hutton JJ. A digital architecture for a network‐based learning health system: integrating chronic care management, quality improvement, and research. EGEMS (Wash DC). 2015;3(1):1168. doi: 10.13063/2327-9214.1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cystic Fibrosis Foundation . CF Smart reports: Using Registry Data to Improve Care. February 20, 2018. Bethesda, MD: Cystic Fibrosis Foundation; 2018. https://www.cfsmartreports.com [Google Scholar]
- 27. Provost L, Murray S. The Health Care Data Guide: Learning from Data for Improvement. San Francisco, CA: Jossey‐Bass; 2011. [Google Scholar]
- 28. Foundation CF . First‐of‐Its‐Kind Study Uses Smartphone to Track Cystic Fibrosis in Real Life. Accessed August 1, 2022. https://www.cff.org/press-releases/2021-06/first-its-kind-study-uses-smartphone-track-cystic-fibrosis-real-life
- 29. Albon D, Thomas L, Hoberg L, et al. Cystic fibrosis learning network telehealth innovation lab during the COVID‐19 pandemic: a success QI story for interdisciplinary care and agenda setting. BMJ Open Qual. 2022;11(2):e001844. doi: 10.1136/bmjoq-2022-001844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jaclyn D, Andrew N, Ryan P, et al. Patient and family perceptions of telehealth as part of the cystic fibrosis care model during COVID‐19. J Cyst Fibros. 2021;20:e23‐e28. doi: 10.1016/j.jcf.2021.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perkins RC, Davis J, NeSmith A, et al. Favorable clinician acceptability of telehealth as part of the cystic fibrosis care model during the COVID‐19 pandemic. Ann Am Thorac Soc. 2021;18:1588‐1592. doi: 10.1513/AnnalsATS.202012-1484RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Compton M, Soper M, Reilly B, et al. A feasibility study of urgent implementation of cystic fibrosis multidisciplinary telemedicine clinic in the face of COVID‐19 pandemic: single‐center experience. Telemed J E Health. 2020;26:978‐984. doi: 10.1089/tmj.2020.0091 [DOI] [PubMed] [Google Scholar]
- 33. Womack C, Farsin R, Farsad M, Chaudary N. Emerging alternatives to conventional clinic visits in the era of COVID‐19: adoption of telehealth at VCU adult cystic fibrosis center. Int J Gen Med. 2020;13:1175‐1186. doi: 10.2147/IJGM.S274193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nay L, Vajda J, McNamara S, Ong T. Sustained reduction in time to data entry in the Cystic Fibrosis Foundation registry. Pediatr Qual Saf. 2022;7(1):e529. doi: 10.1097/pq9.0000000000000529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ong T, Onchiri FM, Britto MT, et al. Impact of guideline‐recommended dietitian assessments on weight gain in infants with cystic fibrosis. J Cyst Fibros. 2021;21:115‐122. doi: 10.1016/j.jcf.2021.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Somerville LAL, List RP, Compton MH, et al. Real‐world outcomes in cystic fibrosis telemedicine clinical care in a time of a global pandemic. Chest. 2022;161(5):1167‐1179. doi: 10.1016/j.chest.2021.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bruschwein HM, Soper M, Jennings D, et al. Mental health screening of patients with cystic fibrosis through telehealth during COVID‐19: evaluation of feasibility and process adoption. Fam Syst Health. 2022;40(3):397‐402. doi: 10.1037/fsh0000698 [DOI] [PubMed] [Google Scholar]
- 38. Compton M, List R, Starheim E, et al. Home spirometry utilisation in telemedicine clinic for cystic fibrosis care during COVID‐19 pandemic: a quality improvement process. BMJ Open Qual. 2021;10(3):e001529. doi: 10.1136/bmjoq-2021-001529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hente E, Weiland J, Mullen L, et al. Assessment of treatment burden and complexity in cystic fibrosis: a quality improvement project. Pediatr Pulmonol. 2021;56(7):1992‐1999. doi: 10.1002/ppul.25361 [DOI] [PubMed] [Google Scholar]
- 40. Gremyr A, Andersson Gäre B, Thor J, Elwyn G, Batalden P, Andersson AC. The role of co‐production in learning health systems. Int J Qual Health Care. 2021;33(Supplement_2):ii26‐ii32. doi: 10.1093/intqhc/mzab072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seid M, Hartley DM, Dellal G, Myers S, Margolis PA. Organizing for collaboration: an actor‐oriented architecture in ImproveCareNow. Learn Health Syst. 2020;4(1):e10205. doi: 10.1002/lrh2.10205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murray DS, Anixt JS, Coury DL, et al. Transforming an autism pediatric research network into a learning health system: lessons learned. Pediatr Qual Saf. 2019;4(2):e152. doi: 10.1097/pq9.0000000000000152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Citters A, Buus‐Frank M, King J, et al. The cystic fibrosis learning network: a mixed methods evaluation of program goals, attributes, and impact. Under Review. [DOI] [PMC free article] [PubMed]
- 44. PCORI . Engagement Resources: Compensation Framework. Updated September 30, 2021. 2022. https://www.pcori.org/engagement/engagement-resources#content-4029
- 45. Seid M, Davis R, Havens M, et al. Building a learning healthcare system network. Engagement and community building. James M. Anderson Center for Health Systems Excellence https://www.cincinnatichildrens.org/research/divisions/j/anderson-center/learning-networks
- 46. Beck AF, Hartley DM, Kahn RS, et al. Rapid, bottom‐up Design of a Regional Learning Health System in response to COVID‐19. Mayo Clin Proc. 2021;96(4):849‐855. doi: 10.1016/j.mayocp.2021.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee JM, Carlson E, Albanese‐O'Neill A, et al. Adoption of telemedicine for type 1 diabetes care during the COVID‐19 pandemic. Diabetes Technol Ther. 2021;23(9):642‐651. doi: 10.1089/dia.2021.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fink AK, Loeffler DR, Marshall BC, Goss CH, Morgan WJ. Data that empower: the success and promise of CF patient registries. Pediatr Pulmonol. 2017;52(S48):S44‐S51. doi: 10.1002/ppul.23790 [DOI] [PubMed] [Google Scholar]
- 49. Schechter MS, Fink AK, Homa K, Goss CH. The Cystic Fibrosis Foundation patient registry as a tool for use in quality improvement. BMJ Qual Saf. 2014;23(Suppl 1):i9‐i14. doi: 10.1136/bmjqs-2013-002378 [DOI] [PubMed] [Google Scholar]
- 50. Dixon‐Woods M, Campbell A, Chang T, et al. A qualitative study of design stakeholders' views of developing and implementing a registry‐based learning health system. Implement Sci. 2020;15(1):16. doi: 10.1186/s13012-020-0976-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Citters AD, Gifford AH, Brady C, et al. Formative evaluation of a dashboard to support coproduction of healthcare services in cystic fibrosis. J Cyst Fibros. 2020;19(5):768‐776. doi: 10.1016/j.jcf.2020.03.009 [DOI] [PubMed] [Google Scholar]
- 52. Cresswell K, Sheikh A, Franklin BD, et al. Interorganizational knowledge sharing to establish digital health learning ecosystems: qualitative evaluation of a National Digital Health Transformation Program in England. J Med Internet Res. 2021;23(8):e23372. doi: 10.2196/23372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McGarry ME, Gibb ER, Oates GR, Schechter MS. Left behind: the potential impact of CFTR modulators on racial and ethnic disparities in cystic fibrosis. Paediatr Respir Rev. 2022;42:35‐42. doi: 10.1016/j.prrv.2021.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McGarry ME, McColley SA. Cystic fibrosis patients of minority race and ethnicity less likely eligible for CFTR modulators based on CFTR genotype. Pediatr Pulmonol. 2021;56(6):1496‐1503. doi: 10.1002/ppul.25285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. DiMango E, Simpson K, Menten E, Keating C, Fan W, Leu CS. Health disparities among adults cared for at an urban cystic fibrosis program. Orphanet J Rare Dis. 2021;16(1):332. doi: 10.1186/s13023-021-01965-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Albon D, Van Citters AD, Ong T, et al. Telehealth use in cystic fibrosis during COVID‐19: association with race, ethnicity, and socioeconomic factors. J Cyst Fibros. 2021;20(Suppl 3):49‐54. doi: 10.1016/j.jcf.2021.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gifford AH, Ong T, Dowd C, et al. Evaluating barriers to and promoters of telehealth during the COVID‐19 pandemic at US cystic fibrosis programs. J Cyst Fibros. 2021;20(Suppl 3):9‐13. doi: 10.1016/j.jcf.2021.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ong T, Van Citters AD, Dowd C, et al. Remote monitoring in telehealth care delivery across the US cystic fibrosis care network. J Cyst Fibros. 2021;20(Suppl 3):57‐63. doi: 10.1016/j.jcf.2021.08.035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A Characteristics of Participating CF Programs
Appendix B Examples of shared purpose/aims across CFLN systems of leadership.
Appendix C Table of family of measures
Appendix D Role descriptions for patient and family partner lead, quality improvement leader, and physician leader
Appendix E Financial construct provided by the CF Foundation
Appendix F Coproduction scales. Ladder for Engagement for Patient and Family Partner Rating and Coproduction Scale for Team Rating.


