ABSTRACT
Purpose:
In the past few decades, candidemia has escalated to worrisome levels, leading to substantial morbidity and mortality in neonates. The rise in anti-fungal drug resistance demands prompt diagnosis and treatment. This study aimed to determine the speciation and susceptibility pattern of Candida species recovered from special care new-born units and identify risk factors for developing candidemia in neonates.
Method:
A total of 580 blood samples from clinically suspected septicemic neonates were collected and subjected to culture. Cultures positive for yeasts were sub-cultured on Sabouraud dextrose agar. Identification of a suspected purified colony of Candida was confirmed to the species level by both conventional and automated techniques matrix-assisted laser desorption and ionization time-of-flight mass spectrometry. Anti-fungal susceptibility of isolates was performed by an automated method (VITEK 2 system) using VITEK 2 cards. Multi-variate logistic regression analysis was used to identify risk factors associated with candidemia.
Result:
A total of 56 (9.66%) isolates of Candida species were recovered from 580 blood cultures. Non-albicans Candida species predominated with 82.14% of cases, whereas 17.86% of cases were caused by Candida albicans. Candida tropicalis (46.42%) was the most common isolate recovered, followed by Candida albicans (17.8%). Risk factor analyses identified a very low birth weight [odds ratio (OR) =4.05, 95% confidence interval (CI) =2.03–8.08] and prolonged antibiotic therapy (OR = 3.79, 95% CI = 1.7–8.7) among others as significant predictors of candidemia. All the Candida isolates showed 100% sensitivity to voriconazole and micafungin, whereas the overall sensitivities for fluconazole, amphotericin B, caspofungin, and flucytosine were 85.71%, 96.43%, 96.43%, and 91.07%, respectively.
Conclusion:
Candidemia is a life-threatening condition in neonates. Identification of Candida species and routine anti-fungal susceptibility is a must to select a suitable and effective anti-fungal therapy to revoke emerging resistance to anti-fungals.
Keywords: Anti-fungal susceptibility tests, candida tropicalis, candidemia, very low birth weight
Introduction
Candida species have emerged as major blood stream infection pathogens associated with pronounced mortality and morbidity in very-low-birth-weight neonates (defined as birth weights <1500 g).[1,2] Successful management of neonatal candidemia includes appropriate anti-fungal therapy and supportive care as well as preventive measures to reduce the risk of systemic Candida infections.
At present, Candida is the fourth most common cause of bloodstream infections in the United States, causing invasive life-threatening fungal infections among hospitalized patients.[3,4] The global incidence of candidemia has increased more than fivefold in the past decade.[5] The incidence of candidemia is about 6.51 cases per 1000 intensive care unit (ICU) admissions in India.[6]
Importance of Candida species in neonatal sepsis and sepsis-related mortality is increasingly being recognized.[7] In particular, among new-borns with a birth weight <1000g, 4–8% will develop candidemia, with a mortality rate of 30%.[8] Common risk factors for neonatal candidemia include prematurity and a very low birth weight [VLBW <1500 g; low birth weight (LBW) <2500 g], central vascular catheterization, parenteral nutrition, use of broad-spectrum antibiotics, H2 blockers and corticosteroids, endotracheal intubation, and prolonged hospital stay.[7,9] New-borns who survive frequently have long-term neurological impairment, including cerebral palsy, blindness, hearing impairment, cognitive deficits, and periventricular leukomalacia.[10]
Furthermore, many regions of the world are witnessing a surge in non-albicans Candida species. Candida tropicalis is the most common non-albicans Candida (NAC) causing blood stream infection (BSI) in hospital settings. In India, it ranks first among all Candida species known to cause BSI.[11,12,13] A similar change in species distribution was also noted in other Asian countries.[14] Therefore, routine screening of Candida isolates to the species level is of crucial importance as this could assist clinicians in promoting adoption of important prophylactic and treatment guidelines for its improved management.
Although diagnostic competency for fungal infections has improved, the critical status and the non-specific presentation of the invasive disease in hospital settings often delay confirmation of etiology and appropriate management.[15] With the rise in anti-fungal resistance, susceptibility tests play an ever-increasing role in the selection of anti-fungal drugs. Knowledge on risk factors to consider when deciding to administer empirical anti-fungal therapy is essential for clinicians to prevent candidemia. Therefore, the present study aimed to assess changing trends of candidemia in neonates admitted to special new-born care units to examine in vitro susceptibility to common anti-fungal drugs and to assess risk factors for Candida septicaemia.
Materials and Methods
This prospective study was performed in the Department of Microbiology, Rajendra Institute of Medical Sciences, Ranchi, from July 2020 to June 2021. A total of 580 blood samples were collected aseptically in blood culture bottles (BD BACTEC Peds Plus) from neonates admitted in special care new-born units (SCNUs) for clinically suspected neonatal sepsis before starting anti-microbial therapy and processed for culture using an automated blood culture system (BD BACTEC FX 100). If BACTEC flagged positive results, then sub-culture was performed on blood agar, Mac Conkey agar, and Sabouraud’s dextrose agar (SDA) with antibiotics. The suspected Candida colonies were identified by Gram stain and further speciated by a panel of tests like germ tube test, color on Hi Chrome Candida differential agar, growth morphology on corn meal agar (Dalmau’s plate method), and sugar assimilation tests. It was further confirmed with an automated identification method using matrix-assisted laser desorption and ionization time-of-flight mass spectrometry (MALDI-TOF MS) (BioMeriux). Candidemia was diagnosed by isolation of Candida species from at least one positive blood culture containing pure growth of Candida species with supportive clinical features. The clinical and laboratory data on all eligible neonates were collected in a structured proforma. The anti-fungal susceptibility tests of the isolates were performed by an automated method (VITEK2 system) with anti-fungal drugs amphotericin B (AMB), caspofungin (CAS), fluconazole (FLU), flucytosine (FC), micafungin (MFG), and voriconazole (VRC) using AST-YS08 card. The drug concentration was in the range of 0.25–16 μg/ml for AMB, 0.25–4 μg/ml for CAS, 1–64 μg/ml for FLU and FC, 0.06–4 μg/ml for MFG, and 0.125–16 μg/ml for VRC. Results of MICs were obtained according to the interpretative criteria provided by the automated systems’ recommendations (CLSI) guidelines.[16] Quality control was performed using the following strains as controls for the evaluation: Candida albicans (ATCC 90028), Candida parapsilosis (ATCC22019), and Candida krusei (ATCC 6258).
Statistical analysis
Data were collected in a structured proforma and were classified, analyzed, and evaluated by using SPSS version 21 for Windows (SPSS Inc., Chicago, IL). We had performed uni-variate logistic regression of known risk factors for neonatal candidemia. All variables with P < 0.05 were selected for inclusion in the multi-variate logistic regression model to identify predisposing risk factors associated with neonatal candidemia.
Result
Out of 580 blood samples, 43.4% (n = 252) were culture-positive. Among positive culture, 54.4% were Gram-negative bacilli, 23.4% were Gram-positive cocci, and 22.2% (n = 56) were Candida isolates. The prevalence of candidemia was 9.66% of total clinically suspected cases of neonatal sepsis. Candidemia was more prevalent in male (62.5%) than in female (37.5%). The male-to-female ratio was 1.66:1. Among neonates with positive blood culture for candidemia, the gestational age ranged from 30 weeks to 40 weeks with a mean age of 34.12 weeks [95% confidence interval (CI) for the mean of 33.26 to 34.99] and the birth weight ranged from 930 g to 2760 g with a mean weight of 1588.30g (95% CI for the mean of 1456.87 to 1719.73). The majority of candidemia episodes occurred in VLBW (<1500 g) neonates (66.07%). The mean duration of the total hospital stay was 16.03 days (95% CI 14.76 to 17.31 days). Candida species were isolated mainly in late-onset sepsis (LOS), 35/56 (62.5%), when compared to early-onset sepsis (EOS), 21/56 (37.5%). Out of 56 isolates, non-albicans Candida (NAC) accounted for 46 isolates (82.14%), whereas Candida albicans were 10 (17.86%) isolates. The most common species isolated was Candida tropicalis 26 (46.42%), as shown in Figure 1.
Figure 1.
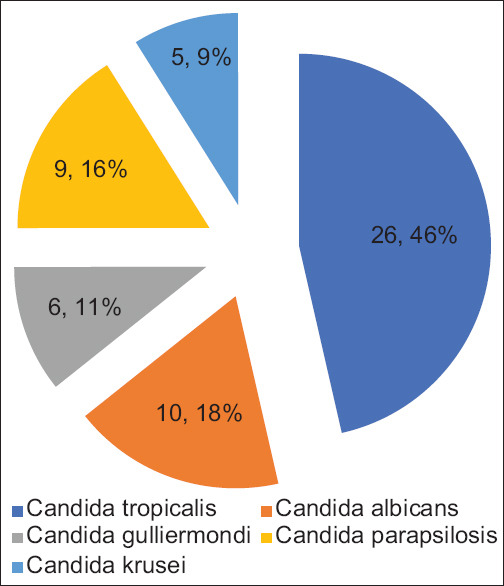
Distribution of Candida species isolated from blood culture
All the Candida isolates showed 100% sensitivity to voriconazole and micafungin, and the overall sensitivity for fluconazole, amphotericin B, caspofungin, and flucytosine is summarized in Figure 2.
Figure 2.
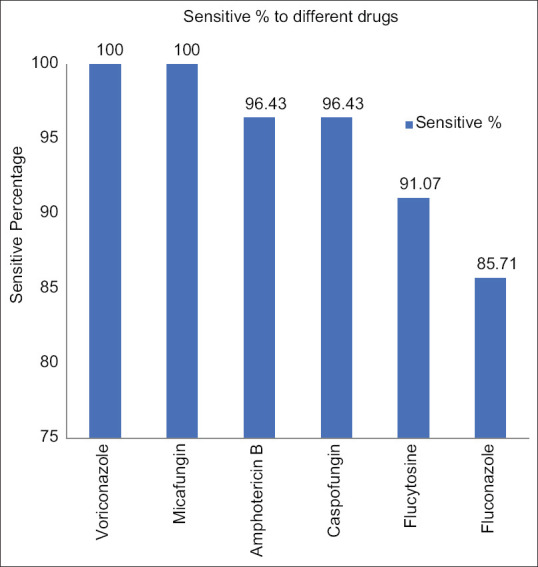
Anti-fungal sensitivity pattern of Candida isolates in the study (n = 56)
The baseline characteristics of the neonates with candidemia are summarized in Table 1.
Table 1.
Baseline characteristics of neonates with candidemia
| Variables | Value | P |
|---|---|---|
| Mean birth weight (g) (mean) | 1921 | <0.0001 |
| Male, gender, n (%) | 35 (62.5%) | - |
| Low birth weight, n (%) | 37 (66.07) | <0.0001 |
| Total parenteral nutrition, n (%) | 36 (64.29%) | 0.0031 |
| Normal vaginal delivery, n (%) | 36 (85.7%) | NS |
| Prolonged use of antibiotics, n (%) | 46 (82.14%) | <0.0001 |
| Prematurity, n (%) | 40 (71.4%) | 0.00013 |
| Ventilator support, n (%) | 26 (46.4%) | <0.0001 |
| Prolonged rupture of membranes (>18 hours), n (%) | 11 (19.64%) | 0.0007 |
| Thrombocytopenia (<1,50,000 cells/mm) | 45 (80.35%) | <0.0001 |
| Post-natal steroid treatment | 15 (26.78%) | 0.0091 |
| Fever, n (%) | 52 (92.85%) | <0.0001 |
| Tachypnea, n (%) | 46 (81.05%) | 0.32 |
The percentage anti-fungal susceptibility of Candida albicans and NAC is shown in Table 2.
Table 2.
Anti-fungal susceptibility profile of Candida isolates by VITEK 2 system
| Organism isolated | Anti-fungals | Susceptibility (%) | Intermediate | Resistance (%) |
|---|---|---|---|---|
| C.tropicalis (n=-26) | Amphotericin B | 26 (100%) | - | 0 |
| Fluconazole | 26 (100%) | - | 0 | |
| Voriconazole | 26 (100%) | - | 0 | |
| Flucytosine | 25 (96%) | - | 1 (4%) | |
| Caspofungin | 26 (100%) | - | 0 | |
| Micafungin | 26 (100%) | - | 0 | |
| C.albicans (n=10) | Amphotericin B | 10 (100%) | - | 0 |
| Fluconazole | 10 (100%) | - | 0 | |
| Voriconazole | 10 (100%) | - | 0 | |
| Flucytosine | 10 (100%) | - | 0 | |
| Caspofungin | 10 (100%) | - | 0 | |
| Micafungin | 10 (100%) | - | 0 | |
| C.gullerimondi (n=6) | Amphotericin B | 6 (100%) | - | 0 |
| Fluconazole | 6 (100%) | - | 0 | |
| Voriconazole | 6 (100%) | - | 0 | |
| Flucytosine | 6 (100%) | - | 0 | |
| Caspofungin | 6 (100%) | - | 0 | |
| Micafungin | 6 (100%) | - | 0 | |
| C.parapsilosis (n=9) | Amphotericin B | 9 (100%) | - | 0 |
| Fluconazole | 6 (66.66%) | - | 3 (33.33%) | |
| Voriconazole | 9 (100%) | - | 0 | |
| Flucytosine | 9 (100%) | - | 0 | |
| Caspofungin | 9 (100%) | - | 0 | |
| Micafungin | 9 (100%) | - | 0 | |
| C.krusei (n=5) | Amphotericin B | 3 (60%) | - | 2 (40%) |
| Fluconazole | 0 | - | 5 (100%) | |
| Voriconazole | 5 (100%) | - | 0 | |
| Flucytosine | 1 (20%) | - | 4 (80%) | |
| Caspofungin | 4 (80%) | - | 1 (20%) | |
| Micafungin | 5 (100%) | - | 0 |
In the uni-variate analysis, the factors significantly associated with candidemia were total parenteral nutrition (P < 0.0001, 95% CI 0.077 to 0.14), VLBW (P < 0.0001, 95% CI 0.29 to 0.37), assisted ventilation (P < 0.0001, 95% CI 0.4337 to 0.5301), pre-term (P = 0.0001, 95% 0.40 to 0.49), central line (P < 0.00010.0015, 95% CI 0.25 to 0.34), and prolonged antibiotic use (P < 0.0001, 95% CI 0.37 to 0.46).
Forward step-wise multi-variate logistic regression was used to evaluate the risk factors for candidemia identified as significant in the uni-variate analyses. The results of this analysis are detailed in Figure 3.
Figure 3.
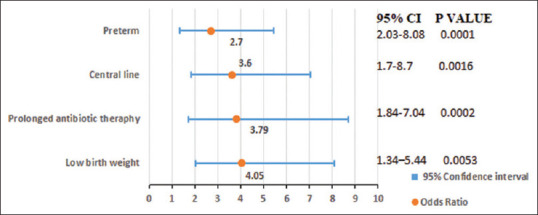
Multi-variate logistic regression analysis to determine predisposing risk factors of neonatal candidemia
Fever was the most common presenting symptom in 92.85%, followed by tachypnea (80.35%.), poor weight gain (69.6%), and lethargy (62.5%) observed in our study. The correlation coefficient of fever with tachypnea was 0.77 (CI 0.74 to 0.80, P < 0.001). The central line was put in 58.93% for inotropic support and proper management of the neonates. Some of the them developed multiple organ dysfunction syndrome (MODS), and the final outcome succumbed to death within 48 hours of candidemia diagnosis. The overall mortality rate in neonates with candidemia was 30.3%.
Discussion
In the present study, candidemia was responsible for 9.65% cases of suspected neonatal sepsis, which is consistent with the observations of Lamba M et al.,[17] Goel et al.,[18] and Benjamin et al.[7] In a study conducted by Chaudhury et al.,[19] from Eastern India, the total cases of neonatal candidemia were 10.4%, which is similar to our study, whereas some authors have observed higher rates (31%) of neonatal candidemia.[12] These variations in isolation rate of Candida could be because of variability in the various risk factors in the study population. Of the 56 neonates with candidemia in the present study, the majority presented with late onset sepsis and only had early sepsis, which is consistent with the findings of Caggiano et al.[20] and Benjamin et al.;[7] they also reported candidemia accounting for 9–13% of blood stream infections in neonates in neonatal ICUs (NICUs).
In our study, Candidemia was more commonly found to be due to NAC species (82.14%), followed by Candida albicans (17.8%), which is similar to several other studies conducted from different regions of India.[17,18,21,22,23] In a 11-year prospective pediatric study in Turkey in 2020, Aslan N et al.[24] showed collective predominance for NAC species in parallel with our study.
As per the previous reports, most cases of neonatal and pediatric candidemia were caused by Candida albicans.[25] However, recent studies from different geographical areas of the world including India have shown changing trends of neonatal candidemia, with emergence of NAC species as an important cause of candidemia.[26,27,28]
The increased incidence of NAC is probably due to the greater use of invasive devices, broadspectrum anti-bacterial agents, more extensive surgical procedures, and selection of less susceptible species by the pressure of anti-fungal agents such as fluconazole.[26]
In our study, the most common species isolated was Candida tropicalis (46.42%), which is comparable to the epidemiological studies carried out in different parts of our country, indicating Candida tropicalis as the common cause of nosocomial candidemia.[6,17]
Increased incidence of C. tropicalis as a cause of candidemia in hospitalized settings may pertain to its presence on the hands of the hospital personnel. The major virulence factor of this organism is the ability to produce clusters. C. tropicalis may be more virulent than C. albicans when introduced in an immunocompromised host and thereby rapidly progress from colonization to invasion.
The multi-variate logistic regression model suggested that VLBW, prolonged use of antibiotics, and central line were the factors most highly associated with increased odds of candidemia. Similar findings have been reported by other studies that demonstrated the association of risk factors with candidemia in the neonates.[3,12,17,18,29,30,31]
In our study, the majority of candidemia episodes occurred in VLBW (<1500 g) neonates (66.07%). VLBW infants are known to be at a high risk of candidemia because of their immature immune system, which may lead to an inability to eliminate pathogens from the bloodstream at the initiation of the anti-fungal system.
Broad-spectrum antibiotic use poses a significant threat for candidemia.[2,9,10] A previous study conducted by Kaufman et al.[32] showed that decreased use of carbapenem may be associated with decreased incidence of invasive fungal infections. The widespread use of anti-bacterial agents may suppress bacterial flora and increase the Candida colonization density.[33] This finding highlights the need to evaluate the anti-microbial burden in local NICUs in India.
Use of various invasive devices, such as catheters and endotracheal tubes, may be to blame for the nosocomial spread of pathogens through the hands of health care workers (HCWs). In this study, we did not obtain samples from potentially colonized HCW in the unit. The hands of HCW and environmental surfaces are recently acknowledged as potential reservoirs for nosocomial strains of Candida. More importantly, prophylactic measures such as the use of filters for parenteral nutrition and implementing a restrictive policy toward antibiotic use to decrease Candida colonization/infection rates will further reduce the morbidity and mortality associated with these infections.
It was observed in the present study that fever was the most common presenting symptom (92.85%), followed by tachypnea (80.35%). Similar findings have been reported by other authors also.[34]
Thrombocytopenia can be a specific marker of fungal sepsis in NICU. In our study, 45 out of 56 neonates (80.35%) had thrombocytopenia, which is comparable to Yunus et al.,[33] who found 66/83 (80%) incidence of thrombocytopenia in patients with invasive fungal sepsis.
In the present study, we found an increase in the anti-fungal drug resistance, especially for the azole group of drugs in NAC species. Many authors have also found increasing trends of fluconazole resistance, reported as 37.5% by Gupta et al.,[35] 36% by Kothari et al.,[36] and 11.7% by Xess et al.[29]
In the study reported by Narain et al.,[37] all fungal isolates were sensitive to amphotericin B, which is in contrast to our study as fungal resistance to amphotericin B was found to be 3.57% among all the Candida isolates. Similar results were reported by Bhatt et al.[38] with resistance to amphotericin B at 8%.
In other studies also, a variable range of resistance to flucytosine was reported, ranging from 37% by Bhatt et al.[38] to 0% by Pahwa et al.[39]
Our study showed increasing incidence of multi-drug-resistant species of Candida krusei (8.9%), which is comparatively higher than findings (1–4%) in previous studies.[6,17,18] This high incidence of Candida krusei could be due to use of fluconazole in empirical treatment of suspected neonatal systemic candidiasis, which might have led to selection of innately resistant to it. Xess et al.[29] reported 3.3% incidence of C. krusei in North India. Only one Indian study by Shrivastava G, et al.[40] reported 38% incidence of C. krusei in Central India. The rise of resistant species is alarming and should be considered thoroughly. It shows the importance of keeping echinocandins as the second-line drugs at hand.
This study has limitations inherent to a single center and a lack of follow-up of neonates who were diagnosed to have candidemia to study the long-term consequence in neonates. More regional data from different hospital settings are needed in future to allow comparison of findings. Further studies on risk factors for candidemia and on newer anti-fungal drugs should be emphasized.
Conclusion
Candida species are important bloodstream pathogens in neonates that are being isolated with increasing frequency. In this population, candidemia due to NAC has been increased dramatically in the past few decades and associated with a poor prognosis. To conclude, emergence of NAC species and their association with higher mortality and longer duration of hospital stay is a cause for concern. Increased incidence and occurrence of multi-drug resistance of C. krusei are alarming. Prevention of risk factors in susceptible neonates with early removal of central line, timely fungal culture, Candida speciation, and susceptibility testing are necessary for appropriate institution of treatment and better outcome. Adequate and accurate laboratory diagnosis with correct species identification can limit the mortality by initiation of timely and cost-effective anti-fungal treatment and will lead to containment of multi-fungal-resistant infection in the community.
Ethical approval
Approved by Institutional Research Board (IRB) and Institutional Ethics Committee (IEC) wide memo no. 31, IEC, Rims, Ranchi, Dated 03-06-2020.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B. Epidemiological, clinical and microbiological characteristics of late-onset sepsis among very low birth weight infants in Israel:A national survey. Pediatrics. 2002;109:34–9. doi: 10.1542/peds.109.1.34. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez D, Almirante B, Park BJ, Cuenca-Estrella M, Planes AM, Sanchez F, et al. Candidemia in neonatal intensive care units:Barcelona, Spain. Pediatr Infect Dis J. 2006;25:224–9. doi: 10.1097/01.inf.0000202127.43695.06. [DOI] [PubMed] [Google Scholar]
- 3.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C, et al. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States:A propensity analysis. Clin Infect Dis. 2005;41:1232–9. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis:A persistent public health problem. ClinMicrobiol Rev. 2007;20:133–63. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barchiesi F, Orsetti E, Mazzanti S, Trave F, Salvi A, Nitti C, et al. Candidemia in the elderly:What does it change? PLoS One. 2017;12:e0176576. doi: 10.1371/journal.pone.0176576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakrabarti A, Sood P, Rudramurthy SM, Chen S, Kaur H, Capoor M, et al. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med. 2015;41:285–95. doi: 10.1007/s00134-014-3603-2. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin DK, Jr, Stoll BJ, Gantz MG, Walsh MC, Sánchez PJ, Das A, et al. Eunice kennedy shriver national institute of child health and human development neonatal research network. Neonatal candidiasis:Epidemiology, risk factors, and clinical judgment. Pediatrics. 2010;126:e865–73. doi: 10.1542/peds.2009-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Testoni D, Hayashi M, Cohen-Wolkowiez M, Benjamin DK, Jr, Lopes RD, Clark RH, et al. Late-onset bloodstream infections in hospitalized term infants. Pediatr Infect Dis J. 2014;33:920–3. doi: 10.1097/INF.0000000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oeser C, Vergnano S, Naidoo R, Anthony M, Chang J, Chow P, et al. Neonatal invasive fungal infection in England 2004-2010. ClinMicrobiol Infect. 2014;20:936–41. doi: 10.1111/1469-0691.12578. [DOI] [PubMed] [Google Scholar]
- 10.Espinel-Ingroff A, Pfaller MA, Bustamante B, Canton E, Fothergill A, Fuller J, et al. Multilaboratory study of epidemiological cutoff values for detection of resistance in eight Candida species to fluconazole, posaconazole, and voriconazole. Antimicrob Agents Chemother. 2014;58:2006–12. doi: 10.1128/AAC.02615-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lass-Flörl C. The changing face of epidemiology of invasive fungal disease in Europe. Mycoses. 2009;52:197–205. doi: 10.1111/j.1439-0507.2009.01691.x. [DOI] [PubMed] [Google Scholar]
- 12.Rani R, Mohapatra NP, Mehta G, Randhawa VS. Changing trends of Candida species in neonatal septicaemia in a tertiary North Indian hospital. Indian J Med Microbiol. 2002;20:42–4. [PubMed] [Google Scholar]
- 13.Verma AK, Prasad KN, Singh M, Dixit AK, Ayyagari A. Candidaemia in patients of a tertiary health care hospital from north India. Indian J Med Res. 2003;117:122–8. [PubMed] [Google Scholar]
- 14.Chakrabarti A, Chander J, Kasturi P, Panigrahi D. Candidaemia:A 10-year study in an Indian teaching hospital. Mycoses. 1992;35:47–51. doi: 10.1111/j.1439-0507.1992.tb00818.x. [DOI] [PubMed] [Google Scholar]
- 15.Falagas ME, Roussos N, Vardakas KZ. Relative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world:A systematic review. Int J Infect Dis. 2010;14:e954–66. doi: 10.1016/j.ijid.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 16.NCCLS. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Second Edition. NCCLS document M27-A2 Pennsylvania 19087-1898 USA; 2002 [Google Scholar]
- 17.Lamba M, Sharma D, Sharma R, Vyas A, Mamoria V. To study the profile of Candida isolates and antifungal susceptibility pattern of neonatal sepsis in a tertiary care hospital of North India. J Matern Fetal Neonatal Med. 2021;34:2655–9. doi: 10.1080/14767058.2019.1670799. [DOI] [PubMed] [Google Scholar]
- 18.Goel N, Ranjan PK, Aggarwal R, Chaudhary U, Sanjeev N. Emergence of nonalbicans Candida in neonatal septicemia and antifungal susceptibility:Experience from a tertiary care center. J Lab Physicians. 2009;1:53–5. doi: 10.4103/0974-2727.59699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhury N, Majumdar M, Dutta A, Das S, Mukherjee T, Ghosh P, et al. Emerging incidence of candidemia in neonatal intensive care unit and sick newborn care unit in a tertiary care hospital of Eastern India. Int J Health Clin Res. 2020;3:60–7. [Google Scholar]
- 20.Caggiano G, Lovero G, De Giglio O, Barbuti G, Montgana O, Laforgia N, et al. candidemia in the neonatal intensive care unit:A retrospective, observational survey and analysis of literature data. Biomed Res Int. 2017;2017:7901763. doi: 10.1155/2017/7901763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takkar VP, Bhakoo ON, Narang A. Scoring system forthe prediction of early neonatal infections. Indian Pediatr. 1974;11:597–600. [PubMed] [Google Scholar]
- 22.Nazir A, Masoodi T. Spectrum of candidal species isolated from neonates admitted in an Intensive Care Unit of teaching hospital of Kashmir, North India. J Lab Physicians. 2018;10:255–9. doi: 10.4103/JLP.JLP_1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juyal D, Sharma M, Pal S, Rathaur VK, Sharma N. Emergence of non-albicans Candida species in neonatal candidemia. N Am J Med Sci. 2013;5:541–5. doi: 10.4103/1947-2714.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aslan N, Yildizdas D, Alabaz D, Horoz OO, Yontem A, Kocabas E. Invasive candida infections in a pediatric intensive care unit in Turkey:Evaluation of an 11-year period. J Pediatr Intensive Care. 2020;9:21–6. doi: 10.1055/s-0039-1695061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capoor MR, Nair D, Deb M, Verma PK, Srivastava L, Aggarwal P. Emergence of non-albicans Candida species and antifungal resistance in a tertiary care hospital. Jpn J Infect Dis. 2005;58:344–8. [PubMed] [Google Scholar]
- 26.Saiman L, Ludington E, P faller M, RangelFrausto S, Wiblin RT, Dawson J, et al. Risk factors for candidemia in neonatal intensive care unit patients. The National Epidemiology of Mycosis Survey Study Group. Pediatr Infect Dis J. 2000;19:31924. doi: 10.1097/00006454-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Clerihew L, Lamagni TL, Brocklehurst P, McGuire W. Invasive fungal infection in very low birthweight infants:National prospective surveillance study. Arch Dis Child Fetal Neonatal Ed. 2006;91:F188–92. doi: 10.1136/adc.2005.082024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang XB, Yu SJ, Yu J×. Retrospective analysis of epidemiology and prognostic factors for candidemia at a hospital in China, 2000–2009. Jpn J Infect Dis. 2012;65:510–5. [PubMed] [Google Scholar]
- 29.Xess I, Jain N, Hasan F, Mandal P, Banerjee U. Epidemiology of candidemia in a tertiary care centre of North India:5-year study. Infection. 2007;35:256–9. doi: 10.1007/s15010-007-6144-6. [DOI] [PubMed] [Google Scholar]
- 30.Lona-Reyes JC, Gómez-Ruiz LM, Cordero-Zamora A, Cortés-González SI, Quiles-Corona M, Pérez-Ramírez RO, et al. Incidence and factors associated with invasive candidiasis in a neonatal intensive care unit in Mexico. An Pediatr (Engl Ed) 2022;97:79–86. doi: 10.1016/j.anpede.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Silvester EJ, Watanabe MMY, Pittet LF, Boast A, Bryant PA, Haeusler GM, et al. Candidemia in children:A 16-year longitudinal epidemiologic study. Pediatr Infect Dis J. 2021;40:537–43. doi: 10.1097/INF.0000000000003082. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman DA. Challenging issues in neonatal candidiasis. Curr Med Res Opin. 2010;26:1769–78. doi: 10.1185/03007995.2010.487799. [DOI] [PubMed] [Google Scholar]
- 33.Yunus M, Agarwal V, Tomer P, Gupta P, Upadhyay A. Epidemiology, clinical spectrum and outcomes of fungal sepsis in neonates in neonatal intensive care unit:A prospective observational study. Int J Contemporary Med Res. 2018;5:1–5. [Google Scholar]
- 34.Oberoi JK, Wattal C, Goel N, Raveendran R, Datta S, Prasad K. Non-albicans Candida species in blood stream infections in a tertiary care hospital at New Delhi, India. Indian J Med Res. 2012;136:997–1003. [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta N, Mittal N, Sood P, Kumar S, Kaur R, Mathur MD, et al. Candidemia in neonatal intensive care unit. Indian J Pathol Microbiol. 2001;44:45–8. [PubMed] [Google Scholar]
- 36.Kothari A, Sagar V. Epidemiology of Candida bloodstream infections in a tertiary care institute in India. Indian J Med Microbiol. 2009;27:171–2. doi: 10.4103/0255-0857.49440. [DOI] [PubMed] [Google Scholar]
- 37.Narain S, Shastri JS, Mathur M, Mehta PR. Neonatal systemic candidiasis in a tertiary care centre. Indian J Med Microbiol. 2003;21:56–8. [PubMed] [Google Scholar]
- 38.Bhatt M, Sarangi G, Paty BP, Mohapatra D, Chayani N, Mahapatra A, et al. Biofilm as a virulence marker in Candida species in nosocomial blood stream infection and its correlation with antifungal resistance. Indian J Med Microbiol. 2015;33((Suppl S1)):112–4. doi: 10.4103/0255-0857.150909. [DOI] [PubMed] [Google Scholar]
- 39.Pahwa N, Kumar R, Nirkhiwale S, Bandi A. Species distribution and drug susceptibility of Candida in clinical isolates from a tertiary care centre at Indore. Indian J Med Microbiol. 2014;32:44–8. doi: 10.4103/0255-0857.124300. [DOI] [PubMed] [Google Scholar]
- 40.Shrivastava G, Bajpai T, Bhatambare G, Chitnis V, Deshmukh A. Neonatal candidemia:Clinical importance of species identification. Int Med J Sifa Univ. 2015;2:37. [Google Scholar]


