Abstract
The varieties and capabilities of artificial intelligence and machine learning in orthopedic surgery are extensively expanding. One promising method is neural networks, emphasizing big data and computer-based learning systems to develop a statistical fracture-detecting model. It derives patterns and rules from outstanding amounts of data to analyze the probabilities of different outcomes using new sets of similar data. The sensitivity and specificity of machine learning in detecting fractures vary from previous studies. AI may be most promising in the diagnosis of less-obvious fractures that are more commonly missed. Future studies are necessary to develop more accurate and effective detection models that can be used clinically.
Keywords: Artificial intelligence, Machine learning, Orthopedics, Trauma, Neural network
Core Tip: Machine learning is currently applied to image-screening assistance, predictive analytics, and intraoperative robotics, specifically in the trauma orthopedics field. Artificial intelligence can be used in the emergency department of trauma centers as a screening tool and aid to orthopedists, helping them improve their sensitivity and specificity and help shorten their diagnosis time. In real-life practice, orthopedic surgeons consider various factors when making a prediction; that is why machine learning-based predictive models include features such as history and physical exam data, along with imaging results. Artificial intelligence application may be able to identify such patterns and increase the chance of optimum results.
INTRODUCTION
Nowadays, artificial intelligence and machine learning are involved significantly in medicine[1-4]. Musculoskeletal trauma is one of the main reasons for emergency department (ED) visits. Due to the nature of trauma, missed injuries are common after the primary assessment of patients[5]. Avoiding missed injuries is essential for the timely and efficient treatment of patients seen in the ED[6,7]. Therefore, identifying errors in medical imaging interpretation that contribute to missed and delayed diagnoses is critical. AI surveillance may be most promising in settings where misinterpretation is most prone to happen. Hallas et al[8] showed that fracture misdiagnoses were most likely to occur between the hours of 8 pm and 2 am.
Machine learning (ML) and artificial intelligence (AI) are poised to assist physicians in faster and more efficient identification of fractures on radiographs taken in the clinic and/or the ED, working through a high volume of images while maintaining high accuracy[9]. The ability of ML to handle large amounts of data and multiple simultaneous variables means that it can identify patterns (injuries) that humans may be more likely to miss. Analyzing big data gives ML the power to be applied for predictive analytics, such as personalized treatment and prediction of surgical outcomes[10].
In this review article, we discuss the developmental frontier of AI applications in commonly used image modalities for identifying orthopedic injuries.
OVERVIEW OF DEEP LEARNING TECHNOLOGY
From being introduced in 1959 until now, AI applications have increased exponentially. Physicians are now beginning to take advantage of this constantly developing tool within their fields. Better central- and graphic-processing units (CPU and GPU) are being designed to have the ability to put into use the ever-growing amounts of data that is now accessible[11].
AI is described as algorithms solving issues that usually require humans to intervene. Machine learning is a subset of AI that allows it to learn without complex programming. The power of ML lies in the learning process, which can be divided into two groups: Supervised learning, which requires instructions from humans, and unsupervised learning which the machine itself learns and classifies the data in patterns it finds itself; and potentially identifying patterns that have not yet been recognized by human rules.
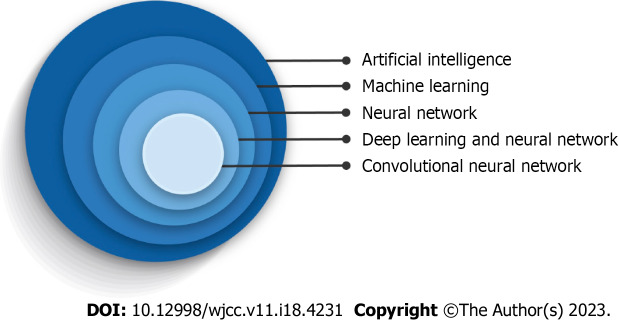
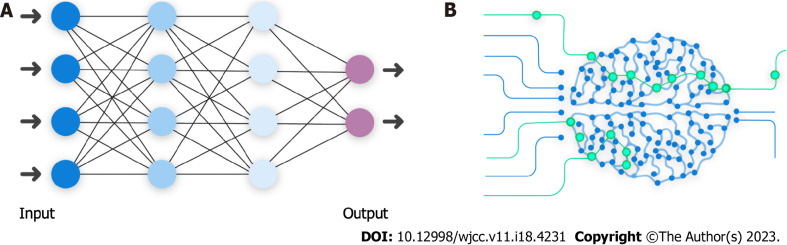
Figure 1 illustrates a hierarchic relationship between these terms with deep learning (DL) being the following subclass. The main difference between classic ML and DL is that, unlike classic algorithms, DL algorithms learn which features are the best for the calculating task rather than human experts choosing them[12,13]. Deep learning algorithms are applied as neural networks that can learn the input data's nonlinear functions. The concept of computer learning pictures lies in convolutional neural networks (CNNs). CNNs contain many layers that are limited in transforming their input with the convolution filters (Figure 2)[14]. Schematic representation illustrated an artificial network inspired by a biological neural system composed of many artificial neurons. As dendrites receive inputs in a neuron cell, an artificial neuron receives signals multiplied by their weights (w) so that output can be determined based on the weighted sum of the input. There is a specific weight for each neuron, and the bias value (b) is to shift the activation function along with the weighted sum of inputs. Here, the activation function is shown as rectified linear unit function to introduce non-linearity to the neuron decision. Here, it chooses the maximum of either z or 0. Considering a collection of connected artificial neurons and when the output of some becomes the inputs of another, arranged in a multilayer complex that is only connected to their adjacent layers (Figure 3).
Figure 1.
Diagram demonstration of convolutional neural networks in the artificial intelligence hierarchy.
Figure 2.
Schematic exemplification of an artificial neuron to highlight its similarity to a biological neuron. Data input (X) is termed weight (W) in an artificial neuron. As in, Xs are multiplied by their Ws, bias value (b) is added to allow the model to fit better, and a nonlinear mathematical formula determines the output (Y) for the next neurons in line.
Figure 3.
Schematic representation illustrated an artificial network inspired by a biological neural system. A: Graphic model of artificial neural network and its similarity to (B) biological neural network; B: Biological neural network. Output of one layer is considered the input of another.
CNNs are a subset of DL algorithms that has surpassed image analysis by acting as an arrangement of layers that simplifies image volume into basic class scores. Using learnable layers that reduce the complexity and parameter requirements per layer starting from the "Dense layer," where all the possible connections between input and output nodes are introduced and classified. Moving on to the "Convolutional layer (CONV layer)," "Pooling layer," and "Dropout layer," which are created to learn more complex features and avoid overfitting, that is when there is a good performance on the training data but poor employment to other data. CONV layer also can be used for determining the exact input volume. CNNs gained enormous popularity in neutral image recognition when they outperformed humans[15].
DL and CNNs can train with input data and its standard labels (for example, fracture or no fracture). Self-learning is a prominent feature of this system, which gives them the advantage of handling novel tasks with less computational power and time, stepping up the interpretation process. It is difficult to determine how a CNN works, but more information on its decision-making has been presented in many related articles[12-14].
Although, medicine as a field has underutilized AI applications so far, its use is increasing[16]. ML is now applied to intraoperative robotics, predictive analytics, and, most importantly, image-screening assistance, specifically in the trauma field[15,17-19].
IMAGE-BASED AI APPLICATION
Physicians have been quick to apply machine learning and AI to fracture detection, given the large number of medical images that must be reviewed and the potential for missed injuries. The image-based DL model is one of the most used AI techniques for fracture detection and has been applied to various modalities such as computed tomography (CT) images, X-Ray, and magnetic resonance imaging.
Given the prevalence of hand and wrist injuries, and scaphoid fractures being the most common carpal bone fractures, Ozkaya et al[20] used CNN for detecting scaphoid fractures, comparing its performance with ED physicians and two orthopedic specialists (one of them being experienced in hand surgery) for detecting scaphoid fractures on anteroposterior wrist radiographs. Even though the experienced orthopedic specialist showed the highest area under the receiver operating curve (AUC) value (0.920), CNN's AUC value was higher than both the untrained orthopedic specialist and ED physicians (0.840 vs 0.820 and 0.760, respectively). CNN also had a significantly higher sensitivity than the ED physicians (72% compared to 62%), even though it showed lower sensitivity than the experienced orthopedic specialist (86%). This article recommended using CNN for detecting scaphoid fractures in centers without experienced hand surgeons available. Oka et al[21] 2021 used image augmentation to increase their training data in an AI model they developed to diagnose distal radius fractures. Their model displayed an excellent diagnostic accuracy at 98% ± 1.6% for detecting distal radius fractures and a 91.1% ± 2.5% diagnostic accuracy for fractures of the ulnar styloid process, despite using a relatively small amount of data. This promising diagnostic rate was achieved by using bi-planar X-ray images. The sensitivity and specificity for distal radius fractures were 98.6% ± 1.8% and 96.7% ± 3.5%, respectively, with the sensitivity and specificity for the styloid process of the ulna being 92.2% ± 5.7% and 90.4% ± 3.9%, respectively.
Liu and colleagues improved an AI algorithm (RetinaNet) and trained it with X-rays of patients with tibial plateau fractures (TPF) to help orthopedic physicians detect TPF. The algorithm's performance was promising; not only was it 16 times faster than the orthopedists, but it also showed a similar accuracy rate (0.91 vs 0.92). Liu suggests that their AI algorithm would perform even better in clinical settings. Humans have been shown to be prone to missed diagnoses when under pressure or overworked, making AI a potentially useful tool in these scenarios[22].
In a study conducted by Small et al[23], C-spine, a CNN developed to detect cervical spine fractures on CT, showed a lower accuracy (92% vs 96%) and sensitivity (79% vs 93%) rate compared to that of radiologists. Nevertheless, CNN was superior to radiologists regarding radiology interpretation times. This decrease in fracture detection time illuminates the possible role of CNN in prioritizing unstable fractures to intervene promptly. Murata et al[24] trained a deep convolutional neural network (DCNN) with plain thoracolumbar radiography (PTLR) to detect vertebral fractures (VF). PTLR is cheaper and more available in primary care centers than CT and MRI, yet PTLRs sensitivity for detecting VF is considerably lower than theirs. The DCNN Murata and his colleagues showed higher sensitivity than orthopedic residents (84.7% vs 72.4%) but lower sensitivity than orthopedic surgeons and spine surgeons (77.5% and 96%, respectively). Their work suggests that DCNN can be used by general and emergency physicians or even orthopedic residents to identify VFs not only early and timely for management but with an 86.0% accuracy rate, higher than the accuracy rate of orthopedic residents (77.5%) and almost equivalent to that of orthopedic surgeons (88%)[24].
In a retrospective study conducted by Mutasa et al[25], CNN was used to not only diagnose but to classify femoral neck fractures (FNF) based on the radiograph-based Garden classification system of FNF[26]. They trained two networks, one to localize the femoral neck on anteroposterior (AP) radiographs, the other to classify the femoral neck into Garden I/II, Garden III/IV, or no fracture groups. Data augmentation improved their CNN performance by providing additional training data. The CNN detected fractures with an accuracy of 92.3%, sensitivity of 0.91, and specificity of 0.93. It also showed a higher sensitivity for detecting and correctly classifying displaced fracture (Garden III or IV) compared to non-displaced fractures (Garden class I or II), with an accuracy rate of 86% vs 80% and a sensitivity of 0.91 vs 0.54, respectively, suggesting that DL using a CNN can help physicians with the timely detection and therefore management of FNFs in the emergency department. More recently, Bae et al[27] used hip and pelvic AP films for training a CNN developed to detect FNFs. They then performed an external validation for their CNN model. This study was conducted in two hospitals. After training and internal validation of one hospital dataset, the test values were 0.999 AUC, 0.986 accuracy, 0.966 sensitivity, and 0.993 specificity. Values of external validation with the other hospital dataset were 0.977, 0.971, 0.939, and 0.982, respectively. Values of the combined hospital dataset were 0.987 AUC, 0.983 accuracy, 0.973 sensitivity, and 0.987 specificity, indicating that even though other hospitals could use the completed model trained with the data set of one hospital for screening FNF, the CNN should also be trained with images from those hospitals, to improve the CNNs performance.
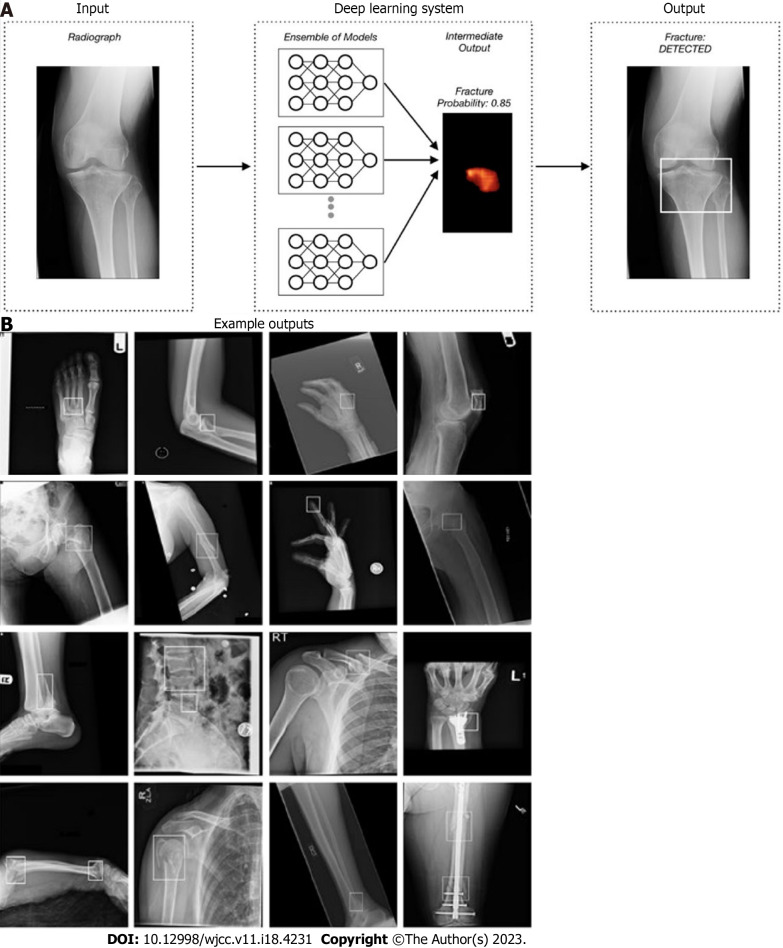
One of the limitations of most algorithms that have been developed is that they are limited to one anatomical area. Therefore, in account to apply them to interact with other algorithms, one interconnected software was needed. Jones et al[28] article using 715343 radiographs is an example of this interaction. This multicentric study, 16 anatomic regions were analyzed using an ensemble of 10 CNNs with mean AUCs above 0.98 for most areas (Figure 4).
Figure 4.
Schematic representation of detection of fracture and localizing it. A: To better outline the fractures, 10 convolutional neural networks were used to predict and generate bounding boxes around them; B: Sixteen anatomical regions and the result of fracture detection in them. Reprinted with permission from Jones et al[28].
Guermazi et al[29] aimed to compare the performance of medical doctors of several fields, four of them being orthopedists, in detecting fractures of various anatomic locations (foot and ankle, knee and leg, hip and pelvis, hand and wrist, elbow and arm, shoulder and clavicle, rib cage and thoracolumbar spine) with and without the assistance of AI, with a minimum washout period of 1 mo. With AI assistance, orthopedists' sensitivity per patient improved by 9.1%, and their specificity per patient enhanced by 2.0%. Their study showed no difference between specialties for sensitivity or specificity per patient improvement with the assistance of AI and that the AI can help clinicians shorten the radiograph reading time by 6.3 per patient. Inoue and colleagues[30] used a CNN model to localize fractures on whole-body CT scans of polytrauma patients and to classify them into pelvic, rib, and spine fractures. The CNN showed 0.839 sensitivity for pelvic fractures, with 0.645 precision. For rib fractures, the sensitivity was 0.713, and the precision was 0.602. In detecting spine fractures, the CNN's sensitivity was 0.780 with a 0.683 precision. Overall, The CNN model demonstrated promising outcomes for detecting all three types of fractures; for the grouped mean values, sensitivity was 0.786, and accuracy was 0.648. They also had their CNN model assist orthopedic surgeons with fracture diagnosis, resulting in increased sensitivity and reduced CT image reading time. The results of these two articles suggest that AI can be used in the emergency department of trauma centers as a screening tool and aid to orthopedists, helping them improve their sensitivity and specificity and help shorten their diagnosis time.
PREDICTIVE ANALYSIS
Even in orthopedic surgery, a field that largely relies on technical devices and imaging modalities, AI use is not limited to fracture detection and surgical robots in the operating room. Predictive modeling in traditional statistical modeling is based on known underlying structures and various hypotheses, but this is not the case for ML[31], which makes ML-based predictive models more efficient. We will review some of the predictive applications of ML in trauma orthopedics.
Orthopedic surgeons use pre-operative data (e.g., imaging information) to choose the best surgery method. But, clinical decision-making in trauma patients is not always straightforward; fractures may not be evident in pre-operative routine assessments (i.e. occult fractures). The study of Hendrickx et al[32] is an example. Tibial shaft fracture complicated with posterior malleolar fracture benefits from the “malleolus first” surgical technique; however, the latter may remain undiagnosed before the surgery. So, the authors used ML methods that accurately predicted posterior malleolar fracture.
Sports medicine is an important topic for predictive medicine. Researchers have compared the performance of ML and traditional regression analysis to predict following-season injuries among 2322 national hockey league players[33]. Advanced ML models outperformed logistic regression in their study. A similar study was also conducted among (American) league baseball players[34]. In another study evaluating soccer players with Achilles tendon rupture[35], 32853 soccer matches were analyzed with ML methods, and pre-injury performance was the best predictor of match participation level after the trauma. Studies have also used ML to predict secondary meniscus tears in 1187 patients who underwent primary anterior cruciate ligament (ACL) reconstruction[32]. They used four ML models, and they all outperformed logistic regression. ML can even identify patients at risk of prolonged opioid use following arthroscopic ACL repair[36].
If it were not for AI, would it be possible to analyze such extensive data while training individualized predictive models?
In general, and with trauma surgeries, studies have also used ML to determine which characteristics would lead to a worse outcome. An example is using ML to predict surgical site infection[37]. The authors analyzed patient and surgical procedure-related factors in 2882526 surgical procedures; the results support the superiority of ML models compared to logistic regression. Other examples include (1) Promising performance to predict delirium after hip fracture fixation in geriatrics (internally and externally validated)[38]; and (2) accurate prediction of short-term outcomes after open reduction and internal fixation in ankle fractures[39]. Martin and colleagues have used ML models to predict ACL revision surgery and developed an in-clinic calculator; in another study, they externally validated their previous findings suggesting that incorporating this tool helps clinicians predict revision risk among these patients[40,41].
The precise predictive ability of ML is advantageous in critical settings where traditional methods may come up short. For example, pertrochanteric fracture surgery in elderly patients accompanies higher morbidity and mortality rates. ML methods were used to predict 1-year mortality after per-trochanteric fracture surgery in 448 patients[42]. ML-based analysis of patients undergoing primary emergency hip fracture surgery accurately predicted 30-d postoperative mortality[43,44].
Orthopedic trauma patients also benefit from long-term rehabilitation, and clinicians assess its success based on patient-reported outcome measures and clinical assessments. ML analyzes these data to determine which factors most likely lead to better outcomes[45]. In a study on hip, knee, and foot trauma patients[46], ML was able to predict rehabilitation success. Hopefully, by applying these findings in the future, trauma patients will receive individualized treatments that provide the optimal outcome of healthier and happier patients.
Studies do not always confirm the superiority of AI and ML-based prediction models in trauma orthopedics[39]. AI-based predictive analysis is an emerging field, but despite potential capabilities, we must address its shortcomings to yield more accurate algorithms. Some limitations are as follows: the predictive model is affected by the type and nature of variables; the exact data size to build a precise model is not clear; only the output can be obtained, and the information and knowledge that lead to an ML algorithm is unknown[47,48]. In real-life practice, orthopedic surgeons consider various factors when making a prediction; that is why ML-based predictive models include features such as history and physical exam data, along with imaging results. It is essential that the ML algorithm considers all the variables necessary for making the correct prediction. These novel predictive models still have a long way to go before they can be successfully implemented in day-to-day practice, however[49] (Table 1).
Table 1.
Stated studies and comparison of performances between artificial intelligence and human experts
|
Ref.
|
Region of interest
|
Modality
|
AI performance
|
Human performance
|
| Niiya et al[33], 2022 | Ribs | CT | 0.93 (sensitivity) | Data has not provided by the authors |
| Meng et al[34], 2021 | Ribs | CT | 0.92 (recall rate); 0.94 (precision) | 0.79, 083 (recall rate of radiologist 1 & 2); 0.88 (precision of radiologist 1 & 2) |
| Ozkaya et al[22], 2022 | Hand & Wrist (Scaphoid) | Radiographs | 0.84 (AUC); 0.72 (sensitivity) | 0.92, 0.76 (AUC of experienced orthopedist, and ED physician, respectively); 0.86, 0.62 (sensitivity of experienced orthopedist, and ED physician, respectively) |
| Oka et al[23], 2021 | Hand & Wrist (Distal of radius & styloid process of the ulna) | Radiographs | 0.98 ± 0.016/0.98 ± 0.018 & 0.91 ± 0.025/0.96 ± 0.035; (accuracy/sensitivity for detecting distal radius fractures & fractures of the ulnar styloid process, respectively) | Data not provided by the authors |
| Liu et al[24], 2021 | Tibial plateau fractures | Radiographs | 0.91 (accuracy) | 0.92 ± 0.03 (accuracy) |
| Small et al[25], 2021 | Vertebrae (Cervical) | CT | 0.92 (accuracy); 0.79 (sensitivity); 3-8 min (report time) | 0.96 (accuracy); 0.93 (sensitivity); 33-43 min (report time) |
| Murata et al[26], 2020 | Vertebrae | Radiographs | 0.86 (accuracy); 0.84 (sensitivity) | 0.77, 0.88 (accuracy rate of orthopedic residents & orthopedic surgeons, respectively); 0.72, 0.77, 0.96 (sensitivity of orthopedic residents, orthopedic, surgeons & spine surgeons, respectively) |
| Mutasa et al[27], 2020 | Femur (Femoral neck) | Radiographs | 0.92 (accuracy); 0.91 (sensitivity); 0.93 (specificity) | Data not provided by the authors |
| Bae et al[29], 2021 | Femur (Femoral neck) | Radiographs | 0.98 (AUC); 0.98 (accuracy); 0.97 (sensitivity); 0.98 (specificity) | Data not provided by the authors |
| Jones et al[30], 2020 | Various anatomic regions | Radiographs | Mean AUCs above 0.98 for most areas | Data not provided by the authors |
| Guermazi et al[31], 2022 | Various anatomic regions | Radiographs | 0.93 (AUC) 0.88 (Sensitivity); 0.88 (specificity) | 0.64 ± 0.09 (sensitivity); 0.90 ± 0.08 (specificity) |
| Inoue et al[32], 2022 | Pelvis, rib, vertebrae | CT | 0.78 (sensitivity); 0.64 (accuracy) | 0.69 (sensitivity of orthopedic surgeon 1); 0.67 (sensitivity of orthopedic surgeon 2); 0.76 (sensitivity of orthopedic surgeon 3) |
AI: Artificial intelligence; CT: Computed tomography; AUC: Area under the curve; ED: Emergency Department.
LIMITATIONS AND STRENGTHS OF THE CURRENT STUDY
The inclusion of search details is not mandatory in narrative reviews, which may compromise the thoroughness and impartiality of the search methods. Selective inclusion of publications that support a particular hypothesis can introduce bias and hinder the exploration of the existing evidence. Narrative reviews often lack descriptions of their selection and review methods, making replication and verification of their results impossible, which conflicts with scientific evidence. These reviews rely on written paragraphs to summarize research findings and do not conduct pooled analyses, which limits objectivity and instead reflects dominant opinions at the time of publication. While narrative reviews may provide a general understanding of a body of evidence, they do not fully explore alternative hypotheses and cannot ensure the correctness of dominant opinions. The aforementioned statements have been added to the manuscript.
CONCLUSION
As the long history of AI inclusion in medicine tells us[50], AI has a great potential to enhance diagnostic accuracy, especially in imaging-related areas[51]. However, has it gained the reliability to act in an emergency in severe trauma patients? A recent study by De Simone et al[49] demonstrated that emergency surgeons have a growing interest in AI implantation in the acute settings of the ED and emphasized that the support of healthcare systems is essential for the progress of AI in this field.
Aside from being used in hospital settings, high-accurate outcome predictors have also been helpful for bedside counseling of elderly patients concerned about trauma[52]. These predictors can help trauma detection before the patient arrives at the hospital.
All of this can give us a picture of how hospitals and Eds may be affected in the next 10 years: considering all of this potential that AI has, user-friendly applications must be developed to guide doctors through the most critical data and imaging available in emergencies. AI applications may be able to identify such patterns and increase the chance of optimum results. It cannot be defined how the future will be precisely, but it is safe to say that AI has not yet been able to do all of the complex tasks that humans do, but it can augment their performance to help them keep up with the ever-increasing workflow.
Footnotes
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior authors or other coauthors who contributed their efforts to this manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 26, 2023
First decision: April 10, 2023
Article in press: May 8, 2023
Specialty type: Orthopedics
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Spain; Jamshidi M, Czech Republic S-Editor: Liu JH L-Editor: Filipodia P-Editor: Zhang XD
Contributor Information
Maryam Salimi, Department of Orthopaedic Surgery, Denver Health Medical Center, Denver, CO 80215, United States.
Joshua A Parry, Department of Orthopaedic Surgery, Denver Health Medical Center, Denver, CO 80215, United States.
Raha Shahrokhi, Student Research Committee, Shiraz University of Medical Sciences, Shiraz 7138433608, Iran.
Seyedarad Mosalamiaghili, Student Research Committee, Shiraz University of Medical Sciences, Shiraz 7138433608, Iran. aradmosalami@gmail.com.
References
- 1.Jamshidi MB, Lalbakhsh A, Talla J, Peroutka Z, Hadjilooei F, Lalbakhsh P, Jamshidi M, Spada L, Mirmozafari M, Dehghani M, Sabet A, Roshani S, Bayat-Makou N, Mohamadzade B, Malek Z, Jamshidi A, Kiani S, Hashemi-Dezaki H, Mohyuddin W. Artificial Intelligence and COVID-19: Deep Learning Approaches for Diagnosis and Treatment. IEEE Access. 2020;8:109581–109595. doi: 10.1109/ACCESS.2020.3001973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moztarzadeh O, Jamshidi M, Sargolzaei S, Jamshidi A, Baghalipour N, Malekzadeh Moghani M, Hauer L. Metaverse and Healthcare: Machine Learning-Enabled Digital Twins of Cancer. Bioengineering. 2023;10:455. doi: 10.3390/bioengineering10040455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jamshidi MB, Daneshfar F. A Hybrid Echo State Network for Hypercomplex Pattern Recognition, Classification, and Big Data Analysis. 2022 12th International Conference on Computer and Knowledge Engineering (ICCKE); 2022: IEEE. [Google Scholar]
- 4.Jamshidi MB, Talla J, Lalbakhsh A, Sharifi-Atashgah MS, Sabet A, Peroutka Z. A conceptual deep learning framework for COVID-19 drug discovery. 2021 IEEE 12th Annual Ubiquitous Computing, Electronics & Mobile Communication Conference (UEMCON); 2021: IEEE. [Google Scholar]
- 5.Yang F, Bai XJ, Li ZF. Analysis of misdiagnosis in patients with multiple trauma. Chin J Traumatol. 2011;14:20–24. [PubMed] [Google Scholar]
- 6.Stinner DJ, Edwards D. Surgical Management of Musculoskeletal Trauma. Surg Clin North Am. 2017;97:1119–1131. doi: 10.1016/j.suc.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Gathen M, Jaenisch M, Fuchs F, Weinhold L, Schmid M, Koob S, Wirtz DC, Wimmer MD. Litigations in orthopedics and trauma surgery: reasons, dynamics, and profiles. Arch Orthop Trauma Surg. 2022;142:3659–3665. doi: 10.1007/s00402-021-03958-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallas P, Ellingsen T. Errors in fracture diagnoses in the emergency department--characteristics of patients and diurnal variation. BMC Emerg Med. 2006;6:4. doi: 10.1186/1471-227X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olczak J, Fahlberg N, Maki A, Razavian AS, Jilert A, Stark A, Sköldenberg O, Gordon M. Artificial intelligence for analyzing orthopedic trauma radiographs. Acta Orthop. 2017;88:581–586. doi: 10.1080/17453674.2017.1344459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehdian R, Howard M. Artificial Intelligence in Trauma and Orthopaedics. In: Lidströmer N, Ashrafian H, editors. Artificial Intelligence in Medicine. Cham: Springer International Publishing; 2020; 1-14. [Google Scholar]
- 11.Shen D, Wu G, Suk HI. Deep Learning in Medical Image Analysis. Annu Rev Biomed Eng. 2017;19:221–248. doi: 10.1146/annurev-bioeng-071516-044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chartrand G, Cheng PM, Vorontsov E, Drozdzal M, Turcotte S, Pal CJ, Kadoury S, Tang A. Deep Learning: A Primer for Radiologists. Radiographics. 2017;37:2113–2131. doi: 10.1148/rg.2017170077. [DOI] [PubMed] [Google Scholar]
- 13.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436–444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 14.Litjens G, Kooi T, Bejnordi BE, Setio AAA, Ciompi F, Ghafoorian M, van der Laak JAWM, van Ginneken B, Sánchez CI. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60–88. doi: 10.1016/j.media.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Hirschmann A, Cyriac J, Stieltjes B, Kober T, Richiardi J, Omoumi P. Artificial Intelligence in Musculoskeletal Imaging: Review of Current Literature, Challenges, and Trends. Semin Musculoskelet Radiol. 2019;23:304–311. doi: 10.1055/s-0039-1684024. [DOI] [PubMed] [Google Scholar]
- 16.Rouzrokh P, Wyles CC, Philbrick KA, Ramazanian T, Weston AD, Cai JC, Taunton MJ, Lewallen DG, Berry DJ, Erickson BJ, Maradit Kremers H. A Deep Learning Tool for Automated Radiographic Measurement of Acetabular Component Inclination and Version After Total Hip Arthroplasty. J Arthroplasty. 2021;36:2510–2517.e6. doi: 10.1016/j.arth.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei J, Li D, Sing DC, Beeram I, Puvanesarajah V, Tornetta P 3rd, Fritz J, Yi PH. Detecting upper extremity native joint dislocations using deep learning: A multicenter study. Clin Imaging. 2022;92:38–43. doi: 10.1016/j.clinimag.2022.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Wei J, Li D, Sing DC, Beeram I, Puvanesarajah V, Tornetta P 3rd, Fritz J, Yi PH. Detecting upper extremity native joint dislocations using deep learning: A multicenter study. Clin Imaging. 2022;92:38–43. doi: 10.1016/j.clinimag.2022.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Chea P, Mandell JC. Current applications and future directions of deep learning in musculoskeletal radiology. Skeletal Radiol. 2020;49:183–197. doi: 10.1007/s00256-019-03284-z. [DOI] [PubMed] [Google Scholar]
- 20.Ozkaya E, Topal FE, Bulut T, Gursoy M, Ozuysal M, Karakaya Z. Evaluation of an artificial intelligence system for diagnosing scaphoid fracture on direct radiography. Eur J Trauma Emerg Surg. 2022;48:585–592. doi: 10.1007/s00068-020-01468-0. [DOI] [PubMed] [Google Scholar]
- 21.Oka K, Shiode R, Yoshii Y, Tanaka H, Iwahashi T, Murase T. Artificial intelligence to diagnosis distal radius fracture using biplane plain X-rays. J Orthop Surg Res. 2021;16:694. doi: 10.1186/s13018-021-02845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu PR, Zhang JY, Xue MD, Duan YY, Hu JL, Liu SX, Xie Y, Wang HL, Wang JW, Huo TT, Ye ZW. Artificial Intelligence to Diagnose Tibial Plateau Fractures: An Intelligent Assistant for Orthopedic Physicians. Curr Med Sci. 2021;41:1158–1164. doi: 10.1007/s11596-021-2501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Small JE, Osler P, Paul AB, Kunst M. CT Cervical Spine Fracture Detection Using a Convolutional Neural Network. AJNR Am J Neuroradiol. 2021;42:1341–1347. doi: 10.3174/ajnr.A7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murata K, Endo K, Aihara T, Suzuki H, Sawaji Y, Matsuoka Y, Nishimura H, Takamatsu T, Konishi T, Maekawa A, Yamauchi H, Kanazawa K, Endo H, Tsuji H, Inoue S, Fukushima N, Kikuchi H, Sato H, Yamamoto K. Artificial intelligence for the detection of vertebral fractures on plain spinal radiography. Sci Rep. 2020;10:20031. doi: 10.1038/s41598-020-76866-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mutasa S, Varada S, Goel A, Wong TT, Rasiej MJ. Advanced Deep Learning Techniques Applied to Automated Femoral Neck Fracture Detection and Classification. J Digit Imaging. 2020;33:1209–1217. doi: 10.1007/s10278-020-00364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garden RS. Low-angle fixation in fractures of the femoral neck. J Bone Joint Surg Br. 1961;43-B:647–663. [Google Scholar]
- 27.Bae J, Yu S, Oh J, Kim TH, Chung JH, Byun H, Yoon MS, Ahn C, Lee DK. External Validation of Deep Learning Algorithm for Detecting and Visualizing Femoral Neck Fracture Including Displaced and Non-displaced Fracture on Plain X-ray. J Digit Imaging. 2021;34:1099–1109. doi: 10.1007/s10278-021-00499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones RM, Sharma A, Hotchkiss R, Sperling JW, Hamburger J, Ledig C, O'Toole R, Gardner M, Venkatesh S, Roberts MM, Sauvestre R, Shatkhin M, Gupta A, Chopra S, Kumaravel M, Daluiski A, Plogger W, Nascone J, Potter HG, Lindsey RV. Assessment of a deep-learning system for fracture detection in musculoskeletal radiographs. NPJ Digit Med. 2020;3:144. doi: 10.1038/s41746-020-00352-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guermazi A, Tannoury C, Kompel AJ, Murakami AM, Ducarouge A, Gillibert A, Li X, Tournier A, Lahoud Y, Jarraya M, Lacave E, Rahimi H, Pourchot A, Parisien RL, Merritt AC, Comeau D, Regnard NE, Hayashi D. Improving Radiographic Fracture Recognition Performance and Efficiency Using Artificial Intelligence. Radiology. 2022;302:627–636. doi: 10.1148/radiol.210937. [DOI] [PubMed] [Google Scholar]
- 30.Inoue T, Maki S, Furuya T, Mikami Y, Mizutani M, Takada I, Okimatsu S, Yunde A, Miura M, Shiratani Y, Nagashima Y, Maruyama J, Shiga Y, Inage K, Orita S, Eguchi Y, Ohtori S. Automated fracture screening using an object detection algorithm on whole-body trauma computed tomography. Sci Rep. 2022;12:16549. doi: 10.1038/s41598-022-20996-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett M, Hayes K, Kleczyk EJ, Mehta R. Similarities and Differences between Machine Learning and Traditional Advanced Statistical Modeling in Healthcare Analytics. arXiv preprint arXiv:220102469. 2022. [Google Scholar]
- 32.Hendrickx LAM, Sobol GL, Langerhuizen DWG, Bulstra AEJ, Hreha J, Sprague S, Sirkin MS, Ring D, Kerkhoffs GMMJ, Jaarsma RL, Doornberg JN Machine Learning Consortium. A Machine Learning Algorithm to Predict the Probability of (Occult) Posterior Malleolar Fractures Associated With Tibial Shaft Fractures to Guide "Malleolus First" Fixation. J Orthop Trauma. 2020;34:131–138. doi: 10.1097/BOT.0000000000001663. [DOI] [PubMed] [Google Scholar]
- 33.Luu BC, Wright AL, Haeberle HS, Karnuta JM, Schickendantz MS, Makhni EC, Nwachukwu BU, Williams RJ 3rd, Ramkumar PN. Machine Learning Outperforms Logistic Regression Analysis to Predict Next-Season NHL Player Injury: An Analysis of 2322 Players From 2007 to 2017. Orthop J Sports Med. 2020;8:2325967120953404. doi: 10.1177/2325967120953404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karnuta JM, Luu BC, Haeberle HS, Saluan PM, Frangiamore SJ, Stearns KL, Farrow LD, Nwachukwu BU, Verma NN, Makhni EC, Schickendantz MS, Ramkumar PN. Machine Learning Outperforms Regression Analysis to Predict Next-Season Major League Baseball Player Injuries: Epidemiology and Validation of 13,982 Player-Years From Performance and Injury Profile Trends, 2000-2017. Orthop J Sports Med. 2020;8:2325967120963046. doi: 10.1177/2325967120963046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diniz P, Abreu M, Lacerda D, Martins A, Pereira H, Ferreira FC, Kerkhoffs GM, Fred A. Pre-injury performance is most important for predicting the level of match participation after Achilles tendon ruptures in elite soccer players: a study using a machine learning classifier. Knee Surg Sports Traumatol Arthrosc. 2022;30:4225–4237. doi: 10.1007/s00167-022-07082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson AB, Grazal CF, Balazs GC, Potter BK, Dickens JF, Forsberg JA. Can Predictive Modeling Tools Identify Patients at High Risk of Prolonged Opioid Use After ACL Reconstruction? Clin Orthop Relat Res. 2020;478:0–1618. doi: 10.1097/CORR.0000000000001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mamlook REA, Wells LJ, Sawyer R. Machine-learning models for predicting surgical site infections using patient pre-operative risk and surgical procedure factors. Am J Infect Control. 2023;51:544–550. doi: 10.1016/j.ajic.2022.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Mamlook REA, Wells LJ, Sawyer R. Machine-learning models for predicting surgical site infections using patient pre-operative risk and surgical procedure factors. Am J Infect Control. 2023;51:544–550. doi: 10.1016/j.ajic.2022.08.013. [DOI] [PubMed] [Google Scholar]
- 39.Merrill RK, Ferrandino RM, Hoffman R, Shaffer GW, Ndu A. Machine Learning Accurately Predicts Short-Term Outcomes Following Open Reduction and Internal Fixation of Ankle Fractures. J Foot Ankle Surg. 2019;58:410–416. doi: 10.1053/j.jfas.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Martin RK, Wastvedt S, Pareek A, Persson A, Visnes H, Fenstad AM, Moatshe G, Wolfson J, Engebretsen L. Predicting Anterior Cruciate Ligament Reconstruction Revision: A Machine Learning Analysis Utilizing the Norwegian Knee Ligament Register. J Bone Joint Surg Am. 2022;104:145–153. doi: 10.2106/JBJS.21.00113. [DOI] [PubMed] [Google Scholar]
- 41.Martin RK, Wastvedt S, Pareek A, Persson A, Visnes H, Fenstad AM, Moatshe G, Wolfson J, Lind M, Engebretsen L. Machine learning algorithm to predict anterior cruciate ligament revision demonstrates external validity. Knee Surg Sports Traumatol Arthrosc. 2022;30:368–375. doi: 10.1007/s00167-021-06828-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Huang L, Liu Y, Chen Q, Li X, Hu J. Prediction of mortality at one year after surgery for pertrochanteric fracture in the elderly via a Bayesian belief network. Injury. 2020;51:407–413. doi: 10.1016/j.injury.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 43.Cao Y, Forssten MP, Mohammad Ismail A, Borg T, Ioannidis I, Montgomery S, Mohseni S. Predictive Values of Preoperative Characteristics for 30-Day Mortality in Traumatic Hip Fracture Patients. J Pers Med. 2021;11 doi: 10.3390/jpm11050353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forssten MP, Bass GA, Ismail AM, Mohseni S, Cao Y. Predicting 1-Year Mortality after Hip Fracture Surgery: An Evaluation of Multiple Machine Learning Approaches. J Pers Med. 2021;11 doi: 10.3390/jpm11080727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu M, Chen W, Hirdes JP, Stolee P. The K-nearest neighbor algorithm predicted rehabilitation potential better than current Clinical Assessment Protocol. J Clin Epidemiol. 2007;60:1015–1021. doi: 10.1016/j.jclinepi.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Tschuggnall M, Grote V, Pirchl M, Holzner B, Rumpold G, Fischer MJ. Machine learning approaches to predict rehabilitation success based on clinical and patient-reported outcome measures. Inform Med Unlocked. 2021;24:100598. [Google Scholar]
- 47.Oosterhoff JHF, Gravesteijn BY, Karhade AV, Jaarsma RL, Kerkhoffs GMMJ, Ring D, Schwab JH, Steyerberg EW, Doornberg JN the Machine Learning Consortium. Feasibility of Machine Learning and Logistic Regression Algorithms to Predict Outcome in Orthopaedic Trauma Surgery. J Bone Joint Surg Am. 2022;104:544–551. doi: 10.2106/JBJS.21.00341. [DOI] [PubMed] [Google Scholar]
- 48.Ramkumar PN, Luu BC, Haeberle HS, Karnuta JM, Nwachukwu BU, Williams RJ. Sports Medicine and Artificial Intelligence: A Primer. Am J Sports Med. 2022;50:1166–1174. doi: 10.1177/03635465211008648. [DOI] [PubMed] [Google Scholar]
- 49.De Simone B, Abu-Zidan FM, Gumbs AA, Chouillard E, Di Saverio S, Sartelli M, Coccolini F, Ansaloni L, Collins T, Kluger Y, Moore EE, Litvin A, Leppaniemi A, Mascagni P, Milone L, Piccoli M, Abu-Hilal M, Sugrue M, Biffl WL, Catena F. Knowledge, attitude, and practice of artificial intelligence in emergency and trauma surgery, the ARIES project: an international web-based survey. World J Emerg Surg. 2022;17:10. doi: 10.1186/s13017-022-00413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kahn CE Jr. Artificial intelligence in radiology: decision support systems. Radiographics. 1994;14:849–861. doi: 10.1148/radiographics.14.4.7938772. [DOI] [PubMed] [Google Scholar]
- 51.Recht M, Bryan RN. Artificial Intelligence: Threat or Boon to Radiologists? J Am Coll Radiol. 2017;14:1476–1480. doi: 10.1016/j.jacr.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 52.El Hechi M, Gebran A, Bouardi HT, Maurer LR, El Moheb M, Zhuo D, Dunn J, Bertsimas D, Velmahos GC, Kaafarani HMA. Validation of the artificial intelligence-based trauma outcomes predictor (TOP) in patients 65 years and older. Surgery. 2022;171:1687–1694. doi: 10.1016/j.surg.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]