Abstract
Background
Molecular point-of-care testing (POCT) used in primary care can inform whether a patient presenting with an acute respiratory infection has influenza. A confirmed clinical diagnosis, particularly early in the disease, could inform better antimicrobial stewardship. Social distancing and lockdowns during the COVID-19 pandemic have disturbed previous patterns of influenza infections in 2021. However, data from samples taken in the last quarter of 2022 suggest that influenza represents 36% of sentinel network positive virology, compared with 24% for respiratory syncytial virus. Problems with integration into the clinical workflow is a known barrier to incorporating technology into routine care.
Objective
This study aims to report the impact of POCT for influenza on antimicrobial prescribing in primary care. We will additionally describe severe outcomes of infection (hospitalization and mortality) and how POCT is integrated into primary care workflows.
Methods
The impact of POCT for influenza on antimicrobial stewardship (PIAMS) in UK primary care is an observational study being conducted between December 2022 and May 2023 and involving 10 practices that contribute data to the English sentinel network. Up to 1000 people who present to participating practices with respiratory symptoms will be swabbed and tested with a rapid molecular POCT analyzer in the practice. Antimicrobial prescribing and other study outcomes will be collected by linking information from the POCT analyzer with data from the patient’s computerized medical record. We will collect data on how POCT is incorporated into practice using data flow diagrams, unified modeling language use case diagrams, and Business Process Modeling Notation.
Results
We will present the crude and adjusted odds of antimicrobial prescribing (all antibiotics and antivirals) given a POCT diagnosis of influenza, stratifying by whether individuals have a respiratory or other relevant diagnosis (eg, bronchiectasis). We will also present the rates of hospital referrals and deaths related to influenza infection in PIAMS study practices compared with a set of matched practices in the sentinel network and the rest of the network. We will describe any difference in implementation models in terms of staff involved and workflow.
Conclusions
This study will generate data on the impact of POCT testing for influenza in primary care as well as help to inform about the feasibility of incorporating POCT into primary care workflows. It will inform the design of future larger studies about the effectiveness and cost-effectiveness of POCT to improve antimicrobial stewardship and any impact on severe outcomes.
International Registered Report Identifier (IRRID)
DERR1-10.2196/46938
Keywords: medical records systems, computerized; influenza point-of-care systems; general practice; RSV; implementation; outcome assessment; health care; antimicrobial stewardship; acute respiratory infection; antimicrobial; influenza; primary care; respiratory symptom
Introduction
Rapid Testing for Influenza in a Postpandemic Health Service
Measures to limit SARS-CoV-2 transmission during the COVID-19 pandemic coincided with a marked decrease in infections caused by other respiratory viruses [1,2].
After the lifting of COVID-19 containment measures, the robust return of influenza, seasonal coronaviruses, parainfluenza virus, and respiratory syncytial virus (RSV) was observed in many countries including the United Kingdom [3].
Figure 1 and Table 1 show data from the English national sentinel surveillance network as of the end of November 2022. Influenza and RSV were the main circulating respiratory viruses, with SARS-CoV-2 making up only 12.4% (15/121) of the total number of laboratory-confirmed cases.
Figure 1.
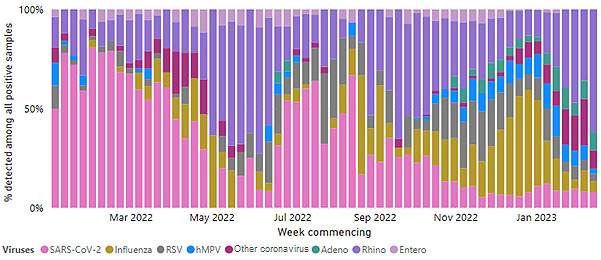
Swab positivity by week in the English national sentinel surveillance network from March 2022 to January 2023 [4]. hMPV: human metapneumovirus; RSV: respiratory syncytial virus.
Table 1.
Swab positivity by week in the English national sentinel surveillance network between September 2022 and November 2022 (n=818).
| Week commencing | Swab positivity | |||||
|
|
Adenovirus (n=54) | hMPVa (n=85) | Influenza (n=215) | Other coronaviruses (n=13) | RSVb (n=255) | SARS-CoV-2 (n=196) |
| 9/26/2022 (n=26), n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 11 (42.3) | 15 (57.7) |
| 10/3/2022 (n=54), n (%) | 0 (0) | 1 (1.9) | 20 (37) | 0 (0) | 6 (11.1) | 27 (50) |
| 10/10/2022 (n=52), n (%) | 0 (0) | 2 (3.8) | 14 (26.9) | 1 (1.9) | 6 (11.5) | 29 (55.8) |
| 10/17/2022 (n=70), n (%) | 0 (0) | 6 (8.6) | 19 (27.1 | 2 (2.9) | 17 (24.3 | 26 (37.1) |
| 10/24/2022 (n=82), n (%) | 0 (0) | 6 (7.3) | 28 (34.1 | 0 (0) | 30 (36.6 | 18 (22) |
| 10/31/2022 (n=94), n (%) | 9 (9.6) | 9 (9.6) | 31 (33) | 1 (1.1) | 24 (25.5 | 20 (21.3) |
| 11/7/2022 (n=95), n (%) | 12 (12.6) | 11 (11.6) | 16 (16.8) | 4 (4.2) | 35 (36.8) | 17 (17.9) |
| 11/14/2022 (n=122), n (%) | 6 (4.9) | 18 (14.8) | 34 (27.9) | 2 (1.6) | 42 (34.4) | 20 (16.4) |
| 11/21/2022 (n=102), n (%) | 12 (11.8) | 14 (13.7) | 27 (26.5) | 0 (0) | 40 (39.2) | 9 (8.8) |
| 11/28/2022 (n=121), n (%) | 15 (12.4) | 18 (14.9) | 26 (21.5) | 3 (2.5) | 44 (36.4) | 15 (12.4) |
ahMPV: human metapneumovirus.
bRSV: respiratory syncytial virus.
In the context of a postpandemic health care service where there are high levels of circulating influenza, accurate, rapid molecular diagnostic test platforms have the potential to (1) improve clinical decision-making regarding the use of antibiotics and antivirals, (2) improve patient outcomes due to the early appropriate use of antivirals, and (3) provide better information to inform sentinel surveillance and clinical research including studies of vaccine effectiveness and real-world trials [5,6].
Specifically, for patients with influenza infection, early diagnosis and administration of antivirals may improve clinical outcomes [7,8]. They may also limit symptom duration and spread to household contacts, and newer antivirals for influenza, such as baloxavir, have been shown to improve the time to resolution of symptoms and reduce complications in high-risk patients [9].
Currently, only a small proportion of patients with respiratory tract infections (RTIs) undergoes diagnostic microbiological testing before receiving treatments in primary care [10], and there is evidence of widespread variations in antimicrobial prescribing practices [11]. This is important, as prescribing in primary care accounts for about 8% of National Health Service (NHS) expenditure, which is equivalent to over £9 billion (US $11.1 billion) per year, with just over £220 million (US $271.5 million) being spent on antimicrobials [12]. Inappropriate prescribing of antimicrobials and unwarranted variation in prescribing can contribute to increase of antimicrobial-resistant strains and patient adverse events in the short and long term [13].
We have previously shown that, in a prepandemic context, it is feasible to undertake point-of-care testing (POCT) for influenza in primary care in the England, with promising impacts on antimicrobial use and estimates of influenza vaccine effectiveness that are comparable with published data [14-16]. However, its impact on more severe outcomes such as hospitalization and mortality following infection has not been reported.
Recent work on in-pandemic prescribing of azithromycin and doxycycline for RTIs across the sentinel network has shown scope for measures to improve antibiotic stewardship in primary care [17].
With the ending of widespread national testing for SARS-CoV-2 and other RTIs in the United Kingdom in March 2022 [18] and with high levels of circulating influenza in a post-COVID-19 health service during the autumn 2022 compared with 2021, there is a need to revisit questions about the feasibility of implementing rapid diagnosis of influenza during the expected peak of viral circulation this winter (2022-2023) season and its impact on clinical management in terms of improved antimicrobial stewardship and severe outcomes (hospitalization and mortality).
Objectives
This study aims to report the impact of POCT for influenza on antimicrobial prescribing in primary care. We will additionally describe severe outcomes postinfection (hospitalization and mortality) and how POCT is integrated into primary care workflows.
Methods
Study Setting and Population
The impact of POCT for influenza on antimicrobial stewardship (PIAMS) study will take place between December 2022 and May 2023, during the influenza season as defined by the UK Health Security Agency (UKHSA). It is nested within the English national sentinel network managed by the Oxford-Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) at the Nuffield Department of Primary Care, University of Oxford. This network of over 1800 primary care practices is the primary infectious disease surveillance network for England, which is generally representative of the English population [19]. It has been providing weekly infection data for over 50 years that are used to monitor trends in infectious disease and investigate real-world vaccine and treatment effectiveness [20].
All practices that contribute data to the English national sentinel network will be invited to participate in the PIAMS study, and 10 practices will be selected to take part in the PIAMS study. We will prioritize practices within the network with capacity to undertake influenza POCT and who had previously been involved in SARS-CoV-2 POCT through the RAPTOR-C19 study [21]. Those practices that have a history of less than 80% complete data returns during the previous winter season will be excluded.
Each participant practice will receive training about the study, including hands-on training on how to administer a swab test and how to use the POCT analyzers.
Case Definition of Eligible Patients
All patients registered within the PIAMS study practice who do not have an opt-out code on their medical record and are showing symptoms of influenza-like illness (ILI), acute respiratory illness (ARI), or fever of higher than 37.5 °C will be eligible for the study if they consent to participate. We will use the European Centre for Disease Prevention and Control case definitions of ILI and ARI for this study. We will use the following exclusion criteria: (1) patient has an opt-out code on their medical record or (2) no informed consent.
Face-to-face Recruitment of Eligible Patients and Point-of-care Testing for Influenza
We will undertake opportunistic swab sampling for this study, with potential participants being identified from those registered patients who present to the study practice with respiratory symptoms described in the case definition. No screening nor eligibility assessment will be undertaken. Blinding of participants and researchers will not be undertaken.
Eligible patients or their parent or legal guardian will be approached by a practice general practitioner (GP) or research nurse to explain the study and ask for consent to take part when they present for a face-to-face consultation at the practice.
After obtaining consent, a nasopharyngeal swab will be taken by a suitably qualified and experienced GP or research nurse. For those who do not attend the practice in person, a self-test kit will be sent to their home.
The cobas Liat POCT analyzer, manufactured by Roche Diagnostics International, will be used for this study [22]. This POCT analyzer is an automated multiplex polymerase chain reaction system, with previous studies demonstrating excellent performance comparable with gold standard laboratory assays, with sensitivity and specificity in the region of 100% and 97.1%-100%, respectively, for influenza A and 97.8%-100% and 99.5%-99.7%, respectively, for influenza B when fresh, prospectively collected samples are tested [23,24]. It has CE-marking in the European Union. In the United States, it is Food and Drug Administration–cleared, and it is also Clinical Laboratory Improvement Amendments–waived for use for rapid influenza testing. This study will not assess the accuracy of the POCT analyzer but will use the machine for its approved purpose.
The swab will be inoculated in a test kit and tested on the cobas Liat analyzer as soon as possible after being taken. The results will be available to the clinician in less than 20 minutes.
Antimicrobial prescribing and other study outcomes for those who have been swabbed will be obtained by linking information from the cobas Liat analyzer with data from the patient’s computerized medical record in primary care. We will demonstrate the feasibility of reporting antimicrobial stewardship and severe outcomes postinfection. Severe outcomes postinfection such as hospitalization and mortality will be obtained by linking the patient’s primary care computerized medical record to secondary care records at the national level including hospitalization and mortality data. A recent study used a linked cohort to study severe outcomes from COVID-19 infection [25].
Virtual Recruitment of Eligible Patients and Home Swabbing of Recruited Patients
We envisage that the majority of eligible patients will be seen face-to-face for this study. Where the eligible patient is not able to attend the practice in person, they will be told about the study remotely, and verbal consent will be taken.
A home swabbing kit will be sent to consented patients who are not able to attend the practice in person. This will include a nasal swab and instructions on how to take a self-test. Further details of our patients self-swabbing at home service have been previously published [26]. Once the nasal swab has been taken, the patient will be asked to place the swab in the included transport container with the manufacturer-approved universal transport media or viral transport media and inform the study practice staff who will arrange collection of the swab sample within 4 hours of being taken for immediate POCT.
Clinical Workflow Integration
Data regarding the utility of the influenza POCT in primary care and the issues and barriers to implementation of influenza POCT in primary care workflows will be collected by ethnographic observation of study practices and by a semistructured survey of clinicians and practice staff at the participating practices. Information will be collected about the following domains previously found to be important to implementation of POCT sampling [27]: usability of the POCT platform, clinical pathways and training, result reporting, clinical governance, costs, monitoring of effectiveness.
The ethnographic observations, semistructured survey, and reported POCT results will be used to assess which practices were more successful at integrating POCTs into their practice processes by comparing business process models of the practices. Business process models are graphical representations of business-oriented processes within an organization. This is helpful to model collaborations and business transactions within health systems. Business processes are typically modeled using Business Process Modeling Notation (BPMN). BPMN can be used to depict the end-to-end flow of a business process. The notation has been specifically designed to coordinate the sequence of processes and the messages that flow between different process participants in a related set of business activities [28,29].
Sample Size Calculation
The primary outcome measure is the proportion of consultations for RTI with all antibiotics prescribed during the winter season. We aim to detect a difference of ≤7% in the proportion of consultations for RTI in which antibiotics were prescribed, which is what we found previously between practices using POCT and the rest of the RCGP network [14,15]. We have assumed that the coefficient of variation between practices within the sentinel network, for the proportion of consultations with antibiotics prescribed, is 0.23 from Ashworth et al [11] with an α of .05 and a power of 80%. To detect a 5% difference in the proportion of consultations at which antibiotics are prescribed, we would need to sample 1023 people with ILI or ARI.
Previous swabbing rates across the network have averaged 6 swabs per practice per week in previous years. Given 10 practices swabbing a maximum of 3 swabs per week for 36 weeks, we would expect to collect up to 1000 face-to-face nasopharyngeal swabs from eligible people with ILI or ARI over the course of the study.
Statistical Methods
We will undertake descriptive analysis of the swabbing rates and swab positivity rates from the study practices. This includes analysis of the demographic and clinical characteristics of those presenting with RTI symptoms and those with confirmed influenza infections in primary care. We will also report the number of patients who have also undertaken swabbing for reference laboratory analysis in addition to POCT testing in study practices. In those study practices that also take part in virological surveillance, these reference laboratory swabs are reported to the UKHSA as part of national disease surveillance.
To answer our primary objective, we will calculate the crude odds of antimicrobial prescribing (all antibiotics and all antivirals regardless of type), given a POCT diagnosis of influenza is the probability of antimicrobial prescribing given a particular POCT test result. We will record whether the antimicrobials were given within 7 days of the POCT diagnosis or within 30 days of diagnosis. We will calculate the crude odds ratio by dividing the odds of antimicrobial prescribing in people who receive a positive influenza POCT result by the odds in people who receive a negative test result.
We will also calculate the adjusted odds ratio using a logistic regression model. We will use the following set of covariates that are known to be associated with the severity of influenza and are collected from the routine data extracted from the electronic health record: age, sex, ethnicity (reported in 5 categories: White, Asian, Black, other, or mixed; maximized using an ontology [30]), socioeconomic status (measured using the index of multiple deprivation [31], which is a nationally available measure assigned based on post code), any of these chronic underlying conditions (chronic pulmonary disease, cardiovascular disease, diabetes, liver disease, renal disease, neurologic or neuromuscular conditions, treatment-induced immunosuppression, and disease-induced immunosuppression), number of GP visits in the 12 months prior to the study period describing a study participant’s health care–seeking behavior, number of hospitalizations in the 12 months prior to the study period (used as proxy for the severity of the chronic conditions), influenza vaccination in previous influenza seasons (at least one), pregnancy, use of influenza antivirals, and pneumococcal and COVID-19 vaccination status.
To answer our secondary objective, we will select a matched set of practices from the rest of the English national sentinel network based on practice size, average age of registered patients, proportion of registered female patients, and number of GPs employed during the study.
The rates of hospital referrals and deaths related to influenza infection within 30 days of diagnosis will be compared between PIAMS study practices and the rest of the network using the ANOVA test.
Ethical Considerations
The study was approved by the English National Research Ethics Committees (Integrated Research Application System reference: 292961; REC reference: 21/YH/0077).
A practice GP or research nurse will undertake informed consent. This person will be suitably qualified and experienced and have been authorized to do so by the principal investigator. Once an eligible patient is identified, they or their parent or legal guardian will be approached by a practice GP or research nurse. Written and verbal versions of the participant information and informed consent will be presented to the patient or their parent or legal guardian detailing no less than the exact nature of the study, what it will involve for the participant, the implications and constraints of the protocol, and the known side effects and any risks involved in taking part. It will also be clearly stated that the participant is free to withdraw from the study at any time for any reason without prejudice to future care, without affecting their legal rights, and with no obligation to give a reason for withdrawal.
The patient will be allowed as much time as wished to consider the information and provided the opportunity to question the investigator, their GP, or other independent parties to decide whether they will participate in the study. Written informed consent will then be obtained by means of a dated signature and the dated signature of the person who presented and obtained the informed consent. A copy of the signed informed consent will be given to the participant. The original signed form will be retained at the study site.
When the eligible patient is not able to attend the practice in person, they will be told about the study remotely by a trained GP or nurse. The trained GP or nurse will go through the remote consent form with the participant over the phone. Once the participant has provided verbal consent to take part in the study, the researcher will sign a copy of the remote consent form and will provide the participant with a copy via a secure method such as NHS email. The patient will then be sent a swab to take at home and return to the practice within 4 hours of being taken for immediate POCT. Collection of the sample will be arranged by study staff.
For the ethnographic observations, we will verbally inform and orally consent the clinicians, a PIAMS researcher will record the consent process by completing a Record of Consent Form and the form will be signed in the presence of the participant to confirm oral consent. We will also provide information about the PIAMS study on practice posters to notify the patients about the observation activities associated with the nested qualitative study. Their consent will be implied by not opting out. A PIAMS researcher will be available to answer any questions they may have about observations undertaken for the nested qualitative study. Both clinicians and patients have the right to withdraw from participation or to decline to participate at any time without reason.
For the semistructured questionnaire, all survey respondents will complete an informed consent question embedded at the beginning of the anonymous questionnaire. If the participants answer “YES” to the first question of the form, this will imply their consent to participate, and the survey will begin. If the participants answer “NO” to the informed consent question, they will directly exit the current page. No participant will be forced to participate in the survey, and their participation will be based on their agreement, which can be withdrawn at any time.
Pseudonymized information about swab samples from consented patients, including participant ID, site ID, and swab result, will be stored in the POCT machines. These data will be extracted weekly from the machines and sent via an encrypted email to the study team to be stored in the research group’s secure network.
Data from study practice medical records will be extracted twice weekly from information systems of the RCGP RSC practices by Apollo, part of Wellbeing Software. These records are pseudonymized as close to the source as possible by Apollo using a nonreversible “hash” algorithm. Patients who decline to share their data are excluded from the extraction process.
Data for the study are held on dedicated secure servers at the RCGP data and analytics hub in the Clinical Informatics and Health Outcomes Research Group, University of Oxford. The research group’s secure network is located behind a firewall within the university’s network; all in-bounded connections are blocked, but out-bounded connections are allowed. Only staff members or associated members of the research group approved by the head of department can access the data from secure workstations or secure laptops with encrypted drive. All staff members of the research group working within the team base work from secure workstations or secure laptops with encrypted drive within the research group’s secure network. The use of personal equipment is not permitted and cannot be connected to the secure network. A risk assessment of the physical security of the research group's offices and server room has been conducted by the building and facilities manager, the faculty information technology (IT) service manager, and the research group's information governance lead.
Survey data for the nested qualitative substudy are securely collected using Jisc (online surveys). The university has a license agreement to use this service and has recommended the service for gathering confidential data. Extraction of the survey data is only available to people with a registered Jisc account, which the university has to approve. Only authorized study researchers will be able to export survey data once logged in to Jisc. Once survey data are extracted, the data will be stored within the secure Oxford-RCGP Clinical Informatics Digital Hub (ORCHID) network. Access to your data is strictly controlled. Only authorized study researchers will have access to only pseudonymized research data from within the aforementioned secure ORCHID network.
The university is compliant with the Data Protection Act and UK GDPR and has systems for technical and organizational controls for information security, including a university-level Information Security and Governance Group chaired by the university senior information risk owner. The research group’s private network has its own system-level security policy and is tested for vulnerabilities annually.
Study practices are provided with a fee for participating in this study to cover the costs of training staff and hosting the study. A small remuneration is also provided to practices for each swab taken to cover the additional time taken during each consultation to undertake swabbing for this study. Patients are not remunerated for taking part in this study.
Results
The study started in December 2022, and we expect to complete the final analysis for the study by June 2023.
Baseline Data From the English National Sentinel Surveillance Network
In terms of context for this study, currently, the English national sentinel network had grown from just over 500 practices in 2019 to 1879 practices by October 2021. This represents 28.6% of English primary care practices and 31% of the population (N=17,560,196). The sentinel network’s population was found to be broadly representative of the national population in terms of age, gender, ethnicity, NHS Region, socioeconomic status, obesity, and smoking habit [19].
Latest data from across the sentinel network, between October 2020 and October 2021, show that practices have collected >8000 virology swab samples for reference laboratory testing [19]. Figure 1 and Table 1 show the latest virology swab positivity by week in the English national sentinel surveillance network prior to the start of the study.
Protocol Amendments
Important protocol amendments will be referred to the English National Research Ethics Committees for ethical approval. Once approved, changes will be communicated directly with the recruiting study practices. The amended protocol will be shared with all relevant parties (eg, investigators and clinical research networks) in a timely manner.
Discussion
Overview
This study will generate data on the impact of POCT testing for influenza in primary care, especially information on its impact on antimicrobial prescribing. Additionally, the study will describe severe outcomes postinfection (hospitalization and mortality) and how POCT is integrated into primary care workflows.
It will be a chance to revisit questions about the feasibility of implementing rapid diagnosis of influenza following the end of widespread national testing for SARS-CoV-2 and other RTIs in the United Kingdom and previous studies about its promising impacts prepandemic [14-16].
Limitations
The nonrandomized design is a limitation of our study. However, we feel that there is still value in examining the objectives within this study using a mixed methods approach as there are many questions about the challenges and barriers to implementation of rapid diagnostic testing in primary care, including the variability of adoption in different practices that are important to understand [15]. Unpacking these factors using a mixed methods design will be important to design a successful experimental study at scale to answer questions related to health outcomes. Additionally, information about influenza subtype is not available from the POCT machines used in this study and will restrict interpretation of our results, especially for calculating influenza vaccine effectiveness. A possible solution to this would have been to ask study practices to send their swabs to the national reference laboratory for additional analysis. However, this would have introduced a further step in the testing procedure in the clinic, which may have increased the risk of aerosol generation. Given that the study was originally proposed during the COVID-19 pandemic, we felt it was important to minimize the risk of generating aerosol droplets and thus did not include this in the study.
Strengths
A strength of the study is that it is conducted among practices that are nested in the RCGP RSC English sentinel surveillance network and had also been involved in POCT sampling in previous years. This ensured that practice staff had experience with using rapid diagnostic machines, minimizing the number of spoiled samples. It will also allow comparisons of the performance of practices using POCT for influenza testing with other practices in the sentinel network that participate in the usual virology sampling program conducted by the UKHSA.
Conclusion
Building on previous work showing the promising impacts of rapid, near-patient testing on antimicrobial use in primary care within the context of a prepandemic health service, this study will help inform about the feasibility of incorporating POCT into primary care workflows. It will inform the design of future larger studies about the effectiveness and cost-effectiveness of POCT to improve antimicrobial stewardship and any impact on severe outcomes.
Acknowledgments
We acknowledge Professor Maria Zambon from the UK Health Security Agency for her advice about the use of point-of-care testing in the National Health Service. We also acknowledge the participating practices for sharing data and patients and their parents or carers for volunteering to participate in this study as well as In Practice Systems and Wellbeing for their collaboration with pseudonymized data extraction. We thank Elissa Robbins, Karen Gilliam, Susie Ochoa, and Babar Javed from Roche Diagnostics for the scientific discussions regarding the design of the study protocol and their support in undertaking this study.
This collaborative study is funded by Roche Diagnostics through an investigator-initiated grant. The manuscript was also reviewed by Roche Diagnostics before submission.
The University of Oxford sponsored this study and reviewed and proposed edits to the study protocol and study documents prior to submission for ethics approval. The Nuffield Department of Primary Care Health Sciences, University of Oxford is the coordinating center.
Abbreviations
- ARI
acute respiratory illness
- BPMN
Business Process Modeling Notation
- GP
general practitioner
- ILI
influenza-like illness
- NHS
National Health Service
- ORCHID
Oxford-Royal College of General Practitioners Clinical Informatics Digital Hub
- PIAMS
impact of POCT for influenza on antimicrobial stewardship
- POCT
point-of-care testing
- RCGP
Royal College of General Practitioners
- RSC
Research and Surveillance Centre
- RSV
respiratory syncytial virus
- RTI
respiratory tract infection
- UKHSA
UK Health Security Agency
Data Availability
Data cannot be shared publicly because the data are owned by the Oxford Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) and its participating practices. Data are available from the University of Oxford Institutional Data Access Committee (contact via ku.ca.xo.chp@nanigsuled.nomis) for researchers who meet the criteria for access to confidential data. The data underlying the results presented in the study are available from [32]. Further enquiries about the RCGP RSC network and data requests can be found at [33] or by emailing ku.ca.xo.chp@seiriuqneecitcarp.
Footnotes
Authors' Contributions: UH, AW, JS, CA, EB, JM, ML, CXX, and SdL contributed to the planning of the study. UH, AW, JS, CA, EB, JM, CO, RB, FF, ML, CXX, MJ, GM, TC, and SdL contributed to the reporting of the study protocol and to the conduct of the study to date.
Conflicts of Interest: SdL is director of the Oxford Royal College of General Practitioners Research and Surveillance Centre. Through his university, he has received funding for vaccine-related research from AstraZeneca, GSK, Sanofi, Seqirus, and Takeda; he has been a member of advisory boards for AstraZeneca, Sanofi, and Seqirus. UH has undertaken continuing professional development podcasts funded by Seqirus and has been a member of advisory boards for Janssen. TC has received grant funding and/or equipment and consumables free of charge for the purposes of independent research from BioFire diagnostics, Biomerieux, QIAGEN and Sherlock Biosciences. They have received speaker fees, honoraria and travel re-imbursement from BioFire diagnostics, BioMerieux, QIAGEN and Janssen. TC has also received consultancy fees from BioMerieux, QIAGEN, Cepheid, Roche, Janssen and Synairgen research. TC has been a member of advisory boards for Cepheid, Roche, Janssen, Shiongi, Sanofi and Seqirus and acted as a member of independent data monitoring committees for trials sponsored by Roche and received remuneration at fair market value for these services. TS is also a member of an independent data monitoring committee for a Roche-sponsored study.
References
- 1.Olsen SJ, Azziz-Baumgartner E, Budd AP, Brammer L, Sullivan S, Pineda RF, Cohen C, Fry AM. Decreased influenza activity during the COVID-19 pandemic - United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep. 2020 Sep 18;69(37):1305–1309. doi: 10.15585/mmwr.mm6937a6. doi: 10.15585/mmwr.mm6937a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Summeren J, Meijer A, Aspelund G, Casalegno J, Erna G, Hoang U, Lina B, VRS study group in Lyon. de Lusignan S, Teirlinck AC, Thors V, Paget J. Low levels of respiratory syncytial virus activity in Europe during the 2020/21 season: what can we expect in the coming summer and autumn/winter? Euro Surveill. 2021 Jul;26(29):2100639. doi: 10.2807/1560-7917.ES.2021.26.29.2100639. https://hal.archives-ouvertes.fr/hal-03681859 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodjat P, Christensen PA, Subedi S, Bernard DW, Olsen RJ, Long SW. The reemergence of seasonal respiratory viruses in Houston, Texas, after relaxing COVID-19 restrictions. Microbiol Spectr. 2021 Oct 31;9(2):e0043021. doi: 10.1128/Spectrum.00430-21. https://journals.asm.org/doi/10.1128/Spectrum.00430-21?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virology Dashboard. Royal College of General Practitioners Research and Surveillance Centre. [2023-02-23]. https://tinyurl.com/mrvd34y3 .
- 5.Leland DS, Ginocchio CC. Role of cell culture for virus detection in the age of technology. Clin Microbiol Rev. 2007 Jan;20(1):49–78. doi: 10.1128/CMR.00002-06. https://europepmc.org/abstract/MED/17223623 .20/1/49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zavattoni M, Percivalle E, Cattaneo E, Revello MG, Torsellini M, Gerna G. Optimized detection of respiratory viruses in nasopharyngeal secretions. New Microbiol. 2003 Apr;26(2):133–40. [PubMed] [Google Scholar]
- 7.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012 Jan;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X.S1473-3099(11)70295-X [DOI] [PubMed] [Google Scholar]
- 8.Jefferson T, Jones M, Doshi P, Del Mar CB, Heneghan CJ, Hama R, Thompson MJ. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2012 Jan 18;1:CD008965. doi: 10.1002/14651858.CD008965.pub3. https://core.ac.uk/reader/189829908?utm_source=linkout . [DOI] [PubMed] [Google Scholar]
- 9.Ison MG, Portsmouth S, Yoshida Y, Shishido T, Hayden F, Uehara T. LB16. Phase 3 trial of baloxavir marboxil in high-risk influenza patients (CAPSTONE-2 study) Open Forum Infectious Diseases. 2018;5(Suppl 1):S764–S765. doi: 10.1093/ofid/ofy229.2190. [DOI] [PubMed] [Google Scholar]
- 10.Linder JA, Nieva HR, Blumentals WA. Antiviral and antibiotic prescribing for influenza in primary care. J Gen Intern Med. 2009 Apr;24(4):504–10. doi: 10.1007/s11606-009-0933-9. https://europepmc.org/abstract/MED/19225847 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashworth M, Charlton J, Ballard K, Latinovic R, Gulliford M. Variations in antibiotic prescribing and consultation rates for acute respiratory infection in UK general practices 1995-2000. Br J Gen Pract. 2005 Aug;55(517):603–8. https://bjgp.org/lookup/pmidlookup?view=long&pmid=16105368 . [PMC free article] [PubMed] [Google Scholar]
- 12.English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) Public Health England. 2018. [2023-05-28]. https://webarchive.nationalarchives.gov.uk/ukgwa/20181130125613/https://www.gov.uk/government/publications/english-surveillance-programme-antimicrobial-utilisation-and-resistance-espaur-report .
- 13.Armstrong D, Ashworth M, Dregan A, White P. The relationship between prior antimicrobial prescription and meningitis: a case-control study. Br J Gen Pract. 2016 Apr;66(645):e228–33. doi: 10.3399/bjgp16X684313. https://bjgp.org/lookup/pmidlookup?view=long&pmid=26965030 .bjgp16X684313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lusignan S, Hoang U, Liyanage H, Tripathy M, Sherlock J, Joy M, Ferreira F, Diez-Domingo J, Clark T. Using point of care testing to estimate influenza vaccine effectiveness in the English primary care sentinel surveillance network. PLoS One. 2021;16(3):e0248123. doi: 10.1371/journal.pone.0248123. https://dx.plos.org/10.1371/journal.pone.0248123 .PONE-D-20-39506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Lusignan S, Hoang U, Liyanage H, Tripathy M, Yonova I, Byford R, Ferreira F, Diez-Domingo J, Clark T. Integrating molecular point-of-care testing for influenza into primary care: a mixed-methods feasibility study. Br J Gen Pract. 2020 Aug;70(697):e555–e562. doi: 10.3399/bjgp20X710897. https://bjgp.org/lookup/pmidlookup?view=long&pmid=32661013 .bjgp20X710897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Lusignan S, Hoang U, Liyanage H, Yonova I, Ferreira F, Diez-Domingo J, Clark T. Feasibility of point-of-care testing for influenza within a national primary care sentinel surveillance network in England: protocol for a mixed methods study. JMIR Res Protoc. 2019 Nov 11;8(11):e14186. doi: 10.2196/14186. https://www.researchprotocols.org/2019/11/e14186/ v8i11e14186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lusignan S, Joy M, Sherlock J, Tripathy M, van Hecke O, Gbinigie K, Williams J, Butler C, Hobbs FR. PRINCIPLE trial demonstrates scope for in-pandemic improvement in primary care antibiotic stewardship: a retrospective sentinel network cohort study. BJGP Open. 2021 Oct;5(5):1. doi: 10.3399/BJGPO.2021.0087. http://bjgpopen.org/lookup/pmidlookup?view=long&pmid=34312163 .BJGPO.2021.0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iacobucci G. Covid-19: Who will be eligible for free testing from 1 April? BMJ. 2022 Mar 25;376:o807. doi: 10.1136/bmj.o807. [DOI] [PubMed] [Google Scholar]
- 19.Leston M, Elson W, Watson C, Lakhani A, Aspden C, Bankhead C, Borrow R, Button E, Byford R, Elliot AJ, Fan X, Hoang U, Linley E, Macartney J, Nicholson BD, Okusi C, Ramsay M, Smith G, Smith S, Thomas M, Todkill D, Tsang RS, Victor W, Williams AJ, Williams J, Zambon M, Howsam G, Amirthalingam G, Lopez-Bernal J, Hobbs FDR, de Lusignan S. Representativeness, vaccination uptake, and COVID-19 clinical outcomes 2020-2021 in the UK Oxford-Royal College of General Practitioners Research and Surveillance Network: cohort profile summary. JMIR Public Health Surveill. 2022 Dec 19;8(12):e39141. doi: 10.2196/39141. https://publichealth.jmir.org/2022/12/e39141/ v8i12e39141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Lusignan S, Correa A, Smith GE, Yonova I, Pebody R, Ferreira F, Elliot AJ, Fleming D. RCGP Research and Surveillance Centre: 50 years' surveillance of influenza, infections, and respiratory conditions. Br J Gen Pract. 2017 Oct;67(663):440–441. doi: 10.3399/bjgp17X692645. https://bjgp.org/lookup/pmidlookup?view=long&pmid=28963401 .67/663/440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholson BD, Hayward G, Turner PJ, Lee JJ, Deeks A, Logan M, Moore A, Seeley A, Fanshawe T, Oke J, Koshiaris C, Sheppard JP, Hoang U, Parimalanathan V, Edwards G, Liyange H, Sherlock J, Byford R, Zambon M, Ellis J, Bernal JL, Amirthalingam G, Linley E, Borrow R, Howsam G, Baines S, Ferreira F, de Lusignan S, Perera R, Hobbs FDR. Rapid community point-of-care testing for COVID-19 (RAPTOR-C19): protocol for a platform diagnostic study. Diagn Progn Res. 2021 Feb 08;5(1):4. doi: 10.1186/s41512-021-00093-8. https://europepmc.org/abstract/MED/33557927 .10.1186/s41512-021-00093-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling L, Kaplan SE, Lopez JC, Stiles J, Lu X, Tang Y. Parallel validation of three molecular devices for simultaneous detection and identification of influenza A and B and respiratory syncytial viruses. J Clin Microbiol. 2018 Mar;56(3):1. doi: 10.1128/JCM.01691-17. https://europepmc.org/abstract/MED/29263204 .JCM.01691-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibson J, Schechter-Perkins EM, Mitchell P, Mace S, Tian Y, Williams K, Luo R, Yen-Lieberman B. Multi-center evaluation of the cobas® Liat® Influenza A/B & RSV assay for rapid point of care diagnosis. J Clin Virol. 2017 Oct;95:5–9. doi: 10.1016/j.jcv.2017.08.004.S1386-6532(17)30221-4 [DOI] [PubMed] [Google Scholar]
- 24.Gosert R, Naegele K, Hirsch HH. Comparing the cobas Liat influenza A/B and respiratory syncytial virus assay with multiplex nucleic acid testing. J Med Virol. 2019 Apr;91(4):582–587. doi: 10.1002/jmv.25344. https://europepmc.org/abstract/MED/30345524 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood A, Denholm R, Hollings S, Cooper J, Ip S, Walker V, Denaxas S, Akbari A, Banerjee A, Whiteley W, Lai A, Sterne J, Sudlow C, CVD-COVID-UK consortium Linked electronic health records for research on a nationwide cohort of more than 54 million people in England: data resource. BMJ. 2021 Apr 07;373:n826. doi: 10.1136/bmj.n826. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=33827854 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lusignan S, Lopez Bernal J, Byford R, Amirthalingam G, Ferreira F, Akinyemi O, Andrews N, Campbell H, Dabrera G, Deeks A, Elliot AJ, Krajenbrink E, Liyanage H, McGagh D, Okusi C, Parimalanathan V, Ramsay M, Smith G, Tripathy M, Williams J, Victor W, Zambon M, Howsam G, Nicholson BD, Tzortziou Brown V, Butler CC, Joy M, Hobbs FDR. Influenza and respiratory virus surveillance, vaccine uptake, and effectiveness at a time of cocirculating COVID-19: protocol for the English primary care sentinel system for 2020-2021. JMIR Public Health Surveill. 2021 Feb 19;7(2):e24341. doi: 10.2196/24341. https://publichealth.jmir.org/2021/2/e24341/ v7i2e24341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Point of care tests for influenza and other respiratory viruses. Public Health England. 2018. [2023-05-28]. https://tinyurl.com/5n77b8v2 .
- 28.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007 Nov;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043.S0091-6749(07)01823-4 [DOI] [PubMed] [Google Scholar]
- 29.Liyanage H, Luzi D, De Lusignan S, Pecoraro F, McNulty R, Tamburis O, Krause P, Rigby M, Blair M. Accessible Modelling of Complexity in Health (AMoCH) and associated data flows: asthma as an exemplar. J Innov Health Inform. 2016 Apr 18;23(1):863. doi: 10.14236/jhi.v23i1.863. https://informatics.bmj.com/lookup/pmidlookup?view=long&pmid=27348490 . [DOI] [PubMed] [Google Scholar]
- 30.Tippu Z, Correa A, Liyanage H, Burleigh D, McGovern A, Van Vlymen J, Jones S, De Lusignan S. Ethnicity recording in primary care computerised medical record systems: an ontological approach. J Innov Health Inform. 2017 Mar 14;23(4):920. doi: 10.14236/jhi.v23i4.920. https://informatics.bmj.com/lookup/pmidlookup?view=long&pmid=28346128 . [DOI] [PubMed] [Google Scholar]
- 31.English indices of deprivation 2019. Ministry of Housing, Communities and Local Government. 2019. Sep 26, [2023-05-28]. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019 .
- 32.Clinical Informatics and Health Outcomes Group. ORCHID. [2023-05-28]. https://orchid.phc.ox.ac.uk/
- 33.Using Oxford-RCGP RSC for observational studies. ORCHID. [2023-05-28]. https://orchid.phc.ox.ac.uk/index.php/orchid-data/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared publicly because the data are owned by the Oxford Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) and its participating practices. Data are available from the University of Oxford Institutional Data Access Committee (contact via ku.ca.xo.chp@nanigsuled.nomis) for researchers who meet the criteria for access to confidential data. The data underlying the results presented in the study are available from [32]. Further enquiries about the RCGP RSC network and data requests can be found at [33] or by emailing ku.ca.xo.chp@seiriuqneecitcarp.


