Abstract
The Papaver rhoeas L. (P. rhoeas) plant, which belongs to the Papaveraceae family, is also used as food and is exploited to treat several health problems. The purpose of this research is to determine the anti-struvite, anti-inflammatory, analgesic, and antidepressant effects of the stem extract (SE) and flower extract (FE) of the plant P. rhoeas. We used polarizing microscopy and Fourier transform infrared spectrometry (FT-IR) to evaluate the anti-struvite effect of our plant. The edema approach induced by the carrageenan molecule was used to study the anti-inflammatory impact of our extracts. The analgesic test was determined by calculating the number of abdominal contractions induced by the intraperitoneal (IP) administration of acetic acid. To evaluate the antidepressant effect of our extracts, we used the forced swimming test (FST). According to the results of the secondary metabolite extraction, both extracts contained high contents of secondary metabolites, while the results of the screening test showed that flavonoids, alkaloids, phenols, tannins, coumarins, saponins, and terpenoids were present. The result of struvite crystallization inhibition observed by polarizing microscopy and FT-IR shows the inhibition of struvite crystal aggregation by SE by decreasing the amount and size of crystals in a manner similar to cystone. The results of anti-inflammatory activity show maximum inhibition of edema after six hours of carrageenan injection in rats (T6) for all extracts, with a maximum value of 86.36% for SE at the dose of 200 mg/kg. Regarding the analgesic effect of our plant, the lowest number of abdominal contractions was observed in rats treated with SE at a dose of 400 mg/kg. The FST results show that the lowest immobilization time was observed in rats treated with FE at a dose of 400 mg/kg. The results obtained show that the flowers and stems of P. rhoeas can constitute a rich source of bioactive molecules with potential pharmaceutical applications.
Keywords: Papaver rhoeas L., Phytochemical screening, Extraction, Alkaloids, Flavonoids, Tannins, Saponosides, Anti-struvite, Anti-inflammatory, Analgesic, Antidepressant
1. Introduction
Morocco is distinguished by a remarkable variety and abundance of medicinal herbs, and it has a long history of using plants for both aesthetic and therapeutic reasons (El-Assri et al., 2021, Taleb, 2017, Tlemcani et al., 2023). A number of infectious, cardiovascular, inflammatory, neurological, and malignant disorders, among others, can be prevented or treated with medicinal plants thanks to their therapeutic benefits. They are also used in the pharmaceutical sector. Alternative medicine is used for various considerations, such as the socio-cultural habits of the populations, the high costs of conventional medicine, and the necessity to find alternatives to conventional plant-based drugs for the treatment of different pathologies (Chahmi et al., 2015). Most medicinal plants are found in food and vegetable plants. Ethnobotany is the branch of botany that studies, documents, and explains the ancestral uses of plants in various societies. By studying every plant cell, researchers in this discipline can find plant species that could be a reserve or source for future drugs. (Bendaoud et al., 2023, Bendaoud et al., 2022, El Khomsi et al., 2022, El-Assri et al., 2021, Hmamou et al., 2023, Hmamou et al., 2022, Hmamou et al., 2022, Khomsi et al., 2022, Plante médicinale, n.d.).
Struvite, also known as Magnesium Ammonium Phosphate Hexahydrate (MgNH4PO46H2O), has been the focus of attention in the last decade for several reasons. Indeed, it crystallizes rapidly in some areas, which causes the clogging of the pipes in the system. It is also the most important representative of infectious urinary lithiasis, which can account for 30% of all urinary lithiasis (Sidorczuk et al., 2020). Urease-producing bacteria, especially species of the genus Proteus, are responsible for this infection (Bichler et al., 2002). They can develop rapidly in a matter of weeks or months and, if not treated properly, can expand to fill the entire intrarenal system. The probability of losing a kidney is 50% in patients with infectious stones who do not undergo treatment(McLean et al., 1988). It is well known that urinary tract infections are linked to inflammation, which is one of the consequences of many different diseases. The inflammatory syndromes are very frequently encountered in everyday practice. Furthermore, the inflammatory reaction can be linked to a wide range of pathogenic phenomena, including thromboembolic diseases, systemic diseases, infections, and cancers (Coussens and Werb, 2002, Dandona et al., 2004, Karin et al., 2006). Thus, although it is essential for the survival of the attacked organism, inflammation is no less dangerous. The treatments used, non-steroidal and steroidal anti-inflammatories, are risky but remain the best-selling drugs (Sene et al., 2016). Depression is a frequent comorbidity of some chronic inflammatory diseases, and some therapies targeting pro-inflammatory cytokines may induce symptoms of depression(Djamila and Emmanuel, 2015). Depression is a varied and difficult psychological disorder. It is due to a disruption of monoamine neurotransmitters in the noradrenergic, dopaminergic, and/or serotonergic nervous systems. Several treatments are available, but their inconvenience discourages people to use them. (Griffin et al., 2013) (Elhwuegi, 2004). Herbal medications have recently shown real promise as a safe alternative therapy for a number of disorders and may eventually be effective in treating depression and anxiety without side effects(Es-Safi et al., 2020).
The P. rhoeas plant, belonging to the Papaveraceous family, is also used as food and is exploited to treat inflammation, respiratory problems, diarrhea, cough, insomnia, asthma, and discomfort. (Çoban et al., 2017, El and Karakaya, 2004, Kalav and Sariyar, 1989, Selen Isbilir and Sagiroglu, 2012) The extracts of P. rhoeas have many pharmacological actions, including antioxidant, antimutagenic, cytoprotective, antibacterial, analgesic, and antiulcerogenic properties (Çoban et al., 2017, Gürbüz et al., 2003, Hasplova et al., 2011, Katarzyna et al., 2021, Osanloo et al., 2016, Pourmotabbed et al., 2004, Todorova et al., 2015), have also been established in recent investigations. Within this framework, the present work aims at the chemical characterization and determination of the anti-struvite, anti-inflammatory, analgesic, antidepressant properties of the P. rhoeas stem and flowers extracts.
2. Material and methods
2.1. Plant material
The P. rhoeas plants were freshly collected in April 2021 in the province of Taounate (34° 32′ 09′′ N, 4° 38′ 24′′ W), Morocco. They were identified by the botanist Eloutassi Nouredinne, professor at the Regional Center for Education and Training in Fez, accredited by the French Association for Plant Protection, and given the number P01/07001. It was then deposited in the herbarium of our laboratory. The two organs (stems and flowers) of P. rhoeas were dried in our laboratory at room temperature for one month, protected from light and humidity.
2.2. Preparation of extracts
We obtained the hydroethanol extracts of P. rhoeas stems and flowers by maceration. For this purpose, 20 g of dried powder (prepared in an electric mill (KRUPS; GX332850)) were macerated in a flask with 0.2L of ethanol–water 70 % (v/v) for 48 h in the laboratory at room temperature. Subsequently, this product has been filtered through the Whatman paper, and the removal of solvents is done with a rotary evaporator type (BUCHI; R-100 Rotavapor®; New Castle, DE, USA). The final product was stored at four °C until use.
2.3. Extraction of flavonoids
Ten g of each plant part were macerated in 200 mL of alcohol for 24 h. The alcoholic product was filtered and dried. The dry product obtained was washed using distilled water and ethyl acetate 50% (v/v).After stirring and decanting, the organic phase was recovered and evaporated, and the aqueous phase was washed with n-butanol. The butanol phase was then evaporated at 60 °C after stirring (Sharma and Janmeda, 2017).
2.4. Extraction of tannins
2.5 g of each organ of our plant powder was macerated for 72 h at room temperature in distilled water and 50 mL of acetone 80% (v/v). To remove pigments and lipids, the aqueous phase was washed with dichloromethane. An extraction of the aqueous phase was performed with ethyl acetate; this operation was performed after the separation of the organic phase. The latter was then evaporated to a dryness of 40 °C (Zhang et al., 2008).
2.5. Extraction of saponosides
After two hours of reflux with 75 mL of pure hexane, five g of powder from each portion of the plant were created. The precipitated product was macerated in 25 mL of ethanol for 24 h. The ethanolic phase was evaporated at 60 °C at the time of filtration. Using 50 mL of petroleum ether/water 50% (v/v), the dry residue was recovered after 30 min of heating in a water bath at 50 °C. Finally, n-butanol was used to remove the components of the aqueous phase for 30 min. After decantation, the resulting product was then purified by evaporation (Ballesteros et al., 2013).
2.6. Extraction of alkaloids
Using the Soxhlet equipment, 150 mL of 100% ethanol was used to extract 10 g of stem and flower (powder) over the course of five hourss. At 60 °C, this ethanol extract was dried out. A 20 mL and a 10 mL amount of chloroform were used to absorb the dry portion of the residue. The dry residue was dissolved in 20 mL of chloroform and then acidified with hydrochloric acid (HCL) (5%, pH 3). After half an hours at ambient temperature, the acidic aqueous phase was extracted with 20 mL of chloroform that had been basified with NaHCO3 (5%) at pH 9. At a temperature of 40 °C, this chloroform phase evaporated (Yubin et al., 2014).
2.7. Phytochemical screening
The phytochemical analyses allow us to highlight the presence of groups of chemical classes in the given extract. Alkaloids were characterized by Wagner, Dragendorff, and Mayer reagents. The ferric chloride (FeCl3) reaction was used to characterize the polyphenols. Flavonoids were searched by the cyanidin reaction. The tannins were analyzed using Stiasny's reagent. The frothing test was used to characterize the saponosides. The Salkowski test was used to characterize terpenoids. Finally, the coumarins are identified by looking at their UV fluorescence at a wavelength of 366 nm. (The presence of coumarins is shown by a bright fluorescence in the tube where ammonia has been introduced) (Hajjaj, 2018, Hama Hamadou et al., 2018, Rahman et al., 2022).
2.8. Anti-struvite activity
2.8.1. In vitro struvite crystallization model
The in vitro crystallization protocol we used is identical to that previously described by Kaloustian et al.(Kaloustian et al., 2003) with some minor modifications. Solution 1 is 0.1 M potassium dihydrogen phosphate (KH2PO4). Solution 2 consisted of ammonium chloride (50 g), magnesium chloride (41 g), 20 mL of ammonium hydroxide supplemented to 50 mL with bi-distilled water and then diluted 10 times. One mL of solution 1 was distributed to test tubes, and then 1 mL of SE, FE, and cystone with three concentrations: 2, 1, and 0.5 mg/ml were added. In place of the sample, for the tubes of negative control, 1 mL of distilled water was added. The tubes were incubated for about half an hours at 37 °C after the addition of 1 mL of solution 2. Then the structure and amount of ammonium magnesium phosphate crystals in each tube were counted with a polarizing microscope (OLYMPUS U-SPT Japan (×500)).
2.8.2. Characterization of struvite crystals
The FT-IR was used to characterize struvite crystals in the tubes after SE, FE, and cystone treatment at the three concentrations (0.5, 1, and 2 mg/mL). Then, we dried the precipitate that formed before examining it. To do this, 5% of the precipitate was diluted in 95% Potassium bromide (KBr). Once the product was obtained, which was in the form of a powder, it was transferred to a granule mold with a diameter of 13 mm. Then, 1 mm thick translucent pellets were formed and subjected to FT-IR analysis (Burker Optic GMBH, Co.KG., Ettlingen, Germany). The investigation utilized a wide wavelength range (4000 and 400 cm−1) (Mammate et al., 2023, Zhang et al., 2015).
2.9. Pharmacological activities
2.9.1. Animals used
To evaluate the pharmacological activities of SE and FE, male Wistar rats weighing 110–160 g were used. In a temperature-controlled room with a 12/12 h dark/light cycle and 55 ± 5% humidity, they were kept in cages with five rats per cage. All criteria outlined in the report “Guide for the care and use of laboratory animals” were met in this investigation (Oubihi et al., 2020). Food and water were available to them. The Institutional Ethics Committee for the Care and Use of Laboratory Animals of the Faculty of Sciences Dhar El Mehraz, Sidi Mohamed Ben Abdallah University of Fez, Morocco, reviewed and approved the present study under the ethical authorization number 07/2021/LIEME. The choice of doses is based on previous studies; indeed, a study shows that the median lethal dose (LD50) of the aerial part P. rhoeas aqueous extract is greater than 2000 mg/kg (Hajjaj, 2018). Moreover, the LD50 of the P. rhoeas petal aqueous, and hydroethanolic extracts is 4000 mg/kg (Rachid Soulimani et al., 2001). For this reason, the doses chosen in this work to evaluate the pharmacological activities were between 150 and 400 mg/kg.
2.9.2. Anti-inflammatory effect
The carrageenan molecule-induced edema technique is applied to investigate the anti-inflammatory action. (Hindawi, n.d., Winter et al., 1962). This test's Wistar rats were separated into six groups (n = 5). Groups 2, 3, 4, and 5 were given both SE and FE at dosages of 200 and 400 mg/kg respectively, whereas group 1 simply received normal saline (0.9% NaCl) (the control). Group six, used as a standard, received a diclofenac (15 mg/kg). Plethysmometer measurements were made of the rats' paw volumes right before the carrageenan injection (V0), as well as 3 h (T3), 4 h (T4), 5 h (T5), and 6 h (T6), afterwards (Vt). The percentage of edema inhibition (EI) induced by carrageenan injection was calculated by the following equation (Saénz et al., 1998):
The volumes before and after carrageenan administration are denoted by the letters V0 and Vt, respectively.
2.9.3. Analgesic activity
The determination of analgesic activity was performed by calculating the number of abdominal contractions caused by intraperitoneal (IP) administration of acetic acid (0.7%). Wistar rats in this test were separated into six groups (n = 5). Groups 2, 3, 4, and 5 received both SE and FE at doses of 200 and 400 mg/kg, respectively, whereas group 1 simply received normal saline (0.9% NaCl) (the control). Group 6, used as a standard, received aspirin (200 mg/kg). After 1.5 h, all rats received 10 mg/mL acetic acid diluted to 0.7% by IP 1.5. After 5 min (lag time), the number of abdominal contractions for each treated rat was counted for the next half hours (Konaté et al., 2012).
2.9.4. Antidepressant activity
To study the antidepressant action of our P. rhoeas extracts in rats, the FST protocol was carried out according to the method described by (Cryan et al., 2005, Rodríguez-Landa et al., 2018). A clear glass aquarium (50 × 30 × 60 cm) containing water with a depth of 25 cm (25 ± 1 °C) was used as the experimental swimming apparatus. Six groups of five rats each were created using rats. Group 1 was treated with a placebo (normal saline), while group 2 took the usual drug (diazepam, 5 mg/kg), and finally, groups three, four, five, and six received our test extracts SE and FE (150 and 300 mg/kg, respectively). Each rat was placed in the tank for six minutes after thirty minutes, where the initial two minutes were the initial adaptation time and the final four minutes were the immobility period.
2.10. Treatment of the results by statistical tests
A comparison of means was performed using Student's t-test and ANOVA one way followed by Tukey post hoc test at p < 0.5, and the criteria for normality were checked. The results were expressed as means ± standard deviation. Minitab 19 statistical software was used to perform all analyses (version 19.1.).
3. Results
3.1. Flavonoids, tannins, saponosides, and alkaloids extraction
The results of the chemical characterization of our samples are presented in Fig. 1. The content of all classes of secondary metabolites in the FE is higher than that found in the SE. The most abundant secondary metabolites in FE and SE are alkaloids 12.41 ± 0.64 and 13.20%±0.91, followed by flavonoids 6.43 ± 0.58 and 7.62%±0.88, respectively.
Fig. 1.
Percentage of secondary metabolite classes found in SE and FE of P. rhoeas. Results are expressed as a mean ± SD; a: indicate that no difference between the two studied parts ((SE): Stem extract; (FE): Flower extract), for each secondary metabolite classes, using Student's t-test at p < 0.05.
3.2. Phytochemical screening
Phytochemical screening tests show that the stems and flowers of P. rhoeas are very rich in phenols, coumarins, tannins, terpenoids, flavonoids, saponins, alkaloids. The Table 1 represents the results obtained.
Table 1.
Qualitative phytochemical analysis of stems and flowers of P. rhoeas.
| Stems | Flowers | |
|---|---|---|
| Alkaloids Dragendroff’s reagent Mayer |
+++ ++ |
++ ++ |
| Phenols | ++ | +++ |
| Flavonoids | ++ | +++ |
| Tannins | ++ | ++ |
| Saponins | ++ | ++ |
| Coumarins | + | ++ |
| Terpenoids | ++ | ++ |
+: Low concentration; ++: Medium concentration; +++: High concentration.
3.3. Anti-struvite effect
3.3.1. Microscope observation
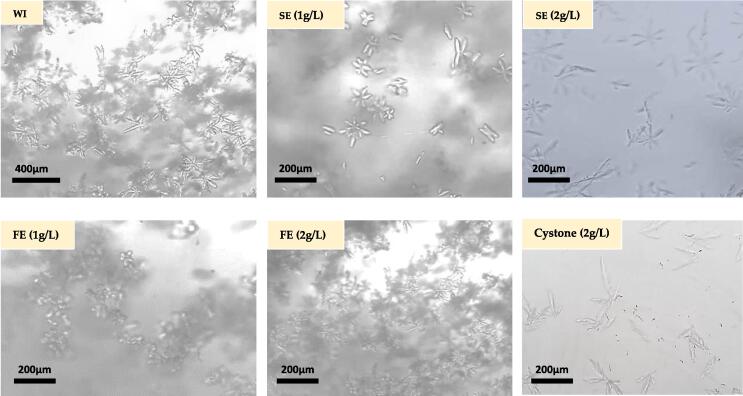
The struvite anti-crystallization activity of SE and FE was evaluated by microscopic observation. The struvite crystals microscopic crystallization is expressed in mm3. The Fig. 2 shows the activity of the studied extracts on struvite crystal size and number. Microscopic observation of the negative control (WI) showed that the size and number of crystals were greater than those of the tests performed with our two samples, SE and FE. Struvite crystals were reduced in number and size more strongly in the presence of SE than in the presence of FE. Cystone shows an anti-struvite effect similar to that of SE. The difference in the anti-struvite effect between the three concentrations of the same sample is not significant.
Fig. 2.
Microscopic observation (500 × ) of struvite crystals after 30 min incubation at 30 °C, with (WI): Without inhibitor; (SE): Stem extract; (FE): Flower extract.
3.3.2. Characterization of crystals
The characterization of the struvite crystal structure was done by FT-IR. The Fig. 3 represents the FT-IR spectra of the negative control (WI), SE, FE, and cystone at three concentrations (0.5, 1, and 2 mg/mL). The domains of the spectra with characteristic struvite bands are shown: Mg-O, PO4 3-, NH4 +, H2O, and O(6)-H(16).
Fig. 3.
FT-IR spectra of struvite crystals after treatment with SE, FE and cystone after 30 min incubation at 30 °C.
3.4. Pharmacological activities
3.4.1. Anti-inflammatory effect
Table 2, Table 3 display the findings of the anti-inflammatory activity of our SE and FE samples. Compared with the control group, the volume of edema induced by carrageenan injection in rats treated with our extracts and diclofenac was significantly reduced (Table 2). The difference in edema volume between our samples and the control is significant (p < 0.05), whereas the difference between our samples and diclofenac is not significant (p greater than 0.05). The maximum percentage inhibition of carrageenan-induced edema was observed after six hours of injection for both extracts. The maximum inhibition of carrageenan-induced edema of P. rhoeas SE and FE with 200 and 400 mg/kg doses was 86.36 ± 1.28, 72.72 ± 5.14, 77.276.43, and 63.63%±7.71, respectively, whereas that of diclofenac was 98.18%±2.57 in T6 (Table 3). This inhibition of all extracts and diclofenac increases by moving from T3 to T6. The difference in percent inhibition of carrageenan-induced edema in all samples was significant compared with the control (p greater than 0.05).
Table 2.
The effect of SE, FE, Diclofenac, and control on carrageenan-induced edema in rats. The data represent the difference in mean edema volume (mean ± SEM), (n = 5), a, b, c, and bc indicate the significant difference between the samples at p < 0.05 using ANOVA one way and Tuckey test.
| T3 | T4 | T5 | T6 | |
|---|---|---|---|---|
| Control | 0.60 ± 00a | 0.70 ± 0.14a | 0.60 ± 0.14a | 0.55 ± 0.07a |
| SE (200 mg/kg) | 0.50 ± 00b | 0.22 ± 0.03b | 0.17 ± 0.03b | 0.07 ± 0.007bc |
| FE (200 mg/kg) | 0.42 ± 0.03b | 0.22 ± 0.03b | 0.13 ± 0.02b | 0.12 ± 0.03bc |
| SE (400 mg/kg) | 0.42 ± 0.03b | 0.36 ± 0.01b | 0.25 ± 0.02b | 0.15 ± 0.02bc |
| FE (400 mg/kg) | 0.50 ± 00b | 0.37 ± 0.03b | 0.30 ± 0.04b | 0.2 ± 0.04b |
| Diclofenac (15 mg/kg) | 0.20 ± 0.02c | 0.20 ± 0.02b | 0.06 ± 0.01b | 0.01 ± 0.01c |
Table 3.
Percentage inhibition of carrageenan-induced edema in SE, and FE, and diclofenac-treated rats; (mean ± SEM), (n = 5); a, b, c, ab, and bc indicate the significant difference between the studied samples at p < 0.05 using ANOVA one way and Tuckey test.
| T3 | T4 | T5 | T6 | |
|---|---|---|---|---|
| SE (200 mg/kg) | 16.66 ± 00b | 67.85 ± 5.05a | 70.83 ± 5.89ab | 86.36 ± 1.28ab |
| FE (200 mg/kg) | 29.16 ± 5.89b | 67.85 ± 5.05a | 77.50 ± 3.54ab | 77.27 ± 6.43bc |
| SE (400 mg/kg) | 29.16 ± 5.89b | 48.57 ± 02.02b | 58.33 ± 4.71bc | 72.72 ± 5.14bc |
| FE (400 mg/kg) | 16.66 ± 00b | 46.42 ± 5.05b | 50 ± 7.07c | 63.63 ± 7.71c |
| Diclofenac (15 mg/kg) | 66.66 ± 4.71a | 70.71 ± 03.03a | 90 ± 2.36a | 98.18 ± 2.57a |
3.4.2. Analgesic activity
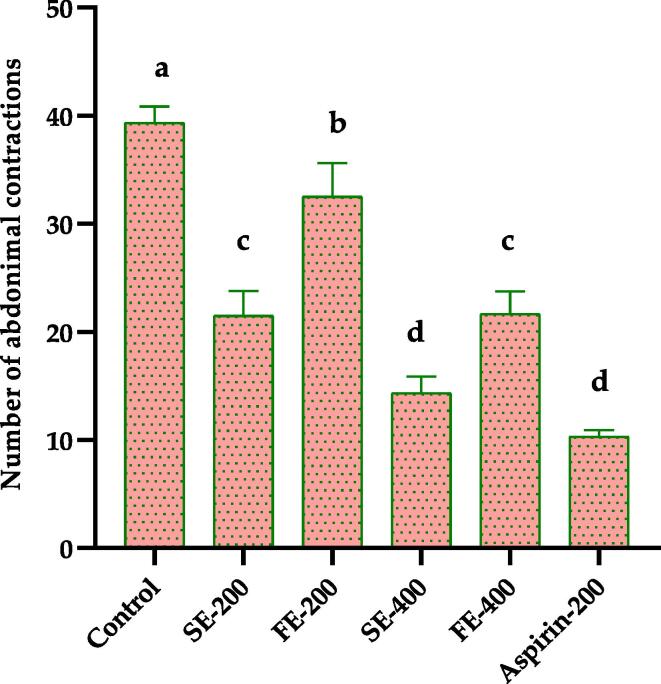
The Fig. 4 depicts the analgesic activity results obtained. The results obtained show that the number of abdominal contractions in rats receiving SE and FE at 200 mg/kg and 400 mg/kg are 21.5 ± 2.29, 14.33 ± 1.52, 32.5 ± 3.12, and 21.66 ± 2.08, respectively. The number of abdominal contractions in aspirin-treated rats is 10.33 ± 0.57. The difference in these results is statistically significant compared with the negative control 39.33 ± 1.52.
Fig. 4.
Effect of P. rhoeas SE and FE on the response of rats to acetic acid-induced abdominal contractions (n = 5), a, b, c and d—values with a significant difference using Student's t-test at p < 0.05. SE-200: stem extract (200 mg/kg); FE-200: flower extract (200 mg/kg); SE-400: stem extract (400 mg/kg); FE-400: flower extract (400 mg/kg); Aspirin-200: Aspirin (200 mg/kg).
3.4.3. Antidepressant activity
In Fig. 5, the results of the forced swim test are illustrated. The immobility time in rats treated with SE and FE at 150 and 300 mg/kg is 133.26 ± 14.39, 119.49 ± 9.20, 62.91 ± 8.86, and 47.19 s ± 2.67 respectively, whereas it is 198 ± 2.82 and 41.25 s ± 2.44, respectively, in the negative control and diazepam (positive control)-treated rats.
Fig. 5.
Impact of SE, FE, and diazepam on immobility time in rats (n = 5), a, b and c—values with a significant difference, using Student's t-test at p < 0.05. SE-150: stem extract (200 mg/kg); FE-150: flower extract (150 mg/kg); SE-300: stem extract (300 mg/kg); FE-300: flower extract (300 mg/kg); Diazepam-15: Diazepam (15 mg/kg).
4. Discussion
According to ethnobotanical studies that we have done in the region of Fez-Meknes, Morocco, P. rhoeas is used to relieve pain and depression. It is also used to treat urinary lithiasis and other health problems among the local population (El-Assri et al., 2021, Tlemcani et al., 2023). The antistruvite activity was evaluated in vitro, and the anti-inflammatory, analgesic, and antidepressant activities were examined in rats using several tests to confirm their properties.
It is demonstrated that P. rhoeas has primary and secondary metabolites, including vitamins, fatty acids, amino acids, carbohydrates, essential oils, phenolic compounds, alkaloids, coumarins, organic acids, flavonoids, and other compounds, hence its use in food and traditional pharmacopoeia (Grauso et al., 2021a). The extracts used in this work are the same extracts we used in our study published in 2022 (Hmamou et al., 2022), in which we quantified the content of total polyphenols and flavonoids. Total polyphenols in SE, and FE are 10.58 and 22.10 mg gallic acid equivalents (GAE)/g extract, respectively, while flavonoids are 4.49 and 4.50 mg quercetin equivalents (QE)/g extract, respectively. In the Papaveraceae family, the most abundant metabolites detected are alkaloids. (+)-rhoeadine was found to be the most abundant alkaloid isolated from the aerial parts of P. rhoeas. The minor alkaloids are (+)-roemerine, (+)-isocorydine, allocryptopine, (+)-berberine, rhoeagenine, coulteropine, coptisine, (-)-sinactine, sanguinarine, and protopine (Grauso et al., 2021b). In terms of pharmacology, these alkaloids, especially those of the isoquinoline type, are the most important (Bournine et al., 2013, Nepali et al., 2014). In 2004, a study showed that P. rhoeas extracts contained the flavonoid glycosides, astragalin, isoquercitrin, and hyperoside (Hillenbrand et al., 2004) as well as flavonoids kaempferols, hypoletin, luteolin, and quercetin,. On the other hand, another study found that extracts of the same plant contained rutin, vitexin, coumarin, luteolinidin, and malvidin (Rachid Soulimani et al., 2001).
Infection lithiasis is a very old disease. The oldest known struvite stone dates back to the Bronze Age. Infection lithiasis is most often related to urease bacteria, which are responsible for the formation of stones composed of ammonia-magnesium phosphate or struvite. More rarely, other germs lacking urease can promote the formation of phosphocalcic and uric acid stones (Masson, n.d.). More than 200 species of bacteria have urease activity (McLean et al., 1988) and use urea as a source of nitrogen. The Proteus genre is the main culprit in stone infection (Griffith, 1978). Crystal aggregation is a crucial step in the formation and progression of urinary stones (Kok and Khan, 1994). Prevention of kidney stone formation relies primarily on inhibition of crystal growth and aggregation. Results of anti-struvite activity suggest that the P. rhoeas plant contains active substances effective in inhibiting struvite crystal formation and aggregation. Previous research has identified comparable structural alterations in struvite crystals (Cahil et al., 2007, Manzoor et al., 2018, Raj et al., 2023, Sidorczuk et al., 2020). In addition, we observe a minor difference in band intensity as a function of concentration in our samples and cystone, which can be explained by the interaction between substances in the extracts and struvite.
Comparing our results of anti-inflammatory activity with those of other studies, aqueous extracts of P. rhoeas aerial parts at doses of 400 and 800 mg/kg showed a maximum percentage of edema inhibition of 87.47 and 87.49%, respectively (Hajjaj, 2018). The model of local inflammation induced by subcutaneous injection of carrageenan is a widely used model in research on anti-inflammatory substances. This injection causes local inflammation, which appears as edema. After injection, carrageenan triggers a biphasic reaction during edema formation(Vinegar et al., 1969). The first stage is characterized by the release of serotonin, kinins, and histamine during the first hour. In contrast, the second stage is characterized by the secretion of prostaglandins in the next 2–3 h, which are the main molecules responsible for acute inflammation (Ricciotti and FitzGerald, 2011). Lipoxygenase indirectly triggers an inflammatory response, whereas the enzyme cyclooxygenase directly contributes to inflammation through prostaglandin synthesis (Islam et al., 2020, Pidgeon et al., 2007). The abundance of polyphenolic components in SE and FE could be the cause of their inhibitory action on cyclooxygenase enzymes. Prostaglandins that promote inflammation are not produced in the presence of polyphenols(Chy et al., 2020). Tannins, saponins, and flavonoids also have an anti-inflammatory impact (Choi et al., 2005, Mostafa et al., 2018, Pérez-Guerrero et al., 2001, Vezza et al., 2016).
Comparing our results of analgesic activity with those of the other studies, the number of abdominal contractions in rats treated with aqueous extracts of P. rhoeas aerial parts at 200 mg/kg and 400 mg/kg is 20.25 ± 3.21, and 12.25 ± 2.40, respectively (Hajjaj, 2018). The results indicate a dose-dependent increase in the peripheral analgesic efficacy of our extracts. An increase in the content of molecules that have an analgesic action at the maximum dose (400 mg/kg) can explain the increase in this analgesic activity by increasing the doses of extracts. Reduction of peripheral nociceptor sensitivity in peritoneal free nerve endings for chemically produced pain and regulation of the production and secretion of many endogenous inflammation-causing molecules may be the potential mechanisms by which both extracts induce peripheral analgesia. These suggested pathways are consistent with the idea that any substance that reduces the magnitude of muscle twitching provides analgesia by preventing the production and secretion of prostaglandins and the spread of peripheral pain (Debebe et al., 2007, Tadiwos et al., 2017).
These results of the present research indicate that oral administration of SE and FE decreases depression in rats, which may suggest that the studied extracts of P. rhoeas enhance at least one of the neuromediators related to depression, such as dopamine, serotonin, or norepinephrine (M.D and Alford, 2009). The benefits of P. rhoeas extracts have been extensively studied, and the current study also found that extracts of this plant possess potent antidepressant properties. Previous research has demonstrated profound effects of P. rhoeas extracts on a variety of animal models(R. Soulimani et al., 2001) (Pourmotabbed et al., 2004, Sahraei et al., 2007, Sahraei et al., 2006). However, recent research has shown the ability of P. rhoeas to reduce stress in male rats. In addition, this extract of P. rhoeas improves memory and prevents stress-related memory loss in mice (Osanloo et al., 2016). Most research on P. rhoeas indicates that its extracts include papaveric acid, papaverine, and muconic acid, three of the most potent substances for affecting brain neurotransmitters. The effects of P. rhoeas extracts on glutamate have also been demonstrated in previous research(Saeed-Abadi et al., 2012). Since glutamate inhibition is now recognized as one of the most effective solutions for treating depression, it would seem that this mechanism is at least largely responsible for the action of this extract.
Many non-steroidal anti-inflammatory drugs (NSAIDs) also have anti-inflammatory, analgesic, and antidepressant properties, as they generally inhibit the cyclooxygenase enzyme responsible for prostaglandin production (Fond, 2016, Fond et al., 2014, Hajjaj, 2018, Köhler et al., 2014). Our study suggests that SE and FE cause anti-inflammatory and analgesic effects similar to those of NSAIDs, which is consistent with the traditional use of the plant by the Moroccan population.
5. Conclusion
The results of the present investigation indicate that extracts from the P. rhoeas stem and flower cause remarkable inhibition of struvite crystallization as well as anti-inflammatory, analgesic, and antidepressant effects. Our results indicate that the P. rhoeas stem and flower are a rich source of bioactive molecules that may have potential pharmaceutical applications.
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R165), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors extend their appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R165), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Anouar Hmamou, Email: anouar.hmamou@usmba.ac.ma.
El-Mehdi El-Assri, Email: elmehdi.elassri@usmba.ac.ma.
Mostafa El Khomsi, Email: elkhomsi.mostafa@uit.ac.ma.
Mohammed Kara, Email: elkhomsi.mostafa@uit.ac.ma.
Samar Zuhair Alshawwa, Email: szalshawwa@pnu.edu.sa.
Omkulthom Al Kamaly, Email: omalkmali@pnu.edu.sa.
Fatima Ezzahra El oumari, Email: Fatimaezzahra.eloumari@usmba.ac.ma.
Noureddine Eloutassi, Email: eloutassinoureddine@gmail.com.
Amal Lahkimi, Email: amal.lahkimi@usmba.ac.ma.
References
- Ballesteros O.J.V., Perea E.M., Méndez J.J., Arango W.M., Noreña D.A. Quantification, chemical and biological characterization of the saponosides material from Sida cordifolia L. (escobilla) Rev. Cubana Plant Med. 2013;18:298–314. [Google Scholar]
- Bendaoud A., Lahkimi A., Kara M., Moubchir T., Assouguem A., Belkhiri A., Allali A., Hmamou A., Almeer R., Sayed A.A., Peluso I., Eloutassi N. Field study and chemical analysis of plant waste in the Fez-Meknes Region, Morocco. Sustainability. 2022;14:6029. doi: 10.3390/su14106029. [DOI] [Google Scholar]
- Bendaoud A., Belkhiri A., Maai M., Moubchir T., Hmamou A., Tlemcani S., Eloutassi N., Lahkimi A. Simple and combined pretreatment of a mixture of forestry and aromatic-medicinal plant waste by chemical, physical and enzymatic methods. J. Ecol. Eng. 2023;24:376–383. doi: 10.12911/22998993/160094. [DOI] [Google Scholar]
- Bichler K.-H., Eipper E., Naber K., Braun V., Zimmermann R., Lahme S. Urinary infection stones. Int. J. Antimicrob. Agents. 2002;19:488–498. doi: 10.1016/S0924-8579(02)00088-2. [DOI] [PubMed] [Google Scholar]
- Bournine L., Bensalem S., Wauters J.-N., Iguer-Ouada M., Maiza-Benabdesselam F., Bedjou F., Castronovo V., Bellahcène A., Tits M., Frédérich M. Identification and quantification of the main active anticancer alkaloids from the root of Glaucium flavum. Int. J. Mol. Sci. 2013;14:23533–23544. doi: 10.3390/ijms141223533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahil, A., Najdoski, M., Stefov, V., 2007. Infrared and Raman spectra of magnesium ammonium phosphate hexahydrate (struvite) and its isomorphous analogues. IV. FTIR spectra of protiated and partially deuterated nickel ammonium phosphate hexahydrate and nickel potassium phosphate hexahydrate. Journal of Molecular Structure, MOLECULAR SPECTROSCOPY AND MOLECULAR STRUCTURE 2006 834–836, 408–413. https://doi.org/10.1016/j.molstruc.2006.11.049.
- Chahmi N., Anissi J., Jennan S., Farah A., Sendide K., Hassouni M.E. Antioxidant activities and total phenol content of Inula viscosa extracts selected from three regions of Morocco. Asian Pac. J. Trop. Biomed. 2015;5:228–233. doi: 10.1016/S2221-1691(15)30010-1. [DOI] [Google Scholar]
- Choi J., Jung H.-J., Lee K.-T., Park H.-J. Antinociceptive and anti-inflammatory effects of the saponin and sapogenins obtained from the stem of Akebia quinata. J. Med. Food. 2005;8:78–85. doi: 10.1089/jmf.2005.8.78. [DOI] [PubMed] [Google Scholar]
- Chy M.N.U., Adnan M., Rauniyar A.K., Amin M.M., Majumder M., Islam M.S., Afrin S., Farhana K., Nesa F., Sany M.A., Tanim M.A.H., Siddique T.I., Paul A. Evaluation of anti-nociceptive and anti-inflammatory activities of Piper sylvaticum (Roxb.) stem by experimental and computational approaches. Adv. Tradit. Med. (ADTM) 2020;20:327–341. doi: 10.1007/s13596-019-00395-9. [DOI] [Google Scholar]
- Çoban İ., Toplan G.G., Özbek B., Gürer Ç.U., Sarıyar G. Variation of alkaloid contents and antimicrobial activities of Papaver rhoeas L. growing in Turkey and northern Cyprus. Pharm. Biol. 2017;55:1894–1898. doi: 10.1080/13880209.2017.1340964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan, J.F., Valentino, R.J., Lucki, I., 2005. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neuroscience & Biobehavioral Reviews, Animal Models of Depression and Antidepressant Activity 29, 547–569. https://doi.org/10.1016/j.neubiorev.2005.03.008 [DOI] [PubMed]
- Dandona P., Aljada A., Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Debebe, E., Makonnen, E., Debella, A., 2007. Antinociceptive effect of the methanolic extract of roots of andrachne aspera in three models of nociception 8.
- Djamila B., Emmanuel H. Inflammation et dépression : revue de la littérature. PSN. 2015;13:7–14. doi: 10.3917/psn.132.0007. [DOI] [Google Scholar]
- El S.N., Karakaya S. Radical scavenging and iron-chelating activities of some greens used as traditional dishes in Mediterranean diet. Int. J. Food Sci. Nutr. 2004;55:67–74. doi: 10.1080/09637480310001642501. [DOI] [PubMed] [Google Scholar]
- El Khomsi M., Kara M., Hmamou A., Assouguem A., Al Kamaly O., Saleh A., Ercisli S., Fidan H., Hmouni D. In vitro studies on the antimicrobial and antioxidant activities of total polyphenol content of Cynara humilis from Moulay Yacoub Area (Morocco) Plants. 2022;11:1200. doi: 10.3390/plants11091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Assri E., Barnossi A.E., Chebaibi M., Hmamou A., Asmi H.E., Bouia A., Eloutassi N. Ethnobotanical survey of medicinal and aromatic plants in Taounate, Pre-Rif of Morocco. Ethnobot. Res. Appl. 2021;22:1–23. [Google Scholar]
- El-Assri E.-M., Eloutassi N., Azeddin E.B., Bakkari F., Hmamou A., Bouia A. Wild Chamomile (Matricaria recutita L) from the Taounate Province, Morocco: Extraction and valorisation of the antibacterial activity of its essential oils. TJNPR. 2021;5:883–888. doi: 10.26538/tjnpr/v5i5.15. [DOI] [Google Scholar]
- Elhwuegi A.S. Central monoamines and their role in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28:435–451. doi: 10.1016/j.pnpbp.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Es-Safi I., Mechchate H., Amaghnouje A., El Moussaoui A., Cerruti P., Avella M., Grafov A., Bousta D. Marketing and legal status of phytomedicines and food supplements in Morocco. J. Complement. Integr. Med. 2020;18:279–285. doi: 10.1515/jcim-2020-0168. [DOI] [PubMed] [Google Scholar]
- Fond G. Causes et conséquences de l’inflammation dans la dépression majeure et applications thérapeutiques potentielles. L’information psychiatrique. 2016;93:803–808. doi: 10.1684/ipe.2016.1560. [DOI] [Google Scholar]
- Fond G., Hamdani N., Kapczinski F., Boukouaci W., Drancourt N., Dargel A., Oliveira J., Le Guen E., Marlinge E., Tamouza R., Leboyer M. Effectiveness and tolerance of anti-inflammatory drugs’ add-on therapy in major mental disorders: a systematic qualitative review. Acta Psychiatr. Scand. 2014;129:163–179. doi: 10.1111/acps.12211. [DOI] [PubMed] [Google Scholar]
- Grauso L., de Falco B., Motti R., Lanzotti V. Corn poppy, Papaver rhoeas L.: a critical review of its botany, phytochemistry and pharmacology. Phytochem. Rev. 2021;20:227–248. [Google Scholar]
- Griffin C.E., Kaye A.M., Bueno F.R., Kaye A.D. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13:214–223. [PMC free article] [PubMed] [Google Scholar]
- Griffith D.P. Struvite stones. Kidney Int. 1978;13:372–382. doi: 10.1038/ki.1978.55. [DOI] [PubMed] [Google Scholar]
- Gürbüz İ., Üstün O., Yesilada E., Sezik E., Kutsal O. Anti-ulcerogenic activity of some plants used as folk remedy in Turkey. J. Ethnopharmacol. 2003;88:93–97. doi: 10.1016/S0378-8741(03)00174-0. [DOI] [PubMed] [Google Scholar]
- Hajjaj G. Analgesic and anti-inflammatory effects of Papaver Rhoeas L. A traditional medicinal plant of Morocco. JONAM. 2018;2 doi: 10.23880/jonam-16000150. [DOI] [Google Scholar]
- Hama Hamadou, H., Moutari, S.K., Maman Manzo, L., Moussa, I., Adamou, R., Khalid, I., 2018. 32 - Criblage phytochimique et dosage des polyphénols du Detarium microcarpum Guill. et Perr. utilisé dans le traitement des maladies parasitaires au Niger.
- Hasplova K., Hudecova A., Miadokova E., Magdolenova Z., Galova E., Vaculcikova L., Gregan F., Dusinska M. Biological activity of plant extract isolated from Papaver rhoeas on human lymfoblastoid cell line. Neoplasma. 2011;58:386–391. doi: 10.4149/neo_2011_05_386. [DOI] [PubMed] [Google Scholar]
- Hillenbrand M., Zapp J., Becker H. Depsides from the Petals of Papaver rhoeas. Planta Med. 2004;70:380–382. doi: 10.1055/s-2004-818956. [DOI] [PubMed] [Google Scholar]
- Hindawi, n.d. In Vivo Potential Anti-Inflammatory Activity of Melissa officinalis L. Essential Oil [WWW Document]. URL https://www.hindawi.com/journals/aps/2013/101759/ (accessed 9.10.22). [DOI] [PMC free article] [PubMed]
- Hmamou, A., Eloutassi, N., Alshawwa, S.Z., Al kamaly, O., Kara, M., Bendaoud, A., El-Assri, E.-M., Tlemcani, S., El Khomsi, M., Lahkimi, A., 2022. Total Phenolic Content and Antioxidant and Antimicrobial Activities of Papaver rhoeas L. Organ Extracts Growing in Taounate Region, Morocco. Molecules 27, 854. https://doi.org/10.3390/molecules27030854 [DOI] [PMC free article] [PubMed]
- Hmamou A., Kara M., Khomsi M.E., Saleh A., Al Kamaly O., Bendaoud A., El Ouadrhiri F., Adachi A., Tlemcani S., Eloutassi N., Lahkimi A. Comparative study on the total phenolics, total flavonoids, and biological activities of Papaver rhoeas L. extracts from different geographical regions of Morocco. Appl. Sci. 2023;13:2695. doi: 10.3390/app13042695. [DOI] [Google Scholar]
- Islam M.E., Islam K.M.D., Billah M.M., Biswas R., Sohrab M.H., Rahman S.M.M. Antioxidant and anti-inflammatory activity of Heritiera fomes (Buch.-Ham), a mangrove plant of the Sundarbans. Adv. Tradit. Med. (ADTM) 2020;20:189–197. doi: 10.1007/s13596-019-00401-0. [DOI] [Google Scholar]
- Kalav Y.N., Sariyar G. Alkaloids from Turkish Papaver rhoeas. Planta Med. 1989;55:488. doi: 10.1055/s-2006-962072. [DOI] [PubMed] [Google Scholar]
- Kaloustian J., El-Moselhy T.F., Portugal H. Determination of calcium oxalate (mono- and dihydrate) in mixtures with magnesium ammonium phosphate or uric acid: the use of simultaneous thermal analysis in urinary calculi. Clin. Chim. Acta. 2003;334:117–129. doi: 10.1016/S0009-8981(03)00228-6. [DOI] [PubMed] [Google Scholar]
- Karin M., Lawrence T., Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Katarzyna J., Karolina J., Patrycja K., Mateusz B., Izabela G. Mineral composition and antioxidant potential in the common poppy (Papaver rhoeas L.) petal infusions. Biol. Trace Elem. Res. 2021;199:371–381. doi: 10.1007/s12011-020-02134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khomsi M.E., Imtara H., Kara M., Hmamou A., Assouguem A., Bourkhiss B., Tarayrah M., AlZain M.N., Alzamel N.M., Noman O., Hmouni D. Antimicrobial and antioxidant properties of total polyphenols of Anchusa italica Retz. Molecules. 2022;27:416. doi: 10.3390/molecules27020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler O., Benros M.E., Nordentoft M., Farkouh M.E., Iyengar R.L., Mors O., Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiat. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- Kok D.J., Khan S.R. Calcium oxalate nephrolithiasis, a free or fixed particle disease. Kidney Int. 1994;46:847–854. doi: 10.1038/ki.1994.341. [DOI] [PubMed] [Google Scholar]
- Konaté K., Bassolé I.H.N., Hilou A., Aworet-Samseny R.R., Souza A., Barro N., Dicko M.H., Datté J.Y., M’Batchi B. Toxicity assessment and analgesic activity investigation of aqueous acetone extracts of Sida acuta Burn f. and Sida cordifolia L. (Malvaceae), medicinal plants of Burkina Faso. BMC Complement. Altern. Med. 2012;12:1–11. doi: 10.1186/1472-6882-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M.D, A.T.B., Alford, B.A., 2009. Depression: Causes and Treatment. University of Pennsylvania Press.
- Mammate, N., El oumari, F.E., Imtara, H., Belchkar, S., Benjelloun Touimi, G., Al-Zharani, M., A. Rudayni, H., Ahmed Qurtam, A., S. Aleissa, M., A. Nasr, F., M. Noman, O., Sqalli Houssaini, T., 2023. Anti-Struvite, Antimicrobial, and Anti-Inflammatory Activities of Aqueous and Ethanolic Extracts of Saussurea costus (Falc) Lipsch Asteraceae. Molecules 28, 667. https://doi.org/10.3390/molecules28020667 [DOI] [PMC free article] [PubMed]
- Manzoor M.A.P., Duwal S.R., Mujeeburahiman M., Rekha P.-D. Vitamin C inhibits crystallization of struvite from artificial urine in the presence of Pseudomonas aeruginosa. Int. Braz. J. Urol. 2018;44:1234–1242. doi: 10.1590/S1677-5538.IBJU.2017.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson, E., n.d. Lithiase urinaire d’infection [WWW Document]. EM-Consulte. URL https://www.em-consulte.com/article/792216/lithiase-urinaire-d-infection (accessed 4.20.23).
- McLean R.J.C., Nickel J.C., Cheng K.-J., Costerton J.W., Banwell J.G. The ecology and pathogenicity of urease-producing bacteria in the urinary tract. CRC Crit. Rev. Microbiol. 1988;16:37–79. doi: 10.3109/10408418809104467. [DOI] [PubMed] [Google Scholar]
- Mostafa N.M., Abd El-Ghffar E.A., Hegazy H.G., Eldahshan O.A. New methoxyflavone from casimiroa sapota and the biological activities of its leaves extract against lead acetate induced hepatotoxicity in rats. Chem. Biodivers. 2018;15:e1700528. doi: 10.1002/cbdv.201700528. [DOI] [PubMed] [Google Scholar]
- Nepali K., Sharma S., Sharma M., Bedi P.M.S., Dhar K.L. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur. J. Med. Chem. 2014;77:422–487. doi: 10.1016/j.ejmech.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Osanloo N., Najafi-Abedi A., Jafari F., Javid F., Pirpiran M., Memar Jafari M.-R., Mousavi Khosravi S.A., Rahimzadeh Behzadi M., Ranjbaran M., Sahraei H. Papaver Rhoeas L. hydroalcoholic extract exacerbates forced swimming test-induced depression in mice. Basic Clin. Neurosci. 2016;7:195–202. doi: 10.15412/J.BCN.03070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oubihi A., Hosni H., Nounah I., Ettouil A., Harhar H., Alaoui K., Ouhssine M., Guessous Z. Phenolic content, antioxidant activity, anti-inflammatory potential, and acute toxicity study of Thymus leptobotrys Murb. extracts. Biochem. Res. Int. 2020;2020:e8823209. doi: 10.1155/2020/8823209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Guerrero C., Herrera M.D., Ortiz R., Alvarez de Sotomayor M., Fernández M.A. A pharmacological study of Cecropia obtusifolia Bertol aqueous extract. J. Ethnopharmacol. 2001;76:279–284. doi: 10.1016/S0378-8741(01)00253-7. [DOI] [PubMed] [Google Scholar]
- Pidgeon G.P., Lysaght J., Krishnamoorthy S., Reynolds J.V., O’Byrne K., Nie D., Honn K.V. Lipoxygenase metabolism: roles in tumor progression and survival. Cancer Metastasis Rev. 2007;26:503–524. doi: 10.1007/s10555-007-9098-3. [DOI] [PubMed] [Google Scholar]
- Plante médicinale : définition, propriétés, liste guide, récolte [WWW Document], n.d. . AquaPortail. URL https://www.aquaportail.com/definition-42-plante-medicinale.html (accessed 4.16.23).
- Pourmotabbed A., Rostamian B., Manouchehri G., Pirzadeh-Jahromi G., Sahraei H., Ghoshooni H., Zardooz H., Kamalnegad M. Effects of Papaver rhoeas extract on the expression and development of morphine-dependence in mice. J. Ethnopharmacol. 2004;95:431–435. doi: 10.1016/j.jep.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Rahman, M., Surag, A.T., Begum, R., Hossain Tusher, M.S., Huda, M.K., 2022. Phytochemical, Antioxidant, Anti-Inflammatory, and Thrombolytic Properties of Cleisomeria lanatum (Lindl.) Lindl. ex G. Don. Scientifica 2022, e5660527. https://doi.org/10.1155/2022/5660527. [DOI] [PMC free article] [PubMed]
- Raj CT, D., Palaninathan, V., James, R.A., 2023. Anti-uropathogenic, antioxidant and struvite crystallization inhibitory potential of fresh and fermented coconut water. Biocatalysis and Agricultural Biotechnology 47, 102555. https://doi.org/10.1016/j.bcab.2022.102555
- Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Landa, J.F., Cueto-Escobedo, J., Flores-Aguilar, L.Á., Rosas-Sánchez, G.U., Rovirosa-Hernández, M. de J., García-Orduña, F., Carro-Juárez, M., 2018. The Aqueous Crude Extracts of Montanoa frutescens and Montanoa grandiflora Reduce Immobility Faster Than Fluoxetine Through GABAA Receptors in Rats Forced to Swim. J Evid Based Complementary Altern Med 23, 2515690X18762953. https://doi.org/10.1177/2515690X18762953. [DOI] [PMC free article] [PubMed]
- Saeed-Abadi S., Ranjbaran M., Jafari F., Najafi-Abedi A., Rahmani B., Esfandiari B., Delfan B., Mojabi N., Ghahramani M., Sahraei H. Effects of Papaver rhoeas (L.) extract on formalin-induced pain and inflammation in mice. Pak. J. Biol. Sci. 2012;15:1041–1044. doi: 10.3923/pjbs.2012.1041.1044. [DOI] [PubMed] [Google Scholar]
- Saénz M.T., García M.D., Fernández M.A. Anti-inflammatory activity and acute toxicity of Anredera leptostachys. Phytomedicine. 1998;5:195–198. doi: 10.1016/S0944-7113(98)80027-5. [DOI] [PubMed] [Google Scholar]
- Sahraei H., Fatemi S.M., Pashaei-Rad S., Faghih-Monzavi Z., Salimi S.H., Kamalinegad M. Effects of Papaver rhoeas extract on the acquisition and expression of morphine-induced conditioned place preference in mice. J. Ethnopharmacol. 2006;103:420–424. doi: 10.1016/j.jep.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Sahraei H., Shams J., Faghih-Monzavi Z., Zardooz H., Pashaei-Rad S., Pourmotabbed A., Ghoshooni H., Kamalinejad M. Effects of Papaver rhoeas. extract on the development and expression of tolerance to morphine-induced locomotor activity in mice. Pharm. Biol. 2007;45:475–480. doi: 10.1080/13880200701389359. [DOI] [Google Scholar]
- Selen Isbilir S., Sagiroglu A. An assessment of in vitro antioxidant activities of different extracts from Papaver rhoeas L. leaves. Int. J. Food Prop. 2012;15:1300–1308. doi: 10.1080/10942912.2010.520542. [DOI] [Google Scholar]
- Sene M., Ndiaye M., Barboza F.S., Sene M., Diatta W., Sarr A., Ndiaye-Sy A., Dieye A.M., Sy G.Y. Activité anti-inflammatoire de l’extrait aqueux des feuilles de Elaeis guineensis Jacq. (ARECACEAE) sur l’oedème aigu de la patte de rat induit par la carraghènine. Int. J. Biol. Chem. Sci. 2016;10:2568–2574. doi: 10.4314/ijbcs.v10i6.13. [DOI] [Google Scholar]
- Sharma V., Janmeda P. Extraction, isolation and identification of flavonoid from Euphorbia neriifolia leaves. Arab. J. Chem. 2017;10:509–514. doi: 10.1016/j.arabjc.2014.08.019. [DOI] [Google Scholar]
- Sidorczuk D., Kozanecki M., Civalleri B., Pernal K., Prywer J. Structural and optical properties of struvite. elucidating structure of infrared spectrum in high frequency range. J. Phys. Chem. A. 2020;124:8668–8678. doi: 10.1021/acs.jpca.0c04707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulimani R., Younos C., Jarmouni-Idrissi S., Bousta D., Khalouki F., Laila A. Behavioral and pharmaco-toxicological study of Papaver rhoeas L. in mice. J. Ethnopharmacol. 2001;74:265–274. doi: 10.1016/S0378-8741(00)00383-4. [DOI] [PubMed] [Google Scholar]
- Soulimani R., Younos C., Jarmouni-Idrissi S., Bousta D., Khallouki F., Khalouki F., Laila A. Behavioral and pharmaco-toxicological study of Papaver rhoeas L. in mice. J. Ethnopharmacol. 2001;74:265–274. doi: 10.1016/s0378-8741(00)00383-4. [DOI] [PubMed] [Google Scholar]
- Tadiwos Y., Nedi T., Engidawork E. Analgesic and anti-inflammatory activities of 80% methanol root extract of Jasminum abyssinicum Hochst. ex. Dc. (Oleaceae) in mice. J. Ethnopharmacol. 2017;202:281–289. doi: 10.1016/j.jep.2017.02.036. [DOI] [PubMed] [Google Scholar]
- Taleb, M.S., 2017. Aromatic and Medicinal Plants in Morocco: Diversity and Socio-Economic Role 11, 5.
- Tlemcani S., Lahkimi A., Eloutassi N., Bendaoud A., Hmamou A., Bekkari H. Ethnobotanical study of medicinal plants in the Fez-Meknes region of Morocco. J. Pharm. Pharmacogn. Res. 2023;11:137–159. doi: 10.56499/jppres22.1459_11.1.137. [DOI] [Google Scholar]
- Todorova T., Pesheva M., Gregan F., Chankova S. Antioxidant, antimutagenic, and anticarcinogenic effects of Papaver rhoeas L. extract on Saccharomyces cerevisiae. J. Med. Food. 2015;18:460–467. doi: 10.1089/jmf.2014.0050. [DOI] [PubMed] [Google Scholar]
- Vezza T., Rodríguez-Nogales A., Algieri F., Utrilla M.P., Rodriguez-Cabezas M.E., Galvez J. Flavonoids in inflammatory bowel disease: A review. Nutrients. 2016;8:211. doi: 10.3390/nu8040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinegar R., Schreiber W., Hugo R. Biphasic development of carrageenin edema in rats. J. Pharmacol. Exp. Ther. 1969;166:96–103. [PubMed] [Google Scholar]
- Winter C.A., Risley E.A., Nuss G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- Yubin, J., Miao, Y., Bing, W., Yao, Z., 2014. The extraction, separation and purification of alkaloids in the natural medicine 8.
- Zhang Y., Tang Y., Xu J., Zhang D., Lu G., Jing W. Modulation of polyepoxysuccinic acid on crystallization of calcium oxalate. J. Solid State Chem. 2015;231:7–12. doi: 10.1016/j.jssc.2015.08.001. [DOI] [Google Scholar]
- Zhang S.-Y., Zheng C.-G., Yan X.-Y., Tian W.-X. Low concentration of condensed tannins from catechu significantly inhibits fatty acid synthase and growth of MCF-7 cells. Biochem. Biophys. Res. Commun. 2008;371:654–658. doi: 10.1016/j.bbrc.2008.04.062. [DOI] [PubMed] [Google Scholar]