Abstract
Although sex differences in learning behaviors are well documented, sexual dimorphism in the synaptic processes of encoding is only recently appreciated. Studies in male rodents have built upon the discovery of long-term potentiation (LTP), and acceptance of this activity-dependent increase in synaptic strength as a mechanism of encoding, to identify synaptic receptors and signaling activities that coordinate the activity-dependent remodeling of the subsynaptic actin cytoskeleton that is critical for enduring potentiation and memory. These molecular substrates together with other features of LTP, as characterized in males, have provided an explanation for a range of memory phenomena including multiple stages of consolidation, the efficacy of spaced training, and the location of engrams at the level of individual synapses. In the present report, we summarize these findings and describe more recent results from our laboratories showing that in females the same actin regulatory mechanisms are required for hippocampal LTP and memory but, in females only, the engagement of both modulatory receptors such as TrkB and synaptic signaling intermediaries including Src and ERK1/2 requires neuron-derived estrogen and signaling through membrane-associated estrogen receptor α (ERα). Moreover, in association with the additional ERα involvement, females exhibit a higher threshold for hippocampal LTP and spatial learning. We propose that the distinct LTP threshold in females contributes to as yet unappreciated sex differences in information processing and features of learning and memory.
Keywords: actin cytoskeleton, estrogen, hippocampus, long-term potentiation, spaced training, spatial memory
1 |. INTRODUCTION: SEX DIFFERENCES IN LEARNING
Investigations into possible sex differences in memory began with the late 19th century monograph by Havelock Ellis (1894). Among a broad array of biological variables, this seminal work included a chapter on intellectual operations in which Ellis suggested that the then available body of work pointed to a female superiority in verbal but not other forms of memory. Numerous studies followed such that by 1974, Maccoby and Jacklin in a groundbreaking book (Maccoby & Jacklin, 1974) were able to summarize results from nearly 1,600 papers on psychological differences between the sexes. They concluded that females are superior in verbal learning while males perform better on visual–spatial problems. Subsequent experimental work has for the most part confirmed these male–female differences (see Andreano & Cahill, (2009) and Koss & Frick, (2017) for reviews). However, it has been argued from meta-analyses of these results, along with those for other psychological variables, that differences between the sexes are generally small with regard to effect sizes (Hyde, 2005). Evaluation is further complicated by the somewhat arbitrary descriptions of spatial learning and verbal fluency. The phenomena in the real-world lack self-evident measurements and in practice are largely defined by the paradigms used to test them. That said, reasonable though by no means complete agreement across an impressively diverse array of experimental designs supports the general idea that males outperform females on problems that involve spatial relationships (Fernandez-Baizan et al., 2019; Gagnon et al., 2018; Piber et al., 2018; Siedlecki et al., 2019; Wierenga et al., 2019). Whether this reflects sex differences in a particular type of memory processing or instead arises from other variables that affect outcomes on spatial problems is open to question. It has for example been argued that the apparent sexual dimorphism can be traced to different strategies (allocentric vs. egocentric) employed by women versus men when dealing with cues about current and future locations (Cherney et al., 2008; Dabbs Jr. et al., 1998; Lawton, 1994; Piber et al., 2018; Sandstrom et al., 1998). Notably, the use of such strategies is dependent on memory and there remains the possibility that each sex selects a behavioral strategy that aligns best with dimorphisms in the encoding and retrieval of information.
The female advantage on verbal problems summarized by Maccoby and Jacklin (1974) has been confirmed in numerous subsequent studies. Women reliably score higher than men when asked to recall words belonging to a particular category (first letter, animals, etc.), suggesting that they have better and/or more readily accessible long-term memory for semantic items. They also perform at a higher level when asked to recall words from a recently presented list (Berenbaum et al., 1997; Kramer et al., 1997; Youngjohn et al., 1991), as expected for superior encoding and retrieval of the original material. Importantly, women also outscore men in tests using faces rather than words (Rehnman & Herlitz, 2007). It is therefore possible that the female advantage is evident in tasks involving serial presentation of items of intrinsic interest rather than being restricted to verbal material. Finally, there are reasons to assume that sex differences in verbal versus spatial memory are manifested in everyday life. Support for this comes from work on episodic memory, an autobiographical form of encoding that incorporates the identity of serial cues, their relative positions, and the order in which they occur (i.e., what, where, and when information) (Dickerson & Eichenbaum, 2010; Eacott & Easton, 2010; Tulving, 1972). Recall of commonplace events, essentially within an episode, is often used to probe the accuracy and completeness of memory. A recent meta-analysis of hundreds of studies published over a 40-year period concluded that women do well in aspects of episodic memory that are verbal in nature or require recall of faces or odors. Men are reportedly superior in those aspects that place heavy demands on spatial memory (Asperholm et al., 2019).
There has been considerable discussion about the origins of sexual dimorphisms in memory and, in particular, whether they result from different life histories as opposed to being the consequence of selective pressures for sex roles (e.g., males as hunters would benefit from enhanced spatial processing) (Ellis, 2011). Related to this, a number of studies indicate that spatial learning by animals is superior in males relative to females (Koss & Frick, 2017) and that the variations in allocentric/egocentric strategies are also present (Hamilton et al., 2007; Vorhees et al., 2008; Vorhees & Williams, 2014). The collection of findings from such work suggests that for spatial learning the male advantage may be common to mammals as a group (Jones et al., 2003; Silverman & Eals, 1992). If so, the sexual dimorphism observed in humans would likely reflect the retention of a sex-linked feature that arose more than 200 million years ago. Whether this dimorphic feature pertains to exploration of extended environments, and thus relates naturally to observed differences in spatial learning, is a challenging and unresolved problem. In any event, the animal research strongly suggests that neurobiological as opposed to experiential variables are responsible for sex differences in spatial learning. These findings accordingly opened the way to experimental work on brain systems that might account for such differences.
Research to date has not described animal effects that might relate to the advantage enjoyed by women in verbal learning paradigms. Indeed, to our knowledge, there is no evidence that female rodents consistently outperform males on any type of memory problem although there are mixed findings for object recognition tasks and evidence that females in proestrus, the high circulating estrogen stage of the estrous cycle, perform better than non-proestrus females and, in some studies, males (Koss & Frick, 2017). The absence of reliable evidence for a female advantage in animal studies of different forms of learning could simply reflect the relative absence of studies searching for such effects, as well as the obvious difficulty of establishing animal analogues of semantic cues. Moreover, human studies rarely involve the practice sessions typically used in animal research to shape behavior toward low variance endpoints. However, we have found that rodents remember individual odors from a recently sampled sequence and do so without training or overt rewards (Cox et al., 2019; Otto, Schottler, et al., 1991; Wang, Cox, et al., 2018). Odors are of innate interest to rodents and sequence tests of the type just noted might be analogous to the face recognition problems in which women are superior to men (faces are inherently interesting to humans). Sequential olfactory paradigms are accordingly a plausible starting point for investigations into potential female advantages in memory processing by rodents. We have developed versions of the olfactory paradigms that use multiple olfactory cues to sample the three basic elements of episodic memory (cue identity, temporal order, and location) and, as in human studies, do not entail repetition or overt rewards (Cox et al., 2019). There is thus the possibility of testing if male and female rodents parallel humans with regard to which aspects of an episode are more easily acquired.
2 |. SYNAPTIC SUBSTRATES FOR MEMORY STORAGE
The mechanisms used by the brain to lay down memory traces constitute a reasonable starting point for investigations into the causes for the sex differences summarized above. It had been suspected since the time of Ramón y Cajal that use-dependent modifications to synapses underlie memory encoding and the discovery of long-term potentiation (LTP) confirmed that connections in brain possess the requisite plasticity (Bliss & Lomo, 1973). LTP is a process wherein brief trains of high frequency or rhythmic neuronal activity can enhance transmission at individual synapses (Bliss et al. 2007; Lynch, 2003; Nicoll, 2017) (Figure 1a,b). It has been described for excitatory glutamatergic synapses throughout the CNS (e.g., cortex, striatum, amygdala, spinal cord) and is particularly well characterized for intrinsic circuitry within hippocampus. Like memory, LTP develops very quickly yet is both extraordinarily persistent and synapse specific; thus, it satisfies the constraints of a putative information storage mechanism imposed by the unusual properties of memory including its enormous capacity (Lynch, 2003, 2004; Morris, 2003; Nicoll, 2017). Initial tests of the hypothesis that LTP underlies memory showed that selective pharmacological suppression of potentiation blocks spatial learning without affecting acquisition of simple cue–response associations (Morris et al., 1986; Staubli et al., 1989). There are now many reports showing that disruption of LTP similarly disrupts memory formation (Lynch, 1998; Rex et al., 2005, 2010) and that facilitation of potentiation enhances memory (Lynch, 2002, 2004; Wang, Cox, et al., 2018). It was then found that learning is accompanied by LTP in the same synapses critical for encoding the information. We first demonstrated this for the system that conveys olfactory cues into the cortex (Roman et al., 1987). The pertinent experiments used discrete 5 Hz (the sniffing frequency) stimulation of the lateral olfactory tract as a positive or negative cue in a two-odor discrimination paradigm. The monosynaptic responses generated in piriform (olfactory) cortex by the stimulation pulses underwent a lasting increase in amplitude as a rat learned that the “electric odor” was either correct or incorrect. Now several studies have shown that synaptic potentiation occurs with learning (Cohen et al., 2008; Whitlock et al., 2006). Together these findings provide compelling evidence that LTP is the synaptic mechanism for encoding many forms of memory.
FIGURE 1.
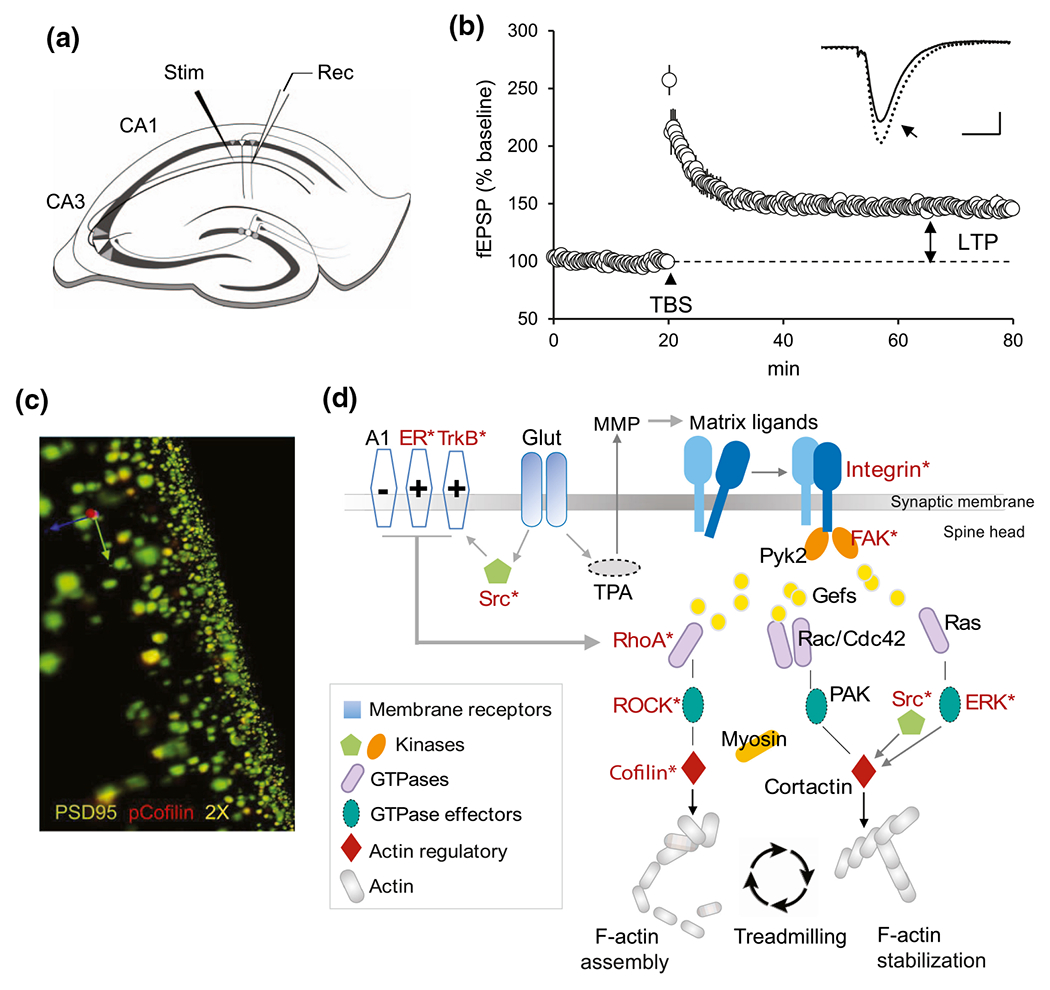
Long-term potentiation (LTP): A synaptic substrate for memory encoding. (a) Schematic of a hippocampal slice (cut perpendicular to the hippocampal long axis) showing the principal cell layers (bold lines) and the glutamatergic projections from field CA3 to field CA1 evaluated in LTP studies. For these analyses, stimulation (Stim; TBS) was applied to the CA3-CA1 projections and field excitatory postsynaptic potential (fEPSP) responses were recorded (Rec) from the CA1 apical dendritic field. (b) Plot shows the size of CA3-CA1 fEPSPs (i.e., the slope of the evoked response) collected over time from before (baseline) to after TBS (applied at arrow head). As shown, stimulation with a single train of 10 theta bursts (4 pulses at 100 Hz, 200 ms intervals) increases the size of the postsynaptic response which then stabilizes at ~150% of the baseline level. The initial increase is both AMPA and NMDA receptor dependent, whereas the continued expression of stable LTP reflects increases in postsynaptic AMPA receptor-gated currents. Representative traces show fEPSP responses collected before (solid) and after (dashed) LTP induction. Bar: 0.5 mV, 10 ms. (c) Photomicrograph shows immunofluorescent labeling of the postsynaptic density protein PSD95 (green) and p-cofilin (red); double-labeled postsynaptic elements appear yellow. This approach was used to detect receptors and signaling activities associated with LTP. (d) Schematic shows the postsynaptic receptors and actin regulatory machinery identified to be critical for enduring LTP. This LTP substrate map includes signaling through the RhoA, Rac/Cdc42, and Ras GTPases that controls the polymerization (RhoA path) and stabilization (Rac/Cdc42, Ras paths) of new spine F-actin. These processes require involvement of synaptic integrins and are influenced by modulatory receptors including TrkB and the estrogen receptors (ER) which both facilitate RhoA signaling with TBS, and the adenosine A1 receptor which inhibits RhoA. ER influences are sexually dimorphic and proposed sex differences in the weight of kinase activation through the estrogen and NMDA receptors contribute to the observed sexually dimorphic responses of downstream elements (those exhibiting sex differences are indicated in red and an asterisk reflecting results in (Wang, Le, et al., 2018))
Early investigations into the cellular mechanisms that induce and express enduring LTP focused on hippocampus and showed that potentiation of the glutamatergic CA3 to CA1 intrahippocampal connections required an increase in postsynaptic calcium (Dunwiddie & Lynch, 1979; Lynch et al., 1983; Wang, Trieu et al., 2016). This result meshed well with the discovery that LTP induction is dependent on calcium permeable NMDA-type glutamate receptors (Muller et al., 1988; Park et al., 2014). The search for an LTP expression mechanism generally also focused on hippocampal field CA1 pyramidal cells as representative of neurons through the cortical telencephalon. These studies revealed that in association with LTP there is a change in the size and shape of postsynaptic dendritic spines (Harris et al., 2003; Lee et al., 1980), a finding subsequently confirmed in numerous studies using progressively more sophisticated techniques (Matsuzaki et al., 2004; Yang et al., 2008), as well as an increase in the area of postsynaptic density (PSD) (Chen et al., 2007). Despite intense interest in the possibility of new spine formation, studies generally indicate that changes in spine and synapse size occur without a significant increase in numbers of postsynaptic elements in the field of potentiation (Chen et al., 2007; Harris, 2020). Given that PSD size is highly correlated with the size of the neurotransmitter receptor pool, PSD expansion provided a straightforward explanation for the enhanced excitatory postsynaptic currents that define LTP. Related experiments demonstrated that in CA1, LTP expression does not affect transmitter release but instead is associated with a selective increase in the ionic currents gated by the postsynaptic AMPA-type glutamate receptors that produce the excitatory postsynaptic response (Kauer et al. 1988; Muller & Lynch, 1988, 1989; Nicoll, 2003). Although these studies focused on male rodents, there is as yet no evidence that fundamental LTP expression mechanisms differ between the sexes.
It then remained to identify the cell biological steps that occur between the rapid triggers for LTP and the structural changes to synapses that express the effect. Results obtained using novel microscopic approaches to visualize filamentous (F-) actin within large numbers of dendritic spines (Kramar et al., 2006; Lin et al., 2005) showed that induction of CA1 LTP was quickly followed by a reorganization of the subsynaptic actin cytoskeleton and that preventing this effect caused potentiation to rapidly decay back to baseline. Pertinent to the LTP-memory association, disruption of the actin cytoskeleton has also been shown to interfere with memory (Lamprecht, 2016; Rudy, 2015). Other lines of investigation, including quantitative synaptic imaging (Figure 1c), identified within dendritic spines a collection of small GTPase-initiated signaling cascades that lead from the very rapid synaptic events that induce LTP to the slower formation and stabilization of actin networks within dendritic spines (Chen et al., 2007; Rex et al., 2009, 2010; Seese et al., 2012). As indicated in the schematic in Figure 1d, this signaling is positively modulated by brain derived neurotrophic factor (BDNF), released during afferent activity and acting through postsynaptic TrkB receptors, and depressed by adenosine acting on postsynaptic adenosine A1 receptors (Rex et al., 2009). Importantly, as discussed below, estrogen also modulates actin regulatory signaling at excitatory synapses (Kramar et al., 2009; Wang, Le, et al., 2018) and these actions have proven critical for sex differences in LTP and learning.
3 |. LTP AS AN EXPLANATORY CONSTRUCT FOR MEMORY PHENOMENOLOGY
The identification of synaptic elements controlling the activity-dependent remodeling of the actin cytoskeleton that supports LTP has provided insight into the neurobiological basis of several well-known memory phenomena. In particular, as described below, the characterization of these synaptic mechanisms in male hippocampal field CA1 has identified constraints on aspects of synaptic plasticity that suggest explanations for the links between memory and particular cortical rhythms, the temporal properties of memory consolidation, and the efficacy of spaced training. Moreover, the identification of synaptic substrates for encoding has provided markers for analyses of the engrams for different forms of learning.
3.1 |. Cortical rhythms are associated with learning
Progress toward identifying the cellular events underlying the production of stable synaptic potentiation substantially increased the explanatory power of the hypothesis that brain networks use LTP to encode new memories. It had been known for some time that many forms of learning are associated with oscillatory activity in brain, and in particular with the 4–7 Hz theta rhythm (Hasselmo et al., 2002; Sakimoto & Sakata, 2020), but the reasons for this were obscure. The situation was clarified by the discovery that afferent stimulation with brief high-frequency bursts spaced apart by the period of the theta wave (i.e., 200 ms between bursts; theta burst stimulation, TBS) is near optimal for inducing robust and stable LTP (Larson et al. 1986; Larson & Munkacsy, 2015). The reasons for this curious link between an EEG rhythm and structural modifications to synapses center on the efficacy of TBS in engaging a mechanism for suppressing the feedforward inhibitory transmission that prevents opening of voltage-sensitive NMDA receptors (NMDARs) at glutamatergic synapses (Larson & Lynch, 1988). Relatedly, the TBS pattern developed in LTP studies subsequently found widespread usage in transcranial magnetic stimulation studies for a variety of human conditions (e.g., depression, substance abuse) (Sanna et al., 2019) including efforts to enhance human memory and cognition.
3.2 |. The rapid phase of memory consolidation
LTP research has also been informative with regard to the much-discussed topic of memory consolidation. The idea that newly acquired information passes through a stabilization period before transferring to long-term storage dates to the late 19th century. Analyses of the effect under controlled conditions became possible with the advent of electroconvulsive shock therapy (Duncan, 1949) and suggested means for studying it in animals (Misanin et al., 1968; Popik et al., 1994). The resultant memory consolidation literature is both large and contentious with regard to potential mechanisms (Babayan et al., 2012; Lynch et al., 2007). The discovery that low-frequency stimulation erases LTP when applied within 15 to 30 min of induction led to novel insights into the processes underlying the rapid phase of both LTP and memory consolidation (Larson et al., 1993; Lynch et al., 2007; Staubli & Scafidi, 1999). Considering cytoskeletal contributions to consolidation provided even greater understanding. In particular, evidence was obtained that the actin filaments that form shortly after LTP-inducing TBS are dynamic (treadmilling) until being stabilized by components of the signaling cascades noted earlier (Figure 1d). Low-frequency stimulation gives rise to increases in extracellular adenosine that engage postsynaptic adenosine A1 receptors which, in turn, suppress actin regulatory signaling (Abraham & Huggett, 1997; Arai et al., 1990; Huang et al., 1999; Rex et al., 2009). If this occurs prior to new F-actin stabilization, the newly formed filaments are lost leading to a rapid decay of potentiation (Kramar et al., 2006). These results help explain why conditions such as electroconvulsive shock and anoxia, that increase extracellular adenosine, cause retrograde amnesia (Huang & Hsu, 2001). Evidence for a second and delayed stage of consolidation is summarized below.
3.3 |. The spaced trials effect
A third area in which advances in the understanding of LTP have suggested substrates for complex memory phenomena involves the ubiquitous spaced trials effect. The advantages of spacing between learning sessions were first described in the classic work of Ebbinghaus (1885) with subsequent hypotheses emphasizing various psychological factors. The possibility that neurobiological timing mechanisms are also involved has received little attention. However, recent studies of male rodents led to the surprising finding that, for the CA3-CA1 projection, delivery of second bout of TBS (“TBS2”) doubled the magnitude of LTP induced by a first bout (“TBS1”) (Figure 2a). A similar elevation was obtained with a third delivery of TBS (Kramar et al., 2012). Remarkably, an elevation of LTP magnitude was only obtained when the successive TBS bouts were separated by about 1 hr; TBS2 had no evident effect when delivered at shorter intervals. These results have been replicated with evidence for modest differences in timing across rat strains (Cao & Harris, 2014). Investigations into the cellular events responsible for the physiological spaced trials effect revealed that hippocampal field CA1 contains a large population of synapses with a high threshold for LTP induction; TBS1 fails to trigger actin polymerization and stable LTP in these contacts. It does however prime them for potentiation in response to TBS2 likely through the mobilization of proteins that lower the activity threshold for structural modifications (Kramar et al., 2012; Lynch et al., 2013). The spaced-stimulation effect on LTP is only relevant to one segment of the wide distribution of intervals over which spaced trials have been shown to improve learning but it nonetheless predicts outcomes in one of the most widely studied paradigms for spatial learning by male mice: Object location memory (OLM) which is known to depend on hippocampal field CA1 (Figure 2b). Specifically, we found the sampling time required to produce robust long-term OLM was drastically reduced when training was divided into three brief periods separated by 1 hr as compared to occurring in one massed session; spaced training using shorter and longer intertrial intervals was ineffective (Seese et al., 2014).
FIGURE 2.
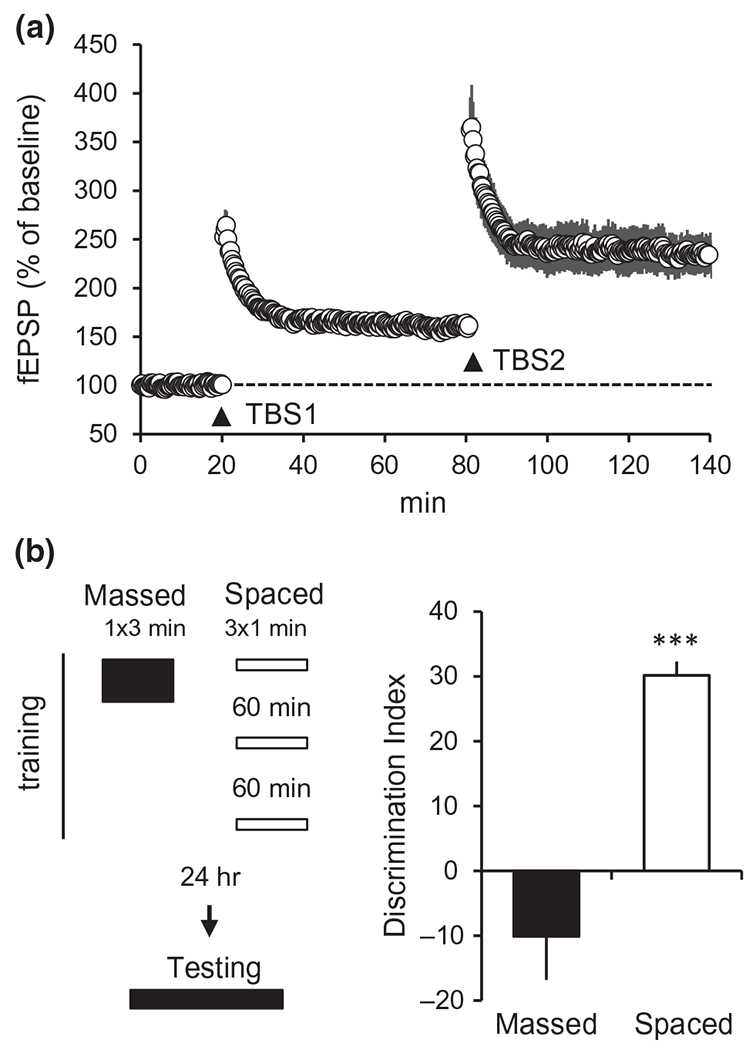
Spacing paradigms facilitate LTP and object location memory (OLM). (a) LTP exhibits a spaced-stimulation effect. In hippocampal slices, a single train of 10 burst TBS (TBS1) applied to CA3-CA1 projections (as in Figure 1a,b) induces LTP in CA1. Application of a second train of TBS (TBS2), 1 hr after the first, further increases the magnitude of the potentiation. The 1 hr delay is critical: if TBS2 is applied <50 min after TBS1 there is no augmentation of potentiation (Kramar et al., 2012). (b) Spaced training facilitates OLM. As illustrated at left, for training mice were allowed to explore a chamber containing two identical objects in one session of 3 min (1 × 3 min; “Massed”) or in three 1-min sessions spaced by 1 hr (3 × 1 min; “Spaced”). To test retention 24 hr later, mice were returned to the chamber with one of the objects displaced away from a corner and allowed to explore for 5 min. The Discrimination Index (DI) is the proportion of total exploration time devoted to the moved object; a higher DI indicates learning of original object location. Mice given spaced training showed robust learning, whereas those given the same amount of training in one massed trial failed to learn object location (***p < 0.001 massed vs. spaced). Modified from Seese et al. (2014)
3.4 |. Multiple stages of memory consolidation
Work on the LTP spaced trials effect led to a second, previously undetected phase of consolidation. Synapses are adhesion junctions and as such include integrins, transmembrane proteins that link extracellular matrix to the internal actin cytoskeleton (Brakebusch & Fassler, 2003; Hynes, 1992). These matrix receptors constitute a principal route whereby extracellular signals reorganize submembrane actin networks and thereby modify intercellular junctions. Studies using ligand-mimetic peptides, selective toxins, genetic manipulations, or neutralizing antisera have shown integrins play a central role in the rapid phase of LTP consolidation (Kramar et al., 2006). As expected from this, TBS causes synaptic integrins to enter their active state for 5–7 min, a period sufficient to trigger the actin polymerization that stabilizes LTP. Surprisingly, TBS2 delivered at any point over the following ~45 min failed to reactivate the integrins or their signaling kinases but was fully effective in this regard after longer delays (Babayan et al., 2012). Infusion of a compound (brefeldin) that disrupts the insertion of integrins into membranes blocked reactivation by TBS2 delivered after a 60-min delay. These results suggest that the initial integrin activation by TBS1 is followed by proteolytic degradation or internalization of the receptors and that recovery requires 45–60 min. It was then found that application of integrin neutralizing antisera or brefeldin after LTP induction, but before integrin recovery, caused a complete erasure of LTP (Babayan et al., 2012; Lynch et al., 2013). The same treatment had no effect when applied after recovery of integrin function. These findings indicate that integrin dynamics generate a second stage of LTP consolidation but are not required to maintain synapses in their potentiated state. One possibility is that the return of functional integrins serves to anchor the synaptic cytoskeleton in its reorganized configuration. Finally, infusions of integrin neutralizing antisera into hippocampus prior to but not after integrin recovery fully blocked the formation of long-term memory in a spatial task (Babayan et al., 2012). Thus, identification of a second stage of LTP stabilization with a description of underlying mechanisms enabled the discovery of a corresponding phase of memory consolidation.
3.5 |. Engrams
Nineteenth century investigators hypothesized that learning results in associations between “nervous elements,” and thus a memory trace, or “engram,” connecting different regions (as described by Schacter (1982)). The search for engrams has largely involved physiological methods and various forms of conditioning (Swain & Thompson, 1993), and analyses of neuronal activation patterns (Govindarajan et al., 2006; Tonegawa et al., 2015) but the description of molecular substrates of LTP (Figure 1d) suggested a means for mapping the distribution of synapses associated with encoding of new memories. A first study of this type asked if exploration of a novel environment produces LTP-related synaptic changes in field CA1, a region important to spatial learning. Numbers of synapses containing an LTP marker (i.e., the transiently phosphorylated (p) protein cofilin) were low in control animals and about 30% higher in the exploration group (Fedulov et al., 2007). Moreover, PSDs associated with p-cofilin were substantially larger than those that were not. For reasons discussed earlier, it can be assumed that p-cofilin-positive synapses were stronger (potentiated) relative to their neighbors. Injections of an NMDAR antagonist, which disrupts LTP, blocked the increase in both synaptic p-cofilin levels and learning in the exploration group. Together these findings indicate that synapses exhibiting increases in p-cofilin with training are undergoing LTP as part of the memory trace. Importantly, only a very small percentage (<1%) of field CA1 synapses contained elevated p-cofilin after training, a finding consistent with the view that a memory system using LTP rules will have the large capacity needed for a lifetime of interactions with dynamic and complex circumstances. Subsequent studies using a different behavioral paradigm found that the increases in synapses associated with an LTP marker occur in discrete subfields of hippocampus rather than being widely distributed (Cox et al., 2014). Extension of this strategy to analyses of neocortex could result in maps of synaptic changes occurring there in association with learning and thus visualization of a cortical engram.
4 |. ESTROGEN PROMOTES LTP AND LEARNING
There is an extensive body of evidence that estrogen promotes the growth of dendritic spines and enhances functional synaptic plasticity in rodent hippocampus (Foy et al., 1999, 2008; Frick et al., 2015, 2018; Gould et al., 1990; Luine & Frankfurt, 2013, 2020). This is clearly the case for both ovariectomized (Cordoba Montoya & Carrer, 1997; Frye et al., 2007) and gonadally intact females (Warren et al., 1995; Woolley et al., 1990); a smaller collection of reports indicate that estrogen infusion improves spatial memory and synaptic plasticity in males as well (Frick et al., 2015; Kampen & Sherwin, 1996; Kato et al., 2020; Luine & Rodriguez, 1994). Moreover, in gonadally intact females, increases in circulating estrogen across the estrous cycle are associated with increases in total numbers of spines, and the proportion of mushroom-shaped spines, in hippocampal field CA1. Conversely, lower estrogen levels are associated with relatively lower spine numbers and proportionately fewer mushroom-shaped spines (Gonzalez-Burgos et al., 2005; Kato et al., 2013; Prang-Kiel et al., 2008; Woolley et al., 1990). Although the high circulating estrogen state is positively correlated with the magnitude of field CA1 LTP (Bi et al., 2001; Warren et al., 1995), it is not known if estrogen effects on spine counts, in particular, contribute to rapid effects of the steroid on synaptic plasticity. Pertinent to this, it is noteworthy that estrogen-induced increases in spine number are NMDAR-dependent, require genomic activity, and in the short term (<1 day), do not entail changes in AMPA receptor currents (Romeo et al. 2005; Smith & McMahon, 2005; Woolley & McEwen, 1994). This contrasts with the rapid effects of E2 on synaptic responses that have been described in many brain regions (Kelly et al., 1976; Nabekura et al., 1986; Wong & Moss, 1991): These occur within minutes, do not depend on protein synthesis or NMDAR function, and reflect increases in AMPA receptor currents (Kramar et al., 2009; Wong & Moss, 1992; Zadran et al., 2009). As will be described below, estrogen also influences synaptic GTPase and kinase signaling activities that are required for induction of LTP (Kramar et al., 2009; Hasegawa et al., 2015; Wang, Le, et al., 2018; Wang, Kantorovich, et al., 2016) and results suggest that these processes account for the rapid effects of estrogen on synaptic potentiation.
In line with estrogen effects on transmission and dendritic spines, the preponderance of findings point to the conclusion that performance on certain learning tasks fluctuates across stages of the human menstrual and rodent estrous cycles. For example, women show better verbal memory when circulating estrogen levels are high, but during the same periods perform more poorly in spatial tasks (Epting & Overman, 1998; Hampson, 1990; Hampson & Kimura, 1988). Studies in rodent initially agreed with evidence for the negative effects of estrogen on spatial learning: Rats in proestrus (high-estrogen state) reportedly performed worse in spatial (Morris Water Maze, radial arm maze) tasks than rats in low estrogen phases (Frye, 1995; Stackman et al., 1997; Warren & Juraska, 1997). However, in object-based spatial tasks, cycling females perform better in the high-estrogen state (Frye et al., 2007; Paris & Frye, 2008); this finding aligns with above-noted evidence that high-estrogen levels facilitate CA1 LTP and increase spine density thereby suggesting a link between these estrogen actions and memory encoding (see, Sheppard et al., 2019 for further review). The seemingly conflicting results, for performance in spatial versus object-based tasks, raise the possibility that stress, which is heightened in Morris Water Maze tasks, may influence the response to estrogen (Rubinow et al., 2004). Alternatively, there may be different brain regions critical for learning object-based as opposed to distant-reference tasks (Luine, 2015) and the nature of estrogen effects may be region specific (Gould et al., 1990; Qi et al., 2016; Woolley et al., 1990).
A recent experiment described an unambiguous instance of estrogen-dependent enhancement for OLM, a CA1-dependent, object-based spatial task (Figure 3a). An important feature of the study was that the period of training (initial cue sampling) was at threshold levels for male acquisition and long-term memory (~5 min) (Stefanko et al., 2009). Females trained outside proestrus (i.e., in low circulating estrogen states) did not recognize the new object location in a retention trial conducted 24 hr after training, whereas males had high retention scores. Notably, the non-proestrus females did successfully encode the location of the objects when given a longer training session, indicating that their deficit relates to the speed at which spatial relationships are learned (unpublished observation). Very different results were obtained with females trained in proestrus: these mice performed as well as males when given 5-min OLM training. Importantly, the amount of time spent sampling the objects during training was not detectably affected by estrous cycle stage (Figure 3a, right), a result suggesting that higher estrogen levels during proestrus improved learning by enhancing encoding and not by influencing attention to the cues.
FIGURE 3.
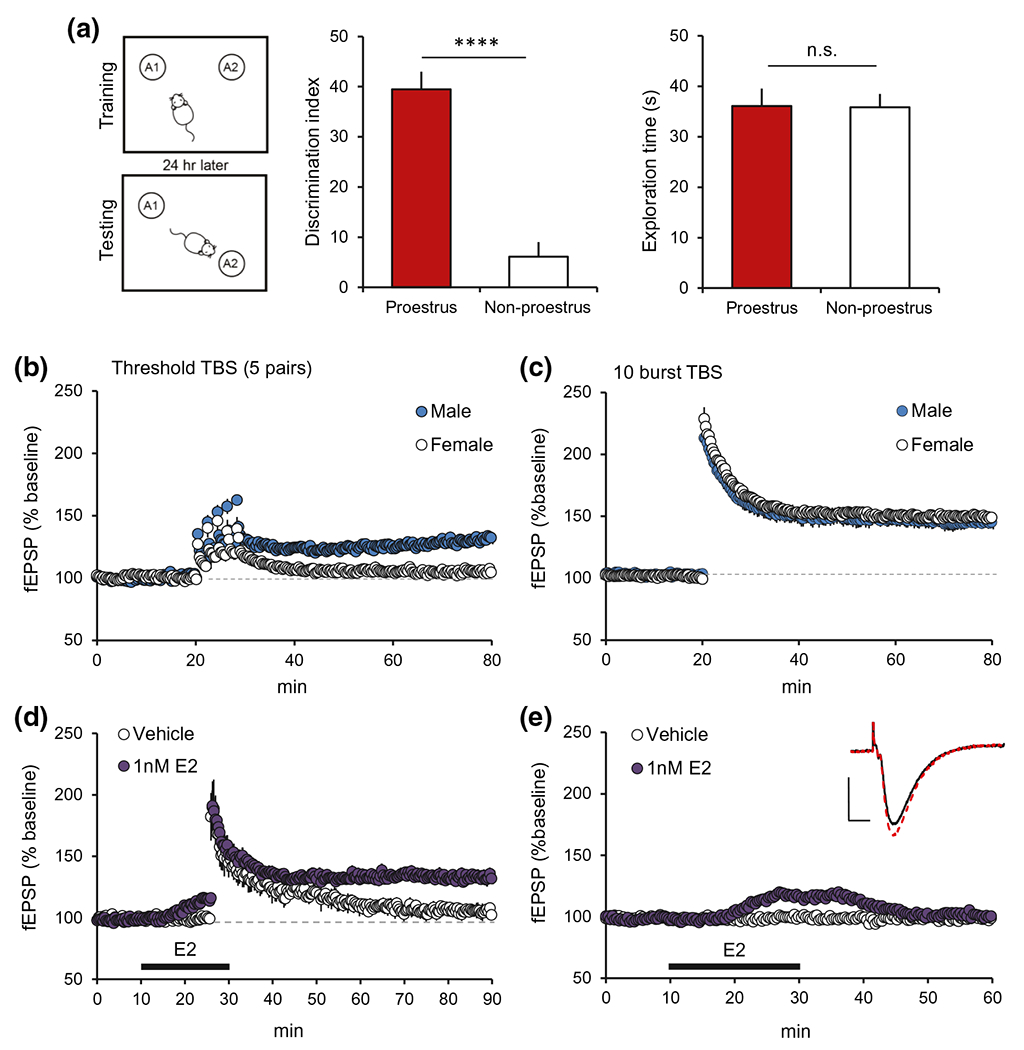
Effects of estradiol (E2) and sex on object-based spatial learning and synaptic plasticity. (a) Schematic of the OLM paradigm: Female mice in proestrus or estrus/diestrus (non-proestrus) were allowed to explore the chamber containing two identical objects for 5 min during “Training.” “Testing” 24 hr later entailed exploration of the same chamber with one object displaced for 5 min. Mice trained in proestrus (high-estrogen) performed significantly better than those in non-proestrus (low-estrogen) (****p < 0.0001, left graph); both groups explored the objects for similar amounts of time during training (n.s. p > 0.05, right graph). (b) Threshold-level TBS stimulation (5 theta burst pairs, 2 min between pairs) induced robust field CA1 LTP in male rats but failed to elicit enduring potentiation in females (p = 0.0005, t(11)=4.86 for male (n = 6) vs. female (n = 7) during last 5 min of recording). (c) A normal 10 theta burst train induced robust and comparable LTP in slices from males and females. (d) In slices from male rats, application of subthreshold TBS (3 theta bursts) induced enduring CA1 LTP in the presence of E2 (bar: 1 nM, 20 min) but not vehicle infusion (p = 0.005, TBS vs. vehicle during last 5 min; n = 5/group). (e) E2 (1 nM) infusion markedly increased the CA1 fEPSP response and this reversed with E2 wash-out; vehicle infusion had no effect on these basal responses. Representative traces: superimposed fEPSPs from the periods before (black line) and immediately after (red line) E2 infusion. Scale bar: 1mV, 5ms. Panels a-c are modified from Wang, Jia, et al., (2018); https://www.jneurosci.org/content/38/37/7935. Panels d and e are from Kramar et al. (2009) Copyright 2009 Society for Neuroscience; https://www.jneurosci.org/content/29/41/12982
Analogous results are reported for estrogen effects on LTP in hippocampal field CA1, a critical region for OLM. Tests using threshold levels of stimulation for inducing robust and stable synaptic potentiation in males (i.e., five paired theta bursts) failed to generate reliable LTP in hippocampal slices from young adult females (Figure 3b). However, a full-length train of 10 theta bursts elicited comparable LTP in males and females (Figure 3c). Moreover, in hippocampal slices (ex vivo), infusion of estradiol (E2, the most potent and prevalent estrogen in brain) at concentrations (1 nM) comparable to those in hippocampus during proestrus (Hojo et al., 2009; Mukai et al., 2010) potently enhanced LTP (Figure 3d) (Kramar et al., 2009). Relatedly, E2 rapidly and reversibly increased the amplitude of AMPA receptor-gated synaptic fEPSPs elicited in male CA1 by single pulse afferent stimulation (Figure 3e); as with LTP, this effect of E2 infusion did not require involvement, or increase the size, of NMDAR-mediated synaptic responses (Kramar et al., 2009). In males, the facilitatory effects of infused E2 were blocked by antagonists of estrogen receptor ß (ERß) but not affected by antagonists for the other two classes of brain estrogen receptors ERα and GPER1 (Kramar et al., 2009; Wang, Kantorovich, et al., 2016). These observations raised the question of whether exogenous E2 activates the actin signaling machinery that leads to LTP (Figure 1d). Indeed, pretreatment with a toxin (latrunculin) that selectively interferes with actin polymerization eliminated E2-induced enhancement of synaptic responses. Moreover, brief E2 infusion activated the small GTPase RhoA and RhoA-associated kinase (ROCK) and increased phosphorylation of cofilin, a downstream target of ROCK. Cofilin is a constitutively active protein that severs newly formed actin filaments. Phosphorylation inactivates cofilin and thereby allowing elongation of actin filaments. As expected from these results, E2 treatment increased spine F-actin, an effect that reversed upon washout of the hormone (Kramar et al., 2009). ROCK inhibition prevented both actin polymerization and facilitation of synaptic responses by E2. In all, brief treatments with physiological concentrations of estradiol activated, through ERß, one the key signaling cascades (RhoA>ROCK>cofilin>actin polymerization) (Figure 1d) that is engaged by TBS and critical for shifting synapses into their potentiated state. Importantly, in these studies of males, brief E2 treatment failed to activate a second signaling pathway engaged by TBS that involves the small GTPases Rac and Cdc42 and their effector PAK. Available evidence suggests that the Rac/Cdc42-to-PAK cascade serves to stabilize and elaborate the actin networks assembled in response to stimulation of the RhoA-ROCK system by TBS (Rex et al., 2009). The absence of the Rac/Cdc42 response after a short exposure to low levels of E2 helps explain why actin polymerization and EPSP enhancement induced by the steroid reverses after washout in marked contrast to the persistence of synaptic potentiation after TBS.
Links between synaptic (i.e., membrane-associated) estrogen receptors and actin regulatory signaling could be reasonably direct because these receptors activate Src, a tyrosine kinase that influences the activity of small GTPases engaged with LTP (Bunda et al., 2014; Luo et al., 2017). However, recent work indicates that more complex molecular interactions are involved. Specifically, E2 infusion causes ß1-family integrins to shift into their activated configuration (Wang, Trieu, et al., 2016), an event that is critical for TBS-induced actin polymerization and LTP (Kramar et al., 2006). Moreover, suppressing ß1 integrin function prevented E2 effects on synaptic responses. Integrins are activated in many circumstances by neighboring receptors via a process referred to as inside-out signaling or, in other fields, ligand-independent transactivation (Lee et al., 2002; Rajagopal & Chao, 2006). While this process could be involved, it is also the case that inhibitors of matrix metalloproteinases (MMPs), extracellular enzymes that generate integrin ligands from the extracellular matrix, block the effects of E2 on baseline synaptic transmission (Wang, Kantorovich, et al., 2016). Thus, estrogen receptor activation may cause the release of factors controlling the MMPs and, consequently, ligation of the synaptic integrins. There is evidence that this MMP-integrin sequence is required for the production of LTP (Babayan et al., 2012; Nagy et al., 2006; Wang, Kantorovich, et al., 2016).
It should be noted that E2 infusion also activates synaptic TrkB receptors for the neurotrophin BDNF. This involves ligand-free transactivation as sequestration of released BDNF does not prevent TrkB activation by the hormone (Wang, Kantorovich, et al., 2016). However, neither BDNF sequestration nor pretreatment with a TrkB antagonist detectably influences E2’s facilitation of excitatory synaptic responses. A potential explanation for the latter result is that TrkB transactivation by estradiol is incomplete and, in particular, fails to initiate TrkB signaling to the GTPases. Alternatively, direct signaling from the estrogen receptor (or estrogen receptors), independent of TrkB, is both sufficient for actin remodeling and functionally occludes further contributions from TrkB.
In summary, exogenous E2 activates some but not all of the actin management systems used to support enduring LTP and thereby produces a weak and transient form of synaptic potentiation. This effect would serve to prime the synaptic mechanisms activated by neuronal activity, and in particular by TBS in the experimental context, and thereby result in the greatly augmented potentiation in the presence of estradiol (Kramar et al., 2009). These observations opened the way for studies testing if this complex machinery varies between males versus females.
5 |. THE SUBSTRATES FOR LTP ARE SEXUALLY DIMORPHIC
Although circulating estrogens pass the blood–brain barrier to influence neuronal activity in the CNS, recent work has shown that estrogens and related steroids are also synthesized by forebrain neurons including those in hippocampus (Hojo et al., 2009). Cytochrome P450 aromatase (AROM), the rate limiting enzyme for E2 synthesis, is present at surprisingly high concentrations in hippocampus and localized to axon terminals (Peterson et al., 2005; Tabatadze et al., 2014). Reflecting local neurosteroid synthesis, E2 levels are several-fold higher in hippocampus than in blood in both sexes, and hippocampal neurons release estrogen (Hojo et al., 2009; Kato et al., 2013; Mukai et al., 2010; Tabatadze et al., 2014). Nevertheless, blocking estrogen production with AROM inhibitors in adults caused a marked reduction in LTP in females only (Figure 4a) (Bender et al., 2017; Vierk et al., 2012; Wang, Le, et al., 2018). Investigations into the mechanisms underlying this striking example of sexual dimorphism began with the assumption that locally released estrogen engages the same signaling pathways as does exogenous E2. This was not the case. Antagonists of ERß (e.g., PHTPP, 3 μM), present at concentrations that blocked the effects of applied E2 on synaptic responses in males (Wang, Kantorovich, et al., 2016), did not disturb female LTP (Figure 4b), whereas an antagonist of ERα (MPP, 3 μM) prevented stable potentiation in females only (Figure 4c,d). Tests using mutant mice with a point mutation that prevents palmitoylation of ERα, and thus insertion of the receptor into membranes, demonstrated a nearly complete loss of stable LTP in females but normal potentiation in males. Mutant females lacking nuclear ERα expressed normal LTP (Wang, Le, et al., 2018). These results indicate that membrane signaling, as opposed to transcriptional effects of the estrogen receptors, is critical for potentiation at least through the first hour after stimulation. This contrasts with the well described and dimorphic effects of E2 on spine integrity (Brandt et al., 2013) and on spinogenesis and NMDAR currents (Smith et al., 2009; Woolley & McEwen, 1994; Woolley et al., 1997) which, as noted above, depend on new protein synthesis and are slower to emerge. Genetic manipulations that prevent AROM expression in hippocampus lead to reductions in neuronal arbors and synaptic density as well as disruption of LTP and memory in both sexes (Lu et al., 2019). However, we found that with briefer manipulations (e.g., AROM inhibition for a few days) synaptic populations were not disturbed, and LTP and memory were impaired in females only (Wang, Le, et al., 2018). These results indicate that in females, but not males, signaling sequences set in motion by TBS are dependent on locally produced and released estrogen acting though membrane ERα.
FIGURE 4.
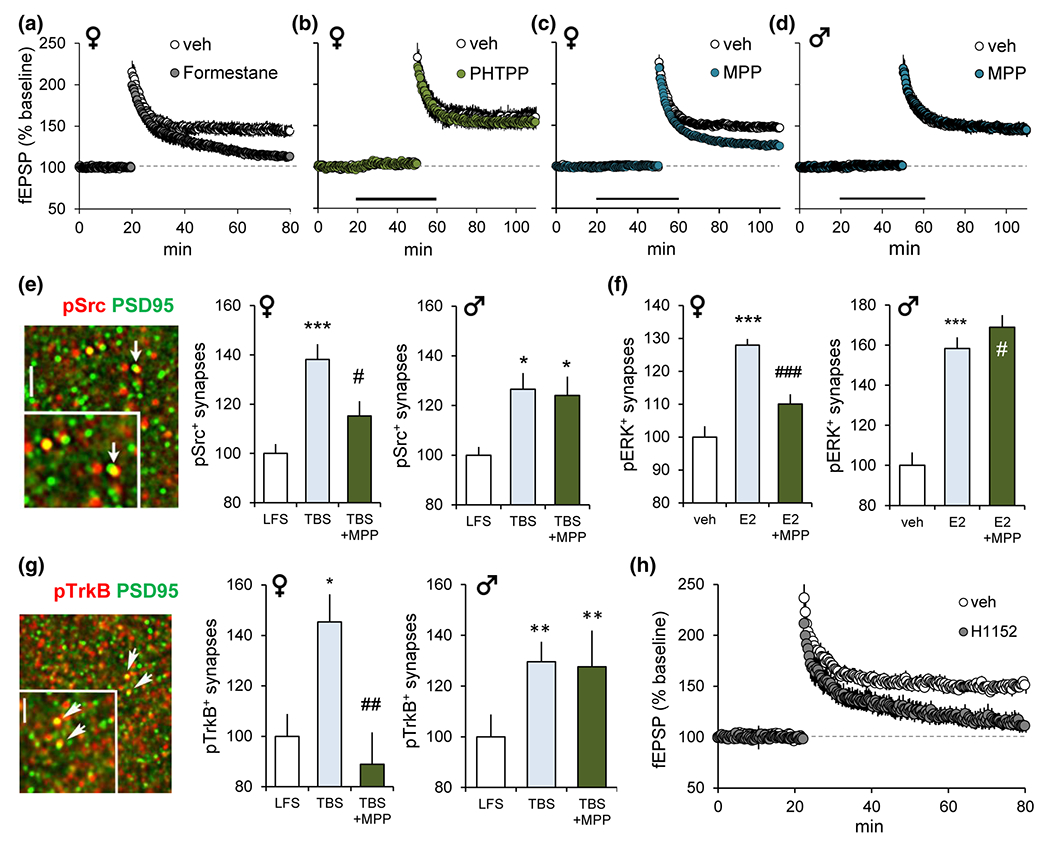
Estrogen and ERα are critical for CA1-LTP and actin regulatory synaptic signaling. (a-d) Plots show effects of manipulations on LTP induced by a single train of 10 burst TBS applied to CA3-CA1 projections in hippocampal slices; control slices received low-frequency stimulation (LFS). (a) Pretreatment of females with P450 aromatase inhibitor formestane for 7 days fully disrupted LTP (p = 0.0026 vehicle (veh) vs. formestane). (b) In females, LTP was not affected by ERß antagonist PHTPP (p = 0.70 vs. veh). (c,d) ERα antagonist MPP (3 μM) reduced LTP magnitude in females (p < 0.0001 vs. veh) but not in males (p = 0.87). (e-g) Quantitative fluorescence deconvolution tomography (Seese et al., 2013, 2014) was used to assess changes in synaptic protein levels in the CA1 field of LTP at 3–4 min after TBS or 15 min after E2 infusion. Graphs show changes in the proportion of postsynaptic (PSD95+) elements containing the signaling protein of interest (normalized to controls). (e) Image shows pSrc (red) and PSD95 (green) immunolabeling (yellow=double-labeled). In females, TBS increased pSrc+synapses (***p < 0.001), and this effect was reduced by MPP (#p < 0.02 TBS vs. TBS+MPP); in males, MPP did not attenuate TBS-induced increases in pSrc (*p < 0.05 vs. LFS). (f) In females, E2 increased synaptic pERK, and MPP attenuated this response (veh vs. E2 ***p < 0.001; E2 vs. E2+MPP ###p < 0.001). In males, MPP did not affect the E2-induced increase in pERK (veh vs. E2 ***p < 0.001; E2 vs. E2+MPP #p > 0.05). (g) Synaptic immunolabeling for pTrkB and PSD95 (arrows/yellow show double labeling). In females MPP completely blocked the TBS-induced increase in synaptic pTrkB (LFS vs. TBS *p < 0.05; TBS vs. TBS+MPP ##p < 0.01). In males, MPP had no effect on TBS-induced increase in pTrkB (**p < 0.01 vs. LFS). (h) In females, ROCK inhibitor, H1152 (100 nM), blocked LTP. Modified from Wang, Le, et al., (2018); https://www.jneurosci.org/content/38/37/7935
In further analyses of the basis of sexual dimorphism in hippocampal field CA1 LTP, we evaluated the physiological response to LTP-inducing TBS. The composite postsynaptic response to the stimulation pulses that comprise a single theta burst was not detectably affected by estrogen receptor antagonists or downregulation of surface ERα. The pronounced facilitation of burst responses that normally occurs during a TBS train was also intact after these manipulations (Wang, Le, et al., 2018). These results rule out the possibility that local neurosteroid estrogen and ERα influence neurotransmitter release or the complex events, including NMDAR activation, that together constitute the postsynaptic response to TBS. However, suppressing ERα function blocked TBS-induced activation of the LTP-critical kinases Src and ERK in females while having no measurable effect on these kinases in males (Figure 4e for Src effects ). These enzymes are present in the postsynaptic element and activated by TBS in an NMDAR-dependent fashion in males, evidently without contribution from locally derived estrogen. In agreement with others, we found the TBS-NMDAR route for kinase activation is operative in females as well but, from the results just described, this process appears to require a “boost” from release of locally produced estrogen (Figure 5). This argument implies that one (or more) feature of NMDAR function is better developed in males so as to not require supplemental estrogen receptor contributions for kinase activation and LTP. Of interest in this regard are recent reports that NMDAR-mediated Src activation involves non-ionic signaling (Dore et al., 2016; Nabavi et al., 2013); a metabotropic route is also suggested to be important for NMDAR-dependent ERK activation (Weilinger et al., 2016). It is therefore possible that non-ionic coupling between the NMDARs and LTP-related kinases is sexually dimorphic, and specifically more potent in males, thereby removing the need for the boost from E2/ERα for induction of LTP (Figure 5). This scenario would suggest that females, like males, retain the dependency of LTP on the ionic (calcium signaling) functions of the NMDARs. We propose that in females the dependence on a local estrogen/ERα step for kinase activation may account for the higher activity threshold for induction of LTP described above (Figure 3b) (Wang, Le, et al., 2018). Brief strings of theta bursts, as used in the LTP threshold studies, resemble conditions occurring during learning (Otto, Eichenbaum, et al., 1991) suggesting these results are plausibly related to sex differences in the threshold for encoding long-term memory.
FIGURE 5.
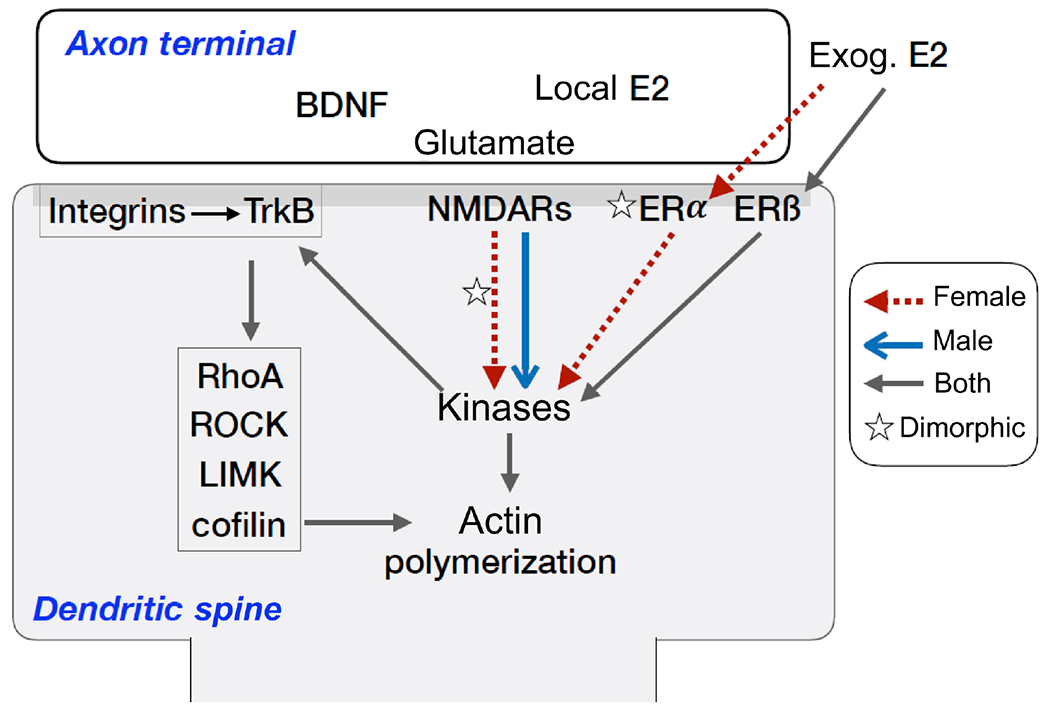
Sexual dimorphism in actin regulation underlying synaptic plasticity. Schematic illustrates proposed mechanisms for dimorphism in activity-dependent regulation of the dendritic spine actin cytoskeleton. First, both males and females express synaptic estrogen receptors, but in females only estrogen, either endogenous or exogenous (thus, circulating), regulates actin regulatory “kinases” (ERK1/2, Src, FAK) through both ERβ and ERα (red-dashed lines, stars); in males, exogenous E2 regulates kinases through ERß, and there was no evidence of activity-induced, endogenous estrogen signaling. Second, synaptic ERα levels are greater in females as compared to males, whereas levels of ERß are comparable between the sexes. Third, the dependence of TBS-induced kinase activation on ERα in females indicates that the drive from the NMDA receptors to the kinases is greater in males (indicated by bold blue arrow); hence, in males there is no need for the ERα boost. Finally, after kinase activation, the mechanisms of actin regulation and TBS-LTP appear to be same between the sexes: both depend on integrins, TrkB, RhoA signaling, and new actin polymerization
To further understand the actions of exogenous, and thus circulating, estrogen on synaptic actin regulatory signaling we evaluated effects of 1 nM E2 infusion on the postsynaptic kinases. The steroid activated postsynaptic ERK (Figure 4f) and Src in both sexes (Bi et al., 2000; Wang, Le, et al., 2018). In males these responses were largely dependent upon ERß with no evident contribution from ERα (Wang, Kantorovich, et al., 2016). In contrast, in females, the effects of E2 infusion were reduced by about half in the presence of ERα antagonist MPP (Figure 4f), indicating that complete kinase activation by applied or circulating E2 requires binding to both classes of estrogen receptor. Evidence that in females the same ERα antagonist significantly attenuated TBS-induced activation of ERK/Src, whereas an ERß blocker had little if any effect (Wang, Le, et al., 2018) indicates that in females locally derived estrogen, released during induction of LTP, acts via synaptic ERα alone without contribution from ERß. The partial efficacy of the ERß blocker with regard to infused E2 raises the possibility that in vivo the two avenues of estradiol action promote LTP during proestrus when both locally derived and circulating hormone levels are high and, specifically, that locally derived and circulating estrogen act through ERα and ERß, respectively, to engage the same downstream effector kinases (Figure 5). This conclusion accounts for the superior spatial learning scores by proestrus females.
There remains the question of why males do not use locally produced estrogen and ERα to boost NMDAR signaling and promote LTP. One possibility is suggested by the observation that males have substantially lower concentrations of synaptic ERα as compared to females (Wang, Le, et al., 2018). An alternative but not exclusive possibility is that signaling from ERα to various effectors is less effective in males, in which case dimorphism would involve sexually differentiated coupling between the two types of receptors (ERα and NMDARs) and downstream kinases. Sex differences in LTP, at least for field CA1, may be restricted to the events just described. Indeed, many of the mechanisms required for LTP in males are also engaged and required in females. In both sexes, TBS activates (α1-integrins and TrkB receptors and both steps are essential for LTP consolidation (Wang, Le, et al., 2018). TrkB autophosphorylation (activation) is dependent on integrin activation (Wang, Kantorovich, et al., 2016) and antagonism of the (α1 integrins blocks LTP in females, again as was found for males. Theta bursts activate signaling to p-cofilin in females, and inhibition of ROCK, an upstream kinase for cofilin phosphorylation, effectively suppresses LTP consolidation in females (Figure 4h) as in males (Wang, Le, et al., 2018). However, activation of synaptic TrkB (Figure 4g) and ß1 integrin is disrupted by ERα antagonists in females only indicating that dimorphism in activities of the membrane receptors account for the majority of sex differences in signaling thus far identified.
It should be noted that although in field CA1 the downstream LTP-related actin management steps thus far tested are comparable between the sexes, critical features in the male LTP substrate map, such as mechanisms of F-actin stabilization and contributions of adenosine receptors, have yet to be investigated in females. Moreover, effects of locally produced E2 and ERα on the synaptic Rac/Cdc42 and Ras cascades have yet to be evaluated for hippocampus although in neocortical neurons E2 activates Ras and Src in an ERα-independent fashion, consistent with our evidence that exogenous E2 acts primarily through ERß (Nethrapalli et al., 2001). Important goals of future research will be to determine if, in females, the local steroid elicits signaling through the F-actin stabilization cascades and thus influences the stability of both LTP and the memory trace, and further to test if synaptic membrane ERα and ERß make similar contributions to postsynaptic actin regulation and plasticity in both sexes.
Finally, although evidence for estrogen involvement in LTP in other brain areas, such as amygdala (Bender et al., 2017), aligns with the sexual dimorphism described for hippocampal field CA1, studies of the dentate gyrus (DG) highlight the fact that estrogen effects are region-, or perhaps cell type-, specific. In particular, well-described effects of circulating and applied E2 on dendritic spines in field CA1 are generally absent in the DG molecular layer (Frankfurt & Luine, 2015; Woolley et al., 1990). Nevertheless, E2 infusion reportedly has greater effects on basal fEPSPs in the DG than in CA1 (Kim et al., 2006). Reported E2 effects on LTP in the DG have been inconsistent. The dentate receives its principal input from entorhinal cortex via the perforant path: the lateral perforant path arises from lateral entorhinal cortex and terminates in the DG outer molecular layer, whereas the medial perforant path arises from medial entorhinal cortex and terminates in the middle molecular layer. Potentiation in the lateral perforant path is reportedly more vulnerable to disruption in proestrus (high estrogen) females compared to those in diestrus (Velisek & Vathy, 2005). Although contrasting with effects in CA1, these findings are consistent with results showing perforant path LTP is depressed by estrogen treatment (Gupta et al., 2001) and weaker in females as compared to males (Maren, 1995). Other groups find that for the medial perforant path, estrogen treatment enhances LTP in ovariectomized females (Nebieridze et al., 2012) and 1 nM E2 infusion enhances LTP in males (Tanaka & Sokabe, 2012). These inconsistent findings could reflect differences in E2 effects on LTP in the medial and lateral perforant paths with the former exhibiting the CA1 response profile and the latter having atypical responses to the steroid. This idea would be consistent with other results from our laboratory showing that the lateral perforant path exhibits an unusual presynaptic and endocannabinoid-dependent form of LTP, whereas potentiation in the medial perforant path relies on the same postsynaptic mechanisms characterized in field CA1 (Wang, Jia, et al., 2018; Wang, Trieu, et al., 2016).
6 |. DISCUSSION
As described, sex differences in LTP are evident in synaptic mechanisms that (a) comprise the earliest steps in the complex sequences that are set in motion by learning-related patterns of afferent activity and (b) underlie lasting modifications to the structure and function of glutamatergic synapses. In females, these mechanisms of plasticity depend on locally produced estrogen that acts on postsynaptic receptors of the ERα subtype that work in concert with the NMDARs to activate kinases involved in reorganization of the submembrane cytoskeleton. Males do not use these ERα mechanisms but more fully rely upon the NMDARs to engage the same kinases to effect actin remodeling. We propose that the requirements for locally produced estrogen mobilization and release, and ERα engagement, are responsible for the higher activity threshold for eliciting stable synaptic LTP in females relative to males. Given that the CA3 to CA1 projection is critical for encoding spatial relationships, it is reasonable to conclude that the sexually dimorphic role of local estrogen transmission is a major contributor to one of the better established sex differences in learning (Andreano & Cahill, 2009; Koss & Frick, 2017).
Results from our research suggest that sex differences in actin signaling and LTP are limited to the estrogen-ERα signaling pathway. This is despite evidence that ERß mediates actions of applied E2 on basic synaptic transmission (Koss & Frick, 2017; Kramar et al., 2009; Oberlander & Woolley, 2016) along with reported contributions from the third estrogen receptor, GPER1 (Kumar & Foster, 2020). Regarding LTP, events downstream from the estrogen receptors, including activation and reliance upon synaptic integrins, TrkB, and the RhoA cascade leading to actin polymerization, were not detectably different between male and female rodents. However, other key features of the male substrate map for LTP-related reorganization of the subsynaptic cytoskeleton have yet to be investigated in females. Prominent among these are the small GTPase-initiated sequences that stabilize and support branching of the actin filaments that assemble shortly after the initial induction of LTP. As illustrated in Figure 1d, these activities reflect signaling downstream from Rac/Cdc42 and Ras. The discovery of these aspects of the substrate map occurred during investigations (Kramar et al., 2006; Rex et al., 2009) into why diverse manipulations, including theta pattern synaptic activation and adenosine infusion, erase potentiation in males but only when applied within minutes after induction (Arai et al., 1990; Huang & Hsu, 2001; Huang et al., 1999; Larson et al., 1993). The presence of a time-dependent window for erasure established that LTP has a rapid consolidation period similar to that described for the formation of memory traces (Lynch et al., 2007, 2013). Whether treatments that disrupt LTP consolidation in males are equally effective and operate over the same time periods in females is a critical issue for future research. Any sex differences would suggest one of two conclusions: (a) the female estrogen-ERα-kinase pathway exerts different effects on stabilization mechanisms than does the male NMDAR-kinase only sequence or (b) there are as yet undiscovered dimorphisms in the LTP substrate map.
Related to the above, recent work has added new dimensions to the description of LTP stabilization in males. In particular, we now know that two striking effects emerge about 1 hr after induction: (a) a second stage of consolidation at already potentiated synapses (Babayan et al., 2012), and (b) a time-dependent capacity to trigger LTP in additional contacts (Kramar et al., 2012; Lynch et al., 2013). The absence of data on whether these phenomena are present in females constitutes a major gap in our understanding of sex differences in memory-related plasticity. A first question with far reaching implications concerns the possibility of a time window during which a second application of near-threshold stimulation enhances the probability of stable potentiation in females. As described, we now know that stimulation that is near threshold for induction of field CA1 LTP in males does not elicit lasting potentiation in adult females. The question of interest here is whether, in females, a first round of near-threshold stimulation would leave a trace in consolidation machinery that can be amplified by a subsequent stimulation episode to the point that potentiation is fully stabilized. Were this to be case, then the elevated activation threshold (for induction of LTP) would result in a female-specific LTP spaced trials effect. Relatedly, we do not know if females express the ~1-hr spaced trials effect that is prominent for LTP and spatial learning in males (Figure 2a) (Cao & Harris, 2014; Kramar et al., 2012; Seese et al., 2014). As discussed, the substrates for the 1-hr rule involve a large percentage of contacts that have a high threshold for being shifted into their potentiated state. In essence, results indicate any given collection of axons that constitutes an input to target cells includes subpopulations of synapses with low and high plasticity thresholds. In males, the first episode of LTP-inducing stimulation elicits potentiation in the low threshold synapses and primes the high threshold synapses for future potentiation (i.e., lowers their potentiation threshold) via cell processes that require 60 min to reach completion (Lynch et al., 2013). Whether and to what degree these complex thresholding events, and their behavioral concomitants, are present in females are essential topics for future research.
Infusions of physiologically realistic concentrations of estrogen produce a dramatic increase in the magnitude of LTP in both males and females (Bi et al., 2000; Foy et al., 1999; Kramar et al., 2009; Smith & McMahon, 2005; Warren et al., 1995), a result that likely relates to better performance on memory tests (Frick & Berger-Sweeney, 2001; Paris & Frye, 2008; Shors et al., 2000) for females within versus outside proestrus (the high circulating estrogen state). Estrogen could raise the LTP ceiling by increasing the amount of potentiation expressed at individual synapses or by acting on those contacts with high plasticity thresholds. Evidence that the synaptic activation threshold for potentiation can be reduced was obtained in studies using positive allosteric modulators of AMPARs to enhance LTP (Arai & Kessler, 2007; Lynch, 2006; Lynch & Gall, 2006; Lynch et al., 2011). Treatment with such drugs (e.g., ampakines) nearly doubled the percent LTP induced by a single TBS train but markedly reduced the added potentiation normally produced by a second train delivered 1 hr later (Lynch et al., 2013). These results predict that memory enhancement produced by facilitation of fast glutamatergic transmission, a result obtained across species and paradigms, may come at the expense of the spaced trials effect for learning. Estrogen promotes LTP through a very different mechanism and it will accordingly be of great interest to determine if it does so without engaging those synapses with high plasticity thresholds.
The research discussed above focused on plasticity within the dense projections arising in hippocampal field CA3 and terminating in the apical dendrites of field CA1, the so-called Schaffer-commissural pathway (Amaral & Witter, 1989). These are among the most thoroughly characterized synapses in the brain, have been the principal target of the vast majority of LTP studies, and are thought to be representative of circuitry throughout the cortical telencephalon. Nevertheless, other forms of LTP-like plasticity are known to be present even within hippocampus. One prominent example of this is found in the lateral perforant path, one of the two primary cortical inputs to the dentate gyrus and field CA3 (van Groen et al., 2003; Witter, 1993). Persistent potentiation in these connections involves endocannabinoid-triggered adjustments to the presynaptic cytoskeleton and associated changes in transmitter release rather than the postsynaptic modifications found in field CA1 (Wang, Jia, et al., 2018; Wang, Trieu, et al., 2016). Very little is known about the effects of endogenous or added estrogen on baseline physiology or the singular form of plasticity expressed by this pathway. Accordingly, there remains the possibility that the nature and magnitude of sexual dimorphisms are regionally differentiated.
In summary, available evidence describes pronounced sexual dimorphism in synaptic mechanisms of learning: Sex differences have been identified in the initial (receptor-related) but not subsequent mechanisms that lead to new actin polymerization required for consolidation of CA1 LTP. Whether sex differences are also present in the equally complex processes that elaborate (e.g., influence branching and thus the breadth) and stabilize the reorganized actin cytoskeleton is currently unknown; the latter point is pertinent to aspects of learning such as the endurance of the memory trace. Nevertheless, the results as they stand raise the question of whether there are adaptive advantages to the synaptic dimorphisms thus far identified. The lower threshold for LTP and spatial learning identified in males would be valuable when the material to be encoded is distinct and not likely to be confused with transient, irrelevant signals. On the other hand, slower acquisition requiring additional sampling to reach threshold for stable encoding in females might be advantageous in situations in which the signal to noise ratio for critical cues is low. According to these arguments, synaptic dimorphism might reflect evolutionary pressures toward optimization of male versus female learning in different types of real-world environments. Testing of these ideas will likely be more compelling once information is gained about possible dimorphisms in secondary features of LTP and the time-dependent facets of learning as discussed above.
Significance.
Analyses of mechanisms of functional synaptic plasticity in rodent hippocampus have identified molecular substrates for enduring changes in synaptic strength associated with memory encoding. In both sexes this involves actin cytoskeletal remodeling but, in females only, locally derived estrogen and estrogen receptor alpha are required to engage synaptic receptors and signaling activities critical for plasticity. The estrogen requirement is associated with a heightened threshold for enduring changes in synaptic strength and memory in females. These sex differences would be expected to give rise to dimorphisms in properties of learning and to differentially shape higher cognitive function.
ACKNOWLEDGMENTS
The work described was supported by the National Institutes of Health grants NS045260, NS085709, and HD089491 to C.M.G. and G.L. and Department of Defense Multidisciplinary University Research Initiative Grant N00014-101-0072 from the Office of Naval Research to G.L. A.A.L. was supported by the NIMH training grant to 5T32MH119049-02.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the Supporting Information section.
REFERENCES
- Abraham WC, & Huggett A (1997). Induction and reversal of long-term potentiation by repeated high-frequency stimulation in rat hippocampal slices. Hippocampus, 7, 137–145. [DOI] [PubMed] [Google Scholar]
- Amaral DG,& Witter MP.(1989). The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience, 31, 571–591. 10.1016/0306-4522(89)90424-7 [DOI] [PubMed] [Google Scholar]
- Andreano JM, & Cahill L (2009). Sex influences on the neurobiology of learning and memory. Learning & Memory, 16, 248–266. 10.1101/lm.918309 [DOI] [PubMed] [Google Scholar]
- Arai AC, & Kessler M (2007). Pharmacology of ampakine modulators: From AMPA receptors to synapses and behavior. Current Drug Targets, 8, 583–602. [DOI] [PubMed] [Google Scholar]
- Arai A, Kessler M, & Lynch G (1990). The effects of adenosine on the development of long-term potentiation. Neuroscience Letters, 119, 41–44. 10.1016/0304-3940(90)90750-4 [DOI] [PubMed] [Google Scholar]
- Asperholm M, Hogman N, Rafi J, & Herlitz A (2019). What did you do yesterday? A meta-analysis of sex differences in episodic memory. Psychological Bulletin, 145, 785–821. 10.1037/bul0000197 [DOI] [PubMed] [Google Scholar]
- Babayan AH, Kramar EA, Barrett RM, Jafari M, Haettig J, Chen LY, Rex CS, Lauterborn JC, Wood MA, Gall CM, & Lynch G (2012). Integrin dynamics produce a delayed stage of long-term potentiation and memory consolidation. Journal of Neuroscience, 32, 12854–12861. 10.1523/JNEUROSCI.2024-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender RA, Zhou L, Vierk R, Brandt N, Keller A, Gee CE, Schafer MK, & Rune GM (2017). Sex-dependent regulation of aromatase-mediated synaptic plasticity in the basolateral amygdala. Journal of Neuroscience, 37, 1532–1545. 10.1523/JNEUROSCI.1532-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum SA, Baxter L,Seidenberg M, & Hermann B. (1997). Role of the hippocampus in sex differences in verbal memory: Memory outcome following left anterior temporal lobectomy. Neuropsychology, 11, 585–591. 10.1037/0894-4105.ll.4.585 [DOI] [PubMed] [Google Scholar]
- Bi R, Broutman G, Foy MR, Thompson RF, & Baudry M (2000). The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 97, 3602–3607. 10.1073/pnas.060034497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, & Baudry M (2001). Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proceedings of the National Academy of Sciences of the United States of America, 98, 13391–13395. 10.1073/pnas.241507698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T, Collingridge GL, & Morris RGM (2007). Synaptic plasticity in the hippocampus. In Anderson P, Morris R, Amaral D, Bliss T, & O’Keefe J (Eds.), The hippocampus book (pp. 343–374). Oxford University Press. [Google Scholar]
- Bliss TVP, & Lomo T (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the anesthetized rabbit following stimulation of the perforant path. Journal of Physiology, 232, 334–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C, & Fassler R (2003). The integrin-actin connection, an eternal love affair. EMBO Journal, 22, 2324–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt N, Vierk R, & Rune GM (2013). Sexual dimorphism in estrogen-induced synaptogenesis in the adult hippocampus. International Journal of Developmental Biology, 57, 351–356. 10.1387/ijdb.120217gr [DOI] [PubMed] [Google Scholar]
- Bunda S, Heir P, Srikumar T, Cook JD, Burrell K, Kano Y, Lee JE, Zadeh G, Raught B, & Ohh M (2014). Src promotes GTPase activity of Ras via tyrosine 32 phosphorylation. Proceedings of the National Academy of Sciences of the United States of America, 111, E3785–E3794. 10.1073/pnas.1406559111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, & Harris KM (2014). Augmenting saturated LTP by broadly spaced episodes of theta-burst stimulation in hippocampal area CA1 of adult rats and mice. Journal of Neurophysiology, 112, 1916–1924. 10.1152/jn.00297.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LY, Rex CS, Casale MS, Gall CM, & Lynch G (2007). Changes in synaptic morphology accompany actin signaling during LTP. Journal of Neuroscience, 27, 5363–5372. 10.1523/JNEUROSCI.0164-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney ID, Brabec CM, & Runco DV (2008). Mapping out spatial ability: Sex differences in way-finding navigation. Perceptual and Motor Skills, 107, 747–760. 10.2466/pms.107.3.747-760 [DOI] [PubMed] [Google Scholar]
- Cohen Y, Reuveni I, Barkai E,& Maroun M. (2008). Olfactory learning-induced long-lasting enhancement of descending and ascending synaptic transmission to the piriform cortex. Journal of Neuroscience, 28, 6664–6669. 10.1523/Jneurosci.0178-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba Montoya DA, & Carrer HF (1997). Estrogen facilitates induction of long term potentiation in the hippocampus of awake rats. Brain Research, 778, 430–438. [DOI] [PubMed] [Google Scholar]
- Cox BM, Cox CD, Gunn BG, Le AA, Inshishian VC, Gall CM, & Lynch G (2019). Acquisition of temporal order requires an intact CA3 commissural/associational (C/A) feedback system in mice. Communications Biology, 2, 251. 10.1038/s42003-019-0494-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CD, Rex CS, Palmer LC, Babayan AH, Pham DT, Corwin SD, Trieu BH, Gall CM, & Lynch G (2014). A map of LTP-related synaptic changes in dorsal hippocampus following unsupervised learning. Journal of Neuroscience, 34, 3033–3041. 10.1523/JNEUROSCI.4159-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs JM Jr., Chang EL, Strong RA, & Milun R (1998). Spatial ability, navigation strategy, and geographic knowledge among men and women. Evolution and Human Behavior, 19, 89–98. 10.1016/S1090-5138(97)00107-4 [DOI] [Google Scholar]
- Dickerson BC, & Eichenbaum H (2010). The episodic memory system: Neurocircuitry and disorders. Neuropsychopharmacology, 35, 86–104. 10.1038/npp.2009.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore K, Aow J, & Malinow R (2016). The emergence of NMDA receptor metabotropic function: Insights from imaging. Frontiers in Synaptic Neuroscience, 8, 20. 10.3389/fnsyn.2016.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan CP (1949). The retroactive effect of electroshock on learning. Journal of Comparative and Physiological Psychology, 42, 32–44. 10.1037/h0058173 [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, & Lynch G (1979). The relationship between extracellular calcium concentrations and the induction of hippocampal long-term potentiation. Brain Research, 169, 103–110. 10.1016/0006-8993(79)90377-9 [DOI] [PubMed] [Google Scholar]
- Eacott MJ, & Easton A (2010). Episodic memory in animals: Remembering which occasion. Neuropsychologia, 48, 2273–2280. 10.1016/j.neuropsychologia.2009.ll.002 [DOI] [PubMed] [Google Scholar]
- Ebbinghaus H (1885). Über das Gedächtnis. Untersuchungen zur experimentellen Psychologie. Duncker & Humblot. [Google Scholar]
- Ellis H (1894). Man and woman; A study of human secondary sexual characteristics. New York, NY: Charles Schribner’s Sons. [Google Scholar]
- Ellis L (2011). Identifying and explaining apparent universal sex differences in cognition and behavior. Personality and Individual Differences, 51, 552–561. 10.1016/j.paid.2011.04.004 [DOI] [Google Scholar]
- Epting LK, & Overman WH (1998). Sex-sensitive tasks in men and women: A search for performance fluctuations across the menstrual cycle. Behavioral Neuroscience, 112, 1304–1317. 10.1037/0735-7044.112.6.1304 [DOI] [PubMed] [Google Scholar]
- Fedulov V, Rex CS, Simmons DA, Palmer L, Gall CM, & Lynch G (2007). Evidence that long-term potentiation occurs within individual hippocampal synapses during learning. Journal of Neuroscience, 27, 8031–8039. 10.1523/JNEUROSCI.2003-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Baizan C, Arias JL, & Mendez M (2019). Spatial memory in young adults: Gender differences in egocentric and allocentric performance. Behavioral Brain Research, 359, 694–700. 10.1016/j.bbr.2018.09.017 [DOI] [PubMed] [Google Scholar]
- Foy MR, Baudry M, Diaz Brinton R, & Thompson RF (2008). Estrogen and hippocampal plasticity in rodent models. Journal of Alzheimer’s Disease, 15, 589–603. 10.3233/JAD-2008-15406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, & Berger TW (1999). 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. Journal of Neurophysiology, 81, 925–929. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, & Luine V (2015). The evolving role of dendritic spines and memory: Interaction(s) with estradiol. Hormones and Behavior, 74, 28–36. 10.1016/j.yhbeh.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, & Berger-Sweeney J (2001). Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behavioral Neuroscience, 115, 229–237. 10.1037/0735-7044.115.1.229 [DOI] [PubMed] [Google Scholar]
- Frick KM, Kim J, Tuscher JJ, & Fortress AM (2015). Sex steroid hormones matter for learning and memory: Estrogenic regulation of hippocampal function in male and female rodents. Learning & Memory, 22, 472–493. 10.1101/lm.037267.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Tuscher JJ, Koss WA, Kim J, & Taxier LR (2018). Estrogenic regulation of memory consolidation: A look beyond the hippocampus, ovaries, and females. Physiology & Behavior, 187, 57–66. 10.1016/j.physbeh.2017.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA (1995). Estrus-associated decrements in a water maze task are limited to acquisition. Physiology & Behavior, 57, 5–14. 10.1016/0031-9384(94)00197-d [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, & Walf AA (2007). Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiology of Learning and Memory, 88, 208–216. 10.1016/j.nlm.2007.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon KT, Thomas BJ, Munion A, Creem-Regehr SH, Cashdan EA, & Stefanucci JK (2018). Not all those who wander are lost: Spatial exploration patterns and their relationship to gender and spatial memory. Cognition, 180, 108–117. 10.1016/j.cognition.2018.06.020 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos I, Alejandre-Gomez M, & Cervantes M (2005). Spine-type densities of hippocampal CA1 neurons vary in proestrus and estrus rats. Neuroscience Letters, 379, 52–54. 10.1016/j.neulet.2004.12.043 [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, & McEwen BS (1990). Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. Journal of Neuroscience, 10, 1286–1291. 10.1523/JNEUROSCI.10-04-01286.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, Kelleher RJ, & Tonegawa S (2006). A clustered plasticity model of long-term memory engrams. Nature Reviews Neuroscience, 7, 575–583. 10.1038/nrnl937 [DOI] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, & Maren S (2001). Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1). Brain Research, 888, 356–365. 10.1016/s0006-8993(00)03116-4 [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Weisend MP, & Sutherland RJ (2007). How do room and apparatus cues control navigation in the Morris water task? Evidence for distinct contributions to a movement vector. Journal of Experimental Psychology: Animal Behavior Processes, 33, 100–114. 10.1037/0097-7403.33.2.100 [DOI] [PubMed] [Google Scholar]
- Hampson E (1990). Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology, 15, 97–111. 10.1016/0306-4530(90)90018-5 [DOI] [PubMed] [Google Scholar]
- Hampson E, & Kimura D (1988). Reciprocal effects of hormonal fluctuations on human motor and perceptual-spatial skills. Behavioral Neuroscience, 102, 456–459. 10.1037//0735-7044.102.3.456 [DOI] [PubMed] [Google Scholar]
- Harris KM (2020). Structural LTP: From synaptogenesis to regulated synapse enlargement and clustering. Current Opinion in Neurobiology, 63, 189–197. 10.1016/j.conb.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Fiala JC, & Ostroff L (2003). Structural changes at dendritic spine synapses during long-term potentiation. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358, 745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Hojo Y, Kojima H, Ikeda M, Hotta K, Sato R, Ooishi Y, Yoshiya M, Chung BC, Yamazaki T, & Kawato S (2015). Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: Involvement of kinase networks. Brain Research, 1621, 147–161. 10.1016/j.brainres.2014.12.056 [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelon C, & Wyble BP (2002). A proposed function for hippocampal theta rhythm: Separate phases of encoding and retrieval enhance reversal of prior learning. Neural Computation, 14, 793–817. 10.1162/089976602317318965 [DOI] [PubMed] [Google Scholar]
- Hojo Y, Higo S, Ishii H, Ooishi Y, Mukai H, Murakami G, Kominami T, Kimoto T, Honma S, Poirier D, & Kawato S (2009). Comparison between hippocampus-synthesized and circulation-derived sex steroids in the hippocampus. Endocrinology, 150, 5106–5112. 10.1210/en.2009-0305 [DOI] [PubMed] [Google Scholar]
- Huang CC, & Hsu KS (2001). Progress in understanding the factors regulating reversibility of long-term potentiation. Reviews in the Neurosciences, 12, 51–68. 10.1515/REVNEURO.2001.12.1.51 [DOI] [PubMed] [Google Scholar]
- Huang CC, Liang YC, & Hsu KS (1999). A role for extracellular adenosine in time-dependent reversal of long-term potentiation by low-frequency stimulation at hippocampal CA1 synapses. Journal of Neuroscience, 19, 9728–9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JS (2005). The gender similarities hypothesis. American Psychologist, 60, 581–592. 10.1037/0003-066X.60.6.581 [DOI] [PubMed] [Google Scholar]
- Hynes R (1992). Integrins, versatility, modulation, and signaling in cell adhesion. Cell, 69, 11–25. 10.1016/0092-8674(92)90115-S [DOI] [PubMed] [Google Scholar]
- Jones CM, Braithwaite VA, & Healy SD (2003). The evolution of sex differences in spatial ability. Behavioral Neuroscience, 117, 403–411. 10.1037/0735-7044.117.3.403 [DOI] [PubMed] [Google Scholar]
- Kampen DL, & Sherwin BB (1996). Estradiol is related to visual memory in healthy young men. Behavioral Neuroscience, 110, 613–617. 10.1037//0735-7044.110.3.613 [DOI] [PubMed] [Google Scholar]
- Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, Uebayashi M, & Kawato S (2013). Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Frontiers in Neural Circuits, 7, 149. 10.3389/fncir.2013.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato AC, Murakami G, Hojo Y, Horie S, & Kawato S (2020). Rapid effects of estradiol on dendritic spines and synaptic plasticity in the male and female hippocampus. In Estrogens and memory: Basic research and clinical implications (pp. 38–47). Oxford, UK: Oxford University Press. [Google Scholar]
- Kauer JA, Malenka RC, & Nicoll RA (1988). A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron, 1, 911–917. 10.1016/0896-6273(88)90148-1 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, & Dudley CA (1976). Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Research, 114, 152–157. 10.1016/0006-8993(76)91017-9 [DOI] [PubMed] [Google Scholar]
- Kim MT, Soussou W, Gholmieh G, Ahuja A, Tanguay A, Berger TW, & Brinton RD (2006). 17beta-Estradiol potentiates field excitatory postsynaptic potentials within each subfield of the hippocampus with greatest potentiation of the associational/commissural afferents of CA3. Neuroscience, 141, 391–406. 10.1016/j.neuroscience.2006.03.075 [DOI] [PubMed] [Google Scholar]
- Koss WA, & Frick KM (2017). Sex differences in hippocampal function. Journal of Neuroscience Research, 95, 539–562. 10.1002/jnr.23864 [DOI] [PubMed] [Google Scholar]
- Kramar EA, Babayan AH, Gavin CF, Cox CD, Jafari M, Gall CM, Rumbaugh G, & Lynch G (2012). Synaptic evidence for the efficacy of spaced learning. Proceedings of the National Academy of Sciences of the United States of America, 109, 5121–5126. 10.1073/pnas.1120700109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, & Lynch G (2009). Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. Journal of Neuroscience, 29, 12982–12993. 10.1523/JNEUROSCI.3059-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Rex CS, Gall CM, & Lynch G (2006). Integrin-driven actin polymerization consolidates long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America, 103, 5579–5584. 10.1073/pnas.0601354103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Delis DC, Kaplan E, O’Donnell L, & Prifitera A (1997). Developmental sex differences in verbal learning. Neuropsychology, 11, 577–584. 10.1037/0894-4105.11.4.577 [DOI] [PubMed] [Google Scholar]
- Kumar A, & Foster TC (2020). G protein-coupled estrogen receptor: Rapid effects on hippocampal-dependent spatial memory and synaptic plasticity. Frontiers in Endocrinology, 11, 385. 10.3389/fendo.2020.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R (2016). The role of actin cytoskeleton in memory formation in amygdala. Frontiers in Molecular Neuroscience, 9, 23. 10.3389/fnmol.2016.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, & Lynch G (1988). Role of N-methyl-d-aspartate receptors in the induction of synaptic potentiation by burst stimulation patterned after the hippocampal theta-rhythm. Brain Research, 441, 111–118. 10.1016/0006-8993(88)91388-1 [DOI] [PubMed] [Google Scholar]
- Larson J, & Munkacsy E (2015). Theta-burst LTP. Brain Research, 1621, 38–50. 10.1016/j.brainres.2014.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Wong D, & Lynch G (1986). Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Research, 368, 347–350. 10.1016/0006-8993(86)90579-2 [DOI] [PubMed] [Google Scholar]
- Larson J, Xiao P, & Lynch G (1993). Reversal of LTP by theta frequency stimulation. Brain Research, 600, 97–102. 10.1016/0006-8993(93)90406- [DOI] [PubMed] [Google Scholar]
- Lawton CA (1994). Gender differences in way-finding strategies—Relationship to spatial ability and spatial anxiety. Sex Roles, 30, 765–779. 10.1007/Bf01544230 [DOI] [Google Scholar]
- Lee FS, Rajagopal R, & Chao MV (2002). Distinctive features of Trk neurotrophin receptor transactivation by G protein-coupled receptors. Cytokine & Growth Factor Reviews, 13, 11–17. [DOI] [PubMed] [Google Scholar]
- Lee K, Schottler F, Oliver M, & Lynch G (1980). Brief bursts of high-frequency stimulation produce two types of structural changes in rat hippocampus. Journal of Neurophysiology, 44, 247–258. [DOI] [PubMed] [Google Scholar]
- Lin B, Kramar EA, Bi X, Brucher FA, Gall CM, & Lynch G (2005). Theta stimulation polymerizes actin in dendritic spines of hippocampus. Journal of Neuroscience, 25, 2062–2069. 10.1523/JNEUROSCI.4283-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Sareddy GR, Wang J, Wang R, Li Y, Dong Y, Zhang Q, Liu J, O’Connor JC, Xu J, Vadlamudi RK, & Brann DW (2019). Neuron-derived estrogen regulates synaptic plasticity and memory. Journal of Neuroscience, 39, 2792–2809. 10.1523/JNEUROSCI.1970-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V (2015). Recognition memory tasks in neuroendocrine research. Behavioural Brain Research, 285, 158–164. 10.1016/j.bbr.2014.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, & Frankfurt M (2013). Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience, 239, 34–45. 10.1016/j.neuroscience.2012.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, & Frankfurt M (2020). Estrogenic regulation of memory: The first 50 years. Hormones and Behavior, 121, ARTN 104711. 10.1016/j.yhbeh.2020.104711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, & Rodriguez M (1994). Effects of estradiol on radial arm maze performance of young and aged rats. Behavioral and Neural Biology, 62, 230–236. 10.1016/s0163-1047(05)80021-4 [DOI] [PubMed] [Google Scholar]
- Luo WF, Janostiak R, Tolde O, Ryzhova LM, Koudelkova L, Dibus M, Brabek J, Hanks SK, & Rosel D (2017). ARHGAP42 is activated by Src-mediated tyrosine phosphorylation to promote cell motility. Journal of Cell Science, 130, 2382–2393. 10.1242/jcs.197434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G (1998). Memory and the brain: Unexpected chemistries and a new pharmacology. Neurobiology of Learning and Memory, 70, 82–100. 10.1006/nlme.1998.3840 [DOI] [PubMed] [Google Scholar]
- Lynch G (2002). Memory enhancement: The search for mechanism-based drugs. Nature Neuroscience, 5(Suppl. 11), 1035–1038. 10.1038/nn935 [DOI] [PubMed] [Google Scholar]
- Lynch G (2003). Long-term potentiation in the Eocene. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358, 625–628. 10.1098/rstb.2002.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G (2004). AMPA receptor modulators as cognitive enhancers. Current Opinion in Pharmacology, 4, 4–11. 10.1016/j.coph.2003.09.009 [DOI] [PubMed] [Google Scholar]
- Lynch G (2006). Glutamate-based therapeutic approaches: Ampakines. Current Opinion in Pharmacology, 6, 82–88. 10.1016/j.coph.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Lynch G, & Gall CM (2006). Ampakines and the threefold path to cognitive enhancement. Trends in Neurosciences, 29, 554–562. 10.1016/j.tins.2006.07.007 [DOI] [PubMed] [Google Scholar]
- Lynch G, Kramar EA, Babayan AH, Rumbaugh G, & Gall CM (2013). Differences between synaptic plasticity thresholds result in new timing rules for maximizing long-term potentiation. Neuropharmacology, 64, 27–36. 10.1016/j.neuropharm.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Larson J, Kelso S, Barrionuevo G, & Schottler F (1983). Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature, 305, 719–721. 10.1038/305719a0 [DOI] [PubMed] [Google Scholar]
- Lynch G, Palmer LC, & Gall CM (2011). The likelihood of cognitive enhancement. Pharmacology, Biochemistry and Behavior, 99, 116–129. 10.1016/j.pbb.2010.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Rex CS, & Gall CM (2007). LTP consolidation: Substrates, explanatory power, and functional significance. Neuropharmacology, 52, 12–23. 10.1016/j.neuropharm.2006.07.027 [DOI] [PubMed] [Google Scholar]
- Lynch MA (2004). Long-term potentiation and memory. Physiological Reviews, 84, 87–136. 10.1152/physrev.00014.2003 [DOI] [PubMed] [Google Scholar]
- Maccoby EE, & Jacklin CN (1974). The psychology of sex differences. Stanford, CA: Stanford University Press. [Google Scholar]
- Maren S (1995). Sexually dimorphic perforant path long-term potentiation (LTP) in urethane-anesthetized rats. Neuroscience Letters, 196, 177–180. 10.1016/0304-3940(95)11869-x [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, & Kasai H (2004). Structural basis of long-term potentiation in single dendritic spines. Nature, 429, 761–766. 10.1038/nature02617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, & Lewis DJ (1968). Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science, 160, 554–555. [DOI] [PubMed] [Google Scholar]
- Morris RG (2003). Long-term potentiation and memory. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358, 643–647. 10.1098/rstb.2002.1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, & Baudry M (1986). Selective impairment of learning and blockade of long-term potentiation by an N-methyl-d-aspartate receptor antagonist, AP5. Nature, 319, 774–776. 10.1038/319774a0 [DOI] [PubMed] [Google Scholar]
- Mukai H, Kimoto T, Hojo Y, Kawato S, Murakami G, Higo S, Hatanaka Y, & Ogiue-Ikeda M (2010). Modulation of synaptic plasticity by brain estrogen in the hippocampus. Biochimica et Biophysica Acta, 1800, 1030–1044. 10.1016/j.bbagen.2009.11.002 [DOI] [PubMed] [Google Scholar]
- Muller D, Joly M, & Lynch G (1988). Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science, 242, 1694–1697. 10.1126/science.2904701 [DOI] [PubMed] [Google Scholar]
- Muller D, & Lynch G (1988). Long-term potentiation differentially affects two components of synaptic responses in hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 85, 9346–9350. 10.1073/pnas.85.23.9346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, & Lynch G (1989). Evidence that changes in presynaptic calcium currents are not responsible for long-term potentiation in hippocampus. Brain Research, 479, 290–299. 10.1016/0006-8993(89)91631-4 [DOI] [PubMed] [Google Scholar]
- Nabavi S, Kessels HW, Alfonso S, Aow J, Fox R, & Malinow R (2013). Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. Proceedings of the National Academy of Sciences of the United States of America, 110, 4027–4032. 10.1073/pnas.1219454110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura J, Oomura Y, Minami T, Mizuno Y, & Fukuda A (1986). Mechanism of the rapid effect of 17 beta-estradiol on medial amygdala neurons. Science, 233, 226–228. 10.1126/science.3726531 [DOI] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J , Costa RM, Silva AJ, Kaczmarek L, & Huntley GW (2006). Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. Journal of Neuroscience, 26, 1923–1934. 10.1523/JNEUROSCI.4359-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebieridze N, Zhang XL, Chachua T, Velisek L, Stanton PK, & Veliskova J (2012). beta-Estradiol unmasks metabotropic receptor-mediated metaplasticity of NMDA receptor transmission in the female rat dentate gyrus. Psychoneuroendocrinology, 37, 1845–1854. 10.1016/j.psyneuen.2012.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethrapalli IS, Singh M, Guan X, Guo Q, Lubahn DB, Korach KS, & Toran-Allerand CD (2001). Estradiol (E2) elicits SRC phosphorylation in the mouse neocortex: The initial event in E2 activation of the MAPK cascade? Endocrinology, 142, 5145–5148. 10.1210/endo.l42.12.8546 [DOI] [PubMed] [Google Scholar]
- Nicoll RA (2003). Expression mechanisms underlying long-term potentiation: A postsynaptic view. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358, 721–726. 10.1098/rstb.2002.1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA (2017). A brief history of long-term potentiation. Neuron, 93, 281–290. 10.1016/j.neuron.2016.12.015 [DOI] [PubMed] [Google Scholar]
- Oberlander JG, & Woolley CS (2016). 17beta-estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. Journal of Neuroscience, 36, 2677–2690. 10.1523/JNEUROSCI.4437-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H, Wiener SI, & Wible CG (1991). Learning-related patterns of CA1 spike trains parallel stimulation parameters optimal for inducing hippocampal long-term potentiation. Hippocampus, 1, 181–192. 10.1002/hipo.450010206 [DOI] [PubMed] [Google Scholar]
- Otto T, Schottler F, Staubli U, Eichenbaum H, & Lynch G (1991). Hippocampus and olfactory discrimination learning: Effects of entorhinal cortex lesions on olfactory learning and memory in a successive-cue, go-no-go task. Behavioral Neuroscience, 105, 111–119. 10.1037/0735-7044.105.l.lll [DOI] [PubMed] [Google Scholar]
- Paris JJ, & Frye CA (2008). Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction, 136, 105–115. 10.1530/REP-07-0512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park P, Volianskis A, Sanderson TM, Bortolotto ZA, Jane DE, Zhuo M, Kaang BK, & Collingridge GL (2014). NMDA receptor-dependent long-term potentiation comprises a family of temporally overlapping forms of synaptic plasticity that are induced by different patterns of stimulation. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 369, 20130131. 10.1098/rstb.2013.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, & Saldanha CJ (2005). Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proceedings of the Royal Society B: Biological Sciences, 272, 2089–2096. 10.1098/rspb.2005.3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piber D, Nowacki J, Mueller SC, Wingenfeld K, & Otte C (2018). Sex effects on spatial learning but not on spatial memory retrieval in healthy young adults. Behavioral Brain Research, 336, 44–50. 10.1016/j.bbr.2017.08.034 [DOI] [PubMed] [Google Scholar]
- Popik P, Mamczarz J, & Vetulani J (1994). The effect of electroconvulsive shock and nifedipine on spatial learning and memory in rats. Biological Psychiatry, 35, 864–869. 10.1016/0006-3223(94)90022-1 [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Jarry H, Schoen M, Kohlmann P, Lohse C, Zhou L, & Rune GM (2008). Gonadotropin-releasing hormone regulates spine density via its regulatory role in hippocampal estrogen synthesis. Journal of Cell Biology, 180, 417–426. 10.1083/jcb.200707043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XQ, Zhang K, Xu T, Yamaki VN, Wei ZS, Huang MF, Rose GM, & Cai X (2016). Sex differences in long-term potentiation at temporoammonic-CA1 synapses: Potential implications for memory consolidation. PLoS One, 11, ARTN e0165891. 10.1371/journal.pone.0165891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal R, & Chao MV (2006). A role for Fyn in Trk receptor transactivation by G-protein-coupled receptor signaling. Molecular and Cellular Neurosciences, 33, 36–46. 10.1016/j.men.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Rehnman J, & Herlitz A (2007). Women remember more faces than men do. Acta Psychologica, 124, 344–355. 10.1016/j.actpsy.2006.04.004 [DOI] [PubMed] [Google Scholar]
- Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, & Lynch G (2009). Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. Journal of Cell Biology, 186, 85–97. 10.1083/jcb.200901084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Gavin CF, Rubio MD, Kramar EA, Chen LY, Jia Y, Huganir RL, Muzyczka N, Gall CM, Miller CA, Lynch G, & Rumbaugh G (2010). Myosin IIb regulates actin dynamics during synaptic plasticity and memory formation. Neuron, 67(4), 603–617. 10.1016/j.neuron.2010.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Kramar EA, Colgin LL, Lin B, Gall CM, & Lynch G (2005). Long-term potentiation is impaired in middle-aged rats: Regional specificity and reversal by adenosine receptor antagonists. Journal of Neuroscience, 25, 5956–5966. 10.1523/JNEUROSCI.0880-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman F, Staubli U, & Lynch G (1987). Evidence for synaptic potentiation in a cortical network during learning. Brain Research, 418, 221–226. 10.1016/0006-8993(87)90089-8 [DOI] [PubMed] [Google Scholar]
- Romeo RD, McCarthy JB, Wang A, Milner TA, & McEwen BS (2005). Sex differences in hippocampal estradiol-induced N-methyl-d-aspartic acid binding and ultrastructural localization of estrogen receptor-alpha. Neuroendocrinology, 81, 391–399. 10.1159/000089557 [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Arseneau LM, Beverly JL, & Juraska JM (2004). Effect of the estrous cycle on water maze acquisition depends on the temperature of the water. Behavioral Neuroscience, 118, 863–868. 10.1037/0735-7044.118.4.863 [DOI] [PubMed] [Google Scholar]
- Rudy JW (2015). Actin dynamics and the evolution of the memory trace. Brain Research, 1621, 17–28. 10.1016/j.brainres.2014.12.007 [DOI] [PubMed] [Google Scholar]
- Sakimoto Y, & Sakata S (2020). The role of the hippocampal theta rhythm in non-spatial discrimination and associative learning task. Neuroscience & Biobehavioral Reviews, 110, 92–99. 10.1016/j.neubiorev.2018.09.016 [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kaufman J, & Huettel SA (1998). Males and females use different distal cues in a virtual environment navigation task. Cognitive Brain Research, 6, 351–360. 10.1016/s0926-6410(98)00002-0 [DOI] [PubMed] [Google Scholar]
- Sanna A, Fattore L, Badas P, Corona G, Cocco V, & Diana MA (2019). Intermittent theta burst stimulation of the prefrontal cortex in cocaine use disorder: A pilot study. Frontiers Neuroscience, 13, 765. 10.3389/fnins.2019.00765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL (1982). Stranger behind the engram: Theories of memory and the psychology of Science. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Seese RR, Babayan AH, Katz AM, Cox CD, Lauterborn JC, Lynch G, & Gall CM (2012). LTP induction translocates cortactin at distant synapses in wild-type but not Fmr1 knock-out mice. Journal of Neuroscience, 32(21), 7403–7413. 10.1523/JNEUROSCI.0968-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seese RR, Chen LY, Cox CD, Schulz D, Babayan AH, Bunney WE, Henn FA, Gall CM, & Lynch G (2013). Synaptic abnormalities in the infralimbic cortex of a model of congenital depression. Journal of Neuroscience, 33, 13441–13448. 10.1523/JNEUROSCI.2434-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seese RR, Wang K, Yao YQ, Lynch G, & Gall CM (2014). Spaced training rescues memory and ERK1/2 signaling in fragile X syndrome model mice. Proceedings of the National Academy of Sciences of the United States of America, 111, 16907–16912. 10.1073/pnas.1413335111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard PAS, Choleris E, & Galea LAM (2019). Structural plasticity of the hippocampus in response to estrogens in female rodents. Molecular Brain, 12, 22. 10.1186/sl3041-019-0442-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Beylin AV, Wood GE, & Gould E (2000). The modulation of Pavlovian memory. Behavioral Brain Research, 110, 39–52. 10.1016/s0166-4328(99)00183-7 [DOI] [PubMed] [Google Scholar]
- Siedlecki KL, Falzarano F, & Salthouse TA (2019). Examining gender differences in neurocognitive functioning across adulthood. Journal of the International Neuropsychological Society, 25, 1051–1060. 10.1017/S1355617719000821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman I, & Eals M (1992). Sex differences in spatial abilities: Evolutionary theory and data. In Barkow JH, Cosmides L, & Tooby J (Eds.), The adapted mind: Evolutional psychology and the generation of culture (pp. 533–549). Oxford University Press. [Google Scholar]
- Smith CC, & McMahon LL (2005). Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. Journal of Neuroscience, 25, 7780–7791. 10.1523/Jneurosci.0762-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, & McMahon LL (2009). Estradiol and the relationship between dendritic spines, NR2B containing NMDA receptors, and the magnitude of long-term potentiation at hippocampal CA3-CA1 synapses. Psychoneuroendocrinology, 34(Suppl. 1), S130–S142. 10.1016/j.psyneuen.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Blasberg ME, Langan CJ, & Clark AS (1997). Stability of spatial working memory across the estrous cycle of Long-Evans rats. Neurobiology of Learning and Memory, 67, 167–171. 10.1006/nlme.1996.3753 [DOI] [PubMed] [Google Scholar]
- Staubli U, & Scafidi J (1999). Time-dependent reversal of long-term potentiation in area CA1 of the freely moving rat induced by theta pulse stimulation. Journal of Neuroscience, 19, 8712–8719. 10.1523/JNEUROSCI.19-19-08712.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Thibault O, DiLorenzo M, & Lynch G (1989). Antagonism of NMDA receptors impairs acquisition but not retention of olfactory memory. Behavioral Neuroscience, 103, 54–60. 10.1037/0735-7044.103.1.54 [DOI] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, & Wood MA (2009). Modulation of long-term memory for object recognition via HDAC inhibition. Proceedings of the National Academy of Sciences of the United States of America, 106, 9447–9452. 10.1073/pnas.0903964106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain RA, & Thompson RF (1993). In search of engrams. Annals of the New York Academy of Sciences, 702, 27–39. 10.1111/j.1749-6632.1993.tbl7240.x [DOI] [PubMed] [Google Scholar]
- Tabatadze N, Sato SM, & Woolley CS (2014). Quantitative analysis of long-form aromatase mRNA in the male and female rat brain. PLoS ONE, 9, e100628. 10.1371/journal.pone.0100628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, & Sokabe M (2012). Continuous de novo synthesis of neurosteroids is required for normal synaptic transmission and plasticity in the dentate gyrus of the rat hippocampus. Neuropharmacology, 62, 2373–2387. 10.1016/j.neuropharm.2012.02.007 [DOI] [PubMed] [Google Scholar]
- Tonegawa S, Pignatelli M, Roy DS, & Ryan TJ (2015). Memory engram storage and retrieval. Current Opinion in Neurobiology, 35, 101–109. 10.1016/j.conb.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Tulving E (1972). Episodic and semantic memory. In Tulving E & Donaldson W (Eds.), Organization of memory (pp. 381–403). Academic Press. [Google Scholar]
- van Groen T, Miettinen P, & Kadish I (2003). The entorhinal cortex of the mouse: Organization of the projection to the hippocampal formation. Hippocampus, 13, 133–149. 10.1002/hipo.10037 [DOI] [PubMed] [Google Scholar]
- Velisek L, & Vathy I (2005). Mifepristone (RU486) inhibits lateral perforant path long-term potentiation in hippocampal slices from prenatally morphine-exposed female rats. International Journal of Developmental Neuroscience, 23, 559–565. 10.1016/j.ijdevneu.2005.08.001 [DOI] [PubMed] [Google Scholar]
- Vierk R, Glassmeier G, Zhou L, Brandt N, Fester L, Dudzinski D, Wilkars W, Bender RA, Lewerenz M, Gloger S, Graser L, Schwarz J, & Rune GM (2012). Aromatase inhibition abolishes LTP generation in female but not in male mice. Journal of Neuroscience, 32, 8116–8126. 10.1523/JNEUROSCI.5319-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, Johnson HL, & Williams MT (2008). Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: Effects of dose and rearing conditions. International Journal of Developmental Neuroscience, 26, 599–610. 10.1016/j.ijdevneu.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, & Williams MT (2014). Assessing spatial learning and memory in rodents. ILAR Journal, 55, 310–332. 10.1093/ilar/ilu013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Cox BM, Jia Y, Le AA, Cox CD, Jung KM, Hou B, Piomelli D, Gall CM, & Lynch G (2018). Treating a novel plasticity defect rescues episodic memory in Fragile X model mice. Molecular Psychiatry, 23, 1798–1806. 10.1038/mp.2017.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Jia Y, Pham DT, Palmer LC, Jung KM, Cox CD, Rumbaugh G, Piomelli D, Gall CM, & Lynch G (2018). Atypical endocannabinoid signaling initiates a new form of memory-related plasticity at a cortical input to hippocampus. Cerebral Cortex, 28, 2253–2266. 10.1093/cercor/bhxl26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Kantorovich S, Babayan AH, Hou B, Gall CM, & Lynch G (2016). Estrogen’s effects on excitatory synaptic transmission entail integrin and TrkB transactivation and depend upon ß1-integrin function. Neuropsychopharmacology, 41, 2723–2732. 10.1038/npp.2016.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Le AA, Hou B, Lauterborn JC, Cox CD, Levin ER, Lynch G, & Gall CM (2018). Memory-related synaptic plasticity is sexually dimorphic in rodent hippocampus. Journal of Neuroscience, 38, 7935–7951. 10.1523/JNEUROSCI.0801-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Trieu BH, Palmer LC, Jia Y, Pham DT, Jung KM, Karsten CA, Merrill CB, Mackie K, Gall CM, Piomelli D, & Lynch G (2016). A primary cortical input to hippocampus expresses a pathway-specific and endocannabinoid-dependent form of long-term potentiation. eNeuro, 3(4), ENEUR0.0160-16.2016. 10.1523/ENEURO.0160-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, & Greenough WT (1995). LTP varies across the estrous cycle: Enhanced synaptic plasticity in proestrus rats. Brain Research, 703, 26–30. 10.1016/0006-8993(95)01059-9 [DOI] [PubMed] [Google Scholar]
- Warren SG, & Juraska JM (1997). Spatial and nonspatial learning across the rat estrous cycle. Behavioral Neuroscience, 111, 259–266. 10.1037/0735-7044.lll.2.259 [DOI] [PubMed] [Google Scholar]
- Weilinger NL, Lohman AW, Rakai BD, Ma EM, Bialecki J, Maslieieva V, Rilea T, Bandet MV, Ikuta NT, Scott L, Colicos MA, Teskey GC, Winship IR, & Thompson RJ (2016). Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nature Neuroscience, 19, 432–442. 10.1038/nn.4236 [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, & Bear MF (2006). Learning induces long-term potentiation in the hippocampus. Science, 313, 1093–1097. 10.1126/science.1128134 [DOI] [PubMed] [Google Scholar]
- Wierenga LM, Bos MGN, van Rossenberg F, & Crone EA (2019). Sex effects on development of brain structure and executive functions: Greater variance than mean effects. Journal of Cognitive Neuroscience, 31, 730–753. 10.1162/jocn_a_01375 [DOI] [PubMed] [Google Scholar]
- Witter MP (1993). Organization of the entorhinal—Hippocampal system: A review of current anatomical data. Hippocampus, 3(Suppl. 1), 33–44. [PubMed] [Google Scholar]
- Wong M, & Moss RL (1991). Electrophysiological evidence for a rapid membrane action of the gonadal steroid, 17 beta-estradiol, on CA1 pyramidal neurons of the rat hippocampus. Brain Research, 543,148–152. 10.1016/0006-8993(91)91057-8 [DOI] [PubMed] [Google Scholar]
- Wong M, & Moss RL (1992). Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. Journal of Neuroscience, 12, 3217–3225. 10.1523/JNEUROSCI.12-08-03217.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, & McEwen BS (1990). Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. Journal of Neuroscience, 10, 4035–4039. 10.1523/JNEUROSCI.10-12-04035.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, & McEwen BS (1994). Estradiol regulates hippocampal dendritic spine density via an N-methyl-d-aspartate receptor-dependent mechanism. Journal of Neuroscience, 14, 7680–7687. 10.1523/JNEUROSCI.14-12-07680.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, & Schwartzkroin PA (1997). Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: Correlation with dendritic spine density. Journal of Neuroscience, 17, 1848–1859. 10.1523/JNEUROSCI.17-05-01848.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang XB, Frerking M, & Zhou Q (2008). Spine expansion and stabilizaiton associated with long-term potentiation. Journal of Neuroscience, 28, 5740–5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngjohn JR, Larrabee GJ, & Crook TH (1991). First-last names and the grocery list selective reminding test: Two computerized measures of everyday verbal learning. Archives of Clinical Neuropsychology, 6, 287–300. [PubMed] [Google Scholar]
- Zadran S, Qin Q, Bi X, Zadran H, Kim Y, Foy MR, Thompson R, & Baudry M (2009). 17-Beta-estradiol increases neuronal excitability through MAP kinase-induced calpain activation. Proceedings of the National Academy of Sciences of the United States of America, 106, 21936–21941. [DOI] [PMC free article] [PubMed] [Google Scholar]


