Prenatal repair of a myelomeningocele is associated with persistent improvement in posterior fossa imaging findings of Chiari II malformation at school age compared with postnatal repair.
Abstract
BACKGROUND AND PURPOSE:
Short-term results demonstrate that prenatal repair of a myelomeningocele is associated with a reduction in hydrocephalus and an increased likelihood of the reversal of Chiari II malformations compared with postnatal repair. The purpose of this study was to identify the long-term imaging findings at school age among subjects who underwent pre- versus postnatal repair of a myelomeningocele.
MATERIALS AND METHODS:
A subset of subjects enrolled in the Management of Myelomeningocele Study who underwent either prenatal (n = 66) or postnatal (n = 63) repair of a lumbosacral myelomeningocele and had follow-up brain MR imaging at school age were included. The prevalence of posterior fossa features of Chiari II malformation and supratentorial abnormalities and the change in these findings from fetal to school-age MR imaging were compared between the 2 groups.
RESULTS:
Prenatal repair of a myelomeningocele was associated with higher rates of normal location of fourth ventricle and lower rates of hindbrain herniation, cerebellar herniation, tectal beaking, brainstem distortion, and kinking at school age compared with postnatal repair (all P < .01). Supratentorial abnormalities, including corpus callosal abnormalities, gyral abnormalities, heterotopia, and hemorrhage, were not significantly different between the 2 groups (all P > .05). The rates of resolution of brainstem kinking, tectal beaking, cerebellar and hindbrain herniation, and normalization of fourth ventricle size from fetal to school age MR imaging were higher among the prenatal compared with postnatal surgery group (all, P < .02).
CONCLUSIONS:
Prenatal repair of a myelomeningocele is associated with persistent improvement in posterior fossa imaging findings of Chiari II malformation at school age compared with postnatal repair.
Spinal dysraphism includes a broad spectrum of congenital anomalies with an incidence of ∼3 per 10,000 live births in the United States.1-3 More than 85% of these are myelomeningoceles (MMCs), an open neural tube defect that consists of a poorly differentiated neural placode extending into a midline dorsal sac containing CSF.1-3 Almost all fetuses with MMCs have associated Chiari II malformations, characterized by a small posterior fossa and caudal displacement with narrowing and elongation of the fourth ventricle, cerebellum, and brainstem.4 It is believed that hindbrain herniation occurs due to leakage of CSF into the sac and amniotic fluid, resulting in chronic CSF hypotension.5 The herniation of the posterior fossa contents can further interfere with normal CSF flow (including narrowing and occlusion of the aqueduct) and the development of structures that absorb CSF, resulting in hydrocephalus.5 Moreover, MMCs are associated with supratentorial developmental abnormalities, including abnormal corpus callosum, abnormal gyration, and periventricular nodular heterotopia (PVNH).6
It is believed that chronic exposure of the poorly developed neural placode and spinal nerves to amniotic fluid results in progressive neuronal loss,7 which, in combination with the abnormally formed spinal cord, leads to lower-extremity motor and sensory dysfunction and loss of bladder and bowel control. The posterior fossa and supratentorial abnormalities can result in impairment in nonverbal reasoning, attention, language functionality,8 and executive function in these children.9
Prenatal repair of an MMC was first reported in 1998, and early studies demonstrated reversal of hindbrain herniation and a decreased need for CSF diversion compared with historical controls.10,11 The Management of Myelomeningocele Study (MOMS), a randomized controlled trial comparing prenatal with the then-standard postnatal repair of MMCs12 demonstrated a significant benefit among the prenatal surgery group with a reduced need for CSF diversion at 12 months13 and improved motor function at 30 months.14 Prenatal repair was associated with lower rates of abnormal posterior fossa imaging findings and syringomyelia at 12 months.12 Additional studies have further confirmed this finding of higher rates of resolution of hindbrain herniation in the prenatal surgery group in the short term.15,16 However, the long-term imaging findings of prenatal repair of MMCs are unknown. MOMS2 was designed as a follow-up to MOMS to assess the long-term outcomes of prenatal surgical repair of an MMC when the children were 5–10 years of age. The purpose of this study was to compare the long-term imaging findings and the change in imaging findings from fetal life to school age between children who underwent fetal and postnatal repair of MMCs in MOMS2.
MATERIALS AND METHODS
The MOMS2 study was conducted at the same 3 sites that participated in MOMS (Children’s Hospital of Philadelphia, Vanderbilt University, and University of California, San Francisco) and the George Washington University Biostatistics Center served as the data-coordinating center. Institutional review board approval was obtained at each clinical site and the data-coordinating center. Caregivers gave written informed consent, and children gave assent per local institutional review board regulations.
Imaging
The protocol of MOMS has been described previously.12 Briefly, fetal brain and spine MR imaging performed at 18–26 weeks before intervention were reviewed by an independent committee comprising of 2 pediatric neuroradiologists and a pediatric radiologist. Reviewers were blinded to the assigned surgery group and scored the brain and spine findings, including cerebellar and hindbrain herniation, cerebellar hypoplasia, brainstem kinking, tectal beaking, size of the fourth ventricle, structural abnormalities including those of the corpus callosum, and the presence of a sac overlying the MMC defect.
Study participation for MOMS2 consisted of a single visit when the child was 5–10 years of age at one of the clinical centers or at home if the parent or caregiver declined travel.17 Children who presented to the clinical center underwent 3T imaging of the brain and spine without sedation or contrast administration. The brain imaging protocol included 3D isotropic T1-weighted MPRAGE, 3D isotropic T2-weighted TSE, and 32-direction DTI. Some patients also had 3D T2 FLAIR imaging and SWI. An independent committee of 3 pediatric neuroradiologists with 9–40 years’ experience reviewed the MR images in consensus and were blinded to treatment group, clinical reads, and any prior MR imaging results. Studies were scored on the basis of consensus review and included the location and size of the fourth ventricle (assessed in all planes), tectal beaking (assessed in the sagittal and axial planes), cerebellar hypoplasia (assessed in all planes), brainstem hypoplasia, and brainstem distortion and kinking (assessed in the sagittal plane). Cerebellar herniation was assessed in the sagittal and coronal planes and categorized as mild (at C1), moderate (at C2), or severe (below C2). Hindbrain herniation was assessed in the sagittal plane and categorized on the basis of the location of the cervicomedullary junction as none (above foramen magnum), mild (between foramen magnum and C1), moderate (between C1 and C2), or severe (below C2). Supratentorial features that were assessed included corpus callosum, callosal ridge, hypothalamic adhesion, anterior commissure, presence of abnormal gyration, heterotopia, deep gray nuclei signal, and hemorrhagic and nonhemorrhagic lesions (white matter atrophy). Spine MR imaging was assessed for the presence of syringomyelia and epidermoid cysts. One of the pediatric neuroradiologists involved in the MOMS image review was available to address questions and ensure consistency in scoring. Due to challenges in imaging assessment of a tethered spinal cord after MMC repair, the need for tethered cord release surgery was used as an estimate of the incidence of symptomatic tethered cord.
Statistical Analyses
Categoric variables were compared using the χ2 test or Fisher exact test, as appropriate. Due to skewed distributions, continuous variables were compared using the Wilcoxon rank-sum test. P values < .05 were considered statistically significant. Statistical analysis used SAS, Version 9.04 (SAS Institute).
RESULTS
Study Population
The mean age at the time of the follow-up visit was 7.5 years in the prenatal and 7.3 years in the postnatal surgery groups (Table 1, P = .46). Most children in both groups were white (92% versus 87%, P = .33), and there was a lower proportion of girls in the prenatal surgery group (44% versus 65%, P = .022), similar to the MOMS results.14 The distribution of the MMC lesion level was similar between the 2 groups (P = .56). Most of the fetuses in both groups had a sac overlying the defect, with a slightly higher prevalence in the postnatal compared with the prenatal surgery group (87.1% versus 72.7%, P = .043).
Table 1:
Demographic variables of the study groupsa
| Variable | Prenatal Surgery (n = 66) | Postnatal Surgery (n = 63) | P Valueb |
|---|---|---|---|
| Age at follow-up (yr) | 7.5 (SD, 1.3) | 7.3 (SD, 1.2) | .460 |
| Female (No.) (%) | 29 (43.9%) | 41 (65.1%) | .022 |
| Race (No.) (%) | .334 | ||
| White | 61 (92.4%) | 55 (87.3%) | |
| African American | 1 (1.5%) | 1 (1.6%) | |
| Hispanic | 2 (3.0%) | 5 (7.9%) | |
| Other | 2 (3.0%) | 2 (3.2%) | |
| Gestational age at birth (week) | 34.4 (SD, 2.6) | 37.5 (SD, 0.8) | <.001 |
| Anatomic lesion level (No.) (%) | .563 | ||
| Thoracic | 2 (3.0%) | 1 (1.6%) | |
| L1–L2 | 9 (13.6%) | 12 (19.1%) | |
| L3–L4 | 33 (50.0%) | 29 (46.0%) | |
| L5–S2 | 22 (33.3%) | 21 (33.3%) | |
| Presence of sac on fetal imaging | 48 (72.7%) | 54 (87.1%) | .043 |
| Tethered cord release | 19 (28.8) | 8 (12.7) | .025 |
Values listed are No. (%) or mean (SD).
P value for race is white versus all others. P value for lesion level is T1–L2 versus L3–S2.
Imaging Findings at School Age
A higher proportion of children in the prenatal surgery group had a normal location of the fourth ventricle and absence of hindbrain herniation, cerebellar herniation, brainstem distortion, brainstem kinking, brainstem hypoplasia, and tectal beaking compared with the postnatal surgery group at school age (all, P ≤ .01, Table 2.
Table 2:
School-age posterior fossa and spine imaging findings in children who underwent prenatal-versus-postnatal surgery
| Imaging | Prenatal Surgery n/N (%) | Postnatal Surgery n/N (%) | RR (95% CI) | P Value |
|---|---|---|---|---|
| Fourth ventricle location | <.001 | |||
| Normal | 19/66 (29) | 6/63 (10) | ||
| Low | 42/66 (64) | 35/63 (56) | ||
| At foramen magnum | 3/66 (5) | 10/63 (16) | ||
| Below foramen magnum | 2/66 (3) | 12/63 (19) | ||
| Tectal beaking | 53/66 (80) | 61/63 (97) | 0.83 (0.73–0.94) | .003 |
| Hindbrain herniation | <.001 | |||
| None | 26/66 (39) | 8/62 (13) | ||
| Mild | 23/66 (35) | 12/62 (19) | ||
| Moderate | 15/66 (23) | 26/62 (40) | ||
| Severe | 2/66 (3) | 17/62 (27) | ||
| Cerebellar herniation degree | <.001 | |||
| None | 33/66 (50) | 8/63 (13) | ||
| Mild | 22/66 (33) | 21/63 (33) | ||
| Moderate | 11/66 (17) | 20/63 (32) | ||
| Severe | 0 | 14/63 (22) | ||
| Cerebellar hypoplasia | 4/66 (6) | 9/63 (14) | 0.42 (0.14–1.31) | .12 |
| Brainstem hypoplasia | 8/66 (12) | 19/63 (30) | 0.40 (0.19–0.85) | .01 |
| Brainstem distortion | 33/66 (50) | 52/63 (83) | 0.61 (0.46–0.79) | <.001 |
| Brainstem kinking | <.001 | |||
| None | 53/66 (80) | 31/63 (49) | ||
| Mild | 11/66 (17) | 20/63 (32) | ||
| Moderate | 2/66 (3) | 11/63 (17) | ||
| Severe | 0 | 1/63 (2) | ||
| Syringomyelia | 37/63 (59) | 48/59 (81) | 0.7 (0.6–0.9) | .007 |
| Epidermoid cyst | 7/63 (11) | 2/58 (3) | 3.2 (0.7–14.9) | .17 |
Note:—RR indicates relative risk.
Supratentorial malformations such as PVNH, abnormal gyration, and abnormal morphology of the corpus callosum or anterior commissure were not significantly different between treatment groups. The presence of supratentorial abnormalities, including hemorrhage, nonhemorrhagic lesions, abnormal signal of the deep gray nuclei, and diminished parahippocampal gyral volume did not differ between the 2 groups (Online Supplemental Data).
The incidence of syringomyelia was higher in the postnatal compared with prenatal surgery group (81% versus 59%, P = .007, Table 2). The prenatal surgery group had a higher rate of tethered cord release surgery (28.8% versus 12.7%, P = .025). There was no significant difference between the groups in the incidence of epidermoid cyst on school-age MR imaging (P = .17).
Change in Imaging Findings
In the prenatal surgery group, 55% of the children with a small fourth ventricle at baseline fetal MR imaging reverted to normal fourth ventricular size by school age, while only 14% of the children in the postnatal surgery group reverted to a normal-sized fourth ventricle at school age (P < .001). There were higher rates of resolution of brainstem kinking, tectal beaking, cerebellar herniation, and hindbrain herniation by school age in the prenatal surgery group compared with the postnatal group (all P < .05, Table 3 and Figure). There was new development of syringomyelia by school age in 58% of children who underwent prenatal surgery compared with 78% among those who underwent postnatal surgery (P = .03). The change in structural abnormalities (abnormal gyration or PVNH) from fetal MR imaging to school age MR imaging was not significantly different among those who underwent prenatal-versus-postnatal surgery (Table 3).
Table 3:
Change in imaging variables from baseline fetal to school age in pre- and postnatal surgery groups
| Imaging Variable | Prenatal Surgery | Postnatal Surgery | P Value |
|---|---|---|---|
| Fourth ventricle size | n = 66 | n = 63 | |
| Changed from small to normal | 36 (55) | 9 (14) | <.001 |
| Remained small | 28 (42) | 51 (81) | |
| Another pattern | 2 (3) | 3 (5) | |
| Tectal beaking | n = 60 | n = 54 | |
| Resolved | 12 (20) | 2 (4) | .008 |
| Not resolved | 48 (80) | 52 (96) | |
| Hindbrain herniation | n = 66 | n = 62 | |
| Resolved | 26 (39) | 8 (13) | .001 |
| Remained the same | 40 (61) | 54 (87) | |
| Cerebellar herniation | n = 65 | n = 62 | |
| Resolved | 32 (49) | 8 (13) | <.001 |
| Remained the same | 32 (49) | 53 (85) | |
| New | 1 (2) | 1 (2) | |
| Cerebellar hypoplasia | n = 61 | n = 54 | |
| Resolved | 44 (72) | 33 (61) | .27 |
| Remained the same | 17 (28) | 20 (37) | |
| New | 0 | 1 (2) | |
| Brainstem kinking | n = 38 | n = 41 | |
| Resolved | 16 (42) | 8 (20) | .02 |
| Remained the same | 20 (53) | 23 (56) | |
| New | 2 (5) | 10 (24) | |
| Structural abnormality (gyral and heterotopia) | n = 64 | n = 60 | |
| Resolved | 2 (3) | 1 (2) | .94 |
| Remained the same | 40 (63) | 40 (67) | |
| New | 22 (34) | 19 (32) |
FIGURE.
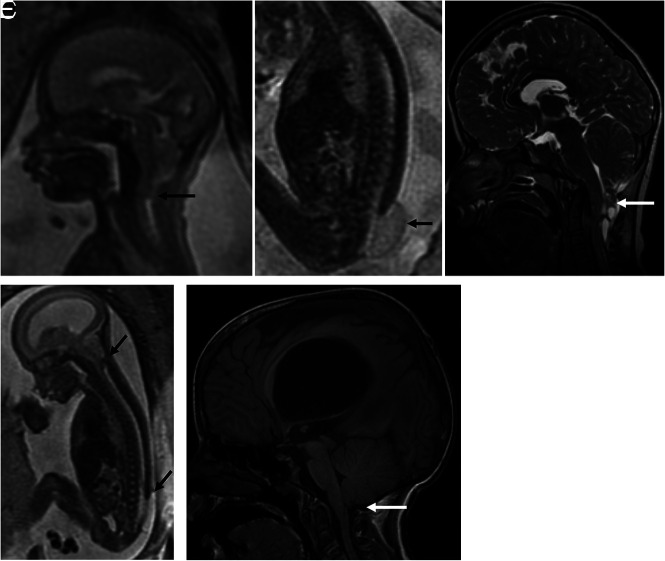
A and B, A 25-week-old fetus with a lumbosacral MMC (arrow) and Chiari II malformation (arrow) with the cerebellum extending to approximately the C3 level. The patient underwent postnatal repair with follow-up MR imaging (C) at 8 years demonstrating persistent brainstem and cerebellar herniation to C2 (white arrow). D, A 21-week 6-day-old fetus with a lower lumbar MMC (arrow) and Chiari II malformation with the cerebellum extending to approximately the C3 level (arrow). E, Status post fetal repair at 22 weeks 5 days with no residual cerebellar or brainstem herniation at 9 years of age (white arrow).
DISCUSSION
Our study demonstrates that the improvement in structural posterior fossa abnormalities from prenatal compared with postnatal repair of an MMC persists into school age. Early studies of open fetal repair of an MMC demonstrated high rates of resolution of hindbrain herniation in 3–6 weeks following the procedure, with a possibly lower rate of progressive hydrocephalus and an increase in posterior fossa subarachnoid spaces in utero.10,18,19 These higher rates of resolution of cerebellar herniation associated with prenatal repair are known to persist in the postnatal period.15,16,19 In MOMS, at 12 months of age, the prenatal surgery group demonstrated improved Chiari II–related posterior fossa findings as well as syringomyelia compared with the postnatal surgery group.12 This is the first study to demonstrate the persistent long-term improvement in Chiari II malformations in school-age children who underwent prenatal compared with postnatal repair of an MMC.
CNS anomalies associated with MMC develop at various gestational ages, with Chiari II malformation being a relatively late manifestation, increasing in prevalence from 31% at 16 weeks to 92% at 16–20 weeks.7 In MOMS, the prevalence of severe preoperative hindbrain herniation was not significantly different between the prenatal and postnatal surgery groups.14 The bony morphology of the posterior fossa is, however, known to change rapidly toward normal after fetal surgery within as early as 1 week postrepair.20 These bony changes continue to improve in the fetal surgery group at 4 weeks postrepair with normalization of the configuration of the posterior fossa, characterized by the clivus-supraocciput angle in the early postnatal period.18,21,22 There is, however, conflicting data on the changes in posterior fossa configuration in the postnatal repair group, with reports of both a lower and relatively normal clivus-supraocciput angle compared with healthy controls.21,22 However, the posterior fossa area is known to remain smaller in neonates who underwent prenatal or postnatal repair compared with healthy controls.18,22 Although there are differences in imaging timepoints and measurement techniques among these studies, these early changes to the bony posterior fossa configuration could potentially explain the persistent improvement in Chiari II manifestations seen at school age in our study.
Prenatal-versus-postnatal surgery had no significant effect on malformations of the supratentorial brain in childhood. The overall prevalence of malformations such as abnormal gyration and PVNH on fetal imaging were low (3%–8%), similar to findings in prior reports.6,18 However, the true prevalence based on childhood imaging is much higher (45%–77%), underscoring the challenges with prenatal identification of these malformations,23 particularly in the setting of MMC and Chiari II malformation.18 The corpus callosum was abnormal in 83%–86% of children, similar to prior reports of 60%–86% on fetal sonography and postnatal MR imaging.6,24,25 Supratentorial malformations in MMC are thought to be due to neuroepithelial and ependymal denudation, and these occur at 16 weeks before the development of a Chiari II malformation and hydrocephalus.26 This early and permanent damage to the ependyma could explain the lack of improvement in the incidence of supratentorial malformations despite prenatal MMC repair, which was typically performed at 19–26 weeks in MOMS.14 However, the effect of the timing of fetal surgery on supratentorial abnormalities remains unknown. Future studies should also assess the effect of prenatal surgery on quantitative structural and connectivity metrics of the supratentorial brain, particularly in the setting of a known lack of improvement in long-term cognitive function with prenatal surgery.17
It is difficult to directly relate the qualitative evaluations of structural impairments to neurodevelopmental outcomes. Assessment of neurodevelopmental outcomes at school age17 did not show marked improvement in adaptive behavior and cognitive skills but did report improvement in fine-motor skills in the prenatal surgery group, which is associated with cerebellar function.27 Quantitative assessment of the tectum and its connectivity with the frontal and parietal regions showed stronger attention-orienting and arousal in association with reduced tectal dysmorphology.28 Because prenatal surgery reduces hindbrain herniation and the findings of Chiari II, there may be an indirect effect leading to improved cognitive and motor functions.
Finally, prenatal surgery was associated with a lower incidence of syringomyelia but higher rates of tethered cord release surgery in our cohort. However, spina bifida registry data as well as recent single-institutional data suggest similar rates of tethered cord release surgery of 13%–18% in both prenatal and postnatal surgery cohorts.29,30 Our study cohort is slightly older than children in these studies, and the need for tethered cord release is known to increase with age.31 Furthermore, practice and indications for detethering can be variable among institutions.31
Our study has several limitations. Blood-sensitive sequences were not available in all patients, limiting the assessment of the frequency of hemorrhagic lesions. The presence or absence of a sac overlying the MMC defect and the level and size of the MMC defect may affect the severity and evolution of Chiari II manifestations,32-34 which was not adjusted for in our study. However, only a small fraction of cases did not have an overlying sac on fetal imaging. Furthermore, the myelocele was more prevalent in the prenatal surgery group, which may be underestimating our results on long-term improvement in imaging findings with prenatal surgery because the absence of overlying sac suggests a more severe phenotype.33 The association of imaging findings with functional outcome was not assessed; however, prenatal repair has been shown to be associated with better mobility and independent functioning at school age.17 Interpretation of ventricular size is complex in the setting of CSF shunts, shunt revisions, and third ventriculostomy procedures,35 and detailed assessment of ventricular size will be the focus of a future study. In the MOMS2 cohort, all patients in the postnatal surgery group had hydrocephalus.35 In the prenatal and postnatal surgery groups, ventricular size has been shown to be larger in those with unshunted hydrocephalus compared with those with shunted hydrocephalus and those with no hydrocephalus in the prenatal surgery group.35 Post-MOMS single-institution data and MOMS2 studies also demonstrated fewer shunt placements and shunt revisions in the prenatal compared with the postnatal surgery group both in the short and long term.15,17 Finally, quantitative assessments of the bony posterior fossa was not included in this study.
CONCLUSIONS
Our study demonstrates that prenatal repair of an MMC is associated with persistent improvement in posterior fossa imaging findings at school age compared with postnatal repair. This outcome along with long-term neurodevelopmental outcome data provides valuable information for parental counseling for prenatal surgery for MMC.
Acknowledgment
In memory of Elizabeth Thom, PhD, who was instrumental in contributing to this work.
ABBREVIATIONS:
- MMC
myelomeningocele
- PVNH
periventricular nodular heterotopia
Footnotes
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U01 HD06854: follow-up of the Management of Myelomeningocele Study), funded by the National Institutes of Health. Elizabeth George was supported by the American Society of Neuroradiology Scholar Award.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Agopian AJ, Canfield MA, Olney RS, et al. ; National Birth Defects Prevention Study. Spina bifida subtypes and sub-phenotypes by maternal race/ethnicity in the National Birth Defects Prevention Study. Am J Med Genet A 2012;158A:109–15 10.1002/ajmg.a.34383 [DOI] [PubMed] [Google Scholar]
- 2.Boulet SL, Yang Q, Mai C, et al. ; National Birth Defects Prevention Network. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res A Clin Mol Teratol 2008;82:527–32 10.1002/bdra.20468 [DOI] [PubMed] [Google Scholar]
- 3.Parker SE, Mai CT, Canfield MA, et al. ; National Birth Defects Prevention Network. Updated national birth prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol 2010;88:1008–16 10.1002/bdra.20735 [DOI] [PubMed] [Google Scholar]
- 4.Rossi A, Gandolfo C, Morana G, et al. Current classification and imaging of congenital spinal abnormalities. Semin Roentgenol 2006;41:250–73 10.1053/j.ro.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 5.McLone DG, Knepper PA. The cause of Chiari II malformation: a unified theory. Pediatr Neurosci 1989;15:1–12 10.1159/000120432 [DOI] [PubMed] [Google Scholar]
- 6.Maurice P, Garel J, Garel C, et al. New insights in cerebral findings associated with fetal myelomeningocele: a retrospective cohort study in a single tertiary centre. BJOG 2021;128:376–83 10.1111/1471-0528.16185 [DOI] [PubMed] [Google Scholar]
- 7.Ben Miled S, Loeuillet L, Duong Van Huyen JP, et al. Severe and progressive neuronal loss in myelomeningocele begins before 16 weeks of pregnancy. Am J Obstet Gynecol 2020;223:256 e1–e9 10.1016/j.ajog.2020.02.052 [DOI] [PubMed] [Google Scholar]
- 8.Vachha B, Adams R. Language differences in young children with myelomeningocele and shunted hydrocephalus. Pediatr Neurosurg 2003;39:184–89 10.1159/000072469 [DOI] [PubMed] [Google Scholar]
- 9.Foss S, Flanders TM, Heuer GG, et al. Neurobehavioral outcomes in patients with myelomeningocele. Neurosurg Focus 2019;47:E6 10.3171/2019.7.FOCUS19445 [DOI] [PubMed] [Google Scholar]
- 10.Johnson MP, Sutton LN, Rintoul N, et al. Fetal myelomeningocele repair: short-term clinical outcomes. Am J Obstet Gynecol 2003;189:482–87 10.1067/s0002-9378(03)00295-3 [DOI] [PubMed] [Google Scholar]
- 11.Tulipan N, Bruner JP, Hernanz-Schulman M, et al. Effect of intrauterine myelomeningocele repair on central nervous system structure and function. Pediatr Neurosurg 1999;31:183–88 10.1159/000028859 [DOI] [PubMed] [Google Scholar]
- 12.Adzick NS, Thom EA, Spong CY, et al. ; MOMS Investigators. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 2011;364:993–1004 10.1056/NEJMoa1014379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tulipan N, Wellons JC 3rd, Thom EA, et al. ; MOMS Investigators. Prenatal surgery for myelomeningocele and the need for cerebrospinal fluid shunt placement. J Neurosurg Pediatr 2015;16:613–20 10.3171/2015.7.PEDS15336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farmer DL, Thom EA, Brock JW 3rd, et al. ; Management of Myelomeningocele Study Investigators. The Management of Myelomeningocele Study: full cohort 30-month pediatric outcomes. Am J Obstet Gynecol 2018;218:256.e13 10.1016/j.ajog.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flanders TM, Heuer GG, Madsen PJ, et al. Detailed analysis of hydrocephalus and hindbrain herniation after prenatal and postnatal myelomeningocele closure: report from a single institution. Neurosurgery 2020;86:637–45 10.1093/neuros/nyz302 [DOI] [PubMed] [Google Scholar]
- 16.Nagaraj UD, Bierbrauer KS, Zhang B, et al. Hindbrain herniation in Chiari II malformation on fetal and postnatal MRI. AJNR Am J Neuroradiol 2017;38:1031–36 10.3174/ajnr.A5116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houtrow AJ, Thom EA, Fletcher JM, et al. Prenatal repair of myelomeningocele and school-age functional outcomes. Pediatrics 2020;145:e2019154 10.1542/peds.2019-1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rethmann C, Scheer I, Meuli M, et al. Evolution of posterior fossa and brain morphology after in utero repair of open neural tube defects assessed by MRI. Eur Radiol 2017;27:4571–80 10.1007/s00330-017-4807-y [DOI] [PubMed] [Google Scholar]
- 19.Sutton LN, Adzick NS, Bilaniuk LT, et al. Improvement in hindbrain herniation demonstrated by serial fetal magnetic resonance imaging following fetal surgery for myelomeningocele. JAMA 1999;282:1826–31 10.1001/jama.282.19.1826 [DOI] [PubMed] [Google Scholar]
- 20.Aertsen M, Verduyckt J, De Keyzer F, et al. Reliability of MR imaging-based posterior fossa and brainstem measurements in open spinal dysraphism in the era of fetal surgery. AJNR Am J Neuroradiol 2019;40:191–98 10.3174/ajnr.A5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.da Costa MDS, Nicacio JM, Dastoli PA, et al. Alterations in skull base anatomy in intrauterine and postnatal repaired myelomeningoceles. Childs Nerv Syst 2020;36:2757–63 10.1007/s00381-020-04587-6 [DOI] [PubMed] [Google Scholar]
- 22.Grant RA, Heuer GG, Carrion GM, et al. Morphometric analysis of posterior fossa after in utero myelomeningocele repair. J Neurosurg Pediatr 2011;7:362–68 10.3171/2011.1.PEDS10234 [DOI] [PubMed] [Google Scholar]
- 23.Glenn OA, Cuneo AA, Barkovich AJ, et al. Malformations of cortical development: diagnostic accuracy of fetal MR imaging. Radiology 2012;263:843–55 10.1148/radiol.12102492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunpalin Y, Richter J, Mufti N, et al. Cranial findings detected by second-trimester ultrasound in fetuses with myelomeningocele: a systematic review. BJOG 2021;128:366–74 10.1111/1471-0528.16496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morais BA, Solla DJ, Yamaki VN, et al. Brain abnormalities in myelomeningocele patients. Childs Nerv Syst 2020;36:1507–13 10.1007/s00381-019-04386-8 [DOI] [PubMed] [Google Scholar]
- 26.de Wit OA, den Dunnen WF, Sollie KM, et al. Pathogenesis of cerebral malformations in human fetuses with meningomyelocele. Cerebrospinal Fluid Res 2008;5:4 10.1186/1743-8454-5-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dennis M, Salman MS, Juranek J, et al. Cerebellar motor function in spina bifida meningomyelocele. Cerebellum 2010;9:484–98 10.1007/s12311-010-0191-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams VJ, Juranek J, Stuebing K, et al. Examination of frontal and parietal tectocortical attention pathways in spina bifida meningomyelocele using probabilistic diffusion tractography. Brain Connect 2013;3:512–22 10.1089/brain.2013.0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cools MJ, Tang AR, Pruthi S, et al. A comparison of MRI appearance and surgical detethering rates between intrauterine and postnatal myelomeningocele closures: a single-center pilot matched cohort study. Childs Nerv Syst 2023;39:647–53 10.1007/s00381-022-05627-z [DOI] [PubMed] [Google Scholar]
- 30.Worley G, Greenberg RG, Rocque BG, et al. Neurosurgical procedures for children with myelomeningocele after fetal or postnatal surgery: a comparative effectiveness study. Dev Med Child Neurol 2021;63:1294–1301 10.1111/dmcn.14792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dias MS, Wang M, Rizk EB, et al. ; National Spina Bifida Patient Registry Group. Tethered spinal cord among individuals with myelomeningocele: an analysis of the National Spina Bifida Patient Registry. J Neurosurg Pediatr 2023;7:1–7 10.3171/2023.5.PEDS20868a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corroenne R, Zhu KH, Johnson E, et al. Impact of the size of the lesion in prenatal neural tube defect repair on imaging, neurosurgical and motor outcomes: a retrospective cohort study. BJOG 2021;128:392–99 10.1111/1471-0528.16316 [DOI] [PubMed] [Google Scholar]
- 33.Nagaraj UD, Bierbrauer KS, Stevenson CB, et al. Myelomeningocele versus myelocele on fetal MR images: are there differences in brain findings? AJR Am J Roentgenol 2018;211:1376–80 10.2214/AJR.18.20088 [DOI] [PubMed] [Google Scholar]
- 34.Fletcher JM, Copeland K, Frederick JA, et al. Spinal lesion level in spina bifida: a source of neural and cognitive heterogeneity. J Neurosurg 2005;102:268–79 10.3171/ped.2005.102.3.0268 [DOI] [PubMed] [Google Scholar]
- 35.Fletcher JM, Houtrow AJ, MacPherson C. Hydrocephalus and school-age neuropsychological outcomes in the Management of Myelomeningocele prenatal surgery trial: a secondary analysis. J Neurosurg Pediatr 2023. Mar 3. [Epub ahead of print] 10.3171/2022.10.PEDS22358 [DOI] [PMC free article] [PubMed] [Google Scholar]