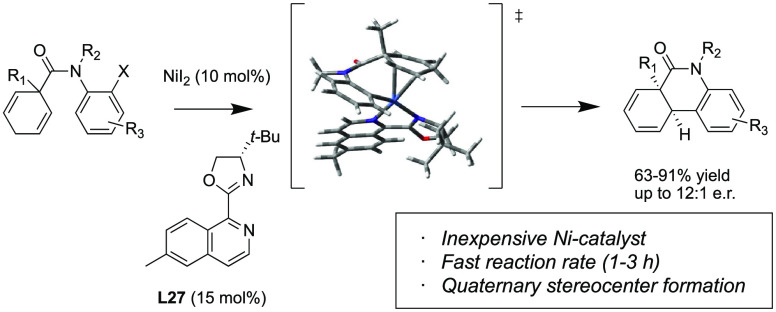
Abstract
A Ni-catalyzed enantioselective intramolecular Mizoroki–Heck reaction has been developed to transform symmetrical 1,4-cyclohexadienes with attached aryl halides into phenanthridinone analogues containing quaternary stereocenters. Herein, we report important advances in reaction optimization enabling control of unwanted proto-dehalogenation and alkene reduction side products. Moreover, this approach provides direct access to six-membered ring heterocyclic systems bearing all-carbon quaternary stereocenters, which have been much more challenging to form enantioselectively with nickel-catalyzed Heck reactions. A wide range of substrates were demonstrated to work in good to excellent yields. Good enantioselectivity was demonstrated using a new synthesized chiral iQuinox-type bidentate ligand (L27). The sustainability, low price of nickel catalysts, and significantly faster reaction rate (1 h) versus that of a recently reported palladium-catalyzed reaction (20 h) make this process an attractive alternative.
Introduction
Palladium has a privileged status in the world of cross-coupling chemistry, having demonstrated a unique versatility and efficiency in a range of synthetic organometallic transformations.1−4 Nevertheless, palladium’s cheaper, more earth abundant, and environmentally friendly group 10 relative nickel has recently attracted significant attention as a worthy replacement or, more frequently, as a complementary transition metal catalyst with unique reactivity.5−9 Nickel’s reactivity, especially its greater range of oxidation states,9,10 creates significant challenges in developing efficient and useful transformations. Despite this, and following the seminal nickel reports of Watson and Jacobsen11 and Nakao,12 there have been a flood of recent reports5−7 with applications of nickel-catalyzed alkene functionalization and other Heck-type reactions illustrating an impressive array of reactivity. In the specific and most challenging realm of intramolecular enantioselective reactions to form quaternary stereocenters, there has also been a variety of impressive reports.13 However, most of these communications report new alkene reaction manifolds such as dicarbofunctionalization;14−16 therefore, although there was early work demonstrating Ni-catalyzed Heck reactions,17,18 more recently there have been many fewer reports of traditional intramolecular19,20 or intermolecular21−24 Heck reaction transformations likely due to the difficulty in achieving selective reactions with nickel, the difficulty of nickel complexes undergoing β-hydride elimination, and the challenges in regenerating Ni(0) from Ni(II) in the Mizoroki–Heck reaction catalytic cycle.
The more popular Ni-catalyzed Heck-type difunctionalization reactions provide a complementary pathway to traditional palladium-catalyzed Heck reactions and overcome the much slower tendency of nickel complexes to undergo β-hydride elimination. Perhaps predictably then, many fewer examples have offered a direct replacement of nickel for palladium in a traditional enantioselective intramolecular Heck reaction. A second perhaps more surprising deficiency in the recent reports of intramolecular nickel-catalyzed Heck reactions, including alkene difunctionalization, is an example of six-membered ring formation with high levels of enantioselectivity. There is an abundance of reports with excellent enantioselectivity to form five-membered rings,25−51 with indolinone or oxindole structures being the most common product formed by far. In contrast, there is only one recent report with a collection of six-membered substrates with excellent enantioselectivity52 and two additional reports citing just one successful enantioselective six-membered ring example.53,54 Otherwise, communications to date have reported modest enantioselectivities below 50% enantiomeric excess (e.r. = 3:1) or no enantioselectivity achieved for six-membered ring examples.26,29,55,56 Given the importance of six-membered rings in organic chemistry and drug development,57,58 the scarcity of examples of enantioselective six-membered ring formation seems a serious omission. Herein, we demonstrate conditions for a new nickel-catalyzed enantioselective intramolecular Heck reaction that substitutes nickel for palladium in an identical Heck transformation that was recently reported by our group.59 In addition to contrasting the nickel-catalyzed conditions for the identical palladium Heck reaction, it also illustrates a rare example of an enantioselective nickel-catalyzed Heck reaction to form a wide selection of six-membered ring structures. The structures formed are quaternary stereocenter-containing analogues of the highly bioactive phenanthridinone structure and therefore should have interest to the drug development community due to the benefits of three-dimensional structures in successful drug architectures.60−62 As with our first report, the process uses a desymmetrization strategy63,64 to generate a chiral quaternary stereocenter. Importantly, the nickel Heck reaction occurs considerably faster than the palladium-catalyzed reaction, illustrating another important benefit of the nickel process consistent with other recent reports highlighting lower catalyst loadings and lower temperatures in a Suzuki–Miyaura reaction.65
Results and Discussion
The substrates 1 for the Ni-catalyzed Heck reactions were synthesized using the previously reported59 three-step sequence that involves a Birch reduction–alkylation process followed by coupling with a corresponding primary or secondary 2-haloaniline derivative (SI-Table 1). As with the Pd-catalyzed case, tertiary amides must be used and N-methyl and N-methoxymethyl (N-MOM) amides were chosen for this work. The N-MOM amides allow subsequent deprotection to reveal the important H-bond donor in the secondary amide of the parent phenanthridinone (SI-Table 2).
In our previous work with Pd-catalyzed enantioselective desymmetrizing Heck reaction, the more sterically demanding alkyl groups at the quaternary center (e.g., -iPr, -iBu, and -Cy) resulted in lower enantioselectivities. Thus, we initially explored Ni as an alternative catalyst choice to achieve higher levels of enantioselectivity with these more sterically demanding substrates. Our preliminary screening of reaction parameters with tertiary aryl bromide 1d in dimethylformamide (DMF) at 80 °C provided promising results using NiCl2 as the catalyst, bipyridine (bipy) as the ligand, and Zn as the reducing agent (Table 1, entry 1). The reaction was completed in an impressively short time (10 min) versus the palladium-catalyzed reaction (24 h), but with significant formation of two major side products: a protodehalogenated 2-1 species and a cyclized alkene 2-2 species. Both, we suspected, were the result of Ni–H intermediates. Further optimization using aryl bromide 1d as the model substrate was performed to increase product yield and decrease the amount of the major side products.
Table 1. Optimization of the Heck Reactiona.
| entry | R | time | T (°C) | NiX2 (mol %) | M0 (equiv) | additive(s) (equiv) | 2 (%)b | 2-1 (%)b | 2-2 (%)b |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Me | 10 min | 80 | NiCl2 (20) | Zn (3.0) | – | 45 | 49 | 6 |
| 2 | Me | 10 min | 80 | NiCl2 (20) | Zn (3.0) | LiI (1.0) | 56 | 40 | 5 |
| 3 | Me | 10 min | 80 | NiCl2 (20) | Zn (3.0) | KI (1.0) | 67 | 33 | 5 |
| 4 | Me | 1.5 h | 80 | NiCl2 (20) | Zn (3.0) | K3PO4 (1.0) | 59 | 33 | 8 |
| 5 | Me | 1 h | 80 | NiCl2 (20) | Zn (1.5) | KI (1.0) | 63 | 29 | 9 |
| 6 | Me | 10 min | 120 | NiCl2 (20) | Zn (3.0) | KI (1.0) | 62 | 28 | 11 |
| 7 | Me | 2 h | 60 | NiCl2 (20) | Zn (3.0) | KI (1.0) | 57 | 36 | 7 |
| 8 | Me | 20 min | 80 | NiCl2 (20) | Zn (3.0) | KI (1.0), 2-cyclohexenone (2.0) | 85 | 9 | 6 |
| 9 | Me | 18 h | 80 | NiCl2 (20) | TDAE (3.0) | KI (1.0), 2-cyclohexenone (2.0) | 80 | 4 | 10 |
| 10 | MOM | 1.5 h | 80 | NiCl2 (20) | Zn (3.0) | KI (1.0), 2-cyclohexenone (2.0) | 72 | 0.2 | 22 |
| 11 | MOM | 3 h | 80 | NiCl2 (20) | Mn (3.0) | KI (1.0), 2-cyclohexenone (2.0) | 73 | 0.3 | 25 |
| 12 | MOM | 24 h | 80 | Ni(acac)2 (20) | Mn (3.0) | KI (1.0), 2-cyclohexenone (3.0) | 79 | 7 | 14 |
| 13 | MOM | 1 h | 80 | NiBr2 (20) | Mn (3.0) | KI (1.0), 2-cyclohexenone (3.0) | 73 | – | 27 |
| 14 | MOM | 1.5 h | 80 | NiI2 (10) | Mn (3.0) | KI (1.0), 2-cyclohexenone (3.0) | 93 | – | 7 |
Conditions: NiX2 (10–20 mol %), bipy (15–20 mol %), M0 (3 equiv), additives (1–3 equiv), DMF (12 mL/mmol of ArX).
Determined by GC analysis.
We first improved the catalytic system outcome with the addition of 1 equiv of LiI (entry 2). Iodide has been shown to improve the reactivity of Ni-catalyzed transformations, although the exact reason for the benefit is not definitively known. It has been proposed that the iodide either facilitates the transfer of electrons from the reducing agent (Zn) to the Ni catalytic complex,66−68 promotes formation of a beneficial nickelate complex,69,70 or facilitates β-hydride elimination.71,72 Alternatively, a nickel-catalyzed halogen exchange73 of the aryl bromide for iodide with the excess iodide can facilitate substrate reactivity toward oxidative addition of the nickel catalyst. Replacing LiI with KI as an external iodide source resulted in a slight decrease in the amount of unwanted protodebromination product 2-1 (entry 3). To test whether the KI improvement was more the result of the potassium cation than the iodide anion, we used K3PO4 as the additive (entry 4). The absence of iodide significantly prolonged the reaction time and increased the amount of cyclized alkene (2-2) versus the desired cyclized diene. Reducing the amount of Zn led to a decreased catalyst turnover rate (entry 5). Increasing or decreasing the temperature had a minimal impact on product yield (entry 3 vs entries 6 and 7). Because both side products, 2-1 and 2-2, are likely the result of Ni–H intermediates, we explored the use of acceptor olefin additives to consume the hydride species. Prior work by Lv74 elegantly demonstrated that the addition of a sacrificial acceptor olefin facilitated the Ni(0)-catalyzed oxidative Heck arylation via a transfer hydrogenation process. To our delight, the reaction efficiency was significantly improved after the addition of 2 equiv of 2-cyclohexen-1-one as a sacrificial alkene (entry 8). Replacing Zn with tetrakis(dimethylamino)ethylene (TDAE) as an organic reductant resulted in a significantly longer reaction time (entry 9). Unfortunately, after employing our initially optimized conditions to a MOM-protected tertiary amide 1e (entry 10), we observed an increased level of formation of cyclized alkene side product 2-2, so further optimization through additional screening of the Ni source, reductants, and additives was performed (entries 11–14). The reaction efficiency was dramatically improved with NiI2 (10 mol %) as the catalyst and Mn as the reductant (entry 14). Because Mn is a stronger two-electron reductant [E° = −1.18 V vs the standard hydrogen electrode (SHE) in water] than Zn (E° = −0.76 V vs SHE in water), it likely accelerates catalyst turnover by reducing the Ni(II)-H species faster, if the Ni(II)-H is not consumed by the sacrificial alkene.
Initially, we explored an enantioselective version of this reaction with a survey of common chiral ligands (L1–18) used in Ni-catalyzed cross-coupling reactions (SI-Table 3). Unfortunately, an array of commercially available bidentate and tridentate N- or P,N-ligands commonly reported with asymmetric Ni-catalyzed reactions75−78 (e.g., pyox, box, pybox, biox, diamine, PHOX, Quinap, and PINAP) did not provide optimal enantioselective results. However, we observed that tBu-pyox- and tBu-iQuinox-type ligands were more enantioselective, and therefore, subsequent studies focused on these ligand types. Thus, we began to synthesize and test novel functionalized tBu-pyox79,80 and tBu-iQuinox (SI-Scheme 1)81,82 chiral ligands (L19–27) (SI-Table 3). To our delight, the enantioselectivity was improved to 9:1 e.r. with (S)-tBu-iQuinox L27, presumably by favorable π-stacking of the substrate aryl group and the isoqunoline ring of the iQuinox ligand.
After establishing the optimal reaction conditions (Table 1, entry 14) and achieving the enantioselectivity with (S)-tBu-iQuinox L27 (SI-Table 3), we proceeded to evaluate the substrate scope of the enantioselective intramolecular Ni-catalyzed Heck reaction with variations at the quaternary center (R1), the amide nitrogen (R2), and the aryl halide (R3) (Table 2). We were pleased to find that a variety of aryl bromides and aryl iodides could undergo this Ni-catalyzed Heck cyclization to furnish the phenanthridinone derivatives in good to excellent yields with good enantioselectivities. Moreover, most reactions were completed within 3 h, which is roughly 7 times faster than with the Pd catalyst.59 Both aryl halides demonstrated compatibility with a simple achiral ligand such as bipy; however, aryl iodides showed better reactivity when employing chiral ligand L27. The higher reactivity of aryl iodides is consistent with the weaker C–I bond being more susceptible to oxidative addition than the C–Br bond of the aryl bromides.83 In addition, we believe it correlates to the complex nature of the iodide effect that seems to increase substrate reactivity.66−68
Table 2. Substrate Scopea.

Unless otherwise noted, the reaction of aryl halide 1 or 1-I (1 equiv) was carried out with NiI2 (10 mol %), bipy or L27 (15 mol %), Mn (3 equiv), KI (1 equiv), 2-cyclohexenone (3 equiv), and DMF (0.08 M) at 80 °C. The yield of 2 is the isolated yield.
At 65 °C.
With L26 (R = F).
A range of alkyl (R1) groups were well-tolerated and showed reactivity comparable to that of the the analogous Pd version,59 including methyl (2a, 2b, 2m, and 2n), ethyl (2c and 2o), and isopropyl (2d, 2e, and 2i–l) groups. A larger scale (1 mmol) enantioselective Heck reaction converting 1c-I to 2c demonstrated a slight reduction in reaction yield and a modest decrease in enantioselectivity. We were particularly delighted to achieve higher enantioselectivity for the isopropyl (2e) derivative (11:1) than with our previous Pd-catalyzed method (7:1). Functional groups such as ether (2f) and ester (2g and 2p) and large benzyl groups (2h) were generally well-tolerated. As with the Pd catalyst report,59 only tertiary amides (R2 = Me or MOM) were successful in this intramolecular Heck reaction. Our previous experimental work with palladium59 suggests the secondary amides experience an ortho effect65,84,85 with a stable six-membered ring chelation event between the amide oxygen and the metal after oxidative addition to the aryl halide. Next, we examined aryl halide substitution (R3) and found a variety of aryl ring substituents were tolerated, including the 2-F, 2-Cl, 2-Me, and 3-Me derivatives (2i–l, respectively). The formation of a protodechlorinated side product was observed with the chloro analogue (2j) in 22% yield for X = Br, which results from oxidative addition of nickel into the C–Cl bond. Formation of this side product was decreased by decreasing the reaction temperature to 65 °C. When X = Br for 2j, a 67% yield was obtained at the lower temperature, and when X = I for 2j, a 91% yield was achieved. The higher yield of the iodo derivative is likely due to the greater difference in reactivity of the Ar–I and Ar–Cl bonds versus the Ar–Br and Ar–Cl bonds. The enantiomeric ratio for 2j was lower, but a similar lower enantiomeric ratio was seen in our studies of the same enantioselective desymmetrizing Heck reaction with palladium.59 Noteworthy was the tolerance of pyridine rings, which afforded 2m–o derivatives in very good yields, albeit with lower er (2o). Interestingly, pyridine bromide 1m delivered exclusively the cyclized alkene product (2m-2). We speculate this may be the result of the initial 1,3-diene product isomerizing to the 1,4-diene product and the resulting conjugation with the pyridine ring promoting reduction with the Ni–H complex before reaction with the cyclohexenone sacrificial alkene. It must be noted that, in our phenanthridinone substrate, the presence of heterocyclic rings was not compatible with the Pd-catalyzed conditions,59 thereby illustrating another benefit of the Ni-catalyzed Heck version. Although enantioselectivity was not studied with bromopyridine derivatives 2m-2 and 2n, iodopyridine 2o did afford enantioselectivity. Lastly, tricyclic 6-5-6 ring system 2p was efficiently formed in good yield.86 Consistent with our previous report,59 removal of the MOM amide protecting group was achieved with TMS-I generated in situ87 for most Heck products, except for pyridine analogues for which BCl3 was used to deprotect the MOM group (SI-Table 4).
Mechanistic Studies
We performed mechanistic studies to shed light on the reaction process (Scheme 1). We first analyzed the role of the reducing metal, Mn primarily, but also the Zn used in the early reaction development. When manganese was removed from the reaction mixture, the reaction was completely hindered, suggesting the catalytic cycle is not initiated by a Ni(II) catalyst (eq 1). Using 10 mol % Ni(COD)2 in the absence of Mn afforded the cyclized diene in low yield (8%) (eq 2), the expected result for one round of catalyst activity. Under identical reaction conditions with the addition of 3 equiv of Mn, 78% of product was formed, which indicates the importance of Mn to turn over the catalytic cycle. In the context of this catalytic process, we speculated that an arylmanganese intermediate (ArMnBr or ArMnI), if formed,88,89 could create an aryl–Ni intermediate via nucleophilic addition instead of direct oxidative addition of Ni into the aryl halide. In addition, insertion of Mn into the Ar–X bond could facilitate the formation of protodehalogenated species. To assess the possibility of ArMnX intermediates, both aryl bromide 1e and aryl iodide 1e-I were used as substrates for stoichiometric reactions with Mn powder (eq 3a). Both reactions were quenched with D2O after 3 h, and the mixtures allowed to stir for an additional 18 h. Deuterated dehalogenated side product D12e-1 was formed in only 13% yield and presumably from an ArMnI intermediate. When stoichiometric reactions were performed with Zn, we observed deuterated side product D12e-I in 82% yield (eq 3b). The additional unknown side product was presumed to be the cyclized alkene, based on the GC retention time and the MS data (Supporting Information). The basis for this cyclization is unclear but may be the result of minor Ni or Pd contamination of the commercial Zn reagent. Overall, these results illustrate protodehalogenation reactions are likely with the aryl iodide substrates, but less relevant to the aryl bromide reactions. Given the short reaction times, it is likely the Mn oxidative addition has a limited impact in hampering the desired Heck product formation. However, insertion of Zn into the Ar–I reaction is likely contributing to some of the protodeiodination side product, 2e-1.90−92 Therefore, the main function of Mn in our reaction is to create a reducing environment for Ni(II) salts to form the necessary zerovalent nickel species, thus making it a better reductant.
Scheme 1. Mechanistic Control Studies.
Reactions were run on a 20–25 mg scale of aryl halide 1 or 1-I and analyzed by GC-MS. s.m. = starting material.
The role of 2-cyclohexenone as a sacrificial alkene was the next reaction component to be analyzed. As noted earlier, it was introduced to remove Ni–H intermediates and reduce the amount of protodehalogenation (2-1) and alkene (2-1) side products. Cyclohexanone was detected and quantified (1.2 equiv) in the gas chromatographic analysis of the reactions, demonstrating that the enone system was reduced. To test for a potential competing reduction of the sacrificial alkene by Mn,93 2-cyclohexenone was stirred and heated at 80 °C with Mn in DMF (eq 4). The formation of cyclohexanone was not observed, which confirms that the Mn is not responsible for the enone reduction. Finally, adding TEMPO (1 equiv) did not impact the reaction, suggesting that the formation of cyclized diene product 2e occurs through a nonradical two-electron pathway (eq 5). Therefore, we propose a traditional two-electron Heck reaction mechanism (Scheme 2). The low-valent Ni(0) species generated under reductive conditions undergoes oxidative addition with aryl halide 1 to afford Ni(II) intermediate A. Ligand association of intermediate A followed by an intramolecular migratory insertion produces alkyl-Ni(II)-Ar species C. A syn-coplanar position of nickel with a β-hydrogen allows for efficient β-hydride elimination to afford product 2 and N(II)–XH species D. The Ni(0) catalyst is regenerated upon Mn reduction. Some of the Ni(II)–H is presumably converted to a Ni–enolate by reaction with 2-cyclohexenone and then subsequently reduced with Mn to Ni(0). Formation of the cyclohexanone, which was detected in our mechanistic studies, could occur by protonation of the Ni–enolate intermediate via a Ni(II)–H complex also present in solution.94
Scheme 2. Proposed Reaction Mechanism.

Computational Study
An understanding of the basis for the enantioselectivity in the catalytic cycle was developed through a computational study of the key 1,2-migratory insertion event taking proposed complex B to C (Scheme 2) for substrate 2a (R1 and R2 = Me; R3 = H). We initially assumed this step would be rate-determining and stereodetermining. The Gibbs free energy of activation for this step with bipyridine ligand was calculated to be quite low at 1.9 kcal/mol, and the reaction is very exothermic (17.5 kcal/mol) and is unlikely to be reversible.
To better understand the asymmetric induction, a study of the transition state of the 1,2-migratory insertion with L27 was conducted considering eight possible geometries: the bidentate chiral ligand in either of two possible binding positions (180° rotated), addition of the aryl group to either alkene of the cyclohexadiene (pro-R or pro-S), and either possible helical twist of the amide in the transition state. These variations were explored for intermediates B and C as well as the transition structure between them. The results are shown in SI-Table 5 and SI-Figure 1.
The lowest-energy structures for intermediate B led to high energy barriers. In fact, of the eight stereoisomeric transition structures, only two had energies of <24 kcal/mol above the global minimum. These two transition structures lay 13.4 and 17.2 kcal/mol above the global minimum for intermediate B, although only 2.2 and 6.0 kcal/mol, respectively, above the corresponding stereoisomers of intermediate B, which were both (coincidentally) 11.2 kcal/mol above the global minimum. These two pathways differed by only a 180° flip of the bidentate chiral ligand and thus corresponded to the same stereochemical outcome of the reaction. The exothermicity (16.3 kcal/mol) closely matched that of the achiral bipyridine case.
Assuming that the barriers for the reaction steps leading up to intermediates B are low, and that the migratory insertion is rate-determining, the pathway having the lowest overall barrier to migratory insertion should dominate. Even if the pathway with the second highest barrier were to contribute, the stereochemical outcome would not be affected. The remaining pathways, leading to different stereochemical outcomes, all involve much higher overall barriers.
The predicted stereochemical outcome from the computational analysis with 2a, R for the quaternary carbon and R for the tertiary bridgehead carbon, with the (S)-L27 ligand (Figure 1) matches in a relative sense the stereochemistry seen from our previous Pd-catalyzed Heck intramolecular desymmetrizing reactions.59 Products generated for both reactions did have the same major enantiomer formed on the basis of chiral HPLC retention times.
Figure 1.
Lowest-energy transition state for 1,2-migratory insertion of B to C.
However, the energy difference between the favored pathway yielding the observed major product and lowest-energy alternate pathway yielding the opposite stereochemistry is notably large (10.9 kcal/mol). If the 1,2-migratory insertion (B to C in Scheme 2) solely controls the stereochemical outcome, as we had supposed, then the selectivity would be far greater than observed. We are thus forced to conclude that this simple explanation of the stereochemical outcome is inadequate, and in fact, there is good reason to suspect as much.
The foregoing analysis presumes that interconversion of the different possible conformations of the initial amide 1 is rapid compared to the rate of reaction (the typical Curtin–Hammett condition). However, this hindered amide is twisted, and axially chiral, although presumably racemic. Furthermore, interconversion of the enantiomers is likely to have a fairly higher barrier, quite likely in the neighborhood of 20 kcal/mol.95 Consequently, the reaction is likely to experience some degree of dynamic kinetic resolution, as detailed below, and has been previously observed for similar Heck reactions.96
The eight aforementioned conformations of intermediate B differ by the orientation of the bidentate ligand, by which alkene the nickel complexes, and by the twist of the amide. If we assume that isomerization of the former two parameters is rapid, but that amide twist interconversion is potentially slow, we arrive at Scheme 3.
Scheme 3. Kinetics of the Reaction in Scheme 2, Assuming That Isomerization of the Amide (k1/k–1 process) Can Be Slow.
Calculated energies are shown in kilocalories per mole (transition state energies for k2B-R, k2BT-S, k2B-S, and k2BT-R, minima for B and BT).
For the sake of simplicity, we do not specify whether the amide twist isomerization occurs in free amide 1, in intermediate A, and/or in intermediate B, because it does not matter for this purpose. The series of structures 1, A, and B have one twist of the amide, and this “B” represents the four isomers of B that were calculated and have this twist (R, RA, RF, and RAF in SI-Table 5 and SI-Figure 1). The series of structures 1T, AT, and BT represent the corresponding structures with the opposite amide twist (including RT, RAT, RFT, and RAFT in SI-Table 5 and SI-Figure 1). The lowest energy of the four “BT” structures is the global minimum, while the lowest energy of the four “B” structures is 1.6 kcal/mol higher.
The BT structures lead via low barriers (13.4 or 17.2 kcal/mol) to the major observed stereochemistry of the product (S,S in this case). Much higher barriers (24.9 or 26.0 kcal/mol) lead to the unobserved product. The B structures, on the contrary, lead preferentially to the minor observed stereochemistry via barriers that are generally fairly high (24.3 or 27.4 kcal/mol above the global minimum), although significantly lower than the barriers leading to the major product stereochemistry (27.4 or 28.4 kcal/mol).
If the interconversion of the different amide twists (k1 and k–1) is rapid compared to k2R, i.e., if the k1 process has a barrier significantly below 24 kcal/mol, then the Curtin–Hammett condition applies, and the reaction would be expected to funnel essentially exclusively through the lowest overall barrier, shown in green, leading exclusively to one stereoisomer product. This is the condition we initially had assumed. On the contrary, if the amide twist interconversion is slow, having a barrier significantly higher than 24 kcal/mol, then one would expect the 50% that had the “desired” twist at the beginning (1T) would proceed to the (S,S) stereochemistry of the product, while the other 50%, having the opposite twist (1), would preferentially yield the (R,R) product. Little if any stereoselectivity would be observed overall. If the rate of the amide twist interconversion is competitive with that of the reaction, i.e., the k1 barrier is in the neighborhood of 24 kcal/mol, then one would expect reduced enantioselectivity, to the extent that 1 cannot isomerize to 1T before reacting through B to yield the (R,R) product. This analysis offers two insights into the reaction process. First, it provides an explanation for why higher enantiomeric ratios are not achieved with the large calculated energy difference (∼11 kcal/mol) between the two diastereomeric transition state complexes in the 1,2-migratory insertion, which might otherwise be expected to afford enantiomeric ratios of >99:1. Second, it leads to the interesting notion that the stereoselectivity of this reaction could be improved by an increase in the barriers for the migratory insertion or, alternatively, a decrease in the barriers for the amide bond rotation. A simultaneous and equivalent change in both the amide rotamer barrier and the migratory insertion barrier would not help, so merely decreasing the temperature is unlikely to improve enantioselectivity. To date, experimental work concurs; attempts to improve the enantioselectivity by decreasing the reaction temperature have failed (data not shown)
Conclusion
In summary, we have developed the first example of an enantioselective intramolecular Ni-catalyzed synthesis of a heterocyclic system with a quaternary stereocenter using the Birch–Heck sequence. This work represents a rare example of a Ni-catalyzed intramolecular Heck reaction to form a six-membered ring with an all-carbon quaternary center and demonstrates the broadest substrate scope to date with good to very good levels of enantioselectivity. In comparison with the Pd-catalyzed version of the reaction, the alternative Ni-catalyzed desymmetrization reaction is much faster (1 h vs 20 h) than the analogous Pd version and can be applied to a wide range of substrates giving good to excellent yields. It affords enantioselectivities comparable to that of the Pd/BINAP Heck reaction59 with the use of a newly synthesized chiral iQuinox-type bidentate ligand L27. Notably, during reaction optimization, we were able to control unwanted Ni–H reductions, including a protodehalogenation side reaction. As such, this work presents a direct and valuable comparison of the performance of nickel and palladium catalysts, which should facilitate the application of Ni catalysis to traditional Heck transformations.
Experimental Section
General Procedures
All reactants and reagents were commercially available and used without further purification unless otherwise indicated. Anhydrous tetrahydrofuran (THF) was obtained by distillation from benzophenone-sodium under argon. All reactions were carried out under an inert atmosphere of argon in flame-dried glassware unless otherwise indicated. Concentrated refers to the removal of solvent with a rotary evaporator at normal water aspirator pressure. Concentrated under high vacuum refers to removal of solvent with a direct-drive rotary vane vacuum pump. Thin layer chromatography (TLC) was performed using silica gel 60 Å precoated aluminum-backed plates (0.25 mm thickness) with a fluorescent indicator. Developed TLC plates were visualized with UV light (254 nm) and KMnO4 spray. Flash column chromatography was conducted with the indicated solvent system using normal phase silica gel (60 Å, 230–400 mesh). Yields refer to chromatographically and spectroscopically pure (>95%) compounds, except as otherwise indicated.
1H and 13C NMR spectra were recorded on a Bruker Avance III 400 instrument at 400 and 100 MHz, respectively. Chemical shifts are reported in δ values (parts per million) relative to an internal reference [0.05% (v/v)] of tetramethylsilane (TMS) for 1H NMR or the solvent signal, chloroform (CDCl3) or DMSO-d6, for 13C NMR. NMR analysis of the tertiary amides was conducted at increased temperatures (77–100 °C) in DMSO-d6 due to the presence of atropisomers. Peak splitting patterns in the 1H NMR spectra are reported as follows: s, singlet; bs, broad singlet; d, doublet; t, triplet; q, quartet; hept, heptet; dd, doublet of doublets; ddd, doublet of doublets of doublets; dt, doublet of triplets; dq, doublet of quartets; m, multiplet. 13C NMR experiments were conducted with the attached proton test (APT) pulse sequence. 13C multiplicities are reported as δu (up) for methyl and methine and δd (down) for methylene and quaternary carbons.
GC-MS analyses were performed with an Agilent 6890 GC instrument and a Hewlett-Packard 5973 EI-MS detector fitted with a 30 m × 0.25 mm column filled with cross-linked 5% PH ME siloxane (0.25 μm film thickness); the gas pressure was 7.63 psi of He. Analysis of samples involved either heating from 70 to 250 °C (10 °C/min) and then being held at 250 °C for 5 min (method A) or heating from 175 to 250 °C (25 °C/min) and then being held at 250 °C for 2 min (method B). Melting points were measured on a Stanford Research Systems MPA160 melting point apparatus and are uncorrected. HPLC analysis was conducted using an Agilent 1100 instrument fitted with a DAD at 254 nm using a CHIRACEL OD-H 4.6 mm × 250 mm, 5 μm column, run under the specified conditions. HRMS were collected at the University of Delaware using a Q-Exactive Orbitrap instrument with an ESI source in positive mode or a Waters GCT Premier instrument equipped with a LIFDI (liquid field desorption ionization). Optical rotations were determined on a PerkinElmer 341 polarimeter at Villanova University at 589 nm and 20.0 °C.
Computational Methods
All calculations were carried out with Gaussian 1697 and using density functional theory (DFT) in the gas phase. We chose the specific configuration applied successfully by Houk, Chen, and co-workers to a related Ni(II) migratory insertion.40 This procedure involves geometry optimization using the B3LYP hybrid functional,98 the def2-SVP basis set,99,100 and the Grimme D3 empirical dispersion correction101 with Becke–Johnson damping,102 followed by a single-point energy correction using the TZVPP basis set. After each optimization [conducted with fopt = (calcfc,tight) or fopt = (calcfc,ts,tight)], a subsequent frequency calculation was performed to confirm the nature of the stationary point as a minimum (NImag = 0) or a transition structure (NImag = 1) and to obtain thermodynamic corrections. Gibbs free energies at 298 K were used for analysis, but essentially the same picture would be obtained using either enthalpies or electronic energies, as the thermodynamic corrections to the energy barriers and differences were small, as would be expected.
The structures computed were those of intermediates B and C in Scheme 2, and the transition structure between them, for the case in which R1 = R2 = CH3, R3 = H, and L–L = (R)-tBu-6-CH3-iQuinox. Eight major stereoisomers were computed, corresponding to the three major factors that could be varied one way or the other: (1) the orientation of the (R)-tBu-6-CH3-iQuinox ligand (a given orientation, or flipped 180° to transpose the positions of the two coordinating nitrogen atoms), (2) which of the two alkenes in the cyclohexadiene unit coordinates to nickel, and (3) whether to twist the amide out of plane in one sense or the other. In a few cases, there were additional orientations of the iQuinox ligand, leading to similar energies. In addition, of course, there would be another eight equivalent structures with the S configuration at the carbon bearing the tert-butyl group, but these would have the same energies and were not computed. The amide functionality was in all cases restricted to the conformation illustrated in Scheme 2. Conformational depictions and calculation details are available in the Supporting Information.
Birch Reduction/Alkylation
General Procedure A
A flame-dried three-necked round-bottom flask with a stir bar, connected to a Dewar condenser, under argon, was charged with benzoic acid (1.0 mmol, 1.0 equiv) that was dissolved in THF (0.4 mL, 2.5 M) and cooled to −78 °C. Ammonia (7 mL, 0.14 M) was distilled into the flask, and lithium (4.0 mmol, 4.0 equiv) was added in small pieces until a dark blue color was maintained for 30 min. Isoprene was added dropwise to quench the excess lithium and produce a bright yellow opaque solution. An alkylating agent (2.0 mmol, 2.0 equiv) was added slowly dropwise. When the addition was complete, the reaction mixture was maintained at −78 °C while the color faded to white/off-white over 1 h. The reaction mixture was then warmed to room temperature, and the ammonia was allowed to evaporate. Once the ammonia had evaporated, the reaction was quenched with water and the mixture washed with diethyl ether. The aqueous layer was acidified with 6 N HCl (until the pH reached ∼1) and then extracted with diethyl ether. The combined organic layers were washed with Na2S2O4 and brine, dried with MgSO4, and concentrated in vacuo.
General Procedure B
A flame-dried flask with a stir bar, connected to a Dewar condenser, under argon, was charged with benzoate ester (1.0 mmol, 1.0 equiv), THF (0.4 mL, 2.5 M), and tBuOH (1.1 mmol, 1.1 equiv) and cooled to −78 °C. Ammonia (7 mL, 0.14 M) was distilled into the flask, and lithium (2.0 mmol, 2.0 equiv) was added in small pieces until a dark blue color was maintained for 30 min. Isoprene was added dropwise to quench the lithium and produce a bright yellow opaque solution. An alkylating agent (1.1 mmol, 1.1 equiv) in THF (0.4 mL, 2.5 M) was added slowly dropwise. When the addition was complete, the reaction mixture was maintained at −78 °C while the color faded to white/off-white over 1 h. The reaction mixture was then warmed to room temperature, and the ammonia was allowed to evaporate under a stream of argon. Once the ammonia had evaporated, the reaction was quenched with water and the mixture extracted with Et2O (5 × 7 mL/mmol). The combined organic layers were washed with brine, dried with MgSO4, concentrated in vacuo, and purified with flash chromatography on silica gel.
1-Methylcyclohexa-2,5-diene-1-carboxylic acid (S1a). Using Birch reduction/alkylation procedure A with benzoic acid (3.00 g, 24.6 mmol, 1.0 equiv) and iodomethane (3.06 mL, 49.1 mmol, 2.0 equiv) in THF (9.8 mL, 2.5 M) afforded S1a (3.18 g, 23.0 mmol) in 94% yield as a white solid: mp 31.2–33.4 °C. Spectral data were in accordance with the literature.103
1-Ethylcyclohexa-2,5-diene-1-carboxylic acid (S1b). Using Birch reduction/alkylation procedure A with benzoic acid (3.03 g, 24.6 mmol, 1.0 equiv) and bromoethane (3.70 mL, 49.6 mmol, 2.0 equiv) in THF (9.8 mL, 2.5 M) provided S1b (3.74 g, 24.6 mmol) in 100% yield as a clear colorless oil. Spectral data were in accordance with the literature.104
1-Isopropylcyclohexa-2,5-diene-1-carboxylic acid (S1c). Using Birch reduction/alkylation procedure A with benzoic acid (3.01 g, 24.6 mmol, 1.0 equiv) and 2-iodopropane (4.82 mL, 49.2 mmol, 2.0 equiv) in THF (9.8 mL, 2.5 M) afforded isopropyl diene acid S1c (7.07 g, 42.5 mmol) in 96% yield as a white solid: mp 74.0–76.0 °C. Spectral data were in accordance with the literature.59
1-(Methoxymethyl)cyclohexa-2,5-diene-1-carboxylic acid (S1d). Using Birch reduction/alkylation procedure A with benzoic acid (3.02 g, 24.76 mmol, 1.0 equiv) and chloromethyl methyl ether (3.76 mL, 49.5 mmol, 2.0 equiv) in THF (9.9 mL, 2.5 M) afforded S1d (3.74 g, 22.2 mmol) in 90% yield as a white solid: mp 66.9–70.5 °C. Spectral data were in accordance with the literature.59
1-(2-Ethoxy-2-oxoethyl)cyclohexa-2,5-diene-1-carboxylic acid (S1e). Using Birch reduction/alkylation procedure A with benzoic acid (2.00 g, 16.4 mmol, 1.0 equiv) and ethyl 2-chloroacetate (3.51 mL, 32.8 mmol, 2.0 equiv) in THF (6.6 mL, 2.5 M) afforded S1e (3.41 g, 16.2 mmol) in 99% yield as a pale-yellow liquid that solidified to white crystals upon cooling: mp 63.5–66 °C. Spectral data were in accordance with the literature.59
1-Benzylcyclohexa-2,5-diene-1-carboxylic acid (S1f). Using Birch reduction/alkylation procedure A (2.33 g, 19.1 mmol, 1.0 equiv) and benzyl chloride (4.38 mL, 38.1 mmol, 2.0 equiv) in THF (7.6 mL, 2.5 M) afforded S1f (3.83 g, 17.9 mmol) in 94% yield as a white crystalline solid: mp 71.9–75.0 °C. Spectral data were in accordance with the literature.104
Ethyl-1-(2-iodobenzyl)cyclohexa-2,5-diene-1-carboxylate (S1g). Using Birch reduction/alkylation procedure B with ethyl benzoate (5.25 g, 35.0 mmol, 1.0 equiv) and 2-iodobenzyl bromide (11.4 g, 38.5 mmol, 1.1 equiv) in THF (14 mL, 2.5 M) afforded S1g (10.98 g, 29.82 mmol) in 85% yield as a clear colorless oil. Spectral data were in accordance with the literature.86
General Aniline Methylation Procedure A
A flame-dried round-bottom flask with a stir bar, under argon, was charged with aniline (1.0 mmol, 1.0 equiv) dissolved in THF (2 mL, 0.5 M). The solution was cooled to −78 °C, and then nBuLi (2.5 M in hexanes, 0.3 mL, 0.7 mmol, 0.7 equiv) was added slowly dropwise over 15 min. After the reaction mixture was stirred at −78 °C for 30 min, iodomethane (1.1 mmol, 1.1 equiv) was added over 5 min. The solution was stirred at −78 °C for 1 h and then at room temperature overnight. The reaction was quenched with water, and the mixture extracted with Et2O (3 × 5 mL/mmol). The organic layers were combined and washed with brine, dried over MgSO4, and concentrated in vacuo. The crude products were purified by column chromatography.
General Aniline Methylation Procedure B
A flame-dried round-bottom flask with a stir bar, under argon, was charged with aniline (1.0 mmol, 1.0 equiv) dissolved in THF (2 mL, 0.5 M) and cooled to −78 °C. Using an automated syringe pump, MeLi (1.6 M in Et2O, 0.6 mL, 1.0 mmol, 1.0 equiv) was added dropwise over 45 min. After the reaction mixture was stirred at −78 °C for 30 min, iodomethane (1.1 mmol, 1.1 equiv) was dissolved in THF (0.3 mL, 3.3 M) and added dropwise to the flask over 10 min at −78 °C. The solution was stirred at −78 °C for 1 h and then warmed to room temperature overnight. The reaction was quenched with saturated aqueous NH4Cl (1.6 mL/mmol), and the mixture extracted with Et2O (3 × 1.6 mL/mmol). The organic layers were combined and washed with brine, dried over MgSO4, and concentrated in vacuo. The crude products were purified by column chromatography.
2-Bromo-N-methylaniline. Using general aniline methylation procedure A with 2-bromoaniline (3.04 g, 17.7 mmol, 1.0 equiv) in THF (35 mL, 0.5 M) afforded the crude product that was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford pure 2-bromo-N-methylaniline (1.98 g, 10.6 mmol) in 60% yield as a clear yellow oil. Spectral data were in accordance with the literature.105
2-Iodo-N-methylaniline. Using general aniline methylation procedure B with 2-iodoaniline (4.0 g, 18.3 mmol, 1.0 equiv) in THF (37 mL, 0.5 M) afforded the crude product that was purified by column chromatography (silica, 60:1 hexanes/EtOAc) to afford pure 2-iodo-N-methylaniline (3.56 g, 15.3 mmol) in 83% yield as a yellow-orange oil. Spectral data were in accordance with the literature.105
General Benzamide Synthesis Procedure
In a round-bottom flask with a stir bar, oxalyl chloride (2.2 mmol, 2.2 equiv) was dissolved in DCM (5.5 mL, 0.4 M) and a catalytic amount of DMF (1.4 μL, 0.02 equiv) was added. The Birch product (1.0 mmol, 1.0 equiv) was dissolved in DCM (2.5 mL, 0.4 M) and added dropwise to the flask. The reaction mixture was refluxed under argon for 1 h until it turned deep yellow. Once the reaction had reached completion, as judged by GC-MS analysis, the mixture was concentrated under vacuum to remove excess oxalyl chloride.
In a round-bottom flask with a stir bar, 2-haloaniline or purified 2-halo-N-methylaniline (1.05–1.3 mmol, 1.05–1.3 equiv) was dissolved in DCM (2.5 mL, 0.4 M) and cooled to 0 °C. Triethylamine (2.5 mmol, 2.5 equiv) was added dropwise followed shortly thereafter by the addition of the acid chloride (1.0 mmol, 1.0 equiv) in DCM (2.5 mL, 0.4 M). The mixture was allowed to warm to room temperature and react overnight. The reaction mixture was diluted with DCM, washed with saturated NaHCO3, 1 N HCl (not used for pyridine- and pyrimidine-containing substrates), and brine, and then dried with MgSO4. The crude product was concentrated under vacuum and purified by column chromatography.
N-(2-Bromophenyl)-N,1-dimethylcyclohexa-2,5-diene-1-carboxamide (1a). Using the general benzamide synthesis procedure, diene acid S1a (1.15g, 7.33 mmol, 1.0 equiv) in DCM (18.3 mL, 0.4 M) reacted with 2-bromo-N-methylaniline (1.77 g, 9.53 mmol, 1.3 equiv) in DCM (23.8 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to afford pure 1a (2.08 g, 6.79 mmol) in 93% yield as a white solid: mp 42.2–44.4 °C; 1H NMR (400 MHz, CDCl3) δ 7.46 (d, J = 7.5 Hz, 1H), 7.23–7.17 (m, 1H), 7.13–7.02 (m, 2H), 5.59 (d, J = 10.1 Hz, 1H), 5.48–5.38 (m, 2H), 4.94 (d, J = 10.1 Hz, 1H), 3.08 (s, 3H), 2.22 (d, J = 23.1 Hz, 1H), 1.83 (d, J = 23.0 Hz, 1H), 1.26 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 133.1, 131.2, 131.1, 128.9, 128.3, 127.8, 123.5, 121.7, 39.0, 29.3; δd 174.2, 143.3, 124.6, 45.8, 25.9; GC (method B) tR = 2.272 min; EI-MS m/z (%) 305 (M+, 1), 214 (45), 185 (18), 134 (100), 105 (13), 93 (53), 77 (46), 65 (8), 51 (8); HRMS (ESI) calcd for C15H17ONBr [M + H]+ 306.0494, found 306.0493.
N-(2-Iodophenyl)-N,1-dimethylcyclohexa-2,5-diene-1-carboxamide (1a-I). Using the general benzamide synthesis procedure, diene acid S1a (1.16 g, 7.42 mmol, 1.0 equiv) in DCM (18.6 mL, 0.4 M) reacted with 2-iodo-N-methylaniline (2.25 g, 9.65 mmol, 1.3 equiv) in DCM (24.1 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to afford pure 1a-I (2.07 g, 5.86 mmol) in 79% yield as a white solid: mp 35.8–38.2 °C; 1H NMR (400 MHz, CDCl3) δ 7.80 (d, J = 7.9 Hz, 1H), 7.27–7.18 (m, 2H), 6.96 (t, J = 7.9 Hz, 1H), 5.64 (s, 1H), 5.56 (d, J = 9.9 Hz, 1H), 5.49 (s, 1H), 5.01 (s, 1H), 3.14 (s, 3H), 2.30 (d, J = 23.2 Hz, 1H), 1.94 (d, J = 23.2 Hz, 1H), 1.35 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 139.4, 131.2, 130.2, 128.9, 128.8, 123.6, 121.6, 39.4, 29.4; δd 174.2, 146.6, 101.8, 45.8, 26.0; GC (method B) tR = 2.608 min; EI-MS m/z (%) 353.0 (M+, 1), 260.9 (92), 243.9 (19), 230.9 (20), 202.8 (7), 182.1 (1), 134.0 (100), 105.0 (30), 91.0 (47), 77.0 (47), 64.0 (11), 51.0 (12); HRMS (ESI) calcd for C15H17ONI [M + H]+ 354.0355, found 354.0350.
N-(2-Bromophenyl)-1-methylcyclohexa-2,5-diene-1-carboxamide (S2b). Using the general benzamide synthesis procedure, diene acid S1a (0.505 g, 3.62 mmol, 1.0 equiv) in DCM (9.0 mL, 0.4 M) reacted with 2-bromoaniline (0.44 mL, 3.87 mmol, 1.1 equiv) in DCM (9.7 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford pure S2b (1.01 g, 3.46 mmol) in 95% yield as a clear colorless oil: 1H NMR (400 MHz, CDCl3) δ 8.32 (dd, J = 8.3, 1.6 Hz, 1H), 8.28 (s, 1H), 7.41 (dd, J = 8.1, 1.5 Hz, 1H), 7.25–7.16 (m, 1H), 6.89–6.80 (m, 1H), 5.97–5.87 (m, 2H), 5.76–5.67 (m, 2H), 2.86–2.65 (m, 2H), 1.34 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 132.2, 129.7, 128.4, 126.1, 124.8, 120.9, 24.7; δd 172.8, 136.1, 113.1, 46.4, 26.0; GC (method B) tR = 2.373 min; EI-MS m/z (%) 291.1 (M + 1+, 5), 276.0 (4), 198.9 (9), 171.0 (11), 120.0 (26), 93.1 (100), 77.1(35), 65.1 (10), 51.1 (5); HRMS (ESI) calcd for C14H15ONBr [M + H]+ 292.0337, found 292.0331.
N-(2-Iodophenyl)-1-methylcyclohexa-2,5-diene-1-carboxamide (S2b-I). Using the general benzamide synthesis procedure, diene acid S1a (0.299 g, 2.16 mmol, 1.0 equiv) in DCM (6.5 mL, 0.4 M) reacted with 2-iodoaniline (0.615 g, 2.81 mmol, 1.3 equiv) in DCM (7 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford pure S2b-I (0.694 g, 2.05 mmol) in 99% yield as an off-white solid: mp 72.7–75.0 °C; 1H NMR (400 MHz, CDCl3) δ 8.33 (dd, J = 8.2, 1.6 Hz, 1H), 8.17 (s, 1H), 7.76 (dd, J = 8.0, 1.5 Hz, 1H), 7.39–7.30 (m, 1H), 6.82 (td, J = 7.6, 1.6 Hz, 1H), 6.09–5.99 (m, 2H), 5.87–5.78 (m, 2H), 3.00–2.76 (m, 2H), 1.44 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 138.8, 129.7, 129.3, 126.1, 125.5, 120.9, 24.8; δd 173.0, 138.6, 89.0, 46.3, 26.2; GC (method B) tR = 2.792 min; EI-MS m/z (%) 339.0 (M+, 13), 324.0 (9), 246.9 (35), 218.9 (20), 202.9 (4), 120.0 (39), 93.0 (100), 77.0 (39), 64.0 (8), 51.0 (5); HRMS (ESI) calcd for C14H15ONI [M + H]+ 340.0198, found 340.0197.
1-Ethyl-N-(2-iodophenyl)cyclohexa-2,5-diene-1-carboxamide (S2c-I). Using the general benzamide synthesis procedure, diene acid S1b (0.501 g, 3.26 mmol, 1.0 equiv) in DCM (8.2 mL, 0.4 M) reacted with 2-iodoaniline (0.935 g, 4.27 mmol, 1.3 equiv) in DCM (10.7 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford pure S2c-I (1.10 g, 3.11 mmol) in 95% yield as a white solid: 1H NMR (400 MHz, CDCl3) δ 8.31 (dd, J = 8.3, 1.6 Hz, 1H), 8.13 (s, 1H), 7.74 (dd, J = 7.9, 1.5 Hz, 1H), 7.32 (ddd, J = 8.5, 7.2, 1.5 Hz, 1H), 6.80 (td, J = 7.6, 1.6 Hz, 1H), 6.16–6.06 (m, 2H), 5.72 (dt, J = 10.4, 2.1 Hz, 2H), 2.96–2.73 (m, 2H), 1.87 (q, J = 7.5 Hz, 2H), 0.86 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 138.8, 129.2, 128.1, 127.8, 125.6, 121.1, 8.9; δd 172.8, 138.6, 89.1, 50.8, 29.5, 26.5; GC (method B) tR = 3.101 min; EI-MS m/z (%) 353.1 (M+, 16), 324 (19), 246.9 (50), 218.9 (29), 202.9 (4), 120 (35), 107.0 (64), 91.0 (33), 79.0 (100), 65.0 (8).
N-(2-Bromophenyl)-1-isopropyl-N-methylcyclohexa-2,5-diene-1-carboxamide (1d). Using the general benzamide synthesis procedure, diene acid S1c (0.501 g, 3.00 mmol, 1.0 equiv) in DCM (7.5 mL, 0.4 M) reacted with 2-bromo-N-methylaniline (0.587 g, 3.153 mmol, 1.05 equiv) in DCM (7.9 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 20:1 hexanes/EtOAc) to afford pure 1d (0.897 g, 2.70 mmol) in 90% yield as a white solid: mp 85.4–86.1 °C; 1H NMR (400 MHz, CDCl3) δ 7.50–7.43 (m, 1H), 7.15–7.01 (m, 3H), 5.54 (d, J = 10.3 Hz, 1H), 5.43 (d, J = 10.4 Hz, 1H), 5.25 (d, J = 10.3 Hz, 1H), 5.02 (d, J = 10.7 Hz, 1H), 3.09 (s, 3H), 2.45 (hept, J = 6.8 Hz, 1H), 2.25 (d, J = 23.2 Hz, 1H), 2.01 (d, J = 20.1 Hz, 1H), 0.80 (d, J = 6.7 Hz, 3H), 0.61 (d, J = 7.0 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 133.0, 130.4, 130.1, 128.9, 128.9, 127.9, 124.9, 123.9, 38.9, 35.9, 17.8, 17.1; δd 174.7, 143.8, 124.3, 54.0, 26.6; GC (method B) tR = 2.692 min; EI-MS m/z (%) 333.1 (M – 1+, 2), 290.0 (23), 214.0 (55), 185.0 (16), 134.0 (100), 121.1 (9), 105.0 (72), 91.0 (9), 77.1 (78), 51.0 (8); HRMS (ESI) calcd for C17H21ONBr [M + H]+ 334.0807, found 334.0806.
N-(2-Iodophenyl)-1-isopropyl-N-methylcyclohexa-2,5-diene-1-carboxamide (1d-I). Using the general benzamide synthesis procedure, diene acid S1c (0.340 g, 2.05 mmol, 1.0 equiv) in DCM (5.1 mL, 0.4 M) reacted with 2-iodo-N-methylaniline (0.620 g, 2.66 mmol, 1.3 equiv) in DCM (6.7 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford pure 1d-I (0.267 g, 1.64 mmol) in 84% yield as a white solid: mp 89.6–90.8 °C; 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 7.9 Hz, 1H), 7.11 (d, J = 3.9 Hz, 2H), 6.93–6.84 (m, 1H), 5.66–5.34 (m, 2H), 5.27 (d, J = 10.4 Hz, 1H), 5.01 (s, 1H), 3.08 (s, 3H), 2.46 (hept, J = 6.9 Hz, 1H), 2.31–1.94 (m, 2H), 0.83 (d, J = 6.8 Hz, 3H), 0.62 (d, J = 6.9 Hz, 2H); 13C{1H} NMR (101 MHz, CDCl3) δu 139.4, 130.3, 129.6, 128.8, 128.8, 125.0, 124.3, 123.8, 39.4, 36.0, 18.1, 17.1; δd 174.8, 147.1, 101.5, 54.0, 26.7; GC (method B) tR = 3.051 min; EI-MS m/z (%) 381.0 (M+, 2), 338.0 (20), 260.9 (75), 244.9 (5), 230.9 (15), 210.1 (14), 134.0 (100), 105.0 (80), 91.0 (9), 77.0 (39), 51.0 (8); HRMS (ESI) calcd for C17H21ONI [M + H]+ 382.0668, found 382.0664.
N-(2-Bromophenyl)-1-isopropylcyclohexa-2,5-diene-1-carboxamide (S2e). Using the general benzamide synthesis procedure, diene acid S1c (0.800 g, 4.81 mmol, 1.0 equiv) in DCM (12.0 mL, 0.4 M) reacted with 2-bromoaniline (1.08 g, 6.25 mmol, 1.3 equiv) in DCM (15.6 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford pure S2e (1.18 g, 3.68 mmol) in 79% yield as a yellow oil: 1H NMR (400 MHz, CDCl3) δ 8.42 (dd, J = 8.3, 1.6 Hz, 1H), 8.35 (s, 1H), 7.50 (dd, J = 8.0, 1.5 Hz, 1H), 7.29 (ddd, J = 8.6, 7.4, 1.5 Hz, 1H), 6.94 (td, J = 7.7, 1.6 Hz, 1H), 6.17–6.07 (m, 2H), 5.78 (dp, J = 10.6, 2.1 Hz, 2H), 2.81 (qd, J = 3.3, 1.8 Hz, 2H), 2.48 (hept, J = 6.9 Hz, 1H), 0.90 (d, J = 6.9 Hz, 6H); 13C{1H} NMR (101 MHz, CDCl3) δu 132.2, 128.5, 128.4, 126.7, 124.9, 121.3, 33.4, 17.7; δd 172.5, 136.1, 113.4, 54.4, 26.8; GC (method B) tR = 2.897 min; EI-MS m/z (%) 321.1 (M + 1+, 8), 277.9 (14), 198.9 (21), 170.9 (19), 121.0 (55), 105.0 (85), 91.0 (25), 79.0 (100), 65.1 (8), 51.0 (7).
N-(2-Iodophenyl)-1-isopropylcyclohexa-2,5-diene-1-carboxamide (S2e-I). Using the general benzamide synthesis procedure, diene acid S1c (0.497 g, 2.99 mmol, 1.0 equiv) in DCM (7.5 mL) reacted with 2-iodoaniline (0.863 g, 3.94 mmol, 1.3 equiv) in DCM (9.9 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to afford pure S2e-I (0.93 g, 2.53 mmol) in 84% yield as a yellow oil: 1H NMR (400 MHz, DMSO-d6) δ 8.22 (s, 1H), 7.90–7.78 (m, 2H), 7.34 (t, J = 7.5 Hz, 1H), 6.93–6.84 (m, 1H), 6.09 (dt, J = 10.7, 3.4 Hz, 2H), 5.82–5.74 (m, 2H), 2.87–2.67 (m, 2H), 2.33 (m, 1H), 0.91–0.76 (d, 6H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 139.3, 129.3, 128.4, 127.2, 126.7, 124.2, 33.9, 18.0; δd 172.5, 139.3, 93.9, 53.5, 26.9; GC (method B) tR = 3.671 min; EI-MS m/z (%) 367.1 (M+, 14), 324.0 (16), 247.0 (53), 218.9 (32), 197.1 (11), 121.1 (64), 105.1 (65), 91.0 (35), 79.1 (100), 65.1 (11), 51.0 (7).
N-(2-Iodophenyl)-1-(methoxymethyl)cyclohexa-2,5-diene-1-carboxamide (S2f-I). Using the general benzamide synthesis procedure, diene acid S1d (0.500 g, 2.97 mmol, 1.0 equiv) in DCM (7.4 mL, 0.4 M) reacted with 2-iodoaniline (0.781 g, 3.57 mmol, 1.2 equiv) in DCM (8.9 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 7:1 hexanes/EtOAc) to afford pure S2f-I (1.06 g, 2.87 mmol) in 97% yield as a yellow oil: 1H NMR (400 MHz, CDCl3) δ 8.25 (dd, J = 8.2, 1.6 Hz, 1H), 8.21 (s, 1H), 7.67 (dd, J = 8.0, 1.5 Hz, 1H), 7.28–7.20 (m, 1H), 6.73 (td, J = 7.6, 1.6 Hz, 1H), 6.11–6.01 (m, 2H), 5.89–5.79 (m, 2H), 3.62 (s, 2H), 3.33 (s, 3H), 2.92–2.68 (m, 2H); 13C{1H} NMR (101 MHz, CDCl3) δu 138.8, 129.2, 128.4, 125.9, 125.7, 121.3, 59.6; δd 171.1, 138.6, 89.1, 77.0, 51.1, 26.7; GC (method B) tR = 3.353 min; EI-MS m/z (%) 369 (M+, 2), 336.0 (5), 227.9 (4), 262.9 (7), 244.9 (35), 232.0 (26), 218.8 (10), 210.0 (5), 196.0 (8), 150.9 (10), 118.9 (14), 105.0 (26), 92.0 (100), 77.0 (13), 63.0 (6); HRMS (ESI) calcd for C15H17INO2 [M + H]+ 370.0304, found 370.0294.
Ethyl 2-{1-[(2-Iodophenyl)(methyl)carbamoyl]cyclohexa-2,5-dien-1-yl}acetate (1g-I). Using the general benzamide synthesis procedure, diene acid S1e (0.502 g, 2.38 mmol, 1.0 equiv) in DCM (6 mL, 0.4 M) reacted with 2-iodo-N-methylaniline (0.721 g, 3.09 mmol, 1.3 equiv) in DCM (7.7 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to afford pure 1g-I (0.84 g, 1.97 mmol) in 83% yield as a tan solid: mp 63.7–64.8 °C; 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 7.8 Hz, 1H), 7.23 (d, J = 7.7 Hz, 1H), 7.14 (d, J = 7.4 Hz, 1H), 6.89 (td, J = 7.6, 1.7 Hz, 1H), 5.78 (d, J = 10.3 Hz, 1H), 5.58 (d, J = 10.2 Hz, 1H), 5.41 (d, J = 10.1 Hz, 1H), 5.10 (d, J = 10.3 Hz, 1H), 4.05 (q, J = 7.1 Hz, 2H), 3.11 (s, 3H), 2.85 (d, J = 16.0 Hz, 1H), 2.52 (d, J = 16.1 Hz, 1H), 2.25 (d, J = 23.3 Hz, 1H), 1.94 (d, J = 22.8 Hz, 1H), 1.17 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 139.5, 130.5, 129.0, 129.0, 128.6, 126.9, 124.6, 123.7, 39.5, 14.3; δd 171.1, 146.4, 101.2, 60.2, 47.7, 46.6, 26.1; GC (method B) tR = 4.145 min; EI-MS m/z (%) 380.1 (M+, 8), 260.0 (45), 232.9 (14), 210.1 (9), 165.1 (62), 134.0 (81), 119.0 (24), 105.0 (35), 91.1 (100), 77.0 (14); HRMS (ESI) calcd for C18H21O3NI [M + H]+ 426.0566, found 426.0580.
1-Benzyl-N-(2-bromophenyl)cyclohexa-2,5-diene-1-carboxamide (S2h). Using the general benzamide synthesis procedure, diene acid S1f (1.01 g, 4.71 mmol, 1.0 equiv) in DCM (11.8 mL, 0.4 M) reacted with 2-bromoaniline (1.05 g, 6.12 mmol, 1.3 equiv) in DCM (15.3 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford pure S2h (1.53 g, 4.15 mmol) in 88% yield as a yellow oil that solidified to white-yellow crystals upon cooling: mp 70.2–72.0 °C; 1H NMR (400 MHz, CDCl3) δ 8.43 (dd, J = 8.3, 1.6 Hz, 1H), 8.31 (s, 1H), 7.48 (dd, J = 8.0, 1.5 Hz, 1H), 7.31 (ddd, J = 8.5, 7.5, 1.5 Hz, 1H), 7.27–7.14 (m, 4H), 6.95 (td, J = 7.7, 1.6 Hz, 1H), 5.99 (dt, J = 10.3, 3.3 Hz, 2H), 5.82 (dt, J = 10.4, 2.0 Hz, 2H), 3.21 (s, 2H), 2.73 (dddd, J = 23.4, 5.5, 3.1, 2.3 Hz, 1H), 2.56 (dtt, J = 23.4, 3.6, 1.8 Hz, 1H); 13C{1H} NMR (101 MHz, CDCl3) δu 132.2, 130.7, 128.4, 127.9, 127.8, 127.6, 126.3, 125.0, 121.2; δd 172.0, 137.4, 136.0, 113.3, 51.3, 43.4, 26.2; GC (method A) tR = 19.251 min; EI-MS m/z (%) 367.1 (M+, 7), 276.0 (15), 196.1 (25), 170.1 (12), 152.1 (4), 120.0 (4), 105.0 (29), 91.1 (100), 77.1 (10), 63.1 (10); HRMS (ESI) calcd for C20H19ONBr [M + H]+ 368.0650, found 368.0657.
1-Benzyl-N-(2-iodophenyl)cyclohexa-2,5-diene-1-carboxamide (S2h-I). Using the general benzamide synthesis procedure, diene acid S1f (0.501 g, 2.33 mmol, 1.0 equiv) in DCM (5.8 mL, 0.4 M) reacted with 2-iodoaniline (0.665 g, 3.03 mmol, 1.3 equiv) in DCM (7.6 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 7:1 hexanes/EtOAc) to afford pure S2h-I (0.868 g, 2.09 mmol) in 90% yield as a yellow solid: mp 68.6–70.4 °C; 1H NMR (400 MHz, CDCl3) δ 8.35 (dd, J = 8.2, 1.6 Hz, 1H), 8.11 (s, 1H), 7.75 (dd, J = 8.0, 1.5 Hz, 1H), 7.41–7.32 (m, 1H), 7.27–7.16 (m, 5H), 6.84 (td, J = 7.6, 1.6 Hz, 1H), 6.03 (dt, J = 10.2, 3.4 Hz, 2H), 5.85 (dt, J = 10.4, 2.0 Hz, 2H), 3.23 (s, 2H), 2.85–2.73 (m, 1H), 2.66–2.53 (m, 1H); 13C{1H} NMR (101 MHz, CDCl3) δu 138.8, 130.7, 129.2, 127.9, 127.8, 127.6, 126.3, 125.8, 121.2; δd 172.2, 138.5, 137.4, 89.3, 51.2, 43.4, 26.3; GC (method B) tR = 19.751 min; EI-MS m/z (%) 415.1 (M+, 7), 324.0 (14), 245.9 (10), 218.9 (8), 196.0 (40), 170.0 (14), 119.0 (11), 105.0 (32), 91.0 (100), 77.1 (11), 65.0 (8); HRMS (ESI) calcd for C20H19ONI [M + H]+ 416.0511, found 416.0508.
N-(2-Bromo-4-fluorophenyl)-1-isopropylcyclohexa-2,5-diene-1-carboxamide (S2i). Using the general benzamide synthesis procedure, diene acid S1c (0.499 g, 3.00 mmol, 1.0 equiv) in DCM (7.5 mL, 0.4 M) reacted with 2-bromo-4-fluoroaniline (0.44 mL, 3.90 mmol, 1.3 equiv) in DCM (9.8 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 20:1 hexanes/EtOAc) to afford pure S2i (0.907 g, 2.68 mmol) in 90% yield as a clear colorless oil: 1H NMR (400 MHz, CDCl3) δ 8.37 (dd, J = 9.2, 5.6 Hz, 1H), 8.23 (s, 1H), 7.26 (dd, J = 7.9, 2.9 Hz, 1H), 7.03 (ddd, J = 9.2, 7.8, 2.9 Hz, 1H), 6.12 (dtd, J = 10.7, 3.4, 1.7 Hz, 2H), 5.77 (dt, J = 10.5, 2.0 Hz, 2H), 2.84–2.76 (m, 2H), 2.47 (hept, J = 6.9 Hz, 1H), 0.90 (d, J = 6.9 Hz, 6H); 13C{1H} NMR (101 MHz, CDCl3) δu 128.5, 126.6, 122.3 (d, 3JC–F = 8.1 Hz), 119.2 (d, 2JC–F = 26.3 Hz), 115.1 (d, 2JC–F = 22.2 Hz), 33.4, 17.7; δd 172.5, 158.3 (d, 1JC–F = 248.5 Hz), 132.6, 54.3, 26.8; 19F NMR (376 MHz, CDCl3) δ −116.66; GC (method B) tR = 2.741 min; EI-MS m/z (%) 337.1 (M – 1+, 7), 294.0 (15), 216.9 (18), 188.9 (17), 138.0 (15), 121.1 (80), 105.0 (58), 79.1 (100), 51.0 (6); HRMS (ESI) calcd for C16H18ONBrF [M + H]+ 338.0556, found 338.0560.
N-(4-Fluoro-2-iodophenyl)-1-isopropylcyclohexa-2,5-diene-1-carboxamide (S2i-I). Using the general benzamide synthesis procedure, diene acid S1c (0.501 g, 3.01 mmol, 1.0 equiv) in DCM (7.5 mL, 0.4 M) reacted with 4-fluoro-2-iodoaniline (0.37 mL, 3.16 mmol, 1.05 equiv) in DCM (7.9 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 20:1 hexanes/EtOAc) to afford pure S2i-I (1.06 g, 2.74 mmol) in 91% yield as an orange solid: mp 62.0–62.5 °C; 1H NMR (400 MHz, CDCl3) δ 8.16 (dd, J = 9.1, 5.5 Hz, 1H), 7.95 (s, 1H), 7.40 (dd, J = 7.7, 2.9 Hz, 1H), 7.00 (ddd, J = 9.1, 7.8, 2.9 Hz, 1H), 6.06 (dt, J = 10.3, 3.3 Hz, 2H), 5.72 (dt, J = 10.5, 2.1 Hz, 2H), 2.84–2.65 (m, 2H), 2.40 (hept, J = 6.9 Hz, 1H), 0.83 (d, J = 6.9 Hz, 6H); 13C{1H} NMR (101 MHz, CDCl3) δu 128.6, 126.7, 125.3 (d, 2JC–F = 24.9 Hz), 122.2 (d, 3JC–F = 7.8 Hz), 115.9 (d, 2JC–F = 21.7 Hz), 33.4, 17.7; δd 172.6, 158.4 (d, 1JC–F = 248.6 Hz), 135.1, 88.7, 54.2, 26.9; 19F NMR (376 MHz, CDCl3) δ −116.70; GC (method B) tR = 3.152 min; EI-MS m/z (%) 385.1 (M+, 18), 342.0 (21), 264.9 (54), 236.9 (29), 215.0 (10), 138.0 (26), 121.1 (100), 105.0 (65), 85.1 (17), 79.0 (99) 51.0 (7); HRMS (ESI) calcd for C16H18ONFI [M + H]+ 386.0417, found 386.0408.
N-(2-Bromo-4-chlorophenyl)-1-isopropylcyclohexa-2,5-diene-1-carboxamide (S2j). Using the general benzamide synthesis procedure, diene acid S1c (0.502 g, 3.02 mmol, 1.0 equiv) in DCM (7.6 mL, 0.4 M) reacted with 2-bromo-4-chloroaniline (0.804 g, 3.90 mmol, 1.3 equiv) in DCM (9.8 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 20:1 hexanes/EtOAc) to afford pure S2j (0.99 g, 2.79 mmol) in 93% yield as a yellow oil: 1H NMR (400 MHz, CDCl3) δ 8.39 (d, J = 8.9 Hz, 1H), 8.32 (s, 1H), 7.50 (d, J = 2.4 Hz, 1H), 7.27 (dd, 1H), 6.15–6.09 (m, 2H), 5.77 (dt, J = 10.5, 2.0 Hz, 2H), 2.82–2.78 (m, 2H), 2.46 (hept, J = 6.9 Hz, 1H), 0.89 (d, J = 6.9 Hz, 6H); 13C{1H} NMR (101 MHz, CDCl3) δu 131.6, 128.6, 128.4, 126.5, 121.8, 116.2, 33.4, 17.7; δd 172.5, 134.9, 129.0, 113.4, 54.4, 26.8; GC (method B) tR = 3.515 min; EI-MS m/z (%) 355.1 (M + 1+, 6), 312.0 (13), 234.9 (16), 206.9 (16), 154.0 (11), 121.1 (90), 105.0 (51), 91.0 (12), 79.1 (100), 51.0 (6).
N-(4-Chloro-2-iodophenyl)-1-isopropylcyclohexa-2,5-diene-1-carboxamide (S2j-I). Using the general benzamide synthesis procedure, diene acid S1c (0.505 g, 3.04 mmol, 1.0 equiv) in DCM (7.6 mL, 0.4 M) reacted with 4-chloro-2-iodoaniline (0.798 g, 3.15 mmol, 1.04 equiv) in DCM (7.9 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 20:1 hexanes/EtOAc) to afford pure S2j-I (0.998 g, 2.48 mmol) in 82% yield as a white solid: mp 85.3–86.5 °C; 1H NMR (400 MHz, CDCl3) δ 8.20 (d, J = 8.9 Hz, 1H), 8.04 (s, 1H), 7.66 (d, J = 2.4 Hz, 1H), 7.23 (dd, J = 8.9, 2.4 Hz, 1H), 6.06 (dt, J = 10.3, 3.4 Hz, 2H), 5.71 (dt, J = 10.5, 2.0 Hz, 2H), 2.84–2.65 (m, 2H), 2.39 (hept, J = 6.9 Hz, 1H), 0.83 (d, J = 6.9 Hz, 6H); 13C{1H} NMR (101 MHz, CDCl3) δu 137.8, 129.2, 128.7, 126.6, 121.6, 33.4, 17.7; δd 172.7, 137.5, 129.5, 89.0, 54.4, 26.9; GC (method B) tR = 4.148 min; EI-MS m/z (%) 401.1 (M+, 13), 358.0 (13), 280.9 (40), 252.9 (23), 231.0 (9), 154.0 (20), 121.1 (100), 105.0 (56), 91.0 (15), 79.0 (90), 63.0 (6), 51.0 (5); HRMS (ESI) calcd for C16H18ONClI [M + H]+ 402.0122, found 402.0119.
N-(2-Bromo-4-methylphenyl)-1-isopropylcyclohexa-2,5-diene-1-carboxamide (S2k). Using the general benzamide synthesis procedure, diene acid S1c (0.504 g, 3.03 mmol, 1.0 equiv) in DCM (7.6 mL, 0.4 M) reacted with 2-bromo-4-methylaniline (0.48 mL, 3.94 mmol, 1.3 equiv) in DCM (9.9 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford pure S2k (0.966 g, 2.89 mmol) in 96% yield as a yellow oil: 1H NMR (400 MHz, CDCl3) δ 8.26 (d, J = 8.3 Hz, 1H), 7.32 (dd, J = 2.0, 0.9 Hz, 1H), 7.09 (dd, J = 8.4, 2.0 Hz, 1H), 6.15–6.05 (m, 2H), 5.78 (dt, J = 10.5, 2.0 Hz, 2H), 2.80 (dp, J = 5.4, 1.9 Hz, 2H), 2.46 (h, J = 7.0 Hz, 1H), 2.28 (s, 3H), 0.90 (d, J = 6.9 Hz, 7H); 13C{1H} NMR (101 MHz, CDCl3) δu 132.4, 128.9, 128.3, 126.7, 121.2, 33.4, 20.5, 17.7; δd 172.4, 134.9, 133.5, 113.3, 54.3, 26.8; GC (method B) tR = 3.296 min; EI-MS m/z (%) 333.1 (M – 1+, 13), 290.0 (19), 212.9 (22), 185.9 (24), 134.1 (60), 122.1 (62), 105.0 (65), 91.0 (15), 79.1 (100), 51.0 (8).
N-(2-Iodo-5-methylphenyl)-1-isopropylcyclohexa-2,5-diene-1-carboxamide (S2l-I). Using the general benzamide synthesis procedure, diene acid S1c (0.501 g, 3.00 mmol, 1.0 equiv) in DCM (7.5 mL, 0.4 M) reacted with 2-iodo-5-methylaniline (0.735 g, 3.15 mmol, 1.05 equiv) in DCM (7.9 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford pure S2l-I (1.13 g, 2.96 mmol) in 99% yield as a white solid: mp 78.2–79.1 °C; 1H NMR (400 MHz, CDCl3) δ 8.11 (d, J = 2.1 Hz, 1H), 8.01 (s, 1H), 7.52 (d, J = 8.1 Hz, 1H), 6.57 (dd, J = 8.1, 1.5 Hz, 1H), 6.05 (dt, J = 10.3, 3.3 Hz, 2H), 5.72 (dt, J = 10.5, 2.0 Hz, 2H), 2.85–2.64 (m, 2H), 2.40 (hept, J = 6.9 Hz, 1H), 2.24 (s, 3H), 0.84 (d, J = 6.9 Hz, 6H); 13C{1H} NMR (101 MHz, CDCl3) δu 138.3, 129.50, 128.5, 126.7, 126.7, 122.0, 33.4, 21.3, 17.8; δd 172.8, 139.6, 138.3, 54.3, 26.9; GC (method B) tR = 3.659 min; EI-MS m/z (%) 381.1 (M+, 26), 338.0 (196), 260.9 (60), 232.9 (50), 211.0 (18), 134.0 (99), 121.0 (81), 105.0 (76), 85 (18), 79.0 (100), 65.0 (5), 51.0 (10); HRMS (ESI) calcd for C17H21ONI [M + H]+ 382.0668, found 382.0658.
N-(2-Bromopyridin-3-yl)-1-methylcyclohexa-2,5-diene-1-carboxamide (S2m). Using the general benzamide synthesis procedure, diene acid S1a (0.501 g, 3.62 mmol, 1.0 equiv) in DCM (9.1 mL, 0.4 M) reacted with 2-bromopyridin-3-amine (0.814 g, 4.71 mmol, 1.3 equiv) in DCM (11.8 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to afford pure S2m (0.899 g, 3.07 mmol) in 85% yield as a white-tan solid: mp 96.0–97.8 °C; 1H NMR (400 MHz, CDCl3) δ 8.69 (dd, J = 8.1, 1.8 Hz, 1H), 8.40 (s, 1H), 8.05 (dd, J = 4.6, 1.8 Hz, 1H), 7.25 (dd, J = 8.2, 4.7 Hz, 1H), 6.05 (dt, J = 10.2, 3.4 Hz, 2H), 5.78 (dt, J = 10.3, 2.1 Hz, 2H), 2.96–2.75 (m, 2H), 1.42 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 144.2, 129.3, 127.8, 126.5, 123.6, 24.5; δd 173.4, 133.9, 132.9, 46.5, 26.0; GC (method B) tR = 2.454 min; EI-MS m/z (%) 292.0 (M – 1+, 1), 199.0 (8), 171.9 (5), 119.0 (6), 93.0 (100), 77.0 (31), 65.0 (8), 51.0 (4).
N-(3-Bromopyridin-2-yl)-1-methylcyclohexa-2,5-diene-1-carboxamide (S2n). Using the general benzamide synthesis procedure, diene acid S1a (0.501 g, 3.62 mmol, 1.0 equiv) in DCM (9.1 mL, 0.4 M) reacted with 2-amino-3-bromopyridine (0.814 g, 4.71 mmol, 1.3 equiv) in DCM (11.8 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to afford pure S2n (0.858 g, 2.93 mmol) in 81% yield as a white solid: mp 96.0–97.8 °C; 1H NMR (400 MHz, CDCl3) δ 8.47 (s, 1H), 8.43 (dd, J = 4.8, 1.6 Hz, 1H), 7.83 (dd, J = 7.9, 1.6 Hz, 1H), 6.94 (dd, J = 7.9, 4.7 Hz, 1H), 6.06–5.96 (m, 2H), 5.82 (dt, J = 10.4, 2.0 Hz, 2H), 2.94–2.75 (m, 2H), 1.43 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 147.6, 141.1, 129.7, 126.1, 120.8, 24.6; δd 171.7, 148.6, 111.0, 46.6, 26.0; GC (method B) tR = 2.695 min; EI-MS m/z (%) 293.0 (M+, 12), 200.9 (42), 171.9 (83), 157.9 (16), 119.0 (6), 93.1 (100), 77.0 (54), 65.0 (14), 51.0 (7); HRMS (ESI) calcd for C13H14ON2Br [M + H]+ 293.0289, found 293.0287.
N-(3-Bromopyridin-2-yl)-1-ethylcyclohexa-2,5-diene-1-carboxamide (S2o). Using the general benzamide synthesis procedure, diene acid S1b (1.11 g, 6.50 mmol, 1.0 equiv) in DCM (16.3 mL, 0.4 M) reacted with 2-amino-3-bromopyridine (1.71 g, 9.88 mmol, 1.5 equiv) in DCM (24.7 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 3:2 hexanes/EtOAc) to afford pure S2o (1.575 g, 5.13 mmol) in 78% yield as a white solid: mp 81.3–84.5 °C; 1H NMR (400 MHz, CDCl3) δ 8.48 (s, 1H), 8.43 (dd, J = 4.7, 1.6 Hz, 1H), 7.83 (dd, J = 7.9, 1.6 Hz, 1H), 6.94 (dd, J = 7.9, 4.7 Hz, 1H), 6.11 (dt, J = 10.3, 3.4 Hz, 2H), 5.74 (dt, J = 10.4, 2.1 Hz, 2H), 2.87–2.79 (m, 2H), 1.91 (q, J = 7.5 Hz, 2H), 0.86 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 147.6, 141.1, 128.0, 127.8, 120.8, 8.8; δd 171.6, 148.6, 111.1, 51.1, 29.3, 26.3; GC (method B) tR = 3.072 min; EI-MS m/z (%) 307.0 (M+, 5), 200.9 (18), 171.9 (49), 157.9 (8), 108.1 (40), 91.1 (30), 79.1 (100), 65.1 (7), 51.1 (5); HRMS (ESI) calcd for C14H16ON2Br [M + H]+ 307.0446, found 307.0447.
N-(3-Iodopyridin-2-yl)-1-ethylcyclohexa-2,5-diene-1-carboxamide (S2o-I). Using the general benzamide synthesis procedure, diene acid S1b (0.561 g, 3.29 mmol, 1.0 equiv) in DCM (8.2 mL, 0.4 M) reacted with 2-amino-3-iodopyridine (0.759 g, 3.45 mmol, 1.05 equiv) in DCM (8.6 mL, 0.4 M) to afford the crude product that was purified by column chromatography (silica, 3:2 hexanes/EtOAc) to afford pure S2o-I (1.04 g, 2.94 mmol) in 89% yield as a white solid: mp 101.3–101.9 °C; 1H NMR (400 MHz, CDCl3) δ 8.38 (dd, J = 4.7, 1.7 Hz, 1H), 8.26 (s, 1H), 7.99 (dd, J = 7.9, 1.6 Hz, 1H), 6.72 (dd, J = 7.9, 4.7 Hz, 1H), 6.09–5.99 (m, 2H), 5.72–5.63 (m, 2H), 2.88–2.67 (m, 2H), 1.84 (q, J = 7.5 Hz, 2H), 0.79 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 148.4, 147.8, 128.1, 127.8, 121.3, 8.8; δd 171.8, 150.9, 86.0, 51.0, 29.3, 26.4; GC (method B) tR = 3.444 min; EI-MS m/z (%) 353.1 (M – 1+, 9), 246.9 (39), 219.9 (100), 203.9 (12), 108.0 (33), 93.0 (21), 79.0 (60), 65.0 (5), 51.0 (4); HRMS (ESI) calcd for C14H16ON2I [M + H]+ 355.0307, found 355.0299.
General Procedure for the Methoxymethyl (MOM) Group Protection of Amide N
A secondary amide (1.0 mmol, 1.0 equiv) was dissolved in THF (7 mL, 0.14 M) and cooled to 0 °C. LiHMDS (1.0 M in hexanes, 1.2 mmol, 1.2 equiv) was added dropwise, and the solution stirred for 10 min. Chloro- or bromomethyl methyl ether (2.0–5.0 mmol, 2.0–5.0 equiv) was added dropwise to the reaction solution, and the mixture was left stirring while slowly warming to rt. Upon completion, the reaction was quenched with saturated NH4Cl, and the mixture diluted with EtOAc. The aqueous layer was extracted twice with EtOAc. The combined organic layers were washed with brine, dried with MgSO4, filtered, and concentrated. The crude product was purified by column chromatography.
N-(2-Bromophenyl)-N-(methoxymethyl)-1-methylcyclohexa-2,5-diene-1-carboxamide (1b). Using the general procedure for MOM group protection, secondary amide S2b (0.444 g, 1.52 mmol, 1.0 equiv) in THF (10.6 mL, 0.14 M) was alkylated with chloromethyl methyl ether (0.248 mL, 3.04 mmol, 2.0 equiv). The crude product was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford 1b (0.389 g, 1.16 mmol) in 76% yield as a clear colorless oil: 1H NMR (400 MHz, DMSO-d6) δ 7.62 (d, J = 7.6 Hz, 1H), 7.34–7.21 (m, 3H), 5.58 (s, 2H), 5.46 (s, 1H), 5.25 (s, 1H), 4.25 (s, 1H), 3.25 (s, 3H), 2.45–2.05 (m, 2H), 1.22 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 133.2, 130.5, 130.1, 128.3, 124.4, 122.7, 56.4, 29.4; δd 174.1, 140.6, 121.4, 80.2, 46.0, 25.9; GC (method B) tR = 2.578 min; EI-MS m/z (%) 335.1 (M + 1+, 1), 305.1 (5), 244.0 (4), 224.1 (19), 211.9 (8), 198.9 (8), 184.9 (70), 164.0 (58), 152.0 (5), 134.0 (6), 105.0 (7), 93.0 (100), 77.0 (39), 65.0 (7), 51.0 (5); HRMS (ESI) calcd for C16H19O2NBr [M + H]+ 336.0599, found 336.0590.
N-(2-Iodophenyl)-N-(methoxymethyl)-1-methylcyclohexa-2,5-diene-1-carboxamide (1b-I). Using the general procedure for MOM group protection, secondary amide S2b-I (0.998 g, 2.95 mmol, 1.0 equiv) in THF (20.7 mL, 0.14 M) was alkylated with chloromethyl methyl ether (0.90 mL, 11.8 mmol, 4.0 equiv). The crude product was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford 1b-I (0.990 g, 2.58 mmol) in 88% yield as a yellow oil: 1H NMR (400 MHz, DMSO-d6) δ 7.90–7.86 (m, 1H), 7.36 (td, J = 7.6, 1.6 Hz, 1H), 7.28 (dd, J = 7.8, 1.8 Hz, 1H), 7.09 (td, J = 7.5, 1.8 Hz, 1H), 5.72–5.55 (m, 3H), 5.50 (d, J = 9.9 Hz, 1H), 5.27 (s, 1H), 4.22 (d, J = 10.1 Hz, 1H), 3.26 (s, 3H), 2.48–2.14 (m, 2H), 1.26 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 139. 9, 139.5, 132.4, 130.8, 129.9, 129.0, 124.2, 122.5, 56.5, 29.5, 26.8; δd 174.1, 150.7, 144.0, 80.6, 46.1, 25.9; GC (method B) tR = 2.935 min; EI-MS m/z (%) 383.1 (M+, 1), 292.0 (4), 259.9 (6), 244.9 (11), 230.9 (100), 224.1 (18), 202.9 (5), 164.0 (28), 134.0 (4), 93.0 (50), 77.0 (24), 65.0 (5), 51.0 (5); HRMS (ESI) calcd for C16H19O2NI [M + H]+ 384.0461, found 384.0447.
1-Ethyl-N-(2-iodophenyl)-N-(methoxymethyl)cyclohexa-2,5-diene-1-carboxamide (1c-I). Using the general procedure for MOM group protection, secondary amide S2c-I (1.10 g, 3.12 mmol, 1.0 equiv) in THF (21.8 mL, 0.14 M) was alkylated with chloromethyl methyl ether (0.47 mL, 6.24 mmol, 2.0 equiv). The crude product was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford 1c-I (0.978 g, 2.46 mmol) in 79% yield as an orange solid: mp 64.9–66.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.84 (dd, J = 7.8, 2.3 Hz, 1H), 7.30 (t, J = 7.6 Hz, 1H), 7.21 (d, J = 7.7 Hz, 1H), 7.09–7.00 (m, 1H), 5.66 (d, J = 10.0 Hz, 1H), 5.52–5.39 (m, 3H), 5.27 (s, 1H), 4.17 (d, J = 9.8 Hz, 1H), 3.22 (s, 3H), 2.37 (d, J = 23.2 Hz, 1H), 2.18 (d, J = 23.3 Hz, 1H), 1.69 (q, J = 6.9 Hz, 2H), 0.70 (q, J = 0.7 Hz, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 139.5, 132.4, 129.8, 129.2, 128.9, 127.2, 125.6, 123.9, 56.6, 8.7; δd 174.0, 144.1, 117.5, 80.5, 50.4, 33.2, 26.3; GC (method B) tR = 3.233 min; EI-MS m/z (%) 397.1 (M+, 1), 365.0 (5), 292.0 (7), 259.9 (13), 239.0 (16), 230.9 (100), 202.9 (4), 164.0 (25), 134.0 (4), 107.0 (26), 90.0 (17), 79.0 (43); HRMS (ESI) calcd for C17H21O2NI [M + H]+ 398.0617, found 398.0611.
N-(2-Bromophenyl)-1-isopropyl-N-(methoxymethyl)cyclohexa-2,5-diene-1-carboxamide (1e). Using the general procedure for MOM group protection, secondary amide S2e (1.10 g, 3.43 mmol, 1.0 equiv) in THF (24 mL, 0.14 M) was alkylated with chloromethyl methyl ether (0.51 mL, 6.86 mmol, 2.0 equiv). The crude product was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford 1e (1.01 g, 2.77 mmol) in 81% yield as an orange solid: mp 70.2–71.5 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.61 (dd, J = 7.6, 1.8 Hz, 1H), 7.33–7.19 (m, 3H), 5.62 (s, 1H), 5.46 (s, 1H), 5.40–5.20 (m, 3H), 4.27 (s, 1H), 3.28 (s, 3H), 2.44–2.34 (m, 1H), 2.33–2.12 (m, 2H), 0.75 (s, 6H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 133.2, 133.1, 130.0, 128.3, 124.5, 56.8, 35.09, 17.8; δd 174.4, 141.1, 124.5, 80.6, 54.4, 26.7; GC (method B) tR = 3.046 min; EI-MS m/z (%) 363.1 (M – 1+, 1), 331.1 (7), 252.1 (10), 213.9 (15), 183.0 (68), 164.1 (64), 121.1 (21), 105.1 (100), 91.0 (15), 79.1 (47), 51.1 (6); HRMS (ESI) calcd for C18H23O2NBr [M + H]+ 364.0912, found 364.0907.
N-(2-Iodophenyl)-1-isopropyl-N-(methoxymethyl)cyclohexa-2,5-diene-1-carboxamide (1e-I). Using the general procedure for MOM group protection, secondary amide S2e-I (0.359 g, 0.978 mmol, 1.0 equiv) in THF (6.8 mL, 0.14 M) was alkylated with bromomethyl methyl ether (0.16 mL, 1.96 mmol, 2.0 equiv). The crude product was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford 1e-I (0.289 g, 0.703 mmol) in 72% yield as a light orange solid: mp 76.9–79.8 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.90 (d, J = 7.9 Hz, 1H), 7.35 (t, J = 7.6 Hz, 1H), 7.24–7.18 (m, 1H), 7.11 (td, J = 7.7, 1.7 Hz, 1H), 5.70 (d, J = 10.2 Hz, 1H), 5.56 (d, J = 9.8 Hz, 1H), 5.41 (m, 2H), 5.27 (d, J = 10.3 Hz, 1H), 4.23 (d, J = 9.9 Hz, 1H), 3.31 (s, 3H), 2.43 (hept, J = 6.7 Hz, 1H), 2.38–2.18 (m, 2H), 0.88 (d, J = 6.6 Hz, 3H), 0.73 (d, J = 6.6 Hz, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 139.5, 132.3, 129.9, 129.6, 129.1, 128.9, 125.9, 124.7, 124.6, 56.8, 35.8, 26.7, 18.3, 17.6; δd 174.3, 144.1, 102.7, 80.6, 54.3, 26.7; GC (method B) tR = 3.451 min; EI-MS m/z (%) 411.1 (M+, 1), 379.1 (4), 292.0 (10), 260.1 (10), 252.1 (12), 230.9 (100), 202.9 (5), 164.0 (34), 121.1 (15), 105.0 (55), 90.0 (10), 79.0 (26), 51.0 (4); HRMS (ESI) calcd for C18H23O2NI [M + H]+ 412.0773, found 412.0767.
N-(2-Iodophenyl)-N,1-bis(methoxymethyl)cyclohexa-2,5-diene-1-carboxamide (1f-I). Using the general procedure for MOM group protection, secondary amide S2f-I (0.503 g, 1.36 mmol, 1.0 equiv) in THF (9.5 mL, 0.14 M) was alkylated with chloromethyl methyl ether (0.41 mL, 5.45 mmol, 4.0 equiv). The crude product was purified by column chromatography (silica, 7:1 hexanes/EtOAc) to afford 1f-I (0.506 g, 1.22 mmol) in 90% yield as a dark yellow oil: 1H NMR (400 MHz, DMSO-d6) δ 7.88 (dt, J = 8.2, 2.0 Hz, 1H), 7.37–7.31 (m, 1H), 7.27–7.20 (m, 1H), 7.12–7.05 (m, 1H), 5.66–5.57 (m, 2H), 5.56–5.42 (m, 2H), 5.32 (s, 1H), 4.21 (d, J = 9.9 Hz, 1H), 3.60 (d, J = 8.7 Hz, 1H), 3.35 (d, J = 8.8 Hz, 1H), 3.28 (s, 3H), 3.24 (s, 3H), 2.49–2.19 (m, 2H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 139.5, 132.6, 129.9, 128.9, 126.9, 126.5, 126.3, 124.3, 59.3, 56.6; δd 172.6, 143.6, 102.6, 80.3, 80.0, 51.3, 26.4; GC (method B) tR 3.399 min; EI-MS m/z (%) 413.1 (M+, 1), 337.0 (7), 289.9 (9), 259.9 (6), 245.1 (9), 231.9 (100), 202.9 (4), 180.0 (4), 164.0 (9), 121.0 (6), 105.0 (44), 90.0 (34), 77.0 (16), 65.0 (4), 51.0 (4); HRMS (ESI) calcd for C17H21O3NI [M + H]+ 414.0566, found 414.0557.
1-Benzyl-N-(2-bromophenyl)-N-(methoxymethyl)cyclohexa-2,5-diene-1-carboxamide (1h). Using the general procedure for MOM group protection, secondary amide S2h (0.996 g, 2.71 mmol, 1.0 equiv) in THF (19 mL, 0.14 M) was alkylated with bromomethyl methyl ether (0.44 mL, 5.41 mmol, 2.0 equiv). The crude product was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford 1h (1.03 g, 2.49 mmol) in 92% yield as a yellow oil: 1H NMR (400 MHz, CDCl3) δ 7.48 (s, 1H), 7.23–7.16 (m, 1H), 7.17–7.11 (m, 3H), 7.11–7.05 (m, 4H), 5.69 (d, J = 10.2 Hz, 1H), 5.63 (s, 1H), 5.52 (s, 1H), 5.41 (s, 1H), 5.01 (s, 1H), 4.28 (d, J = 10.0 Hz, 1H), 3.43 (s, 3H), 3.19 (d, J = 13.1 Hz, 1H), 3.07 (d, J = 13.2 Hz, 1H), 1.93 (s, 2H); 13C{1H} NMR (101 MHz, CDCl3) δu 132.8, 131.4, 129.3, 129.1, 127.3, 126.3, 125.9, 125.5, 123.4, 57.1; δd 174.7, 140.3, 137.4, 124.0, 80.2, 51.4, 46.6, 26.0; GC (method A) tR = 18.724 min; EI-MS m/z (%) 413.2 (M + 1+, 1), 379.1 (4), 288.0 (9), 240.1 (26), 213.9 (8), 184.9 (30), 168.0 (32), 152.0 (6), 105.0 (99), 91.0 (100), 79.0 (16), 65.0 (7); HRMS (ESI) calcd for C22H23O2NBr [M + H]+ 412.0774, found 412.0767.
1-Benzyl-N-(2-iodophenyl)-N-(methoxymethyl)cyclohexa-2,5-diene-1-carboxamide (1h-I). Using the general procedure for MOM group protection, secondary amide S2h-I (0. 501 g, 1.204 mmol, 1.0 equiv) in THF (8.4 mL, 0.14 M) was alkylated with chloromethyl methyl ether (0.37 mL, 4.82 mmol, 4.0 equiv). The crude product was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford 1h-I (0.470 g, 1.02 mmol) in 85% yield as a yellow oil: 1H NMR (400 MHz, DMSO-d6) δ 7.80 (d, J = 8.2 Hz, 1H), 7.24 (t, J = 7.6 Hz, 1H), 7.19–6.97 (m, 7H), 5.59–5.40 (m, 4H), 5.13 (s, 1H), 4.19 (d, J = 9.9 Hz, 1H), 3.24 (s, 3H), 3.02–2.90 (m, 2H), 2.10–1.93 (m, 2H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 139.5, 132.4, 131.5, 129.9, 129.2, 128.8, 127.7, 127.6, 126.3, 125.5, 123.8, 56.7; δd 173.8, 143.9, 137.6, 115.1, 80.7, 60.1, 51.3, 46.8, 26.1; GC (method A) tR = 19.957 min; EI-MS m/z (%) 459 (M+, 1), 427.1 (6), 336.0 (15), 300.0 (6), 292.0 (10), 259.9 (10), 259.9 (20), 240.1 (42), 230.9 (70), 213.1 (4), 196.0 (5), 168.0 (31), 150.9 (7), 105.0 (98), 91.0 (100), 79.0 (18), 65.0 (8); HRMS (ESI) calcd for C22H23O2NI [M + H]+ 459.0695, found 459.0696.
N-(2-Bromo-4-fluorophenyl)-1-isopropyl-N-(methoxymethyl)cyclohexa-2,5-diene-1-carboxamide (1i). Using the general procedure for MOM group protection, secondary amide S2i (0.8434 g, 2.49 mmol, 1.0 equiv) in THF (17.4 mL, 0.14 M) was alkylated with chloromethyl methyl ether (0.38 mL, 4.98 mmol, 2.0 equiv). The crude product was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford 1i (0.828 g, 2.17 mmol) in 87% yield as a white solid: mp 66.8–69.9 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.58 (dd, J = 8.3, 2.9 Hz, 1H), 7.26 (dd, J = 8.9, 5.7 Hz, 1H), 7.19 (td, J = 8.4, 2.9 Hz, 1H), 5.69 (s, 1H), 5.49 (d, J = 9.7 Hz, 1H), 5.43–5.35 (m, 3H), 4.26 (d, J = 9.9 Hz, 1H), 3.30 (s, 3H), 2.47–2.35 (m, 2H), 2.24 (d, J = 23.5 Hz, 1H), 0.82 (d, J = 7.1 Hz, 3H), 0.72 (d, J = 6.9 Hz, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 134.0 (d, 3JC–F = 9.4 Hz), 128.8, 126.0, 125.0, 124.6, 120.2 (d, 2JC–F = 25.7 Hz), 115.3 (d, 2JC–F = 22.1 Hz), 56.8, 35.8, 18.1, 17.5; δd 174.4, 161.7 (d, 1JC–F = 250 Hz), 137.6, 125.1, 80.3, 54.3, 26.7; 19F NMR (376 MHz, DMSO-d6) δ −111.69; GC (method B) tR = 2.815 min; EI-MS m/z (%) 381.1 (M + 1+, 1), 349.1 (5), 262.0 (6), 246.0 (4), 232.0 (10), 201.0 (57), 182.1 (37), 121.1 (26), 105.1 (100), 90.0 (12), 105.1 (100), 51.1 (4); HRMS (ESI) calcd for C18H22O2NBrF [M + H]+ 382.0818, found 382.0813.
N-(4-Fluoro-2-iodophenyl)-1-isopropyl-N-(methoxymethyl)cyclohexa-2,5-diene-1-carboxamide (1i-I). Using the general procedure for MOM group protection, secondary amide S2i-I (0.201 g, 0.519 mmol, 1.0 equiv) in THF (3.6 mL, 0.14 M) was alkylated with chloromethyl methyl ether (0.16 mL, 2.08 mmol, 4.0 equiv). The crude product was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford 1i-I (0.214 g, 0.499 mmol) in 96% yield as a solid: mp 65.1–66.6 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.73 (dd, J = 8.2, 1.9 Hz, 1H), 7.22–7.15 (m, 2H), 5.71 (d, J = 9.7 Hz, 1H), 5.53 (d, J = 9.6 Hz, 1H), 5.38 (dd, J = 19.5, 10.4 Hz, 2H), 4.19 (d, J = 9.6 Hz, 1H), 3.29 (s, 3H), 2.46–2.40 (m, 1H), 2.39–2.19 (m, 2H), 0.86 (d, J = 6.7 Hz, 3H), 0.72 (d, J = 6.9 Hz, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 133.0 (d, 3JC–F = 9.0 Hz), 129.0, 125.9 (d, 2JC–F = 24.9 Hz), 124.8, 124.7, 115.8 (d, 2JC–F = 22.3 Hz), 56.8, 35.8, 18.3, 17.5; δd 174.3, 161.3 (d, 1JC–F = 251 Hz), 140.8, 100.0, 80.5, 54.3, 26.7; 19F NMR (376 MHz, DMSO-d6) δ −113.19; GC (method B) tR = 3.193 min; EI-MS m/z (%) 429.1 (M+, 1), 397.1 (7), 310.0 (9), 278.0 (12), 265.0 (7), 262.0 (4), 248.9 (100), 182.0 (27), 121.1 (18), 105.0 (55), 79.0 (28); HRMS (ESI) calcd for C18H22O2NFI [M + H]+ 430.0679, found 430.0675.
N-(2-Bromo-4-chlorophenyl)-1-isopropyl-N-(methoxymethyl)cyclohexa-2,5-diene-1-carboxamide (1j). Using the general procedure for MOM group protection, secondary amide S2j (0.969 g, 2.73 mmol, 1.0 equiv) in THF (19.1 mL, 0.14 M) was alkylated with chloromethyl methyl ether (0.42 mL, 5.50 mmol, 2.0 equiv). The crude product was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford 1j (0.855 g, 2.14 mmol) in 79% yield as a low-melting point orange solid: 1H NMR (400 MHz, CDCl3) δ 7.57 (t, J = 1.3 Hz, 1H), 7.16 (d, J = 1.3 Hz, 2H), 5.70–5.62 (m, 2H), 5.49 (d, J = 10.2 Hz, 1H), 5.38 (d, J = 10.4 Hz, 1H), 5.25 (s, 1H), 4.24 (d, J = 10.0 Hz, 1H), 3.40 (s, 3H), 2.51 (hept, J = 6.8 Hz, 1H), 2.40 (d, J = 23.4 Hz, 1H), 2.16 (d, J = 23.6 Hz, 1H), 0.87 (d, J = 6.8 Hz, 3H), 0.71 (d, J = 6.9 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 133.1, 132.4, 129.6, 127.7, 125.7, 124.9, 79.9, 57.1, 35.7, 17.8, 17.1; δd 175.2, 139.5, 134.4, 124.9, 79.9, 54.4, 26.7; GC (method B) tR = 3.480 min; EI-MS m/z (%) 399.1 (M + 1+, 1), 367.1 (4), 280.0 (5), 263.9 (4), 247.9 (8), 218.9 (65), 198.0 (42), 121.1 (28), 105.1 (100), 90.0 (12), 79.1 (50), 51.1 (4); HRMS (ESI) calcd for C18H22O2NBrCl [M + H]+ 398.0522, found 398.0515.
N-(4-Chloro-2-iodophenyl)-1-isopropyl-N-(methoxymethyl)cyclohexa-2,5-diene-1-carboxamide (1j-I). Using the general procedure for MOM group protection, secondary amide S2j-I (0.500 g, 1.25 mmol, 1.0 equiv) in THF (8.8 mL, 0.14 M) was alkylated with chloromethyl methyl ether (0.38 mL, 4.98 mmol, 4.0 equiv). The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 1j-I (0.483 g, 1.08 mmol) in 87% yield as a low-melting point tan solid: 1H NMR (400 MHz, DMSO-d6) δ 7.92 (d, J = 2.4 Hz, 1H), 7.40 (dd, J = 8.4, 2.4 Hz, 1H), 7.17 (dd, J = 8.4, 2.0 Hz, 1H), 5.72–5.65 (m, 1H), 5.51 (d, J = 9.8 Hz, 1H), 5.45–5.30 (m, 3H), 4.23 (d, J = 9.8 Hz, 1H), 3.29 (s, 3H), 2.49–2.36 (m, 2H), 2.27 (d, J = 23.4 Hz, 1H), 0.86 (d, J = 6.9 Hz, 3H), 0.74 (d, J = 7.1 Hz, 2H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 138.3, 133.0, 129.0, 128.9, 126.2, 125.2, 124.8, 56.9, 35.8, 18.3, 17.6; δd 174.2, 143.3, 133.4, 103.7, 80.4, 54.3, 26.8; GC (method B) tR = 4.011 min; EI-MS m/z (%) 445.0 (M+, 1), 413.1 (5), 326.0 (6), 293.9 (8), 290.1 (7), 264.9 (100), 198.0 (34), 121.1 (25), 105.1 (80), 90.0 (10), 79.1 (45), 51.0 (4); HRMS (ESI) calcd for C18H22O2NClI [M + H]+ 446.0384, found 446.0373.
N-(2-Bromo-4-methylphenyl)-1-isopropyl-N-(methoxymethyl)cyclohexa-2,5-diene-1-carboxamide (1k). Using the general procedure for MOM group protection, secondary amide S2k (0.99 g, 2.96 mmol, 1.0 equiv) in THF (20.7 mL, 0.14 M) was alkylated with chloromethyl methyl ether (0.45 mL, 5.92 mmol, 2.0 equiv). The crude product was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford 1k (0.857 g, 2.2 mmol) in 77% yield as a tan solid: mp 66.2–68.8 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.48 (s, 1H), 7.11 (s, 2H), 5.67 (d, J = 19.2 Hz, 1H), 5.49 (d, J = 9.9 Hz, 1H), 5.37 (d, J = 10.5 Hz, 2H), 5.30 (s, 1H), 4.24 (d, J = 9.8 Hz, 1H), 3.29 (s, 3H), 2.45–2.36 (m, 1H), 2.36–2.32 (m, 4H), 2.23 (d, J = 23.0 Hz, 1H), 0.82 (d, J = 7.0 Hz, 3H), 0.72 (d, J = 7.0 Hz, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 133.3, 132.6, 130.3, 128.8, 128.0, 126.4, 125.0, 56.8, 35.8, 20.6, 17.9; δd 174.5, 140.2, 138.4, 124.1, 80.6, 54.4, 26.7; GC (method B) tR = 3.480 min; EI-MS m/z (%) 399.1 (M + 1+, 1), 367.1 (4), 280.0 (5), 263.9 (4), 247.9 (8), 218.9 (65), 198.0 (42), 121.1 (28), 105.1 (100), 90.0 (12), 79.1 (50), 51.1 (4); HRMS (ESI) calcd for C19H25O2NBr [M + H]+ 378.1069, found 378.1063.
N-(2-Iodo-5-methylphenyl)-1-isopropyl-N-(methoxymethyl)cyclohexa-2,5-diene-1-carboxamide (1l-I). Using the general procedure for MOM group protection, secondary amide S2l-I (0.883 g, 2.32 mmol, 1.0 equiv) in THF (16.2 mL, 0.14 M) was alkylated with chloromethyl methyl ether (0.53 mL, 6.95 mmol, 3.0 equiv). The crude product was purified by column chromatography (silica, 9:1 hexanes/EtOAc) to afford 1l-I (0.832 g, 1.95 mmol) in 84% yield as a yellow oil that solidified to yellow crystals upon cooling: mp 71.6–72.3 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.73 (d, J = 8.0 Hz, 1H), 7.00 (d, J = 2.2 Hz, 1H), 6.92 (dd, J = 8.1, 2.3 Hz, 1H), 5.72 (d, J = 10.1 Hz, 1H), 5.49 (d, J = 9.6 Hz, 1H), 5.47–5.36 (m, 2H), 5.25 (d, J = 10.1 Hz, 1H), 4.24 (d, J = 9.8 Hz, 1H), 3.30 (s, 3H), 2.47–2.39 (m, 1H), 2.37 (s, 1H), 2.24 (s, 3H), 2.16 (d, J = 23.5 Hz, 1H), 0.87 (d, J = 6.7 Hz, 3H), 0.73 (d, J = 6.7 Hz, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 139.1, 133.1, 130.8, 129.5, 125.7, 124.2, 124.1, 56.9, 35.8, 20.5, 18.3, 17.5; δd 174.4, 143.8, 138.6, 98.6, 80.5, 54.3, 26.7; GC (method B) tR = 3.610 min; EI-MS m/z (%) 425.1 (M+, 1), 266.1 (22), 259.1 (7), 245.0 (100), 178.0 (55), 148.0 (6), 121.1 (11), 105.0 (40), 90.0 (12), 79.0 (22); HRMS (ESI) calcd for C19H25O2NI [M + H]+ 426.0930, found 426.0919.
N-(2-Bromopyridin-3-yl)-N-(methoxymethyl)-1-methylcyclohexa-2,5-diene-1-carboxamide (1m). Using the general procedure for MOM group protection, secondary amide S2m (0.856 g, 2.92 mmol, 1.0 equiv) in THF (20.4 mL, 0.14 M) was alkylated with chloromethyl methyl ether (0.44 mL, 5.84 mmol, 2.0 equiv). The crude product was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to afford 1m (0.822 g, 2.45 mmol) in 84% yield as a yellow solid: mp 59.0–61.3 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.29 (dd, J = 4.7, 1.9 Hz, 1H), 7.68 (dd, J = 7.7, 1.9 Hz, 1H), 7.38 (dd, J = 7.8, 4.7 Hz, 1H), 5.60 (s, 2H), 5.47 (s, 1H), 5.32 (s, 1H), 4.30 (s, 1H), 3.25 (s, 3H), 2.41 (d, J = 24.3 Hz, 1H), 2.07 (d, J = 23.3 Hz, 1H), 1.21 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 149.2, 141.2, 123.8, 56.6, 29.1; δd 173.9, 144.3, 138.0, 80.2, 46.1, 25.9; GC (method B) tR = 2.786 min; EI-MS m/z (%) 339.1 (M + 2+, 1), 306.0 (4), 245.0 (9), 216.0 (32), 200.9 (26), 184.9 (44), 165.0 (25), 137.0 (8), 119.0 (11), 106.0 (8), 93.1 (100), 77.1 (50), 64.0 (10), 51.0 (7); HRMS (ESI) calcd for C15H18O2N2Br [M + H]+ 337.0552, found 337.0555.
N-(3-Bromopyridin-2-yl)-N-(methoxymethyl)-1-methylcyclohexa-2,5-diene-1-carboxamide (1n). Using the general procedure for MOM group protection, secondary amide S2n (0.737 g, 2.51 mmol, 1.0 equiv) in THF (17.6 mL, 0.14 M) was alkylated with bromomethyl methyl ether (0.82 mL, 10.0 mmol, 4.0 equiv). The crude product was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to afford 1n (0.547 g, 1.62 mmol) in 65% yield as a yellow oil: 1H NMR (400 MHz, DMSO-d6) δ 8.43 (dd, J = 4.6, 1.7 Hz, 1H), 8.11 (dd, J = 8.0, 1.7 Hz, 1H), 7.33 (dd, J = 7.9, 4.6 Hz, 1H), 5.60–5.42 (m, 4H), 5.05 (s, 1H), 3.27 (s, 3H), 2.49–2.30 (m, 2H), 1.28 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 148.1, 142.5, 129.8, 125.4, 123.5, 56.7, 29.4; δd 175.0, 153.0, 121.0, 79.6, 46.3, 25.9; GC (method B) tR = 2.740 min; EI-MS m/z (%) 338.1 (M + 1+, 1), 304.0 (6), 245.0 (20), 214.9 (21), 200.9 (41), 185.9 (66), 156.0 (33), 135.0 (3), 120.0 (9), 106.0 (6), 93.1 (100), 77.1 (50), 65.1 (11), 51.1 (8); HRMS (ESI) calcd for C15H18O2N2Br [M + H]+ 337.0552, found 337.0547.
N-(3-Bromopyridin-2-yl)-1-ethyl-N-(methoxymethyl)cyclohexa-2,5-diene-1-carboxamide (1o). Using the general procedure for MOM group protection, secondary amide S2o (1.43 g, 4.66 mmol, 1.0 equiv) in THF (32.6 mL, 0.14 M) was alkylated with bromomethyl methyl ether (2.3 mL, 27.9 mmol, 6.0 equiv). The crude product was purified by column chromatography (silica, 3:2 hexanes/EtOAc) to afford 1o (1.28 g, 3.64 mmol) in 78% yield as a yellow solid: mp 41.9–44.4 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.42 (d, J = 4.6 Hz, 1H), 8.10 (d, J = 8.0 Hz, 1H), 7.32 (dd, J = 8.0, 4.7 Hz, 1H), 5.56 (d, J = 10.2 Hz, 2H), 5.39 (d, J = 10.3 Hz, 2H), 5.05 (s, 2H), 3.28 (s, 3H), 2.45–2.36 (m, 2H), 1.74 (q, J = 7.5 Hz, 2H), 0.74 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 148.1, 142.5, 128.0, 125.4, 125.0, 56.8, 8.6; δd 176.1, 153.0, 121.1, 79.5, 50.7, 33.4, 26.3; GC (method B) tR = 3.027 min; EI-MS m/z (%) 350.1 (M – 1+, 1), 320.1 (6), 245.0 (15), 215.0 (19), 200.9 (33), 186.0 (61), 156.9 (29), 134.0 (12), 107.1 (39), 91.1 (50), 79.1 (100), 65.1 (5), 51.1 (6); HRMS (ESI) calcd for C16H20O2N2Br [M + H]+ 351.0708, found 351.0706.
1-Ethyl-N-(2-iodopyridin-3-yl)cyclohexa-2,5-diene-1-carboxamide (1o-I). Using the general procedure for MOM group protection, secondary amide S2o-I (0.497 g, 1.41 mmol, 1.0 equiv) in THF (9.9 mL, 0.14 M) was alkylated with bromomethyl methyl ether (0.46 mL, 5.65 mmol, 4.0 equiv). The crude product was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to afford 1o-I (0.283 g, 0.711 mmol) in 50% yield as a tan solid: mp 71.3–72.1 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.45–8.38 (m, 1H), 8.28 (dd, J = 7.8, 1.7 Hz, 1H), 7.12 (dd, J = 7.9, 4.6 Hz, 1H), 5.56 (d, J = 10.7 Hz, 2H), 5.42 (d, J = 10.0 Hz, 2H), 5.04 (s, 2H), 3.28 (s, 3H), 2.43 (s, 2H), 1.76 (q, J = 7.7 Hz, 2H), 0.76 (t, J = 7.4 Hz, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δu 148.8, 148.6, 128.1, 124.9, 56.8, 8.7; δd 176.2, 155.9, 97.9, 79.9, 50.7, 33.5, 26.3; GC (method B) tR = 3.496 min; EI-MS m/z (%) 398.1 (M+, 1), 366.1 (5), 291.0 (20), 264.0 (30), 246.9 (36), 231.9 (100), 204.9 (37), 165 (6), 134.0 (10), 107.1 (25), 91.1 (25), 79.0 (58), 51.0 (4); HRMS (ESI) calcd for C16H20O2N2I [M + H]+ 399.0570, found 399.0567.
Mizoroki–Heck General Procedure
A vial with a stir bar was either flame-dried under argon or oven-dried, charged with the aryl halide diene (0.1 mmol, 1.0 equiv), and then placed in a glovebox. Anhydrous nickel salt (0.01 mmol, 10 mol %), a ligand (bipy or L27) (0.015 mmol, 15 mol %), Mn (0.3 mmol, 3.0 equiv), KI (0.1 mmol, 1.0 equiv), 2-cyclohexenone (0.3 mmol, 3.0 equiv), and DMF (1.2 mL, 0.08 M) were added, and the vial was sealed with a pressure relief cap and removed from the glovebox. The contents of the vial were stirred at 80 °C in a pie reactor until the reaction was determined to be complete by GC-MS analysis (typically 1–6 h). GC-MS analysis was optimal because the reactant and the product have quite similar TLC properties. Upon completion, the reaction mixture was filtered through an aluminum oxide plug to remove Ni and Mn metals and the plug was washed with EtOAc. The resulting organic solution was washed with 1 N HCl (twice) or water (for pyridine- and pyrimidine-containing substrates) and brine, dried with MgSO4, and filtered. The crude product was concentrated in vacuo and purified by column chromatography.
(6aR,10aR)-5,6a-Dimethyl-6a,10a-dihydrophenanthridin-6(5H)-one (2a). Racemic Procedure. Using the general procedure detailed above, diene bromide 1a (0.0995 g, 0.327 mmol, 1.0 equiv) in DMF (3.9 mL, 0.08M) or diene iodide 1a-I (0.101 g, 0.283 mmol, 1.0 equiv) in DMF (3.4 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % 2,2′-bipyridine for 1.5 or 3 h, respectively. The crude products were purified by column chromatography (silica, 4:1 hexanes/EtOAc) to afford 2a (0.0626 g, 0.278 mmol) in 85% yield from 1a or in 98% yield (0.0631 g, 0.280 mmol) from 1a-I as a yellow oil.
Enantioselective Procedure. Using the general procedure detailed above, diene iodide 1a-I (0.0449 g, 0.127 mmol, 1.0 equiv) in DMF (1.5 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % tBu-6CH3iQuinox (L27) for 1.5 h. The crude products were purified by column chromatography (silica, 4:1 hexanes/EtOAc) to afford 2a (0.0226 g, 0.100 mmol) with an enantiomeric ratio of 10:1 (82% ee) in 79% yield as a yellow oil. Spectral data were in accordance with the literature:59 [α]D20 = +146 (c 0.95, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.30 (td, J = 8.0, 1.6 Hz, 1H), 7.23 (dd, J = 7.5, 1.6 Hz, 1H), 7.08 (td, J = 7.5, 1.1 Hz, 1H), 6.99 (dd, J = 8.2, 1.1 Hz, 1H), 6.12–6.07 (m, 1H), 6.05–5.99 (m, 1H), 5.91–5.87 (m, 1H), 5.58 (ddt, J = 9.3, 3.0, 1.0 Hz, 1H), 3.52–3.45 (m, 1H), 3.35 (s, 3H), 1.23 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 131. 8, 128. 6, 127.8, 125.2, 124.2, 123.3, 114.3, 44.3, 29.9, 23.4; δd 173.4, 139.0, 126.6, 40.8; GC (method B) tR = 1.940 min; EI-MS m/z (%) 224.1 (M – 1+, 79), 210.1 (100), 195.1 (23), 182.1 (28), 167.1 (30), 152.1 (9), 139.0 (5), 128.0 (5), 115.0 (8), 105.0 (4), 90.9 (7), 77.0 (9), 63.0 (6), 51.1 (6); HPLC (Chiralcel OD-H, 97.5:2.5 n-hexane/isopropanol, flow rate of 1 mL/min, λ = 254 nm) tR1 = 11.39 min (major), tR2 = 12.87 min (minor).
(6aR,10aR)-5-(Methoxymethyl)-6a-methyl-6a,10a-dihydrophenanthridin-6(5H)-one (2b). Racemic Procedure. Using the general procedure detailed above, diene bromide 1b (0.0797 g, 0.237 mmol, 1.0 equiv) in DMF (2.8 mL, 0.08 M) or diene iodide 1b-I (0.0404 g, 0.104 mmol, 1.0 equiv) in DMF (1.2 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % 2,2′-bipyridine for 2 or 4 h, respectively. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2b (0.0459 g, 0.179 mmol) in 76% yield from 1b or in 75% yield (0.0201 g, 0.0787 mmol) from 1b-I as a white solid: mp 136.5–138.3 °C.
Enantioselective Procedure. Using the general procedure detailed above, diene iodide 1b-I (0.0504 g, 0.130 mmol, 1.0 equiv) in DMF (1.6 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % tBu-6CH3iQuinox (L27) for 2.5 h. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2b (0.0281 g, 0.110 mmol) with an enantiomeric ratio of 10:1 (82% ee) in 85% yield as a white solid. Spectral data were in accordance with the literature:59 [α]D20 = +312 (c 0.74, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.23–7.19 (m, 2H), 7.15 (dd, J = 7.4, 1.3 Hz, 1H), 7.07–7.01 (m, 1H), 6.09–6.02 (m, 1H), 5.99–5.92 (m, 1H), 5.86–5.81 (m, 1H), 5.55–5.49 (m, 2H), 5.03 (d, J = 10.7 Hz, 1H), 3.46–3.39 (m, 1H), 3.27 (s, 3H), 1.21 (s, 3H); GC (method B) tR = 2.128; EI-MS m/z (%) 255.1 (35, M+), 240.0 (5), 223.0 (23), 210.0 (100), 192.0 (92), 180.0 (43), 165.0 (41), 152.0 (24), 139.0 (5), 128.0 (6), 115.0 (8), 91.0 (10), 77.0 (8); 13C{1H} NMR (101 MHz, CDCl3) δu 131.6, 128.8, 128.7, 128.0, 125.3, 124.5, 124.0, 115.6, 56.1, 44.5, 23.3; δd 174.8, 137.6, 126.3, 73.7, 40.9; HPLC (Chiralcel OD-H, 97.5:2.5 n-hexane/isopropanol, flow rate of 1 mL/min, λ = 254 nm) tR1 = 9.54 min (major), tR2 = 14.52 min (minor).
(6aR,10aR)-6a-Ethyl-5-(methoxymethyl)-6a,10a-dihydrophenanthridin-6(5H)-one (2c). Racemic Procedure. Using the general procedure detailed above, diene iodide 1c-I (0.135 g, 0.336 mmol, 1.0 equiv) in DMF (4 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % 2,2′-bipyridine for 2 h. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2c (0.0786 g, 0.292 mmol) in 87% yield as a clear colorless oil.
Enantioselective Procedure. Using the general procedure detailed above, diene iodide 1c-I (0.100 g, 0.252 mmol, 1.0 equiv) in DMF (3 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % tBu-6CH3iQuinox (L27) for 3 h. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2c (0.0559 g, 0.208 mmol) with an enantiomeric ratio of 12:1 (84% ee) in 82% yield as a clear colorless oil.
The 1 mmol Scale Enantioselective Procedure. Using the general procedure detailed above, diene iodide 1c-I (0.397 g, 1.0 mmol, 1.0 equiv) in DMF (12 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % tBu-6CH3iQuinox (L27) for 6 h. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2c (0.142 g, 0.527 mmol) with an enantiomeric ratio of 9:1 (80% ee) in 53% yield as a clear colorless oil. Spectral data were in accordance with the literature:59 [α]D20 = +157 (c 0.35, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.31–7.24 (m, 2H), 7.21 (d, 1H), 7.10 (td, J = 6.9, 2.0 Hz, 1H), 6.22–6.14 (m, 1H), 6.04–5.99 (m, 1H), 5.95 (d, J = 9.1 Hz, 1H), 5.63 (d, J = 10.6 Hz, 1H), 5.55 (dd, J = 9.4, 2.5 Hz, 1H), 5.05 (d, J = 10.6 Hz, 1H), 3.70–3.64 (m, 1H), 3.34 (s, 3H), 1.75–1.64 (m, 1H), 1.59–1.46 (m, 1H), 0.91 (t, J = 7.4 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 129.8, 128.9, 128.6, 128.0, 125.2, 125.2, 124.0, 115.5, 56.2, 41.3, 9.1; δd 174.4, 137.8, 126.2, 73.8, 45.2, 27.9; GC (method B) tR = 2.380; EI-MS m/z (%) 269.1 (36, M+), 237.0 (28), 224.0 (100), 208.0 (91), 196.0 (64), 178.0 (82), 167.0 (28), 152.0 (26), 139.0 (5), 115.0 (5), 91.0 (8), 77.0 (8); HPLC (Chiralcel OD-H, 97.5:2.5 n-hexane/isopropanol, flow rate of 1 mL/min, λ = 254 nm) tR1 = 8.27 min (major), tR2 = 14.30 min (minor).
(6aR,10aR)-6a-Isopropyl-5-methyl-6a,10a-dihydrophenanthridin-6(5H)-one (2d). Racemic Procedure. Using the general procedure detailed above, diene bromide 1d (0.0954 g, 0.285 mmol, 1.0 equiv) in DMF (3.4 mL, 0.08 M) or diene iodide 1d-I (0.0993 g, 0.262 mmol, 1.0 equiv) in DMF (3.1 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % 2,2′-bipyridine for 1.5 or 3 h, respectively. The crude products were purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2d (0.0613 g, 0.242 mmol) in 85% yield from 1d or in 90% yield (0.0595 g, 0.235 mmol) from 1d-I as a clear colorless oil.
Enantioselective Procedure. Using the general procedure detailed above, diene iodide 1d-I (0.0499 g, 0.131 mmol, 1.0 equiv) in DMF (1.6 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % tBu-6CH3iQuinox (L27) for 2.5 h. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2d (0.0273 g, 0.108 mmol) with an enantiomeric ratio of 9:1 (80% ee) in 83% yield as a clear colorless oil. Spectral data were in accordance with the literature:59 [α]D20 = +270 (c 0.5, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.23 (ddd, J = 8.1, 7.5, 1.6 Hz, 1H), 7.13 (dd, J = 7.3, 1.6 Hz, 1H), 7.00 (td, J = 7.4, 1.1 Hz, 1H), 6.90 (dd, J = 8.2, 1.1 Hz, 1H), 6.17–6.08 (m, 1H), 6.00–5.86 (m, 2H), 5.48–5.40 (m, 1H), 3.74 (s, 1H), 3.29 (s, 3H), 1.73–1.58 (m, 1H), 0.86 (d, J = 6.9 Hz, 3H), 0.74 (d, J = 6.8 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 129.2, 128.5, 127.8, 127.2, 125.4, 125.2, 123.3, 114.2, 41.09, 29.9, 29.8, 18.5, 18.0; δd 172.6, 139.3, 126.9, 48.5; GC (method B) tR = 2.294; EI-MS m/z (%) 253.1 (12, M+), 210.1 (100), 195.0 (23), 180.0 (7), 167.0 (10), 152.0 (7); HPLC (Chiralcel OD-H, 97.5:2.5 n-hexane/isopropanol, flow rate of 1 mL/min, λ = 254 nm) tR1 = 9.96 min (major), tR2 = 11.76 min (minor).
(6aR,10aR)-6a-Isopropyl-5-(methoxymethyl)-6a,10a-dihydrophenanthridin-6(5H)-one (2e). Racemic Procedure. Using the general procedure detailed above, diene bromide 1e (0.0988 g, 0.275 mmol, 1.0 equiv) in DMF (3.3 mL, 0.08 M) or diene iodide 1e-I (0.103 g, 0.243 mmol, 1.0 equiv) in DMF (2.9 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % 2,2′-bipyridine for 2 or 2.5 h, respectively. The crude products were purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2e (0.0708 g, 0.24 mmol) in 90% yield from 1e or in 91% yield (0.0625 g, 0.221 mmol) from 1e-I as a white solid: mp 104.4–106.5 °C.
Chiral Procedure. Using the general procedure detailed above, diene iodide 1e-I (0.103 g, 0.243 mmol, 1.0 equiv) in DMF (2.9 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % tBu-6CH3iQuinox (L27) for 3 h. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2e (0.0460 g, 0.162 mmol) with an enantiomeric ratio of 11:1 (84% ee) in 67% yield as a tan oil that solidified to white crystals upon cooling: [α]D20 = +160 (c 0.65, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.32–7.22 (m, 2H), 7.19 (dd, J = 7.4, 1.5 Hz, 1H), 7.10 (td, J = 7.2, 1.8 Hz, 1H), 6.22 (ddd, J = 9.6, 5.2, 1.0 Hz, 1H), 6.07–5.95 (m, 2H), 5.66 (d, J = 10.6 Hz, 1H), 5.53 (ddt, J = 9.3, 2.2, 1.0 Hz, 1H), 5.02 (d, J = 10.5 Hz, 1H), 3.81 (t, J = 3.2 Hz, 1H), 3.36 (s, 3H), 1.79 (hept, J = 6.9 Hz, 1H), 0.98 (d, J = 6.9 Hz, 3H), 0.84 (d, J = 6.8 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 129.2, 128.4, 128.0, 126.7, 125.8, 125.4, 124.0, 115.5, 74.1, 56.3, 48.7, 41.3, 40.7, 29.3, 18.5, 17.7; δd 173.8, 138.2, 126.6, 74.1, 48.7; GC (method B) tR = 2.560 min; EI-MS m/z (%) 283.1 (M+, 17), 251.0 (12), 240.0 (17), 224 (10), 208.0 (100), 196.0 (99), 178 (67), 167 (14), 152.0 (17), 139 (4), 115.0 (4), 105.0 (4), 77.0 (5); HRMS (ESI) calcd for C18H22O2N [M + H]+ 284.1651, found 284.1646; HPLC )Chiralcel OD-H, 97.5:2.5 n-hexane/isopropanol, flow rate of 1 mL/min, λ = 254 nm) tR1 = 7.80 min (major), tR2 = 13.46 min (minor).
(6aR,10aR)-5,6a-Bis(methoxymethyl)-6a,10a-dihydrophenanthridin-6(5H)-one (2f). Racemic Procedure. Using the general procedure detailed above, diene iodide 1f-I (0.100 g, 0.242 mmol, 1.0 equiv) in DMF (2.9 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % 2,2′-bipyridine for 3 h. The crude product was purified by column chromatography (silica, 7:1 hexanes/EtOAc) to afford 2f (0.0630 g, 0.221 mmol) in 91% yield as a yellow oil.
Enantioselective Procedure. Using the general procedure detailed above, diene iodide 1f-I (0.0500 g, 0.121 mmol, 1.0 equiv) in DMF (1.5 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % tBu-6CH3iQuinox (L27) for 4 h. The crude product was purified by column chromatography (silica, 7:1 hexanes/EtOAc) to afford 2f (0.0313 g, 0.110 mmol) with an enantiomeric ratio of 12:1 (85% ee) in 91% yield as a yellow oil: [α]D20 = +126 (c 0.21, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.22–7.18 (m, 2H), 7.18–7.14 (m, 1H), 7.06–7.00 (m, 1H), 6.12–6.04 (m, 1H), 6.02–5.92 (m, 1H), 5.86–5.79 (m, 1H), 5.71–5.63 (m, 1H), 5.40 (d, J = 10.6 Hz, 1H), 5.17 (d, J = 10.6 Hz, 1H), 3.88–3.82 (m, 1H), 3.46–3.36 (m, 2H), 3.29 (s, 3H), 3.23 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 128.2, 127.9, 127.9, 127.5, 125.8, 124.8, 124.0, 115.5, 59.5, 56.2, 37.6; δd 171.9, 137.8, 125.8, 74.1, 73.5, 47.1; GC (method B) tR = 2.597 min; EI-MS m/z (%) 285.1 (M+, 1), 240.0 (5), 221.0 (5), 209.0 (100), 195.0 (11), 180.0 (45), 165.0 (10), 152.0 (10); HRMS (ESI) calcd for C17H20O3N [M + H]+ 286.1443, found 286.1442; HPLC (Chiralcel OD-H, 97.5:2.5 n-hexane/isopropanol, flow rate of 1 mL/min, λ = 254 nm) tR1 = 18.33 min (major), tR2 = 24.17 min (minor).
Ethyl 2-[(6aR,10aR)-5-Methyl-6-oxo-5,10a-dihydrophenanthridin-6a(6H)-yl]acetate (2g). Racemic Procedure. Using the general procedure detailed above, diene iodide 1g-I (0.202 g, 0.475 mmol, 1.0 equiv) in DMF (5.7 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % 2,2′-bipyridine for 2 h. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2g (0.104 g, 0.349 mmol) in 74% yield as a clear colorless oil.
Enantioselective Procedure. Using the general procedure detailed above, diene iodide 1g-I (0.0501 g, 0.118 mmol, 1.0 equiv) in DMF (1.4 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % tBu-6CH3iQuinox (L27) for 2 h. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2g (0.0249 g, 0.0837 mmol) with an enantiomeric ratio of 3:1 (49% ee) in 71% yield as a clear colorless oil. Spectral data were in accordance with the literature:59 [α]D20 = +209 (c 0.86, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.31–7.21 (m, 2H), 7.05 (td, J = 7.5, 1.1 Hz, 1H), 6.97 (dd, J = 8.1, 1.1 Hz, 1H), 6.14–5.99 (m, 2H), 5.95–5.85 (m, 1H), 5.85–5.75 (m, 1H), 4.17–4.01 (m, 3H), 3.38 (s, 3H), 2.70 (q, 2H), 1.23 (t, J = 7.1 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 128.5, 127.9, 127.8, 127.7, 125.2, 125.0, 123.3, 114.2, 38.5, 30.2, 14.2; δd 171.4, 170.9, 139.4, 125.8, 60.6, 44.3, 37.8; GC (method B) tR = 3.162 min; EI-MS m/z (%) 296.1 (M+, 1), 252.1 (5), 210.0 (100), 195.0 (10), 180.0 (15), 165.0 (7), 152.0 (8); HPLC (Chiralcel OD-H, 60:40 n-hexane/isopropanol, flow rate of 1 mL/min, λ = 254 nm) tR1 = 18.93 min (major), tR2 = 24.37 min (minor).
(6aR,10aR)-6a-Benzyl-5-(methoxymethyl)-6a,10a-dihydrophenanthridin-6(5H)-one (2h). Racemic Procedure. Using the general procedure detailed above, diene bromide 1h (0.0990 g, 0.241 mmol, 1.0 equiv) in DMF (2.9 mL, 0.08 M) or diene iodide 1h-I (0.0504 g, 0.109 mmol, 1.0 equiv) in DMF (1.3 mL, 0.08M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % 2,2′-bipyridine for 2 or 4 h, respectively. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2h (0.0721 g, 0.218 mmol) in 90% yield from 1h or in 97% yield (0.0625 g, 0.105 mmol) from 1h-I as a yellow oil.
Chiral Procedure. Using the general procedure detailed above, diene iodide 1h-I (0.0503 g, 0.109 mmol, 1.0 equiv) in DMF (1.3 mL, 0.08M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % tBu-6CH3iQuinox (L27) for 3 h. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2h (0.0237 g, 0.0715 mmol) with an enantiomeric ratio of 11:1 (83% ee) in 66% yield as a tan oil. Spectral data were in accordance with the literature:59 [α]D20 = +50 (c 0.23, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.33–7.30 (m, 2H), 7.30–7.26 (m, 1H), 7.25–7.19 (m, 2H), 7.18–7.14 (m, 2H), 7.04–6.99 (m, 2H), 6.10–6.04 (m, 2H), 5.98–5.89 (m, 1H), 5.59 (d, J = 10.6 Hz, 1H), 5.55 (dd, J = 9.4, 2.9 Hz, 1H), 5.18 (d, J = 10.6 Hz, 1H), 3.62–3.58 (m, 1H), 3.39 (s, 3H), 3.01 (d, J = 13.3 Hz, 1H), 2.86 (d, J = 13.3 Hz, 1H); 13C{1H} NMR (101 MHz, CDCl3) δu 130.7, 130.2, 128.7, 128.4, 128.3, 128.1, 126.8, 125.3, 124.7, 124.2, 115.7, 56.4, 40.2; δd 174.1, 137.9, 136.3, 125.9, 74.0, 46.8, 40.9; GC (method A) tR = 17.679 min; EI-MS m/z (%) 331.1 (M+, 1), 239.0 (10), 224.0 (19), 208.0 (100), 196.0 (15), 178.0 (26), 165.0 (6), 152.0 (7), 91.0 (40); HPLC (Chiralcel OD-H, 97.5:2.5 n-hexane/isopropanol, flow rate of 1 mL/min, λ = 254 nm) tR1 = 9.21 min (major), tR2 = 15.29 min (minor).
(6aR,10aR)-2-Fluoro-6a-isopropyl-5-(methoxymethyl)-6a,10a-dihydrophenanthridin-6(5H)-one (2i). Racemic Procedure. Using the general procedure detailed above, diene bromide 1i (0.101 g, 0.262 mmol, 1.0 equiv) in DMF (3.1 mL, 0.08 M) or diene iodide 1i-I (0.0501 g, 0.117 mmol, 1.0 equiv) in DMF (1.4 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % 2,2′-bipyridine for 4 h. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2i (0.0669 g, 0.222 mmol) in 85% yield from 1i or in 61% yield (0.0214 g, 0.0710 mmol) from 1i-I as a white solid: mp 130.3–132.0 °C.
Enantioselective Procedure. Using the general procedure detailed above, diene iodide 1i-I (0.0502 g, 0.117 mmol, 1.0 equiv) in DMF (1.4 mL, 0.08M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % tBu-6CH3iQuinox (L27) for 6 h. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2i (0.0307 g, 0.102 mmol) with an enantiomeric ratio of 10:1 (81% ee) in 87% yield as a white oil that solidified to white crystals upon cooling: [α]D20 = +152 (c 0.21, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.23 (dd, J = 8.9, 4.8 Hz, 1H), 6.97 (td, J = 8.6, 3.0 Hz, 1H), 6.92 (dd, J = 8.2, 2.9 Hz, 1H), 6.26–6.17 (m, 1H), 6.07–5.94 (m, 2H), 5.64 (d, J = 10.7 Hz, 1H), 5.51 (ddt, J = 9.4, 2.2, 1.0 Hz, 1H), 4.97 (d, J = 10.8 Hz, 1H), 3.78 (s, 1H), 3.35 (s, 3H), 1.77 (hept, J = 6.9 Hz, 1H), 0.99 (d, J = 6.9 Hz, 3H), 0.85 (d, J = 6.8 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 128.2, 126.7, 125.8 (d, 3JC–F = 12.1 Hz), 117.0, 116.9, 115.2 (d, 2JC–F = 23.2 Hz), 114.4 (d, 2JC–F = 23.2 Hz), 56.3, 41.4, 29.3, 18.5, 17.9; δd 173.3, 160.5 (d, 1JC–F = 245.5 Hz), 134.4, 128.6, 74.4, 48.5; 19F NMR (376 MHz, CDCl3) δ −119.34; GC (method B) tR = 2.490 min; EI-MS m/z (%) 301.1 (M+, 32), 269 (14), 258.0 (7), 242.0 (9), 226.0 (77), 214.0 (100), 196.0 (58), 185.0 (14), 170.0 (10), 105.0 (5); HRMS (ESI) calcd for C18H21O2NF [M + H]+ 302.1556, found 302.1550; HPLC (Chiralcel OD-H, 97.5:2.5 n-hexane/isopropanol, flow rate of 1 mL/min, λ = 254 nm) tR1 = 9.53 min (major), tR2 = 16.15 min (minor).
(6aR,10aR)-2-Chloro-6a-isopropyl-5-(methoxymethyl)-6a,10a-dihydrophenanthridin-6(5H)-one (2j). Racemic Procedure. Using the general procedure detailed above, diene bromide 1j (0.0999 g, 0.251 mmol, 1.0 equiv) in DMF (3 mL, 0.08 M) or diene iodide 1j-I (0.0502 g, 0.112 mmol, 1.0 equiv) in DMF (1.3 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % 2,2′-bipyridine for 6 h at 65 °C. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2j (0.0531 g, 0.167 mmol) in 67% yield from 1j or in 91% yield (0.0324 g, 0.102 mmol) from 1j-I as a tan solid: mp 101.1–102.9 °C.
Enantioselective Procedure. Using the general procedure detailed above, diene iodide 1j-I (0.0508 g, 0.114 mmol, 1.0 equiv) in DMF (1.4 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % tBu-6CH3iQuinox (L27) for 6 h at 65 °C. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2j (0.0242 g, 0.0761 mmol) with an enantiomeric ratio of 7:1 (74% ee) in 68% yield as a clear oil that solidified to tan crystals on cooling: [α]D20 = +99 (c 0.22, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.20–7.16 (m, 1H), 7.14 (s, 1H), 7.11 (d, J = 2.3 Hz, 1H), 6.19–6.10 (m, 1H), 5.99–5.93 (m, 1H), 5.90 (d, J = 10.7 Hz, 1H), 5.56 (d, J = 10.7 Hz, 1H), 5.47–5.39 (m, 1H), 4.91 (d, J = 10.6 Hz, 1H), 3.74–3.68 (m, 1H), 3.27 (s, 3H), 1.70 (hept, J = 6.7 Hz, 1H), 0.91 (d, J = 6.9 Hz, 3H), 0.79 (d, J = 6.8 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 128.3, 128.2, 127.9, 126.6, 125.9, 125.8, 116.9, 56.3, 41.2, 29.3, 18.5, 17.8; δd 173.4, 136.9, 129.0, 128.5, 74.2, 48.6; GC (method B) tR = 3.135 min; EI-MS m/z (%) 317.1 (M+, 29), 285.1 (10), 272.0 (9), 258.0 (10), 242.0 (86), 230.0 (100), 214.0 (61), 195.0 (12), 180.0 (13), 166.0 (13), 152.0 (14), 139.0 (6), 105.0 (7), 77.0 (5); HRMS (ESI) calcd for C18H21O2NCl [M + H]+ 318.1261, found 318.1260; HPLC (Chiralcel OD-H, 97.5:2.5 n-hexane/isopropanol, flow rate of 1 mL/min, λ = 254 nm) tR1 = 8.98 min (major), tR2 = 13.42 min (minor).
6a-Isopropyl-5-(methoxymethyl)-2-methyl-6a,10a-dihydrophenanthridin-6(5H)-one (2k). Using the general procedure detailed above, diene bromide 1k (0.101 g, 0.264 mmol, 1.0 equiv) in DMF (3.2 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % 2,2′-bipyridine for 3 h. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2k (0.0684 g, 0.229 mmol) in 87% yield as a clear colorless oil: 1H NMR (400 MHz, CDCl3) δ 7.14 (d, J = 8.3 Hz, 1H), 7.08 (dd, J = 8.1, 2.3 Hz, 1H), 6.99 (d, J = 2.1 Hz, 1H), 6.25–6.16 (m, 1H), 6.06–5.94 (m, 2H), 5.63 (d, J = 10.6 Hz, 1H), 5.52 (ddt, J = 9.3, 2.2, 1.1 Hz, 1H), 5.01 (d, J = 10.6 Hz, 1H), 3.76 (d, J = 3.3 Hz, 1H), 3.34 (s, 3H), 2.34 (s, 3H), 1.78 (hept, J = 7.0 Hz, 1H), 0.98 (d, J = 6.9 Hz, 3H), 0.84 (d, J = 6.8 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 129.3, 129.1, 128.4, 126.8, 125.8, 125.3, 115.4, 56.3, 41.3, 29.2, 20.7, 18.5, 17.9; δd 173.7, 135.7, 133.6, 126.4, 74.1, 48.7; GC (method B) tR = 2.836 min; EI-MS m/z (%) 297.1 (M+, 28), 265.1 (5), 254.1 (8), 238.0 (6), 222.0 (98), 210.0 (100), 192.0 (53), 180.0 (14), 165.0 (16), 152.0 (11), 77.0 (4); HRMS (ESI) calcd for C19H24O2N [M + H]+ 298.1807, found 298.1803.
(6aR,10aR)-6a-Isopropyl-5-(methoxymethyl)-3-methyl-6a,10a-dihydrophenanthridin-6(5H)-one (2l). Racemic Procedure. Using the general procedure detailed above, diene iodide 1l-I (0.101 g, 0.235 mmol, 1.0 equiv) in DMF (2.8 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % 2,2′-bipyridine for 2 h. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2l (0.0562 g, 0.189 mmol) in 81% yield as a white cloudy oil.
Enantioselective Procedure. Using the general procedure detailed above, diene iodide 1l-l (0.0501 g, 0.118 mmol, 1.0 equiv) in DMF (1.4 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % tBu-6CH3iQuinox (L27) for 1.5 h. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2l (0.0222 g, 0.0746 mmol) with an enantiomeric ratio of 10:1 (81% ee) in 63% yield as a white cloudy oil: [α]D20 = +130 (c 0.39, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.03–6.97 (m, 2H), 6.88–6.81 (m, 1H), 6.17–6.09 (m, 1H), 5.96–5.88 (m, 2H), 5.58 (d, J = 10.6 Hz, 1H), 5.47–5.43 (m, 1H), 4.93 (d, J = 10.6 Hz, 1H), 3.73–3.65 (m, 1H), 3.30 (s, 3H), 2.30 (s, 3H), 1.72 (hept, J = 6.9 Hz, 1H), 0.90 (d, J = 6.9 Hz, 3H), 0.76 (d, J = 6.8 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 129.6, 128.3, 126.7, 125.8, 125.2, 124.7, 116.2, 56.4, 40.9, 29.2, 21.6, 18.5, 17.9; δd 174.0, 138.1, 137.9, 123.6, 74.1, 48.6; GC (method B) tR = 2.775 min; EI-MS m/z (%) 297.1 (M+, 18), 266.1 (4), 254.1 (22), 238.0 (6), 222.0 (100), 210.0 (68), 192.0 (45), 180.0 (10), 165.0 (12), 152.0 (8), 105.0 (5); HRMS (ESI) calcd for C19H24O2N [M + H]+ 298.1807, found 298.1800; HPLC (Chiralcel OD-H, 97.5:2.5 n-hexane/isopropanol, flow rate of 1 mL/min, λ = 254 nm) tR1 = 7.32 min (major), tR2 = 12.13 min (minor).
5-(Methoxymethyl)-6a-methyl-6a,9,10,10a-tetrahydrobenzo[c][1,5]naphthyridin-6(5H)-one (2m-2). Using the general procedure detailed above, diene bromide 1m (0.100 g, 0.297 mmol, 1.0 equiv) in DMF (3.6 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % 2,2′-bipyridine for 6 h. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2m-2 (0.0635 g, 0.246 mmol) in 85% yield as a yellow oil: 1H NMR (400 MHz, CDCl3) δ 8.30 (dd, J = 4.8, 1.4 Hz, 1H), 7.54 (dd, J = 8.3, 1.4 Hz, 1H), 7.19 (dd, J = 8.3, 4.8 Hz, 1H), 5.91 (d, J = 1.5 Hz, 2H), 5.62 (d, J = 10.8 Hz, 1H), 5.04 (d, J = 10.8 Hz, 1H), 3.37 (s, 3H), 2.91 (dd, J = 12.9, 3.0 Hz, 1H), 2.26–2.20 (m, 1H), 2.19–2.14 (m, 1H), 1.98–1.91 (m, 1H), 1.69–1.62 (m, 1H), 1.27 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 144.4, 129.8, 128.0, 122.4, 56.3, 47.0, 24.9; δd 174.8, 148.5, 133.5, 73.6, 42.5, 26.0, 24.7; GC (method B) tR = 2.260 min; EI-MS m/z (%) 258.1 (M+, 100), 243.0 (99), 227.0 (6), 211.0 (21), 205.0 (13), 197.0 (23), 185.0 (85), 171.0 (25), 156.0 (5), 144.0 (4), 131.0 (5), 91.0 (6), 77.0 (6); HRMS (ESI) calcd for C15H19O2N2 [M + H]+ 259.1447, found 259.1447.
5-(Methoxymethyl)-6a-methyl-6a,10a-dihydrobenzo[c][1,8]naphthyridin-6(5H)-one (2n). Using the general procedure detailed above, diene bromide 1n (0.0895 g, 0.267 mmol, 1.0 equiv) in DMF (3.2 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % 2,2′-bipyridine for 5 h. The crude product was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to afford 2n (0.0542 g, 0.211 mmol) in 80% yield as a tan solid: mp 124.9–126.0 °C; 1H NMR (400 MHz, CDCl3) δ 8.37 (dd, J = 5.0, 1.8 Hz, 1H), 7.60–7.57 (m, 1H), 7.05 (dd, J = 7.4, 4.9 Hz, 1H), 6.17–6.07 (m, 2H), 5.92–5.88 (m, 1H), 5.73 (d, J = 9.6 Hz, 1H), 5.65–5.58 (m, 1H), 5.56 (d, J = 9.7 Hz, 1H), 3.51 (t, J = 3.1 Hz, 1H), 3.41 (s, 3H), 1.33 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 147.0, 136.9, 131.3, 127.4, 125.9, 124.6, 119.3, 57.1, 42.7, 23.3; δd 175.1, 150.0, 121.3, 71.4, 41.2; GC (method B) tR = 2.290 min; EI-MS m/z (%) 256.1 (M+, 13), 241.0 (46), 224.0 (82), 211.0 (71), 195.0 (25), 181.0 (24), 167.0 (100), 153.0 (8), 142.0 (10), 133.0 (18), 129.1 (7), 121.0 (11), 115.0 (12), 109.0 (4), 91.0 (15), 79.0 (6), 65.0 (4); HRMS (ESI) calcd for C15H17O2N2 [M + H]+ 257.1290, found 257.1289.
(6aR,10aR)-6a-Ethyl-5-(methoxymethyl)-6a,10a-dihydrobenzo[c][1,8]naphthyridin-6(5H)-one (2o). Racemic Procedure. Using the general procedure detailed above, diene bromide 1o (0.151 g, 0.427 mmol, 1.0 equiv) in DMF (5.1 mL, 0.08 M) or diene iodide 1o-I (0.100 g, 0.251 mmol, 1.0 equiv) in DMF (3 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % 2,2′-bipyridine for 3 or 4 h, respectively. The crude product was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to afford 2o (0.0873 g, 0.323 mmol) in 76% yield from 1o or in 82% yield (0.0557 g, 0.206 mmol) from 1o-I as a clear colorless oil.
Enantioselective Procedure. Using the general procedure detailed above, diene iodide 1o-I (0.0198 g, 0.0502 mmol, 1.0 equiv) in DMF (0.6 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % tBu-6FiQuinox (L26) for 2.5 h. The crude product was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to afford 2o (0.0103 g, 0.0381 mmol) with an enantiomeric ratio of 7:1 (76% ee) in 76% yield as a clear colorless oil: [α]D20 = +73 (c 0.16, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.27 (dd, J = 4.9, 1.8 Hz, 1H), 7.48 (dd, J = 7.3, 0.6 Hz, 1H), 6.96 (dd, J = 7.4, 4.9 Hz, 1H), 6.16–6.08 (m, 1H), 6.05–5.95 (m, 1H), 5.91–5.83 (m, 1H), 5.65 (d, J = 9.7 Hz, 1H), 5.49–5.40 (m, 2H), 3.62–3.56 (m, 1H), 3.31 (s, 3H), 1.65–1.59 (m, 1H), 1.49–1.43 (m, 1H), 0.85 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 146.9, 136.9, 129.6, 127.6, 125.9, 125.3, 119.3, 57.1, 39.7, 9.1; δd 174.8, 150.1, 121.3, 71.4, 45.4, 28.1; GC (method B) tR = 2.626 min; EI-MS m/z (%) 270.1 (M+, 10), 255.1 (24), 238.1 (100), 225.1 (53), 209.1 (25), 197.0 (52), 181.1 (36), 167.1 (50), 154.0 (16), 140.0 (8), 132.0 (14), 127.0 (19), 121.0 (19), 115.0 (6), 91.0 (10), 77.0 (7); HRMS (ESI) calcd for C16H19O2N2 [M + H]+ 271.1447, found 271.1445; HPLC (Chiralcel OD-H, 85:15 n-hexane/isopropanol, flow rate of 1 mL/min, λ = 254 nm) tR1 = 11.17 min (minor), tR2 = 12.18 min (major).
Ethyl 4b,9-Dihydro-8aH-fluorene-8a-carboxylate (2p). Using the general procedure detailed above diene iodide S1g (0.199 g, 0.543 mmol, 1.0 equiv) in DMF (6.5 mL, 0.08 M) was subjected to the Heck reaction conditions with 10 mol % NiI2/15 mol % 2,2′-bipyridine for 3 h. The crude product was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to afford 2p (0.0947 g, 0.394 mmol) in 73% yield as a clear colorless oil. Spectral data were in accordance with the literature:861H NMR (400 MHz, CDCl3) δ 7.24–7.15 (m, 4H), 6.07–5.98 (m, 1H), 5.95–5.86 (m, 2H), 5.80–5.72 (m, 1H), 4.48 (d, J = 4.9 Hz, 1H), 4.18 (q, J = 7.1 Hz, 2H), 3.58 (d, J = 15.9 Hz, 1H), 3.15 (d, J = 15.9 Hz, 1H), 1.26 (t, J = 7.1 Hz, 4H); 13C{1H} NMR (101 MHz, CDCl3) δu 128.2, 127.1, 127.0, 126.8, 124.0, 123.7, 123.2, 121.1, 46.2, 14.2; δd 175.2, 144.3, 139.8, 61.2, 46.0; GC (method B) tR = 1.676 min; EI-MS m/z (%) 240.1 (M+, 12), 167.1 (100), 152.0 (13), 139.0 (4), 115.0 (4).
MOM (methoxymethyl) Group Deprotection
General Procedure A
A flame-dried round-bottom flask with a stir bar, under argon, was charged with chlorotrimethylsilane (0.45 mmol, 4.5 equiv) dissolved in CH3CN (1.7 mL, 0.06 M). After NaI (0.45 mmol, 4.5 equiv) was added, the resulting heterogeneous solution was stirred for 15 min at room temperature. In a second round-bottom flask, the MOM-protected Heck product (0.1 mmol, 1.0 equiv) was dissolved in CH3CN (1.7 mL, 0.06 M) and cooled to 0 °C. The TMS-Cl/NaI solution was added to the Heck product by syringe. The resulting mixture was stirred at 0 °C for 1 h. The reaction progress was monitored by TLC and GC-MS. Upon completion, the reaction was quenched with 1 M NaOH (17 mL/mmol), and the mixture stirred overnight. The aqueous layer was extracted thrice with Et2O. The combined organic layers were washed with brine, dried over MgSO4, and concentrated under reduced pressure. The crude products were purified by column chromatography.
General Procedure B
A flame-dried round-bottom flask with a stir bar, under argon, was charged with the MOM-protected Heck product (0.1 mmol, 1.0 equiv) dissolved in DCM (5 mL, 0.02 M). After the solution was cooled to −78 °C, BCl3 (1.0 M in DCM, 1 mmol, 10 equiv) was added, and the reaction mixture was stirred for 2–3 h. The reaction progress was monitored by TLC and GC-MS. Upon starting material consumption, the reaction was quenched with saturated NaHCO3 (50 mL/mmol), and the mixture extracted thrice with DCM. The combined organic layers were washed with brine, dried over MgSO4, and concentrated under reduced pressure. The crude intermediate was placed in a vial and redissolved in THF (5 mL, 0.02 M). Then, 1 M NaOH (5 mL, 5 mmol, 50 equiv) was added, and the vial was sealed with a pressure relief cap; the contents were stirred at 65 °C in a pie reactor until the reaction was determined to be complete by GC-MS analysis (typically 1 h). Upon completion of the reaction, the mixture was extracted thrice with Et2O. The combined organic layers were washed with brine, dried over MgSO4, and concentrated under reduced pressure. The crude products were purified by column chromatography.
(6aR,10aR)-6a-Ethyl-6a,10a-dihydrophenanthridin-6(5H)-one (3c). Using general MOM group deprotection procedure A with MOM-protected Heck product 2c (0.0368 g, 0.137 mmol, 1.0 equiv) in CH3CN (4.7 mL, 0.03 M) afforded the crude deprotected product that was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to give pure 3c (0.0253 g, 0.112 mmol) in 82% yield as a white solid: mp 127.4–129.2 °C; 1H NMR (400 MHz, CDCl3) δ 8.22 (s, 1H), 7.17–7.10 (m, 2H), 6.97 (td, J = 7.4, 1.2 Hz, 1H), 6.67 (dd, J = 7.8, 1.4 Hz, 1H), 6.14–6.06 (m, 1H), 5.99–5.90 (m, 1H), 5.87–5.80 (m, 1H), 5.52–5.44 (m, 1H), 3.69–3.63 (m, 1H), 1.71–1.59 (m, 1H), 1.55–1.45 (m, 1H), 0.86 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 128.9, 128.8, 128.5, 127.8, 125.4, 125.0, 124.0, 115.0, 41.2, 9.2; δd 174.5, 135.9, 124.8, 45.2, 28.1; GC (method B) tR = 2.352 min; EI-MS m/z (%) 224.1 (M – 1+,14), 196 (100), 178.0 (49), 167 (17), 152.0 (11), 139.0 (4), 115.0 (4), 77.0 (4); HRMS (ESI) calcd for C15H16ON [M + H]+ 226.1232, found 226.1232.
(6aR,10aR)-6a-Isopropyl-6a,10a-dihydrophenanthridin-6(5H)-one (3e). Using general MOM group deprotection procedure A with MOM-protected Heck product 2e (0.100 g, 0.353 mmol, 1.0 equiv) in CH3CN (12 mL, 0.03 M) afforded the crude deprotected product that was purified by column chromatography (silica, 3:2 hexanes/EtOAc) to give pure 3e (0.0681 g, 0.284 mmol) in 81% yield as a white solid: mp 169.9–172.4 °C; 1H NMR (400 MHz, CDCl3) δ 7.45 (s, 1H), 7.25–7.16 (m, 2H), 7.05 (td, J = 7.5, 1.2 Hz, 1H), 6.68 (dd, J = 7.9, 1.1 Hz, 1H), 6.22 (dd, J = 9.7, 5.0 Hz, 1H), 6.06–5.97 (m, 1H), 5.94 (d, J = 9.2 Hz, 1H), 5.54–5.47 (m, 1H), 3.89 (s, 1H), 1.84 (hept, J = 6.8 Hz, 1H), 1.01 (d, J = 6.8 Hz, 3H), 0.86 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 129.3, 128.6, 127.8, 126.0, 125.9, 125.2, 123.8, 114.7, 41.3, 29.9, 18.5, 17.9; δd 173.6, 136.0, 125.3, 48.7; GC (method B) tR = 2.523 min; EI-MS m/z (%) 239.1 (M+, 6), 196 (100), 178.0 (42), 167 (12), 152.0 (9), 139.0 (4); HRMS (ESI) calcd for C16H18ON [M + H]+ 240.1388, found 240.1383.
(6aR,10aR)-6a-(Methoxymethyl)-6a,10a-dihydrophenanthridin-6(5H)-one (3f). Using general MOM group deprotection procedure A with MOM-protected Heck product 2f (0.0326 g, 0.114 mmol, 1.0 equiv) in CH3CN (3.9 mL, 0.03 M) afforded the crude deprotected product that was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to give pure 3f (0.0268 g, 0.111 mmol) in 97% yield as a clear colorless oil: 1H NMR (400 MHz, CDCl3) δ 8.05 (s, 1H), 7.16 (d, J = 7.5 Hz, 1H), 7.12 (td, J = 7.7, 1.5 Hz, 1H), 6.96 (td, J = 7.5, 1.2 Hz, 1H), 6.64 (dd, J = 7.8, 1.2 Hz, 1H), 6.06 (dd, J = 9.5, 5.1 Hz, 1H), 6.03–5.95 (m, 1H), 5.78 (dd, J = 9.4, 4.3 Hz, 1H), 5.68 (d, J = 9.4 Hz, 1H), 3.98 (dd, J = 4.6, 2.1 Hz, 1H), 3.55 (d, J = 8.9 Hz, 1H), 3.39 (d, J = 8.9 Hz, 1H), 3.27 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 128.0, 127.7, 127.3, 126.2, 126.2, 124.5, 124.5, 123.6, 114.8, 59.5, 36.6; δd 171.8, 135.8, 124.5, 72.8, 47.3; GC (method B) tR = 2.603 min; EI-MS m/z (%) 240.1 (M – 1+, 21), 210.0 (20), 196.0 (100), 177.9 (64), 167.0 (15), 152.0 (16), 138.9 (6), 76.9 (4); HRMS (ESI) calcd for C15H16NO2 [M + H]+ 242.1181, found 242.1174.
(6aR,10aR)-2-Fluoro-6a-isopropyl-6a,10a-dihydrophenanthridin-6(5H)-one (3i). Using general MOM group deprotection procedure A with MOM-protected Heck product 2i (0.0793 g, 0.265 mmol, 1.0 equiv) in CH3CN (9 mL, 0.03 M) afforded the crude deprotected product that was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to give pure 3i (0.0355 g, 0.138 mmol) in 52% yield as a white solid: mp 178.0–180.9 °C; 1H NMR (400 MHz, CDCl3) δ 8.64 (s, 1H), 6.94–6.89 (m, 2H), 6.74–6.70 (m, 1H), 6.26–6.17 (m, 1H), 6.06–5.97 (m, 1H), 5.97–5.90 (m, 1H), 5.51–5.43 (m, 1H), 3.87–3.81 (m, 1H), 1.83 (hept, J = 6.9 Hz, 1H), 1.00 (d, J = 6.9 Hz, 3H), 0.87 (d, J = 6.8 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 128.4, 125.9 (d, 3JC–F = 8.6 Hz), 125.4, 116.2, 116.1, 115.3 (d, 2JC–F = 23.0 Hz), 114.4 (d, 2JC–F = 22.8 Hz), 41.2, 30.0, 18.5, 17.9; δd 173.9, 159.0 (d, 1JC–F = 242.2 Hz) 132.3, 126.9 (d, 3JC–F = 7.3 Hz), 48.3; 19F NMR (376 MHz, CDCl3) δ −119.38; GC (method B) tR = 2.527 min; EI-MS m/z (%) 257.1 (M+, 10), 214.0 (100), 196.0 (43), 185.0 (12), 169.9 (10); HRMS (ESI) calcd for C16H17ONF [M + H]+ 258.1294, found 258.1298.
(6aR,10aR)-2-Chloro-6a-isopropyl-6a,10a-dihydrophenanthridin-6(5H)-oneone (3j). Using general MOM group deprotection procedure A with MOM-protected Heck product 2j (0.0161 g, 0.0507 mmol, 1.0 equiv) in CH3CN (1.7 mL, 0.03 M) afforded the crude deprotected product that was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to give pure 3j (0.0130 g, 0.0475 mmol) in 94% yield as a white solid: mp 182.5–184.4 °C; 1H NMR (400 MHz, CDCl3) δ 8.18 (s, 1H), 7.20–7.15 (m, 2H), 6.67 (d, J = 8.2 Hz, 1H), 6.22 (m, 1H), 6.07–5.98 (m, 1H), 5.93 (m, 1H), 5.51–5.43 (m, 1H), 3.87–3.82 (m, 1H), 1.81 (hept, J = 6.9 Hz, 1H), 1.00 (d, J = 6.9 Hz, 3H), 0.87 (d, J = 6.8 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 128.4, 128.4, 127.8, 127.0, 126.0, 125.8, 125.5, 116.1, 41.1, 30.0, 18.5, 17.9; δd 173.7, 134.8, 128.4, 127.0, 48.5; GC (method B) tR = 3.215 min; EI-MS m/z (%) 273.0 (M+, 10), 230.0 (100), 211.9 (26), 194.9 (12), 177.0 (12), 167.0 (14), 151.9 (6), 138.9 (6); HRMS (ESI) calcd for C16H17ONCl [M + H]+ 274.0999, found 274.0995.
6a-Isopropyl-2-methyl-6a,10a-dihydrophenanthridin-6(5H)-one (3k). Using general MOM group deprotection procedure A with MOM-protected Heck product 2k (0.100 g, 0.336 mmol, 1.0 equiv) in CH3CN (11.4 mL, 0.03 M) afforded the crude deprotected product that was purified by column chromatography (silica, 3:2 hexanes/EtOAc) to give pure 3k (0.0724 g, 0.285 mmol) in 85% yield as a white solid: mp 193.3–194.4 °C; 1H NMR (400 MHz, CDCl3) δ 7.41 (s, 1H), 7.02–6.98 (m, 2H), 6.57 (d, J = 8.5 Hz, 1H), 6.21 (dd, J = 9.7, 5.1 Hz, 1H), 6.05–5.96 (m, 1H), 5.93 (d, J = 9.1 Hz, 1H), 5.50 (ddt, J = 9.3, 1.9, 0.9 Hz, 1H), 3.83 (s, 1H), 2.32 (s, 3H), 1.83 (hept, J = 6.9 Hz, 1H), 1.00 (d, J = 6.9 Hz, 3H), 0.87 (d, J = 6.8 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 129.4, 129.2, 128.3, 126.0, 125.9, 125.1, 114.6, 41.3, 29.9, 20.9, 18.5, 17.9; δd 173.5, 133.5, 133.3, 125.1, 48.7; GC (method B) tR = 2.844 min; EI-MS m/z (%) 253.2 (M+, 18), 210.1 (100), 192.1 (46), 180.1 (10), 165.1 (15), 152.1 (10), 77.1 (4); HRMS (ESI) calcd for C17H20ON [M + H]+ 254.1545, found 254.1545.
6a-Methyl-6a,9,10,10a-tetrahydrobenzo[c][1,5]naphthyridin-6(5H)-one (3m-2). Using general MOM group deprotection procedure B with MOM-protected Heck product 2m-2 (0.0323, 0.125 mmol, 1.0 equiv) in CH3CN (4.3 mL, 0.03 M) afforded the crude deprotected product that was purified by column chromatography (silica, 3:2 hexanes/EtOAc) to give pure 3m-2 (0.0169 g, 0.0789 mmol) in 63% yield as a yellow solid: mp 134.9–137.4 °C; 1H NMR (400 MHz, CDCl3) δ 8.83 (s, 1H), 8.27 (dd, J = 3.8, 2.5 Hz, 1H), 7.15–7.12 (m, 2H), 5.95–5.85 (m, 2H), 2.92 (dd, J = 12.7, 3.1 Hz, 1H), 2.20–2.15 (m, 1H), 1.94–1.83 (m, 1H), 1.80–1.69 (m, 2H), 1.31 (s, 1H), 1.30 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 144.3, 129.2, 128.3, 122.4, 121.9, 47.0, 42.3, 25.2; δd 175.6, 147.6, 131.5, 42.3, 25.7, 24.5; GC (method B) tR = 2.294 min; EI-MS m/z (%) 214.0 (M+, 62), 199.0 (100), 185.0 (13), 171.0 (13), 161.0 (14), 156.0 (6), 131.0 (4), 91.0 (4), 77.0 (4); HRMS (ESI) calcd for C13H15ON2 [M + H]+ 215.1184, found 215.1182.
(6aR,10aR)-6a-Ethyl-6a,10a-dihydrobenzo[c][1,8]naphthyridin-6(5H)-one (3o). Using general MOM group deprotection procedure B with MOM-protected Heck product 2o (0.0156, 0.0577 mmol, 1.0 equiv) in CH3CN (1.9 mL, 0.03 M) afforded the crude deprotected product that was purified by column chromatography (silica, 3:2 hexanes/EtOAc) to give pure 3o (0.0102 g, 0.0451 mmol) in 79% yield as a white solid: mp 163.9–165.5 °C; 1H NMR (400 MHz, CDCl3) δ 9.08 (s, 1H), 8.26 (dd, J = 5.0, 1.7 Hz, 1H), 7.60–7.54 (m, 1H), 7.00 (dd, J = 7.4, 5.0 Hz, 1H), 6.16 (m, 1H), 6.06 (m, 1H), 5.91–5.87 (m, 1H), 5.55 (m,1H), 3.77–3.71 (m, 1H), 1.70–1.61 (m, 2H), 0.95 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 146.8, 136.8, 128.9, 127.2, 125.6, 125.3, 119.2, 39.8, 9.2; δd 174.3, 149.7, 120.2, 45.6, 28.2; GC (method B) tR = 2.237 min; EI-MS m/z (%) 225.0 (M – 1+, 20), 211.0 (4), 197.0 (100), 179.0 (6), 169.0 (11), 154.0 (15), 127.0 (8), 115.0 (4), 77.0 (4); HRMS (ESI) calcd for C13H15ON2 [M + H]+ 227.1184, found 227.1183.
Synthesis of Chiral tBu-iQuinox Ligands (L26 and L27)
General Procedure for the Synthesis of Isoquinoline N-Oxides
In a round-bottom flask with a stir bar, isoquinoline (1.0 mmol, 1.0 equiv) was dissolved in DCM (0.65 mL, 10.8 M) and the mixture cooled to 0 °C. m-Chloroperbenzoic acid (mCPBA, 2.0 mmol, 2.0 equiv) was dissolved in DCM (0.65 mL, 10.8 M), and the mixture added dropwise to the reaction mixture. The mixture was allowed to warm to room temperature and react overnight. The reaction mixture was diluted with DCM, washed with 3 N NaOH and brine, and then dried with MgSO4. The crude product was concentrated under vacuum and purified by column chromatography.
6-Fluoroisoquinoline 2-Oxide (4a). Using the general procedure for the synthesis of isoquinoline N-oxides, 6-fluoroisoquinoline (0.300 g, 2.04 mmol, 1.0 equiv) in DCM (1.3 mL, 10.8 M) reacted with mCPBA (1.01 g, 4.08 mmol, 2.0 equiv) in DCM (2.7 mL, 10.8 M) to afford the crude product that was purified by column chromatography (silica, 10:1 EtOAc/MeOH) to give pure 4a (0.288 g, 1.76 mmol) in 86% yield as a tan solid: mp 225.1–225.6 °C; 1H NMR (400 MHz, CDCl3) δ 8.76 (d, J = 4.0 Hz, 1H), 8.20–8.13 (m, 1H), 7.75 (dd, J = 9.0, 5.2 Hz, 1H), 7.64 (d, J = 7.2 Hz, 1H), 7.48–7.37 (m, 2H); 13C{1H} NMR (101 MHz, CDCl3) δu 137.8, 136.1, 127.7 (d, 3JC–F = 9.2 Hz), 123.6 (d, 4JC–F = 5.5 Hz), 120.2 (d, 2JC–F = 25.7 Hz), 110.9 (d, 2JC–F = 22.1 Hz); δd 162.3 (d, 1JC–F = 253.1 Hz), 130.0 (d, 3JC–F = 9.9 Hz), 126.7; 19F NMR (376 MHz, CDCl3) δ −108.08; HRMS (ESI) calcd for C9H7ONF [M + H]+ 164.0512, found 164.0504.
6-Methylisoquinoline 2-Oxide (4b). Using the general procedure for the synthesis of isoquinoline N-oxides, 6-methylisoquinoline (0.200 g, 1.40 mmol, 1.0 equiv) in DCM (0.9 mL, 10.8 M) reacted with mCPBA (0.688 g, 2.79 mmol, 2.0 equiv) in DCM (1.8 mL, 10.8 M) to afford the crude product that was purified by column chromatography (silica, 10:1 EtOAc/MeOH) to give pure 4b (0.180 g, 1.13 mmol) in 81% yield as a white solid: mp 135.5–135.8 °C; 1H NMR (400 MHz, CDCl3) δ 8.72 (d, J = 1.8 Hz, 1H), 8.11 (dd, J = 7.1, 1.8 Hz, 1H), 7.63 (d, J = 8.5 Hz, 1H), 7.61–7.54 (m, 2H), 7.46 (dd, J = 8.4, 1.6 Hz, 1H), 2.52 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 136.7, 136.0, 131.8, 125.8, 124.8, 123.6; 21.8; δd 139.6, 129.1, 127.7; HRMS (ESI) calcd for C10H10ON [M + H]+ 160.0762, found 160.0757.
General Procedure for the Cyanation of Isoquinoline N-Oxides
A vial with a stir bar was either flame-dried under argon or oven-dried and charged with isoquinoline N-oxide (1.0 mmol, 1.0 equiv) and trimethylsilyl cyanide (2.2 mmol, 2.2 equiv). The vial was sealed with a pressure relief cap, and its contents were stirred at 130 °C in a pie reactor for 1 h. Upon completion of the reaction, the crude reaction mixture was concentrated under vacuum and purified by column chromatography.
6-Fluoroisoquinoline-1-carbonitrile (5a). Using the general procedure for cyanation of isoquinoline N-oxides, N-oxide 4a (0.412 g, 2.53 mmol, 1.0 equiv) reacted with trimethylsilyl cyanide (0.741 mL, 5.56 mmol, 2.2 equiv) to afford the crude product that was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to give pure 5a (0.410 g, 2.38 mmol) in 94% yield as a white solid: mp 123.3–124.0 °C; 1H NMR (400 MHz, CDCl3) δ 8.50 (dd, J = 5.7, 0.8 Hz, 1H), 8.24 (ddt, J = 8.8, 5.2, 0.9 Hz, 1H), 7.71 (dd, J = 5.7, 0.9 Hz, 1H), 7.47–7.37 (m, 2H); 13C{1H} NMR (101 MHz, CDCl3) δu 144.1, 128.8 (d, 3JC–F = 9.9 Hz), 123.9 (d, 4JC–F = 5.6 Hz), 120.8 (d, 2JC–F = 26.2 Hz), 110.8 (d, 2JC–F = 21.4 Hz); δd 163.8 (d, 1JC–F = 256.7 Hz), 137.5 (d, 3JC–F = 10.7 Hz), 126.6, 115.6; 19F NMR (376 MHz, CDCl3) δ −103.47; GC (method A) tR = 8.084 min; EI-MS m/z (%) 171.9 (M+, 100), 144.9 (36), 177.8 (4), 99.8 (4), 93.9 (4), 85.8 (4), 73.9 (3); HRMS (ESI) calcd for C10H6N2F [M + H]+ 173.0515, found 173.0509.
6-Methylisoquinoline-1-carbonitrile (5b). Using the general procedure for cyanation of isoquinoline N-oxides, N-oxide 4b (0.159 g, 1.00 mmol, 1.0 equiv) reacted with trimethylsilyl cyanide (0.744 mL, 2.20 mmol, 2.2 equiv) to afford the crude product that was purified by column chromatography (silica, 10:1 hexanes/EtOAc) to give pure 5b (0.155 g, 0.922 mmol) in 92% yield as a white solid: mp 103.9–104.4 °C; 1H NMR (400 MHz, CDCl3) δ 8.60 (d, J = 5.6 Hz, 1H), 8.24 (d, J = 8.6 Hz, 1H), 7.80 (dd, J = 5.7, 0.9 Hz, 1H), 7.70 (s, 1H), 7.63 (dd, J = 8.5, 1.6 Hz, 1H), 2.60 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δu 143.4, 132.2, 126.1, 125.1, 123.8, 22.1; δd 142.6, 136.2, 134.4, 128.0, 116.0; GC (method B) tR = 1.413 min; EI-MS m/z (%) 168.0 (M+, 100), 140.0 (17), 114.0 (9), 63.0 (4); HRMS (ESI) calcd for C11H9N2 [M + H]+ 169.0766, found 169.0759.
General Procedure for the Synthesis of (S)-4-(tert-Butyl)-2-(isoquinol-1-yl)-4,5-dihydrooxazole [(S)-tert-butyl iQuinox]
A two-necked round-bottom flask with a stir bar was equipped with a reflux condenser and charged with zinc triflate (0.2 mmol, 20 mol %). The flask was placed under high vacuum and heated to 90 °C for 1 h using a mineral oil bath. In a separate flame-dried round-bottom flask with a stir bar, under argon, isoquinoline-1-carbonitrile (1.0 mmol, 1.0 equiv) and (S)-tert-leucinol (1.0 mmol, 1.0 equiv) were dissolved in toluene (4 mL, 0.25 M). After the flask had been cooled with zinc triflate to room temperature, the mixture was added to it, and the new mixture refluxed under argon for 15–24 h. Once the reaction had reached completion, as judged by GC-MS or TLC analysis, the reaction mixture was allowed to cool to room temperature, diluted with EtOAc, washed with saturated NaHCO3 and brine, and then dried with MgSO4. The crude product was concentrated under vacuum and purified by column chromatography.
(S)-4-(tert-Butyl)-2-(6-fluoroisoquinolin-1-yl)-4,5-dihydrooxazole (tBu-6FiQuinox, L26). Using the general procedure for the synthesis of (S)-tert-butyl iQuinox, 5a (0.154 g, 0.893 mmol, 1.0 equiv) reacted with (S)-tert-leucinol (0.105 g, 0.893 mmol, 1.0 equiv) and zinc triflate (0.065 g, 0.179 mmol, 20 mol %) in toluene (3.6 mL, 0.25 M) to afford the crude product that was purified column chromatography (silica, 3:2 hexanes/EtOAc) to give pure L26 (0.154 g, 0.566 mmol) in 63% yield as a white solid: mp 86.9–87.2 °C; [α]D20 = −41 (c 0.40, CHCl3); 1H NMR (400 MHz, CDCl3) δ 9.41–9.32 (m, 1H), 8.54 (d, J = 5.6 Hz, 1H), 7.63 (d, J = 5.6 Hz, 1H), 7.41–7.31 (m, 2H), 4.48–4.36 (m, 1H), 4.30–4.18 (m, 2H), 0.98 (s, 9H); 13C{1H} NMR (101 MHz, CDCl3) δu 142.6, 131.2 (d, 3JC–F = 9.4 Hz), 122.8 (d, 4JC–F = 5.2 Hz), 119.0 (d, 2JC–F = 24.7 Hz), 110.2 (d, 2JC–F = 20.8 Hz), 77.6, 26.1; δd 163.0 (d, 1JC–F = 254.1 Hz), 161.6, 146.3 (d, 4JC–F = 1.6 Hz), 138.5 (d, 3JC–F = 10.4 Hz), 124.7, 68.2, 34.1; 19F NMR (376 MHz, CDCl3) δ −107.09; GC (method B) tR = 2.604 min; EI-MS m/z (%) 272.0 (M+, 12), 257.0 (3), 216.0 (100), 186.0 (61), 172.9 (17), 159.9 (49), 145.9 (81), 132.9 (18), 126.0 (17), 118.9 (8), 98.9 (6), 57.0 (12); HRMS (ESI) calcd for C16H18ON2F [M + H]+ 273.1403, found 273.1397.
(S)-4-(tert-Butyl)-2-(6-methylisoquinolin-1-yl)-4,5-dihydrooxazole (tBu-6CH3iQuinox, L27). Using the general procedure for the synthesis of (S)-tert-butyl iQuinox, 5b (0.124 g, 0.737 mmol, 1.0 equiv) reacted with (S)-tert-leucinol (0.0860 g, 0.737 mmol, 1.0 equiv) and zinc triflate (0.054 g, 0.147 mmol, 20 mol %) in toluene (2.9 mL, 0.25 M) to afford the crude product that was purified column chromatography (silica, 3:2 hexanes/EtOAc) to give pure L27 (0.168 g, 0.626 mmol) in 85% yield as a yellow oil: [α]D20 = −32 (c 0.34, CHCl3); 1H NMR (400 MHz, CDCl3) δ 9.19 (d, J = 8.8 Hz, 1H), 8.58 (d, J = 5.6 Hz, 1H), 7.66 (d, J = 5.6 Hz, 1H), 7.62 (s, 1H), 7.51 (dd, J = 8.8, 1.8 Hz, 1H), 4.55–4.43 (m, 1H), 4.37–4.25 (m, 2H), 2.55 (s, 3H), 1.06 (s, 9H); 13C{1H} NMR (101 MHz, CDCl3) δu 141.9, 130.8, 127.2, 125.9, 122.8, 77.5, 26.1, 21.9; δd 161.9, 146.1, 140.7, 137.1, 125.9, 68.1, 34.1; GC (method B) tR = 3.187 min; EI-MS m/z (%) 268.1 (M+, 28), 253.1 (4), 211.1 (100), 197.0 (4), 183.1 (58), 169.0 (13), 156.0 (48), 142.0 (50), 129.0 (12), 115 (17), 89.0 (4), 57.1 (5); HRMS (ESI) calcd for C17H21ON2 [M + H]+ 269.1654, found 269.1646.
Control Experiment in the Absence of Mn
An oven-dried screw-cap vial, equipped with a magnetic stir bar, was charged with the aryl iodide diene 1e-I (0.020 g, 0.049 mmol, 1.0 equiv) and then placed in a glovebox. Anhydrous NiI2 (0.0015 g, 0.0049 mmol, 10 mol %), bipy (0.0011 g, 0.0074 mmol, 15 mol %), KI (0.0081 g, 0.049 mmol, 1.0 equiv), 2-cyclohexenone (14 μL, 0.0141 g, 0.147 mmol, 3.0 equiv), and DMF (0.6 mL, 0.08 M) were added, and the vial was sealed with a pressure relief cap and removed from the glovebox. The contents of the vial were stirred at 80 °C in a pie reactor for 15 h, filtered through an aluminum oxide plug, diluted with EtOAc, and analyzed by TLC/GC-MS. However, the formation of desired product 2e was not observed, and only starting material 1e-I (>99% as determined by GC) was present in the reaction mixture: GC (method B) tR = 3.438 min; EI-MS m/z (%) 411.1 (M+, 1), 379.1 (4), 292.0 (8), 260.1 (10), 252.1 (8), 230.9 (100), 202.9 (5), 164.0 (34), 121.1 (10), 105.0 (50), 90.0 (7), 79.0 (26), 51.0 (4).
Control Experiment Using Ni(COD)2 in the Absence of Mn
An oven-dried screw-cap vial, equipped with a magnetic stir bar, was charged with aryl iodide diene 1e-I (0.020 g, 0.049 mmol, 1.0 equiv) and then placed in a glovebox. Anhydrous Ni(COD)2 (0.0013 g, 0.0049 mmol, 10 mol %), bipy (0.0011 g, 0.0074 mmol, 15 mol %), KI (0.0081 g, 0.049 mmol, 1.0 equiv), 2-cyclohexenone (14 μL, 0.0141 g, 0.147 mmol, 3.0 equiv), and DMF (0.6 mL, 0.08 M) were added, and the vial was sealed with a pressure relief cap and removed from the glovebox. The contents of the vial were stirred at 80 °C in a pie reactor for 15 h, filtered through an aluminum oxide plug, diluted with EtOAc, and analyzed by TLC/GC-MS. The reaction mixture contained the desired product 2e (8% as determined by GC) and starting material 1e-I (>90% as determined by GC). 2e: GC (method B) tR = 2.546 min; EI-MS m/z (%) 283.1 (M+, 17), 251.0 (12), 240.0 (15), 224 (10), 208.0 (100), 196.0 (99), 178 (67), 167 (10), 152.0 (12), 139 (4), 115.0 (4), 105.0 (4), 77.0 (6). 1e-I: GC (method B) tR = 3.439 min; EI-MS m/z (%) 411.1 (M+, 1), 379.1 (4), 292.0 (8), 260.1 (10), 252.1 (8), 230.9 (100), 202.9 (5), 164.0 (34), 121.1 (10), 105.0 (50), 90.0 (7), 79.0 (26), 51.0 (4).
Control Experiment Using Ni(COD)2 in the Presence of Mn
An oven-dried screw-cap vial, equipped with a magnetic stir bar, was charged with aryl iodide diene 1e-I (0.020 g, 0.049 mmol, 1.0 equiv) and then placed in a glovebox. Anhydrous Ni(COD)2 (0.0013 g, 0.0049 mmol, 10 mol %), bipy (0.0011 g, 0.0074 mmol, 15 mol %), Mn (0.0080 g, 0.147 mmol, 3.0 equiv), KI (0.0081 g, 0.049 mmol, 1.0 equiv), 2-cyclohexenone (14 μL, 0.0141 g, 0.147 mmol, 3.0 equiv), and DMF (0.6 mL, 0.08 M) were added, and the vial was sealed with a pressure relief cap and removed from the glovebox. The contents of the vial were stirred at 80 °C in a pie reactor for 15 h. After the reaction had reached completion, the reaction mixture was filtered through an aluminum oxide plug, diluted with EtOAc, and analyzed by TLC/GC-MS. The reaction mixture contained the desired product 2e (78% as determined by GC): GC (method B) tR = 2.546 min; EI-MS m/z (%) 283.1 (M+, 17), 251.0 (12), 240.0 (15), 224 (10), 208.0 (100), 196.0 (99), 178 (67), 167 (10), 152.0 (12), 139 (4), 115.0 (4), 105.0 (4), 77.0 (6).
Control Experiment Using D2O and Mn with the Aryl Bromide Diene
An oven-dried screw-cap vial, equipped with a magnetic stir bar, was charged with aryl bromide diene 1e (0.020 g, 0.055 mmol, 1.0 equiv) and then placed in a glovebox. Mn (0.0091 g, 0.165 mmol, 3.0 equiv), KI (0.0091 g, 0.055 mmol, 1.0 equiv), and DMF (0.66 mL, 0.08 M) were added, and the vial was sealed with a pressure relief cap and removed from the glovebox. The contents of the vial were stirred at 80 °C. After 3 h, D2O (0.01 mL, 0.55 mmol, 10.0 equiv) was added and the mixture was heated at 80 °C for additional 18 h. The reaction mixture was filtered through an aluminum oxide plug, diluted with EtOAc, and analyzed by TLC/GC-MS. The reaction mixture contained only the starting material 1e (>99% as determined by GC): GC (method B) tR = 3.032 min; EI-MS m/z (%) 363.1 (M – 1+, 1), 331.1 (7), 243.9 (10), 227.9 (4), 213.9 (15), 183.0 (68), 164.1 (64), 121.1 (21), 105.1 (100), 91.0 (12), 79.1 (47), 51.1 (5).
Control Experiment Using D2O and Mn with the Aryl Iodide Diene
An oven-dried screw-cap vial, equipped with a magnetic stir bar, was charged with aryl bromide diene 1e-I (0.020 g, 0.049 mmol, 1.0 equiv) and then placed in a glovebox. Mn (0.0080 g, 0.147 mmol, 3.0 equiv), KI (0.0081 g, 0.049 mmol, 1.0 equiv), and DMF (0.6 mL, 0.08 M) were added, and the vial was sealed with a pressure relief cap and removed from the glovebox. The contents of the vial were stirred at 80 °C. After 3 h, D2O (0.01 mL, 0.49 mmol, 10.0 equiv) was added and the mixture was heated at 80 °C for additional 18 h. The reaction mixture was filtered through an aluminum oxide plug, diluted with EtOAc, and analyzed by TLC/GC-MS. The reaction mixture contained deuterated dehalogenated side product-D12e-1 (13% as determined by GC) and starting material 1e-I (87% as determined by GC): 2e-1: GC (method B) tR = 2.323 min; EI-MS m/z (%) 254.0 (M – 32+, 21), 211.0 (5), 166.0 (30), 150.9 (23), 135.0 (5), 121.0 (20), 106.0 (100), 91.0 (6), 79.0 (36). 51.0 (5). 1e-I: GC (method B) tR = 3.439 min; EI-MS m/z (%) 379.1 (M – 32+, 4), 291.9 (7), 259.9 (12) 252.1 (0), 244.8 (4), 230.9 (100), 202.9 (4), 164.0 (33), 121.1 (12), 105.0 (50), 79.0 (26), 51.0 (4).
Control Experiment Using D2O and Zn with the Aryl Bromide Diene
An oven-dried screw-cap vial, equipped with a magnetic stir bar, was charged with aryl bromide diene 1d (0.025 g, 0.075 mmol, 1.0 equiv) and then placed in a glovebox. Zn (0.0146 g, 0.224 mmol, 3.0 equiv), KI (0.0124 g, 0.075 mmol, 1.0 equiv), and DMF (0.9 mL, 0.08 M) were added, and the vial was sealed with a pressure relief cap and removed from the glovebox. The contents of the vial were stirred at 80 °C. After 3 h, D2O (0.014 mL, 0.75 mmol, 10 equiv) was added and the mixture was heated at 80 °C for additional 18 h. The reaction mixture was filtered through an aluminum oxide plug, diluted with EtOAc, and analyzed by TLC/GC-MS. The reaction mixture contained only starting material 1d (>99% as determined by GC): GC (method B) tR = 2.677 min; EI-MS m/z (%) 333.1 (M – 1+, 3), 290.0 (23), 214.0 (63), 197 (5), 185.0 (17), 134.0 (100), 121.1 (10), 105.0 (74), 91.0 (9), 77.1 (39), 51.0 (6).
Control Experiment Using D2O and Zn with the Aryl Iodide Diene
An oven-dried screw-cap vial, equipped with a magnetic stir bar, was charged with aryl iodide diene 1d-I (0.025 g, 0.066 mmol, 1.0 equiv) and then placed in a glovebox. Zn (0.0129 g, 0.197 mmol, 3.0 equiv), KI (0.0111 g, 0.066 mmol, 1.0 equiv), and DMF (0.8 mL, 0.08 M) were added, and the vial was sealed with a pressure relief cap and removed from the glovebox. The contents of the vial were stirred at 80 °C. After 3 h, D2O (0.012 mL, 0.66 mmol, 10 equiv) was added and the mixture was heated at 80 °C for additional 18 h. The reaction mixture was filtered through an aluminum oxide plug, diluted with EtOAc, and analyzed by TLC/GC-MS. The reaction mixture contained deuterated dehalogenated side product-D12d-1 (82% as determined by GC). The organic layer was washed twice with 1 N HCl and once withbrine, dried with MgSO4, and filtered and concentrated in vacuo. The crude residue was purified by column chromatography (silica, 4:1 hexanes/EtOAc) to afford 2d-1 in 65% yield (0.011 g, 0.043 mmol) as a yellow oil: 1H NMR (400 MHz, CDCl3) δ 7.28–7.20 (m, 3H), 7.15–7.09 (m, 1H), 5.35 (s, 4H), 3.23 (s, 3H), 2.49 (hept, J = 6.9 Hz, 1H), 2.37–2.07 (m, 2H), 0.76 (d, J = 6.9 Hz, 6H); 13C{1H} NMR (101 MHz, CDCl3) δu 128.8, 128.7, 128.4, 127.9, 127.0, 124.1, 40.9, 35.9, 17.5; δd 174.9, 145.0, 128.1, 53.9, 26.3; GC (mthod B) tR = 1.951 min; EI-MS m/z (%) 256.0 (M+, 6), 213.0 (28), 136.0 (100), 121.0 (14), 105.0 (54), 95.0 (10), 79.0 (45), 51.0 (6).
Control Experiment Using 2-Cyclohexenone and Mn
An oven-dried screw-cap vial, equipped with a magnetic stir bar, was charged with 2-cyclohexenone (50 μL, 0.05 g, 0.52 mmol, 1.0 equiv), Mn (0.057 g, 1.04 mmol, 2.0 equiv), and DMF (2 mL, 0.26 M) in a glovebox. The vial was sealed with a pressure relief cap, removed from the glovebox, and stirred at 80 °C for 15 h. The reaction mixture was filtered through an aluminum oxide plug, diluted with EtOAc, and analyzed by TLC/GC-MS. The reaction mixture contained only starting material 2-cyclohexenone (>99% as determined by GC): GC (method B) tR = 1.970 min; EI-MS m/z (%) 96.0 (M+, 41), 73.0 (4), 68.0 (100), 55.0 (5).
Control Experiment Using TEMPO
An oven-dried screw-cap vial, equipped with a magnetic stir bar, was charged with aryl iodide diene 1e-I (0.020 g, 0.049 mmol, 1.0 equiv) and then placed in a glovebox. Anhydrous NiI2 (0.0015 g, 0.0049 mmol, 10 mol %), bipy (0.0011 g, 0.0074 mmol, 15 mol %), Mn (0.0080 g, 0.147 mmol, 3.0 equiv), KI (0.0081 g, 0.049 mmol, 1.0 equiv), 2-cyclohexenone (14 μL, 0.0141 g, 0.147 mmol, 3.0 equiv), TEMPO (0.0077 g, 0.049 mmol, 1.0 equiv), and DMF (0.6 mL, 0.08 M) were added. The vial was sealed with a pressure relief cap and removed from the glovebox. The contents of the vial were stirred at 80 °C in a pie reactor for 15 h. After the reaction had reached completion, the reaction mixture was filtered through an aluminum oxide plug, diluted with EtOAc, and analyzed by TLC/GC-MS. The reaction mixture contained desired product 2e (94% as determined by GC): GC (method B) tR = 2.546 min; EI-MS m/z (%) 283.1 (M+, 17), 251.0 (12), 240.0 (15), 224 (10), 208.0 (100), 196.0 (99), 178 (67), 167 (10), 152.0 (12), 139 (4), 115.0 (4), 105.0 (4), 77.0 (6).
Acknowledgments
The authors are especially thankful to the National Institutes of Health for financial support (NIGMS, 1-R15-GM123475-01A1). Essential financial support was also received from Bryn Mawr College. The authors acknowledge the National Science Foundation for a Major Research Instrumentation Award (CHE-0958996), which funded the acquisition of the NMR spectrometer used in this work. In addition, the authors thank the reviewers for helpful suggestions that improved the computational analysis of the reaction.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.3c00202.
NMR spectra for all new compounds, chiral LC traces for enantioselective Mizoroki–Heck reactions, a table of amide formation (SI-Table 1), secondary amide protection (SI-Table 2), chiral ligand screening (SI-Table 3), MOM group deprotection (SI-Table 4), a scheme for the synthesis of chiral tBu-iQuinox ligands (SI-Scheme 1), GC-MS and NMR data for mechanistic control studies, computational data, including conformation images (SI-Figure 1), and calculation details (SI-Table 5) (PDF)
FAIR data, including the primary NMR FID files, for compounds 1a, 1a-I, S2b, S2b-I, S2c-I, 1d, 1d-I, S2e, S2e-I, S2f-I, 1g-I, S2h, S2h-I, S2i, S2i-I, S2j, S2j-I, S2k, S2l-I, S2m–o, S2o-I, 1b, 1b-I, 1c-I, 1e, 1e-I, 1f-I, 1h, 1h-I, 1i, 1i-I, 1j, 1j-I, 1k, 1l-I, 1m–o, 1o-I, 2a–d, 2d-1, 2e–l, 2m-2, 2n–p, 3c, 3e, 3f, 3i–k, 3m-2, 3o, 4a, 4b, 5a, 5b, L26, and L27 (ZIP)
The authors declare no competing financial interest.
Supplementary Material
References
- Wu X.-F.; Anbarasan P.; Neumann H.; Beller M. From Noble Metal to Nobel Prize: Palladium-Catalyzed Coupling Reactions as Key Methods in Organic Synthesis. Angew. Chem., Int. Ed. 2010, 49 (48), 9047–9050. 10.1002/anie.201006374. [DOI] [PubMed] [Google Scholar]
- Nicolaou K. C.; Bulger P. G.; Sarlah D. Palladium-Catalyzed Cross-Coupling Reactions in Total Synthesis. Angew. Chem., Int. Ed. 2005, 44 (29), 4442–4489. 10.1002/anie.200500368. [DOI] [PubMed] [Google Scholar]
- The Nobel Prize in Chemistry 2010 was awarded jointly to Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki for palladium-catalyzed cross couplings in organic synthesis. 2010. https://www.nobelprize.org/prizes/chemistry/2010/summary/.
- Gildner P. G.; Colacot T. J. Reactions of the 21st Century: Two Decades of Innovative Catalyst Design for Palladium-Catalyzed Cross-Couplings. Organometallics 2015, 34 (23), 5497–5508. 10.1021/acs.organomet.5b00567. [DOI] [Google Scholar]
- Chernyshev V. M.; Ananikov V. P. Nickel and Palladium Catalysis: Stronger Demand than Ever. ACS Catal. 2022, 12 (2), 1180–1200. 10.1021/acscatal.1c04705. [DOI] [Google Scholar]
- Bhakta S.; Ghosh T. Emerging Nickel Catalysis in Heck Reactions: Recent Developments. Adv. Synth. Catal. 2020, 362 (23), 5257–5274. 10.1002/adsc.202000820. [DOI] [Google Scholar]
- Poremba K. E.; Dibrell S. E.; Reisman S. E. Nickel-Catalyzed Enantioselective Reductive Cross-Coupling Reactions. ACS Catal. 2020, 10 (15), 8237–8246. 10.1021/acscatal.0c01842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley E. A.; Tasker S. Z.; Jensen K. L.; Jamison T. F. Nickel Catalysis: Synergy between Method Development and Total Synthesis. Acc. Chem. Res. 2015, 48 (5), 1503–1514. 10.1021/acs.accounts.5b00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker S. Z.; Standley E. A.; Jamison T. F. Recent advances in homogeneous nickel catalysis. Nature 2014, 509 (7500), 299–309. 10.1038/nature13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diccianni J.; Lin Q.; Diao T. Mechanisms of Nickel-Catalyzed Coupling Reactions and Applications in Alkene Functionalization. Acc. Chem. Res. 2020, 53 (4), 906–919. 10.1021/acs.accounts.0c00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M. P.; Jacobsen E. N. Asymmetric Intramolecular Arylcyanation of Unactivated Olefins via C–CN Bond Activation. J. Am. Chem. Soc. 2008, 130 (38), 12594–12595. 10.1021/ja805094j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao Y.; Ebata S.; Yada A.; Hiyama T.; Ikawa M.; Ogoshi S. Intramolecular Arylcyanation of Alkenes Catalyzed by Nickel/AlMe2Cl. J. Am. Chem. Soc. 2008, 130 (39), 12874–12875. 10.1021/ja805088r. [DOI] [PubMed] [Google Scholar]
- Reznikov A. N.; Ashatkina M. A.; Klimochkin Y. N. Recent developments in asymmetric Heck type cyclization reactions for constructions of complex molecules. Org. Biomol. Chem. 2021, 19 (26), 5673–5701. 10.1039/D1OB00496D. [DOI] [PubMed] [Google Scholar]
- Oxtoby L. J.; Vasquez A. M.; Kang T.; Li Z.-Q.; Engle K. M.. 13.04 - Metal-Mediated and Catalyzed Difunctionalization of Unsaturated Organics. In Comprehensive Organometallic Chemistry IV; Parkin G., Meyer K., O’Hare D., Eds.; Elsevier, 2022; pp 132–193. [Google Scholar]
- Zhu S.; Zhao X.; Li H.; Chu L. Catalytic three-component dicarbofunctionalization reactions involving radical capture by nickel. Chem. Soc. Rev. 2021, 50 (19), 10836–10856. 10.1039/D1CS00399B. [DOI] [PubMed] [Google Scholar]
- Li Y.; Wang K.; Ping Y.; Wang Y.; Kong W. Nickel-Catalyzed Domino Heck Cyclization/Suzuki Coupling for the Synthesis of 3,3-Disubstituted Oxindoles. Org. Lett. 2018, 20 (4), 921–924. 10.1021/acs.orglett.7b03713. [DOI] [PubMed] [Google Scholar]
- Lebedev S. A.; Lopatina V. S.; Petrov E. S.; Beletskaya I. P. Condensation of organic bromides with vinyl compounds catalysed by nickel complexes in the presence of zinc. J. Organomet. Chem. 1988, 344 (2), 253–259. 10.1016/0022-328X(88)80484-4. [DOI] [Google Scholar]
- Boldrini G. P.; Savoia D.; Tagliavini E.; Trombini C.; Ronchi A. U. Nickel-catalyzed coupling of activated alkenes with organic halides. J. Organomet. Chem. 1986, 301 (3), C62–C64. 10.1016/0022-328X(86)80050-X. [DOI] [Google Scholar]
- Kwiatkowski M. R.; Alexanian E. J. Nickel-Catalyzed Mizoroki–Heck-Type Reactions of Unactivated Alkyl Bromides. Angew. Chem., Int. Ed. 2018, 57 (51), 16857. 10.1002/anie.201810757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.-L.; Newman S. G. Nickel-Catalyzed Domino Heck-Type Reactions Using Methyl Esters as Cross-Coupling Electrophiles. Angew. Chem., Int. Ed. 2019, 58 (50), 18159–18164. 10.1002/anie.201911372. [DOI] [PubMed] [Google Scholar]
- Gøgsig T. M.; Kleimark J.; Nilsson Lill S. O.; Korsager S.; Lindhardt A. T.; Norrby P.-O.; Skrydstrup T. Mild and Efficient Nickel-Catalyzed Heck Reactions with Electron-Rich Olefins. J. Am. Chem. Soc. 2012, 134 (1), 443–452. 10.1021/ja2084509. [DOI] [PubMed] [Google Scholar]
- Walker B. R.; Sevov C. S. An Electrochemically Promoted, Nickel-Catalyzed Mizoroki–Heck Reaction. ACS Catal. 2019, 9 (8), 7197–7203. 10.1021/acscatal.9b02230. [DOI] [Google Scholar]
- Ehle A. R.; Zhou Q.; Watson M. P. Nickel(0)-Catalyzed Heck Cross-Coupling via Activation of Aryl C–OPiv Bonds. Org. Lett. 2012, 14 (5), 1202–1205. 10.1021/ol203322v. [DOI] [PubMed] [Google Scholar]
- Alisha M.; Philip R. M.; Anilkumar G. Low-Cost Transition Metal-Catalyzed Heck-Type Reactions: An Overview. Eur. J. Org. Chem. 2022, 2022 (7), e202101384. 10.1002/ejoc.202101384. [DOI] [Google Scholar]
- Desrosiers J.-N.; Wen J.; Tcyrulnikov S.; Biswas S.; Qu B.; Hie L.; Kurouski D.; Wu L.; Grinberg N.; Haddad N.; et al. Enantioselective Nickel-Catalyzed Mizoroki–Heck Cyclizations To Generate Quaternary Stereocenters. Org. Lett. 2017, 19 (13), 3338–3341. 10.1021/acs.orglett.7b01054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F.; Jin Y.; Wang C. Nickel-Catalyzed Asymmetric Intramolecular Reductive Heck Reaction of Unactivated Alkenes. Org. Lett. 2019, 21 (17), 6989–6994. 10.1021/acs.orglett.9b02577. [DOI] [PubMed] [Google Scholar]
- Qin X.; Yao Lee M. W.; Zhou J. S. Asymmetric Hydroarylation of Enones via Nickel-Catalyzed 5-Endo-Trig Cyclization. Org. Lett. 2019, 21 (15), 5990–5994. 10.1021/acs.orglett.9b02130. [DOI] [PubMed] [Google Scholar]
- Qin X.; Lee M. W. Y.; Zhou J. S. Nickel-Catalyzed Asymmetric Reductive Heck Cyclization of Aryl Halides to Afford Indolines. Angew. Chem., Int. Ed. 2017, 56 (41), 12723–12726. 10.1002/anie.201707134. [DOI] [PubMed] [Google Scholar]
- Jin Y.; Wang C. Nickel-Catalyzed Asymmetric Reductive Arylalkylation of Unactivated Alkenes. Angew. Chem., Int. Ed. 2019, 58 (20), 6722–6726. 10.1002/anie.201901067. [DOI] [PubMed] [Google Scholar]
- Wang K.; Ding Z.; Zhou Z.; Kong W. Ni-Catalyzed Enantioselective Reductive Diarylation of Activated Alkenes by Domino Cyclization/Cross-Coupling. J. Am. Chem. Soc. 2018, 140 (39), 12364–12368. 10.1021/jacs.8b08190. [DOI] [PubMed] [Google Scholar]
- Wang K.; Kong W. Enantioselective Reductive Diarylation of Alkenes by Ni-Catalyzed Domino Heck Cyclization/Cross Coupling. Synlett 2019, 30 (09), 1008–1014. 10.1055/s-0037-1612214. [DOI] [Google Scholar]
- Ma T.; Chen Y.; Li Y.; Ping Y.; Kong W. Nickel-Catalyzed Enantioselective Reductive Aryl Fluoroalkenylation of Alkenes. ACS Catal. 2019, 9 (10), 9127–9133. 10.1021/acscatal.9b03172. [DOI] [Google Scholar]
- Wu J.; Wang C. Nickel-Catalyzed Asymmetric Reductive Dicarbamoylation of Alkenes. Org. Lett. 2021, 23 (16), 6407–6411. 10.1021/acs.orglett.1c02223. [DOI] [PubMed] [Google Scholar]
- Marchese A. D.; Wollenburg M.; Mirabi B.; Abel-Snape X.; Whyte A.; Glorius F.; Lautens M. Nickel-Catalyzed Enantioselective Carbamoyl Iodination: A Surrogate for Carbamoyl Iodides. ACS Catal. 2020, 10 (8), 4780–4785. 10.1021/acscatal.0c00841. [DOI] [Google Scholar]
- Wu X.; Qu J.; Chen Y. Quinim: A New Ligand Scaffold Enables Nickel-Catalyzed Enantioselective Synthesis of α-Alkylated γ-Lactam. J. Am. Chem. Soc. 2020, 142 (37), 15654–15660. 10.1021/jacs.0c07126. [DOI] [PubMed] [Google Scholar]
- He J.; Xue Y.; Han B.; Zhang C.; Wang Y.; Zhu S. Nickel-Catalyzed Asymmetric Reductive 1,2-Carboamination of Unactivated Alkenes. Angew. Chem., Int. Ed. 2020, 59 (6), 2328–2332. 10.1002/anie.201913743. [DOI] [PubMed] [Google Scholar]
- Yoon H.; Marchese A. D.; Lautens M. Carboiodination Catalyzed by Nickel. J. Am. Chem. Soc. 2018, 140 (35), 10950–10954. 10.1021/jacs.8b06966. [DOI] [PubMed] [Google Scholar]
- Li Y.; Zhang F.-P.; Wang R.-H.; Qi S.-L.; Luan Y.-X.; Ye M. Carbamoyl Fluoride-Enabled Enantioselective Ni-Catalyzed Carbocarbamoylation of Unactivated Alkenes. J. Am. Chem. Soc. 2020, 142 (47), 19844–19849. 10.1021/jacs.0c09949. [DOI] [PubMed] [Google Scholar]
- Fan P.; Lan Y.; Zhang C.; Wang C. Nickel/Photo-Cocatalyzed Asymmetric Acyl-Carbamoylation of Alkenes. J. Am. Chem. Soc. 2020, 142 (5), 2180–2186. 10.1021/jacs.9b12554. [DOI] [PubMed] [Google Scholar]
- Wu X.; Turlik A.; Luan B.; He F.; Qu J.; Houk K. N.; Chen Y. Nickel-Catalyzed Enantioselective Reductive Alkyl-Carbamoylation of Internal Alkenes. Angew. Chem., Int. Ed. 2022, 61 (36), e202207536. 10.1002/anie.202207536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.; Luan B.; Zhao W.; He F.; Wu X.-Y.; Qu J.; Chen Y. Catalytic Desymmetric Dicarbofunctionalization of Unactivated Alkenes. Angew. Chem., Int. Ed. 2022, 61 (26), e202111598. 10.1002/anie.202111598. [DOI] [PubMed] [Google Scholar]
- Qiao J.-B.; Zhang Y.-Q.; Yao Q.-W.; Zhao Z.-Z.; Peng X.; Shu X.-Z. Enantioselective Reductive Divinylation of Unactivated Alkenes by Nickel-Catalyzed Cyclization Coupling Reaction. J. Am. Chem. Soc. 2021, 143 (33), 12961–12967. 10.1021/jacs.1c05670. [DOI] [PubMed] [Google Scholar]
- Ping Y.; Wang K.; Pan Q.; Ding Z.; Zhou Z.; Guo Y.; Kong W. Ni-Catalyzed Regio- and Enantioselective Domino Reductive Cyclization: One-Pot Synthesis of 2,3-Fused Cyclopentannulated Indolines. ACS Catal. 2019, 9 (8), 7335–7342. 10.1021/acscatal.9b02081. [DOI] [Google Scholar]
- Hsieh J.-C.; Ebata S.; Nakao Y.; Hiyama T. Asymmetric Synthesis of Indolines Bearing a Benzylic Quaternary Stereocenter through Intramolecular Arylcyanation of Alkenes. Synlett 2010, 2010 (11), 1709–1711. 10.1055/s-0029-1219964. [DOI] [Google Scholar]
- Wang G.; Shen C.; Ren X.; Dong K. Ni-Catalyzed enantioselective reductive arylcyanation/cyclization of N-(2-iodo-aryl) acrylamide. Chem. Commun. 2022, 58 (8), 1135–1138. 10.1039/D1CC04996H. [DOI] [PubMed] [Google Scholar]
- Zhao T.-Y.; Xiao L.-J.; Zhou Q.-L. Nickel-Catalyzed Desymmetric Reductive Cyclization/Coupling of 1,6-Dienes: An Enantioselective Approach to Chiral Tertiary Alcohol. Angew. Chem., Int. Ed. 2022, 61 (11), e202115702. 10.1002/anie.202115702. [DOI] [PubMed] [Google Scholar]
- Lin Z.; Jin Y.; Hu W.; Wang C. Nickel-catalyzed asymmetric reductive aryl-allylation of unactivated alkenes. Chem. Sci. 2021, 12 (19), 6712–6718. 10.1039/D1SC01115D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang K.; Huang W.; Shan C.; Qu J.; Chen Y. Synthesis of 3,3-Dialkyl-Substituted Isoindolinones Enabled by Nickel-Catalyzed Reductive Dicarbofunctionalization of Enamides. Org. Lett. 2021, 23 (14), 5523–5527. 10.1021/acs.orglett.1c01871. [DOI] [PubMed] [Google Scholar]
- Cerveri A.; Giovanelli R.; Sella D.; Pedrazzani R.; Monari M.; Nieto Faza O.; López C. S.; Bandini M. Enantioselective CO2 Fixation Via a Heck-Coupling/Carboxylation Cascade Catalyzed by Nickel. Chem. - Eur. J. 2021, 27 (28), 7657–7662. 10.1002/chem.202101082. [DOI] [PubMed] [Google Scholar]
- Chen X.-W.; Yue J.-P.; Wang K.; Gui Y.-Y.; Niu Y.-N.; Liu J.; Ran C.-K.; Kong W.; Zhou W.-J.; Yu D.-G. Nickel-Catalyzed Asymmetric Reductive Carbo-Carboxylation of Alkenes with CO2. Angew. Chem., Int. Ed. 2021, 60 (25), 14068–14075. 10.1002/anie.202102769. [DOI] [PubMed] [Google Scholar]
- Harris M. R.; Konev M. O.; Jarvo E. R. Enantiospecific Intramolecular Heck Reactions of Secondary Benzylic Ethers. J. Am. Chem. Soc. 2014, 136 (22), 7825–7828. 10.1021/ja5026485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.-H.; Sun R.-Z.; Yao F.; Hu X.-D.; Xiang L.-X.; Cong H.; Liu W.-B. Enantioselective Nickel-Catalyzed Reductive Aryl/Alkenyl–Cyano Cyclization Coupling to All-Carbon Quaternary Stereocenters. J. Am. Chem. Soc. 2022, 144 (11), 4776–4782. 10.1021/jacs.2c01237. [DOI] [PubMed] [Google Scholar]
- Tian Z.-X.; Qiao J.-B.; Xu G.-L.; Pang X.; Qi L.; Ma W.-Y.; Zhao Z.-Z.; Duan J.; Du Y.-F.; Su P.; et al. Highly Enantioselective Cross-Electrophile Aryl-Alkenylation of Unactivated Alkenes. J. Am. Chem. Soc. 2019, 141 (18), 7637–7643. 10.1021/jacs.9b03863. [DOI] [PubMed] [Google Scholar]
- Li H.; Chen J.; Dong J.; Kong W. Ni-Catalyzed Reductive Arylcyanation of Alkenes. Org. Lett. 2021, 23 (16), 6466–6470. 10.1021/acs.orglett.1c02270. [DOI] [PubMed] [Google Scholar]
- Jin Y.; Yang H.; Wang C. Nickel-Catalyzed Asymmetric Reductive Arylbenzylation of Unactivated Alkenes. Org. Lett. 2020, 22 (7), 2724–2729. 10.1021/acs.orglett.0c00688. [DOI] [PubMed] [Google Scholar]
- Pan Q.; Ping Y.; Wang Y.; Guo Y.; Kong W. Ni-Catalyzed Ligand-Controlled Regiodivergent Reductive Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc. 2021, 143 (27), 10282–10291. 10.1021/jacs.1c03827. [DOI] [PubMed] [Google Scholar]
- Taylor R. D.; MacCoss M.; Lawson A. D. G. Rings in Drugs. J. Med. Chem. 2014, 57 (14), 5845–5859. 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]
- Aldeghi M.; Malhotra S.; Selwood D. L.; Chan A. W. E. Two- and Three-dimensional Rings in Drugs. Chem. Bio. Drug Des. 2014, 83 (4), 450–461. 10.1111/cbdd.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton M.; Malachowski W. P.; Yap G. P. A.; Rachii D.; Feldman G.; Krasley A.; Chen Z.; Tran M.; Wiley K.; Matei A.; et al. Catalytic Enantioselective Birch–Heck Sequence for the Synthesis of Phenanthridinone Derivatives with an All-Carbon Quaternary Stereocenter. J. Org. Chem. 2022, 87 (2), 1154–1172. 10.1021/acs.joc.1c02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering F. Escape from Flatland 2: complexity and promiscuity. MedChemComm 2013, 4 (3), 515–519. 10.1039/c2md20347b. [DOI] [Google Scholar]
- Lovering F.; Bikker J.; Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52 (21), 6752–6756. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- Talele T. T. Opportunities for Tapping into Three-Dimensional Chemical Space through a Quaternary Carbon. J. Med. Chem. 2020, 63 (22), 13291–13315. 10.1021/acs.jmedchem.0c00829. [DOI] [PubMed] [Google Scholar]
- Nájera C.; Foubelo F.; Sansano J. M.; Yus M. Enantioselective desymmetrization reactions in asymmetric catalysis. Tetrahedron 2022, 106–107, 132629. 10.1016/j.tet.2022.132629. [DOI] [Google Scholar]
- Zeng X.-P.; Cao Z.-Y.; Wang Y.-H.; Zhou F.; Zhou J. Catalytic Enantioselective Desymmetrization Reactions to All-Carbon Quaternary Stereocenters. Chem. Rev. 2016, 116 (12), 7330–7396. 10.1021/acs.chemrev.6b00094. [DOI] [PubMed] [Google Scholar]
- Mohadjer Beromi M.; Nova A.; Balcells D.; Brasacchio A. M.; Brudvig G. W.; Guard L. M.; Hazari N.; Vinyard D. J. Mechanistic Study of an Improved Ni Precatalyst for Suzuki–Miyaura Reactions of Aryl Sulfamates: Understanding the Role of Ni(I) Species. J. Am. Chem. Soc. 2017, 139 (2), 922–936. 10.1021/jacs.6b11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitlis P. M.; Haynes A.; James B. R.; Catellani M.; Chiusoli G. P. Iodide effects in transition metal catalyzed reactions. Dalton Trans. 2004, 3409–3419. 10.1039/b410128f. [DOI] [PubMed] [Google Scholar]
- Wang K.; Ding Z.; Zhou Z.; Kong W. Ni-Catalyzed Enantioselective Reductive Diarylation of Activated Alkenes by Domino Cyclization/Cross-Coupling. J. Am. Chem. Soc. 2018, 140 (39), 12364–12368. 10.1021/jacs.8b08190. [DOI] [PubMed] [Google Scholar]
- Colon I.; Kelsey D. R. Coupling of aryl chlorides by nickel and reducing metals. J. Org. Chem. 1986, 51 (14), 2627–2637. 10.1021/jo00364a002. [DOI] [Google Scholar]
- Prinsell M. R.; Everson D. A.; Weix D. J. Nickel-catalyzed, sodium iodide-promoted reductive dimerization of alkyl halides, alkyl pseudohalides, and allylic acetates. Chem. Commun. 2010, 46 (31), 5743–5745. 10.1039/c0cc01716g. [DOI] [PubMed] [Google Scholar]
- Cassar L.; Foà M. Nickel-catalyzed carbonylation of aromatic halides at atmospheric pressure of carbon monoxide. J. Organomet. Chem. 1973, 51, 381–393. 10.1016/S0022-328X(00)93534-4. [DOI] [Google Scholar]
- Bergamini P.; Costa E.; Ganter C.; Orpen A. G.; Pringle P. G. The reaction of trimethylsilyldiazomethane with complexes of the type [PtX(CH3)(diphosphine)] (X = Cl, Br, I). Some observations on β-hydrogen migrations in PtCHRCH3 species and organoplatinum(II)-catalysts for alkene formation from trimethylsilyldiazomethane. J. Chem. Soc., Dalton Trans. 1999, (6), 861–866. 10.1039/a807719c. [DOI] [Google Scholar]
- Fagnou K.; Lautens M. Halide Effects in Transition Metal Catalysis. Angew. Chem., Int. Ed. 2002, 41 (1), 26–47. . [DOI] [PubMed] [Google Scholar]
- Arvela R. K.; Leadbeater N. E. Fast and Easy Halide Exchange in Aryl Halides. Synlett 2003, 2003 (08), 1145–1148. 10.1055/s-2003-39887. [DOI] [Google Scholar]
- Lv H.; Kang H.; Zhou B.; Xue X.; Engle K. M.; Zhao D. Nickel-catalyzed intermolecular oxidative Heck arylation driven by transfer hydrogenation. Nat. Commun. 2019, 10 (1), 5025. 10.1038/s41467-019-12949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diccianni J. B.; Diao T. Mechanisms of Nickel-Catalyzed Cross-Coupling Reactions. Trends Chem. 2019, 1 (9), 830–844. 10.1016/j.trechm.2019.08.004. [DOI] [Google Scholar]
- Yang G.; Zhang W. Renaissance of pyridine-oxazolines as chiral ligands for asymmetric catalysis. Chem. Soc. Rev. 2018, 47, 1783–1810. 10.1039/C7CS00615B. [DOI] [PubMed] [Google Scholar]
- Hu X. Nickel-catalyzed cross coupling of non-activated alkyl halides: a mechanistic perspective. Chem. Sci. 2011, 2 (10), 1867–1886. 10.1039/c1sc00368b. [DOI] [Google Scholar]
- Oliveira C. C.; Pfaltz A.; Correia C. R. D. Quaternary Stereogenic Centers through Enantioselective Heck Arylation of Acyclic Olefins with Aryldiazonium Salts: Application in a Concise Synthesis of (R)-Verapamil. Angew. Chem., Int. Ed. 2015, 54 (47), 14036–14039. 10.1002/anie.201507927. [DOI] [PubMed] [Google Scholar]
- Holder J. C.; Shockley S. E.; Wiesenfeldt M. P.; Shimizu H.; Stoltz B. M. Preparation of (S)- tert -ButylPyOx and Palladium-catalyzed Asymmetric Conjugate Addition of Arylboronic Acids. Org. Synth. 2016, 92. 10.1002/0471264229.os092.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey D. P.; Sandford C.; Rhodes Z.; Gensch T.; Fries L. R.; Sigman M. S.; Minteer S. D. Investigating the Role of Ligand Electronics on Stabilizing Electrocatalytically Relevant Low-Valent Co(I) Intermediates. J. Am. Chem. Soc. 2019, 141 (3), 1382–1392. 10.1021/jacs.8b12634. [DOI] [PubMed] [Google Scholar]
- Sarmah B. K.; Konwar M.; Bhattacharyya D.; Adhikari P.; Das A. Regioselective Cyanation of Six-Membered N -Heteroaromatic Compounds Under Metal-, Activator-, Base- and Solvent-Free Conditions. Adv. Synth. Catal. 2019, 361, 5616–5625. 10.1002/adsc.201901103. [DOI] [Google Scholar]
- Pezzetta C.; Bonifazi D.; Davidson R. W. M. Enantioselective Synthesis of N-Benzylic Heterocycles: A Nickel and Photoredox Dual Catalysis Approach. Org. Lett. 2019, 21 (22), 8957–8961. 10.1021/acs.orglett.9b03338. [DOI] [PubMed] [Google Scholar]
- Anjali B. A.; Suresh C. H. Interpreting Oxidative Addition of Ph–X (X = CH 3, F, Cl, and Br) to Monoligated Pd(0) Catalysts Using Molecular Electrostatic Potential. ACS Omega 2017, 2 (8), 4196–4206. 10.1021/acsomega.7b00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo S.; Laidlaw G.; Kennedy A. R.; Sproules S.; Nelson D. J. Oxidative Addition of Aryl Electrophiles to a Prototypical Nickel(0) Complex: Mechanism and Structure/Reactivity Relationships. Organometallics 2017, 36 (8), 1662–1672. 10.1021/acs.organomet.7b00208. [DOI] [Google Scholar]
- Wada M.; Kusabe K.; Oguro K. Aryl(pentachlorophenyl)nickel(II) complexes. Lack of free rotation about tolyl-nickel bonds and lack of ″ortho effect″ in carbonylation. Inorg. Chem. 1977, 16 (2), 446–449. 10.1021/ic50168a045. [DOI] [Google Scholar]
- Krasley A. T.; Malachowski W. P.; Terz H. M.; Tran Tien S. Catalytic Enantioselective Birch–Heck Sequence for the Synthesis of Tricyclic Structures with All-Carbon Quaternary Stereocenters. Org. Lett. 2018, 20 (7), 1740–1743. 10.1021/acs.orglett.8b00196. [DOI] [PubMed] [Google Scholar]
- Fukuyama T.; Liu G. Stereocontrolled Total Synthesis of (±)-Gelsemine. J. Am. Chem. Soc. 1996, 118, 7426–7427. 10.1021/ja961701s. [DOI] [Google Scholar]
- Peng Z.; Knochel P. Preparation of Functionalized Organomanganese(II) Reagents by Direct Insertion of Manganese to Aromatic and Benzylic Halides. Org. Lett. 2011, 13 (12), 3198–3201. 10.1021/ol201109g. [DOI] [PubMed] [Google Scholar]
- Kakiya H.; Nishimae S.; Shinokubo H.; Oshima K. Preparation of organomanganese reagents from organic halides with activated manganese. Tetrahedron 2001, 57 (42), 8807–8815. 10.1016/S0040-4020(01)00864-X. [DOI] [Google Scholar]
- Knochel P.Organomagnesium and Organozinc Chemistry. In Organometallics in Synthesis, 2013; pp 223–372. [Google Scholar]
- Knochel P.Organozinc Reagents in Science of Synthesis, Vol. 3; Thieme: Stuttgart, Germany, 2004. [Google Scholar]
- Knochel P.; Jones P.. Organozinc reagents: A practical approach; Oxford University Press, 1999. [Google Scholar]
- Magnus P.; Waring M. J.; Scott D. A. Conjugate reduction of α,β-unsaturated ketones using an MnIII catalyst, phenylsilane and isopropyl alcohol. Tetrahedron Lett. 2000, 41 (50), 9731–9733. 10.1016/S0040-4039(00)01728-7. [DOI] [Google Scholar]
- Eberhardt N. A.; Guan H. Nickel Hydride Complexes. Chem. Rev. 2016, 116 (15), 8373–8426. 10.1021/acs.chemrev.6b00259. [DOI] [PubMed] [Google Scholar]
- Lapierre A. J. B.; Geib S. J.; Curran D. P. Low-Temperature Heck Reactions of Axially Chiral o-Iodoacrylanilides Occur with Chirality Transfer: Implications for Catalytic Asymmetric Heck Reactions. J. Am. Chem. Soc. 2007, 129 (3), 494–495. 10.1021/ja067790i. [DOI] [PubMed] [Google Scholar]
- McDermott M. C.; Stephenson G. R.; Hughes D. L.; Walkington A. J. Intramolecular Asymmetric Heck Reactions: Evidence for Dynamic Kinetic Resolution Effects. Org. Lett. 2006, 8 (14), 2917–2920. 10.1021/ol0606132. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; Li X.; Caricato M.; Marenich A. V.; Bloino J.; Janesko B. G.; Gomperts R.; Mennucci B.; Hratchian H. P.; Ortiz J. V.; Izmaylov A. F.; Sonnenberg J. L.; Williams-Young D.; Ding F.; Lipparini F.; Egidi F.; Goings J.; Peng B.; Petrone A.; Henderson T.; Ranasinghe D.; Zakrzewski V. G.; Gao J.; Rega N.; Zheng G.; Liang W.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Throssell K.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M. J.; Heyd J. J.; Brothers E. N.; Kudin K. N.; Staroverov V. N.; Keith T. A.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A. P.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Millam J. M.; Klene M.; Adamo C.; Adamo C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Farkas O.; Foresman J. B.; Fox D. J.. Gaussian 16; Gaussian, Inc.: Willingford, CT, 2016. [Google Scholar]
- Becke A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- Weigend F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8 (9), 1057–1065. 10.1039/b515623h. [DOI] [PubMed] [Google Scholar]
- Weigend F.; Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7 (18), 3297–3305. 10.1039/b508541a. [DOI] [PubMed] [Google Scholar]
- Grimme S.; Antony J.; Ehrlich S.; Krieg H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132 (15), 154104. 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- Grimme S.; Ehrlich S.; Goerigk L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32 (7), 1456–1465. 10.1002/jcc.21759. [DOI] [PubMed] [Google Scholar]
- Joo W.; Wang W.; Mesch R.; Matsuzawa K.; Liu D.; Willson C. G. Synthesis of Unzipping Polyester and a Study of its Photochemistry. J. Am. Chem. Soc. 2019, 141 (37), 14736–14741. 10.1021/jacs.9b06285. [DOI] [PubMed] [Google Scholar]
- Jackson L. V.; Walton J. C. Homolytic dissociation of 1-substituted cyclohexa-2,5-diene-1-carboxylic acids: an EPR spectroscopic study of chain propagation. J. Chem. Soc., Perkin Trans. 2 2001, 2 (9), 1758–1764. 10.1039/b104859g. [DOI] [Google Scholar]
- Peng Q.; Hu J.; Huo J.; Yuan H.; Xu L.; Pan X. Cp*Rh(iii) catalyzed ortho-halogenation of N-nitrosoanilines by solvent-controlled regioselective C–H functionalization. Org. Biomol. Chem. 2018, 16 (24), 4471–4481. 10.1039/C8OB00601F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.