Abstract
Background
Targeting protein kinase B (Akt) and its downstream signaling proteins are promising options in designing novel and potent drug candidates against hepatocellular carcinoma (HCC). The present study explores the anti-HCC potentials of Cannabis sativa (C. sativa) extract via the involvement of Akt using both in silico and in vivo animal models of HCC approaches.
Methods
Phytoconstituents of C. sativa extract obtained from Gas Chromatography Mass-spectrometry (GCSM) were docked into the catalytic domain of Akt-2. The Diethylnitrosamine (DEN) model of HCC was treated with C. sativa extract. The effects of C. sativa extract treatments on DEN model of hepatocellular carcinoma were assessed by One-way analysis of variance (ANOVA) of the treated and untreated groups
Result
The lead phytoconstituents of C. sativa extract, Δ-9-tetrahydrocannabinol (Δ-9-THC) and cannabidiol form stable hydrophobic and hydrogen bond interactions within the catalytic domain of Akt-2. C. sativa extract (15 mg/kg and 30 mg/kg) respectively gives a 3-fold decrease in the activities of liver function enzymes when compared with the positive control (group 2). It also gives a 1.5-fold decrease in hepatic lipid peroxidation and elevates serum antioxidant enzymes’ activities by 1-fold in HCC treated Wistar rats when compared with the positive control (group 2). In an animal model of hepatocellular carcinoma, C. sativa extract significantly downregulated Akt and HIF mRNAs in groups 3, 4, and 5 with 2, 1.5, 2.5-fold decrease relative to group 2. VEGF mRNA was downregulated by 1.5-fold decrease in groups 3-5 when compared to group 2. The expression of XIAP mRNA was downregulated by 1.5, 2, and 1.25-folds in groups 3, 4, and 5 respectively, in comparison with group 2. In comparison to group 2, COX-2 mRNA levels were downregulated by 1.5, 1, and 1-folds in groups 3–5. In groups 3–5, CRP mRNA was downregulated by 2-fold in comparison with group 2. In groups 3–5, p21 mRNA was upregulated by 2, 2.5, and 3-folds, respectively when compared with group 2. It upregulated p53 mRNA by 2.5, 3.5, and 2.5-folds in groups 3–5 in comparison with group 2. It downregulated AFP mRNA by 3.5, 2.5, .2.5-folds in groups 3, 4, and 5 respectively when compared with group 2. Histologic analysis showed that C. sativa extract reduced necrosis and inflammation in HCC.
Conclusion
C. sativa demonstrates anti-hepatocellular carcinoma potentials in an animal model of HCC and with the involvement of Akt. Its anticancer potential is mediated through antiangiogenic, proapoptotic, cycle arrest, and anti-inflammatory mechanisms.
In future studies, the mechanisms of anti-HCC effects of Δ-9-tetrahydrocannabinol (Δ-9- THC) and cannabidiol via the PI3K-Akt signaling pathways should be explored.
Keywords: Anti-angiogenic, Proapoptotic, Anti-inflammatory, Δ-9-tetrahydrocannabinol, Cannabidiol
Background
Hepatocellular carcinoma (HCC) represents over 85% of primary liver cancers. Liver cancers cause about one-fourth of cancer-related mortality (Yang et al. 2019). According to WHO, over 1 million patients will lose their lives to liver cancer in 2030 (World Health Organization 2020). Liver cancer represents the second most lethal neoplasm after pancreatic cancer (Jemal et al. 2004). The incidence of HCC is high in patients with underlying liver disease, mostly due to hepatitis B or C virus (HBV or HCV) infection or alcohol abuse. However, nonalcoholic fatty liver disease (NAFLD), metabolic syndrome, and obesity are increasing the incidence of liver cancer in western nations (Younossi et al. 2019). Liver cancer is most prevalent in Africa and the far East (Franca et al. 2004). In the current state of HCC public health, there is no approved drug for its prevention (Villanueva 2019). Sorafenib, an oral multikinase inhibitor approved in 2007, is under a lot of scrutiny due to its perceived reduced median survival of less than 10 months (Sanoff et al. 2016). For advanced HCC, multiple drug candidates are now available including lenvatinib, regorafenib, cabozantinib, ramucirumab, and nivolumab. However, their short overall survival (OS) end points and their associated side effects (such as seizures, dermatitis, gastrointestinal bleeding, hypertension, pneumonitis, and stomatitis) make them unsuitable for clinical trials.
Targeting the protein kinase B (Akt) and its downstream signaling proteins are promising options in designing novel and potent drug candidates against HCC (Dimri and Satyanarayana 2020). Akt, a serine/threonine kinase is at the crossroad of neoplasm development (Dimri and Satyanarayana 2020). Natural products have been reported to inhibit the PI3K/Akt/mTOR signaling (Huang 2013). C. sativa is also known as hemp or marijuana, it is a specie of the Cannabinaceae family of the plant and have been shown to demonstrate anticancer potentials (Massi et al. 2013, Velasco et al. 2016, Tomco et al. 2020). As far back as the mid-1970s, Munson et al. (1975) discovered that Tetrahydrocannabinol (THC), Δ8-THC and cannabinol inhibit Lewis lung adenocarcinoma growth in mice. In 2006, a pilot clinical trial involving nine glioblastoma patients found that intracranial administration of THC was safe for the treatment of cancer (Guzman et al. 2006). Glioma cells were the most commonly used cellular model in studies of C. sativa’s anticancer effects in the 2000s (Hinz and Ramer 2022). However, a broad range of tumor cell lines from various entities have been examined over time. Numerous mechanisms by which cannabinoids inhibit tumorigenesis have indeed been outlined. Among these, protein kinase B (Akt) inhibition proves to be an essential mechanism. However, there is a paucity of data on cannabinoids' anti-tumoral effects on hepatocellular carcinoma via Akt inhibition.
The present study hereby explores the anticancer potentials of C. sativa extract via the involvement of Akt using both in silico and in vivo animal model of HCC approaches.
Methodology
Extraction and gas chromatography mass-spectrometry (GCMS) of C. sativa
Dried C. sativa https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:306087-2 leaves were obtained with permission from the National Drug Law Enforcement Agency (NDLEA), Headquarters, Abeokuta, Nigeria. Dr. O. A. Obembe, a plant taxonomist at Adekunle Ajasin University, Akungba-Akoko, Nigeria, authenticated the C. sativa leaves with voucher number 195. A voucher specimen was later deposited at the Herbarium of Adekunle Ajasin University (AAUAH). The C. sativa leaves were pulverized into powder. Three hundred and seventy-seven grams (377 g) of the pulverized C. sativa leaves were soaked in 2.5 L of petroleum ether for 48 hours and filtered (Romano and Hazekamp, 2013). The concentration of the filtrate was carried using a rotary evaporator. The GCMS analysis was as earlier reported by Akinloye et al. (2021).
Docking of phytoconstituents against the Akt
The phytoconstituents of C. sativa extract obtained from the GCMS analysis above were retrieved from PubChem. The Akt (PDB, 3E88 and 2.5 Aº) protein receptor was downloaded from the protein databank (http://rcsb.org). C. sativa extract’s phytoconstituents were docked into catalytic domain of 3E88 using the grid coordinate (X = − 23.38, Y = 48.05, Z = − 7.43) of the co-crystallized, aminofurazan compound. Autodock 4.0 was employed for the docking. Molecules of water within the catalytic domain of 3E88 were removed. Validation of the docking scores was carried out by docking the co-crystallized ligand back into the catalytic site of 3E88 and estimate the root mean square deviation of the redocked ligand and the co-crystallized.
Wistar rats
Thirty (30) male Wistar rats between 150 and 180 g were obtained from the Institute of Advanced Medical Research and Training (IAMRAT), College of Medicine, University of Ibadan, Nigeria. They were handled based on the recommendation of the Code of Ethics of the World Medical Association (Declaration of Helsinki). The experimental protocols were endorsed by the Institutional Animal Ethics Committee (IAEC), with ethical approval number, FUNAABIEC/19/03.
Experimental design
The Wistar rats were grouped with respect to their weights into 5 groups of 6 Wistar rats per group.
Group 1, were given Wistar rats food and water only. Groups 2–4, were administered intra-peritoneally with 200 mg/kg of Diethylnitrosamine (DEN) and 0.5 mL/kg of carbon tetrachloride (CCl4) once a week for 3 weeks consecutively (Akinloye et al. 2021). Groups 3–4, were treated p.o (Per os): oral administration) for 3 weeks with 15 mg/kg and 30 mg/kg of C. sativa extract respectively. Group 5 did not receive DEN but were treated concurrently with groups 3–4 with 30 mg/kg of C. sativa extract. The animals were sacrificed through cervical dislocation on week 15 following DEN induction.
Note: The treatment commenced week 12 after the HCC had been confirmed histologically.
This was carried out by removing the liver of a randomly selected Wistar rat. The liver was preserved in buffered formalin at 10% for 24 h. With running water, the fixative was washed away. The liver tissue was dehydrated, washed in methyl-benzoate, and then set in paraffin wax. Hematoxylin and eosin were used to stain the liver sections after it had been cut into 3–5-μ-thick pieces. Light microscopy observations were made of the sections.
Liver function assays
The activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) were evaluated in the serum of the Wistar rats. These were carried out using Randox Kits from Randox Laboratories Ltd. (55 Diamond Road,
Crumlin, County Antrim, BT29 4QY, United Kingdom Ardmore, United Kingdom). The protocols as stated in the manufacturer’s (Randox Laboratories Ltd.) manuals were used.
Evaluation of superoxide dismutase and catalase activities
The activities of superoxide dismutase and catalase were evaluated in the serum. These were carried following the methods reported by Misra and Fridovich, (1972) and Hadwan and Abed (2016) respectively.
Evaluation of lipid peroxidation
The methods described by Farombi et al. (2000) was used to measure the formation of thiobarbituric acid reactive substances (TBARS).
Reverse transcription-polymerase chain reaction
RNAs isolation from the liver, reverse transcription, and quantitative PCR were carried out as earlier reported by Akinloye et al. (2021). The primers are as follows (Table 1):
Table 1.
The nucleotide sequences of the primers used
| Target gene | Forward 5’-3’ | Reverse 5′-3′ |
|---|---|---|
| GAPDH | AAGGGCTCATGACCACAGTC | GGATGCAGGGATGATGTTCT |
| COX 2 | GATGACGAGCGACTGTTCCA | TGGTAACCGCTCAGGTGTTG |
| IL 6 | GACTTCCAGCCAGTTGCCTT | GCAGTGGCTGTCAACAACAT |
| CRP | GCAGTAGGTGGGCCTGAAAT | CCCGTCAAGCCAAAGCTCTA |
| P21 | GAGAACTGGGGAGGGCTTTC | TCCTGAGCCTGTTTCGTGTC |
| VEGR | CGGGCCTCTGAAACCATGAA | GCTTTCTGCTCCCCTTCTGT |
| AKT 2 | ACAGACTGTGCCCTGTCCAC | CGTGGCCTCCAGGTCTTGAT |
| MMP-2 | AGAGGATACCCCAAGCCACT | AAAGGCAGCGTCTACTTGCT |
| P27 | GACTCACTCGCGGCTCC | GGCTCCCGTTAGACACTCTC |
| AFP | CACCATCGAGCTCGGCTATT | TCCCAAAAACTCGCTTGGGT |
| P53 | CCCCTGAAGACTGGATAACTGT | TCTCCTGACTCAGAGGGAGC |
| BAX | AGGACGCATCCACCAAGAAG | CAGTTGAAGTTGCCGTCTGC |
| XIAP | TGCATAATGAGGACTGGGGG | GCCCCGATCAGGAAAAACAC |
| BAD | CTTGAGGAAGTCCGATCCCG | GCTCACTCGGCTCAAACTCT |
Histopathological studies
The Histological analysis of the livers were carried as earlier reported by Akinloye et al. (2021). The livers were kept in 10% buffered formalin for 24 h. The fixative was washed away using running water. The liver tissues were dehydrated, washed in methyl-benzoate, and then embedded in paraffin wax. The liver sections were stained with hematoxylin and eosin after being cut into 3–5-mm-thick pieces. The sections were examined using light microscopy.
Statistical analysis
GraphPad Prism 7 was used for the one-way analysis of variance (ANOVA). Mean ± standard error of the mean (SEM) was used to express data from groups 1 to 5. The P < 0.05 was set as the significance difference between the groups.
Note: GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and mRNA levels (gene expression) were quantified using ImageJ software. The mean and standard error of the mean (SEM) of each gel image were obtained from ImageJ. For each group, the control (GAPDH) and the gene expression were normalized (normalization (Gene/GAPDH) × 100) and their respective SEM were also normalized (normalization (SEM of the gene/GAPDH SEM), that is the error bar on each histogram plot). Prism version 7 was used for plotting the graphs of relative mRNA expression (mean values, Y axis) against the groups (X axis) and the ANOVA analysis.
Results
Δ-9- THC, cannabidiol, and cannabinol as the major components of C. sativa
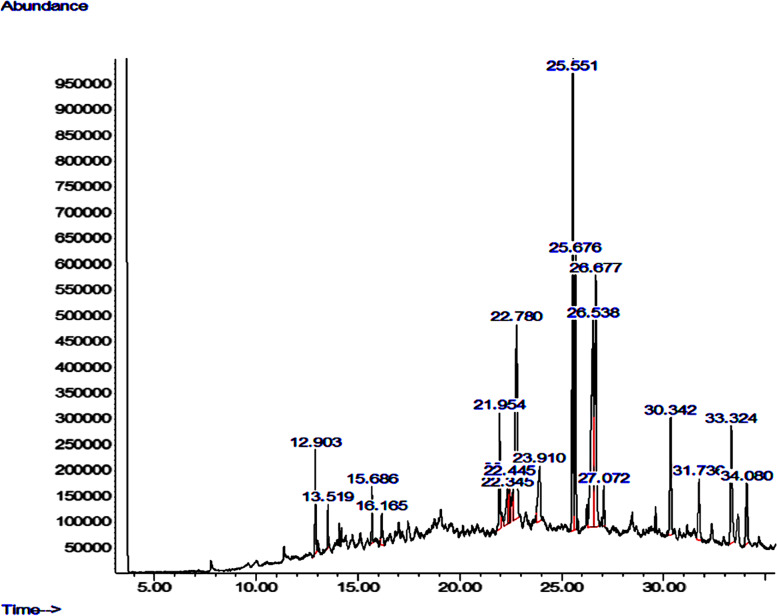
Figure 1 shows the GC-MS chromatogram of the phytoconstituents of the C. sativa extract. Δ-9-tetrahydrocannabinol (Δ-9-THC), cannabidiol, and cannabinol with percentage composition of 16.26%, 14.98%, and 13.486% respectively are the major components of the extract (Table 2).
Fig. 1.
GC-MS chromatogram of C. sativa extract
Table 2.
Gas-chromatography-mass spectrometry profiles of C. sativa extract
| Name | Retention time | Height | Area | Total |
|---|---|---|---|---|
| Caryophyllene | 12.903 | 199885 | 5469672 | 2.421% |
| Humulene | 13.519 | 84897 | 2273244 | 1.006% |
| Caryophyllene oxide | 15.686 | 108877 | 2851290 | 1.262% |
| Octadecanal | 16.165 | 60722 | 2707189 | 1.198% |
| Pentadecane | 21.954 | 222716 | 7159469 | 3.169% |
| 17-Pentatriacontene | 22.345 | 66952 | 4090780 | 1.811% |
| 2-pentadecanone | 22.417 | 92705 | 3880932 | 1.718% |
| Phytol | 22.445 | 85790 | 2238384 | 0.991% |
| Cannabinol | 22.780 | 375981 | 30466852 | 13.486% |
| Eicosanoic acid | 23.910 | 106439 | 8271611 | 3.661% |
| Δ-9-tetrahydrocannabinol | 25.551 | 1018978 | 36738929 | 16.263% |
| Caryophyllenol | 25.676 | 532074 | 17820294 | 7.888% |
| Cannabidiol | 26.538 | 396198 | 33834639 | 14.977% |
| Oleic acid | 26.677 | 484266 | 28258524 | 12.509% |
| Humulene oxide | 27.072 | 77698 | 2848796 | 1.261% |
| Methanone | 30.342 | 227246 | 10497238 | 4.647% |
| Nerolidol | 31.736 | 118114 | 6901660 | 3.055% |
| Cannabichromene | 33.324 | 226087 | 13132800 | 5.813% |
| Cannabicoumaronone | 34.080 | 116888 | 6469606 | 2.864% |
There are 19 phytoconstituents in the C. sativa extract, Δ-9-tetrahydrocannabinol, cannabinol, and cannabidiol constitute 16.263%, 13.486%, and 14.977% respectively of the extract
Phytoconstituents of C. sativa extract demonstrate inhibition of Akt
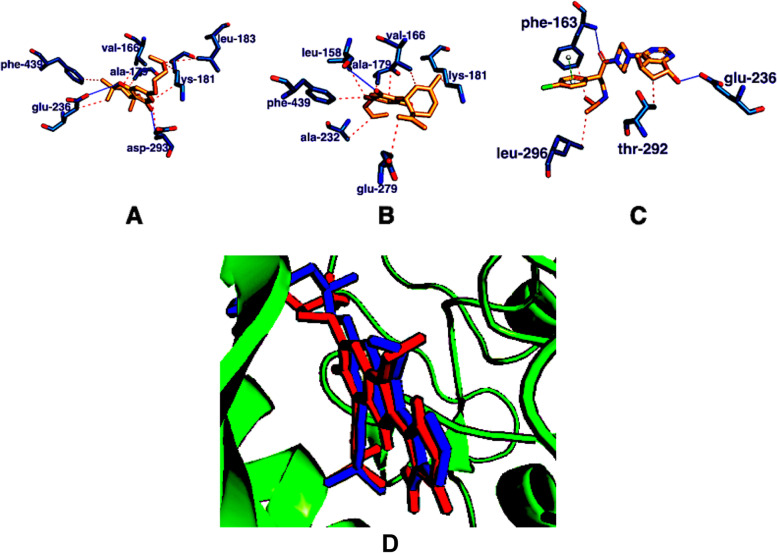
Docking of the phytoconstituents of C. sativa extract against the catalytic domain of Akt-2 shows they are potent inhibitor of Akt (Table 3). Two of the major components of the C. sativa extract, THC and cannabidiol possess the highest binding affinities with Akt-2, with binding energies of -10.3 kcal/mol and -10.0 kcal/mol respectively (Table 3). The lead compounds THC and cannabidiol demonstrate better inhibition of Akt when compared with ipatasertib (standard drug) with a binding energy of − 9.8 kcal/mol. Ipatasertib forms two hydrogen bond interactions (glu-236, phe-163), two hydrophobic interactions (leu-296, thr-292), and one pie stacking interaction (phe-163), THC forms seven hydrophobic interactions (phe-439, glu-236, val-166, ala-179, lys-181, leu-183, asp-293) and two hydrogen bonds (glu-236, asp-293), while cannabidiol form seven hydrophobic interactions (phe-439, leu-158, ala-179, val-166, lys-181, glu-279, ala-232) and one hydrogen bond (Fig. 2).
Table 3.
Virtual high throughput screening of the phytoconstituents of C. sativa extract against the catalytic domain of Akt-2
| S/N | C. sativa | Docking score (kcal/mol) |
|---|---|---|
| 1 | Δ-9-tetrahydrocannabinol | − 10.3 |
| 2 | Caryophyllene oxide | − 10.0 |
| 3 | Cannabidiol | − 10.0 |
| 4 | Caryophyllenol | − 9.9 |
| 5 | Caryophyllene | − 9.8 |
| 6 | Cannabicoumaronone | − 9.6 |
| 7 | Humulene | − 9.5 |
| 8 | Humulene oxide | − 9.3 |
| 9 | Cannabichromene | − 8.9 |
| 10 | Cannabinol | − 8.2 |
| 11 | Pentadecane | − 7.5 |
| 12 | Nerolidol | − 7.1 |
| 13 | phytol | − 6.9 |
| 14 | Oleic acid | − 6.3 |
| 15 | 17-pentatriacontene | − 6.1 |
| 16 | Eicosanoic acid | − 6.0 |
| 17 | 2-pentadecanone | − 5.9 |
| 18 | Octadecanal | − 5.8 |
| 19 | Methanone | − 5.5 |
Fig. 2.
3E88 in combination with A THC (orange), B cannabidiol (orange), and C ipatasertib (orange). The blue lines show hydrogen bond interactions, the red lines depict hydrophobic interactions, and the green line depicts pi stacking interaction. D The binding poses of the re-docked (blue) and the co-crystallized compound (red) within the catalytic domain of 3E88
Validation of docking scores
Validating docking studies requires re-docking of the co-crystallized ligand into the catalytic site of the protein. When the co-crystallized ligand was re-docked into the catalytic site of 3E88, a deviation of 0.301 Å was observed (Fig. 2D). The docking is considered accurate and reliable when the deviation is less than 2.0 Å (Morris et al. 1998).
C. sativa extract attenuated liver enzymes’ activities
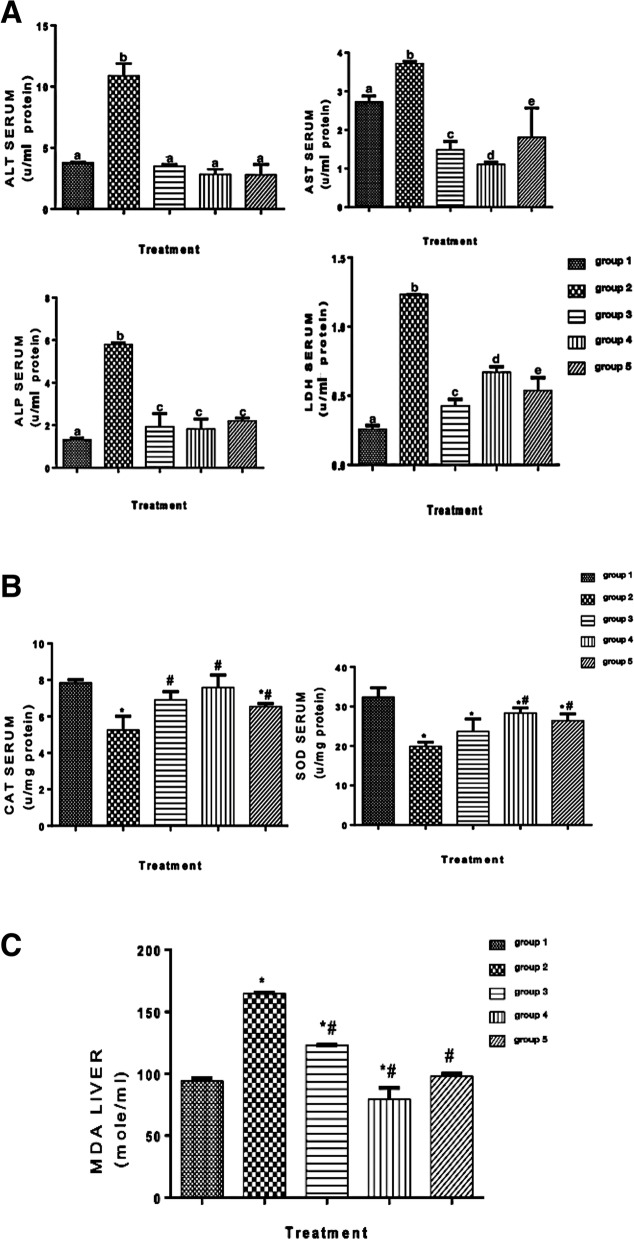
It has been reported that liver enzyme activities play a significant role in the prognosis of HCC (Zhang et al. 2017). In Fig. 3A, the activities of liver functions enzymes (AST, ALT, ALP, and LDH) were significantly attenuated by treating with 15 mg/kg and 30 mg/kg of C. sativa extract (groups 3–5) when compared with the untreated group (group 2).
Fig. 3.
A Activities of liver function enzymes, ALT, AS, ALP, and LDH. Different letters on the bars show significant different. Significance is at p < 0.05. Significance is at p < 0.05. B Catalase and superoxide dismutase activities in the serum. *Significant difference when groups 2–5 are compared with group 1, # significant difference when groups 3–5 are compared with group 2. Significance is at p < 0.05. C MDA concentration in the liver. *Significant difference when groups 2–5 are compared with group 1, # significant difference when groups 3–5 are compared with group 2. Significance is at p < 0.05
C. sativa extract increase the activities of antioxidant enzymes
High oxidative stress and low antioxidant capacities are believed to play a significant role in the development and progression of hepatocellular carcinoma (HCC) (Hsiao et al. 2021).
Catalase and superoxide dismutase activities were significantly increased by treating with 15 mg/kg and 30 mg/kg of C. sativa extract (groups 3-5) when compared with the untreated group (group 2) (Fig. 3B).
C. sativa extract ameliorates lipid peroxidation in HCC
Chronic necroinflammation of the liver leads to lipid peroxidation and oxidative stress, which contribute to hepatocellular carcinoma (HCC). (Feng et al. 2021). The concentration of malondialdehyde (MDA) (lipid peroxidation marker) in the liver was attenuated significantly by treating with 15 mg/kg and 30 mg/kg of C. sativa extract (groups 3–5) when compared with the untreated group (group 2) (Fig. 3C). The concentration of MDA was upregulated significantly in the group that received DEN alone (group 2) when compared with group 1.
C. sativa extract downregulates Akt mRNA
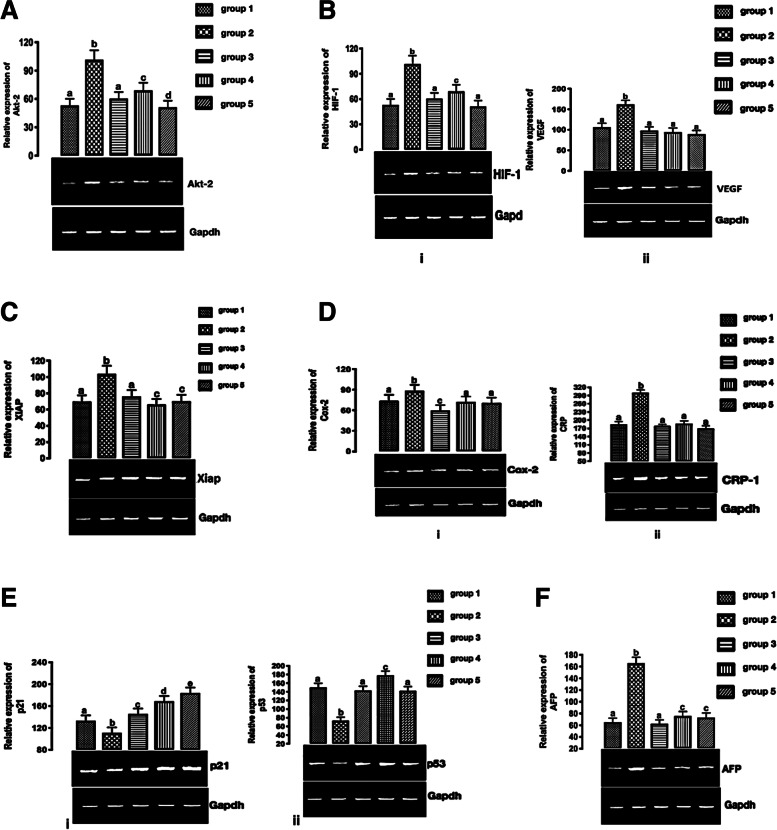
According to Tang et al. (2018), upregulation and activation of the Akt play a role in HCC invasion and metastasis. Direct and indirect anti-tumor activity can be observed when Akt is inhibited (Mroweh et al. 2021). Akt mRNA was upregulated significantly in the group that received DEN alone (group 2) when compared with group 1. The expression of Akt mRNA was significantly increased by treating with 15 mg/kg and 30 mg/kg of C. sativa extract (groups 3–5) when compared with the untreated group (group 2) (Fig. 4A).
Fig. 4.
A Expression of Akt-2 mRNA in HCC. Different letters on the bars show significant different. Significance is at p < 0.05. B (i) HIF-1 and (ii) VEGF mRNAs expression in HCC. Different letters on the bars show significant different. Significance is at p < 0.05. C Expression of XIAP mRNA in HCC. Different letters on the bars show significant different. Significance is at p < 0.05. D COX-2 and (ii) CRP mRNAs expression in HCC. Different letters on the bars show significant different. E (i) p21 and (ii) p53 mRNAs expression. Different letters on the bars show significant different. Significance is at p < 0.05. F AFP mRNA expression. Different letters on the bars show significant different. Significance is at p < 0.05
C. sativa extract downregulate Akt signaling cascade mRNAs
The expression patterns of mRNAs are associated with the signaling effects of Akt (angiogenesis, apoptosis, inflammation, and evasion of cell cycle arrest) were determined.
C. sativa extract downregulates angiogenic-related mRNAs
The hypoxia-inducible transcription factors (HIFs) regulate cellular metabolism, angiogenesis, proliferation, and migration, enabling a cell to respond to hypoxia (Wilson et al. 2014) and HCC is marked by overexpression of VEGF, which is considered the force driving physiological and pathological angiogenesis (ElGhandour et al. 2021). HIF and VEGF mRNAs were significantly upregulated in the group that received DEN alone (group 2) when compared with group 1. The expression of HIF-1 and VEGF mRNAs were significantly downregulated by treating with 15 mg/kg and 30 mg/kg of C. sativa extract (groups 3–5) when compared with the untreated group (group 2) (Fig. 4B).
C. sativa extract downregulate X-linked inhibitor of apoptosis protein (XIAP) mRNA
The overexpression of the X-linked inhibitor of apoptosis (XIAP) protein in hepatocellular carcinoma promotes metastasis and tumor recurrence (Shi et al. 2008).
XIAP mRNA was significantly upregulated in the group that received DEN alone (group 2) when compared with group 1. However, treating with 15 mg/kg and 30 mg/kg of C. sativa extract (groups 3–5) significantly downregulate XIAP mRNA when compared with the untreated group (group 2) (Fig. 4C).
C. sativa extract downregulate pro-inflammatory mRNAs
Carcinogenesis has been linked to cyclooxygenase-2 (COX-2) (Bae et al. 2001) and the COX-2 gene is upregulated in HCC (Chen et al. 2017).
The expression of pro-inflammatory cyclooxygenase-2 (COX-2) and C-reactive protein (CRP) mRNAs were significantly upregulated in the group that received DEN alone (group 2) when compared with group 1. They were significantly downregulated by treating with 15 mg/kg and 30 mg/kg of C. sativa extract (groups 3–5) when compared with the untreated group (group 2)
(Fig. 4D).
C. sativa extract upregulate cell cycle arrest mRNAs
Cell cycle arrest and apoptosis are triggered by increased p21 and p53 transcriptional activities (Engeland 2022).
P21 and p53 mRNAs expression were significantly upregulated in the group that received DEN alone (group 2) when compared with group 1. On the other hand, p21 and p53 mRNAs expression were significantly downregulated by treating with 15 mg/kg and 30 mg/kg of C. sativa extract (groups 3–5) when compared with the untreated group (group 2) (Fig. 4E).
C. sativa extract downregulate AFP mRNA expression
The only tumor biomarker routinely used in the treatment of hepatocellular carcinoma is alpha-fetoprotein (AFP) (HCC). AFP levels are strongly linked to tumor aggressiveness. Its concentrations are linked to poorly differentiated HCC, tumor size, and microvascular invasion. (Muscari and Maulat 2020).
AFP mRNA expression was significantly upregulated in the group that received DEN alone (group 2) when compared with group 1. However, AFP mRNA expression was significantly downregulated by treating with 15 mg/kg and 30 mg/kg of C. sativa extract (groups 3–5) when compared with the untreated group (group 2) (Fig. 4F).
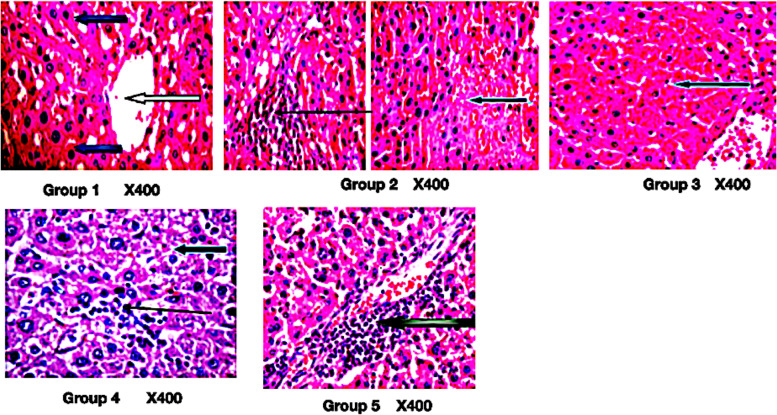
C. sativa extract moderate liver necrosis in HCC
C. sativa extract moderate necrosis and reduce inflammation in HCC (Fig. 5).
Fig. 5.
Photomicrograph of liver section stained by hematoxylin and eosin. Group 1: normal architecture as seen in lower magnification, the central venules appears mildly crowded (white arrows), the sinusoids appear normal (slender arrow) and devoid invasion of inflammatory cells, the hepatocytes revealed morphology that are normal (blue arrow). Group 2: mild vascular congestion (white arrow), there is a focal area of hepatocytes with necrosis (blue arrow) and infiltration of inflammatory cells (slender arrow). Group 3: focal area of moderate necrosis (blue arrow) and mild infiltration of inflammatory cells. Group 4: hepatocytes with fat infiltration (severe macovesicular and necrosis (blue arrow), there is mild portal triditis (white arrow), and sinusoid show mild infiltration (slender arrow). Group 5: portal infiltration of portal triads (black arrow), hepatocytes appear normal (blue arrow), and sinusoids show no infiltration (slender arrow)
Discussion
Liver cancer represents the second most deadly neoplasm (Jemal et al. 2004, Yang et al. 2019). In this study, we showed that C. sativa demonstrates anti-hepatocellular carcinoma potentials in animal model and with the involvement of Akt, a protein that is central to neoplastic development. GCMS analysis of the C. sativa extract revealed delta-9-tetrahydrocannabinol (Δ-9-THC), cannabidiol, and cannabinol with percentage composition of 16.26%, 14.98%, and 13.486% respectively as the major components of the extract. Molecular docking of the phytoconstituents of C. sativa extract against Akt-2 showed they inhibit Akt-2, a promising alternative in the design of novel and potent drug candidates against HCC. THC and cannabidiol possess the highest binding affinities with Akt-2 and also demonstrate better inhibition than ipatasertib. The better inhibition showed when compared to ipatasertib may not be unconnected with the formation of seven hydrophobic interactions and two hydrogen bonds within the Akt-2 catalytic domain by THC and seven hydrophobic interactions and one hydrogen bond by cannabidiol when compared with ipatasertib with two hydrogen bond interactions, two hydrophobic interactions, and one pie stacking interaction. The inhibition of Ak-2 by phytoconstituents of the C. sativa extract as shown by the molecular docking studies herein confirmed the report of Ozaita et al. (2007) that THC increased the phosphorylation of Ak-2. The RMSD of 0.301 Å showed by re-docking the co-crystallized ligand into the active domain of 3E88 further confirmed the validity of the in silico results (Morris et al. 1998).
The activities of liver enzymes are predictive and prognostic of HCC (Zhang et al. 2017). Increased activities of liver function enzymes are characteristics of HCC (Lala et al. 2020), as shown herein. However, treatment of HCC with 15 mg/kg and 30 mg/kg of C. sativa extract (groups 3-5) significantly attenuated the activities of liver function enzymes in group 3-5, when compared with group 2. The attenuation of the activities of the liver function enzymes demonstrated herein corroborated the reported hepatoprotective potentials of C. sativa (Ismail et al. 2018, Stohs and Ray 2020).
The significant decrease shown in the activities of catalase and superoxide dismutase enzymes in group 2 is in tandem with the report of Cheng et al. (2017) that HCC patients have increased oxidative stress and reduced antioxidant enzyme. However, treatment with C. sativa extract (groups 3–5) significantly increase the activities of these enzyme. This may be as a result of the reported antioxidant potentials of C. sativa (Girgih et al. 2011).
Upregulation and activation of the Akt have been implicated in the invasion and metastasis of HCC (Tang et al. 2018). In this study, treatment with C. sativa extract (groups 3–5) significantly downregulated Akt mRNA expression when compared with group 2. Inhibition of Akt as shown in the in silico studies may account for this observation. This also corroborated the report of Ellert-Miklaszewska et al. (2005) that cannabinoids down-regulate PI3K/Akt and Erk signaling. It also confirmed the report of Vara et al. (2011) that carotenoids inhibit the serine-threonine kinase Atk/mammalian target of rapamycin C.
Anti-angiogenic mRNAs HIF-1 and VEGF expression were downregulated significantly with C. sativa extract treatments (groups 3–5). This depicts that the C. sativa extract possesses anti-angiogenic potentials and hitherto confirm the inhibition of Akt, since Akt is known to be at the crossroad of angiogenesis (Dimri and Satyanarayana 2020). The downregulation of the anti-angiogenic mRNAs by the C. sativa extract further confirmed the reported anti-angiogenic properties of C. sativa (Solinas et al. 2012).
The upregulation of p21 and p53 mRNAs and hitherto downregulation of XIAP mRNA by the C. sativa extract treatments (groups 3–5), further corroborated the report of Ellert-Miklaszewska et al. (2005) that cannabinoid-induced inhibition of Akt leads to apoptosis and cell cycle arrest.
The downregulation of the pro-inflammatory mRNAs (COX-2 and CRP) by the C. sativa extract treatments (groups 3–5) corroborated its reported anti-inflammatory potentials (Nagarkatti et al. 2009) that C. sativa demonstrated anti-inflammatory potentials. Also, the downregulation of CRP and COX-2 further gives credence to the anti-HCC potentials of the C. sativa extract because high serum level of CRP has been reported to signify poor prognosis of patients with HCC (Zheng et al. 2013), while COX-2 overexpression has been shown to inhibit tumor cell apoptosis (Wang et al. 2019).
Alpha-fetoprotein (AFP) is still the main biomarker of liver neoplasm (Chan et al. 2017). AFP mRNA expression was upregulated in the group that received DEN alone (group 2) when compared with other groups. Furthermore, treatment with C. sativa extract (groups 3–5) significantly downregulated AFP mRNA. This shows treatment with C. sativa extract (groups 3–5) possess anti-HCC potentials.
Necrosis and inflammation are the hallmarks of HCC (Chan et al. 2017, Yu et al. 2018). The amelioration of necrosis and inflammation by the C. sativa extract further confirmed its anti-HCC potentials.
Conclusion
We established that C. sativa demonstrates anti-hepatocellular carcinoma potentials in an animal model of HCC and with the involvement of Akt. THC and cannabidiol form stable hydrophobic and hydrogen bond interactions within the catalytic domain of Akt-2. C. sativa extract reduced the activities of liver function enzymes. It ameliorates lipid peroxidation and increases the antioxidant enzymes’ activities. It shows anti-angiogenic, proapoptotic, and anti-inflammatory effects. It also demonstrates cell cycle arrest. C. sativa extract further demonstrates its anti-HCC effects by moderating necrosis and reduce inflammation in HCC. In future studies, the mechanisms of anti-HCC effects of Δ-9-tetrahydrocannabinol (Δ-9- THC) and cannabidiol via the PI3K-Akt signaling pathways should be explored. Although preclinical trials have demonstrated the clinical efficacy of C. sativa, clinical trials with cancer patients are lacking. It is imperative to review the results of prospective and randomized studies on the use of C. sativa in cancer treatment before drawing any conclusions.
Acknowledgements
The authors are grateful to the management of NDLEA Headquarters, Abeokuta, Nigeria for the provision of Cannabis sativa used for this study.
Abbreviations
- HCC
Hepatocellular carcinoma
- Akt
Protein kinase B
- GCSM
Gas Chromatography Mass-spectrometry
- DEN
Diethylnitrosamine
- Δ-9-THC
Δ-9-tetrahydrocannabinol
- WHO
World Health Organization
- HBV or HCV
Hepatitis B or C virus
- NAFLD
Nonalcoholic fatty liver disease
- OS
Overall survival
- NDLEA
National Drug Law Enforcement Agency
- IAMRAT
Institute of Advanced Medical Research and Training
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- ALP
Alkaline phosphatase
- LDH
Lactate dehydrogenase
- TBARS
Thiobarbituric acid reactive substances
- RNA
Ribonucleic acid
- ANOVA
One-way analysis of variance
- SEM
Standard error of the mean
- HIF-1
Hypoxia-inducible factor-1
- VEGF
Vascular endothelial growth factor
- XIAP
X-linked inhibitor of apoptosis protein
- COX-2
Cyclooxygenase-2
- CRP
C-reactive protein
- AFP
Alpha-fetoprotein
- H and E
Hematoxylin and eosin
Authors’ contributions
AOA proposed, design, and supervised the study, and he also edited the manuscript. DIA wrote part of the manuscript, carried out biochemical assays, and analyzed the data. MTS, MAL, F. O. O, and MAO carried out the in silico screening, biochemical assays, and analyzed the data. DSM took part in all aspects of the study and wrote the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable
Availability of data and materials
The supporting data are available, contact the corresponding author.
Declarations
Ethics approval and consent to participate
The experimental protocols were endorsed by the Federal University of Agriculture, Abeokuta, Nigeria, animal ethics Committee (FUNAABIAEC), with ethical approval number, FUNAABIEC/19/03.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dorcas I. Akinloye, Email: ibukundi@funaab.edu.ng
Damilohun S. Metibemu, Email: damilohun.s.metibemu@jsums.edu
Mujidat T. Shittu, Email: mujidatshittu40@gmail.com
Mariam A. Lawal, Email: mariamaina69@gmail.com
Faith O. Olatunji, Email: faitho@yahoo.com
Muideen A. Oyediran, Email: oyediran@yahoo.com
Oluseyi A. Akinloye, Email: oaakin@yahoo.com
References
- Akinloye OA, Akinloye DI, Lawal MA, Shittu MT, Metibemu DS. Terpenoids from Azadirachta indica are potent inhibitors of Akt: validation of the anticancer potentials in hepatocellular carcinoma in male Wistar rats. J Food Biochem. 2021;45(1):e13559. doi: 10.1111/jfbc.13559. [DOI] [PubMed] [Google Scholar]
- Bae SH, Jung ES, Park YM, Kim BS, Kim BK, Kim DG, Ryu WS. Expression of cyclooxygenase-2 (COX-2) in hepatocellular carcinoma and growth inhibition of hepatoma cell lines by a COX-2 inhibitor, NS-398. Clin Cancer Res. 2001;7(5):1410–1418. [PubMed] [Google Scholar]
- Chan, S. L., Chan, A. W., & Yu, S. C. H. Alpha-fetoprotein as a biomarker in hepatocellular carcinoma: focus on its role in composition of tumor staging systems and monitoring of treatment response. Biomark Dis, 2017:623-635.
- Chen H, Cai W, Chu ESH, Tang J, Wong CC, Wong SH, Yu J. Hepatic cyclooxygenase-2 overexpression induced spontaneous hepatocellular carcinoma formation in mice. Oncogene. 2017;36(31):4415–4426. doi: 10.1038/onc.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SB, Liu HT, Chen SY, Lin PT, Lai CY, Huang YC. Changes of oxidative stress, glutathione, and its dependent antioxidant enzyme activities in patients with hepatocellular carcinoma before and after tumor resection. PLoS One. 2017;12(1):e0170016. doi: 10.1371/journal.pone.0170016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri M, Satyanarayana A. Molecular signaling pathways and therapeutic targets in hepatocellular carcinoma. Cancers. 2020;12(2):491. doi: 10.3390/cancers12020491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elghandor AM, Bayoumy EM, Ibrahim WA, Sayed MM, Salama AB, Teama NM, Salama MM. Vascular endothelial growth factor in relation to the development of hepatocellular carcinoma in hepatitis C virus patients treated by direct-acting antivirals. Egypt Liver J. 2021;11(1):1–6. [Google Scholar]
- Ellert-Miklaszewska A, Kaminska B, Konarska L. Cannabinoids down-regulate PI3K/Akt and Erk signalling pathways and activate proapoptotic function of Bad protein. Cell Signal. 2005;17(1):25–37. doi: 10.1016/j.cellsig.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Engeland K. Cell cycle regulation: p53–p21-RB signaling. Cell Death Different. 2022;29(5):946–960. doi: 10.1038/s41418-022-00988-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farombi EO, Tahnteng JG, Agboola AO, Nwankwo JO, Emerole GO. Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron—a Garcinia kola seed extract. Food Chem Toxicol. 2000;38(6):535–541. doi: 10.1016/S0278-6915(00)00039-9. [DOI] [PubMed] [Google Scholar]
- Feng YY, Yu J, Huang YH, Lin YH, Yeh CT. The lipid peroxidation derived DNA adduct γ-OHPdG levels in paraneoplastic liver tissues predict postoperative outcomes of hepatoma. J Cancer. 2021;12(13):4064–4074. doi: 10.7150/jca.56982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França AVC, Elias Júnior J, Lima BLGD, Martinelli AL, Carrilho FJ. Diagnosis, staging and treatment of hepatocellular carcinoma. BrazilJ Med Biol Res. 2004;37(11):1689–1705. doi: 10.1590/S0100-879X2004001100015. [DOI] [PubMed] [Google Scholar]
- Girgih AT, Udenigwe CC, Aluko RE. In vitro antioxidant properties of hemp seed (Cannabis sativa L.) protein hydrolysate fractions. J Am Oil Chem Soc. 2011;88(3):381–389. doi: 10.1007/s11746-010-1686-7. [DOI] [Google Scholar]
- Guzman M, Duarte MJ, Blazquez C, Ravina J, Rosa MC, Galve-Roperh I, González-Feria L. A pilot clinical study of Δ9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer. 2006;95(2):197–203. doi: 10.1038/sj.bjc.6603236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwan MH, Abed HN. Data supporting the spectrophotometric method for the estimation of catalase activity. Data Brief. 2016;6:194–199. doi: 10.1016/j.dib.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz, B., & Ramer, R. Cannabinoids as anticancer drugs: current status of preclinical research. Br J Cancer, 2022: 1-13. [DOI] [PMC free article] [PubMed]
- Hsiao YF, Cheng SB, Lai CY, Liu HT, Huang SC, Huang YC. The prognostic role of glutathione and its related antioxidant enzymes in the recurrence of hepatocellular carcinoma. Nutrients. 2021;13(11):4071. doi: 10.3390/nu13114071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. Inhibition of PI3K/Akt/mTOR signaling by natural products. Anti-Cancer Agents Med Chem. 2013;13(7):967–970. doi: 10.2174/1871520611313070001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail M, Hasan H, El-Orfali Y, Ismail H, Khawaja G. Anti-inflammatory, antioxidative, and hepatoprotective effects of trans δ9-tetrahydrocannabinol/sesame oil on adjuvant-induced arthritis in rats. Evid-Based Complement Altern Med. 2018;2018:9365464. doi: 10.1155/2018/9365464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, Edwards BK. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101(1):3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- Lala V, Goyal A, Bansal P, Minter DA. Liver Function Tests.[Updated 2020 Jul 4]. StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2020.
- Massi P, Solinas M, Cinquina V, Parolaro D. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75(2):303–312. doi: 10.1111/j.1365-2125.2012.04298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. doi: 10.1016/S0021-9258(19)45228-9. [DOI] [PubMed] [Google Scholar]
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19:1639–1662. doi: 10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B. [DOI] [Google Scholar]
- Mroweh M, Roth G, Decaens T, Marche PN, Lerat H, Macek Jílková Z. Targeting Akt in hepatocellular carcinoma and its tumor microenvironment. Int J Mol Sci. 2021;22(4):1794. doi: 10.3390/ijms22041794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson AE, Harris LS, Friedman MA, Dewey WL, Carchman RA. Antineoplastic activity of cannabinoids. J Natl Cancer Institute. 1975;55(3):597–602. doi: 10.1093/jnci/55.3.597. [DOI] [PubMed] [Google Scholar]
- Muscari F, Maulat C. Preoperative alpha-fetoprotein (AFP) in hepatocellular carcinoma (HCC): is this 50-year biomarker still up-to-date? Transl Gastroenterol Hepatol. 2020;5:46. doi: 10.21037/tgh.2019.12.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 2009;1(7):1333–1349. doi: 10.4155/fmc.09.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaita A, Puighermanal E, Maldonado R. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J Neurochem. 2007;102(4):1105–1114. doi: 10.1111/j.1471-4159.2007.04642.x. [DOI] [PubMed] [Google Scholar]
- Romano LL, Hazekamp A. Cannabis oil: chemical evaluation of an upcoming cannabis-based medicine. Cannabinoids. 2013;1(1):1–11. 10.7150/ijms.6050.
- Sanoff HK, Chang Y, Lund JL, O’Neil BH, Dusetzina SB. Sorafenib effectiveness in advanced hepatocellular carcinoma. Oncol. 2016;21(9):1113. doi: 10.1634/theoncologist.2015-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YH, Ding WX, Zhou J, He JY, Xu Y, Gambotto AA, Rabinowich H, Fan J, Yin XM. Expression of X-linked inhibitor-of-apoptosis protein in hepatocellular carcinoma promotes metastasis and tumor recurrence. Hepatology (Baltimore, Md.) 2008;48(2):497–507. doi: 10.1002/hep.22393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Massi P, Cantelmo AR, Cattaneo MG, Cammarota R, Bartolini D, Cinquina V, Valenti M, Vicentini LM, Noonan DM, Albini A, Parolaro D. Cannabidiol inhibits angiogenesis by multiple mechanisms. Br J Pharmacol. 2012;167(6):1218–1231. doi: 10.1111/j.1476-5381.2012.02050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohs SJ, Ray SD. Is cannabidiol hepatotoxic or hepatoprotective: a review. Toxicol Res Appl. 2020;4:2397847320922944. [Google Scholar]
- Tang Y, Wang R, Zhang Y, Lin S, Qiao N, Sun Z, Cheng S, Zhou W. Co-upregulation of 14–3-3ζ and P-Akt is associated with oncogenesis and recurrence of hepatocellular carcinoma. Cell Physiol Biochem. 2018;45(3):1097–1107. doi: 10.1159/000487351. [DOI] [PubMed] [Google Scholar]
- Tomko AM, Whynot EG, Ellis LD, Dupré DJ. Anti-cancer potential of cannabinoids, terpenes, and flavonoids present in cannabis. Cancers. 2020;12(7):1985. doi: 10.3390/cancers12071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara D, Salazar M, Olea-Herrero N, Guzman M, Velasco G, Diaz-Laviada I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagy. Cell Death Different. 2011;18(7):1099–1111. doi: 10.1038/cdd.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco G, Hernández-Tiedra S, Dávila D, Lorente M. The use of cannabinoids as anticancer agents. Progress Neuro-psychopharmacol and Biol Psychiatry. 2016;64:259–266. doi: 10.1016/j.pnpbp.2015.05.010. [DOI] [Google Scholar]
- Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–62. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- Wang Q, Lu D, Fan L, Li Y, Liu Y, Yu H, Sun G. COX-2 induces apoptosis-resistance in hepatocellular carcinoma cells via the HIF-1α/PKM2 pathway. Int J Mol Med. 2019;43(1):475–488. doi: 10.3892/ijmm.2018.3936. [DOI] [PubMed] [Google Scholar]
- Wilson GK, Tennant DA, McKeating JA. Hypoxia inducible factors in liver disease and hepatocellular carcinoma: current understanding and future directions. J Hepatol. 2014;61(6):1397–1406. doi: 10.1016/j.jhep.2014.08.025. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Projections of mortality and causes of death, 2016 to 2060. 2020. [Google Scholar]
- Yang, J. D., Hainaut, P., Gores, G. J., Amadou, A., Plymoth, A., & Roberts, L. R. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol, 2019: 1-16. [DOI] [PMC free article] [PubMed]
- Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, Romero-Gomez M. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17(4):748–755. doi: 10.1016/j.cgh.2018.05.057. [DOI] [PubMed] [Google Scholar]
- Yu LX, Ling Y, Wang HY. Role of non-resolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis Oncol. 2018;2(1):6. doi: 10.1038/s41698-018-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wang H, Ning Z, Xu L, Zhuang L, Wang P, Meng Z. Serum liver enzymes serve as prognostic factors in patients with intrahepatic cholangiocarcinoma. OncoTargets Therapy. 2017;10:1441. doi: 10.2147/OTT.S124161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Zhou L, Gao S, Yang Z, Yao J, Zheng S. Prognostic role of C-reactive protein in hepatocellular carcinoma: a systematic review and meta-analysis. Int j Medical Sci. 2013;10(6):653–664. doi: 10.7150/ijms.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The supporting data are available, contact the corresponding author.