Abstract
The synthesis of a range of loline alkaloids is reported. The C(7) and C(7a) stereogenic centers for the targets were formed by the established conjugate addition of lithium (S)-N-benzyl-N-(α-methylbenzyl)amide to tert-butyl 5-benzyloxypent-2-enoate, ensuing enolate oxidation to give an α-hydroxy-β-amino ester, and then formal exchange of the resultant amino and hydroxyl functionalities (via the intermediacy of the corresponding aziridinium ion) to give an α-amino-β-hydroxy ester. Subsequent transformation gave a 3-hydroxyprolinal derivative which was converted to the corresponding N-tert-butylsulfinylimine. Mannich-type reaction with the enolate derived from O-Boc protected methyl glycolate then formed the remaining C(1) and C(2) stereogenic centers for the targets. The 2,7-ether bridge was formed by a displacement reaction, completing construction of the loline alkaloid core. Facile manipulations then gave a range of loline alkaloids, including loline itself.
Introduction
1-Aminopyrrolizidine alkaloids—e.g., absoluline1 (Figure 1)—are a small but important family, all of which display interesting biological activity. Meanwhile, the synthetic analogue SC-531162 (Figure 1) has been established as a potent and selective antagonist for 5-HT4 serotonin receptors. The 1-aminopyrrolizidine core is also present within the structures of the insecticidal loline alkaloids3—e.g., loline4 (Figure 1), the titular compound—which possess additional molecular complexity due to the presence of an ether bridge between C(2) and C(7) of the pyrrolizidine ring.
Figure 1.
Representative 1-aminopyrrolizidine alkaloids and synthetic analogues. Ar1 = 4-methoxyphenyl. Ar2 = 2-methoxy-4-amino-5-chlorophenyl. *The question of configuration at the stereogenic center within the s-Bu side chain of laburnamine remains unaddressed.
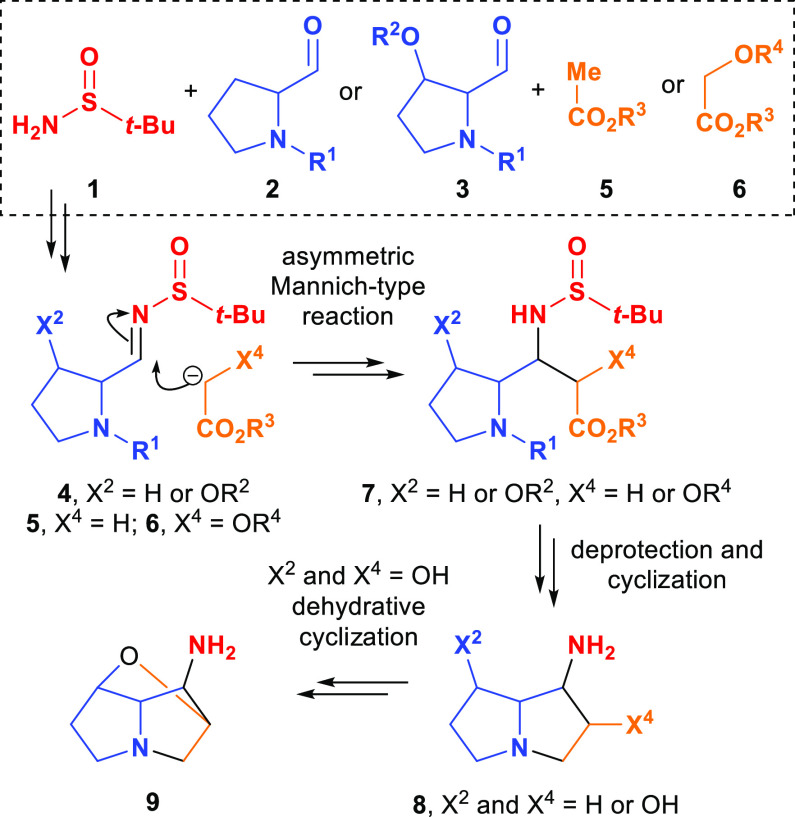
No doubt as a result of their interesting structures and associated biological activities, there has been interest in the development of syntheses of compounds containing the 1-aminopyrrolizidine moiety, with absouline in particular proving a relatively popular target.5 Investigations directed toward the synthesis of the loline alkaloids are in contrast relatively limited,6 especially considering that they have been known for well over a century. The apparently simple structures of these alkaloids belie the relative challenge in their synthesis, which has often necessitated a high step count (particularly when the targets are required in an enantiomerically pure form) and in some cases has precluded synthetic endeavors.7 We envisaged the development of a flexible route to enable access to any of the members of the 1-aminopyrrolizidine alkaloid family (absouline-type or loline-type) and their analogues, including non-natural polyhydroxylated derivatives, based upon applications of an asymmetric Mannich-type reaction of an N-tert-butylsulfinylimine 4 with the enolate of either an acetate ester 5(8) or an O-protected glycolate ester 6(9) (as required).10 The requisite N-tert-butylsulfinylimines 4 would be derived from the condensation of either enantiomer of N-tert-butylsulfinylamide 1 with a suitably protected prolinal derivative 2 or 3-hydroxyprolinal derivative 3.11 The former, 2, would be readily available from either enantiomer of proline itself, whilst the latter, 3, would be available in either enantiomeric form via adaptation of our synthesis of the stereoisomers of 3-hydroxyproline.12 Condensation of either enantiomer of N-tert-butylsulfinylamide 1 with a single enantiomer of the chiral aldehydes 2 and 3 would lead to epimeric (at sulfur) N-tert-butylsulfinylimines 4. Literature precedent suggests that the stereochemical outcome of the Mannich-type reaction of such epimeric N-tert-butylsulfinylimines can be predominantly dictated by the configuration of the stereogenic center at the sulfur atom in both epimers, and so the reaction of both was anticipated to give rise to synthetically useful levels of diastereoselectivity.13,14 Thus, a range of stereoisomeric forms of the adducts 7 should be available via this approach. It was then proposed that these adducts 7 could undergo a deprotection and cyclization sequence of reactions to give a range of 1-aminopyrrolizidines 8, encompassing the core of the absouline-type alkaloids (X2 = X4 = H) and various non-natural hydroxylated analogues thereof (X2 and/or X4 = OH). In the presence of an appropriate stereochemical disposition of hydroxy substituents (X2 and X4 = OH), a formal dehydrative cyclization may provide 9,15 representing the core of the loline alkaloids (Figure 2). Herein, we describe our initial studies in this area that culminate in the synthesis of several members of the loline alkaloid family, including loline itself.
Figure 2.
Proposed key sequence in the synthesis of 1-aminopyrrolizidine alkaloids (absouline-type or loline-type, and non-natural hydroxylated analogues).
Results and Discussion
In order to prepare the requisite N-tert-butylsulfinylimine required for the proposed asymmetric Mannich-type reaction toward the loline alkaloid core, the protected 3-hydroxyprolinate derivative 16 was first prepared in an analogous fashion to that previously described for the enantiomer,12 in five steps from α,β-unsaturated ester 10. Briefly, the conjugate addition of lithium (S)-N-benzyl-N-(α-methylbenzyl)amide to 10 followed by in situ enolate oxidation with (+)-10-camphorsulfonyloxaziridine (CSO) gave α-hydroxy-β-amino ester 11 in 62% yield. Subsequent (formal) exchange of the amino and hydroxy functionalities via the intermediacy of the corresponding aziridinium ion 12 furnished α-amino-β-hydroxy ester 13 in 80% combined yield after three iterations of the reaction.16 Hydrogenolysis of 13 with in situ N-Boc protection gave 14 in quantitative yield, with 14 then undergoing chemoselective activation of the terminal hydroxy group upon treatment with 2-naphthalenesulfonyl chloride, giving 15 in 82% yield. Base-induced cyclization of 15 provided the 3-hydroxyprolinate derivative 16 in 78% yield. The identity, including relative configuration, of 16 was unambiguously established by single-crystal X-ray diffraction analysis (Figure S1, Supporting Information). The Flack x parameter17 for the crystal structure was −0.009(8), which is therefore also consistent with the assigned absolute (R,R)-configuration. A series of N- and O-protecting group manipulations of 16 was next performed, giving 18 (proceeding via 17) in 94% yield over three steps. The N-allyl and O-triisopropylsilyl protecting group combination was chosen in order to avoid ensuing chemoselectivity issues: a series of selective N- and O-deprotections would subsequently be required to enable the requisite cyclization reactions in the construction of the loline alkaloid framework. Conversion of 18 to the requisite N-tert-butylsulfinylimine was planned by initial formation of the corresponding aldehyde 19 followed by condensation with the requisite enantiomer of tert-butylsulfinamide 1. Previous reports concerning the asymmetric Mannich-type reaction of glycolate derivatives9,14,18 suggested that N-tert-butylsulfinylimine (SS)-21 [derived from (S)-tert-butylsulfinamide 1] would be required to give the correct C(1)–C(7a) relative configuration (pyrrolizidine numbering) required for the loline alkaloids. It was, however, of more broad interest to perform the condensation of both enantiomers of 1 with aldehyde 19 and thus, under standard conditions for this conversion, tert-butylsulfinimines (RS)-20 and (SS)-21 were formed as single diastereoisomers (epimeric at the sulfur atom) which were isolated in 74 and 70% yields (from 18), respectively. The configuration of the newly formed double bond within (RS)-20 and (SS)-21 was assigned as (E) in both cases on the basis of the well-established outcome of this condensation process10 (Scheme 1).
Scheme 1. Preparation of N-tert-Butylsulfinylimines (RS)-20 and (SS)-21a.
DTBMP = 2,6-di-tert-butyl-4-methylphenol. Ar = 2-naphthyl.
Combined yield after three iterations of the reaction.
With the requisite N-tert-butylsulfinylimines (RS)-20 and (SS)-21 in hand, the key asymmetric Mannich-type reaction was investigated. In all of the previously reported application of this reaction,9,14,18 the optimal conditions have been found to involve deprotonation of an O-Boc protected glycolate ester (5 equiv) with LiHMDS or LDA (5 equiv), followed by addition to the requisite N-tert-butylsulfinylimine (1 equiv). Following this precedent, deprotonation of O-Boc methyl glycolate 22 with LiHMDS and subsequent reaction with N-tert-butylsulfinylimine (RS)-20 delivered the adduct 25 as a single diastereoisomer (>95:5 dr) that was isolated in 83% yield (Scheme 2). In contrast, addition to (SS)-21 under the same conditions gave a 50:50 mixture of diastereoisomeric adducts 26 and 27, which proved inseparable upon chromatography and were thus isolated in 82% combined yield (Scheme 2). The absolute configurations within 25–27 were all unambiguously assigned by a combination of single-crystal X-ray diffraction analyses and 1H-1H NMR NOE analyses following their conversion to cyclized derivatives (see the Supporting Information for full details). In an effort to increase the diastereoselectivity of the asymmetric Mannich-type reaction, the use of NaHMDS (which has not been reported as a base to promote this reaction prior to this study) as an alternative base was considered. In the event, deprotonation of O-Boc methyl glycolate 22 with NaHMDS and addition to N-tert-butylsulfinylimine (SS)-21 produced the adduct 26 as a single diastereoisomer (>95:5 dr) that was isolated in 76% yield (Scheme 2). The stereochemical outcomes of these asymmetric Mannich-type reactions [of N-tert-butylsulfinylimine (RS)-20 with O-Boc methyl glycolate 22 promoted by LiHMDS and N-tert-butylsulfinylimine (SS)-21 with O-Boc methyl glycolate 22 promoted by NaHMDS] are thus in complete accordance with the established diastereoselectivity of this reaction9,14,18 and indicate, in each case, that the reaction is under the dominant stereocontrol of the N-tert-butylsulfinyl substituent, also consistent with previous studies.14
Scheme 2. Asymmetric Mannich-type Reactions of N-tert-Butylsulfinylimines (RS)-20 and (SS)-21.
The diastereoselectivity of this asymmetric Mannich-type reaction may be influenced by both the diastereoselectivity of the enolization of O-Boc methyl glycolate 22(19) and the diastereofacialselectivity of addition of the intermediate (Z)-enolate 23(20) and/or the (E)-enolate 24(20) to the N-tert-butylsulfinylimines (RS)-20 and (SS)-21. However, none of the previous reports concerning this reaction9,14,18 have provided insight into the diastereoselectivity of the requisite enolization process of O-Boc methyl glycolate, although several transition state models assuming addition of the corresponding (E)-enolate20 to the requisite N-tert-butylsulfinylimine(s) have been proposed. As O-silylation of the intermediate enolates has proven to be a valuable tool to interrogate the ratio of enolates formed under a range of conditions,21 this approach was used to investigate the diastereoselectivity of the enolization of O-Boc methyl glycolate 22 under the conditions employed herein. When O-Boc methyl glycolate 22 was subjected to deprotonation with LiHMDS followed by the addition of TMSCl,19 a 90:10 mixture of silylenolethers corresponding to trapping of the (Z)-enolate 23(20) and (E)-enolate 24,20 respectively, was observed. In contrast, enolization of O-Boc methyl glycolate 22 with NaHMDS followed by treatment with TMSCl under the same conditions19 implied that a 75:25 mixture of (Z)-enolate 23(20) and (E)-enolate 24,20 respectively, was present. Given these results, a simple mechanism involving irreversible enolization of O-Boc methyl glycolate 22 to give the (E)-enolate 24 followed by irreversible addition to the requisite N-tert-butylsulfinylimine is unable to account for the often very high (>95:5 dr) diastereoselectivities of this reaction class, as exemplified by the reaction of O-Boc methyl glycolate 22 with N-tert-butylsulfinylimine (RS)-20 promoted by LiHMDS. On the contrary, a mechanism involving preferential kinetic (irreversible) formation of the (Z)-enolate 23(19) followed by its addition to the requisite N-tert-butylsulfinylimine can rationalize the observed outcome, as can a mechanism involving interconversion of the (Z)-enolate 23(19) and the (E)-enolate 24(19) under the reaction conditions followed by the addition of either or both of the enolates to the requisite N-tert-butylsulfinylimine (Curtin–Hammett control); the latter may be of particular relevance in the case of enolization with NaHMDS.21 Further investigation is therefore required to ascertain the precise mechanistic origin of the observed diastereoselectivity in these reactions although, in any case, the observation that the use of NaHMDS offers improved overall diastereoselectivity is noteworthy and may prove a valuable insight for future applications of this reaction.
With the required adduct 26 available as a single diastereoisomer, elaboration to the loline alkaloids was investigated (Scheme 3). Treatment of 26 with Pd(PPh3)4 and 1,3-dimethylbarbituric acid (DMBA)22 gave pyrrolizidinone 28 in quantitative yield. O-Desilylation of 28 using TBAF gave pyrrolizidinone 29 in 96% yield. The identity (including absolute configuration) of 29 was unambiguously confirmed by single-crystal X-ray diffraction analysis (Figure S2, Supporting Information). Subsequent reduction of 29 with Me2S·BH3 complex gave the borane adduct 30·BH3 in 51% isolated yield after chromatography, which was unambiguously identified by single-crystal X-ray diffraction analysis (Figure S3, Supporting Information). This was quantitatively decomplexed by refluxing in MeOH to give the free pyrrolizidine 30. However, 30 was obtained in 79% yield from 29 when the borane adduct 30·BH3 was refluxed in MeOH without prior purification. The free pyrrolizidine 30 also proved amenable to analysis by single-crystal X-ray diffraction, providing further confirmation of both structure and absolute configuration (Figure S4, Supporting Information). The ether bridge was formed upon initial reaction of 30 with Ms2O and Et3N to give mesylate 31, followed by immediate treatment with K2CO3 in MeOH at 70 °C, which resulted in the formation of N-tert-butylsulfinyl loline 32 in 67% yield. The presence of the ether bridge within 32 was evidenced by a strong correlation between C(2)H and C(7), and reciprocally between C(7)H and C(2) in its 1H-13C HMBC NMR spectrum. N-tert-Butylsulfinyl loline 32 decomposed to a complex mixture of unidentifiable products upon standing at rt. overnight, although its immediate treatment (without purification) with HCl in MeOH at 70 °C resulted in the removal of the N-tert-butylsulfinyl group to give norloline dihydrochloride 33·2HCl in 85% yield from 30. The identity (including absolute configuration) of norloline dihydrochloride 33·2HCl was confirmed by single-crystal X-ray diffraction analysis (Figure S5, Supporting Information). Elaboration of 33·2HCl to a range of the loline alkaloids was readily achieved using well-established procedures. Acetylation of 33·2HCl gave N-acetyl loline 34 in 67% yield, whilst treatment of 33·2HCl with 37% aqueous formaldehyde solution (formalin) and NaBH3CN in MeCN gave N-methyl loline 35, which was isolated as the dihydrochloride 35·2HCl in 66% yield. Mono-N-methylation of 33·2HCl was achieved upon its initial treatment with Boc2O to give N-Boc norloline 36 in 33% yield, followed by subjecting to LiAlH4 to give loline 37, which was isolated as the dihydrochloride 37·2HCl in 35% yield. The alkaloid free bases norloline 33, N-methyl loline 35, and loline 37 were generated in situ in CDCl3 (in order to avoid previously reported handling issues)23 upon neutralization of the corresponding dihydrochloride salts with NaOH. The resultant solutions were then immediately subjected to NMR spectroscopic analysis. The 1H and 13C NMR data for norloline dihydrochloride 33·2HCl, norline free base 33, N-acetyl loline 34, N-methyl loline dihydrochloride 35·2HCl, N-methyl loline free base 35, loline dihydrochloride 37·2HCl, and loline free base 37 showed excellent agreement with those previously reported.24
Scheme 3. Preparation of Loline Alkaloids.
Conclusions
In conclusion, the synthesis of a range of loline alkaloids has been delineated. The pivotal reaction in the synthesis involves an asymmetric Mannich-type reaction of the enolate of O-Boc-protected methyl glycolate with an N-tert-butylsulfinylimine derived from the condensation of enantiopure tert-butylsulfinamide with a 3-hydroxyprolinal derivative. The synthesis should prove to be flexible for the preparation of a range of other compounds containing the 1-aminopyrrolizidine core, such as absouline-type alkaloids and non-natural hydroxylated analogues, simply by varying reaction partners in the asymmetric Mannich-type reaction.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.3c00047.
Experimental details; characterization data; and copies of 1H and 13C NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ikhiri K.; Ahond A.; Poupat C.; Potier P.; Pusset J.; Sévenet T. Plantes de Nouvelle Calédonie, 109. Absouline, Alcaloïde Pyrrolizidinique Nouveau Isole de Hugonia oreogena et Hugonia penicillanthemum. J. Nat. Prod. 1987, 50, 626. 10.1021/np50052a008. [DOI] [Google Scholar]
- Flynn D. L.; Zabrowski D. L.; Becker D. P.; Nosal R.; Villamil C. I.; Gullikson G. W.; Moummi C.; Yang D.-C. SC-53116: The First Selective Agonist at the Newly Identified Serotonin 5-HT4 Receptor Subtype. J. Med. Chem. 1992, 35, 1486. 10.1021/jm00086a019. [DOI] [PubMed] [Google Scholar]
- For a review of the isolation, structural elucidation and synthesis of the loline alkaloids, see:; Schard C. L.; Grossman R. B.; Nagabhyru P.; Faulkner J. P.; Mallik U. P. Loline alkaloids: Currencies of Mutualism. Phytochemistry 2007, 68, 980–996. 10.1016/j.phytochem.2007.01.010. [DOI] [PubMed] [Google Scholar]
- The isolation and structural elucidation of loline (temuline) presents a somewhat involved story. See ref (3) for further details
- For selected previous syntheses of absouline (most recent first), see:; a Ye J.-L.; Chen H.; Zhang Y.-F.; Huang P.-Q. A Versatile Access to Vicinal Diamine Motifs by Highly anti-Selective Asymmetric Vinylogous Mannich Reactions: an Efficient Total Synthesis of (+)-absouline. Org. Chem. Front. 2016, 3, 683. 10.1039/C6QO00022C. [DOI] [Google Scholar]; b Davies S. G.; Fletcher A. M.; Lebee C.; Roberts P. M.; Thomson J. E.; Yin J. Asymmetric Synthesis of (−)-(1R,7aS)-Absouline. Tetrahedron 2013, 69, 1369–1377. 10.1016/j.tet.2012.11.080. [DOI] [Google Scholar]; c Eklund E. J.; Pike R. D.; Scheerer J. R. Synthesis of 1-Aminopyrrolizidine Alkaloid (−)-Absouline by Stereoselective Aminoconjugate Addition. Tetrahedron Lett. 2012, 53, 4644. 10.1016/j.tetlet.2012.06.028. [DOI] [Google Scholar]
- For previous syntheses of members of the loline alkaloid family (most recent first), see:; a Ye J.-L.; Liu Y.; Yang Z.-P.; Huang P.-Q. The Asymmetric Total Synthesis of (+)-N-Acetyl Norloline. Chem. Commun. 2016, 52, 561. 10.1039/C5CC07480K. [DOI] [PubMed] [Google Scholar]; b Miller K. E.; Wright A. J.; Olesen M. K.; Hovey M. T.; Scheerer J. R. Stereoselective Synthesis of (+)-Loline Alkaloid Skeleton. J. Org. Chem. 2015, 80, 1569. 10.1021/jo502493e. [DOI] [PubMed] [Google Scholar]; c Cakmak M.; Mayer P.; Trauner D. An Efficient Synthesis of Loline Alkaloids. Nat. Chem. 2011, 3, 543. 10.1038/nchem.1072. [DOI] [PubMed] [Google Scholar]; d Hovey M. T.; Eklund E. J.; Pike R. D.; Mainkar A. A.; Scheerer J. R. Synthesis of (±)-Acetylnorloline via Stereoselective Tethered Aminohydroxylation. Org. Lett. 2011, 13, 1246. 10.1021/ol200155p. [DOI] [PubMed] [Google Scholar]; e Blakemore P. R.; Kim S.-K.; Schulze V. K.; White J. D.; Yokochi A. F. T. Asymmetric Synthesis of (+)-Loline, a Pyrrolizidine Alkaloid from Rye Grass and Tall Fescue. J. Chem. Soc., Perkin Trans. 1 2001, 1831. 10.1039/b103936a. [DOI] [Google Scholar]; f Blakemore P. R.; Schulze V. K.; White J. D. Asymmetric Synthesis of (+)-Loline. Chem. Commun. 2000, 1263, 1263. 10.1039/b003121f. [DOI] [Google Scholar]; g Tufariello J. J.; Meckler H.; Winzenberg K. Synthesis of the Lolium Alkaloids. J. Org. Chem. 1986, 51, 3557–3557. 10.1021/jo00368a035. [DOI] [Google Scholar]
- a Ye J.-L.; Liu Y.; Zhang Y.-F.; Yang P.-Z.; Huang P.-Q. Studies on the Second-Generation Approach to Loline Alkaloids: Synthesis of N-Bus-norloline through N-tert-Butanesulfinyl Imine Based Asymmetric Vinylogous Mannich Reaction. Synthesis 2016, 48, 1684–1692. 10.1055/s-0035-1561432. [DOI] [Google Scholar]; b Wilson S. R.; Sawicki R. A.; Huffman J. C. Synthetic and Structural Studies of the Lolium Alkaloids. J. Org. Chem. 1981, 46, 3887. 10.1021/jo00332a025. [DOI] [Google Scholar]
- Tang T. P.; Ellman J. A. The tert-Butanesulfinyl Group: An Ideal Chiral Directing Group and Boc-Surrogate for the Asymmetric Synthesis and Applications of β-Amino Acids. J. Org. Chem. 1999, 64, 12–13. 10.1021/jo9820824. [DOI] [PubMed] [Google Scholar]
- Wang Y.; He Q.-F.; Wang H.-W.; Zhou X.; Huang Z.-Y.; Qin Y. Highly Diastereoselective Enolate Addition of O-Protected α-Hydroxyacetate to (SR)-tert-Butanesulfinylimines: Synthesis of Taxol Side Chain. J. Org. Chem. 2006, 71, 1588–1591. 10.1021/jo052298n. [DOI] [PubMed] [Google Scholar]
- For reviews concerning the use of N-tert-butylsulfinylamide in synthesis (including in Mannich-type reactions), see:; a Ellman J. A. Applications of tert-Butanesulfinamide in the Asymmetric Synthesis of Amines. Pure Appl. Chem. 2003, 75, 39. 10.1351/pac200375010039. [DOI] [Google Scholar]; b Robak M. T.; Herbage M. A.; Ellman J. A. Synthesis and Applications of tert-Butanesulfinamide. Chem. Rev. 2010, 110, 3600. 10.1021/cr900382t. [DOI] [PubMed] [Google Scholar]
- For example, see:; a Prakash G. K. S.; Mandal M. Stereoselective Synthesis of Trifluoromethylated Vicinal Ethylenediamines with α-Amino N-tert-Butanesulfinimines and TMSCF3. J. Am. Chem. Soc. 2002, 124, 6538–6539. 10.1021/ja020482+. [DOI] [PubMed] [Google Scholar]; b Morriello G. J.; Mills S. G.; Johnson T.; Reibarkh M.; Chicchi G.; De Martino J.; Kurtz M.; Davies P.; Tsao K. L. C.; Zheng S.; Tong X.; Carlson E.; Townson K.; Tattersall F. D.; Wheeldon A.; Boyce S.; Collinson N.; Rupniak N.; Moore S.; De Vita R. J. Substituted Fused Bicyclic Pyrrolizinones as Potent, Orally Bioavailable hNK1 Antagonists. Bioorg. Med. Chem. Lett. 2010, 20, 2007. 10.1016/j.bmcl.2010.01.065. [DOI] [PubMed] [Google Scholar]; c Song X.-N.; Yao Z.-J. Short Asymmetric Synthesis of (S,S)-PDP Using L-Prolinol Derivative as Economic Starting Material. Tetrahedron 2010, 66, 2589. 10.1016/j.tet.2010.02.048. [DOI] [Google Scholar]
- Davies S. G.; Fletcher A. M.; Linsdall S. M.; Roberts P. M.; Thomson J. E. Asymmetric Syntheses of (2R,3S)-3-Hydroxyproline and (2S,3S)-3-Hydroxyproline. Org. Lett. 2018, 20, 4135. 10.1021/acs.orglett.8b01736. [DOI] [PubMed] [Google Scholar]
- Evans J. W.; Ellman J. A. Stereoselective Synthesis of 1,2-Disubstituted β-Amino Alcohols by Nucleophilic Addition to N-tert-Butanesulfinyl α-Alkoxyaldimines. J. Org. Chem. 2003, 68, 9948–9957. 10.1021/jo035224p. [DOI] [PubMed] [Google Scholar]
- Hjelmgaard T.; Faure S.; Lemoine P.; Viossat B.; Aitken D. J. Rapid Assembly of the Polyhydroxylated β-Amino Acid Constituents of Microsclerodermins C, D, and E. Org. Lett. 2008, 10, 841. 10.1021/ol702962z. [DOI] [PubMed] [Google Scholar]
- Similar cyclisation reactions have been successfully deployed to form the ether bridge in previously reported syntheses of the loline alkaloids. For example, see: ref (6c) and ref (6e)
- Ring-opening of the aziridinium ion 12 results in formation of an ∼55:45 mixture of 11 and 13 (see ref (12)). Initially, this gave 11 in 56% yield and 13 in 44% yield. Resubjection of the recovered 11 to the reaction conditions gave 11 in 55% yield and 13 in 44% yield. A further resubjection of the recovered 11 to the reaction conditions gave 11 in 54% yield and 13 in 39% yield. Thus, the combined overall yield of 13 over the three runs was 80%
- a Flack H. D. On Enantiomorph-Polarity Estimation. Acta Crystallogr., Sect. A: Found. Crystallogr. 1983, 39, 876. 10.1107/S0108767383001762. [DOI] [Google Scholar]; b Flack H. D.; Bernardinelli G. Reporting and Evaluating Absolute-structure and Absolute-configuration Determinations. J. Appl. Crystallogr. 2000, 33, 1143. 10.1107/S0021889800007184. [DOI] [Google Scholar]
- a Ke B.; Qin Y.; Zhao F.; Qu Y. Synthesis and Biological Evaluation of Novel 3′-N-tert-Butylsulfonyl Analogues of Docetaxel. Bioorg. Med. Chem. Lett. 2008, 18, 4783. 10.1016/j.bmcl.2008.07.101. [DOI] [PubMed] [Google Scholar]; b Burnett C. M.; Williams R. M. Asymmetric Synthesis of the Core of AMPTD, the Key Amino Acid of Microsclerodermins F-I. Tetrahedron Lett. 2009, 50, 5449. 10.1016/j.tetlet.2009.06.144. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Okuyama K.; Momoi Y.; Sugimoto K.; Okano K.; Tokuyama H. Synthetic Studies toward Haouamine B: Construction of Indenotetrahydropyridone Skeleton. Synlett 2011, 73–76. 10.1055/s-0030-1259096. [DOI] [Google Scholar]; d Shevchuk M. V.; Kukhar V. P.; Röschenthaler G.-V.; Bassil B. S.; Kawada K.; Soloshonok V. A.; Sorochinsky A. E. New Asymmetric Approach to β-Trifluoromethyl Isoserines. RSC Adv. 2013, 3, 6479. 10.1039/c3ra40687c. [DOI] [Google Scholar]; e Jing Y.; Zhou W.; Li W.; Zhao L.; Wang Y. The Synthesis of Novel Taxoids for Oral Administration. Bioorg. Med. Chem. 2014, 22, 194. 10.1016/j.bmc.2013.11.037. [DOI] [PubMed] [Google Scholar]; f Callebaut G.; Colpaert F.; Nonn M.; Kiss L.; Sillanpä R.; Törnroos K. W.; Fülöp F.; De Kimpe N.; Mangelinckx S. Asymmetric Synthesis of Chloroisothreonine Derivatives via syn-Stereoselective Mannich-type Additions Across N-Sulfinyl-α-chloroimines. Org. Biomol. Chem. 2014, 12, 3393. 10.1039/C4OB00243A. [DOI] [PubMed] [Google Scholar]; g Momoi Y.; Okuyama K.; Toya H.; Sugimoto K.; Okano K.; Tokuyama H. Total Synthesis of (−)-Haouamine B Pentaacetate and Structural Revision of Haouamine B. Angew. Chem., Int. Ed. 2014, 53, 13215. 10.1002/anie.201407686. [DOI] [PubMed] [Google Scholar]
- See the Supporting Information for full experimental details of the enolate trapping studies.
- The (Z) and (E) descriptors for enolates are assigned herein by the generally accepted method, denoting the geometric relationship of the OMetal substituent at one end of the double bond to the higher priority (Cahn-Ingold-Prelog) OBoc substituent at the other. Note that this method for assignment of the (Z) and (E) stereochemical descriptors to enolates has not been universally adopted in the other reports concerning this reaction
- a Stork G.; Hudrlik P. F. Isolation of Ketone Enolates as Trialkylsilyl Ethers. J. Am. Chem. Soc. 1968, 90, 4462. 10.1021/ja01018a051. [DOI] [Google Scholar]; b Godenschwager P. F.; Collum D. B. Lithium Hexamethyldisilazide-Mediated Enolizations: Influence of Triethylamine on E/Z Selectivities and Enolate Reactivities. J. Am. Chem. Soc. 2008, 130, 8726. 10.1021/ja800250q. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Woltornist R. A.; Collum D. B. Ketone Enolization with Sodium Hexamethyldisilazide: Solvent- and Substrate-Dependent E–Z Selectivity and Affiliated Mechanisms. J. Am. Chem. Soc. 2021, 143, 17452. 10.1021/jacs.1c06529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro-Helion F.; Merzouk A.; Guibe F. Mild and Selective Palladium(0)-Catalyzed Deallylation of Allylic Amines. Allylamine and Diallylamine as Very Convenient Ammonia Equivalents for the Synthesis of Primary Amines. J. Org. Chem. 1993, 58, 6109–6113. 10.1021/jo00074a044. [DOI] [Google Scholar]
- Petroski R. J.; Yates S. G.; Weisleder D.; Powell R. G. Isolation, Semi-Synthesis, and NMR Spectral Studies of Loline Alkaloids. J. Nat. Prod. 1989, 52, 810. 10.1021/np50064a023. [DOI] [Google Scholar]
- A full data comparison is provided in the Supporting Information
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.