Abstract
Context:
Literature on treating pediatric spinal deformity with navigation is limited, particularly using large nationally represented cohorts. Further, the comparison of single-institution data to national-level database outcomes is also lacking.
Aim:
(1) To compare navigated versus conventional posterior pediatric deformity surgery based on 30-day outcomes and perioperative factors using the National Surgical Quality Improvement Program (NSQIP) database and (2) to compare the outcomes of the NSQIP navigated group to those of fluoroscopy-only and navigated cases from a single-institution.
Settings and Design:
Retrospective cohort study.
Subjects and Methods:
Pediatric patients who underwent posterior deformity surgery with and without navigation were included. Primary outcomes were 30-day readmission, reoperation, morbidity, and complications. The second part of this study included AIS patients < 18 years old at a single institution between 2015 and 2019. Operative time, length of stay, transfusion rate, and complication rate were compared between single-institution and NSQIP groups.
Statistical Analysis Used:
Univariate analyses with independent t-test and Chi-square or Fisher's exact test was used. Multivariate analyses through the application of binary logistic regression models.
Results:
Part I of the study included 16,950 patients, with navigation utilized in 356 patients (2.1%). In multivariate analysis, navigation predicted reoperation, deep wound infection, and sepsis. After controlling for operative year, navigation no longer predicted reoperation. In Part II of the study, 288 single institution AIS patients were matched to 326 navigation patients from the NSQIP database. Operative time and transfusion rate were significantly higher for the NSQIP group.
Conclusions:
On a national scale, navigation predicted increased odds of reoperation and infectious-related events and yielded greater median relative value units (RVUs) per case but had longer operating room (OR) time and fewer RVUs-per-minute. After controlling for operative year, RVUs-per-minute and reoperation rates were similar between groups. The NSQIP navigated surgery group was associated with significantly higher operative time and transfusion rates compared to the single-institution groups.
Keywords: Adolescent idiopathic scoliosis, complications, navigation, pediatric spinal deformity, reoperation
INTRODUCTION
The frequency of spinal deformity surgery has been increasing due to technological advancements.[1,2,3,4,5] The intricate complexities of spinal anatomy, particularly in deformity surgery, have led to the development of new techniques designed to minimize adverse events.[2,3,6,7,8] Navigation has been developed to maximize pedicle screw placement accuracy and has been of particular benefit in complex spinal deformity cases, with the goal of minimizing complications and hardware failure and reducing blood loss.[2,9,10,11]
While current research has demonstrated positive clinical satisfaction and radiographic accuracy with the use of navigation for treating degenerative disease, literature on treating pediatric deformity with navigation is limited, particularly using large nationally represented cohorts.[11,12] Further, comparison of single-institution data to national-level database outcomes is also limited. Such comparison could provide insight into learning curves and technique-related factors associated with better outcomes.
The purpose of this study was to compare navigated versus conventional posterior pediatric deformity surgery based on 30-day readmission, reoperation, and morbidity and perioperative factors using the NSQIP database and to compare the outcomes of the NSQIP navigated group to those of fluoroscopy-only and navigated cases from a single-institution.
SUBJECTS AND METHODS
Study design and population
This two-part study consists of a retrospective analysis of data from the Pediatric American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database and a comparison to patients from a single institution who were operated on by the senior author. The first part of the project is exempt from Institutional Review Board (IRB) review and informed consent requirement as it utilized a de-identified, publicly available database, and no direct patient involvement occurred. For the second part of the study, IRB approval was obtained at the senior author's home institution (Feinstein Institute for Medical Research at Northwell Health, IRB #21-0165).
Part I
The NSQIP database began in 1994 as an initiative to improve the quality of surgical care in the Veterans Administration and later expanded to all participating hospitals in the United States. At present, there are over 680 participating hospitals with standardized clinical reviewers and routine site audits to ensure the reliable data. The database has frequently been used in the spine literature.[13,14]
Pediatric patients who underwent posterior deformity surgery were identified in the 2012–2018 NSQIP databases using Current Procedural Terminology (CPT) codes 22800-22804. Patients were stratified into groups with and without navigation using CPT code 61873. Patients were excluded if they had preoperative infection, open wound, cerebral hemorrhage, wound class >1, required Cardiopulmonary Resuscitation (CPR), transfusion, or inotropic support prior to surgery, or underwent surgery for revision, lesion, or nonelective or urgent purposes. Patients with missing 30-day outcomes were also excluded. Patients were also excluded if they had anterior fusion, nonelective, or lesion-related CPT codes.
Part II
The second part of this study consisted of an IRB-approved retrospective chart review of AIS patients, <18 years old, at a single institution between 2015 and 2019. Patients were excluded if they had <2 years of follow-up or had undergone previous spine surgery or surgery for a non-idiopathic etiology. The single-institution patients were divided into fluoroscopy (Fluoro) and “technique'n'technology” (TNT) groups and were compared to each other and to the navigated AIS patients from the NSQIP dataset (NAV). All pedicle screws were introduced by senior surgeons through freehand anatomic technique: Screws were placed based on anatomic landmarks, with ball-tipped probes used to palpate the screw tracts. In the Fluoro group, once placed, screw positioning was verified under fluoroscopy before rod placement. Patients operated on between 2015 and 2017 were placed in the Fluoro group. In the TNT group, once screws were placed using the freehand anatomic technique, Airo computed tomography (CT) scan navigation was utilized in lieu of fluoroscopy, whereby a reference clamp was attached to one of the spinous processes in the area of the arthrodesis based on surgeon preference. After clamp positioning, a radiology technician executed the CT scans with the mobile scanner and images were automatically transferred to the workstation. Screw positioning was once again verified before rod placement with no additional image guidance or navigation thereafter. Patients operated on between 2018 and 2019 were placed in the TNT group.
In 2018, the Airo CT scanner (Brainlab AG) was introduced at our institution for spine-related surgeries. The scanner consists of a CT table and image-guidance system that allows for real-time CT navigation. Surgeons conducted Airo procedures using the “TNT” approach.
Outcomes and statistical analyses
Part I
Primary outcomes were 30-day readmission, reoperation, overall morbidity, and specific complications. Readmission includes any inpatient stay to the same or another hospital related to the surgical procedure. Reoperation includes all major surgical procedures requiring return to the operating room for intervention of any kind. Morbidity includes infectious, pulmonary, cardiac, renal, neurological, hematologic, and thromboembolic complications reported in the ACS-NSQIP dataset. In addition, reasons for reoperation were obtained from NSQIP-provided data and were compared between navigated and conventional groups.
Primary outcomes, as well as specific complications, were compared between navigated and conventional groups. Predictors of primary outcomes were analyzed for among the entire cohort. The variables evaluated as potential predictors included patient demographic, comorbidity, laboratory values, and procedural factors [Table 1]. Procedural factors specifically included operative time, length of hospital stay, and relative value units (RVUs) per case and per minute of operative time. The specific complications are provided in Table 2.
Table 1.
Baseline differences in patient demographic, comorbidity, laboratory, and procedural factors, and primary outcomes by presence or absence of computer-assisted surgery
| With CAS (n=356) | Without CAS (n=16,594) | P | Cases available (n=16,950) | |
|---|---|---|---|---|
| Demographics, n (%) | ||||
| Age (years), mean (SD) | 13.8 (2.8) | 13.8 (2.7) | 0.866 | 16,950 |
| African−American race | 39 (11.8) | 2696 (18.2) | 0.003 | 15,120 |
| Hispanic ethnicity | 31 (8.8) | 1625 (10.6) | 0.292 | 15,712 |
| Female gender | 245 (68.8) | 11,575 (69.8) | 0.704 | 16,950 |
| Comorbidities, n (%) | ||||
| Obese | 56 (16.3) | 2416 (15.3) | 0.619 | 16,132 |
| Pulmonary comorbidity | 69 (19.4) | 2795 (16.8) | 0.206 | 16,950 |
| Cardiac comorbidity | 26 (7.3) | 1453 (8.8) | 0.337 | 16,950 |
| Esophageal/GI disease | 33 (9.3) | 1692 (10.2) | 0.567 | 16,950 |
| Developmental delay | 79 (22.2) | 3505 (21.1) | 0.625 | 16,950 |
| Seizure disorder | 31 (8.7) | 1580 (9.5) | 0.605 | 16,950 |
| Cerebral palsy | 30 (8.4) | 1571 (9.5) | 0.507 | 16,950 |
| Structural CNS abnormality | 49 (13.8) | 2202 (13.3) | 0.786 | 16,950 |
| Neuromuscular disorder | 72 (20.2) | 3668 (22.1) | 0.397 | 16,950 |
| Preoperative steroid use | 3 (0.8) | 179 (1.1) | 1.000# | 16,950 |
| Nutritional support | 26 (7.3) | 1274 (7.7) | 0.793 | 16,950 |
| Hematologic disorder | 10 (2.8) | 309 (1.9) | 0.193 | 16,950 |
| Congenital malformation | 86 (24.2) | 5077 (30.6) | 0.009 | 16,950 |
| Childhood malignancy | 5 (1.4) | 166 (1.0) | 0.450 | 16,950 |
| ASA-class ≥3 | 108 (30.4) | 5055 (30.5) | 0.977 | 16,932 |
| Lab values, mean (SD) | ||||
| White cell count | 7.0 (2.3) | 6.9 (2.3) | 0.758 | 14,089 |
| Hematocrit | 40.5 (3.6) | 39.9 (3.4) | 0.005 | 14,481 |
| INR | 1.1 (0.1) | 1.1 (0.1) | 0.672 | 9657 |
| Procedural factors, median (IQR) | ||||
| Operative time (min) | 331 (248–435) | 269 (205–344) | <0.001 | 16,940 |
| LOS (days) | 4 (3–5) | 4 (3–5) | 0.304 | 16,909 |
| Total RVUs | 57.7 (49.3–77.4) | 53.9 (45.6–74.4) | <0.001 | 16,950 |
| RVUs per minute | 0.18 (0.13–0.26) | 0.21 (0.15–0.28) | <0.001 | 16,940 |
| Total RVUs subtracting NAV | 50.9 (42.5–70.6) | 53.9 (45.6–74.4) | 0.238 | 16,948 |
| Unadjusted primary outcomes, n (%) | ||||
| Readmission | 21 (5.9) | 647 (3.9) | 0.055 | 16,950 |
| Reoperation | 22 (6.2) | 513 (3.1) | 0.001 | 16,950 |
| Mean days to reoperation | 16.3 (7.4) | 14.1 (8.4) | 0.229 | |
| Morbidity | 269 (75.6) | 11,199 (67.5) | 0.001 | 16,950 |
#Fisher’s exact test. Bold values indicate significance (P<0.05). Pulmonary comorbidities include ventilator dependence, asthma, chronic lung disease, chronic oxygen support, tracheostomy, or structural pulmonary or airway abnormalities. Cardiac comorbidities include previous cardiac surgery and cardiac risk factors. IQR - Interquartile ranges; ASA - American Society of Anesthesiologists; RVUs - Relative value units; CAS – Computer-assisted surgery; GI - Gastric/intestinal; CNS - Central nervous system; SD - Standard deviation; LOS - Length of stay; INR - International normalized ratio; NAV - Navigated patients from the NSQIP dataset
Table 2.
Univariate and multivariate analysis of specific complication by presence or absence of computer assisted surgery
| Specific complication | With CAS, n (%) | Without CAS, n (%) | Univariate P | OR (95% CI) | Multivariate P |
|---|---|---|---|---|---|
| Any wound complication | 21 (5.9) | 677 (4.1) | 0.087 | ||
| Superficial site infection | 4 (1.1) | 124 (0.7) | 0.417# | ||
| Deep wound infection | 9 (2.5) | 134 (0.8) | 0.003# | 2.926 (1.305–6.563) | 0.009 |
| Organ space infection | 1 (0.3) | 39 (0.2) | 0.573# | ||
| Wound dehiscence | 10 (2.8) | 499 (3.0) | 0.828 | ||
| Pulmonary complication | 4 (1.1) | 269 (1.6) | 0.461 | ||
| Pneumonia | 3 (0.8) | 178 (1.1) | 1.000# | ||
| Unplanned intubation | 1 (0.3) | 134 (0.8) | 0.536# | ||
| Pulmonary embolism | 0 | 4 (0.02) | 1.000# | ||
| Acute kidney injury | 1 (0.3) | 17 (0.1) | 0.318# | ||
| Urinary tract infection | 3 (0.8) | 122 (0.7) | 0.748# | ||
| Stroke/CVA | 0 | 5 (0.03) | 1.000# | ||
| Seizure | 0 | 10 (0.1) | 1.000# | ||
| Nerve injury | 0 | 58 (0.3) | 0.636# | ||
| Cardiac arrest requiring CPR | 1 (0.3) | 23 (0.1) | 0.399# | ||
| Transfusion | 262 (73.6) | 10,938 (65.9) | 0.002 | 0.977 (0.731–1.306) | 0.874 |
| Blood stream infection | 0 | 5 (0.03) | 1.000# | ||
| Sepsis/septic shock | 8 (2.2) | 124 (0.7) | 0.007# | 3.192 (1.324–7.693) | 0.010 |
#Fisher’s exact test. Bold values indicate significance (P<0.05). CPR - Cardiopulmonary resuscitation; CVA - Cerebrovascular accident; CAS – Computer-assisted surgery; OR - Odds ratio; CI - Confidence interval
Demographic, comorbidity, laboratory, and procedural factors were individually analyzed for the baseline differences between navigated and conventional patients using Student's t-test for continuous and Chi-squared or Fisher's exact test for the categorical variables. The above factors were also individually analyzed for association with primary outcomes using univariate logistic regression. Variables significant in the univariate analyses (P < 0.05) were then evaluated for significance (P < 0.05) as independent predictors and control variables in a series of multivariate logistic regression analyses of primary outcomes.
In addition, post hoc analyses controlling for operative year were performed for primary outcomes using multivariate logistic regression and for operative time, RVUs per case, and RVUs per minute, with multivariate analysis performed using quantile (median) regression.
Part II
The fluoro, TNT, and NAV were compared on the basis of operative time, length of stay, transfusion rate, and complication rate. In addition, the Fluoro and TNT groups were compared to each other on the basis of total radiation time and dose, preoperative and postoperative Cobb angle, correction of Cobb, preoperative and postoperative kyphosis, operative time, estimated blood loss, and length of stay.
Shapiro–Wilk test was used to confirm the distribution normality. Data were presented as medians and interquartile (25th–75th percentile) ranges in the continuous variables (Cobb, kyphosis, etc.) and frequency and percentages for the categorical variables (complication, transfusion, etc.). The continuous data were analyzed using Kruskal–Wallis or Wilcoxon rank-sum test and categorical data were analyzed using the Chi-square and Fisher's exact test. Statistical analyses were performed by an independent biostatistician with SAS version 9.3 (SAS Institute, Cary NC). All P values were two tailed, with P < 0.05 considered significant.
RESULTS
Part I
There were 16,950 patients included, with navigation utilized in 356 patients (2.1%). Significant baseline differences were only observed in only 3 of 22 demographic and comorbidity variables [Table 1]. Patients in the navigated group were significantly less likely to be African − American (11.8 vs. 18.2%, P = 0.003) or to have a congenital malformation (24.2 vs. 30.6%, P = 0.009), and had greater mean preoperative hematocrit (40.5 vs. 39.9, P = 0.005), compared to those in the conventional group, respectively.
Navigation was associated with longer median operative times (331 vs. 269 min) and total median RVUs per case (57.7 vs. 53.9), but fewer median RVUs per minute (0.18 vs. 0.21) compared to conventional surgery, respectively (P < 0.001). There was no difference in total median RVUs per case when subtracting the value of RVUs for navigation, 6.81, from the navigated cases (50.9 vs. 53.9, P = 0.238). The median length of stay was similar between groups (4 vs. 4 days, P = 0.304).
In univariate analysis [Tables 1 and 2], navigation was associated with greater rates of reoperation (6.2 vs. 3.1%, P = 0.001) and overall morbidity (75.6 vs. 67.5%, P = 0.001). Navigation was also associated with the greater rates of wound infection (2.5 vs. 0.8%, P = 0.003), transfusion (73.6 vs. 65.9%, P = 0.002), and sepsis/septic shock (2.2 vs. 0.7%, P = 0.007). Readmission rates (5.9 vs. 3.9%, P = 0.055) and mean duration from surgery to reoperation (16.3 vs. 14.1 days, P = 0.229) were similar between groups.
After adjusting for significant baseline differences and predictor variables in multivariate analysis [Tables 2-5], navigation predicted reoperation (odds ratio [OR] = 1.920, P = 0.019, 95% confidence interval [CI95]: 1.115–3.306), deep wound infection (OR = 2.926, P = 0.009, CI95: 1.305–6.563), and sepsis/septic shock (OR = 3.192, P = 0.010, CI95: 1.324–7.693), but no longer predicted overall morbidity (P = 0.955) or transfusion (P = 0.874). Reoperation most commonly occurred due to site-related complications followed by hardware-related events [Figure 1].
Table 5.
Univariate and multivariate analysis of predictors of morbidity
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Morbidity (n=5482) | No morbidity (n=16,282) | P | OR (95% CI) | P | |
| Demographics, n (%) | |||||
| Age (years), mean (SD) | 13.9 (2.5) | 13.5 (3.1) | <0.001 | 1.051 (1.034–1.069) | <0.001 |
| African−American race | 1966 (19.1) | 769 (16.0) | <0.001 | 1.193 (1.068–1.333) | 0.002 |
| Hispanic ethnicity | 1180 (11.0) | 476 (9.6) | 0.008 | 1.401 (1.206–1.628) | <0.001 |
| Female gender | 7826 (68.2) | 3994 (72.9) | <0.001 | 0.959 (0.870–1.058) | 0.403 |
| Comorbidities, n (%) | |||||
| Obese | 1638 (15.1) | 834 (15.8) | 0.264 | ||
| Pulmonary comorbidity | 2132 (18.6) | 732 (13.4) | <0.001 | 1.032 (0.906–1.177) | 0.634 |
| Cardiac comorbidity | 1066 (9.3) | 413 (7.5) | <0.001 | 1.113 (0.950–1.305) | 0.184 |
| Esophageal/GI disease | 1366 (11.9) | 359 (6.5) | <0.001 | 1.247 (1.045–1.487) | 0.014 |
| Developmental delay | 2794 (24.4) | 790 (14.4) | <0.001 | 1.291 (1.113–1.498) | 0.001 |
| Seizure disorder | 1343 (11.7) | 268 (4.9) | <0.001 | 1.384 (1.111–1.723) | 0.004 |
| Cerebral palsy | 1338 (11.7) | 263 (4.8) | <0.001 | 1.078 (0.859–1.353) | 0.519 |
| Structural CNS abnormality | 1621 (14.1) | 630 (11.5) | <0.001 | 0.798 (0.695–0.917) | 0.001 |
| Neuromuscular disorder | 2894 (25.2) | 846 (15.4) | <0.001 | 1.310 (1.150–1.492) | <0.001 |
| Preoperative steroid use | 141 (1.2) | 41 (0.7) | 0.004 | 1.294 (0.829–2.020) | 0.256 |
| Nutritional support | 1088 (9.5) | 212 (3.9) | <0.001 | 1.218 (0.958–1.548) | 0.107 |
| Hematologic disorder | 249 (2.2) | 70 (1.3) | <0.001 | 1.233 (0.885–1.718) | 0.216 |
| Congenital malformation | 3610 (31.5) | 1553 (28.3) | <0.001 | 0.863 (0.779–0.956) | 0.005 |
| Childhood malignancy | 100 (0.9) | 71 (1.3) | 0.010 | 0.579 (0.392–0.855) | 0.006 |
| ASA-class ≥3 | 3847 (33.6) | 1316 (24.0) | <0.001 | 0.904 (0.800–1.022) | 0.106 |
| Lab values, mean (SD) | |||||
| White cell count | 6.9 (2.4) | 6.9 (2.2) | 0.423 | 0.993 (0.975–1.011) | 0.442 |
| Hematocrit | 39.9 (4.4) | 39.9 (4.4) | 0.987 | 0.979 (0.968–0.991) | 0.001 |
| INR | 1.1 (0.1) | 1.1 (0.1) | 0.228 | ||
| Procedural factors | |||||
| Computer assistance | |||||
| With CAS | 269 (75.6a) | 87 | 0.001 | 0.992 (0.738–1.333) | 0.955 |
| Without CAS | 11,199 (67.5b) | 15,947 | |||
| Operative time | 304 (111) | 247 (94) | <0.001 | 1.005 (1.005–1.006) | <0.001 |
| LOS | 5.6 (5.8) | 4.9 (5.7) | <0.001 | 0.999 (0.991–1.007) | 0.776 |
| Total RVUs | 62.9 (27.4) | 54.7 (24.0) | <0.001 | 1.009 (1.007–1.011) | <0.001 |
aPercent of patients with CAS who experienced morbidity; bPercent of patients without CAS who experienced morbidity; #Fisher’s exact test. Bold values indicate significance (P<0.05). Pulmonary comorbidities include ventilator dependence, asthma, chronic lung disease, chronic oxygen support, tracheostomy, or structural pulmonary or airway abnormalities. Cardiac comorbidities include previous cardiac surgery and cardiac risk factors. ASA - American Society of Anesthesiologists; RVUs - Relative value units; CAS - Computer assisted surgery; GI - Gastric/intestinal; CNS - Central nervous system; SD - Standard deviation; INR - International normalized ratio; OR - Odds ratio; CI - Confidence interval; LOS - Length of stay
Figure 1.
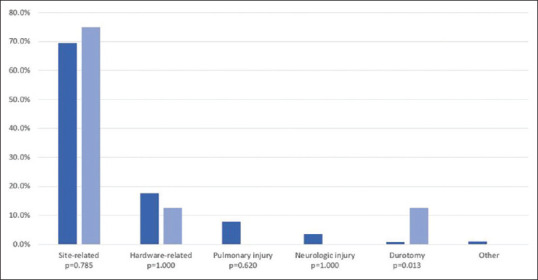
Reasons for reoperations among navigated and conventional pediatric spinal deformity fusion patients
Table 4.
Univariate and multivariate analysis of predictors of reoperation
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Reoperation (n=535) | No reoperation (n=16,415) | P | OR (95% CI) | P | |
| Demographics, n (%) | |||||
| Age (years), mean (SD) | 13.4 (3.1) | 13.8 (2.7) | 0.002 | 1.012 (0.970–1.056) | 0.586 |
| African−American race | 88 (18.3) | 2647 (18.1) | 0.922 | 1.083 (0.813–1.444) | 0.585 |
| Hispanic ethnicity | 53 (10.6) | 1603 (10.5) | 0.989 | ||
| Female gender | 325 (60.7) | 11,495 (70.0) | <0.001 | 0.947 (0.745–1.203) | 0.947 |
| Comorbidities, n (%) | |||||
| Obese | 131 (26.7) | 2341 (15.0) | <0.001 | 2.472 (1.901–3.214) | <0.001 |
| Pulmonary comorbidity | 175 (32.7) | 2689 (16.4) | <0.001 | 1.216 (0.922–1.604) | 0.166 |
| Cardiac comorbidity | 77 (14.4) | 1402 (8.5) | <0.001 | 1.067 (0.764–1.490) | 0.704 |
| Esophageal/GI disease | 119 (22.2) | 1606 (9.8) | <0.001 | 0.847 (0.608–1.179) | 0.326 |
| Developmental delay | 260 (48.6) | 3324 (20.2) | <0.001 | 1.926 (1.414–2.622) | <0.001 |
| Seizure disorder | 132 (24.7) | 1479 (9.0) | <0.001 | 1.230 (0.861–1.758) | 0.255 |
| Cerebral palsy | 125 (23.4) | 1476 (9.0) | <0.001 | 1.064 (0.739–1.534) | 0.738 |
| Structural CNS abnormality | 138 (25.8) | 2180 (12.9) | <0.001 | 1.171 (0.883–1.554) | 0.273 |
| Neuromuscular disorder | 244 (45.6) | 3496 (21.3) | <0.001 | 1.533 (1.162–2.021) | 0.002 |
| Preoperative steroid use | 13 (2.4) | 169 (1.0) | 0.002 | 1.056 (0.491–2.271) | 0.889 |
| Nutritional support | 106 (19.8) | 1194 (7.3) | <0.001 | 0.867 (0.588–1.277) | 0.470 |
| Hematologic disorder | 23 (4.3) | 296 (1.8) | <0.001 | 0.913 (0.487–1.711) | 0.777 |
| Congenital malformation | 272 (50.8) | 4,891 (29.8) | <0.001 | 1.091 (0.846–1.406) | 0.502 |
| Childhood malignancy | 4 (0.7) | 167 (1.0) | 0.539 | ||
| ASA-class ≥3 | 321 (60.0) | 4842 (29.5) | <0.001 | 1.519 (1.123–2.055) | 0.007 |
| Lab values, mean (SD) | |||||
| White cell count | 7.4 (2.7) | 6.9 (2.3) | <0.001 | 1.024 (0.981–1.069) | 0.284 |
| Hematocrit | 39.8 (4.3) | 39.9 (3.8) | 0.551 | 0.982 (0.954–1.010) | 0.195 |
| INR | 1.1 (0.1) | 1.1 (0.1) | 0.882 | ||
| Procedural factors | |||||
| Computer assistance, n (%) | |||||
| With CAS | 22 (6.2a) | 334 | 0.001 | 1.920 (1.115–3.306) | 0.019 |
| Without CAS | 513 (3.1b) | 16,081 | |||
| Operative time | 333 (122) | 284 (108) | <0.001 | 1.002 (1.001–1.003) | <0.001 |
| LOS | 10.7 (12.7) | 5.2 (5.3) | <0.001 | 1.040 (1.031–1.050) | <0.001 |
| Total RVUs | 67.8 (29.4) | 60.0 (26.5) | <0.001 | 1.005 (1.001–1.003) | 0.010 |
aPercent of patients with CAS who returned to the operating room; bPercent of patients without CAS who returned to the operating room; #Fisher’s exact test. Bold values indicate significance (P<0.05). Pulmonary comorbidities include ventilator dependence, asthma, chronic lung disease, chronic oxygen support, tracheostomy, or structural pulmonary or airway abnormalities. Cardiac comorbidities include previous cardiac surgery and cardiac risk factors. ASA - American Society of Anesthesiologists; RVUs - Relative value unit; CAS - Computer-assisted surgery; GI - Gastric/intestinal; CNS - Central nervous system; SD - Standard deviation; OR - Odds ratio; CI - Confidence interval; LOS - Length of stay; INR - International normalized ratio
Post hoc multivariate logistic regression analysis demonstrated that, after controlling for operative year, navigation no longer predicted reoperation (OR = 1.005, P = 0.058, CI95: 1.068–1.230). Multivariate quantile regression revealed that, while navigation predicted a 44-min increase (P < 0.001, CI95: 29–60 min) in median operative time, it also predicted an 8.2 unit increase in median RVUs per case (P < 0.001, CI95: 5.7–10.7 RVUs), yielding a statistically insignificant 0.008 unit decrease in median RVUs per minute (P = 0.293, CI95: −0.022–0.007) compared to conventional surgery.
Medical comorbidities predictive of poorer 30-day outcomes are provided in Tables 3-5. Of note, female gender was protective of readmission (OR = 0.787, P = 0.021), while obesity (OR = 2.010, P < 0.001), pulmonary comorbidity (OR = 1.463, P = 0.001), and developmental delay (OR = 1.637, P < 0.001) predicted readmission. Obesity (OR = 2.472, P < 0.001), developmental delay (OR = 1.926, P < 0.001), operative time (OR = 1.002, P < 0.001), length of stay (OR = 1.040, P < 0.001), and total RVUs (OR = 1.005, P = 0.010) predicted reoperation. Finally, age (OR = 1.051, P < 0.001), African − American race (OR = 1.193, P = 0.002), Hispanic ethnicity (OR = 1.401, P < 0.001), developmental delay (OR = 1.291, P < 0.001), seizure disorder (OR = 1.384, P = 0.004), neuromuscular disorder (OR = 1.310, P < 0.001), operative time (OR = 1.005, P < 0.001), and total RVUs (OR = 1.009, P < 0.001) predicted morbidity.
Table 3.
Univariate and multivariate analysis of predictors of readmission
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Readmitted (n=668), n (%) | Not readmitted (n=16,282), n (%) | P | OR (95% CI) | P | |
| Demographics, n (%) | |||||
| Age (years), mean (SD) | 13.4 (3.2) | 13.8 (2.7) | 0.001 | 0.998 (0.963–1.034) | 0.902 |
| African−American race | 109 (18.0) | 2626 (18.1) | 0.947 | 1.144 (0.895–1.463) | 0.281 |
| Hispanic ethnicity | 76 (12.0) | 1580 (10.5) | 0.209 | ||
| Female gender | 377 (56.4) | 11,443 (70.3) | <0.001 | 0.787 (0.642–0.964) | 0.021 |
| Comorbidities, n (%) | |||||
| Obese | 139 (22.4) | 2333 (15.0) | <0.001 | 2.010 (1.588–2.544) | <0.001 |
| Pulmonary comorbidity | 234 (35.0) | 2630 (16.2) | <0.001 | 1.463 (1.157–1.850) | 0.001 |
| Cardiac comorbidity | 91 (13.6) | 1388 (8.5) | <0.001 | 1.006 (0.750–1.349) | 0.970 |
| Esophageal/GI disease | 186 (27.8) | 1539 (9.5) | <0.001 | 1.417 (1.083–1.854) | 0.011 |
| Developmental delay | 329 (49.3) | 3255 (20.0) | <0.001 | 1.637 (1.247–2.149) | <0.001 |
| Seizure disorder | 176 (26.3) | 1435 (8.8) | <0.001 | 1.381 (1.011–1.888) | 0.043 |
| Cerebral palsy | 170 (25.4) | 1431 (8.8) | <0.001 | 0.949 (0.688–1.309) | 0.749 |
| Structural CNS abnormality | 181 (27.1) | 2070 (12.7) | <0.001 | 1.236 (0.969–1.576) | 0.088 |
| Neuromuscular disorder | 307 (46.0) | 3433 (21.1) | <0.001 | 1.391 (1.090–1.776) | 0.008 |
| Preoperative steroid use | 16 (2.4) | 166 (1.0) | 0.001 | 1.166 (0.619–2.197) | 0.635 |
| Nutritional support | 143 (21.4) | 1157 (7.1) | <0.001 | 0.986 (0.711–1.367) | 0.932 |
| Hematologic disorder | 23 (3.4) | 296 (1.8) | 0.002 | 0.713 (0.386–1.315) | 0.278 |
| Congenital malformation | 330 (49.4) | 4833 (29.7) | <0.001 | 1.083 (0.871–1.348) | 0.474 |
| Childhood malignancy | 11 (1.6) | 1.0) | 0.092 | ||
| ASA-class ≥3 | 395 (59.1) | 4768 (29.3) | <0.001 | 1.513 (1.161–1.970) | 0.002 |
| Lab values, mean (SD) | |||||
| White cell count | 7.3 (2.5) | 6.9 (2.3) | <0.001 | 1.043 (1.007–1.081) | 0.019 |
| Hematocrit | 40.3 (4.2) | 39.9 (3.8) | 0.009 | 0.996 (0.971–1.021) | 0.723 |
| INR | 1.1 (0.1) | 1.1 (0.1) | 0.433 | ||
| Procedural factors | |||||
| Computer assistance, n (%) | |||||
| With CAS | 21 (5.9a) | 335 | 0.055 | 1.388 (0.807–2.388) | 0.236 |
| Without CAS | 647 (3.9b) | 15,947 | |||
| Operative time | 319 (112) | 284 (109) | <0.001 | 1.001 (1.000–1.002) | 0.018 |
| LOS | 6.4 (6.2) | 5.3 (6.8) | <0.001 | 0.992 (0.977–1.007) | 0.303 |
| Total RVUs | 66.1 (28.3) | 60.1 (26.5) | 0.017 | 1.004 (1.001–1.008) | 0.014 |
aPercent of patients with CAS who were readmitted; bPercent of patients without CAS who were readmitted; Bold values indicate significance (P<0.05). Pulmonary comorbidities include ventilator dependence, asthma, chronic lung disease, chronic oxygen support, tracheostomy, or structural pulmonary or airway abnormalities. Cardiac comorbidities include previous cardiac surgery and cardiac risk factors. ASA - American Society of Anesthesiologists; RVUs - Relative value units; CAS – Computer-assisted surgery; GI - Gastric/intestinal; CNS - Central nervous system; SD - Standard deviation; OR - Odds ratio; CI - Confidence interval; LOS - Length of stay; INR - International normalized ratio
Part II
There were 288 AIS patients who underwent posterior spinal fusion at our institution (136 Fluoro, 152 TNT), which were matched and compared to 326 NAV patients from the NSQIP dataset. NAV patients were significantly younger than both Fluoro and TNT patients [Table 6]. 30-day complication rates were similar between all three groups. In the Fluoro group, four patients returned to the OR for superficial site infections, which were all resolved with I&D; one patient required increased pain management; and one patient had desaturation events requiring BiPAP. One patient also developed a DVT, but this occurred after 30 days. In the TNT group, two patients developed superior mesenteric artery syndrome, which resolved through gastric decompression; one patient developed hypotensive shock; and one patient developed wound dehiscence.
Table 6.
Comparison of clinical variables between navigated patients from the NSQIP dataset, fluoroscopy group, and “technique’n’technology” group utilizing the Airo CT navigation technology groups
| NAV (n=326) | Fluoro (n=136) | TNT (n=152) | P a | P b | P 3 | P d | |
|---|---|---|---|---|---|---|---|
| Age (years) | 13.8 (11.1–16.5) | 14.7 (13.3–15.9) | 14.8 (13.4–16.3) | <0.001 | 0.010 | <0.001 | 0.450 |
| Operative time (min) | 323 (240–434) | 304 (259–345) | 247 (219–288) | <0.001 | 0.050 | <0.001 | <0.001 |
| LOS (days) | 4 (3–5) | 4 (3–5) | 3 (2–4) | <0.001 | 0.190 | <0.001 | <0.001 |
| Transfusion, n (%) | 236 (72.4) | 30 (22.2) | 41 (27.2) | <0.001 | <0.001 | <0.001 | 0.335 |
| Complications, n (%) | 22 (6.8) | 7 (5.2) | 4 (2.6) | 0.180 | 0.67 | 0.070 | 0.558 |
aP-value for overall group difference; bP-value for Fluoro to NAV; 3P-value for TNT to NAV; 4P-value for Fluoro to TNT. Data are presented as median and IQRs or counts and percentages when applicable. Bold values indicate significance (P<0.05). Fluoro - Fluoroscopy group; TNT - “Technique’n’technology” group utilizing the Airo CT navigation technology; NAV - Navigated patients from the NSQIP dataset; LOS - Length of stay; IQRs: Interquartile ranges
Median operative time was 323 min for NAV, which was significantly higher than 304 min for Fluoro and 247 min for TNT. Transfusion rates were also significantly higher for the NSQIP NAV group (72.4%) than the Fluoro (22.2%) and TNT (27.2%) groups but were statistically equivalent between the TNT and Fluoro groups. Median LOS for NAV was greater than that for TNT (4 vs. 3 days, P < 0.001).
Analysis of the fluoro versus TNT groups revealed similar pre- and post-operative Cobb angles with similar degrees of Cobb correction [Table 7]. The TNT group had greater postoperative kyphosis than the Fluoro group (27.9 vs. 24.6°, P < 0.001). The TNT group had less radiation time but had a greater radiation dose overall. Both groups had a median 13 levels fused (P = 0.390).
Table 7.
Comparison of radiographic and perioperative outcomes between fluoroscopy group and “technique’n’ technology” group utilizing the Airo CT navigation technology patients
| Fluoro (n=136) | TNT (n=152) | P | |
|---|---|---|---|
| Female, n (%) | 105 (77.2) | 109 (71.7) | 0.290 |
| Total radiation dose (mGy) | 2.9 (1.9–4.3) | 4.2 (1.6–12.9) | 0.040 |
| Total radiation time (s) | 23.0 (15.4–33.0) | 17.0 (9.4–22.6) | 0.001 |
| Preoperative major curve (°) | 56.0 (50.0–63.5) | 58.0 (54.3–63.9) | 0.180 |
| Postoperative major curve (°) | 17.0 (12.2–22.9) | 18.1 (12.7–24.8) | 0.610 |
| Cobb correction (%) | 69.7 (59.9–78.7) | 69.2 (59.4–80.1) | 0.620 |
| Preoperative kyphosis (°) | 25.5 (17.0–36.2) | 26.5 (15.6–37.5) | 0.800 |
| Postoperative kyphosis (°) | 24.6 (18.0–31.0) | 27.9 (18.1–37.6) | 0.001 |
| EBL (mL) | 600 (400–700) | 450 (300–700) | <0.001 |
Data are presented as median and IQRs or counts and percentages when applicable. Bold values indicate significance (P<0.05). Fluoro - Fluoroscopy group; TNT - “Technique’n’ technology” group utilizing the Airo CT navigation technology; IQRs - Interquartile ranges; EBL - Estimate blood loss
DISCUSSION
Improvements in imaging technology and computer-assisted surgery have allowed spine surgeons to operate on increasingly complex deformity cases with greater accuracy.[15,16,17,18] However, short-term outcomes evaluating navigated pediatric deformity surgery remain poorly studied. This is the first large-scale database study to evaluate navigation as a predictor of outcomes in posterior deformity surgery in pediatric patients and to compare the nationally represented navigated cohorts' outcomes to those of fluoroscopy-only and navigated cases performed at a single institution.
In the present study, after adjusting for patient-related and procedural factors, navigation in posterior fusion for pediatric spinal deformity predicted a 92% increase in odds of reoperation as well as a 2.9-times and 3.2-times increase in odds of deep wound infection and sepsis/septic shock, respectively. However, after controlling for operative year in a separate analysis, navigation no longer predicted reoperation, but remained associated with deep wound infection and sepsis-related events. Site-related events were the most common reason for reoperation. While navigation was associated with greater rates of morbidity and transfusion in univariate analysis, adjusted analysis demonstrated similar odds of morbidity and transfusion following navigation.
Compared to our single-institution data utilizing the TNT approach to navigated pedicle screw placement, whereby screws are initially placed under freehand anatomic technique followed by CT-based navigation, the NSQIP NAV group had statistically similar complication rates, but significantly greater operative times and transfusion rates. Notably, the Fluoro and TNT groups both had similar transfusion rates compared to each other, but significantly lower transfusion rates compared to the NAV group. Other studies have found similar levels of blood loss between navigated and nonnavigated surgery.[16] Our findings suggest that, while navigation may be associated with an increased risk of infectious-related events on a national scale, factors on an individual level, such as learning curve, sterile technique, minimized number of CT spins, and operative efficiency and reduced operative time, can have a profound impact on outcomes.[19,20] The factors such as increased operating room personnel, intraoperative O-arm spins and frequent relocations into sub-sterile rooms, and increased setup time may ultimately pose an increased risk of accidental contamination and associated infectious-related events.
Further, while learning curve cannot be evaluated directly in the present study, the finding that reoperation no longer statistically differed between navigated and conventional groups after controlling for operative year suggests that improvements in navigated technology during study period and increased surgeon experience can maximize the benefits of navigation. In a learning curve study of navigated vertebral body tethering, Mathew et al. demonstrated a steep learning curve over a 5-year period whereby operative time, hospital stay, and blood loss decreased and suggested that evolving technology may play a notable role.[19]
Prolonged operative time has been associated with navigation in prior deformity studies.[15,21] In addition, a large meta-analysis found that operative time was about 30-min longer on average in navigated versus nonnavigated surgery.[16] In the present study, multivariate quantile regression demonstrated that navigation independently predicted a 44-min increase in median operative time. Interestingly, in the treatment of single-level degenerative disease, navigation and nonnavigated cases have been found to have similar operative times, suggesting that case complexity likely plays an important role.[22] Compared to the single-surgeon Fluoro and TNT patients, NAV was associated with significantly higher OR time.
Moreover, we found that navigation predicted an 8.2-point increase in median RVUs per case. Unadjusted analysis demonstrated that navigation was associated with fewer RVUs per minute compared to conventional surgery, suggesting that there is a significant mismatch between efficiency and reimbursement. However, when taking into account operative year as well as significant patient-related variables, navigation was no longer associated with RVUs per minute in multivariate analysis. This is the first study to compare RVUs per case and per minute between navigated and nonnavigated surgery.
Comparison between the Fluoro to the TNT groups revealed that the TNT group still had a higher overall radiation dose despite having a lower radiation time. Radiation exposure is of particularly importance in children. Meta-analyses have demonstrated higher radiation dose in navigated surgery.[16] In the pediatric cervical spine literature, nonnavigated O-arm technique has been demonstrated to be effective in evaluating screw malposition without subjecting patients to radiation doses as high as those seen in navigation.[23] Interestingly, the Fluoro and TNT groups both had similar postoperative Cobb angles and degrees of Cobb correction, suggesting that both techniques allow for adequate coronal correction.
An important limitation of this study is the lack of granularity in data inherent to the utilization of a national surgical database, with an inability of the dataset to capture individual surgeon experience.[24,25,26] It is possible that newer, less experienced surgeons, are more frequently utilizing navigated technologies, which could skew outcomes more favorably toward conventional surgery. Other surgeon- and patient-related factors may influence operative time, blood loss, and infection with and without the use of navigation. Nevertheless, the current study provides interesting data regarding the use of navigation in pediatric deformity surgery on the national scale, which is currently lacking in the literature. The NSQIP dataset also does not provide details regarding the technique in which navigation is being utilized or the number of CT spins. In addition, the dataset does not provide specific information about pedicle screw placement (e.g., cortical vs. traditional screws). Although navigated versus conventional pedicle screw accuracy is a well-studied topic, our study did not evaluate for screw accuracy, which would have provided additional discussion points.
CONCLUSIONS
On a national scale, navigation predicted an increased odds of reoperation and infectious-related events and yielded greater median RVUs per case but had longer OR time and fewer RVUs-per-minute. However, after controlling for operative year, RVUs-per-minute and reoperation rates were similar between groups. The NSQIP navigated surgery group was associated with significantly higher median operative time and transfusion rates compared to the single-institution fluoroscopy-only and freehand followed by navigation, or “TNT,” groups. Complication rates were similar between all three groups. These findings suggest that navigated technology could be utilized more efficiently on a national level and that learning curve, surgeon experience and technique, and improvements in technology can maximize the benefits of navigation. In addition, specific comorbidities and demographic factors, such as African − American race and Hispanic ethnicity, predicted poorer outcomes. The identification of such predictors can allow surgeons to identify and potentially target interventions for patients who are at risk.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Safaee MM, Ames CP, Smith JS. Epidemiology and socioeconomic trends in adult spinal deformity care. Neurosurgery. 2020;87:25–32. doi: 10.1093/neuros/nyz454. [DOI] [PubMed] [Google Scholar]
- 2.Boddapati V, Lombardi JM, Urakawa H, Lehman RA. Intraoperative image guidance for the surgical treatment of adult spinal deformity. Ann Transl Med. 2021;9:91. doi: 10.21037/atm-20-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith JS, Shaffrey CI, Ames CP, Lenke LG. Treatment of adult thoracolumbar spinal deformity: Past, present, and future. J Neurosurg Spine. 2019;30:551–67. doi: 10.3171/2019.1.SPINE181494. [DOI] [PubMed] [Google Scholar]
- 4.Hussain I, Fu KM, Uribe JS, Chou D, Mummaneni PV. State of the art advances in minimally invasive surgery for adult spinal deformity. Spine Deform. 2020;8:1143–58. doi: 10.1007/s43390-020-00180-8. [DOI] [PubMed] [Google Scholar]
- 5.Song J, Katz AD, Perfetti D, Job A, Morris M, Goldstein J, et al. Impact of discharge to rehabilitation on postdischarge morbidity following multilevel posterior lumbar fusion. Clin Spine Surg. 2022;35:24–30. doi: 10.1097/BSD.0000000000001174. [DOI] [PubMed] [Google Scholar]
- 6.Nooh A, Aoude A, Fortin M, Aldebeyan S, Abduljabbar FH, Eng PJ, et al. Use of computer assistance in lumbar fusion surgery: Analysis of 15222 patients in the ACS-NSQIP Database. Global Spine J. 2017;7:617–23. doi: 10.1177/2192568217699193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song J, Katz AD, Dalal S, Silber J, Essig D, Qureshi S, et al. Comparison of relative value units and 30-Day Outcomes between primary and revision pediatric spinal deformity surgery. Clin Spine Surg. 2023;36:E40–4. doi: 10.1097/BSD.0000000000001352. [DOI] [PubMed] [Google Scholar]
- 8.Shahi P, Vaishnav A, Araghi K, Shinn D, Song J, Dalal S, et al. robotics reduces radiation exposure in minimally invasive lumbar fusion compared with navigation. Spine (Phila Pa 1976) 2022;47:1279–86. doi: 10.1097/BRS.0000000000004381. [DOI] [PubMed] [Google Scholar]
- 9.Schwab FJ, Blondel B, Bess S, Hostin R, Shaffrey CI, Smith JS, et al. Radiographical spinopelvic parameters and disability in the setting of adult spinal deformity: A prospective multicenter analysis. Spine (Phila Pa 1976) 2013;38:E803–12. doi: 10.1097/BRS.0b013e318292b7b9. [DOI] [PubMed] [Google Scholar]
- 10.Smith JS, Klineberg E, Schwab F, Shaffrey CI, Moal B, Ames CP, et al. Change in classification grade by the SRS-Schwab Adult Spinal Deformity Classification predicts impact on health-related quality of life measures: Prospective analysis of operative and nonoperative treatment. Spine (Phila Pa 1976) 2013;38:1663–71. doi: 10.1097/BRS.0b013e31829ec563. [DOI] [PubMed] [Google Scholar]
- 11.Cui G, Wang Y, Kao TH, Zhang Y, Liu Z, Liu B, et al. Application of intraoperative computed tomography with or without navigation system in surgical correction of spinal deformity: A preliminary result of 59 consecutive human cases. Spine (Phila Pa 1976) 2012;37:891–900. doi: 10.1097/BRS.0b013e31823aff81. [DOI] [PubMed] [Google Scholar]
- 12.Strong MJ, Yee TJ, Khalsa SS, Saadeh YS, Swong KN, Kashlan ON, et al. The feasibility of computer-assisted 3D navigation in multiple-level lateral lumbar interbody fusion in combination with posterior instrumentation for adult spinal deformity. Neurosurg Focus. 2020;49:E4. doi: 10.3171/2020.5.FOCUS20353. [DOI] [PubMed] [Google Scholar]
- 13.Song J, Katz AD, Perfetti D, Job A, Morris M, Virk S, et al. Comparative analysis of 30-Day readmission, reoperation, and morbidity between posterior cervical decompression and fusion performed in inpatient and outpatient settings. Asian Spine J. 2023;17:75–85. doi: 10.31616/asj.2021.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strigenz A, Katz AD, Lee-Seitz M, Shahsavarani S, Song J, Verma RB, et al. The 5-Item modified frailty index independently predicts morbidity in patients undergoing instrumented fusion following extradural tumor removal. Spine Surg Relat Res. 2023;7:19–25. doi: 10.22603/ssrr.2022-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore HG, Samuel AM, Burroughs PJ, Pathak N, Tuason DA, Grauer JN. Use of intraoperative navigation for posterior spinal fusion in adolescent idiopathic scoliosis surgery is safe to consider. Spine Deform. 2021;9:403–10. doi: 10.1007/s43390-020-00218-x. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin KD, Kadiyala M, Talwar D, Sankar WN, Flynn JJ, Anari JB. Does intraoperative CT navigation increase the accuracy of pedicle screw placement in pediatric spinal deformity surgery.A systematic review and meta-analysis? Spine Deform. 2022;10:19–29. doi: 10.1007/s43390-021-00385-5. [DOI] [PubMed] [Google Scholar]
- 17.Morse KW, Otremski H, Page K, Widmann RF. Less invasive pediatric spinal deformity surgery: The case for robotic-assisted placement of pedicle screws. HSS J. 2021;17:317–25. doi: 10.1177/15563316211027828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez D, Ghessese S, Cook D, Hedequist D. Initial intraoperative experience with robotic-assisted pedicle screw placement with stealth navigation in pediatric spine deformity: An evaluation of the first 40 cases. J Robot Surg. 2021;15:687–93. doi: 10.1007/s11701-020-01159-3. [DOI] [PubMed] [Google Scholar]
- 19.Mathew S, Larson AN, Potter DD, Milbrandt TA. Defining the learning curve in CT-guided navigated thoracoscopic vertebral body tethering. Spine Deform. 2021;9:1581–9. doi: 10.1007/s43390-021-00364-w. [DOI] [PubMed] [Google Scholar]
- 20.Bourget-Murray J, Brown GE, Peiro-Garcia A, Earp MA, Parsons DL, Ferri-de-Barros F. Quality, safety, and value of innovating classic operative techniques in scoliosis surgery: Intraoperative traction and navigated sequential drilling. Spine Deform. 2019;7:588–95. doi: 10.1016/j.jspd.2018.09.070. [DOI] [PubMed] [Google Scholar]
- 21.Katz AD, Galina J, Song J, Hasan S, Perfetti D, Virk S, et al. Impact of navigation on 30-Day outcomes for adult spinal deformity surgery. Global Spine J. 2021:21925682211047551. doi: 10.1177/21925682211047551. Doi:101177/21925682211047551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bovonratwet P, Nelson SJ, Ondeck NT, Geddes BJ, Grauer JN. Comparison of 30-Day complications between navigated and conventional single-level instrumented posterior lumbar fusion: A propensity score matched analysis. Spine (Phila Pa 1976) 2018;43:447–53. doi: 10.1097/BRS.0000000000002327. [DOI] [PubMed] [Google Scholar]
- 23.Verhofste BP, Glotzbecker MP, Hresko MT, MacDougall RD, Birch CM, O'Neill NP, et al. Intraoperative use of O-arm in pediatric cervical spine surgery. J Pediatr Orthop. 2020;40:e266–71. doi: 10.1097/BPO.0000000000001416. [DOI] [PubMed] [Google Scholar]
- 24.Katz AD, Song J, Bowles D, Ng T, Neufeld E, Hasan S, et al. What is a better value for your time.Anterior cervical discectomy and fusion versus cervical disc arthroplasty? J Craniovertebr Junction Spine. 2022;13:331–8. doi: 10.4103/jcvjs.jcvjs_69_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz AD, Song J, Virk S, Silber JS, Essig D. Adjunct pelvic fixation in short-to-medium segment degenerative fusion constructs independently predicts readmission and morbidity. J Craniovertebr Junction Spine. 2022;13:182–91. doi: 10.4103/jcvjs.jcvjs_60_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz AD, Song J, Ngan A, Job A, Morris M, Perfetti D, et al. Discharge to rehabilitation predicts increased morbidity in patients undergoing posterior cervical decompression and fusion. Clin Spine Surg. 2022;35:129–36. doi: 10.1097/BSD.0000000000001319. [DOI] [PubMed] [Google Scholar]


