Abstract
Background
Vascular endothelial growth factors (VEGFs) and their receptors (VEGFRs) play complicated oncogenic roles in multiple tumors by initiating and promoting tumor angiogenesis and lymphangiogenesis. The main goal of our study was to comprehensively investigate the oncogenic roles of VEGFs and VEGFRs in stomach adenocarcinoma (STAD).
Methods
The present study applied multiple bioinformatic tools to comprehensively explore the expression levels, prognostic values, genetic alterations and immune infiltrations of VEGFs and VEGFRs in STAD patients.
Results
We found that VEGFA, VEGFC, placenta growth factor, FLT1, KDR, FLT4, and Neuropilin 1 were overexpressed in STAD, while the expression of VEGFB and VEGFD were decreased. Survival analysis revealed that higher transcription levels of VEGF/VEGFRs were obviously correlated with worse clinical outcome in STAD patients. Additionally, high alteration frequencies of VEGFs and VEGFRs (27%) were observed in STAD patients, and alterations of VEGFs and VEGFRs improved their prognosis. The expression of VEGFs and VEGFRs was remarkably associated with immune cell infiltration and immune checkpoint expression in STAD patients.
Conclusion
Our study systematically explored the transcriptome profiles and distinct prognostic values of VEGFs and their receptors in STAD and contributed to a better understanding of the oncogenic roles of VEGF/VEGFR members in STAD.
Keywords: Vascular endothelial growth factors, Vascular endothelial growth factor receptors, Stomach adenocarcinoma, Prognosis, Bioinformatics
1. Introduction
Solid tumor tissue is comprised of neoplastic cells and surrounding stromal cells, and this heterogeneous composition contributes to maintaining the complexity of tumor microenvironment (TME) [1]. Owing to the rapid development rate, tumor cells demand a constant supply of nutrients to maintain a higher metabolic rate. These pathological processes are mainly dependent on the induction of angiogenesis [2]. During physiological processes, angiogenesis is crucial for embryonic processes and wound healing. However, abnormally accelerated angiogenesis processes in TME provide supplemental oxygen and nutrients to tumor cells, which leads to the development of tumor [3]. Besides, the pericyte coverage of tumor vasculature is often lacking or loose, which enhanced the vascular permeability and thereby promoting the metastasis of cancer cells [4]. Therefore, angiogenesis has been recognized as a primary driving force to participate in the pathological process of many severe human diseases, especially malignant tumors, and inhibition of the tumor angiogenesis pathway is a promising strategy for clinical anti-tumor therapy [5].
The process of angiogenesis is mainly modulated by multiple growth factors and their correlated receptor tyrosine kinases (RTKs). Among them, vascular endothelial growth factors (VEGFs) and their receptors (VEGFRs) are especially important [6]. Accumulating studies have indicated that VEGFs and VEGFRs are principal drivers in the initiation and progression of tumor angiogenesis [7,8]. There are five VEGFs in the mammalian genome, including VEGFA (also known as VEGF), VEGFB, VEGFC, VEGFD (also known as FIGF) and placenta growth factor (PGF), which are mainly responsible for the processes of angiogenesis and lymphangiogenesis in physiological or pathological condition [9]. In addition, previously published researches have proved that, with the activation of VEGF/VEGFR pathways, the proliferation, differentiation and migration of tumor cells are significantly promoted [10,11]. In terms of VEGFR, there are two subtypes: tyrosine kinase receptor and non-tyrosine kinase receptor. The former includes VEGFR1 (also known as FLT1), VEGFR2 (also known as KDR), and VEGFR3 (also known as FLT4), and the latter, Neuropilin 1 (NRP1) and NRP2 [12]. VEGFR1, a common receptor for VEGFA, VEGFB and PGF ligands, is mainly expressed on the surface of vascular endothelial cells, macrophages, dendritic cells and tumor cells [13]. The activation of VEGFR1 mediated by VEGFs is related to cellular proliferation, migration, apoptosis, and angiogenesis [14]. VEGFR2, a receptor for VEGFA, VEGFC and VEGFD, is predominantly expressed on vascular endothelium cells, with the activation of VEGFR2 significantly enhanced mitogenesis, angiogenesis, vascular invasion and vascular permeability [15]. VEGFR3 is a tyrosine kinase receptor of VEGFC and VEGFD that is mainly expressed on lymphatic endothelial cells and serves as a vital role in regulating lymphangiogenesis [16]. NRP1 and NRP2 are co-receptors that selectively bind certain VEGFs and regulate the functions of VEGFR2 and VEGFR3, thereby enhancing the migration of endothelial cells in angiogenesis [17].
Stomach adenocarcinoma (STAD) accounts for the fifth most frequently diagnosed cancer, accounting for more than 700,000 cancer-related deaths around the world [18]. In recent years, several anti-angiogenic agents targeting the VEGF/VEGFR signaling and conferring survival benefits in patients with STAD have been approved for clinical treatment of STAD [19]. Nevertheless, only a subset of STAD patients benefited from the clinically approved anti-angiogenic drugs in use currently, and many who initially respond develop resistance over time. Thus, we performed the present study to explore the oncogenic roles of distinct VEGF/VEGFR members in STAD.
2. Materials and methods
2.1. Oncomine
Oncomine (https://www.oncomine.org/) database is a web-based project providing genome-wide expression analysis across diverse cancer types [20]. In the present study, Oncomine project was applied for analyzing the transcription levels of distinct VEGF/VEGFR members in distinct cancers. P-value <0.05, fold change (FC) > 1.5, and gene rank in the top 10% were considered to be statistically significant.
2.2. The Cancer Genome Atlas data
The Cancer Genome Atlas (TCGA, https://www.cancer.gov/tcga) is a public resource containing many types of data from over 20,000 tumor and normal samples. In the present study, we first obtained the sequencing profiles of RNA in Fragments Per Kilobase Million format and clinicopathological parameters of STAD patients from TCGA database. We then analyzed the expression patterns of distinct VEGF/VEGFR members and their correlations with patients’ clinical characteristics in STAD by R software (v4.1.1, https://www.r-project.org/). P-value <0.05 was considered to be statistically significant.
2.3. Kaplan-Meier Plotter
The Kaplan Meier plotter (http://kmplot.com/analysis/) is a web-based platform constructed for evaluating the prognostic values of more than 50,000 genes across 21 types of malignant tumor, including STAD [21,22]. In this study, we applied this platform to explore the influences of distinct VEGF/VEGFR members on clinical outcome of STAD patients, including overall survival (OS), first-progression survival (FP), and post-progression survival (PPS). The hazard ratio (HR) with 95% confidence intervals (CIs) and log-rank p-values were automatically calculated to the best cutoff value, and log-rank p-value <0.05 was recognized statically significant.
2.4. cBioPortal
The c-Bio Cancer Genomics Portal (cBioPortal, v3.7.11, https://www.cbioportal.org/) is an integrated online-based platform that contains multidimensional cancer genomic datasets from TCGA project [23]. We used the Stomach Adenocarcinoma (Pfizer and UHK, Nat Genet 2014) [24] and Stomach Adenocarcinoma (TCGA, PanCancer Atlas) [25] datasets in cBioPortal to explore the genetic alterations of distinct VEGF/VEGFR genes and their prognostic values in STAD patients. A log-rank test p-value <0.05 was recognized statically significant.
2.5. TIMER
Tumor IMmune Estimation Resource (TIMER, https://cistrome.shinyapps.io/timer/) is a platform for systematical exploration of tumor immunological features, which precalculated the infiltrations of several tumor infiltrated immune cells (TIICs) (Neutrophils, B cells, Macrophages, CD4+ T cells, CD8+ T cells, and Dendritic cells) for more than 10,000 tumors from 32 types of malignant tumor [26]. In our study, TIMER database was use for assessing the correlations between the infiltration levels of TIICs and gene expression levels of distinct VEGF/VEGFR members in STAD. Besides, we also evaluated the correlations of immune checkpoint expression level with expression levels of distinct VEGF/VEGFRs members in STAD by TIMER. The relationships were evaluated by Spearman's test, and p-value <0.05 was recognized statically significant.
3. Results
3.1. Transcriptional levels of VEGF/VEGFR members in pan-cancer
We utilized Oncomine project to evaluate the differential transcriptional levels of distinct VEGF/VEGFR members between STAD and corresponding normal samples. As a result, VEGFB, VEGFD, and FLT4 showed significantly downregulated expression levels in STAD compared to control specimens (Fig. 1). Besides, the transcriptional levels of VEGFC, PGF, KDR, NRP1, and NRP2 were obviously elevated in STAD compared to control specimens. However, no differential expression information of VEGFA and FLT1 in STAD was observed in the Oncomine dataset.
Fig. 1.
The transcription levels of VEGF/VEGFR members in different types of cancers (Oncomine). Red represents the target gene is upregulated, and blue indicates that the target gene is downregulated.
3.2. mRNA expression levels of VEGF/VEGFR members and their correlations with clinicopathological characters in patients with STAD
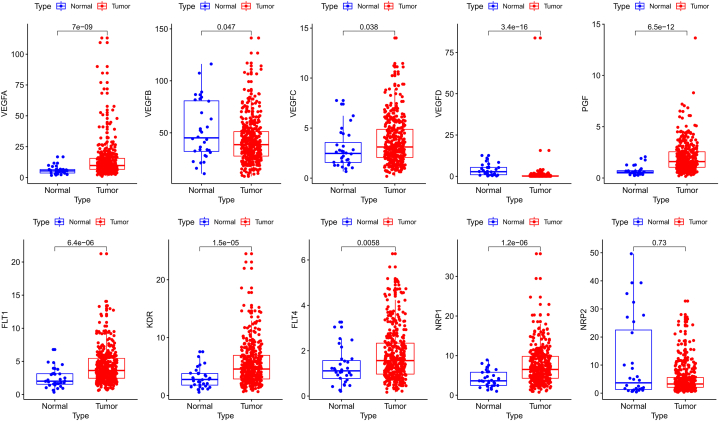
The sequencing data of 407 specimens (including 375 cancerous and 32 normal specimens) and corresponding clinicopathological parameters were retrieved from TCGA. Differential expression analysis found that the mRNA expression levels of VEGFA, VEGFC, PGF, FLT1, KDR, FLT4, and NRP1 were obviously elevated in STAD compared to control specimens, while the mRNA expression levels of VEGFB and VEGFD were significantly downregulated, and no expression difference was found for NRP2 (Fig. 2). We further evaluated the correlation of distinct VEGF/VEGFR members’ expression with the pathological stage of STAD patients. As displayed in Fig. 3, the transcriptome levels of VEGFB, VEGFC, VEGFD and PGF were upregulated in stage II tumors. The mRNA expression of FLT1, NRP1, and NRP2 showed an increasing trend with increasing tumor stage. Contrariwise, VEGFA, KDR, and FLT4 groups did not show significant differences.
Fig. 2.
The mRNA expression levels of VEGF/VEGFR members in STAD (TCGA). The red dots indicate the STAD samples, and the blue dots indicate the normal samples.
Fig. 3.
The correlations between VEGF/VEGFRs expression and tumor stage in STAD patients (TCGA).
3.3. Correlations of the increased expression levels of distinct VEGF/VEGFRs with poor clinical outcome of STAD patients
We applied the Kaplan Meier plotter for determining the influences of distinct VEGF/VEGFR members on clinical outcomes of STAD patients and found that each member of the VEGF/VEGFR family genes were obviously correlated with the clinical outcomes of STAD patients. As shown in Fig. 4, patients with STAD possess higher VEGF/VEGFRs expression showed worse OS, FP, and PPS than those possess low expression (p < 0.05).
Fig. 4.
The prognostic values of distinct VEGF/VEGFR members in patients with STAD (Kaplan-Meier Plotter).
3.4. Genetic alterations of VEGF/VEGFR members in STAD patients
The genetic variations of VEGF/VEGFR members in STAD were explored via cBioPortal project. As a result, the VEGF/VEGFR members were altered in 145 samples of 540 STAD patients from two datasets, and the individual sequence alteration frequencies varied from 0.6 to 6% (Fig. 5A and B). Subsequently, we found that STAD patients in the altered subgroup shared an obviously increased progression-free survival and disease-specific survival compared with those in unaltered subgroup (Fig. 5C and D). However, STAD patients with or without alterations in VEGF/VEGFR members had no relationship with OS and disease-free survival (Fig. 5E and F), and the survival curve of each VEGF/VEGFR member in STAD patients was displayed in Supplementary Fig. 1.
Fig. 5.
The genetic alteration analysis of VEGF/VEGFRs in patients with STAD (cBioPortal). (A) Summary of alterations in VEGF/VEGFRs. (B) OncoPrint tab summary of alteration types and proportions of distinct VEGF/VEGFR members in STAD. Kaplan–Meier plots comparing (C) progression free survival (PFS), (D) disease specific survival (DSS), (E) overall survival (OS) and (F) disease-free survival (DFS) in patients with/without VEGF/VEGFRs gene alterations.
3.5. VEGF/VEGFR members positively correlated with immune cell infiltration in STAD
Considering the VEGF/VEGFR signaling-mediated angiogenesis is connected with immune reaction in TME, we further utilized TIMER for exploring the relationships of distinct VEGF/VEGFR members expression with the abundances of TIICs. As shown in Fig. 6, VEGFA showed a significant negative connection with the Macrophage, B cell, CD4+ T cell, CD8+ T cell, and Dendritic cell abundances in STAD. VEGFB, VEGFC, FLT1, KDR, FLT4, and NRP2 were all positively associated with Macrophage, CD4+ T cell, CD8+ T cell, Dendritic cell, and Neutrophil abundances in STAD patients, but there was no significant correlation with B cell. Additionally, all six host immune cells have an obvious association with FIGF and NRP1 expression in STAD. With regard to PGF, CD4+ T cell and Macrophage showed a positive correlation in STAD, while B cell showed a negative correlation.
Fig. 6.
The correlations between distinct VEGF/VEGFR genes and immune cell infiltration (TIMER).
3.6. Correlations of VEGF/VEGFR members with immune checkpoints in STAD
Studies have reported that abnormal angiogenesis induces immunosuppression in TME by undermining the functions of tumor-infiltrating lymphocytes (TILs). Therefore, the correlations of distinct VEGF/VEGFR members with immunosuppression biomarkers, including PDCD1 (PD1), CD274 (PD-L1), CTLA4 and HAVCR2 (TIM-3), were evaluated by TIMER. As displayed in Fig. 7, VEGFA expression was in connection with CD274 and CTLA4 in STAD. VEGFB and VEGFD were positively connected with PDCD1 and HAVCR2. Besides, expression of PGF was found to be positively associated with HAVCR2 expression. In terms of the rest members, VEGFC, FLT1, KDR, FLT4, NRP1, and NRP2 were all significantly positively correlated with all analyzed immune checkpoints in patients with STAD. These results suggested that VEGF/VEGFR-mediated oncogenic roles in STAD might partially through upregulating immune checkpoint expression to suppress the functions of TIL.
Fig. 7.
The correlations between distinct VEGF/VEGFR genes and immune checkpoint expression (TIMER).
4. Discussion
VEGFs not only induce tumor angiogenesis and increase vascular permeability, but also directly activate downstream signaling pathways of tumor cells by binding to VEGFRs. To date, it is wildly accepted that VEGF/VEGFR-mediated angiogenesis is crucial for the progression of STAD, and several VEGF/VEGFR pathway-targeted drugs, such as bevacizumab, ramucirumab and apatinib, have shown a beneficial effect on STAD treatment [27]. However, typical of a heterogeneous disease, only a few patients with STAD show sensitivity to anti-VEGF/VEGFR treatment, and the clinical application of VEGF/VEGFR pathway-targeted drugs today is still limited. Therefore, we launched the present research to comprehensively analyze the oncogenic roles of distinct VEGF/VEGFR members from the viewpoints of expression characteristics, clinical relevance, prognostic properties, somatic alteration, and immune infiltration. As far as we know, this is the first study to analyze the oncogenic roles of different VEGF/VEGFR members in human STAD. We hope that our work will provide an optimal treatment strategy, and therefore improve the clinical outcome of STAD patients.
VEGFA, also called VEGF, is the prototype member of the VEGF family. VEGFA mainly binds to VEGFR2 and thereby mediates blood vessel formation [8]. Studies have reported that VEGFA was overexpressed in STAD specimens compared with the tumor-adjacent normal specimens. The literature also shows that overexpression of VEGFA in STAD patients was obviously associated with tumor differentiation and vascular invasion [28,29]. In vitro and in vivo analyses found that VEGFA overexpression contributes to cell proliferation and tumor growth of NCI–N87 gastric cancer cells [30]. Moreover, overexpression of VEGFA promoted peritoneal metastasis of gastric cancer cells by activating the ERK/JNK signaling in vivo [31]. Our study revealed that VEGFA was obviously overexpressed in STAD compared to normal specimens. Survival analysis found that patients with STAD possess higher VEGFA expression showed worse OS, FP, and PPS compared to those possess low VEGFA expression. Moreover, VEGFA overexpression was negatively connected with immune infiltrations of CD4+ T cell, B cell, Macrophage, CD8+ T cell, and Dendritic cell, and simultaneously positively correlated with CD274 and CTLA4 expression.
VEGFB is a ligand of VEGFR1, which is mainly expressed on the endotheliocyte, myocardium, and skeletal muscle [32]. To date, the significance of VEGFB in pathological angiogenesis is still extremely controversial and deserves more attention. Several studies indicated that VEGFB predominantly contributes to the development of the cardiovascular system and its function for angiogenesis is not essential [17]. On the contrary, various studies also demonstrated that VEGFB is a vascular remolding molecular boosting cancer metastasis and plays a prognostic marker for cancer patients [33]. In STAD, a recent research reported that VEGFB was highly expressed in cancer specimens, and its overexpression was obviously related to with aggressive histological stage and worse clinical outcome [34]. In our research, we found that VEGFB expression in STAD was reduced compared to normal controls, and high VEGFB expression was remarkably related to short OS, FP, and PPS in STAD patients. Immune infiltration analysis suggested that VEGFB overexpression was obviously positively correlated with Macrophage, CD8+ T cell, Dendritic cell, CD4+ T cell, and Neutrophil infiltration level. Moreover, VEGFB expression is also significantly associated with expression levels of PDCD1, HAVCR2, and CTLA4.
VEGFC has a high affinity for VEGFR3 and is essential for lymphangiogenesis. Recent studies have demonstrated that VEGFC serves as an oncogene in multiple cancer types, including STAD [35]. VEGFC overexpression is a predictive biomarker for lymphatic metastasis for STAD patients, and patients possess higher VEGFC expression shared worse survival rates than those possess low VEGFC expression [36]. Kallistatin has been reported to suppress VEGFC expression to restrain the NF-ҡB signaling, thus inhibiting the lymphangiogenesis and lymphatic metastasis of STAD in vivo [37]. In addition, VEGFC overexpression contributes to distant metastasis and cisplatin resistance of STAD [38]. Our study revealed that VEGFC expression in STAD was obviously elevated, and its expression was significantly varied across different tumor stages. We also found that VEGFC overexpression was remarkably related to worse OS, FP, and PPS in STAD patients. Additionally, our findings suggested that VEGFC expression was significantly associated with immune infiltrations of CD8+ T cell, CD4+ T cell, Macrophage, Dendritic cell, and Neutrophil, and there are positive correlations between VEGFC expression and immune checkpoint biomarkers (PDCD1, CD274, CTLA4, and HAVCR2). These results above are consistent with the idea that VEGFC acts as an oncogene in STAD.
VEGFD, also known as FIGF, presents similar properties to VEGFC and promotes the development of lymphatic vessels in tumors by binding VEGFR3. VEGFD expression was found to be obviously higher in gastric cancer cell lines than in normal controls, and overexpression of VEGFD was remarkably associated with hepatic metastasis in STAD patients undergoing surgery [39,40]. Besides, a meta-analysis across 30 studies revealed that VEGFD serves as a predictive marker for worse clinical outcome in STAD [41]. In vitro, curcumin restrains the lymphangiogenesis of gastric cancer cells via inhibiting the HMGB1/VEGFD axis [42]. Our results indicated that VEGFD expression in STAD was remarkably decreased. However, no significant correlations between VEGFD expression and tumor stage was observed in patients with STAD. Patients with STAD in the high VEGFD expression subgroup showed worse OS, FP, and PPS than those in the low VEGFD subgroup. Besides, VEGFD was found to be remarkably associated with all TIICs’ abundance and immune checkpoint biomarker expression. Given that VEGFC and VEGFD all participate in lymphangiogenesis by binding VEGFR3, targeting the VEGFC/VEGFD-VEGFR3 pathway to inhibit lymphatic metastasis might be an alternative strategy for anti-cancer therapy of STAD, especially for those who have developed resistance to anti-angiogenic therapies.
PGF, also called PLGF, is also a member of the VEGF family and was first identified in human placental tissues. The present study proved that PGF expression was significantly elevated in STAD, and overexpression of PGF was remarkably related to positive lymph node metastases, advanced tumor stage, and decreased survival time in STAD patients [43]. PGF knockdown was demonstrated to suppress migration and induce apoptosis in STAD by depressing PI3K/Akt signaling pathway [44]. In addition, PGF acts as a predictor for ramucirumab treatment, and patients with STAD in the PGF-low group showed higher ramucirumab sensitivity than the PGF-high group [45]. Recent study also determined that PGF expression was remarkably increased in STAD, and PGF overexpression was remarkably connected with worse OS, FP, and PPS in STAD. Besides, high PGF expression was significantly correlated with high CD4+ T cell, Macrophage, and B cell infiltration levels in STAD. PGF expression was also positively connected with PDCD1 and HAVCR2 expression in STAD.
There are three types of VEGFRs: VEGFR1, VEGFR2, and VEGFR3. Studies have indicated that mRNA and protein expression of VEGFR1 were significantly elevated in STAD compared to adjacent non-cancerous specimen, and high VEGFR1 acts as a predictor for poor clinical outcome in STAD patients [32,46]. Blockade of VEGFR1 contributes to enhancing paclitaxel sensitivity in gastric cancer cells [47]. VEGFR2, also called KDR, is an angiogenesis regulatory gene that has been proved to be closely related to tumorigenesis and metastasis in diverse malignant tumors, including STAD [48]. Furthermore, the survival rates of STAD patients possess higher VEGFR2 expression are obviously lower than those possess low VEGFR2 expression [46]. In addition, selectively targeting VEGFR2 contributes to enhancing the T cell-mediated antitumor immunity in STAD [49]. VEGFR3, also called FLT4, is mainly responsible for the development of lymphatic vessels. VEGFR3 overexpression has been found to be significantly associated with depth of invasion and lymphatic metastasis in STAD [50]. Besides, elevated VEGFR3 expression also has been found to be significantly related to unfavourable OS in STAD patients, with the silencing of VEGFR3 significantly inhibiting proliferation and inducing apoptosis in gastric cancer cell lines [46,51]. Similarly, our research revealed that the expression levels of these three VEGFRs in STAD are all remarkably higher than those in normal specimens. In addition, all of these VEGFRs are remarkably correlated with poor OS, FP, and PPS in STAD patients. We also found that these three VEGFRs positively correlate with Macrophage, CD8+ T cell, Dendritic cell, CD4+ T cell, and Neutrophil infiltration levels. Expression of these VEGFRs are remarkably associated with PDCD1, CD274, HAVCR2, and CTLA4, respectively. These results confirmed the oncogenic roles of VEGFRs in human STAD.
NRP1 and NPR2 are transmembrane non-tyrosine kinase glycoproteins that modulate the activity of RTKs by selectively binding to certain isoforms of VEGFR. NRP1 is mainly expressed in vascular endothelial tissue, and dysregulation of NRP1 has been found to be participated in the development of diverse malignant tumors, including STAD [52]. A recent study involving 1225 cases revealed that NRP1 overexpression in STAD was closely related to aggressive clinical stage, low differentiation, and lymph node metastasis [53]. Upregulation of NRP1 predicts worse clinical outcomes and promotes tumor metastasis through epithelial-mesenchymal transition in STAD [54]. NRP2 is specifically expressed in the venous and lymphatic endothelial cells, and NRP2 overexpression increase the proliferation and migration of gastric cancer cells by enhancing VEGF activity [55]. In addition, knockdown of NRP2 remarkably suppressed migration and invasion of gastric cancer cells in vitro and sensitized gastric cancer cells to 5-FU toxicity in vitro via the β-catenin signaling [56]. Likewise, our findings revealed that NRP1 mRNA expression level in STAD is significantly higher than that in non-cancerous controls. However, the difference of NRP2 expression between cancer and control samples with no significance. Interestingly, we found that the expression patterns of NRP1/NRP2 in differential cancer stages was similar to the expression patterns of VEGFR1/VEGFR2/VEGFR3. Survival analysis revealed that high expressions of NRP1 and NRP2 are obviously related to unfavourable OS, FP, and PPS in STAD. Immune infiltration analysis demonstrated that NRP1 and NRP2 are positively correlated with all host immune cells except for B cell. In addition, we also found that NRP1 and NRP2 overexpression correlate with high PDCD1, CD274, HAVCR2 and CTLA4 expression in STAD. Collectively, these results imply that NRP dysregulation in STAD leads to the development of tumors, and the carcinogenic effect of VEGFRs was partially enhanced by NRPs. Co-targeting NRPs might improve the efficiency of anti-VEGF therapy in STAD.
In recent decades, immunotherapy has gained significant progress as an anti-cancer treatment for several malignant tumors. Accumulating studies focused on the association between gene expression and tumor immunity have been published. For example, NFE2L2 has been identified to serve as a crucial role in the progression of brain lower grade glioma by regulating the infiltration of TIL [57]. BRAP has been reported to be overexpressed in liver cancer specimens compared with non-cancerous control, and overexpression of BRAP is remarkably associated with immune infiltrating cells and immune checkpoint biomarkers in liver cancer [58]. As for VEGF/VEGFRs, studies have reported that VEGFA contributes to inhibiting the immunity of T and NK cells via promoting the proliferation of Treg cells, thereby enabling the cancer cells to escape the immunological surveillance in STAD [59]. Besides, studies also found that VEGFC expression was obviously negatively associated with the density of dendritic cells in STAD. VEGFC contributes to immune escape of STAD by regulating the function of dendritic cells [60]. VEGFR2 was highly expressed by effector Treg cells, with the silencing of VEGFR2 significantly enhanced CD8+ T cell infiltration abundances by suppressing VEGFR2-mediated effector Treg cell proliferation [61]. NRP2 was reported to serve as a molecular mediator that links efferocytosis and immunosuppression by regulating phagocytosis in macrophage, knockout of NRP2 in macrophage suppressed the immunosuppressive effects in the myeloid compartment [62]. Likewise, our findings indicated that the expression of VEGF/VEGFRs in STAD was remarkably associated with abundances of TIICs, including B cell, Macrophage, CD4+ T cell, Dendritic cell, CD8+ T cell, and Neutrophil. We also found that the expression of VEGF/VEGFRs was remarkably correlated the immune checkpoint biomarkers, including PDCD1, CD274, CTLA4, and HAVCR2. These results above indicate that VEGF/VEGFRs might mediate the initiation and progression of STAD partially through regulating the abundances of TIICs and immune checkpoint biomarkers expression; therefore, targeting the VEGF/VEGFR signaling might enhance the efficacy of immunotherapy in STAD.
Inevitably, several limitations exist in our study. Firstly, the present study is a retrospective study based on public database, multicenter prospective studies are required to further validate our findings. Besides, clinical and fundamental investigations are needed for exploring the biological mechanisms underlying VEGF/VEGFR-induced tumorigenesis in STAD.
5. Conclusion
In our study, comprehensive bioinformatic analyses were performed to investigate the expression levels, prognostic values, genetic alterations, and immune infiltrations of distinct VEGF/VEGFR members in patients with STAD. We found that the expression of VEGFA, VEGFB, VEGFC, VEGFD, PGF, VEGFR1, VEGFR2, VEGFR3 and NRP1 were significantly dysregulated in STAD. Increased expression of VEGF/VEGFRs is a potential predictive biomarker of poor prognosis in STAD patients. Besides, we found that the expression of VEGF/VEGFRs was remarkably associated with the infiltration of diverse TIICs in STAD. Our findings elucidate the complexity of the VEGF/VEGFR-mediated tumorigenesis and provide reliable evidence for future comprehensive analysis on the biological function and molecular mechanisms of the VEGF/VEGFR pathway in human STAD, which may provide clues to identify novel prognostic biomarkers among VEGF/VEGFRs in STAD.
Authors' contributions
Jianxin Li: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ting Han: Conceived and designed the experiments; Analyzed and interpreted the data. All authors read and approved the final manuscript.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in The Cancer Genome Atlas (TCGA) repository (https://portal.gdc.cancer.gov/repository?facetTab=files&filters=%7B%22op%22%3A%22and%22%2C%22content%22%3A%5B%7B%22op%22%3A%22in%22%2C%22content%22%3A%7B%22field%22%3A%22cases.primary_site%22%2C%22value%22%3A%5B%22stomach%22%5D%7D%7D%2C%7B%22op%22%3A%22in%22%2C%22content%22%3A%7B%22field%22%3A%22cases.project.program.name%22%2C%22value%22%3A%5B%22TCGA%22%5D%7D%7D%2C%7B%22op%22%3A%22in%22%2C%22content%22%3A%7B%22field%22%3A%22cases.project.project_id%22%2C%22value%22%3A%5B%22TCGA-STAD%22%5D%7D%7D%2C%7B%22op%22%3A%22in%22%2C%22content%22%3A%7B%22field%22%3A%22files.data_category%22%2C%22value%22%3A%5B%22transcriptome%20profiling%22%5D%7D%7D%2C%7B%22op%22%3A%22in%22%2C%22content%22%3A%7B%22field%22%3A%22files.data_type%22%2C%22value%22%3A%5B%22Gene%20Expression%20Quantification%22%5D%7D%7D%2C%7B%22op%22%3A%22in%22%2C%22content%22%3A%7B%22field%22%3A%22files.experimental_strategy%22%2C%22value%22%3A%5B%22RNA-Seq%22%5D%7D%7D%5D%7D).
Ethics statement
Not applicable (No human clinical data were used in the study).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17687.
Abbreviations
- VEGF
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptor
- STAD
Stomach adenocarcinoma
- TME
Tumor microenvironment
- RTK
receptor tyrosine kinase
- PGF
Placenta growth factor
- NRP1
Neuropilin 1
- NRP2
Neuropilin 2
- TCGA
The Cancer Genome Atlas
- OS
Overall survival
- FP
First-progression survival
- PPS
Post-progression survival
- HR
Hazard ratio
- CI
Confidence interval
- cBioPortal
c-Bio Cancer Genomics Portal
- TIMER
Tumor IMmune Estimation Resource
- TIL
Tumor-infiltrating lymphocyte
- TIIC
tumor infiltrated immune cell
Appendix A. Supplementary data
The following is the Supplementary data to this article.
figs1
References
- 1.Saharinen P., et al. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol. Med. 2011;17(7):347–362. doi: 10.1016/j.molmed.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar-Cazares D., et al. Contribution of angiogenesis to inflammation and cancer. Front. Oncol. 2019;9:1399. doi: 10.3389/fonc.2019.01399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajabi M., Mousa S.A. The role of angiogenesis in cancer treatment. Biomedicines. 2017;5(2):34. doi: 10.3390/biomedicines5020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aggarwal C., Somaiah N., Simon G. Antiangiogenic agents in the management of non-small cell lung cancer: where do we stand now and where are we headed? Cancer Biol. Ther. 2012;13(5):247–263. doi: 10.4161/cbt.19594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z., et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin. Cancer Biol. 2015;35:S224–S243. doi: 10.1016/j.semcancer.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi H., Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. 2005;109(3):227–241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- 7.Ramjiawan R.R., Griffioen A.W., Duda D.G. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy? Angiogenesis. 2017;20(2):185–204. doi: 10.1007/s10456-017-9552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karaman S., Leppänen V.M., Alitalo K. Vascular endothelial growth factor signaling in development and disease. Development. 2018;145(14) doi: 10.1242/dev.151019. [DOI] [PubMed] [Google Scholar]
- 9.Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 2013;153(1):13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou W., et al. Proliferation and migration of lung cancer could be inhibited by oxymatrine through the regulation for miR-520/VEGF. Am. J. Chin. Med. 2019;47(4):865–878. doi: 10.1142/S0192415X19500459. [DOI] [PubMed] [Google Scholar]
- 11.Ma F., et al. Hypoxic macrophage-derived VEGF promotes proliferation and invasion of gastric cancer cells, dig. Dis. Sci. 2019;64(11):3154–3163. doi: 10.1007/s10620-019-05656-w. [DOI] [PubMed] [Google Scholar]
- 12.Rapisarda A., Melillo G. Role of the VEGF/VEGFR axis in cancer biology and therapy. Adv. Cancer Res. 2012;114:237–267. doi: 10.1016/B978-0-12-386503-8.00006-5. [DOI] [PubMed] [Google Scholar]
- 13.Lacal P.M., Graziani G. Therapeutic implication of vascular endothelial growth factor receptor-1 (VEGFR-1) targeting in cancer cells and tumor microenvironment by competitive and non-competitive inhibitors. Pharmacol. Res. 2018;136:97–107. doi: 10.1016/j.phrs.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Mohammad Rezaei F., et al. Dysregulated KDR and FLT1 gene expression in colorectal cancer patients. Rep. Biochem. Mol. Biol. 2019;8(3):244–252. [PMC free article] [PubMed] [Google Scholar]
- 15.Yang F F., et al. Potential role of soluble VEGFR-1 in antiangiogenesis therapy for cancer. Expert Rev. Anticancer Ther. 2011;11(4):541–549. doi: 10.1586/era.10.171. [DOI] [PubMed] [Google Scholar]
- 16.Song G., Li Y., Jiang G. Role of VEGF/VEGFR in the pathogenesis of leukemias and as treatment targets. Oncol. Rep. 2012;28(6):1935–1944. doi: 10.3892/or.2012.2045. (Review) [DOI] [PubMed] [Google Scholar]
- 17.Melincovici C.S., et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis, Rom. J. Morphol. Embryol. 2018;59(2):455–467. [PubMed] [Google Scholar]
- 18.Bray F., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 19.Hironaka S. Anti-angiogenic therapies for gastric cancer, Asia Pac. J. Clin. Oncol. 2019;15(4):208–217. doi: 10.1111/ajco.13174. [DOI] [PubMed] [Google Scholar]
- 20.Rhodes D.R., et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6(1):1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagy Á., Munkácsy G., Győrffy B. Pancancer survival analysis of cancer hallmark genes. Sci. Rep. 2021;11(1):6047. doi: 10.1038/s41598-021-84787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szász A.M., et al. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016;7(31):49322–49333. doi: 10.18632/oncotarget.10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao J J., et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6(269) doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang K., et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat. Genet. 2014;46(6):573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 25.Hoadley K.A., et al. Cell-of-Origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell. 2018;173(2):291–304. doi: 10.1016/j.cell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li T., et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–e110. doi: 10.1158/0008-5472.CAN-17-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel T.H., Cecchini M. Targeted therapies in advanced gastric cancer. Curr. Treat. Options Oncol. 2020;21(9):70. doi: 10.1007/s11864-020-00774-4. [DOI] [PubMed] [Google Scholar]
- 28.Chen S., et al. VEGF promotes gastric cancer development by upregulating CRMP4. Oncotarget. 2016;7(13):17074–17086. doi: 10.18632/oncotarget.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo K., et al. VEGF-C and VEGF-A synergistically enhance lymph node metastasis of gastric cancer. Biol. Pharm. Bull. 2007;30(4):633–637. doi: 10.1248/bpb.30.633. [DOI] [PubMed] [Google Scholar]
- 30.Park J.H., et al. Lentivirus-mediated VEGF knockdown suppresses gastric cancer cell proliferation and tumor growth in vitro and in vivo. OncoTargets Ther. 2020;13:1331–1341. doi: 10.2147/OTT.S234344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X., et al. Hypoxia-autophagy axis induces VEGFA by peritoneal mesothelial cells to promote gastric cancer peritoneal metastasis through an integrin α5-fibronectin pathway. J. Exp. Clin. Cancer Res. 2020;39(1):221. doi: 10.1186/s13046-020-01703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X., et al. Isoform-specific expression of VEGF-B in normal tissues and tumors. Growth Factors. 2001;19(1):49–59. doi: 10.3109/08977190109001075. [DOI] [PubMed] [Google Scholar]
- 33.Yang X., et al. VEGF-B promotes cancer metastasis through a VEGF-A-independent mechanism and serves as a marker of poor prognosis for cancer patients. Proc. Natl. Acad. Sci. U.S.A. 2015;112(22):E2900–E2909. doi: 10.1073/pnas.1503500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Y., et al. Co-expression of VEGF-B and FLT-1 correlates with malignancy and prognosis of gastric cancer. Biomarkers Med. 2021;15(7):481–488. doi: 10.2217/bmm-2020-0608. [DOI] [PubMed] [Google Scholar]
- 35.Liu X.E., Sun X.D., Wu J.M. Expression and significance of VEGF-C and FLT-4 in gastric cancer. World J. Gastroenterol. 2004;10(3):352–355. doi: 10.3748/wjg.v10.i3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikeda K., et al. Intratumoral lymphangiogenesis and prognostic significance of VEGFC expression in gastric cancer. Anticancer Res. 2014;34(8):3911–3915. [PubMed] [Google Scholar]
- 37.Ma C., et al. Kallistatin inhibits lymphangiogenesis and lymphatic metastasis of gastric cancer by downregulating VEGF-C expression and secretion. Gastric Cancer. 2018;21(4):617–631. doi: 10.1007/s10120-017-0787-5. [DOI] [PubMed] [Google Scholar]
- 38.Cho H.J., et al. VEGF-C mediates RhoGDI2-induced gastric cancer cell metastasis and cisplatin resistance. Int. J. Cancer. 2014;135(7):1553–1563. doi: 10.1002/ijc.28801. [DOI] [PubMed] [Google Scholar]
- 39.Deng J., et al. STAT3 regulation the expression of VEGF-D in HGC-27 gastric cancer cell. Am. J. Transl. Res. 2014;6(6):756–767. [PMC free article] [PubMed] [Google Scholar]
- 40.Deng J., et al. Vascular endothelial growth factor-D is correlated with hepatic metastasis from gastric cancer after radical gastrectomy. Surgery. 2009;146(5):896–905. doi: 10.1016/j.surg.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 41.Peng L., et al. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in gastric cancer: a meta-analysis. Mol. Biol. Rep. 2012;39(10):9473–9484. doi: 10.1007/s11033-012-1812-8. [DOI] [PubMed] [Google Scholar]
- 42.Da W., et al. Curcumin inhibits the lymphangiogenesis of gastric cancer cells by inhibiton of HMGB1/VEGF-D signaling. Int. J. Immunopathol. Pharmacol. 2019;33 doi: 10.1177/2058738419861600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen C.N., et al. The significance of placenta growth factor in angiogenesis and clinical outcome of human gastric cancer. Cancer Lett. 2004;213(1):73–82. doi: 10.1016/j.canlet.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 44.Akrami H., et al. PlGF knockdown inhibited tumor survival and migration in gastric cancer cell via PI3K/Akt and p38MAPK pathways. Cell Biochem. Funct. 2016;34(3):173–180. doi: 10.1002/cbf.3176. [DOI] [PubMed] [Google Scholar]
- 45.Natsume M., et al. Placental growth factor is a predictive biomarker for ramucirumab treatment in advanced gastric cancer, Cancer Chemother. Pharma. 2019;83(6):1037–1046. doi: 10.1007/s00280-019-03817-2. [DOI] [PubMed] [Google Scholar]
- 46.Hirashima Y., et al. Impact of vascular endothelial growth factor receptor 1, 2, and 3 expression on the outcome of patients with gastric cancer. Cancer Sci. 2009;100(2):310–315. doi: 10.1111/j.1349-7006.2008.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang J.E., et al. Blockade of VEGFR-1 and VEGFR-2 enhances paclitaxel sensitivity in gastric cancer cells. Yonsei Med. J. 2013;54(2):374–380. doi: 10.3349/ymj.2013.54.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lian L., et al. VEGFR2 promotes tumorigenesis and metastasis in a pro-angiogenic-independent way in gastric cancer. BMC Cancer. 2019;19(1):183. doi: 10.1186/s12885-019-5322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J., et al. Immune-mediated antitumor effect by VEGFR2 selective inhibitor for gastric cancer. OncoTargets Ther. 2019;12:9757–9765. doi: 10.2147/OTT.S233496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ge H., et al. Prognostic and clinical significance of VEGFR-3 in gastric cancer: a meta-analysis. Clin. Chim. Acta. 2017;474:114–119. doi: 10.1016/j.cca.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Qin X.J., et al. Effect of lentivirus-mediated shRNA targeting VEGFR-3 on proliferation, apoptosis and invasion of gastric cancer cells. Int. J. Mol. Med. 2011;28(5):761–768. doi: 10.3892/ijmm.2011.758. [DOI] [PubMed] [Google Scholar]
- 52.Li L., et al. Neuropilin-1 is associated with clinicopathology of gastric cancer and contributes to cell proliferation and migration as multifunctional co-receptors. J. Exp. Clin. Cancer Res. 2016;35:16. doi: 10.1186/s13046-016-0291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao H., et al. Clinicopathological significance of Neuropilin 1 expression in gastric cancer: a meta-analysis. Dis. Markers. 2020;2020 doi: 10.1155/2020/4763492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin Q., et al. Neuropilin-1 predicts poor prognosis and promotes tumor metastasis through epithelial-mesenchymal transition in gastric cancer. J. Cancer. 2021;12(12):3648–3659. doi: 10.7150/jca.52851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim W.H., et al. Neuropilin2 expressed in gastric cancer endothelial cells increases the proliferation and migration of endothelial cells in response to VEGF. Exp. Cell Res. 2009;315(13):2154–2164. doi: 10.1016/j.yexcr.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 56.Samuel S., et al. Neuropilin-2 mediated β-catenin signaling and survival in human gastro-intestinal cancer cell lines. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0023208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ju Q., et al. NFE2L2 is a potential prognostic biomarker and is correlated with immune infiltration in brain lower grade glioma: a pan-cancer analysis. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/3580719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ju Q., et al. BRCA1-Associated protein is a potential prognostic biomarker and is correlated with immune infiltration in liver hepatocellular carcinoma: a pan-cancer analysis. Front. Mol. Biosci. 2020;7 doi: 10.3389/fmolb.2020.573619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saeed A., Park R., Sun W. The integration of immune checkpoint inhibitors with VEGF targeted agents in advanced gastric and gastroesophageal adenocarcinoma: a review on the rationale and results of early phase trials. J. Hematol. Oncol. 2021;14(1):13. doi: 10.1186/s13045-021-01034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi A., et al. Correlation of vascular endothelial growth factor-C expression with tumor-infiltrating dendritic cells in gastric cancer. Oncology. 2002;62(2):121–127. doi: 10.1159/000048257. [DOI] [PubMed] [Google Scholar]
- 61.Tada Y., et al. Targeting VEGFR2 with Ramucirumab strongly impacts effector/activated regulatory T cells and CD8+ T cells in the tumor microenvironment. J. Immunother. Cancer. 2018;6(1):106. doi: 10.1186/s40425-018-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roy S., et al. Macrophage-derived neuropilin-2 exhibits novel tumor-promoting functions. Cancer Res. 2018;78(19):5600–5617. doi: 10.1158/0008-5472.CAN-18-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
figs1
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in The Cancer Genome Atlas (TCGA) repository (https://portal.gdc.cancer.gov/repository?facetTab=files&filters=%7B%22op%22%3A%22and%22%2C%22content%22%3A%5B%7B%22op%22%3A%22in%22%2C%22content%22%3A%7B%22field%22%3A%22cases.primary_site%22%2C%22value%22%3A%5B%22stomach%22%5D%7D%7D%2C%7B%22op%22%3A%22in%22%2C%22content%22%3A%7B%22field%22%3A%22cases.project.program.name%22%2C%22value%22%3A%5B%22TCGA%22%5D%7D%7D%2C%7B%22op%22%3A%22in%22%2C%22content%22%3A%7B%22field%22%3A%22cases.project.project_id%22%2C%22value%22%3A%5B%22TCGA-STAD%22%5D%7D%7D%2C%7B%22op%22%3A%22in%22%2C%22content%22%3A%7B%22field%22%3A%22files.data_category%22%2C%22value%22%3A%5B%22transcriptome%20profiling%22%5D%7D%7D%2C%7B%22op%22%3A%22in%22%2C%22content%22%3A%7B%22field%22%3A%22files.data_type%22%2C%22value%22%3A%5B%22Gene%20Expression%20Quantification%22%5D%7D%7D%2C%7B%22op%22%3A%22in%22%2C%22content%22%3A%7B%22field%22%3A%22files.experimental_strategy%22%2C%22value%22%3A%5B%22RNA-Seq%22%5D%7D%7D%5D%7D).