Summary:
In this report, we present a 57-year-old man with chronic bilateral lower extremity wounds from nonuremic calciphylaxis, which were successfully reconstructed using a piscine-derived acellular dermal matrix. The acellular dermal matrix incorporated quickly, providing a wound bed that was amenable to skin grafting. We demonstrate that this is an effective off-the-shelf solution for these chronic wounds, resulting in pain reduction and complete closure of the wounds, allowing the patient to return to his previous baseline activities, and improving his quality of life.
Cutaneous calciphylaxis is a debilitating condition caused by calcification of cutaneous blood vessels within the dermis and subcutis, leading to progressive narrowing and eventual occlusion of the vessel lumen and ischemic infarction of supplied tissue.1 Clinical presentation includes livedo reticularis, plaques, nodules, and ulceration.1
Treatment is targeted toward preventing calcification, decalcifying affected vessels, pain management, and wound care.1 Preventing further calcification requires correcting metabolic derangements when present.1,2
Although correcting the underlying problem is essential, this does not address the wounds. Infected or highly exudative wounds are typically treated surgically, whereas dry, uninfected wounds are treated enzymatically.2,3 Aggressive debridement has been associated with worsening ulceration.3 Once the underlying disease is controlled, successful cases have described debridement of deep ulcers, followed by skin grafting.4
Kerecis omega-3 (Kerecis, Isafjordur, Iceland) is a cod-derived acellular dermal matrix (ADM). It has grown in popularity in wounds secondary to diabetes, vascular insufficiency, and burns.5–8 There are also reports of its use in mucogingival procedures and in a critical upper extremity wound.9,10 In vivo, this ADM promotes angiogenesis and collagen synthesis, functioning as an antiinflammatory agent that increases the speed of wound healing.11 There is a paucity of literature regarding the use of this ADM in patients with cutaneous calcific lesions, with a single report of iatrogenic neonatal calcinosis cutis.12 In this report, we describe the use of Kerecis in reconstruction of bilateral lower extremity wounds in a patient with nonuremic calciphylaxis.
CASE DESCRIPTION
An otherwise healthy 57-year-old man presented with bilateral lower extremity ulcerations. He had an extensive workup before presentation, including evaluations by dermatology, nephrology, and rheumatology. He was initially thought to have pyoderma gangrenosum and was started on prednisone. Doppler ultrasonography of his lower extremities ruled out venous insufficiency. Lower extremity imaging demonstrated superficial subcutaneous vascular calcification, consistent with dermatopathological evaluation of tissue samples from his wounds, demonstrating idiopathic nonuremic calcinosis cutis.
The wounds grew larger after multiple bouts of cellulitis requiring inpatient antibiotics. Wound treatment included hyperbaric oxygen, silver sulfadiazine, and negative pressure wound therapy (NPWT).
After a year of local wound care, healing stagnated. His quality-of-life significantly deteriorated, with the exudative wounds becoming his main source of stress and pain, requiring consultation with pain management. The patient was not forced to retire due to the demands of wound care. He no longer enjoyed his hobbies and lost hope for any solution.
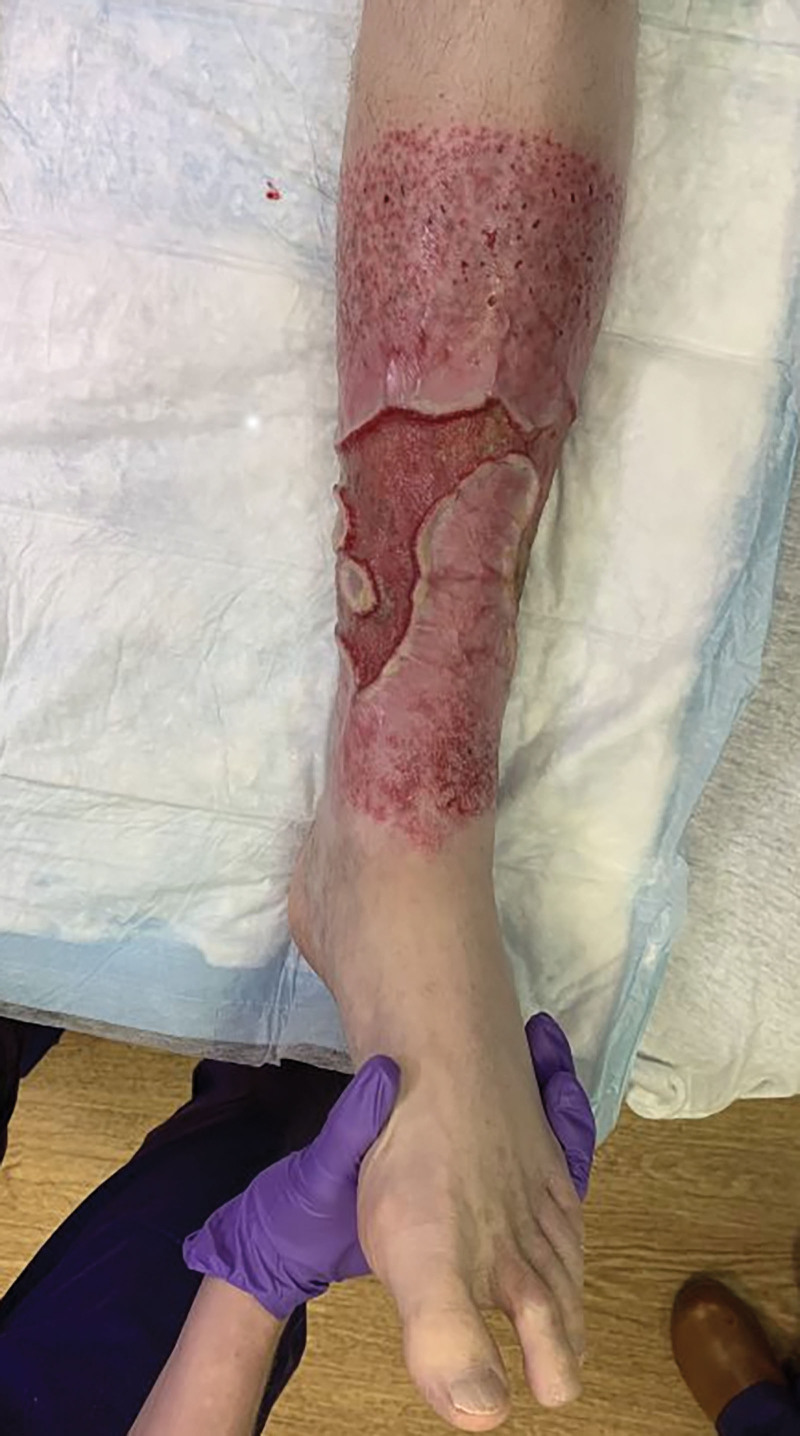
The patient had wounds on his bilateral legs, right measuring 100 cm2 and left with two wounds measuring 96 and 120 cm2 (Fig. 1). (See figure 1, Supplemental Digital Content 1, which shows a preoperative photograph of the posterior right lower extremity. http://links.lww.com/PRSGO/C662.) Wounds were to the deep dermis without muscle or critical structure exposure. There were no signs of infection. His pain was at a six on the visual analog pain scale. We decided to reconstruct the wounds using a staged approach, given the chronicity of the wounds and the extensive workup consistent with idiopathic calcinosis cutis.
Fig. 1.
Preoperative photograph: anterior left lower extremity.
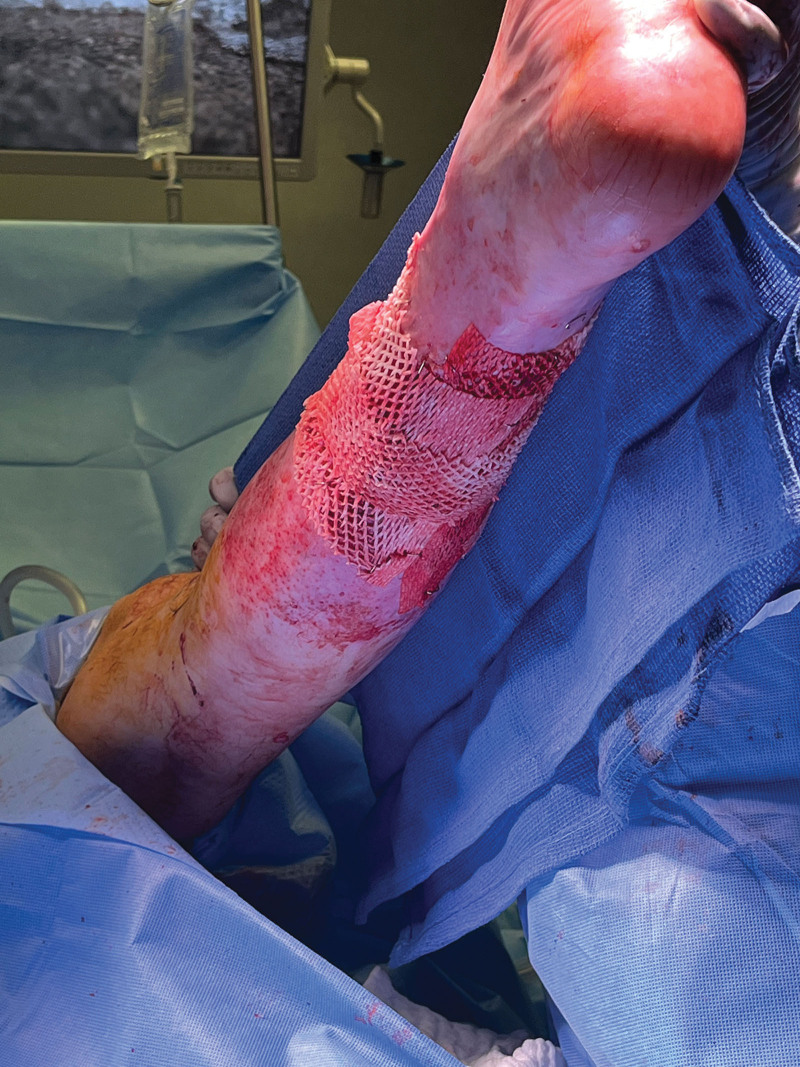
First, he underwent superficial debridement, and Kerecis was applied to the wounds (Fig. 2). NPWT was applied for 1 week, and subsequently changed every 3–5 days (standard at our institution). The wounds were reevaluated at each dressing change for granulation tissue formation.
Fig. 2.
Intraoperative view of the left lower extremity with Kerecis applied.
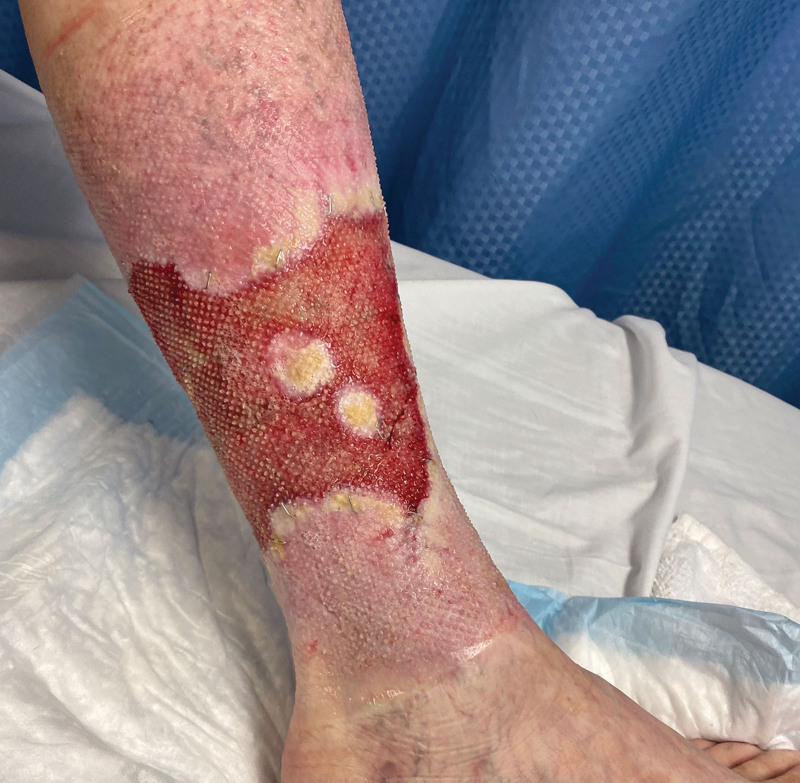
On postoperative day 20, the wound bases were noted to have healthy granulation tissue throughout (Fig. 3). The patient was then taken for second-stage reconstruction with split-thickness skin grafting to resurface the wounds.
Fig. 3.
Left lower extremity wound bed before definitive skin grafting, postoperative day 20 after Kerecis placement.
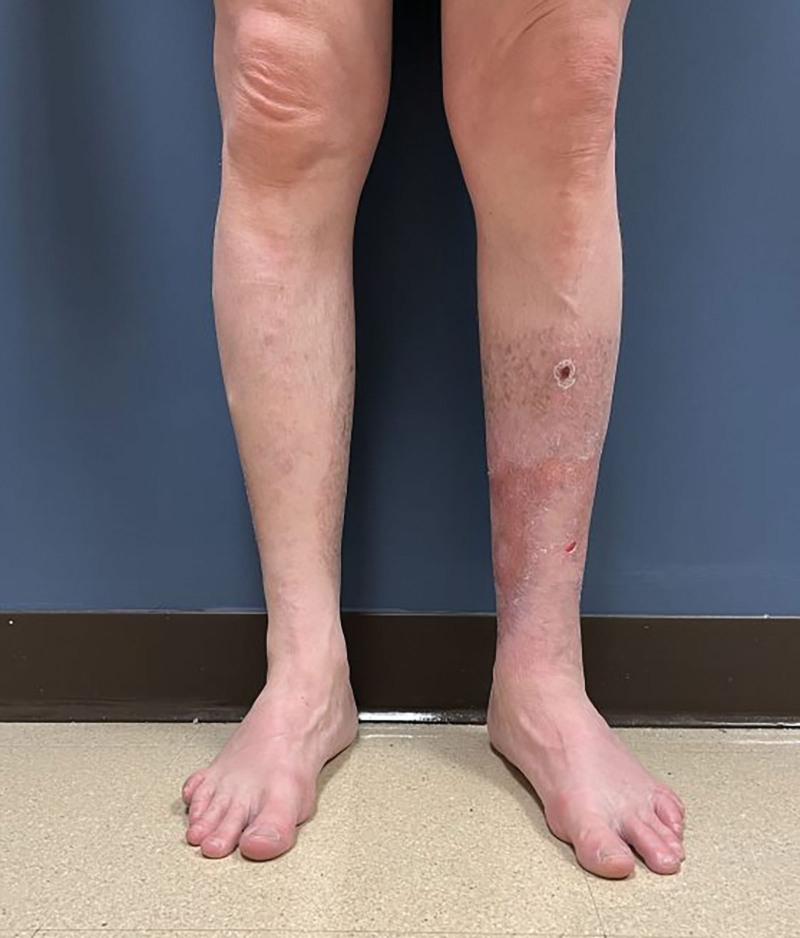
Two months after grafting, the patient’s wounds had complete, stable epithelialization. His pain was significantly improved, recorded as a two on the visual analog pain scale, down from six. The patient was participating in his hobbies, and his quality of life had improved significantly when compared with initial presentation. At 1-year postoperative, he had stable, pain-free coverage of his legs (Fig. 4). (See figure 2, Supplemental Digital Content 2, which shows a 1-year postoperative photograph of the posterior view of bilateral lower extremities. http://links.lww.com/PRSGO/C663.)
Fig. 4.
One-year postoperative photograph: anterior view of bilateral lower extremities.
DISCUSSION
Calciphylaxis is a systemic disease process seen in patients with end-stage renal disease, disequilibrium of calcium and phosphate levels, or vitamin K deficiency.1 Nonulcerating cutaneous calciphylaxis is often representative of early disease progression, whereas ulcerating lesions indicate more severe disease and are associated with high mortality.13
When addressing these wounds, treating the underlying metabolic derangement is essential. A multidisciplinary approach should be used, with the goal of preventing progression of vascular calcification, decalcifying affected vessels, preventing infection, and limiting morbidity. Therefore, it necessitates involvement of various specialties, including nephrology, dermatology, plastic surgery, and nutrition.2
Wound management includes necrotic tissue removal, infection prevention, and wound healing promotion.1 Surgical and nonsurgical approaches have been used to debride these wounds, although concerns about further wound complications (ie, pathergy) have been described.14 This underscores the importance of two key points. First, the underlying condition must be addressed. Second, the wounds must be stable and/or healing. Nonsurgical treatments described include maggot therapy, NPWT, and hyperbaric oxygen.2,15–19
When considering ADMs, it is essential to choose the product that best aligns with the goals of the reconstruction.10 Kerecis has become a useful tool. Unlike bovine and porcine products, there are no known pathogens that are transferred between North Atlantic cod and humans.20 This allows for a gentler processing of the fish skin so it retains more of the native structure and components, including omega-3s.21,22 These properties allow for rapid incorporation and granulation tissue formation, which allowed us to achieve definitive closure of these chronic, painful wounds. Previous studies have evaluated the structure of Kerecis under electron microscopy, demonstrating less unintentional cross-linking of the ADM when compared with human amnion/chorionic membrane allograft, allowing for easier migration of the native fibroblasts through the structure.23 Additionally, Kerecis is less susceptible to infection compared with human amnion or chorion.24
CONCLUSIONS
In this case report, we describe the successful application of Kerecis to help convert wounds associated with idiopathic nonuremic cutaneous calciphylaxis into wound beds amenable to skin grafting. Three months after the initial presentation, our patient reported improvements in pain, cosmesis, and quality of life. As our experience with Kerecis grows and the body of literature expands, we will have a much better understanding of the limitations and indications of this ADM. In the interim, we have included this in our treatment algorithm as a simple solution for many different problems.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
ACKNOWLEDGMENT
This study was carried out under the supervision of our institutional review board.
Supplementary Material
Footnotes
Published online 12 July 2023.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205–215. [DOI] [PubMed] [Google Scholar]
- 2.Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin R. Mysterious calciphylaxis: wounds with eschar—to debride or not to debride? Ostomy Wound Manage. 2004;50:64–66, 68–70; discussion 71. [PubMed] [Google Scholar]
- 4.Wollina U, Helm C, Hansel G, et al. Deep ulcer shaving combined with split-skin transplantation in distal calciphylaxis. Int J Low Extrem Wounds. 2008;7:102–107. [DOI] [PubMed] [Google Scholar]
- 5.Lullove EJ, Liden B, Winters C, et al. A multicenter, blinded, randomized controlled clinical trial evaluating the effect of omega-3–rich fish skin in the treatment of chronic, nonresponsive diabetic foot ulcers. Wounds. 2021;33:169–177. [DOI] [PubMed] [Google Scholar]
- 6.Michael S, Winters C, Khan M. Acellular fish skin graft use for diabetic lower extremitywound healing: a retrospective study of 58 ulcerations and a literature review. Wounds. 2019;31:262–268. [PubMed] [Google Scholar]
- 7.Woodrow T, Chant T, Chant H. Treatment of diabetic foot wounds with acellular fish skin graft rich in omega-3: a prospective evaluation. J Wound Care. 2019;28:76–80. [DOI] [PubMed] [Google Scholar]
- 8.Stone R, II, Saathoff EC, Larson DA, et al. Accelerated wound closure of deep partial thickness burns with acellular fish skin graft. Int J Mol Sci. 2021;22:1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dragan IF, Garcia H AA, Malik R, et al. One-year outcomes of a piscine soft tissue alternative used in mucogingival procedures: a clinical case series. Int J Periodontics Restorative Dent. 2020;40:603–609. [DOI] [PubMed] [Google Scholar]
- 10.Shahriari SRK, Ederle AC, Whisonant C, et al. Successful upper extremity limb salvage using cellular- and tissue-based products in a patient with uncontrolled diabetes. Wounds. 2022;34:e104–e107. [PubMed] [Google Scholar]
- 11.Chen H, Yin B, Hu B, et al. Acellular fish skin enhances wound healing by promoting angiogenesis and collagen deposition. Biomed Mater. 2021;16:045011. [DOI] [PubMed] [Google Scholar]
- 12.Ahn KH, Park ES. A rare case report of neonatal calcinosis cutis induced by distant and delayed extravasation of intravenous calcium gluconate. Arch Plast Surg. 2021;48:641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210–2217. [DOI] [PubMed] [Google Scholar]
- 14.Sato T, Ichioka S. How should we manage multiple skin ulcers associated with calciphylaxis? J Dermatol. 2012;39:966–968. [DOI] [PubMed] [Google Scholar]
- 15.Picazo M, Bover J, de la Fuente J, et al. Larvas estériles como coadyuvantes al tratamiento local en una paciente con calcifilaxis proximal [Sterile maggots as adjuvant procedure for local treatment in a patient with proximal calciphylaxis]. Nefrologia. 2005;25:559–562. [In Spanish.] [PubMed] [Google Scholar]
- 16.Tittelbach J, Graefe T, Wollina U. Painful ulcers in calciphylaxis—combined treatment with maggot therapy and oral pentoxyfillin. J Dermatolog Treat. 2001;12:211–214. [DOI] [PubMed] [Google Scholar]
- 17.Basile C, Montanaro A, Masi M, et al. Hyperbaric oxygen therapy for calcific uremic arteriolopathy: a case series. J Nephrol. 2002;15:676–680. [PubMed] [Google Scholar]
- 18.Deng Y, Xie G, Li C, et al. Calcific uremic arteriolopathy ameliorated by hyperbaric oxygen therapy in high-altitude area. Ren Fail. 2014;36:1139–1141. [DOI] [PubMed] [Google Scholar]
- 19.Podymow T, Wherrett C, Burns KD. Hyperbaric oxygen in the treatment of calciphylaxis: a case series. Nephrol Dial Transplant. 2001;16:2176–2180. [DOI] [PubMed] [Google Scholar]
- 20.Baldursson BT, Kjartansson H, Konradsdottir F, et al. Healing rate and autoimmune safety of full-thickness wounds treated with fish skin acellular dermal matrix versus porcine small-intestine submucosa: a noninferiority study. Int J Low Extrem Wounds. 2015;14:37–43. [DOI] [PubMed] [Google Scholar]
- 21.Da LC, Huang YZ, Xie HQ, et al. Membranous extracellular matrix-based scaffolds for skin wound healing. Pharmaceutics. 2021;13:1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holl J, Kowalewski C, Zimek Z, et al. Chronic diabetic wounds and their treatment with skin substitutes. Cells. 2021;10:655..I [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorweiler B, Trinh TT, Dünschede F, et al. The marine Omega3 wound matrix for treatment of complicated wounds: a multicenter experience report. Gefasschirurgie. 2018;23:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magnusson S, Baldursson BT, Kjartansson H, et al. Regenerative and antibacterial properties of acellular fish skin grafts and human amnion/chorion membrane: implications for tissue preservation in combat casualty care. Mil Med. 2017;182:383–388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.