Abstract
In this research work, hog plum (Spondius mangifera L.) was treated with Aloe vera gel (AVG) coating and paraffin wax-coated box (PWB) packaging and stored at ambient condition (25 ± 3 °C and 80–85% relative humidity) for 12 d to evaluate their impact on postharvest quality and storability. The physicochemical properties, microbiological analysis, and decay evaluation were analyzed throughout the storage period. The AVG and PWB coating treatments both demonstrated a significant effect on the quality of fruits during storage. The results showed that fruits with AVG coating and PWB packaging exhibited lower decay rates, weight loss, color difference, total microbial population, total soluble solids, titratable acidity, and higher fruit firmness and pH than uncoated (control) fruits. The AVG coating was the most effective treatment, followed by the PWB packaging treatment. Our findings show that the AVG coating and PWB packaging treatment can be a promising solution for preserving the quality of hog plums and also helps in increasing the lifetime of hog plums during storage.
Keywords: Aloe vera gel, Edible coating, Hog plum, Paraffin wax-coated box packaging, Post-harvest quality
1. Introduction
Hog plum (Spondius mangifera L.) is considered a healthy fruit as it is abundant in proteins, minerals, dietary fibres, thiamin, riboflavin, and ascorbic acid with minimum calories [1]. Besides these, it is composed of many bioactive compounds like phenolics, flavonoids, and triterpenes, which serve numerous biological functions, such as antimicrobial, hypoglycemic index, antioxidant, and anti-inflammatory activities to the body [2]. Thus, the acceptability of this fruit spreads across different tropical parts of America, Asia, Africa, and Brazil [3]. It is a minor fruit in Bangladesh and is known as Amra. The annual production of hog plum in Bangladesh was 40521 M. Tons in the fiscal year 2018–2019, and production has been increasing every year [4]. It can be used as homemade pickles, chutney, jam, soft drink powder, and even in raw form with salt and chilli powder for consumption [5,6]. The mature fruit of hog plum is abundant in acids and micro-and macronutrients that combine well with sugars and is used to make a range of traditional high-energy and refreshing beverages. Currently, it is regarded as a valuable resource for producing various industrial products and new medicines for various diseases [7].
However, its perishable nature is the major hindrance to its widespread application and makes it vulnerable all over the production chain. The perishability of the fruit causes negative physicochemical changes, including weight loss, flesh softening, quality degradation owing to microbial attack, and changes in sugar and acid content [8]. So, some preservation technologies were employed to extend the shelf-life of hog plum fruits, including irradiation [9], coating with cassava starch [10], and hot air convective drying [11]. Shelf-life enhancement with biodegradable films, edible coatings, and biodegradable packaging has become a novel approach for perishable fruits and vegetables [12]. They act as moisture and oxygen barriers during the processing, handling, and storage of fruits and vegetables, and their natural biocide activity not only delays deterioration but also improves safety [13]. It also helps to modify the environment surrounding fruits and decreases weight loss during transportation and storage [14]. The edible coatings are derived primarily from agricultural products and by-products that can be eaten in conjunction with the coated item [15].
Aloe vera gel (AVG) as an edible coating is gaining popularity in fruit coating since it is edible, eco-friendly, has no chemical reactions with fruits, and retains the taste of the fruits [16]. It also performs some biological functions, such as curing different burns, wounds, and cardiovascular and gastrointestinal issues [17]. The mucilaginous structure of aloe vera gel is abundant in polysaccharides. Among the other polysaccharides, the presence of acetylated-glucomannans in AVG, which provides a thick texture, is believed to be the most suitable for preparing edible coatings [18]. Furthermore, the presence of essential oils in AVG improves the physical appearance of fruits. The shelf life and quality of fruits have also been significantly improved using this gel. From the previous literature, it has been observed that AVG coating significantly reduced firmness losses, weight losses, and color changes in apple [19], blueberry [20], strawberry [21], papaya [22], and table grapes [23] compared to uncoated fruits. Moreover, it is also found that AVG coating resulted in higher antioxidant activity in jujube fruit [24], higher acidity and vitamin C, respiration rate, and antioxidant activity in blueberry [20], higher ascorbate peroxidase, peroxidase, superoxide dismutase, catalase activities, ascorbic acid, DPPH scavenging antioxidants and phenolics in persimmon [25] and lotus root slices [26], higher respiration rate, soluble solids content, titratable acidity in cornelian cherry [27], than uncoated fruits during storage. Moreover, AVG coating inhibited the decay incidence in strawberry and tomato [21,28].
In addition to edible coating, edible films and paper and paperboard packages are renewable source-based packaging materials that draw manufacturers’ interest [29]. Poor barrier characteristics of papers and paperboard packages against moisture, gas, aroma, and grease hinder their broad applicability in the industry, which can be adjusted using coating from various hydrophobic materials [30]. Improving the mechanical properties of papers and paperboards has been found to be achieved by the addition of several polymeric material coatings like alginate, celluloses, lipids, milk proteins, paraffin wax, starch, and zein. Among others, paraffin wax is widely accepted to avoid moisture and water vapor transmission in fresh fruits and vegetables because of its low polar chemical structure [31]. Additionally, paraffin wax coating can potentially preserve fruits and vegetables from being damaged during chilling and enhance their appearance. Its feasibility is related to its concentration during application as a coating on paper and paperboard [32].
To the best of our knowledge, no prior study has been conducted on the effect of AVG coating and paraffin wax-coated box packaging on the post-harvest qualities of hog plums while they are being stored, despite the fact they have potential roles of improving the post-harvest quality of hog plum. Therefore, the main aim of this research was to evaluate the impact of corrugated paperboard packaging coated with paraffin wax and AVG coating on the post-harvest qualities of hog plums during storage at room temperature.
2. Materials and methods
2.1. Sample collecting and preparation
Uniform in maturity (collecting after 8–9 weeks of fruits set, when the endocarp was demarcated), size and shape, disease and damage-free (by visual observation) hog plum (Spondius mangifera L.) fruits were harvested by hand harvesting method from a local orchard in Dinajpur district (25° 38′ 11.6664″ N and 88° 38′ 10.7592″ E), Bangladesh. The pH of the orchard soil was 5.5, and the lime content was 4 kg/decimal, respectively. Fruits were placed in plastic crates and immediately transferred to the Food Engineering and Technology Laboratory of Hajee Mohammad Danesh Science and Technology University (Dinajpur, Bangladesh) under environmental conditions. The fruits were rinsed with running tap water to remove adhering dirt and dried using a gentle blower. The dried hog plum fruits were used for Aloe vera gel and paraffin wax box coating treatments.
2.2. Preparation of aloe vera gel coating
In this work, Aloe vera gel was extracted from mature aloe vera leaves, which are uniform in color, size, and disease-free aloe vera leaves, according to Parven et al. [22], with some modifications. The leaves were first washed under running tap water to eliminate dirt and then soaked for 5 min in 0.1% sodium hypochlorite. After drying, the gelatinous parenchyma was isolated from the leaves using a sharp stainless-steel knife. In a blender, the hydro parenchyma (colorless) was uniformly blended. The resulting mixture was then filtered through a sterile muslin cloth to eliminate fibrous fractions. Finally, the liquid gel fraction was obtained at the end of the process. The obtained gel was diluted with distilled water at a 1:1 (v/v) ratio, according to Hassanpour [33]. Then, the obtained mixture was pasteurized at 70°C for 45 min, and subsequently, we cooled down the mixture to room temperature (25 ± 3 °C). The pH was adjusted to 4.0 with citric acid.
2.3. Paperboard coating and conditioning
The paraffin wax was melted at 80–85 °C. The paraffin wax coating (thickness about 1 mm) was applied onto the inner wall of the corrugated paperboard (thickness −3 mm, single wall, B flute) using the manual coating method. The dimension of paperboard was 9ʺ × 6ʺ × 6ʺ. After coating, the paraffin wax-coated paperboard (PWB) was dried for 24 h at room temperature.
2.4. Experimental design and treatments
The experiment was conducted in a completely randomized design (CRD) with three treatments: without coating (control) treatment, AVG coating treatment, and PWB packaging treatment. Fruits were coated according to the process reported by Chrysargyris et al. [28]. Fruits were washed in 0.05% sodium hypochlorite solution for 5 min before being rewashed with distilled water and dried before coating application at ambient temperature. The fruits were immersed in AVG concentration for 10 min. The coating solution was evenly applied to the entire fruit surface and allowed to dry at room temperature for 30 min. The dried AVG-coated fruits were stored in a paperboard box at ambient temperature. In another treatment, the uncoated fruits were kept in a paraffin wax-coated paperboard box (PWB) and uncoated paperboard box (control) and then stored at room temperature (25 ± 3 °C). The experimental set-up consisted of three treatments × three replications × six fruits per replication × five sampling intervals (with day 0) using 270 fruits. The fruits were stored at ambient conditions (25 ± 3 °C and 80–85% relative humidity) for 12 d. The fruits were sampled on 0 (before coating), 3rd, 6th, 9th, and 12th d of the storage period of application of the coating for various analyses.
2.5. Physical and chemical quality of the fruits
2.5.1. Total soluble solids content, titratable acidity, and pH
Total soluble solids (TSS) of fruits were determined using a portable manual hand refractometer (HI 9601) from the extracted fruit juice of each fruit after removing skin and seeds. The pH of each sample (fruit juice) was determined by a digital pH meter (HI 2211 pH/ORP meter, China) with a glass electrode [34]. Titratable acidity (TA) was analyzed by potentiometric titration of 5 mL of aliquot (5 gm extracted juice was diluted to 100 mL) using 0.1 N NaOH up to pH 8.1 with phenolphthalein indicator (0.1%) until the endpoint was reached. The result was expressed as a percent of citric acid per 100 g on a fresh weight basis.
2.5.2. Weight loss
Weight loss was measured according to the method described by Duan et al. [12]. A digital balance (model with accuracy) was used to measure the weight of each fruit sample on the first day and during each sampling day of storage. The weight loss of fruits was measured in a percentage using the following formula: percent weight loss = (Wo – Wf) × 100/Wo, where Wo is the initial weight of fruits, and Wf is the final weight of the fruit.
2.5.3. Fruits firmness
Firmness was measured using a texture analyzer (Probe TA39, TA-MTP) to measure the resistance of samples to penetration of a rod of 2 mm diameter for fruits. The rod was perpendicularly placed on the fruits and pressed until a gradually visible cut appeared, at which point the fruit firmness (kg/cm2) was recorded [28]. Each fruit was punctured around the equatorial area thrice, and the mean value was reported.
2.5.4. Color value
The color difference (ΔE) of fruit samples was determined following the Hunter color lab system (coordinate L, a, b) using a colorimeter (CR400, Konica Minolta). The results were the averages of three measured values along the equatorial axis for each fruit. The ΔE was calculated according to the following equation
where “o” refers to the color reading of fresh samples used as control.
2.5.5. Decay evaluation of fruits
The severity of fruits’ decay was visually evaluated according to Chrysargyris et al. [28] during the storage period. The degree of fruit surface infection was assessed using a scale of 1–5. (Where: 1 denotes clean, no infection; 2 denotes trace infection; 3 denotes slight infection; 4 denotes moderate infection, and 5 denotes severe infection). Fruits with mycelia growth on the surface were considered to decay. Three different fruits per treatment and storage time were used to conduct the decay analysis.
2.6. Microbial analysis of fruits
10g sample was taken and homogenized in 90 mL of sterile peptone water using a stomacher (MIX 2, AES Laboratoire, Combourg, France). Serial dilutions were performed, and 1 mL of each dilution was poured onto plate count agar to determine the total plate count (total number of microorganisms). Plates were incubated at 37 °C for 24 h in an incubator. A Colony Counter (Stuart Scientific, UK) was used to count the total number of microorganisms over the 24 h incubation period. Serial dilutions were performed in triplicate. Microbes were counted on plates containing 30–300 colonies and multiplied by the dilution factor. The microbial population was expressed as colony-forming units (CFU) per g of the sample [17]. The fruits were sampled for microbial analysis on 0 (before coating), 6th, and 12th d of the storage period of application of the AVG coating.
2.7. Statistical analysis
A single factor completely randomized design (CRD) was used, and all data were analyzed statistically using statistical software SPSS (IBM SPSS Statistic 22). Duncan's new multiple range tests (DMRT) were used to compare the mean difference at p ≤ 0.05. All results were expressed as the mean ± standard deviation.
3. Results and discussion
3.1. Firmness
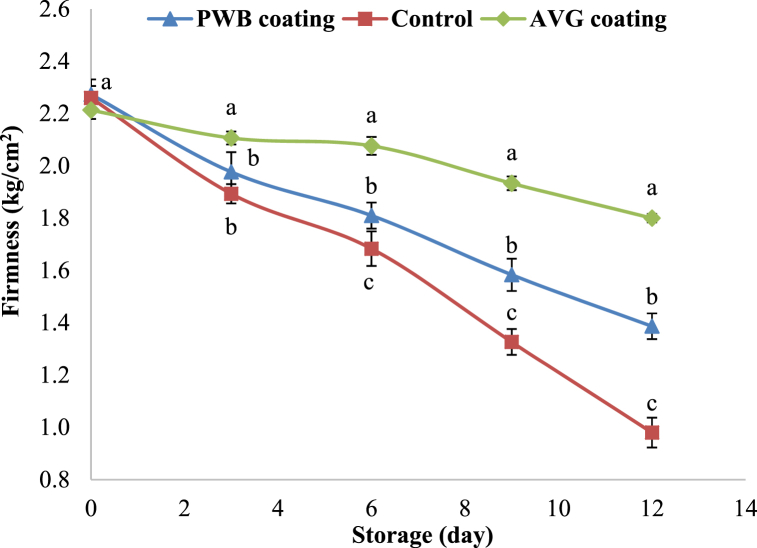
The firmness of the fruit flesh is one of the important quality characteristics that affect the storage ability [35] and consumer preferences [36] of the fruit. Fruits become softer and lose their firmness as a result of biochemical changes in cell wall fractions that involve hydrolytic reactions that lead to the breakdown of cell-wall polymers such as cellulose, hemicelluloses, and pectin, among others [37] and the decrease in turgor pressure in the cell [38] when the maturation is in progress. The hydrolytic enzymes polygalacturonase, pectin methyl esterase, pectate lyase, rhamnogalacturonase, cellulase, galactosidase, and others, cause various hydrolytic reactions [39]. This study also observed that the firmness of fruits was affected by coating types and the storage period, as shown in Fig. 1. The firmness of hog plum decreased with an increase in storage time in both control and treated fruits. The firmness of the hog plum was in the range between 2.27 kg/cm2 and 0.98 kg/cm2. The AVG coating treatment on hog plum possessed significantly higher firmness of 1.69 kg/cm2 compared to PWB packaging treatment (1.39 kg/cm2) and uncoated hog plum (0.98 kg/cm2) at the last day of storage. Application of AVG coating on the fruit's surface could delay the fruit flesh from softening during storage by lowering the activities of the enzymes polygalacturonase, pectin methylesterase, and galactosidase in fruit [17,40]. The coating on the surface of the fruit acts as a barrier to water diffusion and prevents dehydration, resulting in a reduced loss of firmness [41]. Furthermore, the loss of firmness of the stored fruits could be due to the transformation of insoluble pectin into soluble form while storage [42]. Khan et al. [19] and Parven et al. [22] found that the AVG coating on apple and papaya, respectively minimized the loss of firmness during storage. Other similar studies by Rasouli et al. [43] and Totad et al. [44] reported that AVG coating delays the softening of the fruit flesh during storage. In contrast, Islam et al. [24] observed no effect on the fruit flesh firmness after harvest.
Fig. 1.
Firmness changes of hog plum during storage. Different letters show the significant difference between treatments at the specified storage day (P < 0.05).
3.2. Weight loss
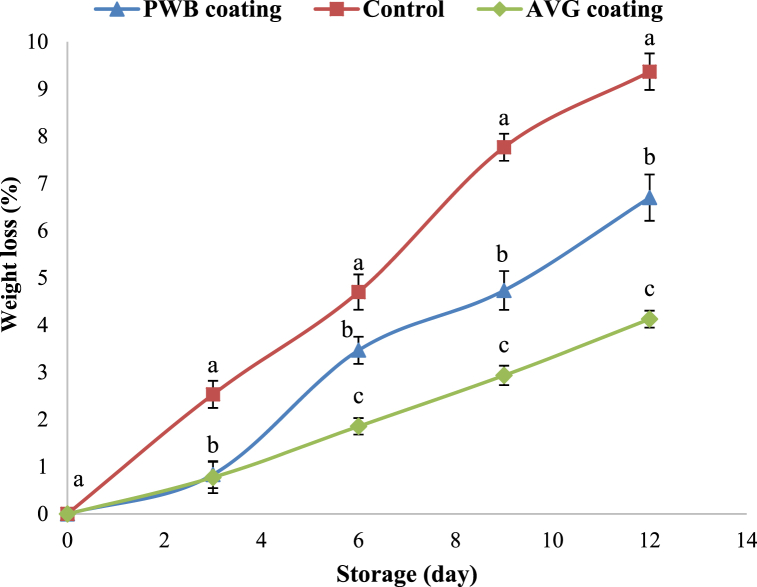
The weight loss of fruits causes economic concerns since it leads to a deterioration in both the structural quality of the fruit and its visual attractiveness. Generally, it is assumed that the weight loss of fresh fruits and vegetables occurs through the peel, which is influenced by the vapor pressure gradient between the fruit tissue and the surrounding atmosphere. This gradient can cause senescence through metabolic reactions such as ethylene production [45]. Water loss in fresh fruit may cause by the decrease of carbon reserves in the fruit due to respiration and transpiration [46]. As a result, water is easily released through the peel by transpiration, which also allows for the removal of several other soluble substrates. As stated in past research [20,22], in this study, the weight loss of fruits gradually increased throughout the storage period for all treatments, as shown in Fig. 2. Coating treatments demonstrated a significant effect on the reduction of weight loss of hog plum compared to control. Percent water loss ranged from 4.13 to 9.37% at the end of the storage. The AVG coating treatment showed a better effect compared to the PWB packaging treatment and control after 12 d of storage. The results in this present study exhibited that AVG coating treatment and PWB packaging treatment effectively reduced the percent weight loss of hog plum compared to the control treatment, probably, the coating may function as a protective barrier on fruits and their surroundings, reducing foreign exchange and moisture loss [47]. The loss of water from fruits might be influenced by light, temperature, maturation, and oxidation, resulting in increased weight loss [22]. The percent weight of hog plums during storage is in line with the findings of Freitas et al. [10]. Silva et al. [48] reported that the edible coating may prevent water loss, sugar accumulation, and starch breakdown. The AVG coating on apples [19] and papaya [22] also demonstrated a similar reduction trend in weight loss of these fruits during storage.
Fig. 2.
Percent weight loss of hog plum during storage. Different letters show the significant difference between treatments at the specified storage day (P < 0.05).
3.3. Total soluble solids
The total soluble solids (TSS) content of fruits has the greatest impact on how it tastes when eaten. With the maturation of the fruit, the TSS increases as a consequence of the hydrolysis of the polysaccharides not dissolved in simple sugars [49]. In our study, the coating treatments and storage time demonstrated a significant effect on the TSS of hog plums. Like previous studies [22,25], in this current study, a gradual increase in TSS content was noticed in all treatments throughout the storage period, as shown in Table 1. The control fuits had a significantly higher level of TSS content of 8.92°Brix compared to the PWB packaging treatment (8.19°Brix) and AVG coating treatment (7.95°Brix) at the end of 12 d of storage. The coating treatments were found to reduce the TSS content during the storage of hog plums. The AVG coating and PWB packaging treatments showed statistically the same effect on the TSS content of fruits throughout the storage. The gel barriers around the fruit and/or the paraffin wax coating on the interior surface of the box may have changed the internal atmosphere by reducing oxygen and/or raising carbon dioxide levels, suppressing ethylene production [22]. According to Barakat et al. [50], increase in TSS content with storage time in climacteric fruits is common. Rodriguez et al. [51] stated that the breakdown of pectin and the transformation of carbohydrates into simple sugars throughout storage due to the metabolic functions of the tissues could lead to an increase in TSS. Shahkoomahally and Ramezanian [52] also reported that the TSS of fruits increases, possibly due to the higher water and weight loss during storage. A similar effect of AVG coating on the TSS content has been observed for strawberry [53] and apples [19].
Table 1.
TSS, TA, and pH of AVG and PWB coated hog plum during storage interval.
| Parameter | Storage (day) |
|||||
|---|---|---|---|---|---|---|
| Treatment | 0 | 3 | 6 | 9 | 12 | |
| TSS (° Brix) | Control | 7.50 ± 0.33Ad | 7.78 ± 0.19Acd | 8.19 ± 0.32Abc | 8.58 ± 0.21Aab | 8.92 ± 0.16Aa |
| PWB coated hog plum | 7.51 ± 0.42Ab | 7.53 ± 0.38Ab | 7.97 ± 0.11Aab | 8.12 ± 0.11Ba | 8.19 ± 0.34Ba | |
| AVG coated hog plum | 7.5 ± 0.05Ab | 7.67 ± 0.18Aab | 7.73 ± 0.22Aab | 7.76 ± 0.08Cab | 7.95 ± 0.13Ba | |
| TA | Control | 0.18 ± 0.03Ad | 0.37 ± 0.05Ac | 0.56 ± 0.06Ab | 0.72 ± 0.04Aa | 0.77 ± 0.05Aa |
| PWB coated hog plum | 0.20 ± 0.03Ad | 0.27 ± 0.04Bd | 0.36 ± 0.03Bc | 0.51 ± 0.04Bb | 0.67 ± 0.05Ba | |
| AVG coated hog plum | 0.19 ± 0.02Ad | 0.23 ± 0.03Bd | 0.30 ± 0.03Bc | 0.38 ± 0.04Cb | 0.47 ± 0.05Ca | |
| pH | Control | 3.20 ± 0.08Aa | 2.92 ± 0.05Bb | 2.73 ± 0.07Bc | 2.68 ± 0.03Bc | 2.54 ± 0.02Bd |
| PWB coated hog plum | 3.17 ± 0.07Aa | 2.93 ± 0.05Bb | 2.81 ± 0.05Bc | 2.71 ± 0.05Bc | 2.60 ± 0.08Bd | |
| AVG coated hog plum | 3.17 ± 0.04Aa | 3.06 ± 0.05Ab | 2.98 ± 0.07Abc | 2.94 ± 0.05Acd | 2.86 ± .03Ad | |
Note: Control: Untreated fruit; AVG: Aloe vera gel; PWB: Paraffin wax-coated paperboard box packaging. Values with different superscript letters in a row (small letter) and a column (Capital letter) are significantly different (P < 0.05). Data are represented as mean ± SD.
3.4. Titratable acidity and pH
Titratable acidity (TA) and pH are two important parameters for determining the freshness of fruit and are directly correlated since the pH value is defined based on acid compounds. In our study, the TA and pH were affected by coating treatments and storage time. All the treatments showed a significant increase in TA and a decrease in pH with the increase in storage period, as summarised in Table 1. At 12 d of storage, the TA values varied from 0.47 to 0.77, where the lowest value of TA (0.47) was found in AVG-coated hog plums. The pH ranged from 2.86 to 2.54 at the end of the storage, with AVG-coated hog plums having the highest pH of 2.86. This finding is similar to Freitas et al. [10], who investigated hog plum coated with cassava starch and PVC film. In general, TA content decreases [24,25] and pH increases [33] during storage, probably due to organic acids serving as a substrate for respiration during the ripening or maturing of the fruit. However, an increase in TA in our study could be caused by anaerobic respiration (increased CO2 concentration and decreased oxygen concentration), which may affect the glycolytic enzyme system, leading to a build-up of acids in fruits [54]. In this current investigation, the AVG coating and PWB packaging treatments were found to reduce the increase of TA and decrease of pH.
3.5. Color values
One of the most important consumer expectations for fruit acceptance is the color of the fruit. Consequently, it is essential to ensure that the fruit keeps its proper color while it is being stored. The increase in the breakdown of chlorophyll, the accumulation of anthocyanins, and the acceleration of the carotenoid synthesis due to a rise in ethylene in the fruit promotes the color change of the fruit after harvest [55]. Applications of edible coatings can alter the fruit surface qualities that prevent discoloration by delaying changes in anthocyanin and total phenolic contents [56]. In our study, the color difference (ΔE) for all treatments was increased gradually from harvest color with the increase in storage period, as presented in Fig. 3. The control demonstrated a higher color difference compared to PWB packaging and AVG coating treatment, but the values were not statistically significant up to nine days of storage due to a high standard deviation from the mean. The total color difference of AVG coating treatments was significantly lower than the control at the end of the experiment (12 d). Chlorophyll transforms into other pigments and synthesizes carotenoids and anthocyanins, which causes color changes [57]. According to another study, color changes and softening occur due to the degradation of cell wall components by hydrolytic enzyme activity during ripening and post-harvest storage [58]. The AVG coating and PWB packaging of fruits modify its gas permeability by acting as a barrier. This modified atmospheric condition may slow down the process of color changing in fruits externally and internally, delaying chlorophyll degradation and carotenoid synthesis [34]. In this experiment, the AVG coating treatments retained the color of hog plum peel throughout the storage better than the PWB coating treatment. A similar effect of AVG coating on the color change of papaya [22] and strawberry [21] has also been reported.
Fig. 3.
Total color difference (ΔE) of AVG and PWB coated hog plum during storage. Different letters on bar chart show the significant difference between treatments at the specified storage day. (P < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.6. Decay evaluation
The visual decay evaluation of the control, treated hog plum is shown in Table 2. There were no evident signs of decay in PWB-packed hog plum and AVG-coated hog plum until 3 d and 6 d. The infection of fruits increased significantly after the third day of storage for PWB-packed hog plum and after six days of storage for AVG-coated hog plum, with soft rot spot growth and shrinkage continuing until the end of the storage period for both treatments. AVG coating, PWB packaging, and control treatment secured 1.97, 2.17, and 2.63 values at the 12 d of storage. The results of decay evaluation exhibited that the AVG coating had a lower infection score than PWB packaging and control throughout the storage period, which is consistent with the previous research showing that the application of AVG coating could increase resistance against decay substances in table grapes [23], strawberry [47], and orange fruit [43].
Table 2.
Effect of AVG coating and PWB packaging on decay incidence of hog plum during storage.
| Treatment | Storage (day) |
||||
|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | |
| Control | 1 ± 0.00Ad | 1 ± 0.00Ad | 1.63 ± 0.06Ac | 2.27 ± 0.12Ab | 2.63 ± 0.12Aa |
| PWB coated hog plum | 1 ± 0.00Ad | 1 ± 0.00Ad | 1.23 ± 0.06Bc | 1.83 ± 0.12Bb | 2.17 ± 0.06Ba |
| AVG coated hog plum | 1 ± 0.00Ac | 1 ± 0.00Ac | 1.03 ± 0.06Cc | 1.53 ± 0.10Cb | 1.97 ± 0.06Ca |
Note: Control: Untreated fruit; AVG: Aloe vera gel; PWB: Paraffin wax-coated paperboard box packaging. Values with different superscript letters in a row (small letter) and a column (Capital letter) are significantly different (P < 0.05). Data are represented as mean ± SD.
3.7. Microbial population
The edible coating can improve food safety by slowing or preventing the growth of microorganisms. Aloe vera gel, as an edible coating, has antimicrobial activity against Gram-positive than Gram-negative bacteria [59] and antifungal activity against Rhizopus stolonifer, Bortrytis cinerea, and Penicillium digitatum [60]. In our research, the AVG coating and PWB coating treatments lowered the growth of microorganisms during storage at room temperature, as summarised in Table 3. After harvesting, the microbial population was found to be 1.2 × 103 CFU/g. Microbial load increased with an increase in storage period. Both coating treatments lowered the microbial growth more than the control at the end of storage, where the AVG coating treatment possessed the better result. After 12 d of storage, the total microbial population in control was 1.5 × 107 CFU/g, whereas in the AVG-coated and PWB-coated hog plum, 1.2 × 105 CFU/g and 1.4 × 106 CFU/g, respectively. According to Albanese et al. [61], an edible coating is effective at delaying microbial proliferation because it creates a layer on the fruit's surface that reduces water activity on the surface of the fruit. A reduction of microbial growth after the application of AVG coating has also been reported for lotus root slices [26], Persian walnut [62], and grapes [17] during storage.
Table 3.
Microbial evaluation of AVG and PWB coated hog plum during storage.
| Treatment | Storage (day) |
||
|---|---|---|---|
| 0 | 6 | 12 | |
| Control | 1.2 × 103 ± 0.08 Ac | 1.4 × 106 ± 0.14 Ab | 1.5 × 107 ± 0.18 Aa |
| PWB coated hog plum | 1.2 × 103 ± 0.08 Ac | 1.3 × 105 ± 0.15 Bb | 1.4 × 106 ± 0.10 Ba |
| AVG coated hog plum | 1.2 × 103 ± 0.08 Ac | 3.3 × 104 ± 0.17 Cb | 1.2 × 105 ± 0.12Ca |
Note: Control: Untreated fruit; AVG: Aloe vera gel; PWB: Paraffin wax-coated paperboard box packaging. Values with different superscript letters in a row (small letter) and a column (Capital letter) are significantly different (P < 0.05). Data are represented as mean ± SD.
4. Conclusion
This research has found that AVG coating and PWB packaging affected hog plum fruit post-harvest quality. AVG coating and PWB coating treatments prevent water loss, loss of firmness, and skin color changes in fruits during storage. The AVG coating treatment was noticed to provide a better effect compared to the PWB coating treatment and control. These results indicate that AVG coating could be used as an environmentally friendly non-chemical treatment to preserve the quality of hog plum fruit and increase its post-harvest life when stored at 25 ± 3 °C. Additional research is required on the coating qualities of AVG, the packaging properties of PWB coating, and the antioxidant characteristics of hog plum fruits. Furthermore, more research is needed to determine the underlying correlation between AVG treatment and hog plum fruit antioxidant capacity. Therefore, the study suggests that the AVG coating and PWB packaging could be considered an eco-friendly treatment to maintain the quality of hog plum fruits and to increase the lifetime at 25 ± 3 °C storage condition.
Author contribution statement
Md Shakil, Sariful Islam: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sabina Yasmin: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Md. Sazzat Hossain Sarker: Conceived and designed the experiments; Wrote the paper.
Fatehatun Noor: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Funding statement
This research was funded by the Institute of Research and Training (IRT, HSTU, Bangladesh), and the project number is 91,2019-2020.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors are grateful to the Department of Food Engineering and Technology, HSTU, Bangladesh, for enabling research environment and laboratory facilities.
References
- 1.Bhuyan M.H.R., Yeasmen N., Islam M.A., Easdani M. Development of sauce from locally available Hog plum (Spondias dulcis) in Bangladesh. Fundam. Appl. Agric. 2017;2:267–270. http://www.f2ffoundation.org/faa/index.php/home/article/view/178 [Google Scholar]
- 2.Schiassi M.C.E.V., de Souza V.R., Lago A.M.T., Campos L.G., Queiroz F. Fruits from the Brazilian Cerrado region: Physico-chemical characterization, bioactive compounds, antioxidant activities, and sensory evaluation. Food Chem. 2018;245:305–311. doi: 10.1016/j.foodchem.2017.10.104. http://www.doi:10.1016/j.foodchem.2017.10.104 [DOI] [PubMed] [Google Scholar]
- 3.Oladunjoye A.O., Eziama S.C. Effect of microwave-assisted alkaline treatment on physicochemical, functional and structural properties of hog plum (Spondias mombin L.) bagasse. LWT--Food Sci. Technol. 2020;132 doi: 10.1016/j.lwt.2020.109821. [DOI] [Google Scholar]
- 4.BBS . Bangladesh Bureau of Statistics, May; 2019. Bangladesh Labor Force Survey-2019. Bangladesh Bureau of Statistics, Statistics and Informatics Division, Ministry of Planning, Government of the People's Republic of Bangladesh.https://bbs.portal.gov.bd/sites/default/files/files/bbs.portal.gov.bd [Google Scholar]
- 5.Akter S., Shahriar S.M.S., Morshed S., Islam M.N. Study on the chemical composition of fresh Mymensingh and Barishal hog-plum (Spondius mangifera) and developed leather and jelly and sensory evaluation. J. Environ. Sci. & Natural Resources. 2012;5:29–36. doi: 10.3329/jesnr.v5i2.14598. [DOI] [Google Scholar]
- 6.Alam M.S., Shakil M., Bari T., Sohany M., Nayem M.F. Formulation and quality evaluation of instant soft drink powder prepared from hog plum (Spondius Mangifera) and mint (Mentha spicata) Int. J. Food Sci. Nutr. 2020;5:33–37. http://www.foodsciencejournal.com/archives/2020/vol5/issue1/4-6-60 [Google Scholar]
- 7.Arif M., Rahman M.A., Imran M., Khalid M., Khushtar M. An insight of Spondias mangifera willd: an underutilized medicinal plant with immense nutraceutical and therapeutic potentials. Int. J. Res. Pharm. Sci. 2015;6:17–26. [Google Scholar]
- 8.Dhall R.K. Advances in edible coatings for fresh fruits and vegetables: a review. Crit. Rev. Food Sci. Nutr. 2013;53:435–450. doi: 10.1080/10408398.2010.541568. [DOI] [PubMed] [Google Scholar]
- 9.Abdullah A.M., Khokon M.M.A., Hasan M.Z., Khan Z.U.M., Mahmud S.A., Rashid H. Radiation preservation of hog-plum (Spondias pinnata) in combination with chemicals. Am. J. Food Technol. 2016;11:221–227. doi: 10.3923/ajft.2016.221.227. [DOI] [Google Scholar]
- 10.Freitas R.V.D.S., Souza P.A.de, Coelho E.L., Souza F.X.de, Beserra H.N.B.R. Storage of mombin fruits coated with cassava starch and pvc film. Rev. Caatinga. 2017;30:244–249. doi: 10.1590/1983-21252017v30n127rc. [DOI] [Google Scholar]
- 11.Ojediran J.O., Okonkwo C.E., Olaniran A.F., Iranloye Y.M., Adewumi A.D., Erinle O., Afolabi Y.T., Adeyi O., Adeyi A. Hot air convective drying of hog plum fruit (Spondias mombin): effects of physical and edible-oil-aided chemical pretreatments on drying and quality characteristics. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e08312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan J., Wu R., Strik B.C., Zhao Y. Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions. Postharvest Biol. Technol. 2011;59:71–79. http://doi:10.1016/j.postharvbio.2010.08.006 [Google Scholar]
- 13.Cha D.S., Chinnan M.S. Biopolymer-based antimicrobial packaging: a review. Crit. Rev. Food Sci. Nutr. 2005;44:223–237. doi: 10.1080/10408690490464276. https://doi:10.1080/10408690490464276 [DOI] [PubMed] [Google Scholar]
- 14.Krochta J.M., Baldwin E.A., Nisperos-Carriedo M.O. Technomic Publ. Co. Inc; USA: 1994. Edible Coatings and Films to Improve Food Quality. [Google Scholar]
- 15.Brishti F.H., Misir J., Sarker A. Effect of biopreservatives on storage life of papaya (Carica papaya L.) Int. J. Food Stud. 2013;2:126–136. doi: 10.7455/ijfs/2.1.2013.a10. [DOI] [Google Scholar]
- 16.Suriati L., Mangku I.G.P., Rudianta I.N. The characteristics of Aloe vera gel as an edible coating. IOP Conference Series: Earth and Enviro Sci. 2018;207 https://doi:10.1088/1755-1315/207/1/012051 [Google Scholar]
- 17.Valverde J.M., Valero D., Martínez-Romero D., Guillén F., Castillo S., Serrano M. Novel edible coating based on Aloe vera gel to maintain table grape quality and safety. J. Agric. Food Chem. 2005;53:7807–7813. doi: 10.1021/jf050962v. https://doi:10.1021/jf050962v [DOI] [PubMed] [Google Scholar]
- 18.Hamman J.H. Composition and applications of Aloe vera leaf gel. Molecules. 2008;13:1599–1616. doi: 10.3390/molecules13081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan N., Riaz A., Rahman Z., Mawa J.U., Begum H. Shelf-life assessment of apple fruit coated with aloe vera gel and calcium chloride. Pure Appl. Biol. 2019;8:1876–1889. [Google Scholar]
- 20.Ates U., Islam A., Ozturk B., Aglar E., Karakaya O., Gun S. Changes in quality traits and phytochemical components of blueberry (Vaccinium Corymbosum Cv. Bluecrop) fruit in response to postharvest Aloe vera treatment. Int. J. Fruit Sci. 2022;22:303–316. doi: 10.1080/15538362.2022.2038341. [DOI] [Google Scholar]
- 21.Qamar J., Ejaz S., Anjum M.A., Nawaz A., Hussain S., Ali S., Saleem S. Effect of Aloe vera gel, chitosan and sodium alginate based edible coatings on postharvest quality of refrigerated strawberry fruits of cv. Chandle, J. Hortic. Sci. Technol. 2018;1:8–16. doi: 10.46653/jhst180101008. [DOI] [Google Scholar]
- 22.Parven A., Sarker M.R., Megharaj M., Meftaul M.I. Prolonging the shelf life of Papaya (Carica papaya L.) using Aloe vera gel at ambient temperature. Sci. Hortic. 2020;265 doi: 10.1016/j.scienta.2020.109228. [DOI] [Google Scholar]
- 23.Nia A.E., Taghipour S., Siahmansour S. Pre-harvest application of chitosan and postharvest Aloe vera gel coating enhances quality of table grape (Vitis vinifera L. cv. ‘Yaghouti’) during postharvest period. Food Chem. 2021;347 doi: 10.1016/j.foodchem.2021.129012. [DOI] [PubMed] [Google Scholar]
- 24.Islam A., Acıkalın R., Ozturk B., Aglar E., Kaiser C. Combined effects of Aloe vera gel and modified atmosphere packaging treatments on fruit quality traits and bioactive compounds of jujube (Ziziphus jujuba Mill.) fruit during cold storage and shelf life. Postharvest Biol. Technol. 2022;187 doi: 10.1016/j.postharvbio.2022.111855. [DOI] [Google Scholar]
- 25.Saleem M.S., Ejaz S., Anjum M.A., Ali S., Hussain S., Nawaz A., Naz S., Maqbool M., Abbas A.M. Aloe vera gel coating delays softening and maintains quality of stored persimmon (Diospyros kaki Thunb.) Fruits. J. Food Sci. Technol. 2022;59:3296–3306. doi: 10.1007/s13197-022-05412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali S., Anjum M.A., Nawaz A., Naz S., Hussain S., Ejaz S., Sardar H. Effect of pre‐storage ascorbic acid and Aloe vera gel coating application on enzymatic browning and quality of lotus root slices. J. Food Biochem. 2020;44 doi: 10.1111/jfbc.13136. [DOI] [PubMed] [Google Scholar]
- 27.Ozturk B., Aglar E. Effects of modified atmosphere packaging (MAP) and Aloe vera treatments on quality characteristics of cornelian cherry fruits during cold storage. Akademik Ziraat Dergisi. 2019:1–8. doi: 10.29278/azd.592897. [DOI] [Google Scholar]
- 28.Chrysargyris A., Nikou A., Tzortzakis N. Effectiveness of Aloe vera gel coating for maintaining tomato fruit quality. N. Z. J. Crop Hortic. Sci. 2016;44:203–217. doi: 10.1080/01140671.2016.1181661. [DOI] [Google Scholar]
- 29.Shakil M., Mahawanich T. Effect of UV-C curing on properties of ferulic acid-added soy protein film. Proceedings of the 24th Food Innovation Asia Conference. 2022:341–348. https://www.researchgate.net/publication/360764325 FIAC 2022. [Google Scholar]
- 30.Kukiatkulchai D. MSc. thesis. Kasetsart University; Thailand: 2007. Effect of Coating Modify Starch and Sizing Agent on Properties of Kraft Liner in Cold Storage Application. [Google Scholar]
- 31.Suppakul P., Miltz J., Sonneveld K., Bigger W.S. Active packaging technologies with an emphasis on antimicrobial packaging and its applications. J. Food Sci. 2003;68:408–420. doi: 10.1111/j.1365-2621.2003.tb05687.x. [DOI] [Google Scholar]
- 32.Aloui H., Khwaldia K. Effects of coating weight and nanoclay content on functional and physical properties of bionanocomposite-coated paper. Cellulose. 2017;24:4493–4507. doi: 10.1007/s10570-017-1436-1. [DOI] [Google Scholar]
- 33.Hassanpour H. Effect of Aloe vera gel coating on antioxidant capacity, antioxidant enzyme activities and decay in raspberry fruit. LWT--Food Sci. Technol. 2015;60:495–501. doi: 10.1016/j.lwt.2014.07.049. [DOI] [Google Scholar]
- 34.Ergun M., Satici F. Use of aloe vera gel as biopreservative for ‘Granny Smith’ and ‘red chief’ apples. J. Anim. Plant Sci. 2012;22:363–368. [Google Scholar]
- 35.Ozturk B., Kucuker E., Karaman S., Ozkan Y. The effects of cold storage and aminoethoxyvinylglycine (AVG) on bioactive compounds of plum fruit (Prunus salicina Lindell cv.‘Black Amber’) Postharvest Biol. Technol. 2012;72:35–41. doi: 10.1016/j.postharvbio.2012.04.015. [DOI] [Google Scholar]
- 36.Chen Y., Y.H. Sun J., Lin H., Hung Y.C., Zhang S., Lin Y., Lin T. Paper based 1-MCP treatment suppresses cell wall metabolism and delays softening of Huanghua pears during storage. J. Sci. Food Agric. 2017;97:2547–2552. doi: 10.1002/jsfa.8072. [DOI] [PubMed] [Google Scholar]
- 37.Wang L., Jin P., Wang J., Jiang L., Shan T., Zheng Y. Effect of β-aminobutyric acid on cell wall modification and senescence in sweet cherry during storage at 20°C. Food Chem. 2015;175:471–477. doi: 10.1016/j.foodchem.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Mannozzi C., Tylewicz U., Chinnici F., Siroli L., Rocculi P., Dalla Rosa M., Romani S. Effects of chitosan based coatings enriched with procyanidin by-product on quality of fresh blueberries during storage. Food Chem. 2018;251:18–24. doi: 10.1016/j.foodchem.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Payasi A., Mishra N.N., Chaves A.L.S., Singh R. Biochemistry of fruit softening: an overview. Physiol. Mol. Biol. Plants. 2009;15:103–113. doi: 10.1007/s12298-009-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Remón S., Venturini M.E., Lopez-Buesa P., Oria R. Burlat cherry quality after long range transport: optimisation of packaging conditions. Innov. Food Sci. Emerg. Technol. 2003;4:425–434. https://doiord/10.1016/S1466-8564(03)00058-4 [Google Scholar]
- 41.Mohebbi M., Ansarifar E., Hasanpour N., Amiryousefi M.R. Suitability of aloe vera and gum tragacanth as edible coatings for extending the shelf life of button mushroom. Food Bioproc. Tech. 2012;5:3193–3202. doi: 10.1007/s11947-011-0709-1. [DOI] [Google Scholar]
- 42.Bayindirli L.A. Density and viscosity of grape juice as a function of concentration and temperature. J. Food Process. Preserv. 1993;17:147–151. doi: 10.1111/j.1745-4549.1993.tb00231.x. [DOI] [Google Scholar]
- 43.Rasouli M., Saba M.K., Ramezanian A. A., Inhibitory effect of salicylic acid and Aloe vera gel edible coating on microbial load and chilling injury of orange fruit. Sci. Hortic. 2019;247:27–34. doi: 10.1016/j.scienta.2018.12.004. [DOI] [Google Scholar]
- 44.Totad M.G., Sharma R.R., Sethi S., Verma M.K. Effect of edible coatings on ‘Misty’blueberry (Vaccinium corymbosum) fruits stored at low temperature. Acta Physiol. Plant. 2019;41:1–7. doi: 10.1007/s11738-019-2973-z. [DOI] [Google Scholar]
- 45.Bai J., Alleyne V., Hagenmaier R.D., Mattheis J.P., Baldwin E.A. Formulation of zein coatings for apples (Malus domestica Borkh) Postharvest Biol. Technol. 2003;28:259–268. doi: 10.1016/S0925-5214(02)00182-5. [DOI] [Google Scholar]
- 46.Saki M., ValizadehKaji B., Abbasifar A., Shahrjerdi I. Effect of chitosan coating combined with thymol essential oil on physicochemical and qualitative properties of fresh fig (Ficus carica L.) fruit during cold storage. J. Food Meas. Charact. 2019;13:1147–1158. doi: 10.1007/s11694-019-00030-w. [DOI] [Google Scholar]
- 47.Sogvar O.B., Saba M.K., Emamifar A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016;114:29–35. doi: 10.1016/j.postharvbio.2015.11.019. [DOI] [Google Scholar]
- 48.Silva G.M.C., Silva W.B., Medeiros D.B., Salvador A.R., Cordeiro M.H.M., da Silva N.M., Santana D.B., Mizobutsi G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017;237:372–378. doi: 10.1016/j.foodchem.2017.05.123. [DOI] [PubMed] [Google Scholar]
- 49.Abd El-Gawad M.G., Zaki Z.A., Ekbal Z.A. Effect of some postharvest treatments on quality of" alphonse" mango fruits during cold storage, Middle East. J. Agric. Res. 2019;8:1067–1079. doi: 10.36632/mejar/2019.8.4.9. [DOI] [Google Scholar]
- 50.Barakat M.R., Mohamed M.A.A., Essa M.A., Zaki Z.A. Effect of using some biological post-harvest treatments on storability of Washington Navel orange fruits compared with Imazalil post-harvest chemical treatments. J. Hort. Sci. & Ornamen. Plants. 2012;4:50–57. [Google Scholar]
- 51.Rodriguez A., Batlle R., Nerin C. The use of natural essential oils as antimicrobial solutions in paper packaging, Part II. Prog. Org. Coat. 2007;60:33–38. doi: 10.1016/j.porgcoat.2007.06.006. [DOI] [Google Scholar]
- 52.Shahkoomahally S., Ramezanian A. Effect of natural aloe vera gel coating combined with calcium chloride and citric acid treatments on grape (Vitis vinifera L. cv. Askari) quality during storage. Am. J. Food Technol. 2014;2:1–5. doi: 10.12691/ajfst-2-1-1. [DOI] [Google Scholar]
- 53.Zafari E., Mohammadkhani A., Roohi V., Fadaei A., Zafari H. Effect of exogenous putrescine and Aloe vera gel coating on post-harvest life of strawberry (Fragaria ananassa Duch.) fruit, cultivar Kamarosa. Int. j. agric. crop sci. 2015;8:578–584. [Google Scholar]
- 54.Sharma P., Shehin V.P., Kaur N., Vyas P. Application of edible coatings on fresh and minimally processed vegetables: a review. Int. J. Veg. Sci. 2019;25:295–314. doi: 10.1080/19315260.2018.1510863. [DOI] [Google Scholar]
- 55.Guerra M., Casquero P.A. Effect of harvest date on cold storage and postharvest quality of plum cv. Green Gage, Postharvest Biol. Technol. 2008;47:325–332. doi: 10.1016/j.postharvbio.2007.07.009. [DOI] [Google Scholar]
- 56.Meighani H., Ghasemnezhad M., Bakhshi D. Effect of different coatings on post-harvest quality and bioactive compounds of pomegranate (Punica granatum L.) fruits. J. Food Sci. Technol. 2015;52:4507–4514. doi: 10.1007/s13197-014-1484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ochiki S., Gesimba M.R., Wolukau J.N. Effect of Aloe vera gel coating on postharvest quality and shelf life of mango (Mangifera indica L.) fruits Var. Ngowe, j. hortic. & for. 2015;7:1–7. doi: 10.5897/jhf2014.0370. [DOI] [Google Scholar]
- 58.Serrano M., Valverde J.M., Guillén F., Castillo S., Martínez-Romero D., Valero D. Use of Aloe vera gel coating preserves the functional properties of table grapes. J. Agric. Food Chem. 2006;54:3882–3886. doi: 10.1021/jf060168p. [DOI] [PubMed] [Google Scholar]
- 59.Saritha V., Anilakumar K.R., Khanum F. Antioxidant and antibacterial activity of Aloe vera gel extracts. Int. J. Pharm. Biol. Arch. 2010;1:376–384. [Google Scholar]
- 60.Navarro D., Díaz-Mula H.M., Guillén F., Zapata P.J., Castillo S., Serrano M., Valero D., Martínez-Romero D. Reduction of nectarine decay caused by Rhizopus stolonifer, Botrytis cinerea and Penicillium digitatum with Aloe vera gel alone or with the addition of thymol. Int. J. Food Microbiol. 2011;151:241–246. doi: 10.1016/j.ijfoodmicro.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Albanese D., Cinquanta L., Di-Matteo M. Effects of an innovative dipping treatment on the cold storage of minimally processed Annurca apples. Food Chemi. 2007;105:1054–1060. [Google Scholar]
- 62.Habibi A., Yazdani N., Chatrabnous N., Koushesh Saba M., Vahdati K. Inhibition of browning via aqueous gel solution of Aloe vera: a new method for preserving fresh fruits as a case study on fresh kernels of Persian walnut. J. Food Sci. Technol. 2021;59:2784–2793. doi: 10.1007/s13197-021-05301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.