Abstract
Introduction
NBOMes and NBOHs are psychoactive drugs derived from phenethylamines and have hallucinogenic effects due to their strong agonism to serotonin 5-HT2A receptors. Although cases of toxicity associated with the recreational use of substituted phenethylamines are frequently reported, there is a lack of information on the possible neurotoxic effects of NBOMe and NBOH in the brain hippocampus, a major neurogenesis region.
Objectives
This study aimed at assessing the phenotypic and molecular effects of prolonged exposure of the hippocampus to the drugs 25H-NBOMe and 25H-NBOH.
Methods
The ex vivo organotypic culture model of hippocampal slices (OHC) was used to investigate, by immunofluorescence and confocal microscopy, and transcriptome analyses, the mechanisms associated with the neurotoxicity of 25H-NBOMe and 25H-NBOH.
Results
Reduction in the density of mature neurons in the OHCs occurred after two and seven days of exposure to 25H-NBOMe and 25H-NBOH, respectively. After the withdrawal of 25H-NBOMe, the density of mature neurons in the OHCs stabilized. In contrast, up to seven days after 25H-NBOH removal from the culture medium, progressive neuron loss was still observed in the OHCs. Interestingly, the exposure to 25H-NBOH induced progenitor cell differentiation, increasing the density of post-mitotic neurons in the OHCs. Corroborating these findings, the functional enrichment analysis of differentially expressed genes in the OHCs exposed to 25H-NBOH revealed the activation of WNT/Beta-catenin pathway components associated with neurogenesis. During and after the exposure to 25H-NBOMe or 25H-NBOH, gene expression patterns related to the activation of synaptic transmission and excitability of neurons were identified. Furthermore, activation of signaling pathways and biological processes related to addiction and oxidative stress and inhibition of the inflammatory response were observed after the period of drug exposure.
Conclusion
25H-NBOMe and 25H-NBOH disrupt the balance between neurogenesis and neuronal death in the hippocampus and, although chemically similar, have distinct neurotoxicity mechanisms.
Keywords: 25H-NBOMe, 25H-NBOH, Phenethylamines, Organotypic hippocampal culture, Neurotoxicity, Neurogenesis
Graphical abstract
Highlights
-
•
25H-NBOMe and 25H-NBOH have different neurotoxic effects on the hippocampus.
-
•
Hippocampal neurogenesis is activated by 25H-NBOH and inhibited by 25H-NBOMe.
-
•
Both drugs activate mechanisms of synaptic transmission and excitability of neurons.
-
•
Mechanisms of addiction and oxidative stress remain activated after drug withdrawal.
1. Introduction
Over the years, several new psychoactive substances (NPS) have been synthesized and became alternatives to the use of lysergic acid diethylamide (LSD) and other amphetamines. Classic psychedelics act as agonists of the serotonin 2A receptor (5-HT2AR) generating hallucinogenic effects that include euphoria, changes in perception, increased sensitivity of the senses and a greater feeling of empathy [1].
According to their structure, they were divided into two families, the indoleamines and the phenylalkylamines. In the latter, there is the group of phenylethylamines that includes substances such as mescaline and 2C. From substitutions in the molecular structure of phenylethylamines and the addition of a benzyl group, with 2C molecules as an intermediate, N-Benzylphenethylamines, such as 25X-NBOMe and 25X-NBOH, were synthesized. In this context, the “X” indicates the substitutions at the 4th carbon of the phenethylamine aromatic ring, usually Bromine (25B-NBOMe) or Iodine (25I-NBOMe) and the “OMe” (25X-NBOMe) or “OH” (25X-NBOH) indicates the substitutions at the benzyl group [2].
Substances of the NBOMe class have been used for pharmacological and neuroimaging studies of serotonin receptors. However, the illicit recreational use of these drugs imposes several challenges to public health. NBOMes/NBOHs can be sold as a powder, in solution, or in blotter paper for oral administration, the latter being the most common. Toxicity associated with the use of NBOMes and NBOHs has been reported and symptoms include panic, fear, confusion, hallucinations, tachycardia, hypertension, agitation, aggression, convulsions and hyperthermia, in addition to the occurrence of deaths, mainly associated with the use of 25I-NBOMe [1,3,4]. However, only a few studies have sought to understand the mechanisms of toxicity of these drugs. A previous work has shown that the 25H-NBOH and 25H-NBOMe are highly toxic to zebrafish embryos in vivo, causing malformation and lethal effects associated with the ability of these substances to bind to DNA [5]. A similar effect was reported to 25C-NBOMe in Artemia salina and zebrafish [6].
The hippocampus is a structure of the central nervous system (CNS) that makes up the limbic system and plays a crucial role in the development of spatial memories, contributing to the formation of a cognitive map of the environment and context-dependent memory [7,8]. Furthermore, the hippocampus is one of the few brain regions where neurogenesis occurs in postnatal life. This study aimed at assessing the effects of prolonged exposure to the drugs 25H-NBOMe and 25H-NBOH on the balance between neurogenesis and neuronal death in the CNS using the organotypic hippocampal culture (OHC) model, which preserves several aspects of tissue architecture, such as the diversity and their interactions [[9], [10], [11]].
2. Material and methods
2.1. Animals
Neonate Wistar rats (7 days old) were used for the production of organotypic hippocampal cultures (OHC). The animals were obtained from the Institute of Science and Technology in Biomodels of FIOCRUZ and transferred to the experimental bioterium at Instituto René Rachou with a lactating female the day before the experiment. Water and food were provided ad libitum, and the animals were maintained under light/dark cycles of 12–12 h and at controlled temperature and humidity (20–26 °C, 40–60%, respectively). This study was approved by the Ethics Committee on the Use of Animals of FIOCRUZ (license LW-23/17).
2.2. Organotypic hippocampal cultures
The OHCs were prepared according to the method described by Stoppini et al. [12] with modifications. After euthanasia by decapitation, the brains were removed and the hippocampi were dissected in culture medium (consisting of MEM Eagle + HEPES medium (Vitrocell Embriolife, Campinas, Brazil) plus 25% Hanks balanced solution 1× (Sigma, Basel, Switzerland)), cooled to 4 °C. Then, the hippocampi were sliced at 400 μm using a McIlwain tissue chopper (Mickle Laboratory Engineering Co Ltd, Gomshal, UK). Six slices from different animals were placed onto each PICM0RG50 organotypic culture insert (Merck Millicell, Darmstadt, Germany) in a six-well culture plate. During the first week, the OHCs were cultured in 1 mL of culture medium plus 25% heat-inactivated equine serum (Bio Nutrientes do Brasil Ltda., Barueri, Brazil). From the beginning of the second week, culture medium without equine serum was used. During the entire experiment, the OHCs were incubated in a humidified atmosphere containing 5% CO2 at 37 °C, changing half the volume of medium every 2 or 3 days. After the stabilization phase (14 days), the OHCs were further incubated with culture medium containing 0.5 μM 25H-NBOMe or 25H-NBOH or culture medium without these drugs (CTRL) for 2, 4 or 7 days (endpoints 2, 4 and 7). 25H-NBOMe and 25H-NBOH were synthesized and characterized in a previous work [13]. For endpoints 9 and 14, after 7 days of culture, the medium was replaced with a new drug-free medium and the OHCs were incubated for another 2 or 7 days.
2.3. Immunofluorescence
The OHCs were immunostained following the method described by Gogolla et al. [14] with adaptations. At the end of each experiment, the OHCs were processed for immunostaining incubating them as follows: ice-cold 4% paraformaldehyde (PFA) (pH 7.2) for 5 min, ice-cold 20% methanol in phosphate-buffered saline (PBS) 1× for 5 min, 0.05% Tween in PBS 1× overnight, and bovine serum albumin (BSA) 20% in PBS 1× overnight. Washes with 1× PBS were performed between incubations. The OHCs were removed from the inserts and incubated overnight with Cy3 conjugate anti-NeuN antibody (1:100, ABN78C3, Merck), to determine the density of mature neurons or anti-NeuroD1 (1:500, AB60704, Abcam) for post-mitotic immature neurons. To the latter, additional incubation (4 h) with a secondary antibody, Alexa Fluor 488 fluorophore conjugate anti-mouse IgG (1:20,000, A10684, ThermoFisher), was performed. Finally, the OHCs were labeled with 4′,6′-diamino-2-phenylindole (DAPI) (1 μg/mL, ThermoFisher) and slides were mounted with ProLong Diamond Antifade Mountant (ThermoFisher).
2.4. Confocal microscopy
Images of the OHCs were captured with a Nikon Eclipse Ti confocal microscope (Nikon, Tokyo, Japan) using a 488/561 fluorescence filter. Stacks of z-series images (∼12 optical images with 2.79 μm thickness each) were obtained at 10× magnification, looking for representative areas with greater cellular tissue coverage. The threshold parameters of each laser were adjusted using a negative control stained with DAPI or DAPI and a secondary antibody, to remove background noise and tissue autofluorescence. For representative photos of details of the OHCs, magnifications of 60× or 100× were used.
The images were analyzed using the NIS-Elements Analysis software (Nikon). The channels corresponding to the NeuN+, NeuroD1+ and DAPI+ markings were treated with the Noise Reduction, Gauss-Laplace Sharpen and Local Contrast tools. The total tissue area was determined by creating an automatic binary mask from the channel with DAPI+ fluorescence, and delimiting the tissue-filled spaces with the Region of Interest Manager (ROI) tool. For counting mature neurons, a binary mask was created based on the DAPI+ field, where only Cy3 channel signal (corresponding to NeuN+ labeling) with fluorescence threshold >10 A U were selected, and individual cell nuclei were counted. The mature neuron density of each OHC was calculated dividing the NeuN+ cell count by the tissue area (mm2). For immature postmitotic neurons counting, a binary layer was created from the NeuroD1+ and DAPI+ labeling, selecting only elements with a fluorescence threshold >10 A U. The value of the selected area was normalized by the tissue area (NeuroD1+ and DAPI+/mm2).
2.5. RNA-seq
Total RNA from three pools of three OHCs from each group (CTRL, 25H-NBOMe and 25H-NBOH) at endpoints 4 and 9 (total 18 samples) was extracted using the miRNeasy Mini kit (217004, Qiagen, Hilden, Germany), according to the manufacturer’s instructions. All samples had their RNA quantified by fluorometry using the Qubit RNA HS Assay kit (Q32852, Invitrogen, Carlsbad, CA) and the Qubit 2.0 Fluorometer (Invitrogen) and evaluated for purity with the NanoDrop (ThermoFisher).
The cDNA libraries were produced using the TruSeq Stranded mRNA kit (Illumina, San Diego, CA) and the indexed fragments were sequenced with a NextSeq 500 (Illumina, San Diego, CA) and a TG NextSeq 500/550 High Output v2 Kit (Illumina, San Diego, CA).
2.6. Bioinformatics analysis
The raw reads were processed with the Trimmomatic software [15], removing adapters, low-quality bases or very short reads (less than 36nt). The filtered reads were aligned to the Rattus norvegicus reference genome (release 94) using the STAR (Spliced Transcripts Alignment to a Reference) software [16] and only reads mapped to a single genome site were used to calculate the number of reads and the value of reads per kilobase of transcript per million reads mapped (RPKM) [17] for each gene. Contrast analysis between groups CTRL vs 25H-NBOMe at endpoint 4 (contrast 1), CTRL vs 25H-NBOH at endpoint 4 (contrast 2), CTRL vs 25H-NBOMe at endpoint 9 (contrast 3), and CTRL vs 25H- NBOH at point 9 (contrast 4) were performed with the DESeq2 package [18]. Genes with Log2 Fold Change equal to or greater than 0.58 (positive or negative) and P-value less than 0.05 in the False Discovery Rate Analysis (FDR) were considered differentially expressed (DEG). Functional enrichment analysis of DEGs was performed with Metacore software (Clarivate Analytics, London, UK) using standard parameters.
2.7. Statistical analysis
Statistical analyzes were performed with GraphPad Prism software (version 8.0.2) (GraphPad Software Inc., Irvine, CA). Data were evaluated for normality using the Anderson-Darling, D’Agostino & Pearson, Shapiro-Wilk and Kolmogorov-Smirnov tests. For comparisons that involved analyzing the effect of two factors (e.g., drug and exposure time), two-way analysis of variance (ANOVA) with Tukey’s multiple comparison post-test was used. Differences with P value less than 0.05 were considered statistically significant.
3. Results
3.1. Drug-induced phenotypic changes
To evaluate the effects of 25H-NBOMe and 25H-NBOH on neuron density in the hippocampus, phenotypic time series analysis was performed using immunofluorescence and confocal microscopy. A significant decrease in the density of mature neurons, expressing the NeuN protein, was observed in the OHCs from the second day of culture with 25H-NBOMe and from the seventh day of culture with 25H-NBOH in relation to the control group. Even seven days after removing the drugs from the culture medium, the neuronal density of the OHCs remained lower than that of the control group. However, the neurotoxic effect of 25H-NBOH persisted even after drug removal from the OHC culture medium (Fig. 1A). 25H-NBOH induced an increase in the differentiation of progenitor cells in postmitotic neurons, expressing NeuroD1, in OHCs. Although this induction occurred only in the presence of 25H-NBOH, the increased density of postmitotic neurons in OHCs was maintained even seven days after drug removal from the culture medium (Fig. 1B). In contrast, 25H-NBOMe did not affect the density of postmitotic neurons expressing NeuroD1. These results show that, although chemically similar, the two drugs induce different neurodegenerative profiles. It is important to highlight that the OHCs did not present significant variations in their total area throughout the time series, indicating that the changes observed in the density of mature and post-mitotic neurons were not due to tissue distention.
Fig. 1.
25H-NBOMe and 25H-NBOH induce distinct phenotypes in organotypic hippocampal cultures. Effect of 25H-NBOMe or 25H-NBOH on the density of mature neurons (A) and progenitor cells (B) in organotypic hippocampal cultures. Group means were compared using the two-way ANOVA test followed by Tukey’s multiple comparison test. Data are represented as means and standard deviations. N: CTRL 2D and 7D = 4; CTRL 14D = 5; 25H-NBOMe 2D, 7D and 14D = 4; 25H-NBOH 2D and 7D = 4; 25H-NBOH 14D = 7. *P < 0,05; **P < 0,01, ***P < 0,001 and ****P < 0,0001. (C) Representative micrographs (10x) at endpoint 14D. Scale bar = 100 μm. Abbreviations: 2D = endpoint 2 (two days with the drug in the culture medium); 7D = endpoint 7 (seven days with the drug in the culture medium); 14D = endpoint 14 (seven days with the drug in the culture medium and additional seven days without the drug).
3.2. Transcriptional profile
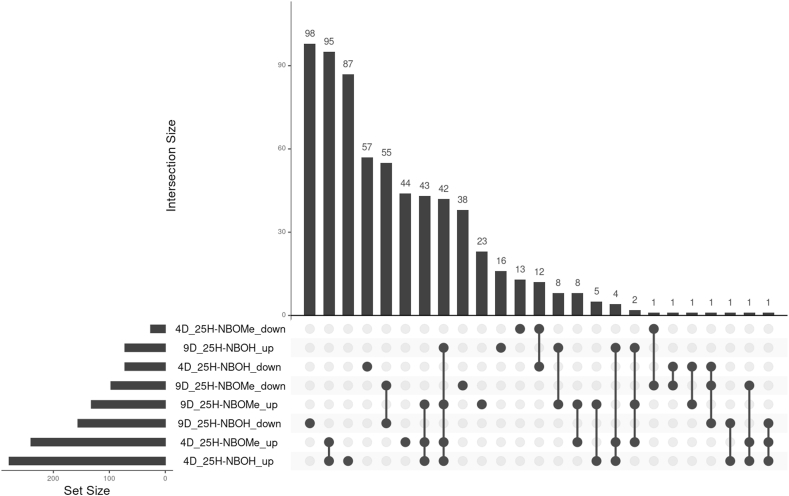
To elucidate the molecular mechanisms underlying the phenotypic changes induced by 25H-NBOMe and 25H-NBOH, the effects of these two drugs on global gene expression in OHC cells were evaluated by RNA-Seq. To identify differentially expressed genes (DEG), the following contrasts were analyzed: 1) CTRL vs 25H-NBOMe at endpoint 4; 2) CTRL vs 25H-NBOH at endpoint 4; 3) CTRL vs 25H-NBOMe at endpoint 9; and 4) CTRL vs 25H-NBOH at endpoint 9. In OHCs cultured with 25H-NBOMe, 266 DEGs (240 upregulated and 26 downregulated) were found in contrast 1 and 229 DEGs (132 upregulated and 97 downregulated) in contrast 3. In OHCs cultured with 25H-NBOH, 351 DEGs (279 upregulated and 72 downregulated) were found in contrast 2 and 228 DEGs (72 upregulated and 156 downregulated) in contrast 4 (Fig. 2).
Fig. 2.
Differentially expressed genes in organotypic hippocampal cultures treated with 25H-NBOMe or 25H-NBOH compared to controls with no drug. Abbreviations: 4D = endpoint 4 (four days with the drug in the culture medium); 9D = endpoint 9 (seven days with the drug in the culture medium and additional two days without the drug); up = upregulated; down = downregulated.
The functional enrichment analysis of the DEGs identified in the contrasts revealed the activation of mechanisms related to synaptic transmission and excitability of neurons in OHCs cultured with 25H-NBOMe and 25H-NBOH at endpoints 4 and 9 (Fig. 3 and Supplementary Materials 1 to 6). However, these mechanisms appear less active in OHCs cultured with 25H-NBOH when compared to 25H-NBOMe at endpoint 9 (Fig. 3 and Supplementary Material 6). At endpoint 4, both drugs regulated progenitor cell differentiation by either inhibiting (25H-NBOMe) or activating (25H-NBOH) gene expression in the WNT/Beta-catenin pathway (Fig. 3 and Supplementary Materials 1 to 3). At endpoint 9, both drugs activated the expression of genes related to addiction, oxidative stress and neurogenesis, and inhibited genes related to the inflammatory response (Fig. 3 and Supplementary Materials 5 and 6).
Fig. 3.
25H-NBOMe and 25H-NBOH effects in transcriptional signatures of organotypic hippocampal cultures. Effect of 25H-NBOMe or 25H-NBOH on transcriptional signature of organotypic hippocampal cultures after 4 (A) or 9 days (B). Dashed line indicates the threshold P value for statistical significance (0.01). Only the top 10 differentially affected pathways or networks in each contrast were used for comparisons. Abbreviations; 4D = endpoint 4 (four days with the drug in the culture medium); 9D = endpoint 9 (seven days with the drug in the culture medium and additional two days without the drug); up = upregulated; down = downregulated.
4. Discussion
The substituted phenethylamines of the NBOMe and NBOH classes quickly spread as recreational drugs because they have a much lower production cost than traditional hallucinogenic drugs. As with other psychedelics, the effects of substituted phenethylamines can vary according to an individual’s mental health and social environment. However, the use of these drugs offers much greater risks, such as tachycardia, high blood pressure, seizures, aggressiveness, metabolic acidosis, anxiety, and, in cases of overdose, it can lead to death [19]. This is the first work to investigate the effects of prolonged exposure of the hippocampus, the main CNS structure responsible for memory consolidation and post-embryonic neurogenesis, to substituted phenethylamines.
The main mechanism mediated by genes overexpressed in OHCs on the fourth day of culture with 25H-NBOMe or 25H-NBOH was synaptic signaling (Fig. 3 and Supplementary Materials 1 to 3). Processes such as the excitability of neurons and increased synaptic levels of NMDA receptors were activated in OHCs exposed to drugs. These findings corroborate previous reports on the impact of others NBOMe derivatives in synaptic transmission. For instance, it has already been shown that 25C-NBOMe to increase the excitability of pyramidal neurons in orbitofrontal cortex of adult rats [20]. Other studies reported that 25B-NBOMe and 25I-NBOMe increase the release of excitatory neurotransmitters in rat cortex, striatum, and nucleus accumbens [21,22]. In the present study, the synaptic transmission induction cascade appears to be mediated by inhibition of the transcription factor GATA-1, which was predicted to be an upstream regulator in contrasts 1 and 2 (Supplementary Material 3). The downregulation of this transcription factor favors the activation of several genes necessary for the formation of synaptic connections [23].
According to a study by Poklis et al. [24], substituted phenethylamines are 5-HT2A serotonin receptor agonists. These receptors mediate physiological processes such as neuronal excitation (probably responsible for psychedelic effects), learning, memory, anxiety, vasoconstriction/vasodilation, and platelet aggregation [25,26]. Activation of the 5-HT2A receptor with LSD-like agonists also produces potent anti-inflammatory effects through the inhibition of TNF-alpha [27,28]. In the present study, on the fourth day of culture with 25H-NBOMe or 25H-NBOH, several genes related to the immune response were downregulated, indicating that substituted phenethylamines may also have an anti-inflammatory effect similar to LSD. It has already been reported that 25I–NBOMe exposure reduces the number of microglia and astrocytes in the rat cortex [29]. Notably, NBOH derivates are the most selective, potent, and highly efficacious 5-HT2A receptor agonists developed to date [[30], [31], [32]]. This could explain why the anti-inflammatory effect was stronger in OHCs treated with 25H-NBOH than 25H-NBOMe at the nineth day (Fig. 3 and Supplementary Materials 4–6).
On the fourth day of culture, OHCs subjected to 25H-NBOMe showed a gene expression profile unfavorable to the differentiation of progenitor cells through the inhibition of elements of the WNT/Beta-catenin pathway such as TCF7, TCF4 (predicted) and SOX18 (Supplementary Material 1) [33,34]. The results also indicated the activation of the enzyme EZH2 (Supplementary Material 1), responsible for the addition of methyl groups to the lysine 27 of histone H3 [35]. The activation of this enzyme would regulate the neurogenesis reduction cascade, inhibiting gene expression through the formation of heterochromatin [36]. Accordingly, the derivate 25I-NBOMe has also been shown to decrease the density of post-mitotic cells in the rat hippocampus, indicating impaired neurogenesis in this brain region [37]. Regarding the OHCs treated with 25H–NBOH, after four days of culture, an inverse mechanism was observed, with the activation of elements of the WNT/Beta-catenin pathway and the activation of the NeuroD1 (predicted) and NeuroD6 genes, favoring neurogenesis (Material Supplementary 2). In this regard, Tsybko and cols [38], demonstrated that 25CN-NBOH increases the mRNA levels of Bdnf, that positively regulates the proliferation and differentiation of neural cells, in the hippocampus. Together, these gene expression profiles corroborate the density phenotypes of mature neurons (NeuN+) and post-mitotic neurons (Neurod1+) of OHCs treated with substituted phenethylamines (Fig. 1).
Even after removing the drugs from the culture medium, the mechanisms of synaptic transmission and excitability of OHC neurons remained activated, as observed on the ninth day of culture (Fig. 3 and Supplementary Materials 4 to 6). However, these mechanisms appear to be much more active in OHCs exposed to 25H-NBOMe (Fig. 3 and Supplementary Material 6). This may be, in part, because 25H-NBOH induces the formation of new neurons that, seemingly, do not advance in the process of cell differentiation, maintaining the phenotype of immature neurons (Neurod1+) without increasing the density of mature neurons expressing the NeuN protein (Fig. 1).
Four mechanisms stood out when the differentially expressed genes were analyzed after the removal of 25H-NBOMe and 25H-NBOH from the culture media: 1) Increased expression of genes from the LRRK2 pathway in neurons in Parkinson’s disease (Fig. 3 and Supplementary Material 6) - the Lrrk2 gene controls neuronal processes such as reuptake of synaptic proteins, plasticity and development of dendritic spines, in addition to being an important molecular substrate responsible for the consolidation of compulsive ethanol consumption [39]; 2) Increased expression of genes related to neurogenesis (Supplementary Material 6) - these findings corroborate the results of immunofluorescence analyzes for NeurodD1 of OHCs exposed to 25H-NBOH (Fig. 1B); 3) Increased expression of genes related to oxidative stress (Fig. 3 and Supplementary Material 6); 4) Decreased expression of genes related to the inflammatory response, being more expressive in OHCs exposed to 25H-NBOH (Fig. 3 and Supplementary Material 6). FOXP3, an important transcription factor in the development and function of regulatory T cells [40], was predicted as an upstream regulator of the decrease in the immune response in OHCs exposed to 25H-NBOMe (Fig. 3 and Supplementary Material 4).
An inherent constraint of the OHC model lies in its inadequacy to assess the systemic effects of the drugs on the entire organism, which includes the interaction with the blood-brain barrier. It is also noteworthy that there may be variations in individual response to the drugs due to differences in mental health and social environment. Additionally, any extrapolation of the study’s results to the human hippocampus must be approached with caution. Further investigations into the impact of these drugs on other brain regions are warranted and may shed additional light on the neurotoxicity of 25H-NBOMe and 25H-NBOH. Another limitation of this study was the use of a single dose of drugs (0.5 μM). While previous studies testing NBOMs in vitro employed much higher concentrations (25–400 μM) [41,42], the dose tested in the present study is high compared to expected levels in humans. NBOMe-linked toxicities have been reported in patients with serum drug concentrations as low as 0.180 ng/mL (∼0.0005 μM) [43].
5. Conclusion
Although structurally similar, the substituted phenethylamines 25H-NBOMe and 25H-NBOH showed different toxicity mechanisms. Phenotypic and molecular analyzes revealed a milder profile of the effects of 25H-NBOH, and it was also able to induce neurogenesis, although without complete differentiation of new neurons that maintained the immature phenotype (Neurod1+). In turn, 25H-NBOMe induced neurodegeneration earlier than 25H-NBOH and activated genes related to epigenetic mechanisms that inhibit neurogenesis. Both drugs stimulated mechanisms of synaptic transmission and excitability of neurons, which remained activated even after the exposure period. Inflammatory response genes had their expression reduced during and after the drug exposure period, suggesting their anti-inflammatory effect. Interestingly, after the period of exposure of OHCs to 25H-NBOMe or 5H-NBOH, genes related to addiction had their expression increased.
Funding sources
This study recieved financial support from FIOCRUZ, Instituto Nacional de Ciência e Tecnologia em Vacinas (INCT-Vacinas), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) [Finance Code 001 - Process #88881.516313/2020-01], Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) [Grant # RED-00042-16] and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [Grant # 429121/2018-0]. ADF is recipient of a research fellowship from CNPq [Grant # 313114/2021-8].
Author contribution statement
Larissa Marcely Gomes Cassiano: Marina da Silva Oliveira: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Wellington Alves de Barros: Performed the experiments.
Ângelo de Fátima: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Roney Santos Coimbra: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
Technical support was provided by the FIOCRUZ Network of Technological Platforms: NGS Sequencing (P01-007), and Bioinformatics (P08-002).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17720.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zawilska J.B., Kacela M., Adamowicz P. NBOMes-highly potent and toxic alternatives of LSD. Front. Neurosci. 2020;14:78. doi: 10.3389/fnins.2020.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poulie C.B.M., Jensen A.A., Halberstadt A.L., Kristensen J.L. DARK classics in chemical neuroscience: NBOMes. ACS Chem. Neurosci. 2019 doi: 10.1021/acschemneuro.9b00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood D.M., Sedefov R., Cunningham A., Dargan P.I. Prevalence of use and acute toxicity associated with the use of NBOMe drugs. Clin. Toxicol. (Phila) 2015;53:85–92. doi: 10.3109/15563650.2015.1004179. [DOI] [PubMed] [Google Scholar]
- 4.Stellpflug S.J., Kealey S.E., Hegarty C.B., Janis G.C. 2-(4-Iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOMe): clinical case with unique confirmatory testing. J. Med. Toxicol. 2014;10:45–50. doi: 10.1007/s13181-013-0314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Barros W.A., Nunes C. da S., da Costa Ribeiro Souza J.A., Nascimento I.J.D.S., Figueiredo I.M., de Aquino T.M., et al. The new psychoactive substances 25H-NBOMe and 25H-NBOH induce abnormal development in the zebrafish embryo and interact in the DNA major groove. Curr. Res. Toxicol. 2021;2:386–398. doi: 10.1016/j.crtox.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Álvarez-Alarcón N., Osorio-Méndez J.J., Ayala-Fajardo A., Garzón-Méndez W.F., Garavito-Aguilar Z.V. Zebrafish and Artemia salina in vivo evaluation of the recreational 25C-NBOMe drug demonstrates its high toxicity. Toxicol. Rep. 2021;8:315–323. doi: 10.1016/j.toxrep.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knierim J.J. The hippocampus. Curr. Biol. 2015;25:R1116–R1121. doi: 10.1016/j.cub.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 8.Aly M., Ranganath C. New perspectives on the hippocampus and memory. Neurosci. Lett. 2018;680:1–3. doi: 10.1016/j.neulet.2018.05.047. [DOI] [PubMed] [Google Scholar]
- 9.Humpel C. Organotypic brain slice cultures: a review. Neuroscience. 2015;305:86–98. doi: 10.1016/j.neuroscience.2015.07.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavaliere F., Benito-Muñoz M., Matute C. Organotypic cultures as a model to study adult neurogenesis in CNS disorders. Stem Cell. Int. 2016;2016 doi: 10.1155/2016/3540568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassiano L.M.G., Oliveira M.S., Pioline J., Salim A.C.M., Coimbra R.S. Neuroinflammation regulates the balance between hippocampal neuron death and neurogenesis in an ex vivo model of thiamine deficiency. J. Neuroinflammation. 2022;19 doi: 10.1186/s12974-022-02624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoppini L., Buchs P.A., Muller D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 13.de Barros W.A., Queiroz M.P., da Silva Neto L., Borges G.M., Martins F.T., de Fátima Â. Synthesis of 25X-BOMes and 25X-NBOHs (X = H, I, Br) for pharmacological studies and as reference standards for forensic purposes. Tetrahedron Lett. 2021;66 doi: 10.1016/j.tetlet.2020.152804. [DOI] [Google Scholar]
- 14.Gogolla N., Galimberti I., DePaola V., Caroni P. Staining protocol for organotypic hippocampal slice cultures. Nat. Protoc. 2006;1:2452–2456. doi: 10.1038/nprot.2006.180. [DOI] [PubMed] [Google Scholar]
- 15.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mortazavi A., Williams B.A., McCue K., Schaeffer L., Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 18.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill S.L., Doris T., Gurung S., Katebe S., Lomas A., Dunn M., et al. Severe clinical toxicity associated with analytically confirmed recreational use of 25I-NBOMe: case series. Clin. Toxicol. (Phila) 2013;51:487–492. doi: 10.3109/15563650.2013.802795. [DOI] [PubMed] [Google Scholar]
- 20.Tang Z.-H., Yu Z.-P., Li Q., Zhang X.-Q., Muhetaer K., Wang Z.-C., et al. The effects of serotonergic psychedelics in synaptic and intrinsic properties of neurons in layer II/III of the orbitofrontal cortex. Psychopharmacology (Berl.) 2023 doi: 10.1007/s00213-023-06366-y. [DOI] [PubMed] [Google Scholar]
- 21.Wojtas A., Herian M., Skawski M., Sobocińska M., González-Marín A., Noworyta-Sokołowska K., et al. Neurochemical and behavioral effects of a new hallucinogenic compound 25B-NBOMe in rats. Neurotox. Res. 2021;39:305–326. doi: 10.1007/s12640-020-00297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herian M., Wojtas A., Kamińska K., Świt P., Wach A., Gołembiowska K. Hallucinogen-like action of the novel designer drug 25I-NBOMe and its effect on cortical neurotransmitters in rats. Neurotox. Res. 2019;36:91–100. doi: 10.1007/s12640-019-00033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi M., Wang S.E., Ko S.Y., Kang H.J., Chae S.Y., Lee S.H., et al. Overexpression of human GATA-1 and GATA-2 interferes with spine formation and produces depressive behavior in rats. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poklis J.L., Raso S.A., Alford K.N., Poklis A., Peace M.R. Analysis of 25I-NBOMe, 25B-NBOMe, 25C-NBOMe and other dimethoxyphenyl-N-[(2-Methoxyphenyl) Methyl]Ethanamine derivatives on blotter paper. J. Anal. Toxicol. 2015;39:617–623. doi: 10.1093/jat/bkv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno J.L., Holloway T., Albizu L., Sealfon S.C., González-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci. Lett. 2011;493:76–79. doi: 10.1016/j.neulet.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel R., Dickenson A.H. Modality selective roles of pro-nociceptive spinal 5-HT2A and 5-HT3 receptors in normal and neuropathic states. Neuropharmacology. 2018;143:29–37. doi: 10.1016/j.neuropharm.2018.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nau F., Yu B., Martin D., Nichols C.D. Serotonin 5-HT2A receptor activation blocks TNF-α mediated inflammation in vivo. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu B., Becnel J., Zerfaoui M., Rohatgi R., Boulares A.H., Nichols C.D. Serotonin 5-hydroxytryptamine(2A) receptor activation suppresses tumor necrosis factor-alpha-induced inflammation with extraordinary potency. J. Pharmacol. Exp. Therapeut. 2008;327:316–323. doi: 10.1124/jpet.108.143461. [DOI] [PubMed] [Google Scholar]
- 29.Herian M., Wojtas A., Maćkowiak M., Wawrzczak-Bargiela A., Solarz A., Bysiek A., et al. Neurotoxicological profile of the hallucinogenic compound 25I-NBOMe. Sci. Rep. 2022;12:2939. doi: 10.1038/s41598-022-07069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen A.A., Halberstadt A.L., Märcher-Rørsted E., Odland A.U., Chatha M., Speth N., et al. The selective 5-HT2A receptor agonist 25CN-NBOH: structure-activity relationship, in vivo pharmacology, and in vitro and ex vivo binding characteristics of [3H]25CN-NBOH. Biochem. Pharmacol. 2020;177 doi: 10.1016/j.bcp.2020.113979. [DOI] [PubMed] [Google Scholar]
- 31.Braden M.R., Parrish J.C., Naylor J.C., Nichols D.E. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol. Pharmacol. 2006;70:1956–1964. doi: 10.1124/mol.106.028720. [DOI] [PubMed] [Google Scholar]
- 32.Poulie C.B.M., Jensen A.A., Halberstadt A.L., Kristensen J.L. DARK classics in chemical neuroscience: NBOMes. ACS Chem. Neurosci. 2020;11:3860–3869. doi: 10.1021/acschemneuro.9b00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arredondo S.B., Valenzuela-Bezanilla D., Mardones M.D., Varela-Nallar L. Role of Wnt signaling in adult hippocampal neurogenesis in health and disease. Front. Cell Dev. Biol. 2020;8:860. doi: 10.3389/fcell.2020.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kormish J.D., Sinner D., Zorn A.M. Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev. Dynam. 2010;239:56–68. doi: 10.1002/dvdy.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao R., Wang L., Wang H., Xia L., Erdjument-Bromage H., Tempst P., et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 36.Pereira J.D., Sansom S.N., Smith J., Dobenecker M.-W., Tarakhovsky A., Livesey F.J. Ezh2, the histone methyltransferase of PRC2, regulates the balance between self-renewal and differentiation in the cerebral cortex. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15957–15962. doi: 10.1073/pnas.1002530107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catlow B.J., Song S., Paredes D.A., Kirstein C.L., Sanchez-Ramos J. Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp. Brain Res. 2013;228:481–491. doi: 10.1007/s00221-013-3579-0. [DOI] [PubMed] [Google Scholar]
- 38.Tsybko A.S., Ilchibaeva T.V., Filimonova E.A., Eremin D.V., Popova N.K., Naumenko V.S. The chronic treatment with 5-HT2A receptor agonists affects the behavior and the BDNF system in mice. Neurochem. Res. 2020;45:3059–3075. doi: 10.1007/s11064-020-03153-5. [DOI] [PubMed] [Google Scholar]
- 39.Paiva I.M., de Carvalho L.M., Di Chiaccio I.M., Lima Assis I de, Naranjo E.S., Bernabé M.G., et al. Inhibition of Lrrk2 reduces ethanol preference in a model of acute exposure in zebrafish. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2020;100 doi: 10.1016/j.pnpbp.2020.109885. [DOI] [PubMed] [Google Scholar]
- 40.Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 41.Xu P., Qiu Q., Li H., Yan S., Yang M., Naman C.B., et al. 25C-NBOMe, a novel designer psychedelic, induces neurotoxicity 50 times more potent than methamphetamine in vitro. Neurotox. Res. 2019;35:993–998. doi: 10.1007/s12640-019-0012-x. [DOI] [PubMed] [Google Scholar]
- 42.Zwartsen A., Hondebrink L., Westerink R.H. Changes in neuronal activity in rat primary cortical cultures induced by illicit drugs and new psychoactive substances (NPS) following prolonged exposure and washout to mimic human exposure scenarios. Neurotoxicology. 2019;74:28–39. doi: 10.1016/j.neuro.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki J., Dekker M.A., Valenti E.S., Arbelo Cruz F.A., Correa A.M., Poklis J.L., et al. Toxicities associated with NBOMe ingestion-a novel class of potent hallucinogens: a review of the literature. Psychosomatics. 2015;56:129–139. doi: 10.1016/j.psym.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.