Abstract
Background
Psoriasis is an immune‐mediated disease with either skin or joints manifestations, or both, and it has a major impact on quality of life. Although there is currently no cure for psoriasis, various treatment strategies allow sustained control of disease signs and symptoms. The relative benefit of these treatments remains unclear due to the limited number of trials comparing them directly head‐to‐head, which is why we chose to conduct a network meta‐analysis.
Objectives
To compare the benefits and harms of non‐biological systemic agents, small molecules, and biologics for people with moderate‐to‐severe psoriasis using a network meta‐analysis, and to provide a ranking of these treatments according to their benefits and harms.
Search methods
For this update of the living systematic review, we updated our searches of the following databases monthly to October 2022: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase.
Selection criteria
Randomised controlled trials (RCTs) of systemic treatments in adults over 18 years with moderate‐to‐severe plaque psoriasis, at any stage of treatment, compared to placebo or another active agent. The primary outcomes were: proportion of participants who achieved clear or almost clear skin, that is, at least Psoriasis Area and Severity Index (PASI) 90; proportion of participants with serious adverse events (SAEs) at induction phase (8 to 24 weeks after randomisation).
Data collection and analysis
We conducted duplicate study selection, data extraction, risk of bias assessment, and analyses. We synthesised data using pairwise and network meta‐analysis (NMA) to compare treatments and rank them according to effectiveness (PASI 90 score) and acceptability (inverse of SAEs).
We assessed the certainty of NMA evidence for the two primary outcomes and all comparisons using CINeMA, as very low, low, moderate, or high. We contacted study authors when data were unclear or missing.
We used the surface under the cumulative ranking curve (SUCRA) to infer treatment hierarchy, from 0% (worst for effectiveness or safety) to 100% (best for effectiveness or safety).
Main results
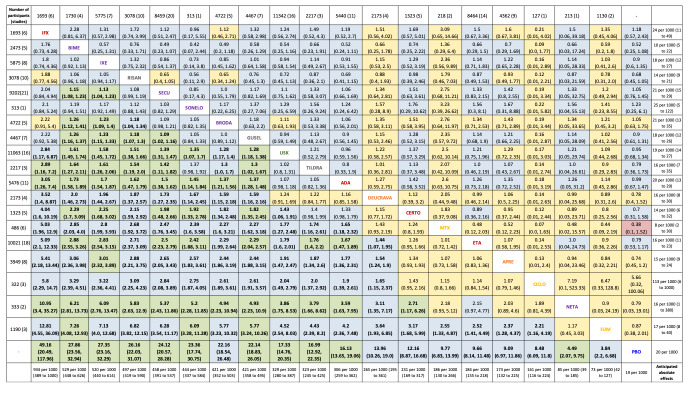
This update includes an additional 12 studies, taking the total number of included studies to 179, and randomised participants to 62,339, 67.1% men, mainly recruited from hospitals. Average age was 44.6 years, mean PASI score at baseline was 20.4 (range: 9.5 to 39). Most studies were placebo‐controlled (56%). We assessed a total of 20 treatments. Most (152) trials were multicentric (two to 231 centres). One‐third of the studies (65/179) had high risk of bias, 24 unclear risk, and most (90) low risk. Most studies (138/179) declared funding by a pharmaceutical company, and 24 studies did not report a funding source.
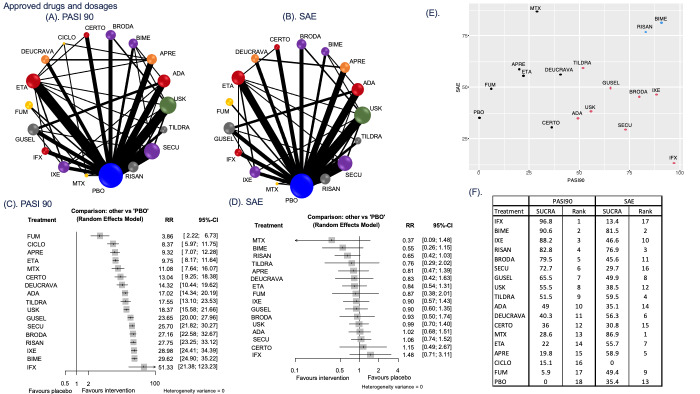
Network meta‐analysis at class level showed that all interventions (non‐biological systemic agents, small molecules, and biological treatments) showed a higher proportion of patients reaching PASI 90 than placebo. Anti‐IL17 treatment showed a higher proportion of patients reaching PASI 90 compared to all the interventions. Biologic treatments anti‐IL17, anti‐IL12/23, anti‐IL23, and anti‐TNF alpha showed a higher proportion of patients reaching PASI 90 than the non‐biological systemic agents.
For reaching PASI 90, the most effective drugs when compared to placebo were (SUCRA rank order, all high‐certainty evidence): infliximab (risk ratio (RR) 49.16, 95% CI 20.49 to 117.95), bimekizumab (RR 27.86, 95% CI 23.56 to 32.94), ixekizumab (RR 27.35, 95% CI 23.15 to 32.29), risankizumab (RR 26.16, 95% CI 22.03 to 31.07). Clinical effectiveness of these drugs was similar when compared against each other. Bimekizumab and ixekizumab were significantly more likely to reach PASI 90 than secukinumab. Bimekizumab, ixekizumab, and risankizumab were significantly more likely to reach PASI 90 than brodalumab and guselkumab. Infliximab, anti‐IL17 drugs (bimekizumab, ixekizumab, secukinumab, and brodalumab), and anti‐IL23 drugs except tildrakizumab were significantly more likely to reach PASI 90 than ustekinumab, three anti‐TNF alpha agents, and deucravacitinib. Ustekinumab was superior to certolizumab. Adalimumab, tildrakizumab, and ustekinumab were superior to etanercept. No significant difference was shown between apremilast and two non‐biological drugs: ciclosporin and methotrexate.
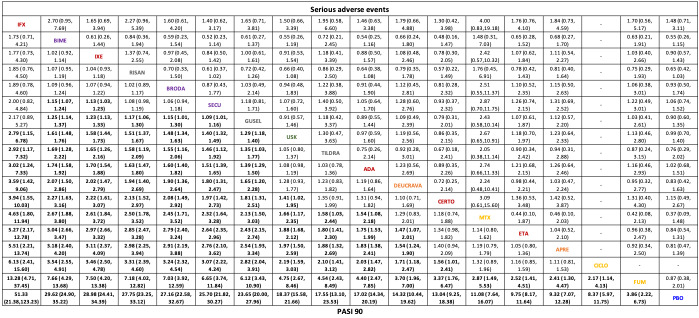
We found no significant difference between any of the interventions and the placebo for the risk of SAEs. The risk of SAEs was significantly lower for participants on methotrexate compared with most of the interventions. Nevertheless, the SAE analyses were based on a very low number of events with very low‐ to moderate‐certainty evidence for all the comparisons. The findings therefore have to be viewed with caution.
For other efficacy outcomes (PASI 75 and Physician Global Assessment (PGA) 0/1), the results were similar to the results for PASI 90. Information on quality of life was often poorly reported and was absent for several of the interventions.
Authors' conclusions
Our review shows that, compared to placebo, the biologics infliximab, bimekizumab, ixekizumab, and risankizumab were the most effective treatments for achieving PASI 90 in people with moderate‐to‐severe psoriasis on the basis of high‐certainty evidence.
This NMA evidence is limited to induction therapy (outcomes measured from 8 to 24 weeks after randomisation), and is not sufficient for evaluating longer‐term outcomes in this chronic disease. Moreover, we found low numbers of studies for some of the interventions, and the young age (mean 44.6 years) and high level of disease severity (PASI 20.4 at baseline) may not be typical of patients seen in daily clinical practice.
We found no significant difference in the assessed interventions and placebo in terms of SAEs, and the safety evidence for most interventions was very low to moderate quality.
More randomised trials directly comparing active agents are needed, and these should include systematic subgroup analyses (sex, age, ethnicity, comorbidities, psoriatic arthritis). To provide long‐term information on the safety of treatments included in this review, an evaluation of non‐randomised studies is needed.
Editorial note: This is a living systematic review. Living systematic reviews offer a new approach to review updating, in which the review is continually updated, incorporating relevant new evidence as it becomes available. Please refer to the Cochrane Database of Systematic Reviews for the current status of this review.
Keywords: Adult, Female, Humans, Male, Biological Products, Biological Products/therapeutic use, Infliximab, Infliximab/therapeutic use, Methotrexate, Methotrexate/therapeutic use, Network Meta-Analysis, Psoriasis, Psoriasis/drug therapy, Systematic Reviews as Topic, Tumor Necrosis Factor-alpha, Ustekinumab, Ustekinumab/therapeutic use
Plain language summary
Which medicines, taken by mouth or injected, work best to treat a skin condition called plaque psoriasis?
Key messages
‐ After six months of treatment, medicines called 'biologics' seem to work best to clear patches of psoriasis on the skin.
‐ Longer studies are needed to assess the benefits and potential harms of longer treatment with medicines that are injected or taken by mouth to treat psoriasis.
‐ More studies are needed that compare these types of medicines directly against each other.
What is psoriasis?
Psoriasis is an immune condition that affects the skin and, sometimes, the joints. Psoriasis speeds up the production of new skin cells, which build up to form raised patches on the skin known as 'plaques'. Plaques can also be flaky, scaly, itchy, and appear red on white skin, and as darker patches on darker skin tones. Plaque psoriasis is the most common form of psoriasis.
How is psoriasis treated?
Treatments for psoriasis depend on how bad the symptoms are. Around 10% to 20% of people with moderate or severe psoriasis will need to take medicines that affect their immune system, to help control the psoriasis. These medicines are called systemic treatments, because they affect the whole body. These are usually taken by mouth (orally) or injected.
Why did we do this Cochrane Review?
There are three different types of systemic medicines to treat psoriasis:
‐ 'biologics' – proteins, such as antibodies, that target interleukins and cytokines (parts of the immune system that affect how cells behave); ‐ small molecules – organic compounds that affect immune cells (examples include apremilast); and ‐ non‐biologic medicines – medicines that have been in use for a long time to treat psoriasis, such as methotrexate, ciclosporin, and retinoids.
We wanted to find out about the benefits and potential harms of taking systemic medicines to treat psoriasis, and to see if some medicines work better than others.
What did we do?
We searched for studies that tested systemic medicines to treat plaque psoriasis.
How up to date is this review?
We included evidence up to October 2022.
What did we find?
We found 179 studies, including 12 new studies since our last search (October 2022). The studies tested 20 different medicines, covering 62,339 adults with psoriasis (average age 44.6 years) and lasted from two to six months. Of 149 studies that reported their source of funding, a pharmaceutical company provided funding for 138 studies and 11 were funded by non‐commercial organisations or academic institutions.
Most studies compared the systemic medicine against a placebo (a 'dummy' treatment that does not contain any medicine but looks identical to the medicine being tested). They used a common measurement scale called the PASI (Psoriasis Area and Severity Index) to compare how well each medicine cleared psoriasis plaques from the skin, looking for a 90% improvement (called 'PASI 90'). Few studies reported on participants' well‐being.
We compared all the medicines with each other using a mathematical method called a network meta‐analysis.
What are the main results of our review?
All the medicines tested worked better than a placebo to treat psoriasis (measured as a 90% improvement in PASI).
Biologic medicines (that targeted interleukins 17, 23 and 12/23, and the cytokine TNF‐alpha) treated psoriasis better than the non‐biologic medicines.
Compared with placebo, four biologic medicines worked best to treat psoriasis, with little difference between them:
‐ infliximab (targets TNF‐alpha);
‐ ixekizumab and bimekizumab (targets interleukin‐17); and
‐ risankizumab (targets interleukin‐23).
We found no significant difference in the numbers of serious unwanted events for all systemic medicines tested when compared with a placebo. However, the studies did not consistently report results about safety, such as serious unwanted events. We therefore could not create a reliable risk profile of systemic medicines.
Limitations of the evidence
We are confident in our results for the four biologic medicines (infliximab, ixekizumab, bimekizumab, and risankizumab) that worked best to treat psoriasis. We are less confident in our results for serious unwanted events, because of the low number of unwanted events reported.
We are also less confident in the results for the non‐biologic medicines because of concerns about how some of the studies were conducted. Further research is likely to change these results.
We did not find many studies for some of the 20 medicines included in our review. Participants in the studies often had severe psoriasis at the start of the study, so our results may not be useful for people whose psoriasis is less severe. Our findings relate only to treatment with systemic medicines for up to six months at most.
Editorial note: This is a living systematic review. Living systematic reviews offer a new approach to review updating, in which the review is continually updated, incorporating relevant new evidence as it becomes available. Please refer to the Cochrane Database of Systematic Reviews for the current status of this review.
Background
Please refer to our glossary (see Table 1).
1. Glossary.
| Term | Definition |
| Antagonist | A substance that interferes with or inhibits the physiological action of another |
| Antigen | A molecule capable of inducing an immune response |
| Anti‐TNF alpha | A pharmaceutical drug that suppresses the physiologic response to tumour necrosis factor (TNF) |
| Biological agent | Therapeutic agents consisting of immune molecules such as soluble receptors, recombinant cytokines, and monoclonal antibodies that target effector molecules or cells of the immune system |
| Biosimilar | Biological agent highly similar to another already‐approved biological medicine |
| CD6 | Cluster of differentiation (CD) 6 is a protein encoded by the CD6 gene |
| Cheilitis | An inflammation of the lips |
| Chimeric protein | A chimeric protein can be made by combining two different genes |
| Complex cyclophilin ‐ciclosporin | Cyclophilins are a family of proteins that bind to ciclosporin, an immunosuppressant agent |
| Creatinine | A compound that is produced by metabolism of creatine and excreted in the urine |
| Cyclic adenosine monophosphate | It is a second messenger important in many biological processes |
| Cytokines | Small proteins produced by a broad range of cells that are important in cell signalling; they are immunomodulating agents |
| Dendritic cells | Antigen‐presenting cells of the immune system |
| Dermis | It is a layer of the skin |
| Epitope | It is a part of an antigen |
| Erythematous | Redness of the skin |
| Folic acid | B vitamin |
| Humanised antibody | Antibodies from non‐human species whose protein sequences have been modified to increase their similarity to antibody variants produced naturally in humans |
| IL‐17A | A pro‐inflammatory cytokine |
| IL‐23R | A cytokine receptor |
| Immune‐mediated | A group of diseases that are characterised by common inflammatory pathways leading to inflammation, and which may result from a dysregulation of the normal immune response |
| Immunogenicity | This is the ability of a particular substance, such as an antigen or epitope, to provoke an immune response in the body of a human or animal |
| Immunoglobulin 1 Fc | An antibody |
| Interferon (IFN)‐c | A protein released by cells, usually in response to a pathogen |
| Interleukin | A kind of cytokine |
| Janus kinase (JAK) inhibitors | A pharmaceutical drug that inhibits the activity of one or more of the Janus kinase family of enzymes |
| Keratinocytes | Epidermal cells that constitute 95% of the epidermis |
| Lymphocyte | A subtype of a white blood cell |
| Lymphoid organ | Part of the body that defends the body against invading pathogens that cause infections or the spread of tumours |
| Metalloproteinases | A protease enzyme |
| Monoclonal antibodies | Antibodies that are made by identical immune cells that are all clones of a unique parent cell |
| Murine sequence | Mouse genomic sequencing |
| Neutrophils | Type of white blood cell involved in the innate immune system |
| p40 | Subunit beta of interleukin 12 and 23 |
| Periumbilical | Around the navel |
| Pharmacological treatments | Drugs |
| Phase I | First‐in‐man studies |
| Phase II | Studies to assess how well the drug works, as well as to continue phase I safety assessments in a larger group of volunteers and participants |
| Phase III | Randomised, controlled, multicentre trials on large patient groups, are aimed at being the definitive assessment of how effective the drug is |
| Phase IV | Post‐marketing trials that involve safety surveillance |
| Phosphodiesterase 4 inhibitors | A pharmaceutical drug used to block the degradative action of phosphodiesterase 4 |
| Progressive multifocal leukoencephalopathy | A rare viral neurological disease characterised by progressive damage of the white matter of the brain at multiple locations |
| Receptor | A protein molecule that receives chemical signals from outside a cell |
| Small molecules | Chemically manufactured molecules (or SMOLs for short) |
| Sphingosine 1‐phosphate receptor agonists | A class of protein‐coupled receptors that are targets of the lipid signalling molecule sphingosine‐1‐phosphate |
| T cells/CD4 T cells | A type of white blood cell that is of key importance to the immune system |
| Th1 and Tc1 cells | A type of T cell |
| Th17 and Tc17 cells | A type of T cell |
| TNF‐alpha | A protein that is part of the inflammatory response |
| Tumour necrosis factor antagonists | Class of biological agents |
| Umbilic | Navel |
| Xerosis | Dry skin |
Description of the condition
Psoriasis is an immune‐mediated disease for which a person can have genetic susceptibility, manifesting in chronic inflammatory effects on either the skin or joints, or both, with a prevalence ranging from 0.14% (East Asia) to 1.99% (Australasia) (Armstrong 2020b; Parisi 2020). The causes of psoriasis are not fully understood. There appears to be interaction between environmental factors and genetic susceptibility. Genome‐wide (or whole genome) association trials found several candidate genes relating to psoriasis (Capon 2017; Yan 2021a). Various environmental factors, including stress, injury, and infections, are suspected of triggering or aggravating the evolution of psoriasis. An inflammatory immune response involving dendritic cells, T cells, keratinocytes, neutrophils, and the cytokines released from immune cells initiates the pathophysiological process (Yan 2021; Yan 2021a).
Diagnosis is made based on clinical findings; skin biopsy is rarely used to diagnose the disease (Armstrong 2020b). Several clinical types of psoriasis exist: plaque, pustular, inverse, and erythrodermic. Plaque psoriasis is the most common form, affecting 90% of people with psoriasis (Griffiths 2007). Plaque psoriasis typically appears as raised erythematous and well‐demarcated areas of inflamed skin covered with silvery‐white, scaly skin (Griffiths 2007). The location of the plaques is usually symmetrical on the elbows, knees, scalp, lower back, and the periumbilical region. For 5% to 25% of people with psoriasis, their joints are also involved (Alinaghi 2019; Helliwell 2005; Zachariae 2003).
Severity
Chronicity characterises the natural history of plaque psoriasis; this means that severity varies over time, from minor localised patches to complete body coverage. The severity of the disease usually fluctuates around the same level for a particular person (Nijsten 2007), but, for each person with this disease, the evolution and duration of remission is unpredictable. The psoriasis is declared clear when there are no lesions.
More than a dozen outcome instruments are used to assess the severity of psoriasis and the efficacy of different treatments (Naldi 2010; Spuls 2010); the Psoriasis Area and Severity Index (PASI) score is one of these instruments (Schmitt 2005). The PASI combines the assessment of the severity of lesions and the area affected into a single score in the range of 0 (no disease) to 72 (maximal disease). Recent clinical trials evaluating biological therapies that have received secondary marketing authorisation by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) used PASI 75, i.e. a 75% improvement in the PASI score, and more recently PASI 90, i.e. a 90% improvement in the PASI score, as primary endpoints. The PASI score has substantial limitations, such as low‐response distribution, no consensus on interpretability, and low responsiveness in mild disease (Spuls 2010). However, PASI 90 is a stringent outcome, as patients reaching PASI 90 are almost clear.
Associated comorbidities, impact, and quality of life
Patients with severe psoriasis or those who develop psoriasis at a young age have a higher risk of cardiometabolic comorbidities than the general population (Armstrong 2020b). The association between psoriasis and metabolic syndrome was confirmed in a population‐based study in the United Kingdom. Moreover, associations with obesity, hypertriglyceridaemia, and hyperglycaemia also increased with severity of psoriasis, independent of other metabolic syndrome components (Langan 2012).
Disease severity alone does not determine the burden of psoriasis. Multiple studies have described an impairment of the quality of life (QoL); others have focused on an evaluation of the stigma people experience; and others have studied the impact on psychosocial life (Kimball 2005).
Impairment of QoL in people with psoriasis, when measured with the 36‐item Short Form Health Survey (SF‐36) questionnaire, is higher than that of people with hypertension, diabetes, or depression (Rapp 1999).
Many tools exist to measure the QoL of people with psoriasis and other skin disorders. These measures may be categorised as psoriasis‐specific (Psoriasis Index of Quality of Life (PSORIQoL), Psoriasis Disability Index (PDI)); skin‐specific (Dermatology Life Quality Index (DLQI), Skindex (a quality of life measure for people with skin disease)); and generic QoL measures (SF‐36). However, methodological weaknesses exist in the use of QoL questionnaires, and there is poor reporting of QoL outcomes in randomised clinical trials (Le Cleach 2008).
Description of the intervention
There is currently no cure for psoriasis, but various treatments can help to control the symptoms; thus, long‐term treatment is usually needed. In daily practice, a treatment strategy needs to be defined, and this usually involves an induction therapy, e.g. the period of time of the initial therapy intended to induce remission of the disease, and a maintenance therapy, e.g. to maintain the remission of the disease.
The therapeutic approach to psoriasis includes topical treatments as a single strategy and a first‐line therapy in the management of minor forms (Mason 2013). Nevertheless, about 20% to 30% of people with psoriasis have a moderate‐to‐severe form requiring a second‐line therapy including phototherapy and non‐biological systemic agents, such as ciclosporin, methotrexate, or acitretin. Among the systemic agents, the choice of drug is not clear. The NICE clinical guidelines in the UK proposed methotrexate as the first choice of systemic agent. Biological agents, such as the tumour necrosis factor (TNF) antagonists (infliximab, etanercept, adalimumab, certolizumab); the monoclonal antibody ustekinumab that targets interleukin‐12 and ‐23 (IL12/23); anti‐IL17 drugs (secukinumab, ixekizumab, brodalumab, or bimekizumab); anti‐IL23 drugs (risankizumab, guselkumab, or tildrakizumab) and new small molecules (apremilast, deucravacitinib) are more recent systemic therapies (Armstrong 2020b; Yan 2021). Many healthcare systems have developed elaborate psoriasis treatment algorithms to address the high cost of newer therapies. Indeed, in Europe and in Canada, there are mandatory reimbursement criteria that patients must meet before being considered for these treatments, due to their high costs (Nast 2015b), such as presenting a moderate‐to‐severe psoriasis after failure, intolerance, or contraindication to at least one or two non‐biological systemic agents (French criteria).
Non‐biological systemic treatments
The oldest oral pharmacological treatments licensed for psoriasis are ciclosporin, methotrexate, acitretin (which is the retinoid of choice for psoriasis), and fumaric acid esters (FAEs), which are licensed for psoriasis in Germany and used off‐licence in other countries (Atwan 2015).
Randomised controlled trials against placebo for both induction and maintenance therapies have demonstrated the efficacy of ciclosporin for psoriasis (Bigby 2004; Christophers 1992; Ellis 1991; Flytström 2008; Heydendael 2003; Ho 1999; Koo 1998; Mahrle 1995; Meffert 1997; Mrowietz 1995; Shupack 1997). In 2008, Saurat and colleagues conducted the only randomised trial comparing the efficacy of methotrexate versus placebo (CHAMPION 2008). Randomised trials against placebo have demonstrated the efficacy of derivatives of vitamin A, the retinoids, in the treatment of plaque psoriasis (Pettit 1979). Fumaric acid esters are an alternative therapy for people with psoriasis, even though the mechanisms of action are not completely understood (Ormerod 2004). A Cochrane Review on FAEs for psoriasis was published in 2015 (Atwan 2015).
Small molecules
Small molecules or target therapies affect molecules inside immune cells. Recently, small molecule drugs have been developed and show potential to treat people with psoriasis not responding to non‐biological treatments. These small molecule drugs include apremilast (Papp 2012c), tofacitinib, a Janus kinase 1/3 inhibitor (Bachelez 2015), and deucravacitinib (Papp 2018). FDA approval for tofacitinib was declined for psoriasis indication based on clinical efficacy and long‐term safety issues, thus we removed this drug from the interventions in the update published in 2022. Deucravacitinib has been approved for psoriasis by the FDA since September 2022.
Biological therapies
Biological therapies use substances made from living organisms, or synthetic versions, to target the immune system. In the 20th century, the development of biological treatments expanded the therapeutic spectrum of systemic treatments for psoriasis. All of the biologics have to be given by infusion or subcutaneous injection, and all have had at least one evaluation of their effectiveness against placebo: etanercept (Leonardi 2003), infliximab (Chaudhari 2001), adalimumab (REVEAL 2008), certolizumab (Reich 2012a), ustekinumab (Lebwohl 2010), secukinumab (Reich 2015), ixekizumab (Leonardi 2012), brodalumab (Papp 2012a), bimekizumab (BE ABLE 1 2018), sonelokimab (Papp 2021), netakimab (PLANETA 2021), guselkumab (Gordon X‐PLORE 2015), mirikizumab (NCT03482011), tildrakizumab (Papp 2015), and risankizumab (IMMhance 2020). Mirikizumab will no longer be submitted for approval for psoriasis (due to competitive space), so it was removed from the interventions in this update. Netakimab has been approved for psoriasis in Russia.
Sonelokimab had not been approved for psoriasis at the time our analyses were done.
How the intervention might work
Dysregulation of the immune system is a critical event in psoriasis, and the evolving knowledge of the role of the immune system in the disease has had an impact on treatment development. Indeed, psoriatic plaque shows marked infiltration by activated T cells, especially CD4+ cells in the dermis. The activated T cells produce several important cytokines, namely, interferon (IFN)‐c, TNF alpha (by Th1 and Tc1 cells), IL17A, and IL23R (by Th17 and Tc17 cells) (Armstrong 2020b; Yan 2021; Yan 2021a).
Non‐biological systemic treatments
Ciclosporin
Ciclosporin is an immunosuppressive agent (a drug that reduces the efficacy of the immune system); it acts by inhibiting the initial phase of the activation of CD4+ T cells, which leads to a block on the synthesis of interleukin 2 by the complex cyclophilin‐ciclosporin, thus preventing T cell proliferation that is key to the pathogenesis of psoriasis (see above) (Ho 1996). This immunosuppression is rapid and reversible. Ciclosporin rapidly reduces the severity of the lesions (over one to three months), but the continuation of treatment is difficult after two years because of the development of adverse effects, such as elevated creatinine levels (Maza 2011). A dose of 5.0 mg/kg/day ciclosporin was significantly more effective than 2.5 mg/kg/day ciclosporin for induction of the remission of psoriasis; however, elevated creatinine was significantly more likely with 5.0 mg/kg/day ciclosporin than with 2.5 mg/kg/day ciclosporin (Christophers 1992).
Methotrexate
Methotrexate is an antimetabolite (an inhibitor of a chemical that is part of normal metabolism), which acts as an antagonist of folic acid (Montaudie 2011). Low doses of methotrexate exert anti‐inflammatory and immunomodulatory activities (Montaudie 2011). The efficacy of methotrexate cannot be assessed earlier than three months; its long‐term safety profile is good. In clinical practice, methotrexate is administered orally at 15 to 25 mg/week (Montaudie 2011).
Retinoids
Retinoids, including acitretin, are involved in the growth and differentiation of skin tissue; they bind to nuclear receptors that belong to the large family of steroid hormone receptors (Sbidian 2011). Retinoids modulate many types of proteins, including epidermal structural proteins, metalloproteinases, and cytokines (Sbidian 2011). The efficacy of retinoids is evaluated after two to three months of treatment, but skin side effects (e.g. xerosis, cheilitis) may limit the ability to increase the dose. Treatment with retinoids is best avoided in women of childbearing age because of risks to a developing foetus and the necessity of using contraception for two years after discontinuation of treatment (Sbidian 2011). People receiving 50 mg/day to 75 mg/day acitretin have significantly improved psoriasis compared with those receiving 10 mg/day to 25 mg/day acitretin (Goldfarb 1988).
FAEs
Fumaric acid esters (FAEs) are chemical compounds derived from the unsaturated dicarboxylic acid (Atwan 2015). Oral preparations of FAEs in psoriasis were developed containing dimethyl fumarate (DMF) and salts of monoethyl fumarate (MEF) as main compounds (Atwan 2015). FAEs produce anti‐inflammatory effects by preventing the proliferation of T cells (Atwan 2015).
FAEs are an effective therapy in people with psoriasis (50% to 70% achieve PASI 75 improvement within four months of treatment). Tolerance is limited by gastrointestinal side effects and flushing of the skin (Atwan 2015). Several case‐series described rare adverse events, such as progressive multifocal leukoencephalopathy (Balak 2016). In clinical practice, FAEs are administered orally. People receive this after a gradual dose incrementation the equivalent of 720 mg of DMF a day.
Small molecules
Small molecule drugs modulate pro‐inflammatory cytokines and selectively inhibit signalling pathways: phosphodiesterase 4 inhibitors (apremilast) and tyrosine kinase 2 (TYK2) inhibitors (deucravacitinib) (Torres 2015).
Apremilast
Apremilast belongs to the phosphodiesterase 4 (PDE4) inhibitors family (Torres 2015). By increasing cyclic adenosine monophosphate (cAMP) levels, PDE4 inhibitors reduce production of pro‐inflammatory TNF alpha and IFNγ in people with psoriasis. Apremilast has been approved for psoriasis; its efficacy seems to be higher than non‐biological systemic therapy, but no randomised controlled trials (RCTs) assessing apremilast versus ciclosporin have been published. However, some RCTs assessing apremilast versus methotrexate or deucravacitinib or risankizumab are ongoing (CTRI/2019/07/020274; NCT04908475; POETYK PSO‐1 2022). The safety of the drug should be detailed in the near future with phase 4 studies. In clinical practice, apremilast is administered orally at 30 mg twice a day (Torres 2015).
Deucravacitinib
Deucravacitinib is a potent oral tyrosine kinase 2 (TYK2) inhibitor that binds to the pseudokinase domain of the enzyme and is functionally more selective than other tyrosine kinase inhibitors. Tyrosine kinase 2 (TYK2) is an intracellular signalling enzyme, which activates signal transducer and activator of transcription (STAT)–dependent gene expression and functional responses of interleukin‐12, interleukin‐23, and type I and III interferon receptors. These cytokine pathways are involved in the pathologic processes associated with psoriasis, and are distinct from responses driven by Janus kinase (JAK) 1 (JAK1), JAK1 and JAK3 in combination, and JAK2. JAK inhibitors target the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, which is pivotal for the downstream signalling of inflammatory cytokines involved in psoriasis. In clinical practice, deucravacitinib is administered orally at 6 mg once daily (Hoy 2022).
Biological therapies
Biological therapies have been developed in recent years and first target and prevent T cell proliferation and then target cytokines involved in psoriasis physiopathology (e.g. anti‐TNF alpha, anti‐IL12/23, anti‐IL23, anti‐IL17).
Anti‐TNF alpha
Three monoclonal antibodies against tumour necrosis factor alpha (TNF‐α) (infliximab, adalimumab, certolizumab) and one recombinant TNF‐α receptor (etanercept) have been developed to inhibit TNF‐α signalling, thus preventing its inflammatory effects, and are approved for psoriasis (Gisondi 2004).
Etanercept is a recombinant TNF‐α receptor and weakly immunogenic (provokes only a mild immune response). Its efficacy is assessed at three months. A 50 mg dose of etanercept is administered subcutaneously twice‐weekly for three months during the induction phase (remission of the psoriasis flare) with 50 mg administered weekly as maintenance therapy (Gisondi 2004).
Infliximab is a chimeric antibody that neutralises the action of TNF‐α. Its efficacy is evaluated after six to eight weeks of treatment. A dose of 5.0 mg/kg infliximab is given as an intravenous (IV) induction regimen at 0, two, and six weeks followed by a maintenance regimen of 5.0 mg/kg every eight weeks. The presence of a murine sequence at recognition sites can lead to the development of anti‐infliximab antibodies that may impair the therapeutic effect (Gisondi 2004).
Adalimumab is a fully humanised antibody with very low immunogenicity. Its efficacy is estimated after eight and 12 weeks of treatment. One dose of 80 mg is administered subcutaneously, followed one week later by a 40 mg subcutaneous dose, which is administered every two weeks (Mossner 2009). Those receiving TNF‐α blockers are potentially exposed to a greater risk of infection and require regular monitoring (Tubach 2009).
Certolizumab is an anti‐TNF alpha with a unique structure that does not contain an Fc (fragment crystallisable) portion as adalimumab or infliximab does, based on the human immunoglobulin G1 Fc. Certolizumab therefore does not display Fc‐mediated effects (improving solubility, increasing drug stability, and decreasing immunogenicity) (Campanati 2017). Treatment starts with a 400 mg dose given as two injections, followed by a further 400 mg dose two and four weeks later. After this, depending on the condition being treated, patients should continue with 200 mg or 400 mg, given as one or two injections every two or four weeks.
Anti‐IL12/23, anti‐IL23, anti‐IL17
Additional monoclonal antibodies have been developed against pro‐inflammatory cytokines; IL‐12, IL‐23, and IL‐17 inhibit the inflammatory pathway at a different point to the anti‐TNF alpha antibodies (Dong 2017).
Interleukin‐12 and IL‐23 share a common domain, p40, which is the target of ustekinumab (which the FDA approved in 2009) (Savage 2015). A 45 mg subcutaneous dose is administered initially (90 mg if body weight is over 100 kg), then 45 mg (or 90 mg) subcutaneously four weeks later, and thereafter 45 mg (or 90 mg) subcutaneously every 12 weeks (Savage 2015). Interleukin‐23 plays an essential role in skin inflammation in psoriasis leading to the development of agents that selectively target the IL‐23p19 subunit (Dong 2017). Drugs targeting the p19 subunit of IL‐23 are guselkumab (a fully human IgG1k monoclonal IL‐23 antagonist), tildrakizumab (a humanised IgG1k monoclonal antibody), and risankizumab (high‐affinity humanised IgG1 monoclonal antibody) (Dong 2017). In July 2017, the FDA approved guselkumab for psoriasis. Guselkumab is given as a 100 mg subcutaneous injection every eight weeks, following two starter doses at week 0 and week four. More recently, both tildrakizumab and risankizumab were approved. The recommended dose for tildrakizumab is one 100 mg injection, followed by a further dose after four weeks and then an injection every 12 weeks. The dose may be increased to 200 mg in certain patients, for example those badly affected by the disease or with bodyweight over 90 kg. The recommended dose for risankizumab is 150 mg, administered by two subcutaneous injections every 12 weeks following two initiation doses at week 0 and four.
Interleukin‐17 inhibitors include secukinumab (a recombinant fully human anti‐IL17A IgG1k monoclonal antibody), ixekizumab (a humanised anti‐IL17 immunoglobulin G4 monoclonal antibody), brodalumab (a human IgG2 monoclonal antibody that decreases the downstream effect of IL‐17 by antagonising the IL‐17RA receptor), bimekizumab (a humanised monoclonal IgG1 antibody that potently and selectively neutralises the biological function of both human IL‐17A and IL‐17F), netakimab (a humanised IgG1 nanobody that targets IL‐17A), and sonelokimab (a trivalent camelid nanobody binding to IL‐17A and IL‐17F) (Dong 2017). The recommended dosage for secukinumab is 300 mg administered subcutaneously at weeks 0, 1, 2, 3, and 4, and then every four weeks thereafter. Ixekizumab is administered at 160 mg (2 x 80 mg injections) at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every four weeks (Dong 2017). The recommended dose for brodalumab is 210 mg given once a week for the first three weeks and then every two weeks. In August 2021, the EMA approved bimekizumab for psoriasis. The recommended dosage for bimekizumab is two injections of 160 mg each (a total of 320 mg) given once every four weeks for 16 weeks, and then every eight weeks thereafter. Netakimab is currently registered in Russia for the treatment of moderate‐to‐severe psoriasis in adults. The recommended dose for netakimab is 120 mg once every four weeks. Sonelokimab had not been approved for psoriasis at the time our analyses were done.
Why it is important to do this review
To determine the treatment pathway in psoriasis, the benefits and harms of each systemic treatment must be determined relative to other therapies. Several RCTs have compared against placebo the efficacy of the different systemic treatments for psoriasis. However, there are few trials comparing non‐biological systemic therapies head‐to‐head, systemic therapies against biological therapies, or biological therapies head‐to‐head. Several previous meta‐analyses or indirect comparison meta‐analyses have been published (Bansback 2009; Brimhall 2008; Fahrbach 2021; Gómez‐García 2017; Gospodarevskaya 2009; Lin 2012; Loveman 2009; Nast 2015a; Nelson 2008; Reich 2008; Reich 2012b; Sawyer 2019; Schmitt 2008; Signorovitch 2010; Signorovitch 2015; Spuls 1997; Strober 2006; Tan 2011; Turner 2009; Woolacott 2006). However, the number of studies included in these publications was low, the searches were not exhaustive, and several trials have been published since their search dates. Also, the publications did not evaluate some systemic treatments. A recent overview of 47 network meta‐analyses (NMA) evaluating the efficacy and safety of systemic treatments in moderate‐to‐severe psoriasis found that there was redundancy in the NMAs included and that the methodological quality of the systematic reviews and NMAs was low (Guelimi 2021).
A network meta‐analysis enables the best use of the direct and indirect information available to determine the relative efficacy of treatments. In other words, a network meta‐analysis will help to highlight the missing key comparisons that are needed to inform clinical practice.
Following the publication of the 2021 update of this review, we are maintaining it as a living systematic review. This means we are continually running the searches and rapidly incorporating any newly identified evidence into the review. We believe a living systematic review approach is appropriate for this review, for three reasons. Firstly, the review addresses an important health issue. The high prevalence of psoriasis (1% to 3% of the world population); the major impact on quality of life for many individuals; the cardiovascular comorbidities associated with significant mortality; the many therapeutic options; and the high costs of these new systemic treatments are reasons, among others, to help physicians in determining which treatment is best suited to a patient. Secondly, an important level of uncertainty remains in the existing evidence in the field of psoriasis, despite searches including the current update (up to 6 October 2022) identifying a total of 179 studies for inclusion in the review. Few head‐to‐head trials have compared systemic treatments against each other. Once the benefit of a treatment has been established against placebo using a high quality of evidence, head‐to‐head trials would be helpful to provide physicians with efficacy estimates between the different biological treatments based on stronger evidence than indirect comparisons. Further head‐to‐head trials are needed to accurately rank drugs according to their risk/benefit ratio. Thirdly, we are aware of ongoing trials in this area of research that will be important to incorporate, and we expect that future research will have an impact on the conclusions. For instance, new molecules have emerged constantly (e.g. since 2017, six new biological treatments for psoriasis have emerged).
The plans for this review were published as a protocol 'Systemic pharmacological treatments for chronic plaque psoriasis' (Sbidian 2015). This review is an update of 'Systemic pharmacological treatments for chronic plaque psoriasis: a network meta‐analysis' (Sbidian 2017; Sbidian 2020; Sbidian 2021; Sbidian 2022).
Objectives
To compare the benefits and harms of non‐biological systemic agents (acitretin, ciclosporin, fumaric acid esters, methotrexate), small molecules (apremilast, deucravacitinib), anti‐TNF alpha (etanercept, infliximab, adalimumab, certolizumab), anti‐IL12/23 (ustekinumab), anti‐IL17 (secukinumab, ixekizumab, brodalumab, bimekizumab, sonelokimab, netakimab), and anti‐IL23 (guselkumab, tildrakizumab, risankizumab) for people with moderate‐to‐severe psoriasis using a network meta‐analysis, and to provide a ranking of these treatments according to their benefits and harms.
A secondary objective is to maintain the currency of the evidence, using a living systematic review approach.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs).
Phase I trials were not eligible because participants, outcomes, dosages, and schema of administration of interventions are too different from phase II, III, and IV studies. Cross‐over trials were not eligible (because of the unpredictable evolution of psoriasis and risk of carry‐over bias). Non‐randomised studies, including follow‐up studies, were not eligible.
Types of participants
We considered trials that included adults (over 18 years of age) with moderate‐to‐severe plaque psoriasis (i.e. needed systemic treatment) or psoriatic arthritis whose skin had been clinically diagnosed with moderate‐to‐severe psoriasis and who were at any stage of treatment.
Types of interventions
Adaptive criteria for considering studies for this review
As a living systematic review, we are continually identifying new evidence for interventions already in the network of trials but also for novel interventions. To provide an update and a useful network of interventions for physicians, we need first to identify new interventions but also, to drop old interventions, which are no longer of interest.
To achieve these goals, we contacted international experts from the EuroGuiDerm Psoriasis guideline group, who would help to provide information on new 'eligible' drugs.
Once a year, a list of all systemic drugs used for psoriasis is proposed to the experts’ group, including:
Drugs already involved in the network.
Marketed drugs, identified using the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) websites (www.accessdata.fda.gov/scripts/cder/drugsatfda and www.ema.europa.eu/ema, respectively).
Drugs under development, identified using the World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) and ISRCTN registry (www.isrctn.com).
The experts’ group select from this list all the systemic drugs needed for the future network. They also add relevant new interventions not proposed in the list. They provide a rationale for all proposed network changes (adding or removing interventions). The experts' group is necessary also to determine which drugs have to be deleted from the network, with clinical practice and market authorisation being different in each country.
It is sufficient to update the interventions network once a year, as we are including phase II and III RCTs. Indeed, the timing between the phase I and the phase II/III for a promising intervention is over one year.
Thus, we contacted the EuroGuiDerm Psoriasis guideline expert group on 30 August 2022. At the time, the group comprised 21 dermatologists. None proposed another drug to be included in the network compared with the list in the previous update (Sbidian 2022). Not approved interventions such as sonelokimab were maintained in the interventions group as well as netakimab, which is licensed in Russia.
For this new update, we considered trials that assessed systemic treatments, irrespective of the dose and duration of treatment, compared with placebo or with an active comparator.
Systemic treatments included the following:
-
Non‐biological treatments
FAEs
Acitretin
Ciclosporin
Methotrexate
-
Small molecules
Apremilast
Deucravacitinib
-
Biologic treatments
-
Anti‐TNF alpha
Infliximab
Etanercept
Adalimumab
Certolizumab
-
Anti‐IL12/23
Ustekinumab
-
Anti‐IL17
Secukinumab
Brodalumab
Ixekizumab
Bimekizumab
Sonelokimab
Netakimab
-
Anti‐IL23
Tildrakizumab
Guselkumab
Risankizumab
-
We were interested to compare both the different drugs (n = 20) and the different classes of drugs (n = 6).
Active comparators include the following:
any of the aforementioned systemic treatments; or
additional treatment not of primary interest but used for the network synthesis, such as topical treatment or phototherapy.
In multi‐arm trials, study groups assessing drugs other than those mentioned above were not eligible. In cases of multi‐dose trials, we grouped together all of the different dose groups as a single arm and performed sensitivity analysis at dose level.
In our Background section, we have referred to ongoing Cochrane Reviews that address some of the systemic treatments administered to adults with plaque psoriasis. We considered these treatments in our review, and we have liaised with each of these teams to harmonise our protocols. However, the Cochrane Review on FAEs, published in 2015, included people with all types of psoriasis and not only plaque‐type psoriasis (Atwan 2015).
In the Data collection and analysis and Assessment of heterogeneity sections, details on what was planned to assess the transitivity assumption for studies, participants and intervention are available.
Types of outcome measures
Psoriasis is a chronic disease; treatments are symptomatic, often with a return to baseline after discontinuation. The core outcome set for psoriasis clinical trials was defined under the auspices of the International Dermatology Outcome Measures group, whereby the authors conducted a Delphi survey and identified the following six domains: (1) skin manifestations of psoriasis (including location), (2) an investigator global assessment, (3) an evaluation of signs and symptoms of both psoriasis and psoriatic arthritis, (4) a patient global assessment of their condition, (5) an assessment of treatment satisfaction, and (6) a measure of health‐related quality of life (Callis Duffin 2018).
As a primary outcome, we chose the first domain (skin manifestations of psoriasis). Confronted with a debilitating and a socially and psychologically highly visible disease, a completely 'clear or almost clear' skin was considered to be a stringent test in the induction phase (i.e. psoriasis flare clearing phase).
Primary outcomes
The proportion of participants who achieved clear or almost clear skin, that is, at least PASI 90 at induction phase.
The proportion of participants with serious adverse events (SAEs) at induction phase. We used the definition of severe adverse events from the International Conference of Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, which includes death, life‐threatening events, initial or prolonged hospitalisation, and adverse events requiring intervention to prevent permanent impairment or damage.
Secondary outcomes
Proportion of participants who achieve PASI 75 at induction phase.
Proportion of participants who achieve a Physician Global Assessment (PGA) value of 0 or 1 at induction phase.
Quality of life measured by a specific scale. Available validated scales are the Dermatology Life Quality Index (DLQI), Skindex, Psoriasis Disability Index (PDI), or Psoriasis Symptom Inventory (PSI) at induction phase.
The proportion of participants with adverse events (AEs) at induction phase ('AE outcome' did not include SAE).
Proportion of participants who achieve PASI 75 at 52 weeks.
Proportion of participants who achieve PASI 90 at 52 weeks.
We defined the induction phase as an evaluation from eight to 24 weeks after the randomisation. In case of multiple time points, we chose the longest one.
To avoid selection of good responders of participants entering into long‐term extension, we selected participants who had been randomised since the induction phase.
We did not include studies that had timings outside of the time ranges stated in our outcomes in our review or analyses. We did not evaluate specific adverse events, just the proportion of participants with at least one adverse event and at least one serious adverse event at induction phase.
Search methods for identification of studies
We aimed to identify all relevant RCTs, regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
For this living systematic review, we revised our search strategies in line with advice from the Guidance for the production and publication of Cochrane living systematic reviews (Living Evidence Network 2019). Details of the search strategies used in the earlier published version of this review are available in Sbidian 2020, Sbidian 2021, and Sbidian 2022.
Since October 2021, the Cochrane Skin Information Specialist has searched the following databases monthly, up to 6 October 2022:
the Cochrane Central Register of Controlled Trials (CENTRAL 2022, Issue 10) in the Cochrane Library using the strategy in Appendix 1;
MEDLINE (via Ovid) from October 2021 to October 2022 using the strategy in Appendix 2; and
Embase (via Ovid) from October 2021 to 2022 week 41, using the strategy in Appendix 3.
Trials registers
We (SA and ES for this update) searched the following trials registers up to 6 October 2022 with the following search terms: psoriasis AND one by one, each drug names listed in Types of interventions:
World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/); and
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov).
Retractions and errata
We undertook a search to identify retraction statements or errata related to our included studies in MEDLINE and Embase on 5 January 2023. We retrieved no new relevant records.
Searching other resources
References from other studies
We checked the bibliographies of included studies and relevant systematic reviews for further references to relevant trials.
Unpublished literature
We contact corresponding authors of ongoing studies as we identify them, and ask them to advise us when trial results are available or to share early or unpublished data. We also contact pharmaceutical companies to attempt to identify unpublished and ongoing trials (see Table 2).
2. Investigators contacted.
| Contact | Requested Information | Contacted | Reply | |
| Missing data | ||||
| Akcali 2014 | Prof. Akcali | Outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs, and SAEs | 8 and 21 November 2016 | No response |
| Al‐Hamamy 2014 | Prof. Al‐Hamamy | Outcomes: PASI 75, PGA 0/1, QoL scale, AEs, and SAEs | 8 and 21 November 2016 | No response |
| Asahina 2010 | Prof. Asahina | Outcome: PASI 90 | 8 November 2016 | Asahina 2010: detailed report |
| Asawanonda 2006 | Prof. Asawanonda | Outcomes: PASI 75, PGA 0/1, AEs, and SAEs | 21 November 2016 15 December 2016 |
Asawanonda 2006: sent detailed report for PASI 75 and AEs. PGA was not collected during this study. |
| FEATURE 2015 | Dr Blauvelt Novartis |
Outcome: QoL scale | 8 and 21 November 2016 | Additional data to the publication not provided |
| Caproni 2009 | Prof. Fabri | Outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs, and SAEs | 8 and 21 November 2016 | Caproni 2009: sent detailed report for PASI 90 and SAEs. Other outcomes (PGA, QoL and AEs) not collected during this study. |
| Dogra 2013 | Prof. Dogra | Outcomes: PGA 0/1, QoL scale, AEs, and SAEs | 8 and 21 November 2016 | No response |
| Dogra 2012 | Prof. Dogra | Outcomes: PGA 0/1, QoL scale, AEs, and SAEs | 8 November 2016 | PGA and QoL scale not collected during this study. AEs and SAEs not provided per arm. |
| Fallah Arani 2011 | Dr Fallah Arani | Outcomes: PASI 90, PGA 0/1 and QoL scale | 8 and 21 November 2016 | Outcomes not collected during this study |
| Flytström 2008 | Prof. Flytström | Outcomes: PGA 0/1 | 12 and 19 January 2017 | Additional data to the publication not provided |
| Gisondi 2008 | Prof. Gisondi | Outcomes: PASI 90, PGA 0/1, QoL scale, AEs, and SAEs | 8 November 2016 | Gisondi 2008: sent detailed report for the requested outcomes except for QoL (not assessed during the study) |
| Gordon 2006 | Prof. Gordon | Outcomes: PGA0/1, AEs | 3 and 12 January 2017 | No response |
| Gottlieb 2012 | Prof. Gottlieb Abbvie |
Outcomes: PASI 90 and QoL scale | 8 November 2016 | Gottlieb 2012: sent detailed report for the requested outcomes |
| Gottlieb 2011 | Prof. Gottlieb Amgen |
Outcomes: PASI 90, PGA 0/1, QoL scale, AEs, and SAEs | 8 November 2016 | Gottlieb 2011: sent detailed report for the requested outcomes |
| ACCEPT 2010 | Prof. Griffiths Janssen |
Outcome: QoL scale | 16 December 2016 | QoL was not collected during this study |
| AMAGINE‐2 2015 | Prof. Lebwohl Valeant Pharmaceuticals NA LLC |
Outcomes: PASI 90 and QoL scale | 8 and 21 November 2016 | AMAGINE‐2 2015: sent detailed report for PASI 90; individual scores and median difference from baseline of QoL were not available |
| AMAGINE‐3 2015 | Prof. Lebwohl Valeant Pharmaceuticals NA LLC |
Outcomes: PASI 90 and QoL scale | 8 and 21 November 2016 | AMAGINE‐3 2015: sent detailed report for PASI 90, individual scores and median difference from baseline of QoL were not available |
| Leonardi 2012 | Prof. Leonardi | Outcomes: QoL scale and AEs | 8 and 21 November 2016 | No response |
| Mahajan 2010 | Prof. Kaur | Outcomes: PASI 90, PGA 0/1, QoL scale, AEs, and SAEs | 8 and 21 November 2016 | No response |
| REVEAL 2008 | Prof. Menter | Outcome: PGA 0/1 | 8 and 21 November 2016 | No response |
| EXPRESS‐II 2007 | Prof. Menter | Outcome: PGA 0/1 | 8 and 21 November 2016 | No response |
| BRIDGE 2017 | Prof. Mrowietz | Outcome: QoL scale | 3 and 12 January 2017 | Additional data to the publication not provided |
| Ortonne 2013 | Prof. Paul Novartis |
Outcome: PASI 90 | 3 January 2017 | Additional data to the publication not provided |
| Papp 2013a | Prof. Papp | Outcome: QoL scale | 22 November 2016 13 December 2016 | Additional data to the publication not provided |
| AMAGINE‐1 2016 | Prof. Papp | Outcome: QoL scale | 22 November 2016 13 December 2016 | Additional data to the publication not provided |
| Papp 2005 | Prof. Papp | Outcome: QoL scale, AEs, and SAEs | 22 November 2016 13 December 2016 | Additional data to the publication not provided |
| Papp 2013b | Prof. Papp | Outcome: PASI 90, PGA0/1, QoL scale | 3 January 2017 | Additional data to the publication not provided |
| JUNCTURE 2015 | Prof. Paul Novartis |
Outcome: QoL scale | 15 December 2016, 2 January 2017 | Additional data to the publication not provided |
| Reich 2015 | Prof. Reich Novartis |
Outcomes: PGA 0/1 and QoL scale | 8 November 2016, 16 December 2016 | Additional data to the publication not provided |
| LIBERATE 2017 | Prof. Reich PelotonAdvantage | Outcome: QoL scale | 4 January 2017 | Additional data to the publication not provided |
| Rich 2013 | Prof. Rich | Outcome: QoL scale | 22 November 2016, 13 December 2016 | No response |
| PRESTA 2010 | Prof. Sterry | Outcomes: PASI 90 and QoL scale | 8 and 21 November 2016 | No response |
| Strober 2011 | Prof. Strober Abbvie |
Outcome: QoL scale | 8 November 2016 | Prof Strober sent detailed report for the requested outcomes |
| CLEAR 2015 | Prof. Thaçi Novartis |
Outcome: QoL scale | 8 and 21 November 2016 | Additional data to the publication not provided |
| Torii 2010 | Prof. Torii | Outcomes: PASI 90 and PGA0/1 | 21 November 2016 | Prof Torii sent detailed report for the requested outcomes |
| Tyring 2006 | Prof. Tyring | Outcomes: PGA 0/1 and QoL scale | 8 and 21 November 2016 | No response |
| Van Bezooijen 2016 | Dr van Bezooijen | Outcomes: PASI 90, adverse effects | 4 and 12 January 2017 | Additional data to the publication not provided |
| Van de Kerkhof 2008 | Prof. van der Kherkhof Pfizer |
Outcome: AEs | 8 and 21 November 2016 | Additional data to the publication not provided |
| LOTUS 2013 | No contact | Outcome: PASI 90 | No | Authors' email not found |
| CLARITY 2018 | Prof Bagel | Outcome: QoL Scale | 24 June 2019 | Email response 1 July 2019 Additional data to the publication not provided |
| ADACCESS 2018 | Prof Blauvelt | Outcome: QoL Scale | 24 June and 1 July 2019 | Email response: 2 July 2019 Additional data to the publication not provided |
| EGALITY 2017 | Prof Gerdes | Outcomes: QoL Scale, AEs, SAEs | 24 June 2019 | Email response 27 June 2019 Additional data to the publication not provided |
| Ikonomidis 2017 | Prof Ikonomidis | Outcomes: PASI 90, 75, PGA0/1, QoL Scale, AEs, SAEs | 24 June and 1 July 2019, 17 August 2020, 8 September 2020 | No response |
| VIP Trial 2018 | Prof Gelfand | Outcome: PASI 90 | 24 June | Email response 24 June 2019 Additional data to the publication not provided |
| SIGNATURE 2019 | Prof. Warren | Outcomes: PGA0/1, SAEs | 24 June 2019, 21 October 2021 | No response |
| NCT02581345 | Dr Caminis | Outcome: QoL scale | 24 June 2019 | Authors' email not found (SHIRE Pharmaceutics). We will contact the authors when the article is published. |
| VOLTAIRE‐PSO 2021 | Prof. Menter | Outcome: QoL scale | 24 June 2019, 21 October 2021 | No response |
| ORION 2020 | Prof. Ferris | Outcome: DLQI | 24 June and 2 July 2019 | No response |
| POLARIS 2020 | Prof. Thaçi | Outcome: PGA0/1 | 24 June 2019, 21 October 2021 | No response |
| SustaIMM 2019 | Prof. Kitamura | Outcome: DLQI | 24 June 2019, 21 October 2021 | Email not received: "The following message to <susumu.kitamura@abbvie.com> was undeliverable." |
| Papp 2017a | Prof. Papp | Outcome: DLQI | 24 June 2019 | Email answer 24 June 2019 Additional data to the publication not provided |
| BE ABLE 1 2018 | Prof. Papp | Outcome: DLQI | 24 June 2019 | Email answer 24 June 2019 Additional data to the publication not provided |
| Papp 2017b | Prof. Papp | Outcome: DLQI | 24 June 2019 | Email answer 24 June 2019 "I am not at liberty to release results that are not in the public domain. Regards, k" |
| Papp 2018 | Prof. Papp | Outcome: DLQI | 24 June 2019 | Email answer 24 June 2019 Additional data to the publication not provided |
| IXORA‐S 2017 | Prof. Reich | Outcome: DLQI | 24 June and 1st July 2019 | Emails not received (email: kreich@dermatologikum.de; kreich@jeruocon.com) |
| TRANSFIGURE 2016 | Prof. Reich | Outcomes: PGA0/1, DLQI | 24 June and 1 July 2019 | Emails not received (email: kreich@dermatologikum.de; kreich@jeruocon.com) |
| PRIME 2017 | Prof. Sticherling | Outcome: DLQI | 24 June and 1 July 2019 | Email answer 2 July 2019 Additional data to the publication not provided |
| CIMPACT 2018 | Prof. Lebwohl | Outcome: DLQI | 24 June and t July 2019 | No response |
| Lee 2016 | Outcomes: PASI 90, DLQI | 24 June and t July 2019 | No response | |
| IMMhance 2020 | Prof. Blauvelt | Outcome: DLQI | 24 June 2019, 21 October 2021 | Email answer 22 October 2021 Additional data to the publication not provided |
| NCT02134210 RaPsOdy | Barbara K Finck, M.D. Coherus Biosciences, Inc |
Outcome: DLQI | 24 June 2019 | No contact. We will contact the authors when the article is published |
| Yu 2019 | Prof. Shi | Outcomes: PGA 0/1, DLQI | 12 August 2020, 8 September 2020 | No response |
| CARIMA 2019 | Prof. von Stebut | Outcomes: PASI 90, 75, IGA 0/1, QoL Scale | 12 August 2020, 8 September 2020 | No response |
| PsOsim 2017 | Prof. Hodge | Outcomes: PASI 90, PGA 0/1, QoL scale | 12 August 2020, 8 September 2020 | No response |
| VIP‐U Trial 2020 | Prof. Gelfand | Outcome: QoL Scale | 12 August 2020 | Email answer 17 August 2020 VIP‐U Trial 2020: sent detailed report for the requested outcome |
| Liu 2020 | Prof. Liu | Outcome: QoL Scale | 12 August 2020 | Email answer 13 August 2020 Liu 2020: sent detailed report for the requested outcome |
| ECLIPSE 2019 | Prof. Reich | Outcomes: QoL scale, AEs, SAEs | 12 August 2020, 8 September 2020 | Email answer 11 September 2020 Additional data to the publication not provided |
| IXORA‐R 2020 | Prof. Blauvelt | Outcomes: PASI 90/75, PGA 0/1, DLQI | 12 August 2020, 15 October 2021 (outcomes at week 24) | Email answer 13 August 2020 Sent detailed report for the requested outcomes except for PASI 75 and DLQI (not disclosed yet) |
| ALLURE 2021 | Prof. Sigurgeirsson | Outcome: DLQI | 12 August 2020, 21 October 2021 | Email answer 25 August 2020 Additional data to the publication not provided |
| Cai 2020 | Prof. Zhang | Outcome: DLQI | 21 October 2021 | No response |
| NCT03055494 ObePso‐S | Prof. Krueger | Outcomes: PASI 75, PGA 1/0, QoL Scale, AEs | 8 September 2020, 9 September 2022 | No contact. We will contact the authors when the article is published. |
| IMMerge 2021 | Prof. Warren | Outcome: QoL scale | 8 September 2020, 21 October 2021 | No response |
| AlMutairi 2021 | Prof. Almutairi | Outcomes: PASI 90/75, QoL | 20 September 2021 | No response |
| BE READY 2021 | Prof. Gordon | Outcomes: QoL, SAE, PASI 75 | 20 September 2021 | No response |
| BE VIVID 2021 | Prof. Reich | Outcomes: QoL, SAE | 20 September 2021 | No response |
| BE RADIANT 2021 | Prof. Reich | Outcomes: QoL, AE, SAE | 20 September 2021 | No response |
| BE SURE 2021 | Prof. Warren | Outcomes: PASI 75, SAE, QoL | 20 September 2021 | Email answer 20 October 2021 Sent detailed report for the requested outcome |
| Seo 2020 | Prof. Lee | Outcomes: SAE, QoL, PASI 90 | 20 September 2021 | No response |
| Ye 2020 | Prof. Chengzhong Zhang | Outcomes: PGA 1/0, AE, SAE, QoL | 20 September 2021 | No response |
| Rathipriyadharshini 2020 | Prof. Srinivasan | Outcomes: PASI 90/75, PGA 1/0, AE, QoL | 20 September 2021 | No response |
| CALYPSO 2018 | Prof. Korotaeva | Outcomes: PGA 1/0, SAE, QoL | 20 September 2021 | Email answer 23 September 2021 CALYPSO 2018: sent detailed report for the requested outcome |
| Singh 2021 | Prof. Sermili Rini Singnarpi | Outcomes: PGA 1/0, SAE, AE, QoL | 20 September 2021 | No response |
| Blauvelt 2021a | Prof. Blauvelt | Outcomes: PASI 75, QoL | 20 September 2021 | Email answer 20 September 2021: "Neither was done" |
| PLANETA 2021 | Prof. Morozova | Outcomes: PGA 1/0, QoL | 20 September 2021 | Email answer 27 September 2021 PLANETA 2021: sent detailed report for the requested outcome |
| Papp 2021 | Prof. Papp | Outcome: QoL | 20 September 2021 | Email answer 20 September 2021 Additional data to the publication not provided |
| AFFIRM 2022 | Prof. Srinivas Shenoy | Outcome: DLQI | 20 September 2021, 9 September 2022 | No response |
| Augustin 2022 | Prof. Augustin | Outcome: DLQI | 20 September 2021, 9 September 2022 | No response |
| NCT03535194 OASIS‐2 | Sponsor: Eli Lilly and Company | Outcome: QoL | 20 September 2021 | No contact. We will contact the authors when the article is published |
| Feldman 2021 | Prof. Stroissnig | Outcomes: PGA 1/0, DLQI, psoriasis worsening as SAE | 9 September 2022, 17 November 2022 | No response |
| POETYK PSO‐1 2022 | Prof. Banerjee | Outcomes: DLQI, psoriasis worsening as SAE | 9 September 2022 | POETYK PSO‐1 2022: sent detailed report for the requested outcomes |
| Cai 2022 | Prof. J. Zhang | Outcomes: PASI 90, DLQI, SAE, psoriasis worsening as SAE, AE | 9 September 2022, 17 November 2022 | No response |
| Ikonomidis 2022 | Prof. Ikonomidis and Prof. Pavlidis | Outcomes: PASI 90, PGA 1/0, QoL, SAE, psoriasis worsening as SAE, AE | 9 September 2022, 17 November 2022 | Ikonomidis 2022: sent detailed report for the requested outcomes |
| Yu 2022 | Prof. Wang | Outcomes: PASI 90, PGA 1/0, QoL, SAE, psoriasis worsening as SAE, AE | 31 October 2022 | Yu 2022: sent detailed report for the requested outcomes |
| IMMpress 2022 | Prof. Odnopozova | Outcome: QoL | 31 October 2022, 17 November 2022 | No response |
| Morita 2022 | Prof. Morita | Outcomes: PASI 90, PASI 75, PGA 1/0, QoL | 31 October 2022 | Morita 2022: sent detailed report for the requested outcomes |
| POETYK PSO‐2 2022 | Prof. Banerjee | Outcomes: psoriasis worsening as SAE, QoL | 31 October 2022 | POETYK PSO‐2 2022: sent detailed report for the requested outcomes |
| SPIRIT‐H2H 2020 | Prof. Kristensen and Prof. De Vlam | Outcomes: PGA 1/0, SAE, psoriasis worsening as SAE, AE, QoL | 31 October 2022, 17 November 2022 | No response |
| Cestari 2021 | Prof. Cestari | Outcomes: psoriasis worsening as SAE, QoL | 17 November 2022 | No response |
| POETYK PSO‐3 2022 | Sponsor: Bristol‐Myers Squibb | Outcomes: QoL | 17 November 2022 | No contact. We will contact the authors when the article is published |
| Awaiting classification studies | ||||
| Chow 2015 | Prof. Chow | Outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs, and SAEs | 8 November 2016, 16 December 2016 | No response |
| Gurel 2015 | Prof. Gurel | Study's protocol and outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs, and SAEs | 17 and 24 January 2017 | Gurel 2015: sent detailed report for the requested outcomes. Finally, the Gurel study was classified in the included studies section. |
| Han 2007 | No contact | Outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs, and SAEs | No | Authors' email not found |
| Krishna 2016 | Prof. Krishna | Asking for study protocol and efficacy/safety results | 5 and 12 January 2017 11 February 2020 |
No response |
| DRKS00000716 | Prof. Jacobi | Asking for study protocol and efficacy/safety results | 12 and 19 January 2017 | No response |
| CTRI/2015/05/005830 | Prof. Shah | Asking for study protocol and efficacy/safety results | 12 and 19 January 2017 11 February 2020 |
No response |
| NCT01088165 | Prof. Holzer | Asking for study protocol and efficacy/safety results | 3 and 24 June 2019 11 February 2020 |
No response |
| NCT02655705 | Prof. Youn | Asking for study protocol and efficacy/safety results | 3 and 24 June 2019 11 February 2020 |
No response |
| EUCTR2010‐020168‐39‐DE | Prof. Anderson | Asking for study protocol and efficacy/safety results | 17 August 2020, 8 September 2020 | No response |
| EUCTR2015‐005279‐25‐DE | Prof. Philipp | Asking for study protocol and efficacy/safety results | 17 August 2020, 8 September 2020, 31 August 2021 | No response |
| Makavos 2020 | Prof. Ikonomidis | Asking for study protocol and efficacy/safety results | 30 October 2020, 10 September 2021 | No response |
| CTRI/2016/10/007345 | Dr Piyush Agarwal, general manager Glenmark Pharmaceuticals Ltd DrPiyush.Agarwal@glenmarkpharma.com Amol.Pendse@glenmarkpharma.com |
Asking for study protocol and efficacy/safety results | 11 February 2020, 30 August 2021 | No response |
| Goldust 2019 | Prof. Goldust | Asking for study protocol and efficacy/safety results | 31 August 2021 | No response |
| NCT01558310 | Dr Yamauchi, Dr Patnaik, Director, Clinical Science Institute | Asking for study protocol and efficacy/safety results | 5 January 2017 | Email response: Additional data to the publication not provided |
| NCT02701205 | Prof Hongzhong Jin | Asking for study protocol and efficacy/safety results | 3 June 2019 11 February 2020, 30 August 2021 |
Email response "This is the mail system at host mta‐8_BSR. Your message could not be delivered to one or more recipients." |
| Abstracts | ||||
| Mrowietz 2005 | Prof. Mrowietz | Study's protocol and outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs, and SAEs | 16 December 2016, 3 January 2017 | Additional data to the publication not provided. Finally, the Mrowietz study was placed in the 'Awaiting classification' section. |
| Ongoing studies | ||||
| EUCTR2017‐001615‐36‐DE | Prof. Gerdes | Asking for study protocol and efficacy/safety results | 17 August 2020, 8 September 2020 | Email answer 8 September 2020: Additional data to the publication not provided |
AE: adverse events; PASI: Psoriasis Area and Severity Index; PGA: Physician Global Assessment; QoL: quality of life; SAE: serious adverse events
Once a year, we manually check additional sources (regulatory agencies and pharmaceutical company trial registries).
We searched reviews submitted to the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for drug registration (using www.accessdata.fda.gov/scripts/cder/drugsatfda and www.ema.europa.eu/ema) up to 6 October 2022.
Adverse events
We did not perform a separate search for rare or delayed adverse events of the target interventions. However, we examined data on adverse events from the included studies we identified.
Annual review of search methods for this living systematic review
Once a year, we revisit our search methods and, if necessary, update the search strategies by adding or removing intervention terms. This ensures the strategies reflect any terminology changes in the topic area, or changes to search terms available in the databases we search.
Data collection and analysis
Selection of studies
We conducted the selection process through Covidence (Covidence 2021), a web tool allowing dual screening of search results based on titles and abstracts, and then full text by independent review authors. Thus, two review authors (SA, ES for this update) independently examined each title and abstract to exclude irrelevant reports. These authors independently examined full‐text articles to determine eligibility. We contacted study authors for clarification when necessary and discussed disagreements to reach consensus. We listed excluded studies and documented the primary reason for exclusion.
As this is a living systematic review, we immediately screened any new citations retrieved by the monthly searches.
Since February 2021, we have used Cochrane’s Screen4Me workflow to help assess the results of the search for RCTs. Screen4Me comprises three components, of which we used two: known assessments (a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labelled as 'RCT' or 'not an RCT'); and the RCT classifier (a machine‐learning model that distinguishes RCTs from non‐RCTs). For more information about Screen4Me and the evaluations that have been done, please go to the Screen4Me webpage on the Cochrane Information Specialist’s portal. In addition, more detailed information regarding evaluations of the Screen4Me components can be found in Marshall 2018 and Noel‐Storr 2021.
Data extraction and management
Two review authors (SA, ES for this update) extracted the data from published and unpublished reports independently, using a standardised form. We pilot‐tested this form (data extraction form) on a set of included trials. We extracted the data to populate the Characteristics of included studies tables in Review Manager 5.4 (RevMan) (Revman 2020).
We extracted the data from the reports of the US FDA when available and, if not, from the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov), and finally from the published reports.
Outcome data
We extracted arm‐level data from each included trial; hence, the total number of participants randomised to each intervention. For binary outcomes, we also extracted the number of participants (if available) who:
reached PASI 90, PASI 75, or PGA 0/1 during the induction phase;
reached PASI 90 or PASI 75 during the maintenance phase (at week 52); and
had at least one SAE/one SAE after excluding flares of psoriasis/one AE during the induction phase.
For quality of life, we extracted from each included trial the mean change score of the study‐specific scale from baseline to follow‐up.
For assessment of quality of life, we recorded all specific quality of life (QoL) scales (Dermatology Life Quality Index (DLQI), Skindex, Psoriasis Disability Index (PDI), and Psoriasis Symptom Inventory (PSI)).
Data on potential effect modifiers
We extracted baseline demographic and clinical characteristics of participants that may have acted as effect modifiers (age, sex, body weight, duration of psoriasis, severity of psoriasis at baseline, previous psoriasis treatment). Two review authors (SA, ES) checked and entered the data into the Review Manager 5 computer software (Revman 2020). We contacted the authors of the trials to request missing data, including missing data for outcomes (see Table 2).
Assessment of risk of bias in included studies
We used Cochrane's risk of bias (RoB) tool to assess the risks of bias. Two review authors (LLC and SA for this update) independently assessed the risk of bias, and one author (ES for this update) resolved any disagreements. For each of the following domains and according to the general principles in section 8.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), we judged the following risk of bias domains as 'low', 'high', or 'unclear'.
-
Selection bias (random sequence generation and allocation concealment items)
Was the allocation sequence adequately generated? We considered randomisation adequate (low risk of bias) if the allocation sequence was generated from a table of random numbers or was computer‐generated. We considered randomisation inadequate (high risk of bias) if sequences could be related to prognosis. We considered randomisation unclear if the paper stated that the trial was randomised, but did not describe the method.
Was allocation adequately concealed? We deemed allocation concealment as adequate if the report stated that it was undertaken by means of sequentially pre‐numbered sealed, opaque envelopes or by a centralised system. We considered a double‐blind double‐dummy process as being at low risk of bias even if the paper did not describe the method of allocation concealment.
-
Performance and detection bias (blinding of participants, and blinding of outcome assessor items)
Was knowledge of the allocated intervention adequately prevented during the study? We evaluated the risk of bias separately for personnel and participants, outcomes assessors, and each outcome.
-
Attrition bias (incomplete outcome data item)
Were incomplete outcome data adequately addressed? We examined if there was imbalance across intervention groups in numbers or reasons for missing data, type of measure undertaken to handle missing data, and whether the analysis was carried out on an intention‐to‐treat (ITT) basis. We assessed the use of strategies to handle missing data.
-
Reporting bias (selective outcome reporting item)
Were reports of the study free of suggestion of selective outcome reporting? We evaluated if each outcome was measured, analysed, and reported. We compared outcomes specified in protocols (if available on the FDA website or ClinicalTrials.gov) and in material and methods with outcomes presented in the Results section. We considered reporting bias inadequate if one specified outcome in the protocols was lacking in the main report.
-
Other risk of bias
We did not address the 'Other risk of bias' item as we did not highlight particular circumstances leading to other risk of bias from particular trial designs, contamination between the experimental and control groups, and particular clinical settings.
Overall risk of bias
To summarise the quality of evidence and to interpret the network results, we used these six RoB criteria (random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessor, incomplete outcome data, and selective outcome reporting) in order to classify each trial.
We would classify the trial as having low risk of bias if we rated none of the domains above as high risk of bias and two or fewer as unclear risk.
We would classify the trial as having moderate risk of bias if we rated one domain as high risk of bias, one or fewer domains as unclear risk, or no domains as high risk of bias, but three or fewer were rated as unclear risk.
All other cases were assumed to pertain to high risk of bias.
Measures of treatment effect
For each pairwise comparison and each dichotomous outcome at each time point, we used risk ratios (RRs) with 95% confidence intervals (CIs) as a measure of treatment effect. For continuous variables (e.g. quality of life scale), we used the standardised mean difference (SMD) with a 95% CI.
For every treatment, we estimated the ranking probabilities of being at each possible rank for all outcomes. We inferred treatment hierarchy using the surface under the cumulative ranking curve (SUCRA) (Salanti 2011). SUCRA was expressed as a percentage between 0 (when it is certain a treatment is the worst) to 100% (when it is certain a treatment is the best). The advantage of SUCRA compared to other ranking measures is that it takes into account the entire distribution of the relative effects (for more information on SUCRA, see Chaimani 2017b; Chaimani 2017c; Veroniki 2018). It should be noted, though, that ranking measures might be of limited value in the presence of large uncertainty in the results, and therefore they should always be reported along with the relative effects.
Unit of analysis issues
The primary unit of analysis was the participant. We did not consider studies with non‐standard design features that would lead to clustering (e.g. cross‐over trials).
We treated comparisons from trials with multiple intervention groups as independent two‐arm studies in the pairwise meta‐analyses. In this analysis, different comparisons were analysed separately, and therefore no study participants were double‐counted. At the network meta‐analysis stage, we properly accounted for the within‐trial correlation.
Dealing with missing data
We extracted, when possible, both the number of randomised and analysed participants in each study arm. We contacted trial authors or sponsors by email to request missing outcome data (numbers of events and numbers of participants for important dichotomous clinical outcomes) when these were not available in study reports that were less than 10 years old (See Table 2). For the main analysis, we assumed that any participant with missing outcome data did not experience clearance (for efficacy outcomes) or did not experience AEs (for safety outcomes), whatever the group. In a sensitivity analysis, we also synthesised the data ignoring the missing participants (complete case analysis), assuming that they were missing at random (Mavridis 2014).
Assessment of heterogeneity
We undertook meta‐analyses only if we judged participants, interventions, comparisons, and outcomes to be sufficiently similar (section 10.10 of the Cochrane Handbook for Systematic Reviews of Interventions) (Deeks 2021). Potential sources of heterogeneity included participants' baseline characteristics (weight, previous systemic treatment or not, treatment doses, co‐interventions, and duration of treatment). When enough data were available, we investigated the distributions of these characteristics across studies and treatment comparisons. The latter allows assessing transitivity, i.e. whether there were important differences between the trials evaluating different comparisons other than the treatments being compared (Salanti 2014). To further reassure the plausibility of the transitivity assumption, we only included in our analyses trials not involving co‐interventions. To better reassure the plausibility of transitivity, we excluded from the main analysis trials including biological‐naïve participants, but assessing efficacy of a biological agent. Indeed, response to biologics is different depending on treatment status (systemic‐naïve or not). The large majority of trials assessing a new biologic did not include as a non‐inclusion criterion being systemic‐naïve participants.
In the classical meta‐analyses, we assessed statistical heterogeneity by visual inspection of the forest plots and using the Q‐test and the I2 statistic. We interpreted the I2 statistic according to the following thresholds (section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions; Deeks 2021): 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% represents considerable heterogeneity.
In the network meta‐analysis, the assessment of statistical heterogeneity in the entire network was based on the estimated heterogeneity variance parameter (τ2) estimated from the network meta‐analysis models (Jackson 2014). We also estimated the prediction intervals to assess how much the estimated heterogeneity affects the relative effects with respect to the additional uncertainly anticipated in future studies (Riley 2011). Where feasible, we would have investigated the possible sources of heterogeneity in subgroup analyses and meta‐regression.
Although we restricted the risk of important heterogeneity in our data by considering eligible only studies without co‐interventions, we investigated differences in heterogeneity across the different analyses. Specifically, we observed whether splitting the nodes of the network and analysing each drug and each dose separately reduced the heterogeneity estimate. We also ran a series of sensitivity analyses (see Sensitivity analysis), and we monitored whether heterogeneity became smaller or larger compared to the primary analysis.
Assessment of reporting biases
To assess reporting biases, we used an adaptation of the funnel plot by subtracting from each study‐specific effect size the mean of meta‐analysis of the study‐specific comparison, which we plotted against the study standard error (Chaimani 2013). We employed this 'comparison‐adjusted funnel plot' for all comparisons of an active treatment against placebo. When we detected substantial funnel plot asymmetry for the two primary outcomes, we investigated the presence of small‐study effects in the network meta‐regression (Chaimani 2012).
Data synthesis
Pairwise meta‐analysis
We conducted pairwise meta‐analyses to synthesise trials comparing one of the treatments against placebo or two treatments against each other. We performed pairwise meta‐analyses for all outcomes and comparisons, provided that at least two studies were available, using a random‐effects model.
Network meta‐analysis
We then employed network meta‐analysis (NMA) for all outcomes and comparisons, to estimate the relative effects for all possible comparisons between any pair of treatments within a frequentist framework, using random‐effects models. We provided a graphical depiction of the evidence network for all outcomes to illustrate the network geometry (Chaimani 2017a). We ran network meta‐analysis using the approach of multivariate meta‐analysis, which treats the different comparisons that appear in studies as different outcomes (White 2012).
We focused on confidence intervals as a finding of uncertainty, as confidence intervals were sufficiently narrow to rule out an important magnitude of effect.
We assessed inconsistency (i.e. the possible disagreement between the different pieces of evidence) locally and globally. Specifically, we used the side‐splitting method (Dias 2010). The comparison of interest showed evidence of inconsistency, when a P value was less than 0.05 when direct and indirect evidence were compared in a z test (Separate Indirect from Direct Evidence (SIDE)). We also fitted the design by treatment interaction model to evaluate the presence of inconsistency in the entire network (Higgins 2012).
We conducted pairwise meta‐analyses using Review Manager 5.4 (Revman 2020), and we performed all other analyses in R software version 4.2.2 using the 'R‐package netmeta' (https://cran.r-project.org/web/packages/netmeta/netmeta.pdf) and 'ggplot2 package' for the network graphs.
As this is a living systematic review, whenever we found new evidence (i.e. studies, data or information) meeting the review inclusion criteria, we extracted the data and assessed risks of bias. For trials identified as completed in clinical trial registries but without posted results or those identified only by a conference proceeding, and for missing outcome data, trained review authors contacted trialists to request complete results. Every six months, we incorporated each newly identified trial in the network. We performed one network for each outcome (PASI‐90, SAEs, PASI‐75, PGA, QoL, and AEs). We re‐analysed the data every six months using the standard approaches outlined in this Data synthesis section, as well as the CiNeMa process. We checked the assumptions of the NMA each time we updated the analysis.
Subgroup analysis and investigation of heterogeneity
We had planned to undertake subgroup analyses and meta‐regressions to investigate potential sources of heterogeneity or inconsistency (such as weight of participants, duration of psoriasis, baseline severity, previous systemic treatments) during the induction phase, but we found no heterogeneity or inconsistency.
Sensitivity analysis
To assess the robustness of our results, we performed the following sensitivity analyses for the two primary outcomes:
running the analysis at dose‐level, considering that each different drug dose is a different intervention;
excluding trials at high risk of bias;
excluding trials with a total sample size smaller than 50 randomised participants;
analysing only the observed participants and assuming that missing participants were missing at random;
analysing only the studies with a short‐term assessment from eight to 16 weeks (to better reassure the plausibility of the transitivity assumption);
including all trials irrespective of the previous systemic treatments received by the participants;
-
analysing only drugs and dosages approved by the European Medicines Agency for plaque psoriasis:
non‐biological systemic treatments: FAEs, acitretin, ciclosporin, methotrexate;
small molecules: apremilast; deucravacitinib;
anti‐TNF alpha: infliximab, etanercept, adalimumab, certolizumab pegol;
anti‐IL12/23: ustekinumab;
anti‐IL17: secukinumab, brodalumab, ixekizumab, bimekizumab;
anti‐IL23: tildrakizumab, guselkumab, risankizumab.
using alternative statistical model (i.e. penalised likelihood regression) that allow the inclusion of studies with zero events in both groups (Evrenoglou 2022); and, lastly
we assessed SAEs after excluding flares of psoriasis.
We undertook this analysis because it has recently been reported that after excluding cases of worsening psoriasis, the risk of occurrence of SAEs is higher in the biologic (especially for anti‐TNF agents) arm than in the placebo arm (Afach 2021).
Summary of findings and assessment of the certainty of the evidence
We did not include summary of findings (SoF) tables because the format of an SoF table does not allow us to present a summary of comparisons across the different drugs. The SoF tables in the previous versions of the review only focused on the comparisons against placebo. However, we presented in Figure 1 all comparison results for the two main outcomes, the anticipated absolute effects and assessment of the certainty of evidence using CINeMA. The anticipated absolute effects were calculated from multiplication of the NMA‐derived relative effects estimates (using a random‐effects model within a frequentist approach) by an assumed control risk based on the weighted control event rate across all studies.
1.
Relative effects of the intervention as estimated from the network meta‐analysis model for Psoriasis Area and Severity Index (PASI) 90 and serious adverse events (SAEs)
Outcomes were all measured at the induction phase (assessment from 8 to 24 weeks after randomisation). Drugs are reported in order of primary benefit ranking. Each cell contains the risk ratio (RR) and 95% confidence interval for the two primary outcomes (PASI 90 and SAEs) of the intervention in the respective column versus the comparator in the respective row. RRs larger than 1 for the lower triangle and smaller than 1 for the upper triangle favour the treatment on the left. Certainty of evidence was assessed for each comparison using CINeMA and classified as high (highlighted in green), moderate (in blue), low (in yellow), and very low (in red). Significant results are highlighted in bold.
The anticipated absolute effects were calculated from multiplication of the NMA‐derived relative effects estimates (using a random‐effects model within a frequentist approach) by an assumed control risk based on the weighted control event rate across all studies.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
We assessed the confidence of the evidence estimates for the two primary outcomes (PASI 90 and SAEs), from network meta‐analysis, based on the CINeMA approach, which is based on the contributions of the direct comparisons to the estimation in the network meta‐analysis (CINeMA 2017; Salanti 2014). CINeMA (Confidence in Network Meta‐Analysis) is a web application that simplifies the evaluation of confidence in the findings from network meta‐analysis.
It is based on six domains: within‐study bias (referring to the impact of risk of bias in the included studies), across‐studies bias (publication or reporting bias), indirectness (relevance to the research question and transitivity), imprecision (comparing the range of treatment effects included in the 95% confidence interval with the range of equivalence), heterogeneity (predictive intervals), and incoherence (if estimates from direct and indirect evidence disagree) (Salanti 2014).
We evaluated the confidence in each NMA RRAB between two given drugs A and B for six domains. The software required some input in each domain in order to recommend whether there were 'major concerns', 'some concerns', or 'no concerns' for the particular domain.
Thus, we finalised threshold values and evaluation rules to be decided through discussions. After determining these rules, the remaining synthesis of confidence in the evidence can be automatically calculated via CINeMA web app; hence, one review author finally input all the data and got the results.
Within‐trial bias: we estimated it as the weighted average of the overall risk of bias of all the trials contributing information to the estimation of RRAB.
Reporting bias: also known as 'publication bias'. We assessed publication bias by considering the comprehensive search strategy that we performed and the risk of publication bias in the specific field. The comparison‐adjusted funnel plots that test the presence of small‐study effects in the network assisted our judgement.
Indirectness: participants in the included studies had a mean age of 45 years, more than 60% were males and had moderate‐to‐severe psoriasis, with an overall mean PASI score at baseline of 20. This young age, the high proportion of males, and the high level of disease severity may not be typical of patients seen in daily clinical practice, thus we judged 'moderate' for any of the evaluated RRAB.
Imprecision: this was rated based on whether the 95% CI of RR was allowing recommendations to be made. We set the margin of equivalent effects (where none of the drugs is favoured) to between RR 0.95 and 1.05. These values were motivated by the fact that assuming 3% response rate (reaching PASI 90) for placebo, then an RRAB of 1.05 indicated a response for drug A higher than that obtained with placebo, which we considered as clinically meaningful. Then, the degree of overlap between the 95% CI of RRAB and the margin of equivalent effects suggests the judgement.
Heterogeneity: this was evaluated by monitoring the agreement between confidence intervals (CIs) and prediction intervals (PIs). CINeMA judges whether the two intervals and their overlap with the margin of equivalent effects provide similar conclusions.
Incoherence: this was evaluated by monitoring the level of disagreement between confidence intervals (CIs) of the direct and indirect RRAB and their overlap with the margin of equivalent effects.
After the judgement for all the six domains, we summarised the overall confidence in evidence for each RR between any two drugs into high, moderate, low, and very low. Starting with high confidence, we downgraded by one level for each ‘major concern’ in any of the six domains; then by two‐thirds of a level down for ‘some concerns’ in ‘within‐study bias’; and by one‐third of a level down for each ‘some concerns’ in any of the other five domains. To obtain the final level, we rounded the number of downgrades to their nearest integer.
For each drug, we calculated the percentage of the four levels based on all comparisons including that drug, for benefits and harms outcomes.
Results
Description of studies
Results of the search
Recent monthly Electronic searches of databases and trials registers for this living systematic review, from 5 October 2021 to 6 October 2022, have identified an additional 814 references to potentially eligible studies. We have also re‐examined 63 studies from the previous version of this review identified as ongoing (42 studies reported in 56 references) or awaiting classification (21 reported in 40 references). We have therefore screened a total of 910 references for this update.
After reviewing the titles and abstracts, we discarded 745 references. We examined the full text of the remaining 165 references. Twenty‐three studies (reported in 24 references) did not meet the inclusion criteria and were excluded (see Characteristics of excluded studies). This increased the total number of excluded studies across all versions of this review to 466. Twenty‐three trials (reported in 30 references) were identified as studies awaiting classification (see Characteristics of studies awaiting classification). We identified 45 studies (reported in 61 references) as ongoing (see Characteristics of ongoing studies). We identified 12 new included studies (reported in 44 references) for this update. We also identified seven references that related to studies previously included in this review.
In total, we have 179 studies reported in 449 references.
For a further summary of our screening process, see the study flow diagram (Figure 2).
2.
Study flow diagram
Included studies
Trial design
All trials used a parallel‐group design. The mean sample size was 348 (range: 10 to 1881). In all, 151 trials were multicentric (2 to 231 centres) and 20 were single‐centre trials (Akcali 2014; Al‐Hamamy 2014; Asawanonda 2006; Chaudhari 2001; Chladek 2005; Dogra 2012; Dogra 2013; Dubertret 1989; Ellis 1991; Gisondi 2008; Gurel 2015; Hunter 1963; Ikonomidis 2017; Khatri 2016; Mahajan 2010; Shehzad 2004; Singh 2021; Van Bezooijen 2016; VIP‐U Trial 2020; Ikonomidis 2022); for eight trials, single‐centre or multicentric status was not clear (Caproni 2009; Engst 1994; Goldfarb 1988; Olsen 1989; Rathipriyadharshini 2020; Ye 2020; Yilmaz 2002; Yu 2019). Most of the trials recruited participants from a hospital setting, but some also from physicians' offices. The trials took place worldwide (n = 71, 40%), in Europe (n = 38, 21%), in Asia (n = 35, 20%), in North America (n = 28, 16%), in the Middle East (n = 2, 1%), or in South America (n = 1, < 1%). The location was not stated for four trials (Caproni 2009; Engst 1994; Goldfarb 1988; Olsen 1989).
In total, 82 trials out of 179 were multi‐arm, including 53 multi‐arm trials that only assessed the same experimental drug at multiple dose levels; 170 multi‐arm trials assessing at least two different drugs; and 12 assessing both the same experimental drug at multiple dose levels and different drugs. In total, 12 trials assessed biosimilars versus original drugs for adalimumab (ADACCESS 2018; AURIEL‐PsO 2020; Cai 2022; CALYPSO 2018; Feldman 2021; NCT02581345; Papp 2017a; PsOsim 2017; VOLTAIRE‐PSO 2021; Yu 2022) and etanercept (EGALITY 2017; NCT02134210 RaPsOdy).
In total, 20 trials had a co‐intervention, mainly with phototherapy (Al‐Hamamy 2014; Asawanonda 2006; Bissonnette 2013; Gottlieb 2012; Gurel 2015; Liu 2020; Lowe 1991; Mahajan 2010; Morita 2022; OPTIMAP 2022; Ruzicka 1990; Saurat 1988; Shehzad 2004; Singh 2021; Sommerburg 1993; Tanew 1991; Van Bezooijen 2016; Ye 2020; Yilmaz 2002; Yu 2019). Only 14 studies were carried out before the year 2000 (Dubertret 1989; Ellis 1991; Engst 1994; Goldfarb 1988; Hunter 1963; Laburte 1994; Lowe 1991; Meffert 1997; Nugteren‐Huying 1990; Olsen 1989; Ruzicka 1990; Saurat 1988; Sommerburg 1993; Tanew 1991).
Characteristics of the participants
This review includes 179 trials (12 new trials for the updated review: Cai 2022; Cestari 2021; Feldman 2021; Ikonomidis 2022; IMMpress 2022; Morita 2022; OPTIMAP 2022; POETYK PSO‐1 2022; POETYK PSO‐2 2022; POETYK PSO‐3 2022; SPIRIT‐H2H 2020; Yu 2022), with a total of 62,339 randomised participants. We summarised the characteristics of the participants in the Characteristics of included studies. The participants were reported to be between 27 and 56.5 years old, with an overall mean age of 44.6; there were more men (41,829) than women (19,805). Age and gender were unreported for, respectively, 1941 and 705 participants (16 and 11 studies). The overall mean weight was 85.4 kg (range: 59 kg to 100.5 kg), and the overall mean Psoriasis Area and Severity Index (PASI) score at baseline was 20.4 (range: 9.5 to 39). The mean duration of psoriasis was 16.5 years (range 4.5 to 21.5).
Characteristics of the comparisons
Trials with two parallel arms (the different dose groups were grouped together in one 'arm')
Intervention versus placebo: 100 trials compared systemic treatments with placebo
-
Twenty‐six trials compared non‐biological systemic treatments versus placebo:
Acitretin (n = 10) (Goldfarb 1988; Gurel 2015; Lowe 1991; Olsen 1989; Ruzicka 1990; Saurat 1988; Sommerburg 1993; Tanew 1991; Yilmaz 2002; Ye 2020).
Fumaric acid esters (FAEs) (n = 4) (AFFIRM 2022; BRIDGE 2017; Nugteren‐Huying 1990; Van Bezooijen 2016).
Ciclosporin (n = 3) (Ellis 1991; Meffert 1997; Singh 2021).
Methotrexate (n = 9) (Al‐Hamamy 2014; Asawanonda 2006; Gottlieb 2012; Hunter 1963; Liu 2020; Mahajan 2010; METOP 2017; OPTIMAP 2022; Shehzad 2004).
-
Nine trials compared small molecule treatments versus placebo:
Apremilast (n = 7) (ESTEEM‐1 2015; ESTEEM‐2 2015; Morita 2022; Ohtsuki 2017; Papp 2012c; Papp 2013b; STYLE 2020).
Deucravacitinib (n = 2) (Papp 2018; POETYK PSO‐3 2022).
-
Sixty‐five trials compared biological treatments versus placebo:
-
Anti‐TNF alpha
Etanercept (n = 9) (Bachelez 2015; Bagel 2012; Gottlieb 2003a; Gottlieb 2011; Leonardi 2003; Papp 2005; Strober 2011; Tyring 2006; Van de Kerkhof 2008).
Adalimumab (n = 7) (Asahina 2010; Bissonnette 2013; Cai 2016; Elewski 2016; Gordon 2006; REVEAL 2008; VIP Trial 2018).
Infliximab (n = 6) (Chaudhari 2001; EXPRESS 2005; EXPRESS‐II 2007; Gottlieb 2004a; Torii 2010; Yang 2012).
Certolizumab (n = 4) (CIMPASI‐1 2018; CIMPASI‐2 2018; Reich 2012a; Umezawa 2021).
-
Anti‐IL12/23
Ustekinumab (n = 7) (Igarashi 2012; Krueger 2007; LOTUS 2013; PEARL 2011; PHOENIX‐1 2008; PHOENIX‐2 2008; VIP‐U Trial 2020).
-
Anti‐IL17
Secukinumab (n = 13) (ALLURE 2021; Cai 2020; ERASURE 2014; FEATURE 2015; JUNCTURE 2015; MATURE 2021; NCT03055494 ObePso‐S; NCT03535194 OASIS‐2; Papp 2013a; Reich 2015; Rich 2013; TRANSFIGURE 2016; VIP‐S trial 2020).
Ixekizumab (n = 3) (Leonardi 2012; NCT03364309; UNCOVER‐1 2016).
Brodalumab (n = 4) (AMAGINE‐1 2016; Nakagawa 2016; Papp 2012a; Seo 2020).
Bimekizumab (n = 2) (BE ABLE 1 2018; BE READY 2021).
Netakimab (n = 2) (NCT02762994; PLANETA 2021).
-
Anti‐IL23
Guselkumab (n = 2) (Ohtsuki 2018; ORION 2020).
Tildrakizumab (n = 2) (Papp 2015; ReSURFACE‐1 2017).
Risankizumab (n = 4) (Blauvelt 2021a; IMMhance 2020; IMMpress 2022; SustaIMM 2019).
-
Intervention versus active comparators: 57 trials compared systemic treatments with systemic treatments
Acitretin versus acitretin (n = 1) (Dogra 2013).
Acitretin versus ciclosporin (n = 1) (Akcali 2014).
Ciclosporin versus methotrexate (n = 4) (Flytström 2008; Heydendael 2003; Piskin 2003; Sandhu 2003).
Ciclosporin versus ciclosporin (n = 3) (Dubertret 1989; Engst 1994; Laburte 1994).
Methotrexate versus methotrexate (n = 2) (Chladek 2005; Dogra 2012).
Methotrexate versus FAEs (n = 1) (Fallah Arani 2011).
Methotrexate versus infliximab (n = 1) (Barker 2011).
Methotrexate versus apremilast (n = 1) (Rathipriyadharshini 2020).
Acitretin versus etanercept (n = 4) (Caproni 2009; Gisondi 2008; Lee 2016; Yu 2019).
FAEs versus secukinumab (n = 1) (PRIME 2017).
FAEs versus guselkumab (n = 1) (POLARIS 2020).
FAEs versus risankizumab (n = 1) (Thaci 2021).
FAEs versus brodalumab (n = 1) (CHANGE 2021).
Etanercept versus etanercept (n = 5) (EGALITY 2017; NCT02134210 RaPsOdy; Ortonne 2013; PRESTA 2010; PRISTINE 2013).
Etanercept versus infliximab (n = 1) (PIECE 2016).
Etanercept versus ustekinumab (n = 1) (ACCEPT 2010).
Adalimumab versus adalimumab (n = 10) (ADACCESS 2018; AURIEL‐PsO 2020; Cai 2022; CALYPSO 2018; Feldman 2021; NCT02581345; Papp 2017a; PsOsim 2017; VOLTAIRE‐PSO 2021; Yu 2022).
Secukinumab versus secukinumab (n = 3) (Augustin 2022; SCULPTURE 2015; SIGNATURE 2019).
Secukinumab versus ustekinumab (n = 2) (CLARITY 2018; CLEAR 2015).
Secukinumab versus guselkumab (n = 1) (ECLIPSE 2019).
Ixekizumab versus ixekizumab (n = 2) (IXORA‐P 2018; Khatri 2016).
Ixekizumab versus ustekinumab (n = 1) (IXORA‐S 2017).
Ixekizumab versus guselkumab (n = 1) (IXORA‐R 2020).
Ixekizumab versus secukinumab (n = 1) (AlMutairi 2021).
Ixekizumab versus adalimumab (n=1) (SPIRIT‐H2H 2020).
Risankizumab versus adalimumab (n = 1) (IMMvent 2019).
Risankizumab versus ustekinumab (n = 1) (Papp 2017b).
Risankizumab versus secukinumab (n = 1) (IMMerge 2021).
Risankizumab versus methotrexate (n=1) (Cestari 2021).
Bimekizumab versus secukinumab (n = 1) (BE RADIANT 2021).
Bimekizumab versus adalimumab (n = 1) (BE SURE 2021).
Trials with three parallel arms (the different dose groups were grouped together in one 'arm')
19 trials compared systemic treatments with systemic treatments and placebo
Methotrexate versus adalimumab versus placebo (n = 1) (CHAMPION 2008).
Etanercept versus ixekizumab versus placebo (n = 2) (UNCOVER‐2 2015; UNCOVER‐3 2015).
Etanercept versus secukinumab versus placebo (n = 1) (FIXTURE 2014).
Etanercept versus apremilast versus placebo (n = 1) (LIBERATE 2017).
Guselkumab versus adalimumab versus placebo (n = 3) (Gordon X‐PLORE 2015; VOYAGE‐1 2016; VOYAGE‐2 2017).
Brodalumab versus ustekinumab versus placebo (n = 2) (AMAGINE‐2 2015; AMAGINE‐3 2015).
Certolizumab versus etanercept versus placebo (n = 1) (CIMPACT 2018).
Tildrakizumab versus etanercept versus placebo (n = 1) (ReSURFACE‐2 2017).
Risankizumab versus ustekinumab versus placebo (n = 2) (UltIMMa‐1 2018; UltIMMa‐2 2018).
Adalimumab versus secukinumab versus placebo (n = 1) (CARIMA 2019).
Bimekizumab versus ustekinumab versus placebo (n = 1) (BE VIVID 2021).
Sonelokimab versus secukinumab versus placebo (n = 1) (Papp 2021).
Deucravacitinib versus apremilast versus placebo (n=2) (POETYK PSO‐1 2022; POETYK PSO‐2 2022).
Three trials compared three systemic treatments.
Apremilast versus etanercept versus ciclosporin (n = 1) (Ikonomidis 2022).
Ixekizumab versus methotrexate versus FAEs (n = 1) (Reich 2020).
Ustekinumab versus etanercept versus ciclosporin (n = 1) (Ikonomidis 2017).
In total, the dataset consisted of 179 studies, which provided information on 317 direct comparisons between 37 different drug dosages, 20 different drugs, six different drug classes, and placebo. For the sensitivity analyses, the different drug doses were divided into approved dosages versus other dosages:
methotrexate, taken orally, ≥ 15 or < 15 mg a week;
ciclosporin, taken orally, ≥ 3 or < 3 mg/kg a day;
acitretin, taken orally, ≥ 35 or < 35 mg a day;
apremilast, taken orally, 30 mg twice a day or other dosages;
deucravacitinib, taken orally, 6 mg once daily or other dosages;
etanercept, subcutaneous (SC), 25 mg twice a week or etanercept 50 mg twice a week;
infliximab, intravenous, 5 mg/kg at week 0, 2, and 4 then every 6 weeks, or other dosages;
adalimumab, SC, 80 mg at week 0, 40 mg at week 1 then 40 mg every other week or other dosages;
certolizumab, SC, 400 mg at week 0, 2, 4 then 400 mg every other week, or other dosages;
secukinumab, SC, 300 mg at week 0, 1, 2, 3, and 4 then every 4 weeks, or other dosages;
ixekizumab, SC, 160 mg at week 0 then 80 mg every other week until week 12 then 80 mg monthly, or other dosages;
brodalumab, SC, 210 mg at week 0, 1, 2, then every other week, or other dosages;
guselkumab, SC, 100 mg at week 0 and 4 then every 8 weeks, or other dosages;
tildrakizumab, SC, 100 mg at week 0 and 4 then every 12 weeks, or other dosages;
risankizumab, SC, 150 mg (2 x 75 mg injections) at week 0, week 4, and every 12 weeks thereafter, or other dosages;
bimekizumab, SC, 320 mg (2 x 160 mg injections) at week 0, 4, 8, 12, 16, and every 8 weeks thereafter, or other dosages.
FAEs (taken orally), ustekinumab (SC) 45 mg or 90 mg according to the weight, sonelokimab (SC), and netakimab (SC) were grouped in one dosage, whatever the dosages.
For each study, we provide details of the dosage in Characteristics of included studies.
Characteristics of the outcomes
For the efficacy outcomes during induction therapy (less than 24 weeks), out of the 179 trials, 146 reported PASI 90, 133 reported on Physician Global Assessment (PGA) 0/1, 1 reported PASI 75, and 79 trials reported assessment of change in quality of life. One hundred and three studies used the dermatology‐specific instrument Dermatology Life Quality Index (DLQI); nine studies used other specific skin instruments (Skindex, PSI, EQ‐5D5L, MGH‐SFQ, and PSS). For all of these studies, the investigators provided citations to reports indicating that the tools had been previously validated. For efficacy outcomes during maintenance phase (52 weeks), 19 trials reported PASI 90 at one year (BE RADIANT 2021; BE VIVID 2021; CLARITY 2018; CLEAR 2015; ECLIPSE 2019; IMMerge 2021; IXORA‐P 2018; IXORA‐S 2017; JUNCTURE 2015; NCT02134210 RaPsOdy; NCT03055494 ObePso‐S; Ohtsuki 2017; Ohtsuki 2018; OPTIMAP 2022; SPIRIT‐H2H 2020; SustaIMM 2019; UltIMMa‐1 2018; UltIMMa‐2 2018; VOYAGE‐1 2016), and 18 reported PASI 75 at one year (BE RADIANT 2021; Cai 2022; CLARITY 2018; CLEAR 2015; ECLIPSE 2019; IMMerge 2021; IXORA‐P 2018; IXORA‐S 2017; JUNCTURE 2015; NCT02134210 RaPsOdy; Ohtsuki 2017; Ohtsuki 2018; OPTIMAP 2022; SPIRIT‐H2H 2020; SustaIMM 2019; UltIMMa‐1 2018; UltIMMa‐2 2018; VOYAGE‐1 2016).
Out of 179 trials, 135 reported the number of participants with adverse events (different from the number of adverse events), and 147 reported the number of serious adverse events.
These outcomes were evaluated between 8 and 24 weeks: eight weeks (seven studies), 10 weeks (seven studies), 12 weeks (77 studies), 13 weeks (2 studies), 15 weeks (one study), 16 weeks (57 studies), 20 weeks (one study), 24 weeks (19 studies), and 26 weeks (three studies). Timing of assessment was unknown or not clearly defined for four studies (Engst 1994; Hunter 1963; Saurat 1988; Shehzad 2004); one study had only a timing of assessment at 52 weeks (IXORA‐P 2018).
Funding
In all, 149 studies declared a source of funding: 138 studies declared pharmaceutical company funding, 11 studies declared unique institutional funding (Chladek 2005; Flytström 2008; Heydendael 2003; Ikonomidis 2017; Liu 2020; OPTIMAP 2022; PIECE 2016; Reich 2020; VIP Trial 2018; VIP‐U Trial 2020; Yu 2019), six studies had no funding source (Akcali 2014; AlMutairi 2021; Asawanonda 2006; Fallah Arani 2011; Gurel 2015; Singh 2021), and 24 studies did not report the source of funding (Al‐Hamamy 2014; Caproni 2009; Dogra 2012; Dogra 2013; Dubertret 1989; Engst 1994; Gisondi 2008; Goldfarb 1988; Hunter 1963; Laburte 1994; Mahajan 2010; Meffert 1997; Nugteren‐Huying 1990; Piskin 2003; Rathipriyadharshini 2020; Ruzicka 1990; Sandhu 2003; Saurat 1988; Shehzad 2004; Sommerburg 1993; Torii 2010; Yang 2012; Ye 2020; Yilmaz 2002).
Excluded studies
We have excluded a total of 466 studies in 475 references throughout the course of this review. We detailed all the reasons for exclusion in the Characteristics of excluded studies and our study flow diagram (Figure 2).
For this update:
We excluded 23 studies (reported in 24 references). The reasons for exclusion were: in 14 studies, the participants did not present with moderate‐to‐severe psoriasis, one study was not a randomised trial, two studies were phase I trials, four studies had a wrong intervention, and two studies had a wrong comparator.
From the previous reviews:
We had excluded 443 full‐text reports from the previous review. The main reasons for exclusion were that the participants did not present with moderate‐to‐severe psoriasis (n = 46), or that another intervention was assessed (n = 117). We excluded 49 reports because they were not trials, three did not include plaque‐type psoriasis, 37 were open‐label extension studies restricted to good responders, and we excluded 191 for other reasons.
In an earlier version of this review (Sbidian 2017), we excluded a number of studies having reviewed the full text, but without creating Characteristics of excluded studies tables (n = 203). The main reason for exclusion of these studies was that the participants did not present with moderate‐to‐severe psoriasis.
In this update, for 10 studies with three arms, one arm was not included, as the intervention was not included in our search:
Saurat 1988: acitretin versus placebo versus etretinate (etretinate arm was not included);
Shehzad 2004: PUVA (psoralen and ultraviolet A) therapy versus methotrexate (methotrexate only was included);
Gottlieb 2011; Strober 2011: briakinumab versus etanercept versus placebo (briakinumab arm was not included);
Gisondi 2008: etanercept versus acitretin versus etanercept plus acitretin (etanercept plus acitretin arm was not included);
Al‐Hamamy 2014: narrowband ultraviolet B phototherapy plus methotrexate versus narrowband ultraviolet B alone and methotrexate alone (arm with methotrexate alone was not included);
VIP Trial 2018: adalimumab versus narrowband ultraviolet B phototherapy versus placebo (arm with narrowband ultraviolet B phototherapy was not included);
Lee 2016: etanercept versus acitretin versus etanercept plus acitretin (arm with etanercept plus acitretin was not included);
Bachelez 2015: tofacitinib versus etanercept versus placebo (tofacitinib arm was not included);
NCT03535194 OASIS‐2: mirikizumab versus placebo versus secukinumab (mirikizumab arm was not included).
Thaçi 2002 compared two different dosages of ciclosporin (a fixed dosage of 200 mg/day and a dosage corresponding to 2.5 mg/kg/day), and we were unable to classify the fixed dosage group either in the ciclosporin ≥ 3 mg/kg/day group or in the ciclosporin < 3 mg/day group for the subgroup meta‐analysis.
Studies awaiting classification
We classified 23 trials reported in 30 references as studies awaiting classification. More details are available in Studies awaiting classification and Table 2. Most of the awaiting studies compared a biological treatment versus another biological treatment or versus non‐biological treatment or versus placebo (n = 13). Two studies assessed a small molecule, and eight assessed non‐biological systemic treatments. Among the 23 trials, eight trials were classified as unpublished (CTRI/2016/10/007345; DRKS00000716; EUCTR2010‐020168‐39‐DE; NCT01088165; NCT01558310; NCT02701205; NCT02714322; NCT02829424).
Ongoing studies
We classified 45 trials (reported in 60 references) as ongoing studies. More details are available in the Characteristics of ongoing studies and Table 2. Most of the ongoing studies compared a biological treatment versus another biological treatment or versus non‐biological treatment or versus placebo (n = 36). Four ongoing studies assessed apremilast or oral tyrosine kinase 2 (TYK2) inhibitor or phosphodiesterase type 4 (PDE4) inhibitor.
Risk of bias in included studies
Figure 3 and Figure 4 summarise the risk of bias assessments. For overall risk of bias across studies, 90 (50%) trials were at low risk of bias. We categorised a third of the studies (65/179, 36%) as being at high risk of bias. We categorised the remaining 24 studies as being at unclear risk of bias. Further details of these assessments are available in the risk of bias table corresponding to each trial in the Characteristics of included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
4.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
In 62 trials, the method of sequence generation was not described at all, or was at best unclear. The remaining studies (n = 117) described the method used to generate the allocation sequence in sufficient detail, and we therefore judged this domain as low risk of bias for these studies. For allocation concealment, most studies (n = 105) received a judgement of low risk of bias. We considered the risk unclear for 73 trials because of the absence of reporting of the method used to guarantee concealment, and one had high risk of bias (Ikonomidis 2022).
Blinding
Blinding of participants and personnel was achieved in 105 studies, whereas 51 studies were at high risk of performance bias. The remaining 23 studies were at unclear risk of performance bias. Blinding of outcome assessment was reported clearly in only 110 of the 179 included studies, whereas 32 studies were at high risk of detection bias. The risk of detection bias was unclear in the remaining 37 studies.
Incomplete outcome data
In more than two‐thirds of the trials (121/179), incomplete outcome data appeared to have been adequately addressed, and any missing outcome data were reasonably well‐balanced across intervention groups, with similar reasons for missing data across the groups. However, in 12 studies the reporting of missing outcome data was largely inadequate because of one or more of the following reasons: the high number of withdrawn participants, an imbalance between groups in the number of withdrawn participants, an imbalance in reasons for missing outcomes, or no intention‐to‐treat (ITT) analysis provided. In 46 studies, this domain was at unclear risk of bias because the following were not reported: the number of participants, reasons for discontinuation, or missing data imputation.
Selective reporting
We considered 14 trials to be at high risk of selective outcome reporting because results for outcomes detailed in the Methods section were not reported in the Results section (Akcali 2014; AMAGINE‐2 2015; AMAGINE‐3 2015; BRIDGE 2017; CARIMA 2019; Engst 1994; Hunter 1963; LIBERATE 2017; Nakagawa 2016; Papp 2005; Papp 2013b; PsOsim 2017; Shehzad 2004; VIP‐U Trial 2020). In all, we considered 116 studies to be at low risk of bias for this domain, as outcome details in the trial register and in the Methods section were reported in the Results section. For the other trials (n = 49), we considered the risk of bias as unclear, because we did not find these trials in any register.
Other potential sources of bias
As detailed in the Methods section, we did not address the 'Other risk of bias' item as we did not highlight particular circumstances leading to other risk of bias from particular trial designs, contamination between the experimental and control groups, and particular clinical settings.
Effects of interventions
Nine trials provided no usable or retrievable data and did not contribute further to the results of this review (Akcali 2014; Chladek 2005; Engst 1994; Ikonomidis 2017; Lowe 1991; Olsen 1989; Piskin 2003; Rathipriyadharshini 2020; Shehzad 2004; see Table 2). The main reason we could not use their data was that these studies addressed none of our outcomes.
Twenty studies, involving 2058 participants (3.3% of the participants in this review), had a co‐intervention and did not contribute further to the results of this review, as we could not assess the specific intervention effect (Al‐Hamamy 2014; Asawanonda 2006; Bissonnette 2013; Gottlieb 2012; Gurel 2015; Liu 2020; Lowe 1991; Mahajan 2010; Morita 2022; OPTIMAP 2022; Ruzicka 1990; Saurat 1988; Shehzad 2004; Singh 2021; Sommerburg 1993; Tanew 1991; Van Bezooijen 2016; Ye 2020; Yilmaz 2002; Yu 2019).
Twelve trials assessed biosimilars versus original drugs for adalimumab (ADACCESS 2018; AURIEL‐PsO 2020; Cai 2022; CALYPSO 2018; Feldman 2021; NCT02581345; Papp 2017a; PsOsim 2017; VOLTAIRE‐PSO 2021; Yu 2022) and etanercept (EGALITY 2017; NCT02134210 RaPsOdy). These were non‐inferiority trials, assessing the same dosage and same administration schema of biosimilar and original drug.
Lowe 1991 and Shehzad 2004 had two reasons for not being included in the network meta‐analysis (both no usable data and co‐interventions). Thus, in total, 39 studies, involving 7524 participants, were not included in the classical or network meta‐analysis (reasons are mentioned above). The interventions of the 39 studies were the following:
acitretin (n = 10) (Akcali 2014; Gurel 2015; Lowe 1991; Olsen 1989; Ruzicka 1990; Saurat 1988; Sommerburg 1993; Tanew 1991; Ye 2020; Yilmaz 2002);
methotrexate (n = 7) (Asawanonda 2006; Al‐Hamamy 2014; Chladek 2005; Gottlieb 2012; Liu 2020; Mahajan 2010; Shehzad 2004);
ciclosporin (n = 3) (Engst 1994; Piskin 2003; Singh 2021);
adalimumab (n = 11) (ADACCESS 2018; AURIEL‐PsO 2020; Bissonnette 2013; Cai 2022; CALYPSO 2018; Feldman 2021; NCT02581345; Papp 2017a; PsOsim 2017; VOLTAIRE‐PSO 2021; Yu 2022);
etanercept (n = 3) (EGALITY 2017; NCT02134210 RaPsOdy; Yu 2019);
others (n = 5) (Ikonomidis 2017; Morita 2022; OPTIMAP 2022; Rathipriyadharshini 2020; Van Bezooijen 2016).
We included a total of 140 studies, involving 54,815 participants (88% participants of this review), in the classical or network meta‐analysis for at least one of the outcomes. We used the total number of studies and participants as a denominator to calculate the proportion of trials and participants used for the quantitative synthesis of each outcome.
One study had only long‐term outcome assessments (IXORA‐P 2018).
Ten studies among the 140, involving 2132 participants (3.4% of the participants in this review), included biological‐naïve participants when assessing the benefit of a biological agent, and did not contribute further to the results of the main analysis, as we could not assume the plausibility of transitivity. Indeed, response to biologics is different depending on treatment status (systemic‐naïve or not). However, these studies were included in the sensitivity analysis (Barker 2011; Caproni 2009; CHAMPION 2008; CHANGE 2021; Gisondi 2008; Lee 2016; POLARIS 2020; PRIME 2017; Reich 2020; Thaci 2021).
Figure 5 and Figure 6 show the network diagrams for all of the outcomes included in the review. The size of the nodes is proportional to the total number of participants allocated to each class‐level (Figure 5) and drug‐level (Figure 6) intervention, with the thickness of the lines proportional to the number of trials evaluating each direct comparison.
5.
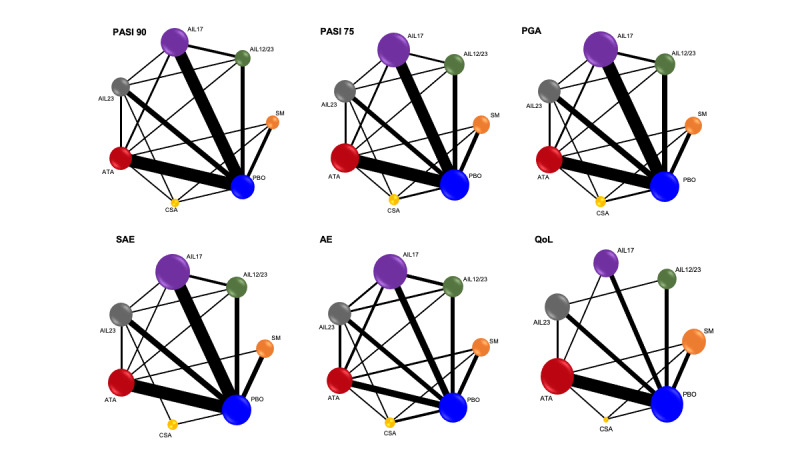
Network plot for all the outcomes at class level
The size of the nodes is proportional to the total number of participants allocated to each intervention and the thickness of the lines proportional to the number of studies evaluating each direct comparison.
AIL12/23: anti‐IL12/23; AIL17: anti‐IL17; AIL23: anti‐IL23, ATA: anti‐TNF alpha; CSA: non‐biological conventional systemic agents; PBO: placebo; SM: small molecules
AE: adverse events; PASI: Psoriasis Area and Severity Index; PGA: Physician Global Assessment; QoL: quality of life; SAE: serious adverse events
6.
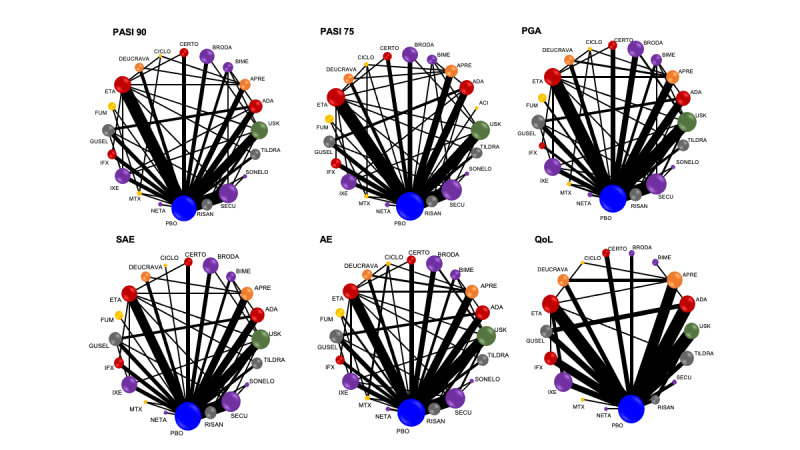
Network plot for all the outcomes at drug level
The size of the nodes is proportional to the total number of participants allocated to each intervention and the thickness of the lines proportional to the number of studies evaluating each direct comparison.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
AE: adverse events; PASI: Psoriasis Area and Severity Index; PGA: Physician Global Assessment; QoL: quality of life; SAE: serious adverse events
Figure 7 shows the network meta‐analysis estimates of all of the outcomes for each comparison at class level.
7.
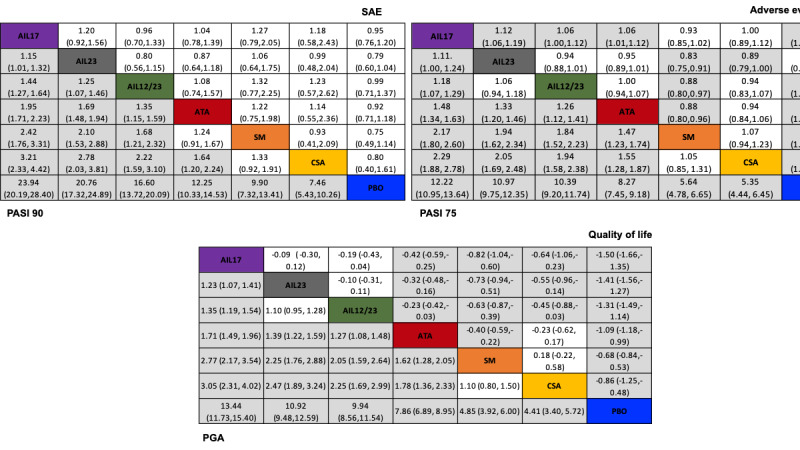
Relative effects of the class‐level intervention as estimated from the network meta‐analysis model
Outcomes were all measured at the induction phase (assessment from 8 to 24 weeks after randomisation). Drugs are reported in order of primary benefit ranking. Each cell contains the risk ratio (RR) (for dichotomous outcomes: PASI 90, serious adverse events, PASI 75, PGA 0/1, adverse events) or the standardised mean difference (SMD) (for the quality of life outcome), plus the 95% confidence interval, of the class level in the respective column versus the class level in the respective row. RRs larger than 1 for the lower triangle and smaller than 1 (or SMDs smaller than zero) for the upper triangle favour the treatment on the left. Significant results are highlighted in grey.
AE: adverse events; PASI: Psoriasis Area and Severity Index; PGA: Physician's Global Assessment; QoL: quality of life; SAE: serious adverse events; SAE without worsening of psoriasis correspond to SAE after exclusion of flares of psoriasis; AIL12/23: anti‐IL12/23; AIL17: anti‐IL17; AIL23: anti‐IL23, ATA: anti‐TNF alpha; CSA: non‐biological conventional systemic agents; PBO: placebo; SM: small molecules
Figure 1, Figure 8, and Figure 9 show the network meta‐analysis estimates of all the outcomes for each comparison at drug level.
8.
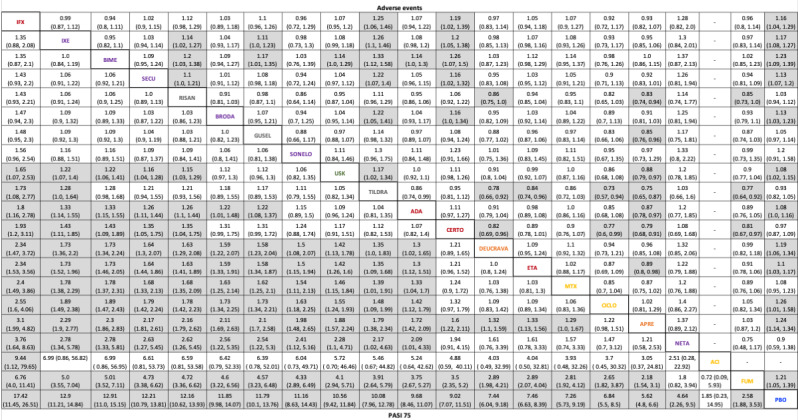
Relative effects of the intervention as estimated from the network meta‐analysis model for Psoriasis Area and Severity Index (PASI) 75 and adverse events (AEs)
Outcomes were all measured at the induction phase (assessment from 8 to 24 weeks after randomisation). Drugs are reported in order of primary benefit ranking. Each cell contains the risk ratio (RR) and 95% confidence interval for the two primary outcomes (PASI 75 and AEs) of the intervention in the respective column versus the comparator in the respective row. RRs larger than 1 for the lower triangle and smaller than 1 for the upper triangle favour the treatment on the left. Significant results are highlighted in grey.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
9.
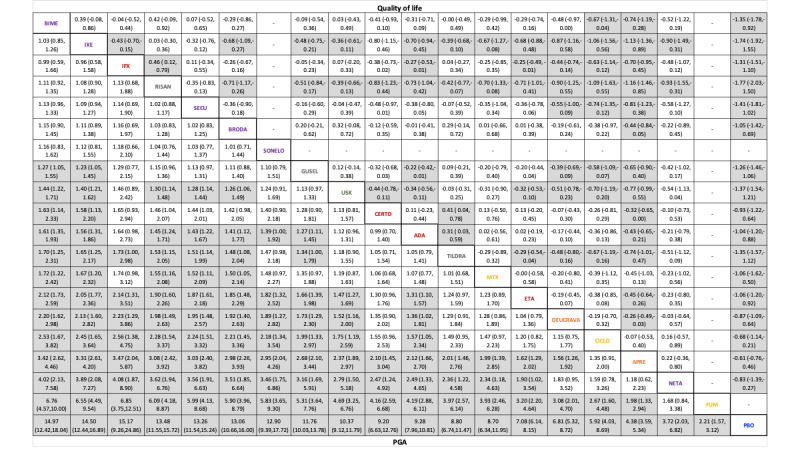
Relative effects of the intervention as estimated from the network meta‐analysis model for Physician's Global Assessment (PGA 0/1) and quality of life (QoL)
Outcomes were all measured at the induction phase (assessment from 8 to 24 weeks after randomisation). Drugs are reported in order of primary benefit ranking. Each cell contains the risk ratio (RR) and 95% confidence interval (PGA 0/1) or standardised mean difference (quality of life) of the intervention in the respective column versus the comparator in the respective row. RRs larger than 1 for the lower triangle and smaller than 1 (or SMDs smaller than zero) for the upper triangle favour the treatment on the left. Significant results are highlighted in grey.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
Figure 10 and Figure 11 show all of the relative effects from the network meta‐analyses against placebo with their 95% confidence and prediction intervals at class and drug level.
10.
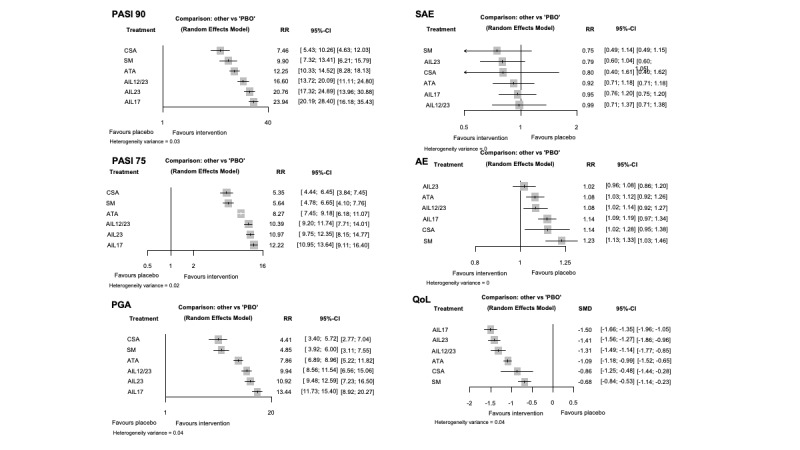
Interval plot. Network meta‐analysis estimates of class‐level versus placebo for all the outcomes
Outcomes were all measured at the induction phase (assessment from 8 to 24 weeks after randomisation).
AE: adverse events; AIL12/23: anti‐IL12/23; AIL17: anti‐IL17; AIL23: anti‐IL23, ATA: anti‐TNF alpha; CI: confidence interval; CSA: non‐biological conventional systemic agents; PGA: Physician Global Assessment; PrI: predictive interval; PBO: placebo; QoL: specific quality of life scale; RR: risk ratio; SAE: serious adverse events; SM: small molecules; SMD: standardised mean difference
11.
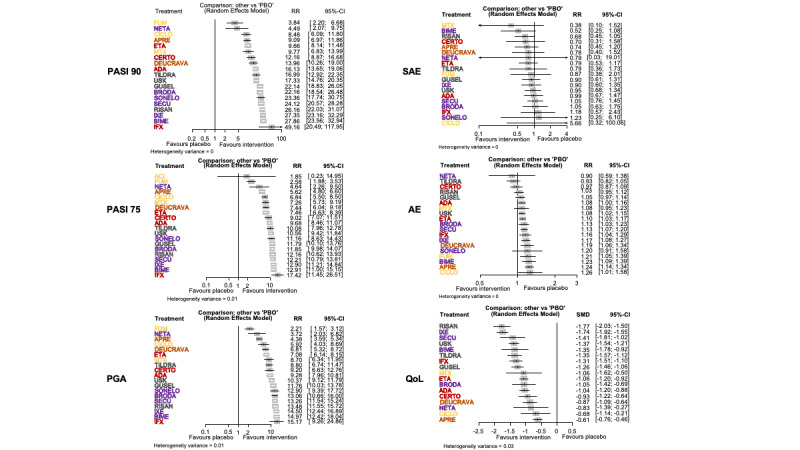
Interval plot. Network meta‐analysis estimates of the interventions versus placebo for the primary outcomes
CI: confidence interval; PrI: predictive interval; RR: risk ratio; SAE: serious adverse events; PGA: Physician Global Assessment; QoL: specific quality of life scale; SMD: standardised mean difference
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
Figure 12 shows a two‐dimensional ranking plot based on surface under the cumulative ranking curve (SUCRA) values for benefit (PASI 90) and acceptability (serious adverse events) at class and drug level. The different colours represent different groups of interventions considering their performance on both outcomes simultaneously. Interventions belonging to the same group were assumed to have a similar performance when the two primary outcomes were considered jointly (Chaimani 2013).
12.
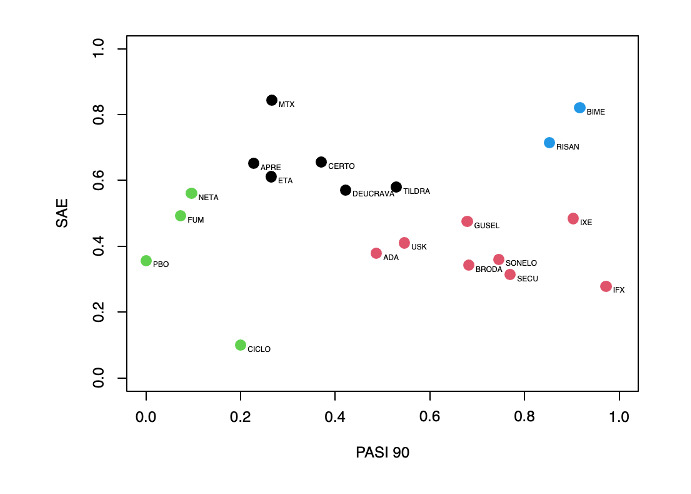
Ranking plot. Ranking plot representing simultaneously the efficacy (x‐axis, PASI 90) and the acceptability (y‐axis, serious adverse events) of all the interventions (drug levels) for patients with moderate‐to‐severe psoriasis. Optimal treatment should be characterised by both high efficacy and acceptability and should be in the right upper corner of this graph.
Outcomes were measured at the induction phase (assessment from 8 to 24 weeks after randomisation).
The different colours represent different groups of interventions considering their performance on both outcomes simultaneously. Interventions belonging to the same group are assumed to have a similar performance when the two primary outcomes are considered jointly.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
PASI: Psoriasis Area and Severity Index; SAE: serious adverse events; SUCRA: surface under the cumulative ranking curve
Figure 13 and Figure 14 show the ranking for all the outcomes at class and drug level, respectively.
13.
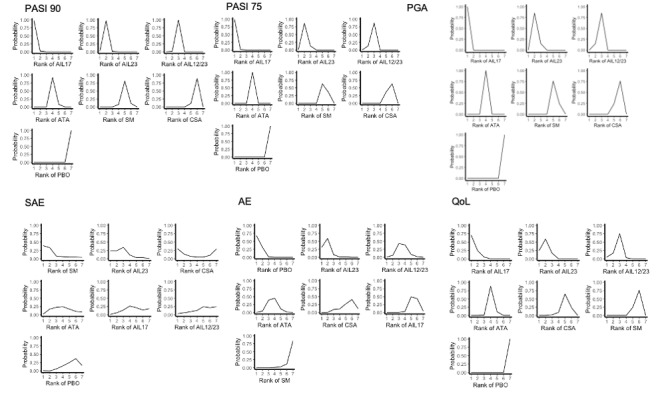
Ranking for all the outcomes at class level
AIL12/23: anti‐IL12/23; AIL17: anti‐IL17; AIL23: anti‐IL23, ATA: anti‐TNF alpha; CSA: non‐biological conventional systemic agents; PBO: placebo; SM: small molecules
AE: adverse events; PASI: Psoriasis Area and Severity Index; PGA: Physician Global Assessment; QoL: quality of life; SAE: serious adverse events
14.
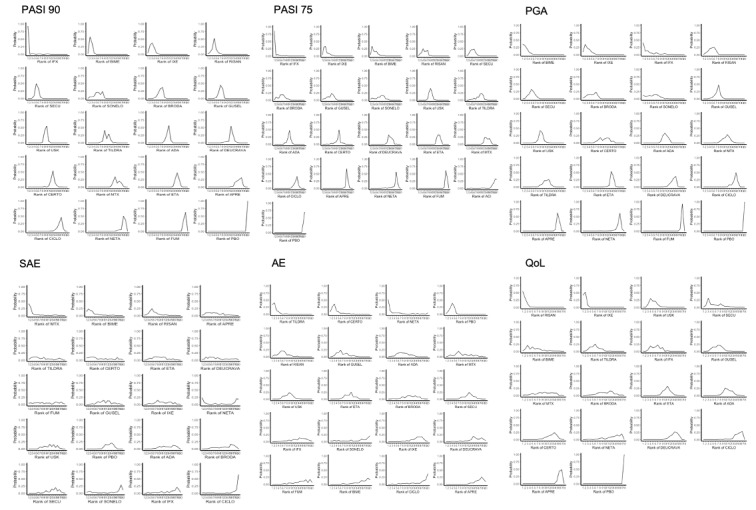
Ranking for all the outcomes at drug level
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
AE: adverse events; PASI: Psoriasis Area and Severity Index; PGA: Physician Global Assessment; QoL: quality of life; SAE: serious adverse events
Since this review does not include summary of findings (SoF) tables, we presentFigure 1instead.Figure 1includes all comparison results for the two main outcomes, but also absolute effects and assessment of the certainty of evidence using CINeMA.
1. Primary outcomes
1.1 The proportion of participants who achieved clear or almost clear skin, e.g. PASI 90
DIRECT EVIDENCE
We report treatment estimates for pairwise meta‐analyses at class and drug level in Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.10; and Analysis 1.9, respectively.
1.1. Analysis.

Comparison 1: Primary outcome ‐ PASI 90, Outcome 1: Non‐biological treatments versus placebo
1.2. Analysis.

Comparison 1: Primary outcome ‐ PASI 90, Outcome 2: Non‐biological treatment 1 versus non‐biological treatment 2
1.3. Analysis.

Comparison 1: Primary outcome ‐ PASI 90, Outcome 3: Anti‐TNF alpha versus placebo
1.4. Analysis.

Comparison 1: Primary outcome ‐ PASI 90, Outcome 4: Anti‐IL12/23 versus placebo
1.5. Analysis.

Comparison 1: Primary outcome ‐ PASI 90, Outcome 5: Anti‐IL17 versus placebo
1.6. Analysis.

Comparison 1: Primary outcome ‐ PASI 90, Outcome 6: Anti‐IL23 versus placebo
1.7. Analysis.

Comparison 1: Primary outcome ‐ PASI 90, Outcome 7: Biologic versus non‐biological treatments
1.8. Analysis.

Comparison 1: Primary outcome ‐ PASI 90, Outcome 8: Biologic 1 versus biologic 2
1.10. Analysis.

Comparison 1: Primary outcome ‐ PASI 90, Outcome 10: Small molecules versus placebo
1.9. Analysis.

Comparison 1: Primary outcome ‐ PASI 90, Outcome 9: Biologic versus small molecules
In terms of reaching PASI 90, anti‐IL17 treatments (secukinumab, ixekizumab, brodalumab, bimekizumab, and sonelokimab) were more effective than placebo (risk ratio at class level (RR) 27.51, 95% confidence interval (CI) 19.19 to 39.46). No significant difference was observed between netakimab and placebo (RR 10.98, 95% CI 0.42 to 288.83). These findings were also confirmed for anti‐IL23 (guselkumab, tildrakizumab, and risankizumab) (class‐level RR 19.96, 95% CI 13.51 to 29.49); anti‐IL12/23 (ustekinumab) (RR 18.37, 95% CI 12.56 to 26.85); anti‐TNF alpha (infliximab, etanercept, adalimumab, and certolizumab) (class‐level RR 13.61, 95% CI 10.65 to 17.41); and small molecules (apremilast, and oral tyrosine kinase 2 (TYK2) inhibitor) (class‐level RR 7.57, 95% CI 5.46 to 10.50). Infliximab, adalimumab, ixekizumab, and risankizumab were more effective than methotrexate (respectively: RR 2.86, 95% CI 2.15 to 3.80; RR 3.73, 95% CI 2.25 to 6.19; RR 2.05, 95% CI 1.43 to 2.94; and RR 2.37, 95% CI 1.59 to 3.54). Secukinumab, ixekizumab, guselkumab, risankizumab, and brodalumab were more effective than FAEs (respectively: RR 8.31, 95% CI 4.23 to 16.35; RR 8.60, 95% CI 3.69 to 20.04; RR 6.02, 95% CI 3.13 to 11.60; RR 8.33, 95% CI 3.87 to 17.95; and RR 3.00, 95% CI 2.04 to 4.42). Ustekinumab, secukinumab, infliximab, ixekizumab, and tildrakizumab were more effective than etanercept. Secukinumab, ixekizumab, brodalumab, risankizumab, and bimekizumab were more effective than ustekinumab. Guselkumab, risankizumab, and bimekizumab were more effective than adalimumab. Secukinumab and ixekizumab were more effective than guselkumab and bimekizumab was more effective than secukinumab. No significant difference was observed between risankizumab and secukinumab, between sonelokimab and secukinumab, between certolizumab and etanercept, or between etanercept and apremilast for this outcome (reaching PASI 90).
NETWORK META‐ANALYSES
The PASI 90 outcome was available in 124 trials, involving 51,034 participants (93% of the participants in the meta‐analysis). For two trials (Nugteren‐Huying 1990; Sandhu 2003), the number of randomised participants was not available, but we added these trials in the complete‐case sensitivity analyses. This outcome was reported in seven trials out of 124 (Augustin 2022; Dogra 2012; Dogra 2013; Khatri 2016; PRISTINE 2013; SCULPTURE 2015; SIGNATURE 2019), comparing different dosages of the same drug in each case. We added these trials to the sensitivity analysis at dose level. This outcome was reported in nine trials out of 124 with biological‐naïve participants that were added to the sensitivity analysis for all trials, whatever previous treatments received by the participants (Barker 2011; Caproni 2009; CHAMPION 2008; CHANGE 2021; Gisondi 2008; POLARIS 2020; PRIME 2017; Reich 2020; Thaci 2021).
Seventy‐two trials, involving 23,817 participants, were placebo‐controlled trials; 34 studies, involving 11,774 participants, were head‐to‐head comparisons; and 18 studies, involving 15,443 participants, had both a placebo and at least two active treatments arms.
PASI 90 was not reported for the remaining 16 trials including IXORA‐P 2018 (only long‐term assessment outcomes), and we were not able to obtain missing information from the trial authors (Table 2).
See Figure 5; Figure 6; Figure 7; Figure 1; Figure 10; Figure 11; Figure 13; Figure 14.
Table 3 summarises the main results of both the direct and indirect evidence and the network meta‐analysis for PASI 90. The summary relative effects from the network meta‐analysis are presented in league tables for both class‐level (Figure 7) and drug‐level (Figure 1) analyses.
3. Direct and indirect evidence and network meta‐analysis results summary table for PASI 90.
| Network meta‐analysis | Direct evidence | Indirect evidence | Separate indirect from direct evidence | |||||
| Comparisons* | RR | 95% CI | RR | 95% CI | RR | 95% CI | z testa | P value |
| Adalimumab versus bimekizumab | 0.58 | (0.53 to 0.63) | 0.6 | (0.51 to 0.71) | 0.57 | (0.51 to 0.63) | 0.61 | 0.54 |
| Adalimumab versus guselkumab | 0.73 | (0.68 to 0.78) | 0.69 | (0.63 to 0.76) | 0.8 | (0.71 to 0.91) | ‐1.98 | 0.05 |
| Adalimumab versus ixekizumab | 0.59 | (0.54 to 0.65) | 0.7 | (0.54 to 0.91) | 0.57 | (0.52 to 0.64) | 1.42 | 0.16 |
| Adalimumab versus placebo | 16.13 | (13.65 to 19.06) | 15.31 | (10.83 to 21.64) | 16.39 | (13.55 to 19.82) | ‐0.34 | 0.74 |
| Adalimumab versus risankizumab | 0.62 | (0.56 to 0.68) | 0.65 | (0.57 to 0.75) | 0.58 | (0.51 to 0.67) | 1.18 | 0.24 |
| Apremilast versus ciclosporin | 1.07 | (0.79 to 1.46) | 1.23 | (0.88 to 1.73) | 0.56 | (0.27 to 1.16) | 1.92 | 0.05 |
| Apremilast versus deucravacitinib | 0.65 | (0.53 to 0.81) | 0.62 | (0.49 to 0.77) | 1.12 | (0.56 to 2.26) | ‐1.59 | 0.11 |
| Apremilast versus etanercept | 0.94 | (0.74 to 1.20) | 1.04 | (0.78 to 1.40) | 0.73 | (0.46 to 1.15) | 1.32 | 0.19 |
| Apremilast versus placebo | 9.09 | (6.97 to 11.86) | 6.04 | (3.89 to 9.36) | 11.52 | (8.25 to 16.09) | ‐2.3 | 0.02 |
| Bimekizumab versus placebo | 27.86 | (23.56 to 32.94) | 25.52 | (11.24 to 57.96) | 27.97 | (23.57 to 33.18) | ‐0.21 | 0.83 |
| Bimekizumab versus secukinumab | 1.15 | (1.08 to 1.23) | 1.15 | (1.07 to 1.24) | 1.17 | (1.03 to 1.32) | ‐0.2 | 0.84 |
| Bimekizumab versus ustekinumab | 1.61 | (1.49 to 1.74) | 1.71 | (1.46 to 2.01) | 1.58 | (1.44 to 1.72) | 0.87 | 0.38 |
| Brodalumab versus placebo | 22.16 | (18.54 to 26.48) | 26.32 | (16.76 to 41.32) | 21.46 | (17.67 to 26.06) | 0.81 | 0.42 |
| Brodalumab versus ustekinumab | 1.28 | (1.17 to 1.40) | 1.27 | (1.16 to 1.39) | 1.62 | (0.95 to 2.77) | ‐0.87 | 0.38 |
| Certolizumab versus etanercept | 1.26 | (0.95 to 1.66) | 1.2 | (0.90 to 1.60) | 2.06 | (0.83 to 5.12) | ‐1.12 | 0.26 |
| Certolizumab versus placebo | 12.16 | (8.87 to 16.68) | 19.73 | (8.28 to 47.02) | 11.3 | (8.05 to 15.86) | 1.17 | 0.24 |
| Ciclosporin versus etanercept | 0.88 | (0.65 to 1.19) | 0.93 | (0.65 to 1.33) | 0.77 | (0.44 to 1.34) | 0.56 | 0.57 |
| Ciclosporin versus methotrexate | 0.87 | (0.60 to 1.25) | 1.01 | (0.61 to 1.68) | 0.73 | (0.43 to 1.25) | 0.85 | 0.4 |
| Deucravacitinib versus placebo | 13.96 | (10.26 to 19.00) | 10.04 | (6.15 to 16.40) | 17.3 | (11.65 to 25.69) | ‐1.69 | 0.09 |
| Etanercept versus infliximab | 0.2 | (0.08 to 0.48) | 0.11 | (0.02 to 0.78) | 0.23 | (0.08 to 0.61) | ‐0.66 | 0.51 |
| Etanercept versus ixekizumab | 0.35 | (0.32 to 0.39) | 0.34 | (0.30 to 0.39) | 0.37 | (0.31 to 0.43) | ‐0.64 | 0.52 |
| Etanercept versus placebo | 9.66 | (8.14 to 11.48) | 11.68 | (8.17 to 16.71) | 9.13 | (7.51 to 11.11) | 1.18 | 0.24 |
| Etanercept versus secukinumab | 0.4 | (0.36 to 0.45) | 0.43 | (0.34 to 0.54) | 0.39 | (0.35 to 0.44) | 0.71 | 0.48 |
| Etanercept versus tildrakizumab | 0.57 | (0.45 to 0.71) | 0.57 | (0.45 to 0.72) | 0.58 | (0.26 to 1.28) | ‐0.04 | 0.97 |
| Etanercept versus ustekinumab | 0.56 | (0.50 to 0.62) | 0.55 | (0.45 to 0.69) | 0.56 | (0.49 to 0.64) | ‐0.05 | 0.96 |
| FAEs versus methotrexate | 0.39 | (0.21 to 0.75) | 0.5 | (0.05 to 5.22) | 0.38 | (0.20 to 0.76) | 0.21 | 0.83 |
| FAEs versus placebo | 3.84 | (2.20 to 6.68) | 3.78 | (2.14 to 6.69) | 4.91 | (0.46 to 52.80) | ‐0.21 | 0.83 |
| Guselkumab versus ixekizumab | 0.81 | (0.75 to 0.87) | 0.77 | (0.70 to 0.85) | 0.88 | (0.78 to 1.00) | ‐1.65 | 0.1 |
| Guselkumab versus placebo | 22.14 | (18.83 to 26.05) | 27.76 | (16.21 to 47.55) | 21.65 | (18.26 to 25.67) | 0.86 | 0.39 |
| Guselkumab versus secukinumab | 0.92 | (0.86 to 0.98) | 0.91 | (0.84 to 0.98) | 0.94 | (0.84 to 1.05) | ‐0.5 | 0.62 |
| Infliximab versus placebo | 49.16 | (20.49 to 117.95) | 42.55 | (16.05 to 112.85) | 89.31 | (12.29 to 649.21) | ‐0.66 | 0.51 |
| Ixekizumab versus placebo | 27.35 | (23.16 to 32.29) | 37.65 | (21.25 to 66.73) | 26.55 | (22.32 to 31.59) | 1.14 | 0.25 |
| Ixekizumab versus ustekinumab | 1.58 | (1.45 to 1.72) | 1.41 | (1.21 to 1.63) | 1.67 | (1.51 to 1.86) | ‐1.87 | 0.06 |
| Methotrexate versus placebo | 9.77 | (6.83 to 13.99) | 5.81 | (0.72 to 46.53) | 9.93 | (6.90 to 14.29) | ‐0.5 | 0.62 |
| Methotrexate versus risankizumab | 0.37 | (0.27 to 0.52) | 0.42 | (0.28 to 0.63) | 0.29 | (0.16 to 0.52) | 1.05 | 0.3 |
| Risankizumab versus placebo | 26.16 | (22.03 to 31.07) | 15.86 | (9.53 to 26.37) | 27.91 | (23.25 to 33.50) | ‐2.05 | 0.04 |
| Secukinumab versus placebo | 24.12 | (20.57 to 28.28) | 22.73 | (15.60 to 33.11) | 24.43 | (20.50 to 29.12) | ‐0.34 | 0.73 |
| Sonelokimab versus placebo | 23.36 | (17.74 to 30.75) | 65.22 | (4.13 to 1031.03) | 23.12 | (17.53 to 30.48) | 0.73 | 0.46 |
| Tildrakizumab versus placebo | 16.99 | (12.92 to 22.35) | 17.25 | (8.26 to 36.02) | 16.95 | (12.61 to 22.77) | 0.04 | 0.97 |
| Ustekinumab versus placebo | 17.33 | (14.76 to 20.35) | 17.86 | (12.97 to 24.60) | 17.16 | (14.25 to 20.66) | 0.21 | 0.83 |
| Risankizumab versus secukinumab | 1.08 | (0.99 to 1.19) | 1.12 | (0.97 to 1.30) | 1.06 | (0.95 to 1.19) | 0.62 | 0.54 |
| Risankizumab versus ustekinumab | 1.51 | (1.38 to 1.66) | 1.65 | (1.42 to 1.92) | 1.43 | (1.27 to 1.60) | 1.52 | 0.13 |
| Secukinumab versus sonelokimab | 1.03 | (0.82 to 1.29) | 1.04 | (0.83 to 1.30) | 0.27 | (0.01 to 9.57) | 0.73 | 0.46 |
| Secukinumab versus ustekinumab | 1.39 | (1.31 to 1.47) | 1.4 | (1.30 to 1.50) | 1.38 | (1.25 to 1.52) | 0.23 | 0.82 |
FAEs: fumaric acid esters; RR: risk ratio; 95% CI: 95% confidence interval
*The comparisons listed in this table were included in at least one direct‐evidence analysis.
az‐value of test for disagreement (direct versus indirect); P value of test for disagreement (direct versus indirect).
All of the interventions appeared superior to placebo in terms of reaching PASI 90. At class level (Figure 7), anti‐IL17 treatment showed a higher proportion of patients reaching PASI 90 compared to all of the interventions versus anti‐IL23 (RR 1.15, 95% CI 1.01 to 1.32); versus anti‐IL12/23 (RR 1.44, 95% CI 1.27 to 1.64); versus anti‐TNF alpha (RR 1.95, 95% CI 1.71 to 2.23); versus small molecules (RR 2.42, 95% CI 1.76 to 3.31); versus non‐biological systemic agents (RR 3.21, 95% CI 2.33 to 4.42). In terms of reaching PASI 90, all of the biologic interventions (anti‐IL17, anti‐IL12/23, anti‐IL23) except anti‐TNF alpha, appeared significantly superior to the small molecule class of treatments. All of the biologic interventions (anti‐IL17, anti‐IL12/23, anti‐IL23, and anti‐TNF alpha) were significantly superior to the non‐biological systemic class of treatments for reaching PASI 90.
Results of comparisons between each of the drugs are available in Figure 1. There was no significant difference between infliximab, bimekizumab, ixekizumab, and risankizumab in terms of reaching PASI 90. Bimekizumab and ixekizumab were significantly more likely to reach PASI 90 than secukinumab. Bimekizumab, ixekizumab, and risankizumab were significantly more likely to reach PASI 90, than brodalumab and guselkumab. Infliximab, anti‐IL17 drugs (bimekizumab, ixekizumab, secukinumab, and brodalumab) and anti‐IL23 drugs (risankizumab and guselkumab) except tildrakizumab were significantly more likely to reach PASI 90 than ustekinumab; three anti‐TNF alpha agents (adalimumab, certolizumab, and etanercept), and deucravacitinib. Ustekinumab was superior to certolizumab (RR 1.43, 95% CI 1.06 to 1.91). Adalimumab, tildrakizumab, and ustekinumab were superior to etanercept (RR 1.67, 95% CI 1.47 to 1.89; RR 1.76, 95% CI 1.40 to 2.20; and RR 1.79, 95% CI 1.60 to 2.01, respectively). No significant difference was shown between apremilast and two non‐biological drugs: ciclosporin and methotrexate.
We assessed the certainty of evidence for each comparison using CINeMA and classified it as high (highlighted in green), moderate (in blue), low (in yellow), and very low (in red) (Figure 1).
Ranking class‐level analysis (Figure 10; Figure 13; Table 4)
4. Ranking findings for all outcomes at class level.
| Class‐level interventions | SUCRA PASI 90 | Rank PASI 90 | SUCRA SAE | Rank SAE |
SUCRA SAE excluded flare of psoriasis |
Rank SAE excluded flare of psoriasis |
SUCRA PASI 75 | Rank PASI 75 | SUCRA AE | Rank AE | SUCRA PGA | Rank PGA | SUCRA QoL | Rank QoL |
| Anti‐IL17 | 99.5 | 1 | 37.6 | 5 | 34.6 | 5 | 99.5 | 1 | 25.1 | 6 | 100 | 1 | 96 | 1 |
| Anti‐IL23 | 83.8 | 2 | 74.3 | 2 | 63.5 | 2 | 80.8 | 2 | 85.5 | 2 | 81.2 | 2 | 83.3 | 2 |
| Anti‐IL12/23 | 66.7 | 3 | 31.4 | 6 | 22.4 | 7 | 69.8 | 3 | 54.6 | 4 | 68.8 | 3 | 70 | 3 |
| Anti‐TNF alpha | 48.7 | 4 | 45.2 | 4 | 29.3 | 6 | 50 | 4 | 57.2 | 3 | 50 | 4 | 48 | 4 |
| Small molecules | 33.3 | 5 | 76.2 | 1 | 85.1 | 1 | 27.9 | 5 | 3.8 | 7 | 28.8 | 5 | 20.1 | 6 |
|
Non‐biological treatments |
18 | 6 | 60.8 | 3 | 53.4 | 4 | 22.1 | 6 | 28.9 | 5 | 21.3 | 6 | 33 | 5 |
| Placebo | 0 | 7 | 24.4 | 7 | 61.7 | 3 | 0 | 7 | 95.1 | 1 | 0 | 7 | 0 | 7 |
AE: adverse events; FAEs: fumaric acid esters; PGA: Physician Global Assessment; QoL: specific quality of life scale; SAE: serious adverse events
Anti‐IL17 class had a better chance of reaching PASI 90 using SUCRA (versus placebo: RR 23.94, 95% CI 20.19 to 28.40; SUCRA = 99.5), followed by anti‐IL23 (versus placebo: RR 20.76, 95% CI 17.32 to 24.89; SUCRA = 83.8), anti‐IL12/23 (versus placebo: RR 16.60, 95% CI 13.72 to 20.09; SUCRA = 66.7), then anti‐TNF alpha (versus placebo: RR 12.25, 95% CI 10.33 to 14.52; SUCRA = 48.7). The heterogeneity τ for this network overall was 0.03, which we considered to be low.
Ranking drug‐level analysis (Figure 11; Figure 14; Table 5)
5. Ranking findings for all outcomes at drug level.
| Drug | SUCRA PASI 90 | Rank PASI 90 | SUCRA SAE | Rank SAE |
SUCRA SAE excluded flare of psoriasis |
Rank SAE excluded flare of psoriasis |
SUCRA PASI 75 | Rank PASI 75 | SUCRA AE | Rank AE | SUCRA PGA | Rank PGA | SUCRA QoL | Rank QoL |
| Infliximab | 96.8 | 1 | 30.2 | 19 | 51.8 | 7 | 97.3 | 1 | 34.9 | 13 | 86 | 3 | 67.9 | 7 |
| Bimekizumab | 92 | 2 | 83.6 | 2 | 85 | 1 | 87 | 3 | 17.2 | 19 | 92.3 | 1 | 70.6 | 6 |
| Ixekizumab | 90.3 | 3 | 47.4 | 12 | 35.6 | 16 | 87.8 | 2 | 29.6 | 14 | 89.8 | 2 | 95.8 | 2 |
| Risankizumab | 85.3 | 4 | 71.7 | 3 | 73.5 | 3 | 79.8 | 4 | 75.1 | 5 | 81.1 | 4 | 96.4 | 1 |
| Secukinumab | 77 | 5 | 30.7 | 18 | 39.7 | 15 | 79.8 | 5 | 41.5 | 12 | 79.3 | 5 | 76.4 | 3 |
| Sonelokimab | 74.4 | 6 | 35.3 | 15 | 42.7 | 11 | 68 | 8 | 28.9 | 15 | 76 | 7 | ‐ | |
| Brodalumab | 68.1 | 7 | 34.3 | 17 | 42.3 | 12 | 75.2 | 6 | 53.7 | 11 | 77.4 | 6 | 43.6 | 10 |
| Guselkumab | 68.1 | 8 | 47.7 | 11 | 46.9 | 10 | 74.8 | 7 | 68.4 | 6 | 66.4 | 8 | 63.1 | 8 |
| Ustekinumab | 54.6 | 9 | 40.8 | 13 | 41.7 | 13 | 59.8 | 9 | 59.6 | 9 | 56.2 | 9 | 75.5 | 4 |
| Tildrakizumab | 52.5 | 10 | 57.6 | 8 | 20.1 | 18 | 56.7 | 10 | 93.6 | 1 | 44.2 | 12 | 72 | 5 |
| Adalimumab | 48.7 | 11 | 36.9 | 14 | 39.8 | 14 | 51.3 | 11 | 60.4 | 7 | 46.7 | 11 | 40.2 | 12 |
| Deucravacitinib | 42.5 | 12 | 58.2 | 7 | 67.1 | 4 | 34 | 13 | 24.7 | 16 | 28.3 | 15 | 25.9 | 15 |
| Certolizumab | 37 | 13 | 64.4 | 5 | 25.1 | 17 | 47.1 | 12 | 87 | 2 | 47.2 | 10 | 30.8 | 13 |
| Methotrexate | 27 | 14 | 85.4 | 1 | ‐ | ‐ | 31.7 | 15 | 59.9 | 8 | 43.4 | 13 | 45.2 | 9 |
| Etanercept | 26.7 | 15 | 59.9 | 6 | 48.2 | 9 | 33.9 | 14 | 58.4 | 10 | 29.4 | 14 | 42.6 | 11 |
| Apremilast | 22.5 | 16 | 66.1 | 4 | 76.1 | 2 | 18.4 | 17 | 14.7 | 20 | 14.6 | 17 | 10.1 | 17 |
| Ciclosporin | 20.1 | 17 | 11.3 | 20 | ‐ | ‐ | 28 | 16 | 18.8 | 18 | 23.4 | 16 | 16.5 | 16 |
| Netakimab | 9.3 | 18 | 52.9 | 9 | 57.3 | 5 | 18.2 | 18 | 81.2 | 4 | 12.7 | 18 | 27.3 | 14 |
| FAEs | 7.2 | 19 | 50.2 | 10 | 51.6 | 8 | 8.5 | 20 | 23.8 | 17 | 5.6 | 19 | ‐ | |
| Placebo | 0 | 20 | 35.2 | 16 | 55.7 | 6 | 1.4 | 21 | 84.8 | 3 | 0 | 20 | 0 | 18 |
| Acitretin | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 11.3 | 19 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
AE: adverse events; FAEs: fumaric acid esters; PASI: Psoriasis Area and Severity Index; PGA: Physician Global Assessment; QoL: specific quality of life scale; SAE: serious adverse events; SUCRA: Surface Under the Cumulative Ranking
At drug‐level, using SUCRA, infliximab had a better chance of reaching PASI 90 at drug level (versus placebo: RR 49.16, 95% CI 20.49 to 117.95; SUCRA = 96.8; high‐certainty evidence), followed by bimekizumab (versus placebo: RR 27.86, 95% CI 23.56 to 32.94; SUCRA = 92; high‐certainty evidence), ixekizumab (versus placebo: RR 27.35, 95% CI 23.15to 32.29; SUCRA = 90.3; high‐certainty evidence), then risankizumab (versus placebo: RR 26.16, 95% CI 22.03 to 31.07; SUCRA = 85.3; high‐certainty evidence). The heterogeneity τ for this network overall was 0, which we considered to be low.
1.2 The proportion of participants with serious adverse events
DIRECT EVIDENCE
We report treatment estimates for pairwise meta‐analyses at class and drug level in Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5; Analysis 2.6; Analysis 2.7; Analysis 2.8; Analysis 2.10; and Analysis 2.9, respectively.
2.1. Analysis.

Comparison 2: Primary outcome ‐ serious adverse events (SAEs), Outcome 1: Non‐biological treatments versus placebo
2.2. Analysis.

Comparison 2: Primary outcome ‐ serious adverse events (SAEs), Outcome 2: Non‐biological treatment 1 versus non‐biological treatment 2
2.3. Analysis.

Comparison 2: Primary outcome ‐ serious adverse events (SAEs), Outcome 3: Anti‐TNF alpha versus placebo
2.4. Analysis.

Comparison 2: Primary outcome ‐ serious adverse events (SAEs), Outcome 4: Anti‐IL12/23 versus placebo
2.5. Analysis.

Comparison 2: Primary outcome ‐ serious adverse events (SAEs), Outcome 5: Anti‐IL17 versus placebo
2.6. Analysis.

Comparison 2: Primary outcome ‐ serious adverse events (SAEs), Outcome 6: Anti‐IL23 versus placebo
2.7. Analysis.

Comparison 2: Primary outcome ‐ serious adverse events (SAEs), Outcome 7: Biologic versus non‐biological treatments
2.8. Analysis.

Comparison 2: Primary outcome ‐ serious adverse events (SAEs), Outcome 8: Biologic 1 versus biologic 2
2.10. Analysis.

Comparison 2: Primary outcome ‐ serious adverse events (SAEs), Outcome 10: Small molecules versus placebo
2.9. Analysis.

Comparison 2: Primary outcome ‐ serious adverse events (SAEs), Outcome 9: Biologic versus small molecules
We found no significant differences between FAEs, etanercept, adalimumab, certolizumab, infliximab, ustekinumab, secukinumab, ixekizumab, brodalumab, bimekizumab, netakimab, sonelokimab, guselkumab, tildrakizumab, risankizumab, apremilast, deucravacitinib, and placebo in the number of participants with serious adverse events (SAEs). The risk of SAEs was significantly lower for participants on methotrexate compared to placebo (RR 0.16, 95% CI 0.03 to 0.88). The risk of SAEs was significantly higher for participants on infliximab compared to methotrexate (RR 2.41, 95% CI 1.04 to 5.59).
There were zero SAEs in the following trials: Fallah Arani 2011 (comparing methotrexate with FAEs); Flytström 2008 (comparing ciclosporin with methotrexate); Heydendael 2003 (comparing ciclosporin with methotrexate); Gisondi 2008; (comparing etanercept with acitretin); Bagel 2012 (comparing etanercept with placebo); Caproni 2009 (comparing etanercept with acitretin); Chaudhari 2001 (comparing infliximab with placebo); Yu 2019 (comparing etanercept with methotrexate); Hunter 1963 (comparing methotrexate with placebo); AlMutairi 2021 (comparing ixekizumab with secukinumab); NCT02762994 (comparing netakimab with placebo); Ikonomidis 2022 (comparing apremilast, etanercept, and ciclosporin); OPTIMAP 2022 (comparing methotrexate versus placebo, co‐intervention anti‐TNF agents).
NETWORK META‐ANALYSES
The SAE outcome was available in 127 trials, involving 51,050 participants (93% of the participants in the meta‐analysis). For one trial (PRESTA 2010), the number of randomised participants was not available. We added this trial to the complete‐cases sensitivity analyses. This outcome was reported in seven trials out of 127 (Khatri 2016; Laburte 1994; Ortonne 2013; PRESTA 2010; PRISTINE 2013; SCULPTURE 2015; SIGNATURE 2019), comparing different dosages of the same drug in each case. We added these studies to the sensitivity analysis at dose level. This outcome was reported in 10 trials out of 127 with biological‐naïve participants that were added to the sensitivity analysis for all trials, whatever previous treatments received by the participants (Barker 2011; Caproni 2009; CHAMPION 2008; CHANGE 2021; Gisondi 2008; Lee 2016; POLARIS 2020; PRIME 2017; Reich 2020; Thaci 2021). Seventy‐five trials, involving 23,636 participants, were placebo‐controlled trials; 34, involving 11,971 participants, were head‐to‐head comparisons; and 18, involving 15,443 participants, had both a placebo and at least two active treatments arms.
SAEs were not reported for the 13 remaining trials, and we were not able to obtain missing information from the trial authors (Table 2).
See Figure 5; Figure 6; Figure 7; Figure 1; Figure 10; Figure 11; Figure 13; Figure 14.
Table 6 summarises the main results of both direct and indirect evidence and the network meta‐analysis for SAEs. We present the summary relative effects from the network meta‐analysis in league tables for both class‐level (Figure 7) and drug‐level (Figure 1) analyses. We found no significant difference between any of the interventions and the placebo for the risk of SAE. This result was verified after excluding flares of psoriasis as SAEs (data not shown). There was no significant difference between all interventions in the number of participants with SAEs (Figure 1). We assessed the certainty of evidence for each comparison using CINeMA and classified it as high (highlighted in green), moderate (in blue), low (in yellow), and very low (in red) (Figure 1).
6. Direct and indirect evidence and network meta‐analysis results summary table for serious adverse events.
| Network meta‐analysis | Direct evidence | Indirect evidence | Separate indirect from direct evidence | |||||
| Comparisons* | RR | 95% CI | RR | 95% CI | RR | 95% CI | z‐testa | P value |
| Adalimumab versus bimekizumab | 1.92 | (0.90 to 4.10) | 2.01 | (0.59 to 6.83) | 1.87 | (0.71 to 4.92) | 0.09 | 0.93 |
| Adalimumab versus guselkumab | 1.11 | (0.69 to 1.78) | 1.1 | (0.54 to 2.22) | 1.12 | (0.59 to 2.12) | ‐0.04 | 0.97 |
| Adalimumab versus placebo | 0.99 | (0.67 to 1.47) | 1.19 | (0.74 to 1.92) | 0.67 | (0.33 to 1.36) | 1.32 | 0.19 |
| Adalimumab versus risankizumab | 1.45 | (0.87 to 2.42) | 0.89 | (0.37 to 2.16) | 1.85 | (0.99 to 3.46) | ‐1.32 | 0.19 |
| Apremilast versus deucravacitinib | 0.94 | (0.44 to 2.00) | 0.77 | (0.27 to 2.19) | 1.17 | (0.40 to 3.47) | ‐0.55 | 0.58 |
| Apremilast versus etanercept | 0.94 | (0.51 to 1.71) | 1.4 | (0.28 to 6.90) | 0.88 | (0.46 to 1.68) | 0.53 | 0.59 |
| Apremilast versus placebo | 0.74 | (0.45 to 1.20) | 0.72 | (0.43 to 1.19) | 1.06 | (0.18 to 6.09) | ‐0.42 | 0.67 |
| Bimekizumab versus placebo | 0.52 | (0.25 to 1.08) | 0.58 | (0.20 to 1.65) | 0.46 | (0.17 to 1.30) | 0.29 | 0.77 |
| Bimekizumab versus ustekinumab | 0.54 | (0.25 to 1.16) | 0.51 | (0.15 to 1.73) | 0.57 | (0.22 to 1.49) | ‐0.14 | 0.89 |
| Brodalumab versus placebo | 1.05 | (0.63 to 1.75) | 0.92 | (0.52 to 1.61) | 1.99 | (0.60 to 6.64) | ‐1.14 | 0.26 |
| Brodalumab versus ustekinumab | 1.11 | (0.63 to 1.93) | 1.5 | (0.63 to 3.55) | 0.89 | (0.43 to 1.85) | 0.91 | 0.36 |
| Certolizumab versus etanercept | 0.89 | (0.36 to 2.16) | 2.56 | (0.30 to 21.74) | 0.71 | (0.27 to 1.89) | 1.07 | 0.29 |
| Certolizumab versus placebo | 0.7 | (0.31 to 1.58) | 0.61 | (0.26 to 1.39) | 33.28 | (0.44 to 2538.26) | ‐1.78 | 0.08 |
| Deucravacitinib versus placebo | 0.78 | (0.40 to 1.52) | 0.68 | (0.34 to 1.37) | 2.92 | (0.34 to 24.95) | ‐1.27 | 0.21 |
| Etanercept versus infliximab | 0.67 | (0.30 to 1.50) | 1.09 | (0.07 to 16.39) | 0.64 | (0.27 to 1.49) | 0.37 | 0.71 |
| Etanercept versus ixekizumab | 0.87 | (0.55 to 1.40) | 0.94 | (0.48 to 1.81) | 0.81 | (0.42 to 1.60) | 0.29 | 0.77 |
| Etanercept versus placebo | 0.79 | (0.53 to 1.17) | 0.74 | (0.46 to 1.18) | 0.92 | (0.45 to 1.87) | ‐0.51 | 0.61 |
| Etanercept versus secukinumab | 0.75 | (0.47 to 1.21) | 0.55 | (0.15 to 1.95) | 0.79 | (0.47 to 1.32) | ‐0.52 | 0.6 |
| Etanercept versus tildrakizumab | 1 | (0.46 to 2.18) | 1.39 | (0.53 to 3.61) | 0.52 | (0.13 to 1.99) | 1.17 | 0.24 |
| Etanercept versus ustekinumab | 0.83 | (0.51 to 1.34) | 0.8 | (0.24 to 2.64) | 0.83 | (0.49 to 1.41) | ‐0.06 | 0.96 |
| Guselkumab versus ixekizumab | 0.99 | (0.63 to 1.57) | 0.91 | (0.47 to 1.77) | 1.07 | (0.58 to 2.01) | ‐0.35 | 0.72 |
| Guselkumab versus placebo | 0.9 | (0.61 to 1.31) | 1.07 | (0.50 to 2.28) | 0.84 | (0.54 to 1.31) | 0.52 | 0.6 |
| Guselkumab versus secukinumab | 0.85 | (0.59 to 1.22) | 0.86 | (0.55 to 1.35) | 0.84 | (0.46 to 1.54) | 0.05 | 0.96 |
| Infliximab versus placebo | 1.18 | (0.57 to 2.43) | 1.22 | (0.58 to 2.59) | 0.72 | (0.05 to 11.13) | 0.37 | 0.71 |
| Ixekizumab versus placebo | 0.9 | (0.60 to 1.35) | 0.95 | (0.54 to 1.68) | 0.86 | (0.48 to 1.52) | 0.25 | 0.81 |
| Ixekizumab versus ustekinumab | 0.94 | (0.58 to 1.54) | 0.73 | (0.18 to 3.01) | 0.98 | (0.58 to 1.64) | ‐0.38 | 0.71 |
| Methotrexate versus placebo | 0.38 | (0.10 to 1.52) | 0.08 | (0.01 to 0.68) | 1.14 | (0.19 to 6.88) | ‐1.86 | 0.06 |
| Methotrexate versus risankizumab | 0.56 | (0.14 to 2.18) | 1.56 | (0.27 to 8.95) | 0.11 | (0.01 to 0.98) | 1.86 | 0.06 |
| Risankizumab versus placebo | 0.68 | (0.45 to 1.05) | 0.55 | (0.29 to 1.07) | 0.8 | (0.46 to 1.41) | ‐0.84 | 0.4 |
| Secukinumab versus placebo | 1.05 | (0.76 to 1.45) | 1.12 | (0.71 to 1.78) | 0.99 | (0.63 to 1.55) | 0.39 | 0.7 |
| Sonelokimab versus placebo | 1.23 | (0.25 to 6.10) | 0.92 | (0.16 to 5.47) | 4.15 | (0.11 to 160.80) | ‐0.72 | 0.47 |
| Tildrakizumab versus placebo | 0.79 | (0.36 to 1.73) | 0.97 | (0.38 to 2.50) | 0.5 | (0.12 to 2.02) | 0.77 | 0.44 |
| Ustekinumab versus placebo | 0.95 | (0.68 to 1.34) | 0.98 | (0.62 to 1.55) | 0.93 | (0.57 to 1.52) | 0.16 | 0.88 |
| Risankizumab versus secukinumab | 0.65 | (0.40 to 1.05) | 1.49 | (0.54 to 4.09) | 0.51 | (0.30 to 0.88) | 1.82 | 0.07 |
| Risankizumab versus ustekinumab | 0.72 | (0.45 to 1.13) | 0.54 | (0.27 to 1.05) | 0.92 | (0.50 to 1.72) | ‐1.16 | 0.25 |
| Secukinumab versus sonelokimab | 0.85 | (0.17 to 4.30) | 0.35 | (0.02 to 6.31) | 1.28 | (0.18 to 9.04) | ‐0.72 | 0.47 |
| Secukinumab versus ustekinumab | 1.1 | (0.75 to 1.62) | 1.26 | (0.70 to 2.30) | 1 | (0.61 to 1.65) | 0.58 | 0.56 |
FAES: fumaric acid esters; RR: risk ratio; 95% CI: 95% confidence interval
*The comparisons listed in this table were included in at least one direct‐evidence analysis.
az‐value of test for disagreement (direct versus indirect); P value of test for disagreement (direct versus indirect).
Ranking class‐level analysis (Figure 10; Figure 13; Table 4)
Small molecules had the highest SUCRA at class level in terms of serious adverse events (versus placebo: RR 0.75, 95% CI 0.49 to 1.14; SUCRA = 76.2), followed by anti‐IL23 (versus placebo: RR 0.79, 95% CI 0.60 to 1.04; SUCRA = 74.3); then non‐biological systemic treatments (versus placebo: RR 0.80, 95% CI 0.40 to 1.61; SUCRA = 60.8), anti‐TNF alpha agents (versus placebo: RR 0.92, 95% CI 0.71 to 1.18; SUCRA = 45.2), and anti‐IL17 (versus placebo: RR 0.95, 95% CI 0.76 to 1.20; SUCRA = 37.6) and anti‐IL12/23 (versus placebo: RR 0.99, 95% CI 0.71 to 1.37; SUCRA = 31.4). The heterogeneity τ for this network overall was 0, which we considered to be low. Placebo ranking changed from 7 (the lowest/worst SUCRA at class level) for serious adverse events to 3 (out of 7) after excluding flares of psoriasis as SAEs.
Ranking drug‐level analysis (Figure 11; Figure 14; Table 5)
Methotrexate had the highest SUCRA at drug level in terms of serious adverse events (versus placebo: RR 0.38, 95% CI 0.10 to 1.52; SUCRA = 85.4; very low‐certainty evidence), followed by bimekizumab (versus placebo: RR 0.52, 95% CI 0.25 to 1.08; SUCRA = 83.6; moderate‐certainty evidence), risankizumab (versus placebo: RR 0.68, 95% CI 0.45 to 1.05; SUCRA = 71.7; moderate‐certainty evidence), apremilast (versus placebo: RR 0.74, 95% CI 0.45 to 1.20; SUCRA = 66.1; low‐certainty evidence), then certolizumab (versus placebo: RR 0.70, 95% CI 0.31 to 1.58; SUCRA = 64.4; moderate‐certainty evidence). However, no significant difference was observed between drugs and placebo. The heterogeneity τ for this network overall was 0, which we considered to be low. After excluding worsening of psoriasis as a SAE, bimekizumab, apremilast, and risankizumab had still the highest SUCRA rank. Methotrexate could not be assessed. Certolizumab ranking changed from 5 to 17 for SAEs after excluding flares of psoriasis as SAEs. Placebo rose from the 16th to the 6th rank.
1.3 Relationship between PASI 90 and serious adverse events
See Figure 12.
We combined together these findings for both efficacy (PASI 90) and acceptability (serious adverse events) in a bivariate ranking plot, where serious adverse events were transformed into acceptability by using the inverse values of the corresponding RRs so that higher values indicated higher acceptability (due to lower SAEs): accordingly, the ideal treatment (highest performance = best efficacy + best acceptability) should appear in the upper right corner of the plot.
At drug level, risankizumab and bimekizumab might be the overall best treatments, considering both outcomes jointly. Other highly effective drugs (ixekizumab and infliximab) had serious adverse events.
2. Secondary outcomes
2.1 Proportion of participants who achieved PASI 75
DIRECT EVIDENCE
We report treatment estimates for pairwise meta‐analyses at class and drug level in Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6; Analysis 3.7; Analysis 3.8; Analysis 3.10; and Analysis 3.9, respectively.
3.1. Analysis.

Comparison 3: Secondary outcome ‐ PASI 75, Outcome 1: Non‐biological treatments versus placebo
3.2. Analysis.

Comparison 3: Secondary outcome ‐ PASI 75, Outcome 2: Non‐biological treatment 1 versus non‐biological treatment 2
3.3. Analysis.

Comparison 3: Secondary outcome ‐ PASI 75, Outcome 3: Anti‐TNF alpha versus placebo
3.4. Analysis.

Comparison 3: Secondary outcome ‐ PASI 75, Outcome 4: Anti‐IL12/23 versus placebo
3.5. Analysis.

Comparison 3: Secondary outcome ‐ PASI 75, Outcome 5: Anti‐IL17 versus placebo
3.6. Analysis.

Comparison 3: Secondary outcome ‐ PASI 75, Outcome 6: Anti‐IL23 versus placebo
3.7. Analysis.

Comparison 3: Secondary outcome ‐ PASI 75, Outcome 7: Biologic versus non‐biological treatments
3.8. Analysis.

Comparison 3: Secondary outcome ‐ PASI 75, Outcome 8: Biologic 1 versus biologic 2
3.10. Analysis.

Comparison 3: Secondary outcome ‐ PASI 75, Outcome 10: Small molecules versus placebo
3.9. Analysis.

Comparison 3: Secondary outcome ‐ PASI 75, Outcome 9: Biologic versus small molecules
NETWORK META‐ANALYSES
The PASI 75 outcome was available in 129 trials, involving 51,335 participants (93.7% of the participants in the meta‐analysis). For one trial (PRESTA 2010), the number of randomised participants was not available. We added these trials to the complete‐case analyses. This outcome was reported in 10 trials out of 129 (Dogra 2012; Dogra 2013; Dubertret 1989; Khatri 2016; Laburte 1994; Ortonne 2013; PRESTA 2010; PRISTINE 2013; SCULPTURE 2015; SIGNATURE 2019), comparing different dosages of the same drug in each case. We added these trials to the sensitivity analysis at dose level. This outcome was reported in 10 trials out of 129 with biological‐naïve participants that were added to the sensitivity analysis for all trials, whatever the previous treatments received by the participants (Barker 2011; Caproni 2009; CHAMPION 2008; CHANGE 2021; Gisondi 2008; Lee 2016; POLARIS 2020; PRIME 2017; Reich 2020; Thaci 2021). Seventy‐four trials, involving 24,001 participants, were placebo‐controlled trials; 37 trials, involving 11,891 participants, were head‐to‐head comparisons; and 18 trials, involving 15,443 participants, had both a placebo and at least two active treatments arms. PASI 75 was not reported for the 11 remaining trials, and we were not able to obtain the missing information from the trial authors (Table 2).
See Figure 5; Figure 6; Figure 7; Figure 8; Figure 10; Figure 11; Figure 13; Figure 14.
We present the summary relative effects from the network meta‐analysis in league tables for both class‐level (Figure 7) and drug‐level (Figure 8) analyses. All of the interventions appeared superior to placebo in terms of reaching PASI 75. At class level, the anti‐IL17 class of drugs was associated with a higher chance of reaching PASI 75 compared to the other classes, except for anti‐IL23 (Figure 7). Anti‐IL17, anti‐IL23, and anti‐IL12/23 appeared significantly superior to the small molecule class; all the biologics (anti‐IL17, anti‐IL23, anti‐IL12/23, anti‐TNF alpha) appeared significantly superior to the non‐biological systemic class, and there was no significant difference between the small molecules and the non‐biological systemic agents in terms of reaching PASI 75. Results of comparisons between each of the drugs are available in Figure 8. Infliximab, anti‐IL17 drugs (ixekizumab, bimekizumab, and secukinumab), and risankizumab were significantly more likely to reach PASI 75, than ustekinumab, other anti‐TNF alpha (adalimumab, certolizumab, and etanercept), and small molecules (apremilast and deucravacitinib).
Ranking class‐level analysis(Figure 10; Figure 13; Table 4)
Ranking analysis performed with SUCRA strongly suggested that anti‐IL17 had a better chance of reaching PASI 75 at class level (versus placebo: RR 12.22, 95% CI 10.95 to 13.64; SUCRA = 99.5), followed by anti‐IL23 (versus placebo: RR 10.97, 95% CI 9.75 to 12.35; SUCRA = 80.8), anti‐IL12/23 (versus placebo: RR 10.39, 95% CI 9.20 to 11.74; SUCRA = 69.8), and anti‐TNF alpha (versus placebo: RR 8.27, 95% CI 7.45 to 9.18; SUCRA = 50). The heterogeneity τ for this network overall was 0.02, which we considered to be low.
Ranking drug‐level analysis(Figure 11; Figure 14; Table 5)
Ranking analysis performed with SUCRA strongly suggested that infliximab had the higher chance of reaching PASI 75 at drug level (versus placebo: RR 17.42, 95% CI 11.45 to 26.51; SUCRA = 97.3), followed by ixekizumab (versus placebo: RR 12.90, 95% CI 11.21 to 14.84; SUCRA = 87.8), bimekizumab (versus placebo: RR 12.91, 95% CI 11.00 to 15.15; SUCRA = 87), risankizumab (versus placebo: RR 12.16, 95% CI 10.62 to 13.93; SUCRA = 79.8) then secukinumab (versus placebo: RR 12.21, 95% CI 10.79 to 13.81; SUCRA = 79.8). The heterogeneity τ for this network overall was 0.01, which we considered to be low.
2.2 Proportion of participants who achieved a Physician Global Assessment (PGA) value of 0 or 1
DIRECT EVIDENCE
We report treatment estimates for pairwise meta‐analyses at class and drug level in Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6; Analysis 4.7; Analysis 4.8; Analysis 4.10; and Analysis 4.9, respectively.
4.1. Analysis.

Comparison 4: Secondary outcome ‐ PGA 0/1, Outcome 1: Non‐biological treatment versus placebo
4.2. Analysis.

Comparison 4: Secondary outcome ‐ PGA 0/1, Outcome 2: Non‐biological treatment 1 versus non‐biological treatment 2
4.3. Analysis.

Comparison 4: Secondary outcome ‐ PGA 0/1, Outcome 3: Anti‐TNF alpha versus placebo
4.4. Analysis.

Comparison 4: Secondary outcome ‐ PGA 0/1, Outcome 4: Anti‐IL12/23 versus placebo
4.5. Analysis.

Comparison 4: Secondary outcome ‐ PGA 0/1, Outcome 5: Anti‐IL17 versus placebo
4.6. Analysis.

Comparison 4: Secondary outcome ‐ PGA 0/1, Outcome 6: Anti‐IL23 versus placebo
4.7. Analysis.

Comparison 4: Secondary outcome ‐ PGA 0/1, Outcome 7: Biologic versus non‐biological treatments
4.8. Analysis.

Comparison 4: Secondary outcome ‐ PGA 0/1, Outcome 8: Biologic 1 versus biologic 2
4.10. Analysis.

Comparison 4: Secondary outcome ‐ PGA 0/1, Outcome 10: Small molecules versus placebo
4.9. Analysis.

Comparison 4: Secondary outcome ‐ PGA 0/1, Outcome 9: Biologic versus small molecules
NETWORK META‐ANALYSES
The PGA 0/1 outcome was available in 117 trials, involving 49,766 participants (91% of the participants in the meta‐analysis). For three studies (Nugteren‐Huying 1990; PRESTA 2010; Sandhu 2003), the number of randomised participants was not available. We added these trials to the complete‐case analyses. This outcome was reported in six trials out of 117 (Augustin 2022; Khatri 2016; Ortonne 2013; PRESTA 2010; PRISTINE 2013; SCULPTURE 2015), comparing different dosages of the same drug. We added these trials to the sensitivity analysis at dose level. This outcome was reported in eight trials out of 117 with biological‐naïve participants that were added to the sensitivity analysis for all trials, whatever the previous treatments received by the participants (Barker 2011; CHAMPION 2008; CHANGE 2021; Gisondi 2008; Lee 2016; PRIME 2017; Reich 2020; Thaci 2021). Sixty‐nine trials, involving 22,388 participants, were placebo‐controlled trials; 30 trials, involving 11,935 participants, were head‐to‐head comparisons; and 18 trials, involving 15,443 participants, had both a placebo and at least two active treatments arms. PGA 0/1 was not reported for the 23 remaining trials, and we were not able to obtain missing information from the trial authors (Table 2).
See Figure 5; Figure 6; Figure 7; Figure 9; Figure 10; Figure 11; Figure 13; Figure 14.
We present the summary relative effects as estimated from the network meta‐analysis in league tables at class level (Figure 7) and drug level (Figure 9). At class level, all of the interventions appeared superior to placebo in terms of reaching PGA 0/1, and anti‐IL17 monoclonal antibodies were associated with a better chance for this outcome compared to the other drug classes (Figure 7). These differences were statistically significant. All of the interventions (anti‐IL17, anti‐IL23, anti‐IL12/23, anti‐TNF alpha) appeared significantly superior to the small molecule and the non‐biological systemic class of treatments. We found no significant difference between small molecule and non‐biological systemic agents. Results of comparisons between each of the drugs are available in Figure 9.
Ranking class‐level analysis(Figure 10; Figure 13; Table 4)
Ranking analysis performed with SUCRA strongly suggested that anti‐IL17 had a better chance of reaching PGA 0/1 at class level (versus placebo: RR 13.44, 95% CI 11.73 to 15.40; SUCRA = 100), followed by anti‐IL23 (versus placebo: RR 10.92, 95% CI 9.48 to 12.59; SUCRA = 81.2), anti‐IL12/23 (versus placebo: RR 9.94, 95% CI 8.56 to 11.54; SUCRA = 68.8), and anti‐TNF alpha (versus placebo: RR 7.86, 95% CI 6.89 to 8.95; SUCRA = 50). The heterogeneity τ for this network overall was 0.04, which we considered to be low.
Ranking drug‐level analysis(Figure 11; Figure 14; Table 5)
Ranking analysis performed with SUCRA strongly suggested that bimekizumab had a better chance of reaching PGA 0/1 at drug level (versus placebo: RR 14.97, 95% CI 12.42 to 18.04; SUCRA = 92.3), followed by ixekizumab (versus placebo: RR 14.50, 95% CI 12.44 to 16.89; SUCRA = 89.8), then infliximab (versus placebo: RR 15.17, 95% CI 9.26 to 24.86; SUCRA = 86), risankizumab (versus placebo: RR 13.48, 95% CI 11.55 to 15.72; SUCRA = 81.1), secukinumab (versus placebo: RR 13.26, 95% CI 11.54 to 15.24; SUCRA = 79.3), then brodalumab (versus placebo: RR 13.06, 95% CI 10.66 to 16.00; SUCRA = 77.4). The heterogeneity τ for this network overall was 0.01, which we considered to be low.
Focusing on efficacy outcomes (PASI 90, PASI 75, and PGA 0/1), the results were similar at class level (Figure 10; Table 4) and at drug level (Figure 11; Table 5).
2.3 Mean difference of quality of life measured by a specific scale
DIRECT EVIDENCE
We report treatment estimates for pair‐wise meta‐analyses at class and drug level in Analysis 5.1; Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5; Analysis 5.6; Analysis 5.7; Analysis 5.8; Analysis 5.9 and Analysis 5.10; respectively.
5.1. Analysis.

Comparison 5: Secondary outcome ‐ quality of life, Outcome 1: Non‐biological treatments versus placebo
5.2. Analysis.

Comparison 5: Secondary outcome ‐ quality of life, Outcome 2: Non‐biological treatment 1 versus non‐biological treatment 2
5.3. Analysis.

Comparison 5: Secondary outcome ‐ quality of life, Outcome 3: Anti‐TNF alpha versus placebo
5.4. Analysis.

Comparison 5: Secondary outcome ‐ quality of life, Outcome 4: Ustekinumab versus placebo
5.5. Analysis.

Comparison 5: Secondary outcome ‐ quality of life, Outcome 5: Anti‐IL17 versus placebo
5.6. Analysis.

Comparison 5: Secondary outcome ‐ quality of life, Outcome 6: Anti‐IL23 versus placebo
5.7. Analysis.

Comparison 5: Secondary outcome ‐ quality of life, Outcome 7: Biologic versus non‐biological treatments
5.8. Analysis.

Comparison 5: Secondary outcome ‐ quality of life, Outcome 8: Biologic 1 versus biologic 2
5.9. Analysis.

Comparison 5: Secondary outcome ‐ quality of life, Outcome 9: Biologic versus small molecules
5.10. Analysis.

Comparison 5: Secondary outcome ‐ quality of life, Outcome 10: Small molecules versus placebo
NETWORK META‐ANALYSES
The quality of life outcome was available in 64 trials, involving 26,265 participants (48% of the participants in the meta‐analysis). This outcome was also reported in five trials (out of 64) (Khatri 2016; Ortonne 2013; PRISTINE 2013; SCULPTURE 2015; SIGNATURE 2019), comparing different dosages of the same drug. We added these trials to the sensitivity analyses at dose level. This outcome was reported in six trials out of 64 with biological‐naïve participants that were added to the sensitivity analysis for all trials, whatever the previous treatments received by the participants (Barker 2011; CHAMPION 2008; CHANGE 2021; POLARIS 2020; Reich 2020; Thaci 2021). The quality of life outcome was not reported for the 76 remaining trials, and we were not able to obtain missing information from the trial authors (Table 2). Forty‐one trials, involving 14,684 participants, were placebo‐controlled trials; 12, involving 2845 participants, were head‐to‐head comparisons; and 11, involving 8736 participants, had both a placebo and at least two active treatments arms.
See Figure 5; Figure 6; Figure 7; Figure 9; Figure 10; Figure 11; Figure 13; Figure 14.
We present the summary relative effects from the network meta‐analysis in league tables for both class‐level (Figure 7) and drug‐level (Figure 9) analyses. All classes of treatments appeared superior to placebo in terms of showing significant improvement on a quality of life scale. Anti‐IL23, anti‐IL12/23, anti‐IL17, and anti‐TNF agents were associated with a higher chance of improving quality of life compared to small molecules (Figure 7). These differences were statistically significant for all of the classes. No significant difference was shown between anti‐IL23, anti‐IL12/23, and anti‐IL17. Anti‐IL23, anti‐IL17, and anti‐IL12/23 were more favourable than anti‐TNF alpha. There were no significant differences between the small molecules and the non‐biological agents. Results of comparisons between each of the drugs are available in Figure 9.
Ranking class‐level analysis(Figure 10; Figure 13; Table 4)
Ranking analysis performed with SUCRA strongly suggested that anti‐IL17 had a better chance of improving quality of life at class level (versus placebo: standardised mean difference (SMD) −1.50, 95% confidence interval (CI) −1.66 to −1.35; SUCRA = 96), followed by anti‐IL23 (versus placebo: SMD −1.41, 95% CI −1.56 to −1.27; SUCRA = 83.3), and anti‐IL12/23 (versus placebo: SMD −1.31, 95% CI −1.49 to −1.14; SUCRA = 70). The heterogeneity τ for this network overall was 0.04, which we considered to be low.
Ranking drug‐level analysis(Figure 11; Figure 14; Table 5)
Ranking analysis for quality of life performed with SUCRA strongly suggested that risankizumab was the best treatment at drug level (versus placebo: SMD −1.77, 95% CI −2.03 to −1.50; SUCRA = 96.4), followed by ixekizumab (versus placebo: SMD −1.74, 95% CI −1.92 to −1.55; SUCRA = 95.8), secukinumab (versus placebo: SMD −1.41, 95% CI −1.81 to −1.02; SUCRA = 76.4), ustekinumab (versus placebo: SMD −1.37, 95% CI −1.54 to −1.21; SUCRA = 75.5), then tildrakizumab (versus placebo: SMD −1.35, 95% CI −1.57 to −1.12; SUCRA = 72). The heterogeneity τ for this network overall was 0.03, which we considered to be low. Moreover, four interventions (acitretin, FAEs, sonelokimab) were not included in the ranking at drug level, due to no available data.
In total, information on quality of life was poorly reported and lacking for almost half of the population included in the NMA, so the results have to be considered with caution.
2.4 The proportion of participants with adverse events
DIRECT EVIDENCE
We report treatment estimates for pairwise meta‐analyses at class and drug level in Analysis 6.1; Analysis 6.2; Analysis 6.3; Analysis 6.4; Analysis 6.5; Analysis 6.6; Analysis 6.7; Analysis 6.8; Analysis 6.10; and Analysis 6.9 respectively.
6.1. Analysis.

Comparison 6: Secondary outcome ‐ adverse events, Outcome 1: Non‐biological treatments versus placebo
6.2. Analysis.

Comparison 6: Secondary outcome ‐ adverse events, Outcome 2: Non‐biological treatment 1 versus non‐biological treatment 2
6.3. Analysis.

Comparison 6: Secondary outcome ‐ adverse events, Outcome 3: Anti‐TNF alpha versus placebo
6.4. Analysis.

Comparison 6: Secondary outcome ‐ adverse events, Outcome 4: Ustekinumab versus placebo
6.5. Analysis.

Comparison 6: Secondary outcome ‐ adverse events, Outcome 5: Anti‐IL17 versus placebo
6.6. Analysis.

Comparison 6: Secondary outcome ‐ adverse events, Outcome 6: Anti‐IL23 versus placebo
6.7. Analysis.

Comparison 6: Secondary outcome ‐ adverse events, Outcome 7: Biologic versus non‐biological treatments
6.8. Analysis.

Comparison 6: Secondary outcome ‐ adverse events, Outcome 8: Biologic 1 versus biologic 2
6.10. Analysis.

Comparison 6: Secondary outcome ‐ adverse events, Outcome 10: Small molecules versus placebo
6.9. Analysis.

Comparison 6: Secondary outcome ‐ adverse events, Outcome 9: Biologic versus small molecules
NETWORK META‐ANALYSES
The adverse events (AEs) outcome was available in 118 trials, involving 48,953 participants (89% of the participants in the meta‐analysis). AEs were not reported for the 22 remaining trials, and we were not able to obtain missing information from the trial authors (Table 2). This outcome was also reported in five trials (out of 118) (Khatri 2016; Ortonne 2013; PRISTINE 2013; SCULPTURE 2015; SIGNATURE 2019), comparing different dosages of the same drug, and were added to the sensitivity analyses at dose level. This outcome was reported in nine trials out of 118 with biological‐naïve participants that were added to the sensitivity analysis for all trials, whatever the previous treatments received by the participants (Barker 2011; CHAMPION 2008; CHANGE 2021; Gisondi 2008; Lee 2016; POLARIS 2020; PRIME 2017; Reich 2020; Thaci 2021). Sixty‐nine trials, involving 22,604 participants, were placebo‐controlled trials; 31, involving 10,906 participants, were head‐to‐head comparisons; and 18, involving 15,443 participants, had both a placebo and at least two active treatments arms.
See Figure 5; Figure 6; Figure 7; Figure 8; Figure 10; Figure 11; Figure 13; Figure 14.
We present the summary relative effects from the network meta‐analysis in league tables for both class‐level (Figure 7) and drug‐level (Figure 8) analyses. At class level, all of the classes of treatments had a more significant risk of AEs compared to placebo, except anti‐IL23. Significant associations were found: anti‐IL17 had a higher risk of AEs compared with anti‐IL23, anti‐IL12/23, and anti‐TNF; anti‐IL23, anti‐IL12/23, and anti‐TNF had a lower risk of AEs compared with small molecules (Figure 7). Results of comparisons between each of the drugs are available in Figure 8.
Ranking class‐level analysis(Figure 10; Figure 13; Table 4)
Placebo had the highest SUCRA (SUCRA 95.1) at class‐level for all adverse events, followed by anti‐IL23 (versus placebo: RR 1.02, 95% CI 0.96 to 1.08; SUCRA = 85.5), anti‐TNF agents (versus placebo: RR 1.08, 95% CI 1.03 to 1.12; SUCRA = 57.2), then anti‐IL12/23 (versus placebo: RR 1.08, 95% CI 1.02 to 1.14; SUCRA = 54.6). The heterogeneity τ for this network overall was 0, which we considered to be low.
Ranking drug‐level analysis(Figure 11; Figure 14; Table 5)
Tildrakizumab had the highest SUCRA at drug‐level for all adverse events (versus placebo: RR 0.93, 95% CI 0.82 to 1.05; SUCRA = 93.6), followed by certolizumab (versus placebo: RR 0.97, 95% CI 0.87 to 1.09; SUCRA = 87), placebo (SUCRA = 84.8), then netakimab (versus placebo: RR 0.90, 95% CI 0.59 to 1.38; SUCRA = 81.2). The heterogeneity τ for this network overall was 0, which we considered to be low.
2.5. Proportion of participants who achieved PASI 90 at 52 weeks
DIRECT EVIDENCE
We report treatment estimates for pairwise meta‐analyses at drug level in Analysis 7.1 and Analysis 7.2.
7.1. Analysis.

Comparison 7: Secondary outcome ‐ PASI 90 at 52 weeks, Outcome 1: Biologic 1 versus biologic 2
7.2. Analysis.

Comparison 7: Secondary outcome ‐ PASI 90 at 52 weeks, Outcome 2: Small molecule 1 versus small molecule 2
Eleven head‐to‐head comparisons compared two different biologics; seven compared two different dosages of secukinumab, guselkumab, ixekizumab, risankizumab, and apremilast, respectively. We produced two meta‐analyses for the comparisons risankizumab versus ustekinumab and secukinumab versus ustekinumab. For reaching PASI 90 at 52 weeks, risankizumab was more effective than ustekinumab (RR 1.73, 95% CI 1.46 to 2.05). Secukinumab was more effective than ustekinumab in reaching PASI 90 at 52 weeks (RR 1.23, 95% CI 1.15 to 1.31); ixekizumab was more effective than ustekinumab in reaching PASI 90 at 52 weeks (RR 1.30, 95% CI 1.11 to 1.52); bimekizumab was more effective than ustekinumab in reaching PASI 90 at 52 weeks (RR 1.47, 95% CI 1.27 to 1.70); risankizumab was more effective than secukinumab in reaching PASI 90 at 52 weeks (RR 1.52, 95% CI 1.31 to 1.76); bimekizumab was more effective than secukinumab in reaching PASI 90 at 52 weeks (RR 1.19, 95% CI 1.09 to 1.28); guselkumab was more effective than adalimumab in reaching PASI 90 at 52 weeks (RR 1.59, 95% CI 1.40 to 1.81); guselkumab was more effective than secukinumab in reaching PASI 90 (RR 1.21, 95% CI 1.13 to 1.29), and ixekizumab was more effective than adalimumab in reaching PASI 90 (RR 1.34, 95% CI 1.04 to 1.74). Ixekizumab every other week was more effective than ixekizumab every four weeks in reaching PASI 90 at 52 weeks (RR 1.06, 95% CI 1.01 to 1.11); and secukinumab 300 mg was more effective than secukinumab 150 mg in reaching PASI 90 at 52 weeks (RR 0.84, 95% CI 0.78 to 0.91).
We did not conduct network meta‐analyses, given the low number of studies for this outcome.
2.6. Proportion of participants who achieved PASI 75 at 52 weeks
DIRECT EVIDENCE
We report treatment estimates for pairwise meta‐analyses at drug level in Analysis 8.1 and Analysis 8.2.
8.1. Analysis.

Comparison 8: Secondary outcome ‐ PASI 75 at 52 weeks, Outcome 1: Biologic 1 versus biologic 2
8.2. Analysis.

Comparison 8: Secondary outcome ‐ PASI 75 at 52 weeks, Outcome 2: Small molecule 1 versus small molecule 2
Ten head‐to‐head comparisons compared two different biologics; seven compared two different dosages of secukinumab, guselkumab, ixekizumab, risankizumab, and apremilast, respectively. We produced two meta‐analysis for the comparison risankizumab versus ustekinumab and secukinumab versus ustekinumab. For reaching PASI 75 at 52 weeks, risankizumab was more effective than ustekinumab (RR 1.26, 95% CI 1.12 to 1.41). Secukinumab was more effective than ustekinumab in reaching PASI 75 at 52 weeks (RR 1.13, 95% CI 1.04 to 1.22); ixekizumab was more effective than ustekinumab in reaching PASI 75 at 52 weeks (RR 1.16, 95% CI 1.05 to 1.29); risankizumab was more effective than secukinumab in reaching PASI 75 at 52 weeks (RR 1.28, 95% CI 1.14 to 1.44); bimekizumab was more effective than secukinumab in reaching PASI 75 at 52 weeks (RR 1.09, 95% CI 1.02 to 1.16); guselkumab was more effective than secukinumab in reaching PASI 75 at 52 weeks (RR 1.06, 95% CI 1.00 to 1.12); guselkumab was more effective than adalimumab in reaching PASI 75 at 52 weeks (RR 1.40, 95% CI 1.28 to 1.54); no difference was observed for ixekizumab and adalimumab in reaching PASI 75 at week 52. Ixekizumab every other week was more effective than ixekizumab every four weeks in reaching PASI 75 at 52 weeks (RR 1.14, 95% CI 1.07 to 1.22); and secukinumab 300 mg was more effective than secukinumab 150 mg in reaching PASI 75 at 52 weeks (RR 0.90, 95% CI 0.85 to 0.94).
We did not conduct network meta‐analyses, given the low number of studies for this outcome.
3. Assessment of heterogeneity and inconsistency
We did not identify important heterogeneity either in direct meta‐analyses or in network meta‐analysis. The common outcome‐specified network heterogeneity and the prediction intervals suggested the presence of low heterogeneity for all outcomes. We investigated differences in heterogeneity between class‐ and drug‐level analysis, and we also investigated differences in heterogeneity between primary and sensitivity analyses for the primary outcomes (see: section 4. Subgroup and sensitivity analyses). The results were very similar.
The distribution of some participant characteristics (age, sex ratio, weight, severity of psoriasis) did not give an indication of important differences in these characteristics across comparisons (see Figure 15; Figure 16).
15.
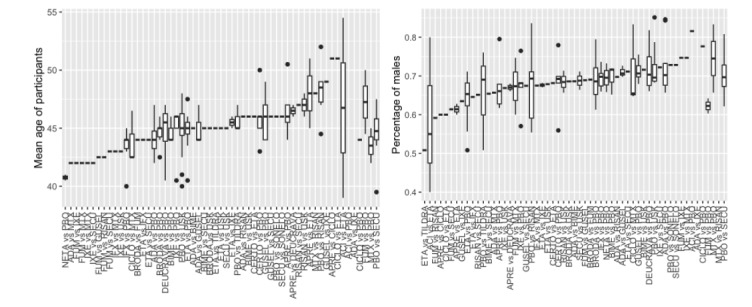
Distributions of age (on the left, mean age in years on the y‐axis) and sex ratio (on the right, percentage of males on the y‐axis) of participants across comparisons (x‐axis)
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
16.
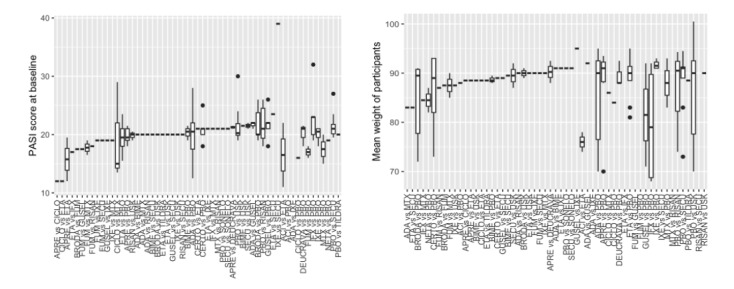
Distributions of PASI score at baseline (on the right, mean PASI on the y‐axis) and weight (on the right, mean weight in kilograms on the y‐axis) of participants across comparisons (x‐axis)
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
At drug‐level analysis, the global test for inconsistency was not significant for any of the outcomes. We detail results of a global test for inconsistency at drug level in Figure 17 and Figure 18 for PASI 90 and SAEs, respectively. The side‐splitting approaches did not indicate inconsistency for SAEs primary outcomes (Table 6). For the PASI 90 primary outcome, the side‐splitting method suggested that there was inconsistency between the direct and indirect evidence on 2 nodes out of 44: apremilast versus placebo (direct evidence RR 6.04, 95% CI 3.89 to 9.36; indirect evidence: RR 11.52, 95% CI 8.25 to 16.09; z‐value of test for disagreement = ‐2.3; P = 0.02) and risankizumab versus placebo (direct evidence RR 15.86, 95% CI 9.53 to 26.37; indirect evidence: RR 27.91, 95% CI 23.25 to 33.50; z‐value of test for disagreement = ‐2.05; P = 0.04). There were a handful of comparisons with statistically significant inconsistency for secondary outcomes, but it did not exceed the expected level of inconsistency that has been suggested by empirical evidence (Veroniki 2013), which is about 10% of the total number of nodes.
17.
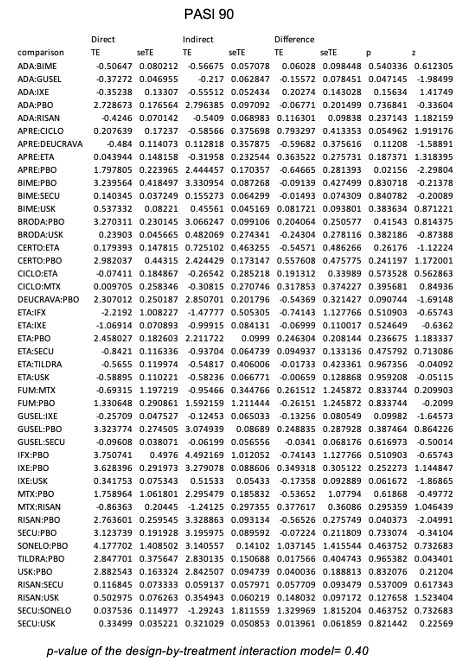
Side‐splitting approach and design‐by‐treatment interaction model for inconsistency for Psoriasis Area and Severity Index (PASI) 90
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
18.
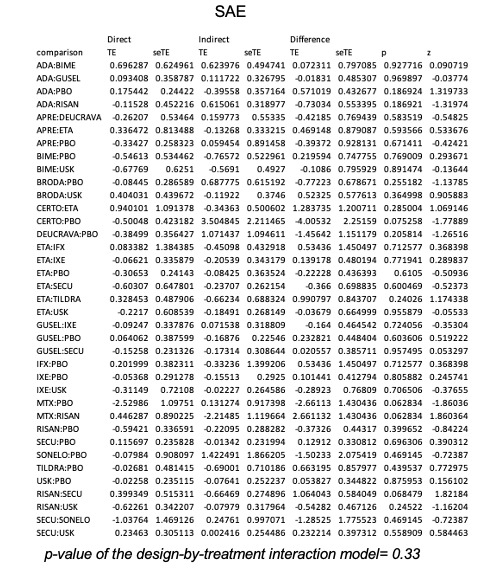
Side‐splitting approach and design‐by‐treatment interaction model for inconsistency for serious adverse events (SAEs)
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
4. Subgroup and sensitivity analyses
As we found no heterogeneity, we did not perform subgroup analyses. From a clinical point of view, it could nevertheless be interesting to have specific efficacy/safety data depending on participants' comorbidities or psoriasis characteristics. However, we did not have enough data for any of the aforementioned characteristics, and were therefore unable to run subgroup analyses and meta‐regressions to investigate their potential effects on the results.
Results of the sensitivity analyses involving the following were similar to those of the main analysis for the two primary outcomes:
excluding studies with fewer than 50 participants (Figure 19) (the heterogeneity τ for this subgroup network was 0 for PASI 90 and SAEs, which we considered to be low);
completers (Figure 20) (the heterogeneity τ for this subgroup network was 0 for PASI 90 and SAEs, respectively, which we considered to be low);
analyses at dose‐level splitting approved dosages versus other dosages for each drug (Figure 21) (the heterogeneity τ for this subgroup network was 0.01 for PASI 90 and 0 for SAEs, which we considered to be low);
excluding studies at high risk of bias (Figure 22) (the heterogeneity τ for this subgroup network was 0 for PASI 90 and SAEs, which we considered to be low);
analysing only the studies with a short‐term assessment from eight to 16 weeks (Figure 23): the heterogeneity τ for this subgroup network was 0 for PASI 90 and SAEs, which we considered to be low;
analysing including trials with systemic treatment‐naïve participants (Figure 24): the heterogeneity τ for this subgroup network was 0.01 and 0 for PASI 90 and SAEs, respectively, which we considered to be low;
-
analysing only drugs and dosages approved by European Medicines Agency for plaque psoriasis (Figure 25; Figure 26):
non‐biological systemic treatments: FAEs, ciclosporin, methotrexate;
small molecules: apremilast, deucravacitinib;
anti‐TNF alpha: infliximab, etanercept, adalimumab, certolizumab pegol;
anti‐IL12/23: ustekinumab;
anti‐IL17: secukinumab, brodalumab, ixekizumab, bimekizumab;
anti‐IL23: tildrakizumab, guselkumab, risankizumab.
19.
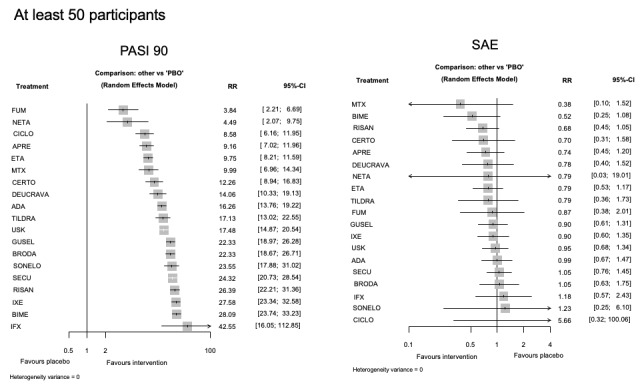
Sensitivity analyses ‐ interval plot. Network meta‐analysis results for primary outcomes (PASI 90 and serious adverse events, left and right forest plot respectively) for trials with at least 50 participants.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
CI: confidence interval; PASI: Psoriasis Area and Severity Index; RR: risk ratio
20.
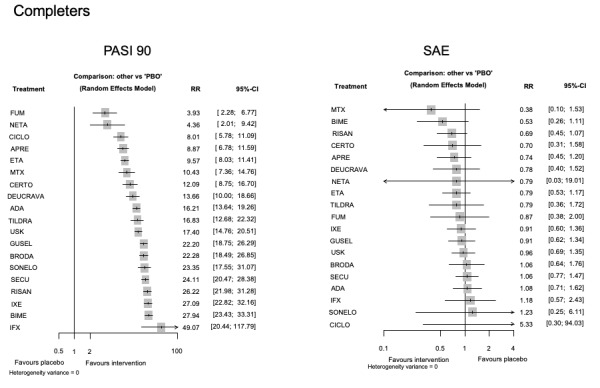
Sensitivity analyses ‐ interval plot. Network meta‐analysis results for primary outcomes (PASI 90 and serious adverse events, left and right forest plot respectively) for the completers.
Outcomes were measured at the induction phase (assessment from 8 to 24 weeks after randomisation).
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
CI: confidence interval; PASI: Psoriasis Area and Severity Index; RR: risk ratio
21.
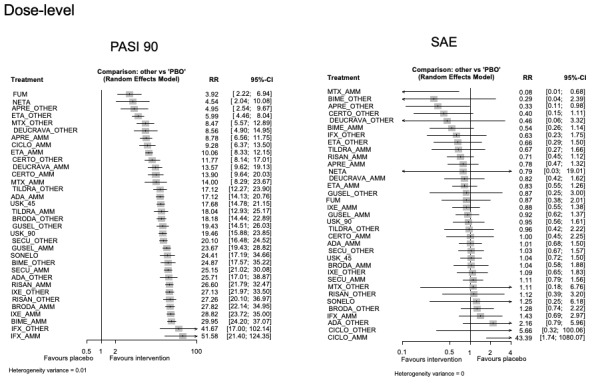
Sensitivity analyses ‐ interval plot. Network meta‐analysis results for primary outcomes (PASI 90 and serious adverse events, left and right forest plot respectively) for all the interventions depending on the doses: approved dosages versus other dosages.
Outcomes were measured at the induction phase (assessment from 8 to 24 weeks after randomisation).
MTX_AMM/Other: methotrexate ≥ 15 mg per week/< 15 mg per week; CICLO_AMM/Other: ciclosporin ≥ 3 mg/kg/day/< 3 mg/kg/day; ACI_AMM/Other: acitretin ≥ 35 mg per day/< 35 mg per day; FUM: fumaric acid esters all dosages; APRE_AMM/Other: apremilast 30 mg twice daily/other dosages; ETA_AMM/Other: etanercept 50 mg twice a week/Other dosage; IFX_AMM/Other: infliximab 5 mg/kg week 0, 2, 4 every 6 weeks/Other dosages; ADA_AMM/Other: adalimumab 80 mg week 0, 40 mg week 1 then 40 mg every other week/Other dosages; CERTO_AMM/Other: certolizumab 400 mg at week 0, 2,4 then 400 mg every other week or other dosages/Other dosages; USK 45/90: ustekinumab 45/90 mg; SECU_AMM/Other: secukinumab 300 mg at week 0, 1, 2, 3, and 4 then every 4 weeks or other dosages/other dosages; IXE_AMM/Other: ixekizumab 160 mg at week 0 then 80 mg every other week until week 12 then 80 mg monthly or other dosages; TILDRA_AMM/Other: tildrakizumab 100 mg at week 0 and 4 then every 12 weeks/Other dosages; GUSEL 100: guselkumab 100 mg per injection; BRODA_AMM/Other: brodalumab 210 mg at week 0, 1, 2 then every other week/other dosages; RISAN_AMM/Other: risankizumab, SC, 150 mg (two 75 mg injections) at week 0, week 4, and every 12 weeks thereafter/other dosages; BIME_AMM/Other: bimekizumab, SC, 320 mg (2 x 160 mg injections) at week 0, 4, 8, 12, 16, and every 8 weeks thereafter/other dosages. DEUCRACA (deucravacitinib), SONELO (sonelokimab), and NETA (netakimab) were grouped in one dosage whatever the dosages.
AMM: 'approved dosage'; CI: confidence interval; PASI: Psoriasis Area and Severity Index; RR: risk ratio
22.
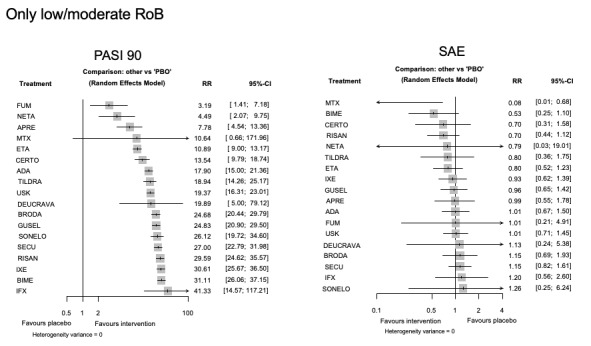
Sensitivity analyses ‐ interval plot. Network meta‐analysis results for primary outcomes (PASI 90 and serious adverse events, left and right forest plot respectively) for all the interventions excluding studies at high risk of bias.
Outcomes were measured at the induction phase (assessment from 8 to 24 weeks after randomisation).
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
CI: confidence interval; PASI: Psoriasis Area and Severity Index; RR: risk ratio
23.
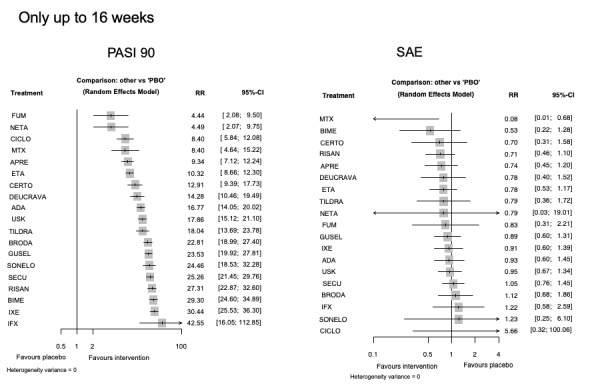
Sensitivity analyses ‐ interval plot. Network meta‐analysis results for primary outcomes (PASI 90 and serious adverse events, left and right forest plot respectively) for all the interventions including studies with a short‐term assessment from 8 to 16 weeks.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
CI: confidence interval; PASI: Psoriasis Area and Severity Index; RR: risk ratio
24.
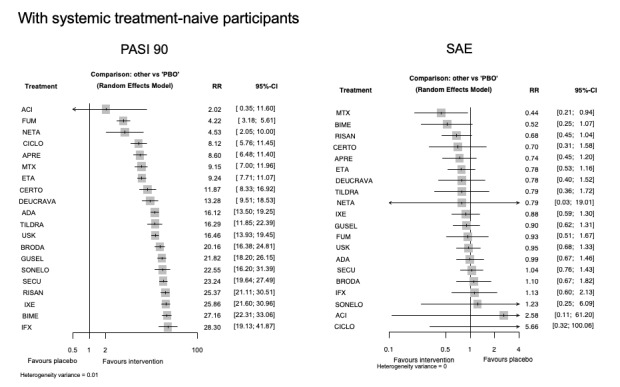
Sensitivity analyses ‐ interval plot. Network meta‐analysis results for primary outcomes (PASI 90 and serious adverse events, left and right forest plot respectively) for all the interventions including studies with systemic treatment‐naive participants.
Outcomes were measured at the induction phase (assessment from 8 to 24 weeks after randomisation).
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
CI: confidence interval; PASI: Psoriasis Area and Severity Index; RR: risk ratio
25.
Network diagrams for PASI 90 (A) and SAE (B) when the analyses were restricted to drugs approved by the European Medicines Agency.
The size of the nodes is proportional to the total number of participants allocated to each intervention and the thickness of the lines proportional to the number of studies evaluating each direct comparison.
Relative effects from the network meta‐analyses against placebo with their 95% confidence interval (C and D).
Interval plot. Network meta‐analysis estimates of the interventions versus placebo for the primary outcomes.
Two‐dimensional ranking plot based on surface under the cumulative ranking curve (SUCRA) values for benefit (PASI 90) and acceptability (serious adverse events) at drug level (E).
Ranking plot representing simultaneously the efficacy (x‐axis, PASI 90) and the acceptability (y‐axis, serious adverse events) of all the interventions (class and drug levels) for patients with moderate‐to‐severe psoriasis. Optimal treatment should be characterised by both high efficacy and acceptability and should be in the right upper corner of this graph.
Outcomes were measured at the induction phase (assessment from 8 to 24 weeks after randomisation).
The different colours represent different groups of interventions considering their performance on both outcomes simultaneously. Interventions belonging to the same group are assumed to have a similar performance when the two primary outcomes are considered jointly.
Ranking analysis for PASI 90 and SAE (F).
CI: confidence interval; PASI: Psoriasis Area and Severity Index; RR: risk ratio; SAE: serious adverse events; SUCRA: surface under the cumulative ranking curve
ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; TILDRA: tildrakizumab; USK: ustekinumab
26.
Relative effects of the intervention as estimated from the network meta‐analysis model for Psoriasis Area and Severity Index (PASI) 90 and serious adverse events (SAEs) for drugs approved by the European Medicines Agency for plaque psoriasis (sensitivity analysis)
Outcomes were all measured at the induction phase (assessment from 8 to 24 weeks after randomisation). Drugs are reported in order of primary benefit ranking. Each cell contains the risk ratio (RR) and 95% confidence interval for the two primary outcomes (PASI 90 and SAEs) of the intervention in the respective column versus the comparator in the respective row. RRs larger than 1 for the lower triangle and smaller than 1 for the upper triangle favour the treatment on the left.
ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; TILDRA: tildrakizumab; USK: ustekinumab
Figure 25 shows the network diagrams for PASI 90 (A) and SAE (B) when the analyses were restricted to drugs and dosages approved by the European Medicines Agency, the relative effects from the network meta‐analyses against placebo with their 95% confidence (C&D), the two‐dimensional ranking plot based on surface under the cumulative ranking curve (SUCRA) values for benefit (PASI 90) and acceptability (serious adverse events) at drug level (E), and the ranking analysis for PASI 90 and SAE (F).
Figure 26 shows the network meta‐analysis estimates of PASI 90 and SAEs for each comparison at drug level. The heterogeneity τ for this subgroup network was 0 for PASI 90 and SAEs, which we considered to be low.
Compared with the principal analyses, when considering the only licensed drugs and dosages, the main difference was that there was no significant difference between infliximab, bimekizumab, ixekizumab, risankizumab, and brodalumab in terms of reaching PASI 90. Bimekizumab and ixekizumab were significantly more likely to reach PASI 90 than secukinumab. Bimekizumab, ixekizumab, risankizumab, brodalumab, and secukinumab were significantly more likely to reach PASI 90 than guselkumab. Infliximab, bimekizumab, ixekizumab, risankizumab, brodalumab, secukinumab, and guselkumab were significantly more likely to reach PASI 90 than ustekinumab, tildrakizumab, the three anti‐TNF alpha agents (adalimumab, certolizumab, and etanercept), and deucravacitinib. Adalimumab, tildrakizumab, ustekinumab, and deucravacitinib were superior to etanercept. No significant difference was shown between apremilast and two non‐biological drugs: ciclosporin and methotrexate.
5. Reporting bias
The comparison‐adjusted funnel plots generally appeared symmetrical, and only the graph for quality of life presented some evidence of small‐study effects, which might be caused by selective outcome reporting (Figure 27). As the funnel plots were symmetrical, we did not consider running meta‐regression.
27.
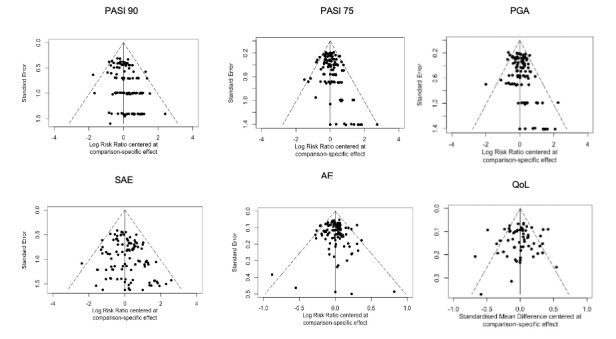
Funnel plot for network meta‐analysis of all the outcomes.
AE: adverse event; lnRR: mean effect size; PASI: Psoriasis Area and Severity Index; QoL: specific quality of life scale; RR: risk ratio; SAE: serious adverse events; SAE without worsening of psoriasis corresponds to SAE after exclusion of flares of psoriasis; SMD: standardised mean difference
6. Grading of the evidence
We present results of evaluation of the certainty of evidence for the primary efficacy and safety outcomes in Table 7; Table 8 and Figure 1; Figure 28; Figure 29.
7. Study bias distribution for PASI 90 using CINeMA.
| Comparison | Number of studies | Within‐study bias | Reporting bias | Indirectness | Imprecision | Heterogeneity | Incoherence | Confidence rating | Reason(s) for downgrading |
| ADA:BIME | 1 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| ADA:GUSEL | 3 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| ADA:IXE | 1 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| ADA:PBO | 8 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| ADA:RISAN | 1 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| APRE:CICLO | 1 | Major concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| APRE:DEUCRAVA | 2 | Major concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| APRE:ETA | 2 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| APRE:PBO | 7 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| BIME:PBO | 3 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| BIME:SECU | 1 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| BIME:USK | 1 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| BRODA:PBO | 5 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| BRODA:USK | 2 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| CERTO:ETA | 1 | Some concerns | Low risk | Some concerns | Some concerns | Some concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision", "Heterogeneity") |
| CERTO:PBO | 5 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| CICLO:ETA | 1 | Major concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CICLO:MTX | 2 | Major concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| DEUCRAVA:PBO | 4 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| ETA:IFX | 1 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| ETA:IXE | 2 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| ETA:PBO | 15 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| ETA:SECU | 1 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| ETA:TILDRA | 1 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| ETA:USK | 1 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| FUM:MTX | 1 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| FUM:PBO | 2 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| GUSEL:IXE | 1 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| GUSEL:PBO | 5 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| GUSEL:SECU | 1 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| IFX:PBO | 5 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| IXE:PBO | 5 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| IXE:USK | 1 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| MTX:PBO | 2 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| MTX:RISAN | 1 | Major concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| NETA:PBO | 2 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| PBO:RISAN | 6 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Low | ("Indirectness") |
| PBO:SECU | 16 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| PBO:SONELO | 1 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| PBO:TILDRA | 3 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| PBO:USK | 11 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| RISAN:SECU | 1 | No concerns | Low risk | Some concerns | Some concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| RISAN:USK | 3 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| SECU:SONELO | 1 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| SECU:USK | 2 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| ADA:APRE | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| ADA:BRODA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| ADA:CERTO | 0 | No concerns | Low risk | Some concerns | Some concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ADA:CICLO | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| ADA:DEUCRAVA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| ADA:ETA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| ADA:FUM | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| ADA:IFX | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| ADA:MTX | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| ADA:NETA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| ADA:SECU | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| ADA:SONELO | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| ADA:TILDRA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ADA:USK | 0 | No concerns | Low risk | Some concerns | Some concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| APRE:BIME | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| APRE:BRODA | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| APRE:CERTO | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| APRE:FUM | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| APRE:GUSEL | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| APRE:IFX | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| APRE:IXE | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Within‐study bias", "Indirectness") |
| APRE:MTX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| APRE:NETA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| APRE:RISAN | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| APRE:SECU | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| APRE:SONELO | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| APRE:TILDRA | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| APRE:USK | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| BIME:BRODA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| BIME:CERTO | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| BIME:CICLO | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| BIME:DEUCRAVA | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| BIME:ETA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| BIME:FUM | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| BIME:GUSEL | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| BIME:IFX | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BIME:IXE | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BIME:MTX | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| BIME:NETA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| BIME:RISAN | 0 | No concerns | Low risk | Some concerns | Some concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BIME:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BIME:TILDRA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| BRODA:CERTO | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| BRODA:CICLO | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| BRODA:DEUCRAVA | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| BRODA:ETA | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| BRODA:FUM | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| BRODA:GUSEL | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BRODA:IFX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BRODA:IXE | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| BRODA:MTX | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| BRODA:NETA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| BRODA:RISAN | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| BRODA:SECU | 0 | No concerns | Low risk | Some concerns | Some concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BRODA:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BRODA:TILDRA | 0 | No concerns | Low risk | Some concerns | No concerns | Some concerns | No concerns | Moderate | ("Indirectness", "Heterogeneity") |
| CERTO:CICLO | 0 | Some concerns | Low risk | Some concerns | Some concerns | Some concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision", "Heterogeneity") |
| CERTO:DEUCRAVA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CERTO:FUM | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| CERTO:GUSEL | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| CERTO:IFX | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| CERTO:IXE | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| CERTO:MTX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CERTO:NETA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| CERTO:RISAN | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| CERTO:SECU | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| CERTO:SONELO | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| CERTO:TILDRA | 0 | No concerns | Low risk | Some concerns | Some concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| CERTO:USK | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| CICLO:DEUCRAVA | 0 | Major concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| CICLO:FUM | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| CICLO:GUSEL | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| CICLO:IFX | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| CICLO:IXE | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| CICLO:NETA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CICLO:PBO | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| CICLO:RISAN | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| CICLO:SECU | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| CICLO:SONELO | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| CICLO:TILDRA | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| CICLO:USK | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| DEUCRAVA:ETA | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| DEUCRAVA:FUM | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| DEUCRAVA:GUSEL | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| DEUCRAVA:IFX | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| DEUCRAVA:IXE | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| DEUCRAVA:MTX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| DEUCRAVA:NETA | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| DEUCRAVA:RISAN | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| DEUCRAVA:SECU | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| DEUCRAVA:SONELO | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| DEUCRAVA:TILDRA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| DEUCRAVA:USK | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| ETA:FUM | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| ETA:GUSEL | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| ETA:MTX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| ETA:NETA | 0 | No concerns | Low risk | Some concerns | Some concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ETA:RISAN | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| ETA:SONELO | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| FUM:GUSEL | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| FUM:IFX | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| FUM:IXE | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| FUM:NETA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| FUM:RISAN | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| FUM:SECU | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| FUM:SONELO | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| FUM:TILDRA | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| FUM:USK | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| GUSEL:IFX | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| GUSEL:MTX | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| GUSEL:NETA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| GUSEL:RISAN | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| GUSEL:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| GUSEL:TILDRA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| GUSEL:USK | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| IFX:IXE | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IFX:MTX | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| IFX:NETA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| IFX:RISAN | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| IFX:SECU | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IFX:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IFX:TILDRA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| IFX:USK | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| IXE:MTX | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| IXE:NETA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| IXE:RISAN | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IXE:SECU | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| IXE:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IXE:TILDRA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| MTX:NETA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| MTX:SECU | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| MTX:SONELO | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| MTX:TILDRA | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| MTX:USK | 0 | Some concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | Moderate | ("Within‐study bias", "Indirectness") |
| NETA:RISAN | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| NETA:SECU | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| NETA:SONELO | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| NETA:TILDRA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| NETA:USK | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| RISAN:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| RISAN:TILDRA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| SECU:TILDRA | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| SONELO:TILDRA | 0 | No concerns | Low risk | Some concerns | Some concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| SONELO:USK | 0 | No concerns | Low risk | Some concerns | No concerns | No concerns | No concerns | High | ("Indirectness") |
| TILDRA:USK | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
8. Study bias distribution for serious adverse events using CINeMA.
| Comparison | Number of studies | Within‐study bias | Reporting bias | Indirectness | Imprecision | Heterogeneity | Incoherence | Confidence rating | Reason(s) for downgrading |
| ADA:BIME | 1 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ADA:GUSEL | 3 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ADA:PBO | 9 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ADA:RISAN | 1 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| APRE:CICLO | 1 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| APRE:DEUCRAVA | 2 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| APRE:ETA | 2 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| APRE:PBO | 9 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BIME:PBO | 3 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BIME:USK | 1 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BRODA:PBO | 5 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BRODA:USK | 2 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CERTO:ETA | 1 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| CERTO:PBO | 5 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| CICLO:ETA | 1 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CICLO:MTX | 2 | Major concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CICLO:PBO | 1 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| DEUCRAVA:PBO | 4 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| ETA:IFX | 1 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ETA:IXE | 2 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ETA:PBO | 13 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ETA:SECU | 1 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ETA:TILDRA | 1 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ETA:USK | 1 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| FUM:MTX | 1 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| FUM:PBO | 2 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| GUSEL:IXE | 1 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| GUSEL:PBO | 5 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| GUSEL:SECU | 1 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IFX:PBO | 6 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IXE:PBO | 5 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IXE:SECU | 1 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IXE:USK | 1 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| MTX:PBO | 2 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | Major concerns | Very low | ("Within‐study bias", "Indirectness", "Imprecision", "Incoherence") |
| MTX:RISAN | 1 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| NETA:PBO | 2 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| PBO:RISAN | 6 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| PBO:SECU | 16 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| PBO:SONELO | 1 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| PBO:TILDRA | 3 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| PBO:USK | 12 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| RISAN:SECU | 1 | No concerns | Low risk | Some concerns | Major concerns | No concerns | Major concerns | Low | ("Indirectness", "Imprecision", "Incoherence") |
| RISAN:USK | 3 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| SECU:SONELO | 1 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| SECU:USK | 2 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ADA:APRE | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ADA:BRODA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ADA:CERTO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ADA:CICLO | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| ADA:DEUCRAVA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| ADA:ETA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ADA:FUM | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| ADA:IFX | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ADA:IXE | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ADA:MTX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| ADA:NETA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ADA:SECU | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ADA:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ADA:TILDRA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ADA:USK | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| APRE:BIME | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| APRE:BRODA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| APRE:CERTO | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| APRE:FUM | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| APRE:GUSEL | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| APRE:IFX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| APRE:IXE | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| APRE:MTX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| APRE:NETA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| APRE:RISAN | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| APRE:SECU | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| APRE:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| APRE:TILDRA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| APRE:USK | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BIME:BRODA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BIME:CERTO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BIME:CICLO | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BIME:DEUCRAVA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BIME:ETA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BIME:FUM | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BIME:GUSEL | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BIME:IFX | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BIME:IXE | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BIME:MTX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BIME:NETA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BIME:RISAN | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BIME:SECU | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BIME:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BIME:TILDRA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BRODA:CERTO | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BRODA:CICLO | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BRODA:DEUCRAVA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BRODA:ETA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BRODA:FUM | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BRODA:GUSEL | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BRODA:IFX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BRODA:IXE | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BRODA:MTX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BRODA:NETA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BRODA:RISAN | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| BRODA:SECU | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BRODA:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| BRODA:TILDRA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| CERTO:CICLO | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CERTO:DEUCRAVA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CERTO:FUM | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CERTO:GUSEL | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| CERTO:IFX | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| CERTO:IXE | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| CERTO:MTX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CERTO:NETA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CERTO:RISAN | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| CERTO:SECU | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| CERTO:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| CERTO:TILDRA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| CERTO:USK | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| CICLO:DEUCRAVA | 0 | Major concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CICLO:FUM | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CICLO:GUSEL | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CICLO:IFX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CICLO:IXE | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CICLO:NETA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CICLO:RISAN | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CICLO:SECU | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CICLO:SONELO | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CICLO:TILDRA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| CICLO:USK | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| DEUCRAVA:ETA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| DEUCRAVA:FUM | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| DEUCRAVA:GUSEL | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| DEUCRAVA:IFX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| DEUCRAVA:IXE | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| DEUCRAVA:MTX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| DEUCRAVA:NETA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| DEUCRAVA:RISAN | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| DEUCRAVA:SECU | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| DEUCRAVA:SONELO | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| DEUCRAVA:TILDRA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| DEUCRAVA:USK | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| ETA:FUM | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| ETA:GUSEL | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Imprecision") |
| ETA:MTX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| ETA:NETA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ETA:RISAN | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| ETA:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| FUM:GUSEL | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| FUM:IFX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| FUM:IXE | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| FUM:NETA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| FUM:RISAN | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| FUM:SECU | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| FUM:SONELO | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| FUM:TILDRA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| FUM:USK | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| GUSEL:IFX | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| GUSEL:MTX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| GUSEL:NETA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| GUSEL:RISAN | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| GUSEL:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| GUSEL:TILDRA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| GUSEL:USK | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IFX:IXE | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IFX:MTX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| IFX:NETA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IFX:RISAN | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IFX:SECU | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IFX:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IFX:TILDRA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IFX:USK | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IXE:MTX | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| IXE:NETA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IXE:RISAN | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IXE:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| IXE:TILDRA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| MTX:NETA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| MTX:SECU | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| MTX:SONELO | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| MTX:TILDRA | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| MTX:USK | 0 | Some concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Low | ("Within‐study bias", "Indirectness", "Imprecision") |
| NETA:RISAN | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| NETA:SECU | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| NETA:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| NETA:TILDRA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| NETA:USK | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| RISAN:SONELO | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| RISAN:TILDRA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| SECU:TILDRA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| SONELO:TILDRA | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| SONELO:USK | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
| TILDRA:USK | 0 | No concerns | Low risk | Some concerns | Major concerns | No concerns | No concerns | Moderate | ("Indirectness", "Imprecision") |
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
28.
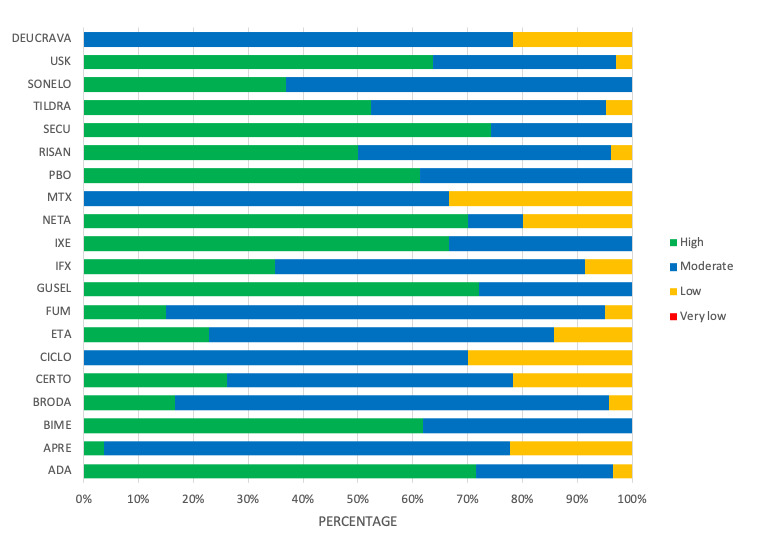
Certainty of evidence per drug for PASI 90 using CINeMA
Each drug is presented as a bar, which indicates the composition of the four‐level confidence of evidence from all comparisons including that drug. Green: high confidence; blue: moderate confidence; yellow: low confidence; red: very low confidence.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; CINeMA: Confidence in Network Meta‐Analysis; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PASI: Psoriasis Area and Severity Index; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
29.
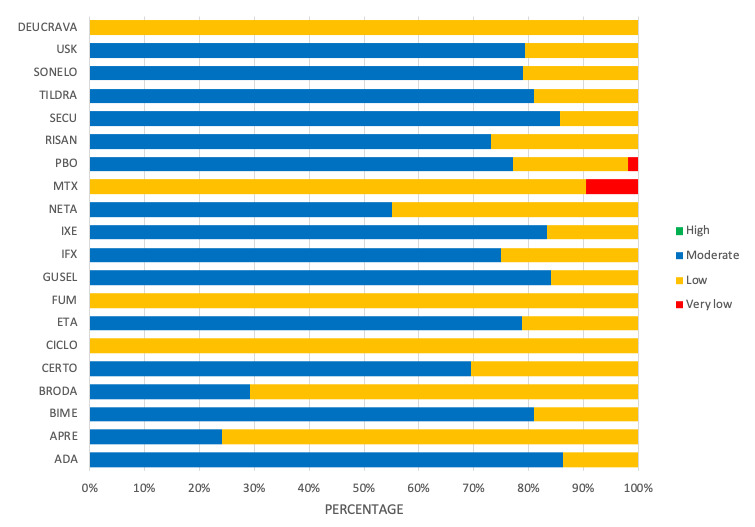
Certainty of evidence per drug for serious adverse events using CINeMA
Each drug is presented as a bar, which indicates the composition of the four‐level confidence of evidence from all comparisons including that drug. Green: high confidence; blue: moderate confidence; yellow: low confidence; red: very low confidence.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BIME: bimekizumab; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; CINeMA: Confidence in Network Meta‐Analysis; DEUCRAVA: deucravacitinib; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; NETA: netakimab; PBO: placebo; RISAN: risankizumab; SECU: secukinumab; SONELO: sonelokimab; TILDRA: tildrakizumab; USK: ustekinumab
Table 7 and Table 8 represent PASI 90 and SAEs, respectively, the evaluation of concerns (no concern, some concerns, or major concerns) for each domain assessed (within‐study bias, reporting bias, indirectness, imprecision, heterogeneity, and incoherence). We detected no reporting bias for any comparison for PASI 90 or SAEs. There were some concerns that indirectness was present for any comparison for PASI 90 or SAEs, as the young age, the high proportion of males, and the high level of disease severity in the network meta‐analysis may not be typical of patients seen in daily clinical practice. After the judgement for all six of the domains, our overall confidence in the evidence for each comparison is rated high, moderate, low, and very low, as described in the Methods section. Results for overall confidence in the evidence are available in Table 7, Table 8, and Figure 1.
Figure 28 and Figure 29 represent by drug the overall percentage of comparisons including that drug assessed as high, moderate, low, and very low certainty of evidence. For PASI 90, the overall certainty of the evidence was moderate to high. None of the comparisons were assessed as very low. For deucravacitinib, certolizumab, methotrexate, and ciclosporin, the certainty of evidence was low for most comparisons including these treatments. For infliximab, risankizumab, sonelokimab, brodalumab, ustekinumab, tildrakizumab, etanercept, apremilast, and FAEs, the certainty of evidence was moderate for most comparisons. For all other drugs, the certainty of evidence was high for most comparisons. Reasons for downgrading to moderate or low certainty were within‐study bias or imprecision, or both. For SAEs, the overall certainty of evidence was low to moderate. None of the comparisons were assessed as very low, except methotrexate versus placebo. For deucravacitinib, certolizumab, methotrexate, apremilast, ciclosporin, netakimab, and FAEs, the certainty of evidence was low. The certainty of evidence was moderate for all other treatments. Reasons for downgrading to moderate or low certainty were within‐study bias or imprecision, or both.
Discussion
Summary of main results
Our review and meta‐analysis compares all systemic pharmacological drugs and systemic drugs undergoing phase II/III trials that are used for moderate‐to‐severe psoriasis in 2022.
This updated review includes 179 studies, involving 62,339 randomised adult participants, which assessed most outcomes during the induction phase (from 8 to 24 weeks after randomisation). Participants in the included studies were young, with a mean age of 44.6 years, and had moderate‐to‐severe psoriasis with an overall mean Psoriasis Area and Severity Index (PASI) score at baseline of 20.4. One hundred trials compared systemic treatment against placebo, 57 were head‐to‐head trials, 19 had both an active comparator and a placebo, and three compared three systemic treatments. Twenty trials had a co‐intervention, mainly phototherapy. Twelve trials assessed biosimilars versus original drugs for adalimumab or etanercept. Finally, 138 studies declared pharmaceutical company funding and 24 studies did not report the source of funding.
We included 140 studies (without co‐intervention and without trials in biosimilar development), involving 54,815 participants (88% of the participants in this review), in the classical or network meta‐analysis (NMA). Non‐biological systemic agents, the oldest class‐level treatment (acitretin, ciclosporin, fumaric acid esters (FAEs), methotrexate); small molecule agents (apremilast, deucravacitinib); anti‐TNF alpha treatments (etanercept, infliximab, adalimumab, certolizumab); an anti‐IL12/23 treatment (ustekinumab); anti‐IL17 treatments (secukinumab, ixekizumab, brodalumab, bimekizumab, sonelokimab ‐ in Russia); and anti‐IL23 (guselkumab, tildrakizumab, risankizumab) have all been approved for psoriasis, except for netakimab, which is a new anti‐IL17 drug with ongoing phase III trials.
The following results are based on network meta‐analysis.
All of the assessed interventions appeared superior to placebo in terms of reaching PASI 90.
At class level, network meta‐analysis showed that the biologics anti‐IL17, anti‐IL23, anti‐IL12/23, and anti‐TNF alpha outperformed the non‐biological agents in reaching PASI 90. Anti‐IL17 treatment showed a higher proportion of patients reaching PASI 90 compared to all of the interventions.
For reaching PASI 90, the most effective drugs when compared to placebo were (in SUCRA (surface under the cumulative ranking curve) rank order): infliximab (high‐certainty evidence), bimekizumab (high‐certainty evidence), ixekizumab (high‐certainty evidence), and risankizumab (high‐certainty evidence). The clinical effectiveness of these drugs was similar when compared against each other; see Figure 1. Bimekizumab and ixekizumab were significantly more likely to reach PASI 90 than secukinumab. Bimekizumab, ixekizumab, and risankizumab were significantly more likely to reach PASI 90 than brodalumab and guselkumab. Infliximab, anti‐IL17 drugs (bimekizumab, ixekizumab, secukinumab, and brodalumab), and anti‐IL23 drugs (risankizumab and guselkumab), except tildrakizumab, were significantly more likely to reach PASI 90 than ustekinumab, three anti‐TNF alpha agents (adalimumab, certolizumab, and etanercept), and deucravacitinib. Ustekinumab was superior to certolizumab. Adalimumab, tildrakizumab, and ustekinumab were superior to etanercept. No significant difference was shown between apremilast and two non‐biological drugs: ciclosporin and methotrexate. Few trials assessed the efficacy of netakimab, sonelokimab, deucravacitinib, acitretin, ciclosporin, fumaric acid esters, and methotrexate in this network, so the results for these drugs have to be interpreted with caution. The results were similar to PASI 90 for the other benefits outcomes (PASI 75 and PGA 0/1).
We found no significant difference between any of the interventions and the placebo for the risk of serious adverse events (SAEs). Methotrexate (very low‐certainty evidence), bimekizumab (moderate‐certainty evidence), risankizumab (moderate‐certainty evidence), apremilast (low‐certainty evidence), and certolizumab (moderate‐certainty evidence) had the highest SUCRA at drug level for all the SAEs.
There was often poor reporting of information about quality of life, and these data were absent for several of the interventions.
Finally, considering both benefit (PASI 90 outcome) and acceptability (SAE outcome), risankizumab and bimekizumab appeared to be the better compromise between benefit and acceptability, bearing in mind the limitations that affect interpretation of the SAE results, such as the very low number of events on which they were based. Other highly effective treatments (ixekizumab and infliximab) had SAEs.
Overall completeness and applicability of evidence
We were able to draw some conclusions on the effectiveness (and ranking) of the systemic treatment options for moderate‐to‐severe chronic plaque psoriasis during the induction phase. Long‐term benefits and harms data are lacking. Specific details are listed below.
Participants
Participants in the included studies had a mean age of 44.6 years and had moderate‐to‐severe psoriasis; more than 60% were males, with an overall mean PASI score at baseline of 20.4 (range: 9.5 to 39) and a duration of psoriasis of 16.5 years (range 4.5 to 21.5). This young age and the high level of disease severity may not be typical of patients seen in daily clinical practice, or those who need a first‐line systemic treatment.
In addition, participants selected for randomised controlled trials (RCTs) generally have few major comorbidities. Almost all studies including one biological arm excluded patients with a history of infectious diseases or malignancies and signs of severe renal, cardiac, hepatic, demyelinating, or other disorders. This may impact the generalisability of these results for clinical practice. However, some participant characteristics (such as being overweight, presence of metabolic syndrome) were reflective of a moderate‐to‐severe psoriasis population, comparable to literature data (Grodner 2021).
Interventions
Evidence on 20 active treatments included in this review was derived from 179 trials (searched for up to October 2022). We included all interventions, irrespective of the dose. Thus, we increased the number of available RCTs for each intervention and had more power to assess SAEs and adverse events (AEs). The number of studies included in the NMA was still low for the following interventions: netakimab, sonelokimab, deucravacitinib, ciclosporin, fumaric acid esters, and methotrexate, meaning that we must be cautious about the conclusions drawn for these drugs. The results from the sensitivity analyses, using (i) a standard dose for each intervention and (ii) only approved drugs and dosages, were similar for PASI 90 (and SAEs) compared to the main analyses, giving us confidence in the results of the main analysis.
Comparisons
Most studies included in the review were only placebo‐controlled (around 56%). Once the benefit of a treatment has been established against placebo using high‐quality evidence, only head‐to‐head trials would be helpful to provide physicians with benefit estimates between the different biologics, based on stronger evidence than indirect comparisons.
Outcomes
Many of the trials included in this review provided evidence for the proportion of participants who reached PASI 90, PASI 75, or Physician Global Assessment (PGA) 0/1, or who experienced SAEs or AEs. We chose PASI 90 as the main benefit outcome. The differences in PASI 90 rates must be balanced against the differences in quality of life improvements that are observed. Results for both outcomes cannot be correlated. On the other hand, patient‐reported outcome (PRO) data were scanty and poorly reported in our review. Moreover, the heterogeneity of the scales used for quality of life in psoriasis trials required using the standardised mean difference (SMD) in the network. The SMD shows the difference in standard deviations of the outcome. It has been suggested that values of 0.2, 0.5, and 0.8 might indicate small, moderate, and large magnitude of the effect size (Cohen 1988). So, from a clinical point of view, the interpretation of the results was difficult: a significant result for a PRO between two drugs did not mean that the result was clinically useful for the patients. Results for SAEs have to be interpreted cautiously, because RCTs do not last long enough and are not powered to be able to detect rare and severe adverse events. The results of our analysis assessing SAEs without psoriasis flares did not differ from those of the primary outcome. We did not summarise individual SAE types or classes of SAE in this review, in part because classification differed across different data sources. This was the subject of a separate detailed assessment of types of SAE, adverse events leading to discontinuation of trial medication, and system‐organ class adverse events (Afach 2021).
Timing
All of the trials included in the NMAs assessed the benefit of the different treatments during the induction treatment phase (from 8 to 24 weeks). Assessment of longer‐term outcomes is also relevant for this chronic disease. The trials were designed to detect differences in the severity of psoriasis in response to therapy over short periods of treatment, and are often underpowered and of insufficient duration to detect rare or long‐term adverse events. It is therefore of interest to conduct studies taking into account the induction of remission but also the long‐term management (long‐term remission) and the long‐term safety of the drug. In order to provide long‐term information on the safety of the treatments included in this review, it will be necessary also to evaluate non‐randomised studies and postmarketing reports released from regulatory agencies.
Due to the large number of ongoing trials (n = 45), it is important to maintain this review as a living review to increase the accuracy of the treatments being tested by incorporating new evidence as it becomes available.
Quality of the evidence
Overall, our confidence in the treatment estimates for PASI 90 is high or moderate for most comparisons involving anti‐IL17, anti‐IL12/23, anti‐IL23, or anti‐TNF alpha agents, and apremilast. We judged our confidence in treatment estimates for PASI 90 to be low for the comparisons involving non‐biological systemic agents and deucravacitinib; we downgraded the certainty of the evidence for risk of bias, imprecision, or both. We judged our confidence in the treatment estimates for SAEs to be low to moderate; we downgraded the certainty of the evidence for imprecision and risk of bias.
Risk of bias
The risks of bias in the included studies appear to be globally low (Figure 3; Figure 4). However, some limitations should be discussed.
There was variation in how well the studies took measures to blind investigators and participants: we rated a third of trials in this review at high or unclear risk of performance bias (69 out of 179). This is an important point to highlight, as the outcomes used for assessing efficacy were subjective. However, the proportion of trials at high risk of bias for blinding used in the network meta‐analyses decreased to 25% (35 out of 140).
The reporting of missing outcome data was largely inadequate in a few studies. Since we chose a likely scenario that any participant with missing outcome data did not experience clearance for the overall analyses, we minimised the risk of overestimating efficacy due to how we reported missing data.
Finally, we rated a few trials at high risk of selective outcome reporting. However, we chose a stringent definition of studies at high risk of selective outcome reporting: we considered reporting bias inadequate if one specified outcome in protocols was lacking in the main report. A large proportion of included trials did not report the patient‐reported outcomes in the main report but only in secondary publications (see Included studies). We extracted outcomes of interest both from main and secondary publications, but this disadvantaged trials that did not report all of the specified outcomes in the main report.
Indirect comparison and network meta‐analyses as standard pairwise meta‐analyses provide 'observational' evidence, since the treatments being compared have not been randomised across studies. However, we considered carefully the assumption underpinning the validity of indirect comparisons, to assure a sufficiently coherent evidence base (Cipriani 2013). The limitations of this review are reflected by CINeMA evaluations.
Heterogeneity (i.e. variation in effect modifiers within comparisons) and inconsistency (imbalance in effect modifiers between comparisons)
We found no evidence of heterogeneity either in direct comparisons or in the entire networks. At drug‐level analysis, the global test for inconsistency was not significant for any of the outcomes.
Imprecision
The number of studies included in the NMA was low for the following interventions (three studies or fewer for each intervention): netakimab, sonelokimab, certolizumab, deucravacitinib, ciclosporin, fumaric acid esters, acitretin, and methotrexate, meaning we must be cautious about the conclusions drawn for these drugs. Indeed, it has been shown that treatment effect estimates differed according to trial sample size, with stronger effect estimates seen in small to moderately sized trials than in the largest trials (Dechartres 2013). Moreover, treatment effects in randomised controlled phase II trials were better than those in matched phase III trials (Liang 2019).
Indirectness or transitivity assumption
We did not find any evidence that important variables, such as age, sex, weight, and duration and severity of psoriasis, varied across comparisons (see Characteristics of included studies and Figure 15; Figure 16). However, the lack of data did not allow us to check the distributions of previous treatments across comparisons, so transitivity cannot properly be assessed statistically.
Several participant characteristics have changed in newer trials, such as participants' exclusion criteria. However, most of the included trials were conducted after 2000, minimising the variability across trial participant characteristics. The location of the trial could also create some differences between participants, as the response to treatment could be related to genetic background (Chiu 2014). To further confirm the plausibility of the transitivity assumption, we only included in our analyses trials not involving co‐interventions and not selecting participants on their previous systemic treatments, and we performed several sensitivity analyses (see Quality of the evidence: Heterogeneity).
Publication bias
We assessed publication bias, considering the comprehensive search strategy we performed and the risk of publication bias in the specific field. The comparison‐adjusted funnel plot for all placebo‐controlled trials for all the outcomes did not indicate any evident risk of publication bias for the two primary outcomes (Figure 27).
Potential biases in the review process
We performed an extensive search for relevant trials. However, we did not contact pharmaceutical companies who do not have publicly available trials databases to enquire whether they had conducted any additional relevant trials. We consider that the probability that we have missed an eligible trial is low, considering our wide search, and this view is supported by the absence of small‐study effects (testing by the comparison‐adjusted funnel plots). However, the fact that 23 studies are awaiting classification and have not yet been incorporated may be a potential source of bias.
We conducted study selection, data extraction, and risk of bias assessments in duplicate and independently, and we reached consensus by discussing any discrepancies. Some published trial reports did not provide enough details to extract outcomes and adequately assess risks of bias, especially those performed before 2000 (i.e. before the International Committee of Medical Journal Editors issued the requirement for trial registration for publication). We contacted the authors of the trials to request missing data, but we cannot avoid some biased assessment in the review process due to incomplete reporting of trial details or results, or both.
We had some departures from the protocol plans (see Differences between protocol and review), especially excluding from the NMA trials with systemic treatment‐naïve participants.
Thus, we added one new sensitivity analysis including only drugs approved by the European Medicines Agency for plaque psoriasis.
We used CINeMA to assess our confidence in the results.
Agreements and disagreements with other studies or reviews
We found 62 network meta‐analyses assessing pharmacological systemic treatment for psoriasis published between 2006 and 2023 (last search on 29 January 2023; search strategies and sources are available in Guelimi 2021). Twenty‐one were published in 2021 and 2022 (Aljefri 2022; Armstrong 2021; Armstrong 2022; Armstrong 2022a; Blauvelt 2021b; Blauvelt 2022; Fahrbach 2021; Fu 2022; Gottlieb 2022; He 2021; Kang 2022; Leonardi 2022; Mrowietz 2021; Pan 2021; Shear 2021; Song 2021; Xie 2021; Xu 2021; Yasmeen 2022; Yu 2022; Zhu 2022). In total, 11/21 were funded by the pharmaceutical industry and 3/21 were funded by academical grants; the funding was unknown for seven reviews.
None of these reviews assessed all biologics, non‐biological treatments, and small molecules. Three assessed both biological, non‐biological treatments, and small molecules, including respectively 13, 16, 17, and 13 interventions (Armstrong 2021; Armstrong 2022a; Shear 2021); Armstrong 2021 included 71 trials in their NMA, Armstrong 2022a 86 trials, and Shear 2021 included 52 trials in their NMA compared with 20 interventions and 179 trials in ours.
Among these 21 NMAs, nine assessed both benefit and harm (Aljefri 2022; Armstrong 2022; Fu 2022; Kang 2022; Song 2021; Xie 2021; Xu 2021; Yu 2022; Zhu 2022); others had only harm or only benefit outcomes.
We compared our findings with the two network meta‐analyses that assessed all classes of interventions (Armstrong 2022a; Shear 2021). Armstrong 2022a included 86 trials (and 34,476 participants) assessing biologic treatments (infliximab, adalimumab, etanercept, certolizumab, ustekinumab, secukinumab, ixekizumab, brodalumab, bimekizumab, risankizumab, guselkumab, and tildrakizumab), apremilast, methotrexate, ciclosporin, acitretin, and FAEs. Armstrong 2022a presented PASI 50, 75, 90, and 100 results at 10 to 16 weeks, and presented their results using the number needed to treat for an additional beneficial outcome (NNTB). Although NNTB is an easily understandable and very useful measure for patients and clinicians, it can be misleading in a network meta‐analysis, since it requires the assumption of a common average control group risk applying to all studies. This is a rather strong assumption, particularly in networks involving head‐to‐head studies without a control group, as here. They also used a standard multinomial analysis for their NMA including a component for baseline risk (placebo rate). However, we have just performed a meta‐analysis of prevalence of PASI 90 responses in the placebo group during the induction phase for patients with psoriasis receiving a systemic treatment, and our findings do not support a fluctuation in placebo response when considering PASI 90 response (Afach 2022). Patients receiving bimekizumab were significantly more likely to achieve PASI 90 than all other biologics in Armstrong 2022a. Our findings, including the analyses using licensed drugs and dosages, did not find a difference in reaching PASI 90 between infliximab, ixekizumab, bimekizumab, and risankizumab. One hypothesis is that the choice of time of evaluation range (from 10 to 16 weeks in Armstrong 2022a and from 8 to 24 weeks in our study) failed to include more trials, such as infliximab trials. Lastly, our review also includes new licensed agents (sonelokimab and deucravacitinib). Shear 2021 presented only harm results.
Authors' conclusions
Implications for practice.
In terms of achieving Psoriasis Area and Severity Index (PASI) 90 with induction therapy (evaluation from 8 to 24 weeks after the randomisation), we found the following results, based on network meta‐analysis.
At class level, all of the assessed interventions (non‐biological systemic agents, small molecules, and biological treatments) showed significant superiority compared with placebo.
At class level, the biologic treatments anti‐IL17, anti‐IL12/23, anti‐IL23, and anti‐TNF alpha showed significant superiority compared with non‐biological systemic agents; anti‐IL17 treatment was associated with a better chance of reaching PASI 90 compared to all of the interventions.
For reaching PASI 90, the most effective drugs when compared to placebo were (in SUCRA (surface under the cumulative ranking curve) rank order): infliximab (high‐certainty evidence), bimekizumab (high‐certainty evidence), ixekizumab (high‐certainty evidence), and risankizumab (high‐certainty evidence). The clinical effectiveness of these drugs was similar when compared against each other. Bimekizumab and ixekizumab were significantly more likely to reach PASI 90 than secukinumab. Bimekizumab, ixekizumab, and risankizumab were significantly more likely to reach PASI 90 than brodalumab and guselkumab.
Infliximab, anti‐IL17 drugs (bimekizumab, ixekizumab, secukinumab, and brodalumab) and anti‐IL23 drugs, except tildrakizumab, were significantly more likely to reach PASI 90 than ustekinumab, three anti‐TNF alpha agents, and deucravacitinib. Ustekinumab was superior to certolizumab. Adalimumab, tildrakizumab, and ustekinumab were superior to etanercept.
No significant difference was shown between apremilast and two non‐biological drugs: ciclosporin and methotrexate.
For the other efficacy outcomes (PASI 75 and PGA 0/1), the results were similar to the results for PASI 90.
For serious adverse events (SAEs), there was no significant difference between any of the assessed interventions and placebo. Nonetheless, the analyses of SAEs were based on a very low number of events with low‐to‐moderate certainty for the majority of the comparisons. The findings therefore have to be viewed with caution. Considering both efficacy (PASI 90 outcome) and acceptability (SAE outcome), highly effective treatments had more SAEs than the other treatments: risankizumab and bimekizumab appeared to be the better compromise between efficacy and acceptability.
Information on quality of life was not well reported and was absent for several of the interventions.
Conservative interpretation is warranted for the results for netakimab, sonelokimab, deucravacitinib, acitretin, ciclosporin, fumaric acid esters, and methotrexate, as these drugs in the NMA have only been evaluated in few trials.
The evidence is limited to a selected trial population (participants were young (mean age of 44.6 years), more than 60% were males, they had a high level of disease severity (with an overall mean score of PASI 20.4 at baseline and long‐time sufferers), and they had few major comorbidities), and the NMA evidence was limited to the induction treatment phase (all results were measured from 8 to 24 weeks after randomisation), which is not relevant enough for a chronic disease, which would require long‐term treatment.
Our main results (i.e. superiority of benefit of the biologic treatments anti‐IL17, anti‐IL12/23, anti‐IL23, and anti‐TNF alpha compared with small molecules and the non‐biological systemic agents) do not reflect the 'real life' management of patients in Europe or Canada, as an example. Currently, biological treatments (as well as apremilast) may be positioned as third‐line therapies by regulatory bodies, with mandatory reimbursement criteria that patients must meet before being considered for these treatments (moderate‐to‐severe disease after failure, intolerance or contraindication to non‐biological systemic agents). Such decisions were based on the lack of long‐term safety knowledge but also take into account economic considerations. In this review, we found insufficient evidence to evaluate long‐term safety, and we did not address economic considerations, so the question of the choice of first‐line treatment for moderate‐to‐severe psoriasis is still debatable.
The first choice of non‐biological systemic agents is still in question, as the limited number of trials assessing non‐biological systemic agents did not allow us to draw robust conclusions; this is also true for some small molecule treatments and biological treatments.
Implications for research.
From a clinical point of view, we need drugs that can be administered long‐term to provide continuous effective control, because continued remission after successful treatment is as important as successful induction of remission. Moreover, treatment should be easy to use, well accepted by patients, have minimal drug‐to‐drug interactions, and should have minimal monitoring requirements, because convenience is also an important issue when dealing with chronic diseases that require prolonged treatments. Finally, the cost of the drug should be affordable by most patients and by any national health service.
Specific questions and issues in the management of psoriasis still remain unmet:
Which non‐biological systemic agents have the best benefit/risk balance?
Which patients are candidates for small molecule treatment?
Which treatments work for subgroups of patients (age, psoriasis severity, previous treatment, psoriatic arthritis, race and ethnicity)?
Which treatments offer the best combination of safety and efficacy in patients with major comorbidities (e.g. hepatitis B/C, latent tuberculosis, HIV, and renal, cardiac, and hepatic impairment) as well as pregnancy?
Adjustment of therapy for patients with stable low disease activity.
Add‐on therapy or switching for patients who failed with a systemic treatment.
Long‐term safety data for all of the treatments.
1. Future trials need to ensure the following.
Participants: enough information about participants is needed to enable systematic subgroup analyses for biological‐naïve patients (or non‐biological systemic agent‐naïve patients); future trials also need to provide an adequate description of data on other important potential effect modifiers such as previous systemic treatments, whether participants are overweight/obese, the duration of a participant's psoriasis, baseline psoriasis severity (efficacy differences could be expected for patients with PASI at 10 and patients with PASI at 40), race and ethnicity, and presence of psoriatic arthritis.
Interventions: high‐quality trials assessing the benefit of non‐biological systemic agents are still needed.
Comparators: once the benefit of a treatment has been established against placebo, only head‐to‐head trials would be helpful to provide physicians with efficacy estimates between the different biologics, with stronger evidence than indirect comparisons. Head‐to‐head comparisons are lacking between the non‐biological systemic agents and small molecules and against each other. More head‐to‐head comparisons between biological agents are also needed (anti‐IL17 versus anti‐IL23, anti‐IL23 versus anti‐IL12/23, anti‐TNF alpha versus anti‐IL12/23).
Outcomes: outcome measure harmonisation is needed for psoriasis.
Timingassessment strategy: all of the trials included in this review were limited to the induction phase (from 8 to 24 weeks). Long‐term efficacy data are critical for chronic diseases. Placebo‐controlled, long‐term trials would not be ethical, due to the suffering it would entail for the people in the placebo group. However, long‐term studies comparing different drugs would be ethical and informative. Such long‐term trials could also assess the adjustment of therapy for patients with stable cleared psoriasis.
2. New trial designs are needed, such as pragmatic trials that permit dose adjustment once in remission, switching, and additional treatments (i.e. adding two or more systemic treatments) as in normal clinical practice. All of this unmet medical need evidence would improve the management of the condition. Lastly, further independent studies are needed to confirm the evidence provided.
3. Finally, evidence‐based decision‐making and management of chronic plaque psoriasis require both benefit AND harm data. As we already know, the limitations of network meta‐analysis and of randomised clinical trials (included in these meta‐analyses) mean we cannot reliably interpret the significance of rare events, given their current design. These studies are designed to detect differences in the severity of psoriasis in response to therapy over short periods of treatment, and are often underpowered and of insufficient duration to detect rare or long‐term adverse events. One way to counter this is to include observational cohort studies/registries in a network observational meta‐analysis.
What's new
| Date | Event | Description |
|---|---|---|
| 11 July 2023 | New citation required but conclusions have not changed | This update includes studies of 20 interventions. Network meta‐analysis shows that infliximab, bimekizumab, ixekizumab, and risankizumab outperformed other drugs when compared to placebo in reaching PASI 90. The clinical effectiveness of these drugs was similar. |
| 11 July 2023 | New search has been performed | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 6 October 2022 are included in the current update (179 included studies). In this update, we have fully incorporated a further 12 new included studies with 3427 additional participants. |
History
Protocol first published: Issue 2, 2015 Review first published: Issue 12, 2017
| Date | Event | Description |
|---|---|---|
| 12 August 2022 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 5 October 2021 are included in the current update (published May 2022, 167 included studies). In addition, the team continues with the monthly screening (last search date 29 July 2022) and have found a further 7 new studies and 13 ongoing studies that will be included in a future update. |
| 20 May 2022 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 5 October 2021 are included in the current update (published May 2022, 167 included studies). In addition, the team continues with the monthly screening (last search date 21 April 2022) and have found a further 5 new studies and 7 ongoing studies that will be included in a future update. |
| 29 April 2022 | New citation required and conclusions have changed | This update included studies of more interventions, assessing two new anti‐IL17 agents (netakimab, sonelokimab). Network meta‐analysis showed that infliximab, bimekizumab, ixekizumab and risankizumab outperformed other drugs when compared to placebo in reaching PASI 90. The clinical effectiveness of these drugs was similar. |
| 29 April 2022 | New search has been performed | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 5 October 2021 are included in the current update (167 included studies). In this update, we have fully incorporated a further 19 new included studies with 5695 additional participants. Ten included studies from the earlier version of this review are excluded because the interventions no longer meet the inclusion criteria (tofacitinib and mirikizumab). |
| 8 October 2021 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 8 September 2020 are included in the current update (published April 2021, 158 included studies). In addition, the team continues with the monthly screening (last search date 5 October 2021) and have found a further 18 new studies and 31 ongoing studies that will be included in a forthcoming update. |
| 28 May 2021 | Amended | There was a mistake in Figure 24 (PASI 90), which we have now rectified. |
| 13 April 2021 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 8 September 2020 are included in the current update (published April 2021, 158 included studies). In addition, the team continues with the monthly screening (last search date 17 March 2021) and have found a further 8 new studies and 15 ongoing studies that will be included in a future update. |
| 13 April 2021 | New citation required and conclusions have changed | This update includes more interventions, including a new anti‐IL23. Network meta‐analysis showed that infliximab, ixekizumab, risankizumab, bimekizumab, secukinumab, guselkumab and brodalumab outperformed other drugs when compared to placebo in reaching PASI 90. The clinical effectiveness of these drugs was similar, except for ixekizumab which had a better chance of reaching PASI 90 compared with secukinumab, guselkumab and brodalumab. |
| 13 April 2021 | New search has been performed | In this update, we have fully incorporated a further 18 new included studies and 13 new ongoing studies from searches up to 8 September 2020, which have been incorporated in an updated network meta‐analysis. This update includes a new biological agent in the network: mirikizumab. |
| 8 March 2021 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 31 January 2019 are included in the current update (published January 2020, 140 included studies). In addition, the team have found a further 18 new included studies and 13 new ongoing studies from searches up to 8 September 2020, to be published in an updated network meta‐analysis. In further searches (up to 20 January 2021) for a future update, the team have found 3 new studies to be included and 14 ongoing studies. |
| 27 January 2021 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 31 January 2019 are included in the current update (published January 2020, 140 included studies). In addition, the team have found a further 18 new included studies and 13 new ongoing studies from searches up to 8 September 2020, to be published in an updated network meta‐analysis. In further searches (up to 14 December 2020) for a future update, the team have found 1 new study to be included and 13 ongoing studies. |
| 13 October 2020 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 31 January 2019 are included in the current update (published January 2020). In addition, the team continues with the monthly screening (last search date 8 September 2020) and has found a further 15 new studies and 13 new ongoing studies that will be included in the next update which is underway. |
| 3 September 2020 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 31 January 2019 are included in the current update (published January 2020). In addition, the team continues with the monthly screening (last search date 22 July 2020) and has found a further 15 new studies and 12 new ongoing studies that will be included in the next update which is underway. |
| 20 July 2020 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 31 January 2019 are included in the current update (published January 2020). In addition, the team continues with the monthly screening (last search date 24 June 2020) and has found a further 14 new studies and 12 new ongoing studies that will be included in the next update which is underway. |
| 6 July 2020 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 31 January 2019 are included in the current update (published January 2020). In addition, the team continues with the monthly screening (last search date 27 May 2020) and has found a further 14 new studies and 12 new ongoing studies that will be included in the next update which is underway. |
| 17 April 2020 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 31 January 2019 are included in the current update (published January 2020). In addition, the team continues with the monthly screening (last search date 10 March 2020) and has found a further 14 new studies and 11 new ongoing studies that will be included in the next update which is underway. |
| 4 March 2020 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 31 January 2019 are included in the current update (published January 2020). In addition, the team continues with the monthly screening (last search date 12 February 2020) and has found a further 14 new studies and 7 new ongoing studies that will be included in the next update which is underway. |
| 12 February 2020 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 31 January 2019 are included in the current update (published January 2020). In addition, the team continues with the monthly screening (last search date 15 January 2020) and has found a further 13 new studies and 7 new ongoing studies that will be included in the next update which is underway. |
| 2 January 2020 | New search has been performed | This update included 31 new studies with 11,867 additional participants. We updated the review in line with the MECIR standards. |
| 2 January 2020 | New citation required and conclusions have changed | This update included studies of more interventions, assessing new anti‐IL17 and anti‐IL23 agents. |
Notes
This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 6 October 2022 are included in the current update (published July 2023, 179 included studies).
Acknowledgements
Acknowledgements from the authors
We would like to thank the EuroGuiDerm psoriasis guideline expert group, which gave us feedback on drug names that should be included in the network.
We would like to thank Dr Ibrahim Yaylali from Cochrane Oral Health for his translation of Gurel 2015 from Turkish into English.
We would like to thank Professor Rintaro Mori from Graduate School of Medicine, Kyoto University, Kyoto, Japan and Professor Erika Ota from Graduate School of Nursing Science, St Luke's International University, Tokyo, Japan, for their translation of Rinsho Iyaku 1991 from Japanese into English.
Liz Doney, the Information Specialist with Cochrane Skin, performed the searches and was an author on this living review update, but has now left her position.
Editorial and peer reviewer contributions
Cochrane Skin supported the authors in the development of this living review update.
The following people conducted the editorial process for this review:
Sign‐off Editor (final editorial decision): Robert Boyle, Cochrane Editorial Board, Imperial College London, UK
Managing Editor (selected peer reviewers, provided comments and editorial guidance to authors, edited the article): Lara Kahale, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks, collated peer reviewer comments, and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy editing and production): Jenny Bellorini, Cochrane Central Production Service
Peer reviewers (provided comments and recommended an editorial decision): Jennifer Hilgart, Cochrane Evidence Production and Methods Directorate (methods), Joanne Platt, Information Specialist, GNOC (search), Francesco Bellinato, Department of Medicine, Section of Dermatology, University of Verona, Verona (clinical), Dr. Anmol Sodhi (clinical), Guillermo Coello Garaicoechea (consumer)
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Library, search strategy
#1 MeSH descriptor: [Psoriasis] this term only #2 psoria*:ti,ab,kw #3 (palmoplantar* next pustulosis):ti,ab,kw #4 pustulosis palmaris et plantaris:ti,ab,kw #5 (pustulosis and palms and soles):ti,ab,kw #6 #1 or #2 or #3 or #4 or #5 #7 MeSH descriptor: [Methotrexate] explode all trees #8 MeSH descriptor: [Fumarates] explode all trees #9 MeSH descriptor: [Etretinate] explode all trees #10 MeSH descriptor: [Acitretin] explode all trees #11 MeSH descriptor: [Isotretinoin] explode all trees #12 MeSH descriptor: [Retinoids] explode all trees #13 MeSH descriptor: [Antibodies, Monoclonal] explode all trees #14 MeSH descriptor: [Interleukin‐12] explode all trees #15 MeSH descriptor: [Interleukin‐23] explode all trees #16 MeSH descriptor: [Interleukin‐12 Subunit p40] explode all trees #17 MeSH descriptor: [Tumor Necrosis Factors] explode all trees #18 MeSH descriptor: [Tumor Necrosis Factor‐alpha] explode all trees #19 MeSH descriptor: [Receptors, Tumor Necrosis Factor, Type II] explode all trees #20 MeSH descriptor: [Receptors, Tumor Necrosis Factor] explode all trees #21 MeSH descriptor: [Receptors, Tumor Necrosis Factor, Type I] explode all trees #22 MeSH descriptor: [TNF‐Related Apoptosis‐Inducing Ligand] explode all trees #23 MeSH descriptor: [Antibodies, Monoclonal] explode all trees #24 MeSH descriptor: [Immunoglobulin Fab Fragments] explode all trees #25 MeSH descriptor: [Phototherapy] explode all trees #26 MeSH descriptor: [Ultraviolet Therapy] explode all trees #27 MeSH descriptor: [PUVA Therapy] explode all trees #28 MeSH descriptor: [Photochemotherapy] explode all trees #29 MeSH descriptor: [Cyclosporine] explode all trees #30 (methotrexate* or amethopterin or mtx or mexate or fumar* or dimethylfumarate or fae or dmf or fumaderm or acitretin or tegison or soriatane or neotigason or ((oral or orally or systemic) and retinoid*) or isotretinoin or accutane or etretin* or ustekinumab or stelara or secukinumab or "CNTO 1275" or "cdp571" or etanercept* or enbrel or adalimumab* or "d2e7" or humira or golimumab or simponi or briakinumab or "ABT‐874" or "psoralen uva" or ciclosporin or cyclosporine or cyclosporine or brodalumab or ixekizumab or phototherap* or ultraviolet or PUVA or photochemotherap* or photodynamic or "light therap*" or photoradiation or "broad band uvb" or "broad band ultraviolet b" or "narrow band uvb" or "narrow band ultraviolet b" or BBUVB or NBUVB or BB‐UVB or NB‐UVB or infliximab* or (monoclonal next antibod*) or remicade or interleukin* or "anti tumour necrosis factor" or "anti tumor necrosis factor" or ("tumour necrosis factor" next antibod*) or ("tumor necrosis factor" next antibod*) or "tnf antibod*" or ("tnf alpha" next antibod*) or "anti tnf" or ("immunoglobulin fab" next fragment*) or "p40 subunit" or "tumor necrosis factor*" or tnf or ("antitumor necrosis" next factor*) or ("antitumour necrosis" next factor*) or ampremilast or guselkumab or certolizumab or tildrakizumab or BMS‐986165 or bimekizumab or rizankizumab or risankizumab or deucravacitinib or hemay005 or sonelokimab or MSB0010841 or netakimab or BCD‐085 or vunakizumab or SHR‐1314):ti,ab,kw #31 {or #7‐#30}
#32 #6 and #31
Searches were date limited by the date a record was added to the database.
Appendix 2. MEDLINE (Ovid) search strategy
Ovid MEDLINE(R) ALL <1946 to October 6, 2022>
1 exp Psoriasis/ or psoria$.ti,ab. 59743 2 palmoplantar$ pustulosis.ti,ab. 621 3 pustulosis palmaris et plantaris.ti,ab. 172 4 (pustulosis and palms and soles).ti,ab. 100 5 or/1‐4 59979 6 Deucravacitinib.ti,ab. 6 7 Hemay005.ti,ab. 1 8 (Sonelokimab or MSB0010841).ti,ab. 2 9 (netakimab or BCD‐085).ti,ab. 10 10 (vunakizumab or SHR‐1314).ti,ab. 1 11 exp Methotrexate/ 39648 12 methotrexate$.mp. 57368 13 amethopterin.mp. 399 14 mtx.ti,ab. 13729 15 mexate.mp. 2 16 exp Fumarates/ 5052 17 (fumar$ and esters).mp. 448 18 dimethylfumarate.mp. 196 19 fae.ti,ab. 975 20 dmf.ti,ab. 8970 21 fumarate$1.mp. 19546 22 fumaderm.mp. 54 23 Etretinate/ 1350 24 Acitretin/ 1226 25 Tegison.mp. 16 26 (Soriatane or Neotigason).mp. 39 27 ((oral or orally or systemic) and retinoid$).ti,ab. 2842 28 Isotretinoin/ 3783 29 Accutane.mp. 200 30 isotretinoin.ti,ab. 3494 31 etretin$.mp. 1739 32 acitretin.mp. 1912 33 Retinoids/ 6148 34 Ustekinumab.mp. 2551 35 stelara.mp. 46 36 secukinumab.mp. 1546 37 apremilast.mp. 870 38 guselkumab.mp. 391 39 BMS‐986165.mp. 8 40 ri?ankizumab.mp. 236 41 CNTO 1275.mp. 16 42 exp antibodies, monoclonal/ 257056 43 monoclonal antibod$.mp. 197471 44 exp Interleukin‐23/ or exp Interleukin‐12/ 16879 45 exp Interleukin‐12 Subunit p40/ or p40 subunit.mp. 1841 46 exp Tumor Necrosis Factors/ or exp Tumor Necrosis Factor‐alpha/ or exp Receptors, Tumor Necrosis Factor, Type II/ or exp Receptors, Tumor Necrosis Factor/ or exp Receptors, Tumor Necrosis Factor, Type I/ or exp TNF‐Related Apoptosis‐Inducing Ligand/ 187882 47 (anti tumour necrosis factor or anti tumor necrosis factor).mp. 5805 48 (tumor necrosis factor‐alpha or tumour necrosis factor‐alpha).mp. 179299 49 anti tnf.mp. 11804 50 (tnf antibod$ or tnf alpha antibod$).mp. 2334 51 (tumour necrosis factor antibod$ or tumor necrosis factor antibod$).mp. 159 52 (antitumor necrosis factor or antitumour necrosis factor).mp. 863 53 exp Immunoglobulin Fab Fragments/ 28497 54 (infliximab$ or monoclonal antibody cA2 or remicade).mp. 16359 55 cdp571.mp. 43 56 (etanercept$ or enbrel).mp. 9268 57 (adalimumab$ or d2e7 or humira).mp. 9776 58 (golimumab or simponi).mp. 1445 59 (Briakinumab or ABT‐874).mp. 75 60 exp Phototherapy/ 47867 61 exp Ultraviolet Therapy/ 8900 62 exp PUVA Therapy/ 4458 63 exp Photochemotherapy/ 23154 64 photodynamic therap$.mp. 23692 65 phototherap$.mp. 17356 66 photochemotherap$.mp. 24110 67 puva.mp. 4665 68 ultraviolet.mp. 187319 69 light therap$.mp. 8939 70 photoradiation therap$.mp. 173 71 BBUVB.mp. 5 72 NBUVB.mp. 194 73 BB‐UVB.mp. 43 74 NB‐UVB.mp. 610 75 broad band uvb.mp. 61 76 broad band ultraviolet b.mp. 13 77 narrow band uvb.mp. 395 78 narrow band ultraviolet b.mp. 424 79 psoralen ultraviolet a.mp. 205 80 psoralen uva.mp. 138 81 Cyclosporine/ 29991 82 (Ciclosporin or cyclosporine or cyclosporin).mp. 58379 83 Bimekizumab.mp. 68 84 brodalumab.mp. 434 85 ixekizumab.mp. 768 86 certolizumab.mp. 1452 87 tildrakizumab.mp. 190 88 or/6‐87 964992 89 randomized controlled trial.pt. 553558 90 controlled clinical trial.pt. 94606 91 randomized.ab. 544046 92 placebo.ab. 224015 93 clinical trials as topic.sh. 198432 94 randomly.ab. 372078 95 trial.ti. 253075 96 89 or 90 or 91 or 92 or 93 or 94 or 95 1413998 97 exp animals/ not humans.sh. 4931018 98 96 not 97 1300832 99 5 and 88 and 98 2965 100 limit 99 to dt=20210508‐20211005 60 101 limit 99 to ed=20210508‐20211005 144 102 100 or 101 193
[Lines 89‐98: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format, from section 3.6.1 in Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, Noel‐Storr A, Rader T, Shokraneh F, Thomas J, Wieland LS. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from: www.training.cochrane.org/handbook]
We time limited results from this database using two different methods: Results were limited by the Create Date (date when the record was added to the database). Results were also limited by the Entry Date (the date processing of the record was completed). Using two date‐limiting fields and combining the results is recommended by the Cochrane Living Evidence Network. See example search syntax below showing limiting with the Create Date (dt) and the Entry Date (ed):
96. 5 and 85 and 95
97. limit 96 to dt=20181031‐20190416
98. limit 96 to ed=20181031‐20190416
99. 97 or 98
Searches are generally run monthly with an overlap of three months to ensure no records are missed.
Appendix 3. Embase (Ovid) search strategy
Embase <1974 to 2022 October 6>
1 exp PSORIASIS/ 97707 2 psoria$.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] 106162 3 palmoplantar$ pustulosis.mp. 880 4 pustulosis palmaris et plantaris.mp. 212 5 (pustulosis and palms and soles).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] 296 6 1 or 2 or 3 or 4 or 5 107474 7 exp deucravacitinib/ 56 8 hemay005.ti,ab. 1 9 exp sonelokimab/ 5 10 exp netakimab/ 53 11 exp vunakizumab/ 5 12 (deucravacitinib or sonelokimab or MSB0010841 or netakimab or BCD‐085 or vunakizumab or SHR‐1314).ti,ab. 63 13 methotrexate/ 190525 14 methotrexate$.ti,ab. 73204 15 amethopterin.ti,ab. 210 16 mtx.ti,ab. 26398 17 mexate.ti,ab. 1 18 fumaric acid derivative/ 1395 19 (fumar$ and esters).ti,ab. 624 20 dimethylfumarate.ti,ab. 430 21 fae.ti,ab. 1220 22 dmf.ti,ab. 12252 23 fumarate$1.ti,ab. 15846 24 fumaderm.ti,ab. 116 25 etretinate/ 4627 26 acitretin.ti,ab. 2570 27 tegison.ti,ab. 16 28 (Soriatane or Neotigason).ti,ab. 65 29 ((oral or orally or systemic) and retinoid$).ti,ab. 4116 30 isotretinoin/ 13500 31 isotretinoin.ti,ab. 5032 32 Accutane.ti,ab. 218 33 etretin$.ti,ab. 1677 34 retinoid/ 15017 35 ustekinumab.ti,ab. 5167 36 ustekinumab/ 9240 37 stelara.ti,ab. 74 38 secukinumab/ 5147 39 secukinumab.ti,ab. 3129 40 ampremilast.ti,ab. 2 41 guselkumab/ 1299 42 guselkumab.ti,ab. 706 43 "CNTO 1275".ti,ab. 20 44 monoclonal antibod$.ti,ab. 242042 45 exp monoclonal antibody/ 657311 46 interleukin 23/ 16120 47 interleukin 12/ 50609 48 interleukin 12p40/ 7665 49 p40 subunit.ti,ab. 709 50 exp tumor necrosis factor/ 176513 51 tumor necrosis factor alpha/ 230800 52 tumor necrosis factor receptor 2/ 4471 53 tumor necrosis factor receptor/ 11694 54 tumor necrosis factor related apoptosis inducing ligand/ 11513 55 (anti tumour necrosis factor or anti tumor necrosis factor).ti,ab. 8268 56 (tumor necrosis factor‐alpha or tumour necrosis factor‐alpha).ti,ab. 109922 57 anti tnf.ti,ab. 24317 58 (tnf antibod$ or tnf alpha antibod$).ti,ab. 3042 59 (tumour necrosis factor antibod$ or tumor necrosis factor antibod$).ti,ab. 215 60 (antitumor necrosis factor or antitumour necrosis factor).ti,ab. 1144 61 "immunoglobulin F(ab) fragment"/ 8803 62 (infliximab$ or monoclonal antibody cA2 or remicade).ti,ab. 28842 63 cdp571.ti,ab. 51 64 (etanercept$ or enbrel).ti,ab. 16088 65 (adalimumab$ or d2e7 or humira).ti,ab. 20918 66 (golimumab or simponi).ti,ab. 4253 67 (Briakinumab or ABT‐874).ti,ab. 111 68 exp phototherapy/ 99752 69 PUVA/ 10150 70 photochemotherapy/ 9015 71 photodynamic therap$.ti,ab. 26880 72 phototherap$.ti,ab. 14260 73 photochemotherap$.ti,ab. 2692 74 puva.ti,ab. 4488 75 ultraviolet.ti,ab. 79701 76 light therap$.ti,ab. 3136 77 photoradiation therap$.ti,ab. 194 78 BBUVB.ti,ab. 15 79 NBUVB.ti,ab. 421 80 BB‐UVB.ti,ab. 59 81 NB‐UVB.ti,ab. 965 82 broad band uvb.ti,ab. 79 83 broad band ultraviolet b.ti,ab. 17 84 narrow band uvb.ti,ab. 625 85 narrow band ultraviolet b.ti,ab. 540 86 psoralen ultraviolet a.ti,ab. 268 87 psoralen uva.ti,ab. 183 88 cyclosporin/ 83639 89 (Ciclosporin or cyclosporine or cyclosporin).ti,ab. 73037 90 brodalumab.ti,ab. 588 91 ixekizumab.ti,ab. 1391 92 ixekizumab/ 2510 93 brodalumab/ 1431 94 certolizumab.mp. 8479 95 tildrakizumab.mp. 720 96 BMS‐986165.ti,ab. 43 97 bimekizumab/ 210 98 Bimekizumab.ti,ab. 112 99 risankizumab/ 727 100 Ri?ankizumab.ti,ab. 337 101 or/7‐100 1570359 102 crossover procedure.sh. 68907 103 double‐blind procedure.sh. 190430 104 single‐blind procedure.sh. 44590 105 (crossover$ or cross over$).tw. 115480 106 placebo$.tw. 335424 107 (doubl$ adj blind$).tw. 225791 108 allocat$.tw. 175009 109 trial.ti. 345044 110 randomized controlled trial.sh. 687002 111 random$.tw. 1732195 112 or/102‐111 2196680 113 exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 29835404 114 human/ or normal human/ 23020899 115 113 and 114 23020899 116 113 not 115 6814505 117 112 not 116 1944616 118 6 and 101 and 117 6216 119 limit 118 to dd=20210508‐20211005 203
[Lines 102‐117: Based on terms suggested for identifying RCTs in Embase (section 3.6.2) in Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, Noel‐Storr A, Rader T, Shokraneh F, Thomas J, Wieland LS. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from: www.training.cochrane.org/handbook]
We time‐limited results from this database by the Date Delivered field (date the citation XML file is created for delivery to Ovid and has a state=’new’). The Date Delivered field is recommended for date limiting in Embase in the Cochrane Information Specialists’ Handbook, section 6.6 Updating searches. See example search syntax below (dd=date delivered):
116. 6 and 99 and 115
117. limit 116 to dd=20181031‐20190416
Searches are generally run monthly with an overlap of three months to ensure no records are missed.
Appendix 4. Living systematic review protocol
Living systematic reviews (LSRs) and living network meta‐analyses (Living NMAs) offer a new approach to review updating in which the review is continually updated, incorporating relevant new evidence as it becomes available (Elliott 2017).
The methods outlined below are specific to maintaining this review as a living systematic review on the Cochrane Library. They will be used immediately upon publication of this update. Core review methods, such as the criteria for considering studies in the review and assessment of risk of bias, are unchanged. As such, below we outline only those areas of the Methods for which additional activities or rules apply.
Six methodological steps will be repeated at regular intervals to update the NMA over time: adaptive search for treatments and trials, screening of reports and selection of trials, data extraction, assessment of risk of bias, update of the network of trials and synthesis, and finally dissemination.
1. Adaptive search for treatments and trials
(1) As a living systematic review, we aim to identify all relevant RCTs, regardless of language or publication status (published, unpublished, in press, or in progress).
Bibliographic databases The Cochrane Skin Information Specialist (ED) will search the following databases every month:
We will limit the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library. Searches of this database by the date a record was added to the database.
MEDLINE via Ovid. We will limit Results sets from this database using two different methods: Results will first be limited by the Create Date (date when the record was added to the database). Results will also be limited by the Entry Date (the date processing of the record was completed) using two date‐limiting fields and combining the results as recommended by the Living Systematic Review Methods Group. See example search syntax below showing limiting with the Create Date (dt) and the Entry Date (ed):
96. 5 and 85 and 95
97. limit 96 to dt=20181031‐20190416
98. limit 96 to ed=20181031‐20190416
99. 97 or 98
Embase via Ovid. We will limit results from this database by the Date Delivered field (date the citation XML file is created for delivery to Ovid and has a state=’new’). The Date Delivered field is recommended for date limiting in Embase in the Cochrane Information Specialists’ Handbook, section 6.6 Updating searches. See example search syntax below (dd=date delivered):
116. 6 and 99 and 115
117. limit 116 to dd=20181031‐20190416
Note that different limit options are proposed for MEDLINE and Embase, because their record fields are different.
For all date‐limiting of bibliographic databases described above, we will apply an overlap of three months with previous searches. This approach is recommended by the Living Systematic Review Methods Group and aims to minimise the risk of missing relevant trials.
The search strategies for these three databases are displayed in Appendix 2 (MEDLINE) and Appendix 3 (Embase). The CENTRAL strategy has been slightly amended to take into account new interventions (sonelokimab and netakimab) and those that are no longer of interest (i.e. tofacitinib and mirikizumab) have been removed, and is shown below:
#1 MeSH descriptor: [Psoriasis] this term only #2 psoria*:ti,ab,kw #3 (palmoplantar* next pustulosis):ti,ab,kw #4 pustulosis palmaris et plantaris:ti,ab,kw #5 (pustulosis and palms and soles):ti,ab,kw #6 #1 or #2 or #3 or #4 or #5 #7 MeSH descriptor: [Methotrexate] explode all trees #8 MeSH descriptor: [Fumarates] explode all trees #9 MeSH descriptor: [Etretinate] explode all trees #10 MeSH descriptor: [Acitretin] explode all trees #11 MeSH descriptor: [Isotretinoin] explode all trees #12 MeSH descriptor: [Retinoids] explode all trees #13 MeSH descriptor: [Antibodies, Monoclonal] explode all trees #14 MeSH descriptor: [Interleukin‐12] explode all trees #15 MeSH descriptor: [Interleukin‐23] explode all trees #16 MeSH descriptor: [Interleukin‐12 Subunit p40] explode all trees #17 MeSH descriptor: [Tumor Necrosis Factors] explode all trees #18 MeSH descriptor: [Tumor Necrosis Factor‐alpha] explode all trees #19 MeSH descriptor: [Receptors, Tumor Necrosis Factor, Type II] explode all trees #20 MeSH descriptor: [Receptors, Tumor Necrosis Factor] explode all trees #21 MeSH descriptor: [Receptors, Tumor Necrosis Factor, Type I] explode all trees #22 MeSH descriptor: [TNF‐Related Apoptosis‐Inducing Ligand] explode all trees #23 MeSH descriptor: [Antibodies, Monoclonal] explode all trees #24 MeSH descriptor: [Immunoglobulin Fab Fragments] explode all trees #25 MeSH descriptor: [Phototherapy] explode all trees #26 MeSH descriptor: [Ultraviolet Therapy] explode all trees #27 MeSH descriptor: [PUVA Therapy] explode all trees #28 MeSH descriptor: [Photochemotherapy] explode all trees #29 MeSH descriptor: [Cyclosporine] explode all trees #30 (methotrexate* or amethopterin or mtx or mexate or fumar* or dimethylfumarate or fae or dmf or fumaderm or acitretin or tegison or soriatane or neotigason or ((oral or orally or systemic) and retinoid*) or isotretinoin or accutane or etretin* or ustekinumab or stelara or secukinumab or "CNTO 1275" or "cdp571" or etanercept* or enbrel or adalimumab* or "d2e7" or humira or golimumab or simponi or briakinumab or "ABT‐874" or "psoralen uva" or ciclosporin or cyclosporine or cyclosporine or brodalumab or ixekizumab or phototherap* or ultraviolet or PUVA or photochemotherap* or photodynamic or "light therap*" or photoradiation or "broad band uvb" or "broad band ultraviolet b" or "narrow band uvb" or "narrow band ultraviolet b" or BBUVB or NBUVB or BB‐UVB or NB‐UVB or infliximab* or (monoclonal next antibod*) or remicade or interleukin* or "anti tumour necrosis factor" or "anti tumor necrosis factor" or ("tumour necrosis factor" next antibod*) or ("tumor necrosis factor" next antibod*) or "tnf antibod*" or ("tnf alpha" next antibod*) or "anti tnf" or ("immunoglobulin fab" next fragment*) or "p40 subunit" or "tumor necrosis factor*" or tnf or ("antitumor necrosis" next factor*) or ("antitumour necrosis" next factor*) or ampremilast or guselkumab or certolizumab or tildrakizumab or BMS‐986165 or bimekizumab or rizankizumab or risankizumab or deucravacitinib or hemay005 or sonelokimab or MSB0010841 or netakimab or BCD‐085 or vunakizumab or SHR‐1314):ti,ab,kw #31 {or #7‐#30}
#32 #6 and #31
Deduplication and preparation the results for primary screening will be performed by the Cochrane Skin Information Specialist (ED)
Trials registers We will search records of RCTs from ClinicalTrials.gov and the WHO’s International Clinical Trials Registry Platform (ICTRP) through CENTRAL, which now includes trial records from these resources. Records are added to CENTRAL on a monthly basis (see relevant sections of ‘How CENTRAL is created’). CENTRAL therefore has a short lag period behind the individual registries.
Unpublished literature
We will search reviews submitted to the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for drug registration (using www.accessdata.fda.gov/scripts/cder/drugsatfda and www.ema.europa.eu/ema) yearly.
Review of search methods We will review search methods and strategies approximately yearly, ensuring they reflect any terminology changes in the topic area or in the databases searched. We will also revisit yearly our search methods and, if necessary, update the search strategies by adding or removing intervention terms.
(2) As a living systematic review, we aim to continually identify new evidence for interventions already in the network of trials but also for novel interventions. Indeed, for the 2019 review update, we identified several new interventions in the ongoing trials section that were not part of the initial network (e.g. risankizumab). To provide an update and useful network of interventions for physicians, we need first to identify new interventions but also, to drop old interventions, which are no longer of interest.
To achieve these goals:
(1) We will create a research community in psoriasis, including international experts in the field who will help to provide information of new 'eligible' drugs.
Once a year, a list of all systemic drugs used for psoriasis will be proposed by the scientific steering committee to the international experts’ group, including:
Drugs already involved in the network.
Marketed drugs, which will be identified using the U.S. FDA and the EMA websites (www.accessdata.fda.gov/scripts/cder/drugsatfda and www.ema.europa.eu/ema, respectively).
Drugs under development, which will be identified using the World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) and ISRCTN registry (www.isrctn.com).
The international experts’ group will select from this list all the systemic drugs needed for the future network. They will also add new interventions not proposed in the list. They will provide a rationale for all proposed network changes (adding or removing interventions). The international experts’ group is necessary also to determine which drugs have to be deleted from the network, with clinical practice and market authorisation being different in each country.
It will be sufficient to update the interventions network once a year, as we will include phase II and III RCTs. Indeed, the timing between the phase I and the phase II/III for a promising intervention is over one year.
(2) At the same time, we will search the different data sources described for the initial NMA with the latest updated search strategy. The Cochrane Skin Group will perform the electronic search.
(2.1) Every month, we will re‐run the search from the date of the last iteration to the following one (covering a 1‐month interval), on an automated basis, for electronic searches, trial registries and conference proceedings. We will use a script file (html extraction by automated http requests) to automatically and simultaneously search multiple sources every month. We will manually screen the reference lists of any newly‐included studies and identified systematic reviews.
(2.2) Every year, two authors (ES, LLC) will check other sources (regulatory agencies and industry trial registries) on a manual basis. We will also update the search strategy by adding or removing interventions. We will also review search methods and strategies approximately yearly, to ensure they reflect any terminology changes in the topic area, or in the databases.
As additional steps to inform the living systematic review, one author (ES) contacts corresponding authors of ongoing studies as they are identified and asks them to advise when results are available, or to share early or unpublished data.
2. Screening of reports and selection of trials
We will immediately screen any new citations retrieved by the monthly searches. We will pay attention to duplicate studies, i.e. the same trial reported in several articles. We will consider using Cochrane’s Screen4Me workflow to help assess the search results, depending on the volume of search results we identify in the first few months. Screen4Me comprises three components: known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labelled as 'an RCT' or as 'Not an RCT'; the RCTclassifier – a machine learning model that distinguishes RCTs from non‐RCTs; and, if appropriate, CochraneCrowd (crowd.cochrane.org) – Cochrane’s citizen science platform where the Crowd help to identify and describe health evidence.
The selection process will then be done through Covidence (Covidence 2021), a web tool allowing a double selection on title, abstract and then full text by independent reviewers.
3. Data synthesis
Whenever we find new evidence (i.e. studies, data or information) meeting the review inclusion criteria, we will extract the data and assess risks of bias. For trials identified as completed in clinical trial registries but without posted results or those identified only by a conference proceeding, and for missing outcome data, trained reviewers will contact trialists to request complete results.
Every three months, we will incorporate each newly identified trial in the network. We will perform one network for each outcome (PASI‐90, SAEs, PASI‐75, PGA, QoL, and AEs). We will re‐analyse the data every three months using the standard approaches outlined in the Data synthesis section as well as the GRADE process.
4. Dissemination
The general principle is that an update is published on the Cochrane Library with an open access each time new findings that impact on review conclusions have been identified.
We will present the results with sufficient information so that the live cumulative NMA becomes a useful tool to help medical decision‐making, taking into account the safety and efficacy of all systemic treatments for chronic plaque psoriasis. The live cumulative NMA will also provide evidence for future guidelines (and updates) on moderate‐to‐severe psoriasis treatment in France but also in Europe (European Dermatology Guidelines) and worldwide.
We will present :
network graphs for each outcome and at each iteration how the networks of evidence evolves over time;
treatment effects in forest plots, league tables and reporting of treatment rankings;
assessments of NMA assumptions and risks of bias for each included trial, to allow readers to assess their level of confidence in the results;
characteristics and results of included trials, to allow for an evaluation of clinical diversity and transitivity.
We will make publicly available in open access to ensure a transparent process:
the protocol (and its amendments);
statistical programmes;
the screening and selection elements (flow diagram, list of included trials, list of excluded trials with reasons for exclusion).
Data and analyses
Comparison 1. Primary outcome ‐ PASI 90.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Non‐biological treatments versus placebo | 5 | 1448 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [1.49, 5.41] |
| 1.1.1 Methotrexate versus placebo | 3 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.53, 7.97] |
| 1.1.2 Fumaric acid esters versus placebo | 2 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 3.78 [2.14, 6.69] |
| 1.2 Non‐biological treatment 1 versus non‐biological treatment 2 | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 Ciclosporin versus methotrexate | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.47, 2.98] |
| 1.2.2 Methotrexate versus fumaric acid esters | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 3.82 [1.65, 8.85] |
| 1.3 Anti‐TNF alpha versus placebo | 33 | 11981 | Risk Ratio (M‐H, Random, 95% CI) | 13.61 [10.65, 17.41] |
| 1.3.1 Etanercept versus placebo | 15 | 5762 | Risk Ratio (M‐H, Random, 95% CI) | 11.69 [8.17, 16.72] |
| 1.3.2 Adalimumab versus placebo | 9 | 3421 | Risk Ratio (M‐H, Random, 95% CI) | 13.13 [8.01, 21.53] |
| 1.3.3 Certolizumab versus placebo | 5 | 1153 | Risk Ratio (M‐H, Random, 95% CI) | 19.77 [8.29, 47.12] |
| 1.3.4 Infliximab versus placebo | 5 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 29.14 [12.22, 69.51] |
| 1.4 Anti‐IL12/23 versus placebo | 11 | 4520 | Risk Ratio (M‐H, Random, 95% CI) | 18.37 [12.56, 26.85] |
| 1.4.1 Ustekinumab versus placebo | 11 | 4520 | Risk Ratio (M‐H, Random, 95% CI) | 18.37 [12.56, 26.85] |
| 1.5 Anti‐IL17 versus placebo | 31 | 14216 | Risk Ratio (M‐H, Random, 95% CI) | 27.51 [19.19, 39.46] |
| 1.5.1 Secukinumab versus placebo | 16 | 4719 | Risk Ratio (M‐H, Random, 95% CI) | 23.09 [15.85, 33.63] |
| 1.5.2 Ixekizumab versus placebo | 5 | 3706 | Risk Ratio (M‐H, Random, 95% CI) | 47.03 [18.81, 117.59] |
| 1.5.3 Brodalumab versus placebo | 5 | 4109 | Risk Ratio (M‐H, Random, 95% CI) | 26.33 [16.77, 41.33] |
| 1.5.4 Bimekizumab versus placebo | 3 | 1089 | Risk Ratio (M‐H, Random, 95% CI) | 29.43 [10.30, 84.15] |
| 1.5.5 Netakimab versus placebo | 2 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 10.98 [0.42, 288.23] |
| 1.5.6 Sonelokimab versus placebo | 1 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 65.68 [4.15, 1038.50] |
| 1.6 Anti‐IL23 versus placebo | 14 | 5353 | Risk Ratio (M‐H, Random, 95% CI) | 19.96 [13.51, 29.49] |
| 1.6.1 Guselkumab versus placebo | 5 | 1767 | Risk Ratio (M‐H, Random, 95% CI) | 27.79 [16.23, 47.60] |
| 1.6.2 Tildrakizumab versus placebo | 3 | 1903 | Risk Ratio (M‐H, Random, 95% CI) | 17.26 [8.27, 36.05] |
| 1.6.3 Risankizumab versus placebo | 6 | 1683 | Risk Ratio (M‐H, Random, 95% CI) | 17.15 [7.28, 40.37] |
| 1.7 Biologic versus non‐biological treatments | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.7.1 Etanercept versus acitretin | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 4.56 [0.81, 25.79] |
| 1.7.2 Adalimumab versus methotrexate | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 3.73 [2.25, 6.19] |
| 1.7.3 Infliximab versus methotrexate | 1 | 868 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [2.15, 3.80] |
| 1.7.4 Ixekizumab versus methotrexate | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [1.43, 2.94] |
| 1.7.5 Risankizumab versus methotrexate | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 2.37 [1.59, 3.54] |
| 1.7.6 Brodalumab versus fumaric acid esters | 1 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [2.04, 4.42] |
| 1.7.7 Guselkumab versus fumaric acid esters | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 6.02 [3.13, 11.60] |
| 1.7.8 Ixekizumab versus fumaric acid esters | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 8.60 [3.69, 20.04] |
| 1.7.9 Risankizumab versus fumaric acid esters | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 8.33 [3.87, 17.95] |
| 1.7.10 Secukinumab versus fumaric acid esters | 1 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 8.31 [4.23, 16.35] |
| 1.7.11 Etanercept versus ciclosporin | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.75, 1.55] |
| 1.8 Biologic 1 versus biologic 2 | 27 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.8.1 Ustekinumab versus etanercept | 1 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [1.45, 2.24] |
| 1.8.2 Secukinumab versus etanercept | 1 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [1.85, 2.92] |
| 1.8.3 Infliximab versus etanercept | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 9.20 [1.28, 66.37] |
| 1.8.4 Ixekizumab versus etanercept | 2 | 2209 | Risk Ratio (M‐H, Random, 95% CI) | 2.98 [2.24, 3.98] |
| 1.8.5 Tildrakizumab versus etanercept | 1 | 934 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [1.39, 2.23] |
| 1.8.6 Certolizumab versus etanercept | 1 | 502 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.90, 1.61] |
| 1.8.7 Secukinumab versus ustekinumab | 2 | 1778 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.30, 1.50] |
| 1.8.8 Ixekizumab versus ustekinumab | 1 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.21, 1.63] |
| 1.8.9 Brodalumab versus ustekinumab | 2 | 3088 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.16, 1.39] |
| 1.8.10 Risankizumab versus ustekinumab | 3 | 965 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.43, 1.93] |
| 1.8.11 Bimekizumab versus ustekinumab | 1 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.46, 2.01] |
| 1.8.12 Guselkumab versus adalimumab | 3 | 1658 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.26, 1.62] |
| 1.8.13 Risankizumab versus adalimumab | 1 | 605 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.33, 1.75] |
| 1.8.14 Bimekizumab versus adalimumab | 1 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.42, 1.94] |
| 1.8.15 Ixekizumab versus adalimumab | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.10, 1.85] |
| 1.8.16 Ixekizumab versus guselkumab | 1 | 1027 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.18, 1.42] |
| 1.8.17 Risankizumab versus secukinumab | 1 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.97, 1.30] |
| 1.8.18 Bimekizumab versus secukinumab | 1 | 743 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.07, 1.24] |
| 1.8.19 Guselkumab versus secukinumab | 1 | 1048 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.98] |
| 1.8.20 Sonelokimab versus secukinumab | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.21] |
| 1.9 Biologic versus small molecules | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.9.1 Etanercept versus apremilast | 2 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.64, 1.64] |
| 1.10 Small molecules versus placebo | 9 | 4623 | Risk Ratio (M‐H, Random, 95% CI) | 7.57 [5.46, 10.50] |
| 1.10.1 Apremilast versus placebo | 7 | 2872 | Risk Ratio (M‐H, Random, 95% CI) | 6.03 [3.89, 9.36] |
| 1.10.2 Deucravacitinib versus placebo | 4 | 1751 | Risk Ratio (M‐H, Random, 95% CI) | 10.04 [6.15, 16.40] |
| 1.11 Small molecule 1 versus small molecule 2 | 2 | 1265 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.30, 2.03] |
| 1.11.1 Deucravacitinib versus apremilast | 2 | 1265 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.30, 2.03] |
| 1.12 Small molecules versus non‐biological treatments | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.88, 1.73] |
| 1.12.1 Apremilast versus ciclosporin | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.88, 1.73] |
1.11. Analysis.

Comparison 1: Primary outcome ‐ PASI 90, Outcome 11: Small molecule 1 versus small molecule 2
1.12. Analysis.

Comparison 1: Primary outcome ‐ PASI 90, Outcome 12: Small molecules versus non‐biological treatments
Comparison 2. Primary outcome ‐ serious adverse events (SAEs).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Non‐biological treatments versus placebo | 5 | 1449 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.21, 1.49] |
| 2.1.1 Methotrexate versus placebo | 3 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.03, 0.88] |
| 2.1.2 Fumaric acid esters versus placebo | 2 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.38, 2.01] |
| 2.2 Non‐biological treatment 1 versus non‐biological treatment 2 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.2.1 Methotrexate versus fumaric acid esters | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.10] |
| 2.3 Anti‐TNF alpha versus placebo | 33 | 10581 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.67, 1.30] |
| 2.3.1 Etanercept versus placebo | 13 | 4265 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.44, 1.23] |
| 2.3.2 Adalimumab versus placebo | 10 | 3485 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.74, 1.89] |
| 2.3.3 Certolizumab versus placebo | 5 | 1153 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.22, 4.24] |
| 2.3.4 Infliximab versus placebo | 6 | 1678 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.58, 2.59] |
| 2.4 Anti‐IL12/23 versus placebo | 12 | 4842 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.62, 1.55] |
| 2.4.1 Ustekinumab versus placebo | 12 | 4842 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.62, 1.55] |
| 2.5 Anti‐IL17 versus placebo | 31 | 14269 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.73, 1.29] |
| 2.5.1 Secukinumab versus placebo | 16 | 4772 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.71, 1.78] |
| 2.5.2 Ixekizumab versus placebo | 5 | 3706 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.45, 1.80] |
| 2.5.3 Brodalumab versus placebo | 5 | 4109 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.52, 1.61] |
| 2.5.4 Bimekizumab versus placebo | 3 | 1089 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.20, 1.65] |
| 2.5.5 Netakimab versus placebo | 2 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.03, 19.17] |
| 2.5.6 Sonelokimab versus placebo | 1 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.15, 10.47] |
| 2.6 Anti‐IL23 versus placebo | 14 | 5354 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.50, 1.21] |
| 2.6.1 Guselkumab versus placebo | 5 | 1767 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.50, 2.28] |
| 2.6.2 Tildrakizumab versus placebo | 3 | 1904 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.37, 2.77] |
| 2.6.3 Risankizumab versus placebo | 6 | 1683 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.29, 1.53] |
| 2.7 Biologic versus non‐biological treatments | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.7.1 Etanercept versus acitretin | 3 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.02] |
| 2.7.2 Adalimumab versus methotrexate | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [0.19, 22.14] |
| 2.7.3 Infliximab versus methotrexate | 1 | 868 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [1.04, 5.59] |
| 2.7.4 Ixekizumab versus methotrexate | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.06, 15.58] |
| 2.7.5 Risankizumab versus methotrexate | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.11, 3.66] |
| 2.7.6 Brodalumab versus fumaric acid esters | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.32, 28.52] |
| 2.7.7 Guselkumab versus fumaric acid esters | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.26, 8.51] |
| 2.7.8 Ixekizumab versus fumaric acid esters | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.10] |
| 2.7.9 Risankizumab versus fumaric acid esters | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 5.37] |
| 2.7.10 Secukinumab versus fumaric acid esters | 1 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.16, 1.75] |
| 2.7.11 Etanercept versus ciclosporin | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.8 Biologic 1 versus biologic 2 | 26 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.8.1 Ustekinumab versus etanercept | 1 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.38, 4.11] |
| 2.8.2 Secukinumab versus etanercept | 1 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.41, 2.82] |
| 2.8.3 Infliximab versus etanercept | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.06, 13.87] |
| 2.8.4 Ixekizumab versus etanercept | 2 | 2209 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.55, 2.06] |
| 2.8.5 Tildrakizumab versus etanercept | 1 | 934 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.28, 1.87] |
| 2.8.6 Certolizumab versus etanercept | 1 | 502 | Risk Ratio (M‐H, Random, 95% CI) | 2.56 [0.30, 21.74] |
| 2.8.7 Secukinumab versus ustekinumab | 2 | 1778 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.70, 2.30] |
| 2.8.8 Ixekizumab versus ustekinumab | 1 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.18, 3.01] |
| 2.8.9 Brodalumab versus ustekinumab | 2 | 3088 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.64, 3.56] |
| 2.8.10 Risankizumab versus ustekinumab | 3 | 965 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.32] |
| 2.8.11 Bimekizumab versus ustekinumab | 1 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.15, 1.73] |
| 2.8.12 Guselkumab versus adalimumab | 3 | 1658 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.45, 1.84] |
| 2.8.13 Risankizumab versus adalimumab | 1 | 605 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.46, 2.72] |
| 2.8.14 Bimekizumab versus adalimumab | 1 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.15, 1.70] |
| 2.8.15 Ixekizumab versus guselkumab | 1 | 1027 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.57, 2.13] |
| 2.8.16 Risankizumab versus secukinumab | 1 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.54, 4.09] |
| 2.8.17 Ixekizumab versus secukinumab | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.8.18 Guselkumab versus secukinumab | 1 | 1048 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.55, 1.35] |
| 2.8.19 Sonelokimab versus secukinumab | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [0.16, 50.61] |
| 2.9 Biologic versus small molecules | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.9.1 Etanercept versus apremilast | 2 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.14] |
| 2.10 Small molecules versus placebo | 11 | 5187 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.47, 1.06] |
| 2.10.1 Apremilast versus placebo | 9 | 3436 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.43, 1.19] |
| 2.10.2 Deucravacitinib versus placebo | 4 | 1751 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.33, 1.49] |
| 2.11 Small molecule 1 versus small molecule 2 | 2 | 1265 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.35, 6.24] |
| 2.11.1 Deucravacitinib versus apremilast | 2 | 1265 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.35, 6.24] |
| 2.12 Small molecules versus non‐biological treatments | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.12.1 Apremilast versus ciclosporin | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
2.11. Analysis.

Comparison 2: Primary outcome ‐ serious adverse events (SAEs), Outcome 11: Small molecule 1 versus small molecule 2
2.12. Analysis.

Comparison 2: Primary outcome ‐ serious adverse events (SAEs), Outcome 12: Small molecules versus non‐biological treatments
Comparison 3. Secondary outcome ‐ PASI 75.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Non‐biological treatments versus placebo | 5 | 1451 | Risk Ratio (M‐H, Random, 95% CI) | 2.34 [1.81, 3.03] |
| 3.1.1 Methotrexate versus placebo | 2 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 2.36 [1.19, 4.68] |
| 3.1.2 Fumaric acid esters versus placebo | 2 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 2.39 [1.78, 3.21] |
| 3.1.3 Acitretin versus placebo | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.23, 14.80] |
| 3.2 Non‐biological treatment 1 versus non‐biological treatment 2 | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.2.1 Ciclosporin versus methotrexate | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.84, 2.23] |
| 3.2.2 Methotrexate versus fumaric acid esters | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [0.74, 7.19] |
| 3.3 Anti‐TNF alpha versus placebo | 35 | 12078 | Risk Ratio (M‐H, Random, 95% CI) | 9.21 [7.78, 10.91] |
| 3.3.1 Etanercept versus placebo | 15 | 5762 | Risk Ratio (M‐H, Random, 95% CI) | 8.56 [7.07, 10.36] |
| 3.3.2 Adalimumab versus placebo | 10 | 3485 | Risk Ratio (M‐H, Random, 95% CI) | 8.25 [6.03, 11.29] |
| 3.3.3 Certolizumab versus placebo | 5 | 1153 | Risk Ratio (M‐H, Random, 95% CI) | 9.55 [6.13, 14.88] |
| 3.3.4 Infliximab versus placebo | 6 | 1678 | Risk Ratio (M‐H, Random, 95% CI) | 18.87 [8.53, 41.75] |
| 3.4 Anti‐IL12/23 versus placebo | 12 | 4842 | Risk Ratio (M‐H, Random, 95% CI) | 11.36 [8.84, 14.61] |
| 3.4.1 Ustekinumab versus placebo | 12 | 4842 | Risk Ratio (M‐H, Random, 95% CI) | 11.36 [8.84, 14.61] |
| 3.5 Anti‐IL17 versus placebo | 30 | 13761 | Risk Ratio (M‐H, Random, 95% CI) | 14.51 [11.58, 18.17] |
| 3.5.1 Secukinumab versus placebo | 15 | 4637 | Risk Ratio (M‐H, Random, 95% CI) | 15.67 [11.54, 21.27] |
| 3.5.2 Ixekizumab versus placebo | 5 | 3706 | Risk Ratio (M‐H, Random, 95% CI) | 15.99 [10.44, 24.47] |
| 3.5.3 Brodalumab versus placebo | 6 | 4171 | Risk Ratio (M‐H, Random, 95% CI) | 13.09 [8.75, 19.57] |
| 3.5.4 Bimekizumab versus placebo | 2 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 13.66 [6.99, 26.71] |
| 3.5.5 Netakimab versus placebo | 2 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 14.12 [0.26, 778.45] |
| 3.5.6 Sonelokimab versus placebo | 1 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 86.98 [5.51, 1373.50] |
| 3.6 Anti‐IL23 versus placebo | 13 | 5197 | Risk Ratio (M‐H, Random, 95% CI) | 11.46 [9.35, 14.05] |
| 3.6.1 Guselkumab versus placebo | 5 | 1767 | Risk Ratio (M‐H, Random, 95% CI) | 12.65 [9.24, 17.31] |
| 3.6.2 Tildrakizumab versus placebo | 3 | 1904 | Risk Ratio (M‐H, Random, 95% CI) | 11.24 [7.33, 17.23] |
| 3.6.3 Risankizumab versus placebo | 5 | 1526 | Risk Ratio (M‐H, Random, 95% CI) | 10.29 [7.20, 14.70] |
| 3.7 Biologic versus non‐biological treatments | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.7.1 Etanercept versus acitretin | 3 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.26, 3.12] |
| 3.7.2 Adalimumab versus methotrexate | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [1.72, 2.94] |
| 3.7.3 Infliximab versus methotrexate | 1 | 868 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [1.58, 2.19] |
| 3.7.4 Ixekizumab versus methotrexate | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.06, 1.56] |
| 3.7.5 Risankizumab versus methotrexate | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.05, 1.50] |
| 3.7.6 Brodalumab versus fumaric acid esters | 1 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [1.64, 2.76] |
| 3.7.7 Guselkumab versus fumaric acid esters | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 3.26 [2.13, 4.99] |
| 3.7.8 Ixekizumab versus fumaric acid esters | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 4.08 [2.46, 6.77] |
| 3.7.9 Risankizumab versus fumaric acid esters | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [2.06, 4.23] |
| 3.7.10 Secukinumab versus fumaric acid esters | 1 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 3.30 [2.36, 4.62] |
| 3.7.11 Etanercept versus ciclosporin | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.89, 1.23] |
| 3.8 Biologic 1 versus biologic 2 | 26 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.8.1 Ustekinumab versus etanercept | 1 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.13, 1.40] |
| 3.8.2 Secukinumab versus etanercept | 1 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.44, 1.88] |
| 3.8.3 Infliximab versus etanercept | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [1.12, 3.81] |
| 3.8.4 Ixekizumab versus etanercept | 2 | 2209 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [1.43, 2.24] |
| 3.8.5 Tildrakizumab versus etanercept | 1 | 934 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.16, 1.50] |
| 3.8.6 Certolizumab versus etanercept | 1 | 502 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.01, 1.40] |
| 3.8.7 Secukinumab versus ustekinumab | 2 | 1778 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.10, 1.19] |
| 3.8.8 Ixekizumab versus ustekinumab | 1 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.02, 1.22] |
| 3.8.9 Brodalumab versus ustekinumab | 2 | 3088 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.04, 1.17] |
| 3.8.10 Risankizumab versus ustekinumab | 3 | 965 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.13, 1.33] |
| 3.8.11 Bimekizumab versus ustekinumab | 1 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.14, 1.39] |
| 3.8.12 Guselkumab versus adalimumab | 3 | 1658 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.14, 1.32] |
| 3.8.13 Risankizumab versus adalimumab | 1 | 605 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.17, 1.37] |
| 3.8.14 Bimekizumab versus adalimumab | 1 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.20, 1.49] |
| 3.8.15 Ixekizumab versus adalimumab | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.00, 1.45] |
| 3.8.16 Risankizumab versus secukinumab | 1 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.05, 1.26] |
| 3.8.17 Bimekizumab versus secukinumab | 1 | 743 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.98, 1.07] |
| 3.8.18 Guselkumab versus secukinumab | 1 | 1048 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 1.01] |
| 3.8.19 Sonelokimab versus secukinumab | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.01] |
| 3.9 Biologic versus small molecules | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.9.1 Etanercept versus apremilast | 2 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.76, 1.43] |
| 3.10 Small molecules versus placebo | 10 | 4884 | Risk Ratio (M‐H, Random, 95% CI) | 4.34 [3.45, 5.46] |
| 3.10.1 Apremilast versus placebo | 8 | 3133 | Risk Ratio (M‐H, Random, 95% CI) | 3.73 [2.81, 4.94] |
| 3.10.2 Deucravacitinib versus placebo | 4 | 1751 | Risk Ratio (M‐H, Random, 95% CI) | 5.54 [4.28, 7.17] |
| 3.11 Small molecule 1 versus small molecule 2 | 2 | 1265 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.19, 1.83] |
| 3.11.1 Deucravacitinib versus apremilast | 2 | 1265 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.19, 1.83] |
| 3.12 Small molecules versus non‐biological treatments | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.95, 1.27] |
| 3.12.1 Apremilast versus ciclosporin | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.95, 1.27] |
3.11. Analysis.

Comparison 3: Secondary outcome ‐ PASI 75, Outcome 11: Small molecule 1 versus small molecule 2
3.12. Analysis.

Comparison 3: Secondary outcome ‐ PASI 75, Outcome 12: Small molecules versus non‐biological treatments
Comparison 4. Secondary outcome ‐ PGA 0/1.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Non‐biological treatment versus placebo | 5 | 1449 | Risk Ratio (M‐H, Random, 95% CI) | 2.35 [1.79, 3.08] |
| 4.1.1 Methotrexate versus placebo | 3 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 3.19 [1.66, 6.16] |
| 4.1.2 Fumaric acid esters versus placebo | 2 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.54, 3.21] |
| 4.2 Non‐biological treatment 1 versus non‐biological treatment 2 | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.2.1 Ciclosporin versus methotrexate | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.47, 1.46] |
| 4.2.2 Methotrexate versus fumaric acid esters | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 3.86 [1.84, 8.09] |
| 4.3 Anti‐TNF alpha versus placebo | 29 | 10194 | Risk Ratio (M‐H, Random, 95% CI) | 8.89 [7.36, 10.74] |
| 4.3.1 Etanercept versus placebo | 13 | 5030 | Risk Ratio (M‐H, Random, 95% CI) | 8.11 [6.35, 10.37] |
| 4.3.2 Adalimumab versus placebo | 9 | 3337 | Risk Ratio (M‐H, Random, 95% CI) | 7.89 [6.13, 10.16] |
| 4.3.3 Certolizumab versus placebo | 5 | 1266 | Risk Ratio (M‐H, Random, 95% CI) | 27.86 [12.17, 63.79] |
| 4.3.4 Infliximab versus placebo | 3 | 561 | Risk Ratio (M‐H, Random, 95% CI) | 13.11 [6.69, 25.69] |
| 4.4 Anti‐IL12/23 versus placebo | 12 | 4842 | Risk Ratio (M‐H, Random, 95% CI) | 10.70 [7.82, 14.66] |
| 4.4.1 Ustekinumab versus placebo | 12 | 4842 | Risk Ratio (M‐H, Random, 95% CI) | 10.70 [7.82, 14.66] |
| 4.5 Anti‐IL17 versus placebo | 28 | 13801 | Risk Ratio (M‐H, Random, 95% CI) | 18.05 [13.08, 24.90] |
| 4.5.1 Secukinumab versus placebo | 12 | 4242 | Risk Ratio (M‐H, Random, 95% CI) | 18.26 [11.34, 29.40] |
| 4.5.2 Ixekizumab versus placebo | 5 | 3706 | Risk Ratio (M‐H, Random, 95% CI) | 18.29 [11.30, 29.61] |
| 4.5.3 Brodalumab versus placebo | 6 | 4171 | Risk Ratio (M‐H, Random, 95% CI) | 19.02 [13.49, 26.81] |
| 4.5.4 Bimekizumab versus placebo | 3 | 1089 | Risk Ratio (M‐H, Random, 95% CI) | 21.60 [9.32, 50.08] |
| 4.5.5 Netakimab versus placebo | 2 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 9.20 [0.36, 232.36] |
| 4.5.6 Sonelokimab versus placebo | 1 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 78.87 [4.99, 1245.88] |
| 4.6 Anti‐IL23 versus placebo | 14 | 5354 | Risk Ratio (M‐H, Random, 95% CI) | 10.43 [8.58, 12.68] |
| 4.6.1 Guselkumab versus placebo | 5 | 1767 | Risk Ratio (M‐H, Random, 95% CI) | 10.87 [8.11, 14.57] |
| 4.6.2 Tildrakizumab versus placebo | 3 | 1904 | Risk Ratio (M‐H, Random, 95% CI) | 10.26 [6.62, 15.91] |
| 4.6.3 Risankizumab versus placebo | 6 | 1683 | Risk Ratio (M‐H, Random, 95% CI) | 9.97 [7.11, 13.98] |
| 4.7 Biologic versus non‐biological treatments | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.7.1 Etanercept versus acitretin | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 4.98 [1.15, 21.49] |
| 4.7.2 Adalimumab versus methotrexate | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [1.79, 3.32] |
| 4.7.3 Infliximab versus methotrexate | 1 | 868 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [1.67, 2.37] |
| 4.7.4 Ixekizumab versus methotrexate | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.24, 2.23] |
| 4.7.5 Risankizumab versus methotrexate | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.11, 1.75] |
| 4.7.6 Brodalumab versus fumaric acid esters | 1 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 3.24 [2.15, 4.87] |
| 4.7.7 Ixekizumab versus fumaric acid esters | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 6.43 [3.19, 12.96] |
| 4.7.8 Risankizumab versus fumaric acid esters | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 2.43 [1.75, 3.38] |
| 4.7.9 Secukinumab versus fumaric acid esters | 1 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 6.16 [3.59, 10.57] |
| 4.7.10 Etanercept versus ciclosporin | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.67, 1.65] |
| 4.8 Biologic 1 versus biologic 2 | 26 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.8.1 Ustekinumab versus etanercept | 1 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.24, 1.58] |
| 4.8.2 Secukinumab versus etanercept | 1 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.73, 2.53] |
| 4.8.3 Infliximab versus etanercept | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 2.50 [1.30, 4.81] |
| 4.8.4 Ixekizumab versus etanercept | 2 | 2209 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [1.74, 2.31] |
| 4.8.5 Tildrakizumab versus etanercept | 1 | 934 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.05, 1.37] |
| 4.8.6 Secukinumab versus ustekinumab | 2 | 1778 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.19, 1.38] |
| 4.8.7 Ixekizumab versus ustekinumab | 1 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.09, 1.39] |
| 4.8.8 Brodalumab versus ustekinumab | 2 | 3088 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.07, 1.27] |
| 4.8.9 Risankizumab versus ustekinumab | 3 | 965 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.23, 1.52] |
| 4.8.10 Bimekizumab versus ustekinumab | 1 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [1.35, 1.83] |
| 4.8.11 Guselkumab versus adalimumab | 3 | 1658 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.19, 1.34] |
| 4.8.12 Risankizumab versus adalimumab | 1 | 605 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.25, 1.54] |
| 4.8.13 Bimekizumab versus adalimumab | 1 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.30, 1.72] |
| 4.8.14 Ixekizumab versus guselkumab | 1 | 1027 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.21, 1.46] |
| 4.8.15 Risankizumab versus secukinumab | 1 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.10, 1.37] |
| 4.8.16 Ixekizumab versus secukinumab | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.81, 1.27] |
| 4.8.17 Bimekizumab versus secukinumab | 1 | 743 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.02, 1.16] |
| 4.8.18 Guselkumab versus secukinumab | 1 | 1048 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.95, 1.05] |
| 4.8.19 Sonelokimab versus secukinumab | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.82, 1.14] |
| 4.9 Biologic versus small molecules | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.9.1 Etanercept versus apremilast | 2 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.78, 1.53] |
| 4.10 Small molecules versus placebo | 10 | 4927 | Risk Ratio (M‐H, Random, 95% CI) | 4.55 [3.52, 5.86] |
| 4.10.1 Apremilast versus placebo | 8 | 3176 | Risk Ratio (M‐H, Random, 95% CI) | 3.68 [2.83, 4.78] |
| 4.10.2 Deucravacitinib versus placebo | 4 | 1751 | Risk Ratio (M‐H, Random, 95% CI) | 6.60 [4.91, 8.88] |
| 4.11 Small molecule 1 versus small molecule 2 | 2 | 1265 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.32, 1.79] |
| 4.11.1 Deucravacitinib versus apremilast | 2 | 1265 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.32, 1.79] |
| 4.12 Small molecules versus non‐biological treatments | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.70, 1.71] |
| 4.12.1 Apremilast versus ciclosporin | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.70, 1.71] |
4.11. Analysis.

Comparison 4: Secondary outcome ‐ PGA 0/1, Outcome 11: Small molecule 1 versus small molecule 2
4.12. Analysis.

Comparison 4: Secondary outcome ‐ PGA 0/1, Outcome 12: Small molecules versus non‐biological treatments
Comparison 5. Secondary outcome ‐ quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Non‐biological treatments versus placebo | 2 | 283 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.40, 0.06] |
| 5.1.1 Methotrexate versus placebo | 2 | 283 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.40, 0.06] |
| 5.2 Non‐biological treatment 1 versus non‐biological treatment 2 | 1 | 108 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.37 [‐1.80, ‐0.95] |
| 5.2.1 Methotrexate versus fumaric acid esters | 1 | 108 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.37 [‐1.80, ‐0.95] |
| 5.3 Anti‐TNF alpha versus placebo | 25 | 8534 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.18, ‐0.96] |
| 5.3.1 Etanercept versus placebo | 8 | 3246 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.34, ‐0.88] |
| 5.3.2 Adalimumab versus placebo | 9 | 3055 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.11, ‐0.85] |
| 5.3.3 Certolizumab versus placebo | 3 | 588 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.13, ‐0.72] |
| 5.3.4 Infliximab versus placebo | 5 | 1645 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐1.48, ‐1.10] |
| 5.4 Ustekinumab versus placebo | 9 | 3359 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐1.54, ‐1.16] |
| 5.5 Anti‐IL17 versus placebo | 9 | 4246 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.47 [‐1.76, ‐1.18] |
| 5.5.1 Ixekizumab versus placebo | 4 | 3564 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.85 [‐2.14, ‐1.55] |
| 5.5.2 Brodalumab versus placebo | 2 | 349 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.44, ‐0.47] |
| 5.5.3 Secukinumab versus placebo | 2 | 213 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐1.71, ‐1.09] |
| 5.5.4 Netakimab versus placebo | 1 | 120 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.26, ‐0.39] |
| 5.6 Anti‐IL23 versus placebo | 9 | 4196 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.46 [‐1.62, ‐1.30] |
| 5.6.1 Guselkumab versus placebo | 3 | 1444 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐1.54, ‐1.18] |
| 5.6.2 Tildrakizumab versus placebo | 3 | 1904 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐1.48, ‐1.23] |
| 5.6.3 Risankizumab versus placebo | 3 | 848 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.82 [‐2.04, ‐1.60] |
| 5.7 Biologic versus non‐biological treatments | 6 | 983 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.97, ‐0.26] |
| 5.7.1 Adalimumab versus methotrexate | 1 | 218 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.65, ‐0.12] |
| 5.7.2 Ixekizumab versus methotrexate | 1 | 108 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.43, 0.33] |
| 5.7.3 Brodalumab versus fumaric acid esters | 1 | 210 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.68, ‐0.13] |
| 5.7.4 Guselkumab versus fumaric acid esters | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.30, ‐0.54] |
| 5.7.5 Ixekizumab versus fumaric acid esters | 1 | 108 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐1.85, ‐1.00] |
| 5.7.6 Risankizumab versus fumaric acid esters | 1 | 120 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.53, ‐0.75] |
| 5.7.7 Etanercept versus ciclosporin | 1 | 100 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.45, 0.34] |
| 5.8 Biologic 1 versus biologic 2 | 9 | 5873 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.40, ‐0.25] |
| 5.8.1 Ixekizumab versus etanercept | 2 | 2209 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.53, ‐0.35] |
| 5.8.2 Tildrakizumab versus etanercept | 1 | 932 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.38, ‐0.10] |
| 5.8.3 Infliximab versus etanercept | 1 | 48 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.25, ‐0.08] |
| 5.8.4 Guselkumab versus adalimumab | 2 | 1407 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.35, ‐0.14] |
| 5.8.5 Bimekizumab versus adalimumab | 1 | 478 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.50, ‐0.12] |
| 5.8.6 Risankizumab versus ustekinumab | 2 | 799 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.46, ‐0.14] |
| 5.9 Biologic versus small molecules | 1 | 100 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.19, 0.60] |
| 5.9.1 Etanercept versus apremilast | 1 | 100 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.19, 0.60] |
| 5.10 Small molecules versus placebo | 7 | 4273 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.71, ‐0.53] |
| 5.10.1 Apremilast versus placebo | 7 | 3009 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.65, ‐0.49] |
| 5.10.2 Deucravacitinib versus placebo | 2 | 1264 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐0.90, ‐0.66] |
| 5.11 Small molecule 1 versus small molecule 2 | 2 | 1265 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.45, ‐0.13] |
| 5.11.1 Deucravacitinib versus apremilast | 2 | 1265 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.45, ‐0.13] |
| 5.12 Small molecules versus non‐biological treatments | 1 | 100 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.65, 0.14] |
| 5.12.1 Apremilast versus ciclosporin | 1 | 100 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.65, 0.14] |
5.11. Analysis.

Comparison 5: Secondary outcome ‐ quality of life, Outcome 11: Small molecule 1 versus small molecule 2
5.12. Analysis.

Comparison 5: Secondary outcome ‐ quality of life, Outcome 12: Small molecules versus non‐biological treatments
Comparison 6. Secondary outcome ‐ adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Non‐biological treatments versus placebo | 5 | 1449 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.86, 1.41] |
| 6.1.1 Methotrexate versus placebo | 3 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.81, 1.10] |
| 6.1.2 Fumaric acid esters versus placebo | 2 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.11, 1.55] |
| 6.2 Non‐biological treatment 1 versus non‐biological treatment 2 | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.2.1 Ciclosporin versus methotrexate | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.90, 1.34] |
| 6.2.2 Methotrexate versus fumaric acid esters | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.90, 1.24] |
| 6.3 Anti‐TNF alpha versus placebo | 28 | 9983 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.01, 1.10] |
| 6.3.1 Etanercept versus placebo | 11 | 4225 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [1.00, 1.16] |
| 6.3.2 Adalimumab versus placebo | 9 | 3338 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.99, 1.12] |
| 6.3.3 Certolizumab versus placebo | 5 | 1153 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.04] |
| 6.3.4 Infliximab versus placebo | 4 | 1267 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.93, 1.36] |
| 6.4 Ustekinumab versus placebo | 12 | 4842 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [1.01, 1.13] |
| 6.5 Anti‐IL17 versus placebo | 30 | 14052 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.13, 1.29] |
| 6.5.1 Secukinumab versus placebo | 14 | 4493 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [1.05, 1.32] |
| 6.5.2 Ixekizumab versus placebo | 5 | 3706 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.10, 1.56] |
| 6.5.3 Brodalumab versus placebo | 6 | 4171 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.01, 1.30] |
| 6.5.4 Bimekizumab versus placebo | 3 | 1089 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.15, 1.69] |
| 6.5.5 Netakimab versus placebo | 2 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.59, 1.37] |
| 6.5.6 Sonelokimab versus placebo | 1 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.86, 1.71] |
| 6.6 Anti‐IL23 versus placebo | 14 | 5354 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 1.00] |
| 6.6.1 Guselkumab versus placebo | 5 | 1767 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.90, 1.11] |
| 6.6.2 Tildrakizumab versus placebo | 3 | 1904 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.72, 1.02] |
| 6.6.3 Risankizumab versus placebo | 6 | 1683 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.07] |
| 6.7 Biologic versus non‐biological treatments | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.7.1 Etanercept versus acitretin | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.72, 1.96] |
| 6.7.2 Adalimumab versus methotrexate | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.05] |
| 6.7.3 Infliximab versus methotrexate | 1 | 868 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.97, 1.20] |
| 6.7.4 Ixekizumab versus methotrexate | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.76, 1.25] |
| 6.7.5 Risankizumab versus methotrexate | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.77, 1.14] |
| 6.7.6 Brodalumab versus fumaric acid esters | 1 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.62, 0.87] |
| 6.7.7 Guselkumab versus fumaric acid esters | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.65, 0.89] |
| 6.7.8 Ixekizumab versus fumaric acid esters | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.74, 1.21] |
| 6.7.9 Risankizumab versus fumaric acid esters | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.75, 0.98] |
| 6.7.10 Secukinumab versus fumaric acid esters | 1 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.71, 0.94] |
| 6.7.11 Etanercept versus ciclosporin | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.56, 1.77] |
| 6.8 Biologic 1 versus biologic 2 | 26 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.8.1 Ustekinumab versus etanercept | 1 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.89, 1.06] |
| 6.8.2 Secukinumab versus etanercept | 1 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.12] |
| 6.8.3 Ixekizumab versus etanercept | 2 | 2209 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.97, 1.15] |
| 6.8.4 Infliximab versus etanercept | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.86, 1.08] |
| 6.8.5 Tildrakizumab versus etanercept | 1 | 934 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.65, 0.86] |
| 6.8.6 Certolizumab versus etanercept | 1 | 502 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.86, 1.28] |
| 6.8.7 Secukinumab versus ustekinumab | 2 | 1778 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.16] |
| 6.8.8 Ixekizumab versus ustekinumab | 1 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.06] |
| 6.8.9 Brodalumab versus ustekinumab | 2 | 3088 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.93, 1.09] |
| 6.8.10 Risankizumab versus ustekinumab | 3 | 965 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.11] |
| 6.8.11 Bimekizumab versus ustekinumab | 1 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.93, 1.32] |
| 6.8.12 Guselkumab versus adalimumab | 3 | 1658 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.09] |
| 6.8.13 Risankizumab versus adalimumab | 1 | 605 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.85, 1.13] |
| 6.8.14 Bimekizumab versus adalimumab | 1 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.90, 1.16] |
| 6.8.15 Ixekizumab versus guselkumab | 1 | 1027 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.92, 1.15] |
| 6.8.16 Risankizumab versus secukinumab | 1 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.87, 1.15] |
| 6.8.17 Ixekizumab versus secukinumab | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.71, 1.52] |
| 6.8.18 Guselkumab versus secukinumab | 1 | 1048 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.90, 1.02] |
| 6.8.19 Sonelokimab versus secukinumab | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.77, 1.42] |
| 6.9 Biologic versus small molecules | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.9.1 Etanercept versus apremilast | 2 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.02, 1.60] |
| 6.10 Small molecules versus placebo | 11 | 5187 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.13, 1.28] |
| 6.10.1 Apremilast versus placebo | 9 | 3436 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.13, 1.32] |
| 6.10.2 Deucravacitinib versus placebo | 4 | 1751 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.04, 1.30] |
| 6.11 Small molecule 1 versus small molecule 2 | 2 | 1265 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.87, 1.07] |
| 6.11.1 Deucravacitinib versus apremilast | 2 | 1265 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.87, 1.07] |
| 6.12 Small molecules versus non‐biological treatments | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.52, 1.68] |
| 6.12.1 Apremilast versus ciclosporin | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.52, 1.68] |
6.11. Analysis.

Comparison 6: Secondary outcome ‐ adverse events, Outcome 11: Small molecule 1 versus small molecule 2
6.12. Analysis.

Comparison 6: Secondary outcome ‐ adverse events, Outcome 12: Small molecules versus non‐biological treatments
Comparison 7. Secondary outcome ‐ PASI 90 at 52 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Biologic 1 versus biologic 2 | 17 | 8729 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.09, 1.32] |
| 7.1.1 Secukinumab versus ustekinumab | 2 | 1778 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.15, 1.31] |
| 7.1.2 Risankizumab versus ustekinumab | 2 | 799 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.46, 2.05] |
| 7.1.3 Ixekizumab versus ustekinumab | 1 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.11, 1.52] |
| 7.1.4 Bimekizumab versus ustekinumab | 1 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.27, 1.70] |
| 7.1.5 Risankizumab versus secukinumab | 1 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.31, 1.76] |
| 7.1.6 Bimekizumab versus secukinumab | 1 | 743 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.09, 1.28] |
| 7.1.7 Guselkumab versus secukinumab | 1 | 1048 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.13, 1.29] |
| 7.1.8 Guselkumab versus adalimumab | 1 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.40, 1.81] |
| 7.1.9 Ixekizumab versus adalimumab | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.04, 1.74] |
| 7.1.10 Secukinumab 150 versus secukinumab 300 | 3 | 1017 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.78, 0.91] |
| 7.1.11 Guselkumab 100 versus guselkumab 50 | 1 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.85, 1.25] |
| 7.1.12 Ixekizumab Q2W versus Ixekizumab Q4W | 1 | 1227 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [1.01, 1.11] |
| 7.1.13 Risankizumab 75 versus risankizumab 150 | 1 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.82, 1.06] |
| 7.2 Small molecule 1 versus small molecule 2 | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.84, 1.86] |
| 7.2.1 Apremilast 30 mg versus apremilast other | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.84, 1.86] |
Comparison 8. Secondary outcome ‐ PASI 75 at 52 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Biologic 1 versus biologic 2 | 16 | 8245 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.02, 1.16] |
| 8.1.1 Secukinumab versus ustekinumab | 2 | 1778 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.04, 1.22] |
| 8.1.2 Risankizumab versus ustekinumab | 2 | 799 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.12, 1.41] |
| 8.1.3 Ixekizumab versus ustekinumab | 1 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.05, 1.29] |
| 8.1.4 Risankizumab versus secukinumab | 1 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.14, 1.44] |
| 8.1.5 Bimekizumab versus secukinumab | 1 | 743 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.02, 1.16] |
| 8.1.6 Guselkumab versus secukinumab | 1 | 1048 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [1.00, 1.12] |
| 8.1.7 Guselkumab versus adalimumab | 1 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.28, 1.54] |
| 8.1.8 Ixekizumab versus adalimumab | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.89, 1.27] |
| 8.1.9 Secukinumab 150 versus secukinumab 300 | 3 | 1017 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.85, 0.94] |
| 8.1.10 Guselkumab 100 versus guselkumab 50 | 1 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.09] |
| 8.1.11 Ixekizumab Q2W versus ixekizumab Q4W | 1 | 1227 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.07, 1.22] |
| 8.1.12 Risankizumab 75 versus risankizumab 150 | 1 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.91, 1.07] |
| 8.2 Small molecule 1 versus small molecule 2 | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.46, 2.78] |
| 8.2.1 Apremilast 30 versus apremilast other | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.46, 2.78] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ACCEPT 2010.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: March 2007 to January 2009 Location: 67 centres in Manchester, UK Phase 3 |
|
| Participants |
Randomised: 903 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 903, mean age 45 years, 613 male Dropouts and withdrawals 24/903 (2.7%)
|
|
| Interventions |
Intervention A. Ustekinumab (n = 209), SC, 45 mg, weeks 0 to 4, 4 weeks Control intervention B. Ustekinumab (n = 347), SC, 90 mg, weeks 0 to 4, 4 weeks C. Etanercept (n = 347), SC, 50 mg x 2/weeks, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p. 127): "Supported by Centocor Research and Development." Declarations of interest: Quote (p. 127): "Dr. Griffiths reports receiving consulting and lecture fees from Abbott, Janssen‐Cilag, Merck Serono, Novartis, Schering‐Plough, and Wyeth and grant support from Merck Serono; Dr. Strober, receiving consulting and lecture fees from Centocor, Johnson & Johnson, Amgen, and Abbott Laboratories and grant support from Amgen and Abbott Laboratories; Dr. van de Kerkhof, receiving consulting fees from Schering‐Plough, Celgene, Centocor, Almirall, UCB, Wyeth, Pfizer, Soffinova, Abbott, Actelion, Galderma, Novartis, Janssen‐Cilag, and Leo Pharma; Dr. Ho, receiving advisory‐board and lecture fees from Schering, Abbott, Janssen‐Ortho, Pfizer, Amgen, and Wyeth and grant support from Centocor, Abbott, Amgen, and Wyeth; Dr. Menter, receiving advisory‐board, consulting, and lecture fees from Abbott, Amgen, Astellas, Biogen Idec, Celgene, Centocor, Genentech, Warner Chilcott, and Wyeth; Drs. Yeilding, Guzzo, Xia, and Dooley and Ms. Li, being employees of Johnson & Johnson and having equity and holding stock options in Johnson & Johnson; Dr. Zhou, being an employee of Johnson & Johnson, having equity and holding stock options in Johnson & Johnson, and having equity in Wyeth; Dr. Fidelus‐Gort, being a former employee of Johnson & Johnson and having equity and holding stock options in Johnson & Johnson; and Dr. Goldstein, receiving consulting fees from Centocor. No other potential conflict of interest relevant to this article was reported." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p. 119): “We randomly assigned...” Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p. 119): “We randomly assigned...” Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 119): “Patients were aware of their treatment assignment”, ... “All study personnel, except those who dispensed or administered a study agent remained unaware of the treatment assignments". Comment: high risk for participants and unclear risk for personnel (no description of means used to avoid communication between participants and personnel and very difficult to avoid) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 119): “All study personnel, except those who dispensed or administered a study agent remained unaware of the treatment assignments". Comment: no description of the method used to assess the primary outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 903 participants underwent randomisation; 903 were analysed Comment: methods for dealing with missing data not specified |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00454584). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
ADACCESS 2018.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: December 2013 and March 2015 Location: 73 study centres in Bulgaria, France, Slovakia, and the USA Phase 3 |
|
| Participants |
Randomised: 465 participants (mean age 46 years, 184 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. GP2017, n = 231 Control intervention B. ref‐ADMB (Humira; AbbVie Ltd, Maidenhead, UK; AbbVie Inc., North Chicago, IL, U.S.A), n = 234 Sourced from Europe or the USA, an initial dose of 80 mg subcutaneous, then followed by 40 mg every other week, starting 1 week after the initial dose until week 15 |
|
| Outcomes |
Assessment at week 16 Primary outcome
Secondary outcomes
|
|
| Notes | Funding source Quote (p 623): "The study was funded by Hexal AG, a Sandoz company. The funder had a role in the study design, data collection, data analysis and manuscript preparation". Declarations of interest Quote (p 623): "A. Blauvelt has served as a scientific adviser and clinical study investigator for AbbVie, Allergan, Amgen, Boehringer Ingelheim, Celgene, Dermira Inc., Eli Lilly and Company, Janssen, Merck Sharp & Dohme, Novartis, Sandoz, UCB Pharma and Valeant; and as a paid speaker for Eli Lilly and Company and Janssen. J.P.L. has served as a clinical study investigator for Sandoz and has received a grant from University Hospital Nice. J.F.F. has served as a clinical study investigator for and has received research grants from Sandoz. J.M.W.served as a clinical study investigator for and has received research grants from Sandoz, and has received research grants and honoraria from Novartis. D.G. has served as a clinical study investigator for Sandoz. E.S., J.J.L. and A. Balfour are employees of Hexal AG (a Novartis Division). C.L.L. has served as a consultant or advisory board member for AbbVie, Amgen, Boehringer Ingelheim, Dermira, Eli Lilly and Company, Janssen, LEO Pharma, Pfizer, Sandoz, VCB and Vitae; as an investigator for Actavis, AbbVie, Amgen, Boehringer Ingelheim, Celgene, Coherus, Cellceutix, Corrona, Dermira, Eli Lilly and Company, Galderma, Glenmark, Janssen, LEO Pharma, Merck, Novartis, Novella, Pfizer, Sandoz, Sienna, Stiefel and Wyeth; and as a participant in speaker bureaus for AbbVie, Celgene, Eli Lilly and Company and Novartis." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 624): "This was a randomized, multicentre phase III confirmatory study consisting of four periods...Randomization was stratified by prior systemic therapy, region and body weight, and was performed centrally". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 624): "This was a randomized, multicentre phase III confirmatory study consisting of four periods...Randomization was stratified by prior systemic therapy, region and body weight, and was performed centrally". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 624): “The study was double blinded; patients, investigator staff and the people performing the study assessments remained blinded to the identity of the given treatments until week 51.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 624): “The study was double blinded; patients, investigator staff and the people performing the study assessments remained blinded to the identity of the given treatments until week 51.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 465 Management of missing data: Quote (supplemental appendix): "No imputation of missing values was performed." Non‐inferiority trial: Quote (p 626): "In line with guidance from the U.S. Food and Drug Administration (FDA), efficacy analyses were conducted using the per protocol analysis set. The per protocol set is considered conservative, as protocol violators who could bias study results towards equivalence are excluded. Supportive analyses were performed using the full analysis set." Table 1: Both per‐protocol and full‐set analyses Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02016105). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results posted on ClinicalTrials.gov |
AFFIRM 2022.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: January 2018 to March 2020 Location: USA (76 sites) Phase 2 |
|
| Participants |
Randomised: 426 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 426, mean of age 50 years, 60% men Dropouts and withdrawals 173/426 (40.6%): tepilamide fumarate 400 mg once a day group (38), tepilamide fumarate 400mg twice a day group (39), tepilamide fumarate 600 mg twice a day group (51), placebo group (45)
|
|
| Interventions |
Intervention A. Tepilamide fumarate 400 mg tablet once a day, n = 105 Control interventions B. Tepilamide fumarate 400 mg tablet twice a day, n = 107 C. Tepilamide fumarate tablets 600 mg twice a day, n = 107 D. Placebo, n = 107 |
|
| Outcomes | At week 24 Primary composite outcome
Secondary outcomes
|
|
| Notes |
Funding source Quote (p 53): "Funding for this study was provided by Dr. Reddy’s Laboratories, SA." Declarations of interest Quote (p 53): "UM has been an advisor and/or received speaker’s honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, Almirall, Aristea, Boehringer‐Ingelheim, Celgene, Dr. Reddy’s, Eli Lilly, Foamix, Formycon, Forward Pharma, Janssen, LEO Pharma, Medac, Novartis, Pierre Fabre, Sanofi‐Aventis, UCB, and Xenoport. LK has received grants and has been an investigator and/or consultant and/or speaker for the following companies: Abbvie, Amgen, Arcutis, BMS, Dermavant, Dr. Reddy’s, Eli Lilly, Leo Pharma, Mayne Pharma, Novartis, OrthoDermatologics, and Pfizer. KR has received grants and/ or personal fees from the following companies: AbbVie, Affibody, Almirall, Amgen, Avillion, Biogen, Boehringer Ingelheim, Bristol‐Myers‐Squibb, Celgene, Centocor, Covagen, Dr. Reddy’s, Forward Pharma, Fresenius Medical Care, Galapagos, GlaxoSmithKline, Janssen‐Cilag, Kyowa Kirin, LEO, Lilly, Medac, Merck Sharp & Dohme, Novartis, Miltenyi Biotec, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB, Valeant, and Xenoport. SM is employed by Dr. Reddy’s Laboratories. SS is a full‐time employee of and owns stocks in Dr. Reddy's Laboratories, Inc., a fully owned subsidiary of Dr Reddy's Laboratories, SA. ML is an employee of Mount Sinai and receives research funds from: Abbvie, Amgen, Arcutis, Boehringer Ingelheim, Dermavant Sciences, Eli Lilly, Incyte, Janssen Research & Development, LLC, Leo Pharmaceuticals, Orth Dermatologics, Pfizer, and UCB, Inc. ML is also a consultant for Aditum Bio, Allergan, Almirall, Arcutis, Inc., Avotres Therapeutics, BirchBioMed, Inc., BMD Skincare, Inc., Boehringer Ingelheim, Bristol‐Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, EMD Serono, Evelo Biosciences, Facilitation of International Dermatology Education, Foundation for Research and Education in Dermatology, Inozyme Pharma, LEO Pharma, Meiji Seika Pharma, Menlo, Mitsubishi, Neuroderm, Pfizer, Primus/Dr. Reddy’s Laboratories, Theravance, and Verrica." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (protocol p. 37) "All subjects who enter into the screening period for the study (defined as the point at which the subject signs the ICF) will receive a subject identification number through the Interactive Web Response System (IWRS)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (article p 54): "Random allocation sequencing was generated by Medidata Solutions (Iselin, NJ), and patients were stratified by prior biologic use (a maximum of 30% of patients with prior, washed‐out biologic use were permitted to enroll) Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (protocol p. 37): "This is a double‐blind study. The investigator, study coordinator(s), subjects and the Sponsor study team and its representatives, will be blinded to the identity of the randomized treatment assignment from the time of randomization until database lock. Randomization data will be kept in strict confidence by the statistician who generated the randomization schedule, the IWRS provider, and the vendor involved in the IP labeling. All active and placebo IP will be of identical appearance, regardless of the dose. Study materials will be packaged and issued in a manner designed to maintain the blind." Quote (article p 54): "This 24‐week, Phase IIb, randomized, double‐blind, placebo‐controlled study was conducted at 74 sites in the United States, primarily dermatology research clinics." Quote (article p 54): "The investigator and all study representatives were blinded to treatment assignments." Comment: adequate process |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (protocol p. 37): "This is a double‐blind study. The investigator, study coordinator(s), subjects and the Sponsor study team and its representatives, will be blinded to the identity of the randomized treatment assignment from the time of randomization until database lock. Randomization data will be kept in strict confidence by the statistician who generated the randomization schedule, the IWRS provider, and the vendor involved in the IP labeling. All active and placebo IP will be of identical appearance, regardless of the dose. Study materials will be packaged and issued in a manner designed to maintain the blind." Quote (article p 54): "This 24‐week, Phase IIb, randomized, double‐blind, placebo‐controlled study was conducted at 74 sites in the United States, primarily dermatology research clinics." Quote (article p 54): "The investigator and all study representatives were blinded to treatment assignments." Comment: adequate process |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dealing with missing data: Quote (article p 55): "Multiple imputation was used to address missing data for the primary efficacy analysis. Modified non‐responder imputation (m‐NRI) and last observation carried forward (LOCF) were also analyzed and used as sensitivity analyses to assess the robustness of efficacy findings. Each tepilamide fumarate dose was compared to placebo... The modified intent‐to‐treat (mITT) population was used for the primary efficacy analysis and included all randomized patients with ≥1 treatment dose and ≥1 post‐dose efficacy assessment. The safety population included all patients who received ≥1 treatment dose." Randomised 426, analysed 406 Comment: 20 participants did not receive at least 1 dose, 426 participants should be involved in the mITT, however 406 participants were analysed for the primary outcome. |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03421197). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results posted on ClinicalTrials.gov |
Akcali 2014.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: January 2008 to January 2009 Location: Gaziantep, Turkey (1 centre) |
|
| Participants |
Randomised: 55 participants (mean age 39 years, 33 male) Inclusion criteria
Exclusion criteria None Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Acitretin (n = 25), orally, 0.3 to 0.5 mg/kg/d Control intervention B. Cyclosporin (n = 21), orally, 3 mg/kg/d |
|
| Outcomes | Assessment at 8 weeks Primary outcome of the trial
Outcomes of the trial
|
|
| Notes |
Funding source Quote (p 1121): "No specific grant". Declarations of interest Quote (p 1121): "The authors declare that there are no conflicts of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p. 1119): "Patients were stratified into one of two groups via a computer‐generated randomisation schedule". Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not stated that it was a blinded trial. Acitretin has visible side effects (muco‐cutaneous dryness). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no independent assessor. Not stated that it was a blind trial. Acitretin has visible side effects. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 55, analysed 46 Management of missing data: not stated |
| Selective reporting (reporting bias) | High risk | Comment: no primary or secondary outcomes stated. No protocol available |
Al‐Hamamy 2014.
| Study characteristics | ||
| Methods | RCT, active‐placebo controlled, open‐label study Date of study: February 2010 to October 2011 Location: Baghdad, Iraq (1 centre) |
|
| Participants |
Randomised: 120 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 120, mean age 41 years, 41 male Dropouts and withdrawals
No more statements regarding time and reasons of follow‐up |
|
| Interventions |
Intervention A. Methotrexate + NBUVB (n = 38), 20 mg/week + 45 mJ/cm2, 3 times/week Control intervention B. NBUVB (n = 38), 45 mJ/cm2, 3 times/week C. Methotrexate (n = 37), 20 mg/week |
|
| Outcomes | Assessment at 6 months Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes |
Funding source: not stated Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 1531): "three groups randomly...” Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not stated that it was a blind trial, probably not blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no independent assessor. Not stated that it was a blind trial, probably not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 120, analysed 113 Management of missing data: not stated |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. The outcomes mentioned in the Methods section appeared to have been reported. |
ALLURE 2021.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: December 2016 to June 2018 Location: worldwide (52 sites) Phase 3 |
|
| Participants |
Randomised: 214 participants Inclusion criteria People eligible for inclusion in this study must fulfil all of the following criteria:
Exclusion criteria
Baseline characteristics N = 214, mean age of 43.5 years and 62% men Dropouts and withdrawals 5/214 (2%): secukinumab 2 mL group (0), secukinumab 1 mL group (2), placebo group (3)
|
|
| Interventions |
Intervention
A. Secukinumab 2 mL form (secukinumab 300 mg/2 mL + 2 x 1 mL placebo SC at randomisation, weeks 1, 3, 4, thereafter 4‐weekly until week 48), n = 72 Control interventions B. Secukinumab 1 mL form (secukinumab 150 mg/1 mL x 2 + 2 mL placebo SC at randomisation, weeks 1, 3, 4, thereafter 4‐weekly until week 48), n = 71 C. Placebo (2 mL + 2 x 1 mL placebo SC at randomisation, weeks 1, 3, and 4, thereafter 4‐weekly until week 48), n = 71 |
|
| Outcomes |
At week 12 Primary composite outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 8): "The study was sponsored by Novartis Pharma AG, Basel, Switzerland." Declarations of interest: Quote (p 7‐8): "Bardur Sigurgeirsson has consulted for Novartis and several other pharmaceutical companies. He has served on an advisory board for Novartis and several other pharmaceutical companies. Knut Schäkel has been advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, Almirall‐Hermal, Amgen, Biogen Idec, Boehringer‐Ingelheim, Chugai Pharma, Celgene, Eli Lilly, Galderma, Janssen, Leo Pharma, Medac, Merck Serono, MSD, Novartis, Pfizer, Polichem SA, Regeneron Pharmaceutical, Sanofi‐Aventis, Schering‐Plough, UCB Pharma, VBL therapeutics. Chih‐Ho Hong is a researcher/consultant/advisor for AbbVie, Amgen, Arcutis, Bausch Health, Boehringer Ingelheim, Bristol‐ Meyers Squibb, Celgene, Dermira, Dermavant, DS Biopharma, Galderma, GlaxoSmithKline, Janssen, LEO Pharma, Lilly, MedImmune, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, and UCB. Isaak Effendy reports no conflict of interest. Waldemar Placek performed clinical trials for Amgen Inc, Maruho Europe Limited, Merck Sharp and Dohme Corp, Mylan, Novartis Poland Sp. z o.o., Johnson and Johnson, Moberg Pharma AB publ, Eli Lilly, Menlo, KYMAB, Bristol Meyers, CTC Team, Boehringer Ingelheim RCV GmbH and Co KG. Phoebe Rich has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from Arcutis Inc., Bristol‐Myers Squibb, Centocor, Dermavant, Eli Lilly, Kadmon, Merck, Novartis, Pfizer, Sun Pharma, and UCB. Deborah Keefe is an employee of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA. Gerard Bruin is an employee of Novartis Institutes for Biomedical Research, Basel, Switzerland. Rong Fu is an employee of Novartis Institute for Biomedical Research, Shanghai, China. Pascal Charef, Isabelle Hampele, and Manmath Patekar are employees of Novartis Pharma AG, Basel, Switzerland." In ClinicalTrials.gov, other prespecified outcomes "such as assess the participant usability and assessment of Dermatology Life Quality Index (DLQI) scores are exploratory in nature and are not reported in these results". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 2): "ALLURE was a 52‐week, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study." Quote (supplemental p. 3): "At Baseline/Randomization visit, all eligible patients were randomized in a 1:1:1 ratio, to one of the 3 treatment arms; described above via Interactive Response Technology (IRT)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 2): "ALLURE was a 52‐week, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study." Quote (supplemental p 3): "Patients, investigators/site personnel and Novartis clinical team reviewing data remained blinded to the identity of the treatment from the time of randomization, using the following methods: (1) randomization data were kept strictly confidential until the time of unbinding, and were not accessible by anyone else involved in the study; (2) the identity of the treatments was concealed by the use of investigational treatment that are all identical in packaging, labeling, appearance, and schedule of administration". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (supplemental p 3): "Patients, investigators/site personnel and Novartis clinical team reviewing data remained blinded to the identity of the treatment from the time of randomization, using the following methods: (1) randomization data were kept strictly confidential until the time of unbinding, and were not accessible by anyone else involved in the study; (2) the identity of the treatments was concealed by the use of investigational treatment that are all identical in packaging, labeling, appearance, and schedule of administration". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (supplemental p. 3): "Patients, investigators/site personnel and Novartis clinical team reviewing data remained blinded to the identity of the treatment from the time of randomization, using the following methods: (1) randomization data were kept strictly confidential until the time of unbinding, and were not accessible by anyone else involved in the study; (2) the identity of the treatments was concealed by the use of investigational treatment that are all identical in packaging, labeling, appearance, and schedule of administration". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (supplemental p. 5): "The co‐primary endpoints PASI 75 and IGA 0 or 1 were analyzed based on the full analysis set (FAS) which comprised of all subjects who were randomized at baseline visit and to whom study treatment was assigned...Multiple imputation was applied as the primary missing data imputation method and non‐responder imputation was done for sensitivity analysis." Randomised 214; analysed 214 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02748863). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported except for DLQI. Results posted on ClinicalTrials.gov |
AlMutairi 2021.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: January 2018 to August 2019 Location: Kuwait (5 sites) Phase ? |
|
| Participants |
Randomised: 54 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 54, mean age of 42 years and 72% men Dropouts and withdrawals Not stated |
|
| Interventions |
Intervention A. Secukinumab, 300 mg subcutaneous injection once weekly for first 4 weeks, followed by once every 4 weeks until week 24, n = 26 Control intervention B. Ixekizumab, 160 mg SC at weeks 0, and then 80 mg SC every 2 weeks until week 12, followed by 80 mg SC every 4 weeks until week 24, n = 82 |
|
| Outcomes |
At week 24
|
|
| Notes |
Funding source: Quote: "This paper is not funded". Declarations of interest : Quote: "The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This is a 24 week, open‐label, randomized controlled study to compare the efficacy and safety of ixekizumab versus secukinumab". Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "This is a 24 week, open‐label, randomized controlled study to compare the efficacy and safety of ixekizumab versus secukinumab". Comment: no description of the method used to guarantee random sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This is a 24 week, open‐label, randomized controlled study to compare the efficacy and safety of ixekizumab versus secukinumab". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This is a 24 week, open‐label, randomized controlled study to compare the efficacy and safety of ixekizumab versus secukinumab". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomised 54, analysed 54 Comment: methods for dealing with missing data not specified |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
AMAGINE‐1 2016.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: August 2012 to March 2014 Location: 73 centres worldwide (Europe, USA and Canada) Phase 3 |
|
| Participants |
Randomised: 661 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 661, mean age 46 years, 484 male Dropouts and withdrawals 33/661(5%); brodalumab 210 (10), brodalumab 140 (11), placebo (12)
|
|
| Interventions |
Intervention A. Brodalumab (n = 222), SC, 210 mg every 2 weeks Control intervention B. Brodalumab (n = 219), SC, 140 mg every 2 weeks C. Placebo (n = 220) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1): "This study was funded by Amgen Inc. & AstraZeneca/MedImmune." Declarations of interest (pp 13‐14): "K.A.P. has served as a consultant, investigator and/or speaker for AbbVie, Amgen Inc., Astellas Pharma, Bayer AG, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Forward Pharma, Galderma, Janssen Biotech Inc., LEO Pharma, Merck, Novartis, Pfizer, Roche and UCB Pharma. K.R. has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Amgen Inc., Biogen‐Idec, Celgene, Centocor, Covagen, Forward Pharma, GSK, Janssen‐Cilag, LEO Pharma, Lilly, Medac, MSD, Novartis, Pfizer, Takeda and Vertex. C.P. has served as a consultant and investigator for Amgen Inc., AbbVie, Boehringer, Janssen‐Cilag, LEO Pharma, Lilly, Novartis and Pfizer. A.B. has served as a consultant and investigator for AbbVie, Amgen Inc., Anacor, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Genentech, Janssen, Merck, Novartis, Pfizer, Regeneron and Sandoz." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (pp. 2 and 3): "Patients were randomized... IP supply was controlled by interactive voice response system and box numbers were assigned at each visit". Comment: no description of the method used to guarantee the random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote (pp. 2 and 3): "Patients were randomized... IP supply was controlled by interactive voice response system and box numbers were assigned at each visit". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 3): "Randomizations remained blinded to all patients and investigators... Throughout the study, patients received placebo as needed to maintain the blind until it was broken." Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 3): "Randomizations remained blinded to all patients and investigators... Throughout the study, patients received placebo as needed to maintain the blind until it was broken." Comment: probably done, placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 661, 661 analysed Management of missing data: quote (pp. 4‐5): "The full analysis set included all randomised patients... Multiple imputations for missing data" Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01708590; AMAGINE‐1). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
AMAGINE‐2 2015.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: August 2012 to September 2014 Location: 142 centres worldwide Phase 3 |
|
| Participants |
Randomised: 1831 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 1831, mean age 45 years, 1258 male Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Brodalumab (n = 610), SC, 140 mg (2 injections week 0, 1 injection eow) Control intervention B. Brodalumab (n = 612), SC, 210 mg (2 injections week 0, 1 injection eow) C. Ustekinumab (n = 300), SC, 45/90 mg (week 0, week 4, and every 12 weeks) D. Placebo (n = 309), orally (same drug administration) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1319): “Amgen funded both studies. ... and Amgen conducted the data analyses. All the authors interpreted the data”. Declarations of interest: Quote (p 1327): "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org." Dr. Lebwohl reported grant support from Amgen, AbbVie, Janssen Biotech, UCB Pharma, Pfizer, Celgene, Eli Lilly, and Novartis outside the submitted work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (protocol): “The randomisation lists will be generated by Amgen using a permuted block design within each strata...via an interactive voice response system”. Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (protocol): “The randomisation lists will be generated by Amgen using a permuted block design within each strata...via an interactive voice response system”. Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (protocol, cf 6. Treatment procedure): “This is a double dummy procedure...” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (protocol, cf 6. Treatment procedure): “This is a double dummy procedure...” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 1831, analysed 1831 Dealing with missing data Quote (protocol and p 1321): "... with missing data imputed as indicating no response" Comment: well described |
| Selective reporting (reporting bias) | High risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01708603). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported, except for participant‐reported outcome. |
AMAGINE‐3 2015.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: September 2012 to August 2014 Location: 142 centres worldwide (no sites that were included in the AMAGINE‐2 study) Phase 3 |
|
| Participants |
Randomised: 1881 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 1881, mean age 45 years, 1288 male Dropouts and withdrawals 65/1881 (3.4%): brodalumab 140 group (25), brodalumab 210 group (16), ustekinumab 45/90 group (10), placebo group (14)
|
|
| Interventions |
Intervention A. Brodalumab (n = 629), SC, 140 mg (2 injections week 0, 1 injection eow) Control interventions B. Brodalumab (n = 624), SC, 210 mg (2 injections week 0, 1 injection eow) C. Ustekinumab (n = 313), SC, 45/90 mg (week 0, week 4, and every 12 weeks) D. Placebo (n = 315), orally (same drug administration) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes |
Funding source Quote (p 1319): “Amgen funded both studies. ... and Amgen conducted the data analyses. All the authors interpreted the data”. Declarations of interest Quote (p 1327): "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org." Dr. Lebwohl reported grant support from Amgen, AbbVie, Janssen Biotech, UCB Pharma, Pfizer, Celgene, Eli Lilly, and Novartis outside the submitted work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (protocol): “The randomisation lists will be generated by Amgen using a permuted block design within each strata..." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (protocol): “The randomisation lists will be generated by Amgen using a permuted block design within each strata...via an interactive voice response system”. Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (protocol, cf 6. Treatment procedure): “This is a double dummy procedure...” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (protocol, cf 6. Treatment procedure): "This is a double dummy procedure...” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 1881, analysed 1881 Dealing with missing data Quote (protocol and p 1321) "...with missing data imputed as indicating no response" Comment: well described |
| Selective reporting (reporting bias) | High risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01708629). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported, except for participant‐reported outcome. |
Asahina 2010.
| Study characteristics | ||
| Methods | RCT, active, placebo‐controlled, double‐blind study Date of study: September 2005 to December 2006 Location: 42 centres in Japan |
|
| Participants |
Randomised: 169 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 169, mean age 45 years, 143 male Dropouts and withdrawals 22 (13%) (A/B/C/D)
|
|
| Interventions |
Intervention A. Adalimumab (n = 38), 40 mg, SC, eow B. Adalimumab (n = 43), 40 mg, SC, 2 injections, week 0, 1 injection eow (week 2) C. Adalimumab (n = 41), 80 mg, SC, eow Control D. Placebo (n = 46), 0.8 mL, SC, eow |
|
| Outcomes | Assessment at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes |
Funding source: support by Abbott (Quote p 309) Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 301): "Patients were randomised..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 301): "Adalimumab 40mg/0.8mL and Placebo 0.8 mL were supplied two‐vial cartons (Adalimumab+Adalimumab, Adalimumab+placebo, Placebo+Placebo)". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no specific description of the method used to guarantee blinding of outcome assessment but considering that this was a placebo‐controlled trial with no known systematic AEs we considered the risk as low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 169, analysed 169 Management of missing data: Quote (p 302): "Patients without evaluation at week 16 were considered non‐responders for the primary analysis". Comment: the report provided sufficient detail about the management of missing data to permit a clear judgement. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. The outcomes mentioned in the Methods section appeared to have been reported. |
Asawanonda 2006.
| Study characteristics | ||
| Methods | RCT, active placebo‐controlled, double‐blind study Date of study: not stated Location: Bangkok, Thailand, Asia |
|
| Participants |
Randomised: 24 participants (mean age 40 years (methotrexate) 48 years (placebo), 15 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate (n = 11), 15 mg/week, orally Control B. Placebo (n = 13), orally Co‐intervention: phototherapy UVB |
|
| Outcomes | Assessment at 24 weeks Primary outcome
Secondary outcome
|
|
| Notes |
Funding source: (quote p 1013) no funding source Declarations of interest: (quote p 1013) "None identified" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1014): "randomized by way of randomization cards" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1014): "to receive either MTX or placebo, which were identical in appearance" Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1015): "PASI scores were given by a investigator blinded to the treatment assignment" Comment: probably done, placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 24, analysed 24 Management of missing data: Comment: no more precision regarding methods for dealing with missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. The outcomes mentioned in the Methods section appeared to have been reported. |
Augustin 2022.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: June 2018 to July 2020 Location: USA, Canada, Czechia, Germany, Hungary, Italy, Russian Federation (worldwide, 67 sites) Phase 3 |
|
| Participants |
Randomised: 331 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 331, mean of age 47 years and 75% men Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Secukinumab 300 mg every 2 weeks Q2, n = 165 Control intervention B. Secukinumab 300 mg every 4 weeks Q4, n = 166 |
|
| Outcomes |
At week 16 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source Quote (p 942): "This study was funded by Novartis Pharma AG." Declarations of interest Quote (p 952): "M.A. has served as a consultant for, or has been a paid speaker for clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including Abbvie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, GSK, Janssen‐Cilag, LEO Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB and Xenoport. K.R. has served as an advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Affibody, Almirall, Amgen, Avillion, Biogen, Boehringer Ingelheim, Bristol‐ Myers Squibb, Celgene, Centocor, Covagen, Dermira, Eli Lilly, Forward Pharma, Fresenius Medical Care, Galapagos, Galderma, GlaxoSmithKline, Janssen‐Cilag, Kyowa Kirin, LEO Pharma, Medac, Merck Sharp & Dohme, Miltenyi Biotec, Novartis, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB, Valeant and Xenoport. P.Y. has served as an investigator for Amgen, Celgene, Dermira, Eli Lilly, Galderma, Janssen, LEO Pharma, MedImmune, Novartis, Pfizer, Regeneron and Sandoz, and has served as an advisor and/or speaker for AbbVie, Amgen, Baxter, Celgene, Dermira, Eli Lilly, Galderma, Janssen, LEO Pharma, Novartis, Pfizer and Regeneron. A.P. has worked as an investigator and/or speaker and/or advisor for AbbVie, Almirall‐Hermal, Amgen, Biogen Idec, Boehringer‐Ingelheim, Celgene, Eli Lilly, GSK, Galderma, Hexal, Janssen, LEO Pharma, MC2, Medac, Merck Serono, Mitsubishi, MSD, Novartis, Pascoe, Pfizer, Regeneron, Roche, San‐ doz Biopharmaceuticals, Sanofi‐Genzyme, Schering‐Plough, Tigercat Pharma and UCB. J.B. has received research funds payable to Psoriasis Treatment Center from AbbVie, Amgen, Arcutis Biotherapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Celgene Corporation, Corrona LLC, Dermavant Sciences Ltd, Dermira, Eli Lilly, Glenmark Pharmaceuticals Ltd, Janssen Biotech, Kadmon Corporation, LEO Pharma, Lycera Corp, Menlo Therapeutics, Novartis, Ortho Dermatologics, Pfi‐ zer, Regeneron Pharmaceuticals, Sun Pharma, Taro Pharmaceutical Industries Ltd and UCB; he has received consultant fees from AbbVie, Amgen, Bristol‐Myers Squibb, Celgene Corporation, Eli Lilly and Company, Janssen Biotech, Novartis, Sun Pharmaceutical Industries Ltd and UCB; and he has received fees for speaking from AbbVie, Celgene Corporation, Eli Lilly, Janssen Biotech and Novartis. S.D. is employed by IQVIA and was funded by Novartis to provide statistical analysis support. R.Y., G.B., J.D., B.P., P.C., M.P. and D.K. are employed by Novartis." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 944): "To mitigate the risk of randomization error at week 16, a sin‐ gle randomization process (for both treatment period 1 and treatment period 2) was performed via Interactive Response Technology (IRT) at the baseline visit as planned in the protocol. Patients were randomized at baseline visit in a 2: 1: 1 ratio into one of three treatment groups......The investigator or his/her delegate will contact the Interactive Response Technology (IRT) after confirming that the subject fulfills all the inclusion/exclusion criteria. The IRT will assign a randomization number to the subject, which will be used to link the subject to a treatment arm and will specify a unique medication number for the package of study drug to be dispensed to the subject"" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 944): "To mitigate the risk of randomization error at week 16, a single randomization process (for both treatment period 1 andtreatment period 2) was performed via Interactive Response Technology (IRT) at the baseline visit as planned in the protocol. Patients were randomized at baseline visit in a 2: 1: 1 ratio into one of three treatment groups....Investigators contacted the IRT team after confirming that the participant had fulfilled all the inclusion/exclusion criteria. The IRT team assigned an unbiased, automated randomization number to the participant, which was used to link the participant to a treatment arm and specified a unique medication number for the package of study drug to be dispensed to the participant. The randomization number was not communicated to the caller." Quote (protocol p. 26): "A patient randomization list will be produced by the IRT provider using a validated system that automates the random assignment of patient numbers to randomization numbers. These randomization numbers are linked to the different treatment arms, which in turn are linked to medication numbers. A separate medication list will be produced by or under the responsibility of Novartis Drug Supply Management using a validated system that automates the random assignment of medication numbers to packs containing the investigational drug(s)." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 944): "Participants, investigators and the sponsor company clinical study team remained blinded to the identity of the study treatment from the time of randomization until the end of the study, except for an independent, unblinded team of clinicians, biostatisticians and programmers involved in the week 16 primary endpoint analysis. Treatment was provided by the sponsor company as a solution in 1 mL (secukinumab 150 mg) prefilled syringes for subcutaneous (s.c.) injection. Placebo solution for s.c. injection in a 1 mL prefilled syringe was also supplied for patients in the Q4W arm to receive alternating treatment and retain patient and investigator blinding." Quote (protocol p. 26): "Subjects, investigator staff and persons performing the assessments, and data analysts will remain blind to the identity of the study treatment from the time of randomization until the end of study database lock: Randomization data are kept strictly confidential until the time of unblinding, and will not be accessible by anyone else involved in the study. The identity of the treatments will be concealed by the use of investigational treatments that are all identical in packaging, labeling, schedule of administration, appearance, taste and odor." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (protocol p. 27‐28): "Subjects, investigator staff and persons performing the assessments, and data analysts will remain blind to the identity of the study treatment from the time of randomization until the end of study database lock: Randomization data are kept strictly confidential until the time of unblinding, and will not be accessible by anyone else involved in the study. The identity of the treatments will be concealed by the use of investigational treatments that are all identical in packaging, labeling, schedule of administration, appearance, taste and odor." Quote (p 944): "Participants, investigators and the sponsor company clinical study team remained blinded to the identity of the study treatment from the time of randomization until the end of the study, except for an independent, unblinded team of clinicians, biostatisticians and programmers involved in the week 16 primary endpoint analysis. Treatment was provided by the sponsor company as a solution in 1 mL (secukinumab 150 mg) prefilled syringes for subcutaneous (s.c.) injection. Placebo solution for s.c. injection in a 1 mL prefilled syringe was also supplied for patients in the Q4W arm to receive alternating treatment and retain patient and investigator blinding." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 945): "Response variables based on PASI andIGA mod 2011 categories were imputed with multiple impu‐tation as the primary method for handling missing value...Multiple imputation is a simulation‐based approach in which missing values are replaced by multiple Bayesian draws from the conditional distribution of missing data given the observed data and covariates. The imputations were performed separately for each treatment group including baseline weight, failure to respond to at least one previous biologic and number of previous systemic therapies as additional covariates. Sensitivity analyses were also conducted using modified nonresponder imputation (mNRI) for PASI and IGA response variables. Using the mNRI method, missing values were imputed with nonresponse regardless of the reason for missing data (e.g. premature study discontinuation, missed visit, administrative issues). Exceptions were applied: firstly, if a participant discontinued the study prior to their last scheduled visit and the participant was a respon‐ der consecutively at least for two preceding visits, then the participant was imputed as a responder for the last scheduled visit. Secondly, if a participant was a responder at the two adjacent visits scheduled 4 weeks or less from the visit with missing data, then the participant was imputed as a responder for the missing visit. A last observation carried forward (LOCF) method was used to analyse DLQI 0/1 response." Randomised 331, analysed 331 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03504852). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results posted on ClinicalTrials.gov |
AURIEL‐PsO 2020.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: February 2016 to December 2017 Location: USA, Bulgaria, Canada, Czechia, Estonia, France, Germany, Hungary, Mexico, Poland, Russian Federation, UK (worldwide) Phase 3 |
|
| Participants |
Randomised: 443 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 443, mean of age 44 years, and 60% men Dropouts and withdrawals 28/443 (6.3%): biosimilar group (9), Humira group (19)
|
|
| Interventions |
Intervention A. MSB11022, SC, biosimilar adalimumab week 0: 80 mg, week 1: 40 mg, then 40 mg eow, n = 222 Control Intervention B. Adalimumab (Humira) week 0: 80 mg, week 1: 40 mg, then 40 mg eow, n = 221 |
|
| Outcomes |
At 16 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 316): "This study was sponsored by Merck. Fresenius Kabi acquired the asset from Merck KGaA". Declarations of interest: Quote (appendix): "J.H. has received honoraria for attendance at advisory boards for Novartis, Eli Lilly, LEO Pharma, Nordic Pharma, UCB, Sanofi Genzyme and Fresenius Kabi; as an investigator for AbbVie, Merck, Amgen, Novartis, Eli Lilly and Pfizer; and as a speaker for AbbVie, Biogen, Eli Lilly, Janssen‐Cilag, LEO Pharma, L'Oréal, Nordic Pharma, Novartis, Pfizer, Pierre Fabre and Sanofi‐Aventis. K.A.P. has received honoraria for attendance at advisory boards for AbbVie, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Dow Pharma, Eli Lilly, Fresenius Kabi, Galderma, Janssen, Merck (MSD), Novartis, Pfizer, Regeneron, Sanofi‐Aventis/Genzyme, UCB and Valeant; as a speaker for AbbVie, Amgen, Celgene, Eli Lilly, Galderma, Janssen, Kyowa Hakka Kirin, LEO, Merck (MSD), Novartis, Pfizer and Valeant; as a consultant for AbbVie, Akros, Amgen, Baxalta, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Coherus, Dermira, Dow Pharma, Eli Lilly, Galderma, Janssen, Kyowa Hakka Kirin, LEO, Merck (MSD), Merck‐Serono, Novartis, Pfizer, Regeneron, Roche, Sanofi‐Aventis/Genzyme, Takeda, UCB and Valeant; and for other activities for AbbVie, Akros, Amgen, Anacor, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Kyowa Hakka Kirin, Merck (MSD), Merck‐Serono, Novartis, Pfizer, Regeneron, Sanofi‐Aventis/Genzyme and Valeant; and has received grants as an investigator for AbbVie, Akros, Amgen, Anacor, Baxalta, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Coherus, Dermira, Dow Pharma, Eli Lilly, Galderma, GSK, Janssen, Kyowa Hakka Kirin, LEO, Merck (MSD), Merck‐Serono, Novartis, Pfizer, Regeneron, Roche, Sanofi‐Aventis/Genzyme, Takeda, UCB and Valeant. V.C. is a former employee of Fresenius Kabi SwissBioSim. M.U. is an employee of Fresenius Kabi SwissBioSim. P.V. has no conflicts of interest to declare. C.J.E. has received honoraria for attendance at advisory boards for AbbVie, Biogen, BMS, Celgene, Fresenius Kabi, GSK, Janssen, Lilly, Mundipharma, Roche and Sanofi; and as a consultant for Anthera, Merck and Samsung Bioepis; and has received grants as an investigator for AbbVie, Biogen and Pfizer". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 319): "The allocation sequence was generated centrally by Cenduit (Nottingham, U.K.) using permuted blocks. The investigators enrolled patients by contacting the central interactive web response system, which assigned patients to their groups according to the allocation sequence." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 319): "The allocation sequence was generated centrally by Cenduit (Nottingham, U.K.) using permuted blocks. The investigators enrolled patients by contacting the central interactive web response system, which assigned patients to their groups according to the allocation sequence." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 318): "AURIEL‐PsO was a multicentre, randomized, double‐blind, parallel‐group trial''. Comment: no description of the method used to guarantee blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 318): "AURIEL‐PsO was a multicentre, randomized, double‐blind, parallel‐group trial''. Comment: no description of the method used to guarantee blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 320): "For the per protocol set, little or no missing data were expected, so no imputation was performed. For the ITT analysis, patients with a missing PASI value at week 16 were classified as nonresponders." Randomised 443, analysed 394 Results posted on ClinicalTrials.gov: per‐protocol analyses (non‐inferiority trial) |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02660580). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results posted on ClinicalTrials.gov. |
Bachelez 2015.
| Study characteristics | ||
| Methods | RCT, active placebo‐controlled, double‐blind study Date of study: November 2010 to September 2012 Location: 122 worldwide excluding the USA and Canada Phase 3 |
|
| Participants |
Randomised: 1106 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 1106, mean of age 46 years, and 41.4% men Dropouts and withdrawals 86/1106 (7.8%); tofacitinib 5 mg group (24), tofacitinib 10 mg twice‐daily group (26), etanercept group (23), placebo group (13)
|
|
| Interventions |
Intervention A. Tofacitinib (n = 330), orally, 5 mg twice daily Control intervention B. Tofacitinib (n = 332) orally, 10 mg twice daily C. Etanercept (n = 336) SC, 50 mg twice‐weekly D. Placebo (n = 108) |
|
| Outcomes | Assessment at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 555): "This study was designed and funded by Pfizer Inc. Study investigators gathered the data, which were maintained in a database by Pfizer." Declarations of interest: Quote (p 560): "HB has provided consultancy services for AbbVie, Amgen, Boehringer, Celgene, Janssen, Leo Pharma, Lilly, Novartis, MSD, Pfizer, and Sandoz. He has also acted as an adviser for AbbVie, Amgen, Boehringer, Celgene, Janssen, Leo Pharma, Lilly, Novartis, Pfizer, and Sandoz; has served on speaker’s bureaus for AbbVie, Amgen, Celgene, Janssen, Leo Pharma, Lilly, Novartis, and Pfizer; and has received a research grant from Pfizer. PCMvdK has provided consultancy services for Celgene, Centocor, Almirall, Amgen, Pfizer, Philips, Abbott, Ely Lilly, Galderma, Novartis, JanssenCilag, Leo Pharma, Sandoz, and Mitsubishi. He has also done clinical trials for Basilea, Pfizer, Ely Lilly, Amgen, AbbVie, Philips Lighting, JanssenCilag, and Leo Pharma. RS has served on speaker’s bureaus for Pfizer, Schülke and Mayr, Lohmann & Rauscher, Meda Pharmaceuticals, Menarini Pharmaceuticals, Stockhausen, and Smith & Nephew; has had consulting agreements with Pfizer, Novartis, Lohmann & Rauscher, Urgo, Chemomedica, Schülke & Mayr, and Pantec Biotechnologies; and has received research and educational grants from Stockhausen, 3M‐Woundcare, Smith & Nephew, Lohmann & Rauscher, Enjo Commercials, Urgo, Chemomedica, and Schülke & Mayr. FV has been a principal investigator, member of a scientific advisory board, or speaker for AbbVie, Janssen, Eli Lilly, Merck, Novartis, and Pfizer. SC has been a consultant and/or speaker for Pfizer, AbbVie, Novartis, Merck, and Janssen‐Cilag. JPa, JPr, PG, HT, MT, HV, and RW are employees of Pfizer Inc. AK, J‐HL, and VY declare no competing interests." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 553): "A computer‐generated randomization schedule was used to assign patients to the treatment groups". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (pp 553‐4): "The study site contacted an interactive voice response system or web‐based interactive response system..." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 553): "For this randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group phase 3 study" Comment: the report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 553): "Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor), Patients and study personnel were masked to treatment assignment: the study drug packaging was labelled.... " Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 1106, 1101 received at least 1 dose of study drug Management of missing data: Quote (p 554): "The primary analysis population for efficacy was the full analysis set, which was defined as all patients who received at least one dose of study drug... We judged patients with missing values for all binary endpoints to be non‐responders in efficacy assessments". Table 2: 1101 analysed participants Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01241591). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Bagel 2012.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: not stated Location: North America |
|
| Participants |
Randomised: 124 participants (median age 39 years (etanercept) and 42 years (placebo), 69 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept (n = 62), SC, 50 mg, twice a week Control intervention B. Placebo (n = 62), SC, twice a week |
|
| Outcomes | Assessment at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Amgen Inc. Declarations of interest Quote p 86: "Dr Bagel receives a salary as founder of the Psoriasis Treatment Center of Central New Jersey. He has received speaker honoraria from Leo Pharma, Galderma, Centocor, Abbott, and Amgen. He has also been compensated as a consultant for Galderma and has served as an investigator for Centocor, Abbott, and Amgen. Dr Lynde has received research grants and honoraria from Amgen, Abbott, Merck, Ortho Biotech, Leo Pharma, and Galderma, for whom he has served as an advisory board member, consultant, and speaker. He has also served as an investigator for Amgen, Abbott, Merck, Ortho Biotech, and Leo Pharma. Dr Tyring has received a research grant and honoraria from Amgen, for whom he has served as a consultant, investigator, and speaker. He has also served as an investigator and/or speaker for Abbott, Leo Pharma, Galderma, GSK, Novartis, Merck, Epiphany, Inhibitex, AiCuris, and Pfizer. Dr Kricorian, Yifei Shi, and Dr Klekotka are employees of Amgen Inc. and have received Amgen stock/stock options." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 87): "Each patient provided written informed consent and received a unique identification number and randomised assignment from an Interactive Web Response System". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 87): "Each patient provided written informed consent and received a unique identification number and randomised assignment from an Interactive Web Response System". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 87): "patients and clinicians were blinded throughout the study as to treatment assignments." Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"patients and clinicians were blinded throughout the study as to treatment assignments." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 124, analysed 124 Dropouts and withdrawals
Quote (p 89): "included in ITT efficacy analysis" Management of missing data: Quote (p 88): "Last observation carried forward imputation was used for missing values" Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. The outcomes mentioned in the Methods section appeared to have been reported except for QoL |
Barker 2011.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: September 2005 to June 2008 Location: 106 centres in Europe Phase 3 |
|
| Participants |
Randomised: 868 participants (mean age 43 years, 586 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
Reasons not stated at week 16 |
|
| Interventions |
Intervention A. Infliximab (n = 653), IV, 5 mg/kg, weeks 0, 2, 6, 14, 22 Control intervention B. Methotrexate (n = 215), orally, 15 mg/week for 22 weeks |
|
| Outcomes | Assessment at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: financial support for this study was provided by Schering‐Plough Research Institute, now Merck, Sharp & Dohme Corporation, Whitehouse Station, NJ, USA Declarations of interest: (Quote Appendix 1): "J.B. has served as a consultant and/or paid speaker for, and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis including Abbott, Celgene, Centocor, Janssen‐Cilag, Johnson and Johnson, Merck, Novartis, Pfizer, Schering‐Plough and Wyeth. M.H. has served as a consultant and/or paid speaker for, and/or has participated in clinical trials sponsored by Abbott, Amgen, Essex, Janssen, Leo, Medac, Novartis, Pfizer, Schering‐Plough and Wyeth. G.W. has no conflicts of interest to disclose. J.‐P.O. has been a consultant for Schering‐Plough, Abbott, Merck‐Serono, Centocor, Wyeth, Janssen‐Cilag, Meda‐Pharma, Pierre‐Fabre and Galderma. H.Z. is an employee of Merck, Sharp & Dohme. H.v.H. was an employee of Merck, Sharp & Dohme at the time of the RESTORE1 study and during the preparation of this manuscript. K.R. has served as a consultant and/or paid speaker for, and/or participated in clinical trials sponsored by Abbott, Celgene, Centocor, Janssen‐Cilag, Leo, Medac and Merck." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1110): “At each eligible subject's baseline visit, study centres telephoned the Interactive Voice REsponse Syste .... for randomisation". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 1110): “At each eligible subject's baseline visit, study centres telephoned the Interactive Voice REsponse Syste .... for randomisation". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 1110): “open‐label trial” Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 1110): “open‐label trial” Comment: no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 868, analysed 868 Quote (p 1110‐11): "Primary and secondary efficacy analyses were based on the ITT population, the ITT population included all randomised patients. At week 16, patients who dropped out early or had missing data for PASI 75 ... were considered nonresponders". Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00251641). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
BE ABLE 1 2018.
| Study characteristics | ||
| Methods | RCT, phase 2, randomised, double‐blinded, placebo‐controlled, parallel‐group, dose‐ranging study Date of study: 25 August 2016 to 1 March 2017 Location: 6 countries (Canada, Czech Republic, Hungary, Japan, Poland, and USA) Phase 2 |
|
| Participants |
Randomised: 250 participants (age 44 years old, 163 males) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention: A. Bimekizumab every 4 weeks at doses of 64 mg, n = 39 Control interventions B. Bimekizumab every 4 weeks at doses of 160 mg, n = 43 C. Bimekizumab every 4 weeks at doses of 160 mg (with 320 mg loading dose at baseline), n = 40 D. Bimekizumab every 4 weeks at doses of 320 mg, n = 43 E. Bimekizumab every 4 weeks at doses of 480 mg, n = 43 F. Placebo, n = 42 |
|
| Outcomes |
At week 12 Primary outcome
Secondary outcomes
|
|
| Notes | Funding source Quote (p 277): "Supported by UCB Pharma." Declarations of interest Quote (p 277): "Dr Papp has received consultant fees from Astellas, AstraZeneca, Baxalta, Baxter, Boehringer Ingelheim, Bristol‐Myers Squibb, CanFite, Celgene, Coherus, Dermira, Dow Pharma, Eli Lilly, Forward Pharma, Galderma, Genentech, Janssen, Kyowa Hakko Kirin, LEO Pharma, Meiji, Seika Pharma, MSD, Merck Serono, Mitsubishi Pharma, Novartis, Pfizer, Regeneron, Roche, Sanofi/Genzyme, Takeda, UCB, and Valeant; investigator fees from Astellas, Baxalta, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Coherus, Dermira, Dow Pharma, Eli Lilly, Galderma, Genentech, GSK, Janssen, Kyowa Hakko Kirin, LEO Pharma, MedImmune, MSD, Merck‐Serono, Novartis, Pfizer, Regeneron, Roche, Sanofi/Genzyme, Takeda, UCB, and Valeant; speaker fees from Astellas, Celgene, Eli Lilly, Galderma, Kyowa Hakko Kirin, LEO Pharma, MSD, Novartis, Pfizer, and Valeant; has participated in advisory boards for Astellas, Baxter, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Dow Pharma, Eli Lilly, Galderma, Janssen, MSD, Novartis, Pfizer, Regeneron, Sanofi/Genzyme, UCB, and Valeant; is a steering committee member for Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Kyowa Hakko Kirin, MSD, Merck‐Serono, Novartis, Pfizer, Regeneron, Sanofi/Genzyme, and Valeant; and is a scientific officer for Kyowa Hakko Kirin. Dr Merola has received honoraria from AbbVie, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Samumed, and UCB. Dr Gottlieb has received consultant fees, advisory board fees, or speaker fees from AbbVie, Allergan, Beiersdorf Inc, Bristol‐Myers Squibb, Celgene, Dermira, Lilly, Incyte, Janssen, Novartis, Reddy Labs, Sun Pharmaceutical Industries, UCB, and Valeant; and research grants from Allergan, Incyte, Janssen, LEO, Eli Lilly and Company, and Novartis. Dr Blauvelt has received consultant fees from Eli Lilly and Company, Janssen, Regeneron, and Sanofi Genzyme; and is a scientific adviser or clinical study investigator for AbbVie, Aclaris, Allergan, Almirall, Amgen, Boehringer Ingelheim, Celgene, Dermavant, Dermira Inc, Eli Lilly and Company, Genentech/Roche, GlaxoSmithKline, Janssen, Leo, Merck Sharp & Dohme, Novartis, Pfizer, Purdue Pharma, Regeneron, Sandoz, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma, UCB Pharma, Valeant, and Vidac. Dr Griffiths has received grants and personal fees from AbbVie, Celgene, LEO, Eli Lilly and Company, Janssen, Novartis, Pfizer, and UCB Pharma; grants from Sandoz; personal fees from Almirall and Galderma. Dr Griffiths has received research grants from AbbVie, Celgene, Novartis, Eli Lilly and Company, Janssen, Sandoz, Pfizer, LEO, and UCB. Mr Patterson and Dr Cioffi own stock in UCB. Dr Cross has no further conflicts to disclose." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p. 279): "An interactive voice or web response system was used for assigning eligible patients to a treatment regimen according to a randomization schedule produced by an independent biostatistician who was not associated with the design or analysis of the study. Treatment assignment was stratified by geographic region and prior biologic exposure." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p. 279): "An interactive voice or web response system was used for assigning eligible patients to a treatment regimen according to a randomization schedule produced by an independent biostatistician who was not associated with the design or analysis of the study. Treatment assignment was stratified by geographic region and prior biologic exposure." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p. 279 and supplemental appendix): "Bimekizumab was provided in single‐use vials containing 160 mg/mL. Due to differences in presentation and to ensure study blinding, bimekizumab and placebo injections were prepared and administered at the investigational sites by unblinded, dedicated study personnel" "Additional details of blinding: Bimekizumab was provided in single‐use vials containing 160 mg/mL. Placebo was supplied as 0.9% saline solution. Treatments were administered as 3 subcutaneous injections (lateral abdominal wall and upper outer thigh). During each dosing visit, each of the 3 injections was administered at a separate injection site, and sites were rotated. Due to differences in presentation and to ensure study blinding, bimekizumab and placebo injections were prepared and administered at the investigational sites by unblinded, dedicated study personnel. The unblinded personnel were not involved in the study in any way other than assuring the medication was taken from the correct kit and administered to patients. All other study personnel remained blinded and did not have access to medication‐related information. To preserve the blinding of treatment doses, each administration consisted of 3 subcutaneous injections". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p. 279 and supplemental appendix): "Bimekizumab was provided in single‐use vials containing 160 mg/mL. Due to differences in presentation and to ensure study blinding, bimekizumab and placebo injections were prepared and administered at the investigational sites by unblinded, dedicated study personnel" "Additional details of blinding: Bimekizumab was provided in single‐use vials containing 160 mg/mL. Placebo was supplied as 0.9% saline solution. Treatments were administered as 3 subcutaneous injections (lateral abdominal wall and upper outer thigh). During each dosing visit, each of the 3 injections was administered at a separate injection site, and sites were rotated. Due to differences in presentation and to ensure study blinding, bimekizumab and placebo injections were prepared and administered at the investigational sites by unblinded, dedicated study personnel. The unblinded personnel were not involved in the study in any way other than assuring the medication was taken from the correct kit and administered to patients. All other study personnel remained blinded and did not have access to medication‐related information. To preserve the blinding of treatment doses, each administration consisted of 3 subcutaneous injections". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data Quote (p. 281): "Efficacy analyses included patients who received 1 dose of study treatment and had a valid measurement of the primary efficacy variable at baseline (full analysis set)...Patients with missing efficacy data were imputed as nonresponders". 250 randomised, 250 analysed Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02905006). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results posted on ClinicalTrials.gov. |
BE RADIANT 2021.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: June 2018 to May 2019 Location: worldwide (77 sites) Phase 3 |
|
| Participants |
Randomised: 743 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 743, mean age of 45 years and 65% men Dropouts and withdrawals 27/743 (3.6%): bimekizumab group (11), secukinumab group (16)
|
|
| Interventions |
Intervention A. Bimekizumab 320 mg every 4 weeks, n = 373 Control intervention B. Secukinumab 300 mg weekly to week 4 followed by once every 4 weeks, n = 370 |
|
| Outcomes |
At week 16 Primary outcome
Secondary outcomes
|
|
| Notes | Funding source: Quote (p 2): "The sponsor, UCB Pharma, funded and designed the trial with the participation of authors employed by the sponsor". Declarations of interest: Quote (disclosure forms): "Dr. Blauvelt reports personal fees and other from AbbVie, personal fees from Aligos, personal fees from Almirall, personal fees and other from Amgen, personal fees and other from Arcutis, personal fees from Arena, personal fees and other from Athenex, personal fees and other from Boehringer Ingelheim, personal fees and other from Bristol Myers Squibb, personal fees and other from Dermavant Sciences, personal fees and other from Eli Lilly, personal fees and other from Evommune, personal fees from Forte, personal fees from Galderma, personal fees and other from Incyte, personal fees and other from Janssen, personal fees and other from Leo, personal fees and other from Novartis, personal fees and other from Pfizer, personal fees from Rapt, personal fees and other from Regeneron, personal fees from Sanofi Genzyme, personal fees and other from Sun Pharma, personal fees and other from UCB Pharma, outside the submitted work." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 2): "This is a phase 3b multicenter, randomized, double‐blind, active‐comparator–controlled, parallel‐group trial conducted across 77 sites..." Quote (p 3): "Randomization was performed with the use of an interactive response technology, stratified by region (North America, Western Europe, Central and Eastern Europe, or Asia and Australia) and previous exposure to biologic agents (yes or no)". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 2): "This is a phase 3b multicenter, randomized, double‐blind, active‐comparator–controlled, parallel‐group trial conducted across 77 sites..." Quote (p 3): "Randomization was performed with the use of an interactive response technology, stratified by region (North America, Western Europe, Central and Eastern Europe, or Asia and Australia) and previous exposure to biologic agents (yes or no)". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 2): "This is a phase 3b multicenter, randomized, double‐blind, active‐comparator–controlled, parallel‐group trial conducted across 77 sites..." Quote (p 3): "To maintain double blinding, patients randomly assigned to the bimekizumab group received placebo at relevant study visits to account for differences in dosing schedules between the treatment groups..". "All sponsor and investigator site personnel involved in the trial were unaware of the treatment assignments except site staff responsible for the preparation and administration of trial treatments and bio‐analytic sample analysis". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 2): "This is a phase 3b multicenter, randomized, double‐blind, active‐comparator–controlled, parallel‐group trial conducted across 77 sites..." Quote (p 3): "Efficacy outcomes were assessed by the investigator, another delegated physician, or an appropriately qualified medical professional, all of whom were unaware of the patients’ treatment assignments". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 5): "For efficacy and quality‐of‐life variables, patients with missing data were considered not to have had a response (nonresponder imputation)." Randomised 743, analysed 743 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03536884). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. No results are posted on ClinicalTrials.gov |
BE READY 2021.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: February 2018 to January 2020 Location: worldwide (77 sites in Australia, Canada, Germany, Hungary, Poland, Russia, South Korea, the UK, and the USA) Phase 3 |
|
| Participants |
Randomised: 435 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 435, mean age of 44.5 years and 72% men Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Bimekizumab 320 mg every 4 weeks, n = 349 Control intervention B. Placebo every 4 weeks, n = 86 |
|
| Outcomes |
At week 16 Primary composite outcome
Secondary outcomes
|
|
| Notes | Funding source: Quote (p 475): "Funding UCB Pharma" Declarations of interest: Quote (p 485): "KBG has received consulting fees from AbbVie, Almirall, Amgen, Boehringer Ingelheim, BMS, Celgene, Dermira, Eli Lilly, Janssen, Novartis, Pfizer, Sun Pharma and UCB Pharma; and research support from AbbVie, BMS, Celgene, Eli Lilly, Janssen, Novartis and UCB Pharma. PF received grant support from AbbVie, Amgen, Celgene,Eli Lilly, Janssen, Merck, Novartis, Pfizer, Sun Pharma, and Sanofi; served as an investigator for AbbVie, Amgen, AstraZeneca, Bristol‐Myers Squibb, Boehringer Ingelheim, Botanix, Celgene, Celtaxsys, CSL, Cutanea, Dermira, Eli Lilly, Galderma, Genentech, Geneseq, GSK, Hexima, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Regeneron, Reistone, Roche, Sanofi Genzyme, Sun Pharma, UCB Pharma, and Valeant/Bausch Health; served on the advisory board of AbbVie, Amgen, Bristol‐Myers Squibb, Celgene, Eli Lilly, Galderma, Janssen, Kyowa Kirin, Leo Pharma, Medac, MSD, Novartis, Ocean Pharma, Pfizer, Samsung Bioepis, Sanofi, SunPharma, Takeda, UCB Pharma, and Xenoport; paid speaker for AbbVie, Almirall, Biogen‐Idec, Celgene, Eli Lilly, Janssen‐Cilag, Leo Pharma, Medac, MSD, Novartis, Sanofi, and Valeant; and has participated in clinical trials sponsored by AbbVie, Affibody, Almirall, Biogen‐Idec, Boehringer Ingelheim, Celgene, Covagen, Eli Lilly, Forward Pharma, Fresenius Medical Care, Galapagos, Galderma, Janssen‐Cilag, Kyowa Kirin, Leo Pharma, Medac, MSD, Miltenyl, Novartis, Ocean Pharma, Pfizer, Sanofi, SunPharma, Takeda, UCB Pharma, and XBiotech. RV served as a consultant, scientific adviser, investigator, or speaker for Amgen, AbbVie, Astellas, Bristol‐ Myers Squibb, Boehringer Ingelheim, Celgene, Dermira, Eli Lilly, Galderma, GSK, Janssen, LEO Pharma, MSD, Merck Serono, Novartis, Pfizer, Regeneron, Roche, Sanofi‐Aventis/Genzyme, Sun Pharma, Takeda, UCB Pharma, and Valeant/Bausch Health. VV, CM, KW, and CC are employees and shareholders of UCB Pharma. AB has served as a scientific adviser for AbbVie, Almirall, Arena, Athenex, Boehringer Ingelheim, Bristol‐Myers Squibb, Dermavant, Eli Lilly, Evommune, Forte, Galderma, Incyte, Janssen, Leo Pharma, Novartis, Pfizer, Rapt, Regeneron, Sanofi Genzyme, Sun Pharma, and UCB Pharma; and as a clinical study investigator for AbbVie, Athenex, Boehringer Ingelheim, Bristol‐Myers Squibb, Dermavant, Eli Lilly, Galderma, Incyte, Janssen, Leo Pharma, Novartis, Pfizer, Regeneron, Sun Pharma, and UCB Pharma." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 476): "BE READY was a phase 3, multicentre, randomised, double‐blind, placebo‐controlled trial done across 77 sites... At week 0, patients were randomly assigned (4:1) to receive either bimekizumab dosed at 320 mg every 4 weeks or placebo every 4 weeks for initial treatment, by use of interactive response technology. The interactive response technology assigned patients on the basis of a predetermined randomisation and packaging schedule provided by the funder. Randomisation was stratified by region (North America, Western Europe, Central or Eastern Europe, and Asia and Australia) and previous biologic exposure (yes vs no)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 476): "BE READY was a phase 3, multicentre, randomised, double‐blind, placebo‐controlled trial done across 77 sites... At week 0, patients were randomly assigned (4:1) to receive either bimekizumab dosed at 320 mg every 4 weeks or placebo every 4 weeks for initial treatment, by use of interactive response technology. The interactive response technology assigned patients on the basis of a predetermined randomisation and packaging schedule provided by the funder. Randomi‐ sation was stratified by region (North America, Western Europe, Central or Eastern Europe, and Asia and Australia) and previous biologic exposure (yes vs no)." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 476): "BE READY was a phase 3, multicentre, randomised, double‐blind, placebo‐controlled trial done across 77 sites... Throughout the study, patients, investigators, and sponsors remained masked to treatment assignment, with the exception of specially designated, unmasked site staff who were responsible for the preparation and administration of study treatments, safety monitoring, or bioanalytical sample analysis." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 476): "BE READY was a phase 3, multicentre, randomised, double‐blind, placebo‐controlled trial done across 77 sites... Throughout the study, patients, investigators, and sponsors remained masked to treatment assignment, with the exception of specially designated, unmasked site staff who were responsible for the preparation and administration of study treatments, safety monitoring, or bioanalytical sample analysis." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 480): "Efficacy analyses of data from the initial treatment period were done in the intention‐to‐treat population, including all randomised patients." Randomly assigned 435, analysed 435 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03410992). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. No results are posted on ClinicalTrials.gov. |
BE SURE 2021.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: January 2018 to February 2020 Location: worldwide (77 sites) Phase 3 |
|
| Participants |
Randomised: 478 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 478, mean age of 45 years and 69% men Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Bimekizumab SC 320 mg every 4 week, n = 319 Control intervention B. Adalimumab SC 40 mg every 2 weeks, n = 159 |
|
| Outcomes |
At week 16 Primary composite outcome
Secondary outcomes
|
|
| Notes | Funding source: Quote (p 2) "The trial was funded by UCB Pharma and de‐ signed by the ninth through the twelfth authors and UCB Pharma." Declarations of interest: Quote (disclosure forms at NEJM.org) "Dr. Warren reports receiving grant support and consulting fees from AbbVie, Almirall, Bristol‐Myers Squibb, Eli Lilly, Janssen, LEO Pharma, Novartis, and UCB Pharma, consulting fees from Amgen, Arena Pharmaceuticals, Avillion, Boehringer Ingelheim, Celgene, Pfizer, Sanofi, Astellas, GlaxoSmithKline, Biogen, DiCE Molecules, Sun Pharma, and Union Therapeutics, and grant support from Medac; Dr. Blauvelt, receiving research funding and consulting fees from AbbVie, Amgen, Arcutis Biotherapeutics, Athenex, Boehringer Ingelheim, Bristol‐Myers Squibb, Dermavant Sciences, Eli Lilly, Evommune, Incyte, Janssen, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sun Pharma, and UCB Pharma and consulting fees from Aligos Therapeutics, Almirall, Arena Pharmaceuticals, Forte Biosciences, Galderma, Rapt Therapeutics, and Sanofi Genzyme; Dr. Bagel, receiving grant support from Arcutis Biotherapeutics, Boehringer Ingelheim, Corrona, Dermavant Sciences, Dermira, Glenmark Pharmaceuticals, Kadmon, LEO Pharma, Lycera, Menlo Therapeutics, Pfizer, Regeneron Pharmaceuticals, Taro Pharmaceutical Industries, and Ortho Dermatologics, grant support, consulting fees, and lecture fees from AbbVie, Celgene, Eli Lilly, Janssen Biotech, and Novartis, and grant support and consulting fees from Amgen, Bristol‐Myers Squibb, Sun Pharmaceutical Industries, and UCB Pharma; Dr. Papp, receiving grant support, consulting fees, fees for serving on a speakers bureau, steering committee fees, and advisory board fees, all paid to his institution, and honoraria from AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Merck (Merck Sharp & Dohme), Novartis, Pfizer, Sanofi Genzyme, and Bausch Health, grant support and consulting fees, all paid to his institution, and honoraria from Akros Pharma, Coherus BioSciences, Mitsubishi Pharma, Takeda, and PRCL Research, grant support, paid to his institution, from Anacor Pharmaceuticals, GlaxoSmithKline, MedImmune, Gilead Sciences, and Moberg Pharma, grant support and consulting fees, all paid to his institution, from Arcutis Biotherapeutics, Baxalta, Can‐Fite BioPharma, Dermira, Genentech, Meiji Seika Pharma, Roche, Evelo Biosciences, Galapagos, Avillion, and DiCE Molecules, grant support, consulting fees, fees for serving on a speakers bureau, and advisory board fees from Astellas, grant support, consulting fees, steering committee fees, and advisory board fees, all paid to his institution, and honoraria from Boehringer Ingelheim and Regeneron Pharmaceuticals, grant support, consulting fees, and advisory board fees, all paid to his institution, from Bristol‐Myers Squibb, Dow Pharma, and Dermavant Sciences, grant support, consulting fees, fees for serving on a speakers bureau, and advisory board fees, all paid to his institution, and honoraria from Galderma, grant support, consulting fees, fees for serving on a speakers bureau, and advisory board fees, all paid to his institution, and honoraria from Kyowa Hakko Kirin, grant support, consulting fees, and fees for serving on a speakers bureau, all paid to his institution, from LEO Pharma and Incyte, grant support, consulting fees, and steering committee fees, all paid to his institution, and honoraria from Merck Serono, grant support, consulting fees, and advisory board fees, all paid to his institution, and honoraria from UCB Pharma, and grant support and advisory board fees, all paid to his institution, from Sun Pharma; Dr. Yamauchi, receiving lecture fees, consulting fees, and investigator fees from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Ortho Dermatologics, Sun Pharma, and UCB Pharma; Dr. Armstrong, receiving grant support, advisory fees, and lecture fees from AbbVie and Regeneron Pharmaceuticals, grant support and advisory fees from Bristol‐Myers Squibb, Dermavant Sciences, Dermira, Eli Lilly, LEO Pharma, Novartis, and UCB Pharma, advisory fees from Janssen, Modernizing Medicine, Ortho Dermatologics, Sanofi Genzyme, Sun Pharma, and Pfizer, grant support from Kyowa Kirin and Galderma, and fees for serving on a data and safety monitoring board from Boehringer Ingelheim and Parexel; Dr. Langley, receiving grant support, advisory board fees, investigator fees, and lecture fees from AbbVie, Amgen, Centocor, Eli Lilly, Janssen, LEO Pharma, Novartis, UCB Pharma, and Celgene and grant support, advisory board fees, and investigator fees from Boehringer Ingelheim, Bristol‐Myers Squibb, Pfizer, and Merck; Ms. Vanvoorden, Drs. De Cuyper and Cioffi, Mr. Peterson, and Dr. Cross, being employed by and owning shares in UCB Pharma; and Dr. Reich, receiving grant support, consulting fees, and lecture fees from AbbVie, Almirall, Biogen Idec, Celgene, Eli Lilly, Janssen‐Cilag, LEO Pharma, Medac, Merck Sharp & Dohme, Novartis, and Sanofi, grant support and consulting fees from Affibody, Boehringer Ingelheim, Covagen, Forward Pharma, Galderma, Kyowa Kirin, Ocean Pharma, Pfizer, Sun Pharma, Takeda, UCB Pharma, and Bristol‐Myers Squibb, consulting fees from Amgen, GlaxoSmithKline, Samsung Bioepis, and XenoPort, lecture fees from Valeant Pharmaceuticals and Sandoz, and grant support from Fresenius Medical Care, Galapagos, Miltenyi Biotec, and XBiotech. No other potential conflict of interest relevant to this article was reported." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 2): "After the screening period, patients were randomly assigned in a 1:1:1 ratio to receive subcutaneous bimekizumab at a dose of 320 mg every 4 weeks for 56 weeks..." Quote (p 3): "Randomization was carried out with the use of interactive‐response technology, stratified according to geographic region (North America, Western Europe, Central and Eastern Europe, or Asia and Australia) and previous exposure to biologic agents (yes or no)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 2): "After the screening period, patients were randomly assigned in a 1:1:1 ratio to receive subcutaneous bimekizumab at a dose of 320 mg every 4 weeks for 56 weeks..." Quote (p 3): "Randomization was carried out with the use of interactive‐response technology, stratified according to geographic region (North America, Western Europe, Central and Eastern Europe, or Asia and Australia) and previous exposure to biologic agents (yes or no)." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 2): "This was a 56‐week, phase 3, multicenter, double‐blind trial of bimekizumab as compared with adalimumab..." Quote (p 3): "To maintain double blinding, patients in all groups received dummy injections at some trial visits to account for the differences in dosing schedules among the treatment groups." "The investigators, other trial‐site personnel, and the sponsor (with the exception of site staff responsible for the preparation and administration of trial treatments and bioanalytic sample analysis) were unaware of the trial‐group assignments." Comment: no detailed description of means used to guarantee absence of communication between blinded and unblinded personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 3) "Efficacy end points were assessed by the investigator, another delegated physician, or an appropriately qualified medical professional, all of whom were unaware of the trial‐group assignments." Comment: no detailed description of means used to guarantee blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 4): "For efficacy variables, imputation of nonresponse was used to account for missing data." Randomised 478, analysed 478 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03412747). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. No results are posted on ClinicalTrials.gov. |
BE VIVID 2021.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: December 2017 to December 2019 Location: worldwide (105 sites) Phase 3 |
|
| Participants |
Randomised: 567 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 567, mean age of 46 years and 72% men Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Bimekizumab 320 mg SC every 4 weeks, n = 321 Control interventions B. Ustekinumab 45 mg or 90 mg SC at weeks 0 and 4, then every 12 weeks, n = 163 C. Placebo SC every 4 weeks, n = 83 |
|
| Outcomes |
At week 16 Primary composite outcome
Secondary outcomes
|
|
| Notes | Funding source: Quote (p 487): "Funding UCB Pharma" Declarations of interest: Quote (p 496‐497): "KR has been an adviser for AbbVie, Affivody, Almirall, Amgen, Biogen‐ Idec, Boehringer Ingelheim, Celgene, Covagen, Eli Lilly, Forward Pharma, Galderma, GSK, Janssen‐Cilag, Kyowa Kirin, LEO Pharma, Medac, MSD, Novartis, Ocean Pharma, Pfizer, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB Pharma, and Xenoport; has been a paid speaker for AbbVie, Almirall, Biogen‐Idec, Celgene, Eli Lilly, Janssen‐ Cilag, LEO Pharma, Medac, MSD, Novartis, Sanofi, and Valeant; and has participated in clinical trials sponsored by AbbVie, Affibody, Almirall,Biogen‐Idec, Boehringer Ingelheim, Celgene, Covagen, Eli Lilly, Forward Pharma, Fresenius Medical Care, Galapagos, Galderma, Janssen‐Cilag, Kyowa Kirin, LEO Pharma, Medac, MSD, Miltenyl, Novartis, Ocean Pharma, Pfizer, Sanofi, Sun Pharma, Takeda, UCB Pharma, and XBiotech, all outside of the submitted work. KAP has been a consultant for AbbVie, Akros, Amgen, Arcutis, Astellas, Bausch Health/Valeant, Baxalta, Boehringer Ingelheim, Bristol Myers Squibb (BMS), Can‐Fite Biopharma, Celgene, Coherus, Dermira, Dice Pharmaceuticals,Dow Pharma, Eli Lilly, Evelo, Galapagos, Galderma, Genentech, Janssen, Kyowa Hakko Kirin, LEO Pharma, Meiji Seika Pharma, MSD, Merck‐Serono, Mitsubishi Pharma, Novartis, Pfizer, PRCL Research, Regeneron, Roche, Sanofi‐Aventis/Genzyme, Takeda, and UCB Pharma; has been on speakers’ bureau for AbbVie, Amgen, Bausch Health/ Valeant, Celgene, Eli Lilly, Galderma, Janssen, Kyowa Hakko Kirin,LEO Pharma, MSD, Novartis, Pfizer, and Sanofi‐Aventis/Genzyme;has received clinical research grants from AbbVie, Akros, Amgen, Anacor, Arcutis, Astellas, Bausch Health/Valeant, Baxalta, Boehringer Ingelheim, BMS, Can‐Fite Biopharma, Celgene, Coherus, Dermira, Dow Pharma, Eli Lilly, Evelo, Galapagos, Galderma, Genentech, Gilead, GSK, Janssen, Kyowa Hakko Kirin, LEO Pharma, Medimmune, MSD, Merck‐Serono, Moberg Pharma, Novartis, Pfizer, PRCL Research, Regeneron, Roche, Sanofi‐Aventis/Genzyme, Sun Pharma, Takeda,for Dermavent, LEO Pharma, and UCB Pharma, all outside of the submitted work. YO has received research grants from Eisai, Torii, Maruho, and Shiseido; has current consulting or advisory board agreements or speakers bureau from AbbVie, Amgen, Boehringer Ingelheim, BMS, Celgene, Eisai, Eli Lilly, Janssen Pharma, Jimro, Kyowa Kirin, LEO Pharma, Maruho, Novartis Pharma, Pfizer, Sanofi, Sun Pharma, Taiho, Tanabe‐Mitsubishi, Torii, and UCB Pharma; and is involved in clinical trials sponsored by AbbVie, Amgen, Boehringer Ingelheim, BMS, Celgene, Eli Lilly, Janssen Pharma, LEO Pharma, Maruho, Pfizer, Sun Pharma, and UCB Pharma, all outside of the submitted work. MW is an employee of UCB Pharma. CM, VV,and CC are employees and shareholders in UCB Pharma. ML is an employee of Mount Sinai Hospital (New York, NY), which receives research funds from AbbVie, Amgen, Arcutis, Boehringer Ingelheim, Dermavant, Eli Lilly, Incyte, Janssen Research & Development,LEO Pharma, Ortho Dermatologics, Pfizer, and UCB Pharma. ML is also a consultant for Aditum Bio, Allergan, Almirall, Arcutis, Avotres Therapeutics, BirchBioMed, BMD Skincare, Boehringer Ingelheim, BMS, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, Evelo, Facilitate International Dermatologic Education, Foundation for Research and Education in Dermatology, Inozyme Pharma, LEO Pharma, Meiji Seika Pharma, Menlo, Mitsubishi, Neuroderm, Pfizer, Promius/Dr Reddy’s Laboratories, Serono, Theravance, and Verrica, all outside of the submitted work." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 488‐9): "BE VIVID was a multicentre, randomised, double‐blind, active comparator and placebo controlled phase 3 trial done across 105 sites... Patients were randomly assigned (4:2:1) to receive bimekizumab, ustekinumab, or placebo, using an interactive response technology, which assigned patients on the basis of a predetermined production randomisation or packaging schedule. Randomisation was stratified by geographical region (North America, Western Europe, Central and Eastern Europe, and Asia and Australia) and previous exposure to biologics (yes or no)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 488‐9): "BE VIVID was a multicentre, randomised, double‐blind, active comparator and placebo controlled phase 3 trial done across 105 sites... Patients were randomly assigned (4:2:1) to receive bimekizumab, ustekinumab, or placebo, using an interactive response technology, which assigned patients on the basis of a predetermined production randomisation or packaging schedule. Randomisation was stratified by geographical region (North America, Western Europe, Central and Eastern Europe, and Asia and Australia) and previous exposure to biologics (yes or no)." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 488‐9): "BE VIVID was a multicentre, randomised, double‐blind, active comparator and placebo controlled phase 3 trial done across 105 sites... To maintain double‐blinding, ustekinumab‐treated patients received placebo to match the bimekizumab dosing regimen (appendix p 6). Throughout the study, patients, investigators, and sponsors remained masked to treatment assignment with the exception of specially designated, unmasked site staff responsible for the preparation and administration of study treatments, safety monitoring, or bioanalytical sample analysis." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 488‐9): "BE VIVID was a multicentre, randomised, double‐blind, active comparator and placebo controlled phase 3 trial done across 105 sites... To maintain double‐blinding, ustekinumab‐treated patients received placebo to match the bimekizumab dosing regimen (appendix p 6). Throughout the study, patients, investigators, and sponsors remained masked to treatment assignment with the exception of specially designated, unmasked site staff responsible for the preparation and administration of study treatments, safety monitoring, or bioanalytical sample analysis." Comment: no detailed description of means used to guarantee absence of communication between blinded and unblinded personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 491): "Efficacy analyses included all randomly assigned patients (intention‐to‐treat population)... For the binary variables reported here, non‐responder imputation was used to account for missing data." Randomly assigned 567, analysed 567 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03370133). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. No results are posted on ClinicalTrials.gov. |
Bissonnette 2013.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, single‐blind study Date of study: May 2009 to June 2011 Location: Montréal, Quebec, Canada (5 centres) Phase 4 |
|
| Participants |
Randomised: 30 participants (median age 56 years (adalimumab) and 57 years (placebo), 23 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Adalimumab (n = 20), SC, 80/40 mg, eow Control intervention B. Topical treatment, phototherapy or no treatment (n = 10) |
|
| Outcomes | Assessment at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Abbott Laboratories Declarations of interest: (quote p 89): "Dr Bissonnette and Dr Bolduc have been investigators, advisors and/ or consultants and received grants and/or honoraria from Abbott, Amgen, Astellas, Novartis, Janssen Ortho, Pfizer, Celgene, and Tribute. Drs Tardif, Harel, Pressacco, and Guertin have no conflicts of interest to declare." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 84): "were randomised a concealed computer generated code created by the sponsor" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 84): "were randomised a concealed computer generated code created by the sponsor" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (pp. 83‐4): "single‐blind (cardiologist and all staff involved in vascular imaging and analysis were blinded to treatment assignment)". Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (pp. 83‐4): "single‐blind (cardiologist and all staff involved in vascular imaging and analysis were blinded to treatment assignment)". Comment: probably done, but no statement about secondary outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 30, analysed 30 Quote (p 84): "For all end points, the analysis was conducted on the ITT population, ... for the PASI 75 end point,... a nonresponder imputation method was used". Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00940862). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Blauvelt 2021a.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: May 2019 to February 2020 Location: USA (38 sites) Phase 3 |
|
| Participants |
Randomised: 157 participants Inclusion criteria:
Exclusion criteria:
Baseline characteristics N = 157, mean of age 49 years and 55% men Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Risankizumab 150 mg SC at weeks 0, 4, and 16, n = 105 Control intervention B. Placebo, n = 52 |
|
| Outcomes |
At week 16 Primary outcomes
Secondary outcomes
|
|
| Notes | Funding source: Quote (p. 8): "This work was supported by AbbVie. AbbVie participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication." Declarations of interest: Quote (p. 8): "Andrew Blauvelt has served as a scientific adviser and/or clinical study investigator for AbbVie, Aligos, Almirall, Amgen, Arcutis, Arena, Athenex, Boehringer Ingelheim, Bristol‐Myers Squibb, Dermavant, Eli Lilly and Company, Evommune, Forte, Galderma, Incyte, Janssen, Leo, Novartis, Pfizer, Rapt, Regeneron, Sanofi Genzyme, Sun Pharma, and UCB Pharma. Kenneth B. Gordon has received honoraria and/or research support from AbbVie, Amgen, Arcutis, Arena Pharma, Bristol Myers Squibb, Dermavant, Dermira, Incyte, Janssen, Kyowa Hakko Kirin, LEO Pharma, Novartis, Pfizer, Sanofi Genzyme, Sun Pharma, and UCB. Patricia Lee does not have any conflicts of interest, but her spouse is a speaker for AbbVie. Jerry Bagel has received research funds pay‐ able to Psoriasis Treatment Center from AbbVie, Amgen, Arcutis Biotherapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Celgene Corporation, Corrona LLC, Dermavant Sciences Ltd, Dermira, UCB, Eli Lilly and Company, Glenmark Pharmaceuticals Ltd, Janssen Biotech, Kadmon Corporation, Leo Pharma, Lycera Corp, Menlo Therapeutics, Novartis, Pfizer, Regeneron Pharmaceuticals, Sun Pharma, Taro Pharmaceutical Industries Ltd, and Ortho Dermatologics; consultant fees from AbbVie, Amgen, Celgene Corporation, Bristol‐Myers Squibb, Eli Lilly and Company, Janssen Biotech, Novartis, Sun Pharmaceutical Industries Ltd, UCB; and fees for speaking from AbbVie, Celgene Corporation, Eli Lilly, Janssen Biotech, and Novartis. Howard Sofen has served as a scientific adviser and/or clinical study investigator for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Janssen, Leo, Novartis, Pfizer, Sanofi Genzyme, Sun Pharma, and UCB. Benjamin Lockshin has served as a speaker, consultant and/or clinical study investigator for AbbVie, Bristol Myers Squibb, Celgene, Corrona registry, Eli Lilly, Incyte, Novartis, Regeneron, Sanofi Genzyme, Sun Pharma, and UCB. Ahmed M. Soliman, Ziqian Geng, Tianyu Zhan, and Gabriela Alperovich are employees of AbbVie Inc. and may hold stock or stock options. Linda Stein Gold has served as a scien‐ tific adviser, speaker and/or clinical study investigator for AbbVie, Almirall, Arcutis, Bristol Myers Squibb, Dermavant, Eli Lilly and Company, Galderma, Incyte, Leo, Novartis, Ortho Derm, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, and UCB Pharma." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p. 2): "Study 1 (NCT03875482) was a multicenter, randomized, double‐blinded, placebo‐controlled, parallel‐group study conducted at 38 sites in the United States..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p. 2): "Study 1 (NCT03875482) was a multicenter, randomized, double‐blinded, placebo‐controlled, parallel‐group study conducted at 38 sites in the United States..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p. 2): "Study 1 (NCT03875482) was a multicenter, randomized, double‐blinded, placebo‐controlled, parallel‐group study conducted at 38 sites in the United States..." Comment: unclear if the process guaranteed blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p. 2): "Study 1 (NCT03875482) was a multicenter, randomized, double‐ blinded, placebo‐controlled, parallel‐group study conducted at 38 sites in the United States..." Comment: unclear if the process guaranteed the blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p. 4): "In study 1, the intent‐to‐treat (ITT) population included all randomized patients; patients were analyzed according to treatment as randomized. The safety analysis population consisted of all patients who received at least one dose of study drug; patients were analyzed according to the first dose of study drug (risankizumab or placebo) received...." "For analysis of both studies, categorical efficacy variables were analyzed using non‐responder imputation (NRI) to handle missing data; mixed‐effect model repeat measurements (MMRMs) of additional efficacy endpoints was used for continuous variables. As observed and modified NRI (mNRI) were conducted as sensitivity analyses for efficacy endpoints (co‐primary and ranked secondary efficacy endpoints in study 1) for the two studies. For mNRI, a patient was considered as a non‐responder for the visit if the patient did not have an evaluation and discontinued study drug due to lack of efficacy or due to an AE of worsening of psoriasis during the visit window". Randomised 157, analysed 157 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03875482). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
BRIDGE 2017.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: November 2012 to November 2015 Location: 57 centres in Austria, Germany, the Netherlands, and Poland Phase 3 |
|
| Participants |
Randomised: 704 participants (mean age 44.5 years, 452 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Dimethyl fumarate (DMF) (n = 280), orally, maximum daily dose of 720 mg DMF Control intervention B. DMF + salt of monoethyl fumarate (n = 286), orally, maximum daily dose of 720 mg DMF C. Placebo (n = 138) |
|
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 1): “This research was funded by Almirall S.A.”. Declarations of interest (p 1): "U.M. has been an advisor and/or received speaker honoraria and/or received grants and/or participated in clinical trials for the following companies: Abbott/AbbVie, Almirall Hermal, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Foamix, Forward Pharma, Galderma, Janssen, LEO Pharma, Lilly, Medac, Miltenyi Biotec, MSD, Novartis, Pfizer, Teva, UCB, VBL and XenoPort. J.C.S. receives advisory board/consulting fees from AbbVie, Biogen, Biogenetica International Laboratories, Egis Pharmaceuticals, Fresenius, LEO Pharma, Lilly, Novartis, Pierre Fabre, Polpharma, Sandoz and Toray Corporation; and receives speaker fees from AbbVie, Actavis, Adamed, Astellas, Berlin‐Chemie Menarini, Fresenius, Janssen‐Cilag, LEO Pharma, Mitsubishi Tanabe Pharma, Novartis, Pierre Fabre, Takeda and Vichy, and clinical trial funding from AbbVie, Actelion, Almirall, Amgen, GlaxoSmithKline, Janssen‐Cilag, Merck, Mitsubishi Tanabe Pharma, Novartis, Regeneron and Takeda. P.V.K. declares consultancy fees for Celgene, Centocor, Almirall, Amgen, Pfizer, Philips, Abbott, Lilly, Galderma, Novartis, Janssen‐Cilag, LEO Pharma, Sandoz and Mitsubishi Tanabe Pharma and carries out clinical trials for Basilea, Pfizer, Lilly, Amgen, AbbVie, Philips Lighting, Janssen‐Cilag and LEO Pharma. R.L." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 2): “Randomisation was performed by the investigators using an interactive web‐based response system.” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 2): “Randomisation was performed by the investigators using an interactive web‐based response system. The randomisation sequence was kept concealed from the investigators during the trial.” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 2): “Treatment was uptitrated over the first 9 weeks, with placebo or up to a maximum daily dose of 720 mg DMF in the LAS41008 or Fumaderm® groups”. Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 2): “Treatment was uptitrated over the first 9 weeks, with placebo or up to a maximum daily dose of 720 mg DMF in the LAS41008 or Fumaderm® groups”. Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 704, analysed 671 Management of missing data: Quote (p 4): “All statistical analyses were based on the full analysis set (FAS) and the per‐protocol set (PPS). As the results of both were consistent, data are presented here only for the FAS. A last‐observation‐carried‐forward approach was used to handle missing data for the PASI‐ and PGA‐derived end points.” DMF/DMF + MEF/placebo Randomised: 280/286/138 Safety set analysis: 279/283/137 (untreated participants excluded) Full set analysis: 267/273/131 (not explained) Comment: not ITT analysis |
| Selective reporting (reporting bias) | High risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01726933). Some prespecified outcomes and those mentioned in the Methods section as DLQI had not been reported |
Cai 2016.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: August 2012 to December 2013 Location: China Phase 3 |
|
| Participants |
Randomised: 425 participants (mean age 43 years, 310 men) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Adalimumab (n = 338), SC, 40 mg, week 0, 2 injections, eow 1 injection Control intervention B. Placebo (n = 87), SC |
|
| Outcomes | Assessment at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes | Funding source: Quote (p 2): "Abbvie Inc participated in the study design, study research, collection, analysis and interpretation of data". Declarations of interest: Quote (p 2): "L Cai, J Gu, J Zheng, M Zheng, G Wang, L‐Y Xi, F Hao, X‐M Liu, Q‐N Sun, Y Wang, W Lai, H Fang, Y‐T Tu, Q Sun, J Chen and X‐H Gao were investigators for this study, and J‐Z Zhang was the principal investigator for this study; all declare no financial, professional or personal relationships that might be perceived as a conflict of interest. Y Gu and HD Teixeira receive a salary as employees of AbbVie and may also receive stock, stock options and/or stock grants. MM Okun is a former AbbVie employee." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 2 and Appendix): "The randomisation schedule was prepared by the Statistics Department of AbbVie, US. Randomization was performed using an adequate block size." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 2 and Appendix): “An interactive voice/web response system determined patient randomisation. The randomisation schedule was prepared by the Statistics Department of AbbVie, US. Randomization was performed using an adequate block size." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 2 and Appendix): “Patients in Period A were randomised 4:1 to receive adalimumab 40 mg every‐other‐week (following a single 80 mg dose), or matching placebo...All AbbVie personnel with direct oversight of the conduct and management of the trial (with the exception of the drug supply team), the investigator, study‐site personnel and the patient remained blinded to each patient’s treatment throughout the 12 week blinded period of the study." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 2 and Appendix): “Patients in Period A were randomised 4: 1 to receive adalimumab 40 mg every‐other‐week (following a single 80 mg dose), or matching placebo...All AbbVie personnel with direct oversight of the conduct and management of the trial (with the exception of the drug supply team), the investigator, study‐site personnel and the patient remained blinded to each patient’s treatment throughout the 12 week blinded period of the study." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned: 425, analysed 425 (ITT) Quote (p 3): "Efficacy was analysed in Period A for all randomised patients [intent‐to‐treat (ITT_A Population)]... Missing data were handled using non‐responder imputation (NRI) for categorical variables and last‐observation‐carried‐forward (LOCF) for continuous variables." Comment: ITT analyses |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01646073). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Cai 2020.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: February 2017 to November 2018 Location: China, Hungary, Malaysia, Turkey, Thailand, Philippines Phase 3 |
|
| Participants |
Randomised: 441 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 441, mean age of 39 years and 79% men Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Secukinumab 150 mg: 150 mg SC at randomisation, weeks 1, 2, 3, 4, and every 4 weeks until week 48, n = 110 Control interventions B. Secukinumab 300 mg: 300 mg SC at randomisation, weeks 1, 2, 3, 4, and every 4 weeks until week 48, n = 221 C. Placebo, n = 110 |
|
| Outcomes |
At week 12 Primary composite outcome
Secondary outcomes
|
|
| Notes | Funding source: Quote (p 2672) :''This study was sponsored by Novartis Pharma AG, Basel, Switzerland.'' Declarations of interest: Quote (p 2672): ''Lin Cai has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from Novartis, AbbVie, Pfizer Inc. Jian‐Zhong Zhang has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Sanofi, La Roche‐Posay China, AbbVie, Bayer, Janssen‐Cilag, Hen‐ lius, Kyowa Kirin, and Pfizer Inc. Xu Yao has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Sanofi, AbbVie, Bayer, Janssen‐ Cilag, and Pfizer Inc. Jun Gu has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Sanofi, La Roche‐Posay China, AbbVie, Bayer, Henlius, and Pfizer Inc. Quan‐Zhong Liu has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from Novartis, La Roche‐Posay China, AbbVie, Bayer, Janssen‐ Cilag, and Pfizer Inc. Min Zheng has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from AbbVie, Janssen‐Cilag, Boehringer Ingelheim, LEO Pharma China, Xian‐Janssen, Novartis, and Pfizer Inc. Shi‐Fa Zhang has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Janssen‐Cilag, Henlius. Jin‐ Hua Xu has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from Novartis, Sanofi, La Roche‐Posay China, AbbVie, Bayer, Kyowa Kirin, and Pfizer Inc. Cheng‐Xin Li has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, AbbVie, Bayer, Janssen‐Cilag, Kyowa Kirin, and Pfizer Inc. Hao Cheng has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, AbbVie, Bayer, Janssen‐Cilag, Henlius, and Pfizer Inc. Qing Guo has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Sanofi, La Roche‐Posay China, AbbVie, Bayer, Janssen‐Cilag, Hen‐ lius, Kyowa Kirin, and Pfizer Inc. Wei‐Li Pan has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Sanofi, La Roche‐Posay China, AbbVie, Bayer, Janssen‐Cilag, Henlius, Kyowa Kirin, and Pfizer Inc. Shen‐Qiu Li has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Sanofi, AbbVie, Bayer, Janssen‐Cilag, and Pfizer Inc. Ruo‐Yu Li has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Bayer, Janssen‐Cilag, MSD, and Pfizer Inc. Zai‐Pei Guo has participated in advisory boards and/ or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Sanofi, AbbVie, Bayer, Janssen‐Cilag, and Pfizer Inc. Zhi‐ Qi Song has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Sanofi, La Roche‐Posay China, AbbVie, Bayer, Janssen‐Cilag, Hen‐ lius, Kyowa Kirin, and Pfizer Inc. Shan‐Shan Li has participated as an investigator and received honoraria from Novartis China. Xiu‐Qin Dong has participated in advisory boards and/or as an investigator and/or speaker and received honoraria from LEO Pharma China, Novartis, Sanofi, AbbVie, Bayer, Janssen‐Cilag. Linda Wang, Rong Fu, Pascaline Regnault, Pascal Charef, Rafal Mazur, and Manmath Patekar are employed by Novartis.'' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 2666): "This study ... was a 52‐week, multicenter, randomized, double‐blind, placebo‐ controlled, parallel‐group, Phase 3 trial." Quote (p 2667): "At Baseline visit, all eligible patients were randomized via interactive response technology (IRT) to one of the treatment arms. The Investigator or his/her delegate contacted the IRT after confirming that the patient fulfilled all the inclusion/exclusion criteria. A patient randomization list was produced by the IRT provider using a validated system that automated the random assignment of patient numbers to randomization numbers. These randomization numbers were linked to the different treatment arms, which in turn were linked to medication numbers for the packages of investigational treatment to be dispensed to the patient (only the medication number, but not the randomization number)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 2666): "This study ... was a 52‐ week, multicenter, randomized, double‐blind, placebo‐ controlled, parallel‐group, Phase 3 trial. ." Quote (p 2667): "At Baseline visit, all eligible patients were randomized via interactive response technology (IRT) to one of the treatment arms. The Investigator or his/her delegate contacted the IRT after confirming that the patient fulfilled all the inclusion/exclusion criteria. A patient randomization list was produced by the IRT provider using a validated system that automated the random assignment of patient numbers to randomization numbers. These randomization numbers were linked to the different treatment arms, which in turn were linked to medication numbers for the packages of investigational treatment to be dispensed to the patient (only the medication number, but not the randomization number)." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (study protocol p 29): "Subjects, investigator staff, persons performing the assessments and data analyst will remain blind to the identity of the treatment from the time of randomization until database lock, using the following methods: (1) Randomization data are kept strictly confidential until the time of unblinding, and will not be accessible by anyone else involved in the study, (2) the identity of the treatments will be concealed by the use of investigational treatment that are all identical in packaging, labeling, schedule of administration, appearance, taste and odor." Quote (p 2666): "This study ... was a 52‐week, multicenter, randomized, double‐blind, placebo‐ controlled, parallel‐group, Phase 3 trial." Quote (p 2666): "Due to treatment blinding, patients received an additional weekly secukinumab or matching placebo dose at Weeks 13, 14, and 15." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (study protocol p 29): "Subjects, investigator staff, persons performing the assessments and data analyst will remain blind to the identity of the treatment from the time of randomization until database lock, using the following methods: (1) Randomization data are kept strictly confidential until the time of unblinding, and will not be accessible by anyone else involved in the study, (2) the identity of the treatments will be concealed by the use of investigational treatment that are all identical in packaging, labeling, schedule of administration, appearance, taste and odor." Quote (p 2666): "This study ... was a 52‐ week, multicenter, randomized, double‐blind, placebo‐ controlled, parallel‐group, Phase 3 trial." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 2667): "The co‐primary endpoints were evaluated using a logistic regression model with treatment group, baseline body weight category, geographical region, and baseline PASI score as exploratory variables and a multiple imputations (MI) method was used for missing values." Randomised 441, analysed 441 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03066609). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
Cai 2022.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: October 2017 to March 2019 Location: China (21 sites) Phase 3 |
|
| Participants |
Randomised: 262 randomised participants, 261 received the control or intervention Inclusion criteria
Exclusion criteria
Baseline characteristics N = 261, median (range) of age 38.0 (18.0 to 74.0) for HLX03 group, median (range) of age 38.5 (19.0 to 71.0) or adalimumab group, and 73% men Dropouts and withdrawals 16/261 (6%): HLX03 group (7), adalimumab group (9)
|
|
| Interventions |
Intervention A. HLX03, SC, biosimilar adalimumab week 0: 80 mg, week 1: 40 mg, then 40 mg eow, n = 131 Control Intervention B. Adalimumab (Humira) week 0: 80 mg, week 1: 40 mg, then 40 mg eow, n = 130 |
|
| Outcomes |
At 16 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 594): "This study was funded by Shang‐ hai Henlius Biotech, Inc., Shanghai, China. The rapid service and open access fees were also sponsored by Shanghai Henlius Biotech, Inc." Declarations of interest: Quote (P 595):"Qingyu Wang and Jun Zhu are employees of Shanghai Henlius Biotech, Inc. Lin Cai, Linfeng Li, Hao Cheng, Yangfeng Ding, Zhenshu Biao, Shifa Zhang, Songmei Geng, Quanzhong Liu, Hong Fang, Zhiqi Song, Yan Lu, Shanshan Li, Qing Guo, Juan Tao, Li He, Jun Gu, Qinping Yang, Xiuping Han, Xinghua Gao, Danqi Deng, Shenqiu Li and Jianzhong Zhang have nothing to disclose." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 586): "This was a randomized, double‐blind, active‐ controlled, parallel‐group study.... A central interactive web response system was used to assign patients 1:1 to receive HLX03 or adalimumab with stratification of prior biological use (yes/no) according to their unique randomization code." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 586): "This was a randomized, double‐blind, active‐ controlled, parallel‐group study.... A central interactive web response system was used to assign patients 1:1 to receive HLX03 or adalimumab with stratification of prior biological use (yes/no) according to their unique randomization code. ....Patients, the investigators, and the study sponsor remained blinded to treatment allocations." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 586): "This was a randomized, double‐blind, active‐controlled, parallel‐group study.... Patients, the investigators, and the study sponsor remained blinded to treatment allocations. Study drugs were administered via subcutaneous injection by an appointed ‘unblinded’ nurse." Comment: no detailed description of means used to guarantee absence of communication between blinded participants and unblinded nurse |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 586): "This was a randomized, double‐blind, active‐controlled, parallel‐group study.... Patients, the investigators, and the study sponsor remained blinded to treatment allocations. Study drugs were administered via subcutaneous injection by an appointed ‘unblinded’ nurse." Comment: no detailed description of means used to guarantee absence of communication between blinded participants and unblinded nurse and patient and investigators performing assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dealing with missing data: Quote (p 587): "Efficacy data were analyzed using the full analysis set (FAS; all randomized patients receiving ≥1 dose of study drug and C ≥ post‐ dose efficacy evaluation) and the per‐protocol set (PPS; all FAS patients without major protocol violations)." Randomised 262, analysed 261 Comment: methods for dealing with missing data not specified |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03316781). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. No results are posted on ClinicalTrials.gov. |
CALYPSO 2018.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: January 2017 to April 2018 Location: Russia (multicentre) Phase 3 |
|
| Participants |
Randomised: 346 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 346, mean of age 42.5 years and 66% men Dropouts and withdrawals
|
|
| Interventions |
Intervention
BCD‐057 (a biosimilar of adalimumab) group includes participants with moderate‐to‐severe plaque psoriasis, who will receive BCD‐057 SC at a dose 80 mg on week 0, then at a dose 40 mg on weeks 1, 3, 5, 7, 9, 11, 13, 15, 17, 19, 21, and 23, n = 174 Control interventions Humira group includes participants with moderate‐to‐severe plaque psoriasis, who will receive Humira SC at a dose 80 mg on week 0, then at a dose 40 mg on weeks 1, 3, 5, 7, 9, 11, 13, 15, 17, 19, 21, and 23, n = 172 |
|
| Outcomes |
At week 16 Primary outcome
Secondary outcomes
|
|
| Notes | Funding source: Quote (clinicaltrials.gov): "Biocad" Declarations of interest: not stated The filling in of the characteristics and ROB tool for this study was made from the article translated from Russian into English. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p. 74): "BCD‐057‐2/CALYPSO (NCT02762955) is a randomized double‐blind clinical study of efficacy and safety of the drug BCD‐057 (international non‐proprietary name, INN: adalimumab, CJSC "BIOCAD", Russia)..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p. 74): "BCD‐057‐2 / CALYPSO (NCT02762955) is a randomized double‐blind clinical study of efficacy and safety of the drug BCD‐057 (international non‐proprietary name, INN: adalimumab, CJSC "BIOCAD", Russia)..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p. 74): "BCD‐057‐2 / CALYPSO (NCT02762955) is a randomized double‐blind clinical study of efficacy and safety of the drug BCD‐057 (international non‐proprietary name, INN: adalimumab, CJSC "BIOCAD", Russia)..." Comment: no description of the method used to guarantee blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p. 74): "BCD‐057‐2 / CALYPSO (NCT02762955) is a randomized double‐blind clinical study of efficacy and safety of the drug BCD‐057 (international non‐proprietary name, INN: adalimumab, CJSC "BIOCAD", Russia)..." Comment: no description of the method used to guarantee blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomised 346, analysed 346 Comment: methods for dealing with missing data not specified, ITT analyses |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02762955). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are not posted on ClinicalTrials.gov. |
Caproni 2009.
| Study characteristics | ||
| Methods | RCT, active‐controlled study Date of study: not stated Location: not stated |
|
| Participants |
Randomised: 60 participants (age range 28 to 67 years (etanercept), 32 to 65 years (acitretin), 24 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept (n = 30), SC, 50 mg, twice a week, 12 weeks Control intervention B. Acitretin (n = 30), orally, 0.4 mg/kg/day, 12 weeks |
|
| Outcomes | Assessment at 12 weeks Primary and secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes | Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p. 211): "Patients were randomly assigned to one of the two groups". Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: probably open‐label trial; term "blind" not used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably open‐label trial; term "blind" not used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no description of the method used to manage the missing data. No ITT analyses mentioned |
| Selective reporting (reporting bias) | Unclear risk | Comment: no primary or secondary outcomes stated |
CARIMA 2019.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: April 2014 to April 2016 Location: Germany (23 sites, multicentre) Phase 3 |
|
| Participants |
Randomised: 151 participants Key inclusion criteria
Key exclusion criteria
Baseline characteristics N = 151, mean age of 51.93 years and 67% men Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Secukinumab 300 (300 mg every week for 4 weeks followed by 300 mg secukinumab every 4 weeks until week 48) (n = 48) Control interventions B. Secukinumab 150 (150 mg every week for 4 weeks followed by 300 mg secukinumab every 4 weeks until week 48) (n = 54) C. Placebo (n = 49) |
|
| Outcomes |
At week 12 Primary outcome
Secondary outcomes
|
|
| Notes | On ClinicalTrials.gov, results submitted without PASI or IGA outcomes Funding source Quote (p. 1061): "The CARIMA study was funded by Novartis Pharma GmbH, Germany. Medical writing assistance was provided by Evelyn Altemeyer, Novartis Ireland Ltd., and funded by Novartis Pharma GmbH, Germany, in line with Good Publication Practice 3 guidelines." Declarations of interest Quote (p. 1061): "EVS received grants from the Deutsche Forschungsgemeinschaft. KR has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Affibody, Amgen, Biogen, Boehringer Ingelheim Pharma, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, Kyowa Kirin, Leo, Lilly, Medac, Merck Sharp & Dohme, Novartis, Ocean Pharma, Pfizer, Regeneron, Sanofi, Takeda, UCB Pharma, and Xenoport. DT has received research support/acted as Principal Investigator (clinical trials) from AbbVie, Almirall, Amgen, Astellas, Biogen‐Idec, Boehringer‐Ingelheim, Celgene, Dignity, Eli Lilly, Forward‐Pharma, GlaxoSmithKline, Leo, Janssen‐Cilag, Maruho, Merck Sharp & Dohme, Mitsubishi Pharma, Novartis, Pfizer, Roche, and Sandoz; has acted as a consultant for AbbVie, Biogen‐Idec, Celgene, Dignity, Maruho, Mitsubishi, Novartis, Pfizer, and Xenoport; has received honoraria from AbbVie, Biogen‐Idec, Celgene, Janssen, Leo, Pfizer, Roche‐Possay, Novartis, and Mundipharma; and has participated in scientific advisory boards for AbbVie, Amgen, Biogen‐Idec, Celgene, Eli Lilly, GlaxoSmithKline, Pfizer, Novartis, Janssen, Mundipharma, and Sandoz. WK served on the executive steering committee of JUPITER and CANTOS; served as a consultant for Amgen, DalCor, Kowa, Novartis, Pfizer, and Sanofi; and has received fees for lectures from Amgen, AstraZeneca, Novartis, Pfizer, and Sanofi. AP is a speaker for AbbVie, Almirall‐Hermal, Amgen, Biogen Idec, Celgene, Eli Lilly, Galderma, Janssen, Leo Pharma, Medac, Novartis, Pfizer, and UCB Pharma; served as an advisor for AbbVie, Almirall‐Hermal, Amgen, Celgene, Eli Lilly, Janssen, Leo Pharma, and Novartis; and has participated in clinical trials funded by AbbVie, Almirall‐Hermal, Amgen, Biogen Idec, Boehringer‐Ingelheim, Celgene, GlaxoSmithKline, Eli Lilly, Galderma, Hexal, Janssen, Leo Pharma, Medac, Merck Serono, Mitsubishi, Merck Sharp & Dohme, Novartis, Pfizer, Tigercat Pharma, Regeneron, Roche, Sandoz Biopharmaceuticals, Schering‐Plough, and UCB Pharma. AK has received honoraria from Novartis, Eli Lilly, Leo Pharma, Almirall, Janssen, UCB Pharma, Merck Sharp & Dohme, and Pfizer and has received fees for board participation from Novartis, Leo Pharma, Janssen, and Eli Lilly. TR has received fees and honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, and Novartis. DY, JF, CS, and NM are employees of Novartis. NNM is a full‐time US government employee. TG has received grant support and speaker honoraria from Abbott Vascular." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 1059): "CARIMA was a multicenter, double‐blind, randomized, placebo‐ controlled, parallel‐group, exploratory trial in patients with plaque‐type psoriasis." Comment: no description |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 1059): "CARIMA was a multicenter, double‐blind, randomized, placebo‐ controlled, parallel‐group, exploratory trial in patients with plaque‐type psoriasis." Comment: no description |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1059): "CARIMA was a multicenter, double‐blind, randomized, placebo‐ controlled, parallel‐group, exploratory trial in patients with plaque‐type psoriasis." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1059): "CARIMA was a multicenter, double‐blind, randomized, placebo‐ controlled, parallel‐group, exploratory trial in patients with plaque‐type psoriasis." Quote (p 1060): "The FMD analysis was performed in a blinded fashion by a core laboratory (University Medical Center Mainz; see Supplementary Materials)." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dealing with missing data: Quote (p 1060‐1): "The full analysis set comprised all randomly assigned patients to whom treatment was administered. All analyses were as observed; missing values were not imputed. " Results for PASI 75 and 90 were reported as a percentage; numbers not reported; impossible to state if all randomised participants were analysed |
| Selective reporting (reporting bias) | High risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02559622). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported, except for IGA. Results posted on ClinicalTrials.gov. |
Cestari 2021.
| Study characteristics | ||
| Methods | RCT, double‐blind, active controlled multicentre study Date of study: July 2018 to November 2021 Location: Brazil (11 sites) Phase 3 |
|
| Participants |
Randomised: 98 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 98, mean of age 48 years, % men not stated Dropouts and withdrawals Not stated |
|
| Interventions |
Intervention A. Risankizumab 150 mg at weeks 0, 4, and every 12 weeks thereafter SC, n = 50 Control intervention B. Methotrexate weekly oral 5 mg may be titrated up to 25 mg, unless achieved ≥ 90% reduction in PASI 90 and sPGA of 0/1 or shown poor tolerability, n = 48 All patients received 5 mg folate weekly |
|
| Outcomes | At 28 weeks Primary outcomes
Secondary outcomes
|
|
| Notes | Risk of bias assessment was done according the abstract, because the article is not published at this time Funding source Quote (clinicaltrials.gov): "AbbVie" Declarations of interest Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (conference abstract): "Risankizumab is an interleukin‐23 antagonist approved for moderate‐to‐severe plaque psoriasis in adults. In this phase 3, multicenter, randomized, double‐blind, double‐dummy, active‐controlled study (NCT03219437)" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (conference abstract): "Risankizumab is an interleukin‐23 antagonist approved for moderate‐to‐severe plaque psoriasis in adults. In this phase 3, multicenter, randomized, double‐blind, double‐dummy, active‐controlled study (NCT03219437)" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (conference abstract): "Risankizumab is an interleukin‐23 antagonist approved for moderate‐to‐severe plaque psoriasis in adults. In this phase 3, multicenter, randomized, double‐blind, double‐dummy, active‐controlled study (NCT03219437)" Comment: no clear description of measures taken to guarantee the blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (conference abstract): "Risankizumab is an interleukin‐23 antagonist approved for moderate‐to‐severe plaque psoriasis in adults. In this phase 3, multicenter, randomized, double‐blind, double‐dummy, active‐controlled study (NCT03219437)" Comment: no description of the method used to guarantee blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomised 98, analysed 98 Comment: no more precision regarding methods for dealing with missing data |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03219437). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results not posted on ClinicalTrials.gov |
CHAMPION 2008.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: not stated Location: multicentre (n = 28) in Europe and Canada Phase 3 |
|
| Participants |
Randomised: 271 participants (mean age 42, 178 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Adalimumab (n = 108), SC, 80 mg at week 0, 40 mg at week 1 and 40 mg eow Control intervention B. Methotrexate (n = 110), orally, 7.5 mg to 25 mg weekly C. Placebo (n = 53), SC and orally (same drug administration) |
|
| Outcomes | Assessments at 16 weeks Primary outcome
Secondary outcomes
|
|
| Notes | Funding source: Quote (p 561): "Abbott Laboratories funded this study and participated in the study design, data collection, data management, data analysis and preparation of the manuscript" Declarations of interest Quote (p 558): "J.‐H.S., G.S., L.D., K.P. and J.‐P.O. have served as consultants for Abbott Laboratories. In addition, they have participated in continuing medical education events supported by unrestricted educational grants from Abbott. R.G.L. reports receiving fees as a consultant or advisory board member for Abbott, Amgen, Astellas, Boehringer‐ Ingelheim, Barrier Therapeutics and Genentech; he has received lecture fees from Abbott, Amgen/ Wyeth and Biogen‐Idec, and has been the principal investigator and received grants from Abbott, Amgen, Astellas, Centocor, Galderma and Genentech. K.U., M.K. and A.C. are employees of Abbott." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 559): "Randomisation was completed through a central computer‐generated scheme stratified by centre, with block sizes of four". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 559): "Patient numbers were centrally assigned by an interactive voice‐response system in consecutive order". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 559): "Adalimumab (Humira; Abbott Laboratories) or matching placebo for SC injection was provided as sterile preservative‐free solution in prefilled syringes. Oral methotrexate tablets were supplied by Wyeth Pharma (Münster, Germany), and placebo tablets were supplied by Abbott GmbH & Co. KG (Ludwigshafen, Germany). Both the methotrexate and placebo tablets were administered as capsules (encapsulated tablets) as a single weekly dose. The capsules for both methotrexate and placebo were supplied by Fisher Clinical Services (Basel, Switzerland)." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 559): "Adalimumab (Humira; Abbott Laboratories) or matching placebo for SC injection was provided as sterile preservative‐free solution in prefilled syringes. Oral methotrexate tablets were supplied by Wyeth Pharma (Münster, Germany), and placebo tablets were supplied by Abbott GmbH & Co. KG (Ludwigshafen, Germany). Both the methotrexate and placebo tablets were administered as capsules (encapsulated tablets) as a single weekly dose. The capsules for both methotrexate and placebo were supplied by Fisher Clinical Services (Basel, Switzerland)." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 271, analysed 271 Management of missing data: Quote (p 562): "Data for 16 patients with missing week 16 assessments for PASI, including the 15 patients who discontinued and one additional patient in the methotrexate group, were imputed as nonresponse." Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00235820). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported, except for DLQI that was published in a second study. |
CHANGE 2021.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study with blinded assessment of the efficacy outcome Date of study: November 2017 to March 2019 Location: Germany (30 sites) Phase 4 |
|
| Participants |
Randomised: 210 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 210, mean of age 44 years, and 69% men Dropouts and withdrawals 61/210 (29%): 14/105 brodalimumab and 47/105 fumaric acid esters
|
|
| Interventions |
Intervention A. Brodalumab (Kyntheum (brodalumab) pre‐filled syringe 210 mg/1.5 mL solution for subcutaneous injections. First 3 injections are administered weekly, and thereafter every 2 weeks (Q2W)), n = 105 Control intervention B. Fumaric acid esters (Fumaderm initial dose tablets (30 mg dimethyl fumarate, 67 mg ethyl hydrogen fumarate calcium salt, 5 mg ethyl hydrogen fumarate magnesium salt, 3 mg ethyl hydrogen fumarate zinc salt) Fumaderm tablets (120 mg dimethyl fumarate, 87 mg ethyl hydrogen fumarate calcium salt, 5 mg ethyl hydrogen fumarate magnesium salt, 3 mg ethyl hydrogen fumarate zinc salt) Fumaderm tablets are administered orally up to 3 times daily in accordance with the dosing scheme in the label), n = 105 |
|
| Outcomes |
At week 24 Primary composite outcome
Secondary outcomes
|
|
| Notes | Funding source: Quote (p 2): "The trial was funded in full by LEO Pharma A/S, Ballerup, Denmark." Declarations of interest: Quote (p 1‐2): "A. Pinter has received honoraria as investigator and/or for consultancy and/or received speakers honoraria and/ or research grants from AbbVie, Almirall Hermal, Amgen, Biogen Idec, BioNTech, Boehringer Ingelheim, Celgene, GSK, Eli Lilly, Galderma, Hexal, Janssen, LEO Pharma, MC2, Medac, Merck Serono, Mitsubishi, MSD, Novartis, Pascoe, Pfizer, Tigercat Pharma, Regeneron, Roche, Sandoz Biopharmaceuticals, Sanofi Genzyme, Schering‐ Plough and UCB Pharma. M. Hoffmann has received honoraria as investigator and/or for consultancy and/or received speaker’s honoraria and/or research grants from AbbVie, Almirall Hermal, Boehringer Ingelheim, Eli Lilly, Janssen, LEO Pharma, Medac, MSD, Novartis, Pfizer and UCB Pharma. K. Reich has received honoraria as investigator and/or for consultancy and/or received speaker’s honoraria and/or research grants from AbbVie, Affibody, Almirall, Amgen, Biogen Idec, Boehringer Ingelheim, Bristol‐Meyers Squibb, Celgene, Covagen, Eli Lilly, Forward Pharma, Fresenius Medical Care, Galapagos, GlaxoSmithKline, Janssen‐Cilag, Kyowa Kirin, LEO Pharma, Medac, Merck Sharp & Dohme Corp., Miltenyi, Novartis, Ocean Pharma, Pfizer, Samsung Bioepis, Sandoz, Sanofi, Sun Pharma, Takeda, UCB Pharma, Valeant, XBiotech and XenoPort. M. Augustin has received honoraria as investigator and/or for consultancy and/or received speaker’s honoraria and/or research grants AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, GSK, Janssen‐Cilag, LEO Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB Pharma and XenoPort. U. Mrowietz has received honoraria as investigator and/or for consultancy and/or received speaker’s honoraria and/or research grants from AbbVie, Almirall, Aristea, Boehringer Ingelheim, Celgene, Dr. Reddy’s, Eli Lilly, Foamix, Formycon, Forward Pharma, Janssen, LEO Pharma, Medac, Novartis, Pierre Fabre, Sanofi‐Aventis, UCB and Xenoport. K. Kaplan, S.D. Gudjonsdottir and T. Delvin are employees of LEO Pharma A/S." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: (p 2): "This was a 24‐week, phase 4, randomized, assessor‐blinded, multi‐centre, open‐label, parallel‐group, active‐controlled trial... Two hundred ten subjects were randomized 1: 1 using an interactive web response system (Bioclinica Trident, Princeton, NJ, USA) to receive either subcutaneous, self‐administered injections of 210 mg brodalumab once weekly at weeks 0, 1 and 2 followed by 210 mg every 2 weeks, or to FAE tablets (Fumaderm" Initial/Fumaderm", Biogen GmbH, Munic, Germany) up to 240 mg three times daily, with individual dose titration according to label". Comment: adequate process |
| Allocation concealment (selection bias) | Low risk | Quote (p 2): "This was a 24‐week, phase 4, randomized, assessor‐blinded, multi‐centre, open‐label, parallel‐group, active‐controlled trial... Two hundred ten subjects were randomized 1: 1 using an interactive web response system (Bioclinica Trident, Princeton, NJ, USA) to receive either subcutaneous, self‐administered injections of 210 mg brodalumab once weekly at weeks 0, 1 and 2 followed by 210 mg every 2 weeks, or to FAE tablets (Fumaderm" Initial/Fumaderm", Biogen GmbH, Munic, Germany) up to 240 mg three times daily, with individual dose titration according to label". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 2‐3): "This was a 24‐week, phase 4, randomized, assessor‐blinded, multi‐centre, open‐label.... Assessment of PASI, static Physician’s Global Assessments (sPGA), BSA and Nail Psoriasis Severity Index (NAPSI) were performed by investigators blinded to the trial treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 2‐3): "This was a 24‐week, phase 4, randomized, assessor‐blinded, multi‐centre, open‐label.... Assessment of PASI, static Physician’s Global Assessments (sPGA), BSA and Nail Psoriasis Severity Index (NAPSI) were performed by investigators blinded to the trial treatment." Comment: no description of the method used to guarantee blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 4): "Efficacy endpoints were analysed for the intention‐to‐treat population (full analysis set). Safety data were analysed for subjects who were exposed to trial treatment and according to the treatment received (safety analysis set). Binary data were analysed using the Cochran–Mantel–Haenszel (CMH) test with stratification by weight group (≥ 100 or < 100 kg) and non‐responder imputation for missing data". Randomised 210, analysed 210 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03331835). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
Chaudhari 2001.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: not stated Location: single‐centre, New Jersey, USA |
|
| Participants |
Randomised: 33 participants (age mean 35 years (infliximab 10), 51 years (infliximab 5), 45 years (placebo), 23 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Infliximab (n = 11), IV, 5 mg/kg, weeks 0, 2, 6, 10 Control intervention B. Infliximab (n = 11), IV, 10 mg/kg, weeks 0, 2, 6, 10 C. Placebo (n = 11), IV, 20 mL, weeks 0, 2, 6, 10 |
|
| Outcomes | Assessment at 10 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Y Johnson and Johnson, Centocor Inc. Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1843): "...were randomly assigned... by means of a lock‐of‐six randomisation scheme" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1843): "Placebo was supplied in a identical manner except that it did not contain IFX... The infliximab infusion solution was given by investigators unaware of treatment assignment..." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1843): "All assessments were done in a masked manner". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 33, analysed 33 Dropouts and withdrawals
Management of missing data: Quote (p 1844): "The primary analysis was done according to ITT, all randomised patients were included". Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Chladek 2005.
| Study characteristics | ||
| Methods | RCT, active‐controlled study Date of study: not stated Location: Prague, Czech Republic |
|
| Participants |
Randomised: 41 participants (mean age 50 years (A), 46 years (B), 44 years (C), 41 years (D), 24 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate (n = 12), 7.5 mg/week, 2.5‐2.5‐2.5 at 12 hours, for 13 weeks Control intervention B. Methotrexate (n = 12), 15 mg/week, 5‐5‐5 at 12 hours, 13 weeks C. Methotrexate (n = 7), 7.5 mg/week, once a week, for 13 weeks D. Methotrexate (n = 10), 15 mg/week, once a week, 13 weeks |
|
| Outcomes | Assessment at 13 weeks Primary or secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes | Funding source: Czech Ministry of Education Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 247): "were randomly assigned" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 247): "were randomly assigned" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: probably open‐label trial, term "blind" not used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably open‐label trial, term "blind" not used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no description of the method used to manage the missing data. No ITT analyses mentioned |
| Selective reporting (reporting bias) | Unclear risk | Comment: no primary or secondary outcomes stated |
CIMPACT 2018.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: January 2015 to December 2016 Location: worldwide Phase 3 |
|
| Participants |
Randomised: 559 participants Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Certolizumab pegol (SC injection 400 mg at weeks 0, 2, 4, followed by certolizumab pegol 200 mg every 2 weeks from week 6 to week 14), n = 165 Control interventions B. Certolizumab pegol (SC injection 400 mg every 2 weeks through week 14), n = 167 C. Etanercept (SC injection 50 mg twice‐weekly through week 12), n = 170 D. Placebo, n = 57 |
|
| Outcomes |
At week 12 Primary outcome
Secondary outcomes
|
|
| Notes | Funding source : Quote (p 226): "Funding sources: Supported by Dermira Inc and UCB Inc. UCB is the regulatory sponsor of certolizumab pegol in psoriasis." Declarations of interest: Quote (p 226): "Dr Lebwohl is an employee of Mount Sinai which receives research funds from AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly, Incyte, Janssen/Johnson & Johnson, Leo Pharmaceutucals, Medimmune/Astra Zeneca, Novartis, Pfizer, Sciderm, UCB, Valeant, and ViDac; and is a consultant for Allergan, Aqua, Boehringer‐Ingelheim, LEO Pharma, Menlo, and Promius. Dr Blauvelt has received honoraria or fees for consulting, serving as a clinical investigator, and/or speaking for AbbVie, Aclaris, Allergan, Almirall, Amgen, Boehringer Ingelheim, Celgene, Dermavant, Dermira Inc, Eli Lilly and Company, Genentech/Roche, GSK, Janssen, LEO Pharma, Merck Sharp & Dohme, Novartis, Pfizer, Purdue Pharma, Regeneron, Sandoz, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma, UCB, Valeant, and Vidac. Dr Paul is a consultant and investigator for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Janssen/Johnson & Johnson, LEO Pharma, Novartis, Pierre Fabre, Pfizer, and Sanofi/Regeneron. Dr Sofen has received honoraria or fees for consulting, serving as a clinical investigator, and/or speaking for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira Inc, Janssen, Eli Lilly, Merck, Novartis, Pfizer, Sun Pharma, UCB, and Valeant. Dr Węgłowska is an investigator and/or speaker for Amgen, Celgene, Coherus, Dermira Inc, Eli Lilly and Company, Galderma, Janssen, LEO Pharma, Merck, Pfizer, Regeneron, Sandoz, and UCB. Dr Piguet has received honoraria or fees for consulting and/or speaking for AbbVie, Almirall, Celgene, Janssen, Novartis, and Pfizer; and has received departmental support for Cardiff University from AbbVie, Almirall, Alliance, Beiersdorf UK Ltd, Biotest, Celgene, Dermal, Eli Lilly, Galderma, Genus Pharma, GlobeMicro, Janssen‐Celag, LaRoche‐Posay, L'Oreal, LEO Pharma, Meda, MSD, Novartis, Pfizer, Sinclair Pharma, Spirit, Stiefel, Samumed, Thornton Ross, TyPham, and UCB. Dr Augustin has received honoraria or fees for consulting and/or speaking for clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly and Company, GSK, Hexal, Janssen‐Cilag, LEO Pharma, Medac, Merck, MSD, Mundipharma, Novartis, Pfizer, Sandoz, UCB BioSciences Inc, and Xenoport. Ms Drew and Dr Burge have received stock options from Dermira Inc. Mr Peterson owns stock in UCB Inc. Dr Rolleri has received stock options from UCB Inc." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 286): "Study drug kits were distributed based on the subject’s interactive voice web response system assigned randomization number; the randomization schedule was produced by an independent biostatistician." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 286): "Study drug kits were distributed based on the subject’s interactive voice web response system assigned randomization number; the randomization schedule was produced by an independent biostatistician." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 268): "Double‐blind CZP and placebo treatments were administered subcutaneously at the study site by study personnel not involved in any other study procedures; etanercept treatment was administered subcutaneously on‐site by unblinded study staff or self‐administered off‐site by the patient after sufficient training. To maintain the single‐blind for etanercept, efficacy assessments were performed by a designated blinded assessor not involved in any other study procedures during blinded study periods." Comment: participants not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 268): "Double‐blind CZP and placebo treatments were administered subcutaneously at the study site by study personnel not involved in any other study procedures; etanercept treatment was administered subcutaneously on‐site by unblinded study staff or self‐administered off‐site by the patient after sufficient training. To maintain the single‐blind for etanercept, efficacy assessments were performed by a designated blinded assessor not involved in any other study procedures during blinded study periods." Comment: assessment by a blinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (p 269): "Analyses were based on the randomized set (all randomized patients)... Imputation of missing data was performed using the Markov chain Monte Carlo method for multiple imputation during the initial period." Included population 559, Table 2 559 Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02346240). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
CIMPASI‐1 2018.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: December 2014 to October 2016 Location: worldwide Phase 3 |
|
| Participants |
Randomised: 234 participants Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Certolizumab pegol (400 mg at weeks 0, 2, 4, followed by certolizumab pegol 200 mg every 2 weeks from week 6 to week 14) (n = 95) Control intervention B. Certolizumab pegol (certolizumab pegol 400 mg every 2 weeks through week 14) (n = 88) C. Placebo (n = 51) |
|
| Outcomes |
At week 16 Primary composite outcome
Secondary outcomes
|
|
| Notes | Funding source Quote (p 302): "Supported by Dermira Inc and UCB Inc." Declarations of interest Quote (p 302): "Dr Gottlieb has consulted and/or received other fees from Janssen Inc, Celgene Corp, Bristol‐Myers Squibb Co, Beiersdorf Inc, AbbVie, UCB, Novartis, Incyte, Eli Lilly, Reddy Labs, Valeant, Dermira Inc, Allergan, and Sun Pharmaceutical Industries; and has received research or educational grants (paid to TuftsMedical Center) from Janssen Incyte, Lilly, Novartis, Allergan, and LEO Pharma. Dr Blauvelt has received honoraria or fees for consulting, being a clinical investigator, and/or speaker for AbbVie, Aclaris, Allergan, Almirall, Amgen, Boehringer Ingelheim, Celgene, Dermavant, Dermira Inc, Eli Lilly, Genentech/Roche, GlaxoSmith‐Kline, Janssen, LEO Pharma,Merck Sharp & Dohme, Novartis, Pfizer, Purdue Pharma, Regeneron, Sandoz, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma, UCB Pharma, Valeant, and Vidac. Dr Leonardi has received fees or honoraria for consulting, speaking, or serving on the advisory board for AbbVie, Actavis, Amgen, Boehringer Ingelheim Pharma, Celgene, Coherus, Corrona, Dermira Inc, Eli Lilly, Galderma, Glenmark, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Sandoz, Stiefel, UCB Pharma, Vitae, and Wyeth. Dr Poulin has received research grants as an investigator for AbbVie, Baxter, Boehringer Ingelheim Pharma, Celgene, Centocor/Janssen, Eli Lilly, EMD Serono, GlaxoSmithKline, LEO Pharma, MedImmune, Merck, Novartis, Pfizer, Regeneron, Takeda, and UCB Pharma; and has received honoraria speaking for AbbVie, Celgene, Janssen, Eli Lilly, LEO Pharma, Novartis, Regeneron, and Sanofi Genzyme. Dr Reich has received speaker’s fees or honoraria from and/or served on the advisory board for AbbVie, Amgen, Biogen, Boehringer Ingelheim Pharma, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, LEO Pharma, Eli Lilly, Medac, Merck Sharp & Dohme, Novartis, Ocean Pharma, Pfizer, Regeneron, Takeda, UCB Pharma, and Xenoport. Dr Thac¸has received research support from AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Dignity, Eli Lilly, Forward‐Pharma, GlaxoSmithKline, LEO Pharma, Janssen‐Cilag, Maruho, Merck Sharp & Dohme, Mitsubishi Pharma, Novartis, Pfizer, Roche, Regeneron, and Sandoz; received honoraria from AbbVie, Biogen, Celgene, Janssen, LEO Pharma, Pfizer, Roche‐Possay, Novartis, and Mundipharma; served as a consultant for AbbVie, Biogen, Celgene, Dignity, Galapagos, Maruho, Mitsubishi, Novartis, Pfizer, and Xenoport; and sat on the scientific advisory boards for AbbVie, Amgen, Biogen, Celgene, Eli Lilly, GlaxoSmithKline, LEO Pharma, Pfizer, Novartis, Janssen, Mundipharma, and Sandoz. Ms Drew and Dr Burge have received stock options fromDermira Inc. Mr Peterson owns stock in UCB Inc. Dr Arendt owns stock in and has received stock options from UCB Inc." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (pp. 303‐4): "CIMPASI‐1 (NCT02326298) and CIMPASI‐2 (NCT02326272) are ongoing, replicate, phase 3, randomized, double‐blinded, multicenter... At the baseline visit, an interactive voice web response system was used to assign patients to... according to the randomization schedule produced by an independent biostatistician (2:2:1, stratified by site)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (pp. 303‐4): "CIMPASI‐1 (NCT02326298) and CIMPASI‐2 (NCT02326272) are ongoing, replicate, phase 3, randomized, double‐blinded, multicenter... At the baseline visit, an interactive voice web response system was used to assign patients to... according to the randomization schedule produced by an independent biostatistician (2:2:1, stratified by site)." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (pp. 303‐4): "CIMPASI‐1 (NCT02326298) and CIMPASI‐2 (NCT02326272) are ongoing, replicate, phase 3, randomized, double‐blinded, multicenter... to assign patients to subcutaneous treatment with CZP 400 mg every 2 weeks, CZP 200 mg every 2 weeks (after loading dose of CZP 400 mg at weeks 0, 2, and 4), or placebo every 2 weeks until week 16 (initial treatment period)". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (pp. 303‐4): "CIMPASI‐1 (NCT02326298) and CIMPASI‐2 (NCT02326272) are ongoing, replicate, phase 3, randomized, double‐blinded, multicenter... to assign patients to subcutaneous treatment with CZP 400 mg every 2 weeks, CZP 200 mg every 2 weeks (after loading dose of CZP 400 mg at weeks 0, 2, and 4), or placebo every 2 weeks until week 16 (initial treatment period)". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 234 Management of missing data: Quote (p 308): "Efficacy analyses were performed on the randomized set (all randomized patients)... The Markov chain Monte Carlo method for multiple imputation was used to account for missing data." Table 2: 234 analysed participants Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02326298). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
CIMPASI‐2 2018.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: December 2014 to December 2016 Location: worldwide Phase 3 |
|
| Participants |
Randomised: 227 participants Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Certolizumab pegol (400 mg at weeks 0, 2, 4, followed by certolizumab pegol 200 mg every 2 weeks from week 6 to week 14) (n = 91) Control intervention B. Certolizumab pegol (certolizumab pegol 400 mg every 2 weeks through week 14) (n = 87) C. Placebo (n = 49) |
|
| Outcomes |
At week 16 Primary composite outcome
Secondary outcomes
|
|
| Notes | Funding source Quote (p 302): "Supported by Dermira Inc and UCB Inc." Declarations of interest Quote (p 302): "Dr Gottlieb has consulted and/or received other fees from Janssen Inc, Celgene Corp, Bristol‐Myers Squibb Co, Beiersdorf Inc, AbbVie, UCB, Novartis, Incyte, Eli Lilly, Reddy Labs, Valeant, Dermira Inc, Allergan, and Sun Pharmaceutical Industries; and has received research or educational grants (paid to TuftsMedical Center) from Janssen Incyte, Lilly, Novartis, Allergan, and LEO Pharma. Dr Blauvelt has received honoraria or fees for consulting, being a clinical investigator, and/or speaker for AbbVie, Aclaris, Allergan, Almirall, Amgen, Boehringer Ingelheim, Celgene, Dermavant, Dermira Inc, Eli Lilly, Genentech/Roche, GlaxoSmith‐Kline, Janssen, LEO Pharma,Merck Sharp & Dohme, Novartis, Pfizer, Purdue Pharma, Regeneron, Sandoz, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma, UCB Pharma, Valeant, and Vidac. Dr Leonardi has received fees or honoraria for consulting, speaking, or serving on the advisory board for AbbVie, Actavis, Amgen, Boehringer Ingelheim Pharma, Celgene, Coherus, Corrona, Dermira Inc, Eli Lilly, Galderma, Glenmark, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Sandoz, Stiefel, UCB Pharma, Vitae, and Wyeth. Dr Poulin has received research grants as an investigator for AbbVie, Baxter, Boehringer Ingelheim Pharma, Celgene, Centocor/Janssen, Eli Lilly, EMD Serono, GlaxoSmithKline, LEO Pharma, MedImmune, Merck, Novartis, Pfizer, Regeneron, Takeda, and UCB Pharma; and has received honoraria speaking for AbbVie, Celgene, Janssen, Eli Lilly, LEO Pharma, Novartis, Regeneron, and Sanofi Genzyme. Dr Reich has received speaker’s fees or honoraria from and/or served on the advisory board for AbbVie, Amgen, Biogen, Boehringer Ingelheim Pharma, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, LEO Pharma, Eli Lilly, Medac, Merck Sharp & Dohme, Novartis, Ocean Pharma, Pfizer, Regeneron, Takeda, UCB Pharma, and Xenoport. Dr Thac¸has received research support from AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Dignity, Eli Lilly, Forward‐Pharma, GlaxoSmithKline, LEO Pharma, Janssen‐Cilag, Maruho, Merck Sharp & Dohme, Mitsubishi Pharma, Novartis, Pfizer, Roche, Regeneron, and Sandoz; received honoraria from AbbVie, Biogen, Celgene, Janssen, LEO Pharma, Pfizer, Roche‐Possay, Novartis, and Mundipharma; served as a consultant for AbbVie, Biogen, Celgene, Dignity, Galapagos, Maruho, Mitsubishi, Novartis, Pfizer, and Xenoport; and sat on the scientific advisory boards for AbbVie, Amgen, Biogen, Celgene, Eli Lilly, GlaxoSmithKline, LEO Pharma, Pfizer, Novartis, Janssen, Mundipharma, and Sandoz. Ms Drew and Dr Burge have received stock options fromDermira Inc. Mr Peterson owns stock in UCB Inc. Dr Arendt owns stock in and has received stock options from UCB Inc." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (pp 303‐4): "CIMPASI‐1 (NCT02326298) and CIMPASI‐2 (NCT02326272) are ongoing, replicate, phase 3, randomized, double‐blinded, multicenter... At the baseline visit, an interactive voice web response system was used to assign patients to... according to the randomization schedule produced by an independent biostatistician (2:2:1, stratified by site)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (pp 303‐4): "CIMPASI‐1 (NCT02326298) and CIMPASI‐2 (NCT02326272) are ongoing, replicate, phase 3, randomized, double‐blinded, multicenter... At the baseline visit, an interactive voice web response system was used to assign patients to... according to the randomization schedule produced by an independent biostatistician (2:2:1, stratified by site)." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (pp 303‐4): "CIMPASI‐1 (NCT02326298) and CIMPASI‐2 (NCT02326272) are ongoing, replicate, phase 3, randomized, double‐blinded, multicenter... to assign patients to subcutaneous treatment with CZP 400 mg every 2 weeks, CZP 200 mg every 2 weeks (after loading dose of CZP 400 mg at weeks 0, 2, and 4), or placebo every 2 weeks until week 16 (initial treatment period)". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (pp 303‐4): "CIMPASI‐1 (NCT02326298) and CIMPASI‐2 (NCT02326272) are ongoing, replicate, phase 3, randomized, double‐blinded, multicenter... to assign patients to subcutaneous treatment with CZP 400 mg every 2 weeks, CZP 200 mg every 2 weeks (after loading dose of CZP 400 mg at weeks 0, 2, and 4), or placebo every 2 weeks until week 16 (initial treatment period)". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 227 Management of missing data: Quote (p 308): "Efficacy analyses were performed on the randomized set (all randomized patients)...The Markov chain Monte Carlo method for multiple imputation was used to account for missing data." Table 2: 227 analysed participants Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02326272). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
CLARITY 2018.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: July 2016 to July 2018 Location: worldwide Phase 3 |
|
| Participants |
Randomised: 1102 participants (mean age 46 years, 458 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Secukinumab 300 (300 mg, SC at randomisation, weeks 1, 2, and 3 and thereafter 4‐weekly until week 48), n = 550 Control intervention B. Ustekinumab 45/90 (45 mg or 90 mg SC based on participant's weight (at randomisation visit) to be administered at randomisation, week 4, 16, 28 and 40), n = 552 |
|
| Outcomes |
Assessment at week 12 Primary composite outcome
Secondary outcomes
|
|
| Notes | Funding source Quote (p 572): "Funding: Novartis Pharma AG, Basel, Switzerland." Declarations of interest: Quote (p 578): "Disclosures. Jerry Bagel is an investigator and/or consultant and/or speaker for AbbVie, Amgen, Boehringer‐Ingelheim, Janssen, Leo, Novartis, Celgene, Eli Lilly, Sun, and Valiant. Manmath Patekar is an employee of Novartis Pharma AG, Basel, Switzerland. Ana de Vera is an employee of Novartis Pharma AG, Basel, Switzerland. Sophie Hugot is an employee ofNovartis Pharma AG, Basel, Switzerland. Isabelle Gilloteau is an employee of Novartis Pharma AG, Basel, Switzerland. Elisa Muscianisi is an employee of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA. Kuan Sheng is an employee of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA. Summer Xia is an employee of Beijing Novartis Pharma Co. Ltd, Shanghai, China. Andrew Blauvelt has served as a scientific consultant and clinical study investigator for AbbVie, Aclaris, Akros, Allergan, Almirall, Amgen, Boehringer Ingelheim, Celgene, Dermavant, Dermira, Eli Lilly and Company, Galderma, Genentech/Roche, GlaxoSmithKline, Janssen, Leo, Meiji, Merck Sharp & Dohme, Novartis, Pfizer, Purdue Pharma, Regeneron, Revance, Sandoz, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma, UCB, Valeant, and Vidac and as a paid speaker for Janssen, Regeneron, and Sanofi Genzyme. Mark Lebwohl is an employee of Mount Sinai which receives research funds from AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly, Incyte, Janssen/Johnson & Johnson, Leo Pharmaceuticals, Medimmune/Astra Zeneca, Novartis, Pfizer, Sciderm, UCB, Valeant, and Vidac. Mark Lebwohl is also a consultant for Allergan, Aqua, Boehringer‐Ingelheim, LEO Pharma, Menlo, and Promius. John Nia and Peter W. Hashim have nothing to disclose." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 572): "CLARITY (NCT02826603) is a multicenter, randomized, double‐blinded, active‐controlled, parallel‐group, phase 3b trial. Eligible patients were randomized 1:1 to receive either..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 572): “CLARITY (NCT02826603) is a multicenter, randomized, double‐blinded, active‐controlled. . .” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 572): “CLARITY (NCT02826603) is a multicenter, randomized, double‐blinded, active‐controlled...” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned: 1102 Management of missing data: Quote (p 573): "Missing values were handled by multiple imputation except for DLQI 0/1, where missing values were handled using last observation carried forward." Table 2: 1101 analysed participants Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02826603). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
CLEAR 2015.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: 27 February 2014 to 11 May 2015 Location: 137 centres in Europe, Australia, and Asia |
|
| Participants |
Randomised: 676 participants (mean age 46 years, 481 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Secukinumab (n = 334), SC, 300 mg weeks 0, 1, 2, 3 then monthly Control intervention B. Ustekinumab (n = 335), SC, 45/90 mg weeks 0, 4 then every 12 weeks |
|
| Outcomes | Assessments at 16 weeks Primary outcome
Secondary outcomes
|
|
| Notes | Funding source: Quote (p 400): "Novartis Pharma supported this study". Declarations of interest (p 400): "Dr Thaçi has served as a consultant, served as an advisory board member, and/or received honoraria for lecturing for AbbVie, Amgen, Biogen‐Idec, Celgene, Eli Lilly, Janssen‐Cilag, Leo Pharma, MSD, Novartis, Pfizer, Regeneron, and Sanofi. Dr Blauvelt has served as a scientific consultant and clinical study investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen Ortho Biotech, Merck, Novartis, Pfizer, and Sandoz. Dr Reich has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis including AbbVie, Amgen, Biogen‐Idec, Celgene, Centocor, Covagen, Eli Lilly, Forward Pharma, GSK, Janssen‐Cilag, Leo Pharma, Medac, MSD, Novartis, Pfizer, Vertex, Takeda, and Xenoport..." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 402): “were randomised via an interactive response technology system". Randomisation was conducted via Interactive Response Technology, which assigned a randomisation number that linked the subject to a treatment arm and specified unique medication pack number. Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 402): “were randomised via an interactive response technology system.“ Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 402): “To maintain blinding, placebo injections matching the secukinumab regimen were given in the ustekinumab group”. Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 402): “To maintain blinding, placebo injections matching the secukinumab regimen were given in the ustekinumab group”. Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 676, analysed 669 Management of missing data: Quote (p 403): “Missing values with respect to response variables based on PASI and IGA mod 2011 scores were imputed as nonresponse (nonresponder imputation)." Comment: it was not an ITT analysis as 7 participants were not taken into account, but low rate of dropout. |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02074982). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Dogra 2012.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: August 2008 to September 2009 Location: Chandigarh, India |
|
| Participants |
Randomised: 60 participants (mean age 37 years, 48 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate (n = 30), orally, 10 mg/week, for 12 weeks Control intervention B. Methotrexate (n = 30), orally, 25 mg/week, for 12 weeks |
|
| Outcomes | Assessment at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes | Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 730): “The randomisation list was generated using a random number table, and the code was kept by an investigator who was not directly involved in the study”. Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 730): “The randomisation list was generated using a random number table, and the code was kept by an investigator who was not directly involved in the study. All tablets were supplied in sealed envelopes bearing the code for any particular patient according to the randomisation list”. Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (pp. 730‐1): “Double blind study, …, the 10 mg group was also given an oral placebo tablet in addition to the MTX to give an equal number of tablets in both groups. The placebo tablets were identical in appearance to the MTX tablets in colour, texture, size, shape and markings. All tablets were supplied in sealed envelopes bearing the code for any particular patient according to the randomisation list”. Comment: clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (pp. 730‐1): “Double blind study, …, the 10 mg group was also given an oral placebo tablet in addition to the MTX to give an equal number of tablets in both groups. The placebo tablets were identical in appearance to the MTX tablets in colour, texture, size, shape and markings. All tablets were supplied in sealed envelopes bearing the code for any particular patient according to the randomisation list”. Comment: clearly described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 60, analysed 51 Dropouts and withdrawals
Management of missing data: no ITT analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Dogra 2013.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: March 2008 to March 2009 Location: Chandigarh, India |
|
| Participants |
Randomised: 61 participants (mean age 37 years, 51 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Acitretin (n = 20), orally, 25 mg/day, for 12 weeks Control intervention B. Acitretin (n = 20), orally, 35 mg/day, for 12 weeks C. Acitretin (n = 21), orally, 50 mg/day, for 12 weeks |
|
| Outcomes | Assessment at 12 weeks Primary outcome
Secondary outcomes
|
|
| Notes | Funding source (quote e305): not stated Declarations of interest (quote e305): not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p e306): “Randomization list was generated using random number table and code was kept with a study coordinator who was not directly involved in assessment of endpoint”. Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p e306): “Randomization list was generated using random number table and code was kept with a study coordinator who was not directly involved in assessment of endpoint”. Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p e306): “double blind” Comment: no description of the method used to guarantee blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p e306): “double blind”, “Randomization list was generated using random number table and code was kept with a study coordinator who was not directly involved in assessment of endpoint”. Comment: no description of the method used to guarantee blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 61, analysed 48 Dropouts and withdrawals:
Not ITT analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Dubertret 1989.
| Study characteristics | ||
| Methods | RCT, active‐controlled study Date of study: July 1987 to January 1988 Location: Paris, France |
|
| Participants |
Randomised: 37 participants (mean age, sex ratio: not stated) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Cyclosporin (n = 18), orally, 2.5 mg/kg/d Control intervention B. Cyclosporin (n = 19), orally, 5 mg/kg/d |
|
| Outcomes | Time to assessment for the primary outcome: not stated Primary outcome
Secondary outcomes
|
|
| Notes | Funding source: not stated, but 1 out of 4 authors was a staff member of Sandoz pharmaceutical company Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 136): "The patients were randomised..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 136): "The patients were randomised..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not specified as blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not specified as blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 37, analysed 37 Dropouts and withdrawals: not stated Management of missing data: no description of the method used to guarantee management of missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
ECLIPSE 2019.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: April 2017 to September 2018 Location: worldwide (142 sites) Phase 3 |
|
| Participants |
Randomised: 1048 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 1048, mean age of 46 years and 67% men Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Guselkumab 100 mg (TREMFYA) SC injection plus placebo (one injection) at weeks 0, 4, 12, and every 8 weeks thereafter until week 44, n = 534 Control intervention B. Secukinumab 300 mg (COSENTYX) administered as two 150 mg SC injections at weeks 0, 1, 2, 3, and 4, and every 4 weeks thereafter until week 44, n = 514 |
|
| Outcomes |
At week 48 Primary outcome
Secondary outcomes
|
|
| Notes | Funding source: Quote (p. 831): "This study was funded by Janssen Research & Development." Declarations of interest: Quote (p. 838): "KR has served as an advisor and paid speaker and has participated in clinical trials for AbbVie, Affibody, Almirall, Amgen, Avillion, Biogen, Boehringer Ingelheim, Celgene, Covagen, Forward Pharma, Fresenius Medical Care, GlaxoSmithKline, Janssen, Janssen‐Cilag, Kyowa Kirin, LEO Pharma, Eli Lilly, Medac, Merck Sharp & Dohme, Novartis, Miltenyi Biotech, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB, Valeant, XBiotech, and Xenoport. AWA has served as a consultant, research investigator, speaker, or data safety board member for AbbVie, Boehringer Ingelheim/Parexel, Bristol‐Myers Squibb, Celgene, Dermavant, Dermira, Eli Lilly, Genentech, GlaxoSmithKline, Janssen, Janssen‐Ortho, Kyowa Hakko Kirin, LEO Pharma, Menlo Therapeutics, Merck, Modernizing Medicine, Novartis Pharmaceutical Corp, Ortho Dermatologics, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, Science 37, UCB Pharma, and Valeant. RGL has served as principle investigator, as a speaker, and on the scientific advisory board for and received compensation in the form of honoraria from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Janssen, LEO Pharma, Eli Lilly, Merck, Novartis, Pizer, Sun, and UCB Pharma. SF, BR, SL, M‐CH, and PB are all employees of Janssen Research & Development and own stock in Johnson & Johnson, of which Janssen is a subsidiary. AB has served as a scientific advisor or clinical study investigator for AbbVie, Aclaris, Allergan, Almirall, Amgen, Arena, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Dermavant, Dermira, Eli Lilly, FLX Bio, Galderma, Genentech/Roche, GlaxoSmithKline, Janssen, LEO Pharma, Meiji, Merck Sharp & Dohme, Novartis, Pfizer, Purdue Pharma, Regeneron, Revance, Sandoz, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma, UCB Pharma, Valeant, and Vidac, and as a paid speaker for Janssen, Regeneron, and Sanofi Genzyme." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p. 833): "Patients were randomly assigned (1:1) to receive either guselkumab or secukinumab. An outside vendor (Paraxel, Waltham, MA, USA) used an interactive web response system to randomly assign patients based on computer‐ generated permuted blocks." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p. 833): "An outside vendor (Paraxel, Waltham, MA, USA) used an interactive web response system to randomly assign patients based on computer‐generated permuted blocks." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p. 832, 833): "A phase 3, multicentre, randomised, double‐blind, comparator‐controlled study (ECLIPSE)... ." "Patients, investigators, and the funder of the study were masked throughout the 56‐week database lock, with the exception of the unmasked site personnel who dispensed or administered the study agent." Comment: unclear if the process guaranteed blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p. 832, 833): "A phase 3, multicentre, randomised, double‐blind, comparator‐controlled study (ECLIPSE)... ." "Patients, investigators, and the funder of the study were masked throughout the 56‐week database lock, with the exception of the unmasked site personnel who dispensed or administered the study agent." Comment: unsure that the process guaranteed the blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p. 834, 835): "For efficacy analyses, we included all patients according to the random treatment allocation (intention‐to‐treat [ITT] population), regardless of the treatment received... Patients with missing data were considered non‐ responders (non‐responder imputation)." Randomly assigned 1048, analysed 1048 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03090100). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
EGALITY 2017.
| Study characteristics | ||
| Methods | Randomised, active‐controlled, double‐blind study Date of study: June 2013 to March 2015 Location: 74 centres in 11 European countries and South Africa Phase 3 |
|
| Participants |
Total sample size: 531 Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. GP2015, n = 264 Control intervention B. Etanercept (Enbrel; Amgen Inc., Thousand Oaks, CA, USA; European Union authorised), n = 267 50 mg subcutaneous injection until week 12 |
|
| Outcomes |
Assessment at week 12 Primary outcome
Secondary outcomes
|
|
| Notes | Funding source: Quote (p 928): "The study was funded by Hexal AG, a Sandoz company. The funder had a role in the study design, data collection, data analysis and manuscript preparation." Declarations of interest Quote (appendix): "Dr Gerdes has been an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials of the following companies: Abbott/AbbVie, Almirall‐Hermal, Amgen, Bayer HealthCare, Biogen Idec, Bioskin, Boehringer‐Ingelheim, Celgene, Centocor, Dermira, Eli Lilly, Foamix, Forward Pharma, Galderma, Hexal AG, Isotechnika, Janssen‐Cilag, Leo Pharma, Medac, Merck Serono, Mitsubishi Tanabe, MSD, Novartis, Pfizer, Sandoz Biopharmaceuticals, Schering‐Plough, Takeda, Teva, UCB Pharma, VBL therapeutics and Wyeth Pharma. Professor Thaci has received research support from Abbvie, Almiral, Amgen, Astellas, Biogen‐Idec, Boehringer‐ Ingelheim, Celgene, Dignity, Elli‐Lilly, Forward‐Pharma, GlaxoSmithKline, Leo, Janssen‐Cilag, Maruho, MSD, Mitsubishi Pharma, Novartis, Pfizer, Roche and Sandoz and honoraria from AbbVie, Biogen‐Idec, Celgene, Janssen, Leo, Mundipharma, Novartis, Pfizer and Roche‐Possay. Professor Thaci has acted as a consultant for Abbvie, Biogen‐Idec, Celgene, Dignity, Galapagos, Maruho, Mitsubishi, Novartis, Pfizer and Xenoport and been part of scientific advisory boards for AbbVie, Amgen, Biogen‐Idec, Celgene, Eli‐Lilly, GlaxoSmithKline, Janssen, Leo‐Pharma, Mundipharma, Novartis, Pfizer and Sandoz. Professor Griffiths has received consultancy/honoraria and/or research funding from Abbvie, Galderma, Janssen, LEO‐Pharma, Lilly, MSD, Novartis, Pfizer, Regeneron, Roche, Sandoz, Sun Pharmaceuticals and UCB Pharma. Professor Arenberger has received grants from Novartis. J Poetzl and H Woehling are employees of Hexal AG. G Wuerth and M Afonso were employees of Hexal AG at the time of the study." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p. 929‐Supplemental Appendix): "EGALITY was a multicentre, randomized, double‐blind, confirmatory efficacy and safety study conducted... In treatment period 1, patients were randomized 1:1 to self‐administer50 mg GP2015 or 50 mg ETN."; "During treatment period 1, patients were randomised via the Interactive Response Technology (IRT) that assigned a unique patient identification number in the IRT system with the treatment arm to which the patient had been assigned. Randomisation was stratified by body weight (< 90 kg; ≥ 90 kg) and prior therapy (no prior systemic therapy, any prior systemic therapy including biologic immunomodulating agents, or prior treatment with a tumour necrosis factor [TNF antagonist])." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p. 929‐Supplemental Appendix): "EGALITY was a multicentre, randomized, double‐blind, confirmatory efficacy and safety study conducted... In treatment period 1, patients were randomized 1:1 to self‐administer50 mg GP2015 or 50 mg ETN."; "During treatment period 1, patients were randomised via the Interactive Response Technology (IRT) that assigned a unique patient identification number in the IRT system with the treatment arm to which the patient had been assigned. Randomisation was stratified by body weight (< 90 kg; ≥ 90 kg) and prior therapy (no prior systemic therapy, any prior systemic therapy including biologic immunomodulating agents, or prior treatment with a tumour necrosis factor [TNF antagonist])". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p. 929): "EGALITY was a multicentre, randomized, double‐blind, confirmatory efficacy and safety study conducted... In treatment period 1, patients were randomized 1:1 to self‐administer 50 mg GP2015 or 50 mg ETN." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p. 929): "EGALITY was a multicentre, randomized, double‐blind, confirmatory efficacy and safety study conducted..In treatment period 1, patients were randomized 1:1 to self‐administer 50 mg GP2015 or 50 mg ETN." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 531 Management of missing data: Quote (Supplemental appendix): "The FAS during treatment period 1 included all randomised patients to whom the study treatment was assigned. For the primary endpoint analysis based on the FAS missing values with respect to the PASI response at week 12 were included as non‐responders regardless of the reason for missing data." Equivalence trial: Quote (p. 931): "The primary efficacy analysis was based on the per protocol set (PPS), which consisted of all patients who completed the study until week 12 without major protocol deviations... The analysis was repeated on the full analysis set (FAS) following the intent‐to‐treat principle as a sensitivity analysis." Table 1: both per‐protocol and full‐set analyses Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01891864). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results posted on ClinicalTrials.gov. |
Elewski 2016.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, double‐blind study Date of study: January 2014 to April 2016 Location: worldwide |
|
| Participants |
Randomised: 217 participants Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Adalimumab, SC, 40 mg, eow for 25 weeks starting 1 week after initial loading dose of 80 mg, n = 109 Control intervention B. Placebo, n = 108 |
|
| Outcomes |
At week 12 mNAPSI 75, PGA of fingernails of clear or minimal PASI 75/90/100 for participants with baseline PASI at 5 |
|
| Notes | Funding source: Quote (p 90): "AbbVie funded this study and participated in the study design; study research; collection, analysis and interpretation of data; and writing, review, and approval of this article. All authors had access to the data and participated in the development, review, and approval of this article and in the decision to submit it for publication." Declarations of interest Quote (p 90): "Dr Elewski has received research funding (paid to her institution) from AbbVie, Amgen, Boehinger Ingelheim, Celgene, Incyte, Lilly, Merck, Novan, Novartis, Pfizer, Valeant, and Viament and honoraria for serving as a consultant to Anacor, Celgene, Lilly, Novartis, Pfizer, and Valeant. Dr Okun has received honoraria for serving on an advisory board and/or as a speaker for AbbVie, Crescendo Biosciences, Gilead Science, and UCB, and he is a former AbbVie employee. Dr Papp has received honoraria for serving on an advisory board or panel, serving as a consultant and speaker for and has received grants (as an investigator) from Allergan, Amgen, Celgene, Centocor, Eli Lilly, Galderma, Genentech, Janssen, LEO Pharma, Merck, Merck‐Serono, Novartis, Pfizer, Schering Plough, and Wyeth. In addition, Dr Papp has received honoraria (as a consultant) and grants (as an investigator) from Astellas, Apotex, Baxter, Boehringer Ingelheim, Kyowa Kirin, Regeneron, and UCB; received honoraria (for serving on an advisory board and panel) from AbbVie, Apotex, Baxter, Boehringer Ingelheim, and UCB; received honoraria (as a consultant) from AbbVie and Bristol‐Myers Squibb; received honoraria (as a speaker) from AbbVie, Astellas, and Janssen‐Cilag; and received grants (as an investigator) from Bristol‐Myers Squibb and GlaxoSmithKline Beecham. Mr Baker has received honoraria (for serving on an advisory board and panel) from Abbvie, Pfizer, Novartis, and Celgene. Dr Crowley has received honoraria (as a consultant and speaker) from AbbVie, Amgen, Celgene, Lilly, and Novartis and has received grants (as an investigator) from AbbVie, Amgen, Astra‐Zeneca, Boehringer Ingelheim, Celgene, Janssen, Lilly, Maruho, Merck, Novartis, Pfizer, Regeneron, and Sandoz. Dr Guillet has received grants (as an investigator) from AbbVie. Dr Sudaram is a former AbbVie employee. Dr Poulin has received grants (as an investigator) and honoraria (as a speaker and for serving on advisory boards) from AbbVie, Amgen, and Centocor/Janssen‐Ortho and has received grants (as an investigator) from Aquinox, Baxter, Boehringer Ingelheim, Bristol‐Myers‐Squibb, Celgene, DS Biopharma, Eli Lilly, Galderma, Genentech, GlaxoSmithKline Beecham, LEO Pharma, Medimmune, Merck, Novartis, Pfizer, Regeneron, Schering Plough, Serono, Takeda, and UCB Pharma. Ms Gu, Dr Geng, and Dr Williams are salaried employees of AbbVie and they receive stocks and stock options. Dr Rich has received honoraria (for serving on an advisory board) from AbbVie, Eli Lilly, Novartis, Sandoz, and Valeant; honoraria (as a consultant) from AbbVie, Novartis, Polichem, and Valeant; and grants (as an investigator) from AbbVie, Allergan, Amgen, Anacor, Cassiopea, Dusa, Eli Lilly, Galderma, Janssen, Leo, Meiji, Merck, Neothetics, Novartis, Pfizer, Psolar, Sandoz, Ranbaxy, and Viamet." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (pp. 91‐2): "This was a phase 3, multicenter, double‐blind, randomized, parallel‐arm, placebo‐controlled trial...Randomization was determined by an interactive voice/web response system." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (pp. 91‐2): "This was a phase 3, multicenter, double‐blind, randomized, parallel‐arm, placebo‐controlled trial...Randomization was determined by an interactive voice/web response system." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (pp. 91‐2): "This was a phase 3, multicenter, double‐blind, randomized, parallel‐arm, placebo‐controlled trial... The investigator, study site, and patients remained blinded to treatment." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (pp. 91‐2): "This was a phase 3, multicenter, double‐blind, randomized, parallel‐arm, placebo‐controlled trial... The investigator, study site, and patients remained blinded to treatment." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 217 Management of missing data: Quote (p 90): "The primary efficacy analysis was performed for the period A intent‐to‐treat population. The primary analysis and ranked secondary end points were tested in ranked order to control multiplicity, and missing data were handled by multiple imputation for all end points." Table 2: 217 analysed participants Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02016482). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results posted on ClinicalTrials.gov. |
Ellis 1991.
| Study characteristics | ||
| Methods | RCT, active, controlled, double‐blind study Date of study: not stated Location: single‐centre (University of Michigan Medical Center, Ann Arbor, USA) |
|
| Participants |
Randomised: 85 participants (mean age 46 years (cyclosporin 3), 42 years (cyclosporin 5), 46 years (cyclosporin 7.5), 43 years (placebo), 66 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ciclosporin (Sandimmun) (n = 15), orally, 7.5 mg/kg, 8 weeks Control intervention B. Ciclosporin (Sandimmun) (n = 20), orally, 5 mg/kg, 8 weeks C. Ciclosporin (Sandimmun) (n = 25), orally, 3 mg/kg, 8 weeks D. Vehicle (Sandimmun oral olive oil) (n = 25), orally, 8 weeks |
|
| Outcomes | Assessment at 8 weeks Primary or secondary outcomes not stated Outcomes
|
|
| Notes | Funding source (p 277): Sandoz Research Institute, the Babcock Dermatologic Endowment (Ann Arbor) and a clinical research centre grant (M01‐RR‐00042) from the National Institutes of Health Declarations of interest: not stated (p 277): "Drs Ellis and Voorhees are consultants to Sandoz Pharmaceuticals corporation (the manufacturer of cyclosporine)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 278): "patients were assigned numbers in consecutive order; each number had been preassigned to one of four treatments groups by means of a computer generated random code in blocks 17". Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 278): "The preparation of cyclosporine and vehicle were identical …patients were blinded to their treatment". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 278): "Other physicians who were blinded to group assignment and laboratory findings evaluated the patient". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 85, analysed not stated Dropouts and withdrawals Not stated Quote (p 279): "In the primary, intention‐to‐treat analysis" Management of missing data: no description of the method used to guarantee management of missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Engst 1994.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: not stated Location: not stated |
|
| Participants |
Randomised: 22 participants (mean age 45.9 years, 18 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ciclosporin A (n = 10), orally, 1.25 mg/kg/d (increase to 2.5 if PASI > 50% of initial PASI), 12 months Control intervention B. Ciclosporin A, (n = 12), orally, 2.5 mg/kg/d (increase to 5 if PASI > 50% of initial PASI), 12 months |
|
| Outcomes | Assessment period: not stated but longer than 16 weeks Primary or secondary outcomes of the trial: not stated Outcomes of the trial
|
|
| Notes | Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 189): "Patients enrolled in the study were randomised..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 189): "Patients enrolled in the study were randomised..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded (open‐label) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not blinded (open‐label) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Dropouts and withdrawals
Management of missing data: no description of the method used to guarantee management of missing data, ITT analyses not mentioned |
| Selective reporting (reporting bias) | High risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section were not reported in Results section. |
ERASURE 2014.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: June 2011 to April 2013 Location: 88 centres worldwide (Erasure) |
|
| Participants |
Randomised: 738 participants mean age 45 years, 509 male Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Secukinumab 300 (n = 245), SC, 300 mg, weeks 0, 1, 2, 3, 4, and every 4 weeks, 12 weeks Control intervention B. Secukinumab 150 (n = 245), SC, 150 mg, weeks 0, 1, 2, 3, 4, and every 4 weeks, 12 weeks C. Placebo (n = 248), SC, weeks 0, 1, 2, 3, 4, and every 4 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes | Funding source, quote (p 326): "funded by Novartis Pharmaceuticals" Declarations of interest (p 337): "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org." Langley received personal fees from Eli Lilly, Leo, Novartis, Janssen, Amgen, AbbVie, Celgene, Merck, Pfizer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (protocol and Appendix): "Randomization numbers were generated by the Interactive Response Technology (IRT) provider using a validated system, which automated the random assignment of subject numbers to randomisation numbers..." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (protocol and Appendix): "Randomization numbers were generated by the Interactive Response Technology (IRT) provider using a validated system, which automated the random assignment of subject numbers to randomisation numbers..." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (protocol and Appendix): “Subjects, investigator staff, persons performing the assessments, and data analysts were blinded to the identity of the treatment from the time of randomisation until primary objective analyses". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (protocol and Appendix): "Subjects, investigator staff, persons performing the assessments, and data analysts were blinded to the identity of the treatment from the time of randomisation until primary objective analyses". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 738 included/738 analysed Quote (p 329): "The analyses of the efficacy end points included all the patients who underwent randomisation according to the treatment assigned at randomisation... Missing values ... were conservatively imputed as nonresponses, regardless the reason of missing data". Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01365455). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
ESTEEM‐1 2015.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: September 2010 to December 2012 Location: 72 centres in the USA, Canada, Australia, Belgium, France, UK, Italy, Germany |
|
| Participants |
Randomised: 844 participants (apremilast (562) mean age 46 years, 379 male; placebo (282) mean age 47 years, 194 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Apremilast (n = 562), orally, 30 mg, twice a day, 16 weeks Control intervention B. Placebo (n = 282), orally, twice a day, 16 weeks |
|
| Outcomes | Assessments at 16 weeks Primary outcomes
Secondary outcomes
|
|
| Notes | Funding source: quote (p 37): "This study was sponsored by Celgene Corporation". Declarations of interest: quote (p 48): "Dr Papp has served as an investigator for Abbott (AbbVie), Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, Centocor, Galderma, Genentech, Incyte, Isotechnika, Janssen, LEO Pharma, Lilly, MedImmune, Merck Sharp & Dohme, Merck‐Serono, Novartis, Pfizer, Stiefel, and Wyeth; a consultant for Abbott, Amgen, Astellas, Biogen Idec, Boehringer Ingelheim, BMS, Celgene, Centocor, Forward Pharma, Galderma, Genentech, Incyte, Isotechnika, Janssen, Johnson &Johnson, Kyowa Kirin, LEO Pharma, Lilly, MedImmune, Merck Sharp & Dohme, Merck‐Serono, Novartis, Pfizer, Takeda Pharmaceuticals, UCB, and Wyeth; and a speaker for Abbott, Amgen, Astellas, Celgene, Centocor, Isotechnika, Janssen, Novartis, and Pfizer. Dr Reich has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis including AbbVie, Amgen, Biogen Idec, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, LEO Pharma, Lilly, Medac, Merck Sharp & Dohme, Novartis, Pfizer, Takeda, and Vertex. Dr Leonardi has served on the advisory board and as an investigator and/or speaker for Abbott, Amgen, Celgene,Centocor, Galderma, Genentech, GlaxoSmithKline, Lilly, Novartis, Novo Nordisk, Pfizer, Sirtris, Stiefel, Vascular Biogenics, and Wyeth." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 38): "ESTEEM 1 was... multicenter, randomised, double‐blind, placebo controlled study". Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 38): "ESTEEM 1 was... multicenter, randomised, double‐blind, placebo controlled study". Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 38): "ESTEEM 1 was... multicenter, randomised, double‐blind, placebo controlled study... Blinding was maintained until all patients discontinued or completed the week 52 visit". Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (pp. 38‐9): "ESTEEM 1 was.. multicenter, randomised, double‐blind, placebo controlled study... Blinding was maintained until all patients discontinued or completed the week 52 visit". Comment: probably done, placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 844 included/844 analysed Quote (p 39): "Efficacy data were assessed for the full analysis set (all randomised patients)... Missing data were handled with the last‐observation‐carried‐forward methodology". Comment: done |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01194219). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported, except the number of participants with a psoriasis flare or rebound during placebo‐controlled phase. |
ESTEEM‐2 2015.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: October 2012 to March 2016 Location: 40 centres in Europe and USA |
|
| Participants |
Randomised: 413 participants (mean age 45 years, 276 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Apremilast (n = 275), orally, 30 mg twice a day until week 32 Control intervention B. Placebo (n = 138), orally (same drug administration) |
|
| Outcomes |
Assessments at 16 weeks Primary outcomes
Secondary outcomes
|
|
| Notes | Funding source: Quote (p 1387): "This study was sponsored by Celgene Corporation". Declarations of interest (Appendix): "C.P. has served as an investigator and consultant for AbbVie, Amgen, Celgene, Eli Lilly, Janssen, LEO Pharma, Novartis and Pfizer. J. Cather has been an investigator for Amgen, Celgene, Galderma, Merck, Novartis and Pfizer, and has served on advisory boards for AbbVie, Janssen, OrthoBiotech and Medac. M.G. has been an investigator for AbbVie, Allergan, Celgene, Dermira, Dr. Reddy’s Laboratories, Eli Lilly, Galderma, Janssen Pharmaceutical, Kythera, Kyowa Hakko Kirin Pharma, LEO Pharma, Merck, Novartis, Pfizer, Regeneron and Takeda, and has served as a speaker for AbbVie, Actelion, Amgen, Astellas, Galderma, Janssen Pharmaceutical, LEO Pharma, Novartis and Pfizer. Y.P. has been an investigator for AbbVie, Amgen, Astellas, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Centocor/Janssen, Eli Lilly, Galderma, Isotechnika, LEO Pharma, Merck, Novartis, Pfizer, Pharmascience, Regeneron, Schering and Stiefel/GSK, and has served as a speaker for AbbVie, Amgen, Galderma, Janssen, LEO Pharma and Novartis. U.M. has been an advisor for and/or received speaker honoraria from and/or received grants from and/or participated in clinical trials for Abbott/AbbVie, Almirall‐Hermal, Amgen, BASF, Biogen Idec, Celgene, Centocor, Eli Lilly, Forward Pharma, Galderma, Janssen, LEO Pharma, Medac, MSD, Miltenyi Biotech, Novartis, Pfizer, Teva, VBL and XenoPort. C.F. has served on the advisory board for and/or received speaker honoraria from Celgene, Novartis, Janssen and AbbVie. J. Crowley has been an investigator for AbbVie, Amgen, AstraZeneca, Celgene, Janssen, Maruho, Merck, Pfizer and Regeneron; has served on the advisory board for AbbVie, Amgen, Celgene and Lilly; and has been a speaker for AbbVie and Amgen." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 1388): "Patient were randomised (2:1) via an interactive voice response system..." Comment: no description of the method used to guarantee the random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote (p 1388): "Patient were randomised (2:1) via an interactive voice response system..." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1388): "identically matching placebo tablets twice daily during the placebo controlled phase" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1388): "double‐blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 413, analysed 411 Management of missing data: Quote (pp. 1389‐90): "Efficacy assessments were conducted for the modified intention‐to‐treat population... The last‐observation‐carried‐forward methodology was used...." Comment: we judged this as a low risk of bias. |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00235820). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
EXPRESS 2005.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: not stated Location: 32 centres in Europe and Canada |
|
| Participants |
Randomised: 378 participants (mean age 43 years, 268 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals (week 24)
|
|
| Interventions |
Intervention A. Infliximab (n = 301), IV, 5 mg/kg weeks 0, 2, 6, and every 8 weeks, 10 weeks Control intervention B. Placebo (n = 77), IV, equivalent, weeks 0, 2, 6, and every 8 weeks, 10 weeks |
|
| Outcomes | Assessments at 10 weeks Primary outcomes
Secondary outcomes
|
|
| Notes | Funding source (p 386): "This study was funded by Centocor, and Schering‐Plough, Kenilworth, NJ, USA". Declarations of interest (p 386): "Consultancies: Dr Reich (Abbott, Biogen Idec, Centocor, Schring‐Plough, Essex, Serano, Wyeth), Dr Nestle (Biogen Idec, Centocor, Schring‐Plough, Genentech, Galderma)..." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1368): "An adaptative treatment allocation was used... The treatment assignment was stored electronically and the stored data were used to allocate future patients in such a way that the imbalance between treatment groups was kept to a minimum". “Randomization was conducted via Interactive Response Technology, which assigned a randomisation number that linked the subject to a treatment arm and specified unique medication pack number". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 1368): "An adaptative treatment allocation was used... The treatment assignment was stored electronically and the stored data were used to allocate future patients in such a way that the imbalance between treatment groups was kept to a minimum". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1368): "The investigators, study site personnel, and patients remained blinded until the database lock at week 50... placebo group". Comment: probably done, placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1368): "The investigators, study site personnel, and patients remained blinded until the database lock at week 50... placebo group". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 378 included/378 analysed Quote (p 1368): "The primary endpoint ... as well as... were analysed on an intention‐to‐treat basis... In patients who discontinued the study agent ... the patients were regarded as not achieving the endpoints for binary responses". Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
EXPRESS‐II 2007.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: not stated Location: 63 centres in Europe, USA, Canada |
|
| Participants |
Randomised: 835 participants (mean age 44 years, 551 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Infliximab (n = 313), IV, 3 mg/kg, weeks 0, 2, 6; 10 weeks Control intervention B. Infliximab (n = 314), IV, 5 mg/kg, weeks 0, 2, 6; 10 weeks C. Placebo (n = 208), IV, weeks 0, 2, 6; 10 weeks |
|
| Outcomes | Assessments at 10 weeks Primary outcomes
Secondary outcomes
|
|
| Notes | Funding source (p 31. e1) by Centocor, Inc, Malvern, Penn, and Schering‐Plough, Kenilworth, NJ Declarations of interest (appendix): "Dr Menter has received consulting, research, and/or speaking support from Abbott Laboratories, Allergan Inc, Allermed, Amgen Inc, Astralis Inc, Berlex Inc, Biogen Idec Inc, Centocor Inc, Cephalon, Collagenex Pharmaceuticals, CombinatoRx, Connetics Corp, Corixa Corporation, Dermik Laboratories, Doak Dermatologics, Dow, Ferndale Laboratories Inc, Fujisawa Healthcare Inc, Galderma, Genentech Inc, Genzyme, GlaxoSmithKline, Ligand Pharmaceuticals, Medicis, Med‐Immune Inc, Novartis Pharmaceuticals, Otsuka Pharmaceutical Inc, Protein Design Labs, QLT USA, Regeneration Pharma AG, Roche Laboratories, Serono, Sinclair, Synta Pharma, Thermosurgery, 3M Pharmaceuticals, Vertex, XOMA, and Zars Inc. Dr Feldman has received consulting, research, and/or speaking support from Amgen, Centocor, and Biogen. Dr Papp's support is as follows: Abbott: Investigator, Consultant; Amgen: Investigator, Consultant, Speaker, Advisory Boards; Centocor: Investigator, Consultant, Speaker, Senior Medical Officer for Canada (non‐compensatory), Advisory Boards; Genentech: Investigator, Consultant, Speaker, Senior Medical Officer for Canada (non‐compensatory), Advisory Boards; Serono: Investigator, Consultant, Speaker, Advisory Boards; Schering: Investigatory, Consultant, Speaker, Advisory Boards; and Wyeth: Speaker, Advisory Boards. Dr Weinstein has received consulting, research, and/or speaking support from Allergan, Amgen, Centocor, Biogen, Genentech, Valeant, Collagenex, CombinatoRx, Fujisawa, Abbott, and QLT. Dr Gottlieb has received research support from and/or is a consultant and/or speaker for Amgen, Inc, BiogenIdec, Inc, Centocor, Inc, Genentech, Inc, Abbott Labs, Ligand Pharmaceuticals, Inc, Beiersdorf, Inc, Fujisawa Healthcare, Inc, Celgene Corp, Bristol Myers Squibb, Inc, Warner Chilcott, Paradigm, Wyeth Pharmaceuticals, Schering‐Plough Corp, Eisai, Roche, Sankyo, Medarex, Kemia, Celera, TEVA, Actelion, and Amarill. At the time of the study, Dr Gottlieb was affiliated with the Clinical Research Center, University of Medicine and Dentistry of New Jersey, Robert Wood Johnson Medical School, New Brunswick, NJ. Dr Guzzo, Dr Dooley, Ms Li, and Ms Arnold are employees of Centocor, Inc. Mr Evans was an employee of Centocor, Inc at the time this study was conducted and is currently an employee of Scios, Inc." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 31; e2): "Randomizations were performed by ClinPhone (Lawrenceville, NJ), allocating patients using a minimization algorithm with a biased coin assignment by means of an interactive voice response system". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 31; e2): "Randomizations were performed by ClinPhone (Lawrenceville, NJ), allocating patients using a minimization algorithm with a biased coin assignment by means of an interactive voice response system". "Patients, investigators, and all study staff except pharmacists were blinded to treatment assignments". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 31. e2): "Patients, investigators, and all study staff except pharmacists were blinded to treatment assignments... to receive IFX 3 mg/kg or 5mg/kg or placebo". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 31. e2): "Patients, investigators, and all study staff except pharmacists were blinded to treatment assignments... to receive IFX 3 mg/kg or 5mg/kg or placebo". Comment: placebo‐controlled, probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 835 included/835 analysed Quote (p 31.e3/4): "For patients who discontinued... these patients were considered as not meeting the respective end‐points regardless of the observed data". Comment: ITT |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Fallah Arani 2011.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: October 2006 to February 2009 Location: Rotterdam/Eindhoven, Netherlands |
|
| Participants |
Randomised: 60 participants (mean age 41 years (methotrexate) and 43 years (fumarate), 36 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate (n = 30), orally, 15 mg/week, Weinstein schema 15 mg weekly in 3 equal doses of 5 mg each 12 hours apart, 16 weeks Control intervention B. Fumarate (n = 30), orally, 720 mg, 30 mg followed by 120 mg and max 720 mg after week 9, 16 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcome
Secondary outcomes
|
|
| Notes | Funding source (p 855): none Declarations of interest (p 855): "none declared" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 856): “patients were randomly assigned ... randomisation was performed centrally according to a computer‐generated randomisation list”. Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 856): “Only the research nurse, who had no contact with the patients before randomisation had insight into the allocation schedule”. Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 856): “could not be blinded because treatment intake differed in both groups” Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 857): “by the same trained assessors (one trained physician and a research nurse in consensus in each site)” Comment: not specified whether "trained assessors" were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 60, analysed 51 Management of missing data: Quote (p 857): “Analysis was by intention‐to‐treat...” Comment: ITT analysis not performed |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
FEATURE 2015.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: May 2012 to January 2013 Location: 32 centres in the USA/Germany/France/Estonia/India/Switzerland |
|
| Participants |
Randomised: 177 participants (mean age 45 years (secukinumab 300 mg), 46 years (secukinumab 150 mg), 47 years (placebo), 117 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Secukinumab (n = 59), SC, 300 mg, weeks 1, 2, 3, 4, 8, 12 B. Secukinumab (n = 59), SC, 150 mg, weeks 1, 2, 3, 4, 8, 12 Control intervention C. Placebo (n = 59), SC, weeks 1, 2, 3, 4, 8, 12 |
|
| Outcomes | Assessment at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes | Funding source: Novartis Pharmaceuticals, Basel, Switzerland Declarations of interest (quote p 484): "A.B. has served as a scientific consultant and clinical study investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer and Sandoz. J.C.P. has served as a consultant, investigator, speaker or advisory board member for Abbott, Biogen‐Idec (formerly Biogen), Centocor, Essex Pharma, Galderma, Janssen‐Cilag/Janssen‐Ortho, Merck‐Serono (formerly Serono), MSD, Novartis, Pfizer and Wyeth, and has received unrestricted research grants from Biogen‐Idec and Wyeth. A.B.G. has served as scientific consultant and/or clinical study investigator for Abbott, Abbvie, Actelion, Akros Pharma, Amgen, Astellas Pharma, Beiersdorf, BMS, Canfite, Celgene, Coronado BioSciences, CSL Behring, GSK, Immune Control, Incyte, Janssen‐Ortho, Lerner Medical Devices, Lilly ICOS, Merck, Novartis, Novo Nordisk, Pfizer, Teva, UCB, Vertex Pharmaceuticals and Xenoport. K.K. has served as a study investigator for Celgene, Hexal, Mitsubishi and Novartis. H.S. has served as a study investigator, consultant and speaker for Novartis. M.R.‐M. has served as a study investigator for Novartis. V.S., R.P., C.P. and S.C. are full‐time employees of Novartis. C.P. and S.C. own stock in Novartis". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 486): “were randomised via interactive response technology to one of the treatment arms...using a validate system that automated the random assignment of subject numbers to randomisation numbers”. Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 486): “were randomised via interactive response technology to one of the treatment arms...using a validate system that automated the random assignment of subject numbers to randomisation numbers”. Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 486): “Subjects, study management team, investigator staff, persons performing the assessments and data analysts were blinded...” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 486): “Subjects, study management team, investigator staff, persons performing the assessments and data analysts were blinded...” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 177, analysed 177 Dropouts and withdrawals
Management of missing data: Quote (supplemental appendix): "Missing values were imputed as non‐response for all efficacy analyses regardless of the reason of missing data". Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01555125). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Feldman 2021.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind Date of study: February 2019 to July 2020 Location: Poland, Estonia, Georgia, Ukraine (20 sites) Phase 3 |
|
| Participants |
Randomised: 413 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 413, mean age of 43.5 years and 61.5% men Dropouts and withdrawals 11/413 (2%): AVT02 group (4), Humira group (7)
|
|
| Interventions |
Intervention A. AVT02 (adalimumab biosimilar) 80 mg (2 × 40 mg) administered subcutaneously (SC), followed by 40 mg once every other week (eow), n = 205 Control intervention B. Humira 80 mg (2 × 40 mg) administered SC, followed by 40 mg eow, n = 207 |
|
| Outcomes |
Assessment at week 16 Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source Quote (p 747): "The study was sponsored by Alvotech Swiss AG. Employees of the sponsor had a role in study design, data analysis and manuscript preparation. Employees of the funder had no role in data collection." Declarations of interest Quote (p 747): "FB, JS, RD, EG, HO, HNH and HS are employees at Alvotech. SF has received research grants from Abbvie, Janssen, Lilly and Novartis and speaker honoraria from Alvotech, Abbvie, Amgen, Lilly, Novartis and Janssen. RK's company has received consultancy fees in relation to this study and in other studies conducted by Alvotech, but no consultancy fees have been received in relation to the writing of this manuscript. NR, GP, KK, and GG declare that they have no conficts of interest that might be relevant to this work." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 737): "Randomisation was performed by an Interactive Voice/Web Response System (IXRS) and subjects were randomised in a 1:1 ratio to receive either AVT02 or originator adalimumab in accordance with the randomisation schedule generated using permuted block randomisation. The randomisation code was prepared by a statistician not otherwise involved in the conduct of the study." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 737):"Randomisation was performed by an Interactive Voice/Web Response System (IXRS) and subjects were randomised in a 1:1 ratio to receive either AVT02 or originator adalimumab in accordance with the randomisation schedule generated using permuted block randomisation. The randomisation code was prepared by a statistician not otherwise involved in the conduct of the study...Subjects, investigators, site staff and the sponsor study team, inclusive of contract research organisation personnel, were unaware of treatment assignment until study closure and database lock" Comment: no description of the method used to guarantee random sequence generation but probably done owing central randomisation process |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 737): "Subjects, investigators, site staff and the sponsor study team, inclusive of contract research organisation personnel, were unaware of treatment assignment until study closure and database lock. The prefilled syringes (PFS) containing either AVT02 or originator adalimumab were masked by packaging. The double blind was maintained by each syringe being surrounded by a masking device. Subjects returned used syringes including the masking device and, while not all masking devices could be confirmed intact, no reports were received to the contrary" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 737): "Subjects, investigators, site staff and the sponsor study team, inclusive of contract research organisation personnel, were unaware of treatment assignment until study closure and database lock. The pre‐filled syringes (PFS) containing either AVT02 or originator adalimumab were masked by packaging. The double blind was maintained by each syringe being surrounded by a masking device. Subjects returned used syringes including the masking device and, while not all masking devices could be confirmed intact, no reports were received to the contrary" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 738 ): "Missing percent improvement was imputed using the last observation carry forward (LOCF) method for subjects with post‐baseline assessment in Stage 1." Quote (p 738): "The primary analysis was performed using the Full Analysis Set (FAS), which, consistent with the intention‐to‐treat principles, is defined as all randomised patients who received at least one dose of randomised study medication. For safety and pharmacokinetic endpoints, the Safety Analysis Set (SAF), defined as all patients who received at least one dose of the investigational product with treatment assignment based on actual treatment received, was analysed based on the actual treatment received." Randomised 413; analysed 402 Comment: for PASI 50, 75, 90: the analysis was done for participants who completed Stage 1 not ITT analysis (supplemental p4). 11 participants did not receive at least 1 dose, 413 participants should be involved in the mITT, but 402 participants were analysed; however, impact on results is unlikely for 11/413 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03849404). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are not posted on ClinicalTrials.gov. |
FIXTURE 2014.
| Study characteristics | ||
| Methods | RCT, active, placebo‐controlled, double‐blind study Date of study: June 2011 to June 2013 Location: 231 centres worldwide (Fixture) |
|
| Participants |
Randomised: 1306 participants, mean age 44 years, 929 male Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Secukinumab 300 (n = 327), SC, 300 mg, weeks 0, 1, 2, 3, 4, and every 4 weeks, 12 weeks Control intervention B. Secukinumab 150 (n = 327), SC, 150 mg, weeks 0, 1, 2, 3, 4, and every 4 weeks, 12 weeks C. Etanercept 50 (n = 326), SC, 50 mg/week twice a week, 12 weeks D. Placebo (n = 326), SC, weeks 0, 1, 2, 3, 4, and every 4 weeks, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes | Funding source, quote (p 326): "funded by Novartis Pharmaceuticals" Declarations of interest (p 337): "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org." Langley received personal fees from Eli Lilly, Leo, Novartis, Janssen, Amgen, AbbVie, Celgene, Merck, Pfizer." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (protocol and Appendix): "Randomization numbers were generated by the Interactive Response Technology (IRT) provider using a validated system, which automated the random assignment of subject numbers to randomisation numbers..." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (protocol and Appendix): “Subjects, investigator staff, persons performing the assessments, and data analysts were blinded to the identity of the treatment from the time of randomisation until primary objective analyses". "Randomization numbers were generated by the Interactive Response Technology (IRT) provider". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (protocol and Appendix): “Subjects, investigator staff, persons performing the assessments, and data analysts were blinded to the identity of the treatment from the time of randomisation until primary objective analyses". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (protocol and Appendix): “Subjects, investigator staff, persons performing the assessments, and data analysts were blinded to the identity of the treatment from the time of randomisation until primary objective analyses." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (p 329): "The analyses of the efficacy end points included all the patients who underwent randomisation according to the treatment assigned at randomisation... Missing values ... were conservatively imputed as nonresponses, regardless the reason of missing data". 1306 included/1306 analysed Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01358578). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Flytström 2008.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: February 2002 to February 2005 Location: multicentre (n = 5), Sweden |
|
| Participants |
Randomised: 84 participants (mean age: 48 years (methotrexate), 46 years (ciclosporin); 55 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate + folic acid (n = 41), orally, 7.5 mg/kg/week (5 mg folic acid except days of methotrexate), 12 weeks Control intervention B. Ciclosporin (n = 43), orally, 3 mg/kg, divided into 2 doses, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcome
Secondary outcomes
|
|
| Notes | Funding source (p 121): "Financial support from the Swedish Psoriasis Association and the Welander foundation" Declarations of interest (p 116): "none declared" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 117): "Randomization was performed with the use of computer‐generated random numbers, numbers by calling a central telephone number". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 117): "Randomization was performed with the use of computer‐generated random numbers, numbers by calling a central telephone number". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 117): "Blinded assessors performed the PASI at baseline and monthly thereafter". Comment: no description of method used to guarantee no communication between caregivers or participants and assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 84, analysed 68 Management of missing data: not ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Gisondi 2008.
| Study characteristics | ||
| Methods | RCT, active‐controlled, investigator‐blinded pilot study Date of study: February 2002 to February 2005 Location: Verona, Italy |
|
| Participants |
Randomised: 60 participants (mean age 55 years (acitretin); 55 years (etanercept), 53 years (acitretin + etanercept), 33 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept (25 mg) and acitretin (0.4 mg/kg) (n = 18), SC (etanercept) and orally (acitretin), twice a week (etanercept) and once a day (acitretin), 24 weeks Control intervention B. Acitretin (n = 20), orally, 0.4 mg/kg, once a day, 24 weeks C. Etanercept (n = 22), SC, 25 mg, twice a week, 24 weeks |
|
| Outcomes | Assessments at 24 weeks Primary outcome
Secondary outcomes
|
|
| Notes | Funding source: not stated Declarations of interest (p 1345): "PG has received lecture fees from Merck‐Serono, Schering‐Plough, Wyeth. GG has received consultation and lecture fees from Abbott, Janssen‐Cilag, Merck‐Serono, Schering‐Plough, Wyeth." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1346): "Randomization was performed with the use of computer‐generated random numbers and block size of four patients". Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 1346): "Randomization was performed with the use of computer‐generated random numbers and block size of four patients". Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 1346): "The PASI assessor was blinded concerning the group allocation of the patient". Comment: acitretin provided visible AEs. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 60, analysed 60 Management of missing data, Quote (p 1346): "An ITT analysis was performed". Comment: no description of the method used to manage the missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Goldfarb 1988.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: not stated Location: not stated |
|
| Participants |
Randomised: 38 participants (mean age 45 to 48 years, 31 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Acitretin (n = 10), orally, 10 to 25 mg/day, 8 weeks B. Acitretin (n = 16), orally, 50 to 75 mg/day, 8 weeks Control intervention C. Placebo (n = 12), orally, daily, 8 weeks |
|
| Outcomes | Assessments at 8 weeks Primary outcomes
Outcomes
|
|
| Notes | Funding source, quote (p 655): "Supported in part by Hoffman‐La Roche Inc., Nutley, NJ, and the Babcock Dermatologic Endowment" Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 656): "21 patients were randomly and equally divided into 4 groups". Comment: no description of the method used to generate the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 656): "21 patients were randomly and equally divided into 4 groups". Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 656): "we have studied 38 patients in a double‐blind fashion". Comment: visible side effect of acitretin |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 656): "we have studied 38 patients in a double‐blind fashion". Comment: visible side effect of acitretin |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 38, analysed 38 No mention of how the missing data were managed |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Gordon 2006.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: March 2003 to June 2004 Location: multicentre (n = 18), in the USA, Canada |
|
| Participants |
Randomised: 148 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 148, mean age 44 years, 99 male Dropouts and withdrawals 8/148 (5%)
|
|
| Interventions |
Intervention A. Adalimumab (n = 46), SC, 40 mg, 12 weeks, week 0: 2 injections, 1 injection eow B. Adalimumab, (n = 50), SC, 40 mg, 12 weeks, week 0, week 1: 2 injections, 1 injection weekly Control intervention C. Placebo (n = 52), SC, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 598): "Supported by Abbott Laboratories" Declarations of interest: Quote (p 598): "Dr Gordon has received research support and honoraria and is a consultant for Abbott. Dr Langley is an investigator and has received research funding to conduct research studies with Abbott. Dr Leonardi is a consultant and speaker for Abbott. Dr Menter has received honoraria and is a consultant for Abbott. Dr Kang is an ad‐hoc consultant for Abbott. Dr Heffernan is a consultant for and has received research funding from Abbott. Drs Zhong, Hoffman, and Okun and Ms Lim are full‐time employees of Abbott." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 599): "Patients were centrally randomised..." Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 599): "Patients were centrally randomised..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 599): "To maintain blinding, prefilled syringes were identically labelled and all patients received the same number of injections at the same time points". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 599): "To maintain blinding, prefilled syringes were identically labelled and all patients received the same number of injections at the same time points". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 148, analysed 147 Dropouts and withdrawals
Management of missing data, Quote (p 601): "modified intent‐to‐treat analysis... a patient with missing data was counted as a nonresponder at that visit". Comment: few lost to follow‐up, well‐balanced numbers and reasons between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Gordon X‐PLORE 2015.
| Study characteristics | ||
| Methods | RCT, active placebo‐controlled, double‐blind study Date of study: October 2011 to August 2013 Location: multicentre (n = 31), Europe and North America Phase 2 |
|
| Participants |
Randomised: 293 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 293, mean age 47 years, 207 male Dropouts and withdrawals 20/293 (6.8%):
|
|
| Interventions |
Intervention A. Guselkumab (n = 41), SC, 5 mg weeks 0, 4, 16 Control intervention B. Guselkumab (n = 41), SC, 15 mg weeks 0, 4, 16 C. Guselkumab(n = 42), SC, 50 mg weeks 0, 4, 16 D. Guselkumab (n = 42), SC, 100 mg weeks 0, 4, 16 E. Guselkumab (n = 42), SC, 200 mg weeks 0, 4, 16 F. Adalimumab (n = 43), SC, 40 mg 2 injections week 0, 1 injection week 1, 1 injection eow G Placebo (n = 42), SC (100 mg weeks 0, 4, 16) |
|
| Outcomes | Assessments at 16 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 137): “This study was sponsored by Janssen Research and Development. Janssen supplied the study agents and collected and analysed the data. All the authors had full access to the data”. Declarations of interest (p 144): "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org." Gordon received grants and personal fees from Abbvie, Amgen, Celgene, Eli Lilly, Novartis; and personal fees from Pfizer and Medac. Reich received personal fees from Celgene, Centocor/Janssen, Forward Pharma, GSK, Janssen Cilag, LEO Pharma, Lilly Medoc, MSD, Novartis, Ocean Pharma, Pfizer, Regeneron, Takeda, Vertex." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 137): “patients were randomised…” Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 137): “patients were randomised…” Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 137, p 143): “double‐blind… Adalimumab was not administered in a blinded, placebo‐controlled manner”, “Another potential issue was to use of a blinded efficacy evaluator at each site instead of the administration of ADA in a blinded manner”. Quote (p 553‐4): "Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor ), Patients and study personnel were masked to treatment assignment: the study drug packaging was labelled.... " Comment: adalimumab group was not double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 137): “to ensure objectivity, all efficacy assessment were performed by an evaluator at each study site who was unaware of the study group”. Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 293, analysed 293 Dropouts and withdrawals
Management of missing data: Quote (p 138): “Patients with missing PGA or PASI score at week 16 were categorized as not having had a response”. Comment: low number of withdrawals, balanced numbers and reasons between groups |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01483599). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Gottlieb 2003a.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: August 2000 to January 2001 Location: multicentre (locations not specified) |
|
| Participants |
Randomised: 112 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 112, mean age 47 years, 70 male Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept (n = 57), SC, auto‐administered, 25 mg twice a week, 24 weeks Control intervention B. Placebo (n = 55), SC, auto‐administered, twice a week, 24 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcome
Secondary outcomes At 4, 8, 12, 24 weeks
|
|
| Notes |
Funding source: Quote (p 1631): "This study was sponsored by Immunex Corp, a subsidiary of Amgem, Inc.)." Declarations of interest: not stated except "Dr Zitnik is an employee of Amgen" (p 1627). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1628): "Patients ... were to be randomised in block of 6 with equal allocation between the treatment group...Patients were assigned numbers based on randomisation tables verified by Immunex Pharmaceutical Planning". Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 1628): "Patients ... were to be randomised in block of 6 with equal allocation between the treatment group...Patients were assigned numbers based on randomisation tables verified by Immunex Pharmaceutical Planning, after which the Immunex Clinical Distribution Department shaped blind‐labelled vials of study drug to the pharmacies". Comment: we do not know whether the investigators were blinded or the numbers of participants per block. This was probably a centralised randomisation, but this was not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1628): "... performed blinded labelling and packaging of the study drug. ... multicenter, randomised, double‐blind" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1628): "... performed blinded labelling and packaging of the study drug. ... multicenter, randomised, double‐blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 112, 112 participants analysed for the primary endpoint Dropouts and withdrawals
Management of missing data: Quote (p 1628): "Patients were analysed on an intent‐to‐treat basis... If a patient discontinued treatment before the end of the study, the last observation was carried forward for efficacy analyses". Comment: high rate of withdrawal in placebo group and imbalanced reasons for withdrawal |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Gottlieb 2004a.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: 2001 to 2003 Location: 24 centres in USA |
|
| Participants |
Randomised: 249 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 249, mean age 44 years, 174 male Dropouts and withdrawals after a 30‐week study period 85/249 (34%)
|
|
| Interventions |
Intervention A. Infliximab (n = 99), IV, 3 mg/kg, weeks 0, 2, 6, for 10 weeks Control intervention B. Infliximab (n = 99), IV, 5 mg/kg, weeks 0, 2, 6, for 10 weeks C. Placebo (n = 51), IV, equivalent, weeks 0, 2, 6, for 10 weeks |
|
| Outcomes | Assessments at 10 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 534): "Supported by Centocor Inc" Declarations of interest: (p 534): "Drs Gottlieb and Menter have received research support from and served as consultants for Centocor Inc. Drs Baker, Bala, Dooley, Evans, Guzzo, and Marano, and Ms Li, are employees of Centocor Inc." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 535): "Randomisation was carried out using adaptive treatment allocation and was stratified by the investigational site". Comment: no description of the method used to generate random sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 535): "Randomisation was carried out using adaptive treatment allocation and was stratified by the investigational site". Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 535): "Patients and investigators were unaware of treatment assignments. Double blind was achieved and maintained by using an independent pharmacist or staff member to prepare all study infusion". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 535): "Patients and investigators were unaware of treatment assignments. Double blind was achieved and maintained by using an independent pharmacist or staff member to prepare all study infusion". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 249 randomised, 249 analysed Methods for dealing with missing data: Quote (p 536): "All randomised patients were included in the efficacy analysis at week 10... Patients who discontinued... were considered to have not achieved the dichotomous end points or were assigned the baseline value for continuous end points after the event occurrence". Comment: done |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Gottlieb 2011.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: June 2008 to March 2009 Location: 33 centres in the USA Phase 3 |
|
| Participants |
Randomised: 209 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 209, mean age 43.5 years, 145 male Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept (n = 141), SC, auto‐administered, 50 mg twice a week, 11 weeks Control intervention B. Placebo (n = 68), SC, auto‐administered, twice a week |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes At 4, 8, 12 weeks
|
|
| Notes |
Funding source: Quote (Appendix 1): "Abbott Laboratories funded this study and participated in the study design, data collection, data management, data analysis and preparation of the manuscript. All of the authors had full access to the data and were involved in the analysis of data, development and revision of the manuscript, and decision to submit the manuscript for publication. The corresponding author takes responsibility for the integrity of the data and the accuracy of the data analysis...)" Declarations of interest: Quote (Appendix 1): "A.B.G. has been a consultant or served on an advisory board for Amgen, Centocor, Celgene, Bristol Myers Squibb, Beiersdorf, Abbott, TEVA, Actelion, UCB, Novo Nordisk, Immune Control, DermiPsor, Incyte, PureTech, Magen Biosciences, Cytokine Pharmasciences, Alnylam, Ono, Pfizer, Schering, Canfite, Schering, UCB, BIND Biosciences and Merck, and has received research/educational grants (paid to Tufts Medical Center) from Centocor, Amgen, Immune Control, Abbott, Novo Nordisk, UCB and Novartis. C.L. has been an investigator for Abbott, Allergan, Altana, Alza, Amgen, Astellas, Celgene, Centocor, Genentech, Bristol Myers, Eli Lilly, Galderma, Genzyme, Pfizer, Incyte, CombinatoRx, 3M Pharmaceuticals, Perrigo Israel Pharmaceutical, ScheringPlough, RTL, Novartis, Vitae and Wyeth; has served on an advisory board and has been a speaker for Abbott, Amgen and Centocor; and has been a consultant for Abbott, Amgen, Centocor and Pfizer. F.K. has been an investigator for Abbott, Centocor, Amgen, Wyeth, Novartis and Merck; and has served on an advisory board and has been a speaker for Abbott, Centocor, Amgen, Eisai, Astellas and Wyeth. S.M. has been an investigator for Abbott, Amgen, Celgene, Centocor, Graceway and Novo Nordisk; and has been a speaker for Abbott. M.O. and D.A.W. are employees of Abbott." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 653): "Patients were randomised..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 653): "Patients were randomised" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 653): “Patients enrolled in the placebo arm received SC injections matching active treatment to maintain the blind. To maintain the blind, all patients received two SC injections at weeks 0 and 4 and one SC injection at week 8, consisting of either briakinumab or matching placebo, depending on the treatment arm. In addition, each patient also received two SC injections biweekly, 3 days apart, week 0 through week 11, consisting of either etanercept or matching placebo, depending on the treatment arm.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 653): “Patients enrolled in the placebo arm received SC injections matching active treatment to maintain the blind. To maintain the blind, all patients received two SC injections at weeks 0 and 4 and one SC injection at week 8, consisting of either briakinumab or matching placebo, depending on the treatment arm. In addition, each patient also received two SC injections biweekly, 3 days apart, week 0 through week 11, consisting of either etanercept or matching placebo, depending on the treatment arm.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 209, analysed 209 Management of missing data: Quote (p 654): “The primary efficacy analysis consisted of four comparisons performed in the intent‐to‐treat population (i.e. all randomised patients), …, Nonresponder imputation was used to handle missing data.” Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00691964). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Gottlieb 2012.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: November 2010 to December 2011 Location: multicentre in Boston, USA Phase 3 |
|
| Participants |
Randomised: 478 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 478, mean age 44 years, 320 male Dropouts and withdrawals 61/478 (12.8%)
|
|
| Interventions |
Intervention A. Methotrexate (n = 239), orally, 15 mg/week 7.5 mg to 10 mg to a maximum of 15 mg, 24 weeks + etanercept, SC, 50 mg x 2/weeks, S1‐S12 and 50 mg/week, S12‐S24, 24 weeks Control intervention B. Placebo (n = 239), orally, 24 weeks + etanercept, SC, 50 mg x 2/weeks, S1‐S12 and 50 mg/week, S12‐S24, 24 weeks |
|
| Outcomes | Assessments at 24 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 649): "This study was funded by Immunex Corporation, a wholly owned subsidiary of Amgen Inc, and by Wyeth, which was acquired by Pfizer..." Declarations of interest: (Appendix): "A.B.G. is a consultant and/or advisory board member for Abbott, Actelion, Amgen, Astellas, Beiersdorf, Bristol‐Myers Squibb, Can‐Fite, Celgene, Centocor (Janssen), Dermipsor, Incyte, Lilly, Merck, Novartis, Novo Nordisk, Pfizer, TEVA, and UCB and is a recipient of research/educational grants paid to Tufts Medical Center by Abbott, Amgen, Celgene, Centocor (Janssen), Immune Control, Novartis, Novo Nordisk, Pfizer, and UCB. R.G.L. has served as an investigator, on the scientific advisory board, and speaker for Abbott, Amgen, Centocor, and Pfizer, and as an advisor and investigator for Celgene, Novartis, and Johnson & Johnson. B.E.S. has served as an advisor, consultant, investigator, and speaker for Abbott, Amgen, and Centocor, and as an advisor, consultant, and investigator for Celgene, Novartis, Maruho, and Pfizer. K.A.P. has been a consultant, advisory board member, and investigator for Abbott, Amgen, Celgene, Centocor, Janssen‐Ortho, MedImmune, Merck, Pfizer, Schering‐Plough, and Wyeth (Wyeth was acquired by Pfizer in October 2009); has consulted for Astellas and UCB; and has served as a speaker for Abbott, Amgen, Celgene, Janssen‐Ortho, Pfizer, Schering‐Plough, and Wyeth. P.K., K.C., E.H.Z.T., M.H., and G.K. are employees and stockholders of Amgen Inc." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 650): "This was a randomised..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 650): "This was a randomised...study" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 650): “double‐blinded placebo‐controlled” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 650): “double‐blinded placebo‐controlled” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 478, analysed 478 Management of missing data: Quote (p 651): “Efficacy analyses were performed using the ITT set (all randomised patients)... Missing post‐baseline data were imputed using last observation carried forward for primary analyses of all efficacy endpoints...” Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01001208). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Gurel 2015.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, single‐blind study Date of study: not stated Location: one centre, Turkey |
|
| Participants |
Randomised: 50 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 50, mean age 43 years, 25 male Dropouts No participants lost to follow‐up |
|
| Interventions |
Intervention A. Acitretin (0.3 to 0.5 mg/kg/day, 25 mg) (n = 25) Control intervention B. Placebo (n = 25) Co‐intervention NBUVB |
|
| Outcomes | Assessment at 12 weeks Primary outcome
Outcomes:
|
|
| Notes |
Funding source: none Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 3): "The physicians were not blinded". Comment: high risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 3): "An independent assessor who is not from the team performed the outcome assessment." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomised 50, analysed 50, no loss to follow‐up during the 12 weeks Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Heydendael 2003.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: October 1998 to June 2000 Location: multicentre (> 1) in Amsterdam/the Netherlands |
|
| Participants |
Randomised: 88 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 88, mean age 40 years, 57 male Dropouts and withdrawals 3/88 (3.4%)
|
|
| Interventions |
Intervention A. Methotrexate (n = 44), orally, 15 mg/week until 4 weeks then increase up to 22.5 mg if reduction from baseline PASI < 25%, 3 divided doses with 12‐h interval, 12 weeks Control intervention B. Ciclosporin (n = 44), orally, 3 mg/kg until 4 weeks then increase up to 5 mg/kg if reduction from baseline PASI < 25%, 2 divided doses, 12 weeks |
|
| Outcomes | Assessments at weeks 16 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 664): "Supported by a grant (OG 97‐009) from the Dutch Health Authorities" Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 660): "Randomisation was performed centrally with the use of computer‐generated random numbers and block size of eight patients". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 660): "Randomisation was performed centrally with the use of computer‐generated random numbers and block size of eight patients". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 660): "The score of the PASI ... was determined... by trained assessors who were unaware of the treatment assignment". Comment: no description of method used to guarantee no communication between caregivers or participants and assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 88 randomised, 85 analysed Quote (pp 660‐1): "If a patient missed a visit, we used the score from the previous visit". Comment: few lost to follow‐up, well‐balanced numbers and reasons between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Hunter 1963.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: not stated Location: 1 centre in London, UK |
|
| Participants |
Randomised: 41 participants (no description of the study population) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate (n = 19), orally, 2.5 mg every day for 1 week and 1 week after Control intervention B. Placebo (n = 17), orally, every day for 1 week and 1 week after |
|
| Outcomes | Assessments not clearly stated (reported at 4 weeks) Primary outcomes
Outcomes
|
|
| Notes |
Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee random sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (pp 1 and 2): “Control tablet of identical appearance... thus neither physician, patient nor pharmacist was aware whether drug or control had been dispensed”. Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (pp 1 and 2): “Control tablet of identical appearance... thus neither physician, patient nor pharmacist was aware whether drug or control had been dispensed”. Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 41 randomised participants and 38 analysed Comment: no description of the method used to manage missing data No ITT analyses |
| Selective reporting (reporting bias) | High risk | No prespecified outcomes mentioned in the Methods section |
Igarashi 2012.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: March 2008 to March 2010 Location: 35 centres in Japan |
|
| Participants |
Randomised: 160 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 160, age median 45 years, 126 male Dropouts and withdrawals 10/160 (6.2%)
|
|
| Interventions |
Intervention A. Ustekinumab (n = 64), SC, 45 mg, weeks 0 to 4, every 12 weeks, 64 weeks Control intervention B. Ustekinumab (n = 62), SC, 90 mg, weeks 0 to 4, every 12 weeks, 64 weeks C. Placebo (n = 32), SC, weeks 0 to 4, every 12 weeks, 64 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 242): "This study was supported by Janssen pharmaceutical KK, a part of the Johnson & Johnson family of companies." Declarations of interest: Quote (p 242): "Igarashi has served as a consultant and speaker for Janssen Pharmaceutical K.K.; H. Nakagawa has served as a consultant for Abbott Japan and Tanabe Mitsubishi, and as a consultant and speaker for Janssen Pharmaceutical K.K.; M. Song is an employee of Centocor Research & Development, Inc., a division of Johnson & Johnson Pharmaceutical Research & Development, L.L.C., and owns stock in Johnson & Johnson; T. Kato and M. Kato are employees of Janssen Pharmaceutical K.K. and own stock in Johnson & Johnson." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 244): “randomised” Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 244): “randomised” Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 243): “double‐blind placebo‐control” Comment: used a placebo without visible side effects |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 243): “double‐blind placebo‐control” Comment: used a placebo without visible side effects |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 160 randomised, 157 analysed (2 did not receive a dose of the drug and 1 was excluded in the placebo group due to lack of efficacy data after receiving a single dose) Methods for dealing with missing data Quote (p 244): “Efficacy analyses were based on all randomised patients with efficacy data after randomisation... Patients who discontinued the study... were considered as treatment failures”. Comment: few lost at follow‐up, well‐balanced numbers and reasons between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Ikonomidis 2017.
| Study characteristics | ||
| Methods | RCT, active‐controlled, single‐blinded study Date of study: January 2013 (still ongoing) Location: 1 centre, Athens, Greece Phase 4 |
|
| Participants |
Randomised: 150 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 150, age median 51 years, 93 male Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ustekinumab 45 mg, SC, at baseline and at 4 and 16 weeks (n = 50) Control interventions B. Etanercept 50 mg SC, 2 days a week for 16 weeks (n = 50) C. Cyclosporine 2.5 to 3 mg/kg daily (n = 50) for 16 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 12): "This study was supported by a grant from the Hellenic Cardiology Society and Hellenic Society of Lipidiology and Atherosclerosis. This study was not funded by any pharmaceutical company and that none of the coauthors received support from the manufacturers of the agents used for treatment". Declarations of interest: Quote (p 12): "none" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 5) "Patients were randomized to receive ... Randomization was performed by an attending dermatologist (E.P.) using a table of random numbers as reproduced from the online randomization software http://www.graphpad.com/quickcalcs/ index.cfm." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 5) "Patients were randomized to receive ... Randomization was performed by an attending dermatologist (E.P.) using a table of random numbers as reproduced from the online randomization software http://www.graphpad.com/quickcalcs/ index.cfm." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 5): "Studies were performed using a Vivid 7 (GE Medical Systems, Horten, Norway) ultrasound system. All studies were digitally stored in a computerized station (Echopac 201; GE Medical Systems, Horten, Norway) and were analyzed by 2 observers, blinded to clinical and laboratory data." Comment: participants not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 5): "Studies were performed using a Vivid 7 (GE Medical Systems, Horten, Norway) ultrasound system. All studies were digitally stored in a computerized station (Echopac 201; GE Medical Systems, Horten, Norway) and were analyzed by 2 observers, blinded to clinical and laboratory data." Comment: participants not blinded. Physicians were blinded for cardiac outcomes, but not for PASI evaluation, so rated high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote (p 6): "All analyses were intention to treat." No statement on amount of missing data and how authors dealt with it |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02144857). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are not posted on ClinicalTrials.gov. |
Ikonomidis 2022.
| Study characteristics | ||
| Methods | RCT, active‐controlled, single‐blinded study Date of study: January 2013 (still ongoing) Location: 1 centre, Athens, Greece Phase 4 |
|
| Participants |
Randomised: 150 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 150, age median 51 years, 60% male Dropouts and withdrawals 0/150: apremilast (0), etanercept (0), cyclosporine (0) |
|
| Interventions |
Intervention A. Apremilast 30 mg twice‐daily, n = 50 Control interventions B. Etanercept 50 mg SC, 2 days a week for 16 weeks n = 50 C. Cyclosporine 2.5 to 3 mg/kg daily for 16 weeks n = 50 |
|
| Outcomes | Assessments at 16 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 12): "This research received no external funding." Declarations of interest: Quote (p 12): "The authors declare no conflict of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 9): "150 patients were randomized to receive apremilast 30 mg twice daily, after an initial 5‐day titration period (n = 50), anti‐tumor necrosis factor‐α, namely etanercept 50 mg subcutaneous, two days per week (n = 50), or cyclosporine 2.5–3 mg/Kg daily (n = 50) for 4 months. According to the standard of care of patients with moderate to severe plaque psoriasis, biologic treatments, such as anti‐TNF‐α agents, and oral medications, including cyclosporine, would be used to start therapy, as previously published [1,20]. Randomization was performed by an attending dermatologist (E.P.) using a random number table as reproduced from the online randomization software http://www.graphpad.com/quickcalcs/index.cfm (accessed on 6 April 2020)." Comment: probably done |
| Allocation concealment (selection bias) | High risk | Quote (p 9):" Randomization was performed by an attending dermatologist (E.P.) using a random number table as reproduced from the online randomization software http://www.graphpad.com/quickcalcs/index.cfm (accessed on 6 April 2020)." Comment: no indication that any measure to guarantee allocation concealment was undertaken |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 9):"Finally, our study was a single‐center trial and not blinded to patients." Quote (p 10): "Echocardiography studies were performed using a Vivid E95 (GE Medical Systems, Horten, Norway) ultrasound system. All studies were digitally stored in a computerized station (EchoPac GE 203) and were analyzed by two investigators (I.I. and G.P.), blinded for clinical and laboratory data." Comment: participants not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 9): "Finally, our study was a single‐center trial and not blinded to patients." Quote (p 10): "Echocardiography studies were performed using a Vivid E95 (GE Medical Systems, Horten, Norway) ultrasound system. All studies were digitally stored in a computerized station (EchoPac GE 203) and were analyzed by two investigators (I.I. and G.P.), blinded for clinical and laboratory data." Comment: participants not blinded. Physicians were blinded for cardiac outcomes, but not for PASI evaluation, so rated high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote (p 11): "We applied intention‐to‐treat analysis." Randomised 150, analysed 150 Comment: no statement on amount of missing data and how authors dealt with it |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02144857). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are not posted on ClinicalTrials.gov. |
IMMerge 2021.
| Study characteristics | ||
| Methods | RCT, active‐controlled, single‐blinded study (outcomes assessor) Date of study: March 2018 to March 2020 Location: worldwide (64 sites) Phase 3 |
|
| Participants |
Randomised: 327 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 327, mean age of 47 years and 65% men Dropouts and withdrawals 46/327 (14%): risankizumab group (15), secukinumab group (31)
|
|
| Interventions |
Intervention A. Risankizumab (2 SC injections of 75 mg (150 mg total) at weeks 0 and 4, and every 12 weeks thereafter until the last dose at week 40, except for participants in France, who received additional doses at weeks 52 and 64 to allow for continuous treatment until it was commercially available for patients in France), n = 164 Control intervention B. Secukinumab (2 SC injections of 150 mg (300 mg total) at weeks 0, 1, 2, 3, and 4, and every 4 weeks thereafter until the last dose at week 48), n = 163 |
|
| Outcomes |
At week 16 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source Quote (p 1): "AbbVie Inc. funded this study, and participated inthe study design, research, analysis, data collection, interpretation of data, reviewing and approval ofthe publication. All authors had access to the data and participated in the development, review, critique and approval of the manuscript throughoutthe editorial process, and approved the final manuscript draft submitted for publication. All authors agree to be accountable for all aspects of the work, ensuring the accuracy and integrity of the publication. Medical writing support was paid for by AbbVie)." Declarations of interest Quote (appendix 1): "R.B.W. has received research grants from and leads clinical trials for AbbVie, Almirall, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, LEO Pharma, Novartis, Pfizer and UCB Pharma; and has received consulting fees from AbbVie, Almirall, Amgen, Arena Pharmaceuticals, Avillion, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Janssen, LEO Pharma, Eli, Lilly, Novartis, Pfizer, Sanofi and UCB Pharma. A.B. has served as a scientific adviser and/or clinical study investigator for AbbVie, Aclaris, Almirall, Arena, Pharmaceuticals, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Dermira, Eli Lilly and Company, Forte, Galderma, Janssen, LEO, Novartis, Ortho, Pfizer, Rapt, Regeneron, Sandoz, Sanofi Genzyme, Sun Pharma and UCB Pharma; and as a paid speaker for AbbVie. Y.P. has received grant funding and honoraria for services as an investigator, speaker and member of advisory boards from AbbVie, Amgen, Bausch, Janssen‐Ortho and UCB Pharma; and has received grant funding as an investigator from Baxter, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galderma, Genentech, GlaxoSmithKline, Incyte, LEO Pharma, MedImmune, Merck, Novartis, Pfizer, Regeneron, Sanofi, Serono and Takeda. C.P. has received grants from and has been a consultant for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Sandoz and UCB Pharma. S.B., M.K., T.W. and Z.G. are full‐time employees of AbbVie Inc. and may hold AbbVie stock and/or stock options." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1): "IMMerge was a phase III, international, multicentre, randomized, ... randomized in a 1:1 ratio via a centralized Interactive Response Technology system to open‐label treatment with risankizumab or secukinumab for up to 64 weeks". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 1): "IMMerge was a phase III, international, multicentre, randomized... randomized in a 1:1 ratio via a centralized Interactive Response Technology system to open‐label treatment with risankizumab or secukinumab for up to 64 weeks". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 1): "open‐label, efficacy–assessor‐blinded, active‐comparator study" Comment: no blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 3): "Efficacy assessments were performed by a qualified physician or designee at each study site at all appropriate study visits. The efficacy assessor was fully trained on the protocol and could not perform efficacy assessments prior to having completed all necessary training. The efficacy assessor remained blinded to each patient’s treatment and clinical laboratory results, and all safety data during the course of the study. The efficacy assessor was instructed to document the dermatological assessments on paper worksheets and was not allowed access to patient electronic case report forms". Comment: clearly defined |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data Quote (p 4): "Missing efficacy data were accounted for using nonresponder imputation, whereby any patient who had a missing value at a study visit was categorized as a nonresponder for that visit, unless the patient was a responder both before and after a specific visit window. Safety analyses were performed on all intent‐to‐treat patients who received at least one dose of study drug (safety population)." Randomised 327, analysed 327 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03478787). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. No results were posted on ClinicalTrials.gov on 21 September 2020. |
IMMhance 2020.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: March 2016 to July 2018 Location: worldwide (60 sites in Australia, Belgium, Canada, Czech Republic, France, Germany, Japan, South Korea, and the US) Phase 3 |
|
| Participants |
Randomised: 507 participants Inclusion criteria
Exclusion criteria:
Baseline characteristics N = 507, mean age of 49.5 years and 70% men Dropouts and withdrawals 7/507 (1.4%): risankizumab group (4), placebo group (3)
|
|
| Interventions |
Intervention A. Risankizumab 150 mg by subcutaneous injection at weeks 0 and 4, n = 407 Control intervention B. Placebo by subcutaneous injection at weeks 0 and 4, n = 100 |
|
| Outcomes |
At week 16 Primary composite outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 658): "Funding for the study was provided by AbbVie and Boehringer Ingelheim." Declarations of interest: Quote (p 657): "Dr Blauvelt has served as a scientific adviser and/or clinical study investigator for AbbVie, Aclaris, Almirall, Arena, Athenex, Boehringer Ingelheim, Bristol‐Myers Squibb, Dermavant, Dermira, Eli Lilly and Company, FLX Bio, Forte, Galderma, Janssen, Leo, Novartis, Ortho, Pfizer, Regeneron, Sandoz, Sanofi Genzyme, Sun Pharma, and UCB Pharma, and as a paid speaker for AbbVie. Dr Leonardi has received honoraria or fees for serving on advisory boards,as a speaker, and as a consultant, as well as grants as an investigator from AbbVie, Actavis, Amgen, Celgene, Coherus, Dermira, Eli Lilly, Galderma, Janssen, Leo, Merck, Novartis, Pfizer, Sandoz, Stiefel, UCB, and Wyeth. Dr Gooderham has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant, as well as grants as an investigator from AbbVie, Amgen, Akros, Arcutis, Boehringer Ingelheim, BMS, Celgene, Coherus, Dermavant, Dermira, Eli Lilly, Galderma, GSK, Janssen, Kyowa Hakko Kirin Pharma, Leo Pharma, MedImmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Sun Pharma, Takeda, UCB, and Valeant. Dr Papp has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant, as well as grants as principal investigator from AbbVie, Amgen, Astellas, Baxalta, Baxter, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Coherus, Dermira, Eli Lilly, Forward Pharma, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa‐Hakko Kirin, Leo Pharma, MedImmune, Merck‐Serono, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Stiefel, Sun Pharma, Takeda, UCB, and Valeant. Dr Philipp has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant, as well as grants as an investigator from AbbVie, Almirall, Amgen, Biogen, BMS GmbH, Boehringer Ingelheim, Celgene, Dermira, Eli Lilly, GSK, Hexal, Janssen Cilag, Leo Pharma, Maruho, MSD, Merck, Mundipharma, Novartis, Pfizer,UCB Pharma, and VBL Therapeutics. Dr J. J. Wu has been an investigator for AbbVie, Amgen, Eli Lilly, Janssen, and Novartis; a paid consultant for AbbVie, Almirall, Amgen, Bristol‐Myers Squibb, Celgene, Dermira, Dr Reddy’s Laboratories, Eli Lilly, Janssen, LEO Pharma, Novartis, Promius Pharma, Regeneron, Sun Pharmaceutical, UCB, and Valeant Pharmaceuticals North America LLC; and a speaker for AbbVie, Amgen, Celgene, Novartis, Regeneron, Sanofi Genzyme, Sun Pharmaceutical, UCB, and Valeant Pharmaceuticals North America LLC.Dr Igarashi has received honoraria or fees for serving on advisory boards, as a speaker, and asa consultant, as well as grants as an investigator from AbbVie, Celgene, Eli Lilly, Kyowa Kirin, Janssen, Maruho and Novartis. Dr Flack is a full‐time employee of Boehringer Ingelheim.Drs Geng, T. Wu, and Williams are full‐time employees of AbbVie and may own stock/options. Dr Camez is a former full‐time employee of AbbVie and may own stock/options. Dr Langley has served as principal investigator for and is a paid member of the scientific advisory board or served as a speaker for AbbVie, Amgen, Celgene, Janssen, Leo, Lilly, Merck, Novartis, Pfizer, and Boehringer Ingelheim". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 650): "The IMMhance study was a 2‐year, phase 3, multinational, double‐blind placebo‐controlled trial with randomized withdrawal and retreatment comparing risankizumab, 150 mg, with placebo. In parts A and B, patients were randomly assigned via interactive response technology using block randomization. Randomizations were stratified by baseline weight (≤ 100 vs > 100 kg) and prior exposure to a tumor necrosis factor α inhibitor (yes vs no)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 650): "The IMMhance study was a 2‐year, phase 3, multinational, double‐blind placebo‐controlled trial with randomized withdrawal and retreatment comparing risankizumab, 150 mg, with placebo. In parts A and B, patients were randomly assigned via interactive response technology using block randomization. Randomizations were stratified by baseline weight (≤100 vs >100 kg) and prior exposure to a tumor necrosis factor α inhibitor (yes vs no)." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 650): "Patients, investigators, and study personnel involved in trial conduct or analysis remained blinded to randomized treatment assignments until study completion. To maintain blinding, risankizumab and its matching placebo were identical in appearance. Following a screening period (1‐6 weeks), patients entered a 16‐week double‐blind treatment period (part A1)." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 650): "Patients, investigators, and study personnel involved in trial conduct or analysis remained blinded to randomized treatment assignments until study completion. To maintain blinding, risankizumab and its matching placebo were identical in appearance. Following a screening period (1‐6 weeks), patients entered a 16‐week double‐blind treatment period (part A1)." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 651): "Efficacy was analyzed in the intention‐to‐treat population." Randomised 507, analysed 507 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02672852). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
IMMpress 2022.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study (IMMpress) Date of study: July 2018 to December 2019 Location: Russia (6 centres) Phase 3 |
|
| Participants |
Randomised: 50 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 50, mean of age 44.5 years, and 54% men Dropouts and withdrawals 2/50 (4%): placebo group (1), risankizumab group (2)
|
|
| Interventions |
Intervention A. Risankizumab 150 mg (2 x 75 mg prefilled syringe) SC, n = 41 Control intervention B. Placebo, n = 9 |
|
| Outcomes | At week 16 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 2073): "AbbVie funded this study and participated in the study design, research, analysis, data collection, and interpretation of data and in the writing, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship. AbbVie funded the journal’s Rapid Service Fee." Declarations of interest: Quote (p 2073‐2074): "Liudmila Odnopozova has no conflict of interest to disclose. Anton Edin has received grant/research support as a principal investigator in clinical trials from AbbVie, Eli Lilly, LEO Pharma, GSK, Bayer, Novartis. Alexey Sukharev has received grant/research support as a principal investigator in clinical trials from AbbVie, Eli Lilly, Novartis, and Pfizer. Tian‐ shuang Wu and Kerstin Aydin are full‐time salaried employees of AbbVie and may own stock/stock options. Maureen Kelly was a full‐ time salaried employee of AbbVie at the time of this research and may own stock/stock options. She has since retired from AbbVie. Alkes Khotko has worked as principal investigator in clinical research for AbbVie, Bristol Myers Squibb, Eli Lilly, Galderma, Janssen, Leo, Novartis, Sanofi, and Amgen over the last 3 years." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 2064): "The IMMpress study (NCT03518047) was a phase 3, randomized, double‐blind, placebo‐controlled study that evaluated the efficacy and safety of risankizumab in patients with moderate to severe plaque psoriasis in the Russian Federation." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 2064): "The IMMpress study (NCT03518047) was a phase 3, randomized, double‐blind, placebo‐controlled study that evaluated the efficacy and safety of risankizumab in patients with moderate to severe plaque psoriasis in the Russian Federation." Comment: no description of the method used to guarantee random sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 2064): "The IMMpress study (NCT03518047) was a phase 3, randomized, double‐blind, placebo‐controlled study that evaluated the efficacy and safety of risankizumab in patients with moderate to severe plaque psoriasis in the Russian Federation." Comment: no description of the method used to guarantee blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 2064): "The IMMpress study (NCT03518047) was a phase 3, randomized, double‐blind, placebo‐controlled study that evaluated the efficacy and safety of risankizumab in patients with moderate to severe plaque psoriasis in the Russian Federation." Comment: no description of the method used to guarantee blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 2075): "The ITT population was used for the analysis of efficacy in period A (double‐blind) and period B (open‐label). The primary endpoint was compared between treatment groups used the Cochran–Mantel–Haenszel test, adjusting for pooled site. For the analysis of categorical variables, nonresponder imputation (NRI) was the primary approach and last observation carried forward (LOCF) was the secondary approach. Continuous variables were analyzed using the LOCF approach." Randomised 50, analysed 50 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03518047). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are not posted on ClinicalTrials.gov. |
IMMvent 2019.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: March 2016 to August 2017 Location: worldwide Phase 3 |
|
| Participants |
Randomised: 605 participants Inclusion criteria
Exclusion criteria Patients with:
Baseline characteristics N = 605, mean of age 46 years and 70% men Dropouts and withdrawals 20/605 (3.3%); risankizumab group (7), adalimumab group (13)
|
|
| Interventions |
Intervention Risankizumab: 150 mg (2 syringes of 75 mg) at weeks 0, 4, and every 12 weeks, n = 301 Control intervention Adalimumab: 80 mg at randomisation; then 40 mg at weeks 1, 3, 5, and every other week, n = 304 |
|
| Outcomes |
At week 16 Primary composite outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1): "Abbvie and Boehringer Ingelheim" Declarations of interest: Quote (p 10): "KR has served as adviser, paid speaker, or participated in clinical trials sponsored by AbbVie, Affibody, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Covagen, Forward Pharma, Fresenius Medical Care, GlaxoSmithKline, Janssen‐Cilag, Kyowa Kirin, Leo, Lilly, Medac, Merck Sharp & Dohme, Novartis, Miltenyi Biotec, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Takeda, UCB, Valeant, and Xenoport. MG has received grant or research support from AbbVie, Akros, Arcutis, Boehringer Ingelheim, BMS, Celgene, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Janssen, Kyowa Kirin, Medimmune, Merck, Novartis, Pfizer, Regeneron, Roche, UCB, and Valeant; has participated in a speaker’s bureau for AbbVie, Actelion, Celgene, Eli Lilly, Galderma, Janssen, Novartis, Pfizer, Regeneron, and Sanofi Genzyme; and has served as a consultant for AbbVie, Amgen, Arcutis, Akros, BoehringerIngelheim, Celgene, KyowaKirin, Novartis, Pfizer, Sanofi Genzyme, and Sun Pharmaceuticals. DT has received grant or research support from AbbVie, Celgene, and Novartis;has participated in a speaker’s bureau for AbbVie, Almirall, Celgene, Eli Lilly, Janssen‐Cilag, Leo Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sandoz/Hexal, Sanofi,andUCB;andhasservedasa consultant for AbbVie, Almirall, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen‐Cilag, Leo Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sandoz/Hexal, Sanofi, and UCB. JJC has received compensation as a speaker, consultant, and investigator for AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, Regeneron, Sanofi‐Aventis, Sun Pharma, and UCB. He has been an investigator for Merck, Maruho, Pfizer, Regeneron, Boehringer Ingelheim, MC‐2, Verrica, and Sandoz. CR has received compensation as a speaker or consultant, or adviser for AbbVie, Janssen, Leo, Lilly, Novartis, and UCB, and has served as a consultant or adviser for AbbVie, Boehinger Ingelheim Dermira, Dr Reddys, Janssen, Leo, Lilly, Novartis, Regeneron‐Sanofi, and UCB. JGK has received honoraria and consulting fees paid to Rockefeller University from AbbVie, Acros, Amgen, BMS, BiogenMA, Boehringer, Innovoderm, Janssen, Kineta, Leo Pharma, Novan, Novartis, Paraxel, Pfizer, Regeneron, Sienna, UCB, and Vitae, and has received consulting fees from Allergan, Asana, Aurigene, BiogenIdec, Escalier, Lilly, Roche, and Valent. T‐FT has served as a consultant for AbbVie, Boehringer Ingelheim, Celgene, Eli‐Lilly, Janssen‐Cilag, Novartis International AG, and Pfizer. MF is a full‐time employee of Boehringer Ingelheim and might hold stock or stock options. YG and DAW are full‐time employees of AbbVie and might hold stock or stock options. EHZT was a full‐time employee of AbbVie when the study was done and might hold stock or stock options. CP has received grants or research support from Pierre Fabre and Sanofi‐Regeneron and has served as a consultant for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Janssen Cilag, Leo, Lilly, Pfizer, Novartis, Pierre Fabre, Sanofi, and UCB." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 2): "IMMvent was a phase 3 randomised, double‐blind, double‐dummy, active‐comparator trial..." Quote (p 3): "In part A and part B of the trial, patients were randomly assigned 1:1 via interactive response technology using block randomisation, which allocated medication to patients through medication numbers randomly generated; double‐blind allocation to each patient was maintained throughout the process". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 2): "IMMvent was a phase 3 randomised, double‐blind, double‐dummy, active‐comparator trial..." Quote (p 3): "In part A and part B of the trial, patients were randomly assigned 1:1 via interactive response technology using block randomisation, which allocated medication to patients through medication numbers randomly generated; double‐blind allocation to each patient was maintained throughout the process" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 2): "IMMvent was a phase 3 randomised, double‐blind, double‐dummy, active‐comparator trial..." Quote (p 3): "Throughout the study, all patients, investigators, and involved study personnel remained masked to treatment assignment. A double‐dummy strategy was used to maintain masking, with patients in each group receiving the same number of injections at each time point. Risankizumab and adalimumab were identical in appearance." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 2): "IMMvent was a phase 3 randomised, double‐blind, double‐dummy, active‐comparator trial..." Quote (p 3): "Throughout the study, all patients, investigators, and involved study personnel remained masked to treatment assignment. A double‐dummy strategy was used to maintain masking, with patients in each group receiving the same number of injections at each time point. Risankizumab and adalimumab were identical in appearance." Comment: no detailed description of means used to guarantee absence of communication between blinded and unblinded personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 5): "Missing efficacy data were handled using non‐responder imputation for categorical variables and last observation carried forward for continuous variables." Randomised 605, analysed 605 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02694523). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results posted on ClinicalTrials.gov: ITT results |
IXORA‐P 2018.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: August 2015 to August 2017 Location: worldwide Phase 3 |
|
| Participants |
Randomised: 1227 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 1227, mean of age 47.5 years and 66% men Dropouts and withdrawals 148/1227 (12.1%): ixekizumab 4‐week group (38), ixekizumab 2‐week group (72), ixekizumab 2/4‐week group (36)
|
|
| Interventions |
Intervention A. Ixekizumab (160 mg ixekizumab given as 2 SC injections at baseline and then 80 mg ixekizumab given as 1 SC injection every 2 weeks to week 52), n = 611 Control interventions B. Ixekizumab (160 mg ixekizumab given as 2 SC injections at baseline and then 80 mg ixekizumab given as 1 SC injection every 4 weeks to week 52), n = 310 C. Ixekizumab (160 mg ixekizumab given as 2 SC injections at baseline and then 80 mg ixekizumab given as 1 SC injection every 4 weeks to week 52, with a dose adjustment to Q2W until week 50 for patients meeting prespecified criteria to which investigators were blinded (Q4W/Q2W dose adjustment), n = 306 |
|
| Outcomes |
At week 52 Primary composite outcome
Secondary outcomes
|
|
| Notes |
Funding source Quote (p 1315): "This study was funded in full by Eli Lilly and Company, Indianapolis, IN, U.S.A". Declarations of interest Quote (p 1323): "R.G.L. has been a consultant and/or scientific adviser and/or investigator and/or scientific officer and/or speaker for AbbVie, Amgen, Celgene, Pfizer, Eli Lilly and Company, Novartis and Boehringer Ingelheim. K.P. has been a consultant and/or scientific adviser and/or investigator and/or scientific officer and/or speaker for Amgen, Anacor, AbbVie, Akros, Allergan, Astellas, AstraZeneca, Baxalta, Baxter, Bristol‐Myers Squibb, Boehringer Ingelheim, Can‐Fite, Celgene, Coherus, Dermira, Dow Pharma, Eli Lilly and Company, Forward Pharma, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa Hakko Kirin, LEO Pharma, Medimmune, Meiji Seika Pharma, Merck (MSD), Merck‐Serono, Mitsubishi Pharma, Novartis, Pfizer, Regeneron, Roche, Sanofi/Genzyme, Takeda, UCB and Valeant. M.G. has been a consultant and/or scientific adviser and/or investigator and/or scientific officer and/or speaker for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Galderma, Janssen, LEOPharma, Novartis, Pfizer, Akros, Dermira, UCB and Coherus. A.B. has been a consultant and/or scientific adviser and/or investigator and/or scientific officer and/or speaker for AbbVie, Aclaris, Allergan, Almirall, Amgen, Boehringer Ingelheim, Celgene, Dermavant, Dermira, Genentech/Roche, GlaxoSmithKline, Janssen, Eli Lilly and Company, LEO Pharma, Merck Sharp& Dohme, Novartis, Pfizer, Purdue Pharma, Regeneron, Sandoz, Sanofi Genzyme, Sun Pharma, Sienna Pharmaceuticals, UCB, Valeant and Vidac. P.F. has been a consultant and/or scientific adviser and/or investigator and/or scientific officer and/or speaker for Abbot/AbbVie, Amgen, Bristol‐Myers Squibb, Boehringer Ingelheim, Celgene, Celtaxsys, Cutanea, Galderma, Genentech, GlaxoSmithKline/Stiefel, Janssen, LEO Pharma, Eli Lilly and Company, Novartis, Regeneron, Roche, Sanofi, Schering‐Plough/Merck,3M/iNova/Valeant, UCB and Wyeth/Pfizer. C.M., L.Z., N.A. and P.P. are employees of/and or own stock in Eli Lilly and Company." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1316): "This multicentre, randomized, double‐blinded, parallel group, phase III trial was conducted...Assignment to dosing regimens was determined by a computer‐generated random sequence using an interactive web response system (IWRS)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 1316): "This multicentre, randomized, double‐blinded, parallel group, phase III trial was conducted...Assignment to dosing regimens was determined by a computer‐generated random sequence using an interactive web response system (IWRS)." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1316): "This multicentre, randomized, double‐blinded, parallel group, phase III trial was conducted...... To maintain investigator blinding, site personnel entered an sPGA score into the IWRS every 4 weeks, beginning at week 0 through week 48." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1316): "This multicentre, randomized, double‐blinded, parallel group, phase III trial was conducted...... To maintain investigator blinding, site personnel entered an sPGA score into the IWRS every 4 weeks, beginning at week 0 through week 48." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (p 1317): "Missing data were imputed as nonresponse (NRI). The multiple imputation (MI) method was also used to impute missing values as a sensitivity analysis..." Included population 1227, table 2 1227 Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02513550). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
IXORA‐R 2020.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: November 2018 to July 2019 Location: 124 sites, USA and Canada Phase 4 |
|
| Participants |
Randomised: 1027 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 1027, mean of age 49 years and 63.5% men Dropouts and withdrawals: Ixekizumab: 32/520, guselkumab 26/507
|
|
| Interventions |
Intervention A. Ixekizumab 160 mg at week 0 then 80 every 2 weeks from weeks 2 to 12, n = 520 Control interventions B. Guselkumab 100 mg at week 0, 4, and 12, n = 507 Participants on guselkumab received placebo injection at weeks 0, 2, 6, 8, and 10 |
|
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source (Quote p 1348): "Funding for this study was provided by Eli Lilly and Company, Indianapolis, IN, U.S.A. Eli Lilly and Company contributed to study design, data collection, data analysis, data interpretation, manuscript preparation and the decision to submit the paper for publication. An advisory committee was involved in the study design and data interpretation, together with authors from Eli Lilly and Company. Authors had full access to all group‐level data in the study, but not individual‐level data that would risk unblinding those authors who were also study investigators. Authors had final responsibility for the decision to submit for publication". Declarations of interest: Quote (Appendix 1): "A.B. has served as a scientific adviser and/or clinical study investigator for AbbVie, Aclaris, Almirall, Arena, Athenex, Boehringer Ingelheim, Bristol‐Myers Squibb, Dermavant, Dermira, Eli Lilly and Company, FLX Bio, Forte, Galderma, Janssen, LEO, Novartis, Ortho, Pfizer, Regeneron, Sandoz, Sanofi Genzyme, Sun Pharma and UCB Pharma, and as a paid speaker for AbbVie. K.P. has served as a scientific adviser and/or clinical study investigator for AbbVie, Akros, Allergan, Almirall, Amgen, Arcutis, Avillion, Bausch Health, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Dermavant, Dermira, Eli Lilly and Company, Galderma, Genentech/Roche, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO, Meiji, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma, Takeda, UCB and Valeant; and as a paid speaker for AbbVie, Akros, Allergan, Almirall, Amgen, Bausch Health, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Dermavant, Dermira, Eli Lilly and Company, Galderma, Genentech/Roche, Janssen, Kyowa Kirin, LEO, Meiji, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma, Takeda, UCB and Valeant. A.G. has served as a consultant or speaker for Janssen, Celgene, Beiersdorf, Bristol‐Myers Squibb, AbbVie, UCB, Novartis, Incyte, Eli Lilly and Company, Allergan, Sun Pharmaceutical Industries, Xbiotech, LEO, Avotres Therapeutics and Boehringer Ingelheim; and received research/educational grants from Janssen, Incyte, Novartis, Xbiotech, UCB and Boehringer Ingelheim. A.J. has served as scientific advisor or clinical study investigator for AbbVie, Asana Biosciences, Castle Biosciences, Inc., Bristol‐Myers Squibb, Celgene, Dermira, Eli Lilly and Company, Galderma, Genentech/Roche, GlaxoSmithKline, LEO Pharma, Novartis, Pfizer, Purdue Pharma, Regeneron, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma and UCB Pharma, and as a paid speaker for Castle Biosciences, Inc., Eli Lilly and Company, Novartis, Regeneron and Sanofi Genzyme. K.R. has served as an advisor and paid speaker and has participated in clinical trials for AbbVie, Affibody, Almirall, Amgen, Avillion, Biogen, Boehringer Ingelheim, Celgene, Covagen, Forward Pharma, Fresenius Medical Care, GlaxoSmithKline, Janssen, Janssen‐Cilag, Kyowa Kirin, LEO Pharma, Eli Lilly and Company, Medac, Merck Sharp & Dohme, Novartis, Miltenyi Biotech, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB, Valeant, XBiotech and Xenoport. C.M. has served as principal investigator, as a speaker or on a scientific advisory board for and received compensation in the form of honoraria from AbbVie, Amgen, Celgene, Janssen, LEO Pharma, GlaxoSmithKline, Bausch Health, Eli Lilly and Company, Novartis, Pfizer and UCB Pharma. K.B.G. has consulting relationships with AbbVie, Amgen, Celgene, Eli Lilly and Company, Janssen, Novartis, Pfizer, Dermira and Boehringer Ingelheim and has received grants from AbbVie, Amgen, Celgene and Janssen. L.K.F. has been an investigator and consultant for Eli Lilly and Company, Janssen and Pfizer; a consultant for UCB; and an investigator for AbbVie, Amgen, Galderma, LEO Pharma and Regeneron. R.G. Langley has served as principal investigator, as a speaker and on the scientific advisory board for and received compensation in the form of honoraria from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Janssen, LEO Pharma, Eli Lilly and Company, Merck, Novartis, Pfizer, Sun and UCB Pharma. Y.T. received grants for research from Maruho, LEO Pharma, Eisai, AbbVie, Kyowa Hakko Kirin, Taiho Pharmaceutical, Celgene, and Eli Lilly and Company, and honoraria for lectures from Torii Pharmaceutical, Maruho, LEO Pharma, Eisai, AbbVie, Kyowa Hakko Kirin, Eli Lilly and Company, Taiho Pharmaceutical, Mitsubishi Tanabe Pharma and Janssen. R.G. Lima, H.E., G.G., L.R., S.Y.P. and R.B. are employees and stockholders of Eli Lilly and Company. J.B. is a speaker and investigator for AbbVie, Celgene, Eli Lilly and Company, Janssen, Novartis and Ortho Dermatologics. He is an investigator for Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb and LEO Pharma." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 3): "Patients were allocated to treatment by a computer‐generated random sequence." Comment: adequate process |
| Allocation concealment (selection bias) | Low risk | Quote: "supplementary material S2 interactive web‐response system (IWRS). The IWRS was used to assign double‐blind investigational product to each patient. The Unblinded Site Personnel at the site confirmed that they located the correct assigned study drug package by entering a confirmation number found on the package into the IWRS. Designated Unblinded Site Personnel were responsible for receipt of study drug shipments, dispensing study drug, administering study drug (ixekizumab, guselkumab, and placebo), recording information in the Study Drug Administration Log, and confirming treatment assignments." Comment: interactive web‐response system guaranteed allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 3): "Patients, investigators and all other personnel involved in the conduct of this ongoing study are to remain blinded to individual treatment assignments until all patients have completed the study." Comment: because the syringes looked different, participants were not allowed to see the syringe before, during, or after the drug administration Comment: not sure that the method was sufficiently efficient to guarantee blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 3): "Patients, investigators and all other personnel involved in the conduct of this ongoing study are to remain blinded to individual treatment assignments until all patients have completed the study. Because the syringes look different, patients were not allowed to see the syringe before, during, or after the drug administration. Unblinded Site Personnel were responsible for maintaining the blind of the patient (e.g. by means of a blindfold or other appropriate physical barrier means communicated to the sponsor for final approval). Designated Unblinded Site Personnel were not involved in any clinical aspects of the study, including clinical evaluations and adverse event assessments." Comment: no detailed description of means used to guarantee absence of communication between blinded and unblinded personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: analysis for primary outcome and major secondary outcome was performed as ITT. Missing data were imputed using a nonresponder imputation method. The number of withdrawals was low and reasons comparable in each group. |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03573323). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
IXORA‐S 2017.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: September 2015 to October 2017 Location: USA (multicentric) Phase 3 |
|
| Participants |
Randomised: 302 participants Inclusion criteria:
Exclusion criteria
Baseline characteristics N = 302, median age 43.5, 202 male Dropouts and withdrawals 12/302 (4%): IXE group (4), USK group (8)
|
|
| Interventions |
Intervention Ixekizumab (160 mg ixekizumab given as 2 SC injections at baseline followed by 80 mg ixekizumab given as a single SC injection once every 2 weeks from week 2 through week 12. After week 12 participants will receive 80 mg ixekizumab every 4 weeks through week 52), n = 136 Control intervention Ustekinumab (45 mg ustekinumab given as SC injection for participants ≤ 100 kg and 90 mg SC injection for participants > 100 kg at weeks 0, 4, 16, 28, and 40), n = 166 |
|
| Outcomes | At week 12 and 24 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source Quote (p 1014): "This study was funded in full by Eli Lilly and Company, Indianapolis, IN, U.S.A." Declarations of interest Quote (Appendix 1): "K.R. has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Amgen, Biogen, Boehringer Ingelheim, Celgene, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, LEO Pharma, Lilly, Medac, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, Takeda, UCB Pharma and Xenoport. A.P. has served as an advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Amgen, Biogen, Boehringer Ingelheim, Celgene, GlaxoSmithKline, Janssen‐Cilag, LEO Pharma, Lilly, Medac, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron and UCB. J.P.L. has served as an advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Amgen, Boehringer Ingelheim, Celgene, Galderma, Janssen, LEO Pharma, Lilly, Merck‐Serono, Novartis, Pfizer, Regeneron, Roche and UCB Pharma. C.F. has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Amgen, Celgene, Centocor, Janssen‐Cilag, LEO Pharma, Lilly, Merck Sharp & Dohme, Novartis and Pfizer. G.M. has served as an investigator for Lilly. L.E.F. has served as an advisor for and/or participated in clinical trials sponsored by AbbVie, Amgen, Celgene, Eli Lily and Company, Galderma, Janssen‐Cilag and Novartis. M.L. has worked as a consultant and/or clinical trial investigator for AbbVie, Allergan Amgen, Anacor, Boehringer Ingelheim, Celgene, Dr Reddy’s, Janssen, LEO Pharma, Lilly, Merck‐Serono, Novartis, Oncobio‐ logics, Pfizer, Regeneron, Roche, Xenon Pharma, Valeant, Bayer, L’Oreal and Galderma. Y.D, C.H., S.W. and S.H. are employees of Eli Lilly and Company, and receive salary from and own stock in the company. C.P. has served as a consultant and/or investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, LEO Pharma, Novartis and Pfizer." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1015): "This 52‐week, phase IIIb, multicentre, controlled, double‐blind, parallel‐group trial (IXORA‐S, NCT02561806) was conducted at 51 sites across 13 countries. Patients were randomized (1:1) via an interactive web‐response system to receive either ixekizumab or ustekinumab. Randomization was stratified by study centre and patient weight (≤ 100 kg vs. > 100 kg)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 1015): "This 52‐week, phase IIIb, multicentre, controlled, double‐blind, parallel‐group trial (IXORA‐S, NCT02561806) was conducted at 51 sites across 13 countries. Patients were randomized (1:1) via an interactive web‐response system to receive either ixekizumab or ustekinumab. Randomization was stratified by study centre and patient weight (≤ 100 kg vs. > 100 kg)." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1015): "To maintain the blinding, patients randomized to ixekizumab received placebo injections matching the ustekinumab dose regimen, and patients in the ustekinumab group received dummy injections of ixekizumab. Unblinded site personnel responsible for ustekinumab and ustekinumab placebo injections were involved in neither the clinical assessments nor the treatment decisions, and kept the patients and investigators blinded from treatment allocation". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1015): "To maintain the blinding, patients randomized to ixekizumab received placebo injections matching the ustekinumab dose regimen, and patients in the ustekinumab group received dummy injections of ixekizumab. Unblinded site personnel responsible for ustekinumab and ustekinumab placebo injections were involved in neither the clinical assessments nor the treatment decisions, and kept the patients and investigators blinded from treatment allocation". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data Quote (p 1016): "Patients were analysed according to the treatment they were assigned at randomization (intention‐to‐treat population). The primary‐analysis model was a logistic regression for the PASI 90 response end point after 12 weeks of treatment, with terms for treatment group, weight and geographical region. Missing data were imputed via nonresponder imputation (NRI), assuming that patients without data had no response". Patients randomised (n = 302), patients analysed (n = 302) Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02561806). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
JUNCTURE 2015.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: June 2012 to January 2013 Location: 38 centres worldwide Phase 3 |
|
| Participants |
Randomised: 182 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 182, mean age 45 years, 125 male Dropouts and withdrawals 5/182 (2.7%)
|
|
| Interventions |
Intervention A. Secukinumab (n = 61), SC, 150 mg weeks 0, 1, 2, 3 then monthly Control intervention B. Secukinumab (n = 60), SC, 300 mg weeks 0, 1, 2, 3 then monthly C. Placebo (n = 61), (same drug administration) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (supplemental file): “The study was sponsored by Novartis Pharma and designed by the scientific steering committee and Novartis personnel. Novartis conducted the data analysis, and all authors had access to the data”. Declarations of interest (p 29): "Dr Paul has served as a consultant for AbbVie Pharmaceuticals, Amgen, Celgene Corporation, Eli Lilly and Company, Janssen Pharmaceuticals, LEO Pharma, Novartis Pharmaceuticals Corporation, Pfizer Inc and Pierre Fabre. Dr Lacour has participated in clinical trials sponsored by Novartis and has received honoraria as a coordinator of clinical trials sponsored by Novartis. Dr Kreutzer has received honoraria for giving speeches for, has received travel grants from, and conducts clinical trials for AbbVie Pharmaceuticals, Biogen, Novartis and Janssen‐Cilag. Dr Jazayeri has served as investigator for and received grants from Novartis. Dr Adams has served as investigator for and received grants from Amgen, Eli Lilly and Company and Novartis. Ms Guindon and Dr Papavassilis are full‐time employees of and own stock in Novartis. Mr You is a full‐time employee of Novartis. Dr Tedremets has no conflicts of interest to declare." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 28 and supplemental file): “were randomly allocated”, "Randomization was conducted via Interactive Response Technology, which assigned a randomization number that linked the subject to a treatment arm and specified unique medication pack number". Comment: no description of the method used to guarantee the random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was conducted via Interactive Response Technology, which assigned a randomization number that linked the subject to a treatment arm and specified unique medication pack number". Comment: well described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1083): “During the induction period, subjects…in the secu 150 mg group were administrated one 150 mg injection and one placebo, ... , in the placebo group... 2 placebo autoinjections”. Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1083): “During the induction period, subjects … in the secu 150 mg group were administrated one 150 mg injection and one placebo, ..., in the placebo group ... 2 placebo autoinjections”. Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 182, analysed 181 Management of missing data: Quote (Supplemental file): “Missing values with respect to response variables based on PASI score or IGA mod 2011 score were imputed as nonresponse regardless of the reason for missing data”. Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01636687). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Khatri 2016.
| Study characteristics | ||
| Methods | Randomised, double‐blind, active‐controlled study Date: April 2015 to August 2016 Location: USA (1 centre: Mount Sinai) Phase 3 |
|
| Participants |
Total sample size: 12 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 12, mean age 47 years, 8 male Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ixekizumab once every 2 weeks, SC, 160 mg 2 injections at week 0 followed by 80 mg ixekizumab given as a single SC injection once every 2 weeks through week 12. After week 12 participants will receive 80 mg ixekizumab every 4 weeks through week 44, n = 6 Control intervention B. Ixekizumab once every 4 weeks, SC, 160 mg, 2 injections at week 0 followed by 80 mg ixekizumab given as a single SC injection once every 4 weeks through week 44, n = 6 |
|
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source Quote (p 33): "Funding provided by Eli Lilly and Company" Declarations of interest: Quote (p 33): "Dr. Khattri has received grant/research support from and is an investigator for Eli Lilly and Company. Dr. Lebwohl is an employee of Mount Sinai, which receives research funds from AbGenomics, Amgen, Anacor, Boehringer Ingelheim, Celgene, Ferndale, Janssen Biotech, Kadmon, LEO Pharma, Eli Lilly and Company, Medimmune, Novartis, Pfizer, Sun Pharma, and Valeant. Dr. Goldblum, Ms. Solotkin, Ms. Ridenour, and Dr. Yang own stock and are employees of Eli Lilly and Company. Dr. Amir and Dr. Min have no conflicts of interest relevant to the content of this article." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 34): "For this 48‐week, randomized, single‐center, open‐label study, patients were randomized at a ratio of 1:1 to receive 80 mg of ixekizumab either every two (Q2W) or four (Q4W) weeks during the induction dosing period (0–12 weeks) following an initial 160 mg dose of ixekizumab." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee random allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 34): "For this 48‐week, randomized, single‐center, open‐label study, patients were randomized at a ratio of 1:1 to receive 80 mg of ixekizumab either every two (Q2W) or four (Q4W) weeks during the induction dosing period (0–12 weeks) following an initial 160 mg dose of ixekizumab." Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 34): "For this 48‐week, randomized, single‐center, open‐label study, patients were randomized at a ratio of 1:1 to receive 80 mg of ixekizumab either every two (Q2W) or four (Q4W) weeks during the induction dosing period (0–12 weeks) following an initial 160 mg dose of ixekizumab." Comment: no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (p 35 ‐ ClinicalTrials.gov): "Response rates were summarized using nonresponder imputation to account for missing data." No missing data at week 12 Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02387801). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Krueger 2007.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: June 2003 to March 2005 Location: 46 centres in Utah, USA Phase 2 |
|
| Participants |
Randomised: 320 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 320, mean age 45 years, 222 male Dropouts and withdrawals 32/320 (8.8%)
|
|
| Interventions |
Intervention A. Ustekinumab 12/23 (n = 64), SC, 45 mg, 45 mg 1 dose, 1 week Control intervention B. Ustekinumab 12/23 (n = 64), SC, 90 mg, 45 mg 1 dose, 1 week C. Ustekinumab 12/23 (n = 64), SC, 45 mg, 45 mg/week, 4 weeks D. Ustekinumab 12/23 (n = 64), SC, 90 mg, 45 mg/week, 4 weeks E. Placebo (n = 64), SC |
|
| Outcomes | Assessments at 12 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source (p 590): "Supported by Centocore, Malvern, PA" Declaration of interest (p 590‐1): "Dr. Krueger reports receiving fees as a consultant or advisory board member for Abbott, Almirall, Alza, Amgen, Astellas, Boehringer Ingelheim, Barrier Therapeutics, Bristol‐Myers Squibb, Centocor, Connetics, and Genentech; Dr. Langley, for Centocor, Abbott, and Amgen/Wyeth; Dr. Leonardi, for Abbott, Amgen, Centocor, and Genentech; and Dr. Lebwohl, for Abbott, Amgen, Astellas, Centocor, Connetics, Galderma, Genentech, Novartis, PharmaDerm, and Warner Chilcott. Dr. Krueger reports receiving lecture fees from Abbott, Amgen, Boehringer Ingelheim, Centocor, and Connetics; Dr. Langley, from Abbott and Amgen/ Wyeth; Dr. Leonardi, from Abbott, Amgen, Centocor, and Genentech; and Dr. Lebwohl, from Abbott, Astellas, Amgen, Centocor, Connetics, Galderma, Genentech, PharmaDerm, and Warner Chilcott. Dr. Krueger reports receiving stipends for a clinical research fellowship from Amgen and Centocor; Dr. Langley, grant support from Centocor, Abbott, and Amgen/Wyeth; Dr. Leonardi, educational grants from Amgen and Genentech; and Dr. Lebwohl, grants from Abbott, Amgen, Astellas, Centocor, Connetics, Galderma, Genentech, PharmaDerm, and Warner Chilcott. Drs. Yeilding, Guzzo, Wang, and Dooley report being employees of Centocor. Dr. Krueger reports owning stock options from ZARS Pharma; Drs. Yeilding, Guzzo, and Dooley report holding stock and stock options in Johnson & Johnson; and Dr. Wang reports being a stockholder in Johnson & Johnson. No other potential conflict of interest relevant to this article was reported." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 581): “Patients ... were randomly assigned”. Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 581): “Patients ... were randomly assigned”. Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 581): “This placebo‐controlled, double‐blind...phase 2 study” Comment: placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 581): “This placebo‐controlled, double‐blind...phase 2 study” Comment: no specific description of the method used to guarantee blinding of outcome assessment, but considering that this was a placebo‐controlled trial with no known systematic AEs we considered the risk as low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 320 included, 320 analysed Quote (p 582): "Efficacy data from all patients who underwent randomisation were analysed... Missing values at week 12 were replaced with the most recently available values for all efficacy variables, missing data at other time points were not imputed". Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00320216). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Laburte 1994.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: not stated Location: 27 centres worldwide |
|
| Participants |
Randomised: 251 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 251, mean age 41 years, 176 male Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ciclosporin A (n = 119), orally, 2.5 mg/kg/d, 12 weeks Control intervention B. Ciclosporin A (n = 132), orally, 5 mg/kg/d, 12 weeks |
|
| Outcomes | Period assessments: 12 weeks Primary or secondary outcomes of the trial:
Outcomes of the trial
|
|
| Notes | Funding source and declarations of interest: not stated, but the first author was employed by Sandoz Pharma Ltd. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 367): "... was an open randomised study in parallel group" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 367): "... was an open randomised study in parallel group" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 367): "... was an open randomised study in parallel group" Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 367): "... was an open randomised study in parallel group" Comment: no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Management of missing data: no description of the method used to guarantee management of missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Lee 2016.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, open‐label study Date of study: July 2009 to April 2011 Location: Korea (multicentric) Phase 4 |
|
| Participants |
Total sample size: 60 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 60, mean age 39 years, 48 male Dropouts and withdrawals 16/60 (26.7%)
|
|
| Interventions |
Intervention A. Etanercept + acitretin (combination of etanercept, 25 mg twice a week and acitretin 10 mg twice a day for 24 weeks), n = 20 Control interventions B. Etanercept, 50 mg twice a week for 12 weeks followed by 25 mg twice a week for 12 weeks, n = 21 C. Acitretin, 10 mg twice a day for 24 weeks, n = 19 |
|
| Outcomes |
At week 24 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source Quote (p 8): "This study was funded by Pfizer Pharmaceuticals Korea Limited; etanercept is a product of Pfizer." Declarations of interest Quote (p 8): "Hyun‐Jeong Yoo is an employee of Pfizer Pharmaceuticals Korea Limited; etanercept is a product of Pfizer. All other authors report no competing interests." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 2): "In this multicenter, randomized, open‐label trial, patients were randomly assigned to one of three treatment groups: (a) etanercept 50 mg twice weekly (BIW) for 12 weeks followed by etanercept 25 mg BIW for a further 12 weeks (ETN–ETN); (b) etanercept 25 mg BIW and acitretin 10 mg twice daily (BID) for 24 weeks (ETN‐ACT); (c) acitretin 10 mg BID for 24 weeks (ACT; Fig. 1)". Comment: no description |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 2): "In this multicenter, randomized, open‐label trial, patients were randomly assigned to one of three treatment groups: (a) etanercept 50 mg twice weekly (BIW) for 12 weeks followed by etanercept 25 mg BIW for a further 12 weeks (ETN–ETN); (b) etanercept 25 mg BIW and acitretin 10 mg twice daily (BID) for 24 weeks (ETN‐ACT); (c) acitretin 10 mg BID for 24 weeks (ACT; Fig. 1)". Comment: no description |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 2): "In this multicenter, randomized, open‐label trial, patients were randomly assigned to one of three treatment groups: (a) etanercept 50 mg twice weekly (BIW) for 12 weeks followed by etanercept 25 mg BIW for a further 12 weeks (ETN–ETN); (b) etanercept 25 mg BIW and acitretin 10 mg twice daily (BID) for 24 weeks (ETN‐ACT); (c) acitretin 10 mg BID for 24 weeks (ACT;Fig. 1)". Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 2): "In this multicenter, randomized, open‐label trial, patients were randomly assigned to one of three treatment groups: (a) etanercept 50 mg twice weekly (BIW) for 12 weeks followed by etanercept 25 mg BIW for a further 12 weeks (ETN–ETN); (b) etanercept 25 mg BIW and acitretin 10 mg twice daily (BID) for 24 weeks (ETN‐ACT); (c) acitretin 10 mg BID for 24 weeks (ACT;Fig. 1)". Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (p 2): "Efficacy evaluation was performed on the modified intent‐to‐treat (mITT) and per protocol (PP) population sets. The mITT population included all randomly assigned patients who received at least one dose of test medication and had both baseline and on‐therapy PASI evaluation...and the patients who did not experience the event were censored at the time of last observation". Included population 60, Table 5 Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00936065). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
Leonardi 2003.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: December 2001 to April 2002 Location: 47 centres in USA |
|
| Participants |
Randomised: 672 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 672, mean age 45 years, 672 male Dropouts and withdrawals 103/672 (15.3%)
|
|
| Interventions |
Intervention A. Etanercept LD (n = 169), SC auto‐administered, 25 mg, once/week, 12 weeks Control interventions B. Etanercept MD (n = 167), SC auto‐administered, 25 mg, twice/week, 12 weeks C. Etanercept HD (n = 168), SC auto‐administered, 50 mg, twice/week, 12 weeks D. Placebo (n = 168), SC, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 2021): "Supported by Immunex, Seattle, a wholly‐owned subsidiary of Agen, Thousand Oaks, Calif" Declarations of interest: Quote (p 2021): "Drs. Leonardi, Powers, Goffe, and Gottlieb report having served as consultants for Amgen, and Drs. Leonardi, Goffe, and Gottlieb report having served as paid lecturers for Amgen. Dr. Gottlieb reports having served as a consultant and paid lecturer for Johnson & Johnson, Genentech, and Biogen; Dr. Leonardi reports having served as a consultant and paid lecturer for Johnson & Johnson and Genentech; Dr. Powers reports having served as a consultant for Genentech and Biogen; and Dr. Goffe reports having served as a consultant and paid lecturer for Biogen. Dr. Zitnik and Ms. Wang report owning equity in Amgen." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 2016): "Patients underwent central randomisation with the use of a permuted block randomisation list, with equal allocation to each of the four treatment groups". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: no description of the method used to guarantee the allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 2015): "Double‐blind... Etanercept ... was supplied to patients in syringes, each containing the contents of one reconstituted vial of etanercept or matching placebo...All patients received two injections per dose of study". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 2015): "Double‐blind... Etanercept ... was supplied to patients in syringes, each containing the contents of one reconstituted vial of etanercept or matching placebo...All patients received two injections per dose of study". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 672 randomised participants, 652 analysed (20 participants did not receive the treatment and were excluded from the analyses) Comment: modified ITT but number of participants not receiving treatment and not included in the analysis low and comparable between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Leonardi 2012.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: April 2010 to May 2011 Location: 23 centres internationally Phase 2 |
|
| Participants |
Randomised: 142 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 142, mean age 46 years, 81 male Dropouts and withdrawals 13/142 (9%) :
|
|
| Interventions |
Intervention A. Placebo (n = 27), SC, 0, 2, 4, 8, 12, 16 weeks, 16 weeks Control intervention B. Ixekizumab (n = 28), SC, 10 mg, 0, 2, 4, 8, 12, 16 weeks, 16 weeks C. Ixekizumab (n = 30), SC, 25 mg, 0, 2, 4, 8, 12, 16 weeks, 16 weeks C. Ixekizumab (n = 29), SC, 75 mg, 0, 2, 4, 8, 12, 16 weeks, 16 weeks C. Ixekizumab (n = 28), SC, 150 mg, 0, 2, 4, 8, 12, 16 weeks, 16 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1190): "Funded by Eli Lilly" Declarations of interest: Quote (p 1198): "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org." Leonardi received personal fees from Abbott, Amgen, Certocor, Eli Lilly, and Pfizer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (protocol p 44): “... from the central randomisation center using an IVRS” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (protocol p 44): “... from the central randomisation center using an IVRS” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (protocol p 22): “The investigators and patients are blinded while the sponsor is unblinded to study assignment”. Comment: placebo‐controlled trial, no systematic AE for the drug, probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (protocol p 22): “The investigators and patients are blinded while the sponsor is unblinded to study assignment”. Comment: placebo‐controlled trial, no systematic AE for the drug, probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Included 142/141 analysed (1 in the placebo group who did not have any post‐baseline assessment) Quote (protocol p 62 and p 1192): "All efficacy and health outcome analyses will be conducted on all patients who received any amount of study drug and have any post‐baseline efficacy assessment....Missing data for the primary timepoint at week 12 will be imputed by the last observation carried forward method". Comment: mITT and 1 participant out of 142 was not included in the analyses. |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01107457). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
LIBERATE 2017.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: October 2012 to April 2016 Location: 82 centres worldwide (USA, Europe, Australia) Phase 3 |
|
| Participants |
Randomised: 250 participants (mean age 45 years, 157 male) Inclusion criteria
Exclusion criteria
Baseline characteristics N = 250, mean age 45 years, 157 male Dropouts and withdrawals 17/250 (6.8%); apremilast (6), etanercept (2), placebo group (9)
|
|
| Interventions |
Intervention A. Apremilast (n = 83), orally, 30 mg twice daily Control intervention B. Etanercept (n = 83), SC, 50 mg weekly D. Placebo (n = 84) |
|
| Outcomes | Assessments at 16 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 2): "This study was sponsored by Celgene Corporation." Declarations of interest: Quote (p 1): "K. Reich has received honoraria as a consultant and/or advisory board member and/or acted as a paid speaker and/or participated in clinical trials sponsored by AbbVie, Amgen, Biogen, Boehringer Ingelheim, Celgene Corporation, Centocor, Covagen, Eli Lilly, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, LEO Pharma, Medac, Merck Sharp & Dohme Corp., Novartis, Ocean Pharma, Pfizer, Regeneron, Takeda, UCB Pharma and XenoPort. M. Gooderham has received honoraria, grants and/or research funding as a speaker, investigator, advisory board member, data safety monitoring board member and/or consultant for AbbVie, Actelion, Amgen, Astellas Pharma US, Boehringer Ingelheim, Celgene Corporation, Dermira, Eli Lilly, Galderma, Janssen, Kyowa Hakko Kirin Pharma, LEO Pharma, MedImmune, Merck & Co., Inc., Novartis, Pfizer, Regeneron, Roche". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 3): "Eligible patients were randomised (1:1:1) via an interactive voice response system to placebo; apremilast oral tablet, 30 mg twice daily; or etanercept subcutaneous injection, 50 mg QW".“Randomization was conducted via Interactive Response Technology, which assigned a randomisation number that linked the subject to a treatment arm and specified unique medication pack number". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 3): "Eligible patients were randomised (1:1:1) via an interactive voice response system to placebo; apremilast oral tablet, 30 mg twice daily; or etanercept subcutaneous injection, 50 mg QW". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 3): "Per the double dummy design, patients received oral tablets (apremilast 30 mg or placebo) twice daily and two subcutaneous injections (etanercept 25 mg each dose or saline placebo) QW." Comment: clearly defined |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 3): "Per the double dummy design, patients received oral tablets (apremilast 30 mg or placebo) twice daily and two subcutaneous injections (etanercept 25 mg each dose or saline placebo) QW." Comment: clearly defined |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 250, 250 analysed Management of missing data: Quote (p 3): "Efficacy assessments were conducted for the modified intent‐to treat (mITT) population (all randomised patients who received ≥1 dose of study medication and had both baseline PASI and ≥ 1 post‐treatment PASI evaluations)... Last‐observation‐carried‐forward (LOCF) methodology was used to impute missing efficacy measurements." Comment: done |
| Selective reporting (reporting bias) | High risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01241591). The prespecified outcomes and those mentioned in the Methods section have not been reported as DLQI. |
Liu 2020.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: August 2014 to October 2016 Location: China (19 centres) Phase 4 |
|
| Participants |
Randomised: 466 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 466, mean age of 43 years and 76% men Dropouts and withdrawals 24/466 (5.15%): methotrexate group (13), placebo group (11)
|
|
| Interventions |
Intervention A. Methotrexate (initial dose of 7.5 mg/week to a maximum dose of 15 mg/week or the maximum tolerated dose within 8 weeks), n = 233 Control intervention B. Placebo, n = 233 Co‐intervention: etanercept (50 mg subcutaneously once weekly) |
|
| Outcomes |
At week 24 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote: "This research was supported by Zhejiang Public Walfare Technology Research Project (Grant number: LGF20H110002). Med‐ ical Health Science and Technology Project of Zhejiang Provincial Health Commission (Grant Number: 2018KY088) and 3SBIO INC." Declarations of interest: Quote: "The authors declare that they have no conflict of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All eligible patients were randomly assigned by a random number created by a computer‐generated coding system to receive either the combination of rhTNFR‐Fc and MTX (combination group) or rhTNFR‐Fc plus placebo (monotherapy group)." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Then patients were randomized 1:1 to receive 50 mg rhTNFR‐Fc subcutaneously once weekly and oral MTX (from an initial dose of 7.5 mg/week to a maximum dose of 15 mg/week or the maximum tolerated dose within 8 weeks) or receive rhTNFR‐Fc (as that in combination group) and a matched placebo (as MTX in combination group) for 24 weeks." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "This was a multicentre, randomized, double‐blind, placebo‐controlled trial of rhTNFR‐Fc..." Comment: no description of the method used to guarantee allocation blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "This was a multicentre, randomized, double‐blind, placebo‐controlled trial of rhTNFR‐Fc..." Comment: no description of the method used to assess the primary outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dealing with missing data: no information on how missing data were handled Quote: "Efficacy analysis was performed using the intent‐to‐treat principle, in which all randomized patients who received any part of the study medication treatment and received at least one evaluation of therapeutic effectiveness were included in the analysis. All results of the efficacy analysis were analysed in the full analysis set (FAS). Safety was analysed in a safety analysis set (SAS), which included all patients who had received at least 1 dose of the study drug." Randomised 466; analysed 466 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (Liu 2020 NCT02313922). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. No results are posted on ClinicalTrials.gov. |
LOTUS 2013.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind (LOTUS) study Date of study: 23 October 2009 to 7 July 2011 Location: 14 centres in China Phase 3 |
|
| Participants |
Randomised: 322 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 322, mean age 40 years, 248 male Dropouts and withdrawals 6/322 (1.86%): ustekinumab group (3), placebo group (3)
|
|
| Interventions |
Intervention A. Ustekinumab (n = 160), SC, 45 mg, week 0, week 4, 4 weeks Control intervention B. Placebo (n = 162), SC, week 0, week 4, 4 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 173): "This study was supported by Janssen Research & Development". Declarations of interest: Quote (p 173): "Drs Zhu, Zang and Wand served as investigators for this Janssen RD‐sponsored study..." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 167): "The LOTUS study is a phase 3, multicenter, randomized, double blind, placebo‐controlled..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 167): "The LOTUS study is a phase 3, multicenter, randomized, double blind, placebo‐controlled..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 167): "The LOTUS study is a phase 3, multicenter, randomized, double blind, placebo‐controlled..." Comment: placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 167): "The LOTUS study is a phase 3, multicenter, randomized, double blind, placebo‐controlled..." Comment: no description of the method used to guarantee blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 322, analysed 322 Quote (p 167): "For efficacy analyses, all randomized patients were included... Patients who discontinued study treatment... were considered treatment failures". Comment: ITT analyses |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01008995). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Lowe 1991.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: not stated Location: 2 centres in Santa Monica and New York City, USA |
|
| Participants |
Randomised: 34 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 34, age range 20 to 75 years, 24 male Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Acitretin (n = 16), orally, 50 mg, daily, 12 weeks Control intervention B. Placebo (n = 18), orally, daily, 12 weeks Co‐intervention: UVB (phototherapy) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 591): "Supported by Roche Dermatologics, Nutley, New Jersey and the Skin Research Foundation of California, Santa Monica, California" Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 592): "Patients receiving UVB phototherapy were randomly assigned". Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 592): "Patients receiving UVB phototherapy were randomly assigned". Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 592): "were randomly assigned to either acitretin or placebo" Comment: no more precision, however adverse effects of acitretin such as cheilitis were visible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 592): "were randomly assigned to either acitretin or placebo... the same observer who was unaware of patient group examined the patients throughout the investigation". Comment: no more precision but adverse effects of acitretin such as cheilitis were visible. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 34 included/34 analysed (Table 2) Comment: no description of the method used to manage the missing data or to perform the analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Mahajan 2010.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: January 2007 to September 2007 Location: 1 centre in Chandighar, India |
|
| Participants |
Randomised: 40 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 40, mean age 37 years, 29 male Dropouts and withdrawals 11/40 (28%)
|
|
| Interventions |
Intervention A. Methotrexate 0.5 mg/kg + folic acid, (n = 20), orally 5 mg/d day‐1; day+1 + NBUVB 3/week max 1200 mJ/cm2 Control intervention B. Placebo + folic acid (n = 20), orally, 5 mg/d day‐1; day+1 + NBUVB 3/week max 1200 mJ/cm2 |
|
| Outcomes | Assessments at 6 months Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: not stated Declarations of interest (p 595): "not declared" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 596): “... were randomised by way of random number table” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 596): “... were randomised by way of random number table” Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 596): “patient‐blinded study” Comment: not double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 596): “patient‐blinded study” Comment: not double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 20/20 included; 20/20 analysed Quote (p 596): “Intention to treat principle was followed for the analysis of the observations”. Comment: no description of the method used to manage the missing data |
| Selective reporting (reporting bias) | Low risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
MATURE 2021.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study (MATURE) Date of study: December 2018 to August 2020 Location: USA, Germany, Spain, Iceland, Poland (worldwide, 22 sites) Phase 3B |
|
| Participants |
Randomised: 122 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 122, mean age of 44 years and 70% men Dropouts and withdrawals 5/122 (4%): secukinumab 2 mL AI (0), secukinumab 2 x 1 mL PFS (2), placebo (3)
|
|
| Interventions |
Intervention A. Secukinumab 300 mg provided in 2 mL auto‐injector (AI), n = 41 Control interventions B. Secukinumab 300 mg provided as 2 x 1 mL prefilled syringe (PFS) of 150 mg/mL, n = 41 C. Placebo, n = 40 |
|
| Outcomes | At week 12 Primary composite outcome
Secondary outcomes
|
|
| Notes |
Funding: Quote (p 1): "This investigation was sponsored by Novartis Pharma AG, Basel, Switzerland." Declaration of interests: Quote (p 9):"Bardur Sigurgeirsson has consulted for Novartis and several other pharmaceutical companies; served on an advisory board for Novartis and several other pharmaceutical companies; and been a speaker for Novartis and several other pharmaceutical companies. John Browning has served on an advisory board for Novartis, Dermira, Dermavant, and Regeneron. He has consulted for Regeneron and Leo Pharmaceuticals. He is a speaker for Regeneron, Pfizer, and Dermira. He is an investigator for Novartis, Amryt, AnaptysBio, Arcutis, Brickell, Chemocentryx, Dermira, Eli Lilly, Forte, Galderma, Pfizer, Regeneron, UCB, and Venthera. Stephen Tyring served as an investigator for Novartis. Jacek C. Szepietowski has served on an advisory board for Leo Pharma, Novartis, Sanofi‐Genzyme, Trevi, Viofor; been a speaker for Abbvie, Leo Pharma, Novartis, Sanofi‐Genzyme, Sunfarm; served as an investigator for Abbvie, BMS, Helm, Galapagos, Galderma, Incyte, InfaRX, Janssen‐Cilag, Novartis, Pfizer, Regeneron, UCH, Trevi. Raquel Rivera‐Díaz participated in advisory boards for AbbVie Laboratories, Janssen Pharmaceuticals Inc., Lilly, and Pfizer; as a speaker for MSD, AbbVie, Janssen, Leo Pharma, Novartis, and Pfizer; as an inves‐ tigator for AbbVie, Pfizer, Janssen, Celgene, Lilly, Novartis, and Leo Pharma; and received travel grants for attending congresses from AbbVie, Janssen, Novartis, Pfizer, Leo Pharma, Celgene, and MSD. Isaak Effendy served as an investigator for AbbVie, Janssen, Leo Pharma, Lilly, Novartis and Pfizer. Deborah Keefe is an employee of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA. Gerard Bruin is an employee of Novartis Institutes for Biomedical Research, Basel, Switzerland. Rong Fu is an employee of Novartis Institute for Biomedical Research, Shanghai, China. Bertrand Paguet, Isabelle Hampele, Maximilian Reinhardt and Manmath Patekar are employees of Novartis Pharma AG, Basel, Switzerland." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (study protocol p. 31): "At Randomization visit, all eligible subjects will be randomized via Interactive Response Technology (IRT) to one of the treatment arms." ... "The IRT will assign one randomization number to the subject, which will be used to link the subject to a treatment arm and will specify a unique medication number for the package of study drug to be dispensed to the subject." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (study protocol p. 31): "The randomization numbers will be generated using the following procedure to ensure that treatment assignment is unbiased and concealed from subjects and investigator staff. A subject randomization list will be produced by the IRT using a validated system that automates the random assignment of subject numbers to randomization numbers. These randomization numbers are linked to the different treatment arms, which in turn are linked to medication numbers. A separate medication list will be produced by or under the responsibility of Novartis Global Clinical Supplies using a validated system that automates the random assignment of medication numbers to packs containing the investigational drug(s)." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (study protocol p. 31‐2): "A double‐dummy design is used to ensure that the identity of the study drug cannot be disguised, as the drug products are visibly different. Subjects, investigator staff and persons performing the assessments, and data analysts will remain blind to the identity of the study treatment from the time of randomization until the end of study database lock, using the following methods: 1. Randomization data are kept strictly confidential until the time of unblinding, and will not be accessible by anyone else involved in the study ... 2. The identity of the treatments will be concealed by the use of investigational treatments that are all identical in packaging, labeling, schedule of administration, appearance, taste and odor." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (study protocol p. 31‐2): "A double‐dummy design is used to ensure that the identity of the study drug cannot be disguised, as the drug products are visibly different. Subjects, investigator staff and persons performing the assessments, and data analysts will remain blind to the identity of the study treatment from the time of randomization until the end of study database lock, using the following methods: 1. Randomization data are kept strictly confidential until the time of unblinding, and will not be accessible by anyone else involved in the study ... 2. The identity of the treatments will be concealed by the use of investigational treatments that are all identical in packaging, labeling, schedule of administration, appearance, taste and odor." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (study protocol p. 68): "The following imputation methods will apply to the missing data: Response variables based on PASI score and IGA mod 2011 categories will be imputed with multiple imputations (MI) method as primary imputation method. MI is a simulation based approach where missing values are replaced by multiple Bayesian draws from the conditional distribution of missing data given the observed data and covariates, creating multiple completed data sets. These completed data sets can then be analyzed using standard methods. Within this analysis the PASI score or IGA mod 2011 categories will be imputed and response variables will be derived based on the imputed scores. In the multiple imputation analysis the response status will be imputed based on the individual treatment arm information. Non‐responder imputation will be used as sensitivity method: Missing values with respect to response variables based on PASI score and IGA mod 2011 categories will be imputed with non‐response regardless to the reason for missing data (e.g. premature study discontinuation, missed visit, administrative issues)". Randomised 122, analysed 122 Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03589885). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov |
Meffert 1997.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: not stated Location: 17 centres in Germany |
|
| Participants |
Randomised: 127 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 127, mean of age and number of male unknown Dropouts and withdrawals 15/128 (12%)
|
|
| Interventions |
Intervention A. Ciclosporin (n = 43), orally, 1.25 mg/kg/d, 10 weeks Control intervention B. Ciclosporin (n = 41), orally, 2.5 mg/kg/d, 10 weeks C. Placebo (n = 44), orally, 10 weeks |
|
| Outcomes | Assessments at 10 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: not stated but 3 out of 4 authors from Sandoz Pharmaceuticals Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 77): "patients were randomised". Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 77): "double blind study period" Comment: no description of the method used to guarantee blinding regarding the need for hypertension and renal function surveillance and modification in ciclosporin groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 77): "double blind study period" Comment: no description of the method used to guarantee blinding, regarding the need for hypertension and renal function surveillance and modification in ciclosporin groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 128 included/120 analysed Comment: methods for dealing with missing data not specified, not ITT analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
METOP 2017.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled study Date of study: February 2013 to May 2015 Location: 13 centres in Europe |
|
| Participants |
Randomised: 120 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 120, mean age 45 years, 100 male Dropouts and withdrawals 21/212 (17.5%), methotrexate n = 14, placebo, n = 7
|
|
| Interventions |
Intervention A. Methotrexate (n = 91), SC, IM, 17.5 to 22.5 mg/week, 12 weeks Control intervention B. Placebo (n = 29) |
|
| Outcomes |
At 16 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 528): "Funding source: Medac. The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and all authors had final responsibility for the decision to submit for publication". Declarations of interest: Quote (p 536): "RBW has received personal fees from AbbVie, Almirall, Amgen, Boehringer Ingelheim Pharma, Celgene, Janssen‐Cilag, Leo, Lilly, Novartis, Pfizer, and Xenoport outside the submitted work. UM has been an advisor to, received speakers honoraria or grants from, or participated in clinical for Abbott/AbbVie, Almirall Hermal, Amgen, BASF, Biogen Idec, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, Foamix, Forward Pharma, Galderma, Janssen, Leo Pharma, Medac, MSD, Miltenyi Biotech, Novartis, Pfizer, Teva, VBL, and Xenoport. RvK has been an investigator, consultant, advisor, or speaker for Abbvie, Almirall, Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, Eli Lilly, GSK, Leo, Janssen‐Cilag, MSD, Novartis, Pfizer, UCB, and VBL Pharma. JN has received grants from Amgen, Novartis, Janssen‐Cilag, LEO, Lilly, Medac, Regeneron, and Dermapharm, outside the submitted work. DW‐T has been an advisor to, received speakers honoraria or grants from, or participated in clinical for Abbvie, Almirall, Amgen, Biogen, Boehringer Ingelheim Pharma, Celgene, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, Leo, Lilly, Medac, Merck Sharp & Dohme, Novartis, Pfizer, UCB Pharma, and VBL. KG has been an advisor to, received speakers honoraria or grants from, or participated in clinical for Abbott/AbbVie, Almirall, Biogen, Boehringer Ingelheim, Celgene, Delenex, Eli Lilly, Galderma, Janssen, Medac, MSD, Novartis, and Pfizer. KR has received personal fees from AbbVie, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, Leo, Lilly, Medac, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, Takeda, UCB Pharma, and Xenoport, outside the submitted work. IZ, TMF, and NB‐S declare no competing interests." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 3): "Eligible patients were randomly assigned (3:1), via computer‐generated random numbers (RandList 1.2) in an ascending order, to receive either methotrexate or placebo injections for the first 16 weeks of the study (phase 1)." Comments: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 3): "Eligible patients were randomly assigned (3:1), via computer‐generated random numbers (RandList 1.2) in an ascending order, to receive either methotrexate or placebo injections for the first 16 weeks of the study (phase 1)." Comments: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 3): "Study phase 1 was done in a double‐blind manner, with group allocation concealed from participants and investigators from the time of randomisation until an interim database lock at week 16...The syringes for placebo and active drug were not distinguishable and were fully coated to prevent identification of colour differences between injections". Comments: clearly defined |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 3): "Study phase 1 was done in a double‐blind manner, with group allocation concealed from participants and investigators from the time of randomisation until an interim database lock at week 16...The syringes for placebo and active drug were not distinguishable and were fully coated to prevent identification of colour differences between injections". Comments: clearly defined |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of randomised participants, n = 120, 120 analysed Quote (p 4): "All outcomes were analysed in the modified intention to‐treat population of patients who had received at least one injection of study drug, with missing data treated as indicating no response (non‐responder imputation)." Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02902861). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Morita 2022.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: November 2018 to February 2020 Location: 4 institutions in Japan Phase ? |
|
| Participants |
Randomised: 42 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 40 analysed, mean of age 61 years, and 70% men Dropouts and withdrawals Not stated |
|
| Interventions |
Intervention A. Apremilast orally administered as 10 mg (in the morning), 20 mg (morning: 10 mg, evening: 10 mg), 30 mg (morning: 10 mg, evening: 20 mg), 40 mg (morning: 20 mg, evening: 20 mg), 50 mg (morning: 20 mg, evening: 30 mg) on days 1, 2, 3, 4, and 5, respectively, followed by 60 mg daily since day 6 (morning: 30 mg, evening: 30 mg) and phototherapy NB‐UVB (311 ± 2 nm) was administered twice a week, n = 27 Control Intervention B. Phototherapy NB‐UVB (311 ± 2 nm) was administered twice a week, n = 13 |
|
| Outcomes |
At 8 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1): "Amgen" Declarations of interest: Quote (p 9): "This research was funded by Amgen K.K. Tokyo, Japan. Akimichi Morita has received research grants, consulting fees, and/or speaker's fee from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Eisai, Janssen, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Nichi‐Iko, Nippon Kayaku, Novartis, Pfizer, Sun Pharmaceutical Industries Taiho Pharmaceutical, Torii Pharmaceutical, Ushio and UCB Pharma. Yukie Yamaguchi declares receiving research grants, and/or consulting fees, and/or speaker's fees from AbbVie, Amgen, Astellas, Boehringer Ingelheim, Eisai, Eli Lilly, Janssen, Kyowa Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Novartis, Sun Pharmaceutical Industries, Taiho Pharmaceutical, Torii Pharmaceutical, and UCB Japan. Chiharu Tateishi has received research grant, consulting fee, and/or speaker's fee from Amgen. Daisuke Hayashi has received research grant, consulting fee from Amgen. Yuko Watanabe has received speaker's fee from AbbVie, Eli Lilly, Maruho, Novartis, Taiho Pharmaceutical and UCB Pharma. Koji Masuda has received research grants from Eli Lilly Japan. Daisuke Tsuruta has re‐ ceived research grants and/or speaker's fee from Abbvie, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Eisai, Janssen, JIMRO Co., Ltd., Kyowa Hakko Kirin, Maruho, Mitsubishi Tanabe, Nippon Kayaku, Novartis, Sun Pharmaceutical Industries, Pfizer, Taiho Pharmaceutical, Teijin Limited, Torii Pharmaceutical, Tsumura & Co. and UCB Pharma. Norito Katoh has received honoraria as a speaker/consultant for Sanofi, Maruho, Abbvie, Ely‐Lilly Japan, Leo Pharma, Jansen Pharma, Mitsubishi Tanabe Pharma, Kyowa Kirin, Celgene Japan and has re‐ ceived grants as an investigator from Maruho, Ely‐Lilly Japan, Sun Pharma, Taiho Pharmaceutical, Torii Pharmaceutical, Boehringer Ingelheim Japan Mitsubishi Tanabe Pharma, Kyowa Kirin, and Leo Pharma. Kyoko Ikumi, Aya Yamamoto, Haruna Nishihara, Yukihiko Watanabe and Ayano Maruyama have nothing to disclose." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 2): "This was a multicenter, randomized, open‐label, parallel‐group, active‐controlled study conducted at four institutions between November 9, 2018, and February 14, 2020" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 2): "This was a multicenter, randomized, open‐label, parallel‐group, active‐controlled study conducted at four institutions between November 9, 2018, and February 14, 2020" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 2): "This was a multicenter, randomized, open‐label, parallel‐group, active‐controlled study conducted at four institutions between November 9, 2018, and February 14, 2020" Comment: participants not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 2): "This was a multicenter, randomized, open‐label, parallel‐group, active‐controlled study conducted at four institutions between November 9, 2018, and February 14, 2020" Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 3): "The efficacy analysis was performed for the full analysis set (FAS) and per‐protocol set (PPS). As a sensitivity analysis, the analyses were performed without and with complementing the missing values using the last‐observation‐carried‐forward (LOCF) method. The secondary end points were analyzed without supplementation of the missing values." Randomised 42, analysed 40 Comment: no description of the number of patients who dropped out in each group |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on Japan Registry of Clinical Trials (jRCTs041180012). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Nakagawa 2016.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: October 2012 to March 2013 Location: multicentre (56) in Japan Phase 2 |
|
| Participants |
Randomised: 151 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 151, mean age 45 years, 120 male Dropouts and withdrawals 6/151 (4%); brodalumab 70 group (2), brodalumab 140 group (0), brodalumab 210 group (0), placebo group (4)
|
|
| Interventions |
Intervention A. Brodalumab (n = 39), SC, 70 mg, 2 injections week 0, 1 injection eow Control intervention B. Brodalumab (n = 37), SC, 140 mg, 2 injections week 0, 1 injection eow C. Brodalumab (n = 37), SC, 210 mg, 2 injections week 0, 1 injection eow D. Placebo (n = 38), orally (same drug administration) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 51): “The study was supported by Kyowa Hakko Kirin Co., Ltd.” Declarations of interest: Quote (p 51): "H. Nakagawa is a consultant and/or received research grants and/or speaker honoraria from for Kyowa Hakko Kirin Co., Ltd., AbbVie, Mitsubishi‐Tanabe Pharma, Janssen Pharmaceutical K.K., Novartis Pharma K.K., Eli Lilly Japan K.K., LEO Pharma Maruho Corporation Limited and MSD K.K.H. Niiro has no conflict of interest to declare. K. Ootaki is an employee of Kyowa Hakko Kirin Co., Ltd." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 45): “were randomised to receive…” Comment: not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 45): “were randomised to receive…” Comment: not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 51): “double‐blind…” Comment: not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of the method used to guarantee blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 151, analysed 151 Comment: no supplementary explanation about the management of missing data |
| Selective reporting (reporting bias) | High risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01748539). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported, except for participant‐reported outcome. |
NCT02134210 RaPsOdy.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: June 2014 to May 2016 Location: worldwide Phase 3 |
|
| Participants |
Randomised: 521 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 521, mean of age 43.5 years and 70% men Dropouts and withdrawals 25/521 (1.4%): CHS‐0214 group (6), Enbrel group (19) Reasons not stated |
|
| Interventions |
Intervention A. CHS‐0214 50 mg twice‐weekly times 12 weeks, n = 261 Control intervention B. Enbrel 50 mg twice‐weekly times 12 weeks, n = 260 |
|
| Outcomes |
At week 12 Primary composite outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (ClinicalTrials.gov): "Coherus Biosciences, Inc." Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (ClinicalTrials.gov): "A Double‐Blind, Randomized, Parallel‐Group, Active‐Control Study to Compare the Efficacy and Safety of CHS‐0214 Versus Enbrel... Allocation: randomized" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (ClinicalTrials.gov): "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (ClinicalTrials.gov): "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dealing with missing data: not stated Results posted on ClinicalTrials.gov: ITT analyses Reasons for treatment discontinuation not stated |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02634801). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
NCT02581345.
| Study characteristics | ||
| Methods | RCT, active‐controlled, triple‐blind study Date of study: September 2015 to April 2017 Location: worldwide Phase 3 |
|
| Participants |
Randomised: 572 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 572, mean of age 45 years and 66% men Dropouts and withdrawals 38/572 (6.7%): biosimilar group (15), Humira group (23)
|
|
| Interventions |
Intervention A. Biological: M923, SC, biosimilar adalimumab week 0: 80 mg, week 1: 40 mg, then 40 mg eow, n = 286 Control Intervention B. Biological: M923, SC, adalimumab (Humira) week 0: 80mg, week 1: 40 mg, then 40 mg eow, n = 286 |
|
| Outcomes |
At 16 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (ClinicalTrials.gov): "Novartis" Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (ClinicalTrials.gov and Statistical analysis plan): "Allocation: randomized... The blocking scheme will be specified in the randomization specifications. Randomization will occur via an Interactive Response Technology (IRT) System until..." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (ClinicalTrials.gov and Statistical analysis plan): "Allocation: randomized... The blocking scheme will be specified in the randomization specifications. Randomization will occur via an Interactive Response Technology (IRT) System until..." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (ClinicalTrials.gov): "Masking: Triple (Participant, Care Provider, Investigator)" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (ClinicalTrials.gov): "Masking: Triple (Participant, Care Provider, Investigator)" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (Statistical analysis plan): "The primary analysis will be based on the non‐responder imputation (NRI) method." Results posted on ClinicalTrials.gov: per‐protocol analyses (non‐inferiority trial) |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02581345). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
NCT02762994.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, multicentre, double‐blind study Date of study: June 2016 to May 2017 Location: Russia Phase 2 |
|
| Participants |
Randomised: 120 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 114, median of age 40 years and 69% men Dropouts and withdrawals 6/114 (5%): BCD‐085 40 mg (1), BCD‐085 80 mg (1), BCD‐085 120 mg (2), placebo (2)
|
|
| Interventions |
Intervention A. BCD‐085 (netakimab anti‐IL17), 40 mg: participant will receive 40 mg of BCD‐085 subcutaneously at weeks 0, 1, 2, 4, 6, 8, 10, n = 30 Control interventions B. BCD‐085, 80 mg: participant will receive 80 mg of BCD‐085 subcutaneously at weeks 0, 1, 2, 4, 6, 8, 10, n = 30 C. BCD‐085, 120 mg: participant will receive 80 mg of BCD‐085 subcutaneously at weeks 0, 1, 2, 4, 6, 8, 10, n = 28 D. Placebo, n = 26 |
|
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (ClinicalTrials.gov): "Biocad" Declarations of interest: not stated RoB completed according to protocol posted on ClinicalTrials.gov |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (study protocol p. 62): "This clinical trial was designed as a multicenter, double‐blind, randomized study of the efficacy and safety...". Quote (study protocol p. 82)"."Randomization in the study will be centralized. Patient included in the study will be randomised within each stratum (block randomization)". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (study protocol p. 85): "During randomization, the BIOCAD's clinical manager allocates the patient to an appropriate stratum, assigns her/his the first free arm number in the block and a 3‐digit randomization number coding this arm (corresponds to the patient's order number in the study). After randomization, the clinical study manager assigns the patient an investigational product lot number (corresponding to treatment arm) and a patient ID. The investigator will know only the subject's ID and investigational product lot number." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (study protocol p. 86): "Neither investigators, not patients will be aware of whether the active treatment or placebo is used in each particular patient. The investigator (the principal investigator, a co‐investigator responsible for the therapy of this patient) receives BCD‐085/placebo in identical secondary packaging (cartons). The drugs differ only by their lot numbers. The lot number is individual for the subject. During therapy, the subject may receive the investigational product of several batches but they will be assigned the same lot number." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (study protocol p. 86): "Neither investigators, not patients will be aware of whether the active treatment or placebo is used in each particular patient. The investigator (the principal investigator, a co‐investigator responsible for the therapy of this patient) receives BCD‐085/placebo in identical secondary packaging (cartons). The drugs differ only by their lot numbers. The lot number is individual for the subject. During therapy, the subject may receive the investigational product of several batches but they will be assigned the same lot number." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (study protocol p. 157‐8): "The safety analysis will include all patients who received at least one dose of the investigational product. Additionally, the SAE analysis will include all randomized patients starting from the ICF signing and until the end of their participation in the study. Per protocol, the efficacy analysis is to include all patients who received at least one dose of BCD‐085/placebo and who attended at least one visit next visit. If no data are available at Week 12, the data from the last assessment are to be used (last‐observation‐carried‐forward method). In addition, these patients should be considered non‐responders and analyzed separately." Randomised 120, analysed 114 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02762994). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
NCT03055494 ObePso‐S.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: April 2017 to February 2019 Location: USA Phase 4 |
|
| Participants |
Randomised: 102 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 82, mean age of 44.5 years and 63% men Dropouts and withdrawals 11/82 (13.4%): secukinumab group (10), placebo group (1)
|
|
| Interventions |
Intervention A. Secukinumab 300 mg SC at randomisation, weeks 1, 2, 3, and 4 followed by monthly dosing up to week 48, n = 54 Control interventions B. Placebo, n = 28 |
|
| Outcomes |
At week 12 Primary composite outcome
Secondary outcome
|
|
| Notes |
Funding source: Quote (ClinicalTrials.gov): "Novartis Pharmaceuticals" Declarations of interest: not stated RoB completed according protocol posted on ClinicalTrials.gov |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (study protocol p 18, p 19): "This study uses a randomized, double‐blind, placebo‐controlled, parallel‐group, multicenter design. At the start of the Double‐blind Treatment Period, eligible patients will be randomized via Interactive Response Technology (IRT) in a 2:1 ratio to one of two treatment arms (secukinumab 300 mg or placebo). Randomization will be stratified by body weight collected at visit 2 (<90kg or >‐ 90kg)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (study protocol p 18, p 19): "This study uses a randomized, double‐blind, placebo‐controlled, parallel‐group, multicenter desing... At the start of the Double‐blind Treatment Period, eligible patients will be randomized via Interactive Response Technology (IRT) in a 2:1 ratio to one of two treatment arms (secukinumab300 mg or placebo). Randomization will be stratified by body weigth collected at visit 2 (< 90kg or >‐ 90kg)." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (study protocol p 32): "Patients, investigators/site staff, persons performing assessments, and Novartis study personnel will remain blinded to individual treatment assignment from the time of randomization until the final database lock at Week 53,using the following methods: 1.Randomization data will be kept strictly confidential until the time of unblinding, and will not be accessible by anyone else involved in the study with the following exceptions: specific vendors whose role in trial conduct requires their unblinding (e.g. IRT), Drug Supply Management (DSM); 2.The identity of secukinumab and placebo prefilled syringes (PFS) will be concealed by identical packaging,labeling, schedule of administration, and appearance." Comment: clearly defined |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (study protocol p 32): "Patients, investigators/site staff, persons performing assessments, and Novartis study personnel will remain blinded to individual treatment assignment from the time of randomization until the final database lock at Week 53,using the following methods: 1.Randomization data will be kept strictly confidential until the time of unblinding, and will not be accessible by anyone else involved in the study with the following exceptions: specific vendors whose role in trial conduct requires their unblinding (e.g. IRT), Drug Supply Management (DSM); 2. The identity of secukinumab and placebo prefilled syringes (PFS) will be concealed by identical packaging,labeling, schedule of administration, and appearance. At the Week 12 primary analysis time point, there will be a database lock after all patients have completed the Week 12 visit. At that time, only the statistician and programmer(s) from the designated CRO will be unblinded in order to perform the analysis." Comment: clearly defined |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dealing with missing data: Quote (study protocol p 65): "For the two primary efficacy variables at Week 12 (and other time points), a patient with a missing assessment will be considered as a non‐responder." Randomised 102, analysed 82 In ClinicalTrials.gov (results section): "a total of 133 patients were screened for the study, with 82 (61.7%) of these completing the screening phase". We are waiting for the publication to compare the number of randomised and analysed participants. |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03055494 ObePso‐S). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
NCT03364309.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: April 2018 to June 2020 Location: China (17 sites) Phase 3 |
|
| Participants |
Randomised: 438 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 438, mean of age 40.5 years and 76.5% men Dropouts and withdrawals
|
|
| Interventions |
Interventions A. Ixekizumab 160 mg at week 0 followed by 80 mg once every 4 weeks (Q4W) SC, n = 174 B. Ixekizumab 160 mg at week 0 followed by 80 mg once every 2 weeks (Q2W) SC, n = 176 Control intervention C. Placebo, n = 88 |
|
| Outcomes | At week 12 Primary composite outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (clinicaltrials.gov)"Eli Lilly and Company" Declarations of interest: not stated RoB completed according to ClinicalTrials.gov protocol. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (study protocol p. 48): "At Week 0 (Visit 2), patients who meet all criteria for enrollment at Visits 1/1A and 2 will be randomized at a 2:2:1 ratio to ixekizumab 80 mg Q2W, ixekizumab 80 mg Q4W, or placebo. Assignment to double‐blind treatment groups will be determined by a computer‐generated random sequence using an interactive web response system (IWRS). The IWRS will be used to assign double‐blind investigational product to each patient. Site personnel will confirm that they have located the correct assigned investigational product package by entering a confirmation number found on the package into the IWRS." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (study protocol p. 48): "At Week 0 (Visit 2), patients who meet all criteria for enrollment at Visits 1/1A and 2 will be randomized at a 2:2:1 ratio to ixekizumab 80 mg Q2W, ixekizumab 80 mg Q4W, or placebo. Assignment to double‐blind treatment groups will be determined by a computer‐generated random sequence using an interactive web response system (IWRS). The IWRS will be used to assign double‐blind investigational product to each patient. Site personnel will confirm that they have located the correct assigned investigational product package by entering a confirmation number found on the package into the IWRS." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (study protocol p. 30): "To maintain blinding, each patient will be administered 2 injections of blinded investigational product subcutaneously at Week 0, and each patient will be administered 1 injection of blinded investigational product subcutaneously Q2W from Weeks 2 through 10 regardless of his/her assigned dose regimen (that is, placebo will be given as necessary to maintain the blind)". Quote (study protocol p. 51): "This is a double‐blind study; patients and study site personnel will be blinded to study treatment until all patients reach Week 60 (Visit 19) or have discontinued from the study (moved into Period 4). To preserve the blinding of the study, a minimum number of sponsor personnel not in direct contact with study sites will see the randomization table and treatment assignments before the study is unblinded". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (study protocol p 30): "To maintain blinding, each patient will be administered 2 injections of blinded investigational product subcutaneously at Week 0, and each patient will be administered 1 injection of blinded investigational product subcutaneously Q2W from Weeks 2 through 10 regardless of his/her assigned dose regimen (that is, placebo will be given as necessary to maintain the blind)". Quote (study protocol p 51): "This is a double‐blind study; patients and study site personnel will be blinded to study treatment until all patients reach Week 60 (Visit 19) or have discontinued from the study (moved into Period 4). To preserve the blinding of the study, a minimum number of sponsor personnel not in direct contact with study sites will see the randomization table and treatment assignments before the study is unblinded." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (study protocol p. 74): "Analysis of categorical efficacy and health outcome variables will be assessed using a NRI method. In both Periods 2 (Induction Dosing Period) and 3 (Maintenance Dosing Period), patients will be considered a non‐responder for the NRI analysis if they do not meet the clinical response criteria or have missing clinical response data at the analysis time point." "A last observation carried forward (LOCF) analysis may be performed on all continuous efficacy and health outcome variables." Randomised 438, analysed 438 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03364309). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results posted on ClinicalTrials.gov |
NCT03535194 OASIS‐2.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: May 2018 to June 2020 Location: worldwide (178 sites) Phase 3 |
|
| Participants |
Randomised: 1484 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 1484, mean age unknown, and 67% men Dropouts and withdrawals 47/1484 (3%): mirikizumab (28), secukinumab (11), placebo (8)
|
|
| Interventions |
Intervention A. Mirikizumab 250 mg SC at weeks 0, 4, 8, and 12, n = 905 Control interventions B. Secukinumab 300 mg SC at weeks 0, 1, 2, 3, 4, 8, and 12, n = 448 C. Placebo, n = 112 |
|
| Outcomes | At week 16 Primary composite outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (ClinicalTrials.gov): "Eli Lilly and Company" Declarations of interest: not stated RoB completed according to ClinicalTrials.gov protocol |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (protocol p. 43): "Assignment to treatment groups will be determined by a computer‐generated random sequence using an interactive web‐response system (IWRS). The IWRS will be used to assign prefilled syringes containing double‐blind investigational product to each patient." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (protocol p. 43): "Assignment to treatment groups will be determined by a computer‐generated random sequence using an interactive web‐response system (IWRS). The IWRS will be used to assign prefilled syringes containing double‐blind investigational product to each patient." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (protocol p. 45): "This is a double‐blind study. The blinding applies to patients, site personnel, and Sponsor personnel. To preserve the blinding of the study, a minimum number of Lilly and site personnel will see the randomization table and treatment assignments before the study is complete." Comment: no description of the method used to guarantee blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (protocol p. 45): "This is a double‐blind study. The blinding applies to patients, site personnel, and Sponsor personnel. To preserve the blinding of the study, a minimum number of Lilly and site personnel will see the randomization table and treatment assignments before the study is complete." Comment: no description of the method used to guarantee blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (protocol p. 72): "The following methods for imputation of missing data will be used: Non‐Responder Imputation (NRI) for binary clinical response and Mixed Model Repeated Measures (MMRM)." Randomised 1465, analysed 1465 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03535194). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results posted on ClinicalTrials.gov |
Nugteren‐Huying 1990.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: not stated Setting: multicentre in the Netherlands |
|
| Participants |
Randomised: 39 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 39, mean age 44 years, 27 male Dropouts and withdrawals 5/39 (12.8%)
|
|
| Interventions |
Intervention A. Dimethylfumarate (n = 12), orally, 120 mg, gradual increase 1 to 6 tablets, once a day, 16 weeks Control intervention B. Octyl hydrogen fumarate (n = 10), orally, 284 mg, gradual increase 1 to 6 tablets, once a day, 16 weeks C. Placebo (n = 12), orally, once a day, 16 weeks |
|
| Outcomes | Assessments at 16 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 331): "The patients were randomly assigned..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 331): "The patients were randomly assigned..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 331): “The double‐blind treatment lasted 16 weeks for each patients... All tablets (provided by Fumapharm AG, Muri, Switzerland) had the same appearance, size and colour”. Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 331): “The double‐blind treatment lasted 16 weeks for each patients... All tablets (provided by Fumapharm AG, Muri, Switzerland) had the same appearance, size and colour”. Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 39, analysed 34 Comment: no description of the method used to perform analyses of the primary outcome and to manage missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Ohtsuki 2017.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: July 201 to December 2015 Location: Japan Phase 2 |
|
| Participants |
Randomised: 254 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 254, mean age of 50.5 years, 202 male Dropouts and withdrawals 37/254 (14.6%): apremilast 30 group (9), apremilast 20 group (16), placebo group (12)
|
|
| Interventions |
Intervention: A. Apremilast (30 mg tablet twice a day for 68 weeks), n = 85 Control intervention: B. Apremilast (20 mg tablet twice a day for 68 weeks), n = 85 C. Placebo, n = 84 |
|
| Outcomes |
At week 16 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 883): "The authors received editorial support in the preparation of the manuscript from Kathy Covino, Ph.D., of Peloton Advantage, LLC, funded by Celgene Corporation. This study was funded by Celgene Corporation." Declarations of interest: Quote (p 883): "Mamitaro Ohtsuki reports consultancy and speaker fees. Yukari Okubo reports consultancy fees. Shinichi Imafuku reports research funds, consultancy fees and speaker fees. Robert M. Day, Peng Chen, Rosemary Petric and Allan Maroli report stock or shares in Celgene Corporation and/or employment by Celgene Corporation. Osamu Nemoto has no relevant financial or personal relationships and no potential conflicts of interest to declare." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 874): "After the screening period, eligible patients began a 16‐week placebo‐controlled period and were randomized via a centralized interactive web response system or interactive voice response system (1:1:1) to placebo, apremilast 20 mg BID. or apremilast 30 mg b.i.d." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 874): "After the screening period, eligible patients began a 16‐week placebo‐controlled period and were randomized via a centralized interactive web response system or interactive voice response system (1:1:1) to placebo, apremilast 20 mg b.i.d. or apremilast 30 mg b.i.d." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 874): "This phase 2b multicenter, randomized, double‐blind, placebo‐controlled study" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 874): "This phase 2b multicenter, randomized, double‐blind, placebo‐controlled study" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 874): "Efficacy and safety assessments were conducted for the modified intent‐to‐treat (mITT) population, which included all patients who were randomized and received at least one dose of study medication; patients not dispensed study medication were excluded from the mITT population... For the primary analysis of PASI‐75, missing values were accounted for using the last observation carried forward methodology; multiple sensitivity analyses (including nonresponder imputation [NRI]) were conducted for the primary end‐point". Randomised 254; analysed 254 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01988103). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Ohtsuki 2018.
| Study characteristics | ||
| Methods | RCT, phase 3, randomised, double‐blind, placebo‐controlled study Date of study: January 2015 to November 2016 Location: Japan (35 sites) Phase 3 |
|
| Participants |
Randomised: 192 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 192, mean age of 49 years, 145 male Dropouts and withdrawals 15/192 (7.8%): guselkumab 100 group (1), guselkumab 50 group (2), placebo group (12)
|
|
| Interventions |
Intervention A. Guselkumab 100 mg with SC injections at weeks 0, 4, and every 8 weeks thereafter (n = 63) Control interventions B. Guselkumab 50 mg with SC injections at weeks 0, 4, and every 8 weeks thereafter (n = 65) C. Placebo (n = 64) |
|
| Outcomes |
At week 16 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 883): "Funding: This study was funded by Janssen Pharmaceutical,Tokyo, Japan." Declarations of interest: Quote (p 1062): "M. O. has received honoraria and/or research grants as a consultant and/or advisory board member and/or paid speaker and/or investigator from Abbvie, Boehringer‐Ingelheim, Celgene, Eisai, Janssen, Kyowa‐Kirin, LEO Pharma, Eli Lilly, Maruho, Novartis, Pfizer, Tanabe‐Mitsubishi, Nichiiko, Torri, Bayer, Pola Pharma, Taiho, Bristol‐Myers Squibb, Astellas, Otsuka, Mochida, Nippon Zoki, Actelion, Sanofi, Kaken Pharmaceuticals, Teijin Pharma, Nippon Kayaku, Shionogi, Ono and Galderma. H. N. has received honoraria and/or research grants as an advisory board member and/or speaker from ABC Pharma, Kyowa Hakko Kirin, Abbvie, Mitsubishi‐Tanabe Pharma, LEO Pharma, Maruho, Eli Lilly Japan, Janssen. H. K., H. M., R. G. and R. Z. are employees of Janssen Pharmaceutical." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1054): "Randomization was performed centrally using a computer‐generated randomization scheme, balanced using randomly permuted blocks and stratified by presence of PsA." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 1054): "Randomization was performed centrally using a computer‐generated randomization scheme, balanced using randomly permuted blocks and stratified by presence of PsA." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1054): "This was a phase 3, randomized, double‐blind, placebo‐controlled study conducted in Japan... Study site personnel, investigators and patients were blinded to treatment allocation until week 52 database lock." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1054): "This was a phase 3, randomized, double‐blind, placebo‐controlled study conducted in Japan... Study site personnel, investigators and patients were blinded to treatment allocation until week 52 database lock." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dealing with missing data: Quote (p 1054): "The randomized analysis set included all randomized patients for efficacy analyses, and data were analyzed by treatment groups...Last observation was carried forward for other patients with missing data." Randomised: 192; analysed: 192 Imbalance reasons and number of withdrawal: Gusel 100 group (1%), Gusel 50 group (2%), Placebo group (20%) |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02325219). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Olsen 1989.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: not stated Location: not stated |
|
| Participants |
Randomised: 15 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 15, age range 23 to 72 years, 11 male Dropouts and withdrawals 3/15 (20%)
|
|
| Interventions |
Intervention A. Acitretin (n = 10), orally, 25/50 mg, daily, 8 weeks Control intervention B. Placebo (n = 5), orally, daily, 8 weeks |
|
| Outcomes | Assessments at 8 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Hoffman‐La Roche Inc. Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 681): "Patients were assigned to... in a random, double‐blind fashion". Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 681): "Patients were assigned to... in a random, double‐blind fashion". Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 681): "Patients were assigned to... in a random, double‐blind fashion". Comment: adverse effects of acitretin such as cheilitis were visible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 681): "Patients were assigned to... in a random, double‐blind fashion". Comment: adverse effects of acitretin such as cheilitis were visible. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15 included/number of participants analysed not stated Comment: no description of the methods used to perform the efficacy analyses and to manage the missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section were reported. |
OPTIMAP 2022.
| Study characteristics | ||
| Methods | RCT, active‐controlled, single‐blind (observer) study Date of study: March 2014 to November 2017 Location: The Netherlands (multicentre) Phase 4 |
|
| Participants |
Randomised: 66 randomised participants, 61 analysed Inclusion criteria
Exclusion criteria
Baseline characteristics N = 61 analysed, mean of age 48 years, 72% men Dropouts and withdrawals At week 49, 29/66 (44%): adalimumab + methotrexate (14), adalimumab (15)
|
|
| Interventions |
Intervention A. Adalimumab 40 mg SC every other week starting 1 week after a loading dose of 80 mg with methotrexate 10 mg weekly, n = 33 randomised, 31 analysed Control intervention B. Adalimumab monotherapy 40 mg SC every other week starting 1 week after a loading dose of 80 mg, n = 33 randomised, 30 analysed |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 2382):"There was no funding source for this study." Declarations of interest: Quote (p 2382): "GvdK was involved in clinical trials for Janssen, AbbVie, Novartis, Pfizer, UCB, Leo Pharma, and Eli Lilly as a subinvestigator. JvdR carries/carried out clinical trials for AbbVie, Celgene, and Janssen and has received speaking fees from AbbVie, Janssen, BMS, and Eli Lilly and reimbursement for attending a symposium from Janssen, Pfizer, Celgene, and AbbVie. All funding is not personal but goes to the independent research fund of the department of dermatology of Radboud UMC (Nijmegen, The Netherlands). SJvB is currently employed at Janssen‐Cilag BV. EP has served as a consultant, advisory board member, speaker, and/or principal investigator for AbbVie, Amgen, AstraZeneca, Baxter, Celgene, ChemoCentryx, Eli Lilly, Galderma, InflaRx, Janssen‐Cilag, Leo Pharma, Novartis, Pfizer, Regeneron, Sandoz, and UCB and received investigator‐initiated grant support (paid to the Erasmus University Rotterdam, Rotterdam, The Netherlands) from AbbVie, AstraZeneca, Celgene, CHDR, Kymera, Novartis, Regeneron, Pfizer, Janssen‐Cilag, and UCB. TR received honoraria for presentations from Pfizer, AbbVie, and Regeneron and a research grant from Genmab. EdJ has received research grants for the independent research fund of the department of dermatology of the Radboud UMC from AbbVie, BMS, Pfizer, Novartis, Janssen Pharmaceutica, UCB, and Leo Pharma and has acted as consultant and/or paid speaker for and/or participated in research sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Amgen, Galapagos, Janssen Pharmaceutica, Novartis, Lilly, Celgene, Leo Pharma, Sanofi, UCB, and Almirall. All funding is not personal but goes to the independent research fund of the department of dermatology of Radboud UMC. MvD is a consultant and advisory board member for AbbVie and reports personal fees from Leopharma; grants and personal fees from Novartis; and personal fees from AbbVie, BMS, Celgene, Lilly, MSD, Pfizer, SanofiGenzyme, and Janssen‐Cilag, outside the submitted work. PS has acted as a consultant for Sanofi and AbbVie (unpaid), received departmental independent research grants for TREAT NL registry from Pharma since December 2019, and received independent research grants in the past (>5 years ago). She is involved in performing clinical trials with many pharmaceutical industries that manufacture drugs used for the treatment of, for example, psoriasis and atopic dermatitis, for which financial compensation is paid to the department/hospital, and is chief investigator of the systemic and phototherapy atopic eczema registry (TREAT NL) for adults and children. The remaining authors state no conflict of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 2381): "Eligible patients were randomized at 1:1 by the treating physician to receive adalimumab (Humira) with MTX or adalimumab (Humira) monotherapy. Randomization was performed by a centralized online randomization service (ALEA) in blocks of eight and stratified by biologic naivety." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 2381): "Eligible patients were randomized at 1:1 by the treating physician to receive adalimumab (Humira) with MTX or adalimumab (Humira) monotherapy. Randomization was performed by a centralized online randomization service (ALEA) in blocks of eight and stratified by biologic naivety." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 2381): "This single‐blinded, randomized controlled trial..... Disease severity (PASI and Investigator Global Assessment) was measured by a blinded outcome assessor at each study visit. The physicians performed all other study procedures and were not blinded. Patients were also not blinded and filled out questionnaires on the QOL (DLQI and Skindex‐29) and disease severity (patient global assessment)". Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 2381): "This single‐blinded, randomized controlled trial..... Disease severity (PASI and Investigator Global Assessment) was measured by a blinded outcome assessor at each study visit. The physicians performed all other study procedures and were not blinded. Patients were also not blinded and filled out questionnaires on the QOL (DLQI and Skindex‐29) and disease severity (patient global assessment)" Comment: no clear description of measures taken to guarantee the blinding of investigators |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dealing with missing data: Quote (p 2379 article): "Missing data are therefore not missing at random. Missing data were imputed by a model‐based imputation, and as a sensitivity analysis, a nonresponse imputation and last observation carried forward were performed" Quote (p 2382): "Analysis for the secondary clinical endpoints was performed on the intention‐to‐treat population, consisting of all patients who had received at least one dose of adalimumab." Randomised 66, analysed 61 Comment: intention‐to‐treat analysis not performed; 5 participants did not receive at least 1 dose, 66 participants should be involved in the ITT, however 61 participants were analysed |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on https://www.clinicaltrialsregister.eu (EudraCT number: 2013‐ 004918‐18). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are not posted. |
ORION 2020.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: March 2017 to February 2018 Location: worldwide Phase 3 |
|
| Participants |
Randomised: 78 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 78, mean of age 46 years, and 68% men Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Guselkumab (100 mg guselkumab administered as a 100 mg/mL solution in a single‐use prefilled syringe (PFS) assembled in a self‐dose device at weeks 0, 4, 12, 20, and 28), n = 62 Control intervention B. Placebo, n = 16 |
|
| Outcomes |
At week 16 Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 7): "Janssen Research & Development, LLC funded this study. Authors employed by Janssen participated in designing the study; collecting, analyzing, and interpreting the data; and in preparing, reviewing, and approving the manuscript. A professional medical writer supported by Janssen provided editorial and submission support." Declarations of interest: Quote (p 7): "Laura K. Ferris has been an investigator and consultant for EliLilly, Janssen, and Pfizer; a consultant for UCB; and an investigator for AbbVie, Amgen, Galderma, Leo Pharma, and Regeneron. H. Chih‐Ho Hong has been an investigator/consultant/or advisory board member for AbbVie, Amgen, Eli Lilly, Galderma, Janssen, Leo Pharma, Merck, Novartis, Pfizer, Regeneron, Sanofi, and UCB. Elyssa Ott, Jingzhi Jiang, Shu Li, and Chenglong Han are employed by Janssen Research & Development, LLC and own stock/stock options in its parent company. Wojciech Baran has been an investigator and consultant for AbbVie, Amgen, Eli Lilly, Janssen, Leo Pharma, Merck, Mylan, Novartis, Pfizer, and Regeneron." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (ClinicalTrials.gov and p 2): "Allocation: randomized"; "ORION (Clinicaltrials.gov identifier: NCT02905331) was a Phase 3, multicentre, double‐blind, placebo‐controlled study in which patients were centrally randomized (4:1) to receive...Randomization employed a computer‐generated permuted block schedule with stratification by country. An interactive web response system assigned a unique treatment code dictating treatment assignment and matching study drug kit. Codes were not provided to investigators. Guselkumab and placebo were delivered by identical devices (see Interventions)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (ClinicalTrials.gov and p 2): "Allocation: randomized"; "ORION (Clinicaltrials.gov identifier: NCT02905331) was a Phase 3, multicentre, double‐blind, placebo‐controlled study in which patients were centrally randomized (4:1) to receive...Randomization employed a computer‐generated permuted block schedule with stratification by country. An interactive web response system assigned a unique treatment code dictating treatment assignment and matching study drug kit. Codes were not provided to investigators. Guselkumab and placebo were delivered by identical devices (see Interventions)." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (ClinicalTrials.gov and p 2): "Double (Participant, Investigator)"; "Patients randomized to guselkumab received placebo at Week 16 to maintain the blind... Guselkumab and placebo were delivered by identical devices (see Interventions)." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (ClinicalTrials.gov and p 2): "Double (Participant, Investigator)"; "Patients randomized to guselkumab received placebo at Week 16 to maintain the blind... Guselkumab and placebo were delivered by identical devices (see Interventions)." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 3): "Efficacy analyses employed all randomized patients who received 1 injection of study agent, analyzed according to assigned treatment groups (full analysis set). The co‐primary endpoints were the proportions of patients achieving IGA 0/1 and PASI 90 responses at Week 16. Patients who met treatment failure criteria (discontinued study agent due to lack of efficacy/an AE of worsening psoriasis or started a protocol‐prohibited treatment before Week 16) were considered nonresponders for the co‐primary endpoints at Week 16, as were patients who did not return for evaluation at Week 16." Randomised 78; analysed 78 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02905331). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Ortonne 2013.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: September 2007 to August 2009 Setting: 17 centres in Austria, France, Greece, and Italy Phase 4 |
|
| Participants |
Randomised: 72 participants randomised Inclusion criteria
Exclusion criteria
Baseline characteristics N = 69 analysed, mean age 46 years, 50 male Dropouts and withdrawals 12/72 (17%), BIW/QW group (7), QW/QW group (5)
|
|
| Interventions |
Intervention A. Etanercept twice‐a‐week/once‐a‐week group (n = 38), 50 mg SC twice a week for 12 weeks then 50 mg once a week to week 24 Control intervention B. Etanercept once‐a‐week/once‐a‐week group (n = 34), 50 mg SC injections once a week for the full 24‐week treatment period |
|
| Outcomes | Assessments at 24 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1080): "Wyeth Research, which was acquired by Pfizer in October 2009, sponsored this clinical trial and was responsible for the collection and analysis of data. Editorial/medical writing assistance was funded by Pfizer Inc." Declarations of interest: Quote (p 1080): "J.P.O. has been an investigator or consultant for Schering‐Plough, Abbott, Merck‐Serono, Centocor, Pfizer, Janssen‐Cilag, Meda‐Pharma, Pierre‐Fabre, Galderma and Leo‐Pharma. C.P. has been an investigator or consultant for Abbott, Amgen, Celgene, Janssen Cilag, Leo Pharma, Novartis and Pfizer Inc. E.B. has no conflicts of interest. V.M., G.G., Y.B. and J.M.G. are employees of Pfizer Inc." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1081): “Patients were randomised by the investigator or other authorized person using an automatic online enrolment system in a 1:1 ratio”. Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 1081): “Patients were randomised by the investigator or other authorized person using an automatic online enrolment system in a 1:1 ratio”. Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 1081): “This was a multicenter, multinational, randomised, open‐label study”. Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 1081): “This was a multicenter, multinational, randomised, open‐label study”. Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 72 included/69 analysed Quote (p 1082): "All efficacy analyses were based on the modified intent‐to treat (mITT) population, which was defined as all patients who had received one or more doses of ETN and had baseline and post baseline data...The MMRM and GEE models have been developed for the analysis of longitudinal categorical data and to handle missing data without any imputation; this kind of model is preferred to the last‐observation‐carried‐forward approach for analysis of longitudinal data". Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00581100). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Papp 2005.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: not stated Location: 50 centres in USA, Canada and Western Europe |
|
| Participants |
Randomised: 611 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 611, mean age 45 years, male 382 out of 583 participants who received 1 dose Dropouts and withdrawals 52/611 (8.5%)
|
|
| Interventions |
Intervention A. Etanercept (n = 204), SC, 25 mg twice a week, 12 weeks Control interventions B. Etanercept (n = 203), SC, 50 mg twice a week, 12 weeks C. Placebo (n = 204), SC, twice a week, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1304): "This study was supported by Immunex Corporation (Seattle, WA, U.S.A)". Declarations of interest: Quote (p 1304): "S.T. has received research support from Amgen; C.E.M.G. has been a paid consultant for Wyeth and Amgen; A.M.N and R.Z. are both full‐time employees of Amgen." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 1305): "Patients were randomly assigned (using an Interactive Voice Response system) to receive placebo or etanercept)" Comment: not stated |
| Allocation concealment (selection bias) | Low risk | Quote (p 1305): "Patients were randomly assigned (using an Interactive Voice Response system) to receive placebo or etanercept)" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1305): “the patients, study site personnel and all sponsor representatives remained blinded to the initial randomisation treatment groups...” Comment: placebo‐controlled, probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1305): “the patients, study site personnel and all sponsor representatives remained blinded to the initial randomisation treatment groups...” Comment: placebo‐controlled, probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 611 randomised participants, 583 analysed (28 participants did not receive the treatment and were excluded from the analyses). Sensitivity analyses (Table 2) were performed with the 611 randomised participants. Management of missing data: Quote: "In the analyses, missing post baseline efficacy data were imputed using last observation carried forward. In addition, a sensitivity analysis was performed on the binary efficacy endpoints to evaluate the robustness of the primary analysis. This sensitivity analysis included all randomised patients. In addition, rather than using LOCF imputation patients with missing data at a given visit were assumed to have not met the response criteria for that endpoint". Comment: the main result (primary outcome) was not an ITT analysis. |
| Selective reporting (reporting bias) | High risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported except for the results of participant‐reported endpoints summarised in a separate publication. |
Papp 2012a.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: December 2009 to April 2010 Location: 23 centres worldwide Phase 3 |
|
| Participants |
Randomised: 198 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 198, mean age 42 years, 107 male Dropouts and withdrawals 10/198 (5%)
|
|
| Interventions |
Intervention A. Brodalumab 70 (n = 39), SC, 70 mg, day 1 and weeks 1, 2, 4, 6, 8, 10; 10 weeks Control intervention B. Brodalumab 140 (n = 39), SC, 140 mg, day 1 and weeks 1, 2, 4, 6, 8, 10; 10 weeks C. Brodalumab 210 (n = 40), SC, 210 mg, day 1 and weeks 1, 2, 4, 6, 8, 10; 10 weeks D. Brodalumab 280 (n = 42), SC, 280 mg, day 1 and weeks 1, 2, 4, 6, 8, 10; 10 weeks E. Placebo (n = 38), SC, day 1 and weeks 1, 2, 4, 6, 8, 10; 10 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1182): "The study was funded by Amgen". Declarations of interest: Quote (pp 1188‐9): "Dr. Papp reports receiving consulting fees from Abbott, Amgen, Astellas, Celgene, Centocor, Eli Lilly, Galderma, Graceway Pharmaceuticals, Janssen, Johnson & Johnson, Merck, Norvartis, Pfizer, and UCB, lecture fees from Abbott, Amgen, Astellas, Celgene, Centocor, Galderma, Janssen, LEO Pharma, Merck, Novartis, Pfizer, and Stiefel, and grant support from Abbott, Amgen, Astellas, Celgene, Centocor, Eli Lilly, Galderma, Glaxo‐SmithKline, Graceway Pharmaceuticals, Janssen, Johnson & Johnson, Medimmune, Merck, Novartis, Pfizer, Stiefel, and UCB; Dr. Leonardi, receiving consulting fees from Abbott, Amgen, Centocor, Eli Lilly, and Pfizer, lecture fees from Abbott and Amgen, and investigator fees from Abbott, Amgen, Celgene, Centocor, Galderma, GlaxoSmithKline, Incyte, Maruho, Novartis, Novo Nordisk, Pfizer, Schering‐Plough (now Merck), Sirtris, Stiefel, Vascular Biogenics, and Wyeth (now Pfizer); Dr. Menter, receiving consulting fees from Abbott, Amgen, Astellas, Centocor, Galderma, Genentech, and Wyeth, lecture fees from Abbott, Amgen, Centocor, Galderma, and Wyeth, and fees for expert testimony from Galderma; Dr. Krueger, receiving consulting fees from Centocor, Eli Lilly, and Pfizer and grant support from Amgen, Centocor, Eli Lilly, Merck, and Pfizer; and Drs. Krikorian, Aras, Li, Russell, Thompson, and Baumgartner being full‐time employees of Amgen. No other potential conflict of interest was relevant to this article was reported." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (protocol p 30): “Randomization: IVRS will be used to randomise subjects into the study. The randomisation list will be generated by Amgen using a permuted block design within each of 4 strata based on BMI at baseline, and participation in the PK study”. Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (protocol p 30): “Randomization: IVRS will be used to randomise subjects into the study." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (protocol p 24 and 50): “double‐blind placebo controlled... Subjects randomised to active drug will receive additional placebo injections as necessary to maintain the blind”. Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (protocol p 39): "PASI assessments will be performed by a blinded assessor. The blinded assessor will be a healthcare professional who has been certified as trained with the standard PASI". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 198 included/198 analysed Quote (p 1183): "The analyses of efficacy endpoints were performed on data from all patients who underwent randomisation (full set analysis), according to the intention‐to‐treat principle... Missing data were handled by means of the baseline‐value‐carried‐forward method or the imputation of no response". Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00307437). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Papp 2012c.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: September 2008 to October 2009 Location: 35 centres in Canada and USA Phase 2 |
|
| Participants |
Randomised: 352 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 352, mean age 44 years, 221 male Dropouts and withdrawals 65/352 (11%) at 16 weeks
|
|
| Interventions |
Intervention A. Apremilast (n = 88), orally, 30 mg, twice a day, 16 weeks Control intervention B. Apremilast (n = 176), orally, 10 mg to 20 mg twice a day, 16 weeks C. Placebo (n = 88), orally, twice a day 16 weeks |
|
| Outcomes | Assessments at 16 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 738): "Funding Celgene Corporation" Declarations of interest: Quote (p 745): "KP has served as an investigator for Abbott, Amgen, Celgene, Centocor, Galderma, Incyte, Isotechnika, Janssen, Lilly, Medimmune, Merck, Novartis, and Pfizer; an adviser for Abbott, Amgen, Astellas, BMS, Celgene, Centocor, Galderma, Incyte, Isotechnika, Janssen, Johnson & Johnson, Lilly, Medimmune, Merck, Novartis, Pfizer, and UCB; and a speaker for Abbott, Amgen, Astellas, Celgene, Centocor, Isotechnika, Janssen, Novartis, and Pfizer. JCC has served as an investigator for Celgene, Centocor, Novartis, and Pfizer; as a speaker for Centocor and Abbott; and as an adviser for Pfizer, Abbott, and Novartis. LR has been a paid investigator for doing clinical trials for Amgen, Genentech, Abbott, Centocor, Basilea, Leo, Isotechnika, Stiefel, GSK, Galderma, 3‐M, Serono, Novartis, Astellas, UCB, Celgene, Johnson & Johnson, and Pfizer. HS has served as an investigator for Abbott, Centocor, Celgene, Amgen, and Pfizer; as a speaker for Abbott and Centocor; and as an adviser for Centocor. RGL has served as an investigator for Abbott, Centocor, Celgene, Amgen, Pfizer, Johnson & Johnson/Ortho Biotech, and Novartis; as a speaker for Abbott, Centocor, Amgen, Pfizer, Johnson & Johnson/Ortho Biotech, and Novartis; and as an adviser for Abbott, Centocor, Celgene, Amgen, Pfizer, Johnson & Johnson/Ortho Biotech, and Novartis. RTM has served as an investigator for Abbott, Centocor, Celgene, Amgen, Novartis, Lilly, Pfizer, Allergan, and Galderma; as a speaker for Centocor and Amgen; and as an adviser for Centocor. CH and RMD are employees of Celgene Corporation." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 739): "Eligible patients were randomly assigned in a 1:1:1:1 ratio to oral apremilast 10 mg twice daily, apremilast 20 mg twice daily, apremilast 30 mg twice daily, or placebo, with a permuted‐block randomisation list generated by an interactive voice response system (ClinPhone, East Windsor, NJ, USA)." Comment: clearly described |
| Allocation concealment (selection bias) | Low risk | Quote (p 739): "Eligible patients were randomly assigned in a 1:1:1:1 ratio to oral apremilast 10 mg twice daily, apremilast 20 mg twice daily, apremilast 30 mg twice daily, or placebo, with a permuted‐block randomisation list generated by an interactive voice response system (ClinPhone, East Windsor, NJ, USA)." Comment: clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 739): "Treatment was double‐blind for the first 16 weeks of the 24‐week treatment phase." Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 739): "Treatment was double‐blind for the first 16 weeks of the 24‐week treatment phase." Comment: probably done, placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 352 included/352 analysed Quote (p 740): "Efficacy data were assessed by intention to treat. Missing data were handled with the last‐observation carried‐forward method." Comment: numbers lost to follow‐up and reasons comparable across groups |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00773734). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Papp 2013a.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: March 2010 to February 2011 Location: 19 international centres Phase 2 |
|
| Participants |
Randomised: 125 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 125, mean age 46 years, 91 male Dropouts and withdrawals 47/125 (38%) at 36 weeks: secukinumab 25 (15): secukinumab 75 (10); secukinumab 225 (4); secukinumab 450 (7); placebo (11)
|
|
| Interventions |
Intervention A. Secukinumab (n = 29), SC, 25 mg, 0, 4, 8 weeks, 12 weeks Control intervention B. Secukinumab (n = 26), SC, 3 x 25 mg, 0, 4, 8 weeks, 12 weeks C. Secukinumab (n = 21), SC, 3 x 75 mg, 0, 4, 8 weeks, 12 weeks D. Secukinumab (n = 27), SC, 3 x 150 mg, 0, 4, 8 weeks, 12 weeks E. Placebo (n = 22), SC, 0, 4, 8 weeks, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes |
Funding source: Quote (p412): "Novartis Pharm AG, Basel, Switzerland" Declarations of interest: Quote (Appendix): "K.A.P. has received honoraria for lecturing at industry‐sponsored meetings and has received industry funding for presentations and consultation at national and international meetings; he has also received research grants from and been a paid consultant to Novartis and other pharmaceutical companies; has served as a scientific officer for pharmaceutical and biotechnology corporations; and is a participant on clinical, scientific and corporate advisory boards. R.G.L. has been a member of scientific advisory boards and served as a clinical investigator for Abbott, Amgen, Celgene, Centocor⁄Johnson & Johnson, Eli Lilly, Fujisawa, Novartis and Pfizer, and has served as a speaker for Abbott, Amgen, Centocor⁄Johnson & Johnson, Fujisawa and Novartis. B.S. has consulted for Novartis and several other pharmaceutical companies; he has been a member of an advisory board for Novartis and several other pharmaceutical companies. S.H., H.J.T., C.P. and H.B.R. are full‐time employees of and own stock in Novartis. M.A., D.R.B. and P.K. declare no conflicts of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 414): “The randomisation numbers were generated by an interactive voice response provider using a validated automated system”. Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 414): “The randomisation numbers were generated by an interactive voice response provider using a validated automated system”. Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (pp 413‐4): “Double‐blind, placebo controlled...Patients, investigator staff, persons performing the assessments and data analysts were blinded ... remained blind until final database lock”. Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (pp 413‐4): “Double‐blind, placebo controlled...Patients, investigator staff, persons performing the assessments and data analysts were blinded ... remained blind until final database lock”. Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 125 included/125 analysed Quote (p 415): "The full analysis set consisted of all patients who were randomised... The missing score was imputed by carrying forward the last non missing post baseline PASI". Comment: very high number of withdrawals (38%) |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01071252). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Papp 2013b.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: April 2006 to May 2007 Location: multicentre (30) in Canada, the Czech Republic, and Germany Phase 2 |
|
| Participants |
Randomised: 260 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 260, mean age 46 years, 163 male Dropouts and withdrawals 47/260 (18%) at 12 weeks
|
|
| Interventions |
Intervention A. Apremilast (n = 173), orally, 10 mg to 20 mg, twice a day, 12 weeks Control intervention B. Placebo (n = 87) |
|
| Outcomes | Assessments at 12 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 27): "This study was sponsored by Celgene Corporation". Declarations of interest: Quote (p 27): "Dr Papp is a consultant and investigator for Celgene Corporation, Abbott, Amgen, Centocor, Janssen‐Ortho, Merck, Novartis and Pfizer and an investigator for Astellas, Leo Pharma and Galderma, receiving honoraria and grants. Dr Kaufmann is an investigator for Abbott, Centocor, Leo, Novartis, Wyeth and Celgene Corporation, but has not received financial compensation. The Department of Dermatology received investigator fees for performing the clinical trials. He served as a speaker for Basilea and Allmiral and received honoraria from each. Dr Thac ¸ is on the advisory board of and is a consultant, investigator and speaker for Abbott, Leo, Novartis, Pfizer, Biogen‐Idec, Janssen‐Cilag and MSD, and received honoraria from each. He is also an investigator for Celgene Corporation. The Department of Dermatology received honoraria ⁄ compensation for conducting studies; no direct compensation was received. Ms Hu receives a salary as an employee of Celgene Corporation. Ms Sutherland receives a salary, stocks and stock options as an employee of Celgene Corporation. Dr Rohane received a salary and stock options as a former employee of Celgene Corporation." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 377): " investigators randomised subjects 1 : 1: 1 to double‐blind treatments for 12 weeks with placebo, apremilast 20 mg QD or apremilast 20 mg twice daily". Comment: no description of the method to guarantee the random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote (p 377): "Using an interactive voice response system, investigators randomised subjects 1 : 1: 1 to double‐blind treatments". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 377): "One capsule of placebo or apremilast was taken orally in the morning before meals, and one capsule of placebo or apremilast was taken in the evening". Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 377): "One capsule of placebo or apremilast was taken orally in the morning before meals, and one capsule of placebo or apremilast was taken in the evening". Comment: probably done, placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 260 included/260 analysed Management of missing data was not described, and substantial number lost to follow‐up (18%) |
| Selective reporting (reporting bias) | High risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00606450). The prespecified outcomes listed on ClinicalTrials.gov were not detailed, the choice of the primary outcome was not clearly defined. In the Methods section, PASI 75 was defined as the primary outcome; no QoL outcomes were listed in the Methods section, although they were in the protocol on ClinicalTrials.gov. |
Papp 2015.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: November 2010 to June 2012 Location: 64 centres in Europe, Asia, and North America Phase 2 |
|
| Participants |
Randomised: 355 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 355, mean age 45 years, 270 male Dropouts and withdrawals 15/355 (4.5%)
|
|
| Interventions |
Intervention A. Tildrakizumab (n = 42), SC, 5 mg weeks 0, 4, every 12 weeks Control interventions B. Tildrakizumab (n = 92), SC, 15 mg weeks 0, 4, every 12 weeks C. Tildrakizumab (n = 89), SC, 50 mg weeks 0, 4, every 12 weeks D. Tildrakizumab (n = 86), SC, 100 mg weeks 0, 4, every 12 weeks E. Tildrakizumab (n = 46), SC, 200 mg weeks 0, 4, every 12 weeks |
|
| Outcomes | Assessments at 16 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 930): “This study was funded by Merck & Co, nc., Kenilworth, NJ, USA”. Declarations of interest: Quote (Appendix 1): "E.P.B., A.M., Q.L., Y.Z. and R.S. are current or former employees of Merck & Co., Inc. K.P. has served as a consultant, advisory board member and/or investigator for Abbott (AbbVie), Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, Centocor, Foreward Pharma, Galderma, Genentech, Incyte, Isotechnika, Janssen, Kyowa Kirin, LEO Pharma, Lilly, Medimmune, Merck Sharp Dome, Merck Serono, Novartis, Regeneron, Stiefel, Takeda, Pfizer and USB. D.T. has served as a consultant, advisory board member and/or investigator for Abbott (AbbVie), Almiral, Amgen, Astellas, Biogen Idec, Boehringer Ingelheim, Celgene, Dignity, Forward Pharma, Galderma, GlaxoSmithKline, Isotechnika, Janssen‐Cilag, LEO Pharma, Lilly, Maruho, Medac, Medimmune, Merck Sharp Dome, Merck Serono, Novartis, Regeneron, Sandoz, Sanofi‐Aventis, Takeda and Pfizer. K.R. has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Amgen, Biogen Idec, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, LEO Pharma, Lilly, Medac, Merck, Novartis, Pfizer, Vertex and Takeda. E.R. has received travel support and nonfinancial support for histology study report preparation from Merck & Co., Inc., and has received speaker’s fees and travel support, or served on advisory boards for Abb‐ Vie, Novartis, Pfizer, Janssen and Amgen. R.G.L. has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Celgene, Centocor, Janssen‐Cilag, LEO Pharma, Merck, MSD (formerly Essex, Schering‐Plough), Novartis and Pfizer (formerly Wyeth). J.G.K. has received personal fees (consulting and/or speaking fees) and grants paid to his institution from Novartis, Pfizer, Janssen, Lilly, Merck, Kadmon, Dermira, Boehringer and BMS; Amgen, Innovaderm, Paraxel and Kyowa have paid grants to J.G.K.’s institution; J.G.K. has also received personal fees from Serono, Biogen Idec, Delenex, AbbVie, Sanofi, Baxter, Xenoport and Kineta. A.B.G. has current consulting/advisory board agreements with Amgen Inc., Astellas, Akros, Centocor (Janssen) Inc., Celgene Corp., Bristol Myers Squibb Co., Beiersdorf Inc., Abbott Labs (AbbVie), TEVA, Actelion, UCB, Novo Nordisk, Novartis, Dermipsor Ltd, Incyte, Pfizer, Canfite, Lilly, Coronado, Vertex, Karyopharm, CSL Behring Biotherapies for Life, GlaxoSmithKline, Xenoport, Catabasis Meiji Seika Pharma Co., Ltd, Takeda, Mitsubishi Tanabe Pharma Development America, Inc, and has received research/educational grants (paid to Tufts Medical Center) from Centocor (Janssen), Amgen, Abbott (Abb‐ Vie), Novartis, Celgene, Pfizer, Lilly, Coronado, Levia, Merck and Xenoport. H.N. has received consultancy/speaker honoraria and/or grants from Novartis, Tanabe Mitsubishi, Maruho, Abbott/AbbVie, Eli Lilly, Merck Sharp & Dohme, Janssen and LEO Pharma." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 931): "Randomisation of treatment and allocation was done centrally by means of an interactive web response system…" Comment: no description of the method used to guarantee the random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote (p 931): “Randomisation of treatment and allocation was done centrally by means of an interactive web response system…” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 931): “double‐blind" Comment: no description of the method used to guarantee blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 932): “double‐blind" Comment: no description of the method used to guarantee blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 355, analysed 352 Management of missing data: Quote (p 932): “The primary analysis was performed on all randomised participants who received at least one or more doses of treatment. Participants who discontinued treatment prior to week 16... were considered to not have achieved PASI 75 at week 16". Comment: low number lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01225731). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Papp 2017a.
| Study characteristics | ||
| Methods | RCT, phase 3, randomised, double‐blind, active‐controlled study Date of study: August 2014 to March 2015 Location: worldwide |
|
| Participants |
Randomised: 350 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 350, mean age of 44 years, 208 male Dropouts and withdrawals 42/350 (12%): biosimilar group (23), Humira 50 group (19)
|
|
| Interventions |
Intervention A. ABP 501 at an initial loading dose of 80 mg subcutaneously on week 1/day 1, followed by 40 mg subcutaneously every other week (starting at week 2) for 16 weeks, n = 175 Control intervention B. Adalimumab, Humira, at an initial loading dose of 80 mg subcutaneously on week 1/day 1, followed by 40 mg subcutaneously every other week (starting at week 2) for 16 weeks, n = 175 |
|
| Outcomes | At week 16 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1093): "Amgen Inc funded this study and participated in the design and conduct of the study; collection, management, analysis, and interpretation of data; and preparation, review, and approval of the manuscript. All authors were involved in the decision to submit the manuscript for publication, and had the right to accept or reject comments or suggestions. A medical writer employed by MedVal Scientific Information Services LLC and funded by Amgen Inc participated in the writing of this manuscript and is acknowledged." Declarations of interest: Quote (p 1093): "Dr Papp has served as a consultant, speaker, scientific officer, steering committee member, investigator,or advisory board member for3M, Abbott, Akesis, Akros, Alza, Amgen, Astellas, Baxter, BMS, Boehringer Ingelheim, CanFite, Celgene, Cipher, Dermira, Eli Lilly, Forward Pharma, Funxional Therapeutics, Galderma, GSK, Isotechnika, Janssen, Johnson & Johnson, Kirin, Kyowa, Lypanosys, MedImmune, Merck‐Serono, Mitsubishi Pharma, MSD, Novartis, Pfizer, Roche, Takeda, UCB, Valeant, and Vertex. Dr Bachelez has served as a consultant, speaker, steering committee member, investigator, or advisory board member for AbbVie, Amgen, Baxalta, Boehringer‐Ingelheim, Celgene, Janssen, LEO Pharma, Lilly, MSD, Novartis, Pfizer, and Takeda, and received grant support from Pfizer. Dr Costanzo has been an investigator/consultant and speaker for AbbVie, Amgen, Celgene, Janssen, Lilly, Novartis, and Pfizer. Dr Foley has served as a consultant, investigator, speaker, and/or advisor for, and/or received travel grants from Galderma, LEOPharma/Peplin, Ascent,Clinuvel, Janssen‐Cilag, Eli Lilly, Australian Ultraviolet Services, Roche, CSL, 3M/iNova/Valeant, GSK/ Stiefel, Abbott/AbbVie, Biogen Idec, Merck Serono, Schering‐Plough/MSD, Wyeth/Pfizer, Amgen, Novartis, Celgene, Aspen, Boehringer Ingelheim, and BMS. Dr Gooderham has been an investigator, consultant, and/or speaker for AbbVie,Amgen, Boehringer Ingelheim, Celgene,Coherus, Dermira, Galderma, Janssen, LEO Pharma, Lilly, Medimmune, Merck Serono, Novartis, Regeneron, Roche, Sanofi Genzyme, Takeda, and Pfizer. Dr Kaur is an Amgen employee and stockholder. Dr Narbutt is an investigator for Amgen. Dr Philipp has been investigator, consultant, and/or speaker for AbbVie, Amgen, Almirall, Biogen, Boehringer‐Ingelheim, BMS, Celgene, Janssen, LEO Pharma, Lilly, MSD, Novartis, Pfizer, and UCB. Dr Spelman has served on advisory boards for Galderma, Novartis, and AbbVie; undertakes sponsored clinical research for AbbVie, Amgen, Anacor, Ascend Biopharmaceuticals, Astellas, Australian Wool Innovation Limited, Blaze Bioscience, Celgene, Dermira, Eli Lilly, Galderma, Genentech, GlaxoSmith Kline, Kythera, LEO Pharma, Merck, Novartis, Phosphagenics, Regeneron, and Trius; and has received sponsored travel from Abbott, Novartis, and Janssen‐Cilag. Dr Weglowska has been an investigator for Amgen, Pfizer, Novartis, Galderma, Eli Lilly, Dermira, Roche, Janssen‐Cilag, Coherus, Genentech, LEO Pharma, Merck, Mylan, and Regeneron. Dr Zhang is an Amgen employee and stockholder. Dr Strober has served on a speakers bureau for AbbVie, receiving honoraria; is a consultant and advisory board member for AbbVie, Amgen, Astra Zeneca, Celgene, Dermira, Forward Pharma, Janssen, LEO Pharma, Eli Lilly, Cutanea‐Maruho, Medac, Novartis, Pfizer, Sun, Stiefel/GlaxoSmithKline, UCB, and Boehringer Ingelheim, receiving honoraria for all; is an investigator for AbbVie, Amgen, GlaxoSmithKline, Novartis, Lilly, Janssen, Merck, XenoPort, Xoma, Celgene (payments to the University of Connecticut); is scientific director for Corrona Psoriasis Registry, receiving a consulting fee; received grant support to the University of Connecticut for a fellowship program from AbbVie and Janssen." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1095): "This randomized, double‐blind, multicenter, active‐controlled phase III trial consisted of a 4‐week screening period, after which eligible patients were randomized 1:1 to receive treatment with ABP 501 or adalimumab...Randomization was carried out by a computer‐generated randomization schedule with stratification by prior biologic use and geographic region. Patients were allocated by an interactive voice and web response system." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 1095): "This randomized, double‐blind, multicenter, active‐controlled phase III trial consisted of a 4‐week screening period, after which eligible patients were randomized 1:1 to receive treatment with ABP 501 or adalimumab...Randomization was carried out by a computer‐generated randomization schedule with stratification by prior biologic use and geographic region. Patients were allocated by an interactive voice and web response system." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1095): "This randomized, double‐blind, multicenter, active‐controlled phase III trial consisted of a 4‐week screening period, after which eligible patients were randomized 1:1 to receive treatment with ABP 501 or adalimumab...During the study, the patients, investigators, study center personnel, and sponsor remained blinded to the patient’s randomized treatment assignment. ABP 501 and adalimumab were administered in identical syringes". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1095): "This randomized, double‐blind, multicenter, active‐controlled phase III trial consisted of a 4‐week screening period, after which eligible patients were randomized 1:1 to receive treatment with ABP 501 or adalimumab...During the study, the patients, investigators, study center personnel, and sponsor remained blinded to the patient’s randomized treatment assignment. ABP 501 and adalimumab were administered in identical syringes". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p. 1096): "Efficacy data were analyzed using the full analysis set, which included all patients initially randomized in the study with missing values imputed using the last observation carried forward method." Randomised 350; analysed 345 (equivalence design) |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01970488). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Papp 2017b.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: February 2014 to July 2015 Location: worldwide Phase 2 |
|
| Participants |
Randomised: 166 participants Inclusion criteria
Exclusion criteria
With regards to TB, the following applies:
Baseline characteristics N = 166, mean age of 46 years, 109 male Dropouts and withdrawals 9/166 (5.4%): risankizumab 18 (4), risankizumab 90 (2), risankizumab 180 (2), USK (1)
|
|
| Interventions |
Intervention A. Risankizumab (low‐dose) (18 mg BI 655066 administered by SC injection plus 2 placebo‐matching BI 655066 injections at week 0, followed by 2 placebo‐matching BI 655066 injections each at weeks 4 and 16), n = 43 Control intervention B. BI 655066 (median‐dose) (90 mg BI 655066 administered by SC injection plus 2 placebo‐matching BI 655066 injections at week 0, followed 90 mg BI 655066 plus 1 placebo‐matching BI 655066 injection at weeks 4 and 16), n = 41 C. BI 655066 (high‐dose) (180 mg BI 655066 administered by SC injection as 2 injections plus a placebo‐matching BI 655066 injection at week 0, followed 180 mg BI 655066 administered as 2 injections at weeks 4 and 16), n = 42 D. Ustekinumab (Stelara administered by SC injection plus 2 saline injections at week 0, Stelara injection plus 1 saline injection at weeks 4 and 16. Stelara dose was 45 mg for participants with body weight ≤ 100 kg at randomisation or 90 mg for participants with body weight > 100 kg at randomisation), n = 40 |
|
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1553): "The trial was funded by Boehringer Ingelheim". Declarations of interest: Quote (p 1560): "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 1552): "This 48‐week, multicenter, randomized, dose‐ranging, phase 2 trial" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 1552): "The trial was double blind within the risankizumab dose groups and single blind (to patients) with regard to drug (ustekinumab or risankizumab). All efficacy assessments were conducted by an assessor who was unaware of the treatment assignments." Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1552): "The trial was double blind within the risankizumab dose groups and single blind (to patients) with regard to drug (ustekinumab or risankizumab). All efficacy assessments were conducted by an assessor who was unaware of the treatment assignments." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data Quote (p 1553): "Primary and other end points were analyzed on an intention‐to‐treat basis... In the primary analyses, last observation carried forward was prespecified in the trial protocol as the method of handling missing data; a sensitivity analysis with nonresponse imputation was also performed". 166 randomised, 166 analysed Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02054481). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results posted on ClinicalTrials.gov |
Papp 2018.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: November 2016 to November 2017 Location: 82 sites in the USA, Japan, Poland, Canada, Germany, Latvia, Mexico, and Australia Phase 2 |
|
| Participants |
Randomised: 267 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 267, mean of age 45 years and 73% men Dropouts and withdrawals 61/267 (15.%): BMS‐986165_3 EOD (10), BMS‐986165_3 (8), BMS‐986165_3*2 (3), BMS‐986165_6*2 (6), BMS‐986165_12 (2), PBO (14)
|
|
| Interventions |
Intervention A. BMS‐986165 3 mg every other day (EOD) (by mouth), n = 44 Control interventions B. BMS‐986165 3 mg a day (by mouth), n = 44 C. BMS‐986165 3 mg*2 a day (by mouth), n = 45 D. BMS‐986165 6 mg*2 a day (by mouth), n = 45 E. BMS‐986165 12 mg a day (by mouth), n = 44 F Placebo, n = 45 |
|
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source Quote (p 1320): "Supported by Bristol‐Myers Squibb" Declarations of interest Quote (p 1320‐21): "Dr. Papp reports receiving grant support, consulting fees, advisory board fees, and fees for serving on a speakers’ bureau from Amgen, AbbVie, Boehringer Ingelheim, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer, UCB, Valeant Pharmaceuticals, and Kyowa Hakko Kirin, grant support, consulting fees, and fees for serving as a scientific officer from Akros Pharma, consulting fees from Can‐Fite BioPharma, grant support, consulting fees, advisory board fees, fees for serving on a speakers’ bureau, and travel support from Celgene, grant support, consulting fees, and advisory board fees from Merck Sharp & Dohme, PRCL Research, and Takeda, grant support from Anacor Pharmaceuticals, GlaxoSmithKline, and Meiji Seika Pharma, and grant support and consulting fees from Coherus BioSciences and Dermira; Dr. Gordon, receiving grant support and consulting fees from AbbVie, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, and UCB and consulting fees from Amgen, Almirall, Dermira, Leo Pharma, Pfizer, and Sun Pharma; Dr. Thaçi, receiving grant support, lecture fees, consulting fees, and advisory board fees from AbbVie, lecture fees, consulting fees, and advisory board fees from Almirall, Pfizer, Sandoz/Hexal, UCB, Regeneron Pharmaceuticals, and Sanofi, consulting fees and advisory board fees from Boehringer Ingelheim, grant support, lecture fees, consulting fees, advisory board fees, and writing assistance from Celgene and Novartis, and lecture fees, consulting fees, advisory board fees, and writing assistance from Eli Lilly, Leo Pharma, and Janssen‐Cilag; Dr. Morita, receiving grant support and lecture fees from AbbVie, Esai, Kyowa Hakko Kirin, Leo Pharma, Maruho, Mitsubishi Tanabe Pharma, Novartis, and Torii Pharmaceutical and lecture fees from Celgene, Eli Lilly Japan, and Janssen Pharmaceutical; Dr. Gooderham, receiving advisory board fees, fees for serving as principal investigator, and lecture fees from AbbVie, Galderma, Leo Pharma, Pfizer, and Regeneron Pharmaceuticals, advisory board fees and lecture fees from Actelion Pharmaceuticals, fees for serving as principal investigator and consulting fees from Akros Pharma, advisory board fees, fees for serving as principal investigator, lecture fees, and consulting fees from Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Novartis Pharmaceuticals, Sanofi Genzyme, and Valeant Pharmaceuticals, fees for serving as principal investigator from Arcutis Pharmaceuticals, Bristol‐Myers Squibb, Dermira, GlaxoSmithKline, MedImmune, Merck, Roche Laboratories, and UCB, and fees for serving as principal investigator and lecture fees from Glenmark; Dr. Foley, receiving grant support, advisory board fees, fees for serving on a speakers’ bureau, and travel support from AbbVie, Celgene, CSL, Galderma, iNova Pharmaceuticals, Janssen, Leo Pharma, Eli Lilly, Novartis, Pfizer, and Sanofi, grant support and advisory board fees from Amgen and Sun Pharma, grant support from Boehringer Ingelheim, Celtaxsys, Cutanea Life Sciences, Dermira, Genentech, and Regeneron Pharmaceuticals, grant support, advisory board fees, and fees for serving on a speakers’ bureau from GlaxoSmithKline, grant support and consulting fees from Bristol‐Myers Squibb, and grant support, fees for serving on a speakers’ bureau, and travel support from Roche; Dr. Kundu, being employed by Bristol‐Myers Squibb; and Dr. Banerjee, being employed by and holding stock in Bristol‐Myers Squibb. No other potential conflict of interest relevant to this article was reported." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1314):"Randomization was stratified according to previous treatment with a biologic agent (yes or no) and geographic region (Japan or the rest of the world), with the use of a central interactive Web‐response system." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 1314):"Randomization was stratified according to previous treatment with a biologic agent (yes or no) and geographic region (Japan or the rest of the world), with the use of a central interactive Web‐response system." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1314): "Patients were randomly assigned to one of five oral doses of BMS‐986165 (3 mg every other day, 3 mg daily, 3 mg twice daily, 6 mg twice daily, or 12 mg daily) or matching oral placebo in a ratio of 1:1:1:1:1:1. Capsules of the active drug (3 mg) or matched placebo were combined as appropriate to provide the required daily dose and were taken each morning and again 12 hours later...Patients, investigators, and the trial sponsor were unaware of the trial‐group assignments." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1314): "Patients were randomly assigned to one of five oral doses of BMS‐986165 (3 mg every other day, 3 mg daily, 3 mg twice daily, 6 mg twice daily, or 12 mg daily) or matching oral placebo in a ratio of 1:1:1:1:1:1. Capsules of the active drug (3 mg) or matched placebo were combined as appropriate to provide the required daily dose and were taken each morning and again 12 hours later...Patients, investigators, and the trial sponsor were unaware of the trial‐group assignments.'' Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data Quote (p 1315): "For the primary end point of PASI 75 and other binary end points (PASI 50, PASI 90, PASI 100, an sPGA score of 0 or 1, and a DLQI score of 0 or 1), patients who discontinued the trial regimen early or who had a missing value at any time point had outcomes imputed as a nonresponse at that time point, regardless of the status of response at the time of discontinuation." Randomised 267, analysed 267 Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02931838). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
Papp 2021.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: August 2018 to March 2015 Location: worldwide (41 sites) Phase 2b |
|
| Participants |
Randomised: 313 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 313, mean of age 46 years, and 73% men Dropouts and withdrawals
|
|
| Interventions |
Intervention A. M1095 (sonelokimab), 30 mg, given at week 0, 2, 4, 8, 12 and every 4 weeks, n = 52 Control interventions B. M1095, 60 mg, given at week 0, 2, 4, 8, 12 and every 4 weeks, n = 52 C. M1095, 120 mg, given at week 0, 2, 4, 8, 12 and every 8 weeks Q8, n = 53 D. M1095, 120 mg, given at week 0, 2, 4, 8, 12 and every 4 weeks Q4, n = 51 E. Secukinumab 300 mg, n = 53 F. Placebo, n = 52 |
|
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1574): "This study was funded by Avillion." Declarations of interest: Quote (p 1574): "MAW is an employee of Avillion (Northbrook, IL, USA). AM is an employee of Avillion (London, UK). KAP reports grants and personal fees from Avillion, during the conduct of the study; personal fees and non‐financial support from Meiji Seika Pharma outside the submitted work; grants and personal fees from AbbVie, Akros, Amgen, Arcutis, Astellas, Baxalta, Boehringer Ingelheim, Bristol‐Myers Squibb, Canfite, Celgene, Centocor, Coherus, Dermira, Dow Pharma, Eli Lilly and Company, Forward Pharma, Galderma, Genentech, Gilead, GlaxoSmithKline, Janssen, Kyowa Hakko Kirin, LEO, MedImmune, Meiji Seika Pharma, Merck Sharpe & Dohme, Merck‐Serono, Mitsubishi Pharma, Moberg Pharma, Novartis, Pfizer, PRCL Research, Regeneron, Roche, Sanofi Aventis/Genzyme, Samsung Bioepsis, Sun Pharma, Takeda, UCB Pharma, and Valeant/Bausch Health outside the submitted work; and being a consultant (no compensation) for AstraZeneca and Meiji Seika Pharma. KR reports personal fees from Avillion, during the conduct of the study; personal fees from Almirall, Amgen, Centocor, Dermira, GlaxoSmithKline, Samsung Bioepsis, Valeant, and Xenoport outside the submitted work; grants from Galapagos, Miltenyi Biotec, Sun Pharma, Regeneron, and Takeda outside the submitted work; grants and personal fees from AbbVie, Affibody, Biogen, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Covagen, Forward Pharma, Fresenius Medical Care, Janssen‐Cilag, Kyowa Kirin, LEO, Eli Lilly and Company, Medac, Merck Sharpe & Dohme, Novartis, Ocean Pharma, Pfizer, Sanofi, and UCB outside the submitted work; and serving as an advisor for, serving as a paid speaker for, or participating in clinical trials sponsored by AbbVie, Affibody, Almirall, Amgen, Avillion, Biogen, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Centocor, Covagen, Dermira, Forward Pharma, Fresenius Medical Care, Galapagos, Galderma, GlaxoSmithKline, Janssen‐Cilag, Kyowa Kirin, Leo, Lilly, Medac, Merck Sharp & Dohme, Novartis, Miltenyi Biotec, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB, Valeant, and Xenoport." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1567): "Randomisation was done at a study level via a centralised interactive response technology system, which provided blinded treatment kit numbers to the investigator". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 1567): "Participants were enrolled by masked investigators and randomly assigned (1:1:1:1:1:1)....". "Randomisation was done at a study level via a centralised interactive response technology system, which provided blinded treatment kit numbers to the investigator". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1567): "Study drug was prepared and administered at the site by a designated unmasked individual at the study site, who had no other involvement in the trial. Participants and all other site personnel were masked to therapy allocation throughout the study. The study sponsor was unmasked after all participants had completed week 24 treatment and the database was locked." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 1567): "Study drug was prepared and administered at the site by a designated unmasked individual at the study site, who had no other involvement in the trial. Participants and all other site personnel were masked to therapy allocation throughout the study. The study sponsor was unmasked after all participants had completed week 24 treatment and the database was locked." Comment: no detailed description of means used to guarantee absence of communication between blinded and unblinded personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 1568): "The primary outcome was analysed in the intention‐to‐ treat (ITT) population; participants with missing data were considered as nonresponders (nonresponder imputation)." ...."Selected sensitivity analyses (missing response imputed with last observation carried forward, using randomised previous biologic use and bodyweight stratum) were done in the ITT population. The safety population was defined as all patients who received the study drug and and was identical to the defined ITT population". Randomised 313, analysed 313 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03384745). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
PEARL 2011.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: December 2008 to March 2010 Location: 13 centres in Taiwan and Korea |
|
| Participants |
Randomised: 121 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 121, mean age 41 years, 103 male Dropouts and withdrawals 9/121 (7.4%): ustekinumab group (4), placebo group (5)
|
|
| Interventions |
Intervention A. Ustekinumab, SC, 45 mg, weeks 0, 4, 16 + placebo week 12, 16 weeks (n = 61) Control intervention B. Placebo, SC, weeks 0 to 4 + ustekinumab 45 mg weeks 12 to 16 (n = 60) |
|
| Outcomes | Assessments at 12 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 162): "This study was supported by Centocore, Inc". Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 155): “Patients were enrolled in this multicenter..., double‐blind, placebo‐controlled study... Randomization was performed via an interactive voice response system based on minimization with bias‐coin assignment...”“Randomization was conducted via Interactive Response Technology, which assigned a randomisation number that linked the subject to a treatment arm and specified unique medication pack number". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 155): “Patients were enrolled in this multicenter..., double‐blind, placebo‐controlled study... Randomization was performed via an interactive voice response system based on minimization with bias‐coin assignment...” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 155): "Patients were enrolled in this multicenter..., double‐blind, placebo‐controlled study..." Comment: placebo trial, probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 155): “"Patients were enrolled in this multicenter..., double‐blind, placebo‐controlled study..." Comment: placebo trial, probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 121, analysed 121 Quote (p 156): “For all efficacy analyses, patients were analysed by assigned treatment groups...Data were analysed by intent‐to‐treat for the primary endpoint... Patients who discontinued study treatment... were judged as non‐responders for binary endpoints”. Comment: ITT analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
PHOENIX‐1 2008.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: December 2005 to September 2007 Location: 48 centres in the USA, Canada, Belgium Phase 3 |
|
| Participants |
Randomised: 766 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 766, mean age of 45 years, 531 male Dropouts and withdrawals 23/766 (3%) :
|
|
| Interventions |
Intervention A. Ustekinumab (n = 255), SC, 45 mg, weeks 0 to 4 and every 12 weeks, 40 weeks Control intervention B. Ustekinumab (n = 256), SC, 90 mg, weeks 0 to 4 and every 12 weeks, 40 weeks C. Placebo (n = 255), SC, weeks 0 to 4, 40 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1665): "Centocor Inc." Declarations of interest: Quote (p 1673): "CLL has served as a consultant for Abbott, Amgen, Centocor, and Genentech, as an investigator for Abbott, Allergan, Altana, Alza, Amgen, Astellas, Celgene, Centocor, Genentech, Bristol Myers, Eli Lilly, Fujisawa, Galderma, CombinatoRx, 3M Pharmaceuticals, Perrigo Isreal Pharamceutical, ScheringPlough, Serono, RTL, Novartis, Vitae, and Wyeth, and as a speaker for Abbott, Amgen, Centocor, Genentech, and Warner Chilcott. ABK has served as an investigator and consultant for Abbott, Amgen, and Centocor and has been a study steering committee member, speaker, and fellowship funding recipient from Centocor. KAP has served as a consultant and advisory board member for Abbott, Alza, Amgen, Celgene, Centocor, Johnson and Johnson, Isotechnika, Janssen Ortho Biotech, Medimmune, MerckSerono, and Wyeth. KBG has served as a consultant for Abbott, Amgen, Astellas, Centocor, and Genentech and has received grant support from Abbott, Astellas, and Centocor. NY, CG, YW, SL, and LTD are employees of Centocor and own stock in Johnson and Johnson." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (pp. 1667‐8): “...via a centralised interactive voice response system” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (pp. 1667‐8): “...via a centralised interactive voice response system” Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (pp. 1666‐7): “This phase 3, double‐blind, placebo‐controlled... Patients received placebo injections as needed to preserve the blind. The study sponsor was unblinded to treatment... Site monitors, investigators, site personnel involved in the study conduct, and patients remained blinded until week 76”. Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (pp. 1666‐7): “This phase 3, double‐blind, placebo‐controlled... Patients received placebo injections as needed to preserve the blind. The study sponsor was unblinded to treatment... Site monitors, investigators, site personnel involved in the study conduct, and patients remained blinded until week 76”. Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Included 255/256/255 Analysed 255/256/255 Quote (p. 1668): "Efficacy data from all randomised patients were analysed according to the assigned treatment group.... Patients who discontinued study treatment... were deemed to be treatment failures". Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00267969). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
PHOENIX‐2 2008.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: March 2006 to September 2007 Location: 70 centres in Europe and North America |
|
| Participants |
Randomised: 1230 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 1230, mean age of 45 years, 840 male Dropouts and withdrawals 33/1230 (2.7%)
|
|
| Interventions |
Intervention A. Ustekinumab (n = 409), SC, 45 mg, weeks 0 to 4 and every 12 weeks, 52 weeks Control intervention B. Ustekinumab (n = 411), SC, 90 mg, weeks 0 to 4 and every 12 weeks, 52 weeks C. Placebo (n = 410), SC, weeks 0 to 4, 4 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes |
Funding source: Centocor Inc (p 1675) Declaration of interest: Quote (p 1684): "KP has served as a consultant and advisory board member for Abbott, Alza, Amgen, Celgene, Centocor, Isotechnika, Janssen Ortho Biotech, Johnson & Johnson, Medimmune, MerckSerono, and Wyeth. RGL has received research grants, served on scientific advisory boards, and has been a speaker for Amgen, Biogen‐Idec, Centocor, Genentech, Novartis, Schering‐Plough, and Serono. ML has received honoraria, served as a speaker and advisory board member for Abbott, Amgen, Centocor, Genentech, and Stiefel, and has served as an advisory board member for Astellas and a consultant for UCB. GK has received fees as a consultant or advisory board member for Abbott, Almirall, Alza, Amgen, Anacor, Astellas, Barrier Therapeutics, Boehringer Ingleheim, Bristol Myers Squibb, Centocor, CombinatoRx, Exelixis, Genentech, Genzyme, Isis, L’Oreal, Lupin Limited, Magen Biosciencs, MedaCorp, Medicis, Novartis, Nova Nordisc, Schering‐Plough, Somagenics, theDerm.org, Synvista, Warner Chilcot, UCB, USANA Health Sciences, and ZARS, owns equities and stock in ZARS, and has received lecture fees from Abbott, Amgen, Astellas, Boehringer Ingleheim, Centocor, Connetics, National Psoriasis Foundation, The Foundation for Better Health Care, and Warner Chilcot, and has received partial stipend support for a clinical research fellowship from Abbott, Amgen, and Centocor. KR has received honoraria as a consultant and advisory board member and acted as a paid speaker for Abbot, Biogen‐Idec, Centocor, Janssen‐Cilag, Schering‐Plough, MerckSerono, UCB, and Wyeth. PS, NY, CG, M‐CH, YW, SL, and LTD are employees of Centocor. PS, NY, CG, YW, SL, and LTD own stock in Johnson and Johnson." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1676): “Patients were randomly assigned... with bias coin assignment via a centralised interactive voice response system (ClinPhone, East Windsor, NJ, USA)”. Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 1676): “Patients were randomly assigned... with bias coin assignment via a centralised interactive voice response system (ClinPhone, East Windsor, NJ, USA)”. Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (pp. 1676‐7): “Double‐blind, ..., placebo‐controlled... Site monitors investigators personnel involved in the study conduct, and patients remained blinded... until W52”. Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (pp. 1676‐7): “Double‐blind, ..., placebo‐controlled... Site monitors investigators personnel involved in the study conduct, and patients remained blinded... until W52”. Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1230 included/1230 analysed Quote (p 1679): "Efficacy data were analysed by the assigned treatment group... Non‐responder status was assigned for binary variables ... for those patients who discontinued study treatment ..." Comment: ITT analyses |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00307437). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
PIECE 2016.
| Study characteristics | ||
| Methods | RCT, active‐controlled study Date of study: April 2009 to June 2011 Location: 5 centres in The Netherlands |
|
| Participants |
Randomised: 48 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 48, mean age of 44 years, 31 male Dropouts and withdrawals 15/50 (30%)
|
|
| Interventions |
Intervention (n = 48) A. Infliximab (n = 25), IV, 5 mg/kg, weeks 0, 2, 6, 15, 22 Control intervention B. Etanercept (n = 23), SC, 50 mg twice‐weekly |
|
| Outcomes | Assessment at 24 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1): "study was funded by a program grant from the Netherlands Organization for Scientific Research‐Medical Sciences (NWO‐MW; project 152001006)." Declaration of interest: Quote: "A.C.Q. de Vries: none reported; H.B. Thio: has been a consultant and invited speaker for Biogen/Idec, Janssen, Abbvie, Pfizer, MSD, Leopharma, Teva and Novartis. He has received educational grants from Abbvie, Janssen, Pfizer and Biogen/Idec.; W.J.A. de Kort: medical advisor for Novartis; B.C. Opmeer: none reported; H.M. van der Stok: Involved in performing clinical trials with Abbvie, Pfizer, Novartis, Janssen, BioClinic, AMGEN and LeoPharma.; E.M.G.J. de Jong: received research grants for the independent research fund of the department of dermatology of University Medical Centre St Radboud Nijmegen, the Netherlands from AbbVie, Pfizer, and Janssen. Has acted as consultant and/or paid speaker for and/or participated in research sponsored by companies that manufacture drugs used for the treatment of psoriasis including AbbVie, Janssen, MSD, and Pfizer.; B. Horvath: Unrestricted Educational Grant from AbbVie, IIS Studies by Janssen, AbbVie, Performing clinical trial Novartis, Solenne B.V., Consultancies: Abbvie, Janssen, Philips, Galderma.; J.J.V.Busschbach: none reported; T.E.C. Nijsten: received research grants for the independent research fund of the department of dermatology of Erasmus MC, Rotterdam, the Netherlands from AbbVie, Leo Pharma, MSD, Pfizer, and Janssen. Has acted as consultant and/or paid speaker for and/or participated in research sponsored by companies that manufacture drugs used for the treatment of psoriasis including AbbVie, Leo Pharma, Galderma, Janssen, MSD, and Pfizer. ; Ph.I. Spuls: consultancies in the past for Leopharma, AbbVie and Novartis. In the past an independent research grant from Schering Plough and from Leopharma. Involved in performing clinical trials with many pharmaceutical industries that manufacture drugs used for the treatment of psoriasis." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (pp. 4 & 8): “...was a multi‐centre, single‐blind, investigator initiated, randomised controlled trial comparing infliximab and etanercept in the treatment of moderate to severe chronic plaque type psoriasis... Adequate generation of an unpredictable allocation sequence and concealment of allocation was achieved by using a secure online internet facility (the TEN‐ALEA Clinical Trial Data Management System, provided by the Trans European Network http://www.tenalea.com/) performed in the coordinating centre by the main investigators. The sequence was generated in random block sizes of two and four to ensure it was unknown and not predictable by the investigators involved in randomising participants." Comment: done |
| Allocation concealment (selection bias) | Low risk | Quote (pp. 4 and 8): “...was a multi‐centre, single‐blind, investigator initiated, randomised controlled trial comparing infliximab and etanercept in the treatment of moderate to severe chronic plaque type psoriasis... Adequate generation of an unpredictable allocation sequence and concealment of allocation was achieved by using a secure online internet facility (the TEN‐ALEA Clinical Trial Data Management System, provided by the Trans European Network http://www.tenalea.com/) performed in the coordinating centre by the main investigators. The sequence was generated in random block sizes of two and four to ensure it was unknown and not predictable by the investigators involved in randomising participants." Comment: done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (pp. 4 & 8): “...was a multi‐centre, single‐blind, investigator initiated, randomised controlled trial comparing infliximab and etanercept in the treatment of moderate to severe chronic plaque type psoriasis..." Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 8): "Efficacy outcomes were carried out by trained assessors who were blinded to treatment allocation." Comment: no clear description of measures taken to guarantee the blinding of investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 50, analysed 48 Quote (pp. 8 and 9): "Missing data on primary endpoint were imputed using last observation carried forward. Analyses were carried out according to intention‐to‐treat (ITT) principle, apart from the longer term data where a per protocol analysis (PPA) was performed" Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | The trial was prospectively registered on the Dutch Trial Register: www.trialregister.nl/trialreg/index.asp; NTR 1559 The prespecified outcomes mentioned in the Methods section appeared to have been reported |
Piskin 2003.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: not stated Location: Amsterdam and throughout the Netherlands, number not stated |
|
| Participants |
Randomised: 10 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 10, mean age of 43 years, 7 male Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ciclosporin (n = 5), orally, 3 mg/kg/d, 16 weeks Control intervention B. Methotrexate (n = 5), orally, 15 mg/kg/week, 16 weeks |
|
| Outcomes | Assessments at 12 weeks Primary and secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes |
Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 559): "Patients were randomised..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 559): "Patients were randomised..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 559): “Laboratory results were obtained in a blinded fashion before randomisation and at week 12 of therapy. The code was broken only after all definitive results were obtained from all participating patients." Comment: open‐label trial, no double dummy used to guarantee blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of the method used to guarantee blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 included/10 analysed Comment: no statistical analyses section; however, the results were available for the 10 participants initially randomised. Methods for dealing with missing data: not applicable |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
PLANETA 2021.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, double‐blind study Date of study: December 2017 to June 2019 Location: Russia (24 sites) Phase 3 |
|
| Participants |
Randomised: 213 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 213, mean of age 61 years, and 73% men Dropouts and withdrawals 3/213 (1.4%): netakimab Q2W (0), netakimab Q4W (2), placebo (1)
|
|
| Interventions |
Interventions A. Netakimab (BCD‐085) Q2W 120 mg (2 SC injections, 60 mg in 1.0 mL each) at week 0, week 1, week 2, week 4, week 6, week 8, and week 10, n = 85 B. Netakimab (BCD‐085) Q4W 120 mg (2 SC injections, 60 mg in 1.0 mL each) at week 0, week 1, week 2, week 6, and week 10. For the purpose of blind design, patients will receive a placebo (2 injections) at week 4 and week 8, n = 84. Control intervention C. Placebo, n = 44 |
|
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p1330): "Sponsorship for this study and the Rapid Service Fee were funded by JSC BIOCAD, Ul. Italianskaya 17, St Petersburg, Russia, 191186." Declarations of interest: Quote (p1331): "Luis Puig has received consultancy/speaker’s honoraria from and/or participated in clinical trials sponsored by Abbvie, Almirall, Amgen, Baxalta, Biogen, Boehringer Ingelheim, Celgene, Gebro, Janssen, JSC BIO‐ CAD, Leo‐Pharma, Lilly, Merck‐Serono, MSD, Mylan, Novartis, Pfizer, Regeneron, Roche, Sandoz, Samsung‐Bioepis, Sanofi and UCB. Andrey L. Bakulev has received consultancy/ speaker’s honoraria from and/or participated in clinical trials sponsored by Abbvie, Amgen, Boehringer Ingelheim, Celgene, Janssen, JSC BIOCAD, Leo‐Pharma, Lilly, MSD, Novartis, Pfizer, Sanofi, UCB and Zeldis Pfarma. Muza M. Kokhan has received consultancy/speaker’s honoraria from and/or participated in clinical trials sponsored by Celgene, Janssen, JSC BIO‐ CAD, Leo‐Pharma, Lilly, Novartis, Pfizer and Sanofi. Alexey V. Samtsov has received consul‐ tancy/speaker’s honoraria from and/or participated in clinical trials sponsored by Celgene, Glenmark, Jadran and JSC BIOCAD. Vladislav R. Khairutdinov has received consultancy/speak‐ er’s honoraria from and/or participated in clin‐ ical trials sponsored by Abbvie, Belupo, Bosnalec, Celgene, Glenmark, Jadran, Janssen, JSC BIOCAD, Leo‐Pharma, Lilly, MSD, Novartis, Pfizer, Sanofi and Sun Pharma. Maria A. Morozova, Antonina V. Artemeva, Arina V. Zinkina‐ Orikhan, Nikita A. Zolkin, Ivan V. Kuryshev and Alexey N. Petrov are JSC BIOCAD employees." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p. 1321): "The randomization was performed with random sequence using an electronic centralized randomization system." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p. 1321): "The randomization was performed with random sequence using an electronic centralized randomization system." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p. 1321): "The study investigators, trial team and patients were blinded to the treatment allocation during the first 12 weeks of the study....During the first 3 weeks, all patients received subcutaneous injections of NTK or placebo (according to the allocation) once a week (induction phase). Patients in the NTK Q2W group then received the study drug at weeks 4, 6, 8 and 10. Subjects in the NTK Q4W group received NTK at weeks 6 and 10 and placebo at weeks 4 and 8 to preserve blinding". Comment: unclear if the process guaranteed blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p1321): "The study investigators, trial team and patients were blinded to the treatment allocation during the first 12 weeks of the study....During the first 3 weeks, all patients received subcutaneous injections of NTK or placebo (according to the allocation) once a week (induction phase). Patients in the NTK Q2W group then received the study drug at weeks 4, 6, 8 and 10. Subjects in the NTK Q4W group received NTK at weeks 6 and 10 and placebo at weeks 4 and 8 to preserve blinding". Comment: unclear if the process guaranteed blinding of outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dealing with missing data: Quote (p. 1322): "The efficacy and safety analyses were performed according to the intention‐to‐treat (ITT) principle and included all patients randomised in the study (n = 213). For dichotomous responder‐type endpoints, missing responses at a post‐baseline visit were imputed as a nonresponder. For continuous endpoints, no missing data imputation rules were applied." Randomised 213, analysed 213 Comment: no rule was applied for continuous endpoints |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03390101). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results posted on ClinicalTrials.gov |
POETYK PSO‐1 2022.
| Study characteristics | ||
| Methods | RCT, double‐blind, parallel‐group, placebo and active comparator, multicentre study Date of study: August 2018 to July 2019 Location: USA, Canada, China, Germany, Japan, Korea, Poland, Russian Federation, Spain, Taiwan, UK (worldwide) Phase 3b |
|
| Participants |
Randomised: 666 participants Inclusion criteria:
Exclusion criteria:
Baseline characteristics N = 666, mean age 46 years, and 68% male Dropouts and withdrawals 68/666 (10%): deucravacitinib group (25), apremilast group (23), placebo group (20)
|
|
| Interventions |
Intervention A. Deucravacitinib 6 mg once daily, n = 332 Control interventions B. Apremilast 30 mg twice daily, n = 168 C. Placebo, n = 166 |
|
| Outcomes |
Assessment at week 16 Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 2): "This clinical trial was sponsored by Bristol Myers Squibb." Declarations of interest: Quote (p 2): "Dr Armstrong has received research grants and personal fees from Bristol Myers Squibb, Eli Lilly, Janssen, Leo Pharma, and Novartis; has received personal fees from Boehringer Ingelheim/Parexel, Celgene, Dermavant, Genentech, GlaxoSmithKline, Menlo Therapeutics, Merck, Modernizing Medicine, Ortho Dermatologics, Pfizer, Regeneron, Sanofi Genzyme, Science, Sun Pharma, and Valeant; and has received grants from Dermira, Kyowa Hakko Kirin, and UCB, outside the submitted work. Dr Gooderham has served on an advisory board and as a principal investigator for, and has received lecture fees from, AbbVie, Galderma, Leo Pharma, Pfizer, and Regeneron; has served on an advisory board for and received lecture fees from Actelion; has served as a principal investigator for and received consulting fees from Akros Pharma; has served on an advisory board and as a principal investigator for and received lecture and consulting fees from Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Novartis, Sanofi Genzyme, and Valeant; has served as a principal investigator for Arcutis, Bristol Myers Squibb, Dermira, GlaxoSmithKline, MedImmune, Merck, Roche Laboratories, and UCB; and has served as a principal investigator for and received lecture fees from Glenmark. Dr Warren has received research grants from AbbVie, Almirall, Amgen, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer, and UCB, and has received consulting fees from AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer, Sanofi, UCB, and UNION. Dr Papp has served on a speakers bureau for AbbVie, Amgen, Astellas, Celgene, Eli Lilly, Galderma, Janssen, Kyowa Hakko Kirin, Leo Pharma, Merck Sharp & Dohme, Novartis, Pfizer, and Valeant; has received grant/research support from AbbVie, Akros, Allergan, Amgen, Anacor, Arcutis, AstraZeneca, Baxalta, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Coherus, Dermira, Dow Pharma, Eli Lilly, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa Hakko Kirin, Leo Pharma, MedImmune, Meiji Seika Pharma, Merck Serono, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Takeda, UCB, and Valeant; has served as a consultant for AbbVie, Akros, Amgen, Arcutis, Astellas, AstraZeneca, Baxalta, Baxter, Boehringer Ingelheim, Bristol Myers Squibb, CanFite, Celgene, Coherus, Dermira, Dow Pharma, Eli Lilly, Forward Pharma, Galderma, Genentech, Janssen, Kyowa Hakko Kirin, Leo Pharma, Meiji Seika Pharma, Merck Serono, Merck Sharp & Dohme, Mitsubishi Pharma, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Takeda, UCB, and Valeant; has received honoraria from AbbVie, Akros, Amgen, Baxter, Boehringer Ingelheim, Celgene, Coherus, Eli Lilly, Forward Pharma, Galderma, GlaxoSmithKline, Janssen, Kyowa Hakko Kirin, Merck Serono, Merck Sharp & Dohme, Novartis, Pfizer, Takeda, UCB, and Valeant; and has served as a scientific officer, on a steering committee, and on an advisory board for AbbVie, Akros, Amgen, Anacor, Astellas, Baxter, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Dow Pharma, Eli Lilly, Galderma, Janssen, Kyowa Hakko Kirin, Merck Serono, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, Sanofi Genzyme, and Valeant. Dr Strober has served as a consultant (honoraria) for AbbVie, Almirall, Amgen, Arcutis, Arena, Aristea, Asana, Boehringer Ingelheim, Bristol Myers Squibb, Connect Biopharma, Dermavant, Equillium, GlaxoSmithKline, Immunic Therapeutics, Janssen, Leo Pharma, Eli Lilly, Maruho, Meiji Seika Pharma, Mindera, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, UCB, Ventyxbio, and vTv Therapeutics; has served as a speaker for AbbVie, Eli Lilly, Janssen, and Sanofi Genzyme; has served as co‐scientific director (consulting fee) for CorEvitas’ (Corrona) Psoriasis Registry; and has served as an investigator for AbbVie, Cara, CorEvitas’ (Corrona) Psoriasis Registry, Dermavant, Dermira, and Novartis. Dr Thaçi has received grant/research support from and served on a scientific advisory board member and a speaker’s bureau for AbbVie, Almirall, Amgen, Biogen Idec, Boehringer Ingelheim, Eli Lilly, Galapagos, Galderma, Janssen‐Cilag, Leo Pharma, Novartis, Pfizer, Regeneron, Roche, Sandoz‐Hexal, Sanofi, Target‐Solution, and UCB. Dr Morita has received honoraria as a meeting chair or lecturer from AbbVie, AYUMI, Boehringer Ingelheim Japan, Celgene K.K., Eisai, Eli Lilly Japan K.K., Inforward, Janssen Pharmaceutical K.K., Kyowa Kirin, Maruho Co., Mitsubishi Tanabe Pharma, Nippon Kayaku, Novartis Pharma K.K., Taiho Pharmaceutical, Torii Pharmaceutical., and Ushio; has received funding from AbbVie GK, Eisai, Eli Lilly Japan K.K., Kyowa Hakko Kirin, Leo Pharma KK, Maruho, Mitsubishi Tanabe Pharma, Novartis Pharma K.K., Taiho Pharmaceutical, and Torii Pharmaceutical; has received consulting fees from AbbVie, Boehringer Ingelheim Japan, Bristol Myers Squibb, Celgene K.K., Eli Lilly Japan K.K., GlaxoSmithKline K.K., Janssen Pharmaceutical K.K., Kyowa Hakko Kirin, Maruho, Mitsubishi Tanabe Pharma, Nichi‐Iko Pharmaceutical, Nippon Kayaku, Novartis Pharma K.K., NPO Health Institute Research of Skin, Pfizer Japan, Sun Pharma, Taiho Pharmaceutical, and UCB Japan. Dr Szepietowski has served as an advisory board member/consultant for AbbVie, Leo Pharma, Novartis, Pierre‐Fabre, Sanofi Genzyme, and Trevi; has served as a speaker for AbbVie, Eli Lilly, Janssen‐Cilag, Leo Pharma, Novartis, and Sanofi Genzyme; and has served as an investigator for AbbVie, Amgen, Bristol Myers Squibb, Galapagos, Galderma, Incyte, InfraRX, Janssen‐Cilag, Menlo Therapeutics, Merck, Novartis, Pfizer, Regeneron, UCB, and Trevi. Dr Imafuku has received grants and personal fees from AbbVie, Eisai, Janssen, Kyowa Kirin, Leo Pharma, Maruho, Sun Pharma, Taiho Yakuhin, Tanabe Mitsubishi, and Torii Yakuhin, and has received personal fees from Amgen (Celgene), Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, Novartis, and UCB. Dr Colston, Dr Throup, Dr Kundu, Dr Schoenfeld, Ms Linaberry, and Dr Banerjee are employees of and shareholders in Bristol Myers Squibb. Dr Blauvelt has served as a speaker/received honoraria from AbbVie and UCB; served as a scientific adviser/received honoraria from AbbVie, Abcentra, Affibody, Aligos, Almirall, Alumis, Amgen, AnaptysBio, Arcutis, Arena, Aslan, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Dermavant, EcoR1, Eli Lilly and Company, Evelo, Evommune, Forte Biosciences, Galderma, HighlightII Pharma, Incyte, Janssen, Landos, Leo Pharma, Merck, Novartis, Pfizer, Rapt, Regeneron, Sanofi Genzyme, Spherix Global Insights, Sun Pharma, TLL Pharmaceutical, TrialSpark, UCB, Vibliome, and Xencor; and has acted as a clinical study investigator/institution has received clinical study funds from AbbVie, Acelyrin, Amgen, Arcutis, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Eli Lilly and Company, Evelo, Galderma, Incyte, Janssen, Leo, Merck, Novartis, Pfizer, Regeneron, Sun Pharma, and UCB." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 10): "POETYK PSO‐1 was a 52‐week, randomized, double‐blinded, double dummy, placebo‐ and active‐comparator controlled trial conducted at 154 sites." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 10): "POETYK PSO‐1 was a 52‐week, randomized, double‐blinded, double dummy, placebo‐ and active‐comparator controlled trial conducted at 154 sites." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 10): "POETYK PSO‐1 was a 52‐week, randomized, double‐blinded, double dummy, placebo‐ and active‐comparator controlled trial conducted at 154 sites... Apremilast was titrated as per label in a blinded manner from 10 mg QD to 30 mg BID over the first 5 days of dosing." Comment: unclear if the process guaranteed blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 10): "POETYK PSO‐1 was a 52‐week, randomized, double‐blinded, double dummy, placebo‐ and active‐comparator controlled trial conducted at 154 sites... Apremilast was titrated as per label in a blinded manner from 10 mg QD to 30 mg BID over the first 5 days of dosing." Comment: unclear if the process guaranteed the blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 12‐13): "Efficacy analyses were performed using the full analysis set (all randomized patients). Missing data were imputed by nonresponder imputation for the coprimary endpoints; note, as this study was conducted during the global SARS‐CoV2 (COVID‐19) pandemic, PASI 75 and sPGA 0/1 analyses during Weeks 24‒52 excluded patients at visits that were missed solely due to COVID‐19, as advised by the US Food and Drug Administration. The modified baseline‐observation‐carried‐forward method was used to impute missing data for continuous secondary endpoints for patients who discontinued study treatment before Week 16 due to lack of efficacy or AEs. Patients who discontinued study treatment before Week 16 for other reasons had their last valid observation carried forward (including the baseline value as applicable)." Randomised 666, analysed 666 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03624127). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are not posted on ClinicalTrials.gov. |
POETYK PSO‐2 2022.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: June 2018 to November 2019 Location: USA, Australia, Canada, Czechia, Finland, France, Germany, Israel, Italy, New Zealand, Poland (worldwide) Phase 3 |
|
| Participants |
Randomised: 1020 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 1020, mean age 47 years and 66% male Dropouts and withdrawals 133/1020 (13%): deucravacitinib group (54), apremilast group (37), placebo group (42)
|
|
| Interventions |
Intervention A. Deucravacitinib: selective tyrosine kinase 2 (TYK2) inhibitor 6 mg once daily, n = 511 Control interventions B. Apremilast 30 mg twice‐daily, n = 254 C. Placebo, n = 255 |
|
| Outcomes |
At week 16 Primary composite outcome
Secondary outcome
|
|
| Notes |
Funding source: Quote (p 2 pre‐proof): "This clinical trial was sponsored by Bristol Myers Squibb". Declarations of interest: Quote (p 2‐5 pre‐proof): "Dr Strober has served as a consultant (honoraria) for AbbVie, Almirall, Amgen, Arcutis, Arena, Aristea, Asana, Boehringer Ingelheim, Bristol Myers Squibb, Connect Biopharma, Dermavant, Eli Lilly, Equillium, GlaxoSmithKline, Immunic Therapeutics, Janssen, Leo Pharma, Maruho, Meiji Seika Pharma, Mindera, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, UCB, Ventyxbio, and vTv Therapeutics; has served as a speaker for AbbVie, Eli Lilly, Janssen, and Sanofi Genzyme; has served as a co‐scientific director (consulting fee) for CorEvitas’ (Corrona) Psoriasis Registry; and has served as an investigator for AbbVie, Cara, CorEvitas' (Corrona) Psoriasis Registry, Dermavant, Dermira, and Novartis. Dr Thaçi has received grant/research support from and served on a scientific advisory board and a speakers bureau for AbbVie, Almirall, Amgen, Biogen Idec, Boehringer Ingelheim, Eli Lilly, Galapagos, Galderma, Janssen‐Cilag, Leo Pharma, Novartis, Pfizer, Regeneron, Roche, Sandoz‐Hexal, Sanofi, Target‐Solution, and UCB. Dr Sofen has served as a linical investigator for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Janssen, Leo Pharma, Novartis, and Sun Pharma. Dr Kircik has received research grants from AbbVie, Allergan, Almirall, Amgen, Arcutis, Boehringer Ingelheim, Breckinridge Pharma, Bristol Myers Squibb, Celgene, Cellceutix, Centocor, Combinatrix, Connetics, Coria, Dermavant, Dermira, Dow Pharma, Dr. Reddy’s Laboratories, Eli Lilly, Galderma, Genentech, GlaxoSmithKline, Idera, Johnson &Johnson, Leo Pharma, Maruho, Merck, Medicis, Novartis AG, Pfizer, PharmaDerm, Promius, Stiefel, Sun Pharma, UCB, Valeant, and XenoPort; has received honoraria from AbbVie, Allergan, Almirall, Amgen, Arcutis, Biogen Idec, Bristol Myers Squibb, Celgene, Cipher, Connetics, Dermavant, Dermira, Dr. Reddy’s Laboratories, Eli Lilly, Galderma, Genentech, GlaxoSmithKline, Johnson & Johnson, Leo Pharma, Merck, Novartis AG, PharmaDerm, Promius, Serono (Merck Serono International SA), Stiefel, Novartis AG, PharmaDerm, Promius, Serono (Merck Serono International SA), Stiefel, Sun Pharma, Taro, UCB, and Valeant. Dr Gordon has received grant support and consulting fees from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, and UCB, and has received consulting fees from Amgen, Almirall, Dermira, Leo Pharma, Pfizer, and Sun Pharma. Dr Foley has received grant support from Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Galderma, Janssen, Leo Pharma, Merck, Novartis, Pfizer, Sanofi, and Sun Pharma; has served as an investigator for AbbVie, Amgen, Arcutis, Argenx, Aslan, AstraZeneca, Boehringer Ingelheim, Botanix, Bristol Myers Squibb, Celgene, Celtaxsys, CSL, Cutanea, Dermira, Eli Lilly, Evelo, Galderma, Genentech, Geneseq, GlaxoSmithKline, Hexima, Janssen, Kymab, Leo Pharma, Merck, Novartis, Pfizer, Regeneron, Reistone, Roche, Sanofi, Sun Pharma, Teva, UCB, and Valeant; has served on advisory boards for AbbVie, Amgen, Aslan, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galderma, GlaxoSmithKline, Janssen, Leo Pharma, Mayne Pharma, MedImmune, Novartis, Pfizer, Sanofi, Sun Pharma, UCB, and Valeant; has served as a consultant for Aslan, Bristol Myers Squibb, Eli Lilly, Galderma, GenesisCare, Hexima, Janssen, Leo Pharma, Mayne Pharma, Novartis, Pfizer, Roche, and UCB; has received travel grants from AbbVie, EliLilly, Galderma, Janssen, Leo Pharma, Merck, Novartis, Pfizer, Roche, Sanofi, and Sun Pharma; and has served as a speaker for or received honoraria from AbbVie, Amgen, Celgene, Eli Lilly, Galderma, GlaxoSmithKline, Janssen, Leo Pharma, Merck, Novartis, Pfizer, Roche, Sanofi, Sun Pharma, and Valeant. Dr Rich has received research grants as a principal investigator on pharmaceutical trials from AbbVie, Arcutis, Bristol Myers Squibb, Dermavant, Eli Lilly, Janssen, Novartis, Sun Pharma, and UCB. Dr Paul has received grants from and has been a consultant for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Leo Pharma, Merck, Mylan, Novartis, Pfizer, Sandoz, and UCB. Dr Bagel has received research funds payable to the Psoriasis Treatment Center of New Jersey from AbbVie, Amgen, Arcutis, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, CorEvitas’ (Corrona) Psoriasis Registry, Dermavant, Dermira/UCB, Eli Lilly, Glenmark, Janssen Biotech, Kadmon, Leo Pharma, Lycera, Menlo Therapeutics, Novartis, Pfizer, Regeneron, SunPharma, Taro, and Valeant; has served as a consultant for AbbVie, Amgen, Celgene, Eli Lilly, Janssen Biotech, Novartis, Sun Pharma, and Valeant; and has served as a speaker for AbbVie, Celgene, Eli Lilly, Janssen Biotech, and Novartis. Dr Colston, DrThroup, Dr Kundu, Dr Sekaran, Ms Linaberry, and Dr Banerjee are employees of and shareholders in Bristol Myers Squibb. Dr Papp has served on a speakers bureau for AbbVie, Amgen, Astellas, Celgene, Eli Lilly, Galderma, Janssen, Kyowa Hakko Kirin, Leo Pharma, Merck Sharp & Dohme, Novartis, Pfizer, Valeant; has received grant/research support from AbbVie, Akros, Allergan, Amgen, Anacor, Arcutis, AstraZeneca, Baxalta, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Coherus, Dermira, Dow Pharma, Eli Lilly, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa Hakko Kirin, Leo Pharma, MedImmune, Meiji Seika Pharma, Merck Serono, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Takeda, UCB, and Valeant; has served as a consultant for AbbVie, Akros, Amgen, Arcutis, Astellas, AstraZeneca, Baxalta, Baxter, Boehringer Ingelheim, Bristol Myers Squibb, CanFite, Celgene, Coherus, Dermira, Dow Pharma, Eli Lilly, Forward Pharma, Galderma, Genentech, Janssen, Kyowa Hakko Kirin, Leo Pharma, Meiji Seika Pharma, Merck Serono, Merck Sharp & Dohme, Mitsubishi Pharma, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Takeda, UCB, and Valeant; has received honoraria from AbbVie, Akros, Amgen, Baxter, Boehringer Ingelheim, Celgene, Coherus, Eli Lilly, Forward Pharma, Galderma, GlaxoSmithKline, Janssen, Kyowa Hakko Kirin, Merck Serono, Merck Sharp& Dohme, Novartis, Pfizer, Takeda, UCB, and Valeant; and has served as scientific officer or on a steering committee/advisory board for AbbVie, Akros, Amgen, Anacor, Astellas, Baxter, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Dow Pharma, Eli Lilly, Galderma, Janssen, Kyowa Hakko Kirin, Merck Serono, Merck Sharp &Dohme, Novartis, Pfizer, Regeneron, Sanofi Genzyme, and Valeant." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 8 pre‐proof): "PSO‐2 was a 52‐week, multicenter, randomized, double‐blinded, double‐dummy, placebo‐ and active comparator‐controlled, phase 3 trial conducted at 191 sites in 16 countries." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 8 pre‐proof): "PSO‐2 was a 52‐week, multicenter, randomized, double‐blinded, double‐dummy, placebo‐ and active comparator‐controlled, phase 3 trial conducted at 191 sites in 16 countries." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 10 pre‐proof): "Throughout the trial, patients, investigators, and sponsors providing oversight remained blinded to treatment assignment and treatment switches." Comment: unclear if the process guaranteed blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 10 pre‐proof): "Throughout the trial, patients, investigators, and sponsors providing oversight remained blinded to treatment assignment and treatment switches." Comment: unclear if the process guaranteed the blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 13 pre‐proof): "Efficacy analyses during the initial treatment period were performed using the full analysis set, which included all randomized patients, and efficacy analyses during the randomized withdrawal and maintenance period were performed using the Week 24 PASI 75 responder population. Nonresponder imputation was used to account for missing data for all binary efficacy endpoints; however, due to the overlap of the conduct of PSO‐2 with the global SARS‐CoV2 (COVID‐19) pandemic, PASI 75 and sPGA 0/1 analyses during Weeks 24–52 excluded patients at visits that were missed solely due to COVID‐19, per US FDA recommendations.14 Modified baseline observation carried forward was used to impute missing data for continuous secondary endpoints for patients who discontinued treatment due to lack of efficacy or AEs." Randomised 1020, analysed 1020 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03611751). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are not posted on ClinicalTrials.gov. |
POETYK PSO‐3 2022.
| Study characteristics | ||
| Methods | RCT, double‐blind, placebo‐controlled, parallel‐arm, multicentric study Date of study: November 2019 to January 2022 Location: China, Taiwan, Korea Phase 3 |
|
| Participants |
Randomised: 220 participants Inclusion criteria:
Exclusion criteria:
Baseline characteristics N = 220, mean of age 40.5 years, 82% men Dropouts and withdrawals 11/220 (5%): deucravacitinib group (5), placebo group (6)
|
|
| Interventions |
Intervention A. Deucravacitinib 6 mg tablet once daily, n = 146 Control intervention B. Placebo, n = 74 |
|
| Outcomes | At week 16 Primary outcomes
Secondary outcomes
|
|
| Notes | Risk of bias was done according to the study protocol published in ClinicalTrials.gov Funding source: Quote (ClinicalTrials.gov): "Bristol‐Myers Squibb" Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (study protocol p 43): "At Week 0 (Day 1), subjects who meet all criteria for enrollment at Screening and Day 1 will be centrally randomized in a 2:1 ratio to BMS‐986165 6 mg QD or placebo as determined by a computer‐generated randomization schedule using the interactive response technology (IRT). The randomization lists will be generated by the IRT vendor using a permuted block design within each stratum level. The randomization in this study will be stratified by country (mainland China vs. non‐mainland China) and previous biologic use for psoriasis, psoriatic arthritis or other inflammatory diseases only (yes/no)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (study protocol p 43): "At Week 0 (Day 1), subjects who meet all criteria for enrollment at Screening and Day 1 will be centrally randomized in a 2:1 ratio to BMS‐986165 6 mg QD or placebo as determined by a computer‐generated randomization schedule using the interactive response technology (IRT). The randomization lists will be generated by the IRT vendor using a permuted block design within each stratum level. The randomization in this study will be stratified by country (mainland China vs. non‐mainland China) and previous biologic use for psoriasis, psoriatic arthritis or other inflammatory diseases only (yes/no)." Quote (study protocol p 43): "A treatment group will be assigned by IRT based on the above‐described randomization schedule and each subject will be assigned a unique randomization number. In addition, a unique kit number will be assigned to the subject corresponding to the treatment assignment. A kit will contain adequate study treatment for a 4‐week supply. At subsequent visits, when new treatment kits need to be provided, the investigative site will access the IRT to obtain the kit number to assign to the subject" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (study protocol p 43): "All tablets are identical in appearance and will be supplied in bottles with each daily dose made up of the appropriate combination of active and/or placebo tablets to provide the correct treatment... Investigative site staff, Sponsor and designee personnel, and subjects and their families will remain blinded to treatment assignments." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (study protocol p 43): "All tablets are identical in appearance and will be supplied in bottles with each daily dose made up of the appropriate combination of active and/or placebo tablets to provide the correct treatment... Investigative site staff, Sponsor and designee personnel, and subjects and their families will remain blinded to treatment assignments." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (study protocol p 66): "Non‐responder imputation (NRI) will be used for coprimary efficacy endpoints for subjects who discontinue treatment or study prior to Week 16". Quote (study protocol p 11): "The primary efficacy analysis population will be the Full Analysis Set (FAS). The FAS will include all subjects who were randomized to receive assigned study treatment." Randomised 220, analysed 218 Comment: the difference between analysed and randomised is due, according to the authors, to the fact that "participants with missing results at week 16 due to COVID‐19 are excluded" (citation: Clinicaltrials.gov) |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT04167462). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results posted on ClinicalTrials.gov |
POLARIS 2020.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: November 2016 to September 2017 Location: Germany (multicentric) Phase 3 |
|
| Participants |
Randomised: 119 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 119, mean of age 42.5 years, and 69% men Dropouts and withdrawals 27/119 (22.7%): guselkumab group (4), FAEs group (23)
|
|
| Interventions |
Intervention A. Guselkumab (100 mg administered as 100 mg/mL solution SC by single‐use prefilled syringe (PFS) at weeks 0, 4, 12 and 20), n = 60 Control intervention B. FAEs (to this aim, FAE doses will be slowly increased beginning with increasing doses of Fumaderm initial (containing 30 mg dimethylfumarate) over the first 3 weeks. Thereafter, participants will be switched to Fumaderm tablets (containing 120 mg dimethylfumarate) starting with 1 tablet a day. Fumaderm dose may be increased to a maximum of 3 x 2 tablets a day), n = 59 |
|
| Outcomes | At week 24 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 265): "Funding for the trial and its publication was provided by Janssen‐Cilag GmbH". Declarations of interest: Quote (p 275): "D.T. has received honoraria as an investigator or consultant for and/or received speakers’ honoraria and/or research grants from AbbVie, Almirall, Amgen, Boeh‐ ringer Ingelheim, Celgene, Dignity, Dr Reddy, Galapagos, GSK, Janssen, LEO, Lilly, Morphosis, MSD, Novartis, Pfizer, Regeneron/Sanofi, Sandoz‐Hexal and UCB. A.P. has received honoraria as an investigator for, and/or received speakers’ honoraria from, and/or received grants from, and/or been an advisor for AbbVie, Almirall‐Hermal, Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, GSK, Eli Lilly, Galderma, Hexal, Janssen, LEO Pharma, Medac, Merck Serono, Mit‐ subishi, MSD, Novartis, Pfizer, Tigercat Pharma, Regeneron, Roche, Sandoz Biopharmaceuticals, Schering‐Plough and UCB Pharma. M. Sebastian has received honoraria as an investigator for, received grants from, and been an advisor or consultant for AbbVie, Boehringer Ingelheim, Celgene, Dr Reddy, GSK, MSD, Mundipharma, Novartis, UCB Pharma, Janssen, Almirall, LEO Pharma, Galderma, Lilly and Regeneron. C.T. has received honoraria as an investigator for, and/or received speakers’ honoraria and/or grants from, and/or been an advisor for Janssen, Almirall, Allergopharma, AbbVie, LEO and UCB. M. Sticherling has received honoraria as an investigator and/or speaker for, has received grants from, and/or has participated in clinical studies for AbbVie, Actelion, Almirall, Amgen Celgene, Galderma, GSK, Janssen, LEO, Lilly, MSD, Mundi‐ pharma, Novartis, Pfizer, Sandoz, Sanofi and UCB Pharma. S.G. has been an advisor for, and/or received speakers’ honoraria from, and/or received grants from, and/or participated in clinical trials for Abbott/AbbVie, Almirall‐Hermal, Amgen, Baxalta, Bayer Health Care, Biogen Idec, Bioskin, Boehringer Ingelheim, Celgene, Centocor, Dermira, Eli Lilly, Foamix, Forward Pharma, Galderma, Hexal AG, Isotechnika, Janssen, LEO Pharma, Medac, Merck Serono, Mitsubishi Tanabe, MSD, Novartis, Pfizer, Polichem SA, Regeneron Pharmaceuticals, Sandoz Biopharmaceuticals, Sanofi‐Aventis, Schering‐Plough, Sienna Biopharmaceuticals, Takeda, Teva, UCB Pharma, VBL Therapeutics and Wyeth Pharma. S.W., S.K., C.R., H.B. and A.M. are employees of Janssen‐Cilag GmbH, Germany. K.E. has received honoraria as an investigator for, and/or received speakers’ honoraria from, and/or received grants from, and/ or been an advisor for Janssen, AbbVie, Celgene, Hexal, LEO, Lilly, Novartis and Sanofi." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 267): "Patients were randomized 1: 1 based on a computer‐generated randomization schedule that was prepared before the start of the study. The randomization was balanced using randomly permuted blocks of four. The interactive web‐based electronic case report forms assigned a unique treatment code, which dictated the treatment assignment at the baseline visit for each patient. The blinded efficacy assessors were not involved in any other study procedure and did not have access to the allocation data." Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 267): "Patients were randomized 1: 1 based on a computer‐generated randomization schedule that was prepared before the start of the study. The randomization was balanced using randomly permuted blocks of four. The interactive web‐based electronic case report forms assigned a unique treatment code, which dictated the treatment assignment at the baseline visit for each patient. The blinded efficacy assessors were not involved in any other study procedure and did not have access to the allocation data." Comment: no description of the method used to guarantee random allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 266): "This multicentre, randomized, open‐label, assessor‐blinded, active‐comparator‐controlled phase IIIb study... ". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 266‐7): "This multicentre, randomized, open‐label, assessor‐blinded, active‐comparator‐controlled phase IIIb study... ". "The blinded efficacy assessors were not involved in any other study procedure and did not have access to the allocation data." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dealing with missing data: Quote (p 268): "For binary endpoints, all patients with missing data were considered nonresponders (nonresponder imputation analysis). For continuous endpoints, the last available observation after baseline was carried forward (last observation carried forward analysis)." Unbalanced discontinuation proportion (< 1% for guselkumab and 39% for FAEs) |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02951533). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results posted on ClinicalTrials.gov |
PRESTA 2010.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: December 2005 to May 2008 Location: centres (n = 98) worldwide |
|
| Participants |
Randomised: 754 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 754, mean age 46 years, 473 male Dropouts and withdrawals 59/754 (8%)
|
|
| Interventions |
Intervention A. Etanercept, SC, 50 mg, twice a week, 12 weeks (n = 379) Control intervention B. Etanercept, SC, 50 mg, once a week, 12 weeks (n = 373) |
|
| Outcomes | Assessments at 12 weeks Primary and secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes |
Funding source: Quote (p 8): "Wyeth Research, which was acquired by Pfizer in October 2009, sponsored this clinical trial and was responsible for the collection and analysis of data..." Declarations of interest: Quote (p 8): "WS has received fees for speaking/consulting from Abbott, Schering‐Plough, Wyeth, and Janssen‐Cilag. J‐PO has received fees for speaking/conferences/consulting from Schering‐Plough, Abbott, Merck‐Serono, Centocor, Wyeth, Janssen‐Cilag, MedPharma, Laboratorios Pierre‐Fabre, Galderma Laboratories, and Leo Pharma. BK has served on advisory boards for Schering‐Plough and Roche; has received funds for research/travel/conferences from Wyeth, Centocor, Abbott, Schering‐Plough, Roche, and Bristol‐Myers Squibb; and has served on a speaker panel for Bristol‐Myers Squibb. OB has received fees from Wyeth, Schering‐Plough, Abbott, Roche, Chugai, and Bristol‐Myers Squibb. DR, RDP, JE, CM, and BF are all employees of Pfizer." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 3): "We randomly assigned participants to ..." Comment: no description of the method used to generate random sequences |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 3): "We randomly assigned participants to ..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 3): "In the double blind period..." Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 3): "In the double blind period..." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 754 included/752 analysed Quote (p 4): "The modified intention‐to‐treat (ITT) population included all randomised participants who took at least one dose of the test drug and at least one post baseline efficacy evaluation... Efficacy analyses used the last observation carried forward method for imputation of missing data". Comment: mITT and only 2 of 754 participants not included in the analysis of the primary outcome |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00245960). The prespecified outcomes mentioned in the Methods section appeared to have been reported, except for the results of participant‐reported endpoints summarised in a separate publication. |
PRIME 2017.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: June 2015 to June 2016 Location: USA (multicentric) Phase 3 |
|
| Participants |
Randomised: 202 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 202, mean age of 43 years, 124 male Dropouts and withdrawals 60/202 (2%): secukinumab group (6), FAEs group (56)
|
|
| Interventions |
Intervention A. Secukinumab (300 mg at weeks 0, 1, 2, 3, 4, 8, 12, 16, and 20), n = 105 Control intervention B. Fumaderm (week 0: 1 tablet of Fumaderm INITIAL in the evening, n = 97 Week 1: 1 tablet Fumaderm INITIAL, in the morning and evening Week 2: 1 tablet Fumaderm INITIAL in the morning, at noon and in the evening until the last tablet of a 40‐tablet‐blister is consumed Week 2 to 3: at the day after the last tablet of the Fumaderm INITIAL 40‐tablet‐blister is consumed and through week 3, 1 tablet of Fumaderm in the evening Week 4: 1 tablet Fumaderm in the morning and evening Week 5: 1 tablet Fumaderm in the morning, at noon, and in the evening Week 6: 1 tablet of Fumaderm in the morning and at noon, 2 tablets of Fumaderm in the evening Week 7: 2 tablets of Fumaderm in the morning, 1 tablet of Fumaderm at noon, 2 tablets of Fumaderm in the evening Weeks 8 to 24: 2 tablets of Fumaderm in the morning, at noon, and in the evening |
|
| Outcomes | At week 24 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1024): "Novartis Pharma GmbH" Declarations of interest: Quote (Appendix): "M.S. is an advisor and/or paid speaker for and/or has participated in clinical trials sponsored by AbbVie, Actelion, Almirall, Biogen, Boehringer Ingelheim, Celgene, GlaxoSmithKline, Janssen Cilag, LEO Pharma, Eli Lilly, Merck Sharp & Dohme, Mibe, Mundipharma, Novartis, Pfizer, Regeneron and Sanofi. U.M. has been an advisor for and/or received speaker honoraria and/or grants from and/or participated in clinical trials sponsored by Abbott/AbbVie, Almirall Hermal, Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, Foamix, Forward Pharma, Janssen Cilag, LEO Pharma, Medac, MSD, Miltenyi Biotech, Novartis, Pfizer, VBL and Xenoport. M.A. has served as a consultant for, or has been a paid speaker for clinical trials sponsored by AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, GlaxoSmithKline, Janssen Cilag, LEO Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB and Xenoport. D.T. is an advisor or consultant for AbbVie, Amgen, Biogen Idec, Cel‐gene, Dignity, Eli Lilly, Galapagos, GlaxoSmithKline, Janssen, LEO Pharma, Maruho, Mitsubishi, Mundipharma, Novartis, Pfizer, Sandoz and Xenoport. He has participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Astellas, Biogen Idec, Boehringer Ingelheim, Celgene, Dignity, Eli Lilly, Forward Pharma, GlaxoSmithKline, LEO Pharma, Janssen Cilag, Maruho, MSD, Mitsubishi Pharma, Novartis, Pfizer, Roche and Sandoz. He has received honoraria from AbbVie, Biogen Idec, Celgene, Janssen Cilag, LEO Pharma, Pfizer, Roche Possay, Novartis and Mundipharma. K.R. has served as an advisor and/or paid speaker for, and/or has participated in clinical trials sponsored by AbbVie, Amgen, Biogen, Boehringer Ingelheim Pharma, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen Cilag, LEO Pharma, Eli Lilly, Medac, Merck Sharp & Dohme, Novartis, Ocean Pharma, Pfizer, Regeneron, Takeda, UCB Pharma and Xenoport. N.M., C.S., C.H. and J.K. are employees of and/or own stock in Novartis". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1025): "This 24‐week, randomized, open‐label, active‐comparator, parallel‐group, superiority study was conducted... Eligible patients were randomized 1:1 to receive subcutaneous injections of secukinumab 300 mg or oral FAEs per label, via an automated randomization list. Randomization numbers were assigned to patients by the investigators in consecutive order, who then assigned the treatment displayed on the card. Randomization lists and sealed envelopes were generated by personnel who were not otherwise involved in the trial." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 1025): "This 24‐week, randomized, open‐label, active‐comparator, parallel‐group, superiority study was conducted... Eligible patients were randomized 1:1 to receive subcutaneous injections of secukinumab 300 mg or oral FAEs per label, via an automated randomization list. Randomization numbers were assigned to patients by the investigators in consecutive order, who then assigned the treatment displayed on the card. Randomization lists and sealed envelopes were generated by personnel who were not otherwise involved in the trial." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 1025): "This 24‐week, randomized, open‐label, active‐comparator, parallel‐group, superiority study was conducted... The blinded assessor and all involved personnel were instructed to desist from any discussions regarding safety, efficacy and treatment allocation of the study and patients in the presence of the blinded assessor. Efficacy parameters were assessed by blinded assessors who were not involved in any other study procedures and who did not have access to the allocation data or case report forms." Comment: participants not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1025): "This 24‐week, randomized, open‐label, active‐comparator, parallel‐group, superiority study was conducted... The blinded assessor and all involved personnel were instructed to desist from any discussions regarding safety, efficacy and treatment allocation of the study and patients in the presence of the blinded assessor. Efficacy parameters were assessed by blinded assessors who were not involved in any other study procedures and who did not have access to the allocation data or case report forms." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dealing with missing data Quote (p 1026): "Efficacy end points were assessed for the full analysis set, consisting of all randomized patients who had received at least one dose of study drug. Between treatments, comparisons were made by logistic regression models adjusted for centre and baseline values of PASI scores. Odds ratios (ORs), 95% confidence intervals (CIs) and P values were derived from these models. Patients with missing assessments were considered responders if they had already met the response criterion at the time of dropout for the primary end point and all other end points where response was investigated. Otherwise they were considered nonresponders". Randomised 202, analysed 201 Unbalanced proportion regarding discontinuation: 5.7% for secukinumab vs 57.7% for FAE |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02474082). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
PRISTINE 2013.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: April 2008 to March 2012 Location: 32 centres in Europe, Latin America and Asia |
|
| Participants |
Randomised: 273 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 273, mean age of 44 years, 190 male Dropouts and withdrawals 25/273 (9%)
|
|
| Interventions |
Intervention A. Etanercept (n = 137), SC, 50 mg, once a week, 24 weeks Control intervention B. Etanercept (n = 136), SC, 50 mg, twice a week, 24 weeks |
|
| Outcomes | Assessments at 24 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 177): "The PRISTINE trial was sponsored by Pfizer Inc..." Declarations of interest: Quote (pp 177‐8): "Robert Strohal has been a paid consultant of and has received research grants from Pfizer Inc, which provided funding for the PRISTINE study. He is also a member of the Pfizer European Expert Board and of the Pfizer Speakers Bureau. Luis Puig has been a paid consultant of and has received research grants from Pfizer; he has served on Pfizer advisory boards and the Speakers Bureau. Edgardo Chouela is a paid consultant and speaker for Pfizer Inc and Galderma and has conducted clinical studies for Novartis, Jannssen, Pfizer and Roche. Tsen‐Fang Tsai has been a paid consultant of Pfizer Inc; he has served as an investigator and received honoraria for serving as an advisor and speaker for Pfizer. Jeffrey Melin, Bruce Freundlich and Charles Molta were previous employees of Wyeth and Pfizer Inc. Joanne Fuiman, Ronald Pedersen and Deborah Robertson are current employees of Pfizer Inc." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 170): "Subjects were randomly assigned to one of the 2 etanercept treatment groups... in 1:1 treatment allocation". Comment: not specified |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 170): "Subjects were randomly assigned to one of the 2 etanercept treatment groups... in 1:1 treatment allocation". Comment: not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 170): "The study consisted of a 12‐week double‐blind treatment period". Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 170): "The study consisted of a 12‐week double‐blind treatment period". Comment: probably done, placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 273 enrolled and randomised, and 270 analysed Quote (p 171): "All efficacy analyses were performed using the modified intent‐to‐treat population which included all randomised subjects who received at least one dose of etanercept and had both baseline and on therapy PASI evaluations. The last observation‐carried‐forward method was used for the imputation of missing data..." Comment: mITT and only 3 of 273 participants not included in the analyses of the primary outcome |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00663052). The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
PsOsim 2017.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: May 2016 to March 2017 Location: multicentre (99 centres worldwide) Phase 3 |
|
| Participants |
Randomised: 545 participants Key inclusion criteria
Key exclusion criteria
Baseline characteristics N = 545, 72% men, mean age unknown Dropouts and withdrawals Total CHS‐1420: 54/274, adalimumab: 19/136 Reasons not reported |
|
| Interventions |
Intervention A. Adalimumab (Humira) 40 mg 2 doses at week 0/day 0, then 1 dose every 2 weeks starting at week 1 until week 15. At week 16 participants initially randomised to adalimumab will be reassigned (1:1) to CHS‐1420 or continue adalimumab treatment, 1 dose every 2 weeks for weeks 17 to 23, n = 274. At week 24 participants will switch to CHS‐1420 open‐label until study end. Control intervention B. CHS‐1420 (Biosimilar) 40 mg 2 doses at week 0/day 0 then 1 dose every 2 weeks starting at week 1 for 23 weeks, n = 271. At week 24 participants will continue on to CHS‐1420 open‐label until study end. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (ClinicalTrials.gov): "Coherus Biosciences, Inc." Declarations of interest: not stated On ClinicalTrials.gov (NCT02489227), waiting for the publication to contact the main author RoB completed according study protocol posted on ClinicalTrials.gov |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (protocol): "Once the subject has signed the ICF at Screening, site personnel will assign a subject identification number (ID). The subject ID will include the site number (3 digits), and 3 digit subject number, assigned sequentially starting with 001." Comment: suggests centrally with the use of computer‐generation but unsure |
| Allocation concealment (selection bias) | Low risk | Quote (protocol): "Once the subject ID has been assigned, the site will contact the Interactive Voice Response System/Interactive Web‐based Response System (IXRS) to register the subject ID". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "This is a double‐blind study. The Humira and CHS‐1420 syringes will be matched in appearance. Blinded study drug will be shipped under appropriate storage conditions to site personnel according to the regulations of the study country". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "This is a double‐blind study. The Humira and CHS‐1420 syringes will be matched in appearance. Blinded study drug will be shipped under appropriate storage conditions to site personnel according to the regulations of the study country". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Subjects who lack a PASI assessment at week 12 will be considered nonresponders in the primary analyses. As a sensitivity analysis, the last available score will be used". Comment: reasons for withdrawal not reported |
| Selective reporting (reporting bias) | High risk | None of the secondary outcomes were reported, but results on ClinicalTrials.gov |
Rathipriyadharshini 2020.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: 2018 to 2019 Location: India |
|
| Participants |
Randomised: 40 participants Inclusion criteria:
Exclusion criteria:
Baseline characteristics N = 40, mean of age 41 years, % of male unknown Dropouts and withdrawals Not stated |
|
| Interventions |
Intervention A. Apremilast 30 mg twice a day from day 6 to 12 weeks after the recommended initial dosage titration from day 1 to day 6, n = 20 Control intervention B. Methotrexate 7.5 mg per week for 12 weeks along with folic acid 5 mg daily, n = 20 |
|
| Outcomes | At week 12 Primary outcome
Secondary outcome
|
|
| Notes |
Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (article): "It is an open‐labelled randomized comparative clinical study". Comment: no description of the methods used to guarantee the random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (article): "It is an open‐labelled randomized comparative clinical study". Comment: no description of the methods used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (article): "It is an open‐labelled randomized comparative clinical study". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (article): "It is an open‐labelled randomized comparative clinical study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no description of the methods used to manage missing data, no description of the methods used to assess the primary outcome (ITT, PP ...) |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol was registered (CTRI/2019/01/017362). The outcomes mentioned in the Results section were not specified in the Methods section. |
Reich 2012a.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: October 2005 to November 2006 Location: 15 centres in France and Germany |
|
| Participants |
Randomised: 176 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 176, mean age 43 years, 123 male Dropouts and withdrawals 28/176 (16%)
|
|
| Interventions |
Intervention A. Certolizumab 200 (n = 59), SC Initial dose of certolizumab pegol (CZP) 400 mg at week 0, followed by 200 mg CZP every other week (Q2W) until week 10 Control intervention B. Certolizumab 400 (n = 58), SC Initial dose of CZP 400 mg at week 0, followed by 400 mg CZP Q2W until week 10 C. Placebo (n = 59), SC, Q2W until week 10 |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 180): "This study was funded by UCB Pharma, Brussels, Belgium". Declarations of interest: Quote (p 180): "K.R. has served as consultant and ⁄ or paid speaker for and ⁄ or has participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including Abbott, Biogen Idec, Celgene, Centocor, Janssen‐Cilag, Leo, Medac, Merck, MSD (formerly Essex, Schering‐Plough), Novartis and Pfizer (formerly Wyeth). J.‐P.O. is a consultant for Abbott, Centocor, Galderma, Janssen‐ Cilag, Leo, Meda Pharma, Merck Serono and UCB Pharma. A.B.G. has current consulting ⁄ advisory board agreements with Amgen, Astellas, Centocor (Janssen), Celgene, Bristol‐Myers Squibb, Beiersdorf, Abbott, TEVA, Actelion, UCB Pharma, Novo Nordisk, Novartis, Dermipsor, Incyte, Pfizer, Canfite, Merck and Lilly. Research ⁄ educational grants paid to Tufts Medical Center: Centocor (Janssen), Amgen, Immune Control, Abbott, Novo Nordisk, UCB Pharma, Novartis, Celgene and Pfizer. I.J.T. and G.C. are full‐time employees of UCB Pharma. C.T. is a former employee of UCB Pharma. P.M. has served as consultant and ⁄ or paid speaker for and has received grants, consulting and ⁄ or speaker fees from Abott Amgen, Biogen Idec, Bristol‐Myers Squibb, Celgene, Janssen, Novartis, Merck, Pfizer and UCB Pharma." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 181): "Eligible patients were randomised to receive... Randomization was centralized using a dynamic allocation procedure... Treatment was assigned using an interactive voice‐response system". "Randomization was conducted via Interactive Response Technology, which assigned a randomisation number that linked the subject treatment arm and specified unique medication pack number." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 181): "Eligible patients were randomised to receive... Randomization was centralized using a dynamic allocation procedure... Treatment was assigned using an interactive voice‐response system". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 181): "CZP... or matching placebo in liquid formulation for subcutaneous injection... Study doses of CZP or placebo were prepared containing the same volume and labelled in the same manner by designed unblinded pharmacists". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 181): "CZP... or matching placebo in liquid formulation for subcutaneous injection... Study doses of CZP or placebo were prepared containing the same volume and labelled in the same manner by designed unblinded pharmacists". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 176 included/176 analysed Quote (p 182): "Co‐primary efficacy assessments were performed on the intention‐to‐treat population... Nonresponder imputations for missing values were used for the primary analysis". Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00245765). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported, except for pharmacokinetic profile of CDP870. |
Reich 2015.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: December 2008 to July 2009 Location: 14 centres in the USA and Canada |
|
| Participants |
Randomised: 100 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 100, mean age 44 years, 100 male Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Secukinumab (n = 30), SC, 3 mg/kg, 1 infusion (day 1) Control intervention B. Secukinumab (n = 29), SC, 10 mg/kg, 1 infusion (day 1) C. Secukinumab (n = 31), SC, 10 mg/kg, 3 infusions (days 1, 15, 29) D. Placebo (n = 10) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 534): "This trial and publication were found by Novartis Pharma AG, Basel, Switzerland." Declarations of interest: Quote (p 534): "KR has served as a consultant or paid speaker for, or participated in clinical trials sponsored by, AbbVie, Amgen, Biogen‐Idec, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, Leo, Lilly, Medac, MSD, Novartis, Pfizer, Takeda and Vertex. KAP has received grants and has consulted and served as an investigator for AbbVie, Amgen, Astellas, Biogen‐Idec, Celgene, Centocor, Eli Lilly, Forward Pharma, Fujisawa, GlaxoSmithKline, Janssen, Kyowa‐Kirin, Leo, MSD, Novartis (outside the submitted work), Pfizer and Takeda. RTM has received grants/clinical trial stipends from Novartis. JHT served as a clinical investigator for Novartis during conduct of this study. RB received grants from Novartis during the conduct of this study and has received grants, personal fees and non‐ financial support from AbbVie, Amgen, Astellas, Celgene, Eli Lilly, Janssen, Pfizer and Tribute. MB has served as a clinical trial sponsor for Amgen, Eli Lilly and Novartis. DG has served as a clinical trial investigator for Novartis. RAK is a member of an advisory board for Novartis and several other pharmaceutical companies. YP has received grants from AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Merck, Pfizer and Novartis (outside the submitted work). LAR, WMB, TMF and NAB‐S declare no conflict of interests. GS has received grants/clinical trial payments from Janssen, MSD and Novartis (unrelated to secukinumab). JMS, US, TP, EK, GAW, FK and CCB are full‐time employees of Novartis. WH and DML are full‐time employees of and own stock in Novartis. MMS was a full‐time employee of Novartis at the time the study was conducted and the manuscript". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (supplemental appendix): "The randomisation scheme was generated by Novartis Drug Supply Management using a validated system. The randomisation scheme was reviewed and approved by the Biostatistics Quality Assurance group of Novartis and was locked after approval. Subjects were assigned randomisation numbers, according to the randomisation schedule. Each site, upon evaluation of a qualified subject for the trial, faxed the enrolment sheet to the clinical trial leader (CTL) at the fax number provided. The CTL then assigned the randomisation number in a sequential manner and faxed it back to the unblinded pharmacist or qualified site personnel at the site, who then prepared and provided the study medication for the clinic in a blinded fashion." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (supplemental appendix): "Each site, upon evaluation of a qualified subject for the trial, faxed the enrolment sheet to the clinical trial leader (CTL) at the fax number provided. The CTL then assigned the randomisation number in a sequential manner and faxed it back to the unblinded pharmacist or qualified site personnel at the site, who then prepared and provided the study medication for the clinic in a blinded fashion... Treatment allocation and clinical assessment of the subjects were blinded. For preparation of the study medication from bulk supplies, treatment allocation cards were sent to the pharmacist or qualified site personnel at the investigator’s site." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (supporting information): "To maintain the blind of the study, the appearance of placebo infusion bags, ready to administer to the subject, was identical to that of active drug infusion bags. Placebo and active medication were prepared by an unblinded pharmacist or qualified site personnel assigned at each site." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (supporting information): "To maintain data integrity, no subject‐level data were circulated; therefore, blinding was maintained at the individual subject level". Comment probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100 randomised participants, 94 analysed for PASI 75 or 90, 87 analysed for primary outcome (change in PASI) Quote (p 530): "Efficacy and pharmacodynamic parameters were evaluated in all subjects who received ≥ 1 dose of study medication and had a major protocol deviations... Subjects lost to follow‐up were considered relapsed on the day of th first visit without available PASI data". Comment: low rate of loss to follow‐up and reasons comparable between groups |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00805480). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Reich 2020.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label, rater‐blinded, parallel‐group study Date of study: January 2016 to December 2016 Location: Germany (28 centres) Phase 3 |
|
| Participants |
Randomised: 162 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 162, mean of age 42 years, and 75% men Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ixekizumab (60 mg ixekizumab given as 2 SC injections followed by 80 mg ixekizumab given SC every 2 weeks until week 12 and then 80 mg ixekizumab given SC every 4 weeks until week 24), n = 54 Control interventions B. FAEs (105 mg FAE given orally followed by 215 mg FAE given orally 1 to 3 times/day until week 24), n = 54 C. Methotrexate (7.5 mg starting dose up to 30 mg methotrexate given orally once a week until week 24), n = 54 |
|
| Outcomes |
At week 24 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p. 869): "This study was supported by Eli Lilly (Indi‐ anapolis, IN, U.S.A.). This study was designed by Lilly Deutschland GmbH." Declarations of interest: Quote (p878‐879): "K.R. has served as an advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Amgen, Biogen, Boehringer Ingelheim Pharma, Cel‐ gene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, LEO Pharma, Eli Lilly and Company, Medac, Merck Sharp & Dohme, Novartis, Ocean Pharma, Pfizer, Regeneron, Sanofi, Takeda, UCB Pharma and Xenoport. M.A. has served as a consultant or paid speaker for clinical trials sponsored by AbbVie, Almirall, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly and Com‐ pany, GSK, Hexal, Janssen, LEO Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB Pharma and Xenoport. D.T. has been an advisor for, received speaker’s honoraria and grant support from, and participated in clinical trials for AbbVie, Almirall, Amgen, Biogen Idec, Bioskin, Boehringer Ingelheim, Celgene, Dignity, Dr Reddy’s, Eli Lilly and Company, Galapagos, GlaxoSmithKline, LEO Pharma, Janssen‐Cilag, Kymab, Merck Sharp & Dohme, Mundipharma, Morphosis, Novartis, Pfizer, Regeneron, Samsung, Sanofi‐Genzyme, Sandoz and UCB Pharma. A.P. has worked as an investigator, speaker and/or advisor for AbbVie, Almirall‐Hermal, Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, GSK, Eli Lilly and Company, Galderma, Hexal, Janssen, LEO Pharma, Medac, Merck Serono, Mitsubishi, MSD, Novartis, Pfizer, Regeneron, Roche, Sandoz Biopharmaceuticals, Schering‐Plough, Tigercat Pharma and UCB Pharma. U.M. has been an advisor for, received speakers honoraria and/or grants from, and/or participated in clinical trials for Abbott/AbbVie, Almirall‐Hermal, Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly and Company, Foamix, Forward Pharma, Janssen, LEO Pharma, Medac, Miltenyi Biotech, MSD, Novartis, Pfizer, VBL and Xenoport. A.L., C.H., E.S., A.S. and M.D. were employees of and minor stockholders in Eli Lilly and Company during the conduct of this study." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 870): "Patients were randomized 1:1:1 to FAEs, methotrexate or ixekizumab via an interactive web response system." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 870‐1): "To ensure that FAE and methotrexate treatments were given according to labels and according to clinical practice (e.g. dose adjustment due to adverse events), the study was conducted open‐label." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 871): "Both patients and investigators were unblinded to treatment allocation." Comment: open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 871): "A blinded rater assessed all clinical outcome measures to minimize bias for the clinical efficacy assessments of each treatment arm". Comment: no clear description of the process followed to guarantee the blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p. 871): "Non‐ responder imputation was used to impute patients with missing data. Continuous end points were tested using ANCOVA with terms for treatment and baseline. Modified imputation using the baseline observation carried forward was used to impute missing values: patients who discontinued due to adverse events were imputed with their baseline observation." Randomised 162, analysed 162 Unbalance discontinuation treatments: IXE group (4), FAEs group (31), methotrexate group (5) |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02634801). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
ReSURFACE‐1 2017.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: December 2012 to October 2015 Location: at 118 sites (including hospital dermatology units, specialty clinics, private practices, and research sites) in Australia, Canada, Japan, the UK, and the USA Phase 3 |
|
| Participants |
Randomised: 772 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 772, mean age of 47 years, 553 male Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Tildrakizumab 200 mg (SC on weeks 0, 4, 16, 28, 40, and 52), n = 308 Control interventions B. Tildrakizumab 100 mg (SC on weeks 0, 4, 16, 28, 40, and 52), n = 309 C. Placebo, n = 155 |
|
| Outcomes | At week 12 Primary outcome (composite outcome)
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 276): "Funding Merck & Co" Declarations of interest: Quote (p 287): "Declaration of interests: KR has served as a consultant or paid speaker for, or participated in clinical trials sponsored by, Abbvie, Amgen, Biogen‐Idec, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, Leo, Lilly, Medac, Merck & Co, Novartis, Pfizer, Vertex, and Takeda. KAP has served as a consultant or paid speaker for, or participated in clinical trials sponsored by Amgen, Anacor, AbbVie, Active Biotech, Allergan, Astellas, AstraZeneca, Basilea, Bayer, Biogen‐Idec, BMS, Boehringer‐Ingelheim, CanFite, Celgene, Dermira, Eli‐Lilly, Forward Pharma, Genentech, GlaxoSmithKline, Janssen, Kyowa Hako Kirin, Kythera, Leo Pharma, Merck & Co, Merck‐Serono, Novartis, Pfizer, Regeneron, Rigel, Roche, Sanofi‐Genzyme, Takeda, UCB, Valeant, Xenon, and Xoma. AB has served as a scientific adviser and clinical study investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Genentech, GSK, Janssen, Lilly, Merck & Co, Novartis, Pfizer, Regeneron, Sandoz, Sanofi Genzyme, Sun, UCB, and Valeant, and as a paid speaker for Lilly. SKT has participated in trials supported by grants from Merck & Co. RS has served as a consultant or paid speaker for, or participated in clinical trials sponsored by, Leo Pharma, Amgen, Novartix, Merck & Co, Celgene, Coherus Biosciences, Janssen, Regeneron, MedImmune, GlaxoSmithKline, Cutanea, Samson Clinical, Boehringer Ingelheim, Pfiizer, MSD, Oncobiologics, Roche, Eli Lilly, and Bayer. DT has served as a consultant, advisory board member, or an investigator for Abbott (AbbVie), Almiral, Amgen, Astellas, Biogen‐Idec, Boehringer Ingelheim, Celgene, Dignity, Forward‐Pharma, Galderma, GlaxoSmithKline, Isotechnika, Janssen‐Cilag, Leo Pharma, Lilly, Maruho, Medac, Medimmune, Merck & Co, Merck‐Serono, Novartis, Pfizer, Regeneron, Sandoz, Sanofi‐Aventis, and Takeda. KN is a former employee of Merck & Co; AM, NC, QL, KL, CLR, and SG are current Merck & Coemployees. ABK is a consultant and investigator for Merck & Co, Amgen, AbbVie, Janssen, Novartis, Dermira, and Pfizer, a consultant for Sun Pharmaceuticals, Bristol‐Myers Squibb, Lilly, and VBL, and has received fellowship funding from Janssen." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 278): "In reSURFACE 1, participants were randomly assigned (2:2:1) to tildrakizumab 200 mg, tildrakizumab 100 mg, or placebo...In reSURFACE 2, participants were randomly assigned (2:2:1:2) to tildrakizumab 200 mg, tildrakizumab 100 mg, placebo, or etanercept 50 mg...Parexel International, the contract research organisation, generated computer generated randomisation sequences, and an interactive voice‐response system and interactive web‐response system was used by Parexel to allocate participants to groups. Randomised treatment assignments on day 1 were done by region". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 278): "In reSURFACE 1, participants were randomly assigned (2:2:1) to tildrakizumab 200 mg, tildrakizumab 100 mg, or placebo...In reSURFACE 2, participants were randomly assigned (2:2:1:2) to tildrakizumab 200 mg, tildrakizumab 100 mg, placebo, or etanercept 50 mg...Parexel International, the contract research organisation, generated computer generated randomisation sequences, and an interactive voice‐response system and interactive web‐response system was used by Parexel to allocate participants to groups. Randomised treatment assignments on day 1 were done by region". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 279): "Investigators, participants, and study personnel were blinded to group allocation and remained blinded until completion of the studies. A double‐masking technique was used, in which tildrakizumab and its matching placebo or etanercept and its matching placebo were identical in appearance and packaging. Additional placebo doses were administered to maintain masking. The team doing the analysis was blinded until the database was locked." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 279): "Investigators, participants, and study personnel were blinded to group allocation and remained blinded until completion of the studies. A double‐masking technique was used, in which tildrakizumab and its matching placebo or etanercept and its matching placebo were identical in appearance and packaging. Additional placebo doses were administered to maintain masking. The team doing the analysis was blinded until the database was locked." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data Quote (pp. 280‐1): "We specified full‐analysis‐set, intention‐to‐treat, and per protocol patient populations in the study protocols...Patients with missing data were treated as nonresponders (nonresponder imputation [NRI])." Randomised 772, analysed 772 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01722331). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
ReSURFACE‐2 2017.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: February 2013 to September 2015 Location: 132 sites in Austria, Belgium, Canada, Czech Republic, Denmark, France, Germany, Hungary, Italy, Israel, Netherlands, Poland, and the USA Phase 3 |
|
| Participants |
Randomised: 1090 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 1090, mean age of 45 years, 554 male Dropouts and withdrawals 64/1090 (5.9%): tildrakizumab 200 (14), tildrakizumab 100 (12), ETA (24), PBO (14)
|
|
| Interventions |
Intervention Tildrakizumab 200 mg (SC on weeks 0, 4, 16, 28, 40, and 52), n = 314 Control interventions Tildrakizumab 100 mg (SC on weeks 0, 4, 16, 28, 40, and 52), n = 307 Etanercept 50 mg (twice‐weekly until week 12 and once weekly from week 12 to week 28), n = 313 Placebo, n = 156 |
|
| Outcomes | At week 12 Primary outcome (composite outcome)
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 276): "Funding Merck & Co" Declarations of interest: Quote (p 287): "Declaration of interests: KR has served as a consultant or paid speaker for, or participated in clinical trials sponsored by, Abbvie, Amgen, Biogen‐Idec, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, Leo, Lilly, Medac, Merck & Co, Novartis, Pfizer, Vertex, and Takeda. KAP has served as a consultant or paid speaker for, or participated in clinical trials sponsored by, Amgen, Anacor, AbbVie, Active Biotech, Allergan, Astellas, AstraZeneca, Basilea, Bayer, Biogen‐Idec, BMS, Boehringer‐Ingelheim, CanFite, Celgene, Dermira, Eli‐Lilly, Forward Pharma, Genentech, GlaxoSmithKline, Janssen, Kyowa Hako Kirin, Kythera, Leo Pharma, Merck & Co, Merck‐Serono, Novartis, Pfizer, Regeneron, Rigel, Roche, Sanofi‐Genzyme, Takeda, UCB, Valeant, Xenon, and Xoma. AB has served as a scientific adviser and clinical study investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Genentech, GSK, Janssen, Lilly, Merck & Co, Novartis, Pfizer, Regeneron, Sandoz, Sanofi Genzyme, Sun, UCB, and Valeant, and as a paid speaker for Lilly. SKT has participated in trials supported by grants from Merck & Co. RS has served as a consultant or paid speaker for, or participated in clinical trials sponsored by, Leo Pharma, Amgen, Novartix, Merck & Co, Celgene, Coherus Biosciences, Janssen, Regeneron, MedImmune, GlaxoSmithKline, Cutanea, Samson Clinical, Boehringer Ingelheim, Pfiizer, MSD, Oncobiologics, Roche, Eli Lilly, and Bayer. DT has served as a consultant, advisory board member, or an investigator for Abbott (AbbVie), Almiral, Amgen, Astellas, Biogen‐Idec, Boehringer Ingelheim, Celgene, Dignity, Forward‐Pharma, Galderma, GlaxoSmithKline, Isotechnika, Janssen‐Cilag, Leo Pharma, Lilly, Maruho, Medac, Medimmune, Merck & Co, Merck‐Serono, Novartis, Pfizer, Regeneron, Sandoz, Sanofi‐Aventis, and Takeda. KN is a former employee of Merck & Co; AM, NC, QL, KL, CLR, and SG are current Merck & Coemployees. ABK is a consultant and investigator for Merck & Co, Amgen, AbbVie, Janssen, Novartis, Dermira, and Pfizer, a consultant for Sun Pharmaceuticals, Bristol‐Myers Squibb, Lilly, and VBL, and has received fellowship funding from Janssen." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 278): "In reSURFACE 1, participants were randomly assigned (2:2:1) to tildrakizumab 200 mg, tildrakizumab 100 mg, or placebo...In reSURFACE 2, participants were randomly assigned (2:2:1:2) to tildrakizumab 200 mg, tildrakizumab 100 mg, placebo, or etanercept 50 mg...Parexel International, the contract research organisation, generated computer generated randomisation sequences, and an interactive voice‐response system and interactive web‐response system was used by Parexel to allocate participants to groups. Randomised treatment assignments on day 1 were done by region". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 278): "In reSURFACE 1, participants were randomly assigned (2:2:1) to tildrakizumab 200 mg, tildrakizumab 100 mg, or placebo...In reSURFACE 2, participants were randomly assigned (2:2:1:2) to tildrakizumab 200 mg, tildrakizumab 100 mg, placebo, or etanercept 50 mg...Parexel International, the contract research organisation, generated computer generated randomisation sequences, and an interactive voice‐response system and interactive web‐response system was used by Parexel to allocate participants to groups. Randomised treatment assignments on day 1 were done by region". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 279): "Investigators, participants, and study personnel were blinded to group allocation and remained blinded until completion of the studies. A double‐masking technique was used, in which tildrakizumab and its matching placebo or etanercept and its matching placebo were identical in appearance and packaging. Additional placebo doses were administered to maintain masking. The team doing the analysis was blinded until the database was locked." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 279): "Investigators, participants, and study personnel were blinded to group allocation and remained blinded until completion of the studies. A double‐masking technique was used, in which tildrakizumab and its matching placebo or etanercept and its matching placebo were identical in appearance and packaging. Additional placebo doses were administered to maintain masking. The team doing the analysis was blinded until the database was locked." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data Quote (pp 280‐1): "We specified full‐analysis‐set, intention‐to‐treat, and per protocol patient populations in the study protocols...Patients with missing data were treated as nonresponders (nonresponder imputation [NRI])." Randomised 1090, analysed 1090 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01729754). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
REVEAL 2008.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: December 2004 to August 2007 Setting: 81 centres (67 + 14) in the USA, Canada |
|
| Participants |
Randomised: 1212 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 1212, mean age 44 years, 803 male Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Adalimumab (n = 814), SC, 40 mg, week 0: 2 injections, week 1: eow, 16 weeks Control intervention B. Placebo, SC (n = 398), week 0: 2 injections/week 1: eow, 16 weeks |
|
| Outcomes | Assessments at 16 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 106): "Supported by Abbott Laboratories" Declarations of interest: Quote (p 106): "Dr Menter has received research support and/or lecture honoraria from Abbott, Amgen, Astellas, Biogen, Centocor, Genentech, and Wyeth. Dr Tyring has received research support from, has consulted for, and is part of the speakers’ bureaus for Abbott. Dr Gordon has received research support and honoraria from Abbott, Amgen, and Centocor. Dr Kimball is an investigator, speaker, and consultant for Abbott, Amgen, Biogen, Centocor, and Genentech. Dr Leonardi is a consultant for Abbott, Amgen, Centocor, and Genentech and is an investigator for Abbott, Allergan, Altana, Amgen, Astellas, Biogen, Bristol Myers, Centocor, Fujisawa, Galderma, Genentech, Serono, CombinatoRx, 3M Pharmaceuticals, Schering Plough, RTL, and Vitae; he also received an educational grant from Amgen and Genentech, and is part of the speakers’ bureaus for Abbott, Amgen, Centocor, Genentech, and Warner Chilcott. Dr Langley is a scientific advisory board member, investigator, and speaker for Abbott, Amgen, Astellas, Centocor, Norvartis, and Wyeth. Dr Strober serves on the advisory boards of, has received honoraria from, and is an investigator for Abbott, Amgen, Astellas, Centocor, Genentech, and Wyeth, and is part of the speakers’ bureaus for Abbott, Amgen, Astellas, Genentech, and Wyeth. Dr Kaul, Ms Gu, and Dr Okun are employees of Abbott Laboratories. Dr Papp is a consultant for and has received honoraria and travel grants from Abbott, Alza, Amgen, Astellas, Celgene, Centocor, Genentech, Isotechnika, Johnson and Johnson, Serono, Schering‐Plough, and UCB." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 107): "Randomization schedules were generated by one of our data management departments before study inception". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 107): "Patients were randomised by centre via an interactive voice response system". "ADA and placebo‐filled syringes were identically labelled and packaged, and self‐administrated by patients". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 107): "Double‐blind, placebo‐controlled... ADA and placebo‐filled syringes were identically labelled and packaged, and self‐administrated by patients". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 107): "Double‐blind, placebo‐controlled... ADA and placebo‐filled syringes were identically labelled and packaged, and self‐administrated by patients". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1212 included/1212 analysed Quote (p 109): "The primary efficacy analyses were conducted on ITT population... a patient with missing data for a visit... had the last observation carried forward". Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT002377887). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported, except for participant‐reported outcome. |
Rich 2013.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: July 2009 to December 2010 Location: 60 centres in Portland, USA |
|
| Participants |
Randomised: 404 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 404, mean age of 44 years, 306 male Dropouts and withdrawals 24/404 (6%)
|
|
| Interventions |
Intervention A. Secukinumab (n = 66), SC, 150 mg, week 0, 12 weeks Control intervention B. Secukinumab (n = 138), SC, 150 mg, weeks 0, 4, 8, 12 weeks C. Secukinumab (n = 133), SC, 150 mg, weeks 0, 1, 2, 4, 12 weeks D. Placebo (n = 67), SC, weeks 0, 1, 2, 4, 8, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 402): "Novartis Pharma AG, Basel, Switzerland" Declarations of interest: Quote (appendix): "P.R. has received honoraria for lecturing in industry‐sponsored meetings and has received research grants from pharmaceutical companies as an investigator. B.S. has consulted for Novartis and several other pharmaceutical companies; he has served on an advisory board for Novartis and several other pharmaceutical companies. D.T. has served as a speaker and served on advisory boards for Abbott, Biogen‐Idec, Janssen‐Cilag, Leo, MSD, Novartis and Pfizer. C. Paul has received honoraria from and has been a paid consultant to Abbott, Amgen, Celgene, Janssen‐Cilag, Novartis and Pierre Fabre. K.R., E.H., A.G., M.M. and C. Papavassilis are full‐time employees of, and own stock in Novartis. J.‐P.O., A.M. and R.E.S. declare no conflicts of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 404): “Randomization numbers were generated by the interactive response technology provider using a validated system that automated the random assignment of patients numbers to randomisation numbers”. Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 404): “Randomization numbers were generated by the interactive response technology provider using a validated system that automated the random assignment of patients numbers to randomisation numbers”. Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 404): “Patients, investigator staff, persons performing the assessments and data analysts were blinded to the identity of treatment from the time of randomisation until primary outcome analysis”. Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 404): “Patients, investigator staff, persons performing the assessment and data analysts were blinded to the identity of treatment from the time of randomisation until primary outcome analysis”. Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 404 included/404 analysed Quote (p 405): "Following the intent‐to‐treat principle, data were analysed... Missing values were replaced using the last‐observation‐carried‐forward approach". Comment: ITT analyses |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00941031). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Ruzicka 1990.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: December 1986 to March 1988 Location: 7 centres in Germany |
|
| Participants |
Randomised: 82 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 82, mean age 44 years, 55 male Dropouts and withdrawals 4/82 (5%)
|
|
| Interventions |
Intervention A. Acitretin, orally, 35 mg, daily, 8 weeks (n = 42) Control intervention B. Placebo, orally, daily, 8 weeks (n = 40) |
|
| Outcomes | Assessments at 8 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 483): "The study was designed as a randomized, double‐blind, placebo‐controlled parallel group trial". Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 483): "The study was designed as a randomized, double‐blind, placebo‐controlled parallel group trial". Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 483): "The study was designed as a randomized, double‐blind, placebo‐controlled parallel group trial". Comment: no description of the method used to guarantee blinding as visible side effects are related to acitretin |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 483): "The study was designed as a randomized, double‐blind, placebo‐controlled parallel group trial... the investigators blinded to treatment assignment". Comment: no description of the method used to guarantee blinding of outcome assessment as visible side effects are related to acitretin |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 82 included/78 analysed Quote (p 483): "... according to the intention‐to‐treat principle... Dropout data were evaluated on the date of dropout". Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Sandhu 2003.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: not stated Location: multicentric (number not stated) in North India |
|
| Participants |
Randomised: 30 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 30, mean age of 42.5 years, 25 male Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate (n = 15), orally, 0.5 mg/kg dose tapered after PASI 75 obtained Control intervention B. Ciclosporin (n = 15), orally, 3 mg/kg increased to 4 if no change or rise of dose tapered after PASI 75 obtained |
|
| Outcomes | Assessments at 12 weeks Primary or secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes |
Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 459): "Patients were randomly assigned to either..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 459): "Patients were randomly assigned to either..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 30 included/30 analysed Methods for dealing with missing data: not stated |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. No primary outcome declared |
Saurat 1988.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: not stated Location: 6 centres in France and Switzerland |
|
| Participants |
Randomised: 42 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 42, mean age of 44.5 years, 32 male Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Acitretin (n = 20), orally, 2 x 25/d 2 weeks and 25/d + UVA 3/week, daily, 10 weeks Control intervention B. Placebo, orally (n = 22), daily, 10 weeks Co‐intervention: UVA 3/week, 10 weeks |
|
| Outcomes | Assessments not clearly stated (reported at 8 weeks) Primary outcomes
Outcomes
|
|
| Notes |
Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 219): "This multicenter study was performed in a double‐blind, parallel fashion... The patients were randomly allocated to ..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 219): "This multicenter study was performed in a double‐blind, parallel fashion... The patients were randomly allocated to ..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 219): "This multicenter study was performed in a double‐blind, parallel fashion...All patients initially received 2 capsules of test medication (placebo, acitretin 2x25 mg, ...." Comment: no description of the method used to guarantee blinding of outcome assessment with visible AEs in both acitretin and etretinate groups |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no description of the method used to guarantee blinding of outcome assessment with visible AEs in both acitretin and etretinate groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote (p 220): "Patients who left the study ... were not included in the evaluation of efficacy". Comment: no ITT analyses (number lost to follow‐up unknown) |
| Selective reporting (reporting bias) | Low risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
SCULPTURE 2015.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: August 2011 to March 2013 Setting: 133 centres in North and South America, Europe and Asia |
|
| Participants |
Randomised: 966 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 966, mean age 46 years, 635 male Dropouts and withdrawals 38/966 (4%):
|
|
| Interventions |
Intervention A. Secukinumab (n = 482), SC, 150 mg weeks 0, 1, 2, 3 then monthly Control intervention B. Secukinumab (n = 484), SC, 300 mg weeks 0, 1, 2, 3 then monthly |
|
| Outcomes | Assessments at 52 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 27): “Study funded by Novartis Pharma... Novartis conducted data analyses, and all authors had access to data”. Declarations of interest (p 27): "The authors received writing and editorial support from Barry Weichman and Jinling Wu in the preparation of the manuscript from BioScience Communications, New York, NY, supported by Novartis. Dr Mrowietz has served as advisor and/or received speaker honoraria and/or received grants and/or participated in clinical trials for Abbott/AbbVie, Almirall, Amgen, BASF, Biogen Idec, Celgene, Centocor, Eli Lilly, Forward Pharma, Galderma, Janssen, Leo Pharma, Medac, MSD, Miltenyi Biotech, Novartis, Pfizer, Teva, VBL, and Xenoport. Dr Leonardi has served as consultant and/or investigator and/or participated in a speaker’s bureau for AbbVie, Amgen, Celgene, Dermira, Eli Lilly, Galderma, Janssen, Leo Pharma, Merck, Novartis, Pfizer, Sandoz, Stiefel, and UCB. Dr Girolomoni has received advisory/speaker honoraria and/or research funding from AbbVie, Almirall, Boehringer Ingelheim, Celgene, Dompe, Eli Lilly, Galderma, Janssen, Leo Pharma, Merck Serono, Maruho, MSD, Novartis, and Pfizer. Dr Toth has served as investigator for Novartis, Amgen, Eli Lilly, Johnson & Johnson, Abbott, Celgene, Merck, Galderma, and Leo Pharma. Dr Morita has served as consultant and/or paid speaker for and/or participated in psoriasis clinical trials sponsored by AbbVie, Mitsubishi Tanabe, Janssen, Novartis, Eli Lilly, Kyowa‐Kirin, Leo Pharma, Maruho, and MSD. Dr Szepietowski has served as advisor and/or received speakers honoraria and/or participated in clinical trials for Abbott/AbbVie, Actavis, Amgen, BASF, Astellas, Berlin‐Chemie/Menarini, Biogenetica International Laboratories, Centocor, Fresenius, Janssen, Leo Pharma, Mitsubishi Tanabe, Novartis, Pierre‐Fabre, Takeda, Toray Corporation, and Vichy. Dr Regnault, Ms Thurston, and Dr Papavassilis are employees of and/or own stock in Novartis. Dr Balki has no conflicts of interest to declare." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 28): “were randomised” Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 28): “administered via 2 150 mg SC injections or one 150 mg SC and one placebo SC injection respectively” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 28): "administered via 2 150 mg SC injections or one 150 mg SC and one placebo SC injection respectively" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 966, analysed 966 Management of missing data: Quote (p 29): “Missing values for PASI or IGA 2011 modified version responses were imputed as nonresponse regardless of the reason for missing data”. Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01406938). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Seo 2020.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind, parallel arms study Date of study: January 2017 to August 2018 Location: Korea (10 sites) |
|
| Participants |
Randomised: 62 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 62, mean age of 44 years and 61% men Dropouts and withdrawals 17/62 (27.5%); placebo (8), brodalumab (9)
|
|
| Interventions |
Intervention A. Brodalumab 210 mg SC injection at weeks 1, 2, 4, 6, 8, 10, and Q2W thereafter, until week 62, n = 40 Control intervention B. Placebo SC injection at weeks 1, 2, 4, 6, 8, 10, and Q2W thereafter, until week 62, n = 22 |
|
| Outcomes |
At week 12 Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 816): "funded by Kyowa Kirin Korea Co., Ltd." Declarations of interest: Quote (p 816): "Haeyoun Jeong is a full‐time employee of Kyowa Kirin Korea Co., Ltd. The other authors have no conflicts of interest to declare." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 808): "This phase III, randomized, multicenter study consisted of a 12‐ week double‐blind phase followed by a 52‐week open‐label extension phase... patients were randomized to receive brodalumab 210 mg Q2W or placebo for 12 weeks at a 2:1 ratio and were stratified by bodyweight at screening (≤ 70 kg, > 70 kg), prior use of biologic agents, and investigative site. Randomization was performed through a dynamic allocation procedure using an IWRS. The IP was administrated after coordination with the IWRS vendor, Cenduit, an IQVIA business (Durham, NC, USA)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 808): "This phase III, randomized, multicenter study consisted of a 12‐week double‐blind phase followed by a 52‐week open‐label extension phase... patients were randomized to receive brodalumab 210 mg Q2W or placebo for 12 weeks at a 2:1 ratio and were stratified by bodyweight at screening (≤ 70 kg, > 70 kg), prior use of biologic agents, and investigative site. Randomization was performed through a dynamic allocation procedure using an IWRS. The IP was administrated after coordination with the IWRS vendor, Cenduit, an IQVIA business (Durham, NC, USA)." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 808): "This phase III, randomized, multicenter study consisted of a 12‐week double‐blind phase followed by a 52‐week open‐label extension phase..." Comment: no description of the method used to guarantee blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 808): "This phase III, randomized, multicenter study consisted of a 12‐week double‐blind phase followed by a 52‐week open‐label extension phase..." Comment: no description of the method used to guarantee blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 810): "The populations analyzed were the FAS, which included all randomized patients; the PPS, which included all patients in the FAS but excluded those who had received no treatment, had no post‐dosing primary efficacy data available, failed to meet major eligibility criteria, or had major protocol deviations ... investigative site to examine the treatment difference in the PASI 75 response and sPGA success at week 12 using the last observation carried forward method or non‐responder imputation method for missing data." Randomised 62, analysed 62 Note high rate of dropout: 27% |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02982005). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. No results are posted on ClinicalTrials.gov. |
Shehzad 2004.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study Date of study: March 2001 to November 2001 Location: 1 centre in Karachi, Pakistan |
|
| Participants |
Randomised: 40 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 40, age from 18 to 50 years, % male unknown Dropouts and withdrawals
|
|
| Interventions |
Intervention A. PUVA therapy (+ psoralen) (n = 20), 4 times/week Control intervention B. Methotrexate (n = 20), orally, 10 mg/week, 5 mg Saturday + Sunday |
|
| Outcomes | Time of assessments: not stated Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Immunex Corporation Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (in the Methods section): “The selected patients ... randomly allocated to...” Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (in the Methods section): “The selected patients ... randomly allocated to...” Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no description of the methods used to manage missing data, no description of the methods used to assess the primary outcome (ITT, PP ...) |
| Selective reporting (reporting bias) | High risk | Comment: no protocol was available. The outcomes mentioned in the Results section were not specified in the Methods section. |
SIGNATURE 2019.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study (SIGNATURE) Date of study: October 2013 to July 2016 Location: UK‐Ireland (53 centres) |
|
| Participants |
Randomised: 235 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 235, mean of age 46 years and 56% men Dropouts and withdrawals 25/235 (10.6%): secukinumab 150 group (13), secukinumab 300 group (12)
|
|
| Interventions |
Intervention A. Biological: secukinumab 150 mg at day 0 (initiation of study drug) and at weeks 1, 2, 3, and 4, n = 116 Control Intervention B. Biological: secukinumab 300 mg at day 0 (initiation of study drug) and at weeks 1, 2, 3, and 4, n = 119 |
|
| Outcomes | At 16 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 60): "This study was funded by Novartis Pharmaceuticals U.K. Ltd." Declarations of interest: Quote (p 60‐1): "R.B.W. has received honoraria and/or research grants from AbbVie, Almirall, Bristol‐Myers Squibb, Celgene, Janssen, Leo, Lilly, MSD, Novar‐ tis, Sun, Xenoport and UCB Pharma. J.N.W.B. has received honoraria and/or research grants from AbbVie, Almirall, Bristol‐Myers Squibb, Celgene, Janssen, Leo, Lilly, Novartis, Samsung, Sun and UCB Pharma. A.Y.F. is joint copyright owner of the Dermatology Life Quality Index (Cardiff University) and A.Y.F. receives royalties from this. A.Y.F. has also received honoraria for lecturing and consultancy from Lilly, Novartis and Sanofi. A.D.B. has received honoraria for lecturing and consultancy from AbbVie, Almirall, Boehringer Ingelheim, Celgene, Leo, Lilly, Novartis, Janssen and UCB Pharma. B.K. has received honoraria and research grants from AbbVie, Almirall, Cel‐ gene, Janssen, Leo, Lilly, MSD, Novartis and UCB Pharma. Y.A., R.W., C.H. and S.K. are all employees of Novartis Pharmaceuticals U.K. Ltd. C.E.M.G. has received honoraria and/or research grants from AbbVie, Almirall, Bristol‐Myers Squibb, Celgene, Galderma, Janssen, Leo, Lilly, MSD, Novartis, Sandoz and UCB Pharma. C.E.M.G. is also a National Institute for Health Research Senior Investigator." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 61): "Patients were randomized in a 1: 1 ratio to receive either secukinumab 300 mg or secukinumab 150 mg using an Interactive Response Technology randomization system." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 61): "Patients were randomized in a 1: 1 ratio to receive either secukinumab 300 mg or secukinumab 150 mg using an Interactive Response Technology randomization system." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p61): "This was a 72‐week, multicentre, open‐label, noncomparator study". Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p61): "This was a 72‐week, multicentre, open‐label, noncomparator study". Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 62): "Missing values for the response variables based on a ‘period‐wise’ analysis for PASI were imputed with nonresponse imputation (NRI), regardless of the reason for the missing data. Analyses were based on the full analysis set (i.e. all treated patients who had a baseline PASI assessment and at least one post‐baseline PASI assessment)." Reasonable rate of withdrawal (10%) and numbers and reasons comparable between groups Randomised 235, analysed 233 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01961609). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. ClinicalTrials.gov: ITT analyses |
Singh 2021.
| Study characteristics | ||
| Methods | RCT, active‐controlled, non‐blinded study Date of study: August 2018 to July 2019 Location: India (single‐centre) |
|
| Participants |
Randomised: 140 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 140, mean of age 38 years, and 73% men Dropouts and withdrawals 18/140 (13%) methotrexate (8), cyclosporine (10)
|
|
| Interventions |
Intervention A. Methotrexate 0.15 mg/kg/week intramuscular injection + cyclosporine 2.5 mg/kg/day, n = 70 Control intervention B. Methotrexate 0.3 mg/kg/week intramuscular injection, n = 70 |
|
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 221): "Nil" Declarations of interest: Quote (p 221): "There are no conflicts of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 215): "The study was a non‐blinded trial. Participants were randomised equally in a 1:1 allocation (unstratified) into two treatment groups by a computer generated random number sequence using the MS Excel software." Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 215): "The study was a non‐blinded trial. Participants were randomised equally in a 1:1 allocation (unstratified) into two treatment groups by a computer generated random number sequence using the MS Excel software." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 215): "The study was a non‐blinded trial. Participants were randomised equally in a 1:1..." Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 215): "The study was a non‐blinded trial. Participants were randomised equally in a 1:1..." Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dealing with missing data: quote (p 216): "The analysis for adverse effects included all patients excluding the ones who were lost to follow‐up. Per‐protocol analysis was initially done. The parameters found to be significant were also subjected to intention to treat analysis (ITT)." Randomised 140, analysed 140 Comment: no description of the method used to guarantee management of missing data |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was not available on ClinicalTrials.gov but on CTRI (CTRI/2018/07/015044). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Sommerburg 1993.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: 1986 to 1988 Location: 7 centres in Germany |
|
| Participants |
Randomised: 88 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 88, mean age of 45 years, 68 male Dropouts and withdrawals 5/88 (6%)
|
|
| Interventions |
Intervention A. Acitretin (n = 44), orally, 50 mg (15 days) then 25 mg, daily, 8 weeks Control intervention B. Placebo (n = 44), orally, daily, 8 weeks Co‐intervention PUVA (8‐methoxypsoralen), orally 0.6 mg/kg, 3 to 5/week, 8 weeks |
|
| Outcomes | Assessments at 8 weeks Primary outcome
Secondary outcome
|
|
| Notes |
Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 310): "The study was designed as a randomised, double‐blind, parallel groups trial... Both investigators and biostatisticians were blinded". Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 310): "The study was designed as a randomised, double‐blind, parallel groups trial... Both investigators and biostatisticians were blinded". Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (pp. 310‐1): "The study was designed as a randomised, double‐blind, parallel group trial... Both investigators and biostatisticians were blinded… however due to well know side effect pattern of acitretin, ..., the possibility of an investigator bias cannot be excluded". Comment: visible AEs in acitretin groups |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (pp. 310‐1): " The study was designed as a randomised, double‐blind, parallel group trial... Both investigators and biostatisticians were blinded… however due to well know side effect pattern of acitretin, ..., the possibility of an investigator bias cannot be excluded". Comment: visible AEs in acitretin groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 88 included/83 analysed Quote (p 311): "Patients who discontinued the trial prematurely were evaluated on the date of discontinuation of therapy". Comment: not ITT, low number of dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
SPIRIT‐H2H 2020.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label, blinded assessor study Date of study: August 2017 to November 2018 Location: worldwide Phase 4 |
|
| Participants |
Randomised: 566 participants of which 100 had a moderate‐to‐severe plaque psoriasis. Inclusion criteria
Exclusion criteria
Baseline characteristics N = 100, mean age and % of men not stated for patients with moderate‐to‐severe plaque psoriasis Dropouts and withdrawals Not stated for the subgroup moderate‐to‐severe plaque psoriasis |
|
| Interventions |
Intervention A. Ixekizumab 160 milligrams (mg) given subcutaneously (SC) at baseline for all participants 80 mg ixekizumab given once every 2 weeks (Q2W) SC from week 2 to week 12 and once every 4 weeks (Q4W) thereafter for participants with moderate‐to‐severe plaque psoriasis Control Intervention B. Adalimumab 80 mg given SC at baseline followed by 40 mg Q2W given SC starting week 1 for participants with moderate‐to‐severe plaque psoriasis 40 mg adalimumab given Q2W SC at baseline followed by 40 mg Q2W starting at week 2 given SC for participants not meeting criteria for moderate‐to‐severe plaque psoriasis |
|
| Outcomes | At week 24 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (ClinicalTrials.gov): "Eli Lilly and Company" Declarations of interest: Quote (abstract Kristensen et al): "L. Kristensen, AbbVie, 2, 8, Amgen Inc., 2, 8, Biogen, 2, 8, BMS, 2, 8, Eli Lilly, 2, 8, Janssen, 2, 8, Novartis, 2, 8, Pfizer, 2, 8, UCB Pharma, 2, 8, Sanofi, 2, 5, 8; M. Okada, Astellas, 8, Eli Lilly and Company, 8; W. Tillett, AbbVie, 5, 8, Amgen, 5, 8, Eli Lilly, 5, 8, Janssen, 5, 8, Novartis, 5, 8, Pfizer, 5, 8, UCB Pharma, 5, 8; S. Liu‐Leage, Eli Lilly and Company, 3, 4; C. El Baou, Eli Lilly and Company, 9; A. Bradley, Eli Lilly and Company, 3; G. Meszaros, Eli Lilly and Company, 1, 3; K. de Vlam, Eli Lilly and Company, 2, 5, 8, Novartis, 2, 5, 8, UCB, 2, 5, 8, Celgene, 2, 5, 8, Pfizer, 2, 5, 8." The RoB was assessed using the article: Mease PJ, Smolen JS, Behrens F, et al. Ann Rheum Dis 2020;79:123–31. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 124): "This study is a 52 week, phase IIIb/IV, multicentre, randomised, open‐label, blinded‐assessor, parallel‐group study evaluating the efficacy and safety of IXE versus ADA in bDMARD‐naïve, csDMARD‐inadequate‐responder patients (based on medical history) with active PsA. Following a 28‐day screening period, participants were randomised 1:1 to open‐label IXE or ADA during a 52‐week open‐label treatment period (weeks 0–52). Randomisation was stratified by concomitant csDMARD use at baseline and moderate‐to‐severe plaque psoriasis involvement (Psoriasis Area and Severity Index (PASI)≥12, BSA ≥10% and static physician’s global assessment (sPGA) ≥3)." Quote (supplementary): "Assignment to treatment groups was determined by a computer‐generated random sequence using an interactive web‐response system (IWRS). Site personnel confirmed the correct investigational product by entering a confirmation number found on the investigational product into the IWRS." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (supplementary): "Assignment to treatment groups was determined by a computer‐generated random sequence using an interactive web‐response system (IWRS). Site personnel confirmed the correct investigational product by entering a confirmation number found on the investigational product into the IWRS." Quote (supplementary): "Blinded assessors were not allowed to know patient allocation or to be otherwise involved in study procedures, and patients were instructed not to communicate with blinded assessors except for communication required to conduct the blinded data assessment. A third person from the study site was present during each procedure conducted by the blinded assessor to observe and document that the blinding of the assessor was maintained. If unintentionally unblinded, the blinded assessor was replaced. Blinded assessors were required to have at least 1 year of experience for administering the outcome instruments." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 124): "This study is a 52 week, phase IIIb/IV, multicentre, randomised, open‐label, blinded‐assessor, parallel‐group study evaluating the efficacy and safety of IXE versus ADA in bDMARD‐naïve, csDMARD‐inadequate‐responder patients (based on medical history) with active PsA. Following a 28‐day screening period, participants were randomised 1:1 to open‐label IXE or ADA during a 52‐week open‐label treatment period (weeks 0–52). Randomisation was stratified by concomitant csDMARD use at baseline and moderate‐to‐severe plaque psoriasis involvement (Psoriasis Area and Severity Index (PASI)≥12, BSA ≥10% and static physician’s global assessment (sPGA) ≥3)." Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (supplementary): "Blinded assessors were not allowed to know patient allocation or to be otherwise involved in study procedures, and patients were instructed not to communicate with blinded assessors except for communication required to conduct the blinded data assessment. A third person from the study site was present during each procedure conducted by the blinded assessor to observe and document that the blinding of the assessor was maintained. If unintentionally unblinded, the blinded assessor was replaced. Blinded assessors were required to have at least 1 year of experience for administering the outcome instruments." Comment: adequate process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (supplementary): "For assessing noninferiority of IXE to ADA, missing data were imputed using the nonresponders imputation (NRI) method. Noninferiority analysis was performed on the intent‐to‐treat (ITT) population using a prespecified fixed margin approach." Randomised 566, analysed 566 Randomised 100, analysed 100 for the subgroup of patients with moderate‐to‐severe psoriasis |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03151551). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are not posted on ClinicalTrials.gov for the subgroup of patients with moderate‐to‐severe psoriasis. |
Strober 2011.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: July 2008 to April 2009 Location: 41 centres in the USA |
|
| Participants |
Randomised: 211 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 211, mean age 45 years, 131 male Dropouts and withdrawals 18/211 (8.5%): etanercept 12, placebo 6
|
|
| Interventions |
Intervention A. Etanercept (n = 139), SC auto‐administered, 50 mg twice a week, 11 weeks Control intervention B. Placebo (n = 72), SC auto‐administered, twice a week |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes At 4, 8, 12 weeks
|
|
| Notes |
Funding source: Quote (Appendix 1): "Abbott Laboratories funded this study and participated in the study design, data collection, data management, data analysis and preparation of the manuscript. All of the authors had full access to the data and were involved in the analysis of data, development and revision of the manuscript, and decision to submit the manuscript for publication. The corresponding author takes responsibility for the integrity of the data and the accuracy of the data analysis." Declarations of interest: Quote (Appendix 1): "B.E.S. has been an investigator, consultant, speaker, and served on an advisory board for Amgen, Abbott and Centocor; and has also been a speaker for Astellas. J.J.C. has received research support from Abbott, Amgen, Centocor, Celgene and Eli Lilly; has been a consultant for Abbott, Amgen and Centocor; and has been a speaker for Abbott. P.S.Y. has served as a consultant, principle investigator, speaker or advisory board member for Abbott, Amgen, Astellas and Centocor. M.O. and D.A.W. are employees of Abbott." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 662): "Patients were randomised..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 662): "Patients were randomised" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 662): “Patients enrolled in the placebo arm received SC injections matching active treatment to maintain the blind. To maintain the blind, all patients received two SC injections at weeks 0 and 4 and one SC injection at week 8, consisting of either briakinumab or matching placebo, depending on the treatment arm. In addition, each patient also received two SC injections biweekly, 3 days apart, week 0 through week 11, consisting of either etanercept or matching placebo, depending on the treatment arm.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 662): “Patients enrolled in the placebo arm received SC injections matching active treatment to maintain the blind. To maintain the blind, all patients received two SC injections at weeks 0 and 4 and one SC injection at week 8, consisting of either briakinumab or matching placebo, depending on the treatment arm. In addition, each patient also received two SC injections biweekly, 3 days apart, week 0 through week 11, consisting of either etanercept or matching placebo, depending on the treatment arm.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 211, analysed 211 Management of missing data: Quote (p 663): “The primary efficacy analysis consisted of four comparisons performed in the intent‐to‐treat population (i.e. all randomised patients), …, Nonresponder imputation was used to handle missing data.” Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00710580). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
STYLE 2020.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: May 2017 to January 2019 Location: 13 sites in Canada and 28 sites the USA Phase 3 |
|
| Participants |
Randomised: 303 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 303, mean age of 46.9 years, and 62% men Dropouts and withdrawals 51/303 (17%): apremilast group (33), placebo group (18)
|
|
| Interventions |
Intervention A. Apremilast 30 mg tablets orally twice a day for 16 weeks Control intervention B. Placebo tablets twice a day for 16 weeks |
|
| Outcomes | At week 16 Primary composite outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 2): "The authors acknowledge financial support for this study from Celgene Corporation. The authors received editorial support in the preparation of this report from Amy Shaberman, PhD, of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, USA, sponsored by Celgene Corporation, Summit, NJ, USA. The authors, however, directed and are fully responsible for all content and editorial decisions for this report." Declarations of interests: Quote (p 3‐4): "ASVV: AbbVie, Allergan, Celgene Corporation, Derm Tech, Dermira, Novartis, and Valeant – honoraria for advisory board and/or consulting; Merck – pension (ex‐spouse). LSG: Celgene Corporation, LEO Pharma, Novartis, Pfizer, and Stiefel/GlaxoSmithKline – investigator and/or consultant. ML: Mount Sinai (which receives funds from Boehringer Ingelheim, Celgene Corporation, Eli Lilly, Janssen/Johnson & Johnson, Kadmon, MedImmune/AstraZeneca, Novartis, Pfizer, and ViDac). BS: AbbVie, Almirall, Amgen, Arena, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene Corporation, Dermavant, Dermira, Eli Lilly, GlaxoSmithKline, Janssen, Kyowa Hakko Kirin, LEO Pharma, Medac, Meiji Seika Pharma, Menlo Therapeutics, Novartis, Ortho Dermatologics/Valeant, Pfizer, Regeneron, Sanofi‐Genzyme, Sebela Pharmaceuticals, Sun Pharma, and UCB Pharma – honoraria as a consultant and advisory board member; AbbVie, Boehringer Ingelheim, Celgene Corporation, Eli Lilly,Galderma, GlaxoSmithKline, Janssen, Merck, Pfizer, and Sienna – payments (to the University of Connecticut) as an investigator; Corrona Psoriasis Registry – fees as a scientific director; AbbVie and Janssen – grant support (to the University of Connecticut for Fellowship Program). CL: AbbVie, Boehringer Ingelheim, Celgene Corporation, Eli Lilly, Janssen, Merck, Novartis, Pfizer, Sun Pharma, and Valeant – principal investigator/consultant. ST: No conflicts or potential conflicts of interest to disclose. AC: AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Celgene Corporation, Dermira, Eli Lilly, Janssen, Maruho, Novartis, Pfizer, Stiefel/ GlaxoSmithKline, Sun Pharma, and UCB – investigator; Celgene Corporation – consultant. HS: Celgene Corporation, Janssen, Lilly, and Novartis – grants received as an investigator. ZZ, MP, & YW: Celgene Corporation – employment." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 9): "For the placebo‐controlled phase, study personnel randomized patients (2:1), using a permuted block randomization and centralized interactive response technology, to receive apremilast 30 mg BID or placebo for 16 weeks." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 9): "For the placebo‐controlled phase, study personnel randomized patients (2:1), using a permuted block randomization and centralized interactive response technology, to receive apremilast 30 mg BID or placebo for 16 weeks." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 9): "The study sponsor, site, contract research organization (CRO) personnel, and patients were blinded to treatment allocation through week 16". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 9): "The study sponsor, site, contract research organization (CRO) personnel, and patients were blinded to treatment allocation through week 16". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 303, analysed 303 Management of missing data: Quote (p 9, 10): "missing values at week 16 were imputed using the MI method... Primary and secondary endpoints were analyzed in the intent‐to‐treat (ITT) population, defined as all randomized patients." Results for PASI and PGA were reported in supplementary appendix. Comment: number of analysed patients not reported for PGA and PASI |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03123471). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
SustaIMM 2019.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: December 2016 to September 2017 Location: multicentre, Japan Phase 2/3 |
|
| Participants |
Randomised: 182 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 171, mean of age years 52, and 84% men Dropouts and withdrawals 7/171 (4%): risankizumab 150 group (1), risankizumab 75 group (2), placebo group (4)
|
|
| Interventions |
Intervention A. Risankizumab 150 mg by SC injection at weeks 0 and 4 (Part A), n = 55 Control interventions B. Risankizumab 75 mg by SC injection at weeks 0 and 4, n = 58 C. Placebo, n = 55 |
|
| Outcomes | At week 16 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 693): "AbbVie and Boehringer Ingelheim funded the SustaIMM (NCT03000075) study..." Declarations of interest: Quote (p 693): "The authors and AbbVie scientists designed the study and analyzed and interpreted the data. All authors contributed to the development of the content, all authors and AbbVie reviewed and approved the manuscript, and the authors maintained control over the final content. M. O. has received honoraria or fees for serving on advisory boards, as a speaker and as a consultant, and grants as an investigator from AbbVie, Celgene, Eisai, Eli Lilly and Company, Janssen, LEO Pharma, Maruho, Mitsubishi‐Tanabe, Novartis and Torii. H. F. has received honoraria or fees for serving on advisory boards and as a speaker and grants as an investigator from AbbVie, Celgene, Eisai, Eli Lilly and Company, Janssen, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi‐Tanabe, Novartis, Taiho and Torii. M. W., K. S. and M. F. are full‐time employees of Boehringer Ingelheim. X. H., S. K. and J. V. are full‐time employees of AbbVie and may own stock/options. A. I. has received honoraria or fees for serving on advisory boards, as a speaker and as a consultant, and grants as an investigator from AbbVie, Celgene, Eli Lilly, Janssen, Kyowa Hakko Kirin, Maruho and Novartis." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 687): "In this double‐blinded, placebo‐controlled, two‐part study of risankizumab, patients were randomized 2:2:1:1 to receive risankizumab 75 mg, risankizumab 150 mg, placebo with cross‐over to risankizumab 75 mg or placebo with cross‐over to risankizumab 150 mg. Randomization was stratified according to concomitant psoriatic arthritis at baseline (yes vs no) and bodyweight (≤ 90 vs > 90 kg)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 687): "In this double‐blinded, placebo‐controlled, two‐part study of risankizumab, patients were randomized 2:2:1:1 to receive risankizumab 75 mg, risankizumab 150 mg, placebo with cross‐over to risankizumab 75 mg or placebo with cross‐over to risankizumab 150 mg. Randomization was stratified according to concomitant psoriatic arthritis at baseline (yes vs no) and bodyweight (≤ 90 vs > 90 kg)." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 687): "In this double‐blinded, placebo‐controlled, two‐part study of risankizumab, patients were randomized 2:2:1:1 to receive risankizumab 75 mg, risankizumab 150 mg, placebo with cross‐over to risankizumab 75 mg or placebo with cross‐over to risankizumab 150 mg." Comment: no description of the method used to guarantee blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 687): "In this double‐blinded, placebo‐controlled, two‐part study of risankizumab, patients were randomized 2:2:1:1 to receive risankizumab 75 mg, risankizumab 150 mg, placebo with cross‐over to risankizumab 75 mg or placebo with cross‐over to risankizumab 150 mg." Comment: no description of the method used to guarantee blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 688): "For all non‐binary end‐points, last observation carried forward was used for missing data. For all binary end‐points, missing data were imputed as non‐response." Randomised 171, analysed 171 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03000075). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results posted on ClinicalTrials.gov: ITT |
Tanew 1991.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: not stated Location: 2 centres in Austria (Vienna, Innsbruck) |
|
| Participants |
Randomised: 60 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 60, mean age 40 years (acitretin), 49 years (placebo), 42 male Dropouts and withdrawals 12/60 (20%)
|
|
| Interventions |
Intervention A. Acitretin (n = 30), orally, 1 mg/kg, daily, 12 weeks or until complete clearing Control intervention B. Placebo (n = 30), orally, daily, 12 weeks Co‐intervention PUVA, phototherapy, 4 times/week, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary and secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes |
Funding source: supported by a grant from Hoffmann La Roche & Co Ltd Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 682): "Only patients ... were included and assigned randomly..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 682): "Only patients ... were included and assigned randomly..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p. 682): "Acitretin ... or placebo..." Comment: no description of the method used to guarantee blinding of participants and personnel as acitretin leads to visible adverse effects (cheilitis) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p. 682): "Acitretin ... or placebo..." Comment: no description of the method used to guarantee blinding of participants and personnel as acitretin leads to visible adverse effects (cheilitis) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 60, analysed 48 Quote (p 683): "Of the 60 patients, 48 completed the study and were included in the statistical analysis". Comment: not ITT |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, no outcomes defined in the Methods section |
Thaci 2021.
| Study characteristics | ||
| Methods | RCT, active‐controlled, open‐label study with blinded assessment of the efficacy outcome Date of study: August 2017 to July 2018 Location: Germany (21 sites) Phase 3 |
|
| Participants |
Randomised: 120 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 120, mean age of 42 years and 59% men Dropouts and withdrawals 13/120 (11%): risankizumab group (0), Fumaderm 300 group (13)
|
|
| Interventions |
Intervention A. Risankizumab 150 mg by subcutaneous injection at weeks 0, 4, and 16, n = 60 Control intervention B. Fumaderm 30 mg administered as a tablet orally once daily from week 0 to week 2, then up to 240 mg, n = 60 |
|
| Outcomes | At week 24 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (article): "AbbVie Inc. funded the research for this study and participated in the study design". Declarations of interest: Quote (article): "DT: received grant/research support from AbbVie Inc., Celgene, Novartis; has participated in a speakers bureau for AbbVie Inc., Amgen, Almirall, Biogen‐Idec, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen‐Cilag, LEO Pharma, Morphosis, Novartis, Pfizer, Regeneron Pharmaceuticals, Sandoz/Hexal, Sanofi, and UCB Pharma; and has served as a consultant/member of scientific board for AbbVie Inc., Almirall, Celgene, Eli Lilly, Janssen‐Cilag, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sandoz/Hexal, Sanofi, and UCB Pharma. KE: Served as a speaker, investigator, and/or advisor for AbbVie Inc., Almirall, Berlin Chemie, BMS, Boehringer Ingelheim, Celgene, Eli Lilly, Galapagos, Hexal, Janssen, Novartis, Sanofi, and UCB Pharma. AP: Served as an investigator, grant recipient, advisor/consultant, and/or speaker for AbbVie Inc., Almirall‐Hermal, Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, GSK, Hexal, Janssen, LEO Pharma, MC2, Medac, Merck Serono, Mitsubishi, MSD, Novartis, Pascoe, Pfizer, Regeneron Pharmaceuticals, Roche, Sandoz Biopharmaceuticals, Schering‐Plough, Tigercat Pharma, and UCB Pharma." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (article): "Patients were randomly assigned via interactive response/web response technology using block randomization and a randomization schedule prepared by the statistics department of the sponsor. Randomization was stratified by prior phototherapy exposure.'' Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (article): "Patients were randomly assigned via interactive response/web response technology using block randomization and a randomization schedule prepared by the statistics department of the sponsor. Randomization was stratified by prior phototherapy exposure." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (article): "This phase 3, randomized, active‐controlled, multicenter, open‐label study with blinded assessment of efficacy" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (article): "This phase 3, randomized, active‐controlled, multicenter, open‐label study with blinded assessment of efficacy" Comment: no description of method used to guarantee no communication between participants and assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dealing with missing data: Quote (article): "Missing efficacy data were imputed using non‐responder imputation (NRI) for categorical endpoints and last observation carried forward for continuous endpoints. An as‐observed case (OC) analysis was used as a secondary approach in the analysis of continuous endpoints and did not impute values for missing evaluations (e.g. those patients who did not have an evaluation at a scheduled visit were excluded from the OC analysis for that visit)." Randomised 120, analysed 120 Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03255382). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
Torii 2010.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: not stated Location: 28 centres in Japan |
|
| Participants |
Randomised: 54 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 54 participants, mean age 46 years, 36 male Dropouts and withdrawals 7/54 (13%) at W14
|
|
| Interventions |
Intervention A. Infliximab (n = 35), IV, 5 mg/kg, weeks 0, 2, 6; 10 weeks Control intervention B. Placebo (n = 19), IV, weeks 0, 2, 6; 10 weeks |
|
| Outcomes | Assessments at 10 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 41): "Eligible patients were randomised in a 2:1 ratio to either... using the dynamic allocation method". Comment: no description of the methods used to guarantee the random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 41): "Eligible patients were randomised in a 2:1 ratio to either... using the dynamic allocation method". Comment: no description of the methods used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 41): "The induction phase of the treatment was .. double‐blind placebo controlled trial... Infliximab or placebo was administered by IV drip infusion over a period of at least 2h ..." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 41): "The induction phase of the treatment was .. double‐blind placebo controlled trial... Infliximab or placebo was administered by intravenous drip infusion over a period of at least 2h ..." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 54, analysed 54 Quote (p 42): "This primary endpoint analysis was performed on an "intent‐to‐treat" basis...Patients who discontinued the study treatment ... were handled as "not improved" in the assessment". Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
TRANSFIGURE 2016.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: November 2013 to January 2017 Location: worldwide Phase 3 |
|
| Participants |
Randomised: 198 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 198, mean age of 44 years, 160 male Dropouts and withdrawals 12/198 (6.1%): secukinumab 150 (4), secukinumab 300 (1), PBO (7)
|
|
| Interventions |
Intervention A. Biological: secukinumab 150 mg weekly for 5 weeks, then once every 4 weeks up to and including week 128, n = 67 ControlIntervention B. Biological: secukinumab 300 mg weekly for 5 weeks, then once every 4 weeks up to and including week 128, n = 66 C. Biological: placebo, n = 65 |
|
| Outcomes | At week 16 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1): "Funding sources: This study was funded by Novartis Pharma AG, Basel, Switzerland." Declarations of interest: Quote (Appendix): "Conflicts of interest. K.R. has participated in clinical trials sponsored by AbbVie, Amgen, Biogen Idec, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, LEO, Lilly, Medac, MSD, Novartis, Pfizer, Takeda and Vertex; and has served as a consultant for AbbVie, Amgen, Biogen Idec, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, LEO, Lilly, Medac, MSD, Novartis, Pfizer, Takeda and Vertex. J.S. has received educational grants from Novartis, AbbVie and Pfizer; and has received consultancy fees from Novartis, AbbVie, Pfizer and Eli Lilly. P.A. has received grants from Novartis. U.M. has received grants and/or participated in clinical trials for Abbott/AbbVie, Almirall, Amgen, BASF, Biogen Idec, Celgene, Centocor, Eli Lilly, Forward Pharma, Galderma, Janssen, LEO Pharma, Medac, MSD, Miltenyi Biotech, Novartis, Pfizer, Teva, VBL and Xenoport; has served as an advisor for and/or received speaker honoraria and/or grants from Abbott/Abb‐ Vie, Almirall, Amgen, BASF, Biogen Idec, Celgene, Centocor, Eli Lilly, Forward Pharma, Galderma, Janssen, LEO Pharma, Medac, MSD, Miltenyi Biotech, Novartis, Pfizer, Teva, VBL and Xenoport; has participated in clinical trials by Novartis, AbbVie, UCB, Valeant, Athenex, MC2 Therapeutics, Dermira, Kadmon, Boehringer Ingelheim, Galderma, Regeneron, Coherus, Tolmar, Amgen, Total, Watson, Sandoz, Xenoport, AbGenomics and Lilly; and has received consulting fees or speaker honoraria from Novartis, Celgene and AbbVie. M.A. has received grants from and/or participated in clinical trials for AbbVie, Almirall, Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, Janssen‐Cilag, LEO, Medac, MSD (formerly Essex, Schering‐Plough), Mundipharma, Novartis, Pfizer (formerly Wyeth), Pohl Boskamp, Sandoz and Xenoport; and has served as an advisor for and/or received speaker honoraria from AbbVie, Almirall, Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, Janssen‐Cilag, LEO, Medac, MSD (formerly Essex, Schering‐Plough), Mundipharma, Novartis, Pfizer (formerly Wyeth), Pohl Boskamp, Sandoz and Xenoport. A.P., P.R., R.Y. and M.M. are full‐time employees of Novartis." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 2): "Randomization was managed via a central interactive randomization system and ensured that an equal number of patients were allocated to secukinumab 300 mg, secukinumab 150 mg or placebo, stratified by body weight (< 90 kg or ≥ 90 kg). At week 16, all patients receiving placebo were re‐randomized 1:1 to receive either 300 mg or 150 mg secukinumab." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 2): "Randomization was managed via a central interactive randomization system and ensured that an equal number of patients were allocated to secukinumab 300 mg, secukinumab 150 mg or placebo, stratified by body weight (< 90 kg or ≥ 90 kg). At week 16, all patients receiving placebo were re‐randomized 1:1 to receive either 300 mg or 150 mg secukinumab." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 2): "TRANSFIGURE was a randomized, double‐blind, placebo‐controlled trial...Patients received subcutaneous treatments of identical appearance once a week for 5 weeks (at baseline and weeks 1, 2, 3 and 4), followed by dosing every 4 weeks, starting at week 4 (appendixes S3 and S4; see Supporting Information)." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 2): "TRANSFIGURE was a randomized, double‐blind, placebo‐controlled trial...Patients received subcutaneous treatments of identical appearance once a week for 5 weeks (at baseline and weeks 1, 2, 3 and 4), followed by dosing every 4 weeks, starting at week 4 (appendixes S3 and S4; see Supporting Information)." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data Quote (p 2): "Missing values for PASI and Investigator’s Global Assessment (IGA) mod 2011 were imputed using multiple imputation. Missing patient reported outcome values were imputed with last observation carried forward". On ClinicalTrials.gov, randomised 198, analysed 198 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01807520). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
Tyring 2006.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: June 2003 to January 2004 Location: 39 centres in Houston, USA and Canada |
|
| Participants |
Randomised: 620 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 620 participants, mean age 46 years, 419 male Dropouts and withdrawals 23/620 (3.7%): etanercept group (6), placebo group (15)
|
|
| Interventions |
Intervention A. Etanercept (n = 311), 50 mg, SC, twice‐weekly, 12 weeks Control intervention B. Placebo (n = 309), SC, twice‐weekly, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 361): "The study was designed by Immunex, S Tyring, and other members of the Etanercept Psoriasis study group (The complete data set was held at the central data‐processing facility at Amgen)." Declarations of interest: Quote (pp 367‐8): "S Tyring has received research support from Amgen. A Gottlieb is a consultant for several companies (Amgen, BiogenIdec, CellGate, Centocor, Genentech, Novartis AG, Wyeth Pharmaceuticals, Schering‐Plough Corporation, Eisai, Celgene, Bristol Myers Squibb, Beiersdorf, Warner Chilcott, Abbott Labs, Allergan, Kemia, Roche, Sankyo, Medarex, Celera, TEVA, Actelion, and Advanced ImmuniT) and is on the speaker’s bureau for Amgen, BiogenIdec, and Wyeth Pharmaceuticals. She has also received research funding from Amgen, BiogenIdec, Centocor, Genentech, Abbott Labs, Ligand Pharmaceuticals, Beiersdorf, Fujisawa Healthcare, Celgene Corp, Synta, Bristol Myers Squibb, Warner‐Chilcott, and Paradigm. K Papp is a consultant, has received research funding, and has served as a speaker for Amgen, BiogenIdec, Centocor, Genentech, Novartis, Wyeth, Schering‐Plough, Abbott, Allergan, Medimmune, Serono, Xoma, Isotechnica, and GlaxoSmithKline. He has also served as a medical or scientific officer for Amgen, Centocor, Genentech, and Serono. K Gordon has received research support and honoraria from Abbott, Amgen, Biogen‐IDEC, Centocor, Genentech, and Synta. C Leonardi is: a consultant, investigator, and speaker for Amgen and Genentech and has received educational grants from these companies; a consultant, investigator, and speaker for Centocor; a consultant and investigator for Serono; and a consultant, investigator, and speaker for Abbott..." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 30): “Randomisation code lists were generated in the Biostatistics Department at Amgen by a designed person with no other association with the study”. Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 30): “Randomisation code lists were generated in the Biostatistics Department at Amgen by a designed person with no other association with the study”. Comment: no precision |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 30): "All patients received 2 injections per dose of investigational product”. Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 30): “To prevent study assessors from being influenced by the presence of an injection site reaction, patients applied dressings to the last three injection sites and to any erythematous injection sites before each psoriasis evaluation”. Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 620, analysed 617 for the primary outcome Management of missing data: quote (p 31): “The primary analyses for all efficacy endpoints included all randomised patients who received at least one dose of investigational product. Missing values were imputed using last observation carried forward”. Comment: only 2 participants did not receive at least 1 dose, 618 participants should be involved in the mITT, however 617 participants were analysed for the primary outcome. |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00111449). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
UltIMMa‐1 2018.
| Study characteristics | ||
| Methods | RCT, placebo/active‐controlled, double‐blind study Date of study: February 2016 to August 2016 Location: worldwide Phase 3 |
|
| Participants |
Randomised: 506 participants Inclusion criteria
Exclusion criteria
Dropouts and withdrawals 10/506 (2%); risankizumab group (5), ustekinumab group (1), placebo group (4)
|
|
| Interventions |
Intervention A. Risankizumab, SC, 150 mg, n = 304 Control interventions B. Ustekinumab, SC, based on weight per label (45 mg for participants with body weight ≤ 100 kg or 90 mg for participants with body weight > 100 kg), n = 100 C. Placebo, n = 102 |
|
| Outcomes | At week 16 Primary composite outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 650): "AbbVie and Boehringer Ingelheim" Declarations of interest: Quote (p 660): "KBG has received honoraria for serving as a consultant and/or grants as an investigator from AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Dermira, Eli Lilly, GlaxoSmithKline, Janssen, Leo Pharma, Novartis, Pfizer, Regeneron, Sanofi‐Aventis, Sun, and UCB. BS has received honoraria as a consultant for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Dermavant, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Janssen,Leo Pharma, Medac, Meiji Seika Pharma, Menlo Therapeutics, Merck, Novartis, Ortho Dermatologics/Valeant, Pfizer, Regeneron, Sanofi Genzyme, Sebela, Sienna, Sirtris, Sun Pharma, and UCB pharma, and as scientific director for the CORRONA‐NPF Psoriasis Registry. He is an investigator for AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly,Galderma, GlaxoSmithKline, Janssen, Merck, Pfizer, and Sienna. ML has received grants as an investigator from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen Research & Development, Kadmon, Leo Pharma, Novartis, Pfizer, and ViDac and has received honoraria for serving as a consultant for Allergan, Aqua, Boehringer Ingelheim, Leo Pharma, Menlo, and Promius. MA has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant; and grants as an investigator from AbbVie, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Hexal, Janssen, Leo Pharma, Eli Lilly, Medac, Mundipharma, MSD, Novartis, Pfizer, Sandoz, UCB, and Xenoport. AB has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant; and grants as an investigator from AbbVie, Aclaris, Akros, Allergan, Almirall, Amgen, Boehringer Ingelheim, Celgene, Dermavant, Dermira, Eli Lilly, Genentech/Roche, GlaxoSmithKline, Janssen, Leo Pharma, Meiji, Merck Sharp & Dohme, Novartis, Pfizer, Purdue Pharma, Regeneron, Sandoz, Sanofi Genzyme, Sienna pharmaceuticals, UCB, Valeant, and Vidac. YP has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant, and grants as an investigator from AbbVie, Amgen, Baxalta, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Janssen/Centocor, Leo Pharma, MedImmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi‐Genzyme, Sun Pharma, Takeda, Valeant, and UCB. KAP has received honoraria or fees for serving on advisory boards, as a speaker, as a consultant, or as a steering committee member or grants as an investigator from AbbVie, Akros, Allergan, Amgen, Anacor, Arcutis, Astellas, AstraZeneca, Baxalta, Baxter, Boehringer Ingelheim, Bristol‐Myers Squibb, CanFite, Celgene, Coherus, Dermira, Eli Lilly, Forward Pharma, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa‐Hakko Kirin, Leo Pharma, MedImmune, Meiji Seika Pharma, Merck (MSD), Merck‐Serono, Mitsubishi Pharma, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Takeda, UCB, and Valeant. HS has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant, and grants as an investigator from AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novartis, and Pfizer. LP has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant, and grants as an investigator from AbbVie, Amgen, Baxalta, Biogen, Boehringer Ingelheim, Eli Lilly, Janssen, Leo Pharma, Merck‐Serono, MSD, Novartis, Pfizer, Regeneron, Roche; Sandoz, and Sanofi Genzyme. PF has received honoraria and/or research grants from and/or served as an investigator and/or advisory board member for AbbVie, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Celtaxsys, CSL, Cutanea, Dermira, Galderma, Genentech, GSK, iNova, Janssen, Leo Pharma, Lilly, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals, Roche, Sanofi, Sun Pharma, UCB Pharma, and Valeant. MO has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant, and grants as an investigator from AbbVie, Actelion, Astellas, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Eisai, Eli Lilly, and Company, Galderma, Janssen, Kaken, Kyowa‐Kirin, Leo Pharma, Maruho, Mochida, Nichi‐Iko, Nippon Kayaku, Nippon Zoki, Novartis, Ono, Ohtsuka, Pola Pharma, Pfizer, Sanofi, Shionogi, Taiho, Tanabe‐Mitsubishi, Teijin, and Torii. MF is a full‐time employee of Boehringer Ingelheim. ZG, YG, and JMV are full‐time employees of AbbVie and own stock or options. EHZT, a former employee of AbbVie, currently owns stock. HB has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant, and grants as an investigator from AbbVie, Almirall, Amgen, Bayer, Baxalta, Biocad, Boehringer Ingelheim, Celgene, Dermavant, Eli Lilly, Janssen, Leo Pharma, Menarini, MSD, Novartis, Pfizer, Pierre Fabre, Sandoz, Sun Pharmaceuticals, and UCB." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 651‐2): "UltIMMa‐1 and UltIMMa‐2 were replicate phase 3,randomised, double‐blind, placebo‐controlled and active comparator‐controlled...In each study, patients were randomly assigned (3:1:1) to receive risankizumab, ustekinumab, or matching placebo (appendix). Randomisation was stratified by weight (≤ 100 kg vs > 100 kg) and previous exposure to tumour necrosis factor (TNF) inhibitor (yes vs no); there was no restriction on the number of patients with prior TNF inhibitor exposure. Interactive response technology was used for randomisation and allocation of double‐blind treatment to each patient." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 651‐2): "UltIMMa‐1 and UltIMMa‐2 were replicate phase 3,randomised, double‐blind, placebo‐controlled and active comparator‐controlled...In each study, patients were randomly assigned (3:1:1) to receive risankizumab, ustekinumab, or matching placebo (appendix). Randomisation was stratified by weight (≤ 100 kg vs > 100 kg) and previous exposure to tumour necrosis factor (TNF) inhibitor (yes vs no); there was no restriction on the number of patients with prior TNF inhibitor exposure. Interactive response technology was used for randomisation and allocation of double‐blind treatment to each patient." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 651‐2): "UltIMMa‐1 and UltIMMa‐2 were replicate phase 3, randomised, double‐blind, placebo‐controlled and active comparator‐controlled...Patients, investigators, and study personnel involved in the trial conduct or analyses remained masked to treatment assignments until study completion. To maintain blinding, the studies utilised a double‐dummy strategy where in risankizumab and its matching placebo or ustekinumab and its matching placebo were identical in appearance." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 651‐2): "UltIMMa‐1 and UltIMMa‐2 were replicate phase 3, randomised, double‐blind, placebo‐controlled and active comparator‐controlled...Patients, investigators, and study personnel involved in the trial conduct or analyses remained masked to treatment assignments until study completion. To maintain blinding, the studies utilised a double‐dummy strategy where in risankizumab and its matching placebo or ustekinumab and its matching placebo were identical in appearance." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 506 Management of missing data: Quote (p 652‐3): "For both UltIMMa‐1 and UltIMMa‐2 studies, efficacy analyses were done in the intention‐to‐treat population (all randomised patients)... Missing efficacy data for categorical variables were handled with non‐responder imputation and for continuous variables with last observation carried forward". Table 2: 506 analysed participants Comment: done |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02684370). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
UltIMMa‐2 2018.
| Study characteristics | ||
| Methods | RCT, placebo/active‐controlled, double‐blind study Date of study: March 2016 to August 2016 Location: worldwide Phase 3 |
|
| Participants |
Randomised: 491 participants Inclusion criteria
Exclusion criteria
Dropouts and withdrawals 9/491 (1.8%); risankizumab group (2), ustekinumab group (3), placebo group (4)
|
|
| Interventions |
Intervention A. Risankizumab, SC, 150 mg, n = 294 Control interventions B. Ustekinumab, SC, based on weight per label (45 mg for patients with body weight ≤ 100 kg or 90 mg for patients with body weight > 100 kg), n = 99 C. Placebo, n = 98 |
|
| Outcomes | At week 16 Primary composite outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 650): "AbbVie and Boehringer Ingelheim" Declarations of interest: Quote (p 660): "KBG has received honoraria for serving as a consultant and/or grants as an investigator from AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Dermira, Eli Lilly, GlaxoSmithKline, Janssen, Leo Pharma, Novartis, Pfizer, Regeneron, Sanofi‐Aventis, Sun, and UCB. BS has received honoraria as a consultant for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Dermavant, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Janssen, Leo Pharma, Medac, Meiji Seika Pharma, Menlo Therapeutics, Merck, Novartis, Ortho Dermatologics/Valeant, Pfizer, Regeneron, Sanofi Genzyme, Sebela, Sienna, Sirtris, Sun Pharma, and UCB pharma, and as scientific director for the CORRONA‐NPF Psoriasis Registry. He is an investigator for AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, GlaxoSmithKline, Janssen, Merck, Pfizer, and Sienna. ML has received grants as an investigator from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen Research & Development, Kadmon, Leo Pharma, Novartis, Pfizer, and ViDac and has received honoraria for serving as a consultant for Allergan, Aqua, Boehringer Ingelheim, Leo Pharma, Menlo, and Promius. MA has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant; and grants as an investigator from AbbVie, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Hexal, Janssen, Leo Pharma, Eli Lilly, Medac, Mundipharma, MSD, Novartis, Pfizer, Sandoz, UCB, and Xenoport. AB has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant; and grants as an investigator from AbbVie, Aclaris, Akros, Allergan, Almirall, Amgen, Boehringer Ingelheim, Celgene, Dermavant, Dermira, Eli Lilly, Genentech/Roche, GlaxoSmithKline, Janssen, Leo Pharma, Meiji, Merck Sharp & Dohme, Novartis, Pfizer, Purdue Pharma, Regeneron, Sandoz, Sanofi Genzyme, Sienna pharmaceuticals, UCB, Valeant, and Vidac. YP has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant, and grants as an investigator from AbbVie, Amgen, Baxalta, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Janssen/Centocor, Leo Pharma, MedImmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi‐Genzyme, Sun Pharma, Takeda, Valeant, and UCB. KAP has received honoraria or fees for serving on advisory boards, as a speaker, as a consultant, or as a steering committee member or grants as an investigator from AbbVie, Akros, Allergan, Amgen, Anacor, Arcutis, Astellas, AstraZeneca, Baxalta, Baxter, Boehringer Ingelheim, Bristol‐Myers Squibb, CanFite, Celgene, Coherus, Dermira, Eli Lilly, Forward Pharma, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa‐Hakko Kirin, Leo Pharma, MedImmune, Meiji Seika Pharma, Merck (MSD), Merck‐Serono, Mitsubishi Pharma, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Takeda, UCB, and Valeant. HS has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant, and grants as an investigator from AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novartis, and Pfizer. LP has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant, and grants as an investigator from AbbVie, Amgen, Baxalta, Biogen, Boehringer Ingelheim, Eli Lilly, Janssen, Leo Pharma, Merck‐Serono, MSD, Novartis, Pfizer, Regeneron, Roche; Sandoz, and Sanofi Genzyme. PF has received honoraria and/or research grants from and/or served as an investigator and/or advisory board member for AbbVie, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Celtaxsys, CSL, Cutanea, Dermira, Galderma, Genentech, GSK, iNova, Janssen, Leo Pharma, Lilly, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals, Roche, Sanofi, Sun Pharma, UCB Pharma, and Valeant. MO has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant, and grants as an investigator from AbbVie, Actelion, Astellas, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Eisai, Eli Lilly, and Company, Galderma, Janssen, Kaken, Kyowa‐Kirin, Leo Pharma, Maruho, Mochida, Nichi‐Iko, Nippon Kayaku, Nippon Zoki, Novartis, Ono, Ohtsuka, Pola Pharma, Pfizer, Sanofi, Shionogi, Taiho, Tanabe‐Mitsubishi, Teijin, and Torii. MF is a full‐time employee of Boehringer Ingelheim. ZG, YG, and JMV are full‐time employees of AbbVie and own stock or options. EHZT, a former employee of AbbVie, currently owns stock. HB has received honoraria or fees for serving on advisory boards, as a speaker, and as a consultant, and grants as an investigator from AbbVie, Almirall, Amgen, Bayer, Baxalta, Biocad, Boehringer Ingelheim, Celgene, Dermavant, Eli Lilly, Janssen, Leo Pharma, Menarini, MSD, Novartis, Pfizer, Pierre Fabre, Sandoz, Sun Pharmaceuticals, and UCB." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (pp 651‐2): "UltIMMa‐1 and UltIMMa‐2 were replicate phase 3, randomised, double‐blind, placebo‐controlled and active comparator‐controlled...In each study, patients were randomly assigned (3:1:1) to receive risankizumab, ustekinumab, or matching placebo (appendix). Randomisation was stratified by weight (≤ 100 kg vs > 100 kg) and previous exposure to tumour necrosis factor (TNF) inhibitor (yes vs no); there was no restriction on the number of patients with prior TNF inhibitor exposure. Interactive response technology was used for randomisation and allocation of double‐blind treatment to each patient." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (pp. 651‐2): "UltIMMa‐1 and UltIMMa‐2 were replicate phase 3, randomised, double‐blind, placebo‐controlled and active comparator‐controlled...In each study, patients were randomly assigned (3:1:1) to receive risankizumab, ustekinumab, or matching placebo (appendix). Randomisation was stratified by weight (≤ 100 kg vs > 100 kg) and previous exposure to tumour necrosis factor (TNF) inhibitor (yes vs no); there was no restriction on the number of patients with prior TNF inhibitor exposure. Interactive response technology was used for randomisation and allocation of double‐blind treatment to each patient." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (pp. 651‐2): "UltIMMa‐1 and UltIMMa‐2 were replicate phase 3, randomised, double‐blind, placebo‐controlled and active comparator‐controlled...Patients, investigators, and study personnel involved in the trial conduct or analyses remained masked to treatment assignments until study completion. To maintain blinding, the studies utilised a double‐dummy strategy where in risankizumab and its matching placebo or ustekinumab and its matching placebo were identical in appearance." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (pp. 651‐2): "UltIMMa‐1 and UltIMMa‐2 were replicate phase 3, randomised, double‐blind, placebo‐controlled and active comparator‐controlled...Patients, investigators, and study personnel involved in the trial conduct or analyses remained masked to treatment assignments until study completion. To maintain blinding, the studies utilised a double‐dummy strategy where in risankizumab and its matching placebo or ustekinumab and its matching placebo were identical in appearance." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 491 Management of missing data: Quote (pp. 652‐3): "For both UltIMMa‐1 and UltIMMa‐2 studies, efficacy analyses were done in the intention‐to‐treat population (all randomised patients)... Missing efficacy data for categorical variables were handled with nonresponder imputation and for continuous variables with last observation carried forward". Table 2: 491 analysed participants Comment: done |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT0268435). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Umezawa 2021.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: February 2017 to January 2019 Location: Japan (33 centres) Phase 2/3 |
|
| Participants |
Randomised: 127 participants Inclusion criteria
For patients with moderate‐to‐severe chronic plaque psoriasis (PSO)
Exclusion criteria
Baseline characteristics N = 127, mean age of 50 years and 62% men Dropouts and withdrawals 7/127 (5.5%): certolizumab pegol 200 group (2), certolizumab pegol 400 group (2), placebo group (3)
|
|
| Interventions |
Intervention A. Certolizumab pegol SC injection 400 mg at weeks 0, 2, 4, followed by certolizumab pegol SC injection 200 mg every 2 weeks (Q2W) with PBO administered to maintain the blind, starting at week 6, n = 48 Control interventions B. Certolizumab pegol SC injection 400 mg every 2 weeks (Q2W), n = 53 C. Placebo SC injection every 2 weeks (Q2W), n = 26 |
|
| Outcomes | At week 16 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 525): "This study was sponsored by UCB Pharma." Declarations of interest: Quote (p 525‐6): "Yoshinori Umezawa hasreceived consulting agreements and/or speaker fees from Maruho Co. Ltd., AbbVie GK, Janssen Pharmaceutical K.K., Kyowa Hakko Kirin Co. Ltd., and UCB Japan Co., Ltd. Shinya Sakurai and Naoki Hoshii are employees of UCB Japan Co., Ltd. Hidemi Nakagawa has received consulting agreements, honoraria and/or speaker fees from Japan Tobacco Inc., LEO Pharma, Maruho Co. Ltd., Kyowa Hakko Kirin Co. Ltd., AbbVie GK, Mitsubishi‐Tanabe Pharma, Torii Pharmaceuticals Co. Ltd., Janssen Pharmaceuticals K.K., Novartis Pharma K.K., Eli Lilly Japan K.K., Bristol‐Myers Squibb, and UCB Japan Co., Ltd." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 515): "This was a phase 2/3, randomized, double‐ blind, placebo‐controlled trial ...". "Following a 2–5 week screening period to confirm eligibility, an interactive response technology (IRT) was used to randomize patients 2:2:1 to CZP 400 mg every 2 weeks (Q2W), CZP 200 mg Q2W (with a loading dose of CZP 400 mg Q2W at weeks 0, 2, and 4), and placebo, according to the randomization schedule produced by the IRT vendor (stratified by prior biologic exposure [yes/no] and concurrent PsA [yes/no])." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 515): "This was a phase 2/3, randomized, double‐ blind, placebo‐controlled trial ...". "Following a 2–5 week screening period to confirm eligibility, an interactive response technology (IRT) was used to randomize patients 2:2:1 to CZP 400 mg every 2 weeks (Q2W), CZP 200 mg Q2W (with a loading dose of CZP 400 mg Q2W at weeks 0, 2, and 4), and placebo, according to the randomization schedule produced by the IRT vendor (stratified by prior biologic exposure [yes/no] and concurrent PsA [yes/no])." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 515): "This was a phase 2/3, randomized, double‐blind, placebo‐controlled trial ...". "All CZP and placebo treatments were administered subcutaneously at the study site by unblinded, trained site personnel not involved in any other study procedures." Comment: uncertainty about the ability of this process to guarantee blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 515): "This was a phase 2/3, randomized, double‐blind, placebo‐controlled trial ...". "All CZP and placebo treatments were administered subcutaneously at the study site by unblinded, trained site personnel not involved in any other study procedures." Comment: uncertainty about the ability of this process to guarantee blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 517): "For PASI and PGA outcomes, missing data were imputed using Markov chain Monte Carlo (MCMC) multiple imputation methodology.". "For DLQI and INRS change from baseline values, missing data were imputed using the last observation carried forward (LOCF) approach". Randomised 127; analysed 127 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03051217). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
UNCOVER‐1 2016.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: November 2011 to June 2014 Location: multicentre (104) in Europe, Australia, North America Phase 3 |
|
| Participants |
Randomised: 1296 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 1296 participants, mean age 45 years, 883 male Dropouts and withdrawals 66/1296 (5%); ixekizumab 4‐week group (24), ixekizumab 2‐week group (18), placebo (24)
|
|
| Interventions |
Intervention A. Ixekizumab (n = 432), SC, 80 mg, 2 injections week 0, 1 injection monthly Control intervention B. Ixekizumab (n = 433), SC, 80 mg, 2 injections week 0, 1 injection eow C. Placebo (n = 431), SC |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 346): “The trials were sponsored by Eli Lilly and were designed by the scientific steering committee and Eli Lilly personnel. The site investigators collected the data, Eli Lilly personnel performed the data analyses, and all the authors had access to the data.” Declarations of interest: Quote (p 355): "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org." Gordon received grants and personal fees from Abbvie, Amgen, Celgene, Eli Lilly, Novartis; and personal fees from Pfizer and Medac". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (supplemental appendix): “Patients were assigned to treatment groups as determined by a computer‐generated random sequence …" Comment: clearly defined |
| Allocation concealment (selection bias) | Low risk | Quote (supplemental appendix): “Patients were assigned to treatment groups as determined by a computer‐generated random sequence using an interactive voice response system (IVRS). Site personnel confirmed that they had located the correct assigned investigational product package by entering a confirmation number found on the package into the IVRS”. Comment: clearly defined |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 346): “double‐blind, placebo‐controlled” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 346): “double‐blind, placebo‐controlled” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 1296, analysed 1296 Management of missing data: Quote (p 348): “Unless otherwise specified, all analyses of efficacy during the induction period were performed according to the intention‐to‐treat principle. Missing values for the PASI and the sPGA score were imputed conservatively as nonresponses, regardless of the reason for the missing data”. Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01474512). The prespecified outcomes mentioned in the protocol and in the Methods section appeared to have been reported. |
UNCOVER‐2 2015.
| Study characteristics | ||
| Methods | RCT, active, placebo‐controlled, double‐blind study Date of study: May 2012 to May 2015 Location: 118 centres in Europe, Australia, North America Phase 3 |
|
| Participants |
Randomised: 1224 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 224 participants, mean age of 45 years, 821 male Dropouts and withdrawals 63/1224 (5%): ixekizumab 4‐week group (19), ixekizumab 2‐week group (9), etanercept group (25), placebo (10)
|
|
| Interventions |
Intervention A. Ixekizumab (n = 347), SC, 80 mg, 2 injections week 0, 1 injection monthly Control intervention B. Ixekizumab (n = 351), SC, 80 mg, 2 injections week 0, 1 injection eow C. Etanercept (n = 358), SC, 50 mg 1 injection twice‐weekly D. Placebo (n = 168), SC |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p. 543): “The funder Eli Lilly. Data were collected by investigators, gathered by Parexel International, and analysed by the funder”. agents and collected and analysed the data. All the authors had full access to the data”. Declarations of interest: Quote (pp. 550‐1): "CEMG has received grants and personal fees from Eli Lilly, Abbvie, Janssen, Novartis, Sandoz, Pfizer, and GlaxoSmithKline; personal fees from Actelion, Amgen, and UCB Pharma; grants from LEO Pharma and Merck Sharp & Dohme; and is president of the International Psoriasis Council. KR has received personal fees from AbbVie, Amgen, Biogen, Celgene, Forward Pharma, Janssen‐Cilag, LEO Pharma, Eli Lilly, Medac, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, and Takeda. ML is an employee of the Mount Sinai Medical Center which receives research funds from AbGenomics, AbbVie, Amgen, Anacor, Aqua, Canfite Biopharma, Celgene, Clinuvel, Coronado Biosciences, Ferndale, Lilly, Janssen Biotech, LEO Pharmaceuticals, Merz, Novartis, Pfizer, Sandoz, and Valeant. PvdK has received grants from Celgene, Centocor, Allmiral, Pfizer, Philips, AbbVie, Eli Lilly, Galderma, Novartis, Janssen Cilag, and Leo Pharma; and has served as a speaker for Amgen, a consultant for Sandoz and Mitisibishu, and a speaker and consultant for Celgene, AbbVie, Eli Lilly, Galderma, Novartis, Janssen Cilag, and Leo Pharma. CP has received grants and personal fees from Amgen, Abbvie, Celgene, Eli Lilly, Novartis, Janssen, Pfizer, and Leo Pharma. KP has received honoraria as consultant and/or scientific officer and/or advisory board and/or steering committee member and/or acted as a paid speaker and/ or participated in clinical trials and/or received clinical research grants sponsored by 3M, Abbott/AbbVie, Akesis, Akros, Allergan, Alza, Amgen, Anacor, Apotex, Astellas, Baxter, Berlex, Biogen, Boehringer Ingelheim, Celgene, Celtic, Centocor, Cipher, Dermira, Dow Pharma, Eli Lilly, Forward Pharma, Fujisawa, Funxional Therapeutics, Galderma, Genentech, Genexion, GlaxoSmithKline, Isotechnika, Janssen, Janssen Biotech, Johnson & Johnson, Kataka, Kirin, Kyowa, Leo Pharma, Lypanosys, Medical Minds, Medimmune, Merck, Mitsubishi, Novartis, NovImmune, Pan Genetics, Pfizer, Roche, Regneron, Merck‐Serono, Stiefel, Takeda, UCB, Vertex, Wyeth/Pfizer, and Xoma. AM has served as an advisory board member and/or consultant and/or investigator and/or speaker and/or received compensation in the form of grants and/or honoraria from AbbVie, Allergan, Amgen, ApoPharma, Boehringer Ingelheim, Celgene, Convoy Therapeutics, Eli Lilly, Genentech, Janssen Biotech, LEO Pharma, Merck, Novartis, Pfizer, Symbio and Maruho, Syntrix, Wyeth, and XenoPort. GSC, JE, LZ, RJS, SB, DKB, OOO, MPH, and BJN were employees of and hold stock in Eli Lilly & Co during the conduct of this study." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 542): “randomly assigned”, “An interactive voice response system" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 542): “An interactive voice response system was used to assign double‐blind investigational product to every patient. Site personnel confirmed that they had located the correct assigned investigational product package by entering a confirmation number found in the package into to IVRS”. Comment: clearly defined |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 542): “Patients, investigators and study personnel were masked to the treatment allocation. A double‐dummy design was used”. Comment: clearly defined |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 542): “Patients, investigators and study personnel were masked to the treatment allocation. A double‐dummy design was used”. Comment: clearly defined |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 1224, analysed 1224 Management of missing data: Quote (p 543): “All missing data were imputed using non‐responder imputation (NRI)”. Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01597245). One prespecified outcome in the protocol missing from the Results section (assessment of efficacy at 60 weeks), but as we assessed outcomes at induction phase (between 8 and 24 weeks), we judged that the risk of selective reporting was low. |
UNCOVER‐3 2015.
| Study characteristics | ||
| Methods | RCT, active, placebo‐controlled, double‐blind study Date of study: July 2012 to January 2016 Location: 101 in Europe, Asia, North and South America Phase 3 |
|
| Participants |
Randomised: 1346 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 1346 participants, mean age of 46 years, 918 male Dropouts and withdrawals 71/1346 (5%): ixekizumab 4‐week group (10), ixekizumab 2‐week group (13), etanercept group (26), placebo (22)
|
|
| Interventions |
Intervention A. Ixekizumab (n = 386), SC, 80 mg, 2 injections week 0, 1 injection monthly Control intervention B. Ixekizumab (n = 385), SC, 80 mg, 2 injections week 0, 1 injection eow C. Etanercept (n = 382), SC, 50 mg 1 injection twice‐weekly D. Placebo (n = 193), SC |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 541): "Funding Eli Lilly and Co." Role of the funding source: Quote (p 543): "This study was designed jointly by consultant experts in psoriasis and representatives of the funder, Eli Lilly. Data were collected by investigators, gathered by Parexel International, and analysed by the funder. Safety data were reviewed at regular intervals by an independent data monitoring committee. All authors had full access to the data. All coauthors participated in manuscript development with medical writing support paid for by the funder. All authors made the decision to submit the manuscript for publication." Declarations of interest: Quote (pp 550‐1): "CEMG has received grants and personal fees from Eli Lilly, Abbvie, Janssen, Novartis, Sandoz, Pfizer, and GlaxoSmithKline; personal fees from Actelion, Amgen, and UCB Pharma; grants from LEO Pharma and Merck Sharp & Dohme; and is president of the International Psoriasis Council. KR has received personal fees from AbbVie, Amgen, Biogen, Celgene, Forward Pharma, Janssen‐Cilag, LEO Pharma, Eli Lilly, Medac, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, and Takeda. ML is an employee of the Mount Sinai Medical Center which receives research funds from AbGenomics, AbbVie, Amgen, Anacor, Aqua, Canfite Biopharma, Celgene, Clinuvel, Coronado Biosciences, Ferndale, Lilly, Janssen Biotech, LEO Pharmaceuticals, Merz, Novartis, Pfizer, Sandoz, and Valeant. PvdK has received grants from Celgene, Centocor, Allmiral, Pfizer, Philips, AbbVie, Eli Lilly, Galderma, Novartis, Janssen Cilag, and Leo Pharma; and has served as a speaker for Amgen, a consultant for Sandoz and Mitisibishu, and a speaker and consultant for Celgene, AbbVie, Eli Lilly, Galderma, Novartis, Janssen Cilag, and Leo Pharma. CP has received grants and personal fees from Amgen, Abbvie, Celgene, Eli Lilly, Novartis, Janssen, Pfizer, and Leo Pharma. KP has received honoraria as consultant and/or scientific officer and/or advisory board and/or steering committee member and/or acted as a paid speaker and/ or participated in clinical trials and/or received clinical research grants sponsored by 3M, Abbott/AbbVie, Akesis, Akros, Allergan, Alza, Amgen, Anacor, Apotex, Astellas, Baxter, Berlex, Biogen, Boehringer Ingelheim, Celgene, Celtic, Centocor, Cipher, Dermira, Dow Pharma, Eli Lilly, Forward Pharma, Fujisawa, Funxional Therapeutics, Galderma, Genentech, Genexion, GlaxoSmithKline, Isotechnika, Janssen, Janssen Biotech, Johnson & Johnson, Kataka, Kirin, Kyowa, Leo Pharma, Lypanosys, Medical Minds, Medimmune, Merck, Mitsubishi, Novartis, NovImmune, Pan Genetics, Pfizer, Roche, Regneron, Merck‐Serono, Stiefel, Takeda, UCB, Vertex, Wyeth/Pfizer, and Xoma. AM has served as an advisory board member and/or consultant and/or investigator and/or speaker and/or received compensation in the form of grants and/or honoraria from AbbVie, Allergan, Amgen, ApoPharma, Boehringer Ingelheim, Celgene, Convoy Therapeutics, Eli Lilly, Genentech, Janssen Biotech, LEO Pharma, Merck, Novartis, Pfizer, Symbio and Maruho, Syntrix, Wyeth, and XenoPort. GSC, JE, LZ, RJS, SB, DKB, OOO, MPH, and BJN were employees of and hold stock in Eli Lilly & Co during the conduct of this study." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 542): “randomly assigned” "An interactive voice response system" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 542): “An interactive voice response system was used to assign double‐blind investigational product to every patient. Site personnel confirmed that they had located the correct assigned investigational product package by entering a confirmation number found in the package into to IVRS”. Comment: clearly defined |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 542): “Patients, investigators and study personnel were masked to the treatment allocation. A double‐dummy design was used”. Comment: clearly defined |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 542): “Patients, investigators and study personnel were masked to the treatment allocation. A double‐dummy design was used”. Comment: clearly defined |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 1346, analysed 1346 Management of missing data: Quote (p 543): “All missing data were imputed using non‐responder imputation (NRI)”. Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01646177). One prespecified outcome in the protocol missing from the Results section (assessment of efficacy at 60 weeks), but as we assessed outcomes at induction phase (between 8 and 24 weeks), we judged that the risk of selective reporting was low. |
Van Bezooijen 2016.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: 2013 to June 2015 Location: single centre in the Netherlands |
|
| Participants |
Randomised: 33 participants Inclusion criteria
Exclusion criteria
Baseline characteristics Not stated Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Fumaric acid (n = 18), from 215 mg once daily up to a maximum of 215 mg 4 times a day, 24 weeks Control intervention B. Placebo Co‐intervention Etanercept (n = 15) (50 mg SC twice‐weekly for 12 weeks followed by 50 mg once weekly for an additional 12 weeks) |
|
| Outcomes | Assessments at 24 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (supplemental appendix): "This investigator‐initiated study was supported by a grant of Pfizer Pharmaceuticals. Pfizer was not involved in any study procedure, but Pfizer was granted the right to read, but not to edit, the manuscript prior to submission for publication." Declarations of interest: Quote (p 413): "Investigator‐initiated project grant from Pfizer. E. Prens has acted as a consultant for AbbVie, Amgen, Astra‐Zeneca, Baxter, Eli Lilly, Galderma, Janssen‐Cilag, Novartis and Pfizer and has received investigator‐initiated research grants (paid to Erasmus MC) from Pfizer, Janssen‐Cilag and AbbVie. M.B.A. van Doorn has acted as a consultant for Abbott, Janssen, LEO Pharma, MSD and Pfizer, and has been an investigator for Eli Lilly, Idera Pharmaceu‐ticals, Cutanea and Novartis. T. van Gelder has been on the speakers’ bureau or worked as consultant for Sandoz, Novartis, Teva, Chiesi, Astellas and Roche". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (supplemental appendix): “Using a computer‐generated randomisation list, patients were randomised at baseline to a 1:1 ratio to receive either etanercept combined with oral fumarates (combination group) or etanercept only (monotherapy group).” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (supplemental appendix): “Using a computer‐generated randomisation list, patients were randomised at baseline to a 1:1 ratio to receive either etanercept combined with oral fumarates (combination group) or etanercept only (monotherapy group).” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (supplemental appendix): "Patients and the study physicians were not blinded for the allocated treatment group.” Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (supplemental appendix): “The independent PASI assessor (E.P.P.) was blinded to treatment throughout the course of the study.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 33, analysed 33 for the primary outcome Management of missing data: Quote (supplemental appendix): “Patients lost to follow‐up were not included in the PASI 75 response and PGA score analyses.” Comment: not ITT analyses, but all randomised participants reached the primary outcome assessment |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol for the study was available on the European Clinical Trials Database (EudraCT) (EudraCT No. 2011‐005685‐38) (not found). The prespecified results mentioned in the Methods section appeared to have been reported. |
Van de Kerkhof 2008.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: June 2006 to May 2007 Location: multicentre (numbers of centres not stated) in Belgium, France, Germany, Hungary, Italy, Netherlands, Poland, Romania, Spain |
|
| Participants |
Randomised: 142 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 142 participants, mean age of 45 years, 84 male Dropouts and withdrawals 16/143 (11%): etanercept group (6), placebo group (10)
|
|
| Interventions |
Intervention A. Etanercept, 50 mg, self‐administered SC, once a week, 12 weeks (n = 96) Control intervention B. Placebo, self‐administered SC, once a week, 12 weeks (n = 46) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1184): "This study was supported financially by Wyeth Pharmaceuticals, Collegeville, PA, USA)". Comments: 3 authors were employed by Wyeth Pharmaceuticals which supported this study financially Declarations of interest: Quote: (p 1177): "C.Z., M.P.B., L.P. and J.W. are employed by Wyeth Pharmaceuticals, which supported this study financially." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1178): "Patients were randomly assigned (using the Clinical Operations Randomization Environment system) ... according to a 2:1 treatment allocation". Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 1178): "Patients were randomly assigned (using the Clinical Operations Randomization Environment system) ... according to a 2:1 treatment allocation". Comment: not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1178): "In both the double blind controlled study..., etanercept was supplied as a sterile lyophilised powder. All study drugs were self‐administrated QW by the patient by subcutaneous injections". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1178): "In both the double blind controlled study..., etanercept was supplied as a sterile lyophilised powder. All study drugs were self‐administrated QW by the patient by subcutaneous injections". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 142, analysed 142 Management of missing data, Quote (p 1179): "The primary population for efficacy and safety analyses ... was the modified intent‐to‐treat population. The last observations were carried forward in cases of missing efficacy data". Comment: done |
| Selective reporting (reporting bias) | Unclear risk | Comment: the specified outcomes mentioned in the Methods section appeared to have been reported, but no protocol was available. |
VIP Trial 2018.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: February 2012 to October 2016 Location: 8 centres in the USA Phase 4 |
|
| Participants |
Randomised: 96 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 96, mean of age 43 years, and 70% men Dropouts and withdrawals 5/96 (12.1%): ADA group (1), UV group (3), placebo group (1)
|
|
| Interventions |
Intervention A. Adalimumab (Humira). Humira will be given at an initial dose of 80 mg followed by 40 mg the 2nd week, subsequent doses will be given at 40 mg and follow FDA dosing schedule, n = 33 Control intervention B. NBUVB phototherapy. Phototherapy will be given 3 times a week according to the Fitzpatrick scale for skin types, n = 33 C. Placebo injection will be given according to the same dose and schedule as the active comparator, n = 1 |
|
| Outcomes | At weeks 12 Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 10): "This study was supported by grants (National Heart, Lung, and Blood Institute R01‐HL111293, K24‐AR‐064310) and by an unrestricted grant from AbbVie (to the Trustees of the University of Pennsylvania). Dr Mehta is supported by National Institutes of Health Intramural Research Program (Z01 HL‐06193). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication." Declarations of interest: Quote (p 10): "Dr Mehta is a full‐time US Government Employee and receives research grants to the National Heart, Lung, and Blood Institute (NHLBI) from AbbVie, Janssen, Celgene, and Novartis. Dr Gelfand in the past 12 months has served as a consultant for Coherus (DSMB), Dermira, Janssen Biologics, Merck (DSMB), Novartis Corp, Regeneron, Dr. Reddy’s Laboratories, Sanofi and Pfizer Inc, receiving honoraria; and receives research grants (to the Trustees of the University of Pennsylvania) from Abbvie, Janssen, Novartis Corp, Regeneron, Sanofi, Celgene, and Pfizer Inc; and received payment for continuing medical education work related to psoriasis that was supported indirectly by Lilly and Abbvie. Dr Gelfand is a copatent holder of resiquimod for treatment of cutaneous T cell lymphoma. Dr Takeshita receives a research grant from Pfizer Inc (to the Trustees of the University of Pennsylvania) and has received payment for continuing medical education work related to psoriasis that was supported indirectly by Eli Lilly. A.B. Troxel is a co‐patent holder of resiquimod for treatment of cutaneous T cell lymphoma. Dr Tyring conducts clinical studies sponsored by the following companies: Abbvie/ BI; Celgene; Coherus; Dermira; Eli Lilly; Janssen; Leo; Merck; Novartis; Pfizer; Regeneron/Sanofi; and Valeant. He is a speaker for Abbvie, Eli Lilly, Janssen, Leo, Novartis, Pfizer, Regeneron/Sanofi, and Valeant. Dr Armstrong has received research grants and honorarium from AbbVie, Celgene, Janssen, Novartis, Eli Lilly, Regeneron, Sanofi, and Valeant and has participated in continuing medical education work related to psoriasis that was indirectly supported by Eli Lilly and AbbVie. Dr Duffin has received grant/research/clinical trial support from Amgen, Abbvie, Celgene, Eli Lilly, Janssen, Bristol‐Myers Squibb, Stiefel, Novartis, and Pfizer over the last 24 months. Additionally, Dr Duffin has served as a consultant/ on the advisory boards for Amgen, Abbvie, Celgene, Eli Lilly, Janssen, Bristol‐Myers Squibb, Stiefel, Novartis, and Pfizer. Dr Chiesa Fuxench has no conflicts of interest. However, she was being funded, at the time, by a research grant from the National Psoriasis Foundation and a training grant from the National Institutes of Health. Dr Hubbard receives grant funding from the National Institutes of Health and Patient‐Centered Outcomes Research Institute. Dr Rader is the co‐founder of Vascular Strategies and holds equity in the company. Dr Kalb has received grants/research funding from AbbVie, Amgen, Boehringer Ingelheim, Janssen‐ Ortho Inc, Merck & Co, Inc, and Novartis Pharmaceuticals Corp over the last 24 months. During this time frame, he has also served as a consultant honoraria for Dermira, Janssen‐Ortho Inc, Sun Pharmaceutical Industries Ltd, and a DSMB member honoraria for Eli Lilly and Co. Dr Simpson has served as a consultant for AbbVie, Anacor, Celgene, Dermira, Genentech, Leo, Glaxo Smith Kline, Pfizer, Regeneron, Sanofi‐Genzyme, Menlo, and Eli Lilly in the last 24 months. During this time frame, he has also acted as the primary investigator for the following sponsored trials: Anacor, Celgene, Chugai, Dermira, Eli Lilly, Genentech, MedImmune, Merck, Novartis, Regeneron, Roivant, Tioga, and Vanda. Dr Torigian is the co‐founder of Quantitative Radiology Solutions LLC. Dr Van Voorhees has served on the advisory board of Celgene, Dermira, Allergan, Merck, Pfizer, Aqua, Astra Zeneca, Jannsen, Amgen, Leo, Allergan, and Lilly. For Novartis and AbbVie, Dr Van Voorhees acts as a consultant as well as serves on the board. Dr Van Voorhees has received a portion of ex‐spouse pension from Merck. Dr Menter in the last 24 months has served on the advisory board for AbbVie, Allergan, Amgen, Boehringer Ingelheim, Eli Lilly, Janssen Biotech, Inc, and LEO Pharma. He has also worked as a consultant for AbbVie, Allergan, Amgen, Eli Lilly, Galderma, Janssen Biotech, Inc, LEO Pharma, Novartis, Pfizer, Vitae, and Xenoport. Additionally, he has acted as an investigator for AbbVie, Allergan, Amgen, Anacor, Boehringer Ingelheim, Celgene, Dermira, Eli Lilly, Janssen Biotech, Inc, LEO Pharma, Merck, Neothetics, Novartis, Pfizer, Regeneron, Symbio/Maruho, and Xenoport. He also serves as a speaker for AbbVie, Amgen, Janssen Biotech, Inc, and LEO Pharma. He has received compensation in the form of grants from AbbVie, Allergan, Amgen, Anacor, Boehringer Ingelheim, Celgene, Dermira, Janssen Biotech, Inc, LEO Pharma, Merck, Neothetics, Novartis, Pfizer, Regeneron, Symbio/Maruho, and Xenoport. He has also received honoraria from AbbVie, Allergan, Amgen, Boehringer Ingelheim, Eli Lilly, Galderma, Janssen Biotech, Inc, LEO Pharma, Novartis, Pfizer, Vitae, and Xenoport. The other authors report no conflicts." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 2): "The study was a multicenter randomized controlled trial designed to enroll 96 patients across 8 centers in the United States with 1:1:1 allocation to..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 3): "Adalimumab (or corresponding placebo) therapy was administered in a double‐blind manner as a subcutaneous injection with an initial 80 mg dose at week 0, followed by maintenance doses of 40 mg every other week, starting from week 1 and then continued throughout the study". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 3): "Adalimumab (or corresponding placebo) therapy was administered in a double‐blind manner as a subcutaneous injection with an initial 80 mg dose at week 0, followed by maintenance doses of 40 mg every other week, starting from week 1 and then continued throughout the study". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomised: 96; analysed 92 Dealing with missing data: not stated but few withdrawals (1/3/0) |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01553058). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
VIP‐S trial 2020.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: February 2016 to February 2018 Location: USA (12 centres) Phase 4 |
|
| Participants |
Randomised: 91 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 91, mean age of 47.5 years and 67% men Dropouts and withdrawals 5/91 (5.5%): secukinumab group (2), placebo group (3)
|
|
| Interventions |
Intervention A. Secukinumab 300 (300 mg once weekly at baseline, weeks 1, 2, 3, and 4 followed by monthly dosing starting at week 8 through week 48 inclusive), n = 46 Control intervention B. Placebo, n = 45 |
|
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote: "This study is funded by Novartis Pharmaceuticals Corporation, East Hanover, NJ." Declarations of interest: Quote: "Dr Gelfand served as a consultant for BMS, Boehringer Ingelheim, Janssen Biologics, Novartis Corp, UCB (DSMB), Sanofi, and Pfizer, receiving honoraria; and receives research grants (to the Trustees of the University of Pennsylvania) from AbbVie, Boehringer Ingelheim, Janssen, Novartis, Celgene, Ortho Dermatologics, and Pfizer; and received payment for continuing medical education work related to psoriasis that was supported indirectly by Lilly, Ortho Dermatologics, and Novartis. Dr Gelfand is a Deputy Editor for the Journal of Investigative Dermatology receiving honoraria from the Society for Investigative Dermatology.Dr Duffin has received research grants from AbbVie, Amgen, Bristol‐Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Sienna Biopharmaceuticals, Stiefel Laboratories, and UCB; and has received consulting fees from AbbVie, Amgen, Bristol‐Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Ortho Dermatologic, Pfizer, Sienna Biopharmaceuticals, Stiefel Laboratories, and UCB; and is on the speaker's bureau for Novartis.Dr Armstrong has served as investigator, advisor, and/or consultant to Leo, AbbVie, UCB, Janssen, Novartis, Eli Lilly, Sun, Dermavant, BMS, Regeneron Pharmaceuticals, Inc., Sanofi U.S., Dermira, Modmed, and Ortho Dermatologics, Inc.Dr Blauvelt has served as a scientific adviser and/or clinical study investigator for AbbVie, Aclaris, Akros, Allergan, Almirall, Amgen, Arena, Athenex, Boehringer Ingelheim, Bristol‐ Myers Squibb, Celgene, Dermavant, Dermira, Eli Lilly, FLX Bio, Forte, Galderma, Genentech/Roche, GlaxoSmithKline, Janssen, Leo, Meiji, Merck Sharp & Dohme, Novartis, Ortho, Pfizer, Purdue Pharma, Regeneron, Revance, Sandoz, Sanofi Genzyme, SiennaPharmaceuticals, Sun Pharma, UCB Pharma, and Vidac and as a paid speaker for AbbVie, Regeneron, and Sanofi Genzyme.Dr Trying has conducted studies sponsored by the producer of secukinumab.Dr Menter has received compensation from or served as an investigator, consultant, advisory board member, or speaker for Abbott Labs, AbbVie, Allergan, Amgen, Anacor, Boehringer Ingelheim, Celgene, Dermira, Eli Lilly, Galderma, Janssen, Leo, Merck & Co, Neothetics, Novartis, Pfizer, Regeneron, Sienna, Symbio/Maruho, UCB, Vitae, and Xenoport. Dr Gottlieb is currently serving as consultant, advisory board member, speaker for Janssen, Celgene, Bristol Myers Squibb, Beiersdorf, Abbvie, UCB, Novartis, Incyte, Lilly, Reddy Labs, Valeant, Dermira, Allergan, Sun Pharmaceutical Industries, Xbiotech, Leo, Avotres Therapeutics. Research/Educational Grants: Janssen, Incyte, UCB, Novartis, Lilly Xbiotech, Boeringer Ingelheim.Dr Lockshin reports personal fees from Lilly, Novartis, Janssen, and Abbott; has served as a speaker for Novartis, Eli Lilly, and Abbvie; conducted research for Celgene, Abbvie, Novartis, Eli Lilly, and Strata, and served as a consultant for Novartis, Lilly, AstraZeneca, Abbive.Dr. Simpson reports grants from Eli Lilly, Kyowa Hakko Kirin, Leo Pharmaceutical, Merck, Pfizer, and Regeneron, and personal fees from Menlo Therapeutics, Valeant, Novartis, Eli Lilly, Galderma, Dermira, Sanofi Genzyme, Pfizer, Regeneron, and Leo Pharmaceuticals. Dr Shin, Dr Ahlman, Dr Playford, Dr Joshi, Dr Dey, Dr Werner and Dr Alavi have nothing to disclose." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "This was a randomized, double‐blinded, placebo‐controlled, parallel‐group, multicenter study in adult patients (≥ 18 years of age) with moderate‐to‐severe chronic plaque psoriasis....Eligible patients were randomized via Interactive Response Technology in a 1:1 ratio to either secukinumab 300 mg or placebo." |
| Allocation concealment (selection bias) | Low risk | Quote: "The Investigator or his/her delegate will contact the IRT after confirming that the subject fulfills all the inclusion/exclusion criteria. The IRT will assign a randomization number to the subject, which will be used to link the subject to a treatment group and will specify a unique medication number for the first box of study treatment to be dispensed to the subject. The randomization number will not be communicated to the caller. The identity of secukinumab and placebo prefilled syringes (PFS) will be concealed by identical packaging, labeling, schedule of administration, and appearance." Comment: adequate procedure to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients, investigators/site staff, persons performing assessments, and Novartis study personnel remained blinded to individual treatment assignment from time of randomization until the final database lock at week 52." Comment: adequate procedure to guarantee blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients, investigators/site staff, persons performing assessments, and Novartis study personnel remained blinded to individual treatment assignment from time of randomization until the final database lock at week 52." Comment: adequate procedure to guarantee blinding of assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote: "The primary analysis was based on the full analysis set. For the primary efficacy variable, data for patients with missing post‐baseline value were not imputed, and patients were included in the analysis if they had both baseline and post‐baseline assessments. The primary analysis was based on the full analysis set. Changes from baseline in each cardiometabolic biomarker were analyzed at each time point using the same ANCOVA model as for the primary efficacy variable; missing data were imputed using the last‐observation‐carried‐forward method." Randomised 91, analysed 91 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (VIP‐S trial 2020). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
VIP‐U Trial 2020.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: July 2014 to September 2018 Location: University of Pennsylvania, USA (40 sites, multicentre) Phase 4 |
|
| Participants |
Randomised: 43 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 43, mean age of 42.5 years, and 70% men Dropouts and withdrawals 8/43 (18.6%): ustekinumab group (2), placebo group (6) Before cross‐over
After cross‐over
|
|
| Interventions |
Intervention A. Ustekinumab (Stelara) subcutaneous injection 45 mg (if person's weight is 100 kg or less) or 90 mg (if person's weight is > 100 kg) at day 0 and week 4 followed by every 12‐week dosing thereafter; participant will receive total of 52 weeks of ustekinumab (12 weeks during RCT phase, 40 weeks post‐RCT phase); n = 22 Control intervention B. Placebo: placebo subcutaneous injection will be given according to the same dose and schedule as the active comparator until week 12 (end of RCT phase). At week 12, ustekinumab will be administered according to the same injection schedule as the active comparator arm for 52 weeks. Patient will receive total of 52 weeks of ustekinumab (0 weeks during RCT phase, 52 weeks post RCT phase); n = 21 |
|
| Outcomes | At week 52 Primary outcome
Secondary outcomes
|
|
| Notes | In ClinicalTrials.gov, the secondary outcomes are different from paper
Funding source: Quote (p. 92): "This study was funded by a grant to the Trustees of the University of Pennsylvania from Janssen Pharmaceuticals (JMG). JMG received additional funding from NIAMS K24AR064310. JT is funded in part by K23 AR068433. NNM received additional funding from NHLBI Intramural Research Program (HL006193‐05). We thank the patients who volunteered for this study and Suzette Baez Vanderbeek for her project management expertise." Declarations of interest: Quote (p. 92): "Outside of the submitted work, JMG served and received honoraria as a consultant for BMS, Boehringer Ingelheim, Janssen Biologics, Novartis Corp, UCB (DSMB), Sanofi, and Pfizer Inc.; and received research grants (to the Trustees of the University of Pennsylvania) from AbbVie, Boehringer Ingelheim, Janssen, Novartis Corp, Celgene, Ortho Dermatologics, and Pfizer Inc.; and received payment for continuing medical education work related to psoriasis that was supported indirectly by Eli Lilly, Ortho Dermatologics, and Novartis. JMG is a co‐patent holder of resiquimod for treatment of cutaneous T‐cell lymphoma, and is a Deputy Editor for the Journal of Investigative Dermatology receiving honoraria from the Society for Investigative Dermatology. DAT is a cofounder of Quantitative Radiology Solutions LLC. MHN receives a research grant via the Trustees of the University of Pennsylvania from Boehringer Ingelheim, and she is supported by a K23‐AR073932 career development award from the National Institute of Arthritis and Musculo‐skeletal and Skin Diseases. MHN has also received payments for work done as in independent contractor from UptoDate and Derm101. JT receives a grant from NIAMS K23‐AR068433 and a research grant (both to the Trustees of the University of Pennsylvania) from Pfizer Inc., and has received payment for continuing medical education work related to psoriasis that was supported indirectly by Eli Lilly and Novartis. NNM is a full time US government employee. NNM has served as a consultant for Amgen, Eli Lilly, and Leo Pharma receiving grants and/or other payments; as a principal investigator and/or investigator for AbbVie, Celgene, Janssen Pharmaceuticals Inc, and Novartis receiving grants and/or research funding; and as a principal investigator for the National Institute of Health receiving grants and/or research funding. All the other authors state no conflict of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p. 89, 91): "The study was a randomized, double‐blind, placebo‐controlled trial designed to enroll 42 patients with allocation ratio of 1:1 to ustekinumab subcutaneous injections or placebo injections at baseline and week 4.... Study group assignment was performed via block randomization (of four and eight), using a computerized system at the Investigational Drug Services, University of Pennsylvania." Comment: adequate procedure |
| Allocation concealment (selection bias) | Unclear risk | Quote (p. 89, 90): "The study was a randomized, double‐blind, placebo‐controlled trial designed to enroll 42 patients with allocation ratio of 1:1 to ustekinumab subcutaneous injections or placebo injections at baseline and week 4... Ustekinumab (or corresponding placebo) therapy was administered in a double‐blind manner as subcutaneous injections." Comment: lack of information on appearance of ustekinumab and placebo, no information on process of treatment dispensation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p. 89, 91): "The study was a randomized, double‐blind, placebo‐controlled trial designed to enroll 42 patients with allocation ratio of 1:1 to ustekinumab subcutaneous injections or placebo injections at baseline and week 4....Study investigators, staff, and patients were blinded to ustekinumab or placebo status during the placebo‐controlled period. All scans were read in a blinded fashion to patient characteristics, treatment allocation, and visit dates (i.e. baseline, week 12, or end of study)." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p. 89, 91): "The study was a randomized, double‐blind, placebo‐controlled trial designed to enroll 42 patients with allocation ratio of 1:1 to uste‐ kinumab subcutaneous injections or placebo injections at baseline and week 4....Study investigators, staff, and patients were blinded to ustekinumab or placebo status during the placebo‐controlled period. All scans were read in a blinded fashion to patient characteristics, treatment allocation, and visit dates (i.e. baseline, week 12, or end of study)." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p. 91): "The missing data were summarized using frequencies for each outcome measure...The primary analyses were restricted to subjects who completed the trial (i.e. had primary outcome measures assessed at baseline and week 12)." "The primary analyses were restricted to subjects who completed the trial (i.e. had primary outcome measures assessed at baseline and week 12). For TBRmax, additional multivariate linear regression models were fitted to assess sensitivity to potential imbalance of covariates (which may occur by chance in smaller randomized controlled trials), such as age, sex, and major cardiovascular disease risk factors (serum glucose, systolic and diastolic blood pressure, tobacco use, family history, serum LDL, HDL, total cholesterol, body mass index, psoriatic arthritis, and PASI). For binary outcomes, the treatment group comparisons were assessed using logistic regression models." Randomly assigned 43 |
| Selective reporting (reporting bias) | High risk | Comment: in clinicaltrials.gov, the secondary outcomes are different from paper; the protocol for the study was available on ClinicalTrials.gov (NCT02187172). Results are posted on ClinicalTrials.gov. |
VOLTAIRE‐PSO 2021.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: August 2016 to January 2018 Location: worldwide (54 sites) Phase 3 |
|
| Participants |
Randomised: 318 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 318, mean age of 43, 203 male Dropouts and withdrawals 43/318 (13.5%): biosimilar group (18), Humira group (25)
|
|
| Interventions |
Intervention A. Biological: BI 695501, SC, biosimilar adalimumab week 0: 80 mg, week 1: 40 mg, then 40 mg eow (n = 159) Control Intervention B. Biological: adalimumab (Humira) week 0: 80 mg, week 1: 40 mg, then 40 mg eow (n = 159) |
|
| Outcomes | At 16 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 95): "Boehringer Ingelheim provided funding and were responsible for the conduct of this study." Declarations of interest: Quote (p 95): "A Menter has held advisory board, consultant, investigator, and speaker roles with, and grants/research funding and honoraria from, Abbott Labs, Amgen, Janssen Biotech, Inc., LEO Pharma and Sienna; advisory board and investigator roles with, and grants/research funding and honoraria from, Boehringer Ingelheim; investigator roles with, and grants/research funding from, Celgene and Merck; consultant and investigator roles with, and honoraria from, Eli Lilly and Novartis; and consultant, investigator and speaker roles with, and honoraria from, UCB Pharma. S Beissert has held advisory board and speaker roles with, and has received honoraria from, AbbVie, Actelion (now part of Johnson&Johnson), Celgene, Galderma, Janssen‐Cilag, Novartis, and MSD; advisory board roles with, and honoraria from, Amgen, LEO Pharma, Eli Lilly, Menlo Therapeutics, Pfizer, and UCB Pharma; speaker roles with, and honoraria from, La Roche Posay, GlaxoSmithKline and BMS; and investigator roles with, and grants/research funding from, Boehringer Ingelheim. A Cauthen has held a speaker role with Otezla (Amgen), and investigator roles with Amgen, Arcutis, AbbVie, Baxter Healthcare, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Kadmon, Pfizer, Prothena, Therapeutics, TOLMAR, and UCB. J Soung has held speaker bureau roles with, and honoraria and travel fees from, Amgen and Celgene; investigator, speaker bureau, and advisory board roles with, and grants/research funding and honoraria from, Eli Lilly; speaker bureau and investigator roles with, and grants/research funding and honoraria from, AbbVie; investigator roles with, and grants/research funding from, Pfizer, Allergan, Galderma, Actavis, Cassiopeia, GlaxoSmithKline, Boehringer Ingelheim, Kadmon, Novan, Dr. Reddy, Kyowa Kirin, and Menlo; investigator and speaker bureau roles with, and grants/research funding from, Ortho Dermatologics; investigator and speaker bureau roles with, and grants/research funding and honoraria from, Actelion; speaker roles with, and honoraria from, the National Psoriasis Foundation; investigator and advisory board roles with, and grants/research funding and honoraria from, LEO Pharma; investigator, speaker bureau and consultant roles with, and grants/research funding and honoraria from, Novartis; speaker bureau roles with, and honoraria from, Regeneron and Dermira; investigator roles with, and grants/research funding and honoraria from, Janssen; and investigator roles with, and honoraria from, UCB Pharma. S Jazayeri has held speaker roles with Novartis and Abbvie; and grants from AbbVie, Amgen, Athenex, AbGenomics, Bausch Health Americas Valeant, Boehringer Ingelheim, Bristol Myers Squibb, Coherus Biosciences, Corrona LLC, DS Biopharma, Eli Lilly and Company, Galderma, Genentech, Health Analytics, Innovaderm, IQVIA Biotech, Janssen Biotech, Kadmon, Leo Pharmaceuticals, Novartis, Novella, Pfizer, Regeneron, Tolmar, UCB Biopharma, Xenoport (Arbor Pharmaceuticals), and Watson. P Weisenseel has held investigator roles with, and honoraria/investigator fees to his institution from, Boehringer Ingelheim; advisory board, investigator and speaker roles with, and honoraria/investigator fees to his institution from, AbbVie; advisory board and speaker roles with, and honoraria from, Hexal, Almirall and Biogen Idec; advisory board, consultant, investigator and speaker roles with, and honoraria from, Janssen; advisory board, investigator and speaker roles with, and honoraria from, Novartis and Celgene; and speaker roles and honoraria from Medac. P Arenberger reports no disclosures. S Balser, N Czeloth, and G Jayadeva are employees of Boehringer Ingelheim. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 88): "This was a randomized, double‐blind, parallel‐arm, multiple‐ dose, active comparator trial. Patients were randomized 1:1 via Interactive Response Technology (IRT; Almac Clinical Technologies, Souderton, PA, USA) to receive either BI 695501 or adalimumab RP (Humira®; AbbVie Inc., North Chicago, IL, USA). Each patient was allocated the lowest sequentially available randomization number, and the randomization code was concealed from personnel throughout the study." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 88): "This was a randomized, double‐blind, parallel‐arm, multiple‐ dose, active comparator trial. Patients were randomized 1:1 via Interactive Response Technology (IRT; Almac Clinical Technologies, Souderton, PA, USA) to receive either BI 695501 or adalimumab RP (Humira®; AbbVie Inc., North Chicago, IL, USA). Each patient was allocated the lowest sequentially available randomization number, and the randomization code was concealed from personnel throughout the study." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 88): "This was a randomized, double‐blind, parallel‐arm, multiple‐ dose, active comparator trial... The packaging of syringes containing either BI 695501 or adalimumab RP was of identical appearance to ensure blinding, while unique medication identification numbers enabled each patient to receive the correct treatment." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 88): "This was a randomized, double‐blind, parallel‐arm, multiple‐ dose, active comparator trial... The packaging of syringes containing either BI 695501 or adalimumab RP was of identical appearance to ensure blinding, while unique medication identification numbers enabled each patient to receive the correct treatment." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dealing with missing data: Quote (p 89‐90): "The full analysis set (FAS) comprised all randomized patients who received at least one dose of study medication and had efficacy measures required for determining PASI 75 response or non‐response... The primary efficacy analysis, performed on the FAS, was based on logistic regression and the Reeve method was used to calculate the 95% CI. A combination of non‐responder imputation (NRI) and multiple imputation (MI) was used for incomplete or missing data. The per‐protocol analysis set (PPS), used for sensitivity analysis of the primary endpoint, contained all patients in the FAS with no important efficacy‐relevant protocol deviations. In this analysis, missing data were imputed using a combination of NRI and Last Observed Carried Forward (LOCF) ... The safety analysis set (SAF) contained all randomized patients who received at least one dose of study medication." Per‐protocol analyses (non‐inferiority trial) Randomly assigned 318, efficacy FAS analysed 315, safety SAF 317 |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02850965). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are posted on ClinicalTrials.gov. |
VOYAGE‐1 2016.
| Study characteristics | ||
| Methods | RCT, active placebo‐controlled, double‐blind study Date of study: December 2014 to April 2016 Location: 101 centres worldwide |
|
| Participants |
Randomised: 837 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 37, mean age 44 years, 608 male Dropouts and withdrawals 24/837 (2.9%): guselkumab (7), adalimumab (10), placebo group (7)
|
|
| Interventions |
Intervention A. Guselkumab (n = 334), SC, 100 mg, weeks 0 and 4, then every 8 weeks Control intervention B. Adalimumab (n = 329), 80 mg week 0, then 40 mg week 1, and every 2 weeks C. Placebo (n = 174) |
|
| Outcomes | Assessment at 16 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 405): "Supported by Janssen Research & Development LLC, Spring House, PA." Declarations of interest: Quote (p 405): "Dr Blauvelt has served as a scientific adviser and clinical study investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Genentech, GSK, Janssen, Eli Lilly, Merck, Novartis, Pfizer, Regeneron, Sandoz, Sanofi‐Genzyme, Sun, UCB, and Valeant, and as a paid speaker for Eli Lilly. Dr Papp has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for AbbVie, Akesis, Akros, Allergan, Alza, Amgen, Anacor, Artax, Astellas, AstraZeneca, Baxalta, Baxter, Biogen, Boehringer Ingelheim, Bristol‐Myers Squibb, CanFite, Celgene, Celtic, Cipher, Dermira, Dow Pharmaceuticals, Eli Lilly, Ferring Pharmaceuticals, Formycon, Forward Pharma, Funxional Therapeutics, Fujisawa, Galderma, Genentech, Genexion, Genzyme, Gilead, GSK, Janssen, Kyowa Hakko Kirin, Leo, Lypanosys, Medimmune, Meiji Seika Pharma, Merck (MSD), Merck‐Serono, Mitsubishi Pharma, Mylan, Novartis, NovImmune, Pan Genetics, Pfizer, Regeneron, Roche, Sanofi‐Aventis, Stiefel, Takeda, UCB, Vertex, and Valeant. Dr Griffiths has received honoraria and/or grants as an investigator, speaker, and/or advisory board member for AbbVie, Eli Lilly, Janssen, Leo, Novartis, Pfizer, Sandoz, and Sun Pharma. Dr Kimball has received honoraria as a consultant for AbbVie, BMS, Dermira, Eli Lilly ICOS LLC, Merck, and Novartis; and received grants and/or funding for research or the residency/fellowship program as a principal investigator for AbbVie, Amgen, Boehringer Ingelheim, Dermira, Janssen, Merck, and Novartis. Drs Randazzo, Wasfi, Shen, and Li are all employees of Janssen Research & Development LLC (subsidiary of Johnson & Johnson) and own stock in Johnson & Johnson." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 3): "Patients were randomised using a permuted block method; central randomisation was implemented using an interactive World Wide Web response system (Perceptive Informatics, East Windsor, NJ)." Comment: clearly defined |
| Allocation concealment (selection bias) | Low risk | Quote (p 3): "Central randomisation was implemented using an interactive World Wide Web response system (Perceptive Informatics, East Windsor, NJ)." Comment: clearly defined |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 3): "To maintain the blind, matching placebos were used." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 3): "To maintain the blind, matching placebos were used." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 837, 837 analysed Management of missing data: quote (page 3): "Patients who discontinued study agent because of lack of efficacy or an AE of psoriasis worsening or who started a protocol‐prohibited psoriasis treatment were considered nonresponders (binary end points) or had baseline values carried over (continuous end points). Other patients with missing data were considered nonresponders for binary end points (nonresponder imputation) and had last observation carried forward for continuous end points (and all PSSD end points)." Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02207231). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
VOYAGE‐2 2017.
| Study characteristics | ||
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: November 2014 to May 2016 Location: 115 centres worldwide Phase 3 |
|
| Participants |
Randomised: 992 participants (mean age 44 years, 692 male) Inclusion criteria
Exclusion criteria
Baseline characteristics N = 992, mean age 44 years, 692 male Dropouts and withdrawals 44/992 (4.4%); guselkumab (18), adalimumab (11), placebo group (15)
|
|
| Interventions |
Intervention A. Guselkumab (n = 496), SC, 100 mg, weeks 0 and 4, then every 8 weeks Control interventions B. Adalimumab (n = 248), 80 mg week 0, then 40 mg week 1, and every 2 weeks C. Placebo (n = 248) |
|
| Outcomes | Assessments at 16 weeks Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 1): "Supported by Janssen Research & Development, LLC." Declarations of interest: Quote (p 1): "Dr Reich has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Amgen, Biogen, Boehringer Ingelheim Pharma, Celgene, Covagen, Eli Lilly, Forward Pharma, GlaxoSmithKline, Janssen, Leo, Medac, Merck Sharp & Dohme, Novartis, Ocean Pharma, Pfizer, Regeneron, Takeda, UCB Pharma, and Xenoport. Dr Armstrong has served as investigator and/or advisor/consultant for AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Merck, Novartis, and Pfizer. Dr Foley has served as a consultant, investigator, speaker, and/or advisor for and/or received travel grants from 3M/iNova/Valeant, Abbott/AbbVie, Amgen, Biogen Idec, BMS, Boehringer Ingelheim, Celtaxsys, Celgene, Cutanea, Eli Lilly, Galderma, GSK/Stiefel, Janssen, LEO/Peplin, Novartis, Regeneron, Schering‐Plough/MSD, UCB, and Wyeth/Pfizer. Dr Gordon has received research support from AbbVie, Amgen, Boeringher Ingelheim, Eli Lilly, and Janssen, and consultant/ honoraria from AbbVie, Amgen, Boeringher Ingelheim, Celgene, Eli Lilly, Janssen, Novartis, and Pfizer. Drs Song, Wasfi, Randazzo, Li, and Shen are all employees of Janssen Research & Development, LLC (subsidiary of Johnson & Johnson) and own stock in Johnson & Johnson." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 3): "Patients were randomized 2:1:1 using a permuted block method at baseline to guselkumab 100 mg at weeks 0, 4, 12, and 20; placebo at weeks 0, 4, and 12, then guselkumab at weeks 16 and 20; or adalimumab 80 mg at week 0, 40 mg at week 1, and every 2 weeks thereafter through week 23 (Fig 1). Central randomization occurred using an interactive web based response system (Perceptive Informatics, East Windsor, NJ)." Comment: clearly defined |
| Allocation concealment (selection bias) | Low risk | Quote (p 3): "Patients were randomized using a permuted block method at baseline in a 2:1:2 ratio to guselkumab 100 mg at weeks 0, 4, 12, and every 8 weeks through week 44; placebo at weeks 0, 4, and 12 followed by guselkumab 100 mg at weeks 16 and 20, and every 8 weeks through week 44; or adalimumab 80 mg at week 0, 40 mg at week 1, and 40 mg every 2 weeks through week 47. Central randomization was implemented using an interactive World Wide Web response system (Perceptive Informatics, East Windsor, NJ)." Comment: clearly defined |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 3): "double‐blind, placebo‐ and adalimumab comparator controlled study" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 3): "double‐blind, placebo‐ and adalimumab comparator controlled study" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 992, 992 analysed Management of missing data: Quote (p 3): "All randomized patients were included in the primary analysis and some secondary efficacy analyses according to their assigned treatment group.... Patients who discontinued treatment due to lack of efficacy or an adverse event [AE] of worsening of psoriasis, or started a protocol‐prohibited medication/therapy to improve psoriasis were considered treatment failures." Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02207244). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. |
Yang 2012.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: February 2009 to February 2010 Location: 9 centres in China |
|
| Participants |
Randomised: 129 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 129; mean age 39 years (infliximab) and 40 years (placebo), 95 male Dropouts and withdrawals 2/129 (1.55%): infliximab group (1), placebo group (1)
|
|
| Interventions |
Intervention A. Infliximab (n = 84), IV, 5 mg/kg, weeks 0, 2, 6, 14, 22; 22 weeks Control intervention B. Placebo (n = 45), IV, weeks 0, 2, 6 then infliximab 5 mg/kg weeks 10, 12, 16 |
|
| Outcomes | Assessments at 10 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 1846): "This randomised, double‐blind, placebo controlled trial... Eligible patients were randomly assigned in a 1:2 ratio to the placebo and infliximab". Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 1846): "This randomised, double‐blind, placebo controlled trial... Eligible patients were randomly assigned in a 1:2 ratio to the placebo and infliximab". Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1846): "This randomised, double‐blind, placebo controlled trial... Eligible patients were randomly assigned in a 1:2 ratio to the placebo and infliximab... Infliximab 5 mg/kg or placebo was administered by intravenous drip infusion over a period of at least 2 hours on the starting day of treatment (week 0) and at weeks 2 and 6 (induction)". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1846): "This randomised, double‐blind, placebo controlled trial... Eligible patients were randomly assigned in a 1:2 ratio to the placebo and infliximab... Infliximab 5 mg/kg or placebo was administered by intravenous drip infusion over a period of at least 2 hours on the starting day of treatment (week 0) and at weeks 2 and 6 (induction)". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 129, 129 analysed Quote: "In the primary efficacy analysis, data from all randomised subjects were analysed according to their assigned treatment group..." Comment: no description of the method used to manage the missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Ye 2020.
| Study characteristics | ||
| Methods | RCT, active‐controlled study Date of study: August 2017 to February 2019 Location: China |
|
| Participants |
Randomised: 150 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 150, mean of age 33 years, and 57% men Dropouts and withdrawals Not stated |
|
| Interventions |
Intervention A. Acitretin per os initial dose 30 mg/d Control intervention B. No treatment Co‐intervention: narrow‐band ultraviolet therapy |
|
| Outcomes | At week 8 Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: not stated Declarations of interest: Quote (p 5074): "None" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 5069): "The patients were randomly divided into the control group (n = 75) and the observation group (n = 75)." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 5069): "The patients were randomly divided into the control group (n = 75) and the observation group (n = 75)." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no description of whether the trial is blinded or open |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no description of whether the trial is blinded or open |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomised 150, analysed 150 Comment: methods for dealing with missing data not specified, ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the Methods section appeared to have been reported. |
Yilmaz 2002.
| Study characteristics | ||
| Methods | RCT, placebo‐controlled, open‐label study Date of study: not stated Location: Turkey |
|
| Participants |
Randomised: 50 participants Inclusion/exclusion criteria
Baseline characteristics No description of the study population Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Acitretin (n = 50), orally, 0.5 to 0.7 mg/kg, daily Control intervention B. Placebo (n = 50) Co‐intervention PUVA, twice‐weekly, 8‐MOP at a dosage of 0.4 to 0.6 g/kg, 2 hours before UVA exposure |
|
| Outcomes | Time of assessments not stated Primary or secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes |
Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (abstract): "The patients were equally allocated to treatment groups in the study". Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (abstract): "The patients were equally allocated to treatment groups in the study". Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (abstract): "We performed an open, controlled study..." Comment: not blinded, subjective outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (abstract): "We performed an open, controlled study..." Comment: not blinded, subjective outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 50 Comment: no description of the number of participants analysed, no description of the method used to manage missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: only an abstract available |
Yu 2019.
| Study characteristics | ||
| Methods | RCT, active‐controlled study Date of study: not stated Location: China |
|
| Participants |
Randomised: 30 participants Key inclusion criteria
Key exclusion criteria
Baseline characteristics N = 30, mean age of 51.93 years and 67% men Dropouts and withdrawals No withdrawals occurred |
|
| Interventions |
Intervention A. Methotrexate (combination of etanercept, SC injection 50 mg weekly and methotrexate, PO 7.5 mg to 15 mg weekly), n = 15 Control intervention B. No treatment n = 15 Co‐intervention Etanercept (SC injection 50 mg every week through week 24) |
|
| Outcomes | At week 24 Outcomes
|
|
| Notes |
Funding source: Quote (p 449): "This work was supported by grants from National Natural Science Foundation of China (No. 81673050, 81872522), the Program of Science and Technology Commission of Shanghai Municipality (No. 18140901800), Innovation Program of Shanghai Municipal Education Commission (No.2019‐01‐07‐00‐07‐E00046), Excellent Subject Leader Program of Shanghai Municipal Commission of Health and Family Planning (No. 2018BR30), Clinical Research Program of Shanghai Hospital Development Center (No. SHDC12018X06)." Declarations of interest: Quote (p 449): "There is no conflicting interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 443): "Randomization was undertaken with the use of computer‐generated random numbers." Comment: adequate process |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 443): "Randomization was undertaken with the use of computer‐generated random numbers." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no description of the method used to guarantee blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 443): "The PASI score was determined by a dermatologist at 2, 6, 12, 18 and 24 weeks of treatment." Comment: physicians were not blinded for PASI evaluation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15 included/15 analysed Comment: no description of the method used to manage the missing data or to perform the analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The prespecified outcomes mentioned in the methods section appeared to have been reported. |
Yu 2022.
| Study characteristics | ||
| Methods | RCT, active‐controlled, double‐blind study Date of study: August 2019 to June 2021 Location: 21 centres in China Phase 3 |
|
| Participants |
Randomised: 367 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 367, mean of age 39 years, and 81% men Dropouts and withdrawals 30/367 (8%): biosimilar SCT630 group (18), adalimumab group (12)
|
|
| Interventions |
Intervention A. SCT630, biosimilar adalimumab administered at an initial loading dose of 80 mg subcutaneously (SC) on week 1/day 1, 40 mg at the 1st days of week 2 and subsequently every 2 weeks (starting at week 3) until week 16, n = 184 Control Intervention B. Adalimumab (Humira) administered at an initial loading dose of 80 mg subcutaneously (SC) on week 1/day 1, 40 mg at the 1st days of week 2 and subsequently every 2 weeks (starting at week 3) until week 16, n = 183 |
|
| Outcomes |
At 16 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Quote (p 7): "The study was funded by Sinocelltech Ltd. and supported by the National Major Scientific and Technological Special Project for “Sig‐ nificant New Drugs Innovation and Development” (grant number 2018ZX09736002)". Declarations of interest: Quote (p 7): "The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 2): "This was a randomized, double‐blind, active‐controlled phase III trial carried out between 29 August 2019 and 17 June 2021 at 21 centers in China" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 2): "This was a randomized, double‐blind, active‐controlled phase III trial carried out between 29 August 2019 and 17 June 2021 at 21 centers in China" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 2): "This was a randomized, double‐blind, active‐controlled phase III trial" Quote (p 2): "Throughout the study, the patients, investigators, study center personnel, and the sponsor were blinded to the treatment assignment." Comment: no description of the measure undertaken to guarantee the blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 2): "This was a randomized, double‐blind, active‐controlled phase III trial" Quote (p 2): "Throughout the study, the patients, investigators, study center personnel, and the sponsor were blinded to the treatment assignment." Comment: no description of the measure undertaken to guarantee the blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dealing with missing data: Quote (p 3): "Efficacy data were analyzed using the full analysis set (FAS) (defined as all randomized patients who had received ≥ 1 dose of study drug) and the per‐protocol set (PPS; a subset of FAS population, defined as patients with all major efficacy evaluations obtained and without major protocol violations). The safety analysis set (SAS) included all randomized patients who had received ≥ 1 dose of study drug and had post‐dose safety data, and the immunogenicity analysis (IA) included patients in SAS who had at least one evaluable antibody test result." Randomised 367, analysed 367 Comment: methods for dealing with missing data not specified |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT03927352). The prespecified outcomes and those mentioned in the Methods section appeared to have been reported. Results are not posted on ClinicalTrials.gov. |
8‐MOP: methoxsalen ABP 501: adalimumab biosimilar ACI: acitretin ACR: American College of Rheumatology ACT: activated clotting time ADA: adalimumab ADMB: adalimumab AEs: adverse events AIN457: secukinumab ALT: alanine aminotransferase ANC: absolute neutrophil count ANCOVA: analysis of covariance AST: aspartate aminotransferase BI695501: adalimumab biosimilar BCD‐057: adalimumab biosimilar BCG: bacille Calmette‐Guérin BI655066: risankizumab BID: two times a day Bime: bimekizumab BIW: twice a week BMS‐986615: deucravacitinib BSA: body surface area CBC: complete blood count Certo: certolizumab CHS‐0214: etanercept biosimilar CI: confidence interval CIN: cervical intraepithelial neoplasia CMH: Cochran‐Mantel‐Haenszel COPD: chronic obstructive pulmonary disease CRO: contract research organisation CRP: c‐reactive protein CS: systemic corticosteroid C‐SSRS: Columbia Suicide Severity Rating Scale CTL: cytotoxic T‐lymphocyte CZP: certolizumab DLQI: Dermatology Life Quality Index DMF: dimethylformamide DSM: drug supply management ECG: electrocardiogram eow: every other week EQ‐5D‐5L: standardised measure of health‐related quality of life ETA: etanercept ETN: etanercept EudraCT: European Union Drug Regulating Authorities Clinical Trials FAE: fumaric acid esters FAS: full analysis set FDA: food drug administration FDG‐PET/CT: fluorodeoxyglucose (FDG)‐positron emission tomography FMD: flow‐mediated dilatation FSH: follicle‐stimulating hormone GCP: good clinical practice GPSS: genital psoriasis symptoms scale HbA1c: haemoglobin A1c HBV: hepatitis B virus hCG: human chorionic gonadotropin HCV: hepatitis C virus HD: high dose HDL: high‐density lipoprotein Hgb: haemoglobin HIV: human immunodeficiency virus HOMA‐IR: Homeostatic Model Assessment for Insulin Resistance ICF: international classification of functioning, disability and health ICH: intracerebral brain haemorrhage ID: identification number IFX: infliximab IGA: Investigator’s Global Assessment IL(‐23/17/12): interleukin‐23/17/12 IM: intramuscular IMP: investigational medicinal product INRS: itch numeric rating scale IP: investigational product IPAQ: International Physical Activity Questionnaire IRT: interactive response technology ITT: intention‐to‐treat IUD: intrauterine device IUS: intrauterine system IV: intravenous IWRS: interactive web response systems IXE: ixekizumab IXRS: interactive voice/web response system LD: low dose LDL: low‐density lipoprotein LFT: live function tests LOCF: last observation carried forward LTBI: latent tuberculosis infection M3(R2): guideline on non‐clinical safety studies for the conduct of human clinical trials and marketing authorisation for pharmaceuticals MCMC: markov chain Monte Carlo MCS: multiple chemical sensitivity MD: medium dose MEDFICTS: dietary assessment instrument MEF: monoethyl fumarate MGH‐SFQ: Massachusetts general hospital‐sexual functioning questionnaire MGUS: monoclonal gammopathy of undetermined significance MI: multiple imputation m‐ITT: modified ITT MK‐3222: tildrakizumab MMRM: mixed‐model repeated measures mNAPSI: modified NAPSI mNRI: modified NRI MTX: methotrexate NAPPA‐CLIN: Nail Assessment in Psoriasis and Psoriatic Arthritis clinician‐reported measure NAPSI: Nail Psoriasis Severity Index NBUVB: narrow‐band UVB NMSC: non melanoma skin cancer NRI: non responder imputation NTK: netakimab NTMB: nontuberculous mycobacteria NYHA: New‐York Heart Association OC: oral contraceptive OR: odds ratio OS: overall survival PASE: Physical Activity Scale for the Elderly PASI: Psoriasis Area and Severity Index PBO: placebo PFS: progression‐free survival PGA(‐G): Physician Global Assessment PHQ‐8: eight‐item Patient Health Questionnaire depression scale PP: per protocol PPASI: palmoplantar psoriasis severity index PPD: purified protein derivative PPGA: Physician Global Assessment PPS: Palliative Performance Scale Ps: performance status PsA: psoriatic arthritis PSGA: Physician Static Global Assessment PSI: Psoriasis Severity Index PSO: psoriasis PSSD: post‐SSRI sexual dysfunction PSSI: Psoriasis Scalp Severity Index PSSQ: Psoriasis Subject Satisfaction Questionnaire PtGA: Patient's Global Assessment PUVA: psoralen plus ultraviolet A Q(2/4)W: every other week/every 4 weeks QFT: quantiFERON‐TB gold QoL: quality of life R2: cf M3(R2) RA: rheumatoid arthritis RCT: randomised controlled trial RFT: renal function tests rhTNFR‐Fc: tumour necrosis factor receptor: fusion protein RoB: risk of bias SAEs: serious adverse events SAF: safety analysis set SAS: statistical analysis system SC: subcutaneous ScPGA: Scalp Physician Global Assessment SF‐36: 36‐item Short Form Health Survey SIAQ: Self‐Injection Assessment Questionnaire SSA: scalp surface area TB: tuberculosis TBR(max): target background ratio TEAE: treatment emergent adverse event Th‐17: T helper‐17 cell TNFα: tumour necrosis factor alpha ULN: upper limit of normal USK: ustekinumab UV: ultraviolet UVB: ultraviolet B VAS: visual analogue scale W14: week 14 WBC: white blood count WOCBP: women of childbearing potential Please note that the term “conventional” in these tables is replaced with “non‐biological treatment” in the main text of this review.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abe 2017 | Post hoc subgroup analyses of an already included trial |
| Abufarag 2010 | Other treatment |
| ACTRN12606000040561 | Wrong intervention |
| Adsit 2017 | Post hoc subgroup analyses of an already included trial |
| Akhyani 2010 | Other treatment |
| Al‐Oudah 2022 | Wrong intervention |
| Altmeyer 1994 | Not plaque‐type psoriasis |
| Angsten 2007 | Not a trial |
| Anonymous 2005 | Not a trial |
| Anonymous 2008 | Not a trial |
| Anonymous 2019 | Not a randomised trial |
| Araujo 2017 | Not moderate‐to‐severe psoriasis |
| Araujo 2019 | Not moderate‐to‐severe psoriasis |
| Arifov 1998 | Not a randomised trial |
| Armati 1972 | Other treatment |
| Asahina 2016 | Other treatment, now excluded in this update because interventions no longer meet inclusion criteria |
| Augustin 2017 | Dose de‐escalation strategy study |
| Avgerinou 2011 | Not a randomised trial |
| Bachelez 2017 | Post hoc subgroup analyses of an already included trial |
| Bagel 2017a | Open‐label extension restricted to good responders |
| Bagel 2017b | Not a randomised trial |
| Bagel 2017c | Not moderate‐to‐severe psoriasis |
| Bagel 2018b | Not a randomised trial |
| Bagherani 2017 | Commentary/editorial |
| Bagot 1994 | Other treatment |
| Banerjee 2021 | Wrong comparator |
| Bartlett 2008 | Not a trial |
| Barzegari 2004 | Other treatment |
| Batchelor 2009 | Not a trial |
| Bayerl 1992 | Other treatment |
| Beissert 2009 | Other treatment |
| Berbis 1989 | Assessment < 8 weeks |
| Bhat 2017 | Post hoc subgroup analyses of an already included trial |
| Bhuiyan 2010 | Other treatment |
| Bian 2018` | Open‐label extension restricted to good responders |
| Bigby 2004 | Not a trial |
| Bissonnette 2006 | Other treatment |
| Bissonnette 2010 | Other treatment |
| Bissonnette 2015 | Other treatment, now excluded in this update because interventions no longer meet inclusion criteria |
| Bissonnette 2017a | Open‐label extension restricted to good responders |
| Bissonnette 2017b | Not moderate‐to‐severe psoriasis |
| Bissonnette 2018 | Not moderate‐to‐severe psoriasis |
| Bjerke 1989 | Other treatment |
| Blauvelt 2016a | Ineligible study design |
| Blauvelt 2016b | Open‐label extension restricted to good responders |
| Blauvelt 2017a | Pooled trials |
| Blauvelt 2017b | Open‐label extension restricted to good responders |
| Blauvelt 2017c | Open‐label extension restricted to good responders |
| Blauvelt 2017d | Open‐label extension restricted to good responders |
| Blauvelt 2017e | Pooled trials |
| Blauvelt 2017f | Ineligible study design |
| Blauvelt 2017g | Open‐label extension restricted to good responders |
| Blauvelt 2017h | Open‐label extension restricted to good responders |
| Blauvelt 2017i | Open‐label extension restricted to good responders |
| Blauvelt 2017j | Pooled trials |
| Blauvelt 2017k | Open‐label extension restricted to good responders |
| Blauvelt 2018a | Not a randomised trial |
| Blauvelt 2018b | Open‐label extension restricted to good responders |
| Blauvelt 2018c | Pooled trials |
| Blauvelt 2018d | Pooled trials |
| Blauvelt 2018e | Pooled trials |
| Blauvelt 2018f | Pooled trials |
| Blauvelt 2018g | Pooled trials |
| Blauvelt 2018h | Pooled trials |
| Blauvelt 2020 | Not moderate‐to‐severe psoriasis (good responders selection) |
| Branigan 2017 | Open‐label extension restricted to good responders |
| Brasil 2012 | Ineligible study design |
| Brasil 2013 | Ineligible patient population |
| Brasil 2016 | Ineligible patient population |
| Buono 2020 | Not a randomised trial |
| Burden 2017 | Commentary/editorial |
| Burkhardt 2017 | Ineligible study design |
| Callis Duffin 2017 | Comparison of the same drug with the same dosages |
| Cassano 2006 | Identical dosing regimens |
| Cassano 2010 | Not a trial |
| Cather 2006 | Dose‐ranging after remission |
| Cather 2018 | Ineligible patient population |
| Chakravadhanula 2017 | Ineligible intervention |
| Chapman 2018 | Ineligible study design |
| ChiCTR‐INR‐16009710 | Assessment at 4 weeks |
| ChiCTR2000030273 2020 | Phase 1 trial |
| Chládek 2002 | Basic science (aim of study: to understand the physiopathology of the disease) |
| Chodorowska 1999a | Not a trial |
| Chodorowska 1999b | Not a trial |
| Choi 2017 | Not moderate‐to‐severe psoriasis |
| Crowley 2018a | Not moderate‐to‐severe psoriasis |
| Crowley 2018b | Open‐label extension restricted to good responders |
| CTRI/2018/01/011373 | 2 different schemas of administration (same drug, same dosage) |
| CTRI/2020/07/026598 2020 | 2 different schemas of administration (same drug, same dosage) |
| CTRI/2020/12/029472 2020 | Wrong intervention |
| CTRI/2020/12/029611 | Same intervention |
| De Jong 2003 | Other treatment |
| De Mendizabal 2017 | Post hoc subgroup analyses of an already included trial |
| Dubiel 1972 | Not a trial |
| Duffin 2016 | Comparison of 2 different ways of drug injection for the same drug and the same dosage |
| Duffin 2017 | Ineligible study design |
| Ecker‐Schlipf 2009 | Other treatment |
| Edson‐Heredia 2013 | Post hoc subgroup analyses of an already included trial |
| Egeberg 2016 | Commentary/editorial |
| Elewski 2007 | Pooled trials |
| Elewski 2017 | Post hoc subgroup analyses of an already included trial |
| Elewski 2018a | Ineligible study design |
| Elewski 2018b | Ineligible study design |
| Ellis 1986 | Assessment < 8 weeks |
| Ellis 2001 | Another intervention |
| Ellis 2002 | Medico‐economic study |
| Ellis 2012 | Other treatment |
| Engst 1989 | Assessment < 8 weeks |
| Erkko 1997 | Basic science (aim of study: to understand the physiopathology of the disease) |
| EUCTR2007‐004328‐18‐FR | Ineligible intervention |
| EUCTR2012‐005685‐35‐DE | Withdrawn trial, NCT01815723 |
| EUCTR2016‐001593‐15‐ES | Withdrawn trial, DEEP Study |
| EUCTR2016‐003592‐21‐GB | Withdrawn trial |
| EUCTR2018‐001021‐10‐SE | Not moderate‐to‐severe psoriasis |
| EUCTR2019‐000817‐35‐DE | Not moderate‐to‐severe psoriasis |
| EUCTR2021‐000542‐18‐LV | Not moderate‐to‐severe psoriasis |
| EUCTR2022‐000695‐19‐LT | Not moderate‐to‐severe psoriasis |
| EXCEED 2021 | RCT dedicated to psoriatic arthritis. The randomisation was not stratified on plaque psoriasis with BSA > 10% or PASI ≥ 10 but on psoriatic plaque of ≥ 2 cm diameter. |
| Ezquerra 2007 | Other treatment |
| Feldman 2017 | Not moderate‐to‐severe psoriasis |
| Fernandes 2013 | Not a trial |
| Fernandez 2017 | Not a randomised trial |
| Finzi 1993 | Other treatment |
| Fitz 2018 | Post hoc subgroup analyses of an already included trial |
| Fleischer 2005 | Other treatment |
| Foley 2017 | Pooled trials |
| Foley 2018 | Pooled trials |
| Fredriksson 1971 | Other treatment |
| Fredriksson 1978 | Other treatment |
| Friedrich 2001 | Other treatment |
| GAIN 2021 | Not moderate‐to‐severe psoriasis |
| Gambichler 2011 | Other treatment |
| Ganguly 2004 | Pooled trials |
| Gil 2003 | Not a randomised trial |
| Gisondi 2020 | Not a randomised trial |
| Glatt 2017 | Ineligible study design |
| Goerz 1978 | Not a trial |
| Gold 2018 | Ineligible study design |
| Goll 2017 | Not moderate‐to‐severe psoriasis |
| Goll 2018 | Ineligible study design |
| Gollnick 1988 | Other treatment |
| Gollnick 1993 | Other treatment |
| Gollnick 2002 | Other treatment |
| Gordon 2014 | Ineligible study design |
| Gordon 2015 | Ineligible study design |
| Gordon 2018a | Open‐label extension restricted to good responders |
| Gordon 2018b | Post hoc subgroup analyses of an already included trial |
| Gordon 2018c | Pooled trials |
| Gordon 2018d | Post hoc subgroup analyses of an already included trial |
| Gottlieb 2002 | Other treatment |
| Gottlieb 2003b | Other treatment |
| Gottlieb 2003c | Open‐label extension restricted to good responders |
| Gottlieb 2004b | Pooled trials |
| Gottlieb 2005 | Other treatment |
| Gottlieb 2006a | Ineligible intervention |
| Gottlieb 2006b | Ineligible intervention |
| Gottlieb 2010 | Cross‐over trial |
| Gottlieb 2016 | Pooled trials |
| Gottlieb 2017a | Not moderate‐to‐severe psoriasis |
| Gottlieb 2017b | Not moderate‐to‐severe psoriasis |
| Gottlieb 2017c | Post hoc subgroup analyses of an already included trial |
| Gottlieb 2017d | Pooled trials |
| Gottlieb 2018a | Pooled trials |
| Gottlieb 2018b | Pooled trials |
| Goupille 1995 | Not a randomised trial |
| Goupille 2018 | Not moderate‐to‐severe psoriasis |
| Griffiths 1998 | Other treatment |
| Griffiths 2002a | Pooled trials |
| Griffiths 2002b | Pooled trials |
| Griffiths 2005 | Pooled trials |
| Griffiths 2010 | Open‐label extension restricted to good responders |
| Griffiths 2016 | Post hoc subgroup analyses of an already included trial |
| Griffiths 2017 | Open‐label extension restricted to good responders |
| Griffiths 2018a | Ineligible study design |
| Griffiths 2018b | Post hoc subgroup analyses of an already included trial |
| Griffiths 2018c | Pooled trials |
| Grim 2000 | Basic science (aim of study: to understand the physiopathology of the disease) |
| Grossman 1994 | Other treatment |
| Guenther 2020 | Not moderate‐to‐severe psoriasis |
| Gulliver 1996 | Not a trial |
| Gupta 2005 | Other treatment |
| Gupta 2007 | Other treatment |
| Gupta 2008 | Other treatment |
| Han 2013 | Other treatment |
| Hashizume 2007 | Comparison of 2 methods of administration |
| Hawkes 2018 | Ineligible study design |
| Heule 1988 | Assessment < 8 weeks |
| Ho 2010 | Other treatment |
| Holzer 2020 | No efficacy or safety assessment ‐ the study assessed cardiovascular outcomes |
| Hsu 2018 | Post hoc subgroup analyses of an already included trial |
| Hunter 1972 | Other treatment |
| Iest 1989 | Not a randomised trial |
| Imafuku 2017 | Post hoc subgroup analyses of an already included trial |
| ISRCTN18043449 | Not moderate‐to‐severe psoriasis |
| Iversen 2018 | Ineligible comparator |
| Jackson 2018 | Ineligible study design |
| Jacobe 2008 | Another intervention |
| JapicCTI‐194706 2019 | Comparison of different schemas of administration (same drug, same dosage) |
| Jin 2017 | Other treatment, now excluded in this update because interventions no longer meet inclusion criteria |
| Joergensen 2022 | Wrong intervention |
| JPRN‐jRCT2071210135 | Wrong population |
| JPRN‐jRCTs041180012 2018 | Not moderate‐to‐severe psoriasis |
| Kaur 2018 | Not moderate‐to‐severe psoriasis |
| Kavanaugh 2009 | Not a randomised trial |
| Kemeny 2019 | Post hoc subgroup analyses of an already included trial |
| Kimball 2008 | Drug withdrawn for safety reasons |
| Kimball 2011 | Drug withdrawn for safety reasons |
| Kimball 2018 | Ineligible study design |
| Kohm 2022 | Not moderate‐to‐severe psoriasis |
| Koo 1998 | Other treatment |
| Kopp 2015 | Phase 1 trial |
| Korotaeva 2021 | Not moderate‐to‐severe psoriasis |
| Kragballe 1989 | Other treatment |
| Krishnan 2005 | Pooled trials |
| Krishnan 2018 | Pooled trials |
| Kristensen 2017 | Not moderate‐to‐severe psoriasis |
| Krueger 1980 | Other treatment |
| Krueger 2002a | Another intervention |
| Krueger 2002b | Not a trial |
| Krueger 2003 | Not a trial |
| Krueger 2012 | Phase 1 trial |
| Krueger 2015 | Phase 1 trial |
| Krueger 2016a | Other treatment, now excluded in this update because interventions no longer meet inclusion criteria |
| Krueger 2016b | Phase I trial |
| Krueger 2022 | Not moderate‐to‐severe psoriasis |
| Krupashankar 2014 | Another intervention |
| Kuijpers 1998 | Other treatment |
| Lajevardi 2015 | Other treatment |
| Lambert 2018 | Post hoc subgroup analyses of an already included trial |
| Langewouters 2005 | Other treatment |
| Langley 2006 | Other treatment |
| Langley 2010 | Other treatment |
| Langley 2016 | Open‐label extension restricted to good responders |
| Langley 2018 | Ineligible study design |
| Langner 2004 | Not plaque‐type psoriasis |
| Lauharanta 1989 | Other treatment |
| Lawrence 1983 | Other treatment |
| Leavell 1970 | Other treatment |
| Lebwohl 2003 | Another intervention |
| Lebwohl 2003a | Pooled trials |
| Lebwohl 2009 | Pooled trials |
| Lebwohl 2012 | Other treatment |
| Lebwohl 2013 | Other treatment |
| Ledo 1988 | Other treatment |
| Legat 2005 | Other treatment |
| Leonardi 2010a | Pooled trials |
| Leonardi 2010b | Not a randomised trial |
| Leonardi 2010c | Pooled trials |
| Leonardi 2011a | Open‐label extension restricted to good responders |
| Leonardi 2011b | Not plaque‐type psoriasis |
| Levell 1995 | Other treatment |
| Li 2018 | Post hoc subgroup analyses of an already included trial |
| Li 2022 | Phase 1 trial |
| Liang 1995 | Assessment < 8 weeks |
| Louw 2017 | Open‐label extension restricted to good responders |
| Lui 2011 | Other treatment |
| Lui 2012 | Other treatment |
| Lynde 2012 | Other treatment |
| Macdonald 1972 | Not a randomised trial |
| Mahrle 1995 | Other treatment |
| Malik 2010 | Other treatment |
| Marecki 2004 | Other treatment |
| Marks 1986 | Not a randomised trial |
| Mate 2017 | Not moderate‐to‐severe psoriasis |
| Mate 2018 | Open‐label extension restricted to good responders |
| McInnes 2013 | Pooled trials |
| McInnes 2017 | Not moderate‐to‐severe psoriasis |
| Mease 2011 | Drug withdrawn for safety reasons |
| Mease 2016a | Not moderate‐to‐severe psoriasis |
| Mease 2016b | Not moderate‐to‐severe psoriasis |
| Mease 2017a | Not moderate‐to‐severe psoriasis |
| Mease 2017b | Not moderate‐to‐severe psoriasis |
| Mease 2017c | Not moderate‐to‐severe psoriasis |
| Mease 2018 | Not moderate‐to‐severe psoriasis |
| Mease 2020 | Not moderate‐to‐severe psoriasis |
| Mease 2022 | Not a randomised study |
| Meffert 1989 | Other treatment |
| Menon 2012 | Basic science (aim of study: to understand the physiopathology of the disease) |
| Menter 2007 | Pooled trials |
| Menter 2014 | Drug withdrawn for safety reasons |
| Menter 2022 | Not moderate‐to‐severe psoriasis |
| Merola 2017 | Post hoc subgroup analyses of an already included trial |
| Merola 2018 | Not moderate‐to‐severe psoriasis |
| Merola 2020 | Not moderate‐to‐severe psoriasis |
| Meyer 2011 | Other treatment |
| Mittal 2009 | Other treatment |
| Moller 2009 | Other treatment |
| Monk 1986 | Not a randomised trial |
| Montgomery 1993 | Other treatment |
| Mrowietz 1991 | The 2 study arms compared the same molecule with the same dosage |
| Mrowietz 2012 | Pooled trials |
| Narang 2012 | Other treatment |
| Nash 2015 | Not moderate‐to‐severe psoriasis |
| NCT00106847 | Dose de‐escalation strategy study |
| NCT00111111 | Dose de‐escalation strategy study |
| NCT00258713 | Ineligible intervention |
| NCT00358670 | Open‐label extension restricted to good responders |
| NCT00377325 | Withdrawn trial |
| NCT00438360 | Open‐label extension restricted to good responders |
| NCT00585650 | Ineligible patient population |
| NCT00645892 | Open‐label extension restricted to good responders |
| NCT00646191 | Open‐label extension restricted to good responders |
| NCT00647400 | Open‐label extension restricted to good responders |
| NCT00832364 | Withdrawn trial |
| NCT01163253 | Not a randomised trial |
| NCT01235442 | Ineligible intervention |
| NCT01276847 | Phase I trial |
| NCT01412944 | Open‐label extension restricted to good responders |
| NCT01443338 | Ineligible comparator |
| NCT01544595 | Open‐label extension restricted to good responders |
| NCT01550744 | Open‐label extension restricted to good responders |
| NCT01624233 | Not a randomised trial |
| NCT01722214 | Not moderate‐to‐severe psoriasis |
| NCT01806597 | Ineligible patient population |
| NCT01815723 | Withdrawn trial |
| NCT01828086 | Phase I trial |
| NCT01936688 | Withdrawn trial |
| NCT02362789 | Withdrawn trial |
| NCT02409667 | Open‐label extension restricted to good responders |
| NCT02798211 | Not moderate‐to‐severe psoriasis |
| NCT03010527 | Open‐label extension restricted to good responders |
| NCT03020199 | Ineligible comparator |
| NCT03025542 | Not moderate‐to‐severe psoriasis at the time of placebo use |
| NCT03073213 | Phase I trial |
| NCT03210259 | Not moderate‐to‐severe psoriasis |
| NCT03482011 | Other treatment, now excluded in this update because interventions no longer meet inclusion criteria |
| NCT03598790 | Not moderate‐to‐severe psoriasis |
| NCT04121143 | Other treatment, now excluded in this update because interventions no longer meet inclusion criteria |
| NCT04488185 | Withdrawn (low recruitment) |
| NCT04614298 | Trial withdrawn for reconsideration of drug's business |
| NCT04839016 | Other treatment, now excluded in this update because interventions no longer meet inclusion criteria |
| NCT04882098 | Not moderate‐to‐severe psoriasis |
| NCT05073315 | Not moderate‐to‐severe psoriasis |
| NCT05184348 | Ineligible intervention |
| NCT05478499 | Not moderate‐to‐severe psoriasis |
| NCT05495568 | Not moderate‐to‐severe psoriasis |
| Nemoto 2018 | Phase I trial |
| Nieboer 1990 | Other treatment |
| Nijsten 2008 | Not a trial |
| Noda 2011 | Not a randomised trial |
| Noor 2017 | Not a randomised trial |
| Novotny 1973 | Not a trial |
| Nyfors 1978 | Not a trial |
| Okubo 2019 | Open‐label extension restricted to good responders |
| Oliver 2021 | Ineligible study design |
| OPT Pivotal‐1 2015 | Other treatment, now excluded in this update because interventions no longer meet inclusion criteria |
| OPT Pivotal‐2 2015 | Other treatment, now excluded in this update because interventions no longer meet inclusion criteria |
| Orfanos 1978 | Other treatment |
| Orfanos 1979 | Other treatment |
| Ortonne 2008 | Comparison of 2 schemes of administration |
| Ortonne 2011 | Other treatment |
| Osamu 2014 | Phase 1 trial |
| Page 2020 | Phase 1 trial |
| Pakozdi 2018 | Post hoc subgroup analyses of an already included trial |
| Papp 2001 | Other treatment |
| Papp 2006 | Other treatment |
| Papp 2008 | Other treatment |
| Papp 2009 | Pooled data |
| Papp 2011a | Pooled trials |
| Papp 2011b | Drug withdrawn for safety reasons |
| Papp 2011c | Drug withdrawn for safety reasons |
| Papp 2012b | Other treatment, now excluded in this update because interventions no longer meet inclusion criteria |
| Papp 2012d | Phase 1 trial |
| Papp 2012e | Pooled trials |
| Papp 2017c | Open‐label extension restricted to good responders |
| Papp 2018a | Ineligible outcomes |
| Papp 2018b | Ineligible outcomes |
| Park 2013 | Other treatment |
| Paul 2012 | Other treatment |
| Paul 2014 | Other treatment |
| Paul 2018 | Pooled trials |
| Perks 2017 | Ineligible study design |
| Pettit 1979 | Assessment < 8 weeks |
| Petzelbauer 1990 | Not a randomised trial |
| Piascik 2003 | Not a trial |
| Ports 2013 | Other treatment |
| Puig 2018 | Ineligible outcomes |
| Punwani 2012 | Other treatment |
| Rabasseda 2012 | Not a trial |
| Radmanesh 2011 | Comparison of 2 schemes of administration |
| Raman 1998 | Other treatment |
| Reich 2004 | Ineligible intervention |
| Reich 2011 | Pooled trials |
| Reich 2014 | Other treatment |
| Reich 2016a | Ineligible study design |
| Reich 2016b | Ineligible study design |
| Reich 2017a | Ineligible study design |
| Reich 2017b | Open‐label extension restricted to good responders |
| Reich 2017c | Pooled trials |
| Reich 2018a | Ineligible outcomes |
| Reich 2018b | Long‐term follow‐up of patients included in UNCOVER‐3 (at baseline not moderate‐to‐severe psoriasis and no control group) |
| Reich 2018c | Open‐label extension restricted to good responders |
| Reich 2019 | Other treatment, now excluded in this update because interventions no longer meet inclusion criteria |
| Reitamo 1999 | Other treatment |
| Reitamo 2001 | Other treatment |
| Rim 2003 | Other treatment |
| Rinsho Iyaku 1991 | Other treatment |
| Ritchlin 2006a | Not a randomised trial |
| Ritchlin 2006b | Not a randomised trial |
| Romiti 2017 | Post hoc subgroup analyses of an already included trial |
| RPCEC00000201 | Ineligible intervention |
| Ryan 2018 | Not moderate‐to‐severe psoriasis |
| Saeki 2017 | Not a randomised trial |
| Salim 2006 | Other treatment |
| Scholl 1981 | Other treatment |
| Schopf 1998 | Other treatment |
| Schulze 1991 | Other treatment |
| Shintani 2011 | Comparison of 2 schemes of administration |
| Shiohara 1992 | Not a trial |
| Shupack 1997 | Not a trial |
| Simonova 2005 | Other treatment |
| Sinclair 2017 | Pooled trials |
| Sofen 2011 | Basic science (aim of study: to understand the physiopathology of the disease) |
| Sofen 2014 | Phase 1 trial |
| Soung 2022 | Not moderate‐to‐severe psoriasis |
| Spadaro 2008 | Not a trial |
| Spuls 2012 | Not a trial |
| Stein Gold 2018 | Not moderate‐to‐severe psoriasis |
| Stein Gold 2021 | Not moderate‐to‐severe psoriasis |
| Sticherling 1994 | Not a trial |
| Strober 2004 | Not a trial |
| Strober 2012 | Not a randomised trial |
| Strober 2017a | Pooled trials |
| Strober 2017b | Not moderate‐to‐severe psoriasis |
| Strober 2017c | Ineligible outcomes |
| Strober 2018 | Ineligible study design |
| Sun 2019 | Not psoriasis |
| Sweetser 2006 | Cross‐over trial |
| Syversen 2020 | NCT03074656 ‐ pragmatic trial according to anti‐TNF dosages |
| Talamonti 2021 | Not a randomised study |
| Talwar 1992 | Not a randomised trial |
| TCTR20190705002 | Comparison of 2 different schemas of administration (same drug same dosage) |
| Tejasvi 2012 | Other treatment |
| Thaçi 2002 | The 2 study arms compared the same molecule with the same dosage |
| Thaçi 2010 | Other treatment |
| Thaçi 2018 | Ineligible outcomes |
| Tong 2008 | Other treatment |
| Tsakok 2018 | Commentary/editorial |
| Vaclavkova 2014 | Another intervention |
| Valenzuela 2017 | Post hoc subgroup analyses of an already included trial |
| Van de Kerkhof 2017 | Post hoc subgroup analyses of an already included trial |
| Van Joost 1988 | Assessment < 8 weeks |
| Vena 2005 | Comparison of 2 schemas of administration |
| Vena 2012 | Other treatment |
| Verma 2021 | Wrong comparator |
| Viglioglia 1978 | Not a trial |
| Viswanath 2022 | Not moderate‐to‐severe psoriasis |
| Witkamp 1995 | Other treatment |
| Wolf 2012 | Other treatment |
| Wright 1966 | Not a randomised trial |
| Wu 2015 | Other treatment |
| Yan 2011 | Another intervention |
| Yesudian 2013 | Other treatment |
| Yiu 2020 | Commentary |
| Yoon 2007 | Dose‐escalation study |
| Yosipovitch 2018 | Not moderate‐to‐severe psoriasis |
| Zachariae 2008 | Other treatment |
| Zhang 2007 | Other treatment |
| Zhang 2009a | Other treatment |
| Zhang 2009b | Other treatment |
| Zhang 2017 | Other treatment, now excluded in this update because interventions no longer meet inclusion criteria |
| Zhang 2020 | Other treatment, now excluded in this update because interventions no longer meet inclusion criteria |
| Zhang 2022 | Wrong intervention |
| Zhu 2009 | Pooled trials |
| Zhuang 2016 | Phase 1 trial |
| Zobel 1987 | Not a trial |
BSA: body surface area PASI: Psoriasis Area Severity Index RCT: randomised controlled trial TNF: tumour necrosis factor
Characteristics of studies awaiting classification [ordered by study ID]
ChiCTR2000034243.
| Methods | RCT, active‐controlled, double‐blind study Date of study: April 2017 Location: China Phase 3 |
| Participants |
Randomised: 320 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention HS626 (infliximab biosimilar) 5 mg/kg at weeks 0, 2, 6, 14, 22, 30, 38, 46 Control intervention Infliximab 5 mg/kg at weeks 0, 2, 6, 14, 22, 30, 38, 46 |
| Outcomes | At 10, 30, and 52 weeks
|
| Notes | Unpublished study Funding: Hisun Pharmaceutical (Hangzhou) Co. Ltd Date last refreshed on: June 2020 Last checked in October 2022 |
Chow 2015.
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: not stated Location: Canada, Germany, and Poland |
| Participants |
Randomised: 455 participants Inclusion criteria
Exclusion criteria
Baseline characteristics N = 455, mean age of 43 years, 313 male |
| Interventions |
Interventions (n = 366) A. Voclosporin 0.8 mg/kg/day B. Ciclosporin 3.0 mg/kg/day Control intervention B. Placebo, n = 89 |
| Outcomes | At week 24 Primary outcome
Secondary outcomes
|
| Notes | Randomised, placebo and ciclosporin controlled study of ISA247 in plaque psoriasis patients (ESSENCE), NCT00408187 Participants in the voclosporin and ciclosporin arms (n = 355) were treated for 24 weeks; these participants were combined into a '24‐week treatment group'. In the placebo group, 89 participants were included. As the authors presented their results grouping ciclosporin and voclosporin together, we asked them to provide the results for the subgroup of participants with ciclosporin treatment arm. Two emails were sent without response (8 November 2016, 16 December 2016) |
CTRI/2015/05/005830.
| Methods | Randomised, parallel‐group, multiple‐arm study Date of study: 10 December 2013 (starting date) Location: India |
| Participants |
Randomised: 75 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention 1: acitretin: orally, 25 to 50 mg/day, daily single dose Total duration: 90 days Intervention 2: ciclosporin: orally 2.5 to 5 mg/kg/day, daily in 2 divided doses Total duration: 90 days Intervention 3: methotrexate: orally 7.5 to 15 mg/week in 3 divided doses Total duration: 90 days Control Intervention 1: palmo‐plantar psoriasis: variant of psoriasis in which only palms and soles are affected Control Intervention 2: psoriasis vulgaris: variant of psoriasis in which lesions appear on body skin |
| Outcomes | At 90 days Primary outcomes
Secondary outcomes
|
| Notes | Starting date: 10 December 2013. Recruitment status: open to recruitment (10 January 2020) Article published November 2020: Safety and efficacy profile of oral cyclosporine vs oral methotrexate vs oral acitretin in palmo‐plantar psoriasis: a hospital‐based prospective investigator blind randomised controlled comparative study, Samkit Shah, 2020 We asked the authors to provide the results for plaque‐type psoriasis Two emails were sent to Prof. Shah without response (5 and 12 January 2017) |
CTRI/2016/10/007345.
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: October 2016 Location: India Phase 3 |
| Participants |
Randomised: 231 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Apremilast 30 mg tablets: administered 1 tablet twice daily for 16 weeks Control intervention B. Placebo tablets: administered 1 tablet twice daily for 16 weeks |
| Outcomes | At week 16 Primary outcome
Secondary outcomes
|
| Notes | Unpublished Last checked in October 2022: last modified on CTRI 19 December 2017 Emails sent to Dr Piyush Agarwal, Amol Pendse (11 February 2020, 30 August 2021) |
CTRI/2020/10/028555.
| Methods | RCT, active‐controlled, investigator‐blinded study Date of study: October 2020 to August 2021 Location: India |
| Participants |
Randomised: 50 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1 A. Methotrexate oral 0.3 to 0.5 mg/kg body weight/week Intervention 2 B. Methotrexate injectable 0.3 to 0.5 mg/kg body weight/week |
| Outcomes | At 7th day, 30th day, 60th, 90th day Primary outcomes
Secondary outcomes
|
| Notes | Dr Pooja Kanumuru, pooja.kanumuru@yahoo.com Last modified on: November 2021 Recruitment status: completed Last check in October 2022 |
DRKS00000716.
| Methods | Randomised, active‐controlled, parallel‐group, simple blind study Date of study: 3 June 2008 (starting date) Location: Germany |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | At week 8: PASI DLQI Immunohistology At week 24: PASI DLQI Immunohistology |
| Notes | Starting date: 3 June 2008, Prof. Arnd Jacobi, Klinik für Dermatologie und Allergologie Philipps‐Universität Marburg Recruitment status on ICTRP search portal: complete; follow‐up complete Study closing (LPLV): 3 October 2010 We emailed Prof. Jacobi (5 January 2017) without response Last check in October 2022 |
EUCTR2010‐020168‐39‐DE.
| Methods | Randomised, placebo‐controlled, parallel‐group, double‐blind study Date of study: September 2010 to January 2012 Location: Germany Phase 2 |
| Participants |
Randomised: 252 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1: FP‐187 (dimethyl fumarate) at a daily dose of 750 mg divided in 3 doses (250 mg 3 times a day) Intervention 2: FP‐187 at a daily dose of 750 mg divided in 2 doses (375 mg twice‐daily) Intervention 3: FP‐187 at a daily dose of 500 mg divided in 2 doses (250 mg twice‐daily) Intervention 4: placebo |
| Outcomes |
Primary outcome:
Secondary outcome
|
| Notes | Recruitment status: completed Last update posted: December 2012 Study completion date on ClinicalTrials.gov: May 2012 Last checked in October 2022 NCT01230138 Contact: Peder M Andersen, MD Forward‐Pharma GmbH |
EUCTR2015‐005279‐25‐DE.
| Methods | Randomised, placebo‐controlled, parallel‐group, double‐blind study Date of study: September 2016 (starting date) Location: Germany Phase 2 |
| Participants |
Total sample size: 36 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. LEO 32731 (phosphodiesterase 4 inhibitor, Orismilast) 30 mg twice a day for 16 weeks Control Intervention B. Placebo |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes | Study completion date on ClinicalTrials.gov July 2017 Last update posted: August 2017 Last checked in October 2022 NCT02888236 Sandra Philipp, PhD, Charite University, Berlin, Germany; hautarzt.philipp@gmail.com Email sent to Pr Sandra Philipp (31 August 2021) without response UNION Therapeutics announces acquisition of Orismilast (UNI50001) compound class from LEO Pharma (LEO32731); we will wait for more information |
EUCTR2021‐003700‐41‐ES.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel study Date of study: December 2021 Location: Canada, Czechia, United Kingdom, Germany, Poland, Spain, Taiwan, France, Korea, United States, Japan Phase 2 |
| Participants |
Randomised: 255 participants Inclusion criteria
Exclusion criteria
|
| Interventions | JNJ‐77242113 (blocks the binding of interleukin 23 (IL‐23) to its receptor) tablet administered orally Intervention 1: JNJ‐77242113 dose 1 once‐daily Intervention 2: JNJ‐77242113 dose 2 once‐daily Intervention 3: JNJ‐77242113 dose 3 once‐daily Intervention 4: JNJ‐77242113 dose 1 twice‐daily Intervention 5: JNJ‐77242113 dose 3 twice‐daily Intervention 6: placebo |
| Outcomes | At week 16 Primary outcome
Secondary outcomes
|
| Notes | Funding: Janssen Research & Development NCT05223868 (FRONTIER 1) Recruitment status: active, not recruiting Waiting for more published information about the new drug JNJ‐77242113 Last check in October 2022 |
Goldust 2019.
| Methods | RCT study 4 were randomly assigned to receive combination therapy (efficacy assessments were performed) |
| Participants | Randomised : 48 patients with moderate‐to‐severe plaque psoriasis |
| Interventions |
Intervention 1: adalimumab SC 80 mg at weeks 1 and 2 then 40 mg every 2 weeks Intervention 2: no intervention or placebo ? Co‐intervention: methotrexate 15 mg to 20 mg a week |
| Outcomes | PASI Hospital Anxiety and Depression Scale |
| Notes | ABSTRACT Contact author: drmgjgoldust@gmail.com Email sent to Pr Goldust (31 August 2021) Last checked in October 2022 |
Han 2007.
| Methods | Randomised, double‐blind, active‐controlled study Date: not stated Location: China |
| Participants | No statement except a total number of participants (n = 144) |
| Interventions |
Intervention A. Recombinant human tumour necrosis factor receptor (50 mg/week) Control intervention B. Methotrexate (7.5 mg/week) |
| Outcomes |
At 12 weeks Proportion of PASI 50, PASI 75, PASI 90 |
| Notes |
Abstract in Journal of Clinical Dermatology 2007 (730‐2): "HAN Ling, FANG Xu, HUANG Qiong, YANG Qin‐ping, FU Wen‐wen, ZHENG Zhi‐zhong, GU Jun, SUN Jiao‐fang, XU Ai‐e (Department of Dermatology, Huashan Hospital, Fudan University, Shanghai 200040, China) Objective: To evaluate the effect of recombinant human tumour necrosis factor receptor (rhTNFR:Fc) in the treatment of moderate‐to‐severe plaque psoriasis on psoriasis area and severity index (PASI). Methods: Using randomised, double‐blind and double‐simulated, parallel‐controlled with positive drug, multicenter, clinical trial was employed to investigate 144 cases of patients with moderate‐to‐severe plaque psoriasis, of which there were 72 cases in both trial group and the control group respectively, to evaluate the effect on PASI. Results: 124 cases of patients had accomplished the 12‐week clinical trial. After 12 weeks the rate of PASI 50, PASI 75, PASI 90 were significantly higher than those of the control group (P < 0.01). The therapeutic effects on trunk and limbs of the trial group were also much better. Conclusion: The effect of rhTNFR:Fc is more quick and significant, especially assessed by PASI score." Abstract not available at the BIUM and United States NLM libraries No email address for the authors available When we searched Google, we found another abstract of the same study: "Chinese Journal of Dermatology 2007, 40(11) 655‐658" manu41.magtech.com.cn/Jwk_cmazp/EN/abstract/abstract11844.shtml#), which had no supplemental information to enable contacting the authors: "Abstract Objective To investigate the efficacy and tolerability of a recombinant human tumour necrosis factor:Fc fusion protein (rhTNFR:Fc,with a trade name of Yisaipu) in the treatment of moderate‐to‐severe psoriasis vulgaris. Methods A multicentre,randomised,double‐blind,and parallel‐controlled trial was performed. One hundred and forty‐four patients with moderate‐to‐severe psoriasis vulgaris from four centres were randomly assigned and treated with either once‐weekly subcutaneous injection of rhTNFR:Fc (50 mg) or oral methotrexate (methotrexate) (7.5 mg) for 12 weeks. Patients were followed up at 2, 4, 8, 12 weeks after the treatment. Results One hundred and twenty‐four patients finished the 12‐week course of treatment. At 12 weeks after the treatment, a 50%, 75%, 90% improvement in psoriasis area and severity index (PASI) was achieved by 86.11%, 76.39%, 52.78% respectively of rhTNFR:Fc‐treated patients,and by 63.89%, 44.44%, 22.22% respectively in methotrexate‐treated patients,and all the three improvement rates were of significant difference between the two groups of patients (all P < 0.01). Physician global assessment (PGA), dermatology life quality index (DLQI) and 10‐cm visual analogue scale (VAS) all reduced more significantly, and more patients were cured or approximately cured in rhTNFR:Fc‐treated patients than in MTX‐treated patients (all P < 0.05). Adverse reactions,mainly including decrease of leucocytes or neutrophils,infection, dysfunction of liver, oedema and pruritus at the injection site etc. occurred in 26.39% of rhTNFR:Fc‐treated patients and 29.17% of MTX‐treated patients (P > 0.05). Conclusion Compared with MTX,rhTNFR:Fc acts more quickly with a higher cure rate and less toxic reactions in the treatment of psoriasis vulgaris." No contact with the authors, as we could not find the authors' emails |
JPRN‐jRCT2061210069.
| Methods | Randomised, placebo‐controlled, parallel‐group, double‐blind study Date of study: November 2021 Location: USA, Canada, Japan Phase 2 |
| Participants |
Randomised: 200 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Interventions A. Cedirogant (ABBV‐157, small molecule inhibitor of RoRγT) receive dose A once daily orally B. Cedirogant (ABBV‐157, small molecule inhibitor of RoRγT) receive dose B once daily orally C. Cedirogant (ABBV‐157, small molecule inhibitor of RoRγT) receive dose C once daily orally Control intervention D. Placebo |
| Outcomes | Assessments at 16 weeks Primary outcome
Secondary outcomes
|
| Notes | Funding: AbbVie Estimated study completion date: March 2023 NCT05044234 Waiting for more published information about the new drug Cedirogant Last check in October 2022 |
KEEPsAKE‐1.
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: March 2019 Location: United States, Argentina, Australia, Belgium, Bosnia and Herzegovina, Brazil, Bulgaria, Canada, Chile, Croatia, Czechia Phase 4 |
| Participants |
Randomised: 964 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Risankizumab receive 150 mg administered by SC injection at week 0, week 4, and week 16 in period 1. At week 24 participants will receive blinded placebo followed by open‐label 150 mg risankizumab at week 28, and every 12 weeks thereafter in period 2 until the final dosing time point at week 208 Control Intervention B. Placebo receive double‐blind placebo at week 0, week 4, and week 16 in period 1. At week 24 participants will receive 150 mg risankizumab followed by open‐label 150 mg risankizumab at week 28, and every 12 weeks thereafter in period 2 until the final dosing time point at week 208. |
| Outcomes | At 24 weeks Primary outcomes Percentage of participants with an American College of Rheumatology 20% (ACR20) response Participants who met the following 3 conditions for improvement from baseline were classified as meeting the ACR20 response criteria:
Secondary outcomes
|
| Notes |
NCT03675308 Estimated study completion date: September 2024 Last Update posted: February 2022, active, not recruiting Funding: AbbVie We are waiting for subgroup analyses for moderate‐to‐severe psoriasis to be published Last check in October 2022 |
KEEPsAKE‐2.
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: March 2019 Location: worldwide Phase 3 |
| Participants |
Randomised: 444 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Risankizumab 150 mg SC injection at week 0, week 4, and week 16 in period 1. At week 24 participants will receive blinded placebo followed by open‐label 150 mg risankizumab at week 28, and every 12 weeks thereafter in period 2 until the final dosing time point at week 208. Control Intervention B. Placebo receive double‐blind placebo at week 0, week 4, and week 16 in period 1. At week 24 participants will receive 150 mg risankizumab followed by open‐label 150 mg risankizumab at week 28, and every 12 weeks thereafter in period 2 until the final dosing time point at week 208. |
| Outcomes | Assessments at 24 weeks Primary outcome Percentage of participants with an American College of Rheumatology 20% (ACR20) response Participants who met the following 3 conditions for improvement from baseline were classified as meeting the ACR20 response criteria:
Secondary outcomes
|
| Notes | Funding, Quote (ClinicalTrials.gov): "AbbVie" Recruitment status: active, not recruiting Estimated study completion date: Mach 2024 Last update posted: March 2, 2022 We are awaiting subgroup analyses for moderate‐to‐severe psoriasis to be published Last check in October 2022 |
Krishna 2016.
| Methods | RCT, active‐controlled, double‐blind study Date of study: November 2013 to January 2015 Location: India Phase 4 |
| Participants |
Randomised: 50 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Methotrexate 10 mg/week Control intervention B. Methotrexate 25 mg/week |
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
| Notes | On ClinicalTrials.gov (NCT02248792) Recruitment status: unknown; verified September 2014 by C. V. Krishna, Narayana Medical College & Hospital Recruitment status was: recruiting Emails sent to Prof. Krishna (5 and 12 January 2017; 11 February 2020) Last checked in October 2022 |
Makavos 2020.
| Methods | RCT, active‐controlled, open study Date of study: not stated Location: not stated |
| Participants |
Randomised: 150 participants, mean age 52; 92 men Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
| Interventions |
Intervention A. Secukinumab, 300 mg SC, W0, 1, 2, 3, 4 and 300 mg once‐monthly Control intervention B. Ciclosporin, 2.5 to 3 mg/kg daily C. Methotrexate (non‐randomised controlled group, n = 50) |
| Outcomes | Assessments at 16 weeks Primary outcome
Secondary outcomes
|
| Notes | Authors were asked whether:
An email was sent without response to Pr Ikonomidis (30 October 2020, 10 September 2021) Last checked in October 2022 |
Mrowietz 2005.
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: not stated Location: not stated |
| Participants |
Randomised: 175 participants (characteristics not stated) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
| Interventions |
Intervention A. Dimethyl fumarate (n = 105), orally, 240 mg, 3 times/day; 16 weeks Control Intervention B. Placebo (n = 70), orally, 2 capsules, 3 times/day; 16 weeks |
| Outcomes | Assessments at 16 weeks Primary outcome
Secondary outcomes
|
| Notes | Funding, quote (abstract) by Biogen Idec, Inc and Fumapharm Abstract: “Results of a phase III study of a novel oral formulation of dimethyl fumarate in the treatment of moderate‐to‐severe plaque psoriasis: efficacy, safety, and quality of life effects” published in 2005 in the JEADV, Suppl. 2 (Poster P/06.97) We asked the study authors to provide the protocol and results by email. Additional data for the publication not provided Finally, as the risk of bias tool assessment was not possible and there were missing data for the results, Mrowietz 2005 was placed in Studies awaiting classification |
NCT01088165.
| Methods | RCT, active‐controlled, triple‐blind study Date of study: May 2010 ‐ end date not reported Location: Austria Phase 4 |
| Participants |
Randomised: 66 participants Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
| Interventions |
Intervention A. Adalimumab treatment arm: day 1: 2 x 40 mg SC, day 8: 40 mg SC, thereafter 40 mg SC at bi‐weekly intervals Control Interventions B. Fumaric acid esters treatment group C. Narrow‐band UVB radiation |
| Outcomes | Assessments at 12 weeks Primary outcomes
Secondary outcomes
|
| Notes | Funding, Quote (ClinicalTrials.gov, NCT01088165): "by Medical University of Vienna" Recruitment status: unknown; verified January 2012 by Gregor Holzer, Medical University of Vienna Estimated study completion date: May 2014 Last update posted: January 2012 We sent an email to Prof. Holzer to be sure whether this trial is still ongoing (3 June 2019 and 11 February 2020) without response Gregor Holzer, MD gregor.holzer@meduniwien.ac.at Last check in October 2022 |
NCT01558310.
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: March 2012 Location: USA Phase 4 |
| Participants |
Randomised: 30 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Ustekinumab (at weeks 0, 4, 16, 28, and week 40) and placebo (at weeks 12 and 52). The participants when assigned to ustekinumab, depending on body weight, will receive either 45 mg or 9 mg ustekinumab doses Control intervention B. Placebo |
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
| Notes | On ClinicalTrials.gov : Recruitment status: unknown; verified July 2012 by Paul Steven Yamauchi, MD, PhD, Yamauchi, Paul Steven, M.D., Ph.D. Not yet recruiting Estimated study completion date: December 2013 We emailed Dr Yamauchi (5 and 12 January 2017) Email response: "Dear Dr Sbidian, Thank you for your kind email, forwarded to me by Dr Paul Yamauchi, MD, PhD. Our 'Study to Evaluate the Effectiveness of STELARA ™ (USTEKINUMAB) in the Treatment of Scalp Psoriasis (NCT 01558310)' completed enrolment in December 2016 and the last subject will complete in December 2017; as such we do not have the final data analysis. What is your absolute cut‐off for publication data? Would an interim analysis report be acceptable? Best regards, Rickie Patnaik Director, Clinical Science Institute" Will be included when published paulyamauchi@yahoo.com Last checked in October 2022 |
NCT02655705.
| Methods | RCT, placebo‐controlled, open‐label study Date of study: September 2015 ‐ end date not reported Location: Korea Phase 4 |
| Participants |
Randomised: 34 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Ciclosporin A (men 200 mg/day, women 150 mg/day for 16 weeks) Control intervention B. Methotrexate (initial dose 10 mg/week, increasing 2.5 mg every 2 weeks up to 15 mg/week) |
| Outcomes | At week 16 Primary outcome
Secondary outcomes
|
| Notes | Published articles without outcomes of interest Last update posted: April 2016 Emails sent to Pr Sang Woong Youn, Seoul National University Hospital (3 June 2019 and 11 February 2020) Last checked in October 2022 |
NCT02701205.
| Methods | RCT, placebo and active‐controlled, double‐blind study Date of study: January 2015 Location: China Phase 3 |
| Participants |
Randomised: 216 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Recombinant human TNF receptor‐Ig Fusion protein for injection (Qiangke®) 50 mg twice a week by subcutaneous injection for 12 weeks. At the end of the first 12 weeks, all subjects will be treated with recombinant human TNF receptor‐Ig fusion protein for injection (Qiangke®) 50 mg once a week for an additional 12 weeks Control intervention B. Recombinant human TNF receptor‐Ig fusion protein for injection (Qiangke®) 25 mg twice a week by subcutaneous injection for 12 weeks. At the end of the first 12 weeks, all participants will be treated with recombinant human TNF receptor‐Ig fusion protein for injection (Qiangke®) 50 mg once a week for an additional 12 weeks C. Placebo |
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
| Notes | Unpublished Recruitment status: unknown Last update posted: March 2016 Estimated study completion date: December 2017 Emails sent to Prof Hongzhong Jin (3 June 2019 and 11 February 2020 (not delivered), 30 August 2021) Last checked in October 2022 |
NCT02714322.
| Methods | RCT, active‐controlled, double‐blind study Date of study: June 2015 Location: Russia, Estonia, Hungary, Poland, Bulgaria Phase 3 |
| Participants |
Randomised: 294 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Biological: MYL‐1401A (adalimumab) initial dose of 80 mg administered SC, followed by 40 mg SC given every other week starting 1 week after the initial dose Control intervention B. Humira® (adalimumab) initial dose of 80 mg administered SC, followed by 40 mg SC given every other week starting 1 week after the initial dose |
| Outcomes | At week 12 Primary outcome
Secondary outcome
|
| Notes | Recruitment status: completed Last update posted: March 2022 Actual study completion date: March 2017 Abhijit Barve, MD Mylan Waiting for results publication Last checked in October 2022 |
NCT02829424.
| Methods | RCT, active‐controlled, double‐blind study Date of study: April 2016 Location: France Phase 4 |
| Participants |
Randomised: 330 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Methotrexate (low dose) Control interventions B. Placebo Co‐intervention: anti‐TNF agent |
| Outcomes | At week 24 Primary outcome
Secondary outcomes
|
| Notes | Unpublished Recruitment status: unknown Last update posted: July 2016 Last checked in October 2022 |
ABT‐874: name of a monoclonal anti–interleukin 12/23 antibody ACR50: American College of Rheumatology response criteria, ACR 50: 50% improvement from baseline ACR ADA: adalimumab AEs: adverse effects AIDS: acquired immunodeficiency syndrome ALT: aspartate transaminase AST: alanine transaminase BCG: bacille Calmette‐Guérin B‐HCG: beta‐human chorionic gonadotropin BID: two times a day BMI: body mass index BSA: body surface area CASPAR: classification criteria for psoriatic arthritis CIN: cervical intraepithelial neoplasia CPDAI: composite psoriatic disease activity index CRP: c‐reactive protein csDMARD: conventional systemic disease‐modifying antirheumatic drugs DAS‐28: disease activity score‐28 (measure of disease activity in rheumatoid arthritis) DLQI: Dermatology Life Quality Index DMF: dimethylformamide DNA: deoxyribonucleic acid ECG: electrocardiogram eow: every other week FAEs: fumaric acid esters FMD: flow‐mediated dilatation FP‐187: forward‐pharma‐187 is a fumaric acid ester FSH: follicle‐stimulating hormone GRAPPA: group for research and assessment of psoriasis and psoriatic arthritis HAQ‐DI: health assessment questionnaire disability index HBcAb: hepatitis B core antibody HBsAb: hepatitis B surface antibody HBsAg: hepatitis B surface antigen HBV: hepatitis B virus HCV: hepatitis C virus HIV: human immunodeficiency virus HS626: infliximab biosimilar ICTRP: international clinical trials registry platform IGA: Investigator's Global Assessment IL(‐12/‐17/‐23): interleukin‐12, ‐17, ‐23 IM: intramuscular IMT: intima‐media thickness ISA247: voclosporin (immunosuppressive agent) IUD: intrauterine device IV: intravenous LAD: left anterior descending coronary artery LDI‐B: Leeds Dactylitis Index‐Basic LEI: Leeds Enthesitis Index LEO 32731: phosphodiesterase‐4 inhibitor MDA: minimal disease activity mPASI: modified PASI MTX: methotrexate MYL‐1401A: adalimumab biosimilar NAPSI: Nail Psoriasis Severity Index NRS: numeric rating scale NYHA: New‐York Heart Association OMERACT: international, informally organised network which is an independent initiative of international stakeholders interested in outcome measurement OPD: outpatient department PaGA: Patient Global Assessment PASI: Psoriasis Area and Severity Index PDE‐4: phosphodiesterase‐4 PGA: Physician's Global Assessment PPD: purified protein derivative PPQOL: palmoplantar quality of life instrument score Ps: psoriasis PsA: psoriatic arthritis PsARC: psoriatic arthritis response criteria PsASon13: unilateral score compromised 13 joints PSS: psoriasis symptom scale PUVA: psoralen plus ultraviolet A Q2W: every other week RCT: randomised controlled trial rhTNFR:FC: tumour necrosis factor receptor: fusion protein SC: subcutaneous SF‐36: short‐form 36 SJC: swollen joint count SKINDEX: quality of life index for patients with skin diseases SmPC: summaries of product characteristics SPARCC: Spondyloarthritis Research Consortium of Canada score SPGA: Static Physician Global Assessment TB: tuberculosis TGP: glutamate‐pyruvate transaminase TID: three times a day TJC: The Joint Commission TNF: tumour necrosis factor ULN: upper limit of normal UVB: ultraviolet B VAG: visual grading analysis VAS: visual analogue scale W (followed by number): week Please note that the term “conventional” in these tables is replaced with “non‐biological treatment” in the main text of this review.
Characteristics of ongoing studies [ordered by study ID]
Alexis 2022.
| Study name | Study design of a phase 3b, multicenter, randomized, double‐blind, placebo‐controlled trial of guselkumab (GUS) in patients with skin of color who have moderate to severe plaque and/or scalp psoriasis (VISIBLE) |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: July 2022 Location: USA (multicentre) Phase 3 |
| Participants |
Randomised: 200 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Guselkumab, dosage not stated Control intervention B. Placebo |
| Outcomes | At week 16 Primary outcomes
Secondary outcomes
|
| Starting date | Actual study start date: July 2022 Estimated primary completion date: September 2023 Last update posted: October 2022, recruiting |
| Contact information | Not stated |
| Notes | Funding: Janssen Research & Development, LLC NCT05272150 Last checked in October 2022 |
ChiCTR2000029262.
| Study name | Clinical study on the relationship between SLC35 gene variation and psoriasis |
| Methods | Randomised, parallel‐group, active‐controlled, open‐label study Date of study: February 2020 Location: China (multicentre) Phase 4 |
| Participants |
Randomised: 360 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1: acitretin Intervention 2: oral methotrexate Intervention 3: ciclosporin |
| Outcomes |
Primary outcomes:
|
| Starting date | Date of study: February 2020 Estimated primary completion date: January 2021 Last modified on: February 2020, not yet recruiting |
| Contact information | Wang Xiaohua wxh_21773@163.com 2 Lujing Road, Yuexiu District, Guangzhou, Guangdong, China |
| Notes | Funding: Dermatology Hospital of Southern Medical University Last checked in October 2022 |
ChiCTR2000036186.
| Study name | A multi‐center clinical study of systemic treatment strategies for psoriasis in Chinese population |
| Methods | Randomised, parallel‐group, active‐controlled study Date of study: January 2021 Location: China (multicentre) Phase 4 |
| Participants |
Randomised: 1600 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1: acitretin Intervention 2: oral methotrexate Intervention 3: NB‐UVB treatment Intervention 4: biologics |
| Outcomes | PASI |
| Starting date | Date of study: January 2021 Estimated primary completion date: — Last modified on: August 2020, not yet recruiting |
| Contact information | Yuling Shi shiyuling1973@126.com 1278 Baode Road, Jing'an District, Shanghai |
| Notes | Funding: Shanghai Skin Disease Hospital Last checked in October 2022 |
ChiCTR2000039699.
| Study name | A phase 4 clinical study to evaluate the efficacy and safety of induction and maintenance therapy of brodalumab (KHK4827) in subjects with moderate to severe plaque psoriasis |
| Methods | Randomised, parallel‐group, placebo‐controlled, double‐blind study Date of study: November 2020 Location: China Phase 4 |
| Participants |
Randomised: 400 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Brodalumab Injection (KHK4827) 210 mg Q2W, n = 300 Control intervention B. Placebo, n = 100 |
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
| Starting date | Date of study: November 2020 Estimated primary completion date: November 2023 Last modified on: February 2021, recruiting |
| Contact information | Zhang Jianzhong rmzjz@126.com |
| Notes | Funding: Peking University People's Hospital Last checked in October 2022 |
ChiCTR2100045970.
| Study name | A phase 3, multicenter, randomized, double‐blind study evaluating the efficacy and safety of QX001S compared with ustekinumab in subjects with moderate to severe plaque psoriasis |
| Methods | RCT, double‐blind, active‐controlled study Date of study: April 2021 Location: China (multicentre) Phase 3 |
| Participants |
Randomised: 216 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. QX001S injection (ustekinumab biosimilar) Control intervention B. Ustekinumab injection |
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
| Starting date | Actual study start date: April 2021 Estimated primary completion date: April 2023 Last update posted: December 2021, recruiting |
| Contact information | Zhang Jianzhong Rmzjz@126.com |
| Notes | Funding: Peking University People's Hospital Last check in October 2022 |
CTRI/2019/07/020274.
| Study name | Comparative efficacy of methotrexate, apremilast and their combination in psoriasis vulgaris |
| Methods | Randomised, parallel‐group, multiple‐arm study Date of study: July 2019 Location: India |
| Participants |
Randomised: 30 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1: apremilast 30 mg twice a day, starting at 10 mg/day with an increment of 10 mg/day over 5 days, for 8 weeks Intervention 2: oral methotrexate 0.2 mg/kg/week, maximum 25 mg/week for 8 weeks Intervention 3: oral methotrexate 0.2 mg/kg/week, maximum 25 mg/week along with oral apremilast 30 mg twice a day, starting at 10 mg/day with an increment of 10 mg/day over 5 days, for 8 weeks |
| Outcomes |
Primary outcome: To compare the efficacy of apremilast and methotrexate and their combination in patients with psoriasis vulgaris by comparing the PASI score before and after start of the therapy at 0, 2, 4, 6, 8 weeks Secondary outcome: To assess the safety of all the 3 treatment modalities by assessing the side effects at 0, 2, 4, 6, 8 weeks |
| Starting date | Date of first enrolment: July 2019 Last modified on: 22 July 2019, estimation duration of trial 1 year, not yet recruiting |
| Contact information | Dr Nainika Goel Government Medical College and Hospital, Chandigarh
Address Department of Dermatology, D block, 5th floor, GMCH, sector 32, Chandigarh
Chandigarh
CHANDIGARH
160030 dr.nainika1311@gmail.com |
| Notes | Last checked in October 2022 |
CTRI/2020/02/023107.
| Study name | Prospective, multi‐center, randomized, double‐blind, two‐arm, parallel group, active control, comparative clinical study to evaluate efficacy and safety of R‐TPR‐046/Stelara® in patients with moderate‐to‐severe plaque psoriasis |
| Methods | Randomized, parallel‐group, active controlled trial Date of trial: September 2020 Location: India Phase 3 |
| Participants |
Randomised : 220 participants Inclusion criteria:
Exclusion criteria
|
| Interventions |
Intervention A. R‐TPR‐046 (ustekinumab biosimilar) at week 0, week 4, week 16, week 28, and week 40 Control intervention B. Ustekinumab at week 0, week 4, week 16, week 28, and week 40 |
| Outcomes | At week 12 Primary outcome
At week 52 Secondary outcomes
|
| Starting date | Date of trial: September 2020 Last modified: March 2022, closed to recruitment of participants |
| Contact information | Dr Supriya Sonowal Reliance Life Sciences Pvt. Ltd. Address : RLS Bio ‐ Product Trials Group, Dhirubhai Ambani Life Sciences Centre (DALC) R‐282, TTC Area of MIDC, Thane ‐ Belapur Road, Rabale, Navi Mumbai ‐ 400701, India Thane MAHARASHTRA 400701, India Thane MAHARASHTRA 400701 India supriya.sonowal@relbio.com |
| Notes | Last checked in October 2022 |
CTRI/2022/01/039825.
| Study name | Comparative study of oral methotrexate vs oral methotrexate along with apremilast in patients with moderate to severe chronic plaque psoriasis (CSOM‐MAiP) |
| Methods | RCT, active‐controlled, open label study Date of study: February 2022 Location: China (one centre) Phase ? |
| Participants |
Randomised: 30 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Oral apremilast (starting from 10 once‐daily increasing up to 30 mg twice‐daily). Control intervention B. No treatment Co‐intervention: oral methotrexate (15 mg/week). If needed doses can be increased up to max of 22.5 mg/week or decreased or stopped |
| Outcomes | At baseline, then monthly up to 16 weeks Primary outcomes
Secondary outcomes
|
| Starting date | Actual study start date: February 2022 Last update posted: January 2022, not yet recruiting |
| Contact information | Dr Aanal Patel GMERS Medical College & Civil Hospital, Sola aanal2607@gmail.com |
| Notes | Funding: GMERS Medical College and CIvil Hospital Sola (government medical college) Last check in October 2022 |
Dong 2020.
| Study name | Pharmacokinetics, tolerability, immunogenicity, dose increasing safety and evaluate of preliminary effect clinical trial on GR1501 injection in patients with plaque psoriasis |
| Methods | Randomised, parallel, double‐blind study Date of study: August 2018 to August 2020 Location: China Phase I/II |
| Participants |
Randomised: 46 participants Inclusion criteria:
Exclusion criteria
|
| Interventions |
Intervention A. GR1501 (monoclonal antibody IL‐17A) Control intervention B. Placebo |
| Outcomes |
Primary outcomes
At week 12 Secondary outcomes
|
| Starting date | Starting date: August 2018 Date last refreshed on: August 2018 (recruiting status: recruiting) |
| Contact information | Yi Fang, phaseistudy@163.com Study leader: Jianzhong Zhang, rmzjz@126.com |
| Notes | Funding: Chinese Society of Academic Degrees and Graduate Education 'the Project of Research of Degree and Master Education' (No:2019YX01) and Genrix (Shanghai) Biopharmaceutical Co Completed according to the protocol ChiCTR1800017956 Last checked in October 2022 |
EUCTR2017‐001615‐36‐DE.
| Study name | Study to evaluate ABY‐035 in subjects with moderate‐to‐severe plaque psoriasis (AFFIRM‐35) |
| Methods | Randomised, placebo‐controlled, parallel‐group, double‐blind study (AFFIRM‐35) Date of study: March 2018 Location: Germany (multicentre) Phase 2 |
| Participants |
Randomised: 108 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention A. 2 mg ABY‐035 (izokibep anti‐IL17A) SC 12 weeks B. 20 mg ABY‐035 SC 12 weeks C. 80 mg ABY‐035 SC 12 weeks D. 160 mg ABY‐035 SC 12 weeks Control Intervention E. Placebo 12 weeks After the first 12 weeks of treatment, the participants randomised to placebo will receive active treatment. The dose levels and dosing intervals are adjusted depending on the absolute PASI score, to obtain an individualised treatment regimen |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Actual study start date: March 2018 Actual study completion date: November 2021 Last update posted: April 2022, completed |
| Contact information | sgerdes@dermatology.uni‐kiel.de Sascha Gerdes, Dr. Med |
| Notes |
NCT03591887 Funding: Affibody Last checked in October 2022 |
EUCTR2017‐001695‐26‐IT.
| Study name | Monitoring the effectiveness and safety of biological drugs for treatment of psoriasis through evaluation of clinical and biological markers |
| Methods | RCT, active‐controlled, open‐label study Date of study: November 2020 Location: Italy (multicentre) Phase 4 |
| Participants |
Randomised: 154 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Etanercept, SC Control intervention B. Ustekinumab, SC |
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
| Starting date | Date of study: November 2020 Trial status: ongoing |
| Contact information | Claudio Bonifati claudio.bonifati@ifo.gov.it |
| Notes | Funding: Hospital Physiotherapy Institutes Last check in October 2022 |
EUCTR2018‐001238‐16‐FR.
| Study name | A study to evaluate further therapeutic strategies with guselkumab in participants with moderate‐to‐severe plaque‐type psoriasis (GUIDE) |
| Methods | RCT, double‐blind, parallel‐group, placebo‐controlled multicentre study Date of study: February 2019 Location: France, Germany Phase 3 |
| Participants |
Randomised: 880 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention A. Guselkumab 100 mg subcutaneously at weeks 0, 4, 12, and 20 Control intervention B. Placebo Then re‐randomisation |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Actual study start date: February 2019 Estimated study completion date: June 2025 Last update posted: October 2022, active, not recruiting |
| Contact information | JNJ.CT@sylogent.com |
| Notes |
NCT03818035 Funding: Jansssen‐Cilag Germany Last checked in October 2022 |
EUCTR2020‐005205‐42‐DE.
| Study name | A study to investigate interchangeability of ABP 654 for the treatment of subjects with moderate‐to‐severe plaque psoriasis |
| Methods | RCT, active‐controlled, double‐blind study Date of study: March 2021 Location: USA, Canada, Estonia, Germany, Georgia, Latvia, Hungary, Poland, Spain (worldwide) Phase 3 |
| Participants |
Randomised: 494 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Switching group (ustekinumab ‐ ABP 654); participants will initially receive injection of ustekinumab up to week 16. Thereafter, starting from week 28, participants will switch between ABP 654 and ustekinumab every 12 weeks up to week 52. Control intervention B. Continued‐use group ustekinumab SC from day 1 to week 52 |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | Study start date: March 2021 Estimated study completion date: March 2023 Last update posted: December 2021, active, not recruiting |
| Contact information | Amgen Call Center 866‐572‐6436medinfo@amgen.com |
| Notes |
NCT04761627 Last check in October 2022 |
EUCTR2021‐003209‐22‐DE.
| Study name | Study to assess the efficacy and safety of orismilast in psoriasis (IASOS) |
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: December 2021 Location: USA, Germany, Poland, United Kingdom Phase 2 |
| Participants |
Randomised: 202 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Interventions A. Orismilast (PDE4 inhibitor) modified release tablets 20 mg, orally, twice‐daily morning and evening B. Orismilast modified release tablets 30 mg, orally, twice‐daily morning and evening C. Orismilast modified release tablets 40 mg, orally, twice‐daily morning and evening Control intervention D. Placebo |
| Outcomes | At week 16 Primary outcome Percent change from baseline in Psoriasis Activity and Severity Index (PASI) score Secondary outcomes
|
| Starting date | Study start date: December 2021 Estimated primary completion date: December 2022 Last update posted: August 2022, active, not recruiting |
| Contact information | P. A., MD UNION therapeutics A/S |
| Notes | Funding: Quote (clinicaltrials.gov): "UNION therapeutics" NCT05190419 Last check in October 2022 |
Holsken 2021.
| Study name | Expectation‐induced enhancement of pain, itch and quality of life in psoriasis patients |
| Methods | RCT, active‐controlled study Date of study: November 2020 Location: Sweden |
| Participants |
Randomised: 120 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Group 1 pharmacological control: secukinumab (as in clinical routine consisting of 2 subcutaneous injections with 150 mg) for 5 weeks (weeks 0 to 4), followed by 2 additional injections 4 (week 8) and 8 (week 12) weeks later, n = 40 Expectation‐LOW group 2: secukinumab 75 mg as 1 injection and 1 injection with NaCl, n = 40 Expectation‐HIGH group 3: secukinumab 75 mg as one injection and an additional positive verbal instruction presented in a standardised manner by the main study physician to strengthen their expectation towards the benefits of the treatment. The salience of this verbal instruction will be reinforced by combining each injection with the ingestion of a newly tasting beverage together with detailed information about the potential beneficial effects of this combination, n = 40 |
| Outcomes | At 16 weeks Primary outcomes
Secondary outcomes
|
| Starting date | Date of study: November 2020‐2023 |
| Contact information | Dr. Wiebke Sondermann; wiebke.sondermann@uk‐essen.de |
| Notes | Funding: "Gefördert durch die Deutsche Forschungsgemeinschaft (DFG)— Projektnummer 422744262—TRR 289—Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project‐ID 422744262—TRR 289)." Declarations of interest: "WS reports grants from medi Bayreuth, personal fees from Janssen, grants and personal fees from Novartis, personal fees from Lilly UCB, Almirall, LEO Pharma and Sanofi Genzyme, outside the submitted work. The other authors declare that they have no competing interests." Last checked in October 2022 |
IRCT20100102002954N26.
| Study name | Comparison of the efficacy of adalimumab and methotrexate in cases of moderate to sever of psoriasis, a double‐blind clinical trial |
| Methods | RCT, active‐controlled, double‐blind study Date of study: March 2022 Location: Iran Phase 2/3 |
| Participants |
Randomised: 92 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Adalimumab 80 mg subcutaneously to begin and then 40 mg a week later as subcutaneously and then 40 mg subcutaneously every 2 weeks for 16 weeks. In addition, routine treatment (including the use of glycerin topical lotion 10% and 25 mg hydroxyzine tablets 3 times a day) Control intervention B. Methotrexate 20 mg intramuscularly to start and then 10 mg intramuscularly 1 week later and then 10 mg intramuscularly administered every 2 weeks for 16 weeks. In addition, routine treatment (including topical 10% glycerin lotion and 25 mg hydroxyzine tablets three times daily) |
| Outcomes | At week 16 Primary outcomes
Secondary outcomes
|
| Starting date | Expected recruitment start date: March 2022 Expected recruitment end date: July 2022 Last update posted: March 2022, recruiting |
| Contact information | Dr. Fateme Torabi Imam Hossein Hospital, End Imam street, Shahroud, Iran. 3616611151 ShahroudIran (Islamic Republic of) Telephone: +98 23 3234 2000 Email: ftrb4524@gmail.com |
| Notes | Funding: Shahroud University of Medical Sciences Last check in October 2022 |
IRCT20120524009844N8.
| Study name | Therapeutic effects of adalimumab in patients with resistant psoriasis patients by DLQI and PASI questionnaires |
| Methods | Randomised, parallel, open, active‐controlled study Date of study: July 2020 Location: Iran Phase 3 |
| Participants |
Randomised: 60 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention A. Adalimumab 80 mg. The next dose will be 1 week later (40 mg) and then every 2 weeks (40 mg) (the total duration of treatment will be 2 months). Control intervention B. Methotrexate and cyclosporine at a specific dose determined by a dermatologist |
| Outcomes |
Primary outcomes
|
| Starting date | Actual recruitment start date: July 2020 Trial completion date: November 2020, recruitment complete Last update: October 2020 |
| Contact information | Pr Mehdi Amirnia amirniam@tbzmed.ac.ir |
| Notes | Funding: Tabriz University of Medical Sciences Last checked in October 2022 |
NCT02258282.
| Study name | Safety and efficacy of etanercept in patients with psoriasis |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: May 2014 Location: China (1 centre) Phase 4 |
| Participants |
Randomised: 80 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Etanercept (participants under the treatment of 50 mg etanercept) Control intervention B. Placebo |
| Outcomes | At week 24 Primary outcome
Secondary outcomes
|
| Starting date | Study start date: May 2014 Estimated primary completion date: December 2022 Last update posted: April 2017, active, not recruiting |
| Contact information | Yang Min, Ph.D, Chengdu PLA General Hospital |
| Notes | Funding: Chengdu PLA General Hospital Last checked in October 2022 |
NCT03478280.
| Study name | Effect of brodalumab compared to placebo on vascular inflammation in moderate‐to‐severe psoriasis |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: September 2018 Location: Aarhus University Hospital, Denmark Phase 4 |
| Participants |
Randomised: 50 participants Inclusion criteria
Exclusion criteria Non‐Danish speaking |
| Interventions |
Intervention A. Participants will receive 210 mg of Kyntheum administered by subcutaneous injection at weeks 0, 1, and 2 followed by 210 mg every other week (eow) thereafter Control interventions B. Placebo |
| Outcomes | At week 16 Primary outcome Average of maximum TBR values (mean TBRmax) of the entire aorta at baseline and at week 16 (aortic wall inflammation) Secondary outcome The splenic inflammation at baseline and at week 16 in brodalumab‐treated psoriasis participants compared to placebo (time frame: 16 weeks); the spleen‐to‐liver ratio (SLR) based on splenic and liver mean standardised uptake values (SUVmean) |
| Starting date | Study start date: September 2018 Estimated study completion date: March 2020 Last update posted: July 2019, unknown |
| Contact information | Contact: Anne Bregnhøj, MD, PhD, Aarhus University Hospital; +45 2183 5720 annebreg@rm.dk Email sent to Pr Anne Bregnhøj (31 August 2020) |
| Notes | Funding: Aarhus University Hospital‐LEO Pharma Last checked in October 2022 |
NCT03897075.
| Study name | Efficacy and safety study of tildrakizumab in the treatment of nail psoriasis |
| Methods | RCT, parallel‐arm, double‐blind, placebo‐controlled multicentric study Date of study: May 2021 Location: USA, Australia Phase 3 |
| Participants |
Randomised: 210 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention A. Tildrakizumab Comparator B. Placebo |
| Outcomes |
Primary outcomes
Secondary outcomes
Other Outcomes
|
| Starting date | Actual study start date: May 2021 Estimated study completion date: February 2024 Last update posted: August 2022, recruiting |
| Contact information | Head, Clinical development; 91 2266455645clinical.trials@sparcmail.com |
| Notes | Funding: Sun Pharma Global FZE Last checked in October 2022 |
NCT03897088.
| Study name | Efficacy and safety of tildrakizumab in the treatment of scalp psoriasis |
| Methods | RCT, multicentre, double‐blind, placebo‐controlled study Date of study: May 2019 Location: USA, Australia Phase 3 |
| Participants |
Randomised: 231 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention A. Tildrakizumab Comparator B. Placebo |
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcome
|
| Starting date | Actual study start date: May 2019 Actual study completion date: February 2022 Last update posted: August 2022, completed |
| Contact information | Head, Clinical Development; +91 2266455645; clinical.trials@sparcmail.com |
| Notes | Funding: Sun Pharma Global FZE Last checked in October 2022 |
NCT03927352.
| Study name | A phase 3, randomized, double‐blind study evaluating the efficacy and safety of SCT630 compared with adalimumab in subjects with moderate to severe plaque psoriasis |
| Methods | RCT, parallel‐arm, active‐controlled, double‐blind study Date of study: September 2019 Location: China (1 centre) Phase 3 |
| Participants |
Randomised: 330 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention A. SCT630 (biosimilar adalimumab) 80 mg subcutaneously on week 1/day 1 (initial loading dose) and 40 mg at week 2 and every 2 weeks thereafter until week 16 Participants with a PASI 50 response at week 16 continued to receive 40 mg SCT630 until week 48 Control Intervention B. Adalimumab 80 mg subcutaneously on week 1/day 1 (initial loading dose) and 40 mg at week 2 and every 2 weeks thereafter until week 16 At week 16 participants with a PASI 50 response were re‐randomised to treatment with adalimumab or were transitioned to SCT630 until week 48 |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Actual study start date: September 2019 Estimated study completion date: December 2022 Last update posted: January 2021, active, not recruiting |
| Contact information | Guo Ming +86‐10‐58628288‐9138; ming_guo@sinocelltech.com |
| Notes | Funding: Sinocelltech Ltd. Last checked in October 2022 |
NCT04102241.
| Study name | Efficacy and safety study of hemay005 in subjects with moderate to severe plaque psoriasis |
| Methods | RCT, double‐blind, placebo‐controlled, parallel‐arm study Location: China (one centre) Phase 2 |
| Participants |
Randomised: 216 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Interventions A. Hemay005 15 mg twice‐daily for 16 weeks B. Hemay005 30 mg twice‐daily for 16 weeks C. Hemay005 60 mg twice‐daily for 16 weeks Control intervention D. Placebo |
| Outcomes | At 16 weeks Primary outcome
Secondary outcomes
|
| Starting date | Actual study start date: May 2019 Actual study completion date: July 2021 Last update posted: October 2021, completed |
| Contact information | Principal Investigator: Min Zheng, Dr Second Affiliated Hospital, School of Medicine, Zhejiang University |
| Notes | Funding: Tianjin Hemay Pharmaceutical Co., Ltd Last check in October 2022 |
NCT04237116.
| Study name | A study of secukinumab treatment in patients with plaque psoriasis and co‐existing non‐alcoholic fatty liver disease (NAFLD) (pINPOINt) |
| Methods | RCT, double‐blind, parallel‐arm, placebo‐controlled, multicentric study Date of study: February 2020 Location: Germany, Spain Phase 3 |
| Participants |
Randomised: 10 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention A. Secukinumab 300 mg SC weekly in first 4 weeks, followed by every 4 weeks up to week 20; and placebo 300 mg SC at weeks 13, 14, and 15 to maintain the blind Control Intervention B. Placebo 300 mg SC weekly in first 4 weeks, followed by every 4 weeks up to week 8; and secukinumab 300 mg SC weekly for 4 weeks starting at week 12, followed by every 4 weeks up to week 20 |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Actual study start date: February 2020 Actual study completion date: July 2021 Last update posted: January 2022, completed |
| Contact information | Novartis Pharmaceuticals, novartis.email@novartis.com |
| Notes | Funding: Novartis Pharmaceuticals Results submitted to ClinicalTrials.gov: June 2022 Last checked in October 2022 |
NCT04306315.
| Study name | Adjusted brodalumab dose compared with standard brodalumab dose in subjects with moderate‐to‐severe plaque psoriasis and ≥ 120 kg body weight (ADJUST) |
| Methods | RCT, placebo‐controlled, double‐blind, parallel‐arm study Date of location: July 2022 Location: Spain Phase 4 |
| Participants |
Randomised: 384 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention A. Brodalumab 210 mg + brodalumab 70 mg add‐on (subcutaneously at week 0, week 1, and week 2, and then once every 2 weeks. Participants not fulfilling a predefined response at any visit with efficacy assessments after week 16 will receive a dose adjustment to 280 mg brodalumab every 2 weeks) Control intervention B. Brodalumab 210 mg + placebo add‐on (subcutaneously at week 0, week 1, and week 2, and then once every 2 weeks. Participants not fulfilling a predefined response at any time visit with efficacy assessments week 16 will receive a dose adjustment to 210 mg brodalumab + placebo every 2 weeks) |
| Outcomes |
Primary outcome :
Secondary outcomes :
|
| Starting date | Actual study start date: June 2022 Estimated study completion date: November 2025 Last update posted: August 2022, recruiting |
| Contact information | LEO Pharma, raleodk@leo‐pharma.com |
| Notes | Funding: LEO Pharma Last checked in October 2022 |
NCT04394936.
| Study name | An explorative psoriasis biomarker study |
| Methods | RCT, double‐blind, parallel‐arm, placebo‐controlled study Date of study: September 2020 Location: Netherlands Phase: 4 |
| Participants |
Randomised: 50 participants Inclusion criteria:
|
| Interventions |
Intervention A. Guselkumab 100 mg/mL, subcutaneous injection, administered on day 0, 28, and 84 Control intervention B. Placebo SC, administered on day 0, 28, and 84 |
| Outcomes |
Primary outcomes
|
| Starting date | Actual study start date: September 2020 Estimated study completion date: December 2022 Last update posted: June 2021, recruiting |
| Contact information | Robert Rissmann, PhD, Centre for Human Drug Research clintrials@chdr.nl |
| Notes | Funding: Janssen Pharmaceuticals Last checked in October 2022 |
NCT04453137.
| Study name | Multicentre, double‐blind, randomised, parallel‐group, study evaluating PK efficacy, safety, and immunogenicity in patients with plaque psoriasis receiving Humira or AVT02 followed by safety extension phase of AVT02 |
| Methods | RCT, active‐controlled, double‐blind trial, parallel arms Date of study: June 2020 Location: Georgia, Iceland, Poland, Russian Federation, Ukraine Phase 3 |
| Participants |
Randomised: 567 participants Inclusion criteria:
Exclusion criteria
|
| Interventions |
Intervention A. AVT02 (adalimumab biosimilar) Control intervention B. Adalimumab (initial dose of 80 mg (2 × 40 mg) administered SC, followed by 40 mg SC given every other week starting 1 week after the initial dose |
| Outcomes | At 26 weeks to 28 weeks Primary outcomes
Secondary outcomes
|
| Starting date | Actual study start date: June 2020 Estimated study completion date: November 2021 Last update posted: May 2022, completed |
| Contact information | Roshan Dias, MSc, roshan.dias@alvotech.com Heimo Stroissnig, MD, heimo.stroissnig@alvotech.com Principal investigator: Steven Feldman, MD, PhD Wake Forest University Health Sciences |
| Notes | Funding: Alvotech Swiss AG Last checked in October 2022 |
NCT04533737.
| Study name | Efficacy and safety of brodalumab compared with guselkumab in the treatment of plaque psoriasis after inadequate response to ustekinumab (COBRA) |
| Methods | RCT, active‐controlled, double‐blind, parallel‐arm study Date of study: November 2020 Location: Austria, Belgium, France, Greece, Italy, Spain, Sweden, Switzerland, UK (worldwide) Phase 4 |
| Participants |
Randomised: 240 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Brodalumab 210 mg (1.5 mL) at weeks 0, 1, 2, and then every 2 weeks + dummy 1 (placebo 1.0 mL) at weeks 0, 4, and then every 8 weeks Control intervention B. Guselkumab 100 mg (1.0 mL) at weeks 0, 4, and then every 8 weeks + dummy 2 (placebo 1.5 mL) at weeks 0, 1, 2, and then every 2 weeks |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Actual study start date: November 2020 Estimated study completion date: March 2024 Last update posted: October 2022, active, not recruiting |
| Contact information | Not stated |
| Notes | Funding: LEO Pharma Last checked in October 2022 |
NCT04595409.
| Study name | A double‐blind study to compare the efficacy, safety, and immunogenicity of the proposed biosimilar ustekinumab FYB202 to Stelara® in patients with moderate‐to‐severe plaque psoriasis (VESPUCCI) |
| Methods | RCT, active‐controlled, double‐blind, parallel‐arm study Date of study: November 2020 Location: Poland, Estonia, Georgia, Ukraine Phase 3 |
| Participants |
Randomised: 392 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. FYB202 (ustekinumab biosimilar), 1 SC injection at week 0 and week 4, followed by 1 SC injection every 12 weeks thereafter for the next 3 consecutive doses Control intervention B. Ustekinumab, 1 SC injection at week 0 and week 4, followed by 1 SC injection every 12 weeks thereafter for the next 3 consecutive doses |
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
| Starting date | Actual study start date: November 2020 Estimated study completion date: Mach 2022 Last update posted: August 2021, active not recruiting |
| Contact information | vespucci@bioeq.com |
| Notes | Funding: Bioeq GmbH Last checked in October 2022 |
NCT04607980.
| Study name | A study to investigate ABP 654 for the treatment of participants with moderate‐to‐severe plaque psoriasis |
| Methods | RCT, active‐controlled, double‐blind, parallel‐arm study Date of study: November 2020 Location: USA, Canada, Estonia, Germany, Hungary, Latvia, Poland, Lithuania (worldwide) Phase 3 |
| Participants |
Randomised: 563 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. ABP 654 (ustekinumab biosimilar), SC, 45 mg (baseline BW less than equal to (≤) 100 kg) or 90 mg (baseline BW greater than > 100 kg) at weeks 0, 4, and 16. Further, from week 28, participants will receive ABP 654 (same dose) every 12 weeks (Q12W) at weeks 28 and 40 or may receive dose intensification Q8W at weeks 28, 36, and 44, depending on PASI score. Control intervention B. Ustekinumab, SC, 45 mg (baseline BW ≤ 100 kg) or 90 mg (baseline BW > 100 kg) at weeks 0, 4, and 16. At week 28, participants will be re‐randomised to continue on ustekinumab (treatment group B1), or to receive ABP 654 (treatment group B2) on weeks 28 and 40. Depending on PASI score, some participants may not be re‐randomised and may receive dose intensification with ustekinumab Q8W at weeks 28, 36, and 44. |
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
| Starting date | Actual study start date: November 2020 Actual study completion date: June 2022 Last update posted: June 2022, completed |
| Contact information | medinfo@amgen.com |
| Notes | Funding: Amgen Last checked in October 2022 |
NCT04673786.
| Study name | A study to compare the efficacy and safety of CT‐P43 to Stelara in patients with plaque psoriasis |
| Methods | RCT, active‐controlled, double‐blind, parallel‐arm study Date of study: January 2021 Location: Estonia Phase 3 |
| Participants |
Randomised: 509 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. CT‐P43 (ustekinumab biosimilar) 45 mg or 90 mg dose SC Control intervention B. Ustekinumab 45 mg or 90 mg dose SC |
| Outcomes | At week 12 Primary outcome
|
| Starting date | Actual study start date: January 2021 Actual study completion date: May 2022 Last update posted: June 2022, completed |
| Contact information | Not stated |
| Notes | Funding: Celltrion Last checked in October 2022 |
NCT04713592.
| Study name | Study of subcutaneous (injected under the skin) risankizumab to assess change in disease symptoms in adult participants with moderate‐to‐severe plaque psoriasis with palmoplantar involvement (IMMprint) |
| Methods | RCT, placebo‐controlled, double‐blind, parallel‐arm study Date of study: February 2021 Location: USA, Canada, Spain, Puerto Rico (worldwide) Phase 3 |
| Participants |
Randomised: 209participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Risankizumab SC for 52 weeks Control intervention B. Placebo SC for 16 weeks followed by risankizumab for 36 weeks |
| Outcomes | At week 16 Primary outcome
Seconday outcomes
|
| Starting date | Actual study start date: February 2021 Estimated study completion date: April 2023 Last update posted: September 2022, active, not recruiting |
| Contact information | abbvieclinicaltrials@abbvie.com |
| Notes | Funding: AbbVie Last checked in October 2022 |
NCT04728360.
| Study name | Comparative study of BAT2206 with Stelara® in patients with moderate‐to‐severe plaque psoriasis |
| Methods | RCT, active‐controlled, double‐blind, parallel‐arm study Date of study: February 2021 Location: China (1 centre) Phase 3 |
| Participants |
Randomised: 556 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. BAT2206 (ustekinumab biosimilar). Patients who weigh ≤ 100 kg: BAT2206 45 mg (1 injection of 45 mg/0.5 mL) by SC injection via PFS. Patients who weigh > 100 kg: EU‐sourced Stelara 90 mg (2 injections of 45 mg/0.5 mL each) by SC injection via PFS Control intervention B. Ustekinumab (EU‐sourced Stelara). Patients who weigh ≤ 100 kg: EU‐sourced Stelara 45 mg (1 injection of 45 mg/0.5 mL) by SC injection via PFS. Patients who weigh > 100 kg: EU‐sourced Stelara 90 mg (2 injections of 45 mg/0.5 mL each) by SC injection via PFS |
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
| Starting date | Actual study start date: July 2021 Estimated study completion date: May 2023 Last update posted: August 2022, active not recruiting |
| Contact information | Min Zheng, Second Affiliated Hospital, School of Medicine, Zhejiang University |
| Notes | Funding: Bio‐Thera Solutions Last checked in October 2022 |
NCT04785326.
| Study name | Efficacy, safety, and immunogenicity of subcutaneous DMB‐3115 versus Stelara® in patients with moderate‐to‐severe chronic plaque psoriasis (Opportuniti) |
| Methods | RCT, active‐controlled, double‐blind, parallel‐arm study Date of study: April 2021 Location: USA (2 centres) Phase 3 |
| Participants |
Randomised: 605 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. DMB‐3115 45 mg or 90 mg SC Control intervention B. Ustekinumab 45 mg or 90 mg SC. Patients randomised to receive ustekinumab at the beginning of the study will be re‐randomised at week 28 in a 1:1 ratio to either continue on Stelara or will be transitioned to receive DMB‐3115. |
| Outcomes | At weeks 8 and 12 Primary outcome
|
| Starting date | Actual study start date: April 2021 Estimated study completion date: November 2022 Last update posted: November 2021, active, not recruiting |
| Contact information | Ji‐Su Song, songjs@donga.co.kr |
| Notes | Funding: Dong‐A ST Co., Ltd. Last checked in October 2022 |
NCT04839328.
| Study name | A phase Ⅲ efficacy and safety study of Hemay005 in subjects with moderate‐to‐severe plaque psoriasis |
| Methods | RCT, placebo‐controlled, double‐blind, multicentre study Date of study: January 2022 Location: China Phase 3 |
| Participants |
Randomised: 306 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Hemay005 60 mg twice‐daily for 16 weeks Control intervention B. Placebo |
| Outcomes | At 16 weeks Primary outcome
Secondary outcomes
|
| Starting date | Actual study start date: January 2022 Estimated study completion date: July 2023 Last update posted: June 2022, recruiting |
| Contact information | Junitng Wu, +8615822778207, hemay1834@126.com |
| Notes | Funding: Tianjin Hemay Pharmaceutical Co. Ltd. Last check in October 2022 |
NCT04908475.
| Study name | Study of subcutaneous risankizumab injection compared to oral apremilast tablets to assess change in disease activity and adverse events in adult participants with moderate plaque psoriasis who are candidates for systemic therapy |
| Methods | RCT, active‐controlled, open‐label, assessor‐blinded study Date of study: June 2021 Location: worldwide (US, Canada, Germany, Israel, Poland) Phase 3 |
| Participants |
Randomised: 351 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Risankizumab Control intervention B. Apremilast |
| Outcomes | At week 16 Primary outcomes
Secondary outcome
|
| Starting date | Actual study start date:June 2021 Estimated study completion date: April 2023 Last update posted: January 2022, active, not recruiting |
| Contact information | Abbvie Call Center 844‐663‐3742, abbvieclinicaltrials@abbvie.com |
| Notes | Funding: AbbVie Last check in October 2022 |
NCT04914429.
| Study name | A study of guselkumab (TREMFYA) in Chinese participants with moderate‐to‐severe plaque psoriasis |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: August 2021 Location: China (28 sites) Phase 4 |
| Participants |
Randomised: 300 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Guselkumab 100 mg SC injection at weeks 0, 4, and then every 8 weeks (Q8W) Control intervention B. Placebo |
| Outcomes | At week 16 Primary outcomes
Secondary outcomes
|
| Starting date | Study start date: August 2021 Estimated study completion date: December 2023 Last update posted: October 2022, recruiting |
| Contact information | Study contact: 844‐434‐4210, JNJ.CT@sylogent.com Investigators Study Director: Janssen Research & Development, LLC Clinical Trial |
| Notes | Funding: Janssen Research & Development, LLC Last check in October 2022 |
NCT04930042.
| Study name | Efficacy, safety, and immunogenicity of AVT04 with moderate‐to‐severe chronic plaque psoriasis |
| Methods | RCT, active‐controlled, double‐blind study Date of study: June 2021 Location: Estonia, Georgia, Poland, Ukraine Phase 3 |
| Participants |
Randomised: 581 participants Inclusion criteria
Exclusion criteria
Table 5.1: Approved/marketed products medication or therapy washout before BL biologic therapies, including but limited to: adalimumab 12 weeks, etanercept 8 weeks, secukinumab 12 weeks, infliximab 12 weeks, certolizumab pegol 24 weeks, alefacept 24 weeks, briakinumab 24 weeks, guselkumab 13 weeks, brodalumab 13 weeks Any kinase inhibitor for any reason (e.g. tofacitinib citrate) 1 day Any phosphodiesterase type 4 inhibitor (e.g. apremilast (Otezla)) 4 weeks Cyclosporine 4 weeks Methotrexate 4 weeks PUVA‐UVA/UVB 4 weeks Topical psoriasis treatments (examples include vitamin D analogs, topical steroids, polifenols, etc) (except low‐ to mid‐potency topical corticosteroids on face, eyes, scalp, palms, soles, and genital area; only) 2 weeks Oral retinoids 4 weeks Corticosteroids IM ‐ IV ‐ oral ‐ intraarticular 4 weeks Drugs that may cause new onset or exacerbation of psoriasis (including, but not limited to, beta blockers, lithium, and anti‐malarials) 6 months1 TCM (oral) 4 weeks TCM (topical) 2 weeks 1 Unless the patient has been on a stable dose for at least 6 months prior to BL visit without exacerbation of psoriasis. Patient has received live or attenuated vaccines during the 4 weeks prior to BL visit or has the intention of receiving a live or attenuated vaccine at any time during the study. Note: Inactivated (non‐live and non‐attenuated) vaccines are allowed.
Patient has an active and serious infection or history of infections as follows: a. Any active infection (including Severe Acute Respiratory Syndrome‐Coronavirus‐2 [SARS‐CoV‐2] infection) i. For which non‐systemic anti‐infectives were used within 4 weeks prior to BL visit. Note: patients receiving topical antibiotics for facial acne do not need to be excluded. ii. Which required hospitalisation/quarantine or systemic anti‐infective within 8 weeks prior to BL visit b. Recurrent or chronic infections or other active infection that, in the opinion of the investigator or designee, might cause this study to be detrimental to the patient c. Invasive fungal infection or mycobacterial infection d. Opportunistic infections, such as listeriosis, legionellosis, or pneumocystis
|
| Interventions |
Intervention A. AVT04 (ustekinumab biosimilar) initial loading dose of 45 mg followed by 45 mg SC once every 12 weeks starting 4 weeks after the initial loading dose administered SC Control intervention B. EU Stelara (ustekinumab) initial loading dose of 45 mg followed by 45 mg SC once every 12 weeks starting 4 weeks after the initial loading dose administered SC |
| Outcomes |
At week 28 Primary outcome
Secondary outcome
|
| Starting date | Estimated study completion date: September 2022 Last update posted: May 2022, active, not recruiting |
| Contact information | Jaak Talli, MD, +3726109434, jaak.talli@innomedica.ee |
| Notes | Funding: Alvotech Swiss AG Last check in October 2022 |
NCT04967508.
| Study name | A study to compare SB17 (proposed ustekinumab biosimilar) to Stelara® in subjects with moderate to severe plaque psoriasis |
| Methods | RCT, active‐controlled, double‐blind study Date of study: July 2021 Location: Estonia, Lithuania, Hungary, Czechia, Poland, Latvia, Lithuania, Korea, Ukraine (multicentre) Phase 3 |
| Participants |
Randomised: 503 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. SB17 (proposed ustekinumab biosimilar) 45 mg SC at week 0, 4, and then every 12 weeks Control intervention B. Stelara® (ustekinumab) 45mg SC at week 0, 4, and then every 12 weeks |
| Outcomes |
At week 12 Primary outcome
|
| Starting date | Actual study start date: June 2021 Estimated study completion date: December 2022 Last update posted: May 2022, active, not recruiting |
| Contact information | Samsung Bioepis, +82 32 728 0371, sbregistry@samsung.com |
| Notes | Funding: Samsung Bioepis Co., Ltd. Last check in October 2022 |
NCT05004727.
| Study name | Multi‐center PAMPA study (PAMPA) Preventing Arthritis in a Multi‐Center Psoriasis At‐Risk Cohort |
| Methods | RCT, double‐blind, placebo‐controlled, multicentre study Date of study: February 2022 Location: USA, Canada Phase 4 |
| Participants |
Randomised: 350 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Guselkumab 100 mg 1 mL liquid formulation in a single‐dose pre‐filled syringe administered by subcutaneous injection at week 0, week 4 and every 8 weeks thereafter (month 0 to month 24 for arm 1; week 24 to month 24 for arm 2) Control intervention B. Placebo |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | Actual study start date: February 2022
Estimated study completion date: September 2025 Last update posted: September 2022, recruiting |
| Contact information | Jose Scher, MD Jose.Scher@nyulangone.org Courtney Pike, Courtney.Pike@nyulangone.org |
| Notes | Funding: NYU Langone Health Last check in October 2022 |
NCT05020249.
| Study name | A study to evaluate the efficacy and safety of bimekizumab in adult Korean study participants with moderate to severe plaque psoriasis |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: September 2021 Location: Korea (9 sites) Phase 3 |
| Participants |
Randomised: 47 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Bimekizumab Control intervention B. Placebo |
| Outcomes |
At week 16 Primary outcomes
Secondary outcomes
|
| Starting date | Study start date: September 2021 Actual study completion date: September 2022 Last update posted: September 2022, completed |
| Contact information | UCBCares@ucb.com |
| Notes | Funding: UCB Biopharma Last checked in October 2022 |
NCT05108766.
| Study name | A phase Ⅲ study to evaluate tildrakizumab in the treatment of Chinese subjects with moderate to severe plaque type psoriasis |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: December 2020 Location: China (1 centre) Phase 3 |
| Participants |
Randomised: 220 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Tildrakizumab 100 mg subcutaneous (SC) at week 0 and week 4, 100 mg subcutaneous placebo at Week 12 Control intervention B. Placebo |
| Outcomes | At week 12 Primary outcome
Secondary outcomes
|
| Starting date | Study start date: December 2020 Estimated study completion date: March 2023 Last update posted: November 2021, active, not recruiting |
| Contact information | Not stated |
| Notes | Funding: Quote (clinicaltrials.gov): Shenzhen Kangzhe Pharmaceutical Co., Ltd. Last checked in October 2022 |
NCT05335356.
| Study name | Comparing efficacy and safety of Bmab 1200 and Stelara in patients with moderate to severe chronic plaque psoriasis (STELLAR‐2) |
| Methods | RCT, double‐blind, active‐controlled study Date of study: June 2022 Location: United States (multicentre) Phase 3 |
| Participants |
Randomised: 384 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Bmab1200 (ustekinumab biosimilar) 45 mg, 90 mg Control intervention B. Ustekinumab (Stelara) 45 mg, 90 mg |
| Outcomes | At week 12 Primary outcomes
Secondary outcomes
|
| Starting date | Actual study start date: June 2022 Estimated study completion date: October 2023 Last update posted: July 2022, recruiting |
| Contact information | Dr Gursharan Singh gursharan.singh@biocon.com |
| Notes | Funding: Biocon Biologics UK Ltd. Last check in October 2022 |
NCT05344248.
| Study name | A randomized, double blinded, multi‐center, placebo controlled, phase ib/ii clinical study to evaluate the safety, tolerability, efficacy and pharmacokinetic profiles of multiple doses of JS005 in patients with moderate to severe psoriasis |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: January 2021 Location: China (multicentre) Part I of study (phase 1b) Part II of study (phase 2) Phase 1b/2 |
| Participants |
Randomised: 166 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. JS005 (biologic, recombinant humanised monoclonal antibody against IL‐17A), SC Phase 1b: 60 mg, 150 mg, 300 mg, 600 mg; each patient can only receive multiple doses at one dose level. Each patient received weekly dosing (QW) at weeks 0, 1, 2, 3, and 4, and quad‐weekly dosing (Q4W) beginning at week 5 through week 12. Phase 2: multiple subcutaneous injections of the study drug and placebo in 2 doses of 300 mg and 150 mg were performed. Each patient can only receive multiple doses at one dose level. Weekly dosing (QW) was given at 0, 1, 2, 3, and 4 weeks, and quad‐weekly dosing (Q4W) was given from 5 weeks to 12 weeks. Control intervention B. Placebo |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | Study start date: January 2021 Estimated study completion date: September 2023 Last update posted: April 2022, recruiting |
| Contact information | Chunyan Meng, Bachelor chunyan_meng@junshipharma.com |
| Notes | Funding: Shanghai Junshi Bioscience Co., Ltd. Last checked in October 2022 |
NCT05536726.
| Study name | A phase 3 study of recombinant anti‐IL‐17a humanized monoclonal antibody in Chinese participants with moderate‐to‐severe plaque psoriasis |
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: December 2022 Location: China (multicentre) Phase 3 |
| Participants |
Randomised: 450 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Drug 608 (recombinant anti‐IL‐17A humanised monoclonal antibody) starting dose of 160 mg at week 0 followed by 80 mg once every 2 weeks (Q2W) by SC injection during induction period (12 weeks) B. Drug 608 160mg once every 4 weeks (Q4W) by SC injection during induction period (12 weeks) Control intervention C. Placebo |
| Outcomes | At week 12 Primary outcomes
Secondary outcomes
|
| Starting date | Study start date: December 2022 Estimated study completion date: September 2024 Last update posted: October 2022, not yet recruiting |
| Contact information | Principal Investigators: Jinhua Xu, MD, xjhhsyy@163.com Shanghai Huanshan Hospital Fudan University‐Dermatology Jing Zhang, MD, zhangj_fudan@163.com Shanghai Huanshan Hospital Fudan University |
| Notes | Funding: Sunshine Guojian Pharmaceutical (Shanghai) Co., Ltd. Last check in October 2022 |
ADA: adalimumab AE: adverse events ALT: alanine transaminase AST: aspartate aminotransferase BL: baseline BMI: body mass index BSA: body surface area BW: body weight CL: chloride Cmax: maximum concentration ECG: electrocardiogram eGFR: estimated glomerular filtration rate eow: every other week FAEs: fumaric acid esters IM: intramuscular IV: intravenous MRI: magnetic resonance imaging Nab: neutralising antibody NAPSI: Nail Psoriasis Severity Index OA: osteoarthritis PASI: Psoriasis Area and Severity Index PD: pharmacodynamics PFS: pre‐filled syringe PGA: Physician's Global Assessment PK: pharmacokinetics PLT: platelet thrombocyte count PUVA: psoralen plus ultraviolet light A QoL: quality of life RA: rheumatoid arthritis RCT: randomised controlled trial SAE: serious adverse event SC: subcutaneous sCr: serum creatinine sPGA: static Physician Global Assessment TCM: traditional Chinese medicine TB: tuberculosis TBR: time below range Tmax: time to maximum plasma concentration ULN: upper limit of normal UVA/B: ultraviolet A/B WBC: white blood cells
Differences between protocol and review
A. Between the previous review (October 2021) and the last update search (October 2022)
1. Methods: Data collection and analysis > Data synthesis
We performed the new update using R‐package netmeta from R software version 4.2.2.
Thus we changed the sentence:
We conducted pairwise meta‐analyses using Review Manager 5.4 (Revman 2020), and we performed all other analyses in Stata 14 using the 'network' (www.stata-journal.com/article.html?article=st0410) and 'network graphs' packages (www.stata-journal.com/article.html?article=st0411)
for:
We conducted pairwise meta‐analyses using Review Manager 5.4 (Revman 2020), and we performed all other analyses in R software version 4.2.2 using the 'R‐package netmeta' (https://cran.r-project.org/web/packages/netmeta/netmeta.pdf) and 'ggplot2 package' for the network graphs.
Using R‐package netmeta, we were not able to perform the loop‐specific approach to assess local inconsistency, thus we focused on the side‐splitting method only.
Thus we changed the sentence:
We assessed inconsistency (i.e. the possible disagreement between the different pieces of evidence) locally and globally. Specifically, we used the loop‐specific approach (Bucher 1997) and the side‐splitting method (Dias 2010). We also fitted the design by treatment interaction model to evaluate the presence of inconsistency in the entire network (Higgins 2012).
for:
We assessed inconsistency (i.e. the possible disagreement between the different pieces of evidence) locally and globally. Specifically, we used the side‐splitting method (Dias 2010). The comparison of interest showed evidence of inconsistency, when a P value was less than 0.05 when direct and indirect evidence were compared in a z test (Separate Indirect from Direct Evidence (SIDE)). We also fitted the design by treatment interaction model to evaluate the presence of inconsistency in the entire network (Higgins 2012).
2. Methods: Data collection and analysis > Sensitivity analysis
Regarding the sensitivity analysis restricted to only drugs approved by European Medicines Agency for plaque psoriasis:
non‐biological systemic treatments: FAEs, acitretin, ciclosporin, methotrexate;
small molecules: apremilast; deucravacitinib;
anti‐TNF alpha: infliximab, etanercept, adalimumab, certolizumab pegol;
anti‐IL12/23: ustekinumab;
anti‐IL17: secukinumab, brodalumab, ixekizumab, bimekizumab;
anti‐IL23: tildrakizumab, guselkumab, risankizumab.
We only included arms with licensed dosage for these drugs.
B. Between the previous review (September 2020) and the last update search (October 2021)
1. Methods: Data collection and analysis > Sensitivity analysis
We added a new sensitivity analysis:
-
Analysing only drugs approved by European Medicines Agency for plaque psoriasis:
non‐biological systemic treatments: FAEs, acitretin, ciclosporin, methotrexate;
small molecules: apremilast;
anti‐TNF alpha: infliximab, etanercept, adalimumab, certolizumab pegol;
anti‐IL12/23: ustekinumab;
anti‐IL17: secukinumab, brodalumab, ixekizumab, bimekizumab;
anti‐IL23: tildrakizumab, guselkumab, risankizumab.
2. Methods: Search methods for identification of studies
In September 2021, following review, we removed from the search strategies the drug names tofacitinib and mirikizumab as these drugs were no longer applicable to psoriasis. We added the following new drug names to the search strategies: deucravacitinib, hemay005, sonelokimab (MSB0010841), netakimab (BCD‐085), vunakizumab (SHR‐1314).
From February 2021, we have used Screen4Me functionality to remove records unlikely to be RCTs from our search results, increasing the efficiency of our screening process.
C. Between the previous review (January 2019) and the last update search (September 2020)
1. Methods: Data collection and analysis > Data synthesis > Network meta‐analysis
We will provide new networks and re‐analyse the data every six months instead of three months, to have enough new data to integrate.
2. Methods: Data collection and analysis > Assessment of heterogeneity
To better reassure the plausibility of transitivity, we excluded from the main analysis trials including biological‐naïve participants, but assessing efficacy of a biological agent.
3. Methods: Data collection and analysis > Sensitivity analysis
We added two new sensitivity analyses: (1) including trials irrespective of the previous treatments received by the participants, and (2) using another definition of the safety primary outcomes: SAEs after excluding flares of psoriasis.
4. Methods: Data collection and analysis > Summary of findings and assessment of certainty of the evidence
We did not include summary of findings (SoF) tables because the format of an SoF table does not allow us to present a summary of comparisons across the different drugs. The SoF tables in the last version of the review only focused on the comparisons against placebo.
We did not use GRADE assessment for the new update of this review, but CINeMA is a tool specifically dedicated to network meta‐analysis.
We therefore explained the methodology, and added in the Methods section:
We assessed the confidence of the evidence estimates from network meta‐analysis, based on the CINeMA approach, which relies on the contributions of the direct comparisons to the estimation in the network meta‐analysis (CINeMA 2017; Salanti 2014). CINeMA (Confidence in Network Meta‐Analysis) is a web application that simplifies the evaluation of confidence in the findings from network meta‐analysis.
It is based on six domains: within‐study bias (referring to the impact of risk of bias in the included studies), across‐studies bias (publication or reporting bias), indirectness (relevance to the research question and transitivity), imprecision (comparing the range of treatment effects included in the 95% confidence interval with the range of equivalence), heterogeneity (predictive intervals), and incoherence (if estimates from direct and indirect evidence disagree) (Salanti 2014).
The confidence in each NMA (network meta‐analysis) RR (risk ratio)AB between two given drugs A and B was evaluated for six domains. The software required some input in each domain in order to recommend whether there were 'major concerns', 'some concerns', or 'no concerns' for the particular domain.
Thus, threshold values and evaluation rules to be decided were finalised through discussions. After determining these rules, the remaining synthesis of confidence in the evidence can automatically be calculated with the CINeMA web app. One review author input all the data and obtained the results.
Within‐trial bias: we estimated it as the weighted average of the overall risk of bias of all the trials contributing information to the estimation of RRAB.
Reporting bias: also known as 'publication bias'. We assessed publication bias by considering the comprehensive search strategy that we performed and the risk of publication bias in the specific field. The comparison‐adjusted funnel plots that test the presence of small‐study effects in the network assisted our judgements.
Indirectness: since the included studies matched the clinical question of the review, we had 'no concern' about any of the evaluated RRAB.
Imprecision: rated based on whether the 95% CI of RR allowed recommendations to be made. We set the margin of equivalent effects (where none of the drugs is favoured) to between RR 0.95 and 1.05. These values were motivated by the fact that assuming 3% response rate (reaching PASI 90) for placebo, then an RRAB of 1.05 indicated a response for drug A higher than those obtained with placebo, which we considered as clinically meaningful. Then, the degree of overlap between the 95% CI of RRAB and the margin of equivalent effects suggests the judgement.
Heterogeneity: this was evaluated by monitoring the agreement between confidence intervals (CIs) and prediction intervals (PIs). CINeMA judges whether the two intervals and their overlap with the margin of equivalent effects provide similar conclusions.
Incoherence: this was evaluated by monitoring the level of disagreement between confidence intervals (CIs) of the direct and indirect RRAB and their overlap with the margin of equivalent effects.
After the judgement for all the six domains, we summarised our overall confidence in the evidence for each or between any two drugs into high, moderate, low, and very low ratings. Starting with high confidence, we downgraded by one level for each ‘major concern’ in any of the six domains; then two‐thirds of a level down for ‘some concerns’ in ‘within‐study bias’; and one‐third of a level down for each rating of ‘some concerns’ in any of the other five domains. To obtain the final level, we rounded the number of downgrades to their nearest integer.
For each drug, we calculated the percentage of the four levels based on all comparisons including that drug, combining both efficacy and acceptability.
It is important to note that the CINeMA tool was also used in the previous version of our review and results were presented with those from GRADE scoring. Evaluation rules were not the same, however, especially for the margin of equivalent effects, which was RR 1.5. We discussed this point and because the margin of effect was too large, we have changed this rule for this update.
D. Between the previous review (Sbidian 2017) and the first update search (January 2019)
1. Background: Why it is important to do this review
We provided a rationale for maintaining the review as a living systematic review (LSR).
This review includes some new methods relevant for living systematic reviews, which are included in the Methods section, and also described in Appendix 4.
2. Methods: Search methods for identification of studies
Changes between search methods in the existing review and the LSR Older versions of this review included searches of the Cochrane Skin Specialised Register and LILACS. The Skin Register is no longer being maintained, so we will not search it separately for the LSR. The Cochrane Skin Information Specialist has analysed the results of previous searches for this review and has established that no unique studies were identified through LILACS. We will not therefore search LILACS for the LSR.
We did not identify unique trials through our previous searches of the trial results databases of various pharmaceutical companies. We will therefore not search these resources regularly for the LSR.
For the existing review, we searched five trials registries:
the ISRCTN registry (www.isrctn.com);
ClinicalTrials.gov (www.clinicaltrials.gov);
the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au);
the World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/); and
the EU Clinical Trials Register (www.clinicaltrialsregister.eu).
For the LSR, we will search only those that are mandatory under the MECIR standards, i.e. ClinicalTrials.gov and WHO ICTRP. WHO ICTRP is an aggregator of the other three trials registries listed.
3. Interventions
Interventions belonging to the systemic conventional treatments, anti‐TNF alpha, and anti IL12/23 classes were identical to the previous review.
Ponesimod (belonging to the small molecules class), itolizumab, and alefacept (belonging to other biologics class) were withdrawn from the updated review as they are no longer used as systemic treatment for psoriasis.
Bimekizumab (anti‐IL17 class), risankizumab, and mirikizumab (anti‐IL23 class), and BMS‐986165 (small molecules class), are new included drugs for the updated review.
We added new molecules to the search strategy for the update and the LSR searches.
4. Outcomes
Primary and secondary outcomes are identical to the previous review, except for one secondary endpoint: 'Proportion of participants who achieve PASI 75 at 52 weeks' and 'Proportion of participants who achieve PASI 90 at 52 weeks'. These replace 'Proportion of participants with at least one relapse in the maintenance phase (between 52 to 104 weeks)' because this outcome was never available in the maintenance‐phase trials, and our replacement outcomes answer the same question.
Secondary endpoints
Proportion of participants who achieve PASI 75 at induction phase
Proportion of participants who achieve a Physician Global Assessment (PGA) value of 0 or 1 at induction phase
Quality of life measured by a specific scale. Available validated scales are the Dermatology Life Quality Index (DLQI), Skindex, Psoriasis Disability Index (PDI), or Psoriasis Symptom Inventory (PSI) at induction phase
Proportion of participants with adverse effects (AEs) at induction phase
Proportion of participants who achieve PASI 75 at 52 weeks
Proportion of participants who achieve PASI 90 at 52 weeks
To avoid selection of good responders from participants entering into long‐term extension, we selected participants who have been randomised since the induction phase.
The timing of outcomes was also slightly edited: primary outcomes were restricted to only being measured during induction phase (from 8 to 24 weeks after randomisation). All secondary outcomes, except proportion of participants who achieve PASI 75 at 52 weeks and proportion of participants who achieve PASI 90 at 52 weeks, were also restricted to the induction phase. We did not include timing outside these ranges. We also clarified that if there were multiple time points within a phase we would use the longest one.
By expanding the timing (in the previous review, we only analysed trials with short‐term assessment defined as 12 to 16 weeks), we aimed to include more trials.
We also clarified that 'Proportion of participants with adverse effects (AE) at induction phase' did not include serious adverse events.
5. Data collection and analysis: Selection of studies
We used Covidence to screen the titles, abstracts, and full texts (Covidence 2021).
6. Data collection and analysis: Assessment of heterogeneity
For the network meta‐analysis, to further assure the plausibility of the transitivity assumption, we only excluded from our analyses trials involving co‐interventions. We kept in our analyses all trials with a short‐term outcome assessment from 8 to 24 weeks, and not only from 12 to 16 weeks, as we had previously. We performed sensitivity analyses including only studies with a short‐term outcome assessment from 12 to 16 weeks. We also performed sensitivity analyses excluding trials of systemic treatment‐naïve participants.
7. Data collection and analysis: Summary of findings table
We used another method to assess confidence in our results.
"We also performed full evaluation of the confidence in the results using the web application CINeMA (CINeMA 2017). CINeMA (Confidence in Network Meta‐Analysis) is a web application that simplifies the evaluation of confidence in the findings from network meta‐analysis. It is based on six domains: within‐study bias (referring to the impact of risk of bias in the included studies), across‐studies bias (publication or reporting bias), indirectness (relevance to the research question and transitivity), imprecision (comparing the range of treatment effects included in the 95% confidence interval with the range of equivalence), heterogeneity (predictive intervals) and incoherence (if estimates from direct and indirect evidence disagree) (Salanti 2014). Judgements across the six domains are then summarised to obtain four levels of confidence for each relative treatment effect, corresponding to the usual GRADE approach: very low, low, moderate or high."
8. Data collection and analysis: Dealing with missing data
We clarified our approach for dealing with missing data for safety outcomes: "For the main analysis, we assumed that any participant with missing outcome data did not experience clearance (for efficacy outcomes) or did not experience AEs (for safety outcomes), whatever the group."
E. Between the first protocol submission (January 2014) and the first search (February 2015)
1. We identified and added in the protocol new systemic therapeutics for psoriasis
Background: Description of the intervention
Oral treatment
Biological therapies
Background: How the intervention might work?
Oral treatment
Biological therapies
Objectives
We expanded our objectives to clarify the types of systemic treatments for psoriasis. We changed: "To assess the effects of systemic pharmacological treatments for chronic plaque psoriasis" to "To compare the efficacy and safety of conventional systemic agents (acitretin, ciclosporin, fumaric acid esters, methotrexate), small molecules (apremilast, tofacitinib, ponesimod), anti‐TNF alpha (etanercept, infliximab, adalimumab, certolizumab), anti‐IL12/23 (ustekinumab), anti‐IL17 (secukinumab, ixekizumab, brodalumab), anti‐IL23 (guselkumab, tildrakizumab), and other biologics (alefacept, itolizumab) for patients with moderate to severe psoriasis and to provide a ranking of these treatments according to their efficacy and safety."
Methods: Types of intervention
We changed: "Systemic treatments include the following: fumaric acid esters, retinoids (acitretin), ciclosporin, methotrexate, infliximab, etanercept, adalimumab, ustekinumab, briakinumab, alefacept, brodalumab, ixekizumab" to the following:
"Systemic treatments included the following:
-
Systemic conventional treatments:
Fumaric acid esters
Acitretin
Ciclosporin
Methotrexate
-
Small molecules
Apremilast
Tofacitinib
Ponesimod
-
Anti‐TNF alpha
Infliximab
Etanercept
Adalimumab
Certolizumab
-
Anti‐IL12/23
Ustekinumab
-
Anti‐IL17
Secukinumab
Brodalumab
Ixekizumab
-
Anti‐IL23
Tildrakizumab
Guselkumab
-
Other biologic treatment
Itolizumab
Alefacept"
A new anti‐IL23 molecule (BI 655066, risankizumab) appeared after we began this review and was not included in this systematic review. However, the ongoing studies of risankizumab have been reported in this review.
2. Background: Why it is important to do this review
We updated the published literature on other systemic reviews and meta‐analyses.
3. Methods: Criteria for considering studies for this review
Selection of trials
We added: "Phase I trials were not eligible because participants, outcomes, dosages, and schema of administration of interventions are too different from phase II, III, and IV studies."
Outcomes
Primary outcome 1
In the Protocol, we wrote, "The proportion of participants who achieved clear or almost clear skin (by clear or almost clear, we mean a Physician Global Assessment (PGA) value of 0 or 1 or a 90/100 PASI)."
In the review, we changed this sentence to "The proportion of participants who achieved clear or almost clear skin, that is, at least PASI 90".
As PASI and PGA are two different scales, we preferred to assess them separately and added as a secondary outcome "Proportion of participants who achieve a Physician Global Assessment (PGA) value of 0 or 1".
Primary outcome 2
We also modified the sentence about serious adverse effects (SAEs). In the protocol, we had said we would use the FDA's definition: "The proportion of participants with serious adverse effects (SAE)." We used the definition of severe adverse effects from the International Conference of Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, which includes "death, life‐threatening events, initial or prolonged hospitalisation, and adverse events requiring intervention to prevent permanent impairment or damage." The definition remains the same.
Secondary outcome 3
For 'Quality of life measured by a specific scale', we listed Dermatology Life Quality Index (DLQI), Skindex, Psoriasis Disability Index (PDI), or Psoriasis Symptom Inventory (PSI). It is not an exhaustive list. Moreover, we had PSI as a validated scale because it was used by some study authors.
Timing
We modified the period of the induction therapy assessment to less than 24 weeks after randomisation instead of 12 to 24 weeks, because Nast 2015b defined the induction period as being of a duration less than 24 weeks.
To avoid duplicating text, we removed the text discussing timing for remission, as published in the protocol, and edited the timing for induction and maintenance therapy to include the relevant short‐ or long‐term remission classification. We also removed the timing given in the protocol for the quality of life outcome for the same reason (we felt the text was duplicative).
We clarified that our inclusion criterion was to only include studies that reported our timing of interest by editing as follows: "We did not include studies that had timings outside of these time ranges in our analyses" to "We did not include studies that had timings outside of these time ranges in our review."
4. Methods: Search methods for identification of studies
We removed the following two sentences from the review:
"We contacted key investigators and experts in the field to identify further published or unpublished data."
"We contacted pharmaceuticals companies producing fumaric acid esters, and retinoids (fumaric acid esters, retinoids (acitretin), ciclosporin, methotrexate, alefacept, infliximab, etanercept, adalimumab, certolizumab, ustekinumab, secukinumab, brodalumab, ixekizumab, tildrakizumab, guselkumab, Itolizumab, apremilast, tofacitinib, ponesimod."
We replaced them with the following:
"We searched in the trial results databases of each company to identify ongoing and unpublished trials."
5. Methods: Data extraction and management
We added some details about the data extraction (outcome data, other data) for greater clarity and added the sentence, "We extracted the data from the reports of the US Food and Drug Administration (FDA) when available, if not from the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov), and finally from the published reports."
6. Methods: Assessment of risk of bias in included studies
We added information about the network meta‐analysis risk of bias assessment (under "Overall risk of bias").
Network meta‐analysis
"To summarise the quality of evidence and to interpret the network results, we used these six RoB criteria (random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessor, incomplete outcome data, and selective outcome reporting) in order to classify each trial.
We would classify the trial as having low risk of bias if we rated none of the domains above as high risk of bias and two or fewer as unclear risk.
We would classify the trial as having moderate risk of bias if we rated one domain as high risk of bias, one or less domains as unclear risk, or no domains as high risk of bias but three or fewer were rated as unclear risk.
All other cases were assumed to pertain to high risk of bias."
7. Methods: Measure of treatment effect
We added an explanation about relative treatment ranking.
8. Methods: Dealing with missing data
We clarified who the authors or sponsors we contacted were: "We contacted trial authors or sponsors by email to request missing outcome data (numbers of events and numbers of participants for important dichotomous clinical outcomes) when these were not available in study reports that were less than 10 years old."
9. Methods: Assessment of reporting bias and assessment of heterogeneity
We added an explanation of the network meta‐analysis:
"We undertook meta‐analyses only if we judged participants, interventions, comparisons, and outcomes to be sufficiently similar (section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2017). Potential sources of heterogeneity included participants' baseline characteristics (weight, the duration of previous treatment, treatment doses, co‐interventions, and duration of treatment. When enough data were available, we investigated the distributions of these characteristics across studies and treatment comparisons. The latter allows assessing transitivity, i.e. whether there were important differences between the trials evaluating different comparisons other than the treatments being compared (Salanti 2014). To further reassure the plausibility of the transitivity assumption, we only included in our analyses trials not involving co‐interventions.
In the classical meta‐analyses, we assessed statistical heterogeneity by visual inspection of the forest plots and using the Q‐test and the I2 statistic. We interpreted the I2 statistic according to the following thresholds (section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2017): 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% represents considerable heterogeneity.
In the network meta‐analysis, the assessment of statistical heterogeneity in the entire network was based on the estimated heterogeneity standard deviation parameter (τ) estimated from the network meta‐analysis models (Jackson 2014). We also estimated the prediction intervals to assess how much the estimated heterogeneity affects the relative effects with respect to the additional uncertainly anticipated in future studies (Riley 2011). Where feasible, we would have investigated the possible sources of heterogeneity in subgroup analyses and meta‐regression.
Although we restricted the risk of important heterogeneity in our data by considering eligible only studies with a follow‐up period between 12 and 16 weeks and without co‐interventions, we investigated differences in heterogeneity across the different analyses. Specifically, we observed whether splitting the nodes of the network and analysing each drug separately reduced the heterogeneity estimate. We also ran a series of sensitivity analyses (see Sensitivity analysis), and we monitored whether heterogeneity became smaller or larger compared to the primary analysis."
Assessment of reporting biases
To assess reporting biases, we used an adaptation of the funnel plot by subtracting from each study‐specific effect size the mean of meta‐analysis of the study‐specific comparison, which we plotted against the study standard error (Chaimani 2013). We employed this 'comparison‐adjusted funnel plot' for all comparisons of an active treatment against placebo. When we detected funnel plot asymmetry for the two primary outcomes, we investigated the presence of small‐study effects in the network meta‐regression (Chaimani 2012).
10. Methods: Data synthesis
We added the software used for the review: "We conducted pairwise meta‐analyses using Review Manager 5 (RevMan 5) (Revman 2020), and we performed all other analyses in Stata 14 using the 'network' (www.stata-journal.com/article.html?article=st0410) and 'network graphs' packages (www.stata-journal.com/article.html?article=st0411)."
11. Methods: Sensitivity analysis
We added "To assess the robustness of our results, we performed the following sensitivity analyses for the two primary outcomes: (1) running the analysis at dose‐level considering that each different drug dose is a different intervention; (2) excluding trials at high risk of bias; (3) excluding trials with a total sample size smaller than 50 randomised participants; and (4) analysing only the observed participants and assuming that missing participants are missing at random."
12. Methods: Summary of findings table
We added a section detailing the methods used to create the summary of findings tables; we also explained how we used GRADE to assess the certainty (quality/confidence) of the evidence.
13. Contributions of authors
We changed or added authors' contributions: LLC, GD, IGD, and ES screened papers against eligibility criteria. LLC, GD, IGD, CH, CM, CD, and ES appraised the quality of papers. LLC, GD, IGD, CH, CM, CD, and ES extracted data for the review and sought additional information about papers. AC responded to the methodological and statistical comments of the referees instead of LT (Ludovic Trinquard was no longer available and was replaced by Anna Chaimani). AC, LLC, and ES worked on the Methods sections instead of LT, ES, and LLC (Ludovic Trinquard was replaced by Anna Chaimani).
Contributions of authors
Emilie Sbidian: conceptualisation, methodology, validation, project administration, investigation, writing ‐ original draft, supervision
Anna Chaimani: methodology, software, validation, writing ‐ review and editing
Robin Guelimi: investigation, writing ‐ review and editing
Ignacio Garcia‐Doval: investigation, writing ‐ review and editing
Camille Hua: investigation, writing ‐ review and editing
Carolyn Hughes: writing ‐ original draft (PLS)
Luigi Naldi: investigation, writing ‐ review and editing
Maria Kinberger: writing ‐ review and editing
Sivam Afach: software, validation, formal analysis, investigation
Laurence Le Cleach: conceptualisation, methodology, investigation, funding acquisition
Department of Health Disclaimer
The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, the Complex Reviews Support Unit, NIHR, NHS, or the Department of Health.
Sources of support
Internal sources
No sources of support provided
External sources
-
The National Institute for Health Research (NIHR), UK
The NIHR, UK, is the largest single funder of Cochrane Skin.
-
The French Society of Dermatology (SFD), France
The funding agencies have no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation and review of the manuscript.
-
French Ministry of Health, France
Grant support was from the Programme Hospitalier de Recherche Clinique (DGOS n°APHP‐180680).
The funding agencies have no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation and review of the manuscript.
Declarations of interest
Emilie Sbidian: has declared no conflict of interest.
Anna Chaimani: has declared no conflict of interest.
Robin Guelimi: has declared no conflict of interest.
Ignacio Garcia‐Doval: reports payment from Novartis for a presentation unrelated to psoriasis; personal payment. IG‐D also reports receiving meeting expenses from Janssen for the Spanish Academy of Dermatology Annual Congress, personal payment; and payment from UCB (Union Chimique Belge), personal payment.
Camille Hua: has declared no conflict of interest.
Carolyn Hughes: has declared no conflict of interest.
Luigi Naldi: is a contracting member of the EMA PSOLAR Registry Steering Committee (Janssen) and RePhlect European Study Steering Committee (BMS). He received honorarium for participation in advisory board activities from BMS, Boehringer Ingelheim, Leo pharma.
Maria Kinberger: has declared no conflict of interest.
Sivem Afach: has declared no conflict of interest.
Laurence Le Cleach: has declared no conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
ACCEPT 2010 {published data only}
- Griffiths CE, Strober BE, Van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. New England Journal of Medicine 2010;362(2):118-28. [CENTRAL: CN-00734856] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Young MS, Horn EJ, Cather JC. The ACCEPT study: ustekinumab versus etanercept in moderate-to-severe psoriasis patients. Expert Review of Clinical Immunology 2011;7(1):9-13. [CENTRAL: CN-00780322] [PMID: ] [DOI] [PubMed] [Google Scholar]
ADACCESS 2018 {published data only}
- Blauvelt A, Lacour J, Fowler JF, Schuck E, Jauch-Lembach J, Balfour A, et al. Long-term efficacy, safety, and immunogenicity data from a phase III confirmatory study comparing GP2017, a proposed biosimilar, with reference adalimumab. United European Gastroenterology Journal 2017;5(Suppl 1):A301. [CENTRAL: CN-01439136] [Google Scholar]
- Blauvelt A, Lacour JP, Fowler JF Jr, Weinberg JM, Gospodinov D, Schuck E, et al. Phase III randomized study of the proposed adalimumab biosimilar GP2017 in psoriasis: impact of multiple switches. British Journal of Dermatology 2018;179(3):623-31. [CENTRAL: CN-01617973] [DOI] [PubMed] [Google Scholar]
- NCT02016105. Study to demonstrate equivalent efficacy and to compare safety of biosimilar adalimumab (GP2017) and Humira (ADACCESS). clinicaltrials.gov/show/nct02016105 (first received 19 December 2013). [CENTRAL: CN-01479872]
AFFIRM 2022 {published data only}
- Mrowietz U, Kircik L, Reich K, Munjal S, Shenoy S, Lebwohl M, et al. Tepilamide fumarate (PPC-06) extended release tablets in patients with moderate-to-severe plaque psoriasis: safety and efficacy results from the randomized, double-blind, placebo-controlled AFFIRM Study. Journal of Clinical and Aesthetic Dermatology 2022;15(1):53-8. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- NCT03421197. A study to assess the efficacy and safety of PPC-06 (tepilamide fumarate). clinicaltrials.gov/show/nct03421197 (first received 5 February 2018).
Akcali 2014 {published data only}
- Akcali C, Guven EH, Kirtak N, Inaloz HS, Ozgoztasi O, Guvenc U. Serum concentrations of interleukin-2 and tumour necrosis factor-alpha under cyclosporine versus acitretin treatment in plaque-type psoriasis. Journal of International Medical Research 2014;42(5):1118-22. [CENTRAL: CN-01114333] [PMID: ] [DOI] [PubMed] [Google Scholar]
Al‐Hamamy 2014 {published data only}
- Al-Hamamy HR, Al-Mashhadani SA, Mustafa IN. Comparative study of the effect of narrowband ultraviolet B phototherapy plus methotrexate vs. narrowband ultraviolet B alone and methotrexate alone in the treatment of plaque-type psoriasis. International Journal of Dermatology 2014;53(12):1531-5. [CENTRAL: CN-01089920] [PMID: ] [DOI] [PubMed] [Google Scholar]
ALLURE 2021 {unpublished data only}
- EUCTR2015-005170-38-BE. Study of efficacy and safety of secukinumab 2 mL pre-filled syringe (300 mg) in subjects with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015-005170-38-BE (first received 29 July 2016).
- NCT02748863. Study of secukinumab with 2 mL pre-filled syringes (ALLURE). clinicaltrials.gov/ct2/show/NCT02748863 (first received 22 April 2016).
- Sigurgeirsson B, Schakel K, Hong CH, Effendy I, Placek W, Rich P, et al. Efficacy, tolerability, patient usability, and satisfaction with a 2 mL pre-filled syringe containing secukinumab 300 mg in patients with moderate to severe plaque psoriasis: results from the phase 3 randomized, double-blind, placebo-controlled ALLURE study. Journal of Dermatological Treatment 2022;33(3):1718‐26. [DOI] [PubMed] [Google Scholar]
- Sigurgeirsson B, Schäkel K, Hong CH, Effendy I, Placek W, Rich P, et al. Efficacy, tolerability, patient usability, and satisfaction with a 2 mL pre-filled syringe containing secukinumab 300 mg in patients with moderate to severe plaque psoriasis: results from the phase 3 randomized, double-blind, placebo-controlled ALLURE study. Journal of Dermatological Treatment 2021 Apr 26 [Epub ahead of print]. [DOI: 10.1080/09546634.2021.1902925] [DOI] [PubMed]
AlMutairi 2021 {published data only}
- AlMutairi N, Eassa BI. A randomized controlled ixekizumab vs secukinumab trial to study the impact on sexual activity in adult patients with genital psoriasis. Expert Opinion on Biological Therapy 2021;21(2):297-8. [DOI] [PubMed] [Google Scholar]
- AlMutairi N, Eassa BI. Comparing the efficacy and safety of IL-17 inhibitors for treatment of moderate-to-severe psoriasis: a randomized double blind pilot study with a review of literature. Postepy Dermatologii i Alergologii 2021;38(2):281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
AMAGINE‐1 2016 {published data only}
- Armstrong A, Strober B, Ehst BD, Jacobson A. 15921 Absolute PASI response up to 52 weeks with brodalumab in patients with moderate to severe plaque psoriasis. Journal of the American Academy of Dermatology 2020;83(6 Suppl):AB164. [Google Scholar]
- Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, et al. A prospective phase III, randomized,double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. British Journal of Dermatology 2016;175(2):273-86. [CENTRAL: CN-01208651] [DOI] [PubMed] [Google Scholar]
AMAGINE‐2 2015 {published data only}
- Lambert J, Hansen JB, Sohrt A, Puig L. Dermatology Life Quality Index in patients with moderate-to-severe plaque psoriasis treated with brodalumab or ustekinumab. Dermatology and Therapy 2021;11(4):1265-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. New England Journal of Medicine 2015;373(14):1318-28. [CENTRAL: CN-01089800] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Menter A, Sobell J, Silverberg JI, Lebwohl M, Rastogi S, Pillai R, et al. Long-term efficacy of brodalumab for the treatment of moderate-to-severe psoriasis: data from a pivotal Phase III clinical trial. Journal of Clinical and Aesthetic Dermatology 2018;11(5 Suppl 1):S26-7. [CENTRAL: CN-01713709] [Google Scholar]
- Papp KA, Lebwohl MG, Green LJ, Yamauchi PS, Rastogi S, Israel R, et al. Maintenance of clinical efficacy in moderate-to-severe plaque psoriasis: a 52-week evaluation of brodalumab in three multicenter, double-blind studies of 4363 subjects. Journal of Clinical and Aesthetic Dermatology 2017;10(5 Suppl 1):S23-4. [CENTRAL: CN-01713074] [Google Scholar]
AMAGINE‐3 2015 {published data only}
- Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. New England Journal of Medicine 2015;373(14):1318-28. [CENTRAL: CN-01089800] [PMID: ] [DOI] [PubMed] [Google Scholar]
Asahina 2010 {published data only}
- Asahina A, Nakagawa H, Etoh T, Ohtsuki M, Adalimumab M04-688 Study Group. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. Journal of Dermatology 2010;37(4):299-310. [CENTRAL: CN-00762123] [PMID: ] [DOI] [PubMed] [Google Scholar]
Asawanonda 2006 {published data only}
- Asawanonda P, Nateetongrungsak Y. Methotrexate plus narrowband UVB phototherapy versus narrowband UVB phototherapy alone in the treatment of plaque-type psoriasis: a randomized, placebo-controlled study. Journal of the American Academy of Dermatology 2006;54(6):1013-8. [CENTRAL: CN-00556468] [PMID: ] [DOI] [PubMed] [Google Scholar]
Augustin 2022 {published data only}
- Augustin M, Reich K, Yamauchi P, Pinter A, Bagel J, Dahale S, et al. Secukinumab dosing every 2 weeks demonstrated superior efficacy compared with dosing every 4 weeks in patients with psoriasis weighing 90 kg or more: results of a randomized controlled trial. British Journal of Dermatology 2022;186(6):942-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT03504852. Efficacy and safety of 2 secukinumab regimens in 90 kg or higher subjects with moderate to severe chronic plaque-type psoriasis. clinicaltrials.gov/show/nct03504852 (first received 20 April 2018).
AURIEL‐PsO 2020 {published data only}
- Hercogova J, Papp KA, Chyrok V, Ullmann M, Vlachos P, Edwards CJ. AURIEL-PsO: a randomised, double-blind phase III equivalence trial to demonstrate the clinical similarity of the proposed biosimilar MSB11022 to reference adalimumab in patients with moderate-to-severe chronic plaque-type psoriasis. British Journal of Dermatology 2020;182(2):316-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02660580. MSB11022 in moderate to severe chronic plaque psoriasis (AURIEL-PsO). clinicaltrials.gov/show/nct02660580 (first received 21 January 2016). [CENTRAL: CN-01555234]
Bachelez 2015 {published data only}
- Bachelez H, Van de Kerkhof PC, Strohal R, Kubanov A, Valenzuela F, Lee JH, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet 2015;386(9993):552-61. [CENTRAL: CN-01091031] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Valenzuela F, Paul C, Mallbris L, Tan H, Papacharalambous J, Valdez H, et al. Tofacitinib versus etanercept or placebo in patients with moderate to severe chronic plaque psoriasis: patient-reported outcomes from a phase 3 study. Journal of the European Academy of Dermatology and Venereology 2016;30(10):1753-9. [CENTRAL: CN-01368560] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bagel 2012 {published data only}
- Bagel J, Kricorian G, Klekotka P, Tyring S. Etanercept therapy for moderate to severe plaque psoriasis with involvement of the scalp. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB150. [CENTRAL: CN-00843659] [DOI] [PubMed] [Google Scholar]
- Bagel J, Lynde C, Tyring S, Kricorian G, Shi Y, Klekotka P. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. Journal of the American Academy of Dermatology 2012;67(1):86-92. [CENTRAL: CN-00870940] [PMID: ] [DOI] [PubMed] [Google Scholar]
Barker 2011 {published data only}
- Barker J, Hoffmann M, Wozel G, Ortonne JP, Zheng H, Van Hoogstraten H, et al. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1). British Journal of Dermatology 2011;165(5):1109-17. [CENTRAL: CN-00805921] [PMID: ] [DOI] [PubMed] [Google Scholar]
BE ABLE 1 2018 {published data only}
- Blauvelt A, Papp KA, Merola JF, Gottlieb AB, Cross N, Madden C, et al. Bimekizumab for patients with moderate-to-severe plaque psoriasis: 60-week results from BE ABLE 2, a randomized, double-blinded, placebo-controlled phase 2b extension study. Journal of the American Academy of Dermatology 2020;83(5):1367-74. [DOI: 10.1016/j.jaad.2020.05.105] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT02905006. Study to evaluate safety and efficacy of different doses of bimekizumab in patients with chronic plaque psoriasis (BE ABLE 1). clinicaltrials.gov/show/nct02905006 (first received 19 September 2016). [CENTRAL: CN-01520880]
- Papp KA, Merola JF, Gottlieb AB, Griffiths CE, Cross N, Peterson L, et al. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. Journal of the American Academy of Dermatology 2018;79(2):277-86.e10. [CENTRAL: CN-01665198] [DOI] [PubMed] [Google Scholar]
BE RADIANT 2021 {published data only}
- Augustin M, Gooderham M, Gottlieb AB. 31069 Bimekizumab versus secukinumab in plaque psoriasis: reduction in body surface area affected by psoriasis translates into benefits in patient-perceived itching, skin pain, and scaling and health-related quality of life in the BE RADIANT phase 3b trial. Journal of the American Academy of Dermatology 2022;87(3 Suppl):AB25. [Google Scholar]
- Augustin M, Gottlieb AB, Laws P, Wu JJ, Sebastian M, Eusebi P, et al. Bimekizumab versus secukinumab in plaque psoriasis: higher efficacy translates into benefits in patient-perceived symptoms and health-related quality of life in the BE RADIANT multicenter, randomized, double blinded phase 3b trial. Journal of Clinical and Aesthetic Dermatology 2022;15(4 Suppl 1):S23-4. [Google Scholar]
- NCT03536884. A study to evaluate the efficacy and safety of bimekizumab compared to an active comparator in adult subjects with moderate to severe chronic plaque psoriasis (BE RADIANT). clinicaltrials.gov/show/nct03536884 (first received 25 May 2018).
- Reich K, Warren RB, Lebwohl M, Gooderham M, Strober B, Langley RG, et al. Bimekizumab versus secukinumab in plaque psoriasis. New England Journal of Medicine 2021;385(2):142-52. [DOI] [PubMed] [Google Scholar]
BE READY 2021 {published data only}
- Blauvelt A, Wu JJ, Reich K, Gooderham M, Lebwohl M, White K, et al. 27380 Bimekizumab efficacy in patients with moderate to severe plaque psoriasis during the randomized withdrawal and retreatment phase of BE READY, a phase 3 trial. Journal of the American Academy of Dermatology 2021;85(3 Suppl):AB139. [Google Scholar]
- Gordon KB, Foley P, Krueger JG, Pinter A, Reich K, Vender R, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet 2021;397(10273):475-86 Erratum in: Lancet 2021; 397(10288); 1182. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT03410992. A study with an initial treatment period followed by a randomized-withdrawal period to evaluate the efficacy and safety of bimekizumab in adult subjects with moderate to severe chronic plaque psoriasis (BE READY). clinicaltrials.gov/show/nct03410992 (first received 25 January 2018).
BE SURE 2021 {published data only}
- NCT03412747. A study to evaluate the efficacy and safety of bimekizumab in adult subjects with moderate to severe chronic plaque psoriasis (BE SURE). clinicaltrials.gov/show/nct03412747 (first received 26 January 2018).
- Warren RB, Blauvelt A, Bagel J, Papp K, Yamauchi P, Armstrong A, et al. Bimekizumab efficacy and safety versus adalimumab in patients with moderate-to-severe plaque psoriasis: results from a multicenter, randomized, double-blinded active comparator-controlled phase III trial (BE SURE). Journal of Clinical and Aesthetic Dermatology 2021;14(5 Suppl 1):S21-2. [Google Scholar]
- Warren RB, Blauvelt A, Bagel J, Papp KA, Yamauchi P, Armstrong A, et al. Bimekizumab versus adalimumab in plaque psoriasis. New England Journal of Medicine 2021;385(2):130-41. [DOI] [PubMed] [Google Scholar]
BE VIVID 2021 {published data only}
- Gordon K, Foley P, Rich P, Duffin K, Pinter A, Griffiths CEM, et al. Bimekizumab versus ustekinumab in plaque psoriasis: lasting efficacy translates to rapid and sustained improvements in quality of life in the BE VIVID multicentre, randomized, double-blinded phase III trial. British Journal of Dermatology 2022;187(1):e38-9. [Google Scholar]
- Gordon K, Foley P, Rich P, Duffn K, Pinter A, Griffths CEM, et al. Bimekizumab versus ustekinumab in plaque psoriasis: lasting efficacy translates to rapid and sustained improvements in quality of life in the BE VIVID multicenter, randomized, double-blinded Phase III trial. Journal of Clinical and Aesthetic Dermatology 2021;14(5 Suppl 1):S23-4. [Google Scholar]
- NCT03370133. A study to evaluate the efficacy and safety of bimekizumab compared to placebo and an active comparator in adult subjects with moderate to severe chronic plaque psoriasis (BE VIVID). clinicaltrials.gov/show/nct03370133 (first received 12 December 2017).
- Papp K, Lebwohl M, Gottlieb AB, Sebastian M, Langley R, Okubo Y, et al. Bimekizumab for the treatment of moderate-to-severe plaque psoriasis with scalp, nail and palmoplantar involvement through 52 weeks: post-hoc analysis from the BE VIVID Phase III trial. Journal of Clinical and Aesthetic Dermatology 2021;14(5 Suppl 1):S22. [Google Scholar]
- Reich K, Papp KA, Blauvelt A, Langley RG, Armstrong A, Warren RB, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet 2021;397(10273):487-98. [DOI] [PubMed] [Google Scholar]
Bissonnette 2013 {published data only}
- Bissonnette R, Tardif J-C, Harel F, Pressacco J, Bolduc C, Guertin MC. Effects of the tumor necrosis factor-alpha antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: results of a randomized controlled trial. Circulation: Cardiovascular Imaging 2013;6(1):83-90. [CENTRAL: CN-00906599] [EMBASE: 2013325307] [DOI] [PubMed] [Google Scholar]
Blauvelt 2021a {published data only}
- Blauvelt A, Gordon KB, Lee P, Bagel J, Sofen H, Lockshin B, et al. Efficacy, safety, usability, and acceptability of risankizumab 150 mg formulation administered by prefilled syringe or by an autoinjector for moderate to severe plaque psoriasis. Journal of Dermatological Treatment 2022;33(4):2085-93. [DOI] [PubMed] [Google Scholar]
- NCT03875482. A study to assess safety and efficacy of risankizumab using a new formulation in participants with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct03875482 (first received 14 March 2019).
BRIDGE 2017 {published data only}
- Mrowietz U, Szepietowski JC, Loewe R, Van de Kerkhof P, Lamarca R, Ocker WG, et al. Efficacy and safety of LAS41008 (dimethyl fumarate) in adults with moderate-to-severe chronic plaque psoriasis: a randomized, double-blind, Fumaderm® - and placebo-controlled trial (BRIDGE). British Journal of Dermatology 2017;176(3):615-23 Corrigendum to: British Journal of Dermatology 2018; 178(1); 308. [CENTRAL: CN-01336917] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Van de Kerkhof PCM, Loewe R, Mrowietz U, Falques M, Pau-Charles I, Szepietowski JC. Quality of life outcomes in adults with moderate-to-severe plaque psoriasis treated with dimethylfumarate (DMF): a post hoc analysis of the BRIDGE study. Journal of the European Academy of Dermatology & Venereology 2020;34(1):119-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Kerkhof PCM, Loewe R, Mrowietz U, Falques M, Pau-Charles I, Szepietowski JC. Quality of life outcomes in adults with moderate-to-severe plaque psoriasis treated with dimethylfumarate (DMF): a post-hoc analysis of the BRIDGE study. Journal of the European Academy of Dermatology & Venereology 2020;34(1):119-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cai 2016 {published data only}
- Cai L, Gu J, Zheng J, Zheng M, Wang G, Xi LY, et al. Efficacy and safety of adalimumab in Chinese patients with moderate-to-severe plaque psoriasis: results from a phase 3, randomized, placebo-controlled, double-blind study. Journal of the European Academy of Dermatology and Venereology 2016;31(1):89-95. [CENTRAL: CN-01248561] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cai 2020 {published data only}
- Anonymous. Secukinumab 300 mg showed faster and higher efficacy in Chinese moderate to severe plaque psoriasis patients. Journal of the American Academy of Dermatology 2019;81(4 Suppl 1):AB445. [Google Scholar]
- Cai L, Zhang JZ, Yao X, Gu J, Liu Q-Z, Zheng M, et al. Secukinumab demonstrates high efficacy and a favorable safety profile over 52 weeks in Chinese patients with moderate to severe plaque psoriasis. Chinese Medical Journal 2020;133(22):2665-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03066609. Study of efficacy and safety of secukinumab in subjects with moderate to severe chronic plaque-type psoriasis. clinicaltrials.gov/show/nct03066609 (first received 28 February 2017).
- TCTR20161028001. A randomised, double-blind, placebo controlled, multicenter study of subcutaneous secukinumab, to demonstrate efficacy after twelve weeks of treatment and to assess safety, tolerability and long-term efficacy up to one year in subjects with moderate to severe chronic plaque-type psoriasis with or without psoriatic arthritis comorbidity. www.thaiclinicaltrials.org/show/TCTR20161028001 (first received 28 February 2017).
Cai 2022 {published data only}
- Cai L, Li L, Cheng H, Ding Y, Biao Z, Zhang S, et al. Efficacy and safety of HLX03, an adalimumab biosimilar, in patients with moderate-to-severe plaque psoriasis: a randomized, double-blind, phase iii study. Advances in Therapy 2022;39(1):583-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
CALYPSO 2018 {published data only}
- CTRI/2018/03/012598. Comparative double-blind study of the efficacy and safety of BCD-057 and Humira® in patients with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/03/012598 (first received 15 March 2018).
- Korotaeva TV, Samtsov AV, Bakulev AL, Kokhan MM, Minullin IK, Vylegzhanina OA, et al. Comparative efficacy and safety of adalimumab biosimilar (BCD-057) and innovator in patients with psoriasis vulgaris. Results of the BCD-057-2/CALYPSO phase III international, multicenter, randomized double-blind clinical trial [Сравнительная эффективностьи безопасность биоаналога адалимумаба (BCD-057) и оригинального адалимумаба у пациентов с вульгарным псориазом. Результаты BCD-057-2/CALYPSO – международного многоцентрового рандомизированного двойного слепого клинического исследования III фазы]. Modern Rheumatology Journal 2018;12(4):71-84. [DOI: 10.14412/1996-7012-2018-4-71-84] [DOI] [Google Scholar]
- NCT02762955. Comparative clinical trial of efficacy and safety of BCD-057 and Humira® in patients with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct02762955 (first received 5 May 2016).
Caproni 2009 {published data only}
- Caproni M, Antiga E, Melani L, Volpi W, Bianco E, Fabbri P. Serum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: a randomized-controlled trial. Journal of Clinical Immunology 2009;29(2):210-4. [CENTRAL: CN-00685566] [PMID: ] [DOI] [PubMed] [Google Scholar]
CARIMA 2019 {unpublished data only}
- NCT02559622. Evaluation of cardiovascular risk markers in psoriasis patients treated with secukinumab (CARIMA). clinicaltrials.gov/ct2/show/NCT02559622 (first received 4 August 2015).
- Von Stebut E, Reich K, Thaçi D, Koenig W, Pinter A, Korber A, et al. Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. Journal of Investigative Dermatology 2019;139(5):1054-62. [DOI] [PubMed] [Google Scholar]
Cestari 2021 {published data only}
- Cestari TF, DaSilva Souza C, Azulay-Abulafia L, Romiti R, Carvalho AVE, Silva de Castro CC, et al. 26197 Efficacy and safety of risankizumab vs methotrexate in patients with moderate-to-severe plaque psoriasis: results from the 28-week randomized, double-blind period of an ongoing phase 3 study in Brazil. Journal of the American Academy of Dermatology 2021;85(3 Suppl):AB88. [Google Scholar]
CHAMPION 2008 {published data only}
- Navarini AA, Poulin Y, Menter A, Gu Y, Teixeira HD. Analysis of body regions and components of PASI scores during adalimumab or methotrexate treatment for patients with moderate-to-severe psoriasis. Journal of Drugs in Dermatology 2014;13(5):554-62. [CENTRAL: CN-00993155] [PMID: ] [PubMed] [Google Scholar]
- Prussick R, Unnebrink K, Valdecantos WC. Efficacy of adalimumab compared with methotrexate or placebo stratified by baseline BMI in a randomized placebo-controlled trial in patients with psoriasis. Journal of Drugs in Dermatology 2015;14(8):864-8. [CENTRAL: CN-01132989] [PMID: ] [PubMed] [Google Scholar]
- Reich K, Signorovitch J, Ramakrishnan K, Yu AP, Wu EQ, Gupta SR, et al. Benefit-risk analysis of adalimumab versus methotrexate and placebo in the treatment of moderate to severe psoriasis: comparison of adverse event-free response days in the CHAMPION trial. Journal of the American Academy of Dermatology 2010;63(6):1011-8. [CENTRAL: CN-00791143] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Revicki D, Willian MK, Saurat JH, Papp KA, Ortonne JP, Sexton C, et al. Impact of adalimumab treatment on health-related quality of life and other patient-reported outcomes: results from a 16-week randomized controlled trial in patients with moderate to severe plaque psoriasis. British Journal of Dermatology 2008;158(3):549-57. [CENTRAL: CN-00628564] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Saurat JH, Langley RG, Reich K, Unnebrink K, Sasso EH, Kampman W. Relationship between methotrexate dosing and clinical response in patients with moderate to severe psoriasis: subanalysis of the CHAMPION study. British Journal of Dermatology 2011;165(2):399-406. [CENTRAL: CN-00800067] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). British Journal of Dermatology 2008;158(3):558-66. [CENTRAL: CN-00628565] [PMID: ] [DOI] [PubMed] [Google Scholar]
CHANGE 2021 {published data only}
- NCT03331835. A trial comparing the efficacy of subcutaneous injections of brodalumab to oral administrations of fumaric acid esters in adults with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct03331835 (first received 6 November 2017).
- Pinter A, Hoffmann M, Reich K, Augustin M, Kaplan K, Gudjonsdottir SD, et al. A phase 4, randomised, head-to-head trial comparing the efficacy of subcutaneous injections of brodalumab to oral administrations of fumaric acid esters in adults with moderate-to-severe plaque psoriasis (CHANGE). Journal of the European Academy of Dermatology & Venereology 2020;16:16. [DOI] [PubMed] [Google Scholar]
- Pinter A, Hoffmann M, Reich K, Augustin M, Kaplan K, Gudjonsdottir SD, et al. A phase 4, randomized, head-to-head trial comparing the efficacy of subcutaneous injections of brodalumab to oral administrations of fumaric acid esters in adults with moderate-to-severe plaque psoriasis (CHANGE). Journal of the European Academy of Dermatology and Venereology 2021;35(3):701-11. [DOI] [PubMed] [Google Scholar]
Chaudhari 2001 {published data only}
- Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet 2001;357(9271):1842-7. [CENTRAL: CN-00348743] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Chaudhari U, Mulcahy LD, Li S, Dooley LT, Baker DG. Infliximab monotherapy provides rapid and sustained benefit for plaque-type psoriasis. Journal of the American Academy of Dermatology 2003;48(6):829-35. [CENTRAL: CN-00438058] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Masud S, Ramamurthi R, Abdulghani A, Romano P, Chaudhari U, et al. Pharmacodynamic and pharmacokinetic response to anti-tumor necrosis factor-alpha monoclonal antibody (infliximab) treatment of moderate to severe psoriasis vulgaris. Journal of the American Academy of Dermatology 2003;48(1):68-75. [CENTRAL: CN-00466030] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chladek 2005 {published data only}
- Chladek J, Grim J, Martinkova J, Simkova M, Vaneckova J. Low-dose methotrexate pharmacokinetics and pharmacodynamics in the therapy of severe psoriasis. Basic & Clinical Pharmacology & Toxicology 2005;96(3):247-8. [CENTRAL: CN-00513064] [PMID: ] [DOI] [PubMed] [Google Scholar]
CIMPACT 2018 {unpublished data only}
- Lebwohl M, Blauvelt A, Paul C, Sofen H, Weglowska J, Piguet V, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks of a phase 3, multicenter, randomized, double-blind, etanercept- and placebo-controlled study (CIMPACT). Journal of the American Academy of Dermatology 2018;79(2):266-76.e5. [CENTRAL: CN-01665155] [DOI] [PubMed] [Google Scholar]
- NCT02346240. Efficacy and safety study of certolizumab pegol (CZP) versus active comparator and placebo in subjects with plaque psoriasis (PSO) (CIMPACT). clinicaltrials.gov/ct2/show/NCT02346240 (first received 20 January 2015). [CENTRAL: CN-01551710]
CIMPASI‐1 2018 {published data only}
- Gottlieb AB, Blauvelt A, Thaçi D, Leonardi CL, Poulin Y, Drew J, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2). Journal of the American Academy of Dermatology 2018;79(2):302-14.e6. [CENTRAL: CN-01665156] [DOI] [PubMed] [Google Scholar]
- NCT02326298. An efficacy and safety study of two dose levels of certolizumab pegol (CZP) in subjects with plaque psoriasis (PSO). clinicaltrials.gov/show/nct02326298 (first received 29 December 2014). [CENTRAL: CN-01575600]
CIMPASI‐2 2018 {published and unpublished data}
- Gottlieb AB, Blauvelt A, Thaçi D, Leonardi CL, Poulin Y, Drew J, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2). Journal of the American Academy of Dermatology 2018;79(2):302-14.e6. [CENTRAL: CN-01665156] [DOI] [PubMed] [Google Scholar]
- NCT02326272. A study to evaluate the efficacy and safety of two dose levels of certolizumab pegol (CZP) in subjects with plaque psoriasis (PSO) (CIMPASI-2). clinicaltrials.gov/ct2/show/NCT02326272 (first received 22 December 2014). [CENTRAL: CN-01575599]
CLARITY 2018 {unpublished data only}
- Anonymous. Secukinumab is superior to ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: CLARITY, a randomized, controlled, phase 3b trial. Journal of the American Academy of Dermatology 2019;81(4 Supp 1):AB274. [Google Scholar]
- Bagel J, Blauvelt A, Nia J, Hashim P, Patekar M, De Vera A, et al. Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY). Journal of the European Academy of Dermatology and Venereology 2020;35(1):135-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagel J, Blauvelt A, Nia J, Hashim P, Patekar M, Vera A, et al. Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY). Journal of the European Academy of Dermatology & Venereology 2020 May 4 [Epub ahead of print]. [DOI: 10.1111/jdv.16558] [DOI] [PMC free article] [PubMed]
- Bagel J, Nia J, Hashim P, Patekar M, De Vera A, Hugot S, et al. Secukinumab is superior to ustekinumab in clearing skin of patients with moderate-to-severe plaque psoriasis: CLARITY, a randomized, controlled, Phase IIIb trial. Journal of Clinical and Aesthetic Dermatology 2018;11(5 Suppl 1):S27-8. [CENTRAL: CN-01713715] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagel J, Nia J, Hashim PW, Patekar M, De Vera A, Hugot S, et al. Secukinumab is superior to ustekinumab in clearing skin in patients with moderate to severe plaque psoriasis (16-week CLARITY results). Dermatology and Therapy 2018;8(4):571-9. [CENTRAL: CN-01667027] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02826603. Study of secukinumab compared to ustekinumab in subjects with plaque psoriasis (CLARITY). clinicaltrials.gov/ct2/show/NCT02826603 (first received 11 July 2016). [CENTRAL: CN-01506686]
CLEAR 2015 {published data only}
- Blauvelt A, Korman N, Mollon P, Zhao Y, Milutinovic M, You R, et al. Secukinumab treatment provides faster and more effective relief from patient-reported quality of life impact than ustekinumab in subjects with moderate to severe plaque psoriasis. Journal of Clinical and Aesthetic Dermatology 2017;10(5 Suppl 1):S15-6. [CENTRAL: CN-01713052] [Google Scholar]
- Blauvelt A, Reich K, Mehlis S, Vanaclocha F, Sofen H, Abramovits W, et al. Secukinumab demonstrates greater sustained improvements in daily activities and personal relationships than ustekinumab in patients with moderate-to-severe plaque psoriasis: 52-week results from the CLEAR study. Journal of the European Academy of Dermatology and Venereology 2017;31(10):1693-9. [CENTRAL: CN-01615823] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauvelt A, Reich K, Tsai TF, Tyring S, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. Journal of the American Academy of Dermatology 2017;76(1):60-9.e9. [CENTRAL: CN-01368612] [DOI] [PubMed] [Google Scholar]
- Herranz Pinto P, Rivera R, Blauvelt A, Thaçi D, Oliver Vigueras J. Secukinumab 300mg is more efficacious than ustekinumab 90mg: analysis of the CLEAR study. Journal of Clinical and Aesthetic Dermatology 2017;10(5 Suppl 1):S18-9. [CENTRAL: CN-01713059] [Google Scholar]
- Spelman L, Pinto PH, Rivera R, Blauvelt A, Thaçi D, Vigueras JO. Secukinumab 300 mgs is more efficacious than ustekinumab 90 mgs: analysis of patients with body weights over 100kg from the CLEAR study. Australasian Journal of Dermatology 2017;58(Suppl 1):86-7. [CENTRAL: CN-01378828] [Google Scholar]
- Thaçi D, Blauvelt A, Reich K, Tsai TF, Vanaclocha F, Kingo K, et al. Secukinumab delivers greater improvement in health-related quality of life compared to ustekinumab in subjects with moderate-to-severe plaque psoriasis: 16-week data from the CLEAR study. Journal of Clinical and Aesthetic Dermatology 2016;9(5 Suppl 1):S17-8. [CENTRAL: CN-01713053] [Google Scholar]
- Thaçi D, Blauvelt A, Reich K, Tsai TF, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. Journal of the American Academy of Dermatology 2015;73(3):400-9. [CENTRAL: CN-01090628] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dogra 2012 {published data only}
- Dogra S, Krishna V, Kanwar AJ. Efficacy and safety of systemic methotrexate in two fixed doses of 10 mg or 25 mg orally once weekly in adult patients with severe plaque-type psoriasis: a prospective, randomized, double-blind, dose-ranging study. Clinical and Experimental Dermatology 2012;37(7):729-34. [CENTRAL: CN-00879485] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dogra 2013 {published data only}
- Dogra S, Jain A, Kanwar AJ. Efficacy and safety of acitretin in three fixed doses of 25, 35 and 50 mg in adult patients with severe plaque type psoriasis: a randomized, double blind, parallel group, dose ranging study. Journal of the European Academy of Dermatology and Venereology 2013;27(3):e305-11. [CENTRAL: CN-00911790] [EMBASE: 2013118368] [DOI] [PubMed] [Google Scholar]
Dubertret 1989 {published data only}
- Dubertret L, Perussel M, Robiola O, Feutren G. Cyclosporin in psoriasis. A long-term randomized study on 37 patients. Acta Dermato-Venereologica 1989;69(146 Suppl):136. [CENTRAL: CN-00064909] [PMID: ] [PubMed] [Google Scholar]
ECLIPSE 2019 {published data only}
- Armstrong A, Blauvelt A, Flavin S, Hsu MC, Randazzo B, Reich K, et al. Guselkumab demonstrates greater efficacy compared to secukinumab across body weight quartiles and body mass index categories: week 48 results from the ECLIPSE trial. Journal of Clinical and Aesthetic Dermatology 2020;12(5 Suppl):S24. [Google Scholar]
- Blauvelt A, Armstrong A W, Langley R G, Gebauer K, Thaci D, Bagel J, et al. Efficacy of guselkumab versus secukinumab in subpopulations of patients with moderate-to-severe plaque psoriasis: results from the ECLIPSE study. Journal of Dermatological Treatment 2022;33(4):2317-24. [DOI] [PubMed] [Google Scholar]
- Langley RG, Ferris L, Gebauer K, Arenberger P, Gooderham M, Guenther L, et al. Consistent responses to guselkumab by disease region at week 48 in the treatment of moderate-to-severe psoriasis: ECLIPSE trial results. Australasian Journal of Dermatology 2021;62 Suppl 1:58. [Google Scholar]
- Langley RG, Probity KG, Arenberger P, Flavin S, Hsu MC, Li S, et al. 15302 Psoriasis area and severity index component improvements at week 48 in patients treated with guselkumab compared with secukinumab: findings from the ECLIPSE study. Journal of the American Academy of Dermatology 2020;83(6 Suppl):AB34. [Google Scholar]
- NCT03090100. A study to evaluate the comparative efficacy of CNTO 1959 (guselkumab) and secukinumab for the treatment of moderate to severe plaque-type psoriasis (ECLIPSE). clinicaltrials.gov/show/nct03090100 (first received 24 March 2017).
- Reich K, Armstrong AW, Langley RG, Flavin S, Randazzo B, Li S, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet 2019;394(10201):831-9. [DOI] [PubMed] [Google Scholar]
EGALITY 2017 {published data only}
- Gerdes S, Thaçi D, Griffiths CE, Arenberger P, Poetzl J, Wuerth G, et al. Multiple switches between GP2015, an etanercept biosimilar, with originator product do not impact efficacy, safety and immunogenicity in patients with chronic plaque-type psoriasis: 30-week results from the phase 3, confirmatory EGALITY study. Journal of the European Academy of Dermatology and Venereology 2018;32(3):420-7. [CENTRAL: CN-01643364] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths CE, Reich K, Thaçi D, Gerdes S, Arenberger P, Kingo K, et al. Switching treatments of etanercept biosimilar GP2015 with originator product does not impact efficacy, safety and immunogenicity in patients with chronic plaque-type psoriasis. Journal of Investigative Dermatology 2017;137(10 Suppl 2):S193. [CENTRAL: CN-01416626] [Google Scholar]
- Griffiths CE, Thaçi D, Gerdes S, Arenberger P, Pulka G, Kingo K, et al. The EGALITY study: a confirmatory, randomized, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, vs. the originator product in patients with moderate-to-severe chronic plaque-type psoriasis. British Journal of Dermatology 2017;176(4):928-38. [CENTRAL: CN-01424735] [DOI] [PubMed] [Google Scholar]
- NCT01891864. Study to demonstrate equivalent efficacy and to compare safety of biosimilar etanercept (GP2015) and Enbrel (EGALITY). clinicaltrials.gov/show/nct01891864 (first received 3 July 2013). [CENTRAL: CN-01597476]
Elewski 2016 {published data only}
- Elewski BE, Okun MM, Papp K, Baker CS, Crowley JJ, Guillet G, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of a phase 3, randomized, placebo-controlled trial. Journal of the American Academy of Dermatology 2018;78(1):90-99.e1. [CENTRAL: CN-01443156] [DOI: 10.1016/j.jaad.2017.08.029] [DOI] [PubMed] [Google Scholar]
- Elewski BE, Rich PA, Behrens F, Guillet G, Geng Z, Reyes Servin O. Primary efficacy and safety of adalimumab in nail psoriasis from the first 26 weeks of a phase-3, randomized, placebo-controlled trial with subanalysis in patients with and without psoriatic arthritis. Annals of the Rheumatic Diseases 2017;76(Suppl 2):1319-20. [CENTRAL: CN-01467694] [DOI: 10.1136/annrheumdis-2017-eular.2148] [DOI] [Google Scholar]
- Elewski BE, Rich PA, Behrens F, Guillet G, Geng Z, Servin OR. Primary efficacy and safety of adalimumab in nail psoriasis from the first 26 weeks of a phase-3, randomized, placebo-controlled trial with subanalysis in patients with and without psoriatic arthritis. Acta Dermato-Venereologica 2018;98(Suppl 219):26-7. [CENTRAL: CN-01620195] [Google Scholar]
- Elewski BE, Rich PA, Okun MM, Papp K, Baker CS, Crowley JJ, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of a phase-3, randomized, placebo-controlled trial. Journal of the European Academy of Dermatology and Venereology 2016;30(Suppl 6):65. [CENTRAL: CN-01786943] [EMBASE: 611235503] [Google Scholar]
Ellis 1991 {published data only}
- Ellis CN, Fradin MS, Messana JM, Brown MD, Siegel MT, Hartley AH, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. New England Journal of Medicine 1991;324(5):277-84. [CENTRAL: CN-00072304] [PMID: ] [DOI] [PubMed] [Google Scholar]
Engst 1994 {published data only}
- Engst RH, Bubl R, Huber J, Schober C, Jessberger B. Long-term cyclosporin A for psoriasis. Acta Dermatovenerologica Alpina, Panonica et Adriatica 1994;3(4):188-92. [CENTRAL: CN-00178929] [EMBASE: 1995061459] [Google Scholar]
ERASURE 2014 {published data only}
- Gottlieb AB, Langley RG, Philipp S, Sigurgeirsson B, Blauvelt A, Martin R, et al. Secukinumab improves physical function in subjects with plaque psoriasis and psoriatic arthritis: results from two randomized, phase 3 trials. Journal of Drugs in Dermatology 2015;14(8):821-33. [CENTRAL: CN-01132993] [PMID: ] [PubMed] [Google Scholar]
- Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis - results of two phase 3 trials. New England Journal of Medicine 2014;371(4):326-38. [CENTRAL: CN-00999505] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ohtsuki M, Morita A, Abe M, Takahashi H, Seko N, Karpov A, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. Journal of Dermatology 2014;41(12):1039-46. [CENTRAL: CN-01037251] [PMID: ] [DOI] [PubMed] [Google Scholar]
ESTEEM‐1 2015 {published data only}
- Bissonnette R, Pariser DM, Wasel NR, Goncalves J, Day RM, Chen R, et al. Apremilast, an oral phosphodiesterase-4 inhibitor, in the treatment of palmoplantar psoriasis: results of a pooled analysis from phase II PSOR-005 and phase III Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis (ESTEEM) clinical trials in patients with moderate to severe psoriasis. Journal of the American Academy of Dermatology 2016;75(1):99-105. [CENTRAL: CN-01470982] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RG, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). Journal of the American Academy of Dermatology 2015;73(1):37-49. [CENTRAL: CN-01085116] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rich P, Gooderham M, Bachelez H, Goncalves J, Day RM, Chen R, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with difficult-to-treat nail and scalp psoriasis: results of 2 phase III randomized, controlled trials (ESTEEM 1 and ESTEEM 2). Journal of the American Academy of Dermatology 2016;74(1):134-42. [CENTRAL: CN-01127546] [PMID: ] [DOI] [PubMed] [Google Scholar]
ESTEEM‐2 2015 {published data only}
- Paul C, Cather J, Gooderham M, Poulin Y, Mrowietz U, Ferrandiz C, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). British Journal of Dermatology 2015;173(6):1387-99. [CENTRAL: CN-01133855] [PMID: ] [DOI] [PubMed] [Google Scholar]
EXPRESS 2005 {published data only}
- Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 2005;366(9494):1367-74. [CENTRAL: CN-00531178] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Reich K, Nestle FO, Papp K, Ortonne JP, Wu Y, Bala M, et al. Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate-to-severe psoriasis: a randomized controlled trial. British Journal of Dermatology 2006;154(6):1161-8. [CENTRAL: CN-00565410] [PMID: ] [DOI] [PubMed] [Google Scholar]
EXPRESS‐II 2007 {published data only}
- Feldman SR, Gottlieb AB, Bala M, Wu Y, Eisenberg D, Guzzo C, et al. Infliximab improves health-related quality of life in the presence of comorbidities among patients with moderate-to-severe psoriasis. British Journal of Dermatology 2008;159(3):704-10. [CENTRAL: CN-00668311] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. Journal of the American Academy of Dermatology 2007;56(1):31.e1-15. [CENTRAL: CN-00576883] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Reich K, Nestle FO, Wu Y, Bala M, Eisenberg D, Guzzo C, et al. Infliximab treatment improves productivity among patients with moderate-to-severe psoriasis. European Journal of Dermatology 2007;17(5):381-6. [CENTRAL: CN-00699412] [DOI] [PubMed] [Google Scholar]
- Reich K, Ortonne JP, Kerkmann U, Wang Y, Saurat JH, Papp K, et al. Skin and nail responses after 1 year of infliximab therapy in patients with moderate-to-severe psoriasis: a retrospective analysis of the EXPRESS trial. Dermatology 2010;221(2):172-8. [CENTRAL: CN-01628937] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fallah Arani 2011 {published data only}
- Fallah Arani S, Neumann H, Hop WC, Thio HB. Fumarates vs. methotrexate in moderate to severe chronic plaque psoriasis: a multicentre prospective randomized controlled clinical trial. British Journal of Dermatology 2011;164(4):855-61. [CENTRAL: CN-00785701] [PMID: ] [DOI] [PubMed] [Google Scholar]
- ISRCTN76608307. A comparison of the efficacy of oral fumarate and methotrexate therapy in the treatment of severe psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN76608307 (first received 1 September 2006).
FEATURE 2015 {published data only}
- Blauvelt A, Prinz JC, Gottlieb AB, Kingo K, Sofen H, Ruer-Mulard M, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). British Journal of Dermatology 2015;172(2):484-93. [CENTRAL: CN-01052626] [PMID: ] [DOI] [PubMed] [Google Scholar]
Feldman 2021 {published data only}
- EUCTR2017-003367-35-PL. A multicenter, double-blind, randomized, parallel-group, active control study to compare the efficacy, safety, and immunogenicity of AVT02 versus Humira® in patients with moderate-to-severe chronic plaque psoriasis (ALVOPAD PS). www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2017-003367-35-PL (first received 27 November 2018).
- Feldman SR, Reznichenko N, Pulka G, Kingo K, Galdava G, Berti F, et al. Efficacy, safety and immunogenicity of AVT02 versus originator adalimumab in subjects with moderate to severe chronic plaque psoriasis: a multicentre, double-blind, randomised, parallel group, active control, phase III study. BioDrugs 2021;35(6):735-48. [DOI: 10.1007/s40259-021-00502-w] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
FIXTURE 2014 {published data only}
- Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis - results of two phase 3 trials. New England Journal of Medicine 2014;371(4):326-38. [CENTRAL: CN-00999505] [PMID: ] [DOI] [PubMed] [Google Scholar]
Flytström 2008 {published data only}
- Flytström I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. British Journal of Dermatology 2008;158(1):116-21. [CENTRAL: CN-00628309] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gisondi 2008 {published data only}
- Gisondi P, Del Giglio M, Cotena C, Girolomoni G. Combining etanercept and acitretin in the therapy of chronic plaque psoriasis: a 24-week, randomized, controlled, investigator-blinded pilot trial. British Journal of Dermatology 2008;158(6):1345-9. [CENTRAL: CN-00638916] [PMID: ] [DOI] [PubMed] [Google Scholar]
Goldfarb 1988 {published data only}
- Goldfarb MT, Ellis CN, Gupta AK, Tincoff T, Hamilton TA, Voorhees JJ. Acitretin improves psoriasis in a dose-dependent fashion. Journal of the American Academy of Dermatology 1988;18(4 Pt 1):655-62. [CENTRAL: CN-00053926] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gupta AK, Goldfarb MT, Ellis CN, Voorhees JJ. Side-effect profile of acitretin therapy in psoriasis. Journal of the American Academy of Dermatology 1989;20(6):1088-93. [CENTRAL: CN-00061373] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gordon 2006 {published data only}
- Gordon KB, Langley RG, Leonardi C, Toth D, Menter MA, Kang S, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. Journal of the American Academy of Dermatology 2006;55(4):598-606. [CENTRAL: CN-00568251] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Shikiar R, Heffernan M, Langley RG, Willian MK, Okun MM, Revicki DA. Adalimumab treatment is associated with improvement in health-related quality of life in psoriasis: patient-reported outcomes from a phase II randomized controlled trial. Journal of Dermatological Treatment 2007;18(1):25-31. [CENTRAL: CN-00579275] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Shikiar R, Willian MK, Okun MM, Thompson CS, Revicki DA. The validity and responsiveness of three quality of life measures in the assessment of psoriasis patients: results of a phase II study. Health and Quality of Life Outcomes 2006;27(4):71. [CENTRAL: CN-00576575] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordon X‐PLORE 2015 {published data only}
- Gordon KB, Duffin KC, Bissonnette R, Prinz JC, Wasfi Y, Li S, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. New England Journal of Medicine 2015;373(2):136-44. [CENTRAL: CN-01076768] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gottlieb 2003a {published data only}
- Gottlieb AB, Matheson RT, Lowe N, Krueger GG, Kang S, Goffe BS, et al. A randomized trial of etanercept as monotherapy for psoriasis. Archives of Dermatology 2003;139(12):1627-32. [CENTRAL: CN-00459604] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gottlieb 2004a {published data only}
- Feldman SR, Gordon KB, Bala M, Evans R, Li S, Dooley LT, et al. Infliximab treatment results in significant improvement in the quality of life of patients with severe psoriasis: a double-blind placebo-controlled trial. British Journal of Dermatology 2005;152(5):954-60. [CENTRAL: CN-00513174] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. Journal of the American Academy of Dermatology 2004;51(4):534-42. [CENTRAL: CN-00501751] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gottlieb 2011 {published data only}
- Gottlieb AB, Leonardi C, Kerdel F, Mehlis S, Olds M, Williams DA. Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. British Journal of Dermatology 2011;165(3):652-60. [CENTRAL: CN-00811739] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gottlieb 2012 {published data only}
- Gottlieb AB, Langley RG, Strober BE, Papp KA, Klekotka P, Creamer K, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. British Journal of Dermatology 2012;167(3):649-57. [CENTRAL: CN-00842357] [22533447] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurel 2015 {published data only}
- Gurel G, Saracotlu ZN, Aksu AE. A single-blind study comparing acitretin and narrow-band UVB with the combination of placebo and narrow-band UVB in the treatment of plaque-type psoriasis [Plak tip psoriasis tedavisinde asitretin ve dar bant UVB ile plasebo ve dar bant UVB kombinasyonunun karsilastirilditi tek kor calisma]. Türkderm 2015;49(1):2-6. [CENTRAL: CN-01102298] [EMBASE: 2015064189] [Google Scholar]
Heydendael 2003 {published data only}
- Heydendael VM, Spuls PI, Opmeer BC, De Borgie CA, Reitsma JB, Goldschmidt WF, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. New England Journal of Medicine 2003;349(7):658-65. [CENTRAL: CN-00439969] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Opmeer BC, Heydendael VM, De Borgie CA, Spuls PI, Bossuyt PM, Bos JD, et al. Costs of treatment in patients with moderate to severe plaque psoriasis: economic analysis in a randomized controlled comparison of methotrexate and cyclosporine. Archives of Dermatology 2004;140(6):685-90. [CENTRAL: CN-00468098] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hunter 1963 {published data only}
- Hunter GA, Turner AN. Methotrexate in the treatment of psoriasis: a controlled clinical trial. Australasian Journal of Dermatology 1963;7(2):91-2. [CENTRAL: CN-01437408] [PMID: ] [DOI] [PubMed] [Google Scholar]
Igarashi 2012 {published data only}
- Igarashi A, Kato T, Kato M, Song M, Nakagawa H, Japanese Ustekinumab Study Group. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. Journal of Dermatology 2012;39(3):242-52. [CENTRAL: CN-00860708] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Schenkel B, Kato M, Kato T, Igarashi A, Japanese Ustekinumab Study Group. Impact of ustekinumab on health-related quality of life in Japanese patients with moderate-to-severe plaque psoriasis: results from a randomized, double-blind, placebo-controlled phase 2/3 trial. Journal of Dermatology 2012;39(9):761-9. [CENTRAL: CN-00860068] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ikonomidis 2017 {published data only}
- Ikonomidis I, Papadavid E, Makavos G, Andreadou I, Varoudi M, Gravanis K, et al. Lowering interleukin-12 activity improves myocardial and vascular function compared with tumor necrosis factor - a antagonism or cyclosporine in psoriasis. Circulation. Cardiovascular imaging 2017;10(9):e006283. [CENTRAL: CN-01412809] [DOI] [PubMed] [Google Scholar]
- Ikonomidis I, Varoudi M, Makavos G, Papadavid E, Kapniari I, Andreadou I, et al. Greater improvement of coronary artery function, left ventricular deformation and twisting by treatment with IL-17A antagonist compared to cyclosporine in psoriasis. European Heart Journal 2017;38(Suppl 1):688. [CENTRAL: CN-01468831] [Google Scholar]
- Ikonomidis I, Varoudi M, Makavos G, Papadavid E, Kapniari I, Andreadou I, et al. Treatment with IL-17A antagonist results in a greater improvement of coronary artery function, left ventricular deformation and twisting than cyclosporine in psoriasis. European Heart Journal - Cardiovascular Imaging 2017;18(Suppl 3):iii341. [CENTRAL: CN-01452103] [DOI: 10.1093/ehjci/jex298] [DOI] [Google Scholar]
Ikonomidis 2022 {published data only}
- Ikonomidis I, Pavlidis G, Kadoglou N, Makavos G, Katogiannis K, Kountouri A, et al. Apremilast improves endothelial glycocalyx integrity, vascular and left ventricular myocardial function in psoriasis. Pharmaceuticals (Basel, Switzerland) 2022;15(2):no-pagination. [DOI] [PMC free article] [PubMed] [Google Scholar]
IMMerge 2021 {published data only}
- NCT03478787. Risankizumab versus secukinumab for subjects with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct03478787 (first received 27 March 2018).
- Warren RB, Blauvelt A, Poulin Y, Beeck S, Kelly M, Wu T, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase 3, randomised, open-label, efficacy assessor-blinded clinical trial. British Journal of Dermatology 2021;184(1):50-9. [DOI: 10.1111/bjd.19341] [DOI] [PMC free article] [PubMed] [Google Scholar]
IMMhance 2020 {unpublished data only}
- Blauvelt A, Leonardi CL, Gooderham M, Papp KA, Philipp S, Wu JJ, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatology 2020;156(6):649-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauvelt A, Papp K, Gooderham M, Langley RG, Leonardi C, Lacour JP, et al. Efficacy and safety of risankizumab, an IL-23 inihibitor in patients with moderate-to-severe chronic plaque psoriasis: 16-week phase 3 IMMhance trial results. Journal der Deutschen Dermatologischen Gesellschaft [Journal of the German Society of Dermatology] 2018;16(Suppl 1):18. [CENTRAL: CN-01467570] [Google Scholar]
- Blauvelt A, Papp KA, Gooderham M, Langley RG, Leonardi C, Lacour JP, et al. Efficacy and safety of risankizumab, an interleukin-23 inhibitor, in patients with moderate-to-severe chronic plaque psoriasis: 16-week results from the phase III IMMhance trial. British Journal of Dermatology 2017;177(5):e248. [CENTRAL: CN-01452512] [Google Scholar]
- Blauvelt A, Papp KA, Gooderham M, Langley RG, Leonardi C, Lacour JP, et al. Risankizumab efficacy/safety in moderate-to-severe plaque psoriasis: 16-week results from IMMhance. Acta Dermato-Venereologica 2018;98(Suppl 219):30. [CENTRAL: CN-01620186] [Google Scholar]
- NCT02672852. BI 655066/ABBV-066 (Risankizumab) in moderate to severe plaque psoriasis with randomized withdrawal and re-treatment. clinicaltrials.gov/ct2/show/NCT02672852 (first received 1 February 2016). [CENTRAL: CN-01555522]
IMMpress 2022 {published data only}
- NCT03518047. Risankizumab therapy versus placebo for subjects with psoriasis in the Russian Federation (IMMPRESS). clinicaltrials.gov/show/nct03518047 (first received 8 May 2018).
- Odnopozova L, Edin A, Sukharev A, Wu T, Aydin K, Kelly M, Khotko A. Risankizumab for the treatment of moderate to severe plaque psoriasis in the Russian Federation. Dermatology and Therapy 2022;12(9):2063-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
IMMvent 2019 {unpublished data only}
- EUCTR2015-003623-65. BI 655066 (risankizumab) versus adalimumab in a randomised, double blind, parallel group trial in moderate to severe plaque psoriasis to assess safety and efficacy after 16 weeks of treatment and after inadequate adalimumab treatment response (IMMvent) - BI 655066 (risankizumab) versus adalimumab. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number%3A2015-003623-65/EUCTR2015-003623-65 (first received 17 May 2016). [CENTRAL: CN-01855355]
- NCT02694523. BI 655066/ABBV-066 (risankizumab) compared to active comparator (adalimumab) in patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/nct02694523 (first received 29 February 2016). [CENTRAL: CN-01556126]
- Reich K, Gooderham M, Thaçi D, Crowley JJ, Ryan C, Krueger JG, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet 2019;394(10198):576-86. [DOI: 10.1016/S0140-6736(19)30952-3] [DOI] [PubMed] [Google Scholar]
IXORA‐P 2018 {unpublished data only}
- Langley RG, Papp K, Gooderham M, Zhang L, Mallinckrodt C, Agada N, et al. Efficacy and safety of continuous every-2-week dosing of ixekizumab over 52 weeks in patients with moderate-to-severe plaque psoriasis in a randomized phase III trial (IXORA-P). British Journal of Dermatology 2018;178(6):1315-23. [CENTRAL: CN-01606242] [DOI] [PubMed] [Google Scholar]
- NCT02513550. A study comparing different dosing regimens of ixekizumab (LY2439821) in participants with moderate to severe plaque psoriasis (IXORA-P). clinicaltrials.gov/ct2/show/NCT02513550 (first received 30 July 2015). [CENTRAL: CN-01491284]
- Papp K, Orasan RI, Polzer P, Hennege C, Nica RD, Wilhelm S, et al. Absolute and relative pasi improvements with ixekizumab treatment: results at week 12 from IXORA-P. Acta Dermato-Venereologica 2018;98(Suppl 219):44-5. [CENTRAL: CN-01620199] [Google Scholar]
IXORA‐R 2020 {published data only}
- Blauvelt A, Leonardi C, Elewski B, Crowley JJ, Guenther LC, Gooderham M, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. British Journal of Dermatology 2020 Sep 2 [Epub ahead of print]. [DOI: 10.1111/bjd.19509] [DOI] [PMC free article] [PubMed]
- Blauvelt A, Maari C, Gottlieb AB, ElMaraghy H, Young S, Lima RG, et al. 14152 Patient-reported outcomes in a head-to-head, randomized, double-blinded clinical trial of ixekizumab and guselkumab in patients with moderate to severe plaque psoriasis. Journal of the American Academy of Dermatology 2020;83(6 Suppl):AB19. [Google Scholar]
- Blauvelt A, Papp K, Gottlieb A, Jarell A, Reich K, Maari C, et al. A head-to-head comparison of ixekizumab versus guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety, and speed of response from a randomized, double-blinded trial. British Journal of Dermatology 2020;182(6):1348-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauvelt A, Papp K, Gottlieb A, Jarell A, Reich K, Maari C, et al. A head-to-head comparison of ixekizumab versus guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety, and speed of response from a randomized, double-blinded trial. British Journal of Dermatology 2020;182(6):1348-58. [DOI: 10.1111/bjd.18851] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauvelt A, Papp K, Jarel A, Reich K, Gottlieb AB, Lima RG, et al. A head-to-head comparison of ixekizumab versus guselkumab in patients with moderate to severe plaque psoriasis: 12-week efficacy, safety, and speed of response from a randomized, double-blind trial. Journal of Clinical and Aesthetic Dermatology 2020;12(5 Suppl):S20-1. [Google Scholar]
- Leonardi C, Warren RB, See K, Burge R, Gallo G, McKean-Matthews M, et al. 649 Validation of the Optimal Psoriasis Assessment Tool (OPAT) as a method of assessing psoriasis severity and impact from physician and patient perspectives. Journal of Investigative Dermatology 2021;141(5 Suppl):S113. [Google Scholar]
- NCT03573323. A study of ixekizumab (LY2439821) compared to guselkumab in participants with moderate-to-severe plaque psoriasis (IXORA-R). clinicaltrials.gov/show/nct03573323 (first received 29 June 2018).
IXORA‐S 2017 {unpublished data only}
- Anonymous. Rapid clinical response predicts consistent long-term response in patients with moderate-to-severe psoriasis: Ixekizumab vs. ustekinumab. Journal of the American Academy of Dermatology 2019;81(4 Suppl 1):AB113. [Google Scholar]
- Blauvelt A, Lomaga M, Burge R, Zhu B, Henneges C, Shen W, et al. Ixekizumab provides greater cumulative benefits versus ustekinumab over 24 weeks for patients with moderate-to-severe psoriasis in a randomized, double-blind phase 3b clinical trial. Acta Dermato-Venereologica 2018;98(Suppl 219):55-6. [CENTRAL: CN-01620173] [Google Scholar]
- Blauvelt A, Lomaga M, Burge R, Zhu B, Shen W, Shrom D, et al. Greater cumulative benefits from ixekizumab versus ustekinumab treatment over 52 weeks for patients with moderate-to-severe psoriasis in a randomized, double-blinded phase 3b clinical trial. Journal of Dermatological Treatment 2020;31(2):141-6. [DOI] [PubMed] [Google Scholar]
- Burge RT, Papadimitropoulos M, Henneges C, Garcia EG, Romiti R. Ixekizumab treatment leads to early resolution of bothersome symptoms versus ustekinumab. Value in Health 2017;20(9):A902. [CENTRAL: CN-01431388] [Google Scholar]
- Burkhardt N, Reich K, Lomaga M, Henneges C, Dossenbach M, Wilhelm S, et al. Efficacy and safety of ixekizumab (IXE) compared to ustekinumab (UST) in patients with moderate-to-severe plaque psoriasis: a randomised head-to-head trial. Australasian Journal of Dermatology 2017;58(Suppl 1):43. [CENTRAL: CN-01378805] [Google Scholar]
- Burkhardt N, Wilhelm S, Riedl E, Kelin K, Henneges C, Smith SD, et al. Comparison of ixekizumab with ustekinumab in patients with baseline PASI > 15 and/or at-least 3-previous-non-biologic-therapies-treatment-failures: 24-week post-hoc-analysis from a randomized trial (IXORA-S;NCT02561806). Australasian Journal of Dermatology 2018;59(Suppl 1):41. [CENTRAL: CN-01606958] [Google Scholar]
- Ghislain PD, Conrad C, Dutronc Y, Henneges C, Calderon DS, Vincent M, et al. Comparison of ixekizumab and ustekinumab efficacy in the treatment of nail lesions of patients with moderate-to-severe plaque psoriasis: 24-week data from a phase 3 trial. Arthritis and Rheumatology 2017;69(Suppl 10):1827. [Google Scholar]
- NCT02561806. A study of Ixekizumab (LY2439821) in participants with moderate-to-severe plaque psoriasis. clinicaltrials.gov/ct2/show/NCT02561806 (first received 25 September 2015). [CENTRAL: CN-01492565]
- Paul C, Griffiths CE, Van de Kerkhof PCM, Puig L, Dutronc Y, Henneges C, et al. Ixekizumab provides superior efficacy compared with ustekinumab over 52 weeks of treatment: results from IXORA-S, a phase 3 study. Journal of the American Academy of Dermatology 2019;80(1):70-9.e3. [CENTRAL: CN-01913461] [DOI] [PubMed] [Google Scholar]
- Paul C, Puig L, Dutronc Y, Henneges C, Reich K. Consistency of response across subgroups of patients with moderate-to-severe plaque psoriasis following 52 weeks of treatment with ixekizumab compared to ustekinumab. Journal of Investigative Dermatology 2018;138(5 Suppl 1):S81. [CENTRAL: CN-01605382] [Google Scholar]
- Paul C, Van De Kerkhof P, Dutronc Y, Henneges C, Dossenbach M, Hollister K, et al. 52-week results from IXORA-S: a randomized head-to-head trial of ixekizumab and ustekinumab in patients with moderate-to-severe plaque psoriasis. British Journal of Dermatology 2017;177(5):e293. [CENTRAL: CN-01452513] [Google Scholar]
- Puig L, Lomaga M, Hollister K, Dutronc Y, Berggren L, Van De Kerkhof P. An analysis of patient-reported outcomes in ixora-s: comparing ixekizumab and ustekinumab over 52 weeks in moderate-to-severe psoriasis. Acta Dermato-Venereologica 2020;100(19):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich K, Pinter A, Lacour JP, Ferrandiz C, Micali G, French LE, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. British Journal of Dermatology 2017;177(4):1014-23. [CENTRAL: CN-01393820] [DOI] [PubMed] [Google Scholar]
JUNCTURE 2015 {published data only}
- Lacour JP, Paul C, Jazayeri S, Papanastasiou P, Xu C, Nyirady J, et al. Secukinumab administration by autoinjector maintains reduction of plaque psoriasis severity over 52 weeks: results of the randomized controlled JUNCTURE trial. Journal of the European Academy of Dermatology and Venereology 2017;31(5):847-56. [CENTRAL: CN-01342022] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Paul C, Lacour JP, Tedremets L, Kreutzer K, Jazayeri S, Adams S, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). Journal of the European Academy of Dermatology and Venereology 2015;29(6):1082-90. [CENTRAL: CN-01043227] [PMID: ] [DOI] [PubMed] [Google Scholar]
Khatri 2016 {published data only}
- Khatri S, Amir Y, Min M, Goldblum O, Solotkin K, Yang F, et al. Early onset of clinical improvement with ixekizumab in patients with moderate-to-severe plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2016;30(S6):73-4. [CENTRAL: CN-01786974] [EMBASE: 611235569] [PMC free article] [PubMed] [Google Scholar]
- Khattri S, Goldblum O, Solotkin K, Amir Y, Min MS, Ridenour T, et al. Early onset of clinical improvement with ixekizumab in a randomized, open-label study of patients with moderate-to-severe plaque psoriasis. Journal of Clinical and Aesthetic Dermatology 2018;11(5):33-7. [CENTRAL: CN-01610571] [PMC free article] [PubMed] [Google Scholar]
Krueger 2007 {published data only}
- Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. New England Journal of Medicine 2007;356(6):580-92. [CENTRAL: CN-00575216] [PMID: ] [DOI] [PubMed] [Google Scholar]
Laburte 1994 {published data only}
- Laburte C, Grossman R, Abi-Rached J, Abeywickrama KH, Dubertret L. Efficacy and safety of oral cyclosporin A (CyA; Sandimmun) for long-term treatment of chronic severe plaque psoriasis. British Journal of Dermatology 1994;130(3):366-75. [CENTRAL: CN-00100273] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2016 {published and unpublished data}
- Lee JH, Youn JI, Kim TY, Choi JH, Park CJ, Choe YB, et al. A multicenter, randomized, open-label pilot trial assessing the efficacy and safety of etanercept 50 mg twice weekly followed by etanercept 25 mg twice weekly, the combination of etanercept 25 mg twice weekly and acitretin, and acitretin alone in patients with moderate to severe psoriasis. BMC Dermatology 2016;16(1):11. [CENTRAL: CN-01177229] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leonardi 2003 {published data only}
- Feldman SR, Kimball AB, Krueger GG, Woolley JM, Lalla D, Jahreis A. Etanercept improves the health-related quality of life of patients with psoriasis: results of a phase III randomized clinical trial. Journal of the American Academy of Dermatology 2005;53(5):887-9. [CENTRAL: CN-00561361] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gordon KB, Gottlieb AB, Leonardi CL, Elewski BE, Wang A, Jahreis A, et al. Clinical response in psoriasis patients discontinued from and then reinitiated on etanercept therapy. Journal of Dermatological Treatment 2006;17(1):9-17 Erratum in: Journal of Dermatological Treatment 2006;17(3):192. [CENTRAL: CN-00555056] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Krueger GG, Elewski B, Papp K, Wang A, Zitnik R, Jahreis A. Patients with psoriasis respond to continuous open-label etanercept treatment after initial incomplete response in a randomized, placebo-controlled trial. Journal of the American Academy of Dermatology 2006;54(3 Suppl 2):S112-9. [CENTRAL: CN-01625470] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, et al. Etanercept as monotherapy in patients with psoriasis. New England Journal of Medicine 2003;349(21):2014-22. [CENTRAL: CN-00459025] [14627786] [DOI] [PubMed] [Google Scholar]
Leonardi 2012 {published data only}
- Edson-Heredia E, Banerjee S, Zhu B, Maeda-Chubachi T, Cameron GS, Shen W, et al. A high level of clinical response is associated with improved patient-reported outcomes in psoriasis: analyses from a phase 2 study in patients treated with ixekizumab. Journal of the European Academy of Dermatology and Venereology 2016;30(5):864-5. [CENTRAL: CN-01601237] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gordon KB, Leonardi CL, Lebwohl M, Blauvelt A, Cameron GS, Braun D, et al. A 52-week, open-label study of the efficacy and safety of ixekizumab, an anti-interleukin-17A monoclonal antibody, in patients with chronic plaque psoriasis. Journal of the American Academy of Dermatology 2014;71(6):1176-82. [CENTRAL: CN-01091643] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Langley RG, Rich P, Menter A, Krueger G, Goldblum O, Dutronc Y, et al. Improvement of scalp and nail lesions with ixekizumab in a phase 2 trial in patients with chronic plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2015;29(9):1763-70. [CENTRAL: CN-01089988] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. New England Journal of Medicine 2012;366(13):1190-9. [CENTRAL: CN-00814008] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Tham LS, Tang CC, Choi SL, Satterwhite JH, Cameron GS, Banerjee S. Population exposure-response model to support dosing evaluation of ixekizumab in patients with chronic plaque psoriasis. Journal of Clinical Pharmacology 2014;54(10):1117-24. [CENTRAL: CN-01048421] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Zhu B, Edson-Heredia E, Cameron GS, Shen W, Erickson J, Shrom D, et al. Early clinical response as a predictor of subsequent response to ixekizumab treatment: results from a phase II study of patients with moderate-to-severe plaque psoriasis. British Journal of Dermatology 2013;169(9):1337-41. [CENTRAL: CN-01121535] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Zhu B, Edson-Heredia E, Guo J, Maeda-Chubachi T, Shen W, Kimball AB. Itching is a significant problem and a mediator between disease severity and quality of life for patients with psoriasis: results from a randomized controlled trial. British Journal of Dermatology 2014;171(5):1215-9. [CENTRAL: CN-01036585] [PMID: ] [DOI] [PubMed] [Google Scholar]
LIBERATE 2017 {published data only}
- Reich K, Gooderham M, Bewley A, Green L, Soung J, Petric R, et al. Safety and efficacy of apremilast through 104 weeks in patients with moderate to severe psoriasis who continued on apremilast or switched from etanercept treatment: findings from the LIBERATE study. Journal of the European Academy of Dermatology and Venereology 2018;32(3):397-402. [CENTRAL: CN-01643202] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich K, Gooderham M, Green L, Bewley A, Zhang Z, Khanskaya I, et al. The efficacy and safety of apremilast, etanercept, and placebo, in patients with moderate to severe plaque psoriasis: 52-week results from a phase 3b, randomized, placebo-controlled trial (LIBERATE). Journal of the European Academy of Dermatology and Venereology 2017;31(3):507-17. [CENTRAL: CN-01285623] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2020 {unpublished data only}
- Liu LF, Chen JS, Gu J, Xu JH, Jin HZ, Pang XW, et al. Etanercept biosimilar (recombinant human tumor necrosis factor-alpha receptor II: IgG Fc fusion protein) and methotrexate combination therapy in Chinese patients with moderate-to-severe plaque psoriasis: a multicentre, randomized, double-blind, placebo-controlled trial. Archives of Dermatological Research 2020;312(6):437-45. [DOI] [PubMed] [Google Scholar]
- NCT02313922. Optimizing psoriasis treatment of etanercept combined methotrexate. clinicaltrials.gov/ct2/show/NCT02313922 (first received 10 December 2014).
LOTUS 2013 {published data only}
- Zheng M, Zhu X-J, Song M, Shen Y-K, Wang B-X. A randomized, double-blind, placebo-controlled study of ustekinumab in Chinese patients with moderate to severe plaque psoriasis: LOTUS trial results. Journal of Dermatology 2012;39(s1):238-9. [CENTRAL: CN-01032271] [EMBASE: 70801108] [Google Scholar]
- Zhu X, Zheng M, Song M, Shen YK, Chan D, Szapary PO, et al. Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque-type psoriasis: results from a phase 3 clinical trial (LOTUS). Journal of Drugs in Dermatology 2013;12(2):166-74. [CENTRAL: CN-00965604] [PMID: ] [PubMed] [Google Scholar]
- Zhu X-J, Zheng M, Song M, Han C, Chan D, Shen Y-K, et al. Ustekinumab improves health-related quality of life in Chinese patients with moderate-to-severe plaque psoriasis: results from the LOTUS trial and curative effect observation. Journal of Clinical Dermatology 2014;43(9):521-6. [CENTRAL: CN-01037236] [EMBASE: 2014949827] [Google Scholar]
Lowe 1991 {published data only}
- Lowe NJ, Prystowsky JH, Bourget T, Edelstein J, Nychay S, Armstrong R. Acitretin plus UVB therapy for psoriasis. Comparisons with placebo plus UVB and acitretin alone. Journal of the American Academy of Dermatology 1991;24(4):591-4. [CENTRAL: CN-00075422] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mahajan 2010 {published data only}
- Mahajan R, Kaur I, Kanwar AJ. Methotrexate/narrowband UVB phototherapy combination vs. narrowband UVB phototherapy in the treatment of chronic plaque-type psoriasis - a randomized single-blinded placebo-controlled study. Journal of the European Academy of Dermatology and Venereology 2010;24(5):595-600. [CENTRAL: CN-00759274] [PMID: ] [DOI] [PubMed] [Google Scholar]
MATURE 2021 {published data only}
- EUCTR2018-000518-39-DE. Study of efficacy and safety of secukinumab 2 mL auto-injector (300 mg) injections in subjects with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018-000518-39-DE (first received 15 November 2018).
- NCT03589885. Study of efficacy and safety of secukinumab 2 mL auto-injector (300 mg) in subjects with moderate to severe plaque psoriasis (MATURE). clinicaltrials.gov/show/nct03589885 (first received 18 July 2018).
- Sigurgeirsson B, Browning J, Tyring S, Szepietowski J C, Rivera-Diaz R, Effendy I, et al. Secukinumab demonstrates efficacy, safety, and tolerability upon administration by 2 ml autoinjector in adult patients with plaque psoriasis: 52-week results from MATURE, a randomized, placebo-controlled trial. Dermatologic Therapy 2022;35(3):e15285. [DOI] [PubMed] [Google Scholar]
- Sigurgeirsson B, Browning J, Tyring S, Szepietowski JC, Rivera-Diaz R, Effendy I, et al. 27599 Secukinumab administration by a 2 mL autoinjector demonstrates high efficacy with comparable safety and tolerability in adult patients with plaque psoriasis: 16-week results from MATURE. Journal of the American Academy of Dermatology 2021;85(3 Suppl):AB154. [Google Scholar]
- Sigurgeirsson B, Browning J, Tyring S, Szepietowski JC, Rivera-Diaz R, Effendy I, et al. Secukinumab demonstrates efficacy, safety, and tolerability upon administration by 2 ml autoinjector in adult patients with plaque psoriasis: 52-week results from MATURE, a randomized, placebo-controlled trial. Dermatolology Therapy 2022;35(3):e15285. [DOI: 10.1111/dth.15285] [PMID: ] [DOI] [PubMed] [Google Scholar]
Meffert 1997 {published data only}
- Meffert H, Bräutigam M, Färber L, Weidinger G. Low-dose (1.25 mg/kg) cyclosporin A: treatment of psoriasis and investigation of the influence on lipid profile. Acta Dermato-Venereologica 1997;77(2):137-41. [CENTRAL: CN-00138820] [PMID: ] [DOI] [PubMed] [Google Scholar]
METOP 2017 {published data only}
- Reich K, Reich JLK, Falk TM, Blödorn-Schlicht N, Mrowietz U, Von Kiedrowski R, et al. Clinical response of psoriasis to subcutaneous methotrexate correlates with inhibition of cutaneous T helper 1 and 17 inflammatory pathways. British Journal of Dermatology 2019;181(4):859-62. [DOI] [PubMed] [Google Scholar]
- Warren RB, Mrowietz U, Von Kiedrowski R, Niesmann J, Wilsmann-Theis D, Ghoreschi K, et al. An intensified dosing schedule of subcutaneous methotrexate in patients with moderate to severe plaque-type psoriasis (METOP): a 52 week, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389(10068):528-37. [CENTRAL: CN-01330160] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morita 2022 {published data only}
- Morita A, Yamaguchi Y, Tateishi C, Ikumi K, Yamamoto A, Nishihara H, et al. Efficacy and safety of apremilast and phototherapy versus phototherapy only in psoriasis vulgaris. Journal of Dermatology 2022;49(12):1211-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakagawa 2016 {published data only}
- Nakagawa H, Niiro H, Ootaki K, Japanese Brodalumab Study Group. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. Journal of Dermatological Science 2016;81(1):44-52. [CENTRAL: CN-01133729] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Umezawa Y, Nakagawa H, Niiro H, Ootaki K, Japanese Brodalumab Study Group. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2016;30(11):1957-60. [CENTRAL: CN-01457411] [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT02134210 RaPsOdy {published data only}
- Kivitz AJ, Papp K, Devani A, Pinter A, Sinclair R, Ziv M, et al. Randomized, double-blind study comparing CHS-0214 with etanercept (ENBREL) in patients with psoriasis and psoriatic arthritis. Arthritis and Rheumatology 2016;68(Suppl 10):2142-3. [CENTRAL: CN-01292943] [Google Scholar]
- Leonardi C, Tang H, Kelleher C, Finck B. Evaluation of CHS-0214 as a proposed biosimilar to etanercept for the treatment of chronic plaque psoriasis: one-year results from a randomized, double-blind global trial. Journal of the American Academy of Dermatology 2017;76(6):AB128. [Google Scholar]
- NCT02134210. Comparison of CHS-0214 to Enbrel (etanercept) in patients with chronic plaque psoriasis (PsO). clinicaltrials.gov/show/nct02134210 (first received 9 May 2014). [CENTRAL: CN-01545599]
NCT02581345 {published data only}
- NCT02581345. Phase 3 study of M923 and Humira® in subjects with chronic plaque-type psoriasis. clinicaltrials.gov/show/nct02581345 (first received 21 October 2015). [CENTRAL: CN-01587601]
NCT02762994 {published data only}
- NCT02762994. International clinical trial to evaluate efficacy and safety of multiple subcutaneous injections of BCD-085 in various doses in patients with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct02762994 (first received 5 May 2016).
NCT03055494 ObePso‐S {published data only}
- Krueger JG, Pariser D, Muscianisi E, Kianifard F, Steadman J, Prasad Sarkar MR, et al. 15354 Response to treatment with secukinumab in obese patients with moderate to severe psoriasis. Journal of the American Academy of Dermatology 2020;83 (6 Suppl):AB38. [Google Scholar]
- Krueger JG, Pariser D, Tyring SK, Bagel J, Alexis AF, Soung J, et al. 15340 Long-term treatment with secukinumab led to sustained clinical improvement and normalization of inflammatory markers in patients with psoriasis. Journal of the American Academy of Dermatology 2020;83 (6 Suppl):AB37. [Google Scholar]
- NCT03055494. Study to explore the effect of secukinumab, compared to placebo, on fat tissue and skin in plaque psoriasis patients (ObePso-S). clinicaltrials.gov/show/nct03055494 (first received 16 February 2017).
NCT03364309 {published data only}
- NCT03364309. A study of ixekizumab (LY2439821) in Chinese participants with moderate-to-severe plaque psoriasis. clinicaltrials.gov/show/nct03364309 (first received 6 December 2017).
- Rui W, Li X, Zheng J, Pan W, Zheng M, Lu Y, et al. 25258 Efficacy and safety of ixekizumab in Chinese patients with moderate-to-severe plaque psoriasis: 12-week results from a phase 3 study. Journal of the American Academy of Dermatology 2021;85 (3 Suppl):AB55. [Google Scholar]
NCT03535194 OASIS‐2 {published data only}
- NCT03535194. A study to assess if mirikizumab is effective and safe compared to secukinumab and placebo in moderate to severe plaque psoriasis (OASIS-2). clinicaltrials.gov/show/nct03535194 (first received 24 May 2018).
- Papp K, Warren RB, Green LJ, Reich K, Langley R, Paul C, et al. Efficacy and safety of mirikizumab versus secukinumab and placebo in the treatment of moderate-to-severe psoriasis: 52-week results from OASIS-2, a multicenter, randomized, double-blind study. Journal of Clinical and Aesthetic Dermatology 2021;14 (5 Suppl):S26. [Google Scholar]
Nugteren‐Huying 1990 {published data only}
- Nugteren-Huying WM, Schroeff JG, Hermans J, Suurmond D. Fumaric acid therapy in psoriasis; a double-blind, placebo-controlled study [Fumaarzuurtherapie tegen psoriasis; een dubbelblind, placebo-gecontroleerd onderzoek]. Nederlands Tijdschrift voor Geneeskunde 1990;134(49):2387-91. [CENTRAL: CN-00072165] [PMID: ] [PubMed] [Google Scholar]
- Nugteren-Huying WM, Van der Schroeff JG, Hermans J, Suurmond D. Fumaric acid therapy for psoriasis: a randomized, double-blind, placebo-controlled study. Journal of the American Academy of Dermatology 1990;22(2 Pt 1):311-2. [CENTRAL: CN-00066354] [DOI] [PubMed] [Google Scholar]
Ohtsuki 2017 {unpublished data only}
- Imafuku S, Nemoto O, Okubo Y, Komine M, Schafer P, Petric R, et al. Pharmacodynamic analysis of apremilast in Japanese patients with moderate to severe psoriasis: results from a phase 2b randomized trial. Journal of Dermatology 2020;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01988103. Efficacy and safety study of two doses of apremilast (CC-10004) in Japanese subjects with moderate-to-severe plaque-type psoriasis. clinicaltrials.gov/ct2/show/NCT01988103 (first received 24 May 2013). [CENTRAL: CN-01479115]
- Ohtsuki M, Okubo Y, Komine M, Imafuku S, Day RM, Chen P, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of Japanese patients with moderate to severe plaque psoriasis: efficacy, safety and tolerability results from a phase 2b randomized controlled trial. Journal of Dermatology 2017;44(8):873-84. [CENTRAL: CN-01600552] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohtsuki 2018 {published data only}
- NCT02325219. An efficacy and safety of CNTO 1959 (Guselkumab) in participants with moderate to severe plaque-type psoriasis. clinicaltrials.gov/ct2/show/NCT02325219 (first received 24 December 2014).
- Ohtsuki M, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. Journal of Dermatology 2018;45(9):1053-62. [CENTRAL: CN-01646020] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olsen 1989 {published data only}
- Olsen EA, Weed WW, Meyer CJ, Cobo LM. A double-blind, placebo-controlled trial of acitretin for the treatment of psoriasis. Journal of the American Academy of Dermatology 1989;21(4 Pt 1):681-6. [CENTRAL: CN-00063370] [PMID: ] [DOI] [PubMed] [Google Scholar]
OPTIMAP 2022 {unpublished data only}
- Busard CI, Menting SP, Van Bezooijen JS, Van den Reek JM, Hutten BA, Prens EP, et al. Optimizing adalimumab treatment in psoriasis with concomitant methotrexate (OPTIMAP): study protocol for a pragmatic, single-blinded, investigator-initiated randomized controlled trial. Trials [Electronic Resource] 2017;18(1):52 Erratum in: Trials 2017; 18(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUCTR2013-004918-18-NL. Optimising adalimumab treatment in psoriasis with concomitant methotrexate - OPTIMAP [Optimising adalimumab treatment in psoriasis with concomitant methotrexate]. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013-004918-18-NL/EUCTR2013-004918-18-NL (first received 12 December 2013).
- Van Der Kraaij G, Busard C, Van Den Reek J, Menting S, De Jong E, De Kort W, et al. Optimizing adalimumab treatment in psoriasis with concomitant methotrexate: a randomized controlled trial. Journal of the European Academy of Dermatology and Venereology 2019;33:25-6. [Google Scholar]
- Kraaij G, Busard C, den Reek J, Menting S, Musters A, Hutten B, et al. Adalimumab with methotrexate vs. adalimumab monotherapy in psoriasis: first-year results of a single-blind randomized controlled trial. Journal of investigative dermatology 2022;142(9):2375‐2383.e6. [DOI] [PubMed] [Google Scholar]
ORION 2020 {unpublished data only}
- Ferris L, Ott E, Hong HC, Baran W. Efficacy and safety of guselkumab administered with a novel self-injection device for the treatment of moderate-to-severe psoriasis: results from the phase III Orion self-dose study through week 16. Journal of the Dermatology Nurses' Association 2020;12(2):No pagination. [Google Scholar]
- Ferris L, Ott E, Jiang G, Chih-Ho Hong H, Baran W. Efficacy and safety of guselkumab administered with a novel self-injection device for the treatment of moderate-to-severe psoriasis: results from the phase III ORION self-dose study through week 16. Acta Dermato-Venereologica 2018;98(Suppl 219):29. [CENTRAL: CN-01620189] [Google Scholar]
- Ferris LK, Ott E, Jiang J, Hong HC, Li S, Han C, et al. Efficacy and safety of guselkumab, administered with a novel patient-controlled injector (One-Press), for moderate-to-severe psoriasis: results from the phase 3 ORION study. Journal of Dermatological Treatment 2020;31(2):152-9. [DOI] [PubMed] [Google Scholar]
- NCT02905331. Efficacy and safety study of guselkumab in the treatment of participants with moderate to severe plaque-type psoriasis. clinicaltrials.gov/ct2/show/NCT02905331 (first received 14 September 2016). [CENTRAL: CN-01520887]
Ortonne 2013 {published data only}
- Ortonne JP, Paul C, Berardesca E, Marino V, Gallo G, Brault Y, et al. A 24-week randomized clinical trial investigating the efficacy and safety of two doses of etanercept in nail psoriasis. British Journal of Dermatology 2013;168(5):1080-7. [CENTRAL: CN-00967538] [PMID: ] [DOI] [PubMed] [Google Scholar]
Papp 2005 {published data only}
- Krueger GG, Langley RG, Finlay AY, Griffiths CE, Woolley JM, Lalla D, et al. Patient-reported outcomes of psoriasis improvement with etanercept therapy: results of a randomized phase III trial. British Journal of Dermatology 2005;153(6):1192-9. [CENTRAL: CN-00553127] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Papp KA, Tyring S, Lahfa M, Prinz J, Griffiths CE, Nakanishi AM, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. British Journal of Dermatology 2005;152(6):1304-12. [CENTRAL: CN-00522450] [PMID: ] [DOI] [PubMed] [Google Scholar]
Papp 2012a {published data only}
- Gordon KB, Kimball AB, Chau D, Viswanathan HN, Li J, Revicki DA, et al. Impact of brodalumab treatment on psoriasis symptoms and health-related quality of life: use of a novel patient-reported outcome measure, the Psoriasis Symptom Inventory. British Journal of Dermatology 2014;170(3):705-15. [CENTRAL: CN-00981224] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp K, Leonardi C, Menter A, Thompson EH, Milmont CE, Kricorian G, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. Journal of the American Academy of Dermatology 2014;71(6):1183-90.e3. [CENTRAL: CN-01107365] [EMBASE: 2015237135] [DOI] [PubMed] [Google Scholar]
- Papp K, Menter A, Strober B, Kricorian G, Thompson EH, Milmont CE, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. Journal of the American Academy of Dermatology 2015;72(3):436-9.e1. [CENTRAL: CN-01111298] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. New England Journal of Medicine 2012;366(13):1181-9. [CENTRAL: CN-00814009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Papp 2012c {published data only}
- Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet 2012;380(9843):738-46. [CENTRAL: CN-00859723] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Strand V, Fiorentino D, Hu C, Day RM, Stevens RM, Papp KA. Improvements in patient-reported outcomes with apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of moderate to severe psoriasis: results from a phase IIb randomized, controlled study. Health and Quality of Life Outcomes 2013;10(11):82. [CENTRAL: CN-00876574] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papp 2013a {published data only}
- Papp KA, Langley RG, Sigurgeirsson B, Abe M, Baker DR, Konno P, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. British Journal of Dermatology 2013;168(2):412-21. [CENTRAL: CN-00967073] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Sigurgeirsson B, Kircik L, Nemoto O, Mikazans I, Haemmerle S, Thurston HJ, et al. Secukinumab improves the signs and symptoms of moderate-to-severe plaque psoriasis in subjects with involvement of hands and/or feet: subanalysis of a randomized, double-blind, placebo-controlled, phase 2 dose-ranging study. Journal of the European Academy of Dermatology and Venereology 2014;28(8):1127-9. [CENTRAL: CN-01041806] [PMID: ] [DOI] [PubMed] [Google Scholar]
Papp 2013b {published data only}
- Papp KA, Kaufmann R, Thaçi D, Hu C, Sutherland D, Rohane P. Efficacy and safety of apremilast in subjects with moderate to severe plaque psoriasis: results from a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison study. Journal of the European Academy of Dermatology and Venereology 2013;27(3):e376-83. [CENTRAL: CN-01124587] [PMID: ] [DOI] [PubMed] [Google Scholar]
Papp 2015 {published data only}
- Papp K, Thaçi D, Reich K, Riedl E, Langley RG, Krueger JG, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. British Journal of Dermatology 2015;173(4):930-9. [CENTRAL: CN-01105188] [PMID: ] [DOI] [PubMed] [Google Scholar]
Papp 2017a {published data only}
- NCT01970488. Study to compare efficacy and safety of ABP 501 and adalimumab (HUMIRA®) in adults with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct01970488 (first received 28 October 2013). [CENTRAL: CN-01478635]
- Papp K, Bachelez H, Costanzo A, Foley P, Gooderham M, Kaur P, et al. Clinical similarity of biosimilar ABP 501 to adalimumab in the treatment of patients with moderate to severe plaque psoriasis: a randomized, double-blind, multicenter, phase III study. Journal of the American Academy of Dermatology 2017;76(6):1093-102. [CENTRAL: CN-01401166] [DOI] [PubMed] [Google Scholar]
Papp 2017b {unpublished data only}
- NCT02054481. BI 655066 dose ranging in psoriasis, active comparator ustekinumab. clinicaltrials.gov/ct2/show/NCT02054481 (first received 3 February 2014).
- Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour JP, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. New England Journal of Medicine 2017;376(16):1551-60. [CENTRAL: CN-01368160] [DOI] [PubMed] [Google Scholar]
Papp 2018 {published data only}
- Catlett IM, Hu S, Banerjee S, Gordon K, Krueger JG. A selective inhibitor of TYK2, BMS-986165, improves molecular, cellular, and clinical biomarkers associated with efficacy in moderate-to-severe psoriasis. Experimental Dermatology 2018;27(Suppl 2):55. [CENTRAL: CN-01791579] [Google Scholar]
- Catlett IM, Hu S, Banerjee S, Gordon K, Krueger JG. A selective inhibitor of tyrosine kinase 2, bms-986165, improves molecular, cellular, and clinical biomarkers associated with efficacy in moderate-to-severe psoriasis. Journal of the Dermatology Nurses' Association (Conference: 24th World Congress of Dermatology, Italy) 2020;12(2):No pagination. [Google Scholar]
- EUCTR2016-002481-31-LV. Study to evaluate effectiveness and safety in subjects with moderate to severe psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016-002481-31-LV (first received 28 November 2016).
- Gooderham M, Papp K, Gordon K, Foley P, Morita A, Thaçi D, et al. Influence of baseline demographics on efficacy of a selective oral TYK2 inhibitor, BMS-986165, in patients with moderate-to-severe plaque psoriasis: a Phase 2, randomized, placebo-controlled trial. Experimental Dermatology 2018;27(Suppl 2):32. [CENTRAL: CN-01791582] [Google Scholar]
- Gordon K, Papp K, Gooderham M, Thaçi D, Foley P, Morita A, et al. Evaluating influence of baseline characteristics on efficacy of a selective oral TYK2 inhibitor, BMS-986165, in patients with moderate-to-severe plaque psoriasis in a phase 2 trial. Experimental Dermatology 2018;27(Suppl 2):29-30. [CENTRAL: CN-01787103] [Google Scholar]
- Gordon K, Papp K, Gooderham M, Thaçi D, Foley P, Morita A, et al. Influence of baseline demographics and disease characteristics on efficacy of an oral, selective TYK2 inhibitor, BMS-986165, in patients with plaque psoriasis in a phase 2 trial. Annals of the Rheumatic Diseases 2019;78(Suppl 2):907-8. [Google Scholar]
- NCT02931838. Study to evaluate effectiveness and safety in subjects with moderate to severe psoriasis. clinicaltrials.gov/show/nct02931838 (first received 13 October 2016). [CENTRAL: CN-01521531]
- Papp K, Gordon K, Thaçi D, Morita A, Gooderham M, Foley P, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. New England Journal of Medicine 2018;379(14):1313-21. [CENTRAL: CN-01652897] [DOI] [PubMed] [Google Scholar]
- Thaci D, Papp K, Gordon KB, Morita A, Gooderham M, Foley P, et al. An oral, selective tyrosine kinase 2 inhibitor, BMS-986165, improves health-related quality of life in psoriasis: results from a phase II trial. Journal of Clinical and Aesthetic Dermatology 2020;12(5 Suppl):S26. [Google Scholar]
- Thaci D, Strober B, Gordon KB, Foley P, Gooderham M, Morita A, et al. Deucravacitinib in moderate to severe psoriasis: clinical and quality-of-life outcomes in a phase 2 trial. Dermatology and Therapy 2022;12(2):495-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaçi D, Papp K, Gordon K, Morita A, Gooderham M, Foley P, et al. Selective oral tyrosine kinase 2 (TYK2) inhibitor (BMS-986165) impact on quality of life (QOL) in patients with moderate to severe plaque psoriasis (PSO) in a phase 2 trial as assessed by the dermatology life quality index (DLQI). Journal of the European Academy of Dermatology and Venereology 2019;33(S3):71-2. [Google Scholar]
Papp 2021 {published data only}
- NCT03384745. A phase 2b study of the efficacy, safety, and tolerability of M1095 in subjects with moderate to severe psoriasis. clinicaltrials.gov/show/nct03384745 (first received 27 December 2017).
- Papp KA, Weinberg MA, Morris A, Reich K. IL17A/F nanobody sonelokimab in patients with plaque psoriasis: a multicentre, randomised, placebo-controlled, phase 2b study. Lancet 2021;397(10284):1564-75 Erratum in Lancet 2021; 397(10290):2150. [DOI] [PubMed] [Google Scholar]
PEARL 2011 {published data only}
- Tsai TF, Ho JC, Song M, Szapary P, Guzzo C, Shen YK, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). Journal of Dermatological Science 2011;63(3):154-63. [CENTRAL: CN-00810821] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Tsai TF, Song M, Shen YK, Schenkel B, Choe YB, Kim NI, et al. Ustekinumab improves health-related quality of life in Korean and Taiwanese patients with moderate to severe psoriasis: results from the PEARL trial. Journal of Drugs in Dermatology 2012;11(8):943-9. [CENTRAL: CN-00859638] [PMID: ] [PubMed] [Google Scholar]
PHOENIX‐1 2008 {published data only}
- Guenther L, Han C, Szapary P, Schenkel B, Poulin Y, Bourcier M, et al. Impact of ustekinumab on health-related quality of life and sexual difficulties associated with psoriasis: results from two phase III clinical trials. Journal of the European Academy of Dermatology and Venereology 2011;25(7):851-7. [CENTRAL: CN-00887488] [EMBASE: 2011360521] [DOI] [PubMed] [Google Scholar]
- Hu C, Szapary PO, Yeilding N, Zhou H. Informative dropout modeling of longitudinal ordered categorical data and model validation: application to exposure-response modeling of physician's global assessment score for ustekinumab in patients with psoriasis. Journal of Pharmacokinetics and Pharmacodynamics 2011;38(2):237-60. [CENTRAL: CN-00787444] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kimball AB, Gordon KB, Fakharzadeh S, Yeilding N, Szapary PO, Schenkel B, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial through up to 3 years. British Journal of Dermatology 2012;166(4):861-72. [CENTRAL: CN-00841277] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kimball AB, Papp KA, Wasfi Y, Chan D, Bissonnette R, Sofen H, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. Journal of the European Academy of Dermatology and Venereology 2013;27(12):1535-45. [CENTRAL: CN-00915003] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lebwohl M, Leonardi C, Griffiths CE, Prinz JC, Szapary PO, Yeilding N, et al. Long-term safety experience of ustekinumab in patients with moderate-to-severe psoriasis (part I of II): results from analyses of general safety parameters from pooled phase 2 and 3 clinical trials. Journal of the American Academy of Dermatology 2012;66(5):731-41. [CENTRAL: CN-00860736] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lebwohl M, Papp K, Han C, Schenkel B, Yeilding N, Wang Y, et al. Ustekinumab improves health-related quality of life in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial. British Journal of Dermatology 2010;162(1):137-46. [CENTRAL: CN-00743778] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008;371(9625):1665-74. [CENTRAL: CN-00631485] [18486739] [DOI] [PubMed] [Google Scholar]
- Papp KA, Griffiths CE, Gordon K, Lebwohl M, Szapary PO, Wasfi Y, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. British Journal of Dermatology 2013;168(4):844-54. [CENTRAL: CN-01616926] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Reich K, Papp KA, Griffiths CE, Szapary PO, Yeilding N, Wasfi Y, et al. An update on the long-term safety experience of ustekinumab: results from the psoriasis clinical development program with up to four years of follow-up. Journal of Drugs in Dermatology 2012;11(3):300-12. [CENTRAL: CN-00860086] [PMID: ] [PubMed] [Google Scholar]
- Rich P, Bourcier M, Sofen H, Fakharzadeh S, Wasfi Y, Wang Y, et al. Ustekinumab improves nail disease in patients with moderate-to-severe psoriasis: results from PHOENIX 1. British Journal of Dermatology 2014;170(2):398-407. [CENTRAL: CN-00982377] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Zhou H, Hu C, Zhu Y, Lu M, Liao S, Yeilding N, et al. Population-based exposure-efficacy modeling of ustekinumab in patients with moderate to severe plaque psoriasis. Journal of Clinical Pharmacology 2010;50(3):257-67. [CENTRAL: CN-00752537] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hu C, Lu M, Liao S, Marini JC, Yohrling J, et al. Population pharmacokinetic modeling of ustekinumab, a human monoclonal antibody targeting IL-12/23p40, in patients with moderate to severe plaque psoriasis. Journal of Clinical Pharmacology 2009;49(2):162-75. [CENTRAL: CN-01753183] [PMID: ] [DOI] [PubMed] [Google Scholar]
PHOENIX‐2 2008 {published data only}
- Langley RG, Feldman SR, Han C, Schenkel B, Szapary P, Hsu MC, et al. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: results from a randomized, double-blind, placebo-controlled phase III trial. Journal of the American Academy of Dermatology 2010;63(3):457-65. [CENTRAL: CN-00761719] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Langley RG, Lebwohl M, Krueger GG, Szapary PO, Wasfi Y, Chan D, et al. Long-term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate-to-severe psoriasis: results from the PHOENIX 2 study through 5 years of follow-up. British Journal of Dermatology 2015;172(5):1371-83. [CENTRAL: CN-01254538] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008;371(9625):1675-84. [CENTRAL: CN-00631486] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Reich K, Schenkel B, Zhao N, Szapary P, Augustin M, Bourcier M, et al. Ustekinumab decreases work limitations, improves work productivity, and reduces work days missed in patients with moderate-to-severe psoriasis: results from PHOENIX 2. Journal of Dermatological Treatment 2011;22(6):337-47. [CENTRAL: CN-00860939] [PMID: ] [DOI] [PubMed] [Google Scholar]
PIECE 2016 {published data only}
- De Vries AC, Thio HB, De Kort WJ, Opmeer BC, Van der Stok HM, De Jong EM, et al. A prospective randomized controlled trial comparing infliximab and etanercept in patients with moderate-to-severe chronic plaque-type psoriasis: the Psoriasis Infliximab vs. Etanercept Comparison Evaluation (PIECE) study. British Journal of Dermatology 2016;176(3):624-33. [CENTRAL: CN-01336979] [PMID: ] [DOI] [PubMed] [Google Scholar]
Piskin 2003 {published data only}
- Piskin G, Heydendael VM, Rie MA, Bos JD, Teunissen MB. Cyclosporin A and methotrexate are equally effective in reducing T cell numbers in psoriatic skin lesions but have no consistent effect on IFN-gamma and IL-4 expression in psoriatic skin in situ. Archives of Dermatological Research 2003;294(12):559-62. [CENTRAL: CN-00456554] [PMID: ] [DOI] [PubMed] [Google Scholar]
PLANETA 2021 {published data only}
- Andrey B, Aleksey S, Antonina A, Ekaterina C, Roman I. Netakimab: 12-week results from Planeta study, a phase III trial of a novel il-17 inhibitor in moderate-to-severe plaque psoriasis. Journal of the Dermatology Nurses' Association 2020;12(2):No pagination. [Google Scholar]
- Puig L, Bakulev AL, Kokhan MM, Samtsov AV, Khairutdinov VR, Morozova MA, et al. Efficacy and safety of netakimab, a novel anti-IL-17 monoclonal antibody, in patients with moderate to severe plaque psoriasis. Results of a 54-week randomized double-blind placebo-controlled PLANETA clinical trial. Dermatology and Therapy 2021;11(4):1319-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig L, Bakulev AL, Kokhan MM, Samtsov AV, Khairutdinov VR, Morozova MA, et al. Efficacy and safety of netakimab, a novel anti-IL-17 monoclonal antibody, in patients with moderate to severe plaque psoriasis. Results of a 54-week randomized double-blind placebo-controlled PLANETA clinical trial. Dermatology and Therapy 2021;31:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
POETYK PSO‐1 2022 {published data only}
- Armstrong AW, Gooderham M, Warren RB, Papp K, Strober B, Thaci D, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. Journal of the American Academy of Dermatology 2022 Jul 9 [Epub ahead of print]. [DOI: 10.1016/j.jaad.2022.07.002] [PMID: ] [DOI] [PubMed]
- Armstrong AW, Gooderham M, Warren RB, Papp K, Strober B, Thaci D, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the phase 3 POETYK PSO-1 study. Annals of the Rheumatic Diseases 2021;80(Suppl 1):795-6. [Google Scholar]
- Augustin M, Strober B, Armstrong A W, Beaumont J, Pham T P, Hudgens S, Zhuo J, Becker B, Banerjee S, Kisa R M, Papp K. 35205 Deucravacitinib improves Dermatology Life Quality Index (DLQI) in patients with moderate to severe psoriasis: results from the phase 3 POETYK PSO-1 and PSO-2 trials. Journal of the American Academy of Dermatology 2022;87(3 Suppl):AB156. [Google Scholar]
- EUCTR2018-001926-25-ES. Efficacy and safety of BMS-986165 versus placebo and active comparator in subjects with psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018-001926-25-ES (first received 25 October 2018).
- Foley P. Deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, versus placebo and apremilast in psoriasis: efficacy analysis by prior treatment in the phase 3 POETYK PSO-1 and PSO-2 trials. Australasian Journal of Dermatology 2022;63(Suppl 1):65. [Google Scholar]
- JPRN-JapicCTI-184213. An investigational study to evaluate experimental medication BMS-986165 compared to placebo and a currently available treatment in participants with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-JapicCTI-184213 (first received 21 November 2018).
- Warren R B, Armstrong A, Gooderham M, Strober B, Thaci D, Imafuku S, et al. Deucravacitinib, an oral, selective tyrosine kinase 2 inhibitor, in moderate to severe plaque psoriasis: 52-week efficacy results from the phase 3 POETYK PSO-1 and POETYK PSO-2 trials. Annals of the Rheumatic Diseases 2022;81(Suppl 1):1570-1. [Google Scholar]
POETYK PSO‐2 2022 {published data only}
- NCT03611751. An investigational study to evaluate experimental medication BMS-986165 compared to placebo and a currently available treatment in participants with moderate-to-severe plaque psoriasis. clinicaltrials.gov/show/nct03611751 (first received 2 August 2018).
- Strober B, Thaci D, Sofen H, Kircik L, Gordon KB, Foley P, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 POETYK PSO-2 trial. Journal of the American Academy of Dermatology 2022;14:14. [DOI] [PubMed] [Google Scholar]
POETYK PSO‐3 2022 {published data only}
- NCT04167462. An investigational study to evaluate experimental medication BMS-986165 compared to placebo in participants with plaque psoriasis in mainland China, Taiwan, and South Korea (POETYK-PSO-3). clinicaltrials.gov/show/NCT04167462 (first received 18 November 2019).
POLARIS 2020 {unpublished data only}
- NCT02951533. A study to compare the efficacy of guselkumab to fumaric acid esters for the treatment of participants with moderate to severe plaque psoriasis (POLARIS). clinicaltrials.gov/ct2/show/NCT02951533 (first received 28 October 2016). [CENTRAL: CN-01559701]
- Thaçi D, Pinter A, Sebastian M, Termeer C, Sticherling M, Gerdes S, et al. Guselkumab is superior to fumaric acid esters in patients with moderate-to-severe plaque psoriasis who are naïve to systemic treatment: results from a randomised, active comparator-controlled phase 3b trial (POLARIS). British Journal of Dermatology 2020;183(2):265-75. [DOI: 10.1111/bjd.18696] [DOI] [PubMed] [Google Scholar]
PRESTA 2010 {published data only}
- Damjanov N, Karpati S, Kemeny L, Bakos N, Bobic B, Majdan M, et al. Efficacy and safety of etanercept in psoriasis and psoriatic arthritis in the PRESTA study: analysis in patients from Central and Eastern Europe. Journal of Dermatological Treatment 2018;29(1):8-12. [CENTRAL: CN-01458628] [DOI] [PubMed] [Google Scholar]
- Gniadecki R, Robertson D, Molta CT, Freundlich B, Pedersen R, Li W, et al. Self-reported health outcomes in patients with psoriasis and psoriatic arthritis randomized to two etanercept regimens. Journal of the European Academy of Dermatology and Venereology 2012;26(11):1436-43. [CENTRAL: CN-00971660] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Sterry W, Brock F, Dilleen M, Stefanidis D, Germain JM, et al. Pattern of response in patients with moderate-to-severe psoriasis treated with etanercept. British Journal of Dermatology 2015;172(1):230-8. [CENTRAL: CN-01116415] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kirkham B, De Vlam K, Li W, Boggs R, Mallbris L, Nab HW, et al. Early treatment of psoriatic arthritis is associated with improved patient-reported outcomes: findings from the etanercept PRESTA trial. Clinical and Experimental Rheumatology 2015;33(1):11-9. [CENTRAL: CN-01090489] [PMID: ] [PubMed] [Google Scholar]
- Prinz JC, Fitzgerald O, Boggs RI, Foehl J, Robertson D, Pedersen R, et al. Combination of skin, joint and quality of life outcomes with etanercept in psoriasis and psoriatic arthritis in the PRESTA trial. Journal of the European Academy of Dermatology and Venereology 2011;25(5):559-64. [CENTRAL: CN-00802619] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Sterry W, Ortonne JP, Kirkham B, Brocq O, Robertson D, Pedersen RD, et al. Comparison of two etanercept regimens for treatment of psoriasis and psoriatic arthritis: PRESTA randomised double blind multicentre trial. BMJ (Clinical Research Ed.) 2010;340:c147. [CENTRAL: CN-00734727] [PMID: ] [DOI] [PubMed] [Google Scholar]
PRIME 2017 {unpublished data only}
- NCT02474082. Study of secukinumab compared to fumaderm® in adults with moderate to severe psoriasis (PRIME). clinicaltrials.gov/ct2/show/NCT02474082 (first received 16 April 2015). [CENTRAL: CN-01552941]
- Sticherling M, Mrowietz U, Augustin M, Thaçi D, Melzer N, Hentschke C, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. British Journal of Dermatology 2017;177(4):1024-32 Corrigendum to British Journal of Dermatology 2017; 177(6):1772. [CENTRAL: CN-01427286] [DOI] [PubMed] [Google Scholar]
PRISTINE 2013 {published data only}
- Puig L, Strohal R, Husni ME, Tsai TF, Noppakun N, Szumski A, et al. Cardiometabolic profile, clinical features, quality of life and treatment outcomes in patients with moderate-to-severe psoriasis and psoriatic arthritis. Journal of Dermatological Treatment 2015;26(1):7-15. [CENTRAL: CN-01051703] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Strohal R, Puig L, Chouela E, Tsai TF, Melin J, Freundlich B, et al. The efficacy and safety of etanercept when used with as-needed adjunctive topical therapy in a randomised, double-blind study in subjects with moderate-to-severe psoriasis (the PRISTINE trial). Journal of Dermatological Treatment 2013;24(3):169-78. [CENTRAL: CN-00881482] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Thaçi D, Galimberti R, Amaya-Guerra M, Rosenbach T, Robertson D, Pedersen R, et al. Improvement in aspects of sleep with etanercept and optional adjunctive topical therapy in patients with moderate-to-severe psoriasis: results from the PRISTINE trial. Journal of the European Academy of Dermatology and Venereology 2014;28(7):900-6. [CENTRAL: CN-01041533] [PMID: ] [DOI] [PubMed] [Google Scholar]
PsOsim 2017 {published data only}
- EUCTR2015-000632-15-EE. A study to compare the efficacy and safety of CHS-1420 against Humira®. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015-000632-15-EE (first received 22 September 2015).
- Hodge J, Tang H, O'Connor P, Finck B. Switching from adalimumab to CHS-1420: a randomized, double-blind global clinical trial in patients with psoriasis and psoriatic arthritis. Arthritis and Rheumatology 2017;69(Suppl 10):2879. [Google Scholar]
- NCT02489227. Comparison of CHS-1420 versus Humira in subjects with chronic plaque psoriasis (PsOsim). clinicaltrials.gov/show/nct02489227 (first received 2 July 2015).
Rathipriyadharshini 2020 {published data only}
- CTRI/2019/01/017362. A study to assess the effects of apremilast and methotrexate in the treatment of patients with psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/01/017362 (first received 31 January 2019).
- Rathipriyadharshini R, Prem Kumar M, Soundarya S, Srinivasan MS. An open-labelled randomised comparative evaluation of therapeutic efficacy and safety of apremilast versus methotrexate in the treatment of patients with chronic plaque psoriasis. Annals of Tropical Medicine and Public Health 2020;23(15):231517. [Google Scholar]
Reich 2012a {published data only}
- Reich K, Ortonne JP, Gottlieb AB, Terpstra IJ, Coteur G, Tasset C, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab' certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. British Journal of Dermatology 2012;167(1):180-90. [CENTRAL: CN-00856435] [PMID: ] [DOI] [PubMed] [Google Scholar]
Reich 2015 {published data only}
- Reich K, Papp KA, Matheson RT, Tu JH, Bissonnette R, Bourcier M, et al. Evidence that a neutrophil-keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis. Experimental Dermatology 2015;24(7):529-35. [CENTRAL: CN-01171151] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reich 2020 {unpublished data only}
- Leutz A, Pinter A, Thaci D, Augustin M, Schuster C, Fotiou K, et al. Efficacy and safety of ixekizumab after switching from fumaric acid esters or methotrexate in patients with moderate-to-severe plaque psoriasis naive to systemic treatment. British Journal of Dermatology 2021;184(3):548-50. [DOI] [PubMed] [Google Scholar]
- NCT02634801. A study of Ixekizumab (LY2439821) in participants with moderate-to-severe plaque psoriasis naive to systemic treatment. clinicaltrials.gov/ct2/show/NCT02325219 (first received 16 December 2015). [CENTRAL: CN-01554547]
- Reich K, Augustin M, Thaci D, Pinter A, Leutz A, Henneges C, et al. A 24-week multicentre, randomised, open-label, parallel-group study comparing the efficacy and safety of ixekizumab to fumaric acid esters and methotrexate in patients with moderate-to-severe plaque psoriasis naïve to systemic treatment. British Journal of Dermatology 2020;182(4):869-79. [DOI: 10.1111/bjd.18384] [DOI] [PMC free article] [PubMed] [Google Scholar]
ReSURFACE‐1 2017 {published data only}
- Anonymous. Erratum: Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials (Lancet 2017; 390(10091):276-288). Lancet 2017;390(10091):230. [DOI] [PubMed] [Google Scholar]
- Blauvelt A, Sofen H, Papp K, Gooderham M, Tyring S, Zhao Y, et al. Tildrakizumab efficacy and impact on quality of life up to 52 weeks in patients with moderate-to-severe psoriasis: a pooled analysis of two randomized controlled trials. Journal of the European Academy of Dermatology and Venereology 2019;33(12):2305-12. [DOI: 10.1111/jdv.15862] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01722331. A study to evaluate the efficacy and safety of subcutaneous MK-3222, followed by an optional long-term safety extension study, in participants with moderate-to-severe chronic plaque psoriasis (MK-3222-010). clinicaltrials.gov/ct2/show/NCT01722331 (first received 16 March 2017).
- Reich K, Blauvelt A, Thaçi D, Papp KA, Kimball A, Sinclair R, et al. Safety and tolerability of tildrakizumab in patients with chronic plaque psoriasis: esults from long-term extensions of 2 phase 3 studies. Australasian Journal of Dermatology 2018;59(Suppl 1):110-1. [CENTRAL: CN-01606961] [Google Scholar]
- Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaçi D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 2017;390(10091):276-88. [CENTRAL: CN-01422560] [DOI] [PubMed] [Google Scholar]
ReSURFACE‐2 2017 {unpublished data only}
- NCT01729754. A study to evaluate the efficacy and safety/tolerability of subcutaneous tildrakizumab (SCH 900222/MK-3222) in participants with moderate-to-severe chronic plaque psoriasis followed by a long-term extension study (MK-3222-011). clinicaltrials.gov/ct2/show/NCT01729754 (first received 13 November 2012). [CENTRAL: CN-01538777]
- Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaçi D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 2017;390(10091):276-88. [CENTRAL: CN-01422560] [DOI] [PubMed] [Google Scholar]
REVEAL 2008 {published data only}
- Armstrong AW, Villanueva Quintero DG, Echeverria CM, Gu Y, Karunaratne M, Reyes Servin O. Body region involvement and quality of life in psoriasis: analysis of a randomized controlled trial of adalimumab. American Journal of Clinical Dermatology 2016;17(6):691-9. [CENTRAL: CN-01286209] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon K, Papp K, Poulin Y, Gu Y, Rozzo S, Sasso EH. Long-term efficacy and safety of adalimumab in patients with moderate to severe psoriasis treated continuously over 3 years: results from an open-label extension study for patients from REVEAL. Journal of the American Academy of Dermatology 2012;66(2):241-51. [CENTRAL: CN-00860821] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kimball AB, Bensimon AG, Guerin A, Yu AP, Wu EQ, Okun MM, et al. Efficacy and safety of adalimumab among patients with moderate to severe psoriasis with co-morbidities: Subanalysis of results from a randomized, double-blind, placebo-controlled, phase III trial. American Journal of Clinical Dermatology 2011;12(1):51-62. [CENTRAL: CN-00779604] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Menter A, Augustin M, Signorovitch J, Yu AP, Wu EQ, Gupta SR, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. Journal of the American Academy of Dermatology 2010;62(5):812-8. [CENTRAL: CN-00743358] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Menter A, Gordon KB, Leonardi CL, Gu Y, Goldblum OM. Efficacy and safety of adalimumab across subgroups of patients with moderate to severe psoriasis. Journal of the American Academy of Dermatology 2010;63(3):448-56. [CENTRAL: CN-00761638] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. Journal of the American Academy of Dermatology 2008;58(1):106-15. [CENTRAL: CN-00703914] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Papp K, Menter A, Poulin Y, Gu Y, Sasso EH. Long-term outcomes of interruption and retreatment vs. continuous therapy with adalimumab for psoriasis: subanalysis of REVEAL and the open-label extension study. Journal of the European Academy of Dermatology and Venereology 2013;27(5):634-42. [CENTRAL: CN-00970158] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Revicki DA, Willian MK, Menter A, Gordon KB, Kimball AB, Leonardi CL, et al. Impact of adalimumab treatment on patient-reported outcomes: results from a Phase III clinical trial in patients with moderate to severe plaque psoriasis. Journal of Dermatological Treatment 2007;18(6):341-50. [CENTRAL: CN-00628656] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rich 2013 {published data only}
- Augustin M, Abeysinghe S, Mallya U, Qureshi A, Roskell N, McBride D, et al. Secukinumab treatment of plaque psoriasis shows early improvement in DLQI response - results of a phase II regimen-finding trial. Journal of the European Academy of Dermatology and Venereology 2016;30(4):645-9. [CENTRAL: CN-01265142] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C, Reich K, Gottlieb AB, Mrowietz U, Philipp S, Nakayama J, et al. Secukinumab improves hand, foot and nail lesions in moderate-to-severe plaque psoriasis: subanalysis of a randomized, double-blind, placebo-controlled, regimen-finding phase 2 trial. Journal of the European Academy of Dermatology and Venereology 2014;28(12):1670-5. [CENTRAL: CN-01119275] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rich P, Sigurgeirsson B, Thaçi D, Ortonne JP, Paul C, Schopf RE, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. British Journal of Dermatology 2013;168(2):402-11. [CENTRAL: CN-00965685] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ruzicka 1990 {published data only}
- Ruzicka T, Sommerburg C, Braun-Falco O, Köster W, Lengen W, Lensing W, et al. Efficiency of acitretin in combination with UV-B in the treatment of severe psoriasis. Archives of Dermatology 1990;126(4):482-6. [CENTRAL: CN-00066767] [PMID: ] [PubMed] [Google Scholar]
Sandhu 2003 {published data only}
- Sandhu K, Kaur I, Kumar B, Saraswat A. Efficacy and safety of cyclosporine versus methotrexate in severe psoriasis: a study from North India. Journal of Dermatology 2003;30(6):458-63. [CENTRAL: CN-00456950] [PMID: ] [DOI] [PubMed] [Google Scholar]
Saurat 1988 {published data only}
- Saurat JH, Geiger JM, Amblard P, Beani JC, Boulanger A, Claudy A, et al. Randomized double-blind multicenter study comparing acitretin-PUVA, etritinate-PUVA and placebo-PUVA in the treatment of severe psoriasis. Dermatologica 1988;177(4):218-24. [CENTRAL: CN-00058056] [PMID: ] [DOI] [PubMed] [Google Scholar]
SCULPTURE 2015 {published data only}
- Mrowietz U, Leonardi CL, Girolomoni G, Toth D, Morita A, Balki SA, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE). Journal of the American Academy of Dermatology 2015;73(1):27-36.e1. [CENTRAL: CN-01109352] [PMID: ] [DOI] [PubMed] [Google Scholar]
Seo 2020 {published data only}
- NCT02982005. A study of KHK4827 (brodalumab) in subjects with moderate to severe psoriasis in Korea. clinicaltrials.gov/ct2/show/NCT02982005 (first received 5 December 2016).
- Seo SJ, Shin BS, Lee J-H, Jeong H. Efficacy and safety of brodalumab in the Korean population for the treatment of moderate to severe plaque psoriasis: a randomized, phase III, double-blind, placebo-controlled study. Journal of Dermatology 2021;48(6):807-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shehzad 2004 {published data only}
- Shehzad T, Dar NR, Zakria M. Efficacy of concomitant use of puva and methotrexate in disease clearance time in plaque type psoriasis. Journal of the Pakistan Medical Association 2004;54(9):453-5. [CENTRAL: CN-00727152] [PMID: ] [PubMed] [Google Scholar]
SIGNATURE 2019 {unpublished data only}
- NCT01961609. Secukinumab in TNF-IR psoriasis patients (SIGNATURE). clinicaltrials.gov/ct2/show/NCT01961609 (first received 10 October 2013). [CENTRAL: CN-01536745]
- Warren RB, Barker JNWB, Finlay AY, Burden AD, Kirby B, Armendariz Y, et al. Secukinumab for patients failing previous tumour necrosis factor-alpha inhibitor therapy: results of a randomized open-label study (SIGNATURE). British Journal of Dermatology 2019;183(1):60-70. [DOI: 10.1111/bjd.18623] [DOI] [PubMed] [Google Scholar]
Singh 2021 {published data only}
- Singh SK, Singnarpi SR. Safety and efficacy of methotrexate (0.3 mg/kg/week) versus a combination of methotrexate (0.15 mg/kg/week) with cyclosporine (2.5 mg/kg/day) in chronic plaque psoriasis: a randomised non-blinded controlled trial. Indian Journal of Dermatology, Venereology and Leprology 2021;87(2):214-22. [DOI] [PubMed] [Google Scholar]
Sommerburg 1993 {published data only}
- Sommerburg C, Kietzmann H, Eichelberg D, Goos M, Heese A, Holzle E, et al. Acitretin in combination with PUVA: a randomized double-blind placebo-controlled study in severe psoriasis. Journal of the European Academy of Dermatology and Venereology 1993;2(4):308-17. [CENTRAL: CN-00180920] [EMBASE: 1993350796] [Google Scholar]
SPIRIT‐H2H 2020 {published data only}
- CTRI/2017/09/009850. Comparison of ixekizumab with adalimumab in patients with psoriatic arthritis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/09/009850 (first received 19 September 2017).
- Edwards CJ, Bradley A, Nassab MH, Moller B, Machold KP, Sapin C, et al. Ixekizumab vs. adalimumab for the treatment of psoriatic arthritis: 52-week efficacy and safety outcomes. Swiss Medical Weekly 2020;150(Suppl 245):7S. [Google Scholar]
- Edwards CJ, Bradley AJ, Nassab MH, Moller B, Machold KP, Sapin C, et al. Ixekizumab (IXE) vs. adalimumab (ADA) for the treatment of PSA: 52-week efficacy and safety outcomes. Rheumatology (United Kingdom) 2020;59 (Suppl 2):ii10-1. [Google Scholar]
- Kavanaugh A, Lubrano E, Muram T, Lin CY, Liu Leage S, Van Den Bosch F, et al. Head-to-head study evaluating the combined ACR50/PASI100 treatment response of ixekizumab versus adalimumab: individual patient data from a randomized, openlabel study in biologic-naive patients with psoriatic arthritis through week 52. Annals of the Rheumatic Diseases 2020;79(Suppl 1):767. [Google Scholar]
- Kleyn CE, Gooderham M, Kristensen LE, De Vlam K, Schuster C, Liu Leage S, et al. Efficacy of ixekizumab vs. adalimumab in patients with psoriatic arthritis with moderate-to-severe psoriasis: 52-week results from a multicentre, randomized, open-label study. British Journal of Dermatology 2020;183 (Suppl 1):70-1. [Google Scholar]
- Kristensen LE, Okada M, Tillett W, Leage SL, El Baou C, Bradley A, et al. Efficacy of ixekizumab versus adalimumab in psoriatic arthritis (PSA) patients with and without moderate-to-severe psoriasis: 52-week results from a multicentre, randomized open-label study. Journal of Clinical Rheumatology 2021;27 (Suppl 1):S140-1. [Google Scholar]
- Kristensen LE, Okada M, Tillett W, Liu-Leage S, El Baou C, Bradley A, et al. Efficacy of ixekizumab versus adalimumab in Psoriatic Arthritis (PsA) patients with and without moderate-to-severe psoriasis: 52-week results from a multicentre, randomised open-label study. Arthritis & Rheumatology 2020;72 (Suppl 10):2723-6. [Google Scholar]
- Mease PJ, Smolen JS, Behrens F, Nash P, Leage SL, Lingnan L, et al. Multicentre, randomised, open-label, assessor-blinded, parallel-group head-to-head comparison of the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naive to biologic disease-modifying anti-rheumatic drugs: 24-week results. Annals of the Rheumatic Diseases 2019;78 Suppl 2:261-2. [Google Scholar]
- Mease PJ, Smolen JS, Behrens F, Nash P, Liu Leage S, Li L, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naive patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Annals of the Rheumatic Diseases 2019;28:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease PJ, Smolen JS, Behrens F, Nash P, Liu Leage S, Li L, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naive patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Annals of the Rheumatic Diseases 2020;79(1):123-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SD, Kristensen LE, Schusters C, Sapin C, Leage SL, Riedl E, et al. 13771 Comparison of ixekizumab and adalimumab in the treatment of nail psoriasis in psoriatic arthritis patients with moderate to severe psoriasis: 24-week results from a multicenter, randomized, open-label, rater-blinded study (SPIRIT-H2H). Journal of the American Academy of Dermatology 2020;83 (6 Suppl):AB12. [Google Scholar]
- Smolen J, Nash P, Tahir H, Schulze-Koops H, Li L, Hojnik M, et al. A head-to-head comparison of ixekizumab and adalimumab in biologic-naïve patients with active psoriatic arthritis: efficacy and safety outcomes from a randomized, open-label, blinded assessor study through 52 weeks. Arthritis and Rheumatology 2019;71:5266-9. [Google Scholar]
- Van Den Bosch F, Kristensen LE, McGonagle D, Rossini M, Liu-Leage S, Sapin C, et al. Patient-reported outcomes from a randomised, open-label, parallel-group study evaluating ixekizumab versus adalimumab in patients with PSA who are biologic DMARD naïve: 24-week results. Arthritis and Rheumatology 2019;71:1437-8. [Google Scholar]
Strober 2011 {published data only}
- Strober BE, Crowley JJ, Yamauchi PS, Olds M, Williams DA. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. British Journal of Dermatology 2011;165(3):661-8. [CENTRAL: CN-00811738] [PMID: ] [DOI] [PubMed] [Google Scholar]
STYLE 2020 {published data only}
- Van Voorhees AS, Stein Gold L, Lebwohl M, Strober B, Lynde C, Tyring S, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. Journal of the American Academy of Dermatology 2020;83(1):96-103. [DOI] [PubMed] [Google Scholar]
SustaIMM 2019 {published data only}
- NCT03000075. BI 655066 (risankizumab) compared to placebo in Japanese patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/nct03000075 (first received 21 December 2016). [CENTRAL: CN-01560779]
- Ohtsuki M, Fujita H, Watanabe M, Suzaki K, Flack M, Huang X, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. Journal of Dermatology 2019;46(8):686-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanew 1991 {published data only}
- Tanew A, Guggenbichler A, Hönigsmann H, Geiger JM, Fritsch P. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study. Journal of the American Academy of Dermatology 1991;25(4):682-4. [CENTRAL: CN-00612571] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thaci 2021 {published data only}
- NCT03255382. A study to assess the efficacy of risankizumab compared to FUMADERM® in subjects with moderate to severe plaque psoriasis who are naive to and candidates for systemic therapy. clinicaltrials.gov/show/nct03255382 (first received 21 August 2017).
- Thaci D, Eyerich K, Pinter A, Sebastian M, Unnebrink K, Rubant S, et al. Direct comparison of risankizumab and fumaric acid esters in systemic therapy-naive patients with moderate-to-severe plaque psoriasis: a randomized controlled trial. British Journal of Dermatology 2022;186(1):30-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaci D, Eyerich K, Pinter A, Sebastian M, Unnebrink K, Rubant S, et al. Direct comparison of risankizumab and fumaric acid esters in systemic-therapy-naive patients with moderate to severe plaque psoriasis: a randomized controlled trial. British Journal of Dermatology 2021;15(186):30-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaci D, Soliman AM, Eyerich K, Pinter A, Sebastian M, Unnebrink K, et al. Patient-reported outcomes with risankizumab versus fumaric acid esters in systemic therapy-naive patients with moderate to severe plaque psoriasis: a phase 3 clinical trial. Journal of the European Academy of Dermatology and Venereology 2021;35(8):1686-91. [DOI] [PubMed] [Google Scholar]
Torii 2010 {published data only}
- JPRN-JapicCTI-060318. Clinical study to evaluate the efficacy and safety of TA-650 in patients with psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-JapicCTI-060318 (first received 1 November 2006).
- Torii H, Nakagawa H, Japanese Infliximab Study investigators. Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. Journal of Dermatological Science 2010;59(1):40-9. [CENTRAL: CN-00760986] [PMID: ] [DOI] [PubMed] [Google Scholar]
TRANSFIGURE 2016 {published and unpublished data}
- Reich K, Sullivan J, Arenberger P, Mrowietz U, Jazayeri S, Augustin M, et al. Secukinumab shows significant efficacy in nail psoriasis: week 32 results from the TRANSFIGURE study. Annals of the Rheumatic Diseases 2016;75(Suppl 2):603-4. [CENTRAL: CN-01761193] [Google Scholar]
Tyring 2006 {published data only}
- Krishnan R, Cella D, Leonardi C, Papp K, Gottlieb AB, Dunn M, et al. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. British Journal of Dermatology 2007;157(6):1275-7. [CENTRAL: CN-00627972] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Tyring S, Bagel J, Lynde C, Klekotka P, Thompson EH, Gandra SR, et al. Patient-reported outcomes in moderate-to-severe plaque psoriasis with scalp involvement: results from a randomized, double-blind, placebo-controlled study of etanercept. Journal of the European Academy of Dermatology and Venereology 2013;27(1):125-8. [CENTRAL: CN-00971136] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Tyring S, Gordon KB, Poulin Y, Langley RG, Gottlieb AB, Dunn M, et al. Long-term safety and efficacy of 50 mg of etanercept twice weekly in patients with psoriasis. Archives of Dermatology 2007;143(6):719-26. [CENTRAL: CN-00589825] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 2006;367(9504):29-35. [CENTRAL: CN-00532672] [PMID: ] [DOI] [PubMed] [Google Scholar]
UltIMMa‐1 2018 {unpublished data only}
- Augustin M, Lambert J, Zema C, Thompson EHZ, Yang M, Wu EQ, et al. Effect of risankizumab on patient-reported outcomes in moderate to severe psoriasis: the UltIMMa-1 and UltIMMa-2 randomized clinical trials. JAMA Dermatology 2020;156(12):1344-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooderham M, Lebwohl M, Gordon K, Wu T, Photowala H, Bachelez H. High and durable clearance through 52 weeks of risankizumab treatment in patients with moderate-to-severe plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2019;33(S3):20. [Google Scholar]
- Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018;392(10148):650-61. [CENTRAL: CN-01649259] [DOI] [PubMed] [Google Scholar]
- Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab: results from two double-blind, placebo-and ustekinumab-controlled, phase 3 trials in moderate-to-severe plaque psoriasis. Acta Dermato-Venereologica 2018;98(Suppl 219):28-9. [DOI] [PubMed] [Google Scholar]
- NCT02684357. BI 655066 compared to placebo & active comparator (Ustekinumab) in patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/ct2/show/NCT02684357 (first received 18 February 2016). [CENTRAL: CN-01555862]
UltIMMa‐2 2018 {unpublished data only}
- Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018;392(10148):650-61. [CENTRAL: CN-01649259] [DOI] [PubMed] [Google Scholar]
- Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab: results from two double-blind, placebo-and ustekinumab-controlled, phase 3 trials in moderate-to-severe plaque psoriasis. Acta Dermato-Venereologica 2018;98(Suppl 219):28-9. [DOI] [PubMed] [Google Scholar]
- NCT02684370. BI 655066 (Risankizumab) compared to placebo and active comparator (Ustekinumab) in patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/ct2/show/NCT02684370 (first received 18 February 2016). [CENTRAL: CN-01555863]
Umezawa 2021 {published data only}
- Imafuku S, Tada Y, Umezawa Y, Sakurai S, Hoshii N, Nakagawa H. Certolizumab pegol in Japanese patients with moderate to severe plaque psoriasis: effect of demographics and baseline disease characteristics on efficacy. Dermatology and Therapy 2022;12(1):121-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03051217. A study to test the efficacy and safety of certolizumab pegol in Japanese subjects with moderate to severe chronic psoriasis. clinicaltrials.gov/show/nct03051217 (first received 13 February 2017).
- Umezawa Y, Asahina A, Imafuku S, Tada Y, Sano S, Morita A, et al. Efficacy and safety of certolizumab pegol in Japanese patients with moderate to severe plaque psoriasis: 52-week results. Dermatology and Therapy 2021;11(3):943-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa Y, Sakurai S, Hoshii N, Nakagawa H, Group PS Study. Certolizumab pegol for the treatment of moderate to severe plaque psoriasis: 16-week results from a phase 2/3 Japanese study. Dermatology and Therapy 2021;11(2):513-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa Y, Sakurai S, Hoshii N, Nakagawa H, Group PS Study. Certolizumab pegol for the treatment of moderate to severe plaque psoriasis: 16-week results from a phase 2/3 Japanese study. Dermatology and Therapy 2021;19:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
UNCOVER‐1 2016 {published data only}
- Armstrong AW, Lynde CW, McBride SR, Stahle M, Edson-Heredia E, Zhu B, et al. Effect of ixekizumab treatment on work productivity for patients with moderate-to-severe plaque psoriasis: analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatology 2016;152(6):661-9. [CENTRAL: CN-01166284] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. New England Journal of Medicine 2016;375(4):345-56. [CENTRAL: CN-01167902] [PMID: ] [DOI] [PubMed] [Google Scholar]
UNCOVER‐2 2015 {published data only}
- Griffiths CE, Reich K, Lebwohl M, Van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015;386(9993):541-51. [CENTRAL: CN-01091029] [PMID: ] [DOI] [PubMed] [Google Scholar]
UNCOVER‐3 2015 {published data only}
- Griffiths CE, Reich K, Lebwohl M, Van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015;386(9993):541-51. [CENTRAL: CN-01091029] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Van de Kerkhof P, Guenther L, Gottlieb AB, Sebastian M, Wu JJ, Foley P, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled and open-label phases of UNCOVER-3. Journal of the European Academy of Dermatology and Venereology 2017;31(3):477-82. [CENTRAL: CN-01287590] [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Bezooijen 2016 {published data only}
- Van Bezooijen JS, Balak DM, Van Doorn MB, Looman CW, Schreurs MW, Koch BC, et al. Combination therapy of etanercept and fumarates versus etanercept monotherapy in psoriasis: a randomized exploratory study. Dermatology 2016;232(4):407-14. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van de Kerkhof 2008 {published data only}
- Reich K, Segaert S, Van de Kerkhof P, Durian C, Boussuge MP, Paolozzi L, et al. Once-weekly administration of etanercept 50 mg improves patient-reported outcomes in patients with moderate-to-severe plaque psoriasis. Dermatology (Basel, Switzerland) 2009;219(3):239-49. [CENTRAL: CN-00730853] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Van de Kerkhof PC, Segaert S, Lahfa M, Luger TA, Karolyi Z, Kaszuba A, et al. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: a randomized controlled trial with open-label extension. British Journal of Dermatology 2008;159(5):1177-85. [CENTRAL: CN-00681015] [PMID: ] [DOI] [PubMed] [Google Scholar]
VIP‐S trial 2020 {unpublished data only}
- Gelfand JM, Shin DB, Duffin KC, Armstrong AW, Blauvelt A, Tyring SK, et al. A randomized placebo controlled trial of secukinumab on aortic vascular inflammation in moderate to severe plaque psoriasis (VIP-S). Journal of Investigative Dermatology 2020;140(9):1784-93.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02690701. Study to evaluate the effect of secukinumab compared to placebo on aortic vascular inflammation in subjects with moderate to severe plaque psoriasis (VIP-S). clinicaltrials.gov/ct2/show/NCT02690701 (first received 24 February 2016).
VIP Trial 2018 {unpublished data only}
- Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circulation. Cardiovascular Imaging 2018;11(6):e007394. [CENTRAL: CN-01652064] [DOI: 10.1161/CIRCIMAGING.117.007394] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01553058. Vascular Inflammation in psoriasis trial (the VIP trial) (VIP). clinicaltrials.gov/ct2/show/NCT01553058 (first received 14 February 2012). [CENTRAL: CN-01591340]
VIP‐U Trial 2020 {published data only}
- Gelfand JM, Shin DB, Alavi A, Torigian DA, Werner T, Papadopoulos M, et al. A phase IV, randomized, double-blind, placebo-controlled crossover study of the effects of ustekinumab on vascular inflammation in psoriasis (the VIP-U Trial). Journal of Investigative Dermatology 2020;140(1):85-93.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02187172. Vascular inflammation in psoriasis-ustekinumab (VIP-U). clinicaltrials.gov/show/nct02187172 (first received 10 July 2014).
VOLTAIRE‐PSO 2021 {published data only}
- Menter A, Arenberger P, Balser S, Beissert S, Cauthen A, Czeloth N, et al. Similar efficacy, safety, and immunogenicity of the biosimilar BI 695501 and adalimumab reference product in patients with moderate-to-severe chronic plaque psoriasis: results from the randomized phase III VOLTAIRE-PSO study. Expert Opinion on Biological Therapy 2021;21(1):87-96. [DOI] [PubMed] [Google Scholar]
- Menter A, Arenberger P, Balser S, Beissert S. 13097 Biosimilar BI 695501 demonstrates clinical equivalence to adalimumab reference product in patients with moderate to severe chronic plaque psoriasis through 24 weeks. Journal of the American Academy of Dermatology 2020;83 (6 Suppl):AB5. [Google Scholar]
- NCT02850965. Efficacy, safety and immunogenicity of BI 695501 versus Humira® in patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/nct02850965 (first received 1 August 2016). [CENTRAL: CN-01507166]
VOYAGE‐1 2016 {published data only}
- Armstrong AW, Reich K, Foley P, Han C, Song M, Shen YK, et al. Improvement in patient-reported outcomes (Dermatology Life Quality Index and the Psoriasis Symptoms and Signs Diary) with guselkumab in moderate-to-severe plaque psoriasis: results from the phase III VOYAGE 1 and VOYAGE 2 studies. American Journal of Clinical Dermatology 2018;20(1):155-64. [CENTRAL: CN-01943968] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauvelt A, Papp KA, Griffiths CE, Randazzo B, Wasfi Y, Shen Y-K, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. Journal of the American Academy of Dermatology 2016;76(3):405-17. [CENTRAL: CN-01341398] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Papp KA, Kimball AB, Randazzo B, Wasfi Y, Li S, et al. Two-year efficacy and safety of guselkumab for treatment of moderate-to-severe psoriasis: phase 3 VOYAGE 1 trial. Annals of the Rheumatic Diseases 2018;77(Suppl 2):1580-1. [CENTRAL: CN-01647499] [Google Scholar]
VOYAGE‐2 2017 {published data only}
- Reich K, Armstrong AW, Foley P, Song M, Miller M, Shen YK, et al. Maintenance of response through up to 4 years of continuous guselkumab treatment of psoriasis in the VOYAGE 2 phase 3 study. American Journal of Clinical Dermatology 2020;21(6):881-90. [DOI] [PubMed] [Google Scholar]
- Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. Journal of the American Academy of Dermatology 2017;76(3):418-31. [CENTRAL: CN-01341399] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yang 2012 {published data only}
- Yang HZ, Wang K, Jin HZ, Gao TW, Xiao SX, Xu JH, et al. Infliximab monotherapy for Chinese patients with moderate to severe plaque psoriasis: a randomized, double-blind, placebo-controlled multicenter trial. Chinese Medical Journal 2012;125(11):1845-51. [CENTRAL: CN-00904898] [PMID: ] [PubMed] [Google Scholar]
Ye 2020 {published data only}
- Ye T, Ye J, Zhang C. The effects of acitretin on patients with psoriasis vulgaris. International Journal of Clinical and Experimental Medicine 2020;13(7):5068-75. [Google Scholar]
Yilmaz 2002 {published data only}
- Yilmaz E, Yilmaz F, Yerebakan O. Re-PUVA therapy for psoriasis vulgaris: an effective choice. Journal of the European Academy of Dermatology and Venereology 2002;16(Suppl S1):258. [CENTRAL: CN-00416979] [Google Scholar]
- Yılmaz E, Yılmaz F, Yerebakan O. Re-PUVA therapy for psoriasis vulgaris: an effective choice [Psoriazis vulgaris tedavisinde etkili bir seçenek: asitretin ile PUVA kombinasyonu (Re-PUVA)]. Türkiye Klinikleri Dermatoloji Dergisi 2002;12(4):204-8. [CENTRAL: CN-01951242] [Google Scholar]
Yu 2019 {published data only}
- Yu Q, Tong Y, Cui L, Zhang L, Gong Y, Diao H, et al. Efficacy and safety of etanercept combined plus methotrexate and comparison of expression of pro-inflammatory factors expression for the treatment of moderate-to-severe plaque psoriasis. International Immunopharmacology 2019;73:442-50. [DOI] [PubMed] [Google Scholar]
Yu 2022 {published data only}
- Yu C, Zhang F, Ding Y, Li Y, Zhao Y, Gu J, et al. A randomized, double-blind phase III study to demonstrate the clinical similarity of biosimilar SCT630 to reference adalimumab in Chinese patients with moderate to severe plaque psoriasis. International Immunopharmacology 2022;112:109248. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abe 2017 {published data only}
- Abe M, Nishigori C, Torii H, Ihn H, Ito K, Nagaoka M, et al. Tofacitinib for the treatment of moderate to severe chronic plaque psoriasis in Japanese patients: subgroup analyses from a randomized, placebo-controlled phase 3 trial. Journal of Dermatology 2017;44(11):1228-37. [CENTRAL: CN-01615689] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abufarag 2010 {published data only}
- Abufarag A, Aigner S, Czeloth N, Dalken B, Koch H, Niemann G, et al. Selective activation of naturally occurring regulatory T cells (Tregs) by the monoclonal antibody BT-061 as a novel therapeutic opportunity in psoriasis: early clinical results after single doses. Journal of Investigative Dermatology 2010;130(Suppl 2):S64. [CENTRAL: CN-00795758] [EMBASE: 70263767] [Google Scholar]
ACTRN12606000040561 {published data only}
- ACTRN12606000040561. The safety and efficacy of Cpn10 in psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12606000040561 (first received 27 January 2006).
Adsit 2017 {published data only}
- Adsit S, Zaldivar ER, Sofen H, Dei-Cas I, Garcia CM, Penaranda EO, et al. Secukinumab is efficacious and safe in Hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four phase 3 trials. Advances in Therapy 2017;34(6):1327-39. [CENTRAL: CN-01443493] [DOI: 10.1007/s12325-017-0521-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Akhyani 2010 {published data only}
- Akhyani M, Chams-Davatchi C, Hemami MR, Fateh S. Efficacy and safety of mycophenolate mofetil vs. methotrexate for the treatment of chronic plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2010;24(12):1447-51. [CENTRAL: CN-00771805] [PMID: ] [DOI] [PubMed] [Google Scholar]
Al‐Oudah 2022 {published data only}
- Al-Oudah G A, Sahib A S, Al-Hattab M K, Al-Ameedee A A. Effect of CoQ10 administration to psoriatic Iraqi patients on biological therapy upon severity index (PASI) and quality of life index (DLQI) before and after therapy. Journal of Population Therapeutics & Clinical Pharmacology 2022;29(2):e52-60. [DOI] [PubMed] [Google Scholar]
Altmeyer 1994 {published data only}
- Altmeyer PJ, Matthes U, Pawlak F, Hoffmann K, Frosch PJ, Ruppert P, et al. Antipsoriatic effect of fumaric acid derivatives. Results of a multicenter double-blind study in 100 patients. Journal of the American Academy of Dermatology 1994;30(6):977-81. [CENTRAL: CN-00101455] [PMID: ] [DOI] [PubMed] [Google Scholar]
Angsten 2007 {published data only}
- Angsten M, Schopf RE. Anti-TNF-alpha-therapy of psoriasis with infliximab or etanercept. Clinical, histological and immunohistochemical course. Aktuelle Dermatologie 2007;33(8-9):310-6. [CENTRAL: CN-00726884] [EMBASE: 2007503340] [Google Scholar]
Anonymous 2005 {published data only}
- Anonymous. Aldalimumab in psoriatic arthritis and as the initial therapy in rheumatoid arthritis [Adalimumab bei psoriasis-arthritis und zur initialtherapie bei rheumatoider arthritis]. Krankenpflege Journal 2005;43(7-10):244. [PMID: ] [PubMed] [Google Scholar]
Anonymous 2008 {published data only}
- Anonymous. Trial watch: novel biologic for psoriasis shows superiority over current best-seller. Nature Reviews. Drug Discovery 2008;7(11):880-1. [PMID: ] [DOI] [PubMed] [Google Scholar]
Anonymous 2019 {published data only}
- Anonymous. Evaluation of effectiveness and safety in two optimization strategies with secukinumab in the treatment of moderate severe psoriasis. Journal of the American Academy of Dermatology 2019;81 (4 Suppl 1):AB60. [Google Scholar]
Araujo 2017 {published data only}
- Araujo EG, Englbrecht M, Hoepken S, Finzel S, Hueber A, Rech J, et al. Ustekinumab is superior to TNF inhibitor treatment in resolving enthesitis in PSA patients with active enthesitis-results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Annals of the Rheumatic Diseases 2017;76(Suppl 2):142. [CENTRAL: CN-01467990] [Google Scholar]
Araujo 2019 {published data only}
- Araujo EG, Englbrecht M, Hoepken S, Finzel S, Kampylafka E, Kleyer A, et al. Effects of ustekinumab versus tumor necrosis factor inhibition on enthesitis: results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Seminars in Arthritis and Rheumatism 2019;48(4):632-7. [CENTRAL: CN-01930220] [DOI] [PubMed] [Google Scholar]
Arifov 1998 {published data only}
- Arifov S, Vaisov A, Ismagilov A, Abidova Z. Acitretin (neotigason) in the treatment of psoriasis. Journal of the European Academy of Dermatology and Venereology 1998;11(Suppl 2):S290. [CENTRAL: CN-00465900] [Google Scholar]
Armati 1972 {published data only}
- Armati RP. Retinoic acid for psoriasis. Australasian Journal of Dermatology 1972;13(2):79-83. [CENTRAL: CN-00008047] [PMID: ] [DOI] [PubMed] [Google Scholar]
Asahina 2016 {published data only}
- Asahina A, Etoh T, Igarashi A, Imafuku S, Saeki H, Shibasaki Y, et al. Oral tofacitinib efficacy, safety and tolerability in Japanese patients with moderate to severe plaque psoriasis and psoriatic arthritis: a randomized, double-blind, phase 3 study. Journal of Dermatology 2016;43(8):869-80. [CENTRAL: CN-01380021] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Augustin 2017 {published data only}
- Augustin M, Blome C, Paul C, Puig L, Luger T, Lambert J, et al. Quality of life and patient benefit following transition from methotrexate to ustekinumab in psoriasis. Journal of the European Academy of Dermatology and Venereology 2017;31(2):294-303. [CENTRAL: CN-01368710] [DOI: 10.1111/jdv.13823] [DOI] [PubMed] [Google Scholar]
Avgerinou 2011 {published data only}
- Avgerinou G, Tousoulis D, Siasos G, Oikonomou E, Maniatis K, Papageorgiou N, et al. Anti-tumor necrosis factor alpha treatment with adalimumab improves significantly endothelial function and decreases inflammatory process in patients with chronic psoriasis. International Journal of Cardiology 2011;151(3):382-3. [CENTRAL: CN-00860819] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bachelez 2017 {published data only}
- Bachelez H, Griffiths CE, Papp K, Hall S, Merola JF, Feldman SR, et al. Effect of tofacitinib on efficacy and patient-reported outcomes in psoriasis patients with baseline psoriatic arthritis: a pooled analysis of 2 phase 3 studies. Arthritis and Rheumatology 2017;69(Suppl 10):613. [CENTRAL: CN-01602915] [Google Scholar]
Bagel 2017a {published data only}
- Bagel J, Duffin KC, Bukhalo M, Bobonich M, Gill A, Zhao F, et al. Ease of use and confidence using auto-injector to administer ixekizumab in a phase 3 trial evaluated with subcutaneous administration assessment questionnaire (SQAAQ). Journal of Clinical and Aesthetic Dermatology 2017;10(5 Suppl 1):S14-5. [CENTRAL: CN-01713047] [Google Scholar]
Bagel 2017b {published data only}
- Bagel J, Tyring S, Rice KC, Collier DH, Kricorian G, Chung J, et al. Open-label study of etanercept treatment in patients with moderate-to-severe plaque psoriasis who lost a satisfactory response to adalimumab. British Journal of Dermatology 2017;177(2):411-8. [CENTRAL: CN-01393822] [DOI: 10.1111/bjd.15381] [DOI] [PubMed] [Google Scholar]
Bagel 2017c {published data only}
- Bagel J, Duffin KC, Moore A, Ferris LK, Siu K, Steadman J, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. Journal of the American Academy of Dermatology 2017;77(4):667-74. [CENTRAL: CN-01412958] [DOI] [PubMed] [Google Scholar]
Bagel 2018b {published data only}
- Bagel J, Samad AS, Stolshek BS, Aras GA, Chung JB, Kricorian G, et al. Open-label study to evaluate the efficacy of etanercept treatment in subjects with moderate to severe plaque psoriasis who have failed therapy with apremilast. Journal of Drugs in Dermatology 2018;17(10):1078-82. [CENTRAL: CN-01664523] [PubMed] [Google Scholar]
Bagherani 2017 {published data only}
- Bagherani N, Smoller BR. Efficacy of topical tofacitinib, a Janus kinase inhibitor, in the treatment of plaque psoriasis. Dermatologic Therapy 2017;30(3):e12467. [CENTRAL: CN-01600736] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bagot 1994 {published data only}
- Bagot M, Grossman R, Pamphile R, Binderup L, Charue D, Revuz J, et al. Additive effects of calcipotriol and cyclosporine A: from in vitro experiments to in vivo applications in the treatment of severe psoriasis. Comptes Rendus de l'Académie des Sciences. Série III, Sciences de la Vie 1994;317(3):282-6. [CENTRAL: CN-00107966] [PMID: ] [PubMed] [Google Scholar]
Banerjee 2021 {published data only}
- Banerjee S, Das S, Roy A, Ghoshal L. Comparative effectiveness and safety of methotrexate versus PUVA in severe chronic stable plaque psoriasis. Indian Journal of Dermatology 2021;66(4):371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartlett 2008 {published data only}
- Bartlett BL, Tyring SK. Ustekinumab for chronic plaque psoriasis. Lancet 2008;371(9625):1639-40. [CENTRAL: CN-00631484] [PMID: ] [DOI] [PubMed] [Google Scholar]
Barzegari 2004 {published data only}
- Barzegari M, Ghaninejad H, Shizarpoor M. Comparison of bath PUVA and acitretin in treatment of psoriatic patients. Iranian Journal of Dermatology 2004;7(4):31-5. [CENTRAL: CN-00509588] [Google Scholar]
Batchelor 2009 {published data only}
- Batchelor JM, Ingram JR, Williams H. Adalimumab vs methotrexate for the treatment of chronic plaque psoriasis. Archives of Dermatology 2009;145(6):704-6. [CENTRAL: CN-01783607] [EMBASE: 2009300916] [DOI] [PubMed] [Google Scholar]
Bayerl 1992 {published data only}
- Bayerl C. Treatment of psoriasis vulgaris with etretinate versus cyclosporin A. Report on a study [Parallelgruppenvergleich etretinat versus cyclosporin A bei psoriasis vulgaris]. Aktuelle Dermatologie 1992;18(1-2):27-31. [CENTRAL: CN-00197510] [EMBASE: 1992096910] [Google Scholar]
Beissert 2009 {published data only}
- Beissert S, Pauser S, Sticherling M, Frieling U, Loske KD, Frosch PJ, et al. A comparison of mycophenolate mofetil with ciclosporine for the treatment of chronic plaque-type psoriasis. Dermatology (Basel, Switzerland) 2009;219(2):126-32. [CENTRAL: CN-00729115] [PMID: ] [DOI] [PubMed] [Google Scholar]
Berbis 1989 {published data only}
- Berbis P, Geiger JM, Vaisse C, Rognin C, Privat Y. Benefit of progressively increasing doses during the initial treatment with acitretin in psoriasis. Dermatologica 1989;178(2):88-92. [CENTRAL: CN-00058682] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bhat 2017 {published data only}
- Bhat RM, Leelavathy B, Aradhya SS, Gopal MG, Pratap DV, Mubashir M, et al. Secukinumab efficacy and safety in Indian patients with moderate-to-severe plaque psoriasis: sub-analysis from FIXTURE, a randomized, placebo-controlled, phase 3 study. Indian Dermatology Online Journal 2017;8(1):16-24. [DOI: 10.4103/2229-5178.198765] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhuiyan 2010 {published data only}
- Bhuiyan MS, Sikder MdA, Rashid MM, Rabin F. Role of oral colchicine in plaque type psoriasis. A randomized clinical trial comparing with oral methotrexate. Journal of Pakistan Association of Dermatologists 2010;20(3):146-51. [CENTRAL: CN-00789712] [EMBASE: 2010613015] [Google Scholar]
Bian 2018` {published data only}
- Bian M, Bissonnette R, Luger T, Thaçi D, Toth D, Xia S, et al. Secukinumab treatment in moderate-to-severe psoriasis patients demonstrates sustained low absolute PASI up to 4 years: results from SCULPTURE extension study. Australasian Journal of Dermatology 2018;59(Suppl 1):39. [CENTRAL: CN-01606960] [DOI: 10.1111/jdv.14878] [DOI] [Google Scholar]
Bigby 2004 {published data only}
- Bigby M. A randomized controlled trial of methotrexate and cyclosporine in the treatment of psoriasis. Archives of Dermatology 2004;140(3):347-8. [CENTRAL: CN-00515754] [EMBASE: 2004120959] [DOI] [PubMed] [Google Scholar]
Bissonnette 2006 {published data only}
- Bissonnette R, Papp K, Poulin Y, Lauzon G, Aspeslet L, Huizinga R, et al. A randomized, multicenter, double-blind, placebo-controlled phase 2 trial of ISA247 in patients with chronic plaque psoriasis. Journal of the American Academy of Dermatology 2006;54(3):472-8. [CENTRAL: CN-00555245] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bissonnette 2010 {published data only}
- Bissonnette R, Papp K, Maari C, Yao Y, Robbie G, White WI, et al. A randomized, double-blind, placebo-controlled, phase I study of medi-545, an anti-interferon-alfa monoclonal antibody, in subjects with chronic psoriasis. Journal of the American Academy of Dermatology 2010;62(3):427-36. [CENTRAL: CN-00734807] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bissonnette 2015 {published data only}
- Bissonnette R, Iversen L, Sofen H, Griffiths CE, Foley P, Romiti R, et al. Tofacitinib withdrawal and retreatment in moderate-to-severe chronic plaque psoriasis: a randomized controlled trial. British Journal of Dermatology 2015;172(5):1395-406. [CENTRAL: CN-01254758] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bissonnette 2017a {published data only}
- Bissonnette R, Luger T, Thaçi D, Toth D, Messina I, You R, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. British Journal of Dermatology 2017;177(4):1033-42. [CENTRAL: CN-01419893] [DOI: 10.1111/bjd.15706] [DOI] [PubMed] [Google Scholar]
Bissonnette 2017b {published data only}
- Bissonnette R, Harel F, Krueger JG, Guertin MC, Chabot-Blanchet M, Gonzalez J, et al. TNF-α antagonist and vascular inflammation in patients with psoriasis vulgaris: a randomized placebo-controlled study. Journal of Investigative Dermatology 2017;137(8):1638-45. [CENTRAL: CN-01410690] [DOI: 10.1016/j.jid.2017.02.977] [DOI] [PubMed] [Google Scholar]
Bissonnette 2018 {published data only}
- Bissonnette R, Haydey R, Rosoph LA, Lynde CW, Bukhalo M, Fowler JF, et al. Apremilast for the treatment of moderate-to-severe palmoplantar psoriasis: results from a double-blind, placebo-controlled, randomized study. Journal of the European Academy of Dermatology and Venereology 2018;32(3):403-10. [CENTRAL: CN-01643330] [DOI: 10.1111/jdv.14647] [DOI] [PubMed] [Google Scholar]
Bjerke 1989 {published data only}
- Bjerke JR, Geiger JM. Acitretin versus etretinate in severe psoriasis. A double-blind randomized Nordic multicenter study in 168 patients. Acta Dermato-Venereologica 1989;146(Suppl):206-7. [CENTRAL: CN-00064911] [PMID: ] [PubMed] [Google Scholar]
Blauvelt 2016a {published data only}
- Blauvelt A, Papp K, Griffiths CE, Mallbris L, Dutronic Y, Ilo D, et al. Efficacy and safety of ixekizumab in patients previously treated with etanercept. Experimental Dermatology 2016;25(S4):38-9. [CENTRAL: CN-01407588] [DOI: 10.1111/exd.13200] [DOI] [Google Scholar]
Blauvelt 2016b {published data only}
- Blauvelt A, Langley R, Leonardi C, Gordon K, Luger T, Ohtsuki M, et al. Ixekizumab, a novel anti-IL-17A antibody, exhibits low immunogenicity during long-term treatment in patients with psoriasis. Journal of Investigative Dermatology 2016;136(9 Suppl 2):S227. [CENTRAL: CN-01785592] [Google Scholar]
Blauvelt 2017a {published data only}
- Blauvelt A, Reich K, Lebwohl M, Burge D, Arendt C, Peterson L, et al. Certolizumab pegol for the treatment of patients with moderate-to-severe chronic plaque psoriasis: an overview of three randomized controlled trials. British Journal of Dermatology 2017;177(5):e249-50. [CENTRAL: CN-01452510] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blauvelt 2017b {published data only}
- Blauvelt A, Papp KA, Sofen H, Augustin M, Yosipovitch G, Katoh N, et al. Continuous dosing versus interrupted therapy with ixekizumab: an integrated analysis of two phase 3 trials in psoriasis. Journal of the European Academy of Dermatology and Venereology 2017;31(6):1004-13. [CENTRAL: CN-01459095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blauvelt 2017c {published data only}
- Blauvelt A, Lacour JP, Fowler JF, Schuck E, Jauch-Lembach J, Balfour A, et al. Long-term efficacy, safety, and immunogenicity data from a phase III confirmatory study comparing GP2017, a proposed biosimilar, with reference adalimumab. American Journal of Gastroenterology 2017;112(Suppl 1):S419. [CENTRAL: CN-01463019] [Google Scholar]
Blauvelt 2017d {published data only}
- Blauvelt A, Reich K, Warren RB, Szepietowski JC, Sigurgeirsson B, Tyring SK, et al. Secukinumab re-initiation achieves regain of high response levels in patients who interrupt treatment for moderate to severe plaque psoriasis. British Journal of Dermatology 2017;177(3):879-81. [CENTRAL: CN-01622237] [DOI] [PubMed] [Google Scholar]
Blauvelt 2017e {published data only}
- Blauvelt A, Papp KA, Lebwohl MG, Green LJ, Hsu S, Bhatt V, et al. Rapid onset of action in patients with moderate-to-severe psoriasis treated with brodalumab: a pooled analysis of data from two phase 3 randomized clinical trials (AMAGINE-2 and AMAGINE-3). Journal of the American Academy of Dermatology 2017;77(2):372-4. [CENTRAL: CN-01622584] [DOI] [PubMed] [Google Scholar]
Blauvelt 2017f {published data only}
- Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, Leonardi CL, et al. Efficacy and safety of continuous ixekizumab treatment for 60 weeks in moderate-to-severe plaque psoriasis: results from the UNCOVER-3 trial. Journal of Clinical and Aesthetic Dermatology 2017;10(5 Suppl 1):S15. [CENTRAL: CN-01713049] [Google Scholar]
Blauvelt 2017g {published data only}
- Blauvelt A, Papp KA, Griffiths CE, Puig L, Weisman J, Dutronc Y, et al. Efficacy and safety of switching to ixekizumab in etanercept non-responders: a subanalysis from two phase III randomized clinical trials in moderate-to-severe plaque psoriasis (UNCOVER-2 and -3). American Journal of Clinical Dermatology 2017;18(2):273-80 Correction in American Journal of Clinical Dermatology 2018; 19(3):457. [CENTRAL: CN-01368347] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blauvelt 2017h {published data only}
- Blauvelt A, Reich K, Warren R, Sigurgeirsson B, Langley R, Papavassilis C, et al. Secukinumab retreatment shows rapid recapture of treatment response: an analysis of a phase 3 extension trial in psoriasis. International Journal of Dermatology 2017;56(11):1264-5. [CENTRAL: CN-01570754] [Google Scholar]
Blauvelt 2017i {published data only}
- Blauvelt A, Ferris LK, Yamauchi PS, Qureshi A, Leonardi CL, Farahi K, et al. Extension of ustekinumab maintenance dosing interval in moderate-to-severe psoriasis: results of a phase IIIb, randomized, double-blinded, active-controlled, multicentre study (PSTELLAR). British Journal of Dermatology 2017;177(6):1552-61. [CENTRAL: CN-01428390] [DOI] [PubMed] [Google Scholar]
Blauvelt 2017j {published data only}
- Blauvelt A, Lebwohl MG, Green LJ, Hsu S, Bhatt V, Rastogi S, et al. Median time to treatment response in patients with moderate-to-severe plaque psoriasis treated with brodalumab 210mg or ustekinumab: a pooled analysis of data from two phase 3 randomized clinical trials (AMAGINE-2 and AMAGINE-3). Journal of Clinical and Aesthetic Dermatology 2017;10(5 Suppl 1):S22-3. [CENTRAL: CN-01628810] [DOI] [PubMed] [Google Scholar]
Blauvelt 2017k {published data only}
- Blauvelt A, Gooderham M, Iversen L, Ball S, Zhang L, Agada N, et al. Efficacy and safety of ixekizumab for the treatment of plaque psoriasis: results through 108 weeks randomised, phase III clinical trial (UNCOVER-3). Journal of Investigative Dermatology 2017;137(10 Suppl 2):S260. [CENTRAL: CN-01416619] [DOI] [PubMed] [Google Scholar]
Blauvelt 2018a {published data only}
- Blauvelt A, Reich K, Papp KA, Tyring SK, Sinclair R, Thaçi D, et al. Predictors of response to tildrakizumab for moderate to severe chronic plaque psoriasis. Acta Dermato-Venereologica 2018;98(Suppl 219):22. [CENTRAL: CN-01620162] [Google Scholar]
Blauvelt 2018b {published data only}
- Blauvelt A, Sofen H, Papp K, Gooderham M, Zhao Y, Lowry S, et al. Tildrakizumab efficacy over time by week 28 response levels in two phase 3 clinical trials in patients with chronic plaque psoriasis. Acta Dermato-Venereologica 2018;98(Suppl 219):49. [CENTRAL: CN-01920779] [Google Scholar]
Blauvelt 2018c {published data only}
- Blauvelt A, Tyring S, Philipp S, Adam D, Song M, Wasf Y, et al. Speed of response of guselkumab compared with adalimumab for the treatment of moderate-to-severe psoriasis: results through week 24 from the phase 3, double-blinded, placebo-and active comparator-controlled voyage 1 and voyage 2 trials. Acta Dermato-Venereologica 2018;98(Suppl 219):21. [CENTRAL: CN-01620164] [Google Scholar]
Blauvelt 2018d {published data only}
- Blauvelt A, Strober B, Langley R, Burge D, Pisenti L, Yassine M, et al. Safety of certolizumab pegol over 48 weeks in chronic plaque psoriasis phase 3 trials. Acta Dermato-Venereologica 2018;98(Suppl 219):21. [CENTRAL: CN-01620163] [Google Scholar]
Blauvelt 2018e {published data only}
- Blauvelt A, Reich K, Lebwohl M, Burge D, Arendt C, Peterson L, et al. Certolizumab pegol for the treatment of patients with moderate-to-severe chronic plaque psoriasis: pooled analysis of week 16 data from three randomized controlled trials. Journal of the European Academy of Dermatology and Venereology 2018;33(3):546-52. [CENTRAL: CN-01915790] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blauvelt 2018f {published data only}
- Blauvelt A, Muram TM, See K, Mallinckrodt CH, Crowley JJ, Van de Kerkhof P. Improvements in psoriasis within different body regions vary over time following treatment with ixekizumab. Journal of Dermatological Treatment 2018;29(3):220-9. [CENTRAL: CN-01604313] [DOI] [PubMed] [Google Scholar]
Blauvelt 2018g {published data only}
- Blauvelt A, Reich K, Papp KA, Kimball AB, Gooderham M, Tyring SK, et al. Safety of tildrakizumab for moderate-to-severe plaque psoriasis: pooled analysis of three randomized controlled trials. British Journal of Dermatology 2018;179(3):615-22. [CENTRAL: CN-01645090] [DOI] [PubMed] [Google Scholar]
Blauvelt 2018h {published data only}
- Blauvelt A, Tyring S, Gooderham M, Koo J, Zhao Y, Lowry S, et al. Better skin clearance is associated with improved quality of life in moderate-to-severe psoriasis patients treated with tildrakizumab. Acta Dermato-Venereologica 2018;98(Suppl 219):31-2. [CENTRAL: CN-01620179] [Google Scholar]
Blauvelt 2020 {published data only}
- Blauvelt A, Merola JF, Papp KA, Gooderham M, Gottlieb AB, Cioffi C, et al. 14020 Durability of responses with bimekizumab, a selective dual inhibitor of interleukin-17A and -17F, in moderate to severe chronic plaque psoriasis in a 60-week randomized, double-blinded, phase 2b study (BE ABLE 2). Journal of the American Academy of Dermatology 2020;83(6 Suppl):AB16. [Google Scholar]
- Blauvelt A, Papp KA, Merola JF, Gottlieb AB, Cross N, Madden C, et al. Bimekizumab for patients with moderate-to-severe plaque psoriasis: 60-week results from BE ABLE 2, a randomized, double-blinded, placebo-controlled phase 2b extension study. Journal of the American Academy of Dermatology 2020;83(5):1367-74. [DOI] [PubMed] [Google Scholar]
Branigan 2017 {published data only}
- Branigan P, Liu X, Chen Y, Scott B, Yao Z, Li S, et al. Sustained response following withdrawal of guselkumab treatment correlates with reduced Th17 and Th22 effector cytokine levels. Journal of Investigative Dermatology 2017;137(10 Suppl 2):S194. [CENTRAL: CN-01416625] [Google Scholar]
Brasil 2012 {published data only}
- Brasil, Ministério da Saúde, Departamento de Gestão e Incorporação de Tecnologias em, Saúde. Medicamentos biológicos (infliximabe, etanercepte, adalimumabe e ustequinumabe) para o tratamento da psoríase moderada a grave em adultos. conitec.gov.br/images/Incorporados/Biologicos-Psoriase-final.pdf 2012 (accessed prior to 17 December 2019).
Brasil 2013 {published data only}
- Brasil, Ministério da Saúde, Departamento de Gestão e Incorporação de Tecnologias em, Saúde. Golimumabe para artrite psoriásica. conitec.gov.br/images/Incorporados/Golimumabe-ArtritePsoriasica-final.pdf 2013 (accessed prior to 17 December 2019).
Brasil 2016 {published data only}
- Brasil, Ministério da Saúde, Comissão Nacional de Incorporação de Tecnologias no SUS. Golimumabe para o tratamento da artrite psoriásica. conitec.gov.br/images/Relatorios/2016/Relatorio_Golimumabe_ArtritePsoriasica_final.pdf 2016 (accessed prior to 17 December 2019).
Buono 2020 {published data only}
- Buono R, Parisi A, Russo R, Mastrobuoni C, Di Monda G, Valentino U, et al. Efficacy and tolerability about ustekinumab in patients withpsoriatic arthritis after 24 months of treatment. Italian Journal of Medicine 2020;14 (Suppl 2):18. [Google Scholar]
Burden 2017 {published data only}
- Burden AD. Etanercept or infliximab for psoriasis? An independent randomized clinical trial. British Journal of Dermatology 2017;176(3):565. [CENTRAL: CN-01336581] [DOI] [PubMed] [Google Scholar]
Burkhardt 2017 {published data only}
- Burkhardt N, Mrowietz U, Carrascosa JM, Fernandez-Penas P, Guede D, Wilhelm S, et al. Absolute and relative PASI over 1 year of treatment with ixekizumab (IXE): descriptive analysis in patients with moderate-to-severe plaque psoriasis. Australasian Journal of Dermatology 2017;58(Suppl 1):42. [CENTRAL: CN-01378807] [Google Scholar]
Callis Duffin 2017 {published data only}
- Callis Duffin K, Bagel J, Bukhalo M, Mercado Clement IJ, Choi SL, Zhao F, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). Journal of the European Academy of Dermatology and Venereology 2017;31(1):107-13. [CENTRAL: CN-01368719] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cassano 2006 {published data only}
- Cassano N, Loconsole F, Galluccio A, Miracapillo A, Pezza M, Vena GA. Once-weekly administration of high-dosage etanercept in patients with plaque psoriasis: results of a pilot experience (power study). International Journal of Immunopathology and Pharmacology 2006;19(1):225-9. [CENTRAL: CN-00563725] [PubMed] [Google Scholar]
Cassano 2010 {published data only}
- Cassano N, Loconsole F, Miracapillo A, Travaglini M, Digiuseppe MD, Congedo M, et al. Treatment of psoriasis with different dosage regimens of etanercept: preliminary results from the Talpharanta Plastic Study Group. International Journal of Immunopathology and Pharmacology 2010;23(3):797-802. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cather 2006 {published data only}
- Cather J, Krueger G, Jackson M, Samstov A. Efficacy and safety of low-dose acitretin for the treatment of moderate to severe plaque-type psoriasis. Journal of the American Academy of Dermatology (American Academy of Dermatology 64th Annual Meeting March 3-7, 2006) 2006;54(3 Suppl):AB217 (abstract P2877). [CENTRAL: CN-00602420] [Google Scholar]
Cather 2018 {published data only}
- Cather JC, Meeuwis K, Burge R, Bleakman AP, Lin CY, Gottlieb A, et al. Ixekizumab improves impact of genital psoriasis on sexual activity: results from a phase 3b study. Acta Dermato-Venereologica 2018;98(Suppl 219):11. [CENTRAL: CN-01620184] [Google Scholar]
Chakravadhanula 2017 {published data only}
- Chakravadhanula U, Chandrashekar BS, Parekh M, Krupashankar DS, Swaroop HS, Pawar D. One-year pilot study to evaluate sequential therapy with ciclosporin and itolizumab in treatment of chronic plaque psoriasis. British Journal of Dermatology 2017;177(5):e291. [CENTRAL: CN-01452514] [Google Scholar]
Chapman 2018 {published data only}
- Chapman M, Cirulli J, McBride S. Sustained improvement in patient-reported outcomes with continued apremilast treatment over 104 weeks in patients with moderate to severe psoriasis. Australasian Journal of Dermatology 2018;59(Suppl 1):46. [CENTRAL: CN-01606954] [Google Scholar]
ChiCTR2000030273 2020 {published data only}
- ChiCTR2000030273. A comparative study to evaluate the pharmacokinetics and safety of BAT2206 injection vs ustekinumab injection (Stelara) in healthy Chinese male subjects. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000030273 (first received 27 February 2020).
ChiCTR‐INR‐16009710 {unpublished data only}
- ChiCTR-INR-16009710. Acitretin plus methotrexate in the treatment of moderate to severe psoriasis vulgaris [The role of keratin 17 in the pathogenesis of psoriasis through PI3K/Akt signal pathways]. www.chictr.org.cn/showprojen.aspx?proj=16444/ChiCTR-INR-16009710 (first received 2 November 2016).
Chládek 2002 {published data only}
- Chládek J, Grim J, Martínková J, Simková M, Vanìèková J, Koudelková V, et al. Pharmacokinetics and pharmacodynamics of low-dose methotrexate in the treatment of psoriasis. British Journal of Clinical Pharmacology 2002;54(2):147-56. [CENTRAL: CN-00409768] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chodorowska 1999a {published data only}
- Chodorowska G, Czelej D, Juszkiewicz-Borowiec M, Pietrzak A, Wojnowska D, Krasowska D. Selected cytokines and acute phase proteins in psoriatic patients treated with cyclosporin A or Re-PUVA methods. Annales Universitatis Mariae Curie-Sklodowska. Section D: Medicina 1999;54:173-80. [CENTRAL: CN-00325819] [PubMed] [Google Scholar]
Chodorowska 1999b {published data only}
- Chodorowska G, Czelej D, Juszkiewicz-Borowiec M, Pietrzak A, Wojnowska D, Krasowska D. Plasma levels of selected cytokines and acute phase proteins in 2 groups of psoriatic patients treated with cyclosporine A or RE-PUVA method. Journal of the European Academy of Dermatology and Venereology 1999;12(Suppl 2):S330. [CENTRAL: CN-00478492] [PubMed] [Google Scholar]
Choi 2017 {published data only}
- Choi CW, Kim BR, Seo E, Youn SW. The objective Psoriasis Area and Severity Index: a randomized controlled pilot study comparing the effectiveness of ciclosporin and methotrexate. British Journal of Dermatology 2017;177(6):1740-1. [CENTRAL: CN-01914246] [DOI] [PubMed] [Google Scholar]
Crowley 2018a {published data only}
- Crowley J, Gisondi P, Geng Z, Servin OR. Long-term safety and efficacy of adalimumab from the phase 3 randomized, placebo-controlled trial in patients with nail and skin psoriasis. Acta Dermato-Venereologica 2018;98(Suppl 219):25. [CENTRAL: CN-01620198] [Google Scholar]
Crowley 2018b {published data only}
- Crowley J, Papp KA, Hong C, Parno J, Mendelsohn AM, Li Q, et al. Efficacy of tildrakizumab in etanercept partial responders or nonresponders. Acta Dermato-Venereologica 2018;98(Suppl 219):29. [CENTRAL: CN-01620188] [Google Scholar]
CTRI/2018/01/011373 {published data only}
- CTRI/2018/01/011373. Comparison of efficacy and safety of oral vs subcutaneous methotrexate in psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/01/011373 (first received 16 January 2018).
CTRI/2020/07/026598 2020 {published data only}
- CTRI/2020/07/026598. A comparative study for the efficacy and side effects in subcutaneous and oral methotrexate in patients of chronic plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/07/026598 (first received 15 July 2020).
CTRI/2020/12/029472 2020 {published data only}
- CTRI/2020/12/029472. Fasting in managment of psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/12/029472 (first received 1 December 2020).
CTRI/2020/12/029611 {published data only}
- CTRI/2020/12/029611. A study to check effectiveness of dimethyl fumarate gastro-resistant hard capsule in patients with moderate to severe chronic plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/12/029611 (first received 7 December 2020).
De Jong 2003 {published data only}
- De Jong EM, Mork NJ, Seijger MM, De La Brassine M, Lauharanta J, Jansen CT, et al. The combination of calcipotriol and methotrexate compared with methotrexate and vehicle in psoriasis: results of a multicentre placebo-controlled randomized trial. British Journal of Dermatology 2003;148(2):318-25. [CENTRAL: CN-00422725] [DOI] [PubMed] [Google Scholar]
De Mendizabal 2017 {published data only}
- De Mendizabal NV, Heathman M, Jackson K. A longitudinal PKPD model describing the effect of ixekizumab on static physician's global assessment score (sPGA) in patients with moderate-to-severe plaque psoriasis. Journal of Pharmacokinetics and Pharmacodynamics 2017;44(1 Suppl 1):S102. [Google Scholar]
Dubiel 1972 {published data only}
- Dubiel W, Happle R. Experimental treatment with fumaric acid monoethylester in psoriasis vulgaris [Behandlungsversuch mit fumarsauremonoathylester bei psoriasis vulgaris]. Zeitschrift fur Haut- und Geschlechtskrankheiten 1972;47(13):545-50. [PMID: ] [PubMed] [Google Scholar]
Duffin 2016 {published data only}
- Duffin KC, Bagel J, Bukhalo M, Clement IJ, Zhao F, Gill A, et al. Comparison of the pharmacokinetics of ixekizumab following subcutaneous administration using a prefilled syringe versus an autoinjector in patients with moderate-to-severe psoriasis. Journal of the American Academy of Dermatology 2016;74(5 Suppl 1):AB242. [EMBASE: 72275926] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffin 2017 {published data only}
- Duffin KC, Papp KA, Bagel J, Pharm DEL, Chen R, Gottlieb AB. Evaluation of the physician global assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. Journal of Drugs in Dermatology 2017;16(2):147-53. [CENTRAL: CN-01416699] [PubMed] [Google Scholar]
Ecker‐Schlipf 2009 {published data only}
- Ecker-Schlipf B. Psoriasis vulgaris: How effective and safe is the calcineurin inhibitor voclosporin? [Psoriasis vulgaris: Wie wirksam und sicher ist der calcineurin- inhibitor voclosporin?]. Arzneimitteltherapie 2009;27(3):97-8. [EMBASE: 2009143373] [Google Scholar]
Edson‐Heredia 2013 {published data only}
- Edson-Heredia E, Banerjee S, Zhu B, Maeda-Chubachi T, Cameron G, Shen W, et al. A PASI ≥ 90 response is associated with improved patient reported outcomes: results from a phase 2 study in patients with psoriasis treated with ixekizumab. Journal of the European Academy of Dermatology and Venereology 2013;27(Suppl 4):25. [DOI: 10.1111/jdv.12186] [DOI] [PubMed] [Google Scholar]
Egeberg 2016 {published data only}
- Egeberg A. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. New England Journal of Medicine 2016;375(21):2101-2. [CENTRAL: CN-01296632] [DOI: 10.1056/NEJMc1610828] [DOI] [PubMed] [Google Scholar]
Elewski 2007 {published data only}
- Elewski B, Leonardi C, Gottlieb AB, Strober BE, Simiens MA, Dunn M, et al. Comparison of clinical and pharmacokinetic profiles of etanercept 25 mg twice weekly and 50 mg once weekly in patients with psoriasis. British Journal of Dermatology 2007;156(1):138-42. [CENTRAL: CN-00577519] [DOI] [PubMed] [Google Scholar]
Elewski 2017 {published data only}
- Elewski BE, Puig L, Mordin M, Gilloteau I, Sherif B, Fox T, et al. Psoriasis patients with Psoriasis Area and Severity Index (PASI) 90 response achieve greater health-related quality-of-life improvements than those with PASI 75-89 response: results from two phase 3 studies of secukinumab. Journal of Dermatological Treatment 2017;28(6):492-9. [CENTRAL: CN-01443627] [DOI] [PubMed] [Google Scholar]
Elewski 2018a {published data only}
- Elewski B, Menter M, Crowley J, Tyring J, Zhao Y, Lowry S, et al. Sustained and improved efficacy of tildrakizumab from week 28 to week 52 in treating moderate-to-severe plaque psoriasis. Journal of Clinical and Aesthetic Dermatology 2018;11(5 Suppl 1):S25. [CENTRAL: CN-01713707] [Google Scholar]
Elewski 2018b {published data only}
- Elewski B, Menter A, Crowley J, Tyring S, Zhao Y, Lowry S, et al. Sustained and improved efficacy of tildrakizumab from week 28 to week 52 in treating moderate-to-severe plaque psoriasis. Acta Dermato-Venereologica 2018;98(Suppl 219):31. [CENTRAL: CN-01620180] [Google Scholar]
Ellis 1986 {published data only}
- Ellis CN, Gorsulowsky DC, Hamilton TA, Billings JK, Brown MD, Headington JT, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA 1986;256(22):3110-6. [CENTRAL: CN-00045553] [PubMed] [Google Scholar]
Ellis 2001 {published data only}
- Ellis CN, Krueger GG. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. New England Journal of Medicine 2001;345(4):248-55. [CENTRAL: CN-00349381] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ellis CN, Mordin MM, Adler EY. Effects of alefacept on health-related quality of life in patients with psoriasis: results from a randomized, placebo-controlled phase II trial. American Journal of Clinical Dermatology 2003;4(2):131-9. [CENTRAL: CN-00435105] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ellis 2002 {published data only}
- Ellis CN, Reiter KL, Bandekar RR, Fendrick AM. Cost-effectiveness comparison of therapy for psoriasis with a methotrexate-based regimen versus a rotation regimen of modified cyclosporine and methotrexate. Journal of the American Academy of Dermatology 2002;46(2):242-50. [CENTRAL: CN-01783592] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ellis 2012 {published data only}
- Chow C, Zhang Z, Goldfarb MT, Simpson MJ, Ellis CN. Evaluation of Psoriasis Area and Severity Index, Static Physician's Global Assessment, and Lattice System - Physician's Global Assessment for assessing severity of psoriasis. Journal of the American Academy of Dermatology 2012;131(Suppl 1):S81. [CENTRAL: CN-00843715] [Google Scholar]
Engst 1989 {published data only}
- Engst R, Huber J. Results of cyclosporin treatment of severe, chronical psoriasis vulgaris. Hautarzt 1989;40(8):486-9. [EMBASE: 1989202264] [PubMed] [Google Scholar]
Erkko 1997 {published data only}
- Erkko P, Granlund H, Nuutinen M, Reitamo S. Comparison of cyclosporin A pharmacokinetics of a new microemulsion formulation and standard oral preparation in patients with psoriasis. British Journal of Dermatology 1997;136(1):82-8. [PMID: ] [PubMed] [Google Scholar]
EUCTR2007‐004328‐18‐FR {published data only}
- EUCTR2007-004328-18-FR. A 12 week double-blind, randomised, placebo-controlled, modified dose-escalation trial to investigate safety, efficacy, and pharmacokinetics of BIRT 2584XX tablets at doses of 100, 300 and 500 mg administered once daily in patients with moderate to severe psoriasis with a 12 week treatment extension for PASI 50 responders. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2007-004328-18-FR (first received 14 November 2007).
EUCTR2012‐005685‐35‐DE {published data only}
- EUCTR2012-005685-35-DE. A study to compare the efficacy of a new developed product, FP187, to a marketed product, to each other but also to placebo, in patients with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012-005685-35-DE (first received 26 July 2013).
EUCTR2016‐001593‐15‐ES {published data only}
- EUCTR2016-001593-15-ES. Study to evaluate the efficacy and safety of high induction doses of adalimumab in moderate to severe psoriasis patients. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016-001593-15-ES (first received 23 August 2016).
EUCTR2016‐003592‐21‐GB {published data only}
- EUCTR2016-003592-21-GB. Evaluating the benefits of using secukinumab rather than standard treatments as the first systemic treatment in moderate to severe psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016-003592-21-GB (first received 17 October 2016).
EUCTR2018‐001021‐10‐SE {published data only}
- EUCTR2018-001021-10-SE. A clinical study with brodalumab for patients suffering from psoriasis and not benefitting the TNF-alpha treatment. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018-001021-10-SE (first received 17 May 2018).
EUCTR2019‐000817‐35‐DE {published data only}
- EUCTR2019-000817-35-DE. An open-label, randomized, phase IV study, to assess the efficacy and safety of tildrakizumab in patients with moderate to severe chronic plaque psoriasis who are non-responders to dimethyl fumarate therapy. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2019-000817-35-DE (first received 6 June 2019).
- NCT04263610. Efficacy and safety of tildrakizumab in participants with moderate-to-severe chronic plaque psoriasis who are non-responders to dimethyl fumarate therapy (TRANSITION). clinicaltrials.gov/show/NCT04263610 (first received 11 February 2020).
EUCTR2021‐000542‐18‐LV {published data only}
- EUCTR2021-000542-18-LV. A study to investigate the impact of switching between ABP 501 and adalimumab for the treatment of subjects with moderate to severe plaque psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2021-000542-18-LV (first received 22 October 2021).
EUCTR2022‐000695‐19‐LT {published data only}
- EUCTR2022-000695-19-LT. Clinical study assessing interchangeability between SB5 and Humira in patients with moderate to severe chronic plaque psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2022-000695-19-LT (first received 2 May 2022).
EXCEED 2021 {published data only}
- Gottlieb AB, Merola JF, Reich K, Behrens F, Nash P, Griffiths CEM, et al. Efficacy of secukinumab and adalimumab in psoriatic arthritis patients with concomitant moderate to severe plaque psoriasis: results from the EXCEED, a randomised, double-blind head-to-head monotherapy study. British Journal of Dermatology 2021 Apr 29 [Epub ahead of print]. [DOI: 10.1111/bjd.20413] [DOI] [PMC free article] [PubMed]
Ezquerra 2007 {published data only}
- Ezquerra GM, Regana MS, Millet PU. Combination of acitretin and oral calcitriol for treatment of plaque-type psoriasis. Acta Dermato-Venereologica 2007;87(5):449-50. [CENTRAL: CN-00619032] [DOI] [PubMed] [Google Scholar]
Feldman 2017 {published data only}
- Feldman SR, Green L, Kimball AB, Siu K, Zhao Y, Herrera V, et al. Secukinumab improves scalp pain, itching, scaling and quality of life in patients with moderate-to-severe scalp psoriasis. Journal of Dermatological Treatment 2017;28(8):716-21. [CENTRAL: CN-01454207] [DOI] [PubMed] [Google Scholar]
Fernandes 2013 {published data only}
- Fernandes IC, Torres T, Selores M. Maintenance treatment of psoriasis with cyclosporine A: comparison between continuous and weekend therapy. Journal of the American Academy of Dermatology 2013;68(2):341-2. [CENTRAL: CN-00841435] [DOI] [PubMed] [Google Scholar]
Fernandez 2017 {published data only}
- Fernandez Penas P, Goldblum O, Berggren L, Burkhardt N, Jullien D. Long-term clinical outcomes after 2 years of ixekizumab treatment in patients with moderate-to-severe psoriasis with a focus on absolute PASI. Journal of Investigative Dermatology 2017;137(5 Suppl 1):S58. [CENTRAL: CN-01375458] [Google Scholar]
Finzi 1993 {published data only}
- Italian Multicenter Study Group on Cyclosporin in Psoriasis. Cyclosporin versus etretinate: Italian multicenter comparative trial in severe plaque-form psoriasis. Dermatology (Basel, Switzerland) 1993;187(Suppl 1):8-18. [CENTRAL: CN-00095652] [DOI] [PubMed] [Google Scholar]
Fitz 2018 {published data only}
- Fitz L, Zhang W, Soderstrom C, Fraser S, Lee J, Quazi A, et al. Association between serum interleukin-17A and clinical response to tofacitinib and etanercept in moderate to severe psoriasis. Clinical and Experimental Dermatology 2018;43(7):790-7. [CENTRAL: CN-01923580] [DOI] [PubMed] [Google Scholar]
Fleischer 2005 {published data only}
- Fleischer AB, Carroll C, Hartle JE, Krejci-Manwaring J, McCarty MA, Feldman SR. A randomized, double-blind, right/left comparative study of the efficacy of acitretin with and without the co-administration of 0.1 percent tacrolimus ointment in the treatment of moderate to severe psoriasis. Journal of Investigative Dermatology 2005;124(4 Suppl):A46. [CENTRAL: CN-00550944] [Google Scholar]
Foley 2017 {published data only}
- Foley P, Song M, Shen YK, You Y, Wasfi Y, Griffiths CE. Guselkumab treatment provided higher frequency of complete skin clearance compared with adalimumab treatment among patients with moderate-to-severe plaque psoriasis. British Journal of Dermatology 2017;177(5):e273-4. [DOI: 10.1111/bjd.16059] [DOI] [Google Scholar]
Foley 2018 {published data only}
- Foley P, Gordon K, Griffiths CE, Wasfi Y, Randazzo B, Song M, et al. Efficacy of guselkumab compared with adalimumab and placebo for psoriasis in specific body regions: a secondary analysis of 2 randomized clinical trials. JAMA Dermatology 2018;154(6):676-83. [CENTRAL: CN-01611616] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fredriksson 1971 {published data only}
- Fredriksson T. Antipsoriatic activity of retinoic acid (vitamin A acid). Dermatologica 1971;142(3):133-6. [CENTRAL: CN-00006362] [DOI] [PubMed] [Google Scholar]
Fredriksson 1978 {published data only}
- Fredriksson T, Pettersson U. Severe psoriasis - oral therapy with a new retinoid. Dermatologica 1978;157(4):238-44. [CENTRAL: CN-00382865] [DOI] [PubMed] [Google Scholar]
Friedrich 2001 {published data only}
- Friedrich M, Sterry W, Klein A, Ruckert R, Docke WD, Asadullah K. Addition of pentoxifylline could reduce the side effects of fumaric acid esters in the treatment of psoriasis. Acta Dermato-Venereologica 2001;81(6):429-30. [CENTRAL: CN-00388555] [DOI] [PubMed] [Google Scholar]
GAIN 2021 {published data only}
- Reich K, Korber A, Mrowietz U, Sticherling M, Sieder C, Fruh J, et al. Secukinumab 2-weekly vs. 4-weekly dosing in patients with plaque-type psoriasis: results from the randomized GAIN study. British Journal of Dermatology 2021;184(5):849-56. [DOI] [PubMed] [Google Scholar]
Gambichler 2011 {published data only}
- Gambichler T, Tigges C, Scola N, Weber J, Skrygan M, Bechara FG, et al. Etanercept plus narrowband ultraviolet b phototherapy of psoriasis is more effective than etanercept monotherapy at 6 weeks. British Journal of Dermatology 2011;164(6):1383-6. [CENTRAL: CN-00812147] [DOI] [PubMed] [Google Scholar]
Ganguly 2004 {published data only}
- Ganguly R, Singh A, Sato R. Etanercept therapy provides clinically meaningful improvement in dermatology quality of life index in patients with chronic plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2004;18(6):807. [CENTRAL: CN-00550919] [Google Scholar]
Gil 2003 {published data only}
- Gil JM, Sanchez-Regana M, PaIazon DB, Cuchillero RO, Ezquerra GM, Millet PU. Association between calcitriol per os and acitretinoin in the treatment of psoriasis. Journal of the European Academy of Dermatology and Venereology 2003;17(Suppl 3):383. [CENTRAL: CN-00478546] [Google Scholar]
Gisondi 2020 {published data only}
- Gisondi P, Bellinato F, Bruni M, De Angelis G, Girolomoni G. Methotrexate vs secukinumab safety in psoriasis patients with metabolic syndrome. Dermatologic Therapy 2020;33(6):e14281. [DOI] [PubMed] [Google Scholar]
Glatt 2017 {published data only}
- Glatt S, Helmer E, Haier B, Strimenopoulou F, Price G, Vajjah P, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. British Journal of Clinical Pharmacology 2017;83(5):991-1001. [CENTRAL: CN-01447402] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuger J, Oliver R, Glatt S, Page M, Edwards H, Garcet S, Dizier B, Maroof A, Shaw S. Bimekizumab in moderate-to-severe plaque psoriasis: evaluation of clinical and molecular evidence to understand maintenance of response. British journal of dermatology 2022;187(1):e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goerz 1978 {published data only}
- Goerz G, Orfanos CE. Systemic treatment of psoriasis with a new aromatic retinoid. Preliminary evaluation of a multicenter controlled study in the Federal Republic of Germany. Dermatologica 1978;157(Suppl 1):38-44. [PMID: ] [PubMed] [Google Scholar]
Gold 2018 {published data only}
- Gold LS, Forman S, Lebwohl M, Jackson JM, Goncalves J, Levi E, et al. Impact on quality of life and satisfaction with apremilast in patients with moderate plaque psoriasis: 52-week results of the UNVEIL study. Journal of Clinical and Aesthetic Dermatology 2018;11(5 Suppl 1):S23-4. [CENTRAL: CN-01713690] [Google Scholar]
Goll 2017 {published data only}
- Goll GL, Jorgensen KK, Sexton J, Olsen IC, Bolstad N, Lorentzen M, et al. Long-term safety and efficacy of biosimilar infliximab (CT-P13) after switching from originator infliximab: results from the 26-week open label extension of a randomized Norwegian trial. Arthritis and Rheumatology 2017;69(Suppl 10):2800. [CENTRAL: CN-01423748] [Google Scholar]
Goll 2018 {published data only}
- Goll GL, Jorgensen KK, Sexton J, Olsen IC, Bolstad N, Lorentzen M, et al. Long-term safety and efficacy of biosimilar infliximab (CT-P13) after switching from originator infliximab: results from the 26-week open label extension of a Norwegian randomised trial. Annals of the Rheumatic Diseases 2018;77(Suppl 2):1383-4. [CENTRAL: CN-01647461] [DOI: 10.1136/annrheumdis-2018-eular.4620] [DOI] [Google Scholar]
Gollnick 1988 {published data only}
- Gollnick H, Bauer R, Brindley C, Orfanos CE, Plewig G, Wokalek H, et al. Acitretin versus etretinate in psoriasis. Clinical and pharmacokinetic results of a German multicenter study. Journal of the American Academy of Dermatology 1988;19(3):458-68. [CENTRAL: CN-00055942] [DOI] [PubMed] [Google Scholar]
Gollnick 1993 {published data only}
- Gollnick HP, Zaun H, Ruzicka T, Sommerburg C, Loew S, Mahrle G, et al. Relapse rate of severe generalized psoriasis after treatment with acitretin or etretinate. Results of the first randomized double-blind multicenter half-year follow-up study. European Journal of Dermatology 1993;3(6):442-6. [CENTRAL: CN-00181049] [Google Scholar]
Gollnick 2002 {published data only}
- Gollnick H, Altmeyer P, Kaufmann R, Ring J, Christophers E, Pavel S, et al. Topical calcipotriol plus oral fumaric acid is more effective and faster acting than oral fumaric acid monotherapy in the treatment of severe chronic plaque psoriasis vulgaris. Dermatology (Basel, Switzerland) 2002;205(1):46-53. [CENTRAL: CN-00397743] [DOI] [PubMed] [Google Scholar]
Gordon 2014 {published data only}
- Gordon K, Leonardi C, Braun D, Cameron G, Erickson J, Lebwohl M, et al. Results after at least 52 weeks of open label treatment with ixekizumab, an anti-IL-17A monoclonal antibody, in a phase 2 study in chronic plaque psoriasis. Journal of the American Academy of Dermatology 2014;70(5 Suppl 1):AB183. [CENTRAL: CN-01057458] [Google Scholar]
Gordon 2015 {published data only}
- Gordon KB, Leonardi C, Lebwohl M, Cameron G, Erickson J, Braun D, et al. Results after at least 52 weeks of open label treatment with ixekizumab, an anti-IL-17A monoclonal antibody, in a phase 2 study in chronic plaque psoriasis. Journal of Clinical and Aesthetic Dermatology 2015;8(5 Suppl 1):S17-8. [CENTRAL: CN-01378778] [Google Scholar]
Gordon 2018a {published data only}
- Gordon K, Armstrong A, Foley P, Wasfi Y, Song M, Shen YK, et al. Long-term efficacy of guselkumab treatment after drug withdrawal and retreatment in patients with moderate-severe plaque psoriasis: results from voyage 2. Acta Dermato-Venereologica 2018;98(Suppl 219):22-3. [CENTRAL: CN-01620161] [DOI: 10.2340/00015555-2978] [DOI] [Google Scholar]
Gordon 2018b {published data only}
- Gordon K, Reich K, Pariser D, Menter A, Tyring S, Sofen H, et al. Efficacy of tildrakizumab in moderate to severe psoriasis patients with prior exposure to apremilast. Acta Dermato-Venereologica 2018;98(Suppl 219):29-30. [CENTRAL: CN-01620187] [DOI: 10.2340/00015555-2978] [DOI] [Google Scholar]
Gordon 2018c {published data only}
- Gordon K, Crowley J, Poulin Y, Mendelsohn A, Parno J, Rozzo S, et al. Disease severity and efficacy insights: patient-level pasi scores in tildrakizumab psoriasis trials. Acta Dermato-Venereologica 2018;98(Suppl 219):30-1. [DOI: 10.2340/00015555-2978] [DOI] [Google Scholar]
Gordon 2018d {published data only}
- Gordon KB, Armstrong AW, Han C, Foley P, Song M, Wasfi Y, et al. Anxiety and depression in patients with moderate-to-severe psoriasis and comparison of change from baseline after treatment with guselkumab vs. adalimumab: results from the phase 3 VOYAGE 2 study. Journal of the European Academy of Dermatology and Venereology 2018;32(11):1940-9. [CENTRAL: CN-01617890] [DOI] [PubMed] [Google Scholar]
Gottlieb 2002 {published data only}
- Gottlieb AB, Vaishnaw K, Rizova E. Alefacept (AMEVIVETM) does not blunt primary or secondary immune responses. Journal of Investigative Dermatology 2002;118(6):1098. [CENTRAL: CN-00790440] [Google Scholar]
Gottlieb 2003b {published data only}
- Gottlieb AB, Casale TB, Frankel E, Goffe B, Lowe N, Ochs HD, et al. CD4+ T-cell-directed antibody responses are maintained in patients with psoriasis receiving alefacept: results of a randomized study. Journal of the American Academy of Dermatology 2003;49(5):816-25. [CENTRAL: CN-00474727] [DOI] [PubMed] [Google Scholar]
Gottlieb 2003c {published data only}
- Gottlieb AB, Feng A, Zitnik R. Prolonged response durability following ENBRELA® (etanercept) monotherapy. Journal of Investigative Dermatology 2003;121(1):68. [CENTRAL: CN-00795277] [Google Scholar]
Gottlieb 2004b {published data only}
- Gottlieb B, Goffe B, Veith J, Stevens S, Nakanishi A. Safety of etanercept in an integrated multistudy database of patients with psoriasis. Journal of Investigative Dermatology 2004;122(3):A55. [CENTRAL: CN-00509648] [Google Scholar]
Gottlieb 2005 {published data only}
- Gottlieb AB, Griffiths CE, Ho VC, Lahfa M, Mrowietz U, Murrell DF, et al. Oral pimecrolimus in the treatment of moderate to severe chronic plaque-type psoriasis: a double-blind, multicentre, randomized, dose-finding trial. British Journal of Dermatology 2005;152(6):1219-27. [CENTRAL: CN-00522446] [DOI] [PubMed] [Google Scholar]
Gottlieb 2006a {published data only}
- Gottlieb A, Zhang Y. A phase II trial of a new anti-inflammatory combination drug, CRx-140, in subjects with severe psoriasis. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB8. [CENTRAL: CN-00602228] [Google Scholar]
Gottlieb 2006b {published data only}
- Gottlieb A, Cather J, Hamilton T, Sherman M. Preliminary clinical safety and efficacy results from an open-label phase 2 study of STA-5326, an oral IL-12/IL-23 inhibitor, in patients with moderate to severe chronic plaque psoriasis. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB10. [CENTRAL: CN-00602166] [Google Scholar]
Gottlieb 2010 {published data only}
- Gottlieb A, Menter A, Mendelsohn A, Shen YK, Li S, Guzzo C, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet 2009;373(9664):633-40. [CENTRAL: CN-00686924] [DOI] [PubMed] [Google Scholar]
Gottlieb 2016 {published data only}
- Gottlieb A, Gerdes S, Lacour J, Korman N, Papp K, Dutronic Y, et al. Efficacy of ixekizumab in moderate-to-severe psoriasis patients who have or have not received prior biologic therapies: an integrated analysis of 3 phase 3 studies. Journal of Investigative Dermatology 2016;136(9 Suppl 2):S169. [CENTRAL: CN-01747590] [Google Scholar]
Gottlieb 2017a {published data only}
- Gottlieb A, Sullivan J, Kubanov A, You R, Regnault P, Frueh J. Secukinumab shows high and sustained efficacy in patients with moderate-to-severe palmoplantar psoriasis: 2.5-year results from the GESTURE study. British Journal of Dermatology 2017;177(5):e261. [CENTRAL: CN-01452515] [DOI] [PubMed] [Google Scholar]
Gottlieb 2017b {published data only}
- Gottlieb A, Sullivan J, Van Doorn M, Kubanov A, You R, Parneix A, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. Journal of the American Academy of Dermatology 2017;76(1):70-80. [CENTRAL: CN-01368596] [DOI] [PubMed] [Google Scholar]
Gottlieb 2017c {published data only}
- Gottlieb AB, Merola JF, Chen R, Levi E, Duffin KC. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and Body Surface Area (PGA x BSA) composite tool: an analysis of apremilast phase 3 ESTEEM data. Journal of the American Academy of Dermatology 2017;77(6):1178-80. [CENTRAL: CN-01443069] [DOI] [PubMed] [Google Scholar]
Gottlieb 2017d {published data only}
- Gottlieb AB, Lacour JP, Korman N, Wilhelm S, Dutronc Y, Schacht A, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have or have not received prior biological therapies: an integrated analysis of two Phase III randomized studies. Journal of the European Academy of Dermatology and Venereology 2017;31(4):679-85. [CENTRAL: CN-01244380] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gottlieb 2018a {published data only}
- Gottlieb AB, Gordon K, Hsu S, Elewski B, Eichenfield LF, Kircik L, et al. Improvement in itch and other psoriasis symptoms with brodalumab in phase 3 randomized controlled trials. Journal of the European Academy of Dermatology and Venereology 2018;32(8):1305-13. [CENTRAL: CN-01572384] [DOI] [PubMed] [Google Scholar]
Gottlieb 2018b {published data only}
- Gottlieb AB, Blauvelt A, Thaçi D, Leonardi C, Poulin Y, Peterson L, et al. Durable reduction in absolute PASI with certolizumab pegol in patients with chronic plaque psoriasis. Acta Dermato-Venereologica 2018;98(Suppl 219):24. [CENTRAL: CN-01620157] [Google Scholar]
Goupille 1995 {published data only}
- Goupille P, Valat JP. Is methotrexate really effective in patients with psoriatic arthritis? Journal of Rheumatology 1995;22(12):2369-70. [PMID: ] [PubMed] [Google Scholar]
Goupille 2018 {published data only}
- Goupille P, Roussou E, Burmester G, Mease PJ, Gottlieb AB, Garces S, et al. Safety of ixekizumab in patients with psoriatic arthritis: results from a pooled analysis of three clinical trials. Annals of the Rheumatic Diseases 2018;77(Suppl 2):1039-40. [DOI: 10.1136/annrheumdis-2018-eular.2132] [DOI] [Google Scholar]
Griffiths 1998 {published data only}
- Griffiths CM, Boffa M, Wishart J, Adam C, Inglesias L, Van de Kerkhof P, et al. A double-blind, randomised trial to compare the effects of oral liarozole with acitretin in the treatment of chronic plaque psoriasis. British Journal of Dermatology 1998;139(Suppl 51):19. [CENTRAL: CN-00415772] [Google Scholar]
Griffiths 2002a {published data only}
- Griffiths CE, Humbert P, Koo J, Ortonne JP, Christophers E. Relationship between clinical response and quality of life in psoriasis patients treated with alefacept. Journal of the European Academy of Dermatology and Venereology 2002;16(Suppl S1):292. [CENTRAL: CN-00478562] [Google Scholar]
Griffiths 2002b {published data only}
- Griffiths CE, Ortonne JP, Christophers E. Effect of alefacept based on patients' response to prior therapy for psoriasis. British Journal of Dermatology 2002;147(Suppl 62):45. [CENTRAL: CN-00406976] [Google Scholar]
Griffiths 2005 {published data only}
- Griffiths CE. A higher treatment standard for patients with moderate to severe psoriasis. Journal of the European Academy of Dermatology and Venereology 2005;19(Suppl 2):7. [CENTRAL: CN-00602410] [Google Scholar]
Griffiths 2010 {published data only}
- Griffiths CE, Menter A, Strober BE, Yeilding N. Ustekinumab treatment in patients with moderate to severe psoriasis who are nonresponders to etanercept: results from a phase III clinical trial. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB137. [CENTRAL: CN-00843739] [DOI: 10.1016/j.jaad.2009.11.527] [DOI] [Google Scholar]
Griffiths 2016 {published data only}
- Griffiths CE, Warren R, Ilo D, Kerr L, Kent T, Mallbris L. Efficacy and safety of ixekizumab in patients with psoriasis who failed initial etanercept treatment: a subanalysis from UNCOVER 2, a randomized, double-blind, multicentre, phase III clinical trial. British Journal of Dermatology 2016;175(Suppl S1):68-9. [CENTRAL: CN-01303311] [DOI: 10.1111/bjd.14524] [DOI] [Google Scholar]
Griffiths 2017 {published data only}
- Griffiths CE, Vender R, Sofen H, Kircik L, Tan H, Rottinghaus ST, et al. Effect of tofacitinib withdrawal and re-treatment on patient-reported outcomes: results from a phase 3 study in patients with moderate to severe chronic plaque psoriasis. Journal of the European Academy of Dermatology & Venereology 2017;31(2):323-32. [CENTRAL: CN-01332752] [DOI: 10.1111/jdv.13808] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffiths 2018a {published data only}
- Griffiths CE, Papp KA, Kimball AB, Randazzo B, Song M, Li S, et al. Long-term efficacy of guselkumab for the treatment of moderate-to-severe psoriasis: results from the phase 3 VOYAGE 1 trial through two years. Journal of Drugs in Dermatology 2018;17(8):826-32. [CENTRAL: CN-01655581] [PubMed] [Google Scholar]
Griffiths 2018b {published data only}
- Griffith C, Radtke MA, Youn SW, Bissonnette R, Song M, Wasfi Y, et al. Clinical response after guselkumab treatment among adalimumab PASI 90 non-responders: results from the Voyage 1 and 2 trials. Acta Dermato-Venereologica 2018;98(Suppl 219):20. [CENTRAL: CN-01620165] [Google Scholar]
Griffiths 2018c {published data only}
- Griffiths C, Blauvelt A, Reich K, Leonardi C, Mehta N, Tsai T, et al. Secukinumab's long-term safety remains favorable up to 5 years of treatment. Acta Dermato-Venereologica 2018;98(Suppl 219):46. [CENTRAL: CN-01920781] [Google Scholar]
Grim 2000 {published data only}
- Grim J, Chladek J, Martinkova J, Simkova M, Vaneckova J, Koudelkova V. Pharmacokinetics (PK) and pharmacodynamics (PD) of low dose methotrexate (LDMTX) in the treatment of psoriasis. British Journal of Clinical Pharmacology 2000;50(4):390-1. [EMBASE: 2000362454] [Google Scholar]
Grossman 1994 {published data only}
- Grossman RM, Thivolet J, Claudy A, Souteyrand P, Guilhou JJ, Thomas P, et al. A novel therapeutic approach to psoriasis with combination calcipotriol ointment and very low-dose cyclosporine: results of a multicenter placebo-controlled study. Journal of the American Academy of Dermatology 1994;31(1):68-74. [CENTRAL: CN-00102638] [DOI] [PubMed] [Google Scholar]
Guenther 2020 {published data only}
- Guenther L, Potts Bleakman A, Weisman J, Poulin Y, Spelman L, Burge R, et al. Ixekizumab results in persistent clinical improvement in moderate-to-severe genital psoriasis during a 52 week randomized, placebo-controlled, phase 3 clinical trial. Acta Dermato-Venereologica 2020;100(1):adv00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gulliver 1996 {published data only}
- Gulliver WP, Murphy GF, Hannaford VA, Primmett DR. Increased bioavailability and improved efficacy, in severe psoriasis, of a new microemulsion formulation of cyclosporin. British Journal of Dermatology 1996;135(s48):35-9. [EMBASE: 1996272533] [DOI] [PubMed] [Google Scholar]
Gupta 2005 {published data only}
- Gupta SK, Dogra A, Kaur G. Comparative efficacy of methotrexate and hydroxyurea in treatment of psoriasis. Journal of Pakistan Association of Dermatologists 2005;15(3):247-51. [CENTRAL: 2005580293] [Google Scholar]
Gupta 2007 {published data only}
- Gupta R, Gupta S. Methotrexate-betamethasone weekly oral pulse in psoriasis. Journal of Dermatological Treatment 2007;18(5):291-4. [CENTRAL: CN-00619338] [DOI] [PubMed] [Google Scholar]
Gupta 2008 {published data only}
- Gupta AK, Langley RG, Lynde C, Barber K, Gulliver W, Lauzon G, et al. ISA247: quality of life results from a phase II, randomized, placebo-controlled study. Journal of Cutaneous Medicine and Surgery 2008;12(6):268-75. [CENTRAL: CN-00683900] [DOI] [PubMed] [Google Scholar]
Han 2013 {published data only}
- Han C, Kavanaugh A, Genovese MC, Hsu B, Deodhar AA, Hsia EC. Sustained improvement in health-related quality of life, work productivity, employability, and reduced healthcare resource utilization of patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis treated with golimumab: 5-year results from 3 phase III studies. Arthritis and Rheumatism 2013;65(S10):S137. [CENTRAL: CN-01062782] [Google Scholar]
Hashizume 2007 {published data only}
- Hashizume H, Ito T, Yagi H, Takigawa M, Kageyama H, Furukawa F, et al. Efficacy and safety of preprandial versus postprandial administration of low-dose cyclosporin microemulsion (Neoral) in patients with psoriasis vulgaris. Journal of Dermatology 2007;34(7):430-4. [CENTRAL: CN-00610280] [DOI] [PubMed] [Google Scholar]
Hawkes 2018 {published data only}
- Hawkes JE, Lebwohl M, Elewski B, Kircik L, Reich K, Muscianisi E, et al. Secukinumab for the treatment of scalp, nail, and palmoplantar psoriasis. Journal of Clinical and Aesthetic Dermatology 2018;11(5 Suppl 1):S28. [Google Scholar]
Heule 1988 {published data only}
- Heule F, Meinardi MM, Van Joost T, Bos JD. Low-dose cyclosporine effective in severe psoriasis: a double-blind study. Transplantation Proceedings 1988;20(3 Suppl 4):32-41. [CENTRAL: CN-00054273] [PubMed] [Google Scholar]
Ho 2010 {published data only}
- Ho SG, Yeung CK, Chan HH. Methotrexate versus traditional Chinese medicine in psoriasis: a randomized, placebo-controlled trial to determine efficacy, safety and quality of life. Clinical and Experimental Dermatology 2010;35(7):717-22. [CENTRAL: CN-00761451] [DOI] [PubMed] [Google Scholar]
Holzer 2020 {published data only}
- Holzer G, Hoke M, Sabeti-Sandor S, Perkmann T, Rauscher A, Strassegger B, et al. Disparate effects of adalimumab and fumaric acid esters on cardiovascular risk factors in psoriasis patients: results from a prospective, randomized, observer-blinded head-to-head trial. Journal of the European Academy of Dermatology and Venereology 2020;35(2):441-9. [DOI] [PubMed] [Google Scholar]
Hsu 2018 {published data only}
- Hsu S, Green L, Keegan BR, Kircik L, Rastogi S, Pillai R, et al. Efficacy of brodalumab in ustekinumab-naive and-experienced patients with moderate-to-severe plaque psoriasis. Journal of Clinical and Aesthetic Dermatology 2018;11(5 Suppl 1):S27. [Google Scholar]
Hunter 1972 {published data only}
- Hunter GA, Simmons IJ, Thomas BM. A clinical trial of hydroxyurea for psoriasis. Australasian Journal of Dermatology 1972;13(3):93-9. [CENTRAL: CN-00008809] [DOI] [PubMed] [Google Scholar]
Iest 1989 {published data only}
- Iest J, Boer J. Combined treatment of psoriasis with acitretin and UVB phototherapy compared with acitretin alone and UVB alone. British Journal of Dermatology 1989;120(5):665-70. [CENTRAL: CN-00568569] [DOI] [PubMed] [Google Scholar]
Imafuku 2017 {published data only}
- Imafuku S, Torisu-Itakura H, Nishikawa A, Zhao F, Cameron GS, Japanese Uncover-Study Group. Efficacy and safety of ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis: subgroup analysis of a placebo-controlled, phase 3 study (UNCOVER-1). Journal of Dermatology 2017;44(11):1285-90. [CENTRAL: CN-01615794] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN18043449 {published data only}
- ISRCTN18043449. A study of pre-clinical joint disease in psoriasis and the imaging response to ustekinumab. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN18043449 (first received 19 September 2012).
Iversen 2018 {published data only}
- Iversen L, Eidsmo L, Austad J, De Rie M, Osmancevic A, Skov L, et al. Secukinumab treatment in new-onset psoriasis: aiming to understand the potential for disease modification - rationale and design of the randomized, multicenter STEPIn study. Journal of the European Academy of Dermatology and Venereology 2018;32(11):1930-9. [CENTRAL: CN-01630270] [DOI] [PubMed] [Google Scholar]
Jackson 2018 {published data only}
- Jackson JM, Alikhan A, Lebwohl M, Stein Gold L, Levi E, Bagel J. Improvement in scalp and nails with apremilast in patients with moderate plaque psoriasis naive to systemic and biologic therapy: 52-week results of the UNVEIL study. Journal of Clinical and Aesthetic Dermatology 2018;11(5 Suppl 1):S20-1. [Google Scholar]
Jacobe 2008 {published data only}
- Jacobe H, Winterfield L, Kim F, Huet-Adams B, Cayce R. The role of narrowband UV-B plus alefacept combination therapy in the treatment of psoriasis. Archives of Dermatology 2008;144(8):1067-8; author reply 1068-9. [CENTRAL: CN-00650560] [PMID: ] [DOI] [PubMed] [Google Scholar]
JapicCTI‐194706 2019 {published data only}
- JPRN-JapicCTI-194706. A multicenter, randomized, open-label study to evaluate the safe and effective use of the prefilled safety syringe or the auto-injector for the subcutaneous self-injection of bimekizumab solution by subjects with moderate to severe chronic plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-JapicCTI-194706 (first received 11 April 2019).
Jin 2017 {published data only}
- Huang YW, Tsai TF. Efficacy of tofacitinib in patients with moderate-to-severe psoriasis who had inadequate responses to prior biologics. Dermatologica Sinica 2019;37(4):205-8. [Google Scholar]
- Jin T, Sun Z, Chen X, Wang Y, Li R, Ji S, et al. Serum human beta-defensin-2 is a possible biomarker for monitoring response to JAK inhibitor in psoriasis patients. Dermatology 2017;233(2-3):164-9. [CENTRAL: CN-01622175] [DOI] [PubMed] [Google Scholar]
Joergensen 2022 {published data only}
- Joergensen K K, Syversen S W, Goll G L, Bjrlykke K H, Sandanger O, Sexton J, Brun M K, Mrk C, Kvien T K, Gehin J E, Olsen I C, Bolstad N, Haavardsholm E A, Jahnsen J. Proactive therapeutic drug monitoring is superior to standard treatment during maintenance therapy with infliximab; results from a 52-week multicentre randomised trial of 450 patients. Gastroenterology 2022;162(7 Suppl):S-44. [Google Scholar]
JPRN‐jRCT2071210135 {published data only}
- JPRN-jRCT2071210135. Bioequivalence of DMB-3115, a usutekinumab biosimilar, and usutekinumab. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-jRCT2071210135 (first received 22 March 2022).
JPRN‐jRCTs041180012 2018 {published data only}
- JPRN-jRCTs041180012. Comparison of phototherapy alone or together with apremilast in psoriasis vulgaris patients. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-jRCTs041180012 (first received 9 November 2018).
Kaur 2018 {published data only}
- Kaur S, Shafiq N, Dogra S, Mittal B, Bahl A, Narang T, et al. A placebo-controlled, randomized trial to assess, using pet scan, systemic and vascular inflammation, and response to therapy, in patients with psoriasis. Journal of the Dermatology Nurses' Association (Conference: 24th World Congress of Dermatology, Italy) 2020;12(2):No-pagination. [Google Scholar]
- Kaur S, Shafiq N, Dogra S, Mittal BR, Attri SV, Bahl A, et al. 18F-fluorodeoxyglucose positron emission tomography-based evaluation of systemic and vascular inflammation and assessment of the effect of systemic treatment on inflammation in patients with moderate-to-severe psoriasis: a randomized placebo-controlled pilot study. Indian Journal of Dermatology, Venereology and Leprology 2018;84(6):660-6. [CENTRAL: CN-01670374] [DOI] [PubMed] [Google Scholar]
Kavanaugh 2009 {published data only}
- Kavanaugh A. The efficacy of ustekinumab on the articular and dermatologic manifestations of psoriatic arthritis. Current Rheumatology Reports 2009;11(4):233-4. [CENTRAL: CN-00958869] [DOI] [PubMed] [Google Scholar]
Kemeny 2019 {published data only}
- Kemeny L, Berggren L, Dossenbach M, Dutronc Y, Paul C. Efficacy and safety of ixekizumab in patients with plaque psoriasis across different degrees of disease severity: results from UNCOVER-2 and UNCOVER-3. Journal of Dermatological Treatment 2019;30(1):19-26. [CENTRAL: CN-01913619] [DOI] [PubMed] [Google Scholar]
Kimball 2008 {published data only}
- Kimball AB, Gordon KB, Langley RG, Menter A, Chartash EK, Valdes J, et al. Safety and efficacy of ABT-874, a fully human interleukin 12/23 monoclonal antibody, in the treatment of moderate to severe chronic plaque psoriasis: results of a randomized, placebo-controlled, phase 2 trial. Archives of Dermatology 2008;144(2):200-7. [CENTRAL: CN-00630180] [DOI] [PubMed] [Google Scholar]
Kimball 2011 {published data only}
- Kimball AB, Gordon KB, Langley RG, Menter A, Perdok RJ, Valdes J. Efficacy and safety of ABT-874, a monoclonal anti-interleukin 12/23 antibody, for the treatment of chronic plaque psoriasis: 36-week observation/retreatment and 60-week open-label extension phases of a randomized phase II trial. Journal of the American Academy of Dermatology 2011;64(2):263-74. [CENTRAL: CN-00770794] [DOI] [PubMed] [Google Scholar]
Kimball 2018 {published data only}
- Kimball AB, Luger T, Gottlieb A, Puig L, Kaufmann R, Burge R, et al. Long-term impact of ixekizumab on psoriasis itch severity: results from a phase III clinical trial and long-term extension. Acta Dermato-Venereologica 2018;98(1):98-102. [CENTRAL: CN-01446779] [DOI] [PubMed] [Google Scholar]
Kohm 2022 {published data only}
- Kohm M, Rossmanith T, Foldenauer AC, Kellner H, Kiltz U, Rech J, et al. Efficacy of UST in active PSA is independent from concomitant MTX use, even in patients with more severe skin psoriasis: subgroup analysis from a randomized placebo-controlled investigator initiated clinical trial. Annals of the Rheumatic Diseases 2022;81(Suppl 1):850-1. [Google Scholar]
Koo 1998 {published data only}
- Koo J, OLP302 Study Group. A randomized, double-blind study comparing the efficacy, safety and optimal dose of two formulations of cyclosporin, Neoral and Sandimmun, in patients with severe psoriasis. British Journal of Dermatology 1998;139(1):88-95. [CENTRAL: CN-00155452] [DOI] [PubMed] [Google Scholar]
Kopp 2015 {published data only}
Korotaeva 2021 {published data only}
- Korotaeva TV, Mazurov VI, Lila AM, Gaydukova IZ, Bakulev AL, Samtsov AV, et al. Efficacy of netakimab in key psoriatic arthritis domains: 54-week results from the phase III BCD-085-8/patera study. Nauchno-Prakticheskaya Revmatologiya 2021;59(1):47-55. [Google Scholar]
Kragballe 1989 {published data only}
- Kragballe K, Jansén CT, Geiger JM, Bjerke JR, Falk ES, Gip L, et al. A double-blind comparison of acitretin and etretinate in the treatment of severe psoriasis. Results of a Nordic multicentre study. Acta Dermato-Venereologica 1989;69(1):35-40. [CENTRAL: CN-00057941] [PubMed] [Google Scholar]
Krishnan 2005 {published data only}
- Krishnan KR, Cella D, Woolley M, Lalla D, Zitnik R, Brajac D. Etanercept improves symptoms of depression and fatigue in patients with psoriasis. In: 158th Annual Meeting of the American Psychiatric Association; 2005 May 21-26; Atlanta, GA. Vol. 158. 2005:NR293. [CENTRAL: CN-00595903]
Krishnan 2018 {published data only}
- Krishnan E, Zhang N, Wang H. Injection site reactions and injection site pain for the adalimumab biosimilar ABP 501: results from two double-blind randomized controlled studies. United European Gastroenterology Journal 2018;6(8 Suppl):A451. [CENTRAL: CN-01787637] [Google Scholar]
Kristensen 2017 {published data only}
- Kristensen LE, Merola JF, Dutz J, Adams DH, Kerr L, Rich P. Ixekizumab improves nail and skin lesions in patients with active psoriatic arthritis and prior TNF inadequate response. Annals of the Rheumatic Diseases 2017;76(Suppl 2):937. [CENTRAL: CN-01467783] [Google Scholar]
Krueger 1980 {published data only}
- Krueger GG, Shelby NJ, Hansen CD, Taylor MB. Comparison of labelling indices of skin involved and uninvolved with psoriasis: placebo and oral retinoid RO 10-9359 vs. time. Clinical Research 1980;28(1):21A. [CENTRAL: CN-00192437] [Google Scholar]
Krueger 2002a {published data only}
- Feldman SR, Menter A, Koo JY. Improved health-related quality of life following a randomized controlled trial of alefacept treatment in patients with chronic plaque psoriasis. British Journal of Dermatology 2004;150(2):317-26. [CENTRAL: CN-00471158] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Krueger GG, Papp KA, Stough DB, Loven KH, Gulliver WP, Ellis CN, et al. A randomized, double-blind, placebo-controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasis. Journal of the American Academy of Dermatology 2002;47(6):821-33. [CENTRAL: CN-00411712] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Krueger GG. Clinical response to alefacept: results of a phase 3 study of intravenous administration of alefacept in patients with chronic plague psoriasis. Journal of the European Academy of Dermatology and Venereology 2003;17(Suppl 2):17-24. [CENTRAL: CN-00456895] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Menter A, Cather JC, Baker D, Farber HF, Lebwohl M, Darif M. The efficacy of multiple courses of alefacept in patients with moderate to severe chronic plaque psoriasis. Journal of the American Academy of Dermatology 2006;54(1):61-3. [CENTRAL: CN-00622887] [EMBASE: 2005584777] [DOI] [PubMed] [Google Scholar]
Krueger 2002b {published data only}
- Krueger G, Vaishnaw A, Rizova E. Pharmacodynamic effects of IM or IV alefacept: selective reductions in memory- effector (CD45RO+) cells are related to clinical improvement in psoriasis. Journal of Investigative Dermatology 2002;118(6):1098. [CENTRAL: CN-00795004] [Google Scholar]
Krueger 2003 {published data only}
- Krueger GG, Gordon KB, Van de Kerkhof P, Sterry W. Repeated courses of IM alefacept in psoriasis: rationale and design of an international study that mimics the clinical practice setting. Journal of Investigative Dermatology 2003;121(2):57. [CENTRAL: CN-00550788] [Google Scholar]
Krueger 2012 {published data only}
- Krueger JG, Fretzin S, Suarez-Farinas M, Haslett PA, Phipps KM, Cameron GS, et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. Journal of Allergy and Clinical Immunology 2012;130(1):145-54.e9. [CENTRAL: CN-00832721] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krueger 2015 {published data only}
- Krueger JG, Ferris LK, Menter A, Wagner F, White A, Visvanathan S, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. Journal of Allergy and Clinical Immunology 2015;136(1):116-24.e7. [CENTRAL: CN-01110170] [PMID: ] [DOI] [PubMed] [Google Scholar]
Krueger 2016a {published data only}
- Krueger J, Clark JD, Suarez-Farinas M, Fuentes-Duculan J, Cueto I, Wang CQ, et al. Tofacitinib attenuates pathologic immune pathways in patients with psoriasis: a randomized phase 2 study. Journal of Allergy and Clinical Immunology 2016;137(4):1079-90. [CENTRAL: CN-01153710] [PMID: ] [DOI] [PubMed] [Google Scholar]
Krueger 2016b {published data only}
- Krueger JG, Wharton K, Schlitt T, Torene R, Jiang X, Wang CQ, et al. Secukinumab, a new anti-IL17A biologic therapy, induces rapid and durable clinical, histological, and molecular resolution of psoriasis plaques over 1 year of administration. Experimental Dermatology 2016;25(Suppl 4):26. [Google Scholar]
Krueger 2022 {published data only}
- Krueger J, Langley R, Nigen S. 35098 Secukinumab versus guselkumab in the treatment of ustekinumab-resistant psoriatic plaques: 16-week randomized, open-label, multicenter ARROW study. Journal of the American Academy of Dermatology 2022;87(3 Suppl):AB108. [Google Scholar]
Krupashankar 2014 {published data only}
- Dogra S, Krupashankar DS, Budamakuntla L, Srinivas CR, Khopkar U, Gupta S, et al. Long-term efficacy and safety of itolizumab in patients with moderate-to-severe chronic plaque psoriasis: a double-blind, randomized-withdrawal, placebo-controlled study. Journal of the American Academy of Dermatology 2015;73(2):331-3.e1. [CENTRAL: CN-01108850] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Krupashankar DS, Dogra S, Kura M, Saraswat A, Budamakuntla L, Sumathy TK, et al. Efficacy and safety of itolizumab, a novel anti-CD6 monoclonal antibody, in patients with moderate to severe chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, phase-III study. Journal of the American Academy of Dermatology 2014;71(3):484-92. [CENTRAL: CN-01002561] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kuijpers 1998 {published data only}
- Kuijpers AL, Van Pelt JP, Bergers M, Boegheim PJ, Den Bakker JE, Siegenthaler G, et al. The effects of oral liarozole on epidermal proliferation and differentiation in severe plaque psoriasis are comparable with those of acitretin. British Journal of Dermatology 1998;139(3):380-9. [CENTRAL: CN-00159672] [DOI] [PubMed] [Google Scholar]
Lajevardi 2015 {published data only}
- Lajevardi V, Hallaji Z, Daklan S, Abedini R, Goodarzi A, Abdolreza M. The efficacy of methotrexate plus pioglitazone vs. methotrexate alone in the management of patients with plaque-type psoriasis: a single-blinded randomized controlled trial. International Journal of Dermatology 2015;54(1):95-101. [CENTRAL: CN-01052011] [DOI] [PubMed] [Google Scholar]
Lambert 2018 {published data only}
- Lambert J, Ghislain P-D, Merola J, Potts-Bleakman A, Brnabic AJ, Burge R, et al. Clinical signs of epithelial surface disruption impact pain and sexual health in patients with moderate-to-severe genital psoriasis. British Journal of Dermatology 2018;179(Suppl 1):36. [CENTRAL: CN-01620064] [Google Scholar]
Langewouters 2005 {published data only}
- Langewouters AM, Bovenschen HJ, De Jong EM, Van Erp PJM, Van de Kerkhof PC. The effect of topical corticosteroids in combination with alefacept on circulating T-cell subsets in psoriasis. Journal of the European Academy of Dermatology and Venereology 2005;19(Suppl 2):240. [CENTRAL: CN-00602421] [DOI] [PubMed] [Google Scholar]
Langley 2006 {published data only}
- Langley R, Leondardi C, Okun M. Long-term safety and efficacy of adalimumab in psoriasis. In: 4th European Association of Dermatology and Venereology (EADV) Spring Symposium Saariselka, Lapland, Finland. February 9-12th, 2006. Vol. Suppl. 2006:P-021. [CENTRAL: CN-00602234]
Langley 2010 {published data only}
- Langley RG, Papp K, Bissonnette R, Toth D, Matheson R, Hultquist M, et al. Safety profile of intravenous and subcutaneous siplizumab, an anti-CD2 monoclonal antibody, for the treatment of plaque psoriasis: results of two randomized, double-blind, placebo-controlled studies. International Journal of Dermatology 2010;49(7):818-28. [CENTRAL: CN-00761682] [DOI] [PubMed] [Google Scholar]
Langley 2016 {published data only}
- Langley R, Feldman S, Paul C, Gordon K, Strand V, Toth D, et al. Treatment with ixekizumab over 60 weeks provides sustained improvements in healthrelated quality of life: results from UNCOVER-1, a randomized phase 3 trial. Journal of Investigative Dermatology 2016;136(9 Suppl 2):S169. [CENTRAL: CN-01383388] [Google Scholar]
Langley 2018 {published data only}
- Langley RG, Tsai TF, Flavin S, Song M, Randazzo B, Wasfi Y, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. British Journal of Dermatology 2018;178(1):114-23. [CENTRAL: CN-01421735] [DOI: 10.1111/bjd.15750] [DOI] [PubMed] [Google Scholar]
Langner 2004 {published data only}
- Langner A, Roszkiewicz J, Baran E, Placek W. Results of a phase II study of a novel oral fumarate, BG-12, in the treatment of severe psoriasis. Journal of the European Academy of Dermatology and Venereology 2004;18(6):798. [CENTRAL: CN-00550917] [Google Scholar]
Lauharanta 1989 {published data only}
- Lauharanta J, Geiger JM. A double-blind comparison of acitretin and etretinate in combination with bath PUVA in the treatment of extensive psoriasis. British Journal of Dermatology 1989;121(1):107-12. [CENTRAL: CN-00061540] [DOI] [PubMed] [Google Scholar]
Lawrence 1983 {published data only}
- Lawrence CM, Marks J, Shuster S. Addition of retinoids to PUVA for psoriasis. Lancet 1983;1(8326 Pt 1):706. [CENTRAL: CN-00030597] [DOI] [PubMed] [Google Scholar]
Leavell 1970 {published data only}
- Leavell UW, Yarbro JW. Hydroxyurea. A new treatment for psoriasis. Archives of Dermatology 1970;102(2):144-50. [CENTRAL: CN-00004668] [DOI] [PubMed] [Google Scholar]
Lebwohl 2003 {published data only}
- Finlay AY, Salek MS, Haney J, Alefacept Clinical Study Group. Intramuscular alefacept improves health-related quality of life in patients with chronic plaque psoriasis. Dermatology (Basel, Switzerland) 2003;206(4):307-15. [CENTRAL: CN-00437818] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lebwohl M, Christophers E, Langley R, Ortonne JP, Roberts J, Griffiths CE, et al. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Archives of Dermatology 2003;139(6):719-27. [CENTRAL: CN-00438439] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ortonne JP, Lebwohl M, Griffiths CE. Alefacept-induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasis. European Journal of Dermatology 2003;13(2):117-23. [CENTRAL: CN-00436640] [PMID: ] [PubMed] [Google Scholar]
- Ortonne JP. Clinical response to alefacept: results of a phase 3 study of intramuscular administration of alefacept in patient with chronic plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2003;17(Suppl 2):12-6. [CENTRAL: CN-00456894] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lebwohl 2003a {published data only}
- Lebwohl M. The effect of psoriasis and its treatments on circulating T-cell subsets: results of alefacept studies. Journal of the European Academy of Dermatology and Venereology 2003;17(Suppl 3):377. [CENTRAL: CN-00478640] [Google Scholar]
Lebwohl 2009 {published data only}
- Lebwohl M, Kimball A, Gordon K, Szapary P. Comparable efficacy and safety of ustekinumab in moderate to severe psoriasis patients previously treated with systemic therapies and treatment-naive patients. Journal of the American Academy of Dermatology 2009;60(3 Suppl 1):AB167. [Google Scholar]
Lebwohl 2012 {published data only}
- Lebwohl MG, Kircik L, Callis Duffin K, Pariser D, Hooper M, Wenkert D, et al. Safety and efficacy of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. Dermatology and Therapy 2012;2:S39. [CENTRAL: CN-01027857] [DOI] [PubMed] [Google Scholar]
Lebwohl 2013 {published data only}
- Lebwohl MG, Kircik L, Callis Duffin K, Pariser D, Hooper M, Wenkert D, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. Journal of the American Academy of Dermatology 2013;69(3):385-92. [CENTRAL: CN-00964028] [DOI] [PubMed] [Google Scholar]
Ledo 1988 {published data only}
- Ledo A, Martin M, Geiger JM, Marron JM. Acitretin (Ro 10-1670) in the treatment of severe psoriasis. A randomized double-blind parallel study comparing acitretin and etretinate. International Journal of Dermatology 1988;27(9):656-60. [CENTRAL: CN-00058382] [DOI] [PubMed] [Google Scholar]
Legat 2005 {published data only}
- Legat LJ, Hofer A, Wackernagel A, Salmhofer W, Kerl H, Wolf P. Alefacept plus 311 nm narrowband ultraviolet B (NB-UVB) phototherapy in the treatment of psoriasis. Journal of Investigative Dermatology 2005;125(1):A4. [CENTRAL: CN-00550842] [Google Scholar]
Leonardi 2010a {published data only}
- Leonardi C, Guenther L, Wasel N, Yeilding N, Szapary PO, Hsu MC, et al. Characterization of infections associated with ustekinumab in moderate to severe psoriasis patients. Journal of the European Academy of Dermatology and Venereology 2010;24(Suppl 4):22. [EMBASE: 70238720] [Google Scholar]
Leonardi 2010b {published data only}
- Leonardi C, Menter A, Gu Y, Okun M. Efficacy and safety of weekly adalimumab in psoriasis patients with a less than PASI 50 response to 40 mg every other week: results from an open-label extension study. Journal of the European Academy of Dermatology and Venereology 2010;24(Suppl 4):9-10. [EMBASE: 70238693] [Google Scholar]
Leonardi 2010c {published data only}
- Leonardi C, Papp K, Asahina A, Gu Y, Rozzo S. Long-term safety of adalimumab for psoriasis: an analysis of all adalimumab exposure in all global clinical trials. Journal of the European Academy of Dermatology and Venereology 2010;24:26. [CENTRAL: 70238729] [Google Scholar]
Leonardi 2011a {published data only}
- Leonardi C, Kimball A, Schenkel B, Papp K. Sustained improvement in skin disease specific quality of life in patients with moderate to severe psoriasis receiving ustekinumab maintenance therapy: long-term results from PHOENIX 1. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB149. [CENTRAL: CN-00843843] [DOI: 10.1016/j.jaad.2010.09.608] [DOI] [Google Scholar]
Leonardi 2011b {published data only}
- Leonardi C, Langley RG, Papp K, Tyring SK, Wasel N, Vender R, et al. Adalimumab for treatment of moderate to severe chronic plaque psoriasis of the hands and feet: efficacy and safety results from REACH, a randomized, placebo-controlled, double-blind trial. Archives of Dermatology 2011;147(4):429-36. [CENTRAL: CN-00785710] [DOI] [PubMed] [Google Scholar]
Levell 1995 {published data only}
- Levell NJ, Shuster S, Munro CS, Friedmann PS. Remission of ordinary psoriasis following a short clearance course of cyclosporin. Acta Dermato-Venereologica 1995;75(1):65-9. [CENTRAL: CN-00113972] [DOI] [PubMed] [Google Scholar]
Li 2018 {published data only}
- Li N, Teeple A, Muser E, McElligott S, You Y, Song M, et al. Work/study productivity gain and indirect cost savings with guselkumab compared with adalimumab in moderate to severe psoriasis: results from the VOYAGE 1 study. Journal of Managed Care and Specialty Pharmacy 2018;24(10 A):S81. [CENTRAL: CN-01670028] [DOI] [PubMed] [Google Scholar]
Li 2022 {published data only}
- Li Q, Qiao J, Jin H, Chen B, He Z, Wang G, Ni X, Wang M, Xia M, Li B, Chen R, Hu P. Population pharmacokinetic/pharmacodynamic analysis of AK111, an IL-17A monoclonal antibody, in subjects with moderate-to-severe plaque psoriasis. Frontiers in pharmacology 2022;13:966176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liang 1995 {published data only}
- Liang GS, Kerdel FA. Combination therapy and the use of an initial dose of intramuscular methotrexate in patients hospitalized for psoriasis. Journal of Dermatological Treatment 1995;6(2):73-6. [CENTRAL: CN-00171665] [Google Scholar]
Louw 2017 {published data only}
- Louw I, Kivitz AJ, Takeuchi T, Tanaka Y, Nakashima S, Hodge J, et al. The long-term safety and durability of response of CHS-0214, a proposed biosimilar to etanercept: an open-label safety extension study. Arthritis and Rheumatology 2017;69(Suppl 10):2492. [CENTRAL: CN-01423778] [Google Scholar]
Lui 2011 {published data only}
- Lui H, Tan J, Shear N, Bissonnette R, Gulliver W. Efficacy and safety of alefacept in combination with narrowband UVB compared to alefacept alone in subjects with moderate to severe psoriasis: results of the Canadian alefacept phototherapy psoriasis study. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB150. [CENTRAL: CN-00843846] [Google Scholar]
Lui 2012 {published data only}
- Lui H, Gulliver W, Tan J, Hong CH, Hull P, Shear NH, et al. A randomized controlled study of combination therapy with alefacept and narrow band UVB phototherapy (UVB) for moderate to severe psoriasis: efficacy, onset, and duration of response. Journal of Drugs in Dermatology 2012;11(8):929-37. [CENTRAL: CN-01164684] [PubMed] [Google Scholar]
Lynde 2012 {published data only}
- Lynde CW, Gupta AK, Guenther L, Poulin Y, Levesque A, Bissonnette R. A randomized study comparing the combination of nbUVB and etanercept to etanercept monotherapy in patients with psoriasis who do not exhibit an excellent response after 12 weeks of etanercept. Journal of Dermatological Treatment 2012;23(4):261-7. [CENTRAL: CN-00972294] [PMID: ] [DOI] [PubMed] [Google Scholar]
Macdonald 1972 {published data only}
- Macdonald A, Fry L. Retinoic acid in the treatment of psoriasis. British Journal of Dermatology 1972;86(5):524-7. [CENTRAL: CN-00007340] [DOI] [PubMed] [Google Scholar]
Mahrle 1995 {published data only}
- Mahrle G, Schulze HJ, Farber L, Weidinger G, Steigleder GK. Low-dose short-term cyclosporine versus etretinate in psoriasis: improvement of skin, nail, and joint involvement. Journal of the American Academy of Dermatology 1995;32(1):78-88. [CENTRAL: CN-00109143] [DOI] [PubMed] [Google Scholar]
Malik 2010 {published data only}
- Malik T, Ejaz A. Comparison of methotrexate and azathioprine in the treatment of psoriasis: a randomized controlled trial. Journal of Pakistan Association of Dermatologists 2010;20(3):152-7. [CENTRAL: CN-00789615] [Google Scholar]
Marecki 2004 {published data only}
- Marecki S, Kirkpatrick P. Efalizumab. Nature Reviews. Drug Discovery 2004;3(6):473-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Marks 1986 {published data only}
- Marks JM. Cyclosporin A treatment of severe psoriasis. British Journal of Dermatology 1986;115(6):745-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mate 2017 {published data only}
- Mate E, Bagel J, Callis-Duffin K, Moore A, Ferris L, Siu K, et al. Secukinumab is efficacious in clearing moderate-tosevere scalp psoriasis: 12 week results of a randomized phase IIIb study. Australasian Journal of Dermatology 2017;58(Suppl 1):71. [CENTRAL: CN-01378822] [Google Scholar]
Mate 2018 {published data only}
- Mate E, Bissonnette R, Luger T, Thaçi D, Toth D, Lacombe A, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile through 5 years of treatment in moderate to severe psoriasis. Australasian Journal of Dermatology 2018;59(Suppl 1):86. [CENTRAL: CN-01919964] [DOI] [PMC free article] [PubMed] [Google Scholar]
McInnes 2013 {published data only}
- McInnes IB, Papp K, Puig L, Reich K, Ritchlin CT, Strober B, et al. Safety of ustekinumab from the placebo-controlled periods of psoriatic arthritis and psoriasis clinical developmental programs. Annals of the rheumatic diseases 2013;72(Suppl 3):No pagination. [DOI: 10.1136/annrheumdis-2013-eular.1992] [DOI] [Google Scholar]
McInnes 2017 {published data only}
- McInnes IB, Mease PJ, Ritchlin CT, Rahman P, Gottlieb AB, Kirkham B, et al. Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatology 2017;56(11):1993-2003. [CENTRAL: CN-01424217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mease 2011 {published data only}
- Mease P, Genovese MC, Gladstein G, Kivitz AJ, Ritchlin C, Tak PP, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis and Rheumatism 2011;63(4):939-48. [CENTRAL: CN-00779390] [DOI] [PubMed] [Google Scholar]
Mease 2016a {published data only}
- Mease PJ, Okada M, Kishimoto M, Shuler CL, Carlier H, Lin CY, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52 week results from a phase 3 study. Arthritis and Rheumatology 2016;68(Suppl 10):1270-1. [CENTRAL: CN-01296553] [DOI] [PubMed] [Google Scholar]
Mease 2016b {published data only}
- Mease P, Van Der Heijde D, Ritchlin C, Cuchacovich R, Shuler C, Lin CY, et al. A randomized, double-blind, active- and placebo-controlled phase 3 study of efficacy and safety of ixekizumab, adalimumab, and placebo therapy in patients naive to biologic disease modifying antirheumatic drugs with active psoriatic arthritis. Journal of Rheumatology 2016;43(6):1169. [CENTRAL: CN-01294588] [Google Scholar]
Mease 2017a {published data only}
- Mease PJ, Van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Annals of the Rheumatic Diseases 2017;76(1):79-87. [CENTRAL: CN-01374947] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mease 2017b {published data only}
- Mease P, Kishimoto M, Okada M, Lee C, Moriarty S, Mou J, et al. 52-week efficacy and safety results from SPIRIT-P1: a phase 3 study of ixekizumab in patients with active psoriatic arthritis. Journal of Rheumatology 2017;44(6):925. [CENTRAL: CN-01398036] [DOI] [PubMed] [Google Scholar]
Mease 2017c {published data only}
- Mease P, Okada M, Kishimoto M, Shuler C, Carlier H, Lin C, et al. Fifty-two-week efficacy and safety results from SPIRIT-P1: a phase 3 study of ixekizumab in patients with active psoriatic arthritis. Journal of Investigative Dermatology 2017;137(10 Suppl 2):S260. [CENTRAL: CN-01416618] [Google Scholar]
Mease 2018 {published data only}
- Mease P, Van der Heijde D, Landewe R, Mpofu S, Rahman P, Tahir H, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Annals of the Rheumatic Diseases 2018;77(6):890-7. [CENTRAL: CN-01606299] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mease 2020 {published data only}
- Mease PJ, Chohan S, Garcia Fructuoso FJ, Gottlieb AB, Chou R, Mendelsohn AM, et al. 15964 Randomized, double-blind, placebo-controlled, multiple-dose, phase 2b study to demonstrate the safety and efficacy of tildrakizumab, a high-affinity anti-interleukin-23p19 monoclonal antibody, in patients with active psoriatic arthritis. Journal of the American Academy of Dermatology 2020;83(6 Suppl):AB165. [Google Scholar]
Mease 2022 {published data only}
- Mease P J, Foley P, Reich K, Chakravarty SD, Shawi M, Yang YW, et al. Targeted safety analyses of guselkumab: long-term results from randomized clinical trials in patients with active psoriatic arthritis and moderate to severe psoriasis. Annals of the Rheumatic Diseases 2022;81(Suppl 1):1572-3. [Google Scholar]
Meffert 1989 {published data only}
- Meffert H, Sönnichsen N. Acitretin in the treatment of severe psoriasis: a randomized double-blind study comparing acitretin and etretinate. Acta Dermato-Venereologica 1989;146(Suppl):176-7. [CENTRAL: CN-00064910] [PubMed] [Google Scholar]
Menon 2012 {published data only}
- Menon S, Boy MG, Wang C, Wilkinson BE, Zwillich SH, Chan G, et al. Single and multiple-dose pharmacokinetics of tofacitinib (CP-690,550) from a double-blind, placebo-controlled, dose-escalation study in medically stable subjects with psoriasis. Clinical Pharmacology and Therapeutics 2012;91:S33. [CENTRAL: CN-01034861] [Google Scholar]
Menter 2007 {published data only}
- Menter A, Guzzo C, Li S, Gottlieb AB. Efficacy of infliximab in patients with severe psoriasis: subgroup analysis from clinical trials. Journal of the American Academy of Dermatology 2007;56(2):AB174. [CENTRAL: CN-00615988] [Google Scholar]
Menter 2014 {published data only}
- Menter A, Papp KA, Tan H, Tyring S, Wolk R, Buonanno M. Efficacy of tofacitinib, an oral janus kinase inhibitor, on clinical signs of moderate-to-severe plaque psoriasis in different body regions. Journal of Drugs in Dermatology 2014;13(3):252-6. [CENTRAL: CN-00985274] [PubMed] [Google Scholar]
Menter 2022 {published data only}
- Menter A, McCabe D, Lang B. 33221 Further clinical outcomes in patients with moderate-to-severe chronic plaque psoriasis receiving adalimumab reference product continuously or switching between BI 695501 and adalimumab RP in the phase III, randomized, interchangeability VOLTAIRE-X t. Journal of the American Academy of Dermatology 2022;87(3 Suppl):AB61. [Google Scholar]
Merola 2017 {published data only}
- Merola JF, Elewski B, Tatulych S, Lan S, Tallman A, Kaur M. Efficacy of tofacitinib for the treatment of nail psoriasis: two 52-week, randomized, controlled phase 3 studies in patients with moderate-to-severe plaque psoriasis. Journal of the American Academy of Dermatology 2017;77(1):79-87.e1. [CENTRAL: CN-01401051] [DOI] [PubMed] [Google Scholar]
Merola 2018 {published data only}
- Merola JF, Kishimoto M, Adams D, Park SY, Thaçi D. Ixekizumab improves nail and skin psoriasis through 52 weeks of treatment in patients with active psoriatic arthritis: results from two randomized, double-blind, phase 3, clinical trials (SPIRIT-P1 and SPIRIT-P2). Acta Dermato-Venereologica 2018;98(Suppl 219):16. [CENTRAL: CN-01620172] [Google Scholar]
Merola 2020 {published data only}
- Merola JF, Ghislain P-D, Dauendorffer JN, Potts Bleakman A, Brnabic AJM, Burge R, et al. Ixekizumab improves secondary lesional signs, pain and sexual health in patients with moderate-to-severe genital psoriasis. Journal of the European Academy of Dermatology and Venereology 2020;34(6):1257-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merola JF, Gottlieb AB, Mease P, Strand V, Kavanaugh A, Liu L, et al. 13350 Psoriasis outcomes in a randomized trial of etanercept and methotrexate as monotherapy or in combination in patients with psoriatic arthritis. Journal of the American Academy of Dermatology 2020;83(6 Suppl):AB118. [Google Scholar]
Meyer 2011 {published data only}
- Meyer MW, Zachariae C, Bendtzen K, Skov L. Immunogenicity of tumour necrosis factor inhibitors in patients with psoriasis receiving long-term treatment. British Journal of Dermatology 2011;165(6):e17. [EMBASE: 70610785] [Google Scholar]
Mittal 2009 {published data only}
- Mittal R, Malhotra S, Pandhi P, Kaur I, Dogra S. Efficacy and safety of combination acitretin and pioglitazone therapy in patients with moderate to severe chronic plaque-type psoriasis: a randomized, double-blind, placebo-controlled clinical trial. Archives of Dermatology 2009;145(4):387-93. [CENTRAL: CN-00682023] [DOI] [PubMed] [Google Scholar]
Moller 2009 {published data only}
- Moller I. Efficacy of leflunomide in patients with psoriatic arthritis [Eficacia del tratamiento con leflunomida en pacientes con artritis psoriasica]. Seminarios de la Fundacion Espanola de Reumatologia 2009;10(2):48-52. [PMID: 2009314647] [Google Scholar]
Monk 1986 {published data only}
- Monk BE. Cyclosporin A and psoriasis. British Journal of Dermatology 1986;115(2):249-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Montgomery 1993 {published data only}
- Montgomery JA, Snyder HW Jr, Walsh DA, Walsh GM. BCX-34. Purine nucleoside phosphorylase (PNP) inhibitor. Drugs of the Future 1993;18(10):887-90. [EMBASE: 1994025323] [Google Scholar]
Mrowietz 1991 {published data only}
- Mrowietz U, Christophers E. Low-dose ciclosporin A (sandimmun) in psoriasis: a multicenter dose-finding study. Zeitschrift fur Hautkrankheiten 1991;66(Suppl 1):25-9. [CENTRAL: CN-00182853] [Google Scholar]
Mrowietz 2012 {published data only}
- Mrowietz U, Reich K, Rozzo S, Gu Y. Achievement of European Consensus Programme treatment goals in three clinical trials of adalimumab in moderate-to-severe psoriasis. Journal of the American Academy of Dermatology 2012;66(4 Suppl 1):AB183. [EMBASE: 70704582] [Google Scholar]
Narang 2012 {published data only}
- Narang T, Dogra S, Handa S. Serendipity opens new avenues: a pilot study to evaluate the efficacy of saxagliptin in combination with cyclosporine and acitretin in diabetic psoriasis patients. Dermatology and Therapy 2012;2(Suppl 1):S36-7. [CENTRAL: CN-01027858] [Google Scholar]
Nash 2015 {published data only}
- Nash P, Gottlieb A, Mease P, McInnes I, Kirkham B, Kavanaugh A, et al. Secukinumab, a human anti-interleukin-17a monoclonal antibody, significantly reduces psoriasis burden in patients with psoriatic arthritis: results from a phase 3 randomized controlled trial. Internal Medicine Journal 2015;45(Suppl 2):42. [CENTRAL: CN-01361215] [Google Scholar]
NCT00106847 {published data only}
- NCT00106847. A study of the safety and effectiveness of infliximab in patients with plaque-type psoriasis. clinicaltrials.gov/ct2/show/NCT00106847 (first received 1 April 2005).
NCT00111111 {published data only}
- NCT00111111. An evaluation of etanercept in the treatment of subjects with psoriasis. clinicaltrials.gov/ct2/show/nct00111111 (first received 18 May 2005).
NCT00258713 {published data only}
- NCT00258713. A 36-week extension to protocol ISA04-03. clinicaltrials.gov/ct2/show/NCT00258713 (first received 28 November 2005).
NCT00358670 {published data only}
- NCT00358670. Long-term effects of infliximab in the treatment of moderate to severe psoriasis [extension of study P04271, NCT00251641] (P04563). clinicaltrials.gov/ct2/show/nct00358670 (first received 1 August 2006).
NCT00377325 {published data only}
- NCT00377325. The effectiveness of lower cyclosporine doses for psoriasis. clinicaltrials.gov/show/nct00377325 (first received 18 September 2006).
NCT00438360 {published data only}
- NCT00438360. Efficacy and safety of cyclosporine A microemulsion in maintenance patients with chronic plaque psoriasis. clinicaltrials.gov/show/nct00438360 (first received 22 February 2007).
NCT00585650 {published data only}
- NCT00585650. Study of tumor necrosis factor receptor fusion protein etanercept (enbrel) in psoriasis of the hands and/or feet. clinicaltrials.gov/show/nct00585650 (first received 3 January 2008).
NCT00645892 {published data only}
- NCT00645892. Extension study of two dosing schedules of adalimumab in subjects with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/nct00645892 (first received 28 March 2008).
NCT00646191 {published data only}
- NCT00646191. Study of the safety and efficacy of adalimumab in subjects with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/nct00646191 (first received 28 March 2008).
NCT00647400 {published data only}
- NCT00647400. Adalimumab in adult Japanese subjects with psoriasis. clinicaltrials.gov/show/nct00647400 (first received 31 March 2008).
NCT00832364 {published data only}
- NCT00832364. Trial of an injectable biologic and U0279 as combination therapy for severe plaque-type psoriasis. clinicaltrials.gov/show/nct00832364 (first received 30 January 2009).
NCT01163253 {published data only}
- NCT01163253. A long term study to evaluate the safety and tolerability of CP-690,550 for patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/nct01163253 (first received 15 July 2010).
NCT01235442 {published data only}
- NCT01235442. Evaluate efficacy, and safety of topical therapy and etanercept in subjects with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct01235442 (first received 5 November 2010).
NCT01276847 {published data only}
- NCT01276847. A study to assess the effect of ustekinumab (Stelara®) and etanercept (Enbrel®) in participants with moderate to severe psoriasis (MK-0000-206). clinicaltrials.gov/show/nct01276847 (first received 13 January 2011).
NCT01412944 {published data only}
- NCT01412944. Efficacy and safety of intravenous and subcutaneous secukinumab in moderate to severe chronic plaque-type psoriasis (STATURE). clinicaltrials.gov/show/nct01412944 (first received 9 August 2011).
NCT01443338 {published data only}
- NCT01443338. Study evaluating the efficacy and safety of triptergium wilfordii and acitretin in psoriasis vulgaris - CHINA201002016-2. clinicaltrials.gov/show/nct01443338 (first received 29 September 2011).
NCT01544595 {published data only}
- NCT01544595. Extension study of secukinumab prefilled syringes in subjects with moderate to severe chronic plaque-type psoriasis completing preceding psoriasis phase III studies with secukinumab. clinicaltrials.gov/show/nct01544595 (first received 6 March 2012).
NCT01550744 {published data only}
- NCT01550744. A study of ustekinumab to evaluate a "subject-tailored" maintenance dosing approach in subjects with moderate-to-severe plaque psoriasis (PSTELLAR). clinicaltrials.gov/show/nct01550744 (first received 12 March 2012).
NCT01624233 {published data only}
- NCT01624233. A study in Japanese participants with moderate-to-severe psoriasis (UNCOVER-J). clinicaltrials.gov/show/nct01624233 (first received 20 June 2012).
NCT01722214 {published data only}
- NCT01722214. Trial on the effect of adalimumab on vascular inflammation in patients with psoriasis. clinicaltrials.gov/show/nct01722214 (first received 6 November 2012).
NCT01806597 {published data only}
- NCT01806597. Study of safety, tolerability, and efficacy of secukinumab in subjects with moderate to severe palmoplantar psoriasis (GESTURE). clinicaltrials.gov/show/nct01806597 (first received 7 March 2013).
NCT01815723 {published data only}
- NCT01815723. Efficacy study on dimethyl fumarate to treat moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct01815723 (first received 21 March 2013).
NCT01828086 {published data only}
- Kaul M, Jarvis P, Rozenberg I, Kolbinger F, Di Padova F, Calonder C, et al. First-in-human study demonstrating the safety and clinical efficacy of novel anti-IL-17A monoclonal antibody CJM112 in moderate to severe plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2021;35(5):1143-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01828086. Single and multiple dose escalation study to assess the safety and tolerability of CJM112 in psoriasis. clinicaltrials.gov/show/nct01828086 (first received 10 April 2013).
NCT01936688 {published data only}
- EUCTR2013-001740-54-HU. A clinical research study of 28 weeks to test the safety/tolerability and effectiveness of an investigational study medication (subcutaneous SCH 900222/MK-3222) in improving the signs and symptoms of moderate-to-severe chronic plaque psoriasis, and to compare it to an approved medication for the treatment of psoriasis called etanercept. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013-001740-54-HU (first received 18 September 2013).
- NCT01936688. A study to evaluate the efficacy and safety/tolerability of subcutaneous MK-3222 in participants with moderate-to-severe chronic plaque psoriasis (MK-3222-012). clinicaltrials.gov/show/nct01936688 (first received 6 September 2013).
NCT02362789 {published data only}
- NCT02362789. Secukinumab study in PSOriasis exploring pruRITUS intensity and lesional biomarkers (PSORITUS). clinicaltrials.gov/show/nct02362789 (first received 13 February 2015).
NCT02409667 {published data only}
- NCT02409667. Plaque psoriasis efficacy and safety with secukinumab (OPTIMISE). clinicaltrials.gov/show/nct02409667 (first received 7 April 2015).
NCT02798211 {published data only}
- NCT02798211. Study to evaluate the safety and efficacy of secukinumab 300 mg and 150 mg in adult patients with active psoriatic arthritis (PsA) after 16 weeks of treatment compared to placebo. clinicaltrials.gov/show/nct02798211 (first received 14 June 2016).
NCT03010527 {published data only}
- NCT03010527. Study to evaluate the long-term safety, tolerability and efficacy of bimekizumab in patients with chronic plaque psoriasis. clinicaltrials.gov/show/nct03010527 (first received 5 January 2017).
NCT03020199 {published data only}
- EUCTR2015-002423-26-FI. Study of the efficacy of early intervention with secukinumab 300 mg s.c. compared to narrow-band UVB in patients with new-onset moderate to severe plaque psoriasis. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015-002423-26-FI (first received 21 October 2016).
- NCT03020199. Study of the efficacy of early intervention with secukinumab 300 mg s.c. compared to narrow-band UVB in patients with new-onset moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct03020199 (first received 13 January 2017).
NCT03025542 {published data only}
- NCT03025542. Study to evaluate the pharmacokinetics (PK), pharmacodynamics (PD), and safety of bimekizumab in patients with chronic plaque psoriasis. clinicaltrials.gov/show/nct03025542 (first received 19 January 2017).
NCT03073213 {published data only}
- NCT03073213. A study of ixekizumab in Chinese participants with psoriasis vulgaris. clinicaltrials.gov/show/nct03073213 (first received 8 March 2017).
NCT03210259 {published data only}
- NCT03210259. The VOLTAIRE-X trial looks at the effect of switching between Humira® and BI 695501 in patients with plaque psoriasis. clinicaltrials.gov/show/nct03210259 (first received 6 July 2017).
NCT03482011 {published data only}
- Anonymous. Efficacy, safety, and quality of life in patients with moderate-to-severe plaque psoriasis treated with mirikizumab (LY3074828) in a phase 2 study. Journal of the American Academy of Dermatology 2018;79(3 Suppl 1):AB126. [Google Scholar]
- NCT03482011. A study to evaluate the efficacy and safety of mirikizumab (LY3074828) in participants with moderate-to-severe plaque psoriasis. clinicaltrials.gov/show/nct03482011 (first received 29 March 2018).
NCT03598790 {published data only}
- NCT03598790. A study to assess the safety, tolerability and efficacy of bimekizumab in adult subjects with moderate to severe chronic plaque psoriasis (BE BRIGHT). clinicaltrials.gov/show/nct03598790 (first received 25 July 2018).
NCT04121143 {published data only}
- NCT04121143. A phase II clinical study of SHR-1314 injection in the treatment of moderate to severe plaque psoriasis in adults. clinicaltrials.gov/show/NCT04121143 (first received 9 October 2019).
NCT04488185 {published data only}
- NCT04488185. An efficacy study of secukinumab in plaque psoriasis patients with subclinical psoriatic arthritis as measured by musculoskeletal ultrasound (INTERCEPT). clinicaltrials.gov/show/NCT04488185 (first received 27 July 2020).
NCT04614298 {published data only}
- NCT04614298. A phase 4 study of brodalumab (KHK4827) in subjects with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT04614298 (first received 3 November 2020).
NCT04839016 {published data only}
- NCT04839016. Efficacy, safety and PK of SHR-1314 in patients with moderate-to-severe plaque psoriasis. clinicaltrials.gov/show/NCT04839016 (first received 9 April 2021).
NCT04882098 {published data only}
- NCT04882098. A study of guselkumab in participants with active psoriatic arthritis. clinicaltrials.gov/show/NCT04882098 (first received 11 May 2021).
NCT05073315 {published data only}
- NCT05073315. A comparative study between ABP 501 and Humira® in participants with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT05073315 (first received 11 October 2021).
NCT05184348 {published data only}
- NCT05184348. Plexin B2 gene expression and polymorphisms in psoriasis: relation to narrow band ultraviolet B, acitretin and combined therapy. clinicaltrials.gov/show/NCT05184348 (first received 11 January 2022).
NCT05478499 {published data only}
- NCT05478499. Efficacy and safety of deucravacitinib versus placebo in participants with moderate-to-severe scalp psoriasis. clinicaltrials.gov/show/NCT05478499 (first received 28 July 2022).
NCT05495568 {published data only}
- NCT05495568. To compare pharmacokinetics, efficacy, and safety of CT-P17 with Humira in patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/NCT05495568 (first received 10 August 2022).
Nemoto 2018 {published data only}
- Nemoto O, Hirose K, Shibata S, Li K, Kubo H. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. British Journal of Dermatology 2018;178(3):689-96. [CENTRAL: CN-01449577] [DOI] [PubMed] [Google Scholar]
Nieboer 1990 {published data only}
- Nieboer C, De Hoop D, Langendijk PN, Van Loenen AC, Gubbels J. Fumaric acid therapy in psoriasis: a double-blind comparison between fumaric acid compound therapy and monotherapy with dimethylfumaric acid ester. Dermatologica 1990;181(1):33-7. [CENTRAL: CN-00351435] [DOI] [PubMed] [Google Scholar]
Nijsten 2008 {published data only}
- Nijsten T, Spuls P, Stern RS. STROBE: a beacon for observational studies. Archives of Dermatology 2008;144(9):1200-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Noda 2011 {published data only}
- Noda S, Mizuno K, Adachi M. Treatment effect of adalimumab and infliximab in Japanese psoriasis patients: results in a single community-based hospital. Journal of Investigative Dermatology 2011;131(Suppl 2):S38. [EMBASE: 70520994] [DOI] [PubMed] [Google Scholar]
Noor 2017 {published data only}
- Noor SM, Ayub N, Paracha MM. Efficacy and safety of methotrexate versus acitretin in chronic plaque psoriasis. Journal of Postgraduate Medical Institute 2017;31(1):4-7. [CENTRAL: CN-01340758] [Google Scholar]
Novotny 1973 {published data only}
- Novotny F. Use of methotrexate in psoriasis. Ceskoslovenska Dermatologie 1973;48(5):301-5. [PMID: ] [PubMed] [Google Scholar]
Nyfors 1978 {published data only}
- Nyfors A. Benefits and adverse drug experiences during long-term methotrexate treatment of 248 psoriatics. Danish Medical Bulletin 1978;25(5):208-11. [PMID: ] [PubMed] [Google Scholar]
Okubo 2019 {published data only}
- Okubo Y, Ohtsuki M, Morita A, Yamaguchi M, Shima T, Tani Y, et al. Long-term efficacy and safety of secukinumab in Japanese patients with moderate to severe plaque psoriasis: 3-year results of a double-blind extension study. Journal of Dermatology 2019;46(3):186-92. [CENTRAL: CN-01794517] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliver 2021 {published data only}
- Oliver R, Krueger JG, Glatt S, Vajjah P, Mistry C, Page M, et al. Bimekizumab for the treatment of moderate to severe plaque psoriasis: efficacy, safety, pharmacokinetics, pharmacodynamics and transcriptomics from a phase 2a randomized, multicenter double-blinded study. British journal of dermatology 2021;186(4):652-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
OPT Pivotal‐1 2015 {published data only}
- Papp KA, Menter MA, Abe M, Elewski B, Feldman SR, Gottlieb AB, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. British Journal of Dermatology 2015;173(4):949-61. [CENTRAL: CN-01105187] [PMID: ] [DOI] [PubMed] [Google Scholar]
OPT Pivotal‐2 2015 {published data only}
- Papp KA, Menter MA, Abe M, Elewski B, Feldman SR, Gottlieb AB, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. British Journal of Dermatology 2015;173(4):949-61. [CENTRAL: CN-01105187] [PMID: ] [DOI] [PubMed] [Google Scholar]
Orfanos 1978 {published data only}
- Orfanos CE, Goerz G. Oral psoriasis treatment with a new aromatic retinoid (Ro 10-9359): a multi-centre controlled study of 291 patients (preliminary results). Deutsche Medizinische Wochenschrift 1978;103(5):195-9. [CENTRAL: CN-00017768] [DOI] [PubMed] [Google Scholar]
Orfanos 1979 {published data only}
- Orfanos CE, Steigleder GK, Pullmann H, Bloch PH. Oral retinoid and UVB radiation: a new, alternative treatment for psoriasis on an out-patient basis. Acta Dermato-Venereologica 1979;59(3):241-4. [CENTRAL: CN-00020394] [PubMed] [Google Scholar]
Ortonne 2008 {published data only}
- Ortonne JP, Griffiths CE, Dauden E, Strohal R, Robertson D, Pedersen R, et al. Efficacy and safety of continuous versus paused etanercept treatment in patients with moderate-to-severe psoriasis over 54 weeks: the CRYSTEL Study. Expert Review of Dermatology 2008;3(6):657-65. [CENTRAL: CN-00754922] [Google Scholar]
Ortonne 2011 {published data only}
- Ortonne JP, Chimenti S, Reich K, Gniadecki R, Sprøgel P, Unnebrink K, et al. Efficacy and safety of adalimumab in patients with psoriasis previously treated with anti-tumour necrosis factor agents: subanalysis of BELIEVE. Journal of the European Academy of Dermatology and Venereology 2011;25(9):1012-20. [CENTRAL: CN-00812830] [DOI] [PubMed] [Google Scholar]
Osamu 2014 {published data only}
- Osamu N, Hirotaka N, Koji S, Kenji T. Clinical pharmacology of the anti-IL-17 receptor antibody brodalumab (KHK4827) in Japanese normal healthy volunteers and Japanese subjects with moderate to severe psoriasis: a randomized, dose-escalation, placebo-controlled study. Journal of Dermatological Science 2014;75(3):201-4. [CENTRAL: CN-00999213] [DOI] [PubMed] [Google Scholar]
Page 2020 {published data only}
- Page KM, Suarez-Farinas M, Suprun M, Zhang W, Garcet S, Fuentes-Duculan J, et al. Molecular and cellular responses to the TYK2/JAK1 inhibitor PF-06700841 reveal reduction of skin inflammation in plaque psoriasis. Journal of Investigative Dermatology 2020;140(8):1546-55.e4. [DOI] [PubMed] [Google Scholar]
Pakozdi 2018 {published data only}
- Pakozdi T, Georgantas RW, Grebe KM, Visvanathan S, Baum P, Davis W. RNA-seq genomic analysis demonstrated the molecular efficacy of risankizumab in a moderate-to-severe plaque psoriasis phase 2 clinical study. Experimental Dermatology 2018;27(Suppl 2):21. [CENTRAL: CN-01793116] [Google Scholar]
Papp 2001 {published data only}
- Papp K, Bissonnette R, Krueger JG, Carey W, Gratton D, Gulliver WP, et al. The treatment of moderate to severe psoriasis with a new anti-CD11a monoclonal antibody. Journal of the American Academy of Dermatology 2001;45(5):665-74. [CENTRAL: CN-00374574] [DOI] [PubMed] [Google Scholar]
Papp 2006 {published data only}
- Papp K, Langley R, Bissonnette R, Rosoph L. A phase III, randomized, multicenter, double-blind, placebo-controlled study of ISA247 in plaque psoriasis patients. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB9. [CENTRAL: CN-00602194] [DOI] [PubMed] [Google Scholar]
Papp 2008 {published data only}
- Papp K, Bissonnette R, Rosoph L, Wasel N, Lynde CW, Searles G, et al. Efficacy of ISA247 in plaque psoriasis: a randomised, multicentre, double-blind, placebo-controlled phase III study. Lancet 2008;371(9521):1337-42. [CENTRAL: CN-00631348] [DOI] [PubMed] [Google Scholar]
Papp 2009 {published data only}
- Papp K, Okun M, Vender R. Adalimumab in the treatment of psoriasis: pooled efficacy and safety results from three pivotal studies. Journal of Cutaneous Medicine and Surgery 2009;13(Suppl 2):S58-66. [PMID: ] [DOI] [PubMed] [Google Scholar]
Papp 2011a {published data only}
- Papp K, Signorovitch J, Mulani P, Bao Y. Comparison of psoriasis sign and symptom reduction and complete clearance with adalimumab versus etanercept. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB153. [CENTRAL: CN-00843873] [Google Scholar]
Papp 2011b {published data only}
- Papp K, Signorovitch J, Sundaram M, Bao Y. Effects of abt-874 treatment on health-related quality of life and work productivity and activity impairment in patients with psoriasis. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB155. [CENTRAL: CN-00843874] [Google Scholar]
Papp 2011c {published data only}
- Papp K, Yu A, Sundaram M, Bao Y. Achieving long-term sustained response is associated with improvements in patient-reported outcomes in patients with psoriasis treated with ABT-874. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB160. [CENTRAL: CN-00843875] [Google Scholar]
Papp 2012b {published data only}
- Bushmakin AG, Mamolo C, Cappelleri JC, Stewart M. The relationship between pruritus and the clinical signs of psoriasis in patients receiving tofacitinib. Journal of Dermatological Treatment 2015;26(1):19-22. [CENTRAL: CN-01051702] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mamolo C, Harness J, Tan H, Menter A. Tofacitinib (CP-690,550), an oral Janus kinase inhibitor, improves patient-reported outcomes in a phase 2b, randomized, double-blind, placebo-controlled study in patients with moderate-to-severe psoriasis. Journal of the European Academy of Dermatology and Venereology 2014;28(2):192-203. [CENTRAL: CN-00959043] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mamolo CM, Bushmakin AG, Cappelleri JC. Application of the Itch Severity Score in patients with moderate-to-severe plaque psoriasis: clinically important difference and responder analyses. Journal of Dermatological Treatment 2015;26(2):121-3. [CENTRAL: CN-01083900] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Papp KA, Menter A, Strober B, Langley RG, Buonanno M, Wolk R, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. British Journal of Dermatology 2012;167(3):668-77. [CENTRAL: CN-00856433] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Strober B, Buonanno M, Clark JD, Kawabata T, Tan H, Wolk R, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. British Journal of Dermatology 2013;169(5):992-9. [CENTRAL: CN-01122547] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Valenzuela F, Papp KA, Pariser D, Tyring SK, Wolk R, Buonanno M, et al. Effects of tofacitinib on lymphocyte sub-populations, CMV and EBV viral load in patients with plaque psoriasis. BMC Dermatology 2015;15:8. [CENTRAL: CN-01109432] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papp 2012d {published data only}
- Papp KA, Reid C, Foley P, Sinclair R, Salinger DH, Williams G, et al. Anti-IL-17 receptor antibody AMG 827 leads to rapid clinical response in subjects with moderate to severe psoriasis: results from a phase I, randomized, placebo-controlled trial. Journal of Investigative Dermatology 2012;132(10):2466-9. [CENTRAL: CN-00854479] [DOI] [PubMed] [Google Scholar]
Papp 2012e {published data only}
- Papp KA, Poulin Y, Bissonnette R, Bourcier M, Toth D, Rosoph L, et al. Assessment of the long-term safety and effectiveness of etanercept for the treatment of psoriasis in an adult population. Journal of the American Academy of Dermatology 2012;66(2):e33-45. [CENTRAL: CN-00883092] [DOI] [PubMed] [Google Scholar]
Papp 2017c {published data only}
- Papp K, Bachelez H, Costanzo A, Foley P, Gooderham M, Kaur P, et al. Clinical similarity of the biosimilar ABP 501 compared with adalimumab after single transition: long-term results from a randomized controlled, double-blind, 52-week, phase III trial in patients with moderate-to-severe plaque psoriasis. British Journal of Dermatology 2017;177(6):1562-74. [CENTRAL: CN-01440056] [DOI] [PubMed] [Google Scholar]
Papp 2018a {published data only}
- Papp K, Kimball A, Blauvelt A, Reich K, Gooderham M, Tyring S, et al. Effect of tildrakizumab on personal relationships in patients with moderate-to-severe chronic plaque psoriasis. Acta Dermato-Venereologica 2018;98(Suppl 219):54. [CENTRAL: CN-01620176] [Google Scholar]
Papp 2018b {published data only}
- Papp KA, Blauvelt A, Kimball AB, Han C, Randazzo B, Wasfi Y, et al. Patient-reported symptoms and signs of moderate-to-severe psoriasis treated with guselkumab or adalimumab: results from the randomized VOYAGE 1 trial. Journal of the European Academy of Dermatology and Venereology 2018;32(9):1515-22. [CENTRAL: CN-01665289] [DOI: 10.1111/jdv.14910] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2013 {published data only}
- Park KK, Wu JJ, Koo J. A randomized, 'head-to-head' pilot study comparing the effects of etanercept monotherapy vs. etanercept and narrowband ultraviolet B (NB-UVB) phototherapy in obese psoriasis patients. Journal of the European Academy of Dermatology and Venereology 2013;27(7):899-906. [CENTRAL: CN-00969013] [DOI] [PubMed] [Google Scholar]
Paul 2012 {published data only}
- Paul C, Van de Kerkhof P, Puig L, Unnebrink K, Goldblum O, Thaçi D. Influence of psoriatic arthritis on the efficacy of adalimumab and on the treatment response of other markers of psoriasis burden: subanalysis of the BELIEVE study. European Journal of Dermatology 2012;22(6):762-9. [CENTRAL: CN-00966715] [DOI] [PubMed] [Google Scholar]
Paul 2014 {published data only}
- Paul C, Puig L, Kragballe K, Luger T, Lambert J, Chimenti S, et al. Transition to ustekinumab in patients with moderate-to-severe psoriasis and inadequate response to methotrexate: a randomized clinical trial (TRANSIT). British Journal of Dermatology 2014;170(2):425-34. [CENTRAL: CN-00982375] [DOI] [PubMed] [Google Scholar]
Paul 2018 {published data only}
- Paul C, Guenther L, Torii H, Sofen H, Burge R, Lin CY, et al. Impact of ixekizumab on facial psoriasis and related quality of life measures in moderate-to-severe psoriasis patients: 12-week results from two phase III trials. Journal of the European Academy of Dermatology and Venereology 2018;32(1):68-72. [CENTRAL: CN-01449415] [DOI: 10.1111/jdv.14581] [DOI] [PubMed] [Google Scholar]
Perks 2017 {published data only}
- Perks B. Randomized non-inferiority trial fails to find inferiority switching from infliximab originator to CT-P13 biosimilar. GaBI Journal 2017;6(4):188-9. [DOI: 10.5639/gabij.2017.0604.042] [DOI] [Google Scholar]
Pettit 1979 {published data only}
- Pettit JH. Oral retinoid for psoriasis. A report of a double blind study. Acta Dermato-Venereologica 1979;59(85 Suppl):133-6. [CENTRAL: CN-00021931] [MEDLINE: ] [PubMed] [Google Scholar]
Petzelbauer 1990 {published data only}
- Petzelbauer P, Honigsmann H, Langer K, Anegg B, Strohal R, Tanew A, et al. Cyclosporin A in combination with photochemotherapy (PUVA) in the treatment of psoriasis. British Journal of Dermatology 1990;123(5):641-7. [CENTRAL: CN-00351600] [DOI] [PubMed] [Google Scholar]
Piascik 2003 {published data only}
- Piascik P. Alefacept, first biologic agent approved for treatment of psoriasis. Journal of the American Pharmacists Association 2003;43(5):649-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ports 2013 {published data only}
- Ports WC, Khan S, Lan S, Lamba M, Bolduc C, Bissonnette R, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. British Journal of Dermatology 2013;169(1):137-45. [CENTRAL: CN-00920320] [DOI] [PMC free article] [PubMed] [Google Scholar]
Puig 2018 {published data only}
- Puig L, Augustin M, Blauvelt A, Gottlieb AB, Vender R, Korman NJ, et al. Effect of secukinumab on quality of life and psoriasis-related symptoms: a comparative analysis versus ustekinumab from the CLEAR 52-week study. Journal of the American Academy of Dermatology 2018;78(4):741-8. [CENTRAL: CN-01463902] [DOI] [PubMed] [Google Scholar]
Punwani 2012 {published data only}
- Punwani N, Scherle P, Flores R, Shi J, Liang J, Yeleswaram S, et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. Journal of the American Academy of Dermatology 2012;67(4):658-64. [CENTRAL: CN-00881387] [DOI] [PubMed] [Google Scholar]
Rabasseda 2012 {published data only}
- Rabasseda X. A report from the American Academy of Dermatology 70th Annual Meeting (March 16-20, 2012; San Diego, California, USA). Drugs of Today 2012;48(5):367-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Radmanesh 2011 {published data only}
- Radmanesh M, Rafiei B, Moosavi ZB, Sina N. Weekly vs. daily administration of oral methotrexate (MTX) for generalized plaque psoriasis: a randomized controlled clinical trial. International Journal of Dermatology 2011;50(10):1291-3. [CENTRAL: CN-00805615] [DOI] [PubMed] [Google Scholar]
Raman 1998 {published data only}
- Raman GV, Campbell SK, Farrer A, Albano JD, Cook J. Modifying effects of amlodipine on cyclosporin A-induced changes in renal function in patients with psoriasis. Journal of Hypertension 1998;16(4 Suppl):S39-41. [CENTRAL: CN-00308573] [PubMed] [Google Scholar]
Reich 2004 {published data only}
- Reich K. Alefacept in the treatment of psoriasis for whom conventional therapies are ineffective or inappropriate. Journal of the European Academy of Dermatology and Venereology 2004;18(6):808. [CENTRAL: CN-00550795] [Google Scholar]
- Sclessinger J, Pariser R, Park S, Wierz G. Evaluation of the efficacy and safety of alefacept in patients for whom conventional psoriasis therapies are ineffective or inappropriate. Journal of the American Academy of Dermatology 2007;56(2):AB192. [CENTRAL: CN-00616055] [Google Scholar]
Reich 2011 {published data only}
- Reich K, Van Hoogstraten BJ, Wozel G, Zheng H, Flint L. Long-term efficacy and safety of maintenance versus intermittent infliximab therapy for moderate to severe plaque-type psoriasis: the Restore2 trial. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB150. [CENTRAL: CN-00843879] [Google Scholar]
Reich 2014 {published data only}
- Reich K, Puig L, Paul C, Kragballe K, Luger T, Lambert J, et al. One-year safety and efficacy of ustekinumab and results of dose adjustment after switching from inadequate methotrexate treatment: the TRANSIT randomized trial in moderate-to-severe plaque psoriasis. British Journal of Dermatology 2014;170(2):435-44. [CENTRAL: CN-00982376] [DOI] [PubMed] [Google Scholar]
Reich 2016a {published data only}
- Reich K, Soung J, Gooderham M, Zhang Z, Nograles K, Goodfield M. LIBERATE trial: sustained efficacy of apremilast in patients with moderate-to-severe psoriasis who continued on apremilast or switched from etanercept treatment. British Journal of Dermatology 2016;175(S1):71-2. [CENTRAL: CN-01303307] [DOI: 10.1111/bjd.14524] [DOI] [Google Scholar]
Reich 2016b {published data only}
- Reich K, Choi SL, Jackson K, Mallbris L, Blauvelt A. Time course of ixekizumab drug levels and the relationship at week 60 to efficacy in patients with moderate-to- severe plaque psoriasis (UNCOVER-3). Experimental Dermatology 2016;25(S4):39. [CENTRAL: CN-01407586] [Google Scholar]
Reich 2017a {published data only}
- Reich K, Goodfield M, Green L, Nograles K, Chen R, Levi E, et al. Efficacy and safety of apremilast through 104 weeks in subjects with moderate to severe psoriasis randomized to placebo, apremilast, or etanercept who continued on or switched to apremilast after week 16 in a phase 3B study. Arthritis and Rheumatology 2017;69(Suppl 10):627. [Google Scholar]
Reich 2017b {published data only}
- Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. Journal of the American Academy of Dermatology 2017;76(3):418-31. [CENTRAL: CN-01341399] [DOI] [PubMed] [Google Scholar]
Reich 2017c {published data only}
- Reich K, Papp K, Armstrong AW, Wasfi Y, Jiang G, Shen YK, et al. Safety of guselkumab in patients with plaque psoriasis through 2 years: a pooled analysis from VOYAGE 1 and VOYAGE 2. British Journal of Dermatology 2017;177(5):e297. [DOI: 10.1111/bjd.16059] [DOI] [PubMed] [Google Scholar]
Reich 2018a {published data only}
- Reich K, Sullivan J, Arenberger P, Mrowietz U, Jazayeri S, Augustin M, et al. Effect of secukinumab on the clinical activity and disease burden of nail psoriasis: 32-week results from the randomized placebo-controlled TRANSFIGURE trial. British Journal of Dermatology 2018 Oct 26 [Epub ahead of print]. [DOI: 10.1111/bjd.17351] [DOI] [PubMed]
Reich 2018b {published data only}
- Reich K, Jackson K, Ball S, Garces S, Kerr L, Chua L, et al. Ixekizumab pharmacokinetics, anti-drug antibodies, and efficacy through 60 weeks of treatment of moderate to severe plaque psoriasis. Journal of Investigative Dermatology 2018;138(10):2168-73. [CENTRAL: CN-01611449] [DOI] [PubMed] [Google Scholar]
Reich 2018c {published data only}
- Reich K, Gooderham M, Thaçi D, Crowley JJ, Ryan C, Krueger JG, et al. Efficacy and safety of risankizumab (RZB) compared with adalimumab (ADA) in patients with moderate-to-severe plaque psoriasis: results from the phase 3 IMMvent trial. Experimental Dermatology 2018;27(Suppl 2):9-10. [CENTRAL: CN-01790123] [Google Scholar]
Reich 2019 {published data only}
- Bissonnette R, Maari C, Menter MA, Ohata C, Papp KA, Tuttle JL, et al. 15328 Efficacy and safety of mirikizumab in patients with moderate to severe plaque psoriasis: 104-week results from a randomized phase 2 study. Journal of the American Academy of Dermatology 2020;83(6 Suppl):AB147. [Google Scholar]
- Gooderham M, Edson-Heredia E, Li J, Patel D, Guo J, Bagel J. Patient-reported improvements in health-related quality of life by improvements in clinician-rated psoriasis severity: a phase 2 study analysis in patients with psoriasis treated with mirikizumab. Journal of the Dermatology Nurses' Association 2020;12(2):No-pagination. [Google Scholar]
- NCT02899988. A study of mirikizumab (LY3074828) in participants with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct02899988 (first received 14 September 2016).
- Papp K, Maari C, Rich P, Klekotka P, Li J, Tuttle J, et al. Response to mirikizumab at week 52 among patients who did not achieve a PASI 90 response at week 16. Journal of Clinical and Aesthetic Dermatology 2019;12(5 Suppl 1):S29. [Google Scholar]
- Reich K, Bissonnette R, Menter A, Klekotka P, Patel D, Li J, et al. Efficacy and safety of mirikizumab (LY3074828) in the treatment of moderate-to-severe plaque psoriasis: results from a phase II study. British Journal of Dermatology 2017;177(5):e249. [DOI] [PubMed] [Google Scholar]
- Reich K, Rich P, Maari C, Bissonnette R, Leonardi C, Menter A, et al. Efficacy and safety of mirikizumab (LY3074828) in the treatment of moderate-to-severe plaque psoriasis: results from a randomized phase II study. British Journal of Dermatology 2019;181(1):88-95. [DOI: 10.1111/bjd.17628] [DOI] [PubMed] [Google Scholar]
- Rich P, Klekotka P, Patel D, Tuttle J, Li J, Papp K, et al. Improvement in psoriasis scalp severity index (PSSI) during maintenance treatment with mirikizumab. Journal of the Dermatology Nurses' Association 2020;12(2):No pagination. [Google Scholar]
Reitamo 1999 {published data only}
- Reitamo S, Spuls P, Sassolas B, Lahfa M, Claudy A, Griffiths C, et al. A double-blind study in patients with severe psoriasis to assess the clinical activity and safety of rapamycin (sirolimus) alone or in association with a reduced dose of cyclosporine. British Journal of Dermatology 1999;141(5):978-9. [CENTRAL: CN-00428747] [Google Scholar]
Reitamo 2001 {published data only}
- Reitamo S, Spuls P, Sassolas B, Lahfa M, Claudy A, Griffiths CE, et al. Efficacy of sirolimus (rapamycin) administered concomitantly with a subtherapeutic dose of cyclosporin in the treatment of severe psoriasis: a randomized controlled trial. British Journal of Dermatology 2001;145(3):438-45. [CENTRAL: CN-00356242] [DOI] [PubMed] [Google Scholar]
Rim 2003 {published data only}
- Rim JH, Park JY, Choe YB, Youn JI. The efficacy of calcipotriol + acitretin combination therapy for psoriasis: comparison with acitretin monotherapy. American Journal of Clinical Dermatology 2003;4(7):507-10. [CENTRAL: CN-00450180] [DOI] [PubMed] [Google Scholar]
Rinsho Iyaku 1991 {published data only}
- Clinical Study Group for Ciclosporin. Clinical efficacy of ciclosporin in the treatment of psoriasis: multicenter double blind study. Rincho Iyaku (Journal of Clinical Therapeutics and Medicines) 1991;7(3):617-33. [CENTRAL: CN-00545330] [Google Scholar]
Ritchlin 2006a {published data only}
- Ritchlin CT. The efficacy and safety of adalimumab in psoriatic arthritis. Current Rheumatology Reports 2006;8(5):329. [CENTRAL: CN-00898612] [DOI] [PubMed] [Google Scholar]
Ritchlin 2006b {published data only}
- Ritchlin CT. The efficacy and safety of alefacept in psoriatic arthritis. Current Rheumatology Reports 2006;8(5):330-1. [CENTRAL: CN-00898611] [DOI] [PubMed] [Google Scholar]
Romiti 2017 {published data only}
- Romiti R, Papadimitropoulos M, Lin C, Burge RT, Zhu B, Garcia EG. Ixekizumab treatment improves itching and health-related quality (HRQOL) of life in psoriasis patients in Latin America. Value in Health 2017;20(9):A807. [CENTRAL: CN-01431329] [Google Scholar]
RPCEC00000201 {unpublished data only}
- RPCEC00000201. Itolizumab for moderate-to-severe psoriasis-phase 3 [Randomized controlled double blind trial to study safety and efficacy of itolizumab (antiCD6) in moderate-to-severe psoriasis]. registroclinico.sld.cu/trials/RPCEC00000201-En (first received 15 October 2015). [CENTRAL: CN-01835365]
Ryan 2018 {published data only}
- Ryan C, Menter A, Guenther L, Blauvelt A, Bissonnette R, Meeuwis K, et al. Efficacy and safety of ixekizumab in a randomized, double-blinded, placebo-controlled phase IIIb study of patients with moderate-to-severe genital psoriasis. British Journal of Dermatology 2018;179(4):844-52. [CENTRAL: CN-01630323] [DOI] [PubMed] [Google Scholar]
Saeki 2017 {published data only}
- Saeki H, Nakagawa H, Nakajo K, Ishii T, Morisaki Y, Aoki T, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). Journal of Dermatology 2017;44(4):355-62. [DOI: 10.1111/1346-8138.13622] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salim 2006 {published data only}
- Salim A, Tan E, Ilchyshyn A, Berth-Jones J. Folic acid supplementation during treatment of psoriasis with methotrexate: a randomized, double-blind, placebo-controlled trial. British Journal of Dermatology 2006;154(6):1169-74. [CENTRAL: CN-00565411] [DOI] [PubMed] [Google Scholar]
Scholl 1981 {published data only}
- Scholl E. Treatment of psoriasis on an outpatient-base using UVB-radiations, oral retinoid and ten percent saline baths. Schweizerische Rundschau für Medizin Praxis 1981;70(41):1806-16. [CENTRAL: CN-00026632] [PubMed] [Google Scholar]
Schopf 1998 {published data only}
- Schopf RE, Hultsch T, Lotz J, Brautigam M. Eosinophils, pruritus and psoriasis: effects of treatment with etretinate or cyclosporin-A. Journal of the European Academy of Dermatology and Venereology 1998;11(3):234-9. [CENTRAL: CN-00158727] [PMID: ] [PubMed] [Google Scholar]
Schulze 1991 {published data only}
- Schulze HJ. Comparative trial of sandimmune and etretinate for plaque-type psoriasis. Zeitschrift fur Hautkrankheiten 1991;66(Suppl 1):33-8. [CENTRAL: CN-00180765] [Google Scholar]
Shintani 2011 {published data only}
- Shintani Y, Kaneko N, Furuhashi T, Saito C, Morita A. Safety and efficacy of a fixed-dose cyclosporin microemulsion (100 mg) for the treatment of psoriasis. Journal of Dermatology 2011;38(10):966-72. [CENTRAL: CN-00811861] [DOI] [PubMed] [Google Scholar]
Shiohara 1992 {published data only}
- Shiohara T, Imanishi K, Sagawa Y, Nagashima M. Differential effects of cyclosporine and etretinate on serum cytokine levels in patients with psoriasis. Journal of the American Academy of Dermatology 1992;27(4):568-74. [CENTRAL: CN-00361111] [DOI] [PubMed] [Google Scholar]
Shupack 1997 {published data only}
- Shupack J, Abel E, Bauer E, Brown M, Drake L, Freinkel R, et al. Cyclosporine as maintenance therapy in patients with severe psoriasis. Journal of the American Academy of Dermatology 1997;36(3 pt 1):423-32. [CENTRAL: CN-00137758] [DOI] [PubMed] [Google Scholar]
Simonova 2005 {published data only}
- Simonova OV, Nemtsov BF. Psoriatic arthritis: combined treatment with prospidin and methotrexate. Terapevticheskii Arkhiv 2005;77(8):60-4. [CENTRAL: CN-00530903] [PubMed] [Google Scholar]
Sinclair 2017 {published data only}
- Sinclair R, Reich K, Papp K, Tyring SS, Thaçi D, Cichanowitz N, et al. Tildrakizumab, a selective IL-23p19 antibody, in the treatment of chronic plaque psoriasis: results from two randomised, controlled, phase 3 trials. Australasian Journal of Dermatology 2017;58(S1):9-10. [CENTRAL: CN-01378810] [DOI: 10.1111/ajd.12652] [DOI] [Google Scholar]
Sofen 2011 {published data only}
- Sofen H, Smith S, Matheson R, Leonardi C, Calderon C, Bouman-Thio E, et al. Results of a single ascending dose study to assess the safety and tolerability of CNTO 1959 following intravenous or subcutaneous administration in healthy subjects and in subjects with moderate to severe psoriasis. British Journal of Dermatology 2011;165(6):e10. [CENTRAL: CN-01020427] [Google Scholar]
Sofen 2014 {published data only}
- Sofen H, Smith S, Matheson RT, Leonardi CL, Calderon C, Brodmerkel C, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. Journal of Allergy and Clinical Immunology 2014;133(4):1032-40. [CENTRAL: CN-00984656] [DOI] [PubMed] [Google Scholar]
Soung 2022 {published data only}
- Soung J, Cather JC, Gooderham M. 33054 Improvement in touch avoidance in patients with genital psoriasis treated with ixekizumab: 52-week results of a phase 3 clinical trial in patients with moderate-to-severe genital psoriasis (IXORA-Q). Journal of the American Academy of Dermatology 2022;87(3 Suppl):AB69. [Google Scholar]
Spadaro 2008 {published data only}
- Spadaro A, Ceccarelli F, Scrivo R, Valesini G. Life-table analysis of etanercept with or without methotrexate in patients with psoriatic arthritis. Annals of the Rheumatic Diseases 2008;67(11):1650-1. [CENTRAL: CN-00651403] [DOI] [PubMed] [Google Scholar]
Spuls 2012 {published data only}
- Spuls PI, Hooft L. Brodalumab and ixekizumab, anti-interleukin-17-receptor antibodies for psoriasis: a critical appraisal. British Journal of Dermatology 2012;167(4):710-3; discussion 714-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stein Gold 2018 {published data only}
- Stein Gold L, Bagel J, Lebwohl M, Jackson JM, Chen R, Goncalves J, et al. Efficacy and safety of apremilast in systemic- and biologic-naive patients with moderate plaque psoriasis: 52-week results of UNVEIL. Journal of Drugs in Dermatology 2018;17(2):221-8. [CENTRAL: CN-01627309] [PubMed] [Google Scholar]
Stein Gold 2021 {published data only}
- Stein Gold L, Papp K, Pariser D, Green L, Bhatia N, Sofen H, et al. Efficacy and safety of apremilast in patients with mild-to-moderate plaque psoriasis: results of a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Journal of the American Academy of Dermatology 2021;31:31. [DOI] [PubMed] [Google Scholar]
Sticherling 1994 {published data only}
- Sticherling M. Therapy of severe psoriasis with Sandimmune. Hautarzt (Symposium of Nurnberg Sandoz AG 13 February 1993, Nurnberg) 1994;45(1):50-2. [PMID: ] [PubMed] [Google Scholar]
Strober 2004 {published data only}
- Strober BE, Clarke S. Etanercept for the treatment of psoriasis: combination therapy with other modalities. Journal of Drugs in Dermatology 2004;3(3):270-2. [PMID: ] [PubMed] [Google Scholar]
Strober 2012 {published data only}
- Strober BE, Sobell JM, Duffin KC, Bao Y, Guerin A, Yang H, et al. Sleep quality and other patient-reported outcomes improve after patients with psoriasis with suboptimal response to other systemic therapies are switched to adalimumab: results from PROGRESS, an open-label phase IIIB trial. British Journal of Dermatology 2012;167(6):1374-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Strober 2017a {published data only}
- Strober B, Gottlieb AB, Sherif B, Mollon P, Gilloteau I, McLeod L, et al. Secukinumab sustains early patient-reported outcome benefits through 1 year: results from 2 phase III randomized placebo-controlled clinical trials comparing secukinumab with etanercept. Journal of the American Academy of Dermatology 2017;76(4):655-61. [CENTRAL: CN-01368337] [DOI: 10.1016/j.jaad.2016.11.043] [DOI] [PubMed] [Google Scholar]
Strober 2017b {published data only}
- Strober B, Bagel J, Lebwohl M, Stein Gold L, Jackson JM, Chen R, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. Journal of Drugs in Dermatology 2017;16(8):801-8. [CENTRAL: CN-01416087] [PubMed] [Google Scholar]
Strober 2017c {published data only}
- Strober B, Alikhan M, Lockshin B, Schafer P. Cytokine effects of apremilast as a mechanism of efficacy in systemic-naive patients with moderate plaque psoriasis: results from the UNVEIL trial. British Journal of Dermatology 2017;177(5):e256-7. [CENTRAL: CN-01452501] [DOI: 10.1111/bjd.16059] [DOI] [PubMed] [Google Scholar]
Strober 2018 {published data only}
- Strober B, Forman S, Bagel J, Lebwohl M, Stein Gold L, Jackson JM, et al. Efficacy and safety of apremilast in systemic- and biologic-naive patients with moderate plaque psoriasis (52-week results of the UNVEIL study). Journal of Clinical and Aesthetic Dermatology 2018;11(5 Suppl 1):S21-2. [CENTRAL: CN-01713688] [PubMed] [Google Scholar]
Sun 2019 {published data only}
- Sun J, Chen G, Wu M, Han Y, Gao H, Zhang T, et al. China-manufactured adalimumab biosimilar, HLX03, demonstrated pharmacokinetic equivalence and comparable safety to adalimumab. Annals of the Rheumatic Diseases 2019;78 Suppl 2:706. [Google Scholar]
Sweetser 2006 {published data only}
- Sweetser M, Ticho B, Swan S. Subcutaneous administration of alefacept is bioequivalent to intramuscular administration: results of a randomized, open-label, crossover study in healthy volunteers. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB224. [CENTRAL: CN-00602513] [PubMed] [Google Scholar]
Syversen 2020 {published data only}
- Syversen SW, Goll GL, Jorgensen KK, Olsen IC, Sandanger O, Gehin JE, et al. Therapeutic drug monitoring of infliximab compared to standard clinical treatment with infliximab: study protocol for a randomised, controlled, open, parallel-group, phase IV study (the NOR-DRUM study). Trials 2020;21(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Talamonti 2021 {published data only}
- Talamonti M, Malara G, Natalini Y, Bardazzi F, Conti A, Chiricozzi A, et al. Secukinumab improves anxiety and depression in patients with moderate-to-severe psoriasis: results from the SUPREME study. Australasian Journal of Dermatology 2021;62 Suppl 1:27-8. [Google Scholar]
Talwar 1992 {published data only}
- Talwar S. Methotrexate-puvasol combination in treatment of psoriasis. Indian Journal of Dermatology, Venereology and Leprology 1992;58(1):15-9. [CENTRAL: CN-00663131] [Google Scholar]
TCTR20190705002 {published data only}
- TCTR20190705002. Comparison of the clinical efficacy of subcutaneous versus oral administration of methotrexate in patients with psoriasis vulgaris. www.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20190705002 (first received 4 July 2019).
Tejasvi 2012 {published data only}
- Tejasvi T, Chow C, Simpson MJ, Ellis CN. Use of clinical trial data to compare psoriasis area and severity index, static physician's global assessment, and lattice system-physician's global assessment in assessing severity of psoriasis. Dermatology and Therapy 2012;2:S55. [CENTRAL: 71025691] [Google Scholar]
Thaçi 2002 {published data only}
- Thaçi D, Bräutigam M, Kaufmann R, Weidinger G, Paul C, Christophers E. Body-weight-independent dosing of cyclosporine micro-emulsion and three times weekly maintenance regimen in severe psoriasis. A randomised study. Dermatology (Basel, Switzerland) 2002;205(4):383-8. [CENTRAL: CN-00411587] [DOI] [PubMed] [Google Scholar]
Thaçi 2010 {published data only}
- Thaçi D, Ortonne JP, Chimenti S, Ghislain PD, Arenberger P, Kragballe K, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. British Journal of Dermatology 2010;163(2):402-11. [CENTRAL: CN-00771848] [DOI] [PubMed] [Google Scholar]
Thaçi 2018 {published data only}
- Thaçi D, Gottlieb AB, Reich K, Bagel J, Peterson L, Purcaru O, et al. Certolizumab pegol improves patient-reported outcomes in chronic plaque psoriasis over 1 year. Acta Dermato-Venereologica 2018;98(Suppl 219):57-8. [CENTRAL: CN-01620168] [DOI: 10.2340/00015555-2978] [DOI] [Google Scholar]
Tong 2008 {published data only}
- Tong PZ, Si RL. Effectiveness observation on acitretin capsule for plaque psoriasis. Modern Journal of Integrated Traditional Chinese and Western Medicine [xian Dai Zhong Xi Yi Jie He za Zhi] 2008;17(3):364-5. [CENTRAL: CN-00792764] [Google Scholar]
Tsakok 2018 {published data only}
- Tsakok T, Jabbar-Lopez ZK, Smith CH. Subcutaneous methotrexate in patients with moderate-to-severe psoriasis: a critical appraisal. British Journal of Dermatology 2018;179(1):50-3. [CENTRAL: CN-01742390] [DOI] [PubMed] [Google Scholar]
Vaclavkova 2014 {published data only}
- Chimenti S, Arenberger P, Karpati S, Sator PG, Vaclavkova A, Burcklen M, et al. A phase II study of ponesimod, an oral, selective sphingosine 1-phosphate receptor-1 modulator, in chronic plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2013;27(Suppl 4):22. [CENTRAL: CN-01025267] [PMID: 23822555]23822555 [Google Scholar]
- Kemeny L, Yankova R, Talamonti M, Vaclavkova A, Burcklen M, Thomas G, et al. A phase II study of ponesimod in chronic plaque psoriasis: improvements in patient-reported outcomes. Journal of the European Academy of Dermatology and Venereology 2013;27(Suppl 4):21. [CENTRAL: CN-01025268] [PMID: 23822555]23822555 [Google Scholar]
- Vaclavkova A, Chimenti S, Arenberger P, Hollo P, Sator PG, Burcklen M, et al. Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet 2014;384(9959):2036-45. [CENTRAL: CN-01040467] [PMID: ] [DOI] [PubMed] [Google Scholar]
Valenzuela 2017 {published data only}
- Valenzuela F, De la Cruz Fernandez C, Galimberti RL, Gurbuz S, McKean-Matthews M, Goncalves L, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis: subgroup analysis of Latin American patients in the phase 3 randomized UNCOVER-3 study. Actas Dermo-Sifiliograficas 2017;108(6):550-63. [CENTRAL: CN-01464996] [DOI] [PubMed] [Google Scholar]
Van de Kerkhof 2017 {published data only}
- Van de Kerkhof P, Guenther L, Gottlieb AB, Sebastian M, Wu JJ, Foley P, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled and open-label phases of UNCOVER-3. Journal of the European Academy of Dermatology and Venereology 2017;31(3):477-82. [CENTRAL: CN-01413633] [DOI: 10.1111/jdv.14033] [DOI] [PubMed] [Google Scholar]
Van Joost 1988 {published data only}
- Van Joost T, Bos JD, Heule F, Meinardi MM. Low-dose cyclosporin A in severe psorasis. A double-blind study. British Journal of Dermatology 1988;118(2):183-90. [CENTRAL: CN-00052789] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vena 2005 {published data only}
- Vena GA, Cassano N, Galluccio A, Loconsole F, Coviello C, Fai D, et al. Evaluation of the efficacy and tolerability of a new intermittent treatment regimen with cyclosporin A in severe psoriasis. Giornale Italiano di Dermatologia e Venereologia 2005;140(5):575-82. [EMBASE: 2006301575] [Google Scholar]
Vena 2012 {published data only}
- Vena GA, Galluccio A, Pezza M, Vestita M, Cassano N. Combined treatment with low-dose cyclosporine and calcipotriol/betamethasone dipropionate ointment for moderate-to-severe plaque psoriasis: a randomized controlled open-label study. Journal of Dermatological Treatment 2012;23(4):255-60. [CENTRAL: CN-00882673] [DOI] [PubMed] [Google Scholar]
Verma 2021 {published data only}
- Verma KK, Kumar P, Bhari N, Gupta S, Kalaivani M. Azathioprine weekly pulse versus methotrexate for the treatment of chronic plaque psoriasis: a randomized controlled trial. Indian Journal of Dermatology, Venereology and Leprology 2021;87(4):509-14. [DOI] [PubMed] [Google Scholar]
Viglioglia 1978 {published data only}
- Viglioglia PA, Barclay A. Oral retinoids and psoriasis. Dermatologica 1978;157(Suppl 1):32-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Viswanath 2022 {published data only}
- Viswanath V, Joshi P, Lawate P, Tare D, Dhoot D, Mahadkar N, et al. An open-label, randomized, prospective, comparative, three-arm clinical trial to evaluate the safety and effectiveness of apremilast with three different titration methods in patients with chronic plaque psoriasis in India. Psoriasis: Targets and Therapy 2022;12:53-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Witkamp 1995 {published data only}
- Witkamp L, Zonneveld IM, Jung EG, Schopf RE, Christophers E, Grossman R, et al. Efficacy and tolerability of multiple-dose SDZ IMM 125 in patients with severe psoriasis. British Journal of Dermatology 1995;133(1):95-103. [CENTRAL: CN-00118050] [DOI] [PubMed] [Google Scholar]
Wolf 2012 {published data only}
- Wolf P, Weger W, Legat FJ, Posch-Fabian T, Gruber-Wackernagel A, Inzinger M, et al. Treatment with 311-nm ultraviolet B enhanced response of psoriatic lesions in ustekinumab-treated patients: a randomized intraindividual trial. British Journal of Dermatology 2012;166(1):147-53. [CENTRAL: CN-00841290] [DOI] [PubMed] [Google Scholar]
Wright 1966 {published data only}
- Wright ET, Wolborsky M, Hamer EE. Human low-dosage parenteral methotrexate therapy. A controlled toxicity study. Archives of Dermatology 1966;93(6):731-6. [PMID: ] [PubMed] [Google Scholar]
Wu 2015 {published data only}
- Wu C, Jin HZ, Shu D, Li F, He CX, Qiao J, et al. Efficacy and safety of tripterygium wilfordii Hook F versus acitretin in moderate to severe psoriasis vulgaris: a randomized clinical trial. Chinese Medical Journal 2015;128(4):443-9. [CENTRAL: CN-01047537] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yan 2011 {published data only}
- Yan H, Tang M, You Y, Yu JB, Zhang JA, Li XH, et al. Treatment of psoriasis with recombinant human LFA3-antibody fusion protein: a multi-center, randomized, double-blind trial in a Chinese population. European Journal of Dermatology 2011;21(5):737-43. [CENTRAL: CN-00810848] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yesudian 2013 {published data only}
- Yesudian PD, Hashim N, Bharati A, Alkali A, Warren RB, Cox T, et al. A prospective, double-blind, randomized controlled trial of folic acid supplementation vs. placebo in patients with chronic plaque psoriasis treated with methotrexate and effects on serum homocysteine. British Journal of Dermatology 2013;169(Suppl 1):59. [CENTRAL: CN-00873113] [Google Scholar]
Yiu 2020 {published data only}
- Yiu ZZN. Repurposing existing trial data to infer relative efficacy of biologics: guselkumab vs. ustekinumab for psoriasis. British Journal of Dermatology 2020;183(2):202-3. [DOI] [PubMed] [Google Scholar]
Yoon 2007 {published data only}
- Yoon HS, Youn JI. A comparison of two cyclosporine dosage regimens for the treatment of severe psoriasis. Journal of Dermatological Treatment 2007;18(5):286-90. [CENTRAL: CN-00619337] [DOI] [PubMed] [Google Scholar]
Yosipovitch 2018 {published data only}
- Yosipovitch G, Foley P, Ryan C, Cather JC, Meeuwis KA, Burge R, et al. Ixekizumab improved patient-reported genital psoriasis symptoms and impact of symptoms on sexual activity vs placebo in a randomized, double-blind study. Journal of Sexual Medicine 2018;15(11):1645-52. [CENTRAL: CN-01667932] [DOI] [PubMed] [Google Scholar]
Zachariae 2008 {published data only}
- Zachariae C, Mork NJ, Reunala T, Lorentzen H, Falk E, Karvonen SL, et al. The combination of etanercept and methotrexate increases the effectiveness of treatment in active psoriasis despite inadequate effect of methotrexate therapy. Acta Dermato-Venereologica 2008;88(5):495-501. [CENTRAL: CN-00669633] [DOI] [PubMed] [Google Scholar]
Zhang 2007 {published data only}
- Zhang M, Zhang Y-Z, Wang S. The effect of acitretin on moderate to severe plaque psoriasis. Journal of Clinical Dermatology 2007;36(9):592-3. [CENTRAL: CN-00642111] [Google Scholar]
Zhang 2009a {published data only}
- Zhang GL, Huang F, Zhang JL, Li XF. A clinical study of leflunomide and methotrexate therapy in psoriatic arthritis. Chung-Hua Nei Ko Tsa Chih (Chinese Journal of Internal Medicine) 2009;48(7):570-4. [CENTRAL: CN-00732533] [PubMed] [Google Scholar]
Zhang 2009b {published data only}
- Zhang LX, Bai YP, Song PH, You LP, Yang DQ. Effect of Chinese herbal medicine combined with acitretin capsule in treating psoriasis of blood-heat syndrome type. Chinese Journal of Integrative Medicine 2009;15(2):141-4. [CENTRAL: CN-00700202] [DOI] [PubMed] [Google Scholar]
Zhang 2017 {published data only}
- NCT01815424. A study evaluating the efficacy and safety of CP-690,550 in Asian subjects with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct01815424 (first received 21 March 2013). [CENTRAL: CN-01541153]
- Zhang J, Tsai TF, Lee MG, Zheng M, Wang G, Jin H, et al. The efficacy and safety of tofacitinib in Asian patients with moderate to severe chronic plaque psoriasis: a phase 3, randomized, double-blind, placebo-controlled study. Journal of Dermatological Science 2017;88(1):36-45. [CENTRAL: CN-01604532] [DOI] [PubMed] [Google Scholar]
Zhang 2020 {published data only}
- Zhang C, Yan K, Diao Q, Guo Q, Jin H, Yang S, et al. A multi-center, randomized, double-blind, placebo-controlled dose-ranging study evaluating efficacy and safety of SHR-1314 in subjects with moderate-to-severe plaque psoriasis. Arthritis & Rheumatology 2020;72 Suppl 10:1730-3. [Google Scholar]
Zhang 2022 {published data only}
- Zhang C, Yan K, Diao Q, Guo Q, Jin H, Yang S, et al. A multicenter, randomized, double-blinded, placebo-controlled, dose-ranging study evaluating the efficacy and safety of vunakizumab in patients with moderate-to-severe plaque psoriasis. Journal of the American Academy of Dermatology 2022;87(1):95-102. [DOI] [PubMed] [Google Scholar]
Zhu 2009 {published data only}
- Zhu Y, Hu C, Lu M, Liao S, Marini JC, Yohrling J, et al. Population pharmacokinetic modeling of ustekinumab, a human monoclonal antibody targeting IL-12/23p40, in patients with moderate to severe plaque psoriasis. Journal of Clinical Pharmacology 2009;49(2):162-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhuang 2016 {published data only}
- Zhuang Y, Calderon C, Marciniak SJ Jr, Bouman-Thio E, Wasfi Y, Szapary P, et al. First-in-human study to assess guselkumab (anti-IL-23 mAb) pharmacokinetics/safety in healthy subjects and patients with moderate-to-severe psoriasis. European Journal of Clinical Pharmacology 2016;72(11):1303-10. [CENTRAL: CN-01307156] [DOI] [PubMed] [Google Scholar]
Zobel 1987 {published data only}
- Zobel AF. Cyclosporin is being tested for treatment of psoriasis. American Druggist 1987;195(3):102. [EMBASE: 1987106040] [Google Scholar]
References to studies awaiting assessment
ChiCTR2000034243 {published data only}
- ChiCTR2000034243. Phase III clinical trial to evaluate efficacy and safety of HS626 and remicade in patients with chronic moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000034243 (first received 29 June 2020).
Chow 2015 {published data only}
- Chow C, Simpson MJ, Luger TA, Chubb H, Ellis CN. Comparison of three methods for measuring psoriasis severity in clinical studies (part 1 of 2): change during therapy in Psoriasis Area and Severity Index, Static Physician's Global Assessment and Lattice System Physician's Global Assessment. Journal of the European Academy of Dermatology and Venereology 2015;29(7):1406-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Chow C, Simpson MJ, Zang Z, Goldfarb MT, Tejasvi T, Ellis CN. Longitudinal effects of active therapy in a clinical trial on Psoriasis Area and Severity Index, Static Physician's Global Assessment and Lattice System-Physician's Global Assessment for assessing severity of psoriasis. British Journal of Dermatology 2011;165(6):e30. [EMBASE: 70610815] [Google Scholar]
- Simpson MJ, Chow C, Morgenstern H, Luger TA, Ellis CN. Comparison of three methods for measuring psoriasis severity in clinical studies (part 2 of 2): use of quality of life to assess construct validity of the Lattice System Physician's Global Assessment, Psoriasis Area and Severity Index and Static Physician's Global Assessment. Journal of the European Academy of Dermatology and Venereology 2015;29(7):1415-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
CTRI/2015/05/005830 {unpublished data only}
- CTRI/2015/05/005830. Role of oral methotrexate, cyclosporine and acitretin in treatment of palmoplantar psoriasis (redcoloured, painful, itchy, fissured lesions on hands and feet) and psoriasis vulgaris (red coloured, scaly, itchy, elevated lesions on skin over body). ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=10246 (first received 29 May 2015).
CTRI/2016/10/007345 {unpublished data only}
- CTRI/2016/10/007345. A randomized, double-blind, placebo-controlled, comparative, prospective, multicentre trial to assess efficacy and safety of apremilast tablets in subjects with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=16164&EncHid=&modid=&compid=%27,%2716164det%27/CTRI/2016/10/007345 (first received 20 October 2016).
CTRI/2020/10/028555 {published data only}
- CTRI/2020/10/028555. Study the efficacy and side effects of subcutaneous v/s oral methotrexate in the management of moderate to severe psoriasis and palmoplantar psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/10/028555 (first received 22 October 2020).
DRKS00000716 {unpublished data only}
- DRKS00000716. Regulatory T-cell function in psoriasis vulgaris. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00000716 (first received 9 February 2011).
EUCTR2010‐020168‐39‐DE {published data only}
- EUCTR2010-020168-39-DE. A randomised, double blind, placebo controlled efficacy and safety trial of different doses/dose regimens of FP187 compared to placebo in moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2010-020168-39-DE (first received 9 June 2010).
EUCTR2015‐005279‐25‐DE {published data only}
- EUCTR2015-005279-25-DE. A research study to evaluate the efficacy of LEO 32731 oral tablet formulation in patients with moderate to severe psoriasis vulgaris. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015-005279-25-DE (first received 10 February 2016).
EUCTR2021‐003700‐41‐ES {published data only}
- EUCTR. A phase 2b multicenter, randomized, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of JNJ-77242113 for the treatment of moderate-to-severe plaque psoriasis - FRONTIER 1: efficacy and safety of JNJ-77242113 in moderate to severe plaque psoriasis. https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2021-003700-41 2021.
Goldust 2019 {published data only}
- Goldust M. Depression and anxiety in patients with moderate-to-severe plaque psoriasis while on methotrexate plus adalimumab vs. methotrexate monotherapy. British Journal of Dermatology 2019;181(Suppl 1):190. [Google Scholar]
Han 2007 {published data only}
- Han L, Fang X, Huang Q, Yang QP, Fu WW, Zheng ZZ, et al. Analysis of the effect of recombinant human tumor necrosis factor receptor in the treatment of moderate to severe plaque psoriasis on PASI score. Journal of Clinical Dermatology 2007;36(11):730-2. [CENTRAL: CN-00708092] [Google Scholar]
JPRN‐jRCT2061210069 {published data only}
- JPRN-jRCT2061210069. A study to assess adverse events and disease activity with cedirogant (ABBV-157) in adult participants with moderate to severe psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-jRCT2061210069 (first received 14 January 2022).
KEEPsAKE‐1 {published data only}
- Papp K, Soliman AM, Kaufmann C, Barcomb L, Wang Z, White D, et al. 33236 Impact of risankizumab on improving symptoms and health-related quality of life and reducing fatigue and pain among psoriatic arthritis patients with moderate-to-severe skin involvement: evidence from 2 phase III trials. Journal of the American Academy of Dermatology 2022;87(3 Suppl):AB174. [Google Scholar]
KEEPsAKE‐2 {published data only}
- NCT03671148. A study comparing risankizumab to placebo in participants with active psoriatic arthritis including those who have a history of inadequate response or intolerance to biologic therapy(ies) (KEEPsAKE2). https://clinicaltrials.gov/ct2/show/NCT03671148 (first received 17 October 2022).
Krishna 2016 {unpublished data only}
- Krishna C, Parmar N. Improvement in the quality of life of patients with severe plaque psoriasis treated with systemic methotrexate: a prospective, randomized, open-label, parallel group study in 60 patients. Journal of the Dermatology Nurses' Association (Conference: 24th World Congress of Dermatology, Italy) 2020;12(2):No pagination. [Google Scholar]
- Krishna CV, Rao AV. Improvement in the quality of life of patients with severe plaque psoriasis treated with systemic methotrexate in fixed doses of 10 mg or 25 mg orally once weekly: a prospective, randomized, double-blind, parallel-group study. British Journal of Dermatology 2016;175(S1):65-6. [Google Scholar]
- NCT02248792. Quality of life of patients with psoriasis treated with methotrexate: prospective, randomized, double-blind, parallel group study. clinicaltrials.gov/ct2/show/NCT02248792 (first received 22 September 2014).
Makavos 2020 {published data only}
- Makavos G, Ikonomidis I, Andreadou I, Varoudi M, Kapniari I, Loukeri E, et al. Effects of interleukin 17A Inhibition on myocardial deformation and vascular function in psoriasis. Canadian Journal of Cardiology 2020;36(1):100-11. [DOI] [PubMed] [Google Scholar]
Mrowietz 2005 {published data only}
- Mrowietz U, Spellman M. Dimethyl fumarate (BG00012) as an oral therapy for moderate to severe psoriasis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Journal of Investigative Dermatology 2005;125(3 Suppl):A69. [CENTRAL: CN-00792615] [Google Scholar]
- Mrowietz U, Spellman MC. Results of a phase III study of a novel oral formulation of dimethylfumarate in the treatment of moderate to severe plaque psoriasis: efficacy, safety, and quality of life effects. Journal of the European Academy of Dermatology and Venereology 2005;19(Suppl 2):187. [CENTRAL: CN-00602493] [Google Scholar]
- Ortonne JP, Van de Kerkhof P, Mrowietz U. A novel oral agent improves quality of life (QOL) in patients with plaque psoriasis. In: 4th European Association of Dermatology and Venereology (EADV) Spring Symposium; 9-12 February 2006; Saariselka, Lapland, Finland. 2006:P-013. [CENTRAL: CN-00602214]
NCT01088165 {published data only}
- NCT01088165. The influence of adalimumab on cardiovascular and metabolic risk in psoriasis. clinicaltrials.gov/show/nct01088165 (first received 17 March 2010).
NCT01558310 {unpublished data only}
- NCT01558310. A study to evaluate the effectiveness of Stelara™ (ustekinumab) in the treatment of scalp psoriasis. clinicaltrials.gov/ct2/show/NCT01558310 (first received 20 March 2012).
NCT02655705 {unpublished data only}
- NCT02655705. Comparison study of psoriasis severity assessment tools. clinicaltrials.gov/ct2/show/NCT02655705 (first received 4 January 2016).
NCT02701205 {published data only}
- NCT02701205. Safety and efficacy study of etanercept (Qiangke®) to treat moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct02701205 (first received 8 March 2016).
NCT02714322 {published data only}
- EUCTR2014-003420-46-BG. A study to evaluate the similarity in efficacy and safety of mylan adalimumab (MYL-1401A) compared with humira® in subjects with moderate-to-severe chronic skin inflammatory disease. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014-003420-46-BG (first received 19 June 2015).
- NCT02714322. MYL-1401A efficacy and safety comparability study to humira®. clinicaltrials.gov/show/nct02714322 (first received 21 March 2016).
NCT02829424 {published data only}
- NCT02829424. Multicenter randomized double blind controlled-study to assess the potential of methotrexate versus placebo to improve and maintain response to anti TNF- alpha agents in adult patients with moderate to severe psoriasis. clinicaltrials.gov/show/nct02829424 (first received 12 July 2016).
References to ongoing studies
Alexis 2022 {published data only}
- Alexis A, Bhutani T, McMichael AJ, Choi O, Chan D, Rowland K, et al. 694 Study design of a phase 3b, multicenter, randomized, double-blind, placebo-controlled trial of guselkumab (GUS) in patients with skin of color who have moderate to severe plaque and/or scalp psoriasis (VISIBLE). Journal of Investigative Dermatology 2022;142(8 Suppl):S119. [Google Scholar]
- NCT05272150. Study of guselkumab in skin of color participants with moderate-to-severe plaque and/or scalp psoriasis. clinicaltrials.gov/show/NCT05272150 (first received 9 March 2022).
ChiCTR2000029262 {published data only}
- ChiCTR2000029262. Clinical study on the relationship between SLC35 gene variation and psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000029262 (first received 20 January 2020).
ChiCTR2000036186 {published data only}
- ChiCTR2000036186. A multi-center clinical study of systemic treatment strategies for psoriasis in Chinese population. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000036186 (first received 21 August 2020).
ChiCTR2000039699 {published data only}
- ChiCTR2000039699. A phase 4 clinical study to evaluate the efficacy and safety of induction and maintenance therapy of brodalumab (KHK4827) in subjects with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000039699 (first received 6 November 2020).
ChiCTR2100045970 {published data only}
- ChiCTR2100045970. A phase 3,multicenter,randomized,double-blind study evaluating the efficacy and safety of QX001S compared with ustekinumab in subjects with moderate to severe plaque psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2100045970 (first received 30 April 2021).
CTRI/2019/07/020274 {published data only}
- CTRI/2019/07/020274. To compare the effect of three drugs- methotrexate, apremilast and their combination in patients suffering from psoriasis vulgaris. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/07/020274 (first received 17 July 2019).
CTRI/2020/02/023107 {published data only}
- CTRI/2020/02/023107. A comparative clinical study to evaluate efficacy and safety of test drug in patients with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/02/023107 (first received 4 February 2020).
CTRI/2022/01/039825 {published data only}
- CTRI/2022/01/039825. Comparison of drug methotrexate against drugs methotrexate with apremilast in psoriasis disorder. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2022/01/039825 (first received 31 January 2022).
Dong 2020 {published data only}
- Dong W, Nie X, Wang J, Xia L, Cai L, Wang W, et al. Randomised, double-blind, multicentre, phase I/II dose escalation and expansion trial of GR1501 in patients with plaque psoriasis: study protocol. BMJ Open 2020;10(11):e039067. [DOI] [PMC free article] [PubMed] [Google Scholar]
EUCTR2017‐001615‐36‐DE {published data only}
- EUCTR2017-001615-36-DE. A double-blind study in subjects with moderate to severe plaque psoriasis to evaluate efficacy, safety, tolerability of four different dose levels of ABY-035 compared to placebo. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2017-001615-36-DE (first received 7 September 2017).
EUCTR2017‐001695‐26‐IT {published data only}
- EUCTR2017-001695-26-IT. Effectiveness and safety of biological drugs in psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2017-001695-26-IT (first received 4 November 2020).
EUCTR2018‐001238‐16‐FR {published data only}
- EUCTR2018-001238-16-FR. A study to evaluate further therapeutic strategies with guselkumab in patients with moderate-to-severe plaque-type psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018-001238-16-FR (first received 10 July 2019).
- Eyerich K, Weisenseel P, Pinter A, Schakel K, Asadullah K, Wegner S, et al. IL-23 blockade with guselkumab potentially modifies psoriasis pathogenesis: rationale and study protocol of a phase 3b, randomised, double-blind, multicentre study in participants with moderate-to-severe plaque-type psoriasis (GUIDE). BMJ Open 2021;11(9):no pagination. [DOI] [PMC free article] [PubMed] [Google Scholar]
EUCTR2020‐005205‐42‐DE {published data only}
- EUCTR2020-005205-42-DE. A study to investigate interchangeability of ABP 654 for the treatment of subjects with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2020-005205-42-DE (first received 16 March 2021).
EUCTR2021‐003209‐22‐DE {published data only}
- EUCTR2021-003209-22-DE. Study to assess the efficacy and safety of orismilast in psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2021-003209-22-DE (first received 8 October 2021).
Holsken 2021 {published data only}
- DRKS00022104. Influence of psychological factors on the response to therapy with secukinumab in psoriasis patients on the subjective and objective level. www.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00022104 (first received 9 June 2020).
- Holsken S, Krefting F, Schedlowski M, Sondermann W. Expectation-induced enhancement of pain, itch and quality of life in psoriasis patients: study protocol of a randomised controlled trial. BMJ Open 2021;11(9):e047099. [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT20100102002954N26 {published data only}
- IRCT20100102002954N26. Comparison of the effect of adalimumab and methotrexate in the treatment of psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20100102002954N26 (first received 28 February 2022).
IRCT20120524009844N8 {published data only}
- IRCT20120524009844N8. Therapeutic effects of adalimumab in patients with resistant psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20120524009844N8 (first received 20 October 2020).
NCT02258282 {unpublished data only}
- NCT02258282. Safety and efficacy of etanercept in patients with psoriasis. clinicaltrials.gov/ct2/show/NCT02258282 (first received 7 October 2014).
NCT03478280 {published data only}
- NCT03478280. Effect of brodalumab compared to placebo on vascular inflammation in moderate-to-severe psoriasis. clinicaltrials.gov/show/nct03478280 (first received 27 March 2018).
NCT03897075 {published data only}
- NCT03897075. Efficacy and safety study of tildrakizumab in the treatment of nail psoriasis. clinicaltrials.gov/show/nct03897075 (first received 1 April 2019).
NCT03897088 {published data only}
- NCT03897088. Efficacy and safety of tildrakizumab in the treatment of scalp psoriasis. clinicaltrials.gov/show/nct03897088 (first received 1 April 2019).
NCT03927352 {published data only}
- NCT03927352. Efficacy and safety of SCT630 and adalimumab (HUMIRA®) in adults with plaque psoriasis. clinicaltrials.gov/show/nct03927352 (first received 25 April 2019).
NCT04102241 {published data only}
- NCT04102241. Efficacy and safety study of hemay005 in subjects with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT04102241 (first received 25 September 2019).
NCT04237116 {published data only}
- EUCTR2019-003168-37-DE. Study in patients with plaque psoriasis with coexisting non-alcoholic fatty liver disease. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2019-003168-37-DE (first received 24 October 2019).
- NCT04237116. A study of secukinumab treatment in patients with plaque psoriasis and coexisting non-alcoholic fatty liver disease (NAFLD) (pINPOINt). clinicaltrials.gov/show/NCT04237116 (first received 23 January 2020).
NCT04306315 {published data only}
- EUCTR2017-004998-13-FR. Adjusted brodalumab dose compared with standard brodalumab dose in subjects with moderate-to-severe plaque psoriasis and ≥120 kg body weight. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2017-004998-13-FR (first received 10 April 2020).
- NCT04306315. Adjusted brodalumab dose compared with standard brodalumab dose in subjects with moderate-to-severe plaque psoriasis and ≥120 kg body weight. clinicaltrials.gov/show/NCT04306315 (first received 12 March 2020).
NCT04394936 {published data only}
- EUCTR2019-002383-27-NL. An explorative psoriasis biomarker study. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2019-002383-27-NL (first received 13 June 2019).
- NCT04394936. An explorative psoriasis biomarker study. clinicaltrials.gov/show/NCT04394936 (first received 20 May 2020).
NCT04453137 {published data only}
- EUCTR2019-002911-25-PL. Multicenter, double-blind, randomized, parallel-group, study evaluating pharmacokinetic, efficacy, safety, and immunogenicity between patients with moderate to severe chronic plaque psoriasis receiving humira® and patients undergoing repeated switches between humira® and AVT02 followed by a safety extension phase of AVT02. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2019-002911-25-PL (first received 28 June 2020).
- NCT04453137. Pharmacokinetic, efficacy, safety, and immunogenicity of AVT02 with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/NCT04453137 (first received 1 July 2020).
NCT04533737 {published data only}
- NCT04533737. Efficacy and safety of brodalumab compared with guselkumab in the treatment of plaque psoriasis after inadequate response to ustekinumab (COBRA). clinicaltrials.gov/show/NCT04533737 (first received 1 September 2020).
NCT04595409 {published data only}
- EUCTR2019-004364-21-PL. A double-blind study to compare the efficacy, safety, and immunogenicity of the proposed biosimilar ustekinumab FYB202 to stelara in patients with moderate-to-severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2019-004364-21-PL (first received 5 March 2020).
- NCT04595409. A double-blind study to compare the efficacy, safety, and immunogenicity of the proposed biosimilar ustekinumab FYB202 to stelara® in patients with moderate-to-severe plaque psoriasis (VESPUCCI). clinicaltrials.gov/show/NCT04595409 (first received 20 October 2020).
NCT04607980 {published data only}
- EUCTR2020-003184-25-EE. A study to investigate ABP 654 for the treatment of subjects with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2020-003184-25-EE (first received 6 November 2020).
- NCT04607980. A study to investigate ABP 654 for the treatment of participants with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT04607980 (first received 29 October 2020).
NCT04673786 {published data only}
- EUCTR2020-001045-39-EE. Study to compare the efficacy and safety of CT-P43 to Stelara in patients with moderate to severe plaque psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-001045-39-EE (first received 1 October 2020).
- NCT04673786. A phase 3 study to compare the efficacy and safety of CT-P43 to Stelara in patients with plaque psoriasis. clinicaltrials.gov/show/NCT04673786 (first received 17 December 2020).
NCT04713592 {published data only}
- NCT04713592. Study of subcutaneous (injected under the skin) risankizumab to assess change in disease symptoms in adult participants with moderate to severe plaque psoriasis with palmoplantar involvement. clinicaltrials.gov/show/NCT04713592 (first received 19 January 2021).
NCT04728360 {published data only}
- EUCTR2020-004504-33-BG. Study to compare efficacy and safety of BAT2206 with Stelara® in patients with moderate to severe plaque psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-004504-33-BG (first received 9 February 2021).
- NCT04728360. Comparative study of BAT2206 with Stelara® in patients with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT04728360 (first received 28 January 2021).
NCT04785326 {published data only}
- EUCTR2020-005108-21-HU. Clinical study comparing DMB-3115 and Stelara® in patients with moderate to severe chronic plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2020-005108-21-HU (first received 1 March 2021).
- NCT04785326. Efficacy, safety, and immunogenicity of subcutaneous DMB-3115 versus stelara® in patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/NCT04785326 (first received 5 March 2021).
NCT04839328 {published data only}
- NCT04839328. A phase Ⅲ efficacy and safety study of hemay005 in subjects with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT04839328 (first received 9 April 2021).
NCT04908475 {published data only}
- EUCTR2020-005512-21-DE. Study of subcutaneous risankizumab injection compared to oral apremilast tablets to assess adverse events and change in disease activity in adult participants with moderate plaque psoriasis who are candidates for systemic therapy. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-005512-21-DE (first received 7 May 2021).
- NCT04908475. Study of subcutaneous risankizumab injection compared to oral apremilast tablets to assess change in disease activity and adverse events in adult participants with moderate plaque psoriasis who are candidates for systemic therapy. clinicaltrials.gov/show/NCT04908475 (first received 1 June 2021).
NCT04914429 {published data only}
- NCT04914429. A study of guselkumab (TREMFYA) in Chinese participants with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT04914429 (first received 4 June 2021).
NCT04930042 {published data only}
- EUCTR2020-004493-22-PL. A randomized, double-blind, multicenter study to demonstrate equivalent efficacy and to compare safety and immunogenicity of a biosimilar ustekinumab (AVT04) and Stelara® in patients with moderate to severe chronic plaque-type psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-004493-22-PL (first received 29 January 2021).
- NCT04930042. Efficacy, safety, and immunogenicity of AVT04 with moderate-to-severe chronic plaque psoriasis. clinicaltrials.gov/show/NCT04930042 (first received 18 June 2021).
NCT04967508 {published data only}
- EUCTR2020-006115-19-CZ. SB17 versus Stelara® in subjects with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2020-006115-19-CZ (first received 31 March 2021).
- NCT04967508. A study to compare SB17 (proposed ustekinumab biosimilar) to Stelara® in subject with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT04967508 (first received 19 July 2021).
NCT05004727 {published data only}
- NCT05004727. Multi-center PAMPA study. clinicaltrials.gov/show/NCT05004727 (first received 13 August 2021).
NCT05020249 {published data only}
- NCT05020249. A study to evaluate the efficacy and safety of bimekizumab in adult Korean study participants with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT05020249 (first received 25 August 2021).
NCT05108766 {published data only}
- NCT05108766. A phase Ⅲ study to evaluate tildrakizumab in the treatment of Chinese subjects with moderate to severe plaquetype psoriasis. clinicaltrials.gov/show/NCT05108766 (first received 5 November 2021).
NCT05335356 {published data only}
- NCT05335356. Comparing efficacy and safety of Bmab 1200 and Stelara in patients with moderate to severe chronic plaque psoriasis (STELLAR-2). clinicaltrials.gov/show/NCT05335356 (first received 19 April 2022).
NCT05344248 {published data only}
- NCT05344248. Safety, tolerance, efficacy and pharmacokinetics of JS005 multiple dosing. clinicaltrials.gov/show/NCT05344248 (first received 25 April 2022).
NCT05536726 {published data only}
- NCT05536726. A phase 3 study of recombinant anti-IL-17A humanized monoclonal antibody in Chinese participants with moderate-to-severe plaque psoriasis. clinicaltrials.gov/show/NCT05536726 (first received 13 September 2022).
Additional references
Afach 2021
- Afach S, Chaimani A, Evrenoglou T, Penso L, Brouste E, Sbidian E, et al. Meta-analysis results do not reflect the real safety of biologics in psoriasis. British Journal of Dermatology 2021;184(3):415-24. [DOI: 10.1111/bjd.19244] [DOI] [PubMed] [Google Scholar]
Afach 2022
Alinaghi 2019
- Alinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. Journal of the American Academy of Dermatology 2019;80(1):251-65.e19. [PMID: ] [DOI] [PubMed] [Google Scholar]
Aljefri 2022
- Aljefri Yara E, Ghaddaf Abdullah A, Alkhunani Tala A, Alkhamisi Taif A, Alahmadi Rana A, Alamri Awadh M, Alraddadi Ali A. Efficacy and safety of apremilast monotherapy in moderate-to-severe plaque psoriasis: A systematic review and meta-analysis. Dermatologic Therapy 2022;35(7):e15544. [DOI] [PubMed] [Google Scholar]
Armstrong 2020b
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA 2020;323(19):1945-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Armstrong 2021
- Armstrong AW, Soliman AM, Betts KA, Wang Y, Gao Y, Puig L, Augustin M. Comparative efficacy and relative ranking of biologics and oral therapies for moderate-to-severe plaque psoriasis: a network meta-analysis. Dermatology and Therapy 2021;11(3):885-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Armstrong 2022
- Armstrong AW, Soliman AM, Betts KA, Wang Y, Gao Y, Stakias V, Puig L. Long-term benefit-risk profiles of treatments for moderate-to-severe plaque psoriasis: a network meta-analysis. Dermatology and Therapy 2022;12(1):167-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Armstrong 2022a
- Armstrong A, Fahrbach K, Leonardi C, Augustin M, Neupane B, Kazmierska P, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatology and Therapy 2022;12(8):1777-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atwan 2015
- Atwan A, Ingram JR, Abbott R, Kelson MJ, Pickles T, Bauer A, et al. Oral fumaric acid esters for psoriasis. Cochrane Database of Systematic Reviews 2015, Issue 8. Art. No: CD010497. [DOI: 10.1002/14651858.CD010497.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Balak 2016
- Balak DM, Fallah Arani S, Hajdarbegovic E, Hagemans CA, Bramer WM, Thio HB, et al. Efficacy, effectiveness and safety of fumaric acid esters in the treatment of psoriasis: a systematic review of randomized and observational studies. British Journal of Dermatology 2016;175(2):250-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bansback 2009
- Bansback N, Sizto S, Sun H, Feldman S, Willian MK, Anis A. Efficacy of systemic treatments for moderate to severe plaque psoriasis: systematic review and meta-analysis. Dermatology 2009;219(3):209-18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Blauvelt 2021b
- Blauvelt A, Burge R, Malatestinic W, Brnabic A, Guo J, Janardhanan M, et al. Cost per cumulative clinical benefit of biologic therapies for patients with plaque psoriasis: a systematic review. Journal of Managed Care & Specialty Pharmacy 2021;27(1):84-94. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blauvelt 2022
- Blauvelt A, Gooderham M, Griffiths CEM, Armstrong AW, Zhu B, Burge R, et al. Cumulative clinical benefits of biologics in the treatment of patients with moderate-to-severe psoriasis over 1 year: a network meta-analysis. Dermatology and Therapy 2022;12(3):727-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brimhall 2008
- Brimhall AK, King LN, Licciardone JC, Jacobe H, Menter A. Safety and efficacy of alefacept, efalizumab, etanercept and infliximab in treating moderate to severe plaque psoriasis: a meta-analysis of randomized controlled trials. British Journal of Dermatology 2008;159(2):274-85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Callis Duffin 2018
- Callis Duffin K, Merola JF, Christensen R, Latella J, Garg A, Gottlieb AB, et al. Identifying a core domain set to assess psoriasis in clinical trials. JAMA Dermatology 2018;154(10):1137-44. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campanati 2017
- Campanati A, Benfaremo D, Luchetti MM, Ganzetti G, Gabrielli A, Offidani A. Certolizumab pegol for the treatment of psoriasis. Expert Opinion on Biological Therapy 2017;17(3):387-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Capon 2017
- Capon F. The genetic basis of psoriasis. International Journal of Molecular Sciences 2017;18(12):E2526. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2012
- Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Vasiliadis HS, Pandis N, Schmid CH, Welton NJ, Salanti G. Effects of study precision and risk of bias in networks of interventions: a network meta-epidemiological study. International Journal of Epidemiology 2013;42(4):1120-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chaimani 2017a
- Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. Journal of Clinical Epidemiology 2017;16(S0895):30775-2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chaimani 2017b
- Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. Journal of Clinical Epidemiology 2017;83:65-74. [DOI] [PubMed] [Google Scholar]
Chaimani 2017c
- Chaimani A, Salanti G, Leucht S, Geddes JR, Cipriani A. Common pitfalls and mistakes in the set-up, analysis and interpretation of results in network meta-analysis: what clinicians should look for in a published article. Evidence-Based Mental Health 2017;20(3):88-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiu 2014
- Chiu HY, Wang TS, Chan CC, Cheng YP, Lin SJ, Tsai TF. Human leucocyte antigen-Cw6 as a predictor for clinical response to ustekinumab, an interleukin-12/23 blocker, in Chinese patients with psoriasis: a retrospective analysis. British Journal of Dermatology 2014;171(5):1181-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Christophers 1992
- Christophers E, Mrowietz U, Henneicke HH, Farber L, Welzel D. Cyclosporine in psoriasis: a multicenter dose-finding study in severe plaque psoriasis. The German Multicenter Study. Journal of the American Academy of Dermatology 1992;26(1):86-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
CINeMA 2017 [Computer program]
- CINeMA: Confidence in Network Meta-Analysis. Bern: Institute of Social and Preventive Medicine, University of Bern, 2017.
Cipriani 2013
- Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Annals of Internal Medicine 2013;159(2):130-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, N.J: L. Erlbaum Associates, 1988. [Google Scholar]
Covidence 2021 [Computer program]
- Covidence. Version accessed prior to 6 October 2021. Melbourne, Australia: Veritas Health Innovation, 2014. Available at covidence.org.
Dechartres 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ (Clinical research ed.) 2013;346:f2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Dias 2010
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Statistics in Medicine 2010;29(7-8):932-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dong 2017
- Dong J, Goldenberg G. New biologics in psoriasis: an update on IL-23 and IL-17 inhibitors. Cutis 2017;99(2):123-7. [PMID: ] [PubMed] [Google Scholar]
Elliott 2017
- Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, McDonald S, et al. Living systematic review: 1. Introduction-the why, what, when, and how. Journal of Clinical Epidemiology 2017;91:23-30. [DOI] [PubMed] [Google Scholar]
Evrenoglou 2022
- Evrenoglou T, White IR, Afach S, Mavridis D, Chaimani A. Network meta-analysis of rare events using penalized likelihood regression. Statistics in Medicine 2022;41(26):5203-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fahrbach 2021
- Fahrbach K, Sarri G, Phillippo DM, Neupane B, Martel SE, Kiri S, et al. Short-term efficacy of biologic therapies in moderate-to-severe plaque psoriasis: a systematic literature review and an enhanced multinomial network meta-analysis. Dermatology and Therapy 2021;11(6):1965-98. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fu 2022
- Fu H, Guo J. Efficacy of guselkumab compared with adalimumab for psoriasis: a meta-analysis of randomized controlled studies.. Postepy Dermatologii i Alergologii 2022;39(5):953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gisondi 2004
- Gisondi P, Gubinelli E, Cocuroccia B, Girolomoni G. Targeting tumor necrosis factor-alpha in the therapy of psoriasis. Current Drug Targets. Inflammation and Allergy 2004;3(2):175-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gospodarevskaya 2009
- Gospodarevskaya E, Picot J, Cooper K, Loveman E, Takeda A. Ustekinumab for the treatment of moderate to severe psoriasis. Health Technology Assessment 2009;13(Suppl 3):61-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gottlieb 2022
- Gottlieb AB, Saure D, Wilhelm S, Dossenbach M, Schuster C, Smith SD, et al. Indirect comparisons of ixekizumab versus three interleukin-23 p19 inhibitors in patients with moderate-to-severe plaque psoriasis - efficacy findings up to week 12. Journal of Dermatological Treatment 2022;33(1):54-61. [DOI] [PubMed] [Google Scholar]
Griffiths 2007
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007;370(9583):263-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grodner 2021
- Grodner C, Sbidian E, Weill A, Mezzarobba M. Epidemiologic study in a real-world analysis of patients with treatment for psoriasis in the French national health insurance database. Journal of the European Academy of Dermatology and Venereology 2021;35(2):411-6. [DOI] [PubMed] [Google Scholar]
Guelimi 2021
Gómez‐García 2017
- Gómez-García F, Epstein D, Isla-Tejera B, Lorente A, Vélez García-Nieto A, Ruano J. Short-term efficacy and safety of new biological agents targeting the interleukin-23-T helper 17 pathway for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis. British Journal of Dermatology 2017;176(3):594-603. [PMID: ] [DOI] [PubMed] [Google Scholar]
He 2021
- He H, Wu W, Zhang Y, Zhang M, Sun N, Zhao L, et al. Model-based meta-analysis in psoriasis: a quantitative comparison of biologics and small targeted molecules. Frontiers in Pharmacology 2021;12:586827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Helliwell 2005
- Helliwell PS, Taylor WJ. Classification and diagnostic criteria for psoriatic arthritis. Annals of the Rheumatic Diseases 2005;64(Suppl 2):ii3-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2012
- Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Research Synthesis Methods 2012;3(2):98-110. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Ho 1996
- Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, et al. The mechanism of action of cyclosporin A and FK506. Clinical Immunology and Immunopathology 1996;80(3 Pt 2):S40-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ho 1999
- Ho VC, Griffiths CE, Albrecht G, Vanaclocha F, Leon-Dorantes G, Atakan N, et al. Intermittent short courses of cyclosporin (Neoral(R)) for psoriasis unresponsive to topical therapy: a 1-year multicentre, randomized study. The PISCES Study Group. British Journal of Dermatology 1999;141(2):283-91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hoy 2022
- Hoy Sheridan M. Deucravacitinib: first approval. Drugs 2022;82(17):1671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 2014
- Jackson D, Barrett JK, Rice S, White IR, Higgins JP. A design-by-treatment interaction model for network meta-analysis with random inconsistency effects. Statistics in Medicine 2014;33(21):3639-54. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2022
- Kang Q, Chen JS, Yang H. Efficacy and safety profile of phosphodiesterase 4 inhibitor in the treatment of psoriasis: a systematic review and meta-analysis of randomized controlled trials. Frontiers in Immunology 2022;13:1021537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kimball 2005
- Kimball AB, Jacobson C, Weiss S, Vreeland MG, Wu Y. The psychosocial burden of psoriasis. American Journal of Clinical Dermatology 2005;6(6):383-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Langan 2012
- Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. Journal of Investigative Dermatology 2012;132(3 Pt 1):556-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Le Cleach 2008
- Le Cleach L, Chassany O, Levy A, Wolkenstein P, Chosidow O. Poor reporting of quality of life outcomes in dermatology randomized controlled clinical trials. Dermatology 2008;216(1):46-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lebwohl 2010
- Lebwohl M, Papp K, Han C, Schenkel B, Yeilding N, Wang Y, et al. Ustekinumab improves health-related quality of life in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial. British Journal of Dermatology 2010;162(1):137-46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leonardi 2022
- Leonardi CL, See K, Burge R, Sun Z, Zhang Y, Mallbris L, et al. Number needed to treat network meta-analysis to compare biologic drugs for moderate-to-severe psoriasis. Advances in Therapy 2022;39(5):2256-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liang 2019
- Liang F, Wu Z, Mo M, Zhou C, Shen J, Wang Z, et al. Comparison of treatment effect from randomised controlled phase II trials and subsequent phase III trials using identical regimens in the same treatment setting. European Journal of Cancer 2019;121:19-28. [DOI] [PubMed] [Google Scholar]
Lin 2012
- Lin VW, Ringold S, Devine EB. Comparison of ustekinumab with other biological agents for the treatment of moderate to severe plaque psoriasis: a Bayesian network meta-analysis. Archives of Dermatology 2012;148(12):1403-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Living Evidence Network 2019
- Guidance for the production and publication of Cochrane living systematic reviews: Cochrane Reviews in living mode. Available from community.cochrane.org/review-production/production-resources/living-systematic-reviews#guidance (accessed prior to 4 April 2022).
Loveman 2009
- Loveman E, Turner D, Hartwell D, Cooper K, Clegg A. Infliximab for the treatment of adults with psoriasis. Health Technology Assessment 2009;13(Suppl 1):55-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel‐Storr A, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner's guide. Research Synthesis Methods 2018;9(4):602-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mason 2013
- Mason AR, Mason J, Cork M, Dooley G, Hancock H. Topical treatments for chronic plaque psoriasis. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD005028. [DOI: 10.1002/14651858.CD005028.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mavridis 2014
- Mavridis D, Chaimani A, Efthimiou O, Leucht S, Salanti G. Addressing missing outcome data in meta-analysis. Evidence-Based Mental Health 2014;17(3):85-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Maza 2011
- Maza A, Montaudie H, Sbidian E, Gallini A, Aractingi S, Aubin F, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2011;25 Suppl 2:19-27. [PMID: ] [DOI] [PubMed] [Google Scholar]
Montaudie 2011
- Montaudie H, Sbidian E, Paul C, Maza A, Gallini A, Aractingi S, et al. Methotrexate in psoriasis: a systematic review of treatment modalities, incidence, risk factors and monitoring of liver toxicity. Journal of the European Academy of Dermatology and Venereology 2011;25 Suppl 2:12-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mossner 2009
- Mossner R, Reich K. Management of severe psoriasis with TNF antagonists. Adalimumab, etanercept and infliximab. Current Problems in Dermatology 2009;38:107-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mrowietz 1995
- Mrowietz U, Farber L, Henneicke-von Zepelin HH, Bachmann H, Welzel D, Christophers E. Long-term maintenance therapy with cyclosporine and posttreatment survey in severe psoriasis: results of a multicenter study. German Multicenter Study. Journal of the American Academy of Dermatology 1995;33(3):470-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mrowietz 2021
- Mrowietz U, Warren RB, Leonardi CL, Saure D, Petto H, Hartz S, et al. Network meta-analysis of biologic treatments for psoriasis using absolute Psoriasis Area and Severity Index values ≤ 1, 2, 3 or 5 derived from a statistical conversion method. Journal of the European Academy of Dermatology and Venereology 2021;35(5):1161-75. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naldi 2010
- Naldi L. Scoring and monitoring the severity of psoriasis. What is the preferred method? What is the ideal method? Is PASI passe? Facts and controversies. Clinics in Dermatology 2010;28(1):67-72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nast 2015a
- Nast A, Jacobs A, Rosumeck S, Werner RN. Efficacy and safety of systemic long-term treatments for moderate-to-severe psoriasis: a systematic review and meta-analysis. Journal of Investigative Dermatology 2015;135(11):2641-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nast 2015b
- Nast A, Jacobs A, Rosumeck S, Werner RN. Methods Report: European S3-Guidelines on the systemic treatment of psoriasis vulgaris - update 2015--EDF in cooperation with EADV and IPC. Journal of the European Academy of Dermatology and Venereology 2015;29(12):e1-22. [DOI: 10.1111/jdv.13353] [DOI] [PubMed] [Google Scholar]
Nelson 2008
- Nelson AA, Pearce DJ, Fleischer AB Jr, Balkrishnan R, Feldman SR. Cost-effectiveness of biologic treatments for psoriasis based on subjective and objective efficacy measures assessed over a 12-week treatment period. Journal of the American Academy of Dermatology 2008;58(1):125-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nijsten 2007
- Nijsten T, Looman CW, Stern RS. Clinical severity of psoriasis in last 20 years of PUVA study. Archives of Dermatology 2007;143(9):1113-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2021
- Noel-Storr A, Dooley G, Elliott J, Steele E, Shemilt I, Mavergames C, et al. An evaluation of Cochrane Crowd found that crowdsourcing produced accurate results in identifying randomized trials. Journal of Clinical Epidemiology 2021;133:130-9. [DOI: 10.1016/j.jclinepi.2021.01.006] [DOI] [PubMed] [Google Scholar]
Ormerod 2004
- Ormerod AD, Mrowietz U. Fumaric acid esters, their place in the treatment of psoriasis. British Journal of Dermatology 2004;150(4):630-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pan 2021
- Pan R, Wang X, Shu M, Das J, Kalra M, Wang Z. Comparative efficacy of secukinumab against adalimumab and infliximab in patients with moderate-to-severe plaque psoriasis. Chinese Medical Journal 2021;135(1):11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parisi 2020
- Parisi R, Iskandar IY, Kontopantelis E, Augustin M, Griffiths CE, Ashcroft DM, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ (Clinical research ed.) 2020;369:m1590. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rapp 1999
- Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. Journal of the American Academy of Dermatology 1999;41(3 Pt 1):401-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reich 2008
- Reich K, Sinclair R, Roberts G, Griffiths CE, Tabberer M, Barker J. Comparative effects of biological therapies on the severity of skin symptoms and health-related quality of life in patients with plaque-type psoriasis: a meta-analysis. Current Medical Research and Opinion 2008;24(5):1237-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reich 2012b
- Reich K, Burden AD, Eaton JN, Hawkins NS. Efficacy of biologics in the treatment of moderate to severe psoriasis: a network meta-analysis of randomized controlled trials. British Journal of Dermatology 2012;166(1):179-88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Revman 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4.1. Nordic Cochrane Centre, The Cochrane Collaboration, 2020. Copenhagen.
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ (Clinical research ed.) 2011;342:d549. [PMID: ] [DOI] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salanti 2014
- Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLOS One 2014;9(7):e99682. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Savage 2015
- Savage LJ, Wittmann M, McGonagle D, Helliwell PS. Ustekinumab in the treatment of psoriasis and psoriatic arthritis. Rheumatology and Therapy 2015;2(1):1-16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sawyer 2019
- Sawyer LM, Cornic L, Levin LÅ, Gibbons C, Møller AH, Jemec GB. Long-term efficacy of novel therapies in moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. Journal of the European Academy of Dermatology and Venereology 2019;33(2):355-66. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sbidian 2011
- Sbidian E, Maza A, Montaudie H, Gallini A, Aractingi S, Aubin F, et al. Efficacy and safety of oral retinoids in different psoriasis subtypes: a systematic literature review. Journal of the European Academy of Dermatology and Venereology 2011;25 Suppl 2:28-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schmitt 2005
- Schmitt J, Wozel G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology 2005;210(3):194-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schmitt 2008
- Schmitt J, Zhang Z, Wozel G, Meurer M, Kirch W. Efficacy and tolerability of biologic and nonbiologic systemic treatments for moderate-to-severe psoriasis: meta-analysis of randomized controlled trials. British Journal of Dermatology 2008;159(3):513-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shear 2021
- Shear NH, Betts KA, Soliman AM, Joshi A, Wang Y, Zhao J, et al. Comparative safety and benefit-risk profile of biologics and oral treatment for moderate-to-severe plaque psoriasis: a network meta-analysis of clinical trial data. Journal of the American Academy of Dermatology 2021;85(3):572-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Signorovitch 2010
- Signorovitch JE, Wu EQ, Yu AP, Gerrits CM, Kantor E, Bao Y, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics 2010;28(10):935-45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Signorovitch 2015
- Signorovitch JE, Betts KA, Yan YS, LeReun C, Sundaram M, Wu EQ, et al. Comparative efficacy of biological treatments for moderate-to-severe psoriasis: a network meta-analysis adjusting for cross-trial differences in reference arm response. British Journal of Dermatology 2015;172(2):504-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Song 2021
- Song GG, Lee YH. Relative efficacy and safety of tofacitinib for treating psoriasis: a Bayesian network meta-analysis of randomized controlled trials. International Journal of Clinical Pharmacology and Therapeutics 2021;59(4):308-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Spuls 1997
- Spuls PI, Witkamp L, Bossuyt PM, Bos JD. A systematic review of five systemic treatments for severe psoriasis. British Journal of Dermatology 1997;137(6):943-9. [MEDLINE: ] [PubMed] [Google Scholar]
Spuls 2010
- Spuls PI, Lecluse LL, Poulsen ML, Bos JD, Stern RS, Nijsten T. How good are clinical severity and outcome measures for psoriasis?: quantitative evaluation in a systematic review. Journal of Investigative Dermatology 2010;130(4):933-43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Strober 2006
- Strober BE, Siu K, Menon K. Conventional systemic agents for psoriasis. A systematic review. Journal of Rheumatology 2006;33(7):1442-6. [MEDLINE: ] [PubMed] [Google Scholar]
Tan 2011
- Tan JY, Li S, Yang K, Ma B, Chen W, Zha C, et al. Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: a meta-analysis. Journal of Dermatological Treatment 2011;22(6):323-36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Torres 2015
- Torres T, Filipe P. Small molecules in the treatment of psoriasis. Drug Development Research 2015;76(5):215-27. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tubach 2009
- Tubach F, Salmon D, Ravaud P, Allanore Y, Goupille P, Breban M, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis and Rheumatism 2009;60(7):1884-94. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2009
- Turner D, Picot J, Cooper K, Loveman E. Adalimumab for the treatment of psoriasis. Health Technology Assessment 2009;13(Suppl 2):49-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Veroniki 2013
- Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. International Journal of Epidemiology 2013;42(1):332-45. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Veroniki 2018
- Veroniki AA, Straus SE, Rücker G, Tricco AC. Is providing uncertainty intervals in treatment ranking helpful in a network meta-analysis? Journal of Clinical Epidemiology 2018;100:122-9. [DOI] [PubMed] [Google Scholar]
White 2012
- White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Research Synthesis Methods 2012;3(2):111-25. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woolacott 2006
- Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Vergel YB, et al. Etanercept and efalizumab for the treatment of psoriasis: a systematic review. Health Technology Assessment 2006;10(46):1-233, i-iv. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Xie 2021
- Xie Yan, Liu Yang, Liu Yi. Are biologics combined with methotrexate better than biologics monotherapy in psoriasis and psoriatic arthritis: a meta-analysis of randomized controlled trials. Dermatologic Therapy 2021;34(3):e14926. [DOI] [PubMed] [Google Scholar]
Xu 2021
- Xu S, Gao X, Deng J, Yang J, Pan F. Comparative efficacy and safety of biologics in moderate to severe plaque psoriasis: a multiple-treatments meta-analysis. Journal der Deutschen Dermatologischen Gesellschaft (Journal of the German Society of Dermatology : JDDG) 2021;19(1):47-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yan 2021
- Yan D, Blauvelt A, Dey AK, Golpanian RS, Hwang ST, Mehta NN, et al. New frontiers in psoriatic disease research, part II: comorbidities and targeted therapies. Journal of Investigative Dermatology 2021;141(10):2328-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yan 2021a
- Yan D, Gudjonsson JE, Le S, Maverakis E, Plazyo O, Ritchlin C, et al. New frontiers in psoriatic disease research, part I: Genetics, environmental triggers, immunology, pathophysiology, and precision medicine. Journal of Investigative Dermatology 2021;141(9):2112-22.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yasmeen 2022
- Yasmeen N, Sawyer LM, Malottki K, Levin LÅ, Didriksen AE, Jemec GB. Targeted therapies for patients with moderate-to-severe psoriasis: a systematic review and network meta-analysis of PASI response at 1 year. Journal of Dermatological Treatment 2022;33(1):204-18. [DOI] [PubMed] [Google Scholar]
Yu 2022
- Yu Q, Ge X, Jing M, Mi X, Guo J, Xiao M, et al. A systematic review with meta-analysis of comparative efficacy and safety of risankizumab and ustekinumab for psoriasis treatment. Journal of Immunology Research 2022;2022:2802892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zachariae 2003
- Zachariae H. Prevalence of joint disease in patients with psoriasis: implications for therapy. American Journal of Clinical Dermatology 2003;4(7):441-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhu 2022
- Zhu T, Ma L. Meta-analysis of the efficacy and safety of interleukin-23-targeted drugs in the treatment of moderate-to-severe psoriasis. Contrast Media & Molecular Imaging 2022;2022:2172980. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Sbidian 2015
- Sbidian E, Le Cleach L, Trinquart L, Do G, Hughes C, Naldi L, et al. Systemic pharmacological treatments for chronic plaque psoriasis. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD011535. [DOI: 10.1002/14651858.CD011535] [DOI] [Google Scholar]
Sbidian 2017
- Sbidian E, Chaimani A, Garcia-Doval I, Do G, Hua C, Mazaud C, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Systematic Reviews 2017, Issue 12. Art. No: CD011535. [DOI: 10.1002/14651858.CD011535.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sbidian 2020
- Sbidian E, Chaimani A, Afach S, Doney L, Dressler C, Hua C, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta‐analysis. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD011535. [DOI: 10.1002/14651858.CD011535.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sbidian 2021
- Sbidian E, Chaimani A, Garcia-Doval I, Doney L, Dressler C, Hua C, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Systematic Reviews 2021, Issue 4. Art. No: CD011535. [DOI: 10.1002/14651858.CD011535.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sbidian 2022
- Sbidian E, Chaimani A, Garcia-Doval I, Doney L, Dressler C, Hua C, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Systematic Reviews 2022, Issue 5. Art. No: CD011535. [DOI: 10.1002/14651858.CD011535.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]