Abstract
Two years into the coronavirus disease 2019 (COVID-19) pandemic, we have now seen three main variant waves. We performed a retrospective cohort study of all pregnant patients with COVID-19 at our institution from March 22, 2020, to February 26, 2022, to evaluate disease severity and perinatal outcomes among the variants. Patients were categorized as pre-Delta (March 22, 2020–May 31, 2021), Delta (July 1, 2021–December 15, 2021), or Omicron (December 16, 2021– February 26, 2022) based on variant tracking from the Centers for Disease Control and Prevention and genotype sequencing at our institution. There were fewer cases of severe–critical disease (1.8% Omicron vs 13.3% pre-Delta and 24.1% Delta) and adverse perinatal outcomes during the Omicron wave compared with the pre-Delta and Delta waves.
INTRODUCTION
We are now 2 years into the coronavirus disease 2019 (COVID-19) pandemic. The Alpha variant (B.1.1.7) predominated at the start of the pandemic, from March 2020 to June 2021. Next, the Delta variant (B.1.617.2) was most common from July 2021 to December 2021. The Omicron (B.1.1.529) variant was first identified in the United States on December 1, 2021, and on December 16, 2021, became the leading variant sequenced at our institution. We previously published perinatal outcomes associated with the Delta variant in pregnancy1 and now seek to report differences in perinatal outcomes among the three main variants to date.
METHODS
After receiving approval from the University of Alabama at Birmingham institutional review board (IRB-300007195), we performed a retrospective cohort study of all pregnant patients with COVID-19 at the University of Alabama at Birmingham from March 22, 2020, to February 26, 2022. Patients were categorized as pre-Delta (March 22, 2020–May 31, 2021), Delta (July 1, 2021–December 15, 2021), or Omicron (December 16, 2021–February 26, 2022). Timing of variant classification was based on Centers for Disease Control and Prevention data for our region and confirmed based on a subset of pregnant patients who underwent viral genome sequencing at our institution.2 Outcomes included disease severity, transfer rates, symptoms, intensive care unit (ICU) admission, death, need for COVID-19 treatment, intubation, extracorporeal membrane oxygenation, venous thromboembolism, admission indication, cesarean delivery for worsening maternal status, delivery complications (preeclampsia, abruption, postpartum hemorrhage, transfusion), stillbirth, preterm delivery, positive neonatal test result for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and neonatal intensive care unit admission. A subanalysis of outcomes based on vaccination status was planned. Outcomes of patients in the Omicron group were compared with outcomes of those in the pre-Delta and Delta groups using appropriate statistical tests.
RESULTS
We noted a peak of pregnant patients with SARS-CoV-2 infection in January 2022, when the Omicron variant was predominant, which was consistent with national data (Fig. 1). Overall, 49.4% and 53.3% of cases were diagnosed on routine preprocedure screening (ie, asymptomatic) during the pre-Delta and Omicron waves, respectively; only 15.2% of the Delta variant cases were diagnosed on routine screening (ie, the remainder were symptomatic). Transfers of patients with COVID-19 to our institution for escalated care accounted for 2.2% for Omicron cases, 5.3% for pre-Delta cases, and 10.8% for Delta cases (Table 1). Severe–critical disease was significantly different among waves and occurred in 1.8% of patients during the Omicron wave compared with 13.3% during the pre-Delta wave and 24.1% during the Delta wave. Rates of ICU admission were significantly different as well—1.3% during the Omicron wave, 8.4% during the pre-Delta wave, and 17.7% during the Delta wave (Table 1). Compared with both the pre-Delta and Delta waves, patients with the Omicron variant were less likely to undergo pharmacologic treatment, respiratory support, or intubation or to develop venous thromboembolism. Compared with patients with the Delta variant, patients with the Omicron variant were also less likely to receive extracorporeal membrane oxygenation, undergo cesarean delivery for worsening maternal status, deliver preterm, or have their newborn admitted to the neonatal intensive care unit. There were no significant differences in preeclampsia, postpartum hemorrhage, transfusion, abruption, stillbirth, or neonatal COVID-19 positivity by variant status (Table 1).
Fig. 1.
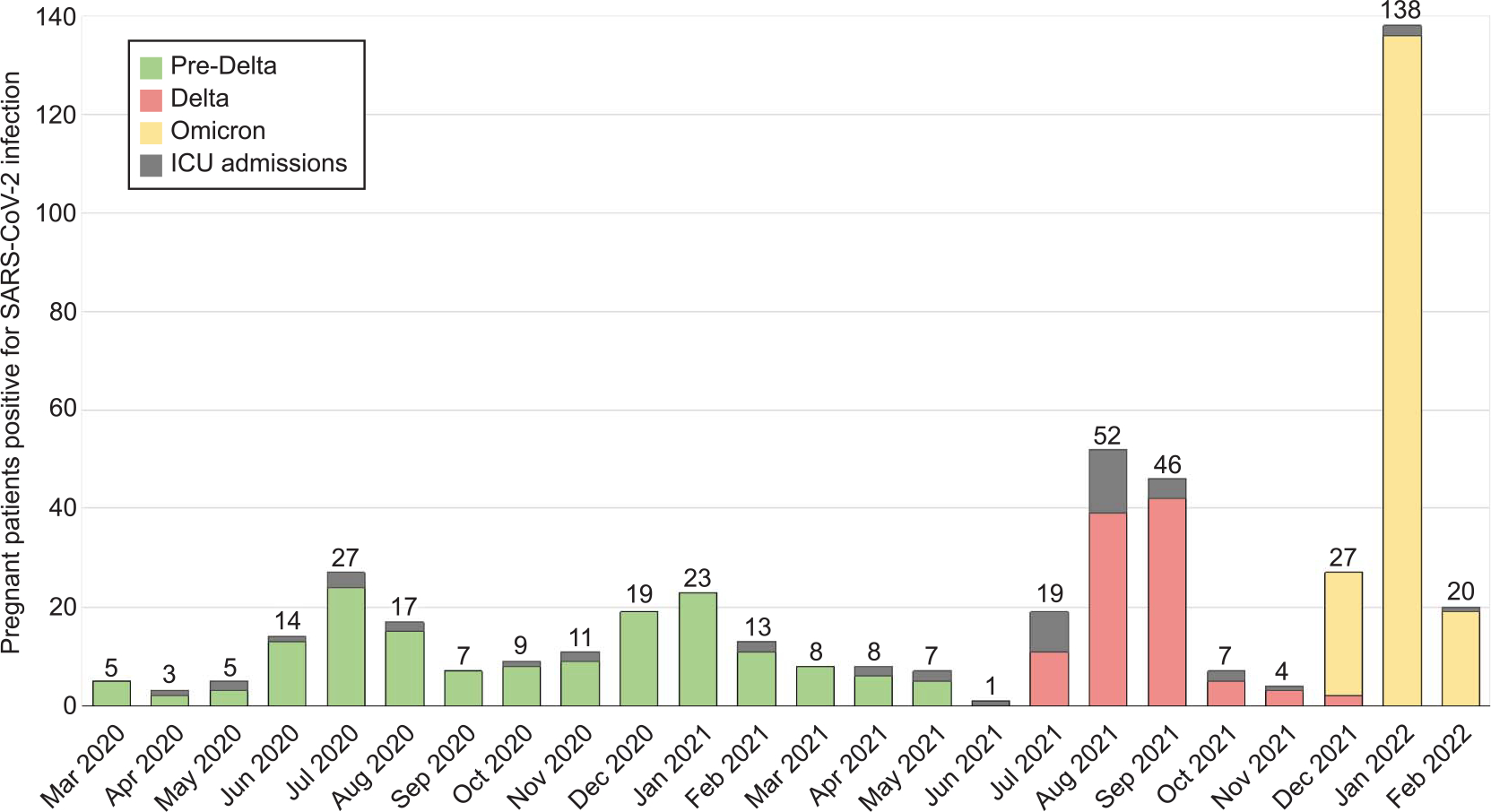
Hospital trends in relationship to the three predominant variants among pregnant patients positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). ICU, intensive care unit.
Seasely. Outcomes Associated With the Omicron Variant of SARS-CoV-2. Obstet Gynecol 2022.
Table 1.
Illness Severity and Perinatal Outcomes of Patients Who Required Delivery During Their Admission With a Positive Test Result for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), According to Variant Type
| Pre-Delta (n=225) | P (Omicron vs Pre-Delta) | Delta (n=158) | P (Omicron vs Delta) | Omicron (n=224) | |
|---|---|---|---|---|---|
|
| |||||
| Disease severity* | <.001 | <.001 | |||
| Asymptomatic | 103 (45.8) | .61 | 31 (19.6) | <.001 | 108 (48.2) |
| Mild–moderate | 92 (40.9) | .05 | 89 (56.3) | .22 | 112 (50.0) |
| Severe–critical | 30 (13.3) | <.001 | 38 (24.1) | <.001 | 4 (1.8) |
| ICU admission | 19 (8.4) | <.001 | 28 (17.7) | <.001 | 3 (1.3) |
| Respiratory support† | 30 (13.4) | <.001 | 42 (26.6) | <.001 | 5 (2.2) |
| Intubation | 13 (5.8) | .001 | 21 (13.3) | <.001 | 1 (0.5) |
| ECMO | 4 (1.8) | .12 | 4 (2.5) | .029 | 0 (0.0) |
| VTE | 6 (2.7) | .030 | 7 (4.4) | .002 | 0 (0.0) |
| Received pharmacologic treatment‡ | 36 (16.0) | <.001 | 53 (33.5) | <.001 | 6 (2.7) |
| Maternal death | 1 (0.4) | >.99 | 3 (1.9) | .07 | 0 (0.0) |
| Transfer rate | 12 (5.3) | .09 | 17 (10.8) | <.001 | 5 (2.2) |
| Received COVID-19 vaccine | 0 (0.0) | <.001 | 6 (3.8) | <.001 | 61 (27.2) |
| Indication for hospital presentation | .001 | <.001 | |||
| Symptomatic COVID-19 or complication from COVID-19 | 57 (31.3) | 87 (64.4) | 81 (41.1) | ||
| Labor or PROM | 61 (33.5) | 18 (13.3) | 34 (17.3) | ||
| Planned procedure or surgery | 28 (15.4) | 8 (5.9) | 25 (12.7) | ||
| Other medical indication | 36 (19.8) | 22 (16.3) | 57 (28.9) | ||
| COVID-19 symptoms | 81 (40.9) | .05 | 111 (74.5) | <.001 | 113 (50.5) |
| Fever, chills, or both | 41 (20.7) | <.001 | 44 (29.5) | <.001 | 19 (8.5) |
| Cough | 49 (24.8) | .019 | 80 (53.7) | <.001 | 35 (15.6) |
| Shortness of breath | 37 (18.7) | .003 | 49 (32.9) | <.001 | 20 (8.9) |
| Fatigue | 8 (4.0) | .67 | 21 (14.1) | .002 | 11 (4.9) |
| Body aches | 19 (9.6) | .94 | 28 (18.8) | .008 | 21 (9.4) |
| Headache | 16 (8.1) | .44 | 23 (15.4) | .14 | 23 (10.3) |
| Loss of taste, smell, or both | 17 (8.6) | <.001 | 17 (11.4) | <.001 | 1 (0.5) |
| Sore throat | 4 (2.0) | .34 | 9 (6.0) | .26 | 8 (3.6) |
| Congestion | 11 (5.6) | .08 | 35 (23.5) | <.001 | 23 (10.3) |
| Nausea, vomiting, diarrhea | 17 (8.6) | .56 | 34 (10.3) | .001 | 23 (10.3) |
| Patients who required delivery | (n=96) | (n=57) | (n=77) | ||
| Gestational age at delivery (wk) | 35.6±5.6 | .29 | 33.6±5.6 | .002 | 36.5±4.9 |
| Cesarean birth | 28 (31.5) | .75 | 28 (49.1) | .07 | 26 (33.8) |
| Indication for cesarean: worsening maternal status | 4 (14.3) | .35 | 14 (50.0) | <.001 | 1 (3.9) |
| Preterm birth (37 wk) | 30 (31.3) | .18 | 34 (59.7) | <.001 | 17 (22.1) |
| NICU admission | 37 (44.1) | .19 | 31 (58.5) | .006 | 25 (33.8) |
| Delivery complications | |||||
| Preeclampsia | 22 (22.9) | .79 | 13 (23.6) | .89 | 19 (24.7) |
| Abruption | 2 (2.1) | .50 | 1 (1.8) | .42 | 0 (0.0) |
| PPH | 10 (10.4) | .21 | 7 (12.7) | .20 | 4 (5.2) |
| Transfusion | 10 (10.4) | .55 | 10 (18.2) | .07 | 6 (7.8) |
| Stillbirth | 5 (5.2) | .46 | 2 (3.5) | >.99 | 2 (2.6) |
| Positive neonatal SARS-CoV-2 test result§ | 0 (0.0) | — | 1 (2.0) | .40 | 0 (0.0) |
ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation; VTE, venous thromboembolism; COVID-19, coronavirus disease 2019; PROM, prelabor rupture of membranes; NICU, neonatal intensive care unit; PPH, postpartum hemorrhage; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data are n (%) or mean±SD unless otherwise specified.
Disease severity based on National Institutes of Health guidelines and categorized by Metz et al.3
Respiratory support included any form of supplemental oxygen (nasal cannula, high-flow, bilevel positive airway pressure, and intubation).
Treatment includes azithromycin, cephalosporins, remdesivir, dexamethasone or convalescent plasma.
Positive neonatal SARS-CoV-2 test result within 48 hours of delivery.
Only 27.2% of patients who tested positive for SARS-CoV-2 infection during the Omicron time-frame were vaccinated. Of the 61 vaccinated patients, 60.7% had mild or moderate disease, and no patients who were vaccinated and positive for the Omicron variant were admitted to the ICU for COVID-19. When considering only unvaccinated patients in the Omicron wave compared with patients in the Delta and pre-Delta waves, findings were consistent with the overall analysis (Appendix 1, available online at http://links.lww.com/AOG/C757).
DISCUSSION
During the Omicron wave of SARS-CoV-2, although the prevalence of SARS-CoV-2 positivity in patients was double that during other waves, there were fewer cases of severe–critical disease and adverse perinatal outcomes compared with the pre-Delta and Delta waves. The increased rate of positive test results obtained on routine screening and the lower transfer rate to a tertiary care center seen with the Omicron variant compared with the Delta variant suggests increased transmissibility and decreased severity of this variant. Limitations of our study include that we did not genotype all patients and were unable to capture data for patients who may have received testing outside of our hospital system, which could bias our results to underreporting in the Omicron surge. Current estimates at our Alabama institution are that 39% of our pregnant patients are vaccinated. Given that vaccines are effective at preventing critical illness, we continue to emphasize the importance of vaccinating all pregnant patients to mitigate severe perinatal morbidity and mortality due to unknown risks of adverse outcomes associated with new and future viral variants.
Supplementary Material
Footnotes
Financial Disclosure
Rachel G. Sinkey reports money was paid to her institution from GestVision, NIH, and ProLab. Alan T. Tita reports money was paid to his institution from the NIH, CDC, and Pfizer for COVID-19 and RSV vaccine in pregnancy studies. Ashley N. Battarbee reports money was paid to her institution from the NIH and CDC for COVID-19 in pregnancy studies. Akila Subramaniam reports money was paid to her institution from the NIH. Jeff M. Szychowski reports money was paid to his institution from the NIH. Jodie Dionne-Odom currently receives grant funding from NIH/NICHD for an unrelated study on infection in pregnancy. Sixto M. Leal Jr. reports money was paid to his institution from the NIH, CDC, ADPH, CNINE, GenMark, mFluiDx, IMMY, SpeeDx, Abnova, Amplyx, IHMA, and JMI. Nitin Arora reports money was paid to his institution from the Congenital and Perinatal Infections Consortium, the UAB Sparkman Center for Global Health, and the Kaul Pediatric Research Institute Grant Program. Alan T. Tita reports money was paid to his institution from Pfizer. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
REFERENCES
- 1.Seasely AR, Blanchard CT, Arora N, Battarbee AN, Casey BM, Dionne-Odom J, et al. Maternal and perinatal outcomes associated with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Delta (B.1.617.2) variant. Obstet Gynecol 2021;138:842–4. doi: 10.1097/AOG.0000000000004607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. COVID data tracker: variant proportions. Accessed March 24, 2022. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
- 3.Metz TD, Clifton RG, Hughes BL, Sandoval G, Saade GR, Grobman WA, et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19). Obstet Gynecol 2021;137:571–80. doi: 10.1097/AOG.0000000000004339 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


