Abstract
Summary
We report the case of a 69-year-old female with systemic mastocytosis, diagnosed based on widespread pigmented papules and macules, elevated serum tryptase levels and confirmatory skin and bone marrow biopsy, on a background of osteoporosis. A CT demonstrated multiple sclerotic lesions within lumbar vertebral bodies, sacrum and ileum, with surrounding osteolysis but no obvious compression fractures. She was treated with the RANK-L inhibitor denosumab, resulting in significant bone mineral density gain over the following 5 years. However, her serum tryptase levels gradually increased during this period despite treatment with the multikinase inhibitor, midostaurin. It is thus conceivable that her rapid increase in bone mineral density may be partly contributed by a predominance of pro-osteoblastic mediators released by abnormal mast cells, suggestive of more advanced disease. This case highlights the complexities of systemic mastocytosis-related bone disease and the interplay of numerous mediators contributing to a phenotype of both increased bone resorption and formation.
Learning points
Systemic mastocytosis is a neoplastic disease of mast cells characterized by abnormal proliferation and accumulation in the skin and other organs. It is most frequently associated with the somatic gain-of-function KIT D816V mutation.
Systemic mastocytosis should be suspected in patients presenting with not only cutaneous symptoms suggestive of mast cell degranulation such as anaphylaxis, flushing or urticaria but also unexplained osteoporosis and gastrointestinal and constitutional symptoms.
The prevalence of osteoporosis in systemic mastocytosis is high. Mast cell activation leads to the secretion of numerous chemical mediators which either promote or inhibit osteoclastic and/or osteoblastic activity, with the balance usually in favour of increased bone resorption. However, in advanced diseases with high mast cell burden, mast-cell-derived cytokines and mediators may promote osteoblastic activity, leading to osteosclerosis and apparent increases in bone mineral density.
Treatment of osteoporosis in systemic mastocytosis involves antiresorptive therapy with bisphosphonates and more recently, denosumab. There are limited data on the role of osteoanabolic agents.
Patient Demographics: Geriatric, Female, White, Australia
Clinical Overview: Bone, Bone
Publication Details: Insight into disease pathogenesis or mechanism of therapy, May, 2023
Background
Systemic mastocytosis (SM) is a rare mast cell neoplasm with a prevalence of 1 in 10,000, arising from the clonal proliferation of abnormal mast cells which accumulate in various tissues including the skin, bone, gastrointestinal tract, spleen and lymph nodes (1). KIT (CD117) is a transmembrane receptor tyrosine kinase expressed by mast cells, haematopoietic progenitor cells, germ cells, melanocytes and gastrointestinal cells (1). Up to 90% of SM present with gain-of-function somatic mutations in KIT, particularly the D816V mutation which permits constitutive activation and auto-phosphorylation of KIT, so that it does not require binding to an activating ligand for its function (2). Clinically, the disease spectrum ranges from cutaneous mastocytosis and indolent SM to more aggressive forms including haematological neoplasms and mast cell leukaemia (1). Clinical symptoms are due to the release of vasoactive mediators (e.g. anaphylaxis, pruritus, urticarial, nausea and vomiting) and mast cell infiltration of organs (lymphadenopathy, hepatosplenomegaly and cytopenia) (3). SM is recognised as a rare cause of secondary osteoporosis, whereby the release of mast cell mediators such as histamine, tryptase, heparin and interleukins may promote osteoclasts and inhibit osteoblasts, leading to bone resorption (4). However, there is a complex interplay between the balance of osteoclastic and osteoblastic drivers, and depending on disease manifestations, some patients may present with osteosclerosis rather than osteoporosis (5). We here describe a case that illustrates the difficulty of managing a patient with SM and significant bone mineral density (BMD) increase whilst on antiresorptive therapy, which may arise due to mast cell-induced osteoblastogenesis.
Case presentation
A 56-year-old female was initially referred for further assessment of bone health, following a traumatic left greater tuberosity fracture sustained after falling down several stairs. Her past medical history included hypertension, hypercholesterolaemia and a benign breast lump. She underwent a thorough osteoporosis review, with her initial dual-energy X-ray absorptiometry (DXA) scan demonstrating L2–4 BMD 1.006 g/cm2, T-score −1.6 s.d., left femoral neck BMD 0.948 g/cm2, T-score −0.3 s.d. and left total hip BMD 1.013 g/cm2, T-score +0.1 s.d. (Fig. 1A and B). A secondary osteoporosis screen including serum calcium, phosphate, renal function, vitamin D, thyroid-stimulating hormone, parathyroid hormone (PTH), coeliac serology and myeloma screen was normal. There were no clinical risk factors for bone loss except minimal dietary calcium intake. The patient was commenced on calcium carbonate 600 mg twice daily, and weight-bearing exercises were encouraged. Her other medications included cholecalciferol 2000 IU daily and irbesartan/hydrochlorothiazide. A repeat DXA scan performed 10 years later at age 66 showed that her L1–3 BMD had declined to 0.926 g/cm2, T-score −2.0 s.d. (with L3 vertebrae T-score: −2.5 s.d. and L4 T-score: −3.3 s.d.), left femoral neck BMD 0.814 g/cm2, T-score −1.6 s.d. and left total hip BMD 0.927 g/cm2, T-score −0.6 s.d. (Fig. 1A and B), in the absence of interim minimal trauma fractures. She received a single dose of intravenous zoledronic acid 4 mg, which she tolerated poorly due to a significant acute phase reaction with a week of myalgias and malaise. Antiresorptive therapy was not continued thereafter, and she was monitored with DXA scans at 2-yearly intervals in our clinic.
Figure 1.
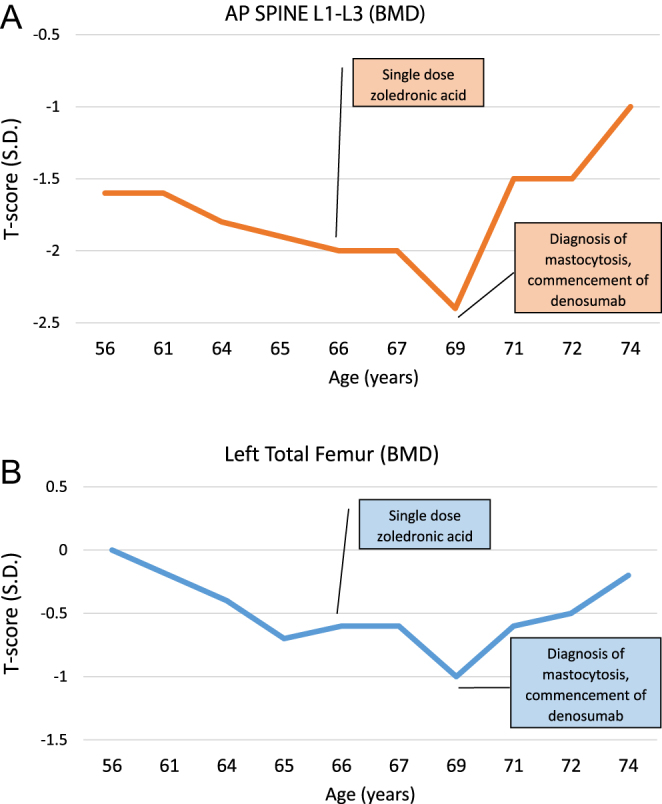
(A and B) DXA scan results AP spine L1–L3 and left total femur.
At the age of 69, she was referred to the dermatology clinic with asymptomatic pigmented macules and papules which arose from her abdomen and gradually spread to affect her face, neck, back and proximal limbs, consistent with urticaria pigmentosa (Fig. 2A and B). Stroking of the lesions evoked urticaria (positive Darier sign), raising suspicion for mast cell degranulation.
Figure 2.
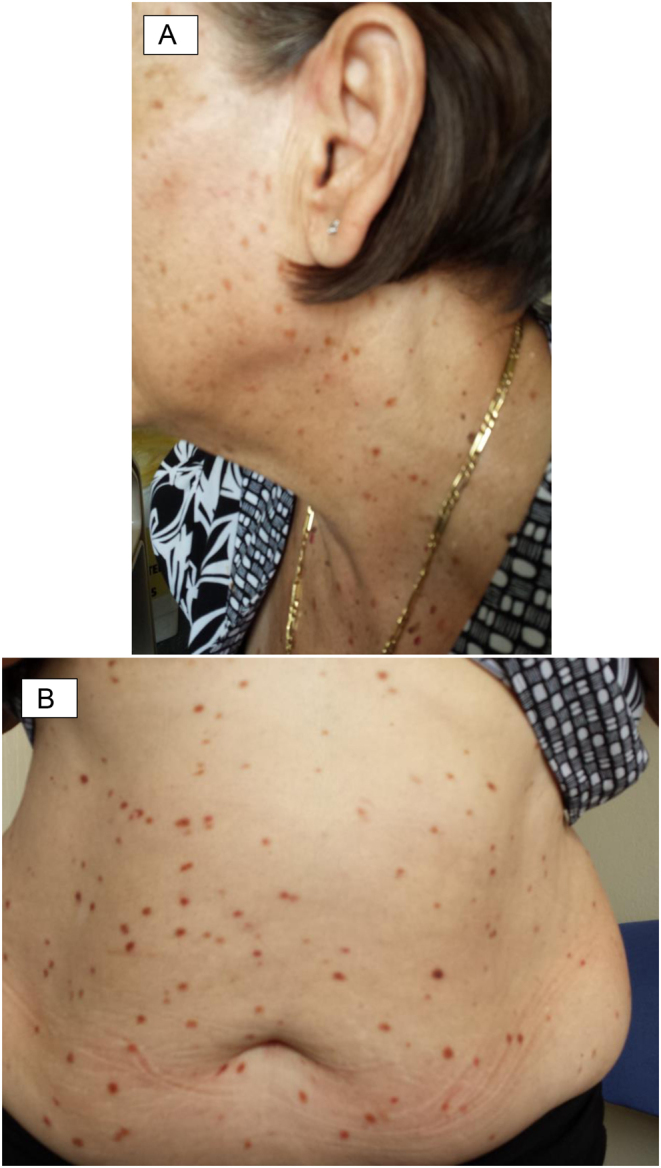
(A and B) Macules and papules over patient’s face, neck and abdomen.
Investigations
The patient underwent a punch biopsy which revealed CD117-positive mast cells, consistent with cutaneous mastocytosis (Fig. 3A, B and C). She also reported increasing lethargy, diarrhoea and intermittent flushing episodes, without weight loss, night sweats or anaphylactic episodes. She did not drink alcohol and thus did not report a history of alcohol-induced wheezing, a typical symptom of mastocytosis. At this point in time, serum tryptase was elevated at 220 mcg/L (normal range <11). A bone marrow biopsy demonstrated 60% infiltration of bone marrow by abnormal mast cells expressing CD117, CD2, CD25 and tryptase (Fig. 4A, B, C and D). Genetic testing for KIT D816V mutation was positive, confirming the diagnosis of SM.
Figure 3.
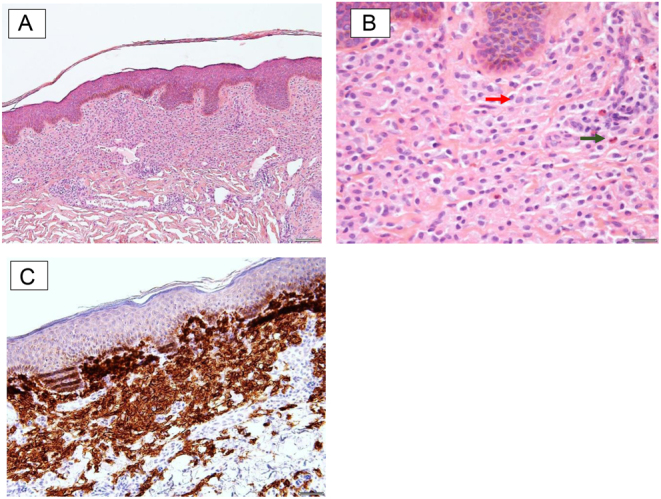
(A–C) Punch biopsy of skin lesion demonstrating cutaneous mastocytosis.
Figure 4.
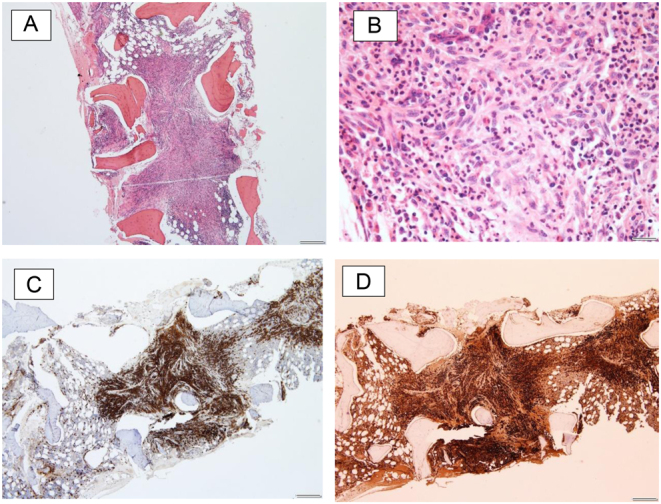
(A–D) Bone marrow biopsy demonstrating infiltration of bone marrow by abnormal mast cells.
Due to her lower back pain, the patient underwent a CT lumbar spine, demonstrating multiple sclerotic foci with surrounding osteolysis within several vertebral bodies, the sacrum and ileum but without evidence of pathological fractures or loss of vertebral height (Fig. 5A, B and C).
Figure 5.
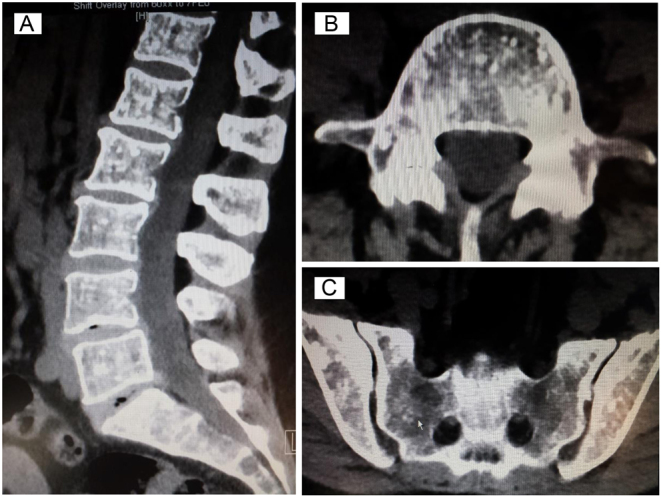
(A–C) CT lumbar spine and sacrum with multiple sclerotic foci within lumbar vertebral bodies and pelvis, with surrounding areas of osteolysis.
Treatment
The patient’s SM was managed within a multidisciplinary setting with input from haematology, endocrinology, immunology and dermatology. She was counselled on environmental and medication triggers (including aspirin, opioids, and general anaesthesia) which could precipitate mast cell degranulation and anaphylaxis, and she received EpiPen education. Her management included antihistamines with cetirizine 20–40 mg daily, ranitidine 150 mg BD, topical corticosteroids and the multikinase inhibitor midostaurin gradually increased to the maximum dose of 100 mg BD.
Outcome and follow-up
A DXA scan was repeated soon after the diagnosis of SM, demonstrating a further decline in bone density at the spine (L1–3 BMD: 0.886 g/cm2, T-score: −2.4 s.d.) and hip (left femoral neck BMD: 0.807 g/cm2, T-score: −1.4 s.d.; total hip BMD: 0.882 g/cm2, T-score: −1 s.d.) (Fig. 1A and B).
Due to osteosclerosis in the lumbar spine, it was difficult to interpret her true BMD, although it was expected to decline further as a result of her SM if not treated with antiresorptive therapy. As zoledronic acid was previously poorly tolerated, denosumab, a RANKL-inhibitor, was considered. There was a theoretical risk of anaphylaxis with denosumab, and thus the patient was closely monitored for side effects following her initial subcutaneous injections. However, she tolerated denosumab without any issues and continued 60 mg every 6 months without dose interruption over the next 5.5 years. Her latest DXA scan demonstrated ongoing gain in bone density, with L1–3 BMD 1.052 g/cm2, T-score −1 s.d. (+18.7% since denosumab commencement), left femoral neck BMD 0.847 g/cm2, T-score −1.1 s.d. and left total hip BMD 0.973 g/cm2, T-score −0.2 s.d. (+10.3%).
Discussion
Mast cells are present in the bone marrow and preferentially involve metabolically active bone tissue (4, 5). Upon activation, mast cells secrete numerous mediators, either promoting osteoclastic (e.g. histamine, heparin, tumour necrosis factor, and interleukin-6) or inhibiting osteoblastic activity (e.g. interleukin-1, interleukin-6 and tumour necrosis factor). Conversely, mediators may also promote osteoblastic (e.g. transforming growth factor-β) or inhibit osteoclastic activity (e.g. interleukin-12, interferon-γ) under different conditions (Fig. 6) (4). Histamine, the most abundant mediator, stimulates osteoclasts and osteoclast precursors and increases RANKL expression in osteoblasts, thus promoting osteoclast recruitment and bone resorption. (6). Increase in osteoprotegerin and RANKL levels were also noted in SM, suggesting the involvement of the RANKL/RANK/OPG pathway (7).
Figure 6.
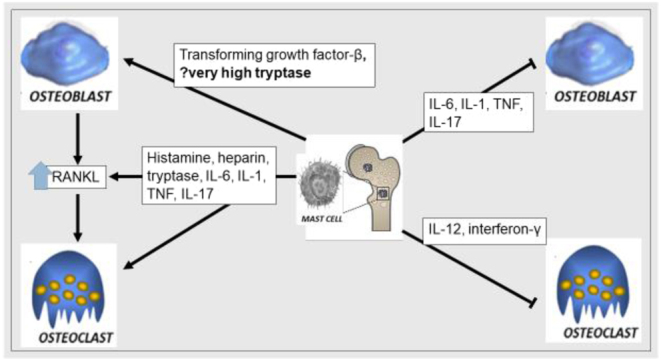
Interplay between pro-osteoclastic and pro-osteoblastic mediators in systemic mastocytosis.
The prevalence of SM in patients with osteoporosis is unknown but is likely underdiagnosed. Skeletal involvement occurs in around 50% of patients and may manifest as osteoporosis, focal sclerotic/lytic bone lesions on imaging, bony pain from marrow infiltration or without any symptoms (5, 8). The prevalence of osteoporosis in SM ranges between 18 and 37%, and vertebral fractures affect up to 20% of patients (5, 8). Osteoporosis is more common at the lumbar spine than hip, likely related to a propensity of mast cells to colonise the more metabolically active trabecular bone (9). There is no difference in the prevalence of osteoporosis in those with or without skin lesions, and thus SM cannot be excluded in the absence of cutaneous mastocytosis (10). Serum tryptase levels are useful in the diagnosis of mastocytosis as a cause of secondary osteoporosis but were not suspected prior to the onset of typical cutaneous manifestations in our patient (1).
Conversely, osteosclerosis and increased bone density can also be observed in 2–19% of SM, with lower reported rates of fragility fractures (5). In these patients, the coexistence of osteolytic and osteosclerotic lesions is seen (5, 6). Serum tryptase and bone turnover markers are highest in patients with osteosclerosis and increased bone density (6). Tryptase levels correlate with markers of bone resorption and formation (11). Thus, mediators favouring osteoclastogenesis may predominate with a moderate increase in mast cells, whereas mediators favouring osteoblastogenesis prevail with a higher mast cell burden (12). Although there was an initial period of tryptase reduction with the commencement of midostaurin, the patient was never able to maintain tryptase levels within the normal range, and recent tests have shown a surge in tryptase levels (Fig. 7).
Figure 7.
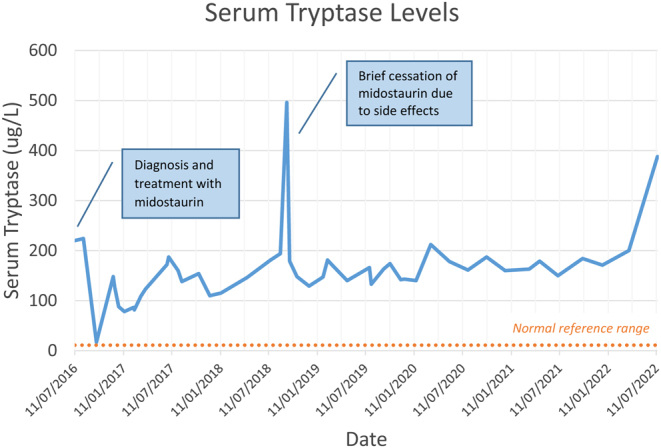
Serum tryptase levels at diagnosis and during treatment.
The FREEDOM extension trial demonstrated increases in BMD of up to 21.7% at the lumbar spine and 9.2% at the total hip after 10 years of denosumab treatment (13). Our patient exhibited increases in BMD of 18.7% at the lumbar spine and 10.3% at the total hip over 5.5 years, and thus the rate of increase appears significantly greater than expected for a denosumab effect alone. Instead, this rapid increase in bone density may also be a signal for increasing osteoblastic activity, particularly as the patient’s SM has gradually become resistant to midostaurin over the years. Studies have shown that while osteoporosis is a common feature in indolent SM, increased bone density is frequently encountered in advanced cases and associated with poorer prognosis (14). Bone marrow mast cell infiltration and serum tryptase levels were both significantly lower in patients with compared to those without osteoporosis (14). In this cohort of 32 patients with advanced SM, 75% of patients exhibited increased bone density (defined as Z-score > +1 s .d.) and 50% demonstrated osteosclerosis (Z-score > +2 s .d.). These patients had higher mast cell burden, serum tryptase levels, alkaline phosphate levels and KIT D816V allele burden even when compared to advanced SM patients without increased BMD (14). Thus, a rapid increase in bone density in this population, which may include our patient (highest Z-score +1.1 s .d. at the total hip) is not necessarily a favourable marker of bone health.
Treatment of osteoporosis in systemic mastocytosis
Predominance of osteoclastic activity and bone resorption is the driver of osteoporosis in patients with SM, and thus antiresorptive agents have been studied in its treatment. Rossini et al. examined the effect of a single dose of intravenous zoledronic acid 5 mg in 25 patients (13 men and 12 postmenopausal women) with SM and bone density T-score ≤−2.5 or fragility fracture without previous bisphosphonate treatment (15). This led to an increase in bone density of 6.0 ± 4.4% at the lumbar spine, 2.4 ± 3.2% at the total hip, a 35% decrease in bone alkaline phosphatase (bALP) and a 56% decrease in C-terminal collagen telopeptide (CTX) at 12 months (15). The only study to investigate the efficacy of denosumab in SM involved four postmenopausal women with BMD T-score ≤−2.5 and a fragility fracture, who had received oral bisphosphonate therapy 3 months before the study (16). Subcutaneous denosumab 60 mg for 6 months led to increases in bone density at the lumbar spine (+0.4–0.5 s.d.) and femoral neck (+0.1–0.6 s.d.) with a reduction in bALP and CTX at 12 months (16). Serum tryptase levels were also noted to decrease in all four patients, raising the hypothesis of denosumab exerting negative feedback on mast cells from suppressed osteoclastogenesis; however, this cannot be concluded due to the small sample size and paucity of data on whether patients were also on disease-modifying treatment such as midostaurin. Tryptase levels in our patient did not decline and in fact steadily increased during the past few years, indicative of ongoing mast cell activation.
There are limited data on the role of newer osteoanabolic agents. The PTH analogue teriparatide may promote further proliferation of mast cells and has not been recommended in this setting (9). Dickkopf-1 (DKK1) and sclerostin, receptor inhibitors of the Wnt pathway, have been studied in patients with SM, with DKK1 levels appearing to be elevated in comparison to controls but not sclerostin (17). It is thus unclear whether the Wnt/β-catenin pathway plays a significant role in the pathophysiology of SM. Nevertheless, in the setting of osteosclerosis and increased bone formation, treatment with a sclerostin inhibitor is not advisable.
In summary, our case highlights the difficulty of managing osteoporosis and osteosclerosis in a patient with progressive SM. While denosumab appeared effective in reversing an initial decline in bone density, the dramatic improvement may also be related to the pro-osteoblastic effects of mediators associated with more advanced disease.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written consent has been obtained from the patient after full explanation of the purpose and nature of all procedures used, and is available upon request.
Ethics and integrity policies
Ethics approval was not required as this is a case report.
Author contribution statement
M Wang was involved in the clinical management of the patient and wrote the manuscript. M Seibel oversaw the clinical management of the patient and assisted with the editing of the manuscript.
Acknowledgements
We would like to acknowledge Timothy Wade and the pathology department for providing photographs of the case.
References
- 1.Pardanani A. Systemic mastocytosis in adults: 2021 Update on diagnosis, risk stratification and management. American Journal of Hematology 202196508–525. ( 10.1002/ajh.26118) [DOI] [PubMed] [Google Scholar]
- 2.Akin C & Metcalfe DD. Systemic mastocytosis. Annual Review of Medicine 200455419–432. ( 10.1146/annurev.med.55.091902.103822) [DOI] [PubMed] [Google Scholar]
- 3.Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O, Awan FT, Hexner E, Mauro MJ, Sternberg DW, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. New England Journal of Medicine 20163742530–2541. ( 10.1056/NEJMoa1513098) [DOI] [PubMed] [Google Scholar]
- 4.Ragipoglu D Dudeck A Haffner-Luntzer M Voss M Kroner J Ignatius A & Fischer V. The role of mast cells in bone metabolism and bone disorders. Frontiers in Immunology 202011 163. ( 10.3389/fimmu.2020.00163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossini M, Zanotti R, Viapiana O, Tripi G, Orsolini G, Idolazzi L, Bonadonna P, Schena D, Escribano L, Adami S, et al. Bone involvement and osteoporosis in mastocytosis. Immunology and Allergy Clinics of North America 201434383–396. ( 10.1016/j.iac.2014.01.011) [DOI] [PubMed] [Google Scholar]
- 6.Greene LW Asadipooya K Corradi PF & Akin C. Endocrine manifestations of systemic mastocytosis in bone. Reviews in Endocrine and Metabolic Disorders 201617419–431. ( 10.1007/s11154-016-9362-3) [DOI] [PubMed] [Google Scholar]
- 7.Rabenhorst A Christopeit B Leja S Gerbaulet A Kleiner S Förster A Raap U Wickenhauser C & Hartmann K. Serum levels of bone cytokines are increased in indolent systemic mastocytosis associated with osteopenia or osteoporosis. Journal of Allergy and Clinical Immunology 20131321234–1237.e7. ( 10.1016/j.jaci.2013.06.019) [DOI] [PubMed] [Google Scholar]
- 8.van der Veer E van der Goot W de Monchy JG Kluin-Nelemans HC & van Doormaal JJ. High prevalence of fractures and osteoporosis in patients with indolent systemic mastocytosis. Allergy 201267431–438. ( 10.1111/j.1398-9995.2011.02780.x) [DOI] [PubMed] [Google Scholar]
- 9.Rossini M, Zanotti R, Orsolini G, Tripi G, Viapiana O, Idolazzi L, Zamò A, Bonadonna P, Kunnathully V, Adami S, et al. Prevalence, pathogenesis, and treatment options for mastocytosis-related osteoporosis. Osteoporosis International 2016272411–2421. ( 10.1007/s00198-016-3539-1) [DOI] [PubMed] [Google Scholar]
- 10.Rossini M, Zanotti R, Bonadonna P, Artuso A, Caruso B, Schena D, Vecchiato D, Bonifacio M, Viapiana O, Gatti D, et al. Bone mineral density, bone turnover markers and fractures in patients with indolent systemic mastocytosis. Bone 201149880–885. ( 10.1016/j.bone.2011.07.004) [DOI] [PubMed] [Google Scholar]
- 11.Guillaume N, Desoutter J, Chandesris O, Merlusca L, Henry I, Georgin-Lavialle S, Barete S, Hirsch I, Bouredji D, Royer B, et al. Bone complications of mastocytosis: a link between clinical and biological characteristics. American Journal of Medicine 201312675.e1–75.e7. ( 10.1016/j.amjmed.2012.07.018) [DOI] [PubMed] [Google Scholar]
- 12.Johansson C Roupe G Lindstedt G & Mellström D. Bone density, bone markers and bone radiological features in mastocytosis. Age and Ageing 1996251–7. ( 10.1093/ageing/25.1.1) [DOI] [PubMed] [Google Scholar]
- 13.Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, Czerwiński E, Fahrleitner-Pammer A, Kendler DL, Lippuner K, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM Trial and open-label extension. Lancet. Diabetes and Endocrinology 20175513–523. ( 10.1016/S2213-8587(1730138-9) [DOI] [PubMed] [Google Scholar]
- 14.Riffel P, Schwaab J, Lutz C, Naumann N, Metzgeroth G, Fabarius A, Schoenberg SO, Hofmann WK, Valent P, Reiter A, et al. An increased bone mineral density is an adverse prognostic factor in patients with systemic mastocytosis. Journal of Cancer Research and Clinical Oncology 2020146945–951. ( 10.1007/s00432-019-03119-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossini M Zanotti R Viapiana O Tripi G Idolazzi L Biondan M Orsolini G Bonadonna P Adami S & Gatti D. Zoledronic acid in osteoporosis secondary to mastocytosis. American Journal of Medicine 20141271127.e1–1127.e4. ( 10.1016/j.amjmed.2014.06.015) [DOI] [PubMed] [Google Scholar]
- 16.Orsolini G Gavioli I Tripi G Viapiana O Gatti D Idolazzi L Zanotti R & Rossini M. Denosumab for the treatment of mastocytosis-related osteoporosis: A case series. Calcified Tissue International 2017100595–598. ( 10.1007/s00223-017-0241-z) [DOI] [PubMed] [Google Scholar]
- 17.Rossini M Viapiana O Zanotti R Tripi G Perbellini O Idolazzi L Bonifacio M Adami S & Gatti D. Dickkopf-1 and sclerostin serum levels in patients with systemic mastocytosis. Calcified Tissue International 201596410–416. ( 10.1007/s00223-015-9969-5) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a