Abstract
Poly(pyrazolyl)borate ligands have been obtained through the reaction of highly reactive haloboranes with in situ formed pyrazolides under very mild conditions. This versatile synthetic method allows the selective synthesis of bis-, tris-, or tetrakis(pyrazolyl)borates. Furthermore, the method is compatible with the use of functional groups on the heterocyclic moieties of the poly(pyrazolyl)borates that were not accessible to date. Strongly encumbered sodium and thallium(I) poly(pyrazolyl)borates with a reduced donating ability have been obtained for the first time.
Introduction
Poly(pyrazolyl)borate ligands were described for the first time by Trofimenko during the 1960s.1 The first generation of these scorpionate ligands provided sandwich complexes of metals presenting octahedral coordination.2 The introduction of bulky substituents on position 3 of the heterocyclic rings was considered the second generation and allowed the isolation of metal complexes bearing only one ligand3 and therefore vacant coordination positions at the metal opening the door to develop catalytic processes. Since then, poly(pyrazolyl)borates of almost any transition metal have been prepared4 and used as enzymatic models,5 for the development of new materials,6 and as power catalysts in reactions as carbene or nitrene C–H insertion, polymerization or carbonyl derivatizations.7 The success of this family of ligands resides in the possibility of fine-tuning the electronic and steric properties of the metal complexes through the introduction of appropriate groups on the heterocyclic rings. However, despite the more than 4200 crystal structures of this type of complexes that have been described,8 the simultaneous pyrazole decoration with bulky and electron-withdrawing substituents has not been possible to date.
The most common route to prepare poly(pyrazolyl)borates is the reaction of a high excess of the desired pyrazole derivative with a metal borohydride in the absence of solvent (Scheme 1a).9 This transformation presents drawbacks: (i) difficult control of the reaction stoichiometry with possible formation of mixtures of dihydrobis(pyrazolyl)borates (Bpx), hydrotris(pyrazolyl)borates (Tpx) and tetrakis(pyrazolyl)borates (Tkpx), (ii) hazardous evolution of hydrogen gas under high temperature conditions,10 (iii) limited functional group scope due to their sensibility under reductant conditions and (iv) pyrazoles containing simultaneously electron-withdrawing and bulky substituents do not react under these conditions due to reduced nucleophilicity and higher steric hindrance. The access to Tpx ligands presenting these characteristics could widen the catalytic applications of their metal complexes due to the increased electrophilicity,11 easier reduction,12 and higher stability of low oxidation states13 of the corresponding metal centers.
Scheme 1. Previous Syntheses of Poly(pyrazolyl)Borate Ligands.
The third generation of tris(pyrazolyl)borates appeared with the substitution of the hydrogen atom with an alkyl or an aryl moiety. Alkyltris(pyrazolyl)borates were prepared from lithium alkylborohydride derivatives,14 very flammable reagents, in a reaction with the same disadvantages related before (Scheme 1b). In this case, addition of a Lewis acid allowed the use of milder conditions but did not avoid the use of hazardous alkylborohydride reagents.15 Aryldihaloboranes were used as an alternative to borohydride compounds for the preparation of aryltris(pyrazolyl)borates (Scheme 1c).16 Although milder reaction conditions were used, this procedure was limited by the poor yield achieved in most cases, the low availability of aryldichloroborane derivatives,17 and the lower stability of the ligands, associated with the lability of the B–N bonds increased by the introduction of an aryl moiety on the boron atom. Good yield was obtained for (Ipc)BCl2 with nonsubstituted sodium pyrazolide.17
Here we present a new practical and direct methodology for the preparation of poly(pyrazolyl)borate ligands under very mild conditions, with wide applicability that yields exclusively the desired poly(pyrazolyl)borate derivative in good to excellent yields (Scheme 2).
Scheme 2. New Route to Poly(pyrazoly)Borate Ligands.
Results and Discussion
Synthesis of Tris(pyrazolyl) Borate Ligands (3)
First, we focused on more extended thalium(I) tris(pyrazolyl)borates (3Tl), the usual entry for metal exchange.4a A dichloroborane dimethylsulfide complex (BHCl2·SMe2) was chosen as the highly reactive boron source and 3-tert-butylpyrazole (1a) as the standard azaheterocycle (Scheme 3). A base must be added to avoid reaction inhibition through protonation of the remaining pyrazole as the reaction proceeds (see SI). A smooth reaction takes place between in situ formed sodium pyrazolide and the chosen boron source with the formation of the sodium salt of the desired hydrotris(3-tert-butylpyrazol-1-yl)borate (3aNa) just using the ca. stoichiometric amounts of all reagents on toluene at room temperature for 24 h. Conditions optimization for tris(pyrazolyl)borate ligands synthesis has been performed and is specified in the Supporting Information (see SI Section 3). Thallium salt 3aTl was prepared in situ through standard procedures to facilitate product purification.14a
Scheme 3. Optimized Conditions for 3aTl.
These optimized conditions were used in the preparation of thallium hydrotris(pyrazolyl)borate complexes using the selection of pyrazole derivatives, as shown in Figure 1. Table 1 summarizes the results obtained for the preparation of TpxTl (3a–jTl) using the optimized reaction conditions. An excellent 90% yield was obtained for complex 3bTl bearing the encumbering tert-butyl group at position 3 and a bromine atom at position 4 of the heterocycle. This method allows for the first time the preparation of boron scorpionate ligands with a strongly electron-withdrawing nitro group in the presence of a bulky substituent such as tert-butyl. Ligand 3cTl was prepared in a satisfactory 93% yield. Introduction of the bulkier adamantyl group at position 3 (3dTl) did not negatively affect the product yield. All attempts to prepare 3bTl, 3cTl and 3dTl presenting electron-withdrawing groups and highly hindering tert-butyl or adamantyl substituents through literature methods were unsuccessful.3a,3b,9a,9b The preparation of TpxTl bearing a mesityl group on position 3 also proceeded smoothly in good yields with a hydrogen (3eTl) or a bromine (3fTl) atom on position 4 of the pyrazole ring.
Figure 1.
Pyrazole derivatives used in the preparation of thallium(I) hydrotris(pyrazolyl) borates.
Table 1. Yield and Regioselectivity of Thallium(I) Hydrotris(pyrazolyl) Borate Complexes (3a–jTl)a.
| Pyrazole | TpxTl | R3 | R4 | R5 | Yield (%)b |
|---|---|---|---|---|---|
| 1a | 3aTl | tBu | H | H | 78, 92c |
| 1b | 3bTl | tBu | Br | H | 96 |
| 1c | 3cTl | tBu | NO2 | H | 93 |
| 1d | 3dTl | Ad | NO2 | H | 51 |
| 1e | 3eTl | Ms | H | H | 81 |
| 1f | 3fTl | Ms | Br | H | 78 |
| 1g | 3gNa | Ph | CHO | H | 79d |
| 1h | 3hNa | Me | NO2 | Me | 86d |
| 1i | 3iTl | iPr | NO2 | iPr | <5e,f |
| 1j | 3jTl | Me/H | H | Me/H | 85 |
Reaction conditions: 1a–j (12.1 mmol), NaH (12.1 mmol), toluene (40 mL) 30 min at 0 °C, BHCl2·SMe2 (4 mmol) 24 h at rt. Evaporation and addition of THF (25 mL), TlOAc (6 mmol), 2 h at rt.
Isolated yield.
Isolated yield obtained in a reaction performed on 5 g scale.
TpxNa complexes were isolated.
24 h at 100 °C.
Conversion into 3iTl calculated by 1H NMR.
We have also explored the compatibility of this new procedure for the synthesis of pyrazolylborates supporting functional groups of special sensibility to reductant environments. We used our standard conditions in the reaction with pyrazole 1g bearing a sensitive aldehyde substituent at position R4. As expected, the reaction proceeded smoothly, and the corresponding sodium complex 3gNa was obtained in 79% yield. Finally, we challenged the scope of the method using as the starting material the trisubstituted pyrazole 1h containing two methyl groups and one electron-withdrawing nitro group. The expected product 3hNa was obtained in a satisfactory 86% yield. For pyrazoles 1g and 1h sodium tris(pyrazolyl)borates were obtained directly, and sodium to thallium exchange was not performed. The introduction of two encumbering isopropyl groups hinders the formation of 3iTl, and only traces of the expected product could be detected even at higher temperatures.
It is noticeable the complete regioselectivity of the reaction with the boron bonded to the nitrogen atom placed farther from the encumbering group for pyrazoles 1a–g (R3 in Table 1), thus providing good control of the potential catalytic pocket for hindered pyrazoles. This is a significant advantage over previous methods that often provide regioisomeric mixtures.3c The regioselectivity achieved is dependent on the size of the substituent at position 3. For methyl-substituted pyrazole 1j, the formation of the four possible regiosiomers was observed. The synthetic usefulness of this procedure was demonstrated by the synthesis of 3aTl on a 5 g scale that provided a remarkable 92% yield.
Synthesis of Bis(pyrazolyl)Borate Ligands (2)
We extended this new methodology to the preparation of less explored thallium dihydrobis(pyrazolyl)borates (2Tl),16,18 difficult to isolate as pure materials.3b,9a,19 The results obtained using a commercially available chloroborane dimethylsulfide complex (BH2Cl·SMe2) for a selection of thallium(I) Bpx complexes are shown in Table 2. Optimized reaction conditions for 3Tl were used, and pyrazole and base equivalents were adjusted to ca. ideal stoichiometric amounts with good results. Pyrazoles 1a–c bearing a highly encumbering tert-butyl group cleanly produced the expected 2a–cTl in good to excellent isolated yields. In these cases, the isolation of compounds 2a–cTl is greatly facilitated by our selection of the boron source that allows full control of the 2:1 stoichiometry, thus avoiding contamination with other pyrazolylborates. However, the use of the standard conditions for 2e–f yielded a complex reaction mixture due to the formation of the desired 2e–fTl besides the two corresponding pyrazaboles with two bridgehead boron atoms (see SI).1a,20 The use of an excess of pyrazolide for 1f prevented the formation of the undesired product and yielded exclusively the formation of 2fTl. Unreacted pyrazole can be easily removed from the reaction crude through Et2O washing before sodium to thallium exchange.
Table 2. Yield and Regioselectivity of Thallium(I) Dihydrobis(pyrazolyl)borate Complexes (2a–fTl)a.
| Pyrazole | R3 | R4 | R5 | BpxTl (2Tl) Yield (%)b |
|---|---|---|---|---|
| 1a | tBu | H | H | 90 |
| 1b | tBu | Br | H | 87 |
| 1c | tBu | NO2 | H | 92 |
| 1f | Ms | Br | H | 76c |
Reaction conditions: 1a–c (10.1 mmol), NaH (10.1 mmol), toluene (50 mL) 30 min at 0 °C, BH2Cl·SMe2 (5 mmol) 24 h at rt. Evaporation and addition of THF (30 mL), TlOAc (7.5 mmol), 2 h at rt.
Isolated yield.
20.1 mmol of 1f and NaH (4.02 equiv) were used.
Synthesis of Tetrakis(pyrazolyl)Borate Ligands (4)
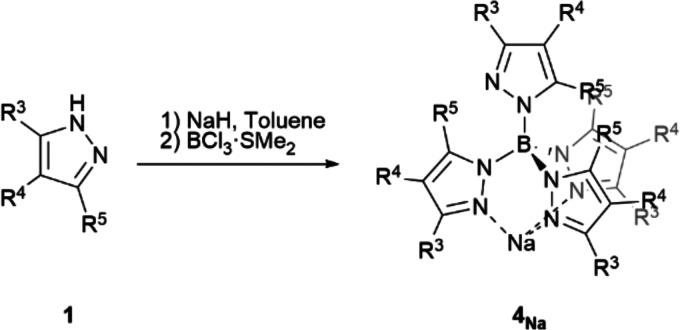
Further, we extended our procedure for the preparation of sterically challenging and rare tetrakis(pyrazolyl)borates.21 Standard conditions were applied to the synthesis of Tkpx ligands increasing pyrazole and base amounts to almost stoichiometric 4.1 equiv to ensure complete conversion of BCl3·SMe2 into the desired tetrakis(pyrazolyl)borate. The scarce examples described of these ligands are almost restricted to those presenting methyl or hydrogen groups on positions 3 and/or 5 of the pyrazole rings due to the difficulty associated with the thermal introduction of the fourth heterocycle.3b,19c,19g,22Table 3 shows the remarkable yields obtained for a variety of TkpxNa (4a–fNa) with highly encumbering substituents. Alkaline salts of these ligands are a common entrance to other metal complexes through metal exchange.22
Table 3. Yield and Regioselectivity of Sodium Tetrakis(pyrazolyl)Borates (4a–fNa)a.
| Pyrazole | R3 | R4 | R5 | TkpxNa (4Na) Yield (%)b |
|---|---|---|---|---|
| 1a | tBu | H | H | 73 |
| 1b | tBu | Br | H | 64 |
| 1c | tBu | NO2 | H | 91 |
| 1f | Ms | Br | H | 93 |
Reaction conditions: 1a–f (4.1 mmol), NaH (4.1 mmol), toluene (15 mL) 30 min at 0 °C, BCl3·SMe2 (1 mmol) 24 h at rt.
Isolated yield.
Molecular Structure Determination
Structures of 2cTl, 3bNa(OH2), and 4fTl were determined by single crystal X-ray diffraction (Figure 2).
Figure 2.

X-ray structures of series 2cTl and 3bNa(OH2).
Conclusions
In conclusion, we have developed a useful and versatile new synthetic procedure for the selective preparation of Bpx, Tpx and Tkpx ligands in a complete selective way under safe and mild conditions. The wide scope of this reaction allows for the first time access to compounds bearing labile functional groups, such as nitro or aldehyde, and including highly hindering and electron-withdrawing substituents simultaneously. Remarkably, a significant additional advantage of the method compared to described procedures is the use of ca. stoichiometric amounts of starting pyrazole derivatives. This method has allowed for the first time the preparation of a challenging new set of poly(pyrazolyl)borate ligands with excellent yields and complete regioselectivity.
Acknowledgments
The authors acknowledge MICIN for financial support through Grant MICIN/AEI/10.13039/501100011033/PID2019-109706RB-I00 ERDF A way of making Europe. The authors also thank Generalitat Valenciana for Grant AICO/2020/215. A.O. thanks MINECO for a Ramon y Cajal fellowship MICIN/AEI/10.13039/501100011033/RYC-2017-22640 ESF Investing in your future.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.3c00761.
Detailed synthetic procedures, characterization of all products and X-ray structural discussion (PDF)
FAIR data, including the primary NMR FID files, for compounds 1a, 1b, 1c, 1d, 1e, 1f, 1g, 1h, 1i, 2aTl, 2bTl, 2cTl, 2fTl, 3aTl, 3bTl, 3cTl, 3dTl, 3eTl, 3fTl, 3gNa, 3hNa, 4aNa, 4bNa, 4cNa, 4fNa, 4fTl, pyrazaboles from 1e and 1f (ZIP)
The authors declare no competing financial interest.
Supplementary Material
References
- See for example:; a Trofimenko S. Boron-Pyrazole Chemistry. J. Am. Chem. Soc. 1966, 88, 1842–1844. 10.1021/ja00960a065. [DOI] [Google Scholar]; b Trofimenko S. Transition Metal Poly(l-pyrazolyl)borates Containing Other Ligands. J. Am. Chem. Soc. 1967, 89, 3904–3905. 10.1021/ja00991a042. [DOI] [Google Scholar]; c Trofimenko S. Boron-Pyrazole Chemistry. IV. Carbon- and Boron-Substituted Poly(1-pyrazolyl)borates. J. Am. Chem. Soc. 1967, 89, 6288–6294. 10.1021/ja01000a053. [DOI] [Google Scholar]
- Jesson J. P.; Trofimenko S.; Eaton D. R. Spectra and Structure of Some Transition Metal Poly(1-pyrazolyl)borates. J. Am. Chem. Soc. 1967, 89, 3148–3158. 10.1021/ja00989a014. [DOI] [Google Scholar]
- a Calabrese J. C.; Trofimenko S.; Thompson J. S. A New Class of Polypyrazolylborate Ligands. J. Chem. Soc. Chem. Commun. 1986, 1122–1123. 10.1039/c39860001122. [DOI] [Google Scholar]; b Trofimenko S.; Calabrese J. C.; Thompson J. S. Novel Polypyrazolylborate Ligands: Coordination Control through 3-Substituents of the Pyrazole Ring. Inorg. Chem. 1987, 26, 1507–1514. 10.1021/ic00257a010. [DOI] [Google Scholar]; c Rheingold A. L.; White C. B.; Trofimenko S. Hydrotris(3-mesityIpyrazol-1 -yl)borate and Hydrobis(3-mesitylpyrazol-l-yl)(5-mesitylpyrazol-l-yl)borate: Symmetric and Asymmetric Ligands with Rotationally Restricted Aryl Substituents. Inorg. Chem. 1993, 32, 3471–3477. 10.1021/ic00068a015. [DOI] [Google Scholar]; d Rheingold A. L.; Ostrander R. L.; Haggerty B. S.; Trofimenko S. Homoscorpionate (Tris(pyrazolyl)borate) Ligands Containing Tethered 3-Phenyl Groups. Inorg. Chem. 1994, 33, 3666–3676. 10.1021/ic00095a009. [DOI] [Google Scholar]
- a Trofimenko S. Recent advances in poly(pyrazolyl)borate (scorpionate) chemistry. Chem. Rev. 1993, 93, 943–980. 10.1021/cr00019a006. [DOI] [Google Scholar]; b Pettinari C.; Pettinari R.; Marchetti F. Golden Jubilee for Scorpionates: Recent Advances in Organometallic Chemistry and Their Role in Catalysis. Adv. Organomet. Chem. 2016, 65, 175–260. 10.1016/bs.adomc.2016.01.002. [DOI] [Google Scholar]
- For recent examples, see:; a Fujisawa K.; Sakuma S.; Ikarugi R.; Jose A.; Solomon E. I. Thermally stable manganese(III) peroxido complexes with hindered N3 tripodal ligands: Structures and their physicochemical properties. J. Inorg. Biochem. 2021, 225, 111597. 10.1016/j.jinorgbio.2021.111597. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hoof S.; Limberg C. The Behavior of Trispyrazolylborato-Metal(II)-Flavonolate Complexes as Functional Models for Bacterial Quercetinase-Assessment of the Metal Impact. Inorg. Chem. 2019, 58, 12843–12853. 10.1021/acs.inorgchem.9b01795. [DOI] [PubMed] [Google Scholar]; c Hoof S.; Limberg C. Bioinspired Trispyrazolylborato Nickel(II) Flavonolate Complexes and Their Reactivity Toward Dioxygen. Z. Anorg. Allg. Chem. 2019, 645, 170–174. 10.1002/zaac.201800457. [DOI] [Google Scholar]; d Pellei M.; Santini C.. Biomimetic Based Applications; George A., Ed.; IntechOpen: 2011; pp 385–428. 10.5772/14854. [DOI] [Google Scholar]
- For recent examples see:; a Noonikara-Poyil A.; Cui H.; Yakovenko A. A.; Stephens P. W.; Lin R.-B.; Wang B.; Chen B.; Dias H. V. R. A Molecular Compound for Highly Selective Purification of Ethylene. Angew. Chem., Int. Ed. 2021, 60, 27184–27188. 10.1002/anie.202109338. [DOI] [PubMed] [Google Scholar]; b Stoessel P.; Auch. A. Metal Complexes. WO2019115423A1, 2019.; c Alexandropoulos D. I.; Schulte K. A.; Vignesh K. R.; Dunbar K. R. Slow magnetic dynamics in a family of mononuclear lanthanide complexes exhibiting the rare cubic coordination geometry. Chem. Commun. 2018, 54, 10136–10139. 10.1039/C8CC04565H. [DOI] [PubMed] [Google Scholar]; d Liddle S. T.; van Slageren J. In Lanthanides and Actinides in Molecular Magnetism; Wiley-VCH: Weinheim, 2015; pp 315–339. 10.1002/9783527673476. [DOI] [Google Scholar]
- For recent examples, see:; a Garcia O. J.; Vendier L.; Etienne M.; Gwaltney S.; Ressler A.; Muñoz-Hernández M.-A. Cyclooctadiene Rh(I) Bis- and Tris(pyrazolyl) aluminate Complexes and Their Catalytic Activity on the Polymerization of Phenylacetylene. Inorg. Chem. 2021, 60, 10757–10763. 10.1021/acs.inorgchem.1c01434. [DOI] [PubMed] [Google Scholar]; b Álvarez M.; Besora M.; Molina F.; Maseras F.; Belderrain T. R.; Pérez P. J. Two Copper-Carbenes from One Diazo Compound. J. Am. Chem. Soc. 2021, 143, 4837–4843. 10.1021/jacs.1c01483. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Kharitonov V. B.; Ostrovskii V. S.; Nelyubina Y. V.; Muratov D. V.; Chusov D.; Loginov D. A. Tris(pyrazolyl) borate rhodium complexes. Application for reductive amination and esterification of aldehydes in the presence of carbon monoxide. J. Organomet. Chem. 2020, 925, 121468. 10.1016/j.jorganchem.2020.121468. [DOI] [Google Scholar]; d Ponduru T. T.; Sun Z.; Cundari T. R.; Dias H. V. R. Nitrene Insertion into Aromatic and Benzylic C-H Bonds Catalyzed by Copper Complexes of Fluorinated Bis- and Tris(pyrazolyl) borates. ChemCatChem. 2019, 11, 4966–4973. 10.1002/cctc.201901087. [DOI] [Google Scholar]
- 4256 crystal structures found in CSD-5.41.; Groom C. R.; Bruno I. J.; Lightfoot M. P.; Ward S. C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179. 10.1107/S2052520616003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Trofimenko S. Boron-pyrazole chemistry. II. Poly(1-pyrazolyl)-borates. J. Am. Chem. Soc. 1967, 89, 3170–3177. 10.1021/ja00989a017. [DOI] [Google Scholar]; b Olmos A.; Pereira A.; Belderrain T. R. P. J.; Pérez T. R. Multigram Synthesis of Thallium Trispyrazolylborate Compounds. Synthesis 2018, 50, 3333–3336. 10.1055/s-0037-1610106. [DOI] [Google Scholar]
- Hydrogen evolution at high temperatures is extremely hazardous. Explosion in our laboratory occurred following the literature procedures using metal borohydrides.
- a Álvarez M.; Molina F.; Pérez P. J. Carbene-Controlled Regioselective Functionalization of Linear Alkanes under Silver Catalysis. J. Am. Chem. Soc. 2022, 144, 23275–23279. 10.1021/jacs.2c11707. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jayaratna N. B.; Pardue D. B.; Ray S.; Yousufuddin M.; Thakur K. G.; Cundari T. R.; Dias H. V. R. Silver(I) complexes of tris(pyrazolyl)borate ligands bearing six trifluoromethyl and three additional electron-withdrawing substituents. Dalton Trans. 2013, 42, 15399–15410. 10.1039/c3dt52152d. [DOI] [PubMed] [Google Scholar]
- a Fischer P. J.; Roe C. B.; Stephenson J. N.; Dunscomb R. J.; Carthy C. L.; Nataro C.; Young V. G. Jr. Exploring opportunities for tuning phenyltris(pyrazol-1-yl)borate donation by varying the extent of phenyl substituent fluorination. Dalton Trans. 2023, 52, 5606–5615. 10.1039/D3DT00735A. [DOI] [PubMed] [Google Scholar]; b Hattori H.; May J. A. Direct bromination and iodination of the trispyrazolyl borate ligand in TpRu(nbd) Cl and the effect of Tp4-X ligands on redox potentials and catalysis. J. Organomet. Chem. 2020, 918, 121288. 10.1016/j.jorganchem.2020.121288. [DOI] [Google Scholar]
- Dias H. V. R.; Lu H.-L.; Kim H.-J.; Polach A. S.; Goh T. K. H. H.; Browning R. G.; Lovely C. J. Copper(I) Ethylene Adducts and Aziridination Catalysts Based on Fluorinated Tris(pyrazolyl)borates [HB(3-(CF3),5-(R) Pz)3]− (where R = CF3, C6H5, H; Pz = pyrazolyl). Organometallics 2002, 21, 1466–1473. 10.1021/om010886v. [DOI] [Google Scholar]
- a Dias H. V. R.; Wang X. Sterically demanding methyl tris(pyrazolyl)borate ligands: synthesis and characterization of thallium(I) complexes supported by [MeB(3-(t-Bu) Pz)3]− and [MeB(3-(Mes) Pz)3]−. Polyhedron 2004, 23, 2533–2539. 10.1016/j.poly.2004.08.023. [DOI] [Google Scholar]; b Graziani O.; Hamon P.; Thépot J.-Y.; Toupet L.; Szilágyi P. Á.; Molnár G.; Bousseksou A.; Tilset M.; Hamon J.-R. Novel tert-Butyl-tris(3-hydrocarbylpyrazol-1-yl) borate Ligands: Synthesis, Spectroscopic Studies, and Coordination Chemistry. Inorg. Chem. 2006, 45, 5661–5674. 10.1021/ic060458n. [DOI] [PubMed] [Google Scholar]; c Dias H. V. R.; Wu J. Organometallics 2012, 31, 1511–1517. 10.1021/om201185v. [DOI] [Google Scholar]
- Chen C.; Jordan R. F. Lewis Acid Catalyzed Synthesis of Poly(pyrazolyl)borate Ligands. Organometallics 2010, 29, 3679–3682. 10.1021/om100590x. [DOI] [Google Scholar]
- a Janiak C.; Braun L.; Girgsdies F. A new route to tris(pyrazolyl)borate ligands and new structural variations in TlTp complexes. J. Chem, Soc., Dalton Trans. 1999, 3133–3136. 10.1039/a902264c. [DOI] [Google Scholar]; b Zagermann J.; Kuchta M.; Merz K.; Metzler-Nolte N. para-Bromophenyl[tris(pyrazolyl)]borate Complexes of Group 1 Metals, Thallium and Magnesium: Synthesis and Characterization of Transfer Agents for “Third-Generation” Tp Ligands. Eur. J. Inorg. Chem. 2009, 2009, 5407–5412. 10.1002/ejic.200900707. [DOI] [Google Scholar]; c Vitze H.; Bolte M.; Lerner H.-W.; Wagner M. Third-Generation Scorpionates [RBpz3]− - How Influential Is the Nondonor Substituent R?. Eur. J. Inorg. Chem. 2016, 2016, 2443–2454. 10.1002/ejic.201500801. [DOI] [Google Scholar]
- Bailey P. J.; Pinho P.; Parsons S. A Chiral Alkyltris(pyrazolyl) borate Ligand: Synthesis of [(Ipc)B(pz)3Mn(CO)3] and [(Ipc) B(pz)3Ru(p-cymene)]PF6 (Ipc = Isopinocampheyl). Inorg. Chem. 2003, 42, 8872–8877. 10.1021/ic035061n. [DOI] [PubMed] [Google Scholar]
- 256 crystal structures found in CSD-5.41.8
- a Trofimenko S. Molybdenum Complexes with Noninert-Gas Configuration. Inorg. Chem. 1970, 9, 2493–2499. 10.1021/ic50093a023. [DOI] [Google Scholar]; b Zaidi S. A. A.; Neyazi M. A. The Synthesis and Ligand Properties of the Dihydro-bis-(1-indazolyl)borate Anion. Transition Met. Chem. 1979, 4, 164–167. 10.1007/BF00619060. [DOI] [Google Scholar]; c Trofimenko S.; Calabrese J. C.; Domaille P. J.; Thompson J. S. Steric Effects in Polypyrazolylborate Ligands. Poly(3-isopropylpyrazolyl)borates: Ligands of Intermediate Steric Requirements. Inorg. Chem. 1989, 28, 1091–1101. 10.1021/ic00305a019. [DOI] [Google Scholar]; d Gorrell I. B.; Looney A.; Parkin G.; Rheingold A. L. {Bis(3-tert-butylpyrazolyl)hydroborato}zinc Alkyl Derivatives: Competitive Reactivity of Zn-C and B-H Bonds. J. Am. Chem. Soc. 1990, 112, 4068–4069. 10.1021/ja00166a070. [DOI] [Google Scholar]; e Looney A.; Han R.; Gorrell I. B.; Cornebise M.; Yoon K.; Parkin G.; Rheingold A. L. Monomeric Alkyl and Hydride Derivatives of Zinc Supported by Poly(pyrazoly)hydroborato Ligation: Synthetic, Structural, and Reactivity Studies. Organometallics 1995, 14, 274–288. 10.1021/om00001a041. [DOI] [Google Scholar]; f Belderrain T. R.; Paneque M.; Carmona E.; Gutiérrez-Puebla E.; Monge M. A.; Ruiz-Valero C. Three-Center, Two-Electron M···H-B Bonds in Complexes of Ni, Co, and Fe and the Dihydrobis(3-tert-butylpyrazolyl)borate Ligand. Inorg. Chem. 2002, 41, 425–428. 10.1021/ic010598r. [DOI] [PubMed] [Google Scholar]; g Adams H.; Batten S. R.; Davies G. M.; Duriska M. B.; Jeffery J. C.; Jensen P.; Lu J.; Motson G. R.; Coles S. J.; Hursthouse M. B.; Ward M. D. New bis-, tris- and tetrakis(pyrazolyl)borate ligands with 3-pyridyl and 4-pyridyl substituents: synthesis and coordination chemistry. Dalton Trans. 2005, 1910–1923. 10.1039/b502892b. [DOI] [PubMed] [Google Scholar]
- a Trofimenko S. Boron-Pyrazole Chemistry. I. Pyrazaboles. J. Am. Chem. Soc. 1967, 89, 3165–3170. 10.1021/ja00989a016. [DOI] [Google Scholar]; b Trofimenko S. Boron-pyrazole chemistry. III. Chemistry of pyrazaboles. J. Am. Chem. Soc. 1967, 89, 4948–4952. 10.1021/ja00995a021. [DOI] [Google Scholar]
- 74 crystal structures found in CSD-5.8
- a Bradley D. C.; Hursthouse M. B.; Newton J.; Walker N. P. C. Some Novel 3,5-Dimethylpyrazolylborato Compounds: Crystal and Molecular Structures of [B{Me2C3N2H}3{Me2C3N2H2}] and [HB{Me2C3N2H2}BH]+ (TaCl6)−. J. Chem. Soc. Chem. Commun. 1984, 188–190. 10.1039/c39840000188. [DOI] [Google Scholar]; b Rheingold A. L.; Yap G. P. A.; Liable-Sands L. M.; Guzei I. A.; Trofimenko S. Coordination Chemistry of Homoscorpionate Ligands with 3-Cyclopropyl Substituents. Inorg. Chem. 1997, 36, 6261–6265. 10.1021/ic970880r. [DOI] [Google Scholar]; c Campo J. A.; Cano M.; Heras J. V.; Pinilla E.; Monge A.; McCleverty J. A. Chemistry of bulky tetrakis(pyrazolyl) borate ligands [B(pzR)4]2 (R = p-CH3OC6H4 or C6H11). J. Chem. Soc., Dalton Trans. 1998, 3065–3070. 10.1039/a803680b. [DOI] [Google Scholar]; d Pettinari C.; Marchetti F.; Pettinari R.; Marinelli A.; Crispini A.; Bellusci A. A sterically hindered tetrakis(pyrazolyl)borate: Synthesis, characterization and coordinative behaviour. Inorg. Chim. Acta 2009, 362, 4593–4598. 10.1016/j.ica.2009.05.026. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.