Abstract
Background
In clinical settings all across the world, including Ethiopia, the irrational use of antibiotics to treat acute diarrhea is common. The main causes of resistance are antibiotic abuse, misuse, and underuse, and among infectious diseases, antibiotic overuse is pervasive in diarrheal infections around the world. As a result, the primary goal of this study was to evaluate antibiotic use patterns for the treatment of acute diarrheal diseases at Debre Tabor Comprehensive Specialized Hospital in North-West Ethiopia.
Methodology
A retrospective cross-sectional study was conducted to assess the antibiotic utilization pattern used to treat acute diarrheal illness, taken from the record cards of 243 patients who received treatment for acute diarrheal illness, employing structured questions from September 1 to September 30, 2022. The results are displayed using percentages and frequency distributions in tables along with figures.
Results
A total of 243 patients were involved, 134 (55.1%) of whom were male and 134 (55.1%) were under the age of five. Out of the 103 cases of acute watery diarrhea, 83 received antibiotics that were given improperly. Additionally, 88 individuals had bloody diarrhea diagnoses, but 58 of them received the currently administered antibiotics. Amoxicillin and co-trimoxazole were the most frequently prescribed medications, with 193 (79.5%) of the 243 cases of patients receiving some form of antibiotic.
Conclusion
The results of the study revealed that there is inappropriate antibiotic use in acute diarrheal disease at Debre Tabor Comprehensive Specialized Hospital, which might be the cause for the overall increase in antimicrobial resistance as well as the associated costs of treatment. The finding is helpful as evidence for prescribers' inappropriate use of antibiotics for the treatment of acute diarrheal sickness.
Keywords: Acute diarrheal disease, Antibiotics utilization, Antibiotics resistance
1. Introduction
1.1. Background
Acute diarrhea is defined as the passage of three or more watery stools per day that lasts up to two weeks. Water sources that have been contaminated, bacterial infections, malnourishment, poor personal hygiene, and poor living conditions all contribute to acute diarrhea in children under the age of five. Acute diarrhea is the second leading cause of death in children under the age of five, particularly in developing nations, even though the cause is easily preventable and treatable [1].
Antibiotics are the medications that are used and abused the most [2]. Reducing improper antibiotic use is regarded to be the most effective method for managing resistance. Overprescribing persists despite rising public knowledge of the harmful effects of antibiotic overuse, in part because of patient demand, professional time restraints, and ambiguous diagnoses [3].
Abuse of antibiotics is pervasive and frequently excessive and unreasonable in all countries. This is particularly problematic in developing countries where the prevalence of infectious diseases is high. Therefore, efficient methods should be developed to guarantee proper antimicrobial medication use [4]. Increased antibiotic use in hospitals is frequently associated with the development of resistance [[5], [6], [7]]. Antibiotic usage patterns have a major impact on the rates of resistance shown in multidrug-resistant nosocomial infections [5,8]. The rising resistance has increased the cost of treatment and increased morbidity and mortality in many individuals [6]. Inappropriate medication use frequently has negative outcomes, such as treatment failure, disease transmission, an increase in antibiotic resistance, and high healthcare costs [5].
Misuse of antibiotics is typical of infectious disorders that cause diarrhea all over the world. When symptoms of diarrhea are new and have not been present for more than 14 days, the condition is considered “acute.” A diarrheal disease called dysentery causes the passing of bloody stools. Enteric bacteria and parasites are more common than viruses in developing nations, and they usually reach their peak during the summer. Viruses typically cause acute diarrhea especially during the winter, in both industrialized and developing nations [9]. These infections exist in a mild and self-limiting fashion and major approaches for treatments are symptomatic treatment such as rehydration of dehydrated patients [10].
There is a high rate of antimicrobial resistance among community pathogens and an estimated 20%–50% of all antibiotic use is inappropriate [11]. As a result, there are more side effects, higher costs, and higher rates of antimicrobial resistance. Antibiotics are most frequently misused for viral and self-limiting illnesses in severe diarrhea. 70% to t of all diarrheal episodes are caused by viral infections, like the rotavirus [12]. Due to the self-limiting nature of the disease and the difficulty and delay in identifying the pathogen, routine use of antimicrobials is not advised [13] in the majority of instances of acute diarrhea.
The joint statement by the World Health Organization (WHO) and United Nations International Children's Emergency Fund (UNICEF) in 2004 recommended the use of low-osmolality ORS along with zinc for the treatment of acute diarrhea in children [14]. Antibiotics are recommended only for acute bloody diarrhea or dysentery. Unfortunately, reports from different parts of the world reported that misuse of antibiotics was common in the treatment of diarrhea [15].
Acute diarrhea is one of the main causes of morbidity in children under the age of five in Ethiopia. The most recent EDHS 2016 data also revealed a 12% nationwide prevalence of acute diarrhea [16] since antibiotics are the most often given medications, their overuse and abuse is a widespread issue. It has been observed that over half of a hospital's overall drug budget in underdeveloped nations is spent on antibiotics. In recent years, there have been numerous reports of antimicrobial drug misuse, and it has been discovered that about half of all prescriptions for antibiotics were poorly chosen [17].
Understanding the extent and pattern of antimicrobial use for acute diarrhea in the community is important for defining a regional intervention program to promote the rational use of antimicrobials, limit the spread of AMR, and reduce the cost of therapy for acute diarrhea. As a result, this study was carried out at the Debre Tabor Comprehensive Specialized Hospital to collect data on the pattern of antibiotic use for the treatment of acute diarrheal diseases.
2. Methods and materials
2.1. Study area and design
The study was conducted at Debre Tabor Comprehensive Specialized Hospital, which is found in the south Gondar zone, Amhara regional state. It is the only specialized hospital in South Gondar Zone, Amhara Region, and North West Ethiopia, and it is specifically found in Debre Tabor Town, which is the capital city of the South Gondar Zone. It is 100 km from Bahir Dar, the capital city of the Amhara regional state, and 666 km from Addis Ababa, which is the capital city of Ethiopia. A cross-sectional retrospective study was carried out on 243 medical cards of patients who were treated for acute diarrheal disease from September 2021 to August 2022.
2.2. Populations
2.2.1. Source and study populations
Selected Patients' charts who were diagnosed and treated for acute diarrheal disease at Debre Tabor Comprehensive Specialized Hospital during the study period were the study populations, and all patient records at Debre Tabor Comprehensive Specialized Hospital were the source populations.
2.2.2. Inclusion and exclusion
All patients who are diagnosed with and recorded as having acute diarrheal disease at Debre Tabor Comprehensive Specialized Hospital were included in the study, and patient charts that are not readable, illegible, or do not contain clear information were excluded from the study.
2.3. Sample size determination and sampling procedures
2.3.1. Sample size determination
The sample size was calculated using a single population proportion formula.
| n = z2p (1 − p)∕d2 |
where n = sample size, Z α/2 = critical value = 1.96, P = estimated performance of population (50%), and d = marginal error = 0.05.
| Then, n = (1.962) × (0.5) (0.5)/(0.05)2 = 384 |
When N < 10,000 sample sizes are adjusted by:
| nf = n ÷ (1 + (n ÷ N)) = nf = 384 ÷ (1 + (384 ÷ 521)) = 221 |
where nf = adjusted sample size, n = the calculated sample size, and N = total patient cards with a diagnosis of acute diarrhea within one year.
The final sample size was 221. By Adding a 10% nonresponse rate, the total number of samples selected was 243.
2.3.2. Sampling technique and procedures
A systematic random sampling technique was followed for study subject identification. Every patient card number diagnosed with acute diarrhea documented in outpatient department (OPD) registered book was counted and recorded first, and then every kth (k = 2) card was selected. The first patient chart was selected using the lottery method from the first up to the second patient chart starting from the time order of the records.
2.4. Data collection tools and procedures
Data were collected using a structured questionnaire, which was prepared from different published literature [[18], [19], [20], [21]] and was initially written in English, then translated into Amharic, and again retranslated back to English to check for any inconsistencies or distortions in the meaning of terms and concepts. Five well-trained pharmacy students using the patient chart, laboratory results, and prescriptions on the chart and the prescriber profile collected the data on the evaluation of antibiotic utilization patterns in the treatment of acute diarrheal diseases retrospectively. The data was recorded in a structured questionnaire.
2.5. Data quality control
The collected data were daily checked for completeness, accuracy, clarity, and consistency by the principal investigator before being entered into SPSS. The pre-test was done to check the feasibility of the study by using SPSS. The findings were presented using frequency distributions and percentages.
2.6. Statistical analysis
The collected data were first checked for completeness, edited, cleared, and entered using SPSS version 25.0. Data analysis was done using the same software, SPSS. Finally, the data were presented in frequency and percentage in tables and figures.
2.7. Operational definition
Antibiotics: A substance that destroys or inhibits the growth of other microorganisms, usually bacteria, and is used in the treatment of infections. That includes penicillin, cephalosporin, and so on, including metronidazole [3,4].
Acute diarrhea: The evacuation of loose stools at least three times a day when symptoms are new and have been present for usually less than seven days but not more than 14 days [1,9].
Chronic diarrhea: Implies an increased frequency of passing looser stools for more than a month [1].
Invasive bacterial diarrhea: Diarrhea caused by bacteria that directly damage gastrointestinal tissue, often characterized by fever, dysentery, and fecal leucocytes [1].
Inappropriate antibiotic use: The inappropriate use of antimicrobials was defined as prescribing antimicrobials for watery, non-bloody diarrhea or not prescribing antimicrobials for an invasive bacterial-type or bloody diarrhea [5,9].
3. Results
3.1. Socio-demographic characteristics of the respondents
There were 521 patient records diagnosed with acute diarrheal diseases within one year (from September 2021 to August 2022). A total of 243 patients' records were selected and studied from September 1 to September 30, 2022. Among the 243 patients, 134 (55.1%) were males and 109 (44.9%) were females. Under five years old, children were around 55.1% and elders were 6.6%, as shown in Table 1.
Table 1.
Socio-demographic characteristics of acute diarrhea patients at Debre Tabor Comprehensive Specialized Hospital, Debre Tabor town, from September 2021 to August 2022.
| Number | Percent (%) | ||
|---|---|---|---|
| Sex of the patient | Male | 134 | 55.1 |
| Female | 109 | 44.9 | |
| Age of the patient | ≤5 years | 134 | 55.1 |
| 6–12 years | 32 | 13.2 | |
| 13–65 years | 61 | 25.1 | |
| >65 years | 16 | 6.6 | |
| Total | 243 | 100.0 | |
3.2. Clinical characteristics
More than half of the patients claimed to have experienced diarrheal symptoms such as fever 100 (41.2%), vomiting 56 (23.0%), and coughing 24 (9.9%) as shown in Fig. 1. Concerning the degree of dehydration, 212 (87.2%) patients had mild to moderate dehydration. Only 31 (12.8%) had severe dehydration, which required intravenous fluid therapy (IV). The diagnosis was dysentery diarrhea in 124 (51.0%) cases, while 119 (49.0%) cases were non-dysentery diarrhea.
Fig. 1.
Number of associated symptoms with diarrhea at Debre Tabor Comprehensive Specialized Hospital, Debre Tabor town, from September 2021 to August 2022.
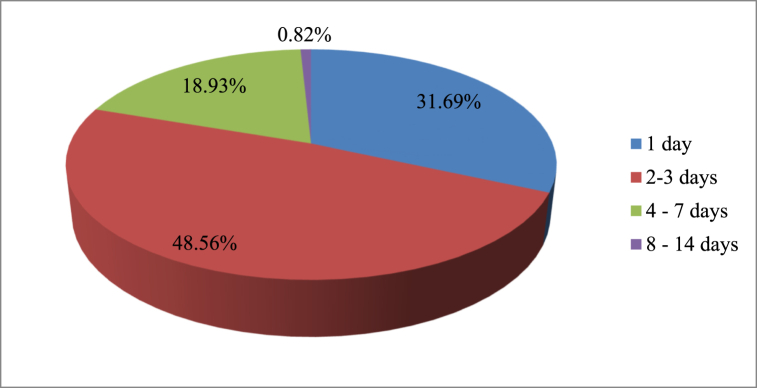
A review of the history of the cases shows that out of 243 patients' cases diagnosed with acute diarrhea, 118 (48.56%) had experienced the illness for 2–3 days, while a small number of the patients (0.82%) had experienced the illness for 8–14 days, as illustrated in Fig. 2.
Fig. 2.
Duration of diarrhea from onset to treatment for patients diagnosed with acute diarrhea at Debre Tabor Comprehensive Specialized Hospital, Debre Tabor town from September 2021 to August 2022.
3.3. Stool characteristics
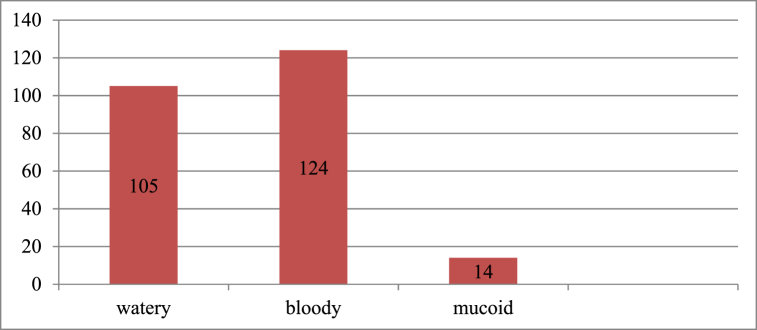
Regarding stool characteristics, all of the patients had a stool examination ordered. Around 119 (49.0%) of the patients had non-bloody diarrhea (watery and mucoid), whereas 124 (51.0%) of the cases were with an invasive bacterial infection type, as seen in Fig. 3.
Fig. 3.
Stool characteristics for acute diarrhea patients at Debre Tabor Comprehensive Specialized Hospital, Debre Tabor town from September 2021 to August 2022.
3.4. Treatment patterns of acute diarrheal diseases
The number of antibiotics administered to single individuals ranged from zero to two, based on the patient's records. As shown in Fig. 4, 50 (20.6%) of the cases during the diarrhea episode received no antibiotic medication, while 193 (79.4%) of the cases received at least one antibiotic medication.
Fig. 4.
Number of antibiotics prescribed for each diarrheal patient in Debre Tabor Comprehensive Specialized Hospital, Debre Tabor town from September 2021 to August 2022.
As seen in Table 2, the patients are given six different types of antimicrobials. Amoxicillin was the most commonly prescribed drug, accounting for 18.5% of all prescriptions, followed by Cotrimoxazole (17.7%), Ciprofloxacin, and metronidazole, which account for the same amount (8.6%), and among combined prescriptions of amoxicillin and Cotrimoxazole, which accounts for 9.5% of all prescriptions.
Table 2.
Antibiotics were prescribed for acute diarrhea patients at Debre Tabor Comprehensive Specialized Hospital, Debre Tabor town from September 2021 to August 2022.
| Antibiotics prescribed for the patients | Number | Percent (%) |
|---|---|---|
| Yes | 193 | 79.4 |
| No | 50 | 20.6 |
| Types of antibiotics prescribed | ||
| Amoxicillin | 45 | 18.5 |
| Ciprofloxacin | 21 | 8.6 |
| Cotrimoxazole | 43 | 17.7 |
| Metronidazole | 21 | 8.6 |
| Cephalexin | 7 | 2.9 |
| Ceftriaxone | 1 | 0.4 |
| Amoxicillin + Ciprofloxacin | 13 | 5.4 |
| Amoxicillin + Cotrimoxazole | 23 | 9.5 |
| Cotrimoxazole + Metronidazole | 4 | 1.6 |
| Amoxicillin + Metronidazole | 15 | 6.2 |
| Total | 193 | 100 |
Of the total, 123 had diarrhea that was watery in type, 113 had improperly administered antibiotics, and 52 of these were children under the age of five. In addition, 24 patients had mucoid diarrhea, and five of them received antibiotic treatment, whereas 96 patients had a bloody form of diarrhea, and thirty of them did not receive antibiotic treatment, as indicated in Table 3.
Table 3.
Antibiotics usage by age groups and stool characteristics for acute diarrheal patients at Debre Tabor Comprehensive Specialized Hospital, Debre Tabor town from September 2021 to August 2022.
| Age groups |
Stool Type and Antibiotics Prescription Pattern |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Watery diarrhea | Antibiotics Prescribed |
Mucoid diarrhea | Antibiotics Prescribed |
Bloody Diarrhea | Antibiotics Prescribed |
Total Antibiotics Prescribed |
|||||
| Yes | No | Yes | No | Yes | No | Yes | No | ||||
| ≤ 5 years | 40 | 35 | 5 | 21 | 1 | 20 | 41 | 35 | 6 | 71 | 31 |
| 6–12 years | 27 | 21 | 6 | 14 | 2 | 12 | 25 | 10 | 15 | 33 | 33 |
| 13–65 years | 24 | 17 | 4 | 10 | 0 | 10 | 12 | 8 | 4 | 25 | 18 |
| >65 years | 22 | 10 | 5 | 7 | 0 | 7 | 10 | 5 | 5 | 15 | 17 |
| Total | 103 | 83 | 20 | 52 | 3 | 49 | 88 | 58 | 30 | 144 | 99 |
3.5. Adherence to standard treatment guidelines
According to treatment guidelines, 162 (66.7%) cases were inappropriately managed, while 81 (33.3%) cases were appropriately treated (see Table 4). Forty-nine cases of bloody diarrhea were not treated with antibiotics.
Table 4.
Antibiotic usage in 243 cases of acute diarrheal diseases at Debre Tabor Comprehensive Specialized Hospital, Debre Tabor town from September 2021 to August 2022.
| Antimicrobial usage | Number | Percent (%) |
|---|---|---|
| Appropriate | 81 | 33.3% |
| Given antimicrobial for bloody diarrhea | 61 | 75.3% |
| Not given antimicrobial for non-bloody diarrhea | 10 | 12.3% |
| Inappropriate | 162 | 66.7% |
| Not given antimicrobial for bloody diarrhea | 79 | 48.8% |
| Given antimicrobial for non-bloody diarrhea | 83 | 51.2% |
3.6. Prescriber profile on acute diarrhea
Physicians were evaluated for their treatment of acute diarrheal patients. An intern prescriber had written prescriptions for about 127 of the 243 patient records. The proportion of proper antibiotic usage among general practitioners (the prescribing profession) was slightly greater than that of the internship, as shown in Table 5.
Table 5.
Antibiotic usage in 243 cases of acute diarrheal diseases with health professionals prescribed at Debre Tabor Comprehensive Specialized Hospital, Debre Tabor town from September 2021 to August 2022.
| Profession of prescriber | Profession of prescriber |
Antibiotic usage |
||||
|---|---|---|---|---|---|---|
| Number | percent | Appropriate |
Inappropriate |
|||
| Number | Percent | number | Percent (%) | |||
| Physician (General Practitioners) | 116 | 47.7% | 85 | 73.3% | 31 | 26.7% |
| Internship | 127 | 52.3% | 87 | 68.5% | 40 | 31.5% |
| Total | 243 | 100% | 172 | 71 | ||
4. Discussion
This study looked at how frequently antibiotics were used overall at Debre Tabor Comprehensive Specialized Hospital to treat acute diarrheal illnesses. It was found that there was evidence of unreasonable antibiotic use in both child and adult patients. Both the World Health Organization's guidelines and Ethiopia's Standard Treatment Guideline advise against using antibiotics regularly. This is because acute diarrhea is often brought on by viruses rather than bacteria. An assessment of their medical histories revealed that the majority of the patients, on average, were unwell for two to three days. Since most illnesses are viral in origin, taking antibiotics won't speed up the disease's progression. However, some of the patients may have taken antibiotics for acute diarrhea when they weren't essential due to the most frequent concomitant symptoms—fever, vomiting, and stomach cramps [10].
The study found that 66.7% of those with severe diarrhea received inappropriate antibiotics, which is greater than the rates found in studies from Thailand, China, and Ujjain, India, where the comparable values were 45.1%, 60.8%, and 71%, respectively [18,22,23]. This difference might be that medical staff in Thailand, China, and India may possess greater knowledge of and adherence to accepted treatment protocols than those at Debre Tabor Comprehensive Specialized Hospital because these countries' healthcare environments are frequently superior to those in Ethiopia.
In this study, 103 patients (42.4%) got ineffective antibiotic therapy, even though 83 (80.6%) of the patients had watery diarrhea, a self-limited form of diarrhea that does not require antibiotic therapy. Inappropriate antibiotic use can lead to resistance, the harmful eradication of healthy flora, protracted disease (like a Salmonella superinfection), protracted carrier status (like delayed Salmonella excretion), the induction of Shiga toxins (from, for example, Shiga toxin-producing E. coli), and an elevated risk of infection costs [24]. This number is lower than that of a study carried out at Chefa-Robit Health Center in Kemissie, North East Amhara, Ethiopia, which found that almost 132 (77% of them) received improper antibiotic treatment, even though 172 (75% of them) had watery diarrhea, a self-limited form of diarrhea that does not require antibiotic therapy [19]. The discrepancy may be due to varying sample sizes, study settings, prescribers' differences, and conditions of population involvement.
On the other hand, 140 (57.6%) of the cases had clinical signs of invasive bacterial type (mucoid and bloody) that necessitated the use of antibiotics; around 61 (75.3%) of them were treated with antibiotics, which was found to be greater than a study carried out in Chefa-Robit Health Center, Kemissie, North East Amhara, Ethiopia, and in Bishoftu general hospital, Ethiopia, where only 25% and 28%, respectively, of the antibiotics were appropriately prescribed [19,20].
The results of the study showed that children under the age of five were most commonly affected by acute diarrheal sickness (approximately 42% of this age group) and that this age group also received the majority of antibiotic recommendations (about 69.6%). The study's findings were nearly identical to those of another study carried out in Ethiopia's Chefa-Robit Health Center, Kemissie, where only 52% of children under the age of five were commonly affected by acute diarrhea, and 76% of the antibiotics were prescribed [19]. This outcome is higher in studies carried out in Puducherry, India, and Delhi, India where 22% and 64% of patients, respectively, received antibiotic prescriptions [21,25]. Given that community in India are comparatively more developed than communities in Ethiopia, particularly in Debre Tabor and the surrounding area, this may be because health professionals in the comprehensive specialized hospital in Debre Tabor may be more compelled by the expectations of the children's parents than parents in the other countries mentioned.
Antibiotics are still administered carelessly in these age groups, however, and careful attention must be paid to their administration [26]. Despite evidence and advice that zinc supplements and oral rehydration salts are crucial for treating, lessening the severity of, and preventing acute diarrheal disease in children, particularly in developing countries, they are not always administered.
In this study, cotrimoxazole (17.7%) and amoxicillin (18.5%) were the two most commonly given medications for the management of acute diarrheal illness. However, research conducted in New Delhi, India, revealed that norfloxacin was more frequently prescribed [27]. A comparable study conducted in Thailand [28] found that Cotrimoxazole was the most often prescribed drug (51%), followed by Norfloxacin (11%), and Nalidixic Acid (0.5%). These differences could be explained by the fact that different countries have different access to drugs, treatment protocols, and techniques for selecting medications.
The study, therefore, discovered a high proportion of inappropriate antibiotic use, which may be attributed to an increase in antimicrobial resistance and its associated costs on a national and international level. The types of antibiotics used in the treatment of acute diarrheal illness were also discovered, as were the prescribing habits of physicians and the incidence of antibiotic use.
5. Limitations of the study
The limitation of the study is that some patients' cards were not fully documented for all information, and no stool culture was done to distinguish between different bacterial specimens. Additionally, because the study was conducted at a single institution, it can be difficult to determine the generalizability of research results.
6. Conclusion
The study's findings showed that Debre Tabor Comprehensive Specialized Hospital continues to utilize antibiotics in the treatment of acute diarrheal sickness inappropriately. Amoxicillin, co-trimoxazole, and ciprofloxacin were the most frequently recommended antibiotics. The majority of incorrect antibiotic prescriptions were made for children under the age of five, who were the age group most commonly impacted by acute diarrheal illness. The finding is helpful as evidence for prescribers' inappropriate use of antibiotics for the treatment of acute diarrheal sickness. Thus, inappropriate use of antibiotics may cause antimicrobial resistance.
Author contribution statement
Getu Tesfaw Addis: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Samuel Berihun Dagnew, Alemnew Anagaw: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper. Teklie Mengie Ayele: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Tesfaye Yimer Tadesse: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data included in article/supp. material/referenced in article.
Funding source
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval statement
Ethical approval for this study was obtained from the ethical review committee of the Debre Tabor University community-based education coordination office with the reference number DTU 1634/2022 and verbal informed consent was obtained from each subject for prospective data collection. Then, a permission letter to conduct the study was obtained from the medical director of Debre Tabor Comprehensive Specialized Hospital with reference number DTH2265/2022.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to express our deepest gratitude to the medical director of Debre Tabor comprehensive specialized hospital for permitting us to collect data and to the nurses for their guidance during data collection.
We also like to acknowledge Debre Tabor University’s community-based education coordination office for giving an ethical approval letter.
Abbreviations/Acronyms
- AMR
Antimicrobial Resistance
- CBE
Community-Based Education
- DTCSH
Debre Tabor Comprehensive Specialized Hospital
- EDHS
Ethiopian Demographic Health Survey
- ORS
Oral Rehydration Solutions
- SFDA
State Food and Drug Administration
- SPSS
Statistical Package for Social Science
- UNICEF
United Nations International Children Emergency Fund
- WHO
World Health Organization
References
- 1.Sanyaolu A.O., Okorie C., Marinkovic A., Jaferi U., Prakash S., Jan A., et al. Global epidemiology and management of acute diarrhea in children from developing countries. Ann. Pediatr. Child Health. 2020 [Google Scholar]
- 2.Bittner C.B., Plach M., Steindl H., Abramov-Sommariva D., Abels C., Kostev K. Prevalence of antibiotic prescription in patients with acute rhinosinusitis treated by general practitioners and otolaryngologists in Germany—a retrospective cohort study. Antibiotics. 2022;11(11):1576. doi: 10.3390/antibiotics11111576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kafle K.K., Karkee S.B., Shrestha N., Prasad R.R., Bhuju G.B., Das P.L., et al. Community intervention to improve knowledge and practice on commonly used drugs. Kathmandu Univ. Med. J. 2010 Jan-Mar;8(29):29–34. doi: 10.3126/kumj.v8i1.3218. [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie A.R., Robertson L., Jappy B., Laing R.B.S., Gould I.M. Audit of antibiotic policy and antimicrobial investigations for treating bactericidal in large teaching hospital. Int. J. Antimicrob. Agents. 2003 Dec;22(6):618–621. doi: 10.1016/j.ijantimicag.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Isturiz R.E., Carbon C. Antibiotic use in developing countries. Infect. Control Hosp. Epidemiol. 2000 Jun;21(6):394–397. doi: 10.1086/501780. [DOI] [PubMed] [Google Scholar]
- 6.Yates R.R. New intervention strategies for reducing antibiotic resistance. Chest. 1999 Mar;115(3 Suppl):24S–27S. doi: 10.1378/chest.115.suppl_1.24s. [DOI] [PubMed] [Google Scholar]
- 7.Patterson J.E. Antibiotic utilization: is there an effect on antimicrobial resistance? Chest. 2001 Feb;119(2 Suppl):426S–430S. doi: 10.1378/chest.119.2_suppl.426s. [DOI] [PubMed] [Google Scholar]
- 8.Coignard B., Siegel J.D., Weinstein R.A., Sohn A.H., Sinkowitz-Cochran R.L., Jarvis W.R. How should we control antimicrobial use? Current practices and controversies. Infect. Control Hosp. Epidemiol. 2000 Dec;21(12):792–795. doi: 10.1086/501733. [DOI] [PubMed] [Google Scholar]
- 9.World Gastroenterology Organization global guideline . World Gastroenterology Organization; February 2012. Acute Diarrhea in Adults and Children a Global Perspective.www.wgo.org [Google Scholar]
- 10.Willyard C. The drug-resistant bacteria that pose the greatest health threats. Nat. News. 2017;543(7643):15. doi: 10.1038/nature.2017.21550. [DOI] [PubMed] [Google Scholar]
- 11.Stainman M.A., Gonzalens R., Linder J.A., Landefeld C.S. Changing use of antibiotics in community based outpatient practice, 1991-1999. Ann. Intern. Med. 2003 Apr 1;138(7):525–533. doi: 10.7326/0003-4819-138-7-200304010-00008. [DOI] [PubMed] [Google Scholar]
- 12.Thapar N., Sanderson I.R. Diarrhea in children: an interface between developing and developed countries. Lancet. 2004 Feb 21;363(9409):641–653. doi: 10.1016/S0140-6736(04)15599-2. [DOI] [PubMed] [Google Scholar]
- 13.Wingate D., Phillips S.F., Lewis S.J., Malagelada J.R., Speelman P., Steffen R., et al. Guidelines for adults on self-medication for the treatment of acute diarrhea. Aliment. Pharmacol. Ther. 2001 Jun;15(6):773–782. doi: 10.1046/j.1365-2036.2001.00993.x. [DOI] [PubMed] [Google Scholar]
- 14.Walker C.L.F., Fontaine O., Young M.W., Black R.E. Zinc and low osmolarity oral dehydration salts for diarrhea: a renewed call to action. Bull. World Health Organ. 2009 Oct;87(10):780–786. doi: 10.2471/blt.08.058990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatnagar S., Lodha R., Choudhury P. IAP Guidelines 2006 on management of acute diarrhea. Indian Pediatr. 2007 May;44(5):380–389. [PubMed] [Google Scholar]
- 16.Natnael T., Lingerew M., Adane M. Prevalence of acute diarrhea and associated factors among children under five in semi-urban areas of northeastern Ethiopia. BMC Pediatr. 2021 doi: 10.1186/s12887-021-02762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al Shimemeri A., Al Ghadeer H., Memish Z. Antibiotic utilization pattern in a general medical ward of a tertiary medical center in Saudi Arabia. Avicenna J Med. 2011 Jul;1(1):8–11. doi: 10.4103/2231-0770.83717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Supcharassaeng S., Suankratay C. Antibiotic prescription for adults with acute diarrhoea at king chulalongkorn memorial hospital, Thailand. J. Med. Assoc. Thail. 2011;94(5):545–550. [PubMed] [Google Scholar]
- 19.Tulu S., Tadesse T., Gube A.A. Assessment of antibiotic utilization pattern in treatment of acute diarrhoea diseases in Bishoftu general Hospital,Oromia Ethiopia. Hindawi Adv. Med. 2018 doi: 10.1155/2018/2376825. 6 page. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misganaw D., Abtew K. Evaluation of antibiotic utilization pattern during acute diarrheal disease at chefa-robit health center, Kemissie, North East Amhara, Ethiopia drug. Healthcare and Patient Safety. 2020;12:169–175. doi: 10.2147/DHPS.S256330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Priyadarshini K., Raj V., Balakrishnan S. Audit of use of antibiotics and zinc supplement in childhood diarrhea. J. Pharmacol. Pharmacother. 2013;4(3):204–205. doi: 10.4103/0976-500X.114601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou F.Q., Wang Y., Li J., Wang G.Q., Liu Y. Management of acute diarrhoea in adults in China: a crosssectional survey. BMC Publ. Health. 2013;13(1):41. doi: 10.1186/1471-2458-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pathak D., Pathak A., Marrone G., Diwan V., Lundborg C.S. Adherence to treatment guidelines for acute diarrhoea in children upto 12 years in Ujjain, India- a cross sectional prescription analysis. BMC Infect. Dis. 2011;11(1) doi: 10.1186/1471-2334-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO . World Health Organization; Geneva: 2001. Global Strategy for Containment of Antimicrobial Resistance: Executive Summary. [Google Scholar]
- 25.Singh J., Bora D., Sachdeva V., Sharma R.S., Verghese T. Prescribing patterns by doctors for acute diarrhoea in children in Delhi, India. J. Diarrhoeal Dis. Res. 1995;13:229–231. [PubMed] [Google Scholar]
- 26.WHO . WHO; Geneva: 2005. The Treatment of Diarrhea: A Manual for Physicians and Other Senior Health Workers. WHO/CDD/SER/80.2.2013. [Google Scholar]
- 27.Anita Kotwani A., Chaudhury R.R., Holloway K. Antibiotics prescribing practice of primary care prescriber for acute diarrhea New Delhi, India. Value Health. 2012 Jan-Feb;15(1 Suppl):S116–S119. doi: 10.1016/j.jval.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Howteerakul N., Higginbotham N., Dibley M.J. Antimicrobial use in children under five years with diarrhea in a central region province, Thailand. Southeast Asian J. Trop. Med. Publ. Health. 2004 Mar;35(1):181–187. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.