Abstract
Background
Many individuals hospitalised with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection experience post-acute sequelae of SARS-CoV-2 infection (PASC), sometimes referred to as “long COVID”. Our objective was to conduct a systematic literature review and meta-analysis to identify PASC-associated symptoms in previously hospitalised patients and determine the frequency and temporal nature of PASC.
Methods
Searches of MEDLINE, Embase, Cochrane Library (2019–2021), World Health Organization International Clinical Trials Registry Platform and reference lists were performed from November to December 2021. Articles were assessed by two reviewers against eligibility criteria and a risk of bias tool. Symptom data were synthesised by random effects meta-analyses.
Results
Of 6942 records, 52 studies with at least 100 patients were analysed; ∼70% were Europe-based studies. Most data were from the first wave of the pandemic. PASC symptoms were analysed from 28 days after hospital discharge. At 1–4 months post-acute SARS-CoV-2 infection, the most frequent individual symptoms were fatigue (29.3% (95% CI 20.1–40.6%)) and dyspnoea (19.6% (95% CI 12.8–28.7%)). Many patients experienced at least one symptom at 4–8 months (73.1% (95% CI 44.2–90.3%)) and 8–12 months (75.0% (95% CI 56.4–87.4%)).
Conclusions
A wide spectrum of persistent PASC-associated symptoms were reported over the 1-year follow-up period in a significant proportion of participants. Further research is needed to better define PASC duration and determine whether factors such as disease severity, vaccination and treatments have an impact on PASC.
Tweetable abstract
PASC is a complex, multisystem condition with multiple clinical phenotypes and prolonged duration. Further research is needed to better define the duration of PASC and determine the impact of factors such as disease severity, vaccination and treatments. https://bit.ly/3Ks39Vx
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can result in severe acute illness and hospitalisation, and although the early phase of the pandemic was associated with high mortality, the majority of individuals survive. However, a significant proportion of survivors have ongoing symptoms after the initial infectious period and experience a prolonged recovery to their baseline health [1–3], often described as “post-acute sequelae of SARS-CoV-2 infection” (PASC) or “long COVID” [2].
A high volume of research, including primary studies and systematic literature reviews (SLRs), has been published on PASC. The variety of symptoms, temporal nature and frequency of PASC are yet to be comprehensively characterised and no consistent definition of PASC has been determined, although proposed definitions include the presence of symptoms from 4 weeks to 3 months after initial SARS-CoV-2 infection [2–5]. Due to inconsistency in the definition of PASC and the design and conduct of studies, the reported prevalence of PASC and its particular symptoms have dramatically varied [6]. As a result, much prior research is subject to challenges or limitations. Owing to the rapid pace of evidence generation for SARS-CoV-2 infection, many literature reviews, especially early in the pandemic, included preprint literature, which may have increased the risk of inclusion of lower quality data [7–11]. In our search of the literature, few previous SLRs examined outcomes at multiple time-points and therefore have been unable to quantify the changing symptom profile of PASC. Furthermore, relatively few literature reviews have included meta-analyses to synthesise outcome data.
To address this knowledge gap, our SLR and meta-analysis aimed to determine the nature and prevalence at multiple time-points of specific classes of PASC in individuals hospitalised for SARS-CoV-2 infection and to contribute knowledge that may help inform a definition of PASC for use in future analytical work.
Methods
The SLR was performed in accordance with a prespecified protocol registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42022306931) on 27 January 2022.
Identification of studies
Studies published from 2019 onwards were identified through electronic database searches conducted on 16 November 2021, in MEDLINE (including MEDLINE In-Process, MEDLINE Daily and MEDLINE Epub Ahead of Print), Embase and Cochrane Library. The bibliographies of relevant SLRs and (network) meta-analyses identified through the electronic database searches were individually reviewed to identify additional studies of relevance. The World Health Organization International Clinical Trials Registry Platform was also searched, on 3 December 2021. Additional grey literature, such as congresses and preprint articles, were not searched due to the recency of the COVID-19 pandemic and a decision to prioritise peer-reviewed literature. Lists of the search terms used in each source are presented in the supplementary material.
Study selection
Each abstract and full text was reviewed against predefined eligibility criteria, developed using the Population, Intervention, Comparison and Outcomes (PICO) framework. In line with accepted practice, each title and abstract was reviewed against the eligibility criteria by one reviewer [12]. A second independent reviewer provided input in cases of uncertainty, and independently reviewed 10% of the excluded articles and all included articles.
Each full-text article was then reviewed by two independent reviewers, with disagreements resolved by discussion until consensus was met. If necessary, a third independent reviewer made the final decision. Studies were required to have an objective of investigating PASC and include patients aged ≥12 years experiencing long-term effects of SARS-CoV-2 infection. Interventional and observational studies were included, while case reports and non-peer-reviewed literature were excluded. Specific criteria were imposed on the study population to increase the reliability of results and reduce heterogeneity. The population was restricted to patients who had been hospitalised for SARS-CoV-2 infection. This ensured that only studies in populations with a definite, confirmed diagnosis of SARS-CoV-2 infection were included, rather than self-diagnosed patients. The required minimum duration of follow-up was 28 days (4 weeks) after SARS-CoV-2 infection diagnosis. Studies that reported outcomes before patients had been discharged from the hospital were excluded based on the clinical definitions used by the Centers for Disease Control and Prevention (CDC) guidelines and the 2021 National Institute for Health and Care Excellence (NICE) rapid guideline (NG188) [3, 13].
In a protocol amendment, due to a large volume of identified evidence, only studies that reported on clinical symptoms associated with PASC were carried through to data extraction. Studies with sample sizes of at least 100 patients were prioritised to improve the precision of outcome estimates.
Data extraction, quality assessment and prioritisation for quantitative synthesis
Data extractions and quality assessments were performed in line with guidelines from the University of York Centre for Reviews and Dissemination by a single individual, with a second individual independently verifying all extracted information (and arbitration by a third individual if necessary) [14]. Top-line information on the included studies was first extracted into a prespecified evidence compendium. Following this, only studies that clearly reported the method of SARS-CoV-2 infection diagnosis were prioritised for quantitative synthesis via a meta-analysis. Subsequently, detailed extractions were conducted to capture information on study characteristics, patient characteristics and PASC symptoms.
Feasibility assessment and meta-analysis
A feasibility assessment was undertaken to assess the suitability of the identified studies for meta-analysis. This consisted of an assessment of the heterogeneity, reported symptoms and follow-up time-points of the included studies. Random effects meta-analyses were conducted to synthesise estimates of the frequency of each symptom reported across studies and pooled estimates were obtained for each symptom at each specific time-point. The estimates generated in the meta-analysis represent the average percentage of patients experiencing the symptoms, while the ranges of symptom frequency (supplementary table S1) provide an indication as to the extent of variation in the percentage of patients experiencing each symptom.
The meta-analyses were run using the metaprop function from the meta package (version 5.1-1) in R software (version 4.1.2) [15]. The metafor package (version 3.4-0) was used to perform a generalised linear mixed model logistic regression [16]. The corresponding 95% confidence intervals were estimated using the Clopper–Pearson method. A 0.5 zero-cell correction was applied in the case of studies reporting zero patients experiencing a specific symptom. Where possible, data from the full study population were used. Where necessary, additional data were digitised from plots and calculations were performed (e.g. pooling of study subgroups). Plots were identified in a systematic manner across all studies and digitised using DigitizeIt software [17]; values obtained via digitisation were checked for quality by an independent reviewer. The approaches used in the feasibility assessment and meta-analysis were validated by clinician feedback.
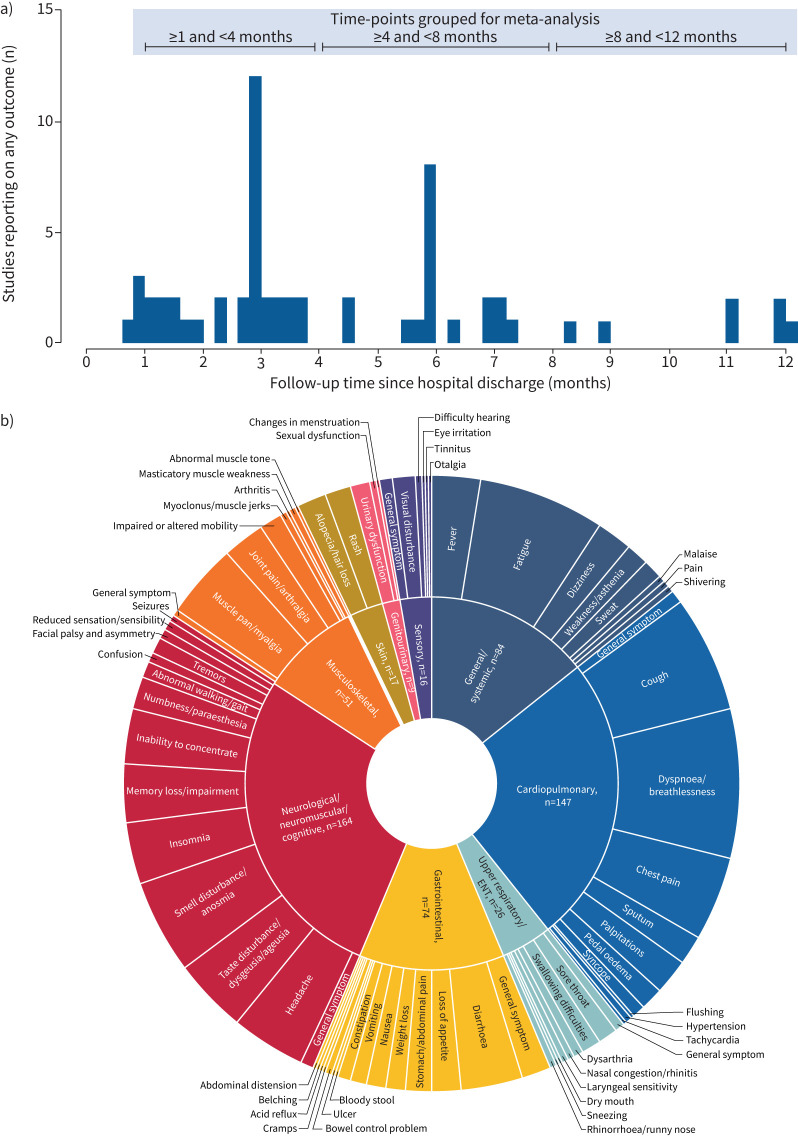
The time-points at which PASC was reported were grouped into three periods for the meta-analysis based on the spread of reported follow-up time-points: ≥1 to <4, ≥4 to <8 and ≥8 to <12 months (figure 1a). The selected lower bound for the first time period was chosen to align with the CDC and NICE definitions of PASC [3, 13].
FIGURE 1.
a) Histogram summarising frequency of reporting of post-acute sequelae of severe acute respiratory syndrome coronavirus 2 infection (PASC) outcomes in extracted studies. Studies that reported on more than one follow-up time-point are included in the plot at each time-point they reported on. b) Sunburst plot summarising frequency of reporting of PASC outcomes in extracted studies. The sunburst plot summarises the frequency of reporting of PASC symptoms across the extracted studies, with the inner ring divided by symptom category and the outer ring reporting all symptoms within each category. The size of each section in both the inner and the outer ring reflects the number of primary publications that reported each symptom. The starburst plot does not contain any information on combined symptoms or the percentage of patients experiencing at least one symptom. COVID-19: coronavirus disease 2019; ENT: ear–nose–throat.
Funnel plots were created and Egger's tests were performed to assess publication bias across individual symptoms. In the funnel plots, the standard error of each study was plotted against the size of the study's treatment effect in the meta-analysis. Egger's test (a linear regression of the treatment effect estimates on their standard errors weighted by their inverse variance) evaluated potential publication bias via funnel plot asymmetry.
Results
Identified studies
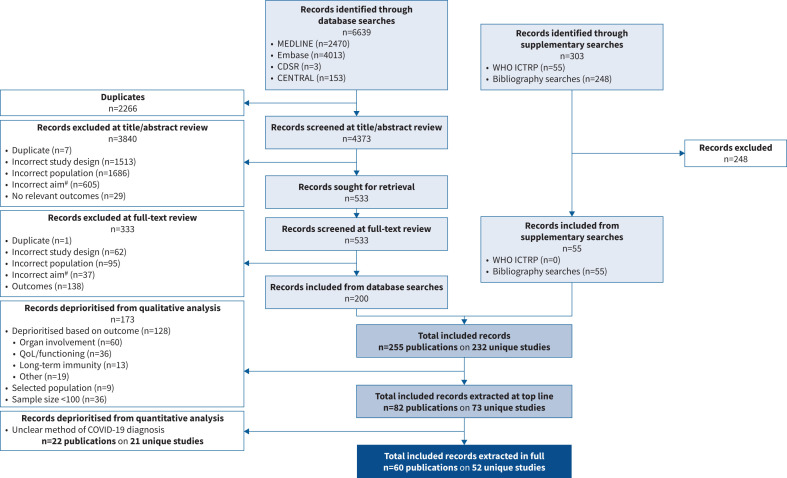
After the removal of duplicates, 4373 records were identified through electronic database searches. After the exclusion of 3840 irrelevant records based on title and abstract, 533 full texts were screened. Following this, 200 records were considered relevant for inclusion in the SLR on the basis that they aimed to characterise an aspect of long-term SARS-CoV-2 infection. An additional 55 records were included from bibliography searches, resulting in 255 total records that met an aspect of the initial PICO criteria. After prioritisation of studies that reported a relevant clinical outcome, had at least 100 patients and reported a clear method for diagnosis of SARS-CoV-2, 60 publications on 52 unique studies were included in the quantitative analysis (figure 2).
FIGURE 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. #: studies were required to have the aim of characterising “long COVID”. CDSR: Cochrane Database of Systematic Reviews; CENTRAL: Cochrane Central Register of Controlled Trials; WHO ICTRP: World Health Organization International Clinical Trials Registry Platform; QoL: quality of life; COVID-19: coronavirus disease 2019.
Study and patient characteristics
Study characteristics varied across the 52 studies (table 1). The majority of studies were prospective cohorts (n=34) and almost all (n=43) used convenience sampling to recruit participants. Almost all studies (n=43) enrolled patients during the first 4 months of 2020, coinciding with the first wave of the pandemic. Study sites spanned Asia, Europe and North America.
TABLE 1.
Summary of study characteristics for extracted studies
| Study | Study design |
Country;
setting |
Sampling method | Population | Sample size (n) | Method of confirming COVID-19 |
| Aranda, 2021 [40] | Prospective cohort | Spain; single centre |
Convenience sampling | Patients >18 years old with COVID-19 and severe COVID-19 pneumonia who had suffered ARDS during hospital admission and survived to hospital discharge | 113 | RT-PCR |
| Asadi-Pooya, 2022 [41] | Prospective cohort | Iran; multicentre |
Convenience sampling | Hospitalised adults (≥18 years) with COVID-19 | 2696 | RT-PCR |
| Aul, 2021 [42] | Prospective cohort | UK; single centre |
Judgemental sampling# | Discharged adults (≥18 years) with COVID-19 | 387 | RT-PCR or clinico-radiological diagnosis |
| Ayoubkhani, 2021 [43] | Retrospective cohort | UK; multicentre |
Judgemental sampling# | Hospitalised patients with a primary diagnosis of COVID-19, identified by ICD-10 codes U07.1 (COVID-19, virus identified) and U07.2 (COVID-19, virus unidentified) | 47 780 | Positive laboratory test or clinical diagnosis |
| Bai, 2021 [44] | Prospective cohort | Italy; single centre |
Convenience sampling | Discharged adults (≥18 years) with COVID-19 | 377 | RT-PCR |
| Caruso, 2021 [45] | Prospective cohort | Italy; single centre |
Convenience sampling | Patients with moderate to severe COVID-19 with a diagnosis of interstitial pneumonia who had undergone baseline chest CT with positive results that was performed at admission | 118 | RT-PCR |
| Clavario, 2021 [46] | Prospective cohort | Italy; single centre |
Convenience sampling | Patients with COVID-19 with a complete CPET evaluation | 200 | RT-PCR |
| DISCOVER [34] | Prospective cohort | UK; single centre |
Convenience sampling | Adult hospitalised patients (≥18 years): 1) typical symptoms of COVID-19 (e.g. influenza-like illness with fever and muscle pain or respiratory illness with cough and shortness of breath) and positive PCR result for SARS-CoV-2, using the established PHE assay in use at the time, or 2) suspected SARS-CoV-2 infection, namely presenting with a) typical symptoms (e.g. influenza-like illness with fever and muscle pain or respiratory illness with cough and shortness of breath); and b) compatible chest radiography findings (consolidation or ground-glass shadowing); and c) alternative causes considered unlikely or excluded (e.g. heart failure, influenza) | 131 | Positive PCR result for SARS-CoV-2 or clinico-radiological diagnosis of COVID-19 |
| Fernández-de-las-Peñas 2021 [47] | Prospective cohort | Spain; multicentre |
Convenience sampling | Discharged adults (≥18 years) with COVID-19 | 1142 | RT-PCR and clinical and radiological findings |
| Fernández-de-las-Peñas, 2022 [48] | Prospective cohort | Spain; multicentre |
Convenience sampling | Hospitalised adults (≥18 years) with COVID-19 | 1969 | RT-PCR and radiological findings |
| Fernández-de-las-Peñas, 2021 [49] | Retrospective case–control/ matched cohort |
Spain; multicentre |
Judgemental sampling# | Hospitalised adults (≥18 years) with COVID-19 | 183 | RT-PCR |
| Fernández-de-las-Peñas, 2021 [28] | Retrospective cohort | Spain; multicentre |
Convenience sampling | Hospitalised adults (≥18 years) with COVID-19 | 1950 | RT-PCR and radiological findings |
| Fernández-de-las-Peñas, 2021 [50] | Prospective case–control/ matched cohort |
Spain; single centre |
Convenience sampling | Hospitalised adults (≥18 years) with COVID-19 | 738 | RT-PCR and consistent clinical and radiological findings |
| FrenchCOVID [51] | Prospective cohort | France; multicentre |
Judgemental sampling# | Discharged adults (≥18 years) with COVID-19 followed up at 6 months | 1137 | RT-PCR |
| Froidure, 2021 [52] | Retrospective cohort | Belgium; single centre |
Convenience sampling | Hospitalised patients with critical or severe COVID-19 who survived and underwent a 3-month follow-up in the study hospital | 134 | Positive PCR on NPS and lung infiltrates on lung HRCT or chest radiography at admission |
| Frontera, 2021 [53] | Prospective cohort | USA; multicentre |
Convenience sampling | Age ≥18 years; hospital admission; survival to discharge; consent to participate in a follow-up interview | 382 | RT-PCR |
| Carfì, 2020 [54] | Prospective cohort | Italy; single centre |
Convenience sampling | Discharged adults (≥18 years) with COVID-19 | 143 | RT-PCR |
| Garrigues, 2020 [55] | Prospective cohort | France; single centre |
Convenience sampling | Patients with positive COVID-19 diagnoses who responded to follow-up questionnaire | 120 | RT-PCR and/or typical abnormalities on chest CT |
| Gautam, 2022 [56] | Retrospective cohort | UK; multicentre |
Convenience sampling | Hospital admission for >3 days, with FIO2 >40% for >6 h; new stroke; pulmonary embolism; DVT; delirium; elevated high-sensitivity troponin levels; residual AKI; tachycardia (pulse rate >100 beats·min−1) at discharge | 200 | Laboratory-confirmed SARS-CoV-2 infection |
| Gherlone, 2021 [57] | Prospective cohort | Italy; single centre |
Convenience sampling | Discharged adults (≥18 years) with COVID-19 | 122 | RT-PCR |
| Gonzalez-Hermosillo, 2021 [58] | Prospective cohort | Mexico; single centre |
Convenience sampling | Adult patients hospitalised with moderate to severe confirmed COVID-19 pneumonia at hospital admission | 130 | Positive real-time RT-PCR test |
| Halpin, 2021 [59] | Retrospective cohort | UK; multicentre |
Convenience sampling | Discharged adults (≥18 years) with COVID-19 | 100 | RT-PCR |
| Han, 2021 [60] | Prospective cohort | China; multicentre |
Judgemental sampling# | Patients ≥18 years old diagnosed with respiratory rate >30 breaths·min−1, SpO2 <90% on room air or severe respiratory distress | 114 | RT-PCR |
| Huang, 2021 [61] | Ambidirectional cohort | China; single centre |
Convenience sampling | Patients who were discharged from the hospital | 1276 | Laboratory confirmed |
| Jacobs, 2020 [62] | Prospective cohort | USA; single centre |
Convenience sampling | Discharged adults (≥18 years) with COVID-19 who had been hospitalised for a duration of at least 3 days | 183 | RT-PCR |
| LinCoS [63] | Prospective cohort | Sweden; multicentre |
Judgemental sampling# | Hospitalised adults (≥18 years) with COVID-19 | 433 | Laboratory confirmed |
| Lindahl, 2021 [64] | Retrospective cohort | Finland; single centre |
Convenience sampling | Hospitalised adults (≥18 years) with COVID-19 | 101 | Laboratory confirmed |
| Lombardo, 2021 [65] | Prospective cohort | Italy; multicentre |
Judgemental sampling# | Discharged adults (≥18 years) with COVID-19 | 303 | RT-PCR |
| Mahajan, 2021 [66] | Prospective cohort | India; single centre |
Convenience sampling | Discharged adults (≥18 years) with COVID-19 | 134 | RT-PCR |
| Meije, 2021 [67] | Prospective cohort | Spain; single centre |
Convenience sampling | Discharged aged >15 years with COVID-19 | 302 | Confirmed cases: met clinical criteria (acute respiratory syndrome), radiological criteria and had a positive PCR; probable cases: met clinical criteria (acute respiratory syndrome), radiological criteria, but with negative or inconclusive PCR |
| Mendez, 2021 [68] | Prospective cohort | Spain; single centre |
Convenience sampling | Discharged adults (≥18 years) with COVID-19 | 171 | Laboratory confirmed |
| Moradian, 2020 [69] | Cross-sectional | Iran; single centre |
Convenience sampling | Hospitalised patients ≥18 years of age with moderate to severe COVID-19 recovered and then discharged 4 weeks earlier | 200 | RT-PCR |
| Morin, 2021 [70] | Prospective cohort | France; single centre |
Convenience sampling | Hospitalised adults (≥18 years) with COVID-19 | 478 | RT-PCR; CT lung scan associated with clinical features; or both |
| Munblit, 2021 [71] | Retrospective cohort | Russia; multicentre |
Convenience sampling | Hospitalised adults (≥18 years) with COVID-19 | 2649 | RT-PCR or clinically confirmed infection |
| Bellan, 2021 [72] | Prospective cohort | Italy; single centre |
Convenience sampling | Discharged adults (≥18 years) with COVID-19 | 238 | RT-PCR (97.5%), bronchial swab, serological testing or suggestive CT results |
| NutriCoviDom [73] | Prospective cohort | France; single centre |
Convenience sampling | Discharged adults (≥18 years) with COVID-19 | 288 | Positive SARS-CoV-2 RT-PCR test on NPS and/or on a typical chest CT scan |
| Ong, 2021 [74] | Prospective cohort | Singapore; multicentre |
Convenience sampling | Hospitalised with COVID-19 | 288 | SARS-CoV-2-specific PCR |
| Pellaud, 2020 [75] | Retrospective cohort | Switzerland; multicentre |
Convenience sampling | Hospitalised with COVID-19 | 196 | RT-PCR |
| PHOSP-COVID [76] | Prospective cohort | UK; multicentre |
Judgemental sampling# | Discharged adults (≥18 years) with COVID-19 | 1077 | Confirmed or clinician-diagnosed COVID-19 |
| PROLUN [77] | Prospective cohort | Norway; multicentre |
Judgemental sampling# | Patients aged >18 years; admitted for >8 h with discharge diagnosis of ICD-10 U07.1 (COVID-19, virus identified), U07.2 (COVID-19, virus unidentified) or J12.x (viral pneumonia) with COVID-19 | 103 | Diagnosis of U07.1 (COVID-19, virus identified), U07.2 (COVID-19, virus unidentified) or J12.x (viral pneumonia, in combination with positive SARS-CoV-2 identification in NPS) |
| Qu, 2021 [78] | Prospective cohort | China; multicentre |
Convenience sampling | Patients hospitalised with fever, respiratory rate >24 breaths·min−1 or cough; clinical type of COVID-19 at hospital admission mild to severe | 540 | Positive results from real-time PCR test for nucleic acid in respiratory or blood samples |
| Rass, 2021 [79] | Prospective cohort | Austria; multicentre |
Convenience sampling | Hospitalised adults (≥18 years) with COVID-19 | 135 | RT-PCR and typical clinical presentation |
| REACT [80] | Retrospective cohort | UK; single centre |
Convenience sampling | Patients aged 18–90 years with a confirmed COVID-19 diagnosis | 101 | PCR testing performed by combined nose and throat swabs |
| Romero-Duarte, 2021 [81] | Retrospective cohort | Spain; multicentre |
Convenience sampling | Hospitalised adults (≥18 years) with COVID-19 | 797 | Positive PCR test for SARS-CoV-2 |
| Sathyamurthy, 2021 [82] | Prospective cohort | India; single centre |
Convenience sampling | Age ≥65 years; hospitalised with acute COVID-19; discharged in a stable condition | 288 | RT-PCR |
| Shang, 2021 [83] | Prospective cohort | China; multicentre |
Convenience sampling | Hospitalised and discharged | 796 | RT-PCR |
| Shoucri, 2021 [84] | Retrospective cohort | USA; single centre |
Convenience sampling | Age ≥18 years; hospitalised | 929 | RT-PCR |
| Suárez-Robles, 2020 [85] | Retrospective cohort | Spain; single centre |
Convenience sampling | Hospitalised adults (≥18 years) with COVID-19 | 134 | RT-PCR |
| Taylor, 2021 [86] | Prospective cohort | UK; multicentre |
Convenience sampling | Presumed or confirmed COVID-19 pneumonia | 675 | NR |
| Weng, 2021 [87] | Retrospective cohort | China; multicentre |
Convenience sampling | Hospitalised patients admitted for respiratory symptoms | 117 | RT-PCR |
| Xiong, 2021 [88] | Prospective cohort | China; single centre |
Convenience sampling | Inpatients aged 20–80 years from Renmin Hospital of Wuhan University (Wuhan, China); diagnosed with COVID-19 and discharged according to WHO interim guidance | 538 | According to WHO interim guidance |
| Zhang, 2021 [89] | Retrospective cohort | China; multicentre |
Convenience sampling | Discharged adults (≥18 years) with COVID-19 | 2433 | Laboratory confirmed |
#: in studies described as employing judgemental sampling, participants were included in studies based on clinicians’ decision, rather than including all eligible patients at a study site. COVID-19: coronavirus disease 2019; ARDS: acute respiratory distress syndrome; RT: reverse transcription; ICD-10: International Classification of Diseases, 10th Revision; CT: computed topography; CPET: cardiopulmonary exercise testing; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; PHE: Public Health England; NPS: nasopharyngeal swab; HRCT: high-resolution computed topography; FIO2: inspiratory oxygen fraction; DVT: deep venous thrombosis; AKI: acute kidney injury; SpO2: peripheral oxygen saturation; NR: not reported; WHO: World Health Organization.
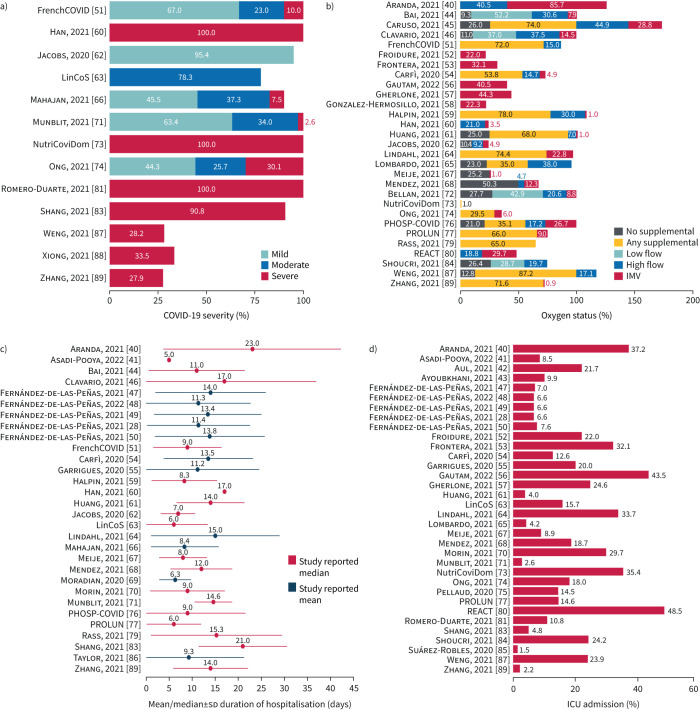
Coronavirus disease 2019 (COVID-19) severity and oxygen status were poorly reported but varied across study populations (figure 3). Of the 13 studies that reported disease severity, seven included only severe cases while four included a mix of mild, moderate and severe cases, based on individual study definitions of severity. Of the 29 studies reporting oxygen status, approximately half (n=14) included patients on supplemental oxygen, although all studies reported mechanical ventilation.
FIGURE 3.
Key study characteristics assessed in feasibility assessment: a) coronavirus disease 2019 (COVID-19) disease severity (reported by 13 out of 52 studies), b) oxygen status (reported by 29 out of 52 studies), c) duration of hospitalisation (reported by 30 out of 52 studies) and d) intensive care unit (ICU) admission (reported by 34 out of 52 studies). Disease severity was recorded either at admission or during the period of hospitalisation; oxygen status was recorded over the period of hospitalisation. Duration of hospitalisation was reported by studies as either mean or median; in the heterogeneity assessment these values were assumed to be equivalent as per standard practice and the standard deviation was calculated from the interquartile range for studies reporting the median. IMV: invasive mechanical ventilation.
The percentage of intensive care unit (ICU) admissions and the duration of hospitalisation were reported by 34 and 30 studies, respectively (figure 3). ICU admissions ranged from 1.5% to 48.5%, while average duration of hospitalisation (reported by studies as either median or mean) ranged from 5 to 23 days. Reported follow-up time-points ranged from 1 to 12 months.
PASC outcomes
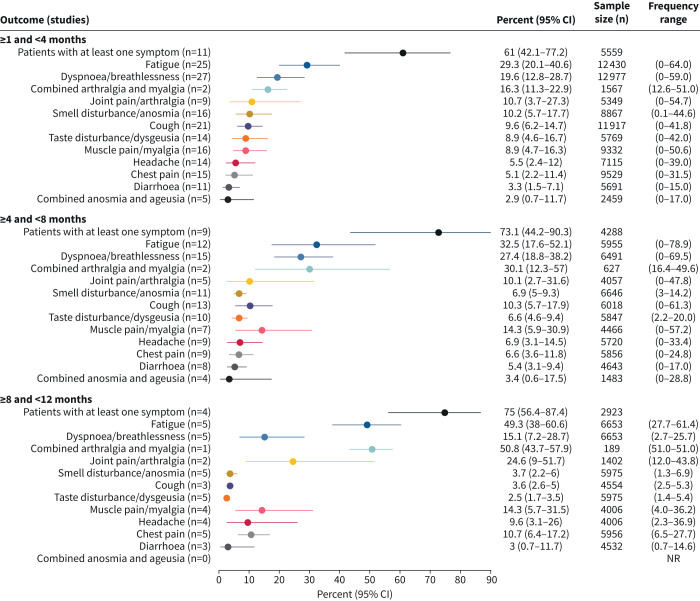
76 PASC symptoms were extracted from the analysed studies. Subsequently, 13 symptoms, including two composite symptoms (combined anosmia/ageusia and combined arthralgia/myalgia), were prioritised for the meta-analysis, selected based on the number of studies that reported on each symptom and based on their clinical relevance (figure 1b). The percentages of patients experiencing each of the 13 symptoms were meta-analysed at each of the three periods of interest and pooled estimates for each meta-analysis were generated (figure 4).
FIGURE 4.
Meta-analysis summary estimates for each of the 13 symptoms of interest at each of the three time periods of interest. Combined anosmia and ageusia and combined arthralgia and myalgia were both extracted as a single outcome for when studies reported both anosmia and ageusia or arthralgia and myalgia as a single outcome. NR: not reported.
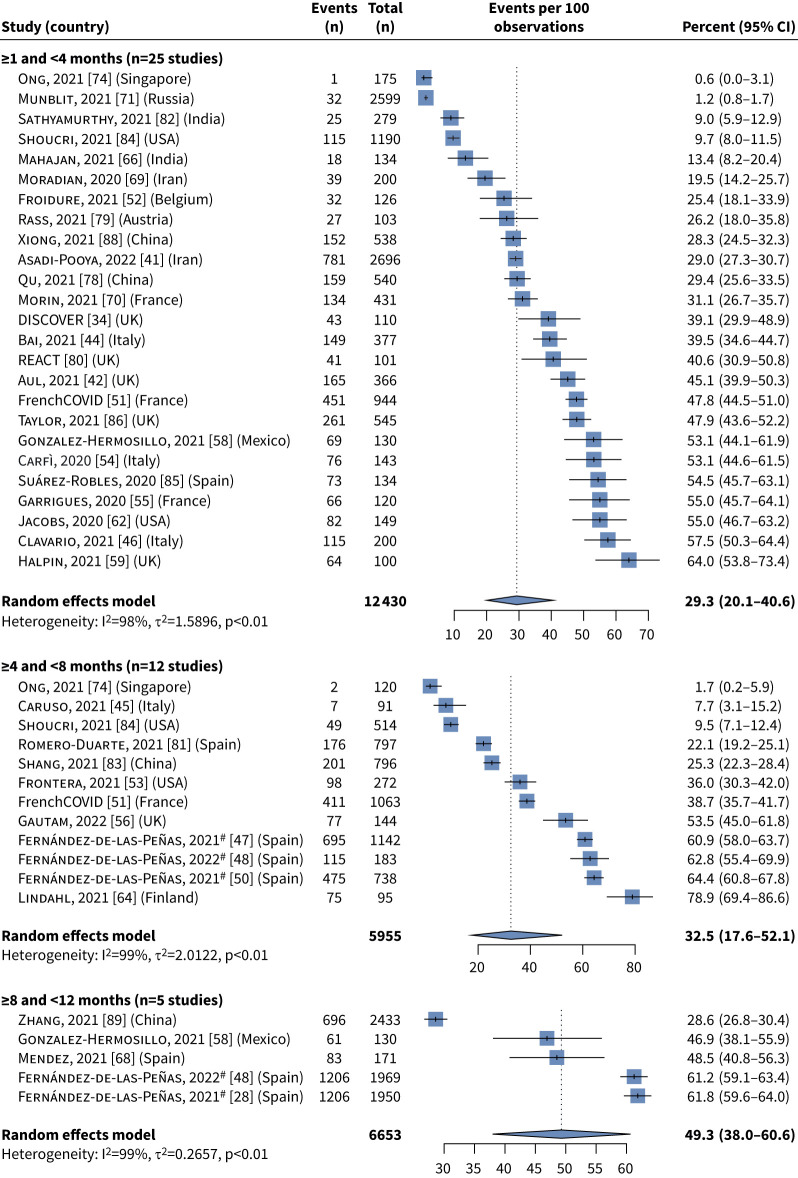
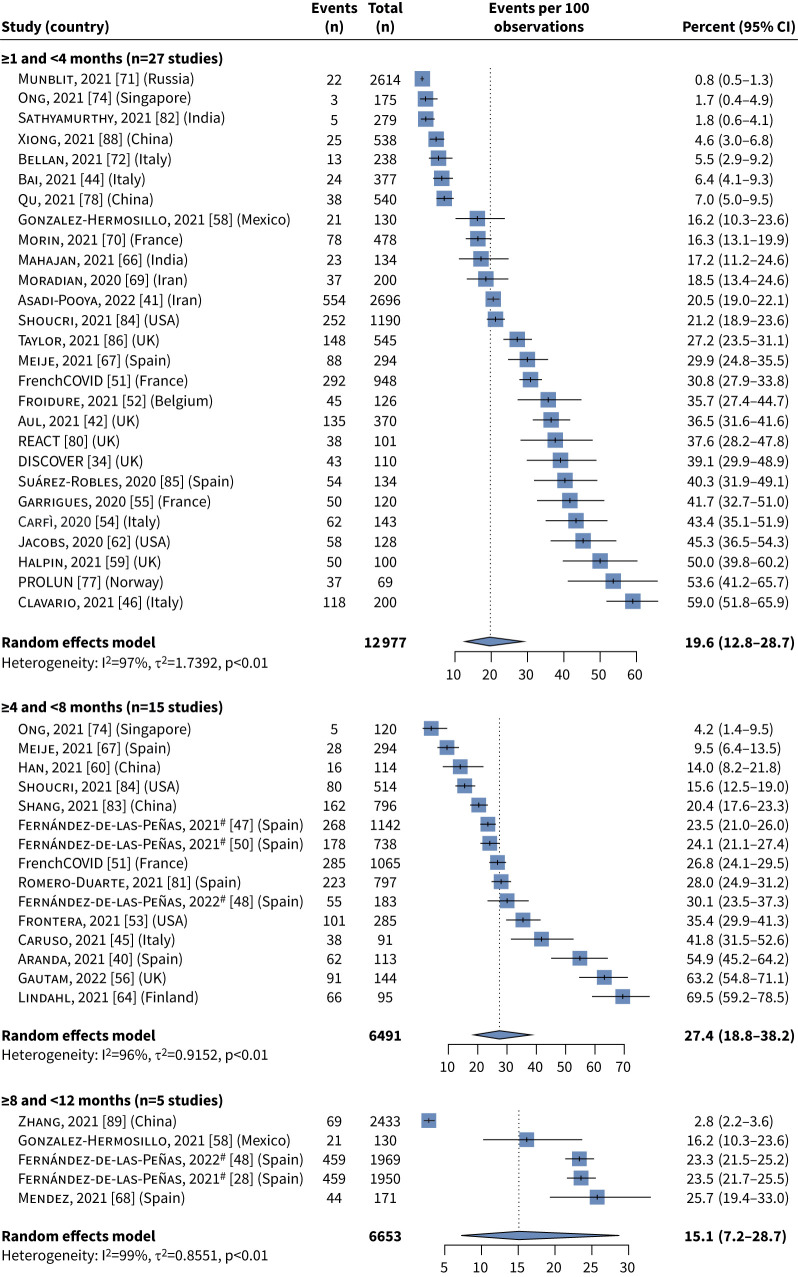
The majority of patients reported at least one symptom at all time-points: 61.0% (95% CI 42.1–77.2%) at ≥1 to <4 months, 73.1% (95% CI 44.2–90.3%) at ≥4 to <8 months and 75.0% (95% CI 56.4–87.4%) at ≥8 to <12 months. Symptom burden generally persisted at ≥4 to <8 and ≥8 to <12 months, and the frequency of some reported symptoms indicated a potential increase over time, although this was not formally tested as most studies only reported data at one time-point (supplementary table S1). The two most frequent individual symptoms were fatigue (≥1 to <4 months: 29.3% (95% CI 20.1–40.6%); ≥4 to <8 months: 32.5% (95% CI 17.6–52.1%); ≥8 to <12 months: 49.3% (95% CI 38.0–60.6%)) and dyspnoea/breathlessness (≥1 to <4 months: 19.6% (95% CI 12.8–28.7%); ≥4 to <8 months: 27.4% (95% CI 18.8–38.2%); ≥8 to <12 months: 15.1% (95% CI 7.2–28.7%)) (figures 5 and 6).
FIGURE 5.
Meta-analysis summary estimates for fatigue at each of the three time periods of interest. #: the Fernández-de-las-Peñas et al. [28, 47, 48, 50] studies were conducted by the same group of authors but included distinct patient groups. I2>50% and p<0.05 indicates substantial heterogeneity.
FIGURE 6.
Meta-analysis summary estimates for dyspnoea/breathlessness at each of the three time periods of interest. #: the Fernández-de-las-Peñas et al. [28, 47, 48, 50] studies were conducted by the same group of authors but included distinct patient groups. I2>50% and p<0.05 indicates substantial heterogeneity.
Heterogeneity and risk of bias
Substantial heterogeneity was found across studies, indicated by I2>50% for each symptom at each time-point. Despite this heterogeneity, no study was identified as an outlier; therefore, no studies warranted exclusion from the meta-analysis.
Studies were of moderate quality and all had at least one element in their design, conduct or analysis that contributed to an increased risk of bias. 32 studies did not have an adequate description of subjects and setting, 37 studies did not have an appropriate participant response rate or did not provide enough information to determine their response rate and 48 studies did not provide enough information to determine whether the sample size was appropriate for the target population. However, all included studies had an appropriate aim of characterising PASC and valid SARS-CoV-2 infection identification methods since these were prespecified inclusion criteria (supplementary figure S1).
Although asymmetry in some funnel plots suggested potential publication bias, all Egger's tests were p>0.05, indicating no significant publication bias for any symptom throughout all time-points (sample funnel plot in supplementary figure S2a), with the exception of diarrhoea at ≥4 to <8 months (p=0.0242) (supplementary figure S2b).
Discussion
In this SLR and meta-analysis, 52 studies of moderate quality reported symptoms of PASC. Most patients reported at least one symptom at all time-points up to 12 months of follow-up. Of 76 different PASC symptoms identified, the most common symptoms were fatigue, dyspnoea/breathlessness and combined arthralgia/myalgia. Other symptoms reported across different time-points included cough, chest pain, palpitations, diarrhoea, smell disturbance, taste disturbance and headache. These symptoms spanned many organ systems without apparent patterns. Where data were available, symptom burden was present at both ≥4 to <8 and ≥8 to <12 months, with 75% of patients reporting at least one symptom at the longest time-point.
The findings of this analysis build upon and are supported by other published literature. Across the wide volume of published SLRs on PASC, the reported symptom profile is varied, both in terms of the number and prevalence of different clinical symptoms identified. Nonetheless, similar symptoms across the literature are consistently cited as being the most common, including fatigue, dyspnoea, headache and chest pain. Some symptoms such as memory or concentration disorder, or “brain fog”, have since become recognised as a common symptom of PASC [1, 18–22]; however, during the early pandemic, the suspected symptoms of PASC were poorly understood and may not have been rigorously captured in these studies.
This work adds value to previously published findings as it considers a wide range of follow-up durations (from 1 to 12 months) and reports results separately at different time-points using appropriate grouping of time periods based on the spread of available data and clinician input. Most other studies had shorter follow-up times of up to 3 or 6 months at the longest and/or did not report results separately at different time-points [18–21, 23]. Our findings are similar to those of a study by Alkodaymi et al. [24], even though they assessed PASC symptoms in a broader study population (hospitalised and non-hospitalised patients) and at slightly different time-points (≥3 to <6, ≥6 to <9, ≥9 to <12 and ≥12 months). The prevalence of most common symptoms was similar during the second follow-up period (fatigue: 33% (95% CI 18–52%) in our study versus 36% (95% CI 27–46%) in Alkodaymi et al. [24]; dyspnoea: 27% (95% CI 19–38%) versus 25% (95% CI 20–30%), respectively) and third follow-up period (fatigue: 49% (95% CI 38–61%) versus 37% (95% CI 16–62%), respectively; dyspnoea: 15% (95% CI 7–29%) versus 21% (95% CI 14–28%), respectively); and the 95% confidence intervals overlap with our results [24]. A meta-analysis by Han et al. [25] focused solely on studies with at least a 1-year follow-up in both hospitalised and non-hospitalised patients. Similarly to our meta-analysis, frequently reported symptoms in Han et al. [25] were fatigue (28% (95% CI 18–39%)), dyspnoea (18% (95% CI 13–24%)) and arthralgia/myalgia (26% (95% CI 8–44%)); the 95% confidence intervals again overlap with our results [25]. Two recent studies, both published after our searches were conducted, report further evidence on the outcomes of previously hospitalised patients. A prospective, observational study found that the most common residual symptoms at 6-month follow-up were dyspnoea (35% of patients), cardiovascular symptoms (including fatigue; 10% of patients) and neurocognitive symptoms (13% of patients) [26]. In addition, an ambidirectional, longitudinal cohort study measured health outcomes of previously hospitalised individuals at three time-points of interest (6 months, 12 months and 2 years after symptom onset) [27]. Fatigue was the most prevalent symptom at all three time-points (6 months: 52%; 12 months: 20%; 2 years: 30%), while dyspnoea was present in 26% of patients at 6 months and 14% of patients after 2 years of follow-up [27]. While there is some variation in symptom prevalence between studies, our findings generally align with those of recently conducted studies, reaffirming the long-term relevance and importance of our analysis within the broader context of research on PASC.
A recently published SLR and meta-analysis reported that the global prevalence of PASC was higher in hospitalised than non-hospitalised patients (54% versus 34%), suggesting that a higher burden may be felt among the former [21]. A meta-analysis by Fernández-de-las-Peñas et al. [28] reported that the most common PASC symptoms among non-hospitalised patients were smell disturbance (∼20%), taste disturbance (∼18%) and dyspnoea (∼16%). Fatigue was not as commonly reported among non-hospitalised patients as observed among hospitalised patients. These and other studies demonstrate ways that select symptoms may differ in prevalence and pattern among hospitalised and non-hospitalised patients [21, 29, 30]. We further categorised studies according to the severity of COVID-19, but not enough data per severity status were available to stratify our results by severity. Future work should examine differences in PASC for patients with differing severity of SARS-CoV-2 infection, as well as for patients whose acute condition was managed in the community rather than in the hospital.
The findings of this and other work reflect that PASC is a complex, multisystem condition with overlapping clinical phenotypes.
Strengths and limitations
The SLR was designed and carried out in accordance with robust, systematic methodology and benefited from valuable clinician input from experts in infectious diseases. A large volume of moderate quality, peer-reviewed literature was identified for hospitalised patients with confirmed SARS-CoV-2 infection. Studies were identified from a diverse spread of geographies and limiting the meta-analysis to studies with 100 patients or higher increased the precision of the estimated prevalence of symptoms.
Similar to other meta-analyses of PASC symptoms [24, 31], several limitations were identified in this work and may limit the generalisability of the findings. All included studies collected data from the early phase of the pandemic and therefore are less representative of the current state of PASC. For example, the findings relate only to the ancestral strain of the SARS-CoV-2 virus rather than other variants and cannot account for the effects of vaccination. While no eligibility criteria were in place to specifically exclude studies with vaccinated patients, vaccines were not yet widely available at the time when the included studies were published, resulting in a lack of data on vaccinated populations. A recent SLR investigated the impact of vaccination on the risk of developing PASC and found a relatively small number of studies, with mixed results: some showed improvements following vaccination, while others showed no change or worsening [32]. Furthermore, the population was limited to hospitalised patients, whereas acute COVID-19 has largely become an outpatient disease [33]. In addition, in order to meet the urgent need for information on SARS-CoV-2, many early studies collected and published data very rapidly, which likely resulted in lower quality and contributed to high between-study heterogeneity, resulting in wide confidence intervals and high I2-values. Along with between-study heterogeneity, Alkodaymi et al. [24] and many others have noted poor reporting of illness severity as a limitation of the PASC evidence base. Indeed, our work found that many studies used inconsistent definitions for levels of severity and limited availability of data per severity status precluded the stratification of results by severity. The lack of stratification further contributed to the level of heterogeneity of patient characteristics within the analyses, complicating the interpretation and generalisation of the pooled results. However, in our SLR the inclusion of only patients who were hospitalised helps to standardise this factor; patients would have been more likely to have illness severe enough to warrant hospitalisation. Patients who recovered well from acute illness may be less likely to remain enrolled in long follow-up studies, resulting in selection bias that may result in overestimation of PASC symptom frequency within general populations. The choice of symptoms measured in different studies and lack of consistent definitions or pooling of different outcomes may also impact the estimates. Measurement bias may also exist in the findings since few studies reported on PASC symptoms from ≥8 to <12 months, limiting the strength of findings for this time interval. Additionally, although three time periods were evaluated, most studies only reported on one time-point and therefore the prevalence of symptoms could not be tracked across time periods for the same group of patients.
Questions for future research
Challenges in establishing a definition of PASC
There is currently no consensus on a definition of PASC [5]. Several challenges in defining PASC have also affected this study. First, the lack of well-defined, objective clinical criteria, such as laboratory tests, radiographic studies or other procedures, for patients who have PASC is a barrier to clinical diagnosis. Therefore, studies rely on subjective reports of symptoms, and the volume of reported symptoms, lack of symptom clustering and heterogeneity in patient population characteristics (including pre-existing conditions and treatment received for acute infection) contribute to the variable presentations of PASC. However, primary research and meta-analyses of PASC symptoms including studies with COVID-19-negative comparator groups have mostly found significant associations between SARS-CoV-2 infection and long-term symptoms [21, 24, 25, 34, 35].
The pathogenic mechanisms underlying PASC have been an area of active research [36] and there is a growing body of literature supporting evidence of viral persistence [37–39]. While it could not be assessed in the current analysis, a useful future approach for defining PASC could involve connecting pathogenic mechanisms to clinical phenotypes, defined by multiple symptoms and test results from a combination of imaging and other diagnostic modalities.
Conclusions
This work has contributed to understanding the natural history and prevalence of PASC for previously hospitalised patients with SARS-CoV-2 infection up to 12 months after hospital discharge. A significant proportion of infected individuals have persistent symptoms for a long period after acute infection. Even in an era of SARS-CoV-2 vaccination and viral evolution, PASC continues to be reported in a substantial proportion of individuals, but lack of symptom patterns and biomarkers have been barriers to defining this clinical entity. Nonetheless, PASC poses a significant clinical, psychosocial and economic burden on society, underscoring the need for deeper clinical phenotyping and more pathogenesis studies, both of which will inform the definition of PASC while developing prevention and treatment strategies. As this study included data only from previously hospitalised patients, future analyses should consider both hospitalised and non-hospitalised patients to ensure that the results are more broadly generalisable to all infected individuals.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0254-2022.SUPPLEMENT (341KB, pdf)
Acknowledgements
The authors acknowledge Jacques Morgan (Costello Medical, London, UK) and Rakshitha Kamath, Sarah Neubert, Zayd Normand and Aashna Shah (Costello Medical, Boston, MA, USA) for contributions to the SLR, and Gene Jerskey and Isabel Haber (Costello Medical, Boston, MA, USA) for medical writing and editorial assistance based on the authors’ input and direction.
Provenance: Submitted article, peer reviewed.
Author contributions: Substantial contributions to study conception and design: J.D. Kelly, T. Curteis, A. Rawal, M. Murton, L.J. Clark, Z. Jafry, R. Shah-Gupta, M. Berry, A. Espinueva, L. Chen, M. Abdelghany, D.A. Sweeney and J.K. Quint. Substantial contributions to analysis and interpretation of the data: J.D. Kelly, T. Curteis, A. Rawal, M. Murton, L.J. Clark, Z. Jafry, R. Shah-Gupta, M. Berry, A. Espinueva, L. Chen, M. Abdelghany, D.A. Sweeney and J.K. Quint. Drafting the article or revising it critically for important intellectual content: J.D. Kelly, T. Curteis, A. Rawal, M. Murton, L.J. Clark, Z. Jafry, R. Shah-Gupta, M. Berry, A. Espinueva, L. Chen, M. Abdelghany, D.A. Sweeney and J.K. Quint. Final approval of the version of the article to be published: J.D. Kelly, T. Curteis, A. Rawal, M. Murton, L.J. Clark, Z. Jafry, R. Shah-Gupta, M. Berry, A. Espinueva, L. Chen, M. Abdelghany, D.A. Sweeney and J.K. Quint.
Conflict of interest: J.D. Kelly received consulting fees from Gilead. T. Curteis, A. Rawal, M. Murton, L.J. Clark and Z. Jafry are employees of Costello Medical, which received payment from Gilead Sciences for analytical services for this study. R. Shah-Gupta is an employee of Gilead Sciences. M. Berry is an employee of, and owns stock in, Gilead Sciences. A. Espinueva was an employee of, and owned stock in, Gilead Sciences at the time of the study. L. Chen is an employee of, and owns stock in, Gilead Sciences and owns stocks in Pfizer. M. Abdelghany is an employee of, and owns stock in, Gilead Sciences. D.A. Sweeney has no conflicts of interest to report. J.K. Quint received consulting fees from Gilead, AstraZeneca and Evidera, and received research grants from HDR UK.
Support statement: This study was sponsored by Gilead Sciences. Support for third-party writing assistance for this article, provided by Gene Jerskey and Isabel Haber (Costello Medical, Boston, MA, USA), was funded by Gilead Sciences in accordance with Good Publication Practice (GPP3) guidelines (www.ismpp.org/gpp3). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Akbarialiabad H, Taghrir MH, Abdollahi A, et al. Long COVID, a comprehensive systematic scoping review. Infection 2021; 49: 1163–1186. doi: 10.1007/s15010-021-01666-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . Post-COVID conditions: information for healthcare providers. 2021. www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html Date last accessed: 9 November 2021.
- 3.National Institute for Health and Care Excellence . COVID-19 rapid guideline: managing the long-term effects of COVID-19. NICE guideline NG188. 2021. www.nice.org.uk/guidance/ng188 Date last accessed: 9 November 2021.
- 4.World Health Organization . WHO coronavirus (COVID-19) dashboard. 2020. https://covid19.who.int Date last accessed: 8 July 2022.
- 5.Raman B, Bluemke DA, Lüscher TF, et al. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J 2022; 43: 1157–1172. doi: 10.1093/eurheartj/ehac031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathieu E, Ritchie H, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). 2020. https://ourworldindata.org/coronavirus Date last accessed: 13 April 2023.
- 7.Wolff D, Nee S, Hickey NS, et al. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection 2021; 49: 15–28. doi: 10.1007/s15010-020-01509-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ludvigsson JF. Children are unlikely to be the main drivers of the COVID-19 pandemic – a systematic review. Acta Paediatr 2020; 109: 1525–1530. doi: 10.1111/apa.15371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagdasarian N, Cross G, Fisher D. Rapid publications risk the integrity of science in the era of COVID-19. BMC Med 2020; 18: 19219. doi: 10.1186/s12916-020-01650-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser N, Brierley L, Dey G, et al. The evolving role of preprints in the dissemination of COVID-19 research and their impact on the science communication landscape. PLoS Biol 2021; 19: e3000959. doi: 10.1371/journal.pbio.3000959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schonhaut L, Costa-Roldan I, Oppenheimer I, et al. Scientific publication speed and retractions of COVID-19 pandemic original articles. Rev Panam Salud Publica 2022; 46: e25. doi: 10.26633/RPSP.2022.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lefebvre C, Glanville J, Briscoe S, et al. Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions, version 6.3 (updated February 2022). London, Cochrane, 2022. https://training.cochrane.org/handbook [Google Scholar]
- 13.Centers for Disease Control and Prevention . Long COVID or post-COVID conditions. 2022. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html Date last accessed: 13 April 2023.
- 14.Centre for Reviews and Dissemination . Systematic Reviews: CRD's Guidance for Undertaking Reviews in Health Care. York, CRD, 2008. [Google Scholar]
- 15.R Core Team . R: A Language and Environment for Statistical Computing. Vienna, R Foundation for Statistical Computing. 2021. [Google Scholar]
- 16.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36: 1–48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 17.Bormann I. DigitizeIt: digitizer software – digitize a scanned graph or chart into (x,y)-data. Version 2.5.9. 2021. www.digitizeit.xyz Date last accessed: 13 April 2023. [Google Scholar]
- 18.Michelen M, Manoharan L, Elkheir N, et al. Characterising long COVID: a living systematic review. BMJ Glob Health 2021; 6: e005427. doi: 10.1136/bmjgh-2021-005427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021; 11: 16144. doi: 10.1038/s41598-021-95565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabrera Martimbianco AL, Pacheco RL, Bagattini M, et al. Frequency, signs and symptoms, and criteria adopted for long COVID-19: a systematic review. Int J Clin Prac 2021; 75: e14357. doi: 10.1111/ijcp.14357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post COVID-19 condition or long COVID: a meta-analysis and systematic review. J Infect Dis 2022; 226: 1593–1607. doi: 10.1093/infdis/jiac136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen NN, Hoang VT, Dao TL, et al. Clinical patterns of somatic symptoms in patients suffering from post-acute long COVID: a systematic review. Eur J Clin Microbiol Infect Dis 2022: 41: 515–545. doi: 10.1007/s10096-022-04417-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Kessel SA, Olde Hartman TC, Lucassen PL, et al. Post-acute and long-COVID-19 symptoms in patients with mild diseases: a systematic review. Fam Pract 2022; 39: 159–167. doi: 10.1093/fampra/cmab076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alkodaymi MS, Omrani OA, Fawzy NA, et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect 2022; 28: 657–666. doi: 10.1016/j.cmi.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han Q, Zheng B, Daines L, et al. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens 2022; 11: 269. doi: 10.3390/pathogens11020269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorusso R, De Piero ME, Mariani S, et al. In-hospital and 6-month outcomes in patients with COVID-19 supported with extracorporeal membrane oxygenation (EuroECMO-COVID): a multicentre, prospective observational study. Lancet Respir Med 2023; 11: 151–162. doi: 10.1016/S2213-2600(22)00403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med 2022; 10: 863–876. doi: 10.1016/S2213-2600(22)00126-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández-de-las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med 2021; 92: 55–70. doi: 10.1016/j.ejim.2021.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernández-de-las-Peñas C, Rodríguez-Jiménez J, Cancela-Cilleruelo I, et al. Post-COVID-19 symptoms 2 years after SARS-CoV-2 infection among hospitalized vs nonhospitalized patients. JAMA Netw Open 2022; 5: e2242106. doi: 10.1001/jamanetworkopen.2022.42106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pérez-González A, Araújo-Ameijeiras A, Fernández-Villar A, et al. Long COVID in hospitalized and non-hospitalized patients in a large cohort in Northwest Spain, a prospective cohort study. Sci Rep 2022; 12: 3369. doi: 10.1038/s41598-022-07414-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez-Ramirez DC, Normand K, Zhaoyun Y, et al. Long-term impact of COVID-19: a systematic review of the literature and meta-analysis. Biomedicines 2021; 9: 900. doi: 10.3390/biomedicines9080900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Notarte KI, Catahay JA, Velasco JV, et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. EClinicalMedicine 2022; 53: 101624. doi: 10.1016/j.eclinm.2022.101624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly JD, Leonard S, Hoggatt KJ, et al. Incidence of severe COVID-19 illness following vaccination and booster with BNT162b2, mRNA-1273, and Ad26.COV2.S vaccines. JAMA 2022; 328: 1427–1437. doi: 10.1001/jama.2022.17985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax 2021; 76: 399–401. doi: 10.1136/thoraxjnl-2020-216086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ballering AV, van Zon SK, Olde Hartman TC, et al. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet 2022; 400: 452–461. doi: 10.1016/S0140-6736(22)01214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peluso MJ, Deeks SG. Early clues regarding the pathogenesis of long-COVID. Trends Immunol 2022; 43: 268–270. doi: 10.1016/j.it.2022.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaebler C, Wang Z, Lorenzi JC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021; 591: 639–644. doi: 10.1038/s41586-021-03207-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peluso MJ, Deeks SG, Mustapic M, et al. SARS-CoV-2 and mitochondrial proteins in neural-derived exosomes of COVID-19. Ann Neurol 2022; 91: 772–781. doi: 10.1002/ana.26350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein S, Ramelli S, Grazioloi A, et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022; 612: 758–763. doi:1038/s41586-022-05542-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aranda J, Oriol I, Martin M, et al. Long-term impact of COVID-19 associated acute respiratory distress syndrome. J Infect 2021; 83: 581–588. doi: 10.1016/j.jinf.2021.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asadi-Pooya AA, Akbari A, Emami A, et al. Long COVID syndrome-associated brain fog. J Med Virol 2022; 94: 979–984. doi: 10.1002/jmv.27404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aul DR, Gates DJ, Draper DA, et al. Complications after discharge with COVID-19 infection and risk factors associated with development of post-COVID pulmonary fibrosis. Respir Med 2021; 188: 106602. doi: 10.1016/j.rmed.2021.106602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with COVID-19: retrospective cohort study. BMJ 2021; 372: n693. doi: 10.1136/bmj.n693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bai Y, Xu J, Chen L, et al. Inflammatory response in lungs and extrapulmonary sites detected by [18F] fluorodeoxyglucose PET/CT in convalescing COVID-19 patients tested negative for coronavirus. Eur J Nucl Med Mol Imaging 2021; 48: 2531–2542. doi: 10.1007/s00259-020-05083-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caruso D, Guido G, Zerunian M, et al. Post-acute sequelae of COVID-19 pneumonia: six-month chest CT follow-up. Radiology 2021; 301: E396–E405. doi: 10.1148/radiol.2021210834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clavario P, De Marzo V, Lotti R, et al. Cardiopulmonary exercise testing in COVID-19 patients at 3 months follow-up. Int J Cardiol 2021; 340: 113–118. doi: 10.1016/j.ijcard.2021.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernández-de-las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. Long-term post-COVID symptoms and associated risk factors in previously hospitalized patients: a multicenter study. J Infect 2021; 83: 237–279. doi: 10.1016/j.jinf.2021.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernández-de-las-Peñas C, Martín-Guerrero J, Navarro-Pardo E, et al. Gastrointestinal symptoms at the acute COVID-19 phase are risk factors for developing gastrointestinal post-COVID symptoms: a multicenter study. Intern Emerg Med 2022; 17: 583–586. doi: 10.1007/s11739-021-02850-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernández-de-las-Peñas C, Torres-Macho J, Velasco-Arribas M, et al. Similar prevalence of long-term post-COVID symptoms in patients with asthma: a case-control study. J Infect 2021; 83: 237–279. doi: 10.1016/j.jinf.2021.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernández-de-las-Peñas C, Rodriguez-Jiménez J, Fuensalida-Novo S, et al. Myalgia as a symptom at hospital admission by severe acute respiratory syndrome coronavirus 2 infection is associated with persistent musculoskeletal pain as long-term post-COVID sequelae: a case-control study. Pain 2021; 162: 2832–2840. doi: 10.1097/j.pain.0000000000002306 [DOI] [PubMed] [Google Scholar]
- 51.Eloy P, Tardivon C, Martin-Blondel G, et al. Severity of self-reported symptoms and psychological burden 6-months after hospital admission for COVID-19: a prospective cohort study. Int J Infect Dis 2021; 112: 247–253. doi: 10.1016/j.ijid.2021.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Froidure A, Mahsouli A, Liistro G, et al. Integrative respiratory follow-up of severe COVID-19 reveals common functional and lung imaging sequelae. Respir Med 2021; 181: 106383. doi: 10.1016/j.rmed.2021.106383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frontera JA, Yang D, Lewis A, et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci 2021; 426: 117486. doi: 10.1016/j.jns.2021.117486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carfì A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324: 603–605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect 2020; 81: e4–e6. doi: 10.1016/j.jinf.2020.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gautam N, Madathil S, Tahani N, et al. Medium-term outcome of severely to critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Clin Infect Dis 2022; 74: 301–308. doi: 10.1093/cid/ciab341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gherlone EF, Polizzi E, Tetè G, et al. Frequent and persistent salivary gland ectasia and oral disease after COVID-19. J Dent Res 2021; 100: 464–471. doi: 10.1177/0022034521997112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzalez-Hermosillo JA, Martinez-Lopez JP, Carrillo-Lampon SA, et al. Post-acute covid-19 symptoms, a potential link with myalgic encephalomyelitis/chronic fatigue syndrome: a 6-month survey in a Mexican cohort. Brain Sci 2021; 11: 760. doi: 10.3390/brainsci11060760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol 2021; 93: 1013–1022. doi: 10.1002/jmv.26368 [DOI] [PubMed] [Google Scholar]
- 60.Han X, Fan Y, Alwalid O, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology 2021; 299: E177–E186. doi: 10.1148/radiol.2021203153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 2021; 398: 747–758. doi: 10.1016/S0140-6736(21)01755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One 2020; 15: e0243882. doi: 10.1371/journal.pone.0243882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Divanoglou A, Samuelsson APK, Sjodahl PER, et al. Rehabilitation needs and mortality associated with the Covid-19 pandemic: a population-based study of all hospitalised and home-healthcare individuals in a Swedish healthcare region. EClinicalMedicine 2021; 36: 100920. doi: 10.1016/j.eclinm.2021.100920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lindahl A, Aro M, Reijula J, et al. Women report more symptoms and impaired quality of life: a survey of Finnish COVID-19 survivors. Infect Dis 2021; 54: 53–62. doi: 10.1080/23744235.2021.1965210 [DOI] [PubMed] [Google Scholar]
- 65.Lombardo MDM, Foppiani A, Peretti GM, et al. Long-term coronavirus disease 2019 complications in inpatients and outpatients: a one-year follow-up cohort study. Open Forum Infect Dis 2021; 8: ofab384. doi: 10.1093/ofid/ofab384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahajan S, Kunal S, Shah B, et al. Left ventricular global longitudinal strain in COVID-19 recovered patients. Echocardiography 2021; 38: 1722–1730. doi: 10.1111/echo.15199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meije Y, Duarte-Borges A, Sanz X, et al. Long-term outcomes of patients following hospitalization for coronavirus disease 2019: a prospective observational study. Clin Microbiol Infect 2021; 27: 1151–1157. doi: 10.1016/j.cmi.2021.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mendez R, Balanza-Martinez V, Luperdi SC, et al. Long-term neuropsychiatric outcomes in COVID-19 survivors: a 1-year longitudinal study. J Intern Med 2021; 29: 247–251. doi: 10.1111/joim.13389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moradian ST, Parandeh A, Khalili R, et al. Delayed symptoms in patients recovered from COVID-19. Iran J Public Health 2020; 49: 2120–2127. doi: 10.18502/ijph.v49i11.4729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morin L, Savale L, Pham T, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA 2021; 325: 1525–1534. doi: 10.1001/jama.2021.3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Munblit D, Bobkova P, Spiridonova E, et al. Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin Exp Allergy 2021; 51: 1107–1120. doi: 10.1111/cea.13997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open 2021; 4: e2036142. doi: 10.1001/jamanetworkopen.2020.36142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quilliot D, Gerard M, Bonsack O, et al. Impact of severe SARS-CoV-2 infection on nutritional status and subjective functional loss in a prospective cohort of COVID-19 survivors. BMJ Open 2021; 11: e048948. doi: 10.1136/bmjopen-2021-048948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ong SWX, Fong SW, Young BE, et al. Persistent symptoms and association with inflammatory cytokine signatures in recovered coronavirus disease 2019 patients. Open Forum Infect Dis 2021; 8: ofab156. doi: 10.1093/ofid/ofab156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pellaud C, Grandmaison G, Pham Huu Thien HP, et al. Characteristics, comorbidities, 30-day outcome and in-hospital mortality of patients hospitalised with COVID-19 in a Swiss area – a retrospective cohort study. Swiss Med Wkly 2020; 150: w20314. doi: 10.4414/smw.2020.20314 [DOI] [PubMed] [Google Scholar]
- 76.Evans RA, McAuley H, Harrison EM, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med 2021; 9: 1275–1287. doi: 10.1016/S2213-2600(21)00383-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lerum TV, Aalokken TM, Bronstad E, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J 2021; 57: 2003448. doi: 10.1183/13993003.03448-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qu G, Zhen Q, Wang W, et al. Health-related quality of life of COVID-19 patients after discharge: a multicenter follow-up study. J Clin Nurs 2021; 30: 1742–1750. doi: 10.1111/jocn.15733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rass V, Beer R, Schiefecker AJ, et al. Neurological outcome and quality of life 3 months after COVID-19: a prospective observational cohort study. Eur J Neurol 2021; 28: 3348–3359. doi: 10.1111/ene.14803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wallis TJM, Heiden E, Horno J, et al. Risk factors for persistent abnormality on chest radiographs at 12-weeks post hospitalisation with PCR confirmed COVID-19. Respir Res 2021; 22: 157. doi: 10.1186/s12931-021-01750-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Romero-Duarte A, Rivera-Izquierdo M, Guerrero-Fernández de Alba I, et al. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: the ANCOHVID multicentre 6-month follow-up study. BMC Med 2021; 19: 129. doi: 10.1186/s12916-021-02003-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sathyamurthy P, Madhavan S, Pandurangan V. Prevalence, pattern and functional outcome of post COVID-19 syndrome in older adults. Cureus 2021; 13: e17189. doi: 10.7759/cureus.17189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shang YF, Liu T, Yu JN, et al. Half-year follow-up of patients recovering from severe COVID-19: analysis of symptoms and their risk factors. J Intern Med 2021; 290: 444–450. doi: 10.1111/joim.13284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shoucri SM, Purpura L, DeLaurentis C, et al. Characterising the long-term clinical outcomes of 1190 hospitalised patients with COVID-19 in New York City: a retrospective case series. BMJ Open 2021; 11: e049488. doi: 10.1136/bmjopen-2021-049488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suárez-Robles M, Iguaran-Bermúdez M, García-Klepizg JL, et al. Ninety days post-hospitalization evaluation of residual COVID-19 symptoms through a phone call check list. Pan Afr Med J 2020; 37: 289. doi: 10.11604/pamj.2020.37.289.27110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Taylor RR, Trivedi B, Patel N, et al. Post-COVID symptoms reported at asynchronous virtual review and stratified follow-up after COVID-19 pneumonia. Clin Med 2021; 21: e384–e391. doi: 10.7861/clinmed.2021-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weng J, Li Y, Li J, et al. Gastrointestinal sequelae 90 days after discharge for COVID-19. Lancet Gastroenterol Hepatol 2021; 6: 344–346. doi: 10.1016/S2468-1253(21)00076-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect 2021; 27: 89–95. doi: 10.1016/j.cmi.2020.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang X, Wang F, Shen Y, et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open 2021; 4: e2127403. doi: 10.1001/jamanetworkopen.2021.27403 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0254-2022.SUPPLEMENT (341KB, pdf)