Abstract
Clinical outcomes from granulocyte transfusion (GTX) are disadvantaged by the short shelf life and compromised function of donor neutrophils. Spontaneous neutrophil death is heterogeneous and mediated by multiple pathways. Leveraging mechanistic knowledge and pharmacological screening, we identified a combined treatment, caspases–lysosomal membrane permeabilization–oxidant–necroptosis inhibition plus granulocyte colony-stimulating factor (CLON-G), which altered neutrophil fate by simultaneously targeting multiple cell death pathways. CLON-G prolonged human and mouse neutrophil half-life in vitro from less than 1 day to greater than 5 days. CLON-G–treated aged neutrophils had equivalent morphology and function to fresh neutrophils, with no impairment to critical effector functions including phagocytosis, bacterial killing, chemotaxis, and reactive oxygen species production. Transfusion with stored CLON-G–treated 3-day-old neutrophils enhanced host defenses, alleviated infection-induced tissue damage, and prolonged survival as effectively as transfusion with fresh neutrophils in a clinically relevant murine GTX model of neutropenia-related bacterial pneumonia and systemic candidiasis. Last, CLON-G treatment prolonged the shelf life and preserved the function of apheresis-collected human GTX products both ex vivo and in vivo in immunodeficient mice. Thus, CLON-G treatment represents an effective and applicable clinical procedure for the storage and application of neutrophils in transfusion medicine, providing a therapeutic strategy for improving GTX efficacy.
INTRODUCTION
Neutropenia and associated bacterial and fungal infections are the most important dose-limiting consequences for patients receiving chemotherapy, radiotherapy, or hematopoietic cell transplantation. Without prompt medical intervention, infections can become life-threatening (1), and not all patients respond to antibiotic therapy (2). An alternative preventative approach is granulocyte colony-stimulating factor (G-CSF) treatment (3). However, this therapy often does not work before the bone marrow has recovered and is associated with side effects such as bone pain, headache, fatigue, and nausea. Long-term use of G-CSF also potentially increases the risk of leukemia (4). Granulocyte transfusion (GTX), also known as neutrophil transfusion, is a therapeutic option for life-threatening bacterial and fungal infections in severely neutropenic patients (5, 6). Large doses of neutrophils can now be easily obtained from healthy donors who have been stimulated with G-CSF. Nevertheless, the clinical benefit of GTX is still compromised by short ex vivo shelf lives and rapid in vivo death of granulocytes (7). Although the efficacy of current GTX practices is controversial (8), GTX is still believed to be of value in appropriate clinical settings, such as when the absolute neutrophil count is <500 cells/μl, when there is evidence of bacterial or fungal infection, or in the case of unresponsiveness to antimicrobial treatment, generally for at least 48 hours (6). The general consensus is that further research and development into granulocyte biology directed at improving neutrophil survival and function in GTX are urgently required.
Neutrophil death is a heterogeneous process mediated by both apoptotic and lytic death pathways (9). We previously showed that reactive oxygen species (ROS) are accumulated in aging neutrophils and ROS-induced phosphatidylinositol 3,4,5-trisphosphate signaling deactivation mediates neutrophil death (10, 11). These ROS have also been shown to be responsible for membrane damage and initiation of cellular demise pathways (12). We also discovered that caspase-3 cleavage and activation are independent of canonical caspase-8– or caspase-9–mediated pathways in aging neutrophils, instead mediated by cytosolic serine protease PR3, which is sequestered in granules in fresh neutrophils and released to the cytosol during aging by ROS-induced lysosomal membrane permeabilization (LMP), leading to pro–caspase-3 cleavage and apoptosis (12). Lytic neutrophil death can be mediated by gasdermin D (GSDMD), which is cleaved and activated to produce an N-terminal fragment (GSDMD-NT) to trigger membrane rupture and subsequent pyroptosis (13). Necroptosis is another programmed form of necrosis, but its role in neutrophil biology is less understood. Human neutrophils were recently reported to undergo necroptosis after exposure to granulocyte-macrophage colony-stimulating factor followed by ligation of adhesion receptors such as CD44, CD11b, CD18, or CD15 (14). Here, we sought to improve the efficacy of neutrophil transfusion by leveraging these multiple neutrophil death mechanisms and prolonging neutrophil survival without compromising their function. First, pharmacological screening revealed a combined treatment that delayed neutrophil death by simultaneously targeting multiple death pathways. CLON-G (caspases-LMP-oxidant-necroptosis inhibition plus G-CSF) treatment altered neutrophil fate, increasing the ex vivo shelf life of collected neutrophils without affecting their in vivo function, improving GTX efficacy in clinically relevant murine models of GTX in neutropenic hosts. These results provide a clinical strategy for the storage and application of neutrophils in transfusion medicine, solidifying CLON-G treatment as a therapeutic approach for improving GTX efficacy.
RESULTS
Pharmacological screening identifies a combined treatment that delays neutrophil death by simultaneously targeting multiple death pathways
Programed neutrophil death is a heterogeneous process mediated by multiple pathways (fig. S1). We treated human or murine neutrophils with various inhibitors of neutrophil death–related pathways including caspases, LMP, serine proteases, ROS/NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) oxidase, and G-CSF (tables S1 and S2). Alone, the pan-caspase inhibitor Q-VD-Oph had the strongest effect on neutrophil death; most other drugs were ineffective. For maximal inhibition, we simultaneously blocked multiple death pathways. Cotreatment with the antioxidant N-acetyl cysteine (NAC), LMP inhibitors Heat shock protein 70 (HSP70) and deferoxamine mesylate (DFO; an iron chelator), phosphoinositide 3-kinase/Akt pathway stimulator G-CSF, and pan-caspase inhibitor Q-VD-Oph prolonged both human (table S1) and mouse (table S2) neutrophil survival. We named this treatment CLO-G (caspases-LMP-oxidant inhibition plus G-CSF).
The full role of necroptosis in neutrophil spontaneous death is unknown. However, necroptosis is often induced when caspases are inhibited, so although treatment with the pan-caspase inhibitor Q-VD-Oph prolonged neutrophil survival, it may also induce necroptosis to ultimately contribute to the death of CLO-G–treated neutrophils. Neutrophil death was further inhibited by combined treatment with CLO-G and a pharmacological inhibitor of necroptosis, Nec-1 s, which is a highly specific small-molecule antagonist of receptor-interacting protein kinase 1. This treatment (Q-VD-Oph, G-CSF, NAC, HSP70, DFO, and Nec-1 s), named CLON-G, showed the best antideath activity, increasing human and mouse neutrophil survival to greater than 90% after 3 days of culture (tables S1 and S2).
It is well known that cell adhesion can trigger integrin-mediated ROS production and subsequently accelerated neutrophil death (15). However, although a Rac guanosine triphosphatase inhibitor, NSC23766 (16), effectively inhibited N-formyl-Met-Leu-Phe (fMLP)–induced neutrophil polarization (fig. S2), it did not alter neutrophil half-life on its own or in combination with other inhibitors, suggesting that cell adhesion may not be a substantial factor in our system in which high serum concentrations kept most neutrophils nonadherent (tables S1 and S2).
CLON-G treatment prolongs the ex vivo half-life of human and mouse neutrophils from less than 24 hours to greater than 5 days
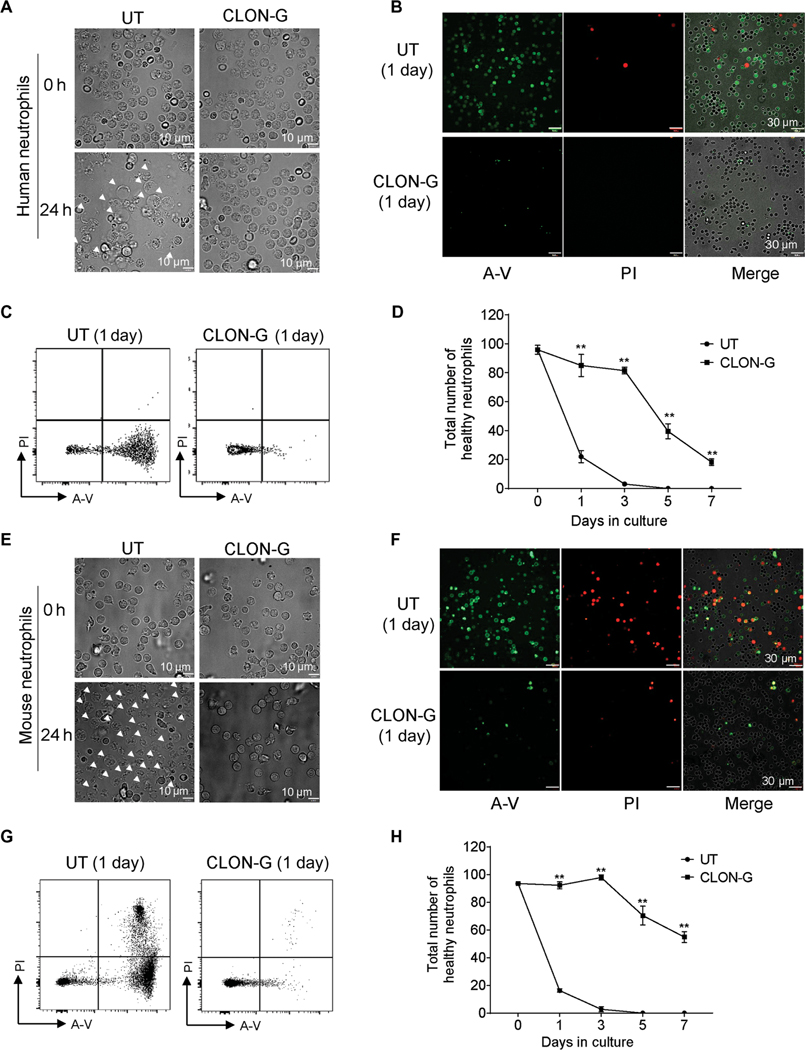
Having determined that CLON-G effectively delayed neutrophil spontaneous death, we next examined its effect on cultured neutrophils in detail. Because many neutrophils died by lytic cell death, the total number of detectable neutrophils, including healthy and dying cells, decreased gradually (Fig. 1A and fig. S3A). For human neutrophils, about 94 and 90% cells remained after 1 and 3 days of CLON-G treatment, respectively, whereas only 68 and 17% cells remained after 1 and 3 days in the untreated group (fig. S3A). We further used fluorescein isothiocyanate (FITC)–annexin V (A-V.) to detect phosphatidylsine (PS) exteriorization and propidium iodide (PI), a membrane-impermeable dye, to monitor cell membrane integrity and to quantify the percentage of dying cells in the remaining intact neutrophils (Fig. 1B and fig. S3B). CLON-G reduced the percentage of dying mouse or human neutrophils (PI+AV+ and PI−AV+) at each time point (Fig. 1C and fig. S3C). Untreated, only <22% healthy human and mouse neutrophils remained after 24 hours, indicating a half-life of less than 24 hours. CLON-G treatment increased neutrophil half-life to more than 5 days, with about 90% survival of neutrophils after 3 days of culture compared to >95% cell disappearance or death in untreated controls (Fig. 1D). Similar results were observed in mouse neutrophils (Fig. 1, E to H, and fig. S3, D and E). The CLON-G effect was not due to augmented cell division because minimal cell proliferation was detected in neutrophil cultures and CLON-G treatment did not influence the proliferating capacity of contaminating neutrophil progenitors (fig. S4). For mouse neutrophils, even after 7 days of culture, >60% of the initial neutrophil count remained in the CLON-G–treated group (Fig. 1H), equivalent to a greater than 7-day half-life.
Fig. 1. CLON-G, a treatment that simultaneously targets multiple death mechanisms, delays neutrophil death, increasing neutrophil half-life from less than 1 day to greater than 5 days.
(A) Freshly isolated human peripheral blood neutrophils were cultured in RPMI 1640 with 20% FBS with or without CLON-G. The morphologies of untreated (UT) or CLON-G–treated (CLON-G) cells were observed after 1 day by light microscopy. White arrowheads indicate dying cells. (B and C) Neutrophils were stained with FITC–annexin V (A-V; green) and PI (red) after culturing for 1 day. Cell death was assessed by confocal fluorescence microscopy (B) and flow cytometry (C). (D) The total number of healthy neutrophils was calculated in untreated and CLON-G– treated cell populations at the indicated time points as the number of remaining cells (intact cells in fig. S3A) multiplied by the proportion of double-negative cells by flow cytometry (shown in fig. S3C). (E) The morphologies of untreated or CLON-G–treated murine neutrophils were observed after 1 day by light microscopy. White arrowheads indicate dying cells. (F and G) Murine neutrophils were stained with FITC–A-V and PI after culturing for 1 day. Cell death was assessed by confocal fluorescence microscopy (F) and flow cytometry (G). (H) The total number of healthy neutrophils was calculated in untreated and CLON-G–treated cell populations at the indicated time points. All data are presented as means ± SD of three experiments. **P < 0.001 compared to the corresponding untreated group.
Previous study showed that G-CSF treatment alone can prolong neutrophil life (15). However, compared to CLON-G treatment, G-CSF only modestly improved neutrophil survival with about 55 and 15% human (fig. S5, A to D) and 40 and 15% mouse (fig. S5, E to H) healthy neutrophils remaining after 1 and 3 days of treatment, respectively. This confirms that the prosurvival effect elicited by CLON-G treatment was not solely due to G-CSF (tables S1 and S2 and fig. S5).
Physical stimuli such as pipetting easily fragment neutrophils undergoing lytic cell death, so we used a recently developed live imaging technology (9) to simultaneously analyze apoptotic and lytic neutrophil death. Mouse neutrophils with enlarged and swollen morphology [“puffed” cells (9)] were a predominant (~15% after 24 hours) population of dead cells (fig. S6A), with two populations observable by microscopy: PI single-positive cells and AV/PI double-positive cells, with PI positivity in all cells suggesting that membrane integrity was always disrupted. Puffed cells were larger (15 to 20 μm) than average neutrophils (~8 to 10 μm). On the basis of these morphological features, puffed cells were distinct from apoptotic neutrophils and likely to be cells undergoing lytic cell death and lost during sample preparation so undetectable by flow cytometry. Flow cytometry analysis mainly detected nonlytic apoptotic neutrophils including PI single-positive cells, AV single-positive cells, and AV/ PI double-positive cells (fig. S6B). There was a progressive increase in both puffed and apoptotic cells during neutrophil aging, and both populations decreased with CLON-G treatment (fig. S6, A and B). A similar effect was also apparent in human neutrophils (fig. S7). Collectively, CLON-G treatment likely suppressed both apoptotic and lytic neutrophil death, consistent with CLON-G–induced inhibition of multiple death pathways. Overall, CLON-G treatment prolonged neutrophil survival in vitro, increasing their half-life from less than 24 hours to greater than 5 days.
Drug removal does not accelerate the death of CLON-G–treated neutrophils
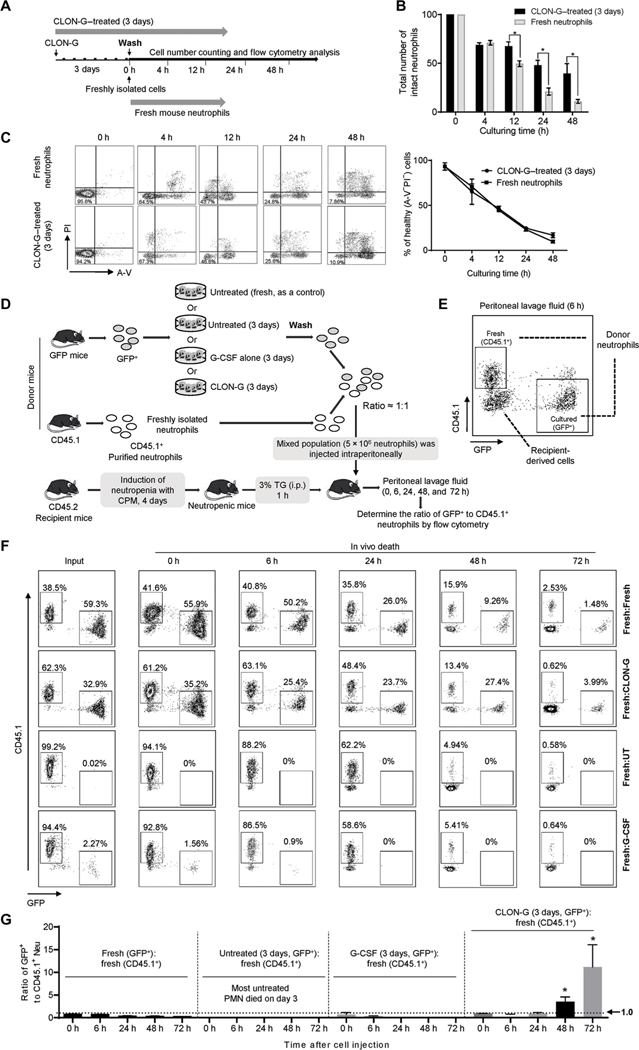
For clinical application, neutrophil life span–prolonging drugs will likely need to be removed before GTX. To establish whether temporary CLON-G treatment sufficiently maintains neutrophil survival, we examined the life span of pharmacologically treated neutrophils in the absence of drugs (Fig. 2A and fig. S8A). After 3 days of CLON-G treatment, washed mouse neutrophils displayed a similar rate of decrease in the total number of healthy cells (Fig. 2B) and a similar percentage of AV−PI− cells (Fig. 2C) as untreated fresh neutrophils in the first 4 hours. However, at and after 12 hours, the number of healthy cells in the 3-day-old CLON-G–treated neutrophil population was higher than in the untreated fresh neutrophil population. This prolonged prosurvival effect was apparent in both mouse (Fig. 2B) and human (fig. S8, B and C) neutrophils, probably because of prolonged intracellular drug retention. To confirm this, we treated fresh neutrophils with CLON-G for 1 hour and then examined the survival of the wash neutrophils over time (fig. S8D). Similarly, the prosurvival effect of CLON-G lasted for more than 3 days after the treated human (fig. S8E) or mouse (fig. S8F) neutrophils were washed with drug-free medium. These results strongly suggest that CLON-G treatment not only can increase the ex vivo shelf life of the collected granulocytes but also may delay in vivo death of transfused granulocytes because of the prolonged intracellular retention of the drugs (or drug effects).
Fig. 2. CLON-G–treated mouse neutrophils display a similar or even longer life span than fresh neutrophils after drug removal.
(A) Mouse neutrophils were treated with CLON-G for 3 days in vitro followed by removing CLON-G by changing the cell culture medium to normal medium (CLON-G–treated, 3 days). At the same time, freshly isolated neutrophils were cultured in the normal medium without CLON-G (Fresh mouse neutrophils). (B) Total numbers of intact neutrophils were counted at the indicated time points. All data are presented as means ± SD of three experiments. *P < 0.01. (C) Cells were stained with FITC–A-V and PI and then analyzed by flow cytometry. The percentage of healthy neutrophils was assessed accordingly. All data are presented as means ± SD of three experiments. (D) Experimental scheme for assessing the relative in vivo death rate of transplanted fresh, G-CSF alone–treated, and CLON-G–treated neutrophils in a mouse peritonitis model. TG, thioglycolate; i.p., intraperitoneal. (E) Cells in peritoneal lavage fluid were stained with PE-CD45.1 and analyzed by flow cytometry. (F) Flow cytometry analysis of donor neutrophils in peritoneal lavage in mice transplanted with indicated neutrophil populations at each time point. Shown are representative flow cytometry plots from one of three independent experiments. (G) The ratio of indicated transplanted neutrophil populations in peritoneal lavage fluid. All data are presented as means ± SD of three experiments. *P < 0.01 compared to 0 hours.
Next, we investigated whether washed, CLON-G–treated 3-day-old and untreated fresh neutrophils had a similar life span at inflammatory sites in vivo. GTX is mainly used clinically for life-threatening bacterial and fungal infections in severely neutropenic patients. To mimic this clinical scenario, we established a mouse model in which neutropenia was induced by the widely used chemotherapy drug cyclophosphamide (CPM) (fig. S9A) (17). On day 4, CPM-treated wild-type mice contained about 95% fewer circulating neutrophils than untreated (day 0) control (fig. S9B). The lower limit of normal neutrophil numbers in humans is 1500 cells/mm3; in patients receiving chemotherapy or radiotherapy, neutrophil counts can be as low as 100/mm3 (93.3% reduction). Thus, the 95% reduction achieved in CPM-induced neutropenia is comparable to neutropenia in patients receiving chemotherapy. In this experiment, the CLON-G–treated 3-day-old neutrophils were isolated from a green fluorescent protein (GFP)–expressing mouse and were thus positive for GFP expression. Untreated fresh neutrophils were prepared from a CD45.1 donor mouse. A mixed population (5 million) was intraperitoneally injected into a CD45.2 recipient mouse (Fig. 2D). Drug-treated and untreated fresh neutrophils were identified by expression of CD45.1 or GFP using flow cytometry analysis (Fig. 2E). At early time points, neutrophil death does not play a major role in inflammation resolution (10), so the ratio of the two neutrophil populations remained unaltered at 6 hours. The relative half-life of untreated and CLON-G–treated neutrophils in the inflamed peritoneal cavity was also assessed at later time points, when considerable neutrophil death had occurred. The ratio of CLON-G–treated 3-day-old neutrophils to fresh neutrophils started to increase at 24 hours and reached a ratio of greater than 10:1 3 days after the adoptive transfer, indicating that the CLON-G–treated 3-day-old neutrophils had a lower death rate than untreated fresh neutrophils at the inflammatory site (Fig. 2, F and G). This result confirms that CLON-G treatment can even promote the survival of transfused granulocytes in vivo due to the prolonged intracellular retention of the drugs. In contrast, consistent with the in vitro results (fig. S5), treatment with G-CSF alone failed to effectively protect neutrophils from aging, with G-CSF–treated 3-day-old neutrophils showing similar survival at the inflammatory site compared to fresh neutrophils (Fig. 2, F and G). In this in vivo assay, the survival of untreated neutrophils in the recipient mice could not be assessed after 3 days of ex vivo culture, when most neutrophils had already died (Fig. 1 and fig. S5).
Stored CLON-G–treated neutrophils function normally and are recruited to the site of infection as efficiently as transfused fresh neutrophils
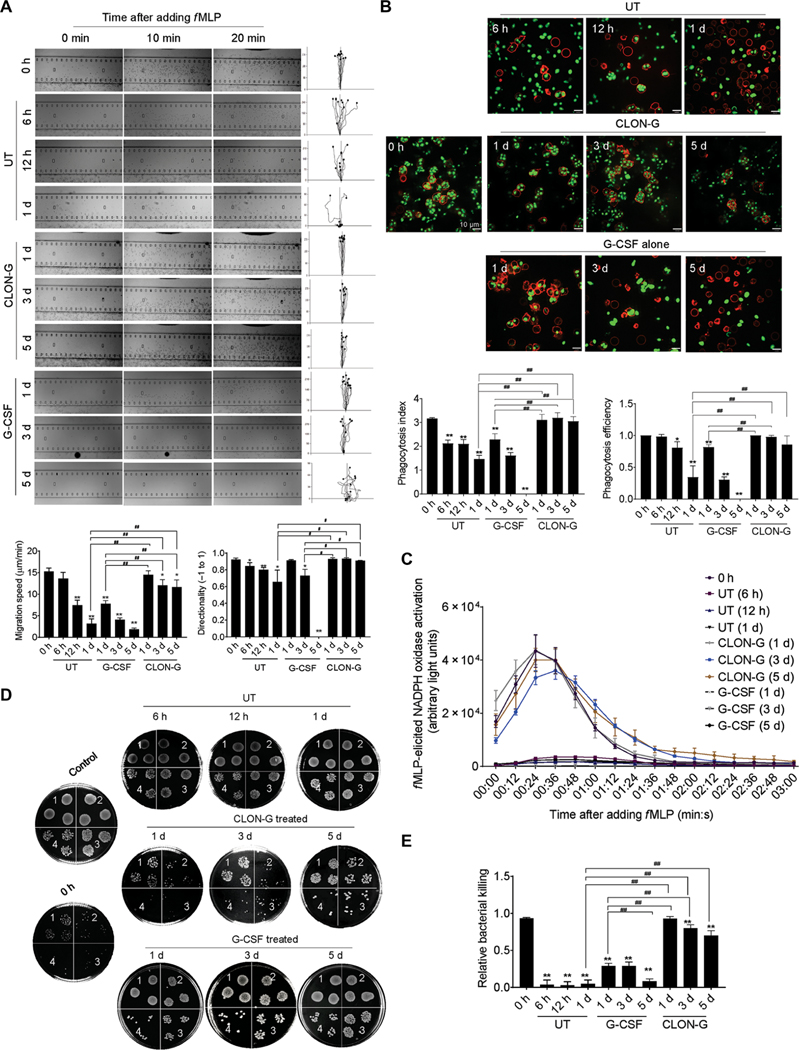
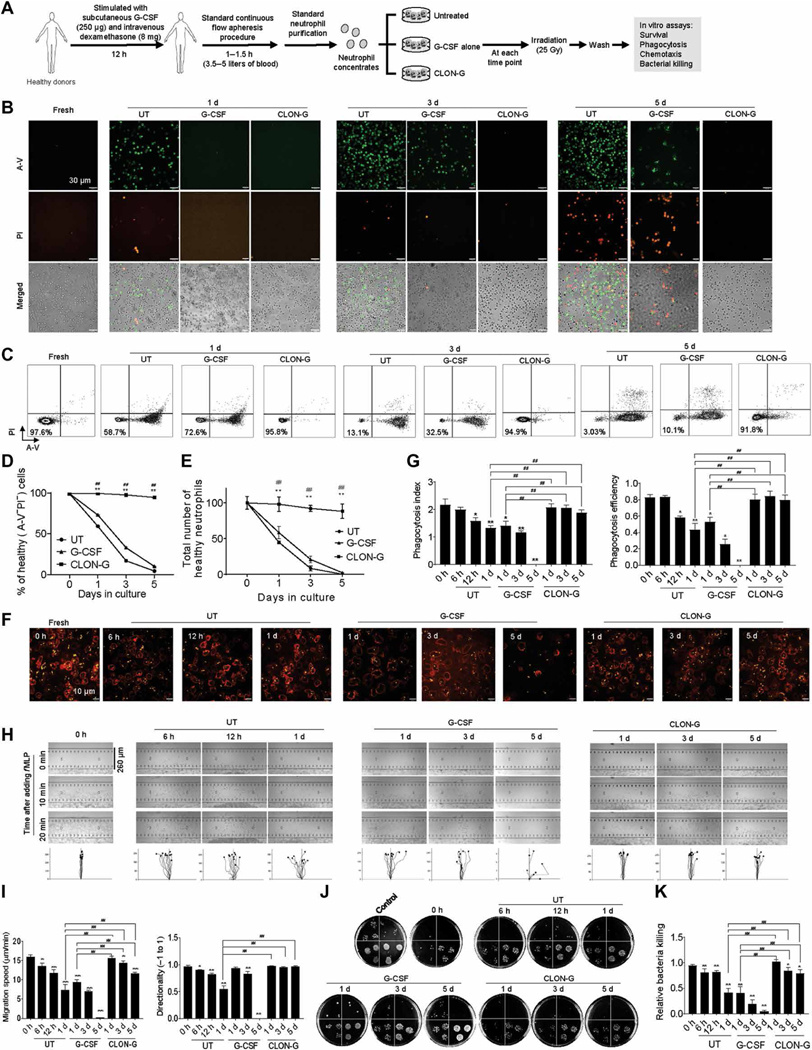
We next explored whether CLON-G treatment affected mouse and human primary neutrophil functions critical for innate immunity and host defenses. First, we investigated whether CLON-G treatment impaired neutrophil chemotaxis using the EZ-TAXIScan apparatus, in which a stable chemoattractant gradient is formed in a 260-μm-wide channel, enabling direct visualization of neutrophil chemotaxis (17, 18). CLON-G–treated neutrophils were exposed to an fMLP gradient, and cell movement was recorded by time-lapse microscopy and analyzed using the Tracking Tool software. Both mouse and human neutrophils displayed the same directionality as freshly isolated neutrophils even after 5 days of CLON-G treatment (Fig. 3A and fig. S10A), and neutrophils treated for 1 day moved at the same speed as untreated fresh neutrophils. Neutrophils treated with CLON-G for 5 days migrated much faster than untreated or G-CSF alone–treated neutrophils cultured for only 1 day (Fig. 3A and fig. S10A).
Fig. 3. CLON-G treatment does not impair the migration and bacterial killing capability of mouse neutrophils.
Freshly isolated mouse bone marrow neutrophils were cultured as described in Fig. 1 and fig. S5. The function of untreated, G-CSF–treated, and CLON-G–treated neutrophils was assessed at indicated time points. (A) The chemotactic migration of neutrophils to fMLP (1 μM) was assessed using an EZ-TAXIScan device. Migration velocity and directionality were calculated. (B) In vitro phagocytosis capacity of neutrophils (APC-Ly6G, red) was measured using zymosan (S. cerevisiae) bioparticles (green). Phagocytosis efficiency was expressed as the percentage of neutrophils that engulfed at least one bioparticle. Phagocytosis index was expressed as the average number of internalized particles per cell. At least 200 cells were assessed for each sample. (C) fMLP-induced NADPH oxidase activation in mouse neutrophils was assessed over time by luminol chemiluminescence. (D) In vitro killing of E. coli by mouse neutrophils. The bacterial killing capabilities were reflected by the decrease in CFU. 1, 2, 3, and 4 indicate serial dilution in the bacterial colony assay (1, 10× dilution; n, 10n× dilution). Control, bacterial suspension without any cells; 0 h, fresh neutrophils. (E) The relative bacterial killing was measured as the proportion of bacteria killed in 60 min. All data are presented as means ± SD of three experiments. *P < 0.05 and **P < 0.001 compared to freshly isolated neutrophil group (0 h). #P < 0.05 compared to untreated (or G-CSF alone–treated) neutrophils cultured for 1 day. ##P < 0.001 compared to untreated (or G-CSF alone–treated) neutrophils cultured for 1 day.
Next, phagocytic capability was assessed by measuring neutrophil engulfment of opsonized fluorescein-conjugated Escherichia coli or zymosan (Saccharomyces cerevisiae) bioparticles (17, 18). CLON-G–treated murine neutrophils had a comparable phagocytosis efficiency (the percentage of cells that engulfed at least one bioparticle) and phagocytosis index (the number of internalized particles per 100 cells) (13, 17–19) to fresh neutrophils, even after 5 days of culture (Fig. 3B). In contrast, the phagocytosis efficiency of untreated or G-CSF alone–treated neutrophils was lower even after 1 day of culture. Thus, CLON-G treatment augmented phagocytosis capability of aging neutrophils. Similar results were observed in human neutrophils (fig. S10B). Phagocytes are known to use NADPH oxidase–dependent ROS release to clear pathogenic organisms during host defense responses. Recent studies have also demonstrated unconventional roles for ROS and NADPH oxidase activation in signal transduction and cell function. Similarly, fMLP-induced NADPH oxidase activation in stored CLON-G–treated cells, but not untreated or G-CSF alone–treated cells, was similar to that in freshly purified neutrophils, even after 5 days of culture (Fig. 3C and fig. S10C).
We also investigated the effect of CLON-G treatment on the in vitro bacterial killing capability of neutrophils by measuring the reduction in colony-forming units (CFU) in the presence of mouse or human neutrophils (13, 17, 18). Stored CLON-G–treated, but not untreated or G-CSF alone–treated, neutrophils were as efficient as untreated fresh neutrophils in clearing live E. coli (Fig. 3, D and E, and fig. S10, D and E).
Last, we investigated in vivo recruitment of transfused neutrophils to the inflammatory site using the peritonitis model. The trafficking of transfused CLON-G–treated 1-day-old GFP+ and untreated fresh CD45.1+ neutrophils was assessed in the same CD45.2 recipient mice (fig. S11A). At early time points (2 and 6 hours after transfusion) when neutrophil death did not play a major role (10), the number of transfused neutrophils in the peritoneal cavity solely depended on the recruitment efficiency. It increased gradually, with ~3 × 104 transfused neutrophils detected in the peritoneal exudate 6 hours after neutrophil intravenous injection (fig. S11B). The ratio of transfused CLON-G–treated to untreated fresh neutrophils in the peritoneal cavity remained unaltered, indicating that the two neutrophil populations were recruited to the inflamed site with a similar efficiency (fig. S11, C and D). Consistent with the ex vivo study, untreated and G-CSF–treated 1-day-old neutrophils exhibited reduced recruitment efficiency compared to fresh neutrophils (fig. S11, C and D).
Transfused CLON-G–treated 3-day-old neutrophils are recruited to inflamed lungs as efficiently as transfused fresh neutrophils in neutropenia-related pneumonia
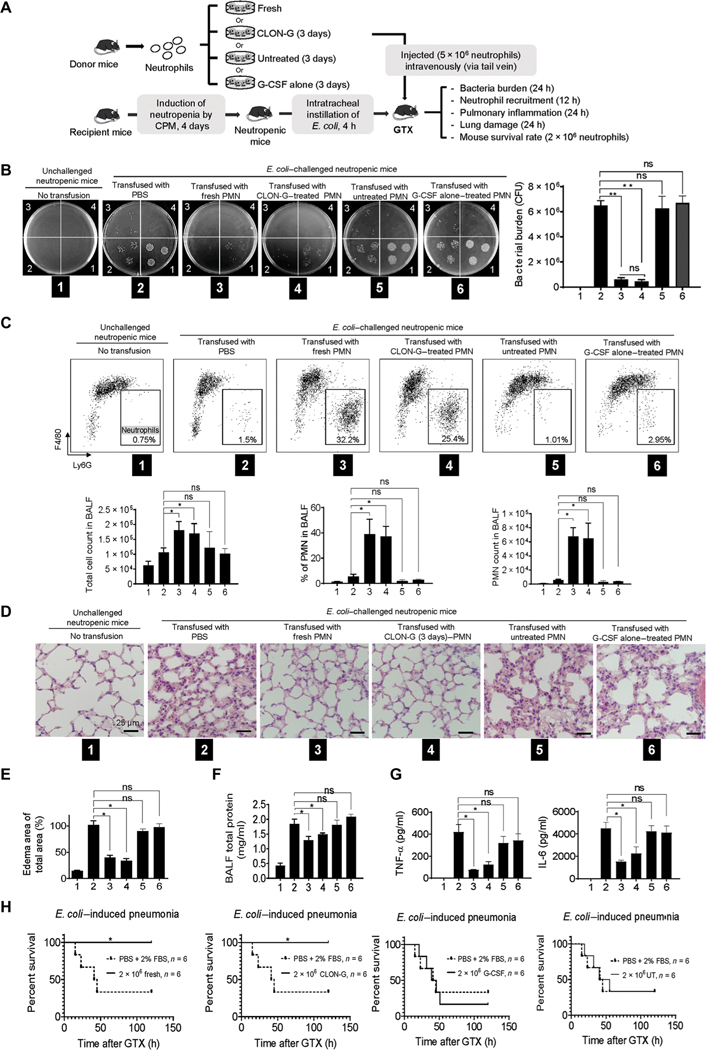
Next, we investigated the survival and function of transfused neutrophils in vivo in a clinically relevant mouse E. coli pneumonia model (fig. S12A) (17, 20). Very few neutrophils were detected in the lungs of unchallenged mice. A total of ~1 × 106 neutrophils were detected in the bronchoalveolar lavage fluid (BALF) 24 hours after bacteria instillation (fig. S12, B and C). Neutrophil recruitment was also apparent when the number of emigrated neutrophils in alveolar air spaces was quantified by histomorphometric analysis of lung sections (fig. S12, D and E). E. coli–induced pulmonary inflammation was associated with edema formation (fig. S12, F and G) and inflammatory cytokine production (fig. S12H).
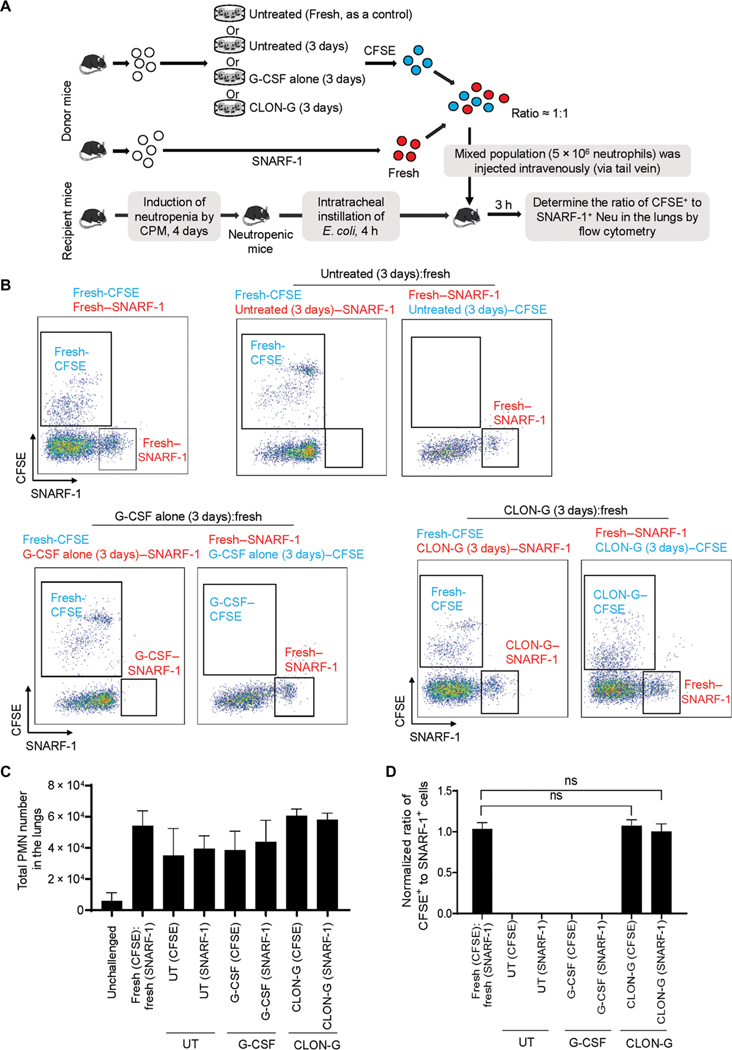
The unsatisfying clinical outcome of GTX is largely due to inefficient accumulation of transfused neutrophils at sites of infection. We therefore examined whether storing neutrophils in CLON-G–containing medium altered the recruitment of transfused neutrophils to inflamed lungs. To distinguish transfused from endogenous neutrophils (about 5 to 10% of normal abundance), we labeled untreated fresh neutrophils with the intracellular fluorescent dye 5-(and −6)-carboxyfluorescein diacetate succinimidyl esters (CFSEs; green) and CLON-G–treated 3-day-old neutrophils with 5-(and −6)-chloromethyl seminaphtorhodafluor-1-acetoxymethylester (SNARF-1) acetate (red) or vice versa. Cell labeling does not alter neutrophil life span or function (17, 21). Clinically, GTX is often administered after an infection has been detected. Accordingly, the mixed (1:1) population was transfused into the same neutropenic recipient mouse 4 hours after E. coli instillation (Fig. 4A), allowing us to compare the trafficking of the two neutrophil types in exactly the same environment. Untreated fresh and CLON-G–treated 3-day-old neutrophils were handled identically before being mixed and studied in parallel, thereby controlling for the effects of ex vivo manipulation. E. coli challenge triggered neutrophil accumulation in the lungs (Fig. 4B). Transfused untreated fresh and CLON-G–treated 3-day-old neutrophils accumulating in the lungs were identified by their distinct fluorescent labels by flow cytometry analysis (Fig. 4C). At an early time point (3 hours), when neutrophil death does not play a major role in inflammation resolution (10), there remained a 1:1 ratio of transfused 3-day-old CLON-G–treated to transfused untreated fresh neutrophils in the lungs (Fig. 4D), suggesting that CLON-G treatment did not compromise recruitment of transfused neutrophils to the lungs in vivo in neutropenic mice. Instead, CLON-G treatment prevented aging-related reduction of neutrophil recruitment capability. As a further comparison, untreated and G-CSF–treated neutrophils exhibited reduced recruitment efficiency compared to fresh neutrophils (Fig. 4, B to D).
Fig. 4. CLON-G treatment does not impair neutrophil recruitment in vivo in a mouse neutropenia-related pneumonia model.
The fresh, untreated, G-CSF alone–treated, and CLON-G–treated neutrophils were prepared as described in Fig. 2D. (A) Neutropenic recipient mice were challenged with E. coli (5 × 103 CFU) and subsequently received transfer of indicated neutrophil populations. (B) The relative recruitment of transfused CFSE+ or SNARF-1+ fresh, untreated, G-CSF alone–treated, and CLON-G–treated neutrophils was assessed by flow cytometry. (C) The total neutrophil number is presented for each group as total cell number multiplied by the percentage of Ly6G+ cells calculated by flow cytometry. (D) The relative recruitment of fresh, untreated, G-CSF alone–treated, and CLON-G–treated neutrophils was calculated as the relative ratio of CFSE+ to SNARF-1+ cells. The ratio of CFSE+ to SNARF-1+ cells in each sample was determined by flow cytometry and normalized to the sample collected from the mice transfused with CFSE+ fresh neutrophils and SNARF-1+ fresh neutrophils. Data are presented as means ± SD of three experiments. ns, no significant difference.
Exaggerated accumulation or hyperactivation of neutrophils can lead to unwanted tissue damage. Induction of neutrophil apoptosis was proposed to be an effective strategy for terminating or resolving deleterious inflammatory responses (22). However, GTX is mainly used clinically for life-threatening bacterial and fungal infections in severely neutropenic patients. Neutrophil-induced tissue damage is less of a concern in neutropenic patients with drastically reduced neutrophil numbers in whom the release of harmful compounds such as oxidants, proteases, and DNA is minimal. Consistently, we did not observe any tissue damage elicited by transfusion of untreated or CLON-G–treated neutrophils in neutropenic recipient mice (fig. S13). In addition, we investigated whether transfusion of CLON-G–treated neutrophils exacerbated lung injury in a lipopolysaccharide-induced acute lung inflammation model. Again, we did not detect any detrimental effect of CLON-G–treated neutrophils after their transfusion to neutropenic hosts (fig. S13).
Transfusion with stored CLON-G–treated neutrophils enhances host defenses and alleviates infection-induced lung damage in neutropenia-related pneumonia
The efficacy of transfused neutrophils in GTX is ultimately reflected by the recipient’s capability to clear invading pathogens. Thus, we examined whether transfusion with stored CLON-G–treated aged neutrophils had a similar bactericidal capacity to untreated fresh neutrophils (Fig. 5A) in mice challenged with 5 × 103 CFU live E. coli. As expected, because of the lack of neutrophils in CPM-induced neutropenic mice, bacterial numbers gradually increased after initial instillation, reaching 6 × 106 after 24 hours (Fig. 5B). GTX enhanced bacterial killing in neutropenic recipient mice. When a substantial number of transfused neutrophils accumulated in the lungs, bacterial burden (the number of live bacteria in the lungs) decreased drastically, reflecting the bacteria-killing capability of transfused neutrophils (Fig. 5, B and C). Transfusion with CLON-G–treated 3-day-old neutrophils enhanced bacteria-killing capacity in neutropenic recipient mice as effectively as transfusion with fresh neutrophils, whereas transfusion with untreated or G-CSF alone–treated neutrophils was ineffective (Fig. 5, B and C).
Fig. 5. Transfusion with stored CLON-G–treated neutrophils enhances host defenses and alleviates infection-induced lung damage as effectively as transfusion with untreated fresh neutrophils.
The fresh, untreated, G-CSF alone–treated, and CLON-G–treated neutrophils were prepared as described in Fig. 2D. (A) Neutropenic mice were challenged with E. coli (5 × 103 CFU) and subsequently received transfer of indicated neutrophil populations. (B) BALF was collected and serially diluted (1, 10× dilution; n, 10n× dilution) with sterile cold water. Aliquots were spread on LB agar plates and incubated overnight at 37°C. Live bacteria were quantified as CFU per lung to determine bacterial killing. Data are presented as means ± SD of three experiments. **P < 0.001. (C) The total number of cells in the lungs was counted with a hemocytometer. Differential cell counts were determined by flow cytometry analysis. Cells were stained with F4/80 FITC and Ly6G APC. The total number of polymorphonuclear neutrophils (PMNs) recruited was calculated as follows: number of PMNs = cell density × volume × % PMN. Data are presented as means (± SD). n ≥ 4 mice in each group. *P < 0.05. (D) Hematoxylin and eosin staining of lung tissues showed pulmonary edema formation in infected lungs. (E) Pulmonary edema formation was quantified as the percentage of edema area in the total parenchymal region using ImageJ software. Data are presented as means ± SD of three experiments. *P < 0.05. (F) Protein accumulation in BALF was measured using a protein assay kit. The standard curve was constructed using bovine serum albumin (BSA). Data are presented as means ± SD of three experiments. *P < 0.05. (G) BALF chemokine and cytokine concentrations were determined by ELISA. Data are presented as means ± SD of three experiments. *P < 0.05. TNF-α, tumor necrosis factor–α. (H) Rate of mortality due to E. coli–induced pneumonia in mice transfused with indicated neutrophil population is shown by a Kaplan-Meier plot. Log-rank tests were used to analyze survival rates. *P < 0.01 as compared to PBS + 2% FBS control treatment.
To assess the severity of neutropenia-related pneumonia, lung tissue sections were examined by microscopy (Fig. 5D). Edema, a sign of lung inflammation, accumulated in the lungs as assessed by microscopy and quantified by morphometry (17, 20). Transfusion with CLON-G–treated 3-day-old or untreated fresh neutrophils inhibited edema formation, with no difference detected between the two populations. However, transfusion with untreated or G-CSF alone–treated neutrophils failed to produce the same protective effect (Fig. 5, D and E). Severe pneumonia is often accompanied by vascular leakage, with increased BALF protein concentrations used as an indicator of vascular leakage and a key parameter of inflammatory lung injury. Similarly, neutrophil transfusion reduced BALF protein concentrations, with no detectable difference in mice transfused with CLON-G–treated 3-day-old neutrophils and mice transfused with fresh neutrophils. BALF protein concentrations remained high when untreated or G-CSF alone–treated neutrophils were transfused (Fig. 5F). Last, the overall inflammatory response was evaluated by assessing concentrations of tumor necrosis factor–α and interleukin-6 (IL-6) in inflamed lungs by enzyme-linked immunosorbent assay (ELISA) as previously described (17, 20). Transfusion with CLON-G–treated 3-day-old neutrophils and untreated fresh neutrophils both reduced inflammatory cytokine concentrations in E. coli–challenged neutropenic recipients. In contrast, transfusion with untreated or G-CSF alone–treated neutrophils was unable to reduce inflammatory cytokine concentrations in E. coli–challenged neutropenic recipients (Fig. 5G).
Patients with neutropenia-related pneumonia can die from severe lung infections. Accordingly, we investigated whether transfusion with stored CLON-G–treated neutrophils increased the survival rate of challenged mice. When challenged with 5 × 103 live E. coli, >60% of neutropenic mice mock treated with phosphate-buffered saline (PBS) died within 2 days. GTX increased the survival rate of challenged mice. Similar to fresh neutrophils, transfusion with stored CLON-G–treated 3-day-old neutrophils, but not untreated or G-CSF alone–treated neutrophils, completely rescued E. coli–challenged neutropenic recipients (Fig. 5H). Together, consistent with the in vitro data, CLON-G treatment did not impair the bacterial killing capability of transfused neutrophils in vivo. Transfusion with stored CLON-G–treated neutrophils enhanced host defenses and alleviated infection-induced tissue damage as effectively as transfusion with fresh neutrophils.
Transfusion with stored CLON-G–treated 3-day-old neutrophils enhances host defenses as effectively as transfusion with fresh neutrophils in neutropenia-related fungal infection
Neutrophils are the principal effector immune cells against Candida albicans infection, which is more frequent in patients with neutropenia (23). Using a mouse model of systemic candidiasis, we assessed whether transfusion with stored CLON-G–treated neutrophils enhanced host defenses in neutropenia-related fungal infection as effectively as transfusion with untreated fresh neutrophils (fig. S14A). GTX increased neutrophil accumulation in the kidney, a major organ contributing to host defenses against C. albicans infection (fig. S14B). Consistently, neutrophil-transfused mice had a decreased fungal burden (fig. S14C), reversal of infection-induced body weight loss (fig. S14D), and improved survival (fig. S14E) compared to mice without GTX. Mice infected with C. albicans developed kidney necrosis and hemorrhage. Mice receiving GTX showed reduced kidney necrosis (fig. S14F), further indicating that GTX conferred a degree of protection against C. albicans–induced sepsis and disease. Transfusion with stored CLON-G–treated 3-day-old neutrophils was as effective as transfusion with fresh neutrophils (fig. S14, B to F). Therefore, CLON-G treatment represents a general strategy to alter neutrophil fate and improve GTX in neutropenia-related infections.
CLON-G treatment prolongs the shelf life of granulocyte apheresis concentrates
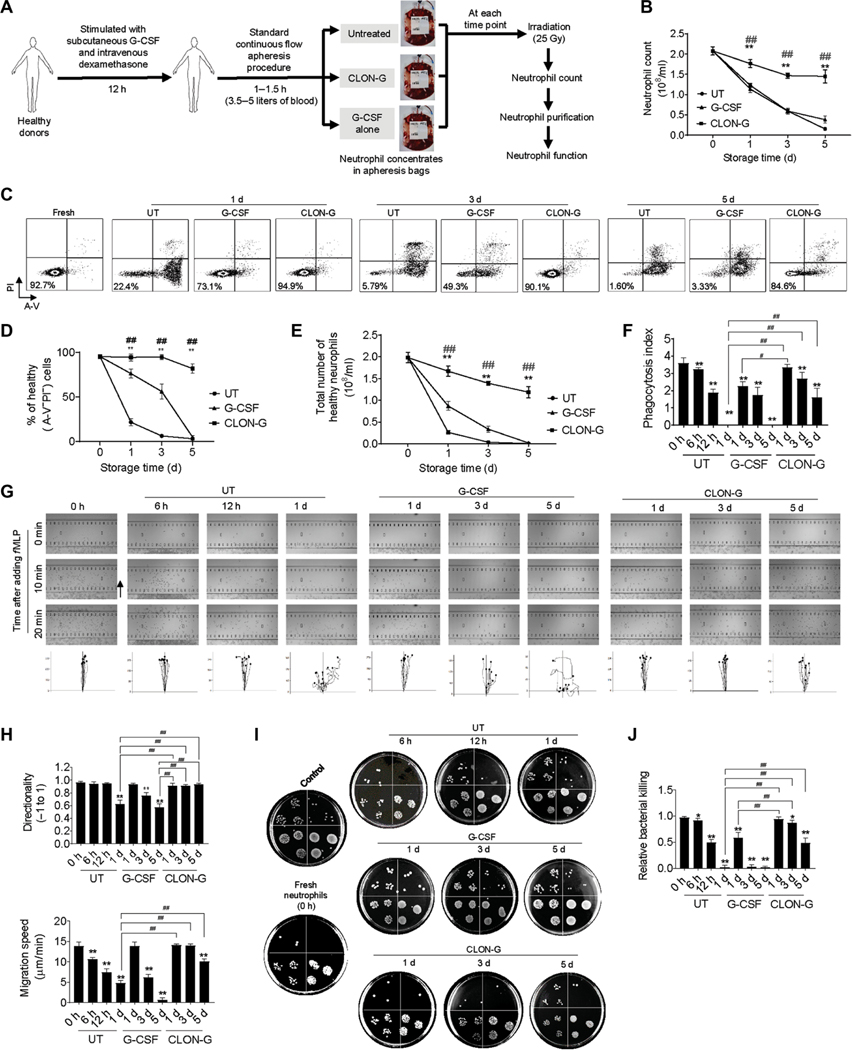
Neutrophils used for GTX are collected from the blood of healthy donors by automatic apheresis. The donors are often stimulated with G-CSF and dexamethasone to increase the abundance of circulating peripheral blood neutrophils by promoting demargination of neutrophils from the endothelial surface of blood vessels and mobilization of neutrophils from the bone marrow (6). In addition, neutrophils collected after the administration of G-CSF appear to be of better quality. We next investigated the effect of CLON-G on the survival and function of neutrophils in the granulocyte apheresis concentrates collected and processed following a standard GTX protocol (Fig. 6A). Clinically, apheresis concentrates are also routinely irradiated [25 grays (Gy)] before transfusion to render lymphocytes in the product incapable of dividing and eliminate the risk of transfusion-associated graft-versus-host disease (6). The irradiation procedure does not interfere with neutrophil function (6). To mimic this clinical situation, we irradiated neutrophils before each experiment (Fig. 6A). CLON-G treatment prolonged the survival of neutrophils in the granulocyte apheresis concentrates (Fig. 6, B to E). Stored CLON-G–treated 3-day-old, but not untreated or G-CSF alone–treated 1-day-old, neutrophils were as efficient as fresh neutrophils in undergoing phagocytosis (Fig. 6, F and G) and chemotaxis (Fig. 6, H and I) and clearing live E. coli (Fig. 6, J and K). Neutrophils retained the capacity to kill bacteria even after 5 days of CLON-G treatment, only showing a moderate 15% reduction in relative bacterial killing capability, compared to a more than 50% reduction in untreated or G-CSF alone–treated neutrophils cultured for only 1 day (Fig. 6K).
Fig. 6. CLON-G treatment delays spontaneous death of human neutrophils isolated from granulocyte apheresis concentrates without impairing their migration and bacterial killing capability.
(A) Granulocyte apheresis concentrates were collected and processed following a standard clinical GTX protocol. (B and C) Neutrophil death was assessed by confocal fluorescence microscopy (B) and flow cytometry (C). Neutrophils isolated from granulocyte apheresis concentrates were cultured in the presence of indicated compounds for the indicated time period and then stained with FITC–A-V and PI. Representative flow cytometry plots from one of three independent experiments are shown in (C). (D) The percentage of healthy neutrophils was calculated in untreated, G-CSF–treated, and CLON-G–treated cell populations at the indicated time points. Data are presented as means ± SD of three experiments. **P < 0.001 compared to the corresponding untreated group. ##P < 0.001 compared to the corresponding G-CSF–treated group. (E) The total number of healthy neutrophils was calculated as the number of remaining cells (intact cells counted using a hemocytometer) multiplied by the proportion of double-negative cells [shown in (D)]. Data are presented as means ± SD. **P < 0.001 compared to the corresponding untreated group. ##P < 0.001 compared to the corresponding G-CSF–treated group. (F) In vitro phagocytosis capacity of neutrophils (APCCD16, red) was measured using fluorescein-conjugated pHrodo–E. coli bioparticles (yellow). (G) Phagocytosis efficiency was expressed as the percentage of neutrophils that engulfed at least one bioparticle. Phagocytosis index was expressed as the average number of internalized particles per cell. At least 200 cells were assessed for each sample. Data are presented as means ± SD of three experiments. *P < 0.05 and **P < 0.001 compared to freshly isolated neutrophil group (0 h); ##P < 0.001 compared to untreated (or G-CSF alone–treated) neutrophils cultured for 1 day. (H and I) The chemotactic migration of neutrophils to fMLP (100 nM) was assessed using an EZ-TAXIScan device (H), and migration velocity and directionality were calculated (I). Data are presented as means ± SD of three experiments. *P < 0.05 and **P < 0.001 compared to freshly isolated neutrophil group (0 h); ##P < 0.001 compared to untreated (or G-CSF alone–treated) neutrophils cultured for 1 day. (J) The bacterial killing capabilities of neutrophils in vitro were reflected by the decrease in CFU of E. coli. Control, bacterial suspension without any cells; 0 h, fresh neutrophils. (K) The relative bacterial killing was measured as the proportion of bacteria killed in 60 min. Data are presented as means ± SD of three experiments. *P < 0.05 and **P < 0.001 compared to freshly isolated neutrophil group (0 h); ##P < 0.001 compared to untreated (or G-CSF alone–treated) neutrophils cultured for 1 day.
Moreover, to examine whether CLON-G treatment prolongs the shelf life of an actual clinical GTX product, we added the compounds directly to apheresis collection bags. Neutrophils in the granulocyte apheresis concentrates were isolated at each time point, and their survival and function were assessed using the same approaches described above (Fig. 7A). Similarly, CLON-G–treated, but not untreated or G-CSF alone–treated, neutrophils showed improved survival (Fig. 7, B to E). Neutrophil functions, including phagocytosis (Fig. 7F), chemotaxis (Fig. 7, G and H), and bacteria clearance (Fig. 7, I and J), were also better preserved in the presence of CLON-G. Thus, CLON-G treatment prolonged the life span of granulocytes in unmanipulated apheresis concentrates. This approach is applicable to the final goal of prolonging the shelf life of the current clinical standard GTX products.
Fig. 7. CLON-G treatment prolongs the shelf life of granulocyte apheresis concentrates.
(A) Granulocyte apheresis concentrates were collected and processed following a standard clinical GTX protocol. The apheresis products were treated with CLON-G without other manipulation. For functional analysis, neutrophils were purified after the CLON-G treatment. (B) Total neutrophil counts in granulocyte apheresis concentrates at indicated time points are shown. Neutrophils isolated from granulocyte apheresis concentrates were counted using a hemocytometer. All data are presented as means ± SD of three experiments. **P < 0.001 compared to the corresponding untreated group. ##P < 0.001 compared to the corresponding G-CSF–treated group. (C) Cell death was assessed by flow cytometry. Neutrophils isolated from granulocyte apheresis concentrates were stained with FITC–A-V and PI. Shown are representative flow cytometry plots from one of three independent experiments. (D) The percentage of healthy neutrophils that was calculated in untreated, G-CSF–treated, and CLON-G–treated granulocyte apheresis concentrates at the indicated time points is shown. All data are presented as means ± SD of three experiments. **P < 0.001 compared to the corresponding untreated group. ##P < 0.001 compared to the corresponding G-CSF–treated group. (E) The total number of healthy neutrophils was calculated as the total number of neutrophils [shown in (B)] multiplied by the proportion of double-negative cells [shown in (D)]. Data are presented as means ± SD. **P < 0.001 compared to the corresponding untreated group. ##P < 0.001 compared to the corresponding G-CSF–treated group. (F) In vitro phagocytosis capacity of neutrophils was measured using fluorescein-conjugated pHrodo–E. coli bioparticles. Phagocytosis index was expressed as the average number of internalized particles per cell. At least 200 cells were assessed for each sample. Data are presented as means ± SD. **P < 0.001 compared to the corresponding untreated group. #P < 0.05 and ##P < 0.001 compared to the corresponding G-CSF–treated group. (G and H) The chemotactic migration of neutrophils to fMLP (100 nM) was assessed using an EZ-TAXIScan device (G), and migration velocity and directionality were calculated (H). Data are presented as means ± SD. **P < 0.001 compared to the corresponding untreated group. ##P < 0.001 compared to the corresponding G-CSF–treated group. (I) The bacterial killing capabilities of neutrophils in vitro were reflected by the decrease in CFU of E. coli. (J) The relative bacterial killing was measured as the proportion of bacteria killed in 60 min. Data are presented as means ± SD of three experiments. *P < 0.05 and **P < 0.001 compared to freshly isolated neutrophil group (0 h); ##P < 0.001 compared to untreated (or G-CSF alone–treated) neutrophils cultured for 1 day.
CLON-G pretreatment prolongs half-life of transfused human neutrophils in vivo in NOD-scid IL2Rgammanull mice
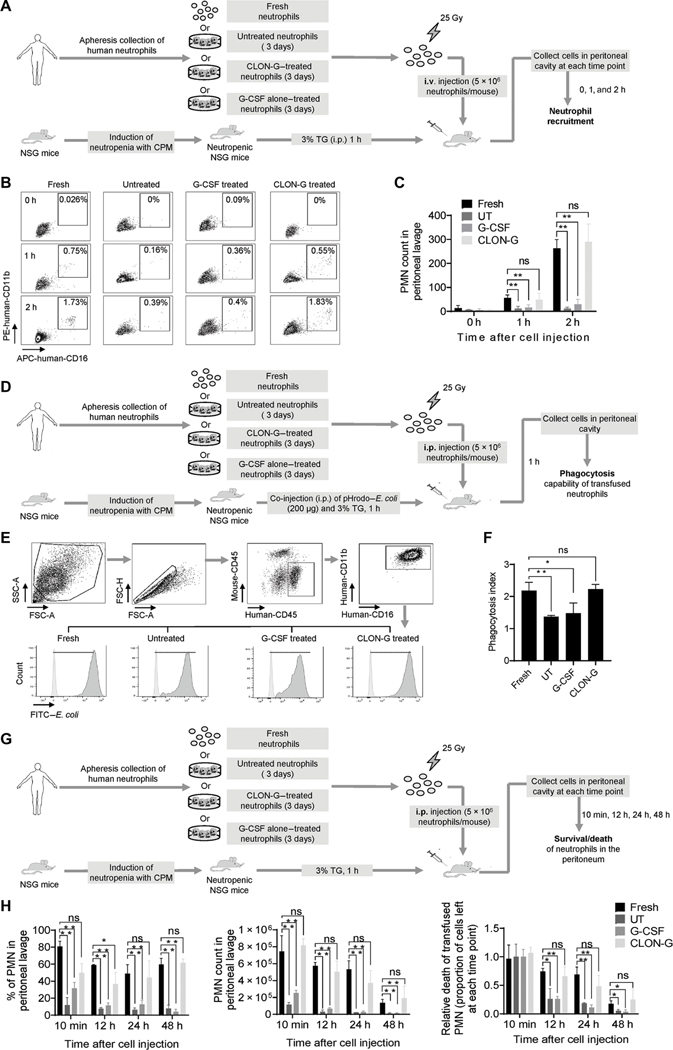
To investigate the in vivo survival and function of transfused human neutrophils, we used a model developed by Trump et al. (24), in which the survival, migration, and phagocytosis of transfused human neutrophils were assessed during peritonitis in neutrophil-depleted NOD-scid IL2Rgammanull (NSG) mice. Human neutrophils were isolated from apheresis-collected granulocyte concentrates (Fig. 8A). The transfused human neutrophils in the NSG recipient mice were identified by their unique surface markers including human-CD16 and human-CD11b (Fig. 8B). Migration of transfused human neutrophils to inflammatory site was assessed at early time points (1 and 2 hours after transfusion) when neutrophil death had not yet started. CLON-G–treated 3-day-old human neutrophils were recruited to the inflamed peritoneal cavity as efficiently as fresh human neutrophils, suggesting that CLON-G treatment did not affect recruitment of human neutrophils. In contrast, because of substantial death of cultured untreated or G-CSF alone–treated human neutrophils, their numbers were lower in the inflamed peritoneal cavity in the NSG recipients (Fig. 8, B and C). The in vivo phagocytosis capability of the transfused human neutrophils was assessed in peritonitis induced by pHrodo–E. coli, a nonviable fluorescently labeled bacterium (Fig. 8D). Transfused CLON-G–treated 3-day-old human neutrophils displayed a similar phagocytosis index compared to transfused fresh human neutrophils. In contrast, the phagocytosis efficiency of transfused untreated or G-CSF alone–treated neutrophils was about 50% lower than that of fresh human neutrophils (Fig. 8, E and F). Last, to measure human neutrophil death at the inflammatory site in recipients, we injected fresh or cultured apheresis-collected granulocyte concentrates into the inflamed peritoneal cavity directly (Fig. 8G). At each time point examined, the numbers of CLON-G–treated 3-day-old human neutrophils left in the peritoneal cavity were comparable to those of fresh neutrophils, whereas the untreated and G-CSF alone–treated human neutrophils showed faster rates of cell death (Fig. 8H). Collectively, these results demonstrated that CLON-G treatment prolongs half-life of human neutrophils. The transfused CLON-G–treated human neutrophils function normally and are recruited to the site of infection as efficiently as transfused fresh neutrophils in vivo in NSG mice.
Fig. 8. Pretreatment with CLON-G prolongs half-life of transfused human neutrophils in vivo in NSG mice.
(A) Experimental scheme for assessing the recruitment of transfused fresh, untreated, G-CSF alone–treated, and CLON-G–treated human neutrophils in NSG mice in a mouse peritonitis model. Human neutrophils were isolated from granulocyte apheresis concentrates as described in Fig. 6 and cultured in the presence of indicated compounds for the indicated time period. Peritonitis in neutropenic NSG mice was induced with 3% TG. i.v., intravenous. (B) Recruitment of transfused human neutrophils to inflamed peritoneal cavities was assessed by flow cytometry. (C) The total recruited human neutrophil number is presented as total cell number multiplied by the percentage of transfused human neutrophils in peritoneal cavities as calculated by flow cytometry. All data are presented as means ± SD of four experiments. **P < 0.001 compared to the corresponding “Fresh neutrophils” group. (D) Experimental scheme for assessing the in vivo phagocytosis capability of transfused human neutrophils in NSG mice in the peritonitis model. (E) Engulfment of pHrodo–E. coli by transfused human neutrophils was analyzed by flow cytometry. FSC-H, forward scatter height; FSC-A, forward scatter area; SSC-A, side scatter area. (F) The phagocytosis index was calculated for indicated samples as mean fluorescence intensity (MFI) fold increase (MFI of treated or untreated human neutrophils at 1 hour/ MFI of the corresponding human neutrophils at time 0). All data are presented as means ± SD of four experiments. *P < 0.05 and **P < 0.001 compared to Fresh neutrophils group. ns, no significant difference (P > 0.05). (G) Experimental scheme for assessing the in vivo death rate of transplanted fresh, untreated, G-CSF alone–treated, and CLON-G–treated human neutrophils in NSG mice in a mouse peritonitis model. (H) The percentage of human neutrophils in peritoneal cavities was calculated by flow cytometry. The number of human neutrophils left at each indicated time point was calculated as total cell number multiplied by the percentage of human neutrophils in the peritoneal cavity. Relative death of transplanted human neutrophils was calculated as the proportion of cells left at each time point. All data are presented as means ± SD of four experiments. *P < 0.05 and **P < 0.001 compared to the corresponding Fresh neutrophils group. ns, no significant difference (P > 0.05).
DISCUSSION
Neutrophils are generally regarded as short-lived cells that die quickly without stimulation (15). Increasing neutrophil viability represents a major challenge for clinical application. One such advance is a recently reported method wherein neutrophil half-life can be increased in vitro from about 1 to 2 days when conditioned for 20 hours at 37°C in anoxic culture medium supplemented with 3 mM glucose and dimethyloxalylglycine (32 mg/ml) (25). Here, by targeting multiple death mechanisms, we developed a treatment, CLON-G treatment, that could alter neutrophil fate and prolong the survival of both human and mouse neutrophils, increasing their half-life from less than 24 hours to greater than 5 days. The morphological, physiological, and bactericidal functions of the neutrophils were unaltered by CLON-G treatment. Transfusion with stored CLON-G–treated neutrophils enhanced host defenses and alleviated infection-induced tissue damage as effectively as transfusion with untreated fresh neutrophils in a clinically relevant murine GTX model in neutropenia-related bacterial pneumonia and systemic candidiasis. We further demonstrated that CLON-G treatment prolonged the shelf life and preserved the function of apheresis-collected human clinical GTX products both ex vivo and in vivo in NSG mice.
In GTX therapy, there is a great need to use stored granulocyte products. Although granulocytes for transfusion are collected on an “as needed” basis, it is nevertheless inevitable that granulocyte concentrates will be stored for a period of time before infusion. First, the time from mobilization and collection of granulocytes to transfusion is rarely less than 12 hours because it takes time to collect, process, and transfuse. It also takes a tremendous effort to get pretransfusion tests, including ABO and RhD typing and testing for transfusion-transmitted disease markers, done in time for GTX. Thus, when preparation is taken into account, using neutrophils stored for 24 hours for GTX is not uncommon. In addition, in many cases, neutrophils used for GTX are collected off-site, delaying transfusion. Currently, in-house granulocyte collection can only be performed in a small number of medical centers. Most granulocyte collections are not carried out in hospitals where they are transfused. It is necessary to ship patient samples from one location to another. Last, developing a product that can be stored for longer time may help more patients get the GTX therapy because leukapheresis donors are difficult to recruit. The possibility of using stored granulocytes could be particularly relevant in small pediatric patients because it would allow concentrates to be split for other patients.
The inability to store the granulocyte products with effective maintenance of neutrophil functional integrity has been well documented and has limited the use of GTX therapy (6, 26). Current GTX practice stipulates using granulocytes collected the same day. Longer storage periods are avoided because of poor neutrophil survival and function. Storage of granulocyte concentrate using the commonly used procedure [in plastic bags, with autologous plasma, citrate anticoagulant, unagitated, and at room temperature (RT)] is associated with impairment in neutrophil function within 24 hours from the time of collection (27). Previous studies examining neutrophils collected by centrifugation leukapheresis demonstrated substantial decreases in neutrophil functions such as ROS production, phagocytosis, bactericidal activity, chemotaxis, and in vivo migration, with storage up to 24 hours (28, 29). These observations led to the current clinical practice in which granulocytes should be transfused as soon as possible after collection but definitely within 24 hours of collection (6). We also revealed that human neutrophils stored for 24 hours have reduced bactericidal capability, which may contribute to the suboptimal and inconsistent outcomes of GTX.
A major goal of the blood bank is to collect, store, and provide an adequate number of functional granulocytes. Optimal methods of granulocyte generation are important. Currently, donor stimulation with a combination of G-CSF and dexamethasone has become the standard procedure to increase the number of circulating neutrophils in leukapheresis donors and, thus, to collect a sufficient number of cells for the preparation of granulocyte concentrates. The granulocytes from the leukapheresis product, obtained after in vivo G-CSF/dexamethasone mobilization, are functionally indistinguishable from those isolated from untreated donors (6, 30). Multiple studies showed that precollection treatment with G-CSF and dexamethasone is an effective way to maintain the functional properties of the collected neutrophils for GTX therapy (31–33). Pretreatment with G-CSF increases granulocyte yield (32, 33) and prolongs granulocyte survival time (34, 35). It was reported that without agitation, granulocyte concentrates can be stored at RT for a maximum of 24 hours (35, 36). Neutrophil morphology after 24 hours of storage showed no differences from that of fresh control cells on cytospins (30). When assessed by means of FluoroBlok inserts (30) or a fluorescence-based assay with 96-well chemotaxis chamber plates (36), neutrophil in vitro chemotaxis capability appeared to be intact after 24 hours of storage. The previous study showed that some functional and biochemical characteristics (such as phorbol 12-myristate 13-acetate–induced ROS production) of G-CSF–mobilized neutrophils can be retained for at least 24 hours of storage by adding additional G-CSF in apheresis bags (35). Nevertheless, these G-CSF–treated 1-day-old neutrophils still displayed a lower platelet-activating factor and fMLP response compared to fresh neutrophils. In addition, using a modified Boyden chamber technique, it was revealed that there was a reduction in nondirected migration after 24 hours of storage although there was no apparent difference in chemotaxis (directed migration) when compared to that at time zero (35). In the current study, using a more sensitive EZ-TAXIScan technique, we showed that neutrophil migration speed decreased, although the directionality was unaffected, when neutrophils were cultured in the presence of G-CSF alone for 24 hours. Moreover, consistent with previous studies (15, 37, 38), we found that G-CSF treatment alone could indeed prolong neutrophil life. However, compared to CLON-G treatment, G-CSF only modestly improved neutrophil survival with most neutrophils still dying within 2 days of treatment.
Our study has limitations. Although we could show that CLON-G treatment can prolong the shelf life and preserve the function of apheresis-collected human GTX products, all of our in vivo experiments were conducted in immunodeficient mice. Whether transfusion with stored CLON-G–treated human neutrophils can effectively enhance host defense in neutropenic patients needs to be further investigated in clinical trials. Another limitation is that we are not able to assess the potential side effect of CLON-G in clinical application. The cellular processes inhibited by CLON-G, including cell death, LMP, and oxidative stress, are common therapeutic targets in many diseases. The related molecules such as caspase inhibitors, necroptosis inhibitors, antioxidants, G-CSF, HSP70, and DFO are either Food and Drug Administration–approved drugs or already in clinical trials. Although these compounds can be individually applied to patients, their combined use may pose a risk of unwanted side effects. To eliminate or minimize these side effects on the recipients, for clinical GTX application, these drugs used to prolong neutrophil life span may need to be removed through rinsing before transfusion. We showed that after drug withdrawal, washed CLON-G–treated 3-day-old mouse neutrophils displayed similar or even longer life spans than untreated fresh neutrophils, which persisted at inflammatory sites in vivo in live animals. This feature was much more prominent in human neutrophils than in mouse neutrophils. The prosurvival effect of CLON-G treatment lasted as long as 5 days after the treated human neutrophils were washed with drug-free medium, potentially because of intracellular drug retention or persistent drug effects. This strongly suggests that CLON-G treatment may not only increase the ex vivo shelf life of human granulocytes but also delay in vivo death of transfused granulocytes and therefore improve GTX efficacy in neutropenic patients.
In summary, both the yield and efficacy of stored granulocytes need to be improved for successful GTX. CLON-G treatment not only facilitates GTX by prolonging the ex vivo shelf life of collected neutrophils but also maintains their function for clinical benefit. Thus, CLON-G represents a therapeutic strategy for improving GTX efficacy and provides an approach for treating neutropenia-related pneumonia and systemic candidiasis, an important and necessary adjunct to current antibiotics and G-CSF therapies. Although we focused on neutropenia-related pneumonia and candidiasis, the same GTX strategy can readily be applied to other neutropenia-related infectious diseases.
MATERIALS AND METHODS
Study design
The objective of this study was to explore therapeutic strategies for improving the ex vivo and in vivo survival of neutrophils in GTX. We first conducted a screening and identified a treatment (CLON-G) that prolonged ex vivo survival of both human and mouse neutrophils, increasing their half-life from about 24 hours to more than 5 days. We next evaluated the function of CLON-G–treated neutrophils and showed that CLON-G-treated neutrophils displayed normal ex vivo functions, including chemotaxis, ROS production, phagocytosis, and bacteria killing. In addition, we assessed the function of transfused neutrophils in vivo in a clinically relevant murine GTX model of neutropenia-related bacterial pneumonia and systemic candidiasis and demonstrated that transfusion with stored CLON-G-treated 3-day-old neutrophils enhanced host defenses, alleviated infection-induced tissue damage, and prolonged survival as effectively as transfusion with fresh neutrophils. Last, we conducted experiments to show that CLON-G treatment can prolong the shelf life and preserve the function of apheresis-collected human GTX products both ex vivo and in vivo in NSG mice. All experiments were performed at least three times independently and successfully reproduced. Reproducibility of the experiments and statistical significance of the results are shown in detail in figure legends or Materials and Methods. Sample size was determined on the basis of prior experience of good sample sizes to ensure adequate data for reliable assessments. The investigators were blinded to group allocation during data collection and analysis. No data were excluded from the analysis. All animal experiments were conducted in accordance with the Animal Welfare Guidelines of the Boston Children’s Hospital. The Boston Children’s Hospital and State Key Laboratory of Experimental Hematology (SKLEH) Animal Care and Use Committee approved and monitored all procedures. No randomization was used in this study because all experimental mice were kept under the same environment. The Ethics Committee of Blood Disease Hospital, Chinese Academy of Medical Sciences approved the protocol for human-related studies. All participating blood donors provided written informed consent for sample collection and data analysis.
Mice
Wild-type C57BL/6 and NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were purchased from the Jackson laboratory. Eight- to 12-week-old mice were used for all experiments. All necessary measures were taken to avoid or minimize any discomfort, pain, or distress to the animals. During pathogen instillation surgery, mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight) in 100 μl of saline. Sufficient anesthesia was verified by frequent monitoring of hindlimb withdrawal and determination of muscular tone or arterial blood pressure when feasible. After surgery, buprenorphine (0.05 to 0.1 mg/kg) was injected subcutaneously every 8 to 12 hours to alleviate pain and distress. In the pneumonia model, mice were euthanized with an overdose of sodium pentobarbital at the conclusion of each experiment. All other mice were euthanized by asphyxiation by inhalation of CO2. In experiments where the survival rates needed to be determined, the death of the experimental mice was determined on the basis of cessation of vital signs (including heartbeat and respiration) or hypothermia (a ventral surface temperature below 27°C) (17, 20). All mice were housed and cared for in approved veterinary facilities located within Boston Children’s Hospital or SKLEH, which provide sterile isolator cages with fresh food, water, and bedding weekly. All animal experiments were conducted in accordance with the Animal Welfare Guidelines of the Boston Children’s Hospital. The Boston Children’s Hospital and SKLEH Animal Care and Use Committee approved and monitored all procedures.
Human neutrophil isolation
Human primary neutrophils were isolated from venous blood from discarded white blood cell filters (Pall Corporation) provided by the Blood Bank Lab at the Boston Children’s Hospital. A total of 1 × 108 to 3 × 108 neutrophils were routinely obtained from one filter [450 ml of blood from a healthy donor (10, 12)]. Collecting neutrophils through a filter (compared to obtaining them by venipuncture and storing in anticoagulant testing tubes) does not impair neutrophil function (such as chemotaxis, phagocytosis, H2O2 production, and the time course of cell death) (12). We coordinated with the blood bank technicians to ensure that all filters were prepared using the same standard procedure and delivered to us in time. All blood was drawn from healthy blood donors. Donors from whom the blood was obtained were unidentifiable, and this research did not involve an intervention or interaction with living individuals or identifiable personal information. Thus, this research is not classified as human subjects research under the Health and Human Services (HHS) human subjects regulations (45 CFR Part 46). The Boston Children’s Hospital and SKLEH Institutional Review Boards approved the protocol.
Preparation of human granulocyte apheresis concentrates
Circulating neutrophil counts in G-CSF (250 μg)– and dexamethasone (8 mg)–stimulated donors are maximal at 12 hours after treatment. Accordingly, the donors were stimulated in the evening, and granulocyte collection was carried out the next morning. Neutrophil harvesting was accomplished through the process of apheresis in which red blood cells (RBCs) and plasma were returned to the donor. To collect enough cells for GTX, standard continuous flow apheresis was performed for 1 to 1.5 hours to process 3.5 to 5 liters of blood. Citrate was used as the anticoagulant during collection, and calcium gluconate was added to the return line to counteract the citrate-induced hypocalcemic symptoms in the donor. Hydroxyethyl starch, a sedimenting agent, was added to the donor’s blood as it enters the centrifuge to facilitate separation of granulocytes from RBCs and improve collection (39). To perform functional assays, neutrophils were purified at indicated time points after the CLON-G treatment as previously described (12). The purity was >97% as determined by both Wright-Giemsa staining and flow cytometry analysis with CD15 and CD16 antibodies. The Wright-Giemsa staining was performed using a Wright-Giemsa Stain Solution (Solarbio, #G1020) following a protocol provided by the manufacturer. For flow cytometry analysis, the isolated neutrophils were suspended in 100 μl of ice-cold PBS and stained with FITC-CD15 (1 μg/ml; BioLegend, MC-480) and allophycocyanin (APC)–CD16 (1 μg/ml; BioLegend, 51.1) at 4°C for 15 min. Samples were examined with a FACSCanto II flow cytometer (Becton Dickinson). Neutrophils were gated by their forward- and side-scatter characteristics and their CD15 and CD16 expression pattern. We did not detect any contamination of hematopoietic progenitor cells (HPCs). Granulocyte-macrophage CFU (CFU-GM) were undetectable in the isolated neutrophil population. The Ethics Committee of Tianjin Blood Disease Hospital approved the study protocol. All participating blood donors provided written informed consent for sample collection and data analysis.
Mouse neutrophil isolation
Mouse bone marrow neutrophils were prepared as previously described (13). Briefly, bone marrow from the femurs and tibias was flushed out with 5 ml of Hanks’ balanced salt solution (HBSS)/ EDTA/bovine serum albumin (BSA) (without Ca2+/Mg2+ salts, 0.5% low endotoxin BSA, and 15 mM EDTA). Cells were spun down (400g, 10 min, RT), resuspended in 1 ml of HBSS/EDTA/BSA, layered over discontinuous Percoll/HBSS gradients (52, 62, and 76%), and centrifuged (1060g, 30 min, RT). The interface between the 62 and 76% layers containing neutrophils was harvested and washed with 5 ml of HBSS/EDTA/BSA. RBCs were removed by resuspension in Histopaque-1119 (Sigma-Aldrich) and centrifuged (1600g, 30 min, RT). The interface between the cell suspension and Histopaque-1119 layers containing neutrophils was harvested and washed with 5 ml of HBSS/EDTA/BSA, and cells were spun down (400g, 5 min, RT) and resuspended in the buffer required for each assay. Using this method, we routinely obtained 10 million to 15 million neutrophils per mouse, >95% of which were morphologically mature (confirmed by flow cytometry analysis using Mac-1 and Ly6G antibodies). For flow cytometry analysis, the isolated neutrophils were suspended with 100 μl of ice-cold PBS and stained with APC–Mac-1 (1 μg/ml; BioLegend, M1/70) and phycoerythrin (PE)–Ly6G (1 μg/ml; BioLegend, 1A8) at 4°C for 15 min. Samples were examined with a FACSCanto II flow cytometer (Becton Dickinson). Cell viability was usually >98%. We did not detect any contamination with HPCs. CFU-GM were undetectable in the isolated neutrophil population.
Neutrophil culture and treatment
Murine or human neutrophils were cultured in RPMI 1640 supplemented with 20% heat-inactivated fetal bovine serum (FBS; Gibco) and 1% penicillin-streptomycin antibiotics at a density of 1 × 106 cells/ml at 37°C in a 5% CO2 incubator. Neutrophils were treated with different concentrations of Z-FA-FMK (Selleckchem, S7391), Z-YVAD-FMK (AdooQ Bioscience, A16317), belnacasan (VX-765) (Selleckchem, S2228), Z-DEVD-FMK (Selleckchem, S7312), Z-IETD-FMK (Selleckchem, S7314), Ac-LEHD-CHO (Sigma Aldrich, SCP0095), Q-VD-Oph (Selleckchem, S7311), Z-VAD-FMK (Selleckchem, S7023), emricasan (Selleckchem, S7775), Ac-DEVD-CHO (Selleckchem, S7901), DFO (Sigma-Aldrich, D9533), mouse Hsp70 (Abcam, ab113187), human Hsp70 (Abcam, ab78427), Diisopropylfluorophosphate (DFP) (Sigma-Aldrich, D0879), Diphenyleneiodonium chloride (DPI) (Sigma-Aldrich, D2926), NAC (Sigma-Aldrich, A9165), G-CSF (Amgen, NEUPOGEN), Nec-1 s (EMD Millipore, 852391–15-2), Necrox-2 (Enzo Life Sciences, ALX-430–166), and Necrox-5 (Enzo Life Sciences, ALX-430–167). CLON-G treatment was defined as combined treatment with Q-VD-Oph (50 μM, caspase inhibitor), DFO (1 μM, LMP inhibitor), Hsp70 (10 pM, LMP inhibitor), NAC (10 μM for human neutrophils and 1 mM for mouse neutrophils), Nec-1 s (10 μM, necroptosis inhibitor), and G-CSF (10 ng/ml).
Statistical analyses
Normality of data distribution was tested and confirmed using the Shapiro-Wilk test. For most experiments, unless stated otherwise, comparisons were made using a two-tailed, unpaired, Student’s t test. Values shown in each figure represent means ± SD. A P value <0.05 was considered statistically significant. In vitro experiments were repeated at least three times. For in vivo experiments, to perform reliable statistical analysis, at least five mice from each treatment group were used for each data point. This number was chosen on the basis of a power analysis that was conducted using Simple Interactive Statistical Analysis http://www.quantitativeskills.com/sisa/. On the basis of our preliminary data and experience, we set the SD to 10% of the average. To detect a 20% difference (average 1 = 100, average 2 = 80, SD1 = 10, SD2 = 8, allocation ratio = 1) with a power level of 90% (90% chance to discover a real difference in the sample) and an α of 0.05, we needed to use n = 5 mice from each genotype or treatment group for sufficient double-sided power. For the survival analysis, we generated Kaplan-Meier survival curves and perform comparisons between groups by log-rank analysis using Prism (GraphPad Software).
Supplementary Material
Acknowledgments:
We thank A. Hsu, L. Ghimire, Q. Hou, and Z. Peng for input and discussions and H. Kambara for technical assistance.
Funding:
H.R.L. is supported by 1 R01 AI142642, 1 R01 AI145274, 1 R01 AI141386, R01HL092020, a grant from Flight Attendant Medical Research Institute (FAMRI) (CIA 123008), and NIH P01 HL095489. This project was directly supported by NIH P01 HL095489 (to H.R.L.). Support was provided by the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2018RC31002 and 2018PT32034 to F.M.), the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (2016-I2M-1–017 to F.M.), and the Natural Science Foundation of Tianjin City (18JCYBJC25700 to F.M.). Support was provided by the National Natural Sciences Foundation of China (81970107 and 31471116 to Y.X.).
Footnotes
Data and materials availability: All data associated with this study are present in the paper or the Supplementary Materials.
Competing interests: The authors declare that they have no competing financial interests.
REFERENCES AND NOTES
- 1.White L, Ybarra M, Neutropenic fever. Hematol. Oncol. Clin. North Am 31, 981–993 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Wingard JR, Eldjerou L, Leather H, Use of antibacterial prophylaxis in patients with chemotherapy-induced neutropenia. Curr. Opin. Hematol. 19, 21–26 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Bennett CL, Djulbegovic B, Norris LB, Armitage JO, Colony-stimulating factors for febrile neutropenia during cancer therapy. N. Engl. J. Med. 368, 1131–1139 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderlini P, Przepiorka D, Champlin R, Korbling M, Biologic and clinical effects of granulocyte colony-stimulating factor in normal individuals. Blood 88, 2819–2825 (1996). [PubMed] [Google Scholar]
- 5.Dale DC, Price TH, Granulocyte transfusion therapy: A new era? Curr. Opin. Hematol. 16, 1–2 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldfinger D, Lu Q, in UpToDate AJ Rosmarin Silvergleid, A. G., Eds. (Wolters Kluwer, 2020), vol. 2020. [Google Scholar]
- 7.Netelenbos T, Massey E, de Wreede LC, Harding K, Hamblin A, Sekhar M, Li A, Ypma PF, Ball L, Zwaginga JJ, Stanworth SJ, The burden of invasive infections in neutropenic patients: Incidence, outcomes, and use of granulocyte transfusions. Transfusion 59, 160–168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price TH, Boeckh M, Harrison RW, McCullough J, Ness PM, Strauss RG, Nichols WG, Hamza TH, Cushing MM, King KE, Young J-AH, Williams E, McFarland J, Holter Chakrabarty J, Sloan SR, Friedman D, Parekh S, Sachais BS, Kiss JE, Assmann SF, Efficacy of transfusion with granulocytes from G-CSF/ dexamethasone-treated donors in neutropenic patients with infection. Blood 126, 2153–2161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teng Y, Luo HR, Kambara H, Heterogeneity of neutrophil spontaneous death. Am. J. Hematol. 92, E156–E159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu D, Hattori H, Jo H, Jia Y, Subramanian KK, Loison F, You J, Le Y, Honczarenko M, Silberstein L, Luo HR, Deactivation of phosphatidylinositol 3,4,5-trisphosphate/Akt signaling mediates neutrophil spontaneous death. Proc. Natl. Acad. Sci. U.S.A. 103, 14836–14841 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, Loison F, Luo HR, Neutrophil spontaneous death is mediated by down-regulation of autocrine signaling through GPCR, PI3Kγ, ROS, and actin. Proc. Natl. Acad. Sci. U.S.A. 107, 2950–2955 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loison F, Zhu H, Karatepe K, Kasorn A, Liu P, Ye K, Zhou J, Cao S, Gong H, Jenne DE, Remold-O’Donnell E, Xu Y, Luo HR, Proteinase 3-dependent caspase-3 cleavage modulates neutrophil death and inflammation. J. Clin. Invest. 124, 4445–4458 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kambara H, Liu F, Zhang X, Liu P, Bajrami B, Teng Y, Zhao L, Zhou S, Yu H, Zhou W, Silberstein LE, Cheng T, Han M, Xu Y, Luo HR, Gasdermin D exerts anti-inflammatory effects by promoting neutrophil death. Cell Rep. 22, 2924–2936 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, He Z, Liu H, Yousefi S, Simon H-U, Neutrophil necroptosis is triggered by ligation of adhesion molecules following GM-CSF priming. 197, 4090–4100 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Luo HR, Loison F, Constitutive neutrophil apoptosis: Mechanisms and regulation. Am. J. Hematol. 83, 288–295 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y, Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. U.S.A. 101, 7618–7623 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Jia Y, Pichavant M, Loison F, Sarraj B, Kasorn A, You J, Robson BE, Umetsu DT, Mizgerd JP, Ye K, Luo HR, Targeted deletion of tumor suppressor PTEN augments neutrophil function and enhances host defense in neutropenia-associated pneumonia. Blood 113, 4930–4941 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad A, Jia Y, Chakraborty A, Li Y, Jain SK, Zhong J, Roy SG, Loison F, Mondal S, Sakai J, Blanchard C, Snyder SH, Luo HR, Inositol hexakisphosphate kinase 1 regulates neutrophil function in innate immunity by inhibiting phosphatidylinositol-(3,4,5)trisphosphate signaling. Nat. Immunol. 12, 752–760 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai J, Li J, Subramanian KK, Mondal S, Bajrami B, Hattori H, Jia Y, Dickinson BC, Zhong J, Ye K, Chang CJ, Ho YS, Zhou J, Luo HR, Reactive oxygen species-induced actin glutathionylation controls actin dynamics in neutrophils. Immunity 37, 1037–1049 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Prasad A, Jia Y, Roy SG, Loison F, Mondal S, Kocjan P, Silberstein LE, Ding S, Luo HR, Pretreatment with phosphatase and tensin homolog deleted on chromosome 10 (PTEN) inhibitor SF1670 augments the efficacy of granulocyte transfusion in a clinically relevant mouse model. Blood 117, 6702–6713 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia Y, Subramanian KK, Erneux C, Pouillon V, Hattori H, Jo H, You J, Zhu D, Schurmans S, Luo HR, Inositol 1,3,4,5-tetrakisphosphate negatively regulates phosphatidylinositol-3,4,5-trisphosphate signaling in neutrophils. Immunity 27, 453–467 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duffin R, Leitch AE, Fox S, Haslett C, Rossi AG, Targeting granulocyte apoptosis: Mechanisms, models, and therapies. Immunol. Rev. 236, 28–40 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Nesher L, Rolston KVI, The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection 42, 5–13 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Trump LR, Nayak RC, Singh AK, Emberesh S, Wellendorf AM, Lutzko CM, Cancelas JA, Neutrophils derived from genetically modified human induced pluripotent stem cells circulate and phagocytose bacteria in vivo. Stem Cells Transl. Med. 8, 557–567 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monceaux V, Chiche-Lapierre C, Chaput C, Witko-Sarsat V, Prevost MC, Taylor CT, Ungeheuer M-N, Sansonetti PJ, Marteyn BS, Anoxia and glucose supplementation preserve neutrophil viability and function. Blood 128, 993–1002 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Marfin AA, Price TH, Granulocyte transfusion therapy. J. Intensive Care Med. 30, 79–88 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Lane TA, Granulocyte storage. Transfus. Med. Rev. 4, 23–34 (1990). [DOI] [PubMed] [Google Scholar]
- 28.Pric THe, Dale DC, Blood kinetics and in vivo chemotaxis of transfused neutrophils: Effect of colllection method, donor corticosteroid treatment, and short-term storage. Blood 54, 977––986. (1979). [PubMed] [Google Scholar]
- 29.Glasser L, Lane TA, McCullough J, Price TH, Panel VII: Neutrophil concentrates: Functional considerations, storage, and quality control. J. Clin. Apher. 1, 179–184 (1983). [DOI] [PubMed] [Google Scholar]
- 30.Drewniak A, Boelens J-J, Vrielink H, Tool ATJ, Bruin MCA, van den Heuvel-Eibrink M, Ball L, van de Wetering MD, Roos D, Kuijpers TW, Granulocyte concentrates: Prolonged functional capacity during storage in the presence of phenotypic changes. Haematologica 93, 1058–1067 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Dale DC, Liles WC, Llewellyn C, Rodger E, Price TH, Neutrophil transfusions: Kinetics and functions of neutrophils mobilized with granulocyte-colony-stimulating factor and dexamethasone. Transfusion 38, 713–721 (1998). [DOI] [PubMed] [Google Scholar]
- 32.Caspar CB, Seger RA, Burger J, Gmur J, Effective stimulation of donors for granulocyte transfusions with recombinant methionyl granulocyte colony-stimulating factor. Blood 81, 2866–2871 (1993). [PubMed] [Google Scholar]
- 33.Bensinger WI, Price TH, Dale DC, Appelbaum FR, Clift R, Lilleby K, Williams B, Storb R, Thomas ED, Buckner CD, The effects of daily recombinant human granulocyte colony-stimulating factor administration on normal granulocyte donors undergoing leukapheresis. Blood 81, 1883–1888 (1993). [PubMed] [Google Scholar]
- 34.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A, Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 80, 2012–2020 (1992). [PubMed] [Google Scholar]
- 35.Leavey PJ, Thurman G, Ambruso DR, Functional characteristics of neutrophils collected and stored after administration of G-CSF. Transfusion 40, 414–419 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Hubel K, Rodger E, Gaviria JM, Price TH, Dale DC, Liles WC, Effective storage of granulocytes collected by centrifugation leukapheresis from donors stimulated with granulocyte-colony-stimulating factor. Transfusion 45, 1876–1889 (2005). [DOI] [PubMed] [Google Scholar]
- 37.van Raam BJ, Drewniak A, Groenewold V, van den Berg TK, Kuijpers TW, Granulocyte colony-stimulating factor delays neutrophil apoptosis by inhibition of calpains upstream of caspase-3. Blood 112, 2046–2054 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maianski NA, Mul FPJ, van Buul JD, Roos D, Kuijpers TW, Granulocyte colony-stimulating factor inhibits the mitochondria-dependent activation of caspase-3 in neutrophils. Blood 99, 672–679 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Goldfinger D, Lu Q, in UpToDate, Post TW, Silvergleid AJ, Rosmarin AG. (Wolters Kluwer, 2020). [Google Scholar]
- 40.Dahlgren C, Karlsson A, Respiratory burst in human neutrophils. J. Immunol. Methods 232, 3–14 (1999). [DOI] [PubMed] [Google Scholar]
- 41.Subramanian KK, Jia Y, Zhu D, Simms BT, Jo H, Hattori H, You J, Mizgerd JP, Luo HR, Tumor suppressor PTEN is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood 109, 4028–4037 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su X, Johansen M, Looney MR, Brown EJ, Matthay MA, CD47 deficiency protects mice from lipopolysaccharide-induced acute lung injury and Escherichia coli pneumonia. J. Immunol 180, 6947–6953 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.