Abstract
Background
Erectile dysfunction (ED) is a multifactorial medical disorder often neglected in clinical practice between elderly men, defined as the inability to achieve and/or maintain a penile erection sufficient for satisfactory sexual intercourse and a common clinical entity among men and associated with impaired quality of life and cardiovascular diseases in elderly men. The aim of this study is to evaluate the association between ED and clinical, demographic and behavioral parameters in elderly men.
Methods
A total of 2436 males aged 60 years and over who participated in the health screening between January 2008 and December 2018 were included in this study. Laboratory exams, clinical and behavior profiles were analyzed. Logistic regression models were used.
Results
Men with ED were older (65.87±5.49 vs. 63.85±4.05 years old. p<0.001), higher prevalence of physical inactivity (23.8 vs. 19%, p = 0.039) and had a higher body mass index (BMI; 28.36±4.06 vs. 27.72±3.89 kg/m2. p<0.001) than men without ED. The multivariate model shown that hypertension (p = 0.001), diabetes mellitus (p<0.001), lower urinary tract symptoms (LUTS), depressive symptoms (p<0.001) and age (p<0.001), were strongly associated with ED.
Conclusion
The main risk factors associated with ED in elderly men were hypertension, diabetes mellitus, LUTS, depressive symptoms and age.
Keywords: age, elderly, erectile dysfunction, obesity, sexual medicine, ED
Introduction
Erectile dysfunction (ED) is a multifactorial medical disorder defined as the inability to achieve and/or maintain a penile erection sufficient for satisfactory sexual intercourse1 and is a common clinical entity among men, with prevalence ranging from 8% in men 20–30 years of age to 37% in men 70–75 years of age.2 This sexual disorder often neglected in clinical practice,3 can significantly impairs quality of life1 and is associated with cardiovascular health4, 5 and risk of cardiovascular events,4 because to increase in systemic arterial damage resultant in occurrence of atherosclerosis.5, 6 Therefore, it is important to identify and reduce ED risk factors, especially the modifiable ones.7
Meta-analyses of cross-sectional population studies8 have shown that behavior and clinical factors were associated with ED and, although the results were inconsistent and somewhat inconclusive, besides most cross-sectional studies were carried out with different age groups and were not adjusted for confounding variables,8 which limits the multifactorial understanding of the relationship between ED, behavior and clinical factors in a specific population.
As age affect the perception and treatment-seeking of ED,9 knowing the epidemiology of ED in elderly men is paramount to planning prevention strategies. To the best of our knowledge, only 2 meta-analyses9,10 have investigated the association between ED and behavior, adjusting for clinical variables, in elderly men. However, Gupta et al10 analyzed data only 740 patients. Nicolosi et al11 and Moreira et al12 investigated the association between ED and various risk factors in men aged between 40–70 years. These studies used validated questionnaires to assess depressive symptoms and lower urinary tract symptoms (LUTS; CES-D and IPSS, respectively) but not to assess ED or physical activity levels, which were both evaluated with single generic questions.11,12 Akkus et al13 observed an increase in the risk of age-related ED. Being over 70 years of age (6.6% of the total sample) presented a risk 171.09 times greater (95% CI: 107.80–271.54) than adults aged between 40 and 49 years. The lack of investigation between ED and various risk factors added to non-use of validate questionnaires to analyze the outcome variables, in elderly aged over 70 years, difficult to understand the behavior of ED in this population. Therefore, the aim of this study is to evaluate the association between ED and clinical, demographic and behavioral parameters in a cohort of 2436 elderly men.
Materials and Methods
Design
This study is a single center based, and retrospective cross-sectional analysis, with primary aim to verify the association between clinical, demographic and behavioral parameters and ED in elderly Brazilian men. Clinical, demographic and behavioral data of men aged 60-years-old or older were collected from a health assessment in the Preventive Medicine Center at Hospital Israelita Albert Einstein between 2008 and 2018. The Ethics Committee of the Hospital Israelita Albert Einstein approved this study (CAAE 94867018.6.0000.0071). A waiver of informed consent was requested and granted due to the observational nature of the study, without adding risks or prejudice to the well-being of research patients. In addition, the waiver of the TCLE is justified by not offering possibilities for regular contact and follow-up. A patient data confidentiality was guaranteed due to our strict internal protocols.
Participants and Settings
Data from 44,395 male check-ups were included. Only the most recent visit was considered in individuals with duplicate data, i.e., more than one preventive medical visit. We excluded individuals with missing data ED, males less 60 years of age, individuals without sexual activity reported in the last year (evaluated with a self-assessment binary question [positive or negative]), and those with penile prostheses. Finally, data from 2436 men over or equal 60 years of age were analyzed.
Clinical Data
Age was registered according preventive medicine assessment. Weight (InBody 230 [Ottoboni®]) and height were evaluated and body mass index (BMI) was calculated. Blood pressure was measured according to the American Heart Association.14
Metabolic syndrome was defined as recommended by the World Health Organization15 and comorbidities (systemic arterial hypertension, diabetes mellitus, dyslipidemia, tobacco use, nonalcoholic fatty liver steatosis [NASH], and continuously used medications) were assessed through medical records. If data were unavailable in medical assessment, we considered presence of hypertension and diabetes when a patient self-reported hypertension or self-reported chronic use of antihypertensive medication and self-reported diabetes mellitus or self-reported chronic use of anti-diabetic medication, respectively.
Laboratory data [glycosylated hemoglobin percentage (%), a standard lipid panel (mg/dL), ultrasensitive c-reactive protein high (mg/dL) and uric acid levels (mg/dL)] were collected after an overnight fast and were standardized criteria for quality control established by the Brazilian Health Ministry.
Behavior factors were assessed using dedicated and validated questionnaires. Alcohol consumption was evaluated with Alcohol Use Disorders Identification Test (AUDIT),16 depressive symptoms with Beck Depressive Inventory (BDI),17 perceived stress with Perceived Stress Scale (PSS),18 physical activity levels with IPAQ19 and ED with IIEF-5.20
Erectile Dysfunction
For presence and levels of ED, we used IIEF-5.20 The questionnaire consists of five questions in two domains (erectile function and intercourse satisfaction) and scored on a five-point ordinal scale (1–5) where lower values represent poorer sexual function. The IIEF-5 scores were: severe (5–7), moderate (8–11), mild to moderate (12–16), mild (17–21), and no ED (22–25).
Data Analyses
Frequencies and percentages were used for categorical variables, while the means and standard deviations (SDs) were used for continuous variables. Shapiro–Wilk test was used to data normality. To compare the categorical variables, we used chi-square test. To compare numerical variables, we used Student’s t-test and the Mann–Whitney test, according to data normality. A logistic regression model was used and the IIEF-5 scores were categorized in ED presence (≤21 points, including severe, moderate, mild to moderate and mild categories) and ED absence (>21 points). A p value < 0.05 was considered significant. Adjusted odds ratios (aOR) and 95% confidence intervals (95% CIs) were computed for the logistic model results. Statistical analyses were conducted using SPSS for Windows Version 24.0 (IBM Corp, Armonk, NY, USA).
Results
Demographic and Clinical Data
We studied 2436 men aged 60 to 91 years. A 1000 of them (41.05% of the participants) reported ED. Among them, 22.3% had mild ED, 9.5% had mild to moderate ED, 4.6% had moderate ED, and 4.7% had severe ED. Table 1 presents the comparison of demographic and clinical data of study participants. Individuals with ED were older (65.87 ± 5.49 vs, 63.85 ± 4.05 years old. p<0.001) and had higher BMI (28.36 ± 4.06 vs. 27.72 ± 3.89 kg/m2. p<0.001) than individuals without ED. Glycosylated hemoglobin (6.01 ± 1.07 vs. 5.84 ± 0.77%, p<0.001) and ultrasensitive c-reactive protein (3.57 ± 9.23 vs. 2.76 ± 5.42 mg/dL, p<0.001) were significantly higher in elderly with ED was higher in elderly with ED. High uric acid and triglycerides levels were not different between the groups (p = 0.85 and 0.74, respectively) and lipid profile results were conflicting. Regarding lipid profiles, high-density lipids (46.15 ± 11.38 vs. 44.61 ± 11.49 mg/dL, p<0.001) were better in elderly without ED, but low-density lipids (105.02 ± 35.06 vs. 111.93 ± 34.62 mg/dL, p<0.001) were worse.
Table 1.
Demographic and Clinical Test Results in Relation to ED in Elderly Men (N = 2436)
| Variable | Mean ± SD | p-value | ||
|---|---|---|---|---|
| All Patients | With ED | Without ED | ||
| Age | 64.68 ± 4.80 | 65.87 ± 5.49 | 63.65 ± 4.05 | <0.001 |
| BMI (kg/m²) | 27.98 ± 3.97 | 28.36 ± 4.06 | 27.72 ± 3.89 | <0.001 |
| HDL (mg/dL) | 45.51 ± 11.45 | 44.61 ± 11.49 | 46.15 ± 11.38 | <0.001 |
| LDL (mg/dL) | 109.09 ± 34.96 | 105.02 ± 35.06 | 111.93 ± 34.62 | <0.001 |
| TG (mg/dL) | 147.62 ± 99.47 | 148.44 ± 84.20 | 147.05 ± 108.88 | 0.74 |
| UA (mg/dL) | 5.95 ±1.62 | 5.94 ± 2.06 | 5.95 ± 1.21 | 0.85 |
| HbA1c (%) | 5.92 ± 0.92 | 6.01 ± 1.07 | 5.84 ± 0.77 | <0.001 |
| Ultrasensitive c-reactive protein high (mg/dL) | 3.04 ± 7.29 | 3.57 ± 9.23 | 2.76 ± 5.42 | <0.001 |
Note: t-Student test: Mann–Whitney test.
Abbreviations: ED, erectile dysfunction; SD, standard deviation; BMI, body mass index; kg/m2, kilogram/ square meter; n, sample size; TC, total cholesterol; HDL, high-density lipids; LDL, low-density lipids; TG, triglycerides; UA, uric acid; HbA1c, glycosylated hemoglobin.
Comorbidities
Table 2 shown the relative frequencies of comorbidities in relation to ED. Elderly men with ED had significantly more hypertension (p<0.001), diabetes mellitus (p<0.001), metabolic syndrome (p = 0.005) and non-alcoholic fatty liver steatosis (p = 0.024), associated with higher cardiovascular risk. Besides, individuals with ED had a significantly higher prevalence of moderate and severe LUTS (p<0.001).
Table 2.
Relative Frequencies (%) of Comorbidities in Relation to ED in Elderly Men (N = 2436)
| Variable | Relative Frequency (%) | p-value | ||
|---|---|---|---|---|
| All Patients | With ED | Without ED | ||
| Hypertension | 51.3 | 58.2 | 46.4 | <0.001 |
| Diabetes mellitus | 20.7 | 27.8 | 15.8 | <0.001 |
| Dyslipidemia | 60.8 | 60.5 | 61.0 | 0.803 |
| Use of hypolipemiant | 39.9 | 42.1 | 38.3 | 0.060 |
| Metabolic syndrome | 18.1 | 20.7 | 16.2 | 0.005 |
| Non-alcoholic fatty liver steatosis | 56.8 | 59.5 | 54.9 | 0.024 |
| Lower urinary tract symptoms | <0.001 | |||
| Absent/mild | 78.3 | 68.5 | 85.0 | |
| Moderate | 18.7 | 26.6 | 13.2 | |
| Severe | 3.0 | 4.9 | 1.8 | |
Notes: Chi-squared test; n = sample size.
Behavioral Assessment
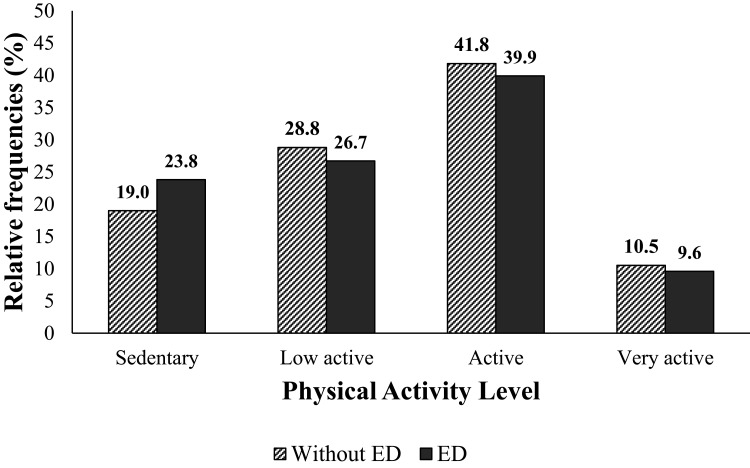
Elderly with ED had a higher prevalence of perceived stress and depressive symptoms (p<0.001 for all comparisons) but not showed differences between tobacco use and alcohol consumption (p = 0.30 and p = 0.51, respectively), as shown in Table 3. The distribution of physical activity levels in individuals with and without ED is shown in Figure 1 (p = 0.039). In total, 21% of the individuals were sedentary, 27.9% were low active, 41% were active and 10.1% were high active.
Table 3.
Relative Frequencies of Tobacco Use, Risky Alcohol Consumption, Perceived Stress and Depressive Symptoms in Relation to ED in Elderly Men (N = 2436)
| Variable | Relative Frequency (%) | p-value | |||
|---|---|---|---|---|---|
| All Patients | With ED | Without ED | |||
| Tobacco use | Never | 53.2 | 51.6 | 54.3 | 0.30 |
| Previous | 38.1 | 40.0 | 36.9 | ||
| Active | 8.7 | 8.5 | 8.9 | ||
| Alcohol consumption | Low-risk | 84.0 | 82.9 | 84.7 | 0.51 |
| Hazardous | 14.1 | 15.1 | 13.5 | ||
| Moderate-severe alcohol use disorder | 1.9 | 2.0 | 1.8 | ||
| Perceived stress | Absent | 83.3 | 79.8 | 85.9 | <0.001 |
| Present | 16.7 | 20.2 | 14.1 | ||
| Depressive symptoms | Absent | 86.1 | 79.9 | 90.4 | <0.001 |
| Present | 13.9 | 20.1 | 9.6 | ||
Notes: Chi-squared test; n = sample size.
Figure 1.
Level of Physical Activity Among Elderly with and without ED (n = 2436).
Predictors of Erectile Dysfunction
Stepwise backward multiple logistic regression was performed, analyzing only the variables associated with statistically significant risk or protection factors for ED in a full multiple logistic regression model (Supplementary Appendix 1). Finally, hypertension, diabetes mellitus, LUTS, depressive symptoms and age were strongly associated with ED, as shown in Table 4.
Table 4.
Predictors of ED in Elderly Men (N = 2436)
| Variable | OR | CI (95%) | p | |
| Hypertension | 1.39 | 1.15 | 1.69 | <0.001 |
| Diabetes mellitus | 1.82 | 1.44 | 2.30 | <0.001 |
| Lower urinary tract symptoms | ||||
| Moderate | 2.27 | 1.77 | 2.90 | <0.001 |
| Severe | 2.84 | 1.62 | 4.98 | <0.001 |
| Depressive symptoms | 2.14 | 1.62 | 2.83 | <0.001 |
| Age | 1.09 | 1.06 | 1.11 | <0.001 |
| Multiple logistic regression (Stepwise backward) | ||||
Discussion
We observed that hypertension, diabetes mellitus, LUTS, depressive symptoms and age were a strong risk of ED in elderly, after controlling for other risk factors. In contrast, we did not find an association between physical activity levels and ED in the elderly population, which differs from the results found in recent real-world cross-sectional population studies.8–10
Our results reinforce previous findings in the literature.4–9,21,22 The high prevalence of ED impairs the elderly’s quality of life21,22 and was associated with cardiovascular complications4–6 and atherosclerosis.4–9 Additionally, we observed that independent risk factors for ED were conditions that are also well-established risk factors for atherosclerosis, such as age, systemic arterial hypertension and diabetes mellitus.23
DM was strongly associated with ED in our study. We hypothesize that diabetic elderly was reported to have an earlier onset of ED that presents with greater severity and poorer response to its treatment,24 besides to worse aspects of synergistic vascular, neurological, and endocrine metabolism and decreased levels of testosterone.25
LUTS were associated with ED in our study.26 We suggest early preventive assessment and follow-up of ED, because LUTS can lead to negative sexual symptoms.
Depressive symptoms were associated with ED, as in other reports.9,21,27,28 Additionally, many anti-depressive drugs increase risk of ED, and patients with ED and depressive symptoms have less adherence to treatment for ED.21 Therefore, special attention should be given to this association when planning and assessing its treatments and also in the association that this variable has with other behavioral and clinical factors.
Aging was associated with ED as previous studies.4,9–11 Age increases the prevalence and severity of ED because decreases testosterone levels, impacting in the process of atherosclerosis, resulting to penile vascular arteriopathy, worsening arterial blood flow and to symptomatic coronary artery disease impacting sexual physical performance,29 besides that libido, sexual function, physical fitness, and mood.30 Furthermore, although ED increases with age, it is not an inevitable outcome of the aging process.
We reinforce those controlling comorbidities and promotion of healthy lifestyle habits. Although physical activity levels were not a protective factor in our study, have been a protective factor in the control of systemic arterial hypertension,31 insulin resistance and diabetes,32 LUTS33 and emotional factors.34
Our study shown several potential limitations and several strengths. First several potential limitations were not collecting data about the use of medication for ED, which could influence in the prevalence of ED and response of risk and protective factors on the disease. Lack of hormonal status, and other conditions associated with ED, such as obstructive sleep apnea presence and gastroenterological disease should be analyzed.
In addition, the sample selection could influence in the inference made in our study. Only elderly with private insurance and who participated in health check-ups were included, what prevents the generalization to the entire elderly Brazilian population.
On the other hand, first several strengths were the important analysis of ED performed in Brazil. In addition, all participants underwent a detailed, complete and validated health examination by a physician, including the assessment of prevalence and severity of ED, behavior factors, physical activity levels, which reinforces the reliability and validity of the demonstrated results. We hope that our study can be used, preventively, in clinical and public health settings. Although our study did not show a causal relationship, this could be an influence on elderly men to use ED prevention strategies and improve their lifestyle.
Conclusion
We did find an association between ED and hypertension, diabetes mellitus, LUTS, depressive symptoms and age the elderly population, however, we did find the same association between ED and physical activity levels. Although, physical activity levels are strongly associated in controlling risk factor in ED as hypertension, diabetes, LUTS and depressive symptoms.
Acknowledgments
We appreciate the Albert Einstein Hospital for their collaboration in the development of the study.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Conterence NC. Impotence: NIH consensus development panel on impotence. JAMA. 1993;270(1):83. doi: 10.1001/jama.1993.03510010089036 [DOI] [PubMed] [Google Scholar]
- 2.Rosen RC, Fisher WA, Eardley I, et al. The multinational Men’s Attitudes to Life Events and Sexuality (MALES) study: i. Prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin. 2004;20(5):607–617. doi: 10.1185/030079904125003467 [DOI] [PubMed] [Google Scholar]
- 3.De Berardis G, Franciosi M, Belfiglio M, et al. Erectile dysfunction and quality of life in type 2 diabetic patients: a serious problem too often overlooked. Diabetes Care. 2002;25:284–291. doi: 10.2337/diacare.25.2.284 [DOI] [PubMed] [Google Scholar]
- 4.Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120(2):151–157. doi: 10.1016/j.amjmed.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 5.Yannas D, Frizza F, Vignozzi L, Corona G, Maggi M, Rastrelli G. Erectile dysfunction is a hallmark of cardiovascular disease: unavoidable matter of fact or opportunity to improve men’s health? J Clin Med. 2021;10(10):2221. PMID: 34065601; PMCID: PMC8161068. doi: 10.3390/jcm10102221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294(23):2996–3002. doi: 10.1001/jama.294.23.2996 [DOI] [PubMed] [Google Scholar]
- 7.Silva AB, Sousa N, Azevedo LF, Martins C. Physical activity and exercise for erectile dysfunction: systematic review and meta-analysis. Br J Sports Med. 2017;51(19):1419–1424. doi: 10.1136/bjsports-2016-096418 [DOI] [PubMed] [Google Scholar]
- 8.Sivaratnam L, Selimin DS, Abd Ghani SR, Nawi HM, Nawi AM. Behavior-related erectile dysfunction: a systematic review and meta-analysis. J Sex Med. 2021;18(1):121–143. PMID: 33223424. doi: 10.1016/j.jsxm.2020.09.009 [DOI] [PubMed] [Google Scholar]
- 9.Cheng JYW, Ng EML, Ko JSN, Chen RYL. Physical activity and erectile dysfunction: meta-analysis of population-based studies. Int J Impot Res. 2007;19(3):245–252. doi: 10.1038/sj.ijir.3901521 [DOI] [PubMed] [Google Scholar]
- 10.Gupta BP, Murad MH, Clifton MM, Prokop L, Nehra A, Kopecky SL. The effect of lifestyle modification and cardiovascular risk factor reduction on erectile dysfunction: a systematic review and meta-analysis. Arch Intern Med. 2011;171(20):1797–1803. doi: 10.1001/archinternmed.2011.440 [DOI] [PubMed] [Google Scholar]
- 11.Nicolosi A, Moreira ED, Shirai M, Ismail Bin Mohd Tambi M, Glasser DB. Epidemiology of erectile dysfunction in four countries: cross-national study of the prevalence and correlates of erectile dysfunction. Urology. 2003;61(1):201–206. doi: 10.1016/S0090-4295(02)02102-7 [DOI] [PubMed] [Google Scholar]
- 12.Moreira ED, Lisboa Lôbo CF, Villa M, Nicolosi A, Glasser DB. Prevalence and correlates of erectile dysfunction in Salvador, northeastern Brazil: a population-based study. Int J Impot Res. 2002;14(S2):S3–S9. doi: 10.1038/sj.ijir.3900892 [DOI] [PubMed] [Google Scholar]
- 13.Akkus E, Kadioglu A, Esen A, et al. Prevalence and correlates of erectile dysfunction in Turkey: a population-based study. Eur Urol. 2002;41(3):298–304. doi: 10.1016/S0302-2838(02)00027-1 [DOI] [PubMed] [Google Scholar]
- 14.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507e520.13. doi: 10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 15.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med J Br Diabet Assoc. 1999;16(5):442–443. [DOI] [PubMed] [Google Scholar]
- 16.Machado PMA, Campelo CL, de Oliveira JVP, Batista RFL, Simões VMF, Santos AMD. Analysis of the AUDIT factor structure in adolescents between 18 and 19 years. Rev Saude Publica. 2021;55:27. doi: 10.11606/s1518-8787.2021055002777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes-Oliveira MH, Gorenstein C, Lotufo Neto F, Andrade LH, Wang YP. Validation of the Brazilian Portuguese version of the Beck Depression Inventory-II in a community sample. Rev Bras Psiquiatr Sao Paulo Braz. 2012;34(4):389–394. doi: 10.1016/j.rbp.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 18.Reis RS, Hino AAF, Añez CRR. Perceived stress scale: reliability and validity study in Brazil. J Health Psychol. 2010;15(1):107–114. doi: 10.1177/1359105309346343 [DOI] [PubMed] [Google Scholar]
- 19.Hallal PC, Simoes E, Reichert FF, et al. Validity and reliability of the telephone-administered international physical activity questionnaire in Brazil. J Phys Act Health. 2010;7(3):402–409. doi: 10.1123/jpah.7.3.402 [DOI] [PubMed] [Google Scholar]
- 20.Gonzáles AI, Sties SW, Wittkopf PG, et al. Validation of the International Index of Erectile Function (IIFE) for use in Brazil. Arq Bras Cardiol. 2013;101(2):176–182. doi: 10.5935/abc.20130141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litwin MS, Nied RJ, Dhanani N. Health-related quality of life in men with erectile dysfunction. J Gen Intern Med. 1998;13(3):159–166. doi: 10.1046/j.1525-1497.1998.00050.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller MJ, Ruof J, Graf-Morgenstern M, Porst H, Benkert O. Quality of partnership in patients with erectile dysfunction after sildenafil treatment. Pharmacopsychiatry. 2001;34(3):91–95. doi: 10.1055/s-2001-14277 [DOI] [PubMed] [Google Scholar]
- 23.Head T, Daunert S, Goldschmidt-Clermont PJ. The Aging Risk and Atherosclerosis: a Fresh Look at Arterial Homeostasis. Front Genet. 2017;8:216. doi: 10.3389/fgene.2017.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penson DF, Latini DM, Lubeck DP, et al. Do impotent men with diabetes have more severe erectile dysfunction and worse quality of life than the general population of impotent patients? Results from the Exploratory Comprehensive Evaluation of Erectile Dysfunction (ExCEED) database. Diabetes Care. 2003;26(4):1093–1099. doi: 10.2337/diacare.26.4.1093 [DOI] [PubMed] [Google Scholar]
- 25.Morano S. Pathophysiology of diabetic sexual dysfunction. J Endocrinol Invest. 2003;26(3 Suppl):65–69. [PubMed] [Google Scholar]
- 26.Rosen RC, Wei JT, Althof SE, et al. Association of sexual dysfunction with lower urinary tract symptoms of BPH and BPH medical therapies: results from the BPH Registry. Urology. 2009;73(3):562–566. doi: 10.1016/j.urology.2008.05.034 [DOI] [PubMed] [Google Scholar]
- 27.Shabsigh R, Klein LT, Seidman S, Kaplan SA, Lehrhoff BJ, Ritter JS. Increased incidence of depressive symptoms in men with erectile dysfunction. Urology. 1998;52(5):848–852. doi: 10.1016/S0090-4295(98)00292-1 [DOI] [PubMed] [Google Scholar]
- 28.Mak R, Backer GD, Kornitzer M, De Meyer JM. Prevalence and correlates of erectile dysfunction in a population-based study in Belgium. Eur Urol. 2002;41(2):132–138. doi: 10.1016/S0302-2838(01)00029-X [DOI] [PubMed] [Google Scholar]
- 29.Oberg K, Sjögren Fugl-Meyer K. On Swedish women’s distressing sexual dysfunctions: some concomitant conditions and life satisfaction. J Sex Med. 2005;2(2):169–180. doi: 10.1111/j.1743-6109.2005.20226.x [DOI] [PubMed] [Google Scholar]
- 30.Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118(4):535–546. doi: 10.1161/CIRCRESAHA.115.307611 [DOI] [PubMed] [Google Scholar]
- 31.Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2(1):e004473. PMID: 23525435; PMCID: PMC3603230. doi: 10.1161/JAHA.112.004473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu S, Cai X, Yin H, et al. Exercise training and endothelial function in patients with type 2 diabetes: a meta-analysis. Cardiovasc Diabetol. 2018;17(1):64. PMID: 29720185; PMCID: PMC5930739. doi: 10.1186/s12933-018-0711-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vrolijks RO, Notenboom-Nas FJM, de Boer D, et al. Exploring pelvic floor muscle activity in men with lower urinary tract symptoms. Neurourol Urodyn. 2020;39(2):732–737. PMID: 31899809; PMCID: PMC7027460. doi: 10.1002/nau.24267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rica RL, Shimojo GL, Gomes MC, et al. Effects of a Kinect-based physical training program on body composition, functional fitness and depression in institutionalized older adults. Geriatr Gerontol Int. 2020;20(3):195–200. PMID: 31923924. doi: 10.1111/ggi.13857 [DOI] [PubMed] [Google Scholar]