Abstract
Background and Aims:
The gastric epithelium undergoes continuous turnover. Corpus epithelial stem cells located in the gastric isthmus serve as a source of tissue self-renewal. We recently identified the transcription factor Mist1 as a marker for this corpus stem cell population that can give rise to cancer. The aim here was to investigate the regulation of the Mist1+ stem cells in the response to gastric injury and inflammation.
Methods:
We used Mist1CreERT;R26-Tdtomato mice in two models of injury and inflammation: the acetic acid-induced ulcer and infection with Helicobacter felis. We analyzed lineage tracing at both early (7–30d) and late (30–90d) time points. Mist1CreERT;R26-Tdtomato;Lgr5DTR-eGFP mice were used to ablate the corpus basal Lgr5+ cell population. Constitutional and conditional Wnt5a knockout mice were used to investigate the role of Wnt5a in wound repair and lineage tracing from the Mist1+ stem cells.
Results:
In both models of gastric injury, Mist1+ isthmus stem cells more rapidly proliferate and trace entire gastric glands compared to the normal state. In regenerating tissue, the number of traced gastric chief cells was significantly reduced, and ablation of Lgr5+ chief cells did not affect Mist1-derived lineage tracing and tissue regeneration. Genetic deletion of Wnt5a impaired proliferation in the gastric isthmus and lineage tracing from Mist1+ stem cells. Similarly, depletion of innate lymphoid cells (ILC2), the main source of Wnt5a, also resulted in reduced proliferation and Mist1+ isthmus cell tracing.
Conclusion:
Gastric Mist1+ isthmus cells are the main supplier of regenerated glands and are activated in part through Wnt5a pathway.
Keywords: Gastric ulcer, stem cells, regeneration, Wnt5a, Helicobacter
Introduction:
Acute epithelial injury results in increased tissue turnover and regeneration through the activation of stem and progenitor cells. Over time, chronic injury can lead to excessive stem cell activation and a higher risk of tumor development [1]. In the stomach, chronic inflammation such as Helicobacter pylori-dependent gastritis represents a risk factor for gastric cancer development [2]. While chronic gastritis is a long-lasting inflammatory condition that is often asymptomatic, peptic ulcer disease represents a more acute gastric injury that can lead to serious complications. At a pathological level, gastric ulcer comprises an epithelial defect that involves the entire mucosal thickness of the stomach. The healing of gastric ulcers consists of changes in both the epithelium and the stroma. Granulation tissue forms at the base as a protective covering and to assist in ulcer healing, while the margins of the ulcer formed by the adjacent epithelium are gradually reduced [3]. In addition, ulcer healing occurs in a series of stages. Immediately after ulcer formation, the healthy gastric glands in the adjacent healing zone become dilated, and epithelial cells on the surface begin to migrate inwards to cover the defect in a process known as restitution. Subsequently, the gastric glands surrounding the ulcer increase their rate of proliferation to expand the epithelial compartment and re-epithelialize the injured area [4].
The gastric epithelium, like other epithelia, has a great capacity for self-renewal and undergoes rapid turnover to maintain homeostasis. This process is maintained by gastric stem cells that reside within a stem cell niche [5]. Since the first description of an undifferentiated, “granule-free”, gastric stem cell [6], several potential markers of stem or progenitor cell populations in the gastric corpus and antrum have been reported, including Lgr5, Sox2, CCK2R, eR1 and Lrig1 [7–12]. A recent multi-color labeling study showed that int the corpus, isthmus stem cell-derived clones more rapidly expand when parietal cells are injured [13]. We previously identified the transcription factor Mist1 (also known as Bhlha15) as a marker of slowly dividing isthmus stem cells, and showed that Mist1+ cells can give rise to cancer in both the gastric corpus and antrum [14, 15]. In addition to its expression in isthmus stem cells, Mist1 also marks gastric chief cells, and several studies have described a potential role of chief cells in the response to injury and the development of metaplasia [16, 17]. While there has been no clear consensus on the role of chief cells in metaplasia and gastric cancer, recent studies have established that mature chief cells may not be required for the development of gastric metaplasia [18–20].
Chronic injury and inflammation represent major risk factors for the development of metaplasia, and are mediated by a complex network of pro- and anti-inflammatory cytokines [21]. A key regulator orchestrating the response of intestinal stem and progenitor cells to injury are the Wnt protein family members [22, 23]. While the function in regeneration of classical (canonical) members of this family is relatively well understood, much less is known about the role of atypical (non-canonical) Wnt family members. Recent data suggest that Wnt5a is particularly crucial for gastrointestinal wound healing [24]. Wnt5a is reported to be essential for the development of the intestine, and Wnt5a-null mice die at birth [25]. In the adult organism, Wnt5a is believed to regulate several cellular functions, including migration and proliferation, in diverse disease states [26]. In gastric cancer, high expression of Wnt5a is associated with more advanced and aggressive tumors [27]. Work from our laboratory has shown that Wnt5a is expressed in innate lymphoid type 2 cells (ILC2) that contribute to the niche for Mist1+ isthmus stem cells in the stomach, with upregulation of Wnt5a contributing to the development of gastric cancer. In this study, we sought to investigate the role of Mist1+ gastric isthmus stem cells in response to injury and ulceration of the stomach, and to determine how this process is mediated by the Wnt5a-dependent stem cell niche.
Methods:
Mice
Mist1-CreERT2 mice [28], Wnt5aflox/flox mice [29], and Wnt5a+/− [30] mice have been described previously and were purchased from Jackson lab. Lgr5-DTR-eGFP mice were provided by Genentech. Cxcr4-eGFP mice were kindly provided by Richard J. Miller (Northwestern University Medical School, USA). Cag-CreERT mice, R26-Tdtomato mice and C57/B6 wild-type (WT) mice were purchased from Jackson Lab. All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at Columbia University.
A detailed description of all methods used in this study can be found in the supplementary information.
Results:
Mist1 lineage tracing increases in response to acute injury and inflammation
We used the acetic acid ulcer model to investigate the response of Mist1+ gastric corpus stem cells to acute injury. Mist1CreERT2;R26-Tdtomato mice were given tamoxifen, and acetic acid was applied to the corpus 7 days later (Fig. S1A). We previously reported that under homeostatic conditions, the average doubling time of Mist1 corpus isthmus stem cells is around 5 days, leading to the first appearance of partially traced glands at 90 days after induction [14]. At d30 after ulcer injury, there was a significant increase in lineage tracing by Mist1;TdT+ cells in the regenerating tissue compared to control glands (Fig. 1A). The first partially traced glands in the regenerating tissue were observed as early as 15 days after injury, with a significantly increased number of traced glands at 30 days post-injury (d15: 16.0 glands/section; d30: 23.0 glands/section). At these time points, uninjured sections from the same animals did not show any partially traced glands (Fig. 1B/C).
Figure 1: Mist1 lineage tracing increases in epithelial regeneration.
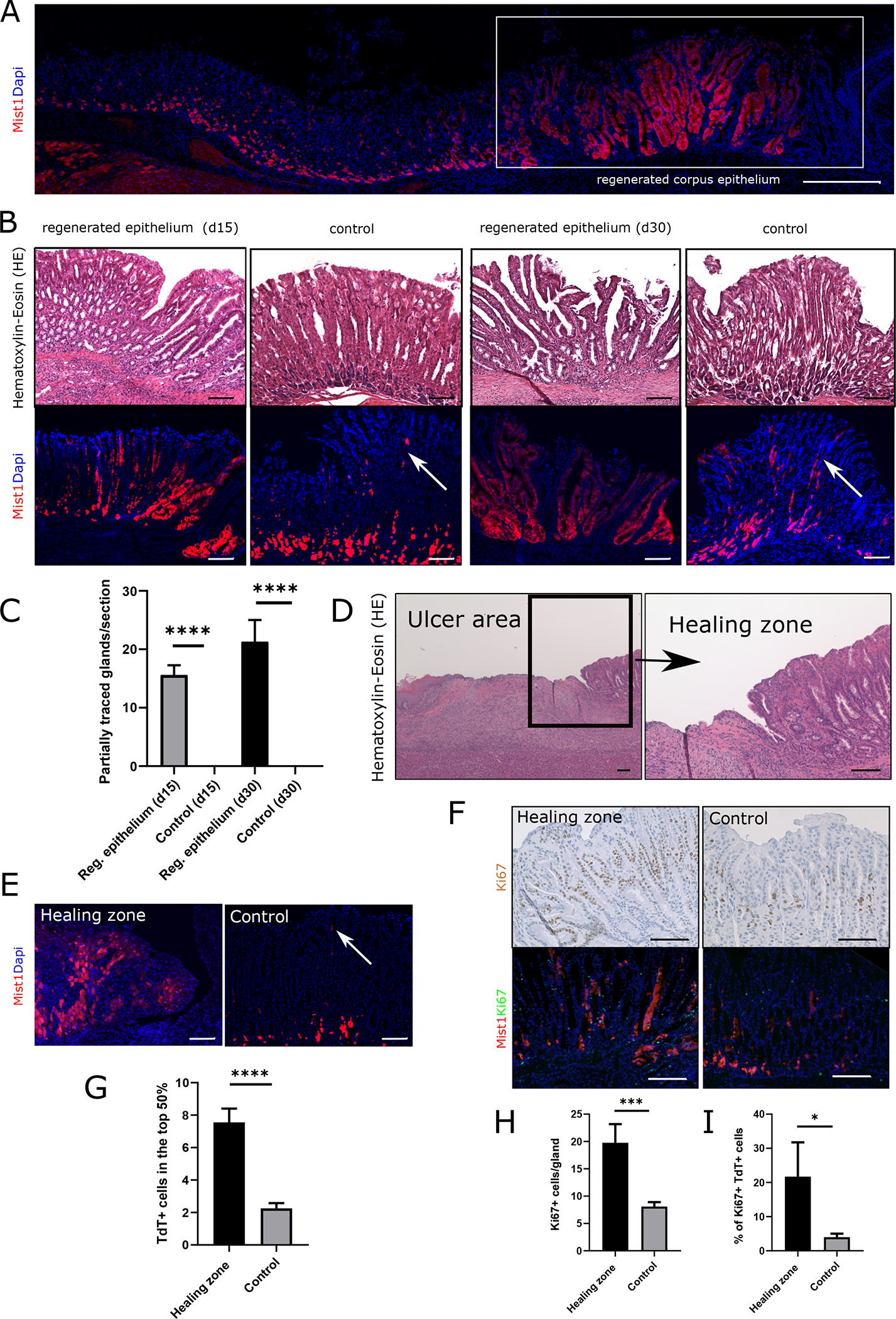
(A) Immunofluorescence of a cross section through the stomach epithelium of Mist1CreERT2;R26-Tdtomato mice at d30 after ulcer injury. Boxed area shows the regenerating epithelium in the corpus. (Scale bar = 500μm) (B) HE staining and immunofluorescence of Mist1CreERT2;R26-Tdtomato mice at d15 and d30 after ulcer injury. White arrows indicate Mist1;TdT+ isthmus stem cells in the control tissue. (Scale bars = 100μm) (C) Quantification of partially traced glands by Mist1;TdT+ cells at d15 and d30 after injury. Results are presented as means +/− SEM, *** p<0.001, * p<0.05 (n=6–7 animals per time point) (D) HE staining of the ulcer area in a WT mice at d7 after injury. Boxed area shows high magnification of healing zone at the wound edge. (Scale bars = 100μm) (E) Immunofluorescence of the healing zone in a Mist1CreERT;R26-Tdtomato mice at d7 after injury and corresponding imaging of uninjured control tissue. The white arrow in the control image indicates Mist1;TdT+ cells in the isthmus. (Scale bars = 100μm) (F) Image panel top: Ki67 staining of the healing zone in a WT mouse at d7 after injury and of corresponding uninjured control tissue. Ki67+ cells are labeled in brown. (Scale bars = 100μm) Image panel bottom: Ki67 staining and immunofluorescence of the healing zone and corresponding control tissue in Mist1CreERT;R26-Tdtomato mice at d7 after ulcer injury. Ki67+ cells are labeled in green. (Scale bars = 50μm) (G) Graph bar quantification of Mist1;TdT+ cells in the top half of the gland. (n=4) (H) Quantification of Ki67+ cells/gland and (I) percentage of Ki67+ Mist1;TdT+ cells in healing zone and control tissue (n=3)
In all figures results are presented as means +/− SEM, **** p<0.0001, *** p<0.001, ** p<0.01, * p<0.05
The process of gastric ulcer healing involves re-epithelialization from the margins followed by expansion of stem and progenitor cells [3]. To investigate the role of isthmus stem cells in this healing zone, Mist1-CreERT2;R26-Tdtomato mice were induced with tamoxifen as described above, and wound edges were examined at d7 after ulcer induction (Fig. 1D). At this early time point, we found an increased number of traced cells derived from isthmus Mist1;TdT+ stem cells in the healing zone at the wound edges (Fig. 1E/G) compared to uninjured tissue. Ki67+ staining revealed a significant increase in proliferation in the isthmus region adjacent to ulcers (Fig. 1F/H). While under homeostatic conditions only a minor percentage of the Mist1;TdT+ isthmus cells are Ki67 positive, this fraction significantly expanded in the healing zone. Co-staining of Ki67 in these Mist1-CreERT2;R26-Tdtomato traced mice showed a significant higher percentage of Ki67+/TdT+ cells compared to controls (Fig. 1I), suggesting increased proliferation by Mist1+ cells in response to acute injury.
The acetic acid ulcer model is a short-term injury model with almost complete epithelial regeneration evident at d30. Nevertheless, long-term changes in the epithelium can be observed after healing of peptic ulcers, particularly in the setting of chronic H. pylori-associated gastritis [31]. To investigate the response of Mist1;TdT+ cells to long-term damage by chronic inflammation, we used the Helicobacter felis infection model. Mist1-CreERT2;R26-Tdtomato mice were induced with tamoxifen and then infected with H. felis (Fig. S1B). Successful infection with H. felis was confirmed by Steiner silver staining at d30 after inoculation (Fig. S2E). Similar to acute injury, chronic inflammation increased lineage tracing from Mist1;TdT+ cells, and partially traced glands could be observed as early as 30 days post injury (Fig. 2A). Under homeostatic conditions, the first partially traced glands were detected at 90 days, but even at this time point, the number of lineage traced glands was significantly higher in Helicobacter-infected mice (Fig. 2B). Similar to the acetic acid ulcer model, we found a significantly higher number of Ki67+/TdT+ cells after H. felis infection compared to uninfected controls (Fig. 2C/E), indicating the presence of increased proliferation by Mist1;TdT+ cells in response to chronic infection. Areas with increased lineage tracing by Mist1;TdT+ cells showed increased staining for CD45+ inflammatory cells, suggesting a correlation between local inflammation and increased tracing activity by Mist1;TdT+ cells (Fig. S2F).
Figure 2: Mist1 lineage tracing increases in response to Helicobacter infection.
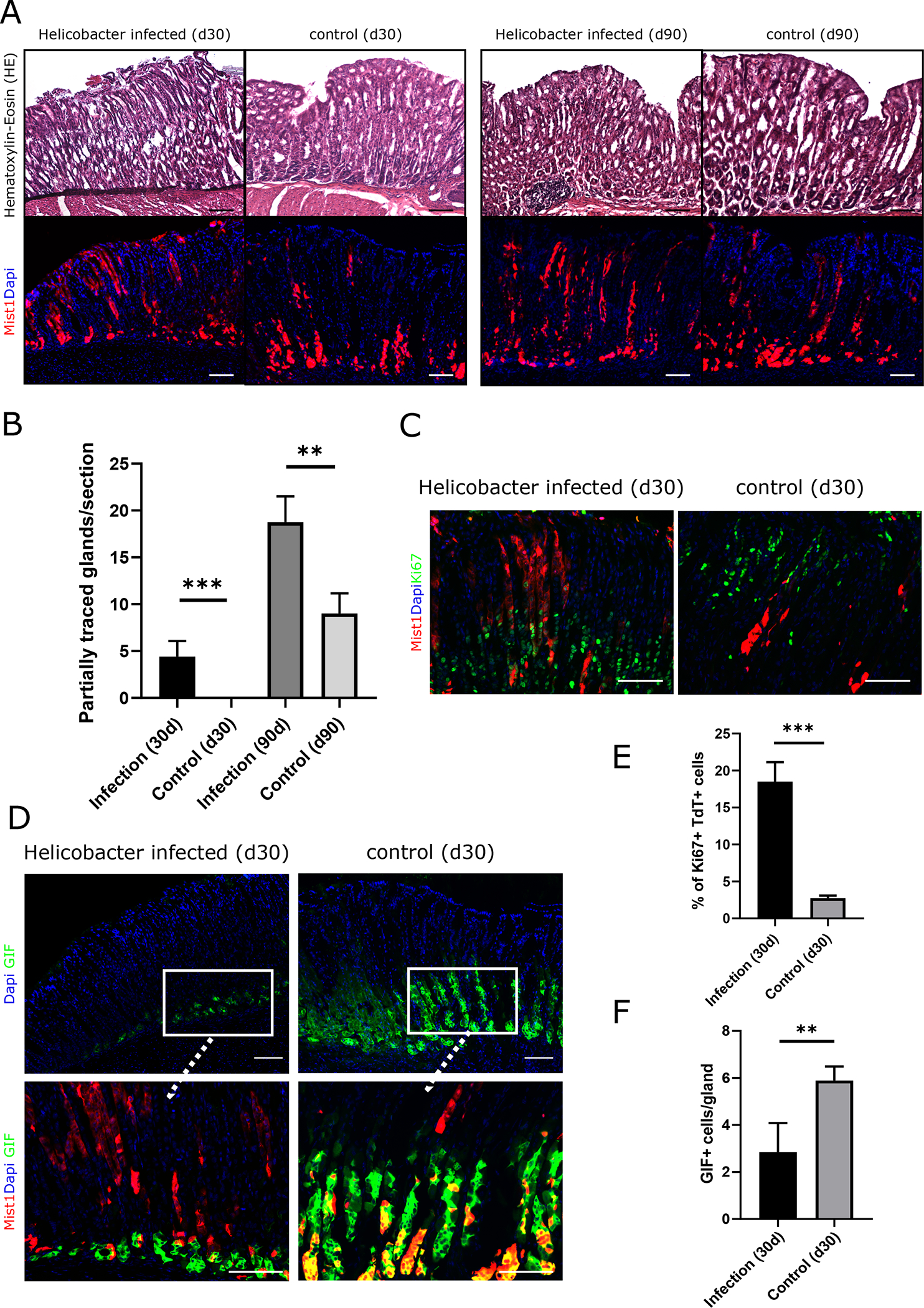
(A) HE staining and immunofluorescence of Mist1CreERT2;R26-Tdtomato mice at d30 and d90 after infection with Helicobacter felis (B) Quantification of partially traced glands by Mist1;TdT+ cells at d30 and d90 after Helicobacter felis infection (n=4–5 animals per timepoint) (C) Ki67 staining and immunofluorescence Helicobacter infected and uninfected Mist1CreERT2;R26-Tdtomato mice at d30 after infection. Ki67+ cells are labeled in green. (D) Top: Immunostaining of sections of the gastric corpus for GIF in Helicobacter infected and uninfected Mist1CreERT2;R26-Tdtomato mice. GIF+ cells are labeled in green. Bottom: High magnification of corresponding co-stainings for GIF (green) with immunofluorescence of Mist1;TdT (red). (E) Bar graphs showing the percentage of Ki67+ Mist1;TdT+ cells and (F) the number of GIF+ cells (left) and in Helicobacter infected and uninfected Mist1CreERT2;R26-Tdtomato mice at d30 post infection (n=4). All scale bars = 100μm
As Mist1 labels not only isthmus stem cells but also gastric chief cells, we quantified the number of Mist1;TdT+ cells at the base of the corpus glands. In H. felis-infected mice, the number of Mist1;TdT+ cells at the gland base was significantly lower compared to uninfected control animals. We validated that these cells were in fact chief cells by immunostaining with gastric intrinsic factor (GIF) antibody, which confirmed the presence of significantly fewer GIF+ cells in the infected epithelium (Fig. 2C/F). Similar findings of decreased traced chief cells were observed in the ulcer area in the corpus of mice with acetic acid-induced injury at d30 after ulcer induction when induced with tamoxifen seven days before the injury (Fig. 3E, see Fig S1A for details).
Figure 3: Lgr5+ chief cells are dispensable for healing and do not affect lineage tracing of isthmus Mist1+ cells.
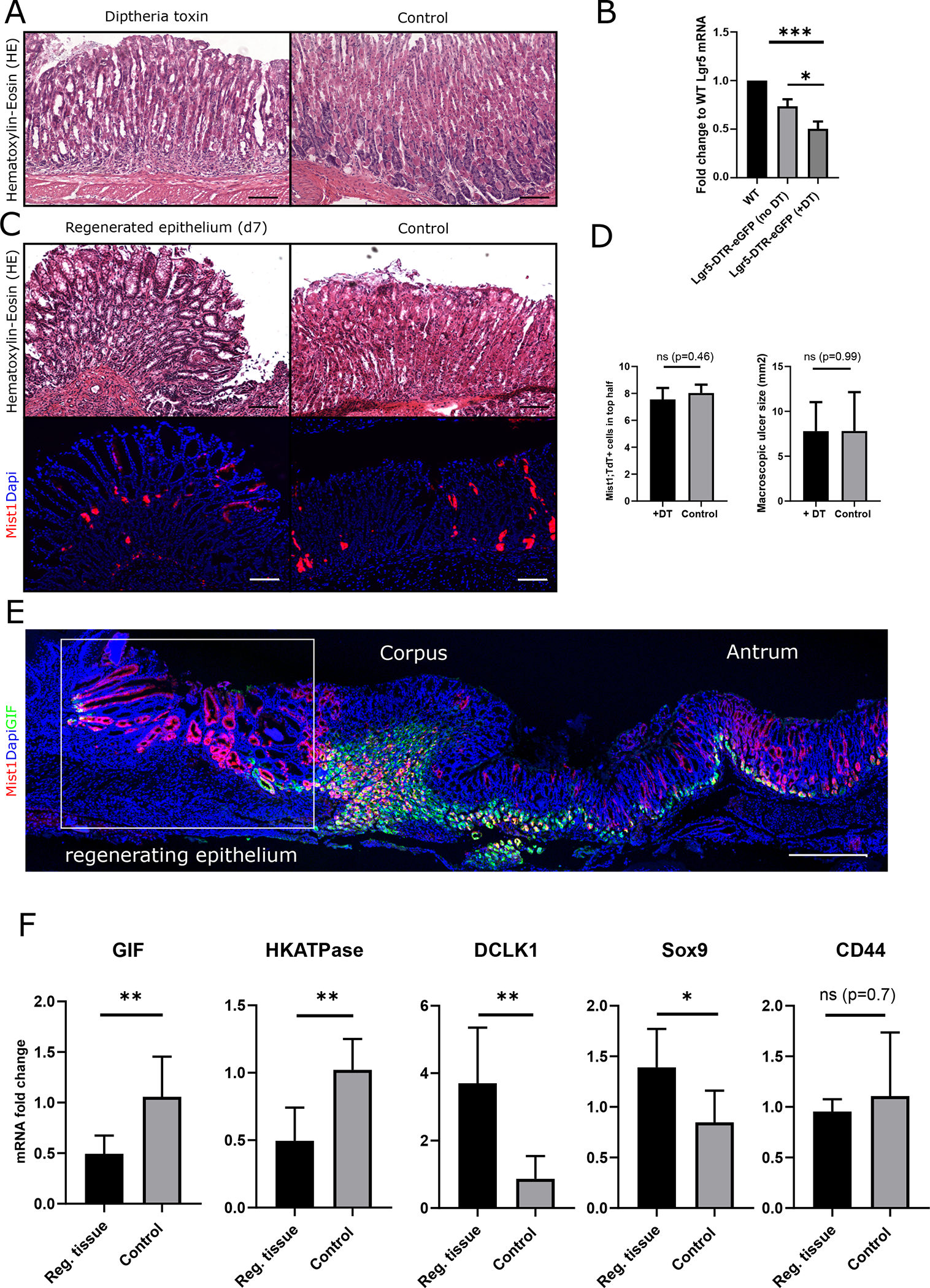
(A) HE stainings of gastric corpus sections in Lgr5-DTR mice at d7 after DT administration and vehicle injected control animals (Scale bars = 100μm). (B) Graph bar showing the mRNA level of Lgr5 from whole corpus tissue in fold change to mRNA levels from WT animals (left bar) in vehicle (middle) and DT injected Lgr5-DTR mice (n=3 per group) (C) HE staining and immunofluorescence of corpus sections from Lgr5-DTR; Mist1CreERT2;R26-Tdtomato mice at d7 after DT (left) and vehicle (right) administration (Scale bars = 100μm). (D) Left: Bar graph quantifying the macroscopic ulcer size at d7 after injury in DT and vehicle injected Lgr5-DTR; Mist1CreERT-R26tdTomato mice (n=4). Right: Quantification of the number of Mist1;TdT+ cells in the healing zone of Mist1CreERT2;R26-Tdtomato;Lgr5-DTR-eGFP mice after DT and vehicle injection (n=3–4). (E) Cross sections of the whole stomach with immunostaining for GIF (green) and immunofluorescence (red) of Mist1;TdT+ cells at d30 after ulcer induction. Boxed area indicates the regenerating area. (Scale bar = 500μm) (F) Graph bars showing the relative mRNA levels of GIF, HKATPase, DCLK-1, Sox9 and CD44 in the regenerating tissue at d30 after ulcer injury in fold change to uninjured control tissue (n=6 per group).
Taken together, these two independent models show a significant increase in lineage tracing of Mist1;TdT+ cells in response to short term injury as well as to chronic inflammation.
Lgr5 ablation does not affect Mist1;TdT+ tracing in regeneration
While Lgr5 marks active stem cells in the intestine [32], in the gastric corpus Lgr5+ expression is localized to the glandular base where it marks chief cells. Under normal conditions, chief cells do not function as progenitors or contribute to the epithelial turnover, but do show some increases in proliferation following injury [8, 33]. To test the contribution of corpus Lgr5+ cells to epithelial regeneration after the ulcer injury, we generated Mist1CreERT2;R26-Tdtomato;Lgr5-DTR-eGFP mice. Lgr5-DTR+ cells were ablated by treatment with diphtheria toxin (DT) (Fig. S1D). Similar to previous reports [8], at 24h after the last DT injection, we found a mild alteration in glandular structure coincident with chief cell loss (Fig. 3A). In DT-treated mice, Lgr5 mRNA levels showed a 50% reduction compared to WT animals (Fig. 3B) and histological analysis revealed a significant reduction in the number of eGFP+ cells (Fig. S2G/H), indicating an successful ablation of Lgr5-DTR+ cells. Notably, we did not observe the dramatic phenotype as previously reported [8]. Similar to the ablation of the Lgr5-DTR+ cells, we observed a significant reduction of Mist1;TdT+ cells at the base of the gland by over 80% (Fig. S2I).
Next, we analyzed gastric wound healing and regeneration in these chief cell-deficient mice at d7 and d15 after ulcer induction. Interestingly, we did not observe any difference in Mist1;TdT+ lineage tracing or macroscopic ulcer size (Fig. 3C/D), suggesting that corpus Lgr5+ cells are dispensable for corpus regeneration following ulcer injury. We next investigated changes in the number of gastric chief cells after injury. In both models, we found a significant decrease in recombined Mist1;TdT+ cells at the base of the corpus glands. This observation was confirmed by first applying ulcer injury to Mist1CreERT2;R26-Tdtomato mice and then later inducing the mice with tamoxifen at d7 after injury. These mice were then sacrificed 36h after tamoxifen induction, and analysis revealed a marked reduction of basal Mist1;TdT+ cells compared to the uninjured control tissue (Fig. S3A). To validate that chief cells are in fact lost in acute injury or inflammation, we performed gastric intrinsic factor (GIF) staining in both models, which showed a significant decrease in the number of GIF+ gastric chief cells (Fig. 3E/F) compared to the uninfected/uninjured control tissue.
In contrast to Lgr5+ zymogenic cells, which did not appear to participate in epithelial regeneration of the gastric corpus, tuft cells marked by the Doublecortin and calcium/calmodulin-dependent protein kinase-like-1 (DCKL1) were significantly expanded in the regenerating tissue (Fig. S3B). Additionally, we found a significant increase in DCLK1 mRNA levels (Fig. 3F), confirming the observation by others that DCLK1+ cells expand during gastric epithelial regeneration [31, 34]. The increased number of DCLK1+ cells and reduced number of GIF+ cells were supported by corresponding changes in gene expression (Fig. 3F). In addition, we confirmed the previously reported findings [31] that parietal cell markers such as H/K-ATPase decrease during gastric corpus regeneration, while SOX9, a marker of progenitor cells in the gastric corpus, and GS-II, a marker of mucus neck cells, are upregulated. In contrast to previous reports, we did not observe a difference in CD44 mRNA expression between healthy and regenerating gastric epithelium (Fig. 3F/S3B).
Wnt5a regulates the process of wound healing in the gastric corpus
In order to identify potential regulators in the response of Mist1;TdT+ corpus stem cells to injury, we measured mRNA levels of all Wnt-ligands in the regenerating epithelium at d5 after ulcer induction. Wnt ligands are known to modulate the response of stem cells to injury and inflammation [35], and have been shown to be essential for epithelial wound healing in the colon [24].
Among all Wnt-ligands, Wnt5a was the highest expressed Wnt-ligand in the regenerating tissue (Fig. 4A). In addition, compared to uninjured control tissue, Wnt5a mRNA-levels were significantly increased in the regenerating tissue (Fig. 4B). Besides Wnt5a, only mRNA expression of Wnt11 was significantly upregulated upon injury (Fig. S4A). To verify the expression of Wnt5a in the regenerating gastric epithelium, we performed in situ hybridization (ISH) for Wnt5a in the healing ulcer at d5 after induction. These ISH studies confirmed the expression of Wnt5a in stromal cells adjacent to the gastric isthmus (Fig. 4E) and revealed a significantly higher number of Wnt5a+ cells in the ulcer area. In addition to the Wnt5a+ cells near the Mist1TdT+ cells in the gastric isthmus (Fig. 4E, boxed area), we found an abundant number of Wnt5a+ cells in the submucosal region (dotted boxed area). Taken together, these results reinforce our previous observations, where Wnt5a was more abundantly expressed in Helicobacter-infected mice [14], supporting that Wnt5a is an important factor regulating the response of Mist1+ isthmus stem cells to injury and inflammation.
Figure 4: Wnt5a regulates the healing of gastric corpus ulcer.
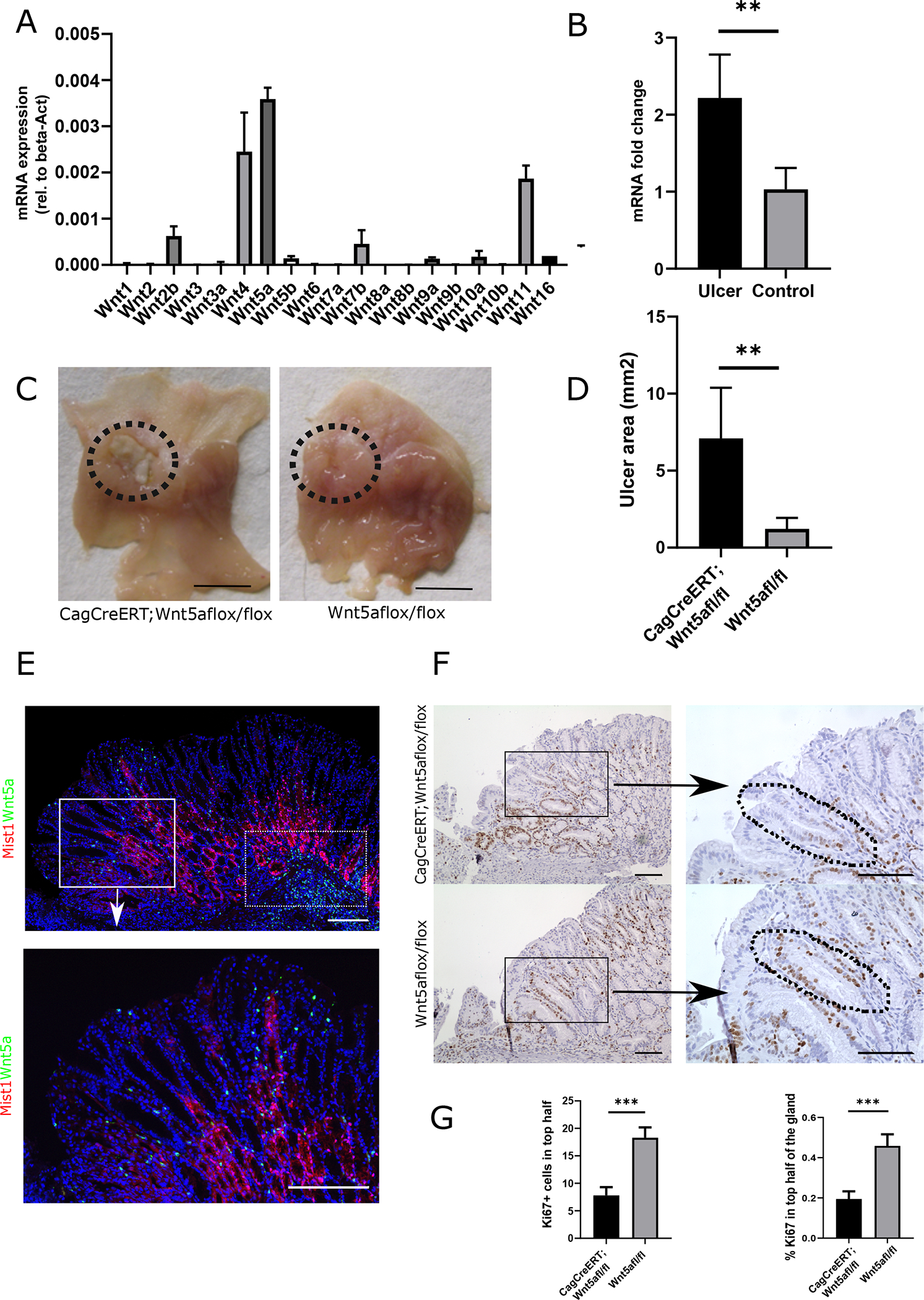
(A) Graph bar showing the mRNA levels of all Wnt ligands (rel. to beta-actin) in the regenerating tissue at d5 after ulcer injury. (WT mice, n=4) (B) Quantification of mRNA levels of Wnt5a from whole tissue of WT mice at d5 after ulcer injury and uninjured control tissue. (n=3) (C) Macroscopic pictures of the ulcer at d10 after injury in CagCreERT;Wnt5a flox/flox (left) and Cre-negative control mice (right). Dotted circle indicates the localization of the ulcer. (scale bars = 1cm). (D) Quantification of the macroscopic ulcer size (in mm2) in CagCreERT;Wnt5a flox/flox (left) and Cre-negative control mice (right). (n=6/per group) (E) In-situ hybridization of the healing zone with a Wnt5a probe (green dots) and immunostaining for RFP (red) Mist1CreERT-R26tdTomato mice at d5 after ulcer injury. Boxed area is shown in high magnification. (Scale bars = 100μm) (F) Ki67+ staining of the healing zone at d10 after ulcer injury in CagCreERT;Wnt5a flox/flox (top) and Cre-negative control mice (bottom). Right panel shows high magnification of the boxed areas. Dotted lines indicates the reduced number of Ki67+ cells in the isthmus region of CagCreERT:Wnt5a flox/flox mice (Scale bars = 100μm) (G) Bar graph showing the number of Ki67+/gland in CagCreERT;Wnt5a flox/flox (left) and Cre-negative control mice (right) (n=6)
We previously reported that in the gastric corpus, innate lymphoid cells (ILC-2) are the primary source of Wnt5a. ILC-2 modulate the restoration of mucosal defects and regulate the response to inflammatory stimuli [36]. Moreover, we showed that ILC-2 express CXCR4, and therefore we investigated the number of CXCR4+ cells in the ulcer using Cxcr4-eGFP mice. We found a significantly higher number of Cxcr4-eGFP+ cells within the injured stomach compared to control (Fig. S4B/C) indicating their putative involvement also in these models of gastric injury. In addition to the increased number of Cxcr4-eGFP+ cells we found an accumulation of other inflammatory cells in the submucosa of the healing zone which was confirmed by CD45 staining (Fig. S4E).
Wnt5a depletion impairs wound healing after acetic ulcer injury
To investigate the role of Wnt5a in ulcer healing, we generated a conditional knock-out of Wnt5a using CagCreERT;Wnt5aflox/flox mice. These mice were induced, and gastric ulcer was created three days later (Fig. S1C). Tamoxifen induction decreased Wnt5a mRNA by over 80% compared to initial levels proofing successful depletion of Wnt5a in this model (Fig. S4D). The natural time course for the healing of acetic acid-induced corpus ulcers showed that mucosal defects with the largest size were found around d7, with a gradual decrease in size thereafter (Fig. S2D). Given the peak of Wnt5a mRNA expression occurred at d5, we investigated macroscopic ulcer size at d10. At this time point, the ulcer size was significantly larger in Wnt5a-deficient animals compared to Cre-negative control animals, underlining that Wnt5a deficiency impairs gastric ulcer healing (Fig. 4C/D and Fig. S6A). While we did not observe an overall difference in Ki67+ cells at the healing wound edge, we did find significantly more Ki67+ cells in the isthmus region of the gastric glands next to the ulcer, where the Wnt5a+ cells reside (Fig. 4F/G). Thus, an increase in proliferation was clearly evident when we analyzed the percentage of Ki67+ cells in this area (Fig. 4G right bar graph).
The main receptor targets for transducing non-canonical Wnt5a activity are the Retinoic acid-related Orphan Receptor 1/2 (Ror 1/2) [37] and the Frizzled 5 receptor (Fzd5) [38, 39]. Through binding to these receptors, Wnt5a has been shown to activate cell motility and actin cytoskeleton reorganization. We investigated the mRNA levels of these three receptors in the whole tissue of the ulcer area at d5 and in the regenerated tissue at d30 after the ulcer injury. Compared to uninjured control tissue we found that all three receptors had a decreased mRNA levels compared to control tissue at d5 (Fig. 5A). These ratios changed at d30, where we found a significant increase in mRNA levels of Ror2 and a trend (p=0.09) towards upregulated Fzd5 levels. The mRNA levels of Ror1 did not show any difference to control tissue (Fig. 5B). When comparing total expression levels of all receptors in the regenerating tissue at d30 after the injury, we found that Fzd5 mRNA was expressed at a significantly higher level compared to Ror1/2 mRNA (Fig. 5C).
Figure 5: Wnt5a receptors are upregulated in the regenerating tissue and Fzd5 is the highest expressed Wnt5a target in Mist1+ cells.
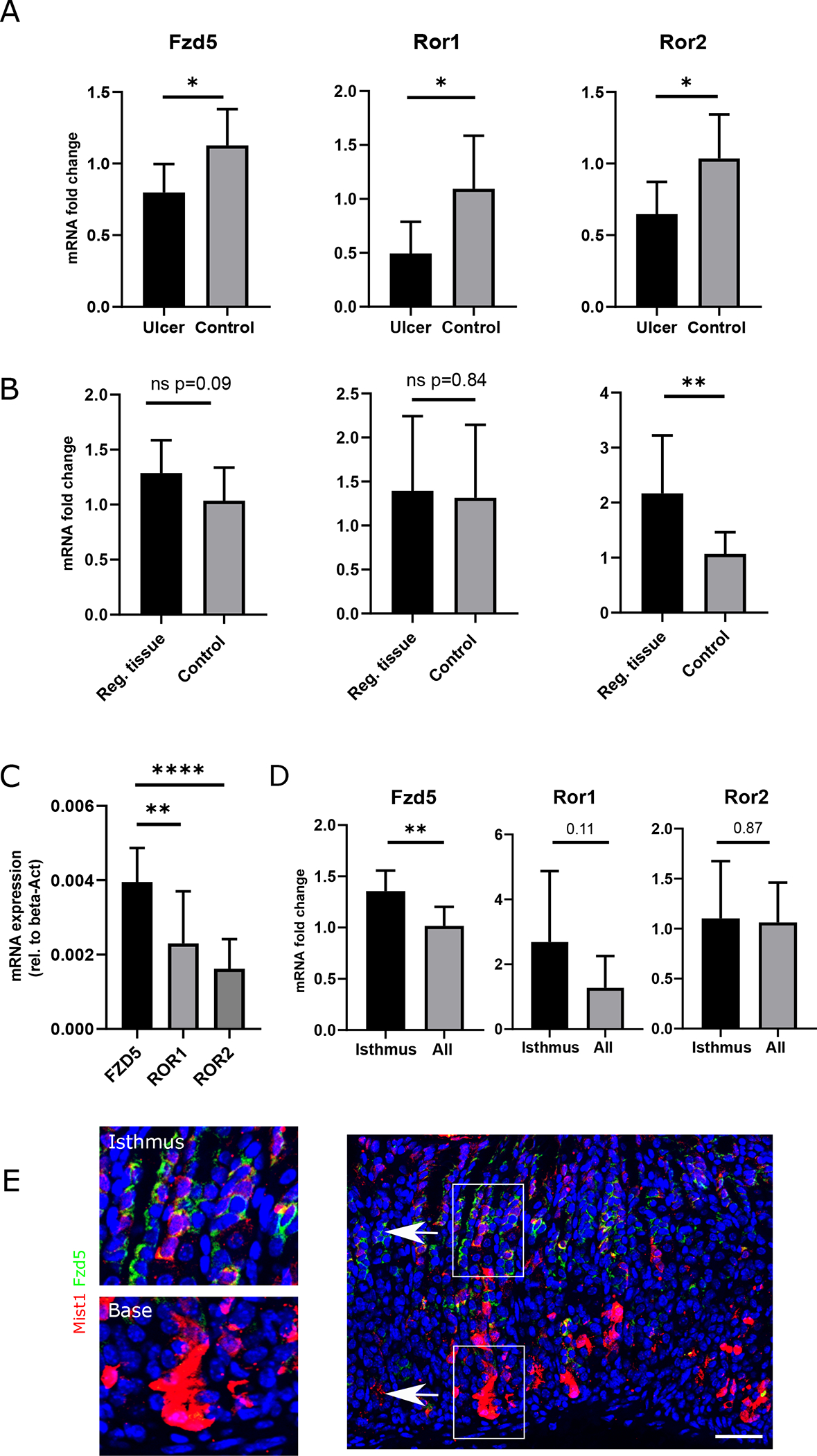
(A) Bar graph showing the fold change in mRNA levels of Fzd5, Ror1 and Ror2 in the ulcer area (whole tissue) compared to control tissue at d5 after ulcer induction. (n=7) (B) Bar graph showing the fold change in mRNA levels of Fzd5, Ror1 and Ror2 in the regenerating tissue (whole tissue) compared to control tissue at d30 after ulcer induction. (n=9) (C) Total mRNA expression (rel. to beta-actin) in whole tissue of Fzd5, Ror1 and Ror2 at d30 after injury (n=9) (D) Bar graph showing the mRNA expression of the indicated Wnt5a receptor in sorted Mist1;TdT+ cells after DT ablation (“Isthmus”) and in vehicle injected Mist1;TdT+ sorted cells (“All”) from Mist1CreERT;TdT:Lgr5-DTR animals (n=8) (E) Immunofluorescence of gastric corpus sections from Mist1CreERT;R26-Tdtomato animals at d3 after induction with tamoxifen. Positive staining for Fzd5 (green) was primarily observed in TdT+ cells the isthmus area (top image left), while TdT+ cells at the base of the gland did not show any staining signal (bottom image left).
In order to investigate the expression levels of these receptors in Mist1+ cells, we sorted Mist1;TdT+ cells from the corpus at d7 after induction with tamoxifen and analyzed the mRNA levels of the Wnt5a receptors (Fig. S5A). Validation of the strategy by RT-PCR for Mist1 mRNA showed a successful enrichment of Mist1 in the sorted cells (Fig. S5B). While this approach included all Mist1;TdT+ cells, (basal Mist1+ chief cells and isthmus cell), we used the above described Mist1CreERT;R26-Tdtomato;Lgr5-DTR mice to ablate the basal Mist1+ cell population. Mice were induced with tamoxifen and received two doses of DT (Fig. S1D). The remaining Mist1;TdT+ cells from the corpus were isolated by FACS. In these sorted cells, which were enriched for isthmus stem cells, we found a significantly (p = 0.01) higher expression of Fzd5, while the other two receptors had decreased mRNA levels comparable to the whole Mist1;TdT+ cell population (Fig. 5D). To confirm that Frizzled5 is mainly expressed by Mist1;TdT+ cells in the isthmus and not by chief cells at the base of the gland, we performed immunostaining for Fzd5 in MistCreERT;R26-Tdtomato animals at d3 after tamoxifen induction. We found that Mist1;TdT+ cells in the isthmus stained positive for Fzd5, while we did not observe any staining at the base of the gland (Fig. 5E). These findings confirm that Mist1+ isthmus stem cells express Fzd5 at significantly higher levels compared to the rest of the Mist1 lineage, rendering them potentially more sensitive to Wnt5a signaling.
We also examined the response of Mist1;TdT+ cells in mice with constitutive, whole body knockout of Wnt5A. As Wnt5a homozygous knock-out mice die at birth [25], we crossed Wnt5a heterozygous knockout mice (Wnt5a+/−) to MistCreERT2;Tdtomato mice. In homeostasis, the gastric mRNA levels of Wnt5a in the hemizygous (Wnt5a+/−) mice were reduced by approximately 40% compared to WT (Wnt5a+/+) animals (Fig. S5C). When comparing baseline Mist1;TdT+ lineage tracing in Wnt5a+/− to Wnt5a+/+ animals, we found a significant reduction in Wnt5a+/− mice (Fig. 6B). This observation supports the theory that Mist1+ isthmus cells are Wnt5a dependent. Furthermore, following Helicobacter felis infection, we did not observe a difference at the first time point (d30) but at the later time point (d90) noted a significant reduction of partially traced glands in the Wnt5a+/− group (Fig. 6A). Nevertheless, we did not find any difference in lineage tracing in the gastric ulcer model either d15 or d30 (Fig. S5C/D), likely because the mRNA levels of Wnt5a in the regenerating tissue of Wnt5a+/− animals are not reduced enough compared to Wnt5a+/+ animals (Fig S5F).
Figure 6: Wnt5a deficiency reduces Mist1 lineage tracing in homeostasis and in Helicobacter infection.
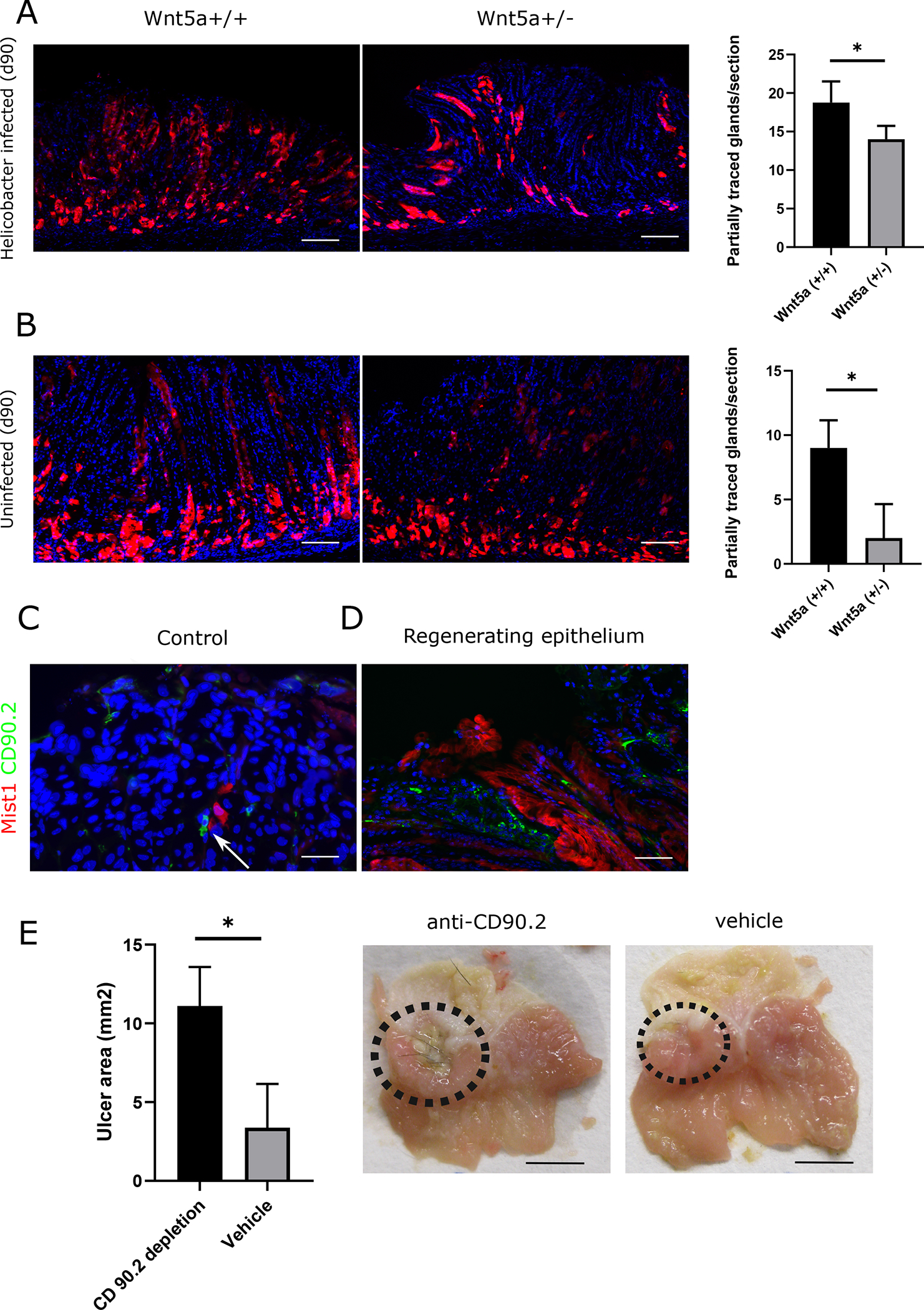
(A) Immunofluorescence of gastric corpus sections from Mist1CreERT;R26-Tdtomato;Wnt5a+/− and Wnt5a(+/+) animals at d90 after infections with Helicobacter felis (top) and without infection (bottom) (B) Bar graph showing quantification of partially traced glands per section in Helicobacter infected (top) and uninfected (bottom) (n=3–4 per group). (C) Immunofluorescence of the gastric corpus (Isthmus area) showing Mist1;TdT+ cells (red) and CD90.2+ cells (green) under homeostatic conditions (D) Immunofluorescence of the gastric corpus (Isthmus area) showing Mist1;TdT (red) and CD90.2+ cells (green) at d15 in the regenerating epithelium (E) bar graph and macroscopic image showing the ulcer area (in mm2) in CD90.2 depleted and vehicle injected control animals (n=3/per group)
As described above one of the main sources of Wnt5a in the stomach are ILC-2 that express CD90.2. As previously reported we found CD90.2+ cells in direct proximity to the Mist1;TdT+ cells in the gastric isthmus (Fig. 6C). In the regenerating tissue we observed an increase in the number of CD90.2+ cells in the gastric isthmus indicating an increase in ILC-2 next to the Mist1;TdT+ cells. To reduce the levels of Wnt5a we depleted the ILC-2 population by application of an anti-CD90.2 antibody as previously described and validated [14]. By this strategy we could significantly reduce the healing of the ulcer compared to vehicle injected control animals (Fig. 6E and Fig. S6B). Even given the fact that CD90.2 does not exclusively label ILC-2, in combination with the data from the Cxcr4-eGFP animals, we can surmise that the depletion of Wnt5a producing ILC-2 impairs wound healing and tissue regeneration.
Discussion:
In this study, we have investigated the role of Mist1+ corpus isthmus stem cells in models of gastric injury and inflammation. As we have shown previously that Mist1+ isthmus cells are regulated by niche ILC-2 cells and can give rise to cancer, the aim of the current study was to investigate the regulation of isthmus stem cells under non-malignant conditions. Using two well-established injury models, we found that Mist+ isthmus cells are activated and show increased lineage tracing in response to injury and inflammation. In contrast, Mist1+ zymogenic cells at the base were mostly lost adjacent to the ulcer and did not show evidence of activation or increased lineage tracing.
In the gastric corpus, Mist1 labels two distinct cell populations: isthmus stem cells and chief cells at the gland base. Over the last decade, several studies have focused on these two distinct populations and their contribution to regeneration, metaplasia and cancer [16, 18, 40]. Our current study reveals that in regenerating tissue, as well as in Helicobacter-dependent gastritis, the number of basal Mist1;TdT+ chief cells significantly decreases while the Mist1+ stem cell population in the isthmus expands. To exclude basal Mist1+ mature chief cells as a possible source of the expanded Mist1-traced population (e.g. through transdifferentiation) [41], we ablated chief cells by DT to Lgr5-DTR mice. In contrast to findings in other studies, we did not observe any significant effect on regeneration or healing following ablation of this cell population. A potential explanation for this discrepancy compared to earlier studies [8] might be the different types of injury models, as the ulcer model represents a different type of injury that may necessitate a greater role by the stem cell compartment. However, we cannot completely rule out by this experiment that some tracing and regeneration comes from a basal Mist1TdT+ cell that is negative for Lgr5, but given all our data in the ulcer model, it is quite unlikely that large portions of the tracing come from basal cells rather than from the described stem cells in the isthmus.
In addition to the ulcer model, we found an increase in lineage tracing following infection with Helicobacter felis. The observation that infection with Helicobacter increases lineage tracing of stem cells has been made in the gastric antrum as well [42]. In the study by Sigal et al., the authors showed an increase in lineage tracing by Lgr5+ cells in the antrum in response to Helicobacter pylori infection. As a further observation, colonization with Helicobacter in the corpus occurred more densely in the isthmus area, raising the possibility of more direct effects. Taken together, these findings point to a model of stem cell activation in the gastrointestinal tract in response to tissue injury and colonization with microbial pathogens.
To further investigate the mechanism of Mist1+ stem cell expansion in response to injury and inflammation, we analyzed the expression of known Wnt ligands, and found Wnt5a to show the highest level of expression. As noted earlier, the physiological roles of Wnt5a have not been fully elucidated yet. Previous reports indicated that expression of Wnt5a in gastric cancer correlated with an aggressive tumor phenotype [27], supporting our previous findings in the mouse model [14]. In other tumors, Wnt5a seems to have opposite effects. In breast cancer, the loss of Wnt5a activity correlates with more advanced disease and rescue of Wnt5a signaling has tumor suppressive effects [43]. However, the role of Wnt5a in response to gastric injury has not been previously studied. Interestingly, in addition to the upregulation of Wnt5a, we found a significant increase in Wnt11 in the regenerating epithelium. Recent studies have suggested similar effects of Wnt5a and Wnt11 during the embryonic development [44] but so far there are no studies on Wnt11 in the gastric epithelium. Future studies are needed to investigate the specific role of Wnt11 in the regeneration of the gastric epithelium.
We have previously shown that in the stomach, Wnt5a is highly expressed in CXCR4+ ILC-2 and contributes to the development of diffuse gastric cancer. CXCR4+ cells have been shown to be recruited to injured tissue areas and facilitate regeneration [45], and we showed in this study an increase in Cxcr4-eGFP+ cells after gastric injury or inflammation. While there is evidence that ILC-2 and Wnt5a can regulate the repair of mucosal defects [24, 36], the receptor(s) Wnt5a is binding and the specific cell types that are responding have yet to be described. In this study, we show that Mist1+ isthmus stem cells highly express the Wnt5a receptor Fzd5, as mRNA levels for this receptor are significantly enriched in the Mist1+ stem cell population. This finding of selective expression of Fzd5 by isthmus cells was confirmed by immunostaining as well. In addition, we provide evidence that Mist1+ isthmus cells are responsive to Wnt5a signaling, as lineage tracing of Mist1+ cells is reduced under Wnt5a deficient conditions. The main source of Wnt5a in the gastric corpus are the ILC-2. When depleting this population, we observed reduced lineage tracing from Mist1+ isthmus stem cells under conditions of ulceration and homeostasis, indicating the importance of Wnt5a in regulation gastric tissue. In addition to ILC-2, there are other potential sources of Wnt5a, as monocytes and macrophages in particular have been reported to produce Wnt5a under inflammatory conditions [46, 47]. While our data point to an important role for Wnt5a in the regulation of Mist1+ stem cells, we would acknowledge that Wnt5a is unlikely to be the sole niche factor for Mist1+ stem cells and that other niche factors like R-spondins may also contribute to stem cell activation and regeneration. Another major limitation of our study is that we cannot rule out that at least some of the tracing comes from basal Mist1TdT+ cells. The accumulated data from different experiments make a large contribution to regeneration by basal cells unlikely, but future studies using newly established genetic engineered mouse models, such as the Kitl-CreERT mice [48], are needed to fully clarify all of the origins of the observed increase in tracing. In addition, acute ulcer healing clearly involves multiple other stromal and epithelial cell types, including both short-term progenitors and terminally differentiated cells. Mist1+ stem cells express high levels of Fzd5 and recent studies have suggested a role of Fzd5 in cancer development [49]. However, the mechanism by which Fzd5 modulates progenitor cell and the intracellular signaling pathways downstream have yet to be defined. In vitro studies using human lymphoma cells have shown that knock-down of Fzd5 leads to a reduced activation of intracellular RhoA [50] which might be one pathway activated by Wnt5.
In summary, we show that Mist1+ isthmus stem cells expand in response to injury and inflammation. Wnt5a secreted by ILC-2 plays an important role in modulating this expansion. These findings point to the importance of ILC-2 in regeneration and tissue repair, thus defining this cell population and secreted Wnt5a as targets for modulating tissue regeneration and repair. Future studies will have to show if Wnt5a and ILC-2 could be used as predictor for tissue regeneration and healing processes in gastric ulcer patients.
Supplementary Material
SFigure 1: (A) Overview of mouse models and treatment regimens used in the described experiments
SFigure 2: (A) Definition of top and bottom region in gastric corpus glands (B) Definition of partially traced and not partially traced gastric corpus glands (C) Macroscopic imaging of macroscopic ulcer size in WT animals at indicated time points after ulcer induction (D) Bar graph showing quantification of macroscopic ulcer size (in mm2) at indicated time points (n=2–3 per time point) (E) Steiner-Silver-Stain showing colonization of Helicobacter felis in the gastric corpus glands at d30 after infection (left image) and uninfected control animals (right image). (F) Immunoflurescence of gastric corpus sections from Mist1CreERT2;R26-Tdtomato mice at d30 after infection with Helicobacter felis stained for CD45 (green). (G) Immunoflurescence of gastric corpus sections from Mist1CreERT2;R26-Tdtomato;Lgr5-DTR-eGFP mice 24h after the last DT (left) or vehicle (right) injection. (H) Bar graph showing the number of eGFP+ cells/gland at 24h after last DT and vehicle injection. (I) Bar graph showing the number of Mist1;TdT+ basal cell per gland in Mist1CreERT2;R26-Tdtomato;Lgr5-DTR-eGFP mice after DT and vehicle injection (n=3–4)
SFigure 3: (A) Immunofluorescence of gastric corpus sections from Mist1CreERT2;R26-Tdtomato mice at d9 after ulcer injury and 36h after tamoxifen induction. (B) Immunofluorescence of HKATPase, DCLK1 and GS-II in WT animals at d30 after the ulcer injury (left panel) and corresponding controls (right panel).
SFigure 4: (A) Bar graphs showing the change in mRNA expression of the indicated Wnt ligands in the gastric epithelium at d5 after ulcer injury (n=4). (B) HE-staining and immunofluorescence of the healing zone at d5 and corresponding control sections from the gastric corpus in CXCR4-eGFP mice. (C) Bar graph showing the quantification of CXCR4-eGFP+ cells analyzed by FACS (top) and corresponding FACS plots (bottom). (D) Bar graph showing the quantification of Wnt5a mRNA in the gastric corpus (whole tissue) in CagCreERT;Wnt5aflox animals and corresponding Cre-negative control animals. (E) Immunofluorescence of gastric corpus sections from Mist1CreERT2;R26-Tdtomato mice at d5 after ulcer injury. Accumulation of CD45+ inflammatory cells was observed in the submucosa at the healing edge of the ulcer (dotted box).
SFigure 5: (A) FACS plot showing the Mist1;TdT+ cell population (B) Bar graph showing the mRNA expression of Mist1 in sorted Mist1;TdT+ cells compared to Mist1;TdT− sorted cells (C) Bar graph showing the mRNA expression of Wnt5a in Wnt5a whole body knock-out animals (Wnt5a+/−) vs. control animals (Wnt5a+/+) (D) Bar graph showing the quantification of partially traced glands by Mist1;TdT+ cells in Mist1CreERT2;R26-Tdtomato;Wnt5a+/− and control mice at d15 and d30 after ulcer injury (E) Corresponding immunofluorescence images (top panel d15, bottom panel d30) (F) Bar graph showing the mRNA levels of Wnt5a in Wnt5a+/− mice compared to Wnt5a +/+ animals at d30 after ulcer injury
SFigure 6: (A) Full set of macroscopic pictures of ulcer healing in CagCreERT;Wnt5a flox/flox (top) and Cre-negative control mice (bottom). (B) Full set of macroscopic pictures of the ulcer healing in Mist1CreERT2;R26-Tdtomato mice treated with anti-CD90.2 (top) and vehicle (bottom). (Scale bars = 1cm).
Short summary.
What is already known about this subject?
Gastric stem cells marked by the transcription factor Mist1 can give rise to cancer in the gastric corpus and antrum in mice.
In the gastric corpus Mist1 labels basal chief cells and a small population of slowly dividing isthmus stem cells.
What are the new findings?
Mist1+ isthmus stem cells but not basal chief cells expand in regeneration and inflammation.
In the regenerating tissue, we observed high levels of Wnt5a and conditional deletion of Wnt5a impairs healing in experimental gastric ulcer
The Wnt5a receptor Frizzled5 (Fzd5) is significantly higher expressed in
Mist1+ isthmus stem cells compared to basal chief cells
How might it impact on clinical practice in the foreseeable future?
The Wnt5a – Fzd5 signaling axis plays an important role in gastric regeneration and inflammation and could be a potential target in patients with peptic ulcer disease
Acknowledgment:
The authors thank Richard Miller (Northwestern University Medical School) for sharing the CxCr4-eGFP mice. We thank Bryana Belin and Madeleine Strait for technical assistance and help with the animal colony, Michael Kissner from the Columbia Stem Cell Initiative (CSCI) for excellent support with FACS, Theresa Swayne and Emilia Munteanu for help with confocal imaging and Victor Guo from the Humane Immune Monitoring Core (HIMC) for help with the RNA in-situ studies.
Grant support/Funding:
This research was supported by NIH grants (R35CA210088, 5R37DK052778 and 5UO1DK103155) to Timothy C. Wang, by a grant of the Deutsche Forschungsgemeinschaft (NI1810/1-1) to Henrik Nienhüser and by a grant of the Deutsche Krebshilfe (70111870) to Moritz Middelhoff.
These studies used the resources of the Cancer Center Flow Core Facility funded in part through Center Grant P30CA013696.
Footnotes
Disclosures: All authors declare no conflict of interest.
References:
- 1.Andersson-Rolf A, Zilbauer M, Koo BK, et al. , Stem Cells in Repair of Gastrointestinal Epithelia. Physiology (Bethesda), 2017. 32(4): p. 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, et al. , Helicobacter pylori infection and the development of gastric cancer. N Engl J Med, 2001. 345(11): p. 784–9. [DOI] [PubMed] [Google Scholar]
- 3.Tarnawski AS, Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci, 2005. 50 Suppl 1: p. S24–33. [DOI] [PubMed] [Google Scholar]
- 4.Tarnawski A, Stachura J, Krause WJ, et al. , Quality of gastric ulcer healing: a new, emerging concept. J Clin Gastroenterol, 1991. 13 Suppl 1: p. S42–7. [DOI] [PubMed] [Google Scholar]
- 5.Mills JC and Shivdasani RA, Gastric epithelial stem cells. Gastroenterology, 2011. 140(2): p. 412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karam SM and Leblond CP, Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec, 1993. 236(2): p. 259–79. [DOI] [PubMed] [Google Scholar]
- 7.Choi E, Lantz TL, Vlacich G, et al. , Lrig1+ gastric isthmal progenitor cells restore normal gastric lineage cells during damage recovery in adult mouse stomach. Gut, 2018. 67(9): p. 1595–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leushacke M, Tan SH, Wong A, et al. , Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol, 2017. 19(7): p. 774–786. [DOI] [PubMed] [Google Scholar]
- 9.Arnold K, Sarkar A, Yram MA, et al. , Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell, 2011. 9(4): p. 317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayakawa Y, Jin G, Wang H, et al. , CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut, 2015. 64(4): p. 544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee Y, Urbanska AM, Hayakawa Y, et al. , Gastrin stimulates a cholecystokinin-2-receptor-expressing cardia progenitor cell and promotes progression of Barrett’s-like esophagus. Oncotarget, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuo J, Kimura S, Yamamura A, et al. , Identification of Stem Cells in the Epithelium of the Stomach Corpus and Antrum of Mice. Gastroenterology, 2017. 152(1): p. 218–231.e14. [DOI] [PubMed] [Google Scholar]
- 13.Han S, Fink J, Jorg DJ, et al. , Defining the Identity and Dynamics of Adult Gastric Isthmus Stem Cells. Cell Stem Cell, 2019. 25(3): p. 342–356 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayakawa Y, Ariyama H, Stancikova J, et al. , Mist1 Expressing Gastric Stem Cells Maintain the Normal and Neoplastic Gastric Epithelium and Are Supported by a Perivascular Stem Cell Niche. Cancer Cell, 2015. 28(6): p. 800–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakitani K, Hayakawa Y, Deng H, et al. , CXCR4-expressing Mist1(+) progenitors in the gastric antrum contribute to gastric cancer development. Oncotarget, 2017. 8(67): p. 111012–111025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nam KT, Lee HJ, Sousa JF, et al. , Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology, 2010. 139(6): p. 2028–2037 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leushacke M, Tan SH, Wong A, et al. , Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol, 2017. [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita H, Hayakawa Y, Niu Z, et al. , Mature gastric chief cells are not required for the development of metaplasia. Am J Physiol Gastrointest Liver Physiol, 2018. 314(5): p. G583–G596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam KT, O’Neal RL, Coffey RJ, et al. , Spasmolytic polypeptide-expressing metaplasia (SPEM) in the gastric oxyntic mucosa does not arise from Lgr5-expressing cells. Gut, 2012. 61(12): p. 1678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayakawa Y, Fox JG and Wang TC, Isthmus Stem Cells Are the Origins of Metaplasia in the Gastric Corpus. Cellular and Molecular Gastroenterology and Hepatology, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bockerstett KA and DiPaolo RJ, Regulation of Gastric Carcinogenesis by Inflammatory Cytokines. Cell Mol Gastroenterol Hepatol, 2017. 4(1): p. 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sigal M, Logan CY, Kapalczynska M, et al. , Stromal R-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature, 2017. 548(7668): p. 451–455. [DOI] [PubMed] [Google Scholar]
- 23.Mah AT, Yan KS and Kuo CJ, Wnt pathway regulation of intestinal stem cells. J Physiol, 2016. 594(17): p. 4837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyoshi H, Ajima R, Luo CT, et al. , Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science, 2012. 338(6103): p. 108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cervantes S, Yamaguchi TP and Hebrok M, Wnt5a is essential for intestinal elongation in mice. Dev Biol, 2009. 326(2): p. 285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kikuchi A, Yamamoto H, Sato A, et al. , Wnt5a: its signalling, functions and implication in diseases. Acta Physiol (Oxf), 2012. 204(1): p. 17–33. [DOI] [PubMed] [Google Scholar]
- 27.Nam S, Chung JW and Yang JY, WNT5A Correlates with Clinicopathological Characteristics in Gastric Cancer: a Meta-Analysis. Cell Physiol Biochem, 2017. 41(1): p. 33–40. [DOI] [PubMed] [Google Scholar]
- 28.Shi G, Zhu L, Sun Y, et al. , Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology, 2009. 136(4): p. 1368–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryu YK, Collins SE, Ho HY, et al. , An autocrine Wnt5a-Ror signaling loop mediates sympathetic target innervation. Dev Biol, 2013. 377(1): p. 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi TP, Bradley A, McMahon AP, et al. , A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development, 1999. 126(6): p. 1211–23. [DOI] [PubMed] [Google Scholar]
- 31.Aihara E, Matthis AL, Karns RA, et al. , Epithelial Regeneration After Gastric Ulceration Causes Prolonged Cell-Type Alterations. Cell Mol Gastroenterol Hepatol, 2016. 2(5): p. 625–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker N, van Es JH, Kuipers J, et al. , Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature, 2007. 449(7165): p. 1003–7. [DOI] [PubMed] [Google Scholar]
- 33.Burclaff J, Willet S, Saenz JB, et al. , Proliferation and Differentiation of Gastric Mucous Neck and Chief Cells During Homeostasis and Injury-induced Metaplasia. Gastroenterology, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kikuchi M, Nagata H, Watanabe N, et al. , Altered expression of a putative progenitor cell marker DCAMKL1 in the rat gastric mucosa in regeneration, metaplasia and dysplasia. BMC Gastroenterol, 2010. 10: p. 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clevers H, Loh KM and Nusse R, Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science, 2014. 346(6205): p. 1248012. [DOI] [PubMed] [Google Scholar]
- 36.Castellanos JG and Longman RS, The balance of power: innate lymphoid cells in tissue inflammation and repair. J Clin Invest, 2019. 129(7): p. 2640–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickinson SC, Sutton CA, Brady K, et al. , The Wnt5a Receptor, Receptor Tyrosine Kinase-Like Orphan Receptor 2, Is a Predictive Cell Surface Marker of Human Mesenchymal Stem Cells with an Enhanced Capacity for Chondrogenic Differentiation. Stem Cells, 2017. 35(11): p. 2280–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blumenthal A, Ehlers S, Lauber J, et al. , The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood, 2006. 108(3): p. 965–73. [DOI] [PubMed] [Google Scholar]
- 39.Buttler K, Becker J, Pukrop T, et al. , Maldevelopment of dermal lymphatics in Wnt5a-knockout-mice. Dev Biol, 2013. 381(2): p. 365–76. [DOI] [PubMed] [Google Scholar]
- 40.Choi E, Hendley AM, Bailey JM, et al. , Expression of Activated Ras in Gastric Chief Cells of Mice Leads to the Full Spectrum of Metaplastic Lineage Transitions. Gastroenterology, 2016. 150(4): p. 918–30 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radyk MD, Burclaff J, Willet SG, et al. , Metaplastic Cells in the Stomach Arise, Independently of Stem Cells, via Dedifferentiation or Transdifferentiation of Chief Cells. Gastroenterology, 2018. 154(4): p. 839–843 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sigal M, Rothenberg ME, Logan CY, et al. , Helicobacter pylori Activates and Expands Lgr5(+) Stem Cells Through Direct Colonization of the Gastric Glands. Gastroenterology, 2015. 148(7): p. 1392–404 e21. [DOI] [PubMed] [Google Scholar]
- 43.Prasad CP, Manchanda M, Mohapatra P, et al. , WNT5A as a therapeutic target in breast cancer. Cancer Metastasis Rev, 2018. 37(4): p. 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santos JMA, Mendes-Silva L, Afonso V, et al. , Exogenous WNT5A and WNT11 proteins rescue CITED2 dysfunction in mouse embryonic stem cells and zebrafish morphants. Cell Death Dis, 2019. 10(8): p. 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller RJ, Banisadr G and Bhattacharyya BJ, CXCR4 signaling in the regulation of stem cell migration and development. J Neuroimmunol, 2008. 198(1–2): p. 31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sessa R, Yuen D, Wan S, et al. , Monocyte-derived Wnt5a regulates inflammatory lymphangiogenesis. Cell Res, 2016. 26(2): p. 262–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao Y, Zheng Q, Wang W, et al. , Biological functions of macrophage-derived Wnt5a, and its roles in human diseases. Oncotarget, 2016. 7(41): p. 67674–67684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hata M, Kinoshita H, Hayakawa Y, et al. , GPR30-Expressing Gastric Chief Cells Do Not Dedifferentiate But Are Eliminated via PDK-Dependent Cell Competition During Development of Metaplasia. Gastroenterology, 2020. 158(6): p. 1650–1666.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinhart Z, Pavlovic Z, Chandrashekhar M, et al. , Genome-wide CRISPR screens reveal a Wnt-FZD5 signaling circuit as a druggable vulnerability of RNF43-mutant pancreatic tumors. Nat Med, 2017. 23(1): p. 60–68. [DOI] [PubMed] [Google Scholar]
- 50.Linke F, Zaunig S, Nietert MM, et al. , WNT5A: a motility-promoting factor in Hodgkin lymphoma. Oncogene, 2017. 36(1): p. 13–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SFigure 1: (A) Overview of mouse models and treatment regimens used in the described experiments
SFigure 2: (A) Definition of top and bottom region in gastric corpus glands (B) Definition of partially traced and not partially traced gastric corpus glands (C) Macroscopic imaging of macroscopic ulcer size in WT animals at indicated time points after ulcer induction (D) Bar graph showing quantification of macroscopic ulcer size (in mm2) at indicated time points (n=2–3 per time point) (E) Steiner-Silver-Stain showing colonization of Helicobacter felis in the gastric corpus glands at d30 after infection (left image) and uninfected control animals (right image). (F) Immunoflurescence of gastric corpus sections from Mist1CreERT2;R26-Tdtomato mice at d30 after infection with Helicobacter felis stained for CD45 (green). (G) Immunoflurescence of gastric corpus sections from Mist1CreERT2;R26-Tdtomato;Lgr5-DTR-eGFP mice 24h after the last DT (left) or vehicle (right) injection. (H) Bar graph showing the number of eGFP+ cells/gland at 24h after last DT and vehicle injection. (I) Bar graph showing the number of Mist1;TdT+ basal cell per gland in Mist1CreERT2;R26-Tdtomato;Lgr5-DTR-eGFP mice after DT and vehicle injection (n=3–4)
SFigure 3: (A) Immunofluorescence of gastric corpus sections from Mist1CreERT2;R26-Tdtomato mice at d9 after ulcer injury and 36h after tamoxifen induction. (B) Immunofluorescence of HKATPase, DCLK1 and GS-II in WT animals at d30 after the ulcer injury (left panel) and corresponding controls (right panel).
SFigure 4: (A) Bar graphs showing the change in mRNA expression of the indicated Wnt ligands in the gastric epithelium at d5 after ulcer injury (n=4). (B) HE-staining and immunofluorescence of the healing zone at d5 and corresponding control sections from the gastric corpus in CXCR4-eGFP mice. (C) Bar graph showing the quantification of CXCR4-eGFP+ cells analyzed by FACS (top) and corresponding FACS plots (bottom). (D) Bar graph showing the quantification of Wnt5a mRNA in the gastric corpus (whole tissue) in CagCreERT;Wnt5aflox animals and corresponding Cre-negative control animals. (E) Immunofluorescence of gastric corpus sections from Mist1CreERT2;R26-Tdtomato mice at d5 after ulcer injury. Accumulation of CD45+ inflammatory cells was observed in the submucosa at the healing edge of the ulcer (dotted box).
SFigure 5: (A) FACS plot showing the Mist1;TdT+ cell population (B) Bar graph showing the mRNA expression of Mist1 in sorted Mist1;TdT+ cells compared to Mist1;TdT− sorted cells (C) Bar graph showing the mRNA expression of Wnt5a in Wnt5a whole body knock-out animals (Wnt5a+/−) vs. control animals (Wnt5a+/+) (D) Bar graph showing the quantification of partially traced glands by Mist1;TdT+ cells in Mist1CreERT2;R26-Tdtomato;Wnt5a+/− and control mice at d15 and d30 after ulcer injury (E) Corresponding immunofluorescence images (top panel d15, bottom panel d30) (F) Bar graph showing the mRNA levels of Wnt5a in Wnt5a+/− mice compared to Wnt5a +/+ animals at d30 after ulcer injury
SFigure 6: (A) Full set of macroscopic pictures of ulcer healing in CagCreERT;Wnt5a flox/flox (top) and Cre-negative control mice (bottom). (B) Full set of macroscopic pictures of the ulcer healing in Mist1CreERT2;R26-Tdtomato mice treated with anti-CD90.2 (top) and vehicle (bottom). (Scale bars = 1cm).


