Abstract
Purpose:
The STudy to Enhance uNderstanding of sTent-associated Symptoms (STENTS) sought to identify risk factors for pain and urinary symptoms, as well as how these symptoms interfere with daily activities after ureteroscopy for stone treatment.
Materials and Methods:
This prospective observational cohort study enrolled patients aged ≥12 years undergoing ureteroscopy with ureteral stent for stone treatment at 4 clinical centers. Participants reported symptoms at baseline; on postoperative days (POD) 1, 3, 5; at stent removal; and day 30 post-stent removal. Outcomes of pain intensity, pain interference, urinary symptoms, and bother were captured with multiple instruments. Multivariable analyses using mixed-effects linear regression models were identified characteristics associated with increased stent-associated symptoms (SAS).
Results:
A total of 424 participants were enrolled. Mean age was 49 years (SD 17); 47% were female. Participants experienced a marked increase in SAS on POD 1. While pain intensity decreased ~50% from POD 1 to POD 5, interference due to pain remained persistently elevated. In multivariable analysis, older age was associated with lower pain intensity(p=0.004). Having chronic pain conditions(p<0.001), prior severe stent pain(p=0.021), and depressive symptoms at baseline(p<0.001) were each associated with higher pain intensity. Neither sex, stone location, ureteral access sheath use, nor stent characteristics were drivers of SAS.
Conclusions:
In this multicenter cohort, interference persisted even as pain intensity decreased. Patient factors (e.g., age, depression) rather than surgical factors were associated with symptom intensity. These findings provide a foundation for patient-centered care and highlight potential targets for efforts to mitigate the burden of SAS.
Keywords: urinary stone disease, ureteroscopy, ureteral stent, stent-associated symptoms, pain
Ureteral stents are an integral element of the treatment of renal and ureteral stones. Stents are placed to ensure ureteral patency and may facilitate ureteral healing in the event of injury,1 but they are typically associated with bothersome pain and urinary symptoms.2 The symptom burden and severity following ureteroscopy (URS) and stent placement is highly variable.3 Although some patients tolerate ureteral stents without complaint, others experience severe symptoms that interfere with many aspects of daily living. Understanding the etiology and mechanism of stent-associated symptoms (SAS) is challenging as there are several potential modifiable and nonmodifiable risk factors that may increase one’s chance for an adverse experience. These include patient demographics, baseline and history of pain and urinary symptoms, psychosocial factors, stone-related factors and prior stone experiences, operative factors including ureteral instrumentation, stent characteristics, and medications.
The inability to identify patients at highest risk for developing increased SAS, and what aspects of the procedure contribute to these symptoms, was the impetus for the STudy to Enhance uNderstanding of sTent-associated Symptoms (STENTS).4 Given the need to gain further understanding of multiple factors that are potentially related to SAS, the Urinary Stone Disease Research Network (USDRN) conducted a prospective, observational cohort study of individuals undergoing URS and stenting for treatment of urolithiasis. The overall aim of STENTS is to improve understanding of SAS after URS to inform future interventional studies. In this study, our specific objectives were to examine the time course of these symptoms, and identify risk factors for increased pain, pain interference, urinary symptoms, and bother.
MATERIALS AND METHODS
Study Design
STENTS is a multicenter prospective observational cohort study of individuals undergoing URS and ureteral stent placement for treatment of a ureteral or renal stone. A description of the STENTS protocol has been published.4
Study Population
Patients aged 12 years and older with a planned unilateral URS for stone treatment were recruited from four clinical centers. All participants were prospectively enrolled after institutional review board approval and informed consent. Participants aged 17 years or younger provided their informed assent, and their parents provided parental permission. Exclusion criteria were an indwelling ureteral stent within the preceding 60 days, concomitant shockwave lithotripsy or percutaneous nephrolithotomy, conditions resulting in neurogenic bladder dysfunction, anatomic urological abnormality resulting in abnormal bladder sensation, and renal transplantation. Vulnerable populations (e.g., prisoners or individuals with cognitive impairment that would impact their ability to participate in the protocol) were also excluded.
Study Procedures
Participants completed baseline questionnaires prior to surgery that recorded individual characteristics, medical and stone history, existing chronic pain conditions (Supplemental Table 1), and medication use. A thorough psychosocial assessment was completed prior to surgery with the following self-reported instruments: 1) Patient-Reported Outcome Measurement Information System (PROMIS) Depression 8a, 2) PROMIS Anxiety 8a, 3) Perceived Stress Scale, 4) Pain Catastrophizing Scale, and 5) Somatic Symptom Scale-8 (Supplemental Table 2). Participants with a prior history of a ureteral stent reported whether they had severe pain or severe urinary symptoms with their previous stent.
Outcomes: Patient Experiences
All participants completed the following questionnaires that assessed pain, urinary symptoms, and the manner and degree to which these symptoms impacted their lives (pain interference and urinary bother): 1) Brief Pain Inventory (BPI) short form,5 which assessed pain severity and pain interference; 2) PROMIS6 measures of pain intensity and pain interference, which allowed for comparing scores to population norms; 3) the Urinary Score of the Ureteral Stent Symptom Questionnaire (USSQ-U),7 which assessed urinary symptoms; and 4) Symptoms of Lower Urinary Tract Dysfunction Research Network Symptom Index-10 (LURN SI-10),8 which assessed urinary symptoms and bother (Supplemental Table 2). These patient-reported symptom scores served as the outcome measures for this study.
Data Collection
Participants completed the above outcome instruments before surgery (baseline), on postoperative days (POD) 1, 3, and 5, on the day of stent removal, and 30 days after stent removal. Questionnaires were self-administered and completed via electronic format, or paper copies if preferred. Trained research coordinators recorded intraoperative data at the time of URS, including stone features, details of ureteral instrumentation, irrigation type, and stent characteristics, which were verified and confirmed by the treating urologist. Postoperative prescriptions were recorded, and participants completed a medication diary during the stent dwell time.
Statistical Analysis
For this prospective observational study, a series of power calculations for the main hypotheses were performed using two-sided t-tests, logistic regressions, correlations, and chi-square tests (with continuity correction); the sample size was sufficient to detect small to moderate effect sizes.4 Descriptive characteristics are reported as means (SDs) and medians (25th, 75th percentiles) for continuous variables depending on the distribution of the data, and as frequencies (percentages) for categorical variables. To test the hypotheses that patient-reported symptom measures (e.g., pain intensity) varied over time, adjusting for important confounders, we used repeated-measures mixed-effects models with random effects for clinical center. The time points in the mixed model were baseline, POD 1, POD 3, POD 5, POD 7–9, and 30 days post-stent removal. Multivariable linear mixed-effects models were adjusted for baseline symptom score, sex, age (per 10 years), body mass index (BMI), chronic pain condition, prior severe stent pain (for models of pain-related outcomes), prior severe urinary symptoms (for models of urinary symptoms–related outcomes), depressive symptoms (PROMIS), location of stone, ureteral access sheath (UAS) use, and URS time. A sensitivity analysis was performed exploring whether inclusion of postoperative medication use (tamsulosin/alpha blockers and oxybutynin/anticholinergics, as well as nonsteroidal anti-inflammatories and opiates) in the multivariable models affected the results. Two-sided significance testing was used with a conventional significance level of 0.05. All statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
The study cohort comprised 424 patients who underwent unilateral URS and stent placement for ureteral and/or kidney stones, of whom 47% were female; mean age was 49 years (SD 17). Of the total, 115 participants (27%) had a history of depression; depressive symptoms at baseline (PROMIS) were none to slight for 342 (83%), mild for 42 (10%), and moderate for 26 (6%); no patients reported severe symptoms. An existing chronic pain condition was reported by 158 patients (37%) (Supplemental Table 1), 170 (40%) had a history of prior stent, 47% of whom reported severe pain with the prior stent. Table 1 further characterizes the study population. Table 2 lists stone and operative factors.
Table 1.
Study population
| Characteristic | Participants (n=424) |
|---|---|
| Mean age, years (SD) | 49 (17) |
| Female | 199 (47%) |
| Race | |
| White | 358 (84%) |
| Black | 32 (8%) |
| Asian | 17 (4%) |
| Other | 16 (4%) |
| Hispanic/Latinx ethnicity | 25 (6%) |
| Mean body mass index, kg/m2 (SD) | 30 (8) |
| Medical history | |
| Depression | 115 (27%) |
| Anxiety | 127 (30%) |
| Mood disorder (other) | 15 (4%) |
| Chronic pain condition | 158 (37%) |
| Prior ureteroscopy | 154 (36%) |
| Prior ureteral stent placement | 170 (40%) |
| Severe pain with prior ureteral stent | 80 (19%) |
| Severe urinary symptoms with prior ureteral stent | 102 (24%) |
| Medication use in 30 days prior to URS | |
| Opioids | 111 (26%) |
| NSAIDs | 155 (37%) |
| Tamsulosin (or alpha blocker) | 136 (32%) |
| Oxybutynin (or anticholinergic) | 9 (2.1%) |
Data shown are n (%) except where indicated. SD = standard deviation; NSAIDs = nonsteroidal anti-inflammatory drugs.
Table 2.
Stone and operative factors
| Variable | Participants (n = 424) |
|---|---|
| Side of treatment: right | 180 (47%) |
| Mean size of dominant treated renal stone, mm | 7.2 (3.8) |
| Mean size of dominant treated ureteral stone, mm | 7.4 (3.1) |
| Dominant stone location* | |
| Renal | 212 (50%) |
| Proximal ureter | 100 (24%) |
| Distal ureter | 103 (24%) |
| Median number of renal stones treated (quartiles) | 3 (2, 4) |
| Operative time, min | 57 (30) |
| Ureteroscopy time, min | 40 (25) |
| Ureteroscope type* | |
| Flexible | 266 (68%) |
| Semirigid | 57 (15%) |
| Both | 71 (18%) |
| Ureteral access sheath use | 197 (47%) |
| Basket extraction | 287 (68%) |
| No. of scope/basket passes | |
| 1–4 | 145 (39%) |
| 5–20 | 152 (41%) |
| >20 | 72 (20%) |
| Irrigation method | |
| Manual | 188 (45%) |
| Constant pressure | 205 (49%) |
| Ureteral stent diameter | |
| 4.7 French | 47 (12%) |
| 6 French | 349 (87%) |
| Ureteral stent length, cm | |
| 22 | 11 (3%) |
| 24 | 127 (32%) |
| 26 | 159 (40%) |
| 28 | 90 (22%) |
| 30 | 13 (3%) |
| Proximal stent curl: complete | 348 (82%) |
| Proximal stent curl location | |
| Renal pelvis | 249 (63%) |
| Calyx | 145 (37%) |
| Stent dwell time, days | 9 (8) |
| Median (quartiles) | 7 (5, 11) |
Note: percentages may not sum to 100 due to rounding.
Data shown are n (%) or mean (SD), except where indicated.
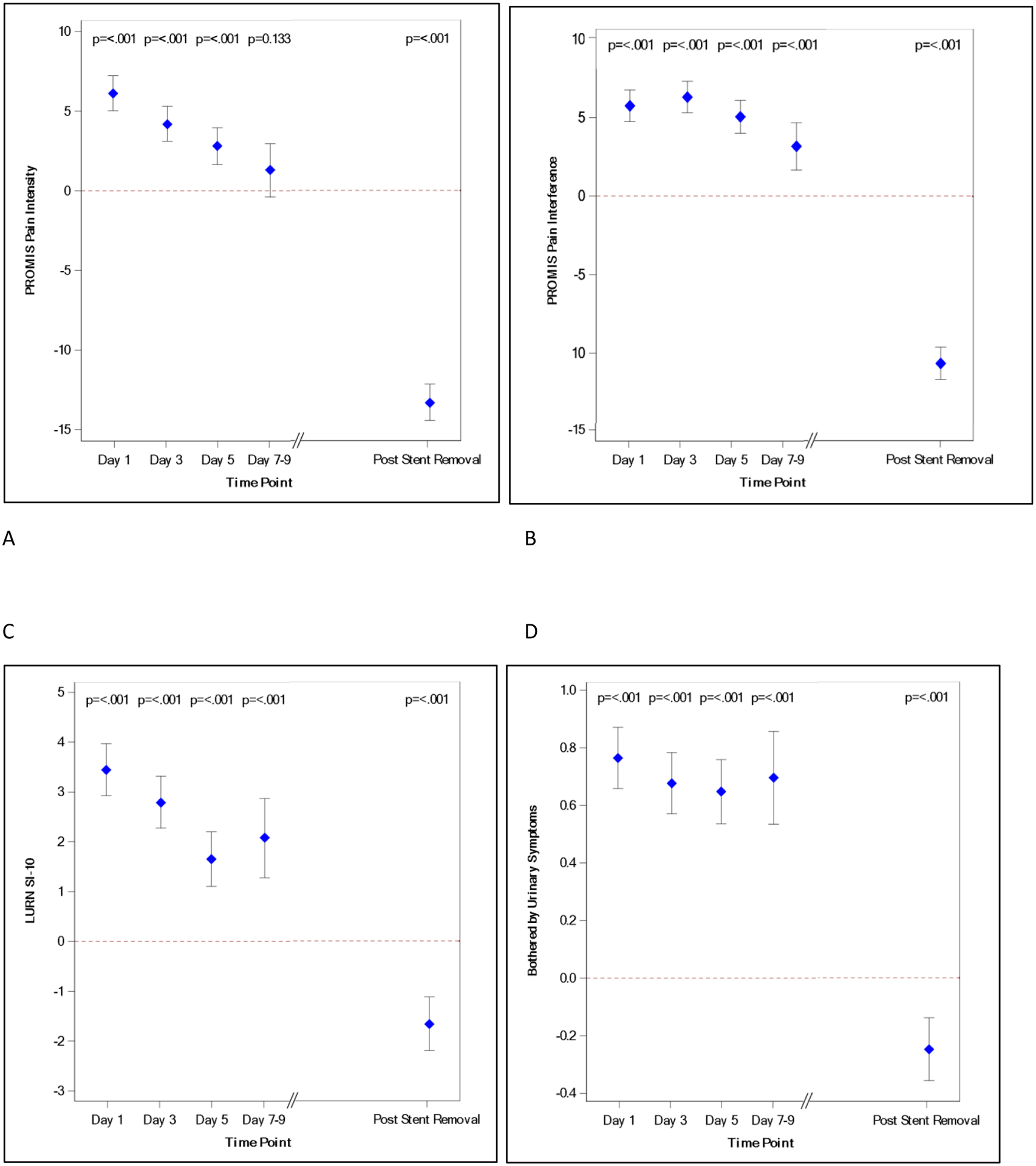
Figure 1 represents the change in SAS over time. There was a sharp increase in all domains of SAS on POD 1. Compared to POD 1, the point estimate for pain intensity decreased over time by approximately 50% on POD 5. Pain intensity continued to decrease and was no longer statistically different from baseline by POD 7–9, while the stent was in place (Fig. 1A, Supplemental Figure 1). Interference due to pain rose on POD 1, however, it remained persistently elevated through POD 5 before decreasing thereafter, lagging behind the decrease in pain intensity (Fig. 1B, Supplemental Figure 2). The trajectory for pain severity and pain interference scores using BPI (Supplemental Table 3) was similar to PROMIS pain intensity and interference. Following a clear peak in urinary symptoms on POD 1, there was a steady decline in symptom score until POD 5, remaining elevated above baseline until after stent removal (LURN SI-10 [Fig. 1C, Supplemental Figure 3] and USSQ-U [Supplemental Table 4]). Like the relationship between pain intensity and pain interference, there was a slower decline in reported urinary bother (Fig. 1D, Supplemental Figure 4), compared to urinary symptoms.
Figure 1:
SAS change over time. A, Pain intensity (PROMIS); B, pain interference (PROMIS); C, urinary symptoms (LURN SI-10); D, urinary bother (LURN SI-10). Mean point estimate and confidence intervals displayed with p values listed for each day. Red dashed line denotes zero change from baseline.
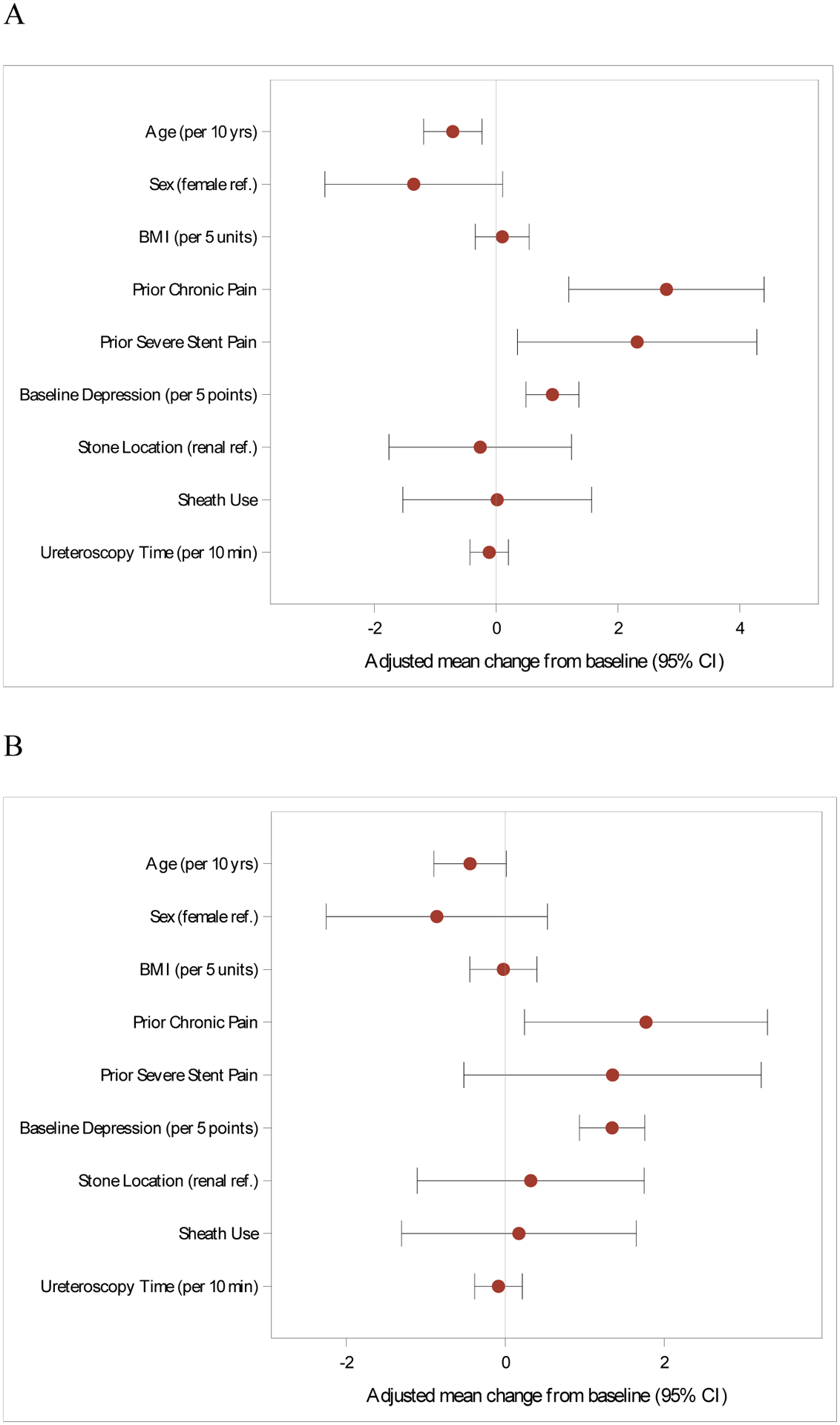
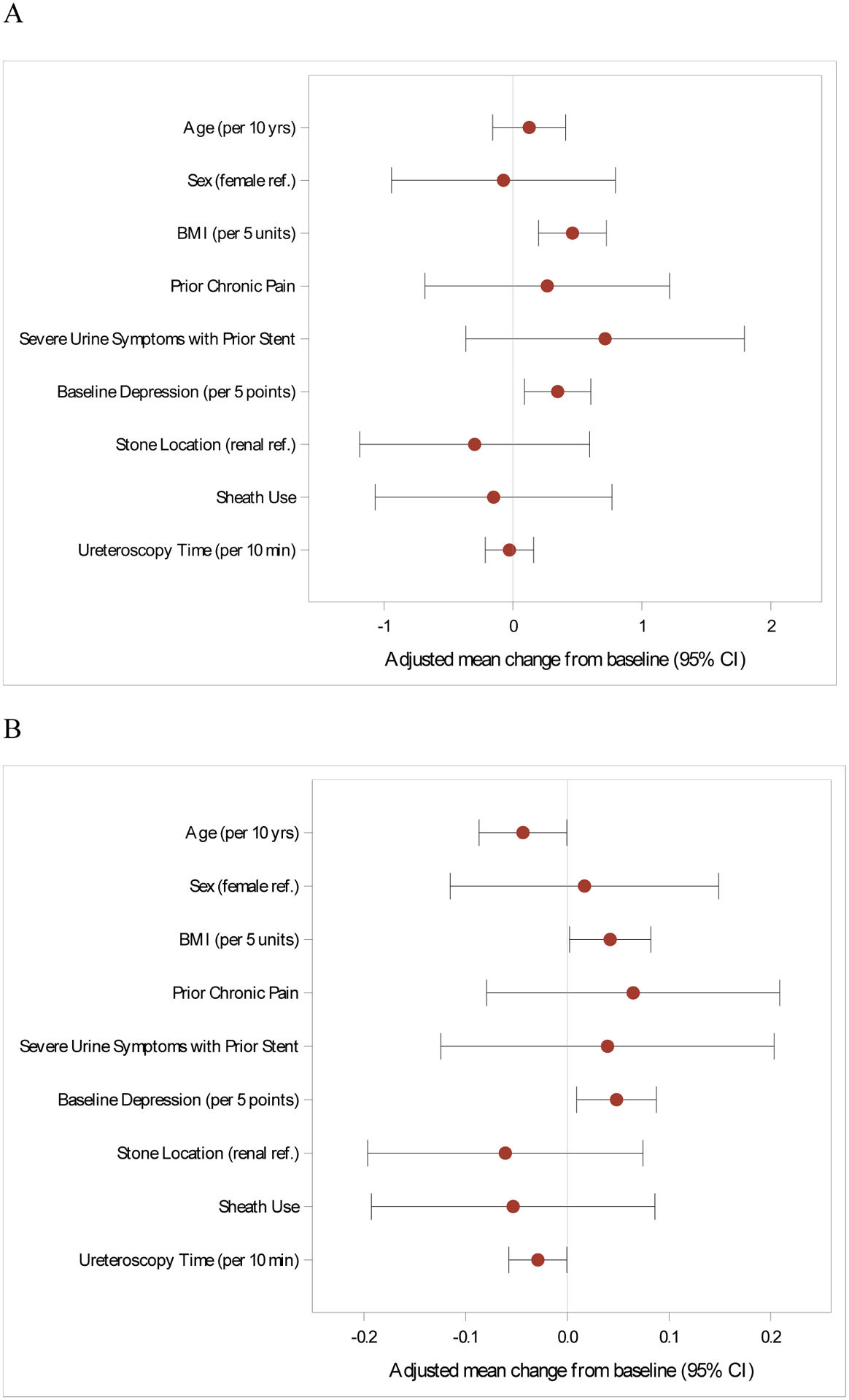
The multivariable models for characteristics associated with increased SAS are shown in Figures 2 (PROMIS pain intensity and interference) and 3 (LURN SI-10 urinary symptoms and bother). Estimates of covariates including results from the BPI and USSQ-U are listed in Supplemental Tables 3 and 4. Younger age, prior severe pain with a stent, existing chronic pain condition, and baseline depressive symptoms were independently associated with higher pain intensity (PROMIS) and severity (BPI). These four risk factors were also associated with higher pain interference using BPI, while chronic pain condition and baseline depressive symptoms were associated with pain interference by PROMIS. In terms of the urinary multivariable models (Figure 3), increasing BMI and depressive symptoms at baseline were each associated with greater urinary symptoms and urinary bother; increasing age was associated with less urinary bother (LURN SI-10). There were no identified risk factors for combined urinary symptoms and bother as measured by the USSQ-U. Factors significantly associated with increased SAS remained the same in a sensitivity analysis that included postoperative medications commonly used to treat SAS.
Figure 2:
Risk factors for increased stent-associated pain intensity (A) and pain interference (B). Measured using PROMIS. Multivariable model.
Figure 3:
Risk factors for increased stent-associated urinary symptoms (A) and urinary bother
(B). Measured using LURN SI-10. Multivariable model.
DISCUSSION
This multicenter prospective study characterizing patients undergoing URS and stent placement for urinary stones revealed multiple important insights: 1) despite postoperative pain intensity decreasing over time, patient-reported interference from pain remained elevated; 2) pain intensity was lower for older patients; there was no difference based on sex; and 3) depressive symptoms, chronic pain condition, and a history of severe pain with a prior stent were risk factors for patients reporting higher pain intensity. Importantly, and contrary to expectations and popular belief, we failed to identify an association of stone and operative factors including ureteral instrumentation and stent characteristics with greater symptoms overall.
The sharp rise in pain intensity on POD 1 is expected, and we have shown previously that SAS peak within the first 2 days, based on a detailed daily assessment.3 The current study shows that the average point estimate of pain intensity decreases by about 50% from POD 1 to POD 5. However, participants continue to report elevated levels of pain interference, similar to POD 1, during this timeframe. This lag in the decline in pain interference has important clinical implications, as we found that patient quality of life and functioning remained negatively impacted despite pain intensity nearing baseline by POD 7–9 after stenting.
Demographic factors such as age and sex may affect the experience after URS and stenting. Although previous data are sparse, the most consistent results are that younger age is associated with greater SAS, unplanned hospital visits, and more use of opioids following stone treatment.9–11 Data are less clear in terms of differences based on sex; whereas two studies reported that women were more likely to report severe pain at various types of stone encounters,12,13 male sex was associated with opioid receipt after URS in the state of Michigan.14 In our study, younger patients were at risk for increased pain intensity and pain interference on multivariable regression; however, the average increase in pain was not different between males and females. Investigations in other specialties regarding aging and its relationship to pain intensity and interference have shown similar findings, with older adults maintaining the ability to function equally or better than their younger counterparts. For example, in a study examining the association of age and pain intensity on interference in orofacial pain patients, findings suggested that at higher levels of pain intensity, its effect on interference decreased with age.15 Although the effect of age on coping with adverse health events has not been thoroughly examined in urinary stones, our findings corroborate the aging theory of socioemotional selectivity, which postulates that with aging, people develop the motivation to maximize positive experiences and minimize the negative.16
Pain perception and experiences may also be influenced by psychosocial factors such as stress, anxiety, depression, social support, and expectations, many of which were comprehensively assessed in STENTS. Given the high degree of correlation between the various psychosocial instruments, only PROMIS depression score was included in the multivariable model due to the potential of collinearity interfering with interpretability. Higher depression score was associated with increased pain intensity and interference; similar findings may be expected with higher scores in other psychosocial domains. A painful past stent experience may lead to anxiety that one will have the same experience the next time. STENTS participants were asked at baseline whether they had severe pain with a previous stent. This simple query was clinically relevant, as a history of severe pain proved to be independently associated with increased pain following a subsequent URS and stent. These findings are not only useful for more comprehensive counseling, they can aid decisions about stentless URS, types of preventative medications or pursuing behavioral therapy, and should contribute to future study design and analyses.
The influence that stone-related and operative factors have on post-ureteroscopic SAS has been unclear. It seems intuitive, and therefore expected, that variables within these domains are important and have an impact on the patient experience. UAS use was of significant interest since there is evidence that it decreases intrarenal pressure17 and therefore may reduce postoperative pain.18 On the other hand, UAS use may result in direct ureteral irritation, inflammation, injury, and potentially ischemia,19,20 and previous studies have associated UAS use with unplanned postoperative encounters,21,22 increased short-term pain,13 and receipt of opioids.14 In STENTS, we did not find that UAS use was associated with increased or decreased SAS, controlling for other variables. Other factors that could affect intrarenal pressure or contribute to ureteral trauma or inflammation—such as high number of scope/basket passes, ureteroscope type, ureteral dilation, or method of irrigation—were likewise not statistically associated with SAS. These findings may indicate that the stent experience has much to do with patient and psychosocial factors and perhaps less to do with surgical technique.
Lastly, compared to pain, we identified fewer risk factors for increased urinary symptoms, although similarities were seen regarding age and depression. Depressive symptoms and higher BMI were associated with an increase in urinary symptoms and bother measured by LURN SI-10. Higher BMI has been shown to be associated with worse urinary symptoms in men and women, so it is possible that high BMI exacerbates the effects of stenting on these symptoms.23 Increasing age was associated with less urinary bother, even though urinary symptom score was similar, another finding that could be explained by improved coping strategies with advancing age. Using the USSQ-U, we did not identify significant risk factors for urinary symptoms. This difference may in part be due to the inclusion of multiple questions on hematuria in USSQ-U, a common symptom during the first several days after surgery. Additional investigation of correlation between the various instruments is planned.
Our findings must be considered in the context of certain limitations. We compare variation in symptoms among individuals, all of whom had a ureteral stent in place. Thus, the study was not designed to compare the separate contributions of the stent and URS. The query at baseline about prior stent experience may be subject to recall bias, although a severe pain experience is less likely to be forgotten. Ureteroscopic interventions were all performed at academic medical centers, and despite the large, prospectively characterized population, these results may not be generalizable to other settings and do not proportionally represent the current US racial demographics. Despite these potential limitations, we believe that the study findings have important implications for understanding and mitigating the burden of ureteral SAS.
CONCLUSIONS
In this multicenter prospective cohort of those undergoing URS and stent for urinary stones, quality of life continued to be affected throughout the stent period, despite the decrease in symptoms over time. Patient factors such as age and depression impacted symptom severity, while surgical factors did not. These findings provide a foundation for patient counseling and highlight potential targets for future efforts to mitigate the burden of SAS and improve the overall patient experience.
Supplementary Material
ACKNOWLEDGMENTS
Urinary Stone Disease Research Network: The following individuals were instrumental in the planning and conduct of the STENTS study at each of the participating institutions:
Clinical Centers
University of Pennsylvania/Children’s Hospital of Pennsylvania, Philadelphia, PA: PI: Peter P Reese, MD, MSCE; Gregory E. Tasian, MD, MSCE. Co-I: Justin Ziemba, MD, MSEd. Study Coordinators: Emily Funsten, Adam Mussell, Rebecca McCune, Salima Shah, Arushi Jain, Antoine Selman-Fermin.
University of Texas Southwestern Medical Center, Dallas, TX: PI: Naim M. Maalouf, MD; Jodi A. Antonelli, MD. Co-I: Brett A. Johnson, MD; Margaret S. Pearle, MD, PhD; Linda A. Baker, MD. Study Coordinators: Brooke Piskator, Joyce Obiaro, Cynthia Rangel, Martinez Hill.
University of Washington, Seattle, WA: PI: Jonathan D. Harper, MD; Hunter Wessells, MD. Co-I: Michele Curatolo, MD; Todd Edwards, PhD; Mathew Sorensen, MD; Collaborator: Robert Sweet, MD. Study Coordinators: Tristan Baxter, Holly Covert, Elsa Ayala, Lisa Flint, Grace Cho, Grace Marshall.
Washington University in St. Louis, St. Louis, MO: PI: Alana C. Desai, MD; H. Henry Lai, MD. Co-I: Kefu Du, MD; Study Coordinators: Susan Mueller, RN; Linda Black, RN; Aleksandra Klim RN.
Scientific Data Research Center
Duke Clinical Research Institute, Duke University, Durham, NC: PI: Charles D. Scales, Jr, MD, MSHS; Co-PI: Hussein R. Al-Khalidi, PhD. Co-I: Amy Corneli, PhD; Bryce Reeve, PhD; Kevin Weinfurt, PhD. Statistician: Hongqiu Yang, PhD. STENTS Project Lead: Davy Andersen. USDRN Project Lead: Laura Johnson. Lead CRA: Andrew Dodd. Data Manager: Omar Thompson. Qualitative Research Core: Carrie Dombeck, Kevin McKenna, Teri Swezey.
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Project Scientist: Ziya Kirkali, MD. Program Official: Christopher Mullins, PhD.
We thank Peter Hoffmann for manuscript preparation and submission. Mr. Hoffmann did not receive compensation beyond his employment by Duke University.
Funding Information
This research was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, as follows: U01DK110961 (UPenn/CHOP – Reese, Tasian), U01KD110986 (Washington Univ. St Louis – Desai, Lai), U01DK110994 (UT Southwestern - Maalouf), U01DK110954 (University of Washington – Harper, Wessells), and U01DK110988 (Duke University – Scales, Al-Khalidi).
Author Disclosure Statement
Tasian: research funding from and service on the Scientific Advisory Board or as a consultant for Dicerna Pharmaceuticals and Alnylam Pharmaceuticals.
Reese: grants from Merck, AbbVie and Gilead to the University of Pennsylvania; consulting provided for VALHealth and (unpaid) eGenesis.
Al-Khalidi: Financial support in terms of grants and DSMB honorarium from NHLBI, NINDS, NIDDK, Mayo Clinic, CSL-Behring, and Medpace.
The remaining authors have no disclosures.
ABBREVIATIONS
- BPI
Brief Pain Inventory
- PROMIS
Patient-Reported Outcome Measurement Information System
- POD
Postoperative days
- SAS
Stent-associated symptoms
- STENTS
STudy to Enhance uNderstanding of sTent-associated Symptoms
- LURN SI-10
Symptoms of Lower Urinary Tract Dysfunction Research Network Symptom Index-10
- UAS
Ureteral access sheath
- URS
Ureteroscopy
- USSQ-U
Urinary Score of the Ureteral Stent Symptom Questionnaire
- USDRN
Urinary Stone Disease Research Network
Data Availability Statement
The data sets generated during the current study are available through the USDRN Scientific Data Research Center (USDRN@duke.edu) upon reasonable request and will be publicly available from the NIDDK Central Repository in the future, in accordance with NIH Data Sharing policies.
References
- 1.Ordonez M, Hwang EC, Borofsky M, Bakker CJ, Gandhi S, Dahm P. Ureteral stent versus no ureteral stent for ureteroscopy in the management of renal and ureteral calculi. Cochrane Database Syst Rev 2019;2:CD012703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi HB, Stainthorpe A, MacDonagh RP, Keeley FX Jr, Timoney AG, Barry MJ. Indwelling ureteral stents: evaluation of symptoms, quality of life and utility. J Urol 2003;169:1065–9. [DOI] [PubMed] [Google Scholar]
- 3.Harper JD, Desai AC, Antonelli JA, et al. Quality of life impact and recovery after ureteroscopy and stent insertion: insights from daily surveys in STENTS. BMC Urol 2022;22:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scales CD Jr, Lai HH, Desai AC, et al. Study to Enhance Understanding of Stent-Associated Symptoms: rationale and study design. J Endourol 2021;35:761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129–38. [PubMed] [Google Scholar]
- 6.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care 2007;45:S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshi HB, Newns N, Stainthorpe A, MacDonagh RP, Keeley FX Jr, Timoney AG. Ureteral stent symptom questionnaire: development and validation of a multidimensional quality of life measure. J Urol 2003;169:1060–4. [DOI] [PubMed] [Google Scholar]
- 8.Cella D, Smith AR, Griffith JW, et al. A new brief clinical assessment of lower urinary tract symptoms for women and men: LURN SI-10. J Urol 2020;203:164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gul Z, Alazem K, Li I, Monga M. Predicting procedural pain after ureteroscopy: does hydrodistention play a role? Int Braz J Urol 2016;42:734–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irani J, Siquier J, Pires C, Lefebvre O, Dore B, Aubert J. Symptom characteristics and the development of tolerance with time in patients with indwelling double-pigtail ureteric stents. BJU Int 1999;84:276–9. [DOI] [PubMed] [Google Scholar]
- 11.Lee FC, Holt SK, Hsi RS, Haynes BM, Harper JD. Preoperative belladonna and opium suppository for ureteral stent pain: a randomized, double-blinded, placebo-controlled study. Urology 2017;100:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borofsky MS, Lane GI, Neises SM, Portis AJ. Patient-Reported Outcomes Measurement System (PROMIS®) for patients with urolithiasis: initial report. J Urol 2017;198:1091–7. [DOI] [PubMed] [Google Scholar]
- 13.Oguz U, Sahin T, Senocak C, et al. Factors associated with postoperative pain after retrograde intrarenal surgery for kidney stones. Turk J Urol 2017;43:303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawken SR, Hiller SC, Daignault-Newton S, et al. Opioid-free discharge is not associated with increased unplanned healthcare encounters after ureteroscopy: results from a statewide quality improvement collaborative. Urology 2021;158:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boggero IA, Geiger PJ, Segerstrom SC, Carlson CR. Pain intensity moderates the relationship between age and pain interference in chronic orofacial pain patients. Exp Aging Res 2015;41:463–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carstensen LL, Fung HH, Charles ST. Socioemotional selectivity theory and the regulation of emotion in the second half of life. Motiv Emot 2003;27:103–23. [Google Scholar]
- 17.Auge BK, Pietrow PK, Lallas CD, Raj GV, Santa-Cruz RW, Preminger GM. Ureteral access sheath provides protection against elevated renal pressures during routine flexible ureteroscopic stone manipulation. J Endourol 2004;18:33–6. [DOI] [PubMed] [Google Scholar]
- 18.Alsyouf M, Abourbih S, West B, Hodgson H, Baldwin DD. Elevated renal pelvic pressures during percutaneous nephrolithotomy risk higher postoperative pain and longer hospital stay. J Urol 2018;199:193–9. [DOI] [PubMed] [Google Scholar]
- 19.Traxer O, Thomas A. Prospective evaluation and classification of ureteral wall injuries resulting from insertion of a ureteral access sheath during retrograde intrarenal surgery. J Urol 2013;189:580–4. [DOI] [PubMed] [Google Scholar]
- 20.Lallas CD, Auge BK, Raj GV, Santa-Cruz R, Madden JF, Preminger GM. Laser Doppler flowmetric determination of ureteral blood flow after ureteral access sheath placement. J Endourol 2002;16:583–90. [DOI] [PubMed] [Google Scholar]
- 21.Du K, Wang RS, Vetter J, et al. Unplanned 30-day encounters after ureterorenoscopy for urolithiasis. J Endourol 2018;32:1100–7. [DOI] [PubMed] [Google Scholar]
- 22.Meier K, Hiller S, Dauw C, et al. Understanding ureteral access sheath use within a statewide collaborative and its effect on surgical and clinical outcomes. J Endourol 2021;35:1340–7. [DOI] [PubMed] [Google Scholar]
- 23.Lai HH, Helmuth ME, Smith AR, et al. Relationship between central obesity, general obesity, overactive bladder syndrome and urinary incontinence among male and female patients seeking care for their lower urinary tract symptoms. Urology 2019;123:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during the current study are available through the USDRN Scientific Data Research Center (USDRN@duke.edu) upon reasonable request and will be publicly available from the NIDDK Central Repository in the future, in accordance with NIH Data Sharing policies.


