Abstract

High-valent iron species have been implicated as key intermediates in catalytic oxidation reactions, both in biological and synthetic systems. Many heteroleptic Fe(IV) complexes have now been prepared and characterized, especially using strongly π-donating oxo, imido, or nitrido ligands. On the other hand, homoleptic examples are scarce. Herein, we investigate the redox chemistry of iron complexes of the dianonic tris-skatylmethylphosphonium (TSMP2–) scorpionate ligand. One-electron oxidation of the tetrahedral, bis-ligated [(TSMP)2FeII]2– leads to the octahedral [(TSMP)2FeIII]−. The latter undergoes thermal spin-cross-over both in the solid state and solution, which we characterize using superconducting quantum inference device (SQUID), Evans method, and paramagnetic nuclear magnetic resonance spectroscopy. Furthermore, [(TSMP)2FeIII]− can be reversibly oxidized to the stable high-valent [(TSMP)2FeIV]0 complex. We use a variety of electrochemical, spectroscopic, and computational techniques as well as SQUID magnetometry to establish a triplet (S = 1) ground state with a metal-centered oxidation and little spin delocalization on the ligand. The complex also has a fairly isotropic g-tensor (giso = 1.97) combined with a positive zero-field splitting (ZFS) parameter D (+19.1 cm–1) and very low rhombicity, in agreement with quantum chemical calculations. This thorough spectroscopic characterization contributes to a general understanding of octahedral Fe(IV) complexes.
Short abstract
The redox chemistry of bis-ligated homoleptic complexes of iron with a dianionic C3-symmetric tris-skatylmethylphosphonium (TSMP2−) scorpionate ligand is investigated. The pseudotetrahedral [(TSMP)2FeII]2− complex can undergo two consecutive one-electron oxidations to give locally octahedral Fe(III) and Fe(IV) compounds. The Fe(III) complex undergoes thermal spin-cross-over between the doublet and sextet states. The Fe(IV) compound has a triplet ground state. Its electronic structure is extensively analyzed by a combination of spectroscopic and computational methods.
Introduction
Fe(IV) compounds, both heme- and non-heme-based,1−4 are crucial intermediates in many biological transformations. As such, they provide inspiration for the development of small-molecule catalysts for green oxidation processes.4−6 Due to their generally highly-oxidizing nature, their isolation and spectroscopic characterization have been challenging. Perhaps, the most studied members of this family involve heteroleptic Fe(IV) complexes stabilized by strong π-donating ligands7 (oxo-,3,5,8,9 imido-,10−12 nitrido-,13−17 isocyanide,18 and ketimide19). In contrast, homoleptic Fe(IV) complexes are relatively scarce. Among these, a [FeF4] species was cryogenically trapped in neon or argon matrices20,21 and, according to DFT calculations, is expected to have a quintet ground state (S = 2). Due to high ionicity of the Fe–F bond, the existence of [FeF4] as a bulk material under normal conditions is thought unlikely. Next, a family of tetrahedral singlet (S = 0) Fe(IV) tetraalkyl complexes is known (A in Chart 1),22−24 which decompose at room temperature in a matter of days. Similar instability has been reported in a distorted square planar singlet complex B,19,23 an extremely rare example of a nontetrahedral FeX4 compound. The triplet (S = 1) dicationic decamethylferrocenium compound C was prepared by oxidation of decamethylferrocene in liquid sulfur dioxide25 and is sufficiently stable for spectroscopic characterization and X-ray crystal structure determination. The latter reveals that the Cp* rings can tilt with respect to one another depending on the counterion, namely, the tilt angle is 0° for the [Sb2F11]− anion but 16.56° for SbF6–, which is caused by coordination of the anion to the Fe(IV) center. Therefore, these complexes can be considered contingently homoleptic. A stable triplet dithiocarbamate complex D was synthesized in 197226,27 and is one of the first reported homoleptic Fe(IV) complexes. Its electronic structure was revisited in great detail more recently28 using a combination of spectroscopic and computational techniques, proving its identity as a true Fe(IV) compound. An indefinitely stable triplet hexahydrazide E that forms in water upon air oxidation29 was extensively characterized as having a triplet spin state and trigonal prismatic geometry. Finally, an NHC-based phenylborate complex F was synthesized recently,30 which has a triplet ground state and local D3d symmetry at the metal center.
Chart 1. Reported Homoleptic Fe(IV) Complexes20,21,30,22−29.
Due to the presence of weakly π-donating hard anionic N-donors, pyrrolide-based ligands have a potential for stabilizing high-valent metal centers.31 As a matter of fact, porphyrine and corrole-based coordination compounds are ubiquitous in nature and often support oxidized reactive intermediates.32,33 Nonetheless, the majority of these systems feature 4-fold planar coordination geometry around the metal, raising the question of what interesting electronic properties and reactivity alternative symmetries could provide. Some examples of such geometries include unconjugated dipyrrolylmethane34−38 and tris-pyrrolylethane39 metal complexes. However, in the case of these molecules, the absence of conjugation on the ligand leads to the relatively energetic aromatic π-orbitals which participate in molecular redox events.
We have previously reported a formally dianionic C3-symmetric tris-skatylmethylphosphonium (TSMP2–) ligand platform that is based on a π-extended pyrrole-based aromatic system, 3-methylindole (skatole).40 It also has a positively charged bridgehead phosphonium atom, which further lowers the energy of its highest occupied molecular orbital (HOMO). Herein, we show that these two aspects are sufficient to access a stable homoleptic, octahedral [(TSMP)2FeIV] species (4, Chart 1) by one-electron oxidation of its isostructural Fe(III) analogue. We use a variety of electrochemical (cyclic voltammetry), spectroscopic (XAS, THz-EPR, 57Fe Mössbauer, paramagnetic NMR and optical spectroscopy), and computational techniques (DFT, TD-DFT, CASSCF/NEVPT2), supported by superconducting quantum inference device (SQUID) magnetometry, to establish a primarily metal-centered oxidation and provide a detailed picture of the electronic structure of 4.
Synthesis and Characterization
The high-spin Fe(II) complex [(TSMP)2FeII]K2 (2a) and its crystallizable benzo-15-crown-5 (B15C5) adduct [(TSMP)2FeII][(B15C5)2K]2 (2b) were synthesized as previously described40 using iron dichloride and two equivalents of the TSMPK2 salt (1) in THF (Scheme 1), followed by addition of benzo-15-crown-5 if needed. Complex 2a is extremely air-sensitive and is immediately converted into the deep-blue Fe(III) compound [(TSMP)2FeIII]K (3a) upon exposure to even trace amounts of oxygen. Compound 3a can also be prepared from 2a by means of one-electron oxidation with ferrocenium or tritylium tetrafluoroborate in acetonitrile with 64.6% isolated yield (Scheme 1). In contrast, repeated attempts to synthesize 3a directly from dipotassium salt 1 and Fe(III) chloride yielded complex mixtures of paramagnetic products with only small amounts of the target complex as indicated by 1H NMR spectroscopy, likely due to ligand oxidation and/or polymerization. The potassium cation in 3a can be easily exchanged for a tetraphenylphosphonium by treatment with PPh4I in dichloromethane (DCM), yielding [(TSMP)2FeIII]PPh4 (3b). Furthermore, the potassium cation can also be complexed with two equivalents of B15C5 to form the [(TSMP)2FeIII][(B15C5)2K] adduct (3c).
Scheme 1. Synthesis of Metal Complexes 2a,b, 3a–c, and 4.

The solid-state structure of 3a was established by X-ray structure determination of single crystals grown from acetonitrile/ether. The compound crystallizes as one-dimensional coordination polymer with potassium atoms intercalated between aromatic rings of adjacent molecules of 3a (Section S3). There are two independent iron centers in the structure, each located on a crystallographic inversion center. The first coordination sphere of the iron atom has approximate octahedral symmetry with N∧Fe∧N angles close to 90° and Fe–N bond lengths within 1.9552(12)–2.0020(12) Å (Figure 1) consistent with a low-spin (S = 1/2) state of the metal center.41,42 The solid-state structures of 3b and 3c are similar to that of 3a (Section S3). Interestingly, the structure of 3c also has two independent iron centers, each possessing exact inversion symmetry and an approximate octahedral environment, but with distinctly different spin states as can be seen from the two sets of Fe–N bond lengths. More specifically, the low-spin (S = 1/2) molecule features bonds within 1.970(2)–2.008(2) Å, while the high-spin (S = 5/2) unit has bonds within 2.123(3)–2.155(2) Å, with these ranges being typical for the assigned spin states.41,42 This discrepancy hints at the possibility of a spin cross-over, which is explored in more detail below.
Figure 1.

Molecular structure of complexes 3a and 4 derived from single crystal X-ray diffraction. Displacement ellipsoids are drawn at 30% probability level. Fused benzene rings are shown in a wireframe style for clarity. Counterions, solvent molecules, and hydrogens are omitted for clarity. Selected bond distances (Å) and angles (degrees): 3a: two molecular fragments in an asymmetric unit, fragment 1: Fe1–N11 1.9885(12), Fe1–N21 2.0019(12), Fe1–N31 1.9805(12), N11∧Fe1∧N21 91.11(5), N21∧Fe1∧N31 90.70(5), N31∧Fe1∧N11 90.73(5); fragment 2: Fe2–N12 1.9889(12), Fe2–N22 1.9885(12), Fe2–N32 1.9552(12), N12∧Fe2∧N22 91.56(5), N22∧Fe2∧N32 90.44(5), N32∧Fe2∧N12 91.16(5); 4: molecule has Ci symmetry, Fe1–N1 1.966(5), Fe1–N2 1.975(6), Fe1–N3 1.966(6), N1∧Fe1∧N2 91.6(2), N2∧Fe1∧N3 91.0(2), N3∧Fe1∧N1 90.7(2).
1H NMR spectra of 3a in
acetonitrile-d3 solution within 233–348 K show only
five paramagnetically
shifted and broadened signals (Section S13.2), whereas six signals would be expected based on the D3d symmetrical solution structure. The effective magnetic
moment (μeff) in solution measured by the Evans method
in this temperature range varies from 3.50 to 4.84 μB, indicating thermal spin cross-over (SCO) (vide infra). It suggests
that the signal of the sixth proton, supposedly the closest one to
the metal center, may be missing due to paramagnetic line broadening,
which may be more pronounced at an increased μeff of the system. This hypothesis is confirmed by the 1H
NMR spectrum of 3b in DCM-d2 at 173 K, at
which temperature most of the complex is in the low-spin S = 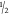 state as indicated by
the fitted effective
solution magnetic moment of 1.89 μB (vide infra).
There, one can observe the sixth signal spanning over the range of
>10 ppm (Figure S22).
state as indicated by
the fitted effective
solution magnetic moment of 1.89 μB (vide infra).
There, one can observe the sixth signal spanning over the range of
>10 ppm (Figure S22).
Complex [(TSMP)2FeIV] (4), for which the Fe(IV) oxidation state is demonstrated below, can be obtained as a bottle-green powder by one-electron oxidation of [(TSMP)2FeIII]PPh4 (3b) using elemental iodine in acetonitrile with 90.1% isolated yield. It is stable both in the solid state and solution for at least six months. Single crystals for X-ray structure determination were grown from pyridine/butyronitrile/ether and contain the electroneutral and roughly octahedral complex with Fe–N distances of 1.966(6)–1.975(5) Å (Figure 1). This is similar but still somewhat shorter on average than the distances in the isostructural anionic unit of 3a (1.9552(12)–2.0020(12) Å). Solution 1H NMR spectra of [(TSMP)2FeIV] (4) in DCM-d2 feature six paramagnetically shifted and broadened signals as expected for the D3d symmetrical solution structure (vide infra). Very moderate solubility of 4 in all conventional NMR solvents precluded us from determining its effective solution magnetic moment by the Evans method. The results of magnetometry in the solid state are discussed below.
Electrochemical Behavior
Cyclic voltammetry (CV) of [(TSMP)2FeII][(B15C5)2K]2 (2b) reveals two couples of redox events: A/D and B/C (Figure 2, left panel). The full CV scan can be repeated at least a hundred times at rates within 50–250 mV/s with no visible changes, which demonstrates chemical reversibility of the redox cycle it represents. Controlled electrolysis at the points A and B of the CV using an optically transparent thin-layer electrochemical (OTTLE) cell43 (Figure 2, middle panel) allows the assignment of event A as the one-electron oxidation of [(TSMP)2FeII]2– (2) to [(TSMP)2FeIII]− (3), whereas event B corresponds to the oxidation of [(TSMP)2FeIII]− (3) to [(TSMP)2FeIV] (4). Correspondingly, their reductive counterparts D and C can be assigned to the same respective processes but in reverse. Importantly, the independently measured CV of [(TSMP)2FeIII]K (3a) is very similar to that of 2b (Section S5.2), which confirms our assignments.
Figure 2.
Cyclic voltammograms of compound 2b (ca. 8 mM solution) in 0.1 M nBu4NPF6 acetonitrile electrolyte. Potentials are referenced with respect to the Fc/Fc+ redox couple. Left panel: overview scans at the rate of 100 mV/s; the full scan starts from an open-circuit potential of −2.12 V). Middle panel: UV–vis of independently synthesized compounds 2b, 3a (in MeCN), and 4 (in DCM) and time-dependent UV–vis spectra of controlled electrolysis at points A (−0.64 V) and B (−0.37 V) indicated on the CV on the left panel. Absorption in spectrum B was truncated due to detector saturation. The asterisks indicate isosbestic points that support a clean conversion between 3a and 4. Right panel: the quasi-reversible redox pair A–C centered at E1/2 = −0.41 V; the inset shows linear dependence of the peak current vs square root of the scan rate.
The pair of redox events A/D is characterized by a large peak separation (ΔEp of 1030 mV at 100 mV/s) and a large full width at half maximum (FWHM) of feature D (>500 mV). These are clear indications of an irreversible electron transfer, suggesting that a substantial reorganization energy for the [(TSMP)2FeII]2– (2)/[(TSMP)2FeIII]− (3) redox couple arises from the different geometries of tetrahedral402 and octahedral 3.
The pair of events B/C can be isolated by means of partial CV scans not involving feature D (Figure 2, left panel). During the first cycle, all [(TSMP)2FeII]2– (2) complex in the electrode diffusion layer is consumed to form [(TSMP)2FeIII]− (3) (event A), followed by oxidation to [(TSMP)2FeIV] (4; event B) with its subsequent reduction back to [(TSMP)2FeIII]− (3) (event C). Since the potential scan window does not involve feature D, corresponding to regeneration of the initial [(TSMP)2FeII]2– (2) ions, by the start of the second cycle, the electrode diffusion layer is depleted of the latter, as indicated by the reduced intensity of feature A, as shown in Figure 2, left panel. The residual intensity is likely due to diffusion of ions of 2 from the outer pool of the [(TSMP)2FeII][(B15C5)2K]2 (2b) complex. This interpretation is confirmed by varying the potential scanning rate (Figure 2, right panel): slower scans result in increased intensity of A, while the faster ones lead to its complete disappearance.
As for the pair of events B/C itself, it represents a redox process centered at E1/2 = −0.41 V with respect to the Fc/Fc+ couple, which lies within the typical range between −1.24 and – 0.03 V for other published Fe(III)/Fe(IV) couples.11,19,29,44,45 Its ΔEp varies with potential scan rate from 110 mV at 50 mV/s to 152 mV at 250 mV/s, while the peak current changes linearly with the square root of the scan rate. These are diagnostic criteria for a quasi-reversible electron transfer,46 implying that the reorganization energy of a transition between [(TSMP)2FeIII]− (3) and [(TSMP)2FeIV] (4) states is rather small, consistently with the similar solid-state geometries of the complexes [(TSMP)2FeIII]K (3a) and [(TSMP)2FeIV] (4) (Figure 1).
SCO in the [(TSMP)2FeIII]− Complex (3)
To probe the electronic structure of [(TSMP)2FeIII]K (3a), we measured the effective magnetic moment (μeff) of a powder sample over a temperature range of 2–400 K with a SQUID. As shown in Figure 3, from 10 to 180 K, μeff is nearly constant at 2.02 ± 0.01 μB. This value is considerably higher than the spin-only value of 1.73 μB that is expected for S = 1/2 complexes, which reflects 3 having appreciable unquenched orbital angular momentum, typical of low-spin ferric complexes.47 Starting from 180 K, μeff increases with temperature and does not get saturated even at 400 K. These experimental findings show that over the course of the SQUID measurement, 3a undergoes a thermally activated SCO from the S = 1/2 ground state to an S = 5/2 state. Of note, because the unsaturated μeff value at 400 K of 3.98 μB exceeds the spin-only value of the quartet S = 3/2 state (3.87 μB), the second spin state cannot be 3/2 but is rather 5/2, anticipated for the distorted octahedral coordination geometry of 3. The variation of μeff was fitted with the domain model of Sorai and Seki,48 and the satisfactory simulations yielded Tc = 450 ± 5 K and nΔH = 15.4 ± 0.2 kJ/mol. Here, Tc represents the critical transition temperature of the SCO, ΔH = HHS – HLS— the enthalpy difference between the high-state and low-spin state, and n — the number of molecules per domain. Below 10 K, the precipitous drop of μeff cannot be simply interpreted as field saturation but primarily arises from weak intermolecular interactions, for which a mean field model49 was invoked to fit the SQUID data and gave zJ = −6 ± 1 cm–1.
Figure 3.
SQUID measurement of 3a under an applied magnetic field of 1 T. The dots represent experimental data, and the solid red line the fit with the following parameters: S = 1/2, giso = 2.30, zJ = −6 cm–1; S = 5/2, D = E = 0, giso = 2.00, and TIP = 0.
The zero-field Mössbauer spectrum (Figure 4) of complex 3a recorded at 80 K displays a well-resolved quadrupole doublet at isotope shift δ = 0.25 mm/s and quadrupole splitting |ΔEQ| = 1.63 mm/s. Both values are characteristic for low-spin ferric centers coordinated by six hard nitrogen donors. DFT calculations performed on both independent anions in the crystal structure of 3a predict isomer shifts (δ = 0.29 and 0.26 mm/s) and quadrupole splittings (|ΔEQ| = 2.19 and 2.18 mm/s) in reasonable agreement with the experiment (see ESI Section S11.1 for details). Furthermore, no other iron species was identified in the spectrum, consistent with the high SCO critical temperature Tc of 450 K.
Figure 4.
Zero-field Mössbauer spectrum of 3a measured on a powder sample at 80 K. The fitted parameters are δ = 0.25 mm/s and |ΔEQ| = 1.63 mm/s.
Interestingly, SQUID magnetometry of the benzo-15-crown-5 adduct [(TSMP)2FeIII][(B15C5)2K] (3c) reveals that at low temperatures, the system has a magnetic moment μeff of 3.61 μB, in between the low- and high-spin states, similarly to 3.88 μB for the case of an intermediate-spin (S = 3/2). The system crosses over toward the high-spin state at only ca. 140 K (Section S8.2). However, an S = 3/2 electronic configuration is unlikely for a [(TSMP)2FeIII]− (3) ion since it would imply significant distortion of the FeN6 environment, leading to strain in the TSMP scaffold, which is expected to be more energetic than the energy gap between the S = 3/2 and other spin states. Indeed, X-ray diffraction on a single crystal of 3c grown from pyridine/n-hexane (Section S3) shows that at 100 K, it exists as a 1:1 mixture of the low-spin and high-spin components with two distinct sets of Fe–N bonds typical for the assigned spin states:42,50,51 1.970(2)–2.008(2) and 2.123(3)–2.155(2) Å, respectively. Although rare for iron(III) coordination compounds, such behavior is not unprecedented and was observed for some tris(dithiocarbamato)50 complexes.
The solid-state behavior of 3 parallels our observations in solution. The SCO between the S = 1/2 and 5/2 states is evident from the strongly temperature-dependent μeff, as measured by Evans method.52−54 The results of such measurements are shown in Figure 5 for potassium salt 3a in acetonitrile-d3 and pyridine-d5, and for tetraphenylphosphonium salt 3b in DCM-d2 as 3a is insoluble in this solvent. The change of the effective solution magnetic moment with temperature is also apparent from the paramagnetic 1H NMR shifts and line broadening, which are analyzed in more detail in ESI Section S13.2.2.
Figure 5.

SCO curve for the [(TSMP)2FeIII]− (3) complex as obtained by the Evans method in different solvents. Data points represent experimental measurements, whereas smooth curves are fits based on the regressive model in eq 1. Explored temperature ranges are limited by the freezing and boiling points of the respective solvents or precipitation of the compound at low temperatures.
The solution SCO behavior can be fitted using eq 1 (Section S6), which allows to extract the enthalpy (ΔH) and entropy (ΔS) of the cross-over as well as the limiting magnetic moments for both low- and high-spin states, μLS and μHS, respectively.
| 1 |
where T is temperature and R is the universal gas constant.
The cross-over curve for 3b in DCM-d2 is the most informative since it spans through both low- and high-μeff regions. A regressive thermodynamic analysis (Section S6.2) provides the following parameters: ΔH and ΔS of 18.1 ± 1.4 kJ/mol and 72.6 ± 5.8 J/(mol·K), respectively, with the critical temperature ΔH/ΔS = Tc of 249 ± 1 K. The limiting magnetic moments μLS and μHS are 1.81 ± 0.07 and 5.35 ± 0.10 μB, respectively, which is close to the spin-only expectation values for the low-spin S = 1/2 (1.73 μB) and high-spin S = 5/2 (5.92 μB) states. A similar analysis for the SCO of 3a in acetonitrile-d3 leads to slightly different values with higher standard errors (Section S6.2) ΔH of 14.4 ± 2.4 kJ/mol, ΔS of 59.6 ± 8.0 J/(mol·K) and Tc of 240 ± 9 K. Because the SCO curve does not cover the low-μeff range, a significant standard error is also associated with the lower limiting value μLS of 2.35 ± 0.45 μB, while the higher one of 5.23 ± 0.08 μB has smaller uncertainty and is similar to that for 3b. Lastly, since 3a in pyridine-d5 has almost completely undergone SCO at the lowest accessible temperature (Figure 5), the corresponding thermodynamic parameters cannot be reliably extracted. Overall, these data indicate that the SCO thermodynamics of 3 is sensitive to solvent and/or counterion, possibly indicating a role of ion pairing in solution.
While the difference of ∼200 K in SCO critical temperatures (Tc) in the solid state and in solution is very high, in some cases, the crystal packing is known to lock some SCO molecules in a fixed spin state,55 slow down the spin transition by anticooperative effects,56,57 or even prevent SCO from happening at all.58
To the best of our knowledge, 3 is the first synthetic complex with an FeIIIN6 core that undergoes thermal SCO. This behavior is likely due to TSMP2– being a relatively weak-field ligand with poor π-accepting properties caused by the presence of low-lying π-orbitals in the extended aromatic systems. Gradual transitions as observed for 3 in the solid state and in solution are commonly observed for SCO in Fe(III) compounds.41 A broad range of critical temperatures Tc (20 K–ca. 400 K) have been observed for Fe(III) compounds with different ligand sets.42 The pronounced difference between 3a and 3c and between solid-state and solution suggests that SCO in anion 3 is strongly sensitive to its environment.
Electronic Structure of [(TSMP)2FeIV] (4)
The presence of 10e̅ conjugated π-systems on the ligand raises the question of the actual electronic structure and metal oxidation state in 4, which we address by the following electrochemical, spectroscopic, and computational studies.
Cyclic Voltammetry
A first insight is provided by comparing cyclic voltammograms of [(TSMP)2FeIII]K (3a) and its isostructural Ga analogue [(TSMP)2GaIII]K (5a) (Figure 6) synthesized using TSMPK2 salt (1) and GaCl3 in acetonitrile (Section S2.1; X-ray crystallographic details in Section S3). If the oxidation event leading from 3a to 4 was mostly ligand-centered, one would expect a small first oxidation potential difference compared with 5a. In contrast, the observed significant difference of 450 mV argues in favor of at least partially metal-centered oxidation of iron in 4.
Figure 6.

Overlay of CVs measured for 3a and 5a. The measurements were performed in ca. 8 mM solution in 0.1 M nBu4NPF6 acetonitrile electrolyte. Potentials are referenced with respect to the Fc+/Fc redox couple. The onsets of oxidation of 3a and 5a (EB and EB′, respectively) are defined as the points of intersection between extrapolated baseline and a tangent to the oxidation feature B/B′.
57Fe Mössbauer Spectroscopy
The electronic structure of the [(TSMP)2FeIV] (4) complex was further probed by zero-field 57Fe Mössbauer spectroscopy at 80 K. The spectrum (Figure 7) shows a very clear quadrupole doublet. The isomer shift (δ) of 0.04 mm/s is substantially lower than 0.25 mm/s measured for the parent Fe(III) compound 3a (Figure 4) and falls within the expected range for a Fe(IV) metal center,59 clearly indicating metal-centered oxidation. The quadrupole splitting |ΔEQ| of 1.96 mm/s suggests a significant deviation of the electronic configuration from the cubic symmetry. The measured isomer shift is similar to that of E(29) (0.045 mm/s) in Chart 1 but different from that of F(30) (−0.23 mm/s), as expected for their distinct donors (N– vs C). At the same time, the measured quadrupole splitting is lower than 2.51 mm/s in E and 3.04 mm/s in F, likely reflecting their slightly different local Fe coordination environments.
Figure 7.
Zero-field Mössbauer spectrum of 4 measured on a powder sample at 80 K. The fitted parameters are δ = 0.04 mm/s and |ΔEQ| = 1.96 mm/s.
XAS Spectroscopy
Our Fe 2p (L2,3) X-ray absorption (XAS) measurements generally agree with the conclusions drawn from the cyclic voltammetry and Mössbauer spectroscopy above. In XAS, a 2p core electron is excited into an empty 3d state, which for a 3d5 iron center in [(TSMP)2FeIII]− (3) leads to a 2p53d6 configuration. The experimental XAS spectra of the potassium salt 3a agree with simulations for anion 3 based on ligand field multiplet theory (Figure 8).60 Given the average excitation lifetime of <1 fs, the spectrum of 3a at 300 K is adequately simulated as a sum of low- and high-spin components in a ratio of 80:20, which is consistent with the solid-state SCO behavior discussed above.
Figure 8.

Experimental (top panel) and simulated (bottom panel) L2,3 edge XAS spectra of the Fe(III) (3a/3) and Fe(IV) (4) complexes.
The experimental XAS spectrum of [(TSMP)2FeIV] (4) is similar but shifted to higher energies by 0.9–1.1 eV compared to 3a (Figure 8, top panel), indicating metal-centered oxidation. This spectrum can be simulated (Figure 8, bottom panel) considering D3d symmetry of 4 with 1a1–1e–2e orbital splitting (see Section VI below), 2-2-0 occupancies, and a 3A2 ground state.60 In the simulation, the two biggest peaks can be approximated as transitions into the 1e and 2e orbitals. However, due to strong 2p3d multiplet effects, this assignment is not completely accurate as significant mixing occurs in the final 2p53d5 state. The shoulder at 710 eV in the experimental spectrum is due to charge transfer, which was not included explicitly in the calculations to limit the number of parameters. These charge transfer excitations were omitted due to the use of the nephelauxetic effect along with reduced electron–electron interactions (factor of 0.85).61 An overview of the exact parameters used in the calculation is given in ESI Section S7.2.
Taken together, the 2p XAS spectra confirm the nature of the [(TSMP)2FeIII]− (3) and [(TSMP)2FeIV] (4) systems. At 300 K, 3a has predominantly a low-spin configuration, while 4 has an 3A2 triplet ground state with a doubly occupied dz2orbital and a half-filled first e(D3d) state (vide infra, Figure 11).
Figure 11.

Quasi-restricted frontier orbitals (isocontour = 0.05) of the ground state of 4 calculated at the B3LYP-D3BJ/def2-TZVP (CP(PPP) for Fe) level of theory using a geometry optimized at the BP86-D3BJ/def2-TZVP level. Orbital energies are given in parentheses.
SQUID Magnetometry
SQUID magnetometry was performed on a microcrystalline sample of 4 in order to determine its spin state and assess its zero-field splitting (ZFS). The fit of the VT measurement (Figure 9, top panel) gives a μeff value of 2.50 μB at room temperature, which is lower but still close to the spin-only expectation value for an intermediate-spin state (S = 1) Fe(IV) center (2.83 μB). The corresponding giso-value is 1.76. The magnetic susceptibility from the VTVH measurement (Figure 9, bottom panel) could be fitted consistently with an axial ZFS parameter D of +15 cm–1. The rhombicity E/D was taken as zero due to the C3 axis of symmetry in the molecule. However, these parameters gave a subpar fit for the measurement at 1 T. We attribute this to small antiferromagnetic intermolecular interactions which compete with the Zeeman effect under low external field (1 T) and become negligible under higher fields (4 and 7 T). These interactions could be caused by the presence of small amounts of paramagnetic impurities or interactions of the sample molecules with one another.
Figure 9.

VT μeff at 0.1 T (top) and VTVH magnetization at 1, 4, and 7 T (bottom) SQUID measurements of 4, fitted using the following spin Hamiltonian parameters: giso = 1.76, D = +15 cm–1, E/D = 0.
Overall, the abnormally low effective magnetic moment (corresponding to the unphysically small giso) and small intermolecular interactions suggest that the measured sample of 4 contains some admixtures, be it paramagnetic impurities or residual solvent. The latter is likely due to the zwitterionic nature of 4, which makes complete solvent removal very difficult even after prolonged drying in vacuo. However, the SQUID measurements still allow to get a qualitative estimate of the zero-field splitting (D ≈ +15 cm–1), as well as a clear determination of S = 1 spin state (3A2 ground state).
THz-EPR Spectroscopy
A more direct way to quantify g-values and ZFS parameters of 4, which does not require the mass of the sample to be precisely known, is through EPR spectroscopy. However, as 4 is an integer-spin (S = 1) system with low rhombicity and an intermediate, positive D-value, the transitions between the mS = 0 sublevel and the non-Kramers doublet mS = ±1 are too energetic to be observed using conventional EPR spectrometers.62 Indeed, X-band measurements on the microcrystalline samples gave no interpretable signal; featureless spectra were also obtained in parallel mode. Therefore, we resorted to high-energy (THz range) frequency-domain Fourier-transform THz-EPR spectroscopy (THz-EPR in short).63
Variable-field THz-EPR spectra of 4 in Figure 10 are shown in relative absorbance
as 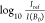 , where Iref is a reference transmittance spectrum measured at
31 K and 0 T,
and I(B0) is a transmittance
spectrum measured at 4.8 K and magnetic field B0 (Figure S14). For B0 = 0, a clear absorption(I) can be observed
at 19.1 cm–1. This feature broadens and splits with
increasing external magnetic field. The field dependence allows for
assignment of the peak at 19.1 cm–1 to the EPR transition
from mS = 0 to the mS = ±1 sublevels of the S = 1 system
(Section S9.2). Thus, the zero-field spectrum
corresponds to an axial ZFS of D = 19.1 cm–1 with vanishing rhombicity (E ≈ 0) due to
the lack of a visible splitting in the zero-field spectrum. From the
linewidth of the signal, E ≤ 0.3 cm–1 can be estimated. Simulations using D = 19.1 cm–1, E = 0 and an isotropic g-value of 1.97 reproduce the zero-field transition energy
as well as the field dependence very well (Figure 10). We note that the spectra feature a lowering
of the relative absorbance around 18.4 cm–1, directly
below the ZFS energy, which is discussed in Section S9.4. Further details of the THz-EPR measurement protocol and
simulations, including demonstration of the unfeasibility of alternative
EPR parameter sets, are discussed in Section S9.
, where Iref is a reference transmittance spectrum measured at
31 K and 0 T,
and I(B0) is a transmittance
spectrum measured at 4.8 K and magnetic field B0 (Figure S14). For B0 = 0, a clear absorption(I) can be observed
at 19.1 cm–1. This feature broadens and splits with
increasing external magnetic field. The field dependence allows for
assignment of the peak at 19.1 cm–1 to the EPR transition
from mS = 0 to the mS = ±1 sublevels of the S = 1 system
(Section S9.2). Thus, the zero-field spectrum
corresponds to an axial ZFS of D = 19.1 cm–1 with vanishing rhombicity (E ≈ 0) due to
the lack of a visible splitting in the zero-field spectrum. From the
linewidth of the signal, E ≤ 0.3 cm–1 can be estimated. Simulations using D = 19.1 cm–1, E = 0 and an isotropic g-value of 1.97 reproduce the zero-field transition energy
as well as the field dependence very well (Figure 10). We note that the spectra feature a lowering
of the relative absorbance around 18.4 cm–1, directly
below the ZFS energy, which is discussed in Section S9.4. Further details of the THz-EPR measurement protocol and
simulations, including demonstration of the unfeasibility of alternative
EPR parameter sets, are discussed in Section S9.
Figure 10.
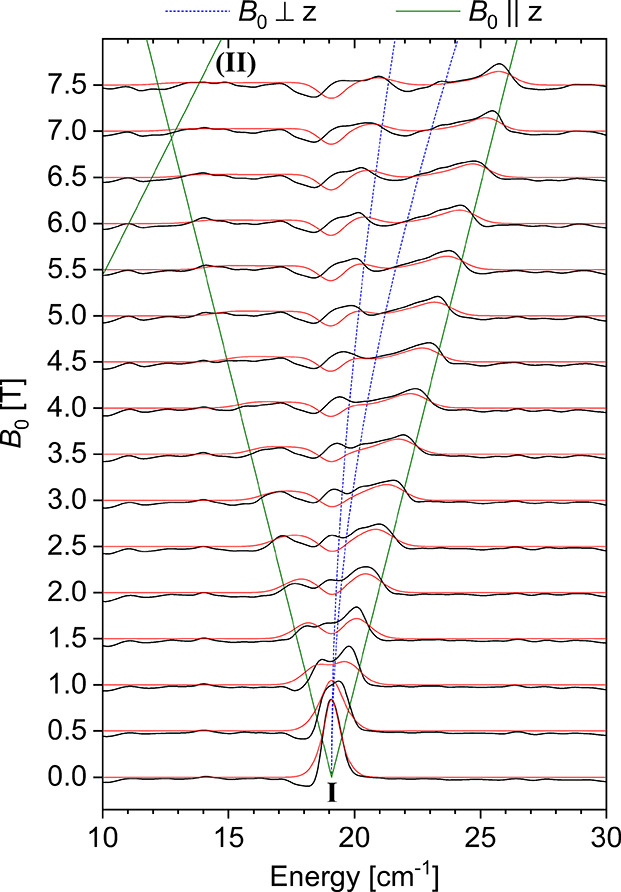
THz-EPR spectra of 4. Relative absorbance spectra (black lines) are offset for the magnetic field B0 at which they were measured. Simulations using D = +19.1 cm–1, E = 0, and an isotropic g-value of 1.97 are shown in red. Calculated transition energies for magnetic fields applied parallel and perpendicular to the main anisotropy axis (z) are shown as solid green and dashed blue lines, respectively. Branch II corresponds to the formally forbidden transitions between the excited mS = ±1 sublevels.
DFT Calculations
Geometries of the alternative spin states of 4 (S = 0, 1, 2) optimized at the BP86-D3BJ/def2-TZVP level of theory show that the S = 1 state is the most energetically favored. Its SCF energy is lower than that of the S = 0 and S = 2 states by 17.8 and 25.0 kcal/mol, respectively. Mössbauer spectral parameters calculated for all three geometries (B3LYP-D3BJ/def2-TZVP level of theory, CP(PPP) basis set for Fe) also support the assignment of the S = 1 spin state (Section S11.2) as derived from SQUID and THz-EPR measurements. More specifically, the calculated isomer shift (δ) of −0.05 mm/s is close to the experimental value of +0.04 mm/s within the uncertainty of computations.64 The calculated quadrupole splitting (ΔEQ) of −2.01 mm/s is also very close to the absolute experimental value of 1.96 mm/s, while the sign of the splitting cannot be inferred from the zero-field measurements.
Given that the experimental Mössbauer parameters are well reproduced by the calculations for the S = 1 state, the computed electron density can be used for closer examination of the electronic structure of 4. Despite the optimized geometry featuring a locally octahedral FeN6 center, the molecule of 4 itself has D3d symmetry with a threefold rotation axis passing through the P–Fe–P atoms. This leads to orbital splitting typical for this kind of symmetry (Figure 11). The doubly occupied 1a1 orbital has almost entirely dz2 character, which can be rationalized based on the irreducible representations of d-orbitals in a D3d point group. More specifically, the dz2 belongs to the representation A1 and therefore cannot mix with the four remaining d-orbitals, (dxy, dx2 – y2) and (dxz, dyz), belonging to the double representation E. At the same time, these four orbitals mix together, forming two degenerate pairs: 1e and 2e (Figure 11). Both these pairs bear an anti-bonding character: 1e along the π-manifold and 2e along the σ-manifold, leaving 1a1 to be the only nonbonding d-orbital. The fact that the 1e pair of SOMOs is primarily localized on the metal center implies that this is also where most of the spin density can be found (Section S11.3).
Considering the discussed orbital manifold, the negative sign of the calculated Mössbauer quadrupole splitting (ΔEQ= −2.01 mm/s) is dominated by the strongly negative valence contribution of the 1a1 orbital. Note that while the 1e SOMOs influence the quadrupole splitting as well, the positive contribution from the dxy and dx2 – y2 orbitals is counterbalanced by the negative one from dxz and dyz. A detailed analysis of the origin of the quadrupole splitting is given in the ESI (Section S10.2).
It is important to note that the doubly occupied 1a1 orbital in Figure 11 points directly at the positively charged phosphonium atoms, which is expected to lower its energy by providing additional electrostatic stabilization. Indeed, comparison of the quasi-restricted orbital energies of 4 and its isoelectronic Si-tethered analogue (G in Chart 2) calculated at the same level of theory reveals that, while the 1e–2e gap is the same in both molecules, the 1a1 orbital in 4 is stabilized by an additional 0.2 eV. Given the close similarity of the molecular geometries of 4 and G (Section S11.4), this difference is most likely due to the electrostatic effects, although subtle influence of the bridgehead atom on π-donating ability of a ligand cannot be excluded.
Chart 2. Comparison of the Quasi-Restricted Orbital (QRO) Energies of 4 and Its Si-tethered Analogue G Calculated at the B3LYP-D3BJ/def2-TZVP (CP(PPP) for Fe) Level of Theory for a Geometry Optimized at the BP86-D3BJ/def2-TZVP Level.
CASSCF Calculations
To gain more insight into the magnetic properties of 4, its excited states were calculated using CASSCF. Because only the d–d excitations are significantly relevant for the ZFS and g-tensor calculations, the active space CAS(4,5) was restricted to only the metal-based d-orbitals. The energies were corrected with strongly-contracted NEVPT2 to recover dynamical correlation.
The results are consistent with the orbital picture given by DFT (Figure 11). They show large splitting between the doubly and singly occupied 1a1 (−11.2 eV) and 1e (−9.5 eV) orbitals and the unoccupied orbital pair 2e (−0.9 eV). The first and second excited states correspond to a single d–d excitation from the doubly occupied nonbonding orbital 1a1 to the π-antibonding pair 1e, and lie at ca. 10850 cm–1 above the ground state. The excitation from the 1a1 orbital to the highly σ-antibonding orbital pair 2e is 19000 cm–1 higher than the ground state (Section S12).
The ZFS and the g-tensor were calculated by the effective Hamiltonian theory (for technical details, see Section S12). The principal axes of the g- and D-tensor coincides with the P–Fe–P C3-axis of rotation. The calculated D-value is +15.3 cm–1, while the rhombicity is zero, consistently with the axial symmetry of the system. The calculated g-tensor exhibits only small anisotropy (g∥ = 2.00, g⊥ = 2.04, giso = 2.03). These parameters are in a good agreement with the data obtained from the SQUID and THz-EPR measurements (vide supra): D = +19.1 cm–1, E ≤ 0.3 cm–1, and giso of 1.97. The moderate ZFS and fairly isotropic g-value are not surprising since the lowest-energy d–d excited state, which is also spin-conserving, lies 10800 cm–1 above the ground state, which is more than 20 times larger than the effective spin-orbit coupling constant of the Fe(IV) ion (515 cm–1).65 Hence, the ground state is fairly isolated and the spin-orbit coupling effect represents a moderate perturbation.
Paramagnetic 1H NMR Spectroscopy
Despite the moderate solubility of 4 in conventional solvents, we were able to acquire its 1H NMR spectra in DCM-d2 (Figure 12). The complex shows six paramagnetically shifted and broadened signals, as expected for the D3d topology in solution. The signals were assigned based on their integral intensity, linewidth and by comparing the spectrum with that of a deuterated (2H3)methylphosphonium analogue, 4-d6 (Sections S13.1 and S13.3.1), which was synthesized independently.
Figure 12.
1H (400 MHz) and 2H (61 MHz) NMR spectra of 4 and its deuterated analogue 4-d6 in DCM at 298 K. Only paramagnetic signals are assigned. The integrals are given in blue and were rounded to the nearest integer.
The 1H NMR spectra of 4 are strongly temperature-dependent (Section S13.3.2), as expected for a paramagnetic compound. Isolation of the hyperfine shifts (δHF) from the observed ones (δobs) by subtracting the diamagnetic contribution (δdia, Section S13.3.2) approximated by an isostructural Ga(III) analogue, [(TSMP)2GaIII]PPh4 (5b), reveals that δHF does not deviate from the Curie behavior (δHFT = const, Figure 13, top panel). This implies the dominance of the Fermi contact shifts, which is in line with a moderate axial ZFS parameter of +19.1 cm–1 and low rhombicity, as derived from the SQUID and THz-EPR studies as well as DFT and CASSCF calculations (vide supra).
Figure 13.

Top panel: variable-temperature 1H NMR (400 MHz) δHFT products of 4 in DCM-d2. Dots show experimental values, straight lines show linear fits (Section S13.3.2). Bottom panel: correlation plots of experimental vs calculated observed chemical shifts for 4 (Section S13.3.3).
The observed 1H chemical shifts can be computationally modeled using a molecular geometry optimized at the PBE-D3BJ/def2-TZVPP level of theory followed by properties calculation at the PBE0-D3BJ/def2-TZVPP level (Section S13.3.3). By using the calculated isotropic proton hyperfine constants (Aiso), experimental giso of 1.97 obtained from THz-EPR (vide supra), diamagnetic shifts (δdia) approximated using 5b, and by substituting them into eq 2, which only takes into account the Fermi contact contribution (δFC), one arrives to the chemical shifts that are >95% accurate with respect to the experiment (Figure 13, bottom panel). This reinforces our prior conclusions as for very strong dominance of the contact shifts, moderate anisotropy, and insignificant spin delocalization in the system.
| 2 |
where δdia −diamagnetic shift; δFC – Fermi contact shift; S – electronic spin quantum number of the complex; μB – Bohr magneton; giso – nuclear g-value; μN – nuclear magneton; k – Boltzmann constant; T – temperature; giso – isotropic electronic g-value of the system; Aiso – isotropic hyperfine coupling constant.
Optical Spectroscopy and TD-DFT
Optical spectra of complexes 2b, 3b, and 4 in solution are shown in Figure 14, top panel. All three compounds display a set of very intense (ε = 3–13·104 cm–1 M–1) absorptions below 375 nm, which we assign to π → π* transitions in aromatic ligands and counterions. Furthermore, both 3b and 4 display a cluster of fairly weak (ε = 2–4·103 cm–1 M–1) absorptions within 480–375 nm (denoted as I), tentatively assigned to higher-lying ligand-to-metal charge transfers (LMCT). Compound 3b also shows a rather strong (ε = 1·104 cm–1 M–1) absorption II at 618 nm, likely being another LMCT due to its intensity and position. Considering that in DCM solution, where the spectrum was measured, 3b exists in a spin-state equilibrium (80.8% of HS component at 298 K, vide supra), we undertook variable-temperature UV–vis studies to ascertain the exact origin of feature II. Cooling to 180 K, where the compound almost entirely exists in an LS state (3.4% of the HS component based on Evans method, vide supra), results in a feature of roughly the same intensity but shifted to 596 nm (Section S14.1). This leads us to conclude that both the LS and HS states of 3b show LMCT within the same optical region. Interestingly, compound 4 displays an intense (ε = 1.8·104 cm–1 M–1) absorption III that, rather unusually, peaks in the near-IR region (λmax = 1234 nm). We interpret it as a higher-oxidation-state counterpart of the LMCT feature II, in line with our assignment of 4 as a true Fe(IV) compound.
Figure 14.

Top panel: UV–vis–NIR spectra of complexes 2b, 3b, and 4 in solution at 298 K. Compound 2b was measured in acetonitrile,403b and 4 were measured in DCM. Bottom panel: experimental and TD-DFT-calculated (first 50 excitations) optical spectra of 4. Calculations were performed at the TPSSh-D3BJ/def2-SVP (def2-TZVP for Fe) level of theory in DCM. Gaussian broadening with FWHM of 170 nm was applied.
In order to reinforce our assignments of the optical spectra of 4, we performed time-dependent density functional theory (TD-DFT) calculations (for details, see Section S14.2). Calculated transitions roughly group in two clusters, which correspond to experimental features I and III (Figure 14, bottom panel). A detailed analysis of these transitions was done using the Natural Transition Orbital (NTO) theory, which performs separate unitary transformations of the occupied and virtual transition molecular orbitals so that only one or very few NTO donor-hole pairs are left, which represent the predominant contribution to the transition.66 According to NTO analysis, transitions in a lower-energy cluster III occur into the metal-based d1e orbitals (Figure 11), while the donor NTOs are combinations of 3-methylindole HOMO or HOMO-1-like orbitals of individual indolide units with different weight for every inversion-related pair (Section S14.2). The higher-energy cluster I is composed of similar transitions into the metal-based d2e orbitals or HOMO-LUMO intra-ligand charge transfer (ICT). In other words, cluster III is mostly π → d1e LMCT, and cluster I is a mixture of π → d2e LMCT and π → π* ICT. As a matter of fact, similar albeit more energetic transitions were observed for a structurally similar octahedral NHC-derived Fe(IV) phenylborate (F in Chart 1).30 There, the counterpart of the feature III in F is 0.74 eV higher in energy, peaking at 715 nm. This observation correlates with the stronger π-accepting properties of the NHC-based borate ligand in F, compared to the indolides in TSMP2–.
Conclusions
In conclusion, we have shown that the tris-skatylmethylphosphonium (TSMP2–) ligand is capable of supporting both Fe(III) and Fe(IV) oxidation states in the respective isostructural complexes 3 and 4. Both compounds are electrochemically connected and can be reversibly converted into one another as well as into the parent 2 by a series of one-electron redox reactions.
Complex 3 undergoes thermal S = 1/2 → 5/2 spin-cross-over both in solution and in the solid state and, to the best of our knowledge, is the only known synthetic system with an FeIIIN6 core capable of doing so. The exact dynamics of this process is highly dependent on the aggregation state, solvent and the counterion. The cross-over is likely possible due to TSMP2– being a relatively weak-field ligand with poor π-accepting properties caused by the absence of low-lying π*-orbitals in the extended aromatic systems.
Compound 4 features an Fe(IV) center with metal-centered oxidation and little spin delocalization on the ligand as shown by a series of electrochemical, spectroscopic, and computational studies. It possesses a triplet (S = 1) ground state, similarly to the related trigonal antiprismatic Fe(IV) hexahydrazide clathrochelate (E in Chart 1)29 and octahedral Fe(IV) tris(NHC)phenylborate (F in Chart 1)30 that were recently reported. All three compounds are C3-symmetrical, possessing an axial ZFS parameter D within the range of 19.1–23.1 cm–1 along with very low rhombicity. Having said that, while 4 features a fairly isotropic g-tensor (giso = 1.97), as indicated by THz-EPR, CASSCF calculations, and NMR spectroscopy, F has significant anisotropy with g∥ = 1.88 and g⊥ = 2.40.30 Remarkably, both compounds have green coloration due to an LCMT transition, which is 0.74 eV less energetic in 4, peaking in the near-IR at 1234 nm. This observation correlates with the lower π-accepting strength of indolides compared to the NHC-based borate ligand in F.
Overall, the findings presented in this study demonstrate the utility of the dianionic TSMP2– scorpionate ligand to access the high-valent Fe(IV) state in an octahedral N6 coordination environment. The self-consistent and detailed spectroscopic and computational characterization of the electronic structure of 4 will provide a valuable reference for the identification of related systems.
Acknowledgments
The authors thank Dr. Serena Busatto and Dr. Celso de Mello Donega for technical support with near-IR spectroscopy and Dr. Andrei Gurinov with Dr. Hugo van Ingen for support with paramagnetic high-field NMR measurements. We thank Dr. Philippe Schollhammer and Dr. Catherine Elleouet for insightful discussion on cyclic voltammetry measurements. We acknowledge Helmholtz Zentrum Berlin für Materialien und Energie for the allocation of synchrotron radiation beamtime at BESSY II. We also thank Dirk Ponwitz (Helmholtz Zentrum Berlin) for technical support with measuring THz-EPR spectra. Andreas Göbels and Bernd Mienert (Max-Planck-Institute for Chemical Energy Conversion) are acknowledged for carrying out SQUID and Mössbauer spectroscopy measurements. We are indebted to late Dr. Eckhard Bill (Max-Planck-Institute for Chemical Energy Conversion) for fruitful discussions on interpretation of EPR, SQUID, and Mössbauer results.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.3c00871.
Synthesis and characterization of 3a-c, 4, as well as their deuterated analogues; details on X-ray crystal structure determination of 3a-c, 4; CV data; details of spin cross-over in 3; additional details on spectroscopic and computational characterization of the electronic structure of 4; and computational coordinates (PDF)
Author Present Address
§§ Institut für Chemie, Humboldt-Universität zu Berlin, 12489 Berlin, Germany
Author Present Address
‡‡ National Institute of Standards and Technology, Materials Measurement Laboratory, Stanford University, Gaithersburg, Maryland 20877, United States) (C.J.T.)
Author Contributions
S.T. and M.E.M. conceived the project. S.T. performed all synthetic work, NMR (including Evans method) measurements, ATR-FTIR, UV–vis, CV, and ESI-MS characterization as well as TD-DFT and some DFT, including hyperfine coupling constant, calculations. S.T. and M.E.M. interpreted the data. M.L. conducted single crystal X-ray diffraction experiments along with solving the structures. C.J.T. carried out synchrotron XAS measurements followed by interpretation by FdG. T.L. and K.H. did synchrotron THz-EPR measurements, which were then interpreted by J.N., T.L., and A.S. M.F.X.T. and S.Y. carried out EPR, Mössbauer, and SQUID measurements, interpreted the results, and performed some DFT and CASSCF calculations. P.Z. and S.Y. performed DFT calculations on compound 3a. S.T., V.N., A.P., and D.A. interpreted paramagnetic NMR spectra. S.T. wrote the tutorial on paramagnetic NMR in Section S13.1. S.T. and M.E.M. wrote the manuscript with input from all authors. All authors read and approved the final manuscript.
This project has received funding from the NoNoMeCat Marie Skłodowska-Curie training network funded by the European Union under the Horizon2020 Program (675020-MSCA-ITN-2015-ETN). The X-ray diffractometer has been financed by the Netherlands Organization for Scientific Research (NWO). The computational work was carried out on the Dutch national e-infrastructure with the support of the SURF Foundation. A.P. acknowledges the Russian Science Foundation (project no. 22-73-00148) for financial support. T.L. acknowledges support by Deutsche Forschungsgemeinschaft (German Research Foundation, Project No. LO 2898/1-1). A.S. and J.N. are grateful for funding from the Max Planck Institute for Chemical Energy Conversion.
The authors declare no competing financial interest.
Supplementary Material
References
- Solomon E. I.; Brunold T. C.; Davis M. I.; Kemsley J. N.; Lee S.-K.; Lehnert N.; Neese F.; Skulan A. J.; Yang Y.-S.; Zhou J. Geometric and Electronic Structure/Function Correlations in Non-Heme Iron Enzymes. Chem. Rev. 2000, 100, 235–350. 10.1021/cr9900275. [DOI] [PubMed] [Google Scholar]
- Costas M.; Mehn M. P.; Jensen M. P.; Que L. Dioxygen Activation at Mononuclear Nonheme Iron Active Sites: Enzymes, Models, and Intermediates. Chem. Rev. 2004, 104, 939–986. 10.1021/cr020628n. [DOI] [PubMed] [Google Scholar]
- Nam W. High-Valent Iron(IV)–Oxo Complexes of Heme and Non-Heme Ligands in Oxygenation Reactions. Acc. Chem. Res. 2007, 40, 522–531. 10.1021/ar700027f. [DOI] [PubMed] [Google Scholar]
- Groves J. T. High-Valent Iron in Chemical and Biological Oxidations. J. Inorg. Biochem. 2006, 100, 434–447. 10.1016/j.jinorgbio.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Que L.; Tolman W. B. Biologically Inspired Oxidation Catalysis. Nature 2008, 455, 333–340. 10.1038/nature07371. [DOI] [PubMed] [Google Scholar]
- Berry J. F.; DeBeer George S.; Neese F. Electronic Structure and Spectroscopy of “Superoxidized” Iron Centers in Model Systems: Theoretical and Experimental Trends. Phys. Chem. Chem. Phys. 2008, 10, 4361–4374. 10.1039/b801803k. [DOI] [PubMed] [Google Scholar]
- Betley T. A.; Surendranath Y.; Childress M. V.; Alliger G. E.; Fu R.; Cummins C. C.; Nocera D. G. A Ligand Field Chemistry of Oxygen Generation by the Oxygen-Evolving Complex and Synthetic Active Sites. Philos. Trans. R. Soc., B 2008, 363, 1293–1303. 10.1098/rstb.2007.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas-Ballesté R.; Que L. Iron-Catalyzed Olefin Epoxidation in the Presence of Acetic Acid: Insights into the Nature of the Metal-Based Oxidant. J. Am. Chem. Soc. 2007, 129, 15964–15972. 10.1021/ja075115i. [DOI] [PubMed] [Google Scholar]
- Kaizer J.; Klinker E. J.; Oh N. Y.; Rohde J.-U.; Song W. J.; Stubna A.; Kim J.; Münck E.; Nam W.; Que L. Nonheme FeIVO Complexes That Can Oxidize the C–H Bonds of Cyclohexane at Room Temperature. J. Am. Chem. Soc. 2004, 126, 472–473. 10.1021/ja037288n. [DOI] [PubMed] [Google Scholar]
- Cramer S. A.; Jenkins D. M. Synthesis of Aziridines from Alkenes and Aryl Azides with a Reusable Macrocyclic Tetracarbene Iron Catalyst. J. Am. Chem. Soc. 2011, 133, 19342–19345. 10.1021/ja2090965. [DOI] [PubMed] [Google Scholar]
- Nieto I.; Ding F.; Bontchev R. P.; Wang H.; Smith J. M. Thermodynamics of Hydrogen Atom Transfer to a High-Valent Iron Imido Complex. J. Am. Chem. Soc. 2008, 130, 2716–2717. 10.1021/ja0776834. [DOI] [PubMed] [Google Scholar]
- Sabenya G.; Gamba I.; Gómez L.; Clémancey M.; Frisch J. R.; Klinker E. J.; Blondin G.; Torelli S.; Que L.; Martin-Diaconescu V.; Latour J.-M.; Lloret-Fillol J.; Costas M. Octahedral Iron(IV)–Tosylimido Complexes Exhibiting Single Electron-Oxidation Reactivity. Chem. Sci. 2019, 10, 9513–9529. 10.1039/C9SC02526J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scepaniak J. J.; Vogel C. S.; Khusniyarov M. M.; Heinemann F. W.; Meyer K.; Smith J. M. Synthesis, Structure, and Reactivity of an Iron(V) Nitride. Science 2011, 331, 1049–1052. 10.1126/science.1198315. [DOI] [PubMed] [Google Scholar]
- Vogel C.; Heinemann F. W.; Sutter J.; Anthon C.; Meyer K. An Iron Nitride Complex. Angew. Chem., Int. Ed. 2008, 47, 2681–2684. 10.1002/anie.200800600. [DOI] [PubMed] [Google Scholar]
- Betley T. A.; Peters J. C. A Tetrahedrally Coordinated L3Fe–Nx Platform That Accommodates Terminal Nitride (FeIV⋮N) and Dinitrogen (FeI–N2 −FeI) Ligands. J. Am. Chem. Soc. 2004, 126, 6252–6254. 10.1021/ja048713v. [DOI] [PubMed] [Google Scholar]
- Scepaniak J. J.; Fulton M. D.; Bontchev R. P.; Duesler E. N.; Kirk M. L.; Smith J. M. Structural and Spectroscopic Characterization of an Electrophilic Iron Nitrido Complex. J. Am. Chem. Soc. 2008, 130, 10515–10517. 10.1021/ja8027372. [DOI] [PubMed] [Google Scholar]
- Bucinsky L.; Breza M.; Lee W.-T.; Hickey A. K.; Dickie D. A.; Nieto I.; DeGayner J. A.; Harris T. D.; Meyer K.; Krzystek J.; Ozarowski A.; Nehrkorn J.; Schnegg A.; Holldack K.; Herber R. H.; Telser J.; Smith J. M. Spectroscopic and Computational Studies of Spin States of Iron(IV) Nitrido and Imido Complexes. Inorg. Chem. 2017, 56, 4751–4768. 10.1021/acs.inorgchem.7b00512. [DOI] [PubMed] [Google Scholar]
- Chanda A.; Popescu D.-L.; de Oliveira F. T.; Bominaar E. L.; Ryabov A. D.; Münck E.; Collins T. J. High-Valent Iron Complexes with Tetraamido Macrocyclic Ligands: Structures, Mössbauer Spectroscopy, and DFT Calculations. J. Inorg. Biochem. 2006, 100, 606–619. 10.1016/j.jinorgbio.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Lewis R. A.; Wu G.; Hayton T. W. Synthesis and Characterization of an Iron(IV) Ketimide Complex. J. Am. Chem. Soc. 2010, 132, 12814–12816. 10.1021/ja104934n. [DOI] [PubMed] [Google Scholar]
- Rau J. V.; Nunziante Cesaro S.; Chilingarov N. S.; Leskiv M. S.; Balducci G.; Sidorov L. N. Mass Spectrometric and FTIR Spectroscopic Identification of FeF4 Molecules in Gaseous Phase. Inorg. Chem. Commun. 2003, 6, 643–645. 10.1016/S1387-7003(03)00070-4. [DOI] [Google Scholar]
- Schlöder T.; Vent-Schmidt T.; Riedel S. A Matrix-Isolation and Quantum-Chemical Investigation of FeF4. Angew. Chem., Int. Ed. 2012, 51, 12063–12067. 10.1002/anie.201206464. [DOI] [PubMed] [Google Scholar]
- Bower B. K.; Tennent H. G. Transition Metal Bicyclo[2.2.1]Hept-1-Yls. J. Am. Chem. Soc. 1972, 94, 2512–2514. 10.1021/ja00762a056. [DOI] [Google Scholar]
- Lewis R. A.; Smiles D. E.; Darmon J. M.; Stieber S. C. E.; Wu G.; Hayton T. W. Reactivity and Mössbauer Spectroscopic Characterization of an Fe(IV) Ketimide Complex and Reinvestigation of an Fe(IV) Norbornyl Complex. Inorg. Chem. 2013, 52, 8218–8227. 10.1021/ic401096p. [DOI] [PubMed] [Google Scholar]
- Casitas A.; Rees J. A.; Goddard R.; Bill E.; DeBeer S.; Fürstner A. Two Exceptional Homoleptic Iron(IV) Tetraalkyl Complexes. Angew. Chem., Int. Ed. 2017, 56, 10108–10113. 10.1002/ange.201612299. [DOI] [PubMed] [Google Scholar]
- Malischewski M.; Adelhardt M.; Sutter J.; Meyer K.; Seppelt K. Isolation and Structural and Electronic Characterization of Salts of the Decamethylferrocene Dication. Science 2016, 353, 678–682. 10.1126/science.aaf6362. [DOI] [PubMed] [Google Scholar]
- Pasek E. A.; Straub D. K. Tris(N,N-Disubstituted Dithiocarbamato)Iron(IV) Tetrafluoroborates. Inorg. Chem. 1972, 11, 259–263. 10.1021/ic50108a012. [DOI] [Google Scholar]
- Petrouleas V.; Petridis D. Moessbauer Quadrupole Splitting Analysis of Iron(IV) Dithio Chelates. Inorg. Chem. 1977, 16, 1306–1309. 10.1021/ic50172a011. [DOI] [Google Scholar]
- Milsmann C.; Sproules S.; Bill E.; Weyhermüller T.; George S. D.; Wieghardt K. Stabilization of High-Valent FeIVS6-Cores by Dithiocarbamate(1−) and 1,2-Dithiolate(2−) Ligands in Octahedral [FeIV(Et2Dtc)3–n(Mnt)n](N–1)– Complexes (N=0, 1, 2, 3): A Spectrosco. Chem. – Eur. J. 2010, 16, 3628–3645. 10.1002/chem.200903381. [DOI] [PubMed] [Google Scholar]
- Tomyn S.; Shylin S. I.; Bykov D.; Ksenofontov V.; Gumienna-Kontecka E.; Bon V.; Fritsky I. O. Indefinitely Stable Iron(IV) Cage Complexes Formed in Water by Air Oxidation. Nat. Commun. 2017, 8, 14099. 10.1038/ncomms14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash O.; Chábera P.; Rosemann N. W.; Huang P.; Häggström L.; Ericsson T.; Strand D.; Persson P.; Bendix J.; Lomoth R.; Wärnmark K. A Stable Homoleptic Organometallic Iron(IV) Complex. Chem. – Eur. J. 2020, 26, 12728–12732. 10.1002/chem.202002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman W. H.; Chang C. J. N2O Activation and Oxidation Reactivity from a Non-Heme Iron Pyrrole Platform. J. Am. Chem. Soc. 2007, 129, 15128–15129. 10.1021/ja076842g. [DOI] [PubMed] [Google Scholar]
- Poulos T. L. Heme Enzyme Structure and Function. Chem. Rev. 2014, 114, 3919–3962. 10.1021/cr400415k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemistry and Biochemistry of B12; Banerjee R., Ed.; Wiley: New York, 1999. [Google Scholar]
- Lee C.-H.; Lindsey S. J. One-Flask Synthesis of Meso-Substituted Dipyrromethanes and Their Application in the Synthesis of Trans-Substituted Porphyrin Building Blocks. Tetrahedron 1994, 50, 11427–11440. 10.1016/S0040-4020(01)89282-6. [DOI] [Google Scholar]
- Swartz D. L.; Odom A. L. Synthesis, Structure, and Hydroamination Kinetics of (2,2‘-Diaryldipyrrolylmethane)- and Bis(2-Arylpyrrolyl)Titanium Complexes. Organometallics 2006, 25, 6125–6133. 10.1021/om0607088. [DOI] [Google Scholar]
- King E. R.; Betley T. A. Unusual Electronic Structure of First Row Transition Metal Complexes Featuring Redox-Active Dipyrromethane Ligands. J. Am. Chem. Soc. 2009, 131, 14374–14380. 10.1021/ja903997a. [DOI] [PubMed] [Google Scholar]
- Novak A.; Blake A. J.; Wilson C.; Love J. B. Titanium and Zirconium Complexes Supported by Dipyrrolide Ligands. Chem. Commun. 2002, 23, 2796–2797. 10.1039/b208751k. [DOI] [PubMed] [Google Scholar]
- Love J. B.; Salyer P. A.; Bailey A. S.; Wilson C.; Blake A. J.; Davies E. S.; Evans D. J. The Dipyrrolide Ligand as a Template for the Spontaneous Formation of a Tetranuclear Iron(II) Complex. Chem. Commun. 2003, 52, 1390–1391. 10.1039/b303485b. [DOI] [PubMed] [Google Scholar]
- Sazama G. T.; Betley T. A. Ligand-Centered Redox Activity: Redox Properties of 3d Transition Metal Ions Ligated by the Weak-Field Tris(Pyrrolyl)Ethane Trianion. Inorg. Chem. 2010, 49, 2512–2524. 10.1021/ic100028y. [DOI] [PubMed] [Google Scholar]
- Tretiakov S.; Damen J. A. M.; Lutz M.; Moret M.-E. A Dianionic C3-Symmetric Scorpionate: Synthesis and Coordination Chemistry. Dalton Trans. 2020, 49, 13549–13556. 10.1039/D0DT02601H. [DOI] [PubMed] [Google Scholar]
- Koningsbruggen P. J.; Maeda Y.; Oshio H.. Iron(III) Spin Crossover Compounds. In Topics in Current Chemistry: Spin Crossover in Transition Metal Compounds I; Springer-Verlag: Berlin, Heidelberg, 2004; Vol. 233, pp 259–324. [Google Scholar]
- Harding D. J.; Harding P.; Phonsri W. Spin Crossover in Iron(III) Complexes. Coord. Chem. Rev. 2016, 313, 38–61. 10.1016/j.ccr.2016.01.006. [DOI] [Google Scholar]
- Krejčik M.; Daněk M.; Hartl F. Simple Construction of an Infrared Optically Transparent Thin-Layer Electrochemical Cell. J. Electroanal. Chem. Interfacial Electrochem. 1991, 317, 179–187. 10.1016/0022-0728(91)85012-E. [DOI] [Google Scholar]
- Knof U.; Weyhermüller T.; Wolter T.; Wieghardt K.; Bill E.; Butzlaff C.; Trautwein A. X. How “Innocent” Are Pentane-2,4-Dionebis(S-Alkylisothiosemicarbazonato) Ligands in Biomimetic FeII and FeIV Complexes?. Angew. Chem., Int. Ed. Engl. 1993, 32, 1635–1638. 10.1002/anie.199316351. [DOI] [Google Scholar]
- Lacy D. C.; Gupta R.; Stone K. L.; Greaves J.; Ziller J. W.; Hendrich M. P.; Borovik A. S. Formation, Structure, and EPR Detection of a High Spin FeIV—Oxo Species Derived from Either an FeIII—Oxo or FeIII—OH Complex. J. Am. Chem. Soc. 2010, 132, 12188–12190. 10.1021/ja1047818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savéant J.-M.Elements of Molecular and Biomolecular Electrochemistry: An Electrochemical Approach to Electron Transfer Chemistry; John Wiley & Sons: Hoboken, NJ, 2006. [Google Scholar]
- Walker F. A. Magnetic Spectroscopic (EPR, ESEEM, Mössbauer, MCD and NMR) Studies of Low-Spin Ferriheme Centers and Their Corresponding Heme Proteins. Coord. Chem. Rev. 1999, 185–186, 471–534. 10.1016/S0010-8545(99)00029-6. [DOI] [Google Scholar]
- Sorai M.; Seki S. Phonon Coupled Cooperative Low-Spin 1A1High-Spin 5T2 Transition in [Fe(Phen)2(NCS)2] and [Fe(Phen)2(NCSe)2] Crystals. J. Phys. Chem. Solids 1974, 35, 555–570. 10.1016/S0022-3697(74)80010-7. [DOI] [Google Scholar]
- Kahn O.Molecular Magnetism; VCH Publishers, Inc.: New York (US), Weinheim (DE), Cambridge (UK), 1993; pp 26–29. [Google Scholar]
- Kahn O.Molecular Magnetism; VCH Publishers, Inc.: New York (US), Weinheim (DE), Cambridge (UK), 1993; p 56. [Google Scholar]
- Kelly C. T.; Griffin M.; Esien K.; Felton S.; Müller-Bunz H.; Morgan G. G. Crystallographic Detection of the Spin State in FeIII Complexes. Cryst. Growth Des. 2022, 22, 6429–6439. 10.1021/acs.cgd.2c00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. F. 400. The Determination of the Paramagnetic Susceptibility of Substances in Solution by Nuclear Magnetic Resonance. J. Chem. Soc. 1959, 2003–2005. 10.1039/jr9590002003. [DOI] [Google Scholar]
- Piguet C. Paramagnetic Susceptibility by NMR: The “Solvent Correction” Removed for Large Paramagnetic Molecules. J. Chem. Educ. 1997, 74, 815. 10.1021/ed074p815. [DOI] [Google Scholar]
- Sur S. K. Measurement of Magnetic Susceptibility and Magnetic Moment of Paramagnetic Molecules in Solution by High-Field Fourier Transform NMR Spectroscopy. J. Magn. Reson. 1989, 82, 169–173. 10.1016/0022-2364(89)90178-9. [DOI] [Google Scholar]
- Nikovskiy I.; Aleshin D. Y.; Novikov V. V.; Polezhaev A. V.; Khakina E. A.; Melnikova E. K.; Nelyubina Y. V. Selective Pathway toward Heteroleptic Spin-Crossover Iron(II) Complexes with Pyridine-Based N-Donor Ligands. Inorg. Chem. 2022, 61, 20866–20877. 10.1021/acs.inorgchem.2c03270. [DOI] [PubMed] [Google Scholar]
- Novikov V. V.; Ananyev I. V.; Pavlov A. A.; Fedin M. V.; Lyssenko K. A.; Voloshin Y. Z. Spin-Crossover Anticooperativity Induced by Weak Intermolecular Interactions. J. Phys. Chem. Lett. 2014, 5, 496–500. 10.1021/jz402678q. [DOI] [PubMed] [Google Scholar]
- Estrader M.; Salinas Uber J.; Barrios L. A.; Garcia J.; Lloyd-Williams P.; Roubeau O.; Teat S. J.; Aromí G. A Magneto-optical Molecular Device: Interplay of Spin Crossover, Luminescence, Photomagnetism, and Photochromism. Angew. Chem., Int. Ed. 2017, 56, 15622–15627. 10.1002/anie.201709136. [DOI] [PubMed] [Google Scholar]
- Nelyubina Y.; Polezhaev A.; Pavlov A.; Aleshin D.; Savkina S.; Efimov N.; Aliev T.; Novikov V. Intramolecular Spin State Locking in Iron(II) 2,6-Di(Pyrazol-3-Yl)Pyridine Complexes by Phenyl Groups: An Experimental Study. Magnetochemistry 2018, 4, 46. 10.3390/magnetochemistry4040046. [DOI] [Google Scholar]
- Datta D.; Saitow M.; Sandhöfer B.; Neese F. 57Fe Mössbauer Parameters from Domain Based Local Pair-Natural Orbital Coupled-Cluster Theory. J. Chem. Phys. 2020, 153, 204101. 10.1063/5.0022215. [DOI] [PubMed] [Google Scholar]
- Stavitski E.; de Groot F. M. F. The CTM4XAS Program for EELS and XAS Spectral Shape Analysis of Transition Metal L Edges. Micron 2010, 41, 687–694. 10.1016/j.micron.2010.06.005. [DOI] [PubMed] [Google Scholar]
- de Groot F.; Kotani A.. Core Level Spectroscopy of Solids, 1st ed.; CRC Press, 2008. [Google Scholar]
- Orchard A. F.Magnetochemistry (Oxford Chemistry Primers, 75); Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Nehrkorn J.; Holldack K.; Bittl R.; Schnegg A. Recent progress in synchrotron-based frequency-domain Fourier-transform THz-EPR. J. Magn. Res. 2017, 280, 10–19. 10.1016/j.jmr.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Römelt M.; Ye S.; Neese F. Calibration of Modern Density Functional Theory Methods for the Prediction of 57Fe Mössbauer Isomer Shifts: Meta-GGA and Double-Hybrid Functionals. Inorg. Chem. 2009, 48, 784–785. 10.1021/ic801535v. [DOI] [PubMed] [Google Scholar]
- Dunn T. M. Spin-Orbit Coupling in the First and Second Transition Series. Trans. Faraday Soc. 1961, 57, 1441–1444. 10.1039/tf9615701441. [DOI] [Google Scholar]
- Martin R. L. Natural Transition Orbitals. J. Chem. Phys. 2003, 118, 4775–4777. 10.1063/1.1558471. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









