ABSTRACT
There is a rising noncommunicable disease (NCD) burden in low- and middle-income countries. Sub-Saharan Africa (SSA) bears a higher burden than the global average with South Africa (SA) enduring the highest regional burden. SA among other southern African countries also bears a high prevalence of HIV and other chronic communicable diseases. Having a perspective on common chronic diseases in the ever-increasing numbers of adult cancer patients in SA will inform our understanding of approaches to better manage them. This commentary reviews regional and national studies and data of low- and middle-income countries and particularly SA on the chronic infectious and NCD multimorbidity burden among adult cancer patients. It also reflects on the considerable health system challenges of managing discordant multimorbidity among adult cancer patients within the SA Public Health System. Despite the critical need to better manage the growing MM burden in general and particularly the high prevalence of discordant multimorbidity among cancer patients, there is a dearth of research into MM management generally and in LMICs particularly.
KEYWORDS: Multimorbidity, noncommunicable diseases, cancer, low- and middle-income countries, South Africa
The changing burden of disease trends is devastating low-and-middle- income-countries (LMICs) including South Africa
Since the Second World War, medical advances have enabled substantial gains in the management of preventable and communicable diseases, resulting in steadily improving global health and increasing life expectancy. This infectious disease predominance is progressively being superseded by a rising noncommunicable disease (NCD) burden with the transition speed corresponding to the Human Development Index (HDI) of global nations [1–3]. This is dramatically illustrated in Figures 1 and 2, (data derived from the Global Burden of Disease Study and Our World in Data tables) [4,5]. By 1990, NCDs constituted the vast majority of the proportional disease burden in high-income countries (HICs), with LMICs beginning their transitions from 1990 onwards. In South Africa (SA), among other HIV endemic regions, the substantial HIV-related communicable, maternal, neonatal, and nutritional diseases (CDs) burden peaked in 2007, but with an effective antiretroviral (ARV) rollout these health burdens have been decreasing [6–9].
Figure 1.
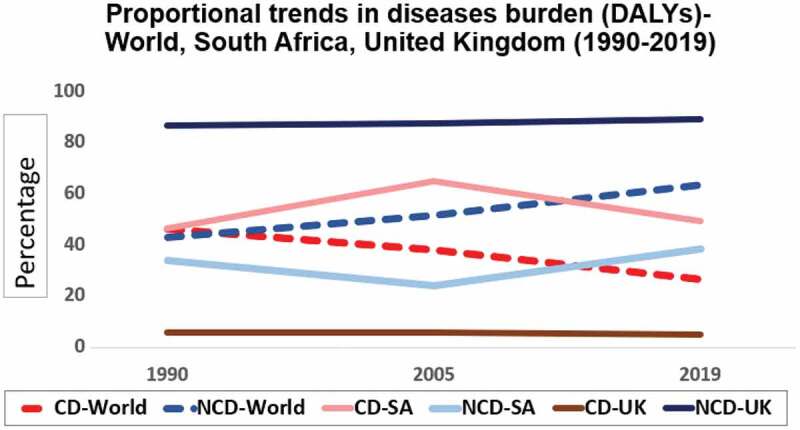
Proportional trends in diseases burden (DALYs)-World, South Africa, United Kingdom (1990–2019).
CD = Communicable, maternal, neonatal and nutritional diseases NCD = Noncommunicable diseases
Derived from Our World in Data tables (2019), https://ourworldindata.org/burden-of-disease
Figure 2.
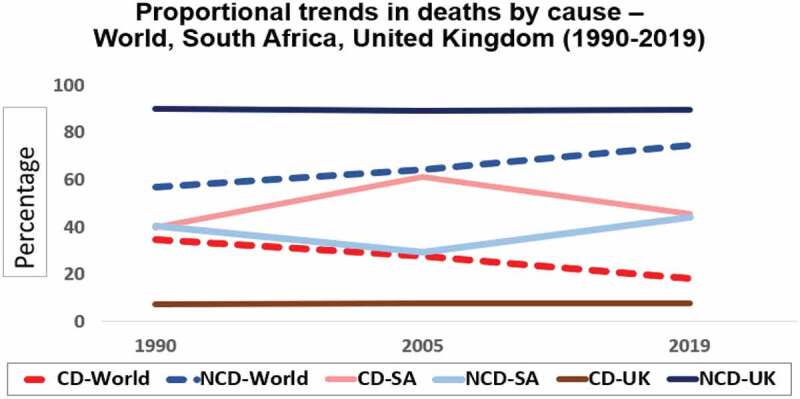
Proportional trends in deaths by cause-World, South Africa, United Kingdom (1990–2019).
CD = Communicable, maternal, neonatal and nutritional diseases NCD = Noncommunicable diseases
Derived from Our World in Data tables (2019), https://ourworldindata.org/burden-of-disease
According to the World Health Organisation (WHO), NCDs kill 41 million people each year, equivalent to 74% of global deaths with over 80% of NCD deaths due to cardiovascular diseases (CVDs), cancers, chronic respiratory diseases and diabetes, and associated kidney disease [10]. Mental illness, especially depression, is the largest contributor to global disability (7.5% of all years lived with disability) with 80% of the disease burden in LMICs [11].
Some 86% of premature NCD deaths (age 30–69 years) occur in LMICs, Sub-Saharan Africa (SSA) bears a higher burden than the global average with SA enduring the highest regional burden [12–15]. Despite SA having the largest global HIV epidemic, its age-standardised death rates (ASM) from NCDs are now higher than those from HIV/AIDS and tuberculosis combined. CVDs are currently the leading category of NCD deaths in SA at 18.9% of all deaths in 2018 [7,16]. This is reflected in the fact that more than 40% of young people are inactive, 70% of women are overweight or obese, around 16% of the population smokes daily and ~18% engage in binge drinking [15]. Concomitantly, SA has the highest prevalence of hypertension (one in three adults) in the world, the diabetes prevalence among adults has tripled to around 13% since 2010 [17], and a large portion of hypertension and diabetes is undiagnosed [18,19]. The prevalence of chronic obstructive pulmonary disease is as high as 24.8% in some urban regions of SA and some 10% of the adult population experience clinical depression at some point in their lives with only around 25% accessing treatment [20].
GLOBOCAN 2020 reports that the global burden of cancer is substantially growing with the cumulative risk of developing cancer by age 75 at 22.6% for males and 18.6% for females [21]. In HICs, cancer has surpassed CVD as the leading cause of premature death3, but the largest percentage increases are now occurring in LMICs [22], and in SSA cancer is among the three leading causes of premature death (ages 30–69 years) [13,23]. In SA, the pathology-based National Cancer Registry (SANCR) provides data for cancer surveillance trends; however, as it is pathology-based, it under-reports the cancer burden, although regional population-based cancer registration has been published since 2017 [24]. Bray et al. derived their incidence estimates for the country based on death reports [13]. SANCR reported a cumulative lifetime risk (before the age of 74) for 2019 of being diagnosed with cancer in males at 16.0% (versus 23.6 from GLOBOCAN) and 12.4% for women (versus 18.7 from GLOBOCAN). In SA the highest incident cancers were estimated to be breast, cervix uteri, and colorectal cancers in females and prostate, lung, and colorectal cancers in males, with cancers with the highest age-standardised mortality rates (ASM) overall being lung, cervix uteri, breast, prostate, and oesophageal cancers [25]. Since the successful rollout of antiretroviral treatments in Southern Africa, the incidence of AIDS-defining illnesses, including AIDS causing (so-called AIDS-defining) malignancies, has been on the decline. Patients are ageing and are thus susceptible to NCDs including non-AIDS-causing (non-AIDS-defining) cancers.
Multimorbidity burden
Unsurprisingly, the high chronic disease burden among global populations has created a perfect storm for multimorbidity (MM), defined as the coexistence in an individual of two or more NCDs (including mental health conditions) and infectious diseases of long duration. It typically occurs in ageing populations, but the global trend is towards a rapidly increasing prevalence at earlier age onset [26–28]. In HICs where the NCD burden is high, NCDs typically cluster together in varying combinations. Whereas in high CD endemic countries, clusters include chronic NCD and CD combinations which often act synergistically physiologically to exacerbate disease development and morbidity (e.g. HIV/ART which causes CVDs, tuberculosis, and diabetes which potentiate each other) [15,29]. Chronic diseases may cluster concordantly (e.g. cardiometabolic diseases such as hypertension and diabetes) where the comorbidities are related to the pathophysiology of the index disease and share the same treatment approaches [30]. They may also be discordant (e.g. cancer, mental health, hypertension, and HIV combinations) requiring separate management of distinct diseases, which poses enormous challenges to the current SA ‘single-disease model’ around which healthcare and research are traditionally organised [29].
Evidence of discordant MM burden in South Africa
Our own evidence of the high discordant MM burden among SSA patients comes from breast and prostate cancer patients newly diagnosed and treated in tertiary academic public health hospital cancer clinics. Our studies span a single site (Chris Hani Baragwanath Academic Hospital, Soweto, Johannesburg), multi-SA (Johannesburg and Kwa-Zulu Natal) site, and a multi-SSA country site (SA, Namibia, Uganda, Zambia, and Nigeria) cohort study [31–39]. We confirmed a high chronic disease and MM burden among these low-, middle- and upper middle-income countries in young (aged 20–49 years) and older women (50–75+ years of age) with breast cancer (BC), with the higher HDI index countries (SA and Namibia) bearing the highest burden [38]. The MM burden among SA women was a high 44% and obesity (52.8%), self-reported hypertension (41.3%), HIV (22.0%) and diabetes (13.7%) were the chronic conditions that occurred most frequently [31,32]. The chronic hypertension and diabetes and overall MM burden among the male prostate cancer cohort,(average age 10 years older than the BC cohort) was also very high, though as expected the HIV prevalence among this group was lower (at 14%) [32]. Multimorbidity is characterised by frailty, functional decline and negatively impacts patients’ capacity to withstand, respond to, and adhere to cancer and other chronic treatments. Patients with MM tend to have poorer health outcomes, decreased quality of life, and greater mental health issues [40]. Our own results confirm this; we showed that HIV negatively impacts response to neoadjuvant chemotherapy, adherence to long-term tamoxifen treatments, and overall survival among BC patients and that multimorbidity burdens of two or more diseases co-existing with BC negatively impacted their survival [34–37,39].
South Africa healthcare system challenges for multimorbidity management among adult cancer patients
The SA quadruple burden of disease has placed an enormous and increasing strain on the country’s under-resourced three-tiered public health system that serves approximately 85% of the population (which is largely socioeconomically disadvantaged and vulnerable), yet with only 20% of the available healthcare resources [41,42]. The impressive successes in integrating HIV and TB care have not been extended to the management of other chronic diseases [27]. The SA public health system is uncoordinated, and disease management is siloed, even at the primary healthcare (PHC) clinical level. This is particularly evident in the management of discordant MM among adult cancer patients. Cancer diagnosis and treatment are typically centralised in tertiary hospitals, already overburdened with the increasing cancer incidence, whereas other chronic diseases such as hypertension, HIV, uncomplicated diabetes, and mental health conditions are diagnosed and managed mainly in primary healthcare clinics, with only complex cases referred for tertiary management. Tertiary and PHC providers do not typically interact, and holistic, patient-centred MM management and shared decision-making are not prioritised. Consequently, cancer patients with MM must navigate polyclinics, general practitioners, and pharmacies in different healthcare facilities. Furthermore, many oncologists do not have clear MM management guidelines and backups to support them. This poses a significant risk for cancer patients’ overall quality of life and care [43].
The current consensus for best practices to manage MM is to focus on MM management at the PHC level, guided by the World Health Organization’s Innovative Care for Chronic Conditions (ICCC) framework [44]. It advocates that PHC providers need to be trained to be expert generalists to manage MM holistically through a tailored patient-centred care approach with skilled communication to cope with complex patient needs, circumstances and competing priorities, varying levels of health literacy and comorbidity of depression or anxiety [45]. The South African Department of Health responded in 2013 with a policy and strategy to re-engineer the PHC system to integrate chronic disease management into PHC services [46].
The United Kingdom National Institute for Health and Care Excellence (NICE) guidelines for MM management call for a re-orientation of care tailored to individual patients’ goals, priorities, and levels of health literacy [47,48]. It is appreciated that the inherent complexity of managing MM needs to be integrated into conceptual frameworks and guidelines that must consider the misalignment between patient needs and services provided [8,40]. There is evidence from work in SA that patients expected workload and capacity impact their healthcare utilisation and health outcomes [8]. Due consideration must also be given to the complex inherent biological interactions of multiple diseases, patient psychosocial, demographic and economic factors. Furthermore, their self-management capacity and capacity to meet health system expectations and demands of them, caregiver burdens and resources for support and quality of life impacts, must be considered. Provider knowledge and skills development in managing MM is required. In addition, health system capacity and re-organisation to manage MM require attention, considering existing community resources to augment scarce resources. Due consideration must be given to assessing patient frailty levels, considering all conditions and treatments simultaneously. Better data and guidelines about drug interactions and effectiveness of commonly prescribed treatments are required. We also require guidelines for stopping limited benefit treatments, introducing non-pharmaceutical treatments, and for coordinating follow-up visits and treatments. The development and recording of individualised management plans and the use of electronic records to facilitate communication between different providers dealing with patients are also fundamental requirements.
Despite the critical need to better manage the growing MM burden, there is a dearth of research into MM management generally and in LMICs particularly [27]. Recent reviews of interventions in HIC PHC settings have had very little impact on patient QoL and mental health [49,50]. Currently, no solutions exist, that we are aware of, that address the integrated management of cancer patients with chronic comorbid diseases. Going forward, we need to achieve many goals:
More epidemiological evidence characterising cancer and MM burden in SA.
Examination of existing policies and best buys, including expert health economic analyses, to determine changes needed to support the development, evaluation, and implementation of various interventions at the community, patient, and healthcare system levels to address the overall burden on the country.
Provision of integrated preventative and disease management services, where the patient, as well as the community, are placed at the centre of care.
Implementation of appropriate electronic tools and resources to enable ongoing surveillance of the burden of cancer and MM to determine trends and evaluate the effectiveness of interventions; and
Activation of community resources and social structures and individuals and patients themselves to actively demand cancer and MM screening/early symptom detection services offered through preventative health services supported by the required policies. These goals are daunting but potentially achievable with effort and coordination across the stakeholder base.
Involvement of all available public and private healthcare resources in so-called ‘private-public partnerships’ (PPPs) to enhance healthcare delivery.
Acknowledgments
We are grateful to the authors who provided the information that we utilised in this commentary.
Responsible Editor Stig Wall
Funding Statement
The article was funded by grants from the National Cancer Institute, R01CA250012 and R01CA19262, the South African Medical Research Council for the Centre for Research on Common Epithelial Cancers and DSI-NRF Centre of Excellence in Human Development at the University of the Witwatersrand, Johannesburg, South Africa.
Authors contributions
MJ – Made substantial contributions to the conception; design of the work; searching for literature; drafted the work and substantively revised it.
OAA – Made substantial contributions to the commentary; and substantively revised the work.
WM – Made substantial contributions to the commentary; and substantively revised it.
PR – Made substantial contributions to the commentary; and substantively revised the work.
HC – Made substantial contributions to the commentary; and substantively revised the work.
NM – Made substantial contributions to the commentary; and substantively revised the work.
SAN – Made substantial contributions to the conception; design of the work; and substantively revised it.
All the authors approved the submitted version and they agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Paper context
In this commentary, we reported on the multimorbidity burden among patients with non-AIDS-defining cancers in South Africa. This is significant because the development and recording of individualised management plans and the use of electronic records to facilitate communication between different providers dealing with patients are fundamental requirements in multimorbidity management.
References
- [1].Boutayeb A, Boutayeb S.. The burden of non communicable diseases in developing countries. Int J Equity Health. 2005;4. doi: 10.1186/1475-9276-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1204–6. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever‐increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029–3030. doi: 10.1002/cncr.33587 [DOI] [PubMed] [Google Scholar]
- [4].Roser M. Burden of disease our world in data. 2021. https://ourworldindata.org/burden-of-disease
- [5].Murray CJL. The global burden of disease study at 30 years. Nat Med. 2022;28:2019–2026. doi: 10.1038/s41591-022-01990-1 [DOI] [PubMed] [Google Scholar]
- [6].Smit M, Olney J, Ford NP, Vitoria M, Gregson S, Vassall A, et al. The growing burden of noncommunicable disease among persons living with HIV in Zimbabwe. AIDS. 2018;32:773–782. doi: 10.1097/QAD.0000000000001754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wong EB, Olivier S, Gunda R, Koole O, Surujdeen A, Gareta D, et al. Convergence of infectious and non-communicable disease epidemics in rural South Africa: a cross-sectional, population-based multimorbidity study. Lancet Glob Health. 2021;9:e967–e976. doi: 10.1016/S2214-109X(21)00176-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Oni T, McGrath N, BeLue R, Roderick P, Colagiuri S, May CR, et al. Chronic diseases and multi-morbidity - a conceptual modification to the WHO ICCC model for countries in health transition. BMC Public Health. 2014;14:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kämpfen F, Wijemunige N, Evangelista B. Aging, non-communicable diseases, and old-age disability in low- and middle-income countries: a challenge for global health. Int J Public Health. 2018;63:1011–1012. doi: 10.1007/s00038-018-1137-z [DOI] [PubMed] [Google Scholar]
- [10].World Health Organisation Noncommunicable diseases . 2022. [cited 2023 Mar 18]. Available from: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases
- [11].Cuadros DF, Tomita A, Vandormael A, Slotow R, Burns JK, Tanser F. Spatial structure of depression in South Africa: a longitudinal panel survey of a nationally representative sample of households. Sci Rep. 2019;9. doi: 10.1038/s41598-018-37791-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hajat C, Stein E. The global burden of multiple chronic conditions: a narrative review. Prev Med Reports. 2018;12:284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bray F, Parkin DM. Cancer in sub-Saharan Africa in 2020: a review of current estimates of the national burden, data gaps, and future needs. Lancet Oncol. 2022;23:719–728. doi: 10.1016/S1470-2045(22)00270-4 [DOI] [PubMed] [Google Scholar]
- [14].Sharma R, Aashima, Nanda M, Fronterre C, Sewagudde P, Ssentongo AE, et al. Mapping cancer in Africa: a comprehensive and comparable characterization of 34 cancer types using estimates from GLOBOCAN 2020. Front Public Health. 2022;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kamkuemah M, Gausi B, Oni T. High prevalence of multimorbidity and non-communicable disease risk factors in South African adolescents and youth living with HIV: implications for integrated prevention. S Afr Med J. 2022;112:259–267. [PubMed] [Google Scholar]
- [16].Mortality and causes of death in South Africa: Findings from death notification . 2018. https://www.statssa.gov.za/publications/P03093/P030932018.pdf
- [17].Grundlingh N, Zewotir TT, Roberts DJ, Manda S. Assessment of prevalence and risk factors of diabetes and pre-diabetes in South Africa. J Heal Popul Nutr. 2022;41(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Micklesfield LK, Kolkenbeck-Ruh A, Mukoma G, Prioreschi A, Said-Mohamed R, Ware LJ, et al. The healthy aging adult South Africa report card: a systematic review of the evidence between 2013 and 2020 for middle-aged South African men and women. Cardiovasc J Afr. 2022;33:38–57. doi: 10.5830/CVJA-2022-015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].The Healthy Aging Adult South Africa Report Card 2022, Adapted from Micklesfield LK, Kolkenbeck-Ruh A, Mukoma G, Prioreschi A, Said-Mohamed R, et al. The healthy aging adult South Africa report card: a systematic review of the evidence between 2013 and 2020 for middle-aged South African men and women. Cardiovasc J Afr. 2022;33:38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Awokola BI, Amusa GA, Jewell CP, Okello G, Stobrink M, Finney LJ, et al. Chronic obstructive pulmonary disease in Sub-Saharan Africa. Int J Tuberc Lung Dis. 2022;26:232–242. doi: 10.5588/ijtld.21.0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].WHO Globocan . 2020. https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf
- [22].Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, Harvey JD, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. 2022;8:420–444. doi: 10.1001/jamaoncol.2021.6987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li Z, Shi J, Li N, Wang M, Jin Y, Zheng ZJ, et al. Temporal trends in the burden of non-communicable diseases in countries with the highest malaria burden, 1990–2019: evaluating the double burden of non-communicable and communicable diseases in epidemiological transition. Global Health. 2022;18. doi: 10.1186/s12992-022-00882-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].South Africa National Cancer Registry . 2019. [cited 2023 Mar 18]. Available from: https://www.nicd.ac.za/wp-content/uploads/2021/12/NCR_Path_2019_Full_Report_8dec2021.pdf
- [25].Global cancer observatory South Africa 2020 . [cited 2023 Mar 18]. Available from: https://cansa.org.za/files/2021/02/IARC-Globocan-SA-2020-Fact-Sheet.pdf
- [26].MacMahon S. The academy of medical sciences. Multimorb. 2018;1–127. https://acmedsci.ac.uk/file-download/82222577577 [Google Scholar]
- [27].Roomaney RA, Van Wyk B, Van Wyk VP. Decolonising multimorbidity? Research gaps in low and middle-income countries. Pan Afr Med J. 2022;41:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Asogwa OA, Boateng D, Marzà-Florensa A, Peters S, Levitt N, van Olmen J, et al. Multimorbidity of non-communicable diseases in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open. 2022;12:e049133. doi: 10.1136/bmjopen-2021-049133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chudasama YV, Khunti K, Davies MJ. Clustering of comorbidities. Futur Healthc J. 2021;8:e224–e229. doi: 10.7861/fhj.2021-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Luijks HD, Lagro-Janssen ALM, van Weel C. Multimorbidity and the primary healthcare perspective. J Comorbidity. 2016;6:46–49. doi: 10.15256/joc.2016.6.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ayeni OA, Joffe M, Cubasch H, Rinaldi S, Taljaard C, Vorster E, et al. Prevalence of comorbidities in women with and without breast cancer in Soweto, South Africa: results from the SABC study. S Afr Med J. 2019;109:264. doi: 10.7196/SAMJ.2019.v109i4.13465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mapanga W, Norris SA, Craig A, Pumpalova Y, Ayeni OA, Chen WC, et al. Prevalence of multimorbidity in men of African descent with and without prostate cancer in Soweto, South Africa. PLoS One. 2022;17:e0276050. doi: 10.1371/journal.pone.0276050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ayeni OA, Norris SA, Joffe M, Cubasch H, Nietz S, Buccimazza I, et al. The multimorbidity profile of South African women newly diagnosed with breast cancer. Int J Cancer. 2019;147(2):361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ayeni OA, O’Neil DS, Pumpalova YS, Chen WC, Nietz S, Phakathi B, et al. Impact of HIV infection on survival among women with stage I‐III breast cancer: results from the South African breast cancer and HIV outcomes study. Int J Cancer. 2022;151:209–221. doi: 10.1002/ijc.33981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ayeni O, Joffe M, Mapanga W, Chen WC, O’Neil DS, Phakathi B, et al. Multimorbidity and overall survival among women with breast cancer: results from the South African breast cancer and HIV outcomes study. Breast Cancer Res. 2023;25:7. doi: 10.1186/s13058-023-01603-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nietz S, O’Neil DS, Ayeni O, Chen WC, Joffe M, Jacobson JS, et al. A comparison of complete pathologic response rates following neoadjuvant chemotherapy among South African breast cancer patients with and without concurrent HIV infection. Breast Cancer Res Treat. 2020;184:861–872. doi: 10.1007/s10549-020-05889-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ayeni O, Chiwambutsa S, Chen WC, Kapungu N, Kanji C, Thelingwani R, et al. The impact of HIV on non-adherence for tamoxifen among women with breast cancer in South Africa. Breast Cancer Res Treat. 2023;197:647–659. doi: 10.1007/s10549-022-06835-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ayeni OA, Norris SA, Joffe M, Cubasch H, Galukande M, Zietsman A, et al. Preexisting morbidity profile of women newly diagnosed with breast cancer in Sub‐Saharan Africa: African breast cancer—Disparities in outcomes study. Int J Cancer. 2012;148:2158–2170. doi: 10.1002/ijc.33387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chasimpha S, McCormack V, Cubasch H, Joffe M, Zietsman A, Galukande M, et al. Disparities in breast cancer survival between women with and without HIV across Sub-Saharan Africa (ABC-DO): a prospective, cohort study. Lancet HIV. 2022;9:e160–e171. doi: 10.1016/S2352-3018(21)00326-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Grembowski D, Schaefer J, Johnson KE, Fischer H, Moore SL, Tai-Seale M, et al. A conceptual model of the role of complexity in the care of patients with multiple chronic conditions. Med Care. 2014;52:S7–S14. doi: 10.1097/MLR.0000000000000045 [DOI] [PubMed] [Google Scholar]
- [41].Samodien E, Abrahams Y, Muller C, Louw J, Chellan N. Non-communicable diseases – A catastrophe for South Africa. S Afr J Sci. 2021;117(5/6):117. [Google Scholar]
- [42].van Rensburg HC. South Africa’s protracted struggle for equal distribution and equitable access – still not there. Hum Resour Health. 2014;12. doi: 10.1186/1478-4491-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Head A, Fleming K, Kypridemos C, Pearson-Stuttard J, O’Flaherty M. Multimorbidity: the case for prevention. J Epidemiol Community Health. 2020;75(3):242–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].World Health Organisation . WHO: innovative care for chronic conditions: building blocks for action. 2002.
- [45].World Health Organisation . Multimorbidity-technical series on safer primary care. 2016. [cited 2023 Mar 18]. Available from: https://apps.who.int/iris/bitstream/handle/10665/252275/9789241511650-eng.pdf
- [46].South African Department of Health . Strategic plan for the prevention and control of noncommunicable diseases 2013-17. 2013.
- [47].Farmer C, Fenu E, O’Flynn N, Guthrie B. Clinical assessment and management of multimorbidity: summary of NICE guidance. BMJ. 2016;354:i4843. [DOI] [PubMed] [Google Scholar]
- [48].NICE . Multimorbidity: clinical assessment and management. 2016. [cited 2023 Mar 18]. Available from: https://www.nice.org.uk/guidance/ng56
- [49].Smith SM, Wallace E, Clyne B, Boland F, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community setting: a systematic review. Syst Rev. 2021;10. doi: 10.1186/s13643-021-01817-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Fortin M, Stewart M, Almirall J, Beaupré P. Challenges in multimorbidity research: lessons learned from the most recent randomized controlled trials in primary care. Front Med. 2022;9. doi: 10.3389/fmed.2022.815783 [DOI] [PMC free article] [PubMed] [Google Scholar]


