Abstract
Background
Treatment targets of ulcerative colitis (UC) have evolved to include not only endoscopic but also histologic remission. However, the concept of histological activity is still in its early days. We aimed to capture the attitudes toward UC histology and the uptake of standardized reporting of endoscopy and histology of UC in daily practice.
Methods
We conducted a cross‐sectional survey of physicians involved in the care of inflammatory bowel disease worldwide. The survey included 21 questions divided into three sections. The first recorded demographics, specialty, and level of experience of participants; the second covered clinical practices and attitudes toward the use and reporting of endoscopy; and the third covered histology.
Results
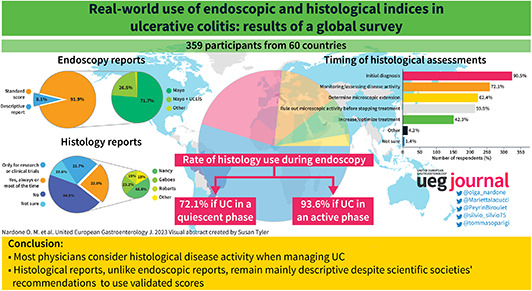
In total, 359 participants from 60 countries and all levels of experience completed the survey. UC histology was used by nearly all respondents (90.5%) for initial diagnosis, by 72% to monitor disease course, by 62.4% to determine the microscopic extension, by 59.9% to confirm deep remission when considering to stop treatment, and 42.3% to increase/optimize treatment. Nevertheless 77.2% of participants reported that no standard histological index was available in their daily practice. Instead, endoscopy reports included the Mayo Endoscopic score in 90% of cases. The majority of respondents welcomed as useful or very useful an artificial intelligence system to automate scoring of endoscopy (69%) or histology (73%).
Conclusion
UC histology reports are less standard than endoscopy reports, although most physicians consider histological activity useful when managing UC and would welcome artificial intelligence systems to automate endoscopic and histological scoring.
Keywords: artificial intelligence, deep remission, endoscopy, histological healing, histological index, histology, mucosal healing, reporting, UC, ulcerative colitis
This survey shows that most physicians consider histological disease activity when managing ulcerative colitis. Nevertheless, histological reports, unlike endoscopic reports, remain mainly descriptive despite recommendations from scientific societies to use validated scores. This is the first estimate of real‐world use of standard reporting in ulcerative colitis.
Key summary.
Summarise the established knowledge on this subject
The standardized assessment of endoscopic and histologic activity of ulcerative colitis (UC) is crucial to manage the disease.
What are the significant and/or new findings of this study?
Despite recommendations, the use of standard validated indices to grade disease severity is modest in histology unlike in endoscopy.
This study can help raise awareness of the need for homogeneity in the histological assessment of UC and suggests ways to achieve it, such as simplified indices and dedicated training.
INTRODUCTION
In the last few years, the targets of treatment in ulcerative colitis (UC) have expanded from symptoms' control and endoscopic remission to include microscopic remission. The absence of inflammation at the histological level, referred to as “histologic remission,” is associated with additional improvement in clinical outcomes, 1 , 2 , 3 and is considered a desirable target of treatment. 4 Furthermore, the combination of endoscopic and histological remission, also called histo‐endoscopic mucosal healing, 5 has become an endpoint of interest in clinical trials starting with the ustekinumab phase 3 trial in UC. 6
However, despite the proven relevance of microscopic disease activity, its assessment remains problematic. Descriptive reports are difficult to compare and lack reproducibility and grading. To overcome these limitations, various scores have been proposed but none have emerged as the de facto standard, as the Mayo Endoscopic Score did in endoscopy. In real‐world practice, the uptake of validated histological indices is thought to be modest, but there is no published estimate of it. To fill this gap and capture differences in practice and attitudes toward endoscopy and histology in UC, we conducted an international survey of physicians.
METHODS
We designed a single‐stage cross‐sectional survey targeted at clinicians involved in the care of inflammatory bowel disease (IBD) worldwide. From November 2022 to January 2023, participants were invited to take a survey hosted on an online platform. The link was sent via email through the mailing lists of IBD‐scope, a webinar platform designed for healthcare professionals interested in IBD, and by personal invitation of physicians with a focus on IBD. Screening questions were included at the beginning of the survey to filter respondents out of the target population and email registration was used to prevent double participation. Responses were collected anonymously and permission for data collection was asked upon survey start. The survey and the invitation email were in English, and responses did not require active writing but only choice selection. The questionnaire comprised 21 questions divided into three parts: the first recorded demographics, specialty, and level of experience of participants (6 questions); the second clinical practices and attitudes toward the use and reporting of endoscopy (8 questions); and the third histology (7 questions). Only demographic questions were mandatory, and the number of respondents was reported for each question to account for missing data. The full questionnaire is available in the Supporting Information S1.
The study was conducted and reported in compliance with the Consensus for Reporting of Survey Studies (CROSS) guidelines; 7 the CROSS checklist is available in Supporting Information S2.
Excel (v16.71, Microsoft) was used to perform descriptive statistics and plot the charts.
RESULTS
Participants
In total, 359 physicians from 60 countries across all continents (54.4% Europe, 24.4% Asia, 12.9% South America, 3.7% North America, 3.2% Africa, and 1.4% Oceania) responded; 87% were gastroenterologists, 7% surgeons, the remaining pediatricians (7/359), internal medicine specialists (5/359), and pathologists (2/359). The majority practiced in academic hospitals (54.5%) or tertiary centers (22.1%), and only around one fifth worked in secondary hospitals (12.8%) or local practices (8.1%). In terms of center size, approximately one third (32.9%) worked in hospitals caring for more than 500 patients with UC per year, 39.3% in institutions caring for 100–500 patients, and 27.9% in smaller centers with up to 100 patients. The average experience was 16.3 years (SD 9.7). More than 87% personally performed endoscopy (Figure 1).
FIGURE 1.
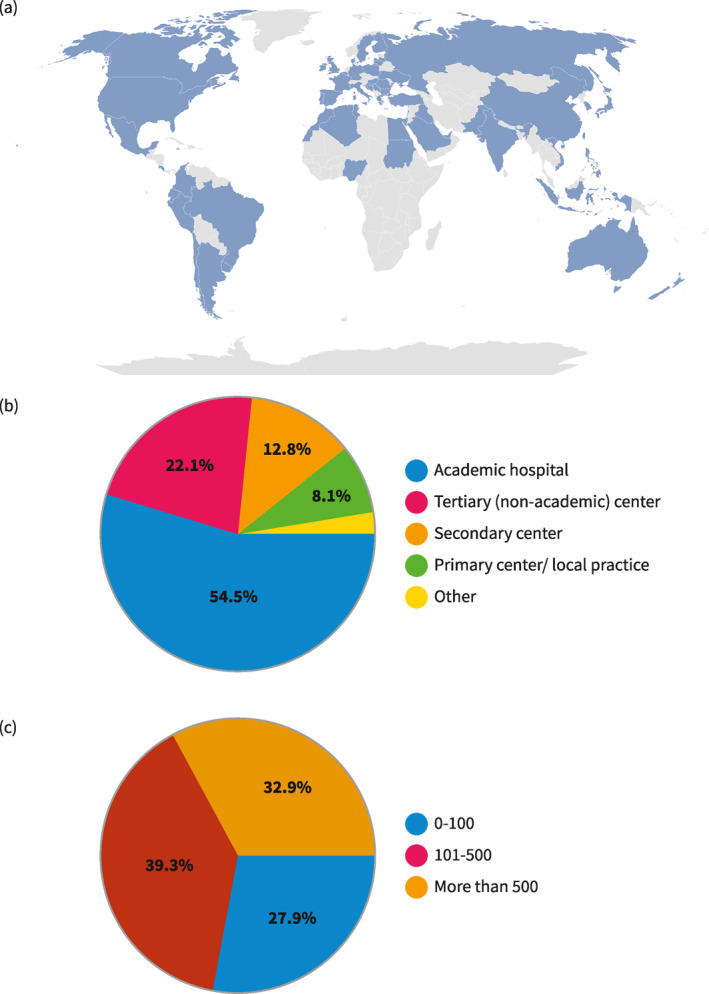
Characteristics of respondents. (a) Geographic representation of survey respondents. Countries in darker blue contributed to more respondents. (b) Place of practice of respondents. (c) Number of patients with ulcerative colitis cared per year in participant's center.
Endoscopy
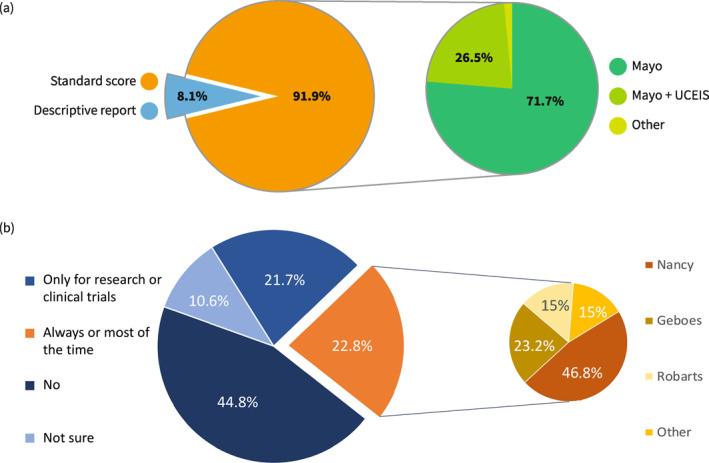
When performing endoscopy in quiescent UC, almost three quarters (72.1%) of respondents reported taking biopsies to assess microscopic activity, while 16.8% only for dysplasia detection, and 10.4% just in case of suspicious lesions; the average number of biopsies was 10.8 (SD 7.34, range 0–36). In the case of active disease, the percentage of operators collecting biopsies increased to 93.6%. Questions on the use of enhanced endoscopy for surveillance provided a mixed picture: 39% used mainly virtual chromoendoscopy (VCE), 12.8% mainly dye‐spray chromoendoscopy (DCE), 21.7% high definition white light, and 21.4% both VCE and DCE. Endoscopy reports included a validated score in 91.9% of cases. Mayo was nearly always used (90%), with a large difference from the second most common score Ulcerative Colitis Endoscopic Index of Severity (UCEIS) (26.5%), while other indices accounted for only 1.7%. Of note, except for 1 respondent, all scores different from Mayo were used in addition to, and not instead of, Mayo. When restricting the analysis to participants working in high‐volume centers (>500 UC patients cared for per year), all but one (117/118) reported using the Mayo score to grade endoscopic activity, and 32% (38/117) used other scores in addition to Mayo, mainly UCEIS (Figure 2).
FIGURE 2.
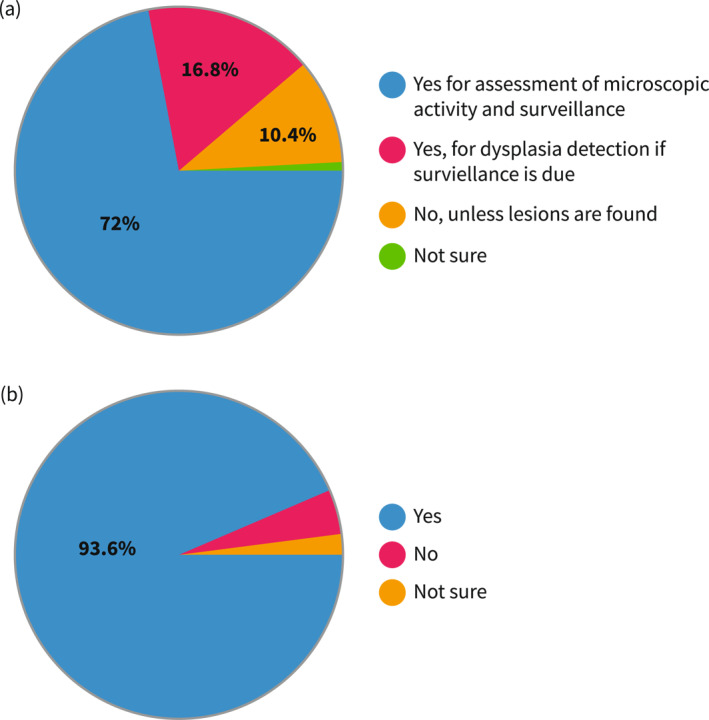
Approaches to biopsy‐taking. (a) Percentages of respondents who usually take (or work in a center where it is usual practice to take) biopsies in endoscopically quiescent UC (Mayo 0–1). (b) Percentages of respondents who usually take (or work in a center where it is usual practice to take) biopsies in endoscopically active UC (Mayo 2–3). UC, ulcerative colitis.
Histology
Centers represented in the survey were roughly equally split between those with a pathologist dedicated to IBD (46.2%) and those without (51%). To measure the UC activity 44.8% of respondents indicated that in their center standard indices were not used, 21.7% reported scores were reported only in selected cases such as clinical trials or research, and 22.8% said scores were used routinely. Regular use of histological score was modestly higher among respondents working in high‐volume centers 28% (33/118) and respondents working in a center with an IBD‐dedicated pathologist 35.1% (58/165) (Figure 3).
FIGURE 3.
Use of validated scores for ulcerative colitis endoscopy (a) and histology (b).
Among those who used (or worked in a center that used) standard scores (160/357) the choice of which index was mixed: 64.4% (103/160) used Nancy Histological Index (NHI), followed by Geboes 31.9% (51/160), and Robarts Histological Index (RHI) 20.6% (33/160), a minority applied other scores such as IBD distribution chronicity index (12/160), Riley (5/160), modified Riley (5/160), Extent, Chronicity, Activity Plus additional findings System (5/160), and others (6/160). Similarly to endoscopy, multiple indices could be selected at the same time (Figure 3).
UC histology was used by nearly all respondents (90.5%) for initial diagnosis. But more interestingly, in 72% of cases to monitor and assess the clinical activity, in 62.4% to determine the microscopic extension, in 59.9% to confirm deep remission when considering whether to stop treatment, and 42.3% to increase/optimize treatment (Figure 4).
FIGURE 4.
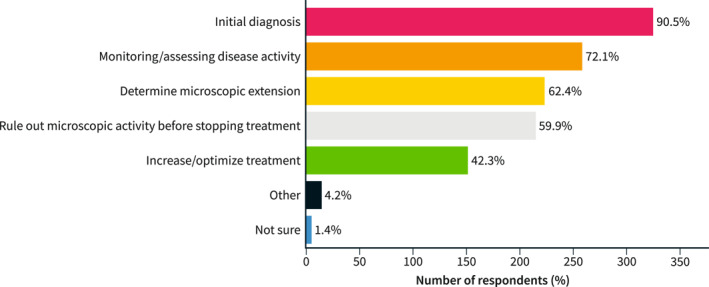
Reasons for using histology in patients with ulcerative colitis.
The disconnect between the recommendation to use validated scores and routine practice is further exemplified by the low proportion of respondents who feel very familiar (0.3%) or at least somewhat familiar (9.3%) with the cutoffs of histological scores.
Finally, two questions investigated the perceived usefulness of a hypothetical artificial intelligence system to automate the scoring of endoscopy and histology. Results were broadly similar with the majority of respondents saying that it would be useful or very useful, 69% (33% and 36%) for endoscopy and 73% (34% and 39%) for histology.
DISCUSSION
In recent years, there has been a growing effort to standardize reporting of endoscopy and histology in IBD with the goal of improving the quality of care and facilitating communication and interdisciplinary management among healthcare professionals. 8 However, the uptake of these recommendations in real‐world practice is yet to be proven.
The present survey evaluated physicians' use of and familiarity with standardized reporting of endoscopy and histology in UC. The large number of respondents and the broad geographic representation support the generalizability of our findings, which, to the best of our knowledge, represent the first estimate of the use of histologic scoring in UC worldwide.
Our results confirm once again that the Mayo Endoscopic Score, albeit imperfect, has become the de facto standard for endoscopic scoring. The situation with histology is far more complicated. As the survey clearly shows, more than half of the physicians use histological information proactively in their clinical decisions, whilst the adoption of indices remains modest and, even when they are used, their variety limits generalization. The disconnect between the recognized importance of histology and the low uptake of standard measures is a gap that needs to be addressed.
We hypothesize different reasons for this. First, historically the role of histology in UC has been mainly focused on diagnosis, exclusion of viral coinfections (i.e., cytomegalovirus or CMV) and assessment of possible dysplasia. The clinical relevance of subtle microscopic inflammation and its association to an increased risk of adverse outcomes compared to endoscopic remission, although described earlier, 9 , 10 has gained ground only in recent years and so has the effort to standardize it. Second, most histological indices require time and some degree of training and are difficult to remember. To date, the vast majority of UC histology reports remain only descriptive, while validated scores are limited to selected settings such as clinical trials or tertiary academic centers.
Scientific societies are stepping up their efforts to provide common guidance. In a recent position paper, the European Crohn and Colitis Organisation suggested the use of RHI for randomized control trials in UC and NHI for both clinical practice and trials. 11 A panel of international experts developed similar recommendations for the use of histopathology in clinical trials of UC. 12 Nevertheless, our survey shows that such recommendations have yet to be translated into clinical practice and the gap between theory and practice remains wide.
The overall picture of UC care emerging from this survey is inevitably affected by the high proportion (54.5%) of respondents from academic centers, which likely leads to an overestimation of all quality measures. However, this possible bias only reinforces our initial point that we need to address the gap between recommendations and practice since the uptake of recommendations is lower in smaller institutions. Another limitation is the availability of the survey solely in English, which might have introduced bias in regions' representation, or skewed participation in favor of academic centers. Some geographic areas, notably North America, and some professionals, particularly pathologists (0.6%), were underrepresented probably due to the mailing list reach; unfortunately, an accurate sampling of medical professionals is extremely difficult to obtain.
Practical obstacles represent a major barrier to the widespread adoption of indices; at the same time, scientific societies agree that the minimum requirement for histological remission is the absence of neutrophils. 11 We previously proposed a simplified index, PICaSSO Histological Remission Index, considering only the presence or absence of neutrophils, 13 this approach could simplify measurement and help standardize reporting without sacrificing prognostic value. Moreover, simplification of indices paves the way for the development of artificial intelligence modules to automate the process. 13 , 14
In conclusion, UC microscopic activity is widely recognized as a treatment target and thus used for clinical management by most physicians; however, its reporting remains largely descriptive and nonstandard. On the contrary, endoscopic severity is almost ubiquitously reported with the Mayo endoscopic score. Scientific societies should continue along the path of a greater harmonization of reports addressing specifically the practical barriers that remain, for instance, by recommending greater recourse to multidisciplinary meetings with pathologists, endoscopists, and clinical gastroenterologists to improve convergence and endorsing the use of biopsies for routine monitoring of UC activity.
AUTHOR CONTRIBUTIONS
Olga Maria Nardone and Tommaso Lorenzo Parigi: conceptualization, questionnaire drafting, data curation, manuscript writing. Marietta Iacucci: project supervision, manuscript reviewing for important intellectual content. Vincenzo Villanacci, Laurent Peyrin‐Biroulet, Subrata Ghosh: manuscript reviewing. Silvio Danese: survey invitation, project supervision, manuscript review for important intellectual content.
CONFLICT OF INTEREST STATEMENT
OMN has served as a speaker for Janssen, Ferring, Takeda, Pfizer, and Fresenius Kabi. MI has received research grants and equipment loans from Pentax USA, Olympus, and Fujifilm and is partially funded by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. LPB reports personal fees from Galapagos, AbbVie, Janssen, Genentech, Ferring, Tillots, Celltrion, Takeda, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Inotrem, Al‐lergan, MSD, Roche, Arena, Gilead, Amgen, BMS, Vifor, Norgine, Mylan, Lilly, Fresenius Kabi, OSE Immunotherapeutics, Enthera, Theravance, Pandion Therapeutics, Gossamer Bio, Viatris, Thermo Fisher; grants from Abbvie, MSD, Takeda, Fresenius Kabi; stock options: CTMA. SG has served as consultant, Steering committee member, Advisory Board Member and Drug Monitoring committee member for Janssen, Pfizer, Takeda, Abbvie, BMS, Gilead, Galapagos, Celltrion, Eli Lilly, Ferring. SD has served as a speaker, consultant and advisory board member for Schering Plough, Abbott (AbbVie) Laboratories, Merck and Co., UCB Pharma, Ferring, Cellerix, Millenium Takeda, Nycomed, Pharmacosmos, Actelion, Alfa Wasserman, Genentech, Grunenthal, Pfizer, AstraZeneca, Novo Nordisk, Vifor and Johnson and Johnson. The remaining authors declare no relevant conflicts of interest.
Supporting information
Supporting Information S1
Supporting Information S2
ACKNOWLEDGMENTS
None.
Open access funding provided by BIBLIOSAN.
Nardone OM, Iacucci M, Villanacci V, Peyrin‐Biroulet L, Ghosh S, Danese S, et al. Real‐world use of endoscopic and histological indices in ulcerative colitis: results of a global survey. United European Gastroenterol J. 2023;11(6):514–19. 10.1002/ueg2.12423
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request to the corresponding author.
REFERENCES
- 1. Bryant RV, Burger DC, Delo J, Walsh AJ, Thomas S, von Herbay A, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow‐up. Gut. 2016;65(3):408–14. 10.1136/gutjnl-2015-309598 [DOI] [PubMed] [Google Scholar]
- 2. Gupta A, Yu A, Peyrin‐Biroulet L, Ananthakrishnan AN. Treat to target: the role of histologic healing in inflammatory bowel diseases: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2021;19(9):1800–13.e4. 10.1016/j.cgh.2020.09.046 [DOI] [PubMed] [Google Scholar]
- 3. Yoon H, Jangi S, Dulai PS, Boland BS, Prokop LJ, Jairath V, et al. Incremental benefit of achieving endoscopic and histologic remission in patients with ulcerative colitis: a systematic review and meta‐analysis. Gastroenterology. 2020;159(4):1262–75.e7. 10.1053/j.gastro.2020.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turner D, Ricciuto A, Lewis A, D’Amico F, Dhaliwal J, Griffiths AM, et al. STRIDE‐II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat‐to‐target strategies in IBD. Gastroenterology. 2021;160(5):1570–83. 10.1053/j.gastro.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 5. Nardone OM, Bazarova A, Bhandari P, Cannatelli R, Daperno M, Ferraz J, et al. PICaSSO virtual electronic chromendoscopy accurately reflects combined endoscopic and histological assessment for prediction of clinical outcomes in ulcerative colitis. United Eur Gastroenterol J. 2022;10(2):147–59. 10.1002/ueg2.12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sands BE, Sandborn WJ, Panaccione R, O’Brien CD, Zhang H, Johanns J, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201–14. 10.1056/nejmoa1900750 [DOI] [PubMed] [Google Scholar]
- 7. Sharma A, Minh Duc NT, Luu Lam Thang T, Nam NH, Ng SJ, Abbas KS, et al. A consensus‐based checklist for reporting of survey studies (CROSS). J Gen Intern Med. 2021;36(10):3179–87. 10.1007/s11606-021-06737-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adamina M, Feakins R, Iacucci M, Spinelli A, Cannatelli R, D’Hoore A, et al. ECCO topical review optimising reporting in surgery, endoscopy, and histopathology. J Crohns Colitis. 2021;15(7):1089–105. 10.1093/ecco-jcc/jjab011 [DOI] [PubMed] [Google Scholar]
- 9. Wright R, Truelove SR. Serial rectal biopsy in ulcerative colitis during the course of a controlled therapeutic trial of various diets. Am J Dig Dis. 1966;11:847–57. 10.1007/bf02233941 [DOI] [PubMed] [Google Scholar]
- 10. Riley SA, Mani V, Goodman MJ, Dutt S, Herd ME. Microscopic activity in ulcerative colitis: what does it mean? Gut. 1991;32(2):174–8. 10.1136/gut.32.2.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Magro F, Doherty G, Peyrin‐Biroulet L, Svrcek M, Borralho P, Walsh A, et al. ECCO position paper: harmonization of the approach to ulcerative colitis histopathology. J Crohns Colitis. 2020;14(11):1503–11. 10.1093/ecco-jcc/jjaa110 [DOI] [PubMed] [Google Scholar]
- 12. Ma C, Sedano R, Almradi A, Vande Casteele N, Parker CE, Guizzetti L, et al. An international consensus to standardize integration of histopathology in ulcerative colitis clinical trials. Gastroenterology. 2021;160(7):2291–302. 10.1053/j.gastro.2021.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gui X, Bazarova A, Del Amor R, Vieth M, de Hertogh G, Villanacci V, et al. PICaSSO Histologic Remission Index (PHRI) in ulcerative colitis: development of a novel simplified histological score for monitoring mucosal healing and predicting clinical outcomes and its applicability in an artificial intelligence system. Gut. 2022;71(5):889–98. 10.1136/gutjnl-2021-326376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iacucci M, Parigi TL, Del Amor R, Meseguer P, Mandelli G, Bozzola A, et al. Artificial Intelligence enabled histological prediction of remission or activity and clinical outcomes in ulcerative colitis. Gastroenterology. 2023;164(7):1180–8.e2. S0016508523002160. 10.1053/j.gastro.2023.02.031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Supporting Information S2
Data Availability Statement
Data are available upon reasonable request to the corresponding author.


