Abstract
Selective serotonin reuptake inhibitors (SSRIs) are the most widely used treatment by women experiencing depression during pregnancy. However, the effects of maternal SSRI use on early offspring development remain poorly understood. Recent studies suggest that SSRIs can modify the gut microbiota and interact directly with particular gut bacteria, raising the question of whether the gut microbiome impacts host responses to SSRIs. In this study, we investigate effects of prenatal SSRI exposure on fetal neurodevelopment and further evaluate potential modulatory influences of the maternal gut microbiome. We demonstrate that maternal treatment with the SSRI fluoxetine induces widespread alterations in the fetal brain transcriptome during midgestation, including increases in the expression of genes relevant to synaptic organization and neuronal signaling and decreases in the expression of genes related to DNA replication and mitosis. Notably, acute maternal fluoxetine treatment has no overt effects on the composition of the maternal gut microbiota. However, maternal pretreatment with antibiotics to deplete the gut microbiome substantially modifies transcriptional responses of the fetal brain to maternal fluoxetine treatment. In particular, maternal fluoxetine treatment elevates localized expression of the opioid binding protein/cell adhesion molecule like gene Opcml in the fetal somatosensory neocortex, thalamus, lateral ganglionic eminence, and striatum, which is prevented by maternal antibiotic treatment. Together, these findings reveal that maternal fluoxetine treatment alters gene expression in the fetal brain through pathways that are impacted, at least in part, by the presence of the maternal gut microbiota.
Keywords: Maternal microbiome, fluoxetine, SSRI, neurodevelopment, Opcml
1. Introduction
Major depression occurs in 8–12% of pregnant women, and the number of pregnant women with symptoms of depression has continued to increase1–3. Among treatment options, selective serotonin reuptake inhibitors (SSRIs) are the most widely used class of antidepressants during pregnancy4. Despite the importance of treating symptoms of depression during pregnancy, maternal use of SSRIs has been associated with adverse obstetric outcomes, including preterm birth, low birth weight, smaller head circumference, poor neonatal adaptation postdelivery and low Apgar scores5–9. Neurodevelopmental abnormalities in the offspring have also been reported: infants of mothers treated with SSRIs during pregnancy exhibited reduced global integration in the frontal brain, increased gray matter in the amygdala and increased white matter connectivity in the insular cortex, compared to matched controls10,11. Consistent with this, some studies have linked maternal SSRI exposure to increased risk for intellectual disability and autism spectrum disorder in the offspring12–14, though the associations remain controversial 15–17. Findings from animal models indicate that maternal SSRI treatment decreases social behavior, increases anxiety-like behavior and alters neurophysiology in the adult offspring18–22. However, exactly how maternal SSRI treatment during pregnancy influences early neurodevelopment to impart lasting alterations in behavior remains poorly understood.
Despite the widespread use of SSRIs as a first-line treatment for depression, patient responses to SSRIs are highly variable23,24. This highlights a need to identify physiological factors that modify responses to SSRIs in order to understand the biological basis of treatment efficacy. The maternal gut microbiome is one such variable that is increasingly recognized for its important roles in the metabolic, immune, and nervous systems25–31 and for its capacity to modulate patient responses to various common medications32–35. Indeed, SSRI use is associated with alterations in the gut microbiome36–38 and select gut bacteria can interact directly with SSRIs39,40, which raises the question of whether the maternal microbiome may modify host responses to SSRI treatment during pregnancy.
This study addresses these open questions by investigating the effects of maternal SSRI treatment on fetal neurodevelopment and by further evaluating roles for the maternal gut microbiome in modulating observed responses to SSRIs. The results reveal widespread influences of maternal fluoxetine treatment on fetal brain gene expression during midgestation, which are modified by the maternal gut microbiota, likely through indirect host-microbial interactions with SSRIs.
2. Materials and methods
2.1. Mice
6–8 week-old specific pathogen-free (SPF) C57BL/6J mice from the Jackson laboratory were group-housed in ventilated cages, with free access to standard rodent chow and water. The holding room maintained a controlled temperature (22–25°C) and humidity, as well as a 12-hour light/dark cycle. Prior to breeding, bedding from all cages was mixed every 3 days over 14 days to normalize the gut microbiota across mice. Mice were randomly divided into four groups: SPF mice treated with saline (SPF + Veh), SPF mice treated with fluoxetine (SPF + FLX), SPF mice pre-treated with antibiotics and treated with saline (ABX + Veh) and SPF mice pre-treated with antibiotics and treated with fluoxetine (ABX + FLX). All experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals using protocols approved by the Institutional Animal Care and Use Committee at UCLA.
2.2. Antibiotic pre-treatment and timed-matings
All male and female mice in the ABX + Veh and ABX + FLX groups were orally gavaged with an antibiotic cocktail of vancomycin (50 mg/kg), neomycin (100 mg/kg), and metronidazole (100 mg/kg) twice daily at 8:00 and 17:00, for 7 days, while being maintained on drinking water supplemented with ampicillin (1 mg/ml). SPF controls were gavaged with saline and maintained on standard drinking water. Males and females in each group were then paired for timed-matings. Mice in the ABX + Veh and ABX + FLX groups were maintained on drinking water supplemented with ampicillin (1 mg/ml), neomycin (1 mg/ml) and vancomycin (0.5 mg/ml). Females were checked daily for vaginal plugs. The day of plug observation was considered embryonic day 0.5 (E0.5), after which dams were separated and monitored for weight gain over 14 days.
2.3. Fluoxetine treatment and tissue collection
On day E7.5, dams that gained 2–3g denoting successful pregnancy were gavaged daily at 8:00 over 8 days with 10 mg/kg fluoxetine hydrochloride7,8 (FLX, Santa Cruz) or saline as the vehicle control (Veh). On E14.5, dams were sacrificed by cervical dislocation to preclude any effects of hypoxic stress from CO2 euthanasia on maternal and fetal physiology. Whole embryos or embryonic brains were collected for downstream analyses.
2.4. Serotonin and tryptophan measurements
Maternal blood samples were collected by cardiac puncture and spun through serum separation tubes (Becton Dickinson) for 10 minutes at 1000 RCF (g) at 4°C. E14.5 fetal brains were sonicated on ice for 10 s at 50 mV in enzyme-linked immunosorbent assay (ELISA) standard buffer supplemented with 10% ascorbic acid (Eagle Biosciences). Serotonin levels were detected by ELISA assay according to the manufacturer’s instructions (Eagle Biosciences, SEU39-K01). Tryptophan levels were measured using the Bridge-IT L-Tryptophan fluorescence assay (Mediomics, 1–1-1001) according to the manufacturer’s protocol. Readings from tissue samples were normalized to total protein content as detected by the 660 nm Protein Assay (Thermo Pierce, 22662). All samples were run in triplicate, and controls and standards were run in duplicate. Optical density was read at 405 nm on a Synergy H1 Hybrid Multimodal plate reader (BioTek). Mean absorbance per sample was calculated based on a standard curve of serial dilutions and triplicates were confirmed to exhibit a coefficient of variability of less than 10%.
2.5. Fluoxetine quantitation
Fluoxetine (1 μM, 10 μM, and 100 μM) was added to water to confirm detection and retention time (RT) = 1.89 min. For standards, fluoxetine (10 μM, 1 μM, 100 nM, and 10 nM) was added to control serum samples before metabolite extraction. 50 μl standard and experimental serum samples were mixed with 50 μL H2O and 400 μL of 100% methanol, the samples were vortexed for 10 sec, placed at −80°C for 20 minutes. Samples were centrifuged and the cell free supernatant was mixed with 300 μl H2O and 400 μl chloroform. The aqueous layer containing fluoxetine was transferred to glass vials (Thermo Fisher Scientific, 13–622-351) and dried in a Genevac EZ-2 Elite evaporator. At the UCLA Metabolomics core facility, dried samples were resuspended in 50% Acetonitrile (ACN):water. Utilizing a Vanquish Flex (Thermo Scientific) UPLC, 1/10th of the sample was loaded onto a Luna 3um NH2 100A (150 × 2.0 mm) column (Phenomenex) equilibrated to 15% mobile phase A (5 mM NH4AcO pH 9.9) and 85% B (ACN). Metabolite was eluted with a 4 min gradient of 15%−90% A at a flow rate of 200 μl/min, followed by re-equilibration to 15% A. Metabolite was detected with a Q Exactive (Thermo Scientific) mass spectrometer in full MS mode with positive ionization mode and at 70K resolution. The data files were then converted to mzXML files with MSConvert and extracted with Maven (v8.1.27.5) for fluoxetine ([M+H]+= 310.14133).
2.6. 16S rRNA gene sequencing and analysis
Fecal samples were collected for the SPF + Veh and SPF + FLX groups on gestational days E3.5, E6.5, E8.5, E11.5 and E14.5, and kept frozen at −80°C. Bacterial genomic DNA was extracted from frozen fecal samples using the Qiagen DNeasy Powersoil Kit, and purified using the QIAquick PCR Purification Kit. The sequencing library was generated according to methods adapted from Caporaso et al.41. The V4 regions of the 16S rRNA gene were amplified by PCR using universal primers barcoded with unique oligonucleotides, Illumina adaptors, and 30 ng of the extracted genomic DNA. The PCR reaction was set up in triplicates, and the product was then purified again using the QIAquick PCR purification kit (Qiagen). DNA concentration was quantified using a BioTek Synergy H1 Multimodal microplate reader, and 250 ng of purified PCR product from each sample was pooled and sequenced by Laragen, Inc. Sequencing was performed using the Illumina MiSeq platform and 2 × 250 bp reagent kit for paired-end sequencing. Operational taxonomic units (OTUs) were chosen by open reference OTU picking based on 99% sequence similarity to the most recent SILVA 132 database42,43. Taxonomy assignment and rarefaction were performed using QIIME2–2020.2.044.
2.7. Fetal brain RNA sequencing and analysis
Embryonic brains were dissected on E14.5 and homogenized in Trizol Reagent (Invitrogen), using three biological replicates per group as the minimum for inferential analysis45. RNA was extracted using the RNAeasy Mini kit with on-column genomic DNA-digest (Qiagen). RNA quality of RIN > 8.0 was confirmed using the 4200 Tapestation system (Agilent). RNA was prepared using the TruSeq RNA Library Prep kit, and 2 × 69 bp paired-end sequencing was performed using the Illumina HiSeq 4000 platform by the UCLA Neuroscience Genomics core facility. FastQC v0.11.8 and HiSAT2 2.1.046,47 were used for quality filtering and mapping. Reads were aligned to UCSC Genome Browser assembly ID: mm10. Differential expression analysis was conducted using DESeq2 1.24.048. Heatmaps were generated using the pheatmap package for R. GO term enrichment analysis of differentially expressed genes with q < 0.05 was conducted using DAVID v6.849. Protein interaction networks were generated using STRING v10.5 using a minimum required interaction score of highest confidence (0.900) and maximum number of interactions of no more than 5 interactors, and line thickness is based on confidence of interaction. Functional enrichments nodes were categorized by GO: biological process, molecular function, and cellular component and/or KEGG pathways using a false discovery rate (FDR) less than 0.05.
2.8. Quantitative RT-PCR
E14.5 brains were dissected and sonicated in Trizol for RNA isolation using the RNAeasy Mini kit with on-column genomic DNA-digest (Qiagen). cDNA synthesis was performed using the qScript cDNA synthesis kit (Quantabio). qRT-PCR was performed on a QuantStudio 5 thermocycler (ThermoFisher Scientific) using SYBR green master mix with Rox passive reference dye and validated primer sets obtained from Primerbank (Harvard).
2.9. Microcomputed tomography (μCT)
Whole embryos were serially dehydrated in ethanol and incubated in 4% (w/v) phosphotungstic acid (EPTA) diluted in 70% EtOH for 4 days at 4°C. Embryos were scanned at 80 kVp/140 μA with 500 ms exposure and a 5-frame average at a resolution of 20 μm using a μCT scanner (HiCT) developed by the Chatziioannou Lab at the Crump Institute for Molecular Imaging at UCLA. 2-dimensional images were reconstructed following dynamic range adjustment using gaussian smoothing. Regions of interest (ROI) for embryo and brain volume measurements were selected manually, and EPTA-stained tissue was segmented based on contrast to give a final embryo and brain volume measurement (mm3) within the ROI. Whole embryo and brain volumes were reconstructed and measured using Amide software (amide.exe 1.0.4).
2.10.1. Fluorescence in situ hybridization
E14.5 embryos were harvested, immediately frozen in liquid nitrogen and then embedded in cryo-embedding medium OCT (Tissue-Tek, VWR). Fetal brains were cut into 15 μm sections, mounted onto SuperFrost Plus slides and post-fixed in 4% paraformaldehyde for 15 min at 4°C. Sections were then serially dehydrated in 50%, 70%, 100% and 100% ethanol for 5 min each at room temperature and processed using the RNAscope Multiplex Fluorescent Kit V2 (Advanced Cell Diagnostics Inc, 323100). Sections were incubated for 2 hrs at 40°C with 3-plex positive control probe (320881), 3-plex negative control probe (320871) or customized target probes for mouse gene Mm-Opcml (824171). Following probe hybridization, sections were washed twice with wash buffer (ACD, 310091), and then sequentially hybridized with amplifier 1, 2, and 3 at 40°C for 30 min, 30 min, and 15 min, respectively. HRP signal was developed and visualized in Opal Dye 690 channel. Sections were then counterstained with the nuclear marker DAPI and mounted using ProLong Gold Antifade Mountant.
2.10.2. Fluorescence in situ hybridization imaging
Slides were imaged using a 20X objective on a Zeiss LSM780 confocal microscope, equipped with a Diode 405 (1%) and HeNe 633 nm at 17%. Images were acquired with 0.7 and 1X zoom, average line 2, pixel dwell of 3.15 μs at a 1024 × 1024 pixel resolution. Scans were exported using the Zen 2.1 (Blue Edition) software.
2.10.3. Fluorescence in situ hybridization image analysis
Images of somatosensory neocortex, thalamus, lateral ganglionic eminence, striatum and hippocampus were deidentified and analyzed using ImageJ (version: 2.0.0-rc-69/1.52p) by a researcher blinded to experimental group. Channels were split into different windows, and the scale was set to 1.2 pixels/ μm. Raw integrated density of Opcml in each image was measured to assess total fluorescence. Then, the DAPI channel of each image was thresholded to 30/255, and the area was measured to ascertain total brain area. Opcml integrated density was divided by the DAPI area for that image, to calculate total raw integrated density per μm2. For somatosensory neocortex, thalamus and striatum, the whole image was quantified, and for lateral ganglionic eminence and hippocampus, an ROI was drawn to isolate the region from adjacent tissue.
2.11.1. Fluorescence immunohistochemistry
E14.5 embryos were quickly collected and fixed in 4% paraformaldehyde for 24 hours at 4°C, after which they are transferred to a 30% sucrose solution for cryoprotection. After a week in sucrose, embryos were frozen in OCT (Tissue-Tek, VWR) and stored at −80°C. Embryos were sectioned sagittally at 10 μm, mounted on Superfrost Ultra Plus glass slides (ThermoFisher Scientific) and stored at −20°C. Slides were incubated in DAKO antigen retrieval solution (Agilent) at 90°C for 2 min, washed with 1X PBS, and then incubated for 1 hour at room temperature with 10% normal donkey serum. Slides were then incubated with primary antibodies for 30 hours at 4°C: anti-5-HT (rat monoclonal, Abcam, ab6336, 1:100), anti-SERT (rabbit polyclonal, Abcam, ab9726, 1:500), or anti-Tph2 (goat polyclonal, US Biological 208476, 1:500). Slides were incubated with the respective secondary antibody for 1 hour at room temperature: donkey anti-rat Alexa Fluor 488, 1:1000; donkey anti-goat, Alexa Fluor 568, 1:1000; donkey anti-rabbit, Alexa Fluor 647, 1:1000 (ThermoFisher Scientific). Slides were mounted with Prolong Gold antifade reagent with DAPI (ThermoFisher Scientific), air-dried for 1 hour, and maintained at 4°C.
2.11.2. Fluorescence immunohistochemistry imaging
Slides were imaged using a 20X objective on a Zeiss LSM780 confocal microscope, equipped with an Argon laser (488 nm) at 14%, a Diode 561 nm at 10% and HeNe 633 nm at 15%. Images were acquired across eight z-sections, scanning a total of 5.31 μm at a 1024 × 1024 pixel resolution. Scans were tiled in the Zen Black Edition software and stitched using the Zen 2.1 (Blue Edition) software.
2.11.3. Fluorescence immunohistochemistry image analysis
To compare 5-HT+, SERT+ and Tph2+ expression in each group, sagittal E13.5 and E15.5 brain sections from the Allen Developing Mouse Brain Atlas (2008) were used as a reference to locate the dorsal raphe nucleus (DRN) and axons. DRN neurons were counted using the ImageJ Puncta Analyzer plugin 50. A DRN standard region of interest (ROI) was used to measure cells that colocalized with 5-HT+ and SERT+ fluorescence, or 5-HT+ and Tph2+ fluorescence in the DRN. Separately, three standard ROIs were used to measure the number of 5-HT+, SERT+ and Tph2+ puncta and integrated density in DRN axon projections. Quantifications were normalized to the area of the ROIs and background noise was subtracted.
2.12. Statistical analysis
Statistical analyses were performed in Prism 8 software. Given that maternal fluoxetine treatment and microbiome status are the primary experimental variables across experiments, biological sample sizes reflect the number of independent dams. Experiments evaluating fetal outcomes include at least 2 randomly selected embryos per dam, where data from offspring from a single dam were averaged to represent the dam as the biological “n”. Group comparisons were assessed using one-way ANOVA with Tukey’s Multiple Comparisons post-hoc test.
3. Results
3.1. Acute maternal fluoxetine treatment has no significant effects on maternal weight, litter size, fetal volume or fetal brain volume.
To first examine effects of acute maternal fluoxetine treatment on gross metrics of maternal and fetal health, conventionally-colonized pregnant dams were orally gavaged with fluoxetine (10 mg/kg; SPF + FLX) or saline as a vehicle control (SPF + Veh) once daily from E7.5 to 14.5 (Fig. 1A). Mass spectrometry-based assessment of maternal serum indicated that acute oral fluoxetine treatment of pregnant dams yielded serum fluoxetine concentrations of 1.597 ± 0.1618 μM (mean ± s.e.m.) on E14.5 (Supplementary Fig. 1A, B). There were no statistically significant differences in maternal weight before or after fluoxetine treatment (Supplementary Fig. 2A–C). Interestingly, pregnant dams that were treated with fluoxetine exhibited modest, though statistically significant, increases in cecal weight on E14.5 (Supplementary Fig. 2D), which may align with expected responses to the proposed antibacterial effects of fluoxetine51. Contrary to a previous report that treated pregnant dams of a different mouse strain (129/SvEvTac) with fluoxetine in drinking water (approximately 10 mg/kg/day) for 14 days52, we observed no statistically significant alterations in average litter size after acute maternal fluoxetine treatment during midgestation (Supplementary Fig. 2E). Microcomputed tomography (μCT)-based imaging indicated no statistically significant effects of maternal fluoxetine treatment on embryo or brain volume (Supplementary Fig. 2F–H). Altogether, these data reveal no overt effects of acute maternal fluoxetine treatment during midgestation on gross metrics of maternal and fetal health.
Figure 1. Maternal fluoxetine treatment alters fetal brain gene expression.
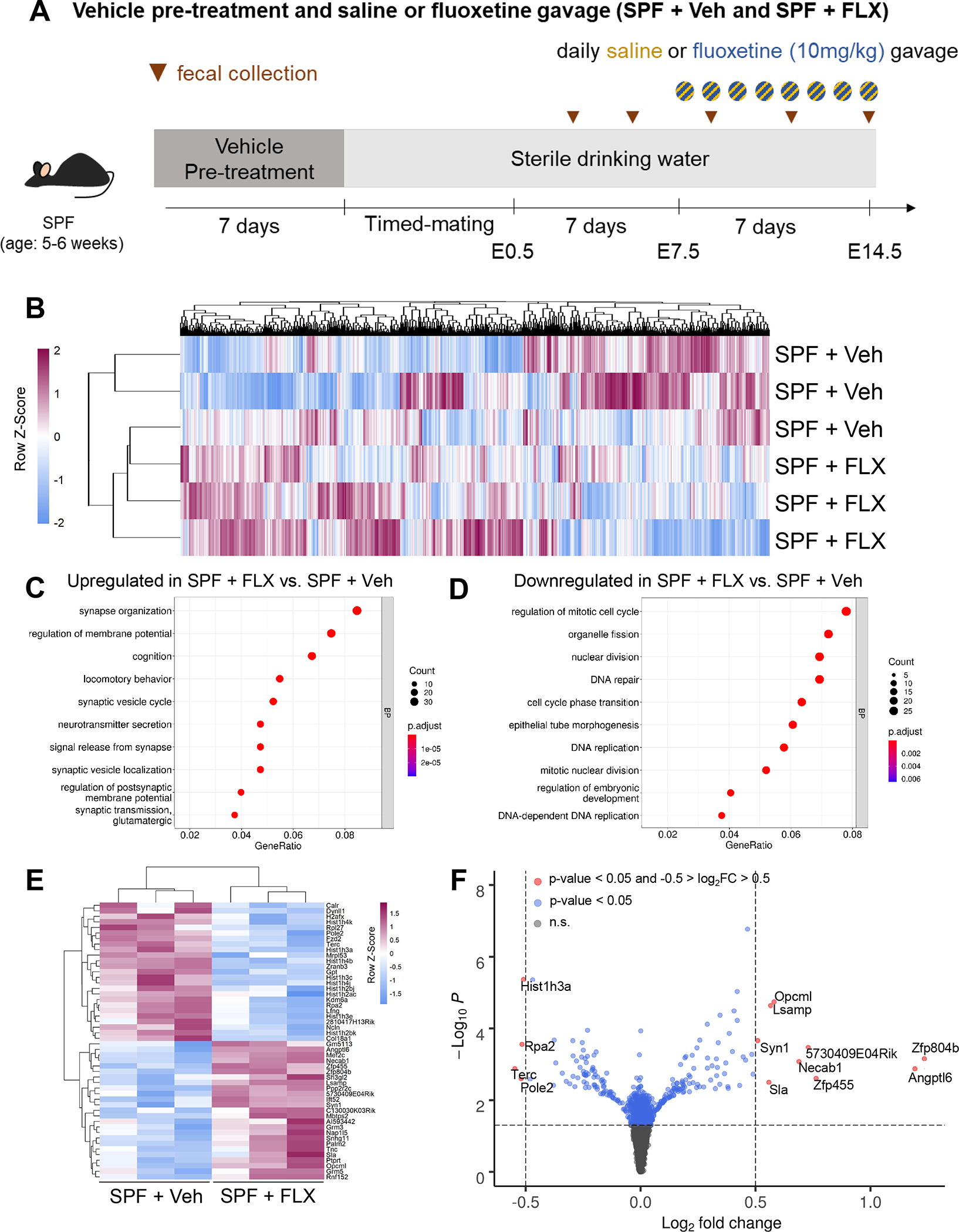
A, Experimental timeline of SPF + Veh and SPF + FLX groups. B, Heatmap of 864 differentially expressed genes (p <0.05) in E14.5 fetal brains from SPF+FLX compared to SPF+Veh dams (Wald test, n = 3, 3, respectively). C, Top 10 biological process (BP) gene ontology (GO) terms that are upregulated in E14.5 fetal brains from SPF+FLX compared to SPF+Veh dams (Fisher’s Exact test, n = 3, 3, respectively). Gene ratio: number of genes present in dataset vs present in GO term. D, GO term enrichment analysis of top 10 biological process (BP) that are downregulated in E14.5 fetal brains of SPF+FLX compared to SPF+Veh dams (Fisher’s Exact test, n = 3, 3, respectively). E, Heatmap of 25 most upregulated and 25 most downregulated differentially expressed genes (p<0.05) in E14.5 fetal brains from SPF+Veh compared to SPF+ FLX dams (Wald test, n = 3, 3, respectively). F, Volcano plot of differentially expressed genes in E14.5 fetal brain of embryos from SPF+FLX compared to SPF+Veh dams. Blue: Differentially expressed genes that are p<0.05. Red: Differentially expressed genes that have log2fold change greater than 0.5 and p<0.05.
3.2. Acute maternal fluoxetine treatment alters the fetal brain transcriptome
In the absence of overt effects of maternal fluoxetine treatment on fetal brain size, we next asked whether maternal fluoxetine treatment alters gene expression in the fetal brain. Fetal brains were harvested on E14.5 from dams treated with fluoxetine or vehicle, and processed for RNA sequencing and differential gene expression analysis. E14.5 was selected as a time point reflecting several active early neurodevelopmental events, including neurogenesis, neuronal migration, axon outgrowth, and synapse formation53. Transcriptomic analysis revealed that maternal fluoxetine treatment significantly altered the expression of 864 genes in the fetal brain, with 451 upregulated and 413 downregulated in fetal brains from offspring of fluoxetine-treated dams relative to those from saline-treated controls (Fig. 1B, Supplementary Table 1). In particular, maternal fluoxetine treatment increased the expression of genes that clustered into pathways related to synapse organization, cognition, locomotory behavior, and neurotransmission in the fetal brain (Fig. 1C, Supplementary Fig. 3A) and decreased the expression of genes most relevant to cell cycle, mitosis, DNA repair and DNA replication pathways in the fetal brain (Fig. 1D, Supplementary Fig. 3B). Among the differentially expressed genes, maternal fluoxetine treatment induced widespread reductions in the expression of several histone-related genes in the fetal brain (Hist1h4k, Hist1h3a, Hist1h4b, Hist1h3c, Hist1h4j, Hist1h3bj, Hist1h2ac, Hist1h3e, and Hist1h2bk), which encode nuclear proteins that play a central role in transcription regulation, DNA repair, DNA replication, and chromosomal stability (Fig. 1E, F). In contrast, maternal fluoxetine treatment increased the expression of select genes that encode for zinc finger proteins (Zfp455 and Zfp804b) and calcium ion binding proteins (Syn1 and Necab1) (Fig. 1E, F). In addition, maternal fluoxetine treatment increased the gene expression of Opcml, which is part of a family of cell adhesion molecules that regulate neurite outgrowth, dendritic arborization and synapse formation1 (Fig. 1E, F). These data reveal that acute maternal fluoxetine treatment elicits global alterations in the fetal brain transcriptome that have the potential to impact early neurodevelopment.
3.3. Acute maternal fluoxetine treatment has no significant effect on the fetal serotonergic system
5-HT is an important signaling molecule in the fetal brain that is derived from both central and peripheral sources54–56. The development of the serotonergic system in the fetal brain begins with the neurogenesis of 5-HT neurons from E9.5–12, followed by a series of neuro-maturational events that continue through the third postnatal week of life57. SSRIs inhibit the 5-HT transporter SERT to modulate the bioavailability of 5-HT and shape key components of the serotonergic system22,58,59. In light of reports that fluoxetine, and other SSRIs, can cross the placenta, we asked if the effects of maternal fluoxetine treatment on fetal brain gene expression could be due to direct effects of fluoxetine on the fetal serotonergic system. To gain insight, we first assessed effects of maternal fluoxetine treatment on levels of 5-HT and its precursor tryptophan in maternal serum and fetal brain at E14.5. As an expected response to the inhibition of SERT-dependent uptake of 5-HT by blood platelets60, maternal fluoxetine treatment significantly decreased maternal serum levels of 5-HT relative to vehicle-treated controls, with no effects on levels of maternal tryptophan (Supplementary Fig. 4A, B). Notably, maternal fluoxetine treatment had no statistically significant effects on levels of 5-HT or tryptophan in the fetal brain, suggesting no direct effects of maternal fluoxetine on the bioavailability of 5-HT in the fetal brain (Supplementary Fig. 4C, D). To further evaluate the possibility for maternal fluoxetine treatment to alter fetal development of the central serotonergic system, serotonergic neurons of the fetal dorsal raphe nucleus (DRN) were stained and imaged for 5-HT, SERT, and Tph2, the rate-limiting enzyme for neuronal 5-HT synthesis. Compared to vehicle-treated controls, there were no statistically significant effects of maternal fluoxetine treatment on the density of 5-HT-, SERT, or Tph2-positive neurons in the DRN (Supplementary Fig. 5A–G), or on the integrated density and number of 5-HT-, SERT-, or Tph2-positive axonal projections from DRN to the prefrontal cortex (Supplementary Fig. 5H–N). Together, these results indicate that maternal fluoxetine treatment has no significant effect on levels of 5-HT or development of serotonergic DRN neurons in the fetal brain. These findings further suggest that the observed transcriptional responses to maternal fluoxetine treatment in the fetal brain are likely not due to direct effects of fluoxetine on the fetal serotonergic system.
3.4. Acute maternal fluoxetine treatment has no overt effects on the maternal gut microbiota
SSRI use is associated with alterations in the composition of the human gut microbiome36–38, and select bacterial members of the gut microbiota can interact directly with fluoxetine39,40,61. To examine effects of acute maternal fluoxetine treatment on the composition of the maternal gut microbiota, we performed 16S rRNA gene sequencing on maternal fecal samples collected before treatment, on E3.5 and E6.5, and during fluoxetine or vehicle treatment on E8.5, E11.5, and E14.5. There was no significant effect of maternal fluoxetine treatment on the alpha diversity of the gut microbiota (Supplementary Fig. 6A). Of note, we observed a modest but not statistically significant reduction in fecal microbial Shannon diversity after 1 and 7 days of maternal fluoxetine treatment (Supplementary Fig. 6A, E8.5 and E14.5), which may align with the modest fluoxetine-associated increases in maternal cecal weight on E14.5 (Supplementary Fig, 2D). There was also no overt effect of maternal fluoxetine treatment on global beta diversity of the gut microbiota, as assessed by principal coordinate analysis of weighted Unifrac distances (Supplementary Fig. 6B). Despite no significant changes in the relative levels of abundant bacterial taxa (Supplementary Fig. 6C, D), select rare microbial taxa were significantly altered in the fecal microbiota of fluoxetine-treated dams compared to vehicle-treated controls (Supplementary Fig. 6E–H). In particular, maternal fluoxetine treatment correlated with significant increases in the relative abundance of Lachnospiraceae bacterium COE1, a short-chain fatty acid-producing bacterium (Supplementary Fig. 6E). The relative abundances of Blautia and Lachnoclostridium were significantly increased on select days after maternal fluoxetine treatment (Supplementary Fig. 6F, G). Lachnospiraceae UCG-006 was increased in the fecal microbiota of dams from the fluoxetine-treated group, compared to the vehicle-treated group, at baseline, before initiating treatments (Supplementary Fig. 6H). Whether these modest alterations in select low-abundance bacterial taxa will be reproducible across independent iterations of maternal fluoxetine treatment is uncertain. Overall, these results reveal that acute maternal fluoxetine treatment has no overt effect on the composition of the maternal gut microbiota.
3.5. Depletion of the maternal gut microbiome modifies fetal brain transcriptomic responses to acute maternal fluoxetine treatment
While the results from this study indicate that acute maternal fluoxetine treatment does not substantially alter the composition of the maternal gut microbiota (Supplementary Fig. 6), whether the presence of a complex gut microbiota impacts fetal responses to maternal fluoxetine treatment is unclear. To address this question, female mice were pre-treated with broad spectrum antibiotics to deplete the microbiota from pre-conception through midgestation, or treated with vehicle as a negative control. Pregnant dams were subjected to oral fluoxetine or saline treatment as in experiments described above, and E14.5 fetal brains were subjected to RNA sequencing and analysis (Fig. 2A). Compared to vehicle controls (Fig. 1), maternal pre-treatment with antibiotics to deplete the gut microbiome induced widespread alterations in fetal brain transcriptomic responses to maternal fluoxetine treatment (Fig. 2B). Notably, the gene expression profiles from fetal brains belonging to antibiotic- and fluoxetine-treated dams (ABX + FLX) clustered closely with those from conventional vehicle-treated controls (SPF + Veh), and apart from antibiotic-treated controls (ABX + Veh), suggesting that the transcriptional changes occur, at least in part, in response to interactions between maternal antibiotic and fluoxetine treatment rather than additive effects of the two independent variables (Fig. 2B). Consistent with this, maternal fluoxetine treatment significantly altered 864 genes in the fetal brain (Fig. 1, 2C), and the differential expression of 264 (~30%) of the 864 genes was prevented by maternal antibiotic pre-treatment (Fig. 2C, D). Of these 264 genes, the 161 that were upregulated in response to maternal fluoxetine treatment and prevented by maternal antibiotic pre-treatment mapped to pathways related to the regulation of membrane potential, synapse organization, and cognition (Fig. 2E, Supplementary Fig. 7A, C), whereas the 101 genes that were downregulated in response to maternal fluoxetine treatment and prevented by maternal antibiotic pre-treatment aligned with pathways related to cell cycle, nuclear division, and DNA repair and replication pathways (Fig. 2F, Supplementary Fig. 7B, D). In addition to these, maternal antibiotic pre-treatment yielded an additional 840 differentially expressed genes in the fetal brains of offspring from fluoxetine-treated dams (Fig. 2C). Notably, maternal fluoxetine exposure also elicited many gene expression alterations in the fetal brain that were non-overlapping: of the 864 genes that were differentially expressed in response to fluoxetine treatment, 600 were not affected by maternal antibiotic treatment (Fig. 2C, Supplementary Fig. 8). Taken together, these data strongly suggest that the presence of the maternal gut microbiota conditions host physiologies that impact fetal responses to maternal fluoxetine exposure during pregnancy.
Figure 2. Depletion of the maternal microbiome modifies the effects of maternal fluoxetine treatment on gene expression in the fetal brain.
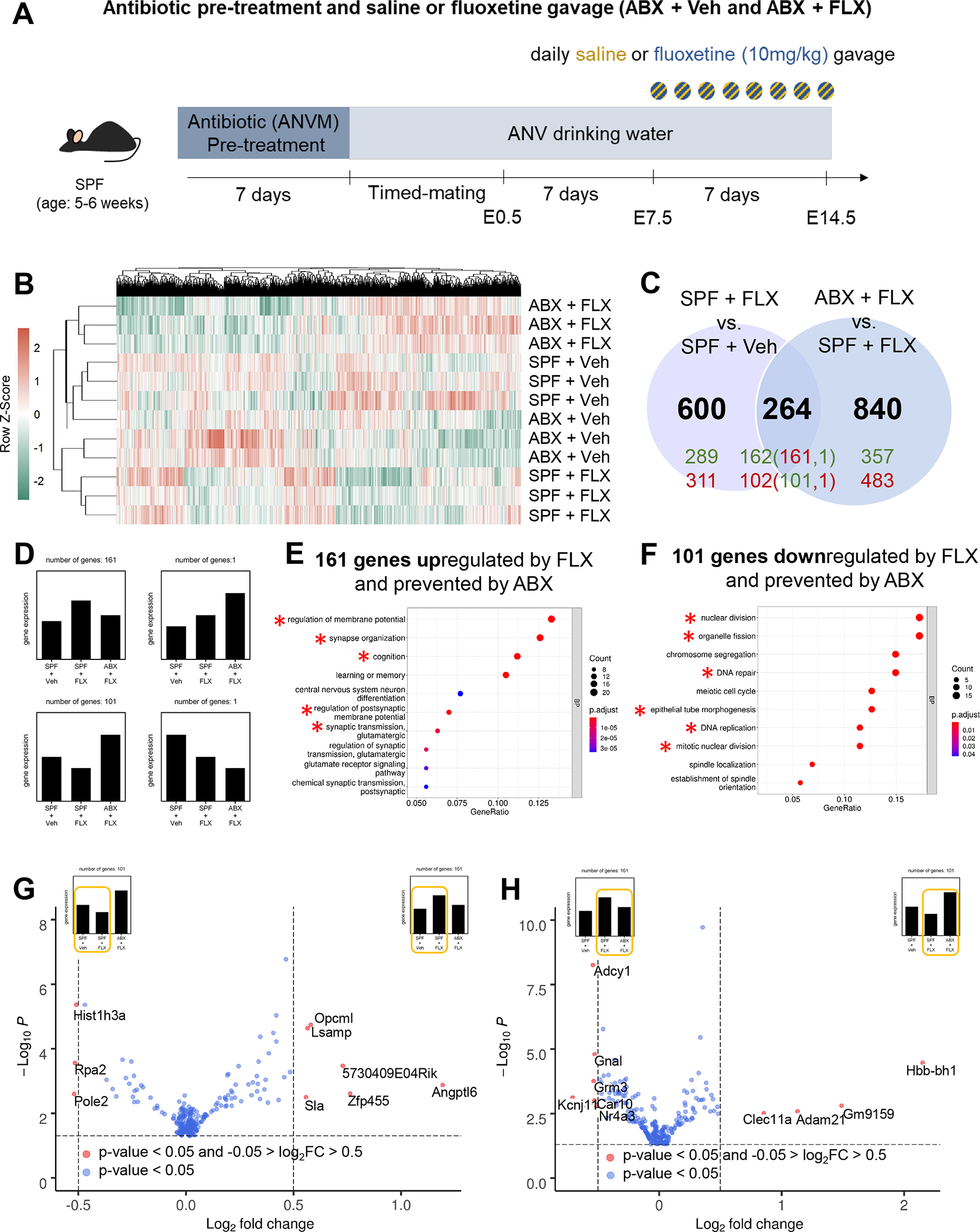
A, Antibiotic depletion treatment and vehicle or fluoxetine exposure during pregnancy. B, Heatmap of differentially expressed genes (p<0.05) in E14.5 fetal brain from SPF+Veh, SPF+FLX, ABX+Veh, and ABX+FLX dams (p<0.05). C, Venn diagram of differentially expressed genes in E14.5 fetal brains from SPF+FLX compared to SPF+Veh dams and ABX+FLX compared to SPF+FLX dams (p<0.05). Black: total genes, Green: Upregulated genes, Red: Downregulated genes. D, Schematic representation of comparative expression levels of genes in the center of the venn diagram. E, Gene ontology analysis of upregulated genes in SPF+FLX compared to both SPF+Veh and ABX+FLX (161 genes). F, Gene ontology analysis of downregulated genes in E14.5 fetal brains from SPF+FLX compared to ABX+FLX and SPF+Veh (101 genes). G, Volcano plot of differentially expressed genes in E14.5 fetal brains from SPF+FLX compared to SPF+Veh dams. Differentially expressed genes that are p<0.05 (blue) and differentially expressed genes that have log2fold change greater than 0.5 (red). H, Volcano plot of differentially expressed genes in E14.5 fetal brains from ABX+FLX compared to SPF+FLX (right) dams. Differentially expressed genes that are p<0.05 (blue) and differentially expressed genes that have log2fold change greater than 0.5 (red). ANVM: ampicillin, neomycin, vancomycin, and metronidazole. ANV: ampicillin, neomycin, and vancomycin.
3.6. Acute maternal fluoxetine treatment elevates Opcml expression, which is prevented by maternal antibiotic treatment
Maternal pre-treatment with antibiotics modified fetal brain transcriptomic responses to maternal fluoxetine treatment, preventing the differential expression of ~30% of fluoxetine-induced alterations in gene expression and further inducing statistically significant changes in an additional 840 genes (Fig. 2, Supplementary Fig. 7). A subset of the genes that were upregulated by maternal fluoxetine treatment and prevented by antibiotic pre-treatment were relevant to pathways for cell adhesion and synapse organization, key processes for neurite outgrowth and circuit wiring (Fig. 2E, G, H, Supplementary Fig. 7A, C, Supplementary Fig. 9). In particular, maternal fluoxetine treatment increased gene expression of the neural adhesion molecule, Opcml, in E14.5 fetal brains, as compared to vehicle-treated controls, which was prevented by maternal antibiotic pre-treatment (Supplementary Fig. 9B). These alterations were confirmed by quantitative RT-PCR of a larger set of fetal brain samples across each experimental group (Supplementary Fig. 9C). Opcml encodes the opioid binding cell adhesion molecule, a member of the IgLON subfamily, which is important for dendritic spine maturation, synaptogenesis and axonal outgrowth62–65.
Neurite outgrowth and synapse formation are critical processes for prenatal neural circuit development4. To further examine effects of maternal fluoxetine treatment and antibiotic pre-treatment on localized expression of Opcml, we performed RNAScope-based in situ hybridization in E14.5 fetal brain sections using transcript-specific probes. Consistent with previous reports62,66, Opcml transcript was distributed prominently within the developing somatosensory neocortex, thalamus, lateral ganglionic eminence, and striatum, and sparsely in the hippocampus (Fig. 3A–E). Consistent with findings from RNA sequencing (Fig. 2G, Supplementary Fig. 9B) and quantitative RT-PCR (Supplementary Fig. 9C), quantification of Opcml integrated density indicated that maternal fluoxetine treatment increased Opcml transcript in the fetal somatosensory neocortex, thalamus, lateral ganglionic eminence and striatum compared to vehicle-treated controls (Fig. 3A–D, F-I), with no significant differences in the hippocampus (Fig. 3E, J). The localized increases in Opcml transcript were prevented by maternal antibiotic pre-treatment (Fig. 3A–D, F–I), indicating that depletion of the maternal microbiome abrogates this particular fetal response to maternal fluoxetine treatment.
Figure 3. Maternal fluoxetine treatment alters Opcml gene expression in the embryonic brain.
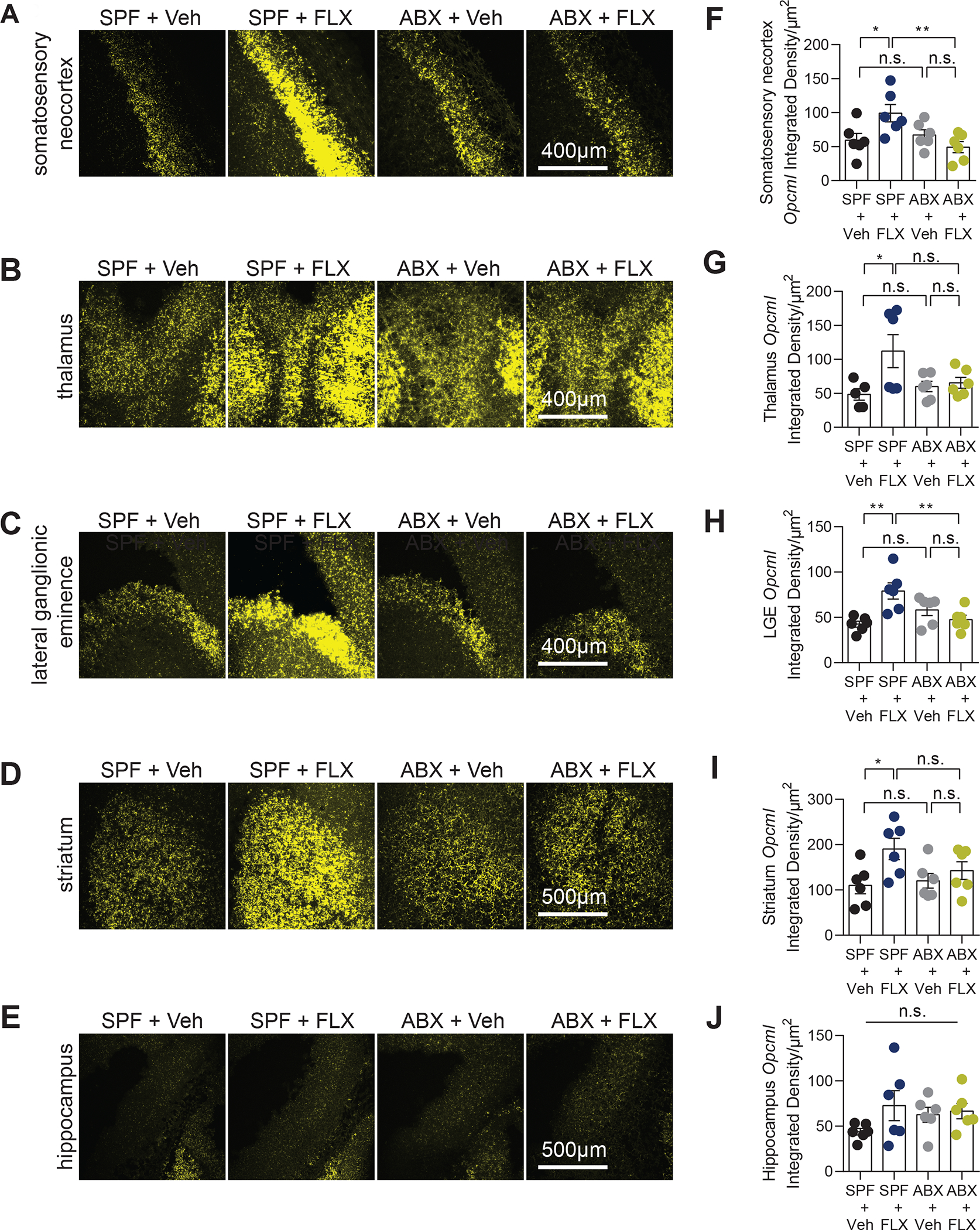
Representative images of Opcml transcript (yellow) in the somatosensory neocortex (A), thalamus (B), lateral ganglionic eminence (C), striatum (D), and hippocampus (E) in E14.5 embryonic brains from SPF + Veh, SPF + FLX, ABX + Veh, and ABX + FLX dams. Quantification of Opcml integrated density normalized to area in the somatosensory neocortex (F), thalamus (G), lateral ganglionic eminence (H), striatum (I), and hippocampus (J) of E14.5 embryonic brains from SPF + Veh, SPF + FLX, ABX + Veh, and ABX + FLX dams (One-way ANOVA with Tukey’s, n = 6, 6, 6, 6 dams). Scale bar: 400μm and 500μm. n.s.: not significant, * p < 0.05, ** p < 0.01, *** p < 0.001
3.7. Antibiotic pre-treatment of fluoxetine-treated dams has no significant effects on gross metrics of maternal and fetal health, maternal serum fluoxetine levels, or components of the fetal serotonergic system
The gut microbiome has the capacity to modify host responses to SSRIs directly or indirectly through myriad mechanisms. Particular gut microbes are reported to respond to, metabolize, or biotransform fluoxetine39,61,67. To assess the possibility of such direct effects of the maternal gut microbiome on the bioavailability of fluoxetine in the host, fluoxetine concentrations were measured by mass spectrometry in fluoxetine-treated dams that were pre-treated with antibiotics. There was no statistically significant effect of maternal antibiotic pre-treatment on E14.5 serum fluoxetine levels in fluoxetine-treated dams (Supplementary Fig. 1A, B). This suggests that the effects of maternal gut microbiome on modifying fetal responses to maternal fluoxetine treatment are likely not due to direct interactions between gut bacteria and antibiotics or fluoxetine. It further suggests that any indirect effects of the maternal gut microbiome on modulating host physiological processes that impact fluoxetine bioavailability, such as host xenobiotic metabolism or transport 32,34,35,68, are also not responsible.
We next considered if the maternal gut microbiome may impact the fetal serotonergic system to modify responses to maternal fluoxetine treatment. Maternal antibiotic pre-treatment had no significant effects on concentrations of 5-HT or tryptophan in the fetal brain (Supplementary Fig. 4C, D), or on numbers of 5-HT-, Tph2-, or SERT-positive neurons in the fetal DRN (Supplementary Fig. 5). This suggests that the maternal gut microbiome does not modify fetal transcriptomic responses to fluoxetine via alterations to the fetal serotonergic system. There were also no significant effects of maternal antibiotic pre-treatment on maternal weight, litter size or fetal volume (Supplementary Fig, 2), suggesting that the ability of the maternal gut microbiota to modify fetal responses to maternal fluoxetine treatment are not due to overt microbial influences on gross metrics of maternal or fetal health. Taken together, these data support the notion that the maternal gut microbiome modifies fetal responses to maternal fluoxetine treatment through indirect effects on host physiologies that impact fetal neurodevelopment.
Discussion
Perinatal SSRI exposures induce various adverse neurophysiological outcomes in animal models18–22 and are associated with increased risk for neurodevelopmental conditions in humans7,69–71. Results from this study demonstrate that effects of maternal SSRI use on offspring development can begin prenatally, as maternal treatment with the common SSRI fluoxetine during pregnancy induces global changes in the fetal brain transcriptome by midgestation. Notably, the influences of maternal fluoxetine treatment on gene expression in the fetal brain occur independently of alterations in the fetal serotonergic system. Although prior studies report that fluoxetine can readily cross the placenta to enter the fetus in humans, mice and rats72–74, findings from our experimental paradigm of acute oral fluoxetine treatment of dams suggest that effects on the fetal brain are likely not due to direct entry of fluoxetine to inhibit fetal SERT. In addition, previous studies in rodents reported that prenatal fluoxetine treatment induces maternal weight loss, reduced live birth rate, decreased litter size, and increased neonatal mortality52,75, which we also did not observe. The discrepancies could be due to differences in length of fluoxetine treatment (previous studies treated for 14 days from early to midgestation or mid to late gestation vs. in our experiments we treated for 7 days from early to midgestation), route of administration (previous studies, drinking water vs. in our experiments, via oral gavage), or dosage (previous studies, 10–12 mg/kg vs. in our experiments, 10 mg/kg reflecting clinically relevant doses of fluoxetine, as scaled to mice). Overall, the lack of overt disruptions to maternal and fetal health in the experimental paradigm used in this study offer the opportunity to examine effects of maternal fluoxetine treatment on fetal neurodevelopment in the absence of potentially confounding developmental alterations.
We observe that acute maternal fluoxetine treatment induces widespread changes in the fetal brain transcriptome during midgestation, characterized by differential expression of genes relevant to synapse organization, cognition, locomotory behavior, regulation of mitotic cell cycle and DNA repair pathways. Interestingly, a previous study of maternally stressed Slc6a4−/− mice, which are deficient in SERT, the molecular target for fluoxetine, reported that E13.5 fetal brains exhibited increased expression of genes involved in neuron projection and forebrain development pathways76. A comparison of the gene expression changes reported in the Slc6a4−/− study relative to those observed herein suggest some common phenotypes, in genes related to synapse organization, synaptic vesicle, cell cycle, and DNA replication, but also many differing phenotypes. For example, we observe substantial alterations in genes related to developmental cell growth, regulation of microtubule organization, ribosome organization and RNA splicing in fetal brains of offspring from dams treated with fluoxetine, which were not reported in offspring of Slc6a4−/− dams. Many factors may contribute to the discrepancies, including developmental influences of constitutive SERT deficiency as opposed to the acute SSRI intervention used in this study. Another potential consideration is the possibility that fluoxetine has physiological effects that occur independently of SERT inhibition. Indeed, fluoxetine is reported to exhibit “off-target” interactions with serotonin 5-HT2B receptor, dopamine D2 receptor, TREK-1 potassium channel, and purine P2X4 receptor77–81 and to influence many physiological systems outside of the nervous system82,83.
The gut microbiome modulates the peripheral serotonergic system, promoting 5-HT biosynthesis from enterochromaffin cells in the gastrointestinal tract 84–87, as well as the central serotonergic system, altering the expression of subsets of 5-HT receptors and levels of 5-HT in select brain regions 88,89. Recent studies suggest that the gut microbiome can also interact with SSRIs, through direct or indirect mechanisms39,90–92. We find that pre-treating dams with antibiotics to deplete the maternal gut microbiome substantially modifies fetal brain transcriptomic responses to maternal fluoxetine treatment. In particular, ~30% of the gene expression changes induced by maternal fluoxetine treatment are prevented by maternal antibiotic pre-treatment, and an additional 840 genes are differentially expressed when fluoxetine-treated dams are pre-treated with antibiotics. Notably, the transcriptomic profiles induced by the combined maternal treatments are distinct from those seen in response to maternal fluoxetine alone or antibiotics alone. This suggests that there are interactions between the two variables, maternal SSRI exposure and maternal gut microbiome, that together alter gene expression in the fetal brain.
Notably, these influences of the maternal gut microbiome appear to be conferred via its homeostatic effects on host physiology, rather than select taxonomic or functional shifts in response to fluoxetine treatment. Indeed, we observe no striking effects of acute maternal fluoxetine treatment on the composition of the gut microbiota, with Lachnospiraceae COE1 being the only taxon that was persistently and significantly increased in response to maternal fluoxetine treatment. While this could align with previous reports that UC Lachnospiraceae is increased with fluoxetine treatment and decreased in depressed patients33,39,91,93, another study suggests that fluoxetine-induced changes in the maternal gut microbiome occur primarily after treatment from gestation through the lactation period6. In the absence of fluoxetine-induced shifts in the composition of the gut microbiota, our data reveal that the presence of the complex maternal gut microbiome, rather than select shifts in the microbiota, influences host responses to maternal fluoxetine treatment. In considering potential pathways involved, we observed no effects of maternal antibiotic treatment on the serum bioavailability of fluoxetine and also no effects of maternal antibiotic treatment on the fetal serotonergic system. These results render unlikely the possibility that the maternal microbiome interacts directly with fluoxetine to alter its downstream actions on the serotonergic system. The finding that maternal antibiotic treatment does not alter fluoxetine bioavailability also renders unlikely the possibility that the maternal microbiome alters pathways for host xenobiotic metabolism of fluoxetine. While exact mechanisms remain unclear, one hypothesis is that the maternal microbiome conditions host physiological states that modify responses to fluoxetine, such as immune homeostasis94 or stress response95,96. Future studies are warranted to identify the mechanisms by which the maternal gut microbiome modifies fetal neurodevelopmental responses to maternal SSRI use.
We observe that maternal fluoxetine treatment induces widespread changes in the fetal brain transcriptome that are modified by maternal antibiotic treatment. We highlight, validate and localize Opcml in particular, based on its key role in regulating synapse formation and neurite outgrowth during fetal neurodevelopment97–99. Notably, recent large-scale genome-wide association studies identify key polymorphisms in Opcml (i.e., rs3016384, rs1941213, and rs132568126) that are associated with schizophrenia100–102. In addition, Opcml deficiency in mice results in immature spine formation and deficits in sensorimotor gating and cognitive behaviors65. These findings suggest that alterations in Opcml during development may contribute to the adverse effects of perinatal fluoxetine treatment on offspring brain development and behavior. Moreover, Opcml is expressed in both neurons and astrocytes62,78,103,104, providing the further opportunity to examine cell-type specific effects of maternal fluoxetine treatment and the maternal gut microbiome on gene expression and cellular function. Overall, understanding effects of the gut microbiome in modifying host responses to SSRI treatment could reveal fundamental insights into the biological bases of SSRI efficacy and the variability therein. Furthermore, examining interactions between the maternal gut microbiome and SSRI use during pregnancy is critical for identifying risks and informing best practices for clinical depression, toward ensuring the health of both mother and offspring.
Supplementary Material
Acknowledgments
We thank members of the Hsiao lab for their critical review of the manuscript; Trent Su, for RNA sequencing advice; Johanna ten Hoeve-Scott for assistance with fluoxetine measurements; and Shili Xu of the Chatziioannou Lab at the UCLA Crump Institute for Molecular Imaging for technical support in μCT image acquisition and analysis.
Funding
Support for this research was provided by the Packard Fellowship in Science and Engineering and Klingenstein-Simons Award to E.Y.H., UPLIFT: UCLA Postdocs’ Longitudinal Investment in Faculty Award # K12 GM106996 and NICHD Pathway to Independence Award #K99 HD101680 to H.E.V., and the NSF Graduate Research Fellowship to E.J.L.C.. E.Y.H. is a New York Stem Cell Foundation – Robertson Investigator. This research was supported in part by the New York Stem Cell Foundation.
References
- 1.Melville JL, Gavin A, Guo Y, Fan MY & Katon WJ Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstet Gynecol 116, 1064–1070, doi: 10.1097/AOG.0b013e3181f60b0a (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavin NI et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 106, 1071–1083, doi: 10.1097/01.AOG.0000183597.31630.db (2005). [DOI] [PubMed] [Google Scholar]
- 3.Haight SC, Byatt N, Moore Simas TA, Robbins CL & Ko JY Recorded Diagnoses of Depression During Delivery Hospitalizations in the United States, 2000–2015. Obstet Gynecol 133, 1216–1223, doi: 10.1097/AOG.0000000000003291 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jimenez-Solem E et al. Prevalence of antidepressant use during pregnancy in Denmark, a nation-wide cohort study. PLoS One 8, e63034, doi: 10.1371/journal.pone.0063034 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes RM et al. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies. Am J Obstet Gynecol 207, 49 e41–49, doi: 10.1016/j.ajog.2012.04.028 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levinson-Castiel R, Merlob P, Linder N, Sirota L & Klinger G Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors in term infants. Arch Pediatr Adolesc Med 160, 173–176, doi: 10.1001/archpedi.160.2.173 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Sujan AC et al. Associations of Maternal Antidepressant Use During the First Trimester of Pregnancy With Preterm Birth, Small for Gestational Age, Autism Spectrum Disorder, and Attention-Deficit/Hyperactivity Disorder in Offspring. JAMA 317, 1553–1562, doi: 10.1001/jama.2017.3413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson S et al. Effect of exposure to selective serotonin reuptake inhibitors in utero on fetal growth: potential role for the IGF-I and HPA axes. Pediatr Res 65, 236–241, doi: 10.1203/PDR.0b013e318193594a (2009). [DOI] [PubMed] [Google Scholar]
- 9.van der Veere CN, de Vries NKS, van Braeckel K & Bos AF Intra-uterine exposure to selective serotonin reuptake inhibitors (SSRIs), maternal psychopathology, and neurodevelopment at age 2.5years - Results from the prospective cohort SMOK study. Early Hum Dev 147, 105075, doi: 10.1016/j.earlhumdev.2020.105075 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Lugo-Candelas C et al. Associations Between Brain Structure and Connectivity in Infants and Exposure to Selective Serotonin Reuptake Inhibitors During Pregnancy. JAMA Pediatr 172, 525–533, doi: 10.1001/jamapediatrics.2017.5227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Videman M et al. Newborn Brain Function Is Affected by Fetal Exposure to Maternal Serotonin Reuptake Inhibitors. Cereb Cortex 27, 3208–3216, doi: 10.1093/cercor/bhw153 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Croen LA, Grether JK, Yoshida CK, Odouli R & Hendrick V Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 68, 1104–1112, doi: 10.1001/archgenpsychiatry.2011.73 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Harrington RA, Lee LC, Crum RM, Zimmerman AW & Hertz-Picciotto I Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics 133, e1241–1248, doi: 10.1542/peds.2013-3406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rai D et al. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ 346, f2059, doi: 10.1136/bmj.f2059 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutchison SM, Masse LC, Brain U & Oberlander TFA 6-year longitudinal study: Are maternal depressive symptoms and Selective Serotonin Reuptake Inhibitor (SSRI) antidepressant treatment during pregnancy associated with everyday measures of executive function in young children? Early Hum Dev 128, 21–26, doi: 10.1016/j.earlhumdev.2018.10.009 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Lupattelli A et al. Effect of Time-Dependent Selective Serotonin Reuptake Inhibitor Antidepressants During Pregnancy on Behavioral, Emotional, and Social Development in Preschool-Aged Children. J Am Acad Child Adolesc Psychiatry 57, 200–208, doi: 10.1016/j.jaac.2017.12.010 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viktorin A et al. Association of Antidepressant Medication Use During Pregnancy With Intellectual Disability in Offspring. JAMA Psychiatry 74, 1031–1038, doi: 10.1001/jamapsychiatry.2017.1727 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bond CM et al. Perinatal fluoxetine exposure results in social deficits and reduced monoamine oxidase gene expression in mice. Brain Res 1727, 146282, doi: 10.1016/j.brainres.2019.06.001 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Boulle F et al. Prenatal stress and early-life exposure to fluoxetine have enduring effects on anxiety and hippocampal BDNF gene expression in adult male offspring. Dev Psychobiol 58, 427–438, doi: 10.1002/dev.21385 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Frazer S, Otomo K & Dayer A Early-life serotonin dysregulation affects the migration and positioning of cortical interneuron subtypes. Transl Psychiatry 5, e644, doi: 10.1038/tp.2015.147 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golebiowska J et al. Serotonin transporter deficiency alters socioemotional ultrasonic communication in rats. Sci Rep 9, 20283, doi: 10.1038/s41598-019-56629-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soiza-Reilly M et al. SSRIs target prefrontal to raphe circuits during development modulating synaptic connectivity and emotional behavior. Mol Psychiatry 24, 726–745, doi: 10.1038/s41380-018-0260-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frodl T Recent advances in predicting responses to antidepressant treatment. F1000Res 6, doi: 10.12688/f1000research.10300.1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preskorn SH Prediction of individual response to antidepressants and antipsychotics: an integrated concept. Dialogues Clin Neurosci 16, 545–554 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ajslev TA, Andersen CS, Gamborg M, Sorensen TI & Jess T Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond) 35, 522–529, doi: 10.1038/ijo.2011.27 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Jostins L et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124, doi: 10.1038/nature11582 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hooper LV, Littman DR & Macpherson AJ Interactions between the microbiota and the immune system. Science 336, 1268–1273, doi: 10.1126/science.1223490 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura I et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 367, doi: 10.1126/science.aaw8429 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Braniste V et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6, 263ra158, doi: 10.1126/scitranslmed.3009759 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erny D et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18, 965–977, doi: 10.1038/nn.4030 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stokholm J, Sevelsted A, Bonnelykke K & Bisgaard H Maternal propensity for infections and risk of childhood asthma: a registry-based cohort study. Lancet Respir Med 2, 631–637, doi: 10.1016/S2213-2600(14)70152-3 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Maini Rekdal V, Bess EN, Bisanz JE, Turnbaugh PJ & Balskus EP Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science 364, doi: 10.1126/science.aau6323 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramsteijn AS, Jasarevic E, Houwing DJ, Bale TL & Olivier JD Antidepressant treatment with fluoxetine during pregnancy and lactation modulates the gut microbiome and metabolome in a rat model relevant to depression. Gut Microbes 11, 735–753, doi: 10.1080/19490976.2019.1705728 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res 29, 787–803, doi: 10.1038/s41422-019-0216-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu H et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 23, 850–858, doi: 10.1038/nm.4345 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Jackson MA et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun 9, 2655, doi: 10.1038/s41467-018-05184-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maier L et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623–628, doi: 10.1038/nature25979 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vich Vila A et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun 11, 362, doi: 10.1038/s41467-019-14177-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fung TC et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol 4, 2064–2073, doi: 10.1038/s41564-019-0540-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munoz-Bellido JL, Munoz-Criado S & Garcia-Rodriguez JA Antimicrobial activity of psychotropic drugs: selective serotonin reuptake inhibitors. Int J Antimicrob Agents 14, 177–180, doi: 10.1016/s0924-8579(99)00154-5 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Caporaso JG et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A 108 Suppl 1, 4516–4522, doi: 10.1073/pnas.1000080107 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quast C et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41, D590–596, doi: 10.1093/nar/gks1219 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yilmaz P et al. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res 42, D643–648, doi: 10.1093/nar/gkt1209 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolyen E et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37, 852–857, doi: 10.1038/s41587-019-0209-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conesa A et al. A survey of best practices for RNA-seq data analysis. Genome Biol 17, 13, doi: 10.1186/s13059-016-0881-8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim D, Langmead B & Salzberg SL HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12, 357–360, doi: 10.1038/nmeth.3317 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pertea M, Kim D, Pertea GM, Leek JT & Salzberg SL Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc 11, 1650–1667, doi: 10.1038/nprot.2016.095 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Love MI, Huber W & Anders S Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550, doi: 10.1186/s13059-014-0550-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang da W, Sherman BT & Lempicki RA Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4, 44–57, doi: 10.1038/nprot.2008.211 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Ippolito DM & Eroglu C Quantifying synapses: an immunocytochemistry-based assay to quantify synapse number. J Vis Exp, doi: 10.3791/2270 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karine de Sousa A et al. New roles of fluoxetine in pharmacology: Antibacterial effect and modulation of antibiotic activity. Microb Pathog 123, 368–371, doi: 10.1016/j.micpath.2018.07.040 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Bauer S, Monk C, Ansorge M, Gyamfi C & Myers M Impact of antenatal selective serotonin reuptake inhibitor exposure on pregnancy outcomes in mice. Am J Obstet Gynecol 203, 375 e371–374, doi: 10.1016/j.ajog.2010.05.008 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Semple BD, Blomgren K, Gimlin K, Ferriero DM & Noble-Haeusslein LJ Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 106–107, 1–16, doi: 10.1016/j.pneurobio.2013.04.001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berger M, Gray JA & Roth BL The expanded biology of serotonin. Annu Rev Med 60, 355–366, doi: 10.1146/annurev.med.60.042307.110802 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daubert EA & Condron BG Serotonin: a regulator of neuronal morphology and circuitry. Trends Neurosci 33, 424–434, doi: 10.1016/j.tins.2010.05.005 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jonnakuty C & Gragnoli C What do we know about serotonin? J Cell Physiol 217, 301–306, doi: 10.1002/jcp.21533 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Deneris E & Gaspar P Serotonin neuron development: shaping molecular and structural identities. Wiley Interdiscip Rev Dev Biol 7, doi: 10.1002/wdev.301 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Velasquez JC et al. In Utero Exposure to Citalopram Mitigates Maternal Stress Effects on Fetal Brain Development. ACS Chem Neurosci 10, 3307–3317, doi: 10.1021/acschemneuro.9b00180 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marcinkiewcz CA et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 537, 97–101, doi: 10.1038/nature19318 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brenner B et al. Plasma serotonin levels and the platelet serotonin transporter. J Neurochem 102, 206–215, doi: 10.1111/j.1471-4159.2007.04542.x (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R & Goodman AL Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 570, 462–467, doi: 10.1038/s41586-019-1291-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hachisuka A, Nakajima O, Yamazaki T & Sawada J Localization of opioid-binding cell adhesion molecule (OBCAM) in adult rat brain. Brain Res 842, 482–486, doi: 10.1016/s0006-8993(99)01831-4 (1999). [DOI] [PubMed] [Google Scholar]
- 63.Sugimoto C, Maekawa S & Miyata S OBCAM, an immunoglobulin superfamily cell adhesion molecule, regulates morphology and proliferation of cerebral astrocytes. J Neurochem 112, 818–828, doi: 10.1111/j.1471-4159.2009.06513.x (2010). [DOI] [PubMed] [Google Scholar]
- 64.Yamada M et al. Synaptic adhesion molecule OBCAM; synaptogenesis and dynamic internalization. Brain Res 1165, 5–14, doi: 10.1016/j.brainres.2007.04.062 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Zhang Z et al. The Schizophrenia Susceptibility Gene OPCML Regulates Spine Maturation and Cognitive Behaviors through Eph-Cofilin Signaling. Cell Rep 29, 49–61 e47, doi: 10.1016/j.celrep.2019.08.091 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Karis K et al. Altered Expression Profile of IgLON Family of Neural Cell Adhesion Molecules in the Dorsolateral Prefrontal Cortex of Schizophrenic Patients. Front Mol Neurosci 11, 8, doi: 10.3389/fnmol.2018.00008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Z et al. Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nat Struct Mol Biol 16, 652–657, doi: 10.1038/nsmb.1602 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carmody RN & Turnbaugh PJ Host-microbial interactions in the metabolism of therapeutic and diet-derived xenobiotics. J Clin Invest 124, 4173–4181, doi: 10.1172/JCI72335 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown AS et al. Association of Selective Serotonin Reuptake Inhibitor Exposure During Pregnancy With Speech, Scholastic, and Motor Disorders in Offspring. JAMA Psychiatry 73, 1163–1170, doi: 10.1001/jamapsychiatry.2016.2594 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Brown HK, Hussain-Shamsy N, Lunsky Y, Dennis CE & Vigod SN The Association Between Antenatal Exposure to Selective Serotonin Reuptake Inhibitors and Autism: A Systematic Review and Meta-Analysis. J Clin Psychiatry 78, e48–e58, doi: 10.4088/JCP.15r10194 (2017). [DOI] [PubMed] [Google Scholar]
- 71.Malm H et al. Infant and childhood neurodevelopmental outcomes following prenatal exposure to selective serotonin reuptake inhibitors: overview and design of a Finnish Register-Based Study (FinESSI). BMC Psychiatry 12, 217, doi: 10.1186/1471-244X-12-217 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Noorlander CW et al. Modulation of serotonin transporter function during fetal development causes dilated heart cardiomyopathy and lifelong behavioral abnormalities. PLoS One 3, e2782, doi: 10.1371/journal.pone.0002782 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olivier JD et al. Fluoxetine administration to pregnant rats increases anxiety-related behavior in the offspring. Psychopharmacology (Berl) 217, 419–432, doi: 10.1007/s00213-011-2299-z (2011). [DOI] [PubMed] [Google Scholar]
- 74.Rampono J et al. Placental transfer of SSRI and SNRI antidepressants and effects on the neonate. Pharmacopsychiatry 42, 95–100, doi: 10.1055/s-0028-1103296 (2009). [DOI] [PubMed] [Google Scholar]
- 75.Vorhees CV et al. A developmental neurotoxicity evaluation of the effects of prenatal exposure to fluoxetine in rats. Fundam Appl Toxicol 23, 194–205, doi: 10.1006/faat.1994.1098 (1994). [DOI] [PubMed] [Google Scholar]
- 76.Sjaarda CP et al. Interplay between maternal Slc6a4 mutation and prenatal stress: a possible mechanism for autistic behavior development. Sci Rep 7, 8735, doi: 10.1038/s41598-017-07405-3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagata K et al. Antidepressants inhibit P2X4 receptor function: a possible involvement in neuropathic pain relief. Mol Pain 5, 20, doi: 10.1186/1744-8069-5-20 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peng L, Gu L, Li B & Hertz L Fluoxetine and all other SSRIs are 5-HT2B Agonists - Importance for their Therapeutic Effects. Curr Neuropharmacol 12, 365–379, doi: 10.2174/1570159X12666140828221720 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Penttila J et al. Effects of fluoxetine on dopamine D2 receptors in the human brain: a positron emission tomography study with [11C]raclopride. Int J Neuropsychopharmacol 7, 431–439, doi: 10.1017/S146114570400450X (2004). [DOI] [PubMed] [Google Scholar]
- 80.Pozzi L, Invernizzi R, Garavaglia C & Samanin R Fluoxetine increases extracellular dopamine in the prefrontal cortex by a mechanism not dependent on serotonin: a comparison with citalopram. J Neurochem 73, 1051–1057, doi: 10.1046/j.1471-4159.1999.0731051.x (1999). [DOI] [PubMed] [Google Scholar]
- 81.Kim CW et al. Dual effects of fluoxetine on mouse early embryonic development. Toxicol Appl Pharmacol 265, 61–72, doi: 10.1016/j.taap.2012.09.020 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Di Rosso ME, Palumbo ML & Genaro AM Immunomodulatory effects of fluoxetine: A new potential pharmacological action for a classic antidepressant drug? Pharmacol Res 109, 101–107, doi: 10.1016/j.phrs.2015.11.021 (2016). [DOI] [PubMed] [Google Scholar]
- 83.Pereira CA et al. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eur J Pharmacol 800, 70–80, doi: 10.1016/j.ejphar.2017.02.029 (2017). [DOI] [PubMed] [Google Scholar]
- 84.Chen Z et al. Interleukin-33 Promotes Serotonin Release from Enterochromaffin Cells for Intestinal Homeostasis. Immunity 54, 151–163 e156, doi: 10.1016/j.immuni.2020.10.014 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mandic AD et al. Clostridium ramosum regulates enterochromaffin cell development and serotonin release. Sci Rep 9, 1177, doi: 10.1038/s41598-018-38018-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reigstad CS et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 29, 1395–1403, doi: 10.1096/fj.14-259598 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yano JM et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276, doi: 10.1016/j.cell.2015.02.047 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clarke G et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 18, 666–673, doi: 10.1038/mp.2012.77 (2013). [DOI] [PubMed] [Google Scholar]
- 89.van de Wouw M et al. Host Microbiota Regulates Central Nervous System Serotonin Receptor 2C Editing in Rodents. ACS Chem Neurosci 10, 3953–3960, doi: 10.1021/acschemneuro.9b00414 (2019). [DOI] [PubMed] [Google Scholar]
- 90.Lukic I et al. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl Psychiatry 9, 133, doi: 10.1038/s41398-019-0466-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lyte M, Daniels KM & Schmitz-Esser S Fluoxetine-induced alteration of murine gut microbial community structure: evidence for a microbial endocrinology-based mechanism of action responsible for fluoxetine-induced side effects. PeerJ 7, e6199, doi: 10.7717/peerj.6199 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Siopi E et al. Changes in Gut Microbiota by Chronic Stress Impair the Efficacy of Fluoxetine. Cell Rep 30, 3682–3690 e3686, doi: 10.1016/j.celrep.2020.02.099 (2020). [DOI] [PubMed] [Google Scholar]
- 93.Naseribafrouei A et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil 26, 1155–1162, doi: 10.1111/nmo.12378 (2014). [DOI] [PubMed] [Google Scholar]
- 94.Pronovost GN & Hsiao EY Perinatal Interactions between the Microbiome, Immunity, and Neurodevelopment. Immunity 50, 18–36, doi: 10.1016/j.immuni.2018.11.016 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Crumeyrolle-Arias M et al. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 42, 207–217, doi: 10.1016/j.psyneuen.2014.01.014 (2014). [DOI] [PubMed] [Google Scholar]
- 96.De Palma G et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat Commun 6, 7735, doi: 10.1038/ncomms8735 (2015). [DOI] [PubMed] [Google Scholar]
- 97.Hashimoto T, Maekawa S & Miyata S IgLON cell adhesion molecules regulate synaptogenesis in hippocampal neurons. Cell Biochem Funct 27, 496–498, doi: 10.1002/cbf.1600 (2009). [DOI] [PubMed] [Google Scholar]
- 98.Sanz RL, Ferraro GB, Girouard MP & Fournier AE Ectodomain shedding of Limbic System-Associated Membrane Protein (LSAMP) by ADAM Metallopeptidases promotes neurite outgrowth in DRG neurons. Sci Rep 7, 7961, doi: 10.1038/s41598-017-08315-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sanz R, Ferraro GB & Fournier AE IgLON cell adhesion molecules are shed from the cell surface of cortical neurons to promote neuronal growth. J Biol Chem 290, 4330–4342, doi: 10.1074/jbc.M114.628438 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lam M et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat Genet 51, 1670–1678, doi: 10.1038/s41588-019-0512-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Athanasiu L et al. Gene variants associated with schizophrenia in a Norwegian genome-wide study are replicated in a large European cohort. J Psychiatr Res 44, 748–753, doi: 10.1016/j.jpsychires.2010.02.002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O’Donovan MC et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet 40, 1053–1055, doi: 10.1038/ng.201 (2008). [DOI] [PubMed] [Google Scholar]
- 103.Zinn K & Ozkan E Neural immunoglobulin superfamily interaction networks. Curr Opin Neurobiol 45, 99–105, doi: 10.1016/j.conb.2017.05.010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kubick N, Brosamle D & Mickael ME Molecular Evolution and Functional Divergence of the IgLON Family. Evol Bioinform Online 14, 1176934318775081, doi: 10.1177/1176934318775081 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


